User login
Hepatitis C virus: Prevention, screening, and interpretation of assays
Screening for hepatitis C virus (HCV) infection in high-risk populations can identify, early on, people at risk of progressive liver disease who may benefit from antiviral therapy and counseling. The US Centers for Disease Control and Prevention (CDC) recommends that all people be assessed for HCV risk factors and that those with risk factors be screened for HCV antibodies (anti-HCV),1 and members of the national societies of gastroenterology and hepatology have endorsed this recommendation.2
Unfortunately, rates at which primary care patients are assessed for risk factors and the rates at which patients at higher risk are screened remain below the goals set by the CDC.3–6 All health care practitioners need to understand how to establish or exclude a diagnosis of HCV infection and to interpret the tests correctly.
WHY SCREEN FOR HCV?
HCV infection is a major public health problem and a leading cause of chronic liver disease. In the United States, an estimated 3.2 million persons (1.3% of the population) have been infected.7 However, in the inner-city primary care setting the rate of HCV infection is as high as 8%, and in Veterans Administration populations it is 17%.8,9 The worldwide prevalence of HCV infection is 2.0%, corresponding to 140 million persons.
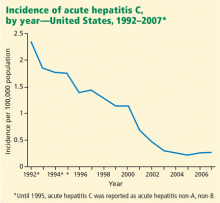
Screening of blood products has led to a decline in the incidence of acute hepatitis C since the late 1980s, although rates have reached a plateau in recent years (Figure 1).10
Approximately 20% of patients infected with HCV develop a serious sequela, such as severe fibrosis, cirrhosis, end-stage liver disease, or hepatocellular carcinoma. Currently, HCV infection causes an estimated 8,000 to 10,000 deaths annually in the United States, and that number is predicted to triple in the next 10 to 20 years. Furthermore, HCV-related disease is the leading indication for liver transplantation in the United States, and it is estimated to cost $600 million to $1 billion annually in medical expenses and loss of work.8
Screening can reduce adverse outcomes
HCV screening has several potential benefits. By detecting HCV infection early, screening facilitates virologic suppression, as treatment earlier in the course of the disease is more effective than later.11,12 Further, early diagnosis together with patient education and subsequent lifestyle modifications may reduce the risk of transmission of HCV infection to other people.13,14
Antiviral therapy with pegylated interferons and ribavirin can cure hepatitis C in up to 90% of cases, depending on the viral genotype15–17 (see discussion of HCV genotypes below). In addition, treatment slows the progression of fibrosis.18 The incidence of hepatocellular carcinoma is lower in patients who achieve a sustained virologic response to antiviral therapy.19 Finally, antiviral therapy prolongs survival.20
New drug therapies are being developed and may, we hope, be even more effective than current drugs. Inhibitors of HCV-specific enzymes such as NS3/4 protease, combined with pegylated interferons and ribavirin, are in phase III clinical trials. These drugs are expected to be available for clinical practice within the next 2 years.21–23 Additionally, nitazoxanide (Alinia), an inducer of eIF2a and PKR phosphorylation, has been shown to increase the treatment response to HCV genotype 4. Studies24 are currently under way in patients infected with HCV genotype 1.
Screening is cost-effective
The National Hepatitis Surveillance Program25 calculated the cost of screening for HCV to be $1,246 per case detected. However, a more vigorous analysis of the same data using several different models to incorporate risk factors based on history revealed costs between $357 and $1,047 per case detected. This compares favorably with the cost of screening for other diseases that physicians routinely screen for.
Antiviral combination therapy for chronic hepatitis C has been shown to be effective in terms of quality-adjusted life-years gained and cost-effectiveness in several studies.26–28
HOW TO SCREEN
To test everyone in the general population would be neither cost-effective nor practical, which is why the CDC recommends that serologic screening for HCV infection be done only in people who have well-established risk factors for it.1,5
Therefore, screening should begin by obtaining a relevant medical history as part of a routine health evaluation. But how should this be done?
McGinn et al29 asked 1,000 patients attending an inner-city clinic to fill out a 27-item questionnaire assessing five “domains” of risk factors for HCV: work, medical, exposure, personal care, and social history. Afterward, they tested all 1,000 patients. They found that the risk factors that best predicted positive results on testing were in three domains: medical (eg, blood transfusions, dialysis, other medical procedures, and elevated liver enzymes), exposure (past contact with another person’s blood), and social history (eg, illicit drug use, incarceration, and sexual activity).
The National Hepatitis Surveillance Program25 explored the cost and yield of several screening strategies for hepatitis C, ie, testing only in patients who had a greater than 7% likelihood of infection based on an empirically derived mathematical model; testing only if significant risk factors were revealed in a simple questionnaire; or testing only if the alanine aminotransferase (ALT) level was elevated. The predictive mathematical model was the most effective and efficient means of deciding who should be tested.
Unfortunately, such a model is too cumbersome to be clinically applicable, and clinical prediction tools for HCV screening have been underused.
GROUPS AT HIGH RISK OF HCV
Groups at risk of HCV infection can be classified as being at high, intermediate, or low risk. The American Association for the Study of Liver Diseases2 rates the level of evidence for screening in all of the following risk groups as class I (ie, there is evidence or general agreement that it is beneficial, useful, and effective) and level B (ie, the data are derived from non-randomized studies).
Intravenous drug abusers
Intravenous drug abuse is the strongest independent risk factor for HCV infection.30–33 It has been the main route of HCV infection over the past decades and currently accounts for 60% of HCV transmission in the United States.7,10,34–37
Hemophilia patients treated with clotting factor concentrates produced before 1987
HCV seroprevalence is very high in patients with hemophilia who received infusions of plasma-derived clotting factor concentrates before 1987.38 In these patients, the HCV genotypes are predominantly 1 and 3, and to a lesser extent genotype 2.39,40 These genotypes likely reflect the prior exposures of the plasma donors.41 (See discussion of HCV genotypes below.) Individuals receiving clotting factor concentrates prepared from plasma pools were at high risk of HCV infection until effective procedures to inactivate viruses were introduced in 1985 (factor VIII) and 1987 (factor IX).42
People infected with HIV
About 25% of people infected with human immunodeficiency virus (HIV) in the Western world also have chronic HCV infection.43 Progression of liver disease is accelerated in HIV-HCV coinfection, and the risk of cirrhosis is twice as high.44
However, about 6% of HIV-positive patients fail to develop HCV antibodies when infected. Thus, HCV RNA should be assessed in HIV patients with unexplained liver disease who are negative for anti-HCV.45
The distribution of HCV genotypes in HIV-infected patients reflects the route of transmission. Genotype 1b accounts for 66% of posttransfusion HCV infections, while genotypes 1a and 3a are more common in intravenous drug users.
GROUPS AT INTERMEDIATE RISK OF HCV
Recipients of blood transfusions before 1992
Before 1992, blood transfusions carried a risk of HCV infection of up to 7% with each unit transfused. Prospective studies of transfusion recipients in the United States found that rates of posttransfusion hepatitis in the 1960s exceeded 20%,36 since most patients received multiple units of blood.
In the mid-1970s, before HCV had been identified, available diagnostic tests indicated that 90% of cases of posttransfusion hepatitis were not caused by hepatitis A or hepatitis B viruses. By this time, the move to all-volunteer blood donors instead of paid donors had reduced the risk of posttransfusion hepatitis to 10%.22,37,46
Although non-A, non-B hepatitis was first recognized because of its association with blood transfusion, population-based sentinel surveillance showed that it accounted for 15% to 20% of cases of community-acquired viral hepatitis in the United States.35 The advent of molecular cloning in 1988 indicated that non-A, non-B hepatitis was primarily caused by HCV.47–52
Screening of blood has reduced the rate of posttransfusion hepatitis C by a factor of about 10,000, to a current rate of 1 per million transfusions.53 The few cases that still occur are due to newly infected people donating blood before they have developed antibodies to the virus, which can take up to 8 weeks.54
Recipients of solid-organ transplants before 1992
Before organ donors were screened for HCV, recipients of solid-organ transplants from infected donors had a high risk of acquiring HCV infection. Transmission rates in different cohorts ranged from 30% to 80%.55 In an attempt to improve the safety of organ transplantation, many transplant centers now screen donors for anti-HCV and test for HCV RNA for verification.
A related problem is pre-existing HCV infection in transplant recipients. Izopet et al56 reported that, in renal transplant recipients with preexisting HCV infection, the HCV RNA titer rose about 10 times (1 log) higher after transplantation, owing to the immunosuppressive drugs that transplant recipients must take. Although this higher viral load does not affect the progression of fibrosis in all patients, the effect of immunosuppressive therapy on liver disease results in a worse outcome for some, and it reduces survival beginning in the second decade after kidney transplantation.56
Additionally, treatment of HCV infection in transplant recipients may pose a challenge, as those receiving immunosuppressive therapy with tacrolimus (Prograf) or cyclosporine (Sandimmune) may develop some degree of renal insufficiency, complicating the use of ribavirin (Rebetol) and subjecting patients to a higher risk of severe anemia. Furthermore, interferon therapy increases the risk of renal allograft rejection and, accordingly, is not often used in renal transplant recipients.
Patients with unexplained elevated aminotransferase levels
HCV infection affects an estimated 1.8% of the general population, but the rate is much higher in people with ALT levels over 40 U/L. Most patients with chronic hepatitis C have no symptoms or only mild symptoms and minimally elevated levels of ALT and aspartate aminotransferase (AST)—ie, two to five times higher than the upper limit of normal.
The first step in the workup of aminotransferase elevations is to confirm the abnormality by repeating the blood test. If an elevation is confirmed, further investigation is warranted. A directed history and physical examination is important and may disclose risk factors, raising clinical suspicion of a particular disease.
Some caveats: The proportion of patients with HCV viremia who have abnormally high aminotransferase levels ranges between only 54% and 66%.57–59 In patients with risk factors for HCV infection and abnormal liver enzyme levels, HCV infection is probable but not certain. Also, liver enzyme tests do not reveal the extent of hepatic injury or reflect the true status of hepatic function.60
Infants born to infected mothers
Children born to HCV-positive women should be tested for anti-HCV no sooner than age 12 months, when passively transferred maternal anti-HCV declines below detectable levels. If earlier diagnosis of HCV infection is desired, a real-time polymerase chain reaction (PCR) test for HCV RNA can be done at or after the infant's first “well-child” visit at age 1 to 2 months.
If positive for either anti-HCV or HCV RNA, children should be evaluated for liver disease, and those with persistently elevated ALT levels should be referred to a specialist for medical management.2,5
GROUPS AT LOW RISK OF HCV
People who have had sexual relations with multiple or infected partners
Sexual activity is associated with a low but measurable risk of transmission of HCV. Large population-based studies, including the National Hepatitis Surveillance Program,25 found an independent association between HCV infection and having sexual relations with multiple partners or with a partner who is infected with HCV.
The CDC reported that 15% to 20% of patients with acute hepatitis C had a history of sexual exposure but no other risk factors. Two-thirds of them had an anti-HCV-positive sexual partner, and one-third reported having had more than two partners in the 6 months before illness.5
More data are needed to determine the risk of and the factors related to transmission of HCV between long-term steady partners as well as in persons with high-risk sexual practices, including whether other sexually transmitted diseases promote transmission of HCV by influencing viral load or modifying mucosal barriers.
Health care workers exposed to HCV, eg, by needlestick
The prevalence of HCV infection in health care workers is no greater than that in the general population, averaging 1% to 2%, and is actually 10 times lower than that of hepatitis B virus infection.47,48,61,62
However, within the disciplines, some groups have a higher prevalence of HCV infection, suggesting that some occupations carry a higher risk. In two US studies, the prevalence of HCV infection was higher in oral surgeons (2.0% and 9.3%) than in other dentists (0.7% and 0.97%).63,64
In a single study that evaluated risk factors for infection, a history of needlestick injury was the only occupational risk factor that was independently associated with HCV infection.65 The average incidence of anti-HCV seroconversion after a needlestick or after an injury with a sharp object contaminated by an HCV-positive source is 1.8% (range 0%–7%).66–69
Although no studies of incidence have documented transmission via mucous membrane or nonintact skin exposures, transmission of HCV from blood splashes to the conjunctiva have been described.70,71
It is worth noting that exposure to blood from unclean needles used in tattooing or body piercing also confers a risk of HCV infection.
SEROLOGIC SCREENING TESTS FOR HCV
- Serologic assays that detect specific antibody to HCV (anti-HCV)
- Molecular assays that detect viral RNA.
Initial serologic screening tests for anti-HCV
Two EIAs are approved for clinical use:
- Abbott HCV EIA 2.0 (Abbott Laboratories, Abbott Park, IL)
- Ortho HCV Version 3.0 enzyme-linked immunosorbent assay (ELISA) (Ortho-Clinical Diagnostics, Rochester, NY).
One enhanced chemiluminescence immunoassay is also approved:
- Vitros Anti-HCV assay (Ortho-Clinical Diagnostics). In practical terms, this test is equivalent to the two EIAs, and the discussion below about EIAs applies to this test as well.
These third-generation tests are highly sensitive (> 99%) and specific (99%) in immunocompetent patients, and eliminate the need for a confirmatory immunoblot assay in patients with clinical liver disease, particularly those with risk factors for HCV infection.
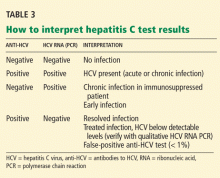
False-positive results are rare now, but they were common with earlier generations of these assays. Most false-positive results occur in patients with autoimmune liver disease or hypergammaglobulinemia who have normal liver enzyme levels and no risk factors for HCV infection. In fact, all positive anti-HCV results should be followed up with an HCV RNA test.
False-negative results are also uncommon, usually occurring only in immunosuppressed patients (eg, organ transplant recipients and HIV-positive patients) and in patients on long-term hemodialysis. Therefore, patients with a history of hemodialysis should be considered for an HCV RNA assay rather than an EIA. Measurement of ALT will not be useful because ALT levels are lower in patients with end-stage renal disease. In most other clinical situations, the HCV EIA is an outstanding screening test for HCV infection because of its high sensitivity and relatively low cost (< $50).
Although the specificity of these tests is good, the predictive value of a positive result varies substantially by the pretest probability of HCV infection. For example, in a group of injection-drug users who are very likely to have ongoing or remote infection, all positive HCV EIA results are likely truly positive.74 On the other hand, in healthy blood donors, up to half of all positive third-generation EIA tests are falsely positive.75
Important points
- A positive anti-HCV antibody test does not distinguish acute from chronic disease or active from past infection, nor is it a sign of immunity or protection.
- A positive anti-HCV EIA requires HCV RNA measurement to discriminate between current infection on the one hand, and either resolved HCV infection or a false-positive result on the other.
- A positive EIA anti-HCV test is a marker that hepatitis C may be present, and it must be followed by confirmatory HCV RNA testing.
- Physicians should be mindful of the potential tribulations associated with false-positive tests. A false-positive test may result in harm to patients that is difficult to measure, such as anxiety, labeling in the medical record, and detrimental effects on close relationships.
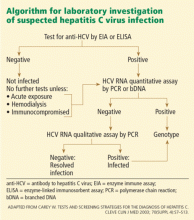
CONFIRMATORY TESTING WITH ASSAYS FOR HCV RNA
As stated above, a positive result on an anti-HCV EIA needs to be confirmed with an assay for HCV RNA, of which there are two types, ie, qualitative and quantitative.
Each involves trade-offs. Qualitative assays are more sensitive and detect more cases, but they provide no information about the amount of virus (viral load). Quantitative assays are less sensitive, so a negative result does not completely exclude hepatitis C, although they can still can detect 95% of cases. They do, however, measure the viral load.
Therefore, the type of test to use depends on the patient’s risk profile, the goals of testing, and the setting in which future care will be provided. The primary objective when a patient has a positive EIA test is to determine whether he or she has ongoing infection, a goal most expeditiously achieved using a qualitative assay. However, since a quantitative assay can detect the vast majority of cases of active HCV infection, many clinicians select this as the test of first choice when the probability of HCV is high (eg, in a patient with risk factors and abnormal liver tests). If the pretest probability is low, a qualitative assay is the better choice.
Many commercial assays are available for detecting (qualitative assays) or measuring (quantitative assays) HCV RNA.
Qualitative HCV RNA assays
The approved qualitative assays are:
- Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics, Pleasanton, CA)
- Cobas Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics)
- Ampliscreen (Roche Molecular Diagnostics)
- Versant HCV RNA Qualitative Assay (Siemens Healthcare Diagnostics, Deerfield, IL)
- Procleix HIV-1/HCV Assay (Chiron, Emeryville, CA).
Quantitative HCV RNA assays
The approved quantitative assays are:
- Amplicor HCV Monitor (Roche Molecular Diagnostics)
- Cobas Amplicor HCV Monitor, version 2.0 (Roche Molecular Diagnostics)
- Versant HCV RNA 3.0 Assay (bDNA) (Siemens Healthcare Diagnostics)
- Cobas Taqman HCV Test (Roche Molecular Diagnostics).
Quantitative tests use target amplification with PCR, transcription-mediated amplification (TMA), or a signal amplification technique such as a branched DNA (bDNA) assay. The sensitivity varies for different types of amplification. TMA assays appear to be the most sensitive for detecting HCV RNA.
The latest innovation is real-time PCR, which shortens the typical time for PCR processing from 1.5 hours to 35 minutes. It may also detect relapsed HCV infection earlier than regular PCR. With the recent availability of real-time PCR assays, which have sensitivities of 10 to 50 IU/mL, many experts feel there is no longer a need for qualitative assays.74 In fact, many laboratories no longer offer qualitative testing. The Cleveland Clinic laboratory has recently stopped offering this test.
Because RNA testing is widely available, the recombinant immunoblot assay (RIBA) has become obsolete in diagnosing HCV infection, except in special circumstances. Currently, the primary purpose of RIBA testing is to distinguish between resolved HCV infection (EIA-positive, HCV RNA-negative, RIBA-positive) and a false-positive EIA (EIA-positive, HCV RNA-negative, RIBA-negative).
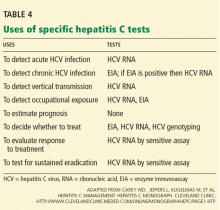
In summary, patients suspected of having acute or chronic HCV infection should first be tested for anti-HCV. Subsequently, HCV RNA testing should be performed in:
- Patients with a positive anti-HCV test
- Patients for whom antiviral treatment is being considered (using a sensitive quantitative assay)
- Patients with unexplained liver disease whose anti-HCV test is negative and who are immunocompromised or suspected of having acute HCV infection.
Significance of the HCV viral load
The significance of the HCV viral load is widely misunderstood. The amount of virus in the blood does not correlate with symptoms, histologic liver injury, or the stage or aggressiveness of disease. Its sole importance is in relation to therapy.
The HCV viral load, measured before treatment, helps predict the likelihood of a treatment response: the lower the pretreatment viral load, the more likely that the patient will respond to current HCV therapies.
Additionally, the pretreatment viral load serves as a baseline for comparison with subsequent measurements during treatment. Patients with HCV genotype 1 who do not achieve more than a 2-log (99%) reduction in viral load by the 12th week of treatment (an early virologic response) have a low response rate, and treatment should generally be stopped, given its cost and side effects.76 However, measuring the viral load to detect an early virologic response is less helpful in patients with HCV genotype 2 or 3 infection, since these patients require only 24 weeks of therapy and most of them clear the virus by week 12 and respond to therapy.
Additionally, patients with genotype 2 or 3 and those with a viral load of less than 600,000 IU/mL have been found to achieve higher rates of sustained virologic response.15 A sustained virologic response is defined as the absence of HCV RNA 24 weeks after stopping treatment and is now considered to be the best predictor of long-term treatment response. A sustained virologic response is generally regarded as a “virologic cure.”
HCV GENOTYPE AFFECTS SUCCESS AND DURATION OF TREATMENT
HCV has at least six major genotypes.1,3–6 Several genotypes are subclassified as “a” or “b” (ie, genotype 1a or 1b); however, these distinctions are of little clinical use.
In the laboratory, HCV genotypes are identified by restriction fragment length polymorphism, by direct sequence analysis, or by reverse hybridization. Once the HCV genotype has been identified, there is no need to repeat the test.
Different genotypes are more common in some areas of the world than in others. Genotype 1 is the one most common in the United States (accounting for 70% to 75% of cases), followed by genotypes 2 and 3 (25%–30%). Genotype 4 is most common in Egypt and the Arabian peninsula.
HCV genotyping is important because it can help predict the likelihood of a response to treatment and in planning the dose and duration of therapy.77 For example, treatment with pegylated interferon plus ribavirin is predicted to work approximately 50% of the time for people with genotype 1, but 80% to 90% of the time for people with genotypes 2 or 3.15–17,78 Additionally, patients with genotype 1 need 12 months of therapy to achieve maximum benefit, whereas those with genotypes 2 and 3 require treatment for only 6 months to achieve maximum benefit.
- Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Intern Med 2004; 141:715–717.
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49:1335–1374.
- Shehab TM, Orrego M, Chunduri R, Lok AS. Identification and management of hepatitis C patients in primary care clinics. Am J Gastroenterol 2003; 98:639–644.
- Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care physicians in the USA: results of a national survey. J Viral Hepat 2001; 8:377–383.
- US Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep 1998; 47:1–39.
- US Centers for Disease Control and Prevention. National prevention strategy: a comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences; summer 2001. http://www.cdc.gov/hepatitis/HCV/Strategy/NatHep-CPrevStrategy.htm. Accessed August 8, 2010.
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–714.
- Kim WR. The burden of hepatitis C in the United States. Hepatology 2002; 36(suppl 1):S30–S34.
- Lau DT, Kleiner DE, Ghany MG, Park Y, Schmid P, Hoofnagle JH. 10-Year follow-up after interferon-alpha therapy for chronic hepatitis C. Hepatology 1998; 28:1121–1127.
- Daniels D, Grytdal S, Wasley A; US Centers for Disease Control and Prevention. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ 2009; 58:1–27.
- Thomson BJ, Kwong G, Ratib S, et al; Trent HCV Study Group. Response rates to combination therapy for chronic HCV infection in a clinical setting and derivation of probability tables for individual patient management. J Viral Hepat 2008; 15:271–278.
- Hayashi N, Takehara T. Antiviral therapy for chronic hepatitis C: past, present, and future. J Gastroenterol 2006; 41:17–27.
- Gordon FD. Cost-effectiveness of screening patients for hepatitis C. Am J Med 1999; 107:36S–40S.
- Hill L, Henry B, Schweikert S; Prevention Practice Committee, American College of Preventive Medicine. Screening for chronic hepatitis C: American College of Preventive Medicine practice policy statement. Am J Prev Med 2005; 28:327–330.
- Hadziyannis SJ, Sette H, Morgan TR, et al; PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140:346–355.
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358:958–965.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Cammà C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology 2004; 39:333–342.
- Yoshida H, Tateishi R, Arakawa Y, et al. Benefit of interferon therapy in hepatocellular carcinoma prevention for individual patients with chronic hepatitis C. Gut 2004; 53:425–430.
- Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology 2002; 123:483–491.
- Hézode C, Forestier N, Dusheiko G, et al; PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009; 360:1839–1850.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Berman K, Kwo PY. Boceprevir, an NS3 protease inhibitor of HCV. Clin Liver Dis 2009; 13:429–439.
- Rossignol JF, Elfert A, Keeffe EB. Treatment of chronic hepatitis C using a 4-week lead-in with nitazoxanide before peginterferon plus nitazoxanide. J Clin Gastroenterol 2009 Dec 31; epub ahead of print.
- Lapane KL, Jakiche AF, Sugano D, Weng CS, Carey WD. Hepatitis C infection risk analysis: who should be screened? Comparison of multiple screening strategies based on the National Hepatitis Surveillance Program. Am J Gastroenterol 1998; 93:591–596.
- Wong JB, Davis GL, McHutchison JG, Manns MP, Albrecht JK; International Hepatitis Interventional Therapy Group. Economic and clinical effects of evaluating rapid viral response to peginterferon alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C. Am J Gastroenterol 2003; 98:2354–2362.
- Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA 2003; 290:228–237.
- Sullivan SD, Jensen DM, Bernstein DE, et al. Cost-effectiveness of combination peginterferon alpha-2a and ribavirin compared with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol 2004; 99:1490–1496.
- McGinn T, O’Connor-Moore N, Alfandre D, Gardenier D, Wisnivesky J. Validation of a hepatitis C screening tool in primary care. Arch Intern Med 2008; 168:2009–2013.
- Kaur S, Rybicki L, Bacon BR, Gollan JL, Rustgi VK, Carey WD. Performance characteristics and results of a large-scale screening program for viral hepatitis and risk factors associated with exposure to viral hepatitis B and C: results of the National Hepatitis Screening Survey. National Hepatitis Surveillance Group. Hepatology 1996; 24:979–986.
- Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol 2000; 95:740–747.
- Austin GE, Jensen B, Leete J, et al. Prevalence of hepatitis C virus seropositivity among hospitalized US veterans. Am J Med Sci 2000; 319:353–359.
- Yawn BP, Wollan P, Gazzuola L, Kim WR. Diagnosis and 10-year follow-up of a community-based hepatitis C cohort. J Fam Pract 2002; 51:135–140.
- Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 18(suppl 1):S11–S19.
- Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis 1997; 1:559–568,
- Alter MJ, Hadler SC, Judson FN, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA 1990; 264:2231–2235.
- Wasley A, Miller JT, Finelli L; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis—United States, 2005. MMWR Surveill Summ 2007; 56:1–24.
- Goedert JJ, Chen BE, Preiss L, Aledort LM, Rosenberg PS. Reconstruction of the hepatitis C virus epidemic in the US hemophilia population, 1940–1990. Am J Epidemiol 2007; 165:1443–1453.
- Eyster ME, Sherman KE, Goedert JJ, Katsoulidou A, Hatzakis A. Prevalence and changes in hepatitis C virus genotypes among multitransfused persons with hemophilia. The Multicenter Hemophilia Cohort Study. J Infect Dis 1999; 179:1062–1069.
- Yee TT, Griffioen A, Sabin CA, Dusheiko G, Lee CA. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut 2000; 47:845–851.
- Lee C, Dusheiko G. The natural history and antiviral treatment of hepatitis C in haemophilia. Haemophilia 2002; 8:322–329.
- Makris M, Garson JA, Ring CJ, Tuke PW, Tedder RS, Preston FE. Hepatitis C viral RNA in clotting factor concentrates and the development of hepatitis in recipients. Blood 1993; 81:1898–1902.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831–837.
- Sulkowski MS. The HIV-coinfected patient: managing viral hepatitis. J Acquir Immune Defic Syndr 2007; 45(suppl 2):S36–S37.
- Bonacini M, Lin HJ, Hollinger FB. Effect of coexisting HIV-1 infection on the diagnosis and evaluation of hepatitis C virus. J Acquir Immune Defic Syndr 2001; 26:340–344.
- Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health 1996; 86:655–661.
- Bell J, Batey RG, Farrell GC, Crewe EB, Cunningham AL, Byth K. Hepatitis C virus in intravenous drug users. Med J Aust 1990; 153:274–276.
- Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol 1997; 35:3274–3277.
- Patrick DM, Tyndall MW, Cornelisse PG, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ 2001; 165:889–895.
- Seeff LB, Wright EC, Zimmerman HJ, McCollum RW. VA cooperative study of post-transfusion hepatitis, 1969-1974: incidence and characteristics of hepatitis and responsible risk factors. Am J Med Sci 1975; 270:355–362.
- Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med 1975; 292:767–770.
- Alter HJ, Holland PV, Purcell RH, et al. Posttransfusion hepatitis after exclusion of commercial and hepatitis-B antigen-positive donors. Ann Intern Med. 1972; 77:691–699.
- Blajchman MA, Vamvakas EC. The continuing risk of transfusion-transmitted infections. N Engl J Med 2006; 355:1303–1305.
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345:41–52.
- Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int 1994; 45:238–244.
- Izopet J, Rostaing L, Sandres K, et al. Longitudinal analysis of hepatitis C virus replication and liver fibrosis progression in renal transplant recipients. J Infect Dis 2000; 181:852–858.
- Dubois F, Desenclos JC, Mariotte N, Goudeau A. Hepatitis C in a French population-based survey, 1994: seroprevalence, frequency of viremia, genotype distribution, and risk factors. The Collaborative Study Group. Hepatology 1997; 25:1490–1496.
- Bellentani S, Pozzato G, Saccoccio G, et al. Clinical course and risk factors of hepatitis C virus related liver disease in the general population: report from the Dionysos study. Gut 1999; 44:874–880.
- Alberti A, Noventa F, Benvegnù L, Boccato S, Gatta A. Prevalence of liver disease in a population of asymptomatic persons with hepatitis C virus infection. Ann Intern Med 2002; 137:961–964.
- Shiffman ML, Diago M, Tran A, et al. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol 2006; 4:645–652.
- Stary A, Kopp W, Hofmann H, Heller-Vitouch C, Kunz C. Seroepidemiologic study of hepatitis C virus in sexually transmitted disease risk groups. Sex Transm Dis 1992; 19:252–258.
- Weinstock HS, Bolan G, Reingold AL, Polish LB. Hepatitis C virus infection among patients attending a clinic for sexually transmitted diseases. JAMA 1993; 269:392–394.
- Thomas DL, Gruninger SE, Siew C, Joy ED, Quinn TC. Occupational risk of hepatitis C infections among general dentists and oral surgeons in North America. Am J Med 1996; 100:41–45.
- Klein RS, Freeman K, Taylor PE, Stevens CE. Occupational risk for hepatitis C virus infection among New York City dentists. Lancet 1991; 338:1539–1542.
- Polish LB, Tong MJ, Co RL, Coleman PJ, Alter MJ. Risk factors for hepatitis C virus infection among health care personnel in a community hospital. Am J Infect Control 1993; 21:196–200.
- Alter MJ. Occupational exposure to hepatitis C virus: a dilemma. Infect Control Hosp Epidemiol 1994; 15:742–744.
- Lanphear BP, Linnemann CC, Cannon CG, DeRonde MM, Pendy L, Kerley LM. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect Control Hosp Epidemiol 1994; 15:745–750.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposures in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control 1995; 23:273–277.
- Mitsui T, Iwano K, Masuko K, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology 1992; 16:1109–1114.
- Sartori M, La Terra G, Aglietta M, Manzin A, Navino C, Verzetti G. Transmission of hepatitis C via blood splash into conjunctiva. Scand J Infect Dis 1993; 25:270–271.
- Ippolito G, Puro V, Petrosillo N, De Carli G, Micheloni G, Magliano E. Simultaneous infection with HIV and hepatitis C virus following occupational conjunctival blood exposure. JAMA 1998; 280:28.
- Carey W. Tests and screening strategies for the diagnosis of hepatitis C. Cleve Clin J Med 2003; 70(suppl 4):S7–S13.
- Carey WD, Jeffers L, Kugelmas M, et al; Hepatitis C management. Hepatitis C Monograph. Cleveland Clinic; www.clevelandclinicmeded.com/online/monograph/hepc/page1.htm. Accessed 7/30/2010.
- Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA 2007; 297:724–732.
- Bowden DS, Berzsenyi MD. Chronic hepatitis C virus infection: genotyping and its clinical role. Future Microbiol 2006; 1:103–112.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Poynard T, McHutchison J, Davis GL, et al. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology 2000; 32:1131–1137.
- Zeuzem S. Interferon-based therapy for chronic hepatitis C: current and future perspectives. Nat Clin Pract Gastroenterol Hepatol 2008; 5:610–622.
Screening for hepatitis C virus (HCV) infection in high-risk populations can identify, early on, people at risk of progressive liver disease who may benefit from antiviral therapy and counseling. The US Centers for Disease Control and Prevention (CDC) recommends that all people be assessed for HCV risk factors and that those with risk factors be screened for HCV antibodies (anti-HCV),1 and members of the national societies of gastroenterology and hepatology have endorsed this recommendation.2
Unfortunately, rates at which primary care patients are assessed for risk factors and the rates at which patients at higher risk are screened remain below the goals set by the CDC.3–6 All health care practitioners need to understand how to establish or exclude a diagnosis of HCV infection and to interpret the tests correctly.
WHY SCREEN FOR HCV?
HCV infection is a major public health problem and a leading cause of chronic liver disease. In the United States, an estimated 3.2 million persons (1.3% of the population) have been infected.7 However, in the inner-city primary care setting the rate of HCV infection is as high as 8%, and in Veterans Administration populations it is 17%.8,9 The worldwide prevalence of HCV infection is 2.0%, corresponding to 140 million persons.
Screening of blood products has led to a decline in the incidence of acute hepatitis C since the late 1980s, although rates have reached a plateau in recent years (Figure 1).10
Approximately 20% of patients infected with HCV develop a serious sequela, such as severe fibrosis, cirrhosis, end-stage liver disease, or hepatocellular carcinoma. Currently, HCV infection causes an estimated 8,000 to 10,000 deaths annually in the United States, and that number is predicted to triple in the next 10 to 20 years. Furthermore, HCV-related disease is the leading indication for liver transplantation in the United States, and it is estimated to cost $600 million to $1 billion annually in medical expenses and loss of work.8
Screening can reduce adverse outcomes
HCV screening has several potential benefits. By detecting HCV infection early, screening facilitates virologic suppression, as treatment earlier in the course of the disease is more effective than later.11,12 Further, early diagnosis together with patient education and subsequent lifestyle modifications may reduce the risk of transmission of HCV infection to other people.13,14
Antiviral therapy with pegylated interferons and ribavirin can cure hepatitis C in up to 90% of cases, depending on the viral genotype15–17 (see discussion of HCV genotypes below). In addition, treatment slows the progression of fibrosis.18 The incidence of hepatocellular carcinoma is lower in patients who achieve a sustained virologic response to antiviral therapy.19 Finally, antiviral therapy prolongs survival.20
New drug therapies are being developed and may, we hope, be even more effective than current drugs. Inhibitors of HCV-specific enzymes such as NS3/4 protease, combined with pegylated interferons and ribavirin, are in phase III clinical trials. These drugs are expected to be available for clinical practice within the next 2 years.21–23 Additionally, nitazoxanide (Alinia), an inducer of eIF2a and PKR phosphorylation, has been shown to increase the treatment response to HCV genotype 4. Studies24 are currently under way in patients infected with HCV genotype 1.
Screening is cost-effective
The National Hepatitis Surveillance Program25 calculated the cost of screening for HCV to be $1,246 per case detected. However, a more vigorous analysis of the same data using several different models to incorporate risk factors based on history revealed costs between $357 and $1,047 per case detected. This compares favorably with the cost of screening for other diseases that physicians routinely screen for.
Antiviral combination therapy for chronic hepatitis C has been shown to be effective in terms of quality-adjusted life-years gained and cost-effectiveness in several studies.26–28
HOW TO SCREEN
To test everyone in the general population would be neither cost-effective nor practical, which is why the CDC recommends that serologic screening for HCV infection be done only in people who have well-established risk factors for it.1,5
Therefore, screening should begin by obtaining a relevant medical history as part of a routine health evaluation. But how should this be done?
McGinn et al29 asked 1,000 patients attending an inner-city clinic to fill out a 27-item questionnaire assessing five “domains” of risk factors for HCV: work, medical, exposure, personal care, and social history. Afterward, they tested all 1,000 patients. They found that the risk factors that best predicted positive results on testing were in three domains: medical (eg, blood transfusions, dialysis, other medical procedures, and elevated liver enzymes), exposure (past contact with another person’s blood), and social history (eg, illicit drug use, incarceration, and sexual activity).
The National Hepatitis Surveillance Program25 explored the cost and yield of several screening strategies for hepatitis C, ie, testing only in patients who had a greater than 7% likelihood of infection based on an empirically derived mathematical model; testing only if significant risk factors were revealed in a simple questionnaire; or testing only if the alanine aminotransferase (ALT) level was elevated. The predictive mathematical model was the most effective and efficient means of deciding who should be tested.
Unfortunately, such a model is too cumbersome to be clinically applicable, and clinical prediction tools for HCV screening have been underused.
GROUPS AT HIGH RISK OF HCV
Groups at risk of HCV infection can be classified as being at high, intermediate, or low risk. The American Association for the Study of Liver Diseases2 rates the level of evidence for screening in all of the following risk groups as class I (ie, there is evidence or general agreement that it is beneficial, useful, and effective) and level B (ie, the data are derived from non-randomized studies).
Intravenous drug abusers
Intravenous drug abuse is the strongest independent risk factor for HCV infection.30–33 It has been the main route of HCV infection over the past decades and currently accounts for 60% of HCV transmission in the United States.7,10,34–37
Hemophilia patients treated with clotting factor concentrates produced before 1987
HCV seroprevalence is very high in patients with hemophilia who received infusions of plasma-derived clotting factor concentrates before 1987.38 In these patients, the HCV genotypes are predominantly 1 and 3, and to a lesser extent genotype 2.39,40 These genotypes likely reflect the prior exposures of the plasma donors.41 (See discussion of HCV genotypes below.) Individuals receiving clotting factor concentrates prepared from plasma pools were at high risk of HCV infection until effective procedures to inactivate viruses were introduced in 1985 (factor VIII) and 1987 (factor IX).42
People infected with HIV
About 25% of people infected with human immunodeficiency virus (HIV) in the Western world also have chronic HCV infection.43 Progression of liver disease is accelerated in HIV-HCV coinfection, and the risk of cirrhosis is twice as high.44
However, about 6% of HIV-positive patients fail to develop HCV antibodies when infected. Thus, HCV RNA should be assessed in HIV patients with unexplained liver disease who are negative for anti-HCV.45
The distribution of HCV genotypes in HIV-infected patients reflects the route of transmission. Genotype 1b accounts for 66% of posttransfusion HCV infections, while genotypes 1a and 3a are more common in intravenous drug users.
GROUPS AT INTERMEDIATE RISK OF HCV
Recipients of blood transfusions before 1992
Before 1992, blood transfusions carried a risk of HCV infection of up to 7% with each unit transfused. Prospective studies of transfusion recipients in the United States found that rates of posttransfusion hepatitis in the 1960s exceeded 20%,36 since most patients received multiple units of blood.
In the mid-1970s, before HCV had been identified, available diagnostic tests indicated that 90% of cases of posttransfusion hepatitis were not caused by hepatitis A or hepatitis B viruses. By this time, the move to all-volunteer blood donors instead of paid donors had reduced the risk of posttransfusion hepatitis to 10%.22,37,46
Although non-A, non-B hepatitis was first recognized because of its association with blood transfusion, population-based sentinel surveillance showed that it accounted for 15% to 20% of cases of community-acquired viral hepatitis in the United States.35 The advent of molecular cloning in 1988 indicated that non-A, non-B hepatitis was primarily caused by HCV.47–52
Screening of blood has reduced the rate of posttransfusion hepatitis C by a factor of about 10,000, to a current rate of 1 per million transfusions.53 The few cases that still occur are due to newly infected people donating blood before they have developed antibodies to the virus, which can take up to 8 weeks.54
Recipients of solid-organ transplants before 1992
Before organ donors were screened for HCV, recipients of solid-organ transplants from infected donors had a high risk of acquiring HCV infection. Transmission rates in different cohorts ranged from 30% to 80%.55 In an attempt to improve the safety of organ transplantation, many transplant centers now screen donors for anti-HCV and test for HCV RNA for verification.
A related problem is pre-existing HCV infection in transplant recipients. Izopet et al56 reported that, in renal transplant recipients with preexisting HCV infection, the HCV RNA titer rose about 10 times (1 log) higher after transplantation, owing to the immunosuppressive drugs that transplant recipients must take. Although this higher viral load does not affect the progression of fibrosis in all patients, the effect of immunosuppressive therapy on liver disease results in a worse outcome for some, and it reduces survival beginning in the second decade after kidney transplantation.56
Additionally, treatment of HCV infection in transplant recipients may pose a challenge, as those receiving immunosuppressive therapy with tacrolimus (Prograf) or cyclosporine (Sandimmune) may develop some degree of renal insufficiency, complicating the use of ribavirin (Rebetol) and subjecting patients to a higher risk of severe anemia. Furthermore, interferon therapy increases the risk of renal allograft rejection and, accordingly, is not often used in renal transplant recipients.
Patients with unexplained elevated aminotransferase levels
HCV infection affects an estimated 1.8% of the general population, but the rate is much higher in people with ALT levels over 40 U/L. Most patients with chronic hepatitis C have no symptoms or only mild symptoms and minimally elevated levels of ALT and aspartate aminotransferase (AST)—ie, two to five times higher than the upper limit of normal.
The first step in the workup of aminotransferase elevations is to confirm the abnormality by repeating the blood test. If an elevation is confirmed, further investigation is warranted. A directed history and physical examination is important and may disclose risk factors, raising clinical suspicion of a particular disease.
Some caveats: The proportion of patients with HCV viremia who have abnormally high aminotransferase levels ranges between only 54% and 66%.57–59 In patients with risk factors for HCV infection and abnormal liver enzyme levels, HCV infection is probable but not certain. Also, liver enzyme tests do not reveal the extent of hepatic injury or reflect the true status of hepatic function.60
Infants born to infected mothers
Children born to HCV-positive women should be tested for anti-HCV no sooner than age 12 months, when passively transferred maternal anti-HCV declines below detectable levels. If earlier diagnosis of HCV infection is desired, a real-time polymerase chain reaction (PCR) test for HCV RNA can be done at or after the infant's first “well-child” visit at age 1 to 2 months.
If positive for either anti-HCV or HCV RNA, children should be evaluated for liver disease, and those with persistently elevated ALT levels should be referred to a specialist for medical management.2,5
GROUPS AT LOW RISK OF HCV
People who have had sexual relations with multiple or infected partners
Sexual activity is associated with a low but measurable risk of transmission of HCV. Large population-based studies, including the National Hepatitis Surveillance Program,25 found an independent association between HCV infection and having sexual relations with multiple partners or with a partner who is infected with HCV.
The CDC reported that 15% to 20% of patients with acute hepatitis C had a history of sexual exposure but no other risk factors. Two-thirds of them had an anti-HCV-positive sexual partner, and one-third reported having had more than two partners in the 6 months before illness.5
More data are needed to determine the risk of and the factors related to transmission of HCV between long-term steady partners as well as in persons with high-risk sexual practices, including whether other sexually transmitted diseases promote transmission of HCV by influencing viral load or modifying mucosal barriers.
Health care workers exposed to HCV, eg, by needlestick
The prevalence of HCV infection in health care workers is no greater than that in the general population, averaging 1% to 2%, and is actually 10 times lower than that of hepatitis B virus infection.47,48,61,62
However, within the disciplines, some groups have a higher prevalence of HCV infection, suggesting that some occupations carry a higher risk. In two US studies, the prevalence of HCV infection was higher in oral surgeons (2.0% and 9.3%) than in other dentists (0.7% and 0.97%).63,64
In a single study that evaluated risk factors for infection, a history of needlestick injury was the only occupational risk factor that was independently associated with HCV infection.65 The average incidence of anti-HCV seroconversion after a needlestick or after an injury with a sharp object contaminated by an HCV-positive source is 1.8% (range 0%–7%).66–69
Although no studies of incidence have documented transmission via mucous membrane or nonintact skin exposures, transmission of HCV from blood splashes to the conjunctiva have been described.70,71
It is worth noting that exposure to blood from unclean needles used in tattooing or body piercing also confers a risk of HCV infection.
SEROLOGIC SCREENING TESTS FOR HCV
- Serologic assays that detect specific antibody to HCV (anti-HCV)
- Molecular assays that detect viral RNA.
Initial serologic screening tests for anti-HCV
Two EIAs are approved for clinical use:
- Abbott HCV EIA 2.0 (Abbott Laboratories, Abbott Park, IL)
- Ortho HCV Version 3.0 enzyme-linked immunosorbent assay (ELISA) (Ortho-Clinical Diagnostics, Rochester, NY).
One enhanced chemiluminescence immunoassay is also approved:
- Vitros Anti-HCV assay (Ortho-Clinical Diagnostics). In practical terms, this test is equivalent to the two EIAs, and the discussion below about EIAs applies to this test as well.
These third-generation tests are highly sensitive (> 99%) and specific (99%) in immunocompetent patients, and eliminate the need for a confirmatory immunoblot assay in patients with clinical liver disease, particularly those with risk factors for HCV infection.
False-positive results are rare now, but they were common with earlier generations of these assays. Most false-positive results occur in patients with autoimmune liver disease or hypergammaglobulinemia who have normal liver enzyme levels and no risk factors for HCV infection. In fact, all positive anti-HCV results should be followed up with an HCV RNA test.
False-negative results are also uncommon, usually occurring only in immunosuppressed patients (eg, organ transplant recipients and HIV-positive patients) and in patients on long-term hemodialysis. Therefore, patients with a history of hemodialysis should be considered for an HCV RNA assay rather than an EIA. Measurement of ALT will not be useful because ALT levels are lower in patients with end-stage renal disease. In most other clinical situations, the HCV EIA is an outstanding screening test for HCV infection because of its high sensitivity and relatively low cost (< $50).
Although the specificity of these tests is good, the predictive value of a positive result varies substantially by the pretest probability of HCV infection. For example, in a group of injection-drug users who are very likely to have ongoing or remote infection, all positive HCV EIA results are likely truly positive.74 On the other hand, in healthy blood donors, up to half of all positive third-generation EIA tests are falsely positive.75
Important points
- A positive anti-HCV antibody test does not distinguish acute from chronic disease or active from past infection, nor is it a sign of immunity or protection.
- A positive anti-HCV EIA requires HCV RNA measurement to discriminate between current infection on the one hand, and either resolved HCV infection or a false-positive result on the other.
- A positive EIA anti-HCV test is a marker that hepatitis C may be present, and it must be followed by confirmatory HCV RNA testing.
- Physicians should be mindful of the potential tribulations associated with false-positive tests. A false-positive test may result in harm to patients that is difficult to measure, such as anxiety, labeling in the medical record, and detrimental effects on close relationships.
CONFIRMATORY TESTING WITH ASSAYS FOR HCV RNA
As stated above, a positive result on an anti-HCV EIA needs to be confirmed with an assay for HCV RNA, of which there are two types, ie, qualitative and quantitative.
Each involves trade-offs. Qualitative assays are more sensitive and detect more cases, but they provide no information about the amount of virus (viral load). Quantitative assays are less sensitive, so a negative result does not completely exclude hepatitis C, although they can still can detect 95% of cases. They do, however, measure the viral load.
Therefore, the type of test to use depends on the patient’s risk profile, the goals of testing, and the setting in which future care will be provided. The primary objective when a patient has a positive EIA test is to determine whether he or she has ongoing infection, a goal most expeditiously achieved using a qualitative assay. However, since a quantitative assay can detect the vast majority of cases of active HCV infection, many clinicians select this as the test of first choice when the probability of HCV is high (eg, in a patient with risk factors and abnormal liver tests). If the pretest probability is low, a qualitative assay is the better choice.
Many commercial assays are available for detecting (qualitative assays) or measuring (quantitative assays) HCV RNA.
Qualitative HCV RNA assays
The approved qualitative assays are:
- Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics, Pleasanton, CA)
- Cobas Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics)
- Ampliscreen (Roche Molecular Diagnostics)
- Versant HCV RNA Qualitative Assay (Siemens Healthcare Diagnostics, Deerfield, IL)
- Procleix HIV-1/HCV Assay (Chiron, Emeryville, CA).
Quantitative HCV RNA assays
The approved quantitative assays are:
- Amplicor HCV Monitor (Roche Molecular Diagnostics)
- Cobas Amplicor HCV Monitor, version 2.0 (Roche Molecular Diagnostics)
- Versant HCV RNA 3.0 Assay (bDNA) (Siemens Healthcare Diagnostics)
- Cobas Taqman HCV Test (Roche Molecular Diagnostics).
Quantitative tests use target amplification with PCR, transcription-mediated amplification (TMA), or a signal amplification technique such as a branched DNA (bDNA) assay. The sensitivity varies for different types of amplification. TMA assays appear to be the most sensitive for detecting HCV RNA.
The latest innovation is real-time PCR, which shortens the typical time for PCR processing from 1.5 hours to 35 minutes. It may also detect relapsed HCV infection earlier than regular PCR. With the recent availability of real-time PCR assays, which have sensitivities of 10 to 50 IU/mL, many experts feel there is no longer a need for qualitative assays.74 In fact, many laboratories no longer offer qualitative testing. The Cleveland Clinic laboratory has recently stopped offering this test.
Because RNA testing is widely available, the recombinant immunoblot assay (RIBA) has become obsolete in diagnosing HCV infection, except in special circumstances. Currently, the primary purpose of RIBA testing is to distinguish between resolved HCV infection (EIA-positive, HCV RNA-negative, RIBA-positive) and a false-positive EIA (EIA-positive, HCV RNA-negative, RIBA-negative).
In summary, patients suspected of having acute or chronic HCV infection should first be tested for anti-HCV. Subsequently, HCV RNA testing should be performed in:
- Patients with a positive anti-HCV test
- Patients for whom antiviral treatment is being considered (using a sensitive quantitative assay)
- Patients with unexplained liver disease whose anti-HCV test is negative and who are immunocompromised or suspected of having acute HCV infection.
Significance of the HCV viral load
The significance of the HCV viral load is widely misunderstood. The amount of virus in the blood does not correlate with symptoms, histologic liver injury, or the stage or aggressiveness of disease. Its sole importance is in relation to therapy.
The HCV viral load, measured before treatment, helps predict the likelihood of a treatment response: the lower the pretreatment viral load, the more likely that the patient will respond to current HCV therapies.
Additionally, the pretreatment viral load serves as a baseline for comparison with subsequent measurements during treatment. Patients with HCV genotype 1 who do not achieve more than a 2-log (99%) reduction in viral load by the 12th week of treatment (an early virologic response) have a low response rate, and treatment should generally be stopped, given its cost and side effects.76 However, measuring the viral load to detect an early virologic response is less helpful in patients with HCV genotype 2 or 3 infection, since these patients require only 24 weeks of therapy and most of them clear the virus by week 12 and respond to therapy.
Additionally, patients with genotype 2 or 3 and those with a viral load of less than 600,000 IU/mL have been found to achieve higher rates of sustained virologic response.15 A sustained virologic response is defined as the absence of HCV RNA 24 weeks after stopping treatment and is now considered to be the best predictor of long-term treatment response. A sustained virologic response is generally regarded as a “virologic cure.”
HCV GENOTYPE AFFECTS SUCCESS AND DURATION OF TREATMENT
HCV has at least six major genotypes.1,3–6 Several genotypes are subclassified as “a” or “b” (ie, genotype 1a or 1b); however, these distinctions are of little clinical use.
In the laboratory, HCV genotypes are identified by restriction fragment length polymorphism, by direct sequence analysis, or by reverse hybridization. Once the HCV genotype has been identified, there is no need to repeat the test.
Different genotypes are more common in some areas of the world than in others. Genotype 1 is the one most common in the United States (accounting for 70% to 75% of cases), followed by genotypes 2 and 3 (25%–30%). Genotype 4 is most common in Egypt and the Arabian peninsula.
HCV genotyping is important because it can help predict the likelihood of a response to treatment and in planning the dose and duration of therapy.77 For example, treatment with pegylated interferon plus ribavirin is predicted to work approximately 50% of the time for people with genotype 1, but 80% to 90% of the time for people with genotypes 2 or 3.15–17,78 Additionally, patients with genotype 1 need 12 months of therapy to achieve maximum benefit, whereas those with genotypes 2 and 3 require treatment for only 6 months to achieve maximum benefit.
Screening for hepatitis C virus (HCV) infection in high-risk populations can identify, early on, people at risk of progressive liver disease who may benefit from antiviral therapy and counseling. The US Centers for Disease Control and Prevention (CDC) recommends that all people be assessed for HCV risk factors and that those with risk factors be screened for HCV antibodies (anti-HCV),1 and members of the national societies of gastroenterology and hepatology have endorsed this recommendation.2
Unfortunately, rates at which primary care patients are assessed for risk factors and the rates at which patients at higher risk are screened remain below the goals set by the CDC.3–6 All health care practitioners need to understand how to establish or exclude a diagnosis of HCV infection and to interpret the tests correctly.
WHY SCREEN FOR HCV?
HCV infection is a major public health problem and a leading cause of chronic liver disease. In the United States, an estimated 3.2 million persons (1.3% of the population) have been infected.7 However, in the inner-city primary care setting the rate of HCV infection is as high as 8%, and in Veterans Administration populations it is 17%.8,9 The worldwide prevalence of HCV infection is 2.0%, corresponding to 140 million persons.
Screening of blood products has led to a decline in the incidence of acute hepatitis C since the late 1980s, although rates have reached a plateau in recent years (Figure 1).10
Approximately 20% of patients infected with HCV develop a serious sequela, such as severe fibrosis, cirrhosis, end-stage liver disease, or hepatocellular carcinoma. Currently, HCV infection causes an estimated 8,000 to 10,000 deaths annually in the United States, and that number is predicted to triple in the next 10 to 20 years. Furthermore, HCV-related disease is the leading indication for liver transplantation in the United States, and it is estimated to cost $600 million to $1 billion annually in medical expenses and loss of work.8
Screening can reduce adverse outcomes
HCV screening has several potential benefits. By detecting HCV infection early, screening facilitates virologic suppression, as treatment earlier in the course of the disease is more effective than later.11,12 Further, early diagnosis together with patient education and subsequent lifestyle modifications may reduce the risk of transmission of HCV infection to other people.13,14
Antiviral therapy with pegylated interferons and ribavirin can cure hepatitis C in up to 90% of cases, depending on the viral genotype15–17 (see discussion of HCV genotypes below). In addition, treatment slows the progression of fibrosis.18 The incidence of hepatocellular carcinoma is lower in patients who achieve a sustained virologic response to antiviral therapy.19 Finally, antiviral therapy prolongs survival.20
New drug therapies are being developed and may, we hope, be even more effective than current drugs. Inhibitors of HCV-specific enzymes such as NS3/4 protease, combined with pegylated interferons and ribavirin, are in phase III clinical trials. These drugs are expected to be available for clinical practice within the next 2 years.21–23 Additionally, nitazoxanide (Alinia), an inducer of eIF2a and PKR phosphorylation, has been shown to increase the treatment response to HCV genotype 4. Studies24 are currently under way in patients infected with HCV genotype 1.
Screening is cost-effective
The National Hepatitis Surveillance Program25 calculated the cost of screening for HCV to be $1,246 per case detected. However, a more vigorous analysis of the same data using several different models to incorporate risk factors based on history revealed costs between $357 and $1,047 per case detected. This compares favorably with the cost of screening for other diseases that physicians routinely screen for.
Antiviral combination therapy for chronic hepatitis C has been shown to be effective in terms of quality-adjusted life-years gained and cost-effectiveness in several studies.26–28
HOW TO SCREEN
To test everyone in the general population would be neither cost-effective nor practical, which is why the CDC recommends that serologic screening for HCV infection be done only in people who have well-established risk factors for it.1,5
Therefore, screening should begin by obtaining a relevant medical history as part of a routine health evaluation. But how should this be done?
McGinn et al29 asked 1,000 patients attending an inner-city clinic to fill out a 27-item questionnaire assessing five “domains” of risk factors for HCV: work, medical, exposure, personal care, and social history. Afterward, they tested all 1,000 patients. They found that the risk factors that best predicted positive results on testing were in three domains: medical (eg, blood transfusions, dialysis, other medical procedures, and elevated liver enzymes), exposure (past contact with another person’s blood), and social history (eg, illicit drug use, incarceration, and sexual activity).
The National Hepatitis Surveillance Program25 explored the cost and yield of several screening strategies for hepatitis C, ie, testing only in patients who had a greater than 7% likelihood of infection based on an empirically derived mathematical model; testing only if significant risk factors were revealed in a simple questionnaire; or testing only if the alanine aminotransferase (ALT) level was elevated. The predictive mathematical model was the most effective and efficient means of deciding who should be tested.
Unfortunately, such a model is too cumbersome to be clinically applicable, and clinical prediction tools for HCV screening have been underused.
GROUPS AT HIGH RISK OF HCV
Groups at risk of HCV infection can be classified as being at high, intermediate, or low risk. The American Association for the Study of Liver Diseases2 rates the level of evidence for screening in all of the following risk groups as class I (ie, there is evidence or general agreement that it is beneficial, useful, and effective) and level B (ie, the data are derived from non-randomized studies).
Intravenous drug abusers
Intravenous drug abuse is the strongest independent risk factor for HCV infection.30–33 It has been the main route of HCV infection over the past decades and currently accounts for 60% of HCV transmission in the United States.7,10,34–37
Hemophilia patients treated with clotting factor concentrates produced before 1987
HCV seroprevalence is very high in patients with hemophilia who received infusions of plasma-derived clotting factor concentrates before 1987.38 In these patients, the HCV genotypes are predominantly 1 and 3, and to a lesser extent genotype 2.39,40 These genotypes likely reflect the prior exposures of the plasma donors.41 (See discussion of HCV genotypes below.) Individuals receiving clotting factor concentrates prepared from plasma pools were at high risk of HCV infection until effective procedures to inactivate viruses were introduced in 1985 (factor VIII) and 1987 (factor IX).42
People infected with HIV
About 25% of people infected with human immunodeficiency virus (HIV) in the Western world also have chronic HCV infection.43 Progression of liver disease is accelerated in HIV-HCV coinfection, and the risk of cirrhosis is twice as high.44
However, about 6% of HIV-positive patients fail to develop HCV antibodies when infected. Thus, HCV RNA should be assessed in HIV patients with unexplained liver disease who are negative for anti-HCV.45
The distribution of HCV genotypes in HIV-infected patients reflects the route of transmission. Genotype 1b accounts for 66% of posttransfusion HCV infections, while genotypes 1a and 3a are more common in intravenous drug users.
GROUPS AT INTERMEDIATE RISK OF HCV
Recipients of blood transfusions before 1992
Before 1992, blood transfusions carried a risk of HCV infection of up to 7% with each unit transfused. Prospective studies of transfusion recipients in the United States found that rates of posttransfusion hepatitis in the 1960s exceeded 20%,36 since most patients received multiple units of blood.
In the mid-1970s, before HCV had been identified, available diagnostic tests indicated that 90% of cases of posttransfusion hepatitis were not caused by hepatitis A or hepatitis B viruses. By this time, the move to all-volunteer blood donors instead of paid donors had reduced the risk of posttransfusion hepatitis to 10%.22,37,46
Although non-A, non-B hepatitis was first recognized because of its association with blood transfusion, population-based sentinel surveillance showed that it accounted for 15% to 20% of cases of community-acquired viral hepatitis in the United States.35 The advent of molecular cloning in 1988 indicated that non-A, non-B hepatitis was primarily caused by HCV.47–52
Screening of blood has reduced the rate of posttransfusion hepatitis C by a factor of about 10,000, to a current rate of 1 per million transfusions.53 The few cases that still occur are due to newly infected people donating blood before they have developed antibodies to the virus, which can take up to 8 weeks.54
Recipients of solid-organ transplants before 1992
Before organ donors were screened for HCV, recipients of solid-organ transplants from infected donors had a high risk of acquiring HCV infection. Transmission rates in different cohorts ranged from 30% to 80%.55 In an attempt to improve the safety of organ transplantation, many transplant centers now screen donors for anti-HCV and test for HCV RNA for verification.
A related problem is pre-existing HCV infection in transplant recipients. Izopet et al56 reported that, in renal transplant recipients with preexisting HCV infection, the HCV RNA titer rose about 10 times (1 log) higher after transplantation, owing to the immunosuppressive drugs that transplant recipients must take. Although this higher viral load does not affect the progression of fibrosis in all patients, the effect of immunosuppressive therapy on liver disease results in a worse outcome for some, and it reduces survival beginning in the second decade after kidney transplantation.56
Additionally, treatment of HCV infection in transplant recipients may pose a challenge, as those receiving immunosuppressive therapy with tacrolimus (Prograf) or cyclosporine (Sandimmune) may develop some degree of renal insufficiency, complicating the use of ribavirin (Rebetol) and subjecting patients to a higher risk of severe anemia. Furthermore, interferon therapy increases the risk of renal allograft rejection and, accordingly, is not often used in renal transplant recipients.
Patients with unexplained elevated aminotransferase levels
HCV infection affects an estimated 1.8% of the general population, but the rate is much higher in people with ALT levels over 40 U/L. Most patients with chronic hepatitis C have no symptoms or only mild symptoms and minimally elevated levels of ALT and aspartate aminotransferase (AST)—ie, two to five times higher than the upper limit of normal.
The first step in the workup of aminotransferase elevations is to confirm the abnormality by repeating the blood test. If an elevation is confirmed, further investigation is warranted. A directed history and physical examination is important and may disclose risk factors, raising clinical suspicion of a particular disease.
Some caveats: The proportion of patients with HCV viremia who have abnormally high aminotransferase levels ranges between only 54% and 66%.57–59 In patients with risk factors for HCV infection and abnormal liver enzyme levels, HCV infection is probable but not certain. Also, liver enzyme tests do not reveal the extent of hepatic injury or reflect the true status of hepatic function.60
Infants born to infected mothers
Children born to HCV-positive women should be tested for anti-HCV no sooner than age 12 months, when passively transferred maternal anti-HCV declines below detectable levels. If earlier diagnosis of HCV infection is desired, a real-time polymerase chain reaction (PCR) test for HCV RNA can be done at or after the infant's first “well-child” visit at age 1 to 2 months.
If positive for either anti-HCV or HCV RNA, children should be evaluated for liver disease, and those with persistently elevated ALT levels should be referred to a specialist for medical management.2,5
GROUPS AT LOW RISK OF HCV
People who have had sexual relations with multiple or infected partners
Sexual activity is associated with a low but measurable risk of transmission of HCV. Large population-based studies, including the National Hepatitis Surveillance Program,25 found an independent association between HCV infection and having sexual relations with multiple partners or with a partner who is infected with HCV.
The CDC reported that 15% to 20% of patients with acute hepatitis C had a history of sexual exposure but no other risk factors. Two-thirds of them had an anti-HCV-positive sexual partner, and one-third reported having had more than two partners in the 6 months before illness.5
More data are needed to determine the risk of and the factors related to transmission of HCV between long-term steady partners as well as in persons with high-risk sexual practices, including whether other sexually transmitted diseases promote transmission of HCV by influencing viral load or modifying mucosal barriers.
Health care workers exposed to HCV, eg, by needlestick
The prevalence of HCV infection in health care workers is no greater than that in the general population, averaging 1% to 2%, and is actually 10 times lower than that of hepatitis B virus infection.47,48,61,62
However, within the disciplines, some groups have a higher prevalence of HCV infection, suggesting that some occupations carry a higher risk. In two US studies, the prevalence of HCV infection was higher in oral surgeons (2.0% and 9.3%) than in other dentists (0.7% and 0.97%).63,64
In a single study that evaluated risk factors for infection, a history of needlestick injury was the only occupational risk factor that was independently associated with HCV infection.65 The average incidence of anti-HCV seroconversion after a needlestick or after an injury with a sharp object contaminated by an HCV-positive source is 1.8% (range 0%–7%).66–69
Although no studies of incidence have documented transmission via mucous membrane or nonintact skin exposures, transmission of HCV from blood splashes to the conjunctiva have been described.70,71
It is worth noting that exposure to blood from unclean needles used in tattooing or body piercing also confers a risk of HCV infection.
SEROLOGIC SCREENING TESTS FOR HCV
- Serologic assays that detect specific antibody to HCV (anti-HCV)
- Molecular assays that detect viral RNA.
Initial serologic screening tests for anti-HCV
Two EIAs are approved for clinical use:
- Abbott HCV EIA 2.0 (Abbott Laboratories, Abbott Park, IL)
- Ortho HCV Version 3.0 enzyme-linked immunosorbent assay (ELISA) (Ortho-Clinical Diagnostics, Rochester, NY).
One enhanced chemiluminescence immunoassay is also approved:
- Vitros Anti-HCV assay (Ortho-Clinical Diagnostics). In practical terms, this test is equivalent to the two EIAs, and the discussion below about EIAs applies to this test as well.
These third-generation tests are highly sensitive (> 99%) and specific (99%) in immunocompetent patients, and eliminate the need for a confirmatory immunoblot assay in patients with clinical liver disease, particularly those with risk factors for HCV infection.
False-positive results are rare now, but they were common with earlier generations of these assays. Most false-positive results occur in patients with autoimmune liver disease or hypergammaglobulinemia who have normal liver enzyme levels and no risk factors for HCV infection. In fact, all positive anti-HCV results should be followed up with an HCV RNA test.
False-negative results are also uncommon, usually occurring only in immunosuppressed patients (eg, organ transplant recipients and HIV-positive patients) and in patients on long-term hemodialysis. Therefore, patients with a history of hemodialysis should be considered for an HCV RNA assay rather than an EIA. Measurement of ALT will not be useful because ALT levels are lower in patients with end-stage renal disease. In most other clinical situations, the HCV EIA is an outstanding screening test for HCV infection because of its high sensitivity and relatively low cost (< $50).
Although the specificity of these tests is good, the predictive value of a positive result varies substantially by the pretest probability of HCV infection. For example, in a group of injection-drug users who are very likely to have ongoing or remote infection, all positive HCV EIA results are likely truly positive.74 On the other hand, in healthy blood donors, up to half of all positive third-generation EIA tests are falsely positive.75
Important points
- A positive anti-HCV antibody test does not distinguish acute from chronic disease or active from past infection, nor is it a sign of immunity or protection.
- A positive anti-HCV EIA requires HCV RNA measurement to discriminate between current infection on the one hand, and either resolved HCV infection or a false-positive result on the other.
- A positive EIA anti-HCV test is a marker that hepatitis C may be present, and it must be followed by confirmatory HCV RNA testing.
- Physicians should be mindful of the potential tribulations associated with false-positive tests. A false-positive test may result in harm to patients that is difficult to measure, such as anxiety, labeling in the medical record, and detrimental effects on close relationships.
CONFIRMATORY TESTING WITH ASSAYS FOR HCV RNA
As stated above, a positive result on an anti-HCV EIA needs to be confirmed with an assay for HCV RNA, of which there are two types, ie, qualitative and quantitative.
Each involves trade-offs. Qualitative assays are more sensitive and detect more cases, but they provide no information about the amount of virus (viral load). Quantitative assays are less sensitive, so a negative result does not completely exclude hepatitis C, although they can still can detect 95% of cases. They do, however, measure the viral load.
Therefore, the type of test to use depends on the patient’s risk profile, the goals of testing, and the setting in which future care will be provided. The primary objective when a patient has a positive EIA test is to determine whether he or she has ongoing infection, a goal most expeditiously achieved using a qualitative assay. However, since a quantitative assay can detect the vast majority of cases of active HCV infection, many clinicians select this as the test of first choice when the probability of HCV is high (eg, in a patient with risk factors and abnormal liver tests). If the pretest probability is low, a qualitative assay is the better choice.
Many commercial assays are available for detecting (qualitative assays) or measuring (quantitative assays) HCV RNA.
Qualitative HCV RNA assays
The approved qualitative assays are:
- Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics, Pleasanton, CA)
- Cobas Amplicor HCV Test, version 2.0 (Roche Molecular Diagnostics)
- Ampliscreen (Roche Molecular Diagnostics)
- Versant HCV RNA Qualitative Assay (Siemens Healthcare Diagnostics, Deerfield, IL)
- Procleix HIV-1/HCV Assay (Chiron, Emeryville, CA).
Quantitative HCV RNA assays
The approved quantitative assays are:
- Amplicor HCV Monitor (Roche Molecular Diagnostics)
- Cobas Amplicor HCV Monitor, version 2.0 (Roche Molecular Diagnostics)
- Versant HCV RNA 3.0 Assay (bDNA) (Siemens Healthcare Diagnostics)
- Cobas Taqman HCV Test (Roche Molecular Diagnostics).
Quantitative tests use target amplification with PCR, transcription-mediated amplification (TMA), or a signal amplification technique such as a branched DNA (bDNA) assay. The sensitivity varies for different types of amplification. TMA assays appear to be the most sensitive for detecting HCV RNA.
The latest innovation is real-time PCR, which shortens the typical time for PCR processing from 1.5 hours to 35 minutes. It may also detect relapsed HCV infection earlier than regular PCR. With the recent availability of real-time PCR assays, which have sensitivities of 10 to 50 IU/mL, many experts feel there is no longer a need for qualitative assays.74 In fact, many laboratories no longer offer qualitative testing. The Cleveland Clinic laboratory has recently stopped offering this test.
Because RNA testing is widely available, the recombinant immunoblot assay (RIBA) has become obsolete in diagnosing HCV infection, except in special circumstances. Currently, the primary purpose of RIBA testing is to distinguish between resolved HCV infection (EIA-positive, HCV RNA-negative, RIBA-positive) and a false-positive EIA (EIA-positive, HCV RNA-negative, RIBA-negative).
In summary, patients suspected of having acute or chronic HCV infection should first be tested for anti-HCV. Subsequently, HCV RNA testing should be performed in:
- Patients with a positive anti-HCV test
- Patients for whom antiviral treatment is being considered (using a sensitive quantitative assay)
- Patients with unexplained liver disease whose anti-HCV test is negative and who are immunocompromised or suspected of having acute HCV infection.
Significance of the HCV viral load
The significance of the HCV viral load is widely misunderstood. The amount of virus in the blood does not correlate with symptoms, histologic liver injury, or the stage or aggressiveness of disease. Its sole importance is in relation to therapy.
The HCV viral load, measured before treatment, helps predict the likelihood of a treatment response: the lower the pretreatment viral load, the more likely that the patient will respond to current HCV therapies.
Additionally, the pretreatment viral load serves as a baseline for comparison with subsequent measurements during treatment. Patients with HCV genotype 1 who do not achieve more than a 2-log (99%) reduction in viral load by the 12th week of treatment (an early virologic response) have a low response rate, and treatment should generally be stopped, given its cost and side effects.76 However, measuring the viral load to detect an early virologic response is less helpful in patients with HCV genotype 2 or 3 infection, since these patients require only 24 weeks of therapy and most of them clear the virus by week 12 and respond to therapy.
Additionally, patients with genotype 2 or 3 and those with a viral load of less than 600,000 IU/mL have been found to achieve higher rates of sustained virologic response.15 A sustained virologic response is defined as the absence of HCV RNA 24 weeks after stopping treatment and is now considered to be the best predictor of long-term treatment response. A sustained virologic response is generally regarded as a “virologic cure.”
HCV GENOTYPE AFFECTS SUCCESS AND DURATION OF TREATMENT
HCV has at least six major genotypes.1,3–6 Several genotypes are subclassified as “a” or “b” (ie, genotype 1a or 1b); however, these distinctions are of little clinical use.
In the laboratory, HCV genotypes are identified by restriction fragment length polymorphism, by direct sequence analysis, or by reverse hybridization. Once the HCV genotype has been identified, there is no need to repeat the test.
Different genotypes are more common in some areas of the world than in others. Genotype 1 is the one most common in the United States (accounting for 70% to 75% of cases), followed by genotypes 2 and 3 (25%–30%). Genotype 4 is most common in Egypt and the Arabian peninsula.
HCV genotyping is important because it can help predict the likelihood of a response to treatment and in planning the dose and duration of therapy.77 For example, treatment with pegylated interferon plus ribavirin is predicted to work approximately 50% of the time for people with genotype 1, but 80% to 90% of the time for people with genotypes 2 or 3.15–17,78 Additionally, patients with genotype 1 need 12 months of therapy to achieve maximum benefit, whereas those with genotypes 2 and 3 require treatment for only 6 months to achieve maximum benefit.
- Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Intern Med 2004; 141:715–717.
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49:1335–1374.
- Shehab TM, Orrego M, Chunduri R, Lok AS. Identification and management of hepatitis C patients in primary care clinics. Am J Gastroenterol 2003; 98:639–644.
- Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care physicians in the USA: results of a national survey. J Viral Hepat 2001; 8:377–383.
- US Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep 1998; 47:1–39.
- US Centers for Disease Control and Prevention. National prevention strategy: a comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences; summer 2001. http://www.cdc.gov/hepatitis/HCV/Strategy/NatHep-CPrevStrategy.htm. Accessed August 8, 2010.
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–714.
- Kim WR. The burden of hepatitis C in the United States. Hepatology 2002; 36(suppl 1):S30–S34.
- Lau DT, Kleiner DE, Ghany MG, Park Y, Schmid P, Hoofnagle JH. 10-Year follow-up after interferon-alpha therapy for chronic hepatitis C. Hepatology 1998; 28:1121–1127.
- Daniels D, Grytdal S, Wasley A; US Centers for Disease Control and Prevention. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ 2009; 58:1–27.
- Thomson BJ, Kwong G, Ratib S, et al; Trent HCV Study Group. Response rates to combination therapy for chronic HCV infection in a clinical setting and derivation of probability tables for individual patient management. J Viral Hepat 2008; 15:271–278.
- Hayashi N, Takehara T. Antiviral therapy for chronic hepatitis C: past, present, and future. J Gastroenterol 2006; 41:17–27.
- Gordon FD. Cost-effectiveness of screening patients for hepatitis C. Am J Med 1999; 107:36S–40S.
- Hill L, Henry B, Schweikert S; Prevention Practice Committee, American College of Preventive Medicine. Screening for chronic hepatitis C: American College of Preventive Medicine practice policy statement. Am J Prev Med 2005; 28:327–330.
- Hadziyannis SJ, Sette H, Morgan TR, et al; PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140:346–355.
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358:958–965.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Cammà C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology 2004; 39:333–342.
- Yoshida H, Tateishi R, Arakawa Y, et al. Benefit of interferon therapy in hepatocellular carcinoma prevention for individual patients with chronic hepatitis C. Gut 2004; 53:425–430.
- Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology 2002; 123:483–491.
- Hézode C, Forestier N, Dusheiko G, et al; PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009; 360:1839–1850.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Berman K, Kwo PY. Boceprevir, an NS3 protease inhibitor of HCV. Clin Liver Dis 2009; 13:429–439.
- Rossignol JF, Elfert A, Keeffe EB. Treatment of chronic hepatitis C using a 4-week lead-in with nitazoxanide before peginterferon plus nitazoxanide. J Clin Gastroenterol 2009 Dec 31; epub ahead of print.
- Lapane KL, Jakiche AF, Sugano D, Weng CS, Carey WD. Hepatitis C infection risk analysis: who should be screened? Comparison of multiple screening strategies based on the National Hepatitis Surveillance Program. Am J Gastroenterol 1998; 93:591–596.
- Wong JB, Davis GL, McHutchison JG, Manns MP, Albrecht JK; International Hepatitis Interventional Therapy Group. Economic and clinical effects of evaluating rapid viral response to peginterferon alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C. Am J Gastroenterol 2003; 98:2354–2362.
- Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA 2003; 290:228–237.
- Sullivan SD, Jensen DM, Bernstein DE, et al. Cost-effectiveness of combination peginterferon alpha-2a and ribavirin compared with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol 2004; 99:1490–1496.
- McGinn T, O’Connor-Moore N, Alfandre D, Gardenier D, Wisnivesky J. Validation of a hepatitis C screening tool in primary care. Arch Intern Med 2008; 168:2009–2013.
- Kaur S, Rybicki L, Bacon BR, Gollan JL, Rustgi VK, Carey WD. Performance characteristics and results of a large-scale screening program for viral hepatitis and risk factors associated with exposure to viral hepatitis B and C: results of the National Hepatitis Screening Survey. National Hepatitis Surveillance Group. Hepatology 1996; 24:979–986.
- Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol 2000; 95:740–747.
- Austin GE, Jensen B, Leete J, et al. Prevalence of hepatitis C virus seropositivity among hospitalized US veterans. Am J Med Sci 2000; 319:353–359.
- Yawn BP, Wollan P, Gazzuola L, Kim WR. Diagnosis and 10-year follow-up of a community-based hepatitis C cohort. J Fam Pract 2002; 51:135–140.
- Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 18(suppl 1):S11–S19.
- Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis 1997; 1:559–568,
- Alter MJ, Hadler SC, Judson FN, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA 1990; 264:2231–2235.
- Wasley A, Miller JT, Finelli L; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis—United States, 2005. MMWR Surveill Summ 2007; 56:1–24.
- Goedert JJ, Chen BE, Preiss L, Aledort LM, Rosenberg PS. Reconstruction of the hepatitis C virus epidemic in the US hemophilia population, 1940–1990. Am J Epidemiol 2007; 165:1443–1453.
- Eyster ME, Sherman KE, Goedert JJ, Katsoulidou A, Hatzakis A. Prevalence and changes in hepatitis C virus genotypes among multitransfused persons with hemophilia. The Multicenter Hemophilia Cohort Study. J Infect Dis 1999; 179:1062–1069.
- Yee TT, Griffioen A, Sabin CA, Dusheiko G, Lee CA. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut 2000; 47:845–851.
- Lee C, Dusheiko G. The natural history and antiviral treatment of hepatitis C in haemophilia. Haemophilia 2002; 8:322–329.
- Makris M, Garson JA, Ring CJ, Tuke PW, Tedder RS, Preston FE. Hepatitis C viral RNA in clotting factor concentrates and the development of hepatitis in recipients. Blood 1993; 81:1898–1902.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831–837.
- Sulkowski MS. The HIV-coinfected patient: managing viral hepatitis. J Acquir Immune Defic Syndr 2007; 45(suppl 2):S36–S37.
- Bonacini M, Lin HJ, Hollinger FB. Effect of coexisting HIV-1 infection on the diagnosis and evaluation of hepatitis C virus. J Acquir Immune Defic Syndr 2001; 26:340–344.
- Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health 1996; 86:655–661.
- Bell J, Batey RG, Farrell GC, Crewe EB, Cunningham AL, Byth K. Hepatitis C virus in intravenous drug users. Med J Aust 1990; 153:274–276.
- Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol 1997; 35:3274–3277.
- Patrick DM, Tyndall MW, Cornelisse PG, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ 2001; 165:889–895.
- Seeff LB, Wright EC, Zimmerman HJ, McCollum RW. VA cooperative study of post-transfusion hepatitis, 1969-1974: incidence and characteristics of hepatitis and responsible risk factors. Am J Med Sci 1975; 270:355–362.
- Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med 1975; 292:767–770.
- Alter HJ, Holland PV, Purcell RH, et al. Posttransfusion hepatitis after exclusion of commercial and hepatitis-B antigen-positive donors. Ann Intern Med. 1972; 77:691–699.
- Blajchman MA, Vamvakas EC. The continuing risk of transfusion-transmitted infections. N Engl J Med 2006; 355:1303–1305.
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345:41–52.
- Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int 1994; 45:238–244.
- Izopet J, Rostaing L, Sandres K, et al. Longitudinal analysis of hepatitis C virus replication and liver fibrosis progression in renal transplant recipients. J Infect Dis 2000; 181:852–858.
- Dubois F, Desenclos JC, Mariotte N, Goudeau A. Hepatitis C in a French population-based survey, 1994: seroprevalence, frequency of viremia, genotype distribution, and risk factors. The Collaborative Study Group. Hepatology 1997; 25:1490–1496.
- Bellentani S, Pozzato G, Saccoccio G, et al. Clinical course and risk factors of hepatitis C virus related liver disease in the general population: report from the Dionysos study. Gut 1999; 44:874–880.
- Alberti A, Noventa F, Benvegnù L, Boccato S, Gatta A. Prevalence of liver disease in a population of asymptomatic persons with hepatitis C virus infection. Ann Intern Med 2002; 137:961–964.
- Shiffman ML, Diago M, Tran A, et al. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol 2006; 4:645–652.
- Stary A, Kopp W, Hofmann H, Heller-Vitouch C, Kunz C. Seroepidemiologic study of hepatitis C virus in sexually transmitted disease risk groups. Sex Transm Dis 1992; 19:252–258.
- Weinstock HS, Bolan G, Reingold AL, Polish LB. Hepatitis C virus infection among patients attending a clinic for sexually transmitted diseases. JAMA 1993; 269:392–394.
- Thomas DL, Gruninger SE, Siew C, Joy ED, Quinn TC. Occupational risk of hepatitis C infections among general dentists and oral surgeons in North America. Am J Med 1996; 100:41–45.
- Klein RS, Freeman K, Taylor PE, Stevens CE. Occupational risk for hepatitis C virus infection among New York City dentists. Lancet 1991; 338:1539–1542.
- Polish LB, Tong MJ, Co RL, Coleman PJ, Alter MJ. Risk factors for hepatitis C virus infection among health care personnel in a community hospital. Am J Infect Control 1993; 21:196–200.
- Alter MJ. Occupational exposure to hepatitis C virus: a dilemma. Infect Control Hosp Epidemiol 1994; 15:742–744.
- Lanphear BP, Linnemann CC, Cannon CG, DeRonde MM, Pendy L, Kerley LM. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect Control Hosp Epidemiol 1994; 15:745–750.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposures in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control 1995; 23:273–277.
- Mitsui T, Iwano K, Masuko K, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology 1992; 16:1109–1114.
- Sartori M, La Terra G, Aglietta M, Manzin A, Navino C, Verzetti G. Transmission of hepatitis C via blood splash into conjunctiva. Scand J Infect Dis 1993; 25:270–271.
- Ippolito G, Puro V, Petrosillo N, De Carli G, Micheloni G, Magliano E. Simultaneous infection with HIV and hepatitis C virus following occupational conjunctival blood exposure. JAMA 1998; 280:28.
- Carey W. Tests and screening strategies for the diagnosis of hepatitis C. Cleve Clin J Med 2003; 70(suppl 4):S7–S13.
- Carey WD, Jeffers L, Kugelmas M, et al; Hepatitis C management. Hepatitis C Monograph. Cleveland Clinic; www.clevelandclinicmeded.com/online/monograph/hepc/page1.htm. Accessed 7/30/2010.
- Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA 2007; 297:724–732.
- Bowden DS, Berzsenyi MD. Chronic hepatitis C virus infection: genotyping and its clinical role. Future Microbiol 2006; 1:103–112.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Poynard T, McHutchison J, Davis GL, et al. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology 2000; 32:1131–1137.
- Zeuzem S. Interferon-based therapy for chronic hepatitis C: current and future perspectives. Nat Clin Pract Gastroenterol Hepatol 2008; 5:610–622.
- Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Intern Med 2004; 141:715–717.
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49:1335–1374.
- Shehab TM, Orrego M, Chunduri R, Lok AS. Identification and management of hepatitis C patients in primary care clinics. Am J Gastroenterol 2003; 98:639–644.
- Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care physicians in the USA: results of a national survey. J Viral Hepat 2001; 8:377–383.
- US Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep 1998; 47:1–39.
- US Centers for Disease Control and Prevention. National prevention strategy: a comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences; summer 2001. http://www.cdc.gov/hepatitis/HCV/Strategy/NatHep-CPrevStrategy.htm. Accessed August 8, 2010.
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–714.
- Kim WR. The burden of hepatitis C in the United States. Hepatology 2002; 36(suppl 1):S30–S34.
- Lau DT, Kleiner DE, Ghany MG, Park Y, Schmid P, Hoofnagle JH. 10-Year follow-up after interferon-alpha therapy for chronic hepatitis C. Hepatology 1998; 28:1121–1127.
- Daniels D, Grytdal S, Wasley A; US Centers for Disease Control and Prevention. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ 2009; 58:1–27.
- Thomson BJ, Kwong G, Ratib S, et al; Trent HCV Study Group. Response rates to combination therapy for chronic HCV infection in a clinical setting and derivation of probability tables for individual patient management. J Viral Hepat 2008; 15:271–278.
- Hayashi N, Takehara T. Antiviral therapy for chronic hepatitis C: past, present, and future. J Gastroenterol 2006; 41:17–27.
- Gordon FD. Cost-effectiveness of screening patients for hepatitis C. Am J Med 1999; 107:36S–40S.
- Hill L, Henry B, Schweikert S; Prevention Practice Committee, American College of Preventive Medicine. Screening for chronic hepatitis C: American College of Preventive Medicine practice policy statement. Am J Prev Med 2005; 28:327–330.
- Hadziyannis SJ, Sette H, Morgan TR, et al; PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140:346–355.
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358:958–965.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Cammà C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology 2004; 39:333–342.
- Yoshida H, Tateishi R, Arakawa Y, et al. Benefit of interferon therapy in hepatocellular carcinoma prevention for individual patients with chronic hepatitis C. Gut 2004; 53:425–430.
- Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology 2002; 123:483–491.
- Hézode C, Forestier N, Dusheiko G, et al; PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009; 360:1839–1850.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Berman K, Kwo PY. Boceprevir, an NS3 protease inhibitor of HCV. Clin Liver Dis 2009; 13:429–439.
- Rossignol JF, Elfert A, Keeffe EB. Treatment of chronic hepatitis C using a 4-week lead-in with nitazoxanide before peginterferon plus nitazoxanide. J Clin Gastroenterol 2009 Dec 31; epub ahead of print.
- Lapane KL, Jakiche AF, Sugano D, Weng CS, Carey WD. Hepatitis C infection risk analysis: who should be screened? Comparison of multiple screening strategies based on the National Hepatitis Surveillance Program. Am J Gastroenterol 1998; 93:591–596.
- Wong JB, Davis GL, McHutchison JG, Manns MP, Albrecht JK; International Hepatitis Interventional Therapy Group. Economic and clinical effects of evaluating rapid viral response to peginterferon alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C. Am J Gastroenterol 2003; 98:2354–2362.
- Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA 2003; 290:228–237.
- Sullivan SD, Jensen DM, Bernstein DE, et al. Cost-effectiveness of combination peginterferon alpha-2a and ribavirin compared with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol 2004; 99:1490–1496.
- McGinn T, O’Connor-Moore N, Alfandre D, Gardenier D, Wisnivesky J. Validation of a hepatitis C screening tool in primary care. Arch Intern Med 2008; 168:2009–2013.
- Kaur S, Rybicki L, Bacon BR, Gollan JL, Rustgi VK, Carey WD. Performance characteristics and results of a large-scale screening program for viral hepatitis and risk factors associated with exposure to viral hepatitis B and C: results of the National Hepatitis Screening Survey. National Hepatitis Surveillance Group. Hepatology 1996; 24:979–986.
- Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol 2000; 95:740–747.
- Austin GE, Jensen B, Leete J, et al. Prevalence of hepatitis C virus seropositivity among hospitalized US veterans. Am J Med Sci 2000; 319:353–359.
- Yawn BP, Wollan P, Gazzuola L, Kim WR. Diagnosis and 10-year follow-up of a community-based hepatitis C cohort. J Fam Pract 2002; 51:135–140.
- Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 18(suppl 1):S11–S19.
- Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis 1997; 1:559–568,
- Alter MJ, Hadler SC, Judson FN, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA 1990; 264:2231–2235.
- Wasley A, Miller JT, Finelli L; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis—United States, 2005. MMWR Surveill Summ 2007; 56:1–24.
- Goedert JJ, Chen BE, Preiss L, Aledort LM, Rosenberg PS. Reconstruction of the hepatitis C virus epidemic in the US hemophilia population, 1940–1990. Am J Epidemiol 2007; 165:1443–1453.
- Eyster ME, Sherman KE, Goedert JJ, Katsoulidou A, Hatzakis A. Prevalence and changes in hepatitis C virus genotypes among multitransfused persons with hemophilia. The Multicenter Hemophilia Cohort Study. J Infect Dis 1999; 179:1062–1069.
- Yee TT, Griffioen A, Sabin CA, Dusheiko G, Lee CA. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut 2000; 47:845–851.
- Lee C, Dusheiko G. The natural history and antiviral treatment of hepatitis C in haemophilia. Haemophilia 2002; 8:322–329.
- Makris M, Garson JA, Ring CJ, Tuke PW, Tedder RS, Preston FE. Hepatitis C viral RNA in clotting factor concentrates and the development of hepatitis in recipients. Blood 1993; 81:1898–1902.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831–837.
- Sulkowski MS. The HIV-coinfected patient: managing viral hepatitis. J Acquir Immune Defic Syndr 2007; 45(suppl 2):S36–S37.
- Bonacini M, Lin HJ, Hollinger FB. Effect of coexisting HIV-1 infection on the diagnosis and evaluation of hepatitis C virus. J Acquir Immune Defic Syndr 2001; 26:340–344.
- Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health 1996; 86:655–661.
- Bell J, Batey RG, Farrell GC, Crewe EB, Cunningham AL, Byth K. Hepatitis C virus in intravenous drug users. Med J Aust 1990; 153:274–276.
- Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol 1997; 35:3274–3277.
- Patrick DM, Tyndall MW, Cornelisse PG, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ 2001; 165:889–895.
- Seeff LB, Wright EC, Zimmerman HJ, McCollum RW. VA cooperative study of post-transfusion hepatitis, 1969-1974: incidence and characteristics of hepatitis and responsible risk factors. Am J Med Sci 1975; 270:355–362.
- Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med 1975; 292:767–770.
- Alter HJ, Holland PV, Purcell RH, et al. Posttransfusion hepatitis after exclusion of commercial and hepatitis-B antigen-positive donors. Ann Intern Med. 1972; 77:691–699.
- Blajchman MA, Vamvakas EC. The continuing risk of transfusion-transmitted infections. N Engl J Med 2006; 355:1303–1305.
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345:41–52.
- Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int 1994; 45:238–244.
- Izopet J, Rostaing L, Sandres K, et al. Longitudinal analysis of hepatitis C virus replication and liver fibrosis progression in renal transplant recipients. J Infect Dis 2000; 181:852–858.
- Dubois F, Desenclos JC, Mariotte N, Goudeau A. Hepatitis C in a French population-based survey, 1994: seroprevalence, frequency of viremia, genotype distribution, and risk factors. The Collaborative Study Group. Hepatology 1997; 25:1490–1496.
- Bellentani S, Pozzato G, Saccoccio G, et al. Clinical course and risk factors of hepatitis C virus related liver disease in the general population: report from the Dionysos study. Gut 1999; 44:874–880.
- Alberti A, Noventa F, Benvegnù L, Boccato S, Gatta A. Prevalence of liver disease in a population of asymptomatic persons with hepatitis C virus infection. Ann Intern Med 2002; 137:961–964.
- Shiffman ML, Diago M, Tran A, et al. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol 2006; 4:645–652.
- Stary A, Kopp W, Hofmann H, Heller-Vitouch C, Kunz C. Seroepidemiologic study of hepatitis C virus in sexually transmitted disease risk groups. Sex Transm Dis 1992; 19:252–258.
- Weinstock HS, Bolan G, Reingold AL, Polish LB. Hepatitis C virus infection among patients attending a clinic for sexually transmitted diseases. JAMA 1993; 269:392–394.
- Thomas DL, Gruninger SE, Siew C, Joy ED, Quinn TC. Occupational risk of hepatitis C infections among general dentists and oral surgeons in North America. Am J Med 1996; 100:41–45.
- Klein RS, Freeman K, Taylor PE, Stevens CE. Occupational risk for hepatitis C virus infection among New York City dentists. Lancet 1991; 338:1539–1542.
- Polish LB, Tong MJ, Co RL, Coleman PJ, Alter MJ. Risk factors for hepatitis C virus infection among health care personnel in a community hospital. Am J Infect Control 1993; 21:196–200.
- Alter MJ. Occupational exposure to hepatitis C virus: a dilemma. Infect Control Hosp Epidemiol 1994; 15:742–744.
- Lanphear BP, Linnemann CC, Cannon CG, DeRonde MM, Pendy L, Kerley LM. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect Control Hosp Epidemiol 1994; 15:745–750.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposures in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control 1995; 23:273–277.
- Mitsui T, Iwano K, Masuko K, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology 1992; 16:1109–1114.
- Sartori M, La Terra G, Aglietta M, Manzin A, Navino C, Verzetti G. Transmission of hepatitis C via blood splash into conjunctiva. Scand J Infect Dis 1993; 25:270–271.
- Ippolito G, Puro V, Petrosillo N, De Carli G, Micheloni G, Magliano E. Simultaneous infection with HIV and hepatitis C virus following occupational conjunctival blood exposure. JAMA 1998; 280:28.
- Carey W. Tests and screening strategies for the diagnosis of hepatitis C. Cleve Clin J Med 2003; 70(suppl 4):S7–S13.
- Carey WD, Jeffers L, Kugelmas M, et al; Hepatitis C management. Hepatitis C Monograph. Cleveland Clinic; www.clevelandclinicmeded.com/online/monograph/hepc/page1.htm. Accessed 7/30/2010.
- Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA 2007; 297:724–732.
- Bowden DS, Berzsenyi MD. Chronic hepatitis C virus infection: genotyping and its clinical role. Future Microbiol 2006; 1:103–112.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982.
- Poynard T, McHutchison J, Davis GL, et al. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology 2000; 32:1131–1137.
- Zeuzem S. Interferon-based therapy for chronic hepatitis C: current and future perspectives. Nat Clin Pract Gastroenterol Hepatol 2008; 5:610–622.
KEY POINTS
- Patients who should be screened include intravenous drug abusers, people infected with human immunodeficiency virus, patients with unexplained elevated alanine aminotransferase levels, infants born to infected mothers, and people with infected sexual partners.
- Patients at risk of HCV infection should be tested for anti-HCV antibody using an enzyme immunoassay (EIA).
- Positive results on anti-HCV EIA testing should be confirmed with an assay for HCV RNA.
- HCV genotyping can help predict the response to therapy. Genotypes 2 or 3 are more likely to respond to therapy than genotype 1.