User login
Worsening dyspnea
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
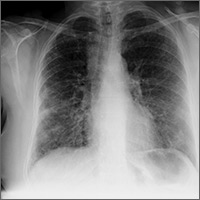
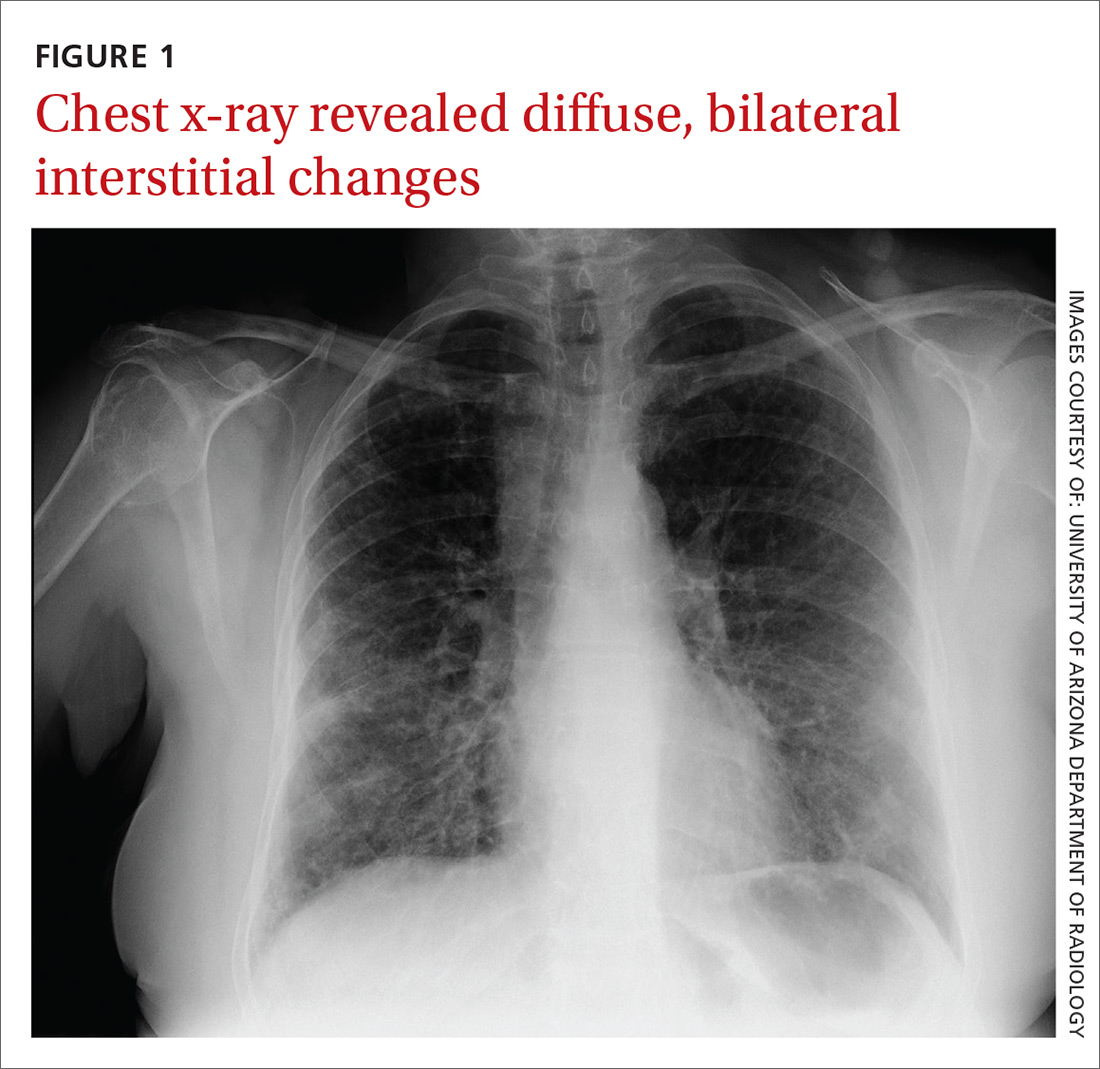
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
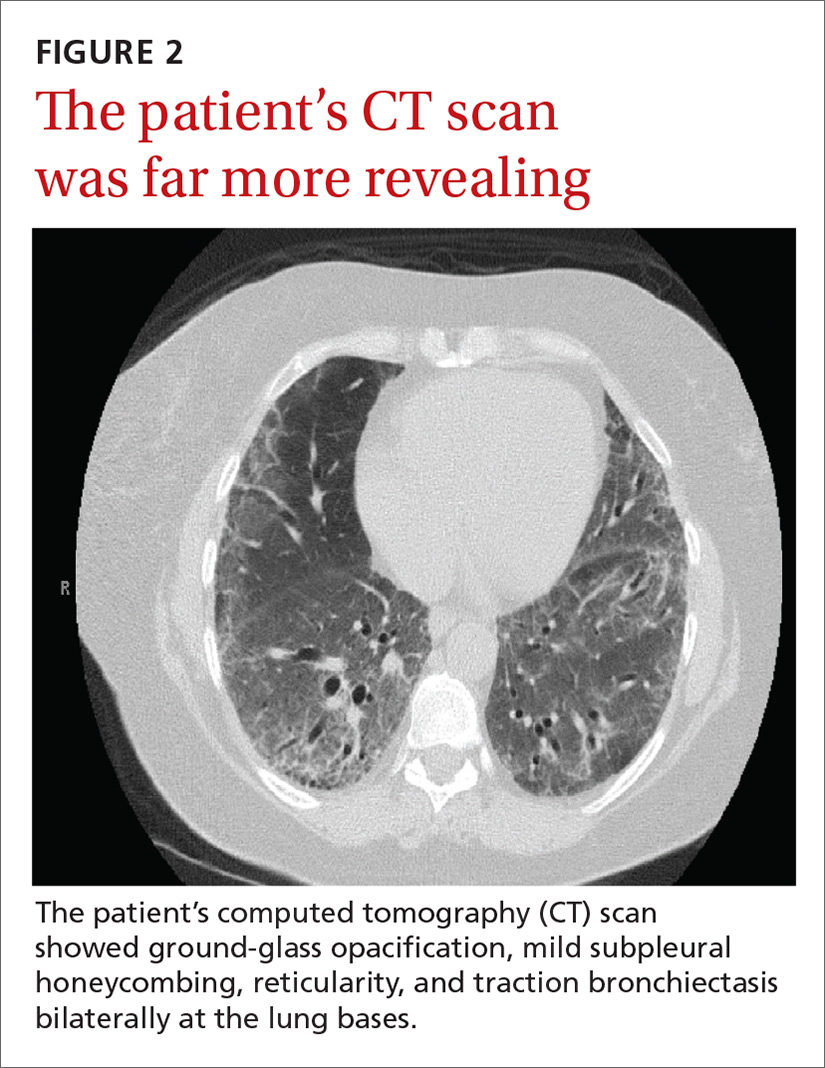
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.
ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
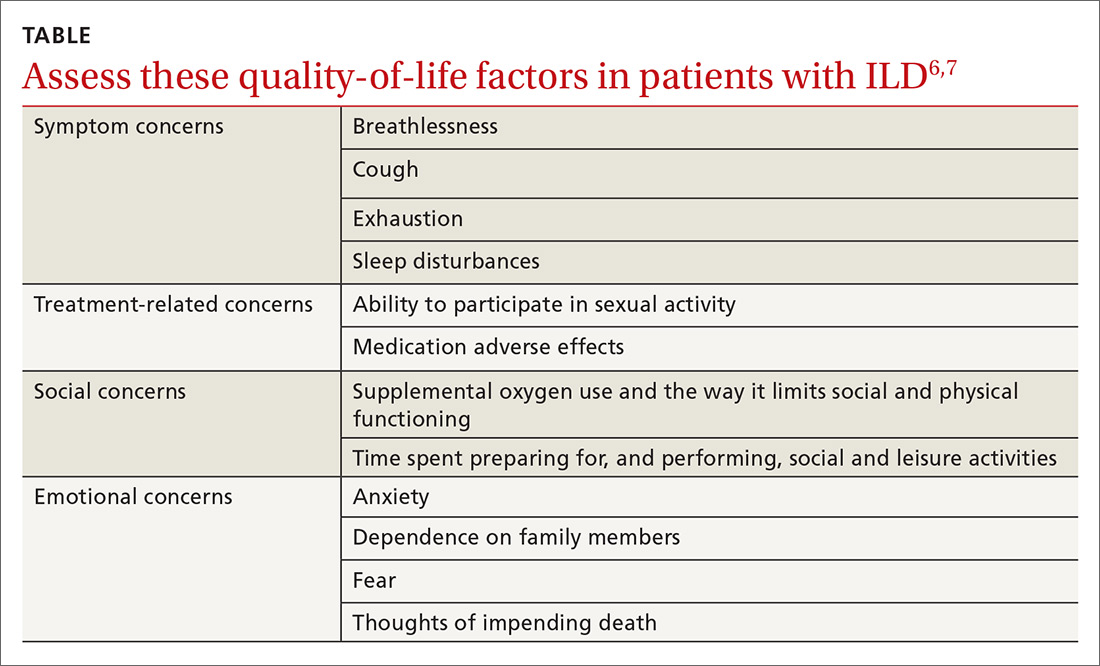
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9
The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; [email protected].
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.

ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9

The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; [email protected].
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.

ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9

The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; [email protected].
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.