User login
Is an SGLT2 inhibitor right for your patient with type 2 diabetes?
› Consider sodium-glucose cotransporter 2 (SGLT2) inhibitors as second-line agents in patients with type 2 diabetes mellitus who need mild hemoglobin A1c reductions (≤1%) and who would benefit from mild to modest weight and blood pressure reductions. A
› Avoid using SGLT2 inhibitors in patients with a history of recurrent genital mycotic or urinary tract infections. B
› Use SGLT2 inhibitors with caution in patients at risk for volume-related adverse effects (dizziness and hypotension), such as the elderly, those with moderate renal dysfunction, and those taking concomitant diuretic therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Joe S is a 41-year-old African-American man who comes to your clinic after his employee health screening revealed elevated triglycerides. The patient has a 3-year history of type 2 diabetes mellitus (T2DM); he also has a history of hypertension, gastroesophageal reflux disease, and obstructive sleep apnea. Mr. S tells you he takes metformin 1000 mg twice daily, but stopped taking his glipizide because he didn’t think it was helping his blood sugar. His last hemoglobin (Hb) A1c result was 8.8%, and he is very resistant to starting insulin therapy.
The patient’s other medications include enalapril 10 mg/d, atorvastatin 10 mg/d, and omeprazole 20 mg/d. Mr. S weighs 255.6 lbs (body mass index=34.7), his BP is 140/88 mm Hg, and his heart rate is 82 beats per minute. Laboratory values include: serum creatinine, 1.01 mg/dL; estimated glomerular filtration rate (eGFR) >100 mL/min/1.73 m2; potassium (K), 4.3 mmol/L; serum phosphorous (Phos), 2.8 mg/dL; magnesium (Mg), 1.9 mg/dL; total cholesterol, 167 mg/dL; low-density lipoprotein (LDL), 78 mg/dL; high-density lipoprotein (HDL), 38 mg/dL; and triglycerides, 256 mg/dL.
CASE 2 › Susan R, a 68-year-old Caucasian woman, returns to your clinic for a follow-up visit 3 months after you prescribed dapagliflozin 10 mg/d for her T2DM. Her glucose levels have improved, but she complains of vaginal pruritus and is worried that she has a yeast infection.
You diagnose vulvovaginal candidiasis in this patient and prescribe a single dose of fluconazole 150 mg. After reviewing her laboratory test results, you notice that since starting the dapagliflozin, her HbA1c level has improved slightly from 9.8% to 9.3%, but is still not where it needs to be. Her eGFR is 49 mL/min/1.73 m2.
What would you recommend to improve control of these patients’ blood glucose levels?
When to consider an SGLT2 inhibitor
Consider therapy with SGLT2 inhibitors in adult patients with T2DM who:3-9,13-15,17-24
- have an HbA1c between 7% and 9%
- would benefit from weight and/or blood pressure reductions
- have metabolic syndrome
- have adequate means to pay for the medication (ie, prescription coverage or the ability to afford it).
In addition, consider an SGLT2 inhibitor as initial monotherapy if metformin is contraindicated or not tolerated, or as add-on therapy to metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase IV inhibitors, or insulin.
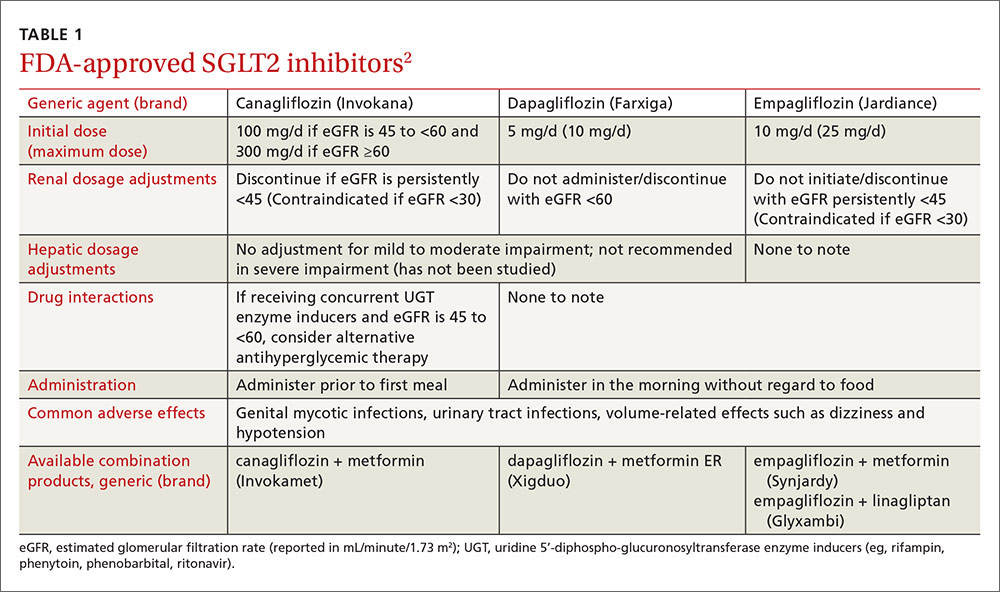
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are the newest class of agents to enter the T2DM management arena. They act in the proximal renal tubules to decrease the reabsorption of glucose by targeting the SGLT2 transmembrane protein, which reabsorbs about 90% of the body’s glucose.1,2 The class is currently made up of 3 agents—canagliflozin, dapagliflozin, and empagliflozin—all of which are approved by the US Food and Drug Administration (FDA) for the treatment of T2DM (TABLE 1).2
The American Diabetes Association and the European Association for the Study of Diabetes published updated guidelines for T2DM management in 2015.1 In addition to lifestyle modifications, the guidelines recommend the use of metformin as first-line therapy unless it is contraindicated or patients are unable to tolerate it (eg, because of gastrointestinal adverse effects). They recommend other pharmacologic therapies as second-line options based on specific patient characteristics. Thus, SGLT2 inhibitors may be used as add-on therapy after metformin, or as a first-line option if metformin is contraindicated or not tolerated. Because the mechanism of action of SGLT2 inhibitors is independent of insulin secretion, these agents may be used at any stage of the diabetes continuum.
SGLT2 agents as monotherapy, or as add-on therapy
All SGLT2 agents have been studied as monotherapy accompanied by diet and exercise and shown to produce HbA1c reductions of 0.34% to 1.11%.3-6 In trials, the effect was similar regardless of study duration (18-104 weeks); generally, higher doses corresponded with larger HbA1c reductions.3-6
SGLT2 inhibitors have also been studied as add-on therapy to several oral agents including metformin, sulfonylureas, thiazolidinediones (TZDs), and the combination of metformin plus sulfonylureas or TZDs or dipeptidyl peptidase IV (DPP-IV) inhibitors.1 When used in any of these combinations, each SGLT2 agent demonstrated a consistent HbA1c lowering effect of 0.62% to 1.19%.7-14
Additionally, SGLT2 inhibitors have been studied in combination with insulin therapy (median or mean daily doses >60 units), which yielded further reductions in HbA1c of 0.58% to 1.02% without significant insulin adjustments or an increase in major hypoglycemia events.15-17 Patients receiving insulin and an SGLT2 inhibitor had lower insulin doses and more weight loss compared to placebo groups.
SGLT2 inhibitors offer additional benefits
Secondary analyses of most studies of SGLT2 inhibitors include changes in BP and weight from baseline as well as minor changes (some positive, some not) in several lipid parameters.3-5,7-9,13-15,17-24 In general, these effects do not appear to be dose-dependent (with the exception of canagliflozin and its associated lipid effects25) and are similar among the 3 medications.3-5,7-9,13-15,17-24 (For more on who would benefit from these agents, see “When to consider an SGLT2 inhibitor” above.)
BP reduction. Although the mean baseline BP was controlled in most studies, SGLT2 inhibitors have been shown to significantly reduce BP. Reductions in BP with all 3 SGLT2 medications range from approximately 2 to 5 mm Hg systolic and 0.5 to 2.5 mm Hg diastolic, which may be due to weight loss and diuresis.4-8,10-16,20-23 While the reductions were modest at best, one study involving empagliflozin reported that more than one-quarter of patients with uncontrolled BP at baseline achieved a BP <130/80 mm Hg 24 weeks later.5 While these agents should not be used solely for their BP lowering effects, they may help a small number of patients with mildly elevated BP achieve their goal without an additional antihypertensive agent.
Weight reduction. Modest weight loss, likely due to the loss of calories through urine, was seen with SGLT2 inhibitors in most studies, with reductions persisting beyond one year of use. In most studies, including those involving obese patients on insulin therapy,15,17,21 patients’ body weights were reduced by approximately 2 to 4 kg from baseline.3-16,18,21-23,26
Lipid effects. Although the mechanism is unclear, use of SGLT2 inhibitors can have varying effects on lipid panels. In most studies, total and LDL cholesterol levels were increased with elevations ranging from 0.7 to 10 mg/dL.3,7,8,18,19,22,23 Conversely, at least one study demonstrated mild reductions in total and LDL cholesterol levels with higher doses of empagliflozin.13 Additionally, modest reductions in triglycerides and increases in HDL across all doses of canagliflozin, dapagliflozin, and empagliflozin have been seen.8,9,13,15,19 While the clinical relevance of these lipid changes is unknown, monitoring is recommended.2
These agents are well tolerated
SGLT2 inhibitors were generally well tolerated in studies. The most common adverse effects include mycotic infections (2.4%-21.6%) and urinary tract infections (UTIs) (4.0%-19.6%) (both with higher incidences in females); volume-related effects such as dizziness and hypotension (0.3%-8.3%); and nasopharyngitis (5.4%-18.3%).4-14,16-23,26-28 Hypoglycemia was observed more often when an SGLT2 inhibitor was used in combination with a sulfonylurea or insulin therapy.4-14,16-23,26-28 The number of times adverse events led to discontinuation was low and similar to that in control groups.4-14,16-23,26-28
Mycotic and urinary infections should be diagnosed and treated according to current standards of care and do not require discontinuation of the SGLT2 inhibitor. Canagli-flozin therapy was associated with electrolyte abnormalities including hyperkalemia, hypermagnesemia, and hyperphosphatemia.25 Thus, levels should be monitored periodically, especially in patients predisposed to elevations due to other conditions or medications.25
Two additional warnings are worth noting
Diabetic ketoacidosis (DKA) has been reported with all 3 agents, and bone fractures have been reported with canagliflozin.
The FDA issued a warning in May 2015 regarding the increased risk of DKA with the use of SGLT2 inhibitor single and combination products.29 This warning was prompted by several case reports of DKA with uncharacteristically mild to moderate glucose elevations in patients with type 1 diabetes mellitus (T1DM) and T2DM who were taking an SGLT2 inhibitor. The absence of significant hyperglycemia delayed diagnosis in many cases. Therefore, patients should be counseled on the signs and symptoms of DKA, as well as when to seek medical attention.
Patients with diabetes and symptoms of ketoacidosis (eg, difficulty breathing, nausea, vomiting, abdominal pain, confusion, and fatigue) should be evaluated regardless of current blood glucose levels, and SGLT2 inhibitors should be discontinued if acidosis is confirmed. Identified potential triggers include illness, reduced food and fluid intake, reduced insulin dose, and history of alcohol intake. Use of SGLT2 inhibitors should be avoided in patients with T1DM until safety and efficacy are established in large randomized controlled trials. The European Medicines Agency announced that a thorough review of all currently approved SGLT2 agents is underway to evaluate the risk for DKA.30
In addition, the FDA called for a revision of the label of canagliflozin to reflect a strengthened warning about an increased risk of bone fractures and decreased bone mineral density (BMD).31 Fractures can occur as early as 12 weeks after initiating treatment and with only minor trauma.31
Over a 2-year period, canagliflozin also significantly decreased BMD in the hip and lower spine compared to placebo.31 Patients should be evaluated for additional risk factors for fracture before taking canagliflozin.31 The FDA is continuing to evaluate whether the other approved SGLT2 inhibitors are associated with an increased risk for fractures.
Drug interactions: Proceed carefully with diuretics
The number of drugs that interact with SGLT2 inhibitors is minimal. Because these agents can cause volume-related effects such as hypotension, dizziness, and osmotic diuresis, patients—particularly the elderly and those with renal impairment—taking concomitant diuretics, especially loop diuretics, may be at increased risk for these effects and should be monitored accordingly.2,25
Canagliflozin is primarily metabolized via glucuronidation by the uridine 5'-diphospho-glucuronosyltransferase (UGT) enzymes. Therefore, UGT enzyme inducers (eg, rifampin, phenytoin, phenobarbital, ritonavir) decrease canagliflozin’s serum concentration. If a patient has an eGFR >60 mL/min/1.73 m2 and is tolerating a dose of 100 mg/d, consider increasing the dose to 300 mg/d during concomitant treatment.
In addition, researchers have found that canagliflozin increases serum levels of digoxin by between 20% and 36%.25 Experts suspect this occurs because canagliflozin inhibits P-glycoprotein efflux of digoxin. Although monitoring of digoxin levels is recommended, this interaction is considered to be minor.25
Cost consideration: SGLT2 inhibitors are more expensive
The SGLT2 inhibitors are available only as brand name products and are more expensive than agents that have generic options (eg, metformin, sulfonylureas, TZDs). The average wholesale cost is approximately $400 for a 30-day supply of all SGLT2 agents.32 When considering an SGLT2 inhibitor, the patient should ideally have medication prescription coverage. Depending on the specific insurance plan, these agents are classified as tier 2 to 4, which is comparable to other oral brand name options.
Research looks at CV outcomes and cancer risk
Cardiovascular (CV) risk reduction. To date, only one study evaluating the effect of SGLT2 inhibitors on CV outcomes is complete.33 Two large randomized controlled trials involving canagliflozin and dapagliflozin designed to evaluate treatment effects on major CV endpoints are ongoing.34,35
In the EMPA-REG OUTCOME trial,33 researchers found that empagliflozin had beneficial effects on CV outcomes, making it one of the only antidiabetic agents on the market to have such benefits. The study, which involved more than 7000 patients with a history of T2DM and existing cardiovascular disease (CVD), found that 10.5% of patients in the empagliflozin group vs 12.1% in the placebo group died from a CV cause or experienced a nonfatal myocardial infarction or stroke over a median of 3.1 years. Results were similar with both doses (10 mg vs 25 mg) of empagliflozin. The mechanisms behind the CV benefits are likely multifactorial and may be related to reductions in weight and BP,33 but additional research is needed to fully elucidate the role of empagliflozin in this population.
Canagliflozin is being evaluated in the Canagliflozin Cardiovascular Assessment Study (CANVAS) for its effect on major CV events—CV death, nonfatal myocardial infarction, and nonfatal stroke—in patients with either a history of CVD or who are at increased risk of CVD and have uncontrolled diabetes.34 The trial is expected to wrap up in June 2017.
And dapagliflozin is being studied in the DECLARE-TIMI 58 trial (the Effect of Dapagliflozin on the Incidence of Cardiovascular Events) in patients with T2DM and either known CVD or at least 2 risk factors for CVD.35 The study is designed to assess dapagliflozin’s effect on the incidence of CV death, myocardial infarction, and ischemic stroke and has an estimated completion date of April 2019, which will provide a median follow-up of 4.5 years.
Cancer. All 3 agents have been examined for any possible carcinogenic links. In 2011, the FDA issued a request for further investigation surrounding the risk of cancer associated with dapagliflozin.36 As of November 2013, 10 of 6045 patients treated with dapagliflozin developed bladder cancer compared to 1 of 3512 controls.36 Furthermore, 9 of 2223 patients treated with dapagliflozin developed breast cancer compared to 1 of 1053 controls.36
Although the trials were not designed to detect an increase in risk, the number of observed cases warranted further investigation. No official warning for breast cancer exists since the characteristics of the malignancies led the FDA to believe dapagliflozin was unlikely the cause.36
Given what we know to date, it appears to be prudent to avoid prescribing SGLT2 inhibitors in patients with active bladder cancer, and to use them with caution in those with a history of the disease.2
Other studies. Initially, animal studies suggested an increased risk of various malignancies associated with canagliflozin use in rats,37 but consistent results were not seen in human studies. Similarly, at least one study found that empagliflozin was associated with lung cancer and melanoma, but closer examination found that most patients who developed these cancers had risk factors.38 Large, long-term studies of these agents in various populations are needed to thoroughly investigate possible carcinogenicity.
Additional considerations: Kidney function, age, and pregnancy
Consider avoiding SGLT2 inhibitors in patients with moderate kidney dysfunction (eGFR 30-59 mL/min/1.73 m2). Studies have shown that SGLT2 inhibitors are not as effective at lowering blood glucose in those with reduced eGFR, although adverse events were similar to those in placebo groups.24,39,40 Dapagliflozin is not recommended in patients with an eGFR <60 mL/min/1.73 m2 due to lack of efficacy.2,24 Empagliflozin does not require dose adjustments if eGFR is ≥45 mL/min/1.73 m2. A lower dose of canagliflozin (ie, 100 mg/d) is recommended in those with an eGFR of 45 to 59 mL/min/1.73 m2.2 All agents are contraindicated in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2).
Older patients are at higher risk for dehydration, hypotension, and falls; therefore, SGLT2 inhibitors should be used with caution in this population. Similarly, they should not be used in patients with T1DM and should be avoided in those with active, or a history of, DKA.
There are no data on the use of SGLT2 inhibitors in pregnancy; thus, these agents should be avoided unless the potential benefits outweigh the potential risks to the unborn fetus.2
CASE 1 › An SGLT2 inhibitor is an acceptable option for Mr. S. Because he is resistant to starting insulin therapy and his HbA1c is <9%, an additional oral medication is reasonable. Adding an SGLT2 inhibitor may reduce his HbA1c up to ~1%, and education on lifestyle modifications may help bring him to goal. An SGLT2 inhibitor may also benefit his BP and weight, both of which could be improved.
Given the drugs he’s taking, drug interactions should not be an issue, and his renal function and pertinent labs (K, Phos, Mg) are within normal limits. Nevertheless, monitor these labs periodically and monitor Mr. S for adverse effects, such as UTIs, although these are more common in women. Canagliflozin is the preferred SGLT2 inhibitor on his insurance formulary, so you could initiate therapy at 100 mg/d, administered prior to the first meal, and increase to 300 mg/d if needed. As an alternative, consider prescribing the metformin/canagliflozin combination agent.
CASE 2 › Ms. R is likely experiencing a yeast infection as an adverse effect of the dapagliflozin. Although one yeast infection is insufficient grounds for discontinuation of the drug, recurrent infections should prompt a risk-to-benefit analysis to determine whether it’s worth continuing the medication. Her recent eGFR (<60 mL/min/1.73 m2) is, however, a contraindication to dapagliflozin, and therapy should be discontinued. Canagliflozin and empagliflozin may be considered since her eGFR is >45 mL/min/1.73 m2, but given her current HbA1c and recent adverse drug event, alternative therapies, such as basal insulin, are more appropriate treatment choices.
CORRESPONDENCE
Katelin M. Lisenby, PharmD, BCPS, University of Alabama College of Community Health Sciences, University Medical Center, Box 870374, Tuscaloosa, AL 35487; [email protected].
1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2015;38:140-149.
2. Canagliflozin, dapagliflozin, empagliflozin. Lexicomp, Inc. (Lexi-Drugs®). Accessed October 12, 2015.
3. Stenlöf K, Cefalu WT, Kim KA, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163-175.
4. Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010:33:2217-2224.
5. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208-219.
6. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015-4021.
7. Wilding JPH, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267-1282.
8. Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467-477.
9. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508-2515.
10. Bristol-Myers Squibb [press release]. New phase III data showed dapagliflozin significantly reduced HbA1c compared to placebo at 24 weeks in patients with type 2 diabetes inadequately controlled with the combination of metformin plus sulfonylurea. Available at: http://news.bms.com/press-release/rd-news/new-phase-iii-data-showed-dapagliflozin-significantly-reduced-hba1c-compared-p&t=635156160653787526. Accessed September 17, 2015.
11. Jabbour SA, Hardy E, Sugg J, et al. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24- week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37:740-750.
12. DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384-393.
13. Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther. 2015;37:1773-1788.
14. Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396-3404.
15. Neal B, Percovik V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium–glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403-411.
16. Wilding JPH, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405-415.
17. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815-1823.
18. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941-950.
19. Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010:375:2223-2233.
20. Bailey CJ, Gross JL, Hennicken D, et al. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43.
21. Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473-1478.
22. Merker L, Häring HU, Christiansen AV, et al. Empagliflozin as add-on to metformin in people with type 2 diabetes. Diabet Med. 2015;32:1555-1567.
23. Ridderstråle M, Anderson KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691-700.
24. Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962-971.
25. Invokana (canagliflozin) tablets [product information]. Titusville, NJ: Janssen Pharmaceuticals Inc. Available at: https://www.invokana.com. Accessed March 15, 2013.
26. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582-2592.
27. Strojek K, Yoon KH, Hruba V, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928-938.
28. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38:355-364.
29. US Food and Drug Administration. FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed July 11, 2016.
30. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638-1642.
31. US Food and Drug Administration. FDA drug safety communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm. Acces-sed July 11, 2016.
32. Canagliflozin, dapagliflozin, empagliflozin. In: RED BOOK [AUHSOP intranet database]. Greenwood Village, CO: Truven Health Analytics; [updated daily]. Available at: http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/BB1644/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/FAF693/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/redbook.ShowProductSearchResults?SearchTerm=JARDIANCE&searchType=redbookProductName&searchTermId=42798&searchContent=%24searchContent&searchFilterAD=filterADActive&searchFilterRepackager=filterExcludeRepackager&searchPattern=%5Ejard. Accessed March 15, 2016.
33. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
34. CANagliflozin cardioVascular Assessment Study (CANVAS). Available at: http://clinicaltrials.gov/show/NCT01032629. Accessed October 12, 2015.
35. Multicenter trial to evaluate the effect of dapagliflozin on the incidence of cardiovascular events (DECLARE-TIMI 58). Available at: http://clinicaltrials.gov/show/NCT01730534. Accessed October 12, 2015.
36. FDA background document. BMS-512148 NDA 202293. In: Proceedings of the US Food and Drug Administration Endocrinologic & Metabolic Drug Advisory Committee Meeting, 2013. Available at: http://www.fda.gov/downloads/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm378079.pdf. Accessed October 12, 2015.
37. Lin HW, Tseng CH. A review of the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol. 2014;2014:719578.
38. Center for Drug Evaluation and Research. Risk assessment and risk mitigation review(s). July 28, 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/ 204629Orig1s000RiskR.pdf. Accessed September 21, 2015.
39. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016-1027.
40. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2: 369-384.
› Consider sodium-glucose cotransporter 2 (SGLT2) inhibitors as second-line agents in patients with type 2 diabetes mellitus who need mild hemoglobin A1c reductions (≤1%) and who would benefit from mild to modest weight and blood pressure reductions. A
› Avoid using SGLT2 inhibitors in patients with a history of recurrent genital mycotic or urinary tract infections. B
› Use SGLT2 inhibitors with caution in patients at risk for volume-related adverse effects (dizziness and hypotension), such as the elderly, those with moderate renal dysfunction, and those taking concomitant diuretic therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Joe S is a 41-year-old African-American man who comes to your clinic after his employee health screening revealed elevated triglycerides. The patient has a 3-year history of type 2 diabetes mellitus (T2DM); he also has a history of hypertension, gastroesophageal reflux disease, and obstructive sleep apnea. Mr. S tells you he takes metformin 1000 mg twice daily, but stopped taking his glipizide because he didn’t think it was helping his blood sugar. His last hemoglobin (Hb) A1c result was 8.8%, and he is very resistant to starting insulin therapy.
The patient’s other medications include enalapril 10 mg/d, atorvastatin 10 mg/d, and omeprazole 20 mg/d. Mr. S weighs 255.6 lbs (body mass index=34.7), his BP is 140/88 mm Hg, and his heart rate is 82 beats per minute. Laboratory values include: serum creatinine, 1.01 mg/dL; estimated glomerular filtration rate (eGFR) >100 mL/min/1.73 m2; potassium (K), 4.3 mmol/L; serum phosphorous (Phos), 2.8 mg/dL; magnesium (Mg), 1.9 mg/dL; total cholesterol, 167 mg/dL; low-density lipoprotein (LDL), 78 mg/dL; high-density lipoprotein (HDL), 38 mg/dL; and triglycerides, 256 mg/dL.
CASE 2 › Susan R, a 68-year-old Caucasian woman, returns to your clinic for a follow-up visit 3 months after you prescribed dapagliflozin 10 mg/d for her T2DM. Her glucose levels have improved, but she complains of vaginal pruritus and is worried that she has a yeast infection.
You diagnose vulvovaginal candidiasis in this patient and prescribe a single dose of fluconazole 150 mg. After reviewing her laboratory test results, you notice that since starting the dapagliflozin, her HbA1c level has improved slightly from 9.8% to 9.3%, but is still not where it needs to be. Her eGFR is 49 mL/min/1.73 m2.
What would you recommend to improve control of these patients’ blood glucose levels?
When to consider an SGLT2 inhibitor
Consider therapy with SGLT2 inhibitors in adult patients with T2DM who:3-9,13-15,17-24
- have an HbA1c between 7% and 9%
- would benefit from weight and/or blood pressure reductions
- have metabolic syndrome
- have adequate means to pay for the medication (ie, prescription coverage or the ability to afford it).
In addition, consider an SGLT2 inhibitor as initial monotherapy if metformin is contraindicated or not tolerated, or as add-on therapy to metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase IV inhibitors, or insulin.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are the newest class of agents to enter the T2DM management arena. They act in the proximal renal tubules to decrease the reabsorption of glucose by targeting the SGLT2 transmembrane protein, which reabsorbs about 90% of the body’s glucose.1,2 The class is currently made up of 3 agents—canagliflozin, dapagliflozin, and empagliflozin—all of which are approved by the US Food and Drug Administration (FDA) for the treatment of T2DM (TABLE 1).2

The American Diabetes Association and the European Association for the Study of Diabetes published updated guidelines for T2DM management in 2015.1 In addition to lifestyle modifications, the guidelines recommend the use of metformin as first-line therapy unless it is contraindicated or patients are unable to tolerate it (eg, because of gastrointestinal adverse effects). They recommend other pharmacologic therapies as second-line options based on specific patient characteristics. Thus, SGLT2 inhibitors may be used as add-on therapy after metformin, or as a first-line option if metformin is contraindicated or not tolerated. Because the mechanism of action of SGLT2 inhibitors is independent of insulin secretion, these agents may be used at any stage of the diabetes continuum.
SGLT2 agents as monotherapy, or as add-on therapy
All SGLT2 agents have been studied as monotherapy accompanied by diet and exercise and shown to produce HbA1c reductions of 0.34% to 1.11%.3-6 In trials, the effect was similar regardless of study duration (18-104 weeks); generally, higher doses corresponded with larger HbA1c reductions.3-6
SGLT2 inhibitors have also been studied as add-on therapy to several oral agents including metformin, sulfonylureas, thiazolidinediones (TZDs), and the combination of metformin plus sulfonylureas or TZDs or dipeptidyl peptidase IV (DPP-IV) inhibitors.1 When used in any of these combinations, each SGLT2 agent demonstrated a consistent HbA1c lowering effect of 0.62% to 1.19%.7-14
Additionally, SGLT2 inhibitors have been studied in combination with insulin therapy (median or mean daily doses >60 units), which yielded further reductions in HbA1c of 0.58% to 1.02% without significant insulin adjustments or an increase in major hypoglycemia events.15-17 Patients receiving insulin and an SGLT2 inhibitor had lower insulin doses and more weight loss compared to placebo groups.
SGLT2 inhibitors offer additional benefits
Secondary analyses of most studies of SGLT2 inhibitors include changes in BP and weight from baseline as well as minor changes (some positive, some not) in several lipid parameters.3-5,7-9,13-15,17-24 In general, these effects do not appear to be dose-dependent (with the exception of canagliflozin and its associated lipid effects25) and are similar among the 3 medications.3-5,7-9,13-15,17-24 (For more on who would benefit from these agents, see “When to consider an SGLT2 inhibitor” above.)
BP reduction. Although the mean baseline BP was controlled in most studies, SGLT2 inhibitors have been shown to significantly reduce BP. Reductions in BP with all 3 SGLT2 medications range from approximately 2 to 5 mm Hg systolic and 0.5 to 2.5 mm Hg diastolic, which may be due to weight loss and diuresis.4-8,10-16,20-23 While the reductions were modest at best, one study involving empagliflozin reported that more than one-quarter of patients with uncontrolled BP at baseline achieved a BP <130/80 mm Hg 24 weeks later.5 While these agents should not be used solely for their BP lowering effects, they may help a small number of patients with mildly elevated BP achieve their goal without an additional antihypertensive agent.
Weight reduction. Modest weight loss, likely due to the loss of calories through urine, was seen with SGLT2 inhibitors in most studies, with reductions persisting beyond one year of use. In most studies, including those involving obese patients on insulin therapy,15,17,21 patients’ body weights were reduced by approximately 2 to 4 kg from baseline.3-16,18,21-23,26
Lipid effects. Although the mechanism is unclear, use of SGLT2 inhibitors can have varying effects on lipid panels. In most studies, total and LDL cholesterol levels were increased with elevations ranging from 0.7 to 10 mg/dL.3,7,8,18,19,22,23 Conversely, at least one study demonstrated mild reductions in total and LDL cholesterol levels with higher doses of empagliflozin.13 Additionally, modest reductions in triglycerides and increases in HDL across all doses of canagliflozin, dapagliflozin, and empagliflozin have been seen.8,9,13,15,19 While the clinical relevance of these lipid changes is unknown, monitoring is recommended.2
These agents are well tolerated
SGLT2 inhibitors were generally well tolerated in studies. The most common adverse effects include mycotic infections (2.4%-21.6%) and urinary tract infections (UTIs) (4.0%-19.6%) (both with higher incidences in females); volume-related effects such as dizziness and hypotension (0.3%-8.3%); and nasopharyngitis (5.4%-18.3%).4-14,16-23,26-28 Hypoglycemia was observed more often when an SGLT2 inhibitor was used in combination with a sulfonylurea or insulin therapy.4-14,16-23,26-28 The number of times adverse events led to discontinuation was low and similar to that in control groups.4-14,16-23,26-28
Mycotic and urinary infections should be diagnosed and treated according to current standards of care and do not require discontinuation of the SGLT2 inhibitor. Canagli-flozin therapy was associated with electrolyte abnormalities including hyperkalemia, hypermagnesemia, and hyperphosphatemia.25 Thus, levels should be monitored periodically, especially in patients predisposed to elevations due to other conditions or medications.25
Two additional warnings are worth noting
Diabetic ketoacidosis (DKA) has been reported with all 3 agents, and bone fractures have been reported with canagliflozin.
The FDA issued a warning in May 2015 regarding the increased risk of DKA with the use of SGLT2 inhibitor single and combination products.29 This warning was prompted by several case reports of DKA with uncharacteristically mild to moderate glucose elevations in patients with type 1 diabetes mellitus (T1DM) and T2DM who were taking an SGLT2 inhibitor. The absence of significant hyperglycemia delayed diagnosis in many cases. Therefore, patients should be counseled on the signs and symptoms of DKA, as well as when to seek medical attention.
Patients with diabetes and symptoms of ketoacidosis (eg, difficulty breathing, nausea, vomiting, abdominal pain, confusion, and fatigue) should be evaluated regardless of current blood glucose levels, and SGLT2 inhibitors should be discontinued if acidosis is confirmed. Identified potential triggers include illness, reduced food and fluid intake, reduced insulin dose, and history of alcohol intake. Use of SGLT2 inhibitors should be avoided in patients with T1DM until safety and efficacy are established in large randomized controlled trials. The European Medicines Agency announced that a thorough review of all currently approved SGLT2 agents is underway to evaluate the risk for DKA.30
In addition, the FDA called for a revision of the label of canagliflozin to reflect a strengthened warning about an increased risk of bone fractures and decreased bone mineral density (BMD).31 Fractures can occur as early as 12 weeks after initiating treatment and with only minor trauma.31
Over a 2-year period, canagliflozin also significantly decreased BMD in the hip and lower spine compared to placebo.31 Patients should be evaluated for additional risk factors for fracture before taking canagliflozin.31 The FDA is continuing to evaluate whether the other approved SGLT2 inhibitors are associated with an increased risk for fractures.
Drug interactions: Proceed carefully with diuretics
The number of drugs that interact with SGLT2 inhibitors is minimal. Because these agents can cause volume-related effects such as hypotension, dizziness, and osmotic diuresis, patients—particularly the elderly and those with renal impairment—taking concomitant diuretics, especially loop diuretics, may be at increased risk for these effects and should be monitored accordingly.2,25
Canagliflozin is primarily metabolized via glucuronidation by the uridine 5'-diphospho-glucuronosyltransferase (UGT) enzymes. Therefore, UGT enzyme inducers (eg, rifampin, phenytoin, phenobarbital, ritonavir) decrease canagliflozin’s serum concentration. If a patient has an eGFR >60 mL/min/1.73 m2 and is tolerating a dose of 100 mg/d, consider increasing the dose to 300 mg/d during concomitant treatment.
In addition, researchers have found that canagliflozin increases serum levels of digoxin by between 20% and 36%.25 Experts suspect this occurs because canagliflozin inhibits P-glycoprotein efflux of digoxin. Although monitoring of digoxin levels is recommended, this interaction is considered to be minor.25
Cost consideration: SGLT2 inhibitors are more expensive
The SGLT2 inhibitors are available only as brand name products and are more expensive than agents that have generic options (eg, metformin, sulfonylureas, TZDs). The average wholesale cost is approximately $400 for a 30-day supply of all SGLT2 agents.32 When considering an SGLT2 inhibitor, the patient should ideally have medication prescription coverage. Depending on the specific insurance plan, these agents are classified as tier 2 to 4, which is comparable to other oral brand name options.
Research looks at CV outcomes and cancer risk
Cardiovascular (CV) risk reduction. To date, only one study evaluating the effect of SGLT2 inhibitors on CV outcomes is complete.33 Two large randomized controlled trials involving canagliflozin and dapagliflozin designed to evaluate treatment effects on major CV endpoints are ongoing.34,35
In the EMPA-REG OUTCOME trial,33 researchers found that empagliflozin had beneficial effects on CV outcomes, making it one of the only antidiabetic agents on the market to have such benefits. The study, which involved more than 7000 patients with a history of T2DM and existing cardiovascular disease (CVD), found that 10.5% of patients in the empagliflozin group vs 12.1% in the placebo group died from a CV cause or experienced a nonfatal myocardial infarction or stroke over a median of 3.1 years. Results were similar with both doses (10 mg vs 25 mg) of empagliflozin. The mechanisms behind the CV benefits are likely multifactorial and may be related to reductions in weight and BP,33 but additional research is needed to fully elucidate the role of empagliflozin in this population.
Canagliflozin is being evaluated in the Canagliflozin Cardiovascular Assessment Study (CANVAS) for its effect on major CV events—CV death, nonfatal myocardial infarction, and nonfatal stroke—in patients with either a history of CVD or who are at increased risk of CVD and have uncontrolled diabetes.34 The trial is expected to wrap up in June 2017.
And dapagliflozin is being studied in the DECLARE-TIMI 58 trial (the Effect of Dapagliflozin on the Incidence of Cardiovascular Events) in patients with T2DM and either known CVD or at least 2 risk factors for CVD.35 The study is designed to assess dapagliflozin’s effect on the incidence of CV death, myocardial infarction, and ischemic stroke and has an estimated completion date of April 2019, which will provide a median follow-up of 4.5 years.
Cancer. All 3 agents have been examined for any possible carcinogenic links. In 2011, the FDA issued a request for further investigation surrounding the risk of cancer associated with dapagliflozin.36 As of November 2013, 10 of 6045 patients treated with dapagliflozin developed bladder cancer compared to 1 of 3512 controls.36 Furthermore, 9 of 2223 patients treated with dapagliflozin developed breast cancer compared to 1 of 1053 controls.36
Although the trials were not designed to detect an increase in risk, the number of observed cases warranted further investigation. No official warning for breast cancer exists since the characteristics of the malignancies led the FDA to believe dapagliflozin was unlikely the cause.36
Given what we know to date, it appears to be prudent to avoid prescribing SGLT2 inhibitors in patients with active bladder cancer, and to use them with caution in those with a history of the disease.2
Other studies. Initially, animal studies suggested an increased risk of various malignancies associated with canagliflozin use in rats,37 but consistent results were not seen in human studies. Similarly, at least one study found that empagliflozin was associated with lung cancer and melanoma, but closer examination found that most patients who developed these cancers had risk factors.38 Large, long-term studies of these agents in various populations are needed to thoroughly investigate possible carcinogenicity.
Additional considerations: Kidney function, age, and pregnancy
Consider avoiding SGLT2 inhibitors in patients with moderate kidney dysfunction (eGFR 30-59 mL/min/1.73 m2). Studies have shown that SGLT2 inhibitors are not as effective at lowering blood glucose in those with reduced eGFR, although adverse events were similar to those in placebo groups.24,39,40 Dapagliflozin is not recommended in patients with an eGFR <60 mL/min/1.73 m2 due to lack of efficacy.2,24 Empagliflozin does not require dose adjustments if eGFR is ≥45 mL/min/1.73 m2. A lower dose of canagliflozin (ie, 100 mg/d) is recommended in those with an eGFR of 45 to 59 mL/min/1.73 m2.2 All agents are contraindicated in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2).
Older patients are at higher risk for dehydration, hypotension, and falls; therefore, SGLT2 inhibitors should be used with caution in this population. Similarly, they should not be used in patients with T1DM and should be avoided in those with active, or a history of, DKA.
There are no data on the use of SGLT2 inhibitors in pregnancy; thus, these agents should be avoided unless the potential benefits outweigh the potential risks to the unborn fetus.2
CASE 1 › An SGLT2 inhibitor is an acceptable option for Mr. S. Because he is resistant to starting insulin therapy and his HbA1c is <9%, an additional oral medication is reasonable. Adding an SGLT2 inhibitor may reduce his HbA1c up to ~1%, and education on lifestyle modifications may help bring him to goal. An SGLT2 inhibitor may also benefit his BP and weight, both of which could be improved.
Given the drugs he’s taking, drug interactions should not be an issue, and his renal function and pertinent labs (K, Phos, Mg) are within normal limits. Nevertheless, monitor these labs periodically and monitor Mr. S for adverse effects, such as UTIs, although these are more common in women. Canagliflozin is the preferred SGLT2 inhibitor on his insurance formulary, so you could initiate therapy at 100 mg/d, administered prior to the first meal, and increase to 300 mg/d if needed. As an alternative, consider prescribing the metformin/canagliflozin combination agent.
CASE 2 › Ms. R is likely experiencing a yeast infection as an adverse effect of the dapagliflozin. Although one yeast infection is insufficient grounds for discontinuation of the drug, recurrent infections should prompt a risk-to-benefit analysis to determine whether it’s worth continuing the medication. Her recent eGFR (<60 mL/min/1.73 m2) is, however, a contraindication to dapagliflozin, and therapy should be discontinued. Canagliflozin and empagliflozin may be considered since her eGFR is >45 mL/min/1.73 m2, but given her current HbA1c and recent adverse drug event, alternative therapies, such as basal insulin, are more appropriate treatment choices.
CORRESPONDENCE
Katelin M. Lisenby, PharmD, BCPS, University of Alabama College of Community Health Sciences, University Medical Center, Box 870374, Tuscaloosa, AL 35487; [email protected].
› Consider sodium-glucose cotransporter 2 (SGLT2) inhibitors as second-line agents in patients with type 2 diabetes mellitus who need mild hemoglobin A1c reductions (≤1%) and who would benefit from mild to modest weight and blood pressure reductions. A
› Avoid using SGLT2 inhibitors in patients with a history of recurrent genital mycotic or urinary tract infections. B
› Use SGLT2 inhibitors with caution in patients at risk for volume-related adverse effects (dizziness and hypotension), such as the elderly, those with moderate renal dysfunction, and those taking concomitant diuretic therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Joe S is a 41-year-old African-American man who comes to your clinic after his employee health screening revealed elevated triglycerides. The patient has a 3-year history of type 2 diabetes mellitus (T2DM); he also has a history of hypertension, gastroesophageal reflux disease, and obstructive sleep apnea. Mr. S tells you he takes metformin 1000 mg twice daily, but stopped taking his glipizide because he didn’t think it was helping his blood sugar. His last hemoglobin (Hb) A1c result was 8.8%, and he is very resistant to starting insulin therapy.
The patient’s other medications include enalapril 10 mg/d, atorvastatin 10 mg/d, and omeprazole 20 mg/d. Mr. S weighs 255.6 lbs (body mass index=34.7), his BP is 140/88 mm Hg, and his heart rate is 82 beats per minute. Laboratory values include: serum creatinine, 1.01 mg/dL; estimated glomerular filtration rate (eGFR) >100 mL/min/1.73 m2; potassium (K), 4.3 mmol/L; serum phosphorous (Phos), 2.8 mg/dL; magnesium (Mg), 1.9 mg/dL; total cholesterol, 167 mg/dL; low-density lipoprotein (LDL), 78 mg/dL; high-density lipoprotein (HDL), 38 mg/dL; and triglycerides, 256 mg/dL.
CASE 2 › Susan R, a 68-year-old Caucasian woman, returns to your clinic for a follow-up visit 3 months after you prescribed dapagliflozin 10 mg/d for her T2DM. Her glucose levels have improved, but she complains of vaginal pruritus and is worried that she has a yeast infection.
You diagnose vulvovaginal candidiasis in this patient and prescribe a single dose of fluconazole 150 mg. After reviewing her laboratory test results, you notice that since starting the dapagliflozin, her HbA1c level has improved slightly from 9.8% to 9.3%, but is still not where it needs to be. Her eGFR is 49 mL/min/1.73 m2.
What would you recommend to improve control of these patients’ blood glucose levels?
When to consider an SGLT2 inhibitor
Consider therapy with SGLT2 inhibitors in adult patients with T2DM who:3-9,13-15,17-24
- have an HbA1c between 7% and 9%
- would benefit from weight and/or blood pressure reductions
- have metabolic syndrome
- have adequate means to pay for the medication (ie, prescription coverage or the ability to afford it).
In addition, consider an SGLT2 inhibitor as initial monotherapy if metformin is contraindicated or not tolerated, or as add-on therapy to metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase IV inhibitors, or insulin.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are the newest class of agents to enter the T2DM management arena. They act in the proximal renal tubules to decrease the reabsorption of glucose by targeting the SGLT2 transmembrane protein, which reabsorbs about 90% of the body’s glucose.1,2 The class is currently made up of 3 agents—canagliflozin, dapagliflozin, and empagliflozin—all of which are approved by the US Food and Drug Administration (FDA) for the treatment of T2DM (TABLE 1).2

The American Diabetes Association and the European Association for the Study of Diabetes published updated guidelines for T2DM management in 2015.1 In addition to lifestyle modifications, the guidelines recommend the use of metformin as first-line therapy unless it is contraindicated or patients are unable to tolerate it (eg, because of gastrointestinal adverse effects). They recommend other pharmacologic therapies as second-line options based on specific patient characteristics. Thus, SGLT2 inhibitors may be used as add-on therapy after metformin, or as a first-line option if metformin is contraindicated or not tolerated. Because the mechanism of action of SGLT2 inhibitors is independent of insulin secretion, these agents may be used at any stage of the diabetes continuum.
SGLT2 agents as monotherapy, or as add-on therapy
All SGLT2 agents have been studied as monotherapy accompanied by diet and exercise and shown to produce HbA1c reductions of 0.34% to 1.11%.3-6 In trials, the effect was similar regardless of study duration (18-104 weeks); generally, higher doses corresponded with larger HbA1c reductions.3-6
SGLT2 inhibitors have also been studied as add-on therapy to several oral agents including metformin, sulfonylureas, thiazolidinediones (TZDs), and the combination of metformin plus sulfonylureas or TZDs or dipeptidyl peptidase IV (DPP-IV) inhibitors.1 When used in any of these combinations, each SGLT2 agent demonstrated a consistent HbA1c lowering effect of 0.62% to 1.19%.7-14
Additionally, SGLT2 inhibitors have been studied in combination with insulin therapy (median or mean daily doses >60 units), which yielded further reductions in HbA1c of 0.58% to 1.02% without significant insulin adjustments or an increase in major hypoglycemia events.15-17 Patients receiving insulin and an SGLT2 inhibitor had lower insulin doses and more weight loss compared to placebo groups.
SGLT2 inhibitors offer additional benefits
Secondary analyses of most studies of SGLT2 inhibitors include changes in BP and weight from baseline as well as minor changes (some positive, some not) in several lipid parameters.3-5,7-9,13-15,17-24 In general, these effects do not appear to be dose-dependent (with the exception of canagliflozin and its associated lipid effects25) and are similar among the 3 medications.3-5,7-9,13-15,17-24 (For more on who would benefit from these agents, see “When to consider an SGLT2 inhibitor” above.)
BP reduction. Although the mean baseline BP was controlled in most studies, SGLT2 inhibitors have been shown to significantly reduce BP. Reductions in BP with all 3 SGLT2 medications range from approximately 2 to 5 mm Hg systolic and 0.5 to 2.5 mm Hg diastolic, which may be due to weight loss and diuresis.4-8,10-16,20-23 While the reductions were modest at best, one study involving empagliflozin reported that more than one-quarter of patients with uncontrolled BP at baseline achieved a BP <130/80 mm Hg 24 weeks later.5 While these agents should not be used solely for their BP lowering effects, they may help a small number of patients with mildly elevated BP achieve their goal without an additional antihypertensive agent.
Weight reduction. Modest weight loss, likely due to the loss of calories through urine, was seen with SGLT2 inhibitors in most studies, with reductions persisting beyond one year of use. In most studies, including those involving obese patients on insulin therapy,15,17,21 patients’ body weights were reduced by approximately 2 to 4 kg from baseline.3-16,18,21-23,26
Lipid effects. Although the mechanism is unclear, use of SGLT2 inhibitors can have varying effects on lipid panels. In most studies, total and LDL cholesterol levels were increased with elevations ranging from 0.7 to 10 mg/dL.3,7,8,18,19,22,23 Conversely, at least one study demonstrated mild reductions in total and LDL cholesterol levels with higher doses of empagliflozin.13 Additionally, modest reductions in triglycerides and increases in HDL across all doses of canagliflozin, dapagliflozin, and empagliflozin have been seen.8,9,13,15,19 While the clinical relevance of these lipid changes is unknown, monitoring is recommended.2
These agents are well tolerated
SGLT2 inhibitors were generally well tolerated in studies. The most common adverse effects include mycotic infections (2.4%-21.6%) and urinary tract infections (UTIs) (4.0%-19.6%) (both with higher incidences in females); volume-related effects such as dizziness and hypotension (0.3%-8.3%); and nasopharyngitis (5.4%-18.3%).4-14,16-23,26-28 Hypoglycemia was observed more often when an SGLT2 inhibitor was used in combination with a sulfonylurea or insulin therapy.4-14,16-23,26-28 The number of times adverse events led to discontinuation was low and similar to that in control groups.4-14,16-23,26-28
Mycotic and urinary infections should be diagnosed and treated according to current standards of care and do not require discontinuation of the SGLT2 inhibitor. Canagli-flozin therapy was associated with electrolyte abnormalities including hyperkalemia, hypermagnesemia, and hyperphosphatemia.25 Thus, levels should be monitored periodically, especially in patients predisposed to elevations due to other conditions or medications.25
Two additional warnings are worth noting
Diabetic ketoacidosis (DKA) has been reported with all 3 agents, and bone fractures have been reported with canagliflozin.
The FDA issued a warning in May 2015 regarding the increased risk of DKA with the use of SGLT2 inhibitor single and combination products.29 This warning was prompted by several case reports of DKA with uncharacteristically mild to moderate glucose elevations in patients with type 1 diabetes mellitus (T1DM) and T2DM who were taking an SGLT2 inhibitor. The absence of significant hyperglycemia delayed diagnosis in many cases. Therefore, patients should be counseled on the signs and symptoms of DKA, as well as when to seek medical attention.
Patients with diabetes and symptoms of ketoacidosis (eg, difficulty breathing, nausea, vomiting, abdominal pain, confusion, and fatigue) should be evaluated regardless of current blood glucose levels, and SGLT2 inhibitors should be discontinued if acidosis is confirmed. Identified potential triggers include illness, reduced food and fluid intake, reduced insulin dose, and history of alcohol intake. Use of SGLT2 inhibitors should be avoided in patients with T1DM until safety and efficacy are established in large randomized controlled trials. The European Medicines Agency announced that a thorough review of all currently approved SGLT2 agents is underway to evaluate the risk for DKA.30
In addition, the FDA called for a revision of the label of canagliflozin to reflect a strengthened warning about an increased risk of bone fractures and decreased bone mineral density (BMD).31 Fractures can occur as early as 12 weeks after initiating treatment and with only minor trauma.31
Over a 2-year period, canagliflozin also significantly decreased BMD in the hip and lower spine compared to placebo.31 Patients should be evaluated for additional risk factors for fracture before taking canagliflozin.31 The FDA is continuing to evaluate whether the other approved SGLT2 inhibitors are associated with an increased risk for fractures.
Drug interactions: Proceed carefully with diuretics
The number of drugs that interact with SGLT2 inhibitors is minimal. Because these agents can cause volume-related effects such as hypotension, dizziness, and osmotic diuresis, patients—particularly the elderly and those with renal impairment—taking concomitant diuretics, especially loop diuretics, may be at increased risk for these effects and should be monitored accordingly.2,25
Canagliflozin is primarily metabolized via glucuronidation by the uridine 5'-diphospho-glucuronosyltransferase (UGT) enzymes. Therefore, UGT enzyme inducers (eg, rifampin, phenytoin, phenobarbital, ritonavir) decrease canagliflozin’s serum concentration. If a patient has an eGFR >60 mL/min/1.73 m2 and is tolerating a dose of 100 mg/d, consider increasing the dose to 300 mg/d during concomitant treatment.
In addition, researchers have found that canagliflozin increases serum levels of digoxin by between 20% and 36%.25 Experts suspect this occurs because canagliflozin inhibits P-glycoprotein efflux of digoxin. Although monitoring of digoxin levels is recommended, this interaction is considered to be minor.25
Cost consideration: SGLT2 inhibitors are more expensive
The SGLT2 inhibitors are available only as brand name products and are more expensive than agents that have generic options (eg, metformin, sulfonylureas, TZDs). The average wholesale cost is approximately $400 for a 30-day supply of all SGLT2 agents.32 When considering an SGLT2 inhibitor, the patient should ideally have medication prescription coverage. Depending on the specific insurance plan, these agents are classified as tier 2 to 4, which is comparable to other oral brand name options.
Research looks at CV outcomes and cancer risk
Cardiovascular (CV) risk reduction. To date, only one study evaluating the effect of SGLT2 inhibitors on CV outcomes is complete.33 Two large randomized controlled trials involving canagliflozin and dapagliflozin designed to evaluate treatment effects on major CV endpoints are ongoing.34,35
In the EMPA-REG OUTCOME trial,33 researchers found that empagliflozin had beneficial effects on CV outcomes, making it one of the only antidiabetic agents on the market to have such benefits. The study, which involved more than 7000 patients with a history of T2DM and existing cardiovascular disease (CVD), found that 10.5% of patients in the empagliflozin group vs 12.1% in the placebo group died from a CV cause or experienced a nonfatal myocardial infarction or stroke over a median of 3.1 years. Results were similar with both doses (10 mg vs 25 mg) of empagliflozin. The mechanisms behind the CV benefits are likely multifactorial and may be related to reductions in weight and BP,33 but additional research is needed to fully elucidate the role of empagliflozin in this population.
Canagliflozin is being evaluated in the Canagliflozin Cardiovascular Assessment Study (CANVAS) for its effect on major CV events—CV death, nonfatal myocardial infarction, and nonfatal stroke—in patients with either a history of CVD or who are at increased risk of CVD and have uncontrolled diabetes.34 The trial is expected to wrap up in June 2017.
And dapagliflozin is being studied in the DECLARE-TIMI 58 trial (the Effect of Dapagliflozin on the Incidence of Cardiovascular Events) in patients with T2DM and either known CVD or at least 2 risk factors for CVD.35 The study is designed to assess dapagliflozin’s effect on the incidence of CV death, myocardial infarction, and ischemic stroke and has an estimated completion date of April 2019, which will provide a median follow-up of 4.5 years.
Cancer. All 3 agents have been examined for any possible carcinogenic links. In 2011, the FDA issued a request for further investigation surrounding the risk of cancer associated with dapagliflozin.36 As of November 2013, 10 of 6045 patients treated with dapagliflozin developed bladder cancer compared to 1 of 3512 controls.36 Furthermore, 9 of 2223 patients treated with dapagliflozin developed breast cancer compared to 1 of 1053 controls.36
Although the trials were not designed to detect an increase in risk, the number of observed cases warranted further investigation. No official warning for breast cancer exists since the characteristics of the malignancies led the FDA to believe dapagliflozin was unlikely the cause.36
Given what we know to date, it appears to be prudent to avoid prescribing SGLT2 inhibitors in patients with active bladder cancer, and to use them with caution in those with a history of the disease.2
Other studies. Initially, animal studies suggested an increased risk of various malignancies associated with canagliflozin use in rats,37 but consistent results were not seen in human studies. Similarly, at least one study found that empagliflozin was associated with lung cancer and melanoma, but closer examination found that most patients who developed these cancers had risk factors.38 Large, long-term studies of these agents in various populations are needed to thoroughly investigate possible carcinogenicity.
Additional considerations: Kidney function, age, and pregnancy
Consider avoiding SGLT2 inhibitors in patients with moderate kidney dysfunction (eGFR 30-59 mL/min/1.73 m2). Studies have shown that SGLT2 inhibitors are not as effective at lowering blood glucose in those with reduced eGFR, although adverse events were similar to those in placebo groups.24,39,40 Dapagliflozin is not recommended in patients with an eGFR <60 mL/min/1.73 m2 due to lack of efficacy.2,24 Empagliflozin does not require dose adjustments if eGFR is ≥45 mL/min/1.73 m2. A lower dose of canagliflozin (ie, 100 mg/d) is recommended in those with an eGFR of 45 to 59 mL/min/1.73 m2.2 All agents are contraindicated in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2).
Older patients are at higher risk for dehydration, hypotension, and falls; therefore, SGLT2 inhibitors should be used with caution in this population. Similarly, they should not be used in patients with T1DM and should be avoided in those with active, or a history of, DKA.
There are no data on the use of SGLT2 inhibitors in pregnancy; thus, these agents should be avoided unless the potential benefits outweigh the potential risks to the unborn fetus.2
CASE 1 › An SGLT2 inhibitor is an acceptable option for Mr. S. Because he is resistant to starting insulin therapy and his HbA1c is <9%, an additional oral medication is reasonable. Adding an SGLT2 inhibitor may reduce his HbA1c up to ~1%, and education on lifestyle modifications may help bring him to goal. An SGLT2 inhibitor may also benefit his BP and weight, both of which could be improved.
Given the drugs he’s taking, drug interactions should not be an issue, and his renal function and pertinent labs (K, Phos, Mg) are within normal limits. Nevertheless, monitor these labs periodically and monitor Mr. S for adverse effects, such as UTIs, although these are more common in women. Canagliflozin is the preferred SGLT2 inhibitor on his insurance formulary, so you could initiate therapy at 100 mg/d, administered prior to the first meal, and increase to 300 mg/d if needed. As an alternative, consider prescribing the metformin/canagliflozin combination agent.
CASE 2 › Ms. R is likely experiencing a yeast infection as an adverse effect of the dapagliflozin. Although one yeast infection is insufficient grounds for discontinuation of the drug, recurrent infections should prompt a risk-to-benefit analysis to determine whether it’s worth continuing the medication. Her recent eGFR (<60 mL/min/1.73 m2) is, however, a contraindication to dapagliflozin, and therapy should be discontinued. Canagliflozin and empagliflozin may be considered since her eGFR is >45 mL/min/1.73 m2, but given her current HbA1c and recent adverse drug event, alternative therapies, such as basal insulin, are more appropriate treatment choices.
CORRESPONDENCE
Katelin M. Lisenby, PharmD, BCPS, University of Alabama College of Community Health Sciences, University Medical Center, Box 870374, Tuscaloosa, AL 35487; [email protected].
1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2015;38:140-149.
2. Canagliflozin, dapagliflozin, empagliflozin. Lexicomp, Inc. (Lexi-Drugs®). Accessed October 12, 2015.
3. Stenlöf K, Cefalu WT, Kim KA, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163-175.
4. Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010:33:2217-2224.
5. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208-219.
6. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015-4021.
7. Wilding JPH, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267-1282.
8. Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467-477.
9. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508-2515.
10. Bristol-Myers Squibb [press release]. New phase III data showed dapagliflozin significantly reduced HbA1c compared to placebo at 24 weeks in patients with type 2 diabetes inadequately controlled with the combination of metformin plus sulfonylurea. Available at: http://news.bms.com/press-release/rd-news/new-phase-iii-data-showed-dapagliflozin-significantly-reduced-hba1c-compared-p&t=635156160653787526. Accessed September 17, 2015.
11. Jabbour SA, Hardy E, Sugg J, et al. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24- week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37:740-750.
12. DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384-393.
13. Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther. 2015;37:1773-1788.
14. Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396-3404.
15. Neal B, Percovik V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium–glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403-411.
16. Wilding JPH, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405-415.
17. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815-1823.
18. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941-950.
19. Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010:375:2223-2233.
20. Bailey CJ, Gross JL, Hennicken D, et al. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43.
21. Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473-1478.
22. Merker L, Häring HU, Christiansen AV, et al. Empagliflozin as add-on to metformin in people with type 2 diabetes. Diabet Med. 2015;32:1555-1567.
23. Ridderstråle M, Anderson KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691-700.
24. Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962-971.
25. Invokana (canagliflozin) tablets [product information]. Titusville, NJ: Janssen Pharmaceuticals Inc. Available at: https://www.invokana.com. Accessed March 15, 2013.
26. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582-2592.
27. Strojek K, Yoon KH, Hruba V, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928-938.
28. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38:355-364.
29. US Food and Drug Administration. FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed July 11, 2016.
30. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638-1642.
31. US Food and Drug Administration. FDA drug safety communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm. Acces-sed July 11, 2016.
32. Canagliflozin, dapagliflozin, empagliflozin. In: RED BOOK [AUHSOP intranet database]. Greenwood Village, CO: Truven Health Analytics; [updated daily]. Available at: http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/BB1644/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/FAF693/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/redbook.ShowProductSearchResults?SearchTerm=JARDIANCE&searchType=redbookProductName&searchTermId=42798&searchContent=%24searchContent&searchFilterAD=filterADActive&searchFilterRepackager=filterExcludeRepackager&searchPattern=%5Ejard. Accessed March 15, 2016.
33. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
34. CANagliflozin cardioVascular Assessment Study (CANVAS). Available at: http://clinicaltrials.gov/show/NCT01032629. Accessed October 12, 2015.
35. Multicenter trial to evaluate the effect of dapagliflozin on the incidence of cardiovascular events (DECLARE-TIMI 58). Available at: http://clinicaltrials.gov/show/NCT01730534. Accessed October 12, 2015.
36. FDA background document. BMS-512148 NDA 202293. In: Proceedings of the US Food and Drug Administration Endocrinologic & Metabolic Drug Advisory Committee Meeting, 2013. Available at: http://www.fda.gov/downloads/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm378079.pdf. Accessed October 12, 2015.
37. Lin HW, Tseng CH. A review of the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol. 2014;2014:719578.
38. Center for Drug Evaluation and Research. Risk assessment and risk mitigation review(s). July 28, 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/ 204629Orig1s000RiskR.pdf. Accessed September 21, 2015.
39. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016-1027.
40. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2: 369-384.
1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2015;38:140-149.
2. Canagliflozin, dapagliflozin, empagliflozin. Lexicomp, Inc. (Lexi-Drugs®). Accessed October 12, 2015.
3. Stenlöf K, Cefalu WT, Kim KA, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163-175.
4. Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010:33:2217-2224.
5. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208-219.
6. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015-4021.
7. Wilding JPH, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267-1282.
8. Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467-477.
9. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508-2515.
10. Bristol-Myers Squibb [press release]. New phase III data showed dapagliflozin significantly reduced HbA1c compared to placebo at 24 weeks in patients with type 2 diabetes inadequately controlled with the combination of metformin plus sulfonylurea. Available at: http://news.bms.com/press-release/rd-news/new-phase-iii-data-showed-dapagliflozin-significantly-reduced-hba1c-compared-p&t=635156160653787526. Accessed September 17, 2015.
11. Jabbour SA, Hardy E, Sugg J, et al. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24- week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37:740-750.
12. DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384-393.
13. Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther. 2015;37:1773-1788.
14. Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396-3404.
15. Neal B, Percovik V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium–glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403-411.
16. Wilding JPH, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405-415.
17. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815-1823.
18. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941-950.
19. Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010:375:2223-2233.
20. Bailey CJ, Gross JL, Hennicken D, et al. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43.
21. Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473-1478.
22. Merker L, Häring HU, Christiansen AV, et al. Empagliflozin as add-on to metformin in people with type 2 diabetes. Diabet Med. 2015;32:1555-1567.
23. Ridderstråle M, Anderson KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691-700.
24. Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962-971.
25. Invokana (canagliflozin) tablets [product information]. Titusville, NJ: Janssen Pharmaceuticals Inc. Available at: https://www.invokana.com. Accessed March 15, 2013.
26. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582-2592.
27. Strojek K, Yoon KH, Hruba V, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928-938.
28. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38:355-364.
29. US Food and Drug Administration. FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed July 11, 2016.
30. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638-1642.
31. US Food and Drug Administration. FDA drug safety communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm. Acces-sed July 11, 2016.
32. Canagliflozin, dapagliflozin, empagliflozin. In: RED BOOK [AUHSOP intranet database]. Greenwood Village, CO: Truven Health Analytics; [updated daily]. Available at: http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/BB1644/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/FAF693/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/redbook.ShowProductSearchResults?SearchTerm=JARDIANCE&searchType=redbookProductName&searchTermId=42798&searchContent=%24searchContent&searchFilterAD=filterADActive&searchFilterRepackager=filterExcludeRepackager&searchPattern=%5Ejard. Accessed March 15, 2016.
33. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
34. CANagliflozin cardioVascular Assessment Study (CANVAS). Available at: http://clinicaltrials.gov/show/NCT01032629. Accessed October 12, 2015.
35. Multicenter trial to evaluate the effect of dapagliflozin on the incidence of cardiovascular events (DECLARE-TIMI 58). Available at: http://clinicaltrials.gov/show/NCT01730534. Accessed October 12, 2015.
36. FDA background document. BMS-512148 NDA 202293. In: Proceedings of the US Food and Drug Administration Endocrinologic & Metabolic Drug Advisory Committee Meeting, 2013. Available at: http://www.fda.gov/downloads/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm378079.pdf. Accessed October 12, 2015.
37. Lin HW, Tseng CH. A review of the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol. 2014;2014:719578.
38. Center for Drug Evaluation and Research. Risk assessment and risk mitigation review(s). July 28, 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/ 204629Orig1s000RiskR.pdf. Accessed September 21, 2015.
39. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016-1027.
40. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2: 369-384.