User login
Patient-Centered HIV Treatment Options: Practical Considerations
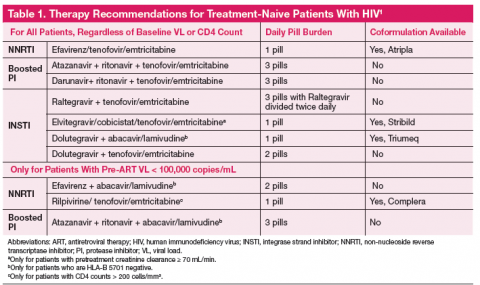
The one pill daily revolution in human immunodeficiency virus (HIV) management continues with the recent FDA approval of Triumeq, a combination tablet with an integrase inhibitor backbone containing dolutegravir, abacavir, and lamivudine. The first all-in-one protease inhibitor (PI) option is on the horizon and might receive approval before this article is published. The Department of Health and Human Services (DHHS) recommends 7 regimens for any treatment-naive patient with HIV, with 3 additional options if the patient has a baseline plasma RNA viral load (VL) < 100,000 copies/mL (Table 1).1 With 10 first-line options and 4 once daily pill regimens, HIV treatment is poised to enter the realm of managed care.
Guidelines recommend treatment of all patients with HIV, both to slow disease progression, and to reduce the risk of HIV transmission.1 Regimens should be individual ized based on vi rologi c efficacy, toxicity, pill burden, dosing frequency, drug interaction potential, drug resistance testing results, comorbid conditions, and cost. Therapy should only be deferred on a case-by-case basis. Patients considering therapy must be willing and able to commit to taking daily medications and understand the risks and benefits of therapy. Each antiretroviral (ARV) class, and each individual drug, has its own set of advantages and disadvantages. This article will review considerations when choosing a regimen, provide a brief overview of the first-line treatment options, and finally touch on cost considerations.
Choosing An Appropriate Regimen
Choosing an antiviral regimen has 2 basic steps. First, a provider must determine what choices are medically appropriate. An appropriate regimen has 3 active agents with acceptable performance and no contraindications. An ARV regimen generally consists of 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus 1 drug from 1 of the following classes: non-nucleoside reverse transcriptase inhibitor (NNRTI); PI boosted with ritonavir or cobicistat; or integrase strand transfer inhibitor (INSTI). The next step is helping the patient determine which regimen will suit him or her best considering dosing, food requirements, and potential adverse effects.
Genotype, Viral Load, and CD4 Testing at Baseline
A primary consideration is, “Will the regimen work?” This question can best be answered by the genotype, plasma RNA VL, and CD4 count. Baseline genotypes should be obtained for all patients and used to eliminate any inferior drug-resistant treatment options. Viral loads > 100,000 copies/mL eliminate 3 treatment options due to inferiority (inability to fully suppress the HIV virus) in patients with high VL (Table 1). Specifically, abacavir is not recommended (unless given with dolutegravir) with VL > 100,000 copies/mL. Rilpivirine is also not recommended for patients with VL > 100,000 copies/mL or CD4 < 200 cells/mm3.
Creatinine Clearance
Many NRTIs require dosage adjustment when creatinine clearance (CrCl) falls below 50 mL/min. Cobicistat is a novel pharmacokinetic enhancer or boosting agent and is only recommended for baseline CrCl > 70 mL/min.2
HLA-B 5701
If a patient has a positive HLA-B 5701 screening test, abacavir is strictly contraindicated due to the propensity for abacavir hypersensitivity, a potentially life-threatening reaction.
Allergies
Darunavir has a sulfonamide moiety and should be avoided in patients with a sulfa allergy.
Drug Interactions
Drug interactions should be a consideration for all patients with an extensive review of their current medications. Pharmaco-enhancers or “boosting agents,” such as ritonavir or cobicistat, are used to increase drug levels of HIV medications. However, since this is accomplished by inhibition of CYP3A there is great potential for interaction with other medications; opiates, amphetamines, sildenafil, most lipid-lowering agents, and numerous other drugs have significant interactions with ritonavir and cobicistat. Looking at current medications may not be enough; some consideration should be made for the potential of adding medications. For example, if a patient has a seizure disorder and is not well controlled on levetiracetam, consideration should be made if there could be a potential need for phenytoin or other agent that commonly interacts with some HIV medications.
Adverse Events
Adverse events (AEs) are a large consideration when choosing ARV therapy (ART) and can either have medical implications or be patient-specific. Some agents have severe AEs like worsening depression and suicidal ideation. Others are less severe and a particular patient may prefer a risk for gastrointestinal (GI) AEs rather than insomnia.
Adherence
Predicting adherence is difficult; however, certain factors may predispose a patient to being less adherent, including active substance abuse, depression or other mood disorder, unstable housing, and inconsistent access to food. Some regimens are more forgiving and require a lower level of adherence and are less likely to have resistance mutations with failure. Both ritonavir-boosted PIs, as well as dolutegravir, seem to have the best outcomes with lower levels of adherence. Tailoring the dosing and food requirements to a patient’s lifestyle can also improve adherence.
Childbearing Age
Pregnancy and potential for pregnancy must be considered in all female patients. Although pregnancy is beyond the scope of this discussion, the perinatal guidelines should be considered in women of childbearing age.3
Custom A Regimen
Convenience is a major consideration when selecting a regimen. Comparator studies done to demonstrate regimen efficacy using intention-to-treat structures highlight this point. Many patients express that a single tablet is their most important priority. Other important considerations for regimen selection are food requirements or restrictions, and whether there are drug coadministration concerns (eg, divalent cations in antacids or vitamins). These will be discussed more with each
individual ARV agent.
Now that regimen considerations have been reviewed from both provider and patient perspectives, we will focus on the individual classes of ARVs to make selection of a regimen more tailored to patient needs.
The NNRTI Class
The NNRTI class has 2 first-line “recommended” agents, efavirenz and rilpivirine, which are both conveniently available as “one-pill-a-day” coformulated regimens with tenofovir and emtricitabine.1 While the 2 NNRTIs are in the same class, they each have unique properties that would lead a clinician to choose one versus the other.
Efavirenz is the only NNRTI recommended to be paired with abacavir and lamivudine (coformulated as Epzicom) first-line nucleosides. However, it is only recommended to be used in patients with VL < 100,000 copies/mL.
Efavirenz is one of the most studied and widely used ARV. Efavirenz/emtricitabine/tenofovir (coformulated as Atripla) was the first once daily regimen and has shown noninferiority or superiority to most ARV regimens. However, AEs are the most common reason for discontinuation. Efavirenz is well known for its CNS AEs, including vivid dreams, somnolence, impaired concentration, headaches, and depression. These AEs typically diminish or abate after several weeks of therapy. However, certain CNS effects may be long term, including depression or suicidal thoughts.
The recommendation of taking efavirenz regimens at bedtime on an empty stomach to reduce CNS AEs is not ideal for every patient. Efavirenz can also have additive CNS AEs with alcohol and potentially lead to blackouts. It can also lead to false positives for marijuana and benzodiazepines on drug screenings. The FDA classifies efavirenz as Pregnancy Category D, so it should be avoided in women of childbearing age.4 However, if pregnancy isdetected while someone is taking efavirenz there is no need to discontinue the agent since the neural tube has formed by that time.3
Rilpivirine is better tolerated than efavirenz, with fewer and less severe CNS AEs. Rilpivirine/emtricitabine/tenofovir (coformulated as Complera) has the convenience of being 1 pill once daily; however, it is not appropriate for all patients. It should be avoided in patients with a pretreatment VL > 100,000 copies/mL or CD4 < 200 cells/mm3. Patients with values beyond these thresholds were more likely to experience early virologic failure in the first months of therapy compared to efavirenz/emtricitabine/tenofovir.5 Because it is a CYP3A4 substrate, rilpivirine has important drug-drug interactions. Proton pump inhibitors are contraindicated, and H2 receptor antagonists, like ranitidine or famotidine, require specific separation intervals, as do antacids and divalent cations. Another item of importance is that it should be administered with a meal.
The NNRTI class is plagued by its low genetic barrier to resistance, with a single mutation that may confer resistance to nearly all NNRTIs. Estimated transmitted HIV drug resistance at baseline in men who have sex with men can be 8% to 10%.6 Transmitted resistance is a concern, a baseline genotype can determine if a patient is at a higher risk for virologic failure with NNRTIs.
Protease Inhibitors
Protease inhibitors have gone from the main stay of HIV treatment to being a less desirable first-line treatment option. Protease inhibitors have the highest pill burden and drug interactions of the first-line agents; they must be taken with food, and as of October 2014 there are no coformulated tablets (other than lopinavir/ritonavir which is not first-line). New combination tablets utilizing cobicistat as a boosting agent may resuscitate the use of the PIs. The coformulations under investigation are atazanavir/cobicistat, darunavir/cobicistat, and darunavir/cobicistat/emtricitabine/tenofovir alafenamide. These combinations will decrease the pill burden for PIs to 1 to 2 pill(s) a day instead of 3 pills a day. Although 1 to 3 pills taken once daily does not sound like a big difference, given a choice, the majority of patients choose the least number of pills.
How Does Cobicistat Compare With Ritonavir?
Cobicistat has no HIV activity and may be better tolerated than ritonavir. Effects on total cholesterol and triglycerides are similar between the 2 boosting agents.7 Cobicistat causes a modest, rapid increase in serum creatinine (SrCr) through inhibition of tubular secretion without impact on the glomerular filtration rate. The coformulated product of cobicistat/elvitegravir is not recommended to be initiated in patients with CrCl < 70 mL/min at baseline.2 It is anticipated that cobicistat-boosted PI regimens will have a similar limitation. The elevation of SrCr from cobicistat makes it difficult to monitor for tenofovir-related renal dysfunction since SrCr is no longer a reliable surrogate marker for renal function.7 Cobicistat and ritonavir inhibit CYP3A4 and have almost identical drug interactions. However, only ritonavir interacts with methadone.
The first-line PIs are better tolerated than other PIs. Gastrointestinal AEs are still common. Scleral icterus and moderate to severe jaundice occur in 5% to 9% of patients taking atazanavir due to inhibition of the indirect metabolism of bilirubin.8 As a sulfa-related drug, darunavir is generally avoided if the patient has a sulfa allergy, but can be used with caution.9 Another limitation for PIs is that both options are required to be taken with food.
Protease inhibitors will always have a place in treatment-experienced patients with drug resistance, but may have a unique role in patients with baseline M184V mutations. A retrospective analysis showed that patients with a M184V mutation alone achieved equivalent viral suppression if the patient was on 3 fully active HIV ARVs or on a boosted PI, lamivudine or emtricitabine, and 1 additional NRTI.10 Patients with only a M184V mutation can still achieve full suppression without adding an additional ARV agent or using 3 HIV drug classes, preserving future treatment options. Boosted-PI regimens have less drug resistance at failure than NNRTI-based regimens. Less than 5% of new infections have baseline (pretreatment) drug resistance to PIs.6
Boosted PIs have a long clinical history and are still appropriate for many treatment-naive and experienced patients. Reduced pill burden with cobicistat formulation may revive utilization when the pill burden decreases from 3 tablets per day to 1 or 2.
Integrase Strand Inhibitors
INSTI-based regimens have had low discontinuation rates overall in clinical trials, and are great options for a majority of patients. There are 3 INSTIs, all of which are on the list of preferred agents according to the DHHS guidelines. INSTIs may be used with any baseline VL or CD4 counts, without concern for potency. Each of the 3 agents has clear benefits and disadvantages when choosing among them.
Raltegravir has no food requirements and has the least amount of drug interactions of nearly all ART regimens since it is not processed via cytochrome P450 enzymes, but it is dosed twice daily. When paired with emtricitabine/tenofovir, it is also the preferred regimen for occupational postexposure prophylaxis.
Dolut egr avi r i s onc e da i ly, ha s no food requirements, and also avoids drug interactions due to cytochrome P450 enzymes. However, this ARV agent does inhibit the renal organic cation transporter, so some clinically relevant interactions exist. Dolutegravir also has the benefit of being paired with either emtricitabine/tenofovir or abacavir/lamivudine, both of which are preferred DHHS regimens. As mentioned previously, dolutegravir/abacavir/lamivudine are now coformulated
and are an additional all-in-one option. Abacavir has conflicting data regarding increased risk for myocardial infarction; this risk should be considered in light of other risk factors for the patient including age, diabetes, etc.11
Elvitegravir is unique as an INSTI. Elvitegravir/cobicistat/emtricitabine/tenofovir must be administered with food and since it utilizes cobicistat as a pharmaco-enhancer to boost elvitegravir’s levels, there are a significant number of potential drug interactions. Cobicistat-containing regimens must not be prescribed for patients with a baseline CrCl <70 mL/min.2
Both dolutegravir and raltegravir can be taken with or without food. However, if the patient is taking a divalent cation (magnesium, iron, or calcium) then the medications must be separated, causing the patient to take medications multiple times per day, or taken with food (and lose the benefit of with or without food).
Cost and the New Era of ARV Generics
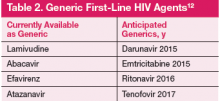
Previously, the majority of HIV regimens were similarly priced and had little impact on prescribing practices. Several ARV are now available as generics. In the next 2 years, generics may have major impact on prescribing practice as first-line options (darunavir, atazanavir, and efavirenz) will be available as generics (Table 2).12
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Author Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of Health and Human Services, the Indian Health Services, or the U.S. Government. Please review complete prescribing information for specific drugs or drug combinations—including but not limited to indications, contraindications, warnings, adverse effects, and drug interactions- before administering pharmacologic therapy to patients.
Journal Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combination—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. HHS Panel on Antiretroviral Guidelines for Adults and Adolescents—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfile/lvguidelines/AdultandAdolescentGL.pdf. Updated November 13, 2014. Accessed December 1, 2014.
2. Stribild [package insert]. Foster City, CA: Gilead Sciences, Inc; 2012.
3. HHS Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Updated March 28, 2014. Accessed October 27, 2014.
4. Atripla [package insert] Princeton, NJ: Bristol-Myers Squibb; Foster City, CA: Gilead Sciences, Inc; 2013.
5. Cohen CJ, Molina JM, Cassetti I, et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS. 2013;27(6):939-950.
6. Bañez Ocfemia MC, Saduvala N, Oster AM, et al. Transmitted HIV-1 drug resistance among men who have sex with men, 11 US jurisdictions, 2008-2011. Presented at: 21st Conference on Retroviruses and Opportunistic Infections; March 3-6, 2014; Boston, MA. Abstract 579.
7. Gallant JE, Koenig E, Andrade-Villaneuva J, et al. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: Week 48 results. J Infect Dis. 2013;208(1):32-39.
8. Reyatoz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
9. Prezista [package insert]. Titusville, NJ: Janssen Pharmaceuicals, Inc; 2013.
10. Hull M, Moore D, Harris M, et al. A lamivudine (3TC)-based backbone in conjunction with a boosted protease inhibitor (PI) is sufficient to achieve virologic suppression in the presence of M184V mutations. Presented at: 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 12-15, 2009; San Francisco, CA. Abstract H-916.
11. Triumeq [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2014.
12. Maxmen A. Generic HIV drugs will widen US treatment net. Nature.
2012;488(7411):267.
The one pill daily revolution in human immunodeficiency virus (HIV) management continues with the recent FDA approval of Triumeq, a combination tablet with an integrase inhibitor backbone containing dolutegravir, abacavir, and lamivudine. The first all-in-one protease inhibitor (PI) option is on the horizon and might receive approval before this article is published. The Department of Health and Human Services (DHHS) recommends 7 regimens for any treatment-naive patient with HIV, with 3 additional options if the patient has a baseline plasma RNA viral load (VL) < 100,000 copies/mL (Table 1).1 With 10 first-line options and 4 once daily pill regimens, HIV treatment is poised to enter the realm of managed care.
Guidelines recommend treatment of all patients with HIV, both to slow disease progression, and to reduce the risk of HIV transmission.1 Regimens should be individual ized based on vi rologi c efficacy, toxicity, pill burden, dosing frequency, drug interaction potential, drug resistance testing results, comorbid conditions, and cost. Therapy should only be deferred on a case-by-case basis. Patients considering therapy must be willing and able to commit to taking daily medications and understand the risks and benefits of therapy. Each antiretroviral (ARV) class, and each individual drug, has its own set of advantages and disadvantages. This article will review considerations when choosing a regimen, provide a brief overview of the first-line treatment options, and finally touch on cost considerations.
Choosing An Appropriate Regimen
Choosing an antiviral regimen has 2 basic steps. First, a provider must determine what choices are medically appropriate. An appropriate regimen has 3 active agents with acceptable performance and no contraindications. An ARV regimen generally consists of 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus 1 drug from 1 of the following classes: non-nucleoside reverse transcriptase inhibitor (NNRTI); PI boosted with ritonavir or cobicistat; or integrase strand transfer inhibitor (INSTI). The next step is helping the patient determine which regimen will suit him or her best considering dosing, food requirements, and potential adverse effects.
Genotype, Viral Load, and CD4 Testing at Baseline
A primary consideration is, “Will the regimen work?” This question can best be answered by the genotype, plasma RNA VL, and CD4 count. Baseline genotypes should be obtained for all patients and used to eliminate any inferior drug-resistant treatment options. Viral loads > 100,000 copies/mL eliminate 3 treatment options due to inferiority (inability to fully suppress the HIV virus) in patients with high VL (Table 1). Specifically, abacavir is not recommended (unless given with dolutegravir) with VL > 100,000 copies/mL. Rilpivirine is also not recommended for patients with VL > 100,000 copies/mL or CD4 < 200 cells/mm3.
Creatinine Clearance
Many NRTIs require dosage adjustment when creatinine clearance (CrCl) falls below 50 mL/min. Cobicistat is a novel pharmacokinetic enhancer or boosting agent and is only recommended for baseline CrCl > 70 mL/min.2
HLA-B 5701
If a patient has a positive HLA-B 5701 screening test, abacavir is strictly contraindicated due to the propensity for abacavir hypersensitivity, a potentially life-threatening reaction.
Allergies
Darunavir has a sulfonamide moiety and should be avoided in patients with a sulfa allergy.
Drug Interactions
Drug interactions should be a consideration for all patients with an extensive review of their current medications. Pharmaco-enhancers or “boosting agents,” such as ritonavir or cobicistat, are used to increase drug levels of HIV medications. However, since this is accomplished by inhibition of CYP3A there is great potential for interaction with other medications; opiates, amphetamines, sildenafil, most lipid-lowering agents, and numerous other drugs have significant interactions with ritonavir and cobicistat. Looking at current medications may not be enough; some consideration should be made for the potential of adding medications. For example, if a patient has a seizure disorder and is not well controlled on levetiracetam, consideration should be made if there could be a potential need for phenytoin or other agent that commonly interacts with some HIV medications.
Adverse Events
Adverse events (AEs) are a large consideration when choosing ARV therapy (ART) and can either have medical implications or be patient-specific. Some agents have severe AEs like worsening depression and suicidal ideation. Others are less severe and a particular patient may prefer a risk for gastrointestinal (GI) AEs rather than insomnia.
Adherence
Predicting adherence is difficult; however, certain factors may predispose a patient to being less adherent, including active substance abuse, depression or other mood disorder, unstable housing, and inconsistent access to food. Some regimens are more forgiving and require a lower level of adherence and are less likely to have resistance mutations with failure. Both ritonavir-boosted PIs, as well as dolutegravir, seem to have the best outcomes with lower levels of adherence. Tailoring the dosing and food requirements to a patient’s lifestyle can also improve adherence.
Childbearing Age
Pregnancy and potential for pregnancy must be considered in all female patients. Although pregnancy is beyond the scope of this discussion, the perinatal guidelines should be considered in women of childbearing age.3
Custom A Regimen
Convenience is a major consideration when selecting a regimen. Comparator studies done to demonstrate regimen efficacy using intention-to-treat structures highlight this point. Many patients express that a single tablet is their most important priority. Other important considerations for regimen selection are food requirements or restrictions, and whether there are drug coadministration concerns (eg, divalent cations in antacids or vitamins). These will be discussed more with each
individual ARV agent.
Now that regimen considerations have been reviewed from both provider and patient perspectives, we will focus on the individual classes of ARVs to make selection of a regimen more tailored to patient needs.
The NNRTI Class
The NNRTI class has 2 first-line “recommended” agents, efavirenz and rilpivirine, which are both conveniently available as “one-pill-a-day” coformulated regimens with tenofovir and emtricitabine.1 While the 2 NNRTIs are in the same class, they each have unique properties that would lead a clinician to choose one versus the other.
Efavirenz is the only NNRTI recommended to be paired with abacavir and lamivudine (coformulated as Epzicom) first-line nucleosides. However, it is only recommended to be used in patients with VL < 100,000 copies/mL.
Efavirenz is one of the most studied and widely used ARV. Efavirenz/emtricitabine/tenofovir (coformulated as Atripla) was the first once daily regimen and has shown noninferiority or superiority to most ARV regimens. However, AEs are the most common reason for discontinuation. Efavirenz is well known for its CNS AEs, including vivid dreams, somnolence, impaired concentration, headaches, and depression. These AEs typically diminish or abate after several weeks of therapy. However, certain CNS effects may be long term, including depression or suicidal thoughts.
The recommendation of taking efavirenz regimens at bedtime on an empty stomach to reduce CNS AEs is not ideal for every patient. Efavirenz can also have additive CNS AEs with alcohol and potentially lead to blackouts. It can also lead to false positives for marijuana and benzodiazepines on drug screenings. The FDA classifies efavirenz as Pregnancy Category D, so it should be avoided in women of childbearing age.4 However, if pregnancy isdetected while someone is taking efavirenz there is no need to discontinue the agent since the neural tube has formed by that time.3
Rilpivirine is better tolerated than efavirenz, with fewer and less severe CNS AEs. Rilpivirine/emtricitabine/tenofovir (coformulated as Complera) has the convenience of being 1 pill once daily; however, it is not appropriate for all patients. It should be avoided in patients with a pretreatment VL > 100,000 copies/mL or CD4 < 200 cells/mm3. Patients with values beyond these thresholds were more likely to experience early virologic failure in the first months of therapy compared to efavirenz/emtricitabine/tenofovir.5 Because it is a CYP3A4 substrate, rilpivirine has important drug-drug interactions. Proton pump inhibitors are contraindicated, and H2 receptor antagonists, like ranitidine or famotidine, require specific separation intervals, as do antacids and divalent cations. Another item of importance is that it should be administered with a meal.
The NNRTI class is plagued by its low genetic barrier to resistance, with a single mutation that may confer resistance to nearly all NNRTIs. Estimated transmitted HIV drug resistance at baseline in men who have sex with men can be 8% to 10%.6 Transmitted resistance is a concern, a baseline genotype can determine if a patient is at a higher risk for virologic failure with NNRTIs.
Protease Inhibitors
Protease inhibitors have gone from the main stay of HIV treatment to being a less desirable first-line treatment option. Protease inhibitors have the highest pill burden and drug interactions of the first-line agents; they must be taken with food, and as of October 2014 there are no coformulated tablets (other than lopinavir/ritonavir which is not first-line). New combination tablets utilizing cobicistat as a boosting agent may resuscitate the use of the PIs. The coformulations under investigation are atazanavir/cobicistat, darunavir/cobicistat, and darunavir/cobicistat/emtricitabine/tenofovir alafenamide. These combinations will decrease the pill burden for PIs to 1 to 2 pill(s) a day instead of 3 pills a day. Although 1 to 3 pills taken once daily does not sound like a big difference, given a choice, the majority of patients choose the least number of pills.
How Does Cobicistat Compare With Ritonavir?
Cobicistat has no HIV activity and may be better tolerated than ritonavir. Effects on total cholesterol and triglycerides are similar between the 2 boosting agents.7 Cobicistat causes a modest, rapid increase in serum creatinine (SrCr) through inhibition of tubular secretion without impact on the glomerular filtration rate. The coformulated product of cobicistat/elvitegravir is not recommended to be initiated in patients with CrCl < 70 mL/min at baseline.2 It is anticipated that cobicistat-boosted PI regimens will have a similar limitation. The elevation of SrCr from cobicistat makes it difficult to monitor for tenofovir-related renal dysfunction since SrCr is no longer a reliable surrogate marker for renal function.7 Cobicistat and ritonavir inhibit CYP3A4 and have almost identical drug interactions. However, only ritonavir interacts with methadone.
The first-line PIs are better tolerated than other PIs. Gastrointestinal AEs are still common. Scleral icterus and moderate to severe jaundice occur in 5% to 9% of patients taking atazanavir due to inhibition of the indirect metabolism of bilirubin.8 As a sulfa-related drug, darunavir is generally avoided if the patient has a sulfa allergy, but can be used with caution.9 Another limitation for PIs is that both options are required to be taken with food.
Protease inhibitors will always have a place in treatment-experienced patients with drug resistance, but may have a unique role in patients with baseline M184V mutations. A retrospective analysis showed that patients with a M184V mutation alone achieved equivalent viral suppression if the patient was on 3 fully active HIV ARVs or on a boosted PI, lamivudine or emtricitabine, and 1 additional NRTI.10 Patients with only a M184V mutation can still achieve full suppression without adding an additional ARV agent or using 3 HIV drug classes, preserving future treatment options. Boosted-PI regimens have less drug resistance at failure than NNRTI-based regimens. Less than 5% of new infections have baseline (pretreatment) drug resistance to PIs.6
Boosted PIs have a long clinical history and are still appropriate for many treatment-naive and experienced patients. Reduced pill burden with cobicistat formulation may revive utilization when the pill burden decreases from 3 tablets per day to 1 or 2.
Integrase Strand Inhibitors
INSTI-based regimens have had low discontinuation rates overall in clinical trials, and are great options for a majority of patients. There are 3 INSTIs, all of which are on the list of preferred agents according to the DHHS guidelines. INSTIs may be used with any baseline VL or CD4 counts, without concern for potency. Each of the 3 agents has clear benefits and disadvantages when choosing among them.
Raltegravir has no food requirements and has the least amount of drug interactions of nearly all ART regimens since it is not processed via cytochrome P450 enzymes, but it is dosed twice daily. When paired with emtricitabine/tenofovir, it is also the preferred regimen for occupational postexposure prophylaxis.
Dolut egr avi r i s onc e da i ly, ha s no food requirements, and also avoids drug interactions due to cytochrome P450 enzymes. However, this ARV agent does inhibit the renal organic cation transporter, so some clinically relevant interactions exist. Dolutegravir also has the benefit of being paired with either emtricitabine/tenofovir or abacavir/lamivudine, both of which are preferred DHHS regimens. As mentioned previously, dolutegravir/abacavir/lamivudine are now coformulated
and are an additional all-in-one option. Abacavir has conflicting data regarding increased risk for myocardial infarction; this risk should be considered in light of other risk factors for the patient including age, diabetes, etc.11
Elvitegravir is unique as an INSTI. Elvitegravir/cobicistat/emtricitabine/tenofovir must be administered with food and since it utilizes cobicistat as a pharmaco-enhancer to boost elvitegravir’s levels, there are a significant number of potential drug interactions. Cobicistat-containing regimens must not be prescribed for patients with a baseline CrCl <70 mL/min.2
Both dolutegravir and raltegravir can be taken with or without food. However, if the patient is taking a divalent cation (magnesium, iron, or calcium) then the medications must be separated, causing the patient to take medications multiple times per day, or taken with food (and lose the benefit of with or without food).
Cost and the New Era of ARV Generics
Previously, the majority of HIV regimens were similarly priced and had little impact on prescribing practices. Several ARV are now available as generics. In the next 2 years, generics may have major impact on prescribing practice as first-line options (darunavir, atazanavir, and efavirenz) will be available as generics (Table 2).12
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Author Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of Health and Human Services, the Indian Health Services, or the U.S. Government. Please review complete prescribing information for specific drugs or drug combinations—including but not limited to indications, contraindications, warnings, adverse effects, and drug interactions- before administering pharmacologic therapy to patients.
Journal Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combination—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The one pill daily revolution in human immunodeficiency virus (HIV) management continues with the recent FDA approval of Triumeq, a combination tablet with an integrase inhibitor backbone containing dolutegravir, abacavir, and lamivudine. The first all-in-one protease inhibitor (PI) option is on the horizon and might receive approval before this article is published. The Department of Health and Human Services (DHHS) recommends 7 regimens for any treatment-naive patient with HIV, with 3 additional options if the patient has a baseline plasma RNA viral load (VL) < 100,000 copies/mL (Table 1).1 With 10 first-line options and 4 once daily pill regimens, HIV treatment is poised to enter the realm of managed care.
Guidelines recommend treatment of all patients with HIV, both to slow disease progression, and to reduce the risk of HIV transmission.1 Regimens should be individual ized based on vi rologi c efficacy, toxicity, pill burden, dosing frequency, drug interaction potential, drug resistance testing results, comorbid conditions, and cost. Therapy should only be deferred on a case-by-case basis. Patients considering therapy must be willing and able to commit to taking daily medications and understand the risks and benefits of therapy. Each antiretroviral (ARV) class, and each individual drug, has its own set of advantages and disadvantages. This article will review considerations when choosing a regimen, provide a brief overview of the first-line treatment options, and finally touch on cost considerations.
Choosing An Appropriate Regimen
Choosing an antiviral regimen has 2 basic steps. First, a provider must determine what choices are medically appropriate. An appropriate regimen has 3 active agents with acceptable performance and no contraindications. An ARV regimen generally consists of 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus 1 drug from 1 of the following classes: non-nucleoside reverse transcriptase inhibitor (NNRTI); PI boosted with ritonavir or cobicistat; or integrase strand transfer inhibitor (INSTI). The next step is helping the patient determine which regimen will suit him or her best considering dosing, food requirements, and potential adverse effects.
Genotype, Viral Load, and CD4 Testing at Baseline
A primary consideration is, “Will the regimen work?” This question can best be answered by the genotype, plasma RNA VL, and CD4 count. Baseline genotypes should be obtained for all patients and used to eliminate any inferior drug-resistant treatment options. Viral loads > 100,000 copies/mL eliminate 3 treatment options due to inferiority (inability to fully suppress the HIV virus) in patients with high VL (Table 1). Specifically, abacavir is not recommended (unless given with dolutegravir) with VL > 100,000 copies/mL. Rilpivirine is also not recommended for patients with VL > 100,000 copies/mL or CD4 < 200 cells/mm3.
Creatinine Clearance
Many NRTIs require dosage adjustment when creatinine clearance (CrCl) falls below 50 mL/min. Cobicistat is a novel pharmacokinetic enhancer or boosting agent and is only recommended for baseline CrCl > 70 mL/min.2
HLA-B 5701
If a patient has a positive HLA-B 5701 screening test, abacavir is strictly contraindicated due to the propensity for abacavir hypersensitivity, a potentially life-threatening reaction.
Allergies
Darunavir has a sulfonamide moiety and should be avoided in patients with a sulfa allergy.
Drug Interactions
Drug interactions should be a consideration for all patients with an extensive review of their current medications. Pharmaco-enhancers or “boosting agents,” such as ritonavir or cobicistat, are used to increase drug levels of HIV medications. However, since this is accomplished by inhibition of CYP3A there is great potential for interaction with other medications; opiates, amphetamines, sildenafil, most lipid-lowering agents, and numerous other drugs have significant interactions with ritonavir and cobicistat. Looking at current medications may not be enough; some consideration should be made for the potential of adding medications. For example, if a patient has a seizure disorder and is not well controlled on levetiracetam, consideration should be made if there could be a potential need for phenytoin or other agent that commonly interacts with some HIV medications.
Adverse Events
Adverse events (AEs) are a large consideration when choosing ARV therapy (ART) and can either have medical implications or be patient-specific. Some agents have severe AEs like worsening depression and suicidal ideation. Others are less severe and a particular patient may prefer a risk for gastrointestinal (GI) AEs rather than insomnia.
Adherence
Predicting adherence is difficult; however, certain factors may predispose a patient to being less adherent, including active substance abuse, depression or other mood disorder, unstable housing, and inconsistent access to food. Some regimens are more forgiving and require a lower level of adherence and are less likely to have resistance mutations with failure. Both ritonavir-boosted PIs, as well as dolutegravir, seem to have the best outcomes with lower levels of adherence. Tailoring the dosing and food requirements to a patient’s lifestyle can also improve adherence.
Childbearing Age
Pregnancy and potential for pregnancy must be considered in all female patients. Although pregnancy is beyond the scope of this discussion, the perinatal guidelines should be considered in women of childbearing age.3
Custom A Regimen
Convenience is a major consideration when selecting a regimen. Comparator studies done to demonstrate regimen efficacy using intention-to-treat structures highlight this point. Many patients express that a single tablet is their most important priority. Other important considerations for regimen selection are food requirements or restrictions, and whether there are drug coadministration concerns (eg, divalent cations in antacids or vitamins). These will be discussed more with each
individual ARV agent.
Now that regimen considerations have been reviewed from both provider and patient perspectives, we will focus on the individual classes of ARVs to make selection of a regimen more tailored to patient needs.
The NNRTI Class
The NNRTI class has 2 first-line “recommended” agents, efavirenz and rilpivirine, which are both conveniently available as “one-pill-a-day” coformulated regimens with tenofovir and emtricitabine.1 While the 2 NNRTIs are in the same class, they each have unique properties that would lead a clinician to choose one versus the other.
Efavirenz is the only NNRTI recommended to be paired with abacavir and lamivudine (coformulated as Epzicom) first-line nucleosides. However, it is only recommended to be used in patients with VL < 100,000 copies/mL.
Efavirenz is one of the most studied and widely used ARV. Efavirenz/emtricitabine/tenofovir (coformulated as Atripla) was the first once daily regimen and has shown noninferiority or superiority to most ARV regimens. However, AEs are the most common reason for discontinuation. Efavirenz is well known for its CNS AEs, including vivid dreams, somnolence, impaired concentration, headaches, and depression. These AEs typically diminish or abate after several weeks of therapy. However, certain CNS effects may be long term, including depression or suicidal thoughts.
The recommendation of taking efavirenz regimens at bedtime on an empty stomach to reduce CNS AEs is not ideal for every patient. Efavirenz can also have additive CNS AEs with alcohol and potentially lead to blackouts. It can also lead to false positives for marijuana and benzodiazepines on drug screenings. The FDA classifies efavirenz as Pregnancy Category D, so it should be avoided in women of childbearing age.4 However, if pregnancy isdetected while someone is taking efavirenz there is no need to discontinue the agent since the neural tube has formed by that time.3
Rilpivirine is better tolerated than efavirenz, with fewer and less severe CNS AEs. Rilpivirine/emtricitabine/tenofovir (coformulated as Complera) has the convenience of being 1 pill once daily; however, it is not appropriate for all patients. It should be avoided in patients with a pretreatment VL > 100,000 copies/mL or CD4 < 200 cells/mm3. Patients with values beyond these thresholds were more likely to experience early virologic failure in the first months of therapy compared to efavirenz/emtricitabine/tenofovir.5 Because it is a CYP3A4 substrate, rilpivirine has important drug-drug interactions. Proton pump inhibitors are contraindicated, and H2 receptor antagonists, like ranitidine or famotidine, require specific separation intervals, as do antacids and divalent cations. Another item of importance is that it should be administered with a meal.
The NNRTI class is plagued by its low genetic barrier to resistance, with a single mutation that may confer resistance to nearly all NNRTIs. Estimated transmitted HIV drug resistance at baseline in men who have sex with men can be 8% to 10%.6 Transmitted resistance is a concern, a baseline genotype can determine if a patient is at a higher risk for virologic failure with NNRTIs.
Protease Inhibitors
Protease inhibitors have gone from the main stay of HIV treatment to being a less desirable first-line treatment option. Protease inhibitors have the highest pill burden and drug interactions of the first-line agents; they must be taken with food, and as of October 2014 there are no coformulated tablets (other than lopinavir/ritonavir which is not first-line). New combination tablets utilizing cobicistat as a boosting agent may resuscitate the use of the PIs. The coformulations under investigation are atazanavir/cobicistat, darunavir/cobicistat, and darunavir/cobicistat/emtricitabine/tenofovir alafenamide. These combinations will decrease the pill burden for PIs to 1 to 2 pill(s) a day instead of 3 pills a day. Although 1 to 3 pills taken once daily does not sound like a big difference, given a choice, the majority of patients choose the least number of pills.
How Does Cobicistat Compare With Ritonavir?
Cobicistat has no HIV activity and may be better tolerated than ritonavir. Effects on total cholesterol and triglycerides are similar between the 2 boosting agents.7 Cobicistat causes a modest, rapid increase in serum creatinine (SrCr) through inhibition of tubular secretion without impact on the glomerular filtration rate. The coformulated product of cobicistat/elvitegravir is not recommended to be initiated in patients with CrCl < 70 mL/min at baseline.2 It is anticipated that cobicistat-boosted PI regimens will have a similar limitation. The elevation of SrCr from cobicistat makes it difficult to monitor for tenofovir-related renal dysfunction since SrCr is no longer a reliable surrogate marker for renal function.7 Cobicistat and ritonavir inhibit CYP3A4 and have almost identical drug interactions. However, only ritonavir interacts with methadone.
The first-line PIs are better tolerated than other PIs. Gastrointestinal AEs are still common. Scleral icterus and moderate to severe jaundice occur in 5% to 9% of patients taking atazanavir due to inhibition of the indirect metabolism of bilirubin.8 As a sulfa-related drug, darunavir is generally avoided if the patient has a sulfa allergy, but can be used with caution.9 Another limitation for PIs is that both options are required to be taken with food.
Protease inhibitors will always have a place in treatment-experienced patients with drug resistance, but may have a unique role in patients with baseline M184V mutations. A retrospective analysis showed that patients with a M184V mutation alone achieved equivalent viral suppression if the patient was on 3 fully active HIV ARVs or on a boosted PI, lamivudine or emtricitabine, and 1 additional NRTI.10 Patients with only a M184V mutation can still achieve full suppression without adding an additional ARV agent or using 3 HIV drug classes, preserving future treatment options. Boosted-PI regimens have less drug resistance at failure than NNRTI-based regimens. Less than 5% of new infections have baseline (pretreatment) drug resistance to PIs.6
Boosted PIs have a long clinical history and are still appropriate for many treatment-naive and experienced patients. Reduced pill burden with cobicistat formulation may revive utilization when the pill burden decreases from 3 tablets per day to 1 or 2.
Integrase Strand Inhibitors
INSTI-based regimens have had low discontinuation rates overall in clinical trials, and are great options for a majority of patients. There are 3 INSTIs, all of which are on the list of preferred agents according to the DHHS guidelines. INSTIs may be used with any baseline VL or CD4 counts, without concern for potency. Each of the 3 agents has clear benefits and disadvantages when choosing among them.
Raltegravir has no food requirements and has the least amount of drug interactions of nearly all ART regimens since it is not processed via cytochrome P450 enzymes, but it is dosed twice daily. When paired with emtricitabine/tenofovir, it is also the preferred regimen for occupational postexposure prophylaxis.
Dolut egr avi r i s onc e da i ly, ha s no food requirements, and also avoids drug interactions due to cytochrome P450 enzymes. However, this ARV agent does inhibit the renal organic cation transporter, so some clinically relevant interactions exist. Dolutegravir also has the benefit of being paired with either emtricitabine/tenofovir or abacavir/lamivudine, both of which are preferred DHHS regimens. As mentioned previously, dolutegravir/abacavir/lamivudine are now coformulated
and are an additional all-in-one option. Abacavir has conflicting data regarding increased risk for myocardial infarction; this risk should be considered in light of other risk factors for the patient including age, diabetes, etc.11
Elvitegravir is unique as an INSTI. Elvitegravir/cobicistat/emtricitabine/tenofovir must be administered with food and since it utilizes cobicistat as a pharmaco-enhancer to boost elvitegravir’s levels, there are a significant number of potential drug interactions. Cobicistat-containing regimens must not be prescribed for patients with a baseline CrCl <70 mL/min.2
Both dolutegravir and raltegravir can be taken with or without food. However, if the patient is taking a divalent cation (magnesium, iron, or calcium) then the medications must be separated, causing the patient to take medications multiple times per day, or taken with food (and lose the benefit of with or without food).
Cost and the New Era of ARV Generics
Previously, the majority of HIV regimens were similarly priced and had little impact on prescribing practices. Several ARV are now available as generics. In the next 2 years, generics may have major impact on prescribing practice as first-line options (darunavir, atazanavir, and efavirenz) will be available as generics (Table 2).12
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Author Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of Health and Human Services, the Indian Health Services, or the U.S. Government. Please review complete prescribing information for specific drugs or drug combinations—including but not limited to indications, contraindications, warnings, adverse effects, and drug interactions- before administering pharmacologic therapy to patients.
Journal Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combination—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. HHS Panel on Antiretroviral Guidelines for Adults and Adolescents—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfile/lvguidelines/AdultandAdolescentGL.pdf. Updated November 13, 2014. Accessed December 1, 2014.
2. Stribild [package insert]. Foster City, CA: Gilead Sciences, Inc; 2012.
3. HHS Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Updated March 28, 2014. Accessed October 27, 2014.
4. Atripla [package insert] Princeton, NJ: Bristol-Myers Squibb; Foster City, CA: Gilead Sciences, Inc; 2013.
5. Cohen CJ, Molina JM, Cassetti I, et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS. 2013;27(6):939-950.
6. Bañez Ocfemia MC, Saduvala N, Oster AM, et al. Transmitted HIV-1 drug resistance among men who have sex with men, 11 US jurisdictions, 2008-2011. Presented at: 21st Conference on Retroviruses and Opportunistic Infections; March 3-6, 2014; Boston, MA. Abstract 579.
7. Gallant JE, Koenig E, Andrade-Villaneuva J, et al. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: Week 48 results. J Infect Dis. 2013;208(1):32-39.
8. Reyatoz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
9. Prezista [package insert]. Titusville, NJ: Janssen Pharmaceuicals, Inc; 2013.
10. Hull M, Moore D, Harris M, et al. A lamivudine (3TC)-based backbone in conjunction with a boosted protease inhibitor (PI) is sufficient to achieve virologic suppression in the presence of M184V mutations. Presented at: 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 12-15, 2009; San Francisco, CA. Abstract H-916.
11. Triumeq [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2014.
12. Maxmen A. Generic HIV drugs will widen US treatment net. Nature.
2012;488(7411):267.
1. HHS Panel on Antiretroviral Guidelines for Adults and Adolescents—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfile/lvguidelines/AdultandAdolescentGL.pdf. Updated November 13, 2014. Accessed December 1, 2014.
2. Stribild [package insert]. Foster City, CA: Gilead Sciences, Inc; 2012.
3. HHS Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Updated March 28, 2014. Accessed October 27, 2014.
4. Atripla [package insert] Princeton, NJ: Bristol-Myers Squibb; Foster City, CA: Gilead Sciences, Inc; 2013.
5. Cohen CJ, Molina JM, Cassetti I, et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS. 2013;27(6):939-950.
6. Bañez Ocfemia MC, Saduvala N, Oster AM, et al. Transmitted HIV-1 drug resistance among men who have sex with men, 11 US jurisdictions, 2008-2011. Presented at: 21st Conference on Retroviruses and Opportunistic Infections; March 3-6, 2014; Boston, MA. Abstract 579.
7. Gallant JE, Koenig E, Andrade-Villaneuva J, et al. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: Week 48 results. J Infect Dis. 2013;208(1):32-39.
8. Reyatoz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
9. Prezista [package insert]. Titusville, NJ: Janssen Pharmaceuicals, Inc; 2013.
10. Hull M, Moore D, Harris M, et al. A lamivudine (3TC)-based backbone in conjunction with a boosted protease inhibitor (PI) is sufficient to achieve virologic suppression in the presence of M184V mutations. Presented at: 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 12-15, 2009; San Francisco, CA. Abstract H-916.
11. Triumeq [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2014.
12. Maxmen A. Generic HIV drugs will widen US treatment net. Nature.
2012;488(7411):267.