User login
2014 Update on sexual dysfunction
Since the last installment of this Update on Sexual Dysfunction, three new drugs have been added to the armamentarium for menopausal symptoms and dyspareunia:
- paroxetine 7.5 mg (Brisdelle)
- conjugated estrogens and bazedoxifene (Duavee)
- ospemifene (Osphena).
In this article, I present a case-based approach to incorporating these drugs into practice and restoring sexual function in the setting of vulvovaginal atrophy and dyspareunia. As is often the case, decision-making requires sifting through multiple layers of information.
How to “tease out” the problem and help the patient regain sexual function
Simon JA, Portman DJ, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Safety profile of paroxetine 7.5 mg in women with moderate-to-severe vasomotor symptoms. Obstet Gynecol. 2014;123(suppl 1):132S–133S.
Conjugated estrogens/bazedoxifene (Duavee) for menopausal symptoms and prevention of osteoporosis. Med Lett Drugs Ther. 2014;56(1441):33–34.
DeGregorio MW, Zerbe RL, Wurz GT. Ospemifene: a first-in-class, non-hormonal selective estrogen receptor modulator approved for the treatment of dyspareunia associated with vulvar and vaginal atrophy [published online ahead of print August 1, 2014]. Steroids. doi:10.1016/j.steroids.2014.07.012.
Goldstein SR, Archer DF, Simon JA, Constantine G. Endometrial safety of ospemifene and the ability of transvaginal ultrasonography to detect small changes in endometrial thickness. Obstet Gynecol. 2014;123(suppl 1):96S–97S.
CASE: LOW DESIRE AND DISCOMFORT DURING INTERCOURSE
Your 58-year-old patient, G2P2, mentions during her annual visit that she’s not that interested in sex anymore. Her children are grown, she’s been happily married for 28 years, and she enjoys her job and denies any symptoms of depression. She says her relationship with her husband is good and, aside from her low desire, she has no worries about the marriage. Her only medication is paroxetine 7.5 mg/day (Brisdelle) for management of her moderate hot flashes, which she initiated at her last annual visit. She reports improvement in her sleep and menopausal symptoms as a result. She has an intact uterus.
You perform a pelvic exam and find atrophic vulva and vagina with mild erythema, and thinned epithelium. When you ask if she has experienced any discomfort, she reports that she needs to use lubrication for intercourse and that, even with lubrication, she has pain upon penetration and a burning sensation that continues throughout intercourse. She also reports that it seems to take her much longer to achieve arousal than in the past, and she often fails to reach orgasm.
How would you manage this patient?
As always, begin with the history
The transition to menopause creates multiple layers of potential symptoms and problems for our patients, and sometimes medical therapy can generate additional ones.
In a patient reporting the onset of low desire and dyspareunia, you would want to first consider her medication history, despite the clear evidence of vaginal atrophy. Begin by asking whether she is taking any new medications prescribed by another provider. In some cases, antihypertensive drugs, psychotropic agents, and other medications can affect sexual function.
This patient has been taking Brisdelle for 1 year and is happy with its effect on her sleep and hot flashes. Simon and colleagues found this nonhormonal agent for moderate to severe vasomotor symptoms to produce no notable effects in weight, libido, or sleep, compared with placebo.
Nevertheless, in this case, because selective serotonin reuptake inhibitors (SSRIs) such as paroxetine can affect arousal and orgasm, it is unclear whether the ultra-low dose of paroxetine she is taking is contributing to her problems. If you were to discontinue the drug to find out, her vasomotor symptoms and sleep disruption would likely recur.
Your decision-making is important here and should involve the patient in an extensive discussion. If there is not enough time for this discussion at the current visit, schedule a follow-up to address her issues fully.
Vulvovaginal atrophy has its own timeline
In many cases, vasomotor symptoms such as hot flashes occur years before the skin begins to atrophy in the vulva and vagina, particularly in women who enter menopause naturally. Among menopausal women who continue to have intercourse on a regular basis, however, these skin changes often are much less troublesome than they are for women who have sex more rarely.
In this patient, one possible scenario is that paroxetine caused a slight reduction in sexual interest, and the frequency of intercourse went down as a result. In women who have little or no intercourse, the vagina begins to shrink and the tissues lose elasticity. This patient may have been undergoing the natural process of menopause, and that process may have been compounded by a decrease in the frequency of sex.
If you were to discontinue the paroxetine, it would still be necessary to treat the vulvovaginal skin and work on manual techniques to gently dilate the introitus.
Option 1: Systemic hormone therapy
Systemic estrogen is the most effective treatment for menopausal vasomotor symptoms, reducing hot flashes by 50% to 100% within 4 weeks of initiation. However, because our patient has an intact uterus, any systemic estrogen she opts to use must be opposed by a progestin for safety reasons.
In terms of estrogen, her options are oral or nonoral formulations. Not only would estrogen manage our patient’s hot flashes but, over time, it would improve her sexual problems and atrophy, which might or might not improve her current complaint of low desire. You likely would need to add a short regimen of topical estrogen and perhaps even a dilator to restore her sexual function completely, however.
Since our patient chose the nonhormonal agent Brisdelle to manage her menopausal symptoms, she may be worried about the increased risk of breast cancer associated with use of a progestin in combination with estrogen. One hormonal option now available that eliminates the need for a progestin is conjugated estrogens and bazedoxefine (Duavee). Bazedoxefine is a third-
generation selective estrogen receptor modulator (SERM). This drug has estrogen-like effects on bone and antiestrogen effects on the uterus.
Duavee is indicated for use in women with a uterus for treatment of:
- moderate to severe vasomotor symptoms of menopause
- prevention of postmenopausal osteoporosis.
Among the risks are an increased risk of venous thromboembolism (VTE) and stroke. It is not approved specifically for the treatment of dyspareunia.
Another hormonal option is ospemifene (Osphena), an estrogen agonist/antagonist indicated for the treatment of moderate to severe dyspareunia in menopausal women. Among the drugs in its class, such as tamoxifen and raloxifene, ospemifene is the only agent that maintains a full estrogenic effect on vaginal tissues. Its risks include VTE and stroke.
Although the labeling includes a warning about the risk of endometrial hyperplasia associated with its use, Goldstein and colleagues found no significant difference in the rate of endometrial thickening greater than 5 mm between women taking ospemifene and those taking placebo after 1 year of daily oral treatment. No carcinomas were found in either group.
Option 2: Local estrogen
If our patient declines all systemic hormone therapy, the topical approach should resolve her vulvovaginal symptoms, and she could continue taking Brisdelle for her menopausal symptoms. Vaginal estrogen would address the skin problems, provided the patient applies it correctly. Many women are afraid to use estrogen creams and compensate by applying them only to the vulva, thinking that, by limiting their use to external tissues, they are avoiding any associated risks.
If she opts for the local approach, this patient should be encouraged to use transvaginal estrogen in small doses to increase the elasticity of the vulvovaginal tissue, even though it may require daily use for a week or two to improve her symptoms, after which once- or twice-weekly administration should suffice.
The use of low-dose vaginal cream for a short duration is unlikely to increase her risks in any way.
Local estrogen is available as a tablet, cream, or ring.
Option 3: A nonhormonal approach
If the patient refuses any hormonal agent—even topical estrogen—I would recommend the use of silicone-based lubricants and a dilator and prescribe more frequent penetration to increase elasticity and reduce pain.
Brisdelle could be continued to address her menopausal symptoms.
Don’t overlook behavioral techniques
Before this patient leaves your office with the option of her choice, a bit of counseling is necessary to instruct her about methods of restoring full sexual function.
Pain is a powerful aversive stimulus. This patient clearly states that she has had less frequent intercourse as a result of dyspareunia. It is not unusual for patients to develop a “habit” of avoidance in response to the behavior that causes their pain.
One recommendation is to talk to this patient about putting sex back into her life by encouraging her to increase sexual activity without penetration until she begins to arouse easily again. Arousal produces physiologic effects, increasing the caliber and length of the vagina as well as lubrication. The use of fingers or dilators may help restore caliber.
The patient can be encouraged to engage in snuggling and cuddling to regain those activities without the fear of pain associated with penetration. Follow-up after 2 weeks of this therapy can confirm the restoration of tissue elasticity, and the green light can be given for penetration to begin again. Couples can be encouraged to plan a “honeymoon weekend” and put some fun back into their sex lives so that this phase of healing doesn’t become an onerous task.
CASE RESOLVED After a discussion of her options, the patient chooses to stick with Brisdelle and use behavioral therapy alone to resolve her dyspareunia. At her follow-up visit 2 weeks later, she reports that she has enjoyed the period of pain-free “sex” and feels ready to add penetration into her activities.
You encourage her to continue sexual intercourse on a regular, relatively frequent basis to prevent a recurrence of dyspareunia. She continues to use silicone-based lubricants.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Since the last installment of this Update on Sexual Dysfunction, three new drugs have been added to the armamentarium for menopausal symptoms and dyspareunia:
- paroxetine 7.5 mg (Brisdelle)
- conjugated estrogens and bazedoxifene (Duavee)
- ospemifene (Osphena).
In this article, I present a case-based approach to incorporating these drugs into practice and restoring sexual function in the setting of vulvovaginal atrophy and dyspareunia. As is often the case, decision-making requires sifting through multiple layers of information.
How to “tease out” the problem and help the patient regain sexual function
Simon JA, Portman DJ, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Safety profile of paroxetine 7.5 mg in women with moderate-to-severe vasomotor symptoms. Obstet Gynecol. 2014;123(suppl 1):132S–133S.
Conjugated estrogens/bazedoxifene (Duavee) for menopausal symptoms and prevention of osteoporosis. Med Lett Drugs Ther. 2014;56(1441):33–34.
DeGregorio MW, Zerbe RL, Wurz GT. Ospemifene: a first-in-class, non-hormonal selective estrogen receptor modulator approved for the treatment of dyspareunia associated with vulvar and vaginal atrophy [published online ahead of print August 1, 2014]. Steroids. doi:10.1016/j.steroids.2014.07.012.
Goldstein SR, Archer DF, Simon JA, Constantine G. Endometrial safety of ospemifene and the ability of transvaginal ultrasonography to detect small changes in endometrial thickness. Obstet Gynecol. 2014;123(suppl 1):96S–97S.
CASE: LOW DESIRE AND DISCOMFORT DURING INTERCOURSE
Your 58-year-old patient, G2P2, mentions during her annual visit that she’s not that interested in sex anymore. Her children are grown, she’s been happily married for 28 years, and she enjoys her job and denies any symptoms of depression. She says her relationship with her husband is good and, aside from her low desire, she has no worries about the marriage. Her only medication is paroxetine 7.5 mg/day (Brisdelle) for management of her moderate hot flashes, which she initiated at her last annual visit. She reports improvement in her sleep and menopausal symptoms as a result. She has an intact uterus.
You perform a pelvic exam and find atrophic vulva and vagina with mild erythema, and thinned epithelium. When you ask if she has experienced any discomfort, she reports that she needs to use lubrication for intercourse and that, even with lubrication, she has pain upon penetration and a burning sensation that continues throughout intercourse. She also reports that it seems to take her much longer to achieve arousal than in the past, and she often fails to reach orgasm.
How would you manage this patient?
As always, begin with the history
The transition to menopause creates multiple layers of potential symptoms and problems for our patients, and sometimes medical therapy can generate additional ones.
In a patient reporting the onset of low desire and dyspareunia, you would want to first consider her medication history, despite the clear evidence of vaginal atrophy. Begin by asking whether she is taking any new medications prescribed by another provider. In some cases, antihypertensive drugs, psychotropic agents, and other medications can affect sexual function.
This patient has been taking Brisdelle for 1 year and is happy with its effect on her sleep and hot flashes. Simon and colleagues found this nonhormonal agent for moderate to severe vasomotor symptoms to produce no notable effects in weight, libido, or sleep, compared with placebo.
Nevertheless, in this case, because selective serotonin reuptake inhibitors (SSRIs) such as paroxetine can affect arousal and orgasm, it is unclear whether the ultra-low dose of paroxetine she is taking is contributing to her problems. If you were to discontinue the drug to find out, her vasomotor symptoms and sleep disruption would likely recur.
Your decision-making is important here and should involve the patient in an extensive discussion. If there is not enough time for this discussion at the current visit, schedule a follow-up to address her issues fully.
Vulvovaginal atrophy has its own timeline
In many cases, vasomotor symptoms such as hot flashes occur years before the skin begins to atrophy in the vulva and vagina, particularly in women who enter menopause naturally. Among menopausal women who continue to have intercourse on a regular basis, however, these skin changes often are much less troublesome than they are for women who have sex more rarely.
In this patient, one possible scenario is that paroxetine caused a slight reduction in sexual interest, and the frequency of intercourse went down as a result. In women who have little or no intercourse, the vagina begins to shrink and the tissues lose elasticity. This patient may have been undergoing the natural process of menopause, and that process may have been compounded by a decrease in the frequency of sex.
If you were to discontinue the paroxetine, it would still be necessary to treat the vulvovaginal skin and work on manual techniques to gently dilate the introitus.
Option 1: Systemic hormone therapy
Systemic estrogen is the most effective treatment for menopausal vasomotor symptoms, reducing hot flashes by 50% to 100% within 4 weeks of initiation. However, because our patient has an intact uterus, any systemic estrogen she opts to use must be opposed by a progestin for safety reasons.
In terms of estrogen, her options are oral or nonoral formulations. Not only would estrogen manage our patient’s hot flashes but, over time, it would improve her sexual problems and atrophy, which might or might not improve her current complaint of low desire. You likely would need to add a short regimen of topical estrogen and perhaps even a dilator to restore her sexual function completely, however.
Since our patient chose the nonhormonal agent Brisdelle to manage her menopausal symptoms, she may be worried about the increased risk of breast cancer associated with use of a progestin in combination with estrogen. One hormonal option now available that eliminates the need for a progestin is conjugated estrogens and bazedoxefine (Duavee). Bazedoxefine is a third-
generation selective estrogen receptor modulator (SERM). This drug has estrogen-like effects on bone and antiestrogen effects on the uterus.
Duavee is indicated for use in women with a uterus for treatment of:
- moderate to severe vasomotor symptoms of menopause
- prevention of postmenopausal osteoporosis.
Among the risks are an increased risk of venous thromboembolism (VTE) and stroke. It is not approved specifically for the treatment of dyspareunia.
Another hormonal option is ospemifene (Osphena), an estrogen agonist/antagonist indicated for the treatment of moderate to severe dyspareunia in menopausal women. Among the drugs in its class, such as tamoxifen and raloxifene, ospemifene is the only agent that maintains a full estrogenic effect on vaginal tissues. Its risks include VTE and stroke.
Although the labeling includes a warning about the risk of endometrial hyperplasia associated with its use, Goldstein and colleagues found no significant difference in the rate of endometrial thickening greater than 5 mm between women taking ospemifene and those taking placebo after 1 year of daily oral treatment. No carcinomas were found in either group.
Option 2: Local estrogen
If our patient declines all systemic hormone therapy, the topical approach should resolve her vulvovaginal symptoms, and she could continue taking Brisdelle for her menopausal symptoms. Vaginal estrogen would address the skin problems, provided the patient applies it correctly. Many women are afraid to use estrogen creams and compensate by applying them only to the vulva, thinking that, by limiting their use to external tissues, they are avoiding any associated risks.
If she opts for the local approach, this patient should be encouraged to use transvaginal estrogen in small doses to increase the elasticity of the vulvovaginal tissue, even though it may require daily use for a week or two to improve her symptoms, after which once- or twice-weekly administration should suffice.
The use of low-dose vaginal cream for a short duration is unlikely to increase her risks in any way.
Local estrogen is available as a tablet, cream, or ring.
Option 3: A nonhormonal approach
If the patient refuses any hormonal agent—even topical estrogen—I would recommend the use of silicone-based lubricants and a dilator and prescribe more frequent penetration to increase elasticity and reduce pain.
Brisdelle could be continued to address her menopausal symptoms.
Don’t overlook behavioral techniques
Before this patient leaves your office with the option of her choice, a bit of counseling is necessary to instruct her about methods of restoring full sexual function.
Pain is a powerful aversive stimulus. This patient clearly states that she has had less frequent intercourse as a result of dyspareunia. It is not unusual for patients to develop a “habit” of avoidance in response to the behavior that causes their pain.
One recommendation is to talk to this patient about putting sex back into her life by encouraging her to increase sexual activity without penetration until she begins to arouse easily again. Arousal produces physiologic effects, increasing the caliber and length of the vagina as well as lubrication. The use of fingers or dilators may help restore caliber.
The patient can be encouraged to engage in snuggling and cuddling to regain those activities without the fear of pain associated with penetration. Follow-up after 2 weeks of this therapy can confirm the restoration of tissue elasticity, and the green light can be given for penetration to begin again. Couples can be encouraged to plan a “honeymoon weekend” and put some fun back into their sex lives so that this phase of healing doesn’t become an onerous task.
CASE RESOLVED After a discussion of her options, the patient chooses to stick with Brisdelle and use behavioral therapy alone to resolve her dyspareunia. At her follow-up visit 2 weeks later, she reports that she has enjoyed the period of pain-free “sex” and feels ready to add penetration into her activities.
You encourage her to continue sexual intercourse on a regular, relatively frequent basis to prevent a recurrence of dyspareunia. She continues to use silicone-based lubricants.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Since the last installment of this Update on Sexual Dysfunction, three new drugs have been added to the armamentarium for menopausal symptoms and dyspareunia:
- paroxetine 7.5 mg (Brisdelle)
- conjugated estrogens and bazedoxifene (Duavee)
- ospemifene (Osphena).
In this article, I present a case-based approach to incorporating these drugs into practice and restoring sexual function in the setting of vulvovaginal atrophy and dyspareunia. As is often the case, decision-making requires sifting through multiple layers of information.
How to “tease out” the problem and help the patient regain sexual function
Simon JA, Portman DJ, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Safety profile of paroxetine 7.5 mg in women with moderate-to-severe vasomotor symptoms. Obstet Gynecol. 2014;123(suppl 1):132S–133S.
Conjugated estrogens/bazedoxifene (Duavee) for menopausal symptoms and prevention of osteoporosis. Med Lett Drugs Ther. 2014;56(1441):33–34.
DeGregorio MW, Zerbe RL, Wurz GT. Ospemifene: a first-in-class, non-hormonal selective estrogen receptor modulator approved for the treatment of dyspareunia associated with vulvar and vaginal atrophy [published online ahead of print August 1, 2014]. Steroids. doi:10.1016/j.steroids.2014.07.012.
Goldstein SR, Archer DF, Simon JA, Constantine G. Endometrial safety of ospemifene and the ability of transvaginal ultrasonography to detect small changes in endometrial thickness. Obstet Gynecol. 2014;123(suppl 1):96S–97S.
CASE: LOW DESIRE AND DISCOMFORT DURING INTERCOURSE
Your 58-year-old patient, G2P2, mentions during her annual visit that she’s not that interested in sex anymore. Her children are grown, she’s been happily married for 28 years, and she enjoys her job and denies any symptoms of depression. She says her relationship with her husband is good and, aside from her low desire, she has no worries about the marriage. Her only medication is paroxetine 7.5 mg/day (Brisdelle) for management of her moderate hot flashes, which she initiated at her last annual visit. She reports improvement in her sleep and menopausal symptoms as a result. She has an intact uterus.
You perform a pelvic exam and find atrophic vulva and vagina with mild erythema, and thinned epithelium. When you ask if she has experienced any discomfort, she reports that she needs to use lubrication for intercourse and that, even with lubrication, she has pain upon penetration and a burning sensation that continues throughout intercourse. She also reports that it seems to take her much longer to achieve arousal than in the past, and she often fails to reach orgasm.
How would you manage this patient?
As always, begin with the history
The transition to menopause creates multiple layers of potential symptoms and problems for our patients, and sometimes medical therapy can generate additional ones.
In a patient reporting the onset of low desire and dyspareunia, you would want to first consider her medication history, despite the clear evidence of vaginal atrophy. Begin by asking whether she is taking any new medications prescribed by another provider. In some cases, antihypertensive drugs, psychotropic agents, and other medications can affect sexual function.
This patient has been taking Brisdelle for 1 year and is happy with its effect on her sleep and hot flashes. Simon and colleagues found this nonhormonal agent for moderate to severe vasomotor symptoms to produce no notable effects in weight, libido, or sleep, compared with placebo.
Nevertheless, in this case, because selective serotonin reuptake inhibitors (SSRIs) such as paroxetine can affect arousal and orgasm, it is unclear whether the ultra-low dose of paroxetine she is taking is contributing to her problems. If you were to discontinue the drug to find out, her vasomotor symptoms and sleep disruption would likely recur.
Your decision-making is important here and should involve the patient in an extensive discussion. If there is not enough time for this discussion at the current visit, schedule a follow-up to address her issues fully.
Vulvovaginal atrophy has its own timeline
In many cases, vasomotor symptoms such as hot flashes occur years before the skin begins to atrophy in the vulva and vagina, particularly in women who enter menopause naturally. Among menopausal women who continue to have intercourse on a regular basis, however, these skin changes often are much less troublesome than they are for women who have sex more rarely.
In this patient, one possible scenario is that paroxetine caused a slight reduction in sexual interest, and the frequency of intercourse went down as a result. In women who have little or no intercourse, the vagina begins to shrink and the tissues lose elasticity. This patient may have been undergoing the natural process of menopause, and that process may have been compounded by a decrease in the frequency of sex.
If you were to discontinue the paroxetine, it would still be necessary to treat the vulvovaginal skin and work on manual techniques to gently dilate the introitus.
Option 1: Systemic hormone therapy
Systemic estrogen is the most effective treatment for menopausal vasomotor symptoms, reducing hot flashes by 50% to 100% within 4 weeks of initiation. However, because our patient has an intact uterus, any systemic estrogen she opts to use must be opposed by a progestin for safety reasons.
In terms of estrogen, her options are oral or nonoral formulations. Not only would estrogen manage our patient’s hot flashes but, over time, it would improve her sexual problems and atrophy, which might or might not improve her current complaint of low desire. You likely would need to add a short regimen of topical estrogen and perhaps even a dilator to restore her sexual function completely, however.
Since our patient chose the nonhormonal agent Brisdelle to manage her menopausal symptoms, she may be worried about the increased risk of breast cancer associated with use of a progestin in combination with estrogen. One hormonal option now available that eliminates the need for a progestin is conjugated estrogens and bazedoxefine (Duavee). Bazedoxefine is a third-
generation selective estrogen receptor modulator (SERM). This drug has estrogen-like effects on bone and antiestrogen effects on the uterus.
Duavee is indicated for use in women with a uterus for treatment of:
- moderate to severe vasomotor symptoms of menopause
- prevention of postmenopausal osteoporosis.
Among the risks are an increased risk of venous thromboembolism (VTE) and stroke. It is not approved specifically for the treatment of dyspareunia.
Another hormonal option is ospemifene (Osphena), an estrogen agonist/antagonist indicated for the treatment of moderate to severe dyspareunia in menopausal women. Among the drugs in its class, such as tamoxifen and raloxifene, ospemifene is the only agent that maintains a full estrogenic effect on vaginal tissues. Its risks include VTE and stroke.
Although the labeling includes a warning about the risk of endometrial hyperplasia associated with its use, Goldstein and colleagues found no significant difference in the rate of endometrial thickening greater than 5 mm between women taking ospemifene and those taking placebo after 1 year of daily oral treatment. No carcinomas were found in either group.
Option 2: Local estrogen
If our patient declines all systemic hormone therapy, the topical approach should resolve her vulvovaginal symptoms, and she could continue taking Brisdelle for her menopausal symptoms. Vaginal estrogen would address the skin problems, provided the patient applies it correctly. Many women are afraid to use estrogen creams and compensate by applying them only to the vulva, thinking that, by limiting their use to external tissues, they are avoiding any associated risks.
If she opts for the local approach, this patient should be encouraged to use transvaginal estrogen in small doses to increase the elasticity of the vulvovaginal tissue, even though it may require daily use for a week or two to improve her symptoms, after which once- or twice-weekly administration should suffice.
The use of low-dose vaginal cream for a short duration is unlikely to increase her risks in any way.
Local estrogen is available as a tablet, cream, or ring.
Option 3: A nonhormonal approach
If the patient refuses any hormonal agent—even topical estrogen—I would recommend the use of silicone-based lubricants and a dilator and prescribe more frequent penetration to increase elasticity and reduce pain.
Brisdelle could be continued to address her menopausal symptoms.
Don’t overlook behavioral techniques
Before this patient leaves your office with the option of her choice, a bit of counseling is necessary to instruct her about methods of restoring full sexual function.
Pain is a powerful aversive stimulus. This patient clearly states that she has had less frequent intercourse as a result of dyspareunia. It is not unusual for patients to develop a “habit” of avoidance in response to the behavior that causes their pain.
One recommendation is to talk to this patient about putting sex back into her life by encouraging her to increase sexual activity without penetration until she begins to arouse easily again. Arousal produces physiologic effects, increasing the caliber and length of the vagina as well as lubrication. The use of fingers or dilators may help restore caliber.
The patient can be encouraged to engage in snuggling and cuddling to regain those activities without the fear of pain associated with penetration. Follow-up after 2 weeks of this therapy can confirm the restoration of tissue elasticity, and the green light can be given for penetration to begin again. Couples can be encouraged to plan a “honeymoon weekend” and put some fun back into their sex lives so that this phase of healing doesn’t become an onerous task.
CASE RESOLVED After a discussion of her options, the patient chooses to stick with Brisdelle and use behavioral therapy alone to resolve her dyspareunia. At her follow-up visit 2 weeks later, she reports that she has enjoyed the period of pain-free “sex” and feels ready to add penetration into her activities.
You encourage her to continue sexual intercourse on a regular, relatively frequent basis to prevent a recurrence of dyspareunia. She continues to use silicone-based lubricants.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
As ICD-10 conversion nears, keep these factors in mind to ensure proper reimbursements in 2014
"I am working hard at my job to ensure high-volume gynecologic surgeons receive payment for services rendered," says Dr. Levy. Speaking at the PEP Practice Management Program at the 2013 Pelvic Anatomy and Gynecologic Surgery Symposium, held December 12-14, in Las Vegas, Dr. Levy offered attendees ways to ensure proper reimbursement for their services rendered.
In this video, Dr. Levy extends key points to viewers.
Related article: As the Affordable Care Act comes of age, a look behind the headlines (Lucia DiVenere, MA; January 2014)
"I am working hard at my job to ensure high-volume gynecologic surgeons receive payment for services rendered," says Dr. Levy. Speaking at the PEP Practice Management Program at the 2013 Pelvic Anatomy and Gynecologic Surgery Symposium, held December 12-14, in Las Vegas, Dr. Levy offered attendees ways to ensure proper reimbursement for their services rendered.
In this video, Dr. Levy extends key points to viewers.
Related article: As the Affordable Care Act comes of age, a look behind the headlines (Lucia DiVenere, MA; January 2014)
"I am working hard at my job to ensure high-volume gynecologic surgeons receive payment for services rendered," says Dr. Levy. Speaking at the PEP Practice Management Program at the 2013 Pelvic Anatomy and Gynecologic Surgery Symposium, held December 12-14, in Las Vegas, Dr. Levy offered attendees ways to ensure proper reimbursement for their services rendered.
In this video, Dr. Levy extends key points to viewers.
Related article: As the Affordable Care Act comes of age, a look behind the headlines (Lucia DiVenere, MA; January 2014)
UPDATE ON TECHNOLOGY
The development of new medical technology continues at a brisk pace—and ObGyns and our patients often are the beneficiaries. Notable technological breakthroughs of the past include hormonal contraception, in vitro fertilization, the application of minimally invasive surgical devices and techniques to gynecologic procedures, and many other innovations.
The leaders in our specialty are innovators themselves, ever vigilant for developments that can help improve health and quality of life for our patients. Regrettably, however, many technologies spread widely before they are fully validated by published studies—or continue to be used long after a superior or less invasive intervention has come along. And many claims about new technology are based on marketing information rather than reliable data.
Another common occurrence in regard to published data: Findings in one well-defined sector of the population are extrapolated to all patients. Even clinicians who are careful about adopting new technology can overlook the fact that it was tested, and proven, in a subset of patients that may not be comparable to all their patients.
In this article, I offer two case studies that illustrate some of the challenges we face when it comes to applying scientific findings to our practice and assessing medical technologies. In both settings, the health of the patient should be our primary focus.
When it comes to technology, patient selection is key
CASE 1: Anovulatory patient requests endometrial ablation
A 34-year-old woman (G2P2) who is moderately obese (body mass index of 32 kg/m2) visits your office to request endometrial ablation to manage her irregular and heavy menses. She reached menarche at age 10, and her periods have been somewhat irregular ever since. She required ovarian stimulation with clomiphene citrate to achieve each of her pregnancies, and both children were delivered by cesarean section. She currently uses condoms for contraception but does not have a steady partner, and she desires no additional pregnancies.
The patient reports that her menses occur every 2 to 6 weeks, with flow lasting from 2 to 8 days. She is housebound for 2 days each cycle because of heavy flow and cramping. She takes no medications and has no other medical conditions.
On exam, she is centrally obese with a tender uterus that is 10- to 12-weeks’ size; no adnexal masses are palpable. All other findings are normal. A recent test for diabetes was negative, but her serum cholesterol and triglyceride levels are borderline elevated.
What intervention would you recommend to address the heavy bleeding?
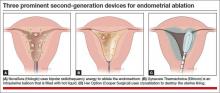
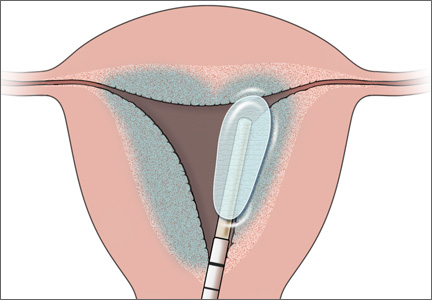
With the advent of second-generation endometrial ablation devices in the late 1990s, women with refractory menorrhagia had a safe, reliable, minimally invasive alternative to hysterectomy that made it possible to treat the endometrial cavity without the technical challenges of resectoscopic surgery. More than 10 million women report menorrhagia each year in the United States—so it is no small problem.1,2 Global endometrial ablation devices make management possible in an office setting, and recovery is significantly shorter than with hysterectomy. The three most prominent nonhysteroscopic ablation devices are (FIGURE):
• NovaSure (Hologic) uses bipolar radiofrequency energy to ablate tissue. Its probe contains stretchable gold-plated fabric that conforms to the endometrial surface.
• Gynecare Thermachoice (Ethicon) consists of an intrauterine balloon that is filled with hot liquid (temperatures of roughly 87°C) to ablate the endometrium
• Her Option (CooperSurgical) is a cryoablation system that consists of an intrauterine probe that forms an ice ball (temperatures of roughly –90°C) that destroys the uterine lining. Several applications are required to treat the majority of the cavity.
Use of these devices is widespread, although a nonsurgical alternative—the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena)—was approved by the US Food and Drug Administration (FDA) in 2009 for the treatment of heavy menstrual bleeding.
Not only is the efficacy of the LNG-IUS for controlling menorrhagia equal to endometrial ablation, but it provides the additional benefits of reliable contraception and management of dysmenorrhea.
6 questions to ask before applying a technology
1. In which population was it studied?
One of the most important issues to consider when you are evaluating technology is the population in which it was studied. In other words, to whom do the findings apply?
Most endometrial ablation technologies were tested in women who had a uterus of normal size (mean, 8 cm) with no structural abnormalities (ie, no polyps or fibroids) and regular but very heavy periods. The findings from these studies now have been extrapolated in many cases to women who have hormonally induced abnormal bleeding, whose periods are irregular. That extrapolation may not be appropriate.
2. What is the diagnosis?
As the manufacturers of global endometrial ablation devices note, heavy menstrual bleeding may arise from an underlying condition, such as endometrial cancer, hormonal imbalance, fibroids, coagulopathy, and so on—and it is important to rule these conditions out before considering endometrial ablation.
For example, in Case 1, the patient is experiencing anovulatory cycles, as evidenced by her irregular menses and the need for ovulation induction to achieve her pregnancies. The success rate of endometrial ablation in the setting of chronic anovulation is unknown. Pivotal trials for all of the nonhysteroscopic endometrial ablation technologies required regular menses or failure of cyclic hormonal therapy prior to enrollment.
In addition, in Case 1, the patient has signs (an enlarged, tender uterus) that suggest the presence of adenomyosis. Endometrial ablation is not recommended as a treatment for women with this condition.
3. Will later surgery be required?
In one study from Kaiser Permanente of Northern California, 21% of women who underwent endometrial ablation for menorrhagia later underwent hysterectomy, and 3.9% underwent other uterine-sparing procedures to alleviate heavy bleeding.3
In that study, women younger than age 45 were 2.1 times more likely to require hysterectomy after endometrial ablation, compared with older women (95% confidence interval [CI], 1.8–2.4). The likelihood of hysterectomy increased with each decreasing stratum of age and exceeded 40% in women aged 40 years or younger.3
In a population-based retrospective cohort study from Scotland, 2,779 (19.7%) of 11,299 women who underwent endometrial ablation for heavy menstrual bleeding required hysterectomy later.4 Again, women who required hysterectomy after endometrial ablation tended to be younger than those who did not. Overall, 26.6% of women undergoing endometrial ablation for heavy menstrual bleeding required further surgery.4
And in a 5-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation, roughly 30% of women required subsequent surgery for heavy menstrual bleeding.5
The need for subsequent surgery following endometrial ablation is likely to be higher among women who do not match the profile of participants in pivotal trials of the devices. All patients considering endometrial ablation should be counseled about the possible need for further surgery.
Related Article The economics of surgical gynecology: How we can not only survive, but thrive, in the 21st Century Barbara S. Levy, MD
4. What is the patient's overall health?
Before selecting an intervention for heavy menstrual bleeding, it is important to consider the patient’s overall health. What comorbidities does she have? Is pain a component of her condition? If so, might she have endometriosis or adenomyosis, as our patient does? If pain is a significant component of her menorrhagia, is it cyclical—that is, does it correspond to the days of heaviest flow? Pain related to the passage of large clots during menses may respond well to endometrial ablation. Pelvic pain occurring before and after the flow probably won’t.
Also keep in mind that the patient in Case 1 has two risk factors for endometrial hyperplasia: anovulatory cycles and obesity. If she undergoes endometrial ablation and subsequently experiences abnormal uterine bleeding, the most prominent sign of endometrial hyperplasia, how will you assess her endometrium 5 years after ablation if she develops abnormal bleeding? Will you be able to adequately sample it?
5. Have less invasive options been tried?
Many of the women studied in pivotal trials of second-generation endometrial ablation devices failed medical therapy prior to undergoing ablation. Because medical therapy is less expensive and noninvasive, it makes sense to offer it before proceeding to surgery. Common pharmacologic approaches include nonsteroidal anti-inflammatory drugs (NSAIDs), oral contraceptives, and tranexamic acid.
In addition, as I mentioned earlier, Mirena is approved for treatment of heavy menstrual bleeding.
Medical therapy and Mirena should be offered prior to surgery because of their noninvasive, reversible nature. Most physicians now consider Mirena to be the first-line option for heavy menstrual bleeding in women who have normal anatomy.6
6. What is the cost of treatment?
With health-care expenses accelerating, we need to be mindful of the cost-effectiveness of the options we recommend.
Let’s assess the expense of managing the patient in Case 1. To address her desire for long-term contraception, we might offer tubal sterilization by laparoscopy followed by endometrial ablation (to address the heavy bleeding), which would require a general anesthetic and management in an ambulatory surgical facility.
If she opts for hysteroscopic sterilization prior to endometrial ablation, the sterilization procedure must be performed at least 3 months prior to ablation (according to FDA labeling) so that tubal occlusion can be demonstrated by hysterosalpingography (HSG) before the uterine cavity is scarred. This means that the patient would require two separate interventions. Although both procedures could be performed in an office setting, the patient would still require time away from work and family.
The cost for sterilization plus ablation in the office without anesthesia would be approximately $4,500 (including HSG) at Medicare rates. For a combined laparoscopic sterilization and endometrial ablation, costs would be higher because of the need for anesthesia and an ambulatory surgical facility. Another option that would address both the heavy bleeding and the need for contraception: oral contraceptives (OCs). The overall cost of this approach over 5 years would be $4,200 (60 months of OCs at $70/month), but it would be fully covered under the Affordable Care Act (ACA), so the patient would have no out-of-pocket expense. The cost of Mirena would be $1,000 ($850 for the device plus $150 for insertion), but it also would be fully covered under the ACA.
CASE 1: Resolved
You discuss the option of cyclic OCs with the patient. This approach would help control her irregular, heavy, and painful menses while providing good contraceptive coverage.
Although sterilization plus endometrial ablation is an option, you counsel the patient that the published “success” rates do not apply to her. Given her young age, obesity, and anovulatory status, endometrial ablation has a greater likelihood of failure. Further, when abnormal bleeding recurs, it may be difficult to assess this high-risk patient’s endometrium.
You also discuss Mirena, which would provide long-term contraception and also manage her menorrhagia and dysmenorrhea; indeed, it is FDA-approved for this indication. In fact, Mirena would address all of this patient’s concerns, offering superb contraception and reliable reductions in bleeding and pelvic pain in the setting of endometriosis and adenomyosis.
Overall, this woman is best served by a highly reliable approach that manages her contraceptive needs and her menorrhagia and dysmenorrhea while reducing her risk for endometrial hyperplasia and cancer. Given the long-term endometrial-sampling problems ablation would create, it is not an optimal solution for this woman.
What this evidence means for practice
When assessing technology, consider which patients it was tested in, your patient’s diagnosis and long-term health risks, the cost and success rate of the technology, and the availability of less invasive options.
How to assess new data on a technology or procedure
Gill SE, Mills BB. Physician opinions regarding elective bilateral salpingectomy with hysterectomy and for sterilization. JMIG. 2013;20(4):517–521.
CASE 2: A healthy patient seeks permanent contraception
At her annual well-woman visit, your 42-year-old patient (G3P3) asks to discuss permanent contraception now that she has completed her family. She has used OCs for more than 10 years without problems. She is a nonsmoker of normal weight (BMI of 22 kg/m2), with normal blood pressure and vital signs. Her family history is remarkable for the health of her parents and siblings: Her mother is 68 years old and well; her father, 70, also is healthy. There is no family history of breast or ovarian cancer, prostate cancer, or significant medical illness.
What options would you offer this patient? Given recent data suggesting that ovarian cancer may originate in the fallopian tubes, would you recommend prophylactic salpingectomy as her contraceptive method of choice?
This patient has many options. In describing them to her, it would be important to focus on the breadth of safe, reversible, long-acting contraceptives now available, including their respective risks, benefits, and long-term outcomes and costs. It also is important to consider the published data on these methods, weighing their relevance to her overall health and medical history. Although some data regarding the origin of ovarian cancer in the fallopian tubes may be especially compelling, it may not be wise to extrapolate it from a specific group of women to all patients, as we shall see.
Option 1: Cyclic OCs
This option should not be excluded, despite the need for daily dosing, because the patient has used it successfully for more than 10 years. OCs are highly effective and have the added benefits of reducing menstrual blood loss, alleviating cramps, and lowering the risks of endometrial and ovarian cancer.7
Option 2: LARC
Long-acting reversible contraceptive (LARC) methods include the LNG-IUS, the copper intrauterine device (IUD; ParaGard, Teva), and the etonogestrel implant (Nexplanon, Merck). Each of these contraceptives is similar to sterilization in terms of efficacy. None requires the interruption of sexual activity or weekly or monthly trips to the pharmacy.
The LNG-IUS and the etonogestrel implant have the added benefits of reducing menstrual blood loss and reducing or eliminating premenstrual symptoms and cramps in most women. In addition, they may reduce the risk of unopposed estrogen stimulation of the endometrium associated with perimenopausal anovulatory cycles.
Option 3: Transcervical tubal sterilization
This hysteroscopic method (Essure, Bayer) avoids the risks of general anesthesia, but successful bilateral placement may not be possible in a small percentage of women. It also requires a 3-month interval between placement and confirmation of tubal occlusion (via HSG). During this interval, alternative contraception must be used. Once occlusion is confirmed, this method is highly reliable.
Option 4: Laparoscopic sterilization
This approach was the method of choice prior to the introduction of Essure in 2002. There now is a resurgence of interest in laparoscopic sterilization due to recent publications describing the distal portion of the fallopian tube as the “true source” of high-grade serous ovarian cancers.
In pathologic analyses of fallopian tubes removed prophylactically from women with a BRCA 1 or 2 mutation, investigators found a significant rate of serous tubal intraepithelial carcinoma.8 Researchers began to study the genetics of these cancers and concluded that, in women with a BRCA mutation, high-grade serous carcinomas may arise from the distal fallopian tube.
Until recently, all of the literature on these tubal carcinomas related only to women with a specific tumor suppressor gene p53 mutation in the BRCA system. Other investigators then reviewed pathology specimens from women without a BRCA mutation who had high-grade serous carcinomas that were peritoneal, tubal, or ovarian in origin. They found serous tubal intraepithelial carcinomas in 33% to 59% of these women.9
“Research suggests that bilateral salpingectomy during hysterectomy for benign indications or as a sterilization procedure may have benefits, such as preventing tubal disease and cancer, without significant risks,” wrote Gill and Mills.10 In conducting a survey of US physicians to determine how widespread prophylactic salpingectomy is during benign hysterectomy or sterilization, they found that 54% of respondents performed bilateral salpingectomy during hysterectomy, usually to lower the risk of cancer (75%) and avoid the need for reoperation (49.1%). Of the 45.5% of respondents who did not perform bilateral salpingectomy during hysterectomy, most (69.4%) believed it has no benefit.10
Although 58% of respondents believed bilateral salpingectomy to be the most effective option for sterilization in women older than 35 years, they reported that they reserve it for women in whom one sterilization procedure has failed or for women who have tubal disease.10
As for the 45.5% of respondents who did not perform prophylactic salpingectomy, they offered as reasons their concern about increased operative time and the risk of complications, as well as a belief that it has no benefit.10
The lifetime risk of ovarian cancer in the general population is 1 in 70 women. Although it is possible that high-grade serous ovarian cancers originate in the distal fallopian tube, as the research to date suggests, it also is possible that we might find in-situ lesions in tissues other than the distal tube, suggesting a more global genetic defect underlying ovarian cancers in the peritoneal and müllerian tissues. Randomized trials are under way in an effort to determine whether excision of the fallopian tubes will prevent the majority of high-grade serous cancers. It will be many years, however, before the results of these trials are available.
CASE 2: Resolved
This patient has used combination OCs for more than 10 years, so her lifetime risk of ovarian cancer has been reduced by approximately 50%. Because her family history indicates that a BRCA mutation is highly unlikely, her lifetime risk of ovarian cancer now has declined from 1:70 to roughly 1:140.
Prophylactic salpingectomy might offer a very small reduction in this patient’s absolute risk of ovarian cancer—from 0.75% to 0.50% lifetime risk—but the current data are not robust enough to suggest that it should be recommended for her. The operation would carry the risks associated with general anesthesia and peritoneal access. Although these risks are small, there is a documented risk of death from laparoscopic sterilization procedures in the United States, from complications related to bowel injury, anesthesia, and hemorrhage.
For these reasons, I would counsel this patient that her best options for contraception are combination OCs, transcervical tubal sterilization, or a long-acting reversible contraceptive such as the IUD or implant.
Tell us what you think, at [email protected]. Please include your name and city and state.
1. Centers for Disease Control and Prevention. Blood disor-ders in women: heavy menstrual bleeding. http://www.cdc.gov/ncbddd/blooddisorders/women/menorrhagia.html. Updated December 12, 2011. Accessed August 15, 2013.
2. Centers for Disease Control and Prevention. Blood disorders in women: research. http://www.cdc.gov/ncbddd/blooddis orders/women/research.html. Updated December 12, 2011. Accessed August 15, 2013.
3. Longinotti K, Jacobson GF, Hung YY, Learman LA. Probability of hysterectomy after endometrial ablation. Obstet Gynecol. 2008;112(6):1214–1220.
4. Cooper K, Lee AJ, Chien P, Raja EA, Timmaraju V, Bhattacharya S. Outcomes following hysterectomy or endometrial ablation for heavy menstrual bleeding: retrospective analysis of hospital episode statistics in Scotland. BJOG. 2011;118(10):1171–1179.
5. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9(4):429–435.
6. Sayre C. Beyond hysterectomy: Alternative options for heavy bleeding. NY Times. http://www.nytimes.com/ref/health/healthguide/esn-menorrhagia-ess.html. Published October 10, 2008. Accessed August 15, 2013.
7. Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer. A systematic review and meta-analysis. Obstet Gynecol. 2013;122(1):139–147.
8. Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007; 31:161–169.
9. Crum CP, McKeon FD, Xian W. The oviduct and ovarian cancer: causality, clinical implications, and “targeted prevention.” Clin Obstet Gynecol. 2012;55(1):24–35.
10. Gill SE, Mills BB. Physician opinions regarding elective bilateral salpingectomy with hysterectomy and for sterilization. JMIG. 2013;20(4):517–521.
The development of new medical technology continues at a brisk pace—and ObGyns and our patients often are the beneficiaries. Notable technological breakthroughs of the past include hormonal contraception, in vitro fertilization, the application of minimally invasive surgical devices and techniques to gynecologic procedures, and many other innovations.
The leaders in our specialty are innovators themselves, ever vigilant for developments that can help improve health and quality of life for our patients. Regrettably, however, many technologies spread widely before they are fully validated by published studies—or continue to be used long after a superior or less invasive intervention has come along. And many claims about new technology are based on marketing information rather than reliable data.
Another common occurrence in regard to published data: Findings in one well-defined sector of the population are extrapolated to all patients. Even clinicians who are careful about adopting new technology can overlook the fact that it was tested, and proven, in a subset of patients that may not be comparable to all their patients.
In this article, I offer two case studies that illustrate some of the challenges we face when it comes to applying scientific findings to our practice and assessing medical technologies. In both settings, the health of the patient should be our primary focus.
When it comes to technology, patient selection is key
CASE 1: Anovulatory patient requests endometrial ablation
A 34-year-old woman (G2P2) who is moderately obese (body mass index of 32 kg/m2) visits your office to request endometrial ablation to manage her irregular and heavy menses. She reached menarche at age 10, and her periods have been somewhat irregular ever since. She required ovarian stimulation with clomiphene citrate to achieve each of her pregnancies, and both children were delivered by cesarean section. She currently uses condoms for contraception but does not have a steady partner, and she desires no additional pregnancies.
The patient reports that her menses occur every 2 to 6 weeks, with flow lasting from 2 to 8 days. She is housebound for 2 days each cycle because of heavy flow and cramping. She takes no medications and has no other medical conditions.
On exam, she is centrally obese with a tender uterus that is 10- to 12-weeks’ size; no adnexal masses are palpable. All other findings are normal. A recent test for diabetes was negative, but her serum cholesterol and triglyceride levels are borderline elevated.
What intervention would you recommend to address the heavy bleeding?
With the advent of second-generation endometrial ablation devices in the late 1990s, women with refractory menorrhagia had a safe, reliable, minimally invasive alternative to hysterectomy that made it possible to treat the endometrial cavity without the technical challenges of resectoscopic surgery. More than 10 million women report menorrhagia each year in the United States—so it is no small problem.1,2 Global endometrial ablation devices make management possible in an office setting, and recovery is significantly shorter than with hysterectomy. The three most prominent nonhysteroscopic ablation devices are (FIGURE):
• NovaSure (Hologic) uses bipolar radiofrequency energy to ablate tissue. Its probe contains stretchable gold-plated fabric that conforms to the endometrial surface.
• Gynecare Thermachoice (Ethicon) consists of an intrauterine balloon that is filled with hot liquid (temperatures of roughly 87°C) to ablate the endometrium
• Her Option (CooperSurgical) is a cryoablation system that consists of an intrauterine probe that forms an ice ball (temperatures of roughly –90°C) that destroys the uterine lining. Several applications are required to treat the majority of the cavity.
Use of these devices is widespread, although a nonsurgical alternative—the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena)—was approved by the US Food and Drug Administration (FDA) in 2009 for the treatment of heavy menstrual bleeding.
Not only is the efficacy of the LNG-IUS for controlling menorrhagia equal to endometrial ablation, but it provides the additional benefits of reliable contraception and management of dysmenorrhea.
6 questions to ask before applying a technology
1. In which population was it studied?
One of the most important issues to consider when you are evaluating technology is the population in which it was studied. In other words, to whom do the findings apply?
Most endometrial ablation technologies were tested in women who had a uterus of normal size (mean, 8 cm) with no structural abnormalities (ie, no polyps or fibroids) and regular but very heavy periods. The findings from these studies now have been extrapolated in many cases to women who have hormonally induced abnormal bleeding, whose periods are irregular. That extrapolation may not be appropriate.
2. What is the diagnosis?
As the manufacturers of global endometrial ablation devices note, heavy menstrual bleeding may arise from an underlying condition, such as endometrial cancer, hormonal imbalance, fibroids, coagulopathy, and so on—and it is important to rule these conditions out before considering endometrial ablation.
For example, in Case 1, the patient is experiencing anovulatory cycles, as evidenced by her irregular menses and the need for ovulation induction to achieve her pregnancies. The success rate of endometrial ablation in the setting of chronic anovulation is unknown. Pivotal trials for all of the nonhysteroscopic endometrial ablation technologies required regular menses or failure of cyclic hormonal therapy prior to enrollment.
In addition, in Case 1, the patient has signs (an enlarged, tender uterus) that suggest the presence of adenomyosis. Endometrial ablation is not recommended as a treatment for women with this condition.
3. Will later surgery be required?
In one study from Kaiser Permanente of Northern California, 21% of women who underwent endometrial ablation for menorrhagia later underwent hysterectomy, and 3.9% underwent other uterine-sparing procedures to alleviate heavy bleeding.3
In that study, women younger than age 45 were 2.1 times more likely to require hysterectomy after endometrial ablation, compared with older women (95% confidence interval [CI], 1.8–2.4). The likelihood of hysterectomy increased with each decreasing stratum of age and exceeded 40% in women aged 40 years or younger.3
In a population-based retrospective cohort study from Scotland, 2,779 (19.7%) of 11,299 women who underwent endometrial ablation for heavy menstrual bleeding required hysterectomy later.4 Again, women who required hysterectomy after endometrial ablation tended to be younger than those who did not. Overall, 26.6% of women undergoing endometrial ablation for heavy menstrual bleeding required further surgery.4
And in a 5-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation, roughly 30% of women required subsequent surgery for heavy menstrual bleeding.5
The need for subsequent surgery following endometrial ablation is likely to be higher among women who do not match the profile of participants in pivotal trials of the devices. All patients considering endometrial ablation should be counseled about the possible need for further surgery.
Related Article The economics of surgical gynecology: How we can not only survive, but thrive, in the 21st Century Barbara S. Levy, MD
4. What is the patient's overall health?
Before selecting an intervention for heavy menstrual bleeding, it is important to consider the patient’s overall health. What comorbidities does she have? Is pain a component of her condition? If so, might she have endometriosis or adenomyosis, as our patient does? If pain is a significant component of her menorrhagia, is it cyclical—that is, does it correspond to the days of heaviest flow? Pain related to the passage of large clots during menses may respond well to endometrial ablation. Pelvic pain occurring before and after the flow probably won’t.
Also keep in mind that the patient in Case 1 has two risk factors for endometrial hyperplasia: anovulatory cycles and obesity. If she undergoes endometrial ablation and subsequently experiences abnormal uterine bleeding, the most prominent sign of endometrial hyperplasia, how will you assess her endometrium 5 years after ablation if she develops abnormal bleeding? Will you be able to adequately sample it?
5. Have less invasive options been tried?
Many of the women studied in pivotal trials of second-generation endometrial ablation devices failed medical therapy prior to undergoing ablation. Because medical therapy is less expensive and noninvasive, it makes sense to offer it before proceeding to surgery. Common pharmacologic approaches include nonsteroidal anti-inflammatory drugs (NSAIDs), oral contraceptives, and tranexamic acid.
In addition, as I mentioned earlier, Mirena is approved for treatment of heavy menstrual bleeding.
Medical therapy and Mirena should be offered prior to surgery because of their noninvasive, reversible nature. Most physicians now consider Mirena to be the first-line option for heavy menstrual bleeding in women who have normal anatomy.6
6. What is the cost of treatment?
With health-care expenses accelerating, we need to be mindful of the cost-effectiveness of the options we recommend.
Let’s assess the expense of managing the patient in Case 1. To address her desire for long-term contraception, we might offer tubal sterilization by laparoscopy followed by endometrial ablation (to address the heavy bleeding), which would require a general anesthetic and management in an ambulatory surgical facility.
If she opts for hysteroscopic sterilization prior to endometrial ablation, the sterilization procedure must be performed at least 3 months prior to ablation (according to FDA labeling) so that tubal occlusion can be demonstrated by hysterosalpingography (HSG) before the uterine cavity is scarred. This means that the patient would require two separate interventions. Although both procedures could be performed in an office setting, the patient would still require time away from work and family.
The cost for sterilization plus ablation in the office without anesthesia would be approximately $4,500 (including HSG) at Medicare rates. For a combined laparoscopic sterilization and endometrial ablation, costs would be higher because of the need for anesthesia and an ambulatory surgical facility. Another option that would address both the heavy bleeding and the need for contraception: oral contraceptives (OCs). The overall cost of this approach over 5 years would be $4,200 (60 months of OCs at $70/month), but it would be fully covered under the Affordable Care Act (ACA), so the patient would have no out-of-pocket expense. The cost of Mirena would be $1,000 ($850 for the device plus $150 for insertion), but it also would be fully covered under the ACA.
CASE 1: Resolved
You discuss the option of cyclic OCs with the patient. This approach would help control her irregular, heavy, and painful menses while providing good contraceptive coverage.
Although sterilization plus endometrial ablation is an option, you counsel the patient that the published “success” rates do not apply to her. Given her young age, obesity, and anovulatory status, endometrial ablation has a greater likelihood of failure. Further, when abnormal bleeding recurs, it may be difficult to assess this high-risk patient’s endometrium.
You also discuss Mirena, which would provide long-term contraception and also manage her menorrhagia and dysmenorrhea; indeed, it is FDA-approved for this indication. In fact, Mirena would address all of this patient’s concerns, offering superb contraception and reliable reductions in bleeding and pelvic pain in the setting of endometriosis and adenomyosis.
Overall, this woman is best served by a highly reliable approach that manages her contraceptive needs and her menorrhagia and dysmenorrhea while reducing her risk for endometrial hyperplasia and cancer. Given the long-term endometrial-sampling problems ablation would create, it is not an optimal solution for this woman.
What this evidence means for practice
When assessing technology, consider which patients it was tested in, your patient’s diagnosis and long-term health risks, the cost and success rate of the technology, and the availability of less invasive options.
How to assess new data on a technology or procedure
Gill SE, Mills BB. Physician opinions regarding elective bilateral salpingectomy with hysterectomy and for sterilization. JMIG. 2013;20(4):517–521.
CASE 2: A healthy patient seeks permanent contraception
At her annual well-woman visit, your 42-year-old patient (G3P3) asks to discuss permanent contraception now that she has completed her family. She has used OCs for more than 10 years without problems. She is a nonsmoker of normal weight (BMI of 22 kg/m2), with normal blood pressure and vital signs. Her family history is remarkable for the health of her parents and siblings: Her mother is 68 years old and well; her father, 70, also is healthy. There is no family history of breast or ovarian cancer, prostate cancer, or significant medical illness.
What options would you offer this patient? Given recent data suggesting that ovarian cancer may originate in the fallopian tubes, would you recommend prophylactic salpingectomy as her contraceptive method of choice?
This patient has many options. In describing them to her, it would be important to focus on the breadth of safe, reversible, long-acting contraceptives now available, including their respective risks, benefits, and long-term outcomes and costs. It also is important to consider the published data on these methods, weighing their relevance to her overall health and medical history. Although some data regarding the origin of ovarian cancer in the fallopian tubes may be especially compelling, it may not be wise to extrapolate it from a specific group of women to all patients, as we shall see.
Option 1: Cyclic OCs
This option should not be excluded, despite the need for daily dosing, because the patient has used it successfully for more than 10 years. OCs are highly effective and have the added benefits of reducing menstrual blood loss, alleviating cramps, and lowering the risks of endometrial and ovarian cancer.7
Option 2: LARC
Long-acting reversible contraceptive (LARC) methods include the LNG-IUS, the copper intrauterine device (IUD; ParaGard, Teva), and the etonogestrel implant (Nexplanon, Merck). Each of these contraceptives is similar to sterilization in terms of efficacy. None requires the interruption of sexual activity or weekly or monthly trips to the pharmacy.
The LNG-IUS and the etonogestrel implant have the added benefits of reducing menstrual blood loss and reducing or eliminating premenstrual symptoms and cramps in most women. In addition, they may reduce the risk of unopposed estrogen stimulation of the endometrium associated with perimenopausal anovulatory cycles.
Option 3: Transcervical tubal sterilization
This hysteroscopic method (Essure, Bayer) avoids the risks of general anesthesia, but successful bilateral placement may not be possible in a small percentage of women. It also requires a 3-month interval between placement and confirmation of tubal occlusion (via HSG). During this interval, alternative contraception must be used. Once occlusion is confirmed, this method is highly reliable.
Option 4: Laparoscopic sterilization
This approach was the method of choice prior to the introduction of Essure in 2002. There now is a resurgence of interest in laparoscopic sterilization due to recent publications describing the distal portion of the fallopian tube as the “true source” of high-grade serous ovarian cancers.
In pathologic analyses of fallopian tubes removed prophylactically from women with a BRCA 1 or 2 mutation, investigators found a significant rate of serous tubal intraepithelial carcinoma.8 Researchers began to study the genetics of these cancers and concluded that, in women with a BRCA mutation, high-grade serous carcinomas may arise from the distal fallopian tube.
Until recently, all of the literature on these tubal carcinomas related only to women with a specific tumor suppressor gene p53 mutation in the BRCA system. Other investigators then reviewed pathology specimens from women without a BRCA mutation who had high-grade serous carcinomas that were peritoneal, tubal, or ovarian in origin. They found serous tubal intraepithelial carcinomas in 33% to 59% of these women.9
“Research suggests that bilateral salpingectomy during hysterectomy for benign indications or as a sterilization procedure may have benefits, such as preventing tubal disease and cancer, without significant risks,” wrote Gill and Mills.10 In conducting a survey of US physicians to determine how widespread prophylactic salpingectomy is during benign hysterectomy or sterilization, they found that 54% of respondents performed bilateral salpingectomy during hysterectomy, usually to lower the risk of cancer (75%) and avoid the need for reoperation (49.1%). Of the 45.5% of respondents who did not perform bilateral salpingectomy during hysterectomy, most (69.4%) believed it has no benefit.10
Although 58% of respondents believed bilateral salpingectomy to be the most effective option for sterilization in women older than 35 years, they reported that they reserve it for women in whom one sterilization procedure has failed or for women who have tubal disease.10
As for the 45.5% of respondents who did not perform prophylactic salpingectomy, they offered as reasons their concern about increased operative time and the risk of complications, as well as a belief that it has no benefit.10
The lifetime risk of ovarian cancer in the general population is 1 in 70 women. Although it is possible that high-grade serous ovarian cancers originate in the distal fallopian tube, as the research to date suggests, it also is possible that we might find in-situ lesions in tissues other than the distal tube, suggesting a more global genetic defect underlying ovarian cancers in the peritoneal and müllerian tissues. Randomized trials are under way in an effort to determine whether excision of the fallopian tubes will prevent the majority of high-grade serous cancers. It will be many years, however, before the results of these trials are available.
CASE 2: Resolved
This patient has used combination OCs for more than 10 years, so her lifetime risk of ovarian cancer has been reduced by approximately 50%. Because her family history indicates that a BRCA mutation is highly unlikely, her lifetime risk of ovarian cancer now has declined from 1:70 to roughly 1:140.
Prophylactic salpingectomy might offer a very small reduction in this patient’s absolute risk of ovarian cancer—from 0.75% to 0.50% lifetime risk—but the current data are not robust enough to suggest that it should be recommended for her. The operation would carry the risks associated with general anesthesia and peritoneal access. Although these risks are small, there is a documented risk of death from laparoscopic sterilization procedures in the United States, from complications related to bowel injury, anesthesia, and hemorrhage.
For these reasons, I would counsel this patient that her best options for contraception are combination OCs, transcervical tubal sterilization, or a long-acting reversible contraceptive such as the IUD or implant.
Tell us what you think, at [email protected]. Please include your name and city and state.
The development of new medical technology continues at a brisk pace—and ObGyns and our patients often are the beneficiaries. Notable technological breakthroughs of the past include hormonal contraception, in vitro fertilization, the application of minimally invasive surgical devices and techniques to gynecologic procedures, and many other innovations.
The leaders in our specialty are innovators themselves, ever vigilant for developments that can help improve health and quality of life for our patients. Regrettably, however, many technologies spread widely before they are fully validated by published studies—or continue to be used long after a superior or less invasive intervention has come along. And many claims about new technology are based on marketing information rather than reliable data.
Another common occurrence in regard to published data: Findings in one well-defined sector of the population are extrapolated to all patients. Even clinicians who are careful about adopting new technology can overlook the fact that it was tested, and proven, in a subset of patients that may not be comparable to all their patients.
In this article, I offer two case studies that illustrate some of the challenges we face when it comes to applying scientific findings to our practice and assessing medical technologies. In both settings, the health of the patient should be our primary focus.
When it comes to technology, patient selection is key
CASE 1: Anovulatory patient requests endometrial ablation
A 34-year-old woman (G2P2) who is moderately obese (body mass index of 32 kg/m2) visits your office to request endometrial ablation to manage her irregular and heavy menses. She reached menarche at age 10, and her periods have been somewhat irregular ever since. She required ovarian stimulation with clomiphene citrate to achieve each of her pregnancies, and both children were delivered by cesarean section. She currently uses condoms for contraception but does not have a steady partner, and she desires no additional pregnancies.
The patient reports that her menses occur every 2 to 6 weeks, with flow lasting from 2 to 8 days. She is housebound for 2 days each cycle because of heavy flow and cramping. She takes no medications and has no other medical conditions.
On exam, she is centrally obese with a tender uterus that is 10- to 12-weeks’ size; no adnexal masses are palpable. All other findings are normal. A recent test for diabetes was negative, but her serum cholesterol and triglyceride levels are borderline elevated.
What intervention would you recommend to address the heavy bleeding?
With the advent of second-generation endometrial ablation devices in the late 1990s, women with refractory menorrhagia had a safe, reliable, minimally invasive alternative to hysterectomy that made it possible to treat the endometrial cavity without the technical challenges of resectoscopic surgery. More than 10 million women report menorrhagia each year in the United States—so it is no small problem.1,2 Global endometrial ablation devices make management possible in an office setting, and recovery is significantly shorter than with hysterectomy. The three most prominent nonhysteroscopic ablation devices are (FIGURE):
• NovaSure (Hologic) uses bipolar radiofrequency energy to ablate tissue. Its probe contains stretchable gold-plated fabric that conforms to the endometrial surface.
• Gynecare Thermachoice (Ethicon) consists of an intrauterine balloon that is filled with hot liquid (temperatures of roughly 87°C) to ablate the endometrium
• Her Option (CooperSurgical) is a cryoablation system that consists of an intrauterine probe that forms an ice ball (temperatures of roughly –90°C) that destroys the uterine lining. Several applications are required to treat the majority of the cavity.
Use of these devices is widespread, although a nonsurgical alternative—the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena)—was approved by the US Food and Drug Administration (FDA) in 2009 for the treatment of heavy menstrual bleeding.
Not only is the efficacy of the LNG-IUS for controlling menorrhagia equal to endometrial ablation, but it provides the additional benefits of reliable contraception and management of dysmenorrhea.
6 questions to ask before applying a technology
1. In which population was it studied?
One of the most important issues to consider when you are evaluating technology is the population in which it was studied. In other words, to whom do the findings apply?
Most endometrial ablation technologies were tested in women who had a uterus of normal size (mean, 8 cm) with no structural abnormalities (ie, no polyps or fibroids) and regular but very heavy periods. The findings from these studies now have been extrapolated in many cases to women who have hormonally induced abnormal bleeding, whose periods are irregular. That extrapolation may not be appropriate.
2. What is the diagnosis?
As the manufacturers of global endometrial ablation devices note, heavy menstrual bleeding may arise from an underlying condition, such as endometrial cancer, hormonal imbalance, fibroids, coagulopathy, and so on—and it is important to rule these conditions out before considering endometrial ablation.
For example, in Case 1, the patient is experiencing anovulatory cycles, as evidenced by her irregular menses and the need for ovulation induction to achieve her pregnancies. The success rate of endometrial ablation in the setting of chronic anovulation is unknown. Pivotal trials for all of the nonhysteroscopic endometrial ablation technologies required regular menses or failure of cyclic hormonal therapy prior to enrollment.
In addition, in Case 1, the patient has signs (an enlarged, tender uterus) that suggest the presence of adenomyosis. Endometrial ablation is not recommended as a treatment for women with this condition.
3. Will later surgery be required?
In one study from Kaiser Permanente of Northern California, 21% of women who underwent endometrial ablation for menorrhagia later underwent hysterectomy, and 3.9% underwent other uterine-sparing procedures to alleviate heavy bleeding.3
In that study, women younger than age 45 were 2.1 times more likely to require hysterectomy after endometrial ablation, compared with older women (95% confidence interval [CI], 1.8–2.4). The likelihood of hysterectomy increased with each decreasing stratum of age and exceeded 40% in women aged 40 years or younger.3
In a population-based retrospective cohort study from Scotland, 2,779 (19.7%) of 11,299 women who underwent endometrial ablation for heavy menstrual bleeding required hysterectomy later.4 Again, women who required hysterectomy after endometrial ablation tended to be younger than those who did not. Overall, 26.6% of women undergoing endometrial ablation for heavy menstrual bleeding required further surgery.4
And in a 5-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation, roughly 30% of women required subsequent surgery for heavy menstrual bleeding.5
The need for subsequent surgery following endometrial ablation is likely to be higher among women who do not match the profile of participants in pivotal trials of the devices. All patients considering endometrial ablation should be counseled about the possible need for further surgery.
Related Article The economics of surgical gynecology: How we can not only survive, but thrive, in the 21st Century Barbara S. Levy, MD
4. What is the patient's overall health?
Before selecting an intervention for heavy menstrual bleeding, it is important to consider the patient’s overall health. What comorbidities does she have? Is pain a component of her condition? If so, might she have endometriosis or adenomyosis, as our patient does? If pain is a significant component of her menorrhagia, is it cyclical—that is, does it correspond to the days of heaviest flow? Pain related to the passage of large clots during menses may respond well to endometrial ablation. Pelvic pain occurring before and after the flow probably won’t.
Also keep in mind that the patient in Case 1 has two risk factors for endometrial hyperplasia: anovulatory cycles and obesity. If she undergoes endometrial ablation and subsequently experiences abnormal uterine bleeding, the most prominent sign of endometrial hyperplasia, how will you assess her endometrium 5 years after ablation if she develops abnormal bleeding? Will you be able to adequately sample it?
5. Have less invasive options been tried?
Many of the women studied in pivotal trials of second-generation endometrial ablation devices failed medical therapy prior to undergoing ablation. Because medical therapy is less expensive and noninvasive, it makes sense to offer it before proceeding to surgery. Common pharmacologic approaches include nonsteroidal anti-inflammatory drugs (NSAIDs), oral contraceptives, and tranexamic acid.
In addition, as I mentioned earlier, Mirena is approved for treatment of heavy menstrual bleeding.
Medical therapy and Mirena should be offered prior to surgery because of their noninvasive, reversible nature. Most physicians now consider Mirena to be the first-line option for heavy menstrual bleeding in women who have normal anatomy.6
6. What is the cost of treatment?
With health-care expenses accelerating, we need to be mindful of the cost-effectiveness of the options we recommend.
Let’s assess the expense of managing the patient in Case 1. To address her desire for long-term contraception, we might offer tubal sterilization by laparoscopy followed by endometrial ablation (to address the heavy bleeding), which would require a general anesthetic and management in an ambulatory surgical facility.
If she opts for hysteroscopic sterilization prior to endometrial ablation, the sterilization procedure must be performed at least 3 months prior to ablation (according to FDA labeling) so that tubal occlusion can be demonstrated by hysterosalpingography (HSG) before the uterine cavity is scarred. This means that the patient would require two separate interventions. Although both procedures could be performed in an office setting, the patient would still require time away from work and family.
The cost for sterilization plus ablation in the office without anesthesia would be approximately $4,500 (including HSG) at Medicare rates. For a combined laparoscopic sterilization and endometrial ablation, costs would be higher because of the need for anesthesia and an ambulatory surgical facility. Another option that would address both the heavy bleeding and the need for contraception: oral contraceptives (OCs). The overall cost of this approach over 5 years would be $4,200 (60 months of OCs at $70/month), but it would be fully covered under the Affordable Care Act (ACA), so the patient would have no out-of-pocket expense. The cost of Mirena would be $1,000 ($850 for the device plus $150 for insertion), but it also would be fully covered under the ACA.
CASE 1: Resolved
You discuss the option of cyclic OCs with the patient. This approach would help control her irregular, heavy, and painful menses while providing good contraceptive coverage.
Although sterilization plus endometrial ablation is an option, you counsel the patient that the published “success” rates do not apply to her. Given her young age, obesity, and anovulatory status, endometrial ablation has a greater likelihood of failure. Further, when abnormal bleeding recurs, it may be difficult to assess this high-risk patient’s endometrium.
You also discuss Mirena, which would provide long-term contraception and also manage her menorrhagia and dysmenorrhea; indeed, it is FDA-approved for this indication. In fact, Mirena would address all of this patient’s concerns, offering superb contraception and reliable reductions in bleeding and pelvic pain in the setting of endometriosis and adenomyosis.
Overall, this woman is best served by a highly reliable approach that manages her contraceptive needs and her menorrhagia and dysmenorrhea while reducing her risk for endometrial hyperplasia and cancer. Given the long-term endometrial-sampling problems ablation would create, it is not an optimal solution for this woman.
What this evidence means for practice
When assessing technology, consider which patients it was tested in, your patient’s diagnosis and long-term health risks, the cost and success rate of the technology, and the availability of less invasive options.
How to assess new data on a technology or procedure
Gill SE, Mills BB. Physician opinions regarding elective bilateral salpingectomy with hysterectomy and for sterilization. JMIG. 2013;20(4):517–521.
CASE 2: A healthy patient seeks permanent contraception
At her annual well-woman visit, your 42-year-old patient (G3P3) asks to discuss permanent contraception now that she has completed her family. She has used OCs for more than 10 years without problems. She is a nonsmoker of normal weight (BMI of 22 kg/m2), with normal blood pressure and vital signs. Her family history is remarkable for the health of her parents and siblings: Her mother is 68 years old and well; her father, 70, also is healthy. There is no family history of breast or ovarian cancer, prostate cancer, or significant medical illness.
What options would you offer this patient? Given recent data suggesting that ovarian cancer may originate in the fallopian tubes, would you recommend prophylactic salpingectomy as her contraceptive method of choice?
This patient has many options. In describing them to her, it would be important to focus on the breadth of safe, reversible, long-acting contraceptives now available, including their respective risks, benefits, and long-term outcomes and costs. It also is important to consider the published data on these methods, weighing their relevance to her overall health and medical history. Although some data regarding the origin of ovarian cancer in the fallopian tubes may be especially compelling, it may not be wise to extrapolate it from a specific group of women to all patients, as we shall see.
Option 1: Cyclic OCs
This option should not be excluded, despite the need for daily dosing, because the patient has used it successfully for more than 10 years. OCs are highly effective and have the added benefits of reducing menstrual blood loss, alleviating cramps, and lowering the risks of endometrial and ovarian cancer.7
Option 2: LARC
Long-acting reversible contraceptive (LARC) methods include the LNG-IUS, the copper intrauterine device (IUD; ParaGard, Teva), and the etonogestrel implant (Nexplanon, Merck). Each of these contraceptives is similar to sterilization in terms of efficacy. None requires the interruption of sexual activity or weekly or monthly trips to the pharmacy.
The LNG-IUS and the etonogestrel implant have the added benefits of reducing menstrual blood loss and reducing or eliminating premenstrual symptoms and cramps in most women. In addition, they may reduce the risk of unopposed estrogen stimulation of the endometrium associated with perimenopausal anovulatory cycles.
Option 3: Transcervical tubal sterilization
This hysteroscopic method (Essure, Bayer) avoids the risks of general anesthesia, but successful bilateral placement may not be possible in a small percentage of women. It also requires a 3-month interval between placement and confirmation of tubal occlusion (via HSG). During this interval, alternative contraception must be used. Once occlusion is confirmed, this method is highly reliable.
Option 4: Laparoscopic sterilization
This approach was the method of choice prior to the introduction of Essure in 2002. There now is a resurgence of interest in laparoscopic sterilization due to recent publications describing the distal portion of the fallopian tube as the “true source” of high-grade serous ovarian cancers.
In pathologic analyses of fallopian tubes removed prophylactically from women with a BRCA 1 or 2 mutation, investigators found a significant rate of serous tubal intraepithelial carcinoma.8 Researchers began to study the genetics of these cancers and concluded that, in women with a BRCA mutation, high-grade serous carcinomas may arise from the distal fallopian tube.
Until recently, all of the literature on these tubal carcinomas related only to women with a specific tumor suppressor gene p53 mutation in the BRCA system. Other investigators then reviewed pathology specimens from women without a BRCA mutation who had high-grade serous carcinomas that were peritoneal, tubal, or ovarian in origin. They found serous tubal intraepithelial carcinomas in 33% to 59% of these women.9
“Research suggests that bilateral salpingectomy during hysterectomy for benign indications or as a sterilization procedure may have benefits, such as preventing tubal disease and cancer, without significant risks,” wrote Gill and Mills.10 In conducting a survey of US physicians to determine how widespread prophylactic salpingectomy is during benign hysterectomy or sterilization, they found that 54% of respondents performed bilateral salpingectomy during hysterectomy, usually to lower the risk of cancer (75%) and avoid the need for reoperation (49.1%). Of the 45.5% of respondents who did not perform bilateral salpingectomy during hysterectomy, most (69.4%) believed it has no benefit.10
Although 58% of respondents believed bilateral salpingectomy to be the most effective option for sterilization in women older than 35 years, they reported that they reserve it for women in whom one sterilization procedure has failed or for women who have tubal disease.10
As for the 45.5% of respondents who did not perform prophylactic salpingectomy, they offered as reasons their concern about increased operative time and the risk of complications, as well as a belief that it has no benefit.10
The lifetime risk of ovarian cancer in the general population is 1 in 70 women. Although it is possible that high-grade serous ovarian cancers originate in the distal fallopian tube, as the research to date suggests, it also is possible that we might find in-situ lesions in tissues other than the distal tube, suggesting a more global genetic defect underlying ovarian cancers in the peritoneal and müllerian tissues. Randomized trials are under way in an effort to determine whether excision of the fallopian tubes will prevent the majority of high-grade serous cancers. It will be many years, however, before the results of these trials are available.
CASE 2: Resolved
This patient has used combination OCs for more than 10 years, so her lifetime risk of ovarian cancer has been reduced by approximately 50%. Because her family history indicates that a BRCA mutation is highly unlikely, her lifetime risk of ovarian cancer now has declined from 1:70 to roughly 1:140.
Prophylactic salpingectomy might offer a very small reduction in this patient’s absolute risk of ovarian cancer—from 0.75% to 0.50% lifetime risk—but the current data are not robust enough to suggest that it should be recommended for her. The operation would carry the risks associated with general anesthesia and peritoneal access. Although these risks are small, there is a documented risk of death from laparoscopic sterilization procedures in the United States, from complications related to bowel injury, anesthesia, and hemorrhage.
For these reasons, I would counsel this patient that her best options for contraception are combination OCs, transcervical tubal sterilization, or a long-acting reversible contraceptive such as the IUD or implant.
Tell us what you think, at [email protected]. Please include your name and city and state.
1. Centers for Disease Control and Prevention. Blood disor-ders in women: heavy menstrual bleeding. http://www.cdc.gov/ncbddd/blooddisorders/women/menorrhagia.html. Updated December 12, 2011. Accessed August 15, 2013.
2. Centers for Disease Control and Prevention. Blood disorders in women: research. http://www.cdc.gov/ncbddd/blooddis orders/women/research.html. Updated December 12, 2011. Accessed August 15, 2013.
3. Longinotti K, Jacobson GF, Hung YY, Learman LA. Probability of hysterectomy after endometrial ablation. Obstet Gynecol. 2008;112(6):1214–1220.
4. Cooper K, Lee AJ, Chien P, Raja EA, Timmaraju V, Bhattacharya S. Outcomes following hysterectomy or endometrial ablation for heavy menstrual bleeding: retrospective analysis of hospital episode statistics in Scotland. BJOG. 2011;118(10):1171–1179.
5. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9(4):429–435.
6. Sayre C. Beyond hysterectomy: Alternative options for heavy bleeding. NY Times. http://www.nytimes.com/ref/health/healthguide/esn-menorrhagia-ess.html. Published October 10, 2008. Accessed August 15, 2013.
7. Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer. A systematic review and meta-analysis. Obstet Gynecol. 2013;122(1):139–147.
8. Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007; 31:161–169.
9. Crum CP, McKeon FD, Xian W. The oviduct and ovarian cancer: causality, clinical implications, and “targeted prevention.” Clin Obstet Gynecol. 2012;55(1):24–35.
10. Gill SE, Mills BB. Physician opinions regarding elective bilateral salpingectomy with hysterectomy and for sterilization. JMIG. 2013;20(4):517–521.
1. Centers for Disease Control and Prevention. Blood disor-ders in women: heavy menstrual bleeding. http://www.cdc.gov/ncbddd/blooddisorders/women/menorrhagia.html. Updated December 12, 2011. Accessed August 15, 2013.
2. Centers for Disease Control and Prevention. Blood disorders in women: research. http://www.cdc.gov/ncbddd/blooddis orders/women/research.html. Updated December 12, 2011. Accessed August 15, 2013.
3. Longinotti K, Jacobson GF, Hung YY, Learman LA. Probability of hysterectomy after endometrial ablation. Obstet Gynecol. 2008;112(6):1214–1220.
4. Cooper K, Lee AJ, Chien P, Raja EA, Timmaraju V, Bhattacharya S. Outcomes following hysterectomy or endometrial ablation for heavy menstrual bleeding: retrospective analysis of hospital episode statistics in Scotland. BJOG. 2011;118(10):1171–1179.
5. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9(4):429–435.
6. Sayre C. Beyond hysterectomy: Alternative options for heavy bleeding. NY Times. http://www.nytimes.com/ref/health/healthguide/esn-menorrhagia-ess.html. Published October 10, 2008. Accessed August 15, 2013.
7. Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer. A systematic review and meta-analysis. Obstet Gynecol. 2013;122(1):139–147.
8. Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007; 31:161–169.
9. Crum CP, McKeon FD, Xian W. The oviduct and ovarian cancer: causality, clinical implications, and “targeted prevention.” Clin Obstet Gynecol. 2012;55(1):24–35.
10. Gill SE, Mills BB. Physician opinions regarding elective bilateral salpingectomy with hysterectomy and for sterilization. JMIG. 2013;20(4):517–521.
We are not getting what we pay for
To read the related Q&A, see The economics of surgical gynecology: How we can not only survive, but thrive, in the 21st Century
To read the related Q&A, see The economics of surgical gynecology: How we can not only survive, but thrive, in the 21st Century
To read the related Q&A, see The economics of surgical gynecology: How we can not only survive, but thrive, in the 21st Century
How to ask about, and manage, the undertreated problem of sexual dysfunction
UPDATE: SEXUAL DYSFUNCTION
New study: ObGyns aren’t fully addressing their patients’ sexual function
(Web News, April 2012)
How to prepare your patient for the many nuances of postpartum sexuality
Roya Rezaee, MD; Sheryl Kingsberg, PhD (January 2012)
Update on sexual dysfunction
Barbara S. Levy, MD (September 2011)
Sexual dysfunction is common among women, with an overall incidence of about 40%—even higher in some populations.1,2 All the more surprising, then, that only a minority of women raise the topic with their physician. In one well-regarded study, for example, only 22% of older women reported having discussed sex with their physician after the age of 50.3
One reason for the lack of communication may be a sense of discomfort around sexuality, among physicians as well as patients. Other reasons may include limited time on the part of physicians, and a lack of clarity about how to evaluate sexual function in women.
How, then, to assess a woman’s sexual function? In this Update, I address this question, and offer numerous others you can discuss with your patients without adding a significant time burden to your day. “A sidebar on page 27 focuses on a few strategies for tackling sexual function in an efficient and timely manner.”
In an ObGyn practice already pressed for time, adding a new domain of concern to the mix can be a challenge. (This is assuming you do not already ask patients routinely about sexual function.) It may not be as challenging as you think, however. One way to start is to add a few basic questions about sexual function to the intake form. This approach serves two purposes:
- It validates sexual function as an important part of health
- It allows the patient to identify any problems without having to raise the subject herself.
The second purpose is especially important because many patients are reluctant to broach the topic of sex.
After reviewing the intake form, you can take a more detailed history, addressing the concerns gently and matter of factly, to determine the scope of the problem, its duration, and any steps the patient has already taken to remedy it. The physical exam then can be more appropriately focused.
Straightforward areas of dysfunction, such as perimenopausal vaginal dryness, usually can be addressed in the same visit. More extensive problems may merit a separate office visit.
Linear model of female sexual function is not clinically useful
The classical linear model that proposes that women progress from sexual excitement to plateau, orgasm, and resolution is not that helpful when we are trying to determine the cause of our patient’s sexual problem and choose a course of treatment. More revealing is the biopsychosocial model, which considers physical, psychological, relational, and situational variables in exploring sexual function. If a physician focuses the history on these four aspects of sexual function, she generally can discover the source of any problem.
According to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), female sexual dysfunction typically falls into one or more categories:
- desire disorders – hypoactive sexual desire disorder (HSDD) and sexual aversion disorder
- problems with arousal – female sexual arousal disorder
- pain disorders – dyspareunia and vaginismus
- orgasmic disorders – female orgasmic disorder.4
If we superimpose this framework over the biopsychosocial model, diagnosis and management can be elucidated further. For example, we might see a 57-year-old patient who complains during her annual exam about decreased libido. We ask about menopausal symptoms and general health and screen for depression and intimate partner violence. On questioning, we discover that her partner was diagnosed with prostate cancer 2 years earlier and is able to achieve an erection on occasion with the use of medication, but there is fear, for both of them, that the erection will not last. This development has changed their sexual behaviors in ways that are not satisfying for either of them.
Clearly, addressing this fairly common scenario as “female sexual dysfunction” will overlook the main issues. This couple needs help communicating their love and support for each other and some direction to help them learn new ways to express themselves sexually that can be satisfying for both. It is important to remain sensitive to the patient’s (and partner’s) social and medical history, age, and physiological status.
Keep in mind, however, that it is common to see overlapping disorders in a patient with sexual dysfunction. For example, a lack of desire can lead to diminished arousal and anorgasmia. For this reason, when one disorder is diagnosed, ask the patient to describe any other problems she may have. Also inquire about the chronology of multiple sexual disorders in order to determine which problem came first.
One last caveat: When you are evaluating a patient’s sexual function, don’t assume that she has a male partner—or any partner at all.
Don’t overlook psychosocial variables when assessing desire disorders
Prevalence of low sexual desire ranges from 26.7% among premenopausal women to 52.4% among naturally menopausal women—no small problem.5
The Female Sexual Function Index (FSFI), a multidimensional self-reporting tool used widely in research, describes sexual desire as “a feeling that includes wanting to have a sexual experience, feeling receptive to a partner’s sexual initiation, and thinking or fantasizing about having sex.”6 The FSFI attempts to discern whether a desire disorder is present by asking about the patient’s feelings over the preceding 4 weeks.
Among the questions it poses are:
- How often have you felt sexual desire?
- How would you rate your level of sexual desire or interest?
These questions may be useful as a starting point when a patient complains of low desire.
Low desire may have multiple causes
Desire disorders may be associated with depression, but they also may arise from experiences and attitudes that occurred during childhood. For example, women who had a strict religious upbringing or were exposed to negative parental attitudes toward sex may suffer lifelong psychological effects. Other deep-seated sources of impaired desire include childhood physical, sexual, and emotional abuse.
These influences may not be readily apparent if the woman is in a new relationship, when powerful hormonal determinants of attraction—driven by phenylethylamine—hold sway. Once the relationship matures, however, and the “lust” begins to recede and a more comfortable, stable relationship emerges—these psychological barriers to physical enjoyment may come to the fore.
Referral is indicated for SAD
Sexual aversion disorder (SAD) is characterized by “persistent or recurrent extreme aversion to and avoidance of all (or almost all) genital sexual contact with a sexual partner,” according to DSM-IV.2 Unlike HSDD, which reflects a lack of interest in sex, SAD may involve physiologic aversion responses such as nausea, revulsion, and shortness of breath.3
SAD is a psychiatric illness that requires management by a qualified mental health professional, preferably a sex therapist. (For information on how to find a qualified therapist, consult the American Association of Sexuality Educators, Counselors, and Therapists at www.aasect.org.)
No FDA-approved drug for HSDD
HSDD is characterized by “a deficiency or absence of sexual fantasies and desire for sexual activity,” according to DSM-IV.4 It may be treated by an experienced psychotherapist without additional training in sexual therapy.
Regrettably, attempts to treat HSDD with pharmacologic agents have been modestly successful at best, and we lack FDA-approved medications. Some trials demonstrated a slight improvement in desire among surgically menopausal women on estrogen therapy when a supraphysiologic dose of testosterone was given. Off-label use of a compounded testosterone or a lower dose of a male testosterone product may be useful.
Avoid laboratory assays for testosterone—serum or salivary—in the diagnosis of HSDD. However, if a commercial testosterone product is given, testing may be useful to prevent excessive levels of testosterone and associated (and sometimes irreversible) physiologic changes, such as male-pattern hair loss and deepening of the voice.
Failure to lubricate is the hallmark of female sexual arousal disorder
The DSM-IV defines female sexual arousal disorder as persistent or recurrent inability to attain, or to maintain until completion of the sexual activity, an adequate physiologic response (lubrication, swelling) of sexual excitement.4 It is analogous to erectile dysfunction in men. In women, however, it may be difficult to distinguish this condition from primary desire disorder, particularly in cases in which a pattern of poor arousal, dryness, and dyspareunia has developed. The hallmark of female sexual arousal disorder is a failure to lubricate.
A few simple questions
Among the questions you might ask the patient:
- Do you feel interested in sexual activity?
- Do you have a problem lubricating well?
- Do you use a lubricant for sexual activity? If so, does it work to make sexual intercourse comfortable?
Other variables to consider
Arousal occurs secondary to genital vasodilation and tissue engorgement. It may be disturbed by any physiologic condition that reduces blood flow, such as smoking, hypertension, diabetes, and hypoestrogenism.
Decreased sensation sometimes may contribute to arousal disorder. For example, when the vagina and external genitalia experience decreased sensation, the cause may be physiologic, neurologic, or supratentorial.
Unlike men, women experience very little direct feedback regarding arousal. A disconnect between the sensory afferent input and higher-level awareness is not unusual. A thorough physical and neurologic exam may be necessary to assess the sensory nerves, integrity of the skin (signs of any inflammation), and blood flow to the genitalia.
Referral to a therapist also may be necessary so that other barriers to intimacy and sexuality can be determined.
As menopause approaches, so do a number of interrelated issues that may affect a woman’s sexual function. Here are five of them:
Body image may deteriorate, leading to feelings of unattractiveness that can lower interest in sexual activity.
Testosterone levels decline throughout a woman’s reproductive life, as does estrogen—but the decline in testosterone is much more gradual without any abrupt reduction at menopause. In addition, some common medications, such as oral contraceptives and oral estrogen formulations, increase sex hormone binding globulin, which can reduce dramatically the free testosterone level. Some women are especially sensitive to the effects of these drugs and may report a rapid loss of sexual desire when these agents are given. To sidestep the problem, give transdermal or transvaginal estrogen, when possible.
Comorbidities take a toll on sexual function in many cases. Arthritis, diabetes, cardiovascular disease, and other impairments may hamper sexual satisfaction. In addition, fatigue, insomnia, and depression are common with age.
Partner issues also play a role in diagnosis and treatment of female sexual dysfunction. For example, poor health in the partner can cause or exacerbate sexual problems.
Pain during sex can trigger desire and arousal disorders
Pain during sexual activity can lead to disorders of desire as well as arousal. When a patient reports pain during sex, pay careful attention to her medical history and perform a detailed physical examination. Patience is vital. Successful treatment of pain disorders requires commitment from the patient and her partner as well as the medical team.
Consider asking the following questions:
- Over the past 4 weeks, how often have you experienced discomfort or pain during vaginal penetration?
- How often have you experienced discomfort or pain following vaginal penetration?
- How would you rate your degree of discomfort or pain during or following vaginal penetration?6
Consider pain as a cause when any patient reports low libido, as pain is a potent suppressor of desire. A meticulous clinical history is required to determine the cause. For example, it is important to uncover whether the pain is of recent onset or of long duration, or whether it is related to childbirth, lactation, or menopausal changes.
Pain upon penetration could be caused by chemical, infectious, or atrophic vulvovaginitis. Dryness and pain upon penetration are often caused by:
- contact dermatitis
- irritation from soaps or scrubbing
- daily use of panty liners
- use of so-called feminine hygiene products
- regular use of swimming pools or hot tubs that contain chemicals.
Another cause of pain to consider is vulvar dystrophy. When lichen sclerosis or hypertrophic dystrophy goes untreated, the result may be fibrosis, lack of elasticity, painful fissures, and loss of normal architecture. These changes usually occur in postmenopausal women, so it is important that treatment address both the fibrosis and the hypoestrogenic atrophy.
If treated early, vulvar vestibulitis may not require surgery
Vulvar vestibulitis is poorly understood. It tends to occur most often in premenopausal women, frequently as a result of vulvar infection or during the postpartum period.
Vulvar vestibulitis involves point tenderness—sometimes experienced as a burning, searing sensation—around the introitus, specifically, the vestibular glands. When this condition is suspected, examine the vulva and vestibule with a moistened cotton swab to assess whether the classic distribution of pain is present. The necks of the vestibular glands may appear inflamed and erythematous.
If the condition is treated early enough with topical steroids and, in some cases, hydroxyzine, surgery may be avoided, provided the patient also avoids topical irritants. In many women, however, vestibulectomy is required to eliminate symptoms.
Vulvodynia may be associated with other pain syndromes
This disorder is a more generalized pain syndrome that involves the entire vulvar region. Like vulvar vestibulitis, it can cause painful penetration. It is also associated with other pain syndromes, including interstitial cystitis and endometriosis. Sensitization to pain at a central level may lead to hyperesthesia and allodynia. Also consider pudendal neuropathy, especially if the patient is a regular bicycle rider.
Treatment usually consists of off-label use of neuromodulators, such as gabapentin, tricyclic antidepressants, or duloxetine. The use of topical local anesthetic creams or gels may also permit pain-free sexual activity.
Vaginismus may indicate a history of sexual abuse
When the perineal muscles surrounding the outer third of the vagina contract involuntarily upon contact with a penis, speculum, or other item, vaginismus may be present. This disorder can be primary or secondary. In primary vaginismus, the patient may be unable to tolerate any vaginal penetration at all, not even a single digit or tampon. When this is the case, the patient may have a history of childhood sexual abuse. Explore her history, including any medical examinations that may have been painful or generated fear and anxiety. Also be aware that women with sexual aversion disorder may present with primary vaginismus.
Secondary vaginismus can occur even after years of satisfying sexual activity when a woman undergoes pelvic reconstructive surgery or develops vulvar dystrophy or vulvovaginal atrophy. Pain or the fear of pain can trigger a powerful reflex spasm of the levator ani musculature. Also keep in mind that secondary vaginismus may not be reproducible during the pelvic examination.
Treatment of both primary and secondary vaginismus includes physical therapy of the pelvic floor using biofeedback. The patient’s partner also needs to attend at least one session to learn techniques to prevent levator spasm and disable the reflex.
In the early phase of treatment, it may be helpful for the couple to agree to participate in a pact to avoid penetration during sexual activity. This approach may help reawaken sexual desire and arousal by eliminating the fear of pain.
The nature of the patient’s relationship with her partner is a powerful determinant of outcome. For example, intimacy and good communication are more likely to resolve the problem, whereas a difficult relationship may inhibit success. Depending on the scenario, counseling and psychological assessment may be necessary in the treatment of vaginismus, especially when a patient has a history of abuse.
Deep dyspareunia may be linked to pelvic pathology
This pain disorder may be associated with poor arousal or with fixation of the pelvic organs as a result of endometriosis, adhesions, or posthysterectomy scarring.
In a “normal” scenario, when arousal is unimpeded, the vagina lengthens by about 30%, and the uterus and cervix lift out of the cul-de-sac. This helps explain why not all women who have retroverted uteri or an obliterated cul-de-sac experience deep dyspareunia.
The patient’s history is a critical component of diagnosis. Ask her about foreplay, lubrication, and arousal prior to penetration. During the physical examination, be vigilant for point tenderness along the vaginal cuff and painful nodularity along the uterosacral ligaments.
To successfully treat deep dyspareunia, you must address any pelvic pathology as well as arousal problems. If penetration occurs prior to adequate arousal, the vagina remains shorter and the uterus has not yet engorged and lifted out of the cul-de-sac, resulting in “bump” dyspareunia. Surgery can elevate and alter any uterine retroversion that is present, but it is very rarely needed when adequate arousal can be achieved.
Pain with orgasm may arise from uterine pathology
At the time of orgasm, the levator ani musculature and myometrium contract strongly. When adenomyosis or degenerating uterine fibroids are present, pain may occur during or after orgasm. Women who have pelvic floor tension myalgia also may experience pelvic pain and aching after orgasm.
To tease out the cause of orgasm-related pain, perform a careful physical examination. To distinguish uterine pain from pain at the pelvic floor, perform a single-digit examination of each pelvic floor muscle before touching the cervix and uterus. Compression applied to a tender uterus often triggers a muscle spasm at the pelvic floor, so it is important to evaluate the pelvic floor muscles for tone and discomfort before performing a bimanual exam.
Treatment of uterine pathology usually entails medical or surgical intervention, whereas pelvic floor tension myalgia is treated with physical therapy and biofeedback.
Female orgasmic disorder may indicate a need for basic education
When a woman reports a persistent delay in or absence of orgasm after an otherwise satisfying episode of sexual activity and excitement, female orgasmic disorder is the likely cause. It may be primary or secondary.
Primary anorgasmia is often related to sexual inexperience and ignorance. Management may require education, use of a vibrator, and permission to engage in self-exploration—or it may necessitate evaluation and management by a trained sexual therapist. Resources for the patient, including educational materials and video, can be found at www.bettersex.com.
Secondary anorgasmia occurs in women who have a history of regular orgasm; the cause is generally drug-related. Among the usual culprits are selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants. Anorgasmia can be difficult to treat in these cases because discontinuation of the antidepressant can worsen depression—and depression is often associated with disorders of desire. One option is switching the class of the antidepressant to one less likely to disrupt orgasm, such as buproprion or trazodone. Off-label use of low-dose sildenafil may reverse the effect of SSRIs on orgasmic function, according to recent evidence.8
We want to hear from you! Tell us what you think.
1. Trompeter SE, Bettencourt R, Barrett-Connor E. Sexual activity and satisfaction in healthy community-dwelling older women. Am J Med. 2012;125(1):37-43.e1.
2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537-544.
3. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text revision. Washington, DC: APA; 2000.
5. West SL, D’Aloisio AA, Agans RP, Kalsbeek WB, Borisov NN, Thorp JM. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168(13):1441-1449.
6. Female Sexual Function Index. http://www.fsfi-questionnaire.com/FSFI%20questionnaire2000.pdf. Published 2000. Accessed August 14 2012.
7. Janata JW, Kingsberg SA. Sexual aversion disorder. In: Balon R Segraves RT, eds. Handbook of Sexual Dysfunction. Boca Raton, FL: Taylor and Francis; 2005.
8. Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-assocated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300(4):395-404.
New study: ObGyns aren’t fully addressing their patients’ sexual function
(Web News, April 2012)
How to prepare your patient for the many nuances of postpartum sexuality
Roya Rezaee, MD; Sheryl Kingsberg, PhD (January 2012)
Update on sexual dysfunction
Barbara S. Levy, MD (September 2011)
Sexual dysfunction is common among women, with an overall incidence of about 40%—even higher in some populations.1,2 All the more surprising, then, that only a minority of women raise the topic with their physician. In one well-regarded study, for example, only 22% of older women reported having discussed sex with their physician after the age of 50.3
One reason for the lack of communication may be a sense of discomfort around sexuality, among physicians as well as patients. Other reasons may include limited time on the part of physicians, and a lack of clarity about how to evaluate sexual function in women.
How, then, to assess a woman’s sexual function? In this Update, I address this question, and offer numerous others you can discuss with your patients without adding a significant time burden to your day. “A sidebar on page 27 focuses on a few strategies for tackling sexual function in an efficient and timely manner.”
In an ObGyn practice already pressed for time, adding a new domain of concern to the mix can be a challenge. (This is assuming you do not already ask patients routinely about sexual function.) It may not be as challenging as you think, however. One way to start is to add a few basic questions about sexual function to the intake form. This approach serves two purposes:
- It validates sexual function as an important part of health
- It allows the patient to identify any problems without having to raise the subject herself.
The second purpose is especially important because many patients are reluctant to broach the topic of sex.
After reviewing the intake form, you can take a more detailed history, addressing the concerns gently and matter of factly, to determine the scope of the problem, its duration, and any steps the patient has already taken to remedy it. The physical exam then can be more appropriately focused.
Straightforward areas of dysfunction, such as perimenopausal vaginal dryness, usually can be addressed in the same visit. More extensive problems may merit a separate office visit.
Linear model of female sexual function is not clinically useful
The classical linear model that proposes that women progress from sexual excitement to plateau, orgasm, and resolution is not that helpful when we are trying to determine the cause of our patient’s sexual problem and choose a course of treatment. More revealing is the biopsychosocial model, which considers physical, psychological, relational, and situational variables in exploring sexual function. If a physician focuses the history on these four aspects of sexual function, she generally can discover the source of any problem.
According to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), female sexual dysfunction typically falls into one or more categories:
- desire disorders – hypoactive sexual desire disorder (HSDD) and sexual aversion disorder
- problems with arousal – female sexual arousal disorder
- pain disorders – dyspareunia and vaginismus
- orgasmic disorders – female orgasmic disorder.4
If we superimpose this framework over the biopsychosocial model, diagnosis and management can be elucidated further. For example, we might see a 57-year-old patient who complains during her annual exam about decreased libido. We ask about menopausal symptoms and general health and screen for depression and intimate partner violence. On questioning, we discover that her partner was diagnosed with prostate cancer 2 years earlier and is able to achieve an erection on occasion with the use of medication, but there is fear, for both of them, that the erection will not last. This development has changed their sexual behaviors in ways that are not satisfying for either of them.
Clearly, addressing this fairly common scenario as “female sexual dysfunction” will overlook the main issues. This couple needs help communicating their love and support for each other and some direction to help them learn new ways to express themselves sexually that can be satisfying for both. It is important to remain sensitive to the patient’s (and partner’s) social and medical history, age, and physiological status.
Keep in mind, however, that it is common to see overlapping disorders in a patient with sexual dysfunction. For example, a lack of desire can lead to diminished arousal and anorgasmia. For this reason, when one disorder is diagnosed, ask the patient to describe any other problems she may have. Also inquire about the chronology of multiple sexual disorders in order to determine which problem came first.
One last caveat: When you are evaluating a patient’s sexual function, don’t assume that she has a male partner—or any partner at all.
Don’t overlook psychosocial variables when assessing desire disorders
Prevalence of low sexual desire ranges from 26.7% among premenopausal women to 52.4% among naturally menopausal women—no small problem.5
The Female Sexual Function Index (FSFI), a multidimensional self-reporting tool used widely in research, describes sexual desire as “a feeling that includes wanting to have a sexual experience, feeling receptive to a partner’s sexual initiation, and thinking or fantasizing about having sex.”6 The FSFI attempts to discern whether a desire disorder is present by asking about the patient’s feelings over the preceding 4 weeks.
Among the questions it poses are:
- How often have you felt sexual desire?
- How would you rate your level of sexual desire or interest?
These questions may be useful as a starting point when a patient complains of low desire.
Low desire may have multiple causes
Desire disorders may be associated with depression, but they also may arise from experiences and attitudes that occurred during childhood. For example, women who had a strict religious upbringing or were exposed to negative parental attitudes toward sex may suffer lifelong psychological effects. Other deep-seated sources of impaired desire include childhood physical, sexual, and emotional abuse.
These influences may not be readily apparent if the woman is in a new relationship, when powerful hormonal determinants of attraction—driven by phenylethylamine—hold sway. Once the relationship matures, however, and the “lust” begins to recede and a more comfortable, stable relationship emerges—these psychological barriers to physical enjoyment may come to the fore.
Referral is indicated for SAD
Sexual aversion disorder (SAD) is characterized by “persistent or recurrent extreme aversion to and avoidance of all (or almost all) genital sexual contact with a sexual partner,” according to DSM-IV.2 Unlike HSDD, which reflects a lack of interest in sex, SAD may involve physiologic aversion responses such as nausea, revulsion, and shortness of breath.3
SAD is a psychiatric illness that requires management by a qualified mental health professional, preferably a sex therapist. (For information on how to find a qualified therapist, consult the American Association of Sexuality Educators, Counselors, and Therapists at www.aasect.org.)
No FDA-approved drug for HSDD
HSDD is characterized by “a deficiency or absence of sexual fantasies and desire for sexual activity,” according to DSM-IV.4 It may be treated by an experienced psychotherapist without additional training in sexual therapy.
Regrettably, attempts to treat HSDD with pharmacologic agents have been modestly successful at best, and we lack FDA-approved medications. Some trials demonstrated a slight improvement in desire among surgically menopausal women on estrogen therapy when a supraphysiologic dose of testosterone was given. Off-label use of a compounded testosterone or a lower dose of a male testosterone product may be useful.
Avoid laboratory assays for testosterone—serum or salivary—in the diagnosis of HSDD. However, if a commercial testosterone product is given, testing may be useful to prevent excessive levels of testosterone and associated (and sometimes irreversible) physiologic changes, such as male-pattern hair loss and deepening of the voice.
Failure to lubricate is the hallmark of female sexual arousal disorder
The DSM-IV defines female sexual arousal disorder as persistent or recurrent inability to attain, or to maintain until completion of the sexual activity, an adequate physiologic response (lubrication, swelling) of sexual excitement.4 It is analogous to erectile dysfunction in men. In women, however, it may be difficult to distinguish this condition from primary desire disorder, particularly in cases in which a pattern of poor arousal, dryness, and dyspareunia has developed. The hallmark of female sexual arousal disorder is a failure to lubricate.
A few simple questions
Among the questions you might ask the patient:
- Do you feel interested in sexual activity?
- Do you have a problem lubricating well?
- Do you use a lubricant for sexual activity? If so, does it work to make sexual intercourse comfortable?
Other variables to consider
Arousal occurs secondary to genital vasodilation and tissue engorgement. It may be disturbed by any physiologic condition that reduces blood flow, such as smoking, hypertension, diabetes, and hypoestrogenism.
Decreased sensation sometimes may contribute to arousal disorder. For example, when the vagina and external genitalia experience decreased sensation, the cause may be physiologic, neurologic, or supratentorial.
Unlike men, women experience very little direct feedback regarding arousal. A disconnect between the sensory afferent input and higher-level awareness is not unusual. A thorough physical and neurologic exam may be necessary to assess the sensory nerves, integrity of the skin (signs of any inflammation), and blood flow to the genitalia.
Referral to a therapist also may be necessary so that other barriers to intimacy and sexuality can be determined.
As menopause approaches, so do a number of interrelated issues that may affect a woman’s sexual function. Here are five of them:
Body image may deteriorate, leading to feelings of unattractiveness that can lower interest in sexual activity.
Testosterone levels decline throughout a woman’s reproductive life, as does estrogen—but the decline in testosterone is much more gradual without any abrupt reduction at menopause. In addition, some common medications, such as oral contraceptives and oral estrogen formulations, increase sex hormone binding globulin, which can reduce dramatically the free testosterone level. Some women are especially sensitive to the effects of these drugs and may report a rapid loss of sexual desire when these agents are given. To sidestep the problem, give transdermal or transvaginal estrogen, when possible.
Comorbidities take a toll on sexual function in many cases. Arthritis, diabetes, cardiovascular disease, and other impairments may hamper sexual satisfaction. In addition, fatigue, insomnia, and depression are common with age.
Partner issues also play a role in diagnosis and treatment of female sexual dysfunction. For example, poor health in the partner can cause or exacerbate sexual problems.
Pain during sex can trigger desire and arousal disorders
Pain during sexual activity can lead to disorders of desire as well as arousal. When a patient reports pain during sex, pay careful attention to her medical history and perform a detailed physical examination. Patience is vital. Successful treatment of pain disorders requires commitment from the patient and her partner as well as the medical team.
Consider asking the following questions:
- Over the past 4 weeks, how often have you experienced discomfort or pain during vaginal penetration?
- How often have you experienced discomfort or pain following vaginal penetration?
- How would you rate your degree of discomfort or pain during or following vaginal penetration?6
Consider pain as a cause when any patient reports low libido, as pain is a potent suppressor of desire. A meticulous clinical history is required to determine the cause. For example, it is important to uncover whether the pain is of recent onset or of long duration, or whether it is related to childbirth, lactation, or menopausal changes.
Pain upon penetration could be caused by chemical, infectious, or atrophic vulvovaginitis. Dryness and pain upon penetration are often caused by:
- contact dermatitis
- irritation from soaps or scrubbing
- daily use of panty liners
- use of so-called feminine hygiene products
- regular use of swimming pools or hot tubs that contain chemicals.
Another cause of pain to consider is vulvar dystrophy. When lichen sclerosis or hypertrophic dystrophy goes untreated, the result may be fibrosis, lack of elasticity, painful fissures, and loss of normal architecture. These changes usually occur in postmenopausal women, so it is important that treatment address both the fibrosis and the hypoestrogenic atrophy.
If treated early, vulvar vestibulitis may not require surgery
Vulvar vestibulitis is poorly understood. It tends to occur most often in premenopausal women, frequently as a result of vulvar infection or during the postpartum period.
Vulvar vestibulitis involves point tenderness—sometimes experienced as a burning, searing sensation—around the introitus, specifically, the vestibular glands. When this condition is suspected, examine the vulva and vestibule with a moistened cotton swab to assess whether the classic distribution of pain is present. The necks of the vestibular glands may appear inflamed and erythematous.
If the condition is treated early enough with topical steroids and, in some cases, hydroxyzine, surgery may be avoided, provided the patient also avoids topical irritants. In many women, however, vestibulectomy is required to eliminate symptoms.
Vulvodynia may be associated with other pain syndromes
This disorder is a more generalized pain syndrome that involves the entire vulvar region. Like vulvar vestibulitis, it can cause painful penetration. It is also associated with other pain syndromes, including interstitial cystitis and endometriosis. Sensitization to pain at a central level may lead to hyperesthesia and allodynia. Also consider pudendal neuropathy, especially if the patient is a regular bicycle rider.
Treatment usually consists of off-label use of neuromodulators, such as gabapentin, tricyclic antidepressants, or duloxetine. The use of topical local anesthetic creams or gels may also permit pain-free sexual activity.
Vaginismus may indicate a history of sexual abuse
When the perineal muscles surrounding the outer third of the vagina contract involuntarily upon contact with a penis, speculum, or other item, vaginismus may be present. This disorder can be primary or secondary. In primary vaginismus, the patient may be unable to tolerate any vaginal penetration at all, not even a single digit or tampon. When this is the case, the patient may have a history of childhood sexual abuse. Explore her history, including any medical examinations that may have been painful or generated fear and anxiety. Also be aware that women with sexual aversion disorder may present with primary vaginismus.
Secondary vaginismus can occur even after years of satisfying sexual activity when a woman undergoes pelvic reconstructive surgery or develops vulvar dystrophy or vulvovaginal atrophy. Pain or the fear of pain can trigger a powerful reflex spasm of the levator ani musculature. Also keep in mind that secondary vaginismus may not be reproducible during the pelvic examination.
Treatment of both primary and secondary vaginismus includes physical therapy of the pelvic floor using biofeedback. The patient’s partner also needs to attend at least one session to learn techniques to prevent levator spasm and disable the reflex.
In the early phase of treatment, it may be helpful for the couple to agree to participate in a pact to avoid penetration during sexual activity. This approach may help reawaken sexual desire and arousal by eliminating the fear of pain.
The nature of the patient’s relationship with her partner is a powerful determinant of outcome. For example, intimacy and good communication are more likely to resolve the problem, whereas a difficult relationship may inhibit success. Depending on the scenario, counseling and psychological assessment may be necessary in the treatment of vaginismus, especially when a patient has a history of abuse.
Deep dyspareunia may be linked to pelvic pathology
This pain disorder may be associated with poor arousal or with fixation of the pelvic organs as a result of endometriosis, adhesions, or posthysterectomy scarring.
In a “normal” scenario, when arousal is unimpeded, the vagina lengthens by about 30%, and the uterus and cervix lift out of the cul-de-sac. This helps explain why not all women who have retroverted uteri or an obliterated cul-de-sac experience deep dyspareunia.
The patient’s history is a critical component of diagnosis. Ask her about foreplay, lubrication, and arousal prior to penetration. During the physical examination, be vigilant for point tenderness along the vaginal cuff and painful nodularity along the uterosacral ligaments.
To successfully treat deep dyspareunia, you must address any pelvic pathology as well as arousal problems. If penetration occurs prior to adequate arousal, the vagina remains shorter and the uterus has not yet engorged and lifted out of the cul-de-sac, resulting in “bump” dyspareunia. Surgery can elevate and alter any uterine retroversion that is present, but it is very rarely needed when adequate arousal can be achieved.
Pain with orgasm may arise from uterine pathology
At the time of orgasm, the levator ani musculature and myometrium contract strongly. When adenomyosis or degenerating uterine fibroids are present, pain may occur during or after orgasm. Women who have pelvic floor tension myalgia also may experience pelvic pain and aching after orgasm.
To tease out the cause of orgasm-related pain, perform a careful physical examination. To distinguish uterine pain from pain at the pelvic floor, perform a single-digit examination of each pelvic floor muscle before touching the cervix and uterus. Compression applied to a tender uterus often triggers a muscle spasm at the pelvic floor, so it is important to evaluate the pelvic floor muscles for tone and discomfort before performing a bimanual exam.
Treatment of uterine pathology usually entails medical or surgical intervention, whereas pelvic floor tension myalgia is treated with physical therapy and biofeedback.
Female orgasmic disorder may indicate a need for basic education
When a woman reports a persistent delay in or absence of orgasm after an otherwise satisfying episode of sexual activity and excitement, female orgasmic disorder is the likely cause. It may be primary or secondary.
Primary anorgasmia is often related to sexual inexperience and ignorance. Management may require education, use of a vibrator, and permission to engage in self-exploration—or it may necessitate evaluation and management by a trained sexual therapist. Resources for the patient, including educational materials and video, can be found at www.bettersex.com.
Secondary anorgasmia occurs in women who have a history of regular orgasm; the cause is generally drug-related. Among the usual culprits are selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants. Anorgasmia can be difficult to treat in these cases because discontinuation of the antidepressant can worsen depression—and depression is often associated with disorders of desire. One option is switching the class of the antidepressant to one less likely to disrupt orgasm, such as buproprion or trazodone. Off-label use of low-dose sildenafil may reverse the effect of SSRIs on orgasmic function, according to recent evidence.8
We want to hear from you! Tell us what you think.
New study: ObGyns aren’t fully addressing their patients’ sexual function
(Web News, April 2012)
How to prepare your patient for the many nuances of postpartum sexuality
Roya Rezaee, MD; Sheryl Kingsberg, PhD (January 2012)
Update on sexual dysfunction
Barbara S. Levy, MD (September 2011)
Sexual dysfunction is common among women, with an overall incidence of about 40%—even higher in some populations.1,2 All the more surprising, then, that only a minority of women raise the topic with their physician. In one well-regarded study, for example, only 22% of older women reported having discussed sex with their physician after the age of 50.3
One reason for the lack of communication may be a sense of discomfort around sexuality, among physicians as well as patients. Other reasons may include limited time on the part of physicians, and a lack of clarity about how to evaluate sexual function in women.
How, then, to assess a woman’s sexual function? In this Update, I address this question, and offer numerous others you can discuss with your patients without adding a significant time burden to your day. “A sidebar on page 27 focuses on a few strategies for tackling sexual function in an efficient and timely manner.”
In an ObGyn practice already pressed for time, adding a new domain of concern to the mix can be a challenge. (This is assuming you do not already ask patients routinely about sexual function.) It may not be as challenging as you think, however. One way to start is to add a few basic questions about sexual function to the intake form. This approach serves two purposes:
- It validates sexual function as an important part of health
- It allows the patient to identify any problems without having to raise the subject herself.
The second purpose is especially important because many patients are reluctant to broach the topic of sex.
After reviewing the intake form, you can take a more detailed history, addressing the concerns gently and matter of factly, to determine the scope of the problem, its duration, and any steps the patient has already taken to remedy it. The physical exam then can be more appropriately focused.
Straightforward areas of dysfunction, such as perimenopausal vaginal dryness, usually can be addressed in the same visit. More extensive problems may merit a separate office visit.
Linear model of female sexual function is not clinically useful
The classical linear model that proposes that women progress from sexual excitement to plateau, orgasm, and resolution is not that helpful when we are trying to determine the cause of our patient’s sexual problem and choose a course of treatment. More revealing is the biopsychosocial model, which considers physical, psychological, relational, and situational variables in exploring sexual function. If a physician focuses the history on these four aspects of sexual function, she generally can discover the source of any problem.
According to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), female sexual dysfunction typically falls into one or more categories:
- desire disorders – hypoactive sexual desire disorder (HSDD) and sexual aversion disorder
- problems with arousal – female sexual arousal disorder
- pain disorders – dyspareunia and vaginismus
- orgasmic disorders – female orgasmic disorder.4
If we superimpose this framework over the biopsychosocial model, diagnosis and management can be elucidated further. For example, we might see a 57-year-old patient who complains during her annual exam about decreased libido. We ask about menopausal symptoms and general health and screen for depression and intimate partner violence. On questioning, we discover that her partner was diagnosed with prostate cancer 2 years earlier and is able to achieve an erection on occasion with the use of medication, but there is fear, for both of them, that the erection will not last. This development has changed their sexual behaviors in ways that are not satisfying for either of them.
Clearly, addressing this fairly common scenario as “female sexual dysfunction” will overlook the main issues. This couple needs help communicating their love and support for each other and some direction to help them learn new ways to express themselves sexually that can be satisfying for both. It is important to remain sensitive to the patient’s (and partner’s) social and medical history, age, and physiological status.
Keep in mind, however, that it is common to see overlapping disorders in a patient with sexual dysfunction. For example, a lack of desire can lead to diminished arousal and anorgasmia. For this reason, when one disorder is diagnosed, ask the patient to describe any other problems she may have. Also inquire about the chronology of multiple sexual disorders in order to determine which problem came first.
One last caveat: When you are evaluating a patient’s sexual function, don’t assume that she has a male partner—or any partner at all.
Don’t overlook psychosocial variables when assessing desire disorders
Prevalence of low sexual desire ranges from 26.7% among premenopausal women to 52.4% among naturally menopausal women—no small problem.5
The Female Sexual Function Index (FSFI), a multidimensional self-reporting tool used widely in research, describes sexual desire as “a feeling that includes wanting to have a sexual experience, feeling receptive to a partner’s sexual initiation, and thinking or fantasizing about having sex.”6 The FSFI attempts to discern whether a desire disorder is present by asking about the patient’s feelings over the preceding 4 weeks.
Among the questions it poses are:
- How often have you felt sexual desire?
- How would you rate your level of sexual desire or interest?
These questions may be useful as a starting point when a patient complains of low desire.
Low desire may have multiple causes
Desire disorders may be associated with depression, but they also may arise from experiences and attitudes that occurred during childhood. For example, women who had a strict religious upbringing or were exposed to negative parental attitudes toward sex may suffer lifelong psychological effects. Other deep-seated sources of impaired desire include childhood physical, sexual, and emotional abuse.
These influences may not be readily apparent if the woman is in a new relationship, when powerful hormonal determinants of attraction—driven by phenylethylamine—hold sway. Once the relationship matures, however, and the “lust” begins to recede and a more comfortable, stable relationship emerges—these psychological barriers to physical enjoyment may come to the fore.
Referral is indicated for SAD
Sexual aversion disorder (SAD) is characterized by “persistent or recurrent extreme aversion to and avoidance of all (or almost all) genital sexual contact with a sexual partner,” according to DSM-IV.2 Unlike HSDD, which reflects a lack of interest in sex, SAD may involve physiologic aversion responses such as nausea, revulsion, and shortness of breath.3
SAD is a psychiatric illness that requires management by a qualified mental health professional, preferably a sex therapist. (For information on how to find a qualified therapist, consult the American Association of Sexuality Educators, Counselors, and Therapists at www.aasect.org.)
No FDA-approved drug for HSDD
HSDD is characterized by “a deficiency or absence of sexual fantasies and desire for sexual activity,” according to DSM-IV.4 It may be treated by an experienced psychotherapist without additional training in sexual therapy.
Regrettably, attempts to treat HSDD with pharmacologic agents have been modestly successful at best, and we lack FDA-approved medications. Some trials demonstrated a slight improvement in desire among surgically menopausal women on estrogen therapy when a supraphysiologic dose of testosterone was given. Off-label use of a compounded testosterone or a lower dose of a male testosterone product may be useful.
Avoid laboratory assays for testosterone—serum or salivary—in the diagnosis of HSDD. However, if a commercial testosterone product is given, testing may be useful to prevent excessive levels of testosterone and associated (and sometimes irreversible) physiologic changes, such as male-pattern hair loss and deepening of the voice.
Failure to lubricate is the hallmark of female sexual arousal disorder
The DSM-IV defines female sexual arousal disorder as persistent or recurrent inability to attain, or to maintain until completion of the sexual activity, an adequate physiologic response (lubrication, swelling) of sexual excitement.4 It is analogous to erectile dysfunction in men. In women, however, it may be difficult to distinguish this condition from primary desire disorder, particularly in cases in which a pattern of poor arousal, dryness, and dyspareunia has developed. The hallmark of female sexual arousal disorder is a failure to lubricate.
A few simple questions
Among the questions you might ask the patient:
- Do you feel interested in sexual activity?
- Do you have a problem lubricating well?
- Do you use a lubricant for sexual activity? If so, does it work to make sexual intercourse comfortable?
Other variables to consider
Arousal occurs secondary to genital vasodilation and tissue engorgement. It may be disturbed by any physiologic condition that reduces blood flow, such as smoking, hypertension, diabetes, and hypoestrogenism.
Decreased sensation sometimes may contribute to arousal disorder. For example, when the vagina and external genitalia experience decreased sensation, the cause may be physiologic, neurologic, or supratentorial.
Unlike men, women experience very little direct feedback regarding arousal. A disconnect between the sensory afferent input and higher-level awareness is not unusual. A thorough physical and neurologic exam may be necessary to assess the sensory nerves, integrity of the skin (signs of any inflammation), and blood flow to the genitalia.
Referral to a therapist also may be necessary so that other barriers to intimacy and sexuality can be determined.
As menopause approaches, so do a number of interrelated issues that may affect a woman’s sexual function. Here are five of them:
Body image may deteriorate, leading to feelings of unattractiveness that can lower interest in sexual activity.
Testosterone levels decline throughout a woman’s reproductive life, as does estrogen—but the decline in testosterone is much more gradual without any abrupt reduction at menopause. In addition, some common medications, such as oral contraceptives and oral estrogen formulations, increase sex hormone binding globulin, which can reduce dramatically the free testosterone level. Some women are especially sensitive to the effects of these drugs and may report a rapid loss of sexual desire when these agents are given. To sidestep the problem, give transdermal or transvaginal estrogen, when possible.
Comorbidities take a toll on sexual function in many cases. Arthritis, diabetes, cardiovascular disease, and other impairments may hamper sexual satisfaction. In addition, fatigue, insomnia, and depression are common with age.
Partner issues also play a role in diagnosis and treatment of female sexual dysfunction. For example, poor health in the partner can cause or exacerbate sexual problems.
Pain during sex can trigger desire and arousal disorders
Pain during sexual activity can lead to disorders of desire as well as arousal. When a patient reports pain during sex, pay careful attention to her medical history and perform a detailed physical examination. Patience is vital. Successful treatment of pain disorders requires commitment from the patient and her partner as well as the medical team.
Consider asking the following questions:
- Over the past 4 weeks, how often have you experienced discomfort or pain during vaginal penetration?
- How often have you experienced discomfort or pain following vaginal penetration?
- How would you rate your degree of discomfort or pain during or following vaginal penetration?6
Consider pain as a cause when any patient reports low libido, as pain is a potent suppressor of desire. A meticulous clinical history is required to determine the cause. For example, it is important to uncover whether the pain is of recent onset or of long duration, or whether it is related to childbirth, lactation, or menopausal changes.
Pain upon penetration could be caused by chemical, infectious, or atrophic vulvovaginitis. Dryness and pain upon penetration are often caused by:
- contact dermatitis
- irritation from soaps or scrubbing
- daily use of panty liners
- use of so-called feminine hygiene products
- regular use of swimming pools or hot tubs that contain chemicals.
Another cause of pain to consider is vulvar dystrophy. When lichen sclerosis or hypertrophic dystrophy goes untreated, the result may be fibrosis, lack of elasticity, painful fissures, and loss of normal architecture. These changes usually occur in postmenopausal women, so it is important that treatment address both the fibrosis and the hypoestrogenic atrophy.
If treated early, vulvar vestibulitis may not require surgery
Vulvar vestibulitis is poorly understood. It tends to occur most often in premenopausal women, frequently as a result of vulvar infection or during the postpartum period.
Vulvar vestibulitis involves point tenderness—sometimes experienced as a burning, searing sensation—around the introitus, specifically, the vestibular glands. When this condition is suspected, examine the vulva and vestibule with a moistened cotton swab to assess whether the classic distribution of pain is present. The necks of the vestibular glands may appear inflamed and erythematous.
If the condition is treated early enough with topical steroids and, in some cases, hydroxyzine, surgery may be avoided, provided the patient also avoids topical irritants. In many women, however, vestibulectomy is required to eliminate symptoms.
Vulvodynia may be associated with other pain syndromes
This disorder is a more generalized pain syndrome that involves the entire vulvar region. Like vulvar vestibulitis, it can cause painful penetration. It is also associated with other pain syndromes, including interstitial cystitis and endometriosis. Sensitization to pain at a central level may lead to hyperesthesia and allodynia. Also consider pudendal neuropathy, especially if the patient is a regular bicycle rider.
Treatment usually consists of off-label use of neuromodulators, such as gabapentin, tricyclic antidepressants, or duloxetine. The use of topical local anesthetic creams or gels may also permit pain-free sexual activity.
Vaginismus may indicate a history of sexual abuse
When the perineal muscles surrounding the outer third of the vagina contract involuntarily upon contact with a penis, speculum, or other item, vaginismus may be present. This disorder can be primary or secondary. In primary vaginismus, the patient may be unable to tolerate any vaginal penetration at all, not even a single digit or tampon. When this is the case, the patient may have a history of childhood sexual abuse. Explore her history, including any medical examinations that may have been painful or generated fear and anxiety. Also be aware that women with sexual aversion disorder may present with primary vaginismus.
Secondary vaginismus can occur even after years of satisfying sexual activity when a woman undergoes pelvic reconstructive surgery or develops vulvar dystrophy or vulvovaginal atrophy. Pain or the fear of pain can trigger a powerful reflex spasm of the levator ani musculature. Also keep in mind that secondary vaginismus may not be reproducible during the pelvic examination.
Treatment of both primary and secondary vaginismus includes physical therapy of the pelvic floor using biofeedback. The patient’s partner also needs to attend at least one session to learn techniques to prevent levator spasm and disable the reflex.
In the early phase of treatment, it may be helpful for the couple to agree to participate in a pact to avoid penetration during sexual activity. This approach may help reawaken sexual desire and arousal by eliminating the fear of pain.
The nature of the patient’s relationship with her partner is a powerful determinant of outcome. For example, intimacy and good communication are more likely to resolve the problem, whereas a difficult relationship may inhibit success. Depending on the scenario, counseling and psychological assessment may be necessary in the treatment of vaginismus, especially when a patient has a history of abuse.
Deep dyspareunia may be linked to pelvic pathology
This pain disorder may be associated with poor arousal or with fixation of the pelvic organs as a result of endometriosis, adhesions, or posthysterectomy scarring.
In a “normal” scenario, when arousal is unimpeded, the vagina lengthens by about 30%, and the uterus and cervix lift out of the cul-de-sac. This helps explain why not all women who have retroverted uteri or an obliterated cul-de-sac experience deep dyspareunia.
The patient’s history is a critical component of diagnosis. Ask her about foreplay, lubrication, and arousal prior to penetration. During the physical examination, be vigilant for point tenderness along the vaginal cuff and painful nodularity along the uterosacral ligaments.
To successfully treat deep dyspareunia, you must address any pelvic pathology as well as arousal problems. If penetration occurs prior to adequate arousal, the vagina remains shorter and the uterus has not yet engorged and lifted out of the cul-de-sac, resulting in “bump” dyspareunia. Surgery can elevate and alter any uterine retroversion that is present, but it is very rarely needed when adequate arousal can be achieved.
Pain with orgasm may arise from uterine pathology
At the time of orgasm, the levator ani musculature and myometrium contract strongly. When adenomyosis or degenerating uterine fibroids are present, pain may occur during or after orgasm. Women who have pelvic floor tension myalgia also may experience pelvic pain and aching after orgasm.
To tease out the cause of orgasm-related pain, perform a careful physical examination. To distinguish uterine pain from pain at the pelvic floor, perform a single-digit examination of each pelvic floor muscle before touching the cervix and uterus. Compression applied to a tender uterus often triggers a muscle spasm at the pelvic floor, so it is important to evaluate the pelvic floor muscles for tone and discomfort before performing a bimanual exam.
Treatment of uterine pathology usually entails medical or surgical intervention, whereas pelvic floor tension myalgia is treated with physical therapy and biofeedback.
Female orgasmic disorder may indicate a need for basic education
When a woman reports a persistent delay in or absence of orgasm after an otherwise satisfying episode of sexual activity and excitement, female orgasmic disorder is the likely cause. It may be primary or secondary.
Primary anorgasmia is often related to sexual inexperience and ignorance. Management may require education, use of a vibrator, and permission to engage in self-exploration—or it may necessitate evaluation and management by a trained sexual therapist. Resources for the patient, including educational materials and video, can be found at www.bettersex.com.
Secondary anorgasmia occurs in women who have a history of regular orgasm; the cause is generally drug-related. Among the usual culprits are selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants. Anorgasmia can be difficult to treat in these cases because discontinuation of the antidepressant can worsen depression—and depression is often associated with disorders of desire. One option is switching the class of the antidepressant to one less likely to disrupt orgasm, such as buproprion or trazodone. Off-label use of low-dose sildenafil may reverse the effect of SSRIs on orgasmic function, according to recent evidence.8
We want to hear from you! Tell us what you think.
1. Trompeter SE, Bettencourt R, Barrett-Connor E. Sexual activity and satisfaction in healthy community-dwelling older women. Am J Med. 2012;125(1):37-43.e1.
2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537-544.
3. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text revision. Washington, DC: APA; 2000.
5. West SL, D’Aloisio AA, Agans RP, Kalsbeek WB, Borisov NN, Thorp JM. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168(13):1441-1449.
6. Female Sexual Function Index. http://www.fsfi-questionnaire.com/FSFI%20questionnaire2000.pdf. Published 2000. Accessed August 14 2012.
7. Janata JW, Kingsberg SA. Sexual aversion disorder. In: Balon R Segraves RT, eds. Handbook of Sexual Dysfunction. Boca Raton, FL: Taylor and Francis; 2005.
8. Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-assocated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300(4):395-404.
1. Trompeter SE, Bettencourt R, Barrett-Connor E. Sexual activity and satisfaction in healthy community-dwelling older women. Am J Med. 2012;125(1):37-43.e1.
2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537-544.
3. Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text revision. Washington, DC: APA; 2000.
5. West SL, D’Aloisio AA, Agans RP, Kalsbeek WB, Borisov NN, Thorp JM. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168(13):1441-1449.
6. Female Sexual Function Index. http://www.fsfi-questionnaire.com/FSFI%20questionnaire2000.pdf. Published 2000. Accessed August 14 2012.
7. Janata JW, Kingsberg SA. Sexual aversion disorder. In: Balon R Segraves RT, eds. Handbook of Sexual Dysfunction. Boca Raton, FL: Taylor and Francis; 2005.
8. Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-assocated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300(4):395-404.
UPDATE ON SEXUAL DYSFUNCTION
- Cancer survivors have many complaints not addressed by their physicians
Janelle Yates, Senior Editor (Web Exclusive, July 2011) - How to talk to patients about sex
Barbara S. Levy, MD (Web Audio, September 2010) - Sexual dysfunction: What can you do for your patients?
Barbara S. Levy, MD (Update, September 2010)
Sexual dysfunction is common among women in the United States. One recent study put the prevalence of distressing sexual dysfunction at 22.2%.1 When cancer enters the picture, that percentage rises—dramatically. A 2010 survey from the Lance Armstrong Foundation found that 46% of people affected by cancer report problems with sex after treatment.2
In this article, I highlight three recent studies that explore the sexual effects of cancer and its treatment:
- a prospective cohort study showing that a majority of women treated for breast cancer experience sexual dysfunction afterward
- two longitudinal studies of women affected by gynecologic cancer, which show significant disruption of sexual function in the short and long term.
Sexual function deteriorates in many women after they are treated for breast Ca
Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med. 2011;8(1):294–302.
According to this prospective cohort study from Australia, a majority of women report significant sexual dysfunction after treatment for breast cancer—even when their sexual function was good, and satisfying, at the time of diagnosis (TABLE).
The Health and Wellbeing after Breast Cancer Study enrolled 1,684 Australian women within 12 months of their first diagnosis of invasive breast cancer. Each woman completed a questionnaire at the time of enrollment, and will complete annual follow-up questionnaires for 5 years to assess the impact of invasive breast cancer on physical, psychological, and socioeconomic wellbeing. Embedded within the 12-month questionnaire was the validated Menopause-Specific Quality of Life Questionnaire (MENQOL), which was used in this study to explore the sexual consequences of the diagnosis and treatment of invasive breast cancer.
Of the initial cohort, 1,011 women completed the 12-month questionnaire. These were women younger than 70 years who had a sexual partner and no evidence of active breast cancer. The authors describe the women in this cohort as representative of all women in Victoria, Australia, who have a new diagnosis of invasive breast cancer, in regard to both age (mean, 59 ± 11 years) and the stage of tumor (stage I, 48%; stage II–IV, 52%) at diagnosis.
Of this group, 70% were treated with lumpectomy and radiation therapy, and 30% were treated with mastectomy (2.6% with bilateral mastectomy). Of the women who underwent mastectomy, 9.6% had reconstructive surgery during the first year after diagnosis.
Forty-nine percent of women were treated with tamoxifen, and 28.2% were treated with an aromatase inhibitor.
After breast Ca, women experience low desire and less frequent sexual activity – as well as distress over both outcomes
| Symptom | Yes | No |
|---|---|---|
| Decreased desire | 71.7% | 19.7% |
| Decreased sexual activity | 72.5% | 21.1% |
| Distressed by sexual function | 49.1% | 8.1% |
| Seeking increase in desire | 64.1% | 19.9% |
| Source: Panjari et al. | ||
More than two thirds of women reported sexual dysfunction 12 months after treatment
At baseline, 83% of women described their prediagnosis sexual function as good and satisfying. Twelve months later, 70% reported significant sexual dysfunction, and 77% reported vasomotor symptoms.
Women who reported new-onset sexual dysfunction were more likely to:
- have become menopausal since diagnosis
- experience hot flashes or night sweats
- be treated with an aromatase inhibitor.
There was no association between sexual dysfunction and stage of disease at diagnosis; type of surgery (lumpectomy or mastectomy); breast reconstruction; lymphedema; or axillary dissection.
Vasomotor symptoms in women taking endocrine therapy were associated with sexual dysfunction
Further analysis demonstrated that, among women who experienced vasomotor symptoms, those taking an aromatase inhibitor were more than three times as likely to report sexual dysfunction (odds ratio [OR], 3.49; 95% confidence interval [CI], 1.72–7.09), compared with women who were not on endocrine therapy—and those taking tamoxifen were almost twice as likely to report sexual dysfunction (OR, 1.73; 95% CI, 1.04–2.89). Chemotherapy was not independently related to sexual dysfunction.
In summary: 70% of women who were free of breast cancer 1 year after enrollment reported bothersome sexual consequences of their disease and its treatment; 77% reported vasomotor symptoms. Women who were rendered menopausal and those who experienced vasomotor symptoms while taking an aromatase inhibitor were at high risk of sexual dysfunction.
Be aware of the side effects of breast cancer and its treatment, and not only prepare your patients for the likely consequences but also make yourself knowledgeable about strategies to ameliorate their vaginal dryness and to improve elasticity and arousal for them.
Proactive stretching, use of vaginal dilators and topical oils, and, most important, psychological strategies to help your patients and their partners adjust to the inevitable physical changes will go a long way toward improving their sexual experiences.
Katie* and Julie* tell typical stories of deep dissatisfaction with the health system after their cancer treatment
Katie: “I wasn’t prepared”
When my doctor told me I had locally advanced breast cancer 3 years ago, when I was 50, I wasn’t that surprised by the cancer diagnosis (I have a strong family history of breast cancer)—only by the fact that the tumor had developed so fast since my previous mammogram 15 months earlier. As treatment, I underwent neoadjuvant chemotherapy and bilateral mastectomy (I had the unaffected breast removed as a preventive measure). I also had breast reconstruction and started taking an aromatase inhibitor.
At the time of my diagnosis, I lost all desire for sexual intimacy—no big surprise there. But even after my treatment was over, my desire did not return. Part of the problem was the fact that chemotherapy rendered me menopausal, and the aromatase inhibitor I was taking compounded the menopausal experience. Quite suddenly, I was experiencing hot flashes, vaginal dryness and itching, pain during intercourse, severe bone and joint aches, weight gain (particularly around my abdomen), and general lethargy.
No one in my family had ever mentioned these effects of cancer. And none of my doctors prepared me, either. In fact, when I raised the subject, they seemed genuinely surprised! They offered no remedy other than a recommendation to apply a “moisturizer”—but they gave no details about what kind or how to use it. My oncologist did say that local estrogen would help relieve the pain of intercourse—but then she recommended strongly against it because my cancer was hormone-receptor positive.
My plastic surgeon did a much better job of explaining the effects and outcome of reconstruction than any of my other physicians, including my ObGyn, did of preparing me for menopause and sexual dysfunction.
All of my physicians strike me as caring, sensitive people, but their underlying attitude, as I perceive it, is that I should be grateful to be alive. In their view, it seems, enjoyment of sex is icing on the cake and, quite frankly, I am plenty lucky to have the cake. My oncologist even told me to let her know if I started “feeling better and having fewer hot flashes” so that she could perform ovarian ablation (and start the whole cycle over again). I was struck by how matter of factly she gave this advice, as though quality of life counts for nothing.
Three years into my postcancer life, I can say I have “adjusted” to my problems rather than overcome them. I am still taking an aromatase inhibitor. Sex is still slightly painful; I still struggle with vaginal dryness; and I sometimes feel like an old woman because of my bone and joint stiffness and pain. I did find out about an over-the-counter vaginal suppository, made with vitamin E and coconut oils, from another breast cancer survivor. And I switched from one aromatase inhibitor to another in an attempt to alleviate my achy joints. It helped.
I am grateful for my life—very much so—and for the expertise of my physicians, who helped to save it. But I wish they had prepared me better for the aftermath of cancer treatment. And I wish there were more remedies for women like me, who cannot take hormones.
Julie: “I’m on my own”
It was more than a surprise when my new doctor told me I had cancer. Until then, I had avoided doctors. That attitude can mean a premature death sentence when it comes to cervical cancer. It was a pretty awful realization that I could have avoided the drastic measures it took to save my life if I had just gotten annual Pap smears and exams. I was 39 at the time of diagnosis.
But after all the surgery and chemo and radiation were finished, the message I received was essentially: “OK, you’re good, for now. Just come back every few months for a check-up.”
What about the aftermath of all that treatment? What about the other aspects of the experience? I found that my doctors had very little to offer outside of surgery and drugs and the quick advice to get counseling or some other support services.
“I’m on my own” is what I’ve been telling concerned folks who ask how I’m doing. I am truly grateful for the skill, medicine, and machinery that made the killing of an invasive tumor possible. But I’m on my own when it comes to finding or inventing ways to cope with the new challenges of a pelvic area damaged by radiation and detoxing from the heavy metal—platinum—that was an ingredient in the chemo I received.
My partner and I have had to be persistently creative, careful, delicate, uncritical, and extremely patient with each other to bring about the return of a “normal” sex life. We have been successful, for the most part, but there is also a slightly new definition of what “normal” is for us. Our latest triumph is that we no longer have to use copious amounts of lubricant to engage in intercourse. Sex is no longer painful, as the vaginal tissues have been slowly, patiently engaged on a regularly scheduled basis. Can you imagine sex on a schedule? Neither could I, but that is what we found worked from as early as 1 week postradiation. As it turns out, this was good advice—really, the only advice I got when it came to the practicalities of restoring function, but it required a fair amount of tweaking and personalization as well.
Another big change in perception that I had to accept as part of my new norm is learning to talk about my most personal areas in a matter of fact way.
The cancer conveyor-belt approach to treatment is a very streamlined, well-run system. I’ve been impressed with the expertise, efficiency, and demeanor of all the professionals I have encountered. Everyone—even receptionists—has been helpful and empathetic, especially my own ObGyn, who has hugged me and cried with me and offered to put herself on the line for me and speak out to the media when I had no health insurance. But for most patients post-treatment, we figuratively walk off a cliff and find ourselves in new territory without any network or structure like we experienced during the “war” on our cancer. This new territory is a place of possibility within the health-care field—one I hope is developing now.

Dr. Barbara S. Levy asks: How do we respond? We physicians are so focused on treating or curing disease that we often lose sight of the woman who has the disease.
Katie’s case is much too common. Women are often reluctant to address their sexuality with us—especially when we have been dismissive. We must recognize this important aspect of quality of life and relationships and be prepared to raise the issue with our patients before they begin therapy.
Educating ourselves and then our patients about strategies to reduce the impact of treatment and menopause on sexual function is the first step. Acknowledging and validating their concerns and being able to offer practical steps to preserve healthy sexual function is something, I think, that all ObGyns should be able to do. Strategies to maintain vulvovaginal elasticity include avoidance of soaps and drying chemicals, daily perineal and vaginal stretching—either manually, with a dilator, or via frequent sexual intercourse. And topical lubricants and moisturizing agents should be recommended to maintain vaginal pH and reduce the dryness, itching, and overall dysesthesia.
As the studies highlighted in this Update on Sexual Dysfunction demonstrate, the sexual consequences of radical hysterectomy are significant. Julie also became menopausal as a result of treatment—yet no one prepared her for the symptoms she would experience, and no member of her treatment team helped her understand the likely impact of therapy. Radiation oncologists do address the need for vaginal dilators or daily intercourse to maintain depth and caliber of the vagina during and after therapy, but they are less likely to prepare a patient for menopausal symptoms.
If our patients are to have an optimal experience, we need to provide coordinated, cross-disciplinary care that includes management not only of immediate side effects of treatment but also psychosocial and long-term hormonal and sexual sequelae of therapy. Julie charted her own course with the help of a very dedicated and sensitive partner to successfully overcome the negative effects of radiation, chemotherapy, surgery, and menopause on her sexuality.
Gynecologic cancer disrupts sexual function, over the short term and the long term
Jensen PT, Groenvold M, Klee MC, Thranov I, Petersen MA, Machin D. Early-stage cervical carcinoma, radical hysterectomy, and sexual function: a longitudinal study. Cancer. 2004;100(1):97–106.
Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause. 2011;18(6):662–669.
These two studies explored sexual function after treatment for gynecologic cancer. The investigators found significant disruption of function.
Jensen et al: Radical hysterectomy for cervical Ca damages sexual function significantly
This was a prospective cohort study of 173 women who had early-stage cervical carcinoma and who underwent radical hysterectomy with pelvic lymphadenectomy (all of them node-negative). A validated questionnaire was administered six times, from 5 weeks to 24 months after surgery. An age-matched group of women without cancer was used for comparison. At the 12-month follow-up, patients were asked to report their sexual function at baseline and compare it to their current status. Overall, the women had a higher level of dissatisfaction with their sexual experiences 12 months after surgery than at baseline.
Details of the study
Women in the Jensen study were 23 to 75 years old (median age, 42.7 years), and 93% were sexually active at the time of diagnosis, reporting an average of one to two sexual activities in a week. Forty-six women (25%) were postmenopausal at diagnosis, compared with 34% of the control group. Only 8% of patients were using HT at study entry, compared with 25% of women in the control group. By 12 months after surgery, however, 25% of gynecologic cancer patients were taking systemic HT.
Findings included low libido and other ills
Severe lack of lubrication and low or no sexual desire were reported by cancer patients throughout the first 2 years after surgery. Patients also reported severe problems achieving orgasm as long as 6 months after surgery, as well as reduced vaginal size; both problems rendered their sexual experiences unsatisfactory. Nevertheless, 40% of patients reported at least some sexual activity by 5 weeks after surgery. By 6 months after surgery, there were almost as many sexually active women among the patient group as there were in the control group. However, at 18 months after treatment, patients reported less interest in intimacy—among both themselves and their partners—than among women in the control group. Overall, women treated for cervical cancer had a higher level of dissatisfaction with their sexual experiences 12 months after surgery than they did before diagnosis.
Although 91% of women who were sexually active before surgery resumed intercourse within 12 months, the frequency of sexual activity declined from one to two times per week to three to four times per month. Major long-term changes occurred in regard to libido (interest in sexual relations), arousal (vaginal lubrication), and vaginal size. Although dyspareunia was a significant problem 5 weeks to 3 months after surgery, it resolved within 1 to 2 years.
Jensen and colleagues concluded that radical hysterectomy for treatment of early-stage cervical cancer has significant negative effects on sexual function in the short and long term. They postulated a neurogenic basis for the sexual complaints and discussed both histologic and clinical studies to support this hypothesis.
They also emphasized the need to discuss the risks and management of sexual dysfunction with patients before and after surgery. Better management of the psychosocial consequences of a cancer diagnosis and the physical effects of radical hysterectomy may help avoid the negative experiences that were reported in this study.
Vaz et al: Rate of dyspareunia was high among women treated for endometrial or cervical Ca
Investigators followed 107 women from initial consultation for radiation therapy through 3 years post-treatment. Although a significant percentage (50%) of the cohort was lost to follow-up—many due to death or tumor recurrence—50% of those who remained reported dyspareunia 3 years after radiotherapy.
Women in this study were 21 to 75 years old (median age, 60 years) and had cervical or endometrial carcinoma. Eighty-nine women (83%) received external pelvic radiation as well as brachytherapy. Before beginning radiation therapy, 37.4% of the cohort underwent surgery for treatment of their cancer. Sixty-four percent of the cohort had stage III or IV disease.
At enrollment, 50% of women reported having a life partner, 82% were postmenopausal, and 11.2% were taking HT. However, only 21.5% of women reported sexual activity. The authors opine that this low rate of sexual activity may have been due to recent surgery, bleeding, or pain related to cancer.
All women were offered “standard” interventions for their dyspareunia, including the use of vaginal dilators twice daily for 2 years, as well as the use of vaginal lubricants. Patients who experienced menopausal symptoms—hot flashes, decreased libido, dyspareunia—were referred to a menopause outpatient clinic.
Before treatment, 20% of women reported dyspareunia. Three years later, 44% of patients reported sexual activity, but 50% had dyspareunia. Twenty-one percent reported lower sexual interest relative to baseline, 8% reported vaginal dryness, and 21% reported vasomotor symptoms. Although there was a trend toward increasing sexual activity with decreasing vaginal dryness, the rise in dyspareunia from 20% to 50% over 3 years is troubling.
Radical hysterectomy and radiation therapy to the pelvis cause neurovascular disruption and sexual consequences quite similar to those found after radical prostate surgery. Sexual arousal and orgasm are dependent on both the parasympathetic and sympathetic nerves supplying the pelvis. these nerves are disrupted in Frankenhauser’s plexus during the parametrial dissection of radical hysterectomy and lie clearly within the radiation treatment field.
Dilators and lubricants may be useful in minimizing actual shrinkage of the vagina. However, the elasticity of vaginal tissues, vasodilation during arousal, and transudation across the vaginal wall may all be lost or significantly compromised.
Advise your patients of the potential for sexual side effects of cervical and endometrial cancer treatment before they undergo that therapy. Proactive management of some of the expected problems, such as reduced elasticity and lubrication, as well as treatment for arousal dysfunction (perhaps with the same pharmacotherapeutic agents that provide improvement for many men after radical prostatectomy), may help your patients avoid the distress and disappointment they often experience after successful treatment of their cancers.
When a patient has undergone treatment for cancer,
ask about her sexual function
A symposium on sexual health yields recommendations for your broader care of cancer patients
How likely are you to encounter a cancer survivor in your practice?
Very.
According to the Centers for Disease Control and Prevention (CDC), there were 6.3 million female cancer survivors in the United States as of 2007—and that number has likely increased by a million or more.3 In fact, the number of cancer survivors is expected to double by 2016.
How likely is that cancer survivor to have sexual dysfunction?
Highly.
According to a 2010 survey by the Lance Armstrong Foundation, 46% of cancer survivors report problems with sexual functioning after treatment—and that’s probably a conservative figure, given that 64% of all people with cancer have a malignancy that directly affects sexual organs.2
One more question for you to ponder: How likely is the cancer patient’s sexual dysfunction to go unaddressed?
Extremely.
According to speakers at the Cancer Survivorship and Sexual Health Symposium, held June 17–19, 2011, in Washington, DC, cancer survivors are ill prepared for many of the symptoms of sexual dysfunction that develop after treatment, and many physicians fail to address this dimension of their health.
The symposium, sponsored by the International Society for Sexual Medicine and the Sexual Medicine Society of North America, was organized to address these gaps in care. During the 3-day conference, speakers from oncology, gynecology, mental health, urology, and other specialties presented data and described their experience managing cancer patients. They also offered recommendations for clinicians:
- Talk about it. Address the “highly prevalent but commonly ignored” adverse sexual effects of malignancy and its treatment. Ask: “How has cancer affected your sex life?”
- Try to prevent it. Consider nerve-sparing strategies during radical hysterectomy, radical trachelectomy, and clitoral preservation, which may lead to improved sexual function
- Encourage and support use of dilators. Advise women who have gynecologic cancer to use dilators to maintain vaginal patency, and be aware that compliance is linked to support from a health-care provider
- Encourage sexual activity, which can help preserve function
- Consider local estrogen. When it is appropriate, prescribe vaginal estrogen, which is minimally absorbed, to reduce vaginal symptoms of menopause. (The safety of local estrogen remains in question for women who have breast cancer.)
- Check for problems at each follow-up appointment, and be prepared to explain function and treatment options more than once
- Promote female genital blood flow. For example, it may be appropriate to begin sexual rehabilitation, such as use of vaginal dilators, during treatment
- Consider referral to a sexual rehabilitation program that includes medical and psychological approaches
- Build a network of psychologists, sex therapists, and other professionals who can assist you in managing your patients’ complaints.
“Discomfort around human sexuality is the main reason the issue doesn’t get raised by health-care providers,” said symposium speaker Sharon L. Bober, PhD, of the Dana-Farber Cancer Institute in Boston. “No one wants to initiate the conversation.” Dr. Bober emphasized the importance of asking about sexual function when a cancer survivor presents for care. “A majority of cancer patients in the community don’t hear this question from their providers.”
—Janelle Yates, Senior Editor
We want to hear from you! Tell us what you think.
1. Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970-978.
2. Rechis R, Boerner L. How cancer has affected post-treatment survivors: a LIVESTRONG report. Austin, Tex: Lance Armstrong Foundation 2011;13-
3. Centers for Disease Control and Prevention. Cancer survivors—United States, 2007. MMWR. 2011;60(9):269-272
- Cancer survivors have many complaints not addressed by their physicians
Janelle Yates, Senior Editor (Web Exclusive, July 2011) - How to talk to patients about sex
Barbara S. Levy, MD (Web Audio, September 2010) - Sexual dysfunction: What can you do for your patients?
Barbara S. Levy, MD (Update, September 2010)
Sexual dysfunction is common among women in the United States. One recent study put the prevalence of distressing sexual dysfunction at 22.2%.1 When cancer enters the picture, that percentage rises—dramatically. A 2010 survey from the Lance Armstrong Foundation found that 46% of people affected by cancer report problems with sex after treatment.2
In this article, I highlight three recent studies that explore the sexual effects of cancer and its treatment:
- a prospective cohort study showing that a majority of women treated for breast cancer experience sexual dysfunction afterward
- two longitudinal studies of women affected by gynecologic cancer, which show significant disruption of sexual function in the short and long term.
Sexual function deteriorates in many women after they are treated for breast Ca
Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med. 2011;8(1):294–302.
According to this prospective cohort study from Australia, a majority of women report significant sexual dysfunction after treatment for breast cancer—even when their sexual function was good, and satisfying, at the time of diagnosis (TABLE).
The Health and Wellbeing after Breast Cancer Study enrolled 1,684 Australian women within 12 months of their first diagnosis of invasive breast cancer. Each woman completed a questionnaire at the time of enrollment, and will complete annual follow-up questionnaires for 5 years to assess the impact of invasive breast cancer on physical, psychological, and socioeconomic wellbeing. Embedded within the 12-month questionnaire was the validated Menopause-Specific Quality of Life Questionnaire (MENQOL), which was used in this study to explore the sexual consequences of the diagnosis and treatment of invasive breast cancer.
Of the initial cohort, 1,011 women completed the 12-month questionnaire. These were women younger than 70 years who had a sexual partner and no evidence of active breast cancer. The authors describe the women in this cohort as representative of all women in Victoria, Australia, who have a new diagnosis of invasive breast cancer, in regard to both age (mean, 59 ± 11 years) and the stage of tumor (stage I, 48%; stage II–IV, 52%) at diagnosis.
Of this group, 70% were treated with lumpectomy and radiation therapy, and 30% were treated with mastectomy (2.6% with bilateral mastectomy). Of the women who underwent mastectomy, 9.6% had reconstructive surgery during the first year after diagnosis.
Forty-nine percent of women were treated with tamoxifen, and 28.2% were treated with an aromatase inhibitor.
After breast Ca, women experience low desire and less frequent sexual activity – as well as distress over both outcomes
| Symptom | Yes | No |
|---|---|---|
| Decreased desire | 71.7% | 19.7% |
| Decreased sexual activity | 72.5% | 21.1% |
| Distressed by sexual function | 49.1% | 8.1% |
| Seeking increase in desire | 64.1% | 19.9% |
| Source: Panjari et al. | ||
More than two thirds of women reported sexual dysfunction 12 months after treatment
At baseline, 83% of women described their prediagnosis sexual function as good and satisfying. Twelve months later, 70% reported significant sexual dysfunction, and 77% reported vasomotor symptoms.
Women who reported new-onset sexual dysfunction were more likely to:
- have become menopausal since diagnosis
- experience hot flashes or night sweats
- be treated with an aromatase inhibitor.
There was no association between sexual dysfunction and stage of disease at diagnosis; type of surgery (lumpectomy or mastectomy); breast reconstruction; lymphedema; or axillary dissection.
Vasomotor symptoms in women taking endocrine therapy were associated with sexual dysfunction
Further analysis demonstrated that, among women who experienced vasomotor symptoms, those taking an aromatase inhibitor were more than three times as likely to report sexual dysfunction (odds ratio [OR], 3.49; 95% confidence interval [CI], 1.72–7.09), compared with women who were not on endocrine therapy—and those taking tamoxifen were almost twice as likely to report sexual dysfunction (OR, 1.73; 95% CI, 1.04–2.89). Chemotherapy was not independently related to sexual dysfunction.
In summary: 70% of women who were free of breast cancer 1 year after enrollment reported bothersome sexual consequences of their disease and its treatment; 77% reported vasomotor symptoms. Women who were rendered menopausal and those who experienced vasomotor symptoms while taking an aromatase inhibitor were at high risk of sexual dysfunction.
Be aware of the side effects of breast cancer and its treatment, and not only prepare your patients for the likely consequences but also make yourself knowledgeable about strategies to ameliorate their vaginal dryness and to improve elasticity and arousal for them.
Proactive stretching, use of vaginal dilators and topical oils, and, most important, psychological strategies to help your patients and their partners adjust to the inevitable physical changes will go a long way toward improving their sexual experiences.
Katie* and Julie* tell typical stories of deep dissatisfaction with the health system after their cancer treatment
Katie: “I wasn’t prepared”
When my doctor told me I had locally advanced breast cancer 3 years ago, when I was 50, I wasn’t that surprised by the cancer diagnosis (I have a strong family history of breast cancer)—only by the fact that the tumor had developed so fast since my previous mammogram 15 months earlier. As treatment, I underwent neoadjuvant chemotherapy and bilateral mastectomy (I had the unaffected breast removed as a preventive measure). I also had breast reconstruction and started taking an aromatase inhibitor.
At the time of my diagnosis, I lost all desire for sexual intimacy—no big surprise there. But even after my treatment was over, my desire did not return. Part of the problem was the fact that chemotherapy rendered me menopausal, and the aromatase inhibitor I was taking compounded the menopausal experience. Quite suddenly, I was experiencing hot flashes, vaginal dryness and itching, pain during intercourse, severe bone and joint aches, weight gain (particularly around my abdomen), and general lethargy.
No one in my family had ever mentioned these effects of cancer. And none of my doctors prepared me, either. In fact, when I raised the subject, they seemed genuinely surprised! They offered no remedy other than a recommendation to apply a “moisturizer”—but they gave no details about what kind or how to use it. My oncologist did say that local estrogen would help relieve the pain of intercourse—but then she recommended strongly against it because my cancer was hormone-receptor positive.
My plastic surgeon did a much better job of explaining the effects and outcome of reconstruction than any of my other physicians, including my ObGyn, did of preparing me for menopause and sexual dysfunction.
All of my physicians strike me as caring, sensitive people, but their underlying attitude, as I perceive it, is that I should be grateful to be alive. In their view, it seems, enjoyment of sex is icing on the cake and, quite frankly, I am plenty lucky to have the cake. My oncologist even told me to let her know if I started “feeling better and having fewer hot flashes” so that she could perform ovarian ablation (and start the whole cycle over again). I was struck by how matter of factly she gave this advice, as though quality of life counts for nothing.
Three years into my postcancer life, I can say I have “adjusted” to my problems rather than overcome them. I am still taking an aromatase inhibitor. Sex is still slightly painful; I still struggle with vaginal dryness; and I sometimes feel like an old woman because of my bone and joint stiffness and pain. I did find out about an over-the-counter vaginal suppository, made with vitamin E and coconut oils, from another breast cancer survivor. And I switched from one aromatase inhibitor to another in an attempt to alleviate my achy joints. It helped.
I am grateful for my life—very much so—and for the expertise of my physicians, who helped to save it. But I wish they had prepared me better for the aftermath of cancer treatment. And I wish there were more remedies for women like me, who cannot take hormones.
Julie: “I’m on my own”
It was more than a surprise when my new doctor told me I had cancer. Until then, I had avoided doctors. That attitude can mean a premature death sentence when it comes to cervical cancer. It was a pretty awful realization that I could have avoided the drastic measures it took to save my life if I had just gotten annual Pap smears and exams. I was 39 at the time of diagnosis.
But after all the surgery and chemo and radiation were finished, the message I received was essentially: “OK, you’re good, for now. Just come back every few months for a check-up.”
What about the aftermath of all that treatment? What about the other aspects of the experience? I found that my doctors had very little to offer outside of surgery and drugs and the quick advice to get counseling or some other support services.
“I’m on my own” is what I’ve been telling concerned folks who ask how I’m doing. I am truly grateful for the skill, medicine, and machinery that made the killing of an invasive tumor possible. But I’m on my own when it comes to finding or inventing ways to cope with the new challenges of a pelvic area damaged by radiation and detoxing from the heavy metal—platinum—that was an ingredient in the chemo I received.
My partner and I have had to be persistently creative, careful, delicate, uncritical, and extremely patient with each other to bring about the return of a “normal” sex life. We have been successful, for the most part, but there is also a slightly new definition of what “normal” is for us. Our latest triumph is that we no longer have to use copious amounts of lubricant to engage in intercourse. Sex is no longer painful, as the vaginal tissues have been slowly, patiently engaged on a regularly scheduled basis. Can you imagine sex on a schedule? Neither could I, but that is what we found worked from as early as 1 week postradiation. As it turns out, this was good advice—really, the only advice I got when it came to the practicalities of restoring function, but it required a fair amount of tweaking and personalization as well.
Another big change in perception that I had to accept as part of my new norm is learning to talk about my most personal areas in a matter of fact way.
The cancer conveyor-belt approach to treatment is a very streamlined, well-run system. I’ve been impressed with the expertise, efficiency, and demeanor of all the professionals I have encountered. Everyone—even receptionists—has been helpful and empathetic, especially my own ObGyn, who has hugged me and cried with me and offered to put herself on the line for me and speak out to the media when I had no health insurance. But for most patients post-treatment, we figuratively walk off a cliff and find ourselves in new territory without any network or structure like we experienced during the “war” on our cancer. This new territory is a place of possibility within the health-care field—one I hope is developing now.

Dr. Barbara S. Levy asks: How do we respond? We physicians are so focused on treating or curing disease that we often lose sight of the woman who has the disease.
Katie’s case is much too common. Women are often reluctant to address their sexuality with us—especially when we have been dismissive. We must recognize this important aspect of quality of life and relationships and be prepared to raise the issue with our patients before they begin therapy.
Educating ourselves and then our patients about strategies to reduce the impact of treatment and menopause on sexual function is the first step. Acknowledging and validating their concerns and being able to offer practical steps to preserve healthy sexual function is something, I think, that all ObGyns should be able to do. Strategies to maintain vulvovaginal elasticity include avoidance of soaps and drying chemicals, daily perineal and vaginal stretching—either manually, with a dilator, or via frequent sexual intercourse. And topical lubricants and moisturizing agents should be recommended to maintain vaginal pH and reduce the dryness, itching, and overall dysesthesia.
As the studies highlighted in this Update on Sexual Dysfunction demonstrate, the sexual consequences of radical hysterectomy are significant. Julie also became menopausal as a result of treatment—yet no one prepared her for the symptoms she would experience, and no member of her treatment team helped her understand the likely impact of therapy. Radiation oncologists do address the need for vaginal dilators or daily intercourse to maintain depth and caliber of the vagina during and after therapy, but they are less likely to prepare a patient for menopausal symptoms.
If our patients are to have an optimal experience, we need to provide coordinated, cross-disciplinary care that includes management not only of immediate side effects of treatment but also psychosocial and long-term hormonal and sexual sequelae of therapy. Julie charted her own course with the help of a very dedicated and sensitive partner to successfully overcome the negative effects of radiation, chemotherapy, surgery, and menopause on her sexuality.
Gynecologic cancer disrupts sexual function, over the short term and the long term
Jensen PT, Groenvold M, Klee MC, Thranov I, Petersen MA, Machin D. Early-stage cervical carcinoma, radical hysterectomy, and sexual function: a longitudinal study. Cancer. 2004;100(1):97–106.
Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause. 2011;18(6):662–669.
These two studies explored sexual function after treatment for gynecologic cancer. The investigators found significant disruption of function.
Jensen et al: Radical hysterectomy for cervical Ca damages sexual function significantly
This was a prospective cohort study of 173 women who had early-stage cervical carcinoma and who underwent radical hysterectomy with pelvic lymphadenectomy (all of them node-negative). A validated questionnaire was administered six times, from 5 weeks to 24 months after surgery. An age-matched group of women without cancer was used for comparison. At the 12-month follow-up, patients were asked to report their sexual function at baseline and compare it to their current status. Overall, the women had a higher level of dissatisfaction with their sexual experiences 12 months after surgery than at baseline.
Details of the study
Women in the Jensen study were 23 to 75 years old (median age, 42.7 years), and 93% were sexually active at the time of diagnosis, reporting an average of one to two sexual activities in a week. Forty-six women (25%) were postmenopausal at diagnosis, compared with 34% of the control group. Only 8% of patients were using HT at study entry, compared with 25% of women in the control group. By 12 months after surgery, however, 25% of gynecologic cancer patients were taking systemic HT.
Findings included low libido and other ills
Severe lack of lubrication and low or no sexual desire were reported by cancer patients throughout the first 2 years after surgery. Patients also reported severe problems achieving orgasm as long as 6 months after surgery, as well as reduced vaginal size; both problems rendered their sexual experiences unsatisfactory. Nevertheless, 40% of patients reported at least some sexual activity by 5 weeks after surgery. By 6 months after surgery, there were almost as many sexually active women among the patient group as there were in the control group. However, at 18 months after treatment, patients reported less interest in intimacy—among both themselves and their partners—than among women in the control group. Overall, women treated for cervical cancer had a higher level of dissatisfaction with their sexual experiences 12 months after surgery than they did before diagnosis.
Although 91% of women who were sexually active before surgery resumed intercourse within 12 months, the frequency of sexual activity declined from one to two times per week to three to four times per month. Major long-term changes occurred in regard to libido (interest in sexual relations), arousal (vaginal lubrication), and vaginal size. Although dyspareunia was a significant problem 5 weeks to 3 months after surgery, it resolved within 1 to 2 years.
Jensen and colleagues concluded that radical hysterectomy for treatment of early-stage cervical cancer has significant negative effects on sexual function in the short and long term. They postulated a neurogenic basis for the sexual complaints and discussed both histologic and clinical studies to support this hypothesis.
They also emphasized the need to discuss the risks and management of sexual dysfunction with patients before and after surgery. Better management of the psychosocial consequences of a cancer diagnosis and the physical effects of radical hysterectomy may help avoid the negative experiences that were reported in this study.
Vaz et al: Rate of dyspareunia was high among women treated for endometrial or cervical Ca
Investigators followed 107 women from initial consultation for radiation therapy through 3 years post-treatment. Although a significant percentage (50%) of the cohort was lost to follow-up—many due to death or tumor recurrence—50% of those who remained reported dyspareunia 3 years after radiotherapy.
Women in this study were 21 to 75 years old (median age, 60 years) and had cervical or endometrial carcinoma. Eighty-nine women (83%) received external pelvic radiation as well as brachytherapy. Before beginning radiation therapy, 37.4% of the cohort underwent surgery for treatment of their cancer. Sixty-four percent of the cohort had stage III or IV disease.
At enrollment, 50% of women reported having a life partner, 82% were postmenopausal, and 11.2% were taking HT. However, only 21.5% of women reported sexual activity. The authors opine that this low rate of sexual activity may have been due to recent surgery, bleeding, or pain related to cancer.
All women were offered “standard” interventions for their dyspareunia, including the use of vaginal dilators twice daily for 2 years, as well as the use of vaginal lubricants. Patients who experienced menopausal symptoms—hot flashes, decreased libido, dyspareunia—were referred to a menopause outpatient clinic.
Before treatment, 20% of women reported dyspareunia. Three years later, 44% of patients reported sexual activity, but 50% had dyspareunia. Twenty-one percent reported lower sexual interest relative to baseline, 8% reported vaginal dryness, and 21% reported vasomotor symptoms. Although there was a trend toward increasing sexual activity with decreasing vaginal dryness, the rise in dyspareunia from 20% to 50% over 3 years is troubling.
Radical hysterectomy and radiation therapy to the pelvis cause neurovascular disruption and sexual consequences quite similar to those found after radical prostate surgery. Sexual arousal and orgasm are dependent on both the parasympathetic and sympathetic nerves supplying the pelvis. these nerves are disrupted in Frankenhauser’s plexus during the parametrial dissection of radical hysterectomy and lie clearly within the radiation treatment field.
Dilators and lubricants may be useful in minimizing actual shrinkage of the vagina. However, the elasticity of vaginal tissues, vasodilation during arousal, and transudation across the vaginal wall may all be lost or significantly compromised.
Advise your patients of the potential for sexual side effects of cervical and endometrial cancer treatment before they undergo that therapy. Proactive management of some of the expected problems, such as reduced elasticity and lubrication, as well as treatment for arousal dysfunction (perhaps with the same pharmacotherapeutic agents that provide improvement for many men after radical prostatectomy), may help your patients avoid the distress and disappointment they often experience after successful treatment of their cancers.
When a patient has undergone treatment for cancer,
ask about her sexual function
A symposium on sexual health yields recommendations for your broader care of cancer patients
How likely are you to encounter a cancer survivor in your practice?
Very.
According to the Centers for Disease Control and Prevention (CDC), there were 6.3 million female cancer survivors in the United States as of 2007—and that number has likely increased by a million or more.3 In fact, the number of cancer survivors is expected to double by 2016.
How likely is that cancer survivor to have sexual dysfunction?
Highly.
According to a 2010 survey by the Lance Armstrong Foundation, 46% of cancer survivors report problems with sexual functioning after treatment—and that’s probably a conservative figure, given that 64% of all people with cancer have a malignancy that directly affects sexual organs.2
One more question for you to ponder: How likely is the cancer patient’s sexual dysfunction to go unaddressed?
Extremely.
According to speakers at the Cancer Survivorship and Sexual Health Symposium, held June 17–19, 2011, in Washington, DC, cancer survivors are ill prepared for many of the symptoms of sexual dysfunction that develop after treatment, and many physicians fail to address this dimension of their health.
The symposium, sponsored by the International Society for Sexual Medicine and the Sexual Medicine Society of North America, was organized to address these gaps in care. During the 3-day conference, speakers from oncology, gynecology, mental health, urology, and other specialties presented data and described their experience managing cancer patients. They also offered recommendations for clinicians:
- Talk about it. Address the “highly prevalent but commonly ignored” adverse sexual effects of malignancy and its treatment. Ask: “How has cancer affected your sex life?”
- Try to prevent it. Consider nerve-sparing strategies during radical hysterectomy, radical trachelectomy, and clitoral preservation, which may lead to improved sexual function
- Encourage and support use of dilators. Advise women who have gynecologic cancer to use dilators to maintain vaginal patency, and be aware that compliance is linked to support from a health-care provider
- Encourage sexual activity, which can help preserve function
- Consider local estrogen. When it is appropriate, prescribe vaginal estrogen, which is minimally absorbed, to reduce vaginal symptoms of menopause. (The safety of local estrogen remains in question for women who have breast cancer.)
- Check for problems at each follow-up appointment, and be prepared to explain function and treatment options more than once
- Promote female genital blood flow. For example, it may be appropriate to begin sexual rehabilitation, such as use of vaginal dilators, during treatment
- Consider referral to a sexual rehabilitation program that includes medical and psychological approaches
- Build a network of psychologists, sex therapists, and other professionals who can assist you in managing your patients’ complaints.
“Discomfort around human sexuality is the main reason the issue doesn’t get raised by health-care providers,” said symposium speaker Sharon L. Bober, PhD, of the Dana-Farber Cancer Institute in Boston. “No one wants to initiate the conversation.” Dr. Bober emphasized the importance of asking about sexual function when a cancer survivor presents for care. “A majority of cancer patients in the community don’t hear this question from their providers.”
—Janelle Yates, Senior Editor
We want to hear from you! Tell us what you think.
- Cancer survivors have many complaints not addressed by their physicians
Janelle Yates, Senior Editor (Web Exclusive, July 2011) - How to talk to patients about sex
Barbara S. Levy, MD (Web Audio, September 2010) - Sexual dysfunction: What can you do for your patients?
Barbara S. Levy, MD (Update, September 2010)
Sexual dysfunction is common among women in the United States. One recent study put the prevalence of distressing sexual dysfunction at 22.2%.1 When cancer enters the picture, that percentage rises—dramatically. A 2010 survey from the Lance Armstrong Foundation found that 46% of people affected by cancer report problems with sex after treatment.2
In this article, I highlight three recent studies that explore the sexual effects of cancer and its treatment:
- a prospective cohort study showing that a majority of women treated for breast cancer experience sexual dysfunction afterward
- two longitudinal studies of women affected by gynecologic cancer, which show significant disruption of sexual function in the short and long term.
Sexual function deteriorates in many women after they are treated for breast Ca
Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med. 2011;8(1):294–302.
According to this prospective cohort study from Australia, a majority of women report significant sexual dysfunction after treatment for breast cancer—even when their sexual function was good, and satisfying, at the time of diagnosis (TABLE).
The Health and Wellbeing after Breast Cancer Study enrolled 1,684 Australian women within 12 months of their first diagnosis of invasive breast cancer. Each woman completed a questionnaire at the time of enrollment, and will complete annual follow-up questionnaires for 5 years to assess the impact of invasive breast cancer on physical, psychological, and socioeconomic wellbeing. Embedded within the 12-month questionnaire was the validated Menopause-Specific Quality of Life Questionnaire (MENQOL), which was used in this study to explore the sexual consequences of the diagnosis and treatment of invasive breast cancer.
Of the initial cohort, 1,011 women completed the 12-month questionnaire. These were women younger than 70 years who had a sexual partner and no evidence of active breast cancer. The authors describe the women in this cohort as representative of all women in Victoria, Australia, who have a new diagnosis of invasive breast cancer, in regard to both age (mean, 59 ± 11 years) and the stage of tumor (stage I, 48%; stage II–IV, 52%) at diagnosis.
Of this group, 70% were treated with lumpectomy and radiation therapy, and 30% were treated with mastectomy (2.6% with bilateral mastectomy). Of the women who underwent mastectomy, 9.6% had reconstructive surgery during the first year after diagnosis.
Forty-nine percent of women were treated with tamoxifen, and 28.2% were treated with an aromatase inhibitor.
After breast Ca, women experience low desire and less frequent sexual activity – as well as distress over both outcomes
| Symptom | Yes | No |
|---|---|---|
| Decreased desire | 71.7% | 19.7% |
| Decreased sexual activity | 72.5% | 21.1% |
| Distressed by sexual function | 49.1% | 8.1% |
| Seeking increase in desire | 64.1% | 19.9% |
| Source: Panjari et al. | ||
More than two thirds of women reported sexual dysfunction 12 months after treatment
At baseline, 83% of women described their prediagnosis sexual function as good and satisfying. Twelve months later, 70% reported significant sexual dysfunction, and 77% reported vasomotor symptoms.
Women who reported new-onset sexual dysfunction were more likely to:
- have become menopausal since diagnosis
- experience hot flashes or night sweats
- be treated with an aromatase inhibitor.
There was no association between sexual dysfunction and stage of disease at diagnosis; type of surgery (lumpectomy or mastectomy); breast reconstruction; lymphedema; or axillary dissection.
Vasomotor symptoms in women taking endocrine therapy were associated with sexual dysfunction
Further analysis demonstrated that, among women who experienced vasomotor symptoms, those taking an aromatase inhibitor were more than three times as likely to report sexual dysfunction (odds ratio [OR], 3.49; 95% confidence interval [CI], 1.72–7.09), compared with women who were not on endocrine therapy—and those taking tamoxifen were almost twice as likely to report sexual dysfunction (OR, 1.73; 95% CI, 1.04–2.89). Chemotherapy was not independently related to sexual dysfunction.
In summary: 70% of women who were free of breast cancer 1 year after enrollment reported bothersome sexual consequences of their disease and its treatment; 77% reported vasomotor symptoms. Women who were rendered menopausal and those who experienced vasomotor symptoms while taking an aromatase inhibitor were at high risk of sexual dysfunction.
Be aware of the side effects of breast cancer and its treatment, and not only prepare your patients for the likely consequences but also make yourself knowledgeable about strategies to ameliorate their vaginal dryness and to improve elasticity and arousal for them.
Proactive stretching, use of vaginal dilators and topical oils, and, most important, psychological strategies to help your patients and their partners adjust to the inevitable physical changes will go a long way toward improving their sexual experiences.
Katie* and Julie* tell typical stories of deep dissatisfaction with the health system after their cancer treatment
Katie: “I wasn’t prepared”
When my doctor told me I had locally advanced breast cancer 3 years ago, when I was 50, I wasn’t that surprised by the cancer diagnosis (I have a strong family history of breast cancer)—only by the fact that the tumor had developed so fast since my previous mammogram 15 months earlier. As treatment, I underwent neoadjuvant chemotherapy and bilateral mastectomy (I had the unaffected breast removed as a preventive measure). I also had breast reconstruction and started taking an aromatase inhibitor.
At the time of my diagnosis, I lost all desire for sexual intimacy—no big surprise there. But even after my treatment was over, my desire did not return. Part of the problem was the fact that chemotherapy rendered me menopausal, and the aromatase inhibitor I was taking compounded the menopausal experience. Quite suddenly, I was experiencing hot flashes, vaginal dryness and itching, pain during intercourse, severe bone and joint aches, weight gain (particularly around my abdomen), and general lethargy.
No one in my family had ever mentioned these effects of cancer. And none of my doctors prepared me, either. In fact, when I raised the subject, they seemed genuinely surprised! They offered no remedy other than a recommendation to apply a “moisturizer”—but they gave no details about what kind or how to use it. My oncologist did say that local estrogen would help relieve the pain of intercourse—but then she recommended strongly against it because my cancer was hormone-receptor positive.
My plastic surgeon did a much better job of explaining the effects and outcome of reconstruction than any of my other physicians, including my ObGyn, did of preparing me for menopause and sexual dysfunction.
All of my physicians strike me as caring, sensitive people, but their underlying attitude, as I perceive it, is that I should be grateful to be alive. In their view, it seems, enjoyment of sex is icing on the cake and, quite frankly, I am plenty lucky to have the cake. My oncologist even told me to let her know if I started “feeling better and having fewer hot flashes” so that she could perform ovarian ablation (and start the whole cycle over again). I was struck by how matter of factly she gave this advice, as though quality of life counts for nothing.
Three years into my postcancer life, I can say I have “adjusted” to my problems rather than overcome them. I am still taking an aromatase inhibitor. Sex is still slightly painful; I still struggle with vaginal dryness; and I sometimes feel like an old woman because of my bone and joint stiffness and pain. I did find out about an over-the-counter vaginal suppository, made with vitamin E and coconut oils, from another breast cancer survivor. And I switched from one aromatase inhibitor to another in an attempt to alleviate my achy joints. It helped.
I am grateful for my life—very much so—and for the expertise of my physicians, who helped to save it. But I wish they had prepared me better for the aftermath of cancer treatment. And I wish there were more remedies for women like me, who cannot take hormones.
Julie: “I’m on my own”
It was more than a surprise when my new doctor told me I had cancer. Until then, I had avoided doctors. That attitude can mean a premature death sentence when it comes to cervical cancer. It was a pretty awful realization that I could have avoided the drastic measures it took to save my life if I had just gotten annual Pap smears and exams. I was 39 at the time of diagnosis.
But after all the surgery and chemo and radiation were finished, the message I received was essentially: “OK, you’re good, for now. Just come back every few months for a check-up.”
What about the aftermath of all that treatment? What about the other aspects of the experience? I found that my doctors had very little to offer outside of surgery and drugs and the quick advice to get counseling or some other support services.
“I’m on my own” is what I’ve been telling concerned folks who ask how I’m doing. I am truly grateful for the skill, medicine, and machinery that made the killing of an invasive tumor possible. But I’m on my own when it comes to finding or inventing ways to cope with the new challenges of a pelvic area damaged by radiation and detoxing from the heavy metal—platinum—that was an ingredient in the chemo I received.
My partner and I have had to be persistently creative, careful, delicate, uncritical, and extremely patient with each other to bring about the return of a “normal” sex life. We have been successful, for the most part, but there is also a slightly new definition of what “normal” is for us. Our latest triumph is that we no longer have to use copious amounts of lubricant to engage in intercourse. Sex is no longer painful, as the vaginal tissues have been slowly, patiently engaged on a regularly scheduled basis. Can you imagine sex on a schedule? Neither could I, but that is what we found worked from as early as 1 week postradiation. As it turns out, this was good advice—really, the only advice I got when it came to the practicalities of restoring function, but it required a fair amount of tweaking and personalization as well.
Another big change in perception that I had to accept as part of my new norm is learning to talk about my most personal areas in a matter of fact way.
The cancer conveyor-belt approach to treatment is a very streamlined, well-run system. I’ve been impressed with the expertise, efficiency, and demeanor of all the professionals I have encountered. Everyone—even receptionists—has been helpful and empathetic, especially my own ObGyn, who has hugged me and cried with me and offered to put herself on the line for me and speak out to the media when I had no health insurance. But for most patients post-treatment, we figuratively walk off a cliff and find ourselves in new territory without any network or structure like we experienced during the “war” on our cancer. This new territory is a place of possibility within the health-care field—one I hope is developing now.

Dr. Barbara S. Levy asks: How do we respond? We physicians are so focused on treating or curing disease that we often lose sight of the woman who has the disease.
Katie’s case is much too common. Women are often reluctant to address their sexuality with us—especially when we have been dismissive. We must recognize this important aspect of quality of life and relationships and be prepared to raise the issue with our patients before they begin therapy.
Educating ourselves and then our patients about strategies to reduce the impact of treatment and menopause on sexual function is the first step. Acknowledging and validating their concerns and being able to offer practical steps to preserve healthy sexual function is something, I think, that all ObGyns should be able to do. Strategies to maintain vulvovaginal elasticity include avoidance of soaps and drying chemicals, daily perineal and vaginal stretching—either manually, with a dilator, or via frequent sexual intercourse. And topical lubricants and moisturizing agents should be recommended to maintain vaginal pH and reduce the dryness, itching, and overall dysesthesia.
As the studies highlighted in this Update on Sexual Dysfunction demonstrate, the sexual consequences of radical hysterectomy are significant. Julie also became menopausal as a result of treatment—yet no one prepared her for the symptoms she would experience, and no member of her treatment team helped her understand the likely impact of therapy. Radiation oncologists do address the need for vaginal dilators or daily intercourse to maintain depth and caliber of the vagina during and after therapy, but they are less likely to prepare a patient for menopausal symptoms.
If our patients are to have an optimal experience, we need to provide coordinated, cross-disciplinary care that includes management not only of immediate side effects of treatment but also psychosocial and long-term hormonal and sexual sequelae of therapy. Julie charted her own course with the help of a very dedicated and sensitive partner to successfully overcome the negative effects of radiation, chemotherapy, surgery, and menopause on her sexuality.
Gynecologic cancer disrupts sexual function, over the short term and the long term
Jensen PT, Groenvold M, Klee MC, Thranov I, Petersen MA, Machin D. Early-stage cervical carcinoma, radical hysterectomy, and sexual function: a longitudinal study. Cancer. 2004;100(1):97–106.
Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause. 2011;18(6):662–669.
These two studies explored sexual function after treatment for gynecologic cancer. The investigators found significant disruption of function.
Jensen et al: Radical hysterectomy for cervical Ca damages sexual function significantly
This was a prospective cohort study of 173 women who had early-stage cervical carcinoma and who underwent radical hysterectomy with pelvic lymphadenectomy (all of them node-negative). A validated questionnaire was administered six times, from 5 weeks to 24 months after surgery. An age-matched group of women without cancer was used for comparison. At the 12-month follow-up, patients were asked to report their sexual function at baseline and compare it to their current status. Overall, the women had a higher level of dissatisfaction with their sexual experiences 12 months after surgery than at baseline.
Details of the study
Women in the Jensen study were 23 to 75 years old (median age, 42.7 years), and 93% were sexually active at the time of diagnosis, reporting an average of one to two sexual activities in a week. Forty-six women (25%) were postmenopausal at diagnosis, compared with 34% of the control group. Only 8% of patients were using HT at study entry, compared with 25% of women in the control group. By 12 months after surgery, however, 25% of gynecologic cancer patients were taking systemic HT.
Findings included low libido and other ills
Severe lack of lubrication and low or no sexual desire were reported by cancer patients throughout the first 2 years after surgery. Patients also reported severe problems achieving orgasm as long as 6 months after surgery, as well as reduced vaginal size; both problems rendered their sexual experiences unsatisfactory. Nevertheless, 40% of patients reported at least some sexual activity by 5 weeks after surgery. By 6 months after surgery, there were almost as many sexually active women among the patient group as there were in the control group. However, at 18 months after treatment, patients reported less interest in intimacy—among both themselves and their partners—than among women in the control group. Overall, women treated for cervical cancer had a higher level of dissatisfaction with their sexual experiences 12 months after surgery than they did before diagnosis.
Although 91% of women who were sexually active before surgery resumed intercourse within 12 months, the frequency of sexual activity declined from one to two times per week to three to four times per month. Major long-term changes occurred in regard to libido (interest in sexual relations), arousal (vaginal lubrication), and vaginal size. Although dyspareunia was a significant problem 5 weeks to 3 months after surgery, it resolved within 1 to 2 years.
Jensen and colleagues concluded that radical hysterectomy for treatment of early-stage cervical cancer has significant negative effects on sexual function in the short and long term. They postulated a neurogenic basis for the sexual complaints and discussed both histologic and clinical studies to support this hypothesis.
They also emphasized the need to discuss the risks and management of sexual dysfunction with patients before and after surgery. Better management of the psychosocial consequences of a cancer diagnosis and the physical effects of radical hysterectomy may help avoid the negative experiences that were reported in this study.
Vaz et al: Rate of dyspareunia was high among women treated for endometrial or cervical Ca
Investigators followed 107 women from initial consultation for radiation therapy through 3 years post-treatment. Although a significant percentage (50%) of the cohort was lost to follow-up—many due to death or tumor recurrence—50% of those who remained reported dyspareunia 3 years after radiotherapy.
Women in this study were 21 to 75 years old (median age, 60 years) and had cervical or endometrial carcinoma. Eighty-nine women (83%) received external pelvic radiation as well as brachytherapy. Before beginning radiation therapy, 37.4% of the cohort underwent surgery for treatment of their cancer. Sixty-four percent of the cohort had stage III or IV disease.
At enrollment, 50% of women reported having a life partner, 82% were postmenopausal, and 11.2% were taking HT. However, only 21.5% of women reported sexual activity. The authors opine that this low rate of sexual activity may have been due to recent surgery, bleeding, or pain related to cancer.
All women were offered “standard” interventions for their dyspareunia, including the use of vaginal dilators twice daily for 2 years, as well as the use of vaginal lubricants. Patients who experienced menopausal symptoms—hot flashes, decreased libido, dyspareunia—were referred to a menopause outpatient clinic.
Before treatment, 20% of women reported dyspareunia. Three years later, 44% of patients reported sexual activity, but 50% had dyspareunia. Twenty-one percent reported lower sexual interest relative to baseline, 8% reported vaginal dryness, and 21% reported vasomotor symptoms. Although there was a trend toward increasing sexual activity with decreasing vaginal dryness, the rise in dyspareunia from 20% to 50% over 3 years is troubling.
Radical hysterectomy and radiation therapy to the pelvis cause neurovascular disruption and sexual consequences quite similar to those found after radical prostate surgery. Sexual arousal and orgasm are dependent on both the parasympathetic and sympathetic nerves supplying the pelvis. these nerves are disrupted in Frankenhauser’s plexus during the parametrial dissection of radical hysterectomy and lie clearly within the radiation treatment field.
Dilators and lubricants may be useful in minimizing actual shrinkage of the vagina. However, the elasticity of vaginal tissues, vasodilation during arousal, and transudation across the vaginal wall may all be lost or significantly compromised.
Advise your patients of the potential for sexual side effects of cervical and endometrial cancer treatment before they undergo that therapy. Proactive management of some of the expected problems, such as reduced elasticity and lubrication, as well as treatment for arousal dysfunction (perhaps with the same pharmacotherapeutic agents that provide improvement for many men after radical prostatectomy), may help your patients avoid the distress and disappointment they often experience after successful treatment of their cancers.
When a patient has undergone treatment for cancer,
ask about her sexual function
A symposium on sexual health yields recommendations for your broader care of cancer patients
How likely are you to encounter a cancer survivor in your practice?
Very.
According to the Centers for Disease Control and Prevention (CDC), there were 6.3 million female cancer survivors in the United States as of 2007—and that number has likely increased by a million or more.3 In fact, the number of cancer survivors is expected to double by 2016.
How likely is that cancer survivor to have sexual dysfunction?
Highly.
According to a 2010 survey by the Lance Armstrong Foundation, 46% of cancer survivors report problems with sexual functioning after treatment—and that’s probably a conservative figure, given that 64% of all people with cancer have a malignancy that directly affects sexual organs.2
One more question for you to ponder: How likely is the cancer patient’s sexual dysfunction to go unaddressed?
Extremely.
According to speakers at the Cancer Survivorship and Sexual Health Symposium, held June 17–19, 2011, in Washington, DC, cancer survivors are ill prepared for many of the symptoms of sexual dysfunction that develop after treatment, and many physicians fail to address this dimension of their health.
The symposium, sponsored by the International Society for Sexual Medicine and the Sexual Medicine Society of North America, was organized to address these gaps in care. During the 3-day conference, speakers from oncology, gynecology, mental health, urology, and other specialties presented data and described their experience managing cancer patients. They also offered recommendations for clinicians:
- Talk about it. Address the “highly prevalent but commonly ignored” adverse sexual effects of malignancy and its treatment. Ask: “How has cancer affected your sex life?”
- Try to prevent it. Consider nerve-sparing strategies during radical hysterectomy, radical trachelectomy, and clitoral preservation, which may lead to improved sexual function
- Encourage and support use of dilators. Advise women who have gynecologic cancer to use dilators to maintain vaginal patency, and be aware that compliance is linked to support from a health-care provider
- Encourage sexual activity, which can help preserve function
- Consider local estrogen. When it is appropriate, prescribe vaginal estrogen, which is minimally absorbed, to reduce vaginal symptoms of menopause. (The safety of local estrogen remains in question for women who have breast cancer.)
- Check for problems at each follow-up appointment, and be prepared to explain function and treatment options more than once
- Promote female genital blood flow. For example, it may be appropriate to begin sexual rehabilitation, such as use of vaginal dilators, during treatment
- Consider referral to a sexual rehabilitation program that includes medical and psychological approaches
- Build a network of psychologists, sex therapists, and other professionals who can assist you in managing your patients’ complaints.
“Discomfort around human sexuality is the main reason the issue doesn’t get raised by health-care providers,” said symposium speaker Sharon L. Bober, PhD, of the Dana-Farber Cancer Institute in Boston. “No one wants to initiate the conversation.” Dr. Bober emphasized the importance of asking about sexual function when a cancer survivor presents for care. “A majority of cancer patients in the community don’t hear this question from their providers.”
—Janelle Yates, Senior Editor
We want to hear from you! Tell us what you think.
1. Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970-978.
2. Rechis R, Boerner L. How cancer has affected post-treatment survivors: a LIVESTRONG report. Austin, Tex: Lance Armstrong Foundation 2011;13-
3. Centers for Disease Control and Prevention. Cancer survivors—United States, 2007. MMWR. 2011;60(9):269-272
1. Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970-978.
2. Rechis R, Boerner L. How cancer has affected post-treatment survivors: a LIVESTRONG report. Austin, Tex: Lance Armstrong Foundation 2011;13-
3. Centers for Disease Control and Prevention. Cancer survivors—United States, 2007. MMWR. 2011;60(9):269-272
Is the annual pelvic exam a relic or a requisite?
The annual pelvic examination has been in the spotlight lately. After a commentary questioning its value was published early this year in the Journal of Women’s Health, the Wall Street Journal highlighted the controversy in its own high-profile article.1,2
At issue is a simple question: Does the pelvic examination deter women from seeking gynecologic care?
Some argue that the exam is so unpleasant that many women avoid their doctor’s office rather than undergo it. Here is the Wall Street Journal’s take on the issue. It’s the first line of its article on the subject:
Of all the indignities that women endure in their lives, one of the most dreaded is the routine pelvic exam.
The authors of the original commentary in the Journal of Women’s Health are a bit more circumspect. Carolyn L. Westhoff, MD, MSc, and colleagues discuss the reasons that a pelvic examination is traditionally performed—to detect sexually transmitted disease, clear a woman for contraception, and screen for cancer—and present evidence suggesting that these indications for the pelvic exam are no longer absolute in asymptomatic women.1
For example, Chlamydia can be identified in a culture obtained from urine or on vaginal swabs collected by the patient herself. Birth control pills can be prescribed without examining the patient as long as she is in good health and reports no problems. And evidence suggests that the pelvic exam—including bimanual palpation—does not increase the detection of ovarian cancer in asymptomatic women.
And now that ACOG has raised the minimum age for cervical cancer screening to 21 years and extended intervals between Pap tests for women who have normal results, there is no reason to examine the cervix every year, Dr. Westhoff and colleagues argue. In young women, annual inspection is unnecessary because cervical dysplasia tends to be transient, and, in older women, cervical inspection is unnecessary because a simple human papillomavirus (HPV) test can yield just as much information as a Pap smear and can be conducted on self-collected vaginal swabs.
So the question remains: Is the annual pelvic exam really required?
I would argue that the answer is “Yes.”
The pelvic examination offers many benefits
I find the pelvic exam to be indispensable in the assessment of the vulva, vagina, pelvic floor, and sexual function—and it yields information I often cannot obtain in any other way. For example, some vulvar lesions produce no symptoms but still pose a risk of cancer or represent a developing problem such as lichen sclerosis, but I cannot identify them unless I see them.
The physical examination of tissue also prompts me to ask focused questions, frequently about things the patient is too embarrassed to bring up herself. For example, I may examine a woman and find a cystocele or urethrocele. If she hasn’t mentioned leaking urine or other difficulties, the discovery prompts me to ask more specifically about these symptoms. When I do, I often uncover a significant source of distress that, for whatever reason, the patient did not report herself.
Other examples: On occasion, during the pelvic examination, I discover vaginismus. That finding prompts me to ask about painful sex. And sometimes a perimenopausal woman has dry vaginal tissue that is not bothersome…yet. By identifying this condition early, I can suggest interventions that prevent the dryness from becoming bothersome.
Validation is another benefit of the pelvic exam. For example, some younger women think they “look weird” or have something wrong with their body, and my reassurance that everything is totally normal can greatly ease their mind.

The power of ritual
In a New York Times article about the need to focus on the actual patient rather than simply the data in her chart, Abraham Verghese, MD, relates the story of an elderly patient whose daughter was resistant to his recommendations until she watched him perform a comprehensive physical exam.3 The medical team discussed the case afterward and concluded that “the daughter witnessing the examination of the patient, that ritual, was the key to earning both their trusts.”3
“I find that patients from almost any culture have deep expectations of a ritual when a doctor sees them,” Dr. Verghese writes, “and they are quick to perceive when he or she gives those procedures short shrift by, say, placing the stethoscope on top of the gown instead of the skin, doing a cursory prod of the belly and wrapping up in 30 seconds.”3
I agree. Patients can be very resistant to our recommendations if they feel they have gotten substandard evaluation. And part of their expectation of evaluation is a physical examination. There is a sense of connection and closeness that patients have come to count on. And they can tell when we are not placing enough emphasis on this aspect of care.
Another argument in favor of the pelvic exam: I see at least one patient every day who is referred to me by her primary care doctor and who has not, over the course of her care for the current complaint, had to take off her clothes for a comprehensive examination. She typically is sent to me because of a finding on magnetic resonance imaging (MRI) or computed tomography (CT). Not uncommonly, women are convinced they have cancer based on these incidental findings. Yet those findings usually turn out to be a perfectly benign entity of little consequence, such as a benign fibroid tumor of the uterus. The power of the physical exam is that it can document things such as fibroids before they turn up on an MRI or CT scan. That is much less stressful for the patient than having a mysterious lesion identified on imaging, necessitating referral to a specialist to identify the problem.
No barriers to care
I don’t think women like the pelvic exam any more than they like mammograms or being physically vulnerable in any way. In my experience, however, patients tend to experience more trepidation about stepping onto the scale than about undergoing a pelvic exam.
Am I going to create a barrier to a patient’s care by insisting on weighing her or on performing a pelvic exam after she voices her desire to avoid these things?
Of course not.
Certainly, if a patient declines either of these options, I honor her wishes. However, I make it a point to have a conversation with her about their intrinsic value.
I try to teach my patients that they are the owners of their bodies, and that everything that occurs in the doctor-patient relationship is collaborative. In my practice, women always have the option to say “No.”
I do agree with Dr. Westhoff and colleagues that some women are turned off by the prospect of a pelvic exam. Adolescents, in particular, are often apprehensive about it. When I see a teenager for her first gynecologic visit, I usually focus on discussion. I try to get to know her and attempt to give her a sense of control over her own body. When it comes to the pelvic exam, I give her a choice—and I honor her decision.
Other patients who may be unwilling to undergo an annual pelvic exam include women who have a history of sexual abuse or molestation. Again, I respect their wishes, but I do talk to them about the value of the physical.
Unlike Dr. Westhoff and colleagues, however, I don’t believe that “no annual pelvic exam” should be our default position. And although there are other components of gynecologic care to see to besides the pelvic exam, I don’t think the exam should be dispensed with in the interest of saving time. In actuality, the pelvic exam is probably the least time-consuming aspect of gynecologic care for an asymptomatic patient.
In my view, the purpose of the annual visit is to identify risks and manage them—and some risks may be overlooked if I don’t have the opportunity to examine the patient. At the annual well woman visit, I have a conversation with the patient about the risks she faces over the next year, depending on her age and overall condition, and I devote quite a bit of time to this discussion. But I don’t do it at the expense of a careful pelvic exam.
Instead, I honor the ritual and the critical information it yields.
INSTANT POLL: A patient arrives for her annual well-woman examination. What do you do?
To read the entire question, enter your response, and see how others have answered, click on the blue title.
We want to hear from you! Tell us what you think.
1. Westhoff CL, Jones HE, Guiahi M. Do new guidelines and technology make the routine pelvic examination obsolete? J Women’s Health. 2011;20(1):5-10.
2. Beck M. Questioning the need for routine pelvic exam. Wall Street Journal. February 15 2011. http://online.wsj.com/article/SB10001424052748703584804576144161119909004.html. Accessed March 10, 2011.
3. Verghese A. Treat the patient not the CT scan. New York Times. February 26, 2011.http://www.nytimes.com/2011/02/27/opinion/27verghese.html. Accessed March 14, 2011.
The annual pelvic examination has been in the spotlight lately. After a commentary questioning its value was published early this year in the Journal of Women’s Health, the Wall Street Journal highlighted the controversy in its own high-profile article.1,2
At issue is a simple question: Does the pelvic examination deter women from seeking gynecologic care?
Some argue that the exam is so unpleasant that many women avoid their doctor’s office rather than undergo it. Here is the Wall Street Journal’s take on the issue. It’s the first line of its article on the subject:
Of all the indignities that women endure in their lives, one of the most dreaded is the routine pelvic exam.
The authors of the original commentary in the Journal of Women’s Health are a bit more circumspect. Carolyn L. Westhoff, MD, MSc, and colleagues discuss the reasons that a pelvic examination is traditionally performed—to detect sexually transmitted disease, clear a woman for contraception, and screen for cancer—and present evidence suggesting that these indications for the pelvic exam are no longer absolute in asymptomatic women.1
For example, Chlamydia can be identified in a culture obtained from urine or on vaginal swabs collected by the patient herself. Birth control pills can be prescribed without examining the patient as long as she is in good health and reports no problems. And evidence suggests that the pelvic exam—including bimanual palpation—does not increase the detection of ovarian cancer in asymptomatic women.
And now that ACOG has raised the minimum age for cervical cancer screening to 21 years and extended intervals between Pap tests for women who have normal results, there is no reason to examine the cervix every year, Dr. Westhoff and colleagues argue. In young women, annual inspection is unnecessary because cervical dysplasia tends to be transient, and, in older women, cervical inspection is unnecessary because a simple human papillomavirus (HPV) test can yield just as much information as a Pap smear and can be conducted on self-collected vaginal swabs.
So the question remains: Is the annual pelvic exam really required?
I would argue that the answer is “Yes.”
The pelvic examination offers many benefits
I find the pelvic exam to be indispensable in the assessment of the vulva, vagina, pelvic floor, and sexual function—and it yields information I often cannot obtain in any other way. For example, some vulvar lesions produce no symptoms but still pose a risk of cancer or represent a developing problem such as lichen sclerosis, but I cannot identify them unless I see them.
The physical examination of tissue also prompts me to ask focused questions, frequently about things the patient is too embarrassed to bring up herself. For example, I may examine a woman and find a cystocele or urethrocele. If she hasn’t mentioned leaking urine or other difficulties, the discovery prompts me to ask more specifically about these symptoms. When I do, I often uncover a significant source of distress that, for whatever reason, the patient did not report herself.
Other examples: On occasion, during the pelvic examination, I discover vaginismus. That finding prompts me to ask about painful sex. And sometimes a perimenopausal woman has dry vaginal tissue that is not bothersome…yet. By identifying this condition early, I can suggest interventions that prevent the dryness from becoming bothersome.
Validation is another benefit of the pelvic exam. For example, some younger women think they “look weird” or have something wrong with their body, and my reassurance that everything is totally normal can greatly ease their mind.

The power of ritual
In a New York Times article about the need to focus on the actual patient rather than simply the data in her chart, Abraham Verghese, MD, relates the story of an elderly patient whose daughter was resistant to his recommendations until she watched him perform a comprehensive physical exam.3 The medical team discussed the case afterward and concluded that “the daughter witnessing the examination of the patient, that ritual, was the key to earning both their trusts.”3
“I find that patients from almost any culture have deep expectations of a ritual when a doctor sees them,” Dr. Verghese writes, “and they are quick to perceive when he or she gives those procedures short shrift by, say, placing the stethoscope on top of the gown instead of the skin, doing a cursory prod of the belly and wrapping up in 30 seconds.”3
I agree. Patients can be very resistant to our recommendations if they feel they have gotten substandard evaluation. And part of their expectation of evaluation is a physical examination. There is a sense of connection and closeness that patients have come to count on. And they can tell when we are not placing enough emphasis on this aspect of care.
Another argument in favor of the pelvic exam: I see at least one patient every day who is referred to me by her primary care doctor and who has not, over the course of her care for the current complaint, had to take off her clothes for a comprehensive examination. She typically is sent to me because of a finding on magnetic resonance imaging (MRI) or computed tomography (CT). Not uncommonly, women are convinced they have cancer based on these incidental findings. Yet those findings usually turn out to be a perfectly benign entity of little consequence, such as a benign fibroid tumor of the uterus. The power of the physical exam is that it can document things such as fibroids before they turn up on an MRI or CT scan. That is much less stressful for the patient than having a mysterious lesion identified on imaging, necessitating referral to a specialist to identify the problem.
No barriers to care
I don’t think women like the pelvic exam any more than they like mammograms or being physically vulnerable in any way. In my experience, however, patients tend to experience more trepidation about stepping onto the scale than about undergoing a pelvic exam.
Am I going to create a barrier to a patient’s care by insisting on weighing her or on performing a pelvic exam after she voices her desire to avoid these things?
Of course not.
Certainly, if a patient declines either of these options, I honor her wishes. However, I make it a point to have a conversation with her about their intrinsic value.
I try to teach my patients that they are the owners of their bodies, and that everything that occurs in the doctor-patient relationship is collaborative. In my practice, women always have the option to say “No.”
I do agree with Dr. Westhoff and colleagues that some women are turned off by the prospect of a pelvic exam. Adolescents, in particular, are often apprehensive about it. When I see a teenager for her first gynecologic visit, I usually focus on discussion. I try to get to know her and attempt to give her a sense of control over her own body. When it comes to the pelvic exam, I give her a choice—and I honor her decision.
Other patients who may be unwilling to undergo an annual pelvic exam include women who have a history of sexual abuse or molestation. Again, I respect their wishes, but I do talk to them about the value of the physical.
Unlike Dr. Westhoff and colleagues, however, I don’t believe that “no annual pelvic exam” should be our default position. And although there are other components of gynecologic care to see to besides the pelvic exam, I don’t think the exam should be dispensed with in the interest of saving time. In actuality, the pelvic exam is probably the least time-consuming aspect of gynecologic care for an asymptomatic patient.
In my view, the purpose of the annual visit is to identify risks and manage them—and some risks may be overlooked if I don’t have the opportunity to examine the patient. At the annual well woman visit, I have a conversation with the patient about the risks she faces over the next year, depending on her age and overall condition, and I devote quite a bit of time to this discussion. But I don’t do it at the expense of a careful pelvic exam.
Instead, I honor the ritual and the critical information it yields.
INSTANT POLL: A patient arrives for her annual well-woman examination. What do you do?
To read the entire question, enter your response, and see how others have answered, click on the blue title.
We want to hear from you! Tell us what you think.
The annual pelvic examination has been in the spotlight lately. After a commentary questioning its value was published early this year in the Journal of Women’s Health, the Wall Street Journal highlighted the controversy in its own high-profile article.1,2
At issue is a simple question: Does the pelvic examination deter women from seeking gynecologic care?
Some argue that the exam is so unpleasant that many women avoid their doctor’s office rather than undergo it. Here is the Wall Street Journal’s take on the issue. It’s the first line of its article on the subject:
Of all the indignities that women endure in their lives, one of the most dreaded is the routine pelvic exam.
The authors of the original commentary in the Journal of Women’s Health are a bit more circumspect. Carolyn L. Westhoff, MD, MSc, and colleagues discuss the reasons that a pelvic examination is traditionally performed—to detect sexually transmitted disease, clear a woman for contraception, and screen for cancer—and present evidence suggesting that these indications for the pelvic exam are no longer absolute in asymptomatic women.1
For example, Chlamydia can be identified in a culture obtained from urine or on vaginal swabs collected by the patient herself. Birth control pills can be prescribed without examining the patient as long as she is in good health and reports no problems. And evidence suggests that the pelvic exam—including bimanual palpation—does not increase the detection of ovarian cancer in asymptomatic women.
And now that ACOG has raised the minimum age for cervical cancer screening to 21 years and extended intervals between Pap tests for women who have normal results, there is no reason to examine the cervix every year, Dr. Westhoff and colleagues argue. In young women, annual inspection is unnecessary because cervical dysplasia tends to be transient, and, in older women, cervical inspection is unnecessary because a simple human papillomavirus (HPV) test can yield just as much information as a Pap smear and can be conducted on self-collected vaginal swabs.
So the question remains: Is the annual pelvic exam really required?
I would argue that the answer is “Yes.”
The pelvic examination offers many benefits
I find the pelvic exam to be indispensable in the assessment of the vulva, vagina, pelvic floor, and sexual function—and it yields information I often cannot obtain in any other way. For example, some vulvar lesions produce no symptoms but still pose a risk of cancer or represent a developing problem such as lichen sclerosis, but I cannot identify them unless I see them.
The physical examination of tissue also prompts me to ask focused questions, frequently about things the patient is too embarrassed to bring up herself. For example, I may examine a woman and find a cystocele or urethrocele. If she hasn’t mentioned leaking urine or other difficulties, the discovery prompts me to ask more specifically about these symptoms. When I do, I often uncover a significant source of distress that, for whatever reason, the patient did not report herself.
Other examples: On occasion, during the pelvic examination, I discover vaginismus. That finding prompts me to ask about painful sex. And sometimes a perimenopausal woman has dry vaginal tissue that is not bothersome…yet. By identifying this condition early, I can suggest interventions that prevent the dryness from becoming bothersome.
Validation is another benefit of the pelvic exam. For example, some younger women think they “look weird” or have something wrong with their body, and my reassurance that everything is totally normal can greatly ease their mind.

The power of ritual
In a New York Times article about the need to focus on the actual patient rather than simply the data in her chart, Abraham Verghese, MD, relates the story of an elderly patient whose daughter was resistant to his recommendations until she watched him perform a comprehensive physical exam.3 The medical team discussed the case afterward and concluded that “the daughter witnessing the examination of the patient, that ritual, was the key to earning both their trusts.”3
“I find that patients from almost any culture have deep expectations of a ritual when a doctor sees them,” Dr. Verghese writes, “and they are quick to perceive when he or she gives those procedures short shrift by, say, placing the stethoscope on top of the gown instead of the skin, doing a cursory prod of the belly and wrapping up in 30 seconds.”3
I agree. Patients can be very resistant to our recommendations if they feel they have gotten substandard evaluation. And part of their expectation of evaluation is a physical examination. There is a sense of connection and closeness that patients have come to count on. And they can tell when we are not placing enough emphasis on this aspect of care.
Another argument in favor of the pelvic exam: I see at least one patient every day who is referred to me by her primary care doctor and who has not, over the course of her care for the current complaint, had to take off her clothes for a comprehensive examination. She typically is sent to me because of a finding on magnetic resonance imaging (MRI) or computed tomography (CT). Not uncommonly, women are convinced they have cancer based on these incidental findings. Yet those findings usually turn out to be a perfectly benign entity of little consequence, such as a benign fibroid tumor of the uterus. The power of the physical exam is that it can document things such as fibroids before they turn up on an MRI or CT scan. That is much less stressful for the patient than having a mysterious lesion identified on imaging, necessitating referral to a specialist to identify the problem.
No barriers to care
I don’t think women like the pelvic exam any more than they like mammograms or being physically vulnerable in any way. In my experience, however, patients tend to experience more trepidation about stepping onto the scale than about undergoing a pelvic exam.
Am I going to create a barrier to a patient’s care by insisting on weighing her or on performing a pelvic exam after she voices her desire to avoid these things?
Of course not.
Certainly, if a patient declines either of these options, I honor her wishes. However, I make it a point to have a conversation with her about their intrinsic value.
I try to teach my patients that they are the owners of their bodies, and that everything that occurs in the doctor-patient relationship is collaborative. In my practice, women always have the option to say “No.”
I do agree with Dr. Westhoff and colleagues that some women are turned off by the prospect of a pelvic exam. Adolescents, in particular, are often apprehensive about it. When I see a teenager for her first gynecologic visit, I usually focus on discussion. I try to get to know her and attempt to give her a sense of control over her own body. When it comes to the pelvic exam, I give her a choice—and I honor her decision.
Other patients who may be unwilling to undergo an annual pelvic exam include women who have a history of sexual abuse or molestation. Again, I respect their wishes, but I do talk to them about the value of the physical.
Unlike Dr. Westhoff and colleagues, however, I don’t believe that “no annual pelvic exam” should be our default position. And although there are other components of gynecologic care to see to besides the pelvic exam, I don’t think the exam should be dispensed with in the interest of saving time. In actuality, the pelvic exam is probably the least time-consuming aspect of gynecologic care for an asymptomatic patient.
In my view, the purpose of the annual visit is to identify risks and manage them—and some risks may be overlooked if I don’t have the opportunity to examine the patient. At the annual well woman visit, I have a conversation with the patient about the risks she faces over the next year, depending on her age and overall condition, and I devote quite a bit of time to this discussion. But I don’t do it at the expense of a careful pelvic exam.
Instead, I honor the ritual and the critical information it yields.
INSTANT POLL: A patient arrives for her annual well-woman examination. What do you do?
To read the entire question, enter your response, and see how others have answered, click on the blue title.
We want to hear from you! Tell us what you think.
1. Westhoff CL, Jones HE, Guiahi M. Do new guidelines and technology make the routine pelvic examination obsolete? J Women’s Health. 2011;20(1):5-10.
2. Beck M. Questioning the need for routine pelvic exam. Wall Street Journal. February 15 2011. http://online.wsj.com/article/SB10001424052748703584804576144161119909004.html. Accessed March 10, 2011.
3. Verghese A. Treat the patient not the CT scan. New York Times. February 26, 2011.http://www.nytimes.com/2011/02/27/opinion/27verghese.html. Accessed March 14, 2011.
1. Westhoff CL, Jones HE, Guiahi M. Do new guidelines and technology make the routine pelvic examination obsolete? J Women’s Health. 2011;20(1):5-10.
2. Beck M. Questioning the need for routine pelvic exam. Wall Street Journal. February 15 2011. http://online.wsj.com/article/SB10001424052748703584804576144161119909004.html. Accessed March 10, 2011.
3. Verghese A. Treat the patient not the CT scan. New York Times. February 26, 2011.http://www.nytimes.com/2011/02/27/opinion/27verghese.html. Accessed March 14, 2011.
UPDATE ON TECHNOLOGY: VESSEL-SEALING DEVICES
When Harry Reich performed the first laparoscopically assisted vaginal hysterectomy in 1989, he advocated the use of sutures for control of the uterine vessels. Monopolar, bipolar, and laser instruments available at that time were inherently risky to use along the pelvic sidewall because of their potential for 1) considerable thermal energy spread beyond the area of treatment (bipolar, NG:YAG laser, monopolar) and 2) unreliable hemostasis (CO2 laser).
Minimally invasive surgical practice has driven meaningful advances in instrumentation and technique over the past 20 years. The constraints inherent in laparoscopic surgery, although somewhat mitigated by robotics, have generated a proliferation of technologies to obtain reliable hemostasis. Every device now on the market claims to “seal” vessels. In this article, I review the mechanism of action of these instruments and compare their strengths and weaknesses, based on high-quality scientific evidence.
Two studies highlight vessel ligation
Newcomb WL, Hope WW, Schmeizer TM, et al. Comparison of blood vessel sealing among new electrosurgical and ultrasonic devices. Surg Endosc. 2009;23:90–96.
Lamberton GR, Hsi RS, Jin DH, et al. Prospective comparison of four laparoscopic vessel ligation devices. J Endourol. 2008;22:2307–2312.
Many energy-delivery systems are available for the gynecologic surgeon; any of them can be used effectively and safely under most circumstances. Before we can make an informed choice about which system is best for our own practice, however, we need to be aware of the strengths and limitations of the systems overall ( TABLE ).
These two studies focus on the following devices:
- Gyrus PK Tissue Management System, PKS Cutting Forceps, and Plasma Trissector (all from Gyrus Medical). These are bipolar electrosurgical devices designed to deliver high current and very low voltage to tissue. Tissue impedance is continuously monitored between the jaws of the instrument, and energy delivery is adjusted accordingly. These systems deliver electrosurgical energy through a series of rapid pulses, thereby allowing the tissue to cool briefly and limiting the heating of adjacent tissue. Protein in the vessel walls is denatured and forms a coagulum, which occludes the lumen.
- Harmonic Scalpel (Ethicon EndoSurgery). This device uses a high-frequency ultrasonic transducer (55,000 cycles/second) to create mechanical vibration of one of the two jaws. The device can be used to vaporize tissue (cut) or achieve hemostasis by coagulation. As with the other devices, protein is denatured and vessels are occluded by formation of a coagulum.
- Ligamax 5 Endoscopic Multiple Clip Applier (Ethicon). This device is a sterile, single-patient-use, 5-mm, endoscopic, multiple-clip applicator that delivers 15 medium or large titanium clips that close to 8.8 mm, the same clip size as the 10-mm applicator. Ligamax 5 has long, thin angled jaws (8.4 mm) that extend beyond vessels and ducts to improve visibility. It includes a long, 33-cm shaft for additional reach, and an anti-clip drop-ratchet mechanism for control over clip closure.
- EnSeal Tissue Sealing and Hemostasis System and EnSeal PTC (SurgRx). EnSeal utilizes nanotechnology to control the energy at the electrode–tissue interface. The jaws contain a temperature-sensitive matrix with embedded conductive carbon spherules designed to “sense” tissue characteristics. It uses extremely high jaw compression to create uniform tissue effects. It does not require a dedicated electrosurgical unit for use; an adapter can be purchased that permits use with most generators.
- LigaSure V (Valleylab). LigaSure is a bipolar electrosurgical device designed to deliver high current and very low voltage to tissue. It monitors tissue impedance between the jaws of the instrument and continuously adjusts the delivery of energy.
TABLE
Rating vessel-sealing devices: 5 measures of success
| Device | Safety: Minimal thermal spread | Reliability: Efficacy on vessels ≤7 mm | Efficiency: Treatment time | Consistency: Independent of user | Utility: Multiple uses |
|---|---|---|---|---|---|
| Harmonic Scalpel | Excellent | Poor | Excellent | Poor | Excellent |
| Gyrus PK | Poor | Poor | Excellent | Fair | Fair |
| LigaSure V | Good | Excellent | Good | Excellent | Fair |
| EnSeal | Fair | Excellent | Poor | Excellent | Poor |
| These ratings were devised by the author based on data from independent studies in living tissue models. | |||||
FIGURE How energy-based vessel-sealing works
(A) When the carotid artery of an animal model was sealed using a standard bipolar forceps, the lumen remained open and a proximal thrombus developed. (B) When LigaSure was used, the artery was fused and the lumen obliterated with one 5-second application. (C and D) When a renal artery was sealed with LigaSure, the lumen of the vessel walls fused completely.
What the studies found
Newcomb and associates compared blood-vessel-sealing ability of the following devices:
- Gyrus 5-mm PKS Cutting Forceps
- Gyrus Plasma Trissector
- Harmonic Scalpel
- EnSeal Tissue Sealing and Hemostasis System
- LigaSure V, using the LigaSure Generator (Valleylab)
- LigaSure V, using the Force Triad Generator (Valleylab)
- Ligamax 5 Endoscopic Multiple Clip Applier.
The authors assessed mean seal times and burst pressures. Used on medium and large vessels, the Harmonic Scalpel and Gyrus products had significant failure rates: 8% to 22% for the Harmonic Scalpel and 41% to 92% for the pulsed, bipolar Gyrus systems.
The shortest sealing times for medium to large vessels were achieved with the LigaSure V using the Force Triad Generator. The Gyrus systems were the fastest devices when vessels were 2 to 3 mm in diameter.
There were no seal failures with the Ligamax, EnSeal, or LigaSure products.
Second study involved repeated applications
Lamberton and colleagues tested 5-mm laparoscopic devices under controlled temperature and humidity using a laparoscopic simulator, focusing on the following systems:
- LigaSure V
- Gyrus PK
- Harmonic Scalpel
- EnSeal Tissue Sealing and Hemostasis System.
The authors used 5-mm bovine arteries to assess sealing time, burst pressure, lateral thermal spread, and smoke production, as well as both subjective and objective effects on visibility.
Each device was used 10 times to determine burst pressure, lateral thermal spread, visibility, and smoke production. The devices were applied 20 times to measure time to seal, based on the devices’ preprogrammed endpoints.
The Harmonic Scalpel produced the lowest thermal spread and least smoke, but also had the lowest mean burst pressure.
The Gyrus PK generated the most smoke and had variable burst pressure. Although it had the fastest sealing times, in three of 10 trials there was a completely open arterial lumen following transection. In addition, 50% of applications involved burst pressures below 50 mm Hg.
Maximum temperatures 2 mm from the device were 49.9°C for the Harmonic Scalpel, 55.5°C for LigaSure, 58.9°C for EnSeal, and 64.5°C for Gyrus PK.
LigaSure was the highest-rated device overall, with the highest burst pressure and fastest sealing time.
EnSeal was the slowest and had variable burst pressures.
Clinical implications of the trials
These studies—neither of which was industry-sponsored—suggest that larger vessels cannot be controlled consistently and effectively using the Harmonic Scalpel or Gyrus systems. The Harmonic Scalpel is most user-dependent.
So what’s the bottom line? In the end, according to Andrew Brill, MD, past president of AAGL, “it’s not the wand, it’s the magician.”
for laparoscopic surgery
When OBG Management surveyed a number of laparoscopic experts and members of our Virtual Board of Editors about their preferred energy sources for laparoscopic surgery, the responses were strikingly similar. The consensus? The best device depends on the case at hand, the skill of the surgeon, economic concerns, and other variables.
As Keith Isaacson, MD, put it, “Because tissue may be thick or thin, moist or desiccated, vascular or avascular, the ideal instrument to achieve a pure cut varies. Because a vessel may be large or small, contain a large amount of collagen or very little collagen, or be on tension or relaxed, the ideal instrument for vessel sealing also varies, depending on the surgical situation.”
All of the devices are roughly equivalent, he added. “Fortunately, almost all of the commercially available energy sources that utilize bipolar radiofrequency or ultrasonic energy will perform our desired function if the surgeon understands the technology and utilizes the instruments properly.”
Here is a summary of recommendations made by the experts we interviewed.
Andrew I. Brill, MD
Director of Minimally Invasive Gynecology,
California Pacific Medical Center,
San Francisco, Calif
Having been actively engaged in advanced laparoscopic surgical training for more than 20 years, I have extensive experience with all of these novel devices. I critically assess any new energy-based device for its ergonomic handedness, propensity for sticking to tissue and production of plume, ability to manipulate and dissect tissue, efficiency in desiccated or fatty tissues, discoloration of tissue by carbon, response to tissue tension, and reliability for hemostasis. Because no device is perfectly suited to all procedures, I customarily rely upon several devices to satisfy my technical needs.
All advanced bipolar energy devices—LigaSure, PK Cutting Forceps, and EnSeal—can be safely used to coagulate and cut all vascular pedicles during hysterectomy and salpingo-oophorectomy. These devices perform best when tissue tension is reduced to maximize vessel sealing. Despite the fact that these devices provide audible feedback to signal the electrosurgical endpoint, I also gauge tissue color, retraction, and the emission of steam before advancing the cutting blade.
The tapered-tip design of the PK Cutting Forceps offers some comparative advantage for fine tissue dissection, but I find that hemostasis is more consistently achieved with less thermal spread using the EnSeal or LigaSure device.
I commonly utilize the Harmonic Scalpel in lieu of any electrosurgical device, understanding that it requires more finesse to achieve equivalent results.
When I anticipate that I will need to manipulate uterine pedicles during a difficult laparoscopic hysterectomy, I employ electrosurgery using the EnSeal device for its superior tissue sealing. A 3-mm curved device by EnSeal, akin to a Pean clamp, works as an exceptionally agile instrument for hysterectomy without sacrificing hemostasis.
Only the LigaSure Advance provides both coaptive coagulation and spark cutting via an electrode on the tip of one blade; therefore, an adjunctive mechanical or energy-based device must be employed to perform culdotomy during total laparoscopic hysterectomy when EnSeal or the PK Cutting Forceps is used.
The ability to efficiently and hemostatically cut through tissue of variable mass with minimal plume, predictable thermal margins, and the retention of tissue color make the Harmonic Scalpel my first choice for laparoscopic resection of endometriosis, extensive adhesiolysis, myomectomy, cervical amputation during supracervical hysterectomy, and culdotomy during total hysterectomy. Although I have similarly utilized the unique capacity of the spatula, J-hook, and needle electrodes by Plasmacision (Gyrus) to cut tissue using bipolar electrosurgery, all have afforded less technical control, not as much hemostasis, and wider thermal margins.
Use of any energy-based device does not preclude the need for skill. Before adding any of these devices to your surgical armamentarium, appropriate training should be acquired in a skills lab using living tissue or with a laparoscopic trainer using a tissue surrogate.
Dr. Brill reports no financial relationships relevant to this review.
Jon I. Einarsson, MD, MPH
Director of Minimally Invasive Gynecologic Surgery,
Brigham and Women’s Hospital,
Assistant Professor of Obstetrics,
Gynecology, and Reproductive Biology,
Harvard Medical School, Boston, Mass
I prefer the Harmonic Scalpel and a Kelly RoBi Bipolar Grasper (Karl Storz). I have been using these instruments for approximately 10 years. I use this combination for all operative laparoscopy cases, including hysterectomy, myomectomy, sacrocolpopexy, resection of endometriosis, and lysis of adhesions.
The Harmonic Scalpel is a versatile instrument that can perform dissection, cutting, and coagulation very efficiently. However, there are better vessel sealers out there, such as the LigaSure and EnSeal devices. In this era of cost containment, it is important to limit the use of multiple disposable energy sources in a single case. By adding the reusable bipolar grasper, vessel sealing becomes efficient and reliable without added cost. In addition, the bipolar grasper functions as a dissector and retractor, thereby minimizing instrument changes.
I watch the bubbles that form during desiccation with the bipolar grasper. Once these bubbles start to “die down,” I know that the tissue is desiccated and safe to transect. It is important to remember that thermal spread with a traditional bipolar grasper is significant (up to 13 mm). For that reason, when I desiccate the uterine vessels with the bipolar grasper, I stay above the rim of the colpotomy ring in order to maintain a safe distance from the ureter during this step.
The Harmonic Scalpel offers several advantages over monopolar electrosurgery for cutting, such as decreased smoke formation and avoidance of complications such as insulation failure, direct coupling, and capacitative coupling.
Dr. Einarsson reports no financial relationships relevant to this review.
Carl F. Giesler, MD
Associate Professor and Director of
Minimally Invasive Surgery,
Department of Obstetrics and Gynecology,
Baylor College of Medicine,
Waco, Tex
I was introduced to laparoscopy as a resident in 1974, and have been teaching laparoscopic surgical techniques to general surgeons and gynecologists since 1988. I began performing laparoscopic hysterectomy in 1991. As an educator and trainer of other laparoscopic surgeons, I have used all available laparoscopic energy sources.
I became an early adapter and user of ultrasonic mechanical energy when it was introduced for laparoscopy in 1995, because of its safety for adjacent tissues and its single-instrument, multiple-task properties.
This energy source was originally named the Ultrasonic Coagulating Shears by Ultracision, but Ethicon EndoSurgery purchased the product in 1995 and changed its name to the Harmonic Scalpel. The current generation is called the Harmonic ACE, and I use it as my primary laparoscopic energy source for all procedures, from management of unruptured ectopic pregnancy to total laparoscopic hysterectomy and extensive adhesiolyis in previously operated abdomens.
The Harmonic ACE allows me to confidently dissect adhesions over loops of bowel, as well as over the ureter, because of its limited lateral thermal spread. It allows nearly bloodless dissection of the retroperitoneal space and space around the bladder. The cavitation effect created by the rapidly moving instrument tip (55,500 cycles/second) creates tissue separation along the areolar tissue planes, where most blood vessels are the size of capillaries and where larger blood vessels are easily identified and controlled by coaptation, coagulation, and cutting.
The protein coagulum is produced at a temperature of 54°C. The sides and back of the active blade have minimal thermal effect because tissue is not held tightly against the moving blade. Minimal char is observed because of the low tissue temperature associated with coagulum formation. Minimal sticking of tissue occurs because the rapid motion of the instrument tip dislodges the tissue.
The ease of use, multiple functions for a single instrument, tissue safety, and minimal residual traumatized tissue are the reasons I prefer the mechanical energy of the Harmonic ACE as my primary laparoscopic energy source.
Dr. Giesler serves as a speaker for Ethicon EndoSurgery.
Cheryl Iglesia, MD
Director, Urogynecology and Reconstructive Pelvic Surgery,
Washington Hospital Center,
Associate Professor, Departments of ObGyn and Urology,
Georgetown University, Washington, DC.
Member, OBG Management Board of Editors
I prefer the Harmonic ACE for straight-stick laparoscopic hysterectomy cases and the PK Cutting Forceps for robotic hysterectomy and sacrocolpopexy.
The Harmonic ACE is fast and has little thermal spread; the PK Cutting Forceps is the only energy source available with reticulating arms for the robot.
I have been using the Harmonic ACE for more than 10 years and the PK Cutting Forceps since 2006.
Dr. Iglesia reports no financial relationships relevant to this review.
Keith Isaacson, MD
Director of Minimally Invasive Gynecologic
Surgery and Infertility,
Director of the AAGL/SRS
Fellowship in Minimally Invasive Gynecologic Surgery,
Newton Wellesley Hospital,
Associate Professor of Obstetrics, Gynecology,
and Reproductive Biology,
Harvard Medical School, Boston, Mass
I have used every bipolar and harmonic tool that is commercially available, including the recently released EnSeal. I like them all. The tools I have chosen were selected in conjunction with our purchasing department and determined to be the optimal tools for the lowest cost and highest efficiency. Our goal is to use as few disposables as possible, but to be as efficient as possible.
The instruments I utilize most frequently are the reusable 5-mm bipolar instruments from Karl Storz (RoBi) and ERBE Medical. The RoBi set includes a variety of tips, ranging from a dissecting Maryland-type tip to a flat, broad-based tip similar to the Kleppinger design. These are all plugged into the ERBE VIO generator. With this assortment of tools, I can seal a vessel of almost any size, whether it be thin-walled, such as a large vein, or a thick-walled artery.
To cut tissue, I prefer the Harmonic Scalpel and the bipolar Laparoscopic Spatula (Gyrus). I use the Harmonic Scalpel during laparoscopic hysterectomy, and the Spatula during laparoscopic myomectomy. Both devices cut tissue well while simultaneously sealing small vessels.
I have been using these instruments for approximately 10 years.
Dr. Isaacson reports that he is a consultant for Karl Storz in hysteroscopy.
Noor Ahmed, MB, ChB, MD
Consultant Gynecologist,
Dundee, Scotland.
Member, OBG Management Virtual
Board of Editors
I am a consultant gynecologist with an interest in fertility, endometriosis surgery, and general gynecologic surgery. Most of my surgical work is performed laparoscopically or vaginally.
My first experience with vessel-sealing technology was in 2000, when I began using the LigaSure Max for difficult vaginal hysterectomies. I was impressed by its ability to clamp and seal and, in later versions, its ability to cut, as well. Since then, I have been using various versions of LigaSure vessel- and tissue-sealing technology for many gynecologic procedures.
I have had some exposure to other vessel- and tissue-sealing technologies, but prefer LigaSure because:
1. It provides an experience similar to that of conventional clamp, cut, and suture through the control offered by its unique combination of pressure and energy, creating vessel and tissue fusion that does not rely on a proximal thrombus and produces minimal thermal spread.
2. The feedback-controlled response system automatically discontinues energy delivery when sealing is complete.
3. It works with clamps of various sizes and shapes, using the same hardware (generator) and saving money and space. Moreover, the nursing staff has to develop familiarity with one machine only.
For laparoscopic procedures, I use LigaSure Lap, LigaSure Atlas Sealer/Divider and, most commonly, the 5-mm LigaSure V Sealer/Divider.
Dr. Ahmed reports no financial relationships relevant to this review.
Michael Kirwin, MD
General Obstetrics and Gynecology, Freehold, NJ.
Member, OBG Management Virtual Board of Editors
With advanced laparoscopic cases, particularly hysterectomy, I use the Harmonic Scalpel and PK Cutting Forceps. I have used the Harmonic Scalpel regularly for approximately 4 years, and it works well for dissection and, particularly, for transecting the cervix in supracervical cases.
Over the past year, I have also begun to use the Gyrus forceps, especially when I expect to encounter larger vessels that require coagulation. Both instruments can be used in 5-mm ports. Both seem relatively reliable and easy for the OR team to use. I would hope that an articulation feature can be added in the future.
Dr. Kirwin reports no financial relationships relevant to this review.
When Harry Reich performed the first laparoscopically assisted vaginal hysterectomy in 1989, he advocated the use of sutures for control of the uterine vessels. Monopolar, bipolar, and laser instruments available at that time were inherently risky to use along the pelvic sidewall because of their potential for 1) considerable thermal energy spread beyond the area of treatment (bipolar, NG:YAG laser, monopolar) and 2) unreliable hemostasis (CO2 laser).
Minimally invasive surgical practice has driven meaningful advances in instrumentation and technique over the past 20 years. The constraints inherent in laparoscopic surgery, although somewhat mitigated by robotics, have generated a proliferation of technologies to obtain reliable hemostasis. Every device now on the market claims to “seal” vessels. In this article, I review the mechanism of action of these instruments and compare their strengths and weaknesses, based on high-quality scientific evidence.
Two studies highlight vessel ligation
Newcomb WL, Hope WW, Schmeizer TM, et al. Comparison of blood vessel sealing among new electrosurgical and ultrasonic devices. Surg Endosc. 2009;23:90–96.
Lamberton GR, Hsi RS, Jin DH, et al. Prospective comparison of four laparoscopic vessel ligation devices. J Endourol. 2008;22:2307–2312.
Many energy-delivery systems are available for the gynecologic surgeon; any of them can be used effectively and safely under most circumstances. Before we can make an informed choice about which system is best for our own practice, however, we need to be aware of the strengths and limitations of the systems overall ( TABLE ).
These two studies focus on the following devices:
- Gyrus PK Tissue Management System, PKS Cutting Forceps, and Plasma Trissector (all from Gyrus Medical). These are bipolar electrosurgical devices designed to deliver high current and very low voltage to tissue. Tissue impedance is continuously monitored between the jaws of the instrument, and energy delivery is adjusted accordingly. These systems deliver electrosurgical energy through a series of rapid pulses, thereby allowing the tissue to cool briefly and limiting the heating of adjacent tissue. Protein in the vessel walls is denatured and forms a coagulum, which occludes the lumen.
- Harmonic Scalpel (Ethicon EndoSurgery). This device uses a high-frequency ultrasonic transducer (55,000 cycles/second) to create mechanical vibration of one of the two jaws. The device can be used to vaporize tissue (cut) or achieve hemostasis by coagulation. As with the other devices, protein is denatured and vessels are occluded by formation of a coagulum.
- Ligamax 5 Endoscopic Multiple Clip Applier (Ethicon). This device is a sterile, single-patient-use, 5-mm, endoscopic, multiple-clip applicator that delivers 15 medium or large titanium clips that close to 8.8 mm, the same clip size as the 10-mm applicator. Ligamax 5 has long, thin angled jaws (8.4 mm) that extend beyond vessels and ducts to improve visibility. It includes a long, 33-cm shaft for additional reach, and an anti-clip drop-ratchet mechanism for control over clip closure.
- EnSeal Tissue Sealing and Hemostasis System and EnSeal PTC (SurgRx). EnSeal utilizes nanotechnology to control the energy at the electrode–tissue interface. The jaws contain a temperature-sensitive matrix with embedded conductive carbon spherules designed to “sense” tissue characteristics. It uses extremely high jaw compression to create uniform tissue effects. It does not require a dedicated electrosurgical unit for use; an adapter can be purchased that permits use with most generators.
- LigaSure V (Valleylab). LigaSure is a bipolar electrosurgical device designed to deliver high current and very low voltage to tissue. It monitors tissue impedance between the jaws of the instrument and continuously adjusts the delivery of energy.
TABLE
Rating vessel-sealing devices: 5 measures of success
| Device | Safety: Minimal thermal spread | Reliability: Efficacy on vessels ≤7 mm | Efficiency: Treatment time | Consistency: Independent of user | Utility: Multiple uses |
|---|---|---|---|---|---|
| Harmonic Scalpel | Excellent | Poor | Excellent | Poor | Excellent |
| Gyrus PK | Poor | Poor | Excellent | Fair | Fair |
| LigaSure V | Good | Excellent | Good | Excellent | Fair |
| EnSeal | Fair | Excellent | Poor | Excellent | Poor |
| These ratings were devised by the author based on data from independent studies in living tissue models. | |||||
FIGURE How energy-based vessel-sealing works
(A) When the carotid artery of an animal model was sealed using a standard bipolar forceps, the lumen remained open and a proximal thrombus developed. (B) When LigaSure was used, the artery was fused and the lumen obliterated with one 5-second application. (C and D) When a renal artery was sealed with LigaSure, the lumen of the vessel walls fused completely.
What the studies found
Newcomb and associates compared blood-vessel-sealing ability of the following devices:
- Gyrus 5-mm PKS Cutting Forceps
- Gyrus Plasma Trissector
- Harmonic Scalpel
- EnSeal Tissue Sealing and Hemostasis System
- LigaSure V, using the LigaSure Generator (Valleylab)
- LigaSure V, using the Force Triad Generator (Valleylab)
- Ligamax 5 Endoscopic Multiple Clip Applier.
The authors assessed mean seal times and burst pressures. Used on medium and large vessels, the Harmonic Scalpel and Gyrus products had significant failure rates: 8% to 22% for the Harmonic Scalpel and 41% to 92% for the pulsed, bipolar Gyrus systems.
The shortest sealing times for medium to large vessels were achieved with the LigaSure V using the Force Triad Generator. The Gyrus systems were the fastest devices when vessels were 2 to 3 mm in diameter.
There were no seal failures with the Ligamax, EnSeal, or LigaSure products.
Second study involved repeated applications
Lamberton and colleagues tested 5-mm laparoscopic devices under controlled temperature and humidity using a laparoscopic simulator, focusing on the following systems:
- LigaSure V
- Gyrus PK
- Harmonic Scalpel
- EnSeal Tissue Sealing and Hemostasis System.
The authors used 5-mm bovine arteries to assess sealing time, burst pressure, lateral thermal spread, and smoke production, as well as both subjective and objective effects on visibility.
Each device was used 10 times to determine burst pressure, lateral thermal spread, visibility, and smoke production. The devices were applied 20 times to measure time to seal, based on the devices’ preprogrammed endpoints.
The Harmonic Scalpel produced the lowest thermal spread and least smoke, but also had the lowest mean burst pressure.
The Gyrus PK generated the most smoke and had variable burst pressure. Although it had the fastest sealing times, in three of 10 trials there was a completely open arterial lumen following transection. In addition, 50% of applications involved burst pressures below 50 mm Hg.
Maximum temperatures 2 mm from the device were 49.9°C for the Harmonic Scalpel, 55.5°C for LigaSure, 58.9°C for EnSeal, and 64.5°C for Gyrus PK.
LigaSure was the highest-rated device overall, with the highest burst pressure and fastest sealing time.
EnSeal was the slowest and had variable burst pressures.
Clinical implications of the trials
These studies—neither of which was industry-sponsored—suggest that larger vessels cannot be controlled consistently and effectively using the Harmonic Scalpel or Gyrus systems. The Harmonic Scalpel is most user-dependent.
So what’s the bottom line? In the end, according to Andrew Brill, MD, past president of AAGL, “it’s not the wand, it’s the magician.”
for laparoscopic surgery
When OBG Management surveyed a number of laparoscopic experts and members of our Virtual Board of Editors about their preferred energy sources for laparoscopic surgery, the responses were strikingly similar. The consensus? The best device depends on the case at hand, the skill of the surgeon, economic concerns, and other variables.
As Keith Isaacson, MD, put it, “Because tissue may be thick or thin, moist or desiccated, vascular or avascular, the ideal instrument to achieve a pure cut varies. Because a vessel may be large or small, contain a large amount of collagen or very little collagen, or be on tension or relaxed, the ideal instrument for vessel sealing also varies, depending on the surgical situation.”
All of the devices are roughly equivalent, he added. “Fortunately, almost all of the commercially available energy sources that utilize bipolar radiofrequency or ultrasonic energy will perform our desired function if the surgeon understands the technology and utilizes the instruments properly.”
Here is a summary of recommendations made by the experts we interviewed.
Andrew I. Brill, MD
Director of Minimally Invasive Gynecology,
California Pacific Medical Center,
San Francisco, Calif
Having been actively engaged in advanced laparoscopic surgical training for more than 20 years, I have extensive experience with all of these novel devices. I critically assess any new energy-based device for its ergonomic handedness, propensity for sticking to tissue and production of plume, ability to manipulate and dissect tissue, efficiency in desiccated or fatty tissues, discoloration of tissue by carbon, response to tissue tension, and reliability for hemostasis. Because no device is perfectly suited to all procedures, I customarily rely upon several devices to satisfy my technical needs.
All advanced bipolar energy devices—LigaSure, PK Cutting Forceps, and EnSeal—can be safely used to coagulate and cut all vascular pedicles during hysterectomy and salpingo-oophorectomy. These devices perform best when tissue tension is reduced to maximize vessel sealing. Despite the fact that these devices provide audible feedback to signal the electrosurgical endpoint, I also gauge tissue color, retraction, and the emission of steam before advancing the cutting blade.
The tapered-tip design of the PK Cutting Forceps offers some comparative advantage for fine tissue dissection, but I find that hemostasis is more consistently achieved with less thermal spread using the EnSeal or LigaSure device.
I commonly utilize the Harmonic Scalpel in lieu of any electrosurgical device, understanding that it requires more finesse to achieve equivalent results.
When I anticipate that I will need to manipulate uterine pedicles during a difficult laparoscopic hysterectomy, I employ electrosurgery using the EnSeal device for its superior tissue sealing. A 3-mm curved device by EnSeal, akin to a Pean clamp, works as an exceptionally agile instrument for hysterectomy without sacrificing hemostasis.
Only the LigaSure Advance provides both coaptive coagulation and spark cutting via an electrode on the tip of one blade; therefore, an adjunctive mechanical or energy-based device must be employed to perform culdotomy during total laparoscopic hysterectomy when EnSeal or the PK Cutting Forceps is used.
The ability to efficiently and hemostatically cut through tissue of variable mass with minimal plume, predictable thermal margins, and the retention of tissue color make the Harmonic Scalpel my first choice for laparoscopic resection of endometriosis, extensive adhesiolysis, myomectomy, cervical amputation during supracervical hysterectomy, and culdotomy during total hysterectomy. Although I have similarly utilized the unique capacity of the spatula, J-hook, and needle electrodes by Plasmacision (Gyrus) to cut tissue using bipolar electrosurgery, all have afforded less technical control, not as much hemostasis, and wider thermal margins.
Use of any energy-based device does not preclude the need for skill. Before adding any of these devices to your surgical armamentarium, appropriate training should be acquired in a skills lab using living tissue or with a laparoscopic trainer using a tissue surrogate.
Dr. Brill reports no financial relationships relevant to this review.
Jon I. Einarsson, MD, MPH
Director of Minimally Invasive Gynecologic Surgery,
Brigham and Women’s Hospital,
Assistant Professor of Obstetrics,
Gynecology, and Reproductive Biology,
Harvard Medical School, Boston, Mass
I prefer the Harmonic Scalpel and a Kelly RoBi Bipolar Grasper (Karl Storz). I have been using these instruments for approximately 10 years. I use this combination for all operative laparoscopy cases, including hysterectomy, myomectomy, sacrocolpopexy, resection of endometriosis, and lysis of adhesions.
The Harmonic Scalpel is a versatile instrument that can perform dissection, cutting, and coagulation very efficiently. However, there are better vessel sealers out there, such as the LigaSure and EnSeal devices. In this era of cost containment, it is important to limit the use of multiple disposable energy sources in a single case. By adding the reusable bipolar grasper, vessel sealing becomes efficient and reliable without added cost. In addition, the bipolar grasper functions as a dissector and retractor, thereby minimizing instrument changes.
I watch the bubbles that form during desiccation with the bipolar grasper. Once these bubbles start to “die down,” I know that the tissue is desiccated and safe to transect. It is important to remember that thermal spread with a traditional bipolar grasper is significant (up to 13 mm). For that reason, when I desiccate the uterine vessels with the bipolar grasper, I stay above the rim of the colpotomy ring in order to maintain a safe distance from the ureter during this step.
The Harmonic Scalpel offers several advantages over monopolar electrosurgery for cutting, such as decreased smoke formation and avoidance of complications such as insulation failure, direct coupling, and capacitative coupling.
Dr. Einarsson reports no financial relationships relevant to this review.
Carl F. Giesler, MD
Associate Professor and Director of
Minimally Invasive Surgery,
Department of Obstetrics and Gynecology,
Baylor College of Medicine,
Waco, Tex
I was introduced to laparoscopy as a resident in 1974, and have been teaching laparoscopic surgical techniques to general surgeons and gynecologists since 1988. I began performing laparoscopic hysterectomy in 1991. As an educator and trainer of other laparoscopic surgeons, I have used all available laparoscopic energy sources.
I became an early adapter and user of ultrasonic mechanical energy when it was introduced for laparoscopy in 1995, because of its safety for adjacent tissues and its single-instrument, multiple-task properties.
This energy source was originally named the Ultrasonic Coagulating Shears by Ultracision, but Ethicon EndoSurgery purchased the product in 1995 and changed its name to the Harmonic Scalpel. The current generation is called the Harmonic ACE, and I use it as my primary laparoscopic energy source for all procedures, from management of unruptured ectopic pregnancy to total laparoscopic hysterectomy and extensive adhesiolyis in previously operated abdomens.
The Harmonic ACE allows me to confidently dissect adhesions over loops of bowel, as well as over the ureter, because of its limited lateral thermal spread. It allows nearly bloodless dissection of the retroperitoneal space and space around the bladder. The cavitation effect created by the rapidly moving instrument tip (55,500 cycles/second) creates tissue separation along the areolar tissue planes, where most blood vessels are the size of capillaries and where larger blood vessels are easily identified and controlled by coaptation, coagulation, and cutting.
The protein coagulum is produced at a temperature of 54°C. The sides and back of the active blade have minimal thermal effect because tissue is not held tightly against the moving blade. Minimal char is observed because of the low tissue temperature associated with coagulum formation. Minimal sticking of tissue occurs because the rapid motion of the instrument tip dislodges the tissue.
The ease of use, multiple functions for a single instrument, tissue safety, and minimal residual traumatized tissue are the reasons I prefer the mechanical energy of the Harmonic ACE as my primary laparoscopic energy source.
Dr. Giesler serves as a speaker for Ethicon EndoSurgery.
Cheryl Iglesia, MD
Director, Urogynecology and Reconstructive Pelvic Surgery,
Washington Hospital Center,
Associate Professor, Departments of ObGyn and Urology,
Georgetown University, Washington, DC.
Member, OBG Management Board of Editors
I prefer the Harmonic ACE for straight-stick laparoscopic hysterectomy cases and the PK Cutting Forceps for robotic hysterectomy and sacrocolpopexy.
The Harmonic ACE is fast and has little thermal spread; the PK Cutting Forceps is the only energy source available with reticulating arms for the robot.
I have been using the Harmonic ACE for more than 10 years and the PK Cutting Forceps since 2006.
Dr. Iglesia reports no financial relationships relevant to this review.
Keith Isaacson, MD
Director of Minimally Invasive Gynecologic
Surgery and Infertility,
Director of the AAGL/SRS
Fellowship in Minimally Invasive Gynecologic Surgery,
Newton Wellesley Hospital,
Associate Professor of Obstetrics, Gynecology,
and Reproductive Biology,
Harvard Medical School, Boston, Mass
I have used every bipolar and harmonic tool that is commercially available, including the recently released EnSeal. I like them all. The tools I have chosen were selected in conjunction with our purchasing department and determined to be the optimal tools for the lowest cost and highest efficiency. Our goal is to use as few disposables as possible, but to be as efficient as possible.
The instruments I utilize most frequently are the reusable 5-mm bipolar instruments from Karl Storz (RoBi) and ERBE Medical. The RoBi set includes a variety of tips, ranging from a dissecting Maryland-type tip to a flat, broad-based tip similar to the Kleppinger design. These are all plugged into the ERBE VIO generator. With this assortment of tools, I can seal a vessel of almost any size, whether it be thin-walled, such as a large vein, or a thick-walled artery.
To cut tissue, I prefer the Harmonic Scalpel and the bipolar Laparoscopic Spatula (Gyrus). I use the Harmonic Scalpel during laparoscopic hysterectomy, and the Spatula during laparoscopic myomectomy. Both devices cut tissue well while simultaneously sealing small vessels.
I have been using these instruments for approximately 10 years.
Dr. Isaacson reports that he is a consultant for Karl Storz in hysteroscopy.
Noor Ahmed, MB, ChB, MD
Consultant Gynecologist,
Dundee, Scotland.
Member, OBG Management Virtual
Board of Editors
I am a consultant gynecologist with an interest in fertility, endometriosis surgery, and general gynecologic surgery. Most of my surgical work is performed laparoscopically or vaginally.
My first experience with vessel-sealing technology was in 2000, when I began using the LigaSure Max for difficult vaginal hysterectomies. I was impressed by its ability to clamp and seal and, in later versions, its ability to cut, as well. Since then, I have been using various versions of LigaSure vessel- and tissue-sealing technology for many gynecologic procedures.
I have had some exposure to other vessel- and tissue-sealing technologies, but prefer LigaSure because:
1. It provides an experience similar to that of conventional clamp, cut, and suture through the control offered by its unique combination of pressure and energy, creating vessel and tissue fusion that does not rely on a proximal thrombus and produces minimal thermal spread.
2. The feedback-controlled response system automatically discontinues energy delivery when sealing is complete.
3. It works with clamps of various sizes and shapes, using the same hardware (generator) and saving money and space. Moreover, the nursing staff has to develop familiarity with one machine only.
For laparoscopic procedures, I use LigaSure Lap, LigaSure Atlas Sealer/Divider and, most commonly, the 5-mm LigaSure V Sealer/Divider.
Dr. Ahmed reports no financial relationships relevant to this review.
Michael Kirwin, MD
General Obstetrics and Gynecology, Freehold, NJ.
Member, OBG Management Virtual Board of Editors
With advanced laparoscopic cases, particularly hysterectomy, I use the Harmonic Scalpel and PK Cutting Forceps. I have used the Harmonic Scalpel regularly for approximately 4 years, and it works well for dissection and, particularly, for transecting the cervix in supracervical cases.
Over the past year, I have also begun to use the Gyrus forceps, especially when I expect to encounter larger vessels that require coagulation. Both instruments can be used in 5-mm ports. Both seem relatively reliable and easy for the OR team to use. I would hope that an articulation feature can be added in the future.
Dr. Kirwin reports no financial relationships relevant to this review.
When Harry Reich performed the first laparoscopically assisted vaginal hysterectomy in 1989, he advocated the use of sutures for control of the uterine vessels. Monopolar, bipolar, and laser instruments available at that time were inherently risky to use along the pelvic sidewall because of their potential for 1) considerable thermal energy spread beyond the area of treatment (bipolar, NG:YAG laser, monopolar) and 2) unreliable hemostasis (CO2 laser).
Minimally invasive surgical practice has driven meaningful advances in instrumentation and technique over the past 20 years. The constraints inherent in laparoscopic surgery, although somewhat mitigated by robotics, have generated a proliferation of technologies to obtain reliable hemostasis. Every device now on the market claims to “seal” vessels. In this article, I review the mechanism of action of these instruments and compare their strengths and weaknesses, based on high-quality scientific evidence.
Two studies highlight vessel ligation
Newcomb WL, Hope WW, Schmeizer TM, et al. Comparison of blood vessel sealing among new electrosurgical and ultrasonic devices. Surg Endosc. 2009;23:90–96.
Lamberton GR, Hsi RS, Jin DH, et al. Prospective comparison of four laparoscopic vessel ligation devices. J Endourol. 2008;22:2307–2312.
Many energy-delivery systems are available for the gynecologic surgeon; any of them can be used effectively and safely under most circumstances. Before we can make an informed choice about which system is best for our own practice, however, we need to be aware of the strengths and limitations of the systems overall ( TABLE ).
These two studies focus on the following devices:
- Gyrus PK Tissue Management System, PKS Cutting Forceps, and Plasma Trissector (all from Gyrus Medical). These are bipolar electrosurgical devices designed to deliver high current and very low voltage to tissue. Tissue impedance is continuously monitored between the jaws of the instrument, and energy delivery is adjusted accordingly. These systems deliver electrosurgical energy through a series of rapid pulses, thereby allowing the tissue to cool briefly and limiting the heating of adjacent tissue. Protein in the vessel walls is denatured and forms a coagulum, which occludes the lumen.
- Harmonic Scalpel (Ethicon EndoSurgery). This device uses a high-frequency ultrasonic transducer (55,000 cycles/second) to create mechanical vibration of one of the two jaws. The device can be used to vaporize tissue (cut) or achieve hemostasis by coagulation. As with the other devices, protein is denatured and vessels are occluded by formation of a coagulum.
- Ligamax 5 Endoscopic Multiple Clip Applier (Ethicon). This device is a sterile, single-patient-use, 5-mm, endoscopic, multiple-clip applicator that delivers 15 medium or large titanium clips that close to 8.8 mm, the same clip size as the 10-mm applicator. Ligamax 5 has long, thin angled jaws (8.4 mm) that extend beyond vessels and ducts to improve visibility. It includes a long, 33-cm shaft for additional reach, and an anti-clip drop-ratchet mechanism for control over clip closure.
- EnSeal Tissue Sealing and Hemostasis System and EnSeal PTC (SurgRx). EnSeal utilizes nanotechnology to control the energy at the electrode–tissue interface. The jaws contain a temperature-sensitive matrix with embedded conductive carbon spherules designed to “sense” tissue characteristics. It uses extremely high jaw compression to create uniform tissue effects. It does not require a dedicated electrosurgical unit for use; an adapter can be purchased that permits use with most generators.
- LigaSure V (Valleylab). LigaSure is a bipolar electrosurgical device designed to deliver high current and very low voltage to tissue. It monitors tissue impedance between the jaws of the instrument and continuously adjusts the delivery of energy.
TABLE
Rating vessel-sealing devices: 5 measures of success
| Device | Safety: Minimal thermal spread | Reliability: Efficacy on vessels ≤7 mm | Efficiency: Treatment time | Consistency: Independent of user | Utility: Multiple uses |
|---|---|---|---|---|---|
| Harmonic Scalpel | Excellent | Poor | Excellent | Poor | Excellent |
| Gyrus PK | Poor | Poor | Excellent | Fair | Fair |
| LigaSure V | Good | Excellent | Good | Excellent | Fair |
| EnSeal | Fair | Excellent | Poor | Excellent | Poor |
| These ratings were devised by the author based on data from independent studies in living tissue models. | |||||
FIGURE How energy-based vessel-sealing works
(A) When the carotid artery of an animal model was sealed using a standard bipolar forceps, the lumen remained open and a proximal thrombus developed. (B) When LigaSure was used, the artery was fused and the lumen obliterated with one 5-second application. (C and D) When a renal artery was sealed with LigaSure, the lumen of the vessel walls fused completely.
What the studies found
Newcomb and associates compared blood-vessel-sealing ability of the following devices:
- Gyrus 5-mm PKS Cutting Forceps
- Gyrus Plasma Trissector
- Harmonic Scalpel
- EnSeal Tissue Sealing and Hemostasis System
- LigaSure V, using the LigaSure Generator (Valleylab)
- LigaSure V, using the Force Triad Generator (Valleylab)
- Ligamax 5 Endoscopic Multiple Clip Applier.
The authors assessed mean seal times and burst pressures. Used on medium and large vessels, the Harmonic Scalpel and Gyrus products had significant failure rates: 8% to 22% for the Harmonic Scalpel and 41% to 92% for the pulsed, bipolar Gyrus systems.
The shortest sealing times for medium to large vessels were achieved with the LigaSure V using the Force Triad Generator. The Gyrus systems were the fastest devices when vessels were 2 to 3 mm in diameter.
There were no seal failures with the Ligamax, EnSeal, or LigaSure products.
Second study involved repeated applications
Lamberton and colleagues tested 5-mm laparoscopic devices under controlled temperature and humidity using a laparoscopic simulator, focusing on the following systems:
- LigaSure V
- Gyrus PK
- Harmonic Scalpel
- EnSeal Tissue Sealing and Hemostasis System.
The authors used 5-mm bovine arteries to assess sealing time, burst pressure, lateral thermal spread, and smoke production, as well as both subjective and objective effects on visibility.
Each device was used 10 times to determine burst pressure, lateral thermal spread, visibility, and smoke production. The devices were applied 20 times to measure time to seal, based on the devices’ preprogrammed endpoints.
The Harmonic Scalpel produced the lowest thermal spread and least smoke, but also had the lowest mean burst pressure.
The Gyrus PK generated the most smoke and had variable burst pressure. Although it had the fastest sealing times, in three of 10 trials there was a completely open arterial lumen following transection. In addition, 50% of applications involved burst pressures below 50 mm Hg.
Maximum temperatures 2 mm from the device were 49.9°C for the Harmonic Scalpel, 55.5°C for LigaSure, 58.9°C for EnSeal, and 64.5°C for Gyrus PK.
LigaSure was the highest-rated device overall, with the highest burst pressure and fastest sealing time.
EnSeal was the slowest and had variable burst pressures.
Clinical implications of the trials
These studies—neither of which was industry-sponsored—suggest that larger vessels cannot be controlled consistently and effectively using the Harmonic Scalpel or Gyrus systems. The Harmonic Scalpel is most user-dependent.
So what’s the bottom line? In the end, according to Andrew Brill, MD, past president of AAGL, “it’s not the wand, it’s the magician.”
for laparoscopic surgery
When OBG Management surveyed a number of laparoscopic experts and members of our Virtual Board of Editors about their preferred energy sources for laparoscopic surgery, the responses were strikingly similar. The consensus? The best device depends on the case at hand, the skill of the surgeon, economic concerns, and other variables.
As Keith Isaacson, MD, put it, “Because tissue may be thick or thin, moist or desiccated, vascular or avascular, the ideal instrument to achieve a pure cut varies. Because a vessel may be large or small, contain a large amount of collagen or very little collagen, or be on tension or relaxed, the ideal instrument for vessel sealing also varies, depending on the surgical situation.”
All of the devices are roughly equivalent, he added. “Fortunately, almost all of the commercially available energy sources that utilize bipolar radiofrequency or ultrasonic energy will perform our desired function if the surgeon understands the technology and utilizes the instruments properly.”
Here is a summary of recommendations made by the experts we interviewed.
Andrew I. Brill, MD
Director of Minimally Invasive Gynecology,
California Pacific Medical Center,
San Francisco, Calif
Having been actively engaged in advanced laparoscopic surgical training for more than 20 years, I have extensive experience with all of these novel devices. I critically assess any new energy-based device for its ergonomic handedness, propensity for sticking to tissue and production of plume, ability to manipulate and dissect tissue, efficiency in desiccated or fatty tissues, discoloration of tissue by carbon, response to tissue tension, and reliability for hemostasis. Because no device is perfectly suited to all procedures, I customarily rely upon several devices to satisfy my technical needs.
All advanced bipolar energy devices—LigaSure, PK Cutting Forceps, and EnSeal—can be safely used to coagulate and cut all vascular pedicles during hysterectomy and salpingo-oophorectomy. These devices perform best when tissue tension is reduced to maximize vessel sealing. Despite the fact that these devices provide audible feedback to signal the electrosurgical endpoint, I also gauge tissue color, retraction, and the emission of steam before advancing the cutting blade.
The tapered-tip design of the PK Cutting Forceps offers some comparative advantage for fine tissue dissection, but I find that hemostasis is more consistently achieved with less thermal spread using the EnSeal or LigaSure device.
I commonly utilize the Harmonic Scalpel in lieu of any electrosurgical device, understanding that it requires more finesse to achieve equivalent results.
When I anticipate that I will need to manipulate uterine pedicles during a difficult laparoscopic hysterectomy, I employ electrosurgery using the EnSeal device for its superior tissue sealing. A 3-mm curved device by EnSeal, akin to a Pean clamp, works as an exceptionally agile instrument for hysterectomy without sacrificing hemostasis.
Only the LigaSure Advance provides both coaptive coagulation and spark cutting via an electrode on the tip of one blade; therefore, an adjunctive mechanical or energy-based device must be employed to perform culdotomy during total laparoscopic hysterectomy when EnSeal or the PK Cutting Forceps is used.
The ability to efficiently and hemostatically cut through tissue of variable mass with minimal plume, predictable thermal margins, and the retention of tissue color make the Harmonic Scalpel my first choice for laparoscopic resection of endometriosis, extensive adhesiolysis, myomectomy, cervical amputation during supracervical hysterectomy, and culdotomy during total hysterectomy. Although I have similarly utilized the unique capacity of the spatula, J-hook, and needle electrodes by Plasmacision (Gyrus) to cut tissue using bipolar electrosurgery, all have afforded less technical control, not as much hemostasis, and wider thermal margins.
Use of any energy-based device does not preclude the need for skill. Before adding any of these devices to your surgical armamentarium, appropriate training should be acquired in a skills lab using living tissue or with a laparoscopic trainer using a tissue surrogate.
Dr. Brill reports no financial relationships relevant to this review.
Jon I. Einarsson, MD, MPH
Director of Minimally Invasive Gynecologic Surgery,
Brigham and Women’s Hospital,
Assistant Professor of Obstetrics,
Gynecology, and Reproductive Biology,
Harvard Medical School, Boston, Mass
I prefer the Harmonic Scalpel and a Kelly RoBi Bipolar Grasper (Karl Storz). I have been using these instruments for approximately 10 years. I use this combination for all operative laparoscopy cases, including hysterectomy, myomectomy, sacrocolpopexy, resection of endometriosis, and lysis of adhesions.
The Harmonic Scalpel is a versatile instrument that can perform dissection, cutting, and coagulation very efficiently. However, there are better vessel sealers out there, such as the LigaSure and EnSeal devices. In this era of cost containment, it is important to limit the use of multiple disposable energy sources in a single case. By adding the reusable bipolar grasper, vessel sealing becomes efficient and reliable without added cost. In addition, the bipolar grasper functions as a dissector and retractor, thereby minimizing instrument changes.
I watch the bubbles that form during desiccation with the bipolar grasper. Once these bubbles start to “die down,” I know that the tissue is desiccated and safe to transect. It is important to remember that thermal spread with a traditional bipolar grasper is significant (up to 13 mm). For that reason, when I desiccate the uterine vessels with the bipolar grasper, I stay above the rim of the colpotomy ring in order to maintain a safe distance from the ureter during this step.
The Harmonic Scalpel offers several advantages over monopolar electrosurgery for cutting, such as decreased smoke formation and avoidance of complications such as insulation failure, direct coupling, and capacitative coupling.
Dr. Einarsson reports no financial relationships relevant to this review.
Carl F. Giesler, MD
Associate Professor and Director of
Minimally Invasive Surgery,
Department of Obstetrics and Gynecology,
Baylor College of Medicine,
Waco, Tex
I was introduced to laparoscopy as a resident in 1974, and have been teaching laparoscopic surgical techniques to general surgeons and gynecologists since 1988. I began performing laparoscopic hysterectomy in 1991. As an educator and trainer of other laparoscopic surgeons, I have used all available laparoscopic energy sources.
I became an early adapter and user of ultrasonic mechanical energy when it was introduced for laparoscopy in 1995, because of its safety for adjacent tissues and its single-instrument, multiple-task properties.
This energy source was originally named the Ultrasonic Coagulating Shears by Ultracision, but Ethicon EndoSurgery purchased the product in 1995 and changed its name to the Harmonic Scalpel. The current generation is called the Harmonic ACE, and I use it as my primary laparoscopic energy source for all procedures, from management of unruptured ectopic pregnancy to total laparoscopic hysterectomy and extensive adhesiolyis in previously operated abdomens.
The Harmonic ACE allows me to confidently dissect adhesions over loops of bowel, as well as over the ureter, because of its limited lateral thermal spread. It allows nearly bloodless dissection of the retroperitoneal space and space around the bladder. The cavitation effect created by the rapidly moving instrument tip (55,500 cycles/second) creates tissue separation along the areolar tissue planes, where most blood vessels are the size of capillaries and where larger blood vessels are easily identified and controlled by coaptation, coagulation, and cutting.
The protein coagulum is produced at a temperature of 54°C. The sides and back of the active blade have minimal thermal effect because tissue is not held tightly against the moving blade. Minimal char is observed because of the low tissue temperature associated with coagulum formation. Minimal sticking of tissue occurs because the rapid motion of the instrument tip dislodges the tissue.
The ease of use, multiple functions for a single instrument, tissue safety, and minimal residual traumatized tissue are the reasons I prefer the mechanical energy of the Harmonic ACE as my primary laparoscopic energy source.
Dr. Giesler serves as a speaker for Ethicon EndoSurgery.
Cheryl Iglesia, MD
Director, Urogynecology and Reconstructive Pelvic Surgery,
Washington Hospital Center,
Associate Professor, Departments of ObGyn and Urology,
Georgetown University, Washington, DC.
Member, OBG Management Board of Editors
I prefer the Harmonic ACE for straight-stick laparoscopic hysterectomy cases and the PK Cutting Forceps for robotic hysterectomy and sacrocolpopexy.
The Harmonic ACE is fast and has little thermal spread; the PK Cutting Forceps is the only energy source available with reticulating arms for the robot.
I have been using the Harmonic ACE for more than 10 years and the PK Cutting Forceps since 2006.
Dr. Iglesia reports no financial relationships relevant to this review.
Keith Isaacson, MD
Director of Minimally Invasive Gynecologic
Surgery and Infertility,
Director of the AAGL/SRS
Fellowship in Minimally Invasive Gynecologic Surgery,
Newton Wellesley Hospital,
Associate Professor of Obstetrics, Gynecology,
and Reproductive Biology,
Harvard Medical School, Boston, Mass
I have used every bipolar and harmonic tool that is commercially available, including the recently released EnSeal. I like them all. The tools I have chosen were selected in conjunction with our purchasing department and determined to be the optimal tools for the lowest cost and highest efficiency. Our goal is to use as few disposables as possible, but to be as efficient as possible.
The instruments I utilize most frequently are the reusable 5-mm bipolar instruments from Karl Storz (RoBi) and ERBE Medical. The RoBi set includes a variety of tips, ranging from a dissecting Maryland-type tip to a flat, broad-based tip similar to the Kleppinger design. These are all plugged into the ERBE VIO generator. With this assortment of tools, I can seal a vessel of almost any size, whether it be thin-walled, such as a large vein, or a thick-walled artery.
To cut tissue, I prefer the Harmonic Scalpel and the bipolar Laparoscopic Spatula (Gyrus). I use the Harmonic Scalpel during laparoscopic hysterectomy, and the Spatula during laparoscopic myomectomy. Both devices cut tissue well while simultaneously sealing small vessels.
I have been using these instruments for approximately 10 years.
Dr. Isaacson reports that he is a consultant for Karl Storz in hysteroscopy.
Noor Ahmed, MB, ChB, MD
Consultant Gynecologist,
Dundee, Scotland.
Member, OBG Management Virtual
Board of Editors
I am a consultant gynecologist with an interest in fertility, endometriosis surgery, and general gynecologic surgery. Most of my surgical work is performed laparoscopically or vaginally.
My first experience with vessel-sealing technology was in 2000, when I began using the LigaSure Max for difficult vaginal hysterectomies. I was impressed by its ability to clamp and seal and, in later versions, its ability to cut, as well. Since then, I have been using various versions of LigaSure vessel- and tissue-sealing technology for many gynecologic procedures.
I have had some exposure to other vessel- and tissue-sealing technologies, but prefer LigaSure because:
1. It provides an experience similar to that of conventional clamp, cut, and suture through the control offered by its unique combination of pressure and energy, creating vessel and tissue fusion that does not rely on a proximal thrombus and produces minimal thermal spread.
2. The feedback-controlled response system automatically discontinues energy delivery when sealing is complete.
3. It works with clamps of various sizes and shapes, using the same hardware (generator) and saving money and space. Moreover, the nursing staff has to develop familiarity with one machine only.
For laparoscopic procedures, I use LigaSure Lap, LigaSure Atlas Sealer/Divider and, most commonly, the 5-mm LigaSure V Sealer/Divider.
Dr. Ahmed reports no financial relationships relevant to this review.
Michael Kirwin, MD
General Obstetrics and Gynecology, Freehold, NJ.
Member, OBG Management Virtual Board of Editors
With advanced laparoscopic cases, particularly hysterectomy, I use the Harmonic Scalpel and PK Cutting Forceps. I have used the Harmonic Scalpel regularly for approximately 4 years, and it works well for dissection and, particularly, for transecting the cervix in supracervical cases.
Over the past year, I have also begun to use the Gyrus forceps, especially when I expect to encounter larger vessels that require coagulation. Both instruments can be used in 5-mm ports. Both seem relatively reliable and easy for the OR team to use. I would hope that an articulation feature can be added in the future.
Dr. Kirwin reports no financial relationships relevant to this review.
Does sildenafil improve antidepressant-related sexual dysfunction in women?
Assessment of female sexual dysfunction is difficult
The four areas of potential abnormality in sexual function, as described in the Diagnostic and Statistical Manual of Mental Disorders–IV, include desire, arousal, orgasm, and pain and are more difficult to differentiate in women than in men. In addition, the variability of the hormonal environment makes it challenging to study the physiologic mechanisms of some of these conditions.
Nurnberg and colleagues sought to eliminate most of the variables by including only premenopausal women who had normal hormone levels at baseline (mean estradiol level, 67 pg/mL; FSH
Selective and nonselective SRIs have been associated with sexual side effects in up to 70% of women—specifically, delayed or absent orgasm, decreased arousal and lubrication, and diminished libido.
Details of the study
This prospective, randomized, double-blind, placebo-controlled trial aimed to assess the efficacy of 50 to 100 mg of sildenafil in reversing new-onset sexual dysfunction in women adequately treated for their underlying major depression. Ninety-eight women (average age, 37 years) were randomized, with 37 and 39 completing the 8-week trial in the placebo and active treatment arms, respectively.
Utilizing an intent-to-treat statistical analysis, the authors found that women taking an average dosage of 91.7 mg of sildenafil experienced significant improvement in overall sexual satisfaction, lubrication, and ability to reach orgasm. Four different scales of sexual function were utilized in the analysis, each of which has been well validated in previous studies. Of note, 28% of women taking sildenafil and 73% of those taking placebo reported no improvement in sexual function throughout the 8-week study.
In this study, responders had slightly (and statistically significantly) higher levels of both free testosterone (1.44 pg/mL vs 1.04 pg/mL) and thyroxine (8.83 ng/dL vs 7.75 ng/dL) than nonresponders in the treatment arm.
These findings are the first to demonstrate efficacy in women
Physiologically, these findings make sense. In estrogen-replete women, type-5 phosphodiesterase inhibitors are thought to increase blood flow, engorgement, and lubrication to the genitalia, resulting in improved sensation and arousal.
By eliminating or controlling for many of the confounders in earlier studies of sildenafil in women, this well-designed trial has demonstrated, for the first time, significant improvement in women who have sexual complaints related to antidepressant medication.
This study shows that treatment with one of the type-5 phosphodiesterase inhibitors for arousal or orgasmic problems, or both, makes sense. It is critical to ensure that baseline hormone levels are well within normal premenopausal ranges and that underlying depression or relationship issues are not contributing to low libido if you are to expect improvement.
Women who have new-onset sexual dysfunction after taking SRIs are most likely to respond. Look for subtle thyroid problems or low testosterone levels in nonresponders.—BARBARA S. LEVY, MD
Assessment of female sexual dysfunction is difficult
The four areas of potential abnormality in sexual function, as described in the Diagnostic and Statistical Manual of Mental Disorders–IV, include desire, arousal, orgasm, and pain and are more difficult to differentiate in women than in men. In addition, the variability of the hormonal environment makes it challenging to study the physiologic mechanisms of some of these conditions.
Nurnberg and colleagues sought to eliminate most of the variables by including only premenopausal women who had normal hormone levels at baseline (mean estradiol level, 67 pg/mL; FSH
Selective and nonselective SRIs have been associated with sexual side effects in up to 70% of women—specifically, delayed or absent orgasm, decreased arousal and lubrication, and diminished libido.
Details of the study
This prospective, randomized, double-blind, placebo-controlled trial aimed to assess the efficacy of 50 to 100 mg of sildenafil in reversing new-onset sexual dysfunction in women adequately treated for their underlying major depression. Ninety-eight women (average age, 37 years) were randomized, with 37 and 39 completing the 8-week trial in the placebo and active treatment arms, respectively.
Utilizing an intent-to-treat statistical analysis, the authors found that women taking an average dosage of 91.7 mg of sildenafil experienced significant improvement in overall sexual satisfaction, lubrication, and ability to reach orgasm. Four different scales of sexual function were utilized in the analysis, each of which has been well validated in previous studies. Of note, 28% of women taking sildenafil and 73% of those taking placebo reported no improvement in sexual function throughout the 8-week study.
In this study, responders had slightly (and statistically significantly) higher levels of both free testosterone (1.44 pg/mL vs 1.04 pg/mL) and thyroxine (8.83 ng/dL vs 7.75 ng/dL) than nonresponders in the treatment arm.
These findings are the first to demonstrate efficacy in women
Physiologically, these findings make sense. In estrogen-replete women, type-5 phosphodiesterase inhibitors are thought to increase blood flow, engorgement, and lubrication to the genitalia, resulting in improved sensation and arousal.
By eliminating or controlling for many of the confounders in earlier studies of sildenafil in women, this well-designed trial has demonstrated, for the first time, significant improvement in women who have sexual complaints related to antidepressant medication.
This study shows that treatment with one of the type-5 phosphodiesterase inhibitors for arousal or orgasmic problems, or both, makes sense. It is critical to ensure that baseline hormone levels are well within normal premenopausal ranges and that underlying depression or relationship issues are not contributing to low libido if you are to expect improvement.
Women who have new-onset sexual dysfunction after taking SRIs are most likely to respond. Look for subtle thyroid problems or low testosterone levels in nonresponders.—BARBARA S. LEVY, MD
Assessment of female sexual dysfunction is difficult
The four areas of potential abnormality in sexual function, as described in the Diagnostic and Statistical Manual of Mental Disorders–IV, include desire, arousal, orgasm, and pain and are more difficult to differentiate in women than in men. In addition, the variability of the hormonal environment makes it challenging to study the physiologic mechanisms of some of these conditions.
Nurnberg and colleagues sought to eliminate most of the variables by including only premenopausal women who had normal hormone levels at baseline (mean estradiol level, 67 pg/mL; FSH
Selective and nonselective SRIs have been associated with sexual side effects in up to 70% of women—specifically, delayed or absent orgasm, decreased arousal and lubrication, and diminished libido.
Details of the study
This prospective, randomized, double-blind, placebo-controlled trial aimed to assess the efficacy of 50 to 100 mg of sildenafil in reversing new-onset sexual dysfunction in women adequately treated for their underlying major depression. Ninety-eight women (average age, 37 years) were randomized, with 37 and 39 completing the 8-week trial in the placebo and active treatment arms, respectively.
Utilizing an intent-to-treat statistical analysis, the authors found that women taking an average dosage of 91.7 mg of sildenafil experienced significant improvement in overall sexual satisfaction, lubrication, and ability to reach orgasm. Four different scales of sexual function were utilized in the analysis, each of which has been well validated in previous studies. Of note, 28% of women taking sildenafil and 73% of those taking placebo reported no improvement in sexual function throughout the 8-week study.
In this study, responders had slightly (and statistically significantly) higher levels of both free testosterone (1.44 pg/mL vs 1.04 pg/mL) and thyroxine (8.83 ng/dL vs 7.75 ng/dL) than nonresponders in the treatment arm.
These findings are the first to demonstrate efficacy in women
Physiologically, these findings make sense. In estrogen-replete women, type-5 phosphodiesterase inhibitors are thought to increase blood flow, engorgement, and lubrication to the genitalia, resulting in improved sensation and arousal.
By eliminating or controlling for many of the confounders in earlier studies of sildenafil in women, this well-designed trial has demonstrated, for the first time, significant improvement in women who have sexual complaints related to antidepressant medication.
This study shows that treatment with one of the type-5 phosphodiesterase inhibitors for arousal or orgasmic problems, or both, makes sense. It is critical to ensure that baseline hormone levels are well within normal premenopausal ranges and that underlying depression or relationship issues are not contributing to low libido if you are to expect improvement.
Women who have new-onset sexual dysfunction after taking SRIs are most likely to respond. Look for subtle thyroid problems or low testosterone levels in nonresponders.—BARBARA S. LEVY, MD