User login
VTE Prevention Guidelines for Inpatients
Patients hospitalized for acute medical illness have more than a 10‐fold increased risk for venous thromboembolism (VTE),[1] with an undeniably dramatic, negative impact on the lives of those afflicted, including fatal pulmonary embolism (PE), which most commonly affects patients on the medical service.[2, 3, 4] Yet estimates for the overall rate of VTE in this population are relatively low, raising questions about which subsets of medical patients warrant the risk and cost of prophylaxis.
Recently, the American College of Physicians published guidelines (ACP‐1)[5] and a supporting review[6] addressing VTE prophylaxis in nonsurgical inpatients, followed by publication of the American College of Chest Physicians (ACCP) 9th Edition of the Chest Guidelines on Antithrombotic Therapy and Prevention of Thrombosis (AT9),[7] which divides VTE prevention into 3 articles,[8, 9, 10] including 1 on nonsurgical patients.[8] Both ACP‐1 and AT9 differ significantly from the 2008 ACCP guidelines (AT8),[11] but took different approaches to methodology, risk assessment, and several other aspects of thromboprophylaxis (Table 1). This narrative review summarizes and compares these recommendations and the methods used to arrive at them, with a final section focusing on implications for improvement teams designing order sets and system changes to address VTE prophylaxis.
| 2008 ACCP VTE Guideline AT8 | 2012 ACCP VTE Guideline AT9 | 2011 ACP Guideline | |
|---|---|---|---|
| |||
| Stance on asymptomatic VTE end points | Because of the strong concordance between asymptomatic DVT and clinically important VTE, we believe that DVT detected by a sensitive screening tesis an appropriate outcome in the early assessment of new thromboprophylaxis interventions. | Use of this surrogate (asymptomatic, screening‐detected thrombosis) creates major problems in making the trade‐off between patient‐important outcomes (thrombosis and serious bleeding). | Surrogate outcomes of asymptomatic screening detected‐thrombosis should not be used. |
| Who should be prophylaxed? | 6.0.0: For acutely ill medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, sepsis, neurologic disease, or inflammatory bowel disease, we recommend thromboprophylaxis with LMWH (1A), UFH (1A), or fondaparinux (1A). | 2.3: For acutely ill hospitalized medical patients at increased risk for thrombosis, we recommend anticoagulant thromboprophylaxis, with LMWH, UFH bid, UFH tid, or fondaparinux (1B). | ACP recommends pharmacologic prophylaxis with heparin or a related drug for venous thromboembolism in medical (including stroke) patients unless the assessed risk for bleeding outweighs the likely benefits (grade: strong recommendation, moderate‐quality evidence). |
| 2.4: For acutely ill hospitalized medical patients at low risk of thrombosis, we recommend against the use of pharmacologic or mechanical prophylaxis. (1B) | |||
| Choice of anticoagulant prophylaxis | There is no compelling evidence that UFH should be administered three times daily in preference to twice daily in medical patients, although these two regimens have never been directly compared. | In choosing the specific anticoagulant drug to be used for pharmacoprophylaxis, choices should be based on patient preference, compliance, and ease of administration (eg, daily vs bid vs tid dosing), as well as on local factors affecting acquisition costs. | [T]he choice of agent for prophylaxis of VTE should be based on ease of use, adverse effect profile, and cost of medication. |
| No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | |
| Mechanical prophylaxis | 1.4.3.1: We recommend that mechanical methods of prophylaxis be used primarily in patients at high risk of bleeding (grade 1A), or possibly as an adjunct to anticoagulant‐based thromboprophylaxis (grade 2A). | 2.7.2: For acutely ill hospitalized medical patients at increased risk of thrombosis who are bleeding or at high risk for major bleeding, we suggest the optimal use of mechanical thromboprophylaxis with GCS (grade 2C) or IPC (grade 2C). | ACP recommends against the use of mechanical prophylaxis with graduated compression stockings for prevention of venous thromboembolism (grade: strong recommendation, moderate‐quality evidence). |
| No strong evidence for IPC vs GCS | |||
| Duration | [T]he optimal duration of thromboprophylaxis remains unclear. To the end of hospitalization for most patients. | 2.8: [W]e suggest against extending the duration of thromboprophylaxis beyond the period of patient immobilization or acute hospital stay (2B). | The optimal duration of heparin prophylaxis is uncertain. |
| Risk Stratification | The approach of individual prophylaxis prescribing based on formal RAMs is not used routinely by most clinicians because it has not been adequately validated and is cumbersome. Individual RAMs may not be worth the effort, because there are only a limited number of thromboprophylaxis options, and one of the principles of effective thromboprophylaxis is to reduce complexity in decision making. | (Noncritical care) No formal risk assessment recommendation. Padua point‐based model is inherent in definitions of baseline VTE risk. | ACP does not support the application of performance measures in medical (including stroke) patients that promotes universal venous thromboembolism prophylaxis regardless of risk. |
| Another approach involves implementation of group‐specific thromboprophylaxis routinely for all patients who belong to each of the major target groups. We support this approach. | There are no validated risk assessment models to stratify VTE risk in critically ill patients. | Many risk assessment tools are available for estimating thromboembolism risk, but the current evidence is insufficient to recommend a validated tool. | |
| [T]he decision is best left to physician judgment, and performance measures targeting all patients are inappropriate. | |||
WHY ARE THE NEW GUIDELINES DIFFERENT?
Major randomized controlled trials (RCTs)[12, 13, 14] of thromboprophylaxis used routine deep vein thrombosis (DVT) surveillance and included both symptomatic (S‐VTE) and asymptomatic VTE (A‐VTE) end points. These studies consistently demonstrated 44% to 63% reductions in VTE without increases in major bleeding.[11] Because of the strong relationship between A‐VTE and S‐VTE outcomes, and a paucity of studies using only S‐VTE outcomes, AT8 judged that A‐VTE outcomes were valid to include, whereas the new guidelines reject the use of asymptomatic VTE end points.[5, 8, 15] To minimize financial and intellectual conflicts of interest, AT9 also used methodologists rather than VTE experts as topic editors, excluded conflicted experts from voting on recommendations, and attempted to estimate patient values and preferences.[15] As a result, AT9 makes fewer strong recommendations (182 1A recommendations in 2008, but only 29 in 2012), replacing them with weak suggestions.
WHAT DO THE NEW GUIDELINES RECOMMEND?
AT8 recommended anticoagulant prophylaxis for acutely ill medical inpatients with known risk factors, but did not recommend routine thromboprophylaxis. However, because of well‐known problems with underprophylaxis,[16, 17, 18, 19] particularly in medical patients, the low risk of bleeding, and difficulties with explicitly defining low‐risk patients, many discounted the need for VTE risk stratification.
Both new guidelines recommend prophylaxis for many nonsurgical patients, but discourage routine thromboprophylaxis for nonsurgical inpatients. AT9 specifically recommends against any thromboprophylaxis for low‐risk medical inpatients, implying that many nonsurgical, non‐critical care patients belong in this category, citing lower estimates of benefit, lower estimates of VTE risk, and potential bleeding risks.
The guidelines[5, 8] agree that, when indicated and absent contraindications, anticoagulant prophylaxis is preferred over mechanical prophylaxis, and agree there is insufficient evidence to recommend 1 anticoagulant over another.
For patients at risk of both VTE and bleeding, ACP‐1 states that intermittent pneumatic compression (IPC) devices are a reasonable option, given the evidence showing benefit in surgical patients. However, ACP‐1 recommends against graduated compression stockings (GCS) in nonsurgical patients based on a meta‐analysis dominated by the CLOTS‐1 (Clots in Legs Or sTockings after Stroke) trial, which found that thigh‐high GCS increased the risk of skin breakdown without reducing VTE[20] in immobilized stroke patients. AT9 does not recommend against GCS for patients facing bleeding and VTE risk. AT9 notes the hazards of generalizing results from stroke patients, and also considers the somewhat contradictory results from the CLOTS‐2 trial in stroke patients, which found a lower rate of VTE with thigh‐high GCS than with knee‐high GCS.[21] AT9 designates a recommendation of 2C for either IPC devices or thigh‐high GCS for those at VTE risk when anticoagulants are contraindicated.
Combination mechanical‐pharmacologic prophylaxis has proven superior in some surgical populations, and many hospitals use combined prophylaxis in high‐risk medical patients. However, combination prophylaxis has not been studied in this population. ACP‐1 does not comment on the practice; AT9 does not recommend for or against it. Institutions that use combination prophylaxis should be aware that although it may seem logical to extrapolate estimates of benefit seen in selected surgical patients, this is not a recommended practice.
RCTs for thromboprophylaxis in nonsurgical inpatients provided prophylaxis for 6 to 21 days. Neither ACP‐1 or AT9 recommend routinely extending prophylaxis beyond the hospital stay, citing an RCT[22] in which the benefit of extended duration low molecular weight heparin was limited to selected subsets of patients and offset by bleeding complications. AT9 suggests prophylaxis for 6 to 21 days, until full mobility is restored, or until dischargewhichever comes first.[8] However, we know of no study that establishes a mobility level at which prophylaxis can be safely discontinued, especially in inpatients with multiple risk factors.
ESTIMATING RISK AND BENEFIT OF PROPHYLAXIS AND LIMITATIONS OF METHODS
Calculating risk/benefit ratios for thromboprophylaxis requires estimates of baseline VTE and bleeding risks, and estimates of the impact of prophylaxis on those baseline risks. Methods to estimate the impact of prophylaxis on S‐VTE from studies relying on A‐VTE all have limitations, as acknowledged by the AT9 introduction.[15]
The ACP‐1 review found the only significant effect of prophylaxis on medical inpatients was a modest reduction in PE and a modest increase in total bleeding events, without effects on major bleeding, DVT, or mortality.[6] The authors summarized the findings as indicative of little or no net benefit for the medical population as a whole. The ACP‐1 review derives estimates of S‐VTE risk, bleeding, and mortality from control (baseline) and interventional arms of RCTs that used routine VTE screening, and included A‐VTE end points. The baseline risk of VTE could potentially be overestimated, because the populations in the trials are not representative of the entire medical population.
On the other hand, pooling trials with screening‐detected VTE to estimate S‐VTE outcomes is a questionable practice that may falsely lower estimates of VTE prophylaxis benefit. Screening‐detected VTE may be treated or declared a study end point before it becomes symptomatic. MEDENOX (Medical Patients With Enoxaparin) is an illustrative example.[12] The 263 placebo recipients suffered 37 A‐VTEs and 4 S‐VTEs. The 272 enoxaparin recipients suffered 17 A‐VTEs and 3 S‐VTEs. Patients at the highest risk of S‐VTE were counted as reaching an end point before they could develop symptoms; this happened more than twice as often in the placebo arm. This decreases both estimates of baseline VTE risk and the measured benefit of prophylaxis for S‐VTE. Screening could conceivably reduce measured effects on mortality as well, because patients begin VTE therapy earlier. Per ACP‐1, the estimated risk for DVT is lower than for PE, running counter to literature experience[8, 16] and raising issues of face validity. The ACP‐1 review accepts all original definitions of major bleeding, including a 2 g/dL drop in hemoglobin,[12] which commonly occurs without any bleeding or clinical consequence, and bleeding events were ascribed to heparins up to 120 days after randomization, long after they could have been responsible.
Previous meta‐analyses of thromboprophylaxis studies[23, 24] shared many of these same limitations, but did not ascribe bleeding complications to heparins for this extended duration, and had point estimates that suggested a larger impact from prophylaxis than ACP‐1. Dentali et al., for example, showed statistically significant impact on PE (relative risk [RR] 0.43), fatal PE (RR 0.38), and a nearly statistically significant large impact on DVT (RR 0.47, 95% confidence interval [CI]: 0.22‐1.00),[24] whereas ACP‐1 estimated a smaller significant impact on PE (RR 0.69), no significant difference in fatal PE, and a much smaller estimate of the impact on DVT (RR 0.78, 95% CI: 0.45‐1.35) (Table 2).
| Baseline Risk | Relative Effect (95% CI) | Absolute Effect per 1000 Patients Treated (95% CI) | |
|---|---|---|---|
| |||
| ACP guideline review (Lederle), UFH or LMWH vs placebo/no treatment, medical patients | |||
| Mortality | 6.6 | OR 0.94 (0.84‐1.04) | 4 fewer (11 fewer to 3 more) |
| Major bleeding | 0.25 | OR 1.49 (0.91‐2.43) | 1 more (no effect to 3 more) |
| Symptomatic DVT | 0.96 | OR 0.78 (0.45‐1.35) | 2 fewer (6 fewer to 4 more) |
| PE | 1.2 | OR 0.69 (0.52‐0.90) | 4 fewer (6 fewer to 1 fewer) |
| Fatal PE | 0.30 | OR 0.77 (0.43‐1.37) | 1 fewer (2 fewer to 1 more) |
| ACCP AT9 (Kahn), non‐critical care medical inpatients, anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Mortality | 4.5 | OR 0.97 (0.79‐1.19) | 1 fewer (9 fewer to 8 more) |
| Major bleeding | 0.40 | OR 1.32 (0.73‐2.37) | 1 more (1 fewer to 6 more) |
| Thrombocytopenia | 0.13 | OR 0.92 (0.54‐1.53) | 1 fewer (6 fewer to 7 more) |
| Symptomatic DVT | |||
| Padua score 4 | 0.2 | RR 0.47 (0.22‐1) | 1 fewer (1 fewer to no effect) |
| Padua score 4 | 6.7 | 34 fewer (51 fewer to no effect) | |
| ACCP AT9 (Kahn) non‐critical care medical inpatients, Anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Nonfatal PE | |||
| Padua score 4 | 0.2 | RR 0.61 (0.23‐1.67) | 1 fewer (1 fewer to 1 more) |
| Padua score 4 | 3.9 | 15 fewer (30 fewer to 36 more) | |
| Fatal PE | 0.4 | RR 0.41 (0.22‐0.76) | 2 fewer (1 fewer to 3 fewer) |
| ACCP AT9 (Kahn), critical care medical inpatients, any heparin (LMWH, UFH) vs placebo/no treatment) | |||
| Mortality | 9.4 | RR 1.01 (0.04‐2.57) | 1 more (56 fewer to 148 more) |
| Major bleeding | 2.7 | RR 2.09 (0.54‐8.16) | 29 more (12 fewer to 190 more) |
| Symptomatic DVT | 5.8 | RR 0.86 (0.59‐1.25) | 4 fewer (12 fewer to 8 more) |
| Pulmonary embolus | 4.2 | RR, 0.73 (0.26‐2.11) | 11 fewer (31 fewer to 47 more) |
AT9 used a variety of methods to estimate each component of the risk/benefit equation. Critical care and non‐critical care estimates were generated independently, but because of limited data, the critical care estimates were highly imprecise. In non‐critical care patients, as in ACP‐1, treatment effects were estimated from RCTs that routinely screened for A‐VTE, and they adapted the Dentali et al. estimate of DVT risk reduction. The baseline risk for bleeding and mortality were derived from the control population of the same meta‐analysis.[24]
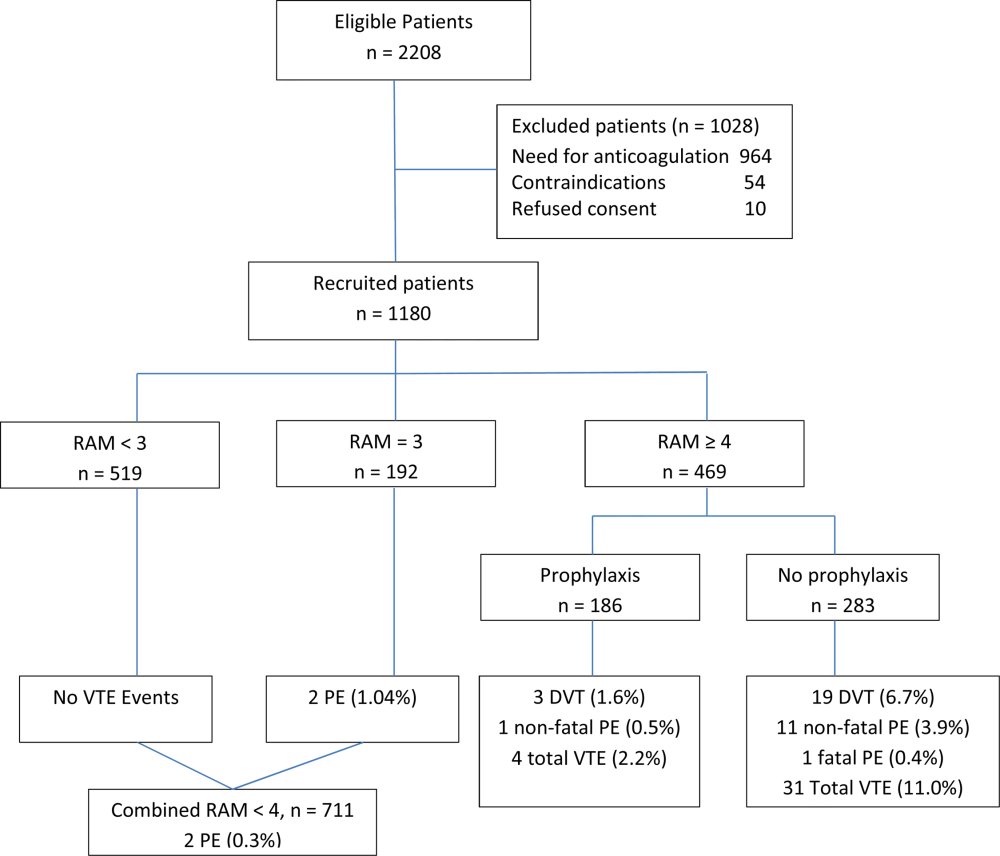
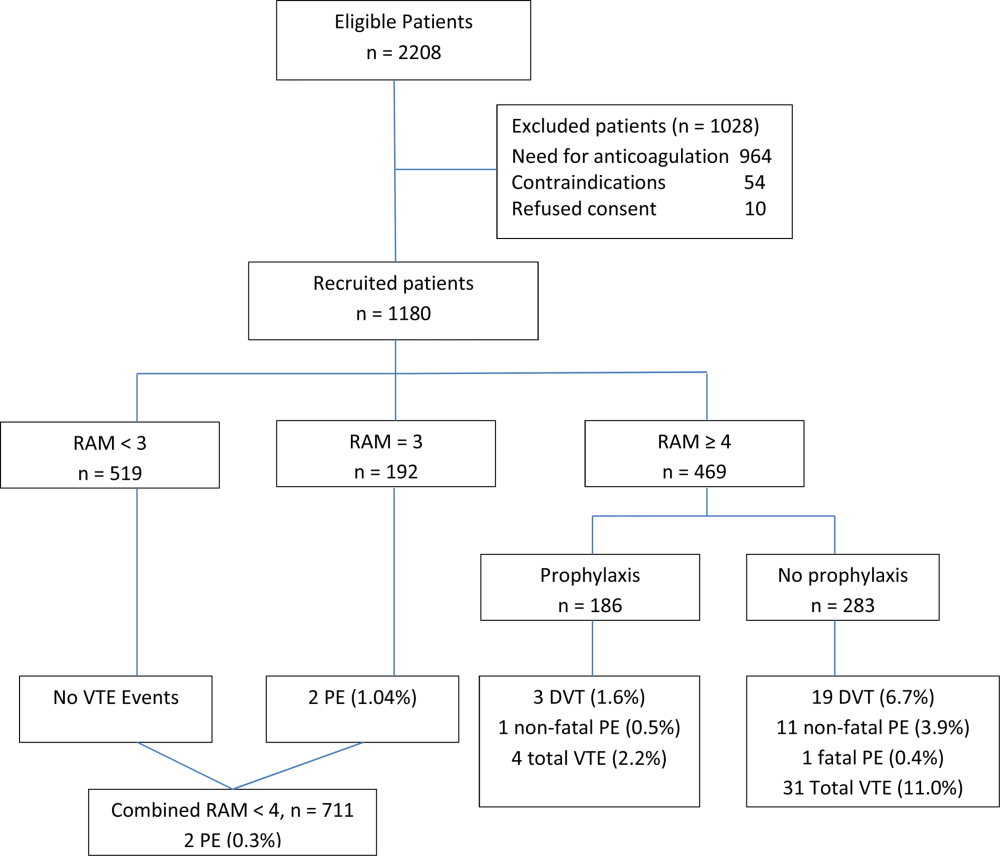
Using a novel approach, AT9 estimated baseline nonsurgical VTE risk from a prospective observational cohort study of 1180 medical inpatients divided into high‐ and low‐risk groups by a point‐scoring system.[25] Deriving risk estimates from an observational cohort has theoretical advantages. Many patients did not receive prophylaxis, allowing for unadjusted risk estimates; they represented a cross‐section of medical inpatients rather than a selected trial population, and risk estimates were not reduced by the culling of screen‐detected A‐VTE.
The Padua risk‐assessment model (RAM) (Table 3) defines high VTE risk as a cumulative score 4. There were 60.3% of patients at low risk and 39.7% at high risk using this threshold. Among unprophylaxed patients, VTE occurred in 11% of high‐risk patients versus 0.3% of low‐risk patients (hazard ratio 32.0, 95% CI: 4.1251.0).
| Baseline Features | Score |
|---|---|
| |
| Active cancer* | 3 |
| Previous VTE (excluding superficial thrombosis) | 3 |
| Reduced mobility | 3 |
| Already known thrombophilic condition | 3 |
| Recent (1 month) trauma and/or surgery | 2 |
| Elderly age (70 years) | 1 |
| Heart and/or respiratory failure | 1 |
| Acute myocardial infarction or stroke | 1 |
| Acute infection and/or rheumatologic disorder | 1 |
| Obesity (BMI 30) | 1 |
| Ongoing hormonal treatment | 1 |
PADUA: A CLOSER LOOK
In the Padua study, 60% of the population appeared to be at such low risk for VTE that prophylaxis would seem unnecessary, but closer scrutiny should raise concern about generalizing these results. Of the 711 low‐risk patients, 1% were immobile, only 6% had cancer, 6% were obese, and only 12% had any acute infection or inflammatory condition, yet their mean length of stay was 7.9 days. These characteristics do not apply to 60% of American inpatients. Furthermore, 964 of 2208 eligible patients (44%) were excluded because they required therapeutic anticoagulation.[25]
Correspondence with the authors revealed that the 2 PEs in patients with Padua scores 4 occurred among 192 patients with a risk score of 3 (Figure 1), a 1% (2/192) risk of PE. This is a very small sample, and the true risk of VTE for medical inpatients with a risk score of 3 may be lower or significantly higher. In the Padua population, a risk score of 3 equated to a VTE risk of 6.9%, whereas those with a score of 0 to 2 had no VTE. For those adapting the Padua model, careful consideration of using a cutoff of 3, versus 4, is warranted.
IMPLICATIONS FOR VTE PROTOCOL IMPLEMENTATION AND IMPROVEMENT TEAMS
AT9 and ACP‐1 sought to focus on S‐VTE, remove bias from recommendations, and highlight potential risks of unnecessary prophylaxis in low‐risk patients. They have largely succeeded in these important goals. However, the complexity of the new guidelines and lack of consensus about VTE risk assessment pose significant challenges to improvement teams tasked with implementing the guidelines in real‐world settings.
CHOOSING A VTE RAM
The fundamental question is: How can hospitals assess VTE risk, assure adequate prophylaxis for patients who need it, while minimizing excess prophylaxis, in a practical, efficient way?
Approach 1: Opt Out Approach
Both guidelines discourage universal prophylaxis for inpatients without contraindications unless the physician opts out. Although the simplicity of this approach is appealing, the low rate of VTE in a substantial segment of the medical inpatient population and known risks of thromboprophylaxis make this strategy suboptimal.
Approach 2: No VTE RAM
ACP‐1 notes that evidence is not sufficient to recommend 1 RAM over another, and essentially advises leaving prophylaxis decisions up to an individual physician's judgment. Although the evidence may not prove which system is best, prophylaxis reliability is dismal when there is no system or when hospitals offer prophylaxis options without guidance.[26, 27] Widespread, well‐documented underprophylaxis[16, 17, 18, 19] is largely the result of relying on unguided physician judgment and relatively passive interventions like educational sessions and pocket cards.[8] This approach also deprives improvement teams of standard definitions of VTE risk, bleeding risk, and adequate prophylaxis necessary to measure and improve VTE prophylaxis. Because of significant gray areas in the literature and varied infrastructure, institutions will not implement identical VTE prevention programs, but institutional standardization remains a cornerstone of improvement.
Approach 3: Buckets of Risk
The AT8 approach to risk assessment was to place patients into VTE risk groups described in the text, rather than have an individualized point‐scoring system.[11] These assessments can be made in seconds with high levels of interobserver agreement, implemented without undue effort, and spur high levels of compliance.[28, 29] Most importantly, implementation was associated with a 40% reduction in hospital‐associated VTE (RR 0.69, 95% CI: 0.470.79) without detectable increases in bleeding or heparin‐induced thrombocytopenia. Although this strategy has not been tested in randomized trials, it has been replicated in multiple real‐world settings that avoid concerns about generalizability due to imperfect trial populations.[28, 30]
The most popular bucket model in common use, derived from a table in the AT8 guidelines, is similar to models presented in UK National Institute for Health and Care Excellence guidelines for medical inpatients.[31] These models are potentially less precise than point‐based systems, but offer simplicity, ease of use, and improved physician acceptance, and thus may be more effective than point‐based models in settings without advanced clinical decision support. The models are flexible to reflect greater or lesser degrees of aggressiveness in defining risk categories, and can be used to approximate some point‐based systems.
Approach 4: Individualized Point‐Based RAM
AT9 authors used the Padua VTE RAM to define low‐ and high‐risk patients for VTE in their recommendation for medical inpatients. The Padua model appears relatively simple, but it does require calculations, and there is a paucity of data for implementation experience with it. As mentioned above, if teams use the Padua model, the optimal cutoff (3 vs 4) for recommending prophylaxis is uncertain, and both should be considered.
The Caprini point‐based system is not mentioned in the guideline for thromboprophylaxis in medical inpatients, but in our collaborative improvement experience, it is perhaps the most commonly used point‐based model for medical inpatients.[28, 30] It is also embedded in AT9 recommendations for prophylaxis in the nonorthopedic surgical population,[9] and thus is tempting to use for both medical and surgical patients. There are several caveats to those considering the use of these more complex point‐based models. Complex point‐based RAM suffer from poor interobserver agreement.[32] They have also had limited ability to exclude low‐risk patients from prophylaxis in validation studies,[33] and have not been tested extensively in medical populations. Although AT9 considers the Caprini RAM relatively easy to use,[9] our experience in collaboratives suggests that for many hospitals, the model is too complex to be used reliably.[28, 30] Clinicians often simply bypass the clinical decision support offered in the tool, rather than checking off all risk factors, adding up the point total, and identifying the appropriate prophylaxis choices based on the point total.[28] Other point‐based RAM (reviewed elsewhere[34, 35, 36]) pose similar implementation challenges.
On the other hand, centers with more sophisticated clinical decision support and a strong improvement framework can overcome some of these challenges to get good results with complex point‐based models. A forcing function can ensure that practitioners complete all risk‐assessment tasks. Providers can check off the VTE risk factors and bleeding risk factors on 1 screen, and several factors like age, body mass index, and renal function can be autopopulated. Instead of asking the provider to add up points, the combination of answers checked off on the first screen can drive behind‐the‐scenes calculations and seamlessly lead providers to prophylaxis choices appropriate for that combination of VTE and bleeding risks. Customized models can be designed for a wide variety of services. Similar strategies can ease adaption with more complex qualitative models as well.[37]
BOTTOM LINE IN CHOOSING A VTE RAM
Many medical inpatients are at high risk for VTE, but others are not at sufficient risk to warrant prophylaxis. VTE risk assessment should be embedded in admission, transfer, and perioperative order sets and may need a hard stop to insure completion. There is a trend to favor individualized point‐based models over models that place patients in groups of risk, but evidence is insufficient to recommend 1 type of RAM over another, and more complex point‐based models often require extensive local customization and algorithmic clinical decision support to effectively implement them. Centers without advanced capability may find the bucket models more effective. We urge improvement teams to trial their RAM with common patient case scenarios, and to make a choice based on an effort‐benefit analysis, feedback from their clinicians, and the level of customization in clinical decision support available to them.
OTHER IMPLEMENTATION STRATEGIES
VTE and bleeding risk change during hospitalization. We have used ongoing daily surveillance and measurement of patients on no prophylaxis to prompt concurrent intervention (ie, measure‐vention) to increase prophylaxis for patients at risk.[28] Improvement teams should focus not only on increasing prophylaxis for those at risk, but should also use measure‐vention, checklists, or other techniques to identify low‐risk (eg, ambulating) patients for cessation of overly aggressive prophylaxis. Efforts to improve early progressive ambulation, limit central venous catheters to those who truly need them, and improve adherence to mechanical prophylaxis can also reduce VTE, as well as benefitting patient populations in other ways.
We recognize there are several approaches to close the implementation gap in delivering thromboprophylaxis judiciously but reliably, and encourage research and publication of varied strategies. Last, we hope efforts to limit unnecessary prophylaxis and challenges inherent in implementing new and complex guidelines do not increase the morbidity and mortality of hospital‐acquired VTE, by derailing the delivery of prophylaxis to those in whom the benefits outweigh the risks.
Disclosures: Dr. Merli has conducted research for Johnson & Johnson, Bristol Myers Squibb, and Portala Scientific and has been a consultant for Johnson & Johnson and Bristol Myers Squibb.
- US Department of Health and Human Services. Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. Available at: http://www.surgeongeneral.gov/topics/deepvein/index.html. Accessed January 29, 2013.
- , , , et al. Incidence of venous thromboembolism in hospitalized patients vs. community residents. Mayo Clin Proc. 2001;76:1102–1110.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , . New onset of venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118(6):1680–1684.
- , , , , . venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625–632.
- , , , . Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2 suppl):e227S–e277S.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- , , , et al. The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry. J Thromb Haemost. 2004;2:1892–1898.
- , , , et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936–945.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , , , et al.; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103(4):736–748.
- , , , et al; CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomized controlled trial. Lancet. 2009;373(9679):1958–1965.
- CLOTS (Clots in Legs Or sTockings after Stroke) Trial Collaboration. Thigh‐length versus below‐knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153(9):553–562.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167(1)476–486.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007; 46(4):278–288.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , . Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes. J Hosp Med. 2009;4(2):81–89.
- . Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? J Hosp Med. 2009;4(2):77–80.
- , . Designing and implementing effective VTE prevention protocols: lessons from collaboratives. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Optimizing prevention of hospital acquired venous thromboembolism: prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10–18.
- , , , et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301–310.
- NHS National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. NICE Clinical Guideline 92. 2010. Available at: http://www.nice.org.uk/guidance/CG92. Accessed April 18, 2013.
- , , , , . Reliability of a point‐based VTE risk assessment tool in the hands of medical residents. J Hosp Med. 2011;6:195–201.
- , , , , , . A validation of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344–350.
- , , , . Risk assessment models for thromboprophylaxis of medical patients. Thromb Res. 2012;129:127–132.
- , , , , . Risk‐assessment models for predicting venous thromboembolism among hospitalized non‐surgical patients: a systematic review. J Thromb Thrombolysis. 2013;35:67–80.
- , , . The use of weighted and scored risk assessment models for venous thromboembolism. Thromb Haemost. 2012;108(6):1072–1076.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
Patients hospitalized for acute medical illness have more than a 10‐fold increased risk for venous thromboembolism (VTE),[1] with an undeniably dramatic, negative impact on the lives of those afflicted, including fatal pulmonary embolism (PE), which most commonly affects patients on the medical service.[2, 3, 4] Yet estimates for the overall rate of VTE in this population are relatively low, raising questions about which subsets of medical patients warrant the risk and cost of prophylaxis.
Recently, the American College of Physicians published guidelines (ACP‐1)[5] and a supporting review[6] addressing VTE prophylaxis in nonsurgical inpatients, followed by publication of the American College of Chest Physicians (ACCP) 9th Edition of the Chest Guidelines on Antithrombotic Therapy and Prevention of Thrombosis (AT9),[7] which divides VTE prevention into 3 articles,[8, 9, 10] including 1 on nonsurgical patients.[8] Both ACP‐1 and AT9 differ significantly from the 2008 ACCP guidelines (AT8),[11] but took different approaches to methodology, risk assessment, and several other aspects of thromboprophylaxis (Table 1). This narrative review summarizes and compares these recommendations and the methods used to arrive at them, with a final section focusing on implications for improvement teams designing order sets and system changes to address VTE prophylaxis.
| 2008 ACCP VTE Guideline AT8 | 2012 ACCP VTE Guideline AT9 | 2011 ACP Guideline | |
|---|---|---|---|
| |||
| Stance on asymptomatic VTE end points | Because of the strong concordance between asymptomatic DVT and clinically important VTE, we believe that DVT detected by a sensitive screening tesis an appropriate outcome in the early assessment of new thromboprophylaxis interventions. | Use of this surrogate (asymptomatic, screening‐detected thrombosis) creates major problems in making the trade‐off between patient‐important outcomes (thrombosis and serious bleeding). | Surrogate outcomes of asymptomatic screening detected‐thrombosis should not be used. |
| Who should be prophylaxed? | 6.0.0: For acutely ill medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, sepsis, neurologic disease, or inflammatory bowel disease, we recommend thromboprophylaxis with LMWH (1A), UFH (1A), or fondaparinux (1A). | 2.3: For acutely ill hospitalized medical patients at increased risk for thrombosis, we recommend anticoagulant thromboprophylaxis, with LMWH, UFH bid, UFH tid, or fondaparinux (1B). | ACP recommends pharmacologic prophylaxis with heparin or a related drug for venous thromboembolism in medical (including stroke) patients unless the assessed risk for bleeding outweighs the likely benefits (grade: strong recommendation, moderate‐quality evidence). |
| 2.4: For acutely ill hospitalized medical patients at low risk of thrombosis, we recommend against the use of pharmacologic or mechanical prophylaxis. (1B) | |||
| Choice of anticoagulant prophylaxis | There is no compelling evidence that UFH should be administered three times daily in preference to twice daily in medical patients, although these two regimens have never been directly compared. | In choosing the specific anticoagulant drug to be used for pharmacoprophylaxis, choices should be based on patient preference, compliance, and ease of administration (eg, daily vs bid vs tid dosing), as well as on local factors affecting acquisition costs. | [T]he choice of agent for prophylaxis of VTE should be based on ease of use, adverse effect profile, and cost of medication. |
| No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | |
| Mechanical prophylaxis | 1.4.3.1: We recommend that mechanical methods of prophylaxis be used primarily in patients at high risk of bleeding (grade 1A), or possibly as an adjunct to anticoagulant‐based thromboprophylaxis (grade 2A). | 2.7.2: For acutely ill hospitalized medical patients at increased risk of thrombosis who are bleeding or at high risk for major bleeding, we suggest the optimal use of mechanical thromboprophylaxis with GCS (grade 2C) or IPC (grade 2C). | ACP recommends against the use of mechanical prophylaxis with graduated compression stockings for prevention of venous thromboembolism (grade: strong recommendation, moderate‐quality evidence). |
| No strong evidence for IPC vs GCS | |||
| Duration | [T]he optimal duration of thromboprophylaxis remains unclear. To the end of hospitalization for most patients. | 2.8: [W]e suggest against extending the duration of thromboprophylaxis beyond the period of patient immobilization or acute hospital stay (2B). | The optimal duration of heparin prophylaxis is uncertain. |
| Risk Stratification | The approach of individual prophylaxis prescribing based on formal RAMs is not used routinely by most clinicians because it has not been adequately validated and is cumbersome. Individual RAMs may not be worth the effort, because there are only a limited number of thromboprophylaxis options, and one of the principles of effective thromboprophylaxis is to reduce complexity in decision making. | (Noncritical care) No formal risk assessment recommendation. Padua point‐based model is inherent in definitions of baseline VTE risk. | ACP does not support the application of performance measures in medical (including stroke) patients that promotes universal venous thromboembolism prophylaxis regardless of risk. |
| Another approach involves implementation of group‐specific thromboprophylaxis routinely for all patients who belong to each of the major target groups. We support this approach. | There are no validated risk assessment models to stratify VTE risk in critically ill patients. | Many risk assessment tools are available for estimating thromboembolism risk, but the current evidence is insufficient to recommend a validated tool. | |
| [T]he decision is best left to physician judgment, and performance measures targeting all patients are inappropriate. | |||
WHY ARE THE NEW GUIDELINES DIFFERENT?
Major randomized controlled trials (RCTs)[12, 13, 14] of thromboprophylaxis used routine deep vein thrombosis (DVT) surveillance and included both symptomatic (S‐VTE) and asymptomatic VTE (A‐VTE) end points. These studies consistently demonstrated 44% to 63% reductions in VTE without increases in major bleeding.[11] Because of the strong relationship between A‐VTE and S‐VTE outcomes, and a paucity of studies using only S‐VTE outcomes, AT8 judged that A‐VTE outcomes were valid to include, whereas the new guidelines reject the use of asymptomatic VTE end points.[5, 8, 15] To minimize financial and intellectual conflicts of interest, AT9 also used methodologists rather than VTE experts as topic editors, excluded conflicted experts from voting on recommendations, and attempted to estimate patient values and preferences.[15] As a result, AT9 makes fewer strong recommendations (182 1A recommendations in 2008, but only 29 in 2012), replacing them with weak suggestions.
WHAT DO THE NEW GUIDELINES RECOMMEND?
AT8 recommended anticoagulant prophylaxis for acutely ill medical inpatients with known risk factors, but did not recommend routine thromboprophylaxis. However, because of well‐known problems with underprophylaxis,[16, 17, 18, 19] particularly in medical patients, the low risk of bleeding, and difficulties with explicitly defining low‐risk patients, many discounted the need for VTE risk stratification.
Both new guidelines recommend prophylaxis for many nonsurgical patients, but discourage routine thromboprophylaxis for nonsurgical inpatients. AT9 specifically recommends against any thromboprophylaxis for low‐risk medical inpatients, implying that many nonsurgical, non‐critical care patients belong in this category, citing lower estimates of benefit, lower estimates of VTE risk, and potential bleeding risks.
The guidelines[5, 8] agree that, when indicated and absent contraindications, anticoagulant prophylaxis is preferred over mechanical prophylaxis, and agree there is insufficient evidence to recommend 1 anticoagulant over another.
For patients at risk of both VTE and bleeding, ACP‐1 states that intermittent pneumatic compression (IPC) devices are a reasonable option, given the evidence showing benefit in surgical patients. However, ACP‐1 recommends against graduated compression stockings (GCS) in nonsurgical patients based on a meta‐analysis dominated by the CLOTS‐1 (Clots in Legs Or sTockings after Stroke) trial, which found that thigh‐high GCS increased the risk of skin breakdown without reducing VTE[20] in immobilized stroke patients. AT9 does not recommend against GCS for patients facing bleeding and VTE risk. AT9 notes the hazards of generalizing results from stroke patients, and also considers the somewhat contradictory results from the CLOTS‐2 trial in stroke patients, which found a lower rate of VTE with thigh‐high GCS than with knee‐high GCS.[21] AT9 designates a recommendation of 2C for either IPC devices or thigh‐high GCS for those at VTE risk when anticoagulants are contraindicated.
Combination mechanical‐pharmacologic prophylaxis has proven superior in some surgical populations, and many hospitals use combined prophylaxis in high‐risk medical patients. However, combination prophylaxis has not been studied in this population. ACP‐1 does not comment on the practice; AT9 does not recommend for or against it. Institutions that use combination prophylaxis should be aware that although it may seem logical to extrapolate estimates of benefit seen in selected surgical patients, this is not a recommended practice.
RCTs for thromboprophylaxis in nonsurgical inpatients provided prophylaxis for 6 to 21 days. Neither ACP‐1 or AT9 recommend routinely extending prophylaxis beyond the hospital stay, citing an RCT[22] in which the benefit of extended duration low molecular weight heparin was limited to selected subsets of patients and offset by bleeding complications. AT9 suggests prophylaxis for 6 to 21 days, until full mobility is restored, or until dischargewhichever comes first.[8] However, we know of no study that establishes a mobility level at which prophylaxis can be safely discontinued, especially in inpatients with multiple risk factors.
ESTIMATING RISK AND BENEFIT OF PROPHYLAXIS AND LIMITATIONS OF METHODS
Calculating risk/benefit ratios for thromboprophylaxis requires estimates of baseline VTE and bleeding risks, and estimates of the impact of prophylaxis on those baseline risks. Methods to estimate the impact of prophylaxis on S‐VTE from studies relying on A‐VTE all have limitations, as acknowledged by the AT9 introduction.[15]
The ACP‐1 review found the only significant effect of prophylaxis on medical inpatients was a modest reduction in PE and a modest increase in total bleeding events, without effects on major bleeding, DVT, or mortality.[6] The authors summarized the findings as indicative of little or no net benefit for the medical population as a whole. The ACP‐1 review derives estimates of S‐VTE risk, bleeding, and mortality from control (baseline) and interventional arms of RCTs that used routine VTE screening, and included A‐VTE end points. The baseline risk of VTE could potentially be overestimated, because the populations in the trials are not representative of the entire medical population.
On the other hand, pooling trials with screening‐detected VTE to estimate S‐VTE outcomes is a questionable practice that may falsely lower estimates of VTE prophylaxis benefit. Screening‐detected VTE may be treated or declared a study end point before it becomes symptomatic. MEDENOX (Medical Patients With Enoxaparin) is an illustrative example.[12] The 263 placebo recipients suffered 37 A‐VTEs and 4 S‐VTEs. The 272 enoxaparin recipients suffered 17 A‐VTEs and 3 S‐VTEs. Patients at the highest risk of S‐VTE were counted as reaching an end point before they could develop symptoms; this happened more than twice as often in the placebo arm. This decreases both estimates of baseline VTE risk and the measured benefit of prophylaxis for S‐VTE. Screening could conceivably reduce measured effects on mortality as well, because patients begin VTE therapy earlier. Per ACP‐1, the estimated risk for DVT is lower than for PE, running counter to literature experience[8, 16] and raising issues of face validity. The ACP‐1 review accepts all original definitions of major bleeding, including a 2 g/dL drop in hemoglobin,[12] which commonly occurs without any bleeding or clinical consequence, and bleeding events were ascribed to heparins up to 120 days after randomization, long after they could have been responsible.
Previous meta‐analyses of thromboprophylaxis studies[23, 24] shared many of these same limitations, but did not ascribe bleeding complications to heparins for this extended duration, and had point estimates that suggested a larger impact from prophylaxis than ACP‐1. Dentali et al., for example, showed statistically significant impact on PE (relative risk [RR] 0.43), fatal PE (RR 0.38), and a nearly statistically significant large impact on DVT (RR 0.47, 95% confidence interval [CI]: 0.22‐1.00),[24] whereas ACP‐1 estimated a smaller significant impact on PE (RR 0.69), no significant difference in fatal PE, and a much smaller estimate of the impact on DVT (RR 0.78, 95% CI: 0.45‐1.35) (Table 2).
| Baseline Risk | Relative Effect (95% CI) | Absolute Effect per 1000 Patients Treated (95% CI) | |
|---|---|---|---|
| |||
| ACP guideline review (Lederle), UFH or LMWH vs placebo/no treatment, medical patients | |||
| Mortality | 6.6 | OR 0.94 (0.84‐1.04) | 4 fewer (11 fewer to 3 more) |
| Major bleeding | 0.25 | OR 1.49 (0.91‐2.43) | 1 more (no effect to 3 more) |
| Symptomatic DVT | 0.96 | OR 0.78 (0.45‐1.35) | 2 fewer (6 fewer to 4 more) |
| PE | 1.2 | OR 0.69 (0.52‐0.90) | 4 fewer (6 fewer to 1 fewer) |
| Fatal PE | 0.30 | OR 0.77 (0.43‐1.37) | 1 fewer (2 fewer to 1 more) |
| ACCP AT9 (Kahn), non‐critical care medical inpatients, anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Mortality | 4.5 | OR 0.97 (0.79‐1.19) | 1 fewer (9 fewer to 8 more) |
| Major bleeding | 0.40 | OR 1.32 (0.73‐2.37) | 1 more (1 fewer to 6 more) |
| Thrombocytopenia | 0.13 | OR 0.92 (0.54‐1.53) | 1 fewer (6 fewer to 7 more) |
| Symptomatic DVT | |||
| Padua score 4 | 0.2 | RR 0.47 (0.22‐1) | 1 fewer (1 fewer to no effect) |
| Padua score 4 | 6.7 | 34 fewer (51 fewer to no effect) | |
| ACCP AT9 (Kahn) non‐critical care medical inpatients, Anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Nonfatal PE | |||
| Padua score 4 | 0.2 | RR 0.61 (0.23‐1.67) | 1 fewer (1 fewer to 1 more) |
| Padua score 4 | 3.9 | 15 fewer (30 fewer to 36 more) | |
| Fatal PE | 0.4 | RR 0.41 (0.22‐0.76) | 2 fewer (1 fewer to 3 fewer) |
| ACCP AT9 (Kahn), critical care medical inpatients, any heparin (LMWH, UFH) vs placebo/no treatment) | |||
| Mortality | 9.4 | RR 1.01 (0.04‐2.57) | 1 more (56 fewer to 148 more) |
| Major bleeding | 2.7 | RR 2.09 (0.54‐8.16) | 29 more (12 fewer to 190 more) |
| Symptomatic DVT | 5.8 | RR 0.86 (0.59‐1.25) | 4 fewer (12 fewer to 8 more) |
| Pulmonary embolus | 4.2 | RR, 0.73 (0.26‐2.11) | 11 fewer (31 fewer to 47 more) |
AT9 used a variety of methods to estimate each component of the risk/benefit equation. Critical care and non‐critical care estimates were generated independently, but because of limited data, the critical care estimates were highly imprecise. In non‐critical care patients, as in ACP‐1, treatment effects were estimated from RCTs that routinely screened for A‐VTE, and they adapted the Dentali et al. estimate of DVT risk reduction. The baseline risk for bleeding and mortality were derived from the control population of the same meta‐analysis.[24]
Using a novel approach, AT9 estimated baseline nonsurgical VTE risk from a prospective observational cohort study of 1180 medical inpatients divided into high‐ and low‐risk groups by a point‐scoring system.[25] Deriving risk estimates from an observational cohort has theoretical advantages. Many patients did not receive prophylaxis, allowing for unadjusted risk estimates; they represented a cross‐section of medical inpatients rather than a selected trial population, and risk estimates were not reduced by the culling of screen‐detected A‐VTE.
The Padua risk‐assessment model (RAM) (Table 3) defines high VTE risk as a cumulative score 4. There were 60.3% of patients at low risk and 39.7% at high risk using this threshold. Among unprophylaxed patients, VTE occurred in 11% of high‐risk patients versus 0.3% of low‐risk patients (hazard ratio 32.0, 95% CI: 4.1251.0).
| Baseline Features | Score |
|---|---|
| |
| Active cancer* | 3 |
| Previous VTE (excluding superficial thrombosis) | 3 |
| Reduced mobility | 3 |
| Already known thrombophilic condition | 3 |
| Recent (1 month) trauma and/or surgery | 2 |
| Elderly age (70 years) | 1 |
| Heart and/or respiratory failure | 1 |
| Acute myocardial infarction or stroke | 1 |
| Acute infection and/or rheumatologic disorder | 1 |
| Obesity (BMI 30) | 1 |
| Ongoing hormonal treatment | 1 |
PADUA: A CLOSER LOOK
In the Padua study, 60% of the population appeared to be at such low risk for VTE that prophylaxis would seem unnecessary, but closer scrutiny should raise concern about generalizing these results. Of the 711 low‐risk patients, 1% were immobile, only 6% had cancer, 6% were obese, and only 12% had any acute infection or inflammatory condition, yet their mean length of stay was 7.9 days. These characteristics do not apply to 60% of American inpatients. Furthermore, 964 of 2208 eligible patients (44%) were excluded because they required therapeutic anticoagulation.[25]
Correspondence with the authors revealed that the 2 PEs in patients with Padua scores 4 occurred among 192 patients with a risk score of 3 (Figure 1), a 1% (2/192) risk of PE. This is a very small sample, and the true risk of VTE for medical inpatients with a risk score of 3 may be lower or significantly higher. In the Padua population, a risk score of 3 equated to a VTE risk of 6.9%, whereas those with a score of 0 to 2 had no VTE. For those adapting the Padua model, careful consideration of using a cutoff of 3, versus 4, is warranted.

IMPLICATIONS FOR VTE PROTOCOL IMPLEMENTATION AND IMPROVEMENT TEAMS
AT9 and ACP‐1 sought to focus on S‐VTE, remove bias from recommendations, and highlight potential risks of unnecessary prophylaxis in low‐risk patients. They have largely succeeded in these important goals. However, the complexity of the new guidelines and lack of consensus about VTE risk assessment pose significant challenges to improvement teams tasked with implementing the guidelines in real‐world settings.
CHOOSING A VTE RAM
The fundamental question is: How can hospitals assess VTE risk, assure adequate prophylaxis for patients who need it, while minimizing excess prophylaxis, in a practical, efficient way?
Approach 1: Opt Out Approach
Both guidelines discourage universal prophylaxis for inpatients without contraindications unless the physician opts out. Although the simplicity of this approach is appealing, the low rate of VTE in a substantial segment of the medical inpatient population and known risks of thromboprophylaxis make this strategy suboptimal.
Approach 2: No VTE RAM
ACP‐1 notes that evidence is not sufficient to recommend 1 RAM over another, and essentially advises leaving prophylaxis decisions up to an individual physician's judgment. Although the evidence may not prove which system is best, prophylaxis reliability is dismal when there is no system or when hospitals offer prophylaxis options without guidance.[26, 27] Widespread, well‐documented underprophylaxis[16, 17, 18, 19] is largely the result of relying on unguided physician judgment and relatively passive interventions like educational sessions and pocket cards.[8] This approach also deprives improvement teams of standard definitions of VTE risk, bleeding risk, and adequate prophylaxis necessary to measure and improve VTE prophylaxis. Because of significant gray areas in the literature and varied infrastructure, institutions will not implement identical VTE prevention programs, but institutional standardization remains a cornerstone of improvement.
Approach 3: Buckets of Risk
The AT8 approach to risk assessment was to place patients into VTE risk groups described in the text, rather than have an individualized point‐scoring system.[11] These assessments can be made in seconds with high levels of interobserver agreement, implemented without undue effort, and spur high levels of compliance.[28, 29] Most importantly, implementation was associated with a 40% reduction in hospital‐associated VTE (RR 0.69, 95% CI: 0.470.79) without detectable increases in bleeding or heparin‐induced thrombocytopenia. Although this strategy has not been tested in randomized trials, it has been replicated in multiple real‐world settings that avoid concerns about generalizability due to imperfect trial populations.[28, 30]
The most popular bucket model in common use, derived from a table in the AT8 guidelines, is similar to models presented in UK National Institute for Health and Care Excellence guidelines for medical inpatients.[31] These models are potentially less precise than point‐based systems, but offer simplicity, ease of use, and improved physician acceptance, and thus may be more effective than point‐based models in settings without advanced clinical decision support. The models are flexible to reflect greater or lesser degrees of aggressiveness in defining risk categories, and can be used to approximate some point‐based systems.
Approach 4: Individualized Point‐Based RAM
AT9 authors used the Padua VTE RAM to define low‐ and high‐risk patients for VTE in their recommendation for medical inpatients. The Padua model appears relatively simple, but it does require calculations, and there is a paucity of data for implementation experience with it. As mentioned above, if teams use the Padua model, the optimal cutoff (3 vs 4) for recommending prophylaxis is uncertain, and both should be considered.
The Caprini point‐based system is not mentioned in the guideline for thromboprophylaxis in medical inpatients, but in our collaborative improvement experience, it is perhaps the most commonly used point‐based model for medical inpatients.[28, 30] It is also embedded in AT9 recommendations for prophylaxis in the nonorthopedic surgical population,[9] and thus is tempting to use for both medical and surgical patients. There are several caveats to those considering the use of these more complex point‐based models. Complex point‐based RAM suffer from poor interobserver agreement.[32] They have also had limited ability to exclude low‐risk patients from prophylaxis in validation studies,[33] and have not been tested extensively in medical populations. Although AT9 considers the Caprini RAM relatively easy to use,[9] our experience in collaboratives suggests that for many hospitals, the model is too complex to be used reliably.[28, 30] Clinicians often simply bypass the clinical decision support offered in the tool, rather than checking off all risk factors, adding up the point total, and identifying the appropriate prophylaxis choices based on the point total.[28] Other point‐based RAM (reviewed elsewhere[34, 35, 36]) pose similar implementation challenges.
On the other hand, centers with more sophisticated clinical decision support and a strong improvement framework can overcome some of these challenges to get good results with complex point‐based models. A forcing function can ensure that practitioners complete all risk‐assessment tasks. Providers can check off the VTE risk factors and bleeding risk factors on 1 screen, and several factors like age, body mass index, and renal function can be autopopulated. Instead of asking the provider to add up points, the combination of answers checked off on the first screen can drive behind‐the‐scenes calculations and seamlessly lead providers to prophylaxis choices appropriate for that combination of VTE and bleeding risks. Customized models can be designed for a wide variety of services. Similar strategies can ease adaption with more complex qualitative models as well.[37]
BOTTOM LINE IN CHOOSING A VTE RAM
Many medical inpatients are at high risk for VTE, but others are not at sufficient risk to warrant prophylaxis. VTE risk assessment should be embedded in admission, transfer, and perioperative order sets and may need a hard stop to insure completion. There is a trend to favor individualized point‐based models over models that place patients in groups of risk, but evidence is insufficient to recommend 1 type of RAM over another, and more complex point‐based models often require extensive local customization and algorithmic clinical decision support to effectively implement them. Centers without advanced capability may find the bucket models more effective. We urge improvement teams to trial their RAM with common patient case scenarios, and to make a choice based on an effort‐benefit analysis, feedback from their clinicians, and the level of customization in clinical decision support available to them.
OTHER IMPLEMENTATION STRATEGIES
VTE and bleeding risk change during hospitalization. We have used ongoing daily surveillance and measurement of patients on no prophylaxis to prompt concurrent intervention (ie, measure‐vention) to increase prophylaxis for patients at risk.[28] Improvement teams should focus not only on increasing prophylaxis for those at risk, but should also use measure‐vention, checklists, or other techniques to identify low‐risk (eg, ambulating) patients for cessation of overly aggressive prophylaxis. Efforts to improve early progressive ambulation, limit central venous catheters to those who truly need them, and improve adherence to mechanical prophylaxis can also reduce VTE, as well as benefitting patient populations in other ways.
We recognize there are several approaches to close the implementation gap in delivering thromboprophylaxis judiciously but reliably, and encourage research and publication of varied strategies. Last, we hope efforts to limit unnecessary prophylaxis and challenges inherent in implementing new and complex guidelines do not increase the morbidity and mortality of hospital‐acquired VTE, by derailing the delivery of prophylaxis to those in whom the benefits outweigh the risks.
Disclosures: Dr. Merli has conducted research for Johnson & Johnson, Bristol Myers Squibb, and Portala Scientific and has been a consultant for Johnson & Johnson and Bristol Myers Squibb.
Patients hospitalized for acute medical illness have more than a 10‐fold increased risk for venous thromboembolism (VTE),[1] with an undeniably dramatic, negative impact on the lives of those afflicted, including fatal pulmonary embolism (PE), which most commonly affects patients on the medical service.[2, 3, 4] Yet estimates for the overall rate of VTE in this population are relatively low, raising questions about which subsets of medical patients warrant the risk and cost of prophylaxis.
Recently, the American College of Physicians published guidelines (ACP‐1)[5] and a supporting review[6] addressing VTE prophylaxis in nonsurgical inpatients, followed by publication of the American College of Chest Physicians (ACCP) 9th Edition of the Chest Guidelines on Antithrombotic Therapy and Prevention of Thrombosis (AT9),[7] which divides VTE prevention into 3 articles,[8, 9, 10] including 1 on nonsurgical patients.[8] Both ACP‐1 and AT9 differ significantly from the 2008 ACCP guidelines (AT8),[11] but took different approaches to methodology, risk assessment, and several other aspects of thromboprophylaxis (Table 1). This narrative review summarizes and compares these recommendations and the methods used to arrive at them, with a final section focusing on implications for improvement teams designing order sets and system changes to address VTE prophylaxis.
| 2008 ACCP VTE Guideline AT8 | 2012 ACCP VTE Guideline AT9 | 2011 ACP Guideline | |
|---|---|---|---|
| |||
| Stance on asymptomatic VTE end points | Because of the strong concordance between asymptomatic DVT and clinically important VTE, we believe that DVT detected by a sensitive screening tesis an appropriate outcome in the early assessment of new thromboprophylaxis interventions. | Use of this surrogate (asymptomatic, screening‐detected thrombosis) creates major problems in making the trade‐off between patient‐important outcomes (thrombosis and serious bleeding). | Surrogate outcomes of asymptomatic screening detected‐thrombosis should not be used. |
| Who should be prophylaxed? | 6.0.0: For acutely ill medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, sepsis, neurologic disease, or inflammatory bowel disease, we recommend thromboprophylaxis with LMWH (1A), UFH (1A), or fondaparinux (1A). | 2.3: For acutely ill hospitalized medical patients at increased risk for thrombosis, we recommend anticoagulant thromboprophylaxis, with LMWH, UFH bid, UFH tid, or fondaparinux (1B). | ACP recommends pharmacologic prophylaxis with heparin or a related drug for venous thromboembolism in medical (including stroke) patients unless the assessed risk for bleeding outweighs the likely benefits (grade: strong recommendation, moderate‐quality evidence). |
| 2.4: For acutely ill hospitalized medical patients at low risk of thrombosis, we recommend against the use of pharmacologic or mechanical prophylaxis. (1B) | |||
| Choice of anticoagulant prophylaxis | There is no compelling evidence that UFH should be administered three times daily in preference to twice daily in medical patients, although these two regimens have never been directly compared. | In choosing the specific anticoagulant drug to be used for pharmacoprophylaxis, choices should be based on patient preference, compliance, and ease of administration (eg, daily vs bid vs tid dosing), as well as on local factors affecting acquisition costs. | [T]he choice of agent for prophylaxis of VTE should be based on ease of use, adverse effect profile, and cost of medication. |
| No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | No strong preference LMWH vs UFH. | |
| Mechanical prophylaxis | 1.4.3.1: We recommend that mechanical methods of prophylaxis be used primarily in patients at high risk of bleeding (grade 1A), or possibly as an adjunct to anticoagulant‐based thromboprophylaxis (grade 2A). | 2.7.2: For acutely ill hospitalized medical patients at increased risk of thrombosis who are bleeding or at high risk for major bleeding, we suggest the optimal use of mechanical thromboprophylaxis with GCS (grade 2C) or IPC (grade 2C). | ACP recommends against the use of mechanical prophylaxis with graduated compression stockings for prevention of venous thromboembolism (grade: strong recommendation, moderate‐quality evidence). |
| No strong evidence for IPC vs GCS | |||
| Duration | [T]he optimal duration of thromboprophylaxis remains unclear. To the end of hospitalization for most patients. | 2.8: [W]e suggest against extending the duration of thromboprophylaxis beyond the period of patient immobilization or acute hospital stay (2B). | The optimal duration of heparin prophylaxis is uncertain. |
| Risk Stratification | The approach of individual prophylaxis prescribing based on formal RAMs is not used routinely by most clinicians because it has not been adequately validated and is cumbersome. Individual RAMs may not be worth the effort, because there are only a limited number of thromboprophylaxis options, and one of the principles of effective thromboprophylaxis is to reduce complexity in decision making. | (Noncritical care) No formal risk assessment recommendation. Padua point‐based model is inherent in definitions of baseline VTE risk. | ACP does not support the application of performance measures in medical (including stroke) patients that promotes universal venous thromboembolism prophylaxis regardless of risk. |
| Another approach involves implementation of group‐specific thromboprophylaxis routinely for all patients who belong to each of the major target groups. We support this approach. | There are no validated risk assessment models to stratify VTE risk in critically ill patients. | Many risk assessment tools are available for estimating thromboembolism risk, but the current evidence is insufficient to recommend a validated tool. | |
| [T]he decision is best left to physician judgment, and performance measures targeting all patients are inappropriate. | |||
WHY ARE THE NEW GUIDELINES DIFFERENT?
Major randomized controlled trials (RCTs)[12, 13, 14] of thromboprophylaxis used routine deep vein thrombosis (DVT) surveillance and included both symptomatic (S‐VTE) and asymptomatic VTE (A‐VTE) end points. These studies consistently demonstrated 44% to 63% reductions in VTE without increases in major bleeding.[11] Because of the strong relationship between A‐VTE and S‐VTE outcomes, and a paucity of studies using only S‐VTE outcomes, AT8 judged that A‐VTE outcomes were valid to include, whereas the new guidelines reject the use of asymptomatic VTE end points.[5, 8, 15] To minimize financial and intellectual conflicts of interest, AT9 also used methodologists rather than VTE experts as topic editors, excluded conflicted experts from voting on recommendations, and attempted to estimate patient values and preferences.[15] As a result, AT9 makes fewer strong recommendations (182 1A recommendations in 2008, but only 29 in 2012), replacing them with weak suggestions.
WHAT DO THE NEW GUIDELINES RECOMMEND?
AT8 recommended anticoagulant prophylaxis for acutely ill medical inpatients with known risk factors, but did not recommend routine thromboprophylaxis. However, because of well‐known problems with underprophylaxis,[16, 17, 18, 19] particularly in medical patients, the low risk of bleeding, and difficulties with explicitly defining low‐risk patients, many discounted the need for VTE risk stratification.
Both new guidelines recommend prophylaxis for many nonsurgical patients, but discourage routine thromboprophylaxis for nonsurgical inpatients. AT9 specifically recommends against any thromboprophylaxis for low‐risk medical inpatients, implying that many nonsurgical, non‐critical care patients belong in this category, citing lower estimates of benefit, lower estimates of VTE risk, and potential bleeding risks.
The guidelines[5, 8] agree that, when indicated and absent contraindications, anticoagulant prophylaxis is preferred over mechanical prophylaxis, and agree there is insufficient evidence to recommend 1 anticoagulant over another.
For patients at risk of both VTE and bleeding, ACP‐1 states that intermittent pneumatic compression (IPC) devices are a reasonable option, given the evidence showing benefit in surgical patients. However, ACP‐1 recommends against graduated compression stockings (GCS) in nonsurgical patients based on a meta‐analysis dominated by the CLOTS‐1 (Clots in Legs Or sTockings after Stroke) trial, which found that thigh‐high GCS increased the risk of skin breakdown without reducing VTE[20] in immobilized stroke patients. AT9 does not recommend against GCS for patients facing bleeding and VTE risk. AT9 notes the hazards of generalizing results from stroke patients, and also considers the somewhat contradictory results from the CLOTS‐2 trial in stroke patients, which found a lower rate of VTE with thigh‐high GCS than with knee‐high GCS.[21] AT9 designates a recommendation of 2C for either IPC devices or thigh‐high GCS for those at VTE risk when anticoagulants are contraindicated.
Combination mechanical‐pharmacologic prophylaxis has proven superior in some surgical populations, and many hospitals use combined prophylaxis in high‐risk medical patients. However, combination prophylaxis has not been studied in this population. ACP‐1 does not comment on the practice; AT9 does not recommend for or against it. Institutions that use combination prophylaxis should be aware that although it may seem logical to extrapolate estimates of benefit seen in selected surgical patients, this is not a recommended practice.
RCTs for thromboprophylaxis in nonsurgical inpatients provided prophylaxis for 6 to 21 days. Neither ACP‐1 or AT9 recommend routinely extending prophylaxis beyond the hospital stay, citing an RCT[22] in which the benefit of extended duration low molecular weight heparin was limited to selected subsets of patients and offset by bleeding complications. AT9 suggests prophylaxis for 6 to 21 days, until full mobility is restored, or until dischargewhichever comes first.[8] However, we know of no study that establishes a mobility level at which prophylaxis can be safely discontinued, especially in inpatients with multiple risk factors.
ESTIMATING RISK AND BENEFIT OF PROPHYLAXIS AND LIMITATIONS OF METHODS
Calculating risk/benefit ratios for thromboprophylaxis requires estimates of baseline VTE and bleeding risks, and estimates of the impact of prophylaxis on those baseline risks. Methods to estimate the impact of prophylaxis on S‐VTE from studies relying on A‐VTE all have limitations, as acknowledged by the AT9 introduction.[15]
The ACP‐1 review found the only significant effect of prophylaxis on medical inpatients was a modest reduction in PE and a modest increase in total bleeding events, without effects on major bleeding, DVT, or mortality.[6] The authors summarized the findings as indicative of little or no net benefit for the medical population as a whole. The ACP‐1 review derives estimates of S‐VTE risk, bleeding, and mortality from control (baseline) and interventional arms of RCTs that used routine VTE screening, and included A‐VTE end points. The baseline risk of VTE could potentially be overestimated, because the populations in the trials are not representative of the entire medical population.
On the other hand, pooling trials with screening‐detected VTE to estimate S‐VTE outcomes is a questionable practice that may falsely lower estimates of VTE prophylaxis benefit. Screening‐detected VTE may be treated or declared a study end point before it becomes symptomatic. MEDENOX (Medical Patients With Enoxaparin) is an illustrative example.[12] The 263 placebo recipients suffered 37 A‐VTEs and 4 S‐VTEs. The 272 enoxaparin recipients suffered 17 A‐VTEs and 3 S‐VTEs. Patients at the highest risk of S‐VTE were counted as reaching an end point before they could develop symptoms; this happened more than twice as often in the placebo arm. This decreases both estimates of baseline VTE risk and the measured benefit of prophylaxis for S‐VTE. Screening could conceivably reduce measured effects on mortality as well, because patients begin VTE therapy earlier. Per ACP‐1, the estimated risk for DVT is lower than for PE, running counter to literature experience[8, 16] and raising issues of face validity. The ACP‐1 review accepts all original definitions of major bleeding, including a 2 g/dL drop in hemoglobin,[12] which commonly occurs without any bleeding or clinical consequence, and bleeding events were ascribed to heparins up to 120 days after randomization, long after they could have been responsible.
Previous meta‐analyses of thromboprophylaxis studies[23, 24] shared many of these same limitations, but did not ascribe bleeding complications to heparins for this extended duration, and had point estimates that suggested a larger impact from prophylaxis than ACP‐1. Dentali et al., for example, showed statistically significant impact on PE (relative risk [RR] 0.43), fatal PE (RR 0.38), and a nearly statistically significant large impact on DVT (RR 0.47, 95% confidence interval [CI]: 0.22‐1.00),[24] whereas ACP‐1 estimated a smaller significant impact on PE (RR 0.69), no significant difference in fatal PE, and a much smaller estimate of the impact on DVT (RR 0.78, 95% CI: 0.45‐1.35) (Table 2).
| Baseline Risk | Relative Effect (95% CI) | Absolute Effect per 1000 Patients Treated (95% CI) | |
|---|---|---|---|
| |||
| ACP guideline review (Lederle), UFH or LMWH vs placebo/no treatment, medical patients | |||
| Mortality | 6.6 | OR 0.94 (0.84‐1.04) | 4 fewer (11 fewer to 3 more) |
| Major bleeding | 0.25 | OR 1.49 (0.91‐2.43) | 1 more (no effect to 3 more) |
| Symptomatic DVT | 0.96 | OR 0.78 (0.45‐1.35) | 2 fewer (6 fewer to 4 more) |
| PE | 1.2 | OR 0.69 (0.52‐0.90) | 4 fewer (6 fewer to 1 fewer) |
| Fatal PE | 0.30 | OR 0.77 (0.43‐1.37) | 1 fewer (2 fewer to 1 more) |
| ACCP AT9 (Kahn), non‐critical care medical inpatients, anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Mortality | 4.5 | OR 0.97 (0.79‐1.19) | 1 fewer (9 fewer to 8 more) |
| Major bleeding | 0.40 | OR 1.32 (0.73‐2.37) | 1 more (1 fewer to 6 more) |
| Thrombocytopenia | 0.13 | OR 0.92 (0.54‐1.53) | 1 fewer (6 fewer to 7 more) |
| Symptomatic DVT | |||
| Padua score 4 | 0.2 | RR 0.47 (0.22‐1) | 1 fewer (1 fewer to no effect) |
| Padua score 4 | 6.7 | 34 fewer (51 fewer to no effect) | |
| ACCP AT9 (Kahn) non‐critical care medical inpatients, Anticoagulant (LMWH, UFH, fondaparinux) vs placebo/no treatment) | |||
| Nonfatal PE | |||
| Padua score 4 | 0.2 | RR 0.61 (0.23‐1.67) | 1 fewer (1 fewer to 1 more) |
| Padua score 4 | 3.9 | 15 fewer (30 fewer to 36 more) | |
| Fatal PE | 0.4 | RR 0.41 (0.22‐0.76) | 2 fewer (1 fewer to 3 fewer) |
| ACCP AT9 (Kahn), critical care medical inpatients, any heparin (LMWH, UFH) vs placebo/no treatment) | |||
| Mortality | 9.4 | RR 1.01 (0.04‐2.57) | 1 more (56 fewer to 148 more) |
| Major bleeding | 2.7 | RR 2.09 (0.54‐8.16) | 29 more (12 fewer to 190 more) |
| Symptomatic DVT | 5.8 | RR 0.86 (0.59‐1.25) | 4 fewer (12 fewer to 8 more) |
| Pulmonary embolus | 4.2 | RR, 0.73 (0.26‐2.11) | 11 fewer (31 fewer to 47 more) |
AT9 used a variety of methods to estimate each component of the risk/benefit equation. Critical care and non‐critical care estimates were generated independently, but because of limited data, the critical care estimates were highly imprecise. In non‐critical care patients, as in ACP‐1, treatment effects were estimated from RCTs that routinely screened for A‐VTE, and they adapted the Dentali et al. estimate of DVT risk reduction. The baseline risk for bleeding and mortality were derived from the control population of the same meta‐analysis.[24]
Using a novel approach, AT9 estimated baseline nonsurgical VTE risk from a prospective observational cohort study of 1180 medical inpatients divided into high‐ and low‐risk groups by a point‐scoring system.[25] Deriving risk estimates from an observational cohort has theoretical advantages. Many patients did not receive prophylaxis, allowing for unadjusted risk estimates; they represented a cross‐section of medical inpatients rather than a selected trial population, and risk estimates were not reduced by the culling of screen‐detected A‐VTE.
The Padua risk‐assessment model (RAM) (Table 3) defines high VTE risk as a cumulative score 4. There were 60.3% of patients at low risk and 39.7% at high risk using this threshold. Among unprophylaxed patients, VTE occurred in 11% of high‐risk patients versus 0.3% of low‐risk patients (hazard ratio 32.0, 95% CI: 4.1251.0).
| Baseline Features | Score |
|---|---|
| |
| Active cancer* | 3 |
| Previous VTE (excluding superficial thrombosis) | 3 |
| Reduced mobility | 3 |
| Already known thrombophilic condition | 3 |
| Recent (1 month) trauma and/or surgery | 2 |
| Elderly age (70 years) | 1 |
| Heart and/or respiratory failure | 1 |
| Acute myocardial infarction or stroke | 1 |
| Acute infection and/or rheumatologic disorder | 1 |
| Obesity (BMI 30) | 1 |
| Ongoing hormonal treatment | 1 |
PADUA: A CLOSER LOOK
In the Padua study, 60% of the population appeared to be at such low risk for VTE that prophylaxis would seem unnecessary, but closer scrutiny should raise concern about generalizing these results. Of the 711 low‐risk patients, 1% were immobile, only 6% had cancer, 6% were obese, and only 12% had any acute infection or inflammatory condition, yet their mean length of stay was 7.9 days. These characteristics do not apply to 60% of American inpatients. Furthermore, 964 of 2208 eligible patients (44%) were excluded because they required therapeutic anticoagulation.[25]
Correspondence with the authors revealed that the 2 PEs in patients with Padua scores 4 occurred among 192 patients with a risk score of 3 (Figure 1), a 1% (2/192) risk of PE. This is a very small sample, and the true risk of VTE for medical inpatients with a risk score of 3 may be lower or significantly higher. In the Padua population, a risk score of 3 equated to a VTE risk of 6.9%, whereas those with a score of 0 to 2 had no VTE. For those adapting the Padua model, careful consideration of using a cutoff of 3, versus 4, is warranted.

IMPLICATIONS FOR VTE PROTOCOL IMPLEMENTATION AND IMPROVEMENT TEAMS
AT9 and ACP‐1 sought to focus on S‐VTE, remove bias from recommendations, and highlight potential risks of unnecessary prophylaxis in low‐risk patients. They have largely succeeded in these important goals. However, the complexity of the new guidelines and lack of consensus about VTE risk assessment pose significant challenges to improvement teams tasked with implementing the guidelines in real‐world settings.
CHOOSING A VTE RAM
The fundamental question is: How can hospitals assess VTE risk, assure adequate prophylaxis for patients who need it, while minimizing excess prophylaxis, in a practical, efficient way?
Approach 1: Opt Out Approach
Both guidelines discourage universal prophylaxis for inpatients without contraindications unless the physician opts out. Although the simplicity of this approach is appealing, the low rate of VTE in a substantial segment of the medical inpatient population and known risks of thromboprophylaxis make this strategy suboptimal.
Approach 2: No VTE RAM
ACP‐1 notes that evidence is not sufficient to recommend 1 RAM over another, and essentially advises leaving prophylaxis decisions up to an individual physician's judgment. Although the evidence may not prove which system is best, prophylaxis reliability is dismal when there is no system or when hospitals offer prophylaxis options without guidance.[26, 27] Widespread, well‐documented underprophylaxis[16, 17, 18, 19] is largely the result of relying on unguided physician judgment and relatively passive interventions like educational sessions and pocket cards.[8] This approach also deprives improvement teams of standard definitions of VTE risk, bleeding risk, and adequate prophylaxis necessary to measure and improve VTE prophylaxis. Because of significant gray areas in the literature and varied infrastructure, institutions will not implement identical VTE prevention programs, but institutional standardization remains a cornerstone of improvement.
Approach 3: Buckets of Risk
The AT8 approach to risk assessment was to place patients into VTE risk groups described in the text, rather than have an individualized point‐scoring system.[11] These assessments can be made in seconds with high levels of interobserver agreement, implemented without undue effort, and spur high levels of compliance.[28, 29] Most importantly, implementation was associated with a 40% reduction in hospital‐associated VTE (RR 0.69, 95% CI: 0.470.79) without detectable increases in bleeding or heparin‐induced thrombocytopenia. Although this strategy has not been tested in randomized trials, it has been replicated in multiple real‐world settings that avoid concerns about generalizability due to imperfect trial populations.[28, 30]
The most popular bucket model in common use, derived from a table in the AT8 guidelines, is similar to models presented in UK National Institute for Health and Care Excellence guidelines for medical inpatients.[31] These models are potentially less precise than point‐based systems, but offer simplicity, ease of use, and improved physician acceptance, and thus may be more effective than point‐based models in settings without advanced clinical decision support. The models are flexible to reflect greater or lesser degrees of aggressiveness in defining risk categories, and can be used to approximate some point‐based systems.
Approach 4: Individualized Point‐Based RAM
AT9 authors used the Padua VTE RAM to define low‐ and high‐risk patients for VTE in their recommendation for medical inpatients. The Padua model appears relatively simple, but it does require calculations, and there is a paucity of data for implementation experience with it. As mentioned above, if teams use the Padua model, the optimal cutoff (3 vs 4) for recommending prophylaxis is uncertain, and both should be considered.
The Caprini point‐based system is not mentioned in the guideline for thromboprophylaxis in medical inpatients, but in our collaborative improvement experience, it is perhaps the most commonly used point‐based model for medical inpatients.[28, 30] It is also embedded in AT9 recommendations for prophylaxis in the nonorthopedic surgical population,[9] and thus is tempting to use for both medical and surgical patients. There are several caveats to those considering the use of these more complex point‐based models. Complex point‐based RAM suffer from poor interobserver agreement.[32] They have also had limited ability to exclude low‐risk patients from prophylaxis in validation studies,[33] and have not been tested extensively in medical populations. Although AT9 considers the Caprini RAM relatively easy to use,[9] our experience in collaboratives suggests that for many hospitals, the model is too complex to be used reliably.[28, 30] Clinicians often simply bypass the clinical decision support offered in the tool, rather than checking off all risk factors, adding up the point total, and identifying the appropriate prophylaxis choices based on the point total.[28] Other point‐based RAM (reviewed elsewhere[34, 35, 36]) pose similar implementation challenges.
On the other hand, centers with more sophisticated clinical decision support and a strong improvement framework can overcome some of these challenges to get good results with complex point‐based models. A forcing function can ensure that practitioners complete all risk‐assessment tasks. Providers can check off the VTE risk factors and bleeding risk factors on 1 screen, and several factors like age, body mass index, and renal function can be autopopulated. Instead of asking the provider to add up points, the combination of answers checked off on the first screen can drive behind‐the‐scenes calculations and seamlessly lead providers to prophylaxis choices appropriate for that combination of VTE and bleeding risks. Customized models can be designed for a wide variety of services. Similar strategies can ease adaption with more complex qualitative models as well.[37]
BOTTOM LINE IN CHOOSING A VTE RAM
Many medical inpatients are at high risk for VTE, but others are not at sufficient risk to warrant prophylaxis. VTE risk assessment should be embedded in admission, transfer, and perioperative order sets and may need a hard stop to insure completion. There is a trend to favor individualized point‐based models over models that place patients in groups of risk, but evidence is insufficient to recommend 1 type of RAM over another, and more complex point‐based models often require extensive local customization and algorithmic clinical decision support to effectively implement them. Centers without advanced capability may find the bucket models more effective. We urge improvement teams to trial their RAM with common patient case scenarios, and to make a choice based on an effort‐benefit analysis, feedback from their clinicians, and the level of customization in clinical decision support available to them.
OTHER IMPLEMENTATION STRATEGIES
VTE and bleeding risk change during hospitalization. We have used ongoing daily surveillance and measurement of patients on no prophylaxis to prompt concurrent intervention (ie, measure‐vention) to increase prophylaxis for patients at risk.[28] Improvement teams should focus not only on increasing prophylaxis for those at risk, but should also use measure‐vention, checklists, or other techniques to identify low‐risk (eg, ambulating) patients for cessation of overly aggressive prophylaxis. Efforts to improve early progressive ambulation, limit central venous catheters to those who truly need them, and improve adherence to mechanical prophylaxis can also reduce VTE, as well as benefitting patient populations in other ways.
We recognize there are several approaches to close the implementation gap in delivering thromboprophylaxis judiciously but reliably, and encourage research and publication of varied strategies. Last, we hope efforts to limit unnecessary prophylaxis and challenges inherent in implementing new and complex guidelines do not increase the morbidity and mortality of hospital‐acquired VTE, by derailing the delivery of prophylaxis to those in whom the benefits outweigh the risks.
Disclosures: Dr. Merli has conducted research for Johnson & Johnson, Bristol Myers Squibb, and Portala Scientific and has been a consultant for Johnson & Johnson and Bristol Myers Squibb.
- US Department of Health and Human Services. Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. Available at: http://www.surgeongeneral.gov/topics/deepvein/index.html. Accessed January 29, 2013.
- , , , et al. Incidence of venous thromboembolism in hospitalized patients vs. community residents. Mayo Clin Proc. 2001;76:1102–1110.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , . New onset of venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118(6):1680–1684.
- , , , , . venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625–632.
- , , , . Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2 suppl):e227S–e277S.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- , , , et al. The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry. J Thromb Haemost. 2004;2:1892–1898.
- , , , et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936–945.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , , , et al.; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103(4):736–748.
- , , , et al; CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomized controlled trial. Lancet. 2009;373(9679):1958–1965.
- CLOTS (Clots in Legs Or sTockings after Stroke) Trial Collaboration. Thigh‐length versus below‐knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153(9):553–562.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167(1)476–486.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007; 46(4):278–288.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , . Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes. J Hosp Med. 2009;4(2):81–89.
- . Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? J Hosp Med. 2009;4(2):77–80.
- , . Designing and implementing effective VTE prevention protocols: lessons from collaboratives. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Optimizing prevention of hospital acquired venous thromboembolism: prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10–18.
- , , , et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301–310.
- NHS National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. NICE Clinical Guideline 92. 2010. Available at: http://www.nice.org.uk/guidance/CG92. Accessed April 18, 2013.
- , , , , . Reliability of a point‐based VTE risk assessment tool in the hands of medical residents. J Hosp Med. 2011;6:195–201.
- , , , , , . A validation of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344–350.
- , , , . Risk assessment models for thromboprophylaxis of medical patients. Thromb Res. 2012;129:127–132.
- , , , , . Risk‐assessment models for predicting venous thromboembolism among hospitalized non‐surgical patients: a systematic review. J Thromb Thrombolysis. 2013;35:67–80.
- , , . The use of weighted and scored risk assessment models for venous thromboembolism. Thromb Haemost. 2012;108(6):1072–1076.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- US Department of Health and Human Services. Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. Available at: http://www.surgeongeneral.gov/topics/deepvein/index.html. Accessed January 29, 2013.
- , , , et al. Incidence of venous thromboembolism in hospitalized patients vs. community residents. Mayo Clin Proc. 2001;76:1102–1110.
- , , , , , . Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809–815.
- , , . New onset of venous thromboembolism among hospitalized patients at Brigham and Women's Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118(6):1680–1684.
- , , , , . venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625–632.
- , , , . Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2 suppl):e227S–e277S.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S–453S.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- , , , et al. The outcome after treatment of venous thromboembolism is different in surgical and acutely ill medical patients. Findings from the RIETE registry. J Thromb Haemost. 2004;2:1892–1898.
- , , , et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936–945.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , , , et al.; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE Global Survey. Thromb Haemost. 2010;103(4):736–748.
- , , , et al; CLOTS Trials Collaboration. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomized controlled trial. Lancet. 2009;373(9679):1958–1965.
- CLOTS (Clots in Legs Or sTockings after Stroke) Trial Collaboration. Thigh‐length versus below‐knee stockings for deep venous thrombosis prophylaxis after stroke: a randomized trial. Ann Intern Med. 2010;153(9):553–562.
- , , , et al. Extended‐duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
- , , , , . Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2007;167(1)476–486.
- , , , , . Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007; 46(4):278–288.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , . Medical admission order sets to improve deep vein thrombosis prophylaxis rates and other outcomes. J Hosp Med. 2009;4(2):81–89.
- . Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? J Hosp Med. 2009;4(2):77–80.
- , . Designing and implementing effective VTE prevention protocols: lessons from collaboratives. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Optimizing prevention of hospital acquired venous thromboembolism: prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10–18.
- , , , et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38(7):301–310.
- NHS National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. NICE Clinical Guideline 92. 2010. Available at: http://www.nice.org.uk/guidance/CG92. Accessed April 18, 2013.
- , , , , . Reliability of a point‐based VTE risk assessment tool in the hands of medical residents. J Hosp Med. 2011;6:195–201.
- , , , , , . A validation of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344–350.
- , , , . Risk assessment models for thromboprophylaxis of medical patients. Thromb Res. 2012;129:127–132.
- , , , , . Risk‐assessment models for predicting venous thromboembolism among hospitalized non‐surgical patients: a systematic review. J Thromb Thrombolysis. 2013;35:67–80.
- , , . The use of weighted and scored risk assessment models for venous thromboembolism. Thromb Haemost. 2012;108(6):1072–1076.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.