User login
Management of Volume Overload
Although patients with left ventricular dysfunction may present with low‐output syndrome and even cardiogenic shock, the majority are admitted with symptoms of congestion.1 The classic symptoms of congestive heart failure reflect fluid overload, that is, orthopnea, paroxysmal nocturnal dyspnea, and peripheral edema; these symptoms can be so dramatic that it is not surprising that patients seek hospitalization.2 Activation of the renin angiotensin system coupled with sympathetic hyperactivity results in marked sodium retention and increased filling pressures in the right and left ventricle that ultimately bring about these congestive symptoms of dyspnea and orthopnea.3 Increased filling pressure precedes admission to the hospital, and filling pressure falls during successful therapy.4, 5 Indeed, normalization of the left ventricular filling pressure much better predicts survival than improved cardiac output.6 However, despite the many advances in the evidence‐based armamentarium for heart failure, the one great deficiency in the evidence base is the lack of data on modalities that can reduce or normalize left ventricular filling pressures. This is not as unexpected as it seems because the symptoms of congestion are so dramatic and, until recently, the tools to mitigate were so few that randomized trials were difficult to conceive. However, the treatment paradigms for acute decompensated heart failure (ADHF) management are changing, and evidence‐based mortality trials for filling pressure reduction and congestion relief continue to evolve.710
DIURETICS
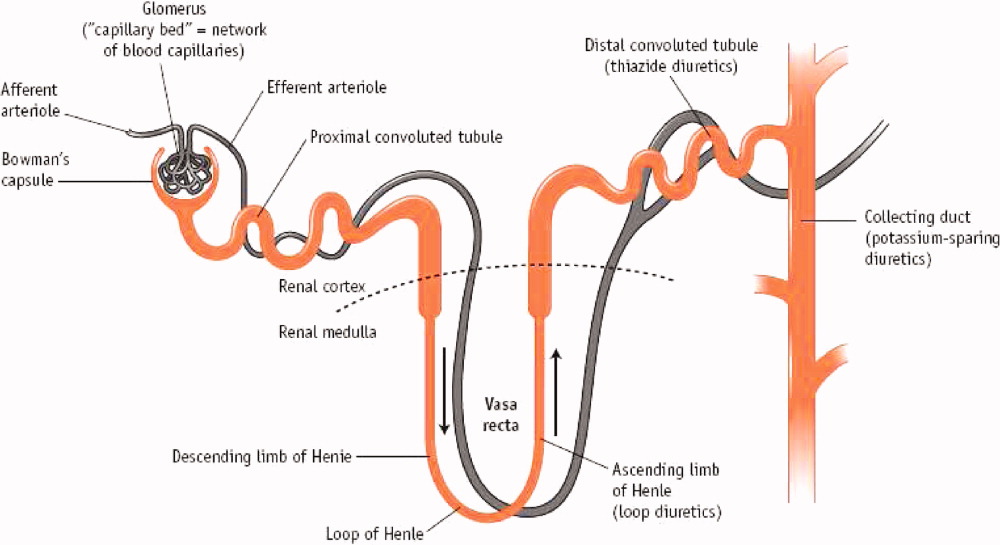
Mercurial diuretics were introduced in the 1920s as the mainstay of therapy for ADHF; the loop diuretics became the foundation of therapy in the 1960s.11, 12 In the Acute Decompensated Heart Failure National Registry database (ADHERE), 88% of patients received intravenous loop diuretics during their hospitalization.13 Loop diuretics act in the thick ascending limb of the loop of Henle to inhibit reabsorption of sodium and chloride by inhibiting the sodium, potassium, and chloride (Na+/K+/2Cl) pump. This blockade causes increased delivery of these solutes to the distal convoluted tubule and collecting duct, resulting in a shift in the balance of osmotic forces toward fluid secretion into the collecting system. Through this mechanism, loop diuretics increase natriuresis and diuresis (Figure 1).14
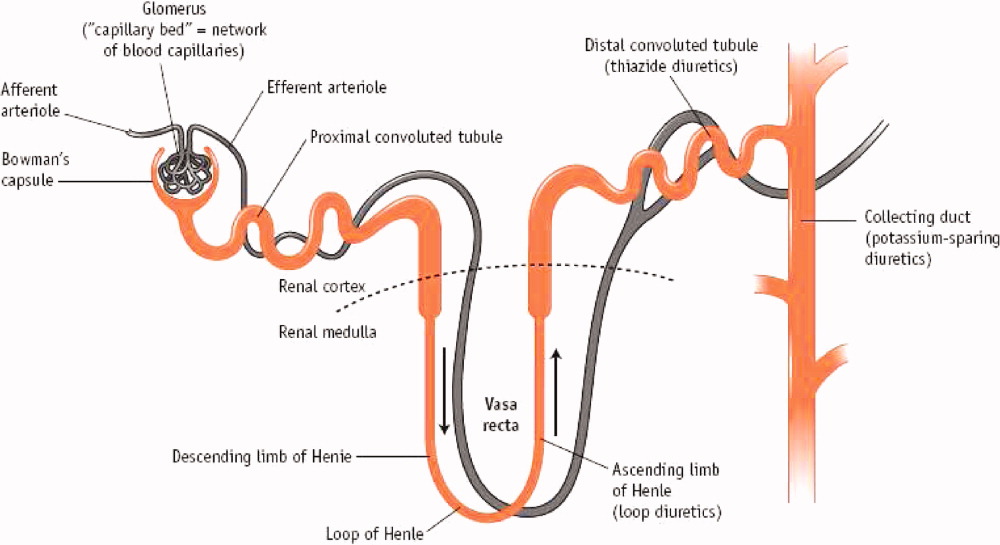
Less commonly used are the thiazide diuretics, which act on the distal convoluted tubule to block Na+, K+‐ATPase and thereby NaCl transport in the distal convoluted tubule.15 Thiazide diuretics are much less powerful than loop diuretics and are rarely used intravenously in the hospital. These do possess a synergistic effect when used with loop diuretics in that sodium reabsorption is blocked in 2 sections of the nephron.16 Extreme care must be taken to avoid overdiuresis, but this combination can be helpful to treat diuretic resistance.17
The third class of agents are the so‐called potassium‐sparing diuretics, which block sodium reuptake in the final portion of the nephron (the collecting ducts), resulting in an obligatory reuptake of potassium. These agents include the aldosterone receptor blocker spironolactone and eplerenone, which act primarily through competitive binding of receptors at the aldosterone‐dependent sodium‐potassium exchange site in the distal convoluted renal tubule. Although weak diuretics, they are the only class of diuretics shown to improve mortality in moderate to severe heart failure,18, 19 presumably by modulating the abnormal neurohormonal activation of the sympathetic nervous system and the renin‐angiotensin‐aldosterone axis.20 Unfortunately, severe hyperkalemia remains a significant side effect and can limit their use.21
Despite the obvious beneficial effects of loop diuretics in the treatment of ADHF, we lack key fundamental information about these most frequently used drugs. For example, what is the correct dose? Escalating the dose of diuretics has been associated with increased mortality in heart failure even when corrected for the severity of the illness.22, 23 Proposed explanations for the increased morality in patients with heart failure include activation of the renin‐angiotensin‐aldosterone system and sympathetic nervous system, decreases in the glomerular filtration rate (GFR) contributing to cardiorenal syndrome, and intravascular volume contraction and decreased left ventricular filling pressure worsening cardiac performance in patients without significant fluid retention.22, 23 It is now recognized that kidney dysfunction plays a vital role in the progress of patients with heart failure, and increases in serum creatinine or blood urea nitrogen are known predictors of mortality.24 Thus, larger doses of diuretics may result in unfavorable outcomes in heart failure patients because of adverse effects on renal function. In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE), there was a clear increase in mortality with escalating loop diuretic doses, especially above 300 mg/day of furosemide (or an equivalent dose of another loop diuretic).25 Although patients with renal dysfunction may require higher doses, prudence would dictate using the lowest dose to gain a reasonable urine output.
How should loop diuretics be given: as a bolus or continuous infusion? Although diuretics have been typically given as a bolus, there are significant theoretical concerns about this method. Furosemide, for example, has a half‐life of approximately 2 hours; when it is given once or twice a day, a breaking phenomenon is seen in which the kidneys start to retain sodium and the effectiveness of the bolus is reduced.26 A Cochrane review has found evidence that a continuous infusion of diuretics produces more diuresis, although the same article suggests that more titration is needed to support this observation.27
Several loop diuretics are used clinically, including furosemide, torsemide, and bumetanide. Which one should be chosen? Furosemide is the least expensive and most widely used, but 2 animal models suggest more favorable cardiac effects (less fibrosis) with torsemide and even mortality benefit.28, 29 There are no comparable human data to guide the clinician, unfortunately. On a milligram per milligram basis, bumetanide produces more natriuresis than either torsemide or furosemide, but again, the clinical significance of this is not known.
VASODILATORS
Nitrates
Nitrates, including nitroglycerin and nitroprusside, have been used in therapy for ADHF primarily as venodilators.30 Thus, they have been shown to reduce right and left ventricular filling pressures, systemic and pulmonary vascular resistance, and, to a lesser extent, systemic blood pressure.31 A serious drawback to the continued use of nitrates is the development of tolerance that can become apparent within hours of their initial use.32 In addition, there have not been large outcome trials to define the duration of benefit or the proper dose.
Nesiritide
A potential role for the natriuretic peptides in heart failure dates back to the 1980s when extracts of the right atrial tissue of rats was shown to produce a brisk natriuresis when given intravenously to a second animal.33 Nesiritide, as the commercially prepared B‐type natriuretic peptide is called, consistently reduced preload and afterload and caused natriuresis in some studies.34, 35 Natriuresis and augmentation of diuresis has not been consistently demonstrated in published reports, however.36, 37 In addition, B‐type natriuretic peptide, when given therapeutically, does suppress aldosterone.38
The largest clinical experience to date with nesiritide came in the Vasodilation in the Management of Acute Congestive Heart Failure (VMAC) trial.39 In this study, nesiritide was compared with intravenous nitroglycerin and placebo when added to standard care for 3 hours in a double‐blind, randomized protocol. Nesiritide reduced filling pressures in comparison with nitroglycerin and placebo and provided greater symptomatic relief in comparison with placebo but not in comparison with nitroglycerin.39 In a retrospective review of consecutive patients, the addition of nesiritide resulted in a decreased length of stay without compromising renal function.40
In 2005, 2 meta‐analyses were published that raised questions about the safety of nesiritide.41, 42 In the first, a review of 5 nesiritide/placebo trials found an increased risk for worsening renal function (specifically, a rise in serum creatinine of 0.5 mg/dL or more).41 This increase occurred in 21% of nesiritide‐treated patients versus 15% of those on placebo (P = .001). Some of these trials in the meta‐analysis employed dosages of nesiritide greater than the currently recommended 0.01 g/kg/minute. When only those patients in the VMAC group that received the recommended 0.01 g/kg/minute dose were analyzed, there was not a significant rise in creatinine.43 Riter et al44 reported a retrospective analysis finding that half or even quarter doses of nesiritide actually produced improvement in renal function compared with the standard dose or no nesiritide use. Higher doses of diuretics, >160 mg of furosemide or its equivalent in conjunction with nesiritide, did increase the risk of renal dysfunction.45 In the Nesiritide Administered Perianesthesia (NAPA) trial, 0.01 g/kg/minute of nesiritide was given as a defined 24‐hour infusion without bolus to high‐risk patients with left ventricular dysfunction undergoing bypass and mitral valve surgery.46 Although serum creatinine increased in both groups following surgery, it increased more so with placebo than nesiritide (P 0.001), despite increased urine output (P 0.001) and shorter length of hospital stay (P = 0.043) in the nesiritide group.46 A smaller study of similar design also noted preservation of renal function with nesiritide compared with placebo in bypass patients.47 Therefore, the current recommendation for nesiritide use is to use no more than 0.01 g/kg/minute. Reduction of diuretic doses would be prudent when nesiritide is used.
Sackner‐Bernstein et al42 combined the results of 3 placebo‐controlled trials and reported an increase in mortality with nesiritide at 30 days. In this study, significant differences were found between the placebo and nesiritide groups in terms of baseline renal function,48 blood pressure, and inotrope use, and this may explain the observed mortality difference.42 Mortality was the same at 180 days. Abraham49 analyzed the 7 available trials and risk‐adjusted the patient populations to avoid group inequalities; with this method, no significant effect of nesiritide use on mortality was seen. The 2 most recent large trialsFollow‐Up Serial Infusions of Nesiritide II (FUSION II) (no effect of nesiritide on mortality) and NAPA (mortality decreased in nesiritide‐treated patients)have provided more safety data concerning the use of nesiritide.46, 50 The most definitive answer will come with the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND‐HF) trial, which will recruit 7000 patients, the largest trial in ADHF to date.
ULTRAFILTRATION (UF)
UF removes water and nonprotein‐bound smallmolecular‐weight and mediummolecular‐weight solutes through the semipermeable membrane when hydrostatic pressure, generated by blood pressure or an external blood pump, exceeds oncotic pressure. The fluid removal rate can be adjusted between 100 and 500 mL/hour. The Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial randomized UF with intravenous diuretic therapy in patients with ADHF and showed that UF produced greater weight and fluid loss at 48 hours versus diuretics and reduced the 90‐day readmission rate for heart failure.51 There was no statistically significant difference noted between the overall mortality and serum creatinine between the 2 groups. Rogers et al52 found in a small, randomized trial that during a 48‐hour period, UF showed no significant difference in the renal hemodynamics (GFR and renal plasma flow in patients with ADHF) versus the standard treatment with intravenous diuretics. The Heart Failure Society of America treatment guidelines for the evaluation and management of patients with ADHF suggest that when congestion fails to improve in response to diuretic therapy, UF may be considered (strength of evidence = C).53
Perhaps the most vexing issue surrounding the use of diuretics, vasodilators, and UF is when to stop intravenous therapy in the hospitalized patient. There are no clear guidelines about this, and perhaps clinical experience is of paramount importance. Theoretically, vasodilator and fluid removal therapy should be continued until the patient is euvolemic, that is, has normal filling pressures (usually associated with normalization of the neck veins and loss of S3) with improvement in symptoms. This was clearly seen in the ESCAPE trial, in which use of either hemodynamic guidance or clinical evaluation of jugular venous pressure resulted in more normal filling pressures.5 This is an extremely important issue because continued fluid removal beyond the point at which the patient is euvolemic may result in renal dysfunction; the latter is a strong predictor of prolonged hospitalization and mortality.54, 55
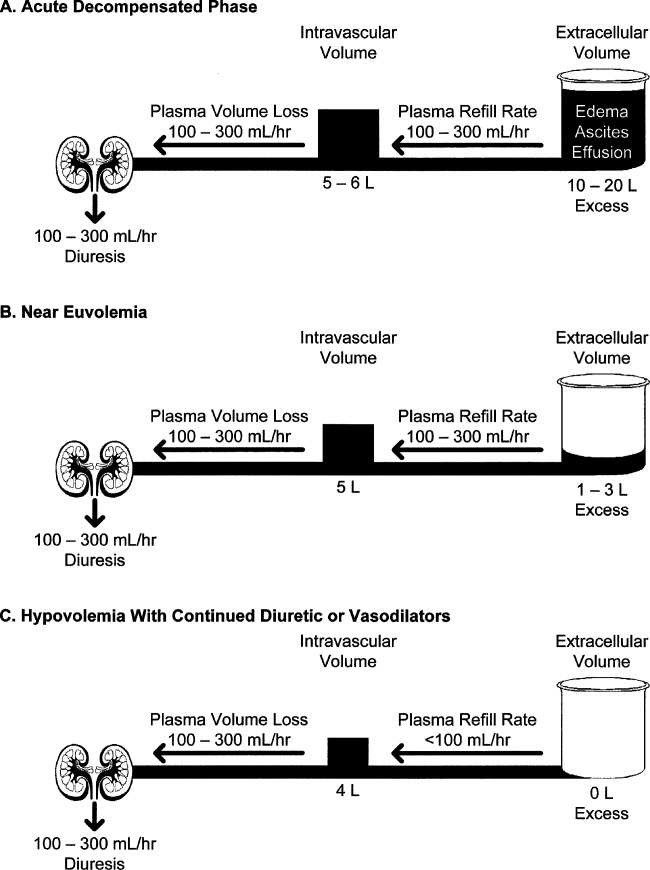
Initial fluid removal can be rapid with either diuretics or UF, and filling pressures fall within minutes with nesiritide. This is beneficial to the patient as long as extracellular sodium and water reenter the vascular bed to maintain preload. Boyle and Sobotka56 emphasized the importance of this plasma refill rate and proposed monitoring the hematocrit as is done in dialysis to prevent excessive fluid removal. As can be seen in Figure 2, the plasma compartment is easily refilled when extracellular volume is increased in edematous patients early in their therapy. However, the plasma refill rate can fall precipitously when this compartment is depleted. Hence, clinicians must be ever alert to this transition period when continued fluid removal or vasodilator therapy results in depletion of vascular, not interstitial, volume with rapid declines in preload and cardiac output. Therefore, these therapies should be stopped when the patient becomes euvolemic, not later when the patient has become hypovolemic with the attendant problems. In the ESCAPE trial, the jugular venous pressure was the best indicator of a normal filling pressure,5, 54 although this is not an infallible guide. Our practice is to stop diuretics and vasodilators when edema has resolved and jugular venous pressure is below 8 cm. In addition, checking blood urea nitrogen and creatinine twice a day can give an early warning of hypovolemia when these rise 25% above baseline.57 Volume status should be carefully evaluated by changes in the physical examination, including postural blood pressure changes and increases in blood urea nitrogen and creatinine. When worsening renal function occurs, judicious fluid replacement with 500 to 1000 mL of normal saline given over 2 to 4 hours can quickly restore euvolemia and may improve renal function in the hypovolemic patient.
INVESTIGATIONAL THERAPIES
Oral Vasopressin Antagonist
Elevation of arginine vasopressin contributes to fluid retention and hyponatremia and is directly proportional to the severity of heart failure.58 Tolvaptan is an oral, nonpeptide, selective vasopressin V2 receptor antagonist whose action on the distal nephron causes loss of electrolyte‐free water (aquaresis).59 The efficacy of tolvaptan was tested in a double‐blind, prospective, randomized international trial, the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST), with a 30 mg/day oral dosage of tolvaptan versus placebo within 48 hours of admission with ADHF.7, 9 Patients receiving tolvaptan showed improvement in dyspnea on day 1 along with body weight and edema reduction on day 7 or discharge in comparison with placebo. The improvement in global clinical assessment was not different between the 2 groups. The serious adverse event frequencies were similar between the groups without excess renal failure or hypotension in the tolvaptan group.7, 9 Unfortunately, this small beneficial effect in the hospital did not result in positive survival benefit.
Adenosine A1‐Receptor (AA1R) Antagonists
The kidney is the only organ in which adenosine is a paracrine vasoconstrictor.60 Dittrich et al61 did a randomized, double‐blind, placebo‐controlled, 2‐way crossover study in patients with heart failure and renal impairment (median GFR = 50 mL/minute) and tested the effectiveness of the AA1R antagonist rolofylline as a renal vasodilator in an outpatient setting. Blockade of these receptors increased vasodilation and GFR. Givertz et al62 evaluated the effect of AA1R antagonists on diuresis and renal function in patients with ADHF and renal impairment or diuretic resistance. A paired, randomized, double‐blind, placebo‐controlled, proof‐of‐concept trial in patients with ADHF and volume overload found that the AA1R antagonist KW‐3092 enhances the response of loop diuretics and may have a renal protective effect. Prophylaxis for Thromboembolism in Critical Care Trials 1 and 2 (PROTECT 1,2) studies investigating KW‐3092 to assess the effects on heart failure and renal function, are currently under way.10
CONCLUSION
The goals in the management of ADHF are deceptively simple: improve symptoms by normalizing filling pressure and volume status efficiently without worsening renal function. Powerful tools, including diuretics, vasodilators, and UF, exist to accomplish these goals, but determining which tools to use in which patients and the precise manner in which to use the tools (alone or in combination and duration) remains more of an art than a science. The concept of euvolemia needs to be more carefully defined and conceptualized in a way that is useful for clinicians. Frequent monitoring of clinical signs, electrolytes, and renal function are our current best guides to assess volume status during therapy. Newer modalities hold promise that early detection of fluid overload may prevent hospitalization and reduce costs. Similarly, new pharmacologic therapies hold promise that their use may improve cardiac function and reduce renal abnormalities, thereby improving outcomes in patients with ADHF.
- .Tailored therapy to hemodynamic goals for advanced heart failure.Eur J Heart Fail.1999;1(3):251–257.
- ,,,,,.Profile for estimating risk of heart failure.Arch Intern Med.1999;159(11):1197–1204.
- .The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure [editorial].J Am Coll Cardiol.1992;20(1):248–254.
- ,,, et al.Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system.J Am Coll Cardiol.2003;41(4):565–571.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Persistently high left ventricular filling pressures predict mortality despite angiotensin converting enzyme inhibition in advanced heart failure [abstract 2624].Circulation.1994;90(4 pt 2):I‐488.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial.JAMA.2007;297(12):1319–1331.
- . Considerations in designing acute decompensated heart failure clinical trials. Available at:http://www.medscape.com/viewarticle/557964. Accessed September 2008.
- ,,, et al.Short‐term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials.JAMA.2007;297(12):1332–1343.
- ,,.Rolofylline (KW‐3902): a new adenosine A1‐receptor antagonist for acute congestive heart failure.Future Cardiol.2008;4(2):117–123.
- ,,,,.Acute haemodynamic effects of frusemide in patients with normal and raised left atrial pressures.Br Heart J.1969;31(6):711–717.
- ,.The diuretic effect of Novasurol and other mercury injections.Wien Klin Wochenschr.2002;33:943–944.
- ,,,,.Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE).Am Heart J.2007;153(6):1021–1028.
- ,,.Optimal use of diuretics in patients with heart failure.Curr Treat Options Cardiovasc Med.2007;9(4):332–342.
- ,,,.Characterization of the thiazide‐sensitive Na(+)‐Cl(−) cotransporter: a new model for ions and diuretics interaction.Am J Physiol Renal Physiol.2000;279(1):F161–F169.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- ,.Combination of high‐dose furosemide and hydrochlorothiazide in the treatment of refractory congestive heart failure.Eur Heart J.1996;17(12):1867–1874.
- ,,, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure.N Engl J Med.1999;341(10):709–717.
- ,,, et al.Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction.N Engl J Med.2003;348(14):1309–1321.
- .Pathophysiologic role of the renin‐angiotensin‐aldosterone and sympathetic nervous systems in heart failure.Am J Health Syst Pharm.2004;61(suppl 2):S4–S13.
- ,,,.Detection of spironolactone‐associated hyperkalaemia following the Randomized Aldactone Evaluation Study (RALES).Drug Saf.2007;30(12):1143–1149.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,.Dose‐dependent association between use of loop diuretics and mortality in advanced systolic heart failure.Am J Cardiol.2006;98(10):1416–1417.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,, et al.Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial.Eur J Heart Fail.2007;9(10):1064–1069.
- ,,.Mechanism of impaired natriuretic response to furosemide during prolonged therapy.Kidney Int.1989;36(4):682–689.
- ,,,.Continuous infusion versus bolus injection of loop diuretics in congestive heart failure.Cochrane Database Syst Rev.2004; (1):CD003178.
- ,,,,,.Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure.J Am Coll Cardiol.2004;43(11):2028–2035.
- ,,, et al.Comparative effects of torasemide and furosemide in rats with heart failure.Biochem Pharmacol.2008;75(3):649–659.
- ,,,.Vasodilators in the management of acute heart failure.Crit Care Med.2008;36(suppl 1):S95–S105.
- .Vasodilators in acute heart failure.Heart Fail Rev.2007;12(2):143–147.
- ,,,,,.Incidence of early tolerance to hemodynamic effects of continuous infusion of nitroglycerin in patients with coronary artery disease and heart failure.Circulation.1987;76(3):577–584.
- ,,.Natriuretic effect of atrial extract on isolated perfused rat kidney.Can J Physiol Pharmacol.1983;61(12):1462–1466.
- ,,, et al.Systemic hemodynamic, neurohormonal, and renal effects of a steady‐state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure.J Card Fail.1998;4(1):37–44.
- ,,, et al.Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure.Circulation.1991;84(4):1581–1588.
- ,,, et al.Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine.Circulation.2004;110(12):1620–1625.
- ,,, et al.Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre‐existing renal dysfunction: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.2007;50(19):1835–1840.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,.Effect of nesiritide on length of hospital stay in patients with decompensated heart failure.J Cardiovasc Pharmacol Ther.2004;9(3):173–177.
- ,,.Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure.Circulation.2005;111(12):1487–1491.
- ,,,.Short‐term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials.JAMA.2005;293(15):1900–1905.
- .Serum creatinine elevations in patients receiving nesiritide are related to starting dose [abstract 248].J Card Fail.2005;11(suppl 6):S156.
- ,,,.Nonhypotensive low‐dose nesiritide has differential renal effects compared with standard‐dose nesiritide in patients with acute decompensated heart failure and renal dysfunction.JAm Coll Cardiol.2006;47(11):2334–2335.
- .Combining nesiritide with high‐dose diuretics may increase the risk of increased serum creatinine [abstract 2180].Circulation.2005;112(17 suppl II):II‐451–II‐452.
- ,,, et al.Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA trial.J Am Coll Cardiol.2007;49(6):716–726.
- ,,,,.Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary‐bypass surgery: a double‐blind placebo‐controlled pilot study.Circulation.2007;116(suppl 11):I‐134–I‐138.
- .Temporal characteristics of serum creatinine elevations in patients receiving nesiritide and nitroglycerin [abstract 2793].Circulation.2008;112(17 suppl II):II‐590.
- .Nesiritide does not increase 30‐day or 6‐month mortality risk [abstract 3169].Circulation.2005;112(17 suppl II):II‐676.
- ,,, et al.Safety and efficacy of outpatient nesiritide in patients with advanced heart failure.Circ Heart Fail.2008;1:9–16.
- ,,, et al.Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure.J Am Coll Cardiol.2007;49(6):675–683.
- ,,, et al.A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure.J Card Fail.2008;14(1):1–5.
- Heart Failure Society of America. Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,, et al.Cardiorenal interactions: insights from the ESCAPE trial.J Am Coll Cardiol.2008;51(13):1268–1274.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,.Redefining the therapeutic objective in decompensated heart failure: hemoconcentration as a surrogate for plasma refill rate.J Card Fail.2006;12(4):247–249.
- .The cardiorenal syndrome: lessons from the ADHERE database and treatment options.Heart Fail Rev.2004;9(3):195–201.
- ,,,.Hyponatremia and vasopressin antagonism in congestive heart failure.Clin Cardiol.2007;30(11):546–551.
- ,.Vasopressin antagonism in heart failure.J Am Coll Cardiol.2005;46(10):1785–1791.
- ,,, et al.BG9719 (CVT‐124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy.Circulation.2002;105(11):1348–1353.
- ,,,,,.The effect of KW‐3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment.J Card Fail.2007;13(8):609–617.
- ,,,,;CKI‐201 and CKI‐202 Investigators.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
Although patients with left ventricular dysfunction may present with low‐output syndrome and even cardiogenic shock, the majority are admitted with symptoms of congestion.1 The classic symptoms of congestive heart failure reflect fluid overload, that is, orthopnea, paroxysmal nocturnal dyspnea, and peripheral edema; these symptoms can be so dramatic that it is not surprising that patients seek hospitalization.2 Activation of the renin angiotensin system coupled with sympathetic hyperactivity results in marked sodium retention and increased filling pressures in the right and left ventricle that ultimately bring about these congestive symptoms of dyspnea and orthopnea.3 Increased filling pressure precedes admission to the hospital, and filling pressure falls during successful therapy.4, 5 Indeed, normalization of the left ventricular filling pressure much better predicts survival than improved cardiac output.6 However, despite the many advances in the evidence‐based armamentarium for heart failure, the one great deficiency in the evidence base is the lack of data on modalities that can reduce or normalize left ventricular filling pressures. This is not as unexpected as it seems because the symptoms of congestion are so dramatic and, until recently, the tools to mitigate were so few that randomized trials were difficult to conceive. However, the treatment paradigms for acute decompensated heart failure (ADHF) management are changing, and evidence‐based mortality trials for filling pressure reduction and congestion relief continue to evolve.710
DIURETICS
Mercurial diuretics were introduced in the 1920s as the mainstay of therapy for ADHF; the loop diuretics became the foundation of therapy in the 1960s.11, 12 In the Acute Decompensated Heart Failure National Registry database (ADHERE), 88% of patients received intravenous loop diuretics during their hospitalization.13 Loop diuretics act in the thick ascending limb of the loop of Henle to inhibit reabsorption of sodium and chloride by inhibiting the sodium, potassium, and chloride (Na+/K+/2Cl) pump. This blockade causes increased delivery of these solutes to the distal convoluted tubule and collecting duct, resulting in a shift in the balance of osmotic forces toward fluid secretion into the collecting system. Through this mechanism, loop diuretics increase natriuresis and diuresis (Figure 1).14

Less commonly used are the thiazide diuretics, which act on the distal convoluted tubule to block Na+, K+‐ATPase and thereby NaCl transport in the distal convoluted tubule.15 Thiazide diuretics are much less powerful than loop diuretics and are rarely used intravenously in the hospital. These do possess a synergistic effect when used with loop diuretics in that sodium reabsorption is blocked in 2 sections of the nephron.16 Extreme care must be taken to avoid overdiuresis, but this combination can be helpful to treat diuretic resistance.17
The third class of agents are the so‐called potassium‐sparing diuretics, which block sodium reuptake in the final portion of the nephron (the collecting ducts), resulting in an obligatory reuptake of potassium. These agents include the aldosterone receptor blocker spironolactone and eplerenone, which act primarily through competitive binding of receptors at the aldosterone‐dependent sodium‐potassium exchange site in the distal convoluted renal tubule. Although weak diuretics, they are the only class of diuretics shown to improve mortality in moderate to severe heart failure,18, 19 presumably by modulating the abnormal neurohormonal activation of the sympathetic nervous system and the renin‐angiotensin‐aldosterone axis.20 Unfortunately, severe hyperkalemia remains a significant side effect and can limit their use.21
Despite the obvious beneficial effects of loop diuretics in the treatment of ADHF, we lack key fundamental information about these most frequently used drugs. For example, what is the correct dose? Escalating the dose of diuretics has been associated with increased mortality in heart failure even when corrected for the severity of the illness.22, 23 Proposed explanations for the increased morality in patients with heart failure include activation of the renin‐angiotensin‐aldosterone system and sympathetic nervous system, decreases in the glomerular filtration rate (GFR) contributing to cardiorenal syndrome, and intravascular volume contraction and decreased left ventricular filling pressure worsening cardiac performance in patients without significant fluid retention.22, 23 It is now recognized that kidney dysfunction plays a vital role in the progress of patients with heart failure, and increases in serum creatinine or blood urea nitrogen are known predictors of mortality.24 Thus, larger doses of diuretics may result in unfavorable outcomes in heart failure patients because of adverse effects on renal function. In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE), there was a clear increase in mortality with escalating loop diuretic doses, especially above 300 mg/day of furosemide (or an equivalent dose of another loop diuretic).25 Although patients with renal dysfunction may require higher doses, prudence would dictate using the lowest dose to gain a reasonable urine output.
How should loop diuretics be given: as a bolus or continuous infusion? Although diuretics have been typically given as a bolus, there are significant theoretical concerns about this method. Furosemide, for example, has a half‐life of approximately 2 hours; when it is given once or twice a day, a breaking phenomenon is seen in which the kidneys start to retain sodium and the effectiveness of the bolus is reduced.26 A Cochrane review has found evidence that a continuous infusion of diuretics produces more diuresis, although the same article suggests that more titration is needed to support this observation.27
Several loop diuretics are used clinically, including furosemide, torsemide, and bumetanide. Which one should be chosen? Furosemide is the least expensive and most widely used, but 2 animal models suggest more favorable cardiac effects (less fibrosis) with torsemide and even mortality benefit.28, 29 There are no comparable human data to guide the clinician, unfortunately. On a milligram per milligram basis, bumetanide produces more natriuresis than either torsemide or furosemide, but again, the clinical significance of this is not known.
VASODILATORS
Nitrates
Nitrates, including nitroglycerin and nitroprusside, have been used in therapy for ADHF primarily as venodilators.30 Thus, they have been shown to reduce right and left ventricular filling pressures, systemic and pulmonary vascular resistance, and, to a lesser extent, systemic blood pressure.31 A serious drawback to the continued use of nitrates is the development of tolerance that can become apparent within hours of their initial use.32 In addition, there have not been large outcome trials to define the duration of benefit or the proper dose.
Nesiritide
A potential role for the natriuretic peptides in heart failure dates back to the 1980s when extracts of the right atrial tissue of rats was shown to produce a brisk natriuresis when given intravenously to a second animal.33 Nesiritide, as the commercially prepared B‐type natriuretic peptide is called, consistently reduced preload and afterload and caused natriuresis in some studies.34, 35 Natriuresis and augmentation of diuresis has not been consistently demonstrated in published reports, however.36, 37 In addition, B‐type natriuretic peptide, when given therapeutically, does suppress aldosterone.38
The largest clinical experience to date with nesiritide came in the Vasodilation in the Management of Acute Congestive Heart Failure (VMAC) trial.39 In this study, nesiritide was compared with intravenous nitroglycerin and placebo when added to standard care for 3 hours in a double‐blind, randomized protocol. Nesiritide reduced filling pressures in comparison with nitroglycerin and placebo and provided greater symptomatic relief in comparison with placebo but not in comparison with nitroglycerin.39 In a retrospective review of consecutive patients, the addition of nesiritide resulted in a decreased length of stay without compromising renal function.40
In 2005, 2 meta‐analyses were published that raised questions about the safety of nesiritide.41, 42 In the first, a review of 5 nesiritide/placebo trials found an increased risk for worsening renal function (specifically, a rise in serum creatinine of 0.5 mg/dL or more).41 This increase occurred in 21% of nesiritide‐treated patients versus 15% of those on placebo (P = .001). Some of these trials in the meta‐analysis employed dosages of nesiritide greater than the currently recommended 0.01 g/kg/minute. When only those patients in the VMAC group that received the recommended 0.01 g/kg/minute dose were analyzed, there was not a significant rise in creatinine.43 Riter et al44 reported a retrospective analysis finding that half or even quarter doses of nesiritide actually produced improvement in renal function compared with the standard dose or no nesiritide use. Higher doses of diuretics, >160 mg of furosemide or its equivalent in conjunction with nesiritide, did increase the risk of renal dysfunction.45 In the Nesiritide Administered Perianesthesia (NAPA) trial, 0.01 g/kg/minute of nesiritide was given as a defined 24‐hour infusion without bolus to high‐risk patients with left ventricular dysfunction undergoing bypass and mitral valve surgery.46 Although serum creatinine increased in both groups following surgery, it increased more so with placebo than nesiritide (P 0.001), despite increased urine output (P 0.001) and shorter length of hospital stay (P = 0.043) in the nesiritide group.46 A smaller study of similar design also noted preservation of renal function with nesiritide compared with placebo in bypass patients.47 Therefore, the current recommendation for nesiritide use is to use no more than 0.01 g/kg/minute. Reduction of diuretic doses would be prudent when nesiritide is used.
Sackner‐Bernstein et al42 combined the results of 3 placebo‐controlled trials and reported an increase in mortality with nesiritide at 30 days. In this study, significant differences were found between the placebo and nesiritide groups in terms of baseline renal function,48 blood pressure, and inotrope use, and this may explain the observed mortality difference.42 Mortality was the same at 180 days. Abraham49 analyzed the 7 available trials and risk‐adjusted the patient populations to avoid group inequalities; with this method, no significant effect of nesiritide use on mortality was seen. The 2 most recent large trialsFollow‐Up Serial Infusions of Nesiritide II (FUSION II) (no effect of nesiritide on mortality) and NAPA (mortality decreased in nesiritide‐treated patients)have provided more safety data concerning the use of nesiritide.46, 50 The most definitive answer will come with the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND‐HF) trial, which will recruit 7000 patients, the largest trial in ADHF to date.
ULTRAFILTRATION (UF)
UF removes water and nonprotein‐bound smallmolecular‐weight and mediummolecular‐weight solutes through the semipermeable membrane when hydrostatic pressure, generated by blood pressure or an external blood pump, exceeds oncotic pressure. The fluid removal rate can be adjusted between 100 and 500 mL/hour. The Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial randomized UF with intravenous diuretic therapy in patients with ADHF and showed that UF produced greater weight and fluid loss at 48 hours versus diuretics and reduced the 90‐day readmission rate for heart failure.51 There was no statistically significant difference noted between the overall mortality and serum creatinine between the 2 groups. Rogers et al52 found in a small, randomized trial that during a 48‐hour period, UF showed no significant difference in the renal hemodynamics (GFR and renal plasma flow in patients with ADHF) versus the standard treatment with intravenous diuretics. The Heart Failure Society of America treatment guidelines for the evaluation and management of patients with ADHF suggest that when congestion fails to improve in response to diuretic therapy, UF may be considered (strength of evidence = C).53
Perhaps the most vexing issue surrounding the use of diuretics, vasodilators, and UF is when to stop intravenous therapy in the hospitalized patient. There are no clear guidelines about this, and perhaps clinical experience is of paramount importance. Theoretically, vasodilator and fluid removal therapy should be continued until the patient is euvolemic, that is, has normal filling pressures (usually associated with normalization of the neck veins and loss of S3) with improvement in symptoms. This was clearly seen in the ESCAPE trial, in which use of either hemodynamic guidance or clinical evaluation of jugular venous pressure resulted in more normal filling pressures.5 This is an extremely important issue because continued fluid removal beyond the point at which the patient is euvolemic may result in renal dysfunction; the latter is a strong predictor of prolonged hospitalization and mortality.54, 55
Initial fluid removal can be rapid with either diuretics or UF, and filling pressures fall within minutes with nesiritide. This is beneficial to the patient as long as extracellular sodium and water reenter the vascular bed to maintain preload. Boyle and Sobotka56 emphasized the importance of this plasma refill rate and proposed monitoring the hematocrit as is done in dialysis to prevent excessive fluid removal. As can be seen in Figure 2, the plasma compartment is easily refilled when extracellular volume is increased in edematous patients early in their therapy. However, the plasma refill rate can fall precipitously when this compartment is depleted. Hence, clinicians must be ever alert to this transition period when continued fluid removal or vasodilator therapy results in depletion of vascular, not interstitial, volume with rapid declines in preload and cardiac output. Therefore, these therapies should be stopped when the patient becomes euvolemic, not later when the patient has become hypovolemic with the attendant problems. In the ESCAPE trial, the jugular venous pressure was the best indicator of a normal filling pressure,5, 54 although this is not an infallible guide. Our practice is to stop diuretics and vasodilators when edema has resolved and jugular venous pressure is below 8 cm. In addition, checking blood urea nitrogen and creatinine twice a day can give an early warning of hypovolemia when these rise 25% above baseline.57 Volume status should be carefully evaluated by changes in the physical examination, including postural blood pressure changes and increases in blood urea nitrogen and creatinine. When worsening renal function occurs, judicious fluid replacement with 500 to 1000 mL of normal saline given over 2 to 4 hours can quickly restore euvolemia and may improve renal function in the hypovolemic patient.

INVESTIGATIONAL THERAPIES
Oral Vasopressin Antagonist
Elevation of arginine vasopressin contributes to fluid retention and hyponatremia and is directly proportional to the severity of heart failure.58 Tolvaptan is an oral, nonpeptide, selective vasopressin V2 receptor antagonist whose action on the distal nephron causes loss of electrolyte‐free water (aquaresis).59 The efficacy of tolvaptan was tested in a double‐blind, prospective, randomized international trial, the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST), with a 30 mg/day oral dosage of tolvaptan versus placebo within 48 hours of admission with ADHF.7, 9 Patients receiving tolvaptan showed improvement in dyspnea on day 1 along with body weight and edema reduction on day 7 or discharge in comparison with placebo. The improvement in global clinical assessment was not different between the 2 groups. The serious adverse event frequencies were similar between the groups without excess renal failure or hypotension in the tolvaptan group.7, 9 Unfortunately, this small beneficial effect in the hospital did not result in positive survival benefit.
Adenosine A1‐Receptor (AA1R) Antagonists
The kidney is the only organ in which adenosine is a paracrine vasoconstrictor.60 Dittrich et al61 did a randomized, double‐blind, placebo‐controlled, 2‐way crossover study in patients with heart failure and renal impairment (median GFR = 50 mL/minute) and tested the effectiveness of the AA1R antagonist rolofylline as a renal vasodilator in an outpatient setting. Blockade of these receptors increased vasodilation and GFR. Givertz et al62 evaluated the effect of AA1R antagonists on diuresis and renal function in patients with ADHF and renal impairment or diuretic resistance. A paired, randomized, double‐blind, placebo‐controlled, proof‐of‐concept trial in patients with ADHF and volume overload found that the AA1R antagonist KW‐3092 enhances the response of loop diuretics and may have a renal protective effect. Prophylaxis for Thromboembolism in Critical Care Trials 1 and 2 (PROTECT 1,2) studies investigating KW‐3092 to assess the effects on heart failure and renal function, are currently under way.10
CONCLUSION
The goals in the management of ADHF are deceptively simple: improve symptoms by normalizing filling pressure and volume status efficiently without worsening renal function. Powerful tools, including diuretics, vasodilators, and UF, exist to accomplish these goals, but determining which tools to use in which patients and the precise manner in which to use the tools (alone or in combination and duration) remains more of an art than a science. The concept of euvolemia needs to be more carefully defined and conceptualized in a way that is useful for clinicians. Frequent monitoring of clinical signs, electrolytes, and renal function are our current best guides to assess volume status during therapy. Newer modalities hold promise that early detection of fluid overload may prevent hospitalization and reduce costs. Similarly, new pharmacologic therapies hold promise that their use may improve cardiac function and reduce renal abnormalities, thereby improving outcomes in patients with ADHF.
Although patients with left ventricular dysfunction may present with low‐output syndrome and even cardiogenic shock, the majority are admitted with symptoms of congestion.1 The classic symptoms of congestive heart failure reflect fluid overload, that is, orthopnea, paroxysmal nocturnal dyspnea, and peripheral edema; these symptoms can be so dramatic that it is not surprising that patients seek hospitalization.2 Activation of the renin angiotensin system coupled with sympathetic hyperactivity results in marked sodium retention and increased filling pressures in the right and left ventricle that ultimately bring about these congestive symptoms of dyspnea and orthopnea.3 Increased filling pressure precedes admission to the hospital, and filling pressure falls during successful therapy.4, 5 Indeed, normalization of the left ventricular filling pressure much better predicts survival than improved cardiac output.6 However, despite the many advances in the evidence‐based armamentarium for heart failure, the one great deficiency in the evidence base is the lack of data on modalities that can reduce or normalize left ventricular filling pressures. This is not as unexpected as it seems because the symptoms of congestion are so dramatic and, until recently, the tools to mitigate were so few that randomized trials were difficult to conceive. However, the treatment paradigms for acute decompensated heart failure (ADHF) management are changing, and evidence‐based mortality trials for filling pressure reduction and congestion relief continue to evolve.710
DIURETICS
Mercurial diuretics were introduced in the 1920s as the mainstay of therapy for ADHF; the loop diuretics became the foundation of therapy in the 1960s.11, 12 In the Acute Decompensated Heart Failure National Registry database (ADHERE), 88% of patients received intravenous loop diuretics during their hospitalization.13 Loop diuretics act in the thick ascending limb of the loop of Henle to inhibit reabsorption of sodium and chloride by inhibiting the sodium, potassium, and chloride (Na+/K+/2Cl) pump. This blockade causes increased delivery of these solutes to the distal convoluted tubule and collecting duct, resulting in a shift in the balance of osmotic forces toward fluid secretion into the collecting system. Through this mechanism, loop diuretics increase natriuresis and diuresis (Figure 1).14

Less commonly used are the thiazide diuretics, which act on the distal convoluted tubule to block Na+, K+‐ATPase and thereby NaCl transport in the distal convoluted tubule.15 Thiazide diuretics are much less powerful than loop diuretics and are rarely used intravenously in the hospital. These do possess a synergistic effect when used with loop diuretics in that sodium reabsorption is blocked in 2 sections of the nephron.16 Extreme care must be taken to avoid overdiuresis, but this combination can be helpful to treat diuretic resistance.17
The third class of agents are the so‐called potassium‐sparing diuretics, which block sodium reuptake in the final portion of the nephron (the collecting ducts), resulting in an obligatory reuptake of potassium. These agents include the aldosterone receptor blocker spironolactone and eplerenone, which act primarily through competitive binding of receptors at the aldosterone‐dependent sodium‐potassium exchange site in the distal convoluted renal tubule. Although weak diuretics, they are the only class of diuretics shown to improve mortality in moderate to severe heart failure,18, 19 presumably by modulating the abnormal neurohormonal activation of the sympathetic nervous system and the renin‐angiotensin‐aldosterone axis.20 Unfortunately, severe hyperkalemia remains a significant side effect and can limit their use.21
Despite the obvious beneficial effects of loop diuretics in the treatment of ADHF, we lack key fundamental information about these most frequently used drugs. For example, what is the correct dose? Escalating the dose of diuretics has been associated with increased mortality in heart failure even when corrected for the severity of the illness.22, 23 Proposed explanations for the increased morality in patients with heart failure include activation of the renin‐angiotensin‐aldosterone system and sympathetic nervous system, decreases in the glomerular filtration rate (GFR) contributing to cardiorenal syndrome, and intravascular volume contraction and decreased left ventricular filling pressure worsening cardiac performance in patients without significant fluid retention.22, 23 It is now recognized that kidney dysfunction plays a vital role in the progress of patients with heart failure, and increases in serum creatinine or blood urea nitrogen are known predictors of mortality.24 Thus, larger doses of diuretics may result in unfavorable outcomes in heart failure patients because of adverse effects on renal function. In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE), there was a clear increase in mortality with escalating loop diuretic doses, especially above 300 mg/day of furosemide (or an equivalent dose of another loop diuretic).25 Although patients with renal dysfunction may require higher doses, prudence would dictate using the lowest dose to gain a reasonable urine output.
How should loop diuretics be given: as a bolus or continuous infusion? Although diuretics have been typically given as a bolus, there are significant theoretical concerns about this method. Furosemide, for example, has a half‐life of approximately 2 hours; when it is given once or twice a day, a breaking phenomenon is seen in which the kidneys start to retain sodium and the effectiveness of the bolus is reduced.26 A Cochrane review has found evidence that a continuous infusion of diuretics produces more diuresis, although the same article suggests that more titration is needed to support this observation.27
Several loop diuretics are used clinically, including furosemide, torsemide, and bumetanide. Which one should be chosen? Furosemide is the least expensive and most widely used, but 2 animal models suggest more favorable cardiac effects (less fibrosis) with torsemide and even mortality benefit.28, 29 There are no comparable human data to guide the clinician, unfortunately. On a milligram per milligram basis, bumetanide produces more natriuresis than either torsemide or furosemide, but again, the clinical significance of this is not known.
VASODILATORS
Nitrates
Nitrates, including nitroglycerin and nitroprusside, have been used in therapy for ADHF primarily as venodilators.30 Thus, they have been shown to reduce right and left ventricular filling pressures, systemic and pulmonary vascular resistance, and, to a lesser extent, systemic blood pressure.31 A serious drawback to the continued use of nitrates is the development of tolerance that can become apparent within hours of their initial use.32 In addition, there have not been large outcome trials to define the duration of benefit or the proper dose.
Nesiritide
A potential role for the natriuretic peptides in heart failure dates back to the 1980s when extracts of the right atrial tissue of rats was shown to produce a brisk natriuresis when given intravenously to a second animal.33 Nesiritide, as the commercially prepared B‐type natriuretic peptide is called, consistently reduced preload and afterload and caused natriuresis in some studies.34, 35 Natriuresis and augmentation of diuresis has not been consistently demonstrated in published reports, however.36, 37 In addition, B‐type natriuretic peptide, when given therapeutically, does suppress aldosterone.38
The largest clinical experience to date with nesiritide came in the Vasodilation in the Management of Acute Congestive Heart Failure (VMAC) trial.39 In this study, nesiritide was compared with intravenous nitroglycerin and placebo when added to standard care for 3 hours in a double‐blind, randomized protocol. Nesiritide reduced filling pressures in comparison with nitroglycerin and placebo and provided greater symptomatic relief in comparison with placebo but not in comparison with nitroglycerin.39 In a retrospective review of consecutive patients, the addition of nesiritide resulted in a decreased length of stay without compromising renal function.40
In 2005, 2 meta‐analyses were published that raised questions about the safety of nesiritide.41, 42 In the first, a review of 5 nesiritide/placebo trials found an increased risk for worsening renal function (specifically, a rise in serum creatinine of 0.5 mg/dL or more).41 This increase occurred in 21% of nesiritide‐treated patients versus 15% of those on placebo (P = .001). Some of these trials in the meta‐analysis employed dosages of nesiritide greater than the currently recommended 0.01 g/kg/minute. When only those patients in the VMAC group that received the recommended 0.01 g/kg/minute dose were analyzed, there was not a significant rise in creatinine.43 Riter et al44 reported a retrospective analysis finding that half or even quarter doses of nesiritide actually produced improvement in renal function compared with the standard dose or no nesiritide use. Higher doses of diuretics, >160 mg of furosemide or its equivalent in conjunction with nesiritide, did increase the risk of renal dysfunction.45 In the Nesiritide Administered Perianesthesia (NAPA) trial, 0.01 g/kg/minute of nesiritide was given as a defined 24‐hour infusion without bolus to high‐risk patients with left ventricular dysfunction undergoing bypass and mitral valve surgery.46 Although serum creatinine increased in both groups following surgery, it increased more so with placebo than nesiritide (P 0.001), despite increased urine output (P 0.001) and shorter length of hospital stay (P = 0.043) in the nesiritide group.46 A smaller study of similar design also noted preservation of renal function with nesiritide compared with placebo in bypass patients.47 Therefore, the current recommendation for nesiritide use is to use no more than 0.01 g/kg/minute. Reduction of diuretic doses would be prudent when nesiritide is used.
Sackner‐Bernstein et al42 combined the results of 3 placebo‐controlled trials and reported an increase in mortality with nesiritide at 30 days. In this study, significant differences were found between the placebo and nesiritide groups in terms of baseline renal function,48 blood pressure, and inotrope use, and this may explain the observed mortality difference.42 Mortality was the same at 180 days. Abraham49 analyzed the 7 available trials and risk‐adjusted the patient populations to avoid group inequalities; with this method, no significant effect of nesiritide use on mortality was seen. The 2 most recent large trialsFollow‐Up Serial Infusions of Nesiritide II (FUSION II) (no effect of nesiritide on mortality) and NAPA (mortality decreased in nesiritide‐treated patients)have provided more safety data concerning the use of nesiritide.46, 50 The most definitive answer will come with the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND‐HF) trial, which will recruit 7000 patients, the largest trial in ADHF to date.
ULTRAFILTRATION (UF)
UF removes water and nonprotein‐bound smallmolecular‐weight and mediummolecular‐weight solutes through the semipermeable membrane when hydrostatic pressure, generated by blood pressure or an external blood pump, exceeds oncotic pressure. The fluid removal rate can be adjusted between 100 and 500 mL/hour. The Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial randomized UF with intravenous diuretic therapy in patients with ADHF and showed that UF produced greater weight and fluid loss at 48 hours versus diuretics and reduced the 90‐day readmission rate for heart failure.51 There was no statistically significant difference noted between the overall mortality and serum creatinine between the 2 groups. Rogers et al52 found in a small, randomized trial that during a 48‐hour period, UF showed no significant difference in the renal hemodynamics (GFR and renal plasma flow in patients with ADHF) versus the standard treatment with intravenous diuretics. The Heart Failure Society of America treatment guidelines for the evaluation and management of patients with ADHF suggest that when congestion fails to improve in response to diuretic therapy, UF may be considered (strength of evidence = C).53
Perhaps the most vexing issue surrounding the use of diuretics, vasodilators, and UF is when to stop intravenous therapy in the hospitalized patient. There are no clear guidelines about this, and perhaps clinical experience is of paramount importance. Theoretically, vasodilator and fluid removal therapy should be continued until the patient is euvolemic, that is, has normal filling pressures (usually associated with normalization of the neck veins and loss of S3) with improvement in symptoms. This was clearly seen in the ESCAPE trial, in which use of either hemodynamic guidance or clinical evaluation of jugular venous pressure resulted in more normal filling pressures.5 This is an extremely important issue because continued fluid removal beyond the point at which the patient is euvolemic may result in renal dysfunction; the latter is a strong predictor of prolonged hospitalization and mortality.54, 55
Initial fluid removal can be rapid with either diuretics or UF, and filling pressures fall within minutes with nesiritide. This is beneficial to the patient as long as extracellular sodium and water reenter the vascular bed to maintain preload. Boyle and Sobotka56 emphasized the importance of this plasma refill rate and proposed monitoring the hematocrit as is done in dialysis to prevent excessive fluid removal. As can be seen in Figure 2, the plasma compartment is easily refilled when extracellular volume is increased in edematous patients early in their therapy. However, the plasma refill rate can fall precipitously when this compartment is depleted. Hence, clinicians must be ever alert to this transition period when continued fluid removal or vasodilator therapy results in depletion of vascular, not interstitial, volume with rapid declines in preload and cardiac output. Therefore, these therapies should be stopped when the patient becomes euvolemic, not later when the patient has become hypovolemic with the attendant problems. In the ESCAPE trial, the jugular venous pressure was the best indicator of a normal filling pressure,5, 54 although this is not an infallible guide. Our practice is to stop diuretics and vasodilators when edema has resolved and jugular venous pressure is below 8 cm. In addition, checking blood urea nitrogen and creatinine twice a day can give an early warning of hypovolemia when these rise 25% above baseline.57 Volume status should be carefully evaluated by changes in the physical examination, including postural blood pressure changes and increases in blood urea nitrogen and creatinine. When worsening renal function occurs, judicious fluid replacement with 500 to 1000 mL of normal saline given over 2 to 4 hours can quickly restore euvolemia and may improve renal function in the hypovolemic patient.

INVESTIGATIONAL THERAPIES
Oral Vasopressin Antagonist
Elevation of arginine vasopressin contributes to fluid retention and hyponatremia and is directly proportional to the severity of heart failure.58 Tolvaptan is an oral, nonpeptide, selective vasopressin V2 receptor antagonist whose action on the distal nephron causes loss of electrolyte‐free water (aquaresis).59 The efficacy of tolvaptan was tested in a double‐blind, prospective, randomized international trial, the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST), with a 30 mg/day oral dosage of tolvaptan versus placebo within 48 hours of admission with ADHF.7, 9 Patients receiving tolvaptan showed improvement in dyspnea on day 1 along with body weight and edema reduction on day 7 or discharge in comparison with placebo. The improvement in global clinical assessment was not different between the 2 groups. The serious adverse event frequencies were similar between the groups without excess renal failure or hypotension in the tolvaptan group.7, 9 Unfortunately, this small beneficial effect in the hospital did not result in positive survival benefit.
Adenosine A1‐Receptor (AA1R) Antagonists
The kidney is the only organ in which adenosine is a paracrine vasoconstrictor.60 Dittrich et al61 did a randomized, double‐blind, placebo‐controlled, 2‐way crossover study in patients with heart failure and renal impairment (median GFR = 50 mL/minute) and tested the effectiveness of the AA1R antagonist rolofylline as a renal vasodilator in an outpatient setting. Blockade of these receptors increased vasodilation and GFR. Givertz et al62 evaluated the effect of AA1R antagonists on diuresis and renal function in patients with ADHF and renal impairment or diuretic resistance. A paired, randomized, double‐blind, placebo‐controlled, proof‐of‐concept trial in patients with ADHF and volume overload found that the AA1R antagonist KW‐3092 enhances the response of loop diuretics and may have a renal protective effect. Prophylaxis for Thromboembolism in Critical Care Trials 1 and 2 (PROTECT 1,2) studies investigating KW‐3092 to assess the effects on heart failure and renal function, are currently under way.10
CONCLUSION
The goals in the management of ADHF are deceptively simple: improve symptoms by normalizing filling pressure and volume status efficiently without worsening renal function. Powerful tools, including diuretics, vasodilators, and UF, exist to accomplish these goals, but determining which tools to use in which patients and the precise manner in which to use the tools (alone or in combination and duration) remains more of an art than a science. The concept of euvolemia needs to be more carefully defined and conceptualized in a way that is useful for clinicians. Frequent monitoring of clinical signs, electrolytes, and renal function are our current best guides to assess volume status during therapy. Newer modalities hold promise that early detection of fluid overload may prevent hospitalization and reduce costs. Similarly, new pharmacologic therapies hold promise that their use may improve cardiac function and reduce renal abnormalities, thereby improving outcomes in patients with ADHF.
- .Tailored therapy to hemodynamic goals for advanced heart failure.Eur J Heart Fail.1999;1(3):251–257.
- ,,,,,.Profile for estimating risk of heart failure.Arch Intern Med.1999;159(11):1197–1204.
- .The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure [editorial].J Am Coll Cardiol.1992;20(1):248–254.
- ,,, et al.Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system.J Am Coll Cardiol.2003;41(4):565–571.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Persistently high left ventricular filling pressures predict mortality despite angiotensin converting enzyme inhibition in advanced heart failure [abstract 2624].Circulation.1994;90(4 pt 2):I‐488.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial.JAMA.2007;297(12):1319–1331.
- . Considerations in designing acute decompensated heart failure clinical trials. Available at:http://www.medscape.com/viewarticle/557964. Accessed September 2008.
- ,,, et al.Short‐term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials.JAMA.2007;297(12):1332–1343.
- ,,.Rolofylline (KW‐3902): a new adenosine A1‐receptor antagonist for acute congestive heart failure.Future Cardiol.2008;4(2):117–123.
- ,,,,.Acute haemodynamic effects of frusemide in patients with normal and raised left atrial pressures.Br Heart J.1969;31(6):711–717.
- ,.The diuretic effect of Novasurol and other mercury injections.Wien Klin Wochenschr.2002;33:943–944.
- ,,,,.Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE).Am Heart J.2007;153(6):1021–1028.
- ,,.Optimal use of diuretics in patients with heart failure.Curr Treat Options Cardiovasc Med.2007;9(4):332–342.
- ,,,.Characterization of the thiazide‐sensitive Na(+)‐Cl(−) cotransporter: a new model for ions and diuretics interaction.Am J Physiol Renal Physiol.2000;279(1):F161–F169.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- ,.Combination of high‐dose furosemide and hydrochlorothiazide in the treatment of refractory congestive heart failure.Eur Heart J.1996;17(12):1867–1874.
- ,,, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure.N Engl J Med.1999;341(10):709–717.
- ,,, et al.Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction.N Engl J Med.2003;348(14):1309–1321.
- .Pathophysiologic role of the renin‐angiotensin‐aldosterone and sympathetic nervous systems in heart failure.Am J Health Syst Pharm.2004;61(suppl 2):S4–S13.
- ,,,.Detection of spironolactone‐associated hyperkalaemia following the Randomized Aldactone Evaluation Study (RALES).Drug Saf.2007;30(12):1143–1149.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,.Dose‐dependent association between use of loop diuretics and mortality in advanced systolic heart failure.Am J Cardiol.2006;98(10):1416–1417.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,, et al.Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial.Eur J Heart Fail.2007;9(10):1064–1069.
- ,,.Mechanism of impaired natriuretic response to furosemide during prolonged therapy.Kidney Int.1989;36(4):682–689.
- ,,,.Continuous infusion versus bolus injection of loop diuretics in congestive heart failure.Cochrane Database Syst Rev.2004; (1):CD003178.
- ,,,,,.Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure.J Am Coll Cardiol.2004;43(11):2028–2035.
- ,,, et al.Comparative effects of torasemide and furosemide in rats with heart failure.Biochem Pharmacol.2008;75(3):649–659.
- ,,,.Vasodilators in the management of acute heart failure.Crit Care Med.2008;36(suppl 1):S95–S105.
- .Vasodilators in acute heart failure.Heart Fail Rev.2007;12(2):143–147.
- ,,,,,.Incidence of early tolerance to hemodynamic effects of continuous infusion of nitroglycerin in patients with coronary artery disease and heart failure.Circulation.1987;76(3):577–584.
- ,,.Natriuretic effect of atrial extract on isolated perfused rat kidney.Can J Physiol Pharmacol.1983;61(12):1462–1466.
- ,,, et al.Systemic hemodynamic, neurohormonal, and renal effects of a steady‐state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure.J Card Fail.1998;4(1):37–44.
- ,,, et al.Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure.Circulation.1991;84(4):1581–1588.
- ,,, et al.Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine.Circulation.2004;110(12):1620–1625.
- ,,, et al.Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre‐existing renal dysfunction: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.2007;50(19):1835–1840.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,.Effect of nesiritide on length of hospital stay in patients with decompensated heart failure.J Cardiovasc Pharmacol Ther.2004;9(3):173–177.
- ,,.Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure.Circulation.2005;111(12):1487–1491.
- ,,,.Short‐term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials.JAMA.2005;293(15):1900–1905.
- .Serum creatinine elevations in patients receiving nesiritide are related to starting dose [abstract 248].J Card Fail.2005;11(suppl 6):S156.
- ,,,.Nonhypotensive low‐dose nesiritide has differential renal effects compared with standard‐dose nesiritide in patients with acute decompensated heart failure and renal dysfunction.JAm Coll Cardiol.2006;47(11):2334–2335.
- .Combining nesiritide with high‐dose diuretics may increase the risk of increased serum creatinine [abstract 2180].Circulation.2005;112(17 suppl II):II‐451–II‐452.
- ,,, et al.Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA trial.J Am Coll Cardiol.2007;49(6):716–726.
- ,,,,.Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary‐bypass surgery: a double‐blind placebo‐controlled pilot study.Circulation.2007;116(suppl 11):I‐134–I‐138.
- .Temporal characteristics of serum creatinine elevations in patients receiving nesiritide and nitroglycerin [abstract 2793].Circulation.2008;112(17 suppl II):II‐590.
- .Nesiritide does not increase 30‐day or 6‐month mortality risk [abstract 3169].Circulation.2005;112(17 suppl II):II‐676.
- ,,, et al.Safety and efficacy of outpatient nesiritide in patients with advanced heart failure.Circ Heart Fail.2008;1:9–16.
- ,,, et al.Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure.J Am Coll Cardiol.2007;49(6):675–683.
- ,,, et al.A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure.J Card Fail.2008;14(1):1–5.
- Heart Failure Society of America. Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,, et al.Cardiorenal interactions: insights from the ESCAPE trial.J Am Coll Cardiol.2008;51(13):1268–1274.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,.Redefining the therapeutic objective in decompensated heart failure: hemoconcentration as a surrogate for plasma refill rate.J Card Fail.2006;12(4):247–249.
- .The cardiorenal syndrome: lessons from the ADHERE database and treatment options.Heart Fail Rev.2004;9(3):195–201.
- ,,,.Hyponatremia and vasopressin antagonism in congestive heart failure.Clin Cardiol.2007;30(11):546–551.
- ,.Vasopressin antagonism in heart failure.J Am Coll Cardiol.2005;46(10):1785–1791.
- ,,, et al.BG9719 (CVT‐124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy.Circulation.2002;105(11):1348–1353.
- ,,,,,.The effect of KW‐3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment.J Card Fail.2007;13(8):609–617.
- ,,,,;CKI‐201 and CKI‐202 Investigators.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
- .Tailored therapy to hemodynamic goals for advanced heart failure.Eur J Heart Fail.1999;1(3):251–257.
- ,,,,,.Profile for estimating risk of heart failure.Arch Intern Med.1999;159(11):1197–1204.
- .The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure [editorial].J Am Coll Cardiol.1992;20(1):248–254.
- ,,, et al.Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system.J Am Coll Cardiol.2003;41(4):565–571.
- ,,, et al.Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial.JAMA.2005;294(13):1625–1633.
- ,,, et al.Persistently high left ventricular filling pressures predict mortality despite angiotensin converting enzyme inhibition in advanced heart failure [abstract 2624].Circulation.1994;90(4 pt 2):I‐488.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial.JAMA.2007;297(12):1319–1331.
- . Considerations in designing acute decompensated heart failure clinical trials. Available at:http://www.medscape.com/viewarticle/557964. Accessed September 2008.
- ,,, et al.Short‐term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials.JAMA.2007;297(12):1332–1343.
- ,,.Rolofylline (KW‐3902): a new adenosine A1‐receptor antagonist for acute congestive heart failure.Future Cardiol.2008;4(2):117–123.
- ,,,,.Acute haemodynamic effects of frusemide in patients with normal and raised left atrial pressures.Br Heart J.1969;31(6):711–717.
- ,.The diuretic effect of Novasurol and other mercury injections.Wien Klin Wochenschr.2002;33:943–944.
- ,,,,.Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE).Am Heart J.2007;153(6):1021–1028.
- ,,.Optimal use of diuretics in patients with heart failure.Curr Treat Options Cardiovasc Med.2007;9(4):332–342.
- ,,,.Characterization of the thiazide‐sensitive Na(+)‐Cl(−) cotransporter: a new model for ions and diuretics interaction.Am J Physiol Renal Physiol.2000;279(1):F161–F169.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- ,.Combination of high‐dose furosemide and hydrochlorothiazide in the treatment of refractory congestive heart failure.Eur Heart J.1996;17(12):1867–1874.
- ,,, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure.N Engl J Med.1999;341(10):709–717.
- ,,, et al.Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction.N Engl J Med.2003;348(14):1309–1321.
- .Pathophysiologic role of the renin‐angiotensin‐aldosterone and sympathetic nervous systems in heart failure.Am J Health Syst Pharm.2004;61(suppl 2):S4–S13.
- ,,,.Detection of spironolactone‐associated hyperkalaemia following the Randomized Aldactone Evaluation Study (RALES).Drug Saf.2007;30(12):1143–1149.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,.Dose‐dependent association between use of loop diuretics and mortality in advanced systolic heart failure.Am J Cardiol.2006;98(10):1416–1417.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,, et al.Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial.Eur J Heart Fail.2007;9(10):1064–1069.
- ,,.Mechanism of impaired natriuretic response to furosemide during prolonged therapy.Kidney Int.1989;36(4):682–689.
- ,,,.Continuous infusion versus bolus injection of loop diuretics in congestive heart failure.Cochrane Database Syst Rev.2004; (1):CD003178.
- ,,,,,.Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure.J Am Coll Cardiol.2004;43(11):2028–2035.
- ,,, et al.Comparative effects of torasemide and furosemide in rats with heart failure.Biochem Pharmacol.2008;75(3):649–659.
- ,,,.Vasodilators in the management of acute heart failure.Crit Care Med.2008;36(suppl 1):S95–S105.
- .Vasodilators in acute heart failure.Heart Fail Rev.2007;12(2):143–147.
- ,,,,,.Incidence of early tolerance to hemodynamic effects of continuous infusion of nitroglycerin in patients with coronary artery disease and heart failure.Circulation.1987;76(3):577–584.
- ,,.Natriuretic effect of atrial extract on isolated perfused rat kidney.Can J Physiol Pharmacol.1983;61(12):1462–1466.
- ,,, et al.Systemic hemodynamic, neurohormonal, and renal effects of a steady‐state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure.J Card Fail.1998;4(1):37–44.
- ,,, et al.Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure.Circulation.1991;84(4):1581–1588.
- ,,, et al.Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine.Circulation.2004;110(12):1620–1625.
- ,,, et al.Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre‐existing renal dysfunction: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.2007;50(19):1835–1840.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,.Effect of nesiritide on length of hospital stay in patients with decompensated heart failure.J Cardiovasc Pharmacol Ther.2004;9(3):173–177.
- ,,.Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure.Circulation.2005;111(12):1487–1491.
- ,,,.Short‐term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials.JAMA.2005;293(15):1900–1905.
- .Serum creatinine elevations in patients receiving nesiritide are related to starting dose [abstract 248].J Card Fail.2005;11(suppl 6):S156.
- ,,,.Nonhypotensive low‐dose nesiritide has differential renal effects compared with standard‐dose nesiritide in patients with acute decompensated heart failure and renal dysfunction.JAm Coll Cardiol.2006;47(11):2334–2335.
- .Combining nesiritide with high‐dose diuretics may increase the risk of increased serum creatinine [abstract 2180].Circulation.2005;112(17 suppl II):II‐451–II‐452.
- ,,, et al.Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA trial.J Am Coll Cardiol.2007;49(6):716–726.
- ,,,,.Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary‐bypass surgery: a double‐blind placebo‐controlled pilot study.Circulation.2007;116(suppl 11):I‐134–I‐138.
- .Temporal characteristics of serum creatinine elevations in patients receiving nesiritide and nitroglycerin [abstract 2793].Circulation.2008;112(17 suppl II):II‐590.
- .Nesiritide does not increase 30‐day or 6‐month mortality risk [abstract 3169].Circulation.2005;112(17 suppl II):II‐676.
- ,,, et al.Safety and efficacy of outpatient nesiritide in patients with advanced heart failure.Circ Heart Fail.2008;1:9–16.
- ,,, et al.Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure.J Am Coll Cardiol.2007;49(6):675–683.
- ,,, et al.A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure.J Card Fail.2008;14(1):1–5.
- Heart Failure Society of America. Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,, et al.Cardiorenal interactions: insights from the ESCAPE trial.J Am Coll Cardiol.2008;51(13):1268–1274.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,.Redefining the therapeutic objective in decompensated heart failure: hemoconcentration as a surrogate for plasma refill rate.J Card Fail.2006;12(4):247–249.
- .The cardiorenal syndrome: lessons from the ADHERE database and treatment options.Heart Fail Rev.2004;9(3):195–201.
- ,,,.Hyponatremia and vasopressin antagonism in congestive heart failure.Clin Cardiol.2007;30(11):546–551.
- ,.Vasopressin antagonism in heart failure.J Am Coll Cardiol.2005;46(10):1785–1791.
- ,,, et al.BG9719 (CVT‐124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy.Circulation.2002;105(11):1348–1353.
- ,,,,,.The effect of KW‐3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment.J Card Fail.2007;13(8):609–617.
- ,,,,;CKI‐201 and CKI‐202 Investigators.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.