User login
Preconception counseling: Make it part of the annual exam
- Supplementing women’s diets with 400 mg folic acid every day reduces the incidence of neural tube defects in their offspring by up to 72% (A).
- Optimizing diabetic glucose control prior to conception is linked to a reduction in birth defects and pregnancy loss (B).
- Limiting caffeine consumption to no more than 200 mg per day may reduce the risk of miscarriage (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
In the United States most women do not seek out prenatal care until the 8th to 12th week of pregnancy, when the crucial period of organogenesis (4 to 10 weeks after fertilization) has already passed. In addition, women whose pregnancies are unintended—up to half of all pregnancies—may delay seeking care even longer.1 Given these realities, family physicians should consider all visits during the reproductive years—especially yearly exams—as opportunities for preconception counseling.
Preconception health care is essential to the health of our nation. Data from the Centers for Disease Control and Prevention (CDC) are cause for concern:2,3
- 12% of infants are born prematurely.
- 31% of pregnancies are complicated by maternal health issues.
- 11% of women smoke during pregnancy.
- 10% drink alcohol during pregnancy.
- 69% of women do not take folate supplements.
- 31% of women are obese.
- 3% of women take medications and supplements that are known teratogens.
The Healthy People 2000 initiative set a goal of 60% of primary caregivers providing preconception care at routine medical visits, but thus far, only about 25% do so.2,3
As a primary care provider, you can have a huge impact on fetal and maternal health by counseling women to choose healthier lifestyles, helping them to manage their chronic conditions, updating their immunizations, and screening for genetic disorders before they become pregnant.
Start with the hard part: Lifestyle modification
Changing the way patients go about their lives—how they eat, how much they exercise, whether they use alcohol or tobacco—has 2 salient characteristics: It’s the most difficult thing to get patients to do, and it has the biggest payoff in improving maternal and fetal health. Lifestyle issues of greatest significance for the preconception patient include:
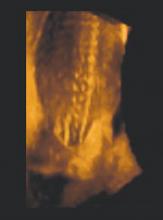
Folic acid supplementation. If your patients are like most women, they may not be aware of the importance of folic acid supplementation. Yet by neglecting to supplement their diet with folates, women are passing up an opportunity to reduce the incidence of neural tube defects (NTDs) such as anencephaly and spina bifida by up to 70%.4
4D ultrasound of an open neural tube defect in a developing fetus
Women considering conception or those who do not use contraception should take 400 mg folic acid, a dosage found in most prenatal vitamins, every day. All women of reproductive age should consider folate supplementation because of the high rate of unplanned pregnancies. Women who have previously had a child with an NTD and women who take anti-epileptic drugs should take 4 mg folate per day.5
Use routine office visits as an opportunity to counsel patients about folic acid supplementation, whether via prenatal vitamins or, if a higher dosage is necessary, by prescription. One group has reported that preconception counseling increases folate use in women planning pregnancy.6
Safer sex counseling. Counseling patients about safer sexual practices may reduce the incidence of human immunodeficiency virus (HIV), herpes, gonorrhea, chlamydia, and syphilis—conditions that may increase the incidence of preterm delivery, fetal malformations, neonatal infection, or developmental abnormalities.3
Identification of patients with HIV is essential, as early treatment with zidovudine (azidothymidine [AZT], Retrovir), reduces the risk of vertical transmission from mother to neonate by up to 70%.7
Infection prevention. Infections with the potential to harm the fetus include parvovirus B-19, cytomegalovirus, toxoplasmosis, and hepatitis B. Vaccinations are available for hepatitis B but not for the others, so it is particularly important to warn patients about avoiding exposure.
Women who work in child care, for example, should avoid direct contact with children who have parvovirus infection (“Fifth disease”) or other viral exanthems. Health care workers should use universal precautions at all times, and all pregnant women should avoid direct exposure to cat feces and consumption of uncooked meats.
Weight control. Obesity is an increasingly serious health problem in the United States. Obesity poses significant risks for pregnant women and their fetuses, including NTDs, diabetes, venous thromboembolism (VTE), premature labor or cesarean delivery, preeclampsia, and macrosomia.8,9 The time to start a program of exercise and weight loss is before conception, because some of the adverse effects of obesity can occur during the first few months of pregnancy.
Diet modification. Many patients considering conception ask about foods that may be unsafe during pregnancy. Like most clinicians, you may want to advise patients to avoid soft cheeses because of the risk of Listeria infection, and not to eat raw or undercooked meats because of the risk of toxoplasmosis.
Women considering conception should not eat fish high in methylmercury, which can affect the neurologic development of a fetus. These include swordfish, tilefish, king mackerel, and shark. Fish with lower levels of methylmercury are shrimp, canned light tuna, pollock, catfish, and salmon.
Women may eat 12 ounces (or 2 average meals) of these safer fish each week. Methylmercury exposure is cumulative; in women with initially high levels, it can take as long as a year after reducing consumption of fish high in methylmercury for levels to return to normal.10 An Environmental Protection Agency fact sheet for clinicians and patients is available at http://www.epa.gov/waterscience/fish/advice/factsheet.html.
Hot tubs and spas. Maternal hyperthermia (core temperature greater than 100.4°F) during the first trimester is associated with an increased risk of NTDs, so tell women who are pregnant or trying to conceive to stay out of hot tubs and heated spas.11
Environmental toxins. Pregnant women should avoid solvents, paint thinners, heavy metals, pesticides, ionizing radiation (unless indicated for necessary health care), alcohol, illicit drugs, and cigarette smoke.
Smoking cessation. Smoking even less than 1 pack a day can be very harmful to the developing fetus.12,13 Smoking increases the risks of miscarriage, stillbirth, and other pregnancy complications, and is also associated with increased neonatal mortality and sudden infant death syndrome. About 11% of pregnant women smoke.14
As a primary care physician, you should use every opportunity to help smokers considering pregnancy to quit. Proven methods of smoking cessation include counseling, medications such as bupropion, and over-the-counter smoking cessation aids, such as nicotine replacement gum and lozenges.15
One intervention shown to be particularly useful for women who smoke fewer than 20 cigarettes a day is the “5 As” method (Ask, Advise, Assess, Assist, and Arrange).16 A review of studies on the effects of smoking during pregnancy that includes cessation interventions such as the 5 As method is available in the American College of Obstetricians and Gynecologists Committee Opinion No. 316.15
Substance abuse. Fetal alcohol spectrum disorders (FASDs) are among the most preventable congenital defects and developmental disabilities. Ask patients trying to conceive about their patterns of alcohol use, and tell them there is no known safe amount of alcohol intake during pregnancy.
The US Preventive Services Task Force recommends screening pregnant patients with either the TWEAK or T-ACE instruments, because these tests can detect relatively low levels of alcohol consumption that may still harm a developing fetus.17 All women contemplating pregnancy need to know that exposure to alcohol can cause FASDs, congenital malformations, intrauterine growth restriction, and miscarriages. Problem drinkers should be referred for treatment.
Illegal drugs also pose significant risks to fetal development. Damage to the placenta caused by cocaine, for example, can lead to abruption, miscarriage, growth restriction, and prematurity. Consider screening all patients for illegal drug use and referring for counseling or methadone management, as indicated.
Caffeine. Recent studies have linked excessive caffeine intake (>200 mg/d) with miscarriages during the first trimester (adjusted hazards ratio=2.23). To reduce the risk of miscarriage, counsel pregnant women to eliminate caffeine or to cut back to less than 200 mg/d.18 Amounts of caffeine in various beverages are listed in TABLE 1 .
TABLE 1
How much caffeine is your patient drinking?30,31
| BEVERAGE | SERVING SIZE (OZ) | CAFFEINE CONTENT (MG) |
|---|---|---|
| Decaffeinated coffee | 8 | 2 |
| Caffeinated coffees | ||
| Starbucks Grande Coffee | 16 | 330 |
| Starbucks Caffe Latte | 16 | 150 |
| Plain, brewed coffee | 8 | 95 |
| Espresso | 1 | 64 |
| Teas | ||
| Decaffeinated tea | 8 | 2 |
| Black tea, brewed | 8 | 47 |
| Snapple iced tea | 16 | 18 |
| Caffeinated soft drinks | ||
| Diet Mountain Dew | 12 | 55 |
| Diet Coke | 12 | 46 |
| Diet Pepsi | 12 | 37 |
| Sam’s Diet Cola | 12 | 13 |
| Energy drinks | ||
| SoBe Adrenaline Rush | 16 | 152 |
| Red Bull | 8.3 | 76 |
Avert trouble: Manage chronic conditions now
A number of maternal health conditions have a potential for adverse consequences to the fetus, but optimizing the mother’s condition before and during pregnancy can often avert problems. Maternal disorders to monitor include:
Diabetes mellitus. Improving glycemic control prior to conception is linked to a 3-fold decrease in the prevalence of birth defects.3 Patients entering pregnancy with hemoglobin A1C levels less than 8.5% have a fetal anomaly rate of 3.4%, whereas women with a hemoglobin A1C of more than 8.5% have an anomaly rate of 22.4%.19
According to the American Association of Clinical Endocrinologists, goals for glucose control during pregnancy include a hemoglobin A1C of less than 6% and blood glucose concentrations of between 60 mg/dL fasting and 120 mg/dL 1 hour after a meal. Achieving these levels may require tighter control than patients are accustomed to. Blood pressure for these patients should not exceed 130/80 mm Hg.
The use of oral hypoglycemic medications during pregnancy is somewhat controversial. For that reason, you may want to refer these patients to an endocrinologist or other expert in diabetes management during pregnancy for consideration of scheduled insulin injections or an insulin pump.20
Patients with diabetes should be screened for retinal disease, renal disease, hypertension, and hyperlipidemia prior to conception, including a 24-hour urine collection for protein and creatinine clearance. Because women with type 1 diabetes have up to a 40% incidence of thyroid dysfunction, consider screening for thyroid disorders as well.21
While counseling patients with diabetes, keep in mind that a blood urea nitrogen concentration of more than 30 mg/dL, current coronary artery disease, and creatinine clearance of less than 30 mL/min are considered contraindications for pregnancy.20 All women who have had diabetes for more than 10 years should have an electrocardiogram (EKG).
Hypothyroidism. Poorly controlled hypothyroidism may cause developmental, growth, and neurologic abnormalities. Patients with thyroid abnormalities should have their medication dosage optimized before they conceive.
Epilepsy. Seizure disorders generally do not worsen during pregnancy, but several antiseizure medications have a potential for harming the fetus ( TABLE 2 ). Counsel patients about the increased risk of congenital anomalies (4%-8%) in neonates born to women with seizure disorders, either because of the disorder itself or as a consequence of antiseizure medication.22
Patients taking anti-epileptic drugs should take 4 mg/d of folate supplementation. According to the American Academy of Neurology, best practice is to use a single agent best suited to the type of seizures the patient experiences, at the lowest effective dose. Avoid multiple anti-epileptic drugs, if possible. Do not change an effective medication regimen if the patient becomes pregnant, but do check drug levels.23 Also counsel patients about the possibility of decreased effectiveness of hormonal contraception while taking enzymeinducing (cytochrome P450) antiepileptic drugs.24
Psychiatric disorders. Approximately 1 of every 7 pregnant women meets the diagnostic criteria for depression.25 Depression, anxiety, and other psychiatric conditions can adversely impact the patient and her developing fetus if the condition is undertreated. Unfortunately, some women stop psychiatric medications when they discover they are pregnant. In 1 study of 201 pregnant women with depression, 43% had a relapse during the course of pregnancy. Patients with relapse included 68% of those who stopped medication during the pregnancy, vs 26% of those who continued taking medication.26
Most antidepressants are relatively safe during pregnancy, with the exception of paroxetine (Paxil), which is associated with fetal cardiac defects.27 When a patient taking psychiatric medications comes in for a preconception visit, consider higher doses of 1 psychotropic medication rather than lower doses of multiple medications. This will decrease fetal medication exposure. If a patient on a stable regimen becomes pregnant, do not switch medications. Always collaborate with the patient’s mental health care team.
Venous thromboembolic disease. Some inherited thrombophilias can lead to a VTE during pregnancy or the postpartum period. Consider testing patients who have had a VTE or have a family history of VTE for anticardiolipin antibodies, protein S deficiency, and other thrombophilias before conception, because some of the lab values that indicate these conditions can change during pregnancy.
Patients with a history of a VTE related to hormone use or pregnancy will require prophylactic anticoagulation. When in doubt, refer to a hematologist or perinatologist. Both heparin and low-molecular-weight heparin are safe during pregnancy. Because warfarin is a suspected teratogen, patients taking warfarin should be converted to either heparin or low-molecular-weight heparin.
Hypertension. Hypertensive disorders may lead to pregnancy-induced hypertension, growth restriction, and renal disease. If patients are taking thiazide diuretics, angiotensin receptor blockers, or angiotensin-converting enzyme inhibitors ( TABLE 2 ), switch to medications such as methyldopa (Aldomet), nifedipine, or labetalol, which are safer during pregnancy.28 Screen patients with long-standing hypertension for cardiac disease (via EKG) and nephropathy before conception.
TABLE 2
Medications that can harm the unborn child32,33
| MEDICATION | POTENTIAL HARM TO FETUS | FDA CATEGORY* |
|---|---|---|
| Cardiovascular | ||
| ACE inhibitors: captopril, enalapril, lisinopril | Cardiovascular malformations, hypotension, anuria, oligohydramnios | C (1st trimester), D (2nd, 3rd trimesters) |
| Statins: atorvastatin, simvastatin | Polydactyly, cleft lip, club foot | X |
| Antidepressants | ||
| SSRIs: citalopram, fluoxetine, paroxetine, sertraline | Pulmonary hypertension, withdrawal syndrome; paroxetine: cardiac malformations, NTDs | C (D, paroxetine) |
| Anxiolytics | ||
| Benzodiazepines: alprazolam, clonazepam, diazepam, lorazepam | Withdrawal syndrome, congenital anomalies (various), floppy infant syndrome | D |
| Anti-epileptic drugs | ||
| Valproic acid and derivatives | NTDs, facial/cardiac defects | D |
| Carbamazepine | See valproic acid | D |
| Phenobarbital | Cardiac defects, hemorrhagic disease of the newborn | D |
| Phenytoin | Fetal hydantoin syndrome | D |
| Other | ||
| Isotretinoin | Hydrocephalus, microcephaly, limb anomalies, preterm labor, increased spontaneous abortions | X |
| Warfarin | Skeletal defects, intrauterine growth restriction, neurologic defects | X |
| Methotrexate | Fetal malformations, spontaneous abortion | X |
| ACE, angiotensin-converting enzyme; NTDs, neural tube defects; SSRIs, selective serotonin reuptake inhibitors. | ||
| *FDA categories describe the relative risk of medication use during pregnancy. The FDA is currently proposing major revisions to the system to upgrade the usefulness of this information. Please reconfirm categories prior to prescribing. | ||
| A: Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities in any trimester of pregnancy. | ||
| B: Animal studies have revealed no evidence of harm to the fetus, but no adequate and well-controlled studies in pregnant women are available OR animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester. | ||
| C: Animal studies have shown an adverse effect and there are no adequate and well-controlled studies in pregnant women OR no animal studies have been conducted and there are no adequate and well-controlled studies in pregnant women. | ||
| D: Adequate well-controlled or observational studies in pregnant women have demonstrated a risk to the fetus. However, the benefits of therapy may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective. | ||
| X: Adequate well-controlled or observational studies in animals or pregnant women have demonstrated positive evidence of fetal abnormalities or risks. The use of the product is contraindicated in women who are or may become pregnant. | ||
| FDA categories source: Meadows M. Pregnancy and the drug dilemma. FDA Consumer Magazine. May-June 2001. Available at www.fda.gov/fdac/features/2001/301_preg.html. Accessed May 6, 2009. | ||
Think ahead: Address immunization status
When a pregnant woman contracts an infectious disease, her developing fetus can be affected. Making sure the immunization status of all your reproductive-age patients is up to date will go a long way toward protecting their offspring from harm.29
Rubella. Also known as German measles, rubella can cause fetal anomalies and spontaneous abortion if contracted during the first half of pregnancy. Because the measles, mumps, and rubella (MMR) vaccine contains live attenuated viruses, susceptible patients should be immunized at least 1 month before they conceive.3
Hepatitis B. Preventing hepatitis B is an important public health issue. Screen patients at risk for hepatitis B infection—health care workers, sex workers, intravenous drug abusers, and nonmonogamous women who do not use barrier protection—with a hepatitis B surface antigen level. Vaccination is safe up to 1 month before conception.3
Varicella. Maternal varicella (chicken pox) can cause fetal harm, particularly if symptoms appear just before or during delivery. Women of reproductive age who have not already had the disease or been vaccinated should be immunized. This is a live virus vaccine and must, therefore, be administered at least 1 month before conception.3
Influenza. Administering influenza vaccine is not contraindicated during pregnancy, although most experts advise waiting until the second trimester. Certainly it is appropriate to administer the vaccine during influenza season or when risk factors such as chronic lung disease are present. Avoid live attenuated vaccine (FluMist) in pregnant women.
Tdap vaccination. The CDC’s Advisory Committee on Immunization Practices recommended in 2008 that susceptible pregnant women receive Tdap during the postpartum period to protect vulnerable infants against pertussis. Tdap is thought to be safe during pregnancy, but it would make sense to administer this vaccine when indicated prior to conception as part of a vaccination screening program.
Screen for genetic conditions prepregnancy
Part of a comprehensive preconception visit includes screening for communicable diseases and genetic conditions.
Communicable diseases. Consider screening all women prior to pregnancy for HIV infection, gonorrhea, chlamydia, hepatitis B, hepatitis C (for health care workers), and syphilis.
Diabetes. Screening guidelines for diabetes are available from the American Association of Clinical Endocrinologists.20 Consider preconception screening for patients who, during a previous pregnancy, had gestational diabetes or who delivered a baby weighing more than 9 pounds.
Genetic screening. Patients from certain ethnic groups are more susceptible to specific genetic mutations. Genetic disorders associated with particular ethnic origins are listed in TABLE 3 . Consider a preconception referral to a genetic counselor or perinatologist when the patient’s family history suggests inherited disorders.
TABLE 3
Genetic disorders: Who to screen, tests to use34
| ETHNIC ORIGIN* | DISORDER | RECOMMENDED TEST |
|---|---|---|
| Ashkenazi Jews | Tay-Sachs disease; Canavan disease | DNA panel, hexosaminidase A |
| African American | Sickle cell trait; beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| French Canadian, Cajun | Tay-Sachs disease | Hexosaminidase A |
| Mediterranean | Alpha-,† beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Indian, Middle Eastern | Sickle cell trait; alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Caucasian | Cystic fibrosis | DNA panel |
| Southeast Asian (Thai, Laotian, Cambodian) | Alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| MCV, mean corpuscular volume. | ||
| *Offer screening to any interested patient. | ||
| †Do not pursue alpha-thalassemia work-up unless patient has a history of pregnancy loss or fetal hydrops. | ||
Correspondence
D. Ashley Hill, MD, Associate Director, Department of Obstetrics and Gynecology, Loch Haven OB/Gyn Group, Florida Hospital Orlando, 235 Princeton Street, Suite 200, Orlando, FL 32804; [email protected]
1. Mayer JP. Unintended childbearing, maternal beliefs, and delay of prenatal care. Birth. 1997;24:247-252.
2. Proceedings of the Preconception Health and Health Care Clinical, Public Health, and Consumer Workgroup Meetings. Atlanta, GA: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities; 2006. Available at: http://www.cdc.gov/ncbddd/preconception/documents/Workgroup%20Proceedings%20June06.pdf. Accessed May 14, 2009.
3. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-06):1-23.Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed May 14, 2009.
4. Lumley J, Watson L, Watson M, et al. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.-
5. Iqbal MM. Prevention of neural tube defects by periconceptional use of folic acid. Pediatr Rev. 2000;21:58-66.
6. de Weerd S, Thomas CM, Cikot RJ, et al. Preconception counseling improves folate status of women planning pregnancy. Obstet Gynecol. 2002;99:45-50.
7. Mofenson LM. Centers for Disease Control and Prevention, US Public Health Service Task Force. US Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Recomm Rep. 2002;51(RR-18):1-38.
8. Robinson HE, O’Connell CM, Joseph KS, et al. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106:1357-1364.
9. Kiel DW, Dodson EA, Artal R, et al. Gestational weight gain and pregnancy outcomes in obese women: How much is enough? Obstet Gynecol. 2007;110:752-758.
10. US Food and Drug Administration. Food safety for moms-to-be. August 24, 2005. Available at: http://www.cfsan.fda.gov/~pregnant/pregnant.html. Accessed September 18, 2008.
11. Cheschier N. ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin. Neural tube defects. Number 44, July 2003. (Replaces committee opinion number 252, March 2001). Int J Gynaecol Obstet. 2003;83:123-133.
12. Castles A, Adams EK, Melvin CL, et al. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16:208-215.
13. US Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. May 27, 2004. Available at: http://www.surgeongeneral.gov/library/smokingconsequences. Accessed July 6, 2008.
14. National Center for Health Statistics. Health, United States, 2004: With Chartbook on Trends in the Health of Americans. Hyattsville, MD: NCHS; 2004. Available at: http://www.cdc.gov/nchs/data/hus/hus04.pdf. Accessed September 18, 2008.
15. ACOG Committee on Health Care for Underdeserved Women; ACOG Committee on Obstetric Practice. ACOG committee opinion. Number 316, October 2005. Smoking cessation during pregnancy. Obstet Gynecol. 2005;106:883-888.
16. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; May 2008. Available at: http://www.ahrq.gov/path/tobacco.htm. Accessed May 14, 2009.
17. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
18. Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198:279.e1-279.e8.
19. Lucas MJ, Leveno KJ, Williams ML, et al. Early pregnancy glycosylated hemoglobin, severity of diabetes, and fetal malformations. Am J Obstet Gynecol. 1989;161:426-431.
20. American Association of Clinical Endocrinologists Diabetes Mellitus Clinical Practice Guidelines Task Force. AACE diabetes mellitus guidelines. Chapter 9: diabetes and pregnancy. Endocr Pract. 2007;13(suppl 1):55-59.
21. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105:675-685.
22. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. ACOG educational bulletin. Seizure disorders in pregnancy. Number 231, December 1996. Int J Gynaecol Obstet. 1997;56:279-286.
23. American Academy of Neurology. Guideline summary for clinicians—management issues for women with epilepsy. Available at: www.aan.com/professionals/practice/pdfs/women_epilepsy.pdf. Accessed May 14, 2009.
24. Carl JS, Weaver SP, Tweed E, et al. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
25. Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799-801.
26. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment [published correction appears in JAMA. 2006;296:170]. JAMA. 2006;295:499-507.
27. FDA public health advisory: paroxetine. December 8, 2005. Available at: http://www.fda.gov/CDER/Drug/advisory/paroxetine200512.htm. Accessed May 6, 2009.
28. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Chronic hypertension in pregnancy. Obstet Gynecol. 2001;98(suppl):177-185.
29. Centers for Disease Control and Prevention. Recommended adult immunization schedule—United States, 2009. MMWR. 2009;57(53):Q-1-Q-4.Available at: http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed May 14, 2009.
30. MayoClinic.Com. How much caffeine is in your daily habit? Available at: http://www.mayoclinic.com/health/caffeine/AN01211. Accessed September 18, 2008.
31. Chou KH, Bell LH. Caffeine content of prepackaged national-brand and private-label carbonated beverages. J Food Sci. 2007;72:C337-C342.
32. Physician’s Desk Reference. Montvale, NJ: Thomson Reuters; 2009.
33. Drugs.com. Available at: http://www.drugs.com. Accessed April 26, 2009.
34. National Society of Genetic Counselors, Prenatal Special Interest Group. Ancestry Based Carrier Screening. Chicago, IL: NSGC; 2005. Available at: http://www.nsgc.org. Accessed September 18, 2008.
- Supplementing women’s diets with 400 mg folic acid every day reduces the incidence of neural tube defects in their offspring by up to 72% (A).
- Optimizing diabetic glucose control prior to conception is linked to a reduction in birth defects and pregnancy loss (B).
- Limiting caffeine consumption to no more than 200 mg per day may reduce the risk of miscarriage (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
In the United States most women do not seek out prenatal care until the 8th to 12th week of pregnancy, when the crucial period of organogenesis (4 to 10 weeks after fertilization) has already passed. In addition, women whose pregnancies are unintended—up to half of all pregnancies—may delay seeking care even longer.1 Given these realities, family physicians should consider all visits during the reproductive years—especially yearly exams—as opportunities for preconception counseling.
Preconception health care is essential to the health of our nation. Data from the Centers for Disease Control and Prevention (CDC) are cause for concern:2,3
- 12% of infants are born prematurely.
- 31% of pregnancies are complicated by maternal health issues.
- 11% of women smoke during pregnancy.
- 10% drink alcohol during pregnancy.
- 69% of women do not take folate supplements.
- 31% of women are obese.
- 3% of women take medications and supplements that are known teratogens.
The Healthy People 2000 initiative set a goal of 60% of primary caregivers providing preconception care at routine medical visits, but thus far, only about 25% do so.2,3
As a primary care provider, you can have a huge impact on fetal and maternal health by counseling women to choose healthier lifestyles, helping them to manage their chronic conditions, updating their immunizations, and screening for genetic disorders before they become pregnant.
Start with the hard part: Lifestyle modification
Changing the way patients go about their lives—how they eat, how much they exercise, whether they use alcohol or tobacco—has 2 salient characteristics: It’s the most difficult thing to get patients to do, and it has the biggest payoff in improving maternal and fetal health. Lifestyle issues of greatest significance for the preconception patient include:
Folic acid supplementation. If your patients are like most women, they may not be aware of the importance of folic acid supplementation. Yet by neglecting to supplement their diet with folates, women are passing up an opportunity to reduce the incidence of neural tube defects (NTDs) such as anencephaly and spina bifida by up to 70%.4
4D ultrasound of an open neural tube defect in a developing fetus
Women considering conception or those who do not use contraception should take 400 mg folic acid, a dosage found in most prenatal vitamins, every day. All women of reproductive age should consider folate supplementation because of the high rate of unplanned pregnancies. Women who have previously had a child with an NTD and women who take anti-epileptic drugs should take 4 mg folate per day.5
Use routine office visits as an opportunity to counsel patients about folic acid supplementation, whether via prenatal vitamins or, if a higher dosage is necessary, by prescription. One group has reported that preconception counseling increases folate use in women planning pregnancy.6
Safer sex counseling. Counseling patients about safer sexual practices may reduce the incidence of human immunodeficiency virus (HIV), herpes, gonorrhea, chlamydia, and syphilis—conditions that may increase the incidence of preterm delivery, fetal malformations, neonatal infection, or developmental abnormalities.3
Identification of patients with HIV is essential, as early treatment with zidovudine (azidothymidine [AZT], Retrovir), reduces the risk of vertical transmission from mother to neonate by up to 70%.7
Infection prevention. Infections with the potential to harm the fetus include parvovirus B-19, cytomegalovirus, toxoplasmosis, and hepatitis B. Vaccinations are available for hepatitis B but not for the others, so it is particularly important to warn patients about avoiding exposure.
Women who work in child care, for example, should avoid direct contact with children who have parvovirus infection (“Fifth disease”) or other viral exanthems. Health care workers should use universal precautions at all times, and all pregnant women should avoid direct exposure to cat feces and consumption of uncooked meats.
Weight control. Obesity is an increasingly serious health problem in the United States. Obesity poses significant risks for pregnant women and their fetuses, including NTDs, diabetes, venous thromboembolism (VTE), premature labor or cesarean delivery, preeclampsia, and macrosomia.8,9 The time to start a program of exercise and weight loss is before conception, because some of the adverse effects of obesity can occur during the first few months of pregnancy.
Diet modification. Many patients considering conception ask about foods that may be unsafe during pregnancy. Like most clinicians, you may want to advise patients to avoid soft cheeses because of the risk of Listeria infection, and not to eat raw or undercooked meats because of the risk of toxoplasmosis.
Women considering conception should not eat fish high in methylmercury, which can affect the neurologic development of a fetus. These include swordfish, tilefish, king mackerel, and shark. Fish with lower levels of methylmercury are shrimp, canned light tuna, pollock, catfish, and salmon.
Women may eat 12 ounces (or 2 average meals) of these safer fish each week. Methylmercury exposure is cumulative; in women with initially high levels, it can take as long as a year after reducing consumption of fish high in methylmercury for levels to return to normal.10 An Environmental Protection Agency fact sheet for clinicians and patients is available at http://www.epa.gov/waterscience/fish/advice/factsheet.html.
Hot tubs and spas. Maternal hyperthermia (core temperature greater than 100.4°F) during the first trimester is associated with an increased risk of NTDs, so tell women who are pregnant or trying to conceive to stay out of hot tubs and heated spas.11
Environmental toxins. Pregnant women should avoid solvents, paint thinners, heavy metals, pesticides, ionizing radiation (unless indicated for necessary health care), alcohol, illicit drugs, and cigarette smoke.
Smoking cessation. Smoking even less than 1 pack a day can be very harmful to the developing fetus.12,13 Smoking increases the risks of miscarriage, stillbirth, and other pregnancy complications, and is also associated with increased neonatal mortality and sudden infant death syndrome. About 11% of pregnant women smoke.14
As a primary care physician, you should use every opportunity to help smokers considering pregnancy to quit. Proven methods of smoking cessation include counseling, medications such as bupropion, and over-the-counter smoking cessation aids, such as nicotine replacement gum and lozenges.15
One intervention shown to be particularly useful for women who smoke fewer than 20 cigarettes a day is the “5 As” method (Ask, Advise, Assess, Assist, and Arrange).16 A review of studies on the effects of smoking during pregnancy that includes cessation interventions such as the 5 As method is available in the American College of Obstetricians and Gynecologists Committee Opinion No. 316.15
Substance abuse. Fetal alcohol spectrum disorders (FASDs) are among the most preventable congenital defects and developmental disabilities. Ask patients trying to conceive about their patterns of alcohol use, and tell them there is no known safe amount of alcohol intake during pregnancy.
The US Preventive Services Task Force recommends screening pregnant patients with either the TWEAK or T-ACE instruments, because these tests can detect relatively low levels of alcohol consumption that may still harm a developing fetus.17 All women contemplating pregnancy need to know that exposure to alcohol can cause FASDs, congenital malformations, intrauterine growth restriction, and miscarriages. Problem drinkers should be referred for treatment.
Illegal drugs also pose significant risks to fetal development. Damage to the placenta caused by cocaine, for example, can lead to abruption, miscarriage, growth restriction, and prematurity. Consider screening all patients for illegal drug use and referring for counseling or methadone management, as indicated.
Caffeine. Recent studies have linked excessive caffeine intake (>200 mg/d) with miscarriages during the first trimester (adjusted hazards ratio=2.23). To reduce the risk of miscarriage, counsel pregnant women to eliminate caffeine or to cut back to less than 200 mg/d.18 Amounts of caffeine in various beverages are listed in TABLE 1 .
TABLE 1
How much caffeine is your patient drinking?30,31
| BEVERAGE | SERVING SIZE (OZ) | CAFFEINE CONTENT (MG) |
|---|---|---|
| Decaffeinated coffee | 8 | 2 |
| Caffeinated coffees | ||
| Starbucks Grande Coffee | 16 | 330 |
| Starbucks Caffe Latte | 16 | 150 |
| Plain, brewed coffee | 8 | 95 |
| Espresso | 1 | 64 |
| Teas | ||
| Decaffeinated tea | 8 | 2 |
| Black tea, brewed | 8 | 47 |
| Snapple iced tea | 16 | 18 |
| Caffeinated soft drinks | ||
| Diet Mountain Dew | 12 | 55 |
| Diet Coke | 12 | 46 |
| Diet Pepsi | 12 | 37 |
| Sam’s Diet Cola | 12 | 13 |
| Energy drinks | ||
| SoBe Adrenaline Rush | 16 | 152 |
| Red Bull | 8.3 | 76 |
Avert trouble: Manage chronic conditions now
A number of maternal health conditions have a potential for adverse consequences to the fetus, but optimizing the mother’s condition before and during pregnancy can often avert problems. Maternal disorders to monitor include:
Diabetes mellitus. Improving glycemic control prior to conception is linked to a 3-fold decrease in the prevalence of birth defects.3 Patients entering pregnancy with hemoglobin A1C levels less than 8.5% have a fetal anomaly rate of 3.4%, whereas women with a hemoglobin A1C of more than 8.5% have an anomaly rate of 22.4%.19
According to the American Association of Clinical Endocrinologists, goals for glucose control during pregnancy include a hemoglobin A1C of less than 6% and blood glucose concentrations of between 60 mg/dL fasting and 120 mg/dL 1 hour after a meal. Achieving these levels may require tighter control than patients are accustomed to. Blood pressure for these patients should not exceed 130/80 mm Hg.
The use of oral hypoglycemic medications during pregnancy is somewhat controversial. For that reason, you may want to refer these patients to an endocrinologist or other expert in diabetes management during pregnancy for consideration of scheduled insulin injections or an insulin pump.20
Patients with diabetes should be screened for retinal disease, renal disease, hypertension, and hyperlipidemia prior to conception, including a 24-hour urine collection for protein and creatinine clearance. Because women with type 1 diabetes have up to a 40% incidence of thyroid dysfunction, consider screening for thyroid disorders as well.21
While counseling patients with diabetes, keep in mind that a blood urea nitrogen concentration of more than 30 mg/dL, current coronary artery disease, and creatinine clearance of less than 30 mL/min are considered contraindications for pregnancy.20 All women who have had diabetes for more than 10 years should have an electrocardiogram (EKG).
Hypothyroidism. Poorly controlled hypothyroidism may cause developmental, growth, and neurologic abnormalities. Patients with thyroid abnormalities should have their medication dosage optimized before they conceive.
Epilepsy. Seizure disorders generally do not worsen during pregnancy, but several antiseizure medications have a potential for harming the fetus ( TABLE 2 ). Counsel patients about the increased risk of congenital anomalies (4%-8%) in neonates born to women with seizure disorders, either because of the disorder itself or as a consequence of antiseizure medication.22
Patients taking anti-epileptic drugs should take 4 mg/d of folate supplementation. According to the American Academy of Neurology, best practice is to use a single agent best suited to the type of seizures the patient experiences, at the lowest effective dose. Avoid multiple anti-epileptic drugs, if possible. Do not change an effective medication regimen if the patient becomes pregnant, but do check drug levels.23 Also counsel patients about the possibility of decreased effectiveness of hormonal contraception while taking enzymeinducing (cytochrome P450) antiepileptic drugs.24
Psychiatric disorders. Approximately 1 of every 7 pregnant women meets the diagnostic criteria for depression.25 Depression, anxiety, and other psychiatric conditions can adversely impact the patient and her developing fetus if the condition is undertreated. Unfortunately, some women stop psychiatric medications when they discover they are pregnant. In 1 study of 201 pregnant women with depression, 43% had a relapse during the course of pregnancy. Patients with relapse included 68% of those who stopped medication during the pregnancy, vs 26% of those who continued taking medication.26
Most antidepressants are relatively safe during pregnancy, with the exception of paroxetine (Paxil), which is associated with fetal cardiac defects.27 When a patient taking psychiatric medications comes in for a preconception visit, consider higher doses of 1 psychotropic medication rather than lower doses of multiple medications. This will decrease fetal medication exposure. If a patient on a stable regimen becomes pregnant, do not switch medications. Always collaborate with the patient’s mental health care team.
Venous thromboembolic disease. Some inherited thrombophilias can lead to a VTE during pregnancy or the postpartum period. Consider testing patients who have had a VTE or have a family history of VTE for anticardiolipin antibodies, protein S deficiency, and other thrombophilias before conception, because some of the lab values that indicate these conditions can change during pregnancy.
Patients with a history of a VTE related to hormone use or pregnancy will require prophylactic anticoagulation. When in doubt, refer to a hematologist or perinatologist. Both heparin and low-molecular-weight heparin are safe during pregnancy. Because warfarin is a suspected teratogen, patients taking warfarin should be converted to either heparin or low-molecular-weight heparin.
Hypertension. Hypertensive disorders may lead to pregnancy-induced hypertension, growth restriction, and renal disease. If patients are taking thiazide diuretics, angiotensin receptor blockers, or angiotensin-converting enzyme inhibitors ( TABLE 2 ), switch to medications such as methyldopa (Aldomet), nifedipine, or labetalol, which are safer during pregnancy.28 Screen patients with long-standing hypertension for cardiac disease (via EKG) and nephropathy before conception.
TABLE 2
Medications that can harm the unborn child32,33
| MEDICATION | POTENTIAL HARM TO FETUS | FDA CATEGORY* |
|---|---|---|
| Cardiovascular | ||
| ACE inhibitors: captopril, enalapril, lisinopril | Cardiovascular malformations, hypotension, anuria, oligohydramnios | C (1st trimester), D (2nd, 3rd trimesters) |
| Statins: atorvastatin, simvastatin | Polydactyly, cleft lip, club foot | X |
| Antidepressants | ||
| SSRIs: citalopram, fluoxetine, paroxetine, sertraline | Pulmonary hypertension, withdrawal syndrome; paroxetine: cardiac malformations, NTDs | C (D, paroxetine) |
| Anxiolytics | ||
| Benzodiazepines: alprazolam, clonazepam, diazepam, lorazepam | Withdrawal syndrome, congenital anomalies (various), floppy infant syndrome | D |
| Anti-epileptic drugs | ||
| Valproic acid and derivatives | NTDs, facial/cardiac defects | D |
| Carbamazepine | See valproic acid | D |
| Phenobarbital | Cardiac defects, hemorrhagic disease of the newborn | D |
| Phenytoin | Fetal hydantoin syndrome | D |
| Other | ||
| Isotretinoin | Hydrocephalus, microcephaly, limb anomalies, preterm labor, increased spontaneous abortions | X |
| Warfarin | Skeletal defects, intrauterine growth restriction, neurologic defects | X |
| Methotrexate | Fetal malformations, spontaneous abortion | X |
| ACE, angiotensin-converting enzyme; NTDs, neural tube defects; SSRIs, selective serotonin reuptake inhibitors. | ||
| *FDA categories describe the relative risk of medication use during pregnancy. The FDA is currently proposing major revisions to the system to upgrade the usefulness of this information. Please reconfirm categories prior to prescribing. | ||
| A: Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities in any trimester of pregnancy. | ||
| B: Animal studies have revealed no evidence of harm to the fetus, but no adequate and well-controlled studies in pregnant women are available OR animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester. | ||
| C: Animal studies have shown an adverse effect and there are no adequate and well-controlled studies in pregnant women OR no animal studies have been conducted and there are no adequate and well-controlled studies in pregnant women. | ||
| D: Adequate well-controlled or observational studies in pregnant women have demonstrated a risk to the fetus. However, the benefits of therapy may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective. | ||
| X: Adequate well-controlled or observational studies in animals or pregnant women have demonstrated positive evidence of fetal abnormalities or risks. The use of the product is contraindicated in women who are or may become pregnant. | ||
| FDA categories source: Meadows M. Pregnancy and the drug dilemma. FDA Consumer Magazine. May-June 2001. Available at www.fda.gov/fdac/features/2001/301_preg.html. Accessed May 6, 2009. | ||
Think ahead: Address immunization status
When a pregnant woman contracts an infectious disease, her developing fetus can be affected. Making sure the immunization status of all your reproductive-age patients is up to date will go a long way toward protecting their offspring from harm.29
Rubella. Also known as German measles, rubella can cause fetal anomalies and spontaneous abortion if contracted during the first half of pregnancy. Because the measles, mumps, and rubella (MMR) vaccine contains live attenuated viruses, susceptible patients should be immunized at least 1 month before they conceive.3
Hepatitis B. Preventing hepatitis B is an important public health issue. Screen patients at risk for hepatitis B infection—health care workers, sex workers, intravenous drug abusers, and nonmonogamous women who do not use barrier protection—with a hepatitis B surface antigen level. Vaccination is safe up to 1 month before conception.3
Varicella. Maternal varicella (chicken pox) can cause fetal harm, particularly if symptoms appear just before or during delivery. Women of reproductive age who have not already had the disease or been vaccinated should be immunized. This is a live virus vaccine and must, therefore, be administered at least 1 month before conception.3
Influenza. Administering influenza vaccine is not contraindicated during pregnancy, although most experts advise waiting until the second trimester. Certainly it is appropriate to administer the vaccine during influenza season or when risk factors such as chronic lung disease are present. Avoid live attenuated vaccine (FluMist) in pregnant women.
Tdap vaccination. The CDC’s Advisory Committee on Immunization Practices recommended in 2008 that susceptible pregnant women receive Tdap during the postpartum period to protect vulnerable infants against pertussis. Tdap is thought to be safe during pregnancy, but it would make sense to administer this vaccine when indicated prior to conception as part of a vaccination screening program.
Screen for genetic conditions prepregnancy
Part of a comprehensive preconception visit includes screening for communicable diseases and genetic conditions.
Communicable diseases. Consider screening all women prior to pregnancy for HIV infection, gonorrhea, chlamydia, hepatitis B, hepatitis C (for health care workers), and syphilis.
Diabetes. Screening guidelines for diabetes are available from the American Association of Clinical Endocrinologists.20 Consider preconception screening for patients who, during a previous pregnancy, had gestational diabetes or who delivered a baby weighing more than 9 pounds.
Genetic screening. Patients from certain ethnic groups are more susceptible to specific genetic mutations. Genetic disorders associated with particular ethnic origins are listed in TABLE 3 . Consider a preconception referral to a genetic counselor or perinatologist when the patient’s family history suggests inherited disorders.
TABLE 3
Genetic disorders: Who to screen, tests to use34
| ETHNIC ORIGIN* | DISORDER | RECOMMENDED TEST |
|---|---|---|
| Ashkenazi Jews | Tay-Sachs disease; Canavan disease | DNA panel, hexosaminidase A |
| African American | Sickle cell trait; beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| French Canadian, Cajun | Tay-Sachs disease | Hexosaminidase A |
| Mediterranean | Alpha-,† beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Indian, Middle Eastern | Sickle cell trait; alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Caucasian | Cystic fibrosis | DNA panel |
| Southeast Asian (Thai, Laotian, Cambodian) | Alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| MCV, mean corpuscular volume. | ||
| *Offer screening to any interested patient. | ||
| †Do not pursue alpha-thalassemia work-up unless patient has a history of pregnancy loss or fetal hydrops. | ||
Correspondence
D. Ashley Hill, MD, Associate Director, Department of Obstetrics and Gynecology, Loch Haven OB/Gyn Group, Florida Hospital Orlando, 235 Princeton Street, Suite 200, Orlando, FL 32804; [email protected]
- Supplementing women’s diets with 400 mg folic acid every day reduces the incidence of neural tube defects in their offspring by up to 72% (A).
- Optimizing diabetic glucose control prior to conception is linked to a reduction in birth defects and pregnancy loss (B).
- Limiting caffeine consumption to no more than 200 mg per day may reduce the risk of miscarriage (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
In the United States most women do not seek out prenatal care until the 8th to 12th week of pregnancy, when the crucial period of organogenesis (4 to 10 weeks after fertilization) has already passed. In addition, women whose pregnancies are unintended—up to half of all pregnancies—may delay seeking care even longer.1 Given these realities, family physicians should consider all visits during the reproductive years—especially yearly exams—as opportunities for preconception counseling.
Preconception health care is essential to the health of our nation. Data from the Centers for Disease Control and Prevention (CDC) are cause for concern:2,3
- 12% of infants are born prematurely.
- 31% of pregnancies are complicated by maternal health issues.
- 11% of women smoke during pregnancy.
- 10% drink alcohol during pregnancy.
- 69% of women do not take folate supplements.
- 31% of women are obese.
- 3% of women take medications and supplements that are known teratogens.
The Healthy People 2000 initiative set a goal of 60% of primary caregivers providing preconception care at routine medical visits, but thus far, only about 25% do so.2,3
As a primary care provider, you can have a huge impact on fetal and maternal health by counseling women to choose healthier lifestyles, helping them to manage their chronic conditions, updating their immunizations, and screening for genetic disorders before they become pregnant.
Start with the hard part: Lifestyle modification
Changing the way patients go about their lives—how they eat, how much they exercise, whether they use alcohol or tobacco—has 2 salient characteristics: It’s the most difficult thing to get patients to do, and it has the biggest payoff in improving maternal and fetal health. Lifestyle issues of greatest significance for the preconception patient include:
Folic acid supplementation. If your patients are like most women, they may not be aware of the importance of folic acid supplementation. Yet by neglecting to supplement their diet with folates, women are passing up an opportunity to reduce the incidence of neural tube defects (NTDs) such as anencephaly and spina bifida by up to 70%.4
4D ultrasound of an open neural tube defect in a developing fetus
Women considering conception or those who do not use contraception should take 400 mg folic acid, a dosage found in most prenatal vitamins, every day. All women of reproductive age should consider folate supplementation because of the high rate of unplanned pregnancies. Women who have previously had a child with an NTD and women who take anti-epileptic drugs should take 4 mg folate per day.5
Use routine office visits as an opportunity to counsel patients about folic acid supplementation, whether via prenatal vitamins or, if a higher dosage is necessary, by prescription. One group has reported that preconception counseling increases folate use in women planning pregnancy.6
Safer sex counseling. Counseling patients about safer sexual practices may reduce the incidence of human immunodeficiency virus (HIV), herpes, gonorrhea, chlamydia, and syphilis—conditions that may increase the incidence of preterm delivery, fetal malformations, neonatal infection, or developmental abnormalities.3
Identification of patients with HIV is essential, as early treatment with zidovudine (azidothymidine [AZT], Retrovir), reduces the risk of vertical transmission from mother to neonate by up to 70%.7
Infection prevention. Infections with the potential to harm the fetus include parvovirus B-19, cytomegalovirus, toxoplasmosis, and hepatitis B. Vaccinations are available for hepatitis B but not for the others, so it is particularly important to warn patients about avoiding exposure.
Women who work in child care, for example, should avoid direct contact with children who have parvovirus infection (“Fifth disease”) or other viral exanthems. Health care workers should use universal precautions at all times, and all pregnant women should avoid direct exposure to cat feces and consumption of uncooked meats.
Weight control. Obesity is an increasingly serious health problem in the United States. Obesity poses significant risks for pregnant women and their fetuses, including NTDs, diabetes, venous thromboembolism (VTE), premature labor or cesarean delivery, preeclampsia, and macrosomia.8,9 The time to start a program of exercise and weight loss is before conception, because some of the adverse effects of obesity can occur during the first few months of pregnancy.
Diet modification. Many patients considering conception ask about foods that may be unsafe during pregnancy. Like most clinicians, you may want to advise patients to avoid soft cheeses because of the risk of Listeria infection, and not to eat raw or undercooked meats because of the risk of toxoplasmosis.
Women considering conception should not eat fish high in methylmercury, which can affect the neurologic development of a fetus. These include swordfish, tilefish, king mackerel, and shark. Fish with lower levels of methylmercury are shrimp, canned light tuna, pollock, catfish, and salmon.
Women may eat 12 ounces (or 2 average meals) of these safer fish each week. Methylmercury exposure is cumulative; in women with initially high levels, it can take as long as a year after reducing consumption of fish high in methylmercury for levels to return to normal.10 An Environmental Protection Agency fact sheet for clinicians and patients is available at http://www.epa.gov/waterscience/fish/advice/factsheet.html.
Hot tubs and spas. Maternal hyperthermia (core temperature greater than 100.4°F) during the first trimester is associated with an increased risk of NTDs, so tell women who are pregnant or trying to conceive to stay out of hot tubs and heated spas.11
Environmental toxins. Pregnant women should avoid solvents, paint thinners, heavy metals, pesticides, ionizing radiation (unless indicated for necessary health care), alcohol, illicit drugs, and cigarette smoke.
Smoking cessation. Smoking even less than 1 pack a day can be very harmful to the developing fetus.12,13 Smoking increases the risks of miscarriage, stillbirth, and other pregnancy complications, and is also associated with increased neonatal mortality and sudden infant death syndrome. About 11% of pregnant women smoke.14
As a primary care physician, you should use every opportunity to help smokers considering pregnancy to quit. Proven methods of smoking cessation include counseling, medications such as bupropion, and over-the-counter smoking cessation aids, such as nicotine replacement gum and lozenges.15
One intervention shown to be particularly useful for women who smoke fewer than 20 cigarettes a day is the “5 As” method (Ask, Advise, Assess, Assist, and Arrange).16 A review of studies on the effects of smoking during pregnancy that includes cessation interventions such as the 5 As method is available in the American College of Obstetricians and Gynecologists Committee Opinion No. 316.15
Substance abuse. Fetal alcohol spectrum disorders (FASDs) are among the most preventable congenital defects and developmental disabilities. Ask patients trying to conceive about their patterns of alcohol use, and tell them there is no known safe amount of alcohol intake during pregnancy.
The US Preventive Services Task Force recommends screening pregnant patients with either the TWEAK or T-ACE instruments, because these tests can detect relatively low levels of alcohol consumption that may still harm a developing fetus.17 All women contemplating pregnancy need to know that exposure to alcohol can cause FASDs, congenital malformations, intrauterine growth restriction, and miscarriages. Problem drinkers should be referred for treatment.
Illegal drugs also pose significant risks to fetal development. Damage to the placenta caused by cocaine, for example, can lead to abruption, miscarriage, growth restriction, and prematurity. Consider screening all patients for illegal drug use and referring for counseling or methadone management, as indicated.
Caffeine. Recent studies have linked excessive caffeine intake (>200 mg/d) with miscarriages during the first trimester (adjusted hazards ratio=2.23). To reduce the risk of miscarriage, counsel pregnant women to eliminate caffeine or to cut back to less than 200 mg/d.18 Amounts of caffeine in various beverages are listed in TABLE 1 .
TABLE 1
How much caffeine is your patient drinking?30,31
| BEVERAGE | SERVING SIZE (OZ) | CAFFEINE CONTENT (MG) |
|---|---|---|
| Decaffeinated coffee | 8 | 2 |
| Caffeinated coffees | ||
| Starbucks Grande Coffee | 16 | 330 |
| Starbucks Caffe Latte | 16 | 150 |
| Plain, brewed coffee | 8 | 95 |
| Espresso | 1 | 64 |
| Teas | ||
| Decaffeinated tea | 8 | 2 |
| Black tea, brewed | 8 | 47 |
| Snapple iced tea | 16 | 18 |
| Caffeinated soft drinks | ||
| Diet Mountain Dew | 12 | 55 |
| Diet Coke | 12 | 46 |
| Diet Pepsi | 12 | 37 |
| Sam’s Diet Cola | 12 | 13 |
| Energy drinks | ||
| SoBe Adrenaline Rush | 16 | 152 |
| Red Bull | 8.3 | 76 |
Avert trouble: Manage chronic conditions now
A number of maternal health conditions have a potential for adverse consequences to the fetus, but optimizing the mother’s condition before and during pregnancy can often avert problems. Maternal disorders to monitor include:
Diabetes mellitus. Improving glycemic control prior to conception is linked to a 3-fold decrease in the prevalence of birth defects.3 Patients entering pregnancy with hemoglobin A1C levels less than 8.5% have a fetal anomaly rate of 3.4%, whereas women with a hemoglobin A1C of more than 8.5% have an anomaly rate of 22.4%.19
According to the American Association of Clinical Endocrinologists, goals for glucose control during pregnancy include a hemoglobin A1C of less than 6% and blood glucose concentrations of between 60 mg/dL fasting and 120 mg/dL 1 hour after a meal. Achieving these levels may require tighter control than patients are accustomed to. Blood pressure for these patients should not exceed 130/80 mm Hg.
The use of oral hypoglycemic medications during pregnancy is somewhat controversial. For that reason, you may want to refer these patients to an endocrinologist or other expert in diabetes management during pregnancy for consideration of scheduled insulin injections or an insulin pump.20
Patients with diabetes should be screened for retinal disease, renal disease, hypertension, and hyperlipidemia prior to conception, including a 24-hour urine collection for protein and creatinine clearance. Because women with type 1 diabetes have up to a 40% incidence of thyroid dysfunction, consider screening for thyroid disorders as well.21
While counseling patients with diabetes, keep in mind that a blood urea nitrogen concentration of more than 30 mg/dL, current coronary artery disease, and creatinine clearance of less than 30 mL/min are considered contraindications for pregnancy.20 All women who have had diabetes for more than 10 years should have an electrocardiogram (EKG).
Hypothyroidism. Poorly controlled hypothyroidism may cause developmental, growth, and neurologic abnormalities. Patients with thyroid abnormalities should have their medication dosage optimized before they conceive.
Epilepsy. Seizure disorders generally do not worsen during pregnancy, but several antiseizure medications have a potential for harming the fetus ( TABLE 2 ). Counsel patients about the increased risk of congenital anomalies (4%-8%) in neonates born to women with seizure disorders, either because of the disorder itself or as a consequence of antiseizure medication.22
Patients taking anti-epileptic drugs should take 4 mg/d of folate supplementation. According to the American Academy of Neurology, best practice is to use a single agent best suited to the type of seizures the patient experiences, at the lowest effective dose. Avoid multiple anti-epileptic drugs, if possible. Do not change an effective medication regimen if the patient becomes pregnant, but do check drug levels.23 Also counsel patients about the possibility of decreased effectiveness of hormonal contraception while taking enzymeinducing (cytochrome P450) antiepileptic drugs.24
Psychiatric disorders. Approximately 1 of every 7 pregnant women meets the diagnostic criteria for depression.25 Depression, anxiety, and other psychiatric conditions can adversely impact the patient and her developing fetus if the condition is undertreated. Unfortunately, some women stop psychiatric medications when they discover they are pregnant. In 1 study of 201 pregnant women with depression, 43% had a relapse during the course of pregnancy. Patients with relapse included 68% of those who stopped medication during the pregnancy, vs 26% of those who continued taking medication.26
Most antidepressants are relatively safe during pregnancy, with the exception of paroxetine (Paxil), which is associated with fetal cardiac defects.27 When a patient taking psychiatric medications comes in for a preconception visit, consider higher doses of 1 psychotropic medication rather than lower doses of multiple medications. This will decrease fetal medication exposure. If a patient on a stable regimen becomes pregnant, do not switch medications. Always collaborate with the patient’s mental health care team.
Venous thromboembolic disease. Some inherited thrombophilias can lead to a VTE during pregnancy or the postpartum period. Consider testing patients who have had a VTE or have a family history of VTE for anticardiolipin antibodies, protein S deficiency, and other thrombophilias before conception, because some of the lab values that indicate these conditions can change during pregnancy.
Patients with a history of a VTE related to hormone use or pregnancy will require prophylactic anticoagulation. When in doubt, refer to a hematologist or perinatologist. Both heparin and low-molecular-weight heparin are safe during pregnancy. Because warfarin is a suspected teratogen, patients taking warfarin should be converted to either heparin or low-molecular-weight heparin.
Hypertension. Hypertensive disorders may lead to pregnancy-induced hypertension, growth restriction, and renal disease. If patients are taking thiazide diuretics, angiotensin receptor blockers, or angiotensin-converting enzyme inhibitors ( TABLE 2 ), switch to medications such as methyldopa (Aldomet), nifedipine, or labetalol, which are safer during pregnancy.28 Screen patients with long-standing hypertension for cardiac disease (via EKG) and nephropathy before conception.
TABLE 2
Medications that can harm the unborn child32,33
| MEDICATION | POTENTIAL HARM TO FETUS | FDA CATEGORY* |
|---|---|---|
| Cardiovascular | ||
| ACE inhibitors: captopril, enalapril, lisinopril | Cardiovascular malformations, hypotension, anuria, oligohydramnios | C (1st trimester), D (2nd, 3rd trimesters) |
| Statins: atorvastatin, simvastatin | Polydactyly, cleft lip, club foot | X |
| Antidepressants | ||
| SSRIs: citalopram, fluoxetine, paroxetine, sertraline | Pulmonary hypertension, withdrawal syndrome; paroxetine: cardiac malformations, NTDs | C (D, paroxetine) |
| Anxiolytics | ||
| Benzodiazepines: alprazolam, clonazepam, diazepam, lorazepam | Withdrawal syndrome, congenital anomalies (various), floppy infant syndrome | D |
| Anti-epileptic drugs | ||
| Valproic acid and derivatives | NTDs, facial/cardiac defects | D |
| Carbamazepine | See valproic acid | D |
| Phenobarbital | Cardiac defects, hemorrhagic disease of the newborn | D |
| Phenytoin | Fetal hydantoin syndrome | D |
| Other | ||
| Isotretinoin | Hydrocephalus, microcephaly, limb anomalies, preterm labor, increased spontaneous abortions | X |
| Warfarin | Skeletal defects, intrauterine growth restriction, neurologic defects | X |
| Methotrexate | Fetal malformations, spontaneous abortion | X |
| ACE, angiotensin-converting enzyme; NTDs, neural tube defects; SSRIs, selective serotonin reuptake inhibitors. | ||
| *FDA categories describe the relative risk of medication use during pregnancy. The FDA is currently proposing major revisions to the system to upgrade the usefulness of this information. Please reconfirm categories prior to prescribing. | ||
| A: Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities in any trimester of pregnancy. | ||
| B: Animal studies have revealed no evidence of harm to the fetus, but no adequate and well-controlled studies in pregnant women are available OR animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester. | ||
| C: Animal studies have shown an adverse effect and there are no adequate and well-controlled studies in pregnant women OR no animal studies have been conducted and there are no adequate and well-controlled studies in pregnant women. | ||
| D: Adequate well-controlled or observational studies in pregnant women have demonstrated a risk to the fetus. However, the benefits of therapy may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective. | ||
| X: Adequate well-controlled or observational studies in animals or pregnant women have demonstrated positive evidence of fetal abnormalities or risks. The use of the product is contraindicated in women who are or may become pregnant. | ||
| FDA categories source: Meadows M. Pregnancy and the drug dilemma. FDA Consumer Magazine. May-June 2001. Available at www.fda.gov/fdac/features/2001/301_preg.html. Accessed May 6, 2009. | ||
Think ahead: Address immunization status
When a pregnant woman contracts an infectious disease, her developing fetus can be affected. Making sure the immunization status of all your reproductive-age patients is up to date will go a long way toward protecting their offspring from harm.29
Rubella. Also known as German measles, rubella can cause fetal anomalies and spontaneous abortion if contracted during the first half of pregnancy. Because the measles, mumps, and rubella (MMR) vaccine contains live attenuated viruses, susceptible patients should be immunized at least 1 month before they conceive.3
Hepatitis B. Preventing hepatitis B is an important public health issue. Screen patients at risk for hepatitis B infection—health care workers, sex workers, intravenous drug abusers, and nonmonogamous women who do not use barrier protection—with a hepatitis B surface antigen level. Vaccination is safe up to 1 month before conception.3
Varicella. Maternal varicella (chicken pox) can cause fetal harm, particularly if symptoms appear just before or during delivery. Women of reproductive age who have not already had the disease or been vaccinated should be immunized. This is a live virus vaccine and must, therefore, be administered at least 1 month before conception.3
Influenza. Administering influenza vaccine is not contraindicated during pregnancy, although most experts advise waiting until the second trimester. Certainly it is appropriate to administer the vaccine during influenza season or when risk factors such as chronic lung disease are present. Avoid live attenuated vaccine (FluMist) in pregnant women.
Tdap vaccination. The CDC’s Advisory Committee on Immunization Practices recommended in 2008 that susceptible pregnant women receive Tdap during the postpartum period to protect vulnerable infants against pertussis. Tdap is thought to be safe during pregnancy, but it would make sense to administer this vaccine when indicated prior to conception as part of a vaccination screening program.
Screen for genetic conditions prepregnancy
Part of a comprehensive preconception visit includes screening for communicable diseases and genetic conditions.
Communicable diseases. Consider screening all women prior to pregnancy for HIV infection, gonorrhea, chlamydia, hepatitis B, hepatitis C (for health care workers), and syphilis.
Diabetes. Screening guidelines for diabetes are available from the American Association of Clinical Endocrinologists.20 Consider preconception screening for patients who, during a previous pregnancy, had gestational diabetes or who delivered a baby weighing more than 9 pounds.
Genetic screening. Patients from certain ethnic groups are more susceptible to specific genetic mutations. Genetic disorders associated with particular ethnic origins are listed in TABLE 3 . Consider a preconception referral to a genetic counselor or perinatologist when the patient’s family history suggests inherited disorders.
TABLE 3
Genetic disorders: Who to screen, tests to use34
| ETHNIC ORIGIN* | DISORDER | RECOMMENDED TEST |
|---|---|---|
| Ashkenazi Jews | Tay-Sachs disease; Canavan disease | DNA panel, hexosaminidase A |
| African American | Sickle cell trait; beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| French Canadian, Cajun | Tay-Sachs disease | Hexosaminidase A |
| Mediterranean | Alpha-,† beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Indian, Middle Eastern | Sickle cell trait; alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Caucasian | Cystic fibrosis | DNA panel |
| Southeast Asian (Thai, Laotian, Cambodian) | Alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| MCV, mean corpuscular volume. | ||
| *Offer screening to any interested patient. | ||
| †Do not pursue alpha-thalassemia work-up unless patient has a history of pregnancy loss or fetal hydrops. | ||
Correspondence
D. Ashley Hill, MD, Associate Director, Department of Obstetrics and Gynecology, Loch Haven OB/Gyn Group, Florida Hospital Orlando, 235 Princeton Street, Suite 200, Orlando, FL 32804; [email protected]
1. Mayer JP. Unintended childbearing, maternal beliefs, and delay of prenatal care. Birth. 1997;24:247-252.
2. Proceedings of the Preconception Health and Health Care Clinical, Public Health, and Consumer Workgroup Meetings. Atlanta, GA: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities; 2006. Available at: http://www.cdc.gov/ncbddd/preconception/documents/Workgroup%20Proceedings%20June06.pdf. Accessed May 14, 2009.
3. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-06):1-23.Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed May 14, 2009.
4. Lumley J, Watson L, Watson M, et al. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.-
5. Iqbal MM. Prevention of neural tube defects by periconceptional use of folic acid. Pediatr Rev. 2000;21:58-66.
6. de Weerd S, Thomas CM, Cikot RJ, et al. Preconception counseling improves folate status of women planning pregnancy. Obstet Gynecol. 2002;99:45-50.
7. Mofenson LM. Centers for Disease Control and Prevention, US Public Health Service Task Force. US Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Recomm Rep. 2002;51(RR-18):1-38.
8. Robinson HE, O’Connell CM, Joseph KS, et al. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106:1357-1364.
9. Kiel DW, Dodson EA, Artal R, et al. Gestational weight gain and pregnancy outcomes in obese women: How much is enough? Obstet Gynecol. 2007;110:752-758.
10. US Food and Drug Administration. Food safety for moms-to-be. August 24, 2005. Available at: http://www.cfsan.fda.gov/~pregnant/pregnant.html. Accessed September 18, 2008.
11. Cheschier N. ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin. Neural tube defects. Number 44, July 2003. (Replaces committee opinion number 252, March 2001). Int J Gynaecol Obstet. 2003;83:123-133.
12. Castles A, Adams EK, Melvin CL, et al. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16:208-215.
13. US Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. May 27, 2004. Available at: http://www.surgeongeneral.gov/library/smokingconsequences. Accessed July 6, 2008.
14. National Center for Health Statistics. Health, United States, 2004: With Chartbook on Trends in the Health of Americans. Hyattsville, MD: NCHS; 2004. Available at: http://www.cdc.gov/nchs/data/hus/hus04.pdf. Accessed September 18, 2008.
15. ACOG Committee on Health Care for Underdeserved Women; ACOG Committee on Obstetric Practice. ACOG committee opinion. Number 316, October 2005. Smoking cessation during pregnancy. Obstet Gynecol. 2005;106:883-888.
16. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; May 2008. Available at: http://www.ahrq.gov/path/tobacco.htm. Accessed May 14, 2009.
17. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
18. Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198:279.e1-279.e8.
19. Lucas MJ, Leveno KJ, Williams ML, et al. Early pregnancy glycosylated hemoglobin, severity of diabetes, and fetal malformations. Am J Obstet Gynecol. 1989;161:426-431.
20. American Association of Clinical Endocrinologists Diabetes Mellitus Clinical Practice Guidelines Task Force. AACE diabetes mellitus guidelines. Chapter 9: diabetes and pregnancy. Endocr Pract. 2007;13(suppl 1):55-59.
21. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105:675-685.
22. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. ACOG educational bulletin. Seizure disorders in pregnancy. Number 231, December 1996. Int J Gynaecol Obstet. 1997;56:279-286.
23. American Academy of Neurology. Guideline summary for clinicians—management issues for women with epilepsy. Available at: www.aan.com/professionals/practice/pdfs/women_epilepsy.pdf. Accessed May 14, 2009.
24. Carl JS, Weaver SP, Tweed E, et al. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
25. Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799-801.
26. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment [published correction appears in JAMA. 2006;296:170]. JAMA. 2006;295:499-507.
27. FDA public health advisory: paroxetine. December 8, 2005. Available at: http://www.fda.gov/CDER/Drug/advisory/paroxetine200512.htm. Accessed May 6, 2009.
28. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Chronic hypertension in pregnancy. Obstet Gynecol. 2001;98(suppl):177-185.
29. Centers for Disease Control and Prevention. Recommended adult immunization schedule—United States, 2009. MMWR. 2009;57(53):Q-1-Q-4.Available at: http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed May 14, 2009.
30. MayoClinic.Com. How much caffeine is in your daily habit? Available at: http://www.mayoclinic.com/health/caffeine/AN01211. Accessed September 18, 2008.
31. Chou KH, Bell LH. Caffeine content of prepackaged national-brand and private-label carbonated beverages. J Food Sci. 2007;72:C337-C342.
32. Physician’s Desk Reference. Montvale, NJ: Thomson Reuters; 2009.
33. Drugs.com. Available at: http://www.drugs.com. Accessed April 26, 2009.
34. National Society of Genetic Counselors, Prenatal Special Interest Group. Ancestry Based Carrier Screening. Chicago, IL: NSGC; 2005. Available at: http://www.nsgc.org. Accessed September 18, 2008.
1. Mayer JP. Unintended childbearing, maternal beliefs, and delay of prenatal care. Birth. 1997;24:247-252.
2. Proceedings of the Preconception Health and Health Care Clinical, Public Health, and Consumer Workgroup Meetings. Atlanta, GA: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities; 2006. Available at: http://www.cdc.gov/ncbddd/preconception/documents/Workgroup%20Proceedings%20June06.pdf. Accessed May 14, 2009.
3. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-06):1-23.Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed May 14, 2009.
4. Lumley J, Watson L, Watson M, et al. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.-
5. Iqbal MM. Prevention of neural tube defects by periconceptional use of folic acid. Pediatr Rev. 2000;21:58-66.
6. de Weerd S, Thomas CM, Cikot RJ, et al. Preconception counseling improves folate status of women planning pregnancy. Obstet Gynecol. 2002;99:45-50.
7. Mofenson LM. Centers for Disease Control and Prevention, US Public Health Service Task Force. US Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Recomm Rep. 2002;51(RR-18):1-38.
8. Robinson HE, O’Connell CM, Joseph KS, et al. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106:1357-1364.
9. Kiel DW, Dodson EA, Artal R, et al. Gestational weight gain and pregnancy outcomes in obese women: How much is enough? Obstet Gynecol. 2007;110:752-758.
10. US Food and Drug Administration. Food safety for moms-to-be. August 24, 2005. Available at: http://www.cfsan.fda.gov/~pregnant/pregnant.html. Accessed September 18, 2008.
11. Cheschier N. ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin. Neural tube defects. Number 44, July 2003. (Replaces committee opinion number 252, March 2001). Int J Gynaecol Obstet. 2003;83:123-133.
12. Castles A, Adams EK, Melvin CL, et al. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16:208-215.
13. US Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. May 27, 2004. Available at: http://www.surgeongeneral.gov/library/smokingconsequences. Accessed July 6, 2008.
14. National Center for Health Statistics. Health, United States, 2004: With Chartbook on Trends in the Health of Americans. Hyattsville, MD: NCHS; 2004. Available at: http://www.cdc.gov/nchs/data/hus/hus04.pdf. Accessed September 18, 2008.
15. ACOG Committee on Health Care for Underdeserved Women; ACOG Committee on Obstetric Practice. ACOG committee opinion. Number 316, October 2005. Smoking cessation during pregnancy. Obstet Gynecol. 2005;106:883-888.
16. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; May 2008. Available at: http://www.ahrq.gov/path/tobacco.htm. Accessed May 14, 2009.
17. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
18. Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198:279.e1-279.e8.
19. Lucas MJ, Leveno KJ, Williams ML, et al. Early pregnancy glycosylated hemoglobin, severity of diabetes, and fetal malformations. Am J Obstet Gynecol. 1989;161:426-431.
20. American Association of Clinical Endocrinologists Diabetes Mellitus Clinical Practice Guidelines Task Force. AACE diabetes mellitus guidelines. Chapter 9: diabetes and pregnancy. Endocr Pract. 2007;13(suppl 1):55-59.
21. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105:675-685.
22. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. ACOG educational bulletin. Seizure disorders in pregnancy. Number 231, December 1996. Int J Gynaecol Obstet. 1997;56:279-286.
23. American Academy of Neurology. Guideline summary for clinicians—management issues for women with epilepsy. Available at: www.aan.com/professionals/practice/pdfs/women_epilepsy.pdf. Accessed May 14, 2009.
24. Carl JS, Weaver SP, Tweed E, et al. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
25. Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799-801.
26. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment [published correction appears in JAMA. 2006;296:170]. JAMA. 2006;295:499-507.
27. FDA public health advisory: paroxetine. December 8, 2005. Available at: http://www.fda.gov/CDER/Drug/advisory/paroxetine200512.htm. Accessed May 6, 2009.
28. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Chronic hypertension in pregnancy. Obstet Gynecol. 2001;98(suppl):177-185.
29. Centers for Disease Control and Prevention. Recommended adult immunization schedule—United States, 2009. MMWR. 2009;57(53):Q-1-Q-4.Available at: http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed May 14, 2009.
30. MayoClinic.Com. How much caffeine is in your daily habit? Available at: http://www.mayoclinic.com/health/caffeine/AN01211. Accessed September 18, 2008.
31. Chou KH, Bell LH. Caffeine content of prepackaged national-brand and private-label carbonated beverages. J Food Sci. 2007;72:C337-C342.
32. Physician’s Desk Reference. Montvale, NJ: Thomson Reuters; 2009.
33. Drugs.com. Available at: http://www.drugs.com. Accessed April 26, 2009.
34. National Society of Genetic Counselors, Prenatal Special Interest Group. Ancestry Based Carrier Screening. Chicago, IL: NSGC; 2005. Available at: http://www.nsgc.org. Accessed September 18, 2008.