User login
Fecal incontinence: Help for patients who suffer silently
› Consider adding a question about fecal incontinence—a condition often unreported by patients and undetected by physicians—to your medical intake form. C
› Use bowel diaries and fecal incontinence grading systems, as needed, to better understand the extent of the problem and assess the effects of treatment. C
› Consider sacral nerve stimulation, the first-line surgical treatment for fecal incontinence, for those who fail to respond to medical therapies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimates suggest that about 18 million adults in the United States suffer from fecal incontinence.1 But because the condition often goes unreported by patients and undetected by physicians, the actual prevalence is not known—and may be considerably higher.
What is known is that fecal incontinence carries a substantial socioeconomic burden. The average annual per patient cost is estimated at $4110.2 But fecal incontinence also exacts a heavy personal toll, and is one of the main reasons elderly individuals are placed in nursing homes.3
But it’s not just the elderly who are affected. A recent study of women ages 45 years and older found that nearly one in 5 had an episode of fecal incontinence at least once a year, and for nearly half, the frequency was once a month or more.4 Less than 3 in 10 reported their symptoms to a clinician, but those who did were most likely to have confided in their primary care physician.5
Fortunately, recent developments—most notably, sacral nerve stimulation, a minimally invasive surgical technique with a high success rate—have changed the outlook for patients with fecal incontinence. Here’s what you need to know to help patients who suffer from this embarrassing condition achieve optimal outcomes.
Risk factors and key causes
Maintaining fecal continence involves a complex series of events, both voluntary and involuntary. Problems at various levels—stool consistency, anatomic and neurologic abnormalities, and psychological problems among them—can disrupt the process.
Those at high risk for fecal incontinence, in addition to the elderly, include patients who are mentally ill and institutionalized, individuals with neurologic disorders, patients who have had anorectal surgery, and women who have had vaginal deliveries.6-8 Obstetric and operative injuries account for most cases of fecal incontinence.9-10
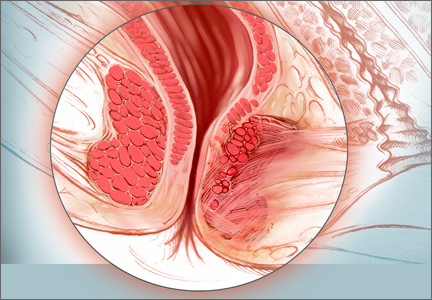
Sphincter defects including attenuation and scarring (shown here), are commonly caused by obstetric and operative injuries.
Risks of vaginal delivery
As many as 25% of women report some degree of fecal incontinence—although often confined to loss of control of flatus—3 months after giving birth.11 Stool incontinence is more frequent among women who sustained third- or fourth-degree perineal tears. Obstetrical risk factors include first vaginal birth, median episiotomy, forceps delivery, vacuum-assisted delivery, and a prolonged second stage of labor.
Asymptomatic sphincter defects. Studies in which women underwent endosonographic examination of the sphincter complex both before and after vaginal delivery have found sphincter defects in anywhere from 7% to 41% of new mothers.12-14 It is important to note, however, that as many as 70% of those with defects detected by sonogram were asymptomatic.15 (Despite the risk of sphincter injury during vaginal delivery and evidence suggesting that the risk of fecal incontinence increases with additional deliveries after a previous perineal tear, prophylactic cesarean section is not recommended.)
Fistula surgery and postop incontinence
Fistula surgery is the primary cause of postoperative incontinence, typically resulting from inadvertent injury to either the internal or external sphincter muscle.16 Other relatively common causes of fecal incontinence are rectal prolapse, trauma, irradiation, neurologic and demyelinating disorders such as multiple sclerosis, neoplasms, stroke, infection (eg, of a perineal wound), and diabetes.17 As diagnostic modalities have improved, much of what was previously termed idiopathic incontinence has been found to have identifiable underlying pathology, such as pudendal and inferior hypogastric neuropathies.18-20
Identifying fecal incontinence starts with a single question
As already noted, most patients with symptoms of bowel leakage do not voluntarily mention it to their physician. Many are likely to acknowledge the problem, however, if they’re specifically asked. While little has been written about how best to screen for fecal incontinence, simply adding it to the checklist on your medical intake form may be a good starting point.
Follow up with a targeted history and physical
When a patient checks fecal incontinence on a form or broaches the subject, it is important to question him or her about medical conditions that may be related. These include urinary incontinence, prolapsing tissue, diabetes, and a history of radiation, as well as childbirth. A medication history is also needed, as certain drugs—including some antacids and laxatives—have been implicated in fecal incontinence.21
Physical assessment should include a general neurologic exam as well as a perineal exam, to look for prolapsing tissue and evidence of scars from prior surgery or obstetrical trauma. Check the anocutaneous reflex by stroking the perianal skin. Absence of the anal wink in a younger patient is likely associated with nerve damage; in an older patient, it may simply indicate muscle weakness. Perform a digital rectal exam to assess for normal resting tone and augmentation with squeeze, regardless of the patient’s age.
Use tools to assess the severity
Anal incontinence can be broadly characterized as complete or partial. Numerous other systems have been proposed for classifying severity, the simplest of which has the following 4 components:
A: Continent of solid, liquid, and flatus (complete continence)
B: Continent of solid and liquid, but not flatus
C: Continent of solid, but not liquid or flatus
D: Continued fecal leakage (complete incontinence).22
Although this classification system may be helpful, it yields little information about the significance of the problem from the patient’s perspective.23 Thus, scales that take into account both the frequency of incontinence episodes and the extent of both the mental and physical impact are used more frequently.
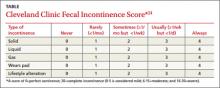
One of the most widely used scales is the Cleveland Clinic Fecal Incontinence Score (TABLE),24 which quantifies both the frequency and type of incontinence and scores the level of severity. Fecal incontinence quality of life scales are available, as well, and include questions about the impact on the patient’s lifestyle, coping behavior, mood, and level of embarrassment.25
Even without a quality of life scale, a couple of targeted questions—(eg, Are you ever afraid to go out? Do you worry about others smelling stool on you?)—will give you an idea of how great an impact fecal incontinence is having on your patient’s life. Asking patients to keep bowel diaries can also be helpful in assessing the extent of the problem and the effect of treatment.
Next steps: Start with modifiable risks
While there are numerous diagnostic tests for fecal incontinence (more about these in a bit), none is necessary for initial treatment, which starts with modifiable risks. Chief among them is smoking.
Smoking cessation. Nicotine is believed to have a direct effect on colonic transit and rectal compliance.26 Thus, smoking is associated with an increased risk for fecal incontinence, independent of chronic cough or chronic obstructive pulmonary disease. Patients should be advised to quit smoking and referred to a smoking cessation program.
Dietary fiber. Diet may be a factor in fecal incontinence, as well. Ask patients to record everything they eat, and advise those with a low intake of dietary fiber to eat more fruits, vegetables, whole grains, and other high-fiber food. Recommend that they avoid caffeine and alcohol, as well.
Some medications may also affect stool form and frequency, and precipitate fecal incontinence. Common offenders, in addition to laxatives and antacids, include antibiotics, proton pump inhibitors, and senna-based colon cleansers.27 Consider a switch to another drug class. A trial with a drug thought to improve bowel continence is recommended, as well.
Prescribe pharmacologic treatment
Kaolin, pectin, bulking agents, bismuth salts, anticholinergics, opium derivatives, diphenoxylate/atropine, and loperamide have all been used to treat fecal incontinence, with variable success. Loperamide, the drug most extensively studied for this purpose, has been found to increase resting anal pressure and improve anal sphincter function and continence by acting directly on the circular and longitudinal muscles of the bowel.28
Amitriptyline has also been used empirically, with some success. It is believed to work by decreasing the frequency and amplitude of rectal motor complexes.29 Clonidine in the form of a transdermal patch has been shown to increase the number of problem-free days and overall quality of life for patients with fecal incontinence.30
Consider biofeedback
Biofeedback training is often the next step after pharmacologic treatment. It has been investigated for the treatment of fecal incontinence, and many patients—particularly if they are highly motivated—have reported improvement.31 Therapy generally has 3 components: exercising the external sphincter complex, training in the discrimination of rectal sensations, and developing synchrony of the internal and external sphincter responses during rectal distension.
The goal is for the patient to learn to contract the sphincter in response to small amounts of rectal distension.
But a significant time commitment on the part of the patient and sophisticated apparatus are necessary to carry out such therapy, and only a few randomized controlled trials (RCTs) have evaluated the effect. The largest RCT had 4 arms: a standard care group; standard care plus instruction on sphincter exercises; standard care with sphincter exercises and biofeedback; and standard care with sphincter exercises, biofeedback, and training at home.32
All 4 groups had similar improvement in symptoms, raising questions about the therapeutic value of biofeedback.32 Long-term studies have found that 60% to 80% of patients will continue to have episodes of incontinence after undergoing biofeedback. A Cochrane review of RCTs concluded that there is not enough evidence to judge whether sphincter exercises and biofeedback are effective in reducing fecal incontinence.33
Still no relief? Order tests and consider surgery
For patients with fecal incontinence refractory to conservative management, more sophisticated diagnostic studies can provide invaluable information for guiding further treatment.
Endoanal ultrasound is considered the gold standard diagnostic test for fecal incontinence. It is superior to electromyography in terms of availability, patient tolerance, and ability to assess the internal anal sphincter, except in cases in which nerve injury is suspected.34
Other tests sometimes used to pinpoint the cause of fecal incontinence include an enema challenge (which can differentiate between liquid and solid incontinence) and anal manometry (which can quantify anal sphincter tone). Defecography (which makes it possible to visualize the rectal emptying process) can be helpful if a diagnosis of rectal prolapse is being considered.
Magnetic resonance imaging is among the most costly diagnostic studies associated with fecal incontinence. But it is the only modality that can depict the morphology of the external sphincter and the presence of muscle atrophy—providing information that has been shown to significantly improve the likelihood of successful sphincter repair.35
A wider range of surgical options
When medical therapy and biofeedback fail to produce adequate results, referral to a colorectal surgeon is appropriate. (Although conservative management is frequently unsuccessful, health plans typically require that they be attempted before surgical intervention is considered.)
Sphincteroplasty, or anterior anal sphincter repair, addresses the most common cause of fecal incontinence—and is still a common surgical procedure.36 Sphincteroplasty generally has good to excellent results, providing there is sufficient muscle mass for a successful repair.37,38
The procedure involves dissecting the sphincter complex from the surrounding anoderm, then overlapping the edges of the sphincter muscle and suturing them together. Continence has been reported nearly 80% of the time, although a longer duration of fecal incontinence and incontinence secondary to operative injury of the sphincter are risk factors for poorer outcomes.39,40
Recent studies have called into question the durability of anterior sphincter repair. A systematic review of 16 studies reporting short- and long-term outcomes for more than 900 patients found that all but one of the studies showed a decline over time in the number of patients who were happy with the outcome.39
Sacral nerve stimulation is first-line surgical treatment
Sacral nerve stimulation (SNS) is the most promising development in the treatment of fecal incontinence. In the last decade, SNS has become the first-line surgical treatment for patients for whom medical and behavioral therapy are unsuccessful.40
A minimally invasive procedure that involves an implantable device, SNS is always preceded by an effectiveness trial in which a finder needle is percutaneously inserted into the third sacral foramen. Stimulation should result in immediate contraction of the pelvic floor and external sphincter and plantar flexion of the big toe.
The next step is the insertion of a temporary stimulator lead, which remains in place for a 2- to 3-week test of low-frequency stimulation. If significant reduction in the number of incontinence episodes during the trial period occurs, the device is inserted (See “Sacral nerve stimulation: A case study” above).
Improvement in fecal continence has been reported to be as high as 100% in some cases, with up to 75% of patients achieving complete continence.41 While the mechanism involved remains unclear, multiple studies have confirmed its effectiveness.42,43
Posterior tibial nerve stimulation is another recent development, in which a small, thin lead is placed at the posterior tibial nerve, then connected to a temporary stimulator. Less data are available for this treatment, but a recent review summarized the findings of 8 published studies and found success rates ranging from 30% to 83%.44
The Secca procedure—a relatively new therapy that delivers radiofrequency energy to the anal sphincter—is another option, believed to work by reducing compliance of the sphincter complex and the level of tolerable rectal distension.45 Procedures using injectable bulking materials and fat grafting around the sphincter complex have demonstrated some promise, as well.46
A number of other surgical modalities are available, and often effective under certain circumstances. Among them are rotational and free muscle transfers, used only in cases in which the bulk of the sphincter complex has been destroyed.47,48 Implantable anal sphincters (made from human muscle and nerve cells) are occasionally used, as well, but frequently need to be removed because of infection.49-51
Regardless of the type of treatment they receive, patients often do not achieve total continence. Anyone who continues to have occasional episodes of fecal incontinence or leakage should be advised to wear incontinence pads, as needed.
Consider colostomy when incontinence is severe
For patients with fecal incontinence severe enough to be disabling—often as a result of irradiation—colostomy remains a tried and true treatment. The rectum can either be left intact or a proctectomy performed in concert with ostomy creation. Most studies evaluating colostomy for the treatment of incontinence have found that it significantly improves the quality of life and that most patients say they would choose to undergo the procedure again.52
1. Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
2. Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586-598.
3. Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the impact of fecal incontinence on nursing home referral. J Am Geriatr Soc. 2010;58:1058-1062.
4. Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66:1101–1108.
5. Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int Clin Pract. 2012;66:1109–1116.
6. Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013;108:113-119.
7. Sundquist JC. Long-term outcome after obstetric injury: a retrospective study. Acta Obstet Gynecol Scand. 2012 Jun;91:715-718.
8. Planting A, Phang PT, Raval MJ, et al. Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg. 2013;56:243-248.
9. Ctercteko GC, Fazio VW, Jagelman DG, et al. Anal sphincter repair: a report of 60 cases and review of the literature. Aust N Z J Surg. 1988;58:703–710.
10. Keighley MRB, Fielding JWL. Management of faecal incontinence and results of surgical treatment. Br J Surg. 1983;70: 463–468.
11. Eason E, Labrecque M, Marcoux S, et al. Anal incontinence after childbirth. CMAJ. 2002;166:326–330.
12. Rieger N, Schloithe A, Saccone G, et al. A prospective study of analsphincter injury due to childbirth. Scand J Gastroenterol. 1998;33:950–955.
13. Zetterstrom J, Mellgren A, Jensen LL, et al. Effect of delivery on anal sphinctermorphology and function. Dis Colon Rectum. 1999;42:1253–1260.
14. Varma A, Gunn J, Gardiner A, et al. Obstetric anal sphincter injury: prospective evaluation of incidence. Dis Colon Rectum. 1999;42:1537–1543.
15. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333–1337.
16. Lindsey I, Jones OM, Smilgin-Humphreys MM, et al. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum. 2004;47:1643–1649.
17. National Digestive Diseases Information Clearinghouse. Fecal
incontinence. Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/fecalincontinence. Accessed October 20, 2013.
18. Roig JV, Villoslada C, Lledo S, et al. Prevalence of pudendal neuropathy in fecal incontinence. Results of a prospective study. Dis Colon Rectum. 1995;38:952–958.
19. Swash M, Gray A, Lubowski DZ, et al. Ultrastructural changes in internal sphincter in neurogenic incontinence. Gut. 1988;29:1692–1698.
20. Rogers J, Henry MM, Misiewicz JJ. Combined sensory and motor deficit in primary fecal incontinence. Gut. 1988;29:5–9.
21. Medline Plus Web site. Bowel incontinence. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003135.htm. Accessed October 20, 2013.
22. Browning GP, Parks AG. Post anal repair for neuropathic fecal incontinence: correlation of clinical result and anal canal pressures. Br J Surg. 1983;70:101–104.
23. Baxter NN, Rothenberger DA, Lowry AC. Measuring fecal incontinence. Dis Colon Rectum. 2003;46:1591–1605.
24. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97.
25. American Society of Colon & Rectal Surgeons Web site. Fecal incontinence quality of life scale. Available at: http://www.fascrs.org/physicians/Fecal_Incontinence_Quality_of_Life_Scale/. Accessed October 20, 2013.
26. Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factor for late onset fecal incontinence: a population based case-control study in women. Gastroenterology. 2010;139:1559-1566.
27. MedlinePlus Web site. Drug-induced diarrhea. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000293.htm. Accessed October 21, 2013.
28. Hallgren T, Fasth S, Delbro DS, et al. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39:2612-2618.
29. Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676-1681.
30. Bharucha AE, Seide BM, Zinsmeister AR, et al. The effects of clonodine on symptoms and anorectal sensoriomotor function in women with faecal incontinence. Aliment Pharmacol Ther. 2010;32:681-688.
31. Engel BT, Nikoomnesh P, Schuster MM. Operant conditioning of rectosphincteric responses in the treatment of fecal incontinence. N Engl J Med. 1974;290:646-649.
32. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329.
33. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of fecal incontinence in adults. Cochrane Database Syst Rev. 2006;(3):CD002111.
34. Sultan AH, Nicholls RJ, Kamm MA, et al. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg. 1993; 80:508–511.
35. Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal MRI adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327.
36. Goetz LH, Lowry AC. Overlapping sphincteroplasty: is it the standard of care? Clin Colon Rectal Surg. 2005;18:22-31.
37. El-Gazzazz G, Zutshi M, Hannaway C, et al. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55:256-261.
38. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55:482-490.
39. Lehto K, Hyoty M, Collin P, et al. Seven-year follow-up after anterior sphincter reconstruction for faecal incontinence. Int J Colorectal Dis. 2013;5:653-658.
40. George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302-306.
41. Jarrett MED, Mowatt G, Glazener CMA, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91:1559–1569.
42. Melenhorst J, Koch SM, Uludag O, et al. Is a morphologically intact anal sphincter necessary for success with sacral nerve modulation in patients with faecal incontinence? Colorectal Dis. 2008;10:257-262.
43. Dudding TC, Pares D, Vaizey CJ, et al. Predictive factors for successful sacral nerve stimulation in the treatment of faecal incontinence: a 10-year cohort analysis. Colorectal Dis. 2008;10:294-256.
44. Findlay JM, Mawell-Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis. 2011;26:265-273.
45. Feretis C, Benakis P, Dailianas A, et al. Implantation of microballoons in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1605–1609.
46. Kenefick NJ, Vaizey CJ, Malouf AJ, et al. Injectable silicone biomaterial for faecal incontinence due to internal anal sphincter dysfunction. Gut. 2002;55:225–228.
47. Konsten J, Baeten CG, Spaans F, et al. Follow-up of anal dynamic graciloplasty for fecal continence. World J Surg. 1993;17:404–409.
48. Baeten C, Spaans F, Fluks A. An implanted neuromuscular stimulator for fecal continence following previously implanted gracilis muscle: report of a case. Dis Colon Rectum. 1988;31:134–137.
49. Wong MT, Meurette G, Stangherlin P, et al. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum. 2011;54:773-779.
50. Parker SC, Spencer MP, Madoff RD, et al. Artificial bowel sphincter: long-term experience at a single institution. Dis Colon Rectum 2003;46:722–729.
51. Takahashi T, Garcia-Osogobio S, Valdovinos MA, et al. Extended two-year results of radio-frequency energy delivery for the treatment of fecal incontinence (Secca procedure). Dis Colon Rectum. 2003;46:711–715.
52. Norton C, Burch J, Kamm MA. Patient’s views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48:1062.
› Consider adding a question about fecal incontinence—a condition often unreported by patients and undetected by physicians—to your medical intake form. C
› Use bowel diaries and fecal incontinence grading systems, as needed, to better understand the extent of the problem and assess the effects of treatment. C
› Consider sacral nerve stimulation, the first-line surgical treatment for fecal incontinence, for those who fail to respond to medical therapies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimates suggest that about 18 million adults in the United States suffer from fecal incontinence.1 But because the condition often goes unreported by patients and undetected by physicians, the actual prevalence is not known—and may be considerably higher.
What is known is that fecal incontinence carries a substantial socioeconomic burden. The average annual per patient cost is estimated at $4110.2 But fecal incontinence also exacts a heavy personal toll, and is one of the main reasons elderly individuals are placed in nursing homes.3
But it’s not just the elderly who are affected. A recent study of women ages 45 years and older found that nearly one in 5 had an episode of fecal incontinence at least once a year, and for nearly half, the frequency was once a month or more.4 Less than 3 in 10 reported their symptoms to a clinician, but those who did were most likely to have confided in their primary care physician.5
Fortunately, recent developments—most notably, sacral nerve stimulation, a minimally invasive surgical technique with a high success rate—have changed the outlook for patients with fecal incontinence. Here’s what you need to know to help patients who suffer from this embarrassing condition achieve optimal outcomes.
Risk factors and key causes
Maintaining fecal continence involves a complex series of events, both voluntary and involuntary. Problems at various levels—stool consistency, anatomic and neurologic abnormalities, and psychological problems among them—can disrupt the process.
Those at high risk for fecal incontinence, in addition to the elderly, include patients who are mentally ill and institutionalized, individuals with neurologic disorders, patients who have had anorectal surgery, and women who have had vaginal deliveries.6-8 Obstetric and operative injuries account for most cases of fecal incontinence.9-10
Sphincter defects including attenuation and scarring (shown here), are commonly caused by obstetric and operative injuries.
Risks of vaginal delivery
As many as 25% of women report some degree of fecal incontinence—although often confined to loss of control of flatus—3 months after giving birth.11 Stool incontinence is more frequent among women who sustained third- or fourth-degree perineal tears. Obstetrical risk factors include first vaginal birth, median episiotomy, forceps delivery, vacuum-assisted delivery, and a prolonged second stage of labor.
Asymptomatic sphincter defects. Studies in which women underwent endosonographic examination of the sphincter complex both before and after vaginal delivery have found sphincter defects in anywhere from 7% to 41% of new mothers.12-14 It is important to note, however, that as many as 70% of those with defects detected by sonogram were asymptomatic.15 (Despite the risk of sphincter injury during vaginal delivery and evidence suggesting that the risk of fecal incontinence increases with additional deliveries after a previous perineal tear, prophylactic cesarean section is not recommended.)
Fistula surgery and postop incontinence
Fistula surgery is the primary cause of postoperative incontinence, typically resulting from inadvertent injury to either the internal or external sphincter muscle.16 Other relatively common causes of fecal incontinence are rectal prolapse, trauma, irradiation, neurologic and demyelinating disorders such as multiple sclerosis, neoplasms, stroke, infection (eg, of a perineal wound), and diabetes.17 As diagnostic modalities have improved, much of what was previously termed idiopathic incontinence has been found to have identifiable underlying pathology, such as pudendal and inferior hypogastric neuropathies.18-20
Identifying fecal incontinence starts with a single question
As already noted, most patients with symptoms of bowel leakage do not voluntarily mention it to their physician. Many are likely to acknowledge the problem, however, if they’re specifically asked. While little has been written about how best to screen for fecal incontinence, simply adding it to the checklist on your medical intake form may be a good starting point.
Follow up with a targeted history and physical
When a patient checks fecal incontinence on a form or broaches the subject, it is important to question him or her about medical conditions that may be related. These include urinary incontinence, prolapsing tissue, diabetes, and a history of radiation, as well as childbirth. A medication history is also needed, as certain drugs—including some antacids and laxatives—have been implicated in fecal incontinence.21
Physical assessment should include a general neurologic exam as well as a perineal exam, to look for prolapsing tissue and evidence of scars from prior surgery or obstetrical trauma. Check the anocutaneous reflex by stroking the perianal skin. Absence of the anal wink in a younger patient is likely associated with nerve damage; in an older patient, it may simply indicate muscle weakness. Perform a digital rectal exam to assess for normal resting tone and augmentation with squeeze, regardless of the patient’s age.
Use tools to assess the severity
Anal incontinence can be broadly characterized as complete or partial. Numerous other systems have been proposed for classifying severity, the simplest of which has the following 4 components:
A: Continent of solid, liquid, and flatus (complete continence)
B: Continent of solid and liquid, but not flatus
C: Continent of solid, but not liquid or flatus
D: Continued fecal leakage (complete incontinence).22
Although this classification system may be helpful, it yields little information about the significance of the problem from the patient’s perspective.23 Thus, scales that take into account both the frequency of incontinence episodes and the extent of both the mental and physical impact are used more frequently.
One of the most widely used scales is the Cleveland Clinic Fecal Incontinence Score (TABLE),24 which quantifies both the frequency and type of incontinence and scores the level of severity. Fecal incontinence quality of life scales are available, as well, and include questions about the impact on the patient’s lifestyle, coping behavior, mood, and level of embarrassment.25
Even without a quality of life scale, a couple of targeted questions—(eg, Are you ever afraid to go out? Do you worry about others smelling stool on you?)—will give you an idea of how great an impact fecal incontinence is having on your patient’s life. Asking patients to keep bowel diaries can also be helpful in assessing the extent of the problem and the effect of treatment.
Next steps: Start with modifiable risks
While there are numerous diagnostic tests for fecal incontinence (more about these in a bit), none is necessary for initial treatment, which starts with modifiable risks. Chief among them is smoking.
Smoking cessation. Nicotine is believed to have a direct effect on colonic transit and rectal compliance.26 Thus, smoking is associated with an increased risk for fecal incontinence, independent of chronic cough or chronic obstructive pulmonary disease. Patients should be advised to quit smoking and referred to a smoking cessation program.
Dietary fiber. Diet may be a factor in fecal incontinence, as well. Ask patients to record everything they eat, and advise those with a low intake of dietary fiber to eat more fruits, vegetables, whole grains, and other high-fiber food. Recommend that they avoid caffeine and alcohol, as well.
Some medications may also affect stool form and frequency, and precipitate fecal incontinence. Common offenders, in addition to laxatives and antacids, include antibiotics, proton pump inhibitors, and senna-based colon cleansers.27 Consider a switch to another drug class. A trial with a drug thought to improve bowel continence is recommended, as well.
Prescribe pharmacologic treatment
Kaolin, pectin, bulking agents, bismuth salts, anticholinergics, opium derivatives, diphenoxylate/atropine, and loperamide have all been used to treat fecal incontinence, with variable success. Loperamide, the drug most extensively studied for this purpose, has been found to increase resting anal pressure and improve anal sphincter function and continence by acting directly on the circular and longitudinal muscles of the bowel.28
Amitriptyline has also been used empirically, with some success. It is believed to work by decreasing the frequency and amplitude of rectal motor complexes.29 Clonidine in the form of a transdermal patch has been shown to increase the number of problem-free days and overall quality of life for patients with fecal incontinence.30
Consider biofeedback
Biofeedback training is often the next step after pharmacologic treatment. It has been investigated for the treatment of fecal incontinence, and many patients—particularly if they are highly motivated—have reported improvement.31 Therapy generally has 3 components: exercising the external sphincter complex, training in the discrimination of rectal sensations, and developing synchrony of the internal and external sphincter responses during rectal distension.
The goal is for the patient to learn to contract the sphincter in response to small amounts of rectal distension.
But a significant time commitment on the part of the patient and sophisticated apparatus are necessary to carry out such therapy, and only a few randomized controlled trials (RCTs) have evaluated the effect. The largest RCT had 4 arms: a standard care group; standard care plus instruction on sphincter exercises; standard care with sphincter exercises and biofeedback; and standard care with sphincter exercises, biofeedback, and training at home.32
All 4 groups had similar improvement in symptoms, raising questions about the therapeutic value of biofeedback.32 Long-term studies have found that 60% to 80% of patients will continue to have episodes of incontinence after undergoing biofeedback. A Cochrane review of RCTs concluded that there is not enough evidence to judge whether sphincter exercises and biofeedback are effective in reducing fecal incontinence.33
Still no relief? Order tests and consider surgery
For patients with fecal incontinence refractory to conservative management, more sophisticated diagnostic studies can provide invaluable information for guiding further treatment.
Endoanal ultrasound is considered the gold standard diagnostic test for fecal incontinence. It is superior to electromyography in terms of availability, patient tolerance, and ability to assess the internal anal sphincter, except in cases in which nerve injury is suspected.34
Other tests sometimes used to pinpoint the cause of fecal incontinence include an enema challenge (which can differentiate between liquid and solid incontinence) and anal manometry (which can quantify anal sphincter tone). Defecography (which makes it possible to visualize the rectal emptying process) can be helpful if a diagnosis of rectal prolapse is being considered.
Magnetic resonance imaging is among the most costly diagnostic studies associated with fecal incontinence. But it is the only modality that can depict the morphology of the external sphincter and the presence of muscle atrophy—providing information that has been shown to significantly improve the likelihood of successful sphincter repair.35
A wider range of surgical options
When medical therapy and biofeedback fail to produce adequate results, referral to a colorectal surgeon is appropriate. (Although conservative management is frequently unsuccessful, health plans typically require that they be attempted before surgical intervention is considered.)
Sphincteroplasty, or anterior anal sphincter repair, addresses the most common cause of fecal incontinence—and is still a common surgical procedure.36 Sphincteroplasty generally has good to excellent results, providing there is sufficient muscle mass for a successful repair.37,38
The procedure involves dissecting the sphincter complex from the surrounding anoderm, then overlapping the edges of the sphincter muscle and suturing them together. Continence has been reported nearly 80% of the time, although a longer duration of fecal incontinence and incontinence secondary to operative injury of the sphincter are risk factors for poorer outcomes.39,40
Recent studies have called into question the durability of anterior sphincter repair. A systematic review of 16 studies reporting short- and long-term outcomes for more than 900 patients found that all but one of the studies showed a decline over time in the number of patients who were happy with the outcome.39
Sacral nerve stimulation is first-line surgical treatment
Sacral nerve stimulation (SNS) is the most promising development in the treatment of fecal incontinence. In the last decade, SNS has become the first-line surgical treatment for patients for whom medical and behavioral therapy are unsuccessful.40
A minimally invasive procedure that involves an implantable device, SNS is always preceded by an effectiveness trial in which a finder needle is percutaneously inserted into the third sacral foramen. Stimulation should result in immediate contraction of the pelvic floor and external sphincter and plantar flexion of the big toe.
The next step is the insertion of a temporary stimulator lead, which remains in place for a 2- to 3-week test of low-frequency stimulation. If significant reduction in the number of incontinence episodes during the trial period occurs, the device is inserted (See “Sacral nerve stimulation: A case study” above).
Improvement in fecal continence has been reported to be as high as 100% in some cases, with up to 75% of patients achieving complete continence.41 While the mechanism involved remains unclear, multiple studies have confirmed its effectiveness.42,43
Posterior tibial nerve stimulation is another recent development, in which a small, thin lead is placed at the posterior tibial nerve, then connected to a temporary stimulator. Less data are available for this treatment, but a recent review summarized the findings of 8 published studies and found success rates ranging from 30% to 83%.44
The Secca procedure—a relatively new therapy that delivers radiofrequency energy to the anal sphincter—is another option, believed to work by reducing compliance of the sphincter complex and the level of tolerable rectal distension.45 Procedures using injectable bulking materials and fat grafting around the sphincter complex have demonstrated some promise, as well.46
A number of other surgical modalities are available, and often effective under certain circumstances. Among them are rotational and free muscle transfers, used only in cases in which the bulk of the sphincter complex has been destroyed.47,48 Implantable anal sphincters (made from human muscle and nerve cells) are occasionally used, as well, but frequently need to be removed because of infection.49-51
Regardless of the type of treatment they receive, patients often do not achieve total continence. Anyone who continues to have occasional episodes of fecal incontinence or leakage should be advised to wear incontinence pads, as needed.
Consider colostomy when incontinence is severe
For patients with fecal incontinence severe enough to be disabling—often as a result of irradiation—colostomy remains a tried and true treatment. The rectum can either be left intact or a proctectomy performed in concert with ostomy creation. Most studies evaluating colostomy for the treatment of incontinence have found that it significantly improves the quality of life and that most patients say they would choose to undergo the procedure again.52
› Consider adding a question about fecal incontinence—a condition often unreported by patients and undetected by physicians—to your medical intake form. C
› Use bowel diaries and fecal incontinence grading systems, as needed, to better understand the extent of the problem and assess the effects of treatment. C
› Consider sacral nerve stimulation, the first-line surgical treatment for fecal incontinence, for those who fail to respond to medical therapies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimates suggest that about 18 million adults in the United States suffer from fecal incontinence.1 But because the condition often goes unreported by patients and undetected by physicians, the actual prevalence is not known—and may be considerably higher.
What is known is that fecal incontinence carries a substantial socioeconomic burden. The average annual per patient cost is estimated at $4110.2 But fecal incontinence also exacts a heavy personal toll, and is one of the main reasons elderly individuals are placed in nursing homes.3
But it’s not just the elderly who are affected. A recent study of women ages 45 years and older found that nearly one in 5 had an episode of fecal incontinence at least once a year, and for nearly half, the frequency was once a month or more.4 Less than 3 in 10 reported their symptoms to a clinician, but those who did were most likely to have confided in their primary care physician.5
Fortunately, recent developments—most notably, sacral nerve stimulation, a minimally invasive surgical technique with a high success rate—have changed the outlook for patients with fecal incontinence. Here’s what you need to know to help patients who suffer from this embarrassing condition achieve optimal outcomes.
Risk factors and key causes
Maintaining fecal continence involves a complex series of events, both voluntary and involuntary. Problems at various levels—stool consistency, anatomic and neurologic abnormalities, and psychological problems among them—can disrupt the process.
Those at high risk for fecal incontinence, in addition to the elderly, include patients who are mentally ill and institutionalized, individuals with neurologic disorders, patients who have had anorectal surgery, and women who have had vaginal deliveries.6-8 Obstetric and operative injuries account for most cases of fecal incontinence.9-10
Sphincter defects including attenuation and scarring (shown here), are commonly caused by obstetric and operative injuries.
Risks of vaginal delivery
As many as 25% of women report some degree of fecal incontinence—although often confined to loss of control of flatus—3 months after giving birth.11 Stool incontinence is more frequent among women who sustained third- or fourth-degree perineal tears. Obstetrical risk factors include first vaginal birth, median episiotomy, forceps delivery, vacuum-assisted delivery, and a prolonged second stage of labor.
Asymptomatic sphincter defects. Studies in which women underwent endosonographic examination of the sphincter complex both before and after vaginal delivery have found sphincter defects in anywhere from 7% to 41% of new mothers.12-14 It is important to note, however, that as many as 70% of those with defects detected by sonogram were asymptomatic.15 (Despite the risk of sphincter injury during vaginal delivery and evidence suggesting that the risk of fecal incontinence increases with additional deliveries after a previous perineal tear, prophylactic cesarean section is not recommended.)
Fistula surgery and postop incontinence
Fistula surgery is the primary cause of postoperative incontinence, typically resulting from inadvertent injury to either the internal or external sphincter muscle.16 Other relatively common causes of fecal incontinence are rectal prolapse, trauma, irradiation, neurologic and demyelinating disorders such as multiple sclerosis, neoplasms, stroke, infection (eg, of a perineal wound), and diabetes.17 As diagnostic modalities have improved, much of what was previously termed idiopathic incontinence has been found to have identifiable underlying pathology, such as pudendal and inferior hypogastric neuropathies.18-20
Identifying fecal incontinence starts with a single question
As already noted, most patients with symptoms of bowel leakage do not voluntarily mention it to their physician. Many are likely to acknowledge the problem, however, if they’re specifically asked. While little has been written about how best to screen for fecal incontinence, simply adding it to the checklist on your medical intake form may be a good starting point.
Follow up with a targeted history and physical
When a patient checks fecal incontinence on a form or broaches the subject, it is important to question him or her about medical conditions that may be related. These include urinary incontinence, prolapsing tissue, diabetes, and a history of radiation, as well as childbirth. A medication history is also needed, as certain drugs—including some antacids and laxatives—have been implicated in fecal incontinence.21
Physical assessment should include a general neurologic exam as well as a perineal exam, to look for prolapsing tissue and evidence of scars from prior surgery or obstetrical trauma. Check the anocutaneous reflex by stroking the perianal skin. Absence of the anal wink in a younger patient is likely associated with nerve damage; in an older patient, it may simply indicate muscle weakness. Perform a digital rectal exam to assess for normal resting tone and augmentation with squeeze, regardless of the patient’s age.
Use tools to assess the severity
Anal incontinence can be broadly characterized as complete or partial. Numerous other systems have been proposed for classifying severity, the simplest of which has the following 4 components:
A: Continent of solid, liquid, and flatus (complete continence)
B: Continent of solid and liquid, but not flatus
C: Continent of solid, but not liquid or flatus
D: Continued fecal leakage (complete incontinence).22
Although this classification system may be helpful, it yields little information about the significance of the problem from the patient’s perspective.23 Thus, scales that take into account both the frequency of incontinence episodes and the extent of both the mental and physical impact are used more frequently.
One of the most widely used scales is the Cleveland Clinic Fecal Incontinence Score (TABLE),24 which quantifies both the frequency and type of incontinence and scores the level of severity. Fecal incontinence quality of life scales are available, as well, and include questions about the impact on the patient’s lifestyle, coping behavior, mood, and level of embarrassment.25
Even without a quality of life scale, a couple of targeted questions—(eg, Are you ever afraid to go out? Do you worry about others smelling stool on you?)—will give you an idea of how great an impact fecal incontinence is having on your patient’s life. Asking patients to keep bowel diaries can also be helpful in assessing the extent of the problem and the effect of treatment.
Next steps: Start with modifiable risks
While there are numerous diagnostic tests for fecal incontinence (more about these in a bit), none is necessary for initial treatment, which starts with modifiable risks. Chief among them is smoking.
Smoking cessation. Nicotine is believed to have a direct effect on colonic transit and rectal compliance.26 Thus, smoking is associated with an increased risk for fecal incontinence, independent of chronic cough or chronic obstructive pulmonary disease. Patients should be advised to quit smoking and referred to a smoking cessation program.
Dietary fiber. Diet may be a factor in fecal incontinence, as well. Ask patients to record everything they eat, and advise those with a low intake of dietary fiber to eat more fruits, vegetables, whole grains, and other high-fiber food. Recommend that they avoid caffeine and alcohol, as well.
Some medications may also affect stool form and frequency, and precipitate fecal incontinence. Common offenders, in addition to laxatives and antacids, include antibiotics, proton pump inhibitors, and senna-based colon cleansers.27 Consider a switch to another drug class. A trial with a drug thought to improve bowel continence is recommended, as well.
Prescribe pharmacologic treatment
Kaolin, pectin, bulking agents, bismuth salts, anticholinergics, opium derivatives, diphenoxylate/atropine, and loperamide have all been used to treat fecal incontinence, with variable success. Loperamide, the drug most extensively studied for this purpose, has been found to increase resting anal pressure and improve anal sphincter function and continence by acting directly on the circular and longitudinal muscles of the bowel.28
Amitriptyline has also been used empirically, with some success. It is believed to work by decreasing the frequency and amplitude of rectal motor complexes.29 Clonidine in the form of a transdermal patch has been shown to increase the number of problem-free days and overall quality of life for patients with fecal incontinence.30
Consider biofeedback
Biofeedback training is often the next step after pharmacologic treatment. It has been investigated for the treatment of fecal incontinence, and many patients—particularly if they are highly motivated—have reported improvement.31 Therapy generally has 3 components: exercising the external sphincter complex, training in the discrimination of rectal sensations, and developing synchrony of the internal and external sphincter responses during rectal distension.
The goal is for the patient to learn to contract the sphincter in response to small amounts of rectal distension.
But a significant time commitment on the part of the patient and sophisticated apparatus are necessary to carry out such therapy, and only a few randomized controlled trials (RCTs) have evaluated the effect. The largest RCT had 4 arms: a standard care group; standard care plus instruction on sphincter exercises; standard care with sphincter exercises and biofeedback; and standard care with sphincter exercises, biofeedback, and training at home.32
All 4 groups had similar improvement in symptoms, raising questions about the therapeutic value of biofeedback.32 Long-term studies have found that 60% to 80% of patients will continue to have episodes of incontinence after undergoing biofeedback. A Cochrane review of RCTs concluded that there is not enough evidence to judge whether sphincter exercises and biofeedback are effective in reducing fecal incontinence.33
Still no relief? Order tests and consider surgery
For patients with fecal incontinence refractory to conservative management, more sophisticated diagnostic studies can provide invaluable information for guiding further treatment.
Endoanal ultrasound is considered the gold standard diagnostic test for fecal incontinence. It is superior to electromyography in terms of availability, patient tolerance, and ability to assess the internal anal sphincter, except in cases in which nerve injury is suspected.34
Other tests sometimes used to pinpoint the cause of fecal incontinence include an enema challenge (which can differentiate between liquid and solid incontinence) and anal manometry (which can quantify anal sphincter tone). Defecography (which makes it possible to visualize the rectal emptying process) can be helpful if a diagnosis of rectal prolapse is being considered.
Magnetic resonance imaging is among the most costly diagnostic studies associated with fecal incontinence. But it is the only modality that can depict the morphology of the external sphincter and the presence of muscle atrophy—providing information that has been shown to significantly improve the likelihood of successful sphincter repair.35
A wider range of surgical options
When medical therapy and biofeedback fail to produce adequate results, referral to a colorectal surgeon is appropriate. (Although conservative management is frequently unsuccessful, health plans typically require that they be attempted before surgical intervention is considered.)
Sphincteroplasty, or anterior anal sphincter repair, addresses the most common cause of fecal incontinence—and is still a common surgical procedure.36 Sphincteroplasty generally has good to excellent results, providing there is sufficient muscle mass for a successful repair.37,38
The procedure involves dissecting the sphincter complex from the surrounding anoderm, then overlapping the edges of the sphincter muscle and suturing them together. Continence has been reported nearly 80% of the time, although a longer duration of fecal incontinence and incontinence secondary to operative injury of the sphincter are risk factors for poorer outcomes.39,40
Recent studies have called into question the durability of anterior sphincter repair. A systematic review of 16 studies reporting short- and long-term outcomes for more than 900 patients found that all but one of the studies showed a decline over time in the number of patients who were happy with the outcome.39
Sacral nerve stimulation is first-line surgical treatment
Sacral nerve stimulation (SNS) is the most promising development in the treatment of fecal incontinence. In the last decade, SNS has become the first-line surgical treatment for patients for whom medical and behavioral therapy are unsuccessful.40
A minimally invasive procedure that involves an implantable device, SNS is always preceded by an effectiveness trial in which a finder needle is percutaneously inserted into the third sacral foramen. Stimulation should result in immediate contraction of the pelvic floor and external sphincter and plantar flexion of the big toe.
The next step is the insertion of a temporary stimulator lead, which remains in place for a 2- to 3-week test of low-frequency stimulation. If significant reduction in the number of incontinence episodes during the trial period occurs, the device is inserted (See “Sacral nerve stimulation: A case study” above).
Improvement in fecal continence has been reported to be as high as 100% in some cases, with up to 75% of patients achieving complete continence.41 While the mechanism involved remains unclear, multiple studies have confirmed its effectiveness.42,43
Posterior tibial nerve stimulation is another recent development, in which a small, thin lead is placed at the posterior tibial nerve, then connected to a temporary stimulator. Less data are available for this treatment, but a recent review summarized the findings of 8 published studies and found success rates ranging from 30% to 83%.44
The Secca procedure—a relatively new therapy that delivers radiofrequency energy to the anal sphincter—is another option, believed to work by reducing compliance of the sphincter complex and the level of tolerable rectal distension.45 Procedures using injectable bulking materials and fat grafting around the sphincter complex have demonstrated some promise, as well.46
A number of other surgical modalities are available, and often effective under certain circumstances. Among them are rotational and free muscle transfers, used only in cases in which the bulk of the sphincter complex has been destroyed.47,48 Implantable anal sphincters (made from human muscle and nerve cells) are occasionally used, as well, but frequently need to be removed because of infection.49-51
Regardless of the type of treatment they receive, patients often do not achieve total continence. Anyone who continues to have occasional episodes of fecal incontinence or leakage should be advised to wear incontinence pads, as needed.
Consider colostomy when incontinence is severe
For patients with fecal incontinence severe enough to be disabling—often as a result of irradiation—colostomy remains a tried and true treatment. The rectum can either be left intact or a proctectomy performed in concert with ostomy creation. Most studies evaluating colostomy for the treatment of incontinence have found that it significantly improves the quality of life and that most patients say they would choose to undergo the procedure again.52
1. Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
2. Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586-598.
3. Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the impact of fecal incontinence on nursing home referral. J Am Geriatr Soc. 2010;58:1058-1062.
4. Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66:1101–1108.
5. Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int Clin Pract. 2012;66:1109–1116.
6. Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013;108:113-119.
7. Sundquist JC. Long-term outcome after obstetric injury: a retrospective study. Acta Obstet Gynecol Scand. 2012 Jun;91:715-718.
8. Planting A, Phang PT, Raval MJ, et al. Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg. 2013;56:243-248.
9. Ctercteko GC, Fazio VW, Jagelman DG, et al. Anal sphincter repair: a report of 60 cases and review of the literature. Aust N Z J Surg. 1988;58:703–710.
10. Keighley MRB, Fielding JWL. Management of faecal incontinence and results of surgical treatment. Br J Surg. 1983;70: 463–468.
11. Eason E, Labrecque M, Marcoux S, et al. Anal incontinence after childbirth. CMAJ. 2002;166:326–330.
12. Rieger N, Schloithe A, Saccone G, et al. A prospective study of analsphincter injury due to childbirth. Scand J Gastroenterol. 1998;33:950–955.
13. Zetterstrom J, Mellgren A, Jensen LL, et al. Effect of delivery on anal sphinctermorphology and function. Dis Colon Rectum. 1999;42:1253–1260.
14. Varma A, Gunn J, Gardiner A, et al. Obstetric anal sphincter injury: prospective evaluation of incidence. Dis Colon Rectum. 1999;42:1537–1543.
15. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333–1337.
16. Lindsey I, Jones OM, Smilgin-Humphreys MM, et al. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum. 2004;47:1643–1649.
17. National Digestive Diseases Information Clearinghouse. Fecal
incontinence. Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/fecalincontinence. Accessed October 20, 2013.
18. Roig JV, Villoslada C, Lledo S, et al. Prevalence of pudendal neuropathy in fecal incontinence. Results of a prospective study. Dis Colon Rectum. 1995;38:952–958.
19. Swash M, Gray A, Lubowski DZ, et al. Ultrastructural changes in internal sphincter in neurogenic incontinence. Gut. 1988;29:1692–1698.
20. Rogers J, Henry MM, Misiewicz JJ. Combined sensory and motor deficit in primary fecal incontinence. Gut. 1988;29:5–9.
21. Medline Plus Web site. Bowel incontinence. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003135.htm. Accessed October 20, 2013.
22. Browning GP, Parks AG. Post anal repair for neuropathic fecal incontinence: correlation of clinical result and anal canal pressures. Br J Surg. 1983;70:101–104.
23. Baxter NN, Rothenberger DA, Lowry AC. Measuring fecal incontinence. Dis Colon Rectum. 2003;46:1591–1605.
24. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97.
25. American Society of Colon & Rectal Surgeons Web site. Fecal incontinence quality of life scale. Available at: http://www.fascrs.org/physicians/Fecal_Incontinence_Quality_of_Life_Scale/. Accessed October 20, 2013.
26. Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factor for late onset fecal incontinence: a population based case-control study in women. Gastroenterology. 2010;139:1559-1566.
27. MedlinePlus Web site. Drug-induced diarrhea. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000293.htm. Accessed October 21, 2013.
28. Hallgren T, Fasth S, Delbro DS, et al. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39:2612-2618.
29. Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676-1681.
30. Bharucha AE, Seide BM, Zinsmeister AR, et al. The effects of clonodine on symptoms and anorectal sensoriomotor function in women with faecal incontinence. Aliment Pharmacol Ther. 2010;32:681-688.
31. Engel BT, Nikoomnesh P, Schuster MM. Operant conditioning of rectosphincteric responses in the treatment of fecal incontinence. N Engl J Med. 1974;290:646-649.
32. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329.
33. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of fecal incontinence in adults. Cochrane Database Syst Rev. 2006;(3):CD002111.
34. Sultan AH, Nicholls RJ, Kamm MA, et al. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg. 1993; 80:508–511.
35. Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal MRI adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327.
36. Goetz LH, Lowry AC. Overlapping sphincteroplasty: is it the standard of care? Clin Colon Rectal Surg. 2005;18:22-31.
37. El-Gazzazz G, Zutshi M, Hannaway C, et al. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55:256-261.
38. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55:482-490.
39. Lehto K, Hyoty M, Collin P, et al. Seven-year follow-up after anterior sphincter reconstruction for faecal incontinence. Int J Colorectal Dis. 2013;5:653-658.
40. George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302-306.
41. Jarrett MED, Mowatt G, Glazener CMA, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91:1559–1569.
42. Melenhorst J, Koch SM, Uludag O, et al. Is a morphologically intact anal sphincter necessary for success with sacral nerve modulation in patients with faecal incontinence? Colorectal Dis. 2008;10:257-262.
43. Dudding TC, Pares D, Vaizey CJ, et al. Predictive factors for successful sacral nerve stimulation in the treatment of faecal incontinence: a 10-year cohort analysis. Colorectal Dis. 2008;10:294-256.
44. Findlay JM, Mawell-Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis. 2011;26:265-273.
45. Feretis C, Benakis P, Dailianas A, et al. Implantation of microballoons in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1605–1609.
46. Kenefick NJ, Vaizey CJ, Malouf AJ, et al. Injectable silicone biomaterial for faecal incontinence due to internal anal sphincter dysfunction. Gut. 2002;55:225–228.
47. Konsten J, Baeten CG, Spaans F, et al. Follow-up of anal dynamic graciloplasty for fecal continence. World J Surg. 1993;17:404–409.
48. Baeten C, Spaans F, Fluks A. An implanted neuromuscular stimulator for fecal continence following previously implanted gracilis muscle: report of a case. Dis Colon Rectum. 1988;31:134–137.
49. Wong MT, Meurette G, Stangherlin P, et al. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum. 2011;54:773-779.
50. Parker SC, Spencer MP, Madoff RD, et al. Artificial bowel sphincter: long-term experience at a single institution. Dis Colon Rectum 2003;46:722–729.
51. Takahashi T, Garcia-Osogobio S, Valdovinos MA, et al. Extended two-year results of radio-frequency energy delivery for the treatment of fecal incontinence (Secca procedure). Dis Colon Rectum. 2003;46:711–715.
52. Norton C, Burch J, Kamm MA. Patient’s views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48:1062.
1. Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
2. Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586-598.
3. Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the impact of fecal incontinence on nursing home referral. J Am Geriatr Soc. 2010;58:1058-1062.
4. Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66:1101–1108.
5. Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int Clin Pract. 2012;66:1109–1116.
6. Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013;108:113-119.
7. Sundquist JC. Long-term outcome after obstetric injury: a retrospective study. Acta Obstet Gynecol Scand. 2012 Jun;91:715-718.
8. Planting A, Phang PT, Raval MJ, et al. Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg. 2013;56:243-248.
9. Ctercteko GC, Fazio VW, Jagelman DG, et al. Anal sphincter repair: a report of 60 cases and review of the literature. Aust N Z J Surg. 1988;58:703–710.
10. Keighley MRB, Fielding JWL. Management of faecal incontinence and results of surgical treatment. Br J Surg. 1983;70: 463–468.
11. Eason E, Labrecque M, Marcoux S, et al. Anal incontinence after childbirth. CMAJ. 2002;166:326–330.
12. Rieger N, Schloithe A, Saccone G, et al. A prospective study of analsphincter injury due to childbirth. Scand J Gastroenterol. 1998;33:950–955.
13. Zetterstrom J, Mellgren A, Jensen LL, et al. Effect of delivery on anal sphinctermorphology and function. Dis Colon Rectum. 1999;42:1253–1260.
14. Varma A, Gunn J, Gardiner A, et al. Obstetric anal sphincter injury: prospective evaluation of incidence. Dis Colon Rectum. 1999;42:1537–1543.
15. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333–1337.
16. Lindsey I, Jones OM, Smilgin-Humphreys MM, et al. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum. 2004;47:1643–1649.
17. National Digestive Diseases Information Clearinghouse. Fecal
incontinence. Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/fecalincontinence. Accessed October 20, 2013.
18. Roig JV, Villoslada C, Lledo S, et al. Prevalence of pudendal neuropathy in fecal incontinence. Results of a prospective study. Dis Colon Rectum. 1995;38:952–958.
19. Swash M, Gray A, Lubowski DZ, et al. Ultrastructural changes in internal sphincter in neurogenic incontinence. Gut. 1988;29:1692–1698.
20. Rogers J, Henry MM, Misiewicz JJ. Combined sensory and motor deficit in primary fecal incontinence. Gut. 1988;29:5–9.
21. Medline Plus Web site. Bowel incontinence. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003135.htm. Accessed October 20, 2013.
22. Browning GP, Parks AG. Post anal repair for neuropathic fecal incontinence: correlation of clinical result and anal canal pressures. Br J Surg. 1983;70:101–104.
23. Baxter NN, Rothenberger DA, Lowry AC. Measuring fecal incontinence. Dis Colon Rectum. 2003;46:1591–1605.
24. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97.
25. American Society of Colon & Rectal Surgeons Web site. Fecal incontinence quality of life scale. Available at: http://www.fascrs.org/physicians/Fecal_Incontinence_Quality_of_Life_Scale/. Accessed October 20, 2013.
26. Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factor for late onset fecal incontinence: a population based case-control study in women. Gastroenterology. 2010;139:1559-1566.
27. MedlinePlus Web site. Drug-induced diarrhea. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000293.htm. Accessed October 21, 2013.
28. Hallgren T, Fasth S, Delbro DS, et al. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39:2612-2618.
29. Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676-1681.
30. Bharucha AE, Seide BM, Zinsmeister AR, et al. The effects of clonodine on symptoms and anorectal sensoriomotor function in women with faecal incontinence. Aliment Pharmacol Ther. 2010;32:681-688.
31. Engel BT, Nikoomnesh P, Schuster MM. Operant conditioning of rectosphincteric responses in the treatment of fecal incontinence. N Engl J Med. 1974;290:646-649.
32. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329.
33. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of fecal incontinence in adults. Cochrane Database Syst Rev. 2006;(3):CD002111.
34. Sultan AH, Nicholls RJ, Kamm MA, et al. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg. 1993; 80:508–511.
35. Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal MRI adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327.
36. Goetz LH, Lowry AC. Overlapping sphincteroplasty: is it the standard of care? Clin Colon Rectal Surg. 2005;18:22-31.
37. El-Gazzazz G, Zutshi M, Hannaway C, et al. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55:256-261.
38. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55:482-490.
39. Lehto K, Hyoty M, Collin P, et al. Seven-year follow-up after anterior sphincter reconstruction for faecal incontinence. Int J Colorectal Dis. 2013;5:653-658.
40. George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302-306.
41. Jarrett MED, Mowatt G, Glazener CMA, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91:1559–1569.
42. Melenhorst J, Koch SM, Uludag O, et al. Is a morphologically intact anal sphincter necessary for success with sacral nerve modulation in patients with faecal incontinence? Colorectal Dis. 2008;10:257-262.
43. Dudding TC, Pares D, Vaizey CJ, et al. Predictive factors for successful sacral nerve stimulation in the treatment of faecal incontinence: a 10-year cohort analysis. Colorectal Dis. 2008;10:294-256.
44. Findlay JM, Mawell-Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis. 2011;26:265-273.
45. Feretis C, Benakis P, Dailianas A, et al. Implantation of microballoons in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1605–1609.
46. Kenefick NJ, Vaizey CJ, Malouf AJ, et al. Injectable silicone biomaterial for faecal incontinence due to internal anal sphincter dysfunction. Gut. 2002;55:225–228.
47. Konsten J, Baeten CG, Spaans F, et al. Follow-up of anal dynamic graciloplasty for fecal continence. World J Surg. 1993;17:404–409.
48. Baeten C, Spaans F, Fluks A. An implanted neuromuscular stimulator for fecal continence following previously implanted gracilis muscle: report of a case. Dis Colon Rectum. 1988;31:134–137.
49. Wong MT, Meurette G, Stangherlin P, et al. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum. 2011;54:773-779.
50. Parker SC, Spencer MP, Madoff RD, et al. Artificial bowel sphincter: long-term experience at a single institution. Dis Colon Rectum 2003;46:722–729.
51. Takahashi T, Garcia-Osogobio S, Valdovinos MA, et al. Extended two-year results of radio-frequency energy delivery for the treatment of fecal incontinence (Secca procedure). Dis Colon Rectum. 2003;46:711–715.
52. Norton C, Burch J, Kamm MA. Patient’s views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48:1062.