User login
Insular Alzheimer disease pathology and the psychometric correlates of mortality
Only a few brain structures have been implicated in the autonomic control of blood pressure and heart rate. Among them are heteromodal association areas in the cortex, especially the insular cortex. Insular infarction has been associated with both cardiac arrhythmias and mortality. However, stroke may not be the only insular pathology with the potential to disrupt autonomic function. Alzheimer disease (AD) is associated with both insular pathology and autonomic dysfunction.
This article presents the hypothesis that autonomic dysfunction reflects subclinical stages of AD pathology affecting the insular cortex and discusses the resulting clinical implications.
AUTONOMIC DYSFUNCTION AS A PRODUCT OF SUBCLINICAL ALZHEIMER DISEASE
Braak and Braak have demonstrated a hierarchical progression of AD pathology that includes the insular cortex.1 This may explain why AD has effects on blood pressure and central autonomic cardioregulatory functions. However, AD reaches the insular cortex at a “preclinical” stage in the Braak and Braak sequence (before “dementia” can be diagnosed). Thus, AD pathology should also be considered as a possible explanation for autonomic morbidity and mortality in nondemented elderly persons.2
Suggestive evidence
The following observations support this possibility:
- Clinical AD is associated with a wide range of dysautonomic phenomena. These can already be demonstrated at the initial diagnosis, which suggests a preclinical onset.
- Only a limited set of brain regions are capable of affecting autonomic control. The insulae are affected at a preclinical stage in the sequence of Braak and Braak (ie, stage III of VI).
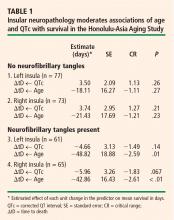
- Neurofibrillary tangle (NFT) counts inside the insulae moderate the association between the heart rate–corrected QT interval (QTc) and survival. This has been demonstrated by my colleagues and I in collaboration with the Honolulu-Asia Aging Study, which is examining the association between insular pathology at autopsy and the slope of premorbid change in the QTc.
Implications of AD-mediated autonomic dysfunction
AD-mediated autonomic dysfunction could have important clinical implications:
- The prevalence of preclinical AD is likely to be higher than the number of demented cases. Many apparently well elderly persons may be affected solely on the basis of subclinical AD pathology.
- Autonomic functions have widespread effects; many cardiac and noncardiac “age-related” changes may actually be related to AD.
- Pharmacologic therapies for AD are known to delay the progression of symptoms and to reduce mortality; these medications may also impact AD-related autonomic problems.
- Conversely, the association between other medications and cardiac arrhythmias/sudden death may be mediated via effects on insular function.
ALZHEIMER DISEASE DISRUPTS AUTONOMIC CONTROL
AD has been associated with a wide variety of dys-autonomic phenomena, including increased pupillary dilation, altered skin conductivity, blunted autonomic response to noxious stimuli, diminished heart rate variability, depressed baroreflex sensitivity, and orthostasis. Autonomic instability has yet to be sought in mild cognitive impairment or even earlier preclinical stages of AD. However, nondemented subjects with mild cognitive impairment and early AD do experience more frequent falls and more gait and balance problems than do age-matched controls.
INSULAR CORTEX: A LIKELY TARGET
The insulae have been specifically implicated in the cortical control of autonomic function.3 The vulnerability of the insular cortex to AD is easy to understand. NFTs appear to spread retrogradely along cortico-cortical and cortico-subcortical connections.4 The insulae are mesiotemporal structures with direct connections to the hippocampus and entorhinal cortex.
Insular lesions result in changes in cardiovascular and autonomic control that are readily detectable by a variety of measures and procedures, including blood pressure, tilt table, balance platform, and electrocardiogram. The electrocardiographic effects of insular pathology include diminished heart rate variability, determined in either the time domain or the frequency domain. Diminished heart rate variability has been associated with increased mortality in cardiovascular disease and type 2 diabetes. It is important to note, however, that the effects of diminished heart rate variability are statistically independent of disease severity in these disorders, and that they can be demonstrated in the absence of clinically significant cardiovascular disease.5
HOLTER MONITOR EVIDENCE
Autopsy studies suggest that as many as 40% of non-demented septuagenarians and octogenarians may have AD pathology that is sufficiently advanced to affect the insular cortex.1 This might explain the high prevalence of supraventricular arrhythmias and longitudinal decreases in heart rate variability among well elderly persons who are free of cardiovascular disease. In fact, unexplained supraventricular arrhythmias are quite common among such individuals. Both tachy-arrhythmias and bradyarrhythmias are common on 24-hour Holter monitor recordings among subjects older than 80 years, and most are unexplained. In a study of the causes of syncope in a large (N = 711) sample of octogenarians, Lipsitz et al confirmed a cardiac etiology in only 21% of cases, whereas 31% of cases were unexplained.6
MORTALITY IN ALZHEIMER DISEASE IS ASSOCIATED WITH RIGHT HEMISPHERE DYSFUNCTION
AD pathology is widely thought to be symmetrically distributed. However, this may not be true of the pre-clinical “limbic” stages of AD.7 Since insular effects on autonomic function are highly lateralized, the side of the brain affected by NFTs may be relevant to effects on cardiac rhythm and, hence, mortality risk.
Interestingly, mortality in AD is specifically associated with right hemisphere metabolic changes by electroencephalography, single-photon emission computed tomography, and positron emission tomography. Mortality can also be specifically associated with tests of constructional praxis. Claus and colleagues found that only the praxis subscore of the Cambridge Cognitive Examination (CAMCOG) was significantly related to survival in patients with early AD (P < .001).8 Its predictive power was based on only two items: copying ability for a spiral and for a three-dimensional house. The effect was independent of age, sex, education, dementia severity, total CAMCOG score, and symptom duration. Similarly, Swan et al found a significant association between performance on the digit symbol substitution test and 5-year mortality among 1,118 subjects (with a mean age of 70.6 years) in the Western Collaborative Group Study.9 In Cox regression analyses, the relative risk for all-cause mortality was 1.44 (95% confidence interval, 1.12 to 1.86) after adjustment for age, education, blood pressure, cancer, cardiovascular/cerebrovascular disease, and smoking.9
RIGHT HEMISPHERE DYSFUNCTION AFFECTS MORTALITY IN OTHER CONDITIONS
The effect of right hemisphere dysfunction on mortality is not limited to AD; it can also be demonstrated in other disorders, including epilepsy, head injury, and stroke.
We have been studying the cognitive correlates of mortality among well elderly septuagenarians and octogenarians living in a single comprehensive care retirement community (CCRC). Once again, visuo-spatial measures have been found to be selectively associated with mortality.10 Clock drawing appears to be the cognitive predictor most strongly correlated with mortality,10 a finding that has been independently replicated in a second CCRC cohort.11 This effect is independent of other cognitive domains, notably executive function.10
SUMMARY
Right hemisphere dysfunction is associated with mortality in AD and other conditions. These associations may be mediated by insular pathology. AD affects the insulae at a preclinical stage, and insular AD pathology may affect as many as 40% of nondemented septuagenarians and octogenarians. This pathology can be shown to affect in vivo cardiac conduction, and may dispose elderly persons to cardiac arrhythmias and sudden death. If so, then AD must be considered a potential cause of cardiac arrhythmia, sudden death, and other autonomic disturbances in nondemented older adults.
Acknowledgments
The author wishes to acknowledge the important cooperation and support received from the Air Force Villages and the Honolulu-Asia Aging Study. This work has been supported by a grant from the National Institute for Neurological Disorders and Stroke (NS45121-01A1).
- Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl 1998; 53:127–140.
- Royall DR, Gao JH, Kellogg DL Jr. Insular Alzheimer’s disease pathology as a cause of “age-related” autonomic dysfunction and mortality in the non-demented elderly. Med Hypotheses 2006; 67:747–758.
- Oppenheimer S. Forebrain lateralization of cardiovascular function: physiology and clinical correlates. Ann Neurol 2001; 49:555–556.
- Pearson RCA, Powell TPS. The neuroanatomy of Alzheimer’s disease. Rev Neurosci 1987; 2:101–122.
- Tasaki H, Serita T, Irita A, et al. A 15-year longitudinal follow-up study of heart rate and heart rate variability in healthy elderly persons. J Gerontol A Biol Sci Med Sci 2000; 55:M744–M749.
- Lipsitz LA, Wei JY, Rowe JW. Syncope in an elderly, institution-alised population: prevalence, incidence, and associated risk. Q J Med 1985; 55:45–54.
- Moossy J, Zubenko GS, Martinez AJ, Rao GR. Bilateral symmetry of morphologic lesions in Alzheimer’s disease. Arch Neurol 1988; 45:251–254.
- Claus JJ, Walstra GJ, Bossuyt PM, Teunisse S, Van Gool WA. A simple test of copying ability and sex define survival in patients with early Alzheimer’s disease. Psychol Med 1999; 29:485–489.
- Swan GE, Carmelli D, LaRue A. Perfomance on the digit symbol substitution test and 5-year mortality in the Western Collaborative Group Study. Am J Epidemiol 1995; 141:32–40.
- Royall DR, Chiodo LK, Mouton C, Polk MJ. Cognitive predictors of mortality in elderly retirees: results from the Freedom House study. Am J Geriatr Psychiatry 2007; 15:243–251.
- Lavery LL, Starenchak SM, Flynn WB, Stoeff MA, Schaffner R, Newman AB. The clock drawing test is an independent predictor of incident use of 24-hour care in a retirement community. J Gerontol A Biol Sci Med Sci 2005; 60:928–932.
Only a few brain structures have been implicated in the autonomic control of blood pressure and heart rate. Among them are heteromodal association areas in the cortex, especially the insular cortex. Insular infarction has been associated with both cardiac arrhythmias and mortality. However, stroke may not be the only insular pathology with the potential to disrupt autonomic function. Alzheimer disease (AD) is associated with both insular pathology and autonomic dysfunction.
This article presents the hypothesis that autonomic dysfunction reflects subclinical stages of AD pathology affecting the insular cortex and discusses the resulting clinical implications.
AUTONOMIC DYSFUNCTION AS A PRODUCT OF SUBCLINICAL ALZHEIMER DISEASE
Braak and Braak have demonstrated a hierarchical progression of AD pathology that includes the insular cortex.1 This may explain why AD has effects on blood pressure and central autonomic cardioregulatory functions. However, AD reaches the insular cortex at a “preclinical” stage in the Braak and Braak sequence (before “dementia” can be diagnosed). Thus, AD pathology should also be considered as a possible explanation for autonomic morbidity and mortality in nondemented elderly persons.2
Suggestive evidence
The following observations support this possibility:
- Clinical AD is associated with a wide range of dysautonomic phenomena. These can already be demonstrated at the initial diagnosis, which suggests a preclinical onset.
- Only a limited set of brain regions are capable of affecting autonomic control. The insulae are affected at a preclinical stage in the sequence of Braak and Braak (ie, stage III of VI).
- Neurofibrillary tangle (NFT) counts inside the insulae moderate the association between the heart rate–corrected QT interval (QTc) and survival. This has been demonstrated by my colleagues and I in collaboration with the Honolulu-Asia Aging Study, which is examining the association between insular pathology at autopsy and the slope of premorbid change in the QTc.
Implications of AD-mediated autonomic dysfunction
AD-mediated autonomic dysfunction could have important clinical implications:
- The prevalence of preclinical AD is likely to be higher than the number of demented cases. Many apparently well elderly persons may be affected solely on the basis of subclinical AD pathology.
- Autonomic functions have widespread effects; many cardiac and noncardiac “age-related” changes may actually be related to AD.
- Pharmacologic therapies for AD are known to delay the progression of symptoms and to reduce mortality; these medications may also impact AD-related autonomic problems.
- Conversely, the association between other medications and cardiac arrhythmias/sudden death may be mediated via effects on insular function.
ALZHEIMER DISEASE DISRUPTS AUTONOMIC CONTROL
AD has been associated with a wide variety of dys-autonomic phenomena, including increased pupillary dilation, altered skin conductivity, blunted autonomic response to noxious stimuli, diminished heart rate variability, depressed baroreflex sensitivity, and orthostasis. Autonomic instability has yet to be sought in mild cognitive impairment or even earlier preclinical stages of AD. However, nondemented subjects with mild cognitive impairment and early AD do experience more frequent falls and more gait and balance problems than do age-matched controls.
INSULAR CORTEX: A LIKELY TARGET
The insulae have been specifically implicated in the cortical control of autonomic function.3 The vulnerability of the insular cortex to AD is easy to understand. NFTs appear to spread retrogradely along cortico-cortical and cortico-subcortical connections.4 The insulae are mesiotemporal structures with direct connections to the hippocampus and entorhinal cortex.
Insular lesions result in changes in cardiovascular and autonomic control that are readily detectable by a variety of measures and procedures, including blood pressure, tilt table, balance platform, and electrocardiogram. The electrocardiographic effects of insular pathology include diminished heart rate variability, determined in either the time domain or the frequency domain. Diminished heart rate variability has been associated with increased mortality in cardiovascular disease and type 2 diabetes. It is important to note, however, that the effects of diminished heart rate variability are statistically independent of disease severity in these disorders, and that they can be demonstrated in the absence of clinically significant cardiovascular disease.5
HOLTER MONITOR EVIDENCE
Autopsy studies suggest that as many as 40% of non-demented septuagenarians and octogenarians may have AD pathology that is sufficiently advanced to affect the insular cortex.1 This might explain the high prevalence of supraventricular arrhythmias and longitudinal decreases in heart rate variability among well elderly persons who are free of cardiovascular disease. In fact, unexplained supraventricular arrhythmias are quite common among such individuals. Both tachy-arrhythmias and bradyarrhythmias are common on 24-hour Holter monitor recordings among subjects older than 80 years, and most are unexplained. In a study of the causes of syncope in a large (N = 711) sample of octogenarians, Lipsitz et al confirmed a cardiac etiology in only 21% of cases, whereas 31% of cases were unexplained.6
MORTALITY IN ALZHEIMER DISEASE IS ASSOCIATED WITH RIGHT HEMISPHERE DYSFUNCTION
AD pathology is widely thought to be symmetrically distributed. However, this may not be true of the pre-clinical “limbic” stages of AD.7 Since insular effects on autonomic function are highly lateralized, the side of the brain affected by NFTs may be relevant to effects on cardiac rhythm and, hence, mortality risk.
Interestingly, mortality in AD is specifically associated with right hemisphere metabolic changes by electroencephalography, single-photon emission computed tomography, and positron emission tomography. Mortality can also be specifically associated with tests of constructional praxis. Claus and colleagues found that only the praxis subscore of the Cambridge Cognitive Examination (CAMCOG) was significantly related to survival in patients with early AD (P < .001).8 Its predictive power was based on only two items: copying ability for a spiral and for a three-dimensional house. The effect was independent of age, sex, education, dementia severity, total CAMCOG score, and symptom duration. Similarly, Swan et al found a significant association between performance on the digit symbol substitution test and 5-year mortality among 1,118 subjects (with a mean age of 70.6 years) in the Western Collaborative Group Study.9 In Cox regression analyses, the relative risk for all-cause mortality was 1.44 (95% confidence interval, 1.12 to 1.86) after adjustment for age, education, blood pressure, cancer, cardiovascular/cerebrovascular disease, and smoking.9
RIGHT HEMISPHERE DYSFUNCTION AFFECTS MORTALITY IN OTHER CONDITIONS
The effect of right hemisphere dysfunction on mortality is not limited to AD; it can also be demonstrated in other disorders, including epilepsy, head injury, and stroke.
We have been studying the cognitive correlates of mortality among well elderly septuagenarians and octogenarians living in a single comprehensive care retirement community (CCRC). Once again, visuo-spatial measures have been found to be selectively associated with mortality.10 Clock drawing appears to be the cognitive predictor most strongly correlated with mortality,10 a finding that has been independently replicated in a second CCRC cohort.11 This effect is independent of other cognitive domains, notably executive function.10
SUMMARY
Right hemisphere dysfunction is associated with mortality in AD and other conditions. These associations may be mediated by insular pathology. AD affects the insulae at a preclinical stage, and insular AD pathology may affect as many as 40% of nondemented septuagenarians and octogenarians. This pathology can be shown to affect in vivo cardiac conduction, and may dispose elderly persons to cardiac arrhythmias and sudden death. If so, then AD must be considered a potential cause of cardiac arrhythmia, sudden death, and other autonomic disturbances in nondemented older adults.
Acknowledgments
The author wishes to acknowledge the important cooperation and support received from the Air Force Villages and the Honolulu-Asia Aging Study. This work has been supported by a grant from the National Institute for Neurological Disorders and Stroke (NS45121-01A1).
Only a few brain structures have been implicated in the autonomic control of blood pressure and heart rate. Among them are heteromodal association areas in the cortex, especially the insular cortex. Insular infarction has been associated with both cardiac arrhythmias and mortality. However, stroke may not be the only insular pathology with the potential to disrupt autonomic function. Alzheimer disease (AD) is associated with both insular pathology and autonomic dysfunction.
This article presents the hypothesis that autonomic dysfunction reflects subclinical stages of AD pathology affecting the insular cortex and discusses the resulting clinical implications.
AUTONOMIC DYSFUNCTION AS A PRODUCT OF SUBCLINICAL ALZHEIMER DISEASE
Braak and Braak have demonstrated a hierarchical progression of AD pathology that includes the insular cortex.1 This may explain why AD has effects on blood pressure and central autonomic cardioregulatory functions. However, AD reaches the insular cortex at a “preclinical” stage in the Braak and Braak sequence (before “dementia” can be diagnosed). Thus, AD pathology should also be considered as a possible explanation for autonomic morbidity and mortality in nondemented elderly persons.2
Suggestive evidence
The following observations support this possibility:
- Clinical AD is associated with a wide range of dysautonomic phenomena. These can already be demonstrated at the initial diagnosis, which suggests a preclinical onset.
- Only a limited set of brain regions are capable of affecting autonomic control. The insulae are affected at a preclinical stage in the sequence of Braak and Braak (ie, stage III of VI).
- Neurofibrillary tangle (NFT) counts inside the insulae moderate the association between the heart rate–corrected QT interval (QTc) and survival. This has been demonstrated by my colleagues and I in collaboration with the Honolulu-Asia Aging Study, which is examining the association between insular pathology at autopsy and the slope of premorbid change in the QTc.
Implications of AD-mediated autonomic dysfunction
AD-mediated autonomic dysfunction could have important clinical implications:
- The prevalence of preclinical AD is likely to be higher than the number of demented cases. Many apparently well elderly persons may be affected solely on the basis of subclinical AD pathology.
- Autonomic functions have widespread effects; many cardiac and noncardiac “age-related” changes may actually be related to AD.
- Pharmacologic therapies for AD are known to delay the progression of symptoms and to reduce mortality; these medications may also impact AD-related autonomic problems.
- Conversely, the association between other medications and cardiac arrhythmias/sudden death may be mediated via effects on insular function.
ALZHEIMER DISEASE DISRUPTS AUTONOMIC CONTROL
AD has been associated with a wide variety of dys-autonomic phenomena, including increased pupillary dilation, altered skin conductivity, blunted autonomic response to noxious stimuli, diminished heart rate variability, depressed baroreflex sensitivity, and orthostasis. Autonomic instability has yet to be sought in mild cognitive impairment or even earlier preclinical stages of AD. However, nondemented subjects with mild cognitive impairment and early AD do experience more frequent falls and more gait and balance problems than do age-matched controls.
INSULAR CORTEX: A LIKELY TARGET
The insulae have been specifically implicated in the cortical control of autonomic function.3 The vulnerability of the insular cortex to AD is easy to understand. NFTs appear to spread retrogradely along cortico-cortical and cortico-subcortical connections.4 The insulae are mesiotemporal structures with direct connections to the hippocampus and entorhinal cortex.
Insular lesions result in changes in cardiovascular and autonomic control that are readily detectable by a variety of measures and procedures, including blood pressure, tilt table, balance platform, and electrocardiogram. The electrocardiographic effects of insular pathology include diminished heart rate variability, determined in either the time domain or the frequency domain. Diminished heart rate variability has been associated with increased mortality in cardiovascular disease and type 2 diabetes. It is important to note, however, that the effects of diminished heart rate variability are statistically independent of disease severity in these disorders, and that they can be demonstrated in the absence of clinically significant cardiovascular disease.5
HOLTER MONITOR EVIDENCE
Autopsy studies suggest that as many as 40% of non-demented septuagenarians and octogenarians may have AD pathology that is sufficiently advanced to affect the insular cortex.1 This might explain the high prevalence of supraventricular arrhythmias and longitudinal decreases in heart rate variability among well elderly persons who are free of cardiovascular disease. In fact, unexplained supraventricular arrhythmias are quite common among such individuals. Both tachy-arrhythmias and bradyarrhythmias are common on 24-hour Holter monitor recordings among subjects older than 80 years, and most are unexplained. In a study of the causes of syncope in a large (N = 711) sample of octogenarians, Lipsitz et al confirmed a cardiac etiology in only 21% of cases, whereas 31% of cases were unexplained.6
MORTALITY IN ALZHEIMER DISEASE IS ASSOCIATED WITH RIGHT HEMISPHERE DYSFUNCTION
AD pathology is widely thought to be symmetrically distributed. However, this may not be true of the pre-clinical “limbic” stages of AD.7 Since insular effects on autonomic function are highly lateralized, the side of the brain affected by NFTs may be relevant to effects on cardiac rhythm and, hence, mortality risk.
Interestingly, mortality in AD is specifically associated with right hemisphere metabolic changes by electroencephalography, single-photon emission computed tomography, and positron emission tomography. Mortality can also be specifically associated with tests of constructional praxis. Claus and colleagues found that only the praxis subscore of the Cambridge Cognitive Examination (CAMCOG) was significantly related to survival in patients with early AD (P < .001).8 Its predictive power was based on only two items: copying ability for a spiral and for a three-dimensional house. The effect was independent of age, sex, education, dementia severity, total CAMCOG score, and symptom duration. Similarly, Swan et al found a significant association between performance on the digit symbol substitution test and 5-year mortality among 1,118 subjects (with a mean age of 70.6 years) in the Western Collaborative Group Study.9 In Cox regression analyses, the relative risk for all-cause mortality was 1.44 (95% confidence interval, 1.12 to 1.86) after adjustment for age, education, blood pressure, cancer, cardiovascular/cerebrovascular disease, and smoking.9
RIGHT HEMISPHERE DYSFUNCTION AFFECTS MORTALITY IN OTHER CONDITIONS
The effect of right hemisphere dysfunction on mortality is not limited to AD; it can also be demonstrated in other disorders, including epilepsy, head injury, and stroke.
We have been studying the cognitive correlates of mortality among well elderly septuagenarians and octogenarians living in a single comprehensive care retirement community (CCRC). Once again, visuo-spatial measures have been found to be selectively associated with mortality.10 Clock drawing appears to be the cognitive predictor most strongly correlated with mortality,10 a finding that has been independently replicated in a second CCRC cohort.11 This effect is independent of other cognitive domains, notably executive function.10
SUMMARY
Right hemisphere dysfunction is associated with mortality in AD and other conditions. These associations may be mediated by insular pathology. AD affects the insulae at a preclinical stage, and insular AD pathology may affect as many as 40% of nondemented septuagenarians and octogenarians. This pathology can be shown to affect in vivo cardiac conduction, and may dispose elderly persons to cardiac arrhythmias and sudden death. If so, then AD must be considered a potential cause of cardiac arrhythmia, sudden death, and other autonomic disturbances in nondemented older adults.
Acknowledgments
The author wishes to acknowledge the important cooperation and support received from the Air Force Villages and the Honolulu-Asia Aging Study. This work has been supported by a grant from the National Institute for Neurological Disorders and Stroke (NS45121-01A1).
- Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl 1998; 53:127–140.
- Royall DR, Gao JH, Kellogg DL Jr. Insular Alzheimer’s disease pathology as a cause of “age-related” autonomic dysfunction and mortality in the non-demented elderly. Med Hypotheses 2006; 67:747–758.
- Oppenheimer S. Forebrain lateralization of cardiovascular function: physiology and clinical correlates. Ann Neurol 2001; 49:555–556.
- Pearson RCA, Powell TPS. The neuroanatomy of Alzheimer’s disease. Rev Neurosci 1987; 2:101–122.
- Tasaki H, Serita T, Irita A, et al. A 15-year longitudinal follow-up study of heart rate and heart rate variability in healthy elderly persons. J Gerontol A Biol Sci Med Sci 2000; 55:M744–M749.
- Lipsitz LA, Wei JY, Rowe JW. Syncope in an elderly, institution-alised population: prevalence, incidence, and associated risk. Q J Med 1985; 55:45–54.
- Moossy J, Zubenko GS, Martinez AJ, Rao GR. Bilateral symmetry of morphologic lesions in Alzheimer’s disease. Arch Neurol 1988; 45:251–254.
- Claus JJ, Walstra GJ, Bossuyt PM, Teunisse S, Van Gool WA. A simple test of copying ability and sex define survival in patients with early Alzheimer’s disease. Psychol Med 1999; 29:485–489.
- Swan GE, Carmelli D, LaRue A. Perfomance on the digit symbol substitution test and 5-year mortality in the Western Collaborative Group Study. Am J Epidemiol 1995; 141:32–40.
- Royall DR, Chiodo LK, Mouton C, Polk MJ. Cognitive predictors of mortality in elderly retirees: results from the Freedom House study. Am J Geriatr Psychiatry 2007; 15:243–251.
- Lavery LL, Starenchak SM, Flynn WB, Stoeff MA, Schaffner R, Newman AB. The clock drawing test is an independent predictor of incident use of 24-hour care in a retirement community. J Gerontol A Biol Sci Med Sci 2005; 60:928–932.
- Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl 1998; 53:127–140.
- Royall DR, Gao JH, Kellogg DL Jr. Insular Alzheimer’s disease pathology as a cause of “age-related” autonomic dysfunction and mortality in the non-demented elderly. Med Hypotheses 2006; 67:747–758.
- Oppenheimer S. Forebrain lateralization of cardiovascular function: physiology and clinical correlates. Ann Neurol 2001; 49:555–556.
- Pearson RCA, Powell TPS. The neuroanatomy of Alzheimer’s disease. Rev Neurosci 1987; 2:101–122.
- Tasaki H, Serita T, Irita A, et al. A 15-year longitudinal follow-up study of heart rate and heart rate variability in healthy elderly persons. J Gerontol A Biol Sci Med Sci 2000; 55:M744–M749.
- Lipsitz LA, Wei JY, Rowe JW. Syncope in an elderly, institution-alised population: prevalence, incidence, and associated risk. Q J Med 1985; 55:45–54.
- Moossy J, Zubenko GS, Martinez AJ, Rao GR. Bilateral symmetry of morphologic lesions in Alzheimer’s disease. Arch Neurol 1988; 45:251–254.
- Claus JJ, Walstra GJ, Bossuyt PM, Teunisse S, Van Gool WA. A simple test of copying ability and sex define survival in patients with early Alzheimer’s disease. Psychol Med 1999; 29:485–489.
- Swan GE, Carmelli D, LaRue A. Perfomance on the digit symbol substitution test and 5-year mortality in the Western Collaborative Group Study. Am J Epidemiol 1995; 141:32–40.
- Royall DR, Chiodo LK, Mouton C, Polk MJ. Cognitive predictors of mortality in elderly retirees: results from the Freedom House study. Am J Geriatr Psychiatry 2007; 15:243–251.
- Lavery LL, Starenchak SM, Flynn WB, Stoeff MA, Schaffner R, Newman AB. The clock drawing test is an independent predictor of incident use of 24-hour care in a retirement community. J Gerontol A Biol Sci Med Sci 2005; 60:928–932.