User login
2016 Update on infectious disease
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
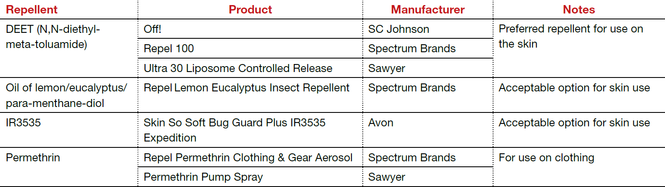
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.
Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.

Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.

Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
In this article
• Azithromycin vs doxycycline for chlamydia
• Insect repellents to prevent Zika virus