User login
Managing community-acquired MRSA lesions: What works?
The author reports no financial disclosure relevant to this article.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) abscesses are best managed surgically; postprocedure antibiotics do not substantially improve outcome. The cure rate with incision and drainage alone is at least 90%.
- If incision and drainage fail to promote healing within 7 days, oral antibiotics of choice are trimethoprim-sulfamethoxazole and tetracycline
- Eradication of nasal carriage of CA-MRSA generally does not help prevent spread of clinical MRSA infection in communities.
CASE: Tender suprapubic lesion
A previously healthy, 22-year-old law school student arrives at your office complaining of “abdominal pain.” She is previously healthy; temperature is normal.
You discover on examination that she has an erythematous, indurated, and tender 3-cm lesion on the suprapubic region. The lesion has no point, but its center is boggy.
Should you prescribe an antibiotic? And should you cover immediately for CA-MRSA? What other factors might influence your decision about treatment?
The incidence of MRSA is increasing in communities across the United States, challenging assumptions about the evaluation and management of skin and soft-tissue infections. In this article, I outline a rational approach to managing patients who have a lesion likely to be caused by CA-MRSA ( TABLE 1 ).
TABLE
Suspect CA-MRSA infection? Consider this treatment scheme
| When a patient meets these criteria… | Provide this management… | And select from these antibiotics |
|---|---|---|
| Lesion nonfluctuant; patient afebrile, healthy (Class 1 infection) | If no drainable abscess, give a common first-line antibiotic for skin and soft-tissue infection; reassess for response | —Semisynthetic penicillin —Oral first- or second-generation cephalosporin —Macrolide —Clindamycin |
| Lesion, fluctuant or pustular, <5 cm in diameter; fever or no fever (Class 2) | Drain abscess surgically if possible; use incision and drainage presumptively for MRSA and monitor closely for response; inpatient management may be indicated | —Trimethoprim sulfamethoxazole —Tetracycline —Clindamycin |
| Lesion, >5 cm in diameter, toxic appearance or at least one unstable comorbidity or a limb-threatening infection (Class 3) | Admit; consider infectious disease consult | Broad-spectrum agent, including vancomycin, for MRSA coverage |
| Sepsis syndrome or life-threatening infection (necrotizing fasciitis)(Class 4) | Admit; institute aggressive surgical debridement; request infectious disease consult | Broad-spectrum agent, including vancomycin, for MRSA coverage |
| Source: Eron et al6 and CDC7 . | ||
When to suspect MRSA skin infection
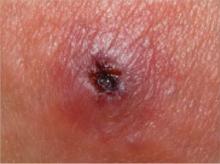
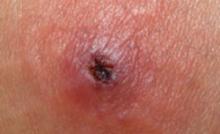
Patients who have a CA-MRSA skin infection often report a “spider bite” because the lesion appears suddenly and unexpectedly in an area where there is no history of trauma.1 Lesions often are pustular with central necrosis; there may be purulent drainage, redness, tenderness, and palpable fluctuance ( FIGURE ).
CA-MRSA skin lesions can occur anywhere on the body, though they appear most often in the axillae or the groin and buttocks. Patients may or may not have a fever.
Persons at increased risk of CA-MRSA disease include users of health clubs, participants in contact sports, men who have sex with men, children younger than 2 years, users of intravenous drugs, military personnel, and prisoners.2,3 Absence of these risk factors in a patient with a skin or soft-tissue infection does not, however, rule out MRSA.4
Regardless of the lesion’s appearance or the patient’s epidemiologic history, consider CA-MRSA if its prevalence in your community has reached 10% to 15%.
CA-MRSA can cause impetigo, but the often-benign nature of this clinical infection makes management decisions less crucial. However, do hospitalize any patient who has a MRSA infection who also exhibits fever or hypothermia, tachycardia >100 bpm, or hypotension with a systolic blood pressure <90 mm Hg or 20 mm Hg below baseline. A skin lesion >5 cm in diameter also likely requires hospitalization and a parenteral antibiotic.5
FIGURE Class-2 CA-MRSA lesion
This raised, red lesion contains a central eschar with dried pus. Such lesions are generally very tender and often fluctuant when palpated.
Incision and drainage are most important
Several management schemes have been proposed to guide the appropriate level of therapy based on presenting characteristics.6,7 If a lesion is clearly fluctuant, incise it and drain the fluid, or refer the patient for surgical consultation. If the lesion is not clearly fluctuant, needle aspiration may help to determine the need for more extensive incision and drainage or to collect a specimen for culture. Although culture of a skin lesion may not have been routine in the past, the advent of CA-MRSA has made it so—particularly given that MRSA lesions may not be clinically distinguishable from those caused by nonresistant S aureus.
Periodic postprocedure follow-up is indicated to ensure resolution of the infection. At the Boston University student health service, CA-MRSA patients return every few days for an appointment with nursing staff for wound irrigation and packing change until the lesion visibly improves. Systemic effects from the infection are monitored as well.
Incision and drainage technique reported. In one study, adult patients were treated with incision and drainage by a surgeon.8 The technique used a#11 blade applied in a “sawing motion” to create a wide opening. The wound cavity was explored for loculations and packed. The identical technique can be used in the office, with one caveat: This study included patients who had an abscess larger than 5 cm in diameter and some whose immune system was compromised—situations not managed routinely in the office.
Are antibiotics indicated after incision and drainage for MRSA?
In the same study,8 the cure rate with incision and drainage alone was just over 90%. The cure rate in the treatment arm of the study, in which patients also received an antibiotic, was 84% (the difference was statistically insignificant), and coverage was inadequate for MRSA. Treatment with cephalexin after incision and drainage resulted in one patient harmed for every 14 treated.
A pediatric study also showed that antibiotics do not affect the outcome of skin lesions following incision and drainage.5 When deciding whether to prescribe postprocedure antibiotics, keep in mind the need to avoid contributing further to bacterial resistance.
Generally, start the patient on trimethoprim (TMP)-sulfamethoxazole (SMX) or tetracycline if incision and drainage fail to promote healing of the MRSA lesion within 7 days. Clindamycin is an option, although resistance is increasingly common. Adjust the choice and dosage of antibiotic as needed once culture and susceptibility testing results are available.
TMP-SMX is generally well tolerated at the recommended dosage of one or two double-strength tablets (160 mg of TMP, 800 mg of SMX) twice daily for adults. If creatinine clearance is 15 to 30 mL/min, halve the dosage. The rate of sulfa allergy with TMP-SMX (3%) is similar to what is seen with other antibiotics.
Tetracycline’s dosing schedule—for adults, 250 or 500 mg, four times daily— makes it difficult to use. Gastrointestinal upset, phototoxicity, and hepatotoxicity can occur. The possibility of tooth discoloration precludes its use in children.
Clindamycin carries a high rate of gastrointestinal-related problems—Clostridium difficile infection in particular (10% incidence, regardless of route). Inducible resistance to clindamycin is 50% in MRSA infections.9 Recent use of antibiotics may increase the likelihood of clindamycin resistance, with erythromycin in particular inducing such resistance. The dosage typically is 150 to 300 mg, every 6 hours.
Doxycycline and minocycline are not recommended. Both carry a 21% failure rate.10
Linezolid is costly and has many drug interactions. In particular, linezolid has the potential to cause serotonin syndrome with agents that affect the serotonergic system. Linezolid may also interact with medications that affect the adrenergic system (pressor agents). Routine use in the community without infectious disease consultation is not advised.
For lesions that are neither fluctuant nor purulent
In such cases, appropriate first-line antibiotics are a semisynthetic penicillin (e.g., dicloxacillin), a first- or second-generation oral cephalosporin, a macrolide, and clindamycin.10 These antibiotics are preferable for group A streptococcal infections, erysipelas (which can be aggressive), and impetigo. Adjustments can be made as culture results become available or if the clinical response is inadequate. There is no particular utility in waiting to administer oral antibiotics in cases of erysipelas or impetigo, although topical antibiotics can often be used for limited cases of impetigo.
CASE RESOLVED
Your patient, who meets criteria for a Class 2 CA-MRSA infection, undergoes incision and drainage of the lesion. No antibiotic is administered.
Two weeks of daily packing of the wound follow—again, without an antibiotic. Subsequently, the wound heals without sign of infection.
Prevention: Simple precautions are the rule
Most CA-MRSA infections result from direct contact with a patient’s wound or from wound drainage on environmental surfaces.
In the medical office. In addition to using sterile technique during incision and drainage, all staff members must wash hands with soap and water or an alcohol-based sanitizer. For the most part, MRSA remains susceptible to triclosan, a topical antiseptic in commercial hand soaps.
Clean equipment as needed with 10% sodium hypochlorite solution or another agent effective against MRSA. Surgical instruments should be disposable or sterilized after each use.
At the patient’s home. Instruct patients to clean the wound, wearing fresh disposable gloves each time, and to cover it with a new, dry dressing. Tell families to avoid sharing linens and clothing unless they have been washed in hot soap and water and dried in a heated dryer. MRSA can live for weeks or months on surfaces exposed to infected wounds11 ; these surfaces can be disinfected with a 10% solution of bleach.
In sports environments. Athletes who have a CA-MRSA infection should not compete unless the wound can be completely covered with a dry dressing. Recommend to those in charge of school and commercial facilities that, in a confirmed case of MRSA infection, they routinely clean locker rooms and sports equipment with either a 10% bleach solution or commercial disinfectant. There is no evidence, however, that more widespread or vigorous cleaning—such as dismantling a training room and all its cardio-fitness equipment for disinfecting—prevents the spread of MRSA.
Encourage athletes to wash their hands properly. Communal towels should be washed in hot water (>140°F) with bleach before reuse. Personal equipment should be cleaned according to the manufacturer’s instructions. Athletes should use a clean towel to provide a barrier between their skin and the surfaces of weight-room and cardio-fitness equipment. They should also clean equipment before and after use with an appropriate cleanser, such as a disinfectant hand wipe.
Screening household contacts for MRSA isn’t useful; attempts to eradicate colonization are generally ineffective. In a large study of military personnel, intranasal mupirocin failed to decrease nasal carriage of MRSA and the incidence of MRSA infections.11 The MRSA nasal colonization rate was 3.9%; 121 persons colonized with MRSA needed to be treated with nasal mupirocin to prevent one MRSA infection in the total study population.
More complex antibiotic regimens are sometimes used in an attempt to eradicate MRSA carriage, but they also have limited effectiveness and carry the general risks of antibiotic use (e.g., gastrointestinal disturbance, allergic reaction). If your office is considering an eradication attempt, consult first with an infectious disease clinician.
Suggested Reading
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. EMERGEncy ID Net Study Group Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
2. Cohen PR. The skin in the gym: a comprehensive review of the cutaneous manifestations of community-acquired methicillin-resistant Staphylococcus aureus infection in athletes. Clin Dermatol. 2008;26:16-26.
3. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8:259-270.
4. Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S aureus infection: a prospective investigation. Clin Infect Dis. 2007;44:471-482.
5. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123-127.
6. Eron LJ, Lipsky BA, Low DE, et al. Expert panel on managing skin and soft tissue infections Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(Suppl 1):i3-i17.
7. Centers for Disease Control and Prevention American Medical Association Infectious Diseases Society of America. Outpatient management of skin and soft tissue infections in the era of community-associated MRSA. September 2007. Available at: http://www.amaassn.org/ama1/pub/upload/mm/36/ca_mrsa_desk_102007.pdf. Accessed November 11, 2008.
8. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
9. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
10. Dellit TH, Duchin J. Guidelines for Evaluation and Management of Community-Associated Methicillin Resistant Staphylococcus aureus Skin and Soft Tissue Infections in Outpatient Settings. December 2007. Available at: http://www.kingcounty.gov/healthservices/health/communicable/providers/~/media/health/
publichealth/documents/communicable/MRSA_guide-lines.ashx. Accessed November 11, 2008.
11. Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51:3591-3598.
The author reports no financial disclosure relevant to this article.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) abscesses are best managed surgically; postprocedure antibiotics do not substantially improve outcome. The cure rate with incision and drainage alone is at least 90%.
- If incision and drainage fail to promote healing within 7 days, oral antibiotics of choice are trimethoprim-sulfamethoxazole and tetracycline
- Eradication of nasal carriage of CA-MRSA generally does not help prevent spread of clinical MRSA infection in communities.
CASE: Tender suprapubic lesion
A previously healthy, 22-year-old law school student arrives at your office complaining of “abdominal pain.” She is previously healthy; temperature is normal.
You discover on examination that she has an erythematous, indurated, and tender 3-cm lesion on the suprapubic region. The lesion has no point, but its center is boggy.
Should you prescribe an antibiotic? And should you cover immediately for CA-MRSA? What other factors might influence your decision about treatment?
The incidence of MRSA is increasing in communities across the United States, challenging assumptions about the evaluation and management of skin and soft-tissue infections. In this article, I outline a rational approach to managing patients who have a lesion likely to be caused by CA-MRSA ( TABLE 1 ).
TABLE
Suspect CA-MRSA infection? Consider this treatment scheme
| When a patient meets these criteria… | Provide this management… | And select from these antibiotics |
|---|---|---|
| Lesion nonfluctuant; patient afebrile, healthy (Class 1 infection) | If no drainable abscess, give a common first-line antibiotic for skin and soft-tissue infection; reassess for response | —Semisynthetic penicillin —Oral first- or second-generation cephalosporin —Macrolide —Clindamycin |
| Lesion, fluctuant or pustular, <5 cm in diameter; fever or no fever (Class 2) | Drain abscess surgically if possible; use incision and drainage presumptively for MRSA and monitor closely for response; inpatient management may be indicated | —Trimethoprim sulfamethoxazole —Tetracycline —Clindamycin |
| Lesion, >5 cm in diameter, toxic appearance or at least one unstable comorbidity or a limb-threatening infection (Class 3) | Admit; consider infectious disease consult | Broad-spectrum agent, including vancomycin, for MRSA coverage |
| Sepsis syndrome or life-threatening infection (necrotizing fasciitis)(Class 4) | Admit; institute aggressive surgical debridement; request infectious disease consult | Broad-spectrum agent, including vancomycin, for MRSA coverage |
| Source: Eron et al6 and CDC7 . | ||
When to suspect MRSA skin infection
Patients who have a CA-MRSA skin infection often report a “spider bite” because the lesion appears suddenly and unexpectedly in an area where there is no history of trauma.1 Lesions often are pustular with central necrosis; there may be purulent drainage, redness, tenderness, and palpable fluctuance ( FIGURE ).
CA-MRSA skin lesions can occur anywhere on the body, though they appear most often in the axillae or the groin and buttocks. Patients may or may not have a fever.
Persons at increased risk of CA-MRSA disease include users of health clubs, participants in contact sports, men who have sex with men, children younger than 2 years, users of intravenous drugs, military personnel, and prisoners.2,3 Absence of these risk factors in a patient with a skin or soft-tissue infection does not, however, rule out MRSA.4
Regardless of the lesion’s appearance or the patient’s epidemiologic history, consider CA-MRSA if its prevalence in your community has reached 10% to 15%.
CA-MRSA can cause impetigo, but the often-benign nature of this clinical infection makes management decisions less crucial. However, do hospitalize any patient who has a MRSA infection who also exhibits fever or hypothermia, tachycardia >100 bpm, or hypotension with a systolic blood pressure <90 mm Hg or 20 mm Hg below baseline. A skin lesion >5 cm in diameter also likely requires hospitalization and a parenteral antibiotic.5
FIGURE Class-2 CA-MRSA lesion
This raised, red lesion contains a central eschar with dried pus. Such lesions are generally very tender and often fluctuant when palpated.
Incision and drainage are most important
Several management schemes have been proposed to guide the appropriate level of therapy based on presenting characteristics.6,7 If a lesion is clearly fluctuant, incise it and drain the fluid, or refer the patient for surgical consultation. If the lesion is not clearly fluctuant, needle aspiration may help to determine the need for more extensive incision and drainage or to collect a specimen for culture. Although culture of a skin lesion may not have been routine in the past, the advent of CA-MRSA has made it so—particularly given that MRSA lesions may not be clinically distinguishable from those caused by nonresistant S aureus.
Periodic postprocedure follow-up is indicated to ensure resolution of the infection. At the Boston University student health service, CA-MRSA patients return every few days for an appointment with nursing staff for wound irrigation and packing change until the lesion visibly improves. Systemic effects from the infection are monitored as well.
Incision and drainage technique reported. In one study, adult patients were treated with incision and drainage by a surgeon.8 The technique used a#11 blade applied in a “sawing motion” to create a wide opening. The wound cavity was explored for loculations and packed. The identical technique can be used in the office, with one caveat: This study included patients who had an abscess larger than 5 cm in diameter and some whose immune system was compromised—situations not managed routinely in the office.
Are antibiotics indicated after incision and drainage for MRSA?
In the same study,8 the cure rate with incision and drainage alone was just over 90%. The cure rate in the treatment arm of the study, in which patients also received an antibiotic, was 84% (the difference was statistically insignificant), and coverage was inadequate for MRSA. Treatment with cephalexin after incision and drainage resulted in one patient harmed for every 14 treated.
A pediatric study also showed that antibiotics do not affect the outcome of skin lesions following incision and drainage.5 When deciding whether to prescribe postprocedure antibiotics, keep in mind the need to avoid contributing further to bacterial resistance.
Generally, start the patient on trimethoprim (TMP)-sulfamethoxazole (SMX) or tetracycline if incision and drainage fail to promote healing of the MRSA lesion within 7 days. Clindamycin is an option, although resistance is increasingly common. Adjust the choice and dosage of antibiotic as needed once culture and susceptibility testing results are available.
TMP-SMX is generally well tolerated at the recommended dosage of one or two double-strength tablets (160 mg of TMP, 800 mg of SMX) twice daily for adults. If creatinine clearance is 15 to 30 mL/min, halve the dosage. The rate of sulfa allergy with TMP-SMX (3%) is similar to what is seen with other antibiotics.
Tetracycline’s dosing schedule—for adults, 250 or 500 mg, four times daily— makes it difficult to use. Gastrointestinal upset, phototoxicity, and hepatotoxicity can occur. The possibility of tooth discoloration precludes its use in children.
Clindamycin carries a high rate of gastrointestinal-related problems—Clostridium difficile infection in particular (10% incidence, regardless of route). Inducible resistance to clindamycin is 50% in MRSA infections.9 Recent use of antibiotics may increase the likelihood of clindamycin resistance, with erythromycin in particular inducing such resistance. The dosage typically is 150 to 300 mg, every 6 hours.
Doxycycline and minocycline are not recommended. Both carry a 21% failure rate.10
Linezolid is costly and has many drug interactions. In particular, linezolid has the potential to cause serotonin syndrome with agents that affect the serotonergic system. Linezolid may also interact with medications that affect the adrenergic system (pressor agents). Routine use in the community without infectious disease consultation is not advised.
For lesions that are neither fluctuant nor purulent
In such cases, appropriate first-line antibiotics are a semisynthetic penicillin (e.g., dicloxacillin), a first- or second-generation oral cephalosporin, a macrolide, and clindamycin.10 These antibiotics are preferable for group A streptococcal infections, erysipelas (which can be aggressive), and impetigo. Adjustments can be made as culture results become available or if the clinical response is inadequate. There is no particular utility in waiting to administer oral antibiotics in cases of erysipelas or impetigo, although topical antibiotics can often be used for limited cases of impetigo.
CASE RESOLVED
Your patient, who meets criteria for a Class 2 CA-MRSA infection, undergoes incision and drainage of the lesion. No antibiotic is administered.
Two weeks of daily packing of the wound follow—again, without an antibiotic. Subsequently, the wound heals without sign of infection.
Prevention: Simple precautions are the rule
Most CA-MRSA infections result from direct contact with a patient’s wound or from wound drainage on environmental surfaces.
In the medical office. In addition to using sterile technique during incision and drainage, all staff members must wash hands with soap and water or an alcohol-based sanitizer. For the most part, MRSA remains susceptible to triclosan, a topical antiseptic in commercial hand soaps.
Clean equipment as needed with 10% sodium hypochlorite solution or another agent effective against MRSA. Surgical instruments should be disposable or sterilized after each use.
At the patient’s home. Instruct patients to clean the wound, wearing fresh disposable gloves each time, and to cover it with a new, dry dressing. Tell families to avoid sharing linens and clothing unless they have been washed in hot soap and water and dried in a heated dryer. MRSA can live for weeks or months on surfaces exposed to infected wounds11 ; these surfaces can be disinfected with a 10% solution of bleach.
In sports environments. Athletes who have a CA-MRSA infection should not compete unless the wound can be completely covered with a dry dressing. Recommend to those in charge of school and commercial facilities that, in a confirmed case of MRSA infection, they routinely clean locker rooms and sports equipment with either a 10% bleach solution or commercial disinfectant. There is no evidence, however, that more widespread or vigorous cleaning—such as dismantling a training room and all its cardio-fitness equipment for disinfecting—prevents the spread of MRSA.
Encourage athletes to wash their hands properly. Communal towels should be washed in hot water (>140°F) with bleach before reuse. Personal equipment should be cleaned according to the manufacturer’s instructions. Athletes should use a clean towel to provide a barrier between their skin and the surfaces of weight-room and cardio-fitness equipment. They should also clean equipment before and after use with an appropriate cleanser, such as a disinfectant hand wipe.
Screening household contacts for MRSA isn’t useful; attempts to eradicate colonization are generally ineffective. In a large study of military personnel, intranasal mupirocin failed to decrease nasal carriage of MRSA and the incidence of MRSA infections.11 The MRSA nasal colonization rate was 3.9%; 121 persons colonized with MRSA needed to be treated with nasal mupirocin to prevent one MRSA infection in the total study population.
More complex antibiotic regimens are sometimes used in an attempt to eradicate MRSA carriage, but they also have limited effectiveness and carry the general risks of antibiotic use (e.g., gastrointestinal disturbance, allergic reaction). If your office is considering an eradication attempt, consult first with an infectious disease clinician.
Suggested Reading
The author reports no financial disclosure relevant to this article.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) abscesses are best managed surgically; postprocedure antibiotics do not substantially improve outcome. The cure rate with incision and drainage alone is at least 90%.
- If incision and drainage fail to promote healing within 7 days, oral antibiotics of choice are trimethoprim-sulfamethoxazole and tetracycline
- Eradication of nasal carriage of CA-MRSA generally does not help prevent spread of clinical MRSA infection in communities.
CASE: Tender suprapubic lesion
A previously healthy, 22-year-old law school student arrives at your office complaining of “abdominal pain.” She is previously healthy; temperature is normal.
You discover on examination that she has an erythematous, indurated, and tender 3-cm lesion on the suprapubic region. The lesion has no point, but its center is boggy.
Should you prescribe an antibiotic? And should you cover immediately for CA-MRSA? What other factors might influence your decision about treatment?
The incidence of MRSA is increasing in communities across the United States, challenging assumptions about the evaluation and management of skin and soft-tissue infections. In this article, I outline a rational approach to managing patients who have a lesion likely to be caused by CA-MRSA ( TABLE 1 ).
TABLE
Suspect CA-MRSA infection? Consider this treatment scheme
| When a patient meets these criteria… | Provide this management… | And select from these antibiotics |
|---|---|---|
| Lesion nonfluctuant; patient afebrile, healthy (Class 1 infection) | If no drainable abscess, give a common first-line antibiotic for skin and soft-tissue infection; reassess for response | —Semisynthetic penicillin —Oral first- or second-generation cephalosporin —Macrolide —Clindamycin |
| Lesion, fluctuant or pustular, <5 cm in diameter; fever or no fever (Class 2) | Drain abscess surgically if possible; use incision and drainage presumptively for MRSA and monitor closely for response; inpatient management may be indicated | —Trimethoprim sulfamethoxazole —Tetracycline —Clindamycin |
| Lesion, >5 cm in diameter, toxic appearance or at least one unstable comorbidity or a limb-threatening infection (Class 3) | Admit; consider infectious disease consult | Broad-spectrum agent, including vancomycin, for MRSA coverage |
| Sepsis syndrome or life-threatening infection (necrotizing fasciitis)(Class 4) | Admit; institute aggressive surgical debridement; request infectious disease consult | Broad-spectrum agent, including vancomycin, for MRSA coverage |
| Source: Eron et al6 and CDC7 . | ||
When to suspect MRSA skin infection
Patients who have a CA-MRSA skin infection often report a “spider bite” because the lesion appears suddenly and unexpectedly in an area where there is no history of trauma.1 Lesions often are pustular with central necrosis; there may be purulent drainage, redness, tenderness, and palpable fluctuance ( FIGURE ).
CA-MRSA skin lesions can occur anywhere on the body, though they appear most often in the axillae or the groin and buttocks. Patients may or may not have a fever.
Persons at increased risk of CA-MRSA disease include users of health clubs, participants in contact sports, men who have sex with men, children younger than 2 years, users of intravenous drugs, military personnel, and prisoners.2,3 Absence of these risk factors in a patient with a skin or soft-tissue infection does not, however, rule out MRSA.4
Regardless of the lesion’s appearance or the patient’s epidemiologic history, consider CA-MRSA if its prevalence in your community has reached 10% to 15%.
CA-MRSA can cause impetigo, but the often-benign nature of this clinical infection makes management decisions less crucial. However, do hospitalize any patient who has a MRSA infection who also exhibits fever or hypothermia, tachycardia >100 bpm, or hypotension with a systolic blood pressure <90 mm Hg or 20 mm Hg below baseline. A skin lesion >5 cm in diameter also likely requires hospitalization and a parenteral antibiotic.5
FIGURE Class-2 CA-MRSA lesion
This raised, red lesion contains a central eschar with dried pus. Such lesions are generally very tender and often fluctuant when palpated.
Incision and drainage are most important
Several management schemes have been proposed to guide the appropriate level of therapy based on presenting characteristics.6,7 If a lesion is clearly fluctuant, incise it and drain the fluid, or refer the patient for surgical consultation. If the lesion is not clearly fluctuant, needle aspiration may help to determine the need for more extensive incision and drainage or to collect a specimen for culture. Although culture of a skin lesion may not have been routine in the past, the advent of CA-MRSA has made it so—particularly given that MRSA lesions may not be clinically distinguishable from those caused by nonresistant S aureus.
Periodic postprocedure follow-up is indicated to ensure resolution of the infection. At the Boston University student health service, CA-MRSA patients return every few days for an appointment with nursing staff for wound irrigation and packing change until the lesion visibly improves. Systemic effects from the infection are monitored as well.
Incision and drainage technique reported. In one study, adult patients were treated with incision and drainage by a surgeon.8 The technique used a#11 blade applied in a “sawing motion” to create a wide opening. The wound cavity was explored for loculations and packed. The identical technique can be used in the office, with one caveat: This study included patients who had an abscess larger than 5 cm in diameter and some whose immune system was compromised—situations not managed routinely in the office.
Are antibiotics indicated after incision and drainage for MRSA?
In the same study,8 the cure rate with incision and drainage alone was just over 90%. The cure rate in the treatment arm of the study, in which patients also received an antibiotic, was 84% (the difference was statistically insignificant), and coverage was inadequate for MRSA. Treatment with cephalexin after incision and drainage resulted in one patient harmed for every 14 treated.
A pediatric study also showed that antibiotics do not affect the outcome of skin lesions following incision and drainage.5 When deciding whether to prescribe postprocedure antibiotics, keep in mind the need to avoid contributing further to bacterial resistance.
Generally, start the patient on trimethoprim (TMP)-sulfamethoxazole (SMX) or tetracycline if incision and drainage fail to promote healing of the MRSA lesion within 7 days. Clindamycin is an option, although resistance is increasingly common. Adjust the choice and dosage of antibiotic as needed once culture and susceptibility testing results are available.
TMP-SMX is generally well tolerated at the recommended dosage of one or two double-strength tablets (160 mg of TMP, 800 mg of SMX) twice daily for adults. If creatinine clearance is 15 to 30 mL/min, halve the dosage. The rate of sulfa allergy with TMP-SMX (3%) is similar to what is seen with other antibiotics.
Tetracycline’s dosing schedule—for adults, 250 or 500 mg, four times daily— makes it difficult to use. Gastrointestinal upset, phototoxicity, and hepatotoxicity can occur. The possibility of tooth discoloration precludes its use in children.
Clindamycin carries a high rate of gastrointestinal-related problems—Clostridium difficile infection in particular (10% incidence, regardless of route). Inducible resistance to clindamycin is 50% in MRSA infections.9 Recent use of antibiotics may increase the likelihood of clindamycin resistance, with erythromycin in particular inducing such resistance. The dosage typically is 150 to 300 mg, every 6 hours.
Doxycycline and minocycline are not recommended. Both carry a 21% failure rate.10
Linezolid is costly and has many drug interactions. In particular, linezolid has the potential to cause serotonin syndrome with agents that affect the serotonergic system. Linezolid may also interact with medications that affect the adrenergic system (pressor agents). Routine use in the community without infectious disease consultation is not advised.
For lesions that are neither fluctuant nor purulent
In such cases, appropriate first-line antibiotics are a semisynthetic penicillin (e.g., dicloxacillin), a first- or second-generation oral cephalosporin, a macrolide, and clindamycin.10 These antibiotics are preferable for group A streptococcal infections, erysipelas (which can be aggressive), and impetigo. Adjustments can be made as culture results become available or if the clinical response is inadequate. There is no particular utility in waiting to administer oral antibiotics in cases of erysipelas or impetigo, although topical antibiotics can often be used for limited cases of impetigo.
CASE RESOLVED
Your patient, who meets criteria for a Class 2 CA-MRSA infection, undergoes incision and drainage of the lesion. No antibiotic is administered.
Two weeks of daily packing of the wound follow—again, without an antibiotic. Subsequently, the wound heals without sign of infection.
Prevention: Simple precautions are the rule
Most CA-MRSA infections result from direct contact with a patient’s wound or from wound drainage on environmental surfaces.
In the medical office. In addition to using sterile technique during incision and drainage, all staff members must wash hands with soap and water or an alcohol-based sanitizer. For the most part, MRSA remains susceptible to triclosan, a topical antiseptic in commercial hand soaps.
Clean equipment as needed with 10% sodium hypochlorite solution or another agent effective against MRSA. Surgical instruments should be disposable or sterilized after each use.
At the patient’s home. Instruct patients to clean the wound, wearing fresh disposable gloves each time, and to cover it with a new, dry dressing. Tell families to avoid sharing linens and clothing unless they have been washed in hot soap and water and dried in a heated dryer. MRSA can live for weeks or months on surfaces exposed to infected wounds11 ; these surfaces can be disinfected with a 10% solution of bleach.
In sports environments. Athletes who have a CA-MRSA infection should not compete unless the wound can be completely covered with a dry dressing. Recommend to those in charge of school and commercial facilities that, in a confirmed case of MRSA infection, they routinely clean locker rooms and sports equipment with either a 10% bleach solution or commercial disinfectant. There is no evidence, however, that more widespread or vigorous cleaning—such as dismantling a training room and all its cardio-fitness equipment for disinfecting—prevents the spread of MRSA.
Encourage athletes to wash their hands properly. Communal towels should be washed in hot water (>140°F) with bleach before reuse. Personal equipment should be cleaned according to the manufacturer’s instructions. Athletes should use a clean towel to provide a barrier between their skin and the surfaces of weight-room and cardio-fitness equipment. They should also clean equipment before and after use with an appropriate cleanser, such as a disinfectant hand wipe.
Screening household contacts for MRSA isn’t useful; attempts to eradicate colonization are generally ineffective. In a large study of military personnel, intranasal mupirocin failed to decrease nasal carriage of MRSA and the incidence of MRSA infections.11 The MRSA nasal colonization rate was 3.9%; 121 persons colonized with MRSA needed to be treated with nasal mupirocin to prevent one MRSA infection in the total study population.
More complex antibiotic regimens are sometimes used in an attempt to eradicate MRSA carriage, but they also have limited effectiveness and carry the general risks of antibiotic use (e.g., gastrointestinal disturbance, allergic reaction). If your office is considering an eradication attempt, consult first with an infectious disease clinician.
Suggested Reading
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. EMERGEncy ID Net Study Group Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
2. Cohen PR. The skin in the gym: a comprehensive review of the cutaneous manifestations of community-acquired methicillin-resistant Staphylococcus aureus infection in athletes. Clin Dermatol. 2008;26:16-26.
3. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8:259-270.
4. Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S aureus infection: a prospective investigation. Clin Infect Dis. 2007;44:471-482.
5. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123-127.
6. Eron LJ, Lipsky BA, Low DE, et al. Expert panel on managing skin and soft tissue infections Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(Suppl 1):i3-i17.
7. Centers for Disease Control and Prevention American Medical Association Infectious Diseases Society of America. Outpatient management of skin and soft tissue infections in the era of community-associated MRSA. September 2007. Available at: http://www.amaassn.org/ama1/pub/upload/mm/36/ca_mrsa_desk_102007.pdf. Accessed November 11, 2008.
8. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
9. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
10. Dellit TH, Duchin J. Guidelines for Evaluation and Management of Community-Associated Methicillin Resistant Staphylococcus aureus Skin and Soft Tissue Infections in Outpatient Settings. December 2007. Available at: http://www.kingcounty.gov/healthservices/health/communicable/providers/~/media/health/
publichealth/documents/communicable/MRSA_guide-lines.ashx. Accessed November 11, 2008.
11. Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51:3591-3598.
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. EMERGEncy ID Net Study Group Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
2. Cohen PR. The skin in the gym: a comprehensive review of the cutaneous manifestations of community-acquired methicillin-resistant Staphylococcus aureus infection in athletes. Clin Dermatol. 2008;26:16-26.
3. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8:259-270.
4. Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S aureus infection: a prospective investigation. Clin Infect Dis. 2007;44:471-482.
5. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123-127.
6. Eron LJ, Lipsky BA, Low DE, et al. Expert panel on managing skin and soft tissue infections Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(Suppl 1):i3-i17.
7. Centers for Disease Control and Prevention American Medical Association Infectious Diseases Society of America. Outpatient management of skin and soft tissue infections in the era of community-associated MRSA. September 2007. Available at: http://www.amaassn.org/ama1/pub/upload/mm/36/ca_mrsa_desk_102007.pdf. Accessed November 11, 2008.
8. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
9. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
10. Dellit TH, Duchin J. Guidelines for Evaluation and Management of Community-Associated Methicillin Resistant Staphylococcus aureus Skin and Soft Tissue Infections in Outpatient Settings. December 2007. Available at: http://www.kingcounty.gov/healthservices/health/communicable/providers/~/media/health/
publichealth/documents/communicable/MRSA_guide-lines.ashx. Accessed November 11, 2008.
11. Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51:3591-3598.
CA-MRSA lesions: What works, what doesn’t
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) abscesses are best managed surgically; postprocedure antibiotics do not substantially improve outcomes. Cure rates with incision and drainage alone are at least 90% (A).
- If incision and drainage fail to promote healing within 7 days, the oral antibiotics of choice are trimethoprim-sulfamethoxazole and tetracycline (C).
- Eradication of nasal carriage of CA-MRSA is generally not useful in preventing spread of clinical MRSA infections in communities (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A previously healthy law student arrives at your office complaining of “abdominal pain.” You discover on examination that she has an erythematous, indurated, and tender 3-cm lesion on her suprapubic region. The lesion has no point, but its center is boggy. The patient’s temperature is normal. Would you give her an antibiotic? Would you cover immediately for community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA)? What other factors might influence your decision?
The incidence of MRSA is increasing in communities across the United States, challenging our assumptions about evaluation and management of skin and soft-tissue infections. In this article, I outline a rational approach to managing patients who have lesions likely to have been caused by CA-MRSA (TABLE).
TABLE
Suspect CA-MRSA? Consider this treatment approach6,7
| CLASS | PATIENT CRITERIA | MANAGEMENT | ANTIBIOTIC CHOICES |
|---|---|---|---|
| 1 | Afebrile and healthy; lesion nonfluctuant | If no drainable abscess, give common first-line antibiotic for SSTI; reassess for response | Semisynthetic penicillin, oral first- or second-generation cephalosporin, macrolide, clindamycin |
| 2 | Fluctuant or pustular lesion <5 cm; with or without fever | Surgical drainage of abscess if possible. Use I&D presumptively for MRSA and monitor closely for response; inpatient management may be indicated | Trimethoprim-sulfamethoxazole, tetracycline, clindamycin |
| 3 | Toxic appearance or at least 1 unstable comorbidity or a limb-threatening infection; lesion >5 cm | Hospital admission with broadspectrum antibiotics for MRSA coverage; consider infectious disease consultation | Broad-spectrum, including vancomycin |
| 4 | Sepsis syndrome or life-threatening infection (necrotizing fasciitis) | Above plus aggressive surgical debridement | Above with infectious disease guidance |
| CA-MRSA, community-acquired methicillin-resistant Staphylococcus aureus; I&D, incision and drainage; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft-tissue infection. | |||
When to suspect MRSA skin infection
Patients with CA-MRSA skin infection often report a “spider bite,” as lesions appear suddenly and unexpectedly in areas without a history of trauma.1 The lesions very often are pustular with central necrosis, and there may be purulent drainage, redness, tenderness, and palpable fluctuance. CA-MRSA can cause impetigo, but the often benign nature of this clinical infection makes management decisions less crucial. CA-MRSA skin lesions can occur anywhere on the body, though most often they appear in the axillae or the groin and buttocks. Patients may or may not have a fever.
Individuals who are at increased risk for CA-MRSA disease include users of health clubs or participants in contact sports, men who have sex with men, children younger than 2 years of age, users of intravenous drugs, military personnel, and prisoners.2,3 However, the absence of these factors in a patient with a skin or soft-tissue infection does not rule out MRSA.4
Regardless of the lesion’s appearance or the patient’s epidemiologic history, consider CA-MRSA if its prevalence in your community reaches 10% to 15%.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) causes up to 74% of purulent skin and soft-tissue infections in communities throughout the United States.1 By definition, this infection occurs in patients who have not been hospitalized and have not undergone medical procedures within the prior year.12
- The annual incidence of CA-MRSA was reported to be 18.0-25.7 cases per 100,000 population between 2001 and 2002.13 Clusters of CA-MRSA have been identified among Alaskan natives, Native American Indians, and Pacific Islanders.12
- Most often this organism causes skin and soft-tissue infections, though cardiac, respiratory, blood, and bone infections can also occur.14
- CA-MRSA species are genotypically distinct from hospital-acquired MRSA. One marker for CA-MRSA, Panton-Valentine leucocidin (PVL), is most often detected in cases of severe and systemic infection, and it may be a virulence factor.15 However, the presence of PVL does not necessarily correlate directly with antibiotic resistance.
- Historically, CA-MRSA was primarily resistant to beta-lactams and erythromycin. More recent strains have also demonstrated resistance to tetracycline and clindamycin.
- Retrospective analyses show that patients with CA-MRSA tend to receive inadequate initial antibiotic coverage, and, independent of this, they tend to have worse clinical outcomes than those infected by methicillin-sensitive strains.16
Hospitalize any patient who exhibits fever or hypothermia, tachycardia greater than 100 beats per minute, or hypotension with a systolic blood pressure <90 mm Hg or 20 mm Hg lower than baseline. A skin lesion >5 cm is also likely to require hospitalization and parenteral antibiotics.5
Treatment: Incision and drainage most important
Several management schemes have been proposed to guide the appropriate level of therapy based on presenting patient characteristics.6,7 If a lesion is clearly fluctuant, incise it and drain the fluid, or refer the patient for surgical consultation. If the lesion is not clearly fluctuant, needle aspiration may help to determine the need for more extensive incision and drainage or to collect a specimen for culture. Although culture of skin lesions may not have been routine in the past, the advent of CA-MRSA has made it so, particularly given that MRSA lesions may not be clinically distinguishable from those caused by nonresistant S aureus.
Periodic postprocedure follow-up is indicated to ensure resolution of the infection. At our health center, patients return every few days for an appointment with nursing staff for wound irrigation and packing change until the lesion visibly improves. Systemic effects from the infection are monitored, as well.
Adult patients in 1 study were treated with incision and drainage by a surgeon.8 The technique described in the article used an 11 blade and a “sawing motion,” creating a wide opening. The wound cavity was explored for loculations and packed. This technique is identical to that used in the office. There is one caveat, though: This study included abscesses larger than 5 cm and patients with compromised immune systems—situations not routinely managed in the primary care office.
Are antibiotics indicated after incision and drainage for MRSA? In this same study, cure rates with incision and drainage alone were just over 90%.8 The cure rate in the treatment arm also receiving an antibiotic was 84% (difference was not statistically significant), and coverage was inadequate for MRSA. Treatment with cephalexin after incision and drainage resulted in 1 patient harmed for every 14 treated (NNH=14). A pediatric study also showed that antibiotics do not affect the outcome of skin lesions following incision and drainage.5 When deciding whether to prescribe postprocedure antibiotics, keep in mind the need to avoid contributing further to bacterial resistance.
Generally if incision and drainage fail to promote healing of the MRSA lesion within 7 days, start the patient on trimethoprim-sulfamethoxazole or tetracycline. Clindamycin is an option, though resistance to it is becoming more common. Adjust the antibiotic choice as needed when culture and sensitivity results become available.
Trimethoprim-sulfamethoxazole is generally well tolerated at the recommended dose of 1 to 2 double-strength tablets (160 mg TMP, 800 mg SMX) twice daily for adults. If a patient’s creatinine clearance is 15 to 30 mL/min, reduce the dose by half. The rate of sulfa allergy is similar to other antibiotics, at 3%.
Tetracycline’s dosing schedule—for adults, 250 or 500 mg 4 times daily—makes it difficult to use. Gastrointestinal upset, phototoxicity, and hepatotoxicity can occur. The possibility of tooth discoloration precludes tetracycline’s use in children.
Clindamycin carries a high rate of gastrointestinal-related problems, Clostridium difficile infection in particular (10% incidence administered in any route). Inducible resistance to clindamycin is 50% in MRSA infections.9 Recent use of antibiotics may increase the likelihood of clindamycin resistance, with erythromycin in particular inducing this resistance. Its dosage typically is 150 to 300 mg every 6 hours.
Doxycycline and minocycline are not recommended, as they carry a 21% failure rate.9
Linezolid is costly and has many drug interactions. In particular, linezolid has the potential to cause serotonin syndrome with agents that affect the serotonergic system. Linezolid may also interact with medications that affect the adrenergic system (pressors). Its routine use in the community without infectious disease consultation is not advised.
For lesions that are not fluctuant or purulent, appropriate first-line antibiotics are semisynthetic penicillins (dicloxacillin), first- or second-generation oral cephalosporins, macrolides, and clindamycin.9 These antibiotics are preferable for group A streptococcal infections, erysipelas (which can be quite aggressive), and impetigo. Adjustments can be made as culture results become available or if the clinical response is inadequate. There is no particular utility in waiting to administer oral antibiotics in cases of erysipelas or impetigo, though topical antibiotics can often be used for limited cases of impetigo.
Prevention: Simple precautions are the rule
Most CA-MRSA infections result from direct contact with a patient’s wound or from wound drainage on environmental surfaces.
In the medical office. In addition to using sterile technique during incision and drainage, be sure that all staff members wash their hands with soap and water or with an alcohol-based sanitizer. For the most part, MRSA remains susceptible to triclosan, a topical antiseptic in commercially available hand soaps.
Clean equipment as needed with 10% sodium hypochlorite solution or another agent effective against MRSA. Surgical instruments should be disposable or sterilized after each use.
At the patient’s home. Instruct patients to clean wounds wearing fresh disposable gloves each time and to cover wounds with new, dry dressings. Tell families to avoid sharing linens and clothing unless they have been washed in hot soap and water and dried in a heated dryer. MRSA can live for weeks to months on surfaces exposed to infected wounds,10 and these surfaces can be disinfected with a 10% bleach solution.
Sports environments. Athletes with CA-MRSA infections should not compete unless the wound can be completely covered with a dry dressing. Recommend to those in charge of school or commercial facilities that, in cases of confirmed MRSA infection, they routinely clean locker rooms and sports equipment with either a 10% bleach solution or commercial disinfectant. There is no evidence, however, that more widespread or vigorous cleaning—such as dismantling a training room and all its cardio-fitness equipment for disinfecting—prevents the spread of MRSA.
Encourage athletes to wash their hands properly. Communal towels should be washed in hot water (>140°F) with bleach before reuse. Personal equipment should be cleaned per the instructions of the manufacturer. Athletes should use a clean towel to provide a barrier between their skin and the surfaces of weight-room or cardio-fitness equipment. They should also clean equipment before and after use with an appropriate cleanser, such as a disinfectant hand-wipe.
Unproductive efforts you can avoid. Screening household contacts for MRSA is not useful, and attempts to eliminate colonization are generally ineffective. In a large military study, use of intranasal mupirocin failed to decrease nasal carriage of MRSA and the incidence of MRSA infections.11 The MRSA nasal colonization rate was 3.9%; 121 individuals with MRSA colonization needed to be treated with nasal mupirocin to prevent 1 MRSA infection in the total study population.
More complex antibiotic regimens are sometimes used in an attempt to eradicate MRSA carriage, though they also have limited effectiveness and carry the general risks of antibiotic use (gastrointestinal disturbance, allergic reaction, etc). If your office is considering an eradication attempt, consult with an infectious disease clinician first.
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
2. Cohen PR. The skin in the gym: a comprehensive review of the cutaneous manifestations of community-acquired methicillin-resistant Staphylococcus aureus infection in athletes. Clin Dermatol. 2008;26:16-26.
3. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8:259-270.
4. Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community associated methicillin resistant Staph aureus infection from methicillin susceptible S aureus infection: a prospective investigation. Clin Infect Dis. 2007;44:471-482.
5. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123-127.
6. Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(suppl S1):i3-i17.
7. CDC, AMA, IDSA, Outpatient management of skin and soft tissue infections in the era of community-associated MRSA. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/36/ca_mrsa_desk_102007.pdf. Accessed June 8, 2008.
8. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
9. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
10. Dellit TH, Ducin J. Guidelines for Evaluation and Management of Community-Associated Methicillin Resistant Staphylococcus aureus Skin and Soft Tissue Infections in Outpatient Settings. Available at: http://www.metrokc.gov/health/providers/epidemiology/MRSA-guidelines.pdf. Accessed August 6, 2008.
11. Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51:3591-3598.
12. Centers for Disease Control and Prevention. Healthcare-associated methicillin resistant Staphylococcus aureus (MRSA). Available at: http://www.cdc.gov/ncidod/dhqp/ar_mrsa.html. Accessed April 14, 2008.
13. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-reistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:2362-a.
14. Active Bacterial Core Surveillance (ABCs) Report, Emerging Infections Program Network methicillin-resistant Staphylococcus aureus, 2006. Available at: http://www.cdc.gov/ncidod/dbmd/abcs/survreports/mrsa06.pdf. Accessed May 5, 2008.
15. Holmes A, Ganner M, McGuane S, et al. Staphylococcus aureus isolates carrying panton-valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol. 2005;43:2384-2390.
16. Davis SL, Perri MB, Donabedian SM, et al. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2007;45:1705-1711.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) abscesses are best managed surgically; postprocedure antibiotics do not substantially improve outcomes. Cure rates with incision and drainage alone are at least 90% (A).
- If incision and drainage fail to promote healing within 7 days, the oral antibiotics of choice are trimethoprim-sulfamethoxazole and tetracycline (C).
- Eradication of nasal carriage of CA-MRSA is generally not useful in preventing spread of clinical MRSA infections in communities (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A previously healthy law student arrives at your office complaining of “abdominal pain.” You discover on examination that she has an erythematous, indurated, and tender 3-cm lesion on her suprapubic region. The lesion has no point, but its center is boggy. The patient’s temperature is normal. Would you give her an antibiotic? Would you cover immediately for community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA)? What other factors might influence your decision?
The incidence of MRSA is increasing in communities across the United States, challenging our assumptions about evaluation and management of skin and soft-tissue infections. In this article, I outline a rational approach to managing patients who have lesions likely to have been caused by CA-MRSA (TABLE).
TABLE
Suspect CA-MRSA? Consider this treatment approach6,7
| CLASS | PATIENT CRITERIA | MANAGEMENT | ANTIBIOTIC CHOICES |
|---|---|---|---|
| 1 | Afebrile and healthy; lesion nonfluctuant | If no drainable abscess, give common first-line antibiotic for SSTI; reassess for response | Semisynthetic penicillin, oral first- or second-generation cephalosporin, macrolide, clindamycin |
| 2 | Fluctuant or pustular lesion <5 cm; with or without fever | Surgical drainage of abscess if possible. Use I&D presumptively for MRSA and monitor closely for response; inpatient management may be indicated | Trimethoprim-sulfamethoxazole, tetracycline, clindamycin |
| 3 | Toxic appearance or at least 1 unstable comorbidity or a limb-threatening infection; lesion >5 cm | Hospital admission with broadspectrum antibiotics for MRSA coverage; consider infectious disease consultation | Broad-spectrum, including vancomycin |
| 4 | Sepsis syndrome or life-threatening infection (necrotizing fasciitis) | Above plus aggressive surgical debridement | Above with infectious disease guidance |
| CA-MRSA, community-acquired methicillin-resistant Staphylococcus aureus; I&D, incision and drainage; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft-tissue infection. | |||
When to suspect MRSA skin infection
Patients with CA-MRSA skin infection often report a “spider bite,” as lesions appear suddenly and unexpectedly in areas without a history of trauma.1 The lesions very often are pustular with central necrosis, and there may be purulent drainage, redness, tenderness, and palpable fluctuance. CA-MRSA can cause impetigo, but the often benign nature of this clinical infection makes management decisions less crucial. CA-MRSA skin lesions can occur anywhere on the body, though most often they appear in the axillae or the groin and buttocks. Patients may or may not have a fever.
Individuals who are at increased risk for CA-MRSA disease include users of health clubs or participants in contact sports, men who have sex with men, children younger than 2 years of age, users of intravenous drugs, military personnel, and prisoners.2,3 However, the absence of these factors in a patient with a skin or soft-tissue infection does not rule out MRSA.4
Regardless of the lesion’s appearance or the patient’s epidemiologic history, consider CA-MRSA if its prevalence in your community reaches 10% to 15%.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) causes up to 74% of purulent skin and soft-tissue infections in communities throughout the United States.1 By definition, this infection occurs in patients who have not been hospitalized and have not undergone medical procedures within the prior year.12
- The annual incidence of CA-MRSA was reported to be 18.0-25.7 cases per 100,000 population between 2001 and 2002.13 Clusters of CA-MRSA have been identified among Alaskan natives, Native American Indians, and Pacific Islanders.12
- Most often this organism causes skin and soft-tissue infections, though cardiac, respiratory, blood, and bone infections can also occur.14
- CA-MRSA species are genotypically distinct from hospital-acquired MRSA. One marker for CA-MRSA, Panton-Valentine leucocidin (PVL), is most often detected in cases of severe and systemic infection, and it may be a virulence factor.15 However, the presence of PVL does not necessarily correlate directly with antibiotic resistance.
- Historically, CA-MRSA was primarily resistant to beta-lactams and erythromycin. More recent strains have also demonstrated resistance to tetracycline and clindamycin.
- Retrospective analyses show that patients with CA-MRSA tend to receive inadequate initial antibiotic coverage, and, independent of this, they tend to have worse clinical outcomes than those infected by methicillin-sensitive strains.16
Hospitalize any patient who exhibits fever or hypothermia, tachycardia greater than 100 beats per minute, or hypotension with a systolic blood pressure <90 mm Hg or 20 mm Hg lower than baseline. A skin lesion >5 cm is also likely to require hospitalization and parenteral antibiotics.5
Treatment: Incision and drainage most important
Several management schemes have been proposed to guide the appropriate level of therapy based on presenting patient characteristics.6,7 If a lesion is clearly fluctuant, incise it and drain the fluid, or refer the patient for surgical consultation. If the lesion is not clearly fluctuant, needle aspiration may help to determine the need for more extensive incision and drainage or to collect a specimen for culture. Although culture of skin lesions may not have been routine in the past, the advent of CA-MRSA has made it so, particularly given that MRSA lesions may not be clinically distinguishable from those caused by nonresistant S aureus.
Periodic postprocedure follow-up is indicated to ensure resolution of the infection. At our health center, patients return every few days for an appointment with nursing staff for wound irrigation and packing change until the lesion visibly improves. Systemic effects from the infection are monitored, as well.
Adult patients in 1 study were treated with incision and drainage by a surgeon.8 The technique described in the article used an 11 blade and a “sawing motion,” creating a wide opening. The wound cavity was explored for loculations and packed. This technique is identical to that used in the office. There is one caveat, though: This study included abscesses larger than 5 cm and patients with compromised immune systems—situations not routinely managed in the primary care office.
Are antibiotics indicated after incision and drainage for MRSA? In this same study, cure rates with incision and drainage alone were just over 90%.8 The cure rate in the treatment arm also receiving an antibiotic was 84% (difference was not statistically significant), and coverage was inadequate for MRSA. Treatment with cephalexin after incision and drainage resulted in 1 patient harmed for every 14 treated (NNH=14). A pediatric study also showed that antibiotics do not affect the outcome of skin lesions following incision and drainage.5 When deciding whether to prescribe postprocedure antibiotics, keep in mind the need to avoid contributing further to bacterial resistance.
Generally if incision and drainage fail to promote healing of the MRSA lesion within 7 days, start the patient on trimethoprim-sulfamethoxazole or tetracycline. Clindamycin is an option, though resistance to it is becoming more common. Adjust the antibiotic choice as needed when culture and sensitivity results become available.
Trimethoprim-sulfamethoxazole is generally well tolerated at the recommended dose of 1 to 2 double-strength tablets (160 mg TMP, 800 mg SMX) twice daily for adults. If a patient’s creatinine clearance is 15 to 30 mL/min, reduce the dose by half. The rate of sulfa allergy is similar to other antibiotics, at 3%.
Tetracycline’s dosing schedule—for adults, 250 or 500 mg 4 times daily—makes it difficult to use. Gastrointestinal upset, phototoxicity, and hepatotoxicity can occur. The possibility of tooth discoloration precludes tetracycline’s use in children.
Clindamycin carries a high rate of gastrointestinal-related problems, Clostridium difficile infection in particular (10% incidence administered in any route). Inducible resistance to clindamycin is 50% in MRSA infections.9 Recent use of antibiotics may increase the likelihood of clindamycin resistance, with erythromycin in particular inducing this resistance. Its dosage typically is 150 to 300 mg every 6 hours.
Doxycycline and minocycline are not recommended, as they carry a 21% failure rate.9
Linezolid is costly and has many drug interactions. In particular, linezolid has the potential to cause serotonin syndrome with agents that affect the serotonergic system. Linezolid may also interact with medications that affect the adrenergic system (pressors). Its routine use in the community without infectious disease consultation is not advised.
For lesions that are not fluctuant or purulent, appropriate first-line antibiotics are semisynthetic penicillins (dicloxacillin), first- or second-generation oral cephalosporins, macrolides, and clindamycin.9 These antibiotics are preferable for group A streptococcal infections, erysipelas (which can be quite aggressive), and impetigo. Adjustments can be made as culture results become available or if the clinical response is inadequate. There is no particular utility in waiting to administer oral antibiotics in cases of erysipelas or impetigo, though topical antibiotics can often be used for limited cases of impetigo.
Prevention: Simple precautions are the rule
Most CA-MRSA infections result from direct contact with a patient’s wound or from wound drainage on environmental surfaces.
In the medical office. In addition to using sterile technique during incision and drainage, be sure that all staff members wash their hands with soap and water or with an alcohol-based sanitizer. For the most part, MRSA remains susceptible to triclosan, a topical antiseptic in commercially available hand soaps.
Clean equipment as needed with 10% sodium hypochlorite solution or another agent effective against MRSA. Surgical instruments should be disposable or sterilized after each use.
At the patient’s home. Instruct patients to clean wounds wearing fresh disposable gloves each time and to cover wounds with new, dry dressings. Tell families to avoid sharing linens and clothing unless they have been washed in hot soap and water and dried in a heated dryer. MRSA can live for weeks to months on surfaces exposed to infected wounds,10 and these surfaces can be disinfected with a 10% bleach solution.
Sports environments. Athletes with CA-MRSA infections should not compete unless the wound can be completely covered with a dry dressing. Recommend to those in charge of school or commercial facilities that, in cases of confirmed MRSA infection, they routinely clean locker rooms and sports equipment with either a 10% bleach solution or commercial disinfectant. There is no evidence, however, that more widespread or vigorous cleaning—such as dismantling a training room and all its cardio-fitness equipment for disinfecting—prevents the spread of MRSA.
Encourage athletes to wash their hands properly. Communal towels should be washed in hot water (>140°F) with bleach before reuse. Personal equipment should be cleaned per the instructions of the manufacturer. Athletes should use a clean towel to provide a barrier between their skin and the surfaces of weight-room or cardio-fitness equipment. They should also clean equipment before and after use with an appropriate cleanser, such as a disinfectant hand-wipe.
Unproductive efforts you can avoid. Screening household contacts for MRSA is not useful, and attempts to eliminate colonization are generally ineffective. In a large military study, use of intranasal mupirocin failed to decrease nasal carriage of MRSA and the incidence of MRSA infections.11 The MRSA nasal colonization rate was 3.9%; 121 individuals with MRSA colonization needed to be treated with nasal mupirocin to prevent 1 MRSA infection in the total study population.
More complex antibiotic regimens are sometimes used in an attempt to eradicate MRSA carriage, though they also have limited effectiveness and carry the general risks of antibiotic use (gastrointestinal disturbance, allergic reaction, etc). If your office is considering an eradication attempt, consult with an infectious disease clinician first.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) abscesses are best managed surgically; postprocedure antibiotics do not substantially improve outcomes. Cure rates with incision and drainage alone are at least 90% (A).
- If incision and drainage fail to promote healing within 7 days, the oral antibiotics of choice are trimethoprim-sulfamethoxazole and tetracycline (C).
- Eradication of nasal carriage of CA-MRSA is generally not useful in preventing spread of clinical MRSA infections in communities (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A previously healthy law student arrives at your office complaining of “abdominal pain.” You discover on examination that she has an erythematous, indurated, and tender 3-cm lesion on her suprapubic region. The lesion has no point, but its center is boggy. The patient’s temperature is normal. Would you give her an antibiotic? Would you cover immediately for community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA)? What other factors might influence your decision?
The incidence of MRSA is increasing in communities across the United States, challenging our assumptions about evaluation and management of skin and soft-tissue infections. In this article, I outline a rational approach to managing patients who have lesions likely to have been caused by CA-MRSA (TABLE).
TABLE
Suspect CA-MRSA? Consider this treatment approach6,7
| CLASS | PATIENT CRITERIA | MANAGEMENT | ANTIBIOTIC CHOICES |
|---|---|---|---|
| 1 | Afebrile and healthy; lesion nonfluctuant | If no drainable abscess, give common first-line antibiotic for SSTI; reassess for response | Semisynthetic penicillin, oral first- or second-generation cephalosporin, macrolide, clindamycin |
| 2 | Fluctuant or pustular lesion <5 cm; with or without fever | Surgical drainage of abscess if possible. Use I&D presumptively for MRSA and monitor closely for response; inpatient management may be indicated | Trimethoprim-sulfamethoxazole, tetracycline, clindamycin |
| 3 | Toxic appearance or at least 1 unstable comorbidity or a limb-threatening infection; lesion >5 cm | Hospital admission with broadspectrum antibiotics for MRSA coverage; consider infectious disease consultation | Broad-spectrum, including vancomycin |
| 4 | Sepsis syndrome or life-threatening infection (necrotizing fasciitis) | Above plus aggressive surgical debridement | Above with infectious disease guidance |
| CA-MRSA, community-acquired methicillin-resistant Staphylococcus aureus; I&D, incision and drainage; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft-tissue infection. | |||
When to suspect MRSA skin infection
Patients with CA-MRSA skin infection often report a “spider bite,” as lesions appear suddenly and unexpectedly in areas without a history of trauma.1 The lesions very often are pustular with central necrosis, and there may be purulent drainage, redness, tenderness, and palpable fluctuance. CA-MRSA can cause impetigo, but the often benign nature of this clinical infection makes management decisions less crucial. CA-MRSA skin lesions can occur anywhere on the body, though most often they appear in the axillae or the groin and buttocks. Patients may or may not have a fever.
Individuals who are at increased risk for CA-MRSA disease include users of health clubs or participants in contact sports, men who have sex with men, children younger than 2 years of age, users of intravenous drugs, military personnel, and prisoners.2,3 However, the absence of these factors in a patient with a skin or soft-tissue infection does not rule out MRSA.4
Regardless of the lesion’s appearance or the patient’s epidemiologic history, consider CA-MRSA if its prevalence in your community reaches 10% to 15%.
- Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) causes up to 74% of purulent skin and soft-tissue infections in communities throughout the United States.1 By definition, this infection occurs in patients who have not been hospitalized and have not undergone medical procedures within the prior year.12
- The annual incidence of CA-MRSA was reported to be 18.0-25.7 cases per 100,000 population between 2001 and 2002.13 Clusters of CA-MRSA have been identified among Alaskan natives, Native American Indians, and Pacific Islanders.12
- Most often this organism causes skin and soft-tissue infections, though cardiac, respiratory, blood, and bone infections can also occur.14
- CA-MRSA species are genotypically distinct from hospital-acquired MRSA. One marker for CA-MRSA, Panton-Valentine leucocidin (PVL), is most often detected in cases of severe and systemic infection, and it may be a virulence factor.15 However, the presence of PVL does not necessarily correlate directly with antibiotic resistance.
- Historically, CA-MRSA was primarily resistant to beta-lactams and erythromycin. More recent strains have also demonstrated resistance to tetracycline and clindamycin.
- Retrospective analyses show that patients with CA-MRSA tend to receive inadequate initial antibiotic coverage, and, independent of this, they tend to have worse clinical outcomes than those infected by methicillin-sensitive strains.16
Hospitalize any patient who exhibits fever or hypothermia, tachycardia greater than 100 beats per minute, or hypotension with a systolic blood pressure <90 mm Hg or 20 mm Hg lower than baseline. A skin lesion >5 cm is also likely to require hospitalization and parenteral antibiotics.5
Treatment: Incision and drainage most important
Several management schemes have been proposed to guide the appropriate level of therapy based on presenting patient characteristics.6,7 If a lesion is clearly fluctuant, incise it and drain the fluid, or refer the patient for surgical consultation. If the lesion is not clearly fluctuant, needle aspiration may help to determine the need for more extensive incision and drainage or to collect a specimen for culture. Although culture of skin lesions may not have been routine in the past, the advent of CA-MRSA has made it so, particularly given that MRSA lesions may not be clinically distinguishable from those caused by nonresistant S aureus.
Periodic postprocedure follow-up is indicated to ensure resolution of the infection. At our health center, patients return every few days for an appointment with nursing staff for wound irrigation and packing change until the lesion visibly improves. Systemic effects from the infection are monitored, as well.
Adult patients in 1 study were treated with incision and drainage by a surgeon.8 The technique described in the article used an 11 blade and a “sawing motion,” creating a wide opening. The wound cavity was explored for loculations and packed. This technique is identical to that used in the office. There is one caveat, though: This study included abscesses larger than 5 cm and patients with compromised immune systems—situations not routinely managed in the primary care office.
Are antibiotics indicated after incision and drainage for MRSA? In this same study, cure rates with incision and drainage alone were just over 90%.8 The cure rate in the treatment arm also receiving an antibiotic was 84% (difference was not statistically significant), and coverage was inadequate for MRSA. Treatment with cephalexin after incision and drainage resulted in 1 patient harmed for every 14 treated (NNH=14). A pediatric study also showed that antibiotics do not affect the outcome of skin lesions following incision and drainage.5 When deciding whether to prescribe postprocedure antibiotics, keep in mind the need to avoid contributing further to bacterial resistance.
Generally if incision and drainage fail to promote healing of the MRSA lesion within 7 days, start the patient on trimethoprim-sulfamethoxazole or tetracycline. Clindamycin is an option, though resistance to it is becoming more common. Adjust the antibiotic choice as needed when culture and sensitivity results become available.
Trimethoprim-sulfamethoxazole is generally well tolerated at the recommended dose of 1 to 2 double-strength tablets (160 mg TMP, 800 mg SMX) twice daily for adults. If a patient’s creatinine clearance is 15 to 30 mL/min, reduce the dose by half. The rate of sulfa allergy is similar to other antibiotics, at 3%.
Tetracycline’s dosing schedule—for adults, 250 or 500 mg 4 times daily—makes it difficult to use. Gastrointestinal upset, phototoxicity, and hepatotoxicity can occur. The possibility of tooth discoloration precludes tetracycline’s use in children.
Clindamycin carries a high rate of gastrointestinal-related problems, Clostridium difficile infection in particular (10% incidence administered in any route). Inducible resistance to clindamycin is 50% in MRSA infections.9 Recent use of antibiotics may increase the likelihood of clindamycin resistance, with erythromycin in particular inducing this resistance. Its dosage typically is 150 to 300 mg every 6 hours.
Doxycycline and minocycline are not recommended, as they carry a 21% failure rate.9
Linezolid is costly and has many drug interactions. In particular, linezolid has the potential to cause serotonin syndrome with agents that affect the serotonergic system. Linezolid may also interact with medications that affect the adrenergic system (pressors). Its routine use in the community without infectious disease consultation is not advised.
For lesions that are not fluctuant or purulent, appropriate first-line antibiotics are semisynthetic penicillins (dicloxacillin), first- or second-generation oral cephalosporins, macrolides, and clindamycin.9 These antibiotics are preferable for group A streptococcal infections, erysipelas (which can be quite aggressive), and impetigo. Adjustments can be made as culture results become available or if the clinical response is inadequate. There is no particular utility in waiting to administer oral antibiotics in cases of erysipelas or impetigo, though topical antibiotics can often be used for limited cases of impetigo.
Prevention: Simple precautions are the rule
Most CA-MRSA infections result from direct contact with a patient’s wound or from wound drainage on environmental surfaces.
In the medical office. In addition to using sterile technique during incision and drainage, be sure that all staff members wash their hands with soap and water or with an alcohol-based sanitizer. For the most part, MRSA remains susceptible to triclosan, a topical antiseptic in commercially available hand soaps.
Clean equipment as needed with 10% sodium hypochlorite solution or another agent effective against MRSA. Surgical instruments should be disposable or sterilized after each use.
At the patient’s home. Instruct patients to clean wounds wearing fresh disposable gloves each time and to cover wounds with new, dry dressings. Tell families to avoid sharing linens and clothing unless they have been washed in hot soap and water and dried in a heated dryer. MRSA can live for weeks to months on surfaces exposed to infected wounds,10 and these surfaces can be disinfected with a 10% bleach solution.
Sports environments. Athletes with CA-MRSA infections should not compete unless the wound can be completely covered with a dry dressing. Recommend to those in charge of school or commercial facilities that, in cases of confirmed MRSA infection, they routinely clean locker rooms and sports equipment with either a 10% bleach solution or commercial disinfectant. There is no evidence, however, that more widespread or vigorous cleaning—such as dismantling a training room and all its cardio-fitness equipment for disinfecting—prevents the spread of MRSA.
Encourage athletes to wash their hands properly. Communal towels should be washed in hot water (>140°F) with bleach before reuse. Personal equipment should be cleaned per the instructions of the manufacturer. Athletes should use a clean towel to provide a barrier between their skin and the surfaces of weight-room or cardio-fitness equipment. They should also clean equipment before and after use with an appropriate cleanser, such as a disinfectant hand-wipe.
Unproductive efforts you can avoid. Screening household contacts for MRSA is not useful, and attempts to eliminate colonization are generally ineffective. In a large military study, use of intranasal mupirocin failed to decrease nasal carriage of MRSA and the incidence of MRSA infections.11 The MRSA nasal colonization rate was 3.9%; 121 individuals with MRSA colonization needed to be treated with nasal mupirocin to prevent 1 MRSA infection in the total study population.
More complex antibiotic regimens are sometimes used in an attempt to eradicate MRSA carriage, though they also have limited effectiveness and carry the general risks of antibiotic use (gastrointestinal disturbance, allergic reaction, etc). If your office is considering an eradication attempt, consult with an infectious disease clinician first.
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
2. Cohen PR. The skin in the gym: a comprehensive review of the cutaneous manifestations of community-acquired methicillin-resistant Staphylococcus aureus infection in athletes. Clin Dermatol. 2008;26:16-26.
3. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8:259-270.
4. Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community associated methicillin resistant Staph aureus infection from methicillin susceptible S aureus infection: a prospective investigation. Clin Infect Dis. 2007;44:471-482.
5. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123-127.
6. Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(suppl S1):i3-i17.
7. CDC, AMA, IDSA, Outpatient management of skin and soft tissue infections in the era of community-associated MRSA. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/36/ca_mrsa_desk_102007.pdf. Accessed June 8, 2008.
8. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
9. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
10. Dellit TH, Ducin J. Guidelines for Evaluation and Management of Community-Associated Methicillin Resistant Staphylococcus aureus Skin and Soft Tissue Infections in Outpatient Settings. Available at: http://www.metrokc.gov/health/providers/epidemiology/MRSA-guidelines.pdf. Accessed August 6, 2008.
11. Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51:3591-3598.
12. Centers for Disease Control and Prevention. Healthcare-associated methicillin resistant Staphylococcus aureus (MRSA). Available at: http://www.cdc.gov/ncidod/dhqp/ar_mrsa.html. Accessed April 14, 2008.
13. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-reistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:2362-a.
14. Active Bacterial Core Surveillance (ABCs) Report, Emerging Infections Program Network methicillin-resistant Staphylococcus aureus, 2006. Available at: http://www.cdc.gov/ncidod/dbmd/abcs/survreports/mrsa06.pdf. Accessed May 5, 2008.
15. Holmes A, Ganner M, McGuane S, et al. Staphylococcus aureus isolates carrying panton-valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol. 2005;43:2384-2390.
16. Davis SL, Perri MB, Donabedian SM, et al. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2007;45:1705-1711.
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666-674.
2. Cohen PR. The skin in the gym: a comprehensive review of the cutaneous manifestations of community-acquired methicillin-resistant Staphylococcus aureus infection in athletes. Clin Dermatol. 2008;26:16-26.
3. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8:259-270.
4. Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community associated methicillin resistant Staph aureus infection from methicillin susceptible S aureus infection: a prospective investigation. Clin Infect Dis. 2007;44:471-482.
5. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123-127.
6. Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(suppl S1):i3-i17.
7. CDC, AMA, IDSA, Outpatient management of skin and soft tissue infections in the era of community-associated MRSA. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/36/ca_mrsa_desk_102007.pdf. Accessed June 8, 2008.
8. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
9. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
10. Dellit TH, Ducin J. Guidelines for Evaluation and Management of Community-Associated Methicillin Resistant Staphylococcus aureus Skin and Soft Tissue Infections in Outpatient Settings. Available at: http://www.metrokc.gov/health/providers/epidemiology/MRSA-guidelines.pdf. Accessed August 6, 2008.
11. Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51:3591-3598.
12. Centers for Disease Control and Prevention. Healthcare-associated methicillin resistant Staphylococcus aureus (MRSA). Available at: http://www.cdc.gov/ncidod/dhqp/ar_mrsa.html. Accessed April 14, 2008.
13. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-reistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:2362-a.
14. Active Bacterial Core Surveillance (ABCs) Report, Emerging Infections Program Network methicillin-resistant Staphylococcus aureus, 2006. Available at: http://www.cdc.gov/ncidod/dbmd/abcs/survreports/mrsa06.pdf. Accessed May 5, 2008.
15. Holmes A, Ganner M, McGuane S, et al. Staphylococcus aureus isolates carrying panton-valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol. 2005;43:2384-2390.
16. Davis SL, Perri MB, Donabedian SM, et al. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2007;45:1705-1711.