User login
Topical hemostasis agents: Some tried and true, others too new
The author reports no financial relationships relevant to this article.
- What clinical problem is this device or drug designed to address?
- Is the problem important clinically for the well-being of my patients?
- What do I know about the basic science and physiology of the problem?
- Are there data supporting improvement in clinical outcomes, or do the studies look only at surrogate endpoints?
- Has the drug or device been studied in gynecologic patients? Is it approved for use in this population?
- Is there evidence that the new technology is more effective, safer, or less expensive than products I use now?
The process of evaluation is critical for us—as advocates for individual patients and as stewards of the diminishing resources available to treat patients in the United States.
With these questions in mind, let’s look at a surgical problem for which there are many new and potentially exciting products being promoted to gynecologic surgeons: the need to achieve hemostasis.
Bleeding is a serious clinical problem during surgery—one that can have a major impact on the well-being of the patient. We generally use sutures and electrosurgical instruments—both bipolar and monopolar—to control major vessels. Topical hemostasis agents may be useful, however, in areas where generalized oozing is present, or where the application of energy may endanger vital structures.
Many products are available for use in these situations. Some are tried and true; others, new or unapproved for use in gynecologic surgery. Your assessment and selection of the optimal product should take into account 1) the cause of bleeding and 2) the mechanism of action of the product.
Surgical bleeding has one of two causes
Although the science of coagulation is complex, bleeding occurs, from a surgical perspective, because of either of two problems:
- failure to control significant arterial and venous sources
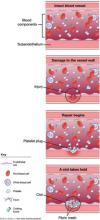
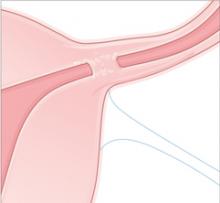
- failure of normal clotting functions, such as vasoconstriction; platelet activation and plugging ( FIGURE ); and activation of the coagulation cascade.
No topical or systemic agent adequately controls Problem #1. To address Problem #2, we have several choices to augment hemostasis, including thermal, chemical, and mechanical means. In addition, newer agents deliver additional coagulation-enhancing products such as platelets and thrombin to the operative site to supplement the patient’s natural clotting process.
FIGURE How a clot forms
Tried and true technologies
Because of long-standing experience with porcine gelatin, oxidized regenerated cellulose (ORC), and microfibrillar collagen, the Food and Drug Administration recategorized them in 2002 as Class-II devices with a clear safety profile.
Porcine gelatin
This class of products (Gelfoam, Surgifoam, Spongistan) has been around since the 1940s. The products have no intrinsic hemostatic action. They absorb 45 times their weight in blood and provide a scaff old on which platelets come into close contact, initiating the release of intrinsic and extrinsic clotting mechanisms.
Oxidized regenerated cellulose
This class (Surgicel, Oxycel) has also been around since the 1940s. The agents have acidic properties, due to their low pH level, and achieve hemostasis via denaturation of blood proteins, mechanical activation of the clotting cascade, and local vasoconstriction.
Because of its low pH, ORC is bactericidal against many common pathogens of the reproductive tract. A few studies have explored laparoscopic application of ORC to achieve hemostasis at sites of uterine perforation and for tubal bleeding secondary to sterilization.1,2 Successful hemostasis of moderate bleeding was achieved without the need for suture or conversion to laparotomy in all cases without brisk arterial bleeding.
Another ORC product (Interceed) is often used in gynecologic surgery as a barrier to adhesion formation. Its degree of oxidation, weave, and pore size differ from those of Surgicel. It requires an absolutely dry operative field to prevent adhesions.
Microfibrillar collagen
Substances derived from bovine collagen were marketed in the 1970s and 1980s. Collagen provides binding sites for platelets, which degranulate, releasing coagulation factors and initiating the clotting cascade.
Microfibrillar collagen products are available in a variety of forms (powder, sheets, loaded syringes for endoscopic placement) and are applied with pressure directly to the bleeding site. Collagen (Instat, Helistat) is supplied as a sponge, whereas microfibrillar collagen (Avitene, Superstat, Actifoam, Helitene, Hemopad, Novacol) is a tenacious powder or sheet. Microfibrillar collagen, like porcine gelatin and ORC, is absorbed by the body over time.
No studies have evaluated microfibrillar collagen in gynecologic surgery, although case reports of successful application to sites of uterine perforation after dilation and curettage and to bleeding sites after vaginal or laparoscopic hysterectomy have been published.3,4
New products are largely unproven
Class-III devices require significantly more study before safety and efficacy can be demonstrated. The two types of products presented here—topical thrombin and tissue sealants—remain largely unproven.
Topical thrombin
This class of products has been available for more than 20 years. As a liquid (Thrombogen), topical thrombin can be supplied in a syringe and sprayed onto oozing sites. A liquid combination of collagen gelatin matrix and bovine thrombin (FloSeal) provides a structure on which clots can form; triggers topical conversion of fibrinogen to fibrin; and activates the clotting cascade. It was approved for use in 1999. CoStasis and Vitagel are similar products, approved in 2000 and 2006, respectively, that add plasma obtained from the patient at the beginning of the surgical procedure.
Thrombin and ORC don’t mix. The acidity of ORC inactivates thrombin; therefore, ORC products should not be used with any product containing bovine or human thrombin.
The theoretical advantage of products that use patients’ plasma is the addition of autologous clotting factors and platelets to the bovine collagen and thrombin mixture. Preliminary studies have shown:
- a reduction in postoperative pain in 20 orthopedic surgery patients randomized to platelet gel, compared with what was seen in 20 women in the control group5
- a reduction in the rate of sternal wound infection in cardiac surgery patients (0.3% with the gel; 1.8% without it)6
- a potentially shorter healing time when platelet gel is applied to surgical wounds.7
The facts. Labeling for topical thrombin specifically states that it is not for use in cases of infection or for postpartum hemorrhage or menorrhagia. Studies of topical thrombin products have used the time to cessation of bleeding as their primary effectiveness end-point. In practical terms, however, studies have demonstrated no reduction in the need for transfusion or chest tube drainage in re-operative cardiac surgery patients.8
Disadvantages of topical thrombin include the cost of the product (including the cost of a plasma-collection device) and the need for operating room staff to collect and combine the product for use. Topical thrombin also exposes the patient to the risk of antibody formation (see Bovine thrombin can trigger risky antibodies), catastrophic bleeding, and, even, death.
Products that contain bovine thrombin have some safety issues with regard to their antigenic reactivity. Patients may develop antibodies to the bovine product that cross-react with human thrombin and factor Va. Associated with all products that contain bovine thrombin is a black box warning that states that the product may be associated with severe bleeding, thrombosis, and, rarely, death, because of antibody formation.
In one case report, a very complicated patient who required systemic anticoagulation for a mechanical aortic valve underwent hysterectomy, with topical thrombin administered at the end of the procedure in an effort to avert postoperative hemorrhage.1 She developed antibodies to the bovine thrombin, which caused significant and severe coagulation defects.
No clinical studies have assessed these products in gynecologic surgery.
Reference
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
This last set of products has been approved for use in cardiopulmonary bypass procedures, in patients who have splenic injury, and to close a colostomy. They are “tissue glues” that also have hemostatic properties.
Tisseel is a combination of human thrombin, human “sealer protein” (fibrinogen), and aprotinin, a synthetic inhibitor of fibrinolysis that prevents premature degradation of a clot once it has formed. In clinical studies, this product has reduced the need for splenectomy in patients who have bleeding that is difficult to control.
Disadvantages of tissue-sealing products. These products have not been studied in gynecologic patients. They have the significant disadvantage of containing products derived from pooled human plasma. Although precautions have been taken to reduce transmission of infectious disease, viral transmission may occur. Anaphylaxis is an additional risk.
Many products are available to help the ObGyn surgeon achieve hemostasis in tough situations. Most of the time, we face generalized oozing after treatment of extensive endometriosis or adhesiolysis; in these cases, older topical agents should serve us well. Patients who experience massive bleeding are not likely to benefit from the use of any of the products described in this article.
Extensive bleeding from uterine incisions—at cesarean section or after myomectomy—might respond to topical thrombin, platelet gel products, or tissue sealants, but these products have not been studied in our patients. They also are expensive and carry some risk for our patients.
Don’t overlook two strategies for extremely high-risk situations:
- Cell-saver technology can help avert transfusion in patients expected to lose a substantial amount of blood
- Intravenous recombinant activated factor VII (NovoSeven) can be life-saving for women who experience postpartum hemorrhage, placenta percreta, or retroperitoneal sarcoma and for whom our standard strategies have failed.—BARBARA S. LEVY, MD
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
2. Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82:221-222.
3. Borten M, Friedman EA. Translaparoscopic hemostasis with microfibrillar collagen in lieu of laparotomy. A report of two cases. J Reprod Med. 1983;28:804-806.
4. Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8:235-238.
5. Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177-182.
6. Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381-386.
7. Hom DB, Linzie MB, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174-183.
8. Wajon P, Gibson J, Calcroft R, Hughes C, Thrift B. Intraoperative plateletpheresis and autologous platelet gel do not reduce chest tube drainage or allogeneic blood transfusion after reoperative coronary artery bypass graft. Anesth Analg. 2001;93:536-542.
The author reports no financial relationships relevant to this article.
- What clinical problem is this device or drug designed to address?
- Is the problem important clinically for the well-being of my patients?
- What do I know about the basic science and physiology of the problem?
- Are there data supporting improvement in clinical outcomes, or do the studies look only at surrogate endpoints?
- Has the drug or device been studied in gynecologic patients? Is it approved for use in this population?
- Is there evidence that the new technology is more effective, safer, or less expensive than products I use now?
The process of evaluation is critical for us—as advocates for individual patients and as stewards of the diminishing resources available to treat patients in the United States.
With these questions in mind, let’s look at a surgical problem for which there are many new and potentially exciting products being promoted to gynecologic surgeons: the need to achieve hemostasis.
Bleeding is a serious clinical problem during surgery—one that can have a major impact on the well-being of the patient. We generally use sutures and electrosurgical instruments—both bipolar and monopolar—to control major vessels. Topical hemostasis agents may be useful, however, in areas where generalized oozing is present, or where the application of energy may endanger vital structures.
Many products are available for use in these situations. Some are tried and true; others, new or unapproved for use in gynecologic surgery. Your assessment and selection of the optimal product should take into account 1) the cause of bleeding and 2) the mechanism of action of the product.
Surgical bleeding has one of two causes
Although the science of coagulation is complex, bleeding occurs, from a surgical perspective, because of either of two problems:
- failure to control significant arterial and venous sources
- failure of normal clotting functions, such as vasoconstriction; platelet activation and plugging ( FIGURE ); and activation of the coagulation cascade.
No topical or systemic agent adequately controls Problem #1. To address Problem #2, we have several choices to augment hemostasis, including thermal, chemical, and mechanical means. In addition, newer agents deliver additional coagulation-enhancing products such as platelets and thrombin to the operative site to supplement the patient’s natural clotting process.
FIGURE How a clot forms
Tried and true technologies
Because of long-standing experience with porcine gelatin, oxidized regenerated cellulose (ORC), and microfibrillar collagen, the Food and Drug Administration recategorized them in 2002 as Class-II devices with a clear safety profile.
Porcine gelatin
This class of products (Gelfoam, Surgifoam, Spongistan) has been around since the 1940s. The products have no intrinsic hemostatic action. They absorb 45 times their weight in blood and provide a scaff old on which platelets come into close contact, initiating the release of intrinsic and extrinsic clotting mechanisms.
Oxidized regenerated cellulose
This class (Surgicel, Oxycel) has also been around since the 1940s. The agents have acidic properties, due to their low pH level, and achieve hemostasis via denaturation of blood proteins, mechanical activation of the clotting cascade, and local vasoconstriction.
Because of its low pH, ORC is bactericidal against many common pathogens of the reproductive tract. A few studies have explored laparoscopic application of ORC to achieve hemostasis at sites of uterine perforation and for tubal bleeding secondary to sterilization.1,2 Successful hemostasis of moderate bleeding was achieved without the need for suture or conversion to laparotomy in all cases without brisk arterial bleeding.
Another ORC product (Interceed) is often used in gynecologic surgery as a barrier to adhesion formation. Its degree of oxidation, weave, and pore size differ from those of Surgicel. It requires an absolutely dry operative field to prevent adhesions.
Microfibrillar collagen
Substances derived from bovine collagen were marketed in the 1970s and 1980s. Collagen provides binding sites for platelets, which degranulate, releasing coagulation factors and initiating the clotting cascade.
Microfibrillar collagen products are available in a variety of forms (powder, sheets, loaded syringes for endoscopic placement) and are applied with pressure directly to the bleeding site. Collagen (Instat, Helistat) is supplied as a sponge, whereas microfibrillar collagen (Avitene, Superstat, Actifoam, Helitene, Hemopad, Novacol) is a tenacious powder or sheet. Microfibrillar collagen, like porcine gelatin and ORC, is absorbed by the body over time.
No studies have evaluated microfibrillar collagen in gynecologic surgery, although case reports of successful application to sites of uterine perforation after dilation and curettage and to bleeding sites after vaginal or laparoscopic hysterectomy have been published.3,4
New products are largely unproven
Class-III devices require significantly more study before safety and efficacy can be demonstrated. The two types of products presented here—topical thrombin and tissue sealants—remain largely unproven.
Topical thrombin
This class of products has been available for more than 20 years. As a liquid (Thrombogen), topical thrombin can be supplied in a syringe and sprayed onto oozing sites. A liquid combination of collagen gelatin matrix and bovine thrombin (FloSeal) provides a structure on which clots can form; triggers topical conversion of fibrinogen to fibrin; and activates the clotting cascade. It was approved for use in 1999. CoStasis and Vitagel are similar products, approved in 2000 and 2006, respectively, that add plasma obtained from the patient at the beginning of the surgical procedure.
Thrombin and ORC don’t mix. The acidity of ORC inactivates thrombin; therefore, ORC products should not be used with any product containing bovine or human thrombin.
The theoretical advantage of products that use patients’ plasma is the addition of autologous clotting factors and platelets to the bovine collagen and thrombin mixture. Preliminary studies have shown:
- a reduction in postoperative pain in 20 orthopedic surgery patients randomized to platelet gel, compared with what was seen in 20 women in the control group5
- a reduction in the rate of sternal wound infection in cardiac surgery patients (0.3% with the gel; 1.8% without it)6
- a potentially shorter healing time when platelet gel is applied to surgical wounds.7
The facts. Labeling for topical thrombin specifically states that it is not for use in cases of infection or for postpartum hemorrhage or menorrhagia. Studies of topical thrombin products have used the time to cessation of bleeding as their primary effectiveness end-point. In practical terms, however, studies have demonstrated no reduction in the need for transfusion or chest tube drainage in re-operative cardiac surgery patients.8
Disadvantages of topical thrombin include the cost of the product (including the cost of a plasma-collection device) and the need for operating room staff to collect and combine the product for use. Topical thrombin also exposes the patient to the risk of antibody formation (see Bovine thrombin can trigger risky antibodies), catastrophic bleeding, and, even, death.
Products that contain bovine thrombin have some safety issues with regard to their antigenic reactivity. Patients may develop antibodies to the bovine product that cross-react with human thrombin and factor Va. Associated with all products that contain bovine thrombin is a black box warning that states that the product may be associated with severe bleeding, thrombosis, and, rarely, death, because of antibody formation.
In one case report, a very complicated patient who required systemic anticoagulation for a mechanical aortic valve underwent hysterectomy, with topical thrombin administered at the end of the procedure in an effort to avert postoperative hemorrhage.1 She developed antibodies to the bovine thrombin, which caused significant and severe coagulation defects.
No clinical studies have assessed these products in gynecologic surgery.
Reference
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
This last set of products has been approved for use in cardiopulmonary bypass procedures, in patients who have splenic injury, and to close a colostomy. They are “tissue glues” that also have hemostatic properties.
Tisseel is a combination of human thrombin, human “sealer protein” (fibrinogen), and aprotinin, a synthetic inhibitor of fibrinolysis that prevents premature degradation of a clot once it has formed. In clinical studies, this product has reduced the need for splenectomy in patients who have bleeding that is difficult to control.
Disadvantages of tissue-sealing products. These products have not been studied in gynecologic patients. They have the significant disadvantage of containing products derived from pooled human plasma. Although precautions have been taken to reduce transmission of infectious disease, viral transmission may occur. Anaphylaxis is an additional risk.
Many products are available to help the ObGyn surgeon achieve hemostasis in tough situations. Most of the time, we face generalized oozing after treatment of extensive endometriosis or adhesiolysis; in these cases, older topical agents should serve us well. Patients who experience massive bleeding are not likely to benefit from the use of any of the products described in this article.
Extensive bleeding from uterine incisions—at cesarean section or after myomectomy—might respond to topical thrombin, platelet gel products, or tissue sealants, but these products have not been studied in our patients. They also are expensive and carry some risk for our patients.
Don’t overlook two strategies for extremely high-risk situations:
- Cell-saver technology can help avert transfusion in patients expected to lose a substantial amount of blood
- Intravenous recombinant activated factor VII (NovoSeven) can be life-saving for women who experience postpartum hemorrhage, placenta percreta, or retroperitoneal sarcoma and for whom our standard strategies have failed.—BARBARA S. LEVY, MD
The author reports no financial relationships relevant to this article.
- What clinical problem is this device or drug designed to address?
- Is the problem important clinically for the well-being of my patients?
- What do I know about the basic science and physiology of the problem?
- Are there data supporting improvement in clinical outcomes, or do the studies look only at surrogate endpoints?
- Has the drug or device been studied in gynecologic patients? Is it approved for use in this population?
- Is there evidence that the new technology is more effective, safer, or less expensive than products I use now?
The process of evaluation is critical for us—as advocates for individual patients and as stewards of the diminishing resources available to treat patients in the United States.
With these questions in mind, let’s look at a surgical problem for which there are many new and potentially exciting products being promoted to gynecologic surgeons: the need to achieve hemostasis.
Bleeding is a serious clinical problem during surgery—one that can have a major impact on the well-being of the patient. We generally use sutures and electrosurgical instruments—both bipolar and monopolar—to control major vessels. Topical hemostasis agents may be useful, however, in areas where generalized oozing is present, or where the application of energy may endanger vital structures.
Many products are available for use in these situations. Some are tried and true; others, new or unapproved for use in gynecologic surgery. Your assessment and selection of the optimal product should take into account 1) the cause of bleeding and 2) the mechanism of action of the product.
Surgical bleeding has one of two causes
Although the science of coagulation is complex, bleeding occurs, from a surgical perspective, because of either of two problems:
- failure to control significant arterial and venous sources
- failure of normal clotting functions, such as vasoconstriction; platelet activation and plugging ( FIGURE ); and activation of the coagulation cascade.
No topical or systemic agent adequately controls Problem #1. To address Problem #2, we have several choices to augment hemostasis, including thermal, chemical, and mechanical means. In addition, newer agents deliver additional coagulation-enhancing products such as platelets and thrombin to the operative site to supplement the patient’s natural clotting process.
FIGURE How a clot forms
Tried and true technologies
Because of long-standing experience with porcine gelatin, oxidized regenerated cellulose (ORC), and microfibrillar collagen, the Food and Drug Administration recategorized them in 2002 as Class-II devices with a clear safety profile.
Porcine gelatin
This class of products (Gelfoam, Surgifoam, Spongistan) has been around since the 1940s. The products have no intrinsic hemostatic action. They absorb 45 times their weight in blood and provide a scaff old on which platelets come into close contact, initiating the release of intrinsic and extrinsic clotting mechanisms.
Oxidized regenerated cellulose
This class (Surgicel, Oxycel) has also been around since the 1940s. The agents have acidic properties, due to their low pH level, and achieve hemostasis via denaturation of blood proteins, mechanical activation of the clotting cascade, and local vasoconstriction.
Because of its low pH, ORC is bactericidal against many common pathogens of the reproductive tract. A few studies have explored laparoscopic application of ORC to achieve hemostasis at sites of uterine perforation and for tubal bleeding secondary to sterilization.1,2 Successful hemostasis of moderate bleeding was achieved without the need for suture or conversion to laparotomy in all cases without brisk arterial bleeding.
Another ORC product (Interceed) is often used in gynecologic surgery as a barrier to adhesion formation. Its degree of oxidation, weave, and pore size differ from those of Surgicel. It requires an absolutely dry operative field to prevent adhesions.
Microfibrillar collagen
Substances derived from bovine collagen were marketed in the 1970s and 1980s. Collagen provides binding sites for platelets, which degranulate, releasing coagulation factors and initiating the clotting cascade.
Microfibrillar collagen products are available in a variety of forms (powder, sheets, loaded syringes for endoscopic placement) and are applied with pressure directly to the bleeding site. Collagen (Instat, Helistat) is supplied as a sponge, whereas microfibrillar collagen (Avitene, Superstat, Actifoam, Helitene, Hemopad, Novacol) is a tenacious powder or sheet. Microfibrillar collagen, like porcine gelatin and ORC, is absorbed by the body over time.
No studies have evaluated microfibrillar collagen in gynecologic surgery, although case reports of successful application to sites of uterine perforation after dilation and curettage and to bleeding sites after vaginal or laparoscopic hysterectomy have been published.3,4
New products are largely unproven
Class-III devices require significantly more study before safety and efficacy can be demonstrated. The two types of products presented here—topical thrombin and tissue sealants—remain largely unproven.
Topical thrombin
This class of products has been available for more than 20 years. As a liquid (Thrombogen), topical thrombin can be supplied in a syringe and sprayed onto oozing sites. A liquid combination of collagen gelatin matrix and bovine thrombin (FloSeal) provides a structure on which clots can form; triggers topical conversion of fibrinogen to fibrin; and activates the clotting cascade. It was approved for use in 1999. CoStasis and Vitagel are similar products, approved in 2000 and 2006, respectively, that add plasma obtained from the patient at the beginning of the surgical procedure.
Thrombin and ORC don’t mix. The acidity of ORC inactivates thrombin; therefore, ORC products should not be used with any product containing bovine or human thrombin.
The theoretical advantage of products that use patients’ plasma is the addition of autologous clotting factors and platelets to the bovine collagen and thrombin mixture. Preliminary studies have shown:
- a reduction in postoperative pain in 20 orthopedic surgery patients randomized to platelet gel, compared with what was seen in 20 women in the control group5
- a reduction in the rate of sternal wound infection in cardiac surgery patients (0.3% with the gel; 1.8% without it)6
- a potentially shorter healing time when platelet gel is applied to surgical wounds.7
The facts. Labeling for topical thrombin specifically states that it is not for use in cases of infection or for postpartum hemorrhage or menorrhagia. Studies of topical thrombin products have used the time to cessation of bleeding as their primary effectiveness end-point. In practical terms, however, studies have demonstrated no reduction in the need for transfusion or chest tube drainage in re-operative cardiac surgery patients.8
Disadvantages of topical thrombin include the cost of the product (including the cost of a plasma-collection device) and the need for operating room staff to collect and combine the product for use. Topical thrombin also exposes the patient to the risk of antibody formation (see Bovine thrombin can trigger risky antibodies), catastrophic bleeding, and, even, death.
Products that contain bovine thrombin have some safety issues with regard to their antigenic reactivity. Patients may develop antibodies to the bovine product that cross-react with human thrombin and factor Va. Associated with all products that contain bovine thrombin is a black box warning that states that the product may be associated with severe bleeding, thrombosis, and, rarely, death, because of antibody formation.
In one case report, a very complicated patient who required systemic anticoagulation for a mechanical aortic valve underwent hysterectomy, with topical thrombin administered at the end of the procedure in an effort to avert postoperative hemorrhage.1 She developed antibodies to the bovine thrombin, which caused significant and severe coagulation defects.
No clinical studies have assessed these products in gynecologic surgery.
Reference
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
This last set of products has been approved for use in cardiopulmonary bypass procedures, in patients who have splenic injury, and to close a colostomy. They are “tissue glues” that also have hemostatic properties.
Tisseel is a combination of human thrombin, human “sealer protein” (fibrinogen), and aprotinin, a synthetic inhibitor of fibrinolysis that prevents premature degradation of a clot once it has formed. In clinical studies, this product has reduced the need for splenectomy in patients who have bleeding that is difficult to control.
Disadvantages of tissue-sealing products. These products have not been studied in gynecologic patients. They have the significant disadvantage of containing products derived from pooled human plasma. Although precautions have been taken to reduce transmission of infectious disease, viral transmission may occur. Anaphylaxis is an additional risk.
Many products are available to help the ObGyn surgeon achieve hemostasis in tough situations. Most of the time, we face generalized oozing after treatment of extensive endometriosis or adhesiolysis; in these cases, older topical agents should serve us well. Patients who experience massive bleeding are not likely to benefit from the use of any of the products described in this article.
Extensive bleeding from uterine incisions—at cesarean section or after myomectomy—might respond to topical thrombin, platelet gel products, or tissue sealants, but these products have not been studied in our patients. They also are expensive and carry some risk for our patients.
Don’t overlook two strategies for extremely high-risk situations:
- Cell-saver technology can help avert transfusion in patients expected to lose a substantial amount of blood
- Intravenous recombinant activated factor VII (NovoSeven) can be life-saving for women who experience postpartum hemorrhage, placenta percreta, or retroperitoneal sarcoma and for whom our standard strategies have failed.—BARBARA S. LEVY, MD
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
2. Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82:221-222.
3. Borten M, Friedman EA. Translaparoscopic hemostasis with microfibrillar collagen in lieu of laparotomy. A report of two cases. J Reprod Med. 1983;28:804-806.
4. Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8:235-238.
5. Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177-182.
6. Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381-386.
7. Hom DB, Linzie MB, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174-183.
8. Wajon P, Gibson J, Calcroft R, Hughes C, Thrift B. Intraoperative plateletpheresis and autologous platelet gel do not reduce chest tube drainage or allogeneic blood transfusion after reoperative coronary artery bypass graft. Anesth Analg. 2001;93:536-542.
1. Sharma JB, Malhotra M, Pundir P. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83:271-275.
2. Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82:221-222.
3. Borten M, Friedman EA. Translaparoscopic hemostasis with microfibrillar collagen in lieu of laparotomy. A report of two cases. J Reprod Med. 1983;28:804-806.
4. Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8:235-238.
5. Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177-182.
6. Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381-386.
7. Hom DB, Linzie MB, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174-183.
8. Wajon P, Gibson J, Calcroft R, Hughes C, Thrift B. Intraoperative plateletpheresis and autologous platelet gel do not reduce chest tube drainage or allogeneic blood transfusion after reoperative coronary artery bypass graft. Anesth Analg. 2001;93:536-542.
The minimally invasive 21st Century, at your fingertips
Sunday morning, and I am sitting at the computer writing this “Editorial” and trying to juggle my tasks for the conclusion of the year—tasks that include over 25,000 miles’ worth of travel to meetings and other events! One of those trips will take me across the country to the “other Washington” for AAGL’s 36th Global Congress of Minimally Invasive Gynecology, November 13 to 17. Although I’m obliged, as presenter and past-president of AAGL, to travel to the congress, I’m pleased to tell you that an exciting collaboration between AAGL and OBG Management will make it possible for you to access key congress events—scientific presentations, industry symposia, expert debates, live telesurgery, surgical tutorials, and even the exhibit hall—from home or office!
The “full flavor” of the congress
Attending the AAGL Congress has always been an adventure for me. What I learn there challenges my thinking about minimally invasive surgical management and pushes me to evaluate new approaches to optimal care. Through the AAGL-OBG Management collaboration, much of that intellectual challenge will be available to surgeons whenever they can get to a computer.
Entering the congress highlights via www.aagl.org, www.aaglcongress.com, or www.obgmanagement.com, you will find an environment where some of the latest tools and tricks displayed at the congress can be viewed. There will be audio and video news coverage of key events from the meeting floor, podcasts, webcasts, and the text of talks with PowerPoint presentations. This trove will give you the full flavor of the meeting.
Keep in mind that I don’t mean “press” coverage or interpretation of activities; rather, these are presentations that have been vetted by AAGL’s scientific program and CME committees. The material will be referenced and organized so that you can focus your attention on subjects of everyday interest and utility.
One plus one is…three, or greater
Both AAGL and OBG Management have been leaders in promoting innovation in gynecologic surgery and providing ObGyns with evidence-based, peer-reviewed scientific papers to help inform our clinical practices. Their collaboration is truly cutting edge: It combines the expertise and integrity of AAGL in teaching minimally invasive gynecology with OBG Management’s technology and expertise in information handling and presentation. The outcome? We’ll be able to learn, more efficiently and more effectively, the most innovative treatments.
To those of you who will attend AAGL’s Global Congress—I look forward to seeing you. To those who cannot be there—welcome to the technology that makes a virtual meeting possible!
Sunday morning, and I am sitting at the computer writing this “Editorial” and trying to juggle my tasks for the conclusion of the year—tasks that include over 25,000 miles’ worth of travel to meetings and other events! One of those trips will take me across the country to the “other Washington” for AAGL’s 36th Global Congress of Minimally Invasive Gynecology, November 13 to 17. Although I’m obliged, as presenter and past-president of AAGL, to travel to the congress, I’m pleased to tell you that an exciting collaboration between AAGL and OBG Management will make it possible for you to access key congress events—scientific presentations, industry symposia, expert debates, live telesurgery, surgical tutorials, and even the exhibit hall—from home or office!
The “full flavor” of the congress
Attending the AAGL Congress has always been an adventure for me. What I learn there challenges my thinking about minimally invasive surgical management and pushes me to evaluate new approaches to optimal care. Through the AAGL-OBG Management collaboration, much of that intellectual challenge will be available to surgeons whenever they can get to a computer.
Entering the congress highlights via www.aagl.org, www.aaglcongress.com, or www.obgmanagement.com, you will find an environment where some of the latest tools and tricks displayed at the congress can be viewed. There will be audio and video news coverage of key events from the meeting floor, podcasts, webcasts, and the text of talks with PowerPoint presentations. This trove will give you the full flavor of the meeting.
Keep in mind that I don’t mean “press” coverage or interpretation of activities; rather, these are presentations that have been vetted by AAGL’s scientific program and CME committees. The material will be referenced and organized so that you can focus your attention on subjects of everyday interest and utility.
One plus one is…three, or greater
Both AAGL and OBG Management have been leaders in promoting innovation in gynecologic surgery and providing ObGyns with evidence-based, peer-reviewed scientific papers to help inform our clinical practices. Their collaboration is truly cutting edge: It combines the expertise and integrity of AAGL in teaching minimally invasive gynecology with OBG Management’s technology and expertise in information handling and presentation. The outcome? We’ll be able to learn, more efficiently and more effectively, the most innovative treatments.
To those of you who will attend AAGL’s Global Congress—I look forward to seeing you. To those who cannot be there—welcome to the technology that makes a virtual meeting possible!
Sunday morning, and I am sitting at the computer writing this “Editorial” and trying to juggle my tasks for the conclusion of the year—tasks that include over 25,000 miles’ worth of travel to meetings and other events! One of those trips will take me across the country to the “other Washington” for AAGL’s 36th Global Congress of Minimally Invasive Gynecology, November 13 to 17. Although I’m obliged, as presenter and past-president of AAGL, to travel to the congress, I’m pleased to tell you that an exciting collaboration between AAGL and OBG Management will make it possible for you to access key congress events—scientific presentations, industry symposia, expert debates, live telesurgery, surgical tutorials, and even the exhibit hall—from home or office!
The “full flavor” of the congress
Attending the AAGL Congress has always been an adventure for me. What I learn there challenges my thinking about minimally invasive surgical management and pushes me to evaluate new approaches to optimal care. Through the AAGL-OBG Management collaboration, much of that intellectual challenge will be available to surgeons whenever they can get to a computer.
Entering the congress highlights via www.aagl.org, www.aaglcongress.com, or www.obgmanagement.com, you will find an environment where some of the latest tools and tricks displayed at the congress can be viewed. There will be audio and video news coverage of key events from the meeting floor, podcasts, webcasts, and the text of talks with PowerPoint presentations. This trove will give you the full flavor of the meeting.
Keep in mind that I don’t mean “press” coverage or interpretation of activities; rather, these are presentations that have been vetted by AAGL’s scientific program and CME committees. The material will be referenced and organized so that you can focus your attention on subjects of everyday interest and utility.
One plus one is…three, or greater
Both AAGL and OBG Management have been leaders in promoting innovation in gynecologic surgery and providing ObGyns with evidence-based, peer-reviewed scientific papers to help inform our clinical practices. Their collaboration is truly cutting edge: It combines the expertise and integrity of AAGL in teaching minimally invasive gynecology with OBG Management’s technology and expertise in information handling and presentation. The outcome? We’ll be able to learn, more efficiently and more effectively, the most innovative treatments.
To those of you who will attend AAGL’s Global Congress—I look forward to seeing you. To those who cannot be there—welcome to the technology that makes a virtual meeting possible!
TECHNOLOGY
Over its history, surgery has been defined by the tools available to practitioners. In our era, opportunities to offer patients minimally invasive surgery have expanded dramatically as methods of establishing visualization, achieving hemostasis, and performing tissue dissection have improved. (I remember trying to treat ectopic pregnancy laparoscopically in the early 1980s without benefit of a camera or suction irrigator!)
For surgeons of my generation, the ability to access the abdominal cavity minimally invasively and to clearly visualize the contents was a significant step forward. Hysteroscopic myomectomy was another tremendous incremental improvement for patients with submucous myomas. But there is much more in store for the coming years.
Where are we headed in the next wave of gynecologic surgery? Will patients require an incision at all? Is there room to advance beyond laparoscopy and hysteroscopy? What innovations will industry offer us in the 21st century?
In this article, I describe something that is fairly familiar to most of us by now, but which is not yet practical for routine gynecologic procedures—robotically assisted endoscopic surgery. I then move on to a phenomenon that, in many respects, is still being imagined—natural orifice transluminal endoscopic surgery, or NOTES.
Robotic systems are best suited for complex surgery
Laparoscopic surgery is limited by the two-dimensional view and need for hand control of long, rigid instruments through ancillary trocar sites. Although these impediments can be overcome with practice and experience, the inability to see in three dimensions and the compromised range of motion hamper optimal management of some surgical procedures.
A number of technological advances may significantly improve our ability to perform suture-intensive or anatomically challenging operations. Several companies are developing camera systems that will permit a three-dimensional view without the need for multiple visual ports. The technology is borrowed from the world of insects, which “see” through multiple lenses within the same eye. The application of such visual processing to optical systems for endoscopic surgery will be a huge advance for laparoscopy—one that is still being perfected by industry. In 2007, the da Vinci robot system (Intuitive Surgical) offers the best opportunity to achieve both three-dimensional visualization and an ability to “feel” tissue and manipulate instruments with markedly increased range of motion.
Cost is the limiting factor
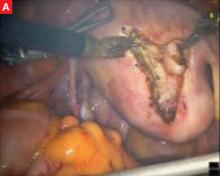
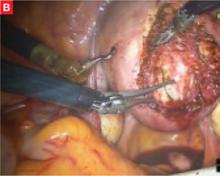
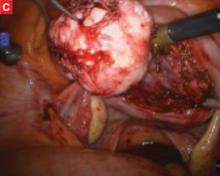
Although the da Vinci system has revolutionized the practice of urology, enabling radical nerve-sparing prostatectomy, its utility in gynecology is still being investigated. Several centers use the robot for a significant percentage of their laparoscopic gynecologic surgery, but the setup time, learning curve, and intraoperative time required make the da Vinci system an impractical tool for many routine procedures. Its true advantage lies in suture-intensive procedures and in surgeries that require meticulous dissection close to major structures. In gynecology, the laparoscopic procedures most likely to benefit from the three-dimensional view and articulating instruments are sacral colpopexy, myomectomy (FIGURE), radical hysterectomy, and lymph node dissection.
Although it is interesting and enjoyable to use robotic technology for routine laparoscopic procedures, I believe the cost is prohibitive—several million dollars for each robot. If the financial barriers are removed, however, this system will be a welcome addition to the toolset for gynecologic laparoscopic surgery. Until then, we need to make intelligent use of this powerful tool.
FIGURE Robotic myomectomy
A: Using the da Vinci robot, the surgeon incises the myometrium down to the fibroid.
B: After grasping the fibroid, the surgeon dissects it away from the surrounding myometrium.
C: As the fibroid is freed, another small tumor becomes apparent at the bottom right, and is also removed.
D: The myometrium is sutured in layers after removal of the fibroids. Photos courtesy of Paul Indman, MD.Just as many of us were able to perform laparoscopic surgery without a three-chip camera and high-tech energy system for hemostasis until the cost of those technologies could be recouped in reduced operating room time and fewer conversions to laparotomy, so will today’s surgeons have to continue performing laparoscopic adnexal surgery, routine hysterectomy, and treatment of ectopic pregnancy the “old-fashioned” way. For complex procedures, however, the da Vinci system is proving to be a major advance in endoscopic surgery.
Look for other, perhaps less expensive, technologies coming down the road that will, at the very least, permit three-dimensional visualization without the need for robotics. In addition, as I discuss in the next section, miniaturization of robotics is on the horizon. Only our imagination limits our thinking about how robotic technology may be used in the not-too-distant future.
ROBOTICS IN GYNECOLOGY
Selected studies
- Bocca S, Stadtmauer L, Oehninger S. Uncomplicated fullterm pregnancy after da Vinci-assisted laparoscopic myomectomy. Reprod Biomed Online. 2007;14:246–249.
- Elliott DS, Chow GK, Gettman M. Current status of robotics in female urology and gynecology. World J Urol. 2006;24:188–192.
- Fiorentino RP, Zepeda MA, Goldstein BH, John CR, Rettenmaier MA. Pilot study assessing robotic laparoscopic hysterectomy and patient outcomes. J Minim Invasive Gynecol. 2006;13:60–63.
- Magrina JF. Robotic surgery in gynecology. Eur J Gynaecol Oncol. 2007;28:77–82.
NOTES takes “minimally invasive” to a new level
Imagine performing surgery for ectopic pregnancy or endometriosis in your office, without anesthesia. Think this is impossible? Think again!
A newer, perhaps better, and definitely less invasive version of endoscopic surgery is on the horizon—natural orifice transluminal endoscopic surgery, or NOTES. In May, a surgeon in Portland, Oregon, performed a cholecystectomy by dropping an endoscope through the patient’s mouth into the stomach, drilling an opening in the gastric wall, and placing small instruments through that opening to perform the surgery. The specimen was then pulled through the small opening in the stomach and retrieved through the patient’s mouth! The stomach was closed with an endoscopic stapling device.
NOTES appears to be the next true advance in minimally invasive surgery. This should come as no surprise to gynecologists. We are the champions of transcervical and transvaginal surgery. General surgeons and gastroenterologists are recognizing what we have long known—that operating through these natural orifices is less uncomfortable for the patient and provides faster, less complicated recovery. They are also recognizing the challenges involved in such an approach.
A new generation of instruments is in the works
Clearly, operating through the vagina, cervix, or stomach necessitates excellent visualization and instruments flexible enough to navigate through tiny openings but strong enough to transect and retrieve tissue. Many of our industry partners are working diligently to create and perfect new instrumentation for NOTES procedures, and research is under way at many centers in this country and overseas into transgastric and transrectal procedures.
Consider what we might be able to achieve with this technology! By eliminating the need for transabdominal access, we can vastly reduce the risk of intestinal and major vessel injury and eliminate the risk of hernia. We can also markedly reduce the discomfort associated with abdominal incisions.
How might this technology be applied in gynecology? I anticipate that ovarian pathology, endometriosis, and ectopic pregnancy will be managed transvaginally or via a small opening in the uterus. Transvaginal hydrolaparoscopy—in which warm saline is used as the distention medium instead of carbon dioxide, and access to the pelvis is achieved through a small culpotomy—has been around for many years but is limited by the rigid instrumentation and restricted visualization now available. With flexible instruments that can “see” around corners yet provide a wide visual field, microrobots that can be placed through a tiny opening and then deployed to accomplish the surgical task, and systems to achieve hemostasis, NOTES may be the next revolution in gynecologic surgery.
Still in a very early stage of development, natural orifice transluminal endoscopic surgery (NOTES) has generated considerable enthusiasm among physicians leading research and development efforts. Hoping to steer these efforts in a responsible direction—and avoid the problems encountered during the early days of laparoscopic surgery, when many inexperienced practitioners began adopting the technique prematurely—a working group from the American Society of Gastrointestinal Endoscopy and the Society of American Gastrointestinal and Endoscopic Surgeons was formed in 2005, calling itself the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR). So far, this group has convened two international conferences and penned two white papers, noting that “the overwhelming sense [at the first international conference]…was that NOTES will develop into a mainstream clinical capability in the near future.”1
Some of the needs NOSCAR has identified are:
- determining the optimal technique and site to achieve access to the peritoneal cavity
- developing a gastric closure method that is 100% reliable
- reducing the risk of intraperitoneal contamination and infection, given the transgastric route that has dominated NOTES so far
- developing the ability to suture
- maintaining spatial orientation during surgery, as well as a multitasking platform that would allow manipulation of tissue, clear visualization, and safe access
- preventing intraperitoneal complications such as bleeding and bowel perforation
- exploring the physiology of pneumoperitoneum in the setting of NOTES
- establishing guidelines for training physicians and reporting both positive and negative outcomes.
In the meantime, NOSCAR recommends that all NOTES procedures in humans be approved by the Institutional Review Board and reported to a registry.
So far, the technology has been used to perform appendectomy and cholecystectomy in humans. Research grants totaling $1.5 million have been pledged by industry.
Reference
1. NOSCAR Working Group. NOTES: gathering momentum. White Paper. May 2006. Available at: http://www.noscar.org/documents/NOTES_White_Paper_May06.pdf. Accessed July 3, 2007.
NATURAL ORIFICE TRANSLUMINAL ENDOSCOPIC SURGERY (NOTES)
Selected studies
To date, 19 abstracts on PubMed discuss the impressive opportunities NOTES will provide. Here is a sample:
- de la Fuente SG, Demaria EJ, Reynolds JD, Portenier DD, Pryor AD. New developments in surgery: natural orifi ce transluminal endoscopic surgery (NOTES). Arch Surg. 2007;142:295–297.
- Fong DG, Pai RD, Thompson CC. Transcolonic endoscopic abdominal exploration: a NOTES survival study in a porcine model. Gastrointest Endosc. 2007;65:312–318.
- Malik A, Mellinger JD, Hazey JW, Dunkin BJ, MacFadyen BV. Endoluminal and transluminal surgery: current status and future possibilities. Surg Endosc. 2006;20:1179–1192.
- McGee MF, Rosen MJ, Marks J, et al. A primer on natural orifi ce transluminal endoscopic surgery: building a new paradigm. Surg Innov. 2006;13:86–93.
- Wilhelm D, Meining A, von Delius S, et al. An innovative, safe and sterile sigmoid access (ISSA) for NOTES. Endoscopy. 2007;39:401–406.
Over its history, surgery has been defined by the tools available to practitioners. In our era, opportunities to offer patients minimally invasive surgery have expanded dramatically as methods of establishing visualization, achieving hemostasis, and performing tissue dissection have improved. (I remember trying to treat ectopic pregnancy laparoscopically in the early 1980s without benefit of a camera or suction irrigator!)
For surgeons of my generation, the ability to access the abdominal cavity minimally invasively and to clearly visualize the contents was a significant step forward. Hysteroscopic myomectomy was another tremendous incremental improvement for patients with submucous myomas. But there is much more in store for the coming years.
Where are we headed in the next wave of gynecologic surgery? Will patients require an incision at all? Is there room to advance beyond laparoscopy and hysteroscopy? What innovations will industry offer us in the 21st century?
In this article, I describe something that is fairly familiar to most of us by now, but which is not yet practical for routine gynecologic procedures—robotically assisted endoscopic surgery. I then move on to a phenomenon that, in many respects, is still being imagined—natural orifice transluminal endoscopic surgery, or NOTES.
Robotic systems are best suited for complex surgery
Laparoscopic surgery is limited by the two-dimensional view and need for hand control of long, rigid instruments through ancillary trocar sites. Although these impediments can be overcome with practice and experience, the inability to see in three dimensions and the compromised range of motion hamper optimal management of some surgical procedures.
A number of technological advances may significantly improve our ability to perform suture-intensive or anatomically challenging operations. Several companies are developing camera systems that will permit a three-dimensional view without the need for multiple visual ports. The technology is borrowed from the world of insects, which “see” through multiple lenses within the same eye. The application of such visual processing to optical systems for endoscopic surgery will be a huge advance for laparoscopy—one that is still being perfected by industry. In 2007, the da Vinci robot system (Intuitive Surgical) offers the best opportunity to achieve both three-dimensional visualization and an ability to “feel” tissue and manipulate instruments with markedly increased range of motion.
Cost is the limiting factor
Although the da Vinci system has revolutionized the practice of urology, enabling radical nerve-sparing prostatectomy, its utility in gynecology is still being investigated. Several centers use the robot for a significant percentage of their laparoscopic gynecologic surgery, but the setup time, learning curve, and intraoperative time required make the da Vinci system an impractical tool for many routine procedures. Its true advantage lies in suture-intensive procedures and in surgeries that require meticulous dissection close to major structures. In gynecology, the laparoscopic procedures most likely to benefit from the three-dimensional view and articulating instruments are sacral colpopexy, myomectomy (FIGURE), radical hysterectomy, and lymph node dissection.
Although it is interesting and enjoyable to use robotic technology for routine laparoscopic procedures, I believe the cost is prohibitive—several million dollars for each robot. If the financial barriers are removed, however, this system will be a welcome addition to the toolset for gynecologic laparoscopic surgery. Until then, we need to make intelligent use of this powerful tool.
FIGURE Robotic myomectomy
A: Using the da Vinci robot, the surgeon incises the myometrium down to the fibroid.
B: After grasping the fibroid, the surgeon dissects it away from the surrounding myometrium.
C: As the fibroid is freed, another small tumor becomes apparent at the bottom right, and is also removed.
D: The myometrium is sutured in layers after removal of the fibroids. Photos courtesy of Paul Indman, MD.Just as many of us were able to perform laparoscopic surgery without a three-chip camera and high-tech energy system for hemostasis until the cost of those technologies could be recouped in reduced operating room time and fewer conversions to laparotomy, so will today’s surgeons have to continue performing laparoscopic adnexal surgery, routine hysterectomy, and treatment of ectopic pregnancy the “old-fashioned” way. For complex procedures, however, the da Vinci system is proving to be a major advance in endoscopic surgery.
Look for other, perhaps less expensive, technologies coming down the road that will, at the very least, permit three-dimensional visualization without the need for robotics. In addition, as I discuss in the next section, miniaturization of robotics is on the horizon. Only our imagination limits our thinking about how robotic technology may be used in the not-too-distant future.
ROBOTICS IN GYNECOLOGY
Selected studies
- Bocca S, Stadtmauer L, Oehninger S. Uncomplicated fullterm pregnancy after da Vinci-assisted laparoscopic myomectomy. Reprod Biomed Online. 2007;14:246–249.
- Elliott DS, Chow GK, Gettman M. Current status of robotics in female urology and gynecology. World J Urol. 2006;24:188–192.
- Fiorentino RP, Zepeda MA, Goldstein BH, John CR, Rettenmaier MA. Pilot study assessing robotic laparoscopic hysterectomy and patient outcomes. J Minim Invasive Gynecol. 2006;13:60–63.
- Magrina JF. Robotic surgery in gynecology. Eur J Gynaecol Oncol. 2007;28:77–82.
NOTES takes “minimally invasive” to a new level
Imagine performing surgery for ectopic pregnancy or endometriosis in your office, without anesthesia. Think this is impossible? Think again!
A newer, perhaps better, and definitely less invasive version of endoscopic surgery is on the horizon—natural orifice transluminal endoscopic surgery, or NOTES. In May, a surgeon in Portland, Oregon, performed a cholecystectomy by dropping an endoscope through the patient’s mouth into the stomach, drilling an opening in the gastric wall, and placing small instruments through that opening to perform the surgery. The specimen was then pulled through the small opening in the stomach and retrieved through the patient’s mouth! The stomach was closed with an endoscopic stapling device.
NOTES appears to be the next true advance in minimally invasive surgery. This should come as no surprise to gynecologists. We are the champions of transcervical and transvaginal surgery. General surgeons and gastroenterologists are recognizing what we have long known—that operating through these natural orifices is less uncomfortable for the patient and provides faster, less complicated recovery. They are also recognizing the challenges involved in such an approach.
A new generation of instruments is in the works
Clearly, operating through the vagina, cervix, or stomach necessitates excellent visualization and instruments flexible enough to navigate through tiny openings but strong enough to transect and retrieve tissue. Many of our industry partners are working diligently to create and perfect new instrumentation for NOTES procedures, and research is under way at many centers in this country and overseas into transgastric and transrectal procedures.
Consider what we might be able to achieve with this technology! By eliminating the need for transabdominal access, we can vastly reduce the risk of intestinal and major vessel injury and eliminate the risk of hernia. We can also markedly reduce the discomfort associated with abdominal incisions.
How might this technology be applied in gynecology? I anticipate that ovarian pathology, endometriosis, and ectopic pregnancy will be managed transvaginally or via a small opening in the uterus. Transvaginal hydrolaparoscopy—in which warm saline is used as the distention medium instead of carbon dioxide, and access to the pelvis is achieved through a small culpotomy—has been around for many years but is limited by the rigid instrumentation and restricted visualization now available. With flexible instruments that can “see” around corners yet provide a wide visual field, microrobots that can be placed through a tiny opening and then deployed to accomplish the surgical task, and systems to achieve hemostasis, NOTES may be the next revolution in gynecologic surgery.
Still in a very early stage of development, natural orifice transluminal endoscopic surgery (NOTES) has generated considerable enthusiasm among physicians leading research and development efforts. Hoping to steer these efforts in a responsible direction—and avoid the problems encountered during the early days of laparoscopic surgery, when many inexperienced practitioners began adopting the technique prematurely—a working group from the American Society of Gastrointestinal Endoscopy and the Society of American Gastrointestinal and Endoscopic Surgeons was formed in 2005, calling itself the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR). So far, this group has convened two international conferences and penned two white papers, noting that “the overwhelming sense [at the first international conference]…was that NOTES will develop into a mainstream clinical capability in the near future.”1
Some of the needs NOSCAR has identified are:
- determining the optimal technique and site to achieve access to the peritoneal cavity
- developing a gastric closure method that is 100% reliable
- reducing the risk of intraperitoneal contamination and infection, given the transgastric route that has dominated NOTES so far
- developing the ability to suture
- maintaining spatial orientation during surgery, as well as a multitasking platform that would allow manipulation of tissue, clear visualization, and safe access
- preventing intraperitoneal complications such as bleeding and bowel perforation
- exploring the physiology of pneumoperitoneum in the setting of NOTES
- establishing guidelines for training physicians and reporting both positive and negative outcomes.
In the meantime, NOSCAR recommends that all NOTES procedures in humans be approved by the Institutional Review Board and reported to a registry.
So far, the technology has been used to perform appendectomy and cholecystectomy in humans. Research grants totaling $1.5 million have been pledged by industry.
Reference
1. NOSCAR Working Group. NOTES: gathering momentum. White Paper. May 2006. Available at: http://www.noscar.org/documents/NOTES_White_Paper_May06.pdf. Accessed July 3, 2007.
NATURAL ORIFICE TRANSLUMINAL ENDOSCOPIC SURGERY (NOTES)
Selected studies
To date, 19 abstracts on PubMed discuss the impressive opportunities NOTES will provide. Here is a sample:
- de la Fuente SG, Demaria EJ, Reynolds JD, Portenier DD, Pryor AD. New developments in surgery: natural orifi ce transluminal endoscopic surgery (NOTES). Arch Surg. 2007;142:295–297.
- Fong DG, Pai RD, Thompson CC. Transcolonic endoscopic abdominal exploration: a NOTES survival study in a porcine model. Gastrointest Endosc. 2007;65:312–318.
- Malik A, Mellinger JD, Hazey JW, Dunkin BJ, MacFadyen BV. Endoluminal and transluminal surgery: current status and future possibilities. Surg Endosc. 2006;20:1179–1192.
- McGee MF, Rosen MJ, Marks J, et al. A primer on natural orifi ce transluminal endoscopic surgery: building a new paradigm. Surg Innov. 2006;13:86–93.
- Wilhelm D, Meining A, von Delius S, et al. An innovative, safe and sterile sigmoid access (ISSA) for NOTES. Endoscopy. 2007;39:401–406.
Over its history, surgery has been defined by the tools available to practitioners. In our era, opportunities to offer patients minimally invasive surgery have expanded dramatically as methods of establishing visualization, achieving hemostasis, and performing tissue dissection have improved. (I remember trying to treat ectopic pregnancy laparoscopically in the early 1980s without benefit of a camera or suction irrigator!)
For surgeons of my generation, the ability to access the abdominal cavity minimally invasively and to clearly visualize the contents was a significant step forward. Hysteroscopic myomectomy was another tremendous incremental improvement for patients with submucous myomas. But there is much more in store for the coming years.
Where are we headed in the next wave of gynecologic surgery? Will patients require an incision at all? Is there room to advance beyond laparoscopy and hysteroscopy? What innovations will industry offer us in the 21st century?
In this article, I describe something that is fairly familiar to most of us by now, but which is not yet practical for routine gynecologic procedures—robotically assisted endoscopic surgery. I then move on to a phenomenon that, in many respects, is still being imagined—natural orifice transluminal endoscopic surgery, or NOTES.
Robotic systems are best suited for complex surgery
Laparoscopic surgery is limited by the two-dimensional view and need for hand control of long, rigid instruments through ancillary trocar sites. Although these impediments can be overcome with practice and experience, the inability to see in three dimensions and the compromised range of motion hamper optimal management of some surgical procedures.
A number of technological advances may significantly improve our ability to perform suture-intensive or anatomically challenging operations. Several companies are developing camera systems that will permit a three-dimensional view without the need for multiple visual ports. The technology is borrowed from the world of insects, which “see” through multiple lenses within the same eye. The application of such visual processing to optical systems for endoscopic surgery will be a huge advance for laparoscopy—one that is still being perfected by industry. In 2007, the da Vinci robot system (Intuitive Surgical) offers the best opportunity to achieve both three-dimensional visualization and an ability to “feel” tissue and manipulate instruments with markedly increased range of motion.
Cost is the limiting factor
Although the da Vinci system has revolutionized the practice of urology, enabling radical nerve-sparing prostatectomy, its utility in gynecology is still being investigated. Several centers use the robot for a significant percentage of their laparoscopic gynecologic surgery, but the setup time, learning curve, and intraoperative time required make the da Vinci system an impractical tool for many routine procedures. Its true advantage lies in suture-intensive procedures and in surgeries that require meticulous dissection close to major structures. In gynecology, the laparoscopic procedures most likely to benefit from the three-dimensional view and articulating instruments are sacral colpopexy, myomectomy (FIGURE), radical hysterectomy, and lymph node dissection.
Although it is interesting and enjoyable to use robotic technology for routine laparoscopic procedures, I believe the cost is prohibitive—several million dollars for each robot. If the financial barriers are removed, however, this system will be a welcome addition to the toolset for gynecologic laparoscopic surgery. Until then, we need to make intelligent use of this powerful tool.
FIGURE Robotic myomectomy
A: Using the da Vinci robot, the surgeon incises the myometrium down to the fibroid.
B: After grasping the fibroid, the surgeon dissects it away from the surrounding myometrium.
C: As the fibroid is freed, another small tumor becomes apparent at the bottom right, and is also removed.
D: The myometrium is sutured in layers after removal of the fibroids. Photos courtesy of Paul Indman, MD.Just as many of us were able to perform laparoscopic surgery without a three-chip camera and high-tech energy system for hemostasis until the cost of those technologies could be recouped in reduced operating room time and fewer conversions to laparotomy, so will today’s surgeons have to continue performing laparoscopic adnexal surgery, routine hysterectomy, and treatment of ectopic pregnancy the “old-fashioned” way. For complex procedures, however, the da Vinci system is proving to be a major advance in endoscopic surgery.
Look for other, perhaps less expensive, technologies coming down the road that will, at the very least, permit three-dimensional visualization without the need for robotics. In addition, as I discuss in the next section, miniaturization of robotics is on the horizon. Only our imagination limits our thinking about how robotic technology may be used in the not-too-distant future.
ROBOTICS IN GYNECOLOGY
Selected studies
- Bocca S, Stadtmauer L, Oehninger S. Uncomplicated fullterm pregnancy after da Vinci-assisted laparoscopic myomectomy. Reprod Biomed Online. 2007;14:246–249.
- Elliott DS, Chow GK, Gettman M. Current status of robotics in female urology and gynecology. World J Urol. 2006;24:188–192.
- Fiorentino RP, Zepeda MA, Goldstein BH, John CR, Rettenmaier MA. Pilot study assessing robotic laparoscopic hysterectomy and patient outcomes. J Minim Invasive Gynecol. 2006;13:60–63.
- Magrina JF. Robotic surgery in gynecology. Eur J Gynaecol Oncol. 2007;28:77–82.
NOTES takes “minimally invasive” to a new level
Imagine performing surgery for ectopic pregnancy or endometriosis in your office, without anesthesia. Think this is impossible? Think again!
A newer, perhaps better, and definitely less invasive version of endoscopic surgery is on the horizon—natural orifice transluminal endoscopic surgery, or NOTES. In May, a surgeon in Portland, Oregon, performed a cholecystectomy by dropping an endoscope through the patient’s mouth into the stomach, drilling an opening in the gastric wall, and placing small instruments through that opening to perform the surgery. The specimen was then pulled through the small opening in the stomach and retrieved through the patient’s mouth! The stomach was closed with an endoscopic stapling device.
NOTES appears to be the next true advance in minimally invasive surgery. This should come as no surprise to gynecologists. We are the champions of transcervical and transvaginal surgery. General surgeons and gastroenterologists are recognizing what we have long known—that operating through these natural orifices is less uncomfortable for the patient and provides faster, less complicated recovery. They are also recognizing the challenges involved in such an approach.
A new generation of instruments is in the works
Clearly, operating through the vagina, cervix, or stomach necessitates excellent visualization and instruments flexible enough to navigate through tiny openings but strong enough to transect and retrieve tissue. Many of our industry partners are working diligently to create and perfect new instrumentation for NOTES procedures, and research is under way at many centers in this country and overseas into transgastric and transrectal procedures.
Consider what we might be able to achieve with this technology! By eliminating the need for transabdominal access, we can vastly reduce the risk of intestinal and major vessel injury and eliminate the risk of hernia. We can also markedly reduce the discomfort associated with abdominal incisions.
How might this technology be applied in gynecology? I anticipate that ovarian pathology, endometriosis, and ectopic pregnancy will be managed transvaginally or via a small opening in the uterus. Transvaginal hydrolaparoscopy—in which warm saline is used as the distention medium instead of carbon dioxide, and access to the pelvis is achieved through a small culpotomy—has been around for many years but is limited by the rigid instrumentation and restricted visualization now available. With flexible instruments that can “see” around corners yet provide a wide visual field, microrobots that can be placed through a tiny opening and then deployed to accomplish the surgical task, and systems to achieve hemostasis, NOTES may be the next revolution in gynecologic surgery.
Still in a very early stage of development, natural orifice transluminal endoscopic surgery (NOTES) has generated considerable enthusiasm among physicians leading research and development efforts. Hoping to steer these efforts in a responsible direction—and avoid the problems encountered during the early days of laparoscopic surgery, when many inexperienced practitioners began adopting the technique prematurely—a working group from the American Society of Gastrointestinal Endoscopy and the Society of American Gastrointestinal and Endoscopic Surgeons was formed in 2005, calling itself the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR). So far, this group has convened two international conferences and penned two white papers, noting that “the overwhelming sense [at the first international conference]…was that NOTES will develop into a mainstream clinical capability in the near future.”1
Some of the needs NOSCAR has identified are:
- determining the optimal technique and site to achieve access to the peritoneal cavity
- developing a gastric closure method that is 100% reliable
- reducing the risk of intraperitoneal contamination and infection, given the transgastric route that has dominated NOTES so far
- developing the ability to suture
- maintaining spatial orientation during surgery, as well as a multitasking platform that would allow manipulation of tissue, clear visualization, and safe access
- preventing intraperitoneal complications such as bleeding and bowel perforation
- exploring the physiology of pneumoperitoneum in the setting of NOTES
- establishing guidelines for training physicians and reporting both positive and negative outcomes.
In the meantime, NOSCAR recommends that all NOTES procedures in humans be approved by the Institutional Review Board and reported to a registry.
So far, the technology has been used to perform appendectomy and cholecystectomy in humans. Research grants totaling $1.5 million have been pledged by industry.
Reference
1. NOSCAR Working Group. NOTES: gathering momentum. White Paper. May 2006. Available at: http://www.noscar.org/documents/NOTES_White_Paper_May06.pdf. Accessed July 3, 2007.
NATURAL ORIFICE TRANSLUMINAL ENDOSCOPIC SURGERY (NOTES)
Selected studies
To date, 19 abstracts on PubMed discuss the impressive opportunities NOTES will provide. Here is a sample:
- de la Fuente SG, Demaria EJ, Reynolds JD, Portenier DD, Pryor AD. New developments in surgery: natural orifi ce transluminal endoscopic surgery (NOTES). Arch Surg. 2007;142:295–297.
- Fong DG, Pai RD, Thompson CC. Transcolonic endoscopic abdominal exploration: a NOTES survival study in a porcine model. Gastrointest Endosc. 2007;65:312–318.
- Malik A, Mellinger JD, Hazey JW, Dunkin BJ, MacFadyen BV. Endoluminal and transluminal surgery: current status and future possibilities. Surg Endosc. 2006;20:1179–1192.
- McGee MF, Rosen MJ, Marks J, et al. A primer on natural orifi ce transluminal endoscopic surgery: building a new paradigm. Surg Innov. 2006;13:86–93.
- Wilhelm D, Meining A, von Delius S, et al. An innovative, safe and sterile sigmoid access (ISSA) for NOTES. Endoscopy. 2007;39:401–406.
It’s time to re-tool the annual exam: Here’s how
Where does this shift in the surveillance strategy for cervical cancer leave us? Implementing new screening intervals gives us a wonderful opportunity to reevaluate the annual exam, and to educate ourselves and patients about interventions that make an impact on health.
Eliminate the annual exam?
Do we still need routine encounters with our patients? In this article, I address 2 topics that can help answer the question: I review the evidence that supports annual “well-woman” visits and outline the interventions that have proven benefit.
Time to retire a time-honored tradition
The utility of an annual health visit—ie, a comprehensive head-to-toe physical exam coupled with a battery of tests for early identification of disease and intervention—came into question with the rise of evidence-based medicine in the mid-1970s and, eventually, became unsupportable. In 1979, the Canadian Task Force on the Periodic Health Examination concluded that the value of only a few preventive interventions was supported by data. In 1989, Oboler and colleagues concluded that “comprehensive annual exams in asymptomatic adults have little screening value…”1
The American College of Physicians, American Medical Association, US Preventive Services Task Force (USPSTF), and US Public Health Service all concur: The routine, annual, comprehensive physical exam is unnecessary. Instead, physicians should institute a selective approach to identifying and preventing health problems in all patients—one based on gender, age, health history, and risk factors.
Some interventions have helped
The incidence of, and mortality from, cervical cancer dropped strikingly in the United States with the advent of annual screening with the Pap smear. Mammography has recently been proved to increase the early detection rate of breast cancer and to reduce the rate of death from breast cancer. The challenge we face, therefore, is to determine which screening tests and interventions are valuable and will translate into improved health outcomes. The USPSTF has set out broad recommendations on 10 areas of screening for women:
- monitor blood pressure
- screen for cervical and colorectal cancers, depression, diabetes, and osteoporosis
- test for chlamydial infection
- measure the cholesterol level
- perform mammography.
New tool helps you develop an exam
Available for you is an excellent online resource developed by the Agency for Healthcare Research and Quality (AHRQ) for adopting the USPSTF screening recommendations. AHRQ has created the “electronic preventive services select” (or ePSS) Web site (http://epss.ahrq.gov), which is searchable by patient sex, age, and behavioral risk factors. The evidence for various preventive services is graded, guiding you on both interventions that are strongly recommended and those that should not be offered routinely because they lack data to support utility.
Make the transition with a systematic approach
We can capitalize on the habit that patients have established and have them come in annually for appropriate, evidence-based services. How do we make the change from the typical ObGyn visit—one that includes a breast and pelvic examination, cervical cancer screening, and mammography—to an evidence-based, annual well-woman visit that can be rapidly implemented and easily documented, using a paper or an electronic medical record?
I recommend creating templates for the annual well-woman visit that are age-specific and include check boxes for the age-appropriate history, physical exam, testing, and counseling that you’ll provide. You can create a distinct form for each of the various age and risk groups or, more simply, devise a single form that includes all guidelines for screening, from which you choose the appropriate areas based, again, on age and risk status.
What should you include on the template that you create? Here are possible items, based on what I use in my practice:
History. Document the patient’s age, allergies, medications, contraceptive method, and risk factors (eg, smoking, a history of infection with high-risk HPV types, and a significant family history of colon, breast, and ovarian cancer and of heart disease and diabetes). Develop a problem list of concerns that the patient, and you, have. Note: I ask the patient to complete a checklist review of systems at every annual visit; doing so helps identify specific health concerns she may want to discuss.
Physical exam. Measure height, weight, body mass index, and blood pressure. Check off items included in the examination of breast, abdominal, and pelvic structures, and elaborate on abnormal findings in a space provided. Include an area on the form for noting “other” concerns, such as findings of skin, musculoskeletal, upper respiratory, and cardiac assessments—any of which is performed as indicated.
Lab testing. Document routine testing with 1) a check box to indicate which tests have been ordered and 2) a line on which to note the tests that were identified as appropriate but were not performed or were deemed inappropriate—and why. Such documentation is helpful when coding pay-for-performance measures.
Counseling. Develop a list that includes smoking cessation, weight loss, exercise, contraception, and prevention of osteoporosis and sexually transmitted infection. The list helps you recall, and discuss, essential areas (TABLE 1).
The goal in developing and using a template? It provides a single, easy-to-use form that is flexible and applicable to all women, and that encourages consistent adherence to guidelines for screening and prevention.
TABLE 1
Remember to provide lifestyle counseling!
| Don’t smoke |
| Drink alcohol in moderation |
| Eat healthy—ie, high-fiber, low-fat foods, including fruits and vegetables |
| Exercise often—ie, aerobic, weight-bearing, and balance activities |
| Maintain healthy weight* |
| Use a condom during sexual intercourse |
| Use a contraceptive |
| *Be prepared to provide strategies for effective, sustainable weight loss to your patients |
With a format in place, screen in 7 areas
What do guidelines recommend that we embrace as interventions to make a difference in patients’ long-term health? Research and consensus have established that the annual well-woman visit be organized around clinical areas of concern, comprising 7 primary intervention areas and 3 optional areas of general health (TABLE 2). In addition, ObGyns are well-positioned to add several areas of counseling, support, and intervention:
- lifelong contraception management and planning
- pre-pregnancy counseling
- prevention of sexually transmitted infection
- identification of sexual concerns
- management of menopause.
Chlamydial infection. “Grade-A” evidence supports annual screening for Chlamydia trachomatis for 1) all sexually active women 25 years and younger and 2) older women who engage in high-risk behavior (eg, more than one sex partner).
Pap smear and HPV typing. ACOG and the American Cancer Society recommend annual Pap smear testing beginning 3 years after the onset of sexual activity and continuing until 30 years of age. Routine testing for high-risk HPV subtypes may be undertaken with the Pap smear for women older than 30 years.
For most women who test negative for HPV and who have negative Pap smear cytology, Pap smear testing should be repeated no more often than every 3 years. Women who are positive for a high-risk HPV type despite a negative Pap smear should continue to be screened annually with cytology and HPV testing.
Breast health. Many groups recommend training women to perform monthly breast self-examination (BSE), although the USPSTF states that there is “insufficient evidence to recommend for or against” BSE. All groups do, however, advise an annual breast examination by a clinician, along with annual or biennial mammography beginning at 40 years of age and annual mammography beginning at 50 years.
Although many women do detect a breast lump when performing a BSE, it is unclear whether BSE improves survival from breast cancer. That’s because many lumps that women discover are benign.
Generally, therefore, I tell patients to pay attention to their breasts as they would other body parts: Don’t ignore an obvious change but don’t feel it is necessary to perform a standardized examination of the breasts monthly; evidence just does not support such a need.
Cardiovascular health. Assess blood pressure in every patient at every visit. Persistently high readings (>130/80 mm Hg) should prompt action—whether lifestyle modification or medication. Many physicians are slow to treat young women with so-called labile or borderline hypertension because the onset of cardiovascular disease is generally at an older age in women, but evidence shows that women suffer from proportionately more strokes at a young age than men do. Aggressive management of persistent hypertension may improve outcome.
- Aspirin therapy is recommended for prevention of stroke in women 45 to 65 years who are at risk. Do not recommend aspirin routinely, however, for women younger than 65 years as a means of preventing myocardial infarction.
- Perform a baseline lipid profile on all women older than 45 years. A woman who has a risk factor for cardiovascular disease—smoking, hypertension, obesity or overweight, a family history of early-onset cardiovascular disease—should be screened at any age.
- Screening may be performed as a random lipid profile to eliminate the barrier of returning after an 8-hour fast. Only women who have a significant abnormality need to return for repeat testing after an overnight fast.
- I usually intervene with lifestyle modification recommendations first—more exercise, weight loss, more monounsaturated fats and omega-3 fats in the diet—and have the patient return for a fasting lipid profile after 3 to 6 months.
Although quality evidence is lacking on the benefit of counseling about weight reduction and exercise, my experience is that providing a message to patients consistently about a healthy lifestyle is more effective than almost any other medical intervention. To have an impact on cardiovascular health, however, it is imperative that we have basic knowledge about nutrition and exercise physiology—which were not taught in medical school.
It is, clearly, not useful to simply tell a patient to lose weight. Evidence does support sustained weight loss when a person participates in an organized program, such as Weight Watchers. Even moderate weight loss is associated with a reduction in the risk of hypertension, an improvement in lipid levels, and a substantial reduction in the risk of breast cancer.
I find that this last statistic—namely, that lifetime physical activity and maintenance of normal body weight is associated with a 20% to 40% reduction in the risk of breast cancer compared with the risk in women who do not exercise or who gain 10 kg or more above their high school weight—is a huge motivator. Why? It’s well-known that women are more concerned about breast cancer than about cardiovascular disease—even though statistics demonstrate that heart disease is the leading cause of death among women.
Diabetes. Women who have a history of gestational diabetes also have a markedly increased risk of type II diabetes within 5 years of the pregnancy. Clearly, these women, as well as those who are obese, have a strong family history of diabetes, or have abnormal lipid levels, should be screened with a random glucose measurement. Women who suffer chronic monilial infection should also be assessed for diabetes.
Colorectal cancer. The second leading cause of cancer death and the fourth most common cancer in the United States carries the same risk for women as it does for men. Polyps and cancers are more likely to present on the right (ascending) side of the colon in women, however, making screening with flexible sigmoidoscopy potentially less useful.
Sixty-five percent of the US population has not been adequately screened for colorectal cancer. This is regrettable, because good-quality data support an association between screening and a reduction in mortality—even simple screening with annual fecal occult blood testing. Ideally, colon cancer testing in people of average risk should begin at 50 years with either
- colonoscopy every 10 years
- flexible sigmoidoscopy every 5 years with or without annual fecal occult blood testing
- dual-contrast barium enema every 5 years
- fecal occult blood testing annually or
- perhaps, virtual colonoscopy or stool-based DNA testing for patients who decline traditional evaluation.
Osteoporosis. For most women, screening for osteoporosis should begin at 65 years with a test of bone mineral density. Younger women who have a significant risk factor (weight, less than 127 pounds; hyperthyroidism; steroid use; a strong family history) might benefit from screening at an earlier age. All women who take more than 7.5 mg of prednisone daily or who have sustained a nontraumatic fracture should be treated to prevent osteoporosis regardless of findings on a dual energy x-ray absortiometry (DEXA) scan.
(Note: It is vital for you to provide osteoporosis screening to Medicare patients because this is 1 of only 2 office-based performance measures in the voluntary Medicare pay-for-performance list for 2007 that are applicable to gynecology practice; the other is screening for incontinence.)
Depression. We know that depression is more common, and tends to present with more physical complaints, in women than in men. Any patient who has vague somatic symptoms, chronic pain, fatigue, decreased libido, and sleep disturbances, or such “hormonal” complaints as premenstrual syndrome and hot flashes, should be screened for depression.
I have found that the Beck Depression Inventory is easy and quick to administer if indicated. This screening instrument can be downloaded from several Web sites (search the terms Beck/Depression/Inventory). For patients who screen positive, provide a resource sheet that includes a listing of specialist referrals and local depression hotline numbers.
TABLE 2
The pillars of an annual primary screening program
| ESSENTIAL | |
|
|
| OPTIONAL | |
|
|
Plus 3 at your discretion
The USPSTF has listed 3 optional areas for annual assessment: thyroid disease, bladder health, and domestic violence.
Thyroid. Because thyroid abnormalities are more common in women and because they may have an impact on the regularity of the menstrual cycle and on weight and hair loss, it seems sensible and appropriate to screen on a selected basis with a test of thyroid-stimulating hormone.
Incontinence. You should definitely include this problem in the review-of-systems questionnaire. Doing so will not only help the patient identify an embarrassing problem that she may be reluctant to bring up, but will also help drive additional services in your practice—such as urodynamic evaluation and surgery.
Build a relationship
The annual visit should reinforce the physician–patient relationship by educating women about the appropriate screening tests and supporting them as active participants in their health care. Take a consistent, balanced approach that complies with guidelines but that also addresses the patient’s concerns by incorporating education and appropriate interventions.
1. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110:214-226.
The author reports no financial relationships relevant to this article.
Where does this shift in the surveillance strategy for cervical cancer leave us? Implementing new screening intervals gives us a wonderful opportunity to reevaluate the annual exam, and to educate ourselves and patients about interventions that make an impact on health.
Eliminate the annual exam?
Do we still need routine encounters with our patients? In this article, I address 2 topics that can help answer the question: I review the evidence that supports annual “well-woman” visits and outline the interventions that have proven benefit.
Time to retire a time-honored tradition
The utility of an annual health visit—ie, a comprehensive head-to-toe physical exam coupled with a battery of tests for early identification of disease and intervention—came into question with the rise of evidence-based medicine in the mid-1970s and, eventually, became unsupportable. In 1979, the Canadian Task Force on the Periodic Health Examination concluded that the value of only a few preventive interventions was supported by data. In 1989, Oboler and colleagues concluded that “comprehensive annual exams in asymptomatic adults have little screening value…”1
The American College of Physicians, American Medical Association, US Preventive Services Task Force (USPSTF), and US Public Health Service all concur: The routine, annual, comprehensive physical exam is unnecessary. Instead, physicians should institute a selective approach to identifying and preventing health problems in all patients—one based on gender, age, health history, and risk factors.
Some interventions have helped
The incidence of, and mortality from, cervical cancer dropped strikingly in the United States with the advent of annual screening with the Pap smear. Mammography has recently been proved to increase the early detection rate of breast cancer and to reduce the rate of death from breast cancer. The challenge we face, therefore, is to determine which screening tests and interventions are valuable and will translate into improved health outcomes. The USPSTF has set out broad recommendations on 10 areas of screening for women:
- monitor blood pressure
- screen for cervical and colorectal cancers, depression, diabetes, and osteoporosis
- test for chlamydial infection
- measure the cholesterol level
- perform mammography.
New tool helps you develop an exam
Available for you is an excellent online resource developed by the Agency for Healthcare Research and Quality (AHRQ) for adopting the USPSTF screening recommendations. AHRQ has created the “electronic preventive services select” (or ePSS) Web site (http://epss.ahrq.gov), which is searchable by patient sex, age, and behavioral risk factors. The evidence for various preventive services is graded, guiding you on both interventions that are strongly recommended and those that should not be offered routinely because they lack data to support utility.
Make the transition with a systematic approach
We can capitalize on the habit that patients have established and have them come in annually for appropriate, evidence-based services. How do we make the change from the typical ObGyn visit—one that includes a breast and pelvic examination, cervical cancer screening, and mammography—to an evidence-based, annual well-woman visit that can be rapidly implemented and easily documented, using a paper or an electronic medical record?
I recommend creating templates for the annual well-woman visit that are age-specific and include check boxes for the age-appropriate history, physical exam, testing, and counseling that you’ll provide. You can create a distinct form for each of the various age and risk groups or, more simply, devise a single form that includes all guidelines for screening, from which you choose the appropriate areas based, again, on age and risk status.
What should you include on the template that you create? Here are possible items, based on what I use in my practice:
History. Document the patient’s age, allergies, medications, contraceptive method, and risk factors (eg, smoking, a history of infection with high-risk HPV types, and a significant family history of colon, breast, and ovarian cancer and of heart disease and diabetes). Develop a problem list of concerns that the patient, and you, have. Note: I ask the patient to complete a checklist review of systems at every annual visit; doing so helps identify specific health concerns she may want to discuss.
Physical exam. Measure height, weight, body mass index, and blood pressure. Check off items included in the examination of breast, abdominal, and pelvic structures, and elaborate on abnormal findings in a space provided. Include an area on the form for noting “other” concerns, such as findings of skin, musculoskeletal, upper respiratory, and cardiac assessments—any of which is performed as indicated.
Lab testing. Document routine testing with 1) a check box to indicate which tests have been ordered and 2) a line on which to note the tests that were identified as appropriate but were not performed or were deemed inappropriate—and why. Such documentation is helpful when coding pay-for-performance measures.
Counseling. Develop a list that includes smoking cessation, weight loss, exercise, contraception, and prevention of osteoporosis and sexually transmitted infection. The list helps you recall, and discuss, essential areas (TABLE 1).
The goal in developing and using a template? It provides a single, easy-to-use form that is flexible and applicable to all women, and that encourages consistent adherence to guidelines for screening and prevention.
TABLE 1
Remember to provide lifestyle counseling!
| Don’t smoke |
| Drink alcohol in moderation |
| Eat healthy—ie, high-fiber, low-fat foods, including fruits and vegetables |
| Exercise often—ie, aerobic, weight-bearing, and balance activities |
| Maintain healthy weight* |
| Use a condom during sexual intercourse |
| Use a contraceptive |
| *Be prepared to provide strategies for effective, sustainable weight loss to your patients |
With a format in place, screen in 7 areas
What do guidelines recommend that we embrace as interventions to make a difference in patients’ long-term health? Research and consensus have established that the annual well-woman visit be organized around clinical areas of concern, comprising 7 primary intervention areas and 3 optional areas of general health (TABLE 2). In addition, ObGyns are well-positioned to add several areas of counseling, support, and intervention:
- lifelong contraception management and planning
- pre-pregnancy counseling
- prevention of sexually transmitted infection
- identification of sexual concerns
- management of menopause.
Chlamydial infection. “Grade-A” evidence supports annual screening for Chlamydia trachomatis for 1) all sexually active women 25 years and younger and 2) older women who engage in high-risk behavior (eg, more than one sex partner).
Pap smear and HPV typing. ACOG and the American Cancer Society recommend annual Pap smear testing beginning 3 years after the onset of sexual activity and continuing until 30 years of age. Routine testing for high-risk HPV subtypes may be undertaken with the Pap smear for women older than 30 years.
For most women who test negative for HPV and who have negative Pap smear cytology, Pap smear testing should be repeated no more often than every 3 years. Women who are positive for a high-risk HPV type despite a negative Pap smear should continue to be screened annually with cytology and HPV testing.
Breast health. Many groups recommend training women to perform monthly breast self-examination (BSE), although the USPSTF states that there is “insufficient evidence to recommend for or against” BSE. All groups do, however, advise an annual breast examination by a clinician, along with annual or biennial mammography beginning at 40 years of age and annual mammography beginning at 50 years.
Although many women do detect a breast lump when performing a BSE, it is unclear whether BSE improves survival from breast cancer. That’s because many lumps that women discover are benign.
Generally, therefore, I tell patients to pay attention to their breasts as they would other body parts: Don’t ignore an obvious change but don’t feel it is necessary to perform a standardized examination of the breasts monthly; evidence just does not support such a need.
Cardiovascular health. Assess blood pressure in every patient at every visit. Persistently high readings (>130/80 mm Hg) should prompt action—whether lifestyle modification or medication. Many physicians are slow to treat young women with so-called labile or borderline hypertension because the onset of cardiovascular disease is generally at an older age in women, but evidence shows that women suffer from proportionately more strokes at a young age than men do. Aggressive management of persistent hypertension may improve outcome.
- Aspirin therapy is recommended for prevention of stroke in women 45 to 65 years who are at risk. Do not recommend aspirin routinely, however, for women younger than 65 years as a means of preventing myocardial infarction.
- Perform a baseline lipid profile on all women older than 45 years. A woman who has a risk factor for cardiovascular disease—smoking, hypertension, obesity or overweight, a family history of early-onset cardiovascular disease—should be screened at any age.
- Screening may be performed as a random lipid profile to eliminate the barrier of returning after an 8-hour fast. Only women who have a significant abnormality need to return for repeat testing after an overnight fast.
- I usually intervene with lifestyle modification recommendations first—more exercise, weight loss, more monounsaturated fats and omega-3 fats in the diet—and have the patient return for a fasting lipid profile after 3 to 6 months.
Although quality evidence is lacking on the benefit of counseling about weight reduction and exercise, my experience is that providing a message to patients consistently about a healthy lifestyle is more effective than almost any other medical intervention. To have an impact on cardiovascular health, however, it is imperative that we have basic knowledge about nutrition and exercise physiology—which were not taught in medical school.
It is, clearly, not useful to simply tell a patient to lose weight. Evidence does support sustained weight loss when a person participates in an organized program, such as Weight Watchers. Even moderate weight loss is associated with a reduction in the risk of hypertension, an improvement in lipid levels, and a substantial reduction in the risk of breast cancer.
I find that this last statistic—namely, that lifetime physical activity and maintenance of normal body weight is associated with a 20% to 40% reduction in the risk of breast cancer compared with the risk in women who do not exercise or who gain 10 kg or more above their high school weight—is a huge motivator. Why? It’s well-known that women are more concerned about breast cancer than about cardiovascular disease—even though statistics demonstrate that heart disease is the leading cause of death among women.
Diabetes. Women who have a history of gestational diabetes also have a markedly increased risk of type II diabetes within 5 years of the pregnancy. Clearly, these women, as well as those who are obese, have a strong family history of diabetes, or have abnormal lipid levels, should be screened with a random glucose measurement. Women who suffer chronic monilial infection should also be assessed for diabetes.
Colorectal cancer. The second leading cause of cancer death and the fourth most common cancer in the United States carries the same risk for women as it does for men. Polyps and cancers are more likely to present on the right (ascending) side of the colon in women, however, making screening with flexible sigmoidoscopy potentially less useful.
Sixty-five percent of the US population has not been adequately screened for colorectal cancer. This is regrettable, because good-quality data support an association between screening and a reduction in mortality—even simple screening with annual fecal occult blood testing. Ideally, colon cancer testing in people of average risk should begin at 50 years with either
- colonoscopy every 10 years
- flexible sigmoidoscopy every 5 years with or without annual fecal occult blood testing
- dual-contrast barium enema every 5 years
- fecal occult blood testing annually or
- perhaps, virtual colonoscopy or stool-based DNA testing for patients who decline traditional evaluation.
Osteoporosis. For most women, screening for osteoporosis should begin at 65 years with a test of bone mineral density. Younger women who have a significant risk factor (weight, less than 127 pounds; hyperthyroidism; steroid use; a strong family history) might benefit from screening at an earlier age. All women who take more than 7.5 mg of prednisone daily or who have sustained a nontraumatic fracture should be treated to prevent osteoporosis regardless of findings on a dual energy x-ray absortiometry (DEXA) scan.
(Note: It is vital for you to provide osteoporosis screening to Medicare patients because this is 1 of only 2 office-based performance measures in the voluntary Medicare pay-for-performance list for 2007 that are applicable to gynecology practice; the other is screening for incontinence.)
Depression. We know that depression is more common, and tends to present with more physical complaints, in women than in men. Any patient who has vague somatic symptoms, chronic pain, fatigue, decreased libido, and sleep disturbances, or such “hormonal” complaints as premenstrual syndrome and hot flashes, should be screened for depression.
I have found that the Beck Depression Inventory is easy and quick to administer if indicated. This screening instrument can be downloaded from several Web sites (search the terms Beck/Depression/Inventory). For patients who screen positive, provide a resource sheet that includes a listing of specialist referrals and local depression hotline numbers.
TABLE 2
The pillars of an annual primary screening program
| ESSENTIAL | |
|
|
| OPTIONAL | |
|
|
Plus 3 at your discretion
The USPSTF has listed 3 optional areas for annual assessment: thyroid disease, bladder health, and domestic violence.
Thyroid. Because thyroid abnormalities are more common in women and because they may have an impact on the regularity of the menstrual cycle and on weight and hair loss, it seems sensible and appropriate to screen on a selected basis with a test of thyroid-stimulating hormone.
Incontinence. You should definitely include this problem in the review-of-systems questionnaire. Doing so will not only help the patient identify an embarrassing problem that she may be reluctant to bring up, but will also help drive additional services in your practice—such as urodynamic evaluation and surgery.
Build a relationship
The annual visit should reinforce the physician–patient relationship by educating women about the appropriate screening tests and supporting them as active participants in their health care. Take a consistent, balanced approach that complies with guidelines but that also addresses the patient’s concerns by incorporating education and appropriate interventions.
Where does this shift in the surveillance strategy for cervical cancer leave us? Implementing new screening intervals gives us a wonderful opportunity to reevaluate the annual exam, and to educate ourselves and patients about interventions that make an impact on health.
Eliminate the annual exam?
Do we still need routine encounters with our patients? In this article, I address 2 topics that can help answer the question: I review the evidence that supports annual “well-woman” visits and outline the interventions that have proven benefit.
Time to retire a time-honored tradition
The utility of an annual health visit—ie, a comprehensive head-to-toe physical exam coupled with a battery of tests for early identification of disease and intervention—came into question with the rise of evidence-based medicine in the mid-1970s and, eventually, became unsupportable. In 1979, the Canadian Task Force on the Periodic Health Examination concluded that the value of only a few preventive interventions was supported by data. In 1989, Oboler and colleagues concluded that “comprehensive annual exams in asymptomatic adults have little screening value…”1
The American College of Physicians, American Medical Association, US Preventive Services Task Force (USPSTF), and US Public Health Service all concur: The routine, annual, comprehensive physical exam is unnecessary. Instead, physicians should institute a selective approach to identifying and preventing health problems in all patients—one based on gender, age, health history, and risk factors.
Some interventions have helped
The incidence of, and mortality from, cervical cancer dropped strikingly in the United States with the advent of annual screening with the Pap smear. Mammography has recently been proved to increase the early detection rate of breast cancer and to reduce the rate of death from breast cancer. The challenge we face, therefore, is to determine which screening tests and interventions are valuable and will translate into improved health outcomes. The USPSTF has set out broad recommendations on 10 areas of screening for women:
- monitor blood pressure
- screen for cervical and colorectal cancers, depression, diabetes, and osteoporosis
- test for chlamydial infection
- measure the cholesterol level
- perform mammography.
New tool helps you develop an exam
Available for you is an excellent online resource developed by the Agency for Healthcare Research and Quality (AHRQ) for adopting the USPSTF screening recommendations. AHRQ has created the “electronic preventive services select” (or ePSS) Web site (http://epss.ahrq.gov), which is searchable by patient sex, age, and behavioral risk factors. The evidence for various preventive services is graded, guiding you on both interventions that are strongly recommended and those that should not be offered routinely because they lack data to support utility.
Make the transition with a systematic approach
We can capitalize on the habit that patients have established and have them come in annually for appropriate, evidence-based services. How do we make the change from the typical ObGyn visit—one that includes a breast and pelvic examination, cervical cancer screening, and mammography—to an evidence-based, annual well-woman visit that can be rapidly implemented and easily documented, using a paper or an electronic medical record?
I recommend creating templates for the annual well-woman visit that are age-specific and include check boxes for the age-appropriate history, physical exam, testing, and counseling that you’ll provide. You can create a distinct form for each of the various age and risk groups or, more simply, devise a single form that includes all guidelines for screening, from which you choose the appropriate areas based, again, on age and risk status.
What should you include on the template that you create? Here are possible items, based on what I use in my practice:
History. Document the patient’s age, allergies, medications, contraceptive method, and risk factors (eg, smoking, a history of infection with high-risk HPV types, and a significant family history of colon, breast, and ovarian cancer and of heart disease and diabetes). Develop a problem list of concerns that the patient, and you, have. Note: I ask the patient to complete a checklist review of systems at every annual visit; doing so helps identify specific health concerns she may want to discuss.
Physical exam. Measure height, weight, body mass index, and blood pressure. Check off items included in the examination of breast, abdominal, and pelvic structures, and elaborate on abnormal findings in a space provided. Include an area on the form for noting “other” concerns, such as findings of skin, musculoskeletal, upper respiratory, and cardiac assessments—any of which is performed as indicated.
Lab testing. Document routine testing with 1) a check box to indicate which tests have been ordered and 2) a line on which to note the tests that were identified as appropriate but were not performed or were deemed inappropriate—and why. Such documentation is helpful when coding pay-for-performance measures.
Counseling. Develop a list that includes smoking cessation, weight loss, exercise, contraception, and prevention of osteoporosis and sexually transmitted infection. The list helps you recall, and discuss, essential areas (TABLE 1).
The goal in developing and using a template? It provides a single, easy-to-use form that is flexible and applicable to all women, and that encourages consistent adherence to guidelines for screening and prevention.
TABLE 1
Remember to provide lifestyle counseling!
| Don’t smoke |
| Drink alcohol in moderation |
| Eat healthy—ie, high-fiber, low-fat foods, including fruits and vegetables |
| Exercise often—ie, aerobic, weight-bearing, and balance activities |
| Maintain healthy weight* |
| Use a condom during sexual intercourse |
| Use a contraceptive |
| *Be prepared to provide strategies for effective, sustainable weight loss to your patients |
With a format in place, screen in 7 areas
What do guidelines recommend that we embrace as interventions to make a difference in patients’ long-term health? Research and consensus have established that the annual well-woman visit be organized around clinical areas of concern, comprising 7 primary intervention areas and 3 optional areas of general health (TABLE 2). In addition, ObGyns are well-positioned to add several areas of counseling, support, and intervention:
- lifelong contraception management and planning
- pre-pregnancy counseling
- prevention of sexually transmitted infection
- identification of sexual concerns
- management of menopause.
Chlamydial infection. “Grade-A” evidence supports annual screening for Chlamydia trachomatis for 1) all sexually active women 25 years and younger and 2) older women who engage in high-risk behavior (eg, more than one sex partner).
Pap smear and HPV typing. ACOG and the American Cancer Society recommend annual Pap smear testing beginning 3 years after the onset of sexual activity and continuing until 30 years of age. Routine testing for high-risk HPV subtypes may be undertaken with the Pap smear for women older than 30 years.
For most women who test negative for HPV and who have negative Pap smear cytology, Pap smear testing should be repeated no more often than every 3 years. Women who are positive for a high-risk HPV type despite a negative Pap smear should continue to be screened annually with cytology and HPV testing.
Breast health. Many groups recommend training women to perform monthly breast self-examination (BSE), although the USPSTF states that there is “insufficient evidence to recommend for or against” BSE. All groups do, however, advise an annual breast examination by a clinician, along with annual or biennial mammography beginning at 40 years of age and annual mammography beginning at 50 years.
Although many women do detect a breast lump when performing a BSE, it is unclear whether BSE improves survival from breast cancer. That’s because many lumps that women discover are benign.
Generally, therefore, I tell patients to pay attention to their breasts as they would other body parts: Don’t ignore an obvious change but don’t feel it is necessary to perform a standardized examination of the breasts monthly; evidence just does not support such a need.
Cardiovascular health. Assess blood pressure in every patient at every visit. Persistently high readings (>130/80 mm Hg) should prompt action—whether lifestyle modification or medication. Many physicians are slow to treat young women with so-called labile or borderline hypertension because the onset of cardiovascular disease is generally at an older age in women, but evidence shows that women suffer from proportionately more strokes at a young age than men do. Aggressive management of persistent hypertension may improve outcome.
- Aspirin therapy is recommended for prevention of stroke in women 45 to 65 years who are at risk. Do not recommend aspirin routinely, however, for women younger than 65 years as a means of preventing myocardial infarction.
- Perform a baseline lipid profile on all women older than 45 years. A woman who has a risk factor for cardiovascular disease—smoking, hypertension, obesity or overweight, a family history of early-onset cardiovascular disease—should be screened at any age.
- Screening may be performed as a random lipid profile to eliminate the barrier of returning after an 8-hour fast. Only women who have a significant abnormality need to return for repeat testing after an overnight fast.
- I usually intervene with lifestyle modification recommendations first—more exercise, weight loss, more monounsaturated fats and omega-3 fats in the diet—and have the patient return for a fasting lipid profile after 3 to 6 months.
Although quality evidence is lacking on the benefit of counseling about weight reduction and exercise, my experience is that providing a message to patients consistently about a healthy lifestyle is more effective than almost any other medical intervention. To have an impact on cardiovascular health, however, it is imperative that we have basic knowledge about nutrition and exercise physiology—which were not taught in medical school.
It is, clearly, not useful to simply tell a patient to lose weight. Evidence does support sustained weight loss when a person participates in an organized program, such as Weight Watchers. Even moderate weight loss is associated with a reduction in the risk of hypertension, an improvement in lipid levels, and a substantial reduction in the risk of breast cancer.
I find that this last statistic—namely, that lifetime physical activity and maintenance of normal body weight is associated with a 20% to 40% reduction in the risk of breast cancer compared with the risk in women who do not exercise or who gain 10 kg or more above their high school weight—is a huge motivator. Why? It’s well-known that women are more concerned about breast cancer than about cardiovascular disease—even though statistics demonstrate that heart disease is the leading cause of death among women.
Diabetes. Women who have a history of gestational diabetes also have a markedly increased risk of type II diabetes within 5 years of the pregnancy. Clearly, these women, as well as those who are obese, have a strong family history of diabetes, or have abnormal lipid levels, should be screened with a random glucose measurement. Women who suffer chronic monilial infection should also be assessed for diabetes.
Colorectal cancer. The second leading cause of cancer death and the fourth most common cancer in the United States carries the same risk for women as it does for men. Polyps and cancers are more likely to present on the right (ascending) side of the colon in women, however, making screening with flexible sigmoidoscopy potentially less useful.
Sixty-five percent of the US population has not been adequately screened for colorectal cancer. This is regrettable, because good-quality data support an association between screening and a reduction in mortality—even simple screening with annual fecal occult blood testing. Ideally, colon cancer testing in people of average risk should begin at 50 years with either
- colonoscopy every 10 years
- flexible sigmoidoscopy every 5 years with or without annual fecal occult blood testing
- dual-contrast barium enema every 5 years
- fecal occult blood testing annually or
- perhaps, virtual colonoscopy or stool-based DNA testing for patients who decline traditional evaluation.
Osteoporosis. For most women, screening for osteoporosis should begin at 65 years with a test of bone mineral density. Younger women who have a significant risk factor (weight, less than 127 pounds; hyperthyroidism; steroid use; a strong family history) might benefit from screening at an earlier age. All women who take more than 7.5 mg of prednisone daily or who have sustained a nontraumatic fracture should be treated to prevent osteoporosis regardless of findings on a dual energy x-ray absortiometry (DEXA) scan.
(Note: It is vital for you to provide osteoporosis screening to Medicare patients because this is 1 of only 2 office-based performance measures in the voluntary Medicare pay-for-performance list for 2007 that are applicable to gynecology practice; the other is screening for incontinence.)
Depression. We know that depression is more common, and tends to present with more physical complaints, in women than in men. Any patient who has vague somatic symptoms, chronic pain, fatigue, decreased libido, and sleep disturbances, or such “hormonal” complaints as premenstrual syndrome and hot flashes, should be screened for depression.
I have found that the Beck Depression Inventory is easy and quick to administer if indicated. This screening instrument can be downloaded from several Web sites (search the terms Beck/Depression/Inventory). For patients who screen positive, provide a resource sheet that includes a listing of specialist referrals and local depression hotline numbers.
TABLE 2
The pillars of an annual primary screening program
| ESSENTIAL | |
|
|
| OPTIONAL | |
|
|
Plus 3 at your discretion
The USPSTF has listed 3 optional areas for annual assessment: thyroid disease, bladder health, and domestic violence.
Thyroid. Because thyroid abnormalities are more common in women and because they may have an impact on the regularity of the menstrual cycle and on weight and hair loss, it seems sensible and appropriate to screen on a selected basis with a test of thyroid-stimulating hormone.
Incontinence. You should definitely include this problem in the review-of-systems questionnaire. Doing so will not only help the patient identify an embarrassing problem that she may be reluctant to bring up, but will also help drive additional services in your practice—such as urodynamic evaluation and surgery.
Build a relationship
The annual visit should reinforce the physician–patient relationship by educating women about the appropriate screening tests and supporting them as active participants in their health care. Take a consistent, balanced approach that complies with guidelines but that also addresses the patient’s concerns by incorporating education and appropriate interventions.
1. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110:214-226.
The author reports no financial relationships relevant to this article.
1. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110:214-226.
The author reports no financial relationships relevant to this article.
Vaginal hysterectomy: 6 challenges, an arsenal of solutions
Newer codes for vaginal hysterectomy capture the work of removing larger uteri without laparoscopy
True or false: When it comes to hysterectomy, surgeons tend to use the route that is safest, least invasive, and most economical.
Sadly, the statement is false. Although vaginal hysterectomy tops all 3 categories, it is the least utilized of surgical routes. The number of vaginal hysterectomies may have increased slightly over the past decade, likely due to the incorporation of laparoscopically assisted vaginal hysterectomy into the mainstream and increased practice with the vaginal component, but fewer than 30% of hysterectomies are performed vaginally.
This article addresses 6 common challenges at vaginal hysterectomy and offers strategies to overcome them.
Laparoscopic strategies ease vaginal hysterectomy, too
Laparoscopic hysterectomy became widely accepted when surgical instruments were developed to overcome the technical challenges inherent in operating with limited access. By incorporating some of the techniques we routinely use for laparoscopic surgery, we can overcome many of the challenges faced during difficult vaginal surgery.
VAGINAL HYSTERECTOMY CHALLENGE 1: Obesity
Unfortunately, our population is increasingly rotund. This is not only a significant risk factor for the patient’s health in general, but it poses some unique challenges for surgeons. I must say that, as tough as it may be to complete a hysterectomy vaginally in a morbidly obese woman, I would much rather approach her pelvic organs through the cul-de-sac, which contains no fat cells, than through the abdominal wall—either laparoscopically or abdominally! The trick is gaining access to the posterior cul-de-sac.
How to enter the cul-de-sac
It seems to be a perverse rule of nature, but a tight upper vaginal ring seems almost universal in obese women. Added to the redundant sidewalls and the large buttocks, this tightness makes entry into the anterior or posterior cul-de-sac problematic. Several tricks make peritoneal access possible:
Position the patient to increase access, with the buttocks well over the edge of the operating table. This brings the operative field a bit closer to the surgeon, and permits the use of long-handled retractors posteriorly.
Use candy-cane stirrups to allow assistants better access to the operative field. Adequate assistance is essential in attempting vaginal surgery in the morbidly obese.
Avoid the Trendelenburg position. Although it might seem that this position would facilitate visualization and placement of a posterior weighted speculum, all it does is allow the patient to slide up on the table, making placement of alternate retractors difficult.
Use the right tools. If the posterior weighted speculum will not stay in place or does not afford access to the cul-de-sac due to an upper vaginal ring, use a narrow Deaver retractor posteriorly (without sidewall or anterior retraction). Use a Jacob’s tenaculum on the posterior lip of the cervix and have your assistant pull straight up on the tenaculum while using the Deaver retractor to see the area between the uterosacral ligaments.
Use the uterosacral ligaments as a guide. Another perversity in morbidly obese women: Despite multiparity, they seem to have little or no apical prolapse but lots of vaginal wall redundancy. The cervix is often elongated, but the uterosacral ligaments are sky high.
I palpate these ligaments, injecting them with a combination of vasopressin diluted 1:5 with bupivacaine and epinephrine (for enhanced hemostasis and preemptive analgesia), then use a pencil electrosurgical electrode to rapidly open the vaginal epithelium between the ligaments.
I then use a long, toothed tissue forceps to tent the peritoneum at 90 degrees to the plane of the posterior cul-de-sac and use Mayo scissors to enter the peritoneal cavity. Usually there is a spurt of fluid to mark appropriate entry into the peritoneum.
I then use the blades of my scissors to stretch the peritoneum between the ligaments and place a moistened 4×4 sponge into the incision.
At the onset of the procedure, inject indigo carmine dye intravenously so that any injury to the bladder will be immediately recognized. I have the circulating nurse empty the bladder while she is prepping the patient, but do not leave an indwelling catheter in place during the operation. I find it cumbersome to work around the catheter.
Problematic entries
When entry into the posterior cul-de-sac is difficult, I stop dissection, place a 4×4 sponge into the incision to reduce bleeding from the vagina, and proceed to attempt anterior entry.
I place the Deaver retractor into the anterior space and move the tenaculum to the anterior lip of the cervix. This gives maximal space for downward traction on the cervix while anterior entry is attempted.
Once again, I inject the tissue with the bupivacaine solution before incising the vaginal epithelium at the level of the internal os. I use sharp dissection only when creating a plane between the lower uterine segment and the bladder.
Ensuring room to move and good visualization
If neither the anterior nor the posterior cul-de-sac can be accessed, it may be time to rethink the vaginal approach—but there is no harm in taking the uterosacral and cardinal ligaments extraperitoneally in an effort to gain some mobility. Hugging the uterus and leaving the anterior retractor in place to lift the bladder superiorly are essential steps to protect the ureters. The cervix can then be split in the midline (12 to 6 o’clock) to easily identify the peritoneal reflections.
Other tips
Sidewall retractors are rarely needed. They significantly impair placement of clamps and sutures by creating a long, narrow, parallel passageway. An alternative trick is to use the suction tip to retract the vaginal sidewall as the surgeon is working.
A disposable, fiberoptic, lighted suction irrigator is another option. The light can be directed precisely where it is needed, and the irrigation helps keep the field clean and tidy, simplifying identification of anatomy.
Skip the sutures whenever possible. Because it is difficult to place sutures with precision in a tight, poorly illuminated space, I use a vessel sealer for all pedicles above the uterosacral ligaments. Some of these instruments were designed specifically for vaginal hysterectomy in the same shape and size as Heaney clamps. They are remarkably efficient and permit the completion of a vaginal procedure when suture placement is difficult.
Use a Heaney needleholder, with the suture loaded precisely in the center of the needle curve, along with the lighted suction irrigator to retract redundant tissue away from the track of the needle, to facilitate suturing high in the pelvis.
VAGINAL HYSTERECTOMY CHALLENGE 2: Nulliparity
Many of us are reluctant to attempt vaginal hysterectomy in a woman who has never had children. Although this situation can be challenging at times, in my experience, the access issues tend to be more difficult in obese, multiparous women.
The same tricks and techniques addressed above will permit the vaginal approach in almost all nulliparous women.
VAGINAL HYSTERECTOMY CHALLENGE 3: Previous cesarean section
A prior cesarean delivery is sometimes considered an indication for laparoscopic hysterectomy. There are 2 concerns here: The patient may have a small pelvis, and there may be significant scar tissue and difficulty gaining access to the anterior cul-de-sac, as a result of repeated dissection between the lower uterine segment and the bladder. Several tricks may be useful:
Examine the patient under anesthesia to ensure that the fundus is not stuck to the anterior abdominal wall. This can occur if the peritoneum was not closed during the last cesarean section.
Empty the bladder before beginning the hysterectomy, and inject indigo carmine dye intravenously with the induction of anesthesia.
Use careful sharp dissection between the bladder and lower uterine segment using fine Metzenbaum scissors, with the tips pointed toward the uterus. Dissect only as far as you can easily see.
Secure the uterosacral, cardinal, and broad ligaments, if necessary, before pursuing entry into the anterior cul-de-sac. It is not essential to gain anterior access before taking these pedicles. The additional mobility and descensus enable safe sharp dissection.
If pedicles have been secured up to the fundus, and the anterior cul-de-sac remains difficult to assess, flip the fundus through the posterior cul-de-sac and reach your finger or an instrument around the top of the fundus to identify the peritoneum anteriorly, then incise it under direct vision.
VAGINAL HYSTERECTOMY CHALLENGE 4: Previous abdominal/pelvic surgery
This history is another often-cited rationale for avoiding the vaginal approach. In reality, the adhesions created from prior surgery tend to arise between the anterior abdominal wall and the omentum or small bowel. This situation makes laparoscopic or open abdominal entry riskier than vaginal peritoneal access through the cul-de-sac.
Two possible exceptions: a patient who has had surgery for deeply infiltrating endometriosis in the cul-de-sac or a woman who has undergone myomectomies with posterior incisions. These patients may have dense scarring in the cul-de-sac, which would preclude a vaginal approach. Whatever the surgical route, appropriate bowel preparation is necessary to permit simple closure of any intestinal injury at the time of hysterectomy. Why not begin vaginally if the exam under anesthesia demonstrates an accessible and reasonably free cul-de-sac?
VAGINAL HYSTERECTOMY CHALLENGE 5: Large myomatous uterus
A uterus of any size can be removed vaginally as long as there is mobility with access to the uterine arteries. The one exception: a patient with a small, normal uterus and a massive pedunculated myoma arising from the top of the fundus. If the fibroid cannot be pulled into the true pelvis for morcellation, it cannot be removed transvaginally. Fortunately, this situation is quite rare.
Keep the uterine serosa intact to maintain orientation.
Split the uterus from 12 to 6 o’clock (bivalving). Protect the bladder with a Deaver retractor anteriorly, and protect the posterior vaginal epithelium with the weighted speculum posteriorly.
Remove chunks of tissue from the inside of the specimen. Orient your scalpel blade so that you are always cutting toward the center of the specimen. That way, if the blade slips, you will not accidentally injure tissue on the pelvic sidewall.
Use Lahey thyroid clamps to place the tissue you plan to remove under tension. Finding the capsule of each myoma and gently separating it from the surrounding myometrium facilitates delivery of larger fibroids into the endometrial cavity. Some myomas require morcellation themselves for removal.
Replace the scalpel blade periodically to keep it sharp. Calcified fibroids can dull the blade rapidly.
Work systematically to remove as much central tissue as possible. Try to keep a clean, sharp margin of tissue around the edges for easy grasping. Torn, irregular tissue is very difficult to grab and may cause significant frustration.
If access becomes limited, try clamping additional pedicles on each side of the specimen. A tiny amount of additional descensus can make a huge difference.
Do not administer GnRH agonists prior to surgery. The uterus may shrink, but the myomas tend to become quite soft and difficult to remove. If the patient is seriously anemic, give norethindrone acetate, 5 to 20 mg daily, to stop bleeding and allow the patient’s red blood cell volume to improve before elective surgery.
Use a vessel-sealing instrument to control the pedicles. This strategy produces optimal hemostasis to permit a dry field during morcellation. Moreover, the seals do not get disrupted when the large uterus is pulled past them. Placing suture around pedicles when there is a large, bulky uterus in the pelvis is challenging at best, and it is frustrating to see significant bleeding after removal of the specimen. This problem does not seem to occur with the sealing devices.
Know when to quit! We should not promise any patient a minimally invasive operation. If there is uncontrolled bleeding or no progress after 5 to 10 minutes, convert to a laparoscopic or abdominal approach.
I schedule cases I know will be challenging as “possible” laparoscopic or open hysterectomy. This alerts the OR staff to have additional equipment ready and nearby should we need it. It is not a surgical failure or complication to convert a minimally invasive hysterectomy to a more invasive technique when appropriate. Better to have tried and failed than never to have tried at all!
VAGINAL HYSTERECTOMY CHALLENGE 6: Avoiding complications
The most common complications of vaginal hysterectomy are bleeding, infection, and injury to the bladder. Ureteral injury is less common at vaginal hysterectomy than with the abdominal or laparoscopic approaches. Thus, I do not think routine cystoscopy is essential after uncomplicated vaginal hysterectomy, although I recommend intravenous administration of indigo carmine dye at the beginning of the procedure to enable rapid recognition of even a small bladder laceration. Sharp, careful dissection of the bladder off the lower uterine segment and the avoidance of finger dissection (especially with a gauze sponge) keep these injuries to a minimum.
Minimize bleeding by using newer vessel-sealing technologies rather than suture for most of the pedicles. I attach the uterosacral–cardinal ligament pedicles to the vaginal cuff at closure with suture. I suture the first pedicle once I have entered the posterior cul-de-sac and hold that suture to stay oriented.
Pay attention to patient positioning. Careful positioning will help you avoid neurological injuries. Avoid hyperflexion at the hips, which stretches the femoral nerve. Large nerves have comparatively little blood supply, so stretching them for prolonged periods can cause hypoxic injury. Although such injuries are almost always rapidly reversible, they are disconcerting for both the patient and her surgeon.
When operating on a very thin woman with a bony sacrum, I like to place egg-crate foam beneath the buttocks to provide some cushioning. I am also very careful with these women to keep their legs in a neutral position, and I watch my surgical assistants to be sure they are not leaning on the patient during the procedure.
Prophylactic antibiotics are a must to avoid postoperative vaginal cuff infections and pelvic abscess. Smokers and women with preexisting bacterial vaginosis are at highest risk for infection. I ask women to discontinue smoking at least 2 weeks prior to surgery and inform all smokers that their risk of infection is heightened. I treat anaerobic overgrowth in the vagina prior to surgery to help prevent infections in women with bacterial vaginosis.
The timing of prophylactic antibiotics is important. Intravenous first-generation cephalosporins must be administered within 60 minutes of the initial incision, but it is important to give them early enough for them to adequately disseminate to tissue before the colpotomy incision.
DVT prophylaxis is especially important for women with large uteri. Routine use of sequential compression stockings is both cost-effective and equivalent to the prophylactic use of subcutaneous heparin, so I use them for all patients undergoing vaginal hysterectomy. Early ambulation (usually within 2 hours of surgery) is also helpful in avoiding thromboses.
90% of hysterectomies can be performed vaginally
Using the techniques described in this article, I have been able to perform over 90% of the hysterectomies in my practice vaginally. More than 50% of my patients are either morbidly obese, nulliparous, or have had previous abdominal surgery of some type.
The instruments I find most useful are the lighted suction irrigator and the vessel-sealing Heaney-type clamp.
Establishing a routine and approaching technically challenging cases with a systematic and standardized set of techniques make the vaginal route possible for the vast majority of patients with benign disease.
Dr. Levy has served as a consultant to ValleyLab.
SUGGESTED READING
1. Abostini A, Vejux N, Colette E, et al. Risk of bladder injury during vaginal hysterectomy in women with a previous cesarean section. J Reprod Med. 2005;50:940-942.
2. Clave H, Barr H, Niccolai P. Painless vaginal hysterectomy with thermal hemostasis (results of a series of 152 cases). Gynecol Surg. 2005;2:101-105.
3. Isik-Akbay EF, Harmanli OH, Panganamamula UR, et al. Hysterectomy in obese women: a comparison of abdominal and vaginal routes. Obstet Gynecol. 2004;104:710-714.
4. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
5. O’Neal MG, Beste T, Shackelford DP. Utility of preemptive local analgesia in vaginal hysterectomy. Am J Obstet Gynecol. 2003;189:1539-1542.
6. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005;(1):CD003677.-
Newer codes for vaginal hysterectomy capture the work of removing larger uteri without laparoscopy
True or false: When it comes to hysterectomy, surgeons tend to use the route that is safest, least invasive, and most economical.
Sadly, the statement is false. Although vaginal hysterectomy tops all 3 categories, it is the least utilized of surgical routes. The number of vaginal hysterectomies may have increased slightly over the past decade, likely due to the incorporation of laparoscopically assisted vaginal hysterectomy into the mainstream and increased practice with the vaginal component, but fewer than 30% of hysterectomies are performed vaginally.
This article addresses 6 common challenges at vaginal hysterectomy and offers strategies to overcome them.
Laparoscopic strategies ease vaginal hysterectomy, too
Laparoscopic hysterectomy became widely accepted when surgical instruments were developed to overcome the technical challenges inherent in operating with limited access. By incorporating some of the techniques we routinely use for laparoscopic surgery, we can overcome many of the challenges faced during difficult vaginal surgery.
VAGINAL HYSTERECTOMY CHALLENGE 1: Obesity
Unfortunately, our population is increasingly rotund. This is not only a significant risk factor for the patient’s health in general, but it poses some unique challenges for surgeons. I must say that, as tough as it may be to complete a hysterectomy vaginally in a morbidly obese woman, I would much rather approach her pelvic organs through the cul-de-sac, which contains no fat cells, than through the abdominal wall—either laparoscopically or abdominally! The trick is gaining access to the posterior cul-de-sac.
How to enter the cul-de-sac
It seems to be a perverse rule of nature, but a tight upper vaginal ring seems almost universal in obese women. Added to the redundant sidewalls and the large buttocks, this tightness makes entry into the anterior or posterior cul-de-sac problematic. Several tricks make peritoneal access possible:
Position the patient to increase access, with the buttocks well over the edge of the operating table. This brings the operative field a bit closer to the surgeon, and permits the use of long-handled retractors posteriorly.
Use candy-cane stirrups to allow assistants better access to the operative field. Adequate assistance is essential in attempting vaginal surgery in the morbidly obese.
Avoid the Trendelenburg position. Although it might seem that this position would facilitate visualization and placement of a posterior weighted speculum, all it does is allow the patient to slide up on the table, making placement of alternate retractors difficult.
Use the right tools. If the posterior weighted speculum will not stay in place or does not afford access to the cul-de-sac due to an upper vaginal ring, use a narrow Deaver retractor posteriorly (without sidewall or anterior retraction). Use a Jacob’s tenaculum on the posterior lip of the cervix and have your assistant pull straight up on the tenaculum while using the Deaver retractor to see the area between the uterosacral ligaments.
Use the uterosacral ligaments as a guide. Another perversity in morbidly obese women: Despite multiparity, they seem to have little or no apical prolapse but lots of vaginal wall redundancy. The cervix is often elongated, but the uterosacral ligaments are sky high.
I palpate these ligaments, injecting them with a combination of vasopressin diluted 1:5 with bupivacaine and epinephrine (for enhanced hemostasis and preemptive analgesia), then use a pencil electrosurgical electrode to rapidly open the vaginal epithelium between the ligaments.
I then use a long, toothed tissue forceps to tent the peritoneum at 90 degrees to the plane of the posterior cul-de-sac and use Mayo scissors to enter the peritoneal cavity. Usually there is a spurt of fluid to mark appropriate entry into the peritoneum.
I then use the blades of my scissors to stretch the peritoneum between the ligaments and place a moistened 4×4 sponge into the incision.
At the onset of the procedure, inject indigo carmine dye intravenously so that any injury to the bladder will be immediately recognized. I have the circulating nurse empty the bladder while she is prepping the patient, but do not leave an indwelling catheter in place during the operation. I find it cumbersome to work around the catheter.
Problematic entries
When entry into the posterior cul-de-sac is difficult, I stop dissection, place a 4×4 sponge into the incision to reduce bleeding from the vagina, and proceed to attempt anterior entry.
I place the Deaver retractor into the anterior space and move the tenaculum to the anterior lip of the cervix. This gives maximal space for downward traction on the cervix while anterior entry is attempted.
Once again, I inject the tissue with the bupivacaine solution before incising the vaginal epithelium at the level of the internal os. I use sharp dissection only when creating a plane between the lower uterine segment and the bladder.
Ensuring room to move and good visualization
If neither the anterior nor the posterior cul-de-sac can be accessed, it may be time to rethink the vaginal approach—but there is no harm in taking the uterosacral and cardinal ligaments extraperitoneally in an effort to gain some mobility. Hugging the uterus and leaving the anterior retractor in place to lift the bladder superiorly are essential steps to protect the ureters. The cervix can then be split in the midline (12 to 6 o’clock) to easily identify the peritoneal reflections.
Other tips
Sidewall retractors are rarely needed. They significantly impair placement of clamps and sutures by creating a long, narrow, parallel passageway. An alternative trick is to use the suction tip to retract the vaginal sidewall as the surgeon is working.
A disposable, fiberoptic, lighted suction irrigator is another option. The light can be directed precisely where it is needed, and the irrigation helps keep the field clean and tidy, simplifying identification of anatomy.
Skip the sutures whenever possible. Because it is difficult to place sutures with precision in a tight, poorly illuminated space, I use a vessel sealer for all pedicles above the uterosacral ligaments. Some of these instruments were designed specifically for vaginal hysterectomy in the same shape and size as Heaney clamps. They are remarkably efficient and permit the completion of a vaginal procedure when suture placement is difficult.
Use a Heaney needleholder, with the suture loaded precisely in the center of the needle curve, along with the lighted suction irrigator to retract redundant tissue away from the track of the needle, to facilitate suturing high in the pelvis.
VAGINAL HYSTERECTOMY CHALLENGE 2: Nulliparity
Many of us are reluctant to attempt vaginal hysterectomy in a woman who has never had children. Although this situation can be challenging at times, in my experience, the access issues tend to be more difficult in obese, multiparous women.
The same tricks and techniques addressed above will permit the vaginal approach in almost all nulliparous women.
VAGINAL HYSTERECTOMY CHALLENGE 3: Previous cesarean section
A prior cesarean delivery is sometimes considered an indication for laparoscopic hysterectomy. There are 2 concerns here: The patient may have a small pelvis, and there may be significant scar tissue and difficulty gaining access to the anterior cul-de-sac, as a result of repeated dissection between the lower uterine segment and the bladder. Several tricks may be useful:
Examine the patient under anesthesia to ensure that the fundus is not stuck to the anterior abdominal wall. This can occur if the peritoneum was not closed during the last cesarean section.
Empty the bladder before beginning the hysterectomy, and inject indigo carmine dye intravenously with the induction of anesthesia.
Use careful sharp dissection between the bladder and lower uterine segment using fine Metzenbaum scissors, with the tips pointed toward the uterus. Dissect only as far as you can easily see.
Secure the uterosacral, cardinal, and broad ligaments, if necessary, before pursuing entry into the anterior cul-de-sac. It is not essential to gain anterior access before taking these pedicles. The additional mobility and descensus enable safe sharp dissection.
If pedicles have been secured up to the fundus, and the anterior cul-de-sac remains difficult to assess, flip the fundus through the posterior cul-de-sac and reach your finger or an instrument around the top of the fundus to identify the peritoneum anteriorly, then incise it under direct vision.
VAGINAL HYSTERECTOMY CHALLENGE 4: Previous abdominal/pelvic surgery
This history is another often-cited rationale for avoiding the vaginal approach. In reality, the adhesions created from prior surgery tend to arise between the anterior abdominal wall and the omentum or small bowel. This situation makes laparoscopic or open abdominal entry riskier than vaginal peritoneal access through the cul-de-sac.
Two possible exceptions: a patient who has had surgery for deeply infiltrating endometriosis in the cul-de-sac or a woman who has undergone myomectomies with posterior incisions. These patients may have dense scarring in the cul-de-sac, which would preclude a vaginal approach. Whatever the surgical route, appropriate bowel preparation is necessary to permit simple closure of any intestinal injury at the time of hysterectomy. Why not begin vaginally if the exam under anesthesia demonstrates an accessible and reasonably free cul-de-sac?
VAGINAL HYSTERECTOMY CHALLENGE 5: Large myomatous uterus
A uterus of any size can be removed vaginally as long as there is mobility with access to the uterine arteries. The one exception: a patient with a small, normal uterus and a massive pedunculated myoma arising from the top of the fundus. If the fibroid cannot be pulled into the true pelvis for morcellation, it cannot be removed transvaginally. Fortunately, this situation is quite rare.
Keep the uterine serosa intact to maintain orientation.
Split the uterus from 12 to 6 o’clock (bivalving). Protect the bladder with a Deaver retractor anteriorly, and protect the posterior vaginal epithelium with the weighted speculum posteriorly.
Remove chunks of tissue from the inside of the specimen. Orient your scalpel blade so that you are always cutting toward the center of the specimen. That way, if the blade slips, you will not accidentally injure tissue on the pelvic sidewall.
Use Lahey thyroid clamps to place the tissue you plan to remove under tension. Finding the capsule of each myoma and gently separating it from the surrounding myometrium facilitates delivery of larger fibroids into the endometrial cavity. Some myomas require morcellation themselves for removal.
Replace the scalpel blade periodically to keep it sharp. Calcified fibroids can dull the blade rapidly.
Work systematically to remove as much central tissue as possible. Try to keep a clean, sharp margin of tissue around the edges for easy grasping. Torn, irregular tissue is very difficult to grab and may cause significant frustration.
If access becomes limited, try clamping additional pedicles on each side of the specimen. A tiny amount of additional descensus can make a huge difference.
Do not administer GnRH agonists prior to surgery. The uterus may shrink, but the myomas tend to become quite soft and difficult to remove. If the patient is seriously anemic, give norethindrone acetate, 5 to 20 mg daily, to stop bleeding and allow the patient’s red blood cell volume to improve before elective surgery.
Use a vessel-sealing instrument to control the pedicles. This strategy produces optimal hemostasis to permit a dry field during morcellation. Moreover, the seals do not get disrupted when the large uterus is pulled past them. Placing suture around pedicles when there is a large, bulky uterus in the pelvis is challenging at best, and it is frustrating to see significant bleeding after removal of the specimen. This problem does not seem to occur with the sealing devices.
Know when to quit! We should not promise any patient a minimally invasive operation. If there is uncontrolled bleeding or no progress after 5 to 10 minutes, convert to a laparoscopic or abdominal approach.
I schedule cases I know will be challenging as “possible” laparoscopic or open hysterectomy. This alerts the OR staff to have additional equipment ready and nearby should we need it. It is not a surgical failure or complication to convert a minimally invasive hysterectomy to a more invasive technique when appropriate. Better to have tried and failed than never to have tried at all!
VAGINAL HYSTERECTOMY CHALLENGE 6: Avoiding complications
The most common complications of vaginal hysterectomy are bleeding, infection, and injury to the bladder. Ureteral injury is less common at vaginal hysterectomy than with the abdominal or laparoscopic approaches. Thus, I do not think routine cystoscopy is essential after uncomplicated vaginal hysterectomy, although I recommend intravenous administration of indigo carmine dye at the beginning of the procedure to enable rapid recognition of even a small bladder laceration. Sharp, careful dissection of the bladder off the lower uterine segment and the avoidance of finger dissection (especially with a gauze sponge) keep these injuries to a minimum.
Minimize bleeding by using newer vessel-sealing technologies rather than suture for most of the pedicles. I attach the uterosacral–cardinal ligament pedicles to the vaginal cuff at closure with suture. I suture the first pedicle once I have entered the posterior cul-de-sac and hold that suture to stay oriented.
Pay attention to patient positioning. Careful positioning will help you avoid neurological injuries. Avoid hyperflexion at the hips, which stretches the femoral nerve. Large nerves have comparatively little blood supply, so stretching them for prolonged periods can cause hypoxic injury. Although such injuries are almost always rapidly reversible, they are disconcerting for both the patient and her surgeon.
When operating on a very thin woman with a bony sacrum, I like to place egg-crate foam beneath the buttocks to provide some cushioning. I am also very careful with these women to keep their legs in a neutral position, and I watch my surgical assistants to be sure they are not leaning on the patient during the procedure.
Prophylactic antibiotics are a must to avoid postoperative vaginal cuff infections and pelvic abscess. Smokers and women with preexisting bacterial vaginosis are at highest risk for infection. I ask women to discontinue smoking at least 2 weeks prior to surgery and inform all smokers that their risk of infection is heightened. I treat anaerobic overgrowth in the vagina prior to surgery to help prevent infections in women with bacterial vaginosis.
The timing of prophylactic antibiotics is important. Intravenous first-generation cephalosporins must be administered within 60 minutes of the initial incision, but it is important to give them early enough for them to adequately disseminate to tissue before the colpotomy incision.
DVT prophylaxis is especially important for women with large uteri. Routine use of sequential compression stockings is both cost-effective and equivalent to the prophylactic use of subcutaneous heparin, so I use them for all patients undergoing vaginal hysterectomy. Early ambulation (usually within 2 hours of surgery) is also helpful in avoiding thromboses.
90% of hysterectomies can be performed vaginally
Using the techniques described in this article, I have been able to perform over 90% of the hysterectomies in my practice vaginally. More than 50% of my patients are either morbidly obese, nulliparous, or have had previous abdominal surgery of some type.
The instruments I find most useful are the lighted suction irrigator and the vessel-sealing Heaney-type clamp.
Establishing a routine and approaching technically challenging cases with a systematic and standardized set of techniques make the vaginal route possible for the vast majority of patients with benign disease.
Dr. Levy has served as a consultant to ValleyLab.
Newer codes for vaginal hysterectomy capture the work of removing larger uteri without laparoscopy
True or false: When it comes to hysterectomy, surgeons tend to use the route that is safest, least invasive, and most economical.
Sadly, the statement is false. Although vaginal hysterectomy tops all 3 categories, it is the least utilized of surgical routes. The number of vaginal hysterectomies may have increased slightly over the past decade, likely due to the incorporation of laparoscopically assisted vaginal hysterectomy into the mainstream and increased practice with the vaginal component, but fewer than 30% of hysterectomies are performed vaginally.
This article addresses 6 common challenges at vaginal hysterectomy and offers strategies to overcome them.
Laparoscopic strategies ease vaginal hysterectomy, too
Laparoscopic hysterectomy became widely accepted when surgical instruments were developed to overcome the technical challenges inherent in operating with limited access. By incorporating some of the techniques we routinely use for laparoscopic surgery, we can overcome many of the challenges faced during difficult vaginal surgery.
VAGINAL HYSTERECTOMY CHALLENGE 1: Obesity
Unfortunately, our population is increasingly rotund. This is not only a significant risk factor for the patient’s health in general, but it poses some unique challenges for surgeons. I must say that, as tough as it may be to complete a hysterectomy vaginally in a morbidly obese woman, I would much rather approach her pelvic organs through the cul-de-sac, which contains no fat cells, than through the abdominal wall—either laparoscopically or abdominally! The trick is gaining access to the posterior cul-de-sac.
How to enter the cul-de-sac
It seems to be a perverse rule of nature, but a tight upper vaginal ring seems almost universal in obese women. Added to the redundant sidewalls and the large buttocks, this tightness makes entry into the anterior or posterior cul-de-sac problematic. Several tricks make peritoneal access possible:
Position the patient to increase access, with the buttocks well over the edge of the operating table. This brings the operative field a bit closer to the surgeon, and permits the use of long-handled retractors posteriorly.
Use candy-cane stirrups to allow assistants better access to the operative field. Adequate assistance is essential in attempting vaginal surgery in the morbidly obese.
Avoid the Trendelenburg position. Although it might seem that this position would facilitate visualization and placement of a posterior weighted speculum, all it does is allow the patient to slide up on the table, making placement of alternate retractors difficult.
Use the right tools. If the posterior weighted speculum will not stay in place or does not afford access to the cul-de-sac due to an upper vaginal ring, use a narrow Deaver retractor posteriorly (without sidewall or anterior retraction). Use a Jacob’s tenaculum on the posterior lip of the cervix and have your assistant pull straight up on the tenaculum while using the Deaver retractor to see the area between the uterosacral ligaments.
Use the uterosacral ligaments as a guide. Another perversity in morbidly obese women: Despite multiparity, they seem to have little or no apical prolapse but lots of vaginal wall redundancy. The cervix is often elongated, but the uterosacral ligaments are sky high.
I palpate these ligaments, injecting them with a combination of vasopressin diluted 1:5 with bupivacaine and epinephrine (for enhanced hemostasis and preemptive analgesia), then use a pencil electrosurgical electrode to rapidly open the vaginal epithelium between the ligaments.
I then use a long, toothed tissue forceps to tent the peritoneum at 90 degrees to the plane of the posterior cul-de-sac and use Mayo scissors to enter the peritoneal cavity. Usually there is a spurt of fluid to mark appropriate entry into the peritoneum.
I then use the blades of my scissors to stretch the peritoneum between the ligaments and place a moistened 4×4 sponge into the incision.
At the onset of the procedure, inject indigo carmine dye intravenously so that any injury to the bladder will be immediately recognized. I have the circulating nurse empty the bladder while she is prepping the patient, but do not leave an indwelling catheter in place during the operation. I find it cumbersome to work around the catheter.
Problematic entries
When entry into the posterior cul-de-sac is difficult, I stop dissection, place a 4×4 sponge into the incision to reduce bleeding from the vagina, and proceed to attempt anterior entry.
I place the Deaver retractor into the anterior space and move the tenaculum to the anterior lip of the cervix. This gives maximal space for downward traction on the cervix while anterior entry is attempted.
Once again, I inject the tissue with the bupivacaine solution before incising the vaginal epithelium at the level of the internal os. I use sharp dissection only when creating a plane between the lower uterine segment and the bladder.
Ensuring room to move and good visualization
If neither the anterior nor the posterior cul-de-sac can be accessed, it may be time to rethink the vaginal approach—but there is no harm in taking the uterosacral and cardinal ligaments extraperitoneally in an effort to gain some mobility. Hugging the uterus and leaving the anterior retractor in place to lift the bladder superiorly are essential steps to protect the ureters. The cervix can then be split in the midline (12 to 6 o’clock) to easily identify the peritoneal reflections.
Other tips
Sidewall retractors are rarely needed. They significantly impair placement of clamps and sutures by creating a long, narrow, parallel passageway. An alternative trick is to use the suction tip to retract the vaginal sidewall as the surgeon is working.
A disposable, fiberoptic, lighted suction irrigator is another option. The light can be directed precisely where it is needed, and the irrigation helps keep the field clean and tidy, simplifying identification of anatomy.
Skip the sutures whenever possible. Because it is difficult to place sutures with precision in a tight, poorly illuminated space, I use a vessel sealer for all pedicles above the uterosacral ligaments. Some of these instruments were designed specifically for vaginal hysterectomy in the same shape and size as Heaney clamps. They are remarkably efficient and permit the completion of a vaginal procedure when suture placement is difficult.
Use a Heaney needleholder, with the suture loaded precisely in the center of the needle curve, along with the lighted suction irrigator to retract redundant tissue away from the track of the needle, to facilitate suturing high in the pelvis.
VAGINAL HYSTERECTOMY CHALLENGE 2: Nulliparity
Many of us are reluctant to attempt vaginal hysterectomy in a woman who has never had children. Although this situation can be challenging at times, in my experience, the access issues tend to be more difficult in obese, multiparous women.
The same tricks and techniques addressed above will permit the vaginal approach in almost all nulliparous women.
VAGINAL HYSTERECTOMY CHALLENGE 3: Previous cesarean section
A prior cesarean delivery is sometimes considered an indication for laparoscopic hysterectomy. There are 2 concerns here: The patient may have a small pelvis, and there may be significant scar tissue and difficulty gaining access to the anterior cul-de-sac, as a result of repeated dissection between the lower uterine segment and the bladder. Several tricks may be useful:
Examine the patient under anesthesia to ensure that the fundus is not stuck to the anterior abdominal wall. This can occur if the peritoneum was not closed during the last cesarean section.
Empty the bladder before beginning the hysterectomy, and inject indigo carmine dye intravenously with the induction of anesthesia.
Use careful sharp dissection between the bladder and lower uterine segment using fine Metzenbaum scissors, with the tips pointed toward the uterus. Dissect only as far as you can easily see.
Secure the uterosacral, cardinal, and broad ligaments, if necessary, before pursuing entry into the anterior cul-de-sac. It is not essential to gain anterior access before taking these pedicles. The additional mobility and descensus enable safe sharp dissection.
If pedicles have been secured up to the fundus, and the anterior cul-de-sac remains difficult to assess, flip the fundus through the posterior cul-de-sac and reach your finger or an instrument around the top of the fundus to identify the peritoneum anteriorly, then incise it under direct vision.
VAGINAL HYSTERECTOMY CHALLENGE 4: Previous abdominal/pelvic surgery
This history is another often-cited rationale for avoiding the vaginal approach. In reality, the adhesions created from prior surgery tend to arise between the anterior abdominal wall and the omentum or small bowel. This situation makes laparoscopic or open abdominal entry riskier than vaginal peritoneal access through the cul-de-sac.
Two possible exceptions: a patient who has had surgery for deeply infiltrating endometriosis in the cul-de-sac or a woman who has undergone myomectomies with posterior incisions. These patients may have dense scarring in the cul-de-sac, which would preclude a vaginal approach. Whatever the surgical route, appropriate bowel preparation is necessary to permit simple closure of any intestinal injury at the time of hysterectomy. Why not begin vaginally if the exam under anesthesia demonstrates an accessible and reasonably free cul-de-sac?
VAGINAL HYSTERECTOMY CHALLENGE 5: Large myomatous uterus
A uterus of any size can be removed vaginally as long as there is mobility with access to the uterine arteries. The one exception: a patient with a small, normal uterus and a massive pedunculated myoma arising from the top of the fundus. If the fibroid cannot be pulled into the true pelvis for morcellation, it cannot be removed transvaginally. Fortunately, this situation is quite rare.
Keep the uterine serosa intact to maintain orientation.
Split the uterus from 12 to 6 o’clock (bivalving). Protect the bladder with a Deaver retractor anteriorly, and protect the posterior vaginal epithelium with the weighted speculum posteriorly.
Remove chunks of tissue from the inside of the specimen. Orient your scalpel blade so that you are always cutting toward the center of the specimen. That way, if the blade slips, you will not accidentally injure tissue on the pelvic sidewall.
Use Lahey thyroid clamps to place the tissue you plan to remove under tension. Finding the capsule of each myoma and gently separating it from the surrounding myometrium facilitates delivery of larger fibroids into the endometrial cavity. Some myomas require morcellation themselves for removal.
Replace the scalpel blade periodically to keep it sharp. Calcified fibroids can dull the blade rapidly.
Work systematically to remove as much central tissue as possible. Try to keep a clean, sharp margin of tissue around the edges for easy grasping. Torn, irregular tissue is very difficult to grab and may cause significant frustration.
If access becomes limited, try clamping additional pedicles on each side of the specimen. A tiny amount of additional descensus can make a huge difference.
Do not administer GnRH agonists prior to surgery. The uterus may shrink, but the myomas tend to become quite soft and difficult to remove. If the patient is seriously anemic, give norethindrone acetate, 5 to 20 mg daily, to stop bleeding and allow the patient’s red blood cell volume to improve before elective surgery.
Use a vessel-sealing instrument to control the pedicles. This strategy produces optimal hemostasis to permit a dry field during morcellation. Moreover, the seals do not get disrupted when the large uterus is pulled past them. Placing suture around pedicles when there is a large, bulky uterus in the pelvis is challenging at best, and it is frustrating to see significant bleeding after removal of the specimen. This problem does not seem to occur with the sealing devices.
Know when to quit! We should not promise any patient a minimally invasive operation. If there is uncontrolled bleeding or no progress after 5 to 10 minutes, convert to a laparoscopic or abdominal approach.
I schedule cases I know will be challenging as “possible” laparoscopic or open hysterectomy. This alerts the OR staff to have additional equipment ready and nearby should we need it. It is not a surgical failure or complication to convert a minimally invasive hysterectomy to a more invasive technique when appropriate. Better to have tried and failed than never to have tried at all!
VAGINAL HYSTERECTOMY CHALLENGE 6: Avoiding complications
The most common complications of vaginal hysterectomy are bleeding, infection, and injury to the bladder. Ureteral injury is less common at vaginal hysterectomy than with the abdominal or laparoscopic approaches. Thus, I do not think routine cystoscopy is essential after uncomplicated vaginal hysterectomy, although I recommend intravenous administration of indigo carmine dye at the beginning of the procedure to enable rapid recognition of even a small bladder laceration. Sharp, careful dissection of the bladder off the lower uterine segment and the avoidance of finger dissection (especially with a gauze sponge) keep these injuries to a minimum.
Minimize bleeding by using newer vessel-sealing technologies rather than suture for most of the pedicles. I attach the uterosacral–cardinal ligament pedicles to the vaginal cuff at closure with suture. I suture the first pedicle once I have entered the posterior cul-de-sac and hold that suture to stay oriented.
Pay attention to patient positioning. Careful positioning will help you avoid neurological injuries. Avoid hyperflexion at the hips, which stretches the femoral nerve. Large nerves have comparatively little blood supply, so stretching them for prolonged periods can cause hypoxic injury. Although such injuries are almost always rapidly reversible, they are disconcerting for both the patient and her surgeon.
When operating on a very thin woman with a bony sacrum, I like to place egg-crate foam beneath the buttocks to provide some cushioning. I am also very careful with these women to keep their legs in a neutral position, and I watch my surgical assistants to be sure they are not leaning on the patient during the procedure.
Prophylactic antibiotics are a must to avoid postoperative vaginal cuff infections and pelvic abscess. Smokers and women with preexisting bacterial vaginosis are at highest risk for infection. I ask women to discontinue smoking at least 2 weeks prior to surgery and inform all smokers that their risk of infection is heightened. I treat anaerobic overgrowth in the vagina prior to surgery to help prevent infections in women with bacterial vaginosis.
The timing of prophylactic antibiotics is important. Intravenous first-generation cephalosporins must be administered within 60 minutes of the initial incision, but it is important to give them early enough for them to adequately disseminate to tissue before the colpotomy incision.
DVT prophylaxis is especially important for women with large uteri. Routine use of sequential compression stockings is both cost-effective and equivalent to the prophylactic use of subcutaneous heparin, so I use them for all patients undergoing vaginal hysterectomy. Early ambulation (usually within 2 hours of surgery) is also helpful in avoiding thromboses.
90% of hysterectomies can be performed vaginally
Using the techniques described in this article, I have been able to perform over 90% of the hysterectomies in my practice vaginally. More than 50% of my patients are either morbidly obese, nulliparous, or have had previous abdominal surgery of some type.
The instruments I find most useful are the lighted suction irrigator and the vessel-sealing Heaney-type clamp.
Establishing a routine and approaching technically challenging cases with a systematic and standardized set of techniques make the vaginal route possible for the vast majority of patients with benign disease.
Dr. Levy has served as a consultant to ValleyLab.
SUGGESTED READING
1. Abostini A, Vejux N, Colette E, et al. Risk of bladder injury during vaginal hysterectomy in women with a previous cesarean section. J Reprod Med. 2005;50:940-942.
2. Clave H, Barr H, Niccolai P. Painless vaginal hysterectomy with thermal hemostasis (results of a series of 152 cases). Gynecol Surg. 2005;2:101-105.
3. Isik-Akbay EF, Harmanli OH, Panganamamula UR, et al. Hysterectomy in obese women: a comparison of abdominal and vaginal routes. Obstet Gynecol. 2004;104:710-714.
4. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
5. O’Neal MG, Beste T, Shackelford DP. Utility of preemptive local analgesia in vaginal hysterectomy. Am J Obstet Gynecol. 2003;189:1539-1542.
6. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005;(1):CD003677.-
SUGGESTED READING
1. Abostini A, Vejux N, Colette E, et al. Risk of bladder injury during vaginal hysterectomy in women with a previous cesarean section. J Reprod Med. 2005;50:940-942.
2. Clave H, Barr H, Niccolai P. Painless vaginal hysterectomy with thermal hemostasis (results of a series of 152 cases). Gynecol Surg. 2005;2:101-105.
3. Isik-Akbay EF, Harmanli OH, Panganamamula UR, et al. Hysterectomy in obese women: a comparison of abdominal and vaginal routes. Obstet Gynecol. 2004;104:710-714.
4. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
5. O’Neal MG, Beste T, Shackelford DP. Utility of preemptive local analgesia in vaginal hysterectomy. Am J Obstet Gynecol. 2003;189:1539-1542.
6. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005;(1):CD003677.-
HYSTEROSCOPIC STERILIZATION
With over 50,000 completed hysteroscopic sterilization procedures worldwide, and 5 years of data, what do we know so far about this innovation? It is now almost 4 years since the FDA approved Essure (Conceptus; San Carlos, Calif), the first hysteroscopic sterilization method available for use in the United States. Two other systems are in the works: Adiana (Adiana; Redwood City, Calif) has completed its Phase III clinical trial and Ovion (American Medical Systems; Minnetonka, Minn) is just beginning its clinical trial this year.
Comparison of the devices
Essure is a disposable delivery system with polyethylene (PET) fibers wound in and around a stainless steel inner coil. An outer coil of nitinol, a superelastic titanium/nickel alloy, is deployed to anchor the device across the uterotubal junction. Wound down, the micro-insert is 0.8 mm in diameter. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Over a period of about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram about 3 months after device placement is required before patients may rely on the device and stop birth control.
Adiana (not yet available in the United States) uses a combination of controlled epithelial destruction and insertion of a porous biomatrix to induce vascularized tissue ingrowth. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice (average less than 1 watt to the endosalpinx). A pushrod then delivers a small porous matrix of material into the tubal lumen. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes.
Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Accessing the tubes
One of the greatest hurdles for occluding the fallopian tubes hysteroscopically is access to the tubes. Both the Adiana clinical trial (not yet published) and the post-market analysis of Essure (not yet published) have demonstrated excellent bilateral placement rates.
Technique is not hard to learn. Both types of devices are inserted through the operating channel of a small hysteroscope. Initial concerns about the ability to access the tubal ostia do not appear to be an issue—at least for those early-adopter physicians performing the procedures. Both clinical trials included gynecologists who were not experienced in operative hysteroscopy. These studies found that cannulation of the tubes is a technique that is easy to learn and rapidly accomplished in most circumstances.
Bilateral placement rates were similar for both devices in the pivotal trials: Adiana 95% (612/655); Essure 90% (464/518).
What are the contraindications?
Approximately 10% of patients have factors that preclude bilateral device placement:
Anatomic factors
- Blocked or stenotic tubes
- Intrauterine adhesions
- Visual field obstructed by polyps, fibroids, or shaggy endometrium
- Lateral tubes
Device or procedure failures due to
- Tubal spasm
- Patient pain/intolerance
- Device malfunction
A second procedure (after correcting the initial problems) will be successful in many women who have what appears to be a technical glitch.
Clinical outcomes, so far
Ultimately, of course, the success of these procedures and the benefits to our patients will be determined by the placement rates in the real world and the ability of a majority of women to rely on the devices for permanent sterilization.
What can we say so far?
Bilateral placement rates
Although the bilateral placement rates for Essure in the pivotal trial were 88% with one attempt, increasing to 92% of all patients enrolled with a second attempt, the data from the postmarket study is even more promising. After FDA approval in November 2002, the manufacturer trained gynecologists in the procedure, and then monitored clinical outcomes in the initial cases performed by these surgeons once they had completed their training and mandatory proctored cases (data not yet published).
Physicians who participated in the clinical trial were excluded from this analysis. The bilateral device placement rate for these women treated by novice users is over 94%. Adiana’s Phase III trial data demonstrate a similar bilateral success rate. It appears that despite the misgivings of many ObGyns, these systems are easy to learn.
I have incorporated the following tips and tricks into my office practice with great results. Patients are thrilled with their experience and leave ready to recruit their friends for the procedure.
- Pretreatment with a nonsteroidal anti-inflammatory drug to block prostaglandin release and uterotubal spasm
- Scheduling the procedure for the early follicular phase of the menstrual cycle to minimize shaggy endometrium, or
- Suppressing the endometrium with progestins from the first day of menses until the scheduled procedure
- Use of warm fluid for uterine distension to reduce spasm
- Placement of topical lidocaine gel into the uterus 10 to 15 minutes prior to the procedure
- Use of a pressure bag to assure adequate uterine distension
Patient safety
Few complications have been reported with either technique. There were the expected rare vasovagal reactions, as well as 2 cases of hypervolemia with Essure and 1 case of hyponatremia in the Adiana trial. Both of these situations should be avoidable with proper monitoring and limiting distension fluids to isotonic solutions. All patients recovered fully. There were no problems with persistent pain or changes in menstrual patterns at 1 year in the Essure trial.
Expulsion of the devices was associated with proximal positioning of the devices in all cases (3%). Patients had no symptoms, and most were able to have a second procedure with excellent placement and retention. Expulsions were identified at the postprocedure scout film or hysterosalpingogram.
Tubal perforation was noted in 0.9% of the patients. Predisposing factors were preexisting tubal occlusion or hydrosalpinx.
Perforations were asymptomatic, as well. Laparoscopic evaluation in 3 cases demonstrated no adhesions or reactions to the tiny perforation sites.
Delivery. An outer coil of nitinol, a superelastic titanium/nickel alloy is deployed to anchor the device across the uterotubal junction. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Occlusion. Over about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram is required before patients may discontinue additional contraception.
Images: Rich Larocco
Anesthesia. Because hysteroscopic sterilization procedures may be performed without general anesthesia and by design avoid the need to access the abdominal cavity, they should be inherently safer for patients than the other available surgical sterilization methods. In the Essure and Adiana trials,1 more than 50% of the patients underwent their procedures under local anesthesia with no additional intravenous sedation. The others had local anesthesia with IV sedation. Only 1 patient (in the Essure trial) underwent general anesthesia.
Patient satisfaction and tolerance of the procedure were excellent; 88% of patients described their experience as good to excellent. Only 4% rated their procedure pain as severe. At discharge (approximately 40 minutes after conclusion of the procedure), 79% of patients had no pain and required no pain medication.
What are the potential complications?
The rare but devastating complications associated with laparoscopic sterilization should be avoidable with the hysteroscopic approach—at least for the 90% of patients for whom access to both tubes is feasible.
Ideal candidates. Hysteroscopic access seems to be the ideal approach for occlusion of the fallopian tubes in patients with medical conditions that may increase the risk of abdominal access, or for whom general anesthesia imposes added risk.
Problem conditions. The following clinical conditions and comorbidities all present significant challenges for the laparoscopic surgeon:
- Cardiac disease
- Thrombophilias
- Immune suppression
- Renal transplant
- Morbid obesity
- Previous abdominal surgery, especially bowel procedures
Delivery. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice. A pushrod then delivers a small porous matrix of material into the tubal lumen.
Occlusion. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes. Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Images: Rich Larocco
How many pregnancies?
There have been no pregnancies in Phase II and III trials of Essure, thus far. In July 2003, Cooper et al1 reported no pregnancies in 7,532 woman-months of use.
Conceptus is aware of 64 pregnancies among more than 50,000 procedures performed worldwide. None appeared to have occurred with proper demonstration of bilateral tubal occlusion after device placement. Most appear to be luteal phase pregnancies present at the time of the sterilization procedure, or failure of either the patient or the physician to assure tubal obstruction prior to stopping additional birth control methods.
There are no documented ectopic pregnancies, although 1 of the 64 reported cases may have been a very early tubal gestation. The patient was treated with methotrexate without firm documentation of the location of the pregnancy.
There are 2 pregnancies among the 605 patients with bilateral Adiana devices (6,860 woman-months of use as of September 2005). One resulted from an error in interpreting the hysterosalpingogram. The other did occur with a properly placed device and occlusion demonstrated on hysterosalpingogram.
It appears that hysteroscopic sterilization with Essure will have acceptable and preventable failure rates (longer term data and postmarket analysis are not yet available for the Adiana device). The calculated 5-year success rate is more than 99% for Essure; this compares favorably with all other surgical sterilization methods.
Do benefits outweigh costs?
The overall cost of hysteroscopic sterilization methods compares favorably with laparoscopic approaches. The expense of the disposable equipment is recouped by avoiding the costs of general anesthesia, and operating room and facility charges.
Advantages of the office setting
Payment for physicians is slightly more than the reimbursement for laparoscopic sterilization performed in a facility.
The real benefit to ObGyns, however, is in moving the procedure into the office environment. This allows us great flexibility in scheduling, and avoids the “down time” required for traveling to a facility, waiting for operating room turnover, anesthesia, and paperwork.
Benefit to healthcare systems. Researchers in closed healthcare systems have analyzed the expenses associated with Essure compared with laparoscopic tubal sterilization. When all costs associated with hysteroscopic sterilization are considered, including the need for additional procedures (when the tubes are not accessible or the procedure fails) and the 3-month hysterosalpingography, there remained a significant savings to the healthcare system for these procedures, compared with laparoscopic techniques.2
Ask women who are currently using contraceptive steroids about their menstrual cycles before they started hormonal birth control. Remind women who had menorrhagia or irregular cycles that no method of sterilization will manage their cycles.
The addition of endometrial ablation to the hysteroscopic sterilization procedure is an option.3 However, only 1 of the global ablation technologies currently has FDA approval for concomitant treatment with Essure: Thermachoice (Ethicon Women’s Health and Urology; Somerville, NJ).
Alternatives to permanent sterilization
In counseling women about permanent sterilization, it is important to cover the alternatives, as well.
IUDs. We should also consider the levonorgestrel-containing intrauterine device (IUD), Mirena (Berlex; Montville, NJ), for patients with menorrhagia who desire long-term contraception. Studies have demonstrated excellent patient satisfaction with this system and reduction in menstrual blood loss equivalent to endometrial ablation.4 Although not a permanent solution, the IUD does provide superb contraception, failure rates are similar to sterilization, and management of menorrhagia is excellent, and the cost is less than 10% of a combined endometrial ablation and sterilization procedure. The ParaGard copper-containing IUD is a good choice for women with normal or light flow, but not for those with heavy cycles.
Systemic hormone methods. For women willing to consider systemic hormones, Depo-Provera or the Implanon implantable rod provide excellent long-term contraception.
Vasectomy. Remember that vasectomy remains an option; however, many women want the assurance that they are in control.
Coding and insurance
The difference in payment for hysteroscopic sterilization can be considerable, depending on the site. When performed anywhere other than your office, payment for use of the facility, medications, personnel, and equipment goes to the facility, whether an ambulatory surgery center or a hospital. When we do these procedures in our offices, our reimbursement reflects the fact that we are using our office space, exam table, equipment, supplies, and personnel.
When considering where to perform hysteroscopic sterilization, remember that no one is paying for our space, personnel, and equipment when we are not in the office. Therefore, there is a great advantage in getting our overhead reimbursed when we perform procedures in the office.
The total relative value units payable to the physician for the new CPT code 58565 (hysteroscopy, surgical, with bilateral fallopian tube cannulation to induce occlusion by placement of permanent implants), which is the code for all systems currently under study:
- 12.12 if performed in a facility
- 57.91 if performed in the office
Most payers cover hysteroscopic sterilization when the policy covers sterilization. Determination of coverage by Medicaid has been secured in at least 36 states.
Past the tipping point
It is time to begin to adopt this technology into routine gynecology practice, for the benefits it offers patients, and practicing surgeons, as well. The data are accumulating on the safety and effectiveness of hysteroscopic sterilization techniques—more than 50,000 procedures have been performed worldwide, and we have 5 years of data.
An apt analogy. Although it is true that we might initiate this approach in up to 10% of women who may ultimately require laparoscopy, there appears to be little downside to the attempt. I would suggest the analogy of attempting an endometrial biopsy in the office in lieu of a D&C under anesthesia for postmenopausal women.
True, we sometimes fail, but for the vast majority of patients, it is clearly beneficial to attempt the office procedure and avoid anesthesia. Similarly, by avoiding abdominal access and general anesthesia for sterilization, we are providing a safer and more pleasant procedure with rapid recovery for our patients. Those few who require a different approach will have invested little time, energy, effort, or risk if we learn to perform hysteroscopic sterilization in the office setting.
Dr. Levy is a consultant to Conceptus, Inc.
1. Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
2. Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Min Invasive Gynecol. 2005;12:318-322.
3. Valle RF, Valdez J, Wright TC, Kenney M. Concomitant Essure tubal sterilization and Thermachoice endometrial ablation: feasibility and safety. Fertil Steril. 2006;86:152-158.
4. Busfield RA, Farquhar CM, Sowter MC, et al. A randomized trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113:257-263.
With over 50,000 completed hysteroscopic sterilization procedures worldwide, and 5 years of data, what do we know so far about this innovation? It is now almost 4 years since the FDA approved Essure (Conceptus; San Carlos, Calif), the first hysteroscopic sterilization method available for use in the United States. Two other systems are in the works: Adiana (Adiana; Redwood City, Calif) has completed its Phase III clinical trial and Ovion (American Medical Systems; Minnetonka, Minn) is just beginning its clinical trial this year.
Comparison of the devices
Essure is a disposable delivery system with polyethylene (PET) fibers wound in and around a stainless steel inner coil. An outer coil of nitinol, a superelastic titanium/nickel alloy, is deployed to anchor the device across the uterotubal junction. Wound down, the micro-insert is 0.8 mm in diameter. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Over a period of about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram about 3 months after device placement is required before patients may rely on the device and stop birth control.
Adiana (not yet available in the United States) uses a combination of controlled epithelial destruction and insertion of a porous biomatrix to induce vascularized tissue ingrowth. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice (average less than 1 watt to the endosalpinx). A pushrod then delivers a small porous matrix of material into the tubal lumen. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes.
Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Accessing the tubes
One of the greatest hurdles for occluding the fallopian tubes hysteroscopically is access to the tubes. Both the Adiana clinical trial (not yet published) and the post-market analysis of Essure (not yet published) have demonstrated excellent bilateral placement rates.
Technique is not hard to learn. Both types of devices are inserted through the operating channel of a small hysteroscope. Initial concerns about the ability to access the tubal ostia do not appear to be an issue—at least for those early-adopter physicians performing the procedures. Both clinical trials included gynecologists who were not experienced in operative hysteroscopy. These studies found that cannulation of the tubes is a technique that is easy to learn and rapidly accomplished in most circumstances.
Bilateral placement rates were similar for both devices in the pivotal trials: Adiana 95% (612/655); Essure 90% (464/518).
What are the contraindications?
Approximately 10% of patients have factors that preclude bilateral device placement:
Anatomic factors
- Blocked or stenotic tubes
- Intrauterine adhesions
- Visual field obstructed by polyps, fibroids, or shaggy endometrium
- Lateral tubes
Device or procedure failures due to
- Tubal spasm
- Patient pain/intolerance
- Device malfunction
A second procedure (after correcting the initial problems) will be successful in many women who have what appears to be a technical glitch.
Clinical outcomes, so far
Ultimately, of course, the success of these procedures and the benefits to our patients will be determined by the placement rates in the real world and the ability of a majority of women to rely on the devices for permanent sterilization.
What can we say so far?
Bilateral placement rates
Although the bilateral placement rates for Essure in the pivotal trial were 88% with one attempt, increasing to 92% of all patients enrolled with a second attempt, the data from the postmarket study is even more promising. After FDA approval in November 2002, the manufacturer trained gynecologists in the procedure, and then monitored clinical outcomes in the initial cases performed by these surgeons once they had completed their training and mandatory proctored cases (data not yet published).
Physicians who participated in the clinical trial were excluded from this analysis. The bilateral device placement rate for these women treated by novice users is over 94%. Adiana’s Phase III trial data demonstrate a similar bilateral success rate. It appears that despite the misgivings of many ObGyns, these systems are easy to learn.
I have incorporated the following tips and tricks into my office practice with great results. Patients are thrilled with their experience and leave ready to recruit their friends for the procedure.
- Pretreatment with a nonsteroidal anti-inflammatory drug to block prostaglandin release and uterotubal spasm
- Scheduling the procedure for the early follicular phase of the menstrual cycle to minimize shaggy endometrium, or
- Suppressing the endometrium with progestins from the first day of menses until the scheduled procedure
- Use of warm fluid for uterine distension to reduce spasm
- Placement of topical lidocaine gel into the uterus 10 to 15 minutes prior to the procedure
- Use of a pressure bag to assure adequate uterine distension
Patient safety
Few complications have been reported with either technique. There were the expected rare vasovagal reactions, as well as 2 cases of hypervolemia with Essure and 1 case of hyponatremia in the Adiana trial. Both of these situations should be avoidable with proper monitoring and limiting distension fluids to isotonic solutions. All patients recovered fully. There were no problems with persistent pain or changes in menstrual patterns at 1 year in the Essure trial.
Expulsion of the devices was associated with proximal positioning of the devices in all cases (3%). Patients had no symptoms, and most were able to have a second procedure with excellent placement and retention. Expulsions were identified at the postprocedure scout film or hysterosalpingogram.
Tubal perforation was noted in 0.9% of the patients. Predisposing factors were preexisting tubal occlusion or hydrosalpinx.
Perforations were asymptomatic, as well. Laparoscopic evaluation in 3 cases demonstrated no adhesions or reactions to the tiny perforation sites.
Delivery. An outer coil of nitinol, a superelastic titanium/nickel alloy is deployed to anchor the device across the uterotubal junction. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Occlusion. Over about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram is required before patients may discontinue additional contraception.
Images: Rich Larocco
Anesthesia. Because hysteroscopic sterilization procedures may be performed without general anesthesia and by design avoid the need to access the abdominal cavity, they should be inherently safer for patients than the other available surgical sterilization methods. In the Essure and Adiana trials,1 more than 50% of the patients underwent their procedures under local anesthesia with no additional intravenous sedation. The others had local anesthesia with IV sedation. Only 1 patient (in the Essure trial) underwent general anesthesia.
Patient satisfaction and tolerance of the procedure were excellent; 88% of patients described their experience as good to excellent. Only 4% rated their procedure pain as severe. At discharge (approximately 40 minutes after conclusion of the procedure), 79% of patients had no pain and required no pain medication.
What are the potential complications?
The rare but devastating complications associated with laparoscopic sterilization should be avoidable with the hysteroscopic approach—at least for the 90% of patients for whom access to both tubes is feasible.
Ideal candidates. Hysteroscopic access seems to be the ideal approach for occlusion of the fallopian tubes in patients with medical conditions that may increase the risk of abdominal access, or for whom general anesthesia imposes added risk.
Problem conditions. The following clinical conditions and comorbidities all present significant challenges for the laparoscopic surgeon:
- Cardiac disease
- Thrombophilias
- Immune suppression
- Renal transplant
- Morbid obesity
- Previous abdominal surgery, especially bowel procedures
Delivery. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice. A pushrod then delivers a small porous matrix of material into the tubal lumen.
Occlusion. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes. Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Images: Rich Larocco
How many pregnancies?
There have been no pregnancies in Phase II and III trials of Essure, thus far. In July 2003, Cooper et al1 reported no pregnancies in 7,532 woman-months of use.
Conceptus is aware of 64 pregnancies among more than 50,000 procedures performed worldwide. None appeared to have occurred with proper demonstration of bilateral tubal occlusion after device placement. Most appear to be luteal phase pregnancies present at the time of the sterilization procedure, or failure of either the patient or the physician to assure tubal obstruction prior to stopping additional birth control methods.
There are no documented ectopic pregnancies, although 1 of the 64 reported cases may have been a very early tubal gestation. The patient was treated with methotrexate without firm documentation of the location of the pregnancy.
There are 2 pregnancies among the 605 patients with bilateral Adiana devices (6,860 woman-months of use as of September 2005). One resulted from an error in interpreting the hysterosalpingogram. The other did occur with a properly placed device and occlusion demonstrated on hysterosalpingogram.
It appears that hysteroscopic sterilization with Essure will have acceptable and preventable failure rates (longer term data and postmarket analysis are not yet available for the Adiana device). The calculated 5-year success rate is more than 99% for Essure; this compares favorably with all other surgical sterilization methods.
Do benefits outweigh costs?
The overall cost of hysteroscopic sterilization methods compares favorably with laparoscopic approaches. The expense of the disposable equipment is recouped by avoiding the costs of general anesthesia, and operating room and facility charges.
Advantages of the office setting
Payment for physicians is slightly more than the reimbursement for laparoscopic sterilization performed in a facility.
The real benefit to ObGyns, however, is in moving the procedure into the office environment. This allows us great flexibility in scheduling, and avoids the “down time” required for traveling to a facility, waiting for operating room turnover, anesthesia, and paperwork.
Benefit to healthcare systems. Researchers in closed healthcare systems have analyzed the expenses associated with Essure compared with laparoscopic tubal sterilization. When all costs associated with hysteroscopic sterilization are considered, including the need for additional procedures (when the tubes are not accessible or the procedure fails) and the 3-month hysterosalpingography, there remained a significant savings to the healthcare system for these procedures, compared with laparoscopic techniques.2
Ask women who are currently using contraceptive steroids about their menstrual cycles before they started hormonal birth control. Remind women who had menorrhagia or irregular cycles that no method of sterilization will manage their cycles.
The addition of endometrial ablation to the hysteroscopic sterilization procedure is an option.3 However, only 1 of the global ablation technologies currently has FDA approval for concomitant treatment with Essure: Thermachoice (Ethicon Women’s Health and Urology; Somerville, NJ).
Alternatives to permanent sterilization
In counseling women about permanent sterilization, it is important to cover the alternatives, as well.
IUDs. We should also consider the levonorgestrel-containing intrauterine device (IUD), Mirena (Berlex; Montville, NJ), for patients with menorrhagia who desire long-term contraception. Studies have demonstrated excellent patient satisfaction with this system and reduction in menstrual blood loss equivalent to endometrial ablation.4 Although not a permanent solution, the IUD does provide superb contraception, failure rates are similar to sterilization, and management of menorrhagia is excellent, and the cost is less than 10% of a combined endometrial ablation and sterilization procedure. The ParaGard copper-containing IUD is a good choice for women with normal or light flow, but not for those with heavy cycles.
Systemic hormone methods. For women willing to consider systemic hormones, Depo-Provera or the Implanon implantable rod provide excellent long-term contraception.
Vasectomy. Remember that vasectomy remains an option; however, many women want the assurance that they are in control.
Coding and insurance
The difference in payment for hysteroscopic sterilization can be considerable, depending on the site. When performed anywhere other than your office, payment for use of the facility, medications, personnel, and equipment goes to the facility, whether an ambulatory surgery center or a hospital. When we do these procedures in our offices, our reimbursement reflects the fact that we are using our office space, exam table, equipment, supplies, and personnel.
When considering where to perform hysteroscopic sterilization, remember that no one is paying for our space, personnel, and equipment when we are not in the office. Therefore, there is a great advantage in getting our overhead reimbursed when we perform procedures in the office.
The total relative value units payable to the physician for the new CPT code 58565 (hysteroscopy, surgical, with bilateral fallopian tube cannulation to induce occlusion by placement of permanent implants), which is the code for all systems currently under study:
- 12.12 if performed in a facility
- 57.91 if performed in the office
Most payers cover hysteroscopic sterilization when the policy covers sterilization. Determination of coverage by Medicaid has been secured in at least 36 states.
Past the tipping point
It is time to begin to adopt this technology into routine gynecology practice, for the benefits it offers patients, and practicing surgeons, as well. The data are accumulating on the safety and effectiveness of hysteroscopic sterilization techniques—more than 50,000 procedures have been performed worldwide, and we have 5 years of data.
An apt analogy. Although it is true that we might initiate this approach in up to 10% of women who may ultimately require laparoscopy, there appears to be little downside to the attempt. I would suggest the analogy of attempting an endometrial biopsy in the office in lieu of a D&C under anesthesia for postmenopausal women.
True, we sometimes fail, but for the vast majority of patients, it is clearly beneficial to attempt the office procedure and avoid anesthesia. Similarly, by avoiding abdominal access and general anesthesia for sterilization, we are providing a safer and more pleasant procedure with rapid recovery for our patients. Those few who require a different approach will have invested little time, energy, effort, or risk if we learn to perform hysteroscopic sterilization in the office setting.
Dr. Levy is a consultant to Conceptus, Inc.
With over 50,000 completed hysteroscopic sterilization procedures worldwide, and 5 years of data, what do we know so far about this innovation? It is now almost 4 years since the FDA approved Essure (Conceptus; San Carlos, Calif), the first hysteroscopic sterilization method available for use in the United States. Two other systems are in the works: Adiana (Adiana; Redwood City, Calif) has completed its Phase III clinical trial and Ovion (American Medical Systems; Minnetonka, Minn) is just beginning its clinical trial this year.
Comparison of the devices
Essure is a disposable delivery system with polyethylene (PET) fibers wound in and around a stainless steel inner coil. An outer coil of nitinol, a superelastic titanium/nickel alloy, is deployed to anchor the device across the uterotubal junction. Wound down, the micro-insert is 0.8 mm in diameter. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Over a period of about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram about 3 months after device placement is required before patients may rely on the device and stop birth control.
Adiana (not yet available in the United States) uses a combination of controlled epithelial destruction and insertion of a porous biomatrix to induce vascularized tissue ingrowth. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice (average less than 1 watt to the endosalpinx). A pushrod then delivers a small porous matrix of material into the tubal lumen. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes.
Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Accessing the tubes
One of the greatest hurdles for occluding the fallopian tubes hysteroscopically is access to the tubes. Both the Adiana clinical trial (not yet published) and the post-market analysis of Essure (not yet published) have demonstrated excellent bilateral placement rates.
Technique is not hard to learn. Both types of devices are inserted through the operating channel of a small hysteroscope. Initial concerns about the ability to access the tubal ostia do not appear to be an issue—at least for those early-adopter physicians performing the procedures. Both clinical trials included gynecologists who were not experienced in operative hysteroscopy. These studies found that cannulation of the tubes is a technique that is easy to learn and rapidly accomplished in most circumstances.
Bilateral placement rates were similar for both devices in the pivotal trials: Adiana 95% (612/655); Essure 90% (464/518).
What are the contraindications?
Approximately 10% of patients have factors that preclude bilateral device placement:
Anatomic factors
- Blocked or stenotic tubes
- Intrauterine adhesions
- Visual field obstructed by polyps, fibroids, or shaggy endometrium
- Lateral tubes
Device or procedure failures due to
- Tubal spasm
- Patient pain/intolerance
- Device malfunction
A second procedure (after correcting the initial problems) will be successful in many women who have what appears to be a technical glitch.
Clinical outcomes, so far
Ultimately, of course, the success of these procedures and the benefits to our patients will be determined by the placement rates in the real world and the ability of a majority of women to rely on the devices for permanent sterilization.
What can we say so far?
Bilateral placement rates
Although the bilateral placement rates for Essure in the pivotal trial were 88% with one attempt, increasing to 92% of all patients enrolled with a second attempt, the data from the postmarket study is even more promising. After FDA approval in November 2002, the manufacturer trained gynecologists in the procedure, and then monitored clinical outcomes in the initial cases performed by these surgeons once they had completed their training and mandatory proctored cases (data not yet published).
Physicians who participated in the clinical trial were excluded from this analysis. The bilateral device placement rate for these women treated by novice users is over 94%. Adiana’s Phase III trial data demonstrate a similar bilateral success rate. It appears that despite the misgivings of many ObGyns, these systems are easy to learn.
I have incorporated the following tips and tricks into my office practice with great results. Patients are thrilled with their experience and leave ready to recruit their friends for the procedure.
- Pretreatment with a nonsteroidal anti-inflammatory drug to block prostaglandin release and uterotubal spasm
- Scheduling the procedure for the early follicular phase of the menstrual cycle to minimize shaggy endometrium, or
- Suppressing the endometrium with progestins from the first day of menses until the scheduled procedure
- Use of warm fluid for uterine distension to reduce spasm
- Placement of topical lidocaine gel into the uterus 10 to 15 minutes prior to the procedure
- Use of a pressure bag to assure adequate uterine distension
Patient safety
Few complications have been reported with either technique. There were the expected rare vasovagal reactions, as well as 2 cases of hypervolemia with Essure and 1 case of hyponatremia in the Adiana trial. Both of these situations should be avoidable with proper monitoring and limiting distension fluids to isotonic solutions. All patients recovered fully. There were no problems with persistent pain or changes in menstrual patterns at 1 year in the Essure trial.
Expulsion of the devices was associated with proximal positioning of the devices in all cases (3%). Patients had no symptoms, and most were able to have a second procedure with excellent placement and retention. Expulsions were identified at the postprocedure scout film or hysterosalpingogram.
Tubal perforation was noted in 0.9% of the patients. Predisposing factors were preexisting tubal occlusion or hydrosalpinx.
Perforations were asymptomatic, as well. Laparoscopic evaluation in 3 cases demonstrated no adhesions or reactions to the tiny perforation sites.
Delivery. An outer coil of nitinol, a superelastic titanium/nickel alloy is deployed to anchor the device across the uterotubal junction. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Occlusion. Over about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram is required before patients may discontinue additional contraception.
Images: Rich Larocco
Anesthesia. Because hysteroscopic sterilization procedures may be performed without general anesthesia and by design avoid the need to access the abdominal cavity, they should be inherently safer for patients than the other available surgical sterilization methods. In the Essure and Adiana trials,1 more than 50% of the patients underwent their procedures under local anesthesia with no additional intravenous sedation. The others had local anesthesia with IV sedation. Only 1 patient (in the Essure trial) underwent general anesthesia.
Patient satisfaction and tolerance of the procedure were excellent; 88% of patients described their experience as good to excellent. Only 4% rated their procedure pain as severe. At discharge (approximately 40 minutes after conclusion of the procedure), 79% of patients had no pain and required no pain medication.
What are the potential complications?
The rare but devastating complications associated with laparoscopic sterilization should be avoidable with the hysteroscopic approach—at least for the 90% of patients for whom access to both tubes is feasible.
Ideal candidates. Hysteroscopic access seems to be the ideal approach for occlusion of the fallopian tubes in patients with medical conditions that may increase the risk of abdominal access, or for whom general anesthesia imposes added risk.
Problem conditions. The following clinical conditions and comorbidities all present significant challenges for the laparoscopic surgeon:
- Cardiac disease
- Thrombophilias
- Immune suppression
- Renal transplant
- Morbid obesity
- Previous abdominal surgery, especially bowel procedures
Delivery. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice. A pushrod then delivers a small porous matrix of material into the tubal lumen.
Occlusion. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes. Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Images: Rich Larocco
How many pregnancies?
There have been no pregnancies in Phase II and III trials of Essure, thus far. In July 2003, Cooper et al1 reported no pregnancies in 7,532 woman-months of use.
Conceptus is aware of 64 pregnancies among more than 50,000 procedures performed worldwide. None appeared to have occurred with proper demonstration of bilateral tubal occlusion after device placement. Most appear to be luteal phase pregnancies present at the time of the sterilization procedure, or failure of either the patient or the physician to assure tubal obstruction prior to stopping additional birth control methods.
There are no documented ectopic pregnancies, although 1 of the 64 reported cases may have been a very early tubal gestation. The patient was treated with methotrexate without firm documentation of the location of the pregnancy.
There are 2 pregnancies among the 605 patients with bilateral Adiana devices (6,860 woman-months of use as of September 2005). One resulted from an error in interpreting the hysterosalpingogram. The other did occur with a properly placed device and occlusion demonstrated on hysterosalpingogram.
It appears that hysteroscopic sterilization with Essure will have acceptable and preventable failure rates (longer term data and postmarket analysis are not yet available for the Adiana device). The calculated 5-year success rate is more than 99% for Essure; this compares favorably with all other surgical sterilization methods.
Do benefits outweigh costs?
The overall cost of hysteroscopic sterilization methods compares favorably with laparoscopic approaches. The expense of the disposable equipment is recouped by avoiding the costs of general anesthesia, and operating room and facility charges.
Advantages of the office setting
Payment for physicians is slightly more than the reimbursement for laparoscopic sterilization performed in a facility.
The real benefit to ObGyns, however, is in moving the procedure into the office environment. This allows us great flexibility in scheduling, and avoids the “down time” required for traveling to a facility, waiting for operating room turnover, anesthesia, and paperwork.
Benefit to healthcare systems. Researchers in closed healthcare systems have analyzed the expenses associated with Essure compared with laparoscopic tubal sterilization. When all costs associated with hysteroscopic sterilization are considered, including the need for additional procedures (when the tubes are not accessible or the procedure fails) and the 3-month hysterosalpingography, there remained a significant savings to the healthcare system for these procedures, compared with laparoscopic techniques.2
Ask women who are currently using contraceptive steroids about their menstrual cycles before they started hormonal birth control. Remind women who had menorrhagia or irregular cycles that no method of sterilization will manage their cycles.
The addition of endometrial ablation to the hysteroscopic sterilization procedure is an option.3 However, only 1 of the global ablation technologies currently has FDA approval for concomitant treatment with Essure: Thermachoice (Ethicon Women’s Health and Urology; Somerville, NJ).
Alternatives to permanent sterilization
In counseling women about permanent sterilization, it is important to cover the alternatives, as well.
IUDs. We should also consider the levonorgestrel-containing intrauterine device (IUD), Mirena (Berlex; Montville, NJ), for patients with menorrhagia who desire long-term contraception. Studies have demonstrated excellent patient satisfaction with this system and reduction in menstrual blood loss equivalent to endometrial ablation.4 Although not a permanent solution, the IUD does provide superb contraception, failure rates are similar to sterilization, and management of menorrhagia is excellent, and the cost is less than 10% of a combined endometrial ablation and sterilization procedure. The ParaGard copper-containing IUD is a good choice for women with normal or light flow, but not for those with heavy cycles.
Systemic hormone methods. For women willing to consider systemic hormones, Depo-Provera or the Implanon implantable rod provide excellent long-term contraception.
Vasectomy. Remember that vasectomy remains an option; however, many women want the assurance that they are in control.
Coding and insurance
The difference in payment for hysteroscopic sterilization can be considerable, depending on the site. When performed anywhere other than your office, payment for use of the facility, medications, personnel, and equipment goes to the facility, whether an ambulatory surgery center or a hospital. When we do these procedures in our offices, our reimbursement reflects the fact that we are using our office space, exam table, equipment, supplies, and personnel.
When considering where to perform hysteroscopic sterilization, remember that no one is paying for our space, personnel, and equipment when we are not in the office. Therefore, there is a great advantage in getting our overhead reimbursed when we perform procedures in the office.
The total relative value units payable to the physician for the new CPT code 58565 (hysteroscopy, surgical, with bilateral fallopian tube cannulation to induce occlusion by placement of permanent implants), which is the code for all systems currently under study:
- 12.12 if performed in a facility
- 57.91 if performed in the office
Most payers cover hysteroscopic sterilization when the policy covers sterilization. Determination of coverage by Medicaid has been secured in at least 36 states.
Past the tipping point
It is time to begin to adopt this technology into routine gynecology practice, for the benefits it offers patients, and practicing surgeons, as well. The data are accumulating on the safety and effectiveness of hysteroscopic sterilization techniques—more than 50,000 procedures have been performed worldwide, and we have 5 years of data.
An apt analogy. Although it is true that we might initiate this approach in up to 10% of women who may ultimately require laparoscopy, there appears to be little downside to the attempt. I would suggest the analogy of attempting an endometrial biopsy in the office in lieu of a D&C under anesthesia for postmenopausal women.
True, we sometimes fail, but for the vast majority of patients, it is clearly beneficial to attempt the office procedure and avoid anesthesia. Similarly, by avoiding abdominal access and general anesthesia for sterilization, we are providing a safer and more pleasant procedure with rapid recovery for our patients. Those few who require a different approach will have invested little time, energy, effort, or risk if we learn to perform hysteroscopic sterilization in the office setting.
Dr. Levy is a consultant to Conceptus, Inc.
1. Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
2. Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Min Invasive Gynecol. 2005;12:318-322.
3. Valle RF, Valdez J, Wright TC, Kenney M. Concomitant Essure tubal sterilization and Thermachoice endometrial ablation: feasibility and safety. Fertil Steril. 2006;86:152-158.
4. Busfield RA, Farquhar CM, Sowter MC, et al. A randomized trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113:257-263.
1. Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
2. Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Min Invasive Gynecol. 2005;12:318-322.
3. Valle RF, Valdez J, Wright TC, Kenney M. Concomitant Essure tubal sterilization and Thermachoice endometrial ablation: feasibility and safety. Fertil Steril. 2006;86:152-158.
4. Busfield RA, Farquhar CM, Sowter MC, et al. A randomized trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113:257-263.
IN THIS ARTICLE
A look at the latest fibroid treatments, including uterine artery embolization, focused ultrasound, and drug therapy.
Not long ago, women with uterine fibroids had to choose between hysterectomy and abdominal myomectomy to alleviate their symptoms. Then came minimally invasive surgeries such as laparoscopic myomectomy and hysteroscopic myoma resection, although even now these surgeries are offered by a limited number of skilled gynecologic surgeons. And despite their substantially shorter recovery times, these procedures are still surgeries, with inherent complications. On top of that, long-term outcomes data are limited.
Enter the next generation of fibroid treatments: uterine artery embolization (UAE), focused ultrasound with magnetic resonance imaging (MRI) guidance, and selective progesterone receptor modulators—though the last option is still in the pipeline. Gynecologists will be seeing advertisements and promotional materials for these interventions in the near and not-so-distant future.
Uterine artery embolization: In the right hands, a worthwhile strategy
Myers ER, Goodwin S, Landow W, et al. Prospective data collection of a new procedure by a specialty society: the FIBROID Registry. Obstet Gynecol. 2005;106:44–51.
Worthington-Kirsch R, Spies JB, Myers ER, et al. The Fibroid Registry for outcomes data (FIBROID) for uterine embolization: short-term outcomes. Obstet Gynecol. 2005;106:52–59.
Congratulations are in order. When the Society of Interventional Radiology created the Fibroid Registry in 2000, it was looking for early data on UAE to share with patients. In its short but impressive life, the registry has collected more data about UAE than we have about “tried-and-true” surgeries.
In the United States, UAE was first used to treat fibroids in 1997. Although numerous studies since then have reported on its safety and effectiveness, many gynecologists continue to question the suitability of UAE for symptomatic women.
The Web-based registry was established with Duke Clinical Research Institute to track short- and long-term outcomes after UAE in various settings.
What we know from the registry
The Fibroid Registry enrolled its first patient in December 2000, and collected data from 72 sites on 3,319 UAE procedures through December 2002. The reports by Myers et al and Worthington-Kirsch and colleagues contain patient demographics, procedural details, and 30-day outcomes.
The registry defined adverse events as any unexpected event that necessitated an unscheduled office or emergency room visit or unanticipated therapy (medical or surgical). Major complications required increased care or additional hospitalization or had permanent adverse sequelae. Minor complications required medical management or no therapy.
Thirty-day outcomes were available for approximately 91% of patients.
A low complication rate
Complications were uncommon during the first 30 days after UAE, with a 1.1% incidence of additional surgery. In fact, complication rates and recovery times compared favorably with myomectomy and abdominal hysterectomy for large fibroids.
The UAE procedure averaged 56 minutes, with 96.2% technical success and a return to normal activities in about 2 weeks.
Other findings:
- 26% of patients had an adverse event, but only 4% had a major event, most commonly emergency care or hospital readmission for pain management (2.1%) or possible infection (<1%).
- 31 women required another procedure within 30 days, including 3 myomectomies, 9 dilatation and curettage procedures for sloughing leiomyomata, 5 hysteroscopic resections, and 3 hysterectomies for unrecorded indications.
- 1 patient was hospitalized for pain 10 days after UAE and underwent exploratory laparotomy with bilateral oophorectomy.
- The most common minor adverse events were hot flushes (5.7%) and pain requiring additional therapy (9.6%).
Predictors of adverse outcomes:
- current or recent smoking (odds ratio [OR] 1.14, 95% confidence interval [CI] 1.007–1.293),
- African-American race (OR 1.129, 95% CI 1.019–1.251),
- prior procedures (OR 1.23, 95% CI 1.02–1.38), and
- duration of procedure (OR 1.004, 95% CI 1.001–1.006).
Interestingly, short-term outcomes did not differ among centers, nor did procedure times, length of stay, and incidence of adverse events during the first 30 days.
What to tell patients
I inform patients with symptomatic fibroids about all available treatments—including the option of doing nothing at all. Most have no interest in UAE, and are distressed by the thought of their bodies reabsorbing dead tissue.
However, I have had several patients whose operative risks were very high. For example, 1 woman was morbidly obese (>250 lb, which required her UAE treatment at a special facility equipped to perform fluoroscopy in morbidly obese patients), hypertensive, diabetic, and hemiparetic after a stroke. She was also a Jehovah’s Witness. Obviously, nonsurgical intervention was to her benefit. Her bleeding stopped almost immediately.
A reasonable alternative
The lay press recently focused on our obligation, as ObGyns, to inform patients about alternative therapies for fibroids. These first reports from the Fibroid Registry are clear: UAE is a reproducible, low-risk procedure—certainly lower in risk than complex surgeries (though situations may arise when myomectomy is preferred, such as a desire for future fertility1).
The registry will continue to provide data we can share with our patients regarding risks, complications, and long-term outcomes. More importantly, our radiology colleagues have taken the lead in developing a voluntary patient registry to track the outcomes of new technology as it disseminates into the general medical community. Our patients would be well served if we developed similar registries to track our surgical outcomes.
Focused ultrasound shrinks fibroids, but has strict eligibility requirements
Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004;183:1713–1719.
Using MRI guidance, a completely noninvasive treatment is now possible: focused ultrasound ablation of uterine fibroids. This procedure, the newest high-tech treatment for symptomatic fibroids, was successfully tested in an earlier pilot study.2 Although it eased symptoms to a remarkable degree during this phase III trial, how many women will ultimately be suitable for the treatment remains unclear.
Selection criteria
This trial of the ExAblate 2000 (InSightec, Tirat Carmel, Israel) recruited symptomatic women who were otherwise suitable candidates for conventional surgeries. Excluded were postmenopausal patients, women weighing more than 250 lb, and women who had a uterus larger than 24 weeks’ size or any single myoma larger than 10 cm. Women with extensive abdominal scars were carefully examined and excluded if the scars lay in the path of the ultrasound beam. The reason: During the earlier study, these scars tended to absorb ultrasound energy, increasing the risk of thermal injury at the skin surface.
Before proceeding, patients underwent MRI or ultrasound to confirm a clear pathway from the anterior abdominal wall to the fibroids without traversing the bladder or bowel.
Hindley et al did not reveal how many women were initially screened, but 109 were finally enrolled at 7 international sites and completed a Uterine Fibroid Symptoms and Quality of Life Questionnaire before and 3 and 6 months after treatment. As for the location of the fibroids: 22% were submucosal, 57% were intramural, and 21% were subserosal.
Up to 4 fibroids were treated per patient—with MRI mapping and thermographic monitoring—with a margin of 1.5 cm from the edge of the ablated area to the edge of the uterus. Conscious sedation was provided as needed, and tolerance of the procedure was measured using a 4-point pain scale. Mean time in the MRI scanner was 202 minutes (range, 90–370 minutes).
Pain stopped when treatment ended
Most women reported mild to moderate pain during the procedure (66%), and 16% complained of severe pain. This discomfort ended immediately when treatment stopped in virtually all patients—only 1% reported severe pain afterwards.
Adverse events
Serious adverse events included: 5 women with heavy menses requiring blood transfusions, 1 patient with pain and bleeding consistent with preprocedure symptoms, 1 woman needing overnight hospitalization for nausea related to opioid analgesia during the procedure, and 1 patient with leg and buttock pain immediately after treatment (it was later discovered that the sciatic nerve was in the far field of the sonication pathway). These symptoms resolved by the follow-up visit.
Two other patients had adverse events unrelated to the procedure.
6-month outcomes
The mean fibroid volume reduction was 13.5%±32%. Although this improvement seems modest, women reported significant relief from fibroid-related symptoms, and the mean severity score on the quality-of-life questionnaire decreased.
Substantial improvement was seen for both mass effect and bleeding symptoms (32.8 points out of 100 for each).
Pros and cons for symptomatic women
Advantages over UAE include the absence of postprocedural pain in almost all patients, which eliminates the need for an overnight stay and likely speeds the return to normal activities.
Disadvantages are that women with major abdominal scarring, anterior myomas underneath the bladder flap, or adhesive disease that causes small or large bowel to lie in the path of the sound waves cannot take advantage of this new technology, nor can women who have myomas close to neurovascular bundles. Add to that the extremely long procedure time (over 3 hours) and the modest reduction in uterine volume.
Six-month outcomes are promising, but this approach is probably best reserved for an academic setting, so careful screening and long-term tracking can continue.
Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–438.
In this comprehensive review, the authors draw from their extensive expertise in endocrinology to describe the rationale behind asoprisnil, a mixed progesterone receptor agonist/antagonist—the most promising pharmaceutical development in gynecology in several decades.
How the drug works
Mifepristone (RU-486) was the first progesterone receptor antagonist. Despite its ability to reduce myoma volume and suppress endometriosis symptoms in small pilot studies, it induces endometrial hyperplasia at doses higher than 5 to 10 mg, probably by acting similarly to unopposed estrogen. In contrast, asoprisnil has antiproliferative effects and no labor-inducing activity. It directly affects blood vessels in the endometrium, creating a local antiproliferative effect that induces amenorrhea despite normal estrogen levels.
What phase II studies reveal
A multicenter, double-blind, placebo-controlled trial involved 5-, 10-, and 25-mg daily doses of oral asoprisnil over 12 weeks. In a dose-dependent manner, asoprisnil induced amenorrhea or significantly suppressed bleeding without causing breakthrough or intermenstrual flow. It also decreased the volume of both the largest fibroid and the uterus as a whole. At 10- and 25-mg doses, pressure symptoms eased substantially over placebo. Adverse effects were minimal and affected the placebo and asoprisnil groups equally.
Phase III trials are now under way to assess the safety and efficacy of asoprisnil in the treatment of menorrhagia and uterine fibroids. Early results in the treatment of endometriosis are also promising.
What this means for fibroid patients
Though asoprisnil is not yet available for use, the phase III trial is winding down and the drug’s impressive potential seems clear.
A useful strategy may be to counsel marginally symptomatic women with fibroids that treatments are in the pipeline that would permit pharmaceutical management of their symptoms. Because this drug induces amenorrhea in the presence of normal circulating estrogen levels, it eliminates hot flushes and, more importantly, the bone loss associated with gonadotropin-releasing hormone agonists.
Disclosure
The author reports no financial relationships relevant to this article.
1. Pron G, Mocarski E, Bennett J, et al. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
2. Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48-54.
Not long ago, women with uterine fibroids had to choose between hysterectomy and abdominal myomectomy to alleviate their symptoms. Then came minimally invasive surgeries such as laparoscopic myomectomy and hysteroscopic myoma resection, although even now these surgeries are offered by a limited number of skilled gynecologic surgeons. And despite their substantially shorter recovery times, these procedures are still surgeries, with inherent complications. On top of that, long-term outcomes data are limited.
Enter the next generation of fibroid treatments: uterine artery embolization (UAE), focused ultrasound with magnetic resonance imaging (MRI) guidance, and selective progesterone receptor modulators—though the last option is still in the pipeline. Gynecologists will be seeing advertisements and promotional materials for these interventions in the near and not-so-distant future.
Uterine artery embolization: In the right hands, a worthwhile strategy
Myers ER, Goodwin S, Landow W, et al. Prospective data collection of a new procedure by a specialty society: the FIBROID Registry. Obstet Gynecol. 2005;106:44–51.
Worthington-Kirsch R, Spies JB, Myers ER, et al. The Fibroid Registry for outcomes data (FIBROID) for uterine embolization: short-term outcomes. Obstet Gynecol. 2005;106:52–59.
Congratulations are in order. When the Society of Interventional Radiology created the Fibroid Registry in 2000, it was looking for early data on UAE to share with patients. In its short but impressive life, the registry has collected more data about UAE than we have about “tried-and-true” surgeries.
In the United States, UAE was first used to treat fibroids in 1997. Although numerous studies since then have reported on its safety and effectiveness, many gynecologists continue to question the suitability of UAE for symptomatic women.
The Web-based registry was established with Duke Clinical Research Institute to track short- and long-term outcomes after UAE in various settings.
What we know from the registry
The Fibroid Registry enrolled its first patient in December 2000, and collected data from 72 sites on 3,319 UAE procedures through December 2002. The reports by Myers et al and Worthington-Kirsch and colleagues contain patient demographics, procedural details, and 30-day outcomes.
The registry defined adverse events as any unexpected event that necessitated an unscheduled office or emergency room visit or unanticipated therapy (medical or surgical). Major complications required increased care or additional hospitalization or had permanent adverse sequelae. Minor complications required medical management or no therapy.
Thirty-day outcomes were available for approximately 91% of patients.
A low complication rate
Complications were uncommon during the first 30 days after UAE, with a 1.1% incidence of additional surgery. In fact, complication rates and recovery times compared favorably with myomectomy and abdominal hysterectomy for large fibroids.
The UAE procedure averaged 56 minutes, with 96.2% technical success and a return to normal activities in about 2 weeks.
Other findings:
- 26% of patients had an adverse event, but only 4% had a major event, most commonly emergency care or hospital readmission for pain management (2.1%) or possible infection (<1%).
- 31 women required another procedure within 30 days, including 3 myomectomies, 9 dilatation and curettage procedures for sloughing leiomyomata, 5 hysteroscopic resections, and 3 hysterectomies for unrecorded indications.
- 1 patient was hospitalized for pain 10 days after UAE and underwent exploratory laparotomy with bilateral oophorectomy.
- The most common minor adverse events were hot flushes (5.7%) and pain requiring additional therapy (9.6%).
Predictors of adverse outcomes:
- current or recent smoking (odds ratio [OR] 1.14, 95% confidence interval [CI] 1.007–1.293),
- African-American race (OR 1.129, 95% CI 1.019–1.251),
- prior procedures (OR 1.23, 95% CI 1.02–1.38), and
- duration of procedure (OR 1.004, 95% CI 1.001–1.006).
Interestingly, short-term outcomes did not differ among centers, nor did procedure times, length of stay, and incidence of adverse events during the first 30 days.
What to tell patients
I inform patients with symptomatic fibroids about all available treatments—including the option of doing nothing at all. Most have no interest in UAE, and are distressed by the thought of their bodies reabsorbing dead tissue.
However, I have had several patients whose operative risks were very high. For example, 1 woman was morbidly obese (>250 lb, which required her UAE treatment at a special facility equipped to perform fluoroscopy in morbidly obese patients), hypertensive, diabetic, and hemiparetic after a stroke. She was also a Jehovah’s Witness. Obviously, nonsurgical intervention was to her benefit. Her bleeding stopped almost immediately.
A reasonable alternative
The lay press recently focused on our obligation, as ObGyns, to inform patients about alternative therapies for fibroids. These first reports from the Fibroid Registry are clear: UAE is a reproducible, low-risk procedure—certainly lower in risk than complex surgeries (though situations may arise when myomectomy is preferred, such as a desire for future fertility1).
The registry will continue to provide data we can share with our patients regarding risks, complications, and long-term outcomes. More importantly, our radiology colleagues have taken the lead in developing a voluntary patient registry to track the outcomes of new technology as it disseminates into the general medical community. Our patients would be well served if we developed similar registries to track our surgical outcomes.
Focused ultrasound shrinks fibroids, but has strict eligibility requirements
Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004;183:1713–1719.
Using MRI guidance, a completely noninvasive treatment is now possible: focused ultrasound ablation of uterine fibroids. This procedure, the newest high-tech treatment for symptomatic fibroids, was successfully tested in an earlier pilot study.2 Although it eased symptoms to a remarkable degree during this phase III trial, how many women will ultimately be suitable for the treatment remains unclear.
Selection criteria
This trial of the ExAblate 2000 (InSightec, Tirat Carmel, Israel) recruited symptomatic women who were otherwise suitable candidates for conventional surgeries. Excluded were postmenopausal patients, women weighing more than 250 lb, and women who had a uterus larger than 24 weeks’ size or any single myoma larger than 10 cm. Women with extensive abdominal scars were carefully examined and excluded if the scars lay in the path of the ultrasound beam. The reason: During the earlier study, these scars tended to absorb ultrasound energy, increasing the risk of thermal injury at the skin surface.
Before proceeding, patients underwent MRI or ultrasound to confirm a clear pathway from the anterior abdominal wall to the fibroids without traversing the bladder or bowel.
Hindley et al did not reveal how many women were initially screened, but 109 were finally enrolled at 7 international sites and completed a Uterine Fibroid Symptoms and Quality of Life Questionnaire before and 3 and 6 months after treatment. As for the location of the fibroids: 22% were submucosal, 57% were intramural, and 21% were subserosal.
Up to 4 fibroids were treated per patient—with MRI mapping and thermographic monitoring—with a margin of 1.5 cm from the edge of the ablated area to the edge of the uterus. Conscious sedation was provided as needed, and tolerance of the procedure was measured using a 4-point pain scale. Mean time in the MRI scanner was 202 minutes (range, 90–370 minutes).
Pain stopped when treatment ended
Most women reported mild to moderate pain during the procedure (66%), and 16% complained of severe pain. This discomfort ended immediately when treatment stopped in virtually all patients—only 1% reported severe pain afterwards.
Adverse events
Serious adverse events included: 5 women with heavy menses requiring blood transfusions, 1 patient with pain and bleeding consistent with preprocedure symptoms, 1 woman needing overnight hospitalization for nausea related to opioid analgesia during the procedure, and 1 patient with leg and buttock pain immediately after treatment (it was later discovered that the sciatic nerve was in the far field of the sonication pathway). These symptoms resolved by the follow-up visit.
Two other patients had adverse events unrelated to the procedure.
6-month outcomes
The mean fibroid volume reduction was 13.5%±32%. Although this improvement seems modest, women reported significant relief from fibroid-related symptoms, and the mean severity score on the quality-of-life questionnaire decreased.
Substantial improvement was seen for both mass effect and bleeding symptoms (32.8 points out of 100 for each).
Pros and cons for symptomatic women
Advantages over UAE include the absence of postprocedural pain in almost all patients, which eliminates the need for an overnight stay and likely speeds the return to normal activities.
Disadvantages are that women with major abdominal scarring, anterior myomas underneath the bladder flap, or adhesive disease that causes small or large bowel to lie in the path of the sound waves cannot take advantage of this new technology, nor can women who have myomas close to neurovascular bundles. Add to that the extremely long procedure time (over 3 hours) and the modest reduction in uterine volume.
Six-month outcomes are promising, but this approach is probably best reserved for an academic setting, so careful screening and long-term tracking can continue.
Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–438.
In this comprehensive review, the authors draw from their extensive expertise in endocrinology to describe the rationale behind asoprisnil, a mixed progesterone receptor agonist/antagonist—the most promising pharmaceutical development in gynecology in several decades.
How the drug works
Mifepristone (RU-486) was the first progesterone receptor antagonist. Despite its ability to reduce myoma volume and suppress endometriosis symptoms in small pilot studies, it induces endometrial hyperplasia at doses higher than 5 to 10 mg, probably by acting similarly to unopposed estrogen. In contrast, asoprisnil has antiproliferative effects and no labor-inducing activity. It directly affects blood vessels in the endometrium, creating a local antiproliferative effect that induces amenorrhea despite normal estrogen levels.
What phase II studies reveal
A multicenter, double-blind, placebo-controlled trial involved 5-, 10-, and 25-mg daily doses of oral asoprisnil over 12 weeks. In a dose-dependent manner, asoprisnil induced amenorrhea or significantly suppressed bleeding without causing breakthrough or intermenstrual flow. It also decreased the volume of both the largest fibroid and the uterus as a whole. At 10- and 25-mg doses, pressure symptoms eased substantially over placebo. Adverse effects were minimal and affected the placebo and asoprisnil groups equally.
Phase III trials are now under way to assess the safety and efficacy of asoprisnil in the treatment of menorrhagia and uterine fibroids. Early results in the treatment of endometriosis are also promising.
What this means for fibroid patients
Though asoprisnil is not yet available for use, the phase III trial is winding down and the drug’s impressive potential seems clear.
A useful strategy may be to counsel marginally symptomatic women with fibroids that treatments are in the pipeline that would permit pharmaceutical management of their symptoms. Because this drug induces amenorrhea in the presence of normal circulating estrogen levels, it eliminates hot flushes and, more importantly, the bone loss associated with gonadotropin-releasing hormone agonists.
Disclosure
The author reports no financial relationships relevant to this article.
Not long ago, women with uterine fibroids had to choose between hysterectomy and abdominal myomectomy to alleviate their symptoms. Then came minimally invasive surgeries such as laparoscopic myomectomy and hysteroscopic myoma resection, although even now these surgeries are offered by a limited number of skilled gynecologic surgeons. And despite their substantially shorter recovery times, these procedures are still surgeries, with inherent complications. On top of that, long-term outcomes data are limited.
Enter the next generation of fibroid treatments: uterine artery embolization (UAE), focused ultrasound with magnetic resonance imaging (MRI) guidance, and selective progesterone receptor modulators—though the last option is still in the pipeline. Gynecologists will be seeing advertisements and promotional materials for these interventions in the near and not-so-distant future.
Uterine artery embolization: In the right hands, a worthwhile strategy
Myers ER, Goodwin S, Landow W, et al. Prospective data collection of a new procedure by a specialty society: the FIBROID Registry. Obstet Gynecol. 2005;106:44–51.
Worthington-Kirsch R, Spies JB, Myers ER, et al. The Fibroid Registry for outcomes data (FIBROID) for uterine embolization: short-term outcomes. Obstet Gynecol. 2005;106:52–59.
Congratulations are in order. When the Society of Interventional Radiology created the Fibroid Registry in 2000, it was looking for early data on UAE to share with patients. In its short but impressive life, the registry has collected more data about UAE than we have about “tried-and-true” surgeries.
In the United States, UAE was first used to treat fibroids in 1997. Although numerous studies since then have reported on its safety and effectiveness, many gynecologists continue to question the suitability of UAE for symptomatic women.
The Web-based registry was established with Duke Clinical Research Institute to track short- and long-term outcomes after UAE in various settings.
What we know from the registry
The Fibroid Registry enrolled its first patient in December 2000, and collected data from 72 sites on 3,319 UAE procedures through December 2002. The reports by Myers et al and Worthington-Kirsch and colleagues contain patient demographics, procedural details, and 30-day outcomes.
The registry defined adverse events as any unexpected event that necessitated an unscheduled office or emergency room visit or unanticipated therapy (medical or surgical). Major complications required increased care or additional hospitalization or had permanent adverse sequelae. Minor complications required medical management or no therapy.
Thirty-day outcomes were available for approximately 91% of patients.
A low complication rate
Complications were uncommon during the first 30 days after UAE, with a 1.1% incidence of additional surgery. In fact, complication rates and recovery times compared favorably with myomectomy and abdominal hysterectomy for large fibroids.
The UAE procedure averaged 56 minutes, with 96.2% technical success and a return to normal activities in about 2 weeks.
Other findings:
- 26% of patients had an adverse event, but only 4% had a major event, most commonly emergency care or hospital readmission for pain management (2.1%) or possible infection (<1%).
- 31 women required another procedure within 30 days, including 3 myomectomies, 9 dilatation and curettage procedures for sloughing leiomyomata, 5 hysteroscopic resections, and 3 hysterectomies for unrecorded indications.
- 1 patient was hospitalized for pain 10 days after UAE and underwent exploratory laparotomy with bilateral oophorectomy.
- The most common minor adverse events were hot flushes (5.7%) and pain requiring additional therapy (9.6%).
Predictors of adverse outcomes:
- current or recent smoking (odds ratio [OR] 1.14, 95% confidence interval [CI] 1.007–1.293),
- African-American race (OR 1.129, 95% CI 1.019–1.251),
- prior procedures (OR 1.23, 95% CI 1.02–1.38), and
- duration of procedure (OR 1.004, 95% CI 1.001–1.006).
Interestingly, short-term outcomes did not differ among centers, nor did procedure times, length of stay, and incidence of adverse events during the first 30 days.
What to tell patients
I inform patients with symptomatic fibroids about all available treatments—including the option of doing nothing at all. Most have no interest in UAE, and are distressed by the thought of their bodies reabsorbing dead tissue.
However, I have had several patients whose operative risks were very high. For example, 1 woman was morbidly obese (>250 lb, which required her UAE treatment at a special facility equipped to perform fluoroscopy in morbidly obese patients), hypertensive, diabetic, and hemiparetic after a stroke. She was also a Jehovah’s Witness. Obviously, nonsurgical intervention was to her benefit. Her bleeding stopped almost immediately.
A reasonable alternative
The lay press recently focused on our obligation, as ObGyns, to inform patients about alternative therapies for fibroids. These first reports from the Fibroid Registry are clear: UAE is a reproducible, low-risk procedure—certainly lower in risk than complex surgeries (though situations may arise when myomectomy is preferred, such as a desire for future fertility1).
The registry will continue to provide data we can share with our patients regarding risks, complications, and long-term outcomes. More importantly, our radiology colleagues have taken the lead in developing a voluntary patient registry to track the outcomes of new technology as it disseminates into the general medical community. Our patients would be well served if we developed similar registries to track our surgical outcomes.
Focused ultrasound shrinks fibroids, but has strict eligibility requirements
Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004;183:1713–1719.
Using MRI guidance, a completely noninvasive treatment is now possible: focused ultrasound ablation of uterine fibroids. This procedure, the newest high-tech treatment for symptomatic fibroids, was successfully tested in an earlier pilot study.2 Although it eased symptoms to a remarkable degree during this phase III trial, how many women will ultimately be suitable for the treatment remains unclear.
Selection criteria
This trial of the ExAblate 2000 (InSightec, Tirat Carmel, Israel) recruited symptomatic women who were otherwise suitable candidates for conventional surgeries. Excluded were postmenopausal patients, women weighing more than 250 lb, and women who had a uterus larger than 24 weeks’ size or any single myoma larger than 10 cm. Women with extensive abdominal scars were carefully examined and excluded if the scars lay in the path of the ultrasound beam. The reason: During the earlier study, these scars tended to absorb ultrasound energy, increasing the risk of thermal injury at the skin surface.
Before proceeding, patients underwent MRI or ultrasound to confirm a clear pathway from the anterior abdominal wall to the fibroids without traversing the bladder or bowel.
Hindley et al did not reveal how many women were initially screened, but 109 were finally enrolled at 7 international sites and completed a Uterine Fibroid Symptoms and Quality of Life Questionnaire before and 3 and 6 months after treatment. As for the location of the fibroids: 22% were submucosal, 57% were intramural, and 21% were subserosal.
Up to 4 fibroids were treated per patient—with MRI mapping and thermographic monitoring—with a margin of 1.5 cm from the edge of the ablated area to the edge of the uterus. Conscious sedation was provided as needed, and tolerance of the procedure was measured using a 4-point pain scale. Mean time in the MRI scanner was 202 minutes (range, 90–370 minutes).
Pain stopped when treatment ended
Most women reported mild to moderate pain during the procedure (66%), and 16% complained of severe pain. This discomfort ended immediately when treatment stopped in virtually all patients—only 1% reported severe pain afterwards.
Adverse events
Serious adverse events included: 5 women with heavy menses requiring blood transfusions, 1 patient with pain and bleeding consistent with preprocedure symptoms, 1 woman needing overnight hospitalization for nausea related to opioid analgesia during the procedure, and 1 patient with leg and buttock pain immediately after treatment (it was later discovered that the sciatic nerve was in the far field of the sonication pathway). These symptoms resolved by the follow-up visit.
Two other patients had adverse events unrelated to the procedure.
6-month outcomes
The mean fibroid volume reduction was 13.5%±32%. Although this improvement seems modest, women reported significant relief from fibroid-related symptoms, and the mean severity score on the quality-of-life questionnaire decreased.
Substantial improvement was seen for both mass effect and bleeding symptoms (32.8 points out of 100 for each).
Pros and cons for symptomatic women
Advantages over UAE include the absence of postprocedural pain in almost all patients, which eliminates the need for an overnight stay and likely speeds the return to normal activities.
Disadvantages are that women with major abdominal scarring, anterior myomas underneath the bladder flap, or adhesive disease that causes small or large bowel to lie in the path of the sound waves cannot take advantage of this new technology, nor can women who have myomas close to neurovascular bundles. Add to that the extremely long procedure time (over 3 hours) and the modest reduction in uterine volume.
Six-month outcomes are promising, but this approach is probably best reserved for an academic setting, so careful screening and long-term tracking can continue.
Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–438.
In this comprehensive review, the authors draw from their extensive expertise in endocrinology to describe the rationale behind asoprisnil, a mixed progesterone receptor agonist/antagonist—the most promising pharmaceutical development in gynecology in several decades.
How the drug works
Mifepristone (RU-486) was the first progesterone receptor antagonist. Despite its ability to reduce myoma volume and suppress endometriosis symptoms in small pilot studies, it induces endometrial hyperplasia at doses higher than 5 to 10 mg, probably by acting similarly to unopposed estrogen. In contrast, asoprisnil has antiproliferative effects and no labor-inducing activity. It directly affects blood vessels in the endometrium, creating a local antiproliferative effect that induces amenorrhea despite normal estrogen levels.
What phase II studies reveal
A multicenter, double-blind, placebo-controlled trial involved 5-, 10-, and 25-mg daily doses of oral asoprisnil over 12 weeks. In a dose-dependent manner, asoprisnil induced amenorrhea or significantly suppressed bleeding without causing breakthrough or intermenstrual flow. It also decreased the volume of both the largest fibroid and the uterus as a whole. At 10- and 25-mg doses, pressure symptoms eased substantially over placebo. Adverse effects were minimal and affected the placebo and asoprisnil groups equally.
Phase III trials are now under way to assess the safety and efficacy of asoprisnil in the treatment of menorrhagia and uterine fibroids. Early results in the treatment of endometriosis are also promising.
What this means for fibroid patients
Though asoprisnil is not yet available for use, the phase III trial is winding down and the drug’s impressive potential seems clear.
A useful strategy may be to counsel marginally symptomatic women with fibroids that treatments are in the pipeline that would permit pharmaceutical management of their symptoms. Because this drug induces amenorrhea in the presence of normal circulating estrogen levels, it eliminates hot flushes and, more importantly, the bone loss associated with gonadotropin-releasing hormone agonists.
Disclosure
The author reports no financial relationships relevant to this article.
1. Pron G, Mocarski E, Bennett J, et al. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
2. Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48-54.
1. Pron G, Mocarski E, Bennett J, et al. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
2. Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48-54.
Subtotal vs total hysterectomy: Does the evidence support saving the cervix?
- Sexual function is not improved more with supracervical than with total hysterectomy.
- Operative morbidity for supracervical and total hysterectomy are similar.
- Pelvic-floor support and urinary incontinence do not seem to be improved with the supracervical approach.
- Cyclic bleeding occurs in 5% to 20% of women after supracervical hysterectomy.
- Reoperation rates for symptoms related to the retained cervix are significant—over 20% in the hands of highly skilled surgeons.
Hysterectomy is an obvious target. The number of hysterectomies performed has not declined substantially since these technologies were introduced, and persists at more than 550,000 per year in the United States. It is still the most widely performed major gynecological procedure.
Technological advances have made possible the use of laparoscopy to facilitate removal of the uterus without a major abdominal incision, with its inherent hazards. Many surgeons, seeking to make the most of new technology, have revisited laparoscopic subtotal hysterectomy, advocating preservation of the cervix to reduce surgical complications, sexual dysfunction, and pelvic-floor defects after hysterectomy.
New data, however—much of it released only in the last 12 to 18 months—tell us there is no difference in sexual function, pelvic floor support, or return to normal activities when the cervix is retained. What’s more, leaving the cervix in place puts the patient at greater risk of reoperation related to hysterectomy.
THEORY
Improved sexual function
EVIDENCE
Recent prospective analyses using validated measures of female sexual function have failed to demonstrate any advantage for supracervical hysterectomy.
Scientific study of sexual function is difficult at best. Many factors influence sexual behavior, and all must be considered when analyzing the effects of hysterectomy. To clearly understand the impact of hysterectomy on female sexual function, prospective studies in which women serve as their own controls provide the best quality evidence. That said, the contention that supracervical hysterectomy results in better sexual function than total hysterectomy stems from the research of a single group, which in 1983 retrospectively compared coital frequency, dyspareunia, libido, and orgasm after “supravaginal uterine amputation” with total hysterectomy.1,2
Simple hysterectomy causes minimal disruption of Frankenhauser’s plexus of autonomic nerves.
They theorized that supracervical operation preserves Frankenhauser’s plexus of autonomic nerves, resulting in better sexual function. However, careful anatomic assessment of the nerve content in the ligaments supporting the uterus has since demonstrated that the rich nerve supply to the uterosacral and cardinal ligaments occurs in the lateral two thirds of these structures. Simple hysterectomy causes minimal disruption of these autonomic nerves, ganglia, and extensions of the inferior hypogastric plexus.3
Thakar et al,4 in a pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom, randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months. Surgical technique was standardized and the endocervix was coagulated in all patients.
The 2 groups were similar in measures of sexual function, including frequency of intercourse, orgasm, and rating of relationship with partner.
The Danish Hysterectomy Group5 randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
There was no change in sexual satisfaction in either group from their prehysterectomy levels. Overall quality of life improved significantly in both groups, in both mental and physical measures.
Roovers et al,6 in a multicenter, nonrandomized trial—powered well to detect 20% differences—compared vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy (technique chosen by the surgeon).
Of the 379 patients recruited (from 13 teaching and nonteaching hospitals in the Netherlands) who had a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
The questionnaire—used to assess sexual pleasure, activity, and problems—specifically addressed lubrication, orgasm, pain, and arousal on a 5-point scale (“not bothered” to “severely bothered”). Their findings:
- Sexual pleasure increased significantly in all groups regardless of type of hysterectomy.
- There was no difference in the incidence of bothersome sexual problems, but a significant number were reported: 43% after vaginal, 41% after subtotal, and 39% after total abdominal hysterectomy (P =.88).
- New sexual problems were reported in 9 patients (23%) after vaginal hysterectomy, 8 patients (24%) after subtotal hysterectomy, and 12 patients (19%) after total abdominal hysterectomy.
- There was a nonsignificant trend toward higher prevalence of arousal and lubrication problems after subtotal hysterectomy and total abdominal hysterectomy, compared with vaginal hysterectomy.
Theory
Improved pelvic floor support, less incontinence
Evidence
Pelvic floor support and urinary incontinence do not seem to be improved with the supracervical approach.
Proponents of supracervical hysterectomy argue that preservation of the cardinal and uterosacral ligaments will reduce the incidence of apical prolapse. In addition, maintenance of the pubocervical ring should lead to less posthysterectomy urinary incontinence.
Our newfound understanding that the nerves are, for the most part, spared at simple hysterectomy should argue against allegations that bowel and urinary function are better preserved by retaining the cervix.
Clearly, long-term outcome studies are required to assess these issues. The randomized trials thus far comparing supracervical with total hysterectomy have not followed patients beyond 2 years.
Nevertheless, at 12 to 24 months, published trials5,7 report an increased incidence of urinary incontinence in patients randomized to supracervical hysterectomy. Prolapse was also reported in a larger number of the patients undergoing subtotal as compared with total hysterectomy (1 to 2% versus 0% at 12 months).
The Total or Supracervical Hysterectomy (TOSH) Research Group7 conducted a multi-center randomized trial with a diverse patient population (78% of women were African American). From January 1998 through April 2000, 135 patients at 4 centers were randomized to supracervical hysterectomy or total abdominal hysterectomy. All patients had benign disease. Surgical technique varied by surgeon as in the general community. Patients and researchers were not blinded as to the technique performed. Subjects were followed for 2 years.
Women undergoing supracervical hysterectomy had a greater incidence of urinary incontinence after surgery.
Both techniques resulted in significant decreases in complaints of urinary incontinence and voiding dysfunction
The Danish Hysterectomy Group5 found that patients who had supracervical hysterectomies had a statistically greater incidence of urinary incontinence after surgery than those undergoing total abdominal hysterectomy (18% versus 9% P = .04). The incidence of new incontinence symptoms was 2.1% in the total abdominal hysterectomy group compared with 7.6% in the subtotal group. There was no change in bowel function in either group.
Thakar et al4 found urinary frequency declined significantly in both groups.
Theory
Fewer injuries and complications, less blood loss
Evidence
Randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Since the creation of a bladder flap and division of the cardinal ligaments is not required in supracervical hysterectomy, we might theoretically expect to see reduced rates of injury to the ureters and bladder. Without the need for colpotomy, blood loss should also be reduced.
Cyclic bleeding may occur after supracervical hysterectomy even when residual endocervical tissue is cored or coagulated.
Given the low incidence of these complications at total hysterectomy, however, a meta-analysis of published randomized trials would be required to properly evaluate this issue. Thus far, randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Thakar et al4 found a significant reduction in operating time as well as a reduction in blood loss (422 mL versus 320 mL) for the supracervical group compared with the total hysterectomy group; however there were no differences in the need for transfusion (5% in each group).
Women who underwent total abdominal hysterectomy had a higher incidence of fever while in the hospital (27% versus 10%), but there was no difference in the rate of infectious morbidity.
Within 1 year of discharge, more patients undergoing supracervical hysterectomy experienced complications: 7% had cyclic bleeding; 2% had cervical prolapse.
The TOSH Research Group7 found no difference in the rate of complications, activity limitations, or symptom improvement between groups. During the first 3 months, there was no difference in missed work, restricting activities, or bed rest. Both techniques resulted in significant decreases in complaints of pelvic pain, pressure, and back pain.
Of women undergoing supracervical hysterectomy, 5% had cyclic vaginal bleeding (only half of the patients in this series had the endocervix ablated).
Further, there were more readmissions related to the hysterectomy in the supracervical group, though this was not statistically significant (relative risk 1.99; 95% confidence interval, 0.58 to 6.8).
Twenty percent of women in the Danish Hysterectomy Group study 5 had persistent vaginal bleeding after subtotal hysterectomy; 2 went on to have trachelectomy for cyclic bleeding.
Prolapse of the cervical stump occurred in 3/136 patients after subtotal hysterectomy, versus no prolapse after total abdominal hysterectomy.
Outcomes after total versus subtotal abdominal hysterectomy. Thakar R, et al. N Engl J Med. 2002;347:1318–1325.4 Level I evidence
CONCLUSION Neither subtotal nor total abdominal hysterectomy adversely affected pelvic organ function at 12 months. Subtotal abdominal hysterectomy resulted in more rapid recovery and fewer short-term complications but infrequently caused cyclical bleeding or cervical prolapse.
- Pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom.
- Randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months.
- Surgical technique was standardized and the endocervix coagulated in all patients.
Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. The Danish Hysterectomy Group. Br J Obstet Gynaecol. 2003;110:1088–1098.5 Level I evidence
CONCLUSION A smaller proportion of women suffered from urinary incontinence after total abdominal hysterectomy than after subtotal abdominal hysterectomy 1 year postoperatively.
- Multicenter, unblinded randomized trial conducted in Denmark.
- Randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
Hysterectomy and sexual well being: Prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Roovers JP, et al. Br Med J. 2003;327:774–779.6 Level II-1 evidence
CONCLUSION Sexual pleasure improved after vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. The persistence and development of bothersome problems during sexual activity were similar for all 3 techniques.
- Multicenter, nonrandomized trial conducted in the Netherlands.
- Investigated sexual function only.
- Compared vaginal, subtotal abdominal, and total abdominal hysterectomy (technique chosen by the surgeon).
- Of the 379 patients with a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
A randomized comparison of total or supracervical hysterectomy: Surgical complications and clinical outcomes. Total or Supracervical Hysterectomy Research Group, Obstet Gynecol. 2003;102:453–462.7 Level I evidence
CONCLUSION We found no statistically significant differences between supracervical hysterectomy and total abdominal hysterectomy in surgical complications and clinical outcomes during 2 years of follow-up.
- Multicenter, unblinded randomized trial conducted in the United States.
- Randomized 135 patients with benign disease to supracervical hysterectomy or total abdominal hysterectomy and followed them for 2 years.
- Surgical technique varied by surgeon as in the general community.
- Only half of the patients had the endocervix ablated.
Long term outcome following laparoscopic supracervical hysterectomy. Okaro EO, et al. Br J Obstet Gynecol. 2001;108:1017–1020.8 Level II-3 evidence
CONCLUSION Symptoms related to the cervical stump requiring further surgery frequently occur following a laparoscopic supracervical hysterectomy.
- Retrospective analysis of case records for 70 patients.
- All subjects were women who would have otherwise undergone abdominal hysterectomy, but agreed to laparoscopic supracervical hysterectomy.
- All surgeries were performed by the same surgeon.
Long-term outcomes: the downside
For a mean of 66 months (range: 4 to 7 years), Okaro et al8 followed 70 patients undergoing laparoscopic supracervical hysterectomy by a single, highly skilled laparoscopic surgeon.
Their findings point out the downside of cervical preservation. Although all patients had the endocervical canal and transition zone cored out, over 24% reported symptoms related to the cervical stump—and all required further surgery. Further, cyclic vaginal bleeding occurred in 11% of women.
One patient developed cervical intraepithelial neoplasia. Dyspareunia and pelvic pain were significant complaints in 19% of the patients. (These women were more likely to have had hysterectomy for endometriosis.) Sixteen of the 17 patients with cervical complaints required trachelectomy within the follow-up time period.
“How empty is theory in the presence of fact!”
Mark Twain, A Connecticut Yankee in King Arthur’s Court
Practice recommendations
We, as clinicians, must accumulate evidence from basic science as well as clinical research, put it all together, and make recommendations based on these data. The data, tell us, in fact, that there is no difference in sexual dysfunction, pelvic floor support, or return to normal activity levels when the cervix is retained, and no evidence to support an advantage to supracervical hysterectomy.
My recommendation is for vaginal hysterectomy when possible. The theoretical advantages of retention of the cervix have driven many clinicians to abandon the vaginal approach in favor of laparoscopic supracervical hysterectomy; no data support this theory.
While not the focus of this article, ample data tell us that the vaginal approach, when technically feasible, is less invasive and carries fewer risks for our patients than laparoscopic or abdominal hysterectomy, and permits excellent access for support of the pelvic floor. I do think that patients who truly believe that their sex lives will be ruined after total hysterectomy or that they will do dramatically better if the cervix remains, may experience this self-fulfilling prophecy.
What I tell patients. I carefully review all the facts with patients in helping them select the appropriate surgical procedure.
I tell patients:
- That overwhelming evidence suggests that sexual function improves in the vast majority of women after hysterectomy, whether or not the cervix is left.
- That there is a real possibility that cyclic bleeding may occur after supracervical hysterectomy, even when the residual endocervical tissue is cored or coagulated. I stress this point to all women who elect hysterectomy.
- That randomized trials demonstrate a significant incidence of reoperation for persistent bleeding.
1. Kikku P. Supravaginal uterine amputation vs. hysterectomy: effects on coital frequency and dyspareunia. Acta Obstet Gynecol Scand. 1983;63:141-145.
2. Kikku P, Gronroos M, Hirvonen T, et al. Supravaginal uterine amputation vs. hysterectomy: effect on libido and orgasm. Acta Obstet Gynecol Scand. 1983;62:147-152.
3. Butler-Manuel SA, Buttery LD, A’Hern RP, et al. Pelvic nerve plexus trauma at radical hysterectomy and simple hysterectomy: the nerve content of the uterine supporting ligaments. Cancer. 2000;89:834-841.
4. Thakar R, Ayers S, Clarkson P, et al. Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med. 2002;347:1318-1325.
5. Gimbel H, Zobbe V, Andersen BM, et al. Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. Br J Obstet Gynaecol. 2003;110:1088-1098.
6. Roovers JP, van der Bom JG, van der Vaart CH, et al. Hysterectomy and sexual wellbeing: prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Br Med J. 2003;327:774-779.
7. Learman LA, Summitt RL, Jr, Varner RE, et al. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
8. Okaro EO, Jones KD, Sutton C. Long term outcome following laparoscopic supracervical hysterectomy. Br J Obstet Gynecol. 2001;108:1017-1020.
- Sexual function is not improved more with supracervical than with total hysterectomy.
- Operative morbidity for supracervical and total hysterectomy are similar.
- Pelvic-floor support and urinary incontinence do not seem to be improved with the supracervical approach.
- Cyclic bleeding occurs in 5% to 20% of women after supracervical hysterectomy.
- Reoperation rates for symptoms related to the retained cervix are significant—over 20% in the hands of highly skilled surgeons.
Hysterectomy is an obvious target. The number of hysterectomies performed has not declined substantially since these technologies were introduced, and persists at more than 550,000 per year in the United States. It is still the most widely performed major gynecological procedure.
Technological advances have made possible the use of laparoscopy to facilitate removal of the uterus without a major abdominal incision, with its inherent hazards. Many surgeons, seeking to make the most of new technology, have revisited laparoscopic subtotal hysterectomy, advocating preservation of the cervix to reduce surgical complications, sexual dysfunction, and pelvic-floor defects after hysterectomy.
New data, however—much of it released only in the last 12 to 18 months—tell us there is no difference in sexual function, pelvic floor support, or return to normal activities when the cervix is retained. What’s more, leaving the cervix in place puts the patient at greater risk of reoperation related to hysterectomy.
THEORY
Improved sexual function
EVIDENCE
Recent prospective analyses using validated measures of female sexual function have failed to demonstrate any advantage for supracervical hysterectomy.
Scientific study of sexual function is difficult at best. Many factors influence sexual behavior, and all must be considered when analyzing the effects of hysterectomy. To clearly understand the impact of hysterectomy on female sexual function, prospective studies in which women serve as their own controls provide the best quality evidence. That said, the contention that supracervical hysterectomy results in better sexual function than total hysterectomy stems from the research of a single group, which in 1983 retrospectively compared coital frequency, dyspareunia, libido, and orgasm after “supravaginal uterine amputation” with total hysterectomy.1,2
Simple hysterectomy causes minimal disruption of Frankenhauser’s plexus of autonomic nerves.
They theorized that supracervical operation preserves Frankenhauser’s plexus of autonomic nerves, resulting in better sexual function. However, careful anatomic assessment of the nerve content in the ligaments supporting the uterus has since demonstrated that the rich nerve supply to the uterosacral and cardinal ligaments occurs in the lateral two thirds of these structures. Simple hysterectomy causes minimal disruption of these autonomic nerves, ganglia, and extensions of the inferior hypogastric plexus.3
Thakar et al,4 in a pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom, randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months. Surgical technique was standardized and the endocervix was coagulated in all patients.
The 2 groups were similar in measures of sexual function, including frequency of intercourse, orgasm, and rating of relationship with partner.
The Danish Hysterectomy Group5 randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
There was no change in sexual satisfaction in either group from their prehysterectomy levels. Overall quality of life improved significantly in both groups, in both mental and physical measures.
Roovers et al,6 in a multicenter, nonrandomized trial—powered well to detect 20% differences—compared vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy (technique chosen by the surgeon).
Of the 379 patients recruited (from 13 teaching and nonteaching hospitals in the Netherlands) who had a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
The questionnaire—used to assess sexual pleasure, activity, and problems—specifically addressed lubrication, orgasm, pain, and arousal on a 5-point scale (“not bothered” to “severely bothered”). Their findings:
- Sexual pleasure increased significantly in all groups regardless of type of hysterectomy.
- There was no difference in the incidence of bothersome sexual problems, but a significant number were reported: 43% after vaginal, 41% after subtotal, and 39% after total abdominal hysterectomy (P =.88).
- New sexual problems were reported in 9 patients (23%) after vaginal hysterectomy, 8 patients (24%) after subtotal hysterectomy, and 12 patients (19%) after total abdominal hysterectomy.
- There was a nonsignificant trend toward higher prevalence of arousal and lubrication problems after subtotal hysterectomy and total abdominal hysterectomy, compared with vaginal hysterectomy.
Theory
Improved pelvic floor support, less incontinence
Evidence
Pelvic floor support and urinary incontinence do not seem to be improved with the supracervical approach.
Proponents of supracervical hysterectomy argue that preservation of the cardinal and uterosacral ligaments will reduce the incidence of apical prolapse. In addition, maintenance of the pubocervical ring should lead to less posthysterectomy urinary incontinence.
Our newfound understanding that the nerves are, for the most part, spared at simple hysterectomy should argue against allegations that bowel and urinary function are better preserved by retaining the cervix.
Clearly, long-term outcome studies are required to assess these issues. The randomized trials thus far comparing supracervical with total hysterectomy have not followed patients beyond 2 years.
Nevertheless, at 12 to 24 months, published trials5,7 report an increased incidence of urinary incontinence in patients randomized to supracervical hysterectomy. Prolapse was also reported in a larger number of the patients undergoing subtotal as compared with total hysterectomy (1 to 2% versus 0% at 12 months).
The Total or Supracervical Hysterectomy (TOSH) Research Group7 conducted a multi-center randomized trial with a diverse patient population (78% of women were African American). From January 1998 through April 2000, 135 patients at 4 centers were randomized to supracervical hysterectomy or total abdominal hysterectomy. All patients had benign disease. Surgical technique varied by surgeon as in the general community. Patients and researchers were not blinded as to the technique performed. Subjects were followed for 2 years.
Women undergoing supracervical hysterectomy had a greater incidence of urinary incontinence after surgery.
Both techniques resulted in significant decreases in complaints of urinary incontinence and voiding dysfunction
The Danish Hysterectomy Group5 found that patients who had supracervical hysterectomies had a statistically greater incidence of urinary incontinence after surgery than those undergoing total abdominal hysterectomy (18% versus 9% P = .04). The incidence of new incontinence symptoms was 2.1% in the total abdominal hysterectomy group compared with 7.6% in the subtotal group. There was no change in bowel function in either group.
Thakar et al4 found urinary frequency declined significantly in both groups.
Theory
Fewer injuries and complications, less blood loss
Evidence
Randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Since the creation of a bladder flap and division of the cardinal ligaments is not required in supracervical hysterectomy, we might theoretically expect to see reduced rates of injury to the ureters and bladder. Without the need for colpotomy, blood loss should also be reduced.
Cyclic bleeding may occur after supracervical hysterectomy even when residual endocervical tissue is cored or coagulated.
Given the low incidence of these complications at total hysterectomy, however, a meta-analysis of published randomized trials would be required to properly evaluate this issue. Thus far, randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Thakar et al4 found a significant reduction in operating time as well as a reduction in blood loss (422 mL versus 320 mL) for the supracervical group compared with the total hysterectomy group; however there were no differences in the need for transfusion (5% in each group).
Women who underwent total abdominal hysterectomy had a higher incidence of fever while in the hospital (27% versus 10%), but there was no difference in the rate of infectious morbidity.
Within 1 year of discharge, more patients undergoing supracervical hysterectomy experienced complications: 7% had cyclic bleeding; 2% had cervical prolapse.
The TOSH Research Group7 found no difference in the rate of complications, activity limitations, or symptom improvement between groups. During the first 3 months, there was no difference in missed work, restricting activities, or bed rest. Both techniques resulted in significant decreases in complaints of pelvic pain, pressure, and back pain.
Of women undergoing supracervical hysterectomy, 5% had cyclic vaginal bleeding (only half of the patients in this series had the endocervix ablated).
Further, there were more readmissions related to the hysterectomy in the supracervical group, though this was not statistically significant (relative risk 1.99; 95% confidence interval, 0.58 to 6.8).
Twenty percent of women in the Danish Hysterectomy Group study 5 had persistent vaginal bleeding after subtotal hysterectomy; 2 went on to have trachelectomy for cyclic bleeding.
Prolapse of the cervical stump occurred in 3/136 patients after subtotal hysterectomy, versus no prolapse after total abdominal hysterectomy.
Outcomes after total versus subtotal abdominal hysterectomy. Thakar R, et al. N Engl J Med. 2002;347:1318–1325.4 Level I evidence
CONCLUSION Neither subtotal nor total abdominal hysterectomy adversely affected pelvic organ function at 12 months. Subtotal abdominal hysterectomy resulted in more rapid recovery and fewer short-term complications but infrequently caused cyclical bleeding or cervical prolapse.
- Pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom.
- Randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months.
- Surgical technique was standardized and the endocervix coagulated in all patients.
Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. The Danish Hysterectomy Group. Br J Obstet Gynaecol. 2003;110:1088–1098.5 Level I evidence
CONCLUSION A smaller proportion of women suffered from urinary incontinence after total abdominal hysterectomy than after subtotal abdominal hysterectomy 1 year postoperatively.
- Multicenter, unblinded randomized trial conducted in Denmark.
- Randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
Hysterectomy and sexual well being: Prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Roovers JP, et al. Br Med J. 2003;327:774–779.6 Level II-1 evidence
CONCLUSION Sexual pleasure improved after vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. The persistence and development of bothersome problems during sexual activity were similar for all 3 techniques.
- Multicenter, nonrandomized trial conducted in the Netherlands.
- Investigated sexual function only.
- Compared vaginal, subtotal abdominal, and total abdominal hysterectomy (technique chosen by the surgeon).
- Of the 379 patients with a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
A randomized comparison of total or supracervical hysterectomy: Surgical complications and clinical outcomes. Total or Supracervical Hysterectomy Research Group, Obstet Gynecol. 2003;102:453–462.7 Level I evidence
CONCLUSION We found no statistically significant differences between supracervical hysterectomy and total abdominal hysterectomy in surgical complications and clinical outcomes during 2 years of follow-up.
- Multicenter, unblinded randomized trial conducted in the United States.
- Randomized 135 patients with benign disease to supracervical hysterectomy or total abdominal hysterectomy and followed them for 2 years.
- Surgical technique varied by surgeon as in the general community.
- Only half of the patients had the endocervix ablated.
Long term outcome following laparoscopic supracervical hysterectomy. Okaro EO, et al. Br J Obstet Gynecol. 2001;108:1017–1020.8 Level II-3 evidence
CONCLUSION Symptoms related to the cervical stump requiring further surgery frequently occur following a laparoscopic supracervical hysterectomy.
- Retrospective analysis of case records for 70 patients.
- All subjects were women who would have otherwise undergone abdominal hysterectomy, but agreed to laparoscopic supracervical hysterectomy.
- All surgeries were performed by the same surgeon.
Long-term outcomes: the downside
For a mean of 66 months (range: 4 to 7 years), Okaro et al8 followed 70 patients undergoing laparoscopic supracervical hysterectomy by a single, highly skilled laparoscopic surgeon.
Their findings point out the downside of cervical preservation. Although all patients had the endocervical canal and transition zone cored out, over 24% reported symptoms related to the cervical stump—and all required further surgery. Further, cyclic vaginal bleeding occurred in 11% of women.
One patient developed cervical intraepithelial neoplasia. Dyspareunia and pelvic pain were significant complaints in 19% of the patients. (These women were more likely to have had hysterectomy for endometriosis.) Sixteen of the 17 patients with cervical complaints required trachelectomy within the follow-up time period.
“How empty is theory in the presence of fact!”
Mark Twain, A Connecticut Yankee in King Arthur’s Court
Practice recommendations
We, as clinicians, must accumulate evidence from basic science as well as clinical research, put it all together, and make recommendations based on these data. The data, tell us, in fact, that there is no difference in sexual dysfunction, pelvic floor support, or return to normal activity levels when the cervix is retained, and no evidence to support an advantage to supracervical hysterectomy.
My recommendation is for vaginal hysterectomy when possible. The theoretical advantages of retention of the cervix have driven many clinicians to abandon the vaginal approach in favor of laparoscopic supracervical hysterectomy; no data support this theory.
While not the focus of this article, ample data tell us that the vaginal approach, when technically feasible, is less invasive and carries fewer risks for our patients than laparoscopic or abdominal hysterectomy, and permits excellent access for support of the pelvic floor. I do think that patients who truly believe that their sex lives will be ruined after total hysterectomy or that they will do dramatically better if the cervix remains, may experience this self-fulfilling prophecy.
What I tell patients. I carefully review all the facts with patients in helping them select the appropriate surgical procedure.
I tell patients:
- That overwhelming evidence suggests that sexual function improves in the vast majority of women after hysterectomy, whether or not the cervix is left.
- That there is a real possibility that cyclic bleeding may occur after supracervical hysterectomy, even when the residual endocervical tissue is cored or coagulated. I stress this point to all women who elect hysterectomy.
- That randomized trials demonstrate a significant incidence of reoperation for persistent bleeding.
- Sexual function is not improved more with supracervical than with total hysterectomy.
- Operative morbidity for supracervical and total hysterectomy are similar.
- Pelvic-floor support and urinary incontinence do not seem to be improved with the supracervical approach.
- Cyclic bleeding occurs in 5% to 20% of women after supracervical hysterectomy.
- Reoperation rates for symptoms related to the retained cervix are significant—over 20% in the hands of highly skilled surgeons.
Hysterectomy is an obvious target. The number of hysterectomies performed has not declined substantially since these technologies were introduced, and persists at more than 550,000 per year in the United States. It is still the most widely performed major gynecological procedure.
Technological advances have made possible the use of laparoscopy to facilitate removal of the uterus without a major abdominal incision, with its inherent hazards. Many surgeons, seeking to make the most of new technology, have revisited laparoscopic subtotal hysterectomy, advocating preservation of the cervix to reduce surgical complications, sexual dysfunction, and pelvic-floor defects after hysterectomy.
New data, however—much of it released only in the last 12 to 18 months—tell us there is no difference in sexual function, pelvic floor support, or return to normal activities when the cervix is retained. What’s more, leaving the cervix in place puts the patient at greater risk of reoperation related to hysterectomy.
THEORY
Improved sexual function
EVIDENCE
Recent prospective analyses using validated measures of female sexual function have failed to demonstrate any advantage for supracervical hysterectomy.
Scientific study of sexual function is difficult at best. Many factors influence sexual behavior, and all must be considered when analyzing the effects of hysterectomy. To clearly understand the impact of hysterectomy on female sexual function, prospective studies in which women serve as their own controls provide the best quality evidence. That said, the contention that supracervical hysterectomy results in better sexual function than total hysterectomy stems from the research of a single group, which in 1983 retrospectively compared coital frequency, dyspareunia, libido, and orgasm after “supravaginal uterine amputation” with total hysterectomy.1,2
Simple hysterectomy causes minimal disruption of Frankenhauser’s plexus of autonomic nerves.
They theorized that supracervical operation preserves Frankenhauser’s plexus of autonomic nerves, resulting in better sexual function. However, careful anatomic assessment of the nerve content in the ligaments supporting the uterus has since demonstrated that the rich nerve supply to the uterosacral and cardinal ligaments occurs in the lateral two thirds of these structures. Simple hysterectomy causes minimal disruption of these autonomic nerves, ganglia, and extensions of the inferior hypogastric plexus.3
Thakar et al,4 in a pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom, randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months. Surgical technique was standardized and the endocervix was coagulated in all patients.
The 2 groups were similar in measures of sexual function, including frequency of intercourse, orgasm, and rating of relationship with partner.
The Danish Hysterectomy Group5 randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
There was no change in sexual satisfaction in either group from their prehysterectomy levels. Overall quality of life improved significantly in both groups, in both mental and physical measures.
Roovers et al,6 in a multicenter, nonrandomized trial—powered well to detect 20% differences—compared vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy (technique chosen by the surgeon).
Of the 379 patients recruited (from 13 teaching and nonteaching hospitals in the Netherlands) who had a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
The questionnaire—used to assess sexual pleasure, activity, and problems—specifically addressed lubrication, orgasm, pain, and arousal on a 5-point scale (“not bothered” to “severely bothered”). Their findings:
- Sexual pleasure increased significantly in all groups regardless of type of hysterectomy.
- There was no difference in the incidence of bothersome sexual problems, but a significant number were reported: 43% after vaginal, 41% after subtotal, and 39% after total abdominal hysterectomy (P =.88).
- New sexual problems were reported in 9 patients (23%) after vaginal hysterectomy, 8 patients (24%) after subtotal hysterectomy, and 12 patients (19%) after total abdominal hysterectomy.
- There was a nonsignificant trend toward higher prevalence of arousal and lubrication problems after subtotal hysterectomy and total abdominal hysterectomy, compared with vaginal hysterectomy.
Theory
Improved pelvic floor support, less incontinence
Evidence
Pelvic floor support and urinary incontinence do not seem to be improved with the supracervical approach.
Proponents of supracervical hysterectomy argue that preservation of the cardinal and uterosacral ligaments will reduce the incidence of apical prolapse. In addition, maintenance of the pubocervical ring should lead to less posthysterectomy urinary incontinence.
Our newfound understanding that the nerves are, for the most part, spared at simple hysterectomy should argue against allegations that bowel and urinary function are better preserved by retaining the cervix.
Clearly, long-term outcome studies are required to assess these issues. The randomized trials thus far comparing supracervical with total hysterectomy have not followed patients beyond 2 years.
Nevertheless, at 12 to 24 months, published trials5,7 report an increased incidence of urinary incontinence in patients randomized to supracervical hysterectomy. Prolapse was also reported in a larger number of the patients undergoing subtotal as compared with total hysterectomy (1 to 2% versus 0% at 12 months).
The Total or Supracervical Hysterectomy (TOSH) Research Group7 conducted a multi-center randomized trial with a diverse patient population (78% of women were African American). From January 1998 through April 2000, 135 patients at 4 centers were randomized to supracervical hysterectomy or total abdominal hysterectomy. All patients had benign disease. Surgical technique varied by surgeon as in the general community. Patients and researchers were not blinded as to the technique performed. Subjects were followed for 2 years.
Women undergoing supracervical hysterectomy had a greater incidence of urinary incontinence after surgery.
Both techniques resulted in significant decreases in complaints of urinary incontinence and voiding dysfunction
The Danish Hysterectomy Group5 found that patients who had supracervical hysterectomies had a statistically greater incidence of urinary incontinence after surgery than those undergoing total abdominal hysterectomy (18% versus 9% P = .04). The incidence of new incontinence symptoms was 2.1% in the total abdominal hysterectomy group compared with 7.6% in the subtotal group. There was no change in bowel function in either group.
Thakar et al4 found urinary frequency declined significantly in both groups.
Theory
Fewer injuries and complications, less blood loss
Evidence
Randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Since the creation of a bladder flap and division of the cardinal ligaments is not required in supracervical hysterectomy, we might theoretically expect to see reduced rates of injury to the ureters and bladder. Without the need for colpotomy, blood loss should also be reduced.
Cyclic bleeding may occur after supracervical hysterectomy even when residual endocervical tissue is cored or coagulated.
Given the low incidence of these complications at total hysterectomy, however, a meta-analysis of published randomized trials would be required to properly evaluate this issue. Thus far, randomized trials have offered no evidence to support a reduction in complication rates or bleeding requiring transfusion.
Thakar et al4 found a significant reduction in operating time as well as a reduction in blood loss (422 mL versus 320 mL) for the supracervical group compared with the total hysterectomy group; however there were no differences in the need for transfusion (5% in each group).
Women who underwent total abdominal hysterectomy had a higher incidence of fever while in the hospital (27% versus 10%), but there was no difference in the rate of infectious morbidity.
Within 1 year of discharge, more patients undergoing supracervical hysterectomy experienced complications: 7% had cyclic bleeding; 2% had cervical prolapse.
The TOSH Research Group7 found no difference in the rate of complications, activity limitations, or symptom improvement between groups. During the first 3 months, there was no difference in missed work, restricting activities, or bed rest. Both techniques resulted in significant decreases in complaints of pelvic pain, pressure, and back pain.
Of women undergoing supracervical hysterectomy, 5% had cyclic vaginal bleeding (only half of the patients in this series had the endocervix ablated).
Further, there were more readmissions related to the hysterectomy in the supracervical group, though this was not statistically significant (relative risk 1.99; 95% confidence interval, 0.58 to 6.8).
Twenty percent of women in the Danish Hysterectomy Group study 5 had persistent vaginal bleeding after subtotal hysterectomy; 2 went on to have trachelectomy for cyclic bleeding.
Prolapse of the cervical stump occurred in 3/136 patients after subtotal hysterectomy, versus no prolapse after total abdominal hysterectomy.
Outcomes after total versus subtotal abdominal hysterectomy. Thakar R, et al. N Engl J Med. 2002;347:1318–1325.4 Level I evidence
CONCLUSION Neither subtotal nor total abdominal hysterectomy adversely affected pelvic organ function at 12 months. Subtotal abdominal hysterectomy resulted in more rapid recovery and fewer short-term complications but infrequently caused cyclical bleeding or cervical prolapse.
- Pivotal multicenter, double-blind, randomized trial conducted in the United Kingdom.
- Randomized 279 patients with benign disease to supracervical or total hysterectomy and followed them for 12 months.
- Surgical technique was standardized and the endocervix coagulated in all patients.
Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. The Danish Hysterectomy Group. Br J Obstet Gynaecol. 2003;110:1088–1098.5 Level I evidence
CONCLUSION A smaller proportion of women suffered from urinary incontinence after total abdominal hysterectomy than after subtotal abdominal hysterectomy 1 year postoperatively.
- Multicenter, unblinded randomized trial conducted in Denmark.
- Randomized 319 patients with benign disease to total abdominal hysterectomy or subtotal abdominal hysterectomy, of whom 276 completed validated mailed questionnaires preoperatively, and at 2, 6, and 12 months postoperatively.
Hysterectomy and sexual well being: Prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Roovers JP, et al. Br Med J. 2003;327:774–779.6 Level II-1 evidence
CONCLUSION Sexual pleasure improved after vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. The persistence and development of bothersome problems during sexual activity were similar for all 3 techniques.
- Multicenter, nonrandomized trial conducted in the Netherlands.
- Investigated sexual function only.
- Compared vaginal, subtotal abdominal, and total abdominal hysterectomy (technique chosen by the surgeon).
- Of the 379 patients with a male partner, 93% completed a validated questionnaire before and 6 months after surgery.
A randomized comparison of total or supracervical hysterectomy: Surgical complications and clinical outcomes. Total or Supracervical Hysterectomy Research Group, Obstet Gynecol. 2003;102:453–462.7 Level I evidence
CONCLUSION We found no statistically significant differences between supracervical hysterectomy and total abdominal hysterectomy in surgical complications and clinical outcomes during 2 years of follow-up.
- Multicenter, unblinded randomized trial conducted in the United States.
- Randomized 135 patients with benign disease to supracervical hysterectomy or total abdominal hysterectomy and followed them for 2 years.
- Surgical technique varied by surgeon as in the general community.
- Only half of the patients had the endocervix ablated.
Long term outcome following laparoscopic supracervical hysterectomy. Okaro EO, et al. Br J Obstet Gynecol. 2001;108:1017–1020.8 Level II-3 evidence
CONCLUSION Symptoms related to the cervical stump requiring further surgery frequently occur following a laparoscopic supracervical hysterectomy.
- Retrospective analysis of case records for 70 patients.
- All subjects were women who would have otherwise undergone abdominal hysterectomy, but agreed to laparoscopic supracervical hysterectomy.
- All surgeries were performed by the same surgeon.
Long-term outcomes: the downside
For a mean of 66 months (range: 4 to 7 years), Okaro et al8 followed 70 patients undergoing laparoscopic supracervical hysterectomy by a single, highly skilled laparoscopic surgeon.
Their findings point out the downside of cervical preservation. Although all patients had the endocervical canal and transition zone cored out, over 24% reported symptoms related to the cervical stump—and all required further surgery. Further, cyclic vaginal bleeding occurred in 11% of women.
One patient developed cervical intraepithelial neoplasia. Dyspareunia and pelvic pain were significant complaints in 19% of the patients. (These women were more likely to have had hysterectomy for endometriosis.) Sixteen of the 17 patients with cervical complaints required trachelectomy within the follow-up time period.
“How empty is theory in the presence of fact!”
Mark Twain, A Connecticut Yankee in King Arthur’s Court
Practice recommendations
We, as clinicians, must accumulate evidence from basic science as well as clinical research, put it all together, and make recommendations based on these data. The data, tell us, in fact, that there is no difference in sexual dysfunction, pelvic floor support, or return to normal activity levels when the cervix is retained, and no evidence to support an advantage to supracervical hysterectomy.
My recommendation is for vaginal hysterectomy when possible. The theoretical advantages of retention of the cervix have driven many clinicians to abandon the vaginal approach in favor of laparoscopic supracervical hysterectomy; no data support this theory.
While not the focus of this article, ample data tell us that the vaginal approach, when technically feasible, is less invasive and carries fewer risks for our patients than laparoscopic or abdominal hysterectomy, and permits excellent access for support of the pelvic floor. I do think that patients who truly believe that their sex lives will be ruined after total hysterectomy or that they will do dramatically better if the cervix remains, may experience this self-fulfilling prophecy.
What I tell patients. I carefully review all the facts with patients in helping them select the appropriate surgical procedure.
I tell patients:
- That overwhelming evidence suggests that sexual function improves in the vast majority of women after hysterectomy, whether or not the cervix is left.
- That there is a real possibility that cyclic bleeding may occur after supracervical hysterectomy, even when the residual endocervical tissue is cored or coagulated. I stress this point to all women who elect hysterectomy.
- That randomized trials demonstrate a significant incidence of reoperation for persistent bleeding.
1. Kikku P. Supravaginal uterine amputation vs. hysterectomy: effects on coital frequency and dyspareunia. Acta Obstet Gynecol Scand. 1983;63:141-145.
2. Kikku P, Gronroos M, Hirvonen T, et al. Supravaginal uterine amputation vs. hysterectomy: effect on libido and orgasm. Acta Obstet Gynecol Scand. 1983;62:147-152.
3. Butler-Manuel SA, Buttery LD, A’Hern RP, et al. Pelvic nerve plexus trauma at radical hysterectomy and simple hysterectomy: the nerve content of the uterine supporting ligaments. Cancer. 2000;89:834-841.
4. Thakar R, Ayers S, Clarkson P, et al. Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med. 2002;347:1318-1325.
5. Gimbel H, Zobbe V, Andersen BM, et al. Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. Br J Obstet Gynaecol. 2003;110:1088-1098.
6. Roovers JP, van der Bom JG, van der Vaart CH, et al. Hysterectomy and sexual wellbeing: prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Br Med J. 2003;327:774-779.
7. Learman LA, Summitt RL, Jr, Varner RE, et al. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
8. Okaro EO, Jones KD, Sutton C. Long term outcome following laparoscopic supracervical hysterectomy. Br J Obstet Gynecol. 2001;108:1017-1020.
1. Kikku P. Supravaginal uterine amputation vs. hysterectomy: effects on coital frequency and dyspareunia. Acta Obstet Gynecol Scand. 1983;63:141-145.
2. Kikku P, Gronroos M, Hirvonen T, et al. Supravaginal uterine amputation vs. hysterectomy: effect on libido and orgasm. Acta Obstet Gynecol Scand. 1983;62:147-152.
3. Butler-Manuel SA, Buttery LD, A’Hern RP, et al. Pelvic nerve plexus trauma at radical hysterectomy and simple hysterectomy: the nerve content of the uterine supporting ligaments. Cancer. 2000;89:834-841.
4. Thakar R, Ayers S, Clarkson P, et al. Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med. 2002;347:1318-1325.
5. Gimbel H, Zobbe V, Andersen BM, et al. Randomized controlled trial of total compared with subtotal hysterectomy with 1-year follow-up results. Br J Obstet Gynaecol. 2003;110:1088-1098.
6. Roovers JP, van der Bom JG, van der Vaart CH, et al. Hysterectomy and sexual wellbeing: prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. Br Med J. 2003;327:774-779.
7. Learman LA, Summitt RL, Jr, Varner RE, et al. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
8. Okaro EO, Jones KD, Sutton C. Long term outcome following laparoscopic supracervical hysterectomy. Br J Obstet Gynecol. 2001;108:1017-1020.
Is HPV DNA testing a reliable alternative to the Pap test?
Context
Screening women with limited access to health care is difficult—in both the United States and the third world. We now know that invasive cervical carcinoma is universally associated with persistent human Papillomavirus (HPV) infection, and that only a small subset of viral types place women at high risk for developing cervical intraepithelial neoplasia (CIN) 3 or carcinoma.
In an effort to increase the sensitivity of the initial encounter with the healthcare system among women with limited resources and access, several researchers and agencies are investigating the use of HPV DNA testing in conjunction with standard Papanicolaou (Pap) smears, thin-layer smears, or HPV testing alone as the primary screening method.
Method and Results
This well-designed prospective trial uses a large Planned Parenthood population to compare the sensitivity, specificity, and frequency of referral for colposcopy in patients undergoing thin-layer Pap smear, HPV testing by polymerase chain reaction (PCR), or HPV testing by liquid-based RNA-DNA hybridization capture with signal amplification.
Unfortunately, the demographics of the study population are not necessarily comparable to those of the average practicing Ob/Gyn. Therefore, it may not be appropriate to extrapolate the results universally. The mean age of participants was 25 years; more than 80% of the eligible women were under age 30. The prevalence of HPV DNA, cervical dysplasia, and carcinoma is significantly different in this age group, compared with women over 30 years of age.
Women in the trial were predominantly Caucasian, with an average of 5 sexual partners in the group under 25 years of age and 9 sexual partners in the over-30 group.
As the authors point out, another limitation of the study is the failure of some patients to return for colposcopy when recommended by the protocol. Only 72.7% of women with abnormal Pap test results and 67.9% of women with high-risk HPV DNA returned for colposcopic evaluation. This limited the reliability of the sensitivity and specificity calculations to some extent.
As expected, HPV DNA testing was more sensitive but less specific than cytology in identifying high-grade dysplasia or cervical carcinoma. HPV DNA by signal amplification identifies more intermediate- and low-risk HPV types than the PCR technique. Therefore, this method has a higher sensitivity but lower specificity for detecting high-grade lesions. All the screening strategies Human Papillomavirus were more sensitive for identifying CIN 3 or higher in women younger than 30 years of age. Specificity, however, was significantly greater for women older than 30 years of age.
Who May Be Affected?
All sexually active women.
Persistence of high-risk HPV DNA types—not the initial presence—is a risk factor for cervical carcinoma.
Expert Commentary
Universal cervical cytology screening of sexually active women has greatly decreased the incidence of invasive cervical carcinoma in developed countries. Recently, commercialization of molecular biology techniques and cytology processing improvements have led to new products for cervical carcinoma screening.
In counseling patients, it is important to remember that it is the persistence of high-risk HPV DNA types—not the initial presence—that is a risk factor for cervical carcinoma. Most HPV infections regress spontaneously, with only about 10% of women remaining infected at 5 years. Young, recently sexually active women are likely to test positive for multiple HPV types. If they can be educated to comply with routine screening recommendations, it will not be clinically useful to employ primary screening with HPV DNA testing.
The downside to universal HPV DNA primary screening is that large numbers of women with transient high-risk HPV infections have normal cytology findings and no “disease.” Labeling them as high risk would create a psychological burden on both the patient and the physician.
Until we have well-studied clinical protocols for managing these patients, we should avoid this dilemma by continuing current Pap screening in women who have good access to care and who are willing to participate in screening at appropriate intervals.
Primary HPV DNA screening is appropriate for women who are unwilling or unable to participate in routine screening.
Bottom Line
Screening strategies for cervical cancer are evolving. Techniques appropriate for young, sexually active women with somewhat limited access to care should not be extrapolated to a low-risk, stable, monogamous population.
If women understand the limitations of cytology screening and the need for repeated tests to capture the majority of true abnormal findings, and if they have financial and geographic access to routine care, it makes sense to use a specific but somewhat less sensitive testing strategy, i.e., cytology screening.
For women with minimal access to routine care, it is essential to use the simplest, most sensitive test to minimize false negatives. It is these women in whom primary screening with HPV DNA with or without cytology makes sense.
Suggested Reading
- Wright TC, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med. 2003;348:489-901.
Context
Screening women with limited access to health care is difficult—in both the United States and the third world. We now know that invasive cervical carcinoma is universally associated with persistent human Papillomavirus (HPV) infection, and that only a small subset of viral types place women at high risk for developing cervical intraepithelial neoplasia (CIN) 3 or carcinoma.
In an effort to increase the sensitivity of the initial encounter with the healthcare system among women with limited resources and access, several researchers and agencies are investigating the use of HPV DNA testing in conjunction with standard Papanicolaou (Pap) smears, thin-layer smears, or HPV testing alone as the primary screening method.
Method and Results
This well-designed prospective trial uses a large Planned Parenthood population to compare the sensitivity, specificity, and frequency of referral for colposcopy in patients undergoing thin-layer Pap smear, HPV testing by polymerase chain reaction (PCR), or HPV testing by liquid-based RNA-DNA hybridization capture with signal amplification.
Unfortunately, the demographics of the study population are not necessarily comparable to those of the average practicing Ob/Gyn. Therefore, it may not be appropriate to extrapolate the results universally. The mean age of participants was 25 years; more than 80% of the eligible women were under age 30. The prevalence of HPV DNA, cervical dysplasia, and carcinoma is significantly different in this age group, compared with women over 30 years of age.
Women in the trial were predominantly Caucasian, with an average of 5 sexual partners in the group under 25 years of age and 9 sexual partners in the over-30 group.
As the authors point out, another limitation of the study is the failure of some patients to return for colposcopy when recommended by the protocol. Only 72.7% of women with abnormal Pap test results and 67.9% of women with high-risk HPV DNA returned for colposcopic evaluation. This limited the reliability of the sensitivity and specificity calculations to some extent.
As expected, HPV DNA testing was more sensitive but less specific than cytology in identifying high-grade dysplasia or cervical carcinoma. HPV DNA by signal amplification identifies more intermediate- and low-risk HPV types than the PCR technique. Therefore, this method has a higher sensitivity but lower specificity for detecting high-grade lesions. All the screening strategies Human Papillomavirus were more sensitive for identifying CIN 3 or higher in women younger than 30 years of age. Specificity, however, was significantly greater for women older than 30 years of age.
Who May Be Affected?
All sexually active women.
Persistence of high-risk HPV DNA types—not the initial presence—is a risk factor for cervical carcinoma.
Expert Commentary
Universal cervical cytology screening of sexually active women has greatly decreased the incidence of invasive cervical carcinoma in developed countries. Recently, commercialization of molecular biology techniques and cytology processing improvements have led to new products for cervical carcinoma screening.
In counseling patients, it is important to remember that it is the persistence of high-risk HPV DNA types—not the initial presence—that is a risk factor for cervical carcinoma. Most HPV infections regress spontaneously, with only about 10% of women remaining infected at 5 years. Young, recently sexually active women are likely to test positive for multiple HPV types. If they can be educated to comply with routine screening recommendations, it will not be clinically useful to employ primary screening with HPV DNA testing.
The downside to universal HPV DNA primary screening is that large numbers of women with transient high-risk HPV infections have normal cytology findings and no “disease.” Labeling them as high risk would create a psychological burden on both the patient and the physician.
Until we have well-studied clinical protocols for managing these patients, we should avoid this dilemma by continuing current Pap screening in women who have good access to care and who are willing to participate in screening at appropriate intervals.
Primary HPV DNA screening is appropriate for women who are unwilling or unable to participate in routine screening.
Bottom Line
Screening strategies for cervical cancer are evolving. Techniques appropriate for young, sexually active women with somewhat limited access to care should not be extrapolated to a low-risk, stable, monogamous population.
If women understand the limitations of cytology screening and the need for repeated tests to capture the majority of true abnormal findings, and if they have financial and geographic access to routine care, it makes sense to use a specific but somewhat less sensitive testing strategy, i.e., cytology screening.
For women with minimal access to routine care, it is essential to use the simplest, most sensitive test to minimize false negatives. It is these women in whom primary screening with HPV DNA with or without cytology makes sense.
Suggested Reading
- Wright TC, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med. 2003;348:489-901.
Context
Screening women with limited access to health care is difficult—in both the United States and the third world. We now know that invasive cervical carcinoma is universally associated with persistent human Papillomavirus (HPV) infection, and that only a small subset of viral types place women at high risk for developing cervical intraepithelial neoplasia (CIN) 3 or carcinoma.
In an effort to increase the sensitivity of the initial encounter with the healthcare system among women with limited resources and access, several researchers and agencies are investigating the use of HPV DNA testing in conjunction with standard Papanicolaou (Pap) smears, thin-layer smears, or HPV testing alone as the primary screening method.
Method and Results
This well-designed prospective trial uses a large Planned Parenthood population to compare the sensitivity, specificity, and frequency of referral for colposcopy in patients undergoing thin-layer Pap smear, HPV testing by polymerase chain reaction (PCR), or HPV testing by liquid-based RNA-DNA hybridization capture with signal amplification.
Unfortunately, the demographics of the study population are not necessarily comparable to those of the average practicing Ob/Gyn. Therefore, it may not be appropriate to extrapolate the results universally. The mean age of participants was 25 years; more than 80% of the eligible women were under age 30. The prevalence of HPV DNA, cervical dysplasia, and carcinoma is significantly different in this age group, compared with women over 30 years of age.
Women in the trial were predominantly Caucasian, with an average of 5 sexual partners in the group under 25 years of age and 9 sexual partners in the over-30 group.
As the authors point out, another limitation of the study is the failure of some patients to return for colposcopy when recommended by the protocol. Only 72.7% of women with abnormal Pap test results and 67.9% of women with high-risk HPV DNA returned for colposcopic evaluation. This limited the reliability of the sensitivity and specificity calculations to some extent.
As expected, HPV DNA testing was more sensitive but less specific than cytology in identifying high-grade dysplasia or cervical carcinoma. HPV DNA by signal amplification identifies more intermediate- and low-risk HPV types than the PCR technique. Therefore, this method has a higher sensitivity but lower specificity for detecting high-grade lesions. All the screening strategies Human Papillomavirus were more sensitive for identifying CIN 3 or higher in women younger than 30 years of age. Specificity, however, was significantly greater for women older than 30 years of age.
Who May Be Affected?
All sexually active women.
Persistence of high-risk HPV DNA types—not the initial presence—is a risk factor for cervical carcinoma.
Expert Commentary
Universal cervical cytology screening of sexually active women has greatly decreased the incidence of invasive cervical carcinoma in developed countries. Recently, commercialization of molecular biology techniques and cytology processing improvements have led to new products for cervical carcinoma screening.
In counseling patients, it is important to remember that it is the persistence of high-risk HPV DNA types—not the initial presence—that is a risk factor for cervical carcinoma. Most HPV infections regress spontaneously, with only about 10% of women remaining infected at 5 years. Young, recently sexually active women are likely to test positive for multiple HPV types. If they can be educated to comply with routine screening recommendations, it will not be clinically useful to employ primary screening with HPV DNA testing.
The downside to universal HPV DNA primary screening is that large numbers of women with transient high-risk HPV infections have normal cytology findings and no “disease.” Labeling them as high risk would create a psychological burden on both the patient and the physician.
Until we have well-studied clinical protocols for managing these patients, we should avoid this dilemma by continuing current Pap screening in women who have good access to care and who are willing to participate in screening at appropriate intervals.
Primary HPV DNA screening is appropriate for women who are unwilling or unable to participate in routine screening.
Bottom Line
Screening strategies for cervical cancer are evolving. Techniques appropriate for young, sexually active women with somewhat limited access to care should not be extrapolated to a low-risk, stable, monogamous population.
If women understand the limitations of cytology screening and the need for repeated tests to capture the majority of true abnormal findings, and if they have financial and geographic access to routine care, it makes sense to use a specific but somewhat less sensitive testing strategy, i.e., cytology screening.
For women with minimal access to routine care, it is essential to use the simplest, most sensitive test to minimize false negatives. It is these women in whom primary screening with HPV DNA with or without cytology makes sense.
Suggested Reading
- Wright TC, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med. 2003;348:489-901.
Break the silence: Discussing sexual dysfunction
- Forty-three percent of U.S. women report being dissatisfied with their sexual functioning.
- Female sexual dysfunction is divided into 4 categories: libido, arousal, orgasm, and pain.
- Factors that contribute to sexual dysfunction are distortion or inflammation of pelvic structures, pelvic or abdominal trauma or surgery, medications, depression, and chronic medical conditions.
- A biopsychosocial model of inquiry is recommended for assessing sexual complaints, emphasizing 4 areas: physical, psychologic, relational, and situational.
In a recent U.S. survey, 43% of female respondents reported being dissatisfied with their sexual functioning, a significantly higher percentage than among male respondents.1 Even more disturbing were separate findings: 71% of adults 25 and older believed their physician would dismiss any sexual concerns they might bring up, while 68% avoided discussing sexual dysfunction with their doctors for fear of embarrassing them.2
A lack of desire for sex is the most common sexual complaint.
These statistics highlight clinicians’ inability to elicit information about and treat sexual disorders in women. Many Ob/Gyns feel they lack the necessary background in fundamental science and psychology to competently evaluate and treat sexual complaints. It is difficult to approach these problems without a complete understanding of the physiology and psychology of female sexual response.
But times are changing. The availability of sildenafil to treat male erectile dysfunction has dramatically increased our patients’ awareness of sexual disorders, as has the open discussion of sexual dissatisfaction on the talkshow circuit. Patients are increasingly likely to expect their health-care providers to evaluate and treat sexual complaints. The following pearls offer a framework for assessing sexual dysfunction, as well as guidelines for therapeutic intervention.
Raising the subject. Although women are gradually opening up about sexual dysfunction, I try not to assume that they will raise the subject themselves. A case in point: Among 308 patients taking selective serotonin reuptake inhibitors (SSRIs), 55% reported sexual dysfunction when the physician asked them about it directly compared with only 14% who reported it spontaneously.3 Many women may not realize sexual complaints are an acceptable subject of discussion for their gynecologic visit, while others may feel uncomfortable talking about sex in general.
I usually begin by asking whether the patient is sexually active and, if she is, whether sex is satisfying to her and her partner. I also ask, “Do you have any concerns about your sexual functioning?” Since this question is sufficiently broad to encompass just about any complaint, it sometimes is helpful in triggering a discussion. If the woman has significant concerns, I follow up with a thorough sexual history.
Assessing your attitudes. As an American College of Obstetricians and Gynecologists (ACOG) technical bulletin points out, the physician should be conscious of any biases he or she holds about certain sexual practices or preferences and should “learn to listen to and discuss ideas and behaviors that conflict with these biases without displaying discomfort.”4 When a patient first begins to talk about her sexual functioning, few things are more troubling than a harried or distracted physician. If you feel that the patient’s concerns require more attention than you are able to provide during that visit, schedule a future appointment to tackle the subject. I usually tell patients that it is too important a subject to try to address in the short time allocated to their current visit.
Exploring the history. A thorough history can make all the difference in pinpointing the underlying cause of a patient’s dysfunction.
Decreased interest in sexual activity is rarely caused by a hormonal imbalance.
I usually have my nurse take a general medical history, including medications. I then meet with the patient in my office (with her clothes on!) and focus on areas such as prior surgeries, endometriosis, prior pelvic surgery or trauma, vaginal or vulvar pain complaints, and depression. Although our intake form asks about domestic violence or a history of physical or sexual abuse, I always make it a point to ask again. Patients are often embarrassed to discuss these issues and will not divulge such sensitive information initially.
The physical exam. I perform a comprehensive physical, including a pelvic exam. This involves checking the introitus for signs of atrophy or vaginitis and palpating the Bartholin’s glands, urethra, and bladder for tenderness. Also, examine episiotomy scars for hypersensitivity and assess the patient for cervical-motion tenderness.
Then evaluate vaginal tone, looking specifically for spasm or difficulty with relaxation of the levator musculature. I want to distinguish between abdominal wall and pelvic-floor muscle tension as potential sources of pain. (The vaginal portion of the examination should be performed with a single digit and only one hand.) Finally, check for masses, and examine the posterior cul-de-sac. Depending on the findings of the examination, I may order laboratory studies or imaging.
Classifying dysfunction. Disorders of female sexual function are divided into 4 areas, described in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (Table 1). They are:
- disorders of libido, which are central in origin, i.e., they originate in the brain and central nervous system;
- disorders of arousal, which are presumably peripheral/physical (frequently caused by vascular disease and diabetes limiting the vascular supply to the genitalia, or by estrogen deficiency);
- an inability to achieve orgasm; and
- pain disorders.
This division, while not necessarily inaccurate, overlooks the complexity of female sexuality. In women, the libido is better described as a striving for emotional closeness and intimacy rather than simply the sexual drive. Women have fewer spontaneous sexual thoughts and fantasies throughout the biological life span than men and are often unaware of or inattentive to signs of their own physical arousal.5-8 Environmental signals such as romance, a feeling of being cherished, and emotional closeness are more likely to call their attention to physical sensations.
A new model. Trying to assess female sexual complaints using only a biological model is unlikely to be successful. The social environment, hormones, drugs, physical abnormalities, and women’s deep psychological issues all have an impact on their sexual encounters. Thus, I find a biopsychosocial model more useful for assessing complaints. I typically explore 4 areas: physical, psychological, relational, and situational. Using these categories of inquiry, I am able to address the complexities of my patients’ complaints and assess each component of sexual dysfunction in the DSM-IV classification.
Disorders of libido or desire. A lack of desire for sex is the most common sexual complaint and the most difficult to assess quickly. It is typically further classified as either hypoactive sexual desire disorder (HSDD) or sexual aversion disorder (SAD). The first is a deficiency or lack of sexual fantasies or thoughts and/or the desire for sexual activity, while SAD is a phobic aversion to and avoidance of sexual contact with a partner.9 HSDD may be caused by psychological or emotional factors or be secondary to endocrine disorders or other medical problems. In contrast, SAD is usually psychological or emotional in origin, frequently deriving from physical or sexual abuse or childhood trauma.
Often the problem may be related to differing expectations between partners regarding the frequency of their desire for sexual contact. Some people are very happy with weekly or monthly sex, while others think 3 times a day is not enough! And despite media hype to the contrary, decreased interest in sexual activity is rarely caused by a hormonal imbalance. Although testosterone levels decline with age, natural menopause does not trigger a dramatic alteration in them. At menopause, estrogen levels decline much more rapidly than ovarian androgen production, decreasing sex hormone binding globulin (SHBG) and effectively increasing free testosterone levels. However, during the perimenopausal anovulatory time frame, as well as with oral estrogen therapy, SHBG is increased, which may reduce free testosterone levels and contribute to a noticeable and rapid decrease in sexual desire in some circumstances.
Premenopausal women may note decreased libido when taking oral contraceptives (OCs) or other medications that suppress ovarian androgen production. However, in these women, the adrenals remain a source of androgen, which may explain why decreased libido is not a universal complaint in this population. Chronic anxiety, stress (both physical and emotional), depression, chronic pain, and longstanding insomnia all deplete the adrenals and are associated with a decrease in libido. Androgen replacement is rarely successful in these patients.
I usually begin my evaluation by asking the patient if she has experienced discomfort with sexual activity. If she reports that sex has become painful when it wasn’t in the past, a careful physiologic assessment is indicated. I look for genital atrophy, tearing, and vaginismus when evaluating patients with decreased sexual desire.
Relational and situational factors are extremely important in evaluating complaints of diminished libido. Many women are exhausted by their roles as mother, daughter, spouse, and productive member of the workforce. Thus, an assessment of the patient’s social situation is critical. Professional counseling may be required to help women learn to limit their commitments and accept the need for “downtime.”
Management strategies for patients with diminished libido incorporate correction of any vulvar and vaginal atrophy, counseling to improve communication between the patient and her partner, and the identification and treatment of any underlying psychiatric problems. The addition of testosterone may be useful for patients on OCs or oral hormone replacement therapy (HRT) and women with surgical menopause or menopause secondary to chemotherapy.9 In other patients with low libido, the benefit of testosterone is less clear. I prefer compounded 1% or 2% testosterone in PLA cream or petrolatum (depending on whether the patient prefers a cream or an ointment). Patients should apply 1/8 teaspoon to thin skin daily. The ointment may be smoothed directly on the genitalia for added lubrication and rapid improvement in atrophic symptoms. Women who are survivors of domestic and/or sexual abuse will require psychotherapy by a trained counselor.
Arousal disorders. In women, arousal disorders are characterized by an inability to achieve or maintain sexual excitement, which manifests itself as a lack of subjective pleasure or a lack of genital or other somatic responses.10 Complicating the diagnosis is the fact that the physiologic changes that occur when women are aroused often are difficult to separate from those linked to desire. In general, however, when diminished desire precedes decreased arousal, HSDD is the diagnosis. Even so, just as in men, diseases affecting blood flow or innervation to the genitals can cause arousal disorders in women. Unfortunately for Ob/Gyns, it is much easier to assess these problems in males, since the physiological lack of responsiveness is quite obvious in men.
Women may complain of dryness or decreased sensation in the genitals, or they may experience pain with intercourse. Any of these may be related to reduced engorgement of the tissues and diminished transudation of lubrication across the vaginal epithelium. Estrogen deficiency is a common cause of recent-onset arousal disturbance in patients with a normal level of sexual desire. In perimenopausal and menopausal women, oral or transdermal estrogen replacement in doses sufficient to relieve vasomotor symptoms may not reach the epithelium of the urogenital tissues to correct atrophic changes. In these women, as well as breastfeeding mothers, topical estrogen may improve vaginal elasticity, lubrication, and engorgement. Women taking OCs or long-acting progestational agents also should be carefully assessed for vaginal atrophy. If it is present, topical estrogen will bring dramatic improvement.
Medications known for causing erectile dysfunction in men also should be assessed in women. These include antihypertensives and some antidepressants. In addition, disease states such as hypertension, diabetes mellitus, and peripheral vascular disease may diminish vasodilation and sensation in women as well as men (Table 2). Drugs such as sildenafil may help increase local blood flow in women when erotic stimuli and the central desire for sexual activity remain intact, although the use of sildenafil in women is still in the experimental stages. Recent studies questioning the effectiveness of sildenafil in women may have been limited by the difficulty of clearly distinguishing arousal from desire disorders. Sildenafil will do nothing to improve libido.
As I mentioned earlier, it often is difficult and not particularly useful clinically to distinguish desire from arousal dysfunction. Also, women may be unaware of vascular congestion and lubrication occurring in the genitals, particularly if they have experienced sexual assault. Dissociation is a common defense mechanism in victims of physical violence, and close physical contact and genital touching may trigger this response.
Women also may be distracted from bodily sensations when other factors—e.g., a crying baby, noisy teenagers, financial concerns, or fatigue—interfere with intimacy. I commonly ask patients how they respond when they are alone with their partners on vacation. If the arousal response is normal under those conditions, I reassure the patient that the cause of her complaint is neither hormonal nor physical. Rather, I encourage her to take time to create opportunities for sensuality and closeness with her partner without external distractions.
Inability to achieve orgasm. Of the 4 categories of dysfunction, this is probably the easiest to assess. First, I ascertain whether the patient has ever experienced orgasm. Women who haven’t will likely require referral to a licensed sexual therapist.
At least 25% of women have been physically or sexually molested at some time during their lives.1 These episodes of violence can create significant barriers to psychological and physical well-being, particularly in women who were molested as young children. Feelings of arousal often go unrecognized because these women are dissociated from their bodies—a defense mechanism learned in early childhood. If they experienced significant pain with their initial sexual experience, their bodies may respond with natural avoidance, muscle spasm, and withdrawal. Even when a relationship is safe and loving, these responses may remain unconscious and difficult to control.
For these reasons, I always inquire about a history of physical or sexual violence, although patients often won’t admit to such a history. For example, they may not define their experience as “abuse” if it involved date-rape, forced sexual intercourse in the context of a steady relationship, or inappropriate touching and molestation without penetration. To explore the subject, I repeat key questions after the nurse takes the initial history, watching for any degree of hesitation or other subtle changes in body language, as these may yield clues about a significant past event. In addition, women who suffered childhood abuse often have multiple tension-related complaints such as irritable bowel syndrome, migraine headaches, urinary frequency, poor sleep, and chronic pelvic pain.
Sometimes I drop the issue of abuse during the history (which I always conduct in my office with the patient fully clothed), but raise the subject again while conducting the physical examination, especially if I note significant embarrassment or difficulty with breast or pelvic exams. I then might say something like: “You know, we often see this kind of tenseness and anxiety with exams in people who have been hurt in the past. Are you sure no one has ever hurt you—perhaps during an exam?” This can defuse the situation and allow a patient to open up, encouraged by your expertise and interest in her. She may feel safe enough to discuss experiences that have haunted her for many years.
—Barbara S. Levy, MD
REFERENCE
1. American College of Obstetricians and Gynecologists. Women’s Health Stats and Facts. Washington, DC: ACOG; 2002;11.-
If a woman has been orgasmic in the past, but complains about anorgasmia, inquire about changes in medications or over-the-counter (OTC) or herbal remedies.
Further, when a patient reports that sex no longer feels like it used to, she should be carefully assessed for depression. There are several brief questionnaires, e.g., the Beck inventory, that can easily be incorporated into a gynecologist’s office routine to screen for depression.
Relational issues can negatively affect orgasmic function as well. Some women may continue to permit sexual activity even when they feel angry, used, or abused by their partner. Sexual activity may even be forced upon them. Similarly, the social situation can interfere with a woman’s ability to achieve orgasm. Women with small children frequently split their attention between the sexual activity and surveillance of the household for crying babies. This inattention to physical sensations can preclude satisfactory arousal and orgasm.
Finally, some women complain about difficulties achieving orgasm during penile-vaginal intercourse. This situation requires some education of both the patient and her partner. While approximately 25% to 30% of women may at times achieve orgasm with intercourse alone, the vast majority require clitoral stimulation. At times, with sufficient mental and physical stimulation, a woman may experience orgasm without direct stimulation, but that is the exception, not the rule. Women anxious about needing clitoral stimulation in order to climax should be reassured that they are sexually normal and functional.
Pain disorders. Pain disorders include dyspareunia, vaginismus, and a new category called “noncoital sexual pain disorders.”10 Dyspareunia may be secondary to medical conditions such as vestibulitis, vaginal atrophy, or infection, or it may be psychologic in origin. It may even contain both physiologic and psychologic elements. In contrast, vaginismus is a conditioned response to fear or pain. It is a reflex contraction of the levator ani muscles in response to attempted penetration and, except in extremely rare cases, is involuntary and out of the patient’s control. It is a learned response to painful attempts at penetration.
In general, pain disorders are easier to evaluate than disorders of libido and arousal. After all, our training emphasizes careful pelvic examination to isolate areas of concern. Unfortunately, if a physician does not understand normal female sexual responses, he or she may mistake an arousal disorder for “bump dyspareunia”—a sensation deep in the pelvis as though something is being hit.
In women, arousal elongates the vagina by approximately 30% and tents the uterus up and out of the cul-de-sac as the tissues engorge, unless the structures are tethered by adhesions and pelvic pathology. Penetration and deep thrusting before a woman is adequately aroused will commonly cause bump dyspareunia. Even in women with significant adhesions or endometriosis, attention to foreplay to assure arousal prior to intercourse often can alleviate discomfort.
I begin my assessment by asking the patient whether the pain occurs with every sexual encounter or is positional or related to the menstrual cycle. Does the pain occur at initial penetration or is it experienced deep in the pelvis? Pain around the time of menses would lead me to suspect endometriosis or adenomyosis as an etiology, whereas pain with penetration is more likely related to vaginismus or vulvovaginal disorders.
An abdominal exam can help identify tense muscles, a clue to a possible history of abuse. I then direct my attention to the vulva, looking for areas of tenderness at the introitus that suggest vulvar vestibulitis. I also look for atrophic changes or signs of chemical irritation. Many women consider their genitalia “dirty” and scrub the vulva with antibacterial soap several times a day. Pain with penetration can be dramatically reduced by paying attention to perineal hygiene and avoiding irritating chemicals, soaps, and commercial products sold to keep women “fresh.”
I examine the vagina initially without a speculum, paying careful attention to the muscle tone of the levators. Women who have a pelvic floor like a rock, i.e., you find yourself fighting the muscles throughout the exam, have been sexually abused until proven otherwise. Involuntary levator contraction can be a withdrawal response precipitated by early painful penetration attempts. (Physical therapy with biofeedback using external sensors along the pelvic floor is highly successful in creating conscious awareness of the muscle tension and in treating vaginismus.)
As the bimanual examination is completed, I look for fixation of the internal genitalia or tender nodules suspicious for endometriosis.
In evaluating the relational component of pain, I ask about any difficulties, particularly discrepancies in expectations between women and their partners, as these can result in complaints of pain.
Management strategies for pain disorders include treatment of underlying gynecologic conditions such as endometriosis, physical therapy to teach relaxation of the pelvicfloor musculature, perineal hygiene to relieve dry, inelastic external genitalia, and estrogen to treat atrophic changes.
TABLE 1
Types of female sexual dysfunction
| TYPE* | SYMPTOMS | TREATMENT | COMMENTS |
|---|---|---|---|
| Disorders of desire or libido: –Hypoactive sexual desire disorder (HSDD) –Sexual aversion disorder (SAD) | HSDD: Deficiency or absence of sexual fantasies or desire SAD: Phobic aversion to and avoidance of sexual contact with a partner | HSDD: Trial of testosterone in deficient women. Modify medications for underlying diseases SAD: Refer for psychologic counseling | Most women with low libido and normal ovarian function will not respond to normal levels of testosterone treatment |
| Disorders of arousal | Inability to attain or maintain sexual excitement | Treat underlying physical disorder. Consider sildenafil, local vasodilating agents, and appropriate estrogen replacement. Refer for psychologic/sexual counseling | Isolated arousal disorders are uncommon in women |
| Orgasmic disorders | Primary: The patient has never experienced orgasm Secondary: The patient has recently become anorgasmic | Correct underlying pharmacologic problem (change of dosage or medication) and/or refer for psychologic/sexual counseling | Look for over-thecounter and herbal supplements as etiologies as well |
| Pain disorders –Dyspareunia –Vaginismus –Noncoital sexual pain | Dyspareunia: Genital pain with intercourse Vaginismus: Involuntary spasm of the muscles comprising the outer third of the vagina Noncoital pain: Genital pain with noncoital sexual stimulation | Correct underlying perineal trauma (eliminate soaps and harsh chemicals) and medical conditions (infection and endometriosis). Try physical therapy (pelvic-floor biofeedback). Refer for counseling | Pelvic pain is multifactorial. Search for history of molestation or abuse in these women |
| *These categories also may be classified according to the etiology of the disorder and whether it is lifelong or acquired, generalized or situational. | |||
TABLE 2
Medications* and medical conditions that may affect sexual functioning
| MEDICATIONS | |
|
|
| MEDICAL AND OTHER CONDITIONS | |
| Organic disorders | Chronic diseases |
|
|
| Conditions | |
| |
| *Source: The Medical Letter. 1992;43(August 7). | |
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
2. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173.-
3. Montejo AI, Llorca G, Izquierdo JA, et al. Sexual dysfunction secondary to SSRIs. A comparative analysis in 308 patients. Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1996;24(6):311-321.
4. American College of Obstetricians and Gynecologists. Sexual dysfunction. ACOG Technical Bulletin #211. Washington, DC: ACOG; 1995.
5. Basson R. Female sexual response revisited. J Soc Obstet Gynecol. 2000;378-83.
6. Laan E, Everaerd W, Van der Velde J, et al. Determinants of subjective experience of sexual arousal in women: feedback from genital arousal and erotic stimulus content. Psychophysiol. 1995;32:444-451.
7. Andersen BL, Cyranowski JM. Women’s sexuality: Behaviors, responses and individual differences. J Consult Clin Psychol. 1995;63:891-906
8. Basson R. Female Sexual Response: The role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
9. Schifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
10. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-893.
- Forty-three percent of U.S. women report being dissatisfied with their sexual functioning.
- Female sexual dysfunction is divided into 4 categories: libido, arousal, orgasm, and pain.
- Factors that contribute to sexual dysfunction are distortion or inflammation of pelvic structures, pelvic or abdominal trauma or surgery, medications, depression, and chronic medical conditions.
- A biopsychosocial model of inquiry is recommended for assessing sexual complaints, emphasizing 4 areas: physical, psychologic, relational, and situational.
In a recent U.S. survey, 43% of female respondents reported being dissatisfied with their sexual functioning, a significantly higher percentage than among male respondents.1 Even more disturbing were separate findings: 71% of adults 25 and older believed their physician would dismiss any sexual concerns they might bring up, while 68% avoided discussing sexual dysfunction with their doctors for fear of embarrassing them.2
A lack of desire for sex is the most common sexual complaint.
These statistics highlight clinicians’ inability to elicit information about and treat sexual disorders in women. Many Ob/Gyns feel they lack the necessary background in fundamental science and psychology to competently evaluate and treat sexual complaints. It is difficult to approach these problems without a complete understanding of the physiology and psychology of female sexual response.
But times are changing. The availability of sildenafil to treat male erectile dysfunction has dramatically increased our patients’ awareness of sexual disorders, as has the open discussion of sexual dissatisfaction on the talkshow circuit. Patients are increasingly likely to expect their health-care providers to evaluate and treat sexual complaints. The following pearls offer a framework for assessing sexual dysfunction, as well as guidelines for therapeutic intervention.
Raising the subject. Although women are gradually opening up about sexual dysfunction, I try not to assume that they will raise the subject themselves. A case in point: Among 308 patients taking selective serotonin reuptake inhibitors (SSRIs), 55% reported sexual dysfunction when the physician asked them about it directly compared with only 14% who reported it spontaneously.3 Many women may not realize sexual complaints are an acceptable subject of discussion for their gynecologic visit, while others may feel uncomfortable talking about sex in general.
I usually begin by asking whether the patient is sexually active and, if she is, whether sex is satisfying to her and her partner. I also ask, “Do you have any concerns about your sexual functioning?” Since this question is sufficiently broad to encompass just about any complaint, it sometimes is helpful in triggering a discussion. If the woman has significant concerns, I follow up with a thorough sexual history.
Assessing your attitudes. As an American College of Obstetricians and Gynecologists (ACOG) technical bulletin points out, the physician should be conscious of any biases he or she holds about certain sexual practices or preferences and should “learn to listen to and discuss ideas and behaviors that conflict with these biases without displaying discomfort.”4 When a patient first begins to talk about her sexual functioning, few things are more troubling than a harried or distracted physician. If you feel that the patient’s concerns require more attention than you are able to provide during that visit, schedule a future appointment to tackle the subject. I usually tell patients that it is too important a subject to try to address in the short time allocated to their current visit.
Exploring the history. A thorough history can make all the difference in pinpointing the underlying cause of a patient’s dysfunction.
Decreased interest in sexual activity is rarely caused by a hormonal imbalance.
I usually have my nurse take a general medical history, including medications. I then meet with the patient in my office (with her clothes on!) and focus on areas such as prior surgeries, endometriosis, prior pelvic surgery or trauma, vaginal or vulvar pain complaints, and depression. Although our intake form asks about domestic violence or a history of physical or sexual abuse, I always make it a point to ask again. Patients are often embarrassed to discuss these issues and will not divulge such sensitive information initially.
The physical exam. I perform a comprehensive physical, including a pelvic exam. This involves checking the introitus for signs of atrophy or vaginitis and palpating the Bartholin’s glands, urethra, and bladder for tenderness. Also, examine episiotomy scars for hypersensitivity and assess the patient for cervical-motion tenderness.
Then evaluate vaginal tone, looking specifically for spasm or difficulty with relaxation of the levator musculature. I want to distinguish between abdominal wall and pelvic-floor muscle tension as potential sources of pain. (The vaginal portion of the examination should be performed with a single digit and only one hand.) Finally, check for masses, and examine the posterior cul-de-sac. Depending on the findings of the examination, I may order laboratory studies or imaging.
Classifying dysfunction. Disorders of female sexual function are divided into 4 areas, described in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (Table 1). They are:
- disorders of libido, which are central in origin, i.e., they originate in the brain and central nervous system;
- disorders of arousal, which are presumably peripheral/physical (frequently caused by vascular disease and diabetes limiting the vascular supply to the genitalia, or by estrogen deficiency);
- an inability to achieve orgasm; and
- pain disorders.
This division, while not necessarily inaccurate, overlooks the complexity of female sexuality. In women, the libido is better described as a striving for emotional closeness and intimacy rather than simply the sexual drive. Women have fewer spontaneous sexual thoughts and fantasies throughout the biological life span than men and are often unaware of or inattentive to signs of their own physical arousal.5-8 Environmental signals such as romance, a feeling of being cherished, and emotional closeness are more likely to call their attention to physical sensations.
A new model. Trying to assess female sexual complaints using only a biological model is unlikely to be successful. The social environment, hormones, drugs, physical abnormalities, and women’s deep psychological issues all have an impact on their sexual encounters. Thus, I find a biopsychosocial model more useful for assessing complaints. I typically explore 4 areas: physical, psychological, relational, and situational. Using these categories of inquiry, I am able to address the complexities of my patients’ complaints and assess each component of sexual dysfunction in the DSM-IV classification.
Disorders of libido or desire. A lack of desire for sex is the most common sexual complaint and the most difficult to assess quickly. It is typically further classified as either hypoactive sexual desire disorder (HSDD) or sexual aversion disorder (SAD). The first is a deficiency or lack of sexual fantasies or thoughts and/or the desire for sexual activity, while SAD is a phobic aversion to and avoidance of sexual contact with a partner.9 HSDD may be caused by psychological or emotional factors or be secondary to endocrine disorders or other medical problems. In contrast, SAD is usually psychological or emotional in origin, frequently deriving from physical or sexual abuse or childhood trauma.
Often the problem may be related to differing expectations between partners regarding the frequency of their desire for sexual contact. Some people are very happy with weekly or monthly sex, while others think 3 times a day is not enough! And despite media hype to the contrary, decreased interest in sexual activity is rarely caused by a hormonal imbalance. Although testosterone levels decline with age, natural menopause does not trigger a dramatic alteration in them. At menopause, estrogen levels decline much more rapidly than ovarian androgen production, decreasing sex hormone binding globulin (SHBG) and effectively increasing free testosterone levels. However, during the perimenopausal anovulatory time frame, as well as with oral estrogen therapy, SHBG is increased, which may reduce free testosterone levels and contribute to a noticeable and rapid decrease in sexual desire in some circumstances.
Premenopausal women may note decreased libido when taking oral contraceptives (OCs) or other medications that suppress ovarian androgen production. However, in these women, the adrenals remain a source of androgen, which may explain why decreased libido is not a universal complaint in this population. Chronic anxiety, stress (both physical and emotional), depression, chronic pain, and longstanding insomnia all deplete the adrenals and are associated with a decrease in libido. Androgen replacement is rarely successful in these patients.
I usually begin my evaluation by asking the patient if she has experienced discomfort with sexual activity. If she reports that sex has become painful when it wasn’t in the past, a careful physiologic assessment is indicated. I look for genital atrophy, tearing, and vaginismus when evaluating patients with decreased sexual desire.
Relational and situational factors are extremely important in evaluating complaints of diminished libido. Many women are exhausted by their roles as mother, daughter, spouse, and productive member of the workforce. Thus, an assessment of the patient’s social situation is critical. Professional counseling may be required to help women learn to limit their commitments and accept the need for “downtime.”
Management strategies for patients with diminished libido incorporate correction of any vulvar and vaginal atrophy, counseling to improve communication between the patient and her partner, and the identification and treatment of any underlying psychiatric problems. The addition of testosterone may be useful for patients on OCs or oral hormone replacement therapy (HRT) and women with surgical menopause or menopause secondary to chemotherapy.9 In other patients with low libido, the benefit of testosterone is less clear. I prefer compounded 1% or 2% testosterone in PLA cream or petrolatum (depending on whether the patient prefers a cream or an ointment). Patients should apply 1/8 teaspoon to thin skin daily. The ointment may be smoothed directly on the genitalia for added lubrication and rapid improvement in atrophic symptoms. Women who are survivors of domestic and/or sexual abuse will require psychotherapy by a trained counselor.
Arousal disorders. In women, arousal disorders are characterized by an inability to achieve or maintain sexual excitement, which manifests itself as a lack of subjective pleasure or a lack of genital or other somatic responses.10 Complicating the diagnosis is the fact that the physiologic changes that occur when women are aroused often are difficult to separate from those linked to desire. In general, however, when diminished desire precedes decreased arousal, HSDD is the diagnosis. Even so, just as in men, diseases affecting blood flow or innervation to the genitals can cause arousal disorders in women. Unfortunately for Ob/Gyns, it is much easier to assess these problems in males, since the physiological lack of responsiveness is quite obvious in men.
Women may complain of dryness or decreased sensation in the genitals, or they may experience pain with intercourse. Any of these may be related to reduced engorgement of the tissues and diminished transudation of lubrication across the vaginal epithelium. Estrogen deficiency is a common cause of recent-onset arousal disturbance in patients with a normal level of sexual desire. In perimenopausal and menopausal women, oral or transdermal estrogen replacement in doses sufficient to relieve vasomotor symptoms may not reach the epithelium of the urogenital tissues to correct atrophic changes. In these women, as well as breastfeeding mothers, topical estrogen may improve vaginal elasticity, lubrication, and engorgement. Women taking OCs or long-acting progestational agents also should be carefully assessed for vaginal atrophy. If it is present, topical estrogen will bring dramatic improvement.
Medications known for causing erectile dysfunction in men also should be assessed in women. These include antihypertensives and some antidepressants. In addition, disease states such as hypertension, diabetes mellitus, and peripheral vascular disease may diminish vasodilation and sensation in women as well as men (Table 2). Drugs such as sildenafil may help increase local blood flow in women when erotic stimuli and the central desire for sexual activity remain intact, although the use of sildenafil in women is still in the experimental stages. Recent studies questioning the effectiveness of sildenafil in women may have been limited by the difficulty of clearly distinguishing arousal from desire disorders. Sildenafil will do nothing to improve libido.
As I mentioned earlier, it often is difficult and not particularly useful clinically to distinguish desire from arousal dysfunction. Also, women may be unaware of vascular congestion and lubrication occurring in the genitals, particularly if they have experienced sexual assault. Dissociation is a common defense mechanism in victims of physical violence, and close physical contact and genital touching may trigger this response.
Women also may be distracted from bodily sensations when other factors—e.g., a crying baby, noisy teenagers, financial concerns, or fatigue—interfere with intimacy. I commonly ask patients how they respond when they are alone with their partners on vacation. If the arousal response is normal under those conditions, I reassure the patient that the cause of her complaint is neither hormonal nor physical. Rather, I encourage her to take time to create opportunities for sensuality and closeness with her partner without external distractions.
Inability to achieve orgasm. Of the 4 categories of dysfunction, this is probably the easiest to assess. First, I ascertain whether the patient has ever experienced orgasm. Women who haven’t will likely require referral to a licensed sexual therapist.
At least 25% of women have been physically or sexually molested at some time during their lives.1 These episodes of violence can create significant barriers to psychological and physical well-being, particularly in women who were molested as young children. Feelings of arousal often go unrecognized because these women are dissociated from their bodies—a defense mechanism learned in early childhood. If they experienced significant pain with their initial sexual experience, their bodies may respond with natural avoidance, muscle spasm, and withdrawal. Even when a relationship is safe and loving, these responses may remain unconscious and difficult to control.
For these reasons, I always inquire about a history of physical or sexual violence, although patients often won’t admit to such a history. For example, they may not define their experience as “abuse” if it involved date-rape, forced sexual intercourse in the context of a steady relationship, or inappropriate touching and molestation without penetration. To explore the subject, I repeat key questions after the nurse takes the initial history, watching for any degree of hesitation or other subtle changes in body language, as these may yield clues about a significant past event. In addition, women who suffered childhood abuse often have multiple tension-related complaints such as irritable bowel syndrome, migraine headaches, urinary frequency, poor sleep, and chronic pelvic pain.
Sometimes I drop the issue of abuse during the history (which I always conduct in my office with the patient fully clothed), but raise the subject again while conducting the physical examination, especially if I note significant embarrassment or difficulty with breast or pelvic exams. I then might say something like: “You know, we often see this kind of tenseness and anxiety with exams in people who have been hurt in the past. Are you sure no one has ever hurt you—perhaps during an exam?” This can defuse the situation and allow a patient to open up, encouraged by your expertise and interest in her. She may feel safe enough to discuss experiences that have haunted her for many years.
—Barbara S. Levy, MD
REFERENCE
1. American College of Obstetricians and Gynecologists. Women’s Health Stats and Facts. Washington, DC: ACOG; 2002;11.-
If a woman has been orgasmic in the past, but complains about anorgasmia, inquire about changes in medications or over-the-counter (OTC) or herbal remedies.
Further, when a patient reports that sex no longer feels like it used to, she should be carefully assessed for depression. There are several brief questionnaires, e.g., the Beck inventory, that can easily be incorporated into a gynecologist’s office routine to screen for depression.
Relational issues can negatively affect orgasmic function as well. Some women may continue to permit sexual activity even when they feel angry, used, or abused by their partner. Sexual activity may even be forced upon them. Similarly, the social situation can interfere with a woman’s ability to achieve orgasm. Women with small children frequently split their attention between the sexual activity and surveillance of the household for crying babies. This inattention to physical sensations can preclude satisfactory arousal and orgasm.
Finally, some women complain about difficulties achieving orgasm during penile-vaginal intercourse. This situation requires some education of both the patient and her partner. While approximately 25% to 30% of women may at times achieve orgasm with intercourse alone, the vast majority require clitoral stimulation. At times, with sufficient mental and physical stimulation, a woman may experience orgasm without direct stimulation, but that is the exception, not the rule. Women anxious about needing clitoral stimulation in order to climax should be reassured that they are sexually normal and functional.
Pain disorders. Pain disorders include dyspareunia, vaginismus, and a new category called “noncoital sexual pain disorders.”10 Dyspareunia may be secondary to medical conditions such as vestibulitis, vaginal atrophy, or infection, or it may be psychologic in origin. It may even contain both physiologic and psychologic elements. In contrast, vaginismus is a conditioned response to fear or pain. It is a reflex contraction of the levator ani muscles in response to attempted penetration and, except in extremely rare cases, is involuntary and out of the patient’s control. It is a learned response to painful attempts at penetration.
In general, pain disorders are easier to evaluate than disorders of libido and arousal. After all, our training emphasizes careful pelvic examination to isolate areas of concern. Unfortunately, if a physician does not understand normal female sexual responses, he or she may mistake an arousal disorder for “bump dyspareunia”—a sensation deep in the pelvis as though something is being hit.
In women, arousal elongates the vagina by approximately 30% and tents the uterus up and out of the cul-de-sac as the tissues engorge, unless the structures are tethered by adhesions and pelvic pathology. Penetration and deep thrusting before a woman is adequately aroused will commonly cause bump dyspareunia. Even in women with significant adhesions or endometriosis, attention to foreplay to assure arousal prior to intercourse often can alleviate discomfort.
I begin my assessment by asking the patient whether the pain occurs with every sexual encounter or is positional or related to the menstrual cycle. Does the pain occur at initial penetration or is it experienced deep in the pelvis? Pain around the time of menses would lead me to suspect endometriosis or adenomyosis as an etiology, whereas pain with penetration is more likely related to vaginismus or vulvovaginal disorders.
An abdominal exam can help identify tense muscles, a clue to a possible history of abuse. I then direct my attention to the vulva, looking for areas of tenderness at the introitus that suggest vulvar vestibulitis. I also look for atrophic changes or signs of chemical irritation. Many women consider their genitalia “dirty” and scrub the vulva with antibacterial soap several times a day. Pain with penetration can be dramatically reduced by paying attention to perineal hygiene and avoiding irritating chemicals, soaps, and commercial products sold to keep women “fresh.”
I examine the vagina initially without a speculum, paying careful attention to the muscle tone of the levators. Women who have a pelvic floor like a rock, i.e., you find yourself fighting the muscles throughout the exam, have been sexually abused until proven otherwise. Involuntary levator contraction can be a withdrawal response precipitated by early painful penetration attempts. (Physical therapy with biofeedback using external sensors along the pelvic floor is highly successful in creating conscious awareness of the muscle tension and in treating vaginismus.)
As the bimanual examination is completed, I look for fixation of the internal genitalia or tender nodules suspicious for endometriosis.
In evaluating the relational component of pain, I ask about any difficulties, particularly discrepancies in expectations between women and their partners, as these can result in complaints of pain.
Management strategies for pain disorders include treatment of underlying gynecologic conditions such as endometriosis, physical therapy to teach relaxation of the pelvicfloor musculature, perineal hygiene to relieve dry, inelastic external genitalia, and estrogen to treat atrophic changes.
TABLE 1
Types of female sexual dysfunction
| TYPE* | SYMPTOMS | TREATMENT | COMMENTS |
|---|---|---|---|
| Disorders of desire or libido: –Hypoactive sexual desire disorder (HSDD) –Sexual aversion disorder (SAD) | HSDD: Deficiency or absence of sexual fantasies or desire SAD: Phobic aversion to and avoidance of sexual contact with a partner | HSDD: Trial of testosterone in deficient women. Modify medications for underlying diseases SAD: Refer for psychologic counseling | Most women with low libido and normal ovarian function will not respond to normal levels of testosterone treatment |
| Disorders of arousal | Inability to attain or maintain sexual excitement | Treat underlying physical disorder. Consider sildenafil, local vasodilating agents, and appropriate estrogen replacement. Refer for psychologic/sexual counseling | Isolated arousal disorders are uncommon in women |
| Orgasmic disorders | Primary: The patient has never experienced orgasm Secondary: The patient has recently become anorgasmic | Correct underlying pharmacologic problem (change of dosage or medication) and/or refer for psychologic/sexual counseling | Look for over-thecounter and herbal supplements as etiologies as well |
| Pain disorders –Dyspareunia –Vaginismus –Noncoital sexual pain | Dyspareunia: Genital pain with intercourse Vaginismus: Involuntary spasm of the muscles comprising the outer third of the vagina Noncoital pain: Genital pain with noncoital sexual stimulation | Correct underlying perineal trauma (eliminate soaps and harsh chemicals) and medical conditions (infection and endometriosis). Try physical therapy (pelvic-floor biofeedback). Refer for counseling | Pelvic pain is multifactorial. Search for history of molestation or abuse in these women |
| *These categories also may be classified according to the etiology of the disorder and whether it is lifelong or acquired, generalized or situational. | |||
TABLE 2
Medications* and medical conditions that may affect sexual functioning
| MEDICATIONS | |
|
|
| MEDICAL AND OTHER CONDITIONS | |
| Organic disorders | Chronic diseases |
|
|
| Conditions | |
| |
| *Source: The Medical Letter. 1992;43(August 7). | |
The author reports no financial relationship with any companies whose products are mentioned in this article.
- Forty-three percent of U.S. women report being dissatisfied with their sexual functioning.
- Female sexual dysfunction is divided into 4 categories: libido, arousal, orgasm, and pain.
- Factors that contribute to sexual dysfunction are distortion or inflammation of pelvic structures, pelvic or abdominal trauma or surgery, medications, depression, and chronic medical conditions.
- A biopsychosocial model of inquiry is recommended for assessing sexual complaints, emphasizing 4 areas: physical, psychologic, relational, and situational.
In a recent U.S. survey, 43% of female respondents reported being dissatisfied with their sexual functioning, a significantly higher percentage than among male respondents.1 Even more disturbing were separate findings: 71% of adults 25 and older believed their physician would dismiss any sexual concerns they might bring up, while 68% avoided discussing sexual dysfunction with their doctors for fear of embarrassing them.2
A lack of desire for sex is the most common sexual complaint.
These statistics highlight clinicians’ inability to elicit information about and treat sexual disorders in women. Many Ob/Gyns feel they lack the necessary background in fundamental science and psychology to competently evaluate and treat sexual complaints. It is difficult to approach these problems without a complete understanding of the physiology and psychology of female sexual response.
But times are changing. The availability of sildenafil to treat male erectile dysfunction has dramatically increased our patients’ awareness of sexual disorders, as has the open discussion of sexual dissatisfaction on the talkshow circuit. Patients are increasingly likely to expect their health-care providers to evaluate and treat sexual complaints. The following pearls offer a framework for assessing sexual dysfunction, as well as guidelines for therapeutic intervention.
Raising the subject. Although women are gradually opening up about sexual dysfunction, I try not to assume that they will raise the subject themselves. A case in point: Among 308 patients taking selective serotonin reuptake inhibitors (SSRIs), 55% reported sexual dysfunction when the physician asked them about it directly compared with only 14% who reported it spontaneously.3 Many women may not realize sexual complaints are an acceptable subject of discussion for their gynecologic visit, while others may feel uncomfortable talking about sex in general.
I usually begin by asking whether the patient is sexually active and, if she is, whether sex is satisfying to her and her partner. I also ask, “Do you have any concerns about your sexual functioning?” Since this question is sufficiently broad to encompass just about any complaint, it sometimes is helpful in triggering a discussion. If the woman has significant concerns, I follow up with a thorough sexual history.
Assessing your attitudes. As an American College of Obstetricians and Gynecologists (ACOG) technical bulletin points out, the physician should be conscious of any biases he or she holds about certain sexual practices or preferences and should “learn to listen to and discuss ideas and behaviors that conflict with these biases without displaying discomfort.”4 When a patient first begins to talk about her sexual functioning, few things are more troubling than a harried or distracted physician. If you feel that the patient’s concerns require more attention than you are able to provide during that visit, schedule a future appointment to tackle the subject. I usually tell patients that it is too important a subject to try to address in the short time allocated to their current visit.
Exploring the history. A thorough history can make all the difference in pinpointing the underlying cause of a patient’s dysfunction.
Decreased interest in sexual activity is rarely caused by a hormonal imbalance.
I usually have my nurse take a general medical history, including medications. I then meet with the patient in my office (with her clothes on!) and focus on areas such as prior surgeries, endometriosis, prior pelvic surgery or trauma, vaginal or vulvar pain complaints, and depression. Although our intake form asks about domestic violence or a history of physical or sexual abuse, I always make it a point to ask again. Patients are often embarrassed to discuss these issues and will not divulge such sensitive information initially.
The physical exam. I perform a comprehensive physical, including a pelvic exam. This involves checking the introitus for signs of atrophy or vaginitis and palpating the Bartholin’s glands, urethra, and bladder for tenderness. Also, examine episiotomy scars for hypersensitivity and assess the patient for cervical-motion tenderness.
Then evaluate vaginal tone, looking specifically for spasm or difficulty with relaxation of the levator musculature. I want to distinguish between abdominal wall and pelvic-floor muscle tension as potential sources of pain. (The vaginal portion of the examination should be performed with a single digit and only one hand.) Finally, check for masses, and examine the posterior cul-de-sac. Depending on the findings of the examination, I may order laboratory studies or imaging.
Classifying dysfunction. Disorders of female sexual function are divided into 4 areas, described in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (Table 1). They are:
- disorders of libido, which are central in origin, i.e., they originate in the brain and central nervous system;
- disorders of arousal, which are presumably peripheral/physical (frequently caused by vascular disease and diabetes limiting the vascular supply to the genitalia, or by estrogen deficiency);
- an inability to achieve orgasm; and
- pain disorders.
This division, while not necessarily inaccurate, overlooks the complexity of female sexuality. In women, the libido is better described as a striving for emotional closeness and intimacy rather than simply the sexual drive. Women have fewer spontaneous sexual thoughts and fantasies throughout the biological life span than men and are often unaware of or inattentive to signs of their own physical arousal.5-8 Environmental signals such as romance, a feeling of being cherished, and emotional closeness are more likely to call their attention to physical sensations.
A new model. Trying to assess female sexual complaints using only a biological model is unlikely to be successful. The social environment, hormones, drugs, physical abnormalities, and women’s deep psychological issues all have an impact on their sexual encounters. Thus, I find a biopsychosocial model more useful for assessing complaints. I typically explore 4 areas: physical, psychological, relational, and situational. Using these categories of inquiry, I am able to address the complexities of my patients’ complaints and assess each component of sexual dysfunction in the DSM-IV classification.
Disorders of libido or desire. A lack of desire for sex is the most common sexual complaint and the most difficult to assess quickly. It is typically further classified as either hypoactive sexual desire disorder (HSDD) or sexual aversion disorder (SAD). The first is a deficiency or lack of sexual fantasies or thoughts and/or the desire for sexual activity, while SAD is a phobic aversion to and avoidance of sexual contact with a partner.9 HSDD may be caused by psychological or emotional factors or be secondary to endocrine disorders or other medical problems. In contrast, SAD is usually psychological or emotional in origin, frequently deriving from physical or sexual abuse or childhood trauma.
Often the problem may be related to differing expectations between partners regarding the frequency of their desire for sexual contact. Some people are very happy with weekly or monthly sex, while others think 3 times a day is not enough! And despite media hype to the contrary, decreased interest in sexual activity is rarely caused by a hormonal imbalance. Although testosterone levels decline with age, natural menopause does not trigger a dramatic alteration in them. At menopause, estrogen levels decline much more rapidly than ovarian androgen production, decreasing sex hormone binding globulin (SHBG) and effectively increasing free testosterone levels. However, during the perimenopausal anovulatory time frame, as well as with oral estrogen therapy, SHBG is increased, which may reduce free testosterone levels and contribute to a noticeable and rapid decrease in sexual desire in some circumstances.
Premenopausal women may note decreased libido when taking oral contraceptives (OCs) or other medications that suppress ovarian androgen production. However, in these women, the adrenals remain a source of androgen, which may explain why decreased libido is not a universal complaint in this population. Chronic anxiety, stress (both physical and emotional), depression, chronic pain, and longstanding insomnia all deplete the adrenals and are associated with a decrease in libido. Androgen replacement is rarely successful in these patients.
I usually begin my evaluation by asking the patient if she has experienced discomfort with sexual activity. If she reports that sex has become painful when it wasn’t in the past, a careful physiologic assessment is indicated. I look for genital atrophy, tearing, and vaginismus when evaluating patients with decreased sexual desire.
Relational and situational factors are extremely important in evaluating complaints of diminished libido. Many women are exhausted by their roles as mother, daughter, spouse, and productive member of the workforce. Thus, an assessment of the patient’s social situation is critical. Professional counseling may be required to help women learn to limit their commitments and accept the need for “downtime.”
Management strategies for patients with diminished libido incorporate correction of any vulvar and vaginal atrophy, counseling to improve communication between the patient and her partner, and the identification and treatment of any underlying psychiatric problems. The addition of testosterone may be useful for patients on OCs or oral hormone replacement therapy (HRT) and women with surgical menopause or menopause secondary to chemotherapy.9 In other patients with low libido, the benefit of testosterone is less clear. I prefer compounded 1% or 2% testosterone in PLA cream or petrolatum (depending on whether the patient prefers a cream or an ointment). Patients should apply 1/8 teaspoon to thin skin daily. The ointment may be smoothed directly on the genitalia for added lubrication and rapid improvement in atrophic symptoms. Women who are survivors of domestic and/or sexual abuse will require psychotherapy by a trained counselor.
Arousal disorders. In women, arousal disorders are characterized by an inability to achieve or maintain sexual excitement, which manifests itself as a lack of subjective pleasure or a lack of genital or other somatic responses.10 Complicating the diagnosis is the fact that the physiologic changes that occur when women are aroused often are difficult to separate from those linked to desire. In general, however, when diminished desire precedes decreased arousal, HSDD is the diagnosis. Even so, just as in men, diseases affecting blood flow or innervation to the genitals can cause arousal disorders in women. Unfortunately for Ob/Gyns, it is much easier to assess these problems in males, since the physiological lack of responsiveness is quite obvious in men.
Women may complain of dryness or decreased sensation in the genitals, or they may experience pain with intercourse. Any of these may be related to reduced engorgement of the tissues and diminished transudation of lubrication across the vaginal epithelium. Estrogen deficiency is a common cause of recent-onset arousal disturbance in patients with a normal level of sexual desire. In perimenopausal and menopausal women, oral or transdermal estrogen replacement in doses sufficient to relieve vasomotor symptoms may not reach the epithelium of the urogenital tissues to correct atrophic changes. In these women, as well as breastfeeding mothers, topical estrogen may improve vaginal elasticity, lubrication, and engorgement. Women taking OCs or long-acting progestational agents also should be carefully assessed for vaginal atrophy. If it is present, topical estrogen will bring dramatic improvement.
Medications known for causing erectile dysfunction in men also should be assessed in women. These include antihypertensives and some antidepressants. In addition, disease states such as hypertension, diabetes mellitus, and peripheral vascular disease may diminish vasodilation and sensation in women as well as men (Table 2). Drugs such as sildenafil may help increase local blood flow in women when erotic stimuli and the central desire for sexual activity remain intact, although the use of sildenafil in women is still in the experimental stages. Recent studies questioning the effectiveness of sildenafil in women may have been limited by the difficulty of clearly distinguishing arousal from desire disorders. Sildenafil will do nothing to improve libido.
As I mentioned earlier, it often is difficult and not particularly useful clinically to distinguish desire from arousal dysfunction. Also, women may be unaware of vascular congestion and lubrication occurring in the genitals, particularly if they have experienced sexual assault. Dissociation is a common defense mechanism in victims of physical violence, and close physical contact and genital touching may trigger this response.
Women also may be distracted from bodily sensations when other factors—e.g., a crying baby, noisy teenagers, financial concerns, or fatigue—interfere with intimacy. I commonly ask patients how they respond when they are alone with their partners on vacation. If the arousal response is normal under those conditions, I reassure the patient that the cause of her complaint is neither hormonal nor physical. Rather, I encourage her to take time to create opportunities for sensuality and closeness with her partner without external distractions.
Inability to achieve orgasm. Of the 4 categories of dysfunction, this is probably the easiest to assess. First, I ascertain whether the patient has ever experienced orgasm. Women who haven’t will likely require referral to a licensed sexual therapist.
At least 25% of women have been physically or sexually molested at some time during their lives.1 These episodes of violence can create significant barriers to psychological and physical well-being, particularly in women who were molested as young children. Feelings of arousal often go unrecognized because these women are dissociated from their bodies—a defense mechanism learned in early childhood. If they experienced significant pain with their initial sexual experience, their bodies may respond with natural avoidance, muscle spasm, and withdrawal. Even when a relationship is safe and loving, these responses may remain unconscious and difficult to control.
For these reasons, I always inquire about a history of physical or sexual violence, although patients often won’t admit to such a history. For example, they may not define their experience as “abuse” if it involved date-rape, forced sexual intercourse in the context of a steady relationship, or inappropriate touching and molestation without penetration. To explore the subject, I repeat key questions after the nurse takes the initial history, watching for any degree of hesitation or other subtle changes in body language, as these may yield clues about a significant past event. In addition, women who suffered childhood abuse often have multiple tension-related complaints such as irritable bowel syndrome, migraine headaches, urinary frequency, poor sleep, and chronic pelvic pain.
Sometimes I drop the issue of abuse during the history (which I always conduct in my office with the patient fully clothed), but raise the subject again while conducting the physical examination, especially if I note significant embarrassment or difficulty with breast or pelvic exams. I then might say something like: “You know, we often see this kind of tenseness and anxiety with exams in people who have been hurt in the past. Are you sure no one has ever hurt you—perhaps during an exam?” This can defuse the situation and allow a patient to open up, encouraged by your expertise and interest in her. She may feel safe enough to discuss experiences that have haunted her for many years.
—Barbara S. Levy, MD
REFERENCE
1. American College of Obstetricians and Gynecologists. Women’s Health Stats and Facts. Washington, DC: ACOG; 2002;11.-
If a woman has been orgasmic in the past, but complains about anorgasmia, inquire about changes in medications or over-the-counter (OTC) or herbal remedies.
Further, when a patient reports that sex no longer feels like it used to, she should be carefully assessed for depression. There are several brief questionnaires, e.g., the Beck inventory, that can easily be incorporated into a gynecologist’s office routine to screen for depression.
Relational issues can negatively affect orgasmic function as well. Some women may continue to permit sexual activity even when they feel angry, used, or abused by their partner. Sexual activity may even be forced upon them. Similarly, the social situation can interfere with a woman’s ability to achieve orgasm. Women with small children frequently split their attention between the sexual activity and surveillance of the household for crying babies. This inattention to physical sensations can preclude satisfactory arousal and orgasm.
Finally, some women complain about difficulties achieving orgasm during penile-vaginal intercourse. This situation requires some education of both the patient and her partner. While approximately 25% to 30% of women may at times achieve orgasm with intercourse alone, the vast majority require clitoral stimulation. At times, with sufficient mental and physical stimulation, a woman may experience orgasm without direct stimulation, but that is the exception, not the rule. Women anxious about needing clitoral stimulation in order to climax should be reassured that they are sexually normal and functional.
Pain disorders. Pain disorders include dyspareunia, vaginismus, and a new category called “noncoital sexual pain disorders.”10 Dyspareunia may be secondary to medical conditions such as vestibulitis, vaginal atrophy, or infection, or it may be psychologic in origin. It may even contain both physiologic and psychologic elements. In contrast, vaginismus is a conditioned response to fear or pain. It is a reflex contraction of the levator ani muscles in response to attempted penetration and, except in extremely rare cases, is involuntary and out of the patient’s control. It is a learned response to painful attempts at penetration.
In general, pain disorders are easier to evaluate than disorders of libido and arousal. After all, our training emphasizes careful pelvic examination to isolate areas of concern. Unfortunately, if a physician does not understand normal female sexual responses, he or she may mistake an arousal disorder for “bump dyspareunia”—a sensation deep in the pelvis as though something is being hit.
In women, arousal elongates the vagina by approximately 30% and tents the uterus up and out of the cul-de-sac as the tissues engorge, unless the structures are tethered by adhesions and pelvic pathology. Penetration and deep thrusting before a woman is adequately aroused will commonly cause bump dyspareunia. Even in women with significant adhesions or endometriosis, attention to foreplay to assure arousal prior to intercourse often can alleviate discomfort.
I begin my assessment by asking the patient whether the pain occurs with every sexual encounter or is positional or related to the menstrual cycle. Does the pain occur at initial penetration or is it experienced deep in the pelvis? Pain around the time of menses would lead me to suspect endometriosis or adenomyosis as an etiology, whereas pain with penetration is more likely related to vaginismus or vulvovaginal disorders.
An abdominal exam can help identify tense muscles, a clue to a possible history of abuse. I then direct my attention to the vulva, looking for areas of tenderness at the introitus that suggest vulvar vestibulitis. I also look for atrophic changes or signs of chemical irritation. Many women consider their genitalia “dirty” and scrub the vulva with antibacterial soap several times a day. Pain with penetration can be dramatically reduced by paying attention to perineal hygiene and avoiding irritating chemicals, soaps, and commercial products sold to keep women “fresh.”
I examine the vagina initially without a speculum, paying careful attention to the muscle tone of the levators. Women who have a pelvic floor like a rock, i.e., you find yourself fighting the muscles throughout the exam, have been sexually abused until proven otherwise. Involuntary levator contraction can be a withdrawal response precipitated by early painful penetration attempts. (Physical therapy with biofeedback using external sensors along the pelvic floor is highly successful in creating conscious awareness of the muscle tension and in treating vaginismus.)
As the bimanual examination is completed, I look for fixation of the internal genitalia or tender nodules suspicious for endometriosis.
In evaluating the relational component of pain, I ask about any difficulties, particularly discrepancies in expectations between women and their partners, as these can result in complaints of pain.
Management strategies for pain disorders include treatment of underlying gynecologic conditions such as endometriosis, physical therapy to teach relaxation of the pelvicfloor musculature, perineal hygiene to relieve dry, inelastic external genitalia, and estrogen to treat atrophic changes.
TABLE 1
Types of female sexual dysfunction
| TYPE* | SYMPTOMS | TREATMENT | COMMENTS |
|---|---|---|---|
| Disorders of desire or libido: –Hypoactive sexual desire disorder (HSDD) –Sexual aversion disorder (SAD) | HSDD: Deficiency or absence of sexual fantasies or desire SAD: Phobic aversion to and avoidance of sexual contact with a partner | HSDD: Trial of testosterone in deficient women. Modify medications for underlying diseases SAD: Refer for psychologic counseling | Most women with low libido and normal ovarian function will not respond to normal levels of testosterone treatment |
| Disorders of arousal | Inability to attain or maintain sexual excitement | Treat underlying physical disorder. Consider sildenafil, local vasodilating agents, and appropriate estrogen replacement. Refer for psychologic/sexual counseling | Isolated arousal disorders are uncommon in women |
| Orgasmic disorders | Primary: The patient has never experienced orgasm Secondary: The patient has recently become anorgasmic | Correct underlying pharmacologic problem (change of dosage or medication) and/or refer for psychologic/sexual counseling | Look for over-thecounter and herbal supplements as etiologies as well |
| Pain disorders –Dyspareunia –Vaginismus –Noncoital sexual pain | Dyspareunia: Genital pain with intercourse Vaginismus: Involuntary spasm of the muscles comprising the outer third of the vagina Noncoital pain: Genital pain with noncoital sexual stimulation | Correct underlying perineal trauma (eliminate soaps and harsh chemicals) and medical conditions (infection and endometriosis). Try physical therapy (pelvic-floor biofeedback). Refer for counseling | Pelvic pain is multifactorial. Search for history of molestation or abuse in these women |
| *These categories also may be classified according to the etiology of the disorder and whether it is lifelong or acquired, generalized or situational. | |||
TABLE 2
Medications* and medical conditions that may affect sexual functioning
| MEDICATIONS | |
|
|
| MEDICAL AND OTHER CONDITIONS | |
| Organic disorders | Chronic diseases |
|
|
| Conditions | |
| |
| *Source: The Medical Letter. 1992;43(August 7). | |
The author reports no financial relationship with any companies whose products are mentioned in this article.
1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
2. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173.-
3. Montejo AI, Llorca G, Izquierdo JA, et al. Sexual dysfunction secondary to SSRIs. A comparative analysis in 308 patients. Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1996;24(6):311-321.
4. American College of Obstetricians and Gynecologists. Sexual dysfunction. ACOG Technical Bulletin #211. Washington, DC: ACOG; 1995.
5. Basson R. Female sexual response revisited. J Soc Obstet Gynecol. 2000;378-83.
6. Laan E, Everaerd W, Van der Velde J, et al. Determinants of subjective experience of sexual arousal in women: feedback from genital arousal and erotic stimulus content. Psychophysiol. 1995;32:444-451.
7. Andersen BL, Cyranowski JM. Women’s sexuality: Behaviors, responses and individual differences. J Consult Clin Psychol. 1995;63:891-906
8. Basson R. Female Sexual Response: The role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
9. Schifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
10. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-893.
1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
2. Marwick C. Survey says patients expect little physician help on sex. JAMA. 1999;281:2173.-
3. Montejo AI, Llorca G, Izquierdo JA, et al. Sexual dysfunction secondary to SSRIs. A comparative analysis in 308 patients. Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1996;24(6):311-321.
4. American College of Obstetricians and Gynecologists. Sexual dysfunction. ACOG Technical Bulletin #211. Washington, DC: ACOG; 1995.
5. Basson R. Female sexual response revisited. J Soc Obstet Gynecol. 2000;378-83.
6. Laan E, Everaerd W, Van der Velde J, et al. Determinants of subjective experience of sexual arousal in women: feedback from genital arousal and erotic stimulus content. Psychophysiol. 1995;32:444-451.
7. Andersen BL, Cyranowski JM. Women’s sexuality: Behaviors, responses and individual differences. J Consult Clin Psychol. 1995;63:891-906
8. Basson R. Female Sexual Response: The role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98:350-353.
9. Schifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
10. Basson R, Berman J, Burnett A, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000;163:888-893.