User login
Nonpruritic erythematous plaques
A 43-year-old man visiting Texas from Hawaii sought care at our dermatology clinic for nonpruritic erythematous plaques on his chest, back, and extremities. The patient reported occasional numbness in his fingers and feet, but denied constitutional symptoms. The patient, who’d had these symptoms for a year, had been previously diagnosed with chronic urticaria and treated with oral antihistamines. He reported that the lesions were never particularly pruritic and he had not responded to previous treatments.
An avid outdoorsman, our patient was born and raised in Texas and had been living in Hawaii. His past medical history was significant for severe hand eczema and when asked about medications he was taking, he listed cetirizine, doxepin, and hydroxyzine.
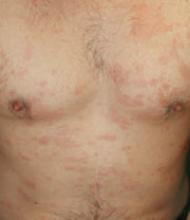
On physical examination the patient had multiple pink to red, nonscaly to minimally scaly flat plaques on his forehead, chest, proximal upper extremities, lower back, and distal lower extremities (FIGURE 1). A 4-mm punch biopsy was taken from a lesion on his lower back and sent for histologic evaluation. The patient’s erythrocyte sedimentation rate, rapid plasma reagin, and complete blood count were all within normal limits.
FIGURE 1
Red plaques
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Borderline lepromatous leprosy
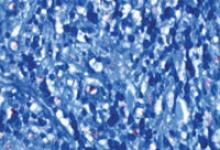
Based on the exam and the results of the biopsy, we diagnosed borderline lepromatous leprosy. Microscopic findings of the punch biopsy showed lymphohistiocytic infiltrate with granulomatous features and numerous acid-fast bacilli (FIGURE 2).
Leprosy traced to a hunting excursion? On further questioning, the patient revealed a very interesting anecdote regarding how he may have contracted leprosy. He reported that approximately 2 to 3 years before his skin symptoms and numbness began, he went hunting in South Texas with some friends. One of the animals he shot was an armadillo. He processed the armadillo, and he and his friends subsequently cooked and ate the meat. Given his history of chronic hand eczema, he wondered whether he might have contracted the disease through an open wound on his hands.
This was possible, given that there are at least 16 case reports of patients with leprosy (also known as Hansen’s disease) in Louisiana, Mississippi, and Texas in which the patient’s contact with armadillos appeared to be the only potential source of transmission.1 That said, leprosy is still a rare condition in the United States: Only 6518 active cases were registered in 2001.2 Eighty percent of these patients were immigrants or frequent travelers to endemic regions.2
FIGURE 2
Punch biopsy
A disease that’s endemic to South America
The earliest reports of leprosy date back to approximately 600 BC.3 Known as a chronic infectious disease caused by Mycobacterium leprae, it is endemic to South America, the Indian subcontinent, and Indochina.4 Early case reports indicate that leprosy was present in North America during the 1700s.1 Other than humans, however, nine-banded armadillos in the Southern United States are the only known endemic host of M leprae.1 The many morbidities that occur in patients with leprosy include ulcers, neuropathies, eye dysfunction, and irreversible neural damage; historically, they also suffered from social seclusion.4
The classification of leprosy is based on the immune response of the host and is categorized along a spectrum.4 On one end, the tuberculoid variant is characterized by a strong cell-mediated immune response; at the other end is the lepromatous variant with a weak cell-mediated immune response.4 Borderline leprosy manifests a combination of clinical and pathologic features of both lepromatous and tuberculoid variants and, depending on the host response, may be classified as borderline lepromatous, borderline borderline, or borderline tuberculoid.2
Rule out other diseases with sensory deficits
Zeroing in on a diagnosis of leprosy requires that you consider other diseases that present with erythematous (or hypopigmented) skin lesions and/or sensory deficits.2 Sensory deficits can be seen in the following diseases:2
- diabetes mellitus
- amyloidosis
- acquired immune deficiency syndrome
- peripheral muscular atrophy
- alcoholism
- nutritional deficiency
- heavy metal poisoning
- syringomyelia
- polio.
Unlike the diseases listed above, leprosy is the only one that produces palpable peripheral nerves.2
The differential for erythematous annular lesions includes tinea corporis, mycosis fungoides, granuloma annulare, psoriasis, sarcoidosis, lichen planus, secondary syphilis, and erythema annulare centrifugum.2
If the patient presents with nodules and plaques, you’ll need to consider sarcoidosis, lymphoma, cutaneous tuberculosis, syphilis, leishmaniasis, and cutaneous systemic mycosis.2
Biopsy clinches the diagnosis
Leprosy lesions characteristically develop in cooler regions of the body and may include macules, papules, nodules, or plaques.2 Temperature sensation is typically the first to be affected by leprosy, followed by touch, pain, and then pressure perception.2 The diagnosis of leprosy is confirmed with skin biopsy.2
A multidrug approach to the treatment of leprosy
The incidence of sulfone antibiotic resistance is increasing; therefore patients with leprosy should be treated with more than 1 drug.5 Standard therapy for leprosy involves rifampin 600 mg daily, dapsone 100 mg daily, and in multibacillary disease, clofazimine 50 mg daily5 (strength of recommendation [SOR]: B). Patients are classified as paucibacillary if they have 1 to 5 skin lesions, and multibacillary if they have more than 5 lesions.6 Other antibiotics used in the treatment of leprosy include ofloxacin, clarithromycin, levofloxacin, and minocycline5 (SOR: B).
Compared with other infectious diseases, such as pulmonary Mycobacterium tuberculosis, leprosy is far less contagious; 95% of the human population has immunity to the disease.5
Our patient received a 3-drug regimen
We reported our patient’s case of leprosy to the Texas Department of Health as well as the National Hansen’s Disease Center. He began treatment shortly after diagnosis at a regional center for Hansen’s disease in San Antonio. He was treated with rifampin, clofazimine, and dapsone, and his lesions improved.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE John M. Martin IV, MD, 7703 Floyd Curl Drive, Dental Building 5.318T Mail Code 7876, San Antonio, TX 78229; [email protected]
1. Truman R. Armadillos as a source of infection for leprosy. South Med J. 2008;101:581-582.
2. Mackowiak PA, Schlossberg MC, Meyer CF, et al. A 49-year-old man with a rash: a case report. Am J Med Sci. 2005;330:134-138.
3. Flower C, Gaskin D, Marquez S. A case of recurrent rash and leg numbness mimicking systemic rheumatic disease. J Clin Rheumatol. 2007;13:143-145.
4. Bhandarkar SS, Cohen C, Kuruvila M, et al. Angiogenesis in cutaneous lesions of leprosy: implications for treatment. Arch Dermatol. 2007;143:1527-1529.
5. U.S. Department of Health and Human Services, Health Resources and Services Administration Web site. National Hansen’s disease program, standard chemotherapy. Available at: www.hrsa.gov/hansens/clinical/chemotherapy/default.htm. Accessed October 10, 2008.
6. Walker SL, Lockwood DNJ. Leprosy. Clin Dermatol. 2007;25:165-172.
A 43-year-old man visiting Texas from Hawaii sought care at our dermatology clinic for nonpruritic erythematous plaques on his chest, back, and extremities. The patient reported occasional numbness in his fingers and feet, but denied constitutional symptoms. The patient, who’d had these symptoms for a year, had been previously diagnosed with chronic urticaria and treated with oral antihistamines. He reported that the lesions were never particularly pruritic and he had not responded to previous treatments.
An avid outdoorsman, our patient was born and raised in Texas and had been living in Hawaii. His past medical history was significant for severe hand eczema and when asked about medications he was taking, he listed cetirizine, doxepin, and hydroxyzine.
On physical examination the patient had multiple pink to red, nonscaly to minimally scaly flat plaques on his forehead, chest, proximal upper extremities, lower back, and distal lower extremities (FIGURE 1). A 4-mm punch biopsy was taken from a lesion on his lower back and sent for histologic evaluation. The patient’s erythrocyte sedimentation rate, rapid plasma reagin, and complete blood count were all within normal limits.
FIGURE 1
Red plaques
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Borderline lepromatous leprosy
Based on the exam and the results of the biopsy, we diagnosed borderline lepromatous leprosy. Microscopic findings of the punch biopsy showed lymphohistiocytic infiltrate with granulomatous features and numerous acid-fast bacilli (FIGURE 2).
Leprosy traced to a hunting excursion? On further questioning, the patient revealed a very interesting anecdote regarding how he may have contracted leprosy. He reported that approximately 2 to 3 years before his skin symptoms and numbness began, he went hunting in South Texas with some friends. One of the animals he shot was an armadillo. He processed the armadillo, and he and his friends subsequently cooked and ate the meat. Given his history of chronic hand eczema, he wondered whether he might have contracted the disease through an open wound on his hands.
This was possible, given that there are at least 16 case reports of patients with leprosy (also known as Hansen’s disease) in Louisiana, Mississippi, and Texas in which the patient’s contact with armadillos appeared to be the only potential source of transmission.1 That said, leprosy is still a rare condition in the United States: Only 6518 active cases were registered in 2001.2 Eighty percent of these patients were immigrants or frequent travelers to endemic regions.2
FIGURE 2
Punch biopsy
A disease that’s endemic to South America
The earliest reports of leprosy date back to approximately 600 BC.3 Known as a chronic infectious disease caused by Mycobacterium leprae, it is endemic to South America, the Indian subcontinent, and Indochina.4 Early case reports indicate that leprosy was present in North America during the 1700s.1 Other than humans, however, nine-banded armadillos in the Southern United States are the only known endemic host of M leprae.1 The many morbidities that occur in patients with leprosy include ulcers, neuropathies, eye dysfunction, and irreversible neural damage; historically, they also suffered from social seclusion.4
The classification of leprosy is based on the immune response of the host and is categorized along a spectrum.4 On one end, the tuberculoid variant is characterized by a strong cell-mediated immune response; at the other end is the lepromatous variant with a weak cell-mediated immune response.4 Borderline leprosy manifests a combination of clinical and pathologic features of both lepromatous and tuberculoid variants and, depending on the host response, may be classified as borderline lepromatous, borderline borderline, or borderline tuberculoid.2
Rule out other diseases with sensory deficits
Zeroing in on a diagnosis of leprosy requires that you consider other diseases that present with erythematous (or hypopigmented) skin lesions and/or sensory deficits.2 Sensory deficits can be seen in the following diseases:2
- diabetes mellitus
- amyloidosis
- acquired immune deficiency syndrome
- peripheral muscular atrophy
- alcoholism
- nutritional deficiency
- heavy metal poisoning
- syringomyelia
- polio.
Unlike the diseases listed above, leprosy is the only one that produces palpable peripheral nerves.2
The differential for erythematous annular lesions includes tinea corporis, mycosis fungoides, granuloma annulare, psoriasis, sarcoidosis, lichen planus, secondary syphilis, and erythema annulare centrifugum.2
If the patient presents with nodules and plaques, you’ll need to consider sarcoidosis, lymphoma, cutaneous tuberculosis, syphilis, leishmaniasis, and cutaneous systemic mycosis.2
Biopsy clinches the diagnosis
Leprosy lesions characteristically develop in cooler regions of the body and may include macules, papules, nodules, or plaques.2 Temperature sensation is typically the first to be affected by leprosy, followed by touch, pain, and then pressure perception.2 The diagnosis of leprosy is confirmed with skin biopsy.2
A multidrug approach to the treatment of leprosy
The incidence of sulfone antibiotic resistance is increasing; therefore patients with leprosy should be treated with more than 1 drug.5 Standard therapy for leprosy involves rifampin 600 mg daily, dapsone 100 mg daily, and in multibacillary disease, clofazimine 50 mg daily5 (strength of recommendation [SOR]: B). Patients are classified as paucibacillary if they have 1 to 5 skin lesions, and multibacillary if they have more than 5 lesions.6 Other antibiotics used in the treatment of leprosy include ofloxacin, clarithromycin, levofloxacin, and minocycline5 (SOR: B).
Compared with other infectious diseases, such as pulmonary Mycobacterium tuberculosis, leprosy is far less contagious; 95% of the human population has immunity to the disease.5
Our patient received a 3-drug regimen
We reported our patient’s case of leprosy to the Texas Department of Health as well as the National Hansen’s Disease Center. He began treatment shortly after diagnosis at a regional center for Hansen’s disease in San Antonio. He was treated with rifampin, clofazimine, and dapsone, and his lesions improved.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE John M. Martin IV, MD, 7703 Floyd Curl Drive, Dental Building 5.318T Mail Code 7876, San Antonio, TX 78229; [email protected]
A 43-year-old man visiting Texas from Hawaii sought care at our dermatology clinic for nonpruritic erythematous plaques on his chest, back, and extremities. The patient reported occasional numbness in his fingers and feet, but denied constitutional symptoms. The patient, who’d had these symptoms for a year, had been previously diagnosed with chronic urticaria and treated with oral antihistamines. He reported that the lesions were never particularly pruritic and he had not responded to previous treatments.
An avid outdoorsman, our patient was born and raised in Texas and had been living in Hawaii. His past medical history was significant for severe hand eczema and when asked about medications he was taking, he listed cetirizine, doxepin, and hydroxyzine.
On physical examination the patient had multiple pink to red, nonscaly to minimally scaly flat plaques on his forehead, chest, proximal upper extremities, lower back, and distal lower extremities (FIGURE 1). A 4-mm punch biopsy was taken from a lesion on his lower back and sent for histologic evaluation. The patient’s erythrocyte sedimentation rate, rapid plasma reagin, and complete blood count were all within normal limits.
FIGURE 1
Red plaques
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Borderline lepromatous leprosy
Based on the exam and the results of the biopsy, we diagnosed borderline lepromatous leprosy. Microscopic findings of the punch biopsy showed lymphohistiocytic infiltrate with granulomatous features and numerous acid-fast bacilli (FIGURE 2).
Leprosy traced to a hunting excursion? On further questioning, the patient revealed a very interesting anecdote regarding how he may have contracted leprosy. He reported that approximately 2 to 3 years before his skin symptoms and numbness began, he went hunting in South Texas with some friends. One of the animals he shot was an armadillo. He processed the armadillo, and he and his friends subsequently cooked and ate the meat. Given his history of chronic hand eczema, he wondered whether he might have contracted the disease through an open wound on his hands.
This was possible, given that there are at least 16 case reports of patients with leprosy (also known as Hansen’s disease) in Louisiana, Mississippi, and Texas in which the patient’s contact with armadillos appeared to be the only potential source of transmission.1 That said, leprosy is still a rare condition in the United States: Only 6518 active cases were registered in 2001.2 Eighty percent of these patients were immigrants or frequent travelers to endemic regions.2
FIGURE 2
Punch biopsy
A disease that’s endemic to South America
The earliest reports of leprosy date back to approximately 600 BC.3 Known as a chronic infectious disease caused by Mycobacterium leprae, it is endemic to South America, the Indian subcontinent, and Indochina.4 Early case reports indicate that leprosy was present in North America during the 1700s.1 Other than humans, however, nine-banded armadillos in the Southern United States are the only known endemic host of M leprae.1 The many morbidities that occur in patients with leprosy include ulcers, neuropathies, eye dysfunction, and irreversible neural damage; historically, they also suffered from social seclusion.4
The classification of leprosy is based on the immune response of the host and is categorized along a spectrum.4 On one end, the tuberculoid variant is characterized by a strong cell-mediated immune response; at the other end is the lepromatous variant with a weak cell-mediated immune response.4 Borderline leprosy manifests a combination of clinical and pathologic features of both lepromatous and tuberculoid variants and, depending on the host response, may be classified as borderline lepromatous, borderline borderline, or borderline tuberculoid.2
Rule out other diseases with sensory deficits
Zeroing in on a diagnosis of leprosy requires that you consider other diseases that present with erythematous (or hypopigmented) skin lesions and/or sensory deficits.2 Sensory deficits can be seen in the following diseases:2
- diabetes mellitus
- amyloidosis
- acquired immune deficiency syndrome
- peripheral muscular atrophy
- alcoholism
- nutritional deficiency
- heavy metal poisoning
- syringomyelia
- polio.
Unlike the diseases listed above, leprosy is the only one that produces palpable peripheral nerves.2
The differential for erythematous annular lesions includes tinea corporis, mycosis fungoides, granuloma annulare, psoriasis, sarcoidosis, lichen planus, secondary syphilis, and erythema annulare centrifugum.2
If the patient presents with nodules and plaques, you’ll need to consider sarcoidosis, lymphoma, cutaneous tuberculosis, syphilis, leishmaniasis, and cutaneous systemic mycosis.2
Biopsy clinches the diagnosis
Leprosy lesions characteristically develop in cooler regions of the body and may include macules, papules, nodules, or plaques.2 Temperature sensation is typically the first to be affected by leprosy, followed by touch, pain, and then pressure perception.2 The diagnosis of leprosy is confirmed with skin biopsy.2
A multidrug approach to the treatment of leprosy
The incidence of sulfone antibiotic resistance is increasing; therefore patients with leprosy should be treated with more than 1 drug.5 Standard therapy for leprosy involves rifampin 600 mg daily, dapsone 100 mg daily, and in multibacillary disease, clofazimine 50 mg daily5 (strength of recommendation [SOR]: B). Patients are classified as paucibacillary if they have 1 to 5 skin lesions, and multibacillary if they have more than 5 lesions.6 Other antibiotics used in the treatment of leprosy include ofloxacin, clarithromycin, levofloxacin, and minocycline5 (SOR: B).
Compared with other infectious diseases, such as pulmonary Mycobacterium tuberculosis, leprosy is far less contagious; 95% of the human population has immunity to the disease.5
Our patient received a 3-drug regimen
We reported our patient’s case of leprosy to the Texas Department of Health as well as the National Hansen’s Disease Center. He began treatment shortly after diagnosis at a regional center for Hansen’s disease in San Antonio. He was treated with rifampin, clofazimine, and dapsone, and his lesions improved.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE John M. Martin IV, MD, 7703 Floyd Curl Drive, Dental Building 5.318T Mail Code 7876, San Antonio, TX 78229; [email protected]
1. Truman R. Armadillos as a source of infection for leprosy. South Med J. 2008;101:581-582.
2. Mackowiak PA, Schlossberg MC, Meyer CF, et al. A 49-year-old man with a rash: a case report. Am J Med Sci. 2005;330:134-138.
3. Flower C, Gaskin D, Marquez S. A case of recurrent rash and leg numbness mimicking systemic rheumatic disease. J Clin Rheumatol. 2007;13:143-145.
4. Bhandarkar SS, Cohen C, Kuruvila M, et al. Angiogenesis in cutaneous lesions of leprosy: implications for treatment. Arch Dermatol. 2007;143:1527-1529.
5. U.S. Department of Health and Human Services, Health Resources and Services Administration Web site. National Hansen’s disease program, standard chemotherapy. Available at: www.hrsa.gov/hansens/clinical/chemotherapy/default.htm. Accessed October 10, 2008.
6. Walker SL, Lockwood DNJ. Leprosy. Clin Dermatol. 2007;25:165-172.
1. Truman R. Armadillos as a source of infection for leprosy. South Med J. 2008;101:581-582.
2. Mackowiak PA, Schlossberg MC, Meyer CF, et al. A 49-year-old man with a rash: a case report. Am J Med Sci. 2005;330:134-138.
3. Flower C, Gaskin D, Marquez S. A case of recurrent rash and leg numbness mimicking systemic rheumatic disease. J Clin Rheumatol. 2007;13:143-145.
4. Bhandarkar SS, Cohen C, Kuruvila M, et al. Angiogenesis in cutaneous lesions of leprosy: implications for treatment. Arch Dermatol. 2007;143:1527-1529.
5. U.S. Department of Health and Human Services, Health Resources and Services Administration Web site. National Hansen’s disease program, standard chemotherapy. Available at: www.hrsa.gov/hansens/clinical/chemotherapy/default.htm. Accessed October 10, 2008.
6. Walker SL, Lockwood DNJ. Leprosy. Clin Dermatol. 2007;25:165-172.