User login
Evaluating brain function in patients with disorders of consciousness
Consciousness has long been a fascinating subject to both philosophers and scientists, yet consciousness has only recently been taken into account by neuroscientists as a topic for research. This article discusses research done over the past 10 years evaluating brain function in patients with disorders of consciousness—specifically those in a vegetative or minimally conscious state. We highlight physiologic, sensory, perceptual, cognitive, and behavioral commonalities and disparities between patients with anoxic and traumatic brain injuries, with the aim of characterizing the neurophysiologic and neuroanatomic differences between these two main causes of disorders of consciousness.
WHAT IS CONSCIOUSNESS?
Although consciousness is difficult to describe, it can be defined as a combination of wakefulness and awareness.1 As for the brain systems supporting these two aspects of consciousness, it has been suggested that the brainstem ascending reticular formation system and its thalamic projections support alertness and the sleep-wake cycle, and that conscious awareness relies on a functional thalamocortical and corticocortical system.
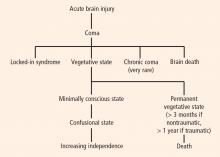
DISORDERS OF CONSCIOUSNESS: A VARIETY OF STATES
Coma: Near-complete unresponsiveness
Coma is a condition of almost complete unresponsiveness in which the patient lies with eyes closed, very limited reflexes, no cyclical wakefulness, and, above all, no signs of awareness. Coma is normally attained after an acute brain insult and may last about 2 weeks, although chronic coma cases have been described, and is usually caused by either temporary or permanent damage to the reticular system.
Vegetative state: Wakefulness without awareness
Following a coma, some patients may enter a vegetative state, which involves a complete absence of consciousness of one’s environment but with preserved sleep-wake cycles and autonomic functions. The vegetative state is easily differentiated from brain death, in which the electroencephalogram shows no brain wave or activity.4 Brain death is the irreversible end of all brain activity and should not be confused with a persistent vegetative state.
The vegetative state is a condition of wakefulness without awareness in which the patient exhibits a partially preserved sleep-wake cycle and a variable portfolio of reflexes and spontaneous nonvolitional behaviors. A patient who has been in a vegetative state for more than 1 month with no improvement is often said to be in a persistent vegetative state. The term permanent vegetative state, implying no chance of recovery, is sometimes used when the vegetative state persists for 3 months after a nontraumatic insult, such as cardiac arrest, or for 1 year after a traumatic brain injury.
Minimally conscious state: Conscious awareness is evident despite impairment
Some patients in a vegetative state may start to recover by entering a minimally conscious state, in which conscious awareness is evident despite profound physical and cognitive impairment. Although communication capabilities are absent, cognitively mediated (or voluntary) behavior occurs in the minimally conscious state, which may be inconsistent but is reproducible enough to be differentiated from reflexive behavior. For example, patients may occasionally be able to smile when asked to do so or follow an object with their eyes. In the minimally conscious state, patients show those basic behaviors seen in the vegetative state along with islands of presumably conscious processing such as inconsistent responses to simple commands and sustained visual pursuit.5 Patients in a minimally conscious state have a better prognosis than those in a persistent or permanent vegetative state.3
Locked-in syndrome: Not a true disorder of consciousness
Another pathology that is often confounded with vegetative or minimally conscious states is the locked-in syndrome, which is characterized by complete paralysis of voluntary muscles in all parts of the body except those controlling eye movements. Individuals with locked-in syndrome are conscious and can think and reason, but they are unable to speak or move. The disorder confines the patient to paralysis and a mute state. Communication may be possible with blinking eye movements.
WHAT CAUSES DISORDERS OF CONSCIOUSNESS?
Disorders of consciousness mostly stem from acute brain insults, which may be caused by hypoxicischemic neural injury or traumatic brain injury. Although traumatic brain injury is currently the most common cause of vegetative and minimally conscious states, nontraumatic causes are becoming more frequent as a result of scientific and technological developments in resuscitation. Nontraumatic causes of disorders of consciousness include stroke, cardiopulmonary arrest, and meningoencephalitis; additionally, patients in the final stage of certain neurodegenerative diseases, including Parkinson, Alzheimer, and Huntington diseases, may lapse into a minimally conscious or vegetative state.6
NEUROLOGIC FINDINGS IN COMATOSE SURVIVORS OF CARDIAC ARREST
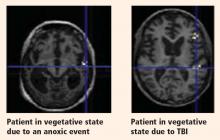
Structural magnetic resonance imaging (MRI) of patients in a vegetative state following cardiac arrest often reveals abnormalities. Most frequently there is a white matter signal in the cerebellum, the thalamus, the frontal and parietal cortices, and the hippocampus. Widespread abnormalities may indicate little to no prospect for recovery. Pupillary light response, corneal reflexes, motor responses to pain, myoclonus status epilepticus, serum neuron-specific enolase, and somatosensory evoked potential studies can assist in predicting efficiently and accurately a poor outcome in comatose patients after cardiopulmonary resuscitation for cardiac arrest.7
DEFINITION PROBLEMS AND MISDIAGNOSIS
The diagnosis of vegetative state emerges from a negative finding—namely, the lack of behaviors that would signal conscious capabilities. Using the nonoccurrence of events as a criterion to establish a fact is inherently problematic, since the causes of a nonoccurrence are theoretically infinite. More specifically, the reasons behind the lack of evidence of voluntary movement in presumably unconscious patients can be classified in terms of malfunctioning of either sensoriperceptual, output/motor, or central processing.
Deficits in sensoriperceptual processing
A patient might have deafness that may lead to a deficit in speech comprehension, or perhaps the auditory pathway and first cortical pathways are spared but the patient is aphasic and cannot process additive events such as speech. In a cohort of 42 patients, we found 17 who lacked the fourth or fifth components of the brain auditory evoked potentials to clicks presented binaurally, signaling severe damage to the auditory pathway.8 It is useless to ask such patients to follow commands, since the sensory input is damaged and the movement (or lack of movement) has no validity for the diagnosis. A similar argument can apply for patients who may show some fixation but exhibit delayed or absent visual evoked potentials when presented with written commands.
Deficits in motor processing
The second type of lesions that may contribute to misdiagnosis in these patients are those found in the effector systems. If the motor voluntary pathways are damaged—either in the motor cortex or in the corticospinal or corticobulbar pathways—then movement might be impaired enough to prevent responses by the patient. Patients of this type are sometimes diagnosed as being in a vegetative state although they might actually have locked-in syndrome,9 with preserved cognition but an inability to initiate voluntary responses as a result of a lesion in the pontine peduncle.
Although the effector systems are difficult to test in unresponsive subjects, some strategies may be tried. Before testing for volition, it is necessary to assess all possible hand, leg, and face reflexes in order to map reflexive behavior. Commands should then specifically target those muscles that showed total or partial preservation of reflexes. To test the output pathways from the cortex to the medulla, a more specialized assessment is needed; the Impaired Consciousness Research Group at the University of Cambridge has developed a simple protocol to assess the ability of the motor cortex to elicit muscle twitches by measuring the motor evoked potentials to simple pulses of transcranial magnetic stimulation. The minimal pulse intensity is determined by electromyographic recordings when transcranial magnetic stimulation pulses are applied to the left or right motor cortices for the hands and feet. The results have shown 2 out of 34 patients to have no detectable motor evoked potentials and 5 patients to have severe delay at maximum pulse intensity [unpublished data]. These results confirm the need for a full neurologic and neurophysiologic assessment in subjects who are unresponsive or show low levels of response, both acutely and more chronically, to minimize the risk of misdiagnosis.
Deficits in central processing
The key element in the assessment of cognitive processing in patients in a vegetative or minimally conscious state is determining deficits in their capacity to process external stimuli in a conscious manner (central processing). This is by far the most difficult characteristic to be determined since the only accepted criteria for awareness are verbal report or voluntary movement, both of which are absent in the vegetative state and are inconsistent and difficult to determine behaviorally in the minimally conscious state.
CLUES TO BRAIN FUNCTION IN DISORDERS OF CONSCIOUSNESS
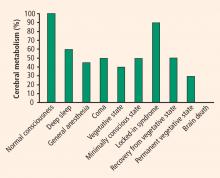
It is important to differentiate a patient in a persistent vegetative state from a patient in a minimally conscious state, as the latter patient has a much higher chance of a favorable outcome. Evaluation of cerebral metabolism and imaging studies can both provide clues to brain function.
Cerebral metabolism
Neuroimaging studies
In the past few years, studies have found that some patients in a vegetative or minimally conscious state can activate cortical networks in response to auditory, visual, and tactile stimuli.10 A challenge in neuroscience is to devise a reliable, objective test to assess awareness without relying on explicit voluntary movements or verbal responses. Such a test would have important theoretical and practical implications. Recent evidence from functional neuroimaging and neurophysiology suggests that some patients with disorders of consciousness exhibit partially preserved conscious processing despite having no clinical or verbal output.11
During a positron emission tomography study, Menon et al showed photographs to a 26-year-old woman who was in a vegetative state 4 months after becoming comatose from an acute febrile illness.12 They found significant activation in the right fusiform gyrus and extrastriate visual association areas when the woman was shown photos of people familiar to her as compared with repixellated versions of the same photos with the faces made unrecognizable. The activation pattern she exhibited was similar to that of healthy volunteers. Interestingly, a few months after this study, the patient became increasingly responsive.
Our group conducted the first evaluation of emotion in the minimally conscious state using functional MRI (fMRI) in a 17-year-old male following a traumatic brain injury.13 The patient was able to localize noxious stimuli, exhibited spontaneous eye opening, and occasionally smiled appropriately and followed people with his eyes. Imaging was performed while he listened to two recordings—one of his mother reading a story about his life, and one of a matched control voice reading the same story. Digital subtraction imaging disclosed strong activation of two areas related to emotion, the amygdala and the bilateral insula, while the recording of the patient’s mother was played. Activation was also evident in the auditory cortex in the superior temporal lobe. The patient recovered 6 months following this study.
Classical conditioning
Classical conditioning represents an alternate approach to MRI for assessing brain function in patients with disordered consciousness.8 Trace conditioning of the eye-blink response is considered to be an objective test of awareness.14 This test involves highly specific learning, requiring an anticipatory electromyographic response to a paired stimulus (eg, a tone followed by an aversive stimulus such as an air puff to the eyes) but not to an unpaired stimulus (eg, a white noise that is not followed by an aversive stimulus). This effect increases in amplitude as the aversive stimulus approaches. Our laboratory is applying this method to study learning and memory in patients with disordered consciousness.
DETERMINING AWARENESS WITHOUT REPORT
The proposed neural correlates of consciousness do not usually take into account the levels of consciousness.15,16 In order to build the framework for a cognitive neuroscience of consciousness, we must consider the content of the consciousness experience in fully awake subjects and patients as well as the cognitive processes occurring in unconscious and conscious subjects.
Two main approaches can be used to assess conscious processing in unresponsive patients. The first is to look for neural correlates in direct intentional actions or imagined actions,11 and the second is to look for physiologic correlates of the cognitive processes required during the conscious processing of stimuli.17
Searching for neural correlates of intended actions
The first approach can have enormous impact in the diagnostic arena (as well as in the legal and ethical arenas), such as in the case reported by Owen et al in which a patient showed brain activity related to imagining actions as prompted by spoken instructions during fMRI evaluation.11 Unfortunately, cases such as these are scarce. Moreover, imagining of actions relies not only on a spared comprehension capacity and preserved memory but also on the subject’s willingness to perform the task. It would seem that only a minority of patients in a vegetative state seem to have the cognitive abilities preserved to accomplish these types of tasks.
Searching for physiologic correlates of cognitive processes
The second approach would tend to work with memory and switching attention capabilities in unresponsive patients, assuming that conscious processing does not exist without these cognitive processes. The evidence for this approach comes from electrophysiology. Cognitive evoked potentials are commonly applied to assess basic auditory or visual cortical processing, automatic attention, and focus attention.18 Both the mismatch negativity wave (a correlate of automatic attention) and the p300 (a correlate of focus attention) are sometimes present,19 specifically in patients in vegetative or minimally conscious states, and they are a good predictor of awakening in stroke, hemorrhage, and traumatic brain injury.20
In day-to-day practice in a neurology clinic or emergency room, it is more feasible to assess cognitive capabilities using event-related potentials than fMRI since they are more widely distributed, more easily validated, shorter, and statistically more powerful in single-subject analysis,21 and because they do not frequently rely on speech comprehension.
NEUROPATHOLOGY AND fMRI
The cause of the brain injury leading to a vegetative or minimally conscious state frequently determines the neuropathology.22 It has also been demonstrated that severely disabled patients (such as those emerging from a minimally conscious state) differ from vegetative state patients in terms of lesions and severity.23
Although residual activity as seen on functional neuroimaging may be unequivocal in some cases, it may represent only fragmentary cognitive processing; it is important not to assume that normal awareness is present. Much still needs to be learned, but results from neuroimaging studies demonstrate that a small proportion of patients in a vegetative or minimally conscious state have some preserved cognitive processes. These findings have ethical and legal implications. For instance, careless bedside chatter among family members or medical personnel is inappropriate and should be avoided. Whether functional neuroimaging can effectively evaluate neuroprocessing in patients in whom cognitive output is difficult to assess remains to be determined. Such evaluation may one day help to predict prognosis. It may also someday help to facilitate communication with patients with locked-in syndrome, who are cognitively intact but are without verbal or motor output.
CONCLUSIONS
It is highly improbable to find patients with preserved cortical connectivity, since structural22 and functional19 studies have demonstrated only a small proportion of patients in a vegetative or minimally conscious state who have relatively preserved brains and cognitive processing. The more we study patients who are unresponsive or show low levels of response, the more complex cognitive processes we find in subpopulations of these patients. Language-related cortical activation is now the most common finding.13,19,24 More recently, a few researchers working with severely damaged patients have started to test paradigms with the aim of uncovering conscious processes that have no need of verbal or movement responses.
The time has come for clinicians in acute care centers to immediately follow their administration of coma scales in unresponsive patients with the use of more sophisticated methodology to assess not only reflexive and intentional behaviors but also these patients’ physiologic and cognitive characteristics. In the field of neurodegenerative disease, it took several years for clinicians to start using more sensitive cognitive tools than just the mini-mental state examination and computed tomography or three-dimensional T1-weighted structural MRI, but nowadays volumetric MRI and detailed cognitive assessments are widely used to diagnose and characterize patients with neurodegenerative disorders. The same path should be taken for patients with severe brain damage. The information yielded by such an approach may one day help to determine a diagnosis or prognosis, guide treatment, or facilitate communication in patients with pathologies of consciousness.
- Jennett B, Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet 1972; 1:734–737.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state.first of two parts. N Engl J Med 1994; 330:1499–1508.
- Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002; 58:349–353.
- Wijdicks EFM. The diagnosis of brain death. N Engl J Med 2000; 344:1215–1221.
- Giacino JT, Trott CT. Rehabilitative management of patients with disorders of consciousness: grand rounds. J Head Trauma Rehabil 2004; 19:254–265.
- Bernat JL. Chronic disorders of consciouness. Lancet 2006; 367: 1181–1192.
- Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 67:203–210.
- Bekinschtein TA. Cognitive Processes in the Vegetative and Minimally Conscious State [thesis]. Buenos Aires, Argentina: University of Buenos Aires; 2006.
- Onofrj M, Thomas A, Paci C, Scesi M, Tombari R. Event related potentials recorded in patients with locked-in syndrome. J Neurol Neurosurg Psychiatry 1997; 63:759–764.
- Schiff ND. Multimodal neuroimaging approaches to disorders of consciousness. J Head Trauma Rehabil 2006; 21:388–397.
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science 2006; 313:1402.
- Menon DK, Owen AM, Williams EJ, et al. Cortical processing in persistent vegetative state. Lancet 1998; 352:200.
- Bekinschtein T, Niklison J, Sigman L, et al. Emotion processing in the minimally conscious state [letter]. J Neurol Neurosurg Psychiatry 2004; 75:788.
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science 1998; 280:77–81.
- Koch C. The Quest for Consciousness: A Neurobiological Approach. Greenwood Village, CO: Roberts & Company Publishers; 2004.
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 2001; 79:1–37.
- Naccache L. Is she conscious? Science 2006; 313:1395–1396.
- Picton TW, Bentin S, Berg P, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology 2000; 37:127–152.
- Kotchoubey B, Lang S, Mezger G, et al. Information processing in severe disorders of consciousness: vegetative state and minimally conscious state. Clin Neurophysiol 2005; 116:2441–2453.
- Daltrozzo J, Wioland N, Mutschler V, Kotchoubey B. Predicting coma and other low responsive patients outcome using event-related brain potentials: a meta-analysis. Clin Neurophysiol 2007; 118:606–614.
- Quian Quiroga R, Garcia H. Single-trial event-related potentials with wavelet denoising. Clin Neurophysiol 2003; 114:376–390.
- Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain 2000; 123:1327–1338.
- Jennett B. Thirty years of the vegetative state: clinical, ethical and legal problems. Prog Brain Res 2005; 150:537–543.
- Schiff ND, Rodriguez-Moreno D, Kamal A, et al. fMRI reveals large-scale network activation in minimally conscious patients. Neurology 2005; 64:514–523.
Consciousness has long been a fascinating subject to both philosophers and scientists, yet consciousness has only recently been taken into account by neuroscientists as a topic for research. This article discusses research done over the past 10 years evaluating brain function in patients with disorders of consciousness—specifically those in a vegetative or minimally conscious state. We highlight physiologic, sensory, perceptual, cognitive, and behavioral commonalities and disparities between patients with anoxic and traumatic brain injuries, with the aim of characterizing the neurophysiologic and neuroanatomic differences between these two main causes of disorders of consciousness.
WHAT IS CONSCIOUSNESS?
Although consciousness is difficult to describe, it can be defined as a combination of wakefulness and awareness.1 As for the brain systems supporting these two aspects of consciousness, it has been suggested that the brainstem ascending reticular formation system and its thalamic projections support alertness and the sleep-wake cycle, and that conscious awareness relies on a functional thalamocortical and corticocortical system.
DISORDERS OF CONSCIOUSNESS: A VARIETY OF STATES
Coma: Near-complete unresponsiveness
Coma is a condition of almost complete unresponsiveness in which the patient lies with eyes closed, very limited reflexes, no cyclical wakefulness, and, above all, no signs of awareness. Coma is normally attained after an acute brain insult and may last about 2 weeks, although chronic coma cases have been described, and is usually caused by either temporary or permanent damage to the reticular system.
Vegetative state: Wakefulness without awareness
Following a coma, some patients may enter a vegetative state, which involves a complete absence of consciousness of one’s environment but with preserved sleep-wake cycles and autonomic functions. The vegetative state is easily differentiated from brain death, in which the electroencephalogram shows no brain wave or activity.4 Brain death is the irreversible end of all brain activity and should not be confused with a persistent vegetative state.
The vegetative state is a condition of wakefulness without awareness in which the patient exhibits a partially preserved sleep-wake cycle and a variable portfolio of reflexes and spontaneous nonvolitional behaviors. A patient who has been in a vegetative state for more than 1 month with no improvement is often said to be in a persistent vegetative state. The term permanent vegetative state, implying no chance of recovery, is sometimes used when the vegetative state persists for 3 months after a nontraumatic insult, such as cardiac arrest, or for 1 year after a traumatic brain injury.
Minimally conscious state: Conscious awareness is evident despite impairment
Some patients in a vegetative state may start to recover by entering a minimally conscious state, in which conscious awareness is evident despite profound physical and cognitive impairment. Although communication capabilities are absent, cognitively mediated (or voluntary) behavior occurs in the minimally conscious state, which may be inconsistent but is reproducible enough to be differentiated from reflexive behavior. For example, patients may occasionally be able to smile when asked to do so or follow an object with their eyes. In the minimally conscious state, patients show those basic behaviors seen in the vegetative state along with islands of presumably conscious processing such as inconsistent responses to simple commands and sustained visual pursuit.5 Patients in a minimally conscious state have a better prognosis than those in a persistent or permanent vegetative state.3
Locked-in syndrome: Not a true disorder of consciousness
Another pathology that is often confounded with vegetative or minimally conscious states is the locked-in syndrome, which is characterized by complete paralysis of voluntary muscles in all parts of the body except those controlling eye movements. Individuals with locked-in syndrome are conscious and can think and reason, but they are unable to speak or move. The disorder confines the patient to paralysis and a mute state. Communication may be possible with blinking eye movements.
WHAT CAUSES DISORDERS OF CONSCIOUSNESS?
Disorders of consciousness mostly stem from acute brain insults, which may be caused by hypoxicischemic neural injury or traumatic brain injury. Although traumatic brain injury is currently the most common cause of vegetative and minimally conscious states, nontraumatic causes are becoming more frequent as a result of scientific and technological developments in resuscitation. Nontraumatic causes of disorders of consciousness include stroke, cardiopulmonary arrest, and meningoencephalitis; additionally, patients in the final stage of certain neurodegenerative diseases, including Parkinson, Alzheimer, and Huntington diseases, may lapse into a minimally conscious or vegetative state.6
NEUROLOGIC FINDINGS IN COMATOSE SURVIVORS OF CARDIAC ARREST
Structural magnetic resonance imaging (MRI) of patients in a vegetative state following cardiac arrest often reveals abnormalities. Most frequently there is a white matter signal in the cerebellum, the thalamus, the frontal and parietal cortices, and the hippocampus. Widespread abnormalities may indicate little to no prospect for recovery. Pupillary light response, corneal reflexes, motor responses to pain, myoclonus status epilepticus, serum neuron-specific enolase, and somatosensory evoked potential studies can assist in predicting efficiently and accurately a poor outcome in comatose patients after cardiopulmonary resuscitation for cardiac arrest.7
DEFINITION PROBLEMS AND MISDIAGNOSIS
The diagnosis of vegetative state emerges from a negative finding—namely, the lack of behaviors that would signal conscious capabilities. Using the nonoccurrence of events as a criterion to establish a fact is inherently problematic, since the causes of a nonoccurrence are theoretically infinite. More specifically, the reasons behind the lack of evidence of voluntary movement in presumably unconscious patients can be classified in terms of malfunctioning of either sensoriperceptual, output/motor, or central processing.
Deficits in sensoriperceptual processing
A patient might have deafness that may lead to a deficit in speech comprehension, or perhaps the auditory pathway and first cortical pathways are spared but the patient is aphasic and cannot process additive events such as speech. In a cohort of 42 patients, we found 17 who lacked the fourth or fifth components of the brain auditory evoked potentials to clicks presented binaurally, signaling severe damage to the auditory pathway.8 It is useless to ask such patients to follow commands, since the sensory input is damaged and the movement (or lack of movement) has no validity for the diagnosis. A similar argument can apply for patients who may show some fixation but exhibit delayed or absent visual evoked potentials when presented with written commands.
Deficits in motor processing
The second type of lesions that may contribute to misdiagnosis in these patients are those found in the effector systems. If the motor voluntary pathways are damaged—either in the motor cortex or in the corticospinal or corticobulbar pathways—then movement might be impaired enough to prevent responses by the patient. Patients of this type are sometimes diagnosed as being in a vegetative state although they might actually have locked-in syndrome,9 with preserved cognition but an inability to initiate voluntary responses as a result of a lesion in the pontine peduncle.
Although the effector systems are difficult to test in unresponsive subjects, some strategies may be tried. Before testing for volition, it is necessary to assess all possible hand, leg, and face reflexes in order to map reflexive behavior. Commands should then specifically target those muscles that showed total or partial preservation of reflexes. To test the output pathways from the cortex to the medulla, a more specialized assessment is needed; the Impaired Consciousness Research Group at the University of Cambridge has developed a simple protocol to assess the ability of the motor cortex to elicit muscle twitches by measuring the motor evoked potentials to simple pulses of transcranial magnetic stimulation. The minimal pulse intensity is determined by electromyographic recordings when transcranial magnetic stimulation pulses are applied to the left or right motor cortices for the hands and feet. The results have shown 2 out of 34 patients to have no detectable motor evoked potentials and 5 patients to have severe delay at maximum pulse intensity [unpublished data]. These results confirm the need for a full neurologic and neurophysiologic assessment in subjects who are unresponsive or show low levels of response, both acutely and more chronically, to minimize the risk of misdiagnosis.
Deficits in central processing
The key element in the assessment of cognitive processing in patients in a vegetative or minimally conscious state is determining deficits in their capacity to process external stimuli in a conscious manner (central processing). This is by far the most difficult characteristic to be determined since the only accepted criteria for awareness are verbal report or voluntary movement, both of which are absent in the vegetative state and are inconsistent and difficult to determine behaviorally in the minimally conscious state.
CLUES TO BRAIN FUNCTION IN DISORDERS OF CONSCIOUSNESS
It is important to differentiate a patient in a persistent vegetative state from a patient in a minimally conscious state, as the latter patient has a much higher chance of a favorable outcome. Evaluation of cerebral metabolism and imaging studies can both provide clues to brain function.
Cerebral metabolism
Neuroimaging studies
In the past few years, studies have found that some patients in a vegetative or minimally conscious state can activate cortical networks in response to auditory, visual, and tactile stimuli.10 A challenge in neuroscience is to devise a reliable, objective test to assess awareness without relying on explicit voluntary movements or verbal responses. Such a test would have important theoretical and practical implications. Recent evidence from functional neuroimaging and neurophysiology suggests that some patients with disorders of consciousness exhibit partially preserved conscious processing despite having no clinical or verbal output.11
During a positron emission tomography study, Menon et al showed photographs to a 26-year-old woman who was in a vegetative state 4 months after becoming comatose from an acute febrile illness.12 They found significant activation in the right fusiform gyrus and extrastriate visual association areas when the woman was shown photos of people familiar to her as compared with repixellated versions of the same photos with the faces made unrecognizable. The activation pattern she exhibited was similar to that of healthy volunteers. Interestingly, a few months after this study, the patient became increasingly responsive.
Our group conducted the first evaluation of emotion in the minimally conscious state using functional MRI (fMRI) in a 17-year-old male following a traumatic brain injury.13 The patient was able to localize noxious stimuli, exhibited spontaneous eye opening, and occasionally smiled appropriately and followed people with his eyes. Imaging was performed while he listened to two recordings—one of his mother reading a story about his life, and one of a matched control voice reading the same story. Digital subtraction imaging disclosed strong activation of two areas related to emotion, the amygdala and the bilateral insula, while the recording of the patient’s mother was played. Activation was also evident in the auditory cortex in the superior temporal lobe. The patient recovered 6 months following this study.
Classical conditioning
Classical conditioning represents an alternate approach to MRI for assessing brain function in patients with disordered consciousness.8 Trace conditioning of the eye-blink response is considered to be an objective test of awareness.14 This test involves highly specific learning, requiring an anticipatory electromyographic response to a paired stimulus (eg, a tone followed by an aversive stimulus such as an air puff to the eyes) but not to an unpaired stimulus (eg, a white noise that is not followed by an aversive stimulus). This effect increases in amplitude as the aversive stimulus approaches. Our laboratory is applying this method to study learning and memory in patients with disordered consciousness.
DETERMINING AWARENESS WITHOUT REPORT
The proposed neural correlates of consciousness do not usually take into account the levels of consciousness.15,16 In order to build the framework for a cognitive neuroscience of consciousness, we must consider the content of the consciousness experience in fully awake subjects and patients as well as the cognitive processes occurring in unconscious and conscious subjects.
Two main approaches can be used to assess conscious processing in unresponsive patients. The first is to look for neural correlates in direct intentional actions or imagined actions,11 and the second is to look for physiologic correlates of the cognitive processes required during the conscious processing of stimuli.17
Searching for neural correlates of intended actions
The first approach can have enormous impact in the diagnostic arena (as well as in the legal and ethical arenas), such as in the case reported by Owen et al in which a patient showed brain activity related to imagining actions as prompted by spoken instructions during fMRI evaluation.11 Unfortunately, cases such as these are scarce. Moreover, imagining of actions relies not only on a spared comprehension capacity and preserved memory but also on the subject’s willingness to perform the task. It would seem that only a minority of patients in a vegetative state seem to have the cognitive abilities preserved to accomplish these types of tasks.
Searching for physiologic correlates of cognitive processes
The second approach would tend to work with memory and switching attention capabilities in unresponsive patients, assuming that conscious processing does not exist without these cognitive processes. The evidence for this approach comes from electrophysiology. Cognitive evoked potentials are commonly applied to assess basic auditory or visual cortical processing, automatic attention, and focus attention.18 Both the mismatch negativity wave (a correlate of automatic attention) and the p300 (a correlate of focus attention) are sometimes present,19 specifically in patients in vegetative or minimally conscious states, and they are a good predictor of awakening in stroke, hemorrhage, and traumatic brain injury.20
In day-to-day practice in a neurology clinic or emergency room, it is more feasible to assess cognitive capabilities using event-related potentials than fMRI since they are more widely distributed, more easily validated, shorter, and statistically more powerful in single-subject analysis,21 and because they do not frequently rely on speech comprehension.
NEUROPATHOLOGY AND fMRI
The cause of the brain injury leading to a vegetative or minimally conscious state frequently determines the neuropathology.22 It has also been demonstrated that severely disabled patients (such as those emerging from a minimally conscious state) differ from vegetative state patients in terms of lesions and severity.23
Although residual activity as seen on functional neuroimaging may be unequivocal in some cases, it may represent only fragmentary cognitive processing; it is important not to assume that normal awareness is present. Much still needs to be learned, but results from neuroimaging studies demonstrate that a small proportion of patients in a vegetative or minimally conscious state have some preserved cognitive processes. These findings have ethical and legal implications. For instance, careless bedside chatter among family members or medical personnel is inappropriate and should be avoided. Whether functional neuroimaging can effectively evaluate neuroprocessing in patients in whom cognitive output is difficult to assess remains to be determined. Such evaluation may one day help to predict prognosis. It may also someday help to facilitate communication with patients with locked-in syndrome, who are cognitively intact but are without verbal or motor output.
CONCLUSIONS
It is highly improbable to find patients with preserved cortical connectivity, since structural22 and functional19 studies have demonstrated only a small proportion of patients in a vegetative or minimally conscious state who have relatively preserved brains and cognitive processing. The more we study patients who are unresponsive or show low levels of response, the more complex cognitive processes we find in subpopulations of these patients. Language-related cortical activation is now the most common finding.13,19,24 More recently, a few researchers working with severely damaged patients have started to test paradigms with the aim of uncovering conscious processes that have no need of verbal or movement responses.
The time has come for clinicians in acute care centers to immediately follow their administration of coma scales in unresponsive patients with the use of more sophisticated methodology to assess not only reflexive and intentional behaviors but also these patients’ physiologic and cognitive characteristics. In the field of neurodegenerative disease, it took several years for clinicians to start using more sensitive cognitive tools than just the mini-mental state examination and computed tomography or three-dimensional T1-weighted structural MRI, but nowadays volumetric MRI and detailed cognitive assessments are widely used to diagnose and characterize patients with neurodegenerative disorders. The same path should be taken for patients with severe brain damage. The information yielded by such an approach may one day help to determine a diagnosis or prognosis, guide treatment, or facilitate communication in patients with pathologies of consciousness.
Consciousness has long been a fascinating subject to both philosophers and scientists, yet consciousness has only recently been taken into account by neuroscientists as a topic for research. This article discusses research done over the past 10 years evaluating brain function in patients with disorders of consciousness—specifically those in a vegetative or minimally conscious state. We highlight physiologic, sensory, perceptual, cognitive, and behavioral commonalities and disparities between patients with anoxic and traumatic brain injuries, with the aim of characterizing the neurophysiologic and neuroanatomic differences between these two main causes of disorders of consciousness.
WHAT IS CONSCIOUSNESS?
Although consciousness is difficult to describe, it can be defined as a combination of wakefulness and awareness.1 As for the brain systems supporting these two aspects of consciousness, it has been suggested that the brainstem ascending reticular formation system and its thalamic projections support alertness and the sleep-wake cycle, and that conscious awareness relies on a functional thalamocortical and corticocortical system.
DISORDERS OF CONSCIOUSNESS: A VARIETY OF STATES
Coma: Near-complete unresponsiveness
Coma is a condition of almost complete unresponsiveness in which the patient lies with eyes closed, very limited reflexes, no cyclical wakefulness, and, above all, no signs of awareness. Coma is normally attained after an acute brain insult and may last about 2 weeks, although chronic coma cases have been described, and is usually caused by either temporary or permanent damage to the reticular system.
Vegetative state: Wakefulness without awareness
Following a coma, some patients may enter a vegetative state, which involves a complete absence of consciousness of one’s environment but with preserved sleep-wake cycles and autonomic functions. The vegetative state is easily differentiated from brain death, in which the electroencephalogram shows no brain wave or activity.4 Brain death is the irreversible end of all brain activity and should not be confused with a persistent vegetative state.
The vegetative state is a condition of wakefulness without awareness in which the patient exhibits a partially preserved sleep-wake cycle and a variable portfolio of reflexes and spontaneous nonvolitional behaviors. A patient who has been in a vegetative state for more than 1 month with no improvement is often said to be in a persistent vegetative state. The term permanent vegetative state, implying no chance of recovery, is sometimes used when the vegetative state persists for 3 months after a nontraumatic insult, such as cardiac arrest, or for 1 year after a traumatic brain injury.
Minimally conscious state: Conscious awareness is evident despite impairment
Some patients in a vegetative state may start to recover by entering a minimally conscious state, in which conscious awareness is evident despite profound physical and cognitive impairment. Although communication capabilities are absent, cognitively mediated (or voluntary) behavior occurs in the minimally conscious state, which may be inconsistent but is reproducible enough to be differentiated from reflexive behavior. For example, patients may occasionally be able to smile when asked to do so or follow an object with their eyes. In the minimally conscious state, patients show those basic behaviors seen in the vegetative state along with islands of presumably conscious processing such as inconsistent responses to simple commands and sustained visual pursuit.5 Patients in a minimally conscious state have a better prognosis than those in a persistent or permanent vegetative state.3
Locked-in syndrome: Not a true disorder of consciousness
Another pathology that is often confounded with vegetative or minimally conscious states is the locked-in syndrome, which is characterized by complete paralysis of voluntary muscles in all parts of the body except those controlling eye movements. Individuals with locked-in syndrome are conscious and can think and reason, but they are unable to speak or move. The disorder confines the patient to paralysis and a mute state. Communication may be possible with blinking eye movements.
WHAT CAUSES DISORDERS OF CONSCIOUSNESS?
Disorders of consciousness mostly stem from acute brain insults, which may be caused by hypoxicischemic neural injury or traumatic brain injury. Although traumatic brain injury is currently the most common cause of vegetative and minimally conscious states, nontraumatic causes are becoming more frequent as a result of scientific and technological developments in resuscitation. Nontraumatic causes of disorders of consciousness include stroke, cardiopulmonary arrest, and meningoencephalitis; additionally, patients in the final stage of certain neurodegenerative diseases, including Parkinson, Alzheimer, and Huntington diseases, may lapse into a minimally conscious or vegetative state.6
NEUROLOGIC FINDINGS IN COMATOSE SURVIVORS OF CARDIAC ARREST
Structural magnetic resonance imaging (MRI) of patients in a vegetative state following cardiac arrest often reveals abnormalities. Most frequently there is a white matter signal in the cerebellum, the thalamus, the frontal and parietal cortices, and the hippocampus. Widespread abnormalities may indicate little to no prospect for recovery. Pupillary light response, corneal reflexes, motor responses to pain, myoclonus status epilepticus, serum neuron-specific enolase, and somatosensory evoked potential studies can assist in predicting efficiently and accurately a poor outcome in comatose patients after cardiopulmonary resuscitation for cardiac arrest.7
DEFINITION PROBLEMS AND MISDIAGNOSIS
The diagnosis of vegetative state emerges from a negative finding—namely, the lack of behaviors that would signal conscious capabilities. Using the nonoccurrence of events as a criterion to establish a fact is inherently problematic, since the causes of a nonoccurrence are theoretically infinite. More specifically, the reasons behind the lack of evidence of voluntary movement in presumably unconscious patients can be classified in terms of malfunctioning of either sensoriperceptual, output/motor, or central processing.
Deficits in sensoriperceptual processing
A patient might have deafness that may lead to a deficit in speech comprehension, or perhaps the auditory pathway and first cortical pathways are spared but the patient is aphasic and cannot process additive events such as speech. In a cohort of 42 patients, we found 17 who lacked the fourth or fifth components of the brain auditory evoked potentials to clicks presented binaurally, signaling severe damage to the auditory pathway.8 It is useless to ask such patients to follow commands, since the sensory input is damaged and the movement (or lack of movement) has no validity for the diagnosis. A similar argument can apply for patients who may show some fixation but exhibit delayed or absent visual evoked potentials when presented with written commands.
Deficits in motor processing
The second type of lesions that may contribute to misdiagnosis in these patients are those found in the effector systems. If the motor voluntary pathways are damaged—either in the motor cortex or in the corticospinal or corticobulbar pathways—then movement might be impaired enough to prevent responses by the patient. Patients of this type are sometimes diagnosed as being in a vegetative state although they might actually have locked-in syndrome,9 with preserved cognition but an inability to initiate voluntary responses as a result of a lesion in the pontine peduncle.
Although the effector systems are difficult to test in unresponsive subjects, some strategies may be tried. Before testing for volition, it is necessary to assess all possible hand, leg, and face reflexes in order to map reflexive behavior. Commands should then specifically target those muscles that showed total or partial preservation of reflexes. To test the output pathways from the cortex to the medulla, a more specialized assessment is needed; the Impaired Consciousness Research Group at the University of Cambridge has developed a simple protocol to assess the ability of the motor cortex to elicit muscle twitches by measuring the motor evoked potentials to simple pulses of transcranial magnetic stimulation. The minimal pulse intensity is determined by electromyographic recordings when transcranial magnetic stimulation pulses are applied to the left or right motor cortices for the hands and feet. The results have shown 2 out of 34 patients to have no detectable motor evoked potentials and 5 patients to have severe delay at maximum pulse intensity [unpublished data]. These results confirm the need for a full neurologic and neurophysiologic assessment in subjects who are unresponsive or show low levels of response, both acutely and more chronically, to minimize the risk of misdiagnosis.
Deficits in central processing
The key element in the assessment of cognitive processing in patients in a vegetative or minimally conscious state is determining deficits in their capacity to process external stimuli in a conscious manner (central processing). This is by far the most difficult characteristic to be determined since the only accepted criteria for awareness are verbal report or voluntary movement, both of which are absent in the vegetative state and are inconsistent and difficult to determine behaviorally in the minimally conscious state.
CLUES TO BRAIN FUNCTION IN DISORDERS OF CONSCIOUSNESS
It is important to differentiate a patient in a persistent vegetative state from a patient in a minimally conscious state, as the latter patient has a much higher chance of a favorable outcome. Evaluation of cerebral metabolism and imaging studies can both provide clues to brain function.
Cerebral metabolism
Neuroimaging studies
In the past few years, studies have found that some patients in a vegetative or minimally conscious state can activate cortical networks in response to auditory, visual, and tactile stimuli.10 A challenge in neuroscience is to devise a reliable, objective test to assess awareness without relying on explicit voluntary movements or verbal responses. Such a test would have important theoretical and practical implications. Recent evidence from functional neuroimaging and neurophysiology suggests that some patients with disorders of consciousness exhibit partially preserved conscious processing despite having no clinical or verbal output.11
During a positron emission tomography study, Menon et al showed photographs to a 26-year-old woman who was in a vegetative state 4 months after becoming comatose from an acute febrile illness.12 They found significant activation in the right fusiform gyrus and extrastriate visual association areas when the woman was shown photos of people familiar to her as compared with repixellated versions of the same photos with the faces made unrecognizable. The activation pattern she exhibited was similar to that of healthy volunteers. Interestingly, a few months after this study, the patient became increasingly responsive.
Our group conducted the first evaluation of emotion in the minimally conscious state using functional MRI (fMRI) in a 17-year-old male following a traumatic brain injury.13 The patient was able to localize noxious stimuli, exhibited spontaneous eye opening, and occasionally smiled appropriately and followed people with his eyes. Imaging was performed while he listened to two recordings—one of his mother reading a story about his life, and one of a matched control voice reading the same story. Digital subtraction imaging disclosed strong activation of two areas related to emotion, the amygdala and the bilateral insula, while the recording of the patient’s mother was played. Activation was also evident in the auditory cortex in the superior temporal lobe. The patient recovered 6 months following this study.
Classical conditioning
Classical conditioning represents an alternate approach to MRI for assessing brain function in patients with disordered consciousness.8 Trace conditioning of the eye-blink response is considered to be an objective test of awareness.14 This test involves highly specific learning, requiring an anticipatory electromyographic response to a paired stimulus (eg, a tone followed by an aversive stimulus such as an air puff to the eyes) but not to an unpaired stimulus (eg, a white noise that is not followed by an aversive stimulus). This effect increases in amplitude as the aversive stimulus approaches. Our laboratory is applying this method to study learning and memory in patients with disordered consciousness.
DETERMINING AWARENESS WITHOUT REPORT
The proposed neural correlates of consciousness do not usually take into account the levels of consciousness.15,16 In order to build the framework for a cognitive neuroscience of consciousness, we must consider the content of the consciousness experience in fully awake subjects and patients as well as the cognitive processes occurring in unconscious and conscious subjects.
Two main approaches can be used to assess conscious processing in unresponsive patients. The first is to look for neural correlates in direct intentional actions or imagined actions,11 and the second is to look for physiologic correlates of the cognitive processes required during the conscious processing of stimuli.17
Searching for neural correlates of intended actions
The first approach can have enormous impact in the diagnostic arena (as well as in the legal and ethical arenas), such as in the case reported by Owen et al in which a patient showed brain activity related to imagining actions as prompted by spoken instructions during fMRI evaluation.11 Unfortunately, cases such as these are scarce. Moreover, imagining of actions relies not only on a spared comprehension capacity and preserved memory but also on the subject’s willingness to perform the task. It would seem that only a minority of patients in a vegetative state seem to have the cognitive abilities preserved to accomplish these types of tasks.
Searching for physiologic correlates of cognitive processes
The second approach would tend to work with memory and switching attention capabilities in unresponsive patients, assuming that conscious processing does not exist without these cognitive processes. The evidence for this approach comes from electrophysiology. Cognitive evoked potentials are commonly applied to assess basic auditory or visual cortical processing, automatic attention, and focus attention.18 Both the mismatch negativity wave (a correlate of automatic attention) and the p300 (a correlate of focus attention) are sometimes present,19 specifically in patients in vegetative or minimally conscious states, and they are a good predictor of awakening in stroke, hemorrhage, and traumatic brain injury.20
In day-to-day practice in a neurology clinic or emergency room, it is more feasible to assess cognitive capabilities using event-related potentials than fMRI since they are more widely distributed, more easily validated, shorter, and statistically more powerful in single-subject analysis,21 and because they do not frequently rely on speech comprehension.
NEUROPATHOLOGY AND fMRI
The cause of the brain injury leading to a vegetative or minimally conscious state frequently determines the neuropathology.22 It has also been demonstrated that severely disabled patients (such as those emerging from a minimally conscious state) differ from vegetative state patients in terms of lesions and severity.23
Although residual activity as seen on functional neuroimaging may be unequivocal in some cases, it may represent only fragmentary cognitive processing; it is important not to assume that normal awareness is present. Much still needs to be learned, but results from neuroimaging studies demonstrate that a small proportion of patients in a vegetative or minimally conscious state have some preserved cognitive processes. These findings have ethical and legal implications. For instance, careless bedside chatter among family members or medical personnel is inappropriate and should be avoided. Whether functional neuroimaging can effectively evaluate neuroprocessing in patients in whom cognitive output is difficult to assess remains to be determined. Such evaluation may one day help to predict prognosis. It may also someday help to facilitate communication with patients with locked-in syndrome, who are cognitively intact but are without verbal or motor output.
CONCLUSIONS
It is highly improbable to find patients with preserved cortical connectivity, since structural22 and functional19 studies have demonstrated only a small proportion of patients in a vegetative or minimally conscious state who have relatively preserved brains and cognitive processing. The more we study patients who are unresponsive or show low levels of response, the more complex cognitive processes we find in subpopulations of these patients. Language-related cortical activation is now the most common finding.13,19,24 More recently, a few researchers working with severely damaged patients have started to test paradigms with the aim of uncovering conscious processes that have no need of verbal or movement responses.
The time has come for clinicians in acute care centers to immediately follow their administration of coma scales in unresponsive patients with the use of more sophisticated methodology to assess not only reflexive and intentional behaviors but also these patients’ physiologic and cognitive characteristics. In the field of neurodegenerative disease, it took several years for clinicians to start using more sensitive cognitive tools than just the mini-mental state examination and computed tomography or three-dimensional T1-weighted structural MRI, but nowadays volumetric MRI and detailed cognitive assessments are widely used to diagnose and characterize patients with neurodegenerative disorders. The same path should be taken for patients with severe brain damage. The information yielded by such an approach may one day help to determine a diagnosis or prognosis, guide treatment, or facilitate communication in patients with pathologies of consciousness.
- Jennett B, Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet 1972; 1:734–737.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state.first of two parts. N Engl J Med 1994; 330:1499–1508.
- Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002; 58:349–353.
- Wijdicks EFM. The diagnosis of brain death. N Engl J Med 2000; 344:1215–1221.
- Giacino JT, Trott CT. Rehabilitative management of patients with disorders of consciousness: grand rounds. J Head Trauma Rehabil 2004; 19:254–265.
- Bernat JL. Chronic disorders of consciouness. Lancet 2006; 367: 1181–1192.
- Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 67:203–210.
- Bekinschtein TA. Cognitive Processes in the Vegetative and Minimally Conscious State [thesis]. Buenos Aires, Argentina: University of Buenos Aires; 2006.
- Onofrj M, Thomas A, Paci C, Scesi M, Tombari R. Event related potentials recorded in patients with locked-in syndrome. J Neurol Neurosurg Psychiatry 1997; 63:759–764.
- Schiff ND. Multimodal neuroimaging approaches to disorders of consciousness. J Head Trauma Rehabil 2006; 21:388–397.
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science 2006; 313:1402.
- Menon DK, Owen AM, Williams EJ, et al. Cortical processing in persistent vegetative state. Lancet 1998; 352:200.
- Bekinschtein T, Niklison J, Sigman L, et al. Emotion processing in the minimally conscious state [letter]. J Neurol Neurosurg Psychiatry 2004; 75:788.
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science 1998; 280:77–81.
- Koch C. The Quest for Consciousness: A Neurobiological Approach. Greenwood Village, CO: Roberts & Company Publishers; 2004.
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 2001; 79:1–37.
- Naccache L. Is she conscious? Science 2006; 313:1395–1396.
- Picton TW, Bentin S, Berg P, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology 2000; 37:127–152.
- Kotchoubey B, Lang S, Mezger G, et al. Information processing in severe disorders of consciousness: vegetative state and minimally conscious state. Clin Neurophysiol 2005; 116:2441–2453.
- Daltrozzo J, Wioland N, Mutschler V, Kotchoubey B. Predicting coma and other low responsive patients outcome using event-related brain potentials: a meta-analysis. Clin Neurophysiol 2007; 118:606–614.
- Quian Quiroga R, Garcia H. Single-trial event-related potentials with wavelet denoising. Clin Neurophysiol 2003; 114:376–390.
- Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain 2000; 123:1327–1338.
- Jennett B. Thirty years of the vegetative state: clinical, ethical and legal problems. Prog Brain Res 2005; 150:537–543.
- Schiff ND, Rodriguez-Moreno D, Kamal A, et al. fMRI reveals large-scale network activation in minimally conscious patients. Neurology 2005; 64:514–523.
- Jennett B, Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet 1972; 1:734–737.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state.first of two parts. N Engl J Med 1994; 330:1499–1508.
- Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002; 58:349–353.
- Wijdicks EFM. The diagnosis of brain death. N Engl J Med 2000; 344:1215–1221.
- Giacino JT, Trott CT. Rehabilitative management of patients with disorders of consciousness: grand rounds. J Head Trauma Rehabil 2004; 19:254–265.
- Bernat JL. Chronic disorders of consciouness. Lancet 2006; 367: 1181–1192.
- Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 67:203–210.
- Bekinschtein TA. Cognitive Processes in the Vegetative and Minimally Conscious State [thesis]. Buenos Aires, Argentina: University of Buenos Aires; 2006.
- Onofrj M, Thomas A, Paci C, Scesi M, Tombari R. Event related potentials recorded in patients with locked-in syndrome. J Neurol Neurosurg Psychiatry 1997; 63:759–764.
- Schiff ND. Multimodal neuroimaging approaches to disorders of consciousness. J Head Trauma Rehabil 2006; 21:388–397.
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science 2006; 313:1402.
- Menon DK, Owen AM, Williams EJ, et al. Cortical processing in persistent vegetative state. Lancet 1998; 352:200.
- Bekinschtein T, Niklison J, Sigman L, et al. Emotion processing in the minimally conscious state [letter]. J Neurol Neurosurg Psychiatry 2004; 75:788.
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science 1998; 280:77–81.
- Koch C. The Quest for Consciousness: A Neurobiological Approach. Greenwood Village, CO: Roberts & Company Publishers; 2004.
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 2001; 79:1–37.
- Naccache L. Is she conscious? Science 2006; 313:1395–1396.
- Picton TW, Bentin S, Berg P, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology 2000; 37:127–152.
- Kotchoubey B, Lang S, Mezger G, et al. Information processing in severe disorders of consciousness: vegetative state and minimally conscious state. Clin Neurophysiol 2005; 116:2441–2453.
- Daltrozzo J, Wioland N, Mutschler V, Kotchoubey B. Predicting coma and other low responsive patients outcome using event-related brain potentials: a meta-analysis. Clin Neurophysiol 2007; 118:606–614.
- Quian Quiroga R, Garcia H. Single-trial event-related potentials with wavelet denoising. Clin Neurophysiol 2003; 114:376–390.
- Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain 2000; 123:1327–1338.
- Jennett B. Thirty years of the vegetative state: clinical, ethical and legal problems. Prog Brain Res 2005; 150:537–543.
- Schiff ND, Rodriguez-Moreno D, Kamal A, et al. fMRI reveals large-scale network activation in minimally conscious patients. Neurology 2005; 64:514–523.