User login
Sublingual buprenorphine plus buprenorphine XR for opioid use disorder
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
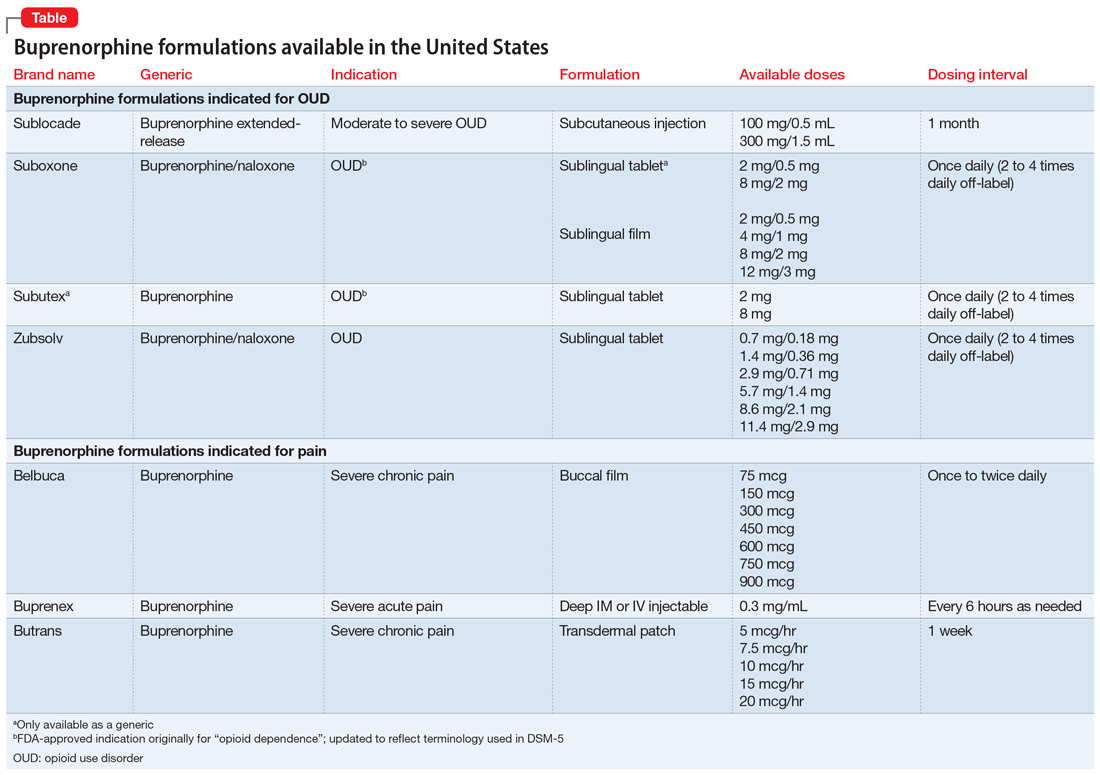
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4
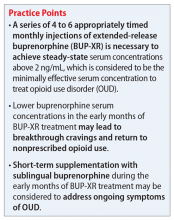
However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4

However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4

However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281