User login
Advances in therapy for type 2 diabetes: GLP–1 receptor agonists and DPP–4 inhibitors
The prevalence of type 2 diabetes mellitus (T2DM) is increasing exponentially worldwide. According to the Centers for Disease Control and Prevention, more than 23 million Americans had diabetes in 2007.1 Globally, the prevalence of diabetes, of which T2DM accounts for 90% to 95% of cases,1 is expected to increase from 171 million in 2000 to 366 million in 2030.2 The National Health and Nutrition Examination Survey (NHANES) showed that about 66% of Americans were overweight or obese between 2003–2004.3 Data from a Swedish National Diabetes Register study showed both overweight and obesity as independent risk factors for cardiovascular disease (CVD) in patients with T2DM.4
This article presents an overview of the evolving concepts of the pathophysiology of T2DM, with a focus on two new therapeutic classes: the glucagon-like peptide–1 (GLP-1) receptor agonists and the dipeptidyl peptidase–4 (DPP-4) inhibitors.
THE PATHOPHYSIOLOGY OF T2DM
The American Association of Clinical Endocrinologists (AACE) describes T2DM as “a progressive, complex metabolic disorder characterized by coexisting defects of multiple organ sites including insulin resistance in muscle and adipose tissue, a progressive decline in pancreatic insulin secretion, unrestrained hepatic glucose production, and other hormonal deficiencies.”5 Other defects include accelerated gastric emptying in patients with T2DM, especially those who are obese or who have the disease for a long duration.6,7
Hormonal deficiencies in T2DM are related to abnormalities in the secretion of the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP).8,9 In addition to the triumvirate of core defects associated with T2DM (involvement of the pancreatic beta cell, muscle, and liver), other mechanisms of disease onset have been advanced, including accelerated lipolysis, hyperglucagonemia, and incretin deficiency/resistance.9 Also, the rate of basal hepatic glucose production is markedly increased in patients with T2DM, which is closely correlated with elevations in fasting plasma glucagon concentration.9
The incretin effect—the intestinal augmentation of secretion of insulin—attributed to GLP-1 and GIP is reduced in patients with T2DM.10 The secretion of GIP may be normal or elevated in patients with T2DM while the secretion of GLP-1 is deficient; however, cellular responsiveness to GLP-1 is preserved while responsiveness to GIP is diminished.11
Both endogenous and exogenous GLP-1 and GIP are degraded in vivo and in vitro by the enzyme DPP-4,12
a ubiquitous, membrane-spanning, cell-surface aminopeptidase that preferentially cleaves peptides with a proline or alanine residue in the second amino-terminal position. DPP-4 is widely expressed (eg, in the liver, lungs, kidney, lymphocytes, epithelial cells, endothelial cells). The role of DPP-4 in the immune system stems from its exopeptidase activity and its interactions with various molecules, including cytokines and chemokines.13
INCRETIN-BASED THERAPIES: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Exenatide is a GLP-1 receptor agonist that is resistant to DPP-4 degradation. Based on preclinical studies, exenatide, which shares a 53% amino acid sequence identity with human GLP-1, is approximately 5,500 times more potent than endogenous GLP-1 in glucose lowering.14,15 Among the acute actions of exenatide is glucose-dependent insulinotropism, the end result of which may be a reduced risk of hypoglycemia.16 This contrasts with insulin secretagogues (eg, sulfonylureas), which increase insulin secretion regardless of glucose concentrations.
Exenatide received US Food and Drug Administration (FDA) approval in 2005 and is indicated for the treatment of patients with T2DM.13,17 Exenatide is administered BID as a subcutaneous (SC) injection in doses of 5 or 10 μg within 1 hour before the two major meals of the day, which should be eaten about 6 hours apart.18
Approved in 2006, sitagliptin was the first DPP-4 inhibitor indicated for adjunctive therapy to lifestyle modifications for the treatment of patients with T2DM.17 The recommended dosage of oral sitagliptin is 100 mg QD. A single-tablet formulation of the combination of sitagliptin and metformin was approved by the FDA in 2007.19 Another DPP-4 inhibitor, saxagliptin, was approved in July 2009 for treatment of patients with T2DM either as monotherapy or in combination with metformin, sulfonylurea, or a thiazolidinedione (TZD).20 The DPP-4 inhibitor vildagliptin is approved in the European Union and Latin America but not in the United States. Vildagliptin is available as a 50- or 100-mg daily dosage; it has been recommended for use at 50 mg QD in combination with a sulfonylurea or at 50 mg BID with either metformin or a TZD.18
GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS IN DEVELOPMENT
Exenatide is currently being evaluated as a once-weekly formulation.21,22 Compared with the BID formulation, exenatide once weekly has been shown to produce significantly greater improvements in glycemic control, with similar reductions in body weight and no increased risk of hypoglycemia.21
Also undergoing regulatory review is the partly DPP-4–resistant acylated GLP-1 receptor agonist liraglutide.13 Liraglutide, a human analogue GLP-1 receptor agonist, has 97% linear amino acid sequence homology to human GLP-1.23,24 Based on its prolonged degradation time and resulting 10- to 14-hour half-life, liraglutide is anticipated to be dosed once daily.13,25,26
Other GLP-1 receptor agonists and DPP-4 inhibitors are in varying stages of development.27 Albiglutide is a long-acting GLP-1 receptor agonist that is generated by the genetic fusion of a DPP-4–resistant GLP-1 to human albumin. Based on pharmacokinetic studies, albiglutide has a half-life of 6 to 8 days. AVE0010, an exendin-4-based GLP-1 receptor agonist, was shown in a 28-day T2DM clinical trial to have an affinity four times greater than native GLP-1 for the human GLP-1 receptor.27 Taspoglutide (R1583), a human analogue GLP-1 receptor agonist, was evaluated in three randomized, placebo-controlled studies as a GLP-1 receptor agonist. Alogliptin, a DPP-4 inhibitor currently in development, has been shown to be safe and effective in studies as monotherapy and in combination with other antidiabetes agents.28–30
CLINICAL TRIALS: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Effects on HbA1c and weight
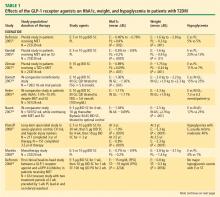
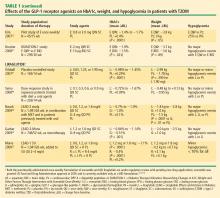
Weight reduction with GLP-1 receptor agonists. In addition to effective glucose lowering, the GLP-1 receptor agonists, particularly exendin-4 agonists, produced beneficial effects on weight (Table 1). Exenatide BID elicited mean weight reductions up to –3.6 kg at 30 weeks21,31,32 and –5.3 kg at 3.5 years.37 Exenatide once weekly resulted in mean weight reductions of up to –3.8 kg at 15 weeks22 and –3.7 kg at 30 weeks.21 Effects on weight with liraglutide varied from a mean reduction of up to –2.99 kg to a slight gain of up to +0.13 kg at 14 weeks40,41 and with weight loss of up to –2.8 kg at 26 weeks23,26 and up to –2.5 kg at 52 weeks.25 In this review, only exenatide has been assessed in insulin-comparator studies, where it was shown to reduce weight compared with the insulin analogues, which led to weight gain.34–36
Hypoglycemia. Patients receiving exenatide experienced lower rates of hypoglycemia (up to 17%) than patients treated with either insulin glargine or insulin aspart (~25%).34,36 The rate of hypoglycemia with exenatide is comparable to that seen with metformin (up to 21%) in a systematic review of oral antidiabetes agents conducted by the Agency for Healthcare Research and Quality.62 No major hypoglycemic events were reported in the liraglutide studies reviewed. The incidence of hypoglycemia reported with DPP-4 inhibitors (Table 2) is also low (2% or less in most studies). The glucose-dependent mechanisms of the incretin-based therapies minimizes the risk of hypoglycemia.
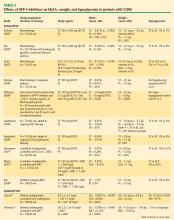
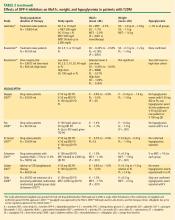
DPP-4 inhibitors and sustained HbA1c reduction. The effects of the DPP-4 inhibitors on HbA1c and weight, either as monotherapy or in combination with other agents, were evaluated in studies ranging in duration from 12 to 52 weeks (Table 2). No studies were identified that compared the glycemic control effects of DPP-4 inhibitors and insulin analogues. Sitagliptin led to a mean reduction in HbA1c from baseline of up to –0.65% at 12 weeks,43,45 up to –0.48% at 18 weeks,44 up to –0.85% at 24 weeks,42,46,47,50 up to –1.0% at 30 weeks,49 and up to –0.67% at 52 weeks.48 Saxagliptin mean reductions in HbA1c ranged from –0.43% to –1.17%.51–54 Data from four 24-week T2DM studies56–60 showed vildagliptin reducing HbA1c up to –1.4% at 24 weeks, with the greatest reduction in a study that involved drug-naïve patients with a relatively short duration of disease (mean, 1.2 years).59 Reductions in HbA1c of –1.0% were sustained in a 52-week study61 and its 52-week extension.58
DPP-4 inhibitors: weight neutral. The DPP-4 inhibitors appear to have a weight-neutral effect (Table 2). The effects of sitagliptin on weight ranged from a loss of –1.5 kg48 at 52 weeks to a gain of +1.8 kg at 24 weeks.50 Weight changes with saxagliptin ranged from a mean reduction of –1.8 kg53 to a gain of +0.7 kg.51 Two vildagliptin studies showed varying effects on weight ranging from a loss of up to –1.8 kg from baseline56 to a gain of up to +1.3 kg57 relative to placebo, both at 24 weeks.
Potential for CV risk reduction
Potentially beneficial effects on CV risk factors, including blood pressure (ie, reduction) and lipid concentrations (ie, differential effects on low-density lipoprotein and high-density lipoprotein cholesterol), were identified in seven GLP-1 receptor studies—three with exenatide (two with exenatide BID,37,38 and one with the investigational exenatide once weekly21) and four with liraglutide.23,25,26,41 For the DPP-4 inhibitors, three studies were identified—two with sitagliptin45,50 and one with vildagliptin61—in which potentially beneficial effects on CV risk factors were demonstrated.The data have been encouraging, although the clinical implications have yet to be fully understood.
Head-to-head comparison
A recent study compared the effects of the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin on postprandial glucose (PPG) concentrations, insulin and glucagon secretion, gastric intake, and caloric intake.39 Although limited by a short treatment duration (2 weeks), the study showed that the GLP-1 receptor agonist had a greater effect than the DPP-4 inhibitor in reducing PPG concentrations, a more potent effect in increasing insulin secretion and decreasing postprandial glucagon secretion, and a relatively greater effect in reducing caloric intake; and that it decreased the rate of gastric emptying (sitagliptin had no effect). These differences suggest that exenatide may provide a greater degree of GLP-1 receptor activation than the more physiologic concentrations of GLP-1 reached with DPP-4 inhibition.39 Results of a scintigraphic study showed that exenatide substantially slows the gastric emptying that is accelerated in patients with T2DM. This could be another beneficial mechanism in treating postprandial glycemia.63
Adverse effects
Exenatide has shown effects on hepatic injury markers (ie, improvement in alanine and aspartate aminotransferases) for up to 3.5 years of treatment.37 For the GLP-1 receptor agonist and DPP-4 inhibitor studies reviewed, the adverse events were generally mild and included nausea and vomiting, nasopharyngitis, and mild hypoglycemia.
Meta-analysis conclusions
The published clinical trial data presented in this review expand the body of evidence on the safety and efficacy of incretin-based therapy in patients with T2DM. These data include the results of a meta-analysis by Amori et al,17 which examined randomized controlled trials of 12 weeks’ or longer duration that compared incretin-based therapy with placebo or other diabetes medications and reported HbA1c changes in adults with T2DM. The meta-analysis showed that incretin-based therapies reduced HbA1c more than placebo (weighted mean difference, –0.97% [95% confidence interval (CI), –1.13% to –0.81%] for GLP-1 receptor agonists and –0.74% [95% CI, –0.85% to –0.62%] for DPP-4 inhibitors) and were noninferior to other antidiabetes agents. Treatment with a GLP-1 receptor agonist (ie, exenatide) caused weight loss (–1.4 kg and –4.8 kg vs placebo and insulin, respectively) while DPP-4 inhibitors (ie, sitagliptin, vildagliptin) were weight neutral.17
Beta-cell function
Evidence regarding the effects of incretin-based therapies, particularly the exendin-4 GLP-1 receptor agonists, on beta-cell function in patients with T2DM continues to accumulate. When assessing long-term (1 year) exenatide treatment in patients with T2DM, a trial (n = 69) comparing exenatide with the basal insulin analogue insulin glargine showed that exenatide and insulin glargine resulted in similar reductions in HbA1c (–0.8% vs –0.7%; P = .55).64 However, exenatide significantly reduced body weight while insulin glargine resulted in weight gain (–3.6 kg vs +1.0 kg; P < .0001). In terms of beta-cell function, arginine-stimulated C-peptide secretion during hyperglycemia increased 2.46-fold from baseline after 52 weeks of exenatide treatment compared with 1.31-fold with insulin glargine treatment (P < .0001).64
With respect to the direct beta-cell effects of liraglutide, a preclinical study reported that liraglutide improved glucose homeostasis in marginal mass islet transplantation in diabetic mice.65 In this study, liraglutide was shown, in a mouse model, to reduce the time to normoglycemia after islet cell transplantation (median time, 1 vs 72.5 days; P < .0001). The effects of liraglutide on beta-cell function also were assessed in 13 patients with T2DM. After 7 days of treatment, liraglutide improved beta-cell function, which was associated with improvement in glucose concentration.66 Liraglutide improved potentiation of insulin secretion during the first meal, owing in part to restoration of the potentiation peak (which is markedly blunted in T2DM), in a phenomenon similar to that observed with exenatide.67
Beneficial effects on beta-cell function have also been reported with DPP-4 inhibitors. In a model-based analysis of patients with T2DM, it was shown that sitagliptin improved basal, static, and dynamic responsiveness of pancreatic beta cells to glucose. The results were observed when sitagliptin was administered both as an add-on to metformin therapy and as monotherapy.68 A 52-week, double-blind, randomized, parallel-group study compared vildagliptin 50 mg/day and placebo in 306 patients with T2DM and mild hyperglycemia (HbA1c, 6.2% to 7.5%). Vildagliptin was shown to significantly increase fasting insulin secretory tone, glucose sensitivity, and rate sensitivity, all of which are aspects of beta-cell function.69
Summary
Based on the ability of incretin-based therapies to address various disease mechanisms, including beta-cell defects (ie, hyperglycemia), hormone-related abnormalities (ie, hyperglucagonemia, incretin deficiency/resistance), and accelerated gastric emptying (especially with GLP-1 receptor agonists); their favorable effects on weight (reduction with GLP-1 receptor agonists and neutral with DPP-4 inhibitors); their beneficial effects on CV risk factors; and their good safety profile (ie, hypoglycemia risk comparable with metformin), these agents could be considered therapeutic advances for the treatment of patients with T2DM.
INCRETIN-BASED THERAPIES IN GUIDELINES AND ALGORITHMS
The 2007 AACE medical guidelines for clinical practice for the management of diabetes recognized the place of the incretin-based therapies and included them among the pharmacologic options.5 Exenatide was specifically recommended for combination therapy with metformin, a sulfonylurea (secretagogue), a sulfonylurea plus metformin, or a TZD. Sitagliptin was recommended for use as monotherapy or in combination with metformin or a TZD.5
In 2009, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes convened a consensus panel to produce an algorithm for the initiation and adjustment of therapy for patients with T2DM. In this algorithm, GLP-1 receptor agonists were considered appropriate in certain clinical scenarios (eg, when hypoglycemia was an issue or weight loss was a major consideration during treatment). However, the groups also noted a need for more data on long-term safety and the cost of treatment with incretin-based therapies.70
The AACE and the American College of Endocrinology recently developed “road maps” for managing patients with T2DM. In patients with T2DM who are naïve to therapy, DPP-4 inhibitors are among the recommended first options when the initial HbA1c is 6.0% to 7.0% and as a combination therapy component when HbA1c reaches 7.0% to 9.0%. In patients who have already received monotherapy for 2 to 3 months and whose HbA1c is 6.5% to 8.5%, treatment options include combination therapy with a DPP-4 inhibitor and metformin or a TZD. Another option includes the initiation of treatment with a GLP-1 receptor agonist in combination with a TZD, with metformin or a sulfonylurea, or with metformin and a sulfonylurea.71
The role of GLP-1 receptor agonist therapies and their incorporation into T2DM treatment algorithms was noted at the 2008 annual meeting of the ADA. In the Banting lecture, Ralph A. DeFronzo, MD, advocated the early use of triple-drug therapy with metformin, exenatide, and a TZD in the management of patients with T2DM.9
CONCLUSION
T2DM, which is linked to weight gain and obesity, is a complex disease that predisposes patients to and is associated with CVD. A better understanding and appreciation of the role of the incretin system in the pathogenesis of T2DM has led to the development of incretin-based therapies, such as the GLP-1 receptor agonists and DPP-4 inhibitors. As more experimental and clinical evidence becomes available, subtle nuances are emerging that distinguish the roles of these two therapeutic classes.
- 2007 National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/diabetes/pubs/estimates07.htm. Updated: July 23, 2008. Accessed September 25, 2009.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Bertin E, Schneider N, Abdelli N, et al. Gastric emptying is accelerated in obese type 2 diabetic patients without autonomic neuropathy. Diabetes Metab 2001; 27:357–364.
- Weytjens C, Keymeulen B, Van Haleweyn C, Somers G, Bossuyt A. Rapid gastric emptying of a liquid meal in long-term type 2 diabetes mellitus. Diabet Med 1998; 15:1022–1027.
- Stonehouse AH, Holcombe JH, Kendall DM. GLP-1 analogues, DPP-IV inhibitors and the metabolic syndrome. In: Fonseca V, ed. Therapeutic Strategies in Metabolic Syndrome. Oxford, UK: Atlas Medical Publishing Ltd; 2008: 137–157.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91:301–307.
- Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 1995; 80:952–957.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696–1705.
- Eng J, Kleinman WA, Singh L, Singh G, Raufman J-P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom: further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992; 267:7402–7405.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:3082–3089.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Davidson JA, Parente EB, Gross JL. Incretin mimetics and dipeptidyl peptidase-4 inhibitors: innovative treatment therapies for type 2 diabetes. Arq Bras Endocrinol Metabol 2008; 52:1039–1049.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Marre M, Shaw J, Brändle M, et al; for the LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabetes Med 2009; 26:268–278.
- Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide 1 with pharmacokinetic properties suitable for once-daily administration. J Med Chem 2000; 43:1664–1669.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Fleck P, Christopher R, Covington P, Wilson C, Mekki Q. Efficacy and safety of alogliptin monotherapy over 12 weeks in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 479-P.
- DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Effect of alogliptin combined with pioglitazone on glycemic control in metformin-treated patients with type 2 diabetes. Paper presented at: 69th Annual Meeting of the American Diabetes Association; June 5–9, 2009; New Orleans, LA. Abstract 2024-PO.
- Nauck M, Ellis G, Fleck P, Wilson C, Mekki Q. Efficacy and safety of alogliptin added to metformin therapy in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 477-P.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008; 30:1448–1460.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 81:161–168.
- Vilsbøll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 2007; 30:1608–1610.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G; for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R; for the CV181-040 Investigators. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract 2009; 63:1395–1406.
- DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 2009; 32:1649–1655.
- Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R; for the CV181-039 Investigators. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 2009; 11:611–622.
- Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R; for the CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin 2009; 25:2401–2411.
- Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naïve patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:376–386.
- Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res 2007; 39:218–223.
- Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007; 9:166–174.
- Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naïve patients with type 2 diabetes: comparison with metformin. Horm Metab Res 2008; 40:892–895.
- Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med 2008; 25:435–441.
- Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract 2007; 76:132–138.
- Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naïve patients with type 2 diabetes. Diabetes Med 2007; 24:955–961.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008; 151:123–129.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Merani S, Truong W, Emamaullee JA, Toso C, Knudsen LB, Shapiro AM. Liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology 2008; 149:4322–4328.
- Mari A, Degn K, Brock B, Rungby J, Ferrannini E, Schmitz O. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care 2007; 30:2032–2033.
- Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A. Mathematical modeling shows exenatide improved beta-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res 2006; 38:838–844.
- Xu L, Man CD, Charbonnel B, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: a model-based approach. Diabetes Obes Metab 2008; 10:1212–1220.
- Mari A, Scherbaum WA, Nilsson PM, et al. Characterization of the influence of vildagliptin on model-assessed b-cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab 2008; 93:103–109.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Jellinger PS, Davidson JA, Blonde L, et al; for the ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus: ACE/AACE Diabetes Road Map Task Force. Endocr Pract 2007; 13:260–268.
The prevalence of type 2 diabetes mellitus (T2DM) is increasing exponentially worldwide. According to the Centers for Disease Control and Prevention, more than 23 million Americans had diabetes in 2007.1 Globally, the prevalence of diabetes, of which T2DM accounts for 90% to 95% of cases,1 is expected to increase from 171 million in 2000 to 366 million in 2030.2 The National Health and Nutrition Examination Survey (NHANES) showed that about 66% of Americans were overweight or obese between 2003–2004.3 Data from a Swedish National Diabetes Register study showed both overweight and obesity as independent risk factors for cardiovascular disease (CVD) in patients with T2DM.4
This article presents an overview of the evolving concepts of the pathophysiology of T2DM, with a focus on two new therapeutic classes: the glucagon-like peptide–1 (GLP-1) receptor agonists and the dipeptidyl peptidase–4 (DPP-4) inhibitors.
THE PATHOPHYSIOLOGY OF T2DM
The American Association of Clinical Endocrinologists (AACE) describes T2DM as “a progressive, complex metabolic disorder characterized by coexisting defects of multiple organ sites including insulin resistance in muscle and adipose tissue, a progressive decline in pancreatic insulin secretion, unrestrained hepatic glucose production, and other hormonal deficiencies.”5 Other defects include accelerated gastric emptying in patients with T2DM, especially those who are obese or who have the disease for a long duration.6,7
Hormonal deficiencies in T2DM are related to abnormalities in the secretion of the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP).8,9 In addition to the triumvirate of core defects associated with T2DM (involvement of the pancreatic beta cell, muscle, and liver), other mechanisms of disease onset have been advanced, including accelerated lipolysis, hyperglucagonemia, and incretin deficiency/resistance.9 Also, the rate of basal hepatic glucose production is markedly increased in patients with T2DM, which is closely correlated with elevations in fasting plasma glucagon concentration.9
The incretin effect—the intestinal augmentation of secretion of insulin—attributed to GLP-1 and GIP is reduced in patients with T2DM.10 The secretion of GIP may be normal or elevated in patients with T2DM while the secretion of GLP-1 is deficient; however, cellular responsiveness to GLP-1 is preserved while responsiveness to GIP is diminished.11
Both endogenous and exogenous GLP-1 and GIP are degraded in vivo and in vitro by the enzyme DPP-4,12
a ubiquitous, membrane-spanning, cell-surface aminopeptidase that preferentially cleaves peptides with a proline or alanine residue in the second amino-terminal position. DPP-4 is widely expressed (eg, in the liver, lungs, kidney, lymphocytes, epithelial cells, endothelial cells). The role of DPP-4 in the immune system stems from its exopeptidase activity and its interactions with various molecules, including cytokines and chemokines.13
INCRETIN-BASED THERAPIES: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Exenatide is a GLP-1 receptor agonist that is resistant to DPP-4 degradation. Based on preclinical studies, exenatide, which shares a 53% amino acid sequence identity with human GLP-1, is approximately 5,500 times more potent than endogenous GLP-1 in glucose lowering.14,15 Among the acute actions of exenatide is glucose-dependent insulinotropism, the end result of which may be a reduced risk of hypoglycemia.16 This contrasts with insulin secretagogues (eg, sulfonylureas), which increase insulin secretion regardless of glucose concentrations.
Exenatide received US Food and Drug Administration (FDA) approval in 2005 and is indicated for the treatment of patients with T2DM.13,17 Exenatide is administered BID as a subcutaneous (SC) injection in doses of 5 or 10 μg within 1 hour before the two major meals of the day, which should be eaten about 6 hours apart.18
Approved in 2006, sitagliptin was the first DPP-4 inhibitor indicated for adjunctive therapy to lifestyle modifications for the treatment of patients with T2DM.17 The recommended dosage of oral sitagliptin is 100 mg QD. A single-tablet formulation of the combination of sitagliptin and metformin was approved by the FDA in 2007.19 Another DPP-4 inhibitor, saxagliptin, was approved in July 2009 for treatment of patients with T2DM either as monotherapy or in combination with metformin, sulfonylurea, or a thiazolidinedione (TZD).20 The DPP-4 inhibitor vildagliptin is approved in the European Union and Latin America but not in the United States. Vildagliptin is available as a 50- or 100-mg daily dosage; it has been recommended for use at 50 mg QD in combination with a sulfonylurea or at 50 mg BID with either metformin or a TZD.18
GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS IN DEVELOPMENT
Exenatide is currently being evaluated as a once-weekly formulation.21,22 Compared with the BID formulation, exenatide once weekly has been shown to produce significantly greater improvements in glycemic control, with similar reductions in body weight and no increased risk of hypoglycemia.21
Also undergoing regulatory review is the partly DPP-4–resistant acylated GLP-1 receptor agonist liraglutide.13 Liraglutide, a human analogue GLP-1 receptor agonist, has 97% linear amino acid sequence homology to human GLP-1.23,24 Based on its prolonged degradation time and resulting 10- to 14-hour half-life, liraglutide is anticipated to be dosed once daily.13,25,26
Other GLP-1 receptor agonists and DPP-4 inhibitors are in varying stages of development.27 Albiglutide is a long-acting GLP-1 receptor agonist that is generated by the genetic fusion of a DPP-4–resistant GLP-1 to human albumin. Based on pharmacokinetic studies, albiglutide has a half-life of 6 to 8 days. AVE0010, an exendin-4-based GLP-1 receptor agonist, was shown in a 28-day T2DM clinical trial to have an affinity four times greater than native GLP-1 for the human GLP-1 receptor.27 Taspoglutide (R1583), a human analogue GLP-1 receptor agonist, was evaluated in three randomized, placebo-controlled studies as a GLP-1 receptor agonist. Alogliptin, a DPP-4 inhibitor currently in development, has been shown to be safe and effective in studies as monotherapy and in combination with other antidiabetes agents.28–30
CLINICAL TRIALS: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Effects on HbA1c and weight
Weight reduction with GLP-1 receptor agonists. In addition to effective glucose lowering, the GLP-1 receptor agonists, particularly exendin-4 agonists, produced beneficial effects on weight (Table 1). Exenatide BID elicited mean weight reductions up to –3.6 kg at 30 weeks21,31,32 and –5.3 kg at 3.5 years.37 Exenatide once weekly resulted in mean weight reductions of up to –3.8 kg at 15 weeks22 and –3.7 kg at 30 weeks.21 Effects on weight with liraglutide varied from a mean reduction of up to –2.99 kg to a slight gain of up to +0.13 kg at 14 weeks40,41 and with weight loss of up to –2.8 kg at 26 weeks23,26 and up to –2.5 kg at 52 weeks.25 In this review, only exenatide has been assessed in insulin-comparator studies, where it was shown to reduce weight compared with the insulin analogues, which led to weight gain.34–36
Hypoglycemia. Patients receiving exenatide experienced lower rates of hypoglycemia (up to 17%) than patients treated with either insulin glargine or insulin aspart (~25%).34,36 The rate of hypoglycemia with exenatide is comparable to that seen with metformin (up to 21%) in a systematic review of oral antidiabetes agents conducted by the Agency for Healthcare Research and Quality.62 No major hypoglycemic events were reported in the liraglutide studies reviewed. The incidence of hypoglycemia reported with DPP-4 inhibitors (Table 2) is also low (2% or less in most studies). The glucose-dependent mechanisms of the incretin-based therapies minimizes the risk of hypoglycemia.
DPP-4 inhibitors and sustained HbA1c reduction. The effects of the DPP-4 inhibitors on HbA1c and weight, either as monotherapy or in combination with other agents, were evaluated in studies ranging in duration from 12 to 52 weeks (Table 2). No studies were identified that compared the glycemic control effects of DPP-4 inhibitors and insulin analogues. Sitagliptin led to a mean reduction in HbA1c from baseline of up to –0.65% at 12 weeks,43,45 up to –0.48% at 18 weeks,44 up to –0.85% at 24 weeks,42,46,47,50 up to –1.0% at 30 weeks,49 and up to –0.67% at 52 weeks.48 Saxagliptin mean reductions in HbA1c ranged from –0.43% to –1.17%.51–54 Data from four 24-week T2DM studies56–60 showed vildagliptin reducing HbA1c up to –1.4% at 24 weeks, with the greatest reduction in a study that involved drug-naïve patients with a relatively short duration of disease (mean, 1.2 years).59 Reductions in HbA1c of –1.0% were sustained in a 52-week study61 and its 52-week extension.58
DPP-4 inhibitors: weight neutral. The DPP-4 inhibitors appear to have a weight-neutral effect (Table 2). The effects of sitagliptin on weight ranged from a loss of –1.5 kg48 at 52 weeks to a gain of +1.8 kg at 24 weeks.50 Weight changes with saxagliptin ranged from a mean reduction of –1.8 kg53 to a gain of +0.7 kg.51 Two vildagliptin studies showed varying effects on weight ranging from a loss of up to –1.8 kg from baseline56 to a gain of up to +1.3 kg57 relative to placebo, both at 24 weeks.
Potential for CV risk reduction
Potentially beneficial effects on CV risk factors, including blood pressure (ie, reduction) and lipid concentrations (ie, differential effects on low-density lipoprotein and high-density lipoprotein cholesterol), were identified in seven GLP-1 receptor studies—three with exenatide (two with exenatide BID,37,38 and one with the investigational exenatide once weekly21) and four with liraglutide.23,25,26,41 For the DPP-4 inhibitors, three studies were identified—two with sitagliptin45,50 and one with vildagliptin61—in which potentially beneficial effects on CV risk factors were demonstrated.The data have been encouraging, although the clinical implications have yet to be fully understood.
Head-to-head comparison
A recent study compared the effects of the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin on postprandial glucose (PPG) concentrations, insulin and glucagon secretion, gastric intake, and caloric intake.39 Although limited by a short treatment duration (2 weeks), the study showed that the GLP-1 receptor agonist had a greater effect than the DPP-4 inhibitor in reducing PPG concentrations, a more potent effect in increasing insulin secretion and decreasing postprandial glucagon secretion, and a relatively greater effect in reducing caloric intake; and that it decreased the rate of gastric emptying (sitagliptin had no effect). These differences suggest that exenatide may provide a greater degree of GLP-1 receptor activation than the more physiologic concentrations of GLP-1 reached with DPP-4 inhibition.39 Results of a scintigraphic study showed that exenatide substantially slows the gastric emptying that is accelerated in patients with T2DM. This could be another beneficial mechanism in treating postprandial glycemia.63
Adverse effects
Exenatide has shown effects on hepatic injury markers (ie, improvement in alanine and aspartate aminotransferases) for up to 3.5 years of treatment.37 For the GLP-1 receptor agonist and DPP-4 inhibitor studies reviewed, the adverse events were generally mild and included nausea and vomiting, nasopharyngitis, and mild hypoglycemia.
Meta-analysis conclusions
The published clinical trial data presented in this review expand the body of evidence on the safety and efficacy of incretin-based therapy in patients with T2DM. These data include the results of a meta-analysis by Amori et al,17 which examined randomized controlled trials of 12 weeks’ or longer duration that compared incretin-based therapy with placebo or other diabetes medications and reported HbA1c changes in adults with T2DM. The meta-analysis showed that incretin-based therapies reduced HbA1c more than placebo (weighted mean difference, –0.97% [95% confidence interval (CI), –1.13% to –0.81%] for GLP-1 receptor agonists and –0.74% [95% CI, –0.85% to –0.62%] for DPP-4 inhibitors) and were noninferior to other antidiabetes agents. Treatment with a GLP-1 receptor agonist (ie, exenatide) caused weight loss (–1.4 kg and –4.8 kg vs placebo and insulin, respectively) while DPP-4 inhibitors (ie, sitagliptin, vildagliptin) were weight neutral.17
Beta-cell function
Evidence regarding the effects of incretin-based therapies, particularly the exendin-4 GLP-1 receptor agonists, on beta-cell function in patients with T2DM continues to accumulate. When assessing long-term (1 year) exenatide treatment in patients with T2DM, a trial (n = 69) comparing exenatide with the basal insulin analogue insulin glargine showed that exenatide and insulin glargine resulted in similar reductions in HbA1c (–0.8% vs –0.7%; P = .55).64 However, exenatide significantly reduced body weight while insulin glargine resulted in weight gain (–3.6 kg vs +1.0 kg; P < .0001). In terms of beta-cell function, arginine-stimulated C-peptide secretion during hyperglycemia increased 2.46-fold from baseline after 52 weeks of exenatide treatment compared with 1.31-fold with insulin glargine treatment (P < .0001).64
With respect to the direct beta-cell effects of liraglutide, a preclinical study reported that liraglutide improved glucose homeostasis in marginal mass islet transplantation in diabetic mice.65 In this study, liraglutide was shown, in a mouse model, to reduce the time to normoglycemia after islet cell transplantation (median time, 1 vs 72.5 days; P < .0001). The effects of liraglutide on beta-cell function also were assessed in 13 patients with T2DM. After 7 days of treatment, liraglutide improved beta-cell function, which was associated with improvement in glucose concentration.66 Liraglutide improved potentiation of insulin secretion during the first meal, owing in part to restoration of the potentiation peak (which is markedly blunted in T2DM), in a phenomenon similar to that observed with exenatide.67
Beneficial effects on beta-cell function have also been reported with DPP-4 inhibitors. In a model-based analysis of patients with T2DM, it was shown that sitagliptin improved basal, static, and dynamic responsiveness of pancreatic beta cells to glucose. The results were observed when sitagliptin was administered both as an add-on to metformin therapy and as monotherapy.68 A 52-week, double-blind, randomized, parallel-group study compared vildagliptin 50 mg/day and placebo in 306 patients with T2DM and mild hyperglycemia (HbA1c, 6.2% to 7.5%). Vildagliptin was shown to significantly increase fasting insulin secretory tone, glucose sensitivity, and rate sensitivity, all of which are aspects of beta-cell function.69
Summary
Based on the ability of incretin-based therapies to address various disease mechanisms, including beta-cell defects (ie, hyperglycemia), hormone-related abnormalities (ie, hyperglucagonemia, incretin deficiency/resistance), and accelerated gastric emptying (especially with GLP-1 receptor agonists); their favorable effects on weight (reduction with GLP-1 receptor agonists and neutral with DPP-4 inhibitors); their beneficial effects on CV risk factors; and their good safety profile (ie, hypoglycemia risk comparable with metformin), these agents could be considered therapeutic advances for the treatment of patients with T2DM.
INCRETIN-BASED THERAPIES IN GUIDELINES AND ALGORITHMS
The 2007 AACE medical guidelines for clinical practice for the management of diabetes recognized the place of the incretin-based therapies and included them among the pharmacologic options.5 Exenatide was specifically recommended for combination therapy with metformin, a sulfonylurea (secretagogue), a sulfonylurea plus metformin, or a TZD. Sitagliptin was recommended for use as monotherapy or in combination with metformin or a TZD.5
In 2009, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes convened a consensus panel to produce an algorithm for the initiation and adjustment of therapy for patients with T2DM. In this algorithm, GLP-1 receptor agonists were considered appropriate in certain clinical scenarios (eg, when hypoglycemia was an issue or weight loss was a major consideration during treatment). However, the groups also noted a need for more data on long-term safety and the cost of treatment with incretin-based therapies.70
The AACE and the American College of Endocrinology recently developed “road maps” for managing patients with T2DM. In patients with T2DM who are naïve to therapy, DPP-4 inhibitors are among the recommended first options when the initial HbA1c is 6.0% to 7.0% and as a combination therapy component when HbA1c reaches 7.0% to 9.0%. In patients who have already received monotherapy for 2 to 3 months and whose HbA1c is 6.5% to 8.5%, treatment options include combination therapy with a DPP-4 inhibitor and metformin or a TZD. Another option includes the initiation of treatment with a GLP-1 receptor agonist in combination with a TZD, with metformin or a sulfonylurea, or with metformin and a sulfonylurea.71
The role of GLP-1 receptor agonist therapies and their incorporation into T2DM treatment algorithms was noted at the 2008 annual meeting of the ADA. In the Banting lecture, Ralph A. DeFronzo, MD, advocated the early use of triple-drug therapy with metformin, exenatide, and a TZD in the management of patients with T2DM.9
CONCLUSION
T2DM, which is linked to weight gain and obesity, is a complex disease that predisposes patients to and is associated with CVD. A better understanding and appreciation of the role of the incretin system in the pathogenesis of T2DM has led to the development of incretin-based therapies, such as the GLP-1 receptor agonists and DPP-4 inhibitors. As more experimental and clinical evidence becomes available, subtle nuances are emerging that distinguish the roles of these two therapeutic classes.
The prevalence of type 2 diabetes mellitus (T2DM) is increasing exponentially worldwide. According to the Centers for Disease Control and Prevention, more than 23 million Americans had diabetes in 2007.1 Globally, the prevalence of diabetes, of which T2DM accounts for 90% to 95% of cases,1 is expected to increase from 171 million in 2000 to 366 million in 2030.2 The National Health and Nutrition Examination Survey (NHANES) showed that about 66% of Americans were overweight or obese between 2003–2004.3 Data from a Swedish National Diabetes Register study showed both overweight and obesity as independent risk factors for cardiovascular disease (CVD) in patients with T2DM.4
This article presents an overview of the evolving concepts of the pathophysiology of T2DM, with a focus on two new therapeutic classes: the glucagon-like peptide–1 (GLP-1) receptor agonists and the dipeptidyl peptidase–4 (DPP-4) inhibitors.
THE PATHOPHYSIOLOGY OF T2DM
The American Association of Clinical Endocrinologists (AACE) describes T2DM as “a progressive, complex metabolic disorder characterized by coexisting defects of multiple organ sites including insulin resistance in muscle and adipose tissue, a progressive decline in pancreatic insulin secretion, unrestrained hepatic glucose production, and other hormonal deficiencies.”5 Other defects include accelerated gastric emptying in patients with T2DM, especially those who are obese or who have the disease for a long duration.6,7
Hormonal deficiencies in T2DM are related to abnormalities in the secretion of the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP).8,9 In addition to the triumvirate of core defects associated with T2DM (involvement of the pancreatic beta cell, muscle, and liver), other mechanisms of disease onset have been advanced, including accelerated lipolysis, hyperglucagonemia, and incretin deficiency/resistance.9 Also, the rate of basal hepatic glucose production is markedly increased in patients with T2DM, which is closely correlated with elevations in fasting plasma glucagon concentration.9
The incretin effect—the intestinal augmentation of secretion of insulin—attributed to GLP-1 and GIP is reduced in patients with T2DM.10 The secretion of GIP may be normal or elevated in patients with T2DM while the secretion of GLP-1 is deficient; however, cellular responsiveness to GLP-1 is preserved while responsiveness to GIP is diminished.11
Both endogenous and exogenous GLP-1 and GIP are degraded in vivo and in vitro by the enzyme DPP-4,12
a ubiquitous, membrane-spanning, cell-surface aminopeptidase that preferentially cleaves peptides with a proline or alanine residue in the second amino-terminal position. DPP-4 is widely expressed (eg, in the liver, lungs, kidney, lymphocytes, epithelial cells, endothelial cells). The role of DPP-4 in the immune system stems from its exopeptidase activity and its interactions with various molecules, including cytokines and chemokines.13
INCRETIN-BASED THERAPIES: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Exenatide is a GLP-1 receptor agonist that is resistant to DPP-4 degradation. Based on preclinical studies, exenatide, which shares a 53% amino acid sequence identity with human GLP-1, is approximately 5,500 times more potent than endogenous GLP-1 in glucose lowering.14,15 Among the acute actions of exenatide is glucose-dependent insulinotropism, the end result of which may be a reduced risk of hypoglycemia.16 This contrasts with insulin secretagogues (eg, sulfonylureas), which increase insulin secretion regardless of glucose concentrations.
Exenatide received US Food and Drug Administration (FDA) approval in 2005 and is indicated for the treatment of patients with T2DM.13,17 Exenatide is administered BID as a subcutaneous (SC) injection in doses of 5 or 10 μg within 1 hour before the two major meals of the day, which should be eaten about 6 hours apart.18
Approved in 2006, sitagliptin was the first DPP-4 inhibitor indicated for adjunctive therapy to lifestyle modifications for the treatment of patients with T2DM.17 The recommended dosage of oral sitagliptin is 100 mg QD. A single-tablet formulation of the combination of sitagliptin and metformin was approved by the FDA in 2007.19 Another DPP-4 inhibitor, saxagliptin, was approved in July 2009 for treatment of patients with T2DM either as monotherapy or in combination with metformin, sulfonylurea, or a thiazolidinedione (TZD).20 The DPP-4 inhibitor vildagliptin is approved in the European Union and Latin America but not in the United States. Vildagliptin is available as a 50- or 100-mg daily dosage; it has been recommended for use at 50 mg QD in combination with a sulfonylurea or at 50 mg BID with either metformin or a TZD.18
GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS IN DEVELOPMENT
Exenatide is currently being evaluated as a once-weekly formulation.21,22 Compared with the BID formulation, exenatide once weekly has been shown to produce significantly greater improvements in glycemic control, with similar reductions in body weight and no increased risk of hypoglycemia.21
Also undergoing regulatory review is the partly DPP-4–resistant acylated GLP-1 receptor agonist liraglutide.13 Liraglutide, a human analogue GLP-1 receptor agonist, has 97% linear amino acid sequence homology to human GLP-1.23,24 Based on its prolonged degradation time and resulting 10- to 14-hour half-life, liraglutide is anticipated to be dosed once daily.13,25,26
Other GLP-1 receptor agonists and DPP-4 inhibitors are in varying stages of development.27 Albiglutide is a long-acting GLP-1 receptor agonist that is generated by the genetic fusion of a DPP-4–resistant GLP-1 to human albumin. Based on pharmacokinetic studies, albiglutide has a half-life of 6 to 8 days. AVE0010, an exendin-4-based GLP-1 receptor agonist, was shown in a 28-day T2DM clinical trial to have an affinity four times greater than native GLP-1 for the human GLP-1 receptor.27 Taspoglutide (R1583), a human analogue GLP-1 receptor agonist, was evaluated in three randomized, placebo-controlled studies as a GLP-1 receptor agonist. Alogliptin, a DPP-4 inhibitor currently in development, has been shown to be safe and effective in studies as monotherapy and in combination with other antidiabetes agents.28–30
CLINICAL TRIALS: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Effects on HbA1c and weight
Weight reduction with GLP-1 receptor agonists. In addition to effective glucose lowering, the GLP-1 receptor agonists, particularly exendin-4 agonists, produced beneficial effects on weight (Table 1). Exenatide BID elicited mean weight reductions up to –3.6 kg at 30 weeks21,31,32 and –5.3 kg at 3.5 years.37 Exenatide once weekly resulted in mean weight reductions of up to –3.8 kg at 15 weeks22 and –3.7 kg at 30 weeks.21 Effects on weight with liraglutide varied from a mean reduction of up to –2.99 kg to a slight gain of up to +0.13 kg at 14 weeks40,41 and with weight loss of up to –2.8 kg at 26 weeks23,26 and up to –2.5 kg at 52 weeks.25 In this review, only exenatide has been assessed in insulin-comparator studies, where it was shown to reduce weight compared with the insulin analogues, which led to weight gain.34–36
Hypoglycemia. Patients receiving exenatide experienced lower rates of hypoglycemia (up to 17%) than patients treated with either insulin glargine or insulin aspart (~25%).34,36 The rate of hypoglycemia with exenatide is comparable to that seen with metformin (up to 21%) in a systematic review of oral antidiabetes agents conducted by the Agency for Healthcare Research and Quality.62 No major hypoglycemic events were reported in the liraglutide studies reviewed. The incidence of hypoglycemia reported with DPP-4 inhibitors (Table 2) is also low (2% or less in most studies). The glucose-dependent mechanisms of the incretin-based therapies minimizes the risk of hypoglycemia.
DPP-4 inhibitors and sustained HbA1c reduction. The effects of the DPP-4 inhibitors on HbA1c and weight, either as monotherapy or in combination with other agents, were evaluated in studies ranging in duration from 12 to 52 weeks (Table 2). No studies were identified that compared the glycemic control effects of DPP-4 inhibitors and insulin analogues. Sitagliptin led to a mean reduction in HbA1c from baseline of up to –0.65% at 12 weeks,43,45 up to –0.48% at 18 weeks,44 up to –0.85% at 24 weeks,42,46,47,50 up to –1.0% at 30 weeks,49 and up to –0.67% at 52 weeks.48 Saxagliptin mean reductions in HbA1c ranged from –0.43% to –1.17%.51–54 Data from four 24-week T2DM studies56–60 showed vildagliptin reducing HbA1c up to –1.4% at 24 weeks, with the greatest reduction in a study that involved drug-naïve patients with a relatively short duration of disease (mean, 1.2 years).59 Reductions in HbA1c of –1.0% were sustained in a 52-week study61 and its 52-week extension.58
DPP-4 inhibitors: weight neutral. The DPP-4 inhibitors appear to have a weight-neutral effect (Table 2). The effects of sitagliptin on weight ranged from a loss of –1.5 kg48 at 52 weeks to a gain of +1.8 kg at 24 weeks.50 Weight changes with saxagliptin ranged from a mean reduction of –1.8 kg53 to a gain of +0.7 kg.51 Two vildagliptin studies showed varying effects on weight ranging from a loss of up to –1.8 kg from baseline56 to a gain of up to +1.3 kg57 relative to placebo, both at 24 weeks.
Potential for CV risk reduction
Potentially beneficial effects on CV risk factors, including blood pressure (ie, reduction) and lipid concentrations (ie, differential effects on low-density lipoprotein and high-density lipoprotein cholesterol), were identified in seven GLP-1 receptor studies—three with exenatide (two with exenatide BID,37,38 and one with the investigational exenatide once weekly21) and four with liraglutide.23,25,26,41 For the DPP-4 inhibitors, three studies were identified—two with sitagliptin45,50 and one with vildagliptin61—in which potentially beneficial effects on CV risk factors were demonstrated.The data have been encouraging, although the clinical implications have yet to be fully understood.
Head-to-head comparison
A recent study compared the effects of the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin on postprandial glucose (PPG) concentrations, insulin and glucagon secretion, gastric intake, and caloric intake.39 Although limited by a short treatment duration (2 weeks), the study showed that the GLP-1 receptor agonist had a greater effect than the DPP-4 inhibitor in reducing PPG concentrations, a more potent effect in increasing insulin secretion and decreasing postprandial glucagon secretion, and a relatively greater effect in reducing caloric intake; and that it decreased the rate of gastric emptying (sitagliptin had no effect). These differences suggest that exenatide may provide a greater degree of GLP-1 receptor activation than the more physiologic concentrations of GLP-1 reached with DPP-4 inhibition.39 Results of a scintigraphic study showed that exenatide substantially slows the gastric emptying that is accelerated in patients with T2DM. This could be another beneficial mechanism in treating postprandial glycemia.63
Adverse effects
Exenatide has shown effects on hepatic injury markers (ie, improvement in alanine and aspartate aminotransferases) for up to 3.5 years of treatment.37 For the GLP-1 receptor agonist and DPP-4 inhibitor studies reviewed, the adverse events were generally mild and included nausea and vomiting, nasopharyngitis, and mild hypoglycemia.
Meta-analysis conclusions
The published clinical trial data presented in this review expand the body of evidence on the safety and efficacy of incretin-based therapy in patients with T2DM. These data include the results of a meta-analysis by Amori et al,17 which examined randomized controlled trials of 12 weeks’ or longer duration that compared incretin-based therapy with placebo or other diabetes medications and reported HbA1c changes in adults with T2DM. The meta-analysis showed that incretin-based therapies reduced HbA1c more than placebo (weighted mean difference, –0.97% [95% confidence interval (CI), –1.13% to –0.81%] for GLP-1 receptor agonists and –0.74% [95% CI, –0.85% to –0.62%] for DPP-4 inhibitors) and were noninferior to other antidiabetes agents. Treatment with a GLP-1 receptor agonist (ie, exenatide) caused weight loss (–1.4 kg and –4.8 kg vs placebo and insulin, respectively) while DPP-4 inhibitors (ie, sitagliptin, vildagliptin) were weight neutral.17
Beta-cell function
Evidence regarding the effects of incretin-based therapies, particularly the exendin-4 GLP-1 receptor agonists, on beta-cell function in patients with T2DM continues to accumulate. When assessing long-term (1 year) exenatide treatment in patients with T2DM, a trial (n = 69) comparing exenatide with the basal insulin analogue insulin glargine showed that exenatide and insulin glargine resulted in similar reductions in HbA1c (–0.8% vs –0.7%; P = .55).64 However, exenatide significantly reduced body weight while insulin glargine resulted in weight gain (–3.6 kg vs +1.0 kg; P < .0001). In terms of beta-cell function, arginine-stimulated C-peptide secretion during hyperglycemia increased 2.46-fold from baseline after 52 weeks of exenatide treatment compared with 1.31-fold with insulin glargine treatment (P < .0001).64
With respect to the direct beta-cell effects of liraglutide, a preclinical study reported that liraglutide improved glucose homeostasis in marginal mass islet transplantation in diabetic mice.65 In this study, liraglutide was shown, in a mouse model, to reduce the time to normoglycemia after islet cell transplantation (median time, 1 vs 72.5 days; P < .0001). The effects of liraglutide on beta-cell function also were assessed in 13 patients with T2DM. After 7 days of treatment, liraglutide improved beta-cell function, which was associated with improvement in glucose concentration.66 Liraglutide improved potentiation of insulin secretion during the first meal, owing in part to restoration of the potentiation peak (which is markedly blunted in T2DM), in a phenomenon similar to that observed with exenatide.67
Beneficial effects on beta-cell function have also been reported with DPP-4 inhibitors. In a model-based analysis of patients with T2DM, it was shown that sitagliptin improved basal, static, and dynamic responsiveness of pancreatic beta cells to glucose. The results were observed when sitagliptin was administered both as an add-on to metformin therapy and as monotherapy.68 A 52-week, double-blind, randomized, parallel-group study compared vildagliptin 50 mg/day and placebo in 306 patients with T2DM and mild hyperglycemia (HbA1c, 6.2% to 7.5%). Vildagliptin was shown to significantly increase fasting insulin secretory tone, glucose sensitivity, and rate sensitivity, all of which are aspects of beta-cell function.69
Summary
Based on the ability of incretin-based therapies to address various disease mechanisms, including beta-cell defects (ie, hyperglycemia), hormone-related abnormalities (ie, hyperglucagonemia, incretin deficiency/resistance), and accelerated gastric emptying (especially with GLP-1 receptor agonists); their favorable effects on weight (reduction with GLP-1 receptor agonists and neutral with DPP-4 inhibitors); their beneficial effects on CV risk factors; and their good safety profile (ie, hypoglycemia risk comparable with metformin), these agents could be considered therapeutic advances for the treatment of patients with T2DM.
INCRETIN-BASED THERAPIES IN GUIDELINES AND ALGORITHMS
The 2007 AACE medical guidelines for clinical practice for the management of diabetes recognized the place of the incretin-based therapies and included them among the pharmacologic options.5 Exenatide was specifically recommended for combination therapy with metformin, a sulfonylurea (secretagogue), a sulfonylurea plus metformin, or a TZD. Sitagliptin was recommended for use as monotherapy or in combination with metformin or a TZD.5
In 2009, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes convened a consensus panel to produce an algorithm for the initiation and adjustment of therapy for patients with T2DM. In this algorithm, GLP-1 receptor agonists were considered appropriate in certain clinical scenarios (eg, when hypoglycemia was an issue or weight loss was a major consideration during treatment). However, the groups also noted a need for more data on long-term safety and the cost of treatment with incretin-based therapies.70
The AACE and the American College of Endocrinology recently developed “road maps” for managing patients with T2DM. In patients with T2DM who are naïve to therapy, DPP-4 inhibitors are among the recommended first options when the initial HbA1c is 6.0% to 7.0% and as a combination therapy component when HbA1c reaches 7.0% to 9.0%. In patients who have already received monotherapy for 2 to 3 months and whose HbA1c is 6.5% to 8.5%, treatment options include combination therapy with a DPP-4 inhibitor and metformin or a TZD. Another option includes the initiation of treatment with a GLP-1 receptor agonist in combination with a TZD, with metformin or a sulfonylurea, or with metformin and a sulfonylurea.71
The role of GLP-1 receptor agonist therapies and their incorporation into T2DM treatment algorithms was noted at the 2008 annual meeting of the ADA. In the Banting lecture, Ralph A. DeFronzo, MD, advocated the early use of triple-drug therapy with metformin, exenatide, and a TZD in the management of patients with T2DM.9
CONCLUSION
T2DM, which is linked to weight gain and obesity, is a complex disease that predisposes patients to and is associated with CVD. A better understanding and appreciation of the role of the incretin system in the pathogenesis of T2DM has led to the development of incretin-based therapies, such as the GLP-1 receptor agonists and DPP-4 inhibitors. As more experimental and clinical evidence becomes available, subtle nuances are emerging that distinguish the roles of these two therapeutic classes.
- 2007 National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/diabetes/pubs/estimates07.htm. Updated: July 23, 2008. Accessed September 25, 2009.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Bertin E, Schneider N, Abdelli N, et al. Gastric emptying is accelerated in obese type 2 diabetic patients without autonomic neuropathy. Diabetes Metab 2001; 27:357–364.
- Weytjens C, Keymeulen B, Van Haleweyn C, Somers G, Bossuyt A. Rapid gastric emptying of a liquid meal in long-term type 2 diabetes mellitus. Diabet Med 1998; 15:1022–1027.
- Stonehouse AH, Holcombe JH, Kendall DM. GLP-1 analogues, DPP-IV inhibitors and the metabolic syndrome. In: Fonseca V, ed. Therapeutic Strategies in Metabolic Syndrome. Oxford, UK: Atlas Medical Publishing Ltd; 2008: 137–157.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91:301–307.
- Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 1995; 80:952–957.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696–1705.
- Eng J, Kleinman WA, Singh L, Singh G, Raufman J-P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom: further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992; 267:7402–7405.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:3082–3089.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Davidson JA, Parente EB, Gross JL. Incretin mimetics and dipeptidyl peptidase-4 inhibitors: innovative treatment therapies for type 2 diabetes. Arq Bras Endocrinol Metabol 2008; 52:1039–1049.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Marre M, Shaw J, Brändle M, et al; for the LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabetes Med 2009; 26:268–278.
- Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide 1 with pharmacokinetic properties suitable for once-daily administration. J Med Chem 2000; 43:1664–1669.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Fleck P, Christopher R, Covington P, Wilson C, Mekki Q. Efficacy and safety of alogliptin monotherapy over 12 weeks in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 479-P.
- DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Effect of alogliptin combined with pioglitazone on glycemic control in metformin-treated patients with type 2 diabetes. Paper presented at: 69th Annual Meeting of the American Diabetes Association; June 5–9, 2009; New Orleans, LA. Abstract 2024-PO.
- Nauck M, Ellis G, Fleck P, Wilson C, Mekki Q. Efficacy and safety of alogliptin added to metformin therapy in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 477-P.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008; 30:1448–1460.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 81:161–168.
- Vilsbøll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 2007; 30:1608–1610.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G; for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R; for the CV181-040 Investigators. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract 2009; 63:1395–1406.
- DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 2009; 32:1649–1655.
- Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R; for the CV181-039 Investigators. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 2009; 11:611–622.
- Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R; for the CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin 2009; 25:2401–2411.
- Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naïve patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:376–386.
- Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res 2007; 39:218–223.
- Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007; 9:166–174.
- Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naïve patients with type 2 diabetes: comparison with metformin. Horm Metab Res 2008; 40:892–895.
- Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med 2008; 25:435–441.
- Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract 2007; 76:132–138.
- Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naïve patients with type 2 diabetes. Diabetes Med 2007; 24:955–961.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008; 151:123–129.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Merani S, Truong W, Emamaullee JA, Toso C, Knudsen LB, Shapiro AM. Liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology 2008; 149:4322–4328.
- Mari A, Degn K, Brock B, Rungby J, Ferrannini E, Schmitz O. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care 2007; 30:2032–2033.
- Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A. Mathematical modeling shows exenatide improved beta-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res 2006; 38:838–844.
- Xu L, Man CD, Charbonnel B, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: a model-based approach. Diabetes Obes Metab 2008; 10:1212–1220.
- Mari A, Scherbaum WA, Nilsson PM, et al. Characterization of the influence of vildagliptin on model-assessed b-cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab 2008; 93:103–109.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Jellinger PS, Davidson JA, Blonde L, et al; for the ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus: ACE/AACE Diabetes Road Map Task Force. Endocr Pract 2007; 13:260–268.
- 2007 National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/diabetes/pubs/estimates07.htm. Updated: July 23, 2008. Accessed September 25, 2009.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Bertin E, Schneider N, Abdelli N, et al. Gastric emptying is accelerated in obese type 2 diabetic patients without autonomic neuropathy. Diabetes Metab 2001; 27:357–364.
- Weytjens C, Keymeulen B, Van Haleweyn C, Somers G, Bossuyt A. Rapid gastric emptying of a liquid meal in long-term type 2 diabetes mellitus. Diabet Med 1998; 15:1022–1027.
- Stonehouse AH, Holcombe JH, Kendall DM. GLP-1 analogues, DPP-IV inhibitors and the metabolic syndrome. In: Fonseca V, ed. Therapeutic Strategies in Metabolic Syndrome. Oxford, UK: Atlas Medical Publishing Ltd; 2008: 137–157.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91:301–307.
- Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 1995; 80:952–957.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696–1705.
- Eng J, Kleinman WA, Singh L, Singh G, Raufman J-P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom: further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992; 267:7402–7405.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:3082–3089.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Davidson JA, Parente EB, Gross JL. Incretin mimetics and dipeptidyl peptidase-4 inhibitors: innovative treatment therapies for type 2 diabetes. Arq Bras Endocrinol Metabol 2008; 52:1039–1049.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Marre M, Shaw J, Brändle M, et al; for the LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabetes Med 2009; 26:268–278.
- Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide 1 with pharmacokinetic properties suitable for once-daily administration. J Med Chem 2000; 43:1664–1669.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Fleck P, Christopher R, Covington P, Wilson C, Mekki Q. Efficacy and safety of alogliptin monotherapy over 12 weeks in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 479-P.
- DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Effect of alogliptin combined with pioglitazone on glycemic control in metformin-treated patients with type 2 diabetes. Paper presented at: 69th Annual Meeting of the American Diabetes Association; June 5–9, 2009; New Orleans, LA. Abstract 2024-PO.
- Nauck M, Ellis G, Fleck P, Wilson C, Mekki Q. Efficacy and safety of alogliptin added to metformin therapy in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 477-P.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008; 30:1448–1460.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 81:161–168.
- Vilsbøll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 2007; 30:1608–1610.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G; for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R; for the CV181-040 Investigators. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract 2009; 63:1395–1406.
- DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 2009; 32:1649–1655.
- Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R; for the CV181-039 Investigators. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 2009; 11:611–622.
- Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R; for the CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin 2009; 25:2401–2411.
- Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naïve patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:376–386.
- Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res 2007; 39:218–223.
- Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007; 9:166–174.
- Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naïve patients with type 2 diabetes: comparison with metformin. Horm Metab Res 2008; 40:892–895.
- Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med 2008; 25:435–441.
- Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract 2007; 76:132–138.
- Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naïve patients with type 2 diabetes. Diabetes Med 2007; 24:955–961.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008; 151:123–129.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Merani S, Truong W, Emamaullee JA, Toso C, Knudsen LB, Shapiro AM. Liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology 2008; 149:4322–4328.
- Mari A, Degn K, Brock B, Rungby J, Ferrannini E, Schmitz O. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care 2007; 30:2032–2033.
- Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A. Mathematical modeling shows exenatide improved beta-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res 2006; 38:838–844.
- Xu L, Man CD, Charbonnel B, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: a model-based approach. Diabetes Obes Metab 2008; 10:1212–1220.
- Mari A, Scherbaum WA, Nilsson PM, et al. Characterization of the influence of vildagliptin on model-assessed b-cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab 2008; 93:103–109.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Jellinger PS, Davidson JA, Blonde L, et al; for the ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus: ACE/AACE Diabetes Road Map Task Force. Endocr Pract 2007; 13:260–268.
KEY POINTS
- Hormonal deficiencies in T2DM are related to abnormalities in the secretion of amylin, glucagon, and incretin hormones.
- In clinical trials, GLP-1 receptor agonists reduced HbA1c levels, had beneficial effects on weight, and caused less hypoglycemia than insulin analogues.
- Both GLP-1 receptor agonists and DPP-4 inhibitors improve pancreatic beta-cell function.
- Incretin-based therapies have been incorporated into recently updated clinical guidelines for treatment of T2DM.