User login
Carcinoid tumors: What should increase our suspicion?
Carcinoid tumors, also known as gastroenteropancreatic neuroendocrine tumors, are neoplasms of neuroendocrine origin. Traditionally considered slow-growing, they are now known to vary in their aggressiveness. Many patients with carcinoid tumors have no symptoms or present with symptoms that have broad differential diagnoses. Unless the primary care physician suspects that the patient has a carcinoid tumor, the appropriate testing is seldom ordered. In addition, these patients are at higher risk of developing other cancers of the genitourinary, gastrointestinal, and respiratory tracts; this makes close follow-up, especially by the primary care provider, extremely important.
This article reviews the epidemiology, pathogenesis, clinical features, management, and prognosis of carcinoid tumors. A better knowledge of these tumors among physicians will facilitate recognition, early diagnosis, and improved outcomes for these patients.
INCIDENCE IS INCREASING
The overall incidence of carcinoid tumors has increased over the last 30 years, due at least in part to improvements in ways to diagnose them.1,2 The incidence over the last decade has been between 2.47 and 4.48 per 100,000 population, depending on race and sex, with the highest rates in black men.1,3
The small intestine is the most frequent location, followed by the lungs/bronchi, rectum, appendix, and stomach3; 67.5% of carcinoid tumors occur somewhere in the gastrointestinal system.
The cause of carcinoid tumors is unknown, but genetic factors may play a role, since these tumors often occur as part of genetic disorders such as multiple endocrine neoplasia type 1, von Hippel-Lindau disease, and neurofibromatosis type 1.1–3
SEROTONERGIC EFFECTS DEPEND ON LOCATION
Carcinoid tumors are classified on the basis of their embryologic origin:
- Foregut (lungs, bronchi, stomach)
- Midgut (small intestine, appendix, proximal large bowel)
- Hindgut (distal large bowel, rectum). Tumors from each of these origins differ clinically, biochemically, and histologically.
Carcinoid tumors secrete several bioactive compounds, including serotonin and bradykinin, and the secretory pattern varies depending on the location of the tumor. Most foregut carcinoid tumors secrete low levels of serotonin, being deficient in the enzyme needed to convert 5-hydroxytryptophan to serotonin. Midgut tumors secrete high levels of serotonin, whereas most hindgut tumors do not secrete 5-hydroxytryptophan or serotonin. These differences in secretory patterns are responsible for the different clinical manifestations and biochemical characteristics of these tumors.
Carcinoid syndrome
The systemic effects of the bioactive compounds secreted by carcinoid tumors are responsible for carcinoid syndrome, which has features that can include bronchospasm (possibly mediated by serotonin or bradykinin), diarrhea (likely mediated by serotonin), cutaneous flushing (which has multiple possible mediators), and right-sided valvular heart lesions (possibly mediated by serotonin).2,4 However, the secretory products of midgut carcinoids are normally inactivated by enzymes in the liver before they enter the systemic circulation. Thus, patients with midgut carcinoid tumors develop the carcinoid syndrome only if they have hepatic metastases.4
In contrast, patients with foregut (bronchial and extraintestinal) carcinoids can present with carcinoid syndrome without hepatic metastases, since their secretory products normally bypass the liver and enter the systemic circulation directly.
Hindgut tumors seldom produce this syndrome, since they do not secrete these products.
Fibrosis
Another effect of carcinoid tumors is desmoplasia, or fibrosis.
The pathogenesis of this phenomenon is poorly understood. Serotonin was thought to mediate this effect, but serotonin antagonists are ineffective in treating it, casting doubt on this hypothesis.2,4 More recently, growth factors such as transforming growth factor beta, platelet-derived growth factor, and basic fibroblast growth factor have been implicated.4
SYMPTOMS ARE OFTEN ABSENT OR NONSPECIFIC
Carcinoid tumors are often clinically silent. Signs and symptoms, when present, represent local effects of the tumor, tumor-induced fibrosis, biological effects of secreted products, or metastases.
Local effects can include abdominal pain, intestinal obstruction, appendicitis, rectal bleeding, and peptic ulcer disease, depending on the site of the tumor. Intestinal obstruction can be caused by intussusception, by the intraluminal effects of the tumor, or by adhesions due to tumor-induced fibrosis.7
Tumor-induced fibrosis can be retroperitoneal and have a myriad of manifestations, for example2,5:
- Hydronephrosis from ureteral obstruction
- Mesenteric ischemia from vascular trapping
- Peyronie disease (an acquired fibrosis of the penis that produces bending or pain of the erect penis).
Classic carcinoid syndrome, caused by the entry of bioactive compounds secreted by the tumor into the systemic circulation, occurs in fewer than 10% of patients with carcinoid tumors.2,6 Most patients who present with this syndrome have a midgut carcinoid tumor.
The hallmark is cutaneous flushing, which typically affects the face, neck, and upper body and lasts from 30 seconds to 30 minutes. This reaction can be unprovoked, but it is often precipitated by foods (eg, bananas, tomatoes, eggplant, kiwi, pineapple, cheese), by alcohol consumption, by exercise, by emotional stimuli, or by anesthesia.4,8 Other symptoms of this syndrome include bronchospasm, secretory diarrhea, and venous telangiectasia.
Carcinoid syndrome variants, caused by differences in secretory products, are seen in some patients with bronchial and gastric carcinoid tumors. Patients with gastric carcinoid variants have flushing that is pruritic and well demarcated, and they have an increased incidence of peptic ulcer. In contrast, flushing in patients with bronchial carcinoid variants can last days and is often associated with changes in mental status.2,9
Carcinoid heart disease refers to endocardial fibrotic plaques that occur in patients with carcinoid tumors, especially those with hepatic metastases. The right side of the heart is generally affected, as the left side is protected by inactivation of the bioactive compounds in the lungs. Tricuspid valve regurgitation is the most common finding, but tricuspid stenosis, pulmonary valve regurgitation, and pulmonary valve stenosis can also occur.10 Left-sided lesions can occur in patients with pulmonary metastases.
Hypoalbuminemia and pellagra. The amino acid tryptophan is a precursor of serotonin and niacin (vitamin B3). In patients with widely metastatic carcinoid tumors, increased conversion of tryptophan to serotonin by the tumor cells can lead to tryptophan deficiency and niacin deficiency.1,2 These manifest as hypoalbuminemia and pellagra (glossitis, scaly skin, and confusion).
IF YOU DON’T THINK ABOUT THEM, YOU WON’T LOOK FOR THEM
Many carcinoid tumors are discovered incidentally during surgery or diagnostic procedures. However, an evaluation for a carcinoid tumor is often prompted by symptoms that suggest the carcinoid syndrome. Of importance, since these symptoms can often be erroneously attributed to other, more common causes (Table 1), a very high level of suspicion is required on the part of the primary care physician to initiate the evaluation for carcinoid tumors.
When evaluating a patient who presents with flushing, the clinician should obtain a detailed history, noting the pattern of flushing, precipitating factors, duration, and associated symptoms, such as diarrhea and bronchospasm. 11
Often, patient recall can be improved by asking the patient to keep a symptom diary. If the diary does not offer any clues to the diagnosis, a trial of abstinence from foods and drugs that increase urinary 5-hydroxyindoleacetic acid (5-HIAA) should be attempted. If the flushing resolves, the foods and drugs can be reintroduced one at a time to identify the culprit.11 However, if symptoms persist, a more extensive workup should be undertaken, including screening for carcinoid tumor.
Biochemical assays
Biochemical screening for carcinoid tumors is performed using an assay for bioamines secreted by the tumor.
Plasma chromogranin A is the preferred screening test because it is very sensitive12; however, it is not very specific. Several conditions (including essential hypertension, proton pump inhibitor use, chronic atrophic gastritis, heart failure, renal failure, and pheochromocytoma) can be associated with elevated levels of chromogranin A. Its specificity for diagnosing carcinoid tumors can be increased to about 84% by raising the cut-off value to 31 or 32 U/L. Its specificity can be increased to 95% by using 84 to 87 U/L as the cut-off value, but this comes at the cost of diminishing its sensitivity (from 75% to about 55%).13 Significantly elevated levels of chromogranin A (> 5,000 U/L) independently predict a poor prognosis.13
Urinary 5-HIAA levels also vary depending on the origin of the carcinoid tumor and are therefore useful in locating the site of the tumor. Foregut tumors lack the decarboxylase enzyme necessary to convert 5-hydroxytryptophan to serotonin, resulting in minimal to no elevation in urinary 5-HIAA levels. Midgut tumors secrete serotonin, resulting in high levels of urinary 5-HIAA. Hindgut tumors secrete neither 5-hydroxytryptophan nor serotonin and thus do not raise urinary 5-HIAA levels.
Other less sensitive markers include bradykinin, human chorionic gonadotropin, and neuropeptide PP.14
Provocative tests
Provocative tests such as the pentagastrin test can be considered if other screening test results are equivocal.12 Such testing must occur in a closely monitored, controlled environment, with intravenous somatostatin available in case of a “carcinoid crisis” (see discussion further below).
Patients with equivocal results from biochemical assays or provocative testing should be followed annually without further testing or evaluated for other causes of their symptoms. If the results of biochemical or provocative testing are abnormal, the tumor topography should be identified with an imaging study.
Imaging tests
Topographic localization of carcinoid tumors is done using various imaging tests, depending on the suspected site of the primary tumor.6
Somatostatin receptor scintigraphy, the preferred imaging test, is based on the principle that some carcinoid tumors have high concentrations of somatostatin receptors. Thus, a radiolabeled form of octreotide, a somatostatin analogue, is used to image these primary tumors and metastases.
Other imaging studies:
- Barium studies
- Computed tomography
- Endoscopy
- Endoscopic ultrasonography.
On imaging studies, in general, the tumor may appear as a smooth submucosal mass, as a target lesion (if it ulcerates), as wall-thickening, or as a cystic or calcified mass.6
MANAGEMENT IS MULTIDISCIPLINARY
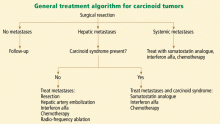
Any patient with a carcinoid tumor should be referred to a medical and surgical oncologist once the diagnosis is established. Referral to additional specialists, such as a gastroenterologist or an interventional radiologist, is based on the location and extent of disease.
Surgery is the cornerstone of therapy
Drug therapy
Somatostatin analogues such as octreotide (Sandostatin) and lanreotide (Somatuline) are useful for treating carcinoid syndrome16 and also have a role in treating systemic metastases. These agents are effective in controlling flushing and diarrhea in 70% to 80% of patients.17 There are data, albeit limited, supporting a role for these agents in inhibiting tumor growth.18 They are the treatment of choice for carcinoid crisis (see discussion below). Important adverse effects include nausea, cramps, diarrhea; cholelithiasis; hypoglycemia or hyperglycemia; cardiac arrhythmias; and gastric atony.16
Interferon alfa is useful as an additive therapy when symptoms of carcinoid syndrome do not resolve with a somatostatin analogue alone. The addition of interferon alfa in these patients is useful for both symptom control (seen in 40% to 50% of those treated) and tumor stabilization (in 20% to 40% of those treated).19 However, it is still unclear whether combination drug therapy is more effective than a somatostatin analogue alone as the initial therapy for carcinoid syndrome.20 Adverse effects of interferon alfa include myelosuppression, depression, flu-like symptoms, and thyroid disturbances.
Supportive therapies
In patients with hepatic metastases who are not candidates for surgery, hepatic artery embolization with chemotherapy or particles can be used as palliative therapy. Severe adverse effects of this procedure include renal failure, hepatic failure, carcinoid crisis, and hepatic abscess and are seen in about 10% of patients.1
Conventional chemotherapy and molecularly targeted therapy are used for patients with rapidly progressive and widely metastatic disease. The precise role and efficacy of these therapies need further study.
Other supportive therapies for patients with carcinoid disease include dietary supplementation with vitamin B3, bronchodilators for bronchospasm, antidiarrheals, diuretics, and valve replacement for carcinoid heart disease.
LONG-TERM PROGNOSIS IS GENERALLY GOOD
The prognosis for patients with carcinoid tumors depends on the location and extent of disease. Tumors of the appendix and rectum have the best prognosis, with 5-year survival rates approaching 100% for localized carcinoid tumors of the appendix.3,21 In contrast, tumors of the small bowel, especially the ileum, are more aggressive and have the worst prognosis (a 5-year survival rate of about 60% to 65%).21
COMPLICATIONS
Other primary malignancies
Carcinoid tumors are often associated with the development of other tumors, not always in the gastrointestinal tract. As many as 52% of patients with small-bowel carcinoid tumors develop another primary tumor.22 This effect is thought to be related to the tumorigenic properties of the bioactive compounds secreted by carcinoid tumors. The other primary malignancy can be synchronous (diagnosed at the same time) or metachronous (diagnosed 1 to 7 years after the carcinoid) and generally arises from the gastrointestinal, genitourinary, or respiratory tract. Adenocarcinoma of the colon is reported as the most common second primary malignancy in patients with carcinoid tumors.22
The best strategy for surveillance in these patients is still unclear. Screening for tumors of the colon, small bowel, lung, cervix, and ovaries at the time of carcinoid tumor diagnosis followed by surveillance for these malignancies has been suggested as a possible approach; however, this comes at the cost of increased patient anxiety, cost, and morbidity from testing. 3
Carcinoid crisis
Carcinoid crisis is a life-threatening emergency caused by release of large amounts of vasoactive compounds from the carcinoid tumor, either spontaneously or provoked by tumor manipulation, surgery, chemotherapy, or hepatic artery embolization.2,5 The syndrome manifests as cardiovascular collapse (severe hypotension or hypertension), tachycardia, and altered mental status.
Treatment differs from those for other causes of shock in that catecholamines and calcium should not be used since they trigger the release of larger amounts of bioactive chemicals from the tumor. In addition, the shock in this situation is refractory to fluid. The mainstay of treatment is the infusion of octreotide and plasma. This crisis can be prevented by giving octreotide before manipulating the tumor.1,2,5
- Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumors. Lancet Oncol 2008; 9:61–72.
- Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005; 128:1717–1751.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003; 97:934–959.
- DeVries H, Verschueren R, Willemse P, Kema I, DeVries E. Diagnostic, surgical, and medical aspect of midgut carcinoids. Cancer Treat Rev 2002; 28:11–25.
- Robertson RG, Griger WJ, Davis NB. Carcinoid tumors. Am Fam Physician 2006; 74:429–434.
- Horton KM, Kamel I, Hofman L, Fishman EK. Carcinoid tumors of the small bowel: a multitechnique imaging approach. AJR Am J Roentgenol 2004; 182:559–567.
- Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999; 340:858–868.
- Bendelow J, Apps E, Jones LE, Poston GJ. Carcinoid syndrome. Eur J Surg Oncol 2008; 34:289–296.
- Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001; 119:1647–1651.
- Bhattacharyya S, Toumpanakis C, Caplin ME, Davar J. Analysis of 150 patients with carcinoid syndrome seen in a single year at one institution in the first decade of the twenty-first century. Am J Cardiol 2008; 101:378–381.
- Nasr C. Flushing: is it carcinoid or something else?Cleveland Clinic Disease Management Project, 2004. Available at http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/endocrinology/flushing/flushing.htm. Accessed 10/25/08.
- Modlin IM, Tang LH. Approaches to the diagnosis of gut neuroendocrine tumors: the last word (today). Gastroenterology 1997; 112:583–590.
- Campana D, Nori F, Piscitelli L, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol 2007; 25:1967–1973.
- Eriksson B, Oberg K. Peptide hormones as tumor markers in neuroendocrine gastrointestinal tumors. Acta Oncol 1991; 30:477–483.
- National Comprehensive Cancer Network. Carcinoid tumors. http://www.nccn.org/professionals/physician_gls/PDF/neuroendocrine.pdf. Accessed 10/25/08.
- Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 2004; 15:966–973.
- Welin SV, Janson ET, Sundin A, et al. High-dose treatment with long-acting somatostatin analogue in patients with advanced midgut carcinoid tumours. Eur J Endocrinol 2004; 151:107–112.
- Arnold R, Trautmann ME, Creutzfeldt W, et al. Somatostatin analogue octreotide and inhibition of tumor growth in metastatic endocrine gastroenteropancreatic tumors. Gut 1996; 38:430–438.
- Bajetta E, Zilembo N, Di Bartolomeo M, et al. Treatment of metastatic carcinoids and other neuroendocrine tumors with recombinant interferon-alpha-2a. A study by the Italian Trials in Medical Oncology Group. Cancer 1993; 72:3099–3105.
- Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon-alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors—the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol 2003; 21:2689–2696.
- Helland SK, Prosch AM, Viste A. Carcinoid tumors in the gastrointestinal tract—a population-based study from Western Norway. Scand J Surg 2006; 95:158–161.
- Habal N, Sims C, Bilchik AJ. Gastrointestinal carcinoid tumors and second primary malignancies. J Surg Oncol 2000; 75:310–316.
Carcinoid tumors, also known as gastroenteropancreatic neuroendocrine tumors, are neoplasms of neuroendocrine origin. Traditionally considered slow-growing, they are now known to vary in their aggressiveness. Many patients with carcinoid tumors have no symptoms or present with symptoms that have broad differential diagnoses. Unless the primary care physician suspects that the patient has a carcinoid tumor, the appropriate testing is seldom ordered. In addition, these patients are at higher risk of developing other cancers of the genitourinary, gastrointestinal, and respiratory tracts; this makes close follow-up, especially by the primary care provider, extremely important.
This article reviews the epidemiology, pathogenesis, clinical features, management, and prognosis of carcinoid tumors. A better knowledge of these tumors among physicians will facilitate recognition, early diagnosis, and improved outcomes for these patients.
INCIDENCE IS INCREASING
The overall incidence of carcinoid tumors has increased over the last 30 years, due at least in part to improvements in ways to diagnose them.1,2 The incidence over the last decade has been between 2.47 and 4.48 per 100,000 population, depending on race and sex, with the highest rates in black men.1,3
The small intestine is the most frequent location, followed by the lungs/bronchi, rectum, appendix, and stomach3; 67.5% of carcinoid tumors occur somewhere in the gastrointestinal system.
The cause of carcinoid tumors is unknown, but genetic factors may play a role, since these tumors often occur as part of genetic disorders such as multiple endocrine neoplasia type 1, von Hippel-Lindau disease, and neurofibromatosis type 1.1–3
SEROTONERGIC EFFECTS DEPEND ON LOCATION
Carcinoid tumors are classified on the basis of their embryologic origin:
- Foregut (lungs, bronchi, stomach)
- Midgut (small intestine, appendix, proximal large bowel)
- Hindgut (distal large bowel, rectum). Tumors from each of these origins differ clinically, biochemically, and histologically.
Carcinoid tumors secrete several bioactive compounds, including serotonin and bradykinin, and the secretory pattern varies depending on the location of the tumor. Most foregut carcinoid tumors secrete low levels of serotonin, being deficient in the enzyme needed to convert 5-hydroxytryptophan to serotonin. Midgut tumors secrete high levels of serotonin, whereas most hindgut tumors do not secrete 5-hydroxytryptophan or serotonin. These differences in secretory patterns are responsible for the different clinical manifestations and biochemical characteristics of these tumors.
Carcinoid syndrome
The systemic effects of the bioactive compounds secreted by carcinoid tumors are responsible for carcinoid syndrome, which has features that can include bronchospasm (possibly mediated by serotonin or bradykinin), diarrhea (likely mediated by serotonin), cutaneous flushing (which has multiple possible mediators), and right-sided valvular heart lesions (possibly mediated by serotonin).2,4 However, the secretory products of midgut carcinoids are normally inactivated by enzymes in the liver before they enter the systemic circulation. Thus, patients with midgut carcinoid tumors develop the carcinoid syndrome only if they have hepatic metastases.4
In contrast, patients with foregut (bronchial and extraintestinal) carcinoids can present with carcinoid syndrome without hepatic metastases, since their secretory products normally bypass the liver and enter the systemic circulation directly.
Hindgut tumors seldom produce this syndrome, since they do not secrete these products.
Fibrosis
Another effect of carcinoid tumors is desmoplasia, or fibrosis.
The pathogenesis of this phenomenon is poorly understood. Serotonin was thought to mediate this effect, but serotonin antagonists are ineffective in treating it, casting doubt on this hypothesis.2,4 More recently, growth factors such as transforming growth factor beta, platelet-derived growth factor, and basic fibroblast growth factor have been implicated.4
SYMPTOMS ARE OFTEN ABSENT OR NONSPECIFIC
Carcinoid tumors are often clinically silent. Signs and symptoms, when present, represent local effects of the tumor, tumor-induced fibrosis, biological effects of secreted products, or metastases.
Local effects can include abdominal pain, intestinal obstruction, appendicitis, rectal bleeding, and peptic ulcer disease, depending on the site of the tumor. Intestinal obstruction can be caused by intussusception, by the intraluminal effects of the tumor, or by adhesions due to tumor-induced fibrosis.7
Tumor-induced fibrosis can be retroperitoneal and have a myriad of manifestations, for example2,5:
- Hydronephrosis from ureteral obstruction
- Mesenteric ischemia from vascular trapping
- Peyronie disease (an acquired fibrosis of the penis that produces bending or pain of the erect penis).
Classic carcinoid syndrome, caused by the entry of bioactive compounds secreted by the tumor into the systemic circulation, occurs in fewer than 10% of patients with carcinoid tumors.2,6 Most patients who present with this syndrome have a midgut carcinoid tumor.
The hallmark is cutaneous flushing, which typically affects the face, neck, and upper body and lasts from 30 seconds to 30 minutes. This reaction can be unprovoked, but it is often precipitated by foods (eg, bananas, tomatoes, eggplant, kiwi, pineapple, cheese), by alcohol consumption, by exercise, by emotional stimuli, or by anesthesia.4,8 Other symptoms of this syndrome include bronchospasm, secretory diarrhea, and venous telangiectasia.
Carcinoid syndrome variants, caused by differences in secretory products, are seen in some patients with bronchial and gastric carcinoid tumors. Patients with gastric carcinoid variants have flushing that is pruritic and well demarcated, and they have an increased incidence of peptic ulcer. In contrast, flushing in patients with bronchial carcinoid variants can last days and is often associated with changes in mental status.2,9
Carcinoid heart disease refers to endocardial fibrotic plaques that occur in patients with carcinoid tumors, especially those with hepatic metastases. The right side of the heart is generally affected, as the left side is protected by inactivation of the bioactive compounds in the lungs. Tricuspid valve regurgitation is the most common finding, but tricuspid stenosis, pulmonary valve regurgitation, and pulmonary valve stenosis can also occur.10 Left-sided lesions can occur in patients with pulmonary metastases.
Hypoalbuminemia and pellagra. The amino acid tryptophan is a precursor of serotonin and niacin (vitamin B3). In patients with widely metastatic carcinoid tumors, increased conversion of tryptophan to serotonin by the tumor cells can lead to tryptophan deficiency and niacin deficiency.1,2 These manifest as hypoalbuminemia and pellagra (glossitis, scaly skin, and confusion).
IF YOU DON’T THINK ABOUT THEM, YOU WON’T LOOK FOR THEM
Many carcinoid tumors are discovered incidentally during surgery or diagnostic procedures. However, an evaluation for a carcinoid tumor is often prompted by symptoms that suggest the carcinoid syndrome. Of importance, since these symptoms can often be erroneously attributed to other, more common causes (Table 1), a very high level of suspicion is required on the part of the primary care physician to initiate the evaluation for carcinoid tumors.
When evaluating a patient who presents with flushing, the clinician should obtain a detailed history, noting the pattern of flushing, precipitating factors, duration, and associated symptoms, such as diarrhea and bronchospasm. 11
Often, patient recall can be improved by asking the patient to keep a symptom diary. If the diary does not offer any clues to the diagnosis, a trial of abstinence from foods and drugs that increase urinary 5-hydroxyindoleacetic acid (5-HIAA) should be attempted. If the flushing resolves, the foods and drugs can be reintroduced one at a time to identify the culprit.11 However, if symptoms persist, a more extensive workup should be undertaken, including screening for carcinoid tumor.
Biochemical assays
Biochemical screening for carcinoid tumors is performed using an assay for bioamines secreted by the tumor.
Plasma chromogranin A is the preferred screening test because it is very sensitive12; however, it is not very specific. Several conditions (including essential hypertension, proton pump inhibitor use, chronic atrophic gastritis, heart failure, renal failure, and pheochromocytoma) can be associated with elevated levels of chromogranin A. Its specificity for diagnosing carcinoid tumors can be increased to about 84% by raising the cut-off value to 31 or 32 U/L. Its specificity can be increased to 95% by using 84 to 87 U/L as the cut-off value, but this comes at the cost of diminishing its sensitivity (from 75% to about 55%).13 Significantly elevated levels of chromogranin A (> 5,000 U/L) independently predict a poor prognosis.13
Urinary 5-HIAA levels also vary depending on the origin of the carcinoid tumor and are therefore useful in locating the site of the tumor. Foregut tumors lack the decarboxylase enzyme necessary to convert 5-hydroxytryptophan to serotonin, resulting in minimal to no elevation in urinary 5-HIAA levels. Midgut tumors secrete serotonin, resulting in high levels of urinary 5-HIAA. Hindgut tumors secrete neither 5-hydroxytryptophan nor serotonin and thus do not raise urinary 5-HIAA levels.
Other less sensitive markers include bradykinin, human chorionic gonadotropin, and neuropeptide PP.14
Provocative tests
Provocative tests such as the pentagastrin test can be considered if other screening test results are equivocal.12 Such testing must occur in a closely monitored, controlled environment, with intravenous somatostatin available in case of a “carcinoid crisis” (see discussion further below).
Patients with equivocal results from biochemical assays or provocative testing should be followed annually without further testing or evaluated for other causes of their symptoms. If the results of biochemical or provocative testing are abnormal, the tumor topography should be identified with an imaging study.
Imaging tests
Topographic localization of carcinoid tumors is done using various imaging tests, depending on the suspected site of the primary tumor.6
Somatostatin receptor scintigraphy, the preferred imaging test, is based on the principle that some carcinoid tumors have high concentrations of somatostatin receptors. Thus, a radiolabeled form of octreotide, a somatostatin analogue, is used to image these primary tumors and metastases.
Other imaging studies:
- Barium studies
- Computed tomography
- Endoscopy
- Endoscopic ultrasonography.
On imaging studies, in general, the tumor may appear as a smooth submucosal mass, as a target lesion (if it ulcerates), as wall-thickening, or as a cystic or calcified mass.6
MANAGEMENT IS MULTIDISCIPLINARY
Any patient with a carcinoid tumor should be referred to a medical and surgical oncologist once the diagnosis is established. Referral to additional specialists, such as a gastroenterologist or an interventional radiologist, is based on the location and extent of disease.
Surgery is the cornerstone of therapy
Drug therapy
Somatostatin analogues such as octreotide (Sandostatin) and lanreotide (Somatuline) are useful for treating carcinoid syndrome16 and also have a role in treating systemic metastases. These agents are effective in controlling flushing and diarrhea in 70% to 80% of patients.17 There are data, albeit limited, supporting a role for these agents in inhibiting tumor growth.18 They are the treatment of choice for carcinoid crisis (see discussion below). Important adverse effects include nausea, cramps, diarrhea; cholelithiasis; hypoglycemia or hyperglycemia; cardiac arrhythmias; and gastric atony.16
Interferon alfa is useful as an additive therapy when symptoms of carcinoid syndrome do not resolve with a somatostatin analogue alone. The addition of interferon alfa in these patients is useful for both symptom control (seen in 40% to 50% of those treated) and tumor stabilization (in 20% to 40% of those treated).19 However, it is still unclear whether combination drug therapy is more effective than a somatostatin analogue alone as the initial therapy for carcinoid syndrome.20 Adverse effects of interferon alfa include myelosuppression, depression, flu-like symptoms, and thyroid disturbances.
Supportive therapies
In patients with hepatic metastases who are not candidates for surgery, hepatic artery embolization with chemotherapy or particles can be used as palliative therapy. Severe adverse effects of this procedure include renal failure, hepatic failure, carcinoid crisis, and hepatic abscess and are seen in about 10% of patients.1
Conventional chemotherapy and molecularly targeted therapy are used for patients with rapidly progressive and widely metastatic disease. The precise role and efficacy of these therapies need further study.
Other supportive therapies for patients with carcinoid disease include dietary supplementation with vitamin B3, bronchodilators for bronchospasm, antidiarrheals, diuretics, and valve replacement for carcinoid heart disease.
LONG-TERM PROGNOSIS IS GENERALLY GOOD
The prognosis for patients with carcinoid tumors depends on the location and extent of disease. Tumors of the appendix and rectum have the best prognosis, with 5-year survival rates approaching 100% for localized carcinoid tumors of the appendix.3,21 In contrast, tumors of the small bowel, especially the ileum, are more aggressive and have the worst prognosis (a 5-year survival rate of about 60% to 65%).21
COMPLICATIONS
Other primary malignancies
Carcinoid tumors are often associated with the development of other tumors, not always in the gastrointestinal tract. As many as 52% of patients with small-bowel carcinoid tumors develop another primary tumor.22 This effect is thought to be related to the tumorigenic properties of the bioactive compounds secreted by carcinoid tumors. The other primary malignancy can be synchronous (diagnosed at the same time) or metachronous (diagnosed 1 to 7 years after the carcinoid) and generally arises from the gastrointestinal, genitourinary, or respiratory tract. Adenocarcinoma of the colon is reported as the most common second primary malignancy in patients with carcinoid tumors.22
The best strategy for surveillance in these patients is still unclear. Screening for tumors of the colon, small bowel, lung, cervix, and ovaries at the time of carcinoid tumor diagnosis followed by surveillance for these malignancies has been suggested as a possible approach; however, this comes at the cost of increased patient anxiety, cost, and morbidity from testing. 3
Carcinoid crisis
Carcinoid crisis is a life-threatening emergency caused by release of large amounts of vasoactive compounds from the carcinoid tumor, either spontaneously or provoked by tumor manipulation, surgery, chemotherapy, or hepatic artery embolization.2,5 The syndrome manifests as cardiovascular collapse (severe hypotension or hypertension), tachycardia, and altered mental status.
Treatment differs from those for other causes of shock in that catecholamines and calcium should not be used since they trigger the release of larger amounts of bioactive chemicals from the tumor. In addition, the shock in this situation is refractory to fluid. The mainstay of treatment is the infusion of octreotide and plasma. This crisis can be prevented by giving octreotide before manipulating the tumor.1,2,5
Carcinoid tumors, also known as gastroenteropancreatic neuroendocrine tumors, are neoplasms of neuroendocrine origin. Traditionally considered slow-growing, they are now known to vary in their aggressiveness. Many patients with carcinoid tumors have no symptoms or present with symptoms that have broad differential diagnoses. Unless the primary care physician suspects that the patient has a carcinoid tumor, the appropriate testing is seldom ordered. In addition, these patients are at higher risk of developing other cancers of the genitourinary, gastrointestinal, and respiratory tracts; this makes close follow-up, especially by the primary care provider, extremely important.
This article reviews the epidemiology, pathogenesis, clinical features, management, and prognosis of carcinoid tumors. A better knowledge of these tumors among physicians will facilitate recognition, early diagnosis, and improved outcomes for these patients.
INCIDENCE IS INCREASING
The overall incidence of carcinoid tumors has increased over the last 30 years, due at least in part to improvements in ways to diagnose them.1,2 The incidence over the last decade has been between 2.47 and 4.48 per 100,000 population, depending on race and sex, with the highest rates in black men.1,3
The small intestine is the most frequent location, followed by the lungs/bronchi, rectum, appendix, and stomach3; 67.5% of carcinoid tumors occur somewhere in the gastrointestinal system.
The cause of carcinoid tumors is unknown, but genetic factors may play a role, since these tumors often occur as part of genetic disorders such as multiple endocrine neoplasia type 1, von Hippel-Lindau disease, and neurofibromatosis type 1.1–3
SEROTONERGIC EFFECTS DEPEND ON LOCATION
Carcinoid tumors are classified on the basis of their embryologic origin:
- Foregut (lungs, bronchi, stomach)
- Midgut (small intestine, appendix, proximal large bowel)
- Hindgut (distal large bowel, rectum). Tumors from each of these origins differ clinically, biochemically, and histologically.
Carcinoid tumors secrete several bioactive compounds, including serotonin and bradykinin, and the secretory pattern varies depending on the location of the tumor. Most foregut carcinoid tumors secrete low levels of serotonin, being deficient in the enzyme needed to convert 5-hydroxytryptophan to serotonin. Midgut tumors secrete high levels of serotonin, whereas most hindgut tumors do not secrete 5-hydroxytryptophan or serotonin. These differences in secretory patterns are responsible for the different clinical manifestations and biochemical characteristics of these tumors.
Carcinoid syndrome
The systemic effects of the bioactive compounds secreted by carcinoid tumors are responsible for carcinoid syndrome, which has features that can include bronchospasm (possibly mediated by serotonin or bradykinin), diarrhea (likely mediated by serotonin), cutaneous flushing (which has multiple possible mediators), and right-sided valvular heart lesions (possibly mediated by serotonin).2,4 However, the secretory products of midgut carcinoids are normally inactivated by enzymes in the liver before they enter the systemic circulation. Thus, patients with midgut carcinoid tumors develop the carcinoid syndrome only if they have hepatic metastases.4
In contrast, patients with foregut (bronchial and extraintestinal) carcinoids can present with carcinoid syndrome without hepatic metastases, since their secretory products normally bypass the liver and enter the systemic circulation directly.
Hindgut tumors seldom produce this syndrome, since they do not secrete these products.
Fibrosis
Another effect of carcinoid tumors is desmoplasia, or fibrosis.
The pathogenesis of this phenomenon is poorly understood. Serotonin was thought to mediate this effect, but serotonin antagonists are ineffective in treating it, casting doubt on this hypothesis.2,4 More recently, growth factors such as transforming growth factor beta, platelet-derived growth factor, and basic fibroblast growth factor have been implicated.4
SYMPTOMS ARE OFTEN ABSENT OR NONSPECIFIC
Carcinoid tumors are often clinically silent. Signs and symptoms, when present, represent local effects of the tumor, tumor-induced fibrosis, biological effects of secreted products, or metastases.
Local effects can include abdominal pain, intestinal obstruction, appendicitis, rectal bleeding, and peptic ulcer disease, depending on the site of the tumor. Intestinal obstruction can be caused by intussusception, by the intraluminal effects of the tumor, or by adhesions due to tumor-induced fibrosis.7
Tumor-induced fibrosis can be retroperitoneal and have a myriad of manifestations, for example2,5:
- Hydronephrosis from ureteral obstruction
- Mesenteric ischemia from vascular trapping
- Peyronie disease (an acquired fibrosis of the penis that produces bending or pain of the erect penis).
Classic carcinoid syndrome, caused by the entry of bioactive compounds secreted by the tumor into the systemic circulation, occurs in fewer than 10% of patients with carcinoid tumors.2,6 Most patients who present with this syndrome have a midgut carcinoid tumor.
The hallmark is cutaneous flushing, which typically affects the face, neck, and upper body and lasts from 30 seconds to 30 minutes. This reaction can be unprovoked, but it is often precipitated by foods (eg, bananas, tomatoes, eggplant, kiwi, pineapple, cheese), by alcohol consumption, by exercise, by emotional stimuli, or by anesthesia.4,8 Other symptoms of this syndrome include bronchospasm, secretory diarrhea, and venous telangiectasia.
Carcinoid syndrome variants, caused by differences in secretory products, are seen in some patients with bronchial and gastric carcinoid tumors. Patients with gastric carcinoid variants have flushing that is pruritic and well demarcated, and they have an increased incidence of peptic ulcer. In contrast, flushing in patients with bronchial carcinoid variants can last days and is often associated with changes in mental status.2,9
Carcinoid heart disease refers to endocardial fibrotic plaques that occur in patients with carcinoid tumors, especially those with hepatic metastases. The right side of the heart is generally affected, as the left side is protected by inactivation of the bioactive compounds in the lungs. Tricuspid valve regurgitation is the most common finding, but tricuspid stenosis, pulmonary valve regurgitation, and pulmonary valve stenosis can also occur.10 Left-sided lesions can occur in patients with pulmonary metastases.
Hypoalbuminemia and pellagra. The amino acid tryptophan is a precursor of serotonin and niacin (vitamin B3). In patients with widely metastatic carcinoid tumors, increased conversion of tryptophan to serotonin by the tumor cells can lead to tryptophan deficiency and niacin deficiency.1,2 These manifest as hypoalbuminemia and pellagra (glossitis, scaly skin, and confusion).
IF YOU DON’T THINK ABOUT THEM, YOU WON’T LOOK FOR THEM
Many carcinoid tumors are discovered incidentally during surgery or diagnostic procedures. However, an evaluation for a carcinoid tumor is often prompted by symptoms that suggest the carcinoid syndrome. Of importance, since these symptoms can often be erroneously attributed to other, more common causes (Table 1), a very high level of suspicion is required on the part of the primary care physician to initiate the evaluation for carcinoid tumors.
When evaluating a patient who presents with flushing, the clinician should obtain a detailed history, noting the pattern of flushing, precipitating factors, duration, and associated symptoms, such as diarrhea and bronchospasm. 11
Often, patient recall can be improved by asking the patient to keep a symptom diary. If the diary does not offer any clues to the diagnosis, a trial of abstinence from foods and drugs that increase urinary 5-hydroxyindoleacetic acid (5-HIAA) should be attempted. If the flushing resolves, the foods and drugs can be reintroduced one at a time to identify the culprit.11 However, if symptoms persist, a more extensive workup should be undertaken, including screening for carcinoid tumor.
Biochemical assays
Biochemical screening for carcinoid tumors is performed using an assay for bioamines secreted by the tumor.
Plasma chromogranin A is the preferred screening test because it is very sensitive12; however, it is not very specific. Several conditions (including essential hypertension, proton pump inhibitor use, chronic atrophic gastritis, heart failure, renal failure, and pheochromocytoma) can be associated with elevated levels of chromogranin A. Its specificity for diagnosing carcinoid tumors can be increased to about 84% by raising the cut-off value to 31 or 32 U/L. Its specificity can be increased to 95% by using 84 to 87 U/L as the cut-off value, but this comes at the cost of diminishing its sensitivity (from 75% to about 55%).13 Significantly elevated levels of chromogranin A (> 5,000 U/L) independently predict a poor prognosis.13
Urinary 5-HIAA levels also vary depending on the origin of the carcinoid tumor and are therefore useful in locating the site of the tumor. Foregut tumors lack the decarboxylase enzyme necessary to convert 5-hydroxytryptophan to serotonin, resulting in minimal to no elevation in urinary 5-HIAA levels. Midgut tumors secrete serotonin, resulting in high levels of urinary 5-HIAA. Hindgut tumors secrete neither 5-hydroxytryptophan nor serotonin and thus do not raise urinary 5-HIAA levels.
Other less sensitive markers include bradykinin, human chorionic gonadotropin, and neuropeptide PP.14
Provocative tests
Provocative tests such as the pentagastrin test can be considered if other screening test results are equivocal.12 Such testing must occur in a closely monitored, controlled environment, with intravenous somatostatin available in case of a “carcinoid crisis” (see discussion further below).
Patients with equivocal results from biochemical assays or provocative testing should be followed annually without further testing or evaluated for other causes of their symptoms. If the results of biochemical or provocative testing are abnormal, the tumor topography should be identified with an imaging study.
Imaging tests
Topographic localization of carcinoid tumors is done using various imaging tests, depending on the suspected site of the primary tumor.6
Somatostatin receptor scintigraphy, the preferred imaging test, is based on the principle that some carcinoid tumors have high concentrations of somatostatin receptors. Thus, a radiolabeled form of octreotide, a somatostatin analogue, is used to image these primary tumors and metastases.
Other imaging studies:
- Barium studies
- Computed tomography
- Endoscopy
- Endoscopic ultrasonography.
On imaging studies, in general, the tumor may appear as a smooth submucosal mass, as a target lesion (if it ulcerates), as wall-thickening, or as a cystic or calcified mass.6
MANAGEMENT IS MULTIDISCIPLINARY
Any patient with a carcinoid tumor should be referred to a medical and surgical oncologist once the diagnosis is established. Referral to additional specialists, such as a gastroenterologist or an interventional radiologist, is based on the location and extent of disease.
Surgery is the cornerstone of therapy
Drug therapy
Somatostatin analogues such as octreotide (Sandostatin) and lanreotide (Somatuline) are useful for treating carcinoid syndrome16 and also have a role in treating systemic metastases. These agents are effective in controlling flushing and diarrhea in 70% to 80% of patients.17 There are data, albeit limited, supporting a role for these agents in inhibiting tumor growth.18 They are the treatment of choice for carcinoid crisis (see discussion below). Important adverse effects include nausea, cramps, diarrhea; cholelithiasis; hypoglycemia or hyperglycemia; cardiac arrhythmias; and gastric atony.16
Interferon alfa is useful as an additive therapy when symptoms of carcinoid syndrome do not resolve with a somatostatin analogue alone. The addition of interferon alfa in these patients is useful for both symptom control (seen in 40% to 50% of those treated) and tumor stabilization (in 20% to 40% of those treated).19 However, it is still unclear whether combination drug therapy is more effective than a somatostatin analogue alone as the initial therapy for carcinoid syndrome.20 Adverse effects of interferon alfa include myelosuppression, depression, flu-like symptoms, and thyroid disturbances.
Supportive therapies
In patients with hepatic metastases who are not candidates for surgery, hepatic artery embolization with chemotherapy or particles can be used as palliative therapy. Severe adverse effects of this procedure include renal failure, hepatic failure, carcinoid crisis, and hepatic abscess and are seen in about 10% of patients.1
Conventional chemotherapy and molecularly targeted therapy are used for patients with rapidly progressive and widely metastatic disease. The precise role and efficacy of these therapies need further study.
Other supportive therapies for patients with carcinoid disease include dietary supplementation with vitamin B3, bronchodilators for bronchospasm, antidiarrheals, diuretics, and valve replacement for carcinoid heart disease.
LONG-TERM PROGNOSIS IS GENERALLY GOOD
The prognosis for patients with carcinoid tumors depends on the location and extent of disease. Tumors of the appendix and rectum have the best prognosis, with 5-year survival rates approaching 100% for localized carcinoid tumors of the appendix.3,21 In contrast, tumors of the small bowel, especially the ileum, are more aggressive and have the worst prognosis (a 5-year survival rate of about 60% to 65%).21
COMPLICATIONS
Other primary malignancies
Carcinoid tumors are often associated with the development of other tumors, not always in the gastrointestinal tract. As many as 52% of patients with small-bowel carcinoid tumors develop another primary tumor.22 This effect is thought to be related to the tumorigenic properties of the bioactive compounds secreted by carcinoid tumors. The other primary malignancy can be synchronous (diagnosed at the same time) or metachronous (diagnosed 1 to 7 years after the carcinoid) and generally arises from the gastrointestinal, genitourinary, or respiratory tract. Adenocarcinoma of the colon is reported as the most common second primary malignancy in patients with carcinoid tumors.22
The best strategy for surveillance in these patients is still unclear. Screening for tumors of the colon, small bowel, lung, cervix, and ovaries at the time of carcinoid tumor diagnosis followed by surveillance for these malignancies has been suggested as a possible approach; however, this comes at the cost of increased patient anxiety, cost, and morbidity from testing. 3
Carcinoid crisis
Carcinoid crisis is a life-threatening emergency caused by release of large amounts of vasoactive compounds from the carcinoid tumor, either spontaneously or provoked by tumor manipulation, surgery, chemotherapy, or hepatic artery embolization.2,5 The syndrome manifests as cardiovascular collapse (severe hypotension or hypertension), tachycardia, and altered mental status.
Treatment differs from those for other causes of shock in that catecholamines and calcium should not be used since they trigger the release of larger amounts of bioactive chemicals from the tumor. In addition, the shock in this situation is refractory to fluid. The mainstay of treatment is the infusion of octreotide and plasma. This crisis can be prevented by giving octreotide before manipulating the tumor.1,2,5
- Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumors. Lancet Oncol 2008; 9:61–72.
- Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005; 128:1717–1751.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003; 97:934–959.
- DeVries H, Verschueren R, Willemse P, Kema I, DeVries E. Diagnostic, surgical, and medical aspect of midgut carcinoids. Cancer Treat Rev 2002; 28:11–25.
- Robertson RG, Griger WJ, Davis NB. Carcinoid tumors. Am Fam Physician 2006; 74:429–434.
- Horton KM, Kamel I, Hofman L, Fishman EK. Carcinoid tumors of the small bowel: a multitechnique imaging approach. AJR Am J Roentgenol 2004; 182:559–567.
- Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999; 340:858–868.
- Bendelow J, Apps E, Jones LE, Poston GJ. Carcinoid syndrome. Eur J Surg Oncol 2008; 34:289–296.
- Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001; 119:1647–1651.
- Bhattacharyya S, Toumpanakis C, Caplin ME, Davar J. Analysis of 150 patients with carcinoid syndrome seen in a single year at one institution in the first decade of the twenty-first century. Am J Cardiol 2008; 101:378–381.
- Nasr C. Flushing: is it carcinoid or something else?Cleveland Clinic Disease Management Project, 2004. Available at http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/endocrinology/flushing/flushing.htm. Accessed 10/25/08.
- Modlin IM, Tang LH. Approaches to the diagnosis of gut neuroendocrine tumors: the last word (today). Gastroenterology 1997; 112:583–590.
- Campana D, Nori F, Piscitelli L, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol 2007; 25:1967–1973.
- Eriksson B, Oberg K. Peptide hormones as tumor markers in neuroendocrine gastrointestinal tumors. Acta Oncol 1991; 30:477–483.
- National Comprehensive Cancer Network. Carcinoid tumors. http://www.nccn.org/professionals/physician_gls/PDF/neuroendocrine.pdf. Accessed 10/25/08.
- Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 2004; 15:966–973.
- Welin SV, Janson ET, Sundin A, et al. High-dose treatment with long-acting somatostatin analogue in patients with advanced midgut carcinoid tumours. Eur J Endocrinol 2004; 151:107–112.
- Arnold R, Trautmann ME, Creutzfeldt W, et al. Somatostatin analogue octreotide and inhibition of tumor growth in metastatic endocrine gastroenteropancreatic tumors. Gut 1996; 38:430–438.
- Bajetta E, Zilembo N, Di Bartolomeo M, et al. Treatment of metastatic carcinoids and other neuroendocrine tumors with recombinant interferon-alpha-2a. A study by the Italian Trials in Medical Oncology Group. Cancer 1993; 72:3099–3105.
- Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon-alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors—the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol 2003; 21:2689–2696.
- Helland SK, Prosch AM, Viste A. Carcinoid tumors in the gastrointestinal tract—a population-based study from Western Norway. Scand J Surg 2006; 95:158–161.
- Habal N, Sims C, Bilchik AJ. Gastrointestinal carcinoid tumors and second primary malignancies. J Surg Oncol 2000; 75:310–316.
- Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumors. Lancet Oncol 2008; 9:61–72.
- Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005; 128:1717–1751.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003; 97:934–959.
- DeVries H, Verschueren R, Willemse P, Kema I, DeVries E. Diagnostic, surgical, and medical aspect of midgut carcinoids. Cancer Treat Rev 2002; 28:11–25.
- Robertson RG, Griger WJ, Davis NB. Carcinoid tumors. Am Fam Physician 2006; 74:429–434.
- Horton KM, Kamel I, Hofman L, Fishman EK. Carcinoid tumors of the small bowel: a multitechnique imaging approach. AJR Am J Roentgenol 2004; 182:559–567.
- Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999; 340:858–868.
- Bendelow J, Apps E, Jones LE, Poston GJ. Carcinoid syndrome. Eur J Surg Oncol 2008; 34:289–296.
- Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001; 119:1647–1651.
- Bhattacharyya S, Toumpanakis C, Caplin ME, Davar J. Analysis of 150 patients with carcinoid syndrome seen in a single year at one institution in the first decade of the twenty-first century. Am J Cardiol 2008; 101:378–381.
- Nasr C. Flushing: is it carcinoid or something else?Cleveland Clinic Disease Management Project, 2004. Available at http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/endocrinology/flushing/flushing.htm. Accessed 10/25/08.
- Modlin IM, Tang LH. Approaches to the diagnosis of gut neuroendocrine tumors: the last word (today). Gastroenterology 1997; 112:583–590.
- Campana D, Nori F, Piscitelli L, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol 2007; 25:1967–1973.
- Eriksson B, Oberg K. Peptide hormones as tumor markers in neuroendocrine gastrointestinal tumors. Acta Oncol 1991; 30:477–483.
- National Comprehensive Cancer Network. Carcinoid tumors. http://www.nccn.org/professionals/physician_gls/PDF/neuroendocrine.pdf. Accessed 10/25/08.
- Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 2004; 15:966–973.
- Welin SV, Janson ET, Sundin A, et al. High-dose treatment with long-acting somatostatin analogue in patients with advanced midgut carcinoid tumours. Eur J Endocrinol 2004; 151:107–112.
- Arnold R, Trautmann ME, Creutzfeldt W, et al. Somatostatin analogue octreotide and inhibition of tumor growth in metastatic endocrine gastroenteropancreatic tumors. Gut 1996; 38:430–438.
- Bajetta E, Zilembo N, Di Bartolomeo M, et al. Treatment of metastatic carcinoids and other neuroendocrine tumors with recombinant interferon-alpha-2a. A study by the Italian Trials in Medical Oncology Group. Cancer 1993; 72:3099–3105.
- Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon-alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors—the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol 2003; 21:2689–2696.
- Helland SK, Prosch AM, Viste A. Carcinoid tumors in the gastrointestinal tract—a population-based study from Western Norway. Scand J Surg 2006; 95:158–161.
- Habal N, Sims C, Bilchik AJ. Gastrointestinal carcinoid tumors and second primary malignancies. J Surg Oncol 2000; 75:310–316.
KEY POINTS
- Bioactive compounds secreted by carcinoid tumors cause carcinoid syndrome—ie, bronchospasm, diarrhea, cutaneous flushing, and right-sided valvular heart lesions.
- Endocardial fibrotic plaques can occur in patients with carcinoid tumors. The right side of the heart is affected more often than the left, as the left side is protected by inactivation of the bioactive compounds in the lungs. Tricuspid valve regurgitation is the most common finding.
- Since carcinoid tumors have high concentrations of somatostatin receptors, octreotide scanning offers a distinct advantage in imaging them: use of radiolabeled octreotide, a somatostatin analogue, enables imaging of primary tumors and metastases.