User login
Difficulty swallowing solid foods; food ‘getting stuck in the chest’
A 61-year-old woman presents to her primary care physician because for the last 4 weeks she has had difficulty swallowing solid food and a feeling of food “getting stuck in the chest.” She also reports having nausea, mild epigastric pain, and heartburn. She denies having fevers, chills, night sweats, weight loss, vomiting, diarrhea, hematochezia, or melena.
Medical history
For the past 20 years, she has had gastroesophageal reflux disease (GERD), intermittently treated with a proton pump inhibitor. She also has arthritis, hyperlipidemia, hypertension, and asthma, and she has undergone right hip replacement for a hip fracture. She has no known allergies.
She lives in the Midwest region of the United States and is on disability due to her arthritis. She is divorced and has three children.
She quit smoking 3 years ago after smoking half a pack per day for 30 years. She drinks socially; she has never used recreational drugs.
She recalls that an uncle had cancer, but she does not know the specific type.
Physical examination
The patient’s temperature is 96.7°F (35.9°C), heart rate 86 per minute, blood pressure 150/92 mm Hg, respiratory rate 16 per minute, and oxygen saturation 100% on room air.
Differential diagnosis
Although the differential diagnosis at this stage is broad, a few conditions that commonly present like this are:
- Esophageal cancer
- Esophageal stricture
- Esophageal webs
- Esophagitis (infectious, inflammatory)
- Peptic ulcer disease.
WHICH TEST SHOULD BE ORDERED?
1. Which test will you order now for this patient?
- Endoscopy (esophagogastroduodenoscopy)
- Serum Helicobacter pylori antibody testing
- Wireless pH monitoring
- Barium swallow
Endoscopy would be the best test to order. Esophageal cancer and esophageal stricture must be ruled out, in view of her long history of GERD, gastritis, and smoking and her alarming symptoms of difficulty swallowing and food sticking. In this situation, endoscopy is the first test recommended. In addition to its diagnostic value, it offers an opportunity to obtain tissue samples and to perform a therapeutic intervention, if necessary.1,2
H pyloriantibody testing is used in the “test-and-treat approach” for H pylori infection, an established management strategy for patients who have uninvestigated dyspepsia and who are younger than 55 years and have no “alarm features,” ie, red flags for cancer. The alarm features commonly described are anemia, early satiety, unexplained weight loss, bleeding, odynophagia, progressive dysphagia, unexplained vomiting, and a family history or prior history of gastrointestinal malignancy.3
Our patient’s symptoms raise the possibility of cancer, so that H pylori testing would not be the best test to order at this point.
Ambulatory wireless pH monitoring with a wireless pH capsule is useful for confirming GERD in those with persistent symptoms (whether typical or atypical) who do not have evidence of mucosal damage on initial endoscopy, particularly if a trial of acid suppression has failed.4–6
Barium swallow is an x-ray examination of the esophagus with contrast. It can show both the anatomy and the function of the esophagus, and it would be the initial diagnostic procedure of choice for patients with dysphagia who have no alarm symptoms.7 However, our patient does have alarm symptoms.
First highlight point
- Endoscopy is the first test in patients with dysphagia with alarm symptoms.
CASE CONTINUES: ENDOSCOPY
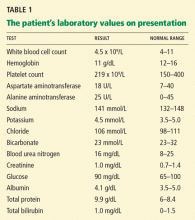
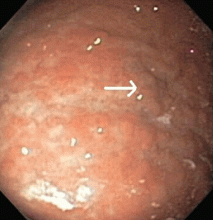
Multiple biopsies of the nodules show atypical lymphoid infiltrates with small cleaved lymphocytes that are mostly positive for CD5, CD20, and CD43 and negative for CD10 and CD23 by flow cytometry. In addition, a staining test for H pylori is positive.
Comment. Our patient has had GERD for the past 20 years, intermittently treated with a proton pump inhibitor. Acid suppressive therapy with a proton pump inhibitor is the standard of care of patients with erosive esophagitis. In standard doses, these drugs control symptoms in most cases and heal esophagitis in almost 90% of cases within 4 to 8 weeks.9 Proton pump inhibitors are also effective for maintaining healing of esophagitis and controlling symptoms in patients who respond to an acute course of therapy for a period of 6 to 12 months.10
WHAT IS THE DIAGNOSIS?
2. Which is the most likely diagnosis for our patient?
- Fundic gland polyps
- Gastric hyperplastic polyps
- Gastric adenomas
- Mucosa-associated lymphoid tissue (MALT) lymphoma
Fundic gland polyps are small (0.1–0.8 cm), hyperemic, sessile, flat, nodular lesions that have a smooth surface. They occur exclusively in the gastric corpus and are composed of normal gastric corpus-type epithelium arranged in a disorderly or microcystic configuration. 11 This pattern does not match our patient’s findings.
Gastric hyperplastic polyps are elongated, cystic, and distorted foveolar epithelium with marked regeneration. Other histologic findings are stromal inflammation, edema, and smooth muscle hyperplasia.12 This does not match our patient’s findings.
Adenomas can be flat or polypoid and range in size from a few millimeters to several centimeters. Endoscopically, adenomatous polyps have a velvety, lobulated appearance. Most are solitary (82% of cases), located in the antrum, and less than 2 cm in diameter.13 This does not match our patient’s findings.
MALT lymphoma, the correct answer, is characterized by small cleaved lymphocytes positive for CD4, CD20, and CD43. Although CD5 positivity is not characteristic, rare cases of MALT lymphoma may be CD5-positive and may be more aggressive.14
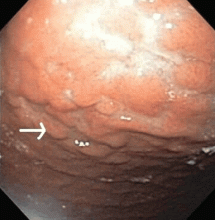
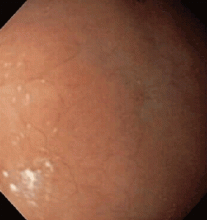
Other common features of MALT lymphoma are erosions, small nodules, thickening of gastric folds—generally suggesting a benign condition—or hyperemic or even normal gastric mucosa.15 Our patient’s complaint of food sticking in her chest and difficulty swallowing was most likely related to the erosive esophagitis found on endoscopy.
A TYPE OF NON-HODGKIN LYMPHOMA
Normal gastric mucosa contains no lymphoid tissue.16,17 Primary gastric lymphoma, of which MALT lymphoma is a subtype, accounts for around 5% of gastric malignancies, with an annual incidence rate of 0.5 per 100,000 people. 18–20 Although rare, it accounts for 60% to 70% of cases of non-Hodgkin lymphoma of the gastrointestinal tract and can involve the perigastric or abdominal lymph nodes or both.21–23 Although earlier studies suggested that its incidence was increasing, recent data indicate the incidence may be decreasing, thanks to active H pylori treatment.24–26
Two subtypes of primary gastric non-Hodgkin lymphoma commonly described are MALT lymphoma and diffuse large B-cell (DLBC) lymphoma. In the Revised European-American Lymphoma Classification, high-grade MALT lymphoma is comparable to DLBC lymphoma and may have transformed from low-grade MALT lymphoma.27,28 Another reported subtype, mantle cell lymphoma with MALT lymphoma features, should be considered in the differential diagnosis, although it is rare.29
MALT lymphoma is linked to H pylori
H pylori infection is a factor in the development of MALT lymphoma,30 as multiple lines of evidence show:
- H pylori infection has been reported in more than 90% of patients with MALT lymphoma.31–35
- H pylori antibodies have been found in stored serum drawn from patients who subsequently developed MALT lymphoma.35
- In response to H pylori antigens, T cells from MALT lymphoma proliferate and cause an increase in tumor immunoglobulin production.36
- In animals experimentally infected with H pylori, around one-third develop lymphoid follicles and lymphoepithelial lesions including B cells, which are similar to human MALT lymphoma.37
However, only a minority of patients with H pylori develop lymphoma, owing to a host immune response that is not well defined.
Second highlight point
- Gastric MALT lymphoma is associated with H pylori.
Associated genetic translocations
Three translocations, t(11;18), t(1;14), and t(14;18), are specifically associated with MALT lymphoma, and the genes involved have been characterized.
The t(11;18) translocation, seen in gastric and nongastric MALT lymphoma, is not seen in H pylori gastritis.38 This translocation is usually associated with extension of the disease outside the stomach (ie, to regional lymph nodes or distal sites).27 Most cases that do not respond to H pylori eradication involve the t(11;18) and t(1;14) translocations.28
Clinical presentation of gastric MALT lymphoma
The average age at presentation with gastric MALT lymphoma is 54 to 58 years.
The most common complaint is nonspecific abdominal pain in the epigastric region, sometimes accompanied by weight loss, nausea, vomiting, and, in a quarter of cases, acute or chronic bleeding.39–41 Weight loss is common, and its extent is associated with the location and the grade of the disease.
Most cases of MALT lymphoma are found serendipitously during endoscopy, on which the appearance of the lesions ranges from small ulcerations to polypoid masses with infiltrated, thickened folds involving predominantly the antrum or prepyloric region.15,41
MANAGING MALT LYMPHOMA
Our patient undergoes endoscopic ultrasonography, which reveals she has stage I disease, ie, it is limited to the stomach without involving the lymph nodes (stage II), adjacent organs (stage III), or distant organs (stage IV).
3. How will you treat this patient, given the present information?
- Chemotherapy
- Radiation therapy
- Surgery
- Antibiotics with a proton pump inhibitor
Antibiotics with a proton pump inhibitor would be best. According to the Maastricht III Consensus Report,42H pylori eradication is the treatment of first choice for H pylori infection in patients with stage I low-grade gastric MALT lymphoma. This therapy can induce complete histologic remission in 80% to 100% of patients with MALT lymphoma. 43 Several studies have shown regression44 or complete remission of low-grade gastric MALT lymphoma after eradication of H pylori with antibiotics, making it a reasonable initial treatment.45–49
Several regimens are used. The first choice in populations in which the prevalence of resistance to clarithromycin (Biaxin) is less than 15% to 20% is a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole (Flagyl). (Metronidazole is preferable to amoxicillin if the prevalence of resistance to metronidazole is less than 40%.)
Sequential treatment—ie, 5 days of a proton pump inhibitor plus amoxicillin followed by 5 additional days of a proton pump inhibitor plus clarithromycin plus tinidazole (Tindamax)— may be better than a 7-day course of the combination of a proton pump inhibitor, amoxicillin, and clarithromycin.50,51
Treatment with a proton pump inhibitor, clarithromycin (500 mg twice a day), and either amoxicillin (1,000 mg twice a day) or metronidazole (400 or 500 mg twice a day) for 14 days is more effective than treatment for 7 days.52
H pylori reinfection in the general population is quite rare, with an estimated yearly rate as low as 2%.53 Recurrence of the infection is a risk factor for lymphoma relapse.17,54
Several predictors of the response of MALT lymphoma to eradication therapy have been recognized: H pylori positivity, stage I, lymphoma confined to the stomach; gastric wall invasion confined to mucosa and submucosa, and the absence of the t(11;18) translocation.
The time between H pylori eradication and complete remission of primary gastric lymphoma varies and can be longer than 12 months.55
Chemotherapy. In a single study,56 complete remission was achieved with oral cyclophosphamide (Cytoxan) in cases of antibiotic-refractory gastric MALT lymphoma. Comparable results were achieved after radiation therapy (see below); hence, oral monotherapy with cyclophosphamide might also be a suitable second-line therapy.57
The regimen of cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone (CHOP) has been recommended for patients with stage III and IV disease.41,58
Rituximab (Rituxan) has been proven effective in treating t(11;18)-positive MALT lymphoma.59
Radiation therapy. Two studies have shown a 100% complete response rate after radiation therapy with a median dose of 30 Gy.57,60 Tsang et al61 reported complete remission in up to 90% of patients receiving radiation therapy alone, with excellent 5-year progression-free and overall survival rates of 98% and 77%, respectively.
Although surgery, radiotherapy, and chemotherapy have been used in cases in which eradication therapy failed and in more advanced stages of MALT lymphoma, there is no consensus about their use, so therapy must be individualized.
Fourth highlight point
- Antibiotic treatment for eradication of H pylori infection is the recommended treatment only for stage I low-grade MALT lymphoma.
FOLOW-UP
4. How should you follow patients with MALT lymphoma?
- Endoscopy
- H pylori testing
- Computed tomography and magnetic resonance imaging
- No surveillance required after treatment
Endoscopy is the correct answer. As initial diagnostic biopsies do not exclude aggressive lymphoma, careful endoscopic follow-up is recommended. A recommended schedule is a breath test for H pylori every 2 months in conjunction with repeat endoscopy with biopsies every 3 to 6 months for the first 2 years, and then annually.62
Although H pylori may be eradicated within 1 month of drug therapy, lymphoma may take several months to disappear histologically. In patients with stage I disease with residual lymphoma after H pylori eradication therapy, a simple wait-and-watch strategy is a suitable alternative to oncologic therapy.63,64
Local relapse may occur after many years of complete remission; thus, patients should be followed closely long-term with endoscopy and possibly endoscopic ultrasonography. 47–49,63
Patients who fail to attain a complete remission within 12 months should undergo radiation therapy, with or without chemotherapy. The same therapy should be started as soon as possible in patients with progressive disease after antibiotic therapy. Patients negative for H pylori, patients with stage II disease, and patients with t(11;18) translocation should receive antibiotic treatment with endoscopic surveillance every 3 months.
Fifth highlight point
- Surveillance endoscopy is recommended for follow-up of MALT lymphoma.
CASE CONTINUES: HER CONDITION IMPROVES, THEN WORSENS
Computed tomography of the chest, abdomen, and pelvis reveals no evidence of additional sites of tumor. Positron emission tomography reveals increased uptake in the left tonsillar region, for which she has undergoes an ear, nose, and throat evaluation, and no pathology is found.
Due to recurrence of her marginal zone Bcell lymphoma of MALT type of the stomach, the patient is referred to an oncology service. She is treated with radiation, receiving 15 sessions of 30 Gy localized to the stomach. Three months after radiation therapy, she undergoes endoscopy again, which shows no evidence of the previously described nodules. Repeat biopsies are negative for H pylori and MALT lymphoma.
Annual surveillance endoscopy and computed tomography for the past 3 years have been negative for any tumor recurrence.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808–1825.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Furlow B. Barium swallow. Radiol Technol 2004; 76:49–58.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Havelund T, Laursen LS, Skoubo-Kristensen E, et al. Omeprazole and ranitidine in treatment of reflux oesophagitis: double blind comparative trial. Br Med J (Clin Res Ed) 1988; 296:89–92.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol 1996; 27:896–903.
- Snover DC. Benign epithelial polyps of the stomach. Pathol Annu 1985; 20:303–329.
- Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009; 6:331–341.
- Wenzel C, Dieckmann K, Fiebiger W, Mannhalter C, Chott A, Raderer M. CD5 expression in a lymphoma of the mucosa-associated lymphoid tissue (MALT)-type as a marker for early dissemination and aggressive clinical behaviour. Leuk Lymphoma 2001; 42:823–829.
- Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol 2003; 98:975–986.
- Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med 1999; 131:88–95.
- Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002; 50(suppl 3):III19–III24.
- Ducreux M, Boutron MC, Piard F, Carli PM, Faivre J. A 15-year series of gastrointestinal non-Hodgkin’s lymphomas: a population-based study. Br J Cancer 1998; 77:511–514.
- Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma in a population-based registry. Br J Cancer 1999; 79:1929–1934.
- d’Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994; 12:1673–1684.
- Koch P, del Valle F, Berdel WE, et al; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 19:3861–3873.
- Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma 2006; 47:2140–2146.
- Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology 1992; 20:29–34.
- Wotherspoon AC. Choosing the right MALT. Gut 1996; 39:617–618.
- Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003; 97:2462–2473.
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2009; epub ahead of print.
- Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001; 98:1182–1187.
- Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357:39–40.
- Shibata K, Shimamoto Y, Nakano S, Miyahara M, Nakano H, Yamaguchi M. Mantle cell lymphoma with the features of mucosa-associated lymphoid tissue (MALT) lymphoma in an HTLV-I-seropositive patient. Ann Hematol 1995; 70:47–51.
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 2005; 23:6370–6378.
- de Jong D, Boot H, van Heerde P, Hart GA, Taal BG. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology 1997; 112:1466–1474.
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338:1175–1176.
- Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol 1994; 47:436–439.
- Doglioni C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339:834–835.
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330:1267–1271.
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993; 342:571–574.
- Lee A, O’Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations—mouse models of gastric lymphoma. Recent Results Cancer Res 2000; 156:42–51.
- Auer IA, Gascoyne RD, Connors JM, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997; 8:979–985.
- Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Malignant tumors of the stomach. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori. Gastroenterol Clin North Am 2000; 29:593–607.
- Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d’Etude des Lymphomes Digestifs. Gastroenterology 1993; 105:1662–1671.
- Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 1991; 101:1159–1170.
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56:772–781.
- Boot H, de Jong D. Gastric lymphoma: the revolution of the past decade. Scand J Gastroenterol Suppl 2002; 236:27–36.
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
- Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995; 345:1591–1594.
- Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med 1995; 122:767–769.
- Ruskoné-Fourmestraux A. Gastrointestinal lymphomas: the French experience of the Groupe d’Étude des Lymphomes Digestifs (GELD). Recent Results Cancer Res 2000; 156:99–103.
- Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 2005; 23:8018–8024.
- Wündisch T, Mösch C, Neubauer A, Stolte M. Helicobacter pylori eradication in gastric mucosa-associated lymphoid tissue lymphoma: results of a 196-patient series. Leuk Lymphoma 2006; 47:2110–2114.
- De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther 2004; 19:407–414.
- Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719–726.
- Paoluzi P, Iacopini F, Crispino P, et al. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 2006; 11:562–568.
- Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther 2006; 23:275–280.
- Bayerdörffer E, Morgner A. Gastric marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: management of the disease. Dig Liver Dis 2000; 32:192–194.
- Savio A, Zamboni G, Capelli P, et al. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Long-term post-treatment follow-up of a multicenter trial in the north-east of Italy and evaluation of the diagnostic protocol’s adequacy. Recent Results Cancer Res 2000; 156:116–124.
- Nakamura S, Matsumoto T, Suekane H, et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005; 104:532–540.
- Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998; 16:1916–1921.
- Solidoro A, Payet C, Sanchez-Lihon J, Montalbetti JA. Gastric lymphomas: chemotherapy as a primary treatment. Semin Surg Oncol 1990; 6:218–225.
- Lévy M, Copie-Bergman C, Molinier-Frenkel V, et al. Treatment of t(11;18)-positive gastric mucosa-associated lymphoid tissue lymphoma with rituximab and chlorambucil: clinical, histological, and molecular follow-up. Leuk Lymphoma 2010; 51:284–290.
- Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol 2001; 80(suppl 3):B100–B105.
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosaassociated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21:4157–4164.
- Hung PD, Schubert ML, Mihas AA. Marginal zone B-cell lymphoma (MALT lymphoma). Curr Treat Options Gastroenterol 2004; 7:133–138.
- Zucca E, Cavalli F. Are antibiotics the treatment of choice for gastric lymphoma? Curr Hematol Rep 2004; 3:11–16.
- Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al; EGI LS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut 2007; 56:1685–1687.
A 61-year-old woman presents to her primary care physician because for the last 4 weeks she has had difficulty swallowing solid food and a feeling of food “getting stuck in the chest.” She also reports having nausea, mild epigastric pain, and heartburn. She denies having fevers, chills, night sweats, weight loss, vomiting, diarrhea, hematochezia, or melena.
Medical history
For the past 20 years, she has had gastroesophageal reflux disease (GERD), intermittently treated with a proton pump inhibitor. She also has arthritis, hyperlipidemia, hypertension, and asthma, and she has undergone right hip replacement for a hip fracture. She has no known allergies.
She lives in the Midwest region of the United States and is on disability due to her arthritis. She is divorced and has three children.
She quit smoking 3 years ago after smoking half a pack per day for 30 years. She drinks socially; she has never used recreational drugs.
She recalls that an uncle had cancer, but she does not know the specific type.
Physical examination
The patient’s temperature is 96.7°F (35.9°C), heart rate 86 per minute, blood pressure 150/92 mm Hg, respiratory rate 16 per minute, and oxygen saturation 100% on room air.
Differential diagnosis
Although the differential diagnosis at this stage is broad, a few conditions that commonly present like this are:
- Esophageal cancer
- Esophageal stricture
- Esophageal webs
- Esophagitis (infectious, inflammatory)
- Peptic ulcer disease.
WHICH TEST SHOULD BE ORDERED?
1. Which test will you order now for this patient?
- Endoscopy (esophagogastroduodenoscopy)
- Serum Helicobacter pylori antibody testing
- Wireless pH monitoring
- Barium swallow
Endoscopy would be the best test to order. Esophageal cancer and esophageal stricture must be ruled out, in view of her long history of GERD, gastritis, and smoking and her alarming symptoms of difficulty swallowing and food sticking. In this situation, endoscopy is the first test recommended. In addition to its diagnostic value, it offers an opportunity to obtain tissue samples and to perform a therapeutic intervention, if necessary.1,2
H pyloriantibody testing is used in the “test-and-treat approach” for H pylori infection, an established management strategy for patients who have uninvestigated dyspepsia and who are younger than 55 years and have no “alarm features,” ie, red flags for cancer. The alarm features commonly described are anemia, early satiety, unexplained weight loss, bleeding, odynophagia, progressive dysphagia, unexplained vomiting, and a family history or prior history of gastrointestinal malignancy.3
Our patient’s symptoms raise the possibility of cancer, so that H pylori testing would not be the best test to order at this point.
Ambulatory wireless pH monitoring with a wireless pH capsule is useful for confirming GERD in those with persistent symptoms (whether typical or atypical) who do not have evidence of mucosal damage on initial endoscopy, particularly if a trial of acid suppression has failed.4–6
Barium swallow is an x-ray examination of the esophagus with contrast. It can show both the anatomy and the function of the esophagus, and it would be the initial diagnostic procedure of choice for patients with dysphagia who have no alarm symptoms.7 However, our patient does have alarm symptoms.
First highlight point
- Endoscopy is the first test in patients with dysphagia with alarm symptoms.
CASE CONTINUES: ENDOSCOPY
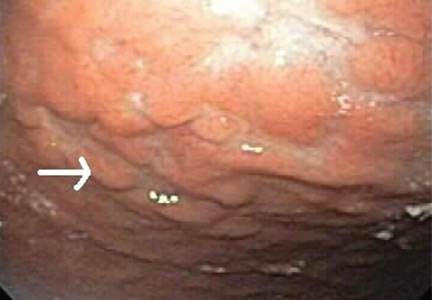
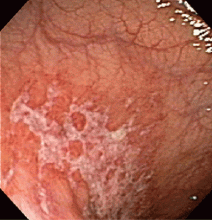
Multiple biopsies of the nodules show atypical lymphoid infiltrates with small cleaved lymphocytes that are mostly positive for CD5, CD20, and CD43 and negative for CD10 and CD23 by flow cytometry. In addition, a staining test for H pylori is positive.
Comment. Our patient has had GERD for the past 20 years, intermittently treated with a proton pump inhibitor. Acid suppressive therapy with a proton pump inhibitor is the standard of care of patients with erosive esophagitis. In standard doses, these drugs control symptoms in most cases and heal esophagitis in almost 90% of cases within 4 to 8 weeks.9 Proton pump inhibitors are also effective for maintaining healing of esophagitis and controlling symptoms in patients who respond to an acute course of therapy for a period of 6 to 12 months.10
WHAT IS THE DIAGNOSIS?
2. Which is the most likely diagnosis for our patient?
- Fundic gland polyps
- Gastric hyperplastic polyps
- Gastric adenomas
- Mucosa-associated lymphoid tissue (MALT) lymphoma
Fundic gland polyps are small (0.1–0.8 cm), hyperemic, sessile, flat, nodular lesions that have a smooth surface. They occur exclusively in the gastric corpus and are composed of normal gastric corpus-type epithelium arranged in a disorderly or microcystic configuration. 11 This pattern does not match our patient’s findings.
Gastric hyperplastic polyps are elongated, cystic, and distorted foveolar epithelium with marked regeneration. Other histologic findings are stromal inflammation, edema, and smooth muscle hyperplasia.12 This does not match our patient’s findings.
Adenomas can be flat or polypoid and range in size from a few millimeters to several centimeters. Endoscopically, adenomatous polyps have a velvety, lobulated appearance. Most are solitary (82% of cases), located in the antrum, and less than 2 cm in diameter.13 This does not match our patient’s findings.
MALT lymphoma, the correct answer, is characterized by small cleaved lymphocytes positive for CD4, CD20, and CD43. Although CD5 positivity is not characteristic, rare cases of MALT lymphoma may be CD5-positive and may be more aggressive.14
Other common features of MALT lymphoma are erosions, small nodules, thickening of gastric folds—generally suggesting a benign condition—or hyperemic or even normal gastric mucosa.15 Our patient’s complaint of food sticking in her chest and difficulty swallowing was most likely related to the erosive esophagitis found on endoscopy.
A TYPE OF NON-HODGKIN LYMPHOMA
Normal gastric mucosa contains no lymphoid tissue.16,17 Primary gastric lymphoma, of which MALT lymphoma is a subtype, accounts for around 5% of gastric malignancies, with an annual incidence rate of 0.5 per 100,000 people. 18–20 Although rare, it accounts for 60% to 70% of cases of non-Hodgkin lymphoma of the gastrointestinal tract and can involve the perigastric or abdominal lymph nodes or both.21–23 Although earlier studies suggested that its incidence was increasing, recent data indicate the incidence may be decreasing, thanks to active H pylori treatment.24–26
Two subtypes of primary gastric non-Hodgkin lymphoma commonly described are MALT lymphoma and diffuse large B-cell (DLBC) lymphoma. In the Revised European-American Lymphoma Classification, high-grade MALT lymphoma is comparable to DLBC lymphoma and may have transformed from low-grade MALT lymphoma.27,28 Another reported subtype, mantle cell lymphoma with MALT lymphoma features, should be considered in the differential diagnosis, although it is rare.29
MALT lymphoma is linked to H pylori
H pylori infection is a factor in the development of MALT lymphoma,30 as multiple lines of evidence show:
- H pylori infection has been reported in more than 90% of patients with MALT lymphoma.31–35
- H pylori antibodies have been found in stored serum drawn from patients who subsequently developed MALT lymphoma.35
- In response to H pylori antigens, T cells from MALT lymphoma proliferate and cause an increase in tumor immunoglobulin production.36
- In animals experimentally infected with H pylori, around one-third develop lymphoid follicles and lymphoepithelial lesions including B cells, which are similar to human MALT lymphoma.37
However, only a minority of patients with H pylori develop lymphoma, owing to a host immune response that is not well defined.
Second highlight point
- Gastric MALT lymphoma is associated with H pylori.
Associated genetic translocations
Three translocations, t(11;18), t(1;14), and t(14;18), are specifically associated with MALT lymphoma, and the genes involved have been characterized.
The t(11;18) translocation, seen in gastric and nongastric MALT lymphoma, is not seen in H pylori gastritis.38 This translocation is usually associated with extension of the disease outside the stomach (ie, to regional lymph nodes or distal sites).27 Most cases that do not respond to H pylori eradication involve the t(11;18) and t(1;14) translocations.28
Clinical presentation of gastric MALT lymphoma
The average age at presentation with gastric MALT lymphoma is 54 to 58 years.
The most common complaint is nonspecific abdominal pain in the epigastric region, sometimes accompanied by weight loss, nausea, vomiting, and, in a quarter of cases, acute or chronic bleeding.39–41 Weight loss is common, and its extent is associated with the location and the grade of the disease.
Most cases of MALT lymphoma are found serendipitously during endoscopy, on which the appearance of the lesions ranges from small ulcerations to polypoid masses with infiltrated, thickened folds involving predominantly the antrum or prepyloric region.15,41
MANAGING MALT LYMPHOMA
Our patient undergoes endoscopic ultrasonography, which reveals she has stage I disease, ie, it is limited to the stomach without involving the lymph nodes (stage II), adjacent organs (stage III), or distant organs (stage IV).
3. How will you treat this patient, given the present information?
- Chemotherapy
- Radiation therapy
- Surgery
- Antibiotics with a proton pump inhibitor
Antibiotics with a proton pump inhibitor would be best. According to the Maastricht III Consensus Report,42H pylori eradication is the treatment of first choice for H pylori infection in patients with stage I low-grade gastric MALT lymphoma. This therapy can induce complete histologic remission in 80% to 100% of patients with MALT lymphoma. 43 Several studies have shown regression44 or complete remission of low-grade gastric MALT lymphoma after eradication of H pylori with antibiotics, making it a reasonable initial treatment.45–49
Several regimens are used. The first choice in populations in which the prevalence of resistance to clarithromycin (Biaxin) is less than 15% to 20% is a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole (Flagyl). (Metronidazole is preferable to amoxicillin if the prevalence of resistance to metronidazole is less than 40%.)
Sequential treatment—ie, 5 days of a proton pump inhibitor plus amoxicillin followed by 5 additional days of a proton pump inhibitor plus clarithromycin plus tinidazole (Tindamax)— may be better than a 7-day course of the combination of a proton pump inhibitor, amoxicillin, and clarithromycin.50,51
Treatment with a proton pump inhibitor, clarithromycin (500 mg twice a day), and either amoxicillin (1,000 mg twice a day) or metronidazole (400 or 500 mg twice a day) for 14 days is more effective than treatment for 7 days.52
H pylori reinfection in the general population is quite rare, with an estimated yearly rate as low as 2%.53 Recurrence of the infection is a risk factor for lymphoma relapse.17,54
Several predictors of the response of MALT lymphoma to eradication therapy have been recognized: H pylori positivity, stage I, lymphoma confined to the stomach; gastric wall invasion confined to mucosa and submucosa, and the absence of the t(11;18) translocation.
The time between H pylori eradication and complete remission of primary gastric lymphoma varies and can be longer than 12 months.55
Chemotherapy. In a single study,56 complete remission was achieved with oral cyclophosphamide (Cytoxan) in cases of antibiotic-refractory gastric MALT lymphoma. Comparable results were achieved after radiation therapy (see below); hence, oral monotherapy with cyclophosphamide might also be a suitable second-line therapy.57
The regimen of cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone (CHOP) has been recommended for patients with stage III and IV disease.41,58
Rituximab (Rituxan) has been proven effective in treating t(11;18)-positive MALT lymphoma.59
Radiation therapy. Two studies have shown a 100% complete response rate after radiation therapy with a median dose of 30 Gy.57,60 Tsang et al61 reported complete remission in up to 90% of patients receiving radiation therapy alone, with excellent 5-year progression-free and overall survival rates of 98% and 77%, respectively.
Although surgery, radiotherapy, and chemotherapy have been used in cases in which eradication therapy failed and in more advanced stages of MALT lymphoma, there is no consensus about their use, so therapy must be individualized.
Fourth highlight point
- Antibiotic treatment for eradication of H pylori infection is the recommended treatment only for stage I low-grade MALT lymphoma.
FOLOW-UP
4. How should you follow patients with MALT lymphoma?
- Endoscopy
- H pylori testing
- Computed tomography and magnetic resonance imaging
- No surveillance required after treatment
Endoscopy is the correct answer. As initial diagnostic biopsies do not exclude aggressive lymphoma, careful endoscopic follow-up is recommended. A recommended schedule is a breath test for H pylori every 2 months in conjunction with repeat endoscopy with biopsies every 3 to 6 months for the first 2 years, and then annually.62
Although H pylori may be eradicated within 1 month of drug therapy, lymphoma may take several months to disappear histologically. In patients with stage I disease with residual lymphoma after H pylori eradication therapy, a simple wait-and-watch strategy is a suitable alternative to oncologic therapy.63,64
Local relapse may occur after many years of complete remission; thus, patients should be followed closely long-term with endoscopy and possibly endoscopic ultrasonography. 47–49,63
Patients who fail to attain a complete remission within 12 months should undergo radiation therapy, with or without chemotherapy. The same therapy should be started as soon as possible in patients with progressive disease after antibiotic therapy. Patients negative for H pylori, patients with stage II disease, and patients with t(11;18) translocation should receive antibiotic treatment with endoscopic surveillance every 3 months.
Fifth highlight point
- Surveillance endoscopy is recommended for follow-up of MALT lymphoma.
CASE CONTINUES: HER CONDITION IMPROVES, THEN WORSENS
Computed tomography of the chest, abdomen, and pelvis reveals no evidence of additional sites of tumor. Positron emission tomography reveals increased uptake in the left tonsillar region, for which she has undergoes an ear, nose, and throat evaluation, and no pathology is found.
Due to recurrence of her marginal zone Bcell lymphoma of MALT type of the stomach, the patient is referred to an oncology service. She is treated with radiation, receiving 15 sessions of 30 Gy localized to the stomach. Three months after radiation therapy, she undergoes endoscopy again, which shows no evidence of the previously described nodules. Repeat biopsies are negative for H pylori and MALT lymphoma.
Annual surveillance endoscopy and computed tomography for the past 3 years have been negative for any tumor recurrence.
A 61-year-old woman presents to her primary care physician because for the last 4 weeks she has had difficulty swallowing solid food and a feeling of food “getting stuck in the chest.” She also reports having nausea, mild epigastric pain, and heartburn. She denies having fevers, chills, night sweats, weight loss, vomiting, diarrhea, hematochezia, or melena.
Medical history
For the past 20 years, she has had gastroesophageal reflux disease (GERD), intermittently treated with a proton pump inhibitor. She also has arthritis, hyperlipidemia, hypertension, and asthma, and she has undergone right hip replacement for a hip fracture. She has no known allergies.
She lives in the Midwest region of the United States and is on disability due to her arthritis. She is divorced and has three children.
She quit smoking 3 years ago after smoking half a pack per day for 30 years. She drinks socially; she has never used recreational drugs.
She recalls that an uncle had cancer, but she does not know the specific type.
Physical examination
The patient’s temperature is 96.7°F (35.9°C), heart rate 86 per minute, blood pressure 150/92 mm Hg, respiratory rate 16 per minute, and oxygen saturation 100% on room air.
Differential diagnosis
Although the differential diagnosis at this stage is broad, a few conditions that commonly present like this are:
- Esophageal cancer
- Esophageal stricture
- Esophageal webs
- Esophagitis (infectious, inflammatory)
- Peptic ulcer disease.
WHICH TEST SHOULD BE ORDERED?
1. Which test will you order now for this patient?
- Endoscopy (esophagogastroduodenoscopy)
- Serum Helicobacter pylori antibody testing
- Wireless pH monitoring
- Barium swallow
Endoscopy would be the best test to order. Esophageal cancer and esophageal stricture must be ruled out, in view of her long history of GERD, gastritis, and smoking and her alarming symptoms of difficulty swallowing and food sticking. In this situation, endoscopy is the first test recommended. In addition to its diagnostic value, it offers an opportunity to obtain tissue samples and to perform a therapeutic intervention, if necessary.1,2
H pyloriantibody testing is used in the “test-and-treat approach” for H pylori infection, an established management strategy for patients who have uninvestigated dyspepsia and who are younger than 55 years and have no “alarm features,” ie, red flags for cancer. The alarm features commonly described are anemia, early satiety, unexplained weight loss, bleeding, odynophagia, progressive dysphagia, unexplained vomiting, and a family history or prior history of gastrointestinal malignancy.3
Our patient’s symptoms raise the possibility of cancer, so that H pylori testing would not be the best test to order at this point.
Ambulatory wireless pH monitoring with a wireless pH capsule is useful for confirming GERD in those with persistent symptoms (whether typical or atypical) who do not have evidence of mucosal damage on initial endoscopy, particularly if a trial of acid suppression has failed.4–6
Barium swallow is an x-ray examination of the esophagus with contrast. It can show both the anatomy and the function of the esophagus, and it would be the initial diagnostic procedure of choice for patients with dysphagia who have no alarm symptoms.7 However, our patient does have alarm symptoms.
First highlight point
- Endoscopy is the first test in patients with dysphagia with alarm symptoms.
CASE CONTINUES: ENDOSCOPY
Multiple biopsies of the nodules show atypical lymphoid infiltrates with small cleaved lymphocytes that are mostly positive for CD5, CD20, and CD43 and negative for CD10 and CD23 by flow cytometry. In addition, a staining test for H pylori is positive.
Comment. Our patient has had GERD for the past 20 years, intermittently treated with a proton pump inhibitor. Acid suppressive therapy with a proton pump inhibitor is the standard of care of patients with erosive esophagitis. In standard doses, these drugs control symptoms in most cases and heal esophagitis in almost 90% of cases within 4 to 8 weeks.9 Proton pump inhibitors are also effective for maintaining healing of esophagitis and controlling symptoms in patients who respond to an acute course of therapy for a period of 6 to 12 months.10
WHAT IS THE DIAGNOSIS?
2. Which is the most likely diagnosis for our patient?
- Fundic gland polyps
- Gastric hyperplastic polyps
- Gastric adenomas
- Mucosa-associated lymphoid tissue (MALT) lymphoma
Fundic gland polyps are small (0.1–0.8 cm), hyperemic, sessile, flat, nodular lesions that have a smooth surface. They occur exclusively in the gastric corpus and are composed of normal gastric corpus-type epithelium arranged in a disorderly or microcystic configuration. 11 This pattern does not match our patient’s findings.
Gastric hyperplastic polyps are elongated, cystic, and distorted foveolar epithelium with marked regeneration. Other histologic findings are stromal inflammation, edema, and smooth muscle hyperplasia.12 This does not match our patient’s findings.
Adenomas can be flat or polypoid and range in size from a few millimeters to several centimeters. Endoscopically, adenomatous polyps have a velvety, lobulated appearance. Most are solitary (82% of cases), located in the antrum, and less than 2 cm in diameter.13 This does not match our patient’s findings.
MALT lymphoma, the correct answer, is characterized by small cleaved lymphocytes positive for CD4, CD20, and CD43. Although CD5 positivity is not characteristic, rare cases of MALT lymphoma may be CD5-positive and may be more aggressive.14
Other common features of MALT lymphoma are erosions, small nodules, thickening of gastric folds—generally suggesting a benign condition—or hyperemic or even normal gastric mucosa.15 Our patient’s complaint of food sticking in her chest and difficulty swallowing was most likely related to the erosive esophagitis found on endoscopy.
A TYPE OF NON-HODGKIN LYMPHOMA
Normal gastric mucosa contains no lymphoid tissue.16,17 Primary gastric lymphoma, of which MALT lymphoma is a subtype, accounts for around 5% of gastric malignancies, with an annual incidence rate of 0.5 per 100,000 people. 18–20 Although rare, it accounts for 60% to 70% of cases of non-Hodgkin lymphoma of the gastrointestinal tract and can involve the perigastric or abdominal lymph nodes or both.21–23 Although earlier studies suggested that its incidence was increasing, recent data indicate the incidence may be decreasing, thanks to active H pylori treatment.24–26
Two subtypes of primary gastric non-Hodgkin lymphoma commonly described are MALT lymphoma and diffuse large B-cell (DLBC) lymphoma. In the Revised European-American Lymphoma Classification, high-grade MALT lymphoma is comparable to DLBC lymphoma and may have transformed from low-grade MALT lymphoma.27,28 Another reported subtype, mantle cell lymphoma with MALT lymphoma features, should be considered in the differential diagnosis, although it is rare.29
MALT lymphoma is linked to H pylori
H pylori infection is a factor in the development of MALT lymphoma,30 as multiple lines of evidence show:
- H pylori infection has been reported in more than 90% of patients with MALT lymphoma.31–35
- H pylori antibodies have been found in stored serum drawn from patients who subsequently developed MALT lymphoma.35
- In response to H pylori antigens, T cells from MALT lymphoma proliferate and cause an increase in tumor immunoglobulin production.36
- In animals experimentally infected with H pylori, around one-third develop lymphoid follicles and lymphoepithelial lesions including B cells, which are similar to human MALT lymphoma.37
However, only a minority of patients with H pylori develop lymphoma, owing to a host immune response that is not well defined.
Second highlight point
- Gastric MALT lymphoma is associated with H pylori.
Associated genetic translocations
Three translocations, t(11;18), t(1;14), and t(14;18), are specifically associated with MALT lymphoma, and the genes involved have been characterized.
The t(11;18) translocation, seen in gastric and nongastric MALT lymphoma, is not seen in H pylori gastritis.38 This translocation is usually associated with extension of the disease outside the stomach (ie, to regional lymph nodes or distal sites).27 Most cases that do not respond to H pylori eradication involve the t(11;18) and t(1;14) translocations.28
Clinical presentation of gastric MALT lymphoma
The average age at presentation with gastric MALT lymphoma is 54 to 58 years.
The most common complaint is nonspecific abdominal pain in the epigastric region, sometimes accompanied by weight loss, nausea, vomiting, and, in a quarter of cases, acute or chronic bleeding.39–41 Weight loss is common, and its extent is associated with the location and the grade of the disease.
Most cases of MALT lymphoma are found serendipitously during endoscopy, on which the appearance of the lesions ranges from small ulcerations to polypoid masses with infiltrated, thickened folds involving predominantly the antrum or prepyloric region.15,41
MANAGING MALT LYMPHOMA
Our patient undergoes endoscopic ultrasonography, which reveals she has stage I disease, ie, it is limited to the stomach without involving the lymph nodes (stage II), adjacent organs (stage III), or distant organs (stage IV).
3. How will you treat this patient, given the present information?
- Chemotherapy
- Radiation therapy
- Surgery
- Antibiotics with a proton pump inhibitor
Antibiotics with a proton pump inhibitor would be best. According to the Maastricht III Consensus Report,42H pylori eradication is the treatment of first choice for H pylori infection in patients with stage I low-grade gastric MALT lymphoma. This therapy can induce complete histologic remission in 80% to 100% of patients with MALT lymphoma. 43 Several studies have shown regression44 or complete remission of low-grade gastric MALT lymphoma after eradication of H pylori with antibiotics, making it a reasonable initial treatment.45–49
Several regimens are used. The first choice in populations in which the prevalence of resistance to clarithromycin (Biaxin) is less than 15% to 20% is a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole (Flagyl). (Metronidazole is preferable to amoxicillin if the prevalence of resistance to metronidazole is less than 40%.)
Sequential treatment—ie, 5 days of a proton pump inhibitor plus amoxicillin followed by 5 additional days of a proton pump inhibitor plus clarithromycin plus tinidazole (Tindamax)— may be better than a 7-day course of the combination of a proton pump inhibitor, amoxicillin, and clarithromycin.50,51
Treatment with a proton pump inhibitor, clarithromycin (500 mg twice a day), and either amoxicillin (1,000 mg twice a day) or metronidazole (400 or 500 mg twice a day) for 14 days is more effective than treatment for 7 days.52
H pylori reinfection in the general population is quite rare, with an estimated yearly rate as low as 2%.53 Recurrence of the infection is a risk factor for lymphoma relapse.17,54
Several predictors of the response of MALT lymphoma to eradication therapy have been recognized: H pylori positivity, stage I, lymphoma confined to the stomach; gastric wall invasion confined to mucosa and submucosa, and the absence of the t(11;18) translocation.
The time between H pylori eradication and complete remission of primary gastric lymphoma varies and can be longer than 12 months.55
Chemotherapy. In a single study,56 complete remission was achieved with oral cyclophosphamide (Cytoxan) in cases of antibiotic-refractory gastric MALT lymphoma. Comparable results were achieved after radiation therapy (see below); hence, oral monotherapy with cyclophosphamide might also be a suitable second-line therapy.57
The regimen of cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone (CHOP) has been recommended for patients with stage III and IV disease.41,58
Rituximab (Rituxan) has been proven effective in treating t(11;18)-positive MALT lymphoma.59
Radiation therapy. Two studies have shown a 100% complete response rate after radiation therapy with a median dose of 30 Gy.57,60 Tsang et al61 reported complete remission in up to 90% of patients receiving radiation therapy alone, with excellent 5-year progression-free and overall survival rates of 98% and 77%, respectively.
Although surgery, radiotherapy, and chemotherapy have been used in cases in which eradication therapy failed and in more advanced stages of MALT lymphoma, there is no consensus about their use, so therapy must be individualized.
Fourth highlight point
- Antibiotic treatment for eradication of H pylori infection is the recommended treatment only for stage I low-grade MALT lymphoma.
FOLOW-UP
4. How should you follow patients with MALT lymphoma?
- Endoscopy
- H pylori testing
- Computed tomography and magnetic resonance imaging
- No surveillance required after treatment
Endoscopy is the correct answer. As initial diagnostic biopsies do not exclude aggressive lymphoma, careful endoscopic follow-up is recommended. A recommended schedule is a breath test for H pylori every 2 months in conjunction with repeat endoscopy with biopsies every 3 to 6 months for the first 2 years, and then annually.62
Although H pylori may be eradicated within 1 month of drug therapy, lymphoma may take several months to disappear histologically. In patients with stage I disease with residual lymphoma after H pylori eradication therapy, a simple wait-and-watch strategy is a suitable alternative to oncologic therapy.63,64
Local relapse may occur after many years of complete remission; thus, patients should be followed closely long-term with endoscopy and possibly endoscopic ultrasonography. 47–49,63
Patients who fail to attain a complete remission within 12 months should undergo radiation therapy, with or without chemotherapy. The same therapy should be started as soon as possible in patients with progressive disease after antibiotic therapy. Patients negative for H pylori, patients with stage II disease, and patients with t(11;18) translocation should receive antibiotic treatment with endoscopic surveillance every 3 months.
Fifth highlight point
- Surveillance endoscopy is recommended for follow-up of MALT lymphoma.
CASE CONTINUES: HER CONDITION IMPROVES, THEN WORSENS
Computed tomography of the chest, abdomen, and pelvis reveals no evidence of additional sites of tumor. Positron emission tomography reveals increased uptake in the left tonsillar region, for which she has undergoes an ear, nose, and throat evaluation, and no pathology is found.
Due to recurrence of her marginal zone Bcell lymphoma of MALT type of the stomach, the patient is referred to an oncology service. She is treated with radiation, receiving 15 sessions of 30 Gy localized to the stomach. Three months after radiation therapy, she undergoes endoscopy again, which shows no evidence of the previously described nodules. Repeat biopsies are negative for H pylori and MALT lymphoma.
Annual surveillance endoscopy and computed tomography for the past 3 years have been negative for any tumor recurrence.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808–1825.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Furlow B. Barium swallow. Radiol Technol 2004; 76:49–58.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Havelund T, Laursen LS, Skoubo-Kristensen E, et al. Omeprazole and ranitidine in treatment of reflux oesophagitis: double blind comparative trial. Br Med J (Clin Res Ed) 1988; 296:89–92.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol 1996; 27:896–903.
- Snover DC. Benign epithelial polyps of the stomach. Pathol Annu 1985; 20:303–329.
- Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009; 6:331–341.
- Wenzel C, Dieckmann K, Fiebiger W, Mannhalter C, Chott A, Raderer M. CD5 expression in a lymphoma of the mucosa-associated lymphoid tissue (MALT)-type as a marker for early dissemination and aggressive clinical behaviour. Leuk Lymphoma 2001; 42:823–829.
- Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol 2003; 98:975–986.
- Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med 1999; 131:88–95.
- Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002; 50(suppl 3):III19–III24.
- Ducreux M, Boutron MC, Piard F, Carli PM, Faivre J. A 15-year series of gastrointestinal non-Hodgkin’s lymphomas: a population-based study. Br J Cancer 1998; 77:511–514.
- Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma in a population-based registry. Br J Cancer 1999; 79:1929–1934.
- d’Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994; 12:1673–1684.
- Koch P, del Valle F, Berdel WE, et al; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 19:3861–3873.
- Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma 2006; 47:2140–2146.
- Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology 1992; 20:29–34.
- Wotherspoon AC. Choosing the right MALT. Gut 1996; 39:617–618.
- Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003; 97:2462–2473.
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2009; epub ahead of print.
- Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001; 98:1182–1187.
- Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357:39–40.
- Shibata K, Shimamoto Y, Nakano S, Miyahara M, Nakano H, Yamaguchi M. Mantle cell lymphoma with the features of mucosa-associated lymphoid tissue (MALT) lymphoma in an HTLV-I-seropositive patient. Ann Hematol 1995; 70:47–51.
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 2005; 23:6370–6378.
- de Jong D, Boot H, van Heerde P, Hart GA, Taal BG. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology 1997; 112:1466–1474.
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338:1175–1176.
- Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol 1994; 47:436–439.
- Doglioni C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339:834–835.
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330:1267–1271.
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993; 342:571–574.
- Lee A, O’Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations—mouse models of gastric lymphoma. Recent Results Cancer Res 2000; 156:42–51.
- Auer IA, Gascoyne RD, Connors JM, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997; 8:979–985.
- Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Malignant tumors of the stomach. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori. Gastroenterol Clin North Am 2000; 29:593–607.
- Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d’Etude des Lymphomes Digestifs. Gastroenterology 1993; 105:1662–1671.
- Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 1991; 101:1159–1170.
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56:772–781.
- Boot H, de Jong D. Gastric lymphoma: the revolution of the past decade. Scand J Gastroenterol Suppl 2002; 236:27–36.
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
- Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995; 345:1591–1594.
- Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med 1995; 122:767–769.
- Ruskoné-Fourmestraux A. Gastrointestinal lymphomas: the French experience of the Groupe d’Étude des Lymphomes Digestifs (GELD). Recent Results Cancer Res 2000; 156:99–103.
- Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 2005; 23:8018–8024.
- Wündisch T, Mösch C, Neubauer A, Stolte M. Helicobacter pylori eradication in gastric mucosa-associated lymphoid tissue lymphoma: results of a 196-patient series. Leuk Lymphoma 2006; 47:2110–2114.
- De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther 2004; 19:407–414.
- Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719–726.
- Paoluzi P, Iacopini F, Crispino P, et al. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 2006; 11:562–568.
- Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther 2006; 23:275–280.
- Bayerdörffer E, Morgner A. Gastric marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: management of the disease. Dig Liver Dis 2000; 32:192–194.
- Savio A, Zamboni G, Capelli P, et al. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Long-term post-treatment follow-up of a multicenter trial in the north-east of Italy and evaluation of the diagnostic protocol’s adequacy. Recent Results Cancer Res 2000; 156:116–124.
- Nakamura S, Matsumoto T, Suekane H, et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005; 104:532–540.
- Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998; 16:1916–1921.
- Solidoro A, Payet C, Sanchez-Lihon J, Montalbetti JA. Gastric lymphomas: chemotherapy as a primary treatment. Semin Surg Oncol 1990; 6:218–225.
- Lévy M, Copie-Bergman C, Molinier-Frenkel V, et al. Treatment of t(11;18)-positive gastric mucosa-associated lymphoid tissue lymphoma with rituximab and chlorambucil: clinical, histological, and molecular follow-up. Leuk Lymphoma 2010; 51:284–290.
- Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol 2001; 80(suppl 3):B100–B105.
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosaassociated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21:4157–4164.
- Hung PD, Schubert ML, Mihas AA. Marginal zone B-cell lymphoma (MALT lymphoma). Curr Treat Options Gastroenterol 2004; 7:133–138.
- Zucca E, Cavalli F. Are antibiotics the treatment of choice for gastric lymphoma? Curr Hematol Rep 2004; 3:11–16.
- Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al; EGI LS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut 2007; 56:1685–1687.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808–1825.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Furlow B. Barium swallow. Radiol Technol 2004; 76:49–58.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Havelund T, Laursen LS, Skoubo-Kristensen E, et al. Omeprazole and ranitidine in treatment of reflux oesophagitis: double blind comparative trial. Br Med J (Clin Res Ed) 1988; 296:89–92.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol 1996; 27:896–903.
- Snover DC. Benign epithelial polyps of the stomach. Pathol Annu 1985; 20:303–329.
- Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009; 6:331–341.
- Wenzel C, Dieckmann K, Fiebiger W, Mannhalter C, Chott A, Raderer M. CD5 expression in a lymphoma of the mucosa-associated lymphoid tissue (MALT)-type as a marker for early dissemination and aggressive clinical behaviour. Leuk Lymphoma 2001; 42:823–829.
- Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol 2003; 98:975–986.
- Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med 1999; 131:88–95.
- Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002; 50(suppl 3):III19–III24.
- Ducreux M, Boutron MC, Piard F, Carli PM, Faivre J. A 15-year series of gastrointestinal non-Hodgkin’s lymphomas: a population-based study. Br J Cancer 1998; 77:511–514.
- Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma in a population-based registry. Br J Cancer 1999; 79:1929–1934.
- d’Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994; 12:1673–1684.
- Koch P, del Valle F, Berdel WE, et al; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 19:3861–3873.
- Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma 2006; 47:2140–2146.
- Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology 1992; 20:29–34.
- Wotherspoon AC. Choosing the right MALT. Gut 1996; 39:617–618.
- Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003; 97:2462–2473.
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2009; epub ahead of print.
- Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001; 98:1182–1187.
- Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357:39–40.
- Shibata K, Shimamoto Y, Nakano S, Miyahara M, Nakano H, Yamaguchi M. Mantle cell lymphoma with the features of mucosa-associated lymphoid tissue (MALT) lymphoma in an HTLV-I-seropositive patient. Ann Hematol 1995; 70:47–51.
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 2005; 23:6370–6378.
- de Jong D, Boot H, van Heerde P, Hart GA, Taal BG. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology 1997; 112:1466–1474.
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338:1175–1176.
- Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol 1994; 47:436–439.
- Doglioni C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339:834–835.
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330:1267–1271.
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993; 342:571–574.
- Lee A, O’Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations—mouse models of gastric lymphoma. Recent Results Cancer Res 2000; 156:42–51.
- Auer IA, Gascoyne RD, Connors JM, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997; 8:979–985.
- Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Malignant tumors of the stomach. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori. Gastroenterol Clin North Am 2000; 29:593–607.
- Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d’Etude des Lymphomes Digestifs. Gastroenterology 1993; 105:1662–1671.
- Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 1991; 101:1159–1170.
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56:772–781.
- Boot H, de Jong D. Gastric lymphoma: the revolution of the past decade. Scand J Gastroenterol Suppl 2002; 236:27–36.
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
- Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995; 345:1591–1594.
- Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med 1995; 122:767–769.
- Ruskoné-Fourmestraux A. Gastrointestinal lymphomas: the French experience of the Groupe d’Étude des Lymphomes Digestifs (GELD). Recent Results Cancer Res 2000; 156:99–103.
- Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 2005; 23:8018–8024.
- Wündisch T, Mösch C, Neubauer A, Stolte M. Helicobacter pylori eradication in gastric mucosa-associated lymphoid tissue lymphoma: results of a 196-patient series. Leuk Lymphoma 2006; 47:2110–2114.
- De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther 2004; 19:407–414.
- Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719–726.
- Paoluzi P, Iacopini F, Crispino P, et al. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 2006; 11:562–568.
- Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther 2006; 23:275–280.
- Bayerdörffer E, Morgner A. Gastric marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: management of the disease. Dig Liver Dis 2000; 32:192–194.
- Savio A, Zamboni G, Capelli P, et al. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Long-term post-treatment follow-up of a multicenter trial in the north-east of Italy and evaluation of the diagnostic protocol’s adequacy. Recent Results Cancer Res 2000; 156:116–124.
- Nakamura S, Matsumoto T, Suekane H, et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005; 104:532–540.
- Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998; 16:1916–1921.
- Solidoro A, Payet C, Sanchez-Lihon J, Montalbetti JA. Gastric lymphomas: chemotherapy as a primary treatment. Semin Surg Oncol 1990; 6:218–225.
- Lévy M, Copie-Bergman C, Molinier-Frenkel V, et al. Treatment of t(11;18)-positive gastric mucosa-associated lymphoid tissue lymphoma with rituximab and chlorambucil: clinical, histological, and molecular follow-up. Leuk Lymphoma 2010; 51:284–290.
- Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol 2001; 80(suppl 3):B100–B105.
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosaassociated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21:4157–4164.
- Hung PD, Schubert ML, Mihas AA. Marginal zone B-cell lymphoma (MALT lymphoma). Curr Treat Options Gastroenterol 2004; 7:133–138.
- Zucca E, Cavalli F. Are antibiotics the treatment of choice for gastric lymphoma? Curr Hematol Rep 2004; 3:11–16.
- Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al; EGI LS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut 2007; 56:1685–1687.
Clinical approach to colonic ischemia
Ischemic colitis is one of the diagnoses that should be considered when patients present with abdominal pain, diarrhea, and intestinal bleeding (others are infectious colitis, inflammatory bowel disease, diverticulitis, and colon cancer). Its incidence is difficult to determine, as many mild cases are transient and are either not reported or misdiagnosed. However, it is the most common type of intestinal ischemia1 and is responsible for an estimated 1 in 2,000 hospital admissions.2
In this review, we review the main causes of and risk factors for colonic ischemia and discuss how to diagnose and treat it.
BLOOD SUPPLY IS TENUOUS IN ‘WATERSHED’ AREAS
The superior and inferior mesenteric arteries have an extensive network of collateral blood vessels at both the base and border of the mesentery, called the arch of Riolan and the marginal artery of Drummond, respectively.
MANY POSSIBLE CAUSES AND FACTORS
Age. Ischemic colitis most often occurs in elderly people; the average age is 70 years.6 Binns and Isaacson7 suggest that age-related tortuosity of the colonic arteries increases vascular resistance and contributes to colonic ischemia in elderly patients.
Hypotension and hypovolemia are the most common mechanisms of colonic ischemia. Hypotension can be due to sepsis or impaired left ventricular function, and hypovolemia can be caused by dehydration or bleeding. These result in systemic hypoperfusion, triggering a mesenteric vasoconstrictive reflex. Once the hypoperfusion resolves and blood flow to the ulcerated portions resumes, bleeding develops from reperfusion injury.8
Cardiac thromboembolism can also contribute to colonic ischemia. Hourmand-Ollivier et al9 found a cardiac source of embolism in almost one-third of patients who had ischemic colitis, suggesting the need for routine screening with electrocardiography, Holter monitoring, and transthoracic echocardiography.
Myocardial infarction. Cappell10 found, upon colonoscopic examination, that about 14% of patients who developed hematochezia after a myocardial infarction had ischemic colitis. These patients had more complications and a worse in-hospital prognosis than did patients who had ischemic colitis due to other causes.11
Major vascular surgical procedures can disrupt the colonic blood supply, and in two case series,12,13 up to 7% of patients who underwent endoscopy after open aortoiliac reconstructive surgery had evidence of ischemic colitis. In other series,14,15 the segment most often affected was the distal left colon, and the cause was iatrogenic ligation of the inferior mesenteric artery or intraoperative hypoperfusion in patients with chronic occlusion of this artery. Endovascular repair of aortoiliac aneurysm also carries a risk of ischemic colitis, though this risk is smaller (< 2%).16
Hypercoagulable states. The role of acquired or hereditary hypercoagulable states in colonic ischemia has not been extensively investigated and remains poorly understood.
Conditions that increase clotting can cause thrombotic occlusion of small vessels that supply the colon, leading to ischemia. In small retrospective studies and case reports,17–26 28% to 74% of patients who had ischemic colitis had abnormal results on tests for protein C deficiency, protein S deficiency, antithrombin III deficiency, antiphospholipid antibodies, the factor V Leiden mutation, and the prothrombin G20210A mutation. However, in what percentage of cases the abnormality actually caused the ischemic colitis remains unknown.
Arnott et al27 reported that 9 of 24 patients with ischemic colitis had abnormal results on testing for hypercoagulable conditions. Three patients had mildly persistent elevation in anticardiolipin antibodies, but none had the factor V Leiden mutation or a deficiency of protein C, protein S, or antithrombin.
Koutroubakis et al20 reported significantly higher prevalences of antiphospholipid antibodies and heterogeneity for the factor V Leiden mutation in 35 patients with a history of ischemic colitis than in 18 patients with diverticulitis and 52 healthy controls (19.4% vs 0 and 1.9%, 22.2% vs 0 and 3.8%, respectively). Overall, 26 (72%) of 36 patients had at least one abnormal hypercoagulable test result.
Most patients with ischemic colitis are relatively old (over 60 years), and many have multiple concomitant vascular risk factors, suggesting that many factors contribute to ischemic colitis and that thrombophilia is not necessarily the direct cause. Hypercoagulable states may play a more important role in young, healthy patients who present with chronic or recurrent colonic ischemia.
Because no large clinical trials have been done and data are scarce and limited to case reports and small retrospective studies, a hypercoagulable evaluation is reserved for younger patients and those with recurrent, unexplained ischemic colitis.
Even if we detect thrombophilia, nobody yet knows what the appropriate medical treatment should be. Although some cases of ischemic colitis with associated thrombophilia have been successfully treated with anticoagulants,28,29 the benefit of diagnosing and treating a hypercoagulable state in ischemic colitis is still unproven. Therefore, oral anticoagulation should be used only in those in whom a hypercoagulable state is the most likely cause of severe or recurrent colonic ischemia.
There are no official guidelines on the duration of anticoagulation in such patients, but we treat for 6 months and consider indefinite treatment if the ischemic colitis recurs.
Medications that should always be considered as possible culprits include:
- Alosetron (Lotronex), which was temporarily withdrawn by the US Food and Drug Administration because of its association with ischemic colitis when prescribed to treat diarrhea-predominant irritable bowel syndrome.30 It was later reinstated, with some restrictions.
- Digitalis
- Diuretics
- Estrogens
- Danazol (Danocrine)
- Nonsteroidal anti-inflammatory drugs
- Tegaserod (Zelnorm)
- Paclitaxel (Abraxane)
- Carboplatin (Paraplatin)
- Sumatriptan (Imitrex)
- Simvastatin (Zocor)
- Interferon-ribavirin31
- Pseudoephedrine (eg, Sudafed).32
Endoscopic retrograde cholangiopancreatography can cause ischemic colitis if the rare life-threatening complication of mesenteric hematoma occurs.33
Chronic constipation can lead to colonic ischemia by increasing intraluminal pressure, which hinders blood flow and reduces the arteriovenous oxygen gradient in the colonic wall.34,35
Long-distance running can cause sustained bouts of ischemia, likely due to shunting of blood away from the splanchnic circulation, along with dehydration and electrolyte abnormalities. Affected runners present with lower abdominal pain and hematochezia. The colitis usually resolves without sequelae with rehydration and electrolyte correction.36
Vasospasm has been described as a cause of ischemia. During systemic hypoperfusion, vasoactive substances shunt blood from the gut to the brain through mesenteric vasoconstriction.37 This phenomenon can occur in dehydration-induced hypotension, heart failure, septic shock, or exposure to drugs such as antihypertensive medications, digoxin, or cocaine. Necrosis of the villous layer and transmural infarctions can occur with uninterrupted ischemia lasting more than 8 hours.38
Snake venom. The bite of Agkistrodon blomhoffii brevicaudus, a pit viper found in China and Korea, was recently reported to cause transient ischemic colitis due to disseminated intravascular coagulation. The condition resolved in about 10 days after treatment with polyvalent antivenom solution, transfusion of platelets and fresh frozen plasma, and empirically chosen antibiotics, ie, ampicillin-sulbactam (Unasyn) and metronidazole (Flagyl).39
CLINICAL MANIFESTATIONS
As stated above, ischemic colitis should be included in the differential diagnosis when assessing patients with abdominal pain, diarrhea, or bloody stools.
Typical presentation
The typical presentation of acute colonic ischemia includes:
- Rapid onset of mild abdominal pain
- Tenderness over the affected bowel area, usually on the left side near the splenic flexure or the rectosigmoid junction
- Mild to moderate hematochezia beginning within 1 day of the onset of abdominal pain. The bleeding is often not profuse and does not cause hemodynamic instability or require transfusion.40
Differentiate from mesenteric ischemia
It is important to differentiate between ischemic colitis and mesenteric ischemia, which is more serious and affects the small bowel.
Most patients with acute mesenteric ischemia complain of sudden onset of severe abdominal pain out of proportion to the tenderness on physical examination, they appear profoundly ill, and they do not have bloody stools until the late stages. They need urgent mesenteric angiography or another fast imaging test.4
In contrast, many patients with chronic mesenteric ischemia (or “abdominal angina”) report recurrent severe postprandial abdominal pain, leading to fear of food and weight loss.
Varies in severity
The severity of ischemic colitis varies widely, with hypoperfusion affecting as little as a single segment or as much as the entire colon. Over three-fourths of cases are the milder, nongangrenous form, which is temporary and rarely causes long-term complications such as persistent segmental colitis or strictures.41 In contrast, gangrenous colonic ischemia, which accounts for about 15% of cases, can be life-threatening.
Colonic ischemia can be categorized according to its severity and clinical presentation42:
- Reversible colonopathy (submucosal or intramural hemorrhage)
- Transient colitis (45% of cases were reversible or transient in a 1978 report by Boley et al43)
- Chronic colitis (19% of cases)
- Stricture (13%)
- Gangrene (19%)
- Fulminant universal colitis.
The resulting ischemic injury can range from superficial mucosal damage to mural or even full-thickness transmural infarction.44
Although most cases involve the left colon, about one-fourth involve the right. Right-sided colonic ischemia tends to be more severe: about 60% of patients require surgery (five times more than with colitis of other regions), and the death rate is twice as high (close to 23%).45
DIAGNOSIS DEPENDS ON SUSPICION
The diagnosis of colonic ischemia largely depends on clinical suspicion, especially since many other conditions (eg, infectious colitis, inflammatory bowel disease, diverticulitis, colon cancer) present with abdominal pain, diarrhea, and hematochezia. One study showed that a clinical presentation of lower abdominal pain or bleeding, or both, was 100% predictive of ischemic colitis when accompanied by four or more of the following risk factors: age over 60, hemodialysis, hypertension, hypoalbuminemia, diabetes mellitus, or drug-induced constipation. 46
Stool studies can identify organisms
Invasive infections with Salmonella, Shigella, and Campylobacter species and Eschericia coli O157:H7 should be identified early with stool studies if the patient presents as an outpatient or has been hospitalized less than 72 hours. Parasites such as Entamoeba histolytica and Angiostrongylus costaricensis and viruses such as cytomegalovirus should be considered in the differential diagnosis, as they can cause ischemic colitis.41,47Clostridium difficile should be excluded in those exposed to antibiotics.
Blood tests may indicate tissue injury
Although no laboratory marker is specific for ischemic colitis, elevated serum levels of lactate, lactate dehydrogenase, creatine kinase, or amylase may indicate tissue injury. The combination of abdominal pain, a white blood cell count greater than 20 × 109/L, and metabolic acidosis suggests intestinal ischemia and infarction.
Endoscopy is the test of choice
Endoscopy has become the diagnostic test of choice in establishing the diagnosis of ischemic colitis, although computed tomography (CT) can provide suggestive findings and exclude other illnesses. Colonoscopy has almost completely replaced radiography with bariumenema contrast as a diagnostic tool because it is more sensitive for detecting mucosal changes, it directly visualizes the mucosa, and it can be used to obtain biopsy specimens.48
Colonoscopy is performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics. In addition, the scope should not be advanced beyond the affected area, and minimal air insufflation should be used to prevent perforation.
Endoscopic findings can help differentiate ischemic colitis from other, clinically similar diseases. For instance, unlike irritable bowel disease, ischemic colitis tends to affect a discrete segment with a clear delineation between affected and normal mucosa, it spares the rectum, the mucosa heals rapidly as seen on serial colonoscopic examinations, and a single linear ulcer, termed the “single-stripe” sign, runs along the longitudinal axis of the colon.49,50
Biopsy features are not specific, as findings of hemorrhage, capillary thrombosis, granulation tissue with crypt abscesses, and pseudopolyps can also be seen in other conditions, such as Crohn disease.54,55
Imaging studies are not specific
Imaging studies are often used, but the findings lack specificity.
Plain abdominal radiography can help only in advanced ischemia, in which distention or pneumatosis can be seen.
CT with contrast can reveal thickening of the colon wall in a segmental pattern in ischemic colitis, but this finding also can be present in infectious and Crohn colitis. CT findings of colonic ischemia also include pericolic streakiness and free fluid. Pneumatosis coli often signifies infarcted bowel.56 However, CT findings can be completely normal in mild cases or if done early in the course.
Angiography in severe cases
Since colonic ischemia is most often transient, mesenteric angiography is not indicated in mild cases. Angiography is only considered in more severe cases, especially when only the right colon is involved, the diagnosis of colonic ischemia has not yet been determined, and acute mesenteric ischemia needs to be excluded. A focal lesion is often seen in mesenteric ischemia, but not often in colonic ischemia.
Looking for the underlying cause
Once the diagnosis of ischemic colitis is made, an effort should be made to identify the cause (Table 1). The initial step can be to remove or treat reversible causes such as medications or infections. As mentioned earlier, electrocardiography, Holter monitoring, and transthoracic echocardiography should be considered in patients with ischemic colitis to rule out cardiac embolic sources.9 A hypercoagulable workup can be done, but only in young patients without other clear causes or patients with recurrent events.
CONSERVATIVE TREATMENT IS ENOUGH FOR MOST
Empirically chosen broad-spectrum antibiotics that cover both aerobic and anaerobic coliform bacteria are reserved for patients with moderate to severe colitis to minimize bacterial translocation and sepsis.
Whenever symptomatic ileus is present, a nasogastric tube should be placed to alleviate vomiting and abdominal discomfort.
Antiplatelet agents have not been evaluated in treating ischemic colitis and are generally not used. As mentioned earlier, anticoagulation has been used in patients who have been proven to have hypercoagulable conditions,28,29 but its benefit is not yet proven. Currently, if the coagulation profile is abnormal, anticoagulation should be used only in cases of recurrent colonic ischemia or in young patients with severe cases in the absence of a clear cause. Anticoagulation should also be used in confirmed cases of cardiac embolization.
Surgery for some
Exploratory laparotomy with possible subtotal or segmental colectomy may be needed in acute, subacute, or chronic settings.42 Acute indications include peritoneal signs, massive bleeding, and fulminant ischemic colitis. Subacute indications are lack of resolution, with symptoms that persist for more than 2 or 3 weeks, or malnutrition or hypoalbuminemia due to protein-losing colonopathy. Colon stricture can be chronic and becomes an indication for surgery only when symptomatic, as some strictures resolve with time (months to years).
Right hemicolectomy and primary anastomosis of viable remaining colon is performed for right-sided colonic ischemia and necrosis, while left-sided colonic ischemia is managed with a proximal stoma and distal mucous fistula, or Hartmann procedure. Re-anastomosis and ostomy closure are usually done after 4 to 6 months.57 However, resection and primary anastomosis can also be an option for patients with isolated ischemia of the sigmoid colon.58 Transendoscopic dilation or stenting of short strictures can be an alternative to surgery, although experience with this is limited.59,60
THE PROGNOSIS IS USUALLY GOOD
The prognosis depends on the extent of injury and comorbidities. Transient, self-limited ischemia involving the mucosa and submucosa has a good prognosis, while fulminant ischemia with transmural infarction carries a poor one, as it can progress to necrosis and death.
Although up to 85% of cases of ischemic colitis managed conservatively improve within 1 or 2 days and resolve completely within 1 or 2 weeks, close to one-fifth of patients develop peritonitis or deteriorate clinically and require surgery.61,62 Surgical resection is required when irreversible ischemic injury and chronic colitis develop, as both can lead to bacteremia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhea, and protein-losing enteropathy.40
- Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19:729–738.
- Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 7th ed. Philadelphia, PA: Saunders; 2002.
- Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum 1996; 39:88–100.
- Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am 2001; 30:445–473.
- Reeders JW, Tytgat GN, Rosenbusch G, et al. Ischaemic colitis. The Hague: Martinus Nijhoff, 1984;17.
- Brandt L, Boley S, Goldberg L, Mitsudo S, Berman A. Colitis in the elderly. A reappraisal. Am J Gastroenterol 1981; 76:239–245.
- Binns JC, Isaacson P. Age-related changes in the colonic blood supply: their relevance to ischaemic colitis. Gut 1978; 19:384–390.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72:65–83.
- Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol 2003; 98:1573–1577.
- Cappell MS. Safety and efficacy of colonoscopy after myocardial infarction: an analysis of 100 study patients and 100 control patients at two tertiary cardiac referral hospitals. Gastrointest Endosc 2004; 60:901–909.
- Cappell MS, Mahajan D, Kurupath V. Characterization of ischemic colitis associated with myocardial infarction: an analysis of 23 patients. Am J Med 2006; 119:527.e1–e9.
- Hagihara PF, Ernst CB, Griffen WO. Incidence of ischemic colitis following abdominal aortic reconstruction. Surg Gynecol Obstet 1979; 149:571–573.
- Brewster DC, Franklin DP, Cambria RP, et al. Intestinal ischemia complicating abdominal aortic surgery. Surgery 1991; 109:447–454.
- Piotrowski JJ, Ripepi AJ, Yuhas JP, Alexander JJ, Brandt CP. Colonic ischemia: the Achilles heel of ruptured aortic aneurysm repair. Am Surg 1996; 62:557–560.
- Ernst CB. Colonic ischemia following aortic reconstruction. In: Rutherford RB, editor. Vascular Surgery. 4th ed. Philadelphia, PA: WB Saunders; 1995:1312–1320.
- Geroghty PS, Sanchez LA, Rubin BG, et al. Overt ischemic colitis after endovascular repair of aortoiliac aneurysm. J Vasc Surg 2004; 40:413–418.
- Klestzick HN, McPhedran P, Cipolla D, Berry WA, DiCorato M, Denowitz J. The antiphospholipid syndrome and ischemic colitis. Gastroenterologist 1995; 3:249–256.
- Knot EA, ten Cate JW, Bruin T, Iburg AH, Tytgat GN. Antithrombin III metabolism in two colitis patients with acquired antithrombin III deficiency. Gastroenterology 1985; 89:421–425.
- Maloisel F. Role of coagulation disorders in mesenteric ischemia. J Chir (Paris) 1996; 133:442–447.
- Koutroubakis IE, Sfiridaki A, Theodoropoulou A, Kouroumalis EA. Role of acquired and hereditary thrombotic risk factors in colon ischemia of ambulatory patients. Gastroenterology 2001; 121:561–565.
- Midian-Singh R, Polen A, Durishin C, Crock RD, Whittier FC, Fahmy N. Ischemic colitis revisited: a prospective study identifying hypercoagulability as a risk factor. South Med J 2004; 97:120–123.
- Blanc P, Bories P, Donadio D, et al. Ischemic colitis and recurrent venous thrombosis caused by familial protein S deficiency. Gastroenterol Clin Biol 1989; 13:945.
- Verger P, Blanc C, Feydy P, Boey S. Ischemic colitis caused by protein S deficiency. Presse Med 1996; 25:1350.
- Ludwig D, Stahl M, David-Walek T, et al. Ischemic colitis, pulmonary embolism, and atrial thrombosis in a patient with inherited resistance to activated protein C. Dig Dis Sci 1998; 43:1362–1367.
- Yee NS, Guerry D, Lichtenstein GR. Ischemic colitis associated with factor V Leiden mutation. Ann Intern Med 2000; 132:595–596.
- Balian A, Veyradier A, Naveau S, et al. Prothrombin 20210G/A mutation in two patients with mesenteric ischemia. Dig Dis Sci 1999; 44:1910–1913.
- Arnott ID, Ghosh S, Ferguson A. The spectrum of ischaemic colitis. Eur J Gastroenterol Hepatol 1999; 11:295–303.
- Chin BW, Greenberg D, Wilson RB, Meredith CG. A case of ischemic colitis associated with factor V Leiden mutation: successful treatment with anticoagulation. Gastrointest Endosc 2007; 66:416–418.
- Heyn J, Buhmann S, Ladurner R, et al. Recurrent ischemic colitis in a patient with Leiden factor V mutation and systemic lupus erythematosus with antiphospholipid syndrome. Eur J Med Res 2008; 13:182–184.
- Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 2006; 101:1069–1079.
- Punnam SR, Pothula VR, Gourineni N, Punnam A, Ranganathan V. Interferon-ribavirin-associated ischemic colitis. J Clin Gastroenterol 2008; 42:323–325.
- Dowd J, Bailey D, Moussa K, Nair S, Doyle R, Culpepper-Morgan JA. Ischemic colitis associated with pseudoephedrine: four cases. Am J Gastroenterol 1999; 94:2430–2434.
- Kingsley DD, Schermer CR, Jamal MM. Rare complications of endoscopic retrograde cholangiopancreatography: two case reports. JSLS 2001; 5:171–173.
- Boley SJ, Agrawal GP, Warren AR, et al. Pathophysiologic effects of bowel distension on intestinal blood flow. Am J Surg 1969; 117:228–234.
- Reinus JF, Brandt LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19:319–343.
- Moses FM. Exercise-associated intestinal ischemia. Curr Sports Med Rep 2005; 4:91–95.
- Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am 1997; 77:289–306.
- Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand 1987; 153:321–324.
- Kim MK, Cho YS, Kim HK, Kim JS, Kim SS, Chae HS. Transient ischemic colitis after a pit viper bite (Agkistrodon blomhoffii brevicaudus). J Clin Gastroenterol 2008; 42:111–112.
- Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am 1998; 27:827–860.
- Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol 1998; 27:122–128.
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology 2000; 118:954–968.
- Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg 1978; 15:1–85.
- Schuler JG, Hudlin MM. Cecal necrosis: infrequent variant of ischemic colitis. Report of five cases. Dis Colon Rectum 2000; 43:708–712.
- Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007; 102:2247–2252.
- Park CJ, Jang MK, Shin WG, et al. Can we predict the development of ischemic colitis among patients with lower abdominal pain? Dis Colon Rectum 2007; 50:232–238.
- Su C, Brandt LJ, Sigal SH, et al. The immunohistological diagnosis of E. coli 0157:H7 colitis: possible association with colonic ischemia. Am J Gastroenterol 1998; 93:1055–1059.
- Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc 1981; 27:156–161.
- Rogers AI, David S. Intestinal blood flow and diseases of vascular impairment. In: Haubrich WS, Schaffner F, Berk JE, editors. Gastroenterology. 5th ed. Philadelphia: WB Saunders; 1995:1212–1234.
- Zuckerman GR, Prakash C, Merriman RB, Sawhney MS, DeSchryver-Kecskemeti K, Clouse RE. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol 2003; 98:2018–2022.
- Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J 2005; 98:217–222.
- Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003; 70:920–930.
- Habu Y, Tahashi Y, Kiyota K, et al. Reevaluation of clinical features of ischemic colitis: analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31:881–886.
- Mitsudo S, Brandt LJ. Pathology of intestinal ischemia. Surg Clin North Am 1992; 72:43–63.
- Price AB. Ischaemic colitis. Curr Top Pathol 1990; 81:229–246.
- Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology 1999; 211:381–388.
- Mosdell DM, Doberneck RC. Morbidity and mortality of ostomy closure. Am J Surg 1991; 162:633–636.
- Iqbal T, Zarin M, Iqbal A, et al. Results of primary closure in the management of gangrenous and viable sigmoid volvulus. Pak J Surg 2007; 23:118–121.
- Oz MC, Forde KA. Endoscopic alternatives in the management of colonic strictures. Surgery 1990; 108:513–519.
- Profili S, Bifulco V, Meloni GB, Demelas L, Niolu P, Manzoni MA. A case of ischemic stenosis of the colon-sigmoid treated with self-expandable uncoated metallic prosthesis. Radiol Med 1996; 91:665–667.
- Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992; 72:203–229.
- Boley SJ. 1989 David H. Sun lecture. Colonic ischemia—25 years later. Am J Gastroenterol 1990; 85:931–934.
Ischemic colitis is one of the diagnoses that should be considered when patients present with abdominal pain, diarrhea, and intestinal bleeding (others are infectious colitis, inflammatory bowel disease, diverticulitis, and colon cancer). Its incidence is difficult to determine, as many mild cases are transient and are either not reported or misdiagnosed. However, it is the most common type of intestinal ischemia1 and is responsible for an estimated 1 in 2,000 hospital admissions.2
In this review, we review the main causes of and risk factors for colonic ischemia and discuss how to diagnose and treat it.
BLOOD SUPPLY IS TENUOUS IN ‘WATERSHED’ AREAS
The superior and inferior mesenteric arteries have an extensive network of collateral blood vessels at both the base and border of the mesentery, called the arch of Riolan and the marginal artery of Drummond, respectively.
MANY POSSIBLE CAUSES AND FACTORS
Age. Ischemic colitis most often occurs in elderly people; the average age is 70 years.6 Binns and Isaacson7 suggest that age-related tortuosity of the colonic arteries increases vascular resistance and contributes to colonic ischemia in elderly patients.
Hypotension and hypovolemia are the most common mechanisms of colonic ischemia. Hypotension can be due to sepsis or impaired left ventricular function, and hypovolemia can be caused by dehydration or bleeding. These result in systemic hypoperfusion, triggering a mesenteric vasoconstrictive reflex. Once the hypoperfusion resolves and blood flow to the ulcerated portions resumes, bleeding develops from reperfusion injury.8
Cardiac thromboembolism can also contribute to colonic ischemia. Hourmand-Ollivier et al9 found a cardiac source of embolism in almost one-third of patients who had ischemic colitis, suggesting the need for routine screening with electrocardiography, Holter monitoring, and transthoracic echocardiography.
Myocardial infarction. Cappell10 found, upon colonoscopic examination, that about 14% of patients who developed hematochezia after a myocardial infarction had ischemic colitis. These patients had more complications and a worse in-hospital prognosis than did patients who had ischemic colitis due to other causes.11
Major vascular surgical procedures can disrupt the colonic blood supply, and in two case series,12,13 up to 7% of patients who underwent endoscopy after open aortoiliac reconstructive surgery had evidence of ischemic colitis. In other series,14,15 the segment most often affected was the distal left colon, and the cause was iatrogenic ligation of the inferior mesenteric artery or intraoperative hypoperfusion in patients with chronic occlusion of this artery. Endovascular repair of aortoiliac aneurysm also carries a risk of ischemic colitis, though this risk is smaller (< 2%).16
Hypercoagulable states. The role of acquired or hereditary hypercoagulable states in colonic ischemia has not been extensively investigated and remains poorly understood.
Conditions that increase clotting can cause thrombotic occlusion of small vessels that supply the colon, leading to ischemia. In small retrospective studies and case reports,17–26 28% to 74% of patients who had ischemic colitis had abnormal results on tests for protein C deficiency, protein S deficiency, antithrombin III deficiency, antiphospholipid antibodies, the factor V Leiden mutation, and the prothrombin G20210A mutation. However, in what percentage of cases the abnormality actually caused the ischemic colitis remains unknown.
Arnott et al27 reported that 9 of 24 patients with ischemic colitis had abnormal results on testing for hypercoagulable conditions. Three patients had mildly persistent elevation in anticardiolipin antibodies, but none had the factor V Leiden mutation or a deficiency of protein C, protein S, or antithrombin.
Koutroubakis et al20 reported significantly higher prevalences of antiphospholipid antibodies and heterogeneity for the factor V Leiden mutation in 35 patients with a history of ischemic colitis than in 18 patients with diverticulitis and 52 healthy controls (19.4% vs 0 and 1.9%, 22.2% vs 0 and 3.8%, respectively). Overall, 26 (72%) of 36 patients had at least one abnormal hypercoagulable test result.
Most patients with ischemic colitis are relatively old (over 60 years), and many have multiple concomitant vascular risk factors, suggesting that many factors contribute to ischemic colitis and that thrombophilia is not necessarily the direct cause. Hypercoagulable states may play a more important role in young, healthy patients who present with chronic or recurrent colonic ischemia.
Because no large clinical trials have been done and data are scarce and limited to case reports and small retrospective studies, a hypercoagulable evaluation is reserved for younger patients and those with recurrent, unexplained ischemic colitis.
Even if we detect thrombophilia, nobody yet knows what the appropriate medical treatment should be. Although some cases of ischemic colitis with associated thrombophilia have been successfully treated with anticoagulants,28,29 the benefit of diagnosing and treating a hypercoagulable state in ischemic colitis is still unproven. Therefore, oral anticoagulation should be used only in those in whom a hypercoagulable state is the most likely cause of severe or recurrent colonic ischemia.
There are no official guidelines on the duration of anticoagulation in such patients, but we treat for 6 months and consider indefinite treatment if the ischemic colitis recurs.
Medications that should always be considered as possible culprits include:
- Alosetron (Lotronex), which was temporarily withdrawn by the US Food and Drug Administration because of its association with ischemic colitis when prescribed to treat diarrhea-predominant irritable bowel syndrome.30 It was later reinstated, with some restrictions.
- Digitalis
- Diuretics
- Estrogens
- Danazol (Danocrine)
- Nonsteroidal anti-inflammatory drugs
- Tegaserod (Zelnorm)
- Paclitaxel (Abraxane)
- Carboplatin (Paraplatin)
- Sumatriptan (Imitrex)
- Simvastatin (Zocor)
- Interferon-ribavirin31
- Pseudoephedrine (eg, Sudafed).32
Endoscopic retrograde cholangiopancreatography can cause ischemic colitis if the rare life-threatening complication of mesenteric hematoma occurs.33
Chronic constipation can lead to colonic ischemia by increasing intraluminal pressure, which hinders blood flow and reduces the arteriovenous oxygen gradient in the colonic wall.34,35
Long-distance running can cause sustained bouts of ischemia, likely due to shunting of blood away from the splanchnic circulation, along with dehydration and electrolyte abnormalities. Affected runners present with lower abdominal pain and hematochezia. The colitis usually resolves without sequelae with rehydration and electrolyte correction.36
Vasospasm has been described as a cause of ischemia. During systemic hypoperfusion, vasoactive substances shunt blood from the gut to the brain through mesenteric vasoconstriction.37 This phenomenon can occur in dehydration-induced hypotension, heart failure, septic shock, or exposure to drugs such as antihypertensive medications, digoxin, or cocaine. Necrosis of the villous layer and transmural infarctions can occur with uninterrupted ischemia lasting more than 8 hours.38
Snake venom. The bite of Agkistrodon blomhoffii brevicaudus, a pit viper found in China and Korea, was recently reported to cause transient ischemic colitis due to disseminated intravascular coagulation. The condition resolved in about 10 days after treatment with polyvalent antivenom solution, transfusion of platelets and fresh frozen plasma, and empirically chosen antibiotics, ie, ampicillin-sulbactam (Unasyn) and metronidazole (Flagyl).39
CLINICAL MANIFESTATIONS
As stated above, ischemic colitis should be included in the differential diagnosis when assessing patients with abdominal pain, diarrhea, or bloody stools.
Typical presentation
The typical presentation of acute colonic ischemia includes:
- Rapid onset of mild abdominal pain
- Tenderness over the affected bowel area, usually on the left side near the splenic flexure or the rectosigmoid junction
- Mild to moderate hematochezia beginning within 1 day of the onset of abdominal pain. The bleeding is often not profuse and does not cause hemodynamic instability or require transfusion.40
Differentiate from mesenteric ischemia
It is important to differentiate between ischemic colitis and mesenteric ischemia, which is more serious and affects the small bowel.
Most patients with acute mesenteric ischemia complain of sudden onset of severe abdominal pain out of proportion to the tenderness on physical examination, they appear profoundly ill, and they do not have bloody stools until the late stages. They need urgent mesenteric angiography or another fast imaging test.4
In contrast, many patients with chronic mesenteric ischemia (or “abdominal angina”) report recurrent severe postprandial abdominal pain, leading to fear of food and weight loss.
Varies in severity
The severity of ischemic colitis varies widely, with hypoperfusion affecting as little as a single segment or as much as the entire colon. Over three-fourths of cases are the milder, nongangrenous form, which is temporary and rarely causes long-term complications such as persistent segmental colitis or strictures.41 In contrast, gangrenous colonic ischemia, which accounts for about 15% of cases, can be life-threatening.
Colonic ischemia can be categorized according to its severity and clinical presentation42:
- Reversible colonopathy (submucosal or intramural hemorrhage)
- Transient colitis (45% of cases were reversible or transient in a 1978 report by Boley et al43)
- Chronic colitis (19% of cases)
- Stricture (13%)
- Gangrene (19%)
- Fulminant universal colitis.
The resulting ischemic injury can range from superficial mucosal damage to mural or even full-thickness transmural infarction.44
Although most cases involve the left colon, about one-fourth involve the right. Right-sided colonic ischemia tends to be more severe: about 60% of patients require surgery (five times more than with colitis of other regions), and the death rate is twice as high (close to 23%).45
DIAGNOSIS DEPENDS ON SUSPICION
The diagnosis of colonic ischemia largely depends on clinical suspicion, especially since many other conditions (eg, infectious colitis, inflammatory bowel disease, diverticulitis, colon cancer) present with abdominal pain, diarrhea, and hematochezia. One study showed that a clinical presentation of lower abdominal pain or bleeding, or both, was 100% predictive of ischemic colitis when accompanied by four or more of the following risk factors: age over 60, hemodialysis, hypertension, hypoalbuminemia, diabetes mellitus, or drug-induced constipation. 46
Stool studies can identify organisms
Invasive infections with Salmonella, Shigella, and Campylobacter species and Eschericia coli O157:H7 should be identified early with stool studies if the patient presents as an outpatient or has been hospitalized less than 72 hours. Parasites such as Entamoeba histolytica and Angiostrongylus costaricensis and viruses such as cytomegalovirus should be considered in the differential diagnosis, as they can cause ischemic colitis.41,47Clostridium difficile should be excluded in those exposed to antibiotics.
Blood tests may indicate tissue injury
Although no laboratory marker is specific for ischemic colitis, elevated serum levels of lactate, lactate dehydrogenase, creatine kinase, or amylase may indicate tissue injury. The combination of abdominal pain, a white blood cell count greater than 20 × 109/L, and metabolic acidosis suggests intestinal ischemia and infarction.
Endoscopy is the test of choice
Endoscopy has become the diagnostic test of choice in establishing the diagnosis of ischemic colitis, although computed tomography (CT) can provide suggestive findings and exclude other illnesses. Colonoscopy has almost completely replaced radiography with bariumenema contrast as a diagnostic tool because it is more sensitive for detecting mucosal changes, it directly visualizes the mucosa, and it can be used to obtain biopsy specimens.48
Colonoscopy is performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics. In addition, the scope should not be advanced beyond the affected area, and minimal air insufflation should be used to prevent perforation.
Endoscopic findings can help differentiate ischemic colitis from other, clinically similar diseases. For instance, unlike irritable bowel disease, ischemic colitis tends to affect a discrete segment with a clear delineation between affected and normal mucosa, it spares the rectum, the mucosa heals rapidly as seen on serial colonoscopic examinations, and a single linear ulcer, termed the “single-stripe” sign, runs along the longitudinal axis of the colon.49,50
Biopsy features are not specific, as findings of hemorrhage, capillary thrombosis, granulation tissue with crypt abscesses, and pseudopolyps can also be seen in other conditions, such as Crohn disease.54,55
Imaging studies are not specific
Imaging studies are often used, but the findings lack specificity.
Plain abdominal radiography can help only in advanced ischemia, in which distention or pneumatosis can be seen.
CT with contrast can reveal thickening of the colon wall in a segmental pattern in ischemic colitis, but this finding also can be present in infectious and Crohn colitis. CT findings of colonic ischemia also include pericolic streakiness and free fluid. Pneumatosis coli often signifies infarcted bowel.56 However, CT findings can be completely normal in mild cases or if done early in the course.
Angiography in severe cases
Since colonic ischemia is most often transient, mesenteric angiography is not indicated in mild cases. Angiography is only considered in more severe cases, especially when only the right colon is involved, the diagnosis of colonic ischemia has not yet been determined, and acute mesenteric ischemia needs to be excluded. A focal lesion is often seen in mesenteric ischemia, but not often in colonic ischemia.
Looking for the underlying cause
Once the diagnosis of ischemic colitis is made, an effort should be made to identify the cause (Table 1). The initial step can be to remove or treat reversible causes such as medications or infections. As mentioned earlier, electrocardiography, Holter monitoring, and transthoracic echocardiography should be considered in patients with ischemic colitis to rule out cardiac embolic sources.9 A hypercoagulable workup can be done, but only in young patients without other clear causes or patients with recurrent events.
CONSERVATIVE TREATMENT IS ENOUGH FOR MOST
Empirically chosen broad-spectrum antibiotics that cover both aerobic and anaerobic coliform bacteria are reserved for patients with moderate to severe colitis to minimize bacterial translocation and sepsis.
Whenever symptomatic ileus is present, a nasogastric tube should be placed to alleviate vomiting and abdominal discomfort.
Antiplatelet agents have not been evaluated in treating ischemic colitis and are generally not used. As mentioned earlier, anticoagulation has been used in patients who have been proven to have hypercoagulable conditions,28,29 but its benefit is not yet proven. Currently, if the coagulation profile is abnormal, anticoagulation should be used only in cases of recurrent colonic ischemia or in young patients with severe cases in the absence of a clear cause. Anticoagulation should also be used in confirmed cases of cardiac embolization.
Surgery for some
Exploratory laparotomy with possible subtotal or segmental colectomy may be needed in acute, subacute, or chronic settings.42 Acute indications include peritoneal signs, massive bleeding, and fulminant ischemic colitis. Subacute indications are lack of resolution, with symptoms that persist for more than 2 or 3 weeks, or malnutrition or hypoalbuminemia due to protein-losing colonopathy. Colon stricture can be chronic and becomes an indication for surgery only when symptomatic, as some strictures resolve with time (months to years).
Right hemicolectomy and primary anastomosis of viable remaining colon is performed for right-sided colonic ischemia and necrosis, while left-sided colonic ischemia is managed with a proximal stoma and distal mucous fistula, or Hartmann procedure. Re-anastomosis and ostomy closure are usually done after 4 to 6 months.57 However, resection and primary anastomosis can also be an option for patients with isolated ischemia of the sigmoid colon.58 Transendoscopic dilation or stenting of short strictures can be an alternative to surgery, although experience with this is limited.59,60
THE PROGNOSIS IS USUALLY GOOD
The prognosis depends on the extent of injury and comorbidities. Transient, self-limited ischemia involving the mucosa and submucosa has a good prognosis, while fulminant ischemia with transmural infarction carries a poor one, as it can progress to necrosis and death.
Although up to 85% of cases of ischemic colitis managed conservatively improve within 1 or 2 days and resolve completely within 1 or 2 weeks, close to one-fifth of patients develop peritonitis or deteriorate clinically and require surgery.61,62 Surgical resection is required when irreversible ischemic injury and chronic colitis develop, as both can lead to bacteremia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhea, and protein-losing enteropathy.40
Ischemic colitis is one of the diagnoses that should be considered when patients present with abdominal pain, diarrhea, and intestinal bleeding (others are infectious colitis, inflammatory bowel disease, diverticulitis, and colon cancer). Its incidence is difficult to determine, as many mild cases are transient and are either not reported or misdiagnosed. However, it is the most common type of intestinal ischemia1 and is responsible for an estimated 1 in 2,000 hospital admissions.2
In this review, we review the main causes of and risk factors for colonic ischemia and discuss how to diagnose and treat it.
BLOOD SUPPLY IS TENUOUS IN ‘WATERSHED’ AREAS
The superior and inferior mesenteric arteries have an extensive network of collateral blood vessels at both the base and border of the mesentery, called the arch of Riolan and the marginal artery of Drummond, respectively.
MANY POSSIBLE CAUSES AND FACTORS
Age. Ischemic colitis most often occurs in elderly people; the average age is 70 years.6 Binns and Isaacson7 suggest that age-related tortuosity of the colonic arteries increases vascular resistance and contributes to colonic ischemia in elderly patients.
Hypotension and hypovolemia are the most common mechanisms of colonic ischemia. Hypotension can be due to sepsis or impaired left ventricular function, and hypovolemia can be caused by dehydration or bleeding. These result in systemic hypoperfusion, triggering a mesenteric vasoconstrictive reflex. Once the hypoperfusion resolves and blood flow to the ulcerated portions resumes, bleeding develops from reperfusion injury.8
Cardiac thromboembolism can also contribute to colonic ischemia. Hourmand-Ollivier et al9 found a cardiac source of embolism in almost one-third of patients who had ischemic colitis, suggesting the need for routine screening with electrocardiography, Holter monitoring, and transthoracic echocardiography.
Myocardial infarction. Cappell10 found, upon colonoscopic examination, that about 14% of patients who developed hematochezia after a myocardial infarction had ischemic colitis. These patients had more complications and a worse in-hospital prognosis than did patients who had ischemic colitis due to other causes.11
Major vascular surgical procedures can disrupt the colonic blood supply, and in two case series,12,13 up to 7% of patients who underwent endoscopy after open aortoiliac reconstructive surgery had evidence of ischemic colitis. In other series,14,15 the segment most often affected was the distal left colon, and the cause was iatrogenic ligation of the inferior mesenteric artery or intraoperative hypoperfusion in patients with chronic occlusion of this artery. Endovascular repair of aortoiliac aneurysm also carries a risk of ischemic colitis, though this risk is smaller (< 2%).16
Hypercoagulable states. The role of acquired or hereditary hypercoagulable states in colonic ischemia has not been extensively investigated and remains poorly understood.
Conditions that increase clotting can cause thrombotic occlusion of small vessels that supply the colon, leading to ischemia. In small retrospective studies and case reports,17–26 28% to 74% of patients who had ischemic colitis had abnormal results on tests for protein C deficiency, protein S deficiency, antithrombin III deficiency, antiphospholipid antibodies, the factor V Leiden mutation, and the prothrombin G20210A mutation. However, in what percentage of cases the abnormality actually caused the ischemic colitis remains unknown.
Arnott et al27 reported that 9 of 24 patients with ischemic colitis had abnormal results on testing for hypercoagulable conditions. Three patients had mildly persistent elevation in anticardiolipin antibodies, but none had the factor V Leiden mutation or a deficiency of protein C, protein S, or antithrombin.
Koutroubakis et al20 reported significantly higher prevalences of antiphospholipid antibodies and heterogeneity for the factor V Leiden mutation in 35 patients with a history of ischemic colitis than in 18 patients with diverticulitis and 52 healthy controls (19.4% vs 0 and 1.9%, 22.2% vs 0 and 3.8%, respectively). Overall, 26 (72%) of 36 patients had at least one abnormal hypercoagulable test result.
Most patients with ischemic colitis are relatively old (over 60 years), and many have multiple concomitant vascular risk factors, suggesting that many factors contribute to ischemic colitis and that thrombophilia is not necessarily the direct cause. Hypercoagulable states may play a more important role in young, healthy patients who present with chronic or recurrent colonic ischemia.
Because no large clinical trials have been done and data are scarce and limited to case reports and small retrospective studies, a hypercoagulable evaluation is reserved for younger patients and those with recurrent, unexplained ischemic colitis.
Even if we detect thrombophilia, nobody yet knows what the appropriate medical treatment should be. Although some cases of ischemic colitis with associated thrombophilia have been successfully treated with anticoagulants,28,29 the benefit of diagnosing and treating a hypercoagulable state in ischemic colitis is still unproven. Therefore, oral anticoagulation should be used only in those in whom a hypercoagulable state is the most likely cause of severe or recurrent colonic ischemia.
There are no official guidelines on the duration of anticoagulation in such patients, but we treat for 6 months and consider indefinite treatment if the ischemic colitis recurs.
Medications that should always be considered as possible culprits include:
- Alosetron (Lotronex), which was temporarily withdrawn by the US Food and Drug Administration because of its association with ischemic colitis when prescribed to treat diarrhea-predominant irritable bowel syndrome.30 It was later reinstated, with some restrictions.
- Digitalis
- Diuretics
- Estrogens
- Danazol (Danocrine)
- Nonsteroidal anti-inflammatory drugs
- Tegaserod (Zelnorm)
- Paclitaxel (Abraxane)
- Carboplatin (Paraplatin)
- Sumatriptan (Imitrex)
- Simvastatin (Zocor)
- Interferon-ribavirin31
- Pseudoephedrine (eg, Sudafed).32
Endoscopic retrograde cholangiopancreatography can cause ischemic colitis if the rare life-threatening complication of mesenteric hematoma occurs.33
Chronic constipation can lead to colonic ischemia by increasing intraluminal pressure, which hinders blood flow and reduces the arteriovenous oxygen gradient in the colonic wall.34,35
Long-distance running can cause sustained bouts of ischemia, likely due to shunting of blood away from the splanchnic circulation, along with dehydration and electrolyte abnormalities. Affected runners present with lower abdominal pain and hematochezia. The colitis usually resolves without sequelae with rehydration and electrolyte correction.36
Vasospasm has been described as a cause of ischemia. During systemic hypoperfusion, vasoactive substances shunt blood from the gut to the brain through mesenteric vasoconstriction.37 This phenomenon can occur in dehydration-induced hypotension, heart failure, septic shock, or exposure to drugs such as antihypertensive medications, digoxin, or cocaine. Necrosis of the villous layer and transmural infarctions can occur with uninterrupted ischemia lasting more than 8 hours.38
Snake venom. The bite of Agkistrodon blomhoffii brevicaudus, a pit viper found in China and Korea, was recently reported to cause transient ischemic colitis due to disseminated intravascular coagulation. The condition resolved in about 10 days after treatment with polyvalent antivenom solution, transfusion of platelets and fresh frozen plasma, and empirically chosen antibiotics, ie, ampicillin-sulbactam (Unasyn) and metronidazole (Flagyl).39
CLINICAL MANIFESTATIONS
As stated above, ischemic colitis should be included in the differential diagnosis when assessing patients with abdominal pain, diarrhea, or bloody stools.
Typical presentation
The typical presentation of acute colonic ischemia includes:
- Rapid onset of mild abdominal pain
- Tenderness over the affected bowel area, usually on the left side near the splenic flexure or the rectosigmoid junction
- Mild to moderate hematochezia beginning within 1 day of the onset of abdominal pain. The bleeding is often not profuse and does not cause hemodynamic instability or require transfusion.40
Differentiate from mesenteric ischemia
It is important to differentiate between ischemic colitis and mesenteric ischemia, which is more serious and affects the small bowel.
Most patients with acute mesenteric ischemia complain of sudden onset of severe abdominal pain out of proportion to the tenderness on physical examination, they appear profoundly ill, and they do not have bloody stools until the late stages. They need urgent mesenteric angiography or another fast imaging test.4
In contrast, many patients with chronic mesenteric ischemia (or “abdominal angina”) report recurrent severe postprandial abdominal pain, leading to fear of food and weight loss.
Varies in severity
The severity of ischemic colitis varies widely, with hypoperfusion affecting as little as a single segment or as much as the entire colon. Over three-fourths of cases are the milder, nongangrenous form, which is temporary and rarely causes long-term complications such as persistent segmental colitis or strictures.41 In contrast, gangrenous colonic ischemia, which accounts for about 15% of cases, can be life-threatening.
Colonic ischemia can be categorized according to its severity and clinical presentation42:
- Reversible colonopathy (submucosal or intramural hemorrhage)
- Transient colitis (45% of cases were reversible or transient in a 1978 report by Boley et al43)
- Chronic colitis (19% of cases)
- Stricture (13%)
- Gangrene (19%)
- Fulminant universal colitis.
The resulting ischemic injury can range from superficial mucosal damage to mural or even full-thickness transmural infarction.44
Although most cases involve the left colon, about one-fourth involve the right. Right-sided colonic ischemia tends to be more severe: about 60% of patients require surgery (five times more than with colitis of other regions), and the death rate is twice as high (close to 23%).45
DIAGNOSIS DEPENDS ON SUSPICION
The diagnosis of colonic ischemia largely depends on clinical suspicion, especially since many other conditions (eg, infectious colitis, inflammatory bowel disease, diverticulitis, colon cancer) present with abdominal pain, diarrhea, and hematochezia. One study showed that a clinical presentation of lower abdominal pain or bleeding, or both, was 100% predictive of ischemic colitis when accompanied by four or more of the following risk factors: age over 60, hemodialysis, hypertension, hypoalbuminemia, diabetes mellitus, or drug-induced constipation. 46
Stool studies can identify organisms
Invasive infections with Salmonella, Shigella, and Campylobacter species and Eschericia coli O157:H7 should be identified early with stool studies if the patient presents as an outpatient or has been hospitalized less than 72 hours. Parasites such as Entamoeba histolytica and Angiostrongylus costaricensis and viruses such as cytomegalovirus should be considered in the differential diagnosis, as they can cause ischemic colitis.41,47Clostridium difficile should be excluded in those exposed to antibiotics.
Blood tests may indicate tissue injury
Although no laboratory marker is specific for ischemic colitis, elevated serum levels of lactate, lactate dehydrogenase, creatine kinase, or amylase may indicate tissue injury. The combination of abdominal pain, a white blood cell count greater than 20 × 109/L, and metabolic acidosis suggests intestinal ischemia and infarction.
Endoscopy is the test of choice
Endoscopy has become the diagnostic test of choice in establishing the diagnosis of ischemic colitis, although computed tomography (CT) can provide suggestive findings and exclude other illnesses. Colonoscopy has almost completely replaced radiography with bariumenema contrast as a diagnostic tool because it is more sensitive for detecting mucosal changes, it directly visualizes the mucosa, and it can be used to obtain biopsy specimens.48
Colonoscopy is performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics. In addition, the scope should not be advanced beyond the affected area, and minimal air insufflation should be used to prevent perforation.
Endoscopic findings can help differentiate ischemic colitis from other, clinically similar diseases. For instance, unlike irritable bowel disease, ischemic colitis tends to affect a discrete segment with a clear delineation between affected and normal mucosa, it spares the rectum, the mucosa heals rapidly as seen on serial colonoscopic examinations, and a single linear ulcer, termed the “single-stripe” sign, runs along the longitudinal axis of the colon.49,50
Biopsy features are not specific, as findings of hemorrhage, capillary thrombosis, granulation tissue with crypt abscesses, and pseudopolyps can also be seen in other conditions, such as Crohn disease.54,55
Imaging studies are not specific
Imaging studies are often used, but the findings lack specificity.
Plain abdominal radiography can help only in advanced ischemia, in which distention or pneumatosis can be seen.
CT with contrast can reveal thickening of the colon wall in a segmental pattern in ischemic colitis, but this finding also can be present in infectious and Crohn colitis. CT findings of colonic ischemia also include pericolic streakiness and free fluid. Pneumatosis coli often signifies infarcted bowel.56 However, CT findings can be completely normal in mild cases or if done early in the course.
Angiography in severe cases
Since colonic ischemia is most often transient, mesenteric angiography is not indicated in mild cases. Angiography is only considered in more severe cases, especially when only the right colon is involved, the diagnosis of colonic ischemia has not yet been determined, and acute mesenteric ischemia needs to be excluded. A focal lesion is often seen in mesenteric ischemia, but not often in colonic ischemia.
Looking for the underlying cause
Once the diagnosis of ischemic colitis is made, an effort should be made to identify the cause (Table 1). The initial step can be to remove or treat reversible causes such as medications or infections. As mentioned earlier, electrocardiography, Holter monitoring, and transthoracic echocardiography should be considered in patients with ischemic colitis to rule out cardiac embolic sources.9 A hypercoagulable workup can be done, but only in young patients without other clear causes or patients with recurrent events.
CONSERVATIVE TREATMENT IS ENOUGH FOR MOST
Empirically chosen broad-spectrum antibiotics that cover both aerobic and anaerobic coliform bacteria are reserved for patients with moderate to severe colitis to minimize bacterial translocation and sepsis.
Whenever symptomatic ileus is present, a nasogastric tube should be placed to alleviate vomiting and abdominal discomfort.
Antiplatelet agents have not been evaluated in treating ischemic colitis and are generally not used. As mentioned earlier, anticoagulation has been used in patients who have been proven to have hypercoagulable conditions,28,29 but its benefit is not yet proven. Currently, if the coagulation profile is abnormal, anticoagulation should be used only in cases of recurrent colonic ischemia or in young patients with severe cases in the absence of a clear cause. Anticoagulation should also be used in confirmed cases of cardiac embolization.
Surgery for some
Exploratory laparotomy with possible subtotal or segmental colectomy may be needed in acute, subacute, or chronic settings.42 Acute indications include peritoneal signs, massive bleeding, and fulminant ischemic colitis. Subacute indications are lack of resolution, with symptoms that persist for more than 2 or 3 weeks, or malnutrition or hypoalbuminemia due to protein-losing colonopathy. Colon stricture can be chronic and becomes an indication for surgery only when symptomatic, as some strictures resolve with time (months to years).
Right hemicolectomy and primary anastomosis of viable remaining colon is performed for right-sided colonic ischemia and necrosis, while left-sided colonic ischemia is managed with a proximal stoma and distal mucous fistula, or Hartmann procedure. Re-anastomosis and ostomy closure are usually done after 4 to 6 months.57 However, resection and primary anastomosis can also be an option for patients with isolated ischemia of the sigmoid colon.58 Transendoscopic dilation or stenting of short strictures can be an alternative to surgery, although experience with this is limited.59,60
THE PROGNOSIS IS USUALLY GOOD
The prognosis depends on the extent of injury and comorbidities. Transient, self-limited ischemia involving the mucosa and submucosa has a good prognosis, while fulminant ischemia with transmural infarction carries a poor one, as it can progress to necrosis and death.
Although up to 85% of cases of ischemic colitis managed conservatively improve within 1 or 2 days and resolve completely within 1 or 2 weeks, close to one-fifth of patients develop peritonitis or deteriorate clinically and require surgery.61,62 Surgical resection is required when irreversible ischemic injury and chronic colitis develop, as both can lead to bacteremia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhea, and protein-losing enteropathy.40
- Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19:729–738.
- Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 7th ed. Philadelphia, PA: Saunders; 2002.
- Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum 1996; 39:88–100.
- Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am 2001; 30:445–473.
- Reeders JW, Tytgat GN, Rosenbusch G, et al. Ischaemic colitis. The Hague: Martinus Nijhoff, 1984;17.
- Brandt L, Boley S, Goldberg L, Mitsudo S, Berman A. Colitis in the elderly. A reappraisal. Am J Gastroenterol 1981; 76:239–245.
- Binns JC, Isaacson P. Age-related changes in the colonic blood supply: their relevance to ischaemic colitis. Gut 1978; 19:384–390.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72:65–83.
- Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol 2003; 98:1573–1577.
- Cappell MS. Safety and efficacy of colonoscopy after myocardial infarction: an analysis of 100 study patients and 100 control patients at two tertiary cardiac referral hospitals. Gastrointest Endosc 2004; 60:901–909.
- Cappell MS, Mahajan D, Kurupath V. Characterization of ischemic colitis associated with myocardial infarction: an analysis of 23 patients. Am J Med 2006; 119:527.e1–e9.
- Hagihara PF, Ernst CB, Griffen WO. Incidence of ischemic colitis following abdominal aortic reconstruction. Surg Gynecol Obstet 1979; 149:571–573.
- Brewster DC, Franklin DP, Cambria RP, et al. Intestinal ischemia complicating abdominal aortic surgery. Surgery 1991; 109:447–454.
- Piotrowski JJ, Ripepi AJ, Yuhas JP, Alexander JJ, Brandt CP. Colonic ischemia: the Achilles heel of ruptured aortic aneurysm repair. Am Surg 1996; 62:557–560.
- Ernst CB. Colonic ischemia following aortic reconstruction. In: Rutherford RB, editor. Vascular Surgery. 4th ed. Philadelphia, PA: WB Saunders; 1995:1312–1320.
- Geroghty PS, Sanchez LA, Rubin BG, et al. Overt ischemic colitis after endovascular repair of aortoiliac aneurysm. J Vasc Surg 2004; 40:413–418.
- Klestzick HN, McPhedran P, Cipolla D, Berry WA, DiCorato M, Denowitz J. The antiphospholipid syndrome and ischemic colitis. Gastroenterologist 1995; 3:249–256.
- Knot EA, ten Cate JW, Bruin T, Iburg AH, Tytgat GN. Antithrombin III metabolism in two colitis patients with acquired antithrombin III deficiency. Gastroenterology 1985; 89:421–425.
- Maloisel F. Role of coagulation disorders in mesenteric ischemia. J Chir (Paris) 1996; 133:442–447.
- Koutroubakis IE, Sfiridaki A, Theodoropoulou A, Kouroumalis EA. Role of acquired and hereditary thrombotic risk factors in colon ischemia of ambulatory patients. Gastroenterology 2001; 121:561–565.
- Midian-Singh R, Polen A, Durishin C, Crock RD, Whittier FC, Fahmy N. Ischemic colitis revisited: a prospective study identifying hypercoagulability as a risk factor. South Med J 2004; 97:120–123.
- Blanc P, Bories P, Donadio D, et al. Ischemic colitis and recurrent venous thrombosis caused by familial protein S deficiency. Gastroenterol Clin Biol 1989; 13:945.
- Verger P, Blanc C, Feydy P, Boey S. Ischemic colitis caused by protein S deficiency. Presse Med 1996; 25:1350.
- Ludwig D, Stahl M, David-Walek T, et al. Ischemic colitis, pulmonary embolism, and atrial thrombosis in a patient with inherited resistance to activated protein C. Dig Dis Sci 1998; 43:1362–1367.
- Yee NS, Guerry D, Lichtenstein GR. Ischemic colitis associated with factor V Leiden mutation. Ann Intern Med 2000; 132:595–596.
- Balian A, Veyradier A, Naveau S, et al. Prothrombin 20210G/A mutation in two patients with mesenteric ischemia. Dig Dis Sci 1999; 44:1910–1913.
- Arnott ID, Ghosh S, Ferguson A. The spectrum of ischaemic colitis. Eur J Gastroenterol Hepatol 1999; 11:295–303.
- Chin BW, Greenberg D, Wilson RB, Meredith CG. A case of ischemic colitis associated with factor V Leiden mutation: successful treatment with anticoagulation. Gastrointest Endosc 2007; 66:416–418.
- Heyn J, Buhmann S, Ladurner R, et al. Recurrent ischemic colitis in a patient with Leiden factor V mutation and systemic lupus erythematosus with antiphospholipid syndrome. Eur J Med Res 2008; 13:182–184.
- Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 2006; 101:1069–1079.
- Punnam SR, Pothula VR, Gourineni N, Punnam A, Ranganathan V. Interferon-ribavirin-associated ischemic colitis. J Clin Gastroenterol 2008; 42:323–325.
- Dowd J, Bailey D, Moussa K, Nair S, Doyle R, Culpepper-Morgan JA. Ischemic colitis associated with pseudoephedrine: four cases. Am J Gastroenterol 1999; 94:2430–2434.
- Kingsley DD, Schermer CR, Jamal MM. Rare complications of endoscopic retrograde cholangiopancreatography: two case reports. JSLS 2001; 5:171–173.
- Boley SJ, Agrawal GP, Warren AR, et al. Pathophysiologic effects of bowel distension on intestinal blood flow. Am J Surg 1969; 117:228–234.
- Reinus JF, Brandt LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19:319–343.
- Moses FM. Exercise-associated intestinal ischemia. Curr Sports Med Rep 2005; 4:91–95.
- Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am 1997; 77:289–306.
- Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand 1987; 153:321–324.
- Kim MK, Cho YS, Kim HK, Kim JS, Kim SS, Chae HS. Transient ischemic colitis after a pit viper bite (Agkistrodon blomhoffii brevicaudus). J Clin Gastroenterol 2008; 42:111–112.
- Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am 1998; 27:827–860.
- Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol 1998; 27:122–128.
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology 2000; 118:954–968.
- Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg 1978; 15:1–85.
- Schuler JG, Hudlin MM. Cecal necrosis: infrequent variant of ischemic colitis. Report of five cases. Dis Colon Rectum 2000; 43:708–712.
- Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007; 102:2247–2252.
- Park CJ, Jang MK, Shin WG, et al. Can we predict the development of ischemic colitis among patients with lower abdominal pain? Dis Colon Rectum 2007; 50:232–238.
- Su C, Brandt LJ, Sigal SH, et al. The immunohistological diagnosis of E. coli 0157:H7 colitis: possible association with colonic ischemia. Am J Gastroenterol 1998; 93:1055–1059.
- Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc 1981; 27:156–161.
- Rogers AI, David S. Intestinal blood flow and diseases of vascular impairment. In: Haubrich WS, Schaffner F, Berk JE, editors. Gastroenterology. 5th ed. Philadelphia: WB Saunders; 1995:1212–1234.
- Zuckerman GR, Prakash C, Merriman RB, Sawhney MS, DeSchryver-Kecskemeti K, Clouse RE. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol 2003; 98:2018–2022.
- Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J 2005; 98:217–222.
- Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003; 70:920–930.
- Habu Y, Tahashi Y, Kiyota K, et al. Reevaluation of clinical features of ischemic colitis: analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31:881–886.
- Mitsudo S, Brandt LJ. Pathology of intestinal ischemia. Surg Clin North Am 1992; 72:43–63.
- Price AB. Ischaemic colitis. Curr Top Pathol 1990; 81:229–246.
- Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology 1999; 211:381–388.
- Mosdell DM, Doberneck RC. Morbidity and mortality of ostomy closure. Am J Surg 1991; 162:633–636.
- Iqbal T, Zarin M, Iqbal A, et al. Results of primary closure in the management of gangrenous and viable sigmoid volvulus. Pak J Surg 2007; 23:118–121.
- Oz MC, Forde KA. Endoscopic alternatives in the management of colonic strictures. Surgery 1990; 108:513–519.
- Profili S, Bifulco V, Meloni GB, Demelas L, Niolu P, Manzoni MA. A case of ischemic stenosis of the colon-sigmoid treated with self-expandable uncoated metallic prosthesis. Radiol Med 1996; 91:665–667.
- Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992; 72:203–229.
- Boley SJ. 1989 David H. Sun lecture. Colonic ischemia—25 years later. Am J Gastroenterol 1990; 85:931–934.
- Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19:729–738.
- Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 7th ed. Philadelphia, PA: Saunders; 2002.
- Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum 1996; 39:88–100.
- Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am 2001; 30:445–473.
- Reeders JW, Tytgat GN, Rosenbusch G, et al. Ischaemic colitis. The Hague: Martinus Nijhoff, 1984;17.
- Brandt L, Boley S, Goldberg L, Mitsudo S, Berman A. Colitis in the elderly. A reappraisal. Am J Gastroenterol 1981; 76:239–245.
- Binns JC, Isaacson P. Age-related changes in the colonic blood supply: their relevance to ischaemic colitis. Gut 1978; 19:384–390.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72:65–83.
- Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol 2003; 98:1573–1577.
- Cappell MS. Safety and efficacy of colonoscopy after myocardial infarction: an analysis of 100 study patients and 100 control patients at two tertiary cardiac referral hospitals. Gastrointest Endosc 2004; 60:901–909.
- Cappell MS, Mahajan D, Kurupath V. Characterization of ischemic colitis associated with myocardial infarction: an analysis of 23 patients. Am J Med 2006; 119:527.e1–e9.
- Hagihara PF, Ernst CB, Griffen WO. Incidence of ischemic colitis following abdominal aortic reconstruction. Surg Gynecol Obstet 1979; 149:571–573.
- Brewster DC, Franklin DP, Cambria RP, et al. Intestinal ischemia complicating abdominal aortic surgery. Surgery 1991; 109:447–454.
- Piotrowski JJ, Ripepi AJ, Yuhas JP, Alexander JJ, Brandt CP. Colonic ischemia: the Achilles heel of ruptured aortic aneurysm repair. Am Surg 1996; 62:557–560.
- Ernst CB. Colonic ischemia following aortic reconstruction. In: Rutherford RB, editor. Vascular Surgery. 4th ed. Philadelphia, PA: WB Saunders; 1995:1312–1320.
- Geroghty PS, Sanchez LA, Rubin BG, et al. Overt ischemic colitis after endovascular repair of aortoiliac aneurysm. J Vasc Surg 2004; 40:413–418.
- Klestzick HN, McPhedran P, Cipolla D, Berry WA, DiCorato M, Denowitz J. The antiphospholipid syndrome and ischemic colitis. Gastroenterologist 1995; 3:249–256.
- Knot EA, ten Cate JW, Bruin T, Iburg AH, Tytgat GN. Antithrombin III metabolism in two colitis patients with acquired antithrombin III deficiency. Gastroenterology 1985; 89:421–425.
- Maloisel F. Role of coagulation disorders in mesenteric ischemia. J Chir (Paris) 1996; 133:442–447.
- Koutroubakis IE, Sfiridaki A, Theodoropoulou A, Kouroumalis EA. Role of acquired and hereditary thrombotic risk factors in colon ischemia of ambulatory patients. Gastroenterology 2001; 121:561–565.
- Midian-Singh R, Polen A, Durishin C, Crock RD, Whittier FC, Fahmy N. Ischemic colitis revisited: a prospective study identifying hypercoagulability as a risk factor. South Med J 2004; 97:120–123.
- Blanc P, Bories P, Donadio D, et al. Ischemic colitis and recurrent venous thrombosis caused by familial protein S deficiency. Gastroenterol Clin Biol 1989; 13:945.
- Verger P, Blanc C, Feydy P, Boey S. Ischemic colitis caused by protein S deficiency. Presse Med 1996; 25:1350.
- Ludwig D, Stahl M, David-Walek T, et al. Ischemic colitis, pulmonary embolism, and atrial thrombosis in a patient with inherited resistance to activated protein C. Dig Dis Sci 1998; 43:1362–1367.
- Yee NS, Guerry D, Lichtenstein GR. Ischemic colitis associated with factor V Leiden mutation. Ann Intern Med 2000; 132:595–596.
- Balian A, Veyradier A, Naveau S, et al. Prothrombin 20210G/A mutation in two patients with mesenteric ischemia. Dig Dis Sci 1999; 44:1910–1913.
- Arnott ID, Ghosh S, Ferguson A. The spectrum of ischaemic colitis. Eur J Gastroenterol Hepatol 1999; 11:295–303.
- Chin BW, Greenberg D, Wilson RB, Meredith CG. A case of ischemic colitis associated with factor V Leiden mutation: successful treatment with anticoagulation. Gastrointest Endosc 2007; 66:416–418.
- Heyn J, Buhmann S, Ladurner R, et al. Recurrent ischemic colitis in a patient with Leiden factor V mutation and systemic lupus erythematosus with antiphospholipid syndrome. Eur J Med Res 2008; 13:182–184.
- Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 2006; 101:1069–1079.
- Punnam SR, Pothula VR, Gourineni N, Punnam A, Ranganathan V. Interferon-ribavirin-associated ischemic colitis. J Clin Gastroenterol 2008; 42:323–325.
- Dowd J, Bailey D, Moussa K, Nair S, Doyle R, Culpepper-Morgan JA. Ischemic colitis associated with pseudoephedrine: four cases. Am J Gastroenterol 1999; 94:2430–2434.
- Kingsley DD, Schermer CR, Jamal MM. Rare complications of endoscopic retrograde cholangiopancreatography: two case reports. JSLS 2001; 5:171–173.
- Boley SJ, Agrawal GP, Warren AR, et al. Pathophysiologic effects of bowel distension on intestinal blood flow. Am J Surg 1969; 117:228–234.
- Reinus JF, Brandt LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19:319–343.
- Moses FM. Exercise-associated intestinal ischemia. Curr Sports Med Rep 2005; 4:91–95.
- Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am 1997; 77:289–306.
- Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand 1987; 153:321–324.
- Kim MK, Cho YS, Kim HK, Kim JS, Kim SS, Chae HS. Transient ischemic colitis after a pit viper bite (Agkistrodon blomhoffii brevicaudus). J Clin Gastroenterol 2008; 42:111–112.
- Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am 1998; 27:827–860.
- Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol 1998; 27:122–128.
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology 2000; 118:954–968.
- Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg 1978; 15:1–85.
- Schuler JG, Hudlin MM. Cecal necrosis: infrequent variant of ischemic colitis. Report of five cases. Dis Colon Rectum 2000; 43:708–712.
- Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007; 102:2247–2252.
- Park CJ, Jang MK, Shin WG, et al. Can we predict the development of ischemic colitis among patients with lower abdominal pain? Dis Colon Rectum 2007; 50:232–238.
- Su C, Brandt LJ, Sigal SH, et al. The immunohistological diagnosis of E. coli 0157:H7 colitis: possible association with colonic ischemia. Am J Gastroenterol 1998; 93:1055–1059.
- Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc 1981; 27:156–161.
- Rogers AI, David S. Intestinal blood flow and diseases of vascular impairment. In: Haubrich WS, Schaffner F, Berk JE, editors. Gastroenterology. 5th ed. Philadelphia: WB Saunders; 1995:1212–1234.
- Zuckerman GR, Prakash C, Merriman RB, Sawhney MS, DeSchryver-Kecskemeti K, Clouse RE. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol 2003; 98:2018–2022.
- Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J 2005; 98:217–222.
- Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003; 70:920–930.
- Habu Y, Tahashi Y, Kiyota K, et al. Reevaluation of clinical features of ischemic colitis: analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31:881–886.
- Mitsudo S, Brandt LJ. Pathology of intestinal ischemia. Surg Clin North Am 1992; 72:43–63.
- Price AB. Ischaemic colitis. Curr Top Pathol 1990; 81:229–246.
- Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology 1999; 211:381–388.
- Mosdell DM, Doberneck RC. Morbidity and mortality of ostomy closure. Am J Surg 1991; 162:633–636.
- Iqbal T, Zarin M, Iqbal A, et al. Results of primary closure in the management of gangrenous and viable sigmoid volvulus. Pak J Surg 2007; 23:118–121.
- Oz MC, Forde KA. Endoscopic alternatives in the management of colonic strictures. Surgery 1990; 108:513–519.
- Profili S, Bifulco V, Meloni GB, Demelas L, Niolu P, Manzoni MA. A case of ischemic stenosis of the colon-sigmoid treated with self-expandable uncoated metallic prosthesis. Radiol Med 1996; 91:665–667.
- Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992; 72:203–229.
- Boley SJ. 1989 David H. Sun lecture. Colonic ischemia—25 years later. Am J Gastroenterol 1990; 85:931–934.
KEY POINTS
- The incidence of colonic ischemia is difficult to ascertain, as most cases are transient and either not reported or misdiagnosed.
- Most cases are in the elderly.
- The clinical presentation is not specific, as other conditions also present with abdominal pain and hematochezia.
- The most common mechanisms are hypotension and hypovolemia caused by dehydration or bleeding that results in systemic hypoperfusion.
- Endoscopy has become the diagnostic procedure of choice.
- Although most patients can be treated conservatively with intravenous fluids, bowel rest, and antibiotics, some develop peritonitis or clinically deteriorate and require surgery.