User login
Delving Deeper
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 32-year-old, previously healthy woman presented to the emergency department (ED) with 3 days of nasal pain, congestion, and cough. A day prior, she had consulted with her primary care provider by phone and had been prescribed amoxicillin-clavulanate for presumed bacterial sinusitis. She subsequently developed fever (39 oC) and pleuritic, left-upper-quadrant abdominal pain. In the ED, chest radiograph demonstrated right hilar opacification. Laboratory studies and computed tomography (CT) of the abdomen and pelvis did not identify a cause for her pain. Given the pleuritic nature of her left-upper-quadrant pain, CT pulmonary angiography was ordered. The CT revealed “mass-like” right hilar opacification and lymphadenopathy. No pulmonary emboli were identified. Levofloxacin was prescribed for presumed pneumonia, and the patient was discharged home. The following week, mediastinal biopsy was arranged for evaluation of the right hilar abnormality.
This is a young woman presenting with upper respiratory symptoms, abdominal pain, fever, and hilar lymphadenopathy. Upper respiratory symptoms are common and usually indicate an inflammatory response to allergens or infection, though autoimmune disorders may affect the upper airways. Fever and hilar lymphadenopathy likely also signify an inflammatory response. Taken together, these findings can be associated with mycobacterial or fungal infection, malignancy, and, particularly in a young woman, sarcoidosis, which could explain her abdominal pain if her presentation included splenomegaly. At this point she likely has a systemic illness involving at least the upper, and possibly the lower, respiratory tract.
Within days, her symptoms resolved. Mediastinal biopsy of the hilar node revealed scant pus. Pathology demonstrated suppurative granulomata. Gram stain; bacterial, mycobacterial, and fungal cultures; and 16S ribosomal analyses for bacteria and fungi from the biopsy were unrevealing. For unclear reasons, prior to the biopsy, she was given intramuscular Haemophilus influenzae type B and tetanus, diphtheria, and pertussis vaccines. Two weeks later, she presented again with fever and left-upper-quadrant pain as well as painful skin nodules at her biopsy and vaccination sites. She was admitted for further evaluation. Chest CT showed expansion of the mediastinal lesion and splenic enlargement. Biopsy of a skin lesion revealed suppurative granulomatous dermatitis and panniculitis. Repeat blood cultures were negative, though serum β-D-glucan was weakly positive at 173 pg/mL (reference range, <60 pg/mL). Tissue cultures and Gram, acid-fast, Fite, and Warthin-Starry stains from the skin biopsy were negative. She was discharged on fluconazole and then readmitted 2 days later with dyspnea, fever, and leukocytosis.
The young woman’s symptoms resolved, only to recur days later; her granulomatous hilar lesions grew larger, and new cutaneous and splenic findings appeared. The granulomatous lesions prompt consideration of infectious, malignant, and immune-mediated processes. The negative cultures make infection less likely, although the elevated β-D-glucan may suggest fungal infection. By description, the skin lesions are consistent with pathergy, a phenomenon characterized by trauma-provoked cutaneous lesions or ulcers, which is associated with numerous syndromes, including Behçet syndrome, inflammatory bowel disease, and neutrophilic dermatoses such as pyoderma gangrenosum (PG) and Sweet syndrome. In addition to details about her medical history, it is important to seek evidence of oral ulcers or vasculitis, as Behçet syndrome may be associated with cutaneous, visceral, and ophthalmologic vasculitis.
Her medical history included hypertension and active, 10-pack-year cigarette use. During childhood, she had occasional ingrown hairs and folliculitis. She did not take medications prior to this acute illness. Family history was notable for cardiovascular disease. She rarely consumed alcohol and did not use illicit drugs. She lived in a rural town in the mid–Willamette Valley of Oregon and worked as an administrative assistant. She spent time outdoors, including trail running and golfing. A case of tularemia was recently reported in an area near her home. Her only travel outside of Oregon was to Puerto Vallarta, Mexico, 16 years previously. She grew up on a farm and had no known tuberculosis exposure.
Tularemia is an interesting diagnostic consideration and could explain her fever, cutaneous lesions, and hilar adenopathy. It is plausible that she had clinically mild pneumonic tularemia at the outset and that her cutaneous lesions are variants of ulceroglandular tularemia. Positive antibodies for Francisella tularensis would be expected if this were the cause of her illness. The ingrown hairs raise the possibility of a primary immune deficiency syndrome predisposing her to abscesses. However, they seem to have been of trivial significance to her, making an immune deficiency syndrome unlikely.
On readmission, she was afebrile, normotensive, and tachycardic (114 beats/min), with a normal respiratory rate and oxygen saturation. She was not ill appearing. She had noninjected conjunctiva and no oral lesions. Apart from tachycardia, cardiovascular examination was unremarkable. Abdominal examination was notable for mild distension and a palpable, tender spleen. Musculoskeletal and neurologic examinations were normal. Her skin was notable for various sized (8 cm × 4 cm to 10 cm × 15 cm) painful ulcers with violaceous, friable borders—some with fluctuance and purulent drainage—on her right hand, bilateral arms, right axilla, sternum, and legs (Figure 1).

Laboratory studies were notable for normocytic anemia (hemoglobin, 8.9 g/dL; range, 12.0-16.0 g/dL), leukocytosis (white blood cells, 24,900/µL; range, 4500-11,000/µL), thrombocytosis (platelet count, 690,000/µL; range, 150,000-400,000/µL), and elevated inflammatory markers (C-reactive protein, 33 mg/dL; range, <0.5 mg/dL; erythrocyte sedimentation rate, 78 mm/h; range, <20 mm/h). A complete metabolic panel was within normal limits. Repeat blood cultures and β -D-glucan and 16S ribosomal assays were negative. Polymerase chain reaction testing for Bartonella henselae was negative. Urine probes for Neisseria gonorrhoeae and Chlamydia trachomatis were negative. Rapid plasma regain (RPR) was negative. Antibodies to toxoplasmosis, histoplasmosis, blastomycosis, and aspergillosis were unrevealing. A Coccidioides test by immunodiffusion was negative. Serum antigen tests for Cryptococcus and Epstein-Barr virus (EBV) were negative. EBV, HIV, and hepatitis antibody tests were negative. Rheumatologic studies, including antinuclear, anti-double-stranded DNA, anti-Smith, anti–Sjögren syndrome antigens A and B, anticentromere, anti-topoisomerase (anti-Scl-70), anti-histidyl-transfer-RNA-synthetase (anti-Jo-1), and anti-nucleosome (anti-chromatic) antibodies, were unrevealing. Levels of angiotensin-converting enzyme, rheumatoid factor, complement, cytoplasmic, and perinuclear antineutrophil cytoplasmic antibodies were also normal. A neutrophil oxidative burst test was negative. In addition, peripheral flow cytology and serum and urine protein electrophoresis were negative. Chest CT revealed bilateral lower lobe consolidations concerning for necrotizing pneumonia, splenic enlargement, numerous hypodense splenic lesions, and a 1.3-cm right hilar node, which had decreased in size compared with 1 month prior.
In summary, the patient presented with recurrent upper respiratory symptoms, fever, and abdominal pain; expanding granulomatous hilar lesions, splenomegaly, and cutaneous lesions consistent with pathergy; elevated inflammatory markers and leukocytosis; and a possible exposure to F tularensis. She has had extensive negative infectious workups, except for a weakly positive β-D-glucan, and completed several courses of apparently unhelpful antimicrobials. At this point, the most notable findings are her splenomegaly and inflammatory masses suggesting an inflammatory process, which may be autoimmune in nature. Both vasculitis and sarcoidosis remain possibilities, and malignancy is possible. Given her possible exposure to F tularensis, obtaining serum antibodies to F tularensis, in addition to biopsies of the skin lesions, is advisable.
Laboratory studies revealed a positive F tularensis antibody with a titer of 1:320 and an IgM of 7 U/mL and IgG of 30 U/mL. This was repeated, revealing a titer of 1:540 and an IgM and IgG of 5 U/mL and 20 U/mL, respectively. Given the potential exposure history, the clinical syndrome compatible with tularemia, and an otherwise extensive yet unrevealing evaluation, she was treated with a 10-day course of streptomycin. Her fever persisted, and the splenic lesions increased in size and number, prompting addition of moxifloxacin without apparent benefit. Skin biopsies taken from the patient’s arm were notable for nodular, suppurative, neutrophilic infiltrates and histiocytes in the medium and deep dermis without multinucleated histiocytes or evidence of vasculitis. Fungal, mycobacterial, and bacterial stains from the biopsy were negative. The findings were consistent with but not diagnostic of an acute neutrophilic dermatosis.
At this point, the patient has a confirmed exposure to F tularensis; she also has persistent fever, progressive splenomegaly, and new skin biopsies consistent with neutrophilic dermatosis. Despite the F tularensis antibody positivity, her negative cultures and lack of improvement with multiple courses of antimicrobials argue against an infectious etiology. Accordingly, malignancy should be considered but seems less likely given that no laboratory, imaging, or tissue samples support it. This leaves immune-mediated etiologies, especially autoimmune conditions associated with neutrophilic dermatoses, as the most likely explanation of her inflammatory syndrome. Neutrophilic dermatoses include some vasculitides, Sweet syndrome, PG, Behçet syndrome, and other inflammatory entities. She has no evidence of vasculitis on biopsy. Given the evidence of inflammation and the history of pathergy, Behçet syndrome and PG should be seriously considered.
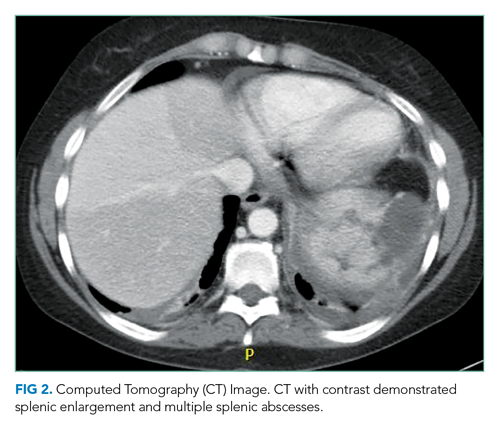
She underwent incision and drainage of the left leg and mediastinal lesions. A follow-up chest CT revealed stable cutaneous and deep tissue lesions and continued splenic enlargement. She was started on prednisone and dapsone for presumed cutaneous and visceral PG. The lesions improved dramatically and, following a month-long hospitalization, she was discharged on dapsone and a slow prednisone taper. Three weeks after discharge, while on dapsone and prednisone, she developed a new skin lesion. Cyclosporine was added, with improvement. Eight weeks after discharge, she developed fever, acute left-upper-quadrant pain, and marked splenomegaly with abscesses seen on CT imaging (Figure 2).
This continues to be a very puzzling case, and it is worth revisiting her clinical course once again. This is a previously healthy 32-year-old woman with multiple hospital presentations for upper-respiratory symptoms, persistent fever, abdominal pain, and painful cutaneous lesions consistent with pathergy; she was found to have granulomatous hilar lesions, progressive splenomegaly, and skin biopsies consistent with neutrophilic dermatosis. Exhaustive infectious and rheumatologic workup was negative, and no evident malignancy was found. Finally, despite multiple courses of antimicrobials, including standard treatments for tularemia (for which she had positive antibodies), her clinical course failed to improve until the addition of systemic anti-inflammatory agents, which resulted in rapid improvement. She then presented 8 weeks later with recurrent fever and splenomegaly. Given the recurrence and the severity of the splenic pathology, a diagnostic splenectomy is advisable for what appears to be visceral PG. In addition, attempting to identify a trigger of her syndrome is important. PG can be associated with inflammatory bowel disease, hematologic disorders (eg, leukemia, myeloma, myelodysplastic syndrome, and myelofibrosis), and autoimmune diseases, especially inflammatory arthritis.1 Therefore, a diagnostic colonoscopy and bone marrow biopsy should be considered. With no history or examination supporting inflammatory arthritis and a broad, unrevealing workup, her rheumatologic evaluation is sufficient.
The patient underwent splenectomy. Gross description of the spleen was notable for multiple abscesses, consisting on microscopy of large areas of necrosis with islands of dense neutrophil collections (Figure 3). Microscopic examination failed to demonstrate microorganisms on multiple stains, and there was no microscopic or flow cytometric evidence of lymphoma. The final pathologic diagnosis was multiple sterile splenic abscesses with siderosis, which, in the context of her overall syndrome, was consistent with an entity termed aseptic abscess syndrome (AAS). After discharge, she underwent a slow steroid taper and was ultimately maintained on daily low-dose prednisone. Cyclosporine and dapsone were discontinued in favor of infliximab infusions. She underwent additional diagnostic workup, including an unremarkable colonoscopy and a bone marrow biopsy, which showed monoclonal gammopathy of undetermined significance (MGUS) with an insignificant IgA monoclonal gammopathy. All cutaneous lesions healed. Three years after the splenectomy, while still on infliximab and prednisone, she developed a new aseptic lung abscess, which resolved after increasing her prednisone dose. Six years after splenectomy, she developed an aseptic liver abscess, which resolved after again increasing the frequency of her infliximab infusions.

DISCUSSION
Diagnostic uncertainty is an intrinsic feature of medical practice—in part because patients often present with undifferentiated and evolving symptoms.2 When faced with uncertainty, clinicians are well served by prioritizing a thoughtful differential diagnosis, adopting a stepwise management strategy, and engaging in iterative reassessments of the patient. In this case, a 32-year-old, previously healthy woman presented with an array of symptoms, including abdominal pain, fever, leukocytosis, necrotic skin lesions, necrotizing mediastinal lymphadenitis, pathergy, and splenomegaly. Elements of the history, examination, and diagnostic studies supported a differential diagnosis of tularemia, PG, and AAS. Through stepwise management and ongoing reassessment, she was ultimately diagnosed with AAS.
Tularemia was initially an important diagnostic consideration in this patient, given her potential exposure and positive F tularensis serum antibodies. Francisella tularensis is a Gram-negative coccobacillus found in more than 250 species of fish, ticks, birds, and mammals. In humans, an incubation period of 3 to 5 days is typical. Although clinical manifestations vary, they often include fever, headache, and malaise.3 Other findings may include lymphadenopathy with or without ulcerative cutaneous lesions (glandular or ulceroglandular tularemia) and cough, dyspnea, pleuritic chest pain, and hilar adenopathy (pneumonic tularemia). As noted by the discussant, a pneumonic tularemia syndrome could have explained this patient’s fever, respiratory symptoms, and hilar adenopathy; ulceroglandular tularemia might have explained her cutaneous lesions. Since splenomegaly may be seen in tularemia, this finding was also consistent with the diagnosis. Serum antibody testing is supportive of the diagnosis, while culture confirms it. Standard treatment consists of a 10- to 14-day course of streptomycin, and combination therapy with a fluoroquinolone is recommended in severe cases.4 In this patient, however, F tularensis was not demonstrated on culture. Furthermore, she did not experience the expected clinical improvement with treatment. Finally, because both IgG and IgM tularemia antibodies may co-occur up to 10 years following infection, her positive F tularensis serum antibodies did not provide evidence of acute infection.5
Recognizing inconsistencies in the diagnosis of tularemia, the focus shifted to PG owing to the patient’s neutrophilic cutaneous lesions, negative infectious workup, and pathergy. Pyoderma gangrenosum is a neutrophilic dermatosis—one of a heterogeneous group of skin conditions characterized by perivascular and diffuse neutrophilic infiltrates without an identifiable infectious agent.6 It is a chronic, recurrent cutaneous disease with several variants.7 The classic presentation includes painful lower-extremity ulcers with violaceous undermined borders and may be associated with pathergy. Guiding principles for the management of PG include controlling inflammation, optimizing wound healing, and minimizing exacerbating factors.1 As such, treatment mainstays include local and systemic anti-inflammatory agents and wound care. As the discussant highlighted, in this case the inflammatory skin lesions were suggestive of PG. However, other features of the case, notably, splenomegaly, splenic abscesses, and necrotizing mediastinal lymphadenitis, were more consistent with another diagnosis: AAS. Aseptic abscess syndrome is an autoinflammatory disorder defined by deep, noninfectious abscesses that preferentially affect the spleen.8 Additional clinical manifestations include weight loss, fever, abdominal pain, and leukocytosis. Lesions may also affect bone, kidney, liver, lung, lymph node, and skin. In one case series, neutrophilic dermatoses were seen in 20% of AAS cases.8 In all cases of AAS, extensive infectious workup is unrevealing, and antibiotics are ineffective. The pathophysiology of AAS is unknown.
Similar to PG, the majority of AAS cases are associated with inflammatory bowel disease, especially Crohn disease.9 However, AAS also has associations with conditions such as MGUS, rheumatoid arthritis, spondyloarthritis, and relapsing polychondritis. Histologically, early lesions demonstrate a necrotic core of neutrophils, with or without surrounding palisading histiocytes, and giant cells. In older lesions, neutrophils may be absent; fibrous tissue may be present.8 Treatment regimens include splenectomy, corticosteroids, colchicine, thalidomide, tumor necrosis factor (TNF) antagonists, and cyclophosphamide. The discussant astutely recommended a splenectomy for this patient, which was both diagnostic and therapeutic. As in this case, relapse is common. Optimal maintenance therapy is yet to be determined.9
Given the overlapping clinical manifestations, shared disease associations, and similar responsiveness to immunosuppression, it is unclear whether AAS represents a new disease entity or a variant of known autoinflammatory disorders. Aseptic abscess syndrome is likely part of a spectrum of autoinflammatory disorders with inflammatory bowel diseases, neutrophilic dermatoses, and other similar diseases.8 While infectious visceral abscesses remain more common, this case highlights the clinical manifestation of an emerging and likely underrecognized entity.
TEACHING POINTS
- Aseptic abscess syndrome should be considered in patients who present with visceral (particularly splenic) abscesses and negative infectious workup.
- Aseptic abscess syndrome is commonly associated with other autoinflammatory disorders; the majority of reported cases are associated with inflammatory bowel disease, especially Crohn disease.
- Up to 20% of AAS cases are associated with neutrophilic dermatoses such as PG.
- The initial treatment for this syndrome is high-dose intravenous glucocorticoids; maintenance treatment regimens include corticosteroids, colchicine, thalidomide, TNF antagonists, and cyclophosphamide.
Acknowledgments
The authors would thank Dr Bob Pelz and Dr John Townes for their contributions to the case.
1. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191-211. https://doi.org/10.2165/11595240-000000000-00000
2. Bhise V, Rajan SS, Sittig DF, Morgan RO, Chaudhary P, Singh H. Defining and measuring diagnostic uncertainty in medicine: a systematic review. J Gen Intern Med. 2018;33(1):103-115. https://doi.org/10.1007/s11606-017-4164-1
3. Penn RL. Francisella tualerensis (Tularemia). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Elsevier Saunders; 2015:2590-2602.
4. Eliasson H, Broman T, Forsman M, Bäck E. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am. 2006;20(2):289-311. https://doi.org/10.1016/j.idc.2006.03.002
5. Bevanger L, Maeland JA, Kvan AI. Comparative analysis of antibodies to Francisella tularensis antigens during the acute phase of tularemia and eight years later. Clin Diagn Lab Immunol. 1994;1(2):238-240.
6. Moschella SL, Davis MDP. Neutrophilic dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Saunders; 2012:424-438.
7. Dabade TS, Davis MDP. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet’s syndrome). Dermatol Ther. 2011;24(2):273-284. https://doi/org/10.1111/j.1529-8019.2011.01403.x
8. André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86(3):145-161. https://doi/org/10.1097/md.0b013e18064f9f3
9. Fillman H, Riquelme P, Sullivan PD, Mansoor AM. Aseptic abscess syndrome. BMJ Case Rep. 2020;13(10):e236437. https://doi.org/10.1136/bcr-2020-236437
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 32-year-old, previously healthy woman presented to the emergency department (ED) with 3 days of nasal pain, congestion, and cough. A day prior, she had consulted with her primary care provider by phone and had been prescribed amoxicillin-clavulanate for presumed bacterial sinusitis. She subsequently developed fever (39 oC) and pleuritic, left-upper-quadrant abdominal pain. In the ED, chest radiograph demonstrated right hilar opacification. Laboratory studies and computed tomography (CT) of the abdomen and pelvis did not identify a cause for her pain. Given the pleuritic nature of her left-upper-quadrant pain, CT pulmonary angiography was ordered. The CT revealed “mass-like” right hilar opacification and lymphadenopathy. No pulmonary emboli were identified. Levofloxacin was prescribed for presumed pneumonia, and the patient was discharged home. The following week, mediastinal biopsy was arranged for evaluation of the right hilar abnormality.
This is a young woman presenting with upper respiratory symptoms, abdominal pain, fever, and hilar lymphadenopathy. Upper respiratory symptoms are common and usually indicate an inflammatory response to allergens or infection, though autoimmune disorders may affect the upper airways. Fever and hilar lymphadenopathy likely also signify an inflammatory response. Taken together, these findings can be associated with mycobacterial or fungal infection, malignancy, and, particularly in a young woman, sarcoidosis, which could explain her abdominal pain if her presentation included splenomegaly. At this point she likely has a systemic illness involving at least the upper, and possibly the lower, respiratory tract.
Within days, her symptoms resolved. Mediastinal biopsy of the hilar node revealed scant pus. Pathology demonstrated suppurative granulomata. Gram stain; bacterial, mycobacterial, and fungal cultures; and 16S ribosomal analyses for bacteria and fungi from the biopsy were unrevealing. For unclear reasons, prior to the biopsy, she was given intramuscular Haemophilus influenzae type B and tetanus, diphtheria, and pertussis vaccines. Two weeks later, she presented again with fever and left-upper-quadrant pain as well as painful skin nodules at her biopsy and vaccination sites. She was admitted for further evaluation. Chest CT showed expansion of the mediastinal lesion and splenic enlargement. Biopsy of a skin lesion revealed suppurative granulomatous dermatitis and panniculitis. Repeat blood cultures were negative, though serum β-D-glucan was weakly positive at 173 pg/mL (reference range, <60 pg/mL). Tissue cultures and Gram, acid-fast, Fite, and Warthin-Starry stains from the skin biopsy were negative. She was discharged on fluconazole and then readmitted 2 days later with dyspnea, fever, and leukocytosis.
The young woman’s symptoms resolved, only to recur days later; her granulomatous hilar lesions grew larger, and new cutaneous and splenic findings appeared. The granulomatous lesions prompt consideration of infectious, malignant, and immune-mediated processes. The negative cultures make infection less likely, although the elevated β-D-glucan may suggest fungal infection. By description, the skin lesions are consistent with pathergy, a phenomenon characterized by trauma-provoked cutaneous lesions or ulcers, which is associated with numerous syndromes, including Behçet syndrome, inflammatory bowel disease, and neutrophilic dermatoses such as pyoderma gangrenosum (PG) and Sweet syndrome. In addition to details about her medical history, it is important to seek evidence of oral ulcers or vasculitis, as Behçet syndrome may be associated with cutaneous, visceral, and ophthalmologic vasculitis.
Her medical history included hypertension and active, 10-pack-year cigarette use. During childhood, she had occasional ingrown hairs and folliculitis. She did not take medications prior to this acute illness. Family history was notable for cardiovascular disease. She rarely consumed alcohol and did not use illicit drugs. She lived in a rural town in the mid–Willamette Valley of Oregon and worked as an administrative assistant. She spent time outdoors, including trail running and golfing. A case of tularemia was recently reported in an area near her home. Her only travel outside of Oregon was to Puerto Vallarta, Mexico, 16 years previously. She grew up on a farm and had no known tuberculosis exposure.
Tularemia is an interesting diagnostic consideration and could explain her fever, cutaneous lesions, and hilar adenopathy. It is plausible that she had clinically mild pneumonic tularemia at the outset and that her cutaneous lesions are variants of ulceroglandular tularemia. Positive antibodies for Francisella tularensis would be expected if this were the cause of her illness. The ingrown hairs raise the possibility of a primary immune deficiency syndrome predisposing her to abscesses. However, they seem to have been of trivial significance to her, making an immune deficiency syndrome unlikely.
On readmission, she was afebrile, normotensive, and tachycardic (114 beats/min), with a normal respiratory rate and oxygen saturation. She was not ill appearing. She had noninjected conjunctiva and no oral lesions. Apart from tachycardia, cardiovascular examination was unremarkable. Abdominal examination was notable for mild distension and a palpable, tender spleen. Musculoskeletal and neurologic examinations were normal. Her skin was notable for various sized (8 cm × 4 cm to 10 cm × 15 cm) painful ulcers with violaceous, friable borders—some with fluctuance and purulent drainage—on her right hand, bilateral arms, right axilla, sternum, and legs (Figure 1).

Laboratory studies were notable for normocytic anemia (hemoglobin, 8.9 g/dL; range, 12.0-16.0 g/dL), leukocytosis (white blood cells, 24,900/µL; range, 4500-11,000/µL), thrombocytosis (platelet count, 690,000/µL; range, 150,000-400,000/µL), and elevated inflammatory markers (C-reactive protein, 33 mg/dL; range, <0.5 mg/dL; erythrocyte sedimentation rate, 78 mm/h; range, <20 mm/h). A complete metabolic panel was within normal limits. Repeat blood cultures and β -D-glucan and 16S ribosomal assays were negative. Polymerase chain reaction testing for Bartonella henselae was negative. Urine probes for Neisseria gonorrhoeae and Chlamydia trachomatis were negative. Rapid plasma regain (RPR) was negative. Antibodies to toxoplasmosis, histoplasmosis, blastomycosis, and aspergillosis were unrevealing. A Coccidioides test by immunodiffusion was negative. Serum antigen tests for Cryptococcus and Epstein-Barr virus (EBV) were negative. EBV, HIV, and hepatitis antibody tests were negative. Rheumatologic studies, including antinuclear, anti-double-stranded DNA, anti-Smith, anti–Sjögren syndrome antigens A and B, anticentromere, anti-topoisomerase (anti-Scl-70), anti-histidyl-transfer-RNA-synthetase (anti-Jo-1), and anti-nucleosome (anti-chromatic) antibodies, were unrevealing. Levels of angiotensin-converting enzyme, rheumatoid factor, complement, cytoplasmic, and perinuclear antineutrophil cytoplasmic antibodies were also normal. A neutrophil oxidative burst test was negative. In addition, peripheral flow cytology and serum and urine protein electrophoresis were negative. Chest CT revealed bilateral lower lobe consolidations concerning for necrotizing pneumonia, splenic enlargement, numerous hypodense splenic lesions, and a 1.3-cm right hilar node, which had decreased in size compared with 1 month prior.
In summary, the patient presented with recurrent upper respiratory symptoms, fever, and abdominal pain; expanding granulomatous hilar lesions, splenomegaly, and cutaneous lesions consistent with pathergy; elevated inflammatory markers and leukocytosis; and a possible exposure to F tularensis. She has had extensive negative infectious workups, except for a weakly positive β-D-glucan, and completed several courses of apparently unhelpful antimicrobials. At this point, the most notable findings are her splenomegaly and inflammatory masses suggesting an inflammatory process, which may be autoimmune in nature. Both vasculitis and sarcoidosis remain possibilities, and malignancy is possible. Given her possible exposure to F tularensis, obtaining serum antibodies to F tularensis, in addition to biopsies of the skin lesions, is advisable.
Laboratory studies revealed a positive F tularensis antibody with a titer of 1:320 and an IgM of 7 U/mL and IgG of 30 U/mL. This was repeated, revealing a titer of 1:540 and an IgM and IgG of 5 U/mL and 20 U/mL, respectively. Given the potential exposure history, the clinical syndrome compatible with tularemia, and an otherwise extensive yet unrevealing evaluation, she was treated with a 10-day course of streptomycin. Her fever persisted, and the splenic lesions increased in size and number, prompting addition of moxifloxacin without apparent benefit. Skin biopsies taken from the patient’s arm were notable for nodular, suppurative, neutrophilic infiltrates and histiocytes in the medium and deep dermis without multinucleated histiocytes or evidence of vasculitis. Fungal, mycobacterial, and bacterial stains from the biopsy were negative. The findings were consistent with but not diagnostic of an acute neutrophilic dermatosis.
At this point, the patient has a confirmed exposure to F tularensis; she also has persistent fever, progressive splenomegaly, and new skin biopsies consistent with neutrophilic dermatosis. Despite the F tularensis antibody positivity, her negative cultures and lack of improvement with multiple courses of antimicrobials argue against an infectious etiology. Accordingly, malignancy should be considered but seems less likely given that no laboratory, imaging, or tissue samples support it. This leaves immune-mediated etiologies, especially autoimmune conditions associated with neutrophilic dermatoses, as the most likely explanation of her inflammatory syndrome. Neutrophilic dermatoses include some vasculitides, Sweet syndrome, PG, Behçet syndrome, and other inflammatory entities. She has no evidence of vasculitis on biopsy. Given the evidence of inflammation and the history of pathergy, Behçet syndrome and PG should be seriously considered.
She underwent incision and drainage of the left leg and mediastinal lesions. A follow-up chest CT revealed stable cutaneous and deep tissue lesions and continued splenic enlargement. She was started on prednisone and dapsone for presumed cutaneous and visceral PG. The lesions improved dramatically and, following a month-long hospitalization, she was discharged on dapsone and a slow prednisone taper. Three weeks after discharge, while on dapsone and prednisone, she developed a new skin lesion. Cyclosporine was added, with improvement. Eight weeks after discharge, she developed fever, acute left-upper-quadrant pain, and marked splenomegaly with abscesses seen on CT imaging (Figure 2).

This continues to be a very puzzling case, and it is worth revisiting her clinical course once again. This is a previously healthy 32-year-old woman with multiple hospital presentations for upper-respiratory symptoms, persistent fever, abdominal pain, and painful cutaneous lesions consistent with pathergy; she was found to have granulomatous hilar lesions, progressive splenomegaly, and skin biopsies consistent with neutrophilic dermatosis. Exhaustive infectious and rheumatologic workup was negative, and no evident malignancy was found. Finally, despite multiple courses of antimicrobials, including standard treatments for tularemia (for which she had positive antibodies), her clinical course failed to improve until the addition of systemic anti-inflammatory agents, which resulted in rapid improvement. She then presented 8 weeks later with recurrent fever and splenomegaly. Given the recurrence and the severity of the splenic pathology, a diagnostic splenectomy is advisable for what appears to be visceral PG. In addition, attempting to identify a trigger of her syndrome is important. PG can be associated with inflammatory bowel disease, hematologic disorders (eg, leukemia, myeloma, myelodysplastic syndrome, and myelofibrosis), and autoimmune diseases, especially inflammatory arthritis.1 Therefore, a diagnostic colonoscopy and bone marrow biopsy should be considered. With no history or examination supporting inflammatory arthritis and a broad, unrevealing workup, her rheumatologic evaluation is sufficient.
The patient underwent splenectomy. Gross description of the spleen was notable for multiple abscesses, consisting on microscopy of large areas of necrosis with islands of dense neutrophil collections (Figure 3). Microscopic examination failed to demonstrate microorganisms on multiple stains, and there was no microscopic or flow cytometric evidence of lymphoma. The final pathologic diagnosis was multiple sterile splenic abscesses with siderosis, which, in the context of her overall syndrome, was consistent with an entity termed aseptic abscess syndrome (AAS). After discharge, she underwent a slow steroid taper and was ultimately maintained on daily low-dose prednisone. Cyclosporine and dapsone were discontinued in favor of infliximab infusions. She underwent additional diagnostic workup, including an unremarkable colonoscopy and a bone marrow biopsy, which showed monoclonal gammopathy of undetermined significance (MGUS) with an insignificant IgA monoclonal gammopathy. All cutaneous lesions healed. Three years after the splenectomy, while still on infliximab and prednisone, she developed a new aseptic lung abscess, which resolved after increasing her prednisone dose. Six years after splenectomy, she developed an aseptic liver abscess, which resolved after again increasing the frequency of her infliximab infusions.

DISCUSSION
Diagnostic uncertainty is an intrinsic feature of medical practice—in part because patients often present with undifferentiated and evolving symptoms.2 When faced with uncertainty, clinicians are well served by prioritizing a thoughtful differential diagnosis, adopting a stepwise management strategy, and engaging in iterative reassessments of the patient. In this case, a 32-year-old, previously healthy woman presented with an array of symptoms, including abdominal pain, fever, leukocytosis, necrotic skin lesions, necrotizing mediastinal lymphadenitis, pathergy, and splenomegaly. Elements of the history, examination, and diagnostic studies supported a differential diagnosis of tularemia, PG, and AAS. Through stepwise management and ongoing reassessment, she was ultimately diagnosed with AAS.
Tularemia was initially an important diagnostic consideration in this patient, given her potential exposure and positive F tularensis serum antibodies. Francisella tularensis is a Gram-negative coccobacillus found in more than 250 species of fish, ticks, birds, and mammals. In humans, an incubation period of 3 to 5 days is typical. Although clinical manifestations vary, they often include fever, headache, and malaise.3 Other findings may include lymphadenopathy with or without ulcerative cutaneous lesions (glandular or ulceroglandular tularemia) and cough, dyspnea, pleuritic chest pain, and hilar adenopathy (pneumonic tularemia). As noted by the discussant, a pneumonic tularemia syndrome could have explained this patient’s fever, respiratory symptoms, and hilar adenopathy; ulceroglandular tularemia might have explained her cutaneous lesions. Since splenomegaly may be seen in tularemia, this finding was also consistent with the diagnosis. Serum antibody testing is supportive of the diagnosis, while culture confirms it. Standard treatment consists of a 10- to 14-day course of streptomycin, and combination therapy with a fluoroquinolone is recommended in severe cases.4 In this patient, however, F tularensis was not demonstrated on culture. Furthermore, she did not experience the expected clinical improvement with treatment. Finally, because both IgG and IgM tularemia antibodies may co-occur up to 10 years following infection, her positive F tularensis serum antibodies did not provide evidence of acute infection.5
Recognizing inconsistencies in the diagnosis of tularemia, the focus shifted to PG owing to the patient’s neutrophilic cutaneous lesions, negative infectious workup, and pathergy. Pyoderma gangrenosum is a neutrophilic dermatosis—one of a heterogeneous group of skin conditions characterized by perivascular and diffuse neutrophilic infiltrates without an identifiable infectious agent.6 It is a chronic, recurrent cutaneous disease with several variants.7 The classic presentation includes painful lower-extremity ulcers with violaceous undermined borders and may be associated with pathergy. Guiding principles for the management of PG include controlling inflammation, optimizing wound healing, and minimizing exacerbating factors.1 As such, treatment mainstays include local and systemic anti-inflammatory agents and wound care. As the discussant highlighted, in this case the inflammatory skin lesions were suggestive of PG. However, other features of the case, notably, splenomegaly, splenic abscesses, and necrotizing mediastinal lymphadenitis, were more consistent with another diagnosis: AAS. Aseptic abscess syndrome is an autoinflammatory disorder defined by deep, noninfectious abscesses that preferentially affect the spleen.8 Additional clinical manifestations include weight loss, fever, abdominal pain, and leukocytosis. Lesions may also affect bone, kidney, liver, lung, lymph node, and skin. In one case series, neutrophilic dermatoses were seen in 20% of AAS cases.8 In all cases of AAS, extensive infectious workup is unrevealing, and antibiotics are ineffective. The pathophysiology of AAS is unknown.
Similar to PG, the majority of AAS cases are associated with inflammatory bowel disease, especially Crohn disease.9 However, AAS also has associations with conditions such as MGUS, rheumatoid arthritis, spondyloarthritis, and relapsing polychondritis. Histologically, early lesions demonstrate a necrotic core of neutrophils, with or without surrounding palisading histiocytes, and giant cells. In older lesions, neutrophils may be absent; fibrous tissue may be present.8 Treatment regimens include splenectomy, corticosteroids, colchicine, thalidomide, tumor necrosis factor (TNF) antagonists, and cyclophosphamide. The discussant astutely recommended a splenectomy for this patient, which was both diagnostic and therapeutic. As in this case, relapse is common. Optimal maintenance therapy is yet to be determined.9
Given the overlapping clinical manifestations, shared disease associations, and similar responsiveness to immunosuppression, it is unclear whether AAS represents a new disease entity or a variant of known autoinflammatory disorders. Aseptic abscess syndrome is likely part of a spectrum of autoinflammatory disorders with inflammatory bowel diseases, neutrophilic dermatoses, and other similar diseases.8 While infectious visceral abscesses remain more common, this case highlights the clinical manifestation of an emerging and likely underrecognized entity.
TEACHING POINTS
- Aseptic abscess syndrome should be considered in patients who present with visceral (particularly splenic) abscesses and negative infectious workup.
- Aseptic abscess syndrome is commonly associated with other autoinflammatory disorders; the majority of reported cases are associated with inflammatory bowel disease, especially Crohn disease.
- Up to 20% of AAS cases are associated with neutrophilic dermatoses such as PG.
- The initial treatment for this syndrome is high-dose intravenous glucocorticoids; maintenance treatment regimens include corticosteroids, colchicine, thalidomide, TNF antagonists, and cyclophosphamide.
Acknowledgments
The authors would thank Dr Bob Pelz and Dr John Townes for their contributions to the case.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 32-year-old, previously healthy woman presented to the emergency department (ED) with 3 days of nasal pain, congestion, and cough. A day prior, she had consulted with her primary care provider by phone and had been prescribed amoxicillin-clavulanate for presumed bacterial sinusitis. She subsequently developed fever (39 oC) and pleuritic, left-upper-quadrant abdominal pain. In the ED, chest radiograph demonstrated right hilar opacification. Laboratory studies and computed tomography (CT) of the abdomen and pelvis did not identify a cause for her pain. Given the pleuritic nature of her left-upper-quadrant pain, CT pulmonary angiography was ordered. The CT revealed “mass-like” right hilar opacification and lymphadenopathy. No pulmonary emboli were identified. Levofloxacin was prescribed for presumed pneumonia, and the patient was discharged home. The following week, mediastinal biopsy was arranged for evaluation of the right hilar abnormality.
This is a young woman presenting with upper respiratory symptoms, abdominal pain, fever, and hilar lymphadenopathy. Upper respiratory symptoms are common and usually indicate an inflammatory response to allergens or infection, though autoimmune disorders may affect the upper airways. Fever and hilar lymphadenopathy likely also signify an inflammatory response. Taken together, these findings can be associated with mycobacterial or fungal infection, malignancy, and, particularly in a young woman, sarcoidosis, which could explain her abdominal pain if her presentation included splenomegaly. At this point she likely has a systemic illness involving at least the upper, and possibly the lower, respiratory tract.
Within days, her symptoms resolved. Mediastinal biopsy of the hilar node revealed scant pus. Pathology demonstrated suppurative granulomata. Gram stain; bacterial, mycobacterial, and fungal cultures; and 16S ribosomal analyses for bacteria and fungi from the biopsy were unrevealing. For unclear reasons, prior to the biopsy, she was given intramuscular Haemophilus influenzae type B and tetanus, diphtheria, and pertussis vaccines. Two weeks later, she presented again with fever and left-upper-quadrant pain as well as painful skin nodules at her biopsy and vaccination sites. She was admitted for further evaluation. Chest CT showed expansion of the mediastinal lesion and splenic enlargement. Biopsy of a skin lesion revealed suppurative granulomatous dermatitis and panniculitis. Repeat blood cultures were negative, though serum β-D-glucan was weakly positive at 173 pg/mL (reference range, <60 pg/mL). Tissue cultures and Gram, acid-fast, Fite, and Warthin-Starry stains from the skin biopsy were negative. She was discharged on fluconazole and then readmitted 2 days later with dyspnea, fever, and leukocytosis.
The young woman’s symptoms resolved, only to recur days later; her granulomatous hilar lesions grew larger, and new cutaneous and splenic findings appeared. The granulomatous lesions prompt consideration of infectious, malignant, and immune-mediated processes. The negative cultures make infection less likely, although the elevated β-D-glucan may suggest fungal infection. By description, the skin lesions are consistent with pathergy, a phenomenon characterized by trauma-provoked cutaneous lesions or ulcers, which is associated with numerous syndromes, including Behçet syndrome, inflammatory bowel disease, and neutrophilic dermatoses such as pyoderma gangrenosum (PG) and Sweet syndrome. In addition to details about her medical history, it is important to seek evidence of oral ulcers or vasculitis, as Behçet syndrome may be associated with cutaneous, visceral, and ophthalmologic vasculitis.
Her medical history included hypertension and active, 10-pack-year cigarette use. During childhood, she had occasional ingrown hairs and folliculitis. She did not take medications prior to this acute illness. Family history was notable for cardiovascular disease. She rarely consumed alcohol and did not use illicit drugs. She lived in a rural town in the mid–Willamette Valley of Oregon and worked as an administrative assistant. She spent time outdoors, including trail running and golfing. A case of tularemia was recently reported in an area near her home. Her only travel outside of Oregon was to Puerto Vallarta, Mexico, 16 years previously. She grew up on a farm and had no known tuberculosis exposure.
Tularemia is an interesting diagnostic consideration and could explain her fever, cutaneous lesions, and hilar adenopathy. It is plausible that she had clinically mild pneumonic tularemia at the outset and that her cutaneous lesions are variants of ulceroglandular tularemia. Positive antibodies for Francisella tularensis would be expected if this were the cause of her illness. The ingrown hairs raise the possibility of a primary immune deficiency syndrome predisposing her to abscesses. However, they seem to have been of trivial significance to her, making an immune deficiency syndrome unlikely.
On readmission, she was afebrile, normotensive, and tachycardic (114 beats/min), with a normal respiratory rate and oxygen saturation. She was not ill appearing. She had noninjected conjunctiva and no oral lesions. Apart from tachycardia, cardiovascular examination was unremarkable. Abdominal examination was notable for mild distension and a palpable, tender spleen. Musculoskeletal and neurologic examinations were normal. Her skin was notable for various sized (8 cm × 4 cm to 10 cm × 15 cm) painful ulcers with violaceous, friable borders—some with fluctuance and purulent drainage—on her right hand, bilateral arms, right axilla, sternum, and legs (Figure 1).

Laboratory studies were notable for normocytic anemia (hemoglobin, 8.9 g/dL; range, 12.0-16.0 g/dL), leukocytosis (white blood cells, 24,900/µL; range, 4500-11,000/µL), thrombocytosis (platelet count, 690,000/µL; range, 150,000-400,000/µL), and elevated inflammatory markers (C-reactive protein, 33 mg/dL; range, <0.5 mg/dL; erythrocyte sedimentation rate, 78 mm/h; range, <20 mm/h). A complete metabolic panel was within normal limits. Repeat blood cultures and β -D-glucan and 16S ribosomal assays were negative. Polymerase chain reaction testing for Bartonella henselae was negative. Urine probes for Neisseria gonorrhoeae and Chlamydia trachomatis were negative. Rapid plasma regain (RPR) was negative. Antibodies to toxoplasmosis, histoplasmosis, blastomycosis, and aspergillosis were unrevealing. A Coccidioides test by immunodiffusion was negative. Serum antigen tests for Cryptococcus and Epstein-Barr virus (EBV) were negative. EBV, HIV, and hepatitis antibody tests were negative. Rheumatologic studies, including antinuclear, anti-double-stranded DNA, anti-Smith, anti–Sjögren syndrome antigens A and B, anticentromere, anti-topoisomerase (anti-Scl-70), anti-histidyl-transfer-RNA-synthetase (anti-Jo-1), and anti-nucleosome (anti-chromatic) antibodies, were unrevealing. Levels of angiotensin-converting enzyme, rheumatoid factor, complement, cytoplasmic, and perinuclear antineutrophil cytoplasmic antibodies were also normal. A neutrophil oxidative burst test was negative. In addition, peripheral flow cytology and serum and urine protein electrophoresis were negative. Chest CT revealed bilateral lower lobe consolidations concerning for necrotizing pneumonia, splenic enlargement, numerous hypodense splenic lesions, and a 1.3-cm right hilar node, which had decreased in size compared with 1 month prior.
In summary, the patient presented with recurrent upper respiratory symptoms, fever, and abdominal pain; expanding granulomatous hilar lesions, splenomegaly, and cutaneous lesions consistent with pathergy; elevated inflammatory markers and leukocytosis; and a possible exposure to F tularensis. She has had extensive negative infectious workups, except for a weakly positive β-D-glucan, and completed several courses of apparently unhelpful antimicrobials. At this point, the most notable findings are her splenomegaly and inflammatory masses suggesting an inflammatory process, which may be autoimmune in nature. Both vasculitis and sarcoidosis remain possibilities, and malignancy is possible. Given her possible exposure to F tularensis, obtaining serum antibodies to F tularensis, in addition to biopsies of the skin lesions, is advisable.
Laboratory studies revealed a positive F tularensis antibody with a titer of 1:320 and an IgM of 7 U/mL and IgG of 30 U/mL. This was repeated, revealing a titer of 1:540 and an IgM and IgG of 5 U/mL and 20 U/mL, respectively. Given the potential exposure history, the clinical syndrome compatible with tularemia, and an otherwise extensive yet unrevealing evaluation, she was treated with a 10-day course of streptomycin. Her fever persisted, and the splenic lesions increased in size and number, prompting addition of moxifloxacin without apparent benefit. Skin biopsies taken from the patient’s arm were notable for nodular, suppurative, neutrophilic infiltrates and histiocytes in the medium and deep dermis without multinucleated histiocytes or evidence of vasculitis. Fungal, mycobacterial, and bacterial stains from the biopsy were negative. The findings were consistent with but not diagnostic of an acute neutrophilic dermatosis.
At this point, the patient has a confirmed exposure to F tularensis; she also has persistent fever, progressive splenomegaly, and new skin biopsies consistent with neutrophilic dermatosis. Despite the F tularensis antibody positivity, her negative cultures and lack of improvement with multiple courses of antimicrobials argue against an infectious etiology. Accordingly, malignancy should be considered but seems less likely given that no laboratory, imaging, or tissue samples support it. This leaves immune-mediated etiologies, especially autoimmune conditions associated with neutrophilic dermatoses, as the most likely explanation of her inflammatory syndrome. Neutrophilic dermatoses include some vasculitides, Sweet syndrome, PG, Behçet syndrome, and other inflammatory entities. She has no evidence of vasculitis on biopsy. Given the evidence of inflammation and the history of pathergy, Behçet syndrome and PG should be seriously considered.
She underwent incision and drainage of the left leg and mediastinal lesions. A follow-up chest CT revealed stable cutaneous and deep tissue lesions and continued splenic enlargement. She was started on prednisone and dapsone for presumed cutaneous and visceral PG. The lesions improved dramatically and, following a month-long hospitalization, she was discharged on dapsone and a slow prednisone taper. Three weeks after discharge, while on dapsone and prednisone, she developed a new skin lesion. Cyclosporine was added, with improvement. Eight weeks after discharge, she developed fever, acute left-upper-quadrant pain, and marked splenomegaly with abscesses seen on CT imaging (Figure 2).

This continues to be a very puzzling case, and it is worth revisiting her clinical course once again. This is a previously healthy 32-year-old woman with multiple hospital presentations for upper-respiratory symptoms, persistent fever, abdominal pain, and painful cutaneous lesions consistent with pathergy; she was found to have granulomatous hilar lesions, progressive splenomegaly, and skin biopsies consistent with neutrophilic dermatosis. Exhaustive infectious and rheumatologic workup was negative, and no evident malignancy was found. Finally, despite multiple courses of antimicrobials, including standard treatments for tularemia (for which she had positive antibodies), her clinical course failed to improve until the addition of systemic anti-inflammatory agents, which resulted in rapid improvement. She then presented 8 weeks later with recurrent fever and splenomegaly. Given the recurrence and the severity of the splenic pathology, a diagnostic splenectomy is advisable for what appears to be visceral PG. In addition, attempting to identify a trigger of her syndrome is important. PG can be associated with inflammatory bowel disease, hematologic disorders (eg, leukemia, myeloma, myelodysplastic syndrome, and myelofibrosis), and autoimmune diseases, especially inflammatory arthritis.1 Therefore, a diagnostic colonoscopy and bone marrow biopsy should be considered. With no history or examination supporting inflammatory arthritis and a broad, unrevealing workup, her rheumatologic evaluation is sufficient.
The patient underwent splenectomy. Gross description of the spleen was notable for multiple abscesses, consisting on microscopy of large areas of necrosis with islands of dense neutrophil collections (Figure 3). Microscopic examination failed to demonstrate microorganisms on multiple stains, and there was no microscopic or flow cytometric evidence of lymphoma. The final pathologic diagnosis was multiple sterile splenic abscesses with siderosis, which, in the context of her overall syndrome, was consistent with an entity termed aseptic abscess syndrome (AAS). After discharge, she underwent a slow steroid taper and was ultimately maintained on daily low-dose prednisone. Cyclosporine and dapsone were discontinued in favor of infliximab infusions. She underwent additional diagnostic workup, including an unremarkable colonoscopy and a bone marrow biopsy, which showed monoclonal gammopathy of undetermined significance (MGUS) with an insignificant IgA monoclonal gammopathy. All cutaneous lesions healed. Three years after the splenectomy, while still on infliximab and prednisone, she developed a new aseptic lung abscess, which resolved after increasing her prednisone dose. Six years after splenectomy, she developed an aseptic liver abscess, which resolved after again increasing the frequency of her infliximab infusions.

DISCUSSION
Diagnostic uncertainty is an intrinsic feature of medical practice—in part because patients often present with undifferentiated and evolving symptoms.2 When faced with uncertainty, clinicians are well served by prioritizing a thoughtful differential diagnosis, adopting a stepwise management strategy, and engaging in iterative reassessments of the patient. In this case, a 32-year-old, previously healthy woman presented with an array of symptoms, including abdominal pain, fever, leukocytosis, necrotic skin lesions, necrotizing mediastinal lymphadenitis, pathergy, and splenomegaly. Elements of the history, examination, and diagnostic studies supported a differential diagnosis of tularemia, PG, and AAS. Through stepwise management and ongoing reassessment, she was ultimately diagnosed with AAS.
Tularemia was initially an important diagnostic consideration in this patient, given her potential exposure and positive F tularensis serum antibodies. Francisella tularensis is a Gram-negative coccobacillus found in more than 250 species of fish, ticks, birds, and mammals. In humans, an incubation period of 3 to 5 days is typical. Although clinical manifestations vary, they often include fever, headache, and malaise.3 Other findings may include lymphadenopathy with or without ulcerative cutaneous lesions (glandular or ulceroglandular tularemia) and cough, dyspnea, pleuritic chest pain, and hilar adenopathy (pneumonic tularemia). As noted by the discussant, a pneumonic tularemia syndrome could have explained this patient’s fever, respiratory symptoms, and hilar adenopathy; ulceroglandular tularemia might have explained her cutaneous lesions. Since splenomegaly may be seen in tularemia, this finding was also consistent with the diagnosis. Serum antibody testing is supportive of the diagnosis, while culture confirms it. Standard treatment consists of a 10- to 14-day course of streptomycin, and combination therapy with a fluoroquinolone is recommended in severe cases.4 In this patient, however, F tularensis was not demonstrated on culture. Furthermore, she did not experience the expected clinical improvement with treatment. Finally, because both IgG and IgM tularemia antibodies may co-occur up to 10 years following infection, her positive F tularensis serum antibodies did not provide evidence of acute infection.5
Recognizing inconsistencies in the diagnosis of tularemia, the focus shifted to PG owing to the patient’s neutrophilic cutaneous lesions, negative infectious workup, and pathergy. Pyoderma gangrenosum is a neutrophilic dermatosis—one of a heterogeneous group of skin conditions characterized by perivascular and diffuse neutrophilic infiltrates without an identifiable infectious agent.6 It is a chronic, recurrent cutaneous disease with several variants.7 The classic presentation includes painful lower-extremity ulcers with violaceous undermined borders and may be associated with pathergy. Guiding principles for the management of PG include controlling inflammation, optimizing wound healing, and minimizing exacerbating factors.1 As such, treatment mainstays include local and systemic anti-inflammatory agents and wound care. As the discussant highlighted, in this case the inflammatory skin lesions were suggestive of PG. However, other features of the case, notably, splenomegaly, splenic abscesses, and necrotizing mediastinal lymphadenitis, were more consistent with another diagnosis: AAS. Aseptic abscess syndrome is an autoinflammatory disorder defined by deep, noninfectious abscesses that preferentially affect the spleen.8 Additional clinical manifestations include weight loss, fever, abdominal pain, and leukocytosis. Lesions may also affect bone, kidney, liver, lung, lymph node, and skin. In one case series, neutrophilic dermatoses were seen in 20% of AAS cases.8 In all cases of AAS, extensive infectious workup is unrevealing, and antibiotics are ineffective. The pathophysiology of AAS is unknown.
Similar to PG, the majority of AAS cases are associated with inflammatory bowel disease, especially Crohn disease.9 However, AAS also has associations with conditions such as MGUS, rheumatoid arthritis, spondyloarthritis, and relapsing polychondritis. Histologically, early lesions demonstrate a necrotic core of neutrophils, with or without surrounding palisading histiocytes, and giant cells. In older lesions, neutrophils may be absent; fibrous tissue may be present.8 Treatment regimens include splenectomy, corticosteroids, colchicine, thalidomide, tumor necrosis factor (TNF) antagonists, and cyclophosphamide. The discussant astutely recommended a splenectomy for this patient, which was both diagnostic and therapeutic. As in this case, relapse is common. Optimal maintenance therapy is yet to be determined.9
Given the overlapping clinical manifestations, shared disease associations, and similar responsiveness to immunosuppression, it is unclear whether AAS represents a new disease entity or a variant of known autoinflammatory disorders. Aseptic abscess syndrome is likely part of a spectrum of autoinflammatory disorders with inflammatory bowel diseases, neutrophilic dermatoses, and other similar diseases.8 While infectious visceral abscesses remain more common, this case highlights the clinical manifestation of an emerging and likely underrecognized entity.
TEACHING POINTS
- Aseptic abscess syndrome should be considered in patients who present with visceral (particularly splenic) abscesses and negative infectious workup.
- Aseptic abscess syndrome is commonly associated with other autoinflammatory disorders; the majority of reported cases are associated with inflammatory bowel disease, especially Crohn disease.
- Up to 20% of AAS cases are associated with neutrophilic dermatoses such as PG.
- The initial treatment for this syndrome is high-dose intravenous glucocorticoids; maintenance treatment regimens include corticosteroids, colchicine, thalidomide, TNF antagonists, and cyclophosphamide.
Acknowledgments
The authors would thank Dr Bob Pelz and Dr John Townes for their contributions to the case.
1. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191-211. https://doi.org/10.2165/11595240-000000000-00000
2. Bhise V, Rajan SS, Sittig DF, Morgan RO, Chaudhary P, Singh H. Defining and measuring diagnostic uncertainty in medicine: a systematic review. J Gen Intern Med. 2018;33(1):103-115. https://doi.org/10.1007/s11606-017-4164-1
3. Penn RL. Francisella tualerensis (Tularemia). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Elsevier Saunders; 2015:2590-2602.
4. Eliasson H, Broman T, Forsman M, Bäck E. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am. 2006;20(2):289-311. https://doi.org/10.1016/j.idc.2006.03.002
5. Bevanger L, Maeland JA, Kvan AI. Comparative analysis of antibodies to Francisella tularensis antigens during the acute phase of tularemia and eight years later. Clin Diagn Lab Immunol. 1994;1(2):238-240.
6. Moschella SL, Davis MDP. Neutrophilic dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Saunders; 2012:424-438.
7. Dabade TS, Davis MDP. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet’s syndrome). Dermatol Ther. 2011;24(2):273-284. https://doi/org/10.1111/j.1529-8019.2011.01403.x
8. André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86(3):145-161. https://doi/org/10.1097/md.0b013e18064f9f3
9. Fillman H, Riquelme P, Sullivan PD, Mansoor AM. Aseptic abscess syndrome. BMJ Case Rep. 2020;13(10):e236437. https://doi.org/10.1136/bcr-2020-236437
1. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191-211. https://doi.org/10.2165/11595240-000000000-00000
2. Bhise V, Rajan SS, Sittig DF, Morgan RO, Chaudhary P, Singh H. Defining and measuring diagnostic uncertainty in medicine: a systematic review. J Gen Intern Med. 2018;33(1):103-115. https://doi.org/10.1007/s11606-017-4164-1
3. Penn RL. Francisella tualerensis (Tularemia). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Elsevier Saunders; 2015:2590-2602.
4. Eliasson H, Broman T, Forsman M, Bäck E. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am. 2006;20(2):289-311. https://doi.org/10.1016/j.idc.2006.03.002
5. Bevanger L, Maeland JA, Kvan AI. Comparative analysis of antibodies to Francisella tularensis antigens during the acute phase of tularemia and eight years later. Clin Diagn Lab Immunol. 1994;1(2):238-240.
6. Moschella SL, Davis MDP. Neutrophilic dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Saunders; 2012:424-438.
7. Dabade TS, Davis MDP. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet’s syndrome). Dermatol Ther. 2011;24(2):273-284. https://doi/org/10.1111/j.1529-8019.2011.01403.x
8. André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86(3):145-161. https://doi/org/10.1097/md.0b013e18064f9f3
9. Fillman H, Riquelme P, Sullivan PD, Mansoor AM. Aseptic abscess syndrome. BMJ Case Rep. 2020;13(10):e236437. https://doi.org/10.1136/bcr-2020-236437
© 2021 Society of Hospital Medicine