User login
HIV-associated PML: Changing epidemiology and clinical approach
The appearance of progressive multifocal leukoencephalopathy (PML) as a complication of human immunodeficiency virus (HIV) infection dates to shortly after the first description of acquired immunodeficiency syndrome (AIDS). The advent of highly active antiretroviral therapy (HAART) dramatically altered the nature of HIV infection, resulting in a substantial decline in mortality and, in essence, turning AIDS into a chronic disease. As patients lived longer with HIV infection, one consequence was an increased incidence of neurologic complications. By the early 1980s, AIDS was well recognized as an underlying disorder that predisposed to PML.
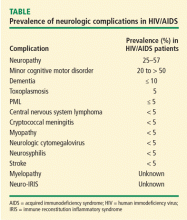
As many as 70% of HIV patients will eventually have involvement of either the peripheral or central nervous system (CNS). Most patients with HIV are managed by primary care clinicians, including those in the fields of family practice, internal medicine, or infectious disease, and the complexity of the neurologic disorders associated with HIV often results in either delayed diagnosis or misdiagnosis. For example, the evolution of HIV in the plasma, where most clinicians measure it, may differ from its evolution in the spinal fluid and brain. An emerging issue is that of hepatitis C coinfection, which may itself be associated with central and peripheral neurologic complications.
Treatment of HIV with antiretroviral agents has numerous neurologic implications. These include the potential ability of these agents to penetrate the blood-brain barrier, their efficacy in both treating and preventing cognitive impairment and other CNS disorders, and their toxic effects in the CNS and peripheral nervous system.
NEUROLOGIC COMPLICATIONS OF AIDS
Neurologic disease in AIDS patients can be classified in several ways. One of the most logical, particularly for primary care clinicians, is the separation of primary from secondary neurologic disorders:
- Primary neurologic disorders are enigmatic and difficult to characterize; they include HIV-associated neurocognitive disorders in adults, encephalopathy in children, myelopathy or spinal cord disease, and peripheral neuropathy.
- Secondary complications are related to progressive immunosuppression. These include opportunistic infections such as cytomegalovirus, toxoplasmosis, or cryptococcal meningitis; and neoplasms such as primary CNS lymphoma. Opportunistic infections and neoplasms have declined in incidence in the HAART era.
AT-RISK POOLS FOR PML
The AIDS epidemic significantly changed the epidemiology of PML, turning a formerly rare disease into a much more common one. In South Florida, the incidence of PML in patients with AIDS increased by 12 times from the 5-year period 1981 to 1984 compared with 1991 to 1994. Only two non-AIDS cases of PML were reported in South Florida during this 15-year period.1
At present, nonimmunosuppressed, healthy individuals account for fewer than 1% of all cases of PML. Non-HIV–related PML represents 10% to 20% of all PML cases. Cancer survivors and patients with rheumatoid arthritis who are treated with immunotherapy constitute the largest at-risk pools among this group. PML related to HIV represents 80% to 90% of PML cases, drawing from a pool of 1.2 million HIV-infected individuals in the United States.
UNIQUE PRESENTATION OF HIV-ASSOCIATED PML
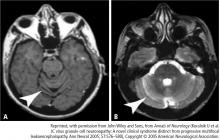
The brain lesion in PML is classically a nonenhancing focal lesion, preferentially in white matter, but lesion characteristics often depart from this characteristic picture. For example, relatively faint contrast enhancement of lesions on magnetic resonance imaging has been observed, as well as involvement of white matter and gray matter. The distribution and character of brain lesions in PML may also differ from the classic picture. For example, the lesion may not be focal, particularly when PML is combined with the symmetric white matter abnormalities that are seen in HIV encephalopathy; this nonclassic presentation can cause difficulty in radiologic differentiation of PML and HIV encephalopathy.
Cerebellar degeneration
A unique presentation of PML is possible in HIV-infected patients. In 1998, Tagliati et al2 described a syndrome of degeneration of the cerebellum in 10 HIV-infected patients. One patient had JC virus (JCV) detected by polymerase chain reaction (PCR) in cerebellar biopsy tissue. The authors proposed the possibility of latent JCV infection of cerebellar granular cells in HIV-infected patients with cerebellar atrophy, lacking further evidence of other features of PML.
MANAGEMENT OF HIV-ASSOCIATED PML
Optimize HAART
A suppressed plasma HIV viral load is the strongest prognostic factor for an improved course in PML.4 In the pre-HAART era, the mean survival of HIV-associated PML was 3 to 6 months, with long-term survival estimated at 10%.5 The use of HAART has achieved a dramatic improvement in long-term survival, to upwards of 50%.6 Neurologic deficits are often irreversible even with HAART, but most HAART recipients show stability in neurologic status for years.
Other key characteristics associated with improved survival in HIV-associated PML appear to be younger age, PML as the heralding manifestation of AIDS, initiation of HAART upon diagnosis of PML, higher CD4 count, and absence of severe neurologic impairment.5–7
Investigational therapies
Specific antiviral drug regimens targeting JCV have been tested empirically in case studies and in clinical trials in patients with AIDS- and non–AIDS-related PML.
Cytosine arabinoside (Ara-C). Ara-C is a nucleoside analog used as an antineoplastic agent; it terminates chain elongation and inhibits DNA polymerase to confer antiviral activity. Ara-C decreased JCV replication in vitro.8 Based on anecdotal reports of efficacy in cancer-related cases of PML,9 Ara-C was tested in a multicenter trial of 57 patients with HIV and biopsy-confirmed PML.10 Neither intravenous nor intrathecal Ara-C combined with established antiviral therapy for AIDS improved the prognosis of these patients, and Ara-C has since been abandoned as a strategy to treat HIV-related PML.
Cidofovir. The noncyclic nucleoside phosphonate cidofovir garnered attention as a potential treatment for PML based on case reports of efficacy in HIV as well as non-HIV patients. Subsequently, a large multicenter study failed to detect any significant added benefit with cidofovir beyond that of HAART.11 Retrospective European studies confirmed the lack of clinical benefit with cidofovir.6,7,12
Interferon alfa. Case reports with interferon alfa-2a and -2b for the treatment of PML show conflicting results with respect to clinical response, symptomatic improvement, and survival, but toxicity has been substantial. In a series of 97 patients with AIDS-related PML, Geschwind et al determined that interferon alfa had no effect on survival beyond that of HAART.13
Mirtazapine. Serotonin receptor antagonists such as mirtazapine can block JCV entry into glial cells via serotonin 5-hydroxytryptamine receptors, providing a rationale for their use as a potential treatment for PML. Verma et al describe a case of clinical improvement (stable neurologic deficit) and PML lesion regression in a 63-year-old bedbound woman with polycythemia vera with biopsy-proven non–HIV-related PML that had progressed to quadriparesis.14
Mefloquine. The antimalarial drug mefloquine inhibits viral replication in cultured human glial cells and astrocytes, inhibits JC viral DNA replication, and showed efficacy against two JCV strains in cell culture.15 A randomized study to assess the effectiveness of mefloquine for treatment of PML has been completed and its results await publication.
SUMMARY
The incidence of PML has remained unchanged from the pre-HAART to the HAART era, but the prognosis is greatly improved. The clinical presentation of PML in AIDS patients may deviate from the classic triad of progressive, multifocal, white matter disease. It may be static and unifocal, and it may involve gray matter and neurons as well as white matter. The number of neurologic manifestations is vast and can include the cerebellar syndrome. Lumbar puncture with a PCR negative for JCV does not confirm the absence of PML.
The standard of care for HIV-associated PML is HAART, with the goal of achieving immunologic recovery and optimal virologic control. Whether therapeutic results obtained in patients with HIV-associated PML can be translated to the setting of non–HIV-associated PML is unclear.
DISCUSSION
Dr. Simpson: As a followup to the Ara-C trial that was published,10 PML confirmed by brain biopsy was one of the enrolling criteria, and the planned study population was 65 patients. Longitudinal examination of viral load in cerebrospinal fluid (CSF) was a part of the study, and we found that the lower the viral load, the better the prognosis. Fifty-two patients were enrolled before the trial was stopped because it was clear that Ara-C was not producing a benefit. The patients had multifocal disease but, because Ara-C does not effectively cross the blood-brain barrier, penetration in the brain was minimal even with the use of an intrathecal shunt in this study.
Dr. Major: Do you think viral load in CSF is a predictor of disease severity and outcome in PML?
Dr. Rudick: Generally speaking, that’s probably true. We have found, as have many of our colleagues who run a lot of CSF samples, that high viral loads are not a good thing.
Dr. Bennett: How is it that the incidence of PML has not changed from the pre-HAART to the post-HAART era? How do you account for this in terms of the change in patients’ T-cell function from pre- to post-HAART?
Dr. Simpson: I don’t know. Intuitively, why do patients treated with HAART, who are relatively immune reconstituted, develop PML? The problem is that not everyone is immune reconstituted. HAART fails in some patients. Further, PML remains a disease that is more common in late-stage HIV among patients with low CD4 counts and high viral loads, meaning that a large population of patients is available to develop this disease. With that said, it is perplexing that the incidence has not gone down more than it has.
Dr. Major: There’s a phenomenon called “unmasking PML with HAART,” in which individuals have no signs of PML upon initiation of HAART, but then very shortly after, PML is diagnosed.
Dr. Berger: You’re talking about PML immune reconstitution inflammatory syndrome (IRIS).
Dr. Major: IRIS can occur before PML, or PML and IRIS can be concurrent. In some patients, once the infection starts, it persists; this suggests that the virus is carried to the brain through the infected lymphocyte populations and may explain why the incidence of PML has not changed from the pre-HAART to the HAART era.
Dr. Calabrese: In patients with HIV who develop PML within the first 6 months of HAART, are we seeing the IRIS phenomenon or is it a presenting sign of advanced HIV?
Dr. Simpson: It’s well known that a number of opportunistic infections can develop in the setting of HAART. In fact, whether one should delay HAART when initiating therapy for opportunistic infections has been debated for just this reason. Most people presume IRIS to be a massive immunologic hit to all organ systems, as CD4 counts rise dramatically to produce hyperimmune-mediated phenomena such as Guillain-Barré syndrome. To what extent immunologic recovery is or is not linked to PML and why it happens are fascinating questions.
Dr. Berger: Opportunistic infections, PML among them, that occur following the initiation of HAART and recovery of the immune system are almost always an IRIS-mediated phenomenon in which the disease has been smoldering and then surfaces because of the release of an inflammatory response.
Dr. Calabrese: In patients with cerebellar degeneration, do you typically detect JCV in PCR in the spinal fluid?
Dr. Simpson: Not in the early stages, but in some patients with later-stage disease,3 the answer is yes. Certainly, PCR of CSF samples to look for JCV is the diagnostic test of choice. But in the early days, when we had no idea what caused this cerebellar syndrome, we were doing cerebellar biopsies.
- Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol 1998; 4:59–68.
- Tagliati M, Simpson D, Margello S, Clifford D, Schwartz RL, Berger JR. Cerebellar degeneration associated with human immunodeficiency virus infection. Neurology 1998; 50:244–251.
- Koralnik I, Wüthrich C, Dang X, et al. JC virus granule cell neuronopathy: a novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol 2005; 57:576–580.
- Clifford DB, Yiannoutsos C, Glicksman M, et al. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology 1999; 52:623–625.
- Berger JR, Levy RM, Flomenhoff D, Dobbs M. Predictive factors for prolonged survival in acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. Ann Neurol 1998; 44:341–349.
- Antinori A, Cingolani A, Lorenzini P, et al. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA). J Neurovirol 2003; 9( suppl 1):47–53.
- Berenguer J, Miralles P, Arrizabalaga J, et al. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clin Infect Dis 2003; 36:1047–1052.
- Hou J, Major EO. The efficacy of nucleoside analogs against JC virus multiplication in a persistently infected human fetal brain cell line. J Neurovirol 1998; 4:451–456.
- Aksamit A. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J Neurovirol 2001; 7:386–390.
- Hall C, Dafni U, Simpson D, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med 1998; 338:1345–1351.
- Marra CM, Rajicic N, Barker DE, et al. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS 2002; 16:1791–1797.
- Gasnault J, Kousignian P, Kahraman M, et al. Cidofovir in AIDS-associated progressive multifocal leukoencephalopathy: a monocenter observational study with clinical and JC virus load monitoring. J Neurovirol 2001; 7:375–381.
- Geschwind MD, Skolasky RI, Royal WS, McArthur JC. The relative contributions of HAART and alpha-interferon for therapy of progressive multifocal leukoencephalopathy in AIDS. J Neurovirol 2001; 7:353–357.
- Verma S, Cikurel K, Koralnik IJ, et al. Mirtazapine in progressive multifocal leukoencephalopathy. J Infect Dis 2007; 196:709–711.
- Brickelmaier M, Lugovskoy A, Kartikeyan R, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother 2009; 53:1840–1849.
The appearance of progressive multifocal leukoencephalopathy (PML) as a complication of human immunodeficiency virus (HIV) infection dates to shortly after the first description of acquired immunodeficiency syndrome (AIDS). The advent of highly active antiretroviral therapy (HAART) dramatically altered the nature of HIV infection, resulting in a substantial decline in mortality and, in essence, turning AIDS into a chronic disease. As patients lived longer with HIV infection, one consequence was an increased incidence of neurologic complications. By the early 1980s, AIDS was well recognized as an underlying disorder that predisposed to PML.
As many as 70% of HIV patients will eventually have involvement of either the peripheral or central nervous system (CNS). Most patients with HIV are managed by primary care clinicians, including those in the fields of family practice, internal medicine, or infectious disease, and the complexity of the neurologic disorders associated with HIV often results in either delayed diagnosis or misdiagnosis. For example, the evolution of HIV in the plasma, where most clinicians measure it, may differ from its evolution in the spinal fluid and brain. An emerging issue is that of hepatitis C coinfection, which may itself be associated with central and peripheral neurologic complications.
Treatment of HIV with antiretroviral agents has numerous neurologic implications. These include the potential ability of these agents to penetrate the blood-brain barrier, their efficacy in both treating and preventing cognitive impairment and other CNS disorders, and their toxic effects in the CNS and peripheral nervous system.
NEUROLOGIC COMPLICATIONS OF AIDS
Neurologic disease in AIDS patients can be classified in several ways. One of the most logical, particularly for primary care clinicians, is the separation of primary from secondary neurologic disorders:
- Primary neurologic disorders are enigmatic and difficult to characterize; they include HIV-associated neurocognitive disorders in adults, encephalopathy in children, myelopathy or spinal cord disease, and peripheral neuropathy.
- Secondary complications are related to progressive immunosuppression. These include opportunistic infections such as cytomegalovirus, toxoplasmosis, or cryptococcal meningitis; and neoplasms such as primary CNS lymphoma. Opportunistic infections and neoplasms have declined in incidence in the HAART era.
AT-RISK POOLS FOR PML
The AIDS epidemic significantly changed the epidemiology of PML, turning a formerly rare disease into a much more common one. In South Florida, the incidence of PML in patients with AIDS increased by 12 times from the 5-year period 1981 to 1984 compared with 1991 to 1994. Only two non-AIDS cases of PML were reported in South Florida during this 15-year period.1
At present, nonimmunosuppressed, healthy individuals account for fewer than 1% of all cases of PML. Non-HIV–related PML represents 10% to 20% of all PML cases. Cancer survivors and patients with rheumatoid arthritis who are treated with immunotherapy constitute the largest at-risk pools among this group. PML related to HIV represents 80% to 90% of PML cases, drawing from a pool of 1.2 million HIV-infected individuals in the United States.
UNIQUE PRESENTATION OF HIV-ASSOCIATED PML
The brain lesion in PML is classically a nonenhancing focal lesion, preferentially in white matter, but lesion characteristics often depart from this characteristic picture. For example, relatively faint contrast enhancement of lesions on magnetic resonance imaging has been observed, as well as involvement of white matter and gray matter. The distribution and character of brain lesions in PML may also differ from the classic picture. For example, the lesion may not be focal, particularly when PML is combined with the symmetric white matter abnormalities that are seen in HIV encephalopathy; this nonclassic presentation can cause difficulty in radiologic differentiation of PML and HIV encephalopathy.
Cerebellar degeneration
A unique presentation of PML is possible in HIV-infected patients. In 1998, Tagliati et al2 described a syndrome of degeneration of the cerebellum in 10 HIV-infected patients. One patient had JC virus (JCV) detected by polymerase chain reaction (PCR) in cerebellar biopsy tissue. The authors proposed the possibility of latent JCV infection of cerebellar granular cells in HIV-infected patients with cerebellar atrophy, lacking further evidence of other features of PML.
MANAGEMENT OF HIV-ASSOCIATED PML
Optimize HAART
A suppressed plasma HIV viral load is the strongest prognostic factor for an improved course in PML.4 In the pre-HAART era, the mean survival of HIV-associated PML was 3 to 6 months, with long-term survival estimated at 10%.5 The use of HAART has achieved a dramatic improvement in long-term survival, to upwards of 50%.6 Neurologic deficits are often irreversible even with HAART, but most HAART recipients show stability in neurologic status for years.
Other key characteristics associated with improved survival in HIV-associated PML appear to be younger age, PML as the heralding manifestation of AIDS, initiation of HAART upon diagnosis of PML, higher CD4 count, and absence of severe neurologic impairment.5–7
Investigational therapies
Specific antiviral drug regimens targeting JCV have been tested empirically in case studies and in clinical trials in patients with AIDS- and non–AIDS-related PML.
Cytosine arabinoside (Ara-C). Ara-C is a nucleoside analog used as an antineoplastic agent; it terminates chain elongation and inhibits DNA polymerase to confer antiviral activity. Ara-C decreased JCV replication in vitro.8 Based on anecdotal reports of efficacy in cancer-related cases of PML,9 Ara-C was tested in a multicenter trial of 57 patients with HIV and biopsy-confirmed PML.10 Neither intravenous nor intrathecal Ara-C combined with established antiviral therapy for AIDS improved the prognosis of these patients, and Ara-C has since been abandoned as a strategy to treat HIV-related PML.
Cidofovir. The noncyclic nucleoside phosphonate cidofovir garnered attention as a potential treatment for PML based on case reports of efficacy in HIV as well as non-HIV patients. Subsequently, a large multicenter study failed to detect any significant added benefit with cidofovir beyond that of HAART.11 Retrospective European studies confirmed the lack of clinical benefit with cidofovir.6,7,12
Interferon alfa. Case reports with interferon alfa-2a and -2b for the treatment of PML show conflicting results with respect to clinical response, symptomatic improvement, and survival, but toxicity has been substantial. In a series of 97 patients with AIDS-related PML, Geschwind et al determined that interferon alfa had no effect on survival beyond that of HAART.13
Mirtazapine. Serotonin receptor antagonists such as mirtazapine can block JCV entry into glial cells via serotonin 5-hydroxytryptamine receptors, providing a rationale for their use as a potential treatment for PML. Verma et al describe a case of clinical improvement (stable neurologic deficit) and PML lesion regression in a 63-year-old bedbound woman with polycythemia vera with biopsy-proven non–HIV-related PML that had progressed to quadriparesis.14
Mefloquine. The antimalarial drug mefloquine inhibits viral replication in cultured human glial cells and astrocytes, inhibits JC viral DNA replication, and showed efficacy against two JCV strains in cell culture.15 A randomized study to assess the effectiveness of mefloquine for treatment of PML has been completed and its results await publication.
SUMMARY
The incidence of PML has remained unchanged from the pre-HAART to the HAART era, but the prognosis is greatly improved. The clinical presentation of PML in AIDS patients may deviate from the classic triad of progressive, multifocal, white matter disease. It may be static and unifocal, and it may involve gray matter and neurons as well as white matter. The number of neurologic manifestations is vast and can include the cerebellar syndrome. Lumbar puncture with a PCR negative for JCV does not confirm the absence of PML.
The standard of care for HIV-associated PML is HAART, with the goal of achieving immunologic recovery and optimal virologic control. Whether therapeutic results obtained in patients with HIV-associated PML can be translated to the setting of non–HIV-associated PML is unclear.
DISCUSSION
Dr. Simpson: As a followup to the Ara-C trial that was published,10 PML confirmed by brain biopsy was one of the enrolling criteria, and the planned study population was 65 patients. Longitudinal examination of viral load in cerebrospinal fluid (CSF) was a part of the study, and we found that the lower the viral load, the better the prognosis. Fifty-two patients were enrolled before the trial was stopped because it was clear that Ara-C was not producing a benefit. The patients had multifocal disease but, because Ara-C does not effectively cross the blood-brain barrier, penetration in the brain was minimal even with the use of an intrathecal shunt in this study.
Dr. Major: Do you think viral load in CSF is a predictor of disease severity and outcome in PML?
Dr. Rudick: Generally speaking, that’s probably true. We have found, as have many of our colleagues who run a lot of CSF samples, that high viral loads are not a good thing.
Dr. Bennett: How is it that the incidence of PML has not changed from the pre-HAART to the post-HAART era? How do you account for this in terms of the change in patients’ T-cell function from pre- to post-HAART?
Dr. Simpson: I don’t know. Intuitively, why do patients treated with HAART, who are relatively immune reconstituted, develop PML? The problem is that not everyone is immune reconstituted. HAART fails in some patients. Further, PML remains a disease that is more common in late-stage HIV among patients with low CD4 counts and high viral loads, meaning that a large population of patients is available to develop this disease. With that said, it is perplexing that the incidence has not gone down more than it has.
Dr. Major: There’s a phenomenon called “unmasking PML with HAART,” in which individuals have no signs of PML upon initiation of HAART, but then very shortly after, PML is diagnosed.
Dr. Berger: You’re talking about PML immune reconstitution inflammatory syndrome (IRIS).
Dr. Major: IRIS can occur before PML, or PML and IRIS can be concurrent. In some patients, once the infection starts, it persists; this suggests that the virus is carried to the brain through the infected lymphocyte populations and may explain why the incidence of PML has not changed from the pre-HAART to the HAART era.
Dr. Calabrese: In patients with HIV who develop PML within the first 6 months of HAART, are we seeing the IRIS phenomenon or is it a presenting sign of advanced HIV?
Dr. Simpson: It’s well known that a number of opportunistic infections can develop in the setting of HAART. In fact, whether one should delay HAART when initiating therapy for opportunistic infections has been debated for just this reason. Most people presume IRIS to be a massive immunologic hit to all organ systems, as CD4 counts rise dramatically to produce hyperimmune-mediated phenomena such as Guillain-Barré syndrome. To what extent immunologic recovery is or is not linked to PML and why it happens are fascinating questions.
Dr. Berger: Opportunistic infections, PML among them, that occur following the initiation of HAART and recovery of the immune system are almost always an IRIS-mediated phenomenon in which the disease has been smoldering and then surfaces because of the release of an inflammatory response.
Dr. Calabrese: In patients with cerebellar degeneration, do you typically detect JCV in PCR in the spinal fluid?
Dr. Simpson: Not in the early stages, but in some patients with later-stage disease,3 the answer is yes. Certainly, PCR of CSF samples to look for JCV is the diagnostic test of choice. But in the early days, when we had no idea what caused this cerebellar syndrome, we were doing cerebellar biopsies.
The appearance of progressive multifocal leukoencephalopathy (PML) as a complication of human immunodeficiency virus (HIV) infection dates to shortly after the first description of acquired immunodeficiency syndrome (AIDS). The advent of highly active antiretroviral therapy (HAART) dramatically altered the nature of HIV infection, resulting in a substantial decline in mortality and, in essence, turning AIDS into a chronic disease. As patients lived longer with HIV infection, one consequence was an increased incidence of neurologic complications. By the early 1980s, AIDS was well recognized as an underlying disorder that predisposed to PML.
As many as 70% of HIV patients will eventually have involvement of either the peripheral or central nervous system (CNS). Most patients with HIV are managed by primary care clinicians, including those in the fields of family practice, internal medicine, or infectious disease, and the complexity of the neurologic disorders associated with HIV often results in either delayed diagnosis or misdiagnosis. For example, the evolution of HIV in the plasma, where most clinicians measure it, may differ from its evolution in the spinal fluid and brain. An emerging issue is that of hepatitis C coinfection, which may itself be associated with central and peripheral neurologic complications.
Treatment of HIV with antiretroviral agents has numerous neurologic implications. These include the potential ability of these agents to penetrate the blood-brain barrier, their efficacy in both treating and preventing cognitive impairment and other CNS disorders, and their toxic effects in the CNS and peripheral nervous system.
NEUROLOGIC COMPLICATIONS OF AIDS
Neurologic disease in AIDS patients can be classified in several ways. One of the most logical, particularly for primary care clinicians, is the separation of primary from secondary neurologic disorders:
- Primary neurologic disorders are enigmatic and difficult to characterize; they include HIV-associated neurocognitive disorders in adults, encephalopathy in children, myelopathy or spinal cord disease, and peripheral neuropathy.
- Secondary complications are related to progressive immunosuppression. These include opportunistic infections such as cytomegalovirus, toxoplasmosis, or cryptococcal meningitis; and neoplasms such as primary CNS lymphoma. Opportunistic infections and neoplasms have declined in incidence in the HAART era.
AT-RISK POOLS FOR PML
The AIDS epidemic significantly changed the epidemiology of PML, turning a formerly rare disease into a much more common one. In South Florida, the incidence of PML in patients with AIDS increased by 12 times from the 5-year period 1981 to 1984 compared with 1991 to 1994. Only two non-AIDS cases of PML were reported in South Florida during this 15-year period.1
At present, nonimmunosuppressed, healthy individuals account for fewer than 1% of all cases of PML. Non-HIV–related PML represents 10% to 20% of all PML cases. Cancer survivors and patients with rheumatoid arthritis who are treated with immunotherapy constitute the largest at-risk pools among this group. PML related to HIV represents 80% to 90% of PML cases, drawing from a pool of 1.2 million HIV-infected individuals in the United States.
UNIQUE PRESENTATION OF HIV-ASSOCIATED PML
The brain lesion in PML is classically a nonenhancing focal lesion, preferentially in white matter, but lesion characteristics often depart from this characteristic picture. For example, relatively faint contrast enhancement of lesions on magnetic resonance imaging has been observed, as well as involvement of white matter and gray matter. The distribution and character of brain lesions in PML may also differ from the classic picture. For example, the lesion may not be focal, particularly when PML is combined with the symmetric white matter abnormalities that are seen in HIV encephalopathy; this nonclassic presentation can cause difficulty in radiologic differentiation of PML and HIV encephalopathy.
Cerebellar degeneration
A unique presentation of PML is possible in HIV-infected patients. In 1998, Tagliati et al2 described a syndrome of degeneration of the cerebellum in 10 HIV-infected patients. One patient had JC virus (JCV) detected by polymerase chain reaction (PCR) in cerebellar biopsy tissue. The authors proposed the possibility of latent JCV infection of cerebellar granular cells in HIV-infected patients with cerebellar atrophy, lacking further evidence of other features of PML.
MANAGEMENT OF HIV-ASSOCIATED PML
Optimize HAART
A suppressed plasma HIV viral load is the strongest prognostic factor for an improved course in PML.4 In the pre-HAART era, the mean survival of HIV-associated PML was 3 to 6 months, with long-term survival estimated at 10%.5 The use of HAART has achieved a dramatic improvement in long-term survival, to upwards of 50%.6 Neurologic deficits are often irreversible even with HAART, but most HAART recipients show stability in neurologic status for years.
Other key characteristics associated with improved survival in HIV-associated PML appear to be younger age, PML as the heralding manifestation of AIDS, initiation of HAART upon diagnosis of PML, higher CD4 count, and absence of severe neurologic impairment.5–7
Investigational therapies
Specific antiviral drug regimens targeting JCV have been tested empirically in case studies and in clinical trials in patients with AIDS- and non–AIDS-related PML.
Cytosine arabinoside (Ara-C). Ara-C is a nucleoside analog used as an antineoplastic agent; it terminates chain elongation and inhibits DNA polymerase to confer antiviral activity. Ara-C decreased JCV replication in vitro.8 Based on anecdotal reports of efficacy in cancer-related cases of PML,9 Ara-C was tested in a multicenter trial of 57 patients with HIV and biopsy-confirmed PML.10 Neither intravenous nor intrathecal Ara-C combined with established antiviral therapy for AIDS improved the prognosis of these patients, and Ara-C has since been abandoned as a strategy to treat HIV-related PML.
Cidofovir. The noncyclic nucleoside phosphonate cidofovir garnered attention as a potential treatment for PML based on case reports of efficacy in HIV as well as non-HIV patients. Subsequently, a large multicenter study failed to detect any significant added benefit with cidofovir beyond that of HAART.11 Retrospective European studies confirmed the lack of clinical benefit with cidofovir.6,7,12
Interferon alfa. Case reports with interferon alfa-2a and -2b for the treatment of PML show conflicting results with respect to clinical response, symptomatic improvement, and survival, but toxicity has been substantial. In a series of 97 patients with AIDS-related PML, Geschwind et al determined that interferon alfa had no effect on survival beyond that of HAART.13
Mirtazapine. Serotonin receptor antagonists such as mirtazapine can block JCV entry into glial cells via serotonin 5-hydroxytryptamine receptors, providing a rationale for their use as a potential treatment for PML. Verma et al describe a case of clinical improvement (stable neurologic deficit) and PML lesion regression in a 63-year-old bedbound woman with polycythemia vera with biopsy-proven non–HIV-related PML that had progressed to quadriparesis.14
Mefloquine. The antimalarial drug mefloquine inhibits viral replication in cultured human glial cells and astrocytes, inhibits JC viral DNA replication, and showed efficacy against two JCV strains in cell culture.15 A randomized study to assess the effectiveness of mefloquine for treatment of PML has been completed and its results await publication.
SUMMARY
The incidence of PML has remained unchanged from the pre-HAART to the HAART era, but the prognosis is greatly improved. The clinical presentation of PML in AIDS patients may deviate from the classic triad of progressive, multifocal, white matter disease. It may be static and unifocal, and it may involve gray matter and neurons as well as white matter. The number of neurologic manifestations is vast and can include the cerebellar syndrome. Lumbar puncture with a PCR negative for JCV does not confirm the absence of PML.
The standard of care for HIV-associated PML is HAART, with the goal of achieving immunologic recovery and optimal virologic control. Whether therapeutic results obtained in patients with HIV-associated PML can be translated to the setting of non–HIV-associated PML is unclear.
DISCUSSION
Dr. Simpson: As a followup to the Ara-C trial that was published,10 PML confirmed by brain biopsy was one of the enrolling criteria, and the planned study population was 65 patients. Longitudinal examination of viral load in cerebrospinal fluid (CSF) was a part of the study, and we found that the lower the viral load, the better the prognosis. Fifty-two patients were enrolled before the trial was stopped because it was clear that Ara-C was not producing a benefit. The patients had multifocal disease but, because Ara-C does not effectively cross the blood-brain barrier, penetration in the brain was minimal even with the use of an intrathecal shunt in this study.
Dr. Major: Do you think viral load in CSF is a predictor of disease severity and outcome in PML?
Dr. Rudick: Generally speaking, that’s probably true. We have found, as have many of our colleagues who run a lot of CSF samples, that high viral loads are not a good thing.
Dr. Bennett: How is it that the incidence of PML has not changed from the pre-HAART to the post-HAART era? How do you account for this in terms of the change in patients’ T-cell function from pre- to post-HAART?
Dr. Simpson: I don’t know. Intuitively, why do patients treated with HAART, who are relatively immune reconstituted, develop PML? The problem is that not everyone is immune reconstituted. HAART fails in some patients. Further, PML remains a disease that is more common in late-stage HIV among patients with low CD4 counts and high viral loads, meaning that a large population of patients is available to develop this disease. With that said, it is perplexing that the incidence has not gone down more than it has.
Dr. Major: There’s a phenomenon called “unmasking PML with HAART,” in which individuals have no signs of PML upon initiation of HAART, but then very shortly after, PML is diagnosed.
Dr. Berger: You’re talking about PML immune reconstitution inflammatory syndrome (IRIS).
Dr. Major: IRIS can occur before PML, or PML and IRIS can be concurrent. In some patients, once the infection starts, it persists; this suggests that the virus is carried to the brain through the infected lymphocyte populations and may explain why the incidence of PML has not changed from the pre-HAART to the HAART era.
Dr. Calabrese: In patients with HIV who develop PML within the first 6 months of HAART, are we seeing the IRIS phenomenon or is it a presenting sign of advanced HIV?
Dr. Simpson: It’s well known that a number of opportunistic infections can develop in the setting of HAART. In fact, whether one should delay HAART when initiating therapy for opportunistic infections has been debated for just this reason. Most people presume IRIS to be a massive immunologic hit to all organ systems, as CD4 counts rise dramatically to produce hyperimmune-mediated phenomena such as Guillain-Barré syndrome. To what extent immunologic recovery is or is not linked to PML and why it happens are fascinating questions.
Dr. Berger: Opportunistic infections, PML among them, that occur following the initiation of HAART and recovery of the immune system are almost always an IRIS-mediated phenomenon in which the disease has been smoldering and then surfaces because of the release of an inflammatory response.
Dr. Calabrese: In patients with cerebellar degeneration, do you typically detect JCV in PCR in the spinal fluid?
Dr. Simpson: Not in the early stages, but in some patients with later-stage disease,3 the answer is yes. Certainly, PCR of CSF samples to look for JCV is the diagnostic test of choice. But in the early days, when we had no idea what caused this cerebellar syndrome, we were doing cerebellar biopsies.
- Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol 1998; 4:59–68.
- Tagliati M, Simpson D, Margello S, Clifford D, Schwartz RL, Berger JR. Cerebellar degeneration associated with human immunodeficiency virus infection. Neurology 1998; 50:244–251.
- Koralnik I, Wüthrich C, Dang X, et al. JC virus granule cell neuronopathy: a novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol 2005; 57:576–580.
- Clifford DB, Yiannoutsos C, Glicksman M, et al. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology 1999; 52:623–625.
- Berger JR, Levy RM, Flomenhoff D, Dobbs M. Predictive factors for prolonged survival in acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. Ann Neurol 1998; 44:341–349.
- Antinori A, Cingolani A, Lorenzini P, et al. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA). J Neurovirol 2003; 9( suppl 1):47–53.
- Berenguer J, Miralles P, Arrizabalaga J, et al. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clin Infect Dis 2003; 36:1047–1052.
- Hou J, Major EO. The efficacy of nucleoside analogs against JC virus multiplication in a persistently infected human fetal brain cell line. J Neurovirol 1998; 4:451–456.
- Aksamit A. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J Neurovirol 2001; 7:386–390.
- Hall C, Dafni U, Simpson D, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med 1998; 338:1345–1351.
- Marra CM, Rajicic N, Barker DE, et al. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS 2002; 16:1791–1797.
- Gasnault J, Kousignian P, Kahraman M, et al. Cidofovir in AIDS-associated progressive multifocal leukoencephalopathy: a monocenter observational study with clinical and JC virus load monitoring. J Neurovirol 2001; 7:375–381.
- Geschwind MD, Skolasky RI, Royal WS, McArthur JC. The relative contributions of HAART and alpha-interferon for therapy of progressive multifocal leukoencephalopathy in AIDS. J Neurovirol 2001; 7:353–357.
- Verma S, Cikurel K, Koralnik IJ, et al. Mirtazapine in progressive multifocal leukoencephalopathy. J Infect Dis 2007; 196:709–711.
- Brickelmaier M, Lugovskoy A, Kartikeyan R, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother 2009; 53:1840–1849.
- Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol 1998; 4:59–68.
- Tagliati M, Simpson D, Margello S, Clifford D, Schwartz RL, Berger JR. Cerebellar degeneration associated with human immunodeficiency virus infection. Neurology 1998; 50:244–251.
- Koralnik I, Wüthrich C, Dang X, et al. JC virus granule cell neuronopathy: a novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol 2005; 57:576–580.
- Clifford DB, Yiannoutsos C, Glicksman M, et al. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology 1999; 52:623–625.
- Berger JR, Levy RM, Flomenhoff D, Dobbs M. Predictive factors for prolonged survival in acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. Ann Neurol 1998; 44:341–349.
- Antinori A, Cingolani A, Lorenzini P, et al. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA). J Neurovirol 2003; 9( suppl 1):47–53.
- Berenguer J, Miralles P, Arrizabalaga J, et al. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clin Infect Dis 2003; 36:1047–1052.
- Hou J, Major EO. The efficacy of nucleoside analogs against JC virus multiplication in a persistently infected human fetal brain cell line. J Neurovirol 1998; 4:451–456.
- Aksamit A. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J Neurovirol 2001; 7:386–390.
- Hall C, Dafni U, Simpson D, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med 1998; 338:1345–1351.
- Marra CM, Rajicic N, Barker DE, et al. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS 2002; 16:1791–1797.
- Gasnault J, Kousignian P, Kahraman M, et al. Cidofovir in AIDS-associated progressive multifocal leukoencephalopathy: a monocenter observational study with clinical and JC virus load monitoring. J Neurovirol 2001; 7:375–381.
- Geschwind MD, Skolasky RI, Royal WS, McArthur JC. The relative contributions of HAART and alpha-interferon for therapy of progressive multifocal leukoencephalopathy in AIDS. J Neurovirol 2001; 7:353–357.
- Verma S, Cikurel K, Koralnik IJ, et al. Mirtazapine in progressive multifocal leukoencephalopathy. J Infect Dis 2007; 196:709–711.
- Brickelmaier M, Lugovskoy A, Kartikeyan R, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother 2009; 53:1840–1849.