User login
‘High’-pertension
Bridging the Evidence/Practice Gap
Optimizing quality of care in patients with acute decompensated heart failure (ADHF) is crucial, given both the frequency and cost of hospitalization for this disorder. Several quality improvement strategies have been identified, including provider education; provider reminder systems and decision support; audit and feedback; patient education; organizational change; and financial incentives, regulation, and policy.1
To assist hospitalists in implementing these strategies, this article briefly reviews evidence‐based guidelines for the treatment of ADHF, presents a practical algorithm for patient assessment and treatment derived from these guidelines and personal experience, and discusses systems to enhance the ultimate transition of patient care from the inpatient to outpatient setting.
EVIDENCE‐BASED GUIDELINES
Evidence‐based guidelines are created in an attempt to promote optimal management of a condition or disorder based on expert analysis of all available relevant scientific data. Current guidelines for the assessment and treatment of ADHF have been developed by a national group purchasing organization,2 the European Society of Cardiology,3 the Heart Failure Society of America,4 and the American College of Emergency Physicians.5 Relevant components of these guidelines will be discussed in the patient assessment and treatment section below.
Publication of guidelines, in and of itself, however, is inadequate to ensure their acceptance and use.1 Data from the American Heart Association (AHA)/American Stroke Association (ASA) Get With The GuidelinesHeart Failure (GWTG‐HF) program continue to demonstrate a substantial gap between guideline recommendations and current care of patients with ADHF.6, 7 One way to promote systemwide adherence with published guidelines is to directly involve healthcare professionals in the implementation process. Consequently, development of local, hospital‐based procedures derived from national or international guidelines may be more effective than the simple dissemination of the guidelines themselves.1 Hospitalists have a unique insight into both patient care and the hospital setting and are frequently involved in evaluating hospital policies and procedures and implementing clinical pathways and guidelines.8 In addition, hospitalist care has been associated with greater compliance with disease‐specific guidelines compared to nonhospitalist care.9 As a result, hospitalists are uniquely suited to play a key role in the development of these procedures.
PATIENT ASSESSMENT AND TREATMENT
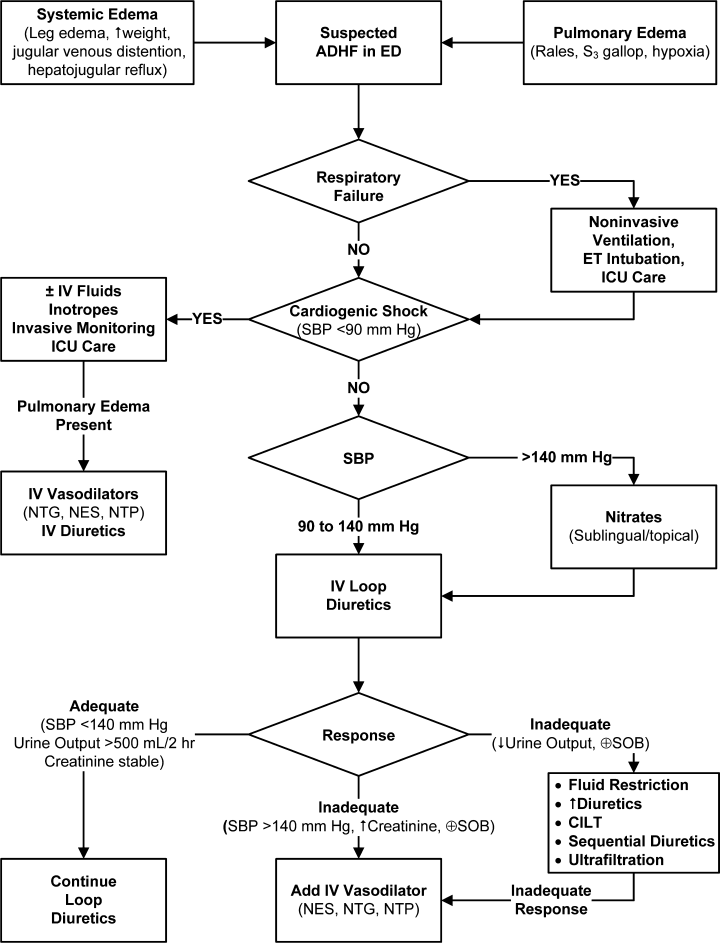
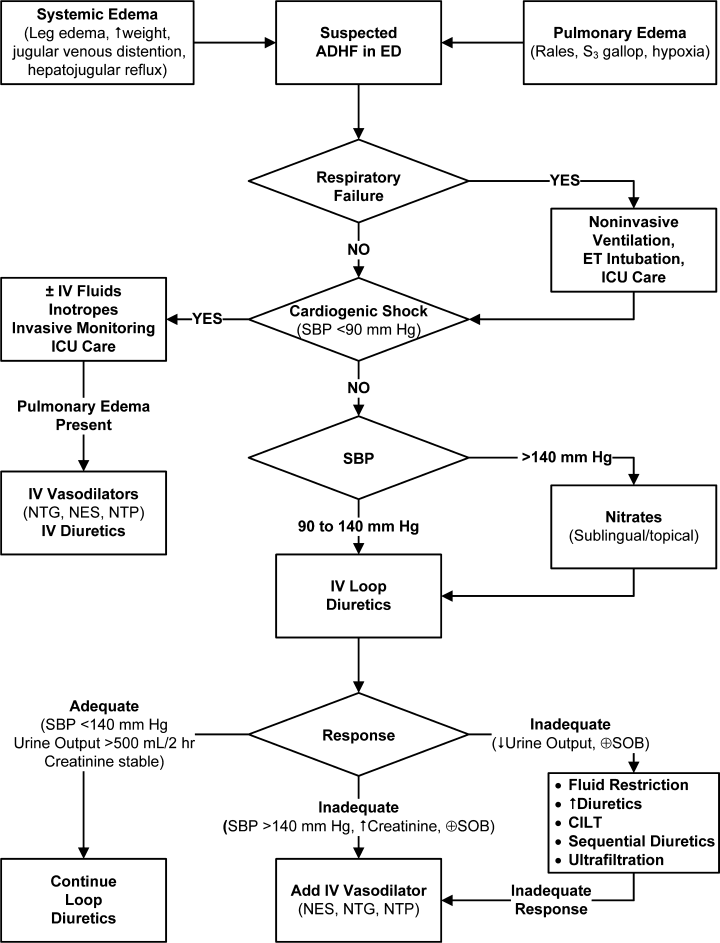
The differential diagnosis of any individual presenting to the emergency department (ED) with signs of systemic or pulmonary edema should include ADHF (Figure 1).25 These individuals require a rapid initial assessment to (1) establish the diagnosis, (2) determine the best location for subsequent treatment, and (3) institute the most appropriate initial therapy.
Treatment Location
Effective and efficient management of ADHF requires determining proper treatment location. Inpatient management of ADHF is expensive, accounting for approximately 60% of the $31.7 billion spent annually on heart failure care in the United States.10 Clearly, patients with impending respiratory failure requiring ventilation assistance and patients with cardiogenic shock requiring inotropic agents and invasive monitoring are best cared for in an intensive care unit (ICU) setting. However, these patients constitute the minority of patients with ADHF. For example, systolic blood pressure (SBP) 90 mm Hg was present in only 2.3% of patients in the Acute Decompensated Heart Failure National Registry (ADHERE), a registry designed to study characteristics, management, and outcomes in a broad sample of patients hospitalized with ADHF.11
Most patients with ADHF present with congestion, not respiratory failure or cardiogenic shock,11, 12 and a select subgroup of these patients will respond to treatment within 1224 hours.13 Although this may be an inordinate amount of time to keep patients in an ED, it is not long enough to generally require full hospital admission. Instead, these patients can be effectively managed in an observation unit (OU).14 The goal of these units is to provide the required level of care over a 12‐ to 24‐hour period while simultaneously reducing costs by eliminating the need for hospital admission. Selecting patients who will respond to therapy during this time frame is a critical component in instituting effective OU management of ADHF. Key entry and exclusion criteria are listed in Table 1.14 In patients who meet these criteria, management in an OU has been shown to yield outcomes comparable to inpatient care, but at a lower cost.1416
| Entry criteria |
|---|
|
| History (at least one of the following) |
| Dyspnea on exertion |
| Paroxysmal nocturnal dyspnea |
| Shortness of breath |
| Edema of legs or abdomen |
| Weight gain |
| Physical examination (at least one of the following) |
| Jugular venous distention or elevation in pulsation |
| Positive abdominal jugular reflux |
| S3/S4 gallop |
| Inspiratory rales |
| Peripheral edema |
| Chest x‐ray (at least one of the following) |
| Cardiomegaly |
| Pulmonary vascular congestion |
| Kerley B lines |
| Pulmonary edema |
| Pleural effusion |
| Exclusion criteria |
| Unstable vital signs (BP >220/120 mm Hg, respiratory rate >25 breath/min, heart rate >130 beats/min) |
| Temperature >38.5C |
| Unstable airway or need for >4 L/min supplemental O2 to keep O2 saturation >90% |
| Peak flow 50% of predicted with wheezing |
| Clinically significant arrhythmia or sustained ventricular tachycardia |
| Any ECG with diagnostic criteria for AMI or ischemia |
| Chest x‐ray with pulmonary infiltrates |
| Any CK‐MB >8.8 ng/mL |
| Any troponin T >0.1 g/L (>0.5 g/L if creatinine >2.0 mg/dL) |
| Requirement for continuous vasoactive medication to stabilize hemodynamics |
| Complex decompensation: concomitant end‐organ hypoperfusion, volume overload, and systemic vasoconstriction |
| Requirement for care guided by pulmonary artery catheter |
| Severe electrolyte imbalance |
| Chronic renal failure requiring dialysis |
| Acute mental status abnormality |
Early Initiation of Therapy
Early institution of effective therapy has been shown to improve outcomes. Consequently, selection of initial therapy should occur concurrently with determination of proper treatment location. In the Prospective Randomized Outcomes Study of Acutely Decompensated Congestive Heart Failure Treated Initially as Outpatients with Nesiritide (PROACTION) trial, initiation of nesiritide in the ED/OU was associated with an 11% reduction in hospital admissions at the index visit (P = .436), a 57% reduction in hospitalizations within 30 days after discharge from the index hospitalization (P = .058), and a 62% reduction in median duration of rehospitalization (P = .032).17 The incidence of symptomatic hypotension was low and did not differ between the groups.17 Likewise, in separate analyses of data from ADHERE, ED initiation of intravenous (IV) vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine significantly reduced the risk of requiring transfer to an ICU and reduced ICU length of stay and total hospital length of stay compared with inpatient initiation of these same therapies.18, 19
Treatment Algorithm
Treatment of ADHF should proceed along a logical care pathway governed by both clinical status and response to prior therapies (Figure 1). One must first consider whether there is evidence of respiratory failure or impending respiratory failure.20 If so, patients should receive immediate ventilatory support via continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), or endotracheal intubation, depending on the degree of respiratory impairment.3, 5 In prospective controlled evaluations, patients with acute respiratory failure secondary to pulmonary edema who were randomized to treatment with CPAP demonstrated significant improvement in cardiopulmonary indices21, 22 and significant reductions in need for endotracheal intubation21 and short‐term mortality22 when compared with similar patients who received standard therapy without CPAP.
Once potential respiratory issues have been addressed, the next items for consideration are circulation and perfusion. Patients with low cardiac output and hypotension (cardiogenic shock) are at risk for developing critical end‐organ dysfunction. In these patients, insertion of a pulmonary artery catheter may aid in assessment of hemodynamic status and response to therapy.4 Patients with low cardiac output and low filling pressures should receive IV fluid loading.3 In contrast, for patients with low cardiac output and high filling pressures, inotropic agents should be considered.3, 4 Also, these patients may require IV vasodilators and/or IV diuretics to treat pulmonary edema once blood pressure (BP) and cardiac output have been stabilized.2, 3, 20
For patients with ADHF who present with symptoms of congestion, but not respiratory failure or cardiogenic shock, the initial therapeutic decision is governed by their BP. Approximately 50% of patients with ADHF will have an SBP > 140 mm Hg.12, 23 These patients tend to be older and to have diastolic rather than systolic dysfunction.12, 20, 23 Symptoms typically have been present for only a short period of time (2448 hours) and are more often due to maldistribution of fluid producing pulmonary edema than total body fluid overload. Consequently, initial treatment should focus on aggressive BP control to relieve this edema. Sublingual or topical nitrates are recommended as a first step, and initial diuretic use should be minimal to avoid intravascular volume depletion leading to renal dysfunction.20 In contrast, patients presenting with SBP between 90 mm Hg and 140 mm Hg are more likely to have some degree of systolic dysfunction, leading to a gradual worsening of their heart failure symptoms and total body fluid overload over a period of weeks.20 These patients require aggressive diuresis. Although the efficacy of IV loop diuretics has not been established in randomized, controlled clinical trials, observational experience demonstrates that they can effectively reduce filling pressures, relieve volume overload, and decrease symptoms of congestion.4 They are currently the mainstay of therapy for ADHF secondary to fluid retention, and their use is recommended in all 4 guidelines.25
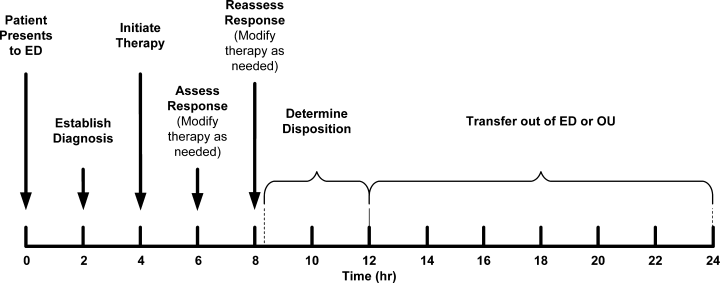
This initial therapeutic choice, however, is only the starting point, and it is important not to stop at this stage. No single definitive therapy for ADHF exists, and not all patients will respond to initial treatment. Optimal management requires early recognition and addressing of both an inadequate response to therapy and any adverse affects induced by this therapy. Frequent reevaluations are an essential component of treating patients with ADHF. For example, the timeline in one of the guidelines calls for assessing the patient's response at 2 and 4 hours after initiation of IV therapy and adjusting treatment as indicated based on these assessments (Figure 2).2
If the patient has an adequate response to initial therapy, defined as SBP 140 mm Hg, stable renal function, and urine output >500 mL over 2 hours (>250 mL if serum creatinine >2.5 mg/dL), this therapy can continue unchanged, and focus shifts to long‐term management issues.2, 4, 14 However, if the response is inadequate, it is important to identify and treat the cause of this inadequate response.
Inadequate urine output secondary to diuretic resistance is common in patients with ADHF, especially in those on long‐term diuretic therapy.3 Despite a 90% prevalence of IV diuretic use in ADHERE, 70% of patients either gained weight or lost fewer than 5 pounds during hospitalization, and 42% were discharged with unresolved symptoms.24 Clearly, diuretic therapy did not produce the desired effect in many of these patients. This inadequate response to loop diuretics is a direct result of their pharmacologic properties, especially as they relate to patients with heart failure. The physiologic effects of loop diuretics are directly related to their concentration in the lumen of the nephron. This concentration depends on both the patient's renal function and the dose and half‐life of the administered diuretic.25 Comorbid renal dysfunction is common in patients with ADHF.26 In addition, even in the absence of this dysfunction, the short half‐life of loop diuretics limits the amount of time that their luminal concentration is in the effective range, and rebound sodium retention can occur whenever the diuretic concentration is below this range.25 Furthermore, the dose‐response curve of loop diuretics is S‐shaped. As a result, a threshold concentration exists beyond which no further augmentation in urine output occurs; ie, there is a maximum physiologic response that is reduced in patients with heart failure.25 Guideline recommendations for patients with diuretic resistance attempt to address these physiologic and pharmacologic limitations. These recommendations include fluid restriction to decrease the overall volume of diuresis necessary, increasing diuretic dose or instituting continuous infusion loop diuretic therapy to increase the amount of time during which the luminal concentration is within the effective range, sequential diuretic blockade to take advantage of the different mechanisms of action of the various diuretic classes to affect different components of the nephron, bypassing the kidney through the use of ultrafiltration, and when these are inadequate, adding a vasodilator in an attempt to augment cardiac output and renal perfusion.3, 4, 24, 25, 2729
Addition of an IV vasodilator is the primary means of addressing an inadequate response typified by hypertension, worsening renal function, and/or persistent symptoms, rather than diuretic resistance. Approximately 25% of patients with ADHF receive IV vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine, although predominantly vasodilators, at some point during their hospitalization.11, 12 These agents improve hemodynamics and reduce symptoms of ADHF.5, 3032 Their use, in combination with low‐dose diuretics, has been shown to be more efficacious than high‐dose diuretics alone.3, 27 Adding a vasodilator may reduce adverse, diuretic‐induced, neurohormonal activation. In an animal model, combining nesiritide with IV furosemide significantly attenuated the rise in plasma aldosterone produced by IV furosemide alone,33 and this finding has been subsequently confirmed in patients with heart failure.34 Finally, vasodilators have proven to be a safer alternative than inotropes in patients with ADHF. In an analysis of data from ADHERE, covariate‐adjusted and propensity‐adjusted mortality risk was >50% lower for patients receiving nitroglycerin or nesiritide compared with those receiving dobutamine,11 and in an analysis of data from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, which evaluated patients with advanced heart failure, the risk‐adjusted mortality hazard ratio (HR) was significantly increased for inotropes (HR: 2.14; 95% confidence interval [CI]: 1.104.15) but not for vasodilators in the absence of inotropes (HR: 1.39; 95% CI: 0.643.00).35
Performance Measures
In addition to instituting effective therapy for the acute decompensation, it is important to implement measures that may improve long‐term outcomes. The Joint Commission on Accreditation of Healthcare Organizations and the AHA/ASA have identified a series of 5 core performance measures that should be completed during hospitalization for AHDF: discharge instructions relevant to patient's education, documentation of left ventricular systolic function evaluation, prescription of angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at discharge in patients with left ventricular systolic dysfunction, adult smoking cessation advice/counseling, and prescription of ‐blocker at discharge (Table 2).36, 37 In an analysis of data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry, prescription of a ‐blocker at discharge significantly reduced the risk‐adjusted odds ratio (OR) for mortality (OR: 0.48; 95% CI: 0.300.79), and prescription of an ACE inhibitor or ARB at discharge significantly reduced the risk‐adjusted OR for rehospitalization or death (OR: 0.51; 95% CI: 0.340.78) at 6090 days.38 Although no correlation was detected between outcomes and the other 3 core performance measures in this evaluation, the 60‐day to 90‐day time frame may have been too short to identify the full effects of smoking cessation counseling and left ventricular function assessment. Failure to detect a beneficial effect of discharge instructions is disappointing, especially given the proven benefit of disease management programs (see below) and may reflect a limitation of this measure, as currently implemented, to determine the thoroughness and patient understanding of the instructions provided.38, 39
| Measure | Description | Source |
|---|---|---|
| HF‐1 | Discharge instructions relevant to patient education | JCAHO; AHA/ASA |
| HF‐2 | Documentation of left ventricular systolic function evaluation | JCAHO; AHA/ASA |
| HF‐3 | Prescription of ACE inhibitor or ARB at discharge in patients with left ventricular systolic dysfunction | JCAHO; AHA/ASA |
| HF‐4 | Adult smoking cessation advice/counseling | JCAHO; AHA/ASA |
| HF‐5 | Prescription of ‐blocker at discharge | AHA/ASA |
TRANSITION OF CARE
Lastly, optimal management of ADHF requires successful transition of care from an inpatient to an outpatient setting. Recidivism is both common and costly. Approximately 2% of patients with ADHF are readmitted within 2 days, 20% within 1 month, and 50% within 6 months of hospital discharge.40 Frequently, these readmissions are caused by nonadherence to the therapeutic regimen following discharge.41 In an evaluation of patients hospitalized for ADHF at a large urban medical center, noncompliance with prescribed diet and/or drugs was the most common precipitating factor for admission (64% of patients), followed by uncontrolled hypertension (44%), cardiac arrhythmia (29%), environmental factors (19%), and inadequate therapy (17%).42 Similarly, in a prospective evaluation of elderly patients hospitalized for ADHF, 53% of readmissions occurring within 90 days of discharge were deemed to be preventable, with the most common contributing factors being noncompliance with medications and/or diet (33%), inadequate discharge planning (15%), inadequate follow‐up (20%), insufficient support system (21%), and failure to seek medical attention promptly when symptoms recurred (20%).43 Consequently, patient education and arrangement for appropriate follow‐up are crucial components of successfully transitioning care to an outpatient setting.
Effective patient education is time‐consuming. Patients must be taught when, how, and why to take their medication. They need to understand their dietary guidelines and the reasons for these guidelines. They need to know how to use daily weigh‐ins as a means of monitoring their fluid status and what to do in response to a change in weight or symptoms. Finally, they need to be cognizant of what constitutes appropriate exercise and the need for this exercise.4447 To enhance understanding and retention, this information should be presented to the patient over the course of the hospitalization. Comprehension should be tested continually and education repeated until appropriate understanding is ensured. Patient education provided in a rushed or perfunctory manner at the moment of discharge is unlikely to be retained or effective.38, 39
Ideally, the patient should be referred to a comprehensive heart failure disease management program for postdischarge care. Numerous evaluations have established the effectiveness of these programs in enhancing use of appropriate medications, improving functional status, reducing readmissions and mortality, and decreasing costs.4454 For example, in separate evaluations, the prevalences of appropriate vasodilator use (93% vs. 61%; P .001),51 ‐blocker use (71% vs. 40%; P .001),50 and ACE inhibitor use (84% vs. 59%; P .001)52 were significantly greater for disease management program participants compared with nonparticipants. In addition, participation in a disease management program was associated with a 52% reduction in the risk of hospitalization for cardiovascular causes (P .001) and a 72% reduction in ED visits (P .01) in 1 evaluation,45 a 36% reduction (95% CI: 16.7%50.9%) in the risk of heart failure admission or death in another,53 and a 67% reduction (95% CI: 41%82%) in the adjusted risk of death in yet another evaluation.52 Unfortunately, recent data indicate that these programs must be ongoing to sustain these benefits. In a prospective evaluation, patients with heart failure were randomized to either standard care or a multidisciplinary disease management program for 6 months followed by standard care.55, 56 Significantly fewer patients in the disease management group required readmission to the hospital (HR: 0.55; 95% CI: 0.350.88) during the 6‐month period in which they actively participated in the disease management program.56 However, by the end of follow‐up (mean 2.8 years), there was no significant difference between treatment groups in all‐cause mortality (HR: 1.09; 95% CI: 0.691.72) or the composite endpoint of death, ED visit, or hospitalization (HR: 1.01; 95% CI: 0.751.37).55
CONCLUSION
A gap between evidence‐based guidelines and current management of patients with ADHF exists. Multiple strategies to bridge this gap in patient management can be employed. Patients with ADHF require rapid assessment to determine appropriate treatment location and initial therapy. Clinical status should guide treatment selection. Once effective acute therapy has been established, strategies to improve long‐term outcomes should be implemented. These strategies include ensuring that care complies with established core performance measures, providing patient education in a manner suited to ensure comprehension and retention, and arranging for appropriate outpatient follow‐up, ideally in a comprehensive heart failure disease management program. Increasing the awareness of the gap between evidence‐based guidelines and current management, as well as strategies to bridge this gap, is crucial to improving the outcomes of patients with ADHF.
- .Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process.J Gen Intern Med.2007;22(12):1762–1770.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- Heart Failure Society of America.Executive summary: HFSA 2006 comprehensive heart failure practice guideline.J Card Fail.2006;12(1):10–38.
- ,,,,,American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,, et al.Influence of the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program on emerging performance measures for patients hospitalized with heart failure.Circulation.2006;114:II–572. Abstract 2740.
- ,,, et al.Quality of care for heart failure patients with concomitant kidney disease in the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program.Circulation.2006;114:II–859. Abstract 3993.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,.Preliminary experience with an emergency department observation unit protocol for heart failure.Acad Emerg Med.2000;7(10):1171. Abstract 33.
- ,,,,,.Effective observation unit treatment of decompensated heart failure.Congest Heart Fail.2002;8(2):68–73.
- ,.Patient outcome and costs following an acute heart failure (HF) management program in an emergency department (ED) observation unit (OU).J Heart Lung Transplant.1999;18(1):92. Abstract 240.
- ,,,,,.Inpatient versus emergency department observation unit management of heart failure.Ann Emerg Med.1998;32(3 part 2):S46–S47. Abstract 180.
- ,,, et al.Observation unit treatment of heart failure with nesiritide: results from the proaction trial.J Emerg Med.2005;29(3):243–252.
- ,,,.Early initiation of IV vasoactive therapy improves heart failure outcomes: an analysis from the ADHERE™ registry database.Ann Emerg Med.2003;42(4 suppl):S26. Abstract 92.
- ,,, et al.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- ,,,,,.Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department.Ann Emerg Med.2008;51(1):45–57.
- ,,,,,.Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema. Short‐term results and long‐term follow‐up.Chest.1995;107(5):1379–1386.
- ,,, et al.Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients.Intensive Care Med.2004;30(5):882–888.
- ,,,,.Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database.J Am Coll Cardiol.2006;47(1):76–84.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- .Diuretic therapy in congestive heart failure.Congest Heart Fail.2000;6(4):197–201.
- ,,, et al.High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database.J Card Fail.2007;13(6):422–430.
- ,,, et al.Randomised trial of high‐dose isosorbide dinitrate plus low‐dose furosemide versus high‐dose furosemide plus low‐dose isosorbide dinitrate in severe pulmonary oedema.Lancet.1998;351(9100):389–393.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- .Management of diuretic‐refractory, volume‐overloaded patients with acutely decompensated heart failure.Curr Cardiol Rep.2005;7(3):204–210.
- ,,, et al.Sustained hemodynamic effects of an infusion of nesiritide (human b‐type natriuretic peptide) in heart failure: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.1999;34(1):155–162.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,, et al.Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide‐induced aldosterone activation in experimental heart failure.Circulation.2004;109(13):1680–1685.
- ,,.Nesiritide appears to inhibit the rise in plasma aldosterone associated with furosemide diuresis.J Card Fail.2006;12(6 suppl 1):S85–S86. Abstract 275.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- Joint Commission on Accreditation of Healthcare Organizations. Specifications Manual for National Implementation of Hospital Quality Measures. Version 2.3b. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm. Accessed October 10,2008.
- American Heart Association/American Stroke Association. Get with the guidelines–heart failure. Fact sheet. http://www.americanheart.org/downloadable/heart/1163802072170HFFactSheet.pdf. Accessed October 10,2008.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,, et al.Evaluating quality of care for patients with heart failure.Circulation.2000;101(12):e122–e140.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,,.Precipitating factors leading to decompensation of heart failure. Traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Prospective evaluation of an outpatient heart failure management program.J Card Fail.2001;7(1):64–74.
- ,,, et al.Improved outcomes from a comprehensive management system for heart failure.Eur J Heart Fail.2001;3(5):619–625.
- ,,, et al.The benefit of implementing a heart failure disease management program.Arch Intern Med.2001;161(18):2223–2228.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141(2):254–258.
- ,,, et al.Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day‐hospital and usual care.J Am Coll Cardiol.2002;40(7):1259–1266.
- ,,, et al.A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission.J Am Coll Cardiol.2002;39(3):471–480.
- ,,, et al.Mortality benefit of a comprehensive heart failure disease management program in indigent patients.Am Heart J.2006;151(2):478–483.
- ,,, et al.Two‐year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure.J Cardiovasc Med (Hagerstown).2007;8(5):324–329.
- ,,,,.Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort.Prog Cardiovasc Nurs.2007;22(4):196–200.
- ,,, et al.Lack of long‐term benefits of a 6‐month heart failure disease management program.J Card Fail.2007;13(4):287–293.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
Optimizing quality of care in patients with acute decompensated heart failure (ADHF) is crucial, given both the frequency and cost of hospitalization for this disorder. Several quality improvement strategies have been identified, including provider education; provider reminder systems and decision support; audit and feedback; patient education; organizational change; and financial incentives, regulation, and policy.1
To assist hospitalists in implementing these strategies, this article briefly reviews evidence‐based guidelines for the treatment of ADHF, presents a practical algorithm for patient assessment and treatment derived from these guidelines and personal experience, and discusses systems to enhance the ultimate transition of patient care from the inpatient to outpatient setting.
EVIDENCE‐BASED GUIDELINES
Evidence‐based guidelines are created in an attempt to promote optimal management of a condition or disorder based on expert analysis of all available relevant scientific data. Current guidelines for the assessment and treatment of ADHF have been developed by a national group purchasing organization,2 the European Society of Cardiology,3 the Heart Failure Society of America,4 and the American College of Emergency Physicians.5 Relevant components of these guidelines will be discussed in the patient assessment and treatment section below.
Publication of guidelines, in and of itself, however, is inadequate to ensure their acceptance and use.1 Data from the American Heart Association (AHA)/American Stroke Association (ASA) Get With The GuidelinesHeart Failure (GWTG‐HF) program continue to demonstrate a substantial gap between guideline recommendations and current care of patients with ADHF.6, 7 One way to promote systemwide adherence with published guidelines is to directly involve healthcare professionals in the implementation process. Consequently, development of local, hospital‐based procedures derived from national or international guidelines may be more effective than the simple dissemination of the guidelines themselves.1 Hospitalists have a unique insight into both patient care and the hospital setting and are frequently involved in evaluating hospital policies and procedures and implementing clinical pathways and guidelines.8 In addition, hospitalist care has been associated with greater compliance with disease‐specific guidelines compared to nonhospitalist care.9 As a result, hospitalists are uniquely suited to play a key role in the development of these procedures.
PATIENT ASSESSMENT AND TREATMENT
The differential diagnosis of any individual presenting to the emergency department (ED) with signs of systemic or pulmonary edema should include ADHF (Figure 1).25 These individuals require a rapid initial assessment to (1) establish the diagnosis, (2) determine the best location for subsequent treatment, and (3) institute the most appropriate initial therapy.

Treatment Location
Effective and efficient management of ADHF requires determining proper treatment location. Inpatient management of ADHF is expensive, accounting for approximately 60% of the $31.7 billion spent annually on heart failure care in the United States.10 Clearly, patients with impending respiratory failure requiring ventilation assistance and patients with cardiogenic shock requiring inotropic agents and invasive monitoring are best cared for in an intensive care unit (ICU) setting. However, these patients constitute the minority of patients with ADHF. For example, systolic blood pressure (SBP) 90 mm Hg was present in only 2.3% of patients in the Acute Decompensated Heart Failure National Registry (ADHERE), a registry designed to study characteristics, management, and outcomes in a broad sample of patients hospitalized with ADHF.11
Most patients with ADHF present with congestion, not respiratory failure or cardiogenic shock,11, 12 and a select subgroup of these patients will respond to treatment within 1224 hours.13 Although this may be an inordinate amount of time to keep patients in an ED, it is not long enough to generally require full hospital admission. Instead, these patients can be effectively managed in an observation unit (OU).14 The goal of these units is to provide the required level of care over a 12‐ to 24‐hour period while simultaneously reducing costs by eliminating the need for hospital admission. Selecting patients who will respond to therapy during this time frame is a critical component in instituting effective OU management of ADHF. Key entry and exclusion criteria are listed in Table 1.14 In patients who meet these criteria, management in an OU has been shown to yield outcomes comparable to inpatient care, but at a lower cost.1416
| Entry criteria |
|---|
|
| History (at least one of the following) |
| Dyspnea on exertion |
| Paroxysmal nocturnal dyspnea |
| Shortness of breath |
| Edema of legs or abdomen |
| Weight gain |
| Physical examination (at least one of the following) |
| Jugular venous distention or elevation in pulsation |
| Positive abdominal jugular reflux |
| S3/S4 gallop |
| Inspiratory rales |
| Peripheral edema |
| Chest x‐ray (at least one of the following) |
| Cardiomegaly |
| Pulmonary vascular congestion |
| Kerley B lines |
| Pulmonary edema |
| Pleural effusion |
| Exclusion criteria |
| Unstable vital signs (BP >220/120 mm Hg, respiratory rate >25 breath/min, heart rate >130 beats/min) |
| Temperature >38.5C |
| Unstable airway or need for >4 L/min supplemental O2 to keep O2 saturation >90% |
| Peak flow 50% of predicted with wheezing |
| Clinically significant arrhythmia or sustained ventricular tachycardia |
| Any ECG with diagnostic criteria for AMI or ischemia |
| Chest x‐ray with pulmonary infiltrates |
| Any CK‐MB >8.8 ng/mL |
| Any troponin T >0.1 g/L (>0.5 g/L if creatinine >2.0 mg/dL) |
| Requirement for continuous vasoactive medication to stabilize hemodynamics |
| Complex decompensation: concomitant end‐organ hypoperfusion, volume overload, and systemic vasoconstriction |
| Requirement for care guided by pulmonary artery catheter |
| Severe electrolyte imbalance |
| Chronic renal failure requiring dialysis |
| Acute mental status abnormality |
Early Initiation of Therapy
Early institution of effective therapy has been shown to improve outcomes. Consequently, selection of initial therapy should occur concurrently with determination of proper treatment location. In the Prospective Randomized Outcomes Study of Acutely Decompensated Congestive Heart Failure Treated Initially as Outpatients with Nesiritide (PROACTION) trial, initiation of nesiritide in the ED/OU was associated with an 11% reduction in hospital admissions at the index visit (P = .436), a 57% reduction in hospitalizations within 30 days after discharge from the index hospitalization (P = .058), and a 62% reduction in median duration of rehospitalization (P = .032).17 The incidence of symptomatic hypotension was low and did not differ between the groups.17 Likewise, in separate analyses of data from ADHERE, ED initiation of intravenous (IV) vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine significantly reduced the risk of requiring transfer to an ICU and reduced ICU length of stay and total hospital length of stay compared with inpatient initiation of these same therapies.18, 19
Treatment Algorithm
Treatment of ADHF should proceed along a logical care pathway governed by both clinical status and response to prior therapies (Figure 1). One must first consider whether there is evidence of respiratory failure or impending respiratory failure.20 If so, patients should receive immediate ventilatory support via continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), or endotracheal intubation, depending on the degree of respiratory impairment.3, 5 In prospective controlled evaluations, patients with acute respiratory failure secondary to pulmonary edema who were randomized to treatment with CPAP demonstrated significant improvement in cardiopulmonary indices21, 22 and significant reductions in need for endotracheal intubation21 and short‐term mortality22 when compared with similar patients who received standard therapy without CPAP.
Once potential respiratory issues have been addressed, the next items for consideration are circulation and perfusion. Patients with low cardiac output and hypotension (cardiogenic shock) are at risk for developing critical end‐organ dysfunction. In these patients, insertion of a pulmonary artery catheter may aid in assessment of hemodynamic status and response to therapy.4 Patients with low cardiac output and low filling pressures should receive IV fluid loading.3 In contrast, for patients with low cardiac output and high filling pressures, inotropic agents should be considered.3, 4 Also, these patients may require IV vasodilators and/or IV diuretics to treat pulmonary edema once blood pressure (BP) and cardiac output have been stabilized.2, 3, 20
For patients with ADHF who present with symptoms of congestion, but not respiratory failure or cardiogenic shock, the initial therapeutic decision is governed by their BP. Approximately 50% of patients with ADHF will have an SBP > 140 mm Hg.12, 23 These patients tend to be older and to have diastolic rather than systolic dysfunction.12, 20, 23 Symptoms typically have been present for only a short period of time (2448 hours) and are more often due to maldistribution of fluid producing pulmonary edema than total body fluid overload. Consequently, initial treatment should focus on aggressive BP control to relieve this edema. Sublingual or topical nitrates are recommended as a first step, and initial diuretic use should be minimal to avoid intravascular volume depletion leading to renal dysfunction.20 In contrast, patients presenting with SBP between 90 mm Hg and 140 mm Hg are more likely to have some degree of systolic dysfunction, leading to a gradual worsening of their heart failure symptoms and total body fluid overload over a period of weeks.20 These patients require aggressive diuresis. Although the efficacy of IV loop diuretics has not been established in randomized, controlled clinical trials, observational experience demonstrates that they can effectively reduce filling pressures, relieve volume overload, and decrease symptoms of congestion.4 They are currently the mainstay of therapy for ADHF secondary to fluid retention, and their use is recommended in all 4 guidelines.25
This initial therapeutic choice, however, is only the starting point, and it is important not to stop at this stage. No single definitive therapy for ADHF exists, and not all patients will respond to initial treatment. Optimal management requires early recognition and addressing of both an inadequate response to therapy and any adverse affects induced by this therapy. Frequent reevaluations are an essential component of treating patients with ADHF. For example, the timeline in one of the guidelines calls for assessing the patient's response at 2 and 4 hours after initiation of IV therapy and adjusting treatment as indicated based on these assessments (Figure 2).2

If the patient has an adequate response to initial therapy, defined as SBP 140 mm Hg, stable renal function, and urine output >500 mL over 2 hours (>250 mL if serum creatinine >2.5 mg/dL), this therapy can continue unchanged, and focus shifts to long‐term management issues.2, 4, 14 However, if the response is inadequate, it is important to identify and treat the cause of this inadequate response.
Inadequate urine output secondary to diuretic resistance is common in patients with ADHF, especially in those on long‐term diuretic therapy.3 Despite a 90% prevalence of IV diuretic use in ADHERE, 70% of patients either gained weight or lost fewer than 5 pounds during hospitalization, and 42% were discharged with unresolved symptoms.24 Clearly, diuretic therapy did not produce the desired effect in many of these patients. This inadequate response to loop diuretics is a direct result of their pharmacologic properties, especially as they relate to patients with heart failure. The physiologic effects of loop diuretics are directly related to their concentration in the lumen of the nephron. This concentration depends on both the patient's renal function and the dose and half‐life of the administered diuretic.25 Comorbid renal dysfunction is common in patients with ADHF.26 In addition, even in the absence of this dysfunction, the short half‐life of loop diuretics limits the amount of time that their luminal concentration is in the effective range, and rebound sodium retention can occur whenever the diuretic concentration is below this range.25 Furthermore, the dose‐response curve of loop diuretics is S‐shaped. As a result, a threshold concentration exists beyond which no further augmentation in urine output occurs; ie, there is a maximum physiologic response that is reduced in patients with heart failure.25 Guideline recommendations for patients with diuretic resistance attempt to address these physiologic and pharmacologic limitations. These recommendations include fluid restriction to decrease the overall volume of diuresis necessary, increasing diuretic dose or instituting continuous infusion loop diuretic therapy to increase the amount of time during which the luminal concentration is within the effective range, sequential diuretic blockade to take advantage of the different mechanisms of action of the various diuretic classes to affect different components of the nephron, bypassing the kidney through the use of ultrafiltration, and when these are inadequate, adding a vasodilator in an attempt to augment cardiac output and renal perfusion.3, 4, 24, 25, 2729
Addition of an IV vasodilator is the primary means of addressing an inadequate response typified by hypertension, worsening renal function, and/or persistent symptoms, rather than diuretic resistance. Approximately 25% of patients with ADHF receive IV vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine, although predominantly vasodilators, at some point during their hospitalization.11, 12 These agents improve hemodynamics and reduce symptoms of ADHF.5, 3032 Their use, in combination with low‐dose diuretics, has been shown to be more efficacious than high‐dose diuretics alone.3, 27 Adding a vasodilator may reduce adverse, diuretic‐induced, neurohormonal activation. In an animal model, combining nesiritide with IV furosemide significantly attenuated the rise in plasma aldosterone produced by IV furosemide alone,33 and this finding has been subsequently confirmed in patients with heart failure.34 Finally, vasodilators have proven to be a safer alternative than inotropes in patients with ADHF. In an analysis of data from ADHERE, covariate‐adjusted and propensity‐adjusted mortality risk was >50% lower for patients receiving nitroglycerin or nesiritide compared with those receiving dobutamine,11 and in an analysis of data from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, which evaluated patients with advanced heart failure, the risk‐adjusted mortality hazard ratio (HR) was significantly increased for inotropes (HR: 2.14; 95% confidence interval [CI]: 1.104.15) but not for vasodilators in the absence of inotropes (HR: 1.39; 95% CI: 0.643.00).35
Performance Measures
In addition to instituting effective therapy for the acute decompensation, it is important to implement measures that may improve long‐term outcomes. The Joint Commission on Accreditation of Healthcare Organizations and the AHA/ASA have identified a series of 5 core performance measures that should be completed during hospitalization for AHDF: discharge instructions relevant to patient's education, documentation of left ventricular systolic function evaluation, prescription of angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at discharge in patients with left ventricular systolic dysfunction, adult smoking cessation advice/counseling, and prescription of ‐blocker at discharge (Table 2).36, 37 In an analysis of data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry, prescription of a ‐blocker at discharge significantly reduced the risk‐adjusted odds ratio (OR) for mortality (OR: 0.48; 95% CI: 0.300.79), and prescription of an ACE inhibitor or ARB at discharge significantly reduced the risk‐adjusted OR for rehospitalization or death (OR: 0.51; 95% CI: 0.340.78) at 6090 days.38 Although no correlation was detected between outcomes and the other 3 core performance measures in this evaluation, the 60‐day to 90‐day time frame may have been too short to identify the full effects of smoking cessation counseling and left ventricular function assessment. Failure to detect a beneficial effect of discharge instructions is disappointing, especially given the proven benefit of disease management programs (see below) and may reflect a limitation of this measure, as currently implemented, to determine the thoroughness and patient understanding of the instructions provided.38, 39
| Measure | Description | Source |
|---|---|---|
| HF‐1 | Discharge instructions relevant to patient education | JCAHO; AHA/ASA |
| HF‐2 | Documentation of left ventricular systolic function evaluation | JCAHO; AHA/ASA |
| HF‐3 | Prescription of ACE inhibitor or ARB at discharge in patients with left ventricular systolic dysfunction | JCAHO; AHA/ASA |
| HF‐4 | Adult smoking cessation advice/counseling | JCAHO; AHA/ASA |
| HF‐5 | Prescription of ‐blocker at discharge | AHA/ASA |
TRANSITION OF CARE
Lastly, optimal management of ADHF requires successful transition of care from an inpatient to an outpatient setting. Recidivism is both common and costly. Approximately 2% of patients with ADHF are readmitted within 2 days, 20% within 1 month, and 50% within 6 months of hospital discharge.40 Frequently, these readmissions are caused by nonadherence to the therapeutic regimen following discharge.41 In an evaluation of patients hospitalized for ADHF at a large urban medical center, noncompliance with prescribed diet and/or drugs was the most common precipitating factor for admission (64% of patients), followed by uncontrolled hypertension (44%), cardiac arrhythmia (29%), environmental factors (19%), and inadequate therapy (17%).42 Similarly, in a prospective evaluation of elderly patients hospitalized for ADHF, 53% of readmissions occurring within 90 days of discharge were deemed to be preventable, with the most common contributing factors being noncompliance with medications and/or diet (33%), inadequate discharge planning (15%), inadequate follow‐up (20%), insufficient support system (21%), and failure to seek medical attention promptly when symptoms recurred (20%).43 Consequently, patient education and arrangement for appropriate follow‐up are crucial components of successfully transitioning care to an outpatient setting.
Effective patient education is time‐consuming. Patients must be taught when, how, and why to take their medication. They need to understand their dietary guidelines and the reasons for these guidelines. They need to know how to use daily weigh‐ins as a means of monitoring their fluid status and what to do in response to a change in weight or symptoms. Finally, they need to be cognizant of what constitutes appropriate exercise and the need for this exercise.4447 To enhance understanding and retention, this information should be presented to the patient over the course of the hospitalization. Comprehension should be tested continually and education repeated until appropriate understanding is ensured. Patient education provided in a rushed or perfunctory manner at the moment of discharge is unlikely to be retained or effective.38, 39
Ideally, the patient should be referred to a comprehensive heart failure disease management program for postdischarge care. Numerous evaluations have established the effectiveness of these programs in enhancing use of appropriate medications, improving functional status, reducing readmissions and mortality, and decreasing costs.4454 For example, in separate evaluations, the prevalences of appropriate vasodilator use (93% vs. 61%; P .001),51 ‐blocker use (71% vs. 40%; P .001),50 and ACE inhibitor use (84% vs. 59%; P .001)52 were significantly greater for disease management program participants compared with nonparticipants. In addition, participation in a disease management program was associated with a 52% reduction in the risk of hospitalization for cardiovascular causes (P .001) and a 72% reduction in ED visits (P .01) in 1 evaluation,45 a 36% reduction (95% CI: 16.7%50.9%) in the risk of heart failure admission or death in another,53 and a 67% reduction (95% CI: 41%82%) in the adjusted risk of death in yet another evaluation.52 Unfortunately, recent data indicate that these programs must be ongoing to sustain these benefits. In a prospective evaluation, patients with heart failure were randomized to either standard care or a multidisciplinary disease management program for 6 months followed by standard care.55, 56 Significantly fewer patients in the disease management group required readmission to the hospital (HR: 0.55; 95% CI: 0.350.88) during the 6‐month period in which they actively participated in the disease management program.56 However, by the end of follow‐up (mean 2.8 years), there was no significant difference between treatment groups in all‐cause mortality (HR: 1.09; 95% CI: 0.691.72) or the composite endpoint of death, ED visit, or hospitalization (HR: 1.01; 95% CI: 0.751.37).55
CONCLUSION
A gap between evidence‐based guidelines and current management of patients with ADHF exists. Multiple strategies to bridge this gap in patient management can be employed. Patients with ADHF require rapid assessment to determine appropriate treatment location and initial therapy. Clinical status should guide treatment selection. Once effective acute therapy has been established, strategies to improve long‐term outcomes should be implemented. These strategies include ensuring that care complies with established core performance measures, providing patient education in a manner suited to ensure comprehension and retention, and arranging for appropriate outpatient follow‐up, ideally in a comprehensive heart failure disease management program. Increasing the awareness of the gap between evidence‐based guidelines and current management, as well as strategies to bridge this gap, is crucial to improving the outcomes of patients with ADHF.
Optimizing quality of care in patients with acute decompensated heart failure (ADHF) is crucial, given both the frequency and cost of hospitalization for this disorder. Several quality improvement strategies have been identified, including provider education; provider reminder systems and decision support; audit and feedback; patient education; organizational change; and financial incentives, regulation, and policy.1
To assist hospitalists in implementing these strategies, this article briefly reviews evidence‐based guidelines for the treatment of ADHF, presents a practical algorithm for patient assessment and treatment derived from these guidelines and personal experience, and discusses systems to enhance the ultimate transition of patient care from the inpatient to outpatient setting.
EVIDENCE‐BASED GUIDELINES
Evidence‐based guidelines are created in an attempt to promote optimal management of a condition or disorder based on expert analysis of all available relevant scientific data. Current guidelines for the assessment and treatment of ADHF have been developed by a national group purchasing organization,2 the European Society of Cardiology,3 the Heart Failure Society of America,4 and the American College of Emergency Physicians.5 Relevant components of these guidelines will be discussed in the patient assessment and treatment section below.
Publication of guidelines, in and of itself, however, is inadequate to ensure their acceptance and use.1 Data from the American Heart Association (AHA)/American Stroke Association (ASA) Get With The GuidelinesHeart Failure (GWTG‐HF) program continue to demonstrate a substantial gap between guideline recommendations and current care of patients with ADHF.6, 7 One way to promote systemwide adherence with published guidelines is to directly involve healthcare professionals in the implementation process. Consequently, development of local, hospital‐based procedures derived from national or international guidelines may be more effective than the simple dissemination of the guidelines themselves.1 Hospitalists have a unique insight into both patient care and the hospital setting and are frequently involved in evaluating hospital policies and procedures and implementing clinical pathways and guidelines.8 In addition, hospitalist care has been associated with greater compliance with disease‐specific guidelines compared to nonhospitalist care.9 As a result, hospitalists are uniquely suited to play a key role in the development of these procedures.
PATIENT ASSESSMENT AND TREATMENT
The differential diagnosis of any individual presenting to the emergency department (ED) with signs of systemic or pulmonary edema should include ADHF (Figure 1).25 These individuals require a rapid initial assessment to (1) establish the diagnosis, (2) determine the best location for subsequent treatment, and (3) institute the most appropriate initial therapy.

Treatment Location
Effective and efficient management of ADHF requires determining proper treatment location. Inpatient management of ADHF is expensive, accounting for approximately 60% of the $31.7 billion spent annually on heart failure care in the United States.10 Clearly, patients with impending respiratory failure requiring ventilation assistance and patients with cardiogenic shock requiring inotropic agents and invasive monitoring are best cared for in an intensive care unit (ICU) setting. However, these patients constitute the minority of patients with ADHF. For example, systolic blood pressure (SBP) 90 mm Hg was present in only 2.3% of patients in the Acute Decompensated Heart Failure National Registry (ADHERE), a registry designed to study characteristics, management, and outcomes in a broad sample of patients hospitalized with ADHF.11
Most patients with ADHF present with congestion, not respiratory failure or cardiogenic shock,11, 12 and a select subgroup of these patients will respond to treatment within 1224 hours.13 Although this may be an inordinate amount of time to keep patients in an ED, it is not long enough to generally require full hospital admission. Instead, these patients can be effectively managed in an observation unit (OU).14 The goal of these units is to provide the required level of care over a 12‐ to 24‐hour period while simultaneously reducing costs by eliminating the need for hospital admission. Selecting patients who will respond to therapy during this time frame is a critical component in instituting effective OU management of ADHF. Key entry and exclusion criteria are listed in Table 1.14 In patients who meet these criteria, management in an OU has been shown to yield outcomes comparable to inpatient care, but at a lower cost.1416
| Entry criteria |
|---|
|
| History (at least one of the following) |
| Dyspnea on exertion |
| Paroxysmal nocturnal dyspnea |
| Shortness of breath |
| Edema of legs or abdomen |
| Weight gain |
| Physical examination (at least one of the following) |
| Jugular venous distention or elevation in pulsation |
| Positive abdominal jugular reflux |
| S3/S4 gallop |
| Inspiratory rales |
| Peripheral edema |
| Chest x‐ray (at least one of the following) |
| Cardiomegaly |
| Pulmonary vascular congestion |
| Kerley B lines |
| Pulmonary edema |
| Pleural effusion |
| Exclusion criteria |
| Unstable vital signs (BP >220/120 mm Hg, respiratory rate >25 breath/min, heart rate >130 beats/min) |
| Temperature >38.5C |
| Unstable airway or need for >4 L/min supplemental O2 to keep O2 saturation >90% |
| Peak flow 50% of predicted with wheezing |
| Clinically significant arrhythmia or sustained ventricular tachycardia |
| Any ECG with diagnostic criteria for AMI or ischemia |
| Chest x‐ray with pulmonary infiltrates |
| Any CK‐MB >8.8 ng/mL |
| Any troponin T >0.1 g/L (>0.5 g/L if creatinine >2.0 mg/dL) |
| Requirement for continuous vasoactive medication to stabilize hemodynamics |
| Complex decompensation: concomitant end‐organ hypoperfusion, volume overload, and systemic vasoconstriction |
| Requirement for care guided by pulmonary artery catheter |
| Severe electrolyte imbalance |
| Chronic renal failure requiring dialysis |
| Acute mental status abnormality |
Early Initiation of Therapy
Early institution of effective therapy has been shown to improve outcomes. Consequently, selection of initial therapy should occur concurrently with determination of proper treatment location. In the Prospective Randomized Outcomes Study of Acutely Decompensated Congestive Heart Failure Treated Initially as Outpatients with Nesiritide (PROACTION) trial, initiation of nesiritide in the ED/OU was associated with an 11% reduction in hospital admissions at the index visit (P = .436), a 57% reduction in hospitalizations within 30 days after discharge from the index hospitalization (P = .058), and a 62% reduction in median duration of rehospitalization (P = .032).17 The incidence of symptomatic hypotension was low and did not differ between the groups.17 Likewise, in separate analyses of data from ADHERE, ED initiation of intravenous (IV) vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine significantly reduced the risk of requiring transfer to an ICU and reduced ICU length of stay and total hospital length of stay compared with inpatient initiation of these same therapies.18, 19
Treatment Algorithm
Treatment of ADHF should proceed along a logical care pathway governed by both clinical status and response to prior therapies (Figure 1). One must first consider whether there is evidence of respiratory failure or impending respiratory failure.20 If so, patients should receive immediate ventilatory support via continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), or endotracheal intubation, depending on the degree of respiratory impairment.3, 5 In prospective controlled evaluations, patients with acute respiratory failure secondary to pulmonary edema who were randomized to treatment with CPAP demonstrated significant improvement in cardiopulmonary indices21, 22 and significant reductions in need for endotracheal intubation21 and short‐term mortality22 when compared with similar patients who received standard therapy without CPAP.
Once potential respiratory issues have been addressed, the next items for consideration are circulation and perfusion. Patients with low cardiac output and hypotension (cardiogenic shock) are at risk for developing critical end‐organ dysfunction. In these patients, insertion of a pulmonary artery catheter may aid in assessment of hemodynamic status and response to therapy.4 Patients with low cardiac output and low filling pressures should receive IV fluid loading.3 In contrast, for patients with low cardiac output and high filling pressures, inotropic agents should be considered.3, 4 Also, these patients may require IV vasodilators and/or IV diuretics to treat pulmonary edema once blood pressure (BP) and cardiac output have been stabilized.2, 3, 20
For patients with ADHF who present with symptoms of congestion, but not respiratory failure or cardiogenic shock, the initial therapeutic decision is governed by their BP. Approximately 50% of patients with ADHF will have an SBP > 140 mm Hg.12, 23 These patients tend to be older and to have diastolic rather than systolic dysfunction.12, 20, 23 Symptoms typically have been present for only a short period of time (2448 hours) and are more often due to maldistribution of fluid producing pulmonary edema than total body fluid overload. Consequently, initial treatment should focus on aggressive BP control to relieve this edema. Sublingual or topical nitrates are recommended as a first step, and initial diuretic use should be minimal to avoid intravascular volume depletion leading to renal dysfunction.20 In contrast, patients presenting with SBP between 90 mm Hg and 140 mm Hg are more likely to have some degree of systolic dysfunction, leading to a gradual worsening of their heart failure symptoms and total body fluid overload over a period of weeks.20 These patients require aggressive diuresis. Although the efficacy of IV loop diuretics has not been established in randomized, controlled clinical trials, observational experience demonstrates that they can effectively reduce filling pressures, relieve volume overload, and decrease symptoms of congestion.4 They are currently the mainstay of therapy for ADHF secondary to fluid retention, and their use is recommended in all 4 guidelines.25
This initial therapeutic choice, however, is only the starting point, and it is important not to stop at this stage. No single definitive therapy for ADHF exists, and not all patients will respond to initial treatment. Optimal management requires early recognition and addressing of both an inadequate response to therapy and any adverse affects induced by this therapy. Frequent reevaluations are an essential component of treating patients with ADHF. For example, the timeline in one of the guidelines calls for assessing the patient's response at 2 and 4 hours after initiation of IV therapy and adjusting treatment as indicated based on these assessments (Figure 2).2

If the patient has an adequate response to initial therapy, defined as SBP 140 mm Hg, stable renal function, and urine output >500 mL over 2 hours (>250 mL if serum creatinine >2.5 mg/dL), this therapy can continue unchanged, and focus shifts to long‐term management issues.2, 4, 14 However, if the response is inadequate, it is important to identify and treat the cause of this inadequate response.
Inadequate urine output secondary to diuretic resistance is common in patients with ADHF, especially in those on long‐term diuretic therapy.3 Despite a 90% prevalence of IV diuretic use in ADHERE, 70% of patients either gained weight or lost fewer than 5 pounds during hospitalization, and 42% were discharged with unresolved symptoms.24 Clearly, diuretic therapy did not produce the desired effect in many of these patients. This inadequate response to loop diuretics is a direct result of their pharmacologic properties, especially as they relate to patients with heart failure. The physiologic effects of loop diuretics are directly related to their concentration in the lumen of the nephron. This concentration depends on both the patient's renal function and the dose and half‐life of the administered diuretic.25 Comorbid renal dysfunction is common in patients with ADHF.26 In addition, even in the absence of this dysfunction, the short half‐life of loop diuretics limits the amount of time that their luminal concentration is in the effective range, and rebound sodium retention can occur whenever the diuretic concentration is below this range.25 Furthermore, the dose‐response curve of loop diuretics is S‐shaped. As a result, a threshold concentration exists beyond which no further augmentation in urine output occurs; ie, there is a maximum physiologic response that is reduced in patients with heart failure.25 Guideline recommendations for patients with diuretic resistance attempt to address these physiologic and pharmacologic limitations. These recommendations include fluid restriction to decrease the overall volume of diuresis necessary, increasing diuretic dose or instituting continuous infusion loop diuretic therapy to increase the amount of time during which the luminal concentration is within the effective range, sequential diuretic blockade to take advantage of the different mechanisms of action of the various diuretic classes to affect different components of the nephron, bypassing the kidney through the use of ultrafiltration, and when these are inadequate, adding a vasodilator in an attempt to augment cardiac output and renal perfusion.3, 4, 24, 25, 2729
Addition of an IV vasodilator is the primary means of addressing an inadequate response typified by hypertension, worsening renal function, and/or persistent symptoms, rather than diuretic resistance. Approximately 25% of patients with ADHF receive IV vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine, although predominantly vasodilators, at some point during their hospitalization.11, 12 These agents improve hemodynamics and reduce symptoms of ADHF.5, 3032 Their use, in combination with low‐dose diuretics, has been shown to be more efficacious than high‐dose diuretics alone.3, 27 Adding a vasodilator may reduce adverse, diuretic‐induced, neurohormonal activation. In an animal model, combining nesiritide with IV furosemide significantly attenuated the rise in plasma aldosterone produced by IV furosemide alone,33 and this finding has been subsequently confirmed in patients with heart failure.34 Finally, vasodilators have proven to be a safer alternative than inotropes in patients with ADHF. In an analysis of data from ADHERE, covariate‐adjusted and propensity‐adjusted mortality risk was >50% lower for patients receiving nitroglycerin or nesiritide compared with those receiving dobutamine,11 and in an analysis of data from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, which evaluated patients with advanced heart failure, the risk‐adjusted mortality hazard ratio (HR) was significantly increased for inotropes (HR: 2.14; 95% confidence interval [CI]: 1.104.15) but not for vasodilators in the absence of inotropes (HR: 1.39; 95% CI: 0.643.00).35
Performance Measures
In addition to instituting effective therapy for the acute decompensation, it is important to implement measures that may improve long‐term outcomes. The Joint Commission on Accreditation of Healthcare Organizations and the AHA/ASA have identified a series of 5 core performance measures that should be completed during hospitalization for AHDF: discharge instructions relevant to patient's education, documentation of left ventricular systolic function evaluation, prescription of angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at discharge in patients with left ventricular systolic dysfunction, adult smoking cessation advice/counseling, and prescription of ‐blocker at discharge (Table 2).36, 37 In an analysis of data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry, prescription of a ‐blocker at discharge significantly reduced the risk‐adjusted odds ratio (OR) for mortality (OR: 0.48; 95% CI: 0.300.79), and prescription of an ACE inhibitor or ARB at discharge significantly reduced the risk‐adjusted OR for rehospitalization or death (OR: 0.51; 95% CI: 0.340.78) at 6090 days.38 Although no correlation was detected between outcomes and the other 3 core performance measures in this evaluation, the 60‐day to 90‐day time frame may have been too short to identify the full effects of smoking cessation counseling and left ventricular function assessment. Failure to detect a beneficial effect of discharge instructions is disappointing, especially given the proven benefit of disease management programs (see below) and may reflect a limitation of this measure, as currently implemented, to determine the thoroughness and patient understanding of the instructions provided.38, 39
| Measure | Description | Source |
|---|---|---|
| HF‐1 | Discharge instructions relevant to patient education | JCAHO; AHA/ASA |
| HF‐2 | Documentation of left ventricular systolic function evaluation | JCAHO; AHA/ASA |
| HF‐3 | Prescription of ACE inhibitor or ARB at discharge in patients with left ventricular systolic dysfunction | JCAHO; AHA/ASA |
| HF‐4 | Adult smoking cessation advice/counseling | JCAHO; AHA/ASA |
| HF‐5 | Prescription of ‐blocker at discharge | AHA/ASA |
TRANSITION OF CARE
Lastly, optimal management of ADHF requires successful transition of care from an inpatient to an outpatient setting. Recidivism is both common and costly. Approximately 2% of patients with ADHF are readmitted within 2 days, 20% within 1 month, and 50% within 6 months of hospital discharge.40 Frequently, these readmissions are caused by nonadherence to the therapeutic regimen following discharge.41 In an evaluation of patients hospitalized for ADHF at a large urban medical center, noncompliance with prescribed diet and/or drugs was the most common precipitating factor for admission (64% of patients), followed by uncontrolled hypertension (44%), cardiac arrhythmia (29%), environmental factors (19%), and inadequate therapy (17%).42 Similarly, in a prospective evaluation of elderly patients hospitalized for ADHF, 53% of readmissions occurring within 90 days of discharge were deemed to be preventable, with the most common contributing factors being noncompliance with medications and/or diet (33%), inadequate discharge planning (15%), inadequate follow‐up (20%), insufficient support system (21%), and failure to seek medical attention promptly when symptoms recurred (20%).43 Consequently, patient education and arrangement for appropriate follow‐up are crucial components of successfully transitioning care to an outpatient setting.
Effective patient education is time‐consuming. Patients must be taught when, how, and why to take their medication. They need to understand their dietary guidelines and the reasons for these guidelines. They need to know how to use daily weigh‐ins as a means of monitoring their fluid status and what to do in response to a change in weight or symptoms. Finally, they need to be cognizant of what constitutes appropriate exercise and the need for this exercise.4447 To enhance understanding and retention, this information should be presented to the patient over the course of the hospitalization. Comprehension should be tested continually and education repeated until appropriate understanding is ensured. Patient education provided in a rushed or perfunctory manner at the moment of discharge is unlikely to be retained or effective.38, 39
Ideally, the patient should be referred to a comprehensive heart failure disease management program for postdischarge care. Numerous evaluations have established the effectiveness of these programs in enhancing use of appropriate medications, improving functional status, reducing readmissions and mortality, and decreasing costs.4454 For example, in separate evaluations, the prevalences of appropriate vasodilator use (93% vs. 61%; P .001),51 ‐blocker use (71% vs. 40%; P .001),50 and ACE inhibitor use (84% vs. 59%; P .001)52 were significantly greater for disease management program participants compared with nonparticipants. In addition, participation in a disease management program was associated with a 52% reduction in the risk of hospitalization for cardiovascular causes (P .001) and a 72% reduction in ED visits (P .01) in 1 evaluation,45 a 36% reduction (95% CI: 16.7%50.9%) in the risk of heart failure admission or death in another,53 and a 67% reduction (95% CI: 41%82%) in the adjusted risk of death in yet another evaluation.52 Unfortunately, recent data indicate that these programs must be ongoing to sustain these benefits. In a prospective evaluation, patients with heart failure were randomized to either standard care or a multidisciplinary disease management program for 6 months followed by standard care.55, 56 Significantly fewer patients in the disease management group required readmission to the hospital (HR: 0.55; 95% CI: 0.350.88) during the 6‐month period in which they actively participated in the disease management program.56 However, by the end of follow‐up (mean 2.8 years), there was no significant difference between treatment groups in all‐cause mortality (HR: 1.09; 95% CI: 0.691.72) or the composite endpoint of death, ED visit, or hospitalization (HR: 1.01; 95% CI: 0.751.37).55
CONCLUSION
A gap between evidence‐based guidelines and current management of patients with ADHF exists. Multiple strategies to bridge this gap in patient management can be employed. Patients with ADHF require rapid assessment to determine appropriate treatment location and initial therapy. Clinical status should guide treatment selection. Once effective acute therapy has been established, strategies to improve long‐term outcomes should be implemented. These strategies include ensuring that care complies with established core performance measures, providing patient education in a manner suited to ensure comprehension and retention, and arranging for appropriate outpatient follow‐up, ideally in a comprehensive heart failure disease management program. Increasing the awareness of the gap between evidence‐based guidelines and current management, as well as strategies to bridge this gap, is crucial to improving the outcomes of patients with ADHF.
- .Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process.J Gen Intern Med.2007;22(12):1762–1770.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- Heart Failure Society of America.Executive summary: HFSA 2006 comprehensive heart failure practice guideline.J Card Fail.2006;12(1):10–38.
- ,,,,,American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,, et al.Influence of the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program on emerging performance measures for patients hospitalized with heart failure.Circulation.2006;114:II–572. Abstract 2740.
- ,,, et al.Quality of care for heart failure patients with concomitant kidney disease in the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program.Circulation.2006;114:II–859. Abstract 3993.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,.Preliminary experience with an emergency department observation unit protocol for heart failure.Acad Emerg Med.2000;7(10):1171. Abstract 33.
- ,,,,,.Effective observation unit treatment of decompensated heart failure.Congest Heart Fail.2002;8(2):68–73.
- ,.Patient outcome and costs following an acute heart failure (HF) management program in an emergency department (ED) observation unit (OU).J Heart Lung Transplant.1999;18(1):92. Abstract 240.
- ,,,,,.Inpatient versus emergency department observation unit management of heart failure.Ann Emerg Med.1998;32(3 part 2):S46–S47. Abstract 180.
- ,,, et al.Observation unit treatment of heart failure with nesiritide: results from the proaction trial.J Emerg Med.2005;29(3):243–252.
- ,,,.Early initiation of IV vasoactive therapy improves heart failure outcomes: an analysis from the ADHERE™ registry database.Ann Emerg Med.2003;42(4 suppl):S26. Abstract 92.
- ,,, et al.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- ,,,,,.Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department.Ann Emerg Med.2008;51(1):45–57.
- ,,,,,.Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema. Short‐term results and long‐term follow‐up.Chest.1995;107(5):1379–1386.
- ,,, et al.Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients.Intensive Care Med.2004;30(5):882–888.
- ,,,,.Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database.J Am Coll Cardiol.2006;47(1):76–84.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- .Diuretic therapy in congestive heart failure.Congest Heart Fail.2000;6(4):197–201.
- ,,, et al.High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database.J Card Fail.2007;13(6):422–430.
- ,,, et al.Randomised trial of high‐dose isosorbide dinitrate plus low‐dose furosemide versus high‐dose furosemide plus low‐dose isosorbide dinitrate in severe pulmonary oedema.Lancet.1998;351(9100):389–393.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- .Management of diuretic‐refractory, volume‐overloaded patients with acutely decompensated heart failure.Curr Cardiol Rep.2005;7(3):204–210.
- ,,, et al.Sustained hemodynamic effects of an infusion of nesiritide (human b‐type natriuretic peptide) in heart failure: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.1999;34(1):155–162.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,, et al.Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide‐induced aldosterone activation in experimental heart failure.Circulation.2004;109(13):1680–1685.
- ,,.Nesiritide appears to inhibit the rise in plasma aldosterone associated with furosemide diuresis.J Card Fail.2006;12(6 suppl 1):S85–S86. Abstract 275.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- Joint Commission on Accreditation of Healthcare Organizations. Specifications Manual for National Implementation of Hospital Quality Measures. Version 2.3b. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm. Accessed October 10,2008.
- American Heart Association/American Stroke Association. Get with the guidelines–heart failure. Fact sheet. http://www.americanheart.org/downloadable/heart/1163802072170HFFactSheet.pdf. Accessed October 10,2008.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,, et al.Evaluating quality of care for patients with heart failure.Circulation.2000;101(12):e122–e140.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,,.Precipitating factors leading to decompensation of heart failure. Traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Prospective evaluation of an outpatient heart failure management program.J Card Fail.2001;7(1):64–74.
- ,,, et al.Improved outcomes from a comprehensive management system for heart failure.Eur J Heart Fail.2001;3(5):619–625.
- ,,, et al.The benefit of implementing a heart failure disease management program.Arch Intern Med.2001;161(18):2223–2228.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141(2):254–258.
- ,,, et al.Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day‐hospital and usual care.J Am Coll Cardiol.2002;40(7):1259–1266.
- ,,, et al.A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission.J Am Coll Cardiol.2002;39(3):471–480.
- ,,, et al.Mortality benefit of a comprehensive heart failure disease management program in indigent patients.Am Heart J.2006;151(2):478–483.
- ,,, et al.Two‐year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure.J Cardiovasc Med (Hagerstown).2007;8(5):324–329.
- ,,,,.Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort.Prog Cardiovasc Nurs.2007;22(4):196–200.
- ,,, et al.Lack of long‐term benefits of a 6‐month heart failure disease management program.J Card Fail.2007;13(4):287–293.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
- .Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process.J Gen Intern Med.2007;22(12):1762–1770.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- Heart Failure Society of America.Executive summary: HFSA 2006 comprehensive heart failure practice guideline.J Card Fail.2006;12(1):10–38.
- ,,,,,American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,, et al.Influence of the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program on emerging performance measures for patients hospitalized with heart failure.Circulation.2006;114:II–572. Abstract 2740.
- ,,, et al.Quality of care for heart failure patients with concomitant kidney disease in the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program.Circulation.2006;114:II–859. Abstract 3993.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,.Preliminary experience with an emergency department observation unit protocol for heart failure.Acad Emerg Med.2000;7(10):1171. Abstract 33.
- ,,,,,.Effective observation unit treatment of decompensated heart failure.Congest Heart Fail.2002;8(2):68–73.
- ,.Patient outcome and costs following an acute heart failure (HF) management program in an emergency department (ED) observation unit (OU).J Heart Lung Transplant.1999;18(1):92. Abstract 240.
- ,,,,,.Inpatient versus emergency department observation unit management of heart failure.Ann Emerg Med.1998;32(3 part 2):S46–S47. Abstract 180.
- ,,, et al.Observation unit treatment of heart failure with nesiritide: results from the proaction trial.J Emerg Med.2005;29(3):243–252.
- ,,,.Early initiation of IV vasoactive therapy improves heart failure outcomes: an analysis from the ADHERE™ registry database.Ann Emerg Med.2003;42(4 suppl):S26. Abstract 92.
- ,,, et al.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- ,,,,,.Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department.Ann Emerg Med.2008;51(1):45–57.
- ,,,,,.Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema. Short‐term results and long‐term follow‐up.Chest.1995;107(5):1379–1386.
- ,,, et al.Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients.Intensive Care Med.2004;30(5):882–888.
- ,,,,.Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database.J Am Coll Cardiol.2006;47(1):76–84.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- .Diuretic therapy in congestive heart failure.Congest Heart Fail.2000;6(4):197–201.
- ,,, et al.High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database.J Card Fail.2007;13(6):422–430.
- ,,, et al.Randomised trial of high‐dose isosorbide dinitrate plus low‐dose furosemide versus high‐dose furosemide plus low‐dose isosorbide dinitrate in severe pulmonary oedema.Lancet.1998;351(9100):389–393.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- .Management of diuretic‐refractory, volume‐overloaded patients with acutely decompensated heart failure.Curr Cardiol Rep.2005;7(3):204–210.
- ,,, et al.Sustained hemodynamic effects of an infusion of nesiritide (human b‐type natriuretic peptide) in heart failure: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.1999;34(1):155–162.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,, et al.Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide‐induced aldosterone activation in experimental heart failure.Circulation.2004;109(13):1680–1685.
- ,,.Nesiritide appears to inhibit the rise in plasma aldosterone associated with furosemide diuresis.J Card Fail.2006;12(6 suppl 1):S85–S86. Abstract 275.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- Joint Commission on Accreditation of Healthcare Organizations. Specifications Manual for National Implementation of Hospital Quality Measures. Version 2.3b. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm. Accessed October 10,2008.
- American Heart Association/American Stroke Association. Get with the guidelines–heart failure. Fact sheet. http://www.americanheart.org/downloadable/heart/1163802072170HFFactSheet.pdf. Accessed October 10,2008.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,, et al.Evaluating quality of care for patients with heart failure.Circulation.2000;101(12):e122–e140.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,,.Precipitating factors leading to decompensation of heart failure. Traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Prospective evaluation of an outpatient heart failure management program.J Card Fail.2001;7(1):64–74.
- ,,, et al.Improved outcomes from a comprehensive management system for heart failure.Eur J Heart Fail.2001;3(5):619–625.
- ,,, et al.The benefit of implementing a heart failure disease management program.Arch Intern Med.2001;161(18):2223–2228.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141(2):254–258.
- ,,, et al.Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day‐hospital and usual care.J Am Coll Cardiol.2002;40(7):1259–1266.
- ,,, et al.A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission.J Am Coll Cardiol.2002;39(3):471–480.
- ,,, et al.Mortality benefit of a comprehensive heart failure disease management program in indigent patients.Am Heart J.2006;151(2):478–483.
- ,,, et al.Two‐year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure.J Cardiovasc Med (Hagerstown).2007;8(5):324–329.
- ,,,,.Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort.Prog Cardiovasc Nurs.2007;22(4):196–200.
- ,,, et al.Lack of long‐term benefits of a 6‐month heart failure disease management program.J Card Fail.2007;13(4):287–293.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.