User login
Facilitated Peer Mentoring: Filling a Critical Gap in Academic Hospital Medicine
Traditional mentorship models may not be adequate for academic hospitalists. The traditional dyadic mentorship model, in which a senior principal investigator and research mentee collaborate for career advancement, is well suited for basic science or clinical research. In contrast, areas of academic hospital medicine such as quality improvement, medical education, hospital operations, point-of-care ultrasound, and clinical expertise may be less suited to this traditional mentoring model. In addition, experienced mentors are limited and those available are often overcommitted or have inadequate time due to responsibilities with other leadership roles. Senior mentors may also be limited because of our specialty’s focus on clinical practice rather than longitudinal research or projects.9 There are other limitations of traditional mentorship that are applicable to all fields of academic medicine, including disparate goals, expectations, levels of commitment, and the inherent power differential between the mentor and mentee.10
In this perspective, we discuss our experience with implementing an alternative and complementary mentorship strategy called facilitated peer mentorship with junior faculty hospitalists in the Division of General Internal Medicine at New York–Presbyterian/Weill Cornell Medical Center.
In facilitated peer mentoring programs, faculty typically work collaboratively in groups of three to five with other faculty who are of similar rank, and a faculty member of a higher academic rank works with the group in meeting their scholarly goals.11 The role of the facilitator is to ensure a safe and respectful learning environment, foster peer collaboration, and redirect the group to draw upon their own experiences. Each junior faculty member serves as both a mentor and mentee for each other with bidirectional feedback, guidance, and support in a group setting. This model emphasizes collaboration, peer networking, empowerment, and the development of personal awareness.10 A number of academic medical centers have used peer mentoring as a response to the challenges encountered in the traditional dyad model.12 To our knowledge, the only published example of a peer mentoring model in academic hospital medicine is in the form of a research-in-progress conference.13 While this example addresses peer-mentored research, there is a gap in other areas of academic hospital medicine with mentoring needs—most of all in personal development and career satisfaction.
We piloted a 12-month facilitated peer mentoring program for new hospitalists. The goal of the program was for junior faculty hospitalists to develop a better understanding of their own identity and core values that would enable them to more confidently navigate career choices, enhance their work vitality and career satisfaction, and develop their potential for leadership roles in academic hospital medicine. Each year, a cohort of four to five incoming hospitalists from different backgrounds, interests, and experience were grouped with a more experienced colleague at an associate professor rank who expressed interest and was selected by our section chief to lead the program. The program was required for new hospitalists and consisted of six 90-minute sessions every two months. The attendance rate was 100% and was ensured by scheduling all sessions at the beginning of the academic year with dates agreed upon by all participants. An e-mail reminder was sent one week prior to each session. Each session had assigned readings and an agenda for discussions (see Table for details).
Our evaluation of the program after two years with two separate cohorts included qualitative feedback through an anonymous survey for participants; in addition, qualitative feedback was collected in a one-hour, in-person discussion and reflection with each cohort. We learned several lessons from the feedback we received from program participants. First, our impression was that the career experience of the junior faculty member had a significant impact on the perceived value of group meetings. For those who entered the hospitalist workforce immediately upon completing their terminal training in internal medicine, the exercise of considering different career versions of themselves had added value in promoting thinking outside-the-box for career opportunities within hospital medicine. Academic hospitalists and general internists more broadly, tend to have broad interests that fuel their passions but may also make it more difficult to define long-term goals. One junior faculty member paired her life interests in global medical education with building an international collaboration with other academic hospitalist programs; another faculty member gained confidence and expanded her network of collaborators by designing a research pilot study on hospitalist-initiated end-of-life discussions. In both cases, the junior faculty identified the facilitated peer mentoring program as a strong influence in finding these opportunities. Peer mentoring at the time of entry into the field of hospital medicine, when many have undefined career goals, can be helpful for navigating this issue at the start of a career. On the other hand, those who had already worked as a hospitalist for one or two years and joined the program found less value in career planning exercises.
Second, junior faculty differed in their desire for scope and depth of the curriculum. Some preferred more frequent sessions with more premeeting readings and self-assessments in fewer topics that were covered more longitudinally. A proposed example of a longitudinal topic was defining and refining existing mentoring relationships. Others found it useful to cover more ground with a potpourri of themes; they wanted to cover different knowledge, skills, and attitudes considered important for personal growth and career development, such as negotiation, leading teams, and managing conflict. We recommend the goals of the peer group be defined collaboratively at the beginning of new groups to respond to the needs of the group.
Third, junior faculty varied in how they viewed the goal of the program on a spectrum ranging from social support to mentorship. On one end of the spectrum, the program provided a safe venue for colleagues to convene periodically to discuss work challenges; this group found the support from peers to be helpful. On the other end, some found value in the coaching and mentoring from peers and the experienced facilitator that guided personal growth and career development.
Our pilot program has several limitations. This is a single-center program with a relatively small number of participants; thus, our experience may be unique to our institution and not representative of all academic hospital medicine programs. We also did not obtain any quantitative metrics of evaluation—mixed methods should be used in the future for more rigorous program evaluation. Finally, our peer mentoring model may not cover all domains of mentoring such as sponsorship for career advancement, provision of resources, and promotion of scholarship, though we mentioned an anecdote of scholarship that resulted from networking and redefining of goals that were facilitated through this program. Scholarship is certainly an important feature of academic medicine—other peer mentoring programs may consider forming groups based on research interests to address this gap. A tailored curriculum toward research and scholarship may garner more interest and benefit from participants interested in advancement of scholarship activities.
Overall, the field of hospital medicine is growing rapidly with junior faculty who need effective mentorship. Facilitated peer mentorship among small groups of junior faculty is a feasible and pragmatic mentorship model that can complement more traditional mentorship models. We discovered wide-ranging and contrasting experiences in our program, which suggests that peer mentorship is not a one-size-fits-all approach. However, facilitated peer mentorship can be a highly adaptable and alternative approach to mentorship for diverse groups of hospitalists, including general internal medicine, pediatrics, and other sub
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836.
2. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5.
3. Cumbler E, Rendón P, Yirdaw E, et al. Keys to career success: resources and barriers identified by early career academic hospitalists. J Gen Intern Med. 2018;33(5):588-589. https://doi.org/10.1007/s11606-018-4336-7.
4. Sambunjak D, Straus SE, Marusić A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103-1115. https://doi.org/10.1001/jama.296.9.1103.
5. Pololi LH, Evans AT, Civian JT, et al. Faculty vitality-surviving the challenges facing academic health centers: a national survey of medical faculty. Acad Med. 2015;90(7):930-936. https://doi.org/10.1097/ACM.0000000000000674.
6. Nagarur A, O’Neill RM, Lawton D, Greenwald JL. Supporting faculty development in hospital medicine: design and implementation of a personalized structured mentoring program. J Hosp Med. 2018;13(2):96-99. https://doi.org/10.12788/jhm.2854.
7. Wachter RM. The state of hospital medicine in 2008. Med Clin North Am. 2008;92(2):265-273, vii. https://doi.org/10.1016/j.mcna.2007.10.008.
8. Wiese J, Centor R. The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6(1):1-2.
9. Rogers JC, Holloway RL, Miller SM. Academic mentoring and family medicine’s research productivity. Fam Med. 1990;22(3):186-190.
10. Pololi L, Knight S. Mentoring faculty in academic medicine. A new paradigm? J Gen Intern Med. 2005;20(9):866-870.
11. Varkey P, Jatoi A, Williams A, et al. The positive impact of a facilitated peer mentoring program on academic skills of women faculty. BMC Med Educ. 2012;12:14. https://doi.org/10.1186/1472-6920-12-14.
12. Pololi LH, Evans AT. Group peer mentoring: an answer to the faculty mentoring problem? A successful program at a large academic department of medicine. J Contin Educ Health Prof. 2015;35(3):192-200. https://doi.org/10.1002/chp.21296.
13. Abougergi MS, Wright SM, Landis R, Howell EE. Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43-46. https://doi.org/10.1002/jhm.865.
Traditional mentorship models may not be adequate for academic hospitalists. The traditional dyadic mentorship model, in which a senior principal investigator and research mentee collaborate for career advancement, is well suited for basic science or clinical research. In contrast, areas of academic hospital medicine such as quality improvement, medical education, hospital operations, point-of-care ultrasound, and clinical expertise may be less suited to this traditional mentoring model. In addition, experienced mentors are limited and those available are often overcommitted or have inadequate time due to responsibilities with other leadership roles. Senior mentors may also be limited because of our specialty’s focus on clinical practice rather than longitudinal research or projects.9 There are other limitations of traditional mentorship that are applicable to all fields of academic medicine, including disparate goals, expectations, levels of commitment, and the inherent power differential between the mentor and mentee.10
In this perspective, we discuss our experience with implementing an alternative and complementary mentorship strategy called facilitated peer mentorship with junior faculty hospitalists in the Division of General Internal Medicine at New York–Presbyterian/Weill Cornell Medical Center.
In facilitated peer mentoring programs, faculty typically work collaboratively in groups of three to five with other faculty who are of similar rank, and a faculty member of a higher academic rank works with the group in meeting their scholarly goals.11 The role of the facilitator is to ensure a safe and respectful learning environment, foster peer collaboration, and redirect the group to draw upon their own experiences. Each junior faculty member serves as both a mentor and mentee for each other with bidirectional feedback, guidance, and support in a group setting. This model emphasizes collaboration, peer networking, empowerment, and the development of personal awareness.10 A number of academic medical centers have used peer mentoring as a response to the challenges encountered in the traditional dyad model.12 To our knowledge, the only published example of a peer mentoring model in academic hospital medicine is in the form of a research-in-progress conference.13 While this example addresses peer-mentored research, there is a gap in other areas of academic hospital medicine with mentoring needs—most of all in personal development and career satisfaction.
We piloted a 12-month facilitated peer mentoring program for new hospitalists. The goal of the program was for junior faculty hospitalists to develop a better understanding of their own identity and core values that would enable them to more confidently navigate career choices, enhance their work vitality and career satisfaction, and develop their potential for leadership roles in academic hospital medicine. Each year, a cohort of four to five incoming hospitalists from different backgrounds, interests, and experience were grouped with a more experienced colleague at an associate professor rank who expressed interest and was selected by our section chief to lead the program. The program was required for new hospitalists and consisted of six 90-minute sessions every two months. The attendance rate was 100% and was ensured by scheduling all sessions at the beginning of the academic year with dates agreed upon by all participants. An e-mail reminder was sent one week prior to each session. Each session had assigned readings and an agenda for discussions (see Table for details).
Our evaluation of the program after two years with two separate cohorts included qualitative feedback through an anonymous survey for participants; in addition, qualitative feedback was collected in a one-hour, in-person discussion and reflection with each cohort. We learned several lessons from the feedback we received from program participants. First, our impression was that the career experience of the junior faculty member had a significant impact on the perceived value of group meetings. For those who entered the hospitalist workforce immediately upon completing their terminal training in internal medicine, the exercise of considering different career versions of themselves had added value in promoting thinking outside-the-box for career opportunities within hospital medicine. Academic hospitalists and general internists more broadly, tend to have broad interests that fuel their passions but may also make it more difficult to define long-term goals. One junior faculty member paired her life interests in global medical education with building an international collaboration with other academic hospitalist programs; another faculty member gained confidence and expanded her network of collaborators by designing a research pilot study on hospitalist-initiated end-of-life discussions. In both cases, the junior faculty identified the facilitated peer mentoring program as a strong influence in finding these opportunities. Peer mentoring at the time of entry into the field of hospital medicine, when many have undefined career goals, can be helpful for navigating this issue at the start of a career. On the other hand, those who had already worked as a hospitalist for one or two years and joined the program found less value in career planning exercises.
Second, junior faculty differed in their desire for scope and depth of the curriculum. Some preferred more frequent sessions with more premeeting readings and self-assessments in fewer topics that were covered more longitudinally. A proposed example of a longitudinal topic was defining and refining existing mentoring relationships. Others found it useful to cover more ground with a potpourri of themes; they wanted to cover different knowledge, skills, and attitudes considered important for personal growth and career development, such as negotiation, leading teams, and managing conflict. We recommend the goals of the peer group be defined collaboratively at the beginning of new groups to respond to the needs of the group.
Third, junior faculty varied in how they viewed the goal of the program on a spectrum ranging from social support to mentorship. On one end of the spectrum, the program provided a safe venue for colleagues to convene periodically to discuss work challenges; this group found the support from peers to be helpful. On the other end, some found value in the coaching and mentoring from peers and the experienced facilitator that guided personal growth and career development.
Our pilot program has several limitations. This is a single-center program with a relatively small number of participants; thus, our experience may be unique to our institution and not representative of all academic hospital medicine programs. We also did not obtain any quantitative metrics of evaluation—mixed methods should be used in the future for more rigorous program evaluation. Finally, our peer mentoring model may not cover all domains of mentoring such as sponsorship for career advancement, provision of resources, and promotion of scholarship, though we mentioned an anecdote of scholarship that resulted from networking and redefining of goals that were facilitated through this program. Scholarship is certainly an important feature of academic medicine—other peer mentoring programs may consider forming groups based on research interests to address this gap. A tailored curriculum toward research and scholarship may garner more interest and benefit from participants interested in advancement of scholarship activities.
Overall, the field of hospital medicine is growing rapidly with junior faculty who need effective mentorship. Facilitated peer mentorship among small groups of junior faculty is a feasible and pragmatic mentorship model that can complement more traditional mentorship models. We discovered wide-ranging and contrasting experiences in our program, which suggests that peer mentorship is not a one-size-fits-all approach. However, facilitated peer mentorship can be a highly adaptable and alternative approach to mentorship for diverse groups of hospitalists, including general internal medicine, pediatrics, and other sub
Traditional mentorship models may not be adequate for academic hospitalists. The traditional dyadic mentorship model, in which a senior principal investigator and research mentee collaborate for career advancement, is well suited for basic science or clinical research. In contrast, areas of academic hospital medicine such as quality improvement, medical education, hospital operations, point-of-care ultrasound, and clinical expertise may be less suited to this traditional mentoring model. In addition, experienced mentors are limited and those available are often overcommitted or have inadequate time due to responsibilities with other leadership roles. Senior mentors may also be limited because of our specialty’s focus on clinical practice rather than longitudinal research or projects.9 There are other limitations of traditional mentorship that are applicable to all fields of academic medicine, including disparate goals, expectations, levels of commitment, and the inherent power differential between the mentor and mentee.10
In this perspective, we discuss our experience with implementing an alternative and complementary mentorship strategy called facilitated peer mentorship with junior faculty hospitalists in the Division of General Internal Medicine at New York–Presbyterian/Weill Cornell Medical Center.
In facilitated peer mentoring programs, faculty typically work collaboratively in groups of three to five with other faculty who are of similar rank, and a faculty member of a higher academic rank works with the group in meeting their scholarly goals.11 The role of the facilitator is to ensure a safe and respectful learning environment, foster peer collaboration, and redirect the group to draw upon their own experiences. Each junior faculty member serves as both a mentor and mentee for each other with bidirectional feedback, guidance, and support in a group setting. This model emphasizes collaboration, peer networking, empowerment, and the development of personal awareness.10 A number of academic medical centers have used peer mentoring as a response to the challenges encountered in the traditional dyad model.12 To our knowledge, the only published example of a peer mentoring model in academic hospital medicine is in the form of a research-in-progress conference.13 While this example addresses peer-mentored research, there is a gap in other areas of academic hospital medicine with mentoring needs—most of all in personal development and career satisfaction.
We piloted a 12-month facilitated peer mentoring program for new hospitalists. The goal of the program was for junior faculty hospitalists to develop a better understanding of their own identity and core values that would enable them to more confidently navigate career choices, enhance their work vitality and career satisfaction, and develop their potential for leadership roles in academic hospital medicine. Each year, a cohort of four to five incoming hospitalists from different backgrounds, interests, and experience were grouped with a more experienced colleague at an associate professor rank who expressed interest and was selected by our section chief to lead the program. The program was required for new hospitalists and consisted of six 90-minute sessions every two months. The attendance rate was 100% and was ensured by scheduling all sessions at the beginning of the academic year with dates agreed upon by all participants. An e-mail reminder was sent one week prior to each session. Each session had assigned readings and an agenda for discussions (see Table for details).
Our evaluation of the program after two years with two separate cohorts included qualitative feedback through an anonymous survey for participants; in addition, qualitative feedback was collected in a one-hour, in-person discussion and reflection with each cohort. We learned several lessons from the feedback we received from program participants. First, our impression was that the career experience of the junior faculty member had a significant impact on the perceived value of group meetings. For those who entered the hospitalist workforce immediately upon completing their terminal training in internal medicine, the exercise of considering different career versions of themselves had added value in promoting thinking outside-the-box for career opportunities within hospital medicine. Academic hospitalists and general internists more broadly, tend to have broad interests that fuel their passions but may also make it more difficult to define long-term goals. One junior faculty member paired her life interests in global medical education with building an international collaboration with other academic hospitalist programs; another faculty member gained confidence and expanded her network of collaborators by designing a research pilot study on hospitalist-initiated end-of-life discussions. In both cases, the junior faculty identified the facilitated peer mentoring program as a strong influence in finding these opportunities. Peer mentoring at the time of entry into the field of hospital medicine, when many have undefined career goals, can be helpful for navigating this issue at the start of a career. On the other hand, those who had already worked as a hospitalist for one or two years and joined the program found less value in career planning exercises.
Second, junior faculty differed in their desire for scope and depth of the curriculum. Some preferred more frequent sessions with more premeeting readings and self-assessments in fewer topics that were covered more longitudinally. A proposed example of a longitudinal topic was defining and refining existing mentoring relationships. Others found it useful to cover more ground with a potpourri of themes; they wanted to cover different knowledge, skills, and attitudes considered important for personal growth and career development, such as negotiation, leading teams, and managing conflict. We recommend the goals of the peer group be defined collaboratively at the beginning of new groups to respond to the needs of the group.
Third, junior faculty varied in how they viewed the goal of the program on a spectrum ranging from social support to mentorship. On one end of the spectrum, the program provided a safe venue for colleagues to convene periodically to discuss work challenges; this group found the support from peers to be helpful. On the other end, some found value in the coaching and mentoring from peers and the experienced facilitator that guided personal growth and career development.
Our pilot program has several limitations. This is a single-center program with a relatively small number of participants; thus, our experience may be unique to our institution and not representative of all academic hospital medicine programs. We also did not obtain any quantitative metrics of evaluation—mixed methods should be used in the future for more rigorous program evaluation. Finally, our peer mentoring model may not cover all domains of mentoring such as sponsorship for career advancement, provision of resources, and promotion of scholarship, though we mentioned an anecdote of scholarship that resulted from networking and redefining of goals that were facilitated through this program. Scholarship is certainly an important feature of academic medicine—other peer mentoring programs may consider forming groups based on research interests to address this gap. A tailored curriculum toward research and scholarship may garner more interest and benefit from participants interested in advancement of scholarship activities.
Overall, the field of hospital medicine is growing rapidly with junior faculty who need effective mentorship. Facilitated peer mentorship among small groups of junior faculty is a feasible and pragmatic mentorship model that can complement more traditional mentorship models. We discovered wide-ranging and contrasting experiences in our program, which suggests that peer mentorship is not a one-size-fits-all approach. However, facilitated peer mentorship can be a highly adaptable and alternative approach to mentorship for diverse groups of hospitalists, including general internal medicine, pediatrics, and other sub
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836.
2. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5.
3. Cumbler E, Rendón P, Yirdaw E, et al. Keys to career success: resources and barriers identified by early career academic hospitalists. J Gen Intern Med. 2018;33(5):588-589. https://doi.org/10.1007/s11606-018-4336-7.
4. Sambunjak D, Straus SE, Marusić A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103-1115. https://doi.org/10.1001/jama.296.9.1103.
5. Pololi LH, Evans AT, Civian JT, et al. Faculty vitality-surviving the challenges facing academic health centers: a national survey of medical faculty. Acad Med. 2015;90(7):930-936. https://doi.org/10.1097/ACM.0000000000000674.
6. Nagarur A, O’Neill RM, Lawton D, Greenwald JL. Supporting faculty development in hospital medicine: design and implementation of a personalized structured mentoring program. J Hosp Med. 2018;13(2):96-99. https://doi.org/10.12788/jhm.2854.
7. Wachter RM. The state of hospital medicine in 2008. Med Clin North Am. 2008;92(2):265-273, vii. https://doi.org/10.1016/j.mcna.2007.10.008.
8. Wiese J, Centor R. The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6(1):1-2.
9. Rogers JC, Holloway RL, Miller SM. Academic mentoring and family medicine’s research productivity. Fam Med. 1990;22(3):186-190.
10. Pololi L, Knight S. Mentoring faculty in academic medicine. A new paradigm? J Gen Intern Med. 2005;20(9):866-870.
11. Varkey P, Jatoi A, Williams A, et al. The positive impact of a facilitated peer mentoring program on academic skills of women faculty. BMC Med Educ. 2012;12:14. https://doi.org/10.1186/1472-6920-12-14.
12. Pololi LH, Evans AT. Group peer mentoring: an answer to the faculty mentoring problem? A successful program at a large academic department of medicine. J Contin Educ Health Prof. 2015;35(3):192-200. https://doi.org/10.1002/chp.21296.
13. Abougergi MS, Wright SM, Landis R, Howell EE. Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43-46. https://doi.org/10.1002/jhm.865.
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836.
2. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5.
3. Cumbler E, Rendón P, Yirdaw E, et al. Keys to career success: resources and barriers identified by early career academic hospitalists. J Gen Intern Med. 2018;33(5):588-589. https://doi.org/10.1007/s11606-018-4336-7.
4. Sambunjak D, Straus SE, Marusić A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103-1115. https://doi.org/10.1001/jama.296.9.1103.
5. Pololi LH, Evans AT, Civian JT, et al. Faculty vitality-surviving the challenges facing academic health centers: a national survey of medical faculty. Acad Med. 2015;90(7):930-936. https://doi.org/10.1097/ACM.0000000000000674.
6. Nagarur A, O’Neill RM, Lawton D, Greenwald JL. Supporting faculty development in hospital medicine: design and implementation of a personalized structured mentoring program. J Hosp Med. 2018;13(2):96-99. https://doi.org/10.12788/jhm.2854.
7. Wachter RM. The state of hospital medicine in 2008. Med Clin North Am. 2008;92(2):265-273, vii. https://doi.org/10.1016/j.mcna.2007.10.008.
8. Wiese J, Centor R. The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6(1):1-2.
9. Rogers JC, Holloway RL, Miller SM. Academic mentoring and family medicine’s research productivity. Fam Med. 1990;22(3):186-190.
10. Pololi L, Knight S. Mentoring faculty in academic medicine. A new paradigm? J Gen Intern Med. 2005;20(9):866-870.
11. Varkey P, Jatoi A, Williams A, et al. The positive impact of a facilitated peer mentoring program on academic skills of women faculty. BMC Med Educ. 2012;12:14. https://doi.org/10.1186/1472-6920-12-14.
12. Pololi LH, Evans AT. Group peer mentoring: an answer to the faculty mentoring problem? A successful program at a large academic department of medicine. J Contin Educ Health Prof. 2015;35(3):192-200. https://doi.org/10.1002/chp.21296.
13. Abougergi MS, Wright SM, Landis R, Howell EE. Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43-46. https://doi.org/10.1002/jhm.865.
© 2020 Society of Hospital Medicine
Clinical Progress Note: Procalcitonin in the Diagnosis and Management of Community-Acquired Pneumonia in Hospitalized Adults
Community-acquired pneumonia (CAP) accounts for more than 1.5 million adult hospitalizations and 100,000 deaths each year in the United States.1 Antibiotic overuse in the hospital setting is an important contributor to the rise of antibiotic resistance, prompting increased efforts to limit inappropriate antibiotic use in hospitals.2 Procalcitonin, a precursor of the hormone calcitonin, is upregulated in bacterial infections and downregulated in viral infections. The US Food and Drug Administration has approved it as a serum biomarker to assist clinicians with decisions about using antibiotics.3
There is no consensus on how to best use procalcitonin in the management of CAP. We provide a practical update that includes a review of recent literature, added secondary analysis, and expert opinion surrounding the use of procalcitonin in the diagnosis and management of CAP in hospitalized adults.
INITIATION OF ANTIBIOTICS
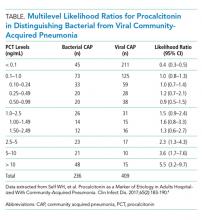
Initial procalcitonin levels do not sufficiently exclude bacterial etiologies of CAP to withhold antibiotic prescription safely. The largest diagnostic accuracy study of procalcitonin in the diagnosis of CAP was a subanalysis of the Etiology of Pneumonia in the Community Study.4 A total of 1,735 adults hospitalized with CAP received procalcitonin testing along with systematic pathogen testing. The area under the receiver operating characteristic curve for procalcitonin in discriminating bacterial pathogens from viral pathogens was 0.73 (95% CI, 0.69-0.77). A procalcitonin cut-off of 0.1 ng/mL resulted in 80.9% (95% CI, 75.3%-85.7%) sensitivity and 51.6% (95% CI, 46.6%-56.5%) specificity for identification of any bacterial pathogen.
In a secondary analysis of this study, we calculated multilevel likelihood ratios (LRs) for ranges of procalcitonin values to determine the diagnostic accuracy of procalcitonin in distinguishing bacterial from viral etiologies of CAP (Table). Multilevel LRs offer more useful diagnostic information than dichotomizing at specified cut-points.5 A procalcitonin result less than 0.1 ng/mL has a negative LR of 0.4 (95% CI, 0.3-0.5), which is not low enough to rule out bacterial CAP effectively when starting with intermediate or high pretest probability. For a low result (<0.1 ng/mL) to be useful in ruling out bacterial CAP, for example having less than a 10% posttest probability of bacterial CAP, the pretest probability would have to be no greater than 22%. Even then, a 10% posttest probability of bacterial CAP may still be too high for clinicians to withhold initial antibiotics. For procalcitonin values between 0.1 ng/mL and 1.0 ng/mL, the probability of bacterial CAP does not change significantly, with an LR of 1.0 (95% CI, 0.8-1.3). Procalcitonin values up to 5 ng/mL reach a modest positive LR of 2.3 (95% CI, 0.8-4.3). Very high values, such as those >10 ng/mL, yield a positive LR of 5.5 (95% CI, 3.2-9.7), are potentially useful in decisions to initiate antibiotics in situations of very low pretest probability of bacterial CAP. For example, a 9% pretest probability of bacterial CAP is likely below many physicians’ threshold for starting antibiotics. A procalcitonin of 12 ng/mL in this patient would increase the posttest probability to 35%, a value that would prompt many physicians to initiate antibiotics.
Overall, there is insufficient evidence to support the use of procalcitonin as a stand-alone test for ruling out bacterial CAP, limiting its use in withholding antibiotics in patients with suspected bacterial CAP.
DISCONTINUATION OF ANTIBIOTICS
While initial procalcitonin measurements may not affect the initial antibiotic treatment decision, procalcitonin levels thereafter can guide the duration of therapy. A meta-analysis of procalcitonin-guided treatment in patients with upper or lower respiratory tract infection (LRTI) showed that procalcitonin guidance reduces antibiotic exposure and antibiotic-related adverse effects and improves survival, albeit a small absolute mortality difference of 1.4 percentage points, primarily observed in the intensive care unit setting.6 Most patients included in this meta-analysis were diagnosed with LRTI (91%), and CAP was the predominant subtype of LRTI (43%). The main effect of procalcitonin guidance for patients with CAP was earlier discontinuation of antibiotic treatment. Procalcitonin-guided algorithms in these trials discouraged, or strongly discouraged, antibiotics if procalcitonin was <0.25 ng/mL or <0.1 ng/mL, respectively. In addition, serial procalcitonin measurements were used to guide discontinuation of antibiotics if procalcitonin dropped below 0.25 ng/mL, or by 80% to 90% from the peak value. This approach safely shortened the duration of therapy in patients with CAP.
There are several limitations in the interpretation and generalizability of this meta-analysis. There is large heterogeneity across the included clinical trials in design, procalcitonin protocols, clinical setting, and respiratory infection type, including bronchitis, acute exacerbation of chronic obstructive pulmonary disease (AECOPD), and CAP. Results were consistent only in one moderate- to high-quality randomized trial specifically studying CAP in the inpatient setting.7 Additionally, most of these trials were conducted in Europe. Antibiotic prescribing practices may be different in the US, and prescribing practices on both continents may have changed over the years with greater awareness and appreciation of antibiotic stewardship.
PROCALCITONIN-GUIDED ALGORITHMS
The ProACT trial, the largest randomized, US multicenter trial to evaluate a procalcitonin-based algorithm to assist with antibiotic decision making, included over 1,600 emergency department patients at 14 academic medical centers.8 Procalcitonin guidance in this trial did not reduce antibiotic exposure compared with usual care for patients with suspected LRTI. However, its applicability to the practice of hospitalists and the inpatient setting is limited. First, only 48% of the study participants required hospitalization. Second, this study included all LRTIs, with CAP comprising just 20% of all final diagnoses. Third, the average number of antibiotic days during hospitalization for CAP was short in both groups (3.9 days in the procalcitonin group and 4.1 days in the usual care group). This relatively short antibiotic duration makes it difficult for any intervention to decrease antibiotic days meaningfully.
In a prepost controlled intervention study for inpatients at a single US tertiary care hospital, procalcitonin guidance in hospitalized patients safely reduced antibiotic use in LRTI, specifically for the discontinuation of antibiotics.9 The greatest benefit of procalcitonin guidance in antibiotic discontinuation was found in patients with AECOPD and patients with an admitting diagnosis of CAP, but with mild illness and a low procalcitonin. Although this prepost study suggested a safe reduction of antibiotic use due to implementation of procalcitonin guidance, the lack of randomization and the absence of a contemporaneous control group are important limitations. Given the mixed findings on the effectiveness of procalcitonin guidance for hospitalized CAP patients in the US, further investigation will be needed with large clinical trials in the inpatient setting for CAP.
CONCLUSIONS
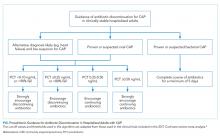
There is insufficient evidence to support the use of serum procalcitonin to withhold initial antibiotics in patients with a clinical syndrome consistent with bacterial CAP. However, the literature supports the use of procalcitonin for the early discontinuation of antibiotics for cases in which the probability of bacterial CAP is low, and procalcitonin remains below 0.1 ng/mL (Figure).
Serial measurements of procalcitonin every one to two days may also be used when clinical uncertainty remains regarding the need for antibiotics. Very low or significantly decreasing procalcitonin levels in patients with CAP and no identified bacterial pathogen likely indicate the infection was not bacterial or was bacterial, but has now been adequately treated with antibiotics. For cases of proven bacterial etiology or high clinical suspicion of bacterial CAP, there is insufficient evidence to recommend the early discontinuation of antibiotics based on procalcitonin levels short of the recommended five-day course according to current guidelines.10 Future clinical trials are needed to determine if procalcitonin guidance can safely decrease the duration of antibiotic therapy for confirmed bacterial CAP to less than five days.
There are discrepancies between the apparent test characteristics of procalcitonin and the recommended antibiotic decisions in many procalcitonin algorithms. For example, algorithms discourage antibiotics when procalcitonin values are 0.1-0.24 ng/mL, and encourage (or even strongly encourage) antibiotic use for higher procalcitonin values of 0.25-1.0 ng/mL. However, the LRs for these ranges are identical and are approximately 1.0 (Table), suggesting that decision-making should be similar across the entire procalcitonin range of 0.1 to 1.0. Future clinical trials should study revised algorithms with different cut-points, including the thresholds found in our secondary analysis of multilevel LRs. Until then, we believe there is insufficient evidence to deviate from current antibiotic decision recommendations at the traditional cut-points.
While procalcitonin is an imperfect biomarker for discriminating bacterial and nonbacterial etiologies of CAP, it may still provide helpful information for the hospitalist in antibiotic decision-making in the same way we apply other commonly used clinical variables such as fever, white blood cell count, band count, and the pattern of infiltrate in chest imaging.
Procalcitonin should be interpreted cautiously in certain populations in which it has not been extensively studied (eg, immunocompromised) or in noninfectious conditions that may elevate procalcitonin, such as major physiologic stress (eg, surgery, trauma, burns) and end-stage renal disease.12-14 Further investigation is needed to determine the efficacy and safety of procalcitonin-guided antibiotic therapy in these populations.
RECOMMENDATIONS
- Based on currently available data, a low procalcitonin value should not be used as a stand-alone test to withhold antibiotics in a patient with CAP.
- Serum procalcitonin measurements may help guide the early discontinuation of antibiotics for patients who the treating clinician judges the risks of bacterial etiology and clinical deterioration to be low.
- Interpret procalcitonin cautiously in immunocompromised patients, undergoing severe physiologic stress, or have underlying end-stage renal disease.
- Serum procalcitonin serves as an adjunct to, rather than a substitute for, clinical judgment.
Disclosures
Dr Choi, Dr Evans, and Dr Glesby have nothing to disclose. Dr Self reports receiving prior research funding from BRAHMS/Thermo-Fisher and BioMerieux for studies on procalcitonin. Dr Self reports personal fees from Inflammatix, grants from Axis Shield, Rapid Pathogen Screening, and BioMerieux, all outside the submitted work. Dr McCarthy reports receiving research funding from Allergan outside the submitted work. Dr Simon reports receiving consulting fees from Roche Diagnostics.
1. Ramirez JA, Wiemken TL, Peyrani P, et al. adults hospitalized with pneumonia in the united states: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. https://doi.org/10.1093/cid/cix647.
2. Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972-978. https://doi.org/10.1001/archinte.163.8.972.
3. Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. https://doi.org/10.1093/ofid/ofw249.
4. Self WH, Balk RA, Grijalva CG, et al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia. Clin Infect Dis. 2017;65(2):183-190. https://doi.org/10.1093/cid/cix317.
5. Straus SE, Richardson WS, Glasziou P, Haynes RB. Evidence-Based Medicine: How to Practice and Teach It (4th Edition). Fourth Edition ed. London, England: Elsevier Churchill Livingstone; 2010.
6. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498. https://doi.org/10.1164/rccm.200512-1922OC.
7. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93. https://doi.org/10.1056/NEJMoa1802670.
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. https://doi.org/10.1056/NEJMoa1802670
10. Townsend J, Adams V, Galiatsatos P, et al. Procalcitonin-guided antibiotic therapy reduces antibiotic use for lower respiratory tract infections in a United States medical center: results of a clinical trial. Open Forum Infect Dis. 2018;5(12):ofy327. https://doi.org/10.1093/ofid/ofy327.
11. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72. https://doi.org/10.1086/511159.
12. Seoane L, Pértega S, Galeiras R, Astola I, Bouza T. Procalcitonin in the burn unit and the diagnosis of infection. Burns. 2014;40(2):223-229. https://doi.org/10.1016/j.burns.2013.11.018.
13. Dahaba AA, Rehak PH, List WF. Procalcitonin and C-reactive protein plasma concentrations in nonseptic uremic patients undergoing hemodialysis. Intensive Care Med. 2003;29(4):579-583. https://doi.org/10.1007/s00134-003-1664-8.
14. Ghabra H, White W, Townsend M, Boysen P, Nossaman B. Use of biomarkers in the prediction of culture-proven infection in the surgical intensive care unit. J Crit Care. 2019;49:149-154. https://doi.org/10.1016/j.jcrc.2018.10.023.
15. Hoshino K, Irie Y, Mizunuma M, Kawano K, Kitamura T, Ishikura H. Incidence of elevated procalcitonin and presepsin levels after severe trauma: a pilot cohort study. Anaesth Intensive Care. 2017;45(5):600-604. https://doi.org/10.1177/0310057X1704500510.
Community-acquired pneumonia (CAP) accounts for more than 1.5 million adult hospitalizations and 100,000 deaths each year in the United States.1 Antibiotic overuse in the hospital setting is an important contributor to the rise of antibiotic resistance, prompting increased efforts to limit inappropriate antibiotic use in hospitals.2 Procalcitonin, a precursor of the hormone calcitonin, is upregulated in bacterial infections and downregulated in viral infections. The US Food and Drug Administration has approved it as a serum biomarker to assist clinicians with decisions about using antibiotics.3
There is no consensus on how to best use procalcitonin in the management of CAP. We provide a practical update that includes a review of recent literature, added secondary analysis, and expert opinion surrounding the use of procalcitonin in the diagnosis and management of CAP in hospitalized adults.
INITIATION OF ANTIBIOTICS
Initial procalcitonin levels do not sufficiently exclude bacterial etiologies of CAP to withhold antibiotic prescription safely. The largest diagnostic accuracy study of procalcitonin in the diagnosis of CAP was a subanalysis of the Etiology of Pneumonia in the Community Study.4 A total of 1,735 adults hospitalized with CAP received procalcitonin testing along with systematic pathogen testing. The area under the receiver operating characteristic curve for procalcitonin in discriminating bacterial pathogens from viral pathogens was 0.73 (95% CI, 0.69-0.77). A procalcitonin cut-off of 0.1 ng/mL resulted in 80.9% (95% CI, 75.3%-85.7%) sensitivity and 51.6% (95% CI, 46.6%-56.5%) specificity for identification of any bacterial pathogen.
In a secondary analysis of this study, we calculated multilevel likelihood ratios (LRs) for ranges of procalcitonin values to determine the diagnostic accuracy of procalcitonin in distinguishing bacterial from viral etiologies of CAP (Table). Multilevel LRs offer more useful diagnostic information than dichotomizing at specified cut-points.5 A procalcitonin result less than 0.1 ng/mL has a negative LR of 0.4 (95% CI, 0.3-0.5), which is not low enough to rule out bacterial CAP effectively when starting with intermediate or high pretest probability. For a low result (<0.1 ng/mL) to be useful in ruling out bacterial CAP, for example having less than a 10% posttest probability of bacterial CAP, the pretest probability would have to be no greater than 22%. Even then, a 10% posttest probability of bacterial CAP may still be too high for clinicians to withhold initial antibiotics. For procalcitonin values between 0.1 ng/mL and 1.0 ng/mL, the probability of bacterial CAP does not change significantly, with an LR of 1.0 (95% CI, 0.8-1.3). Procalcitonin values up to 5 ng/mL reach a modest positive LR of 2.3 (95% CI, 0.8-4.3). Very high values, such as those >10 ng/mL, yield a positive LR of 5.5 (95% CI, 3.2-9.7), are potentially useful in decisions to initiate antibiotics in situations of very low pretest probability of bacterial CAP. For example, a 9% pretest probability of bacterial CAP is likely below many physicians’ threshold for starting antibiotics. A procalcitonin of 12 ng/mL in this patient would increase the posttest probability to 35%, a value that would prompt many physicians to initiate antibiotics.
Overall, there is insufficient evidence to support the use of procalcitonin as a stand-alone test for ruling out bacterial CAP, limiting its use in withholding antibiotics in patients with suspected bacterial CAP.
DISCONTINUATION OF ANTIBIOTICS
While initial procalcitonin measurements may not affect the initial antibiotic treatment decision, procalcitonin levels thereafter can guide the duration of therapy. A meta-analysis of procalcitonin-guided treatment in patients with upper or lower respiratory tract infection (LRTI) showed that procalcitonin guidance reduces antibiotic exposure and antibiotic-related adverse effects and improves survival, albeit a small absolute mortality difference of 1.4 percentage points, primarily observed in the intensive care unit setting.6 Most patients included in this meta-analysis were diagnosed with LRTI (91%), and CAP was the predominant subtype of LRTI (43%). The main effect of procalcitonin guidance for patients with CAP was earlier discontinuation of antibiotic treatment. Procalcitonin-guided algorithms in these trials discouraged, or strongly discouraged, antibiotics if procalcitonin was <0.25 ng/mL or <0.1 ng/mL, respectively. In addition, serial procalcitonin measurements were used to guide discontinuation of antibiotics if procalcitonin dropped below 0.25 ng/mL, or by 80% to 90% from the peak value. This approach safely shortened the duration of therapy in patients with CAP.
There are several limitations in the interpretation and generalizability of this meta-analysis. There is large heterogeneity across the included clinical trials in design, procalcitonin protocols, clinical setting, and respiratory infection type, including bronchitis, acute exacerbation of chronic obstructive pulmonary disease (AECOPD), and CAP. Results were consistent only in one moderate- to high-quality randomized trial specifically studying CAP in the inpatient setting.7 Additionally, most of these trials were conducted in Europe. Antibiotic prescribing practices may be different in the US, and prescribing practices on both continents may have changed over the years with greater awareness and appreciation of antibiotic stewardship.
PROCALCITONIN-GUIDED ALGORITHMS
The ProACT trial, the largest randomized, US multicenter trial to evaluate a procalcitonin-based algorithm to assist with antibiotic decision making, included over 1,600 emergency department patients at 14 academic medical centers.8 Procalcitonin guidance in this trial did not reduce antibiotic exposure compared with usual care for patients with suspected LRTI. However, its applicability to the practice of hospitalists and the inpatient setting is limited. First, only 48% of the study participants required hospitalization. Second, this study included all LRTIs, with CAP comprising just 20% of all final diagnoses. Third, the average number of antibiotic days during hospitalization for CAP was short in both groups (3.9 days in the procalcitonin group and 4.1 days in the usual care group). This relatively short antibiotic duration makes it difficult for any intervention to decrease antibiotic days meaningfully.
In a prepost controlled intervention study for inpatients at a single US tertiary care hospital, procalcitonin guidance in hospitalized patients safely reduced antibiotic use in LRTI, specifically for the discontinuation of antibiotics.9 The greatest benefit of procalcitonin guidance in antibiotic discontinuation was found in patients with AECOPD and patients with an admitting diagnosis of CAP, but with mild illness and a low procalcitonin. Although this prepost study suggested a safe reduction of antibiotic use due to implementation of procalcitonin guidance, the lack of randomization and the absence of a contemporaneous control group are important limitations. Given the mixed findings on the effectiveness of procalcitonin guidance for hospitalized CAP patients in the US, further investigation will be needed with large clinical trials in the inpatient setting for CAP.
CONCLUSIONS
There is insufficient evidence to support the use of serum procalcitonin to withhold initial antibiotics in patients with a clinical syndrome consistent with bacterial CAP. However, the literature supports the use of procalcitonin for the early discontinuation of antibiotics for cases in which the probability of bacterial CAP is low, and procalcitonin remains below 0.1 ng/mL (Figure).
Serial measurements of procalcitonin every one to two days may also be used when clinical uncertainty remains regarding the need for antibiotics. Very low or significantly decreasing procalcitonin levels in patients with CAP and no identified bacterial pathogen likely indicate the infection was not bacterial or was bacterial, but has now been adequately treated with antibiotics. For cases of proven bacterial etiology or high clinical suspicion of bacterial CAP, there is insufficient evidence to recommend the early discontinuation of antibiotics based on procalcitonin levels short of the recommended five-day course according to current guidelines.10 Future clinical trials are needed to determine if procalcitonin guidance can safely decrease the duration of antibiotic therapy for confirmed bacterial CAP to less than five days.
There are discrepancies between the apparent test characteristics of procalcitonin and the recommended antibiotic decisions in many procalcitonin algorithms. For example, algorithms discourage antibiotics when procalcitonin values are 0.1-0.24 ng/mL, and encourage (or even strongly encourage) antibiotic use for higher procalcitonin values of 0.25-1.0 ng/mL. However, the LRs for these ranges are identical and are approximately 1.0 (Table), suggesting that decision-making should be similar across the entire procalcitonin range of 0.1 to 1.0. Future clinical trials should study revised algorithms with different cut-points, including the thresholds found in our secondary analysis of multilevel LRs. Until then, we believe there is insufficient evidence to deviate from current antibiotic decision recommendations at the traditional cut-points.
While procalcitonin is an imperfect biomarker for discriminating bacterial and nonbacterial etiologies of CAP, it may still provide helpful information for the hospitalist in antibiotic decision-making in the same way we apply other commonly used clinical variables such as fever, white blood cell count, band count, and the pattern of infiltrate in chest imaging.
Procalcitonin should be interpreted cautiously in certain populations in which it has not been extensively studied (eg, immunocompromised) or in noninfectious conditions that may elevate procalcitonin, such as major physiologic stress (eg, surgery, trauma, burns) and end-stage renal disease.12-14 Further investigation is needed to determine the efficacy and safety of procalcitonin-guided antibiotic therapy in these populations.
RECOMMENDATIONS
- Based on currently available data, a low procalcitonin value should not be used as a stand-alone test to withhold antibiotics in a patient with CAP.
- Serum procalcitonin measurements may help guide the early discontinuation of antibiotics for patients who the treating clinician judges the risks of bacterial etiology and clinical deterioration to be low.
- Interpret procalcitonin cautiously in immunocompromised patients, undergoing severe physiologic stress, or have underlying end-stage renal disease.
- Serum procalcitonin serves as an adjunct to, rather than a substitute for, clinical judgment.
Disclosures
Dr Choi, Dr Evans, and Dr Glesby have nothing to disclose. Dr Self reports receiving prior research funding from BRAHMS/Thermo-Fisher and BioMerieux for studies on procalcitonin. Dr Self reports personal fees from Inflammatix, grants from Axis Shield, Rapid Pathogen Screening, and BioMerieux, all outside the submitted work. Dr McCarthy reports receiving research funding from Allergan outside the submitted work. Dr Simon reports receiving consulting fees from Roche Diagnostics.
Community-acquired pneumonia (CAP) accounts for more than 1.5 million adult hospitalizations and 100,000 deaths each year in the United States.1 Antibiotic overuse in the hospital setting is an important contributor to the rise of antibiotic resistance, prompting increased efforts to limit inappropriate antibiotic use in hospitals.2 Procalcitonin, a precursor of the hormone calcitonin, is upregulated in bacterial infections and downregulated in viral infections. The US Food and Drug Administration has approved it as a serum biomarker to assist clinicians with decisions about using antibiotics.3
There is no consensus on how to best use procalcitonin in the management of CAP. We provide a practical update that includes a review of recent literature, added secondary analysis, and expert opinion surrounding the use of procalcitonin in the diagnosis and management of CAP in hospitalized adults.
INITIATION OF ANTIBIOTICS
Initial procalcitonin levels do not sufficiently exclude bacterial etiologies of CAP to withhold antibiotic prescription safely. The largest diagnostic accuracy study of procalcitonin in the diagnosis of CAP was a subanalysis of the Etiology of Pneumonia in the Community Study.4 A total of 1,735 adults hospitalized with CAP received procalcitonin testing along with systematic pathogen testing. The area under the receiver operating characteristic curve for procalcitonin in discriminating bacterial pathogens from viral pathogens was 0.73 (95% CI, 0.69-0.77). A procalcitonin cut-off of 0.1 ng/mL resulted in 80.9% (95% CI, 75.3%-85.7%) sensitivity and 51.6% (95% CI, 46.6%-56.5%) specificity for identification of any bacterial pathogen.
In a secondary analysis of this study, we calculated multilevel likelihood ratios (LRs) for ranges of procalcitonin values to determine the diagnostic accuracy of procalcitonin in distinguishing bacterial from viral etiologies of CAP (Table). Multilevel LRs offer more useful diagnostic information than dichotomizing at specified cut-points.5 A procalcitonin result less than 0.1 ng/mL has a negative LR of 0.4 (95% CI, 0.3-0.5), which is not low enough to rule out bacterial CAP effectively when starting with intermediate or high pretest probability. For a low result (<0.1 ng/mL) to be useful in ruling out bacterial CAP, for example having less than a 10% posttest probability of bacterial CAP, the pretest probability would have to be no greater than 22%. Even then, a 10% posttest probability of bacterial CAP may still be too high for clinicians to withhold initial antibiotics. For procalcitonin values between 0.1 ng/mL and 1.0 ng/mL, the probability of bacterial CAP does not change significantly, with an LR of 1.0 (95% CI, 0.8-1.3). Procalcitonin values up to 5 ng/mL reach a modest positive LR of 2.3 (95% CI, 0.8-4.3). Very high values, such as those >10 ng/mL, yield a positive LR of 5.5 (95% CI, 3.2-9.7), are potentially useful in decisions to initiate antibiotics in situations of very low pretest probability of bacterial CAP. For example, a 9% pretest probability of bacterial CAP is likely below many physicians’ threshold for starting antibiotics. A procalcitonin of 12 ng/mL in this patient would increase the posttest probability to 35%, a value that would prompt many physicians to initiate antibiotics.
Overall, there is insufficient evidence to support the use of procalcitonin as a stand-alone test for ruling out bacterial CAP, limiting its use in withholding antibiotics in patients with suspected bacterial CAP.
DISCONTINUATION OF ANTIBIOTICS
While initial procalcitonin measurements may not affect the initial antibiotic treatment decision, procalcitonin levels thereafter can guide the duration of therapy. A meta-analysis of procalcitonin-guided treatment in patients with upper or lower respiratory tract infection (LRTI) showed that procalcitonin guidance reduces antibiotic exposure and antibiotic-related adverse effects and improves survival, albeit a small absolute mortality difference of 1.4 percentage points, primarily observed in the intensive care unit setting.6 Most patients included in this meta-analysis were diagnosed with LRTI (91%), and CAP was the predominant subtype of LRTI (43%). The main effect of procalcitonin guidance for patients with CAP was earlier discontinuation of antibiotic treatment. Procalcitonin-guided algorithms in these trials discouraged, or strongly discouraged, antibiotics if procalcitonin was <0.25 ng/mL or <0.1 ng/mL, respectively. In addition, serial procalcitonin measurements were used to guide discontinuation of antibiotics if procalcitonin dropped below 0.25 ng/mL, or by 80% to 90% from the peak value. This approach safely shortened the duration of therapy in patients with CAP.
There are several limitations in the interpretation and generalizability of this meta-analysis. There is large heterogeneity across the included clinical trials in design, procalcitonin protocols, clinical setting, and respiratory infection type, including bronchitis, acute exacerbation of chronic obstructive pulmonary disease (AECOPD), and CAP. Results were consistent only in one moderate- to high-quality randomized trial specifically studying CAP in the inpatient setting.7 Additionally, most of these trials were conducted in Europe. Antibiotic prescribing practices may be different in the US, and prescribing practices on both continents may have changed over the years with greater awareness and appreciation of antibiotic stewardship.
PROCALCITONIN-GUIDED ALGORITHMS
The ProACT trial, the largest randomized, US multicenter trial to evaluate a procalcitonin-based algorithm to assist with antibiotic decision making, included over 1,600 emergency department patients at 14 academic medical centers.8 Procalcitonin guidance in this trial did not reduce antibiotic exposure compared with usual care for patients with suspected LRTI. However, its applicability to the practice of hospitalists and the inpatient setting is limited. First, only 48% of the study participants required hospitalization. Second, this study included all LRTIs, with CAP comprising just 20% of all final diagnoses. Third, the average number of antibiotic days during hospitalization for CAP was short in both groups (3.9 days in the procalcitonin group and 4.1 days in the usual care group). This relatively short antibiotic duration makes it difficult for any intervention to decrease antibiotic days meaningfully.
In a prepost controlled intervention study for inpatients at a single US tertiary care hospital, procalcitonin guidance in hospitalized patients safely reduced antibiotic use in LRTI, specifically for the discontinuation of antibiotics.9 The greatest benefit of procalcitonin guidance in antibiotic discontinuation was found in patients with AECOPD and patients with an admitting diagnosis of CAP, but with mild illness and a low procalcitonin. Although this prepost study suggested a safe reduction of antibiotic use due to implementation of procalcitonin guidance, the lack of randomization and the absence of a contemporaneous control group are important limitations. Given the mixed findings on the effectiveness of procalcitonin guidance for hospitalized CAP patients in the US, further investigation will be needed with large clinical trials in the inpatient setting for CAP.
CONCLUSIONS
There is insufficient evidence to support the use of serum procalcitonin to withhold initial antibiotics in patients with a clinical syndrome consistent with bacterial CAP. However, the literature supports the use of procalcitonin for the early discontinuation of antibiotics for cases in which the probability of bacterial CAP is low, and procalcitonin remains below 0.1 ng/mL (Figure).
Serial measurements of procalcitonin every one to two days may also be used when clinical uncertainty remains regarding the need for antibiotics. Very low or significantly decreasing procalcitonin levels in patients with CAP and no identified bacterial pathogen likely indicate the infection was not bacterial or was bacterial, but has now been adequately treated with antibiotics. For cases of proven bacterial etiology or high clinical suspicion of bacterial CAP, there is insufficient evidence to recommend the early discontinuation of antibiotics based on procalcitonin levels short of the recommended five-day course according to current guidelines.10 Future clinical trials are needed to determine if procalcitonin guidance can safely decrease the duration of antibiotic therapy for confirmed bacterial CAP to less than five days.
There are discrepancies between the apparent test characteristics of procalcitonin and the recommended antibiotic decisions in many procalcitonin algorithms. For example, algorithms discourage antibiotics when procalcitonin values are 0.1-0.24 ng/mL, and encourage (or even strongly encourage) antibiotic use for higher procalcitonin values of 0.25-1.0 ng/mL. However, the LRs for these ranges are identical and are approximately 1.0 (Table), suggesting that decision-making should be similar across the entire procalcitonin range of 0.1 to 1.0. Future clinical trials should study revised algorithms with different cut-points, including the thresholds found in our secondary analysis of multilevel LRs. Until then, we believe there is insufficient evidence to deviate from current antibiotic decision recommendations at the traditional cut-points.
While procalcitonin is an imperfect biomarker for discriminating bacterial and nonbacterial etiologies of CAP, it may still provide helpful information for the hospitalist in antibiotic decision-making in the same way we apply other commonly used clinical variables such as fever, white blood cell count, band count, and the pattern of infiltrate in chest imaging.
Procalcitonin should be interpreted cautiously in certain populations in which it has not been extensively studied (eg, immunocompromised) or in noninfectious conditions that may elevate procalcitonin, such as major physiologic stress (eg, surgery, trauma, burns) and end-stage renal disease.12-14 Further investigation is needed to determine the efficacy and safety of procalcitonin-guided antibiotic therapy in these populations.
RECOMMENDATIONS
- Based on currently available data, a low procalcitonin value should not be used as a stand-alone test to withhold antibiotics in a patient with CAP.
- Serum procalcitonin measurements may help guide the early discontinuation of antibiotics for patients who the treating clinician judges the risks of bacterial etiology and clinical deterioration to be low.
- Interpret procalcitonin cautiously in immunocompromised patients, undergoing severe physiologic stress, or have underlying end-stage renal disease.
- Serum procalcitonin serves as an adjunct to, rather than a substitute for, clinical judgment.
Disclosures
Dr Choi, Dr Evans, and Dr Glesby have nothing to disclose. Dr Self reports receiving prior research funding from BRAHMS/Thermo-Fisher and BioMerieux for studies on procalcitonin. Dr Self reports personal fees from Inflammatix, grants from Axis Shield, Rapid Pathogen Screening, and BioMerieux, all outside the submitted work. Dr McCarthy reports receiving research funding from Allergan outside the submitted work. Dr Simon reports receiving consulting fees from Roche Diagnostics.
1. Ramirez JA, Wiemken TL, Peyrani P, et al. adults hospitalized with pneumonia in the united states: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. https://doi.org/10.1093/cid/cix647.
2. Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972-978. https://doi.org/10.1001/archinte.163.8.972.
3. Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. https://doi.org/10.1093/ofid/ofw249.
4. Self WH, Balk RA, Grijalva CG, et al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia. Clin Infect Dis. 2017;65(2):183-190. https://doi.org/10.1093/cid/cix317.
5. Straus SE, Richardson WS, Glasziou P, Haynes RB. Evidence-Based Medicine: How to Practice and Teach It (4th Edition). Fourth Edition ed. London, England: Elsevier Churchill Livingstone; 2010.
6. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498. https://doi.org/10.1164/rccm.200512-1922OC.
7. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93. https://doi.org/10.1056/NEJMoa1802670.
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. https://doi.org/10.1056/NEJMoa1802670
10. Townsend J, Adams V, Galiatsatos P, et al. Procalcitonin-guided antibiotic therapy reduces antibiotic use for lower respiratory tract infections in a United States medical center: results of a clinical trial. Open Forum Infect Dis. 2018;5(12):ofy327. https://doi.org/10.1093/ofid/ofy327.
11. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72. https://doi.org/10.1086/511159.
12. Seoane L, Pértega S, Galeiras R, Astola I, Bouza T. Procalcitonin in the burn unit and the diagnosis of infection. Burns. 2014;40(2):223-229. https://doi.org/10.1016/j.burns.2013.11.018.
13. Dahaba AA, Rehak PH, List WF. Procalcitonin and C-reactive protein plasma concentrations in nonseptic uremic patients undergoing hemodialysis. Intensive Care Med. 2003;29(4):579-583. https://doi.org/10.1007/s00134-003-1664-8.
14. Ghabra H, White W, Townsend M, Boysen P, Nossaman B. Use of biomarkers in the prediction of culture-proven infection in the surgical intensive care unit. J Crit Care. 2019;49:149-154. https://doi.org/10.1016/j.jcrc.2018.10.023.
15. Hoshino K, Irie Y, Mizunuma M, Kawano K, Kitamura T, Ishikura H. Incidence of elevated procalcitonin and presepsin levels after severe trauma: a pilot cohort study. Anaesth Intensive Care. 2017;45(5):600-604. https://doi.org/10.1177/0310057X1704500510.
1. Ramirez JA, Wiemken TL, Peyrani P, et al. adults hospitalized with pneumonia in the united states: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. https://doi.org/10.1093/cid/cix647.
2. Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972-978. https://doi.org/10.1001/archinte.163.8.972.
3. Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. https://doi.org/10.1093/ofid/ofw249.
4. Self WH, Balk RA, Grijalva CG, et al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia. Clin Infect Dis. 2017;65(2):183-190. https://doi.org/10.1093/cid/cix317.
5. Straus SE, Richardson WS, Glasziou P, Haynes RB. Evidence-Based Medicine: How to Practice and Teach It (4th Edition). Fourth Edition ed. London, England: Elsevier Churchill Livingstone; 2010.
6. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498. https://doi.org/10.1164/rccm.200512-1922OC.
7. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93. https://doi.org/10.1056/NEJMoa1802670.
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. https://doi.org/10.1056/NEJMoa1802670
10. Townsend J, Adams V, Galiatsatos P, et al. Procalcitonin-guided antibiotic therapy reduces antibiotic use for lower respiratory tract infections in a United States medical center: results of a clinical trial. Open Forum Infect Dis. 2018;5(12):ofy327. https://doi.org/10.1093/ofid/ofy327.
11. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72. https://doi.org/10.1086/511159.
12. Seoane L, Pértega S, Galeiras R, Astola I, Bouza T. Procalcitonin in the burn unit and the diagnosis of infection. Burns. 2014;40(2):223-229. https://doi.org/10.1016/j.burns.2013.11.018.
13. Dahaba AA, Rehak PH, List WF. Procalcitonin and C-reactive protein plasma concentrations in nonseptic uremic patients undergoing hemodialysis. Intensive Care Med. 2003;29(4):579-583. https://doi.org/10.1007/s00134-003-1664-8.
14. Ghabra H, White W, Townsend M, Boysen P, Nossaman B. Use of biomarkers in the prediction of culture-proven infection in the surgical intensive care unit. J Crit Care. 2019;49:149-154. https://doi.org/10.1016/j.jcrc.2018.10.023.
15. Hoshino K, Irie Y, Mizunuma M, Kawano K, Kitamura T, Ishikura H. Incidence of elevated procalcitonin and presepsin levels after severe trauma: a pilot cohort study. Anaesth Intensive Care. 2017;45(5):600-604. https://doi.org/10.1177/0310057X1704500510.
© 2019 Society of Hospital Medicine