User login
Pinacidil induces vascular dilation and hyperemia in vivo and does not impact biophysical properties of neurons and astrocytes in vitro
Interactions between the brain and blood are essential to health. Metabolic supply of the brain is provided through the vasculature, and disruptions of this relationship, in extreme cases such as stroke, is a key characteristic of neurologic disease. Neuro-hemodynamic coupling is also demonstrated in healthy individuals on faster time scales in functional hyperemia, the local increase in blood flow and volume that accompanies neural activity.1,2
We have recently proposed a further level of interdependence between the two systems —ie, the hemoneural hypothesis—which predicts that hemodynamic events such as functional hyperemia will modulate neural activity.3 An impact of hemodynamics on neurons could occur through a number of mechanisms, including the activation of mechanoreceptors on astrocytes or neurons, a thermal impact of increased blood flow on ion channels and vesicle release, and the local increase and diffusion of blood-borne factors such as nitric oxide.4,5 Astrocytes are predicted to play a key role in hemo-neural modulation, as they are tightly coupled to the vascular system and participate in a number of neural functions.6,7 Through these mechanisms and others, hemodynamics could shift the “state” of the local neural circuit, thereby impacting information processing. This regulation of neural dynamics could also provide a homeostatic mechanism for promoting healthy brain function (eg, prevention of kindling).
To study the impact of hyperemia on neural and astrocytic activity in vivo, it is essential to independently control blood flow in the brain with means that do not directly impact neurons or astrocytes. Pinacidil is a sulfonylurea receptor agonist that opens the SUR2B potassium-sensitive ATP channel.8 In the telencephalon, SUR1 receptors are localized to neurons and glia.9,10 In contrast, SUR2 receptors are localized to vasculature, with SUR2A in cardiac and skeletal muscle, and SUR2B in vascular smooth muscle, with primary expression in smaller arteries, arterioles, and capillaries.11 By opening the SUR2B channel, pinacidil hyperpolarizes and relaxes smooth muscle, causing vasodilation. Pinacidil is a potent and selective SUR2B agonist, with a dissociation constant of 135 nM and a half maximal effective concentation (EC50) value of 680 nM.12 This agonist is approximately 5 times more specific for SUR2B than for SUR2A and shows approximately 5 orders of magnitude lower affinity for SUR1 (in the mM range).12–15 Previous studies have demonstrated the efficacy of this agent as a vasodilator.16–18
In the present study, we systematically examined the utility of pinacidil for the selective induction of hyperemia. First, we quantified the vasodilation induced by pinacidil in vivo, and examined local increases in blood volume in the parenchyma. These studies were conducted in anesthetized rats and awake mice. Second, we used in vitro slice recordings to examine whether direct application of relatively high concentrations of pinacidil would have any impact on the physiology of neurons and astrocytes. We found that (1) in vivo, pinacidil induces a level of vasodilation and increased local blood volume consistent with natural functional hyperemia across a variety of preparations, and (2) in vitro, pinacidil has no detectable impact on intrinsic biophysical measures in neurons and astrocytes.
METHODS
Animal preparation in vivo
To probe the impact of pinacidil on arterial diameter and parenchymal blood volume in vivo, we measured the effects of topical application to the primary somatosensory cortex (SI) of rats and mice. Sprague-Dawley rats (250–500 g) and C57BL/6 mice (~25 g) were anaesthetized with pentobarbital (50 mg/kg intraperitoneally initial dose, followed by 5-mg supplements as needed for maintenance). Animals were maintained at approximately 37°C by a heating blanket. Craniotomy (diameter of ~2 mm in rats, ~1 mm in mice) and durotomy were performed over SI, and the cortex was protected with Kwik-Cast silicone elastomer sealant (WPI, Sarasota, FL) while an imaging chamber was attached with dental cement. Kwik-Cast was removed, and the chamber filled with 0.9% saline and sealed with a round cover glass (avoiding bubbles) secured with cyanoacrylate.
Controlling visualization during drug delivery in vivo
To minimize brain motion and flow artifacts during visualization of hemodynamics in the rat preparation, we constructed a customized pressurized chamber with inflow and outflow for constant perfusion. The volume of the chamber was approximately 0.3 mL, and the flow through the system averaged about 2 mL/min. The chamber consisted of a plastic ring 1 cm in diameter and 3 mm high with a flat-top profile and a base shaped to the angle of the lateral skull edge over SI. In the wall of this chamber, three large holes were drilled and patched with pieces cut from rubber NMR septa (VWR International, West Chester, PA) to create resealable ports for drug application and bubble removal. Three additional permanent holes were drilled in the chamber walls, through which blunted 1-cm lengths of 18-gauge stainless steel needles were wedged and affixed with Super glue: one for artificial cerebrospinal fluid (ACSF) inflow, one for combined outflow, and the third for pressure regulation. The overall pressure of the chamber was regulated by a small vertical tube whose height (and thus fluid level) could be adjusted on a manipulator stand, and whose other end was open to the atmosphere. Inflow and outflow were controlled via regulators on a gravity feed system. In the mouse preparation, the need to control visualization was addressed by maintaining a constant rate of wicking in a smaller-profile open chamber, and a microfluidic switch with 12 μL of dead space was added to minimize propulsive impact and delay due to switching between solutions. Drug and ethanol solutions were delivered to rat and mouse chambers after being heated to physiological temperature (37°C).
Optical measurement of hemodynamics in vivo
We used a charge-coupled device camera (the Roper 512B, Princeton Instruments, Trenton, NJ) to image the cortical surface at a frame rate of approximately 4 Hz, with illumination from a voltage-regulated xenon arc lamp. A green band-pass filter (550 nm) was used to maximize imaging near the isosbestic point of hemoglobin, providing optimal vessel contrast and a surrogate measure for blood volume change in the parenchyma. Lenses (50 and 125 mm) were arranged in series to form a macroscope.
Pinacidil administration in vivo
Pinacidil is hydrophobic and was therefore dissolved in ethanol at approximately 12 mg/kg and then diluted 1:100 in ACSF to achieve a 400-μM solution in 1% ethanol. Stock solutions were stored at −20°C and diluted in fresh ACSF for each experiment. For each run, the cortex was imaged for 3 minutes to establish baseline. For the pressurized rat chamber, at the end of the baseline period, 0.1 to 0.3 mL of 400 μM pinacidil in 1% ethanol in ACSF or saline would be pumped into the 0.3-mL chamber (taking about 1 second). Simultaneously, an equivalent volume was drawn out to balance pressure by a push-pull pump with access through two of the resealable rubber ports.
Pinacidil administration in vitro
Coronal slices were prepared from Sprague-Dawley rats at postnatal day 14 to 40 and maintained in a submersion chamber at 27°C for recording. Solutions were prepared in ACSF: 125 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 1.25 mM NaH2PO4, 2 mM CaCl2, 25 mM NaHCO3, and 25 mM d-glucose). The applied solutions were 1% ethanol, ~400 μM pinacidil in 1% ethanol, and ACSF, all perfused with 5% CO2 in 95% O2 (carbogen).
For astrocyte recordings, slices were incubated immediately after cutting for 20 minutes in ACSF containing 50 μM sulforhodamine 101 (SR101), a water-soluble fluorescent dye specifically taken up by astrocytes.19 The slices were then allowed to rest for an hour before recording as usual. For fluorescence, the light source was a 100-W mercury arc lamp, with excitation and barrier filters and dichroic mirrors tailored to the spectral characteristics of SR101 (excitation ~586 nm, emission ~605 nm)
Slices were imaged under differential interference contrast optics with infrared illumination. Cells in layers 2/3 and in the same field and plane of view as a blood vessel greater than 20 μm in diameter were targeted, and vessel expansion was monitored during intracellular recording at approximately 4 Hz with a cooled charge-coupled device camera (Retiga EX, QImaging, Surrey, BC, Canada) connected to the microscope via a parfocal C-mount. This configuration also enabled imaging of neurons and astrocytes in the slice.
Drug/control solutions were switched every 90 to 180 seconds. Recording pipettes were filled with 120 mM KGlu, 10 mM NaCl, 20 mM KCl, 10 mM HEPES, 2mM Mg-ATP, 0.3 mM Na-GTP, 0.5 mM EGTA, and 0.3% to 1% biocytin (wt/vol) for subsequent visualization of the neurons.
RESULTS
Pinacidil induces vasodilation in anesthetized rats and awake mice
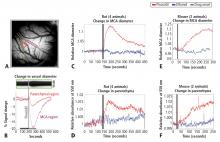
Figure 1A shows the cortical surface over SI. The green bisection line over the MCA shows the point of sampling for the darkness plot in the rectangular box in Figure 1B. The width of this dark band is the width of the MCA over time, showing expansion after pinacidil addition (gray bar). The point at which dilation is observed corresponds to a darkening in a parenchymal region (red triangle in Figure 1A) and over the MCA and surrounding cortex (purple rectangle in Figure 1A, reflecting expansion). Vasodilation and parenchymal signal increases were consistently observed on the first trial in all experiments (4 first runs from 4 anesthetized rats, Figure 1C and 1D). Dilation began less than 10 seconds after drug arrival, with maximal dilation and parenchymal darkening at an approximately 50- to 60-second latency. Ethanol in a 1:100 solution with ACSF under the same conditions evoked a nonsignificant reduction in vessel diameter and no change in parenchymal darkening. Following the first presentation, subsequent pinacidil effects were less reliable.
As shown in Figure 1E and 1F, the hemodynamic impact of pinacidil in anesthetized rats was replicated in awake, head-posted mice (2 mice, 2 runs each). Presentation of 220 or 440 μM pinacidil evoked comparable mean increases in arterial diameter (peak diameter increase of ~20%) and parenchymal darkening (peak increase of ~2%), effects that were repeatable within subjects in a single session (N = 2).
Pinacidil does not have direct effect on nonvascular tissue
Pinacidil does not impact spiking probability or input resistance in neurons or membrane potential in neurons and astrocytes
In recordings from regular-spiking neurons of pyramidal shape (N = 30), we saw no change in any metric measured. At 50 seconds after application, approximately the time of peak vasodilatory effects in vivo, the resting membrane potential did not change (variation of −0.5 ± 0.9 mV standard deviation; Figure 2E), the spike rate induced by current injection did not change (variation of 0.1 ± 1.1 spikes/stimulation; Figure 2F), and input resistance did not change (variation of −0.7 ± 6.5 MΩ; Figure 2D). Similarly, in a limited subset of recordings from fast-spiking interneurons (N = 3), we did not observe any impact of pinacidil application. All significance tests were paired t tests (P > .10).
Astrocytes (N = 35) also showed no significant effects of pinacidil application, demonstrating only a slow depolarization during application of ethanol (1.3 ± 1.7 mV at 50 seconds after drug application) and pinacidil with ethanol (0.5 ± 2.1 mV) (Figure 2B). When we plotted the change in membrane potential at 10-second intervals since drug application (0 to 80 seconds post-drug), we found no trends in astrocytic response to either ACSF or pinacidil (Figure 2C).
During recordings at double our typical application dose (800 μM), we observed 2 pyramidal cells (out of 8) that showed depolarization (peak of 10 to 15 mV) and a loss of spike initiation capability. Following washout, these cells recovered membrane potential but spiking responses to current injection remained impaired. We did not evaluate the impact of this dose on vascular tone or rhythmic vasomotion.
In contrast to the absence of a detectable impact of pinacidil, we found that the membrane potentials of neurons and astrocytes were sensitive to flow rate. Decreasing flow rate caused a consistent depolarization of up to approximately 10 mV that showed an immediate onset, reaching a new baseline within 2 to 5 seconds; increasing flow rate had the opposite effect. In preliminary experiments, we observed two astrocytes that depolarized on switching to the pinacidil solution. These two recordings were obtained prior to placement of an inline pressure meter in the flow pathway that allowed us to monitor and exclude trials that showed flow changes. In the 35 subsequent recordings that did not have flow changes, we never observed a detectable impact of either ethanol or pinacidil on astroctyes or neurons. We also noted that neurons and astrocytes were more likely to die and/or to lose recording quality during a cycle of ethanol or pinacidil presentation, as opposed to ACSF presentation.
DISCUSSION
Pinacidil provides an effective means of inducing vasodilation in vivo. At concentrations less than 400 μM, pinacidil is also selective for cortical vascular smooth muscle, exhibiting no direct effect on intrinsic properties of neurons or astrocytes. As an independent means to induce increased vasodilation and blood volume in a manner analogous to that seen in functional hyperemia, pinacidil provides a viable method for testing the impact of hyperemic events on neural or astrocytic activity. Pinacidil may also be a selective means of emulating other normal hemodynamic phenomena and could have therapeutic applications, such as targeted administration of pinacidil in response to acute vessel obstruction to maintain sufficient perfusion.
The hemodynamic effects induced by pinacidil are similar to natural functional hyperemia. In SI during sensory stimulation in rodents, increases in total oxygenated hemoglobin during sensory stimulation— analogous to our measurement of cortical darkening at 550 nm—peak in a range of 2% to 5%,20–22 and arteries/arterioles dilate 10% to 20%.23 The time course of pinacidil’s effects also parallels the sustained response to continued sensory drive. Arterial diameter in rodent SI and the blood oxygen level–dependent response on functional magnetic resonance imaging in humans and rodents remain high when tactile input is sustained for periods lasting tens of seconds,23,24 as they do under pinacidil application.
Although pinacidil represents an important step forward in our ability to control blood flow while probing the impact of hemodynamics in cortex, it has limitations. The drug is only capable of producing vasodilation; drugs in the same family that block the SUR2B channels to create vasoconstriction (such as diazoxide or glibenclamide) or thromboxane receptor agonists18,25,26 are unfortunately known to be nonspecific, affecting neurons as well as blood vessels. Pinacidil is also not water-soluble, requiring its dissolution in ethanol or DMSO, agents that can have confounding impacts on the system. Applied in vivo, pinacidil also does not appear to wash out fully, or its impact on smooth muscles persists, so that the first trial in each animal is the most consistent and effective one. These limitations stated, this pharmacological approach nevertheless represents a unique means of selective hyperemia induction in vivo.
- Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol 1890; 11:85–108,158-7–158-17.
- Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA 1998; 95:765–772.
- Moore CI, Cao R. The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol 2008; 99:2035–2047.
- Garthwaite G, Bartus K, Malcolm D, et al. Signaling from blood vessels to CNS axons through nitric oxide. J Neurosci 2006; 26:7730–7740.
- Kozlov AS, Angulo MC, Audinat E, Charpak S. Target cellspecific modulation of neuronal activity by astrocytes. Proc Natl Acad Sci USA 2006; 103:10058–10063.
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 2003; 26:523–530.
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 2006; 86:1009–1031.
- Ashcroft FM, Gribble FM. New windows on the mechanism of action of K(ATP) channel openers. Trends Pharmacol Sci 2000; 21:439–445.
- Levin BE, Dunn-Meynell AA, Routh VH. Brain glucosensing and the K(ATP) channel. Nat Neurosci 2001; 4:459–460.
- Zawar C, Plant TD, Schirra C, Konnerth A, Neumcke B. Celltype specific expression of ATP-sensitive potassium channels in the rat hippocampus. J Physiol 1999; 514:327–341.
- Li L, Wu J, Jiang C. Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. J Membr Biol 2003; 196:61–69.
- Schwanstecher M, Sieverding C, Dorschner H, et al. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J 1998; 17:5529–5535.
- Shindo T, Yamada M, Isomoto S, Horio Y, Kurachi Y. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br J Pharmacol 1998; 124:985–991.
- Russ U, Lange U, Loffler-Walz C, Hambrock A, Quast U. Binding and effect of KATP channel openers in the absence of Mg2+. Br J Pharmacol 2003; 139:368–380.
- Higdon NR, Khan SA, Buchanan LV, Meisheri KD. Tissue and species variation in the vascular receptor binding of 3H-P1075, a potent KATP opener vasodilator. J Pharmacol Exp Ther 1997; 280:255–260.
- Wahl M. The effects of pinacidil and tolbutamide in feline pial arteries in situ. Pflugers Arch 1989; 415:250–252.
- Hempelmann RG, Barth HL, Mehdorn HM, Pradel RH, Ziegler A. Effects of potassium channel openers in isolated human cerebral arteries. Neurosurgery 1995; 37:1146–1153.
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 1997; 77:1165–1232.
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 2004; 1:31–37.
- Kong Y, Zheng Y, Johnston D, et al. A model of the dynamic relationship between blood flow and volume changes during brain activation. J Cereb Blood Flow Metab 2004; 24:1382–1392.
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage 2006; 32:33–48.
- Jones M, Devonshire IM, Berwick J, Martin C, Redgrave P, Mayhew J. Alterered neurovascular coupling during information-processing states. Eur J Neurosci 2008; 27:2758–2772.
- Woolsey TA, Rovainen CM, Cox SB, et al. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex 1996; 6:647–660.
- Moore CI, Stern CE, Corkin S, et al. Segregation of somatosensory activation in the human rolandic cortex using fMRI. J Neurophysiol 2000; 84:558–569.
- Crépel V, Krnjević K, Ben-Ari Y. Sulphonylureas reduce the slowly inactivating D-type outward current in rat hippocampal neurons. J Physiol 1993; 466:39–54.
- Lovick TA, Brown LA, Key BJ. Neuronal activity-related coupling in cortical arterioles: involvement of astrocyte-derived factors. Exp Physiol 2005; 90:131–140.
Interactions between the brain and blood are essential to health. Metabolic supply of the brain is provided through the vasculature, and disruptions of this relationship, in extreme cases such as stroke, is a key characteristic of neurologic disease. Neuro-hemodynamic coupling is also demonstrated in healthy individuals on faster time scales in functional hyperemia, the local increase in blood flow and volume that accompanies neural activity.1,2
We have recently proposed a further level of interdependence between the two systems —ie, the hemoneural hypothesis—which predicts that hemodynamic events such as functional hyperemia will modulate neural activity.3 An impact of hemodynamics on neurons could occur through a number of mechanisms, including the activation of mechanoreceptors on astrocytes or neurons, a thermal impact of increased blood flow on ion channels and vesicle release, and the local increase and diffusion of blood-borne factors such as nitric oxide.4,5 Astrocytes are predicted to play a key role in hemo-neural modulation, as they are tightly coupled to the vascular system and participate in a number of neural functions.6,7 Through these mechanisms and others, hemodynamics could shift the “state” of the local neural circuit, thereby impacting information processing. This regulation of neural dynamics could also provide a homeostatic mechanism for promoting healthy brain function (eg, prevention of kindling).
To study the impact of hyperemia on neural and astrocytic activity in vivo, it is essential to independently control blood flow in the brain with means that do not directly impact neurons or astrocytes. Pinacidil is a sulfonylurea receptor agonist that opens the SUR2B potassium-sensitive ATP channel.8 In the telencephalon, SUR1 receptors are localized to neurons and glia.9,10 In contrast, SUR2 receptors are localized to vasculature, with SUR2A in cardiac and skeletal muscle, and SUR2B in vascular smooth muscle, with primary expression in smaller arteries, arterioles, and capillaries.11 By opening the SUR2B channel, pinacidil hyperpolarizes and relaxes smooth muscle, causing vasodilation. Pinacidil is a potent and selective SUR2B agonist, with a dissociation constant of 135 nM and a half maximal effective concentation (EC50) value of 680 nM.12 This agonist is approximately 5 times more specific for SUR2B than for SUR2A and shows approximately 5 orders of magnitude lower affinity for SUR1 (in the mM range).12–15 Previous studies have demonstrated the efficacy of this agent as a vasodilator.16–18
In the present study, we systematically examined the utility of pinacidil for the selective induction of hyperemia. First, we quantified the vasodilation induced by pinacidil in vivo, and examined local increases in blood volume in the parenchyma. These studies were conducted in anesthetized rats and awake mice. Second, we used in vitro slice recordings to examine whether direct application of relatively high concentrations of pinacidil would have any impact on the physiology of neurons and astrocytes. We found that (1) in vivo, pinacidil induces a level of vasodilation and increased local blood volume consistent with natural functional hyperemia across a variety of preparations, and (2) in vitro, pinacidil has no detectable impact on intrinsic biophysical measures in neurons and astrocytes.
METHODS
Animal preparation in vivo
To probe the impact of pinacidil on arterial diameter and parenchymal blood volume in vivo, we measured the effects of topical application to the primary somatosensory cortex (SI) of rats and mice. Sprague-Dawley rats (250–500 g) and C57BL/6 mice (~25 g) were anaesthetized with pentobarbital (50 mg/kg intraperitoneally initial dose, followed by 5-mg supplements as needed for maintenance). Animals were maintained at approximately 37°C by a heating blanket. Craniotomy (diameter of ~2 mm in rats, ~1 mm in mice) and durotomy were performed over SI, and the cortex was protected with Kwik-Cast silicone elastomer sealant (WPI, Sarasota, FL) while an imaging chamber was attached with dental cement. Kwik-Cast was removed, and the chamber filled with 0.9% saline and sealed with a round cover glass (avoiding bubbles) secured with cyanoacrylate.
Controlling visualization during drug delivery in vivo
To minimize brain motion and flow artifacts during visualization of hemodynamics in the rat preparation, we constructed a customized pressurized chamber with inflow and outflow for constant perfusion. The volume of the chamber was approximately 0.3 mL, and the flow through the system averaged about 2 mL/min. The chamber consisted of a plastic ring 1 cm in diameter and 3 mm high with a flat-top profile and a base shaped to the angle of the lateral skull edge over SI. In the wall of this chamber, three large holes were drilled and patched with pieces cut from rubber NMR septa (VWR International, West Chester, PA) to create resealable ports for drug application and bubble removal. Three additional permanent holes were drilled in the chamber walls, through which blunted 1-cm lengths of 18-gauge stainless steel needles were wedged and affixed with Super glue: one for artificial cerebrospinal fluid (ACSF) inflow, one for combined outflow, and the third for pressure regulation. The overall pressure of the chamber was regulated by a small vertical tube whose height (and thus fluid level) could be adjusted on a manipulator stand, and whose other end was open to the atmosphere. Inflow and outflow were controlled via regulators on a gravity feed system. In the mouse preparation, the need to control visualization was addressed by maintaining a constant rate of wicking in a smaller-profile open chamber, and a microfluidic switch with 12 μL of dead space was added to minimize propulsive impact and delay due to switching between solutions. Drug and ethanol solutions were delivered to rat and mouse chambers after being heated to physiological temperature (37°C).
Optical measurement of hemodynamics in vivo
We used a charge-coupled device camera (the Roper 512B, Princeton Instruments, Trenton, NJ) to image the cortical surface at a frame rate of approximately 4 Hz, with illumination from a voltage-regulated xenon arc lamp. A green band-pass filter (550 nm) was used to maximize imaging near the isosbestic point of hemoglobin, providing optimal vessel contrast and a surrogate measure for blood volume change in the parenchyma. Lenses (50 and 125 mm) were arranged in series to form a macroscope.
Pinacidil administration in vivo
Pinacidil is hydrophobic and was therefore dissolved in ethanol at approximately 12 mg/kg and then diluted 1:100 in ACSF to achieve a 400-μM solution in 1% ethanol. Stock solutions were stored at −20°C and diluted in fresh ACSF for each experiment. For each run, the cortex was imaged for 3 minutes to establish baseline. For the pressurized rat chamber, at the end of the baseline period, 0.1 to 0.3 mL of 400 μM pinacidil in 1% ethanol in ACSF or saline would be pumped into the 0.3-mL chamber (taking about 1 second). Simultaneously, an equivalent volume was drawn out to balance pressure by a push-pull pump with access through two of the resealable rubber ports.
Pinacidil administration in vitro
Coronal slices were prepared from Sprague-Dawley rats at postnatal day 14 to 40 and maintained in a submersion chamber at 27°C for recording. Solutions were prepared in ACSF: 125 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 1.25 mM NaH2PO4, 2 mM CaCl2, 25 mM NaHCO3, and 25 mM d-glucose). The applied solutions were 1% ethanol, ~400 μM pinacidil in 1% ethanol, and ACSF, all perfused with 5% CO2 in 95% O2 (carbogen).
For astrocyte recordings, slices were incubated immediately after cutting for 20 minutes in ACSF containing 50 μM sulforhodamine 101 (SR101), a water-soluble fluorescent dye specifically taken up by astrocytes.19 The slices were then allowed to rest for an hour before recording as usual. For fluorescence, the light source was a 100-W mercury arc lamp, with excitation and barrier filters and dichroic mirrors tailored to the spectral characteristics of SR101 (excitation ~586 nm, emission ~605 nm)
Slices were imaged under differential interference contrast optics with infrared illumination. Cells in layers 2/3 and in the same field and plane of view as a blood vessel greater than 20 μm in diameter were targeted, and vessel expansion was monitored during intracellular recording at approximately 4 Hz with a cooled charge-coupled device camera (Retiga EX, QImaging, Surrey, BC, Canada) connected to the microscope via a parfocal C-mount. This configuration also enabled imaging of neurons and astrocytes in the slice.
Drug/control solutions were switched every 90 to 180 seconds. Recording pipettes were filled with 120 mM KGlu, 10 mM NaCl, 20 mM KCl, 10 mM HEPES, 2mM Mg-ATP, 0.3 mM Na-GTP, 0.5 mM EGTA, and 0.3% to 1% biocytin (wt/vol) for subsequent visualization of the neurons.
RESULTS
Pinacidil induces vasodilation in anesthetized rats and awake mice
Figure 1A shows the cortical surface over SI. The green bisection line over the MCA shows the point of sampling for the darkness plot in the rectangular box in Figure 1B. The width of this dark band is the width of the MCA over time, showing expansion after pinacidil addition (gray bar). The point at which dilation is observed corresponds to a darkening in a parenchymal region (red triangle in Figure 1A) and over the MCA and surrounding cortex (purple rectangle in Figure 1A, reflecting expansion). Vasodilation and parenchymal signal increases were consistently observed on the first trial in all experiments (4 first runs from 4 anesthetized rats, Figure 1C and 1D). Dilation began less than 10 seconds after drug arrival, with maximal dilation and parenchymal darkening at an approximately 50- to 60-second latency. Ethanol in a 1:100 solution with ACSF under the same conditions evoked a nonsignificant reduction in vessel diameter and no change in parenchymal darkening. Following the first presentation, subsequent pinacidil effects were less reliable.
As shown in Figure 1E and 1F, the hemodynamic impact of pinacidil in anesthetized rats was replicated in awake, head-posted mice (2 mice, 2 runs each). Presentation of 220 or 440 μM pinacidil evoked comparable mean increases in arterial diameter (peak diameter increase of ~20%) and parenchymal darkening (peak increase of ~2%), effects that were repeatable within subjects in a single session (N = 2).
Pinacidil does not have direct effect on nonvascular tissue
Pinacidil does not impact spiking probability or input resistance in neurons or membrane potential in neurons and astrocytes
In recordings from regular-spiking neurons of pyramidal shape (N = 30), we saw no change in any metric measured. At 50 seconds after application, approximately the time of peak vasodilatory effects in vivo, the resting membrane potential did not change (variation of −0.5 ± 0.9 mV standard deviation; Figure 2E), the spike rate induced by current injection did not change (variation of 0.1 ± 1.1 spikes/stimulation; Figure 2F), and input resistance did not change (variation of −0.7 ± 6.5 MΩ; Figure 2D). Similarly, in a limited subset of recordings from fast-spiking interneurons (N = 3), we did not observe any impact of pinacidil application. All significance tests were paired t tests (P > .10).
Astrocytes (N = 35) also showed no significant effects of pinacidil application, demonstrating only a slow depolarization during application of ethanol (1.3 ± 1.7 mV at 50 seconds after drug application) and pinacidil with ethanol (0.5 ± 2.1 mV) (Figure 2B). When we plotted the change in membrane potential at 10-second intervals since drug application (0 to 80 seconds post-drug), we found no trends in astrocytic response to either ACSF or pinacidil (Figure 2C).
During recordings at double our typical application dose (800 μM), we observed 2 pyramidal cells (out of 8) that showed depolarization (peak of 10 to 15 mV) and a loss of spike initiation capability. Following washout, these cells recovered membrane potential but spiking responses to current injection remained impaired. We did not evaluate the impact of this dose on vascular tone or rhythmic vasomotion.
In contrast to the absence of a detectable impact of pinacidil, we found that the membrane potentials of neurons and astrocytes were sensitive to flow rate. Decreasing flow rate caused a consistent depolarization of up to approximately 10 mV that showed an immediate onset, reaching a new baseline within 2 to 5 seconds; increasing flow rate had the opposite effect. In preliminary experiments, we observed two astrocytes that depolarized on switching to the pinacidil solution. These two recordings were obtained prior to placement of an inline pressure meter in the flow pathway that allowed us to monitor and exclude trials that showed flow changes. In the 35 subsequent recordings that did not have flow changes, we never observed a detectable impact of either ethanol or pinacidil on astroctyes or neurons. We also noted that neurons and astrocytes were more likely to die and/or to lose recording quality during a cycle of ethanol or pinacidil presentation, as opposed to ACSF presentation.
DISCUSSION
Pinacidil provides an effective means of inducing vasodilation in vivo. At concentrations less than 400 μM, pinacidil is also selective for cortical vascular smooth muscle, exhibiting no direct effect on intrinsic properties of neurons or astrocytes. As an independent means to induce increased vasodilation and blood volume in a manner analogous to that seen in functional hyperemia, pinacidil provides a viable method for testing the impact of hyperemic events on neural or astrocytic activity. Pinacidil may also be a selective means of emulating other normal hemodynamic phenomena and could have therapeutic applications, such as targeted administration of pinacidil in response to acute vessel obstruction to maintain sufficient perfusion.
The hemodynamic effects induced by pinacidil are similar to natural functional hyperemia. In SI during sensory stimulation in rodents, increases in total oxygenated hemoglobin during sensory stimulation— analogous to our measurement of cortical darkening at 550 nm—peak in a range of 2% to 5%,20–22 and arteries/arterioles dilate 10% to 20%.23 The time course of pinacidil’s effects also parallels the sustained response to continued sensory drive. Arterial diameter in rodent SI and the blood oxygen level–dependent response on functional magnetic resonance imaging in humans and rodents remain high when tactile input is sustained for periods lasting tens of seconds,23,24 as they do under pinacidil application.
Although pinacidil represents an important step forward in our ability to control blood flow while probing the impact of hemodynamics in cortex, it has limitations. The drug is only capable of producing vasodilation; drugs in the same family that block the SUR2B channels to create vasoconstriction (such as diazoxide or glibenclamide) or thromboxane receptor agonists18,25,26 are unfortunately known to be nonspecific, affecting neurons as well as blood vessels. Pinacidil is also not water-soluble, requiring its dissolution in ethanol or DMSO, agents that can have confounding impacts on the system. Applied in vivo, pinacidil also does not appear to wash out fully, or its impact on smooth muscles persists, so that the first trial in each animal is the most consistent and effective one. These limitations stated, this pharmacological approach nevertheless represents a unique means of selective hyperemia induction in vivo.
Interactions between the brain and blood are essential to health. Metabolic supply of the brain is provided through the vasculature, and disruptions of this relationship, in extreme cases such as stroke, is a key characteristic of neurologic disease. Neuro-hemodynamic coupling is also demonstrated in healthy individuals on faster time scales in functional hyperemia, the local increase in blood flow and volume that accompanies neural activity.1,2
We have recently proposed a further level of interdependence between the two systems —ie, the hemoneural hypothesis—which predicts that hemodynamic events such as functional hyperemia will modulate neural activity.3 An impact of hemodynamics on neurons could occur through a number of mechanisms, including the activation of mechanoreceptors on astrocytes or neurons, a thermal impact of increased blood flow on ion channels and vesicle release, and the local increase and diffusion of blood-borne factors such as nitric oxide.4,5 Astrocytes are predicted to play a key role in hemo-neural modulation, as they are tightly coupled to the vascular system and participate in a number of neural functions.6,7 Through these mechanisms and others, hemodynamics could shift the “state” of the local neural circuit, thereby impacting information processing. This regulation of neural dynamics could also provide a homeostatic mechanism for promoting healthy brain function (eg, prevention of kindling).
To study the impact of hyperemia on neural and astrocytic activity in vivo, it is essential to independently control blood flow in the brain with means that do not directly impact neurons or astrocytes. Pinacidil is a sulfonylurea receptor agonist that opens the SUR2B potassium-sensitive ATP channel.8 In the telencephalon, SUR1 receptors are localized to neurons and glia.9,10 In contrast, SUR2 receptors are localized to vasculature, with SUR2A in cardiac and skeletal muscle, and SUR2B in vascular smooth muscle, with primary expression in smaller arteries, arterioles, and capillaries.11 By opening the SUR2B channel, pinacidil hyperpolarizes and relaxes smooth muscle, causing vasodilation. Pinacidil is a potent and selective SUR2B agonist, with a dissociation constant of 135 nM and a half maximal effective concentation (EC50) value of 680 nM.12 This agonist is approximately 5 times more specific for SUR2B than for SUR2A and shows approximately 5 orders of magnitude lower affinity for SUR1 (in the mM range).12–15 Previous studies have demonstrated the efficacy of this agent as a vasodilator.16–18
In the present study, we systematically examined the utility of pinacidil for the selective induction of hyperemia. First, we quantified the vasodilation induced by pinacidil in vivo, and examined local increases in blood volume in the parenchyma. These studies were conducted in anesthetized rats and awake mice. Second, we used in vitro slice recordings to examine whether direct application of relatively high concentrations of pinacidil would have any impact on the physiology of neurons and astrocytes. We found that (1) in vivo, pinacidil induces a level of vasodilation and increased local blood volume consistent with natural functional hyperemia across a variety of preparations, and (2) in vitro, pinacidil has no detectable impact on intrinsic biophysical measures in neurons and astrocytes.
METHODS
Animal preparation in vivo
To probe the impact of pinacidil on arterial diameter and parenchymal blood volume in vivo, we measured the effects of topical application to the primary somatosensory cortex (SI) of rats and mice. Sprague-Dawley rats (250–500 g) and C57BL/6 mice (~25 g) were anaesthetized with pentobarbital (50 mg/kg intraperitoneally initial dose, followed by 5-mg supplements as needed for maintenance). Animals were maintained at approximately 37°C by a heating blanket. Craniotomy (diameter of ~2 mm in rats, ~1 mm in mice) and durotomy were performed over SI, and the cortex was protected with Kwik-Cast silicone elastomer sealant (WPI, Sarasota, FL) while an imaging chamber was attached with dental cement. Kwik-Cast was removed, and the chamber filled with 0.9% saline and sealed with a round cover glass (avoiding bubbles) secured with cyanoacrylate.
Controlling visualization during drug delivery in vivo
To minimize brain motion and flow artifacts during visualization of hemodynamics in the rat preparation, we constructed a customized pressurized chamber with inflow and outflow for constant perfusion. The volume of the chamber was approximately 0.3 mL, and the flow through the system averaged about 2 mL/min. The chamber consisted of a plastic ring 1 cm in diameter and 3 mm high with a flat-top profile and a base shaped to the angle of the lateral skull edge over SI. In the wall of this chamber, three large holes were drilled and patched with pieces cut from rubber NMR septa (VWR International, West Chester, PA) to create resealable ports for drug application and bubble removal. Three additional permanent holes were drilled in the chamber walls, through which blunted 1-cm lengths of 18-gauge stainless steel needles were wedged and affixed with Super glue: one for artificial cerebrospinal fluid (ACSF) inflow, one for combined outflow, and the third for pressure regulation. The overall pressure of the chamber was regulated by a small vertical tube whose height (and thus fluid level) could be adjusted on a manipulator stand, and whose other end was open to the atmosphere. Inflow and outflow were controlled via regulators on a gravity feed system. In the mouse preparation, the need to control visualization was addressed by maintaining a constant rate of wicking in a smaller-profile open chamber, and a microfluidic switch with 12 μL of dead space was added to minimize propulsive impact and delay due to switching between solutions. Drug and ethanol solutions were delivered to rat and mouse chambers after being heated to physiological temperature (37°C).
Optical measurement of hemodynamics in vivo
We used a charge-coupled device camera (the Roper 512B, Princeton Instruments, Trenton, NJ) to image the cortical surface at a frame rate of approximately 4 Hz, with illumination from a voltage-regulated xenon arc lamp. A green band-pass filter (550 nm) was used to maximize imaging near the isosbestic point of hemoglobin, providing optimal vessel contrast and a surrogate measure for blood volume change in the parenchyma. Lenses (50 and 125 mm) were arranged in series to form a macroscope.
Pinacidil administration in vivo
Pinacidil is hydrophobic and was therefore dissolved in ethanol at approximately 12 mg/kg and then diluted 1:100 in ACSF to achieve a 400-μM solution in 1% ethanol. Stock solutions were stored at −20°C and diluted in fresh ACSF for each experiment. For each run, the cortex was imaged for 3 minutes to establish baseline. For the pressurized rat chamber, at the end of the baseline period, 0.1 to 0.3 mL of 400 μM pinacidil in 1% ethanol in ACSF or saline would be pumped into the 0.3-mL chamber (taking about 1 second). Simultaneously, an equivalent volume was drawn out to balance pressure by a push-pull pump with access through two of the resealable rubber ports.
Pinacidil administration in vitro
Coronal slices were prepared from Sprague-Dawley rats at postnatal day 14 to 40 and maintained in a submersion chamber at 27°C for recording. Solutions were prepared in ACSF: 125 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 1.25 mM NaH2PO4, 2 mM CaCl2, 25 mM NaHCO3, and 25 mM d-glucose). The applied solutions were 1% ethanol, ~400 μM pinacidil in 1% ethanol, and ACSF, all perfused with 5% CO2 in 95% O2 (carbogen).
For astrocyte recordings, slices were incubated immediately after cutting for 20 minutes in ACSF containing 50 μM sulforhodamine 101 (SR101), a water-soluble fluorescent dye specifically taken up by astrocytes.19 The slices were then allowed to rest for an hour before recording as usual. For fluorescence, the light source was a 100-W mercury arc lamp, with excitation and barrier filters and dichroic mirrors tailored to the spectral characteristics of SR101 (excitation ~586 nm, emission ~605 nm)
Slices were imaged under differential interference contrast optics with infrared illumination. Cells in layers 2/3 and in the same field and plane of view as a blood vessel greater than 20 μm in diameter were targeted, and vessel expansion was monitored during intracellular recording at approximately 4 Hz with a cooled charge-coupled device camera (Retiga EX, QImaging, Surrey, BC, Canada) connected to the microscope via a parfocal C-mount. This configuration also enabled imaging of neurons and astrocytes in the slice.
Drug/control solutions were switched every 90 to 180 seconds. Recording pipettes were filled with 120 mM KGlu, 10 mM NaCl, 20 mM KCl, 10 mM HEPES, 2mM Mg-ATP, 0.3 mM Na-GTP, 0.5 mM EGTA, and 0.3% to 1% biocytin (wt/vol) for subsequent visualization of the neurons.
RESULTS
Pinacidil induces vasodilation in anesthetized rats and awake mice
Figure 1A shows the cortical surface over SI. The green bisection line over the MCA shows the point of sampling for the darkness plot in the rectangular box in Figure 1B. The width of this dark band is the width of the MCA over time, showing expansion after pinacidil addition (gray bar). The point at which dilation is observed corresponds to a darkening in a parenchymal region (red triangle in Figure 1A) and over the MCA and surrounding cortex (purple rectangle in Figure 1A, reflecting expansion). Vasodilation and parenchymal signal increases were consistently observed on the first trial in all experiments (4 first runs from 4 anesthetized rats, Figure 1C and 1D). Dilation began less than 10 seconds after drug arrival, with maximal dilation and parenchymal darkening at an approximately 50- to 60-second latency. Ethanol in a 1:100 solution with ACSF under the same conditions evoked a nonsignificant reduction in vessel diameter and no change in parenchymal darkening. Following the first presentation, subsequent pinacidil effects were less reliable.
As shown in Figure 1E and 1F, the hemodynamic impact of pinacidil in anesthetized rats was replicated in awake, head-posted mice (2 mice, 2 runs each). Presentation of 220 or 440 μM pinacidil evoked comparable mean increases in arterial diameter (peak diameter increase of ~20%) and parenchymal darkening (peak increase of ~2%), effects that were repeatable within subjects in a single session (N = 2).
Pinacidil does not have direct effect on nonvascular tissue
Pinacidil does not impact spiking probability or input resistance in neurons or membrane potential in neurons and astrocytes
In recordings from regular-spiking neurons of pyramidal shape (N = 30), we saw no change in any metric measured. At 50 seconds after application, approximately the time of peak vasodilatory effects in vivo, the resting membrane potential did not change (variation of −0.5 ± 0.9 mV standard deviation; Figure 2E), the spike rate induced by current injection did not change (variation of 0.1 ± 1.1 spikes/stimulation; Figure 2F), and input resistance did not change (variation of −0.7 ± 6.5 MΩ; Figure 2D). Similarly, in a limited subset of recordings from fast-spiking interneurons (N = 3), we did not observe any impact of pinacidil application. All significance tests were paired t tests (P > .10).
Astrocytes (N = 35) also showed no significant effects of pinacidil application, demonstrating only a slow depolarization during application of ethanol (1.3 ± 1.7 mV at 50 seconds after drug application) and pinacidil with ethanol (0.5 ± 2.1 mV) (Figure 2B). When we plotted the change in membrane potential at 10-second intervals since drug application (0 to 80 seconds post-drug), we found no trends in astrocytic response to either ACSF or pinacidil (Figure 2C).
During recordings at double our typical application dose (800 μM), we observed 2 pyramidal cells (out of 8) that showed depolarization (peak of 10 to 15 mV) and a loss of spike initiation capability. Following washout, these cells recovered membrane potential but spiking responses to current injection remained impaired. We did not evaluate the impact of this dose on vascular tone or rhythmic vasomotion.
In contrast to the absence of a detectable impact of pinacidil, we found that the membrane potentials of neurons and astrocytes were sensitive to flow rate. Decreasing flow rate caused a consistent depolarization of up to approximately 10 mV that showed an immediate onset, reaching a new baseline within 2 to 5 seconds; increasing flow rate had the opposite effect. In preliminary experiments, we observed two astrocytes that depolarized on switching to the pinacidil solution. These two recordings were obtained prior to placement of an inline pressure meter in the flow pathway that allowed us to monitor and exclude trials that showed flow changes. In the 35 subsequent recordings that did not have flow changes, we never observed a detectable impact of either ethanol or pinacidil on astroctyes or neurons. We also noted that neurons and astrocytes were more likely to die and/or to lose recording quality during a cycle of ethanol or pinacidil presentation, as opposed to ACSF presentation.
DISCUSSION
Pinacidil provides an effective means of inducing vasodilation in vivo. At concentrations less than 400 μM, pinacidil is also selective for cortical vascular smooth muscle, exhibiting no direct effect on intrinsic properties of neurons or astrocytes. As an independent means to induce increased vasodilation and blood volume in a manner analogous to that seen in functional hyperemia, pinacidil provides a viable method for testing the impact of hyperemic events on neural or astrocytic activity. Pinacidil may also be a selective means of emulating other normal hemodynamic phenomena and could have therapeutic applications, such as targeted administration of pinacidil in response to acute vessel obstruction to maintain sufficient perfusion.
The hemodynamic effects induced by pinacidil are similar to natural functional hyperemia. In SI during sensory stimulation in rodents, increases in total oxygenated hemoglobin during sensory stimulation— analogous to our measurement of cortical darkening at 550 nm—peak in a range of 2% to 5%,20–22 and arteries/arterioles dilate 10% to 20%.23 The time course of pinacidil’s effects also parallels the sustained response to continued sensory drive. Arterial diameter in rodent SI and the blood oxygen level–dependent response on functional magnetic resonance imaging in humans and rodents remain high when tactile input is sustained for periods lasting tens of seconds,23,24 as they do under pinacidil application.
Although pinacidil represents an important step forward in our ability to control blood flow while probing the impact of hemodynamics in cortex, it has limitations. The drug is only capable of producing vasodilation; drugs in the same family that block the SUR2B channels to create vasoconstriction (such as diazoxide or glibenclamide) or thromboxane receptor agonists18,25,26 are unfortunately known to be nonspecific, affecting neurons as well as blood vessels. Pinacidil is also not water-soluble, requiring its dissolution in ethanol or DMSO, agents that can have confounding impacts on the system. Applied in vivo, pinacidil also does not appear to wash out fully, or its impact on smooth muscles persists, so that the first trial in each animal is the most consistent and effective one. These limitations stated, this pharmacological approach nevertheless represents a unique means of selective hyperemia induction in vivo.
- Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol 1890; 11:85–108,158-7–158-17.
- Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA 1998; 95:765–772.
- Moore CI, Cao R. The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol 2008; 99:2035–2047.
- Garthwaite G, Bartus K, Malcolm D, et al. Signaling from blood vessels to CNS axons through nitric oxide. J Neurosci 2006; 26:7730–7740.
- Kozlov AS, Angulo MC, Audinat E, Charpak S. Target cellspecific modulation of neuronal activity by astrocytes. Proc Natl Acad Sci USA 2006; 103:10058–10063.
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 2003; 26:523–530.
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 2006; 86:1009–1031.
- Ashcroft FM, Gribble FM. New windows on the mechanism of action of K(ATP) channel openers. Trends Pharmacol Sci 2000; 21:439–445.
- Levin BE, Dunn-Meynell AA, Routh VH. Brain glucosensing and the K(ATP) channel. Nat Neurosci 2001; 4:459–460.
- Zawar C, Plant TD, Schirra C, Konnerth A, Neumcke B. Celltype specific expression of ATP-sensitive potassium channels in the rat hippocampus. J Physiol 1999; 514:327–341.
- Li L, Wu J, Jiang C. Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. J Membr Biol 2003; 196:61–69.
- Schwanstecher M, Sieverding C, Dorschner H, et al. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J 1998; 17:5529–5535.
- Shindo T, Yamada M, Isomoto S, Horio Y, Kurachi Y. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br J Pharmacol 1998; 124:985–991.
- Russ U, Lange U, Loffler-Walz C, Hambrock A, Quast U. Binding and effect of KATP channel openers in the absence of Mg2+. Br J Pharmacol 2003; 139:368–380.
- Higdon NR, Khan SA, Buchanan LV, Meisheri KD. Tissue and species variation in the vascular receptor binding of 3H-P1075, a potent KATP opener vasodilator. J Pharmacol Exp Ther 1997; 280:255–260.
- Wahl M. The effects of pinacidil and tolbutamide in feline pial arteries in situ. Pflugers Arch 1989; 415:250–252.
- Hempelmann RG, Barth HL, Mehdorn HM, Pradel RH, Ziegler A. Effects of potassium channel openers in isolated human cerebral arteries. Neurosurgery 1995; 37:1146–1153.
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 1997; 77:1165–1232.
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 2004; 1:31–37.
- Kong Y, Zheng Y, Johnston D, et al. A model of the dynamic relationship between blood flow and volume changes during brain activation. J Cereb Blood Flow Metab 2004; 24:1382–1392.
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage 2006; 32:33–48.
- Jones M, Devonshire IM, Berwick J, Martin C, Redgrave P, Mayhew J. Alterered neurovascular coupling during information-processing states. Eur J Neurosci 2008; 27:2758–2772.
- Woolsey TA, Rovainen CM, Cox SB, et al. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex 1996; 6:647–660.
- Moore CI, Stern CE, Corkin S, et al. Segregation of somatosensory activation in the human rolandic cortex using fMRI. J Neurophysiol 2000; 84:558–569.
- Crépel V, Krnjević K, Ben-Ari Y. Sulphonylureas reduce the slowly inactivating D-type outward current in rat hippocampal neurons. J Physiol 1993; 466:39–54.
- Lovick TA, Brown LA, Key BJ. Neuronal activity-related coupling in cortical arterioles: involvement of astrocyte-derived factors. Exp Physiol 2005; 90:131–140.
- Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol 1890; 11:85–108,158-7–158-17.
- Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA 1998; 95:765–772.
- Moore CI, Cao R. The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol 2008; 99:2035–2047.
- Garthwaite G, Bartus K, Malcolm D, et al. Signaling from blood vessels to CNS axons through nitric oxide. J Neurosci 2006; 26:7730–7740.
- Kozlov AS, Angulo MC, Audinat E, Charpak S. Target cellspecific modulation of neuronal activity by astrocytes. Proc Natl Acad Sci USA 2006; 103:10058–10063.
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 2003; 26:523–530.
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 2006; 86:1009–1031.
- Ashcroft FM, Gribble FM. New windows on the mechanism of action of K(ATP) channel openers. Trends Pharmacol Sci 2000; 21:439–445.
- Levin BE, Dunn-Meynell AA, Routh VH. Brain glucosensing and the K(ATP) channel. Nat Neurosci 2001; 4:459–460.
- Zawar C, Plant TD, Schirra C, Konnerth A, Neumcke B. Celltype specific expression of ATP-sensitive potassium channels in the rat hippocampus. J Physiol 1999; 514:327–341.
- Li L, Wu J, Jiang C. Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. J Membr Biol 2003; 196:61–69.
- Schwanstecher M, Sieverding C, Dorschner H, et al. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J 1998; 17:5529–5535.
- Shindo T, Yamada M, Isomoto S, Horio Y, Kurachi Y. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br J Pharmacol 1998; 124:985–991.
- Russ U, Lange U, Loffler-Walz C, Hambrock A, Quast U. Binding and effect of KATP channel openers in the absence of Mg2+. Br J Pharmacol 2003; 139:368–380.
- Higdon NR, Khan SA, Buchanan LV, Meisheri KD. Tissue and species variation in the vascular receptor binding of 3H-P1075, a potent KATP opener vasodilator. J Pharmacol Exp Ther 1997; 280:255–260.
- Wahl M. The effects of pinacidil and tolbutamide in feline pial arteries in situ. Pflugers Arch 1989; 415:250–252.
- Hempelmann RG, Barth HL, Mehdorn HM, Pradel RH, Ziegler A. Effects of potassium channel openers in isolated human cerebral arteries. Neurosurgery 1995; 37:1146–1153.
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 1997; 77:1165–1232.
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 2004; 1:31–37.
- Kong Y, Zheng Y, Johnston D, et al. A model of the dynamic relationship between blood flow and volume changes during brain activation. J Cereb Blood Flow Metab 2004; 24:1382–1392.
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage 2006; 32:33–48.
- Jones M, Devonshire IM, Berwick J, Martin C, Redgrave P, Mayhew J. Alterered neurovascular coupling during information-processing states. Eur J Neurosci 2008; 27:2758–2772.
- Woolsey TA, Rovainen CM, Cox SB, et al. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex 1996; 6:647–660.
- Moore CI, Stern CE, Corkin S, et al. Segregation of somatosensory activation in the human rolandic cortex using fMRI. J Neurophysiol 2000; 84:558–569.
- Crépel V, Krnjević K, Ben-Ari Y. Sulphonylureas reduce the slowly inactivating D-type outward current in rat hippocampal neurons. J Physiol 1993; 466:39–54.
- Lovick TA, Brown LA, Key BJ. Neuronal activity-related coupling in cortical arterioles: involvement of astrocyte-derived factors. Exp Physiol 2005; 90:131–140.