User login
Knee osteoarthritis: Should your patient opt for hyaluronic acid injection?
- Hyaluronic acid (HA) injection may provide short-term relief of pain and improved functionality for patients with osteoarthritis of the knee, but benefits do not last beyond 6 months.
- Examine the HA option from a cost-benefit perspective on a case-by-case basis. Based on our meta-analysis, there is no sufficient reason to recommend or not recommend HA injection for treatment of osteoarthritis of the knee.
- You may want to help select patients weigh the possible benefit of HA therapy against its cost.
Though hyaluronic acid may reduce symptoms related to osteoarthritis of the knee, the relatively small and transient response in the population studied in our analysis does not provide sufficient reason to recommend or not recommend this therapy.
Those who might want to opt for hyaluronic acid injections
With the relatively low risk of complications, some patients may still opt to try hyaluronic acid injections as opposed to other osteoarthritis management strategies. Potential candidates include those whose only other option is surgery, in the hope that HA injection might postpone having to make that decision. Also, those whose pain or stiffness have not been relieved with other therapies might want to consider HA.
Two instruments for assessing osteoarthritis
There are many claims to the efficacy of hyaluronic acid injections for decreasing the pain associated with osteoarthritis of the knee. This meta-analysis was an attempt to collapse the data for hyaluronic acid treatment, using a reduction in score on the Western Ontario McMaster Universities Index (WOMAC) or the Lequesne index as its outcome measurement.
The WOMAC is a disease-specific, self-administered instrument for patients with osteoarthritis of the knee or hip. It has 3 separate dimensions (with 24 individual scenarios), measuring pain (5 scenarios), stiffness (2 scenarios), and physical function (17 scenarios). It may be administered using a 100 mm/10 cm visual analog scale (VAS) (where 0=none, 100 or 10=extreme) or a Likert scale (0 to 4, where 0=none, 4=extreme). These results are then scored on a 0 to 20 scale for pain, a 0 to 8 scale for stiffness, and a 0 to 68 scale for physical function. Lower scores for both scales indicate a lesser degree of pain, stiffness, or physical dysfunction. In a double-blind, randomized controlled trial, WOMAC was found to be a valid and reliable tool for determining self-reported status for osteoarthritis of the knee or hip.12
Osteoarthritis is a degenerative, debilitating disease that affects approximately 20.7 million adults in the United States.1 It is the degeneration of the articular cartilage at synovial joints. There seems to be a genetic predisposition to developing osteoarthritis, although most people tend to experience some pain in their joints as they age, usually starting in their 30s or 40s.2 Some elite athletes have even been reported as having arthritic changes in their 20s.3 Unfortunately, many factors may contribute to the pathological state of each person affected, making each case unique. It can be the result of general wear and tear at the joint, structural malalignments, or injury.4 With the surge in the elderly population, a more effective management strategy for osteoarthritis will improve quality of life and reduce health costs for many.
Hyaluronic acid, a normal component of synovial joints, the linear repeating polysaccharide that forms the central axis of proteoglycan aggregates, which are necessary for functional integrity of the articular cartilage.5 It is involved in joint lubrication and nutrition. Native hyaluronic acid increases the viscosity of the extracellular matrix, thereby increasing the load-dispersion properties of the articular cartilage. Arthritic articular cartilage tends to have a decreased concentration of naturally occurring hyaluronic acid.
Unfortunately, injection of hyaluronic acid does not appear to restore the properties that native hyaluronic acid provides to the articular cartilage. A treatment series of intra-articular injections of hyaluronic acid has been reported to decrease the pain associated with osteoarthritis and provide patient relief.6 It has also been reported that residual benefits may last for months after the last injection.7-9 One of the first reported trials of hyaluronic acid injections for treatment of osteoarthritis came in 1974.10 Subsequently, in 1982, a report was published on the therapeutic effect as a result of hyaluronic acid injection into the knee.11 By 1991, some of the first randomized, controlled trials of hyaluronic acid were reported.
Several different chemical compositions of hyaluronic acid are used for the treatment. Though similar, dosage depends on the specific chemical properties of each. A usual dosage is 3 to 5 injections, with the patient receiving 1 injection per week.
The Lequesne index is a 10-question interview-style survey given to patients with osteoarthritis of the knee. It has 5 questions pertaining to pain or discomfort, 1 question dealing with maximum distance walked, and 4 questions about activities of daily living. The total questionnaire is scored on a 0 to 24 scale, with lower scores meaning less functional impairment. A study by Faucher et al13 found the Lequesne index to be a reliable questionnaire.
Methods
Selection of studies
Two researchers (JM and AT) performed a computerized literature search of PubMed (1950–2004), CINAHL (1982–2004), and Medline (1966–2004) to identify citations concerning the efficacy of hyaluronic acid injection for management of osteoarthritis of the knee. Four separate searches were conducted. The first used the terms knee, osteoarthritis, WOMAC, and hyaluronic acid. The second used the same terms as the first, replacing WOMAC with Lequesne. The third and fourth searched WOMAC and validity and Lequesne and validity, respectively. All 4 searches were limited to human randomized clinical trials, in English-language journal reports. A hand search of the reference lists of all retrieved studies was performed to ensure that no eligible studies were excluded.
Studies were selected independently by the same 2 researchers. The search was performed independently to ensure an exhaustive review of the literature. All studies were considered eligible until disqualified based on exclusion criteria.
Studies were eligible for inclusion if they addressed hyaluronic acid injection for osteoarthritis at the knee and used the WOMAC or Lequesne indexes as outcomes measurements. It was also necessary that they provided means and standard deviations in order to perform statistical analysis. We attempted to contact authors who did not provide necessary statistics for meta-analysis; however, we received no responses.
Assessment of methodological quality and data abstraction
The methodological quality of each study was assessed independently by the reviewers using the Physiological Evidence Database (PEDro) rating scale.14 It was determined that studies must include a control group that used placebo saline injections, provide means and standard deviations at baseline for the WOMAC or Lequesne, and also means and standard deviations after the intervention for both the treatment and control groups.
All abstracted data were converted into a percentage of the total possible score for each outcome measurement using a method described by a statistical consultant and an algorithm developed by one investigator (JM) using MATLAB 7.0 (MathWorks, Inc, Natick, Mass). This allowed for comparison between results of the WOMAC and Lequesne studies. This also allowed for comparison to the results of the Wang et al15 meta-analysis.
Statistical analysis
Homogeneity of the included studies, as determined by inclusion and exclusion criteria, allowed for meta-analysis. One meta-analysis was performed on the 3 studies that used the WOMAC scale,16-18 2 on the 4 studies that used the Lequesne index.7-9,19 Most studies that used the Lequesne index reported outcomes for a variety of follow-up points (4, 5, 8, 20, 24, 26, 49, 52 weeks); however, there was not a consistent reporting timeline among these studies. We grouped the follow-up reports into “up to 6 months post-treatment” and “6 months or greater post-treatment.” The first Lequesne analysis evaluated the difference between treatment and placebo groups at with data points less than 6 months post-treatment. The second Lequesne analysis examined the difference between groups at 6 months and greater post-treatment. In the study by Karlsson,7 2 different but similar types of hyaluronic acid were used. These groups were separated and analyzed as independent studies; 95% confidence intervals were calculated for all analyses. Effect sizes were also calculated to determine the between-group effect of the treatment and placebo.
Results
Selection of studies
The literature search resulted in 35 potentially eligible studies, of which 7 were selected after application of the inclusion criteria.7-9,16-19 Three of the selected studies used the WOMAC as an outcome assessment tool,16-18 4 used the Lequesne index.7-9,19 The remaining 28 studies were excluded. TABLE W1 details the rejection of studies.
Methodological quality and study characteristics
Each investigator then independently scored the methodological quality for the remaining studies by using the PEDro scale.14 There was total agreement for all studies in regards to the PEDro score. No study received a score under 5. The average PEDro rating among the studies was 8.2 out of 10.
Homogeneity
All groups across all included studies were similar at baseline. All patients had mild to severe osteoarthritis of the knee. Mean ages of the groups were similar (treatment groups=67.1 years, placebo=66.7 years). Not all patients received treatment as per manufacturer’s recommendations ( TABLES 1 AND 2 ). A saline placebo was used for control groups in all studies. The volume of saline placebo for each study was equal to volume of hyaluronic acid injection plus saline vehicle used for that study. All studies used either the WOMAC scale or the Lequesne Functional Index as an outcome measurement tool. All studies included a follow-up outcome measurement; however, the range of follow-up time periods was wide (4 to 52 weeks).
Meta-analysis
The analysis for the outcome measurements on the WOMAC scale revealed no significant difference between groups in regards to pain or disability (95% confidence interval [CI] for pain, –0.6043 to 5.4755; for disability, –0.8282 to 4.8619). The outcome measurement for stiffness demonstrated a significant difference between treatment and control (95% CI for stiffness, 2.1780 to 8.7955).
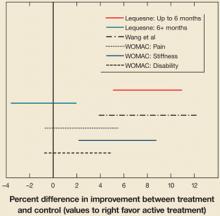
The analysis for the outcome measurement on the Lequesne index revealed a significant difference between groups for measurements taken up to 6 months post-treatment (95% CI, 1.2315 to 2.6268). However, these differences were not seen in the 6+ month outcomes analysis (95% CI, –.8489 to .04787]. Confidence intervals are presented in FIGURE 1 . Mean differences for each outcome at baseline and post-treatment between the active treatment and placebo are presented in TABLE 3 .
TABLE 1
Hyaluronic acid: Manufacturer’s recommendations
| HA BRAND | ARTZAL | SYNVISC | HYALGAN | DUROLANE | SUPLASYN | NRD101 |
|---|---|---|---|---|---|---|
| Number of injections (given 1 week apart) | 5 | 3 | 5 | 1 | 3 | N/A |
| Concomitant local anesthetic injection recommended | Yes | No* | Yes | NR | N/A | N/A |
| Aspiration recommended prior to injection | Yes | Yes | Yes | Yes | No | N/A |
| Dosage per injection (mg) | 25.0 | 32.0 | 40.0 | 60.0 | 20.0 | 25.0 |
| Molecular weight (kDa) | 620–1170 | 6000 | 500–730 | N/A | N/A | 1900 |
| Cross-linked | No | Yes | No | Yes | No | N/A |
| HA, hyaluronic acid; NR, not reported; N/A, not available. | ||||||
| * Purported to alter the efficacy of Synvisc. | ||||||
TABLE 2
Details of included studies
| STUDY | KARLSSON ET AL7 | DOUGADOS ET AL8 | HUSKISSON9 | DAY ET AL16 | ALTMAN ET AL17 | PETRELLA ET AL18 | PHAM ET AL19 | |
|---|---|---|---|---|---|---|---|---|
| Brand | Artzal | Synvisc | Hyalgan | Hyalgan | Artzal | Durolane | Suplasyn | NRD101 |
| Number of injections | ||||||||
| given 1 wk apart | 3 | 3 | 4 | 5 | 5 | 1 | 3 | 3* |
| Mfr’s recommendations | 5 | 3 | 5 | 5 | 5 | 1 | 3 | NA |
| Concomitant local anaesthetic injection | NR | NR | NR | NR | Yes | NR | NR | Yes |
| Aspiration prior to injection | NR | NR | Yes | NR | Yes | NR | NR | Yes |
| Dosage per injection (mg) + vehicle† | 25/2.5 mL saline | 32/2.5 mL saline | 20/2.0 mL saline | 20/2.0 mL saline | 25/2.5 mL saline | 60/3.0 mL saline | 20/2.0 mL saline | 25/2.5 mL saline |
| Cross-linked | No | Yes | No | No | No | Yes | NR | NR |
| NR=not reported | ||||||||
| * Given as 3 courses every 3 months of 3 weekly injections | ||||||||
| † Volume of saline placebo for each study equal to volume of hyaluronic acid injection plus saline vehicle used for that study | ||||||||
FIGURE 1
Hyaluronic acid vs control: 95% confidence intervals
Confidence intervals that cross the 0-value represent a nonsignificant difference between active treatment and control. Also included is a result from the Wang et al meta-analysis15 for comparison.
TABLE 3
Mean improvements: treatment vs. control
| BASELINE | POST-TREATMENT | |||
|---|---|---|---|---|
| HA | PLACEBO | HA | PLACEBO | |
| LEQUESNE | 12.7 (3.0) | 12.44 (2.7) | 8.7 (4.4) | 8.91 (4.4) |
| % Lequesne* | 53.0 (12.8) | 51.83 (11.2) | 36.3 (18.5) | 37.1 (18.1) |
| WOMAC | ||||
| Pain* | 40.5 (17.0) | 43.9 (19.0) | 26.8 (18.3) | 30.9 (21.6) |
| Stiffness* | 47.0 (17.6) | 50.9 (21.1) | 33.0 (21.4) | 42.8 (24.0) |
| Disability* | 42.5 (18.6) | 46.8 (22.2) | 27.9 (19.0) | 33.3 (21.8) |
| The mean (standard deviation) change among included studies in outcomes scores between baseline and post-treatment at the final collection point for hyaluronic acid and placebo. An increase in outcome score represents improvement. | ||||
| * Normalized to a maximal score of 100 possible points | ||||
FIGURE 2
Comparing effect sizes of hyaluronic acid therapy and saline placebo
Across studies, effect sizes were similar for hyaluronic acid therapy and placebo.
Discussion: Benefits are limited
Based on this meta-analysis, we cannot conclude that hyaluronic acid performs better than saline placebo for a reduction of pain or disability on the WOMAC. Some indication may be warranted for reduction in stiffness. It is notable that hyaluronic acid injection and saline placebo groups both experienced an improvement in pain, stiffness, and disability scores on the WOMAC.
We also cannot conclude that hyaluronic acid enhances functionality as evaluated on the Lequesne Index past 6 months post-treatment. In 2 studies,7,19 patients with placebo actually showed reduced disability compared with patients in the hyaluronic acid group. While a short-term improvement in functionality was observed in other studies, this effect was not seen in follow-up reports greater than 6 months.
Comparisons with other meta-analyses
A recently published meta-analysis by Modawal et al20 also pooled data to compare hyaluronic acid treatment to saline placebo. That meta-analysis was similar to ours in that it had relatively small sample size (n=11) and examined self-reported patient outcomes as the item of interest. The difference between this meta-analysis and ours is that the length of the evaluation periods for the other analysis was fairly short; the longest follow-up was 26 weeks. Our study examined the pooled effects of hyaluronic acid carried out to at least 24 weeks and up to 52 weeks. Their results for the short-term outcomes demonstrated significant decrease in pain in the active treatment group; however, these results diminished considerably when controlled for study quality.
Our meta-analysis was not consistent with a previous meta-analysis of hyaluronic acid treatment with regard to pain relief and reduction of disability. This study15 revealed that hyaluronic acid has a therapeutic effect on osteoarthritis of the knee, specifically decreasing pain and disability. The analysis included 20 studies, but differed from ours in that it failed to control for between-group heterogeneity and used an original formula to derive mean differences in efficacy scores between hyaluronic acid and placebo. Each study in our meta-analysis used an identical method for reporting outcome scores. Our study also synthesized the original data values as reported in each original study.
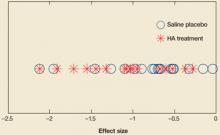
In addition, our results have to be interpreted with attention to a large effect of saline injection, which were much greater than the expected effect of placebo. Calculated effect sizes demonstrated similar effects for both treatment and placebo groups, with no clustering that was different between groups ( FIGURE 2 ). Treatment and placebo groups showed equal response when evaluated for greatest effect.
Refocusing research
The effects of hyaluronic acid injection appear to be transient and offer only slight improvement for older patients with mild to advanced osteoarthritis. Attention should be given to other groups, including younger patients and patients with mild degenerative changes. These studies should attempt to better stratify patient groups to identify those who are most likely to benefit from intervention. In addition, there must be better reporting of treatment protocols and results will allow clinicians to make rational decisions regarding treatments.
Cost and side effects
Consider the degree of benefit and costs when making treatment decisions. The average treatment cost was approximately $900 in 2003.21 Obviously one issue is whether a patient’s insurance provider will pay for the treatment.
Side effects are uncommon, and complications with hyaluronic acid injections have been relatively mild.
A few cases of hyaluronic acid allergy have been reported,22 including sweating, paleness, feelings of pressure in the chest and stomach, skin turning blue, or a decrease in blood pressure.22
Acknowledgments
The authors would like to thank Esra Akdeniz from the Statistical Consulting Center at the Pennsylvania State University for assistance with the methods for performing statistical meta-analysis.
CORRESPONDENCE
Jennifer M. Medina, 146 Recreation Building, University Park, PA 16802. E-mail: [email protected]
1. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998;41:778-799.
2. Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol 2006;18:147-156.
3. Neyret P, Donell ST, Dejour H. Results of partial meniscectomy related to the state of the anterior cruciate ligament. Review at 20 to 35 years. J Bone Joint Surg Br 1993;75:36-40.
4. Moskowitz RW, Kelly MA, Lewallen DG. Understanding osteoarthritis of the knee-causes and effects. Am J Orthop 2004;33:5-9.
5. Walsh DA, Sledge CB, Blanke DR. Biology of the normal joint. In: Kelley WN, Ruddy W, Harris ED Jr, Sledge CB, eds. Textbook of Rheumatology. Vol 1. 5th ed. Philadelphia, PA: W.B. Saunders Company;1997:1-26.
6. Leardini G, Mattara L, Franceschini M, Perbellini A. Intra-articular treatment of knee osteoarthritis. A comparative study between hyaluronic acid and –methyl prednisolone acetate. Clin Exp Rheumatol 1991;9:375-381.
7. Karlsson J, Sjogren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology. 2002;41:1240-1248.
8. Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1 year placebo-controlled trial. Osteoarthritis Cartilage 1993;1:97-103.
9. Huskisson EC, Donnelly S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology 1999;38:602-607.
10. Peyron JG, Balazs EA. Preliminary clinical assessment of Na-hyaluronate injection into human arthritic joints. PatholBiol (Paris) 1974;22:731-736.
11. Namiki O, Toyoshima H, Morisaki N. Therapeutic effect of intra-articular injection of high molecular weight hyaluronic acid on osteoarthritis of the knee. Int J Clin Pharmacol Ther Toxicol 1982;20:501-507.
12. Bellamy N, Buchanan W, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1999;15:1833-1840.
13. Faucher M, Poiraudeau S, Lefevre-Colau MM, Rannou F, Fermanian F, Revel M. Algo-functional assessment of knee osteoarthritis: comparison of the test-retest reliability and construct validity of the WOMAC and Lequesne indexes. Osteoarth Cartil 2002;10:602-610.
14. PEDro Scale. Physiotherapy Evidence Database Web site. Available at: www.pedro.fhs.usyd.edu.au/scale_item.html. Accessed on February 10, 2005.
15. Wang C, Lin J, Chang C, Lin Y, Hou S. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. J Bone Joint Surg 2004;86A:538-545.
16. Day R, Brooks P, Conaghan PG, Petersen M. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intra-articular hyaluronan in osteoarthritis of the knee. J Rheumatol 2004;31:775-782.
17. Altman RD, Akermark C, Beaulieu AD, Schnitzer T. Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartilage 2004;12:642-649.
18. Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning on osteoarthritis of the knee. Arch Intern Med 2002;162:292-298.
19. Pham T, Le Henanff A, Ravaud P, Dieppe P, Paolozzi L, Dougados M. Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomized controlled study in symptomatic knee osteoarthritis. Ann Rhem Dis 2004;63:1611-1617.
20. Modawal A, Ferrer M, Choi HK, Castle JA. Hyaluronic acid injections relieve knee pain. J Fam Pract 2005;54:758-677.
21. Kahan A, Lleu P, Salin L. Prospective randomized study comparing the medicoeconomic benefits of Hylan GF-20 vs. conventional treatment in knee osteoarthritis. Joint Bone Spine 2003;70:276-281.
22. Brandt KD, Block JA, Michalski JP, Moreland LW, Caldwell JR, Lavin PT. Efficacy and safety of intra-articular sodium hyaluronate in knee osteoarthritis. Clin Orthop 2001;385:130-143.
- Hyaluronic acid (HA) injection may provide short-term relief of pain and improved functionality for patients with osteoarthritis of the knee, but benefits do not last beyond 6 months.
- Examine the HA option from a cost-benefit perspective on a case-by-case basis. Based on our meta-analysis, there is no sufficient reason to recommend or not recommend HA injection for treatment of osteoarthritis of the knee.
- You may want to help select patients weigh the possible benefit of HA therapy against its cost.
Though hyaluronic acid may reduce symptoms related to osteoarthritis of the knee, the relatively small and transient response in the population studied in our analysis does not provide sufficient reason to recommend or not recommend this therapy.
Those who might want to opt for hyaluronic acid injections
With the relatively low risk of complications, some patients may still opt to try hyaluronic acid injections as opposed to other osteoarthritis management strategies. Potential candidates include those whose only other option is surgery, in the hope that HA injection might postpone having to make that decision. Also, those whose pain or stiffness have not been relieved with other therapies might want to consider HA.
Two instruments for assessing osteoarthritis
There are many claims to the efficacy of hyaluronic acid injections for decreasing the pain associated with osteoarthritis of the knee. This meta-analysis was an attempt to collapse the data for hyaluronic acid treatment, using a reduction in score on the Western Ontario McMaster Universities Index (WOMAC) or the Lequesne index as its outcome measurement.
The WOMAC is a disease-specific, self-administered instrument for patients with osteoarthritis of the knee or hip. It has 3 separate dimensions (with 24 individual scenarios), measuring pain (5 scenarios), stiffness (2 scenarios), and physical function (17 scenarios). It may be administered using a 100 mm/10 cm visual analog scale (VAS) (where 0=none, 100 or 10=extreme) or a Likert scale (0 to 4, where 0=none, 4=extreme). These results are then scored on a 0 to 20 scale for pain, a 0 to 8 scale for stiffness, and a 0 to 68 scale for physical function. Lower scores for both scales indicate a lesser degree of pain, stiffness, or physical dysfunction. In a double-blind, randomized controlled trial, WOMAC was found to be a valid and reliable tool for determining self-reported status for osteoarthritis of the knee or hip.12
Osteoarthritis is a degenerative, debilitating disease that affects approximately 20.7 million adults in the United States.1 It is the degeneration of the articular cartilage at synovial joints. There seems to be a genetic predisposition to developing osteoarthritis, although most people tend to experience some pain in their joints as they age, usually starting in their 30s or 40s.2 Some elite athletes have even been reported as having arthritic changes in their 20s.3 Unfortunately, many factors may contribute to the pathological state of each person affected, making each case unique. It can be the result of general wear and tear at the joint, structural malalignments, or injury.4 With the surge in the elderly population, a more effective management strategy for osteoarthritis will improve quality of life and reduce health costs for many.
Hyaluronic acid, a normal component of synovial joints, the linear repeating polysaccharide that forms the central axis of proteoglycan aggregates, which are necessary for functional integrity of the articular cartilage.5 It is involved in joint lubrication and nutrition. Native hyaluronic acid increases the viscosity of the extracellular matrix, thereby increasing the load-dispersion properties of the articular cartilage. Arthritic articular cartilage tends to have a decreased concentration of naturally occurring hyaluronic acid.
Unfortunately, injection of hyaluronic acid does not appear to restore the properties that native hyaluronic acid provides to the articular cartilage. A treatment series of intra-articular injections of hyaluronic acid has been reported to decrease the pain associated with osteoarthritis and provide patient relief.6 It has also been reported that residual benefits may last for months after the last injection.7-9 One of the first reported trials of hyaluronic acid injections for treatment of osteoarthritis came in 1974.10 Subsequently, in 1982, a report was published on the therapeutic effect as a result of hyaluronic acid injection into the knee.11 By 1991, some of the first randomized, controlled trials of hyaluronic acid were reported.
Several different chemical compositions of hyaluronic acid are used for the treatment. Though similar, dosage depends on the specific chemical properties of each. A usual dosage is 3 to 5 injections, with the patient receiving 1 injection per week.
The Lequesne index is a 10-question interview-style survey given to patients with osteoarthritis of the knee. It has 5 questions pertaining to pain or discomfort, 1 question dealing with maximum distance walked, and 4 questions about activities of daily living. The total questionnaire is scored on a 0 to 24 scale, with lower scores meaning less functional impairment. A study by Faucher et al13 found the Lequesne index to be a reliable questionnaire.
Methods
Selection of studies
Two researchers (JM and AT) performed a computerized literature search of PubMed (1950–2004), CINAHL (1982–2004), and Medline (1966–2004) to identify citations concerning the efficacy of hyaluronic acid injection for management of osteoarthritis of the knee. Four separate searches were conducted. The first used the terms knee, osteoarthritis, WOMAC, and hyaluronic acid. The second used the same terms as the first, replacing WOMAC with Lequesne. The third and fourth searched WOMAC and validity and Lequesne and validity, respectively. All 4 searches were limited to human randomized clinical trials, in English-language journal reports. A hand search of the reference lists of all retrieved studies was performed to ensure that no eligible studies were excluded.
Studies were selected independently by the same 2 researchers. The search was performed independently to ensure an exhaustive review of the literature. All studies were considered eligible until disqualified based on exclusion criteria.
Studies were eligible for inclusion if they addressed hyaluronic acid injection for osteoarthritis at the knee and used the WOMAC or Lequesne indexes as outcomes measurements. It was also necessary that they provided means and standard deviations in order to perform statistical analysis. We attempted to contact authors who did not provide necessary statistics for meta-analysis; however, we received no responses.
Assessment of methodological quality and data abstraction
The methodological quality of each study was assessed independently by the reviewers using the Physiological Evidence Database (PEDro) rating scale.14 It was determined that studies must include a control group that used placebo saline injections, provide means and standard deviations at baseline for the WOMAC or Lequesne, and also means and standard deviations after the intervention for both the treatment and control groups.
All abstracted data were converted into a percentage of the total possible score for each outcome measurement using a method described by a statistical consultant and an algorithm developed by one investigator (JM) using MATLAB 7.0 (MathWorks, Inc, Natick, Mass). This allowed for comparison between results of the WOMAC and Lequesne studies. This also allowed for comparison to the results of the Wang et al15 meta-analysis.
Statistical analysis
Homogeneity of the included studies, as determined by inclusion and exclusion criteria, allowed for meta-analysis. One meta-analysis was performed on the 3 studies that used the WOMAC scale,16-18 2 on the 4 studies that used the Lequesne index.7-9,19 Most studies that used the Lequesne index reported outcomes for a variety of follow-up points (4, 5, 8, 20, 24, 26, 49, 52 weeks); however, there was not a consistent reporting timeline among these studies. We grouped the follow-up reports into “up to 6 months post-treatment” and “6 months or greater post-treatment.” The first Lequesne analysis evaluated the difference between treatment and placebo groups at with data points less than 6 months post-treatment. The second Lequesne analysis examined the difference between groups at 6 months and greater post-treatment. In the study by Karlsson,7 2 different but similar types of hyaluronic acid were used. These groups were separated and analyzed as independent studies; 95% confidence intervals were calculated for all analyses. Effect sizes were also calculated to determine the between-group effect of the treatment and placebo.
Results
Selection of studies
The literature search resulted in 35 potentially eligible studies, of which 7 were selected after application of the inclusion criteria.7-9,16-19 Three of the selected studies used the WOMAC as an outcome assessment tool,16-18 4 used the Lequesne index.7-9,19 The remaining 28 studies were excluded. TABLE W1 details the rejection of studies.
Methodological quality and study characteristics
Each investigator then independently scored the methodological quality for the remaining studies by using the PEDro scale.14 There was total agreement for all studies in regards to the PEDro score. No study received a score under 5. The average PEDro rating among the studies was 8.2 out of 10.
Homogeneity
All groups across all included studies were similar at baseline. All patients had mild to severe osteoarthritis of the knee. Mean ages of the groups were similar (treatment groups=67.1 years, placebo=66.7 years). Not all patients received treatment as per manufacturer’s recommendations ( TABLES 1 AND 2 ). A saline placebo was used for control groups in all studies. The volume of saline placebo for each study was equal to volume of hyaluronic acid injection plus saline vehicle used for that study. All studies used either the WOMAC scale or the Lequesne Functional Index as an outcome measurement tool. All studies included a follow-up outcome measurement; however, the range of follow-up time periods was wide (4 to 52 weeks).
Meta-analysis
The analysis for the outcome measurements on the WOMAC scale revealed no significant difference between groups in regards to pain or disability (95% confidence interval [CI] for pain, –0.6043 to 5.4755; for disability, –0.8282 to 4.8619). The outcome measurement for stiffness demonstrated a significant difference between treatment and control (95% CI for stiffness, 2.1780 to 8.7955).
The analysis for the outcome measurement on the Lequesne index revealed a significant difference between groups for measurements taken up to 6 months post-treatment (95% CI, 1.2315 to 2.6268). However, these differences were not seen in the 6+ month outcomes analysis (95% CI, –.8489 to .04787]. Confidence intervals are presented in FIGURE 1 . Mean differences for each outcome at baseline and post-treatment between the active treatment and placebo are presented in TABLE 3 .
TABLE 1
Hyaluronic acid: Manufacturer’s recommendations
| HA BRAND | ARTZAL | SYNVISC | HYALGAN | DUROLANE | SUPLASYN | NRD101 |
|---|---|---|---|---|---|---|
| Number of injections (given 1 week apart) | 5 | 3 | 5 | 1 | 3 | N/A |
| Concomitant local anesthetic injection recommended | Yes | No* | Yes | NR | N/A | N/A |
| Aspiration recommended prior to injection | Yes | Yes | Yes | Yes | No | N/A |
| Dosage per injection (mg) | 25.0 | 32.0 | 40.0 | 60.0 | 20.0 | 25.0 |
| Molecular weight (kDa) | 620–1170 | 6000 | 500–730 | N/A | N/A | 1900 |
| Cross-linked | No | Yes | No | Yes | No | N/A |
| HA, hyaluronic acid; NR, not reported; N/A, not available. | ||||||
| * Purported to alter the efficacy of Synvisc. | ||||||
TABLE 2
Details of included studies
| STUDY | KARLSSON ET AL7 | DOUGADOS ET AL8 | HUSKISSON9 | DAY ET AL16 | ALTMAN ET AL17 | PETRELLA ET AL18 | PHAM ET AL19 | |
|---|---|---|---|---|---|---|---|---|
| Brand | Artzal | Synvisc | Hyalgan | Hyalgan | Artzal | Durolane | Suplasyn | NRD101 |
| Number of injections | ||||||||
| given 1 wk apart | 3 | 3 | 4 | 5 | 5 | 1 | 3 | 3* |
| Mfr’s recommendations | 5 | 3 | 5 | 5 | 5 | 1 | 3 | NA |
| Concomitant local anaesthetic injection | NR | NR | NR | NR | Yes | NR | NR | Yes |
| Aspiration prior to injection | NR | NR | Yes | NR | Yes | NR | NR | Yes |
| Dosage per injection (mg) + vehicle† | 25/2.5 mL saline | 32/2.5 mL saline | 20/2.0 mL saline | 20/2.0 mL saline | 25/2.5 mL saline | 60/3.0 mL saline | 20/2.0 mL saline | 25/2.5 mL saline |
| Cross-linked | No | Yes | No | No | No | Yes | NR | NR |
| NR=not reported | ||||||||
| * Given as 3 courses every 3 months of 3 weekly injections | ||||||||
| † Volume of saline placebo for each study equal to volume of hyaluronic acid injection plus saline vehicle used for that study | ||||||||
FIGURE 1
Hyaluronic acid vs control: 95% confidence intervals
Confidence intervals that cross the 0-value represent a nonsignificant difference between active treatment and control. Also included is a result from the Wang et al meta-analysis15 for comparison.
TABLE 3
Mean improvements: treatment vs. control
| BASELINE | POST-TREATMENT | |||
|---|---|---|---|---|
| HA | PLACEBO | HA | PLACEBO | |
| LEQUESNE | 12.7 (3.0) | 12.44 (2.7) | 8.7 (4.4) | 8.91 (4.4) |
| % Lequesne* | 53.0 (12.8) | 51.83 (11.2) | 36.3 (18.5) | 37.1 (18.1) |
| WOMAC | ||||
| Pain* | 40.5 (17.0) | 43.9 (19.0) | 26.8 (18.3) | 30.9 (21.6) |
| Stiffness* | 47.0 (17.6) | 50.9 (21.1) | 33.0 (21.4) | 42.8 (24.0) |
| Disability* | 42.5 (18.6) | 46.8 (22.2) | 27.9 (19.0) | 33.3 (21.8) |
| The mean (standard deviation) change among included studies in outcomes scores between baseline and post-treatment at the final collection point for hyaluronic acid and placebo. An increase in outcome score represents improvement. | ||||
| * Normalized to a maximal score of 100 possible points | ||||
FIGURE 2
Comparing effect sizes of hyaluronic acid therapy and saline placebo
Across studies, effect sizes were similar for hyaluronic acid therapy and placebo.
Discussion: Benefits are limited
Based on this meta-analysis, we cannot conclude that hyaluronic acid performs better than saline placebo for a reduction of pain or disability on the WOMAC. Some indication may be warranted for reduction in stiffness. It is notable that hyaluronic acid injection and saline placebo groups both experienced an improvement in pain, stiffness, and disability scores on the WOMAC.
We also cannot conclude that hyaluronic acid enhances functionality as evaluated on the Lequesne Index past 6 months post-treatment. In 2 studies,7,19 patients with placebo actually showed reduced disability compared with patients in the hyaluronic acid group. While a short-term improvement in functionality was observed in other studies, this effect was not seen in follow-up reports greater than 6 months.
Comparisons with other meta-analyses
A recently published meta-analysis by Modawal et al20 also pooled data to compare hyaluronic acid treatment to saline placebo. That meta-analysis was similar to ours in that it had relatively small sample size (n=11) and examined self-reported patient outcomes as the item of interest. The difference between this meta-analysis and ours is that the length of the evaluation periods for the other analysis was fairly short; the longest follow-up was 26 weeks. Our study examined the pooled effects of hyaluronic acid carried out to at least 24 weeks and up to 52 weeks. Their results for the short-term outcomes demonstrated significant decrease in pain in the active treatment group; however, these results diminished considerably when controlled for study quality.
Our meta-analysis was not consistent with a previous meta-analysis of hyaluronic acid treatment with regard to pain relief and reduction of disability. This study15 revealed that hyaluronic acid has a therapeutic effect on osteoarthritis of the knee, specifically decreasing pain and disability. The analysis included 20 studies, but differed from ours in that it failed to control for between-group heterogeneity and used an original formula to derive mean differences in efficacy scores between hyaluronic acid and placebo. Each study in our meta-analysis used an identical method for reporting outcome scores. Our study also synthesized the original data values as reported in each original study.
In addition, our results have to be interpreted with attention to a large effect of saline injection, which were much greater than the expected effect of placebo. Calculated effect sizes demonstrated similar effects for both treatment and placebo groups, with no clustering that was different between groups ( FIGURE 2 ). Treatment and placebo groups showed equal response when evaluated for greatest effect.
Refocusing research
The effects of hyaluronic acid injection appear to be transient and offer only slight improvement for older patients with mild to advanced osteoarthritis. Attention should be given to other groups, including younger patients and patients with mild degenerative changes. These studies should attempt to better stratify patient groups to identify those who are most likely to benefit from intervention. In addition, there must be better reporting of treatment protocols and results will allow clinicians to make rational decisions regarding treatments.
Cost and side effects
Consider the degree of benefit and costs when making treatment decisions. The average treatment cost was approximately $900 in 2003.21 Obviously one issue is whether a patient’s insurance provider will pay for the treatment.
Side effects are uncommon, and complications with hyaluronic acid injections have been relatively mild.
A few cases of hyaluronic acid allergy have been reported,22 including sweating, paleness, feelings of pressure in the chest and stomach, skin turning blue, or a decrease in blood pressure.22
Acknowledgments
The authors would like to thank Esra Akdeniz from the Statistical Consulting Center at the Pennsylvania State University for assistance with the methods for performing statistical meta-analysis.
CORRESPONDENCE
Jennifer M. Medina, 146 Recreation Building, University Park, PA 16802. E-mail: [email protected]
- Hyaluronic acid (HA) injection may provide short-term relief of pain and improved functionality for patients with osteoarthritis of the knee, but benefits do not last beyond 6 months.
- Examine the HA option from a cost-benefit perspective on a case-by-case basis. Based on our meta-analysis, there is no sufficient reason to recommend or not recommend HA injection for treatment of osteoarthritis of the knee.
- You may want to help select patients weigh the possible benefit of HA therapy against its cost.
Though hyaluronic acid may reduce symptoms related to osteoarthritis of the knee, the relatively small and transient response in the population studied in our analysis does not provide sufficient reason to recommend or not recommend this therapy.
Those who might want to opt for hyaluronic acid injections
With the relatively low risk of complications, some patients may still opt to try hyaluronic acid injections as opposed to other osteoarthritis management strategies. Potential candidates include those whose only other option is surgery, in the hope that HA injection might postpone having to make that decision. Also, those whose pain or stiffness have not been relieved with other therapies might want to consider HA.
Two instruments for assessing osteoarthritis
There are many claims to the efficacy of hyaluronic acid injections for decreasing the pain associated with osteoarthritis of the knee. This meta-analysis was an attempt to collapse the data for hyaluronic acid treatment, using a reduction in score on the Western Ontario McMaster Universities Index (WOMAC) or the Lequesne index as its outcome measurement.
The WOMAC is a disease-specific, self-administered instrument for patients with osteoarthritis of the knee or hip. It has 3 separate dimensions (with 24 individual scenarios), measuring pain (5 scenarios), stiffness (2 scenarios), and physical function (17 scenarios). It may be administered using a 100 mm/10 cm visual analog scale (VAS) (where 0=none, 100 or 10=extreme) or a Likert scale (0 to 4, where 0=none, 4=extreme). These results are then scored on a 0 to 20 scale for pain, a 0 to 8 scale for stiffness, and a 0 to 68 scale for physical function. Lower scores for both scales indicate a lesser degree of pain, stiffness, or physical dysfunction. In a double-blind, randomized controlled trial, WOMAC was found to be a valid and reliable tool for determining self-reported status for osteoarthritis of the knee or hip.12
Osteoarthritis is a degenerative, debilitating disease that affects approximately 20.7 million adults in the United States.1 It is the degeneration of the articular cartilage at synovial joints. There seems to be a genetic predisposition to developing osteoarthritis, although most people tend to experience some pain in their joints as they age, usually starting in their 30s or 40s.2 Some elite athletes have even been reported as having arthritic changes in their 20s.3 Unfortunately, many factors may contribute to the pathological state of each person affected, making each case unique. It can be the result of general wear and tear at the joint, structural malalignments, or injury.4 With the surge in the elderly population, a more effective management strategy for osteoarthritis will improve quality of life and reduce health costs for many.
Hyaluronic acid, a normal component of synovial joints, the linear repeating polysaccharide that forms the central axis of proteoglycan aggregates, which are necessary for functional integrity of the articular cartilage.5 It is involved in joint lubrication and nutrition. Native hyaluronic acid increases the viscosity of the extracellular matrix, thereby increasing the load-dispersion properties of the articular cartilage. Arthritic articular cartilage tends to have a decreased concentration of naturally occurring hyaluronic acid.
Unfortunately, injection of hyaluronic acid does not appear to restore the properties that native hyaluronic acid provides to the articular cartilage. A treatment series of intra-articular injections of hyaluronic acid has been reported to decrease the pain associated with osteoarthritis and provide patient relief.6 It has also been reported that residual benefits may last for months after the last injection.7-9 One of the first reported trials of hyaluronic acid injections for treatment of osteoarthritis came in 1974.10 Subsequently, in 1982, a report was published on the therapeutic effect as a result of hyaluronic acid injection into the knee.11 By 1991, some of the first randomized, controlled trials of hyaluronic acid were reported.
Several different chemical compositions of hyaluronic acid are used for the treatment. Though similar, dosage depends on the specific chemical properties of each. A usual dosage is 3 to 5 injections, with the patient receiving 1 injection per week.
The Lequesne index is a 10-question interview-style survey given to patients with osteoarthritis of the knee. It has 5 questions pertaining to pain or discomfort, 1 question dealing with maximum distance walked, and 4 questions about activities of daily living. The total questionnaire is scored on a 0 to 24 scale, with lower scores meaning less functional impairment. A study by Faucher et al13 found the Lequesne index to be a reliable questionnaire.
Methods
Selection of studies
Two researchers (JM and AT) performed a computerized literature search of PubMed (1950–2004), CINAHL (1982–2004), and Medline (1966–2004) to identify citations concerning the efficacy of hyaluronic acid injection for management of osteoarthritis of the knee. Four separate searches were conducted. The first used the terms knee, osteoarthritis, WOMAC, and hyaluronic acid. The second used the same terms as the first, replacing WOMAC with Lequesne. The third and fourth searched WOMAC and validity and Lequesne and validity, respectively. All 4 searches were limited to human randomized clinical trials, in English-language journal reports. A hand search of the reference lists of all retrieved studies was performed to ensure that no eligible studies were excluded.
Studies were selected independently by the same 2 researchers. The search was performed independently to ensure an exhaustive review of the literature. All studies were considered eligible until disqualified based on exclusion criteria.
Studies were eligible for inclusion if they addressed hyaluronic acid injection for osteoarthritis at the knee and used the WOMAC or Lequesne indexes as outcomes measurements. It was also necessary that they provided means and standard deviations in order to perform statistical analysis. We attempted to contact authors who did not provide necessary statistics for meta-analysis; however, we received no responses.
Assessment of methodological quality and data abstraction
The methodological quality of each study was assessed independently by the reviewers using the Physiological Evidence Database (PEDro) rating scale.14 It was determined that studies must include a control group that used placebo saline injections, provide means and standard deviations at baseline for the WOMAC or Lequesne, and also means and standard deviations after the intervention for both the treatment and control groups.
All abstracted data were converted into a percentage of the total possible score for each outcome measurement using a method described by a statistical consultant and an algorithm developed by one investigator (JM) using MATLAB 7.0 (MathWorks, Inc, Natick, Mass). This allowed for comparison between results of the WOMAC and Lequesne studies. This also allowed for comparison to the results of the Wang et al15 meta-analysis.
Statistical analysis
Homogeneity of the included studies, as determined by inclusion and exclusion criteria, allowed for meta-analysis. One meta-analysis was performed on the 3 studies that used the WOMAC scale,16-18 2 on the 4 studies that used the Lequesne index.7-9,19 Most studies that used the Lequesne index reported outcomes for a variety of follow-up points (4, 5, 8, 20, 24, 26, 49, 52 weeks); however, there was not a consistent reporting timeline among these studies. We grouped the follow-up reports into “up to 6 months post-treatment” and “6 months or greater post-treatment.” The first Lequesne analysis evaluated the difference between treatment and placebo groups at with data points less than 6 months post-treatment. The second Lequesne analysis examined the difference between groups at 6 months and greater post-treatment. In the study by Karlsson,7 2 different but similar types of hyaluronic acid were used. These groups were separated and analyzed as independent studies; 95% confidence intervals were calculated for all analyses. Effect sizes were also calculated to determine the between-group effect of the treatment and placebo.
Results
Selection of studies
The literature search resulted in 35 potentially eligible studies, of which 7 were selected after application of the inclusion criteria.7-9,16-19 Three of the selected studies used the WOMAC as an outcome assessment tool,16-18 4 used the Lequesne index.7-9,19 The remaining 28 studies were excluded. TABLE W1 details the rejection of studies.
Methodological quality and study characteristics
Each investigator then independently scored the methodological quality for the remaining studies by using the PEDro scale.14 There was total agreement for all studies in regards to the PEDro score. No study received a score under 5. The average PEDro rating among the studies was 8.2 out of 10.
Homogeneity
All groups across all included studies were similar at baseline. All patients had mild to severe osteoarthritis of the knee. Mean ages of the groups were similar (treatment groups=67.1 years, placebo=66.7 years). Not all patients received treatment as per manufacturer’s recommendations ( TABLES 1 AND 2 ). A saline placebo was used for control groups in all studies. The volume of saline placebo for each study was equal to volume of hyaluronic acid injection plus saline vehicle used for that study. All studies used either the WOMAC scale or the Lequesne Functional Index as an outcome measurement tool. All studies included a follow-up outcome measurement; however, the range of follow-up time periods was wide (4 to 52 weeks).
Meta-analysis
The analysis for the outcome measurements on the WOMAC scale revealed no significant difference between groups in regards to pain or disability (95% confidence interval [CI] for pain, –0.6043 to 5.4755; for disability, –0.8282 to 4.8619). The outcome measurement for stiffness demonstrated a significant difference between treatment and control (95% CI for stiffness, 2.1780 to 8.7955).
The analysis for the outcome measurement on the Lequesne index revealed a significant difference between groups for measurements taken up to 6 months post-treatment (95% CI, 1.2315 to 2.6268). However, these differences were not seen in the 6+ month outcomes analysis (95% CI, –.8489 to .04787]. Confidence intervals are presented in FIGURE 1 . Mean differences for each outcome at baseline and post-treatment between the active treatment and placebo are presented in TABLE 3 .
TABLE 1
Hyaluronic acid: Manufacturer’s recommendations
| HA BRAND | ARTZAL | SYNVISC | HYALGAN | DUROLANE | SUPLASYN | NRD101 |
|---|---|---|---|---|---|---|
| Number of injections (given 1 week apart) | 5 | 3 | 5 | 1 | 3 | N/A |
| Concomitant local anesthetic injection recommended | Yes | No* | Yes | NR | N/A | N/A |
| Aspiration recommended prior to injection | Yes | Yes | Yes | Yes | No | N/A |
| Dosage per injection (mg) | 25.0 | 32.0 | 40.0 | 60.0 | 20.0 | 25.0 |
| Molecular weight (kDa) | 620–1170 | 6000 | 500–730 | N/A | N/A | 1900 |
| Cross-linked | No | Yes | No | Yes | No | N/A |
| HA, hyaluronic acid; NR, not reported; N/A, not available. | ||||||
| * Purported to alter the efficacy of Synvisc. | ||||||
TABLE 2
Details of included studies
| STUDY | KARLSSON ET AL7 | DOUGADOS ET AL8 | HUSKISSON9 | DAY ET AL16 | ALTMAN ET AL17 | PETRELLA ET AL18 | PHAM ET AL19 | |
|---|---|---|---|---|---|---|---|---|
| Brand | Artzal | Synvisc | Hyalgan | Hyalgan | Artzal | Durolane | Suplasyn | NRD101 |
| Number of injections | ||||||||
| given 1 wk apart | 3 | 3 | 4 | 5 | 5 | 1 | 3 | 3* |
| Mfr’s recommendations | 5 | 3 | 5 | 5 | 5 | 1 | 3 | NA |
| Concomitant local anaesthetic injection | NR | NR | NR | NR | Yes | NR | NR | Yes |
| Aspiration prior to injection | NR | NR | Yes | NR | Yes | NR | NR | Yes |
| Dosage per injection (mg) + vehicle† | 25/2.5 mL saline | 32/2.5 mL saline | 20/2.0 mL saline | 20/2.0 mL saline | 25/2.5 mL saline | 60/3.0 mL saline | 20/2.0 mL saline | 25/2.5 mL saline |
| Cross-linked | No | Yes | No | No | No | Yes | NR | NR |
| NR=not reported | ||||||||
| * Given as 3 courses every 3 months of 3 weekly injections | ||||||||
| † Volume of saline placebo for each study equal to volume of hyaluronic acid injection plus saline vehicle used for that study | ||||||||
FIGURE 1
Hyaluronic acid vs control: 95% confidence intervals
Confidence intervals that cross the 0-value represent a nonsignificant difference between active treatment and control. Also included is a result from the Wang et al meta-analysis15 for comparison.
TABLE 3
Mean improvements: treatment vs. control
| BASELINE | POST-TREATMENT | |||
|---|---|---|---|---|
| HA | PLACEBO | HA | PLACEBO | |
| LEQUESNE | 12.7 (3.0) | 12.44 (2.7) | 8.7 (4.4) | 8.91 (4.4) |
| % Lequesne* | 53.0 (12.8) | 51.83 (11.2) | 36.3 (18.5) | 37.1 (18.1) |
| WOMAC | ||||
| Pain* | 40.5 (17.0) | 43.9 (19.0) | 26.8 (18.3) | 30.9 (21.6) |
| Stiffness* | 47.0 (17.6) | 50.9 (21.1) | 33.0 (21.4) | 42.8 (24.0) |
| Disability* | 42.5 (18.6) | 46.8 (22.2) | 27.9 (19.0) | 33.3 (21.8) |
| The mean (standard deviation) change among included studies in outcomes scores between baseline and post-treatment at the final collection point for hyaluronic acid and placebo. An increase in outcome score represents improvement. | ||||
| * Normalized to a maximal score of 100 possible points | ||||
FIGURE 2
Comparing effect sizes of hyaluronic acid therapy and saline placebo
Across studies, effect sizes were similar for hyaluronic acid therapy and placebo.
Discussion: Benefits are limited
Based on this meta-analysis, we cannot conclude that hyaluronic acid performs better than saline placebo for a reduction of pain or disability on the WOMAC. Some indication may be warranted for reduction in stiffness. It is notable that hyaluronic acid injection and saline placebo groups both experienced an improvement in pain, stiffness, and disability scores on the WOMAC.
We also cannot conclude that hyaluronic acid enhances functionality as evaluated on the Lequesne Index past 6 months post-treatment. In 2 studies,7,19 patients with placebo actually showed reduced disability compared with patients in the hyaluronic acid group. While a short-term improvement in functionality was observed in other studies, this effect was not seen in follow-up reports greater than 6 months.
Comparisons with other meta-analyses
A recently published meta-analysis by Modawal et al20 also pooled data to compare hyaluronic acid treatment to saline placebo. That meta-analysis was similar to ours in that it had relatively small sample size (n=11) and examined self-reported patient outcomes as the item of interest. The difference between this meta-analysis and ours is that the length of the evaluation periods for the other analysis was fairly short; the longest follow-up was 26 weeks. Our study examined the pooled effects of hyaluronic acid carried out to at least 24 weeks and up to 52 weeks. Their results for the short-term outcomes demonstrated significant decrease in pain in the active treatment group; however, these results diminished considerably when controlled for study quality.
Our meta-analysis was not consistent with a previous meta-analysis of hyaluronic acid treatment with regard to pain relief and reduction of disability. This study15 revealed that hyaluronic acid has a therapeutic effect on osteoarthritis of the knee, specifically decreasing pain and disability. The analysis included 20 studies, but differed from ours in that it failed to control for between-group heterogeneity and used an original formula to derive mean differences in efficacy scores between hyaluronic acid and placebo. Each study in our meta-analysis used an identical method for reporting outcome scores. Our study also synthesized the original data values as reported in each original study.
In addition, our results have to be interpreted with attention to a large effect of saline injection, which were much greater than the expected effect of placebo. Calculated effect sizes demonstrated similar effects for both treatment and placebo groups, with no clustering that was different between groups ( FIGURE 2 ). Treatment and placebo groups showed equal response when evaluated for greatest effect.
Refocusing research
The effects of hyaluronic acid injection appear to be transient and offer only slight improvement for older patients with mild to advanced osteoarthritis. Attention should be given to other groups, including younger patients and patients with mild degenerative changes. These studies should attempt to better stratify patient groups to identify those who are most likely to benefit from intervention. In addition, there must be better reporting of treatment protocols and results will allow clinicians to make rational decisions regarding treatments.
Cost and side effects
Consider the degree of benefit and costs when making treatment decisions. The average treatment cost was approximately $900 in 2003.21 Obviously one issue is whether a patient’s insurance provider will pay for the treatment.
Side effects are uncommon, and complications with hyaluronic acid injections have been relatively mild.
A few cases of hyaluronic acid allergy have been reported,22 including sweating, paleness, feelings of pressure in the chest and stomach, skin turning blue, or a decrease in blood pressure.22
Acknowledgments
The authors would like to thank Esra Akdeniz from the Statistical Consulting Center at the Pennsylvania State University for assistance with the methods for performing statistical meta-analysis.
CORRESPONDENCE
Jennifer M. Medina, 146 Recreation Building, University Park, PA 16802. E-mail: [email protected]
1. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998;41:778-799.
2. Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol 2006;18:147-156.
3. Neyret P, Donell ST, Dejour H. Results of partial meniscectomy related to the state of the anterior cruciate ligament. Review at 20 to 35 years. J Bone Joint Surg Br 1993;75:36-40.
4. Moskowitz RW, Kelly MA, Lewallen DG. Understanding osteoarthritis of the knee-causes and effects. Am J Orthop 2004;33:5-9.
5. Walsh DA, Sledge CB, Blanke DR. Biology of the normal joint. In: Kelley WN, Ruddy W, Harris ED Jr, Sledge CB, eds. Textbook of Rheumatology. Vol 1. 5th ed. Philadelphia, PA: W.B. Saunders Company;1997:1-26.
6. Leardini G, Mattara L, Franceschini M, Perbellini A. Intra-articular treatment of knee osteoarthritis. A comparative study between hyaluronic acid and –methyl prednisolone acetate. Clin Exp Rheumatol 1991;9:375-381.
7. Karlsson J, Sjogren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology. 2002;41:1240-1248.
8. Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1 year placebo-controlled trial. Osteoarthritis Cartilage 1993;1:97-103.
9. Huskisson EC, Donnelly S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology 1999;38:602-607.
10. Peyron JG, Balazs EA. Preliminary clinical assessment of Na-hyaluronate injection into human arthritic joints. PatholBiol (Paris) 1974;22:731-736.
11. Namiki O, Toyoshima H, Morisaki N. Therapeutic effect of intra-articular injection of high molecular weight hyaluronic acid on osteoarthritis of the knee. Int J Clin Pharmacol Ther Toxicol 1982;20:501-507.
12. Bellamy N, Buchanan W, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1999;15:1833-1840.
13. Faucher M, Poiraudeau S, Lefevre-Colau MM, Rannou F, Fermanian F, Revel M. Algo-functional assessment of knee osteoarthritis: comparison of the test-retest reliability and construct validity of the WOMAC and Lequesne indexes. Osteoarth Cartil 2002;10:602-610.
14. PEDro Scale. Physiotherapy Evidence Database Web site. Available at: www.pedro.fhs.usyd.edu.au/scale_item.html. Accessed on February 10, 2005.
15. Wang C, Lin J, Chang C, Lin Y, Hou S. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. J Bone Joint Surg 2004;86A:538-545.
16. Day R, Brooks P, Conaghan PG, Petersen M. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intra-articular hyaluronan in osteoarthritis of the knee. J Rheumatol 2004;31:775-782.
17. Altman RD, Akermark C, Beaulieu AD, Schnitzer T. Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartilage 2004;12:642-649.
18. Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning on osteoarthritis of the knee. Arch Intern Med 2002;162:292-298.
19. Pham T, Le Henanff A, Ravaud P, Dieppe P, Paolozzi L, Dougados M. Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomized controlled study in symptomatic knee osteoarthritis. Ann Rhem Dis 2004;63:1611-1617.
20. Modawal A, Ferrer M, Choi HK, Castle JA. Hyaluronic acid injections relieve knee pain. J Fam Pract 2005;54:758-677.
21. Kahan A, Lleu P, Salin L. Prospective randomized study comparing the medicoeconomic benefits of Hylan GF-20 vs. conventional treatment in knee osteoarthritis. Joint Bone Spine 2003;70:276-281.
22. Brandt KD, Block JA, Michalski JP, Moreland LW, Caldwell JR, Lavin PT. Efficacy and safety of intra-articular sodium hyaluronate in knee osteoarthritis. Clin Orthop 2001;385:130-143.
1. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998;41:778-799.
2. Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol 2006;18:147-156.
3. Neyret P, Donell ST, Dejour H. Results of partial meniscectomy related to the state of the anterior cruciate ligament. Review at 20 to 35 years. J Bone Joint Surg Br 1993;75:36-40.
4. Moskowitz RW, Kelly MA, Lewallen DG. Understanding osteoarthritis of the knee-causes and effects. Am J Orthop 2004;33:5-9.
5. Walsh DA, Sledge CB, Blanke DR. Biology of the normal joint. In: Kelley WN, Ruddy W, Harris ED Jr, Sledge CB, eds. Textbook of Rheumatology. Vol 1. 5th ed. Philadelphia, PA: W.B. Saunders Company;1997:1-26.
6. Leardini G, Mattara L, Franceschini M, Perbellini A. Intra-articular treatment of knee osteoarthritis. A comparative study between hyaluronic acid and –methyl prednisolone acetate. Clin Exp Rheumatol 1991;9:375-381.
7. Karlsson J, Sjogren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology. 2002;41:1240-1248.
8. Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1 year placebo-controlled trial. Osteoarthritis Cartilage 1993;1:97-103.
9. Huskisson EC, Donnelly S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology 1999;38:602-607.
10. Peyron JG, Balazs EA. Preliminary clinical assessment of Na-hyaluronate injection into human arthritic joints. PatholBiol (Paris) 1974;22:731-736.
11. Namiki O, Toyoshima H, Morisaki N. Therapeutic effect of intra-articular injection of high molecular weight hyaluronic acid on osteoarthritis of the knee. Int J Clin Pharmacol Ther Toxicol 1982;20:501-507.
12. Bellamy N, Buchanan W, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1999;15:1833-1840.
13. Faucher M, Poiraudeau S, Lefevre-Colau MM, Rannou F, Fermanian F, Revel M. Algo-functional assessment of knee osteoarthritis: comparison of the test-retest reliability and construct validity of the WOMAC and Lequesne indexes. Osteoarth Cartil 2002;10:602-610.
14. PEDro Scale. Physiotherapy Evidence Database Web site. Available at: www.pedro.fhs.usyd.edu.au/scale_item.html. Accessed on February 10, 2005.
15. Wang C, Lin J, Chang C, Lin Y, Hou S. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. J Bone Joint Surg 2004;86A:538-545.
16. Day R, Brooks P, Conaghan PG, Petersen M. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intra-articular hyaluronan in osteoarthritis of the knee. J Rheumatol 2004;31:775-782.
17. Altman RD, Akermark C, Beaulieu AD, Schnitzer T. Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartilage 2004;12:642-649.
18. Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning on osteoarthritis of the knee. Arch Intern Med 2002;162:292-298.
19. Pham T, Le Henanff A, Ravaud P, Dieppe P, Paolozzi L, Dougados M. Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomized controlled study in symptomatic knee osteoarthritis. Ann Rhem Dis 2004;63:1611-1617.
20. Modawal A, Ferrer M, Choi HK, Castle JA. Hyaluronic acid injections relieve knee pain. J Fam Pract 2005;54:758-677.
21. Kahan A, Lleu P, Salin L. Prospective randomized study comparing the medicoeconomic benefits of Hylan GF-20 vs. conventional treatment in knee osteoarthritis. Joint Bone Spine 2003;70:276-281.
22. Brandt KD, Block JA, Michalski JP, Moreland LW, Caldwell JR, Lavin PT. Efficacy and safety of intra-articular sodium hyaluronate in knee osteoarthritis. Clin Orthop 2001;385:130-143.