User login
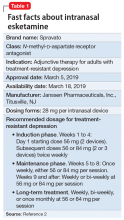
Intranasal esketamine
Treatment-resistant depression (TRD) is a common clinical struggle that practicing clinicians address on a daily basis. Major depressive disorder affects nearly 1 in 5 Americans at some point in their life and, by definition, impairs social and occupational functioning. Historic treatments have focused on the monoamine theories of depression—modulating the monoamines serotonin, norepinephrine, and/or dopamine. Limitations of currently available antidepressants include delayed onset of effect and low remission rates. To further complicate the matter, numerous studies have shown that with each subsequent antidepressant trial, patients have a decreasing likelihood of responding to subsequent antidepressant treatment options. For example, in the classic STAR*D trial, by the time a patient had not responded to the first 2 antidepressant options, the chance that they would respond to a third or fourth antidepressant had decreased to approximately 15% per antidepressant treatment course.1
To address the need for new treatments for patients with TRD, on March 5, 2019 the FDA-approved intranasal
How it works
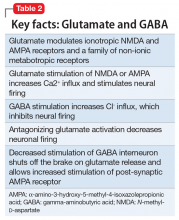
Modern research has looked beyond the monoamine system to explore the neuro-modulatory effects of glutamate and gamma-aminobutyric acid (GABA).3 The yin and yang of glutamate and GABA revolves around neural excitation vs neural inhibition at a local synaptic level. The primary effects of the glutamate and GABA systems (Table 2) can be broken down into several key areas of understanding.
Glutamate modulates ionotropic N-methyl-
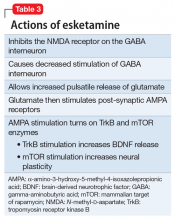
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and has been developed as an intranasal adjunctive treatment for TRD. Esketamine blocks NMDA receptors on GABA interneurons. This allows for increased pulsatile release of glutamate into the synapse. Intrasynaptic glutamate then stimulates postsynaptic AMPA receptors. Glutamate stimulation of postsynaptic AMPA receptors results in an intracellular cascade that activates the enzymes tropomyosin receptor kinase B (TrkB) and mammalian target of rapamycin (mTOR). TrkB stimulation results in increased production and release of BDNF. mTor stimulation increases neuronal membrane protein formation with subsequent increased neural plasticity. Taken together, preclinical models show that esketamine’s inhibition of the NMDA receptor on the GABA interneuron results in a cascade of increased BDNF release and synaptogenesis with increased neuroplasticity (Table 3).
Clinical implications
Treatment-resistant depression affects nearly one-third of patients currently receiving standard antidepressant treatment. Major depressive disorder is currently the second leading cause of disability for working adults within the United States and one of the largest causes of disability worldwide. The esketamine nasal spray could be beneficial for patients who have experienced TRD with standard monoamine antidepressants.
Supporting evidence
Clinical trials examining intranasal esketamine include both short- and long-term studies of patients with TRD.
Continue to: Esketamine was evaluated...
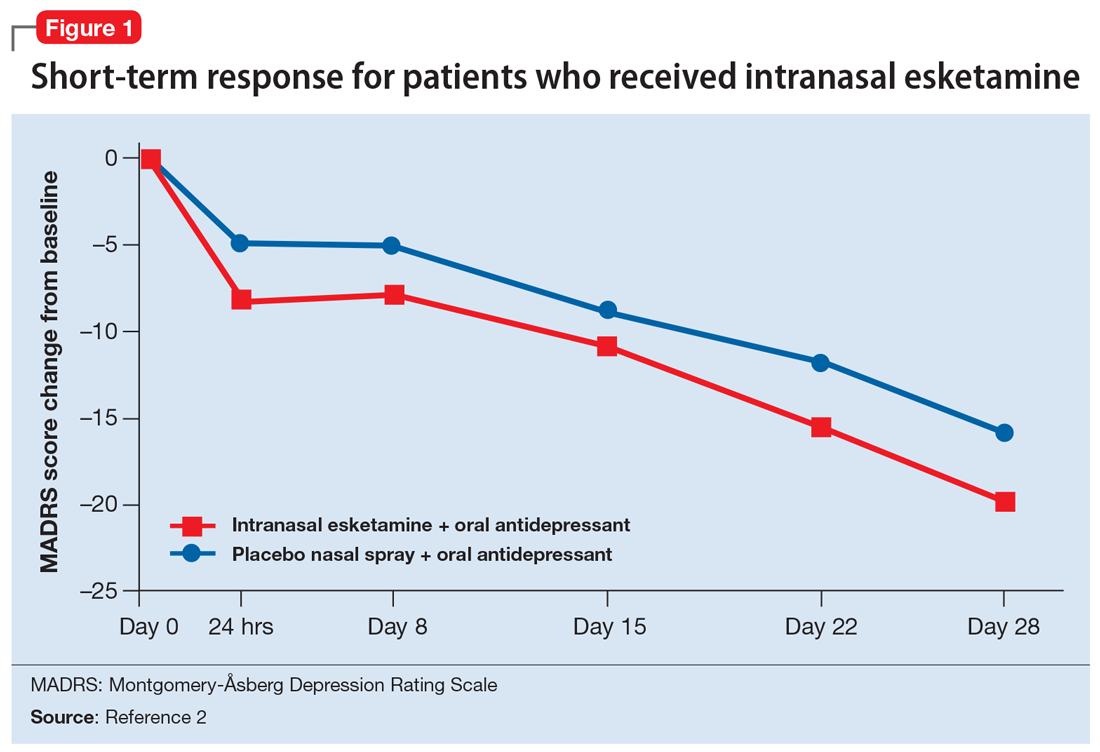
Esketamine was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week) phase III study in adult patients age 18 to 65 with TRD (they had not responded to at least 2 different antidepressants of adequate dose and duration).4 After discontinuing prior antidepressant treatments, all patients were started on a newly initiated antidepressant and were also randomized to concomitant intranasal esketamine or intranasal placebo as follows:
- 114 patients were randomized to the intranasal esketamine plus newly initiated oral antidepressant arm
- 109 patients were randomized to the placebo nasal spray plus newly initiated oral antidepressant arm
- The mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score for each group was 37 (ie, moderately to severely depressed).
Newly started antidepressants included esc
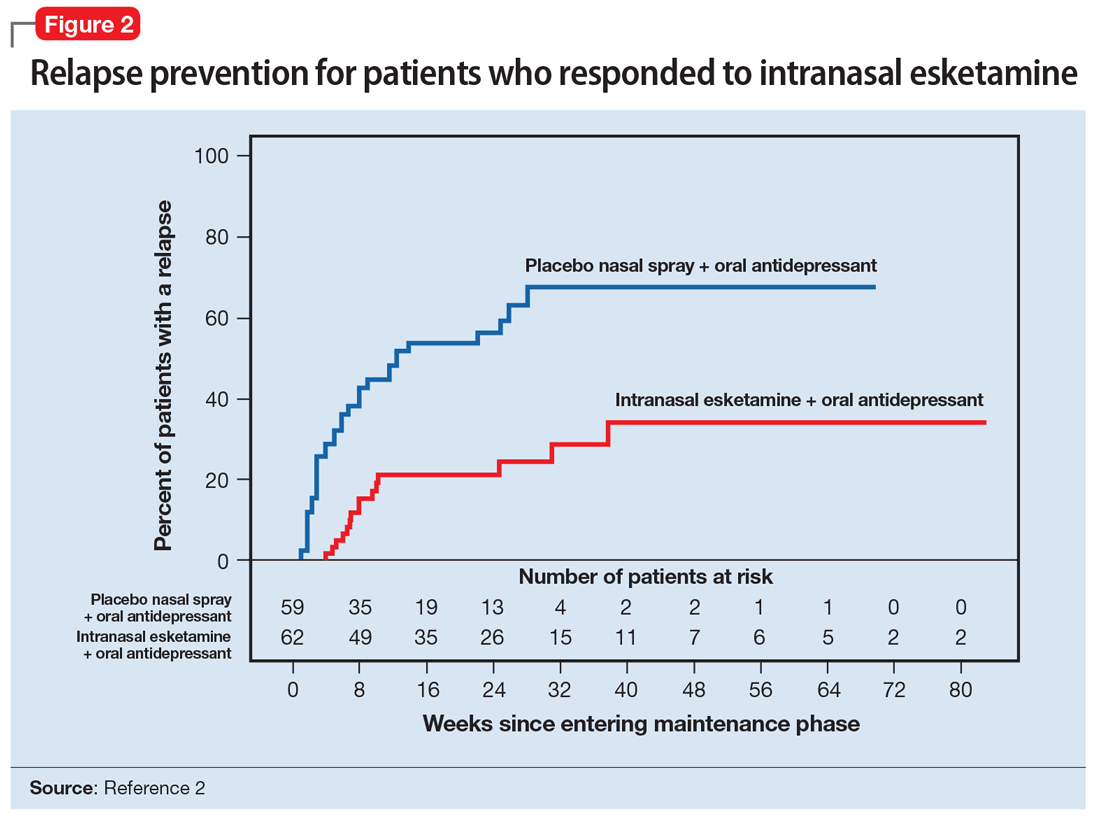
A long-term, double-blind multicenter maintenance-of-effect trial examined adults age 18 to 65 with TRD.5-6 Patients in this study were responders in 1 of 2 short-term studies or in an open-label direct enrollment study. Stable remission was defined as a MADRS total score <12 for at least 3 of the last 4 weeks of the study, and stable response was defined as a MADRS reduction of >50% but not in remission. After 16 weeks of intranasal esketamine plus an oral antidepressant, stable remitters and stable responders were then randomized separately to continue intranasal esketamine or switch to placebo nasal spray, with both groups continuing on their concomitant oral antidepressant. The primary study endpoint was time to relapse. Relapse was defined as a MADRS total score >22 for more than 2 consecutive weeks, hospitalization for worsening of depression, or any other clinically relevant event. The median age was 48, 66% were female, 90% were White and 4% were black. Patients in stable response or stable remission experienced a significantly longer time to relapse compared with patients who continued their oral antidepressant but were switched to placebo intranasal spray. In this remission response study, patients could receive intranasal treatment weekly or bi-weekly based on symptom severity (Figure 22).
Impact on driving. Two studies examined the impact of esketamine on driving performance. One examined adults with major depressive disorder and the other examined healthy participants. The effects of a single 84-mg dose of esketamine nasal spray on a patient’s ability to drive was assessed in 23 healthy adults. In this study, mirt
A second study evaluated the effects of repeated esketamine administration on driving performance in 25 adults with major depressive disorder. In this study, an ethanol-containing beverage was used as an active control. After administration of a single 84-mg dose of intranasal esketamine, driving performance was the same as a placebo at 18 hours. In the multiple dose phase, standard driving performance was similar for esketamine nasal spray and placebo at 6 hours postdose on Days 11, 18, and 25.
Continue to: Pharmacologic profile
Pharmacologic profile
Adverse events. The most common adverse events in patients treated with esketamine nasal spray were dissociation (41%), dizziness (29%), nausea (28%), sedation (23%), and vertigo (23%).2 The majority of these effects were short-term and resolved during the 2-hour observation period.
In addition to spontaneously reported events, sedation and dissociation were further monitored with specific scales. Sedation was measured with the Modified Observer’s Alertness and Sedation Scale. Using this scale, 50% of patients receiving 56 mg and 61% of patients receiving 84 mg of esketamine met criteria for sedation.
Similarly, dissociation/perceptional changes were measured with spontaneously reported events and also with the Clinician Administered Dissociative State Scale. On this scale, 61% of patients receiving the 56-mg dose, and 69% of patients receiving the 84-mg dose met criteria for dissociation/perceptional changes after dose administration.
Increases in blod pressure. Esketamine intranasal spray was associated with a 7 to 9 mm Hg increase in systolic blood pressure and a 4 to 6 mm Hg increase in diastolic blood pressure, both of which peaked 40 minutes post-dose.
Nausea and vomiting. Intranasal esketamine was associated with a 27% rate of nausea at 56 mg, and 32% at 84 mg, with a 6% rate of vomiting at 56 mg and 12% at 84 mg.
Continue to: Pharmacokinetics
Pharmacokinetics
Esketamine exposure increases from 28 to 84 mg in a fairly dose-proportional range. No accumulation of esketamine was observed in the plasma following twice-weekly administration. Bioavailability is approximately 48% following nasal administration. The Tmax for esketamine plasma concentration is 20 to 40 minutes after the last nasal spray. Protein binding of esketamine is approximately 43% to 45%. The brain-to-plasma ratio of noresketamine is 4 to 6 times lower than that of esketamine. The half-life of esketamine ranged from 7 to 12 hours. The mean half-life of nore
Potential drug interactions
Central nervous system depressants. Concomitant use of esketamine and other CNS depressants (ie, benzodiazepines, opioids, alcohol) may increase sedation. Patients receiving esketamine with concomitant use of other CNS depressants should be closely monitored for sedation.
Psychostimulants. Concomitant use of esketamine and psychostimulants (ie, amphetamines, methylphenidates, moda
Monoamine oxidase inhibitors. Concomitant use of esketamine with monoamine oxidase inhibitors may increase blood pressure. Closely monitor blood pressure with concomitant use of esketamine and monoamine oxidase inhibitors.
Use in special populations. Because of concerns of increased sedation, intranasal esketamine should be administered cautiously in patients receiving other CNS depressants, such as benzodiazepines. In patients with psychosis or a prior history of psychosis, esketamine should be used with increased caution and the risk/benefit ratio should be carefully considered.
Continue to: Because of potential teratogenicity...
Because of potential teratogenicity, esketamine is not recommended in women who are pregnant, may become pregnant, or who are currently nursing.
Intranasal esketamine was examined in a phase III trial of 194 patients age ≥65. At the end of 4 weeks, there was no statistically significant difference in groups on the MADRS, the primary efficacy endpoint. There were no overall differences in the safety profile in patients >65 years compared with younger patients; however, the mean esketamine Cmax and area under the curve were higher in older patients compared with younger adults. The mean esketamine half-life was longer in patients with moderate hepatic impairment.
Abuse liability
Esketamine is a CIII controlled substance and concerns about abuse, misuse, and diversion have been taken into account within the REMS drug safety program.2 Patients with a prior history of substance abuse or misuse should be considered with regard to the risk/benefit ratio.
The REMS drug safety program
Due to the nature of its usually transient adverse effects, including sedation, dissociation, hypertension, and nausea, intranasal esketamine will be administered through a REMS drug safety program at certified REMS treatment centers. Certified REMS treatment centers will receive training on how to safely and effectively counsel and monitor patients. Prior to treatment, patients will receive blood pressure monitoring and anticipated adverse effects will be discussed. Patients will be instructed to not eat solid food for 2 hours pre-dose and to not drink anything for 30 minutes prior.
A treatment session consists of nasal administration and a minimum 2-hour post-administration observation period. Blood pressure must be assessed prior to administration and if elevated, (ie, systolic blood pressure >140 mm Hg, diastolic >90 mm Hg), clinicians should consider the risk of short-term increases in blood pressure that may occur. Do not administer if increases in blood pressure or intracranial pressure pose a serious risk.
Continue to: After each intranasal...
After each intranasal administration the patient will be observed for 5 minutes before the second nasal inhaler is utilized and for another 5 minutes when the patient is receiving 84 mg (ie, each inhaler equals 28 mg). After administering, blood pressure should be reassessed at approximately 40 minutes, which corresponds to the Cmax of intranasal esketamine, and periodically thereafter as warranted.
The patient will then be monitored in a quiet environment for a minimum of 2 hours to make sure that dissociative phenomenon, sedation, and hypertensive reactions have normalized prior to discharge from a certified REMS treatment center.
Dosing and administration
Each intranasal device is primed for 2 infusions (1 in each nostril) for a total dose of 28 mg of esketamine. Combinations of devices can be used to adjust the dose as appropriate for individual patients. The recommended starting dose is 56 mg (ie, 2 devices, with a 5-minute gap between devices). The dose can be increased to 84 mg (ie, 3 intranasal devices spaced at 5-minute intervals) by the second dose based on clinical judgment.
The patient will be instructed to recline the head to a 45° angle, clear his or her nostrils prior to the first treatment, and then self-administer a dose to each nostril while holding the reciprocal nostril closed and inhaling. This process is then repeated every 5 minutes for each subsequent device, with a maximum total dose of 3 devices, or 84 mg (Figure 32). The patient will then be monitored for blood pressure, heart rate, and signs of psychologic or physiologic changes for the next 2 hours. Patients may not drive a car or operate any type of motor equipment until the following day after receiving a normal night’s sleep. Patients will be released from the REMS treatment center after 2 hours if both psychological and physical adverse effects have normalized.
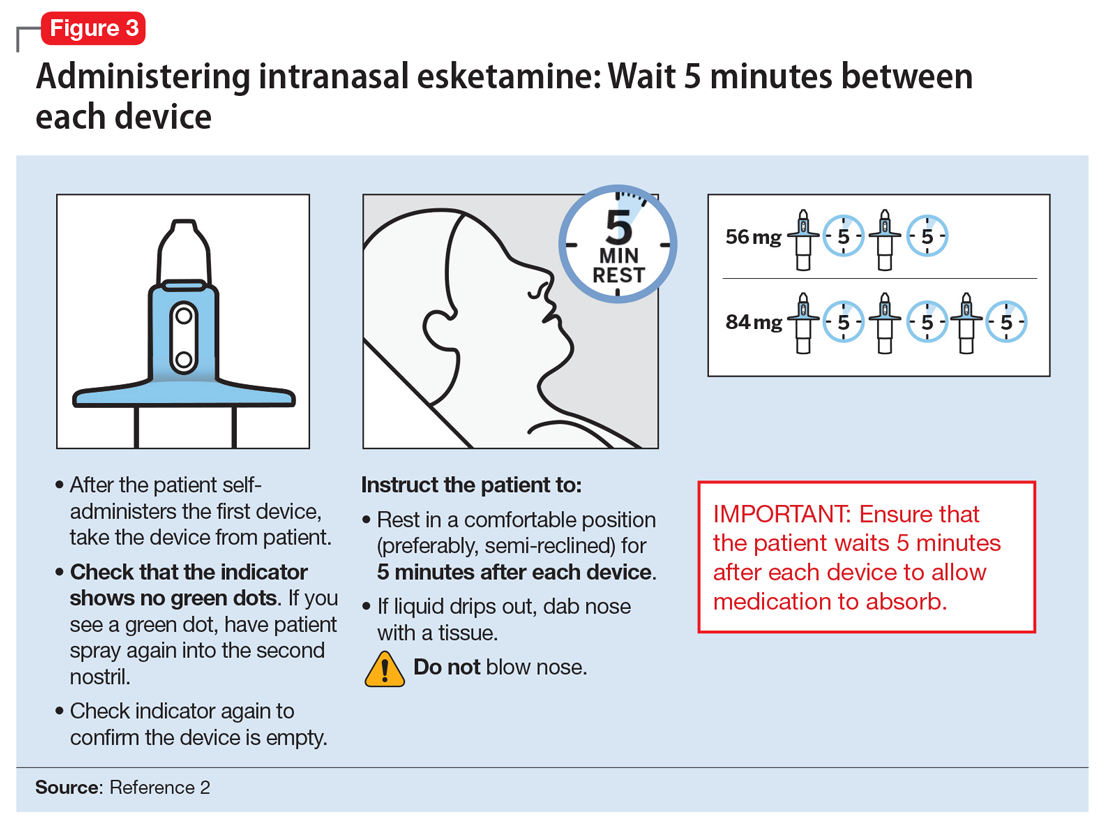
Missed treatment sessions. If a patient misses a treatment session and there is worsening of depressive symptoms, consider returning the patient to the previous dosing schedule (ie, every 2 weeks to once weekly, or weekly to twice weekly).
Continue to: Contraindications for...
Contraindications for intranasal esketamine include:
- aneurysmal vascular disease, including thoracic and abdominal aortic, intracranial, and peripheral arterial vessels, or arterial venous malformations
- history of intracerebral hemorrhage
- hypersensitivity to esketamine, ketamine, or any of the excipients.
Clinical considerations
Intranasal esketamine represents a unique delivery system for the first glutamatergic treatment approved for patients with TRD.
Why Rx? Treatment-resistant depression is found in nearly 1 out of 3 patients with currently available monoaminergic antidepressant treatment options. Patients with TRD are at increased risk of physical and psychological impairment, subsequent worsening of their condition, and social and occupational disability.
Bottom Line
Intranasal esketamine is the first glutamatergic treatment option FDA-approved for patients with treatment-resistant depression who have not responded to standard antidepressant treatment options. In short-term trials, intranasal esketamine significantly improved depressive symptoms as quickly as 24 hours after treatment, with significant improvement maintained through 4 weeks of ongoing administration. In addition, intranasal esketamine was shown to significantly decrease time to relapse for patients who had achieved stable remission or stable response.
Related Resource
- Sullivan MG. FDA approves intranasal esketamine for refractory major depressive disorder. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/195712/depression/fda-approves-intranasal-esketamine-refractory-major-depressive. Published March 5, 2019.
Drug Brand Names
Armodafinil • Nuvigil
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Mirtazapine • Remeron
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Rush AG, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR D Report. Am J Psychiatry. 2006;163(11):1905-1917.
2. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting anti-depression. Nat Med. 2016;22(3):238-249.
4. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
5. Daly EJ, Trivedi M, Janik A, et al. A randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
6. Wajs E, Aluisio L, Morrison R, et al. Long-term safety of esketamine nasal spray plus oral antidepressants in patients with treatment-resistant depression: phase III open-label safety and efficacy study. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
Treatment-resistant depression (TRD) is a common clinical struggle that practicing clinicians address on a daily basis. Major depressive disorder affects nearly 1 in 5 Americans at some point in their life and, by definition, impairs social and occupational functioning. Historic treatments have focused on the monoamine theories of depression—modulating the monoamines serotonin, norepinephrine, and/or dopamine. Limitations of currently available antidepressants include delayed onset of effect and low remission rates. To further complicate the matter, numerous studies have shown that with each subsequent antidepressant trial, patients have a decreasing likelihood of responding to subsequent antidepressant treatment options. For example, in the classic STAR*D trial, by the time a patient had not responded to the first 2 antidepressant options, the chance that they would respond to a third or fourth antidepressant had decreased to approximately 15% per antidepressant treatment course.1
To address the need for new treatments for patients with TRD, on March 5, 2019 the FDA-approved intranasal
How it works
Modern research has looked beyond the monoamine system to explore the neuro-modulatory effects of glutamate and gamma-aminobutyric acid (GABA).3 The yin and yang of glutamate and GABA revolves around neural excitation vs neural inhibition at a local synaptic level. The primary effects of the glutamate and GABA systems (Table 2) can be broken down into several key areas of understanding.
Glutamate modulates ionotropic N-methyl-
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and has been developed as an intranasal adjunctive treatment for TRD. Esketamine blocks NMDA receptors on GABA interneurons. This allows for increased pulsatile release of glutamate into the synapse. Intrasynaptic glutamate then stimulates postsynaptic AMPA receptors. Glutamate stimulation of postsynaptic AMPA receptors results in an intracellular cascade that activates the enzymes tropomyosin receptor kinase B (TrkB) and mammalian target of rapamycin (mTOR). TrkB stimulation results in increased production and release of BDNF. mTor stimulation increases neuronal membrane protein formation with subsequent increased neural plasticity. Taken together, preclinical models show that esketamine’s inhibition of the NMDA receptor on the GABA interneuron results in a cascade of increased BDNF release and synaptogenesis with increased neuroplasticity (Table 3).
Clinical implications
Treatment-resistant depression affects nearly one-third of patients currently receiving standard antidepressant treatment. Major depressive disorder is currently the second leading cause of disability for working adults within the United States and one of the largest causes of disability worldwide. The esketamine nasal spray could be beneficial for patients who have experienced TRD with standard monoamine antidepressants.
Supporting evidence
Clinical trials examining intranasal esketamine include both short- and long-term studies of patients with TRD.
Continue to: Esketamine was evaluated...
Esketamine was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week) phase III study in adult patients age 18 to 65 with TRD (they had not responded to at least 2 different antidepressants of adequate dose and duration).4 After discontinuing prior antidepressant treatments, all patients were started on a newly initiated antidepressant and were also randomized to concomitant intranasal esketamine or intranasal placebo as follows:
- 114 patients were randomized to the intranasal esketamine plus newly initiated oral antidepressant arm
- 109 patients were randomized to the placebo nasal spray plus newly initiated oral antidepressant arm
- The mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score for each group was 37 (ie, moderately to severely depressed).
Newly started antidepressants included esc

A long-term, double-blind multicenter maintenance-of-effect trial examined adults age 18 to 65 with TRD.5-6 Patients in this study were responders in 1 of 2 short-term studies or in an open-label direct enrollment study. Stable remission was defined as a MADRS total score <12 for at least 3 of the last 4 weeks of the study, and stable response was defined as a MADRS reduction of >50% but not in remission. After 16 weeks of intranasal esketamine plus an oral antidepressant, stable remitters and stable responders were then randomized separately to continue intranasal esketamine or switch to placebo nasal spray, with both groups continuing on their concomitant oral antidepressant. The primary study endpoint was time to relapse. Relapse was defined as a MADRS total score >22 for more than 2 consecutive weeks, hospitalization for worsening of depression, or any other clinically relevant event. The median age was 48, 66% were female, 90% were White and 4% were black. Patients in stable response or stable remission experienced a significantly longer time to relapse compared with patients who continued their oral antidepressant but were switched to placebo intranasal spray. In this remission response study, patients could receive intranasal treatment weekly or bi-weekly based on symptom severity (Figure 22).

Impact on driving. Two studies examined the impact of esketamine on driving performance. One examined adults with major depressive disorder and the other examined healthy participants. The effects of a single 84-mg dose of esketamine nasal spray on a patient’s ability to drive was assessed in 23 healthy adults. In this study, mirt
A second study evaluated the effects of repeated esketamine administration on driving performance in 25 adults with major depressive disorder. In this study, an ethanol-containing beverage was used as an active control. After administration of a single 84-mg dose of intranasal esketamine, driving performance was the same as a placebo at 18 hours. In the multiple dose phase, standard driving performance was similar for esketamine nasal spray and placebo at 6 hours postdose on Days 11, 18, and 25.
Continue to: Pharmacologic profile
Pharmacologic profile
Adverse events. The most common adverse events in patients treated with esketamine nasal spray were dissociation (41%), dizziness (29%), nausea (28%), sedation (23%), and vertigo (23%).2 The majority of these effects were short-term and resolved during the 2-hour observation period.
In addition to spontaneously reported events, sedation and dissociation were further monitored with specific scales. Sedation was measured with the Modified Observer’s Alertness and Sedation Scale. Using this scale, 50% of patients receiving 56 mg and 61% of patients receiving 84 mg of esketamine met criteria for sedation.
Similarly, dissociation/perceptional changes were measured with spontaneously reported events and also with the Clinician Administered Dissociative State Scale. On this scale, 61% of patients receiving the 56-mg dose, and 69% of patients receiving the 84-mg dose met criteria for dissociation/perceptional changes after dose administration.
Increases in blod pressure. Esketamine intranasal spray was associated with a 7 to 9 mm Hg increase in systolic blood pressure and a 4 to 6 mm Hg increase in diastolic blood pressure, both of which peaked 40 minutes post-dose.
Nausea and vomiting. Intranasal esketamine was associated with a 27% rate of nausea at 56 mg, and 32% at 84 mg, with a 6% rate of vomiting at 56 mg and 12% at 84 mg.
Continue to: Pharmacokinetics
Pharmacokinetics
Esketamine exposure increases from 28 to 84 mg in a fairly dose-proportional range. No accumulation of esketamine was observed in the plasma following twice-weekly administration. Bioavailability is approximately 48% following nasal administration. The Tmax for esketamine plasma concentration is 20 to 40 minutes after the last nasal spray. Protein binding of esketamine is approximately 43% to 45%. The brain-to-plasma ratio of noresketamine is 4 to 6 times lower than that of esketamine. The half-life of esketamine ranged from 7 to 12 hours. The mean half-life of nore
Potential drug interactions
Central nervous system depressants. Concomitant use of esketamine and other CNS depressants (ie, benzodiazepines, opioids, alcohol) may increase sedation. Patients receiving esketamine with concomitant use of other CNS depressants should be closely monitored for sedation.
Psychostimulants. Concomitant use of esketamine and psychostimulants (ie, amphetamines, methylphenidates, moda
Monoamine oxidase inhibitors. Concomitant use of esketamine with monoamine oxidase inhibitors may increase blood pressure. Closely monitor blood pressure with concomitant use of esketamine and monoamine oxidase inhibitors.
Use in special populations. Because of concerns of increased sedation, intranasal esketamine should be administered cautiously in patients receiving other CNS depressants, such as benzodiazepines. In patients with psychosis or a prior history of psychosis, esketamine should be used with increased caution and the risk/benefit ratio should be carefully considered.
Continue to: Because of potential teratogenicity...
Because of potential teratogenicity, esketamine is not recommended in women who are pregnant, may become pregnant, or who are currently nursing.
Intranasal esketamine was examined in a phase III trial of 194 patients age ≥65. At the end of 4 weeks, there was no statistically significant difference in groups on the MADRS, the primary efficacy endpoint. There were no overall differences in the safety profile in patients >65 years compared with younger patients; however, the mean esketamine Cmax and area under the curve were higher in older patients compared with younger adults. The mean esketamine half-life was longer in patients with moderate hepatic impairment.
Abuse liability
Esketamine is a CIII controlled substance and concerns about abuse, misuse, and diversion have been taken into account within the REMS drug safety program.2 Patients with a prior history of substance abuse or misuse should be considered with regard to the risk/benefit ratio.
The REMS drug safety program
Due to the nature of its usually transient adverse effects, including sedation, dissociation, hypertension, and nausea, intranasal esketamine will be administered through a REMS drug safety program at certified REMS treatment centers. Certified REMS treatment centers will receive training on how to safely and effectively counsel and monitor patients. Prior to treatment, patients will receive blood pressure monitoring and anticipated adverse effects will be discussed. Patients will be instructed to not eat solid food for 2 hours pre-dose and to not drink anything for 30 minutes prior.
A treatment session consists of nasal administration and a minimum 2-hour post-administration observation period. Blood pressure must be assessed prior to administration and if elevated, (ie, systolic blood pressure >140 mm Hg, diastolic >90 mm Hg), clinicians should consider the risk of short-term increases in blood pressure that may occur. Do not administer if increases in blood pressure or intracranial pressure pose a serious risk.
Continue to: After each intranasal...
After each intranasal administration the patient will be observed for 5 minutes before the second nasal inhaler is utilized and for another 5 minutes when the patient is receiving 84 mg (ie, each inhaler equals 28 mg). After administering, blood pressure should be reassessed at approximately 40 minutes, which corresponds to the Cmax of intranasal esketamine, and periodically thereafter as warranted.
The patient will then be monitored in a quiet environment for a minimum of 2 hours to make sure that dissociative phenomenon, sedation, and hypertensive reactions have normalized prior to discharge from a certified REMS treatment center.
Dosing and administration
Each intranasal device is primed for 2 infusions (1 in each nostril) for a total dose of 28 mg of esketamine. Combinations of devices can be used to adjust the dose as appropriate for individual patients. The recommended starting dose is 56 mg (ie, 2 devices, with a 5-minute gap between devices). The dose can be increased to 84 mg (ie, 3 intranasal devices spaced at 5-minute intervals) by the second dose based on clinical judgment.
The patient will be instructed to recline the head to a 45° angle, clear his or her nostrils prior to the first treatment, and then self-administer a dose to each nostril while holding the reciprocal nostril closed and inhaling. This process is then repeated every 5 minutes for each subsequent device, with a maximum total dose of 3 devices, or 84 mg (Figure 32). The patient will then be monitored for blood pressure, heart rate, and signs of psychologic or physiologic changes for the next 2 hours. Patients may not drive a car or operate any type of motor equipment until the following day after receiving a normal night’s sleep. Patients will be released from the REMS treatment center after 2 hours if both psychological and physical adverse effects have normalized.

Missed treatment sessions. If a patient misses a treatment session and there is worsening of depressive symptoms, consider returning the patient to the previous dosing schedule (ie, every 2 weeks to once weekly, or weekly to twice weekly).
Continue to: Contraindications for...
Contraindications for intranasal esketamine include:
- aneurysmal vascular disease, including thoracic and abdominal aortic, intracranial, and peripheral arterial vessels, or arterial venous malformations
- history of intracerebral hemorrhage
- hypersensitivity to esketamine, ketamine, or any of the excipients.
Clinical considerations
Intranasal esketamine represents a unique delivery system for the first glutamatergic treatment approved for patients with TRD.
Why Rx? Treatment-resistant depression is found in nearly 1 out of 3 patients with currently available monoaminergic antidepressant treatment options. Patients with TRD are at increased risk of physical and psychological impairment, subsequent worsening of their condition, and social and occupational disability.
Bottom Line
Intranasal esketamine is the first glutamatergic treatment option FDA-approved for patients with treatment-resistant depression who have not responded to standard antidepressant treatment options. In short-term trials, intranasal esketamine significantly improved depressive symptoms as quickly as 24 hours after treatment, with significant improvement maintained through 4 weeks of ongoing administration. In addition, intranasal esketamine was shown to significantly decrease time to relapse for patients who had achieved stable remission or stable response.
Related Resource
- Sullivan MG. FDA approves intranasal esketamine for refractory major depressive disorder. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/195712/depression/fda-approves-intranasal-esketamine-refractory-major-depressive. Published March 5, 2019.
Drug Brand Names
Armodafinil • Nuvigil
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Mirtazapine • Remeron
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
Treatment-resistant depression (TRD) is a common clinical struggle that practicing clinicians address on a daily basis. Major depressive disorder affects nearly 1 in 5 Americans at some point in their life and, by definition, impairs social and occupational functioning. Historic treatments have focused on the monoamine theories of depression—modulating the monoamines serotonin, norepinephrine, and/or dopamine. Limitations of currently available antidepressants include delayed onset of effect and low remission rates. To further complicate the matter, numerous studies have shown that with each subsequent antidepressant trial, patients have a decreasing likelihood of responding to subsequent antidepressant treatment options. For example, in the classic STAR*D trial, by the time a patient had not responded to the first 2 antidepressant options, the chance that they would respond to a third or fourth antidepressant had decreased to approximately 15% per antidepressant treatment course.1
To address the need for new treatments for patients with TRD, on March 5, 2019 the FDA-approved intranasal
How it works
Modern research has looked beyond the monoamine system to explore the neuro-modulatory effects of glutamate and gamma-aminobutyric acid (GABA).3 The yin and yang of glutamate and GABA revolves around neural excitation vs neural inhibition at a local synaptic level. The primary effects of the glutamate and GABA systems (Table 2) can be broken down into several key areas of understanding.
Glutamate modulates ionotropic N-methyl-
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and has been developed as an intranasal adjunctive treatment for TRD. Esketamine blocks NMDA receptors on GABA interneurons. This allows for increased pulsatile release of glutamate into the synapse. Intrasynaptic glutamate then stimulates postsynaptic AMPA receptors. Glutamate stimulation of postsynaptic AMPA receptors results in an intracellular cascade that activates the enzymes tropomyosin receptor kinase B (TrkB) and mammalian target of rapamycin (mTOR). TrkB stimulation results in increased production and release of BDNF. mTor stimulation increases neuronal membrane protein formation with subsequent increased neural plasticity. Taken together, preclinical models show that esketamine’s inhibition of the NMDA receptor on the GABA interneuron results in a cascade of increased BDNF release and synaptogenesis with increased neuroplasticity (Table 3).
Clinical implications
Treatment-resistant depression affects nearly one-third of patients currently receiving standard antidepressant treatment. Major depressive disorder is currently the second leading cause of disability for working adults within the United States and one of the largest causes of disability worldwide. The esketamine nasal spray could be beneficial for patients who have experienced TRD with standard monoamine antidepressants.
Supporting evidence
Clinical trials examining intranasal esketamine include both short- and long-term studies of patients with TRD.
Continue to: Esketamine was evaluated...
Esketamine was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week) phase III study in adult patients age 18 to 65 with TRD (they had not responded to at least 2 different antidepressants of adequate dose and duration).4 After discontinuing prior antidepressant treatments, all patients were started on a newly initiated antidepressant and were also randomized to concomitant intranasal esketamine or intranasal placebo as follows:
- 114 patients were randomized to the intranasal esketamine plus newly initiated oral antidepressant arm
- 109 patients were randomized to the placebo nasal spray plus newly initiated oral antidepressant arm
- The mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score for each group was 37 (ie, moderately to severely depressed).
Newly started antidepressants included esc

A long-term, double-blind multicenter maintenance-of-effect trial examined adults age 18 to 65 with TRD.5-6 Patients in this study were responders in 1 of 2 short-term studies or in an open-label direct enrollment study. Stable remission was defined as a MADRS total score <12 for at least 3 of the last 4 weeks of the study, and stable response was defined as a MADRS reduction of >50% but not in remission. After 16 weeks of intranasal esketamine plus an oral antidepressant, stable remitters and stable responders were then randomized separately to continue intranasal esketamine or switch to placebo nasal spray, with both groups continuing on their concomitant oral antidepressant. The primary study endpoint was time to relapse. Relapse was defined as a MADRS total score >22 for more than 2 consecutive weeks, hospitalization for worsening of depression, or any other clinically relevant event. The median age was 48, 66% were female, 90% were White and 4% were black. Patients in stable response or stable remission experienced a significantly longer time to relapse compared with patients who continued their oral antidepressant but were switched to placebo intranasal spray. In this remission response study, patients could receive intranasal treatment weekly or bi-weekly based on symptom severity (Figure 22).

Impact on driving. Two studies examined the impact of esketamine on driving performance. One examined adults with major depressive disorder and the other examined healthy participants. The effects of a single 84-mg dose of esketamine nasal spray on a patient’s ability to drive was assessed in 23 healthy adults. In this study, mirt
A second study evaluated the effects of repeated esketamine administration on driving performance in 25 adults with major depressive disorder. In this study, an ethanol-containing beverage was used as an active control. After administration of a single 84-mg dose of intranasal esketamine, driving performance was the same as a placebo at 18 hours. In the multiple dose phase, standard driving performance was similar for esketamine nasal spray and placebo at 6 hours postdose on Days 11, 18, and 25.
Continue to: Pharmacologic profile
Pharmacologic profile
Adverse events. The most common adverse events in patients treated with esketamine nasal spray were dissociation (41%), dizziness (29%), nausea (28%), sedation (23%), and vertigo (23%).2 The majority of these effects were short-term and resolved during the 2-hour observation period.
In addition to spontaneously reported events, sedation and dissociation were further monitored with specific scales. Sedation was measured with the Modified Observer’s Alertness and Sedation Scale. Using this scale, 50% of patients receiving 56 mg and 61% of patients receiving 84 mg of esketamine met criteria for sedation.
Similarly, dissociation/perceptional changes were measured with spontaneously reported events and also with the Clinician Administered Dissociative State Scale. On this scale, 61% of patients receiving the 56-mg dose, and 69% of patients receiving the 84-mg dose met criteria for dissociation/perceptional changes after dose administration.
Increases in blod pressure. Esketamine intranasal spray was associated with a 7 to 9 mm Hg increase in systolic blood pressure and a 4 to 6 mm Hg increase in diastolic blood pressure, both of which peaked 40 minutes post-dose.
Nausea and vomiting. Intranasal esketamine was associated with a 27% rate of nausea at 56 mg, and 32% at 84 mg, with a 6% rate of vomiting at 56 mg and 12% at 84 mg.
Continue to: Pharmacokinetics
Pharmacokinetics
Esketamine exposure increases from 28 to 84 mg in a fairly dose-proportional range. No accumulation of esketamine was observed in the plasma following twice-weekly administration. Bioavailability is approximately 48% following nasal administration. The Tmax for esketamine plasma concentration is 20 to 40 minutes after the last nasal spray. Protein binding of esketamine is approximately 43% to 45%. The brain-to-plasma ratio of noresketamine is 4 to 6 times lower than that of esketamine. The half-life of esketamine ranged from 7 to 12 hours. The mean half-life of nore
Potential drug interactions
Central nervous system depressants. Concomitant use of esketamine and other CNS depressants (ie, benzodiazepines, opioids, alcohol) may increase sedation. Patients receiving esketamine with concomitant use of other CNS depressants should be closely monitored for sedation.
Psychostimulants. Concomitant use of esketamine and psychostimulants (ie, amphetamines, methylphenidates, moda
Monoamine oxidase inhibitors. Concomitant use of esketamine with monoamine oxidase inhibitors may increase blood pressure. Closely monitor blood pressure with concomitant use of esketamine and monoamine oxidase inhibitors.
Use in special populations. Because of concerns of increased sedation, intranasal esketamine should be administered cautiously in patients receiving other CNS depressants, such as benzodiazepines. In patients with psychosis or a prior history of psychosis, esketamine should be used with increased caution and the risk/benefit ratio should be carefully considered.
Continue to: Because of potential teratogenicity...
Because of potential teratogenicity, esketamine is not recommended in women who are pregnant, may become pregnant, or who are currently nursing.
Intranasal esketamine was examined in a phase III trial of 194 patients age ≥65. At the end of 4 weeks, there was no statistically significant difference in groups on the MADRS, the primary efficacy endpoint. There were no overall differences in the safety profile in patients >65 years compared with younger patients; however, the mean esketamine Cmax and area under the curve were higher in older patients compared with younger adults. The mean esketamine half-life was longer in patients with moderate hepatic impairment.
Abuse liability
Esketamine is a CIII controlled substance and concerns about abuse, misuse, and diversion have been taken into account within the REMS drug safety program.2 Patients with a prior history of substance abuse or misuse should be considered with regard to the risk/benefit ratio.
The REMS drug safety program
Due to the nature of its usually transient adverse effects, including sedation, dissociation, hypertension, and nausea, intranasal esketamine will be administered through a REMS drug safety program at certified REMS treatment centers. Certified REMS treatment centers will receive training on how to safely and effectively counsel and monitor patients. Prior to treatment, patients will receive blood pressure monitoring and anticipated adverse effects will be discussed. Patients will be instructed to not eat solid food for 2 hours pre-dose and to not drink anything for 30 minutes prior.
A treatment session consists of nasal administration and a minimum 2-hour post-administration observation period. Blood pressure must be assessed prior to administration and if elevated, (ie, systolic blood pressure >140 mm Hg, diastolic >90 mm Hg), clinicians should consider the risk of short-term increases in blood pressure that may occur. Do not administer if increases in blood pressure or intracranial pressure pose a serious risk.
Continue to: After each intranasal...
After each intranasal administration the patient will be observed for 5 minutes before the second nasal inhaler is utilized and for another 5 minutes when the patient is receiving 84 mg (ie, each inhaler equals 28 mg). After administering, blood pressure should be reassessed at approximately 40 minutes, which corresponds to the Cmax of intranasal esketamine, and periodically thereafter as warranted.
The patient will then be monitored in a quiet environment for a minimum of 2 hours to make sure that dissociative phenomenon, sedation, and hypertensive reactions have normalized prior to discharge from a certified REMS treatment center.
Dosing and administration
Each intranasal device is primed for 2 infusions (1 in each nostril) for a total dose of 28 mg of esketamine. Combinations of devices can be used to adjust the dose as appropriate for individual patients. The recommended starting dose is 56 mg (ie, 2 devices, with a 5-minute gap between devices). The dose can be increased to 84 mg (ie, 3 intranasal devices spaced at 5-minute intervals) by the second dose based on clinical judgment.
The patient will be instructed to recline the head to a 45° angle, clear his or her nostrils prior to the first treatment, and then self-administer a dose to each nostril while holding the reciprocal nostril closed and inhaling. This process is then repeated every 5 minutes for each subsequent device, with a maximum total dose of 3 devices, or 84 mg (Figure 32). The patient will then be monitored for blood pressure, heart rate, and signs of psychologic or physiologic changes for the next 2 hours. Patients may not drive a car or operate any type of motor equipment until the following day after receiving a normal night’s sleep. Patients will be released from the REMS treatment center after 2 hours if both psychological and physical adverse effects have normalized.

Missed treatment sessions. If a patient misses a treatment session and there is worsening of depressive symptoms, consider returning the patient to the previous dosing schedule (ie, every 2 weeks to once weekly, or weekly to twice weekly).
Continue to: Contraindications for...
Contraindications for intranasal esketamine include:
- aneurysmal vascular disease, including thoracic and abdominal aortic, intracranial, and peripheral arterial vessels, or arterial venous malformations
- history of intracerebral hemorrhage
- hypersensitivity to esketamine, ketamine, or any of the excipients.
Clinical considerations
Intranasal esketamine represents a unique delivery system for the first glutamatergic treatment approved for patients with TRD.
Why Rx? Treatment-resistant depression is found in nearly 1 out of 3 patients with currently available monoaminergic antidepressant treatment options. Patients with TRD are at increased risk of physical and psychological impairment, subsequent worsening of their condition, and social and occupational disability.
Bottom Line
Intranasal esketamine is the first glutamatergic treatment option FDA-approved for patients with treatment-resistant depression who have not responded to standard antidepressant treatment options. In short-term trials, intranasal esketamine significantly improved depressive symptoms as quickly as 24 hours after treatment, with significant improvement maintained through 4 weeks of ongoing administration. In addition, intranasal esketamine was shown to significantly decrease time to relapse for patients who had achieved stable remission or stable response.
Related Resource
- Sullivan MG. FDA approves intranasal esketamine for refractory major depressive disorder. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/195712/depression/fda-approves-intranasal-esketamine-refractory-major-depressive. Published March 5, 2019.
Drug Brand Names
Armodafinil • Nuvigil
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Mirtazapine • Remeron
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Rush AG, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR D Report. Am J Psychiatry. 2006;163(11):1905-1917.
2. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting anti-depression. Nat Med. 2016;22(3):238-249.
4. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
5. Daly EJ, Trivedi M, Janik A, et al. A randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
6. Wajs E, Aluisio L, Morrison R, et al. Long-term safety of esketamine nasal spray plus oral antidepressants in patients with treatment-resistant depression: phase III open-label safety and efficacy study. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
1. Rush AG, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR D Report. Am J Psychiatry. 2006;163(11):1905-1917.
2. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting anti-depression. Nat Med. 2016;22(3):238-249.
4. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
5. Daly EJ, Trivedi M, Janik A, et al. A randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
6. Wajs E, Aluisio L, Morrison R, et al. Long-term safety of esketamine nasal spray plus oral antidepressants in patients with treatment-resistant depression: phase III open-label safety and efficacy study. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.