User login
Debates in Hospital Medicine
At a hospital at which I work, every patient who presents to the emergency department with a suspected stroke or transient ischemic attack is evaluated by the stroke team. Per protocol, the team rapidly assesses each patient, orders diagnostic and therapeutic interventions and then refers each and every patient to the hospitalist service for admission and medical comanagement. At no point is any consideration given to whether the patients actually have medical comorbidities, or if a hospitalist will have anything meaningful to add to the care. The firmly set expectation is that hospitalists admit all stroke patients for the purposes of comanagement, while the neurologists consult.
Comanagement has become a mainstay of hospital medicine.1 It is predicated upon the assumption that surgical and specialty patients benefit when their medical comorbidities are managed by hospitalists. It differs conceptually from traditional medical consultation in that hospitalists collaboratively manage patients with surgeons or specialists, sharing responsibility and authority. In practice, however, comanagement varies widely, ranging from a model of care indistinguishable from traditional medical consultation to one where hospitalists admit and assume primary responsibility for surgical and specialty patients. This variability makes it difficult to study and make generalizations about the role and impact of hospitalist comanagement. Nonetheless, recent evidence suggests that hospitalist consultation and comanagement may not be as effective as originally anticipated.
In a 2008 observational cohort study of patients undergoing surgery at an academic medical center, Auerbach et al demonstrated that medical consultation (provided by hospitalists) did not improve glycemic control or increase the likelihood of perioperative beta‐blockade and venous thromboembolism prophylaxis.2 Patients who received consultation had longer adjusted lengths of stay (12.98% longer; 95% confidence interval, 1.61%‐25.61%) and higher adjusted costs (24.36% higher; 95% confidence interval, 13.54%‐36.34%). Notwithstanding the limited generalizability of this study to community hospitals, it has raised concerns that hospitalist consultation does not automatically improve quality of care or cost effectiveness.3
Several other recent trials have also helped to define where hospitalist comanagement may work well and where it may not. In 2004, Huddleston et al published the Hospitalist Orthopedic Team (HOT) trial, the first randomized prospective trial comparing hospitalist‐surgical comanagement to standard care.4 A total of 526 patients undergoing elective hip or knee replacement surgery at the Mayo Clinic were randomized to either standard orthopedic care with consultation as needed, or immediate hospitalist comanagement. The outcomes were disappointing. Hospitalist comanagement reduced minor complications (such as incidence of urinary tract infections, fever, and hyponatremia) but had no effect on moderate or major complications. The HOT intervention modestly reduced adjusted length of stay (LOS), defined as the point at which patients were deemed stable for discharge, by 0.5 days, but had no impact on actual LOS or cost per case. Not surprisingly, orthopedic surgeons and nurses preferred the HOT model of care over the standard model. One year later, Phy et al analyzed outcomes for patients admitted with hip fracture at the same institution.5 This retrospective cohort study compared patients who were admitted to either a standard orthopedic service or to a hospitalist team. In contrast to the HOT trial, hospitalist comanagement of hip fracture patients decreased time to surgery and lowered LOS by 2.2 days without compromising patient outcomes.
How did two trials that occurred roughly simultaneously at the same hospital, involving the same hospitalists and orthopedic surgeons generate such different outcomes? A likely answer is patient selection. Patients who undergo elective joint replacement are usually relatively healthy. They are almost always ambulatory and their comorbidities, when present, are generally reasonably compensated. As a rule, they fare well postoperatively, as evidenced by the 1.3% major complication rate demonstrated in the HOT trial.3 In contrast, hip fracture patients are older, have greater comorbidity and are at remarkably high risk for developing perioperative delirium.3, 4, 6 By definition, their urgent/emergent hip surgery stratifies them to a higher operative risk category than patients who undergo elective joint replacement.7 Half of hip fracture patients do not return to premorbid levels of function, and the 1‐year mortality rate has been estimated to be as high as 25%.6, 8 Given these differences, it is not surprising that hip fracture patients are more likely than elective joint replacement patients to respond favorably to hospitalist comanagement.
In 2007, Simon et al published a retrospective study of 739 pediatric spinal fusion patients at Childrens' Hospital in Denver.9 Beginning in 2004, hospitalists comanaged selected, high‐risk surgical patients (14 of 115 spinal fusion patients, or 12%). Over the course of the study, the mean LOS for low‐risk patients decreased by 21% but the mean LOS for the high‐risk, hospitalist‐comanaged patients decreased by 28%; a 33% relative reduction favoring hospitalist‐managed patients. By targeting selected high‐risk patients, pediatric hospitalists were able to improve upon LOS reductions that occurred systemically across the entire spinal fusion program. Also in 2007, Southern et al compared outcomes for 2,913 patients admitted by full‐time teaching hospitalists vs 6,124 patients admitted by nonhospitalists at Montefiore Medical Center, Bronx, New York.10 Mean LOS for patients admitted to the hospitalist service was 5.01 days vs 5.87 days for the nonhospitalists. Subgroup analysis demonstrated the greatest LOS differentials for patients requiring close clinical monitoring (heart failure, stroke, asthma, or pneumonia) or complex discharge planning.
Although these studies, performed at large academic medical centers, may have limited generalizability, they support the common‐sense notion that hospitalists most benefit patients who are sick, frail, and medically or socially complex. As a corollary, hospitalists probably offer relatively little benefit to surgical and specialty patients who are young or have compensated medical comorbidities and/or straightforward disposition plans. The enormous variability across healthcare institutions makes it difficult if not impossible to define a patient acuity or complexity cutoff below which hospitalist comanagement is unlikely to be beneficial. Nonetheless, some degree of common sense can be applied. As a case in point, a hospitalist probably adds little value to the care of a basically healthy patient with a hemodynamically stable upper gastrointestinal bleed. Despite this, in many institutions, hospitalists admit or comanage all gastroenterology patients, irrespective of their diagnosis, acuity, or complexity.11
One can even hypothesize that hospitalist comanagement may potentially inject risk into patient care. Admitting that patient with a stable upper gastrointestinal bleed to a hospitalist service may delay the gastroenterologist's involvement and initiation of the necessary endoscopy. Having assumed that the hospitalist is running the show, the gastroenterologist may pay insufficient attention to the patient. The hospitalist and gastroenterologist may give conflicting orders and reports that confuse patients, families, and hospital staff, ultimately increasing the likelihood of medical errors.
Ultimately, the risks inherent in adding complexity into patient care must be balanced against the potential benefits. For patients who are sick, frail, or complicated, the risk‐benefit ratio probably tilts in favor of comanagement. However, for generally healthy patients, it is conceivable that adding complexity negates (or worse yet, exceeds) the putative benefits of comanagement.
Given the potential limitations of hospitalist comanagement, why are hospitalists admitting or managing broad and unselected populations of surgical and specialty patients? Hospital leaders have suggested that hospitalist comanagement may protect overstretched surgeons and specialists and extend their capacity. A hospital with only one neurosurgeon on staff might reasonably ask its hospitalists to primarily manage carefully selected low‐acuity neurosurgical patients, allowing the neurosurgeon to serve as a consultant. However, in communities where specialists and surgeons are abundant, this justification is less credible. In such cases, it is difficult not to suspect that the primary reason that hospitalists admit surgical and specialty patients is to enhance the income and quality of life of the surgeons and specialists.
Expanding hospitalist comanagement services for no other reason than to keep specialists and surgeons happy might be justifiable if hospital medicine was not faced with its own critical manpower shortage. Hospital medicine is expected to grow from approximately 20,000 current practitioners to more than 40,000 within a decade.12 The growing shortage of qualified hospitalists has become a preoccupation for hospitalist employers across the country.13 At its 2006 strategic planning retreat, the Board of Directors of the Society of Hospital Medicine identified this issue as one of the greatest threats to the future health of hospital medicine.14 Demand for hospitalists will not abate for at least a decade, which will leave many hospitalist programs significantly understaffed for the foreseeable future. Understaffing forces hospitalist programs to lower hiring standards, jeopardizes patient care, accelerates physician burnout, and may ultimately destabilize hospital medicine.15 Understaffed hospitalist programs should be very circumspect about how and where they expand their clinical coverage.
Another principle underlying hospitalist comanagement is that it improves care by allowing surgeons and specialists to focus on their areas of expertise. Surgeons and specialists who do not have to manage their patients' medical issues can presumably spend more time focusing on their own disciplines. Although this argument is conceptually appealing, there is no evidence that this actually occurs. In fact, it is equally conceivable that hospitalist comanagement could jeopardize care by disengaging surgeons and specialists from their patients' progress (or lack thereof). Furthermore, evidence suggests that hospitalists are underprepared to manage diagnoses that have historically been the purview of surgeons and specialists. Practicing hospitalists who manage acute neurological and neurosurgical conditions, orthopedic trauma, and acute psychiatric illnesses have reported relative undertraining in all of these disease states.10, 16 Generally, hospitalists are expected to deliver this care in the absence of any regime to assess their competence, provide targeted training to fill knowledge gaps, and monitor their progress. At minimum, this should raise concerns about the quality and consistency of care that hospitalists provide to nonmedical patients.
Finally, working collaboratively with other specialties should be a major professional benefit of comanagement. In well‐designed comanagement arrangements, hospitalists and specialists work equitably under clearly defined and mutually agreed upon rules of engagement. They share responsibility for patients, collaborate to improve care, and teach and learn from each other. Unfortunately, in many instances, the power structure becomes lopsided, with surgeons and specialists dictating how, when, and why hospitalists manage their patients.17 Emergency departments have learned to default surgical and specialty patient admissions to hospitalists when surgeons and specialists balk. Hospital administrations may tacitly or overtly expect their financially subsidized hospitalists to cheerfully accept any and all referrals, irrespective of how inappropriate they may be. Practicing hospitalists frequently complain about their subordinate status and inability to control their working conditions, both of which are identified risk factors for career dissatisfaction and burnout.14, 16, 18 Once again, as a specialty facing a critical manpower shortage, hospitalist programs should be very attuned to defining work conditions that foster career satisfaction and physician retention.
REFRAMING COMANAGEMENT
The history of healthcare is laden with examples of new ideas that were widely and indiscriminately adopted only to subsequently fail to withstand rigorous scrutiny.19, 20 The unchecked expansion of hospitalist comanagement has the potential to become another case in point. In the absence of clear definitions of comanagement and good evidence to define best practices, hospitalists are left to use their best judgment to define the parameters of their comanagement services. At minimum, hospitalist leaders should ask some basic questions as they ponder potential comanagement relationships:
-
Why are we being asked to provide this service?
-
Do the patients have comorbidities that require our input?
-
Is there a legitimate quality or efficiency case to be made to support our participation?
-
Do we have the manpower to provide the service? If not, what will suffer as a result?
-
Will the relationship be equitable?
-
What might go wrong?
Comanagement is an appealing construct that has grown to fill many niches of healthcare delivery.10 Given compelling reasons to be skeptical about the purported benefits of comanagement, hospitalists should be circumspect about how and where they offer such services. Comanagement should be applied carefully and methodically, paying close attention to the consequences, intended and unintended. Applying comanagement in a rational, evidence‐based, and sustainable fashion will ultimately better serve patients, the healthcare community, and hospital medicine.
- Society of Hospital Medicine. The Society of Hospital Medicine 2005–2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement. Published by the, 2006. Executive summary available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2167(21):2338–2344.
- .Exceed acceptable: new studies challenge hospitalists to prove our value.Hospitalist.2008;12(2):63.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,.Treatment and survival among elderly Americans with hip fractures: a population‐based study.Am J Public Health.1994;84:1287–1291.
- ,,, et al.Predicting cardiac complications in patients undergoing non‐cardiac surgery.J Gen Intern Med.1986;1:211–219.
- ,,,,.Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study.J Gerontol.1990;45(3):M101–M107.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2:23–29.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,,,,.The spectrum of community‐based hospitalist practice, a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNation wide/Growth_of_Hospital_M.htm. Accessed September 2,2008.
- ,,, et al.Rebuilding the future of the private practice of hospital medicine.The Phoenix Group, May2007.
- Society of Hospital Medicine Board of Directors Strategic Planning Retreat: November 28‐29,2006.
- ,,,,,,for the SGIM Career Satisfaction Study Group.Physician stress: results from the physician worklife study.Stress Health.2001;18(1):37–42.
- ,,, et al.Hospitalist's perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- .Feeling pressure to admit surgical patients? Hospitalists work to set limits on co‐management arrangements.Today's Hospitalist. January2008.
- Society of Hospital Medicine. Career Satisfaction White Paper. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources321:406–412.
- .Pulmonary‐artery catheters—peace at last?N Engl J Med.2006;354(21):2273–2274.
At a hospital at which I work, every patient who presents to the emergency department with a suspected stroke or transient ischemic attack is evaluated by the stroke team. Per protocol, the team rapidly assesses each patient, orders diagnostic and therapeutic interventions and then refers each and every patient to the hospitalist service for admission and medical comanagement. At no point is any consideration given to whether the patients actually have medical comorbidities, or if a hospitalist will have anything meaningful to add to the care. The firmly set expectation is that hospitalists admit all stroke patients for the purposes of comanagement, while the neurologists consult.
Comanagement has become a mainstay of hospital medicine.1 It is predicated upon the assumption that surgical and specialty patients benefit when their medical comorbidities are managed by hospitalists. It differs conceptually from traditional medical consultation in that hospitalists collaboratively manage patients with surgeons or specialists, sharing responsibility and authority. In practice, however, comanagement varies widely, ranging from a model of care indistinguishable from traditional medical consultation to one where hospitalists admit and assume primary responsibility for surgical and specialty patients. This variability makes it difficult to study and make generalizations about the role and impact of hospitalist comanagement. Nonetheless, recent evidence suggests that hospitalist consultation and comanagement may not be as effective as originally anticipated.
In a 2008 observational cohort study of patients undergoing surgery at an academic medical center, Auerbach et al demonstrated that medical consultation (provided by hospitalists) did not improve glycemic control or increase the likelihood of perioperative beta‐blockade and venous thromboembolism prophylaxis.2 Patients who received consultation had longer adjusted lengths of stay (12.98% longer; 95% confidence interval, 1.61%‐25.61%) and higher adjusted costs (24.36% higher; 95% confidence interval, 13.54%‐36.34%). Notwithstanding the limited generalizability of this study to community hospitals, it has raised concerns that hospitalist consultation does not automatically improve quality of care or cost effectiveness.3
Several other recent trials have also helped to define where hospitalist comanagement may work well and where it may not. In 2004, Huddleston et al published the Hospitalist Orthopedic Team (HOT) trial, the first randomized prospective trial comparing hospitalist‐surgical comanagement to standard care.4 A total of 526 patients undergoing elective hip or knee replacement surgery at the Mayo Clinic were randomized to either standard orthopedic care with consultation as needed, or immediate hospitalist comanagement. The outcomes were disappointing. Hospitalist comanagement reduced minor complications (such as incidence of urinary tract infections, fever, and hyponatremia) but had no effect on moderate or major complications. The HOT intervention modestly reduced adjusted length of stay (LOS), defined as the point at which patients were deemed stable for discharge, by 0.5 days, but had no impact on actual LOS or cost per case. Not surprisingly, orthopedic surgeons and nurses preferred the HOT model of care over the standard model. One year later, Phy et al analyzed outcomes for patients admitted with hip fracture at the same institution.5 This retrospective cohort study compared patients who were admitted to either a standard orthopedic service or to a hospitalist team. In contrast to the HOT trial, hospitalist comanagement of hip fracture patients decreased time to surgery and lowered LOS by 2.2 days without compromising patient outcomes.
How did two trials that occurred roughly simultaneously at the same hospital, involving the same hospitalists and orthopedic surgeons generate such different outcomes? A likely answer is patient selection. Patients who undergo elective joint replacement are usually relatively healthy. They are almost always ambulatory and their comorbidities, when present, are generally reasonably compensated. As a rule, they fare well postoperatively, as evidenced by the 1.3% major complication rate demonstrated in the HOT trial.3 In contrast, hip fracture patients are older, have greater comorbidity and are at remarkably high risk for developing perioperative delirium.3, 4, 6 By definition, their urgent/emergent hip surgery stratifies them to a higher operative risk category than patients who undergo elective joint replacement.7 Half of hip fracture patients do not return to premorbid levels of function, and the 1‐year mortality rate has been estimated to be as high as 25%.6, 8 Given these differences, it is not surprising that hip fracture patients are more likely than elective joint replacement patients to respond favorably to hospitalist comanagement.
In 2007, Simon et al published a retrospective study of 739 pediatric spinal fusion patients at Childrens' Hospital in Denver.9 Beginning in 2004, hospitalists comanaged selected, high‐risk surgical patients (14 of 115 spinal fusion patients, or 12%). Over the course of the study, the mean LOS for low‐risk patients decreased by 21% but the mean LOS for the high‐risk, hospitalist‐comanaged patients decreased by 28%; a 33% relative reduction favoring hospitalist‐managed patients. By targeting selected high‐risk patients, pediatric hospitalists were able to improve upon LOS reductions that occurred systemically across the entire spinal fusion program. Also in 2007, Southern et al compared outcomes for 2,913 patients admitted by full‐time teaching hospitalists vs 6,124 patients admitted by nonhospitalists at Montefiore Medical Center, Bronx, New York.10 Mean LOS for patients admitted to the hospitalist service was 5.01 days vs 5.87 days for the nonhospitalists. Subgroup analysis demonstrated the greatest LOS differentials for patients requiring close clinical monitoring (heart failure, stroke, asthma, or pneumonia) or complex discharge planning.
Although these studies, performed at large academic medical centers, may have limited generalizability, they support the common‐sense notion that hospitalists most benefit patients who are sick, frail, and medically or socially complex. As a corollary, hospitalists probably offer relatively little benefit to surgical and specialty patients who are young or have compensated medical comorbidities and/or straightforward disposition plans. The enormous variability across healthcare institutions makes it difficult if not impossible to define a patient acuity or complexity cutoff below which hospitalist comanagement is unlikely to be beneficial. Nonetheless, some degree of common sense can be applied. As a case in point, a hospitalist probably adds little value to the care of a basically healthy patient with a hemodynamically stable upper gastrointestinal bleed. Despite this, in many institutions, hospitalists admit or comanage all gastroenterology patients, irrespective of their diagnosis, acuity, or complexity.11
One can even hypothesize that hospitalist comanagement may potentially inject risk into patient care. Admitting that patient with a stable upper gastrointestinal bleed to a hospitalist service may delay the gastroenterologist's involvement and initiation of the necessary endoscopy. Having assumed that the hospitalist is running the show, the gastroenterologist may pay insufficient attention to the patient. The hospitalist and gastroenterologist may give conflicting orders and reports that confuse patients, families, and hospital staff, ultimately increasing the likelihood of medical errors.
Ultimately, the risks inherent in adding complexity into patient care must be balanced against the potential benefits. For patients who are sick, frail, or complicated, the risk‐benefit ratio probably tilts in favor of comanagement. However, for generally healthy patients, it is conceivable that adding complexity negates (or worse yet, exceeds) the putative benefits of comanagement.
Given the potential limitations of hospitalist comanagement, why are hospitalists admitting or managing broad and unselected populations of surgical and specialty patients? Hospital leaders have suggested that hospitalist comanagement may protect overstretched surgeons and specialists and extend their capacity. A hospital with only one neurosurgeon on staff might reasonably ask its hospitalists to primarily manage carefully selected low‐acuity neurosurgical patients, allowing the neurosurgeon to serve as a consultant. However, in communities where specialists and surgeons are abundant, this justification is less credible. In such cases, it is difficult not to suspect that the primary reason that hospitalists admit surgical and specialty patients is to enhance the income and quality of life of the surgeons and specialists.
Expanding hospitalist comanagement services for no other reason than to keep specialists and surgeons happy might be justifiable if hospital medicine was not faced with its own critical manpower shortage. Hospital medicine is expected to grow from approximately 20,000 current practitioners to more than 40,000 within a decade.12 The growing shortage of qualified hospitalists has become a preoccupation for hospitalist employers across the country.13 At its 2006 strategic planning retreat, the Board of Directors of the Society of Hospital Medicine identified this issue as one of the greatest threats to the future health of hospital medicine.14 Demand for hospitalists will not abate for at least a decade, which will leave many hospitalist programs significantly understaffed for the foreseeable future. Understaffing forces hospitalist programs to lower hiring standards, jeopardizes patient care, accelerates physician burnout, and may ultimately destabilize hospital medicine.15 Understaffed hospitalist programs should be very circumspect about how and where they expand their clinical coverage.
Another principle underlying hospitalist comanagement is that it improves care by allowing surgeons and specialists to focus on their areas of expertise. Surgeons and specialists who do not have to manage their patients' medical issues can presumably spend more time focusing on their own disciplines. Although this argument is conceptually appealing, there is no evidence that this actually occurs. In fact, it is equally conceivable that hospitalist comanagement could jeopardize care by disengaging surgeons and specialists from their patients' progress (or lack thereof). Furthermore, evidence suggests that hospitalists are underprepared to manage diagnoses that have historically been the purview of surgeons and specialists. Practicing hospitalists who manage acute neurological and neurosurgical conditions, orthopedic trauma, and acute psychiatric illnesses have reported relative undertraining in all of these disease states.10, 16 Generally, hospitalists are expected to deliver this care in the absence of any regime to assess their competence, provide targeted training to fill knowledge gaps, and monitor their progress. At minimum, this should raise concerns about the quality and consistency of care that hospitalists provide to nonmedical patients.
Finally, working collaboratively with other specialties should be a major professional benefit of comanagement. In well‐designed comanagement arrangements, hospitalists and specialists work equitably under clearly defined and mutually agreed upon rules of engagement. They share responsibility for patients, collaborate to improve care, and teach and learn from each other. Unfortunately, in many instances, the power structure becomes lopsided, with surgeons and specialists dictating how, when, and why hospitalists manage their patients.17 Emergency departments have learned to default surgical and specialty patient admissions to hospitalists when surgeons and specialists balk. Hospital administrations may tacitly or overtly expect their financially subsidized hospitalists to cheerfully accept any and all referrals, irrespective of how inappropriate they may be. Practicing hospitalists frequently complain about their subordinate status and inability to control their working conditions, both of which are identified risk factors for career dissatisfaction and burnout.14, 16, 18 Once again, as a specialty facing a critical manpower shortage, hospitalist programs should be very attuned to defining work conditions that foster career satisfaction and physician retention.
REFRAMING COMANAGEMENT
The history of healthcare is laden with examples of new ideas that were widely and indiscriminately adopted only to subsequently fail to withstand rigorous scrutiny.19, 20 The unchecked expansion of hospitalist comanagement has the potential to become another case in point. In the absence of clear definitions of comanagement and good evidence to define best practices, hospitalists are left to use their best judgment to define the parameters of their comanagement services. At minimum, hospitalist leaders should ask some basic questions as they ponder potential comanagement relationships:
-
Why are we being asked to provide this service?
-
Do the patients have comorbidities that require our input?
-
Is there a legitimate quality or efficiency case to be made to support our participation?
-
Do we have the manpower to provide the service? If not, what will suffer as a result?
-
Will the relationship be equitable?
-
What might go wrong?
Comanagement is an appealing construct that has grown to fill many niches of healthcare delivery.10 Given compelling reasons to be skeptical about the purported benefits of comanagement, hospitalists should be circumspect about how and where they offer such services. Comanagement should be applied carefully and methodically, paying close attention to the consequences, intended and unintended. Applying comanagement in a rational, evidence‐based, and sustainable fashion will ultimately better serve patients, the healthcare community, and hospital medicine.
At a hospital at which I work, every patient who presents to the emergency department with a suspected stroke or transient ischemic attack is evaluated by the stroke team. Per protocol, the team rapidly assesses each patient, orders diagnostic and therapeutic interventions and then refers each and every patient to the hospitalist service for admission and medical comanagement. At no point is any consideration given to whether the patients actually have medical comorbidities, or if a hospitalist will have anything meaningful to add to the care. The firmly set expectation is that hospitalists admit all stroke patients for the purposes of comanagement, while the neurologists consult.
Comanagement has become a mainstay of hospital medicine.1 It is predicated upon the assumption that surgical and specialty patients benefit when their medical comorbidities are managed by hospitalists. It differs conceptually from traditional medical consultation in that hospitalists collaboratively manage patients with surgeons or specialists, sharing responsibility and authority. In practice, however, comanagement varies widely, ranging from a model of care indistinguishable from traditional medical consultation to one where hospitalists admit and assume primary responsibility for surgical and specialty patients. This variability makes it difficult to study and make generalizations about the role and impact of hospitalist comanagement. Nonetheless, recent evidence suggests that hospitalist consultation and comanagement may not be as effective as originally anticipated.
In a 2008 observational cohort study of patients undergoing surgery at an academic medical center, Auerbach et al demonstrated that medical consultation (provided by hospitalists) did not improve glycemic control or increase the likelihood of perioperative beta‐blockade and venous thromboembolism prophylaxis.2 Patients who received consultation had longer adjusted lengths of stay (12.98% longer; 95% confidence interval, 1.61%‐25.61%) and higher adjusted costs (24.36% higher; 95% confidence interval, 13.54%‐36.34%). Notwithstanding the limited generalizability of this study to community hospitals, it has raised concerns that hospitalist consultation does not automatically improve quality of care or cost effectiveness.3
Several other recent trials have also helped to define where hospitalist comanagement may work well and where it may not. In 2004, Huddleston et al published the Hospitalist Orthopedic Team (HOT) trial, the first randomized prospective trial comparing hospitalist‐surgical comanagement to standard care.4 A total of 526 patients undergoing elective hip or knee replacement surgery at the Mayo Clinic were randomized to either standard orthopedic care with consultation as needed, or immediate hospitalist comanagement. The outcomes were disappointing. Hospitalist comanagement reduced minor complications (such as incidence of urinary tract infections, fever, and hyponatremia) but had no effect on moderate or major complications. The HOT intervention modestly reduced adjusted length of stay (LOS), defined as the point at which patients were deemed stable for discharge, by 0.5 days, but had no impact on actual LOS or cost per case. Not surprisingly, orthopedic surgeons and nurses preferred the HOT model of care over the standard model. One year later, Phy et al analyzed outcomes for patients admitted with hip fracture at the same institution.5 This retrospective cohort study compared patients who were admitted to either a standard orthopedic service or to a hospitalist team. In contrast to the HOT trial, hospitalist comanagement of hip fracture patients decreased time to surgery and lowered LOS by 2.2 days without compromising patient outcomes.
How did two trials that occurred roughly simultaneously at the same hospital, involving the same hospitalists and orthopedic surgeons generate such different outcomes? A likely answer is patient selection. Patients who undergo elective joint replacement are usually relatively healthy. They are almost always ambulatory and their comorbidities, when present, are generally reasonably compensated. As a rule, they fare well postoperatively, as evidenced by the 1.3% major complication rate demonstrated in the HOT trial.3 In contrast, hip fracture patients are older, have greater comorbidity and are at remarkably high risk for developing perioperative delirium.3, 4, 6 By definition, their urgent/emergent hip surgery stratifies them to a higher operative risk category than patients who undergo elective joint replacement.7 Half of hip fracture patients do not return to premorbid levels of function, and the 1‐year mortality rate has been estimated to be as high as 25%.6, 8 Given these differences, it is not surprising that hip fracture patients are more likely than elective joint replacement patients to respond favorably to hospitalist comanagement.
In 2007, Simon et al published a retrospective study of 739 pediatric spinal fusion patients at Childrens' Hospital in Denver.9 Beginning in 2004, hospitalists comanaged selected, high‐risk surgical patients (14 of 115 spinal fusion patients, or 12%). Over the course of the study, the mean LOS for low‐risk patients decreased by 21% but the mean LOS for the high‐risk, hospitalist‐comanaged patients decreased by 28%; a 33% relative reduction favoring hospitalist‐managed patients. By targeting selected high‐risk patients, pediatric hospitalists were able to improve upon LOS reductions that occurred systemically across the entire spinal fusion program. Also in 2007, Southern et al compared outcomes for 2,913 patients admitted by full‐time teaching hospitalists vs 6,124 patients admitted by nonhospitalists at Montefiore Medical Center, Bronx, New York.10 Mean LOS for patients admitted to the hospitalist service was 5.01 days vs 5.87 days for the nonhospitalists. Subgroup analysis demonstrated the greatest LOS differentials for patients requiring close clinical monitoring (heart failure, stroke, asthma, or pneumonia) or complex discharge planning.
Although these studies, performed at large academic medical centers, may have limited generalizability, they support the common‐sense notion that hospitalists most benefit patients who are sick, frail, and medically or socially complex. As a corollary, hospitalists probably offer relatively little benefit to surgical and specialty patients who are young or have compensated medical comorbidities and/or straightforward disposition plans. The enormous variability across healthcare institutions makes it difficult if not impossible to define a patient acuity or complexity cutoff below which hospitalist comanagement is unlikely to be beneficial. Nonetheless, some degree of common sense can be applied. As a case in point, a hospitalist probably adds little value to the care of a basically healthy patient with a hemodynamically stable upper gastrointestinal bleed. Despite this, in many institutions, hospitalists admit or comanage all gastroenterology patients, irrespective of their diagnosis, acuity, or complexity.11
One can even hypothesize that hospitalist comanagement may potentially inject risk into patient care. Admitting that patient with a stable upper gastrointestinal bleed to a hospitalist service may delay the gastroenterologist's involvement and initiation of the necessary endoscopy. Having assumed that the hospitalist is running the show, the gastroenterologist may pay insufficient attention to the patient. The hospitalist and gastroenterologist may give conflicting orders and reports that confuse patients, families, and hospital staff, ultimately increasing the likelihood of medical errors.
Ultimately, the risks inherent in adding complexity into patient care must be balanced against the potential benefits. For patients who are sick, frail, or complicated, the risk‐benefit ratio probably tilts in favor of comanagement. However, for generally healthy patients, it is conceivable that adding complexity negates (or worse yet, exceeds) the putative benefits of comanagement.
Given the potential limitations of hospitalist comanagement, why are hospitalists admitting or managing broad and unselected populations of surgical and specialty patients? Hospital leaders have suggested that hospitalist comanagement may protect overstretched surgeons and specialists and extend their capacity. A hospital with only one neurosurgeon on staff might reasonably ask its hospitalists to primarily manage carefully selected low‐acuity neurosurgical patients, allowing the neurosurgeon to serve as a consultant. However, in communities where specialists and surgeons are abundant, this justification is less credible. In such cases, it is difficult not to suspect that the primary reason that hospitalists admit surgical and specialty patients is to enhance the income and quality of life of the surgeons and specialists.
Expanding hospitalist comanagement services for no other reason than to keep specialists and surgeons happy might be justifiable if hospital medicine was not faced with its own critical manpower shortage. Hospital medicine is expected to grow from approximately 20,000 current practitioners to more than 40,000 within a decade.12 The growing shortage of qualified hospitalists has become a preoccupation for hospitalist employers across the country.13 At its 2006 strategic planning retreat, the Board of Directors of the Society of Hospital Medicine identified this issue as one of the greatest threats to the future health of hospital medicine.14 Demand for hospitalists will not abate for at least a decade, which will leave many hospitalist programs significantly understaffed for the foreseeable future. Understaffing forces hospitalist programs to lower hiring standards, jeopardizes patient care, accelerates physician burnout, and may ultimately destabilize hospital medicine.15 Understaffed hospitalist programs should be very circumspect about how and where they expand their clinical coverage.
Another principle underlying hospitalist comanagement is that it improves care by allowing surgeons and specialists to focus on their areas of expertise. Surgeons and specialists who do not have to manage their patients' medical issues can presumably spend more time focusing on their own disciplines. Although this argument is conceptually appealing, there is no evidence that this actually occurs. In fact, it is equally conceivable that hospitalist comanagement could jeopardize care by disengaging surgeons and specialists from their patients' progress (or lack thereof). Furthermore, evidence suggests that hospitalists are underprepared to manage diagnoses that have historically been the purview of surgeons and specialists. Practicing hospitalists who manage acute neurological and neurosurgical conditions, orthopedic trauma, and acute psychiatric illnesses have reported relative undertraining in all of these disease states.10, 16 Generally, hospitalists are expected to deliver this care in the absence of any regime to assess their competence, provide targeted training to fill knowledge gaps, and monitor their progress. At minimum, this should raise concerns about the quality and consistency of care that hospitalists provide to nonmedical patients.
Finally, working collaboratively with other specialties should be a major professional benefit of comanagement. In well‐designed comanagement arrangements, hospitalists and specialists work equitably under clearly defined and mutually agreed upon rules of engagement. They share responsibility for patients, collaborate to improve care, and teach and learn from each other. Unfortunately, in many instances, the power structure becomes lopsided, with surgeons and specialists dictating how, when, and why hospitalists manage their patients.17 Emergency departments have learned to default surgical and specialty patient admissions to hospitalists when surgeons and specialists balk. Hospital administrations may tacitly or overtly expect their financially subsidized hospitalists to cheerfully accept any and all referrals, irrespective of how inappropriate they may be. Practicing hospitalists frequently complain about their subordinate status and inability to control their working conditions, both of which are identified risk factors for career dissatisfaction and burnout.14, 16, 18 Once again, as a specialty facing a critical manpower shortage, hospitalist programs should be very attuned to defining work conditions that foster career satisfaction and physician retention.
REFRAMING COMANAGEMENT
The history of healthcare is laden with examples of new ideas that were widely and indiscriminately adopted only to subsequently fail to withstand rigorous scrutiny.19, 20 The unchecked expansion of hospitalist comanagement has the potential to become another case in point. In the absence of clear definitions of comanagement and good evidence to define best practices, hospitalists are left to use their best judgment to define the parameters of their comanagement services. At minimum, hospitalist leaders should ask some basic questions as they ponder potential comanagement relationships:
-
Why are we being asked to provide this service?
-
Do the patients have comorbidities that require our input?
-
Is there a legitimate quality or efficiency case to be made to support our participation?
-
Do we have the manpower to provide the service? If not, what will suffer as a result?
-
Will the relationship be equitable?
-
What might go wrong?
Comanagement is an appealing construct that has grown to fill many niches of healthcare delivery.10 Given compelling reasons to be skeptical about the purported benefits of comanagement, hospitalists should be circumspect about how and where they offer such services. Comanagement should be applied carefully and methodically, paying close attention to the consequences, intended and unintended. Applying comanagement in a rational, evidence‐based, and sustainable fashion will ultimately better serve patients, the healthcare community, and hospital medicine.
- Society of Hospital Medicine. The Society of Hospital Medicine 2005–2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement. Published by the, 2006. Executive summary available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2167(21):2338–2344.
- .Exceed acceptable: new studies challenge hospitalists to prove our value.Hospitalist.2008;12(2):63.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,.Treatment and survival among elderly Americans with hip fractures: a population‐based study.Am J Public Health.1994;84:1287–1291.
- ,,, et al.Predicting cardiac complications in patients undergoing non‐cardiac surgery.J Gen Intern Med.1986;1:211–219.
- ,,,,.Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study.J Gerontol.1990;45(3):M101–M107.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2:23–29.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,,,,.The spectrum of community‐based hospitalist practice, a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNation wide/Growth_of_Hospital_M.htm. Accessed September 2,2008.
- ,,, et al.Rebuilding the future of the private practice of hospital medicine.The Phoenix Group, May2007.
- Society of Hospital Medicine Board of Directors Strategic Planning Retreat: November 28‐29,2006.
- ,,,,,,for the SGIM Career Satisfaction Study Group.Physician stress: results from the physician worklife study.Stress Health.2001;18(1):37–42.
- ,,, et al.Hospitalist's perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- .Feeling pressure to admit surgical patients? Hospitalists work to set limits on co‐management arrangements.Today's Hospitalist. January2008.
- Society of Hospital Medicine. Career Satisfaction White Paper. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources321:406–412.
- .Pulmonary‐artery catheters—peace at last?N Engl J Med.2006;354(21):2273–2274.
- Society of Hospital Medicine. The Society of Hospital Medicine 2005–2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement. Published by the, 2006. Executive summary available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2167(21):2338–2344.
- .Exceed acceptable: new studies challenge hospitalists to prove our value.Hospitalist.2008;12(2):63.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,.Treatment and survival among elderly Americans with hip fractures: a population‐based study.Am J Public Health.1994;84:1287–1291.
- ,,, et al.Predicting cardiac complications in patients undergoing non‐cardiac surgery.J Gen Intern Med.1986;1:211–219.
- ,,,,.Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study.J Gerontol.1990;45(3):M101–M107.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2:23–29.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,,,,.The spectrum of community‐based hospitalist practice, a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNation wide/Growth_of_Hospital_M.htm. Accessed September 2,2008.
- ,,, et al.Rebuilding the future of the private practice of hospital medicine.The Phoenix Group, May2007.
- Society of Hospital Medicine Board of Directors Strategic Planning Retreat: November 28‐29,2006.
- ,,,,,,for the SGIM Career Satisfaction Study Group.Physician stress: results from the physician worklife study.Stress Health.2001;18(1):37–42.
- ,,, et al.Hospitalist's perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- .Feeling pressure to admit surgical patients? Hospitalists work to set limits on co‐management arrangements.Today's Hospitalist. January2008.
- Society of Hospital Medicine. Career Satisfaction White Paper. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources321:406–412.
- .Pulmonary‐artery catheters—peace at last?N Engl J Med.2006;354(21):2273–2274.
Acute Aortic Dissection
Aortic dissection is an uncommon but highly lethal disease with an incidence of approximately 2,000 cases per year in the United States.1 It is often mistaken for less serious pathology. In one series, aortic dissection was missed in 38% of patients at presentation, with 28% of patients first diagnosed at autopsy.2 Early recognition and management are crucial. If untreated, the mortality rate for acute aortic dissection increases by approximately 1% per hour over the first 48 hours and may reach 70% at 1 week. As many as 90% of untreated patients who suffer aortic dissection die within 3 months of presentation.3, 4 Generally, cardiothoracic surgeons or cardiologists experienced with managing aortic dissection should direct patient evaluation and treatment. Hospitalists, however, are increasingly assuming responsibility for the initial triage and management of patients with acute chest pain syndromes and therefore must be able to rapidly identify aortic dissection, initiate supportive therapy, and refer patients to appropriate specialty care.
PATHOPHYSIOLOGY
Aortic dissection occurs when layers of the aortic wall separate because of infiltration of high‐pressure arterial blood. The proximate causes are elevated shear stress across the aortic lumen in the setting of a concomitant defect in the aortic media. Shear stress is caused by the rapid increase in luminal pressure per unit of time (dP/dt) that results from cardiac systole. As the aorta traverses away from the heart, an increasing proportion of the kinetic energy of left ventricular systole is stored in the aortic wall as potential energy, which facilitates anterograde propagation of cardiac output during diastole. This conversion of kinetic to potential energy also attenuates shear stress. As the proximal aorta is subject to the steepest fluctuations in pressure, it is at the highest risk of dissection. Degeneration of the aortic media is part of the normal aging process but is accelerated in persons with a bicuspid aortic valve, Turner's syndrome, inflammatory arteritis, or inherited diseases of collagen formation.
Once the aortic intima is compromised, blood dissects longitudinally through the aortic media and propagates proximally or distally, creating a false lumen that may communicate with the true lumen of the aorta. Blood may flow through the true lumen, the false lumen, or both. Propagation of the dissection causes much of the morbidity associated with aortic dissection by disrupting blood flow across branch vessels or by directly compromising the pericardium or aortic valve. Over time, the dissection may traverse the entire aortic wall, causing aortic rupture and exsanguination.
CLASSIFICATION
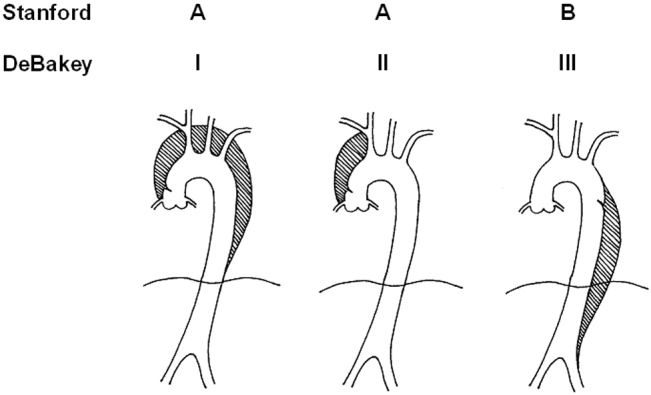
Acute aortic dissection is classified as any aortic dissection diagnosed within 2 weeks of the onset of symptoms, which is the period of highest risk of mortality. Patients who survive more than 2 weeks without treatment are considered to have chronic dissection. Aortic dissections are further classified according to their anatomic location. The fundamental distinction is whether the dissection is proximal (involving the aortic root or ascending aorta) or distal (below the left subclavian artery). The Stanford and DeBakey classification systems are the classification systems most commonly used (Figure 1).
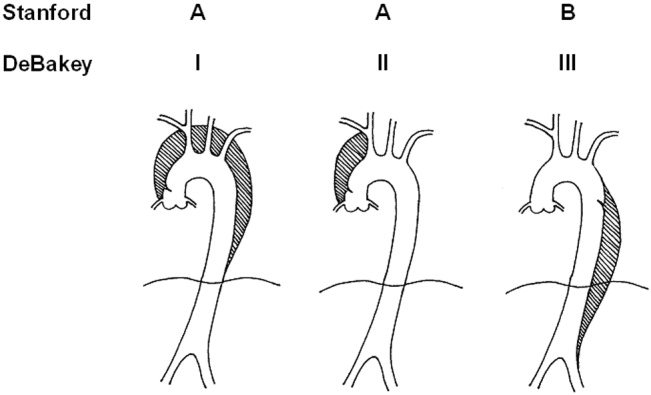
Some variants of aortic dissection are not described in either the Stanford or DeBakey systems. Aortic intramural hematomas (IMH) are caused by intramural hemorrhage of the vasa vasorum without an identifiable intimal tear.57 Penetrating atherosclerotic ulcers (PAUs) are focal defects in the aortic wall with surrounding hematoma but no longitudinal dissection across tissue planes, typically resulting from advanced atherosclerotic disease.8 The pathophysiologic distinctions between IMH, PAU, and classic aortic dissection remain somewhat controversial. Both IMH and PAU may progress to aortic aneurysm formation, frank dissection, or aortic rupture, suggesting that these entities represent a spectrum of diseases with broad overlap (Table 1).9, 10
| Acuity | |
| Acute 2 weeks after onset | |
| Chronic: >2 weeks after onset | |
| Anatomic location: | |
| Ascending aorta: | Stanford Type A, Debakey Type II |
| Ascending and descending aorta: | Stanford Type A, Debakey Type I |
| Descending aorta: | Stanford Type B, Debakey Type III |
| Pathophysiology: | |
| Class 1: Classical aortic dissection with initimal flap between true and false lumen | |
| Class 2: Aortic intramural hematoma without identifiable intimal flap | |
| Class 3: Intimal tear without hematoma (limited dissection) | |
| Class 4: Atherosclerotic plaque rupture with aortic penetrating ulcer | |
| Class 5: Iatrogenic or traumatic aortic dissection (intra‐aortic catherterization, high‐speed deceleration injury, blunt chest trauma) | |
EPIDEMIOLOGY
Aortic dissection is a rare disease, with an estimated incidence of approximately 5‐30 cases per 1 million people per year.1114 Fewer than 0.5% of patients presenting to an emergency department with chest or back pain suffer from aortic dissection.15 Two thirds of patients are male, with an average age at presentation of approximately 65 years. A history of systemic hypertension, found in up to 72% of patients, is by far the most common risk factor.2, 14, 16 Atherosclerosis, a history of prior cardiac surgery, and known aortic aneurysm are other major risk factors.14 The epidemiology of aortic dissection is substantially different in young patients (40 years of age). Hypertension and atherosclerosis become significantly less common, as other risk factors, such as Marfan syndrome, take precedence17 (Table 2). Other risk factors for aortic dissection include:
-
Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos): In the International Registry of Acute Aortic Dissection (IRAD), the largest prospective analysis of aortic dissection to date, 50% of the young patients presenting with aortic dissection had Marfan syndrome.17
-
Bicuspid aortic valve (BAV): Individuals with BAV are 5‐18 times more likely to suffer aortic dissection than those with a trileaflet valve.18, 19 In one survey, 52% of asymptomatic young men with BAV were found to have aortic root dilatation, a frequent precursor of dissection.20 Vascular tissue in individuals with BAV has been found to have increased levels of matrix metalloproteinases, which may degrade elastic matrix components and accelerate medial necrosis.21
-
Aortic coarctation: Aortic coarctation is associated with upper extremity hypertension, BAV and aortic dilatation, all of which predispose to aortic dissection.
-
Turner syndrome: Aortic root dilatation with or without dissection has been incidentally noted in 6%‐9% of patients with Turner syndrome.22, 23
-
Strenuous exercise: Multiple case reports have associated aortic dissection with high‐intensity weightlifting. Many affected individuals were subsequently found to have at least one other risk factor, including hypertension, anabolic steroid abuse, and cocaine abuse.2426
-
Large vessel arteritis: Large vessel arteritides, specifically giant cell arteritis, Takayasu's disease, and tertiary syphilis have long been associated with aortic dilatation and dissection.
-
Cocaine and methamphetamine ingestion: Sympathomimetic drugs cause rapid increases in heart rate and blood pressure, markedly increasing aortic intraluminal shear stress. Furthermore, cocaine is thought to be directly toxic to vascular endothelium and may accelerate medial necrosis.2730
-
Third trimester pregnancy, especially in patients with diseases of collagen31; The significance of pregnancy has recently been called into question by data from the IRAD trial. Of 346 enrolled women with aortic dissection, only 2 were pregnant, suggesting that the previously held association of pregnancy with aortic dissection may be an artifact of selective reporting.1
-
Blunt chest trauma or high‐speed deceleration injury.
-
Iatrogenic injury, typically from intra‐aortic catheterization.
| Hypertension |
| Atherosclerotic disease |
| History of cardiac surgery |
| Aortic aneurysm |
| Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos) |
| Bicuspid aortic valve (BAV) |
| Aortic coarctation |
| Turner syndrome |
| Strenuous exercise |
| Large vessel arteritis: giant cell, Takayasu's, syphilis |
| Cocaine and methamphetamine ingestion |
| Third‐trimester pregnancy |
| Blunt chest trauma or high‐speed deceleration injury |
| Iatrogenic injury, typically from intra‐aortic catheterization |
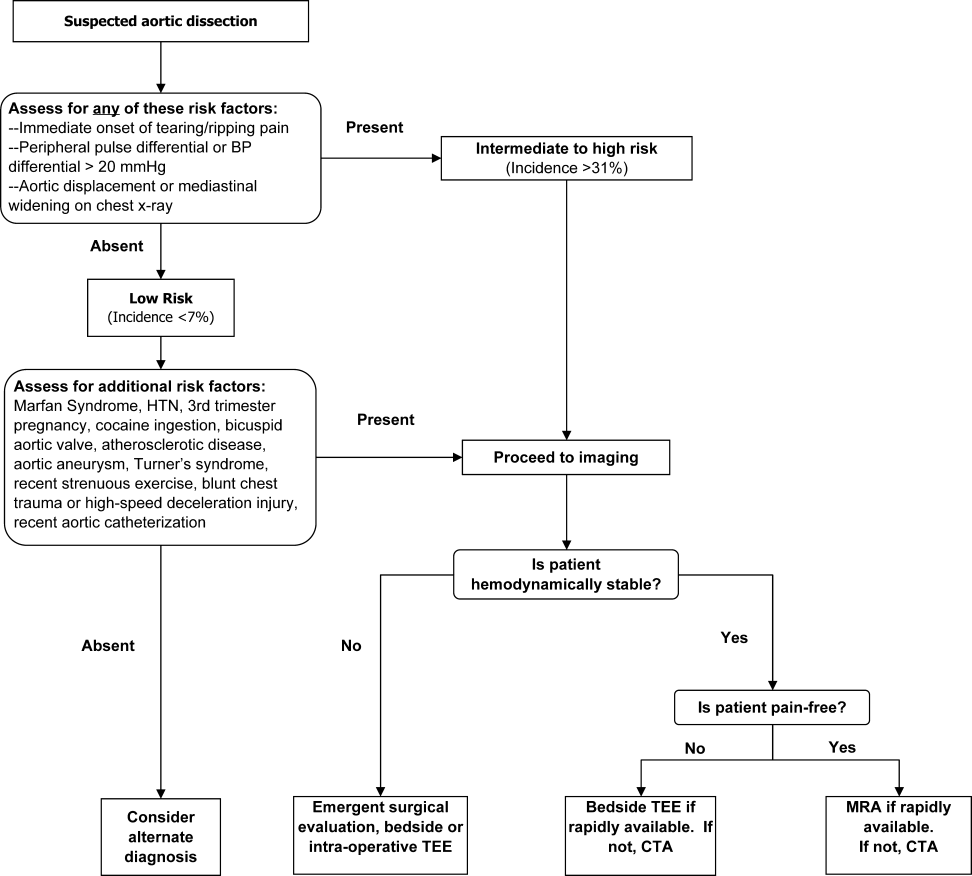
INITIAL EVALUATION
The differential diagnosis for acute aortic dissection includes acute coronary syndrome, pulmonary embolus, pneumothorax, pneumonia, musculoskeletal pain, acute cholecystitis, esophageal spasm or rupture, acute pancreatitis, and acute pericarditis. Acute aortic dissections are rarely asymptomatic; in fact, the absence of sudden‐onset chest pain decreases the likelihood of dissection (negative LR 0.3).32 In the IRAD trial, approximately 95% of patients with aortic dissection complained of pain in the chest, back, or abdomen, with 90% characterizing their pain as either severe or the worst ever and 64% describing it as sharp.14 Although the presence of tearing or ripping chest or back pain suggests aortic dissection (positive LR 1.2‐10.8), its absence does not reliably exclude this diagnosis.32 The wide variability in the presentation of aortic dissection increases the challenge of establishing a diagnosis. Clinical findings depend largely on the anatomical location of the dissection and may include pulse deficits, neurologic deficits, hypotension, hypertension, and end‐organ ischemia. Women who develop aortic dissection are generally older and present later than men. Their symptoms are less typical and are likely to be confounded by altered mental status.1 A diagnosis of aortic dissection should be strongly considered for patients presenting with acute chest or back pain and otherwise unexplained aortic insufficiency, focal neurologic deficits, pulse deficits, or end‐organ injury (Table 3).
| Hypotension or shock due to: |
| a. Hemopericardium and pericardial tamponade |
| b. Acute aortic insufficiency due to dilatation of the aortic annulus |
| c. Aortic rupture |
| d. Lactic acidosis |
| e. Spinal shock |
| Acute myocardial ischemia/emnfarction due to coronary ostial occlusion |
| Pericardial friction rub due to hemopericardium |
| Syncope |
| Pleural effusion or frank hemothorax |
| Acute renal failure due to dissection across renal arteries |
| Mesenteric ischemia due to dissection across intra‐abdominal arteries |
| Neurologic deficits: |
| a. Stroke due to occlusion of arch vessels |
| b. Limb weakness |
| c. Spinal cord deficits due to cord ischemia |
| d. Horner syndrome due to compression of superior sympathetic ganglion. |
| e. Hoarseness due to compression of left recurrent laryngeal nerve |
Electrocardiogram: Electrocardiographic abnormalities are commonly seen in aortic dissection and may include ST‐segment or T‐wave abnormalities or left ventricular hypertrophy.14 Proximal aortic dissections may compromise coronary artery perfusion, generating electrocardiogram (ECG) findings compatible with acute myocardial infarction, which may lead the clinician to diagnose and treat myocardial infarction while missing the underlying diagnosis.33 In a recent survey, 9 of 44 patients (21%) presenting with acute aortic dissection were initially diagnosed with acute coronary syndrome and anticoagulated, with 2 deaths.34 ECGs must therefore be interpreted with extreme caution in aortic dissection.
Chest x‐ray: In the emergency department, chest radiography is a mainstay of the evaluation of acute chest pain. Unfortunately, plain‐film radiography has limited utility for diagnosing aortic dissection.35 In the IRAD trial, mediastinal widening (>8 cm) and abnormal aortic contour, the classic radiographic findings in aortic dissection, were present in only 50%‐60% of cases. Twelve percent of patients had a completely normal chest x‐ray.14 A pooled analysis of previous studies demonstrated that the sensitivity of widened mediastinum and abnormal aortic contour was 65% and 71%, respectively.32 Nonspecific radiographic findings, most notably pleural effusion, were common.36 Thus, if the index of suspicion for aortic dissection is elevated, a confirmatory study must be obtained (Figure 2).
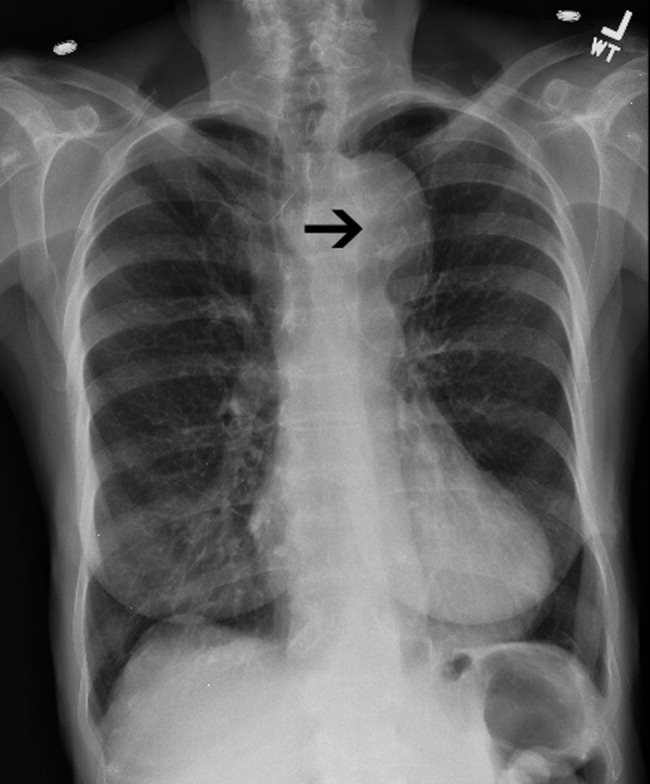
Clinical Prediction Tool
Three clinical features were demonstrated to be effective in identifying aortic dissection in patients presenting with acute chest or back pain: immediate onset of tearing or ripping chest pain, mediastinal widening or aortic enlargement/displacement observed on chest x‐ray, and arm pulse or blood pressure differential exceeding 20 mm Hg. When all 3 findings were absent, dissection was unlikely (7% probability, negative LR 0.07 [CI 0.03‐0.17]). If either chest pain or radiographic findings were present, the likelihood was intermediate (31%‐39% probability). With any other combination of findings, dissection was likely (83‐100% probability). This prediction tool effectively identified 96% of all patients who presented to an emergency department with acute aortic dissection.15 However, 4% of patients categorized as low risk were ultimately diagnosed with aortic dissection. Given the exceptionally high mortality resulting from a missed diagnosis, a 4% false‐negative rate is unacceptably high. Thus, the absence of any of the aforementioned findings should not dissuade the clinician from obtaining a confirmatory imaging study if the pretest probability for acute aortic dissection is elevated.
CONFIRMATORY IMAGING STUDIES
The ideal confirmatory imaging modality should identify aortic dissection with high sensitivity and specificity. It should also identify the entry and exit points of the dissection and provide information about the extent of compromise of the aortic valve, pericardium, and great vessels. Four imaging modalities sufficiently meet these criteria in order to be considered diagnostically useful.
Aortography: Previously the gold standard for diagnosing aortic dissection, aortography is no longer a first‐line imaging modality. The sensitivity and specificity of aortography are at best equivalent and probably inferior to less invasive imaging modalities.37, 38 False negatives may occur if both the true and false lumens opacify equally with contrast, or if the false lumen is sufficiently thrombosed to preclude any instillation of contrast. Aortography cannot identify aortic intramural hematomas, is invasive and highly operator dependent, requires nephrotoxic contrast, and generally takes longer to obtain than other modalities.39
Aortography uniquely offers excellent visualization of the coronary arteries and great vessels and is preferred when such information is necessary. Percutaneous aortic endovascular stent grafting has been recently employed to repair distal aortic dissections.4043 As a result, aortography is gaining new life as a therapeutic modality.
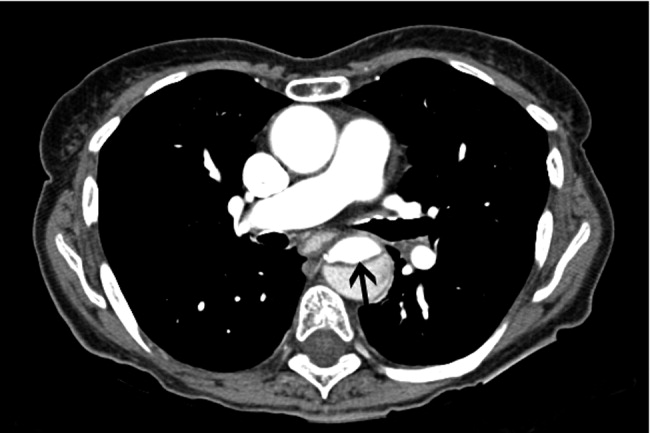
CT angiography: Spiral CT angiography (CTA) is the most commonly used modality for diagnosing aortic dissection.44 It is emergently available at most hospitals, and images can be obtained in minutes. Sensitivity and specificity may approach 100%, and CTA may be more sensitive than MRA or TEE in evaluating arch vessel involvement.4547 Like conventional angiography, CTA requires administration of nephrotoxic contrast. It frequently cannot visualize the entry and exit sites (intimal flaps) of a dissection and provides limited information about the coronary arteries and no information about the competency of the aortic valve.48, 49 Thus, if aortic dissection is identified by CTA, a second study may be needed to provide further diagnostic information and to guide surgical intervention (Figures 3 and 4).

Magnetic Resonance Angiography: Magnetic resonance angiography (MRA) offers excellent noninvasive evaluation of the thoracic aorta. Sensitivity and specificity are probably superior to spiral CTA, and MRA generally identifies the location of the intimal tear and provides some functional information about the aortic valve.44, 50, 51 MRA is not emergently available at many hospitals. Scanning is time intensive, requiring the patient to remain motionless and relatively inaccessible for up to an hour. Furthermore, patient claustrophobia and the presence of implanted devices such as pacemakers or ferromagnetic foreign bodies may preclude MRA.
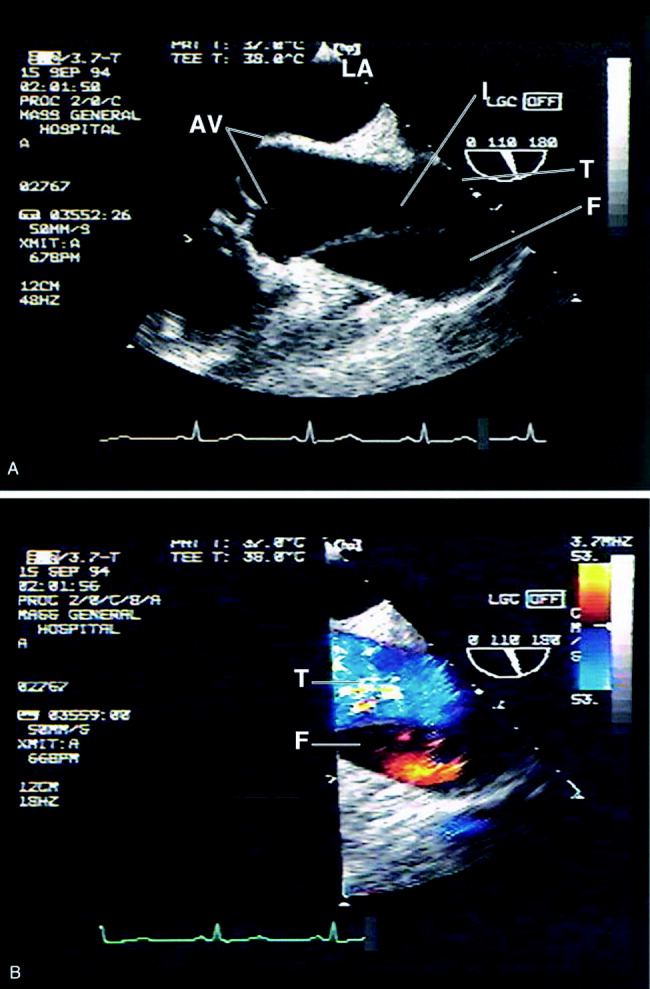
Transesophageal echocardiography: The sensitivity and specificity of transesophageal echocardiography (TEE) are also excellenton a par with CTA and MRA. In addition to providing excellent visualization of the thoracic aorta, TEE provides superb images of the pericardium and detailed assessment of aortic valve function.52 It also is extremely effective at visualizing the aortic intimal flap.44, 49, 53 A significant advantage of TEE is its portability, allowing rapid diagnosis at the bedside. For this reason, it is particularly useful for evaluation of patients who are hemodynamically unstable and are suspected to have an aortic dissection. Because of the anatomic relationship of the aorta with the esophagus and the trachea, TEE more effectively identifies proximal than distal dissections.43 TEE is also somewhat invasive, usually requires patient sedation, and is highly operator dependent, requiring the availability of an experienced and technically skilled operator (Figure 5).
Transthoracic echocardiography: Although it is an excellent tool for the evaluation of many aspects of cardiac anatomy and function, surface echocardiography can reliably visualize only limited portions of the ascending and descending aorta.54, 55 As a consequence, it is neither sensitive nor specific enough to diagnose aortic dissection. Transthoracic echocardiography (TTE) does, however, play a role in rapidly assessing patients at the bedside for aortic valve or pericardial compromise when these complications are suspected.
Recommendations
CTA, MRA, and TEE are all highly sensitive and specific modalities for diagnosing aortic dissection. Therefore, the condition of the patient, the information needed, and the resources and expertise immediately available should drive the choice of study. MRA is considered the gold standard diagnostic study and is the preferred modality for hemodynamically stable patients with suspected aortic dissection. Because of slow data acquisition and the inaccessibility of patients in the scanner, it is generally unsuited for unstable patients, including those with ongoing pain. Bedside TEE is an excellent choice for patients who are too unstable for MRA but is less effective at visualizing distal dissections. Arch aortography is generally reserved for the confirmation of questionable diagnoses or to image specific branch arteries (Tables 4 and 5).
| Overall | Proximal | Distal | |
|---|---|---|---|
| |||
| TEE | 88% | 90% | 80% |
| CTA | 93% | 93% | 93% |
| MRA | 100% | 100% | 100% |
| Aortogram | 87% | 87% | 87% |
| TEE | CTA | MRA | Aortography | |
|---|---|---|---|---|
| ||||
| Sensitivity | ++ | ++ | +++ | ++ |
| Specificity | +++ | ++ | +++ | ++ |
| Classification | +++ | ++ | ++ | + |
| Intimal flap | +++ | ‐ | ++ | + |
| Aortic regurgitation | +++ | ++ | ++ | |
| Pericardial effusion | +++ | ++ | ++ | |
| Branch vessel involvement | + | ++ | ++ | +++ |
| Coronary artery involvement | ++ | + | + | +++ |
Most trials comparing CTA, MRA, and TEE were performed in the early 1990s. Computed tomography has evolved significantly over the intervening decade, and some of the diagnostic limitations previously ascribed to CTA, such as the inability to generate 3‐D reconstructed images, no longer exist. Furthermore, CT angiography is widely available and is gaining increasing acceptance as a first‐line imaging modality for patients with noncardiac chest pain.48 Medical centers that maintain round‐the‐clock CT capability may have limited or delayed access to TEE, MRA, or aortography. Given the potential for rapid and dramatic patient deterioration, it is imperative that a diagnosis be established quickly when aortic dissection is suspected. Thus, when the choice is obtaining an immediate CTA or a delayed TEE or MRA, CTA is generally the better choice (Figure 6).
MANAGEMENT
Acute Management:
Approximately half of all patients who present with acute aortic dissection are acutely hypertensive.14 Hypertensive aortic dissection is a hypertensive emergency that mandates immediate decrease in blood pressure to the lowest level that maintains organ perfusion. As a rule, short‐acting, parenteral, titratable antihypertensive agents should be used (Table 6). Intravenous beta‐adrenergic blockers are the mainstay of acute and chronic therapy. Their negative inotropic and chronotropic effects decrease shear stress across the aortic lumen and decrease the likelihood of dissection propagation and aortic dilatation.56, 57 Parenteral vasodilators (eg, nitroprusside and nitroglycerin) should be initiated if beta‐blockers prove insufficient for lowering blood pressure. They should never be used alone, as they may cause reflex tachycardia and consequently may increase intraluminal shear stress. The use of opiates for analgesia and benzodiazepines for anxiolysis further decreases blood pressure by controlling the severe pain and anxiety often associated with acute dissection.
| Name | Mechanism | Dose | Cautions/contraindications |
|---|---|---|---|
| Esmolol | Cardioselective beta‐1 blocker | Load: 500 g/kg IV | Asthma or bronchospasm |
| Drip: 50 g kg1 min1 IV. | Bradycardia | ||
| Increase by increments of 50 g/min | 2nd‐ or 3rd‐degree AV block | ||
| Cocaine or methamphetamine abuse | |||
| Labetalol | Nonselective beta 1,2 blocker | Load: 20 mg IV | Asthma or bronchospasm |
| Selective alpha‐1 blocker | Drip: 2 mg/min IV | Bradycardia | |
| 2nd or 3rd degree AV block | |||
| Cocaine or methamphetamine abuse | |||
| Enalaprilat | ACE inhibitor | 0.625‐1.25 mg IV q 6 hours. | Angioedema |
| Max dose: 5 mg q 6 hours. | Pregnancy | ||
| Renal artery stenosis | |||
| Severe renal insufficiency | |||
| Nitroprusside | Direct arterial vasodilator | Begin at 0.3 g kg1 min1 IV. | May cause reflex tachycardia |
| Max dose 10 g kg1 min1 | Cyanide/thiocyanate toxicityespecially in renal or hepatic insufficiency | ||
| Nitroglycerin | Vascular smooth muscle relaxation | 5‐200 g/min IV | Decreases preloadcontraindicated in tamponade or other preload‐dependent states |
| Concomitant use of sildenafil or similar agents |
Hypotension or shock, which develop in 15%‐30% of patients with acute aortic dissection, are ominous findings that frequently portends impending hemodynamic collapse.14, 58 Patients who develop hypotension are at a fivefold increased risk of death (55.0% vs. 10.3%) and are at markedly increased risk of developing neurologic deficits, as well as myocardial, mesenteric, and limb ischemia. Hypotension may result from pump failure (due to acute aortic insufficiency, pericardial tamponade, or myocardial ischemia), aortic rupture, systemic lactic acidosis, or spinal shock. Bedside transthoracic echocardiography may be particularly useful for the evaluation of hypotensive patients, as it can be used to quickly and noninvasively determine the integrity of the aortic valve and pericardium. Although hypotension may transiently respond to volume resuscitation, all hypotensive patients with aortic dissection, regardless of type, should be immediately referred for emergent surgical evaluation. Pericardiocentesis in the setting of pericardial tamponade remains controversial; a small study suggested that decompression of the pericardial sac may hasten hemodynamic collapse by accelerating blood loss.59
Facilities that do not maintain urgent cardiopulmonary bypass capability should emergently transport patients with aortic dissection to a facility that provides a higher level of care. Transfer should not be delayed to confirm a questionable diagnosis. Proximal aortic dissection frequently compromises the pericardium, aortic valve, and arch vessels, and therefore emergent surgical repair is indicated. When treated medically, proximal dissection carries a dismal 60% in‐hospital mortality rate.14, 60 In contrast, distal aortic dissection is generally treated medically, with surgical intervention generally reserved for patients with an expanding aortic aneurysm, elevated risk of aortic rupture, refractory hypertension, intractable pain, visceral hypoperfusion, and limb ischemia or paresis.11, 61, 62 Individual branch vessel occlusion may be effectively ameliorated with conventional arterial stenting or balloon fenestration.
Endovascular stent grafting has been used successfully in lieu of surgery for patients with acute or chronic distal (type B) aortic dissections.39, 4042, 63 The stent graft is deployed across the proximal intimal tear, obliterating the false lumen and facilitating aortic healing. Early studies suggested that endovascular stent grafting may be safer and more efficacious than conventional surgical repair of distal dissection.41 A recent meta‐analysis of published trials of endovascular aortic stenting found procedural success rates exceeding 95% and a major complication rate of 11%. Thirty‐day mortality was approximately 5%, with 6‐, 12‐ and 24‐month mortality rates plateauing at 10%. Centers with high patient volume had fewer complications and much lower acute mortality rates.14, 64 These medium‐term outcomes compare favorably with conventional therapy. Endovascular stenting has not been prospectively compared against conventional therapy in randomized trials, and it therefore remains unclear who should be referred for endovascular stenting instead of conventional therapy.
Long‐term Management
Survivors of aortic dissection, especially those with diseases of collagen, have a systemic disease that predisposes them to further aortic and great vessel events. Almost one third of survivors of acute aortic dissection will develop dissection propagation or aortic rupture or will require aortic surgery within 5 years of presentation.41, 60 Young patients who present for aortic dissection should be screened for Marfan syndrome according to the Gent nosology.65 To reduce shear stress to the aortic lumen, all patients should be treated with beta‐blockers for life, with blood pressure targeted to be below 135/80.60, 66 Patients who do not tolerate beta blockade may benefit from treatment with diltiazem or verapamil. Progression to aortic aneurysm is common, and patients should undergo serial imaging of the aorta at 1, 3, 6, and 12 months after discharge and annually thereafter. Dilatation of the proximal aorta to >5.0 cm and of the distal aorta to >6.0 cm should prompt referral for surgical or possibly endovascular repair.41, 67 Although supporting data are limited, it is generally accepted that patients should moderate their physical activity to avoid extremes of tachycardia and blood pressure elevation. Sports that involve high speed or sudden deceleration, such as ice hockey, downhill skiing, and football, should be strictly avoided. Patients should be warned to seek immediate medical attention if they develop recurrent chest or back pain or focal neurologic deficits.
PROGNOSIS
Despite significant medical and surgical advances, aortic dissection remains exceptionally lethal. Patients with proximal dissections are more likely to die than those with distal dissections. Using data from the IRAD trial, Mehta et al determined that age 70 years (OR, 1.70), abrupt onset of chest pain (OR 2.60), hypotension/shock/tamponade (OR, 2.97), renal failure (OR, 4.77), pulse deficit (OR, 2.03), and abnormal ECG (OR, 1.77) were independent determinants of death.59 Medical treatment of proximal dissection is generally reserved for patients too ill, unstable, or frail to undergo surgery. In contrast, most patients with distal dissection are managed medically, with surgery generally reserved for those with acute complications. Hence, patients with proximal dissections who are managed medically and those with distal dissections who are managed surgically have the worst outcomes. Outcomes for women are worse than those for men, which is probably attributable to several factors. Women dissect at an older age, present later after the onset of symptoms, and are more likely to have confounding symptoms that may delay timely diagnosis1 (Table 7).
| Proximal (DeBakey I, II; Stanford A) | Distal (DeBakey III; Stanford B) | |||
|---|---|---|---|---|
| Surgical | Medical | Surgical | Medical | |
| In‐hospital mortality | 26% | 58% | 31% | 11% |
| Average | 35% | 15% | ||
CONCLUSION
Aortic dissection is a rare and acutely life‐threatening cause of acute chest and back pain. Delays in diagnosis and misdiagnoses are common, frequently with catastrophic consequences. The key to diagnosis is maintaining a high index of suspicion for dissection, especially in patients who present with acute severe chest, back, or abdominal pain in the setting of unexplained acute pulse deficits, neurologic deficits, or acute end‐organ injury. Three clinical findings have been shown to be diagnostically useful: immediate onset of tearing or ripping chest or back pain, mediastinal widening or abnormal aortic contour on chest radiograph, and peripheral pulse deficits or variable pulse pressure (>20 mm Hg). If all 3 findings are absent, acute aortic dissection is unlikely. The presence of any of these findings should prompt further workup. A normal chest radiograph does not rule out aortic dissection. Only TEE, CT, and MR angiography are sufficiently specific to rule out dissection. Aortography is rarely used as a first‐line diagnostic tool but may be useful as a confirmatory test or to provide additional anatomic information. Patients who present with proximal aortic dissection or with any aortic dissection with concomitant hypotension are at exceptionally high risk of death and should be immediately referred for surgical evaluation. Beta‐blockers are the mainstay of acute and chronic therapy of aortic dissection. Survivors of aortic dissection are at a markedly elevated risk for further aortic events and should be followed vigilantly posthospitalization.
- ,,, et al.Gender‐related differences in acute aortic dissection.Circulation.2004;109:3014–3021.
- ,,, et al.Clinical features and differential diagnosis of aortic dissection: Experience with 236 cases.Mayo Clin Proc.1993;68:642–651.
- ,.The natural history of thoracic aortic aneurysm disease: an overview.J Card Surg.1997;12(suppl):270–278.
- ,,.Dissecting aneurysms of the aorta: a review of 505 cases.Medicine.1958;37:217–279.
- ,,,.Intimal tear without hematoma; an important variant of aortic dissection that can elude current imaging techniques.Circulation.1999;99:1331–1336.
- ,,, et al.Intramural hemorrhage of the thoracic aorta: diagnostic and therapeutic implications.Circulation.1995;92:1465–1472.
- ,.Acute aortic dissection and its variants; towards a common diagnostic and therapeutic approach.Circulation.1995;92:1376–1378.
- ,,.The penetrating aortic ulcer: pathologic manifestations, diagnosis and management.Mayo Clinic Proc.1988;63:718–725.
- ,,, et al.Acute intramural hematoma of the aorta—a mystery in evolution.Circulation.2005;111:1063–1070.
- ,.Intramural hematoma in acute aortic syndrome; more than one variant of dissection?Circulation.2002;106:284–285.
- ,,,,,,.Acute aortic dissection: population‐based incidence compared with degenerative aortic aneurysm rupture.Mayo Clin Proc.2004;79(2):176–180.
- ,,,,,,.Epidemiology and clinicopathology of aortic dissection.Chest.2000;117:1271–1278.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD).JAMA.2000;283:897–903.
- ,,.Clinical prediction of acute aortic dissection.Arch Intern Med.2000;160:2977–2982.
- ,.Risk factors for aortic dissection: a necropsy study of 161 cases.Am J Cardiol.1984;53:849–855.
- ,,, et al.Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD).J Am Coll Cardiol.2004;43:665–669.
- ,,,.Association of aortic dilatation with regurgitant, stenotic and functionally normal bicuspid aortic valves.J Am Coll Cardiol.1992;19:283–288.
- .Clinical significance of the bicuspid aortic valve.Heart.2000;83:81–85.
- ,,,.Aortic root dilatation in young men with normally functioning bicuspid aortic valves.Heart.1999;82:19–22.
- ,,, et al.Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation.J Thorac Cardiovasc Surg.2003;126:797–806.
- ,,.Further delineation of aortic dilation, dissection, and rupture in patients with Turner syndrome.Pediatrics.1998;102(1):e12.
- ,,, et al.Aortic dilatation, dissection and rupture in patients with Turner syndrome.J Pediatr.1986;109:820–826.
- ,.Weight lifting and type II aortic dissection. A case report.J Sports Med Phys Fitness.2004;44:424–427.
- ,,.Recreational weight lifting and aortic dissection: case report.J Vasc Surg.1993;17:774–776.
- ,,,,,,.Ascending aortic dissection in weight lifters with cystic medial degeneration.Ann Thoracic Surg.1990;49:638–642.
- ,,,,.Acute aortic dissection related to crack cocaine.Circulation.2002;105:1592–1595.
- ,.Thoracic aortic dissection secondary to crack cocaine ingestion.Am J Emerg Med.1997;15:507–509.
- ,.Cocaine‐associated dissection of the thoracic aorta.J Emerg Med.1992;10:723–727.
- ,.Methamphetamine as a risk factor for acute aortic dissection.J Forensic Sci.1999;44(1):23–26.
- ,,,,,.Arterial dissections associated with pregnancy.J Vasc Surg.1995;21:515–520.
- .Does this patient have an acute thoracic aortic dissection?JAMA.2002;287:2262–2272.
- ,,,.Fatal haemostatic complications due to thrombolytic therapy in patients falsely diagnosed as acute myocardial infarction.Eur Heart J.1992;13:840–843.
- ,,,,.The inadvertent administration of anticoagulants to ED patients ultimately diagnosed with thoracic aortic dissection.Am J Emerg Med.2005;23:439–442..
- ,,, et al.Chest radiography for the diagnosis of acute aortic syndrome.Am J Med.2004;116(2):73–77.
- ,,, et al.Clinical significance of pleural effusion in acute aortic dissection.Chest.2002;121:825–830.
- ,,,.Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography.J Am Coll Cardiol.1995;25:1393–1401.
- ,.Digital subtraction angiography of aortic dissection.Am J Roentgenol.1983;141:157–161.
- ,,, et al.Spectrum of conditions initially suggesting acute aortic dissection but with negative aortograms.Am J Cardiol.1986;57:322–326.
- ,,, et al.Nonsurgical reconstruction of thoracic aortic dissection by stent‐graft placement.N Engl J Med.1999;340:1539–1545.
- ,,, et al.Endovascular stent‐graft placement for the treatment of aortic dissection.N Engl J Med.1999;340:1546–1552.
- ,.Aortic dissection: New frontiers in diagnosis and management. Part II: Therapeutic management and follow‐up.Circulation.2003;108:772–778.
- ,,,,,.Aortic dissection: percutaneous management of ischemic complications with endovascular stents and balloon fenestration.J Vasc Surg.1996;23:241–251.
- ,,, et al.Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD).Am J Cardiol.2002;89:1235–1238.
- ,,, et al.Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging.Radiology.1996;199:347–352.
- ,,,.Assessment of the thoracic aorta by spiral CT.AJR Am J Roengenol.1992;158:1127–1130.
- ,Radiologic evaluation of aortic dissection.Radiology.1991;180:297–305.
- ,,,,,.Computed tomography of thoracic aortic dissection: accuracy and pitfalls.J Comput Assist Tomogr.1986;10:211–215.
- ,,.Spiral CT in acute non‐cardiac chest pain.Clin Radiol.1999;54(1):38–45.
- ,,, et al.Diagnosis of thoracic aortic dissection. Magnetic resonance imaging versus transesophageal echocardiography.Circulation.1992;85:434–447.
- ,,,,.Comparison of conventional and transesophageal echocardiography with magnetic resonance imaging for anatomical mapping of thoracic aortic dissection: a dual noninvasive imaging study with anatomical and/or angiographic validation.Int J Card Imaging.1994;10:1–14.
- ,.Transesophageal echocardiography in the diagnosis of diseases of the thoracic aorta: part I. Aortic dissection, aortic intramural hematoma and penetrating atherosclerotic ulcer of the aorta.Chest.1999;116:1772–1779.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328:1–9.
- ,,,,,.Echocardiography in diagnosis of aortic dissection.Lancet.1989;1:457–461.
- ,,,,.A prospectus on the prevention of aortic rupture in the Marfan syndrome with data on survivorship without treatment.Johns Hopkins Med J.1971;129:123–129.
- ,,,.Progression of aortic dilatation and the benefit of long‐term beta‐adrenergic blockade in Marfan's syndrome.N Engl J Med.1994;330:1335–1341.
- ,,, et al.Clinical characteristics of hypotension in patients with acute aortic dissection.Am J Cardiol.2005;95:48–52.
- ,,.Cardiac tamponade complicating proximal aortic dissection: is pericardiocentesis harmful?Circulation.1994;90:2375–2379.
- ,,, et al.Predicting in‐hospital mortality in acute type A aortic dissection.Circulation.2002;105:200–206.
- ,,, et al.Diagnosis and management of aortic dissection: task force report of the European Society of Cardiology.Eur Heart J.2001;22:1642–1681.
- ,,,,,.Clinical profiles and outcomes of acute type B aortic dissection in the current era: Lessons from the international registry of aortic dissection.Circulation.2003;108(suppl II):II‐312–II‐317.
- ,,, et al.Endovascular stent‐graft treatment of aortic dissection: determinants of post‐interventional outcome.Eur Heart J.2005;26:489–497.
- ,,, et al.Endovascular stent‐graft placement in aortic dissection: a meta‐analysis [advance access online].Eur Heart J.2005. Published online October 14, 2005.
- ,,,,.Revised diagnostic criteria for the Marfan syndrome.Am J Med Genet.1996;62:417–426.
- ,,,.Treatment of dissecting aneurysms of the aorta without surgery.J Thorac Cardiovasc Surg.1965;50:364–373.
- ,,,,,.Unoperated aortic aneurysms: a survey of 170 patients.Ann Thorac Surg.1995;59:1204–1209.
Aortic dissection is an uncommon but highly lethal disease with an incidence of approximately 2,000 cases per year in the United States.1 It is often mistaken for less serious pathology. In one series, aortic dissection was missed in 38% of patients at presentation, with 28% of patients first diagnosed at autopsy.2 Early recognition and management are crucial. If untreated, the mortality rate for acute aortic dissection increases by approximately 1% per hour over the first 48 hours and may reach 70% at 1 week. As many as 90% of untreated patients who suffer aortic dissection die within 3 months of presentation.3, 4 Generally, cardiothoracic surgeons or cardiologists experienced with managing aortic dissection should direct patient evaluation and treatment. Hospitalists, however, are increasingly assuming responsibility for the initial triage and management of patients with acute chest pain syndromes and therefore must be able to rapidly identify aortic dissection, initiate supportive therapy, and refer patients to appropriate specialty care.
PATHOPHYSIOLOGY
Aortic dissection occurs when layers of the aortic wall separate because of infiltration of high‐pressure arterial blood. The proximate causes are elevated shear stress across the aortic lumen in the setting of a concomitant defect in the aortic media. Shear stress is caused by the rapid increase in luminal pressure per unit of time (dP/dt) that results from cardiac systole. As the aorta traverses away from the heart, an increasing proportion of the kinetic energy of left ventricular systole is stored in the aortic wall as potential energy, which facilitates anterograde propagation of cardiac output during diastole. This conversion of kinetic to potential energy also attenuates shear stress. As the proximal aorta is subject to the steepest fluctuations in pressure, it is at the highest risk of dissection. Degeneration of the aortic media is part of the normal aging process but is accelerated in persons with a bicuspid aortic valve, Turner's syndrome, inflammatory arteritis, or inherited diseases of collagen formation.
Once the aortic intima is compromised, blood dissects longitudinally through the aortic media and propagates proximally or distally, creating a false lumen that may communicate with the true lumen of the aorta. Blood may flow through the true lumen, the false lumen, or both. Propagation of the dissection causes much of the morbidity associated with aortic dissection by disrupting blood flow across branch vessels or by directly compromising the pericardium or aortic valve. Over time, the dissection may traverse the entire aortic wall, causing aortic rupture and exsanguination.
CLASSIFICATION
Acute aortic dissection is classified as any aortic dissection diagnosed within 2 weeks of the onset of symptoms, which is the period of highest risk of mortality. Patients who survive more than 2 weeks without treatment are considered to have chronic dissection. Aortic dissections are further classified according to their anatomic location. The fundamental distinction is whether the dissection is proximal (involving the aortic root or ascending aorta) or distal (below the left subclavian artery). The Stanford and DeBakey classification systems are the classification systems most commonly used (Figure 1).

Some variants of aortic dissection are not described in either the Stanford or DeBakey systems. Aortic intramural hematomas (IMH) are caused by intramural hemorrhage of the vasa vasorum without an identifiable intimal tear.57 Penetrating atherosclerotic ulcers (PAUs) are focal defects in the aortic wall with surrounding hematoma but no longitudinal dissection across tissue planes, typically resulting from advanced atherosclerotic disease.8 The pathophysiologic distinctions between IMH, PAU, and classic aortic dissection remain somewhat controversial. Both IMH and PAU may progress to aortic aneurysm formation, frank dissection, or aortic rupture, suggesting that these entities represent a spectrum of diseases with broad overlap (Table 1).9, 10
| Acuity | |
| Acute 2 weeks after onset | |
| Chronic: >2 weeks after onset | |
| Anatomic location: | |
| Ascending aorta: | Stanford Type A, Debakey Type II |
| Ascending and descending aorta: | Stanford Type A, Debakey Type I |
| Descending aorta: | Stanford Type B, Debakey Type III |
| Pathophysiology: | |
| Class 1: Classical aortic dissection with initimal flap between true and false lumen | |
| Class 2: Aortic intramural hematoma without identifiable intimal flap | |
| Class 3: Intimal tear without hematoma (limited dissection) | |
| Class 4: Atherosclerotic plaque rupture with aortic penetrating ulcer | |
| Class 5: Iatrogenic or traumatic aortic dissection (intra‐aortic catherterization, high‐speed deceleration injury, blunt chest trauma) | |
EPIDEMIOLOGY
Aortic dissection is a rare disease, with an estimated incidence of approximately 5‐30 cases per 1 million people per year.1114 Fewer than 0.5% of patients presenting to an emergency department with chest or back pain suffer from aortic dissection.15 Two thirds of patients are male, with an average age at presentation of approximately 65 years. A history of systemic hypertension, found in up to 72% of patients, is by far the most common risk factor.2, 14, 16 Atherosclerosis, a history of prior cardiac surgery, and known aortic aneurysm are other major risk factors.14 The epidemiology of aortic dissection is substantially different in young patients (40 years of age). Hypertension and atherosclerosis become significantly less common, as other risk factors, such as Marfan syndrome, take precedence17 (Table 2). Other risk factors for aortic dissection include:
-
Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos): In the International Registry of Acute Aortic Dissection (IRAD), the largest prospective analysis of aortic dissection to date, 50% of the young patients presenting with aortic dissection had Marfan syndrome.17
-
Bicuspid aortic valve (BAV): Individuals with BAV are 5‐18 times more likely to suffer aortic dissection than those with a trileaflet valve.18, 19 In one survey, 52% of asymptomatic young men with BAV were found to have aortic root dilatation, a frequent precursor of dissection.20 Vascular tissue in individuals with BAV has been found to have increased levels of matrix metalloproteinases, which may degrade elastic matrix components and accelerate medial necrosis.21
-
Aortic coarctation: Aortic coarctation is associated with upper extremity hypertension, BAV and aortic dilatation, all of which predispose to aortic dissection.
-
Turner syndrome: Aortic root dilatation with or without dissection has been incidentally noted in 6%‐9% of patients with Turner syndrome.22, 23
-
Strenuous exercise: Multiple case reports have associated aortic dissection with high‐intensity weightlifting. Many affected individuals were subsequently found to have at least one other risk factor, including hypertension, anabolic steroid abuse, and cocaine abuse.2426
-
Large vessel arteritis: Large vessel arteritides, specifically giant cell arteritis, Takayasu's disease, and tertiary syphilis have long been associated with aortic dilatation and dissection.
-
Cocaine and methamphetamine ingestion: Sympathomimetic drugs cause rapid increases in heart rate and blood pressure, markedly increasing aortic intraluminal shear stress. Furthermore, cocaine is thought to be directly toxic to vascular endothelium and may accelerate medial necrosis.2730
-
Third trimester pregnancy, especially in patients with diseases of collagen31; The significance of pregnancy has recently been called into question by data from the IRAD trial. Of 346 enrolled women with aortic dissection, only 2 were pregnant, suggesting that the previously held association of pregnancy with aortic dissection may be an artifact of selective reporting.1
-
Blunt chest trauma or high‐speed deceleration injury.
-
Iatrogenic injury, typically from intra‐aortic catheterization.
| Hypertension |
| Atherosclerotic disease |
| History of cardiac surgery |
| Aortic aneurysm |
| Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos) |
| Bicuspid aortic valve (BAV) |
| Aortic coarctation |
| Turner syndrome |
| Strenuous exercise |
| Large vessel arteritis: giant cell, Takayasu's, syphilis |
| Cocaine and methamphetamine ingestion |
| Third‐trimester pregnancy |
| Blunt chest trauma or high‐speed deceleration injury |
| Iatrogenic injury, typically from intra‐aortic catheterization |
INITIAL EVALUATION
The differential diagnosis for acute aortic dissection includes acute coronary syndrome, pulmonary embolus, pneumothorax, pneumonia, musculoskeletal pain, acute cholecystitis, esophageal spasm or rupture, acute pancreatitis, and acute pericarditis. Acute aortic dissections are rarely asymptomatic; in fact, the absence of sudden‐onset chest pain decreases the likelihood of dissection (negative LR 0.3).32 In the IRAD trial, approximately 95% of patients with aortic dissection complained of pain in the chest, back, or abdomen, with 90% characterizing their pain as either severe or the worst ever and 64% describing it as sharp.14 Although the presence of tearing or ripping chest or back pain suggests aortic dissection (positive LR 1.2‐10.8), its absence does not reliably exclude this diagnosis.32 The wide variability in the presentation of aortic dissection increases the challenge of establishing a diagnosis. Clinical findings depend largely on the anatomical location of the dissection and may include pulse deficits, neurologic deficits, hypotension, hypertension, and end‐organ ischemia. Women who develop aortic dissection are generally older and present later than men. Their symptoms are less typical and are likely to be confounded by altered mental status.1 A diagnosis of aortic dissection should be strongly considered for patients presenting with acute chest or back pain and otherwise unexplained aortic insufficiency, focal neurologic deficits, pulse deficits, or end‐organ injury (Table 3).
| Hypotension or shock due to: |
| a. Hemopericardium and pericardial tamponade |
| b. Acute aortic insufficiency due to dilatation of the aortic annulus |
| c. Aortic rupture |
| d. Lactic acidosis |
| e. Spinal shock |
| Acute myocardial ischemia/emnfarction due to coronary ostial occlusion |
| Pericardial friction rub due to hemopericardium |
| Syncope |
| Pleural effusion or frank hemothorax |
| Acute renal failure due to dissection across renal arteries |
| Mesenteric ischemia due to dissection across intra‐abdominal arteries |
| Neurologic deficits: |
| a. Stroke due to occlusion of arch vessels |
| b. Limb weakness |
| c. Spinal cord deficits due to cord ischemia |
| d. Horner syndrome due to compression of superior sympathetic ganglion. |
| e. Hoarseness due to compression of left recurrent laryngeal nerve |
Electrocardiogram: Electrocardiographic abnormalities are commonly seen in aortic dissection and may include ST‐segment or T‐wave abnormalities or left ventricular hypertrophy.14 Proximal aortic dissections may compromise coronary artery perfusion, generating electrocardiogram (ECG) findings compatible with acute myocardial infarction, which may lead the clinician to diagnose and treat myocardial infarction while missing the underlying diagnosis.33 In a recent survey, 9 of 44 patients (21%) presenting with acute aortic dissection were initially diagnosed with acute coronary syndrome and anticoagulated, with 2 deaths.34 ECGs must therefore be interpreted with extreme caution in aortic dissection.
Chest x‐ray: In the emergency department, chest radiography is a mainstay of the evaluation of acute chest pain. Unfortunately, plain‐film radiography has limited utility for diagnosing aortic dissection.35 In the IRAD trial, mediastinal widening (>8 cm) and abnormal aortic contour, the classic radiographic findings in aortic dissection, were present in only 50%‐60% of cases. Twelve percent of patients had a completely normal chest x‐ray.14 A pooled analysis of previous studies demonstrated that the sensitivity of widened mediastinum and abnormal aortic contour was 65% and 71%, respectively.32 Nonspecific radiographic findings, most notably pleural effusion, were common.36 Thus, if the index of suspicion for aortic dissection is elevated, a confirmatory study must be obtained (Figure 2).

Clinical Prediction Tool
Three clinical features were demonstrated to be effective in identifying aortic dissection in patients presenting with acute chest or back pain: immediate onset of tearing or ripping chest pain, mediastinal widening or aortic enlargement/displacement observed on chest x‐ray, and arm pulse or blood pressure differential exceeding 20 mm Hg. When all 3 findings were absent, dissection was unlikely (7% probability, negative LR 0.07 [CI 0.03‐0.17]). If either chest pain or radiographic findings were present, the likelihood was intermediate (31%‐39% probability). With any other combination of findings, dissection was likely (83‐100% probability). This prediction tool effectively identified 96% of all patients who presented to an emergency department with acute aortic dissection.15 However, 4% of patients categorized as low risk were ultimately diagnosed with aortic dissection. Given the exceptionally high mortality resulting from a missed diagnosis, a 4% false‐negative rate is unacceptably high. Thus, the absence of any of the aforementioned findings should not dissuade the clinician from obtaining a confirmatory imaging study if the pretest probability for acute aortic dissection is elevated.
CONFIRMATORY IMAGING STUDIES
The ideal confirmatory imaging modality should identify aortic dissection with high sensitivity and specificity. It should also identify the entry and exit points of the dissection and provide information about the extent of compromise of the aortic valve, pericardium, and great vessels. Four imaging modalities sufficiently meet these criteria in order to be considered diagnostically useful.
Aortography: Previously the gold standard for diagnosing aortic dissection, aortography is no longer a first‐line imaging modality. The sensitivity and specificity of aortography are at best equivalent and probably inferior to less invasive imaging modalities.37, 38 False negatives may occur if both the true and false lumens opacify equally with contrast, or if the false lumen is sufficiently thrombosed to preclude any instillation of contrast. Aortography cannot identify aortic intramural hematomas, is invasive and highly operator dependent, requires nephrotoxic contrast, and generally takes longer to obtain than other modalities.39
Aortography uniquely offers excellent visualization of the coronary arteries and great vessels and is preferred when such information is necessary. Percutaneous aortic endovascular stent grafting has been recently employed to repair distal aortic dissections.4043 As a result, aortography is gaining new life as a therapeutic modality.
CT angiography: Spiral CT angiography (CTA) is the most commonly used modality for diagnosing aortic dissection.44 It is emergently available at most hospitals, and images can be obtained in minutes. Sensitivity and specificity may approach 100%, and CTA may be more sensitive than MRA or TEE in evaluating arch vessel involvement.4547 Like conventional angiography, CTA requires administration of nephrotoxic contrast. It frequently cannot visualize the entry and exit sites (intimal flaps) of a dissection and provides limited information about the coronary arteries and no information about the competency of the aortic valve.48, 49 Thus, if aortic dissection is identified by CTA, a second study may be needed to provide further diagnostic information and to guide surgical intervention (Figures 3 and 4).


Magnetic Resonance Angiography: Magnetic resonance angiography (MRA) offers excellent noninvasive evaluation of the thoracic aorta. Sensitivity and specificity are probably superior to spiral CTA, and MRA generally identifies the location of the intimal tear and provides some functional information about the aortic valve.44, 50, 51 MRA is not emergently available at many hospitals. Scanning is time intensive, requiring the patient to remain motionless and relatively inaccessible for up to an hour. Furthermore, patient claustrophobia and the presence of implanted devices such as pacemakers or ferromagnetic foreign bodies may preclude MRA.
Transesophageal echocardiography: The sensitivity and specificity of transesophageal echocardiography (TEE) are also excellenton a par with CTA and MRA. In addition to providing excellent visualization of the thoracic aorta, TEE provides superb images of the pericardium and detailed assessment of aortic valve function.52 It also is extremely effective at visualizing the aortic intimal flap.44, 49, 53 A significant advantage of TEE is its portability, allowing rapid diagnosis at the bedside. For this reason, it is particularly useful for evaluation of patients who are hemodynamically unstable and are suspected to have an aortic dissection. Because of the anatomic relationship of the aorta with the esophagus and the trachea, TEE more effectively identifies proximal than distal dissections.43 TEE is also somewhat invasive, usually requires patient sedation, and is highly operator dependent, requiring the availability of an experienced and technically skilled operator (Figure 5).

Transthoracic echocardiography: Although it is an excellent tool for the evaluation of many aspects of cardiac anatomy and function, surface echocardiography can reliably visualize only limited portions of the ascending and descending aorta.54, 55 As a consequence, it is neither sensitive nor specific enough to diagnose aortic dissection. Transthoracic echocardiography (TTE) does, however, play a role in rapidly assessing patients at the bedside for aortic valve or pericardial compromise when these complications are suspected.
Recommendations
CTA, MRA, and TEE are all highly sensitive and specific modalities for diagnosing aortic dissection. Therefore, the condition of the patient, the information needed, and the resources and expertise immediately available should drive the choice of study. MRA is considered the gold standard diagnostic study and is the preferred modality for hemodynamically stable patients with suspected aortic dissection. Because of slow data acquisition and the inaccessibility of patients in the scanner, it is generally unsuited for unstable patients, including those with ongoing pain. Bedside TEE is an excellent choice for patients who are too unstable for MRA but is less effective at visualizing distal dissections. Arch aortography is generally reserved for the confirmation of questionable diagnoses or to image specific branch arteries (Tables 4 and 5).
| Overall | Proximal | Distal | |
|---|---|---|---|
| |||
| TEE | 88% | 90% | 80% |
| CTA | 93% | 93% | 93% |
| MRA | 100% | 100% | 100% |
| Aortogram | 87% | 87% | 87% |
| TEE | CTA | MRA | Aortography | |
|---|---|---|---|---|
| ||||
| Sensitivity | ++ | ++ | +++ | ++ |
| Specificity | +++ | ++ | +++ | ++ |
| Classification | +++ | ++ | ++ | + |
| Intimal flap | +++ | ‐ | ++ | + |
| Aortic regurgitation | +++ | ++ | ++ | |
| Pericardial effusion | +++ | ++ | ++ | |
| Branch vessel involvement | + | ++ | ++ | +++ |
| Coronary artery involvement | ++ | + | + | +++ |
Most trials comparing CTA, MRA, and TEE were performed in the early 1990s. Computed tomography has evolved significantly over the intervening decade, and some of the diagnostic limitations previously ascribed to CTA, such as the inability to generate 3‐D reconstructed images, no longer exist. Furthermore, CT angiography is widely available and is gaining increasing acceptance as a first‐line imaging modality for patients with noncardiac chest pain.48 Medical centers that maintain round‐the‐clock CT capability may have limited or delayed access to TEE, MRA, or aortography. Given the potential for rapid and dramatic patient deterioration, it is imperative that a diagnosis be established quickly when aortic dissection is suspected. Thus, when the choice is obtaining an immediate CTA or a delayed TEE or MRA, CTA is generally the better choice (Figure 6).

MANAGEMENT
Acute Management:
Approximately half of all patients who present with acute aortic dissection are acutely hypertensive.14 Hypertensive aortic dissection is a hypertensive emergency that mandates immediate decrease in blood pressure to the lowest level that maintains organ perfusion. As a rule, short‐acting, parenteral, titratable antihypertensive agents should be used (Table 6). Intravenous beta‐adrenergic blockers are the mainstay of acute and chronic therapy. Their negative inotropic and chronotropic effects decrease shear stress across the aortic lumen and decrease the likelihood of dissection propagation and aortic dilatation.56, 57 Parenteral vasodilators (eg, nitroprusside and nitroglycerin) should be initiated if beta‐blockers prove insufficient for lowering blood pressure. They should never be used alone, as they may cause reflex tachycardia and consequently may increase intraluminal shear stress. The use of opiates for analgesia and benzodiazepines for anxiolysis further decreases blood pressure by controlling the severe pain and anxiety often associated with acute dissection.
| Name | Mechanism | Dose | Cautions/contraindications |
|---|---|---|---|
| Esmolol | Cardioselective beta‐1 blocker | Load: 500 g/kg IV | Asthma or bronchospasm |
| Drip: 50 g kg1 min1 IV. | Bradycardia | ||
| Increase by increments of 50 g/min | 2nd‐ or 3rd‐degree AV block | ||
| Cocaine or methamphetamine abuse | |||
| Labetalol | Nonselective beta 1,2 blocker | Load: 20 mg IV | Asthma or bronchospasm |
| Selective alpha‐1 blocker | Drip: 2 mg/min IV | Bradycardia | |
| 2nd or 3rd degree AV block | |||
| Cocaine or methamphetamine abuse | |||
| Enalaprilat | ACE inhibitor | 0.625‐1.25 mg IV q 6 hours. | Angioedema |
| Max dose: 5 mg q 6 hours. | Pregnancy | ||
| Renal artery stenosis | |||
| Severe renal insufficiency | |||
| Nitroprusside | Direct arterial vasodilator | Begin at 0.3 g kg1 min1 IV. | May cause reflex tachycardia |
| Max dose 10 g kg1 min1 | Cyanide/thiocyanate toxicityespecially in renal or hepatic insufficiency | ||
| Nitroglycerin | Vascular smooth muscle relaxation | 5‐200 g/min IV | Decreases preloadcontraindicated in tamponade or other preload‐dependent states |
| Concomitant use of sildenafil or similar agents |
Hypotension or shock, which develop in 15%‐30% of patients with acute aortic dissection, are ominous findings that frequently portends impending hemodynamic collapse.14, 58 Patients who develop hypotension are at a fivefold increased risk of death (55.0% vs. 10.3%) and are at markedly increased risk of developing neurologic deficits, as well as myocardial, mesenteric, and limb ischemia. Hypotension may result from pump failure (due to acute aortic insufficiency, pericardial tamponade, or myocardial ischemia), aortic rupture, systemic lactic acidosis, or spinal shock. Bedside transthoracic echocardiography may be particularly useful for the evaluation of hypotensive patients, as it can be used to quickly and noninvasively determine the integrity of the aortic valve and pericardium. Although hypotension may transiently respond to volume resuscitation, all hypotensive patients with aortic dissection, regardless of type, should be immediately referred for emergent surgical evaluation. Pericardiocentesis in the setting of pericardial tamponade remains controversial; a small study suggested that decompression of the pericardial sac may hasten hemodynamic collapse by accelerating blood loss.59
Facilities that do not maintain urgent cardiopulmonary bypass capability should emergently transport patients with aortic dissection to a facility that provides a higher level of care. Transfer should not be delayed to confirm a questionable diagnosis. Proximal aortic dissection frequently compromises the pericardium, aortic valve, and arch vessels, and therefore emergent surgical repair is indicated. When treated medically, proximal dissection carries a dismal 60% in‐hospital mortality rate.14, 60 In contrast, distal aortic dissection is generally treated medically, with surgical intervention generally reserved for patients with an expanding aortic aneurysm, elevated risk of aortic rupture, refractory hypertension, intractable pain, visceral hypoperfusion, and limb ischemia or paresis.11, 61, 62 Individual branch vessel occlusion may be effectively ameliorated with conventional arterial stenting or balloon fenestration.
Endovascular stent grafting has been used successfully in lieu of surgery for patients with acute or chronic distal (type B) aortic dissections.39, 4042, 63 The stent graft is deployed across the proximal intimal tear, obliterating the false lumen and facilitating aortic healing. Early studies suggested that endovascular stent grafting may be safer and more efficacious than conventional surgical repair of distal dissection.41 A recent meta‐analysis of published trials of endovascular aortic stenting found procedural success rates exceeding 95% and a major complication rate of 11%. Thirty‐day mortality was approximately 5%, with 6‐, 12‐ and 24‐month mortality rates plateauing at 10%. Centers with high patient volume had fewer complications and much lower acute mortality rates.14, 64 These medium‐term outcomes compare favorably with conventional therapy. Endovascular stenting has not been prospectively compared against conventional therapy in randomized trials, and it therefore remains unclear who should be referred for endovascular stenting instead of conventional therapy.
Long‐term Management
Survivors of aortic dissection, especially those with diseases of collagen, have a systemic disease that predisposes them to further aortic and great vessel events. Almost one third of survivors of acute aortic dissection will develop dissection propagation or aortic rupture or will require aortic surgery within 5 years of presentation.41, 60 Young patients who present for aortic dissection should be screened for Marfan syndrome according to the Gent nosology.65 To reduce shear stress to the aortic lumen, all patients should be treated with beta‐blockers for life, with blood pressure targeted to be below 135/80.60, 66 Patients who do not tolerate beta blockade may benefit from treatment with diltiazem or verapamil. Progression to aortic aneurysm is common, and patients should undergo serial imaging of the aorta at 1, 3, 6, and 12 months after discharge and annually thereafter. Dilatation of the proximal aorta to >5.0 cm and of the distal aorta to >6.0 cm should prompt referral for surgical or possibly endovascular repair.41, 67 Although supporting data are limited, it is generally accepted that patients should moderate their physical activity to avoid extremes of tachycardia and blood pressure elevation. Sports that involve high speed or sudden deceleration, such as ice hockey, downhill skiing, and football, should be strictly avoided. Patients should be warned to seek immediate medical attention if they develop recurrent chest or back pain or focal neurologic deficits.
PROGNOSIS
Despite significant medical and surgical advances, aortic dissection remains exceptionally lethal. Patients with proximal dissections are more likely to die than those with distal dissections. Using data from the IRAD trial, Mehta et al determined that age 70 years (OR, 1.70), abrupt onset of chest pain (OR 2.60), hypotension/shock/tamponade (OR, 2.97), renal failure (OR, 4.77), pulse deficit (OR, 2.03), and abnormal ECG (OR, 1.77) were independent determinants of death.59 Medical treatment of proximal dissection is generally reserved for patients too ill, unstable, or frail to undergo surgery. In contrast, most patients with distal dissection are managed medically, with surgery generally reserved for those with acute complications. Hence, patients with proximal dissections who are managed medically and those with distal dissections who are managed surgically have the worst outcomes. Outcomes for women are worse than those for men, which is probably attributable to several factors. Women dissect at an older age, present later after the onset of symptoms, and are more likely to have confounding symptoms that may delay timely diagnosis1 (Table 7).
| Proximal (DeBakey I, II; Stanford A) | Distal (DeBakey III; Stanford B) | |||
|---|---|---|---|---|
| Surgical | Medical | Surgical | Medical | |
| In‐hospital mortality | 26% | 58% | 31% | 11% |
| Average | 35% | 15% | ||
CONCLUSION
Aortic dissection is a rare and acutely life‐threatening cause of acute chest and back pain. Delays in diagnosis and misdiagnoses are common, frequently with catastrophic consequences. The key to diagnosis is maintaining a high index of suspicion for dissection, especially in patients who present with acute severe chest, back, or abdominal pain in the setting of unexplained acute pulse deficits, neurologic deficits, or acute end‐organ injury. Three clinical findings have been shown to be diagnostically useful: immediate onset of tearing or ripping chest or back pain, mediastinal widening or abnormal aortic contour on chest radiograph, and peripheral pulse deficits or variable pulse pressure (>20 mm Hg). If all 3 findings are absent, acute aortic dissection is unlikely. The presence of any of these findings should prompt further workup. A normal chest radiograph does not rule out aortic dissection. Only TEE, CT, and MR angiography are sufficiently specific to rule out dissection. Aortography is rarely used as a first‐line diagnostic tool but may be useful as a confirmatory test or to provide additional anatomic information. Patients who present with proximal aortic dissection or with any aortic dissection with concomitant hypotension are at exceptionally high risk of death and should be immediately referred for surgical evaluation. Beta‐blockers are the mainstay of acute and chronic therapy of aortic dissection. Survivors of aortic dissection are at a markedly elevated risk for further aortic events and should be followed vigilantly posthospitalization.
Aortic dissection is an uncommon but highly lethal disease with an incidence of approximately 2,000 cases per year in the United States.1 It is often mistaken for less serious pathology. In one series, aortic dissection was missed in 38% of patients at presentation, with 28% of patients first diagnosed at autopsy.2 Early recognition and management are crucial. If untreated, the mortality rate for acute aortic dissection increases by approximately 1% per hour over the first 48 hours and may reach 70% at 1 week. As many as 90% of untreated patients who suffer aortic dissection die within 3 months of presentation.3, 4 Generally, cardiothoracic surgeons or cardiologists experienced with managing aortic dissection should direct patient evaluation and treatment. Hospitalists, however, are increasingly assuming responsibility for the initial triage and management of patients with acute chest pain syndromes and therefore must be able to rapidly identify aortic dissection, initiate supportive therapy, and refer patients to appropriate specialty care.
PATHOPHYSIOLOGY
Aortic dissection occurs when layers of the aortic wall separate because of infiltration of high‐pressure arterial blood. The proximate causes are elevated shear stress across the aortic lumen in the setting of a concomitant defect in the aortic media. Shear stress is caused by the rapid increase in luminal pressure per unit of time (dP/dt) that results from cardiac systole. As the aorta traverses away from the heart, an increasing proportion of the kinetic energy of left ventricular systole is stored in the aortic wall as potential energy, which facilitates anterograde propagation of cardiac output during diastole. This conversion of kinetic to potential energy also attenuates shear stress. As the proximal aorta is subject to the steepest fluctuations in pressure, it is at the highest risk of dissection. Degeneration of the aortic media is part of the normal aging process but is accelerated in persons with a bicuspid aortic valve, Turner's syndrome, inflammatory arteritis, or inherited diseases of collagen formation.
Once the aortic intima is compromised, blood dissects longitudinally through the aortic media and propagates proximally or distally, creating a false lumen that may communicate with the true lumen of the aorta. Blood may flow through the true lumen, the false lumen, or both. Propagation of the dissection causes much of the morbidity associated with aortic dissection by disrupting blood flow across branch vessels or by directly compromising the pericardium or aortic valve. Over time, the dissection may traverse the entire aortic wall, causing aortic rupture and exsanguination.
CLASSIFICATION
Acute aortic dissection is classified as any aortic dissection diagnosed within 2 weeks of the onset of symptoms, which is the period of highest risk of mortality. Patients who survive more than 2 weeks without treatment are considered to have chronic dissection. Aortic dissections are further classified according to their anatomic location. The fundamental distinction is whether the dissection is proximal (involving the aortic root or ascending aorta) or distal (below the left subclavian artery). The Stanford and DeBakey classification systems are the classification systems most commonly used (Figure 1).

Some variants of aortic dissection are not described in either the Stanford or DeBakey systems. Aortic intramural hematomas (IMH) are caused by intramural hemorrhage of the vasa vasorum without an identifiable intimal tear.57 Penetrating atherosclerotic ulcers (PAUs) are focal defects in the aortic wall with surrounding hematoma but no longitudinal dissection across tissue planes, typically resulting from advanced atherosclerotic disease.8 The pathophysiologic distinctions between IMH, PAU, and classic aortic dissection remain somewhat controversial. Both IMH and PAU may progress to aortic aneurysm formation, frank dissection, or aortic rupture, suggesting that these entities represent a spectrum of diseases with broad overlap (Table 1).9, 10
| Acuity | |
| Acute 2 weeks after onset | |
| Chronic: >2 weeks after onset | |
| Anatomic location: | |
| Ascending aorta: | Stanford Type A, Debakey Type II |
| Ascending and descending aorta: | Stanford Type A, Debakey Type I |
| Descending aorta: | Stanford Type B, Debakey Type III |
| Pathophysiology: | |
| Class 1: Classical aortic dissection with initimal flap between true and false lumen | |
| Class 2: Aortic intramural hematoma without identifiable intimal flap | |
| Class 3: Intimal tear without hematoma (limited dissection) | |
| Class 4: Atherosclerotic plaque rupture with aortic penetrating ulcer | |
| Class 5: Iatrogenic or traumatic aortic dissection (intra‐aortic catherterization, high‐speed deceleration injury, blunt chest trauma) | |
EPIDEMIOLOGY
Aortic dissection is a rare disease, with an estimated incidence of approximately 5‐30 cases per 1 million people per year.1114 Fewer than 0.5% of patients presenting to an emergency department with chest or back pain suffer from aortic dissection.15 Two thirds of patients are male, with an average age at presentation of approximately 65 years. A history of systemic hypertension, found in up to 72% of patients, is by far the most common risk factor.2, 14, 16 Atherosclerosis, a history of prior cardiac surgery, and known aortic aneurysm are other major risk factors.14 The epidemiology of aortic dissection is substantially different in young patients (40 years of age). Hypertension and atherosclerosis become significantly less common, as other risk factors, such as Marfan syndrome, take precedence17 (Table 2). Other risk factors for aortic dissection include:
-
Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos): In the International Registry of Acute Aortic Dissection (IRAD), the largest prospective analysis of aortic dissection to date, 50% of the young patients presenting with aortic dissection had Marfan syndrome.17
-
Bicuspid aortic valve (BAV): Individuals with BAV are 5‐18 times more likely to suffer aortic dissection than those with a trileaflet valve.18, 19 In one survey, 52% of asymptomatic young men with BAV were found to have aortic root dilatation, a frequent precursor of dissection.20 Vascular tissue in individuals with BAV has been found to have increased levels of matrix metalloproteinases, which may degrade elastic matrix components and accelerate medial necrosis.21
-
Aortic coarctation: Aortic coarctation is associated with upper extremity hypertension, BAV and aortic dilatation, all of which predispose to aortic dissection.
-
Turner syndrome: Aortic root dilatation with or without dissection has been incidentally noted in 6%‐9% of patients with Turner syndrome.22, 23
-
Strenuous exercise: Multiple case reports have associated aortic dissection with high‐intensity weightlifting. Many affected individuals were subsequently found to have at least one other risk factor, including hypertension, anabolic steroid abuse, and cocaine abuse.2426
-
Large vessel arteritis: Large vessel arteritides, specifically giant cell arteritis, Takayasu's disease, and tertiary syphilis have long been associated with aortic dilatation and dissection.
-
Cocaine and methamphetamine ingestion: Sympathomimetic drugs cause rapid increases in heart rate and blood pressure, markedly increasing aortic intraluminal shear stress. Furthermore, cocaine is thought to be directly toxic to vascular endothelium and may accelerate medial necrosis.2730
-
Third trimester pregnancy, especially in patients with diseases of collagen31; The significance of pregnancy has recently been called into question by data from the IRAD trial. Of 346 enrolled women with aortic dissection, only 2 were pregnant, suggesting that the previously held association of pregnancy with aortic dissection may be an artifact of selective reporting.1
-
Blunt chest trauma or high‐speed deceleration injury.
-
Iatrogenic injury, typically from intra‐aortic catheterization.
| Hypertension |
| Atherosclerotic disease |
| History of cardiac surgery |
| Aortic aneurysm |
| Collagen diseases (eg, Marfan syndrome and Ehlers‐Danlos) |
| Bicuspid aortic valve (BAV) |
| Aortic coarctation |
| Turner syndrome |
| Strenuous exercise |
| Large vessel arteritis: giant cell, Takayasu's, syphilis |
| Cocaine and methamphetamine ingestion |
| Third‐trimester pregnancy |
| Blunt chest trauma or high‐speed deceleration injury |
| Iatrogenic injury, typically from intra‐aortic catheterization |
INITIAL EVALUATION
The differential diagnosis for acute aortic dissection includes acute coronary syndrome, pulmonary embolus, pneumothorax, pneumonia, musculoskeletal pain, acute cholecystitis, esophageal spasm or rupture, acute pancreatitis, and acute pericarditis. Acute aortic dissections are rarely asymptomatic; in fact, the absence of sudden‐onset chest pain decreases the likelihood of dissection (negative LR 0.3).32 In the IRAD trial, approximately 95% of patients with aortic dissection complained of pain in the chest, back, or abdomen, with 90% characterizing their pain as either severe or the worst ever and 64% describing it as sharp.14 Although the presence of tearing or ripping chest or back pain suggests aortic dissection (positive LR 1.2‐10.8), its absence does not reliably exclude this diagnosis.32 The wide variability in the presentation of aortic dissection increases the challenge of establishing a diagnosis. Clinical findings depend largely on the anatomical location of the dissection and may include pulse deficits, neurologic deficits, hypotension, hypertension, and end‐organ ischemia. Women who develop aortic dissection are generally older and present later than men. Their symptoms are less typical and are likely to be confounded by altered mental status.1 A diagnosis of aortic dissection should be strongly considered for patients presenting with acute chest or back pain and otherwise unexplained aortic insufficiency, focal neurologic deficits, pulse deficits, or end‐organ injury (Table 3).
| Hypotension or shock due to: |
| a. Hemopericardium and pericardial tamponade |
| b. Acute aortic insufficiency due to dilatation of the aortic annulus |
| c. Aortic rupture |
| d. Lactic acidosis |
| e. Spinal shock |
| Acute myocardial ischemia/emnfarction due to coronary ostial occlusion |
| Pericardial friction rub due to hemopericardium |
| Syncope |
| Pleural effusion or frank hemothorax |
| Acute renal failure due to dissection across renal arteries |
| Mesenteric ischemia due to dissection across intra‐abdominal arteries |
| Neurologic deficits: |
| a. Stroke due to occlusion of arch vessels |
| b. Limb weakness |
| c. Spinal cord deficits due to cord ischemia |
| d. Horner syndrome due to compression of superior sympathetic ganglion. |
| e. Hoarseness due to compression of left recurrent laryngeal nerve |
Electrocardiogram: Electrocardiographic abnormalities are commonly seen in aortic dissection and may include ST‐segment or T‐wave abnormalities or left ventricular hypertrophy.14 Proximal aortic dissections may compromise coronary artery perfusion, generating electrocardiogram (ECG) findings compatible with acute myocardial infarction, which may lead the clinician to diagnose and treat myocardial infarction while missing the underlying diagnosis.33 In a recent survey, 9 of 44 patients (21%) presenting with acute aortic dissection were initially diagnosed with acute coronary syndrome and anticoagulated, with 2 deaths.34 ECGs must therefore be interpreted with extreme caution in aortic dissection.
Chest x‐ray: In the emergency department, chest radiography is a mainstay of the evaluation of acute chest pain. Unfortunately, plain‐film radiography has limited utility for diagnosing aortic dissection.35 In the IRAD trial, mediastinal widening (>8 cm) and abnormal aortic contour, the classic radiographic findings in aortic dissection, were present in only 50%‐60% of cases. Twelve percent of patients had a completely normal chest x‐ray.14 A pooled analysis of previous studies demonstrated that the sensitivity of widened mediastinum and abnormal aortic contour was 65% and 71%, respectively.32 Nonspecific radiographic findings, most notably pleural effusion, were common.36 Thus, if the index of suspicion for aortic dissection is elevated, a confirmatory study must be obtained (Figure 2).

Clinical Prediction Tool
Three clinical features were demonstrated to be effective in identifying aortic dissection in patients presenting with acute chest or back pain: immediate onset of tearing or ripping chest pain, mediastinal widening or aortic enlargement/displacement observed on chest x‐ray, and arm pulse or blood pressure differential exceeding 20 mm Hg. When all 3 findings were absent, dissection was unlikely (7% probability, negative LR 0.07 [CI 0.03‐0.17]). If either chest pain or radiographic findings were present, the likelihood was intermediate (31%‐39% probability). With any other combination of findings, dissection was likely (83‐100% probability). This prediction tool effectively identified 96% of all patients who presented to an emergency department with acute aortic dissection.15 However, 4% of patients categorized as low risk were ultimately diagnosed with aortic dissection. Given the exceptionally high mortality resulting from a missed diagnosis, a 4% false‐negative rate is unacceptably high. Thus, the absence of any of the aforementioned findings should not dissuade the clinician from obtaining a confirmatory imaging study if the pretest probability for acute aortic dissection is elevated.
CONFIRMATORY IMAGING STUDIES
The ideal confirmatory imaging modality should identify aortic dissection with high sensitivity and specificity. It should also identify the entry and exit points of the dissection and provide information about the extent of compromise of the aortic valve, pericardium, and great vessels. Four imaging modalities sufficiently meet these criteria in order to be considered diagnostically useful.
Aortography: Previously the gold standard for diagnosing aortic dissection, aortography is no longer a first‐line imaging modality. The sensitivity and specificity of aortography are at best equivalent and probably inferior to less invasive imaging modalities.37, 38 False negatives may occur if both the true and false lumens opacify equally with contrast, or if the false lumen is sufficiently thrombosed to preclude any instillation of contrast. Aortography cannot identify aortic intramural hematomas, is invasive and highly operator dependent, requires nephrotoxic contrast, and generally takes longer to obtain than other modalities.39
Aortography uniquely offers excellent visualization of the coronary arteries and great vessels and is preferred when such information is necessary. Percutaneous aortic endovascular stent grafting has been recently employed to repair distal aortic dissections.4043 As a result, aortography is gaining new life as a therapeutic modality.
CT angiography: Spiral CT angiography (CTA) is the most commonly used modality for diagnosing aortic dissection.44 It is emergently available at most hospitals, and images can be obtained in minutes. Sensitivity and specificity may approach 100%, and CTA may be more sensitive than MRA or TEE in evaluating arch vessel involvement.4547 Like conventional angiography, CTA requires administration of nephrotoxic contrast. It frequently cannot visualize the entry and exit sites (intimal flaps) of a dissection and provides limited information about the coronary arteries and no information about the competency of the aortic valve.48, 49 Thus, if aortic dissection is identified by CTA, a second study may be needed to provide further diagnostic information and to guide surgical intervention (Figures 3 and 4).


Magnetic Resonance Angiography: Magnetic resonance angiography (MRA) offers excellent noninvasive evaluation of the thoracic aorta. Sensitivity and specificity are probably superior to spiral CTA, and MRA generally identifies the location of the intimal tear and provides some functional information about the aortic valve.44, 50, 51 MRA is not emergently available at many hospitals. Scanning is time intensive, requiring the patient to remain motionless and relatively inaccessible for up to an hour. Furthermore, patient claustrophobia and the presence of implanted devices such as pacemakers or ferromagnetic foreign bodies may preclude MRA.
Transesophageal echocardiography: The sensitivity and specificity of transesophageal echocardiography (TEE) are also excellenton a par with CTA and MRA. In addition to providing excellent visualization of the thoracic aorta, TEE provides superb images of the pericardium and detailed assessment of aortic valve function.52 It also is extremely effective at visualizing the aortic intimal flap.44, 49, 53 A significant advantage of TEE is its portability, allowing rapid diagnosis at the bedside. For this reason, it is particularly useful for evaluation of patients who are hemodynamically unstable and are suspected to have an aortic dissection. Because of the anatomic relationship of the aorta with the esophagus and the trachea, TEE more effectively identifies proximal than distal dissections.43 TEE is also somewhat invasive, usually requires patient sedation, and is highly operator dependent, requiring the availability of an experienced and technically skilled operator (Figure 5).

Transthoracic echocardiography: Although it is an excellent tool for the evaluation of many aspects of cardiac anatomy and function, surface echocardiography can reliably visualize only limited portions of the ascending and descending aorta.54, 55 As a consequence, it is neither sensitive nor specific enough to diagnose aortic dissection. Transthoracic echocardiography (TTE) does, however, play a role in rapidly assessing patients at the bedside for aortic valve or pericardial compromise when these complications are suspected.
Recommendations
CTA, MRA, and TEE are all highly sensitive and specific modalities for diagnosing aortic dissection. Therefore, the condition of the patient, the information needed, and the resources and expertise immediately available should drive the choice of study. MRA is considered the gold standard diagnostic study and is the preferred modality for hemodynamically stable patients with suspected aortic dissection. Because of slow data acquisition and the inaccessibility of patients in the scanner, it is generally unsuited for unstable patients, including those with ongoing pain. Bedside TEE is an excellent choice for patients who are too unstable for MRA but is less effective at visualizing distal dissections. Arch aortography is generally reserved for the confirmation of questionable diagnoses or to image specific branch arteries (Tables 4 and 5).
| Overall | Proximal | Distal | |
|---|---|---|---|
| |||
| TEE | 88% | 90% | 80% |
| CTA | 93% | 93% | 93% |
| MRA | 100% | 100% | 100% |
| Aortogram | 87% | 87% | 87% |
| TEE | CTA | MRA | Aortography | |
|---|---|---|---|---|
| ||||
| Sensitivity | ++ | ++ | +++ | ++ |
| Specificity | +++ | ++ | +++ | ++ |
| Classification | +++ | ++ | ++ | + |
| Intimal flap | +++ | ‐ | ++ | + |
| Aortic regurgitation | +++ | ++ | ++ | |
| Pericardial effusion | +++ | ++ | ++ | |
| Branch vessel involvement | + | ++ | ++ | +++ |
| Coronary artery involvement | ++ | + | + | +++ |
Most trials comparing CTA, MRA, and TEE were performed in the early 1990s. Computed tomography has evolved significantly over the intervening decade, and some of the diagnostic limitations previously ascribed to CTA, such as the inability to generate 3‐D reconstructed images, no longer exist. Furthermore, CT angiography is widely available and is gaining increasing acceptance as a first‐line imaging modality for patients with noncardiac chest pain.48 Medical centers that maintain round‐the‐clock CT capability may have limited or delayed access to TEE, MRA, or aortography. Given the potential for rapid and dramatic patient deterioration, it is imperative that a diagnosis be established quickly when aortic dissection is suspected. Thus, when the choice is obtaining an immediate CTA or a delayed TEE or MRA, CTA is generally the better choice (Figure 6).

MANAGEMENT
Acute Management:
Approximately half of all patients who present with acute aortic dissection are acutely hypertensive.14 Hypertensive aortic dissection is a hypertensive emergency that mandates immediate decrease in blood pressure to the lowest level that maintains organ perfusion. As a rule, short‐acting, parenteral, titratable antihypertensive agents should be used (Table 6). Intravenous beta‐adrenergic blockers are the mainstay of acute and chronic therapy. Their negative inotropic and chronotropic effects decrease shear stress across the aortic lumen and decrease the likelihood of dissection propagation and aortic dilatation.56, 57 Parenteral vasodilators (eg, nitroprusside and nitroglycerin) should be initiated if beta‐blockers prove insufficient for lowering blood pressure. They should never be used alone, as they may cause reflex tachycardia and consequently may increase intraluminal shear stress. The use of opiates for analgesia and benzodiazepines for anxiolysis further decreases blood pressure by controlling the severe pain and anxiety often associated with acute dissection.
| Name | Mechanism | Dose | Cautions/contraindications |
|---|---|---|---|
| Esmolol | Cardioselective beta‐1 blocker | Load: 500 g/kg IV | Asthma or bronchospasm |
| Drip: 50 g kg1 min1 IV. | Bradycardia | ||
| Increase by increments of 50 g/min | 2nd‐ or 3rd‐degree AV block | ||
| Cocaine or methamphetamine abuse | |||
| Labetalol | Nonselective beta 1,2 blocker | Load: 20 mg IV | Asthma or bronchospasm |
| Selective alpha‐1 blocker | Drip: 2 mg/min IV | Bradycardia | |
| 2nd or 3rd degree AV block | |||
| Cocaine or methamphetamine abuse | |||
| Enalaprilat | ACE inhibitor | 0.625‐1.25 mg IV q 6 hours. | Angioedema |
| Max dose: 5 mg q 6 hours. | Pregnancy | ||
| Renal artery stenosis | |||
| Severe renal insufficiency | |||
| Nitroprusside | Direct arterial vasodilator | Begin at 0.3 g kg1 min1 IV. | May cause reflex tachycardia |
| Max dose 10 g kg1 min1 | Cyanide/thiocyanate toxicityespecially in renal or hepatic insufficiency | ||
| Nitroglycerin | Vascular smooth muscle relaxation | 5‐200 g/min IV | Decreases preloadcontraindicated in tamponade or other preload‐dependent states |
| Concomitant use of sildenafil or similar agents |
Hypotension or shock, which develop in 15%‐30% of patients with acute aortic dissection, are ominous findings that frequently portends impending hemodynamic collapse.14, 58 Patients who develop hypotension are at a fivefold increased risk of death (55.0% vs. 10.3%) and are at markedly increased risk of developing neurologic deficits, as well as myocardial, mesenteric, and limb ischemia. Hypotension may result from pump failure (due to acute aortic insufficiency, pericardial tamponade, or myocardial ischemia), aortic rupture, systemic lactic acidosis, or spinal shock. Bedside transthoracic echocardiography may be particularly useful for the evaluation of hypotensive patients, as it can be used to quickly and noninvasively determine the integrity of the aortic valve and pericardium. Although hypotension may transiently respond to volume resuscitation, all hypotensive patients with aortic dissection, regardless of type, should be immediately referred for emergent surgical evaluation. Pericardiocentesis in the setting of pericardial tamponade remains controversial; a small study suggested that decompression of the pericardial sac may hasten hemodynamic collapse by accelerating blood loss.59
Facilities that do not maintain urgent cardiopulmonary bypass capability should emergently transport patients with aortic dissection to a facility that provides a higher level of care. Transfer should not be delayed to confirm a questionable diagnosis. Proximal aortic dissection frequently compromises the pericardium, aortic valve, and arch vessels, and therefore emergent surgical repair is indicated. When treated medically, proximal dissection carries a dismal 60% in‐hospital mortality rate.14, 60 In contrast, distal aortic dissection is generally treated medically, with surgical intervention generally reserved for patients with an expanding aortic aneurysm, elevated risk of aortic rupture, refractory hypertension, intractable pain, visceral hypoperfusion, and limb ischemia or paresis.11, 61, 62 Individual branch vessel occlusion may be effectively ameliorated with conventional arterial stenting or balloon fenestration.
Endovascular stent grafting has been used successfully in lieu of surgery for patients with acute or chronic distal (type B) aortic dissections.39, 4042, 63 The stent graft is deployed across the proximal intimal tear, obliterating the false lumen and facilitating aortic healing. Early studies suggested that endovascular stent grafting may be safer and more efficacious than conventional surgical repair of distal dissection.41 A recent meta‐analysis of published trials of endovascular aortic stenting found procedural success rates exceeding 95% and a major complication rate of 11%. Thirty‐day mortality was approximately 5%, with 6‐, 12‐ and 24‐month mortality rates plateauing at 10%. Centers with high patient volume had fewer complications and much lower acute mortality rates.14, 64 These medium‐term outcomes compare favorably with conventional therapy. Endovascular stenting has not been prospectively compared against conventional therapy in randomized trials, and it therefore remains unclear who should be referred for endovascular stenting instead of conventional therapy.
Long‐term Management
Survivors of aortic dissection, especially those with diseases of collagen, have a systemic disease that predisposes them to further aortic and great vessel events. Almost one third of survivors of acute aortic dissection will develop dissection propagation or aortic rupture or will require aortic surgery within 5 years of presentation.41, 60 Young patients who present for aortic dissection should be screened for Marfan syndrome according to the Gent nosology.65 To reduce shear stress to the aortic lumen, all patients should be treated with beta‐blockers for life, with blood pressure targeted to be below 135/80.60, 66 Patients who do not tolerate beta blockade may benefit from treatment with diltiazem or verapamil. Progression to aortic aneurysm is common, and patients should undergo serial imaging of the aorta at 1, 3, 6, and 12 months after discharge and annually thereafter. Dilatation of the proximal aorta to >5.0 cm and of the distal aorta to >6.0 cm should prompt referral for surgical or possibly endovascular repair.41, 67 Although supporting data are limited, it is generally accepted that patients should moderate their physical activity to avoid extremes of tachycardia and blood pressure elevation. Sports that involve high speed or sudden deceleration, such as ice hockey, downhill skiing, and football, should be strictly avoided. Patients should be warned to seek immediate medical attention if they develop recurrent chest or back pain or focal neurologic deficits.
PROGNOSIS
Despite significant medical and surgical advances, aortic dissection remains exceptionally lethal. Patients with proximal dissections are more likely to die than those with distal dissections. Using data from the IRAD trial, Mehta et al determined that age 70 years (OR, 1.70), abrupt onset of chest pain (OR 2.60), hypotension/shock/tamponade (OR, 2.97), renal failure (OR, 4.77), pulse deficit (OR, 2.03), and abnormal ECG (OR, 1.77) were independent determinants of death.59 Medical treatment of proximal dissection is generally reserved for patients too ill, unstable, or frail to undergo surgery. In contrast, most patients with distal dissection are managed medically, with surgery generally reserved for those with acute complications. Hence, patients with proximal dissections who are managed medically and those with distal dissections who are managed surgically have the worst outcomes. Outcomes for women are worse than those for men, which is probably attributable to several factors. Women dissect at an older age, present later after the onset of symptoms, and are more likely to have confounding symptoms that may delay timely diagnosis1 (Table 7).
| Proximal (DeBakey I, II; Stanford A) | Distal (DeBakey III; Stanford B) | |||
|---|---|---|---|---|
| Surgical | Medical | Surgical | Medical | |
| In‐hospital mortality | 26% | 58% | 31% | 11% |
| Average | 35% | 15% | ||
CONCLUSION
Aortic dissection is a rare and acutely life‐threatening cause of acute chest and back pain. Delays in diagnosis and misdiagnoses are common, frequently with catastrophic consequences. The key to diagnosis is maintaining a high index of suspicion for dissection, especially in patients who present with acute severe chest, back, or abdominal pain in the setting of unexplained acute pulse deficits, neurologic deficits, or acute end‐organ injury. Three clinical findings have been shown to be diagnostically useful: immediate onset of tearing or ripping chest or back pain, mediastinal widening or abnormal aortic contour on chest radiograph, and peripheral pulse deficits or variable pulse pressure (>20 mm Hg). If all 3 findings are absent, acute aortic dissection is unlikely. The presence of any of these findings should prompt further workup. A normal chest radiograph does not rule out aortic dissection. Only TEE, CT, and MR angiography are sufficiently specific to rule out dissection. Aortography is rarely used as a first‐line diagnostic tool but may be useful as a confirmatory test or to provide additional anatomic information. Patients who present with proximal aortic dissection or with any aortic dissection with concomitant hypotension are at exceptionally high risk of death and should be immediately referred for surgical evaluation. Beta‐blockers are the mainstay of acute and chronic therapy of aortic dissection. Survivors of aortic dissection are at a markedly elevated risk for further aortic events and should be followed vigilantly posthospitalization.
- ,,, et al.Gender‐related differences in acute aortic dissection.Circulation.2004;109:3014–3021.
- ,,, et al.Clinical features and differential diagnosis of aortic dissection: Experience with 236 cases.Mayo Clin Proc.1993;68:642–651.
- ,.The natural history of thoracic aortic aneurysm disease: an overview.J Card Surg.1997;12(suppl):270–278.
- ,,.Dissecting aneurysms of the aorta: a review of 505 cases.Medicine.1958;37:217–279.
- ,,,.Intimal tear without hematoma; an important variant of aortic dissection that can elude current imaging techniques.Circulation.1999;99:1331–1336.
- ,,, et al.Intramural hemorrhage of the thoracic aorta: diagnostic and therapeutic implications.Circulation.1995;92:1465–1472.
- ,.Acute aortic dissection and its variants; towards a common diagnostic and therapeutic approach.Circulation.1995;92:1376–1378.
- ,,.The penetrating aortic ulcer: pathologic manifestations, diagnosis and management.Mayo Clinic Proc.1988;63:718–725.
- ,,, et al.Acute intramural hematoma of the aorta—a mystery in evolution.Circulation.2005;111:1063–1070.
- ,.Intramural hematoma in acute aortic syndrome; more than one variant of dissection?Circulation.2002;106:284–285.
- ,,,,,,.Acute aortic dissection: population‐based incidence compared with degenerative aortic aneurysm rupture.Mayo Clin Proc.2004;79(2):176–180.
- ,,,,,,.Epidemiology and clinicopathology of aortic dissection.Chest.2000;117:1271–1278.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD).JAMA.2000;283:897–903.
- ,,.Clinical prediction of acute aortic dissection.Arch Intern Med.2000;160:2977–2982.
- ,.Risk factors for aortic dissection: a necropsy study of 161 cases.Am J Cardiol.1984;53:849–855.
- ,,, et al.Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD).J Am Coll Cardiol.2004;43:665–669.
- ,,,.Association of aortic dilatation with regurgitant, stenotic and functionally normal bicuspid aortic valves.J Am Coll Cardiol.1992;19:283–288.
- .Clinical significance of the bicuspid aortic valve.Heart.2000;83:81–85.
- ,,,.Aortic root dilatation in young men with normally functioning bicuspid aortic valves.Heart.1999;82:19–22.
- ,,, et al.Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation.J Thorac Cardiovasc Surg.2003;126:797–806.
- ,,.Further delineation of aortic dilation, dissection, and rupture in patients with Turner syndrome.Pediatrics.1998;102(1):e12.
- ,,, et al.Aortic dilatation, dissection and rupture in patients with Turner syndrome.J Pediatr.1986;109:820–826.
- ,.Weight lifting and type II aortic dissection. A case report.J Sports Med Phys Fitness.2004;44:424–427.
- ,,.Recreational weight lifting and aortic dissection: case report.J Vasc Surg.1993;17:774–776.
- ,,,,,,.Ascending aortic dissection in weight lifters with cystic medial degeneration.Ann Thoracic Surg.1990;49:638–642.
- ,,,,.Acute aortic dissection related to crack cocaine.Circulation.2002;105:1592–1595.
- ,.Thoracic aortic dissection secondary to crack cocaine ingestion.Am J Emerg Med.1997;15:507–509.
- ,.Cocaine‐associated dissection of the thoracic aorta.J Emerg Med.1992;10:723–727.
- ,.Methamphetamine as a risk factor for acute aortic dissection.J Forensic Sci.1999;44(1):23–26.
- ,,,,,.Arterial dissections associated with pregnancy.J Vasc Surg.1995;21:515–520.
- .Does this patient have an acute thoracic aortic dissection?JAMA.2002;287:2262–2272.
- ,,,.Fatal haemostatic complications due to thrombolytic therapy in patients falsely diagnosed as acute myocardial infarction.Eur Heart J.1992;13:840–843.
- ,,,,.The inadvertent administration of anticoagulants to ED patients ultimately diagnosed with thoracic aortic dissection.Am J Emerg Med.2005;23:439–442..
- ,,, et al.Chest radiography for the diagnosis of acute aortic syndrome.Am J Med.2004;116(2):73–77.
- ,,, et al.Clinical significance of pleural effusion in acute aortic dissection.Chest.2002;121:825–830.
- ,,,.Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography.J Am Coll Cardiol.1995;25:1393–1401.
- ,.Digital subtraction angiography of aortic dissection.Am J Roentgenol.1983;141:157–161.
- ,,, et al.Spectrum of conditions initially suggesting acute aortic dissection but with negative aortograms.Am J Cardiol.1986;57:322–326.
- ,,, et al.Nonsurgical reconstruction of thoracic aortic dissection by stent‐graft placement.N Engl J Med.1999;340:1539–1545.
- ,,, et al.Endovascular stent‐graft placement for the treatment of aortic dissection.N Engl J Med.1999;340:1546–1552.
- ,.Aortic dissection: New frontiers in diagnosis and management. Part II: Therapeutic management and follow‐up.Circulation.2003;108:772–778.
- ,,,,,.Aortic dissection: percutaneous management of ischemic complications with endovascular stents and balloon fenestration.J Vasc Surg.1996;23:241–251.
- ,,, et al.Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD).Am J Cardiol.2002;89:1235–1238.
- ,,, et al.Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging.Radiology.1996;199:347–352.
- ,,,.Assessment of the thoracic aorta by spiral CT.AJR Am J Roengenol.1992;158:1127–1130.
- ,Radiologic evaluation of aortic dissection.Radiology.1991;180:297–305.
- ,,,,,.Computed tomography of thoracic aortic dissection: accuracy and pitfalls.J Comput Assist Tomogr.1986;10:211–215.
- ,,.Spiral CT in acute non‐cardiac chest pain.Clin Radiol.1999;54(1):38–45.
- ,,, et al.Diagnosis of thoracic aortic dissection. Magnetic resonance imaging versus transesophageal echocardiography.Circulation.1992;85:434–447.
- ,,,,.Comparison of conventional and transesophageal echocardiography with magnetic resonance imaging for anatomical mapping of thoracic aortic dissection: a dual noninvasive imaging study with anatomical and/or angiographic validation.Int J Card Imaging.1994;10:1–14.
- ,.Transesophageal echocardiography in the diagnosis of diseases of the thoracic aorta: part I. Aortic dissection, aortic intramural hematoma and penetrating atherosclerotic ulcer of the aorta.Chest.1999;116:1772–1779.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328:1–9.
- ,,,,,.Echocardiography in diagnosis of aortic dissection.Lancet.1989;1:457–461.
- ,,,,.A prospectus on the prevention of aortic rupture in the Marfan syndrome with data on survivorship without treatment.Johns Hopkins Med J.1971;129:123–129.
- ,,,.Progression of aortic dilatation and the benefit of long‐term beta‐adrenergic blockade in Marfan's syndrome.N Engl J Med.1994;330:1335–1341.
- ,,, et al.Clinical characteristics of hypotension in patients with acute aortic dissection.Am J Cardiol.2005;95:48–52.
- ,,.Cardiac tamponade complicating proximal aortic dissection: is pericardiocentesis harmful?Circulation.1994;90:2375–2379.
- ,,, et al.Predicting in‐hospital mortality in acute type A aortic dissection.Circulation.2002;105:200–206.
- ,,, et al.Diagnosis and management of aortic dissection: task force report of the European Society of Cardiology.Eur Heart J.2001;22:1642–1681.
- ,,,,,.Clinical profiles and outcomes of acute type B aortic dissection in the current era: Lessons from the international registry of aortic dissection.Circulation.2003;108(suppl II):II‐312–II‐317.
- ,,, et al.Endovascular stent‐graft treatment of aortic dissection: determinants of post‐interventional outcome.Eur Heart J.2005;26:489–497.
- ,,, et al.Endovascular stent‐graft placement in aortic dissection: a meta‐analysis [advance access online].Eur Heart J.2005. Published online October 14, 2005.
- ,,,,.Revised diagnostic criteria for the Marfan syndrome.Am J Med Genet.1996;62:417–426.
- ,,,.Treatment of dissecting aneurysms of the aorta without surgery.J Thorac Cardiovasc Surg.1965;50:364–373.
- ,,,,,.Unoperated aortic aneurysms: a survey of 170 patients.Ann Thorac Surg.1995;59:1204–1209.
- ,,, et al.Gender‐related differences in acute aortic dissection.Circulation.2004;109:3014–3021.
- ,,, et al.Clinical features and differential diagnosis of aortic dissection: Experience with 236 cases.Mayo Clin Proc.1993;68:642–651.
- ,.The natural history of thoracic aortic aneurysm disease: an overview.J Card Surg.1997;12(suppl):270–278.
- ,,.Dissecting aneurysms of the aorta: a review of 505 cases.Medicine.1958;37:217–279.
- ,,,.Intimal tear without hematoma; an important variant of aortic dissection that can elude current imaging techniques.Circulation.1999;99:1331–1336.
- ,,, et al.Intramural hemorrhage of the thoracic aorta: diagnostic and therapeutic implications.Circulation.1995;92:1465–1472.
- ,.Acute aortic dissection and its variants; towards a common diagnostic and therapeutic approach.Circulation.1995;92:1376–1378.
- ,,.The penetrating aortic ulcer: pathologic manifestations, diagnosis and management.Mayo Clinic Proc.1988;63:718–725.
- ,,, et al.Acute intramural hematoma of the aorta—a mystery in evolution.Circulation.2005;111:1063–1070.
- ,.Intramural hematoma in acute aortic syndrome; more than one variant of dissection?Circulation.2002;106:284–285.
- ,,,,,,.Acute aortic dissection: population‐based incidence compared with degenerative aortic aneurysm rupture.Mayo Clin Proc.2004;79(2):176–180.
- ,,,,,,.Epidemiology and clinicopathology of aortic dissection.Chest.2000;117:1271–1278.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD).JAMA.2000;283:897–903.
- ,,.Clinical prediction of acute aortic dissection.Arch Intern Med.2000;160:2977–2982.
- ,.Risk factors for aortic dissection: a necropsy study of 161 cases.Am J Cardiol.1984;53:849–855.
- ,,, et al.Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD).J Am Coll Cardiol.2004;43:665–669.
- ,,,.Association of aortic dilatation with regurgitant, stenotic and functionally normal bicuspid aortic valves.J Am Coll Cardiol.1992;19:283–288.
- .Clinical significance of the bicuspid aortic valve.Heart.2000;83:81–85.
- ,,,.Aortic root dilatation in young men with normally functioning bicuspid aortic valves.Heart.1999;82:19–22.
- ,,, et al.Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation.J Thorac Cardiovasc Surg.2003;126:797–806.
- ,,.Further delineation of aortic dilation, dissection, and rupture in patients with Turner syndrome.Pediatrics.1998;102(1):e12.
- ,,, et al.Aortic dilatation, dissection and rupture in patients with Turner syndrome.J Pediatr.1986;109:820–826.
- ,.Weight lifting and type II aortic dissection. A case report.J Sports Med Phys Fitness.2004;44:424–427.
- ,,.Recreational weight lifting and aortic dissection: case report.J Vasc Surg.1993;17:774–776.
- ,,,,,,.Ascending aortic dissection in weight lifters with cystic medial degeneration.Ann Thoracic Surg.1990;49:638–642.
- ,,,,.Acute aortic dissection related to crack cocaine.Circulation.2002;105:1592–1595.
- ,.Thoracic aortic dissection secondary to crack cocaine ingestion.Am J Emerg Med.1997;15:507–509.
- ,.Cocaine‐associated dissection of the thoracic aorta.J Emerg Med.1992;10:723–727.
- ,.Methamphetamine as a risk factor for acute aortic dissection.J Forensic Sci.1999;44(1):23–26.
- ,,,,,.Arterial dissections associated with pregnancy.J Vasc Surg.1995;21:515–520.
- .Does this patient have an acute thoracic aortic dissection?JAMA.2002;287:2262–2272.
- ,,,.Fatal haemostatic complications due to thrombolytic therapy in patients falsely diagnosed as acute myocardial infarction.Eur Heart J.1992;13:840–843.
- ,,,,.The inadvertent administration of anticoagulants to ED patients ultimately diagnosed with thoracic aortic dissection.Am J Emerg Med.2005;23:439–442..
- ,,, et al.Chest radiography for the diagnosis of acute aortic syndrome.Am J Med.2004;116(2):73–77.
- ,,, et al.Clinical significance of pleural effusion in acute aortic dissection.Chest.2002;121:825–830.
- ,,,.Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography.J Am Coll Cardiol.1995;25:1393–1401.
- ,.Digital subtraction angiography of aortic dissection.Am J Roentgenol.1983;141:157–161.
- ,,, et al.Spectrum of conditions initially suggesting acute aortic dissection but with negative aortograms.Am J Cardiol.1986;57:322–326.
- ,,, et al.Nonsurgical reconstruction of thoracic aortic dissection by stent‐graft placement.N Engl J Med.1999;340:1539–1545.
- ,,, et al.Endovascular stent‐graft placement for the treatment of aortic dissection.N Engl J Med.1999;340:1546–1552.
- ,.Aortic dissection: New frontiers in diagnosis and management. Part II: Therapeutic management and follow‐up.Circulation.2003;108:772–778.
- ,,,,,.Aortic dissection: percutaneous management of ischemic complications with endovascular stents and balloon fenestration.J Vasc Surg.1996;23:241–251.
- ,,, et al.Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD).Am J Cardiol.2002;89:1235–1238.
- ,,, et al.Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging.Radiology.1996;199:347–352.
- ,,,.Assessment of the thoracic aorta by spiral CT.AJR Am J Roengenol.1992;158:1127–1130.
- ,Radiologic evaluation of aortic dissection.Radiology.1991;180:297–305.
- ,,,,,.Computed tomography of thoracic aortic dissection: accuracy and pitfalls.J Comput Assist Tomogr.1986;10:211–215.
- ,,.Spiral CT in acute non‐cardiac chest pain.Clin Radiol.1999;54(1):38–45.
- ,,, et al.Diagnosis of thoracic aortic dissection. Magnetic resonance imaging versus transesophageal echocardiography.Circulation.1992;85:434–447.
- ,,,,.Comparison of conventional and transesophageal echocardiography with magnetic resonance imaging for anatomical mapping of thoracic aortic dissection: a dual noninvasive imaging study with anatomical and/or angiographic validation.Int J Card Imaging.1994;10:1–14.
- ,.Transesophageal echocardiography in the diagnosis of diseases of the thoracic aorta: part I. Aortic dissection, aortic intramural hematoma and penetrating atherosclerotic ulcer of the aorta.Chest.1999;116:1772–1779.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328:1–9.
- ,,,,,.Echocardiography in diagnosis of aortic dissection.Lancet.1989;1:457–461.
- ,,,,.A prospectus on the prevention of aortic rupture in the Marfan syndrome with data on survivorship without treatment.Johns Hopkins Med J.1971;129:123–129.
- ,,,.Progression of aortic dilatation and the benefit of long‐term beta‐adrenergic blockade in Marfan's syndrome.N Engl J Med.1994;330:1335–1341.
- ,,, et al.Clinical characteristics of hypotension in patients with acute aortic dissection.Am J Cardiol.2005;95:48–52.
- ,,.Cardiac tamponade complicating proximal aortic dissection: is pericardiocentesis harmful?Circulation.1994;90:2375–2379.
- ,,, et al.Predicting in‐hospital mortality in acute type A aortic dissection.Circulation.2002;105:200–206.
- ,,, et al.Diagnosis and management of aortic dissection: task force report of the European Society of Cardiology.Eur Heart J.2001;22:1642–1681.
- ,,,,,.Clinical profiles and outcomes of acute type B aortic dissection in the current era: Lessons from the international registry of aortic dissection.Circulation.2003;108(suppl II):II‐312–II‐317.
- ,,, et al.Endovascular stent‐graft treatment of aortic dissection: determinants of post‐interventional outcome.Eur Heart J.2005;26:489–497.
- ,,, et al.Endovascular stent‐graft placement in aortic dissection: a meta‐analysis [advance access online].Eur Heart J.2005. Published online October 14, 2005.
- ,,,,.Revised diagnostic criteria for the Marfan syndrome.Am J Med Genet.1996;62:417–426.
- ,,,.Treatment of dissecting aneurysms of the aorta without surgery.J Thorac Cardiovasc Surg.1965;50:364–373.
- ,,,,,.Unoperated aortic aneurysms: a survey of 170 patients.Ann Thorac Surg.1995;59:1204–1209.