User login
4 drugs can improve autism’s repetitive behaviors
Autism’s repetitive behaviors and restricted interests interfere with adaptive functioning, social interactions, and learning. No medications are FDA-approved for autistic disorder, but some selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics and an anticonvulsant have reduced repetitive behaviors in controlled trials. We discuss how that evidence shapes our approach to patients with or without a comorbid family history of bipolar disorder.
Evidence for SSRIs
Repetitive behaviors and restricted interests are autism’s third core domain, as defined by DSM-IVTR criteria.1 For an autistic disorder diagnosis, a patient must show at least one of these behaviors:
- encompassing preoccupations with stereotyped or restricted patterns of interest
- inflexible routines or rituals
- stereotyped, repetitive motor mannerisms
- or persistent preoccupation with parts of objects.
As in obsessive-compulsive disorder (OCD), rituals and restricted interests are thought to decrease anxiety in autism, whereas self-stimulatory behaviors and stereotypy may regulate arousal. The behaviors persist2,3 but may change across the lifespan.
Because SSRIs improve OCD’s repetitive behaviors, clinicians have also used them to treat autism’s repetitive behaviors, though without supporting data. Recently, however, fluoxetine and fluvoxamine have shown efficacy for autism’s repetitive behaviors in randomized, controlled trials. Results indicate:
- In children, fluoxetine is probably better-tolerated than available dosing forms of fluvoxamine.
- In adults, fluvoxamine is well-tolerated and can improve repetitive behavior.
SSRIs and suicidal ideation in autism. The increased risk of suicidality reported with SSRIs when treating depression and OCD has not been seen in children with autism. But because fewer children with autism have been treated with SSRIs, we recommend that you try to assess suicidal ideation during SSRI treatment in those able to express such concerns (Box). Starting with low SSRI dosages (Table 1) and increasing slowly may help prevent behavioral activation, a possible risk factor for suicidality.
Suicidal ideation has not been reported in studies of selective serotonin reuptake inhibitors (SSRIs) in autism. Even so, children and adolescents with autistic disorder are not excluded from the FDA black-box warning of increased risk of suicidality with SSRIs.
Children with obsessive-compulsive disorder (OCD) treated with SSRIs have shown evidence of suicidal thoughts. Thus, higher-functioning children and adults with autism might think about suicide when they become aware of their deficits.
For lower-functioning patients (generally, those who receive medication), we need markers of possible suicidal ideation other than their reports of symptoms. In clinical trials, investigators measure behavioral activation symptoms as risk factors for suicidality.
Thus, when you start an SSRI in a patient with autistic disorder, educate the caregivers to watch for agitation, increased energy, poor sleep, disinhibition, or new hyperactivity. Encourage them to contact you immediately if these signs of activation occur.
Ask higher-functioning patients taking SSRIs about suicidal thinking in a step-wise fashion: thoughts of death, thoughts of their own death, intent, plan, and finally possible attempts.
Table 1
4 drugs with evidence of benefit for autism’s repetitive behaviors*
| Medication | Suggested target daily dosage |
|---|---|
| Fluoxetine7 | Children: Start at 2.5 mg/d; maximum 20 mg/d |
| Adults: Start at 10 to 20 mg/d; maximum 60 mg/d | |
| Fluvoxamine10 | Children: Not first-line; start at 12.5 mg/d; maximum 150 to200 mg/d |
| Adults: Start at 25 mg/d; maximum 300 mg/d | |
| Risperidone13,16 | Children: Start at 0.25 mg/d; maximum 3 mg/d |
| Adults: Start at 2 mg/d; maximum 4 mg/d | |
| Valproate20 | Children: Start at 125 mg (sprinkles); titrate to clinical effect and blood levels of 50 of 120 mcg/mL |
| Adults: Start at 250 mg; increase by 250mg/week to clinical effect and blood levels of 50 to 120 mcg/mL | |
| *Data from randomized, placebo-controlled trials | |
Fluoxetine. In the first open-label study of fluoxetine in children and adults with autistic disorder, global functioning improved significantly in 15 of 23 patients, as measured by the Clinical Global Impressions (CGI) scale.4 Autism symptoms also improved in follow-up, open-label trials, but these did not target repetitive behaviors specifically.5,6
Our group conducted the first randomized, placebo-controlled study of fluoxetine’s effect on repetitive behaviors in children with autism.7 We measured obsessions and compulsions in 45 children, ages 5 to 16, with the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS). This 10-item, clinician-rated questionnaire uses a 5-point scale to rate repetitive behaviors by time spent, distress, interference, resistance, and control.
Using a crossover design—two 8-week phases of active or placebo treatment separated by a 4-week washout—we started liquid fluoxetine at 2.5 mg/d and slowly increased the dosage to clinical effect or a maximum of 0.8 mg/kg/day. Mean final dosage was 9.9 (±4.35) mg/d.
Repetitive behaviors improved, even though we used relatively low dosages to avoid side effects. The mean baseline CY-BOCS compulsion score of 13.15 dropped to 11.6 with fluoxetine and to 12.9 with placebo. Fluoxetine’s effect size was moderate to large, and we found no suicidal ideation with this SSRI.
Fluvoxamine. Repetitive behaviors did not change—as measured with the CY-BOCS—when 18 children with autism received fluvoxamine, 1.5 mg/kg/day, in a 10-week prospective, open-label trial.8 Most patients (72%) reported at least one side effect, and 3 discontinued the SSRI because of behavioral activation. Ten completed the trial. Likewise in a randomized trial, children who received fluvoxamine experienced troublesome side effects and limited benefit.9
Compared with outcomes in children, fluvoxamine has shown greater efficacy in adults. In a 12-week, double-blind, placebo-controlled trial of 30 adults with autism, 8 of 15 treated with fluvoxamine (50 mg/d initially and titrated to 300 mg/d) were rated as responders, compared with none of 15 receiving placebo.
Repetitive behaviors and adaptive functioning improved significantly with fluvoxamine, as measured with the Yale-Brown Obsessive Compulsive Scale (YBOCS) and Vineland Adaptive Behavior Scale, respectively.10 The SSRI was well-tolerated, with only mild nausea and sedation reported.
Thus, fluvoxamine may be useful for treating repetitive behaviors but probably is not a first choice for children with autism. Results might be more favorable in children if fluvoxamine were available in doses <12.5 mg.
Citalopram. Open-label data support using citalopram in autism.11 In a retrospective chart review, 10 of 15 children (73%) were reported “much improved” with citalopram (mean dosage 16.9 mg/d [+/-12.1]), but the review did not specifically address repetitive behaviors. Two patients stopped taking the SSRI because of side effects; agitation, aggressiveness, sedation, and lip dyskinesia were reported.
The National Institutes of Health is sponsoring a multicenter trial of citalopram (starting dosage 2.5 mg/d, up to 20 mg/d) for repetitive behaviors in 144 children with autism, Results are expected in 2007 (see Related resources).
Escitalopram. Some early, open-label evidence suggests that escitalopram may be well-tolerated in autism, but its efficacy for treating repetitive behaviors has not been studied.
Sertraline. One of three reported open-label studies of sertraline in patients with autism measured repetitive behaviors.12 In this study, 42 adults with autism spectrum disorder were treated for 12 weeks with sertraline, 50 to 200 mg/d. One-half were rated “much improved”—mostly in aggressive and repetitive behaviors—with the CGI improvement scale. Sertraline was well-tolerated, although 3 patients dropped out because of persistent agitation.
No randomized trials have examined sertraline in autism.
Clinical recommendations
Family history of bipolar disorder guides our treatment of patients with an autism spectrum disorder and disabling repetitive behaviors (Algorithm).
Algorithm Suggested medications to treat repetitive behaviors in autism
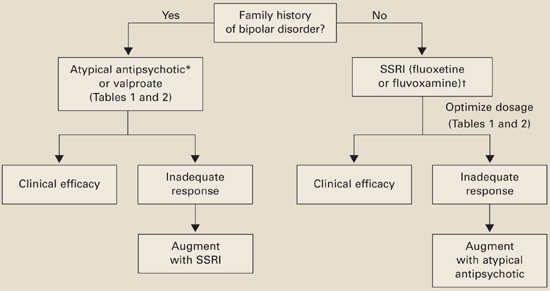
*Risperidone has the most evidence of efficacy, but aripiprazole may be useful for patients with weight-gain problems.
†For children, controlled data support using liquid fluoxetine, starting at 2.5 mg/d.Without bipolar history. SSRIs are usually first-line therapy (although patients with significant irritability and aggression may be an exception and require an atypical antipsychotic first).
If you reach the maximal SSRI dosage without a desired effect, consider adding an atypical (risperidone has the strongest supporting data) or valproate. If behavioral activation symptoms emerge and a lower dosage does not ameliorate them or reduces the clinical effect, consider switching to an atypical or valproate.
With bipolar history. Consider starting with an atypical or valproate; augment with an SSRI if needed.
Monitor for side effects with each medication (Table 2).
Table 2
Medication side effects and recommended monitoring
| Medication | Side effects | Recommended monitoring |
|---|---|---|
| Fluoxetine | Anxiety, insomnia, GI disturbance, appetite and weight changes, mania/hypomania activation, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Fluvoxamine | Somnolence, nervousness, insomnia, agitation, GI disturbance, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Risperidone | Drowsiness, weight gain, hyperglycemia, GI disturbance, extrapyramidal symptoms, neuroleptic malignant syndrome | Obtain metabolic profile, including serum glucose and lipids |
| Monitor for weight gain and clinical signs of extrapyramidal symptoms | ||
| Valproate | Rash, headaches, weight gain, ataxia, alopecia, GI disturbance, hyperammonemic encephalopathy, sedation, thrombocytopenia, polycystic ovary syndrome, pancreatitis, liver failure, teratogenic effects | CBC with platelets, liver function tests, valproate levels |
| Therapeutic blood levels: 50 to 120 mcg/mL |
Evidence for atypical antipsychotics
Atypicals have been used in autistic disorder to treat irritability and impulsive aggression. Risperidone also has been shown to reduce repetitive behaviors in a controlled trial.13 No evidence or only open-label trials support the use of other atypicals in patients with autism.
Risperidone’s side effects may include metabolic syndrome. No studies have examined lipid profiles and insulin resistance after risperidone treatment in patients with autism, but weight gain has been reported in the Research Units on Pediatric Psychopharmacology (RUPP) trial andothers.13-15 Carefully assess the risk-benefit ratio when you consider using risperidone to treat repetitive behaviors in patients with autism.
Atypical antipsychotics also may increase dyskinesia risk, although extrapyramidal symptoms (EPS) have not been reported in studies of patients with autism and repetitive behaviors. Because EPS could develop after clinical trials are completed, long-term naturalistic studies are needed to address this concern.
Risperidone. The double-blind, placebo-controlled RUPP trial examined risperidone’s efficacy in treating autism’s core symptoms (primarily irritability) in 101 children.13 Mean dosages after 8 weeks and during a 16-week open-label extension for 63 children were 2 and 2.1 mg/d, respectively.
Repetitive behavior—as measured with the CY-BOCS, using RUPP trial data16—improved significantly with risperidone compared with placebo. During the 8-week controlled trial, CY-BOCS scores improved from 15.51 (SD±2.73) to 11.65 (SD±4.02) in the risperidone group, compared with 15.18 (SD±3.88) to 14.21 (SD±4.81) in the placebo group. This response was maintained through the open-label trial.
Side effects included weight gain, fatigue, drowsiness, and drooling. No children receiving risperidone dropped out because of side effects. No EPS were reported, based on weekly Abnormal Involuntary Movement scale and Simpson-Angus scale scores.
Olanzapine. Only open-label studies have examined olanzapine in autism, and one systematically measured repetitive behaviors.17 Eight children with autism or other pervasive developmental disorders were given olanzapine, mean dosage 7.8 (±4.7) mg/d at the end of the 12-week trial.
Repetitive behaviors did not change significantly, as measured with YBOCS. Seven of eight patients completed the trial. Mean weight for the group after 12 weeks was 156±55 lbs, compared with 137±56 lbs at baseline.
Quetiapine. No data support using quetiapine for autism’s repetitive behaviors. Quetiapine, 100 to 350 mg/d (1.6 to 5.2 mg/kg/day) was poorly tolerated in a 16-week open-label safety and efficacy trial among 6 mentally retarded boys with autistic disorder. Side effects included a possible seizure, behavioral activation, increased appetite, and weight gain (0.9 to 8.2 kg). Two patients completed the trial.18
Ziprasidone. Small open-label studies and anecdotal reports of ziprasidone in autism have not examined this drug’s effect on repetitive behaviors.
Aripiprazole. Anecdotal information suggests that clinicians are using this medication to treat patients with autism, but no supporting data exist.
Evidence for valproate
Preliminary trials by our group suggest that valproate may reduce repetitive behaviors in autism. In a retrospective, open-label study, 14 patients (mean age 17) with autism spectrum disorder received divalproex sodium (mean 768±582 mg/d) for a mean 11 months. Ten patients (71%) showed sustained improvement in function, as measured by the CGI-improvement scale, and valproate was generally well-tolerated.19
We then measured valproate’s effect on repetitive behaviors in an 8-week, double-blind, placebo-controlled study of 13 patients (mean age 9) with autism spectrum disorder. Repetitive behaviors improved significantly compared with placebo, as measured by the CY-BOCS, in those who received divalproex (mean 833.93±326.21 mg/d).20
Further studies are needed to replicate this finding. Although it is too early to make general recommendations, valproate may be a reasonable choice for children with autism and epilepsy or affective instability.
- Hollander E, Phillips AT, Yeh CC. Targeted treatments for symptom domains in child and adolescent autism. Lancet 2003;362:732-4.
- Hollander E (ed). Autism spectrum disorders. New York: Marcel Decker; 2003.
- National Institute of Health multicenter study of citalopram for repetitive behaviors in autism. http://www.clinicaltrials.gov/ct/show/nct00086645?order=2
Drug brand names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Valproic acid • Depakote, Depakene
- Ziprasidone • Geodon
Disclosures
Dr. Anagnostou reports no financial interest with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Hollander receives research/grant support from and is a consultant to Abbott Laboratories. He also receives support from the National Institutes of Health (STAART Center) to investigate orphan drug status for fluoxetine in treating autism symptoms.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC; American Psychiatric Association; 2000.
2. Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. MRDD Research Reviews 2004;10:234-47.
3. Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004;45:212-29.
4. Cook EH, Rowlett R, Jaselinkis C, Leventhal BL. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am Acad Child Adolesc Psychiatry 1992;31:739-45.
5. DeLong GR, Teague LA, Kamran MM. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol 1998;40:551-62.
6. DeLong GR, Ritch CR, Burch S. Fluoxetine response in children with autistic spectrum disorders: correlation with familial major affective disorder and intellectual achievement. Dev Med Child Neurol 2002;44:652-9.
7. Hollander E, Phillips A, Chaplin W, et al. A placebo-controlled cross over trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology 2005;30:582-9.
8. Martin A, Koenig K, Anderson GM, Scahill L. Low-dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: a prospective, open-label study. J Autism Dev Disord 2003;33:77-85.
9. McDougle CJ, Kresch LE, Posey DJ. Repetitive thoughts and behavior in pervasive developmental disorders: treatment with serotonin reuptake inhibitors. J Autism Dev Disord 2000;30:427-35.
10. McDougle CJ, Naylor ST, Cohen DJ, et al. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996;53:1001-8.
11. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr. 2003;24(2):104-8.
12. McDougle CJ, Brodkin ES, Naylor ST, et al. Sertraline in adults with pervasive developmental disorders: a prospective open-label investigation. J Clin Psychopharmacol 1998;18:62-6.
13. McCracken JT, McGough J, Shah B, et al. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347:314-21.
14. Troost PW, Lahuis BE, Steenhuis MP, et al. Long-term effects of risperidone in children with autism spectrum disorders: A placebo discontinuation study. J Am Acad Child Adolesc Psychiatry 2005;44:1137-44.
15. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 2004;114:e634-e641.
16. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162:1142-8.
17. Potenza MN, Holmes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol 1999;19:37-44.
18. Martin A, Koenig K, Scahill L, Bregman J. Open-label quetiapine in the treatment of children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol 1999;9:99-107.
19. Hollander E, Dolgoff-Kaspar R, Cartwright C, et al. An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry 2001;62:530-4.
20. Hollander E, Soorya LV, Wasserman S, et al. Divalproex sodium vs. placebo in the treatment of repetitive behaviours in autism spectrum disorder. Int J Neuropsychopharmacol 2006;9:209-13.
Autism’s repetitive behaviors and restricted interests interfere with adaptive functioning, social interactions, and learning. No medications are FDA-approved for autistic disorder, but some selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics and an anticonvulsant have reduced repetitive behaviors in controlled trials. We discuss how that evidence shapes our approach to patients with or without a comorbid family history of bipolar disorder.
Evidence for SSRIs
Repetitive behaviors and restricted interests are autism’s third core domain, as defined by DSM-IVTR criteria.1 For an autistic disorder diagnosis, a patient must show at least one of these behaviors:
- encompassing preoccupations with stereotyped or restricted patterns of interest
- inflexible routines or rituals
- stereotyped, repetitive motor mannerisms
- or persistent preoccupation with parts of objects.
As in obsessive-compulsive disorder (OCD), rituals and restricted interests are thought to decrease anxiety in autism, whereas self-stimulatory behaviors and stereotypy may regulate arousal. The behaviors persist2,3 but may change across the lifespan.
Because SSRIs improve OCD’s repetitive behaviors, clinicians have also used them to treat autism’s repetitive behaviors, though without supporting data. Recently, however, fluoxetine and fluvoxamine have shown efficacy for autism’s repetitive behaviors in randomized, controlled trials. Results indicate:
- In children, fluoxetine is probably better-tolerated than available dosing forms of fluvoxamine.
- In adults, fluvoxamine is well-tolerated and can improve repetitive behavior.
SSRIs and suicidal ideation in autism. The increased risk of suicidality reported with SSRIs when treating depression and OCD has not been seen in children with autism. But because fewer children with autism have been treated with SSRIs, we recommend that you try to assess suicidal ideation during SSRI treatment in those able to express such concerns (Box). Starting with low SSRI dosages (Table 1) and increasing slowly may help prevent behavioral activation, a possible risk factor for suicidality.
Suicidal ideation has not been reported in studies of selective serotonin reuptake inhibitors (SSRIs) in autism. Even so, children and adolescents with autistic disorder are not excluded from the FDA black-box warning of increased risk of suicidality with SSRIs.
Children with obsessive-compulsive disorder (OCD) treated with SSRIs have shown evidence of suicidal thoughts. Thus, higher-functioning children and adults with autism might think about suicide when they become aware of their deficits.
For lower-functioning patients (generally, those who receive medication), we need markers of possible suicidal ideation other than their reports of symptoms. In clinical trials, investigators measure behavioral activation symptoms as risk factors for suicidality.
Thus, when you start an SSRI in a patient with autistic disorder, educate the caregivers to watch for agitation, increased energy, poor sleep, disinhibition, or new hyperactivity. Encourage them to contact you immediately if these signs of activation occur.
Ask higher-functioning patients taking SSRIs about suicidal thinking in a step-wise fashion: thoughts of death, thoughts of their own death, intent, plan, and finally possible attempts.
Table 1
4 drugs with evidence of benefit for autism’s repetitive behaviors*
| Medication | Suggested target daily dosage |
|---|---|
| Fluoxetine7 | Children: Start at 2.5 mg/d; maximum 20 mg/d |
| Adults: Start at 10 to 20 mg/d; maximum 60 mg/d | |
| Fluvoxamine10 | Children: Not first-line; start at 12.5 mg/d; maximum 150 to200 mg/d |
| Adults: Start at 25 mg/d; maximum 300 mg/d | |
| Risperidone13,16 | Children: Start at 0.25 mg/d; maximum 3 mg/d |
| Adults: Start at 2 mg/d; maximum 4 mg/d | |
| Valproate20 | Children: Start at 125 mg (sprinkles); titrate to clinical effect and blood levels of 50 of 120 mcg/mL |
| Adults: Start at 250 mg; increase by 250mg/week to clinical effect and blood levels of 50 to 120 mcg/mL | |
| *Data from randomized, placebo-controlled trials | |
Fluoxetine. In the first open-label study of fluoxetine in children and adults with autistic disorder, global functioning improved significantly in 15 of 23 patients, as measured by the Clinical Global Impressions (CGI) scale.4 Autism symptoms also improved in follow-up, open-label trials, but these did not target repetitive behaviors specifically.5,6
Our group conducted the first randomized, placebo-controlled study of fluoxetine’s effect on repetitive behaviors in children with autism.7 We measured obsessions and compulsions in 45 children, ages 5 to 16, with the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS). This 10-item, clinician-rated questionnaire uses a 5-point scale to rate repetitive behaviors by time spent, distress, interference, resistance, and control.
Using a crossover design—two 8-week phases of active or placebo treatment separated by a 4-week washout—we started liquid fluoxetine at 2.5 mg/d and slowly increased the dosage to clinical effect or a maximum of 0.8 mg/kg/day. Mean final dosage was 9.9 (±4.35) mg/d.
Repetitive behaviors improved, even though we used relatively low dosages to avoid side effects. The mean baseline CY-BOCS compulsion score of 13.15 dropped to 11.6 with fluoxetine and to 12.9 with placebo. Fluoxetine’s effect size was moderate to large, and we found no suicidal ideation with this SSRI.
Fluvoxamine. Repetitive behaviors did not change—as measured with the CY-BOCS—when 18 children with autism received fluvoxamine, 1.5 mg/kg/day, in a 10-week prospective, open-label trial.8 Most patients (72%) reported at least one side effect, and 3 discontinued the SSRI because of behavioral activation. Ten completed the trial. Likewise in a randomized trial, children who received fluvoxamine experienced troublesome side effects and limited benefit.9
Compared with outcomes in children, fluvoxamine has shown greater efficacy in adults. In a 12-week, double-blind, placebo-controlled trial of 30 adults with autism, 8 of 15 treated with fluvoxamine (50 mg/d initially and titrated to 300 mg/d) were rated as responders, compared with none of 15 receiving placebo.
Repetitive behaviors and adaptive functioning improved significantly with fluvoxamine, as measured with the Yale-Brown Obsessive Compulsive Scale (YBOCS) and Vineland Adaptive Behavior Scale, respectively.10 The SSRI was well-tolerated, with only mild nausea and sedation reported.
Thus, fluvoxamine may be useful for treating repetitive behaviors but probably is not a first choice for children with autism. Results might be more favorable in children if fluvoxamine were available in doses <12.5 mg.
Citalopram. Open-label data support using citalopram in autism.11 In a retrospective chart review, 10 of 15 children (73%) were reported “much improved” with citalopram (mean dosage 16.9 mg/d [+/-12.1]), but the review did not specifically address repetitive behaviors. Two patients stopped taking the SSRI because of side effects; agitation, aggressiveness, sedation, and lip dyskinesia were reported.
The National Institutes of Health is sponsoring a multicenter trial of citalopram (starting dosage 2.5 mg/d, up to 20 mg/d) for repetitive behaviors in 144 children with autism, Results are expected in 2007 (see Related resources).
Escitalopram. Some early, open-label evidence suggests that escitalopram may be well-tolerated in autism, but its efficacy for treating repetitive behaviors has not been studied.
Sertraline. One of three reported open-label studies of sertraline in patients with autism measured repetitive behaviors.12 In this study, 42 adults with autism spectrum disorder were treated for 12 weeks with sertraline, 50 to 200 mg/d. One-half were rated “much improved”—mostly in aggressive and repetitive behaviors—with the CGI improvement scale. Sertraline was well-tolerated, although 3 patients dropped out because of persistent agitation.
No randomized trials have examined sertraline in autism.
Clinical recommendations
Family history of bipolar disorder guides our treatment of patients with an autism spectrum disorder and disabling repetitive behaviors (Algorithm).
Algorithm Suggested medications to treat repetitive behaviors in autism

*Risperidone has the most evidence of efficacy, but aripiprazole may be useful for patients with weight-gain problems.
†For children, controlled data support using liquid fluoxetine, starting at 2.5 mg/d.Without bipolar history. SSRIs are usually first-line therapy (although patients with significant irritability and aggression may be an exception and require an atypical antipsychotic first).
If you reach the maximal SSRI dosage without a desired effect, consider adding an atypical (risperidone has the strongest supporting data) or valproate. If behavioral activation symptoms emerge and a lower dosage does not ameliorate them or reduces the clinical effect, consider switching to an atypical or valproate.
With bipolar history. Consider starting with an atypical or valproate; augment with an SSRI if needed.
Monitor for side effects with each medication (Table 2).
Table 2
Medication side effects and recommended monitoring
| Medication | Side effects | Recommended monitoring |
|---|---|---|
| Fluoxetine | Anxiety, insomnia, GI disturbance, appetite and weight changes, mania/hypomania activation, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Fluvoxamine | Somnolence, nervousness, insomnia, agitation, GI disturbance, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Risperidone | Drowsiness, weight gain, hyperglycemia, GI disturbance, extrapyramidal symptoms, neuroleptic malignant syndrome | Obtain metabolic profile, including serum glucose and lipids |
| Monitor for weight gain and clinical signs of extrapyramidal symptoms | ||
| Valproate | Rash, headaches, weight gain, ataxia, alopecia, GI disturbance, hyperammonemic encephalopathy, sedation, thrombocytopenia, polycystic ovary syndrome, pancreatitis, liver failure, teratogenic effects | CBC with platelets, liver function tests, valproate levels |
| Therapeutic blood levels: 50 to 120 mcg/mL |
Evidence for atypical antipsychotics
Atypicals have been used in autistic disorder to treat irritability and impulsive aggression. Risperidone also has been shown to reduce repetitive behaviors in a controlled trial.13 No evidence or only open-label trials support the use of other atypicals in patients with autism.
Risperidone’s side effects may include metabolic syndrome. No studies have examined lipid profiles and insulin resistance after risperidone treatment in patients with autism, but weight gain has been reported in the Research Units on Pediatric Psychopharmacology (RUPP) trial andothers.13-15 Carefully assess the risk-benefit ratio when you consider using risperidone to treat repetitive behaviors in patients with autism.
Atypical antipsychotics also may increase dyskinesia risk, although extrapyramidal symptoms (EPS) have not been reported in studies of patients with autism and repetitive behaviors. Because EPS could develop after clinical trials are completed, long-term naturalistic studies are needed to address this concern.
Risperidone. The double-blind, placebo-controlled RUPP trial examined risperidone’s efficacy in treating autism’s core symptoms (primarily irritability) in 101 children.13 Mean dosages after 8 weeks and during a 16-week open-label extension for 63 children were 2 and 2.1 mg/d, respectively.
Repetitive behavior—as measured with the CY-BOCS, using RUPP trial data16—improved significantly with risperidone compared with placebo. During the 8-week controlled trial, CY-BOCS scores improved from 15.51 (SD±2.73) to 11.65 (SD±4.02) in the risperidone group, compared with 15.18 (SD±3.88) to 14.21 (SD±4.81) in the placebo group. This response was maintained through the open-label trial.
Side effects included weight gain, fatigue, drowsiness, and drooling. No children receiving risperidone dropped out because of side effects. No EPS were reported, based on weekly Abnormal Involuntary Movement scale and Simpson-Angus scale scores.
Olanzapine. Only open-label studies have examined olanzapine in autism, and one systematically measured repetitive behaviors.17 Eight children with autism or other pervasive developmental disorders were given olanzapine, mean dosage 7.8 (±4.7) mg/d at the end of the 12-week trial.
Repetitive behaviors did not change significantly, as measured with YBOCS. Seven of eight patients completed the trial. Mean weight for the group after 12 weeks was 156±55 lbs, compared with 137±56 lbs at baseline.
Quetiapine. No data support using quetiapine for autism’s repetitive behaviors. Quetiapine, 100 to 350 mg/d (1.6 to 5.2 mg/kg/day) was poorly tolerated in a 16-week open-label safety and efficacy trial among 6 mentally retarded boys with autistic disorder. Side effects included a possible seizure, behavioral activation, increased appetite, and weight gain (0.9 to 8.2 kg). Two patients completed the trial.18
Ziprasidone. Small open-label studies and anecdotal reports of ziprasidone in autism have not examined this drug’s effect on repetitive behaviors.
Aripiprazole. Anecdotal information suggests that clinicians are using this medication to treat patients with autism, but no supporting data exist.
Evidence for valproate
Preliminary trials by our group suggest that valproate may reduce repetitive behaviors in autism. In a retrospective, open-label study, 14 patients (mean age 17) with autism spectrum disorder received divalproex sodium (mean 768±582 mg/d) for a mean 11 months. Ten patients (71%) showed sustained improvement in function, as measured by the CGI-improvement scale, and valproate was generally well-tolerated.19
We then measured valproate’s effect on repetitive behaviors in an 8-week, double-blind, placebo-controlled study of 13 patients (mean age 9) with autism spectrum disorder. Repetitive behaviors improved significantly compared with placebo, as measured by the CY-BOCS, in those who received divalproex (mean 833.93±326.21 mg/d).20
Further studies are needed to replicate this finding. Although it is too early to make general recommendations, valproate may be a reasonable choice for children with autism and epilepsy or affective instability.
- Hollander E, Phillips AT, Yeh CC. Targeted treatments for symptom domains in child and adolescent autism. Lancet 2003;362:732-4.
- Hollander E (ed). Autism spectrum disorders. New York: Marcel Decker; 2003.
- National Institute of Health multicenter study of citalopram for repetitive behaviors in autism. http://www.clinicaltrials.gov/ct/show/nct00086645?order=2
Drug brand names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Valproic acid • Depakote, Depakene
- Ziprasidone • Geodon
Disclosures
Dr. Anagnostou reports no financial interest with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Hollander receives research/grant support from and is a consultant to Abbott Laboratories. He also receives support from the National Institutes of Health (STAART Center) to investigate orphan drug status for fluoxetine in treating autism symptoms.
Autism’s repetitive behaviors and restricted interests interfere with adaptive functioning, social interactions, and learning. No medications are FDA-approved for autistic disorder, but some selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics and an anticonvulsant have reduced repetitive behaviors in controlled trials. We discuss how that evidence shapes our approach to patients with or without a comorbid family history of bipolar disorder.
Evidence for SSRIs
Repetitive behaviors and restricted interests are autism’s third core domain, as defined by DSM-IVTR criteria.1 For an autistic disorder diagnosis, a patient must show at least one of these behaviors:
- encompassing preoccupations with stereotyped or restricted patterns of interest
- inflexible routines or rituals
- stereotyped, repetitive motor mannerisms
- or persistent preoccupation with parts of objects.
As in obsessive-compulsive disorder (OCD), rituals and restricted interests are thought to decrease anxiety in autism, whereas self-stimulatory behaviors and stereotypy may regulate arousal. The behaviors persist2,3 but may change across the lifespan.
Because SSRIs improve OCD’s repetitive behaviors, clinicians have also used them to treat autism’s repetitive behaviors, though without supporting data. Recently, however, fluoxetine and fluvoxamine have shown efficacy for autism’s repetitive behaviors in randomized, controlled trials. Results indicate:
- In children, fluoxetine is probably better-tolerated than available dosing forms of fluvoxamine.
- In adults, fluvoxamine is well-tolerated and can improve repetitive behavior.
SSRIs and suicidal ideation in autism. The increased risk of suicidality reported with SSRIs when treating depression and OCD has not been seen in children with autism. But because fewer children with autism have been treated with SSRIs, we recommend that you try to assess suicidal ideation during SSRI treatment in those able to express such concerns (Box). Starting with low SSRI dosages (Table 1) and increasing slowly may help prevent behavioral activation, a possible risk factor for suicidality.
Suicidal ideation has not been reported in studies of selective serotonin reuptake inhibitors (SSRIs) in autism. Even so, children and adolescents with autistic disorder are not excluded from the FDA black-box warning of increased risk of suicidality with SSRIs.
Children with obsessive-compulsive disorder (OCD) treated with SSRIs have shown evidence of suicidal thoughts. Thus, higher-functioning children and adults with autism might think about suicide when they become aware of their deficits.
For lower-functioning patients (generally, those who receive medication), we need markers of possible suicidal ideation other than their reports of symptoms. In clinical trials, investigators measure behavioral activation symptoms as risk factors for suicidality.
Thus, when you start an SSRI in a patient with autistic disorder, educate the caregivers to watch for agitation, increased energy, poor sleep, disinhibition, or new hyperactivity. Encourage them to contact you immediately if these signs of activation occur.
Ask higher-functioning patients taking SSRIs about suicidal thinking in a step-wise fashion: thoughts of death, thoughts of their own death, intent, plan, and finally possible attempts.
Table 1
4 drugs with evidence of benefit for autism’s repetitive behaviors*
| Medication | Suggested target daily dosage |
|---|---|
| Fluoxetine7 | Children: Start at 2.5 mg/d; maximum 20 mg/d |
| Adults: Start at 10 to 20 mg/d; maximum 60 mg/d | |
| Fluvoxamine10 | Children: Not first-line; start at 12.5 mg/d; maximum 150 to200 mg/d |
| Adults: Start at 25 mg/d; maximum 300 mg/d | |
| Risperidone13,16 | Children: Start at 0.25 mg/d; maximum 3 mg/d |
| Adults: Start at 2 mg/d; maximum 4 mg/d | |
| Valproate20 | Children: Start at 125 mg (sprinkles); titrate to clinical effect and blood levels of 50 of 120 mcg/mL |
| Adults: Start at 250 mg; increase by 250mg/week to clinical effect and blood levels of 50 to 120 mcg/mL | |
| *Data from randomized, placebo-controlled trials | |
Fluoxetine. In the first open-label study of fluoxetine in children and adults with autistic disorder, global functioning improved significantly in 15 of 23 patients, as measured by the Clinical Global Impressions (CGI) scale.4 Autism symptoms also improved in follow-up, open-label trials, but these did not target repetitive behaviors specifically.5,6
Our group conducted the first randomized, placebo-controlled study of fluoxetine’s effect on repetitive behaviors in children with autism.7 We measured obsessions and compulsions in 45 children, ages 5 to 16, with the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS). This 10-item, clinician-rated questionnaire uses a 5-point scale to rate repetitive behaviors by time spent, distress, interference, resistance, and control.
Using a crossover design—two 8-week phases of active or placebo treatment separated by a 4-week washout—we started liquid fluoxetine at 2.5 mg/d and slowly increased the dosage to clinical effect or a maximum of 0.8 mg/kg/day. Mean final dosage was 9.9 (±4.35) mg/d.
Repetitive behaviors improved, even though we used relatively low dosages to avoid side effects. The mean baseline CY-BOCS compulsion score of 13.15 dropped to 11.6 with fluoxetine and to 12.9 with placebo. Fluoxetine’s effect size was moderate to large, and we found no suicidal ideation with this SSRI.
Fluvoxamine. Repetitive behaviors did not change—as measured with the CY-BOCS—when 18 children with autism received fluvoxamine, 1.5 mg/kg/day, in a 10-week prospective, open-label trial.8 Most patients (72%) reported at least one side effect, and 3 discontinued the SSRI because of behavioral activation. Ten completed the trial. Likewise in a randomized trial, children who received fluvoxamine experienced troublesome side effects and limited benefit.9
Compared with outcomes in children, fluvoxamine has shown greater efficacy in adults. In a 12-week, double-blind, placebo-controlled trial of 30 adults with autism, 8 of 15 treated with fluvoxamine (50 mg/d initially and titrated to 300 mg/d) were rated as responders, compared with none of 15 receiving placebo.
Repetitive behaviors and adaptive functioning improved significantly with fluvoxamine, as measured with the Yale-Brown Obsessive Compulsive Scale (YBOCS) and Vineland Adaptive Behavior Scale, respectively.10 The SSRI was well-tolerated, with only mild nausea and sedation reported.
Thus, fluvoxamine may be useful for treating repetitive behaviors but probably is not a first choice for children with autism. Results might be more favorable in children if fluvoxamine were available in doses <12.5 mg.
Citalopram. Open-label data support using citalopram in autism.11 In a retrospective chart review, 10 of 15 children (73%) were reported “much improved” with citalopram (mean dosage 16.9 mg/d [+/-12.1]), but the review did not specifically address repetitive behaviors. Two patients stopped taking the SSRI because of side effects; agitation, aggressiveness, sedation, and lip dyskinesia were reported.
The National Institutes of Health is sponsoring a multicenter trial of citalopram (starting dosage 2.5 mg/d, up to 20 mg/d) for repetitive behaviors in 144 children with autism, Results are expected in 2007 (see Related resources).
Escitalopram. Some early, open-label evidence suggests that escitalopram may be well-tolerated in autism, but its efficacy for treating repetitive behaviors has not been studied.
Sertraline. One of three reported open-label studies of sertraline in patients with autism measured repetitive behaviors.12 In this study, 42 adults with autism spectrum disorder were treated for 12 weeks with sertraline, 50 to 200 mg/d. One-half were rated “much improved”—mostly in aggressive and repetitive behaviors—with the CGI improvement scale. Sertraline was well-tolerated, although 3 patients dropped out because of persistent agitation.
No randomized trials have examined sertraline in autism.
Clinical recommendations
Family history of bipolar disorder guides our treatment of patients with an autism spectrum disorder and disabling repetitive behaviors (Algorithm).
Algorithm Suggested medications to treat repetitive behaviors in autism

*Risperidone has the most evidence of efficacy, but aripiprazole may be useful for patients with weight-gain problems.
†For children, controlled data support using liquid fluoxetine, starting at 2.5 mg/d.Without bipolar history. SSRIs are usually first-line therapy (although patients with significant irritability and aggression may be an exception and require an atypical antipsychotic first).
If you reach the maximal SSRI dosage without a desired effect, consider adding an atypical (risperidone has the strongest supporting data) or valproate. If behavioral activation symptoms emerge and a lower dosage does not ameliorate them or reduces the clinical effect, consider switching to an atypical or valproate.
With bipolar history. Consider starting with an atypical or valproate; augment with an SSRI if needed.
Monitor for side effects with each medication (Table 2).
Table 2
Medication side effects and recommended monitoring
| Medication | Side effects | Recommended monitoring |
|---|---|---|
| Fluoxetine | Anxiety, insomnia, GI disturbance, appetite and weight changes, mania/hypomania activation, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Fluvoxamine | Somnolence, nervousness, insomnia, agitation, GI disturbance, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Risperidone | Drowsiness, weight gain, hyperglycemia, GI disturbance, extrapyramidal symptoms, neuroleptic malignant syndrome | Obtain metabolic profile, including serum glucose and lipids |
| Monitor for weight gain and clinical signs of extrapyramidal symptoms | ||
| Valproate | Rash, headaches, weight gain, ataxia, alopecia, GI disturbance, hyperammonemic encephalopathy, sedation, thrombocytopenia, polycystic ovary syndrome, pancreatitis, liver failure, teratogenic effects | CBC with platelets, liver function tests, valproate levels |
| Therapeutic blood levels: 50 to 120 mcg/mL |
Evidence for atypical antipsychotics
Atypicals have been used in autistic disorder to treat irritability and impulsive aggression. Risperidone also has been shown to reduce repetitive behaviors in a controlled trial.13 No evidence or only open-label trials support the use of other atypicals in patients with autism.
Risperidone’s side effects may include metabolic syndrome. No studies have examined lipid profiles and insulin resistance after risperidone treatment in patients with autism, but weight gain has been reported in the Research Units on Pediatric Psychopharmacology (RUPP) trial andothers.13-15 Carefully assess the risk-benefit ratio when you consider using risperidone to treat repetitive behaviors in patients with autism.
Atypical antipsychotics also may increase dyskinesia risk, although extrapyramidal symptoms (EPS) have not been reported in studies of patients with autism and repetitive behaviors. Because EPS could develop after clinical trials are completed, long-term naturalistic studies are needed to address this concern.
Risperidone. The double-blind, placebo-controlled RUPP trial examined risperidone’s efficacy in treating autism’s core symptoms (primarily irritability) in 101 children.13 Mean dosages after 8 weeks and during a 16-week open-label extension for 63 children were 2 and 2.1 mg/d, respectively.
Repetitive behavior—as measured with the CY-BOCS, using RUPP trial data16—improved significantly with risperidone compared with placebo. During the 8-week controlled trial, CY-BOCS scores improved from 15.51 (SD±2.73) to 11.65 (SD±4.02) in the risperidone group, compared with 15.18 (SD±3.88) to 14.21 (SD±4.81) in the placebo group. This response was maintained through the open-label trial.
Side effects included weight gain, fatigue, drowsiness, and drooling. No children receiving risperidone dropped out because of side effects. No EPS were reported, based on weekly Abnormal Involuntary Movement scale and Simpson-Angus scale scores.
Olanzapine. Only open-label studies have examined olanzapine in autism, and one systematically measured repetitive behaviors.17 Eight children with autism or other pervasive developmental disorders were given olanzapine, mean dosage 7.8 (±4.7) mg/d at the end of the 12-week trial.
Repetitive behaviors did not change significantly, as measured with YBOCS. Seven of eight patients completed the trial. Mean weight for the group after 12 weeks was 156±55 lbs, compared with 137±56 lbs at baseline.
Quetiapine. No data support using quetiapine for autism’s repetitive behaviors. Quetiapine, 100 to 350 mg/d (1.6 to 5.2 mg/kg/day) was poorly tolerated in a 16-week open-label safety and efficacy trial among 6 mentally retarded boys with autistic disorder. Side effects included a possible seizure, behavioral activation, increased appetite, and weight gain (0.9 to 8.2 kg). Two patients completed the trial.18
Ziprasidone. Small open-label studies and anecdotal reports of ziprasidone in autism have not examined this drug’s effect on repetitive behaviors.
Aripiprazole. Anecdotal information suggests that clinicians are using this medication to treat patients with autism, but no supporting data exist.
Evidence for valproate
Preliminary trials by our group suggest that valproate may reduce repetitive behaviors in autism. In a retrospective, open-label study, 14 patients (mean age 17) with autism spectrum disorder received divalproex sodium (mean 768±582 mg/d) for a mean 11 months. Ten patients (71%) showed sustained improvement in function, as measured by the CGI-improvement scale, and valproate was generally well-tolerated.19
We then measured valproate’s effect on repetitive behaviors in an 8-week, double-blind, placebo-controlled study of 13 patients (mean age 9) with autism spectrum disorder. Repetitive behaviors improved significantly compared with placebo, as measured by the CY-BOCS, in those who received divalproex (mean 833.93±326.21 mg/d).20
Further studies are needed to replicate this finding. Although it is too early to make general recommendations, valproate may be a reasonable choice for children with autism and epilepsy or affective instability.
- Hollander E, Phillips AT, Yeh CC. Targeted treatments for symptom domains in child and adolescent autism. Lancet 2003;362:732-4.
- Hollander E (ed). Autism spectrum disorders. New York: Marcel Decker; 2003.
- National Institute of Health multicenter study of citalopram for repetitive behaviors in autism. http://www.clinicaltrials.gov/ct/show/nct00086645?order=2
Drug brand names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Valproic acid • Depakote, Depakene
- Ziprasidone • Geodon
Disclosures
Dr. Anagnostou reports no financial interest with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Hollander receives research/grant support from and is a consultant to Abbott Laboratories. He also receives support from the National Institutes of Health (STAART Center) to investigate orphan drug status for fluoxetine in treating autism symptoms.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC; American Psychiatric Association; 2000.
2. Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. MRDD Research Reviews 2004;10:234-47.
3. Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004;45:212-29.
4. Cook EH, Rowlett R, Jaselinkis C, Leventhal BL. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am Acad Child Adolesc Psychiatry 1992;31:739-45.
5. DeLong GR, Teague LA, Kamran MM. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol 1998;40:551-62.
6. DeLong GR, Ritch CR, Burch S. Fluoxetine response in children with autistic spectrum disorders: correlation with familial major affective disorder and intellectual achievement. Dev Med Child Neurol 2002;44:652-9.
7. Hollander E, Phillips A, Chaplin W, et al. A placebo-controlled cross over trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology 2005;30:582-9.
8. Martin A, Koenig K, Anderson GM, Scahill L. Low-dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: a prospective, open-label study. J Autism Dev Disord 2003;33:77-85.
9. McDougle CJ, Kresch LE, Posey DJ. Repetitive thoughts and behavior in pervasive developmental disorders: treatment with serotonin reuptake inhibitors. J Autism Dev Disord 2000;30:427-35.
10. McDougle CJ, Naylor ST, Cohen DJ, et al. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996;53:1001-8.
11. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr. 2003;24(2):104-8.
12. McDougle CJ, Brodkin ES, Naylor ST, et al. Sertraline in adults with pervasive developmental disorders: a prospective open-label investigation. J Clin Psychopharmacol 1998;18:62-6.
13. McCracken JT, McGough J, Shah B, et al. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347:314-21.
14. Troost PW, Lahuis BE, Steenhuis MP, et al. Long-term effects of risperidone in children with autism spectrum disorders: A placebo discontinuation study. J Am Acad Child Adolesc Psychiatry 2005;44:1137-44.
15. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 2004;114:e634-e641.
16. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162:1142-8.
17. Potenza MN, Holmes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol 1999;19:37-44.
18. Martin A, Koenig K, Scahill L, Bregman J. Open-label quetiapine in the treatment of children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol 1999;9:99-107.
19. Hollander E, Dolgoff-Kaspar R, Cartwright C, et al. An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry 2001;62:530-4.
20. Hollander E, Soorya LV, Wasserman S, et al. Divalproex sodium vs. placebo in the treatment of repetitive behaviours in autism spectrum disorder. Int J Neuropsychopharmacol 2006;9:209-13.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC; American Psychiatric Association; 2000.
2. Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. MRDD Research Reviews 2004;10:234-47.
3. Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004;45:212-29.
4. Cook EH, Rowlett R, Jaselinkis C, Leventhal BL. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am Acad Child Adolesc Psychiatry 1992;31:739-45.
5. DeLong GR, Teague LA, Kamran MM. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol 1998;40:551-62.
6. DeLong GR, Ritch CR, Burch S. Fluoxetine response in children with autistic spectrum disorders: correlation with familial major affective disorder and intellectual achievement. Dev Med Child Neurol 2002;44:652-9.
7. Hollander E, Phillips A, Chaplin W, et al. A placebo-controlled cross over trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology 2005;30:582-9.
8. Martin A, Koenig K, Anderson GM, Scahill L. Low-dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: a prospective, open-label study. J Autism Dev Disord 2003;33:77-85.
9. McDougle CJ, Kresch LE, Posey DJ. Repetitive thoughts and behavior in pervasive developmental disorders: treatment with serotonin reuptake inhibitors. J Autism Dev Disord 2000;30:427-35.
10. McDougle CJ, Naylor ST, Cohen DJ, et al. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996;53:1001-8.
11. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr. 2003;24(2):104-8.
12. McDougle CJ, Brodkin ES, Naylor ST, et al. Sertraline in adults with pervasive developmental disorders: a prospective open-label investigation. J Clin Psychopharmacol 1998;18:62-6.
13. McCracken JT, McGough J, Shah B, et al. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347:314-21.
14. Troost PW, Lahuis BE, Steenhuis MP, et al. Long-term effects of risperidone in children with autism spectrum disorders: A placebo discontinuation study. J Am Acad Child Adolesc Psychiatry 2005;44:1137-44.
15. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 2004;114:e634-e641.
16. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162:1142-8.
17. Potenza MN, Holmes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol 1999;19:37-44.
18. Martin A, Koenig K, Scahill L, Bregman J. Open-label quetiapine in the treatment of children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol 1999;9:99-107.
19. Hollander E, Dolgoff-Kaspar R, Cartwright C, et al. An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry 2001;62:530-4.
20. Hollander E, Soorya LV, Wasserman S, et al. Divalproex sodium vs. placebo in the treatment of repetitive behaviours in autism spectrum disorder. Int J Neuropsychopharmacol 2006;9:209-13.