User login
Vascular Access Emergencies in the Dialysis Patient
According to the National Institute of Diabetes and Digestive and Kidney Diseases, approximately 468,000 persons in the United States are on dialysis—a number that continues to grow annually.1 The 1-year rate for hemorrhagic complications from arteriovenous fistulas (AVFs) is estimated to be 0.4%.2 One study by Ellingson et al3 reported 1,654 deaths secondary to fatal vascular access hemorrhage over a 6-year period, accounting for 0.4% of all deaths of hemodialysis (HD) patients in that study.3
Nonhemorrhagic vascular access-related complications also contribute to the morbidity and mortality associated with AVFs and arteriovenous grafts (AVGs). Venous stenosis resulting in thrombosis has been estimated to occur in 24.7% of AVGs and 9.0% of AVFs, both of which are common causes of access failure.
Infection is reported to be the second leading cause of death in dialysis patients, and vascular access-related infection rates are reported to occur in 9.5% of AVGs vs 0.4% to 0.9% of AVFs.2,4 Pseudoaneurysms and aneurysms range from 30% to 60% for AVFs,2,5 and contribute to morbidity by limiting available areas to cannulate for dialysis, occasionally requiring surgical revision to restore access function or prevent access rupture.
Steal phenomena, including dialysis access-induced steal syndrome (DASS) and ischemic monomelic neuropathy, as well as heart failure secondary to high output are additional contributors to morbidity and mortality.
With the growing rate of end-stage renal disease (ESRD) in the United States and the contribution to morbidity and mortality by bleeding and other complications, it is essential to understand how to evaluate and treat these patients in the ED. This article reviews the evaluation and treatment of vascular access emergencies, as well as risk factors that contribute to complications in the ESRD patient population.
Hemorrhagic Complications of Vascular Access
Risk Factors
Many patients with ESRD have multiple comorbidities such as coronary artery disease and atrial fibrillation that require anticoagulation, antiplatelet medications, or both. Studies have shown that ESRD patients taking warfarin have an increase in major bleeding episodes of 3.1% per person-year and 4.4% per person-year for those taking aspirin alone, while those taking both medications have an increased bleeding risk of 6.3% per person-year.6 A recent systematic review by Elliott et al7 has suggested a 2-fold increase in bleeding rates in HD patients anticoagulated with warfarin as compared to HD patients not on warfarin.
While uremia secondary to chronic kidney disease (CKD) is a well-known facilitator of bleeding complications, the underlying pathophysiology is not yet completely delineated. However, there are some general underlying principles that may help in understanding the best treatment modalities available at this time. As the kidneys fail, uremic toxins accumulate in the bloodstream. These toxins include urea, creatinine, and phenolic acids, which are believed to interfere with primary hemostasis by effecting platelet adherence to endothelium, platelet activation, and aggregation.8 Functional defects are created in the interactions between the glycoprotein Ib (GPIb) receptor and von Willebrand factor (vWF), which are essential to endothelial adhesion of platelets.9 Additionally, these toxins impair the up regulation of the GPIIbIIIa receptor which is integral to platelet aggregation.10 Platelet activation normally leads to platelet aggregation by increasing production of thromboxane A2 (TXA2) and serotonin that are released from storage granules.10 Some toxins may increase nitric oxide (NO) synthesis, effectively reducing aggregation by decreasing TXA2 and adenosine diphosphate (ADP) levels.11 In addition, elevated levels of fibrinogen fragments have also recently been shown to inhibit platelet function by competing with fibrinogen for the GPIIbIIIa receptor with decreasing levels demonstrated after HD.12
Finally, increased pressure in the venous outflow segment also increases persistent bleeding from puncture sites. These pressures may be exaggerated secondary to venous thrombosis, venous stenosis, pseudoaneurysm, aneurysm, or infection.13 The following sections further describe the evaluation and treatment of these complications.
Clinical Presentation
Patients presenting with bleeding from the vascular access site may present with slow continuous oozing from the needle puncture-site itself or with life-threatening hemorrhage secondary to AVF or AVG rupture.14 The incidence of vascular access rupture is unknown, but it appears the majority of ruptures occur in patients with AVG vs AVF.3 However, several case reports have also described hemorrhagic complications of AVF ruptures.15-17 The risk of rupture may be associated with the development of aneurysms or pseudoaneuryms.18 Possible impending perforation may be signaled by skin thinning or a shiny appearance overlying the aneurysm, or evidence of infection overlying the access site.3 Many patients were shown to have complications such as stenosis, thrombosis, or infection within 6 months prior to rupture.3 Education of patients is also important as most hemorrhages occur prior to hospital arrival.3,19
Evaluation in the ED
As with any patient presenting to the ED, the initial evaluation of an unstable patient experiencing bleeding from a vascular access site includes assessing the airway, breathing, and circulation as a first priority—paying special attention to the area of bleeding while simultaneously preparing for possible intervention. It is also important to determine when the patient last underwent dialysis and if he or she was able to complete HD. This information will identify patients who are candidates for reversing the heparin load likely given during dialysis.
It is also important to note that some patients undergoing HD who have already been identified as having an increased risk of bleeding may not receive heparin or may undergo local heparinization, minimal heparinization, or regional citrate anticoagulation during dialysis, in which case protamine is not indicated.14 The emergency physician (EP) must also determine if the patient is on any antiplatelet or anticoagulation agents.
The vascular access site should be inspected for evidence of aneurysmal changes, infection, and skin thinning as these factors increase the risk of bleeding and vascular rupture. Additionally auscultation and palpation of the vascular access site should be performed to evaluate for other potential complications such as stenosis and thrombosis. Lastly, the EP should anticipate the patient’s need for HD in the setting of a potentially unavailable AVG or AVF to determine whether the patient may need an alternative access.
Treatment and Management
The primary responsibility during the initial treatment of a bleeding access site is to stop further blood loss by utilizing methods that employ direct pressure or, in extreme cases, application of a tourniquet, followed by other interventions such as fluid and blood-product resuscitation; coagulopathy reversal; consideration of desmopressin, cryoprecipitate, tranexamic acid (TXA); HD; and vascular repair.
Control of a bleeding dialysis access-site is a balancing act of adequately controlling the bleeding while maintaining the integrity of the fistula. Overly aggressive management may cause thrombosis in the vascular access site, which is associated with morbidity—eg, site revisions, potential for the need to create a new access site. On the opposite end of the spectrum, failing to adequately control bleeding can lead to significant anemia ranging from minimal symptoms to hemodynamic compromise and death.
Peripheral Venous Access
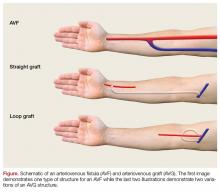
While peripheral venous access is notoriously difficult in patients with ESRD, it is essential for the resuscitation of hemorrhaging patients. Ideally, two large bore peripheral intravenous (IV) lines should be placed in the proximal upper extremities. If peripheral venous access is not achieved, central venous access or interosseous access placement is indicated (Figure).
Direct Pressure
With low-volume bleeding, the first attempt to control the bleeding is simple direct pressure. Except in the instance of trauma or self-inflicted injury, bleeding usually occurs at the site of cannulation of the vascular access post-HD. Direct pressure should be light and limited to as small of an area as possible to prevent thrombosis; the force and area encompassed by direct pressure can be expanded as needed for bleeding that is more difficult to control. In cases of higher volume bleeding, pressure should be placed both proximally and distally to the shunt due to its bidirectional flow. Another possible temporizing measure is to place an upright gallipot or cup over the bleeding site on top of a folded piece of gauze and then securing it with tape.21
Topical Hemostatic Agents
Second simple direct pressure, topical hemostatic agents may be a good adjunct to help obtain hemostasis. There is a wide range of products available, from procoagulants (eg, Combat Gauze, topical thrombin) to factor concentrators (eg, QuikClot). These can be used directly on the bleeding site and only in conjunction with direct pressure.
In addition to topical hemostatic agents, another option is skin glue, which should be applied generously after bleeding has been temporized, with pressure both proximally and distally to the site.
Anticoagulation Reversal
As previously mentioned, it is important to determine when the patient’s last HD was. Heparin is used during dialysis to prevent clotting within the circuit, and although clotting times are monitored during dialysis to guide anticoagulation, it is possible that a patient bleeding after dialysis could still have therapeutic levels of heparin requiring reversal with protamine.
The recommended dose of protamine is 1 mg for every 100 U of heparin given during dialysis; protamine should be administered over 10 minutes. Alternatively, a 10- to 20-mg dose of protamine can be given if the amount of heparin administered during HD is unknown. Additionally, the patient’s medication list, as with any ED presentation, should be carefully reviewed as many dialysis patients have comorbidities requiring anticoagulation with potentially reversible agents.
Hemodialysis to Improve Platelet Dysfunction
It is thought that long-term exposure of platelets to the dialysis membrane can lead to chronic platelet activation leading to platelet dysfunction. There is conflicting data regarding the effects of HD on improving bleeding in renal patients.9,22,23 Hemodialysis is thought to be beneficial, at least partially, through reversing uremia, thus improving platelet function.24 Therefore, in the stable bleeding patient who missed a scheduled dialysis, initiating HD in the ED setting could be beneficial. If the vascular access site is deemed unsafe for HD, another access site must be obtained, for example, by placing a temporary central venous catheter that will allow for successful HD.
Desmopressin
Desmopressin acetate has been shown to reduce bleeding time in uremic patients by releasing vWF and factor VIII into plasma, taking effect within 1 hour and lasting 4 to 8 hours.25-27 Desmopressin has also been shown to reduce blood loss and bleeding times in patients with platelet dysfunction undergoing cardiac surgery.28 While the underlying mechanism is unclear, desmopressin acetate is thought to help with platelet adhesion to the endothelial wall.
Alternatively, one study by Soslau et al29 has suggested that desmopressin may increase serotonin uptake by platelets and increase adenosine triphosphate release, thereby facilitating platelet aggregation. The dosing of desmopressin is 0.2 to 0.3 mcg/kg IV.30 Adverse effects include facial flushing, mild headache, and transient small decrease in blood pressure (BP) with increase in heart rate. Historically, it was thought that desmopressin could lead to water retention, volume overload, congestive heart failure, and hyponatremia; however, these adverse effects have not been seen in uremic patients.30 Tachyphylaxis may occur after just a few doses of desmopressin are given.31 Additionally, hyponatremia and seizures have been seen after repeated administration in children.31
Anemia and Low Hematocrit
As mentioned earlier, anemia and low hematocrit (HCT) may actually exacerbate bleeding tendencies by decreasing the number of platelets exposed to the vessel wall. Red blood cells (RBCs) also produce TXA2 and ADP, both of which play vital roles in normal platelet aggregation. Secondly, RBCs have been shown to increase NO uptake. Nitric oxide is a potent vasodilator and inhibitor of platelet aggregation. The degree of uptake appears to be augmented by increasing HCT levels.32 A goal HCT of greater than 30% has been suggested and demonstrated benefit.33
Cryoprecipitate
Cryoprecipitate is rich in fibrinogen and vWF. Its mechanism is thought to be secondary to increasing functional vWF levels and possibly fibrinogen levels. While the overall effects appear to be variable, studies suggest 10 U of cryoprecipitate is adequate to reverse significant bleeding with resolution of effect at 24 hours.34,35 Given the risks of adverse reactions, variable responses, and risks of hepatitis C and HIV transmission, this therapy must be used cautiously with risk-benefit analysis.
Tranexamic Acid
Tranexamic acid is an antifibrinolytic agent that binds to fibrinogen as a competitive inhibitor of plasmin, inhibiting plasminogen activation. The trauma literature has shown TXA to significantly reduce all-cause mortality.36 It has also been shown to be beneficial in the bleeding uremic patient.37-39
However, it is important to keep in mind that the clearance of TXA in patients with renal disease is unclear. One study by Andersson et al40 demonstrated that TXA has increased plasma concentrations in patients with renal impairment, and a generally accepted practice is to renal-dose this medication. This study recommended a dose of 10 mg/kg IV at varying intervals, such as once daily, twice daily, or every 48 hours depending on the creatinine value, compared to patients with no renal impairment.40 Another study by Sabovic et al39 that evaluated the effects of TXA on gastrointestinal bleeding in patients with renal impairment used a 20-mg IV loading dose of TXA followed by 10 mg/kg orally every 48 hours. Though no adverse events occurred in this study, the study group was small. Other studies have not shown an increase in thromboembolic risk in patients who have no renal disease.36,41
At this time, there is no consensus on the exact dosing of TXA in this patient population. Therefore, this therapy should only be considered if others have failed and the patient continues to have significant blood loss.
Life-threatening Hemorrhage
If a patient is experiencing life-threatening blood loss, more aggressive measures must be employed regardless of risk of damage to the access. In such cases, a consultation with vascular surgery services should be obtained as early as possible. If none of the previously discussed measures are ineffective, the EP may be required to place sutures in the vascular access itself or apply a tourniquet. Again, these interventions may cause permanent damage to the access; however, in the setting of life-threatening hemorrhage such interventions clearly outweigh the risks associated with continued blood loss.
As blood can flow bidirectionally within a fistula, a tourniquet should be placed both proximally and distally to the fistula to obtain adequate hemostasis. Once the tourniquets are in place, if there is no immediate surgical consultation available, the EP may need to temporarily repair the defect to allow minimal tourniquet time. There are a few considerations when placing sutures. Ideally, a noncutting needle should be used to minimize damage. An adequate-sized suture, such as a 3-0 nylon suture, should be used to maintain strength in the high-pressure system. A figure-8 suture or purse-string suture may be placed around the defect. Adequate repair should allow for tourniquet removal.
Hemodynamic Status
The EP must remain aware of the patient’s hemodynamic status. Massive transfusion protocols may need to be initiated. Best current evidence dictates that this should be done in a 1:1:1 ratio of packed red blood cells, platelets, and fresh frozen plasma respectively.42 In our experience, the EP should consider permissive hypotension as aggressive resuscitation and increasing BP can compromise the vascular repair.
Lastly, transfer for definitive management should be arranged if not available at the EP’s institution. The patient should travel with tourniquets in place (although not tightened) in the event of further bleeding.
Nonhemorrhagic Complications of AVF or AVG
Stenosis/Thrombosis
Prolonged bleeding from the cannulation site may suggest outflow stenosis.43 Stenosis with or without subsequent thrombosis is a common cause of vascular access failure. Access failure has also been implicated secondary to poor vascular mapping, resulting in undetected pre-existing stenosis of the inflow artery, outflow vein, or juxta-anastomosis. However, development of stenosis may occur at any time throughout the life of the vascular access. One study by Schild et al4 reported thrombosis rates of 24.7% for grafts and 9.0% for fistula. Additionally, AVGs have a higher reported stenosis rate than AVFs, which is a risk factor for thrombosis.44,45
There has been much debate regarding routine surveillance to prevent clinically significant stenosis with subsequent thrombosis. Surveillance includes a clinical examination, Doppler imaging studies, and flow measurements during dialysis. A recent systematic review from 2016 by Ravani et al,46 demonstrated no difference in risk of access loss in preemptive stenosis correction in AVF or AVG without evidence of access dysfunction. However, on subgroup analysis this review did demonstrate a small benefit regarding risk of thrombosis and access loss in the AVF group.46
The physical examination may indicate evidence of vascular access stenosis or thrombosis. Evidence of stenosis may be indicated by failure of the outflow vein to collapse on arm raise test (distal stenosis), hyperpulsatility or hypopulsatility, loss of the diastolic component of the normal continuous thrill and bruit with only systolic components appreciated, and arm edema (central vein stenosis).43,47 Thrombosis of the vein may be evidenced by complete loss of the thrill and pulsatility on palpation. Sensitivity and specificity of the physical examination for inflow or outflow stenosis has been reported to be between 70% to 92% and 71% to 100%, respectively.48-50
While evidence may or may not support preemptive correction of stenosis, interventions are usually required when the stenosis is more than 50% and interferes with dialysis, decreased flow, abnormal physical examination, or elevated venous pressures.51 If stenosis is associated with interference of effective dialysis or thrombosis is suspected, ultrasound imaging and consultation with a vascular surgeon or interventional radiologist are indicated. Treatment of AVF or AVG stenosis and thrombosis includes percutaneous and surgical interventions.52
A systematic review by Tanner and da Silva53 evaluating adjuvant medical treatments for increasing patency rates of AVF and AVG found no therapy had any improvement in patency rates at 1 month. Another review from 2015 by Palmer et al54 suggested antiplatelet therapy may be protective for stenosis and thrombosis in AVF, but not AVG.
Infection
Infection in patients with ESRD is a major cause of morbidity and mortality, and 24% of these infections may be attributed to the vascular access itself, including central venous catheters (CVC).55 Central venous catheters are associated with the highest rate of infection, followed by AVGs, then AVFs.4,54 Studies have reported 9.5% vs 0.4% to 0.9% infection rates for AVG and AVF, respectively.2,4 These infections are usually due to gram-positive organisms, with the Staphylococcus species being the most common organism involved.55-57 However, infections caused by gram-negative organisms are possible, and broad-spectrum antibiotics should be initiated in the ED if infection is suspected. Patients may present with localized infection with increased risk of rupture of access to profound sepsis. Definitive treatment of an infected graft or fistula usually requires removal of the infected access or at least partial excision with possible interposition of additional graft material.58
Pseudoaneurysm/Aneurysm
Pseudoaneuryms are usually caused by hematoma development after needle puncture or in juxta-anastomic segments postoperatively. Pseudoaneurysms do not have a true wall and may secondarily become infected.59 Pseudoaneurysms occur more frequently in AVG, and are usually reported along with true aneurysms. One study by Al-Thani et al60 detected pseudoaneurysms in 15% of clinically significant aneurysms.
Approximately 30% to 60% of patients with AVFs will develop an aneurysm.2,5 One study by Al-Thani et al60 reported the need for surgical intervention in 31% of patients with an AVF in whom an aneurysm was detected. The risk for developing an aneurysm is highest for those patients on high flux membrane type HD and polycystic kidney disease.5 As discussed earlier in this article, cannulation sites and techniques may also influence aneurysmal changes in the fistula. Aneurysm formation at the site of previous cannulation site should not be re-cannulated.18 Aneurysmal changes can contribute to other complications including high-output heart failure, thrombosis with fistula or graft failure, increased risk of bleeding, ineffective HD when associated with thrombosis or stenosis, pain and peripheral neuropathies secondary to compression of nearby nerves, and interference with functional HD.
Many asymptomatic aneurysmal changes to vascular access may not compromise access function. If a patient is identified with a vascular access pseudoaneurysm or aneurysmal changes with high-risk features, early referral to vascular surgeon for surgical interventions is imperative. High-risk features include any of the complications previously discussed—infection, threatened overlying skin, or shiny appearance. The EP should consider duplex imaging to assist with evaluation. Treatment may include ligation of the AVF, partial resection, stenting, or grafting of the aneurysm with hopes of salvaging the vascular access.61,62
Ischemic Monomelic Neuropathy
Ischemic monomelic neuropathy may result secondary to a type of steal phenomenon, thereby inducing ischemia to supplied nerves. Ischemic monomelic neuropathy has been described in many case reports and narrative reviews.63-67 It has been described as ischemia or infarction of the blood supply to the nerves (vasa nervosa) in the lower arm.68 Ischemic monomelic neuropathy typically occurs immediately after the vascular access creation in the postoperative period. Therefore, it is unlikely to be seen in the ED but as patients may have sequelae of this complication, EPs should be aware of its existence. Patients with ischemic monomelic neuropathy will have severe pain, paresthesia, and weakness immediately after placement of a vascular access. Patients also typically have sensorimotor deficits in the radial, ulnar, and median nerves. Pulses should be preserved. Severe neuropathic pain will develop and may limit the examination. Clinical diagnosis may be difficult immediately after surgery because patients will often have minor deficits secondary to the surgical procedure itself or secondary to the regional block provided by anesthesia, but nerve-conduction studies usually reveal the diagnosis. The treatment is ligation of the access immediately and prognosis is variable, depending on the severity and duration of ischemia, and may result in complete loss of function of the hand.
Steal Syndrome
Dialysis access-associated steal syndrome is a type of distal ischemia secondary to the vascular access site with a reported incidence of 6.2%, and appears to occur more frequently in AVF than AVGs.69,70 Diabetes appears to be a strong risk factor for developing DASS.71 Patients with DASS can present with classic ischemic symptoms such as pain, paresthesia, claudication, pallor, and diminished or absent arterial pulse. Pain may be present only while undergoing dialysis or exercising, or symptoms may be persistent.68,72 There are several possible causes of DASS, including arterial occlusion or insufficiency proximal or distal to the anastomosis, increased flow through the conduit (true steal), or increased flow diverted through collateral vessels.73,74 One clue to the diagnosis is a diminished or absent radial pulse that should improve with compression of the access site.
Once DASS is suspected, diagnosis should be confirmed using venous duplex scanning with finger pressure waveform analysis or arteriogram. Definitive management is surgical intervention with ligation of the access or banding.
High-Output Heart Failure
Changes in cardiac output (CO) are a well-documented effect of AVF placement, with one small study by Korsheed et al75 demonstrating an average increase in CO of 17% only 2 weeks after AVF placement. The increase in CO is thought to be secondary to alterations in systemic vascular resistance and sympathetic activity. While an increase in CO can ultimately lead to high-output heart failure, this is typically only seen in patients with pre-existing cardiac dysfunction.76 Patients are at an increased risk of high-output heart failure when flow through the AVF exceeds 2 L/min; flows below this rate are typically not associated with adverse cardiac effects.77 Another objective measurement for identifying patients at risk of high-output heart failure is the ratio of flow in the fistula (Qa) to cardiac output ratio. Patients with a Qa:CO ratio greater than 0.3 have a significantly increased risk of high-output heart failure.78 There is thought to be no difference in risk of heart failure between AVF and AVG.79
Once overt heart failure has developed, it should be treated in the usual fashion, with IV fluid management and standard pharmacological therapies. If standard conservative heart failure treatment is ineffective, several surgical options are available, including banding, changing the location of the anastomosis, and ultimately closing the fistula.80
Conclusion
While life-threatening bleeding and vascular access rupture are uncommon complications of AVFs and AVGs, it is essential for the EP to rapidly treat the potentially catastrophic hemorrhagic vascular access complications. Depending on the severity and stability of the patient, it is reasonable to begin in a stepwise fashion as presented in this article for patients with minor bleeding, while more severe or persistent bleeding may require several interventions simultaneously to gain control of the bleeding.
Patients with hemodynamic instability requiring transfusion will need a vascular surgery consult and admission. Disposition for stable patients, without evidence of impending aneurysmal related rupture and concern for overlying infection or other complication requiring immediate intervention, will depend on clinical judgment, patient-specific factors and family support, follow-up, and proximity of the patient to medical care.
1. National Institute of Diabetes and Digestive and Kidney Diseases. Kidney disease statistics for the United States. https://www.niddk.nih.gov/health-information/health-statistics/Pages/kidney-disease-statistics-united-states.aspx. Accessed August 24, 2017.
2. Salahi H, Fazelzadeh A, Mehdizadeh A, Razmkon A, Malek-Hosseini SA. Complications of arteriovenous fistula in dialysis patients. Transplant Proc. 2006;38(5):1261-1264. doi:10.1016/j.transproceed.2006.02.066.
3. Ellingson KD, Palekar RS, Lucero CA, et al. Vascular access hemorrhages contribute to deaths among hemodialysis patients. Kidney Int. 2012;82(6):686-692. doi:10.1038/ki.2012.185.
4. Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A. Arteriovenous fistulae vs. arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases. J Vasc Access. 2008;9(4):231-235.
5. Jankovic A, Donfrid B, Adam J, et al. Arteriovenous fistula aneurysm in patients on regular hemodialysis: prevalence and risk factors. Nephron Clin Pract. 2013;124(1-2):94-98. doi:10.1159/000355548.
6. Holden RM, Harman GJ, Wang M, Holland D, Day AG. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008 Jan;3(1):105-110. doi:10.2215/CJN.01810407.
7. Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007;50(3):433-440. doi:10.1053/j.ajkd.2007.06.017.
8. Jubelirer SJ. Hemostatic abnormalities in renal disease. Am J Kidney Dis. 1985;5(5):219-225.
9. Salvati F, Liani M. Role of platelet surface receptor abnormalities in the bleeding and thrombotic diathesis of uremic patients on hemodialysis and peritoneal dialysis. Int J Artif Organs. 2001;24(3):131-135.
10. Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19(4):317-322. doi:10.1111/j.1525-139X.2006.00179.x.
11. Hedges SJ, Dehoney SB, Hooper JS, Amanzadeh J, Busti AJ. Evidence-based treatment recommendations for uremic bleeding. Nat Clin Pract Nephrol. 2007;3(3):138-153. doi:10.1038/ncpneph0421.
12. Thekkedath UR, Chirananthavat T, Leypoldt JK, Cheung AK, Mohammad SF. Elevated fibrinogen fragment levels in uremic plasma inhibit platelet function and expression of glycoprotein IIb-IIIa. Am J Hematol. 2006;81(12):915-926. doi:10.1002/ajh.20720.
13. Padberg FT, Calligaro KD, Sidawy AN. Complications of arteriovenous hemodialysis access: recognition and management. J Vasc Surg. 2008;48(5 Suppl):S55-S80. doi:10.1016/j.jvs.2008.08.067.
14. Lohr JW, Schwab SJ. Minimizing hemorrhagic complications in dialysis patients. J Am Soc Nephrol. 1991;2(5):961-975.
15. Yang TH, Lee CH, Tsai CS, Tsai YT. Successful surgical treatment of a rupture to an arteriovenous fistula aneurysm. Cardiovasc J Afr. 2009;20(3):196-197.
16. Caksen HH, Odabaş D, Arslan S, Kaya A. Spontaneous rupture of arteriovenous fistula in a chronic dialysis patient. J Emerg Med. 2003;24(2):224-225. doi:10.1016/S0736-4679(02)00744-8.
17. Saeed F, Kousar N, Sinnakirouchenan R, Ramalingam VS, Johnson PB, Holley JL. Blood loss through AV fistula: a case report and literature review. Int J Nephrol. 2011;2011:350870. doi:10.4061/2011/350870.
18. NKF KDOQI Guidelines. Clinical practice guidelines for vascular access. Guideline 5. Treatment of fistula complications. Available at http://www2.kidney.org/professionals/kdoqi/guideline_uphd_pd_va/va_guide5.htm. Accessed August 24, 2017.
19. Gill JR, Storck K, Kelly S. Fatal exsanguination from hemodialysis vascular access sites. Forensic Sci Med Pathol. 2012;8(3):259-262. doi:10.1007/s12024-011-9303-0.
20. Manning MA. Use of dialysis access in emergent situations. J Emerg Nurs. 2008;34(1):37-40. doi:10.1016/j.jen.2007.03.018.
21. Reddy VM, Bagul A, Qureshi AA, Nicholson ML. A simple technique to control a bleeding arteriovenous fistula. Ann R Coll Surg Engl. 2006;88(6):592-593. doi:10.1308/003588406X130714f.
22. Oudemans-van Straaten HM. Hemostasis and thrombosis in continuous renal replacement treatment. Semin Thromb Hemost. 2015;41(1):91-98. doi:10.1055/s-0034-1398384.
23. Casserly LF, Dember LM. Thrombosis in end-stage renal disease. Semin Dial. 2003;16(3):245-256. doi:10.1046/j.1525-139X.2003.16048.x.
24. Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30(5):579-589. doi:10.1055/s-2004-835678.
25. Mannucci PM, Remuzzi G, Pusineri F, et al. Deamino-8-D-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308(1):8-12. doi:10.1056/NEJM198301063080102.
26. Ho SJ, Gemmell R, Brighton TA. Platelet function testing in uraemic patients. Hematology. 2008;13(1):49-58. doi:10.1179/102453308X315834.
27. Showalter J, Nguyen ND, Baba S, et al. Platelet aggregometry cannot identify uremic platelet dysfunction in heart failure patients prior to cardiac surgery. J Clin Lab Anal. 2016:1-5. doi:10.1002/jcla.22084.1308/003588406X130714f.
28. Wademan BH, Galvin SD. Desmopressin for reducing postoperative blood loss and transfusion requirements following cardiac surgery in adults. Interact Cardiovasc Thorac Surg. 2014;18(3):360-370. doi:10.1093/icvts/ivt491.
29. Soslau G, Schwartz AB, Putatunda B, et al. Desmopressin-induced improvement in bleeding times in chronic renal failure patients correlates with platelet serotonin uptake and ATP release. Am J Med Sci. 1990;300(6):372-379. http://www.ncbi.nlm.nih.gov/pubmed/2264575. Accessed January 31, 2017.
30. Lethagen S. Desmopressin (DDAVP) and hemostasis. Ann Hematol. 1994;69(4):173-180.
31. Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339(4):245-253. doi:10.1056/NEJM199807233390407.
32. Azarov I, Huang KT, Basu S, Gladwin MT, Hogg N, Kim-Shapiro DB. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280(47):39024-39032. doi:10.1074/jbc.M509045200.
33. Livio M, Marchesi D, Remuzzi G, Gotti E, Mecca G, De Gaetano G. Uraemic bleeding: role of anaemia and beneficial effect of red cell transfusions. Lancet. 1982;320(8306):1013-1015. doi:10.1016/S0140-6736(82)90050-2.
34. Janson PA, Jubelirer SJ, Weinstein MJ, Deykin D. Treatment of the bleeding tendency in uremia with cryoprecipitate. N Engl J Med. 1980;303(23):1318-1322. doi:10.1056/NEJM198012043032302.
35. Triulzi DJ, Blumberg N. Variability in response to cryoprecipitate treatment for hemostatic defects in uremia. Yale J Biol Med. 1990;63(1):1-7.
36. Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Heal Technol Assess. 2013;17(10):1-79. doi:10.3310/hta17100.
37. Mezzano D, Panes O, Muñoz B, et al. Tranexamic acid inhibits fibrinolysis, shortens the bleeding time and improves platelet function in patients with chronic renal failure.Thromb Haemost. 1999;82(4):1250-1254.
38. Mezzano D, Muñoz B, Pais E, Downey P, Pereira J. Fast decrease of bleeding time by tranexamic acid in uremia. Thromb Haemost. 2000;83(5):785.
39. Sabovic M, Lavre J, Vujkovac B. Tranexamic acid is beneficial as adjunctive therapy in treating major upper gastrointestinal bleeding in dialysis patients. Nephrol Dial Transplant. 2003;18(7):1388-1391. doi:10.1093/ndt/gfg117.
40. Andersson L, Eriksson O, Hedlund PO, Kjellman H, Lindqvist B. Special considerations with regard to the dosage of tranexamic acid in patients with chronic renal diseases. Urol Res. 1978;6(2):83-88.
41. Bennett C, Klingenberg SL, Langholz E, Gluud LL. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2014;(11):CD006640. doi: 10.1002/14651858.CD006640.pub3.
42. Holcomb JB, Tilley BC, Baraniuk S, et al; PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi:10.1001/jama.2015.12.
43. Salman L, Beathard G. Interventional nephrology: physical examination as a tool for surveillance for the hemodialysis arteriovenous access. Clin J Am Soc Nephrol. 2013;8(7):1220-1227. doi:10.2215/CJN.00740113.
44. Maya ID, Oser R, Saddekni S, Barker J, Allon M. Vascular access stenosis: comparison of arteriovenous grafts and fistulas. Am J Kidney Dis. 2004;44(5):859-865.
45. Ocak G, Verduijn M, Vossen CY, et al. Chronic kidney disease stages 1-3 increase the risk of venous thrombosis. J Thromb Haemost. 2010;8(11):2428-2435. doi:10.1111/j.1538-7836.2010.04048.x.
46. Ravani P, Quinn RR, Oliver MJ, et al. Pre-emptive correction for haemodialysis arteriovenous access stenosis. Cochrane Database Syst Rev. 2016;(1):CD010709. doi:10.1002/14651858.CD010709.pub2.
47. Pietryga JA, Little MD, Robbin ML. Sonography of arteriovenous fistulas and grafts. Semin Dial. 2017;30(4):309-318. doi:10.1111/sdi.12599.
48. Asif A, Leon C, Orozco-Vargas LC, et al. Accuracy of physical examination in the detection of arteriovenous fistula stenosis. Clin J Am Soc Nephrol. 2007;2(6):1191-1194. doi:10.2215/CJN.02400607.
49. Tessitore N, Bedogna V, Melilli E, et al. In search of an optimal bedside screening program for arteriovenous fistula stenosis. Clin J Am Soc Nephrol. 2011;6(4):819-826. doi:10.2215/CJN.06220710.
50. Dhamija R, Nash SK, Nguyen SV, Slack K, Tadeo J. Monitoring and surveillance of hemodialysis vascular access using StenTec and physical exam. Semin Dial. 2015;28(3):299-304. doi:10.1111/sdi.12311.
51. NKF KDOQI Guidelines. Clinical practice guidelines for hemodialysis adequacy, update 2006. Available at http://kidneyfoundation.cachefly.net/professionals/KDOQI/guideline_upHD_PD_VA/index.htm. Accessed August 12, 2017.
52. Gelbfish GA. Surgical versus percutaneous care of arteriovenous access. Semin Vasc Surg. 2007;20(3):167-174. doi:10.1053/j.semvascsurg.2007.07.011.
53. Tanner NC, da Silva AF. Medical adjuvant treatment to improve the patency of arteriovenous fistulae and grafts: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2016;52(2):243-252. doi:10.1016/j.ejvs.2016.04.016.
54. Palmer SC, Di Micco L, Razavian M, et al. Antiplatelet therapy to prevent hemodialysis vascular access failure: systematic review and meta-analysis. Am J Kidney Dis. 2013;61(1):112-122. doi:10.1053/j.ajkd.2012.08.031.
55. Lafrance JP, Rahme E, Lelorier J, Iqbal S. Vascular access-related infections: definitions, incidence rates, and risk factors. Am J Kidney Dis. 2008;52(5):982-993. doi:10.1053/j.ajkd.2008.06.014.
56. Piraino B. Staphylococcus aureus infections in dialysis patients: focus on prevention. ASAIO J. 46(6):S13-S17.
57. Minga TE, Flanagan KH, Allon M. Clinical consequences of infected arteriovenous grafts in hemodialysis patients. Am J Kidney Dis. 2001;38(5):975-978. doi:10.1053/ajkd.2001.28583.
58. Benrashid E, Youngwirth LM, Mureebe L, Lawson JH. Operative and perioperative management of infected arteriovenous grafts. J Vasc Access. 2017;18(1):13-21. doi:10.5301/jva.5000613.
59. Lazarides MK, Georgiadis GS, Argyriou C. Aneurysm formation and infection in AV prosthesis. J Vasc Access. 2014;15 Suppl 7(Suppl. 7):S120-S124. doi:10.5301/jva.5000228.
60. Al-Thani H, El-Menyar A, Al-Thani N, et al. Characteristics, management, and outcomes of surgically treated arteriovenous fistula aneurysm in patients on regular hemodialysis. Ann Vasc Surg. 2017;41:46-55. doi:10.1016/j.avsg.2016.08.046.
61. Mudoni A, Cornacchiari M, Gallieni M, et al. Aneurysms and pseudoaneurysms in dialysis access. Clin Kidney J. 2015;8(4):363-367. doi:10.1093/ckj/sfv042.
62. Georgiadis GS, Lazarides MK, Panagoutsos SA, et al. Surgical revision of complicated false and true vascular access–related aneurysms. J Vasc Surg. 2008;47(6):1284-1291. doi:10.1016/j.jvs.2008.01.051.
63. Singh V, Qaisar H, Masud A, et al. Ischemic monomelic neuropathy: a long-term follow-up of two cases. J Vasc Access. 2017:0. [Epub ahead of print] doi:10.5301/jva.5000743.
64. Sheetal S, Byju P, Manoj P. Ischemic monomelic neuropathy. J Postgrad Med. 2017;63(1):42-43. doi:10.4103/0022-3859.194221.
65. Rabbani MA, Ahmad B, Shah SM, Ahmad A. Ischemic monomelic neuropathy: a complication of vascular access procedure. J Pak Med Assoc. 2005;55(9):400-401.
66. Hye RJ, Wolf YG. Ischemic monomelic neuropathy: an under-recognized complication of hemodialysis access. Ann Vasc Surg. 1994;8(6):578-582. doi:10.1007/BF02017415.
67. Thimmisetty RK, Pedavally S, Rossi NF, Fernandes JAM, Fixley J. Ischemic monomelic neuropathy: diagnosis, pathophysiology, and management. Kidney Int Reports. 2017;2(1):76-79. doi:10.1016/j.ekir.2016.08.013.
68. MacRae JM, Dipchand C, Oliver M, et al. Arteriovenous access: infection, neuropathy, and other complications. Can J Kidney Heal Dis. 2016;3:2054358116669127. doi:10.1177/2054358116669127.
69. Davidson D, Louridas G, Guzman R, et al. Steal syndrome complicating upper extremity hemoaccess procedures: incidence and risk factors. Can J Surg. 2003;46(6):408-412.
70. Kokkosis AA, Abramowitz SD, Schwitzer J, Nowakowski S, Teodorescu VJ, Schanzer H. Inflow stenosis as a contributing factor in the etiology of AV access-induced ischemic steal. J Vasc Access. 2014;15(4):286-290. doi:10.5301/jva.5000205.
71. Rocha A, Silva F, Queirós J, Malheiro J, Cabrita A. Predictors of steal syndrome in hemodialysis patients. Hemodial Int. 2012;16(4):539-544. doi:10.1111/j.1542-4758.2012.00684.x.
72. Mwipatayi BP, Bowles T, Balakrishnan S, Callaghan J, Haluszkiewicz E, Sieunarine K. Ischemic steal syndrome: a case series and review of current management. Curr Surg. 2006;63(2):130-135. doi:10.1016/j.cursur.2005.04.017.
73. Raml NM. Irreversible sequela in an arterial venous fistula with steal syndrome: A case study. J Vasc Nurs. 2012;30(3):94-97. doi:10.1016/j.jvn.2012.02.001.
74. Malik J, Tuka V, Kasalova Z, et al. Understanding the dialysis access steal syndrome. A review of the etiologies, diagnosis, prevention and treatment strategies. J Vasc Access. 2008;9(3):155-166.
75. Korsheed S, Eldehni MT, John SG, Fluck RJ, McIntyre CW. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol Dial Transplant. 2011;26(10):3296-3302. doi:10.1093/ndt/gfq851.
76. Lazarides MK, Staramos DN, Panagopoulos GN, Tzilalis VD, Eleftheriou GJ, Dayantas JN. Indications for surgical treatment of angioaccess-induced arterial "steal". J Am Coll Surg. 1998;187(4):422-426.
77. Basile C, Lomonte C, Vernaglione L, Casucci F, Antonelli M, Losurdo N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant. 2008;23(1):282-287. doi:10.1093/ndt/gfm549.
78. Wijnen E, Keuter XH, Planken NR, et al. The relation between vascular access flow and different types of vascular access with systemic hemodynamics in hemodialysis patients. Artif Organs. 2005;29(12):960-964. doi:10.1111/j.1525-1594.2005.00165.x.
79. Keuter XH, Kooman JP, Habets J, et al. Effect of upper arm brachial basilic and prosthetic forearm arteriovenous fistula on left ventricular hypertrophy. J Vasc Access. 2007;8(4):296-301.
80. Miller GA, Hwang WW. Challenges and management of high-flow arteriovenous fistulae. Semin Nephrol. 2012;32(6):545-550. doi:10.1016/j.semnephrol.2012.10.005.
According to the National Institute of Diabetes and Digestive and Kidney Diseases, approximately 468,000 persons in the United States are on dialysis—a number that continues to grow annually.1 The 1-year rate for hemorrhagic complications from arteriovenous fistulas (AVFs) is estimated to be 0.4%.2 One study by Ellingson et al3 reported 1,654 deaths secondary to fatal vascular access hemorrhage over a 6-year period, accounting for 0.4% of all deaths of hemodialysis (HD) patients in that study.3
Nonhemorrhagic vascular access-related complications also contribute to the morbidity and mortality associated with AVFs and arteriovenous grafts (AVGs). Venous stenosis resulting in thrombosis has been estimated to occur in 24.7% of AVGs and 9.0% of AVFs, both of which are common causes of access failure.
Infection is reported to be the second leading cause of death in dialysis patients, and vascular access-related infection rates are reported to occur in 9.5% of AVGs vs 0.4% to 0.9% of AVFs.2,4 Pseudoaneurysms and aneurysms range from 30% to 60% for AVFs,2,5 and contribute to morbidity by limiting available areas to cannulate for dialysis, occasionally requiring surgical revision to restore access function or prevent access rupture.
Steal phenomena, including dialysis access-induced steal syndrome (DASS) and ischemic monomelic neuropathy, as well as heart failure secondary to high output are additional contributors to morbidity and mortality.
With the growing rate of end-stage renal disease (ESRD) in the United States and the contribution to morbidity and mortality by bleeding and other complications, it is essential to understand how to evaluate and treat these patients in the ED. This article reviews the evaluation and treatment of vascular access emergencies, as well as risk factors that contribute to complications in the ESRD patient population.
Hemorrhagic Complications of Vascular Access
Risk Factors
Many patients with ESRD have multiple comorbidities such as coronary artery disease and atrial fibrillation that require anticoagulation, antiplatelet medications, or both. Studies have shown that ESRD patients taking warfarin have an increase in major bleeding episodes of 3.1% per person-year and 4.4% per person-year for those taking aspirin alone, while those taking both medications have an increased bleeding risk of 6.3% per person-year.6 A recent systematic review by Elliott et al7 has suggested a 2-fold increase in bleeding rates in HD patients anticoagulated with warfarin as compared to HD patients not on warfarin.
While uremia secondary to chronic kidney disease (CKD) is a well-known facilitator of bleeding complications, the underlying pathophysiology is not yet completely delineated. However, there are some general underlying principles that may help in understanding the best treatment modalities available at this time. As the kidneys fail, uremic toxins accumulate in the bloodstream. These toxins include urea, creatinine, and phenolic acids, which are believed to interfere with primary hemostasis by effecting platelet adherence to endothelium, platelet activation, and aggregation.8 Functional defects are created in the interactions between the glycoprotein Ib (GPIb) receptor and von Willebrand factor (vWF), which are essential to endothelial adhesion of platelets.9 Additionally, these toxins impair the up regulation of the GPIIbIIIa receptor which is integral to platelet aggregation.10 Platelet activation normally leads to platelet aggregation by increasing production of thromboxane A2 (TXA2) and serotonin that are released from storage granules.10 Some toxins may increase nitric oxide (NO) synthesis, effectively reducing aggregation by decreasing TXA2 and adenosine diphosphate (ADP) levels.11 In addition, elevated levels of fibrinogen fragments have also recently been shown to inhibit platelet function by competing with fibrinogen for the GPIIbIIIa receptor with decreasing levels demonstrated after HD.12
Finally, increased pressure in the venous outflow segment also increases persistent bleeding from puncture sites. These pressures may be exaggerated secondary to venous thrombosis, venous stenosis, pseudoaneurysm, aneurysm, or infection.13 The following sections further describe the evaluation and treatment of these complications.
Clinical Presentation
Patients presenting with bleeding from the vascular access site may present with slow continuous oozing from the needle puncture-site itself or with life-threatening hemorrhage secondary to AVF or AVG rupture.14 The incidence of vascular access rupture is unknown, but it appears the majority of ruptures occur in patients with AVG vs AVF.3 However, several case reports have also described hemorrhagic complications of AVF ruptures.15-17 The risk of rupture may be associated with the development of aneurysms or pseudoaneuryms.18 Possible impending perforation may be signaled by skin thinning or a shiny appearance overlying the aneurysm, or evidence of infection overlying the access site.3 Many patients were shown to have complications such as stenosis, thrombosis, or infection within 6 months prior to rupture.3 Education of patients is also important as most hemorrhages occur prior to hospital arrival.3,19
Evaluation in the ED
As with any patient presenting to the ED, the initial evaluation of an unstable patient experiencing bleeding from a vascular access site includes assessing the airway, breathing, and circulation as a first priority—paying special attention to the area of bleeding while simultaneously preparing for possible intervention. It is also important to determine when the patient last underwent dialysis and if he or she was able to complete HD. This information will identify patients who are candidates for reversing the heparin load likely given during dialysis.
It is also important to note that some patients undergoing HD who have already been identified as having an increased risk of bleeding may not receive heparin or may undergo local heparinization, minimal heparinization, or regional citrate anticoagulation during dialysis, in which case protamine is not indicated.14 The emergency physician (EP) must also determine if the patient is on any antiplatelet or anticoagulation agents.
The vascular access site should be inspected for evidence of aneurysmal changes, infection, and skin thinning as these factors increase the risk of bleeding and vascular rupture. Additionally auscultation and palpation of the vascular access site should be performed to evaluate for other potential complications such as stenosis and thrombosis. Lastly, the EP should anticipate the patient’s need for HD in the setting of a potentially unavailable AVG or AVF to determine whether the patient may need an alternative access.
Treatment and Management
The primary responsibility during the initial treatment of a bleeding access site is to stop further blood loss by utilizing methods that employ direct pressure or, in extreme cases, application of a tourniquet, followed by other interventions such as fluid and blood-product resuscitation; coagulopathy reversal; consideration of desmopressin, cryoprecipitate, tranexamic acid (TXA); HD; and vascular repair.
Control of a bleeding dialysis access-site is a balancing act of adequately controlling the bleeding while maintaining the integrity of the fistula. Overly aggressive management may cause thrombosis in the vascular access site, which is associated with morbidity—eg, site revisions, potential for the need to create a new access site. On the opposite end of the spectrum, failing to adequately control bleeding can lead to significant anemia ranging from minimal symptoms to hemodynamic compromise and death.
Peripheral Venous Access
While peripheral venous access is notoriously difficult in patients with ESRD, it is essential for the resuscitation of hemorrhaging patients. Ideally, two large bore peripheral intravenous (IV) lines should be placed in the proximal upper extremities. If peripheral venous access is not achieved, central venous access or interosseous access placement is indicated (Figure).
Direct Pressure
With low-volume bleeding, the first attempt to control the bleeding is simple direct pressure. Except in the instance of trauma or self-inflicted injury, bleeding usually occurs at the site of cannulation of the vascular access post-HD. Direct pressure should be light and limited to as small of an area as possible to prevent thrombosis; the force and area encompassed by direct pressure can be expanded as needed for bleeding that is more difficult to control. In cases of higher volume bleeding, pressure should be placed both proximally and distally to the shunt due to its bidirectional flow. Another possible temporizing measure is to place an upright gallipot or cup over the bleeding site on top of a folded piece of gauze and then securing it with tape.21
Topical Hemostatic Agents
Second simple direct pressure, topical hemostatic agents may be a good adjunct to help obtain hemostasis. There is a wide range of products available, from procoagulants (eg, Combat Gauze, topical thrombin) to factor concentrators (eg, QuikClot). These can be used directly on the bleeding site and only in conjunction with direct pressure.
In addition to topical hemostatic agents, another option is skin glue, which should be applied generously after bleeding has been temporized, with pressure both proximally and distally to the site.
Anticoagulation Reversal
As previously mentioned, it is important to determine when the patient’s last HD was. Heparin is used during dialysis to prevent clotting within the circuit, and although clotting times are monitored during dialysis to guide anticoagulation, it is possible that a patient bleeding after dialysis could still have therapeutic levels of heparin requiring reversal with protamine.
The recommended dose of protamine is 1 mg for every 100 U of heparin given during dialysis; protamine should be administered over 10 minutes. Alternatively, a 10- to 20-mg dose of protamine can be given if the amount of heparin administered during HD is unknown. Additionally, the patient’s medication list, as with any ED presentation, should be carefully reviewed as many dialysis patients have comorbidities requiring anticoagulation with potentially reversible agents.
Hemodialysis to Improve Platelet Dysfunction
It is thought that long-term exposure of platelets to the dialysis membrane can lead to chronic platelet activation leading to platelet dysfunction. There is conflicting data regarding the effects of HD on improving bleeding in renal patients.9,22,23 Hemodialysis is thought to be beneficial, at least partially, through reversing uremia, thus improving platelet function.24 Therefore, in the stable bleeding patient who missed a scheduled dialysis, initiating HD in the ED setting could be beneficial. If the vascular access site is deemed unsafe for HD, another access site must be obtained, for example, by placing a temporary central venous catheter that will allow for successful HD.
Desmopressin
Desmopressin acetate has been shown to reduce bleeding time in uremic patients by releasing vWF and factor VIII into plasma, taking effect within 1 hour and lasting 4 to 8 hours.25-27 Desmopressin has also been shown to reduce blood loss and bleeding times in patients with platelet dysfunction undergoing cardiac surgery.28 While the underlying mechanism is unclear, desmopressin acetate is thought to help with platelet adhesion to the endothelial wall.
Alternatively, one study by Soslau et al29 has suggested that desmopressin may increase serotonin uptake by platelets and increase adenosine triphosphate release, thereby facilitating platelet aggregation. The dosing of desmopressin is 0.2 to 0.3 mcg/kg IV.30 Adverse effects include facial flushing, mild headache, and transient small decrease in blood pressure (BP) with increase in heart rate. Historically, it was thought that desmopressin could lead to water retention, volume overload, congestive heart failure, and hyponatremia; however, these adverse effects have not been seen in uremic patients.30 Tachyphylaxis may occur after just a few doses of desmopressin are given.31 Additionally, hyponatremia and seizures have been seen after repeated administration in children.31
Anemia and Low Hematocrit
As mentioned earlier, anemia and low hematocrit (HCT) may actually exacerbate bleeding tendencies by decreasing the number of platelets exposed to the vessel wall. Red blood cells (RBCs) also produce TXA2 and ADP, both of which play vital roles in normal platelet aggregation. Secondly, RBCs have been shown to increase NO uptake. Nitric oxide is a potent vasodilator and inhibitor of platelet aggregation. The degree of uptake appears to be augmented by increasing HCT levels.32 A goal HCT of greater than 30% has been suggested and demonstrated benefit.33
Cryoprecipitate
Cryoprecipitate is rich in fibrinogen and vWF. Its mechanism is thought to be secondary to increasing functional vWF levels and possibly fibrinogen levels. While the overall effects appear to be variable, studies suggest 10 U of cryoprecipitate is adequate to reverse significant bleeding with resolution of effect at 24 hours.34,35 Given the risks of adverse reactions, variable responses, and risks of hepatitis C and HIV transmission, this therapy must be used cautiously with risk-benefit analysis.
Tranexamic Acid
Tranexamic acid is an antifibrinolytic agent that binds to fibrinogen as a competitive inhibitor of plasmin, inhibiting plasminogen activation. The trauma literature has shown TXA to significantly reduce all-cause mortality.36 It has also been shown to be beneficial in the bleeding uremic patient.37-39
However, it is important to keep in mind that the clearance of TXA in patients with renal disease is unclear. One study by Andersson et al40 demonstrated that TXA has increased plasma concentrations in patients with renal impairment, and a generally accepted practice is to renal-dose this medication. This study recommended a dose of 10 mg/kg IV at varying intervals, such as once daily, twice daily, or every 48 hours depending on the creatinine value, compared to patients with no renal impairment.40 Another study by Sabovic et al39 that evaluated the effects of TXA on gastrointestinal bleeding in patients with renal impairment used a 20-mg IV loading dose of TXA followed by 10 mg/kg orally every 48 hours. Though no adverse events occurred in this study, the study group was small. Other studies have not shown an increase in thromboembolic risk in patients who have no renal disease.36,41
At this time, there is no consensus on the exact dosing of TXA in this patient population. Therefore, this therapy should only be considered if others have failed and the patient continues to have significant blood loss.
Life-threatening Hemorrhage
If a patient is experiencing life-threatening blood loss, more aggressive measures must be employed regardless of risk of damage to the access. In such cases, a consultation with vascular surgery services should be obtained as early as possible. If none of the previously discussed measures are ineffective, the EP may be required to place sutures in the vascular access itself or apply a tourniquet. Again, these interventions may cause permanent damage to the access; however, in the setting of life-threatening hemorrhage such interventions clearly outweigh the risks associated with continued blood loss.
As blood can flow bidirectionally within a fistula, a tourniquet should be placed both proximally and distally to the fistula to obtain adequate hemostasis. Once the tourniquets are in place, if there is no immediate surgical consultation available, the EP may need to temporarily repair the defect to allow minimal tourniquet time. There are a few considerations when placing sutures. Ideally, a noncutting needle should be used to minimize damage. An adequate-sized suture, such as a 3-0 nylon suture, should be used to maintain strength in the high-pressure system. A figure-8 suture or purse-string suture may be placed around the defect. Adequate repair should allow for tourniquet removal.
Hemodynamic Status
The EP must remain aware of the patient’s hemodynamic status. Massive transfusion protocols may need to be initiated. Best current evidence dictates that this should be done in a 1:1:1 ratio of packed red blood cells, platelets, and fresh frozen plasma respectively.42 In our experience, the EP should consider permissive hypotension as aggressive resuscitation and increasing BP can compromise the vascular repair.
Lastly, transfer for definitive management should be arranged if not available at the EP’s institution. The patient should travel with tourniquets in place (although not tightened) in the event of further bleeding.
Nonhemorrhagic Complications of AVF or AVG
Stenosis/Thrombosis
Prolonged bleeding from the cannulation site may suggest outflow stenosis.43 Stenosis with or without subsequent thrombosis is a common cause of vascular access failure. Access failure has also been implicated secondary to poor vascular mapping, resulting in undetected pre-existing stenosis of the inflow artery, outflow vein, or juxta-anastomosis. However, development of stenosis may occur at any time throughout the life of the vascular access. One study by Schild et al4 reported thrombosis rates of 24.7% for grafts and 9.0% for fistula. Additionally, AVGs have a higher reported stenosis rate than AVFs, which is a risk factor for thrombosis.44,45
There has been much debate regarding routine surveillance to prevent clinically significant stenosis with subsequent thrombosis. Surveillance includes a clinical examination, Doppler imaging studies, and flow measurements during dialysis. A recent systematic review from 2016 by Ravani et al,46 demonstrated no difference in risk of access loss in preemptive stenosis correction in AVF or AVG without evidence of access dysfunction. However, on subgroup analysis this review did demonstrate a small benefit regarding risk of thrombosis and access loss in the AVF group.46
The physical examination may indicate evidence of vascular access stenosis or thrombosis. Evidence of stenosis may be indicated by failure of the outflow vein to collapse on arm raise test (distal stenosis), hyperpulsatility or hypopulsatility, loss of the diastolic component of the normal continuous thrill and bruit with only systolic components appreciated, and arm edema (central vein stenosis).43,47 Thrombosis of the vein may be evidenced by complete loss of the thrill and pulsatility on palpation. Sensitivity and specificity of the physical examination for inflow or outflow stenosis has been reported to be between 70% to 92% and 71% to 100%, respectively.48-50
While evidence may or may not support preemptive correction of stenosis, interventions are usually required when the stenosis is more than 50% and interferes with dialysis, decreased flow, abnormal physical examination, or elevated venous pressures.51 If stenosis is associated with interference of effective dialysis or thrombosis is suspected, ultrasound imaging and consultation with a vascular surgeon or interventional radiologist are indicated. Treatment of AVF or AVG stenosis and thrombosis includes percutaneous and surgical interventions.52
A systematic review by Tanner and da Silva53 evaluating adjuvant medical treatments for increasing patency rates of AVF and AVG found no therapy had any improvement in patency rates at 1 month. Another review from 2015 by Palmer et al54 suggested antiplatelet therapy may be protective for stenosis and thrombosis in AVF, but not AVG.
Infection
Infection in patients with ESRD is a major cause of morbidity and mortality, and 24% of these infections may be attributed to the vascular access itself, including central venous catheters (CVC).55 Central venous catheters are associated with the highest rate of infection, followed by AVGs, then AVFs.4,54 Studies have reported 9.5% vs 0.4% to 0.9% infection rates for AVG and AVF, respectively.2,4 These infections are usually due to gram-positive organisms, with the Staphylococcus species being the most common organism involved.55-57 However, infections caused by gram-negative organisms are possible, and broad-spectrum antibiotics should be initiated in the ED if infection is suspected. Patients may present with localized infection with increased risk of rupture of access to profound sepsis. Definitive treatment of an infected graft or fistula usually requires removal of the infected access or at least partial excision with possible interposition of additional graft material.58
Pseudoaneurysm/Aneurysm
Pseudoaneuryms are usually caused by hematoma development after needle puncture or in juxta-anastomic segments postoperatively. Pseudoaneurysms do not have a true wall and may secondarily become infected.59 Pseudoaneurysms occur more frequently in AVG, and are usually reported along with true aneurysms. One study by Al-Thani et al60 detected pseudoaneurysms in 15% of clinically significant aneurysms.
Approximately 30% to 60% of patients with AVFs will develop an aneurysm.2,5 One study by Al-Thani et al60 reported the need for surgical intervention in 31% of patients with an AVF in whom an aneurysm was detected. The risk for developing an aneurysm is highest for those patients on high flux membrane type HD and polycystic kidney disease.5 As discussed earlier in this article, cannulation sites and techniques may also influence aneurysmal changes in the fistula. Aneurysm formation at the site of previous cannulation site should not be re-cannulated.18 Aneurysmal changes can contribute to other complications including high-output heart failure, thrombosis with fistula or graft failure, increased risk of bleeding, ineffective HD when associated with thrombosis or stenosis, pain and peripheral neuropathies secondary to compression of nearby nerves, and interference with functional HD.
Many asymptomatic aneurysmal changes to vascular access may not compromise access function. If a patient is identified with a vascular access pseudoaneurysm or aneurysmal changes with high-risk features, early referral to vascular surgeon for surgical interventions is imperative. High-risk features include any of the complications previously discussed—infection, threatened overlying skin, or shiny appearance. The EP should consider duplex imaging to assist with evaluation. Treatment may include ligation of the AVF, partial resection, stenting, or grafting of the aneurysm with hopes of salvaging the vascular access.61,62
Ischemic Monomelic Neuropathy
Ischemic monomelic neuropathy may result secondary to a type of steal phenomenon, thereby inducing ischemia to supplied nerves. Ischemic monomelic neuropathy has been described in many case reports and narrative reviews.63-67 It has been described as ischemia or infarction of the blood supply to the nerves (vasa nervosa) in the lower arm.68 Ischemic monomelic neuropathy typically occurs immediately after the vascular access creation in the postoperative period. Therefore, it is unlikely to be seen in the ED but as patients may have sequelae of this complication, EPs should be aware of its existence. Patients with ischemic monomelic neuropathy will have severe pain, paresthesia, and weakness immediately after placement of a vascular access. Patients also typically have sensorimotor deficits in the radial, ulnar, and median nerves. Pulses should be preserved. Severe neuropathic pain will develop and may limit the examination. Clinical diagnosis may be difficult immediately after surgery because patients will often have minor deficits secondary to the surgical procedure itself or secondary to the regional block provided by anesthesia, but nerve-conduction studies usually reveal the diagnosis. The treatment is ligation of the access immediately and prognosis is variable, depending on the severity and duration of ischemia, and may result in complete loss of function of the hand.
Steal Syndrome
Dialysis access-associated steal syndrome is a type of distal ischemia secondary to the vascular access site with a reported incidence of 6.2%, and appears to occur more frequently in AVF than AVGs.69,70 Diabetes appears to be a strong risk factor for developing DASS.71 Patients with DASS can present with classic ischemic symptoms such as pain, paresthesia, claudication, pallor, and diminished or absent arterial pulse. Pain may be present only while undergoing dialysis or exercising, or symptoms may be persistent.68,72 There are several possible causes of DASS, including arterial occlusion or insufficiency proximal or distal to the anastomosis, increased flow through the conduit (true steal), or increased flow diverted through collateral vessels.73,74 One clue to the diagnosis is a diminished or absent radial pulse that should improve with compression of the access site.
Once DASS is suspected, diagnosis should be confirmed using venous duplex scanning with finger pressure waveform analysis or arteriogram. Definitive management is surgical intervention with ligation of the access or banding.
High-Output Heart Failure
Changes in cardiac output (CO) are a well-documented effect of AVF placement, with one small study by Korsheed et al75 demonstrating an average increase in CO of 17% only 2 weeks after AVF placement. The increase in CO is thought to be secondary to alterations in systemic vascular resistance and sympathetic activity. While an increase in CO can ultimately lead to high-output heart failure, this is typically only seen in patients with pre-existing cardiac dysfunction.76 Patients are at an increased risk of high-output heart failure when flow through the AVF exceeds 2 L/min; flows below this rate are typically not associated with adverse cardiac effects.77 Another objective measurement for identifying patients at risk of high-output heart failure is the ratio of flow in the fistula (Qa) to cardiac output ratio. Patients with a Qa:CO ratio greater than 0.3 have a significantly increased risk of high-output heart failure.78 There is thought to be no difference in risk of heart failure between AVF and AVG.79
Once overt heart failure has developed, it should be treated in the usual fashion, with IV fluid management and standard pharmacological therapies. If standard conservative heart failure treatment is ineffective, several surgical options are available, including banding, changing the location of the anastomosis, and ultimately closing the fistula.80
Conclusion
While life-threatening bleeding and vascular access rupture are uncommon complications of AVFs and AVGs, it is essential for the EP to rapidly treat the potentially catastrophic hemorrhagic vascular access complications. Depending on the severity and stability of the patient, it is reasonable to begin in a stepwise fashion as presented in this article for patients with minor bleeding, while more severe or persistent bleeding may require several interventions simultaneously to gain control of the bleeding.
Patients with hemodynamic instability requiring transfusion will need a vascular surgery consult and admission. Disposition for stable patients, without evidence of impending aneurysmal related rupture and concern for overlying infection or other complication requiring immediate intervention, will depend on clinical judgment, patient-specific factors and family support, follow-up, and proximity of the patient to medical care.
According to the National Institute of Diabetes and Digestive and Kidney Diseases, approximately 468,000 persons in the United States are on dialysis—a number that continues to grow annually.1 The 1-year rate for hemorrhagic complications from arteriovenous fistulas (AVFs) is estimated to be 0.4%.2 One study by Ellingson et al3 reported 1,654 deaths secondary to fatal vascular access hemorrhage over a 6-year period, accounting for 0.4% of all deaths of hemodialysis (HD) patients in that study.3
Nonhemorrhagic vascular access-related complications also contribute to the morbidity and mortality associated with AVFs and arteriovenous grafts (AVGs). Venous stenosis resulting in thrombosis has been estimated to occur in 24.7% of AVGs and 9.0% of AVFs, both of which are common causes of access failure.
Infection is reported to be the second leading cause of death in dialysis patients, and vascular access-related infection rates are reported to occur in 9.5% of AVGs vs 0.4% to 0.9% of AVFs.2,4 Pseudoaneurysms and aneurysms range from 30% to 60% for AVFs,2,5 and contribute to morbidity by limiting available areas to cannulate for dialysis, occasionally requiring surgical revision to restore access function or prevent access rupture.
Steal phenomena, including dialysis access-induced steal syndrome (DASS) and ischemic monomelic neuropathy, as well as heart failure secondary to high output are additional contributors to morbidity and mortality.
With the growing rate of end-stage renal disease (ESRD) in the United States and the contribution to morbidity and mortality by bleeding and other complications, it is essential to understand how to evaluate and treat these patients in the ED. This article reviews the evaluation and treatment of vascular access emergencies, as well as risk factors that contribute to complications in the ESRD patient population.
Hemorrhagic Complications of Vascular Access
Risk Factors
Many patients with ESRD have multiple comorbidities such as coronary artery disease and atrial fibrillation that require anticoagulation, antiplatelet medications, or both. Studies have shown that ESRD patients taking warfarin have an increase in major bleeding episodes of 3.1% per person-year and 4.4% per person-year for those taking aspirin alone, while those taking both medications have an increased bleeding risk of 6.3% per person-year.6 A recent systematic review by Elliott et al7 has suggested a 2-fold increase in bleeding rates in HD patients anticoagulated with warfarin as compared to HD patients not on warfarin.
While uremia secondary to chronic kidney disease (CKD) is a well-known facilitator of bleeding complications, the underlying pathophysiology is not yet completely delineated. However, there are some general underlying principles that may help in understanding the best treatment modalities available at this time. As the kidneys fail, uremic toxins accumulate in the bloodstream. These toxins include urea, creatinine, and phenolic acids, which are believed to interfere with primary hemostasis by effecting platelet adherence to endothelium, platelet activation, and aggregation.8 Functional defects are created in the interactions between the glycoprotein Ib (GPIb) receptor and von Willebrand factor (vWF), which are essential to endothelial adhesion of platelets.9 Additionally, these toxins impair the up regulation of the GPIIbIIIa receptor which is integral to platelet aggregation.10 Platelet activation normally leads to platelet aggregation by increasing production of thromboxane A2 (TXA2) and serotonin that are released from storage granules.10 Some toxins may increase nitric oxide (NO) synthesis, effectively reducing aggregation by decreasing TXA2 and adenosine diphosphate (ADP) levels.11 In addition, elevated levels of fibrinogen fragments have also recently been shown to inhibit platelet function by competing with fibrinogen for the GPIIbIIIa receptor with decreasing levels demonstrated after HD.12
Finally, increased pressure in the venous outflow segment also increases persistent bleeding from puncture sites. These pressures may be exaggerated secondary to venous thrombosis, venous stenosis, pseudoaneurysm, aneurysm, or infection.13 The following sections further describe the evaluation and treatment of these complications.
Clinical Presentation
Patients presenting with bleeding from the vascular access site may present with slow continuous oozing from the needle puncture-site itself or with life-threatening hemorrhage secondary to AVF or AVG rupture.14 The incidence of vascular access rupture is unknown, but it appears the majority of ruptures occur in patients with AVG vs AVF.3 However, several case reports have also described hemorrhagic complications of AVF ruptures.15-17 The risk of rupture may be associated with the development of aneurysms or pseudoaneuryms.18 Possible impending perforation may be signaled by skin thinning or a shiny appearance overlying the aneurysm, or evidence of infection overlying the access site.3 Many patients were shown to have complications such as stenosis, thrombosis, or infection within 6 months prior to rupture.3 Education of patients is also important as most hemorrhages occur prior to hospital arrival.3,19
Evaluation in the ED
As with any patient presenting to the ED, the initial evaluation of an unstable patient experiencing bleeding from a vascular access site includes assessing the airway, breathing, and circulation as a first priority—paying special attention to the area of bleeding while simultaneously preparing for possible intervention. It is also important to determine when the patient last underwent dialysis and if he or she was able to complete HD. This information will identify patients who are candidates for reversing the heparin load likely given during dialysis.
It is also important to note that some patients undergoing HD who have already been identified as having an increased risk of bleeding may not receive heparin or may undergo local heparinization, minimal heparinization, or regional citrate anticoagulation during dialysis, in which case protamine is not indicated.14 The emergency physician (EP) must also determine if the patient is on any antiplatelet or anticoagulation agents.
The vascular access site should be inspected for evidence of aneurysmal changes, infection, and skin thinning as these factors increase the risk of bleeding and vascular rupture. Additionally auscultation and palpation of the vascular access site should be performed to evaluate for other potential complications such as stenosis and thrombosis. Lastly, the EP should anticipate the patient’s need for HD in the setting of a potentially unavailable AVG or AVF to determine whether the patient may need an alternative access.
Treatment and Management
The primary responsibility during the initial treatment of a bleeding access site is to stop further blood loss by utilizing methods that employ direct pressure or, in extreme cases, application of a tourniquet, followed by other interventions such as fluid and blood-product resuscitation; coagulopathy reversal; consideration of desmopressin, cryoprecipitate, tranexamic acid (TXA); HD; and vascular repair.
Control of a bleeding dialysis access-site is a balancing act of adequately controlling the bleeding while maintaining the integrity of the fistula. Overly aggressive management may cause thrombosis in the vascular access site, which is associated with morbidity—eg, site revisions, potential for the need to create a new access site. On the opposite end of the spectrum, failing to adequately control bleeding can lead to significant anemia ranging from minimal symptoms to hemodynamic compromise and death.
Peripheral Venous Access
While peripheral venous access is notoriously difficult in patients with ESRD, it is essential for the resuscitation of hemorrhaging patients. Ideally, two large bore peripheral intravenous (IV) lines should be placed in the proximal upper extremities. If peripheral venous access is not achieved, central venous access or interosseous access placement is indicated (Figure).
Direct Pressure
With low-volume bleeding, the first attempt to control the bleeding is simple direct pressure. Except in the instance of trauma or self-inflicted injury, bleeding usually occurs at the site of cannulation of the vascular access post-HD. Direct pressure should be light and limited to as small of an area as possible to prevent thrombosis; the force and area encompassed by direct pressure can be expanded as needed for bleeding that is more difficult to control. In cases of higher volume bleeding, pressure should be placed both proximally and distally to the shunt due to its bidirectional flow. Another possible temporizing measure is to place an upright gallipot or cup over the bleeding site on top of a folded piece of gauze and then securing it with tape.21
Topical Hemostatic Agents
Second simple direct pressure, topical hemostatic agents may be a good adjunct to help obtain hemostasis. There is a wide range of products available, from procoagulants (eg, Combat Gauze, topical thrombin) to factor concentrators (eg, QuikClot). These can be used directly on the bleeding site and only in conjunction with direct pressure.
In addition to topical hemostatic agents, another option is skin glue, which should be applied generously after bleeding has been temporized, with pressure both proximally and distally to the site.
Anticoagulation Reversal
As previously mentioned, it is important to determine when the patient’s last HD was. Heparin is used during dialysis to prevent clotting within the circuit, and although clotting times are monitored during dialysis to guide anticoagulation, it is possible that a patient bleeding after dialysis could still have therapeutic levels of heparin requiring reversal with protamine.
The recommended dose of protamine is 1 mg for every 100 U of heparin given during dialysis; protamine should be administered over 10 minutes. Alternatively, a 10- to 20-mg dose of protamine can be given if the amount of heparin administered during HD is unknown. Additionally, the patient’s medication list, as with any ED presentation, should be carefully reviewed as many dialysis patients have comorbidities requiring anticoagulation with potentially reversible agents.
Hemodialysis to Improve Platelet Dysfunction
It is thought that long-term exposure of platelets to the dialysis membrane can lead to chronic platelet activation leading to platelet dysfunction. There is conflicting data regarding the effects of HD on improving bleeding in renal patients.9,22,23 Hemodialysis is thought to be beneficial, at least partially, through reversing uremia, thus improving platelet function.24 Therefore, in the stable bleeding patient who missed a scheduled dialysis, initiating HD in the ED setting could be beneficial. If the vascular access site is deemed unsafe for HD, another access site must be obtained, for example, by placing a temporary central venous catheter that will allow for successful HD.
Desmopressin
Desmopressin acetate has been shown to reduce bleeding time in uremic patients by releasing vWF and factor VIII into plasma, taking effect within 1 hour and lasting 4 to 8 hours.25-27 Desmopressin has also been shown to reduce blood loss and bleeding times in patients with platelet dysfunction undergoing cardiac surgery.28 While the underlying mechanism is unclear, desmopressin acetate is thought to help with platelet adhesion to the endothelial wall.
Alternatively, one study by Soslau et al29 has suggested that desmopressin may increase serotonin uptake by platelets and increase adenosine triphosphate release, thereby facilitating platelet aggregation. The dosing of desmopressin is 0.2 to 0.3 mcg/kg IV.30 Adverse effects include facial flushing, mild headache, and transient small decrease in blood pressure (BP) with increase in heart rate. Historically, it was thought that desmopressin could lead to water retention, volume overload, congestive heart failure, and hyponatremia; however, these adverse effects have not been seen in uremic patients.30 Tachyphylaxis may occur after just a few doses of desmopressin are given.31 Additionally, hyponatremia and seizures have been seen after repeated administration in children.31
Anemia and Low Hematocrit
As mentioned earlier, anemia and low hematocrit (HCT) may actually exacerbate bleeding tendencies by decreasing the number of platelets exposed to the vessel wall. Red blood cells (RBCs) also produce TXA2 and ADP, both of which play vital roles in normal platelet aggregation. Secondly, RBCs have been shown to increase NO uptake. Nitric oxide is a potent vasodilator and inhibitor of platelet aggregation. The degree of uptake appears to be augmented by increasing HCT levels.32 A goal HCT of greater than 30% has been suggested and demonstrated benefit.33
Cryoprecipitate
Cryoprecipitate is rich in fibrinogen and vWF. Its mechanism is thought to be secondary to increasing functional vWF levels and possibly fibrinogen levels. While the overall effects appear to be variable, studies suggest 10 U of cryoprecipitate is adequate to reverse significant bleeding with resolution of effect at 24 hours.34,35 Given the risks of adverse reactions, variable responses, and risks of hepatitis C and HIV transmission, this therapy must be used cautiously with risk-benefit analysis.
Tranexamic Acid
Tranexamic acid is an antifibrinolytic agent that binds to fibrinogen as a competitive inhibitor of plasmin, inhibiting plasminogen activation. The trauma literature has shown TXA to significantly reduce all-cause mortality.36 It has also been shown to be beneficial in the bleeding uremic patient.37-39
However, it is important to keep in mind that the clearance of TXA in patients with renal disease is unclear. One study by Andersson et al40 demonstrated that TXA has increased plasma concentrations in patients with renal impairment, and a generally accepted practice is to renal-dose this medication. This study recommended a dose of 10 mg/kg IV at varying intervals, such as once daily, twice daily, or every 48 hours depending on the creatinine value, compared to patients with no renal impairment.40 Another study by Sabovic et al39 that evaluated the effects of TXA on gastrointestinal bleeding in patients with renal impairment used a 20-mg IV loading dose of TXA followed by 10 mg/kg orally every 48 hours. Though no adverse events occurred in this study, the study group was small. Other studies have not shown an increase in thromboembolic risk in patients who have no renal disease.36,41
At this time, there is no consensus on the exact dosing of TXA in this patient population. Therefore, this therapy should only be considered if others have failed and the patient continues to have significant blood loss.
Life-threatening Hemorrhage
If a patient is experiencing life-threatening blood loss, more aggressive measures must be employed regardless of risk of damage to the access. In such cases, a consultation with vascular surgery services should be obtained as early as possible. If none of the previously discussed measures are ineffective, the EP may be required to place sutures in the vascular access itself or apply a tourniquet. Again, these interventions may cause permanent damage to the access; however, in the setting of life-threatening hemorrhage such interventions clearly outweigh the risks associated with continued blood loss.
As blood can flow bidirectionally within a fistula, a tourniquet should be placed both proximally and distally to the fistula to obtain adequate hemostasis. Once the tourniquets are in place, if there is no immediate surgical consultation available, the EP may need to temporarily repair the defect to allow minimal tourniquet time. There are a few considerations when placing sutures. Ideally, a noncutting needle should be used to minimize damage. An adequate-sized suture, such as a 3-0 nylon suture, should be used to maintain strength in the high-pressure system. A figure-8 suture or purse-string suture may be placed around the defect. Adequate repair should allow for tourniquet removal.
Hemodynamic Status
The EP must remain aware of the patient’s hemodynamic status. Massive transfusion protocols may need to be initiated. Best current evidence dictates that this should be done in a 1:1:1 ratio of packed red blood cells, platelets, and fresh frozen plasma respectively.42 In our experience, the EP should consider permissive hypotension as aggressive resuscitation and increasing BP can compromise the vascular repair.
Lastly, transfer for definitive management should be arranged if not available at the EP’s institution. The patient should travel with tourniquets in place (although not tightened) in the event of further bleeding.
Nonhemorrhagic Complications of AVF or AVG
Stenosis/Thrombosis
Prolonged bleeding from the cannulation site may suggest outflow stenosis.43 Stenosis with or without subsequent thrombosis is a common cause of vascular access failure. Access failure has also been implicated secondary to poor vascular mapping, resulting in undetected pre-existing stenosis of the inflow artery, outflow vein, or juxta-anastomosis. However, development of stenosis may occur at any time throughout the life of the vascular access. One study by Schild et al4 reported thrombosis rates of 24.7% for grafts and 9.0% for fistula. Additionally, AVGs have a higher reported stenosis rate than AVFs, which is a risk factor for thrombosis.44,45
There has been much debate regarding routine surveillance to prevent clinically significant stenosis with subsequent thrombosis. Surveillance includes a clinical examination, Doppler imaging studies, and flow measurements during dialysis. A recent systematic review from 2016 by Ravani et al,46 demonstrated no difference in risk of access loss in preemptive stenosis correction in AVF or AVG without evidence of access dysfunction. However, on subgroup analysis this review did demonstrate a small benefit regarding risk of thrombosis and access loss in the AVF group.46
The physical examination may indicate evidence of vascular access stenosis or thrombosis. Evidence of stenosis may be indicated by failure of the outflow vein to collapse on arm raise test (distal stenosis), hyperpulsatility or hypopulsatility, loss of the diastolic component of the normal continuous thrill and bruit with only systolic components appreciated, and arm edema (central vein stenosis).43,47 Thrombosis of the vein may be evidenced by complete loss of the thrill and pulsatility on palpation. Sensitivity and specificity of the physical examination for inflow or outflow stenosis has been reported to be between 70% to 92% and 71% to 100%, respectively.48-50
While evidence may or may not support preemptive correction of stenosis, interventions are usually required when the stenosis is more than 50% and interferes with dialysis, decreased flow, abnormal physical examination, or elevated venous pressures.51 If stenosis is associated with interference of effective dialysis or thrombosis is suspected, ultrasound imaging and consultation with a vascular surgeon or interventional radiologist are indicated. Treatment of AVF or AVG stenosis and thrombosis includes percutaneous and surgical interventions.52
A systematic review by Tanner and da Silva53 evaluating adjuvant medical treatments for increasing patency rates of AVF and AVG found no therapy had any improvement in patency rates at 1 month. Another review from 2015 by Palmer et al54 suggested antiplatelet therapy may be protective for stenosis and thrombosis in AVF, but not AVG.
Infection
Infection in patients with ESRD is a major cause of morbidity and mortality, and 24% of these infections may be attributed to the vascular access itself, including central venous catheters (CVC).55 Central venous catheters are associated with the highest rate of infection, followed by AVGs, then AVFs.4,54 Studies have reported 9.5% vs 0.4% to 0.9% infection rates for AVG and AVF, respectively.2,4 These infections are usually due to gram-positive organisms, with the Staphylococcus species being the most common organism involved.55-57 However, infections caused by gram-negative organisms are possible, and broad-spectrum antibiotics should be initiated in the ED if infection is suspected. Patients may present with localized infection with increased risk of rupture of access to profound sepsis. Definitive treatment of an infected graft or fistula usually requires removal of the infected access or at least partial excision with possible interposition of additional graft material.58
Pseudoaneurysm/Aneurysm
Pseudoaneuryms are usually caused by hematoma development after needle puncture or in juxta-anastomic segments postoperatively. Pseudoaneurysms do not have a true wall and may secondarily become infected.59 Pseudoaneurysms occur more frequently in AVG, and are usually reported along with true aneurysms. One study by Al-Thani et al60 detected pseudoaneurysms in 15% of clinically significant aneurysms.
Approximately 30% to 60% of patients with AVFs will develop an aneurysm.2,5 One study by Al-Thani et al60 reported the need for surgical intervention in 31% of patients with an AVF in whom an aneurysm was detected. The risk for developing an aneurysm is highest for those patients on high flux membrane type HD and polycystic kidney disease.5 As discussed earlier in this article, cannulation sites and techniques may also influence aneurysmal changes in the fistula. Aneurysm formation at the site of previous cannulation site should not be re-cannulated.18 Aneurysmal changes can contribute to other complications including high-output heart failure, thrombosis with fistula or graft failure, increased risk of bleeding, ineffective HD when associated with thrombosis or stenosis, pain and peripheral neuropathies secondary to compression of nearby nerves, and interference with functional HD.
Many asymptomatic aneurysmal changes to vascular access may not compromise access function. If a patient is identified with a vascular access pseudoaneurysm or aneurysmal changes with high-risk features, early referral to vascular surgeon for surgical interventions is imperative. High-risk features include any of the complications previously discussed—infection, threatened overlying skin, or shiny appearance. The EP should consider duplex imaging to assist with evaluation. Treatment may include ligation of the AVF, partial resection, stenting, or grafting of the aneurysm with hopes of salvaging the vascular access.61,62
Ischemic Monomelic Neuropathy
Ischemic monomelic neuropathy may result secondary to a type of steal phenomenon, thereby inducing ischemia to supplied nerves. Ischemic monomelic neuropathy has been described in many case reports and narrative reviews.63-67 It has been described as ischemia or infarction of the blood supply to the nerves (vasa nervosa) in the lower arm.68 Ischemic monomelic neuropathy typically occurs immediately after the vascular access creation in the postoperative period. Therefore, it is unlikely to be seen in the ED but as patients may have sequelae of this complication, EPs should be aware of its existence. Patients with ischemic monomelic neuropathy will have severe pain, paresthesia, and weakness immediately after placement of a vascular access. Patients also typically have sensorimotor deficits in the radial, ulnar, and median nerves. Pulses should be preserved. Severe neuropathic pain will develop and may limit the examination. Clinical diagnosis may be difficult immediately after surgery because patients will often have minor deficits secondary to the surgical procedure itself or secondary to the regional block provided by anesthesia, but nerve-conduction studies usually reveal the diagnosis. The treatment is ligation of the access immediately and prognosis is variable, depending on the severity and duration of ischemia, and may result in complete loss of function of the hand.
Steal Syndrome
Dialysis access-associated steal syndrome is a type of distal ischemia secondary to the vascular access site with a reported incidence of 6.2%, and appears to occur more frequently in AVF than AVGs.69,70 Diabetes appears to be a strong risk factor for developing DASS.71 Patients with DASS can present with classic ischemic symptoms such as pain, paresthesia, claudication, pallor, and diminished or absent arterial pulse. Pain may be present only while undergoing dialysis or exercising, or symptoms may be persistent.68,72 There are several possible causes of DASS, including arterial occlusion or insufficiency proximal or distal to the anastomosis, increased flow through the conduit (true steal), or increased flow diverted through collateral vessels.73,74 One clue to the diagnosis is a diminished or absent radial pulse that should improve with compression of the access site.
Once DASS is suspected, diagnosis should be confirmed using venous duplex scanning with finger pressure waveform analysis or arteriogram. Definitive management is surgical intervention with ligation of the access or banding.
High-Output Heart Failure
Changes in cardiac output (CO) are a well-documented effect of AVF placement, with one small study by Korsheed et al75 demonstrating an average increase in CO of 17% only 2 weeks after AVF placement. The increase in CO is thought to be secondary to alterations in systemic vascular resistance and sympathetic activity. While an increase in CO can ultimately lead to high-output heart failure, this is typically only seen in patients with pre-existing cardiac dysfunction.76 Patients are at an increased risk of high-output heart failure when flow through the AVF exceeds 2 L/min; flows below this rate are typically not associated with adverse cardiac effects.77 Another objective measurement for identifying patients at risk of high-output heart failure is the ratio of flow in the fistula (Qa) to cardiac output ratio. Patients with a Qa:CO ratio greater than 0.3 have a significantly increased risk of high-output heart failure.78 There is thought to be no difference in risk of heart failure between AVF and AVG.79
Once overt heart failure has developed, it should be treated in the usual fashion, with IV fluid management and standard pharmacological therapies. If standard conservative heart failure treatment is ineffective, several surgical options are available, including banding, changing the location of the anastomosis, and ultimately closing the fistula.80
Conclusion
While life-threatening bleeding and vascular access rupture are uncommon complications of AVFs and AVGs, it is essential for the EP to rapidly treat the potentially catastrophic hemorrhagic vascular access complications. Depending on the severity and stability of the patient, it is reasonable to begin in a stepwise fashion as presented in this article for patients with minor bleeding, while more severe or persistent bleeding may require several interventions simultaneously to gain control of the bleeding.
Patients with hemodynamic instability requiring transfusion will need a vascular surgery consult and admission. Disposition for stable patients, without evidence of impending aneurysmal related rupture and concern for overlying infection or other complication requiring immediate intervention, will depend on clinical judgment, patient-specific factors and family support, follow-up, and proximity of the patient to medical care.
1. National Institute of Diabetes and Digestive and Kidney Diseases. Kidney disease statistics for the United States. https://www.niddk.nih.gov/health-information/health-statistics/Pages/kidney-disease-statistics-united-states.aspx. Accessed August 24, 2017.
2. Salahi H, Fazelzadeh A, Mehdizadeh A, Razmkon A, Malek-Hosseini SA. Complications of arteriovenous fistula in dialysis patients. Transplant Proc. 2006;38(5):1261-1264. doi:10.1016/j.transproceed.2006.02.066.
3. Ellingson KD, Palekar RS, Lucero CA, et al. Vascular access hemorrhages contribute to deaths among hemodialysis patients. Kidney Int. 2012;82(6):686-692. doi:10.1038/ki.2012.185.
4. Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A. Arteriovenous fistulae vs. arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases. J Vasc Access. 2008;9(4):231-235.
5. Jankovic A, Donfrid B, Adam J, et al. Arteriovenous fistula aneurysm in patients on regular hemodialysis: prevalence and risk factors. Nephron Clin Pract. 2013;124(1-2):94-98. doi:10.1159/000355548.
6. Holden RM, Harman GJ, Wang M, Holland D, Day AG. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008 Jan;3(1):105-110. doi:10.2215/CJN.01810407.
7. Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007;50(3):433-440. doi:10.1053/j.ajkd.2007.06.017.
8. Jubelirer SJ. Hemostatic abnormalities in renal disease. Am J Kidney Dis. 1985;5(5):219-225.
9. Salvati F, Liani M. Role of platelet surface receptor abnormalities in the bleeding and thrombotic diathesis of uremic patients on hemodialysis and peritoneal dialysis. Int J Artif Organs. 2001;24(3):131-135.
10. Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19(4):317-322. doi:10.1111/j.1525-139X.2006.00179.x.
11. Hedges SJ, Dehoney SB, Hooper JS, Amanzadeh J, Busti AJ. Evidence-based treatment recommendations for uremic bleeding. Nat Clin Pract Nephrol. 2007;3(3):138-153. doi:10.1038/ncpneph0421.
12. Thekkedath UR, Chirananthavat T, Leypoldt JK, Cheung AK, Mohammad SF. Elevated fibrinogen fragment levels in uremic plasma inhibit platelet function and expression of glycoprotein IIb-IIIa. Am J Hematol. 2006;81(12):915-926. doi:10.1002/ajh.20720.
13. Padberg FT, Calligaro KD, Sidawy AN. Complications of arteriovenous hemodialysis access: recognition and management. J Vasc Surg. 2008;48(5 Suppl):S55-S80. doi:10.1016/j.jvs.2008.08.067.
14. Lohr JW, Schwab SJ. Minimizing hemorrhagic complications in dialysis patients. J Am Soc Nephrol. 1991;2(5):961-975.
15. Yang TH, Lee CH, Tsai CS, Tsai YT. Successful surgical treatment of a rupture to an arteriovenous fistula aneurysm. Cardiovasc J Afr. 2009;20(3):196-197.
16. Caksen HH, Odabaş D, Arslan S, Kaya A. Spontaneous rupture of arteriovenous fistula in a chronic dialysis patient. J Emerg Med. 2003;24(2):224-225. doi:10.1016/S0736-4679(02)00744-8.
17. Saeed F, Kousar N, Sinnakirouchenan R, Ramalingam VS, Johnson PB, Holley JL. Blood loss through AV fistula: a case report and literature review. Int J Nephrol. 2011;2011:350870. doi:10.4061/2011/350870.
18. NKF KDOQI Guidelines. Clinical practice guidelines for vascular access. Guideline 5. Treatment of fistula complications. Available at http://www2.kidney.org/professionals/kdoqi/guideline_uphd_pd_va/va_guide5.htm. Accessed August 24, 2017.
19. Gill JR, Storck K, Kelly S. Fatal exsanguination from hemodialysis vascular access sites. Forensic Sci Med Pathol. 2012;8(3):259-262. doi:10.1007/s12024-011-9303-0.
20. Manning MA. Use of dialysis access in emergent situations. J Emerg Nurs. 2008;34(1):37-40. doi:10.1016/j.jen.2007.03.018.
21. Reddy VM, Bagul A, Qureshi AA, Nicholson ML. A simple technique to control a bleeding arteriovenous fistula. Ann R Coll Surg Engl. 2006;88(6):592-593. doi:10.1308/003588406X130714f.
22. Oudemans-van Straaten HM. Hemostasis and thrombosis in continuous renal replacement treatment. Semin Thromb Hemost. 2015;41(1):91-98. doi:10.1055/s-0034-1398384.
23. Casserly LF, Dember LM. Thrombosis in end-stage renal disease. Semin Dial. 2003;16(3):245-256. doi:10.1046/j.1525-139X.2003.16048.x.
24. Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30(5):579-589. doi:10.1055/s-2004-835678.
25. Mannucci PM, Remuzzi G, Pusineri F, et al. Deamino-8-D-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308(1):8-12. doi:10.1056/NEJM198301063080102.
26. Ho SJ, Gemmell R, Brighton TA. Platelet function testing in uraemic patients. Hematology. 2008;13(1):49-58. doi:10.1179/102453308X315834.
27. Showalter J, Nguyen ND, Baba S, et al. Platelet aggregometry cannot identify uremic platelet dysfunction in heart failure patients prior to cardiac surgery. J Clin Lab Anal. 2016:1-5. doi:10.1002/jcla.22084.1308/003588406X130714f.
28. Wademan BH, Galvin SD. Desmopressin for reducing postoperative blood loss and transfusion requirements following cardiac surgery in adults. Interact Cardiovasc Thorac Surg. 2014;18(3):360-370. doi:10.1093/icvts/ivt491.
29. Soslau G, Schwartz AB, Putatunda B, et al. Desmopressin-induced improvement in bleeding times in chronic renal failure patients correlates with platelet serotonin uptake and ATP release. Am J Med Sci. 1990;300(6):372-379. http://www.ncbi.nlm.nih.gov/pubmed/2264575. Accessed January 31, 2017.
30. Lethagen S. Desmopressin (DDAVP) and hemostasis. Ann Hematol. 1994;69(4):173-180.
31. Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339(4):245-253. doi:10.1056/NEJM199807233390407.
32. Azarov I, Huang KT, Basu S, Gladwin MT, Hogg N, Kim-Shapiro DB. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280(47):39024-39032. doi:10.1074/jbc.M509045200.
33. Livio M, Marchesi D, Remuzzi G, Gotti E, Mecca G, De Gaetano G. Uraemic bleeding: role of anaemia and beneficial effect of red cell transfusions. Lancet. 1982;320(8306):1013-1015. doi:10.1016/S0140-6736(82)90050-2.
34. Janson PA, Jubelirer SJ, Weinstein MJ, Deykin D. Treatment of the bleeding tendency in uremia with cryoprecipitate. N Engl J Med. 1980;303(23):1318-1322. doi:10.1056/NEJM198012043032302.
35. Triulzi DJ, Blumberg N. Variability in response to cryoprecipitate treatment for hemostatic defects in uremia. Yale J Biol Med. 1990;63(1):1-7.
36. Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Heal Technol Assess. 2013;17(10):1-79. doi:10.3310/hta17100.
37. Mezzano D, Panes O, Muñoz B, et al. Tranexamic acid inhibits fibrinolysis, shortens the bleeding time and improves platelet function in patients with chronic renal failure.Thromb Haemost. 1999;82(4):1250-1254.
38. Mezzano D, Muñoz B, Pais E, Downey P, Pereira J. Fast decrease of bleeding time by tranexamic acid in uremia. Thromb Haemost. 2000;83(5):785.
39. Sabovic M, Lavre J, Vujkovac B. Tranexamic acid is beneficial as adjunctive therapy in treating major upper gastrointestinal bleeding in dialysis patients. Nephrol Dial Transplant. 2003;18(7):1388-1391. doi:10.1093/ndt/gfg117.
40. Andersson L, Eriksson O, Hedlund PO, Kjellman H, Lindqvist B. Special considerations with regard to the dosage of tranexamic acid in patients with chronic renal diseases. Urol Res. 1978;6(2):83-88.
41. Bennett C, Klingenberg SL, Langholz E, Gluud LL. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2014;(11):CD006640. doi: 10.1002/14651858.CD006640.pub3.
42. Holcomb JB, Tilley BC, Baraniuk S, et al; PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi:10.1001/jama.2015.12.
43. Salman L, Beathard G. Interventional nephrology: physical examination as a tool for surveillance for the hemodialysis arteriovenous access. Clin J Am Soc Nephrol. 2013;8(7):1220-1227. doi:10.2215/CJN.00740113.
44. Maya ID, Oser R, Saddekni S, Barker J, Allon M. Vascular access stenosis: comparison of arteriovenous grafts and fistulas. Am J Kidney Dis. 2004;44(5):859-865.
45. Ocak G, Verduijn M, Vossen CY, et al. Chronic kidney disease stages 1-3 increase the risk of venous thrombosis. J Thromb Haemost. 2010;8(11):2428-2435. doi:10.1111/j.1538-7836.2010.04048.x.
46. Ravani P, Quinn RR, Oliver MJ, et al. Pre-emptive correction for haemodialysis arteriovenous access stenosis. Cochrane Database Syst Rev. 2016;(1):CD010709. doi:10.1002/14651858.CD010709.pub2.
47. Pietryga JA, Little MD, Robbin ML. Sonography of arteriovenous fistulas and grafts. Semin Dial. 2017;30(4):309-318. doi:10.1111/sdi.12599.
48. Asif A, Leon C, Orozco-Vargas LC, et al. Accuracy of physical examination in the detection of arteriovenous fistula stenosis. Clin J Am Soc Nephrol. 2007;2(6):1191-1194. doi:10.2215/CJN.02400607.
49. Tessitore N, Bedogna V, Melilli E, et al. In search of an optimal bedside screening program for arteriovenous fistula stenosis. Clin J Am Soc Nephrol. 2011;6(4):819-826. doi:10.2215/CJN.06220710.
50. Dhamija R, Nash SK, Nguyen SV, Slack K, Tadeo J. Monitoring and surveillance of hemodialysis vascular access using StenTec and physical exam. Semin Dial. 2015;28(3):299-304. doi:10.1111/sdi.12311.
51. NKF KDOQI Guidelines. Clinical practice guidelines for hemodialysis adequacy, update 2006. Available at http://kidneyfoundation.cachefly.net/professionals/KDOQI/guideline_upHD_PD_VA/index.htm. Accessed August 12, 2017.
52. Gelbfish GA. Surgical versus percutaneous care of arteriovenous access. Semin Vasc Surg. 2007;20(3):167-174. doi:10.1053/j.semvascsurg.2007.07.011.
53. Tanner NC, da Silva AF. Medical adjuvant treatment to improve the patency of arteriovenous fistulae and grafts: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2016;52(2):243-252. doi:10.1016/j.ejvs.2016.04.016.
54. Palmer SC, Di Micco L, Razavian M, et al. Antiplatelet therapy to prevent hemodialysis vascular access failure: systematic review and meta-analysis. Am J Kidney Dis. 2013;61(1):112-122. doi:10.1053/j.ajkd.2012.08.031.
55. Lafrance JP, Rahme E, Lelorier J, Iqbal S. Vascular access-related infections: definitions, incidence rates, and risk factors. Am J Kidney Dis. 2008;52(5):982-993. doi:10.1053/j.ajkd.2008.06.014.
56. Piraino B. Staphylococcus aureus infections in dialysis patients: focus on prevention. ASAIO J. 46(6):S13-S17.
57. Minga TE, Flanagan KH, Allon M. Clinical consequences of infected arteriovenous grafts in hemodialysis patients. Am J Kidney Dis. 2001;38(5):975-978. doi:10.1053/ajkd.2001.28583.
58. Benrashid E, Youngwirth LM, Mureebe L, Lawson JH. Operative and perioperative management of infected arteriovenous grafts. J Vasc Access. 2017;18(1):13-21. doi:10.5301/jva.5000613.
59. Lazarides MK, Georgiadis GS, Argyriou C. Aneurysm formation and infection in AV prosthesis. J Vasc Access. 2014;15 Suppl 7(Suppl. 7):S120-S124. doi:10.5301/jva.5000228.
60. Al-Thani H, El-Menyar A, Al-Thani N, et al. Characteristics, management, and outcomes of surgically treated arteriovenous fistula aneurysm in patients on regular hemodialysis. Ann Vasc Surg. 2017;41:46-55. doi:10.1016/j.avsg.2016.08.046.
61. Mudoni A, Cornacchiari M, Gallieni M, et al. Aneurysms and pseudoaneurysms in dialysis access. Clin Kidney J. 2015;8(4):363-367. doi:10.1093/ckj/sfv042.
62. Georgiadis GS, Lazarides MK, Panagoutsos SA, et al. Surgical revision of complicated false and true vascular access–related aneurysms. J Vasc Surg. 2008;47(6):1284-1291. doi:10.1016/j.jvs.2008.01.051.
63. Singh V, Qaisar H, Masud A, et al. Ischemic monomelic neuropathy: a long-term follow-up of two cases. J Vasc Access. 2017:0. [Epub ahead of print] doi:10.5301/jva.5000743.
64. Sheetal S, Byju P, Manoj P. Ischemic monomelic neuropathy. J Postgrad Med. 2017;63(1):42-43. doi:10.4103/0022-3859.194221.
65. Rabbani MA, Ahmad B, Shah SM, Ahmad A. Ischemic monomelic neuropathy: a complication of vascular access procedure. J Pak Med Assoc. 2005;55(9):400-401.
66. Hye RJ, Wolf YG. Ischemic monomelic neuropathy: an under-recognized complication of hemodialysis access. Ann Vasc Surg. 1994;8(6):578-582. doi:10.1007/BF02017415.
67. Thimmisetty RK, Pedavally S, Rossi NF, Fernandes JAM, Fixley J. Ischemic monomelic neuropathy: diagnosis, pathophysiology, and management. Kidney Int Reports. 2017;2(1):76-79. doi:10.1016/j.ekir.2016.08.013.
68. MacRae JM, Dipchand C, Oliver M, et al. Arteriovenous access: infection, neuropathy, and other complications. Can J Kidney Heal Dis. 2016;3:2054358116669127. doi:10.1177/2054358116669127.
69. Davidson D, Louridas G, Guzman R, et al. Steal syndrome complicating upper extremity hemoaccess procedures: incidence and risk factors. Can J Surg. 2003;46(6):408-412.
70. Kokkosis AA, Abramowitz SD, Schwitzer J, Nowakowski S, Teodorescu VJ, Schanzer H. Inflow stenosis as a contributing factor in the etiology of AV access-induced ischemic steal. J Vasc Access. 2014;15(4):286-290. doi:10.5301/jva.5000205.
71. Rocha A, Silva F, Queirós J, Malheiro J, Cabrita A. Predictors of steal syndrome in hemodialysis patients. Hemodial Int. 2012;16(4):539-544. doi:10.1111/j.1542-4758.2012.00684.x.
72. Mwipatayi BP, Bowles T, Balakrishnan S, Callaghan J, Haluszkiewicz E, Sieunarine K. Ischemic steal syndrome: a case series and review of current management. Curr Surg. 2006;63(2):130-135. doi:10.1016/j.cursur.2005.04.017.
73. Raml NM. Irreversible sequela in an arterial venous fistula with steal syndrome: A case study. J Vasc Nurs. 2012;30(3):94-97. doi:10.1016/j.jvn.2012.02.001.
74. Malik J, Tuka V, Kasalova Z, et al. Understanding the dialysis access steal syndrome. A review of the etiologies, diagnosis, prevention and treatment strategies. J Vasc Access. 2008;9(3):155-166.
75. Korsheed S, Eldehni MT, John SG, Fluck RJ, McIntyre CW. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol Dial Transplant. 2011;26(10):3296-3302. doi:10.1093/ndt/gfq851.
76. Lazarides MK, Staramos DN, Panagopoulos GN, Tzilalis VD, Eleftheriou GJ, Dayantas JN. Indications for surgical treatment of angioaccess-induced arterial "steal". J Am Coll Surg. 1998;187(4):422-426.
77. Basile C, Lomonte C, Vernaglione L, Casucci F, Antonelli M, Losurdo N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant. 2008;23(1):282-287. doi:10.1093/ndt/gfm549.
78. Wijnen E, Keuter XH, Planken NR, et al. The relation between vascular access flow and different types of vascular access with systemic hemodynamics in hemodialysis patients. Artif Organs. 2005;29(12):960-964. doi:10.1111/j.1525-1594.2005.00165.x.
79. Keuter XH, Kooman JP, Habets J, et al. Effect of upper arm brachial basilic and prosthetic forearm arteriovenous fistula on left ventricular hypertrophy. J Vasc Access. 2007;8(4):296-301.
80. Miller GA, Hwang WW. Challenges and management of high-flow arteriovenous fistulae. Semin Nephrol. 2012;32(6):545-550. doi:10.1016/j.semnephrol.2012.10.005.
1. National Institute of Diabetes and Digestive and Kidney Diseases. Kidney disease statistics for the United States. https://www.niddk.nih.gov/health-information/health-statistics/Pages/kidney-disease-statistics-united-states.aspx. Accessed August 24, 2017.
2. Salahi H, Fazelzadeh A, Mehdizadeh A, Razmkon A, Malek-Hosseini SA. Complications of arteriovenous fistula in dialysis patients. Transplant Proc. 2006;38(5):1261-1264. doi:10.1016/j.transproceed.2006.02.066.
3. Ellingson KD, Palekar RS, Lucero CA, et al. Vascular access hemorrhages contribute to deaths among hemodialysis patients. Kidney Int. 2012;82(6):686-692. doi:10.1038/ki.2012.185.
4. Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A. Arteriovenous fistulae vs. arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases. J Vasc Access. 2008;9(4):231-235.
5. Jankovic A, Donfrid B, Adam J, et al. Arteriovenous fistula aneurysm in patients on regular hemodialysis: prevalence and risk factors. Nephron Clin Pract. 2013;124(1-2):94-98. doi:10.1159/000355548.
6. Holden RM, Harman GJ, Wang M, Holland D, Day AG. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008 Jan;3(1):105-110. doi:10.2215/CJN.01810407.
7. Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007;50(3):433-440. doi:10.1053/j.ajkd.2007.06.017.
8. Jubelirer SJ. Hemostatic abnormalities in renal disease. Am J Kidney Dis. 1985;5(5):219-225.
9. Salvati F, Liani M. Role of platelet surface receptor abnormalities in the bleeding and thrombotic diathesis of uremic patients on hemodialysis and peritoneal dialysis. Int J Artif Organs. 2001;24(3):131-135.
10. Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19(4):317-322. doi:10.1111/j.1525-139X.2006.00179.x.
11. Hedges SJ, Dehoney SB, Hooper JS, Amanzadeh J, Busti AJ. Evidence-based treatment recommendations for uremic bleeding. Nat Clin Pract Nephrol. 2007;3(3):138-153. doi:10.1038/ncpneph0421.
12. Thekkedath UR, Chirananthavat T, Leypoldt JK, Cheung AK, Mohammad SF. Elevated fibrinogen fragment levels in uremic plasma inhibit platelet function and expression of glycoprotein IIb-IIIa. Am J Hematol. 2006;81(12):915-926. doi:10.1002/ajh.20720.
13. Padberg FT, Calligaro KD, Sidawy AN. Complications of arteriovenous hemodialysis access: recognition and management. J Vasc Surg. 2008;48(5 Suppl):S55-S80. doi:10.1016/j.jvs.2008.08.067.
14. Lohr JW, Schwab SJ. Minimizing hemorrhagic complications in dialysis patients. J Am Soc Nephrol. 1991;2(5):961-975.
15. Yang TH, Lee CH, Tsai CS, Tsai YT. Successful surgical treatment of a rupture to an arteriovenous fistula aneurysm. Cardiovasc J Afr. 2009;20(3):196-197.
16. Caksen HH, Odabaş D, Arslan S, Kaya A. Spontaneous rupture of arteriovenous fistula in a chronic dialysis patient. J Emerg Med. 2003;24(2):224-225. doi:10.1016/S0736-4679(02)00744-8.
17. Saeed F, Kousar N, Sinnakirouchenan R, Ramalingam VS, Johnson PB, Holley JL. Blood loss through AV fistula: a case report and literature review. Int J Nephrol. 2011;2011:350870. doi:10.4061/2011/350870.
18. NKF KDOQI Guidelines. Clinical practice guidelines for vascular access. Guideline 5. Treatment of fistula complications. Available at http://www2.kidney.org/professionals/kdoqi/guideline_uphd_pd_va/va_guide5.htm. Accessed August 24, 2017.
19. Gill JR, Storck K, Kelly S. Fatal exsanguination from hemodialysis vascular access sites. Forensic Sci Med Pathol. 2012;8(3):259-262. doi:10.1007/s12024-011-9303-0.
20. Manning MA. Use of dialysis access in emergent situations. J Emerg Nurs. 2008;34(1):37-40. doi:10.1016/j.jen.2007.03.018.
21. Reddy VM, Bagul A, Qureshi AA, Nicholson ML. A simple technique to control a bleeding arteriovenous fistula. Ann R Coll Surg Engl. 2006;88(6):592-593. doi:10.1308/003588406X130714f.
22. Oudemans-van Straaten HM. Hemostasis and thrombosis in continuous renal replacement treatment. Semin Thromb Hemost. 2015;41(1):91-98. doi:10.1055/s-0034-1398384.
23. Casserly LF, Dember LM. Thrombosis in end-stage renal disease. Semin Dial. 2003;16(3):245-256. doi:10.1046/j.1525-139X.2003.16048.x.
24. Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30(5):579-589. doi:10.1055/s-2004-835678.
25. Mannucci PM, Remuzzi G, Pusineri F, et al. Deamino-8-D-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308(1):8-12. doi:10.1056/NEJM198301063080102.
26. Ho SJ, Gemmell R, Brighton TA. Platelet function testing in uraemic patients. Hematology. 2008;13(1):49-58. doi:10.1179/102453308X315834.
27. Showalter J, Nguyen ND, Baba S, et al. Platelet aggregometry cannot identify uremic platelet dysfunction in heart failure patients prior to cardiac surgery. J Clin Lab Anal. 2016:1-5. doi:10.1002/jcla.22084.1308/003588406X130714f.
28. Wademan BH, Galvin SD. Desmopressin for reducing postoperative blood loss and transfusion requirements following cardiac surgery in adults. Interact Cardiovasc Thorac Surg. 2014;18(3):360-370. doi:10.1093/icvts/ivt491.
29. Soslau G, Schwartz AB, Putatunda B, et al. Desmopressin-induced improvement in bleeding times in chronic renal failure patients correlates with platelet serotonin uptake and ATP release. Am J Med Sci. 1990;300(6):372-379. http://www.ncbi.nlm.nih.gov/pubmed/2264575. Accessed January 31, 2017.
30. Lethagen S. Desmopressin (DDAVP) and hemostasis. Ann Hematol. 1994;69(4):173-180.
31. Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339(4):245-253. doi:10.1056/NEJM199807233390407.
32. Azarov I, Huang KT, Basu S, Gladwin MT, Hogg N, Kim-Shapiro DB. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280(47):39024-39032. doi:10.1074/jbc.M509045200.
33. Livio M, Marchesi D, Remuzzi G, Gotti E, Mecca G, De Gaetano G. Uraemic bleeding: role of anaemia and beneficial effect of red cell transfusions. Lancet. 1982;320(8306):1013-1015. doi:10.1016/S0140-6736(82)90050-2.
34. Janson PA, Jubelirer SJ, Weinstein MJ, Deykin D. Treatment of the bleeding tendency in uremia with cryoprecipitate. N Engl J Med. 1980;303(23):1318-1322. doi:10.1056/NEJM198012043032302.
35. Triulzi DJ, Blumberg N. Variability in response to cryoprecipitate treatment for hemostatic defects in uremia. Yale J Biol Med. 1990;63(1):1-7.
36. Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Heal Technol Assess. 2013;17(10):1-79. doi:10.3310/hta17100.
37. Mezzano D, Panes O, Muñoz B, et al. Tranexamic acid inhibits fibrinolysis, shortens the bleeding time and improves platelet function in patients with chronic renal failure.Thromb Haemost. 1999;82(4):1250-1254.
38. Mezzano D, Muñoz B, Pais E, Downey P, Pereira J. Fast decrease of bleeding time by tranexamic acid in uremia. Thromb Haemost. 2000;83(5):785.
39. Sabovic M, Lavre J, Vujkovac B. Tranexamic acid is beneficial as adjunctive therapy in treating major upper gastrointestinal bleeding in dialysis patients. Nephrol Dial Transplant. 2003;18(7):1388-1391. doi:10.1093/ndt/gfg117.
40. Andersson L, Eriksson O, Hedlund PO, Kjellman H, Lindqvist B. Special considerations with regard to the dosage of tranexamic acid in patients with chronic renal diseases. Urol Res. 1978;6(2):83-88.
41. Bennett C, Klingenberg SL, Langholz E, Gluud LL. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2014;(11):CD006640. doi: 10.1002/14651858.CD006640.pub3.
42. Holcomb JB, Tilley BC, Baraniuk S, et al; PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi:10.1001/jama.2015.12.
43. Salman L, Beathard G. Interventional nephrology: physical examination as a tool for surveillance for the hemodialysis arteriovenous access. Clin J Am Soc Nephrol. 2013;8(7):1220-1227. doi:10.2215/CJN.00740113.
44. Maya ID, Oser R, Saddekni S, Barker J, Allon M. Vascular access stenosis: comparison of arteriovenous grafts and fistulas. Am J Kidney Dis. 2004;44(5):859-865.
45. Ocak G, Verduijn M, Vossen CY, et al. Chronic kidney disease stages 1-3 increase the risk of venous thrombosis. J Thromb Haemost. 2010;8(11):2428-2435. doi:10.1111/j.1538-7836.2010.04048.x.
46. Ravani P, Quinn RR, Oliver MJ, et al. Pre-emptive correction for haemodialysis arteriovenous access stenosis. Cochrane Database Syst Rev. 2016;(1):CD010709. doi:10.1002/14651858.CD010709.pub2.
47. Pietryga JA, Little MD, Robbin ML. Sonography of arteriovenous fistulas and grafts. Semin Dial. 2017;30(4):309-318. doi:10.1111/sdi.12599.
48. Asif A, Leon C, Orozco-Vargas LC, et al. Accuracy of physical examination in the detection of arteriovenous fistula stenosis. Clin J Am Soc Nephrol. 2007;2(6):1191-1194. doi:10.2215/CJN.02400607.
49. Tessitore N, Bedogna V, Melilli E, et al. In search of an optimal bedside screening program for arteriovenous fistula stenosis. Clin J Am Soc Nephrol. 2011;6(4):819-826. doi:10.2215/CJN.06220710.
50. Dhamija R, Nash SK, Nguyen SV, Slack K, Tadeo J. Monitoring and surveillance of hemodialysis vascular access using StenTec and physical exam. Semin Dial. 2015;28(3):299-304. doi:10.1111/sdi.12311.
51. NKF KDOQI Guidelines. Clinical practice guidelines for hemodialysis adequacy, update 2006. Available at http://kidneyfoundation.cachefly.net/professionals/KDOQI/guideline_upHD_PD_VA/index.htm. Accessed August 12, 2017.
52. Gelbfish GA. Surgical versus percutaneous care of arteriovenous access. Semin Vasc Surg. 2007;20(3):167-174. doi:10.1053/j.semvascsurg.2007.07.011.
53. Tanner NC, da Silva AF. Medical adjuvant treatment to improve the patency of arteriovenous fistulae and grafts: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2016;52(2):243-252. doi:10.1016/j.ejvs.2016.04.016.
54. Palmer SC, Di Micco L, Razavian M, et al. Antiplatelet therapy to prevent hemodialysis vascular access failure: systematic review and meta-analysis. Am J Kidney Dis. 2013;61(1):112-122. doi:10.1053/j.ajkd.2012.08.031.
55. Lafrance JP, Rahme E, Lelorier J, Iqbal S. Vascular access-related infections: definitions, incidence rates, and risk factors. Am J Kidney Dis. 2008;52(5):982-993. doi:10.1053/j.ajkd.2008.06.014.
56. Piraino B. Staphylococcus aureus infections in dialysis patients: focus on prevention. ASAIO J. 46(6):S13-S17.
57. Minga TE, Flanagan KH, Allon M. Clinical consequences of infected arteriovenous grafts in hemodialysis patients. Am J Kidney Dis. 2001;38(5):975-978. doi:10.1053/ajkd.2001.28583.
58. Benrashid E, Youngwirth LM, Mureebe L, Lawson JH. Operative and perioperative management of infected arteriovenous grafts. J Vasc Access. 2017;18(1):13-21. doi:10.5301/jva.5000613.
59. Lazarides MK, Georgiadis GS, Argyriou C. Aneurysm formation and infection in AV prosthesis. J Vasc Access. 2014;15 Suppl 7(Suppl. 7):S120-S124. doi:10.5301/jva.5000228.
60. Al-Thani H, El-Menyar A, Al-Thani N, et al. Characteristics, management, and outcomes of surgically treated arteriovenous fistula aneurysm in patients on regular hemodialysis. Ann Vasc Surg. 2017;41:46-55. doi:10.1016/j.avsg.2016.08.046.
61. Mudoni A, Cornacchiari M, Gallieni M, et al. Aneurysms and pseudoaneurysms in dialysis access. Clin Kidney J. 2015;8(4):363-367. doi:10.1093/ckj/sfv042.
62. Georgiadis GS, Lazarides MK, Panagoutsos SA, et al. Surgical revision of complicated false and true vascular access–related aneurysms. J Vasc Surg. 2008;47(6):1284-1291. doi:10.1016/j.jvs.2008.01.051.
63. Singh V, Qaisar H, Masud A, et al. Ischemic monomelic neuropathy: a long-term follow-up of two cases. J Vasc Access. 2017:0. [Epub ahead of print] doi:10.5301/jva.5000743.
64. Sheetal S, Byju P, Manoj P. Ischemic monomelic neuropathy. J Postgrad Med. 2017;63(1):42-43. doi:10.4103/0022-3859.194221.
65. Rabbani MA, Ahmad B, Shah SM, Ahmad A. Ischemic monomelic neuropathy: a complication of vascular access procedure. J Pak Med Assoc. 2005;55(9):400-401.
66. Hye RJ, Wolf YG. Ischemic monomelic neuropathy: an under-recognized complication of hemodialysis access. Ann Vasc Surg. 1994;8(6):578-582. doi:10.1007/BF02017415.
67. Thimmisetty RK, Pedavally S, Rossi NF, Fernandes JAM, Fixley J. Ischemic monomelic neuropathy: diagnosis, pathophysiology, and management. Kidney Int Reports. 2017;2(1):76-79. doi:10.1016/j.ekir.2016.08.013.
68. MacRae JM, Dipchand C, Oliver M, et al. Arteriovenous access: infection, neuropathy, and other complications. Can J Kidney Heal Dis. 2016;3:2054358116669127. doi:10.1177/2054358116669127.
69. Davidson D, Louridas G, Guzman R, et al. Steal syndrome complicating upper extremity hemoaccess procedures: incidence and risk factors. Can J Surg. 2003;46(6):408-412.
70. Kokkosis AA, Abramowitz SD, Schwitzer J, Nowakowski S, Teodorescu VJ, Schanzer H. Inflow stenosis as a contributing factor in the etiology of AV access-induced ischemic steal. J Vasc Access. 2014;15(4):286-290. doi:10.5301/jva.5000205.
71. Rocha A, Silva F, Queirós J, Malheiro J, Cabrita A. Predictors of steal syndrome in hemodialysis patients. Hemodial Int. 2012;16(4):539-544. doi:10.1111/j.1542-4758.2012.00684.x.
72. Mwipatayi BP, Bowles T, Balakrishnan S, Callaghan J, Haluszkiewicz E, Sieunarine K. Ischemic steal syndrome: a case series and review of current management. Curr Surg. 2006;63(2):130-135. doi:10.1016/j.cursur.2005.04.017.
73. Raml NM. Irreversible sequela in an arterial venous fistula with steal syndrome: A case study. J Vasc Nurs. 2012;30(3):94-97. doi:10.1016/j.jvn.2012.02.001.
74. Malik J, Tuka V, Kasalova Z, et al. Understanding the dialysis access steal syndrome. A review of the etiologies, diagnosis, prevention and treatment strategies. J Vasc Access. 2008;9(3):155-166.
75. Korsheed S, Eldehni MT, John SG, Fluck RJ, McIntyre CW. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol Dial Transplant. 2011;26(10):3296-3302. doi:10.1093/ndt/gfq851.
76. Lazarides MK, Staramos DN, Panagopoulos GN, Tzilalis VD, Eleftheriou GJ, Dayantas JN. Indications for surgical treatment of angioaccess-induced arterial "steal". J Am Coll Surg. 1998;187(4):422-426.
77. Basile C, Lomonte C, Vernaglione L, Casucci F, Antonelli M, Losurdo N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant. 2008;23(1):282-287. doi:10.1093/ndt/gfm549.
78. Wijnen E, Keuter XH, Planken NR, et al. The relation between vascular access flow and different types of vascular access with systemic hemodynamics in hemodialysis patients. Artif Organs. 2005;29(12):960-964. doi:10.1111/j.1525-1594.2005.00165.x.
79. Keuter XH, Kooman JP, Habets J, et al. Effect of upper arm brachial basilic and prosthetic forearm arteriovenous fistula on left ventricular hypertrophy. J Vasc Access. 2007;8(4):296-301.
80. Miller GA, Hwang WW. Challenges and management of high-flow arteriovenous fistulae. Semin Nephrol. 2012;32(6):545-550. doi:10.1016/j.semnephrol.2012.10.005.