User login
Irritable bowel syndrome: Minimize testing, let symptoms guide treatment
- For patients aged <50 years without alarm symptoms, diagnostic testing is unnecessary. Consider celiac sprue testing for patients with diarrhea (C).
- Treatment is indicated when both the patient with irritable bowel syndrome and the physician agree that quality of life has been diminished (C). The goal of therapy is to alleviate global IBS symptoms (abdominal discomfort, bloating, and altered bowel habits that are life-impacting) (C).
- Tegaserod, a 5HT4 receptor agonist, is more effective than placebo at relieving global IBS symptoms in women with constipation (A). Its effectiveness in men is unknown.
- Alosetron, a 5HT3 receptor antagonist, is more effective than placebo at relieving global IBS symptoms in women with diarrhea (A).
- Behavior therapy–relaxation therapy, hypnotherapy, or cognitive therapy–is more effective than placebo at relieving individual symptoms, but no data are available for quality-of-life improvement (B).
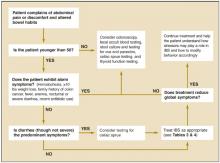
An extensive and expensive evaluation for irritable bowel syndrome (IBS) can be avoided if your patient is aged <50 years and is not experiencing alarm symptoms (hematochezia, 10 lbs weight loss, fever, anemia, nocturnal or severe diarrhea), has not recently taken antibiotics, and has no family history of colon cancer. An algorithm ( Figure ) indicates when work-up is needed and what it should entail.
Newer medications that act on 5HT receptors have proven effective in improving quality of life (global symptom reduction). Evidence supports the use of several traditional medications to reduce individual symptoms of IBS, but not for global symptom reduction.
FIGURE
Evaluating possible irritable bowel syndrome
Who gets irritable bowel syndrome?
Ten percent to 15% of the North American population has IBS, and twice as many women as men have it.1 Symptoms usually begin before the age of 35 years, and many patients can trace their symptoms back to childhood.2 Onset in the elderly is rare.3 The disorder is responsible for approximately 50% of referrals to gastroenterologists.4
The company IBS keeps
Comorbid psychiatric illness is common with IBS, but few patients seek psychiatric care.5 Depression, anxiety, and somatoform disorders are seen in 94% of patients with IBS. IBS is common in patients with chronic fatigue syndrome (51%), fibromyalgia (49%), temporomandibular joint syndrome (64%), and chronic pelvic pain (50%).6 IBS often follows stressful life events,5,7,8 such as a death in the family or divorce. It tends to be chronic, intermittent, and relapsing.3
The symptoms of IBS can overlap with those of other illnesses, including thyroid dysfunction (diarrhea or constipation), functional dyspepsia (abdominal pain), Crohn’s disease or ulcerative colitis (diarrhea, abdominal pain), celiac sprue (diarrhea), polyps and cancers (constipation or abdominal pain), and infectious diarrhea.
Elusive physiologic mechanism
Several physiologic mechanisms have been proposed for IBS symptoms: altered gut reactivity in response to luminal stimuli, hypersensitive gut with enhanced pain response, and altered brain-gut biochemical axis.9 Though the symptoms of irritable bowel syndrome appear to have a physiologic basis, there are no structural or biochemical markers for the disease.
Use symptom-based criteria for diagnosis
Consider a diagnosis of IBS when a patient complains of abdominal discomfort and altered bowel habits. In the absence of a structural or biochemical marker, IBS must be diagnosed according to symptom-based criteria–such as Manning, Rome I, or Rome II–which have been developed for research and epidemiologic purposes. Though their clinical utility remains unproven, these criteria (delineated in Table 1 ) are the crux of clinical diagnosis for IBS.4,10-14 Subtypes of IBS have been described (diarrheapredominant IBS or constipation-predominant IBS), but they are not diagnostically useful, since the treatment goal is improved quality of life.
TABLE 1
Symptom-based criteria for irritable bowel syndrome
| Symptom-based criteria | Symptoms | Sn | Sp | PV+ |
|---|---|---|---|---|
| Manning4,10,13,14 |
| 42%–90% | 70%–100% | 74% |
| Rome I4,10,13 |
| 65%–84% | 100% | 69%–100% |
| Rome II11-13 |
| 49%-65%* | 100%* | 69%-100%* |
| Supportive symptoms | ||||
| Fewer than 3 bowel movements per week | ||||
| More than 3 bowel movements per day | ||||
| Hard or lumpy stools | ||||
| *Found to have similar sensitivity and specificity to Rome I.14 | ||||
| Sn, sensitivity; Sp, specificity; PV+, positive predictive value | ||||
Dubious value of diagnostic tests
The literature regarding the value of diagnostic testing for IBS is controversial. Symptom-based criteria have varied in many studies, as have the criteria used to enroll patients and the measured outcomes of treatment (reduction in abdominal pain, in diarrhea, or in constipation, or improvement in quality of life). Because of these discrepancies, it is difficult to apply the literature clinically. Of the 6 landmark studies that considered the value of diagnostic testing for IBS patients,15-20 only 2 compared IBS patients with groups of healthy controls.17,19
Test results yield little. Most research in this area has compared the prevalence of specific illnesses in the general population with the yield of positive test results for these illnesses among persons meeting the symptom-based diagnostic criteria for IBS.
Two studies15,16 determined the incidence of abnormal test results in patients who met the Manning or Rome I criteria for IBS. In these studies, most diagnostic tests yielded positive results in 2% (range, 0%-8.2%) of patients or less, except for thyroid and lactose intolerance testing. That is equivalent to the incidence in the general population. The prevalence of thyroid disorders and lactose malabsorption was higher in IBS patients (6% and 22%-26%, respectively), but prevalence in the general population is similarly higher (5%-9% and 25%). Based on these results, testing for thyroid disease or lactose malabsorption is indicated only for patients exhibiting symptoms of these disorders (fatigue/weight change or diarrhea related to diertary intake of dairy products, respectively).
An exception. Some clinicians propose that diagnostic testing for patients with IBS symptoms should be driven by the pretest probability of organic disease (prevalence) compared with the general population. Cash21 found the pretest probability of inflammatory bowel disease, colorectal cancer, and infectious diarrhea is less than 1% among IBS patients without alarm symptoms ( Table 2 ). He demonstrated that patients with IBS had a 5% pretest probability of celiac sprue compared with healthy patients (<1% prevalence). Therefore, testing for celiac sprue (eg, complete blood count, antiendomysial antibody, and antigliadin antibody) may be considered for patients with diarrhea.6,21,22 Sigmoidoscopy,15,17 rectal biopsy,17 and abdominal ultrasound18 have low positive yield in patients meeting the diagnostic criteria for IBS.
TABLE 2
Probability of organic disease in irritable bowel syndrome patients
| Disease | Pretest probability-IBS patients (%) | Prevalence-general population (%) | Comments |
|---|---|---|---|
| Colitis/inflammatory inflammatory bowel disease | 0.51-0.98 | 0.3-1.2 | Structural colon lesions were detected with barium enema, colonscopy, sigmoidoscopy |
| Colon cancer | 0-0.51 | 4-6 | Structural colon lesions were detected with barium enema, colonoscopy, sigmoidoscopy |
| Celiac disease | 4.67 | 0.25-0.5 | Note: celiac disease prevalence higher than in general population. |
| Gastrointestinal infection | 0-1.7 | N/A | |
| Thyroid dysfunction | 6 | 5-9 | Prevalence high in both groups |
| Lactose malabsorption | 22-26 | 25 | Prevalence high in both groups |
| Adapted from: Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002; 97:2812-2819. | |||
| Results are from multiple studies: n=125-306. | |||
How to proceed
Those under 50 years of age who have no alarm symptoms can forgo further testing. Testing for celiac sprue and lactose malabsorption might be considered for patients with diarrhea that improves or worsens with change in diet (strength of recommendation [SOR]: C).
Threshold for treatment
Treatment for IBS is indicated when both patient and physician believe global symptoms (abdominal discomfort, bloating, altered bowel habits) have diminished the quality of life (SOR: C). The goal of treatment is to alleviate all IBS symptoms (SOR: C). Treating altered bowel habits (constipation, diarrhea, and fecal urgency) without addressing other IBS symptoms (eg, abdominal pain) is inferior treatment.23,24
Treatment options for IBS
Treatments for IBS include medications, behavior therapy, and complimentary and alternative therapies. Medications traditionally prescribed include bulking agents, anticholinergics/antispasmodics, antidiarrheals, and antidepressants. A 5HT3 receptor antagonist and a 5HT4 receptor partial agonist are now available. Table 3 summarizes the traditional treatments in terms of efficacy, strength of recommendations, and outcomes measured. Alternative and complimentary therapies appear in Table 4 .
As Brandt24 has noted, the evidence for treatment effectiveness is difficult to review and summarize, because the quality of studies has been poor. Most studies did not use healthy control groups, and the numbers of participants were small. Many studies did not use blinded placebo groups. Outcomes measured varied among the studies, with most of them measuring reductions of individual bowel symptoms (eg, diarrhea or constipation). Quality-of-life tools were used in other studies to measure reduction in global IBS symptoms (eg, IBS Quality of Life 25 ). Because of these discrepancies, there is no sound evidence for traditional therapies.
TABLE 3
Treatments for irritable bowel syndrome
| Treatment | Efficacy (NNT) | SOR (studies) | Outcomes measured | Comments |
|---|---|---|---|---|
| 5HT4 receptor agonist (tegaserod)23,24,26-30 | More effective than placebo at relieving global IBS symptoms in women with constipation (3.9-17) | A (4) | Global IBS symptoms, individual IBS symptoms | 83%-100% of study participants were women with IBS and constipation. Rome I and II criteria for entry. May cause diarrhea |
| 5HT4 receptor agonist(alosetron)23,24,26- 35 | More effective than placebo at relieving global IBS symptoms in women with diarrhea (2.5-8.3) | A (4) | Global IBS symptoms, individual IBS symptoms, adverse events | 82%-93% of study participants were women. Rome I and II criteria for entry. May cause severe constipation; restricted use |
| Tricylic antidepressants (trimipramine, desipramine)23,24,36- 39 | Reduces abdominal pain. No more effective than placebo at relieving gloal IBS symptoms (3.2-5) | B (6) | GI symptoms | May cause constipation; no studies done with SSRIs |
| Loperamide23,24,36-39 | Relieves diarrhea. No more effective than placebo at relieving global IBS symptoms (3.2-5) | B (3) | Global IBS symptoms, diarrhea | Constipation or paralytic ileus can occur |
| Bulking agents (corn fiber, wheat bran, psyllium, ispaghula husks, calcium polycabophil)23,24,31,40-42 | Improves constipation. No more effective than placebo in studies considering global symptom improvement (2.2-8.6) | B (13) | GI symptoms, global IBS symptoms | May increase bloating. All studiessmall numbers of patients |
| Anti-spasmodics (hyoscyamine dicyclomine)23,24,26-30 | No evidence on improvement of global IBS symptoms (5.9) | B (3) | Individual IBS and global symptoms | Studies were short, small numbers, inconsistent effectiveness. Could worsen constipation; 15 additional studies done on drugs not available in the US |
| Behavioral therapies (hypnotherapy, relaxation therapy, psychotherapy, biofeedback)23,24,44, 52-57 | More effective than placebo at relieving individuals IBS symptoms (1.4-1.9) | B (16) | GI symptoms, psychological sypmtoms | None measures global IBS symptom improvement. Small numbers of patients |
| SSRI antidepressants (paroxtetine, fluoxetine)23,24, 50-51 | Improved quality of life, decreased abdominal pain | B (16) | Abdominal | One study severe IBS, other study only 10 participants quality of life |
| SOR, strength of recommendation; IBS, irritable bowel syndrome; GI, gastrointestinal; SSRI, selective serotonin reuptake inhibitor. For an explanation of SORs. | ||||
TABLE 4
Complementary and alternative treatments for irritable bowel syndrome
| Treatment | Efficacy | SOR | Outcomes measured | Comments |
|---|---|---|---|---|
| Neomycin 20 | Treatment for 1 week improved symptoms of abdominal pain, diarrhea, and constipation | A | Abdominal pain, diarrhea, or constipation | Studies measuring global symptom improvement lacking |
| Peppermint oil 31,4849 | Some demonstrated improvement in abdominal pain | B | Individual IBS symptoms | Studies measuring global symptom improvement lacking |
| Guar gum 44 | Improved abdominal pain and bowel alterations | B | Study compared fiber to guar gum–equal affect on abdominal pain. Gum was better toleratEd | No placebo-controlled trials |
| Probiotics48 (lactobacillus) | Improvement of abdominal pain and flatulence | C | Abdominal pain, flatulence | Two studies with small numbers |
| Elimination diets 48 | Improvement of diarrhea | C | Diarrhea | Milk, wheat, eggs eliminated; 15%-71% improvement of diarrhea |
| Lactose and fructose avoidance 48 | Conflicting evidence results | D | No controlled studies available | |
| Pancreatic enzymes 48 | No evidence | D | Evidence lacking | |
| Ginger 48 | No evidence | D | No studies |
Medications
Strength of recommendation: A. The recently approved 5HT4 receptor agonist tegaserod (Zelnorm) is more effective than placebo at relieving global symptoms in women with constipation (number needed to treat [NNT]=3.9-17).26-30 Diarrhea can be a serious side effect.
The 5HT3 receptor antagonist alosetron (Lotronex) is more effective than placebo at relieving global IBS symptoms in women with diarrhea (NNT=2.5-8.3).31-35 Severe constipation can be an adverse effect. The prescribing of alosetron is currently restricted to physicians who participate in the manufacturer’s risk management program.
In addition to these serotoninergic agents, others in this class are being developed and undergoing clinical trials. The knowledge being gained about 5HT receptors may revolutionize the care of patients with IBS.
Strength of recommendation: B. Tricyclic antidepressants are no more effective than placebo at relieving global IBS symptoms, but they do decrease abdominal pain (NNT=3.2-5).36-39
Loperamide is no more effective than placebo at relieving IBS global symptoms, but it may be used to treat diarrhea (NNT=2.3-5).31,40-42
Bulking agents (such as calcium polycarbophil or psyllium) are no more effective than placebo at relieving IBS global symptoms, but they may decrease constipation (NNT=2.2-8.6).31,36,43-47
Peppermint oil may be helpful for abdominal pain, but global symptom reduction has not been demonstrated.31,48-49 Only a few studies have looked at the use of antispasmodic agents for IBS. They are of poor quality and used small numbers with no placebo controls.23,31,36,43
Strength of recommendation: C. There are limited studies evaluating the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and paroxetine. Paroxetine was shown in 1 study to improve quality of life.50 Fluoxetine reduced abdominal pain, but did not improve quality of life.51
Behavioral and complementary/alternative therapies
Relaxation therapy, hypnotherapy, and cognitive therapy are effective at relieving individual IBS symptoms, but have not been shown to reduce global IBS symptoms (SOR: B).52-57 Other alternative therapies (eg, guar gum44 [SOR: B], ginger48 [SOR: B], and pancreatic enzymes48 [SOR: C]) have been studied, but high-quality studies considering global improvement have not been published.
The position statement of the American College of Gastroenterology on the management of IBS23 and Brandt’s systematic review of this subject24 were the starting points for this review. The majority of the references from these sources were reviewed and a Medline search was completed to identify new evidence. The Oxford Centre for Evidence-Based Medicine grades of recommendations were applied to this evidence, a care algorithm was created, summary tables were developed, and numbers needed to treat were calculated.
Promote self-awareness
Quality-of-life assessment should be done routinely in the care of IBS patients. Provide support, empathy, and basic behavior modification tools. Educate patients and their families on the theoretical biochemical basis of this illness, and help them connect symptoms with stressors, to facilitate lifestyle modification.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Saito YA, Schoenfeld P, Locke R. The epidemiology of irritable bowel syndrome in North America: A systematic review. Am J Gastrenterol 2002;97:1910-1915.
2. Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 1995;109:1736-1741.
3. Maxwell PR, Mendall MA, Kumar D. Irritable bowel syndrome. Lancet 1997;350:1691-1695.
4. Fass R, Longstreth GF, Pimental M, et al. Evidence- and consensus-based practice guidelines for the diagnosis of irritable bowel syndrome. Arch Intern Med 2001;161:2081-2088.
5. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry 1997;42:835-840.
6. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology 2002;122:1140-1156.
7. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am 2000;84:1313-1327.
8. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 2000;160:221-227.
9. Camilleri M, Prather CM. The irritable bowel syndrome: mechanisms and a practical approach to management. Ann Internal Med 1992;116:1001-1008.
10. Paterson WG, Thompson WG, Vanner SJ, et al. Recommendations for the management of irritable bowel syndrome in family practice. JAMC 1999;161:154-160.
11. Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology 2002;122:1701-1714.
12. Tosetti C, Stanghellini V, Corinaldesi R. The Rome II criteria for patients with functional gastroduodenal disorders. J Clin Gastroenterol 2003;37:92-93.
13. Chey WD, Olden K, Carter E, Boyle J, Drossman D, Chang L. Utility of the Rome I and Rome II criteria for irritable bowel syndrome in US women. Am J Gastroenterol 2002;97:2803-2811.
14. Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol 1999;94:2912-2917.
15. Hamm LR, Sorrells SC, Harding JP, et al. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am J Gastroenterol 1999;94:1279-1282.
16. Tolliver BA, Herrara JL, DiPalma JA. Evaluation of patients who meet clinical criteria for irritable bowel syndrome. Am J Gastroenterol 1994;89:176-178.
17. MacIntosh DG, Thompson WG, Patel DP, Barr R, Guindi M. Is rectal biopsy necessary in irritable bowel syndrome? Am J Gastroenterol 1992;87:1407-1409.
18. Francis CY, Duffy JN, Whorwell PJ, Martin DF. Does routine ultrasound enhance diagnostic accuracy in irritable bowel syndrome? Am J Gastroenterol 1996;91:1348-1350.
19. Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult celiac disease with irritable bowel syndrome: a case-control study in patients fulfilling Rome II criteria referred to secondary care. Lancet 2001;358:1504-1508.
20. Pimental M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome; a double blind, randomized, placebo-controlled trial. Am J Gastroenterol 2003;98:412-419.
21. Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002;97:2812-2819.
22. O’Leary CO, Wieneke P, Buckley S, et al. Celiac disease and irritable bowel-type symptoms. Am J Gastroenterol 2002;97:1463-1467.
23. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastrenterol 2002;97:s1-s5.
24. Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, et al. Systematic review on the management of irritable bowel syndrome in north America. Am J Gastroenterol 2002;97:s7-s26.
25. Drossman DA, Patrick DL, Whitehead WE, NE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol 2000;95:999-1007.
26. Jones BW, Moore DJ, Robinson SM, Song F. A systematic review of tegaserod for the treatment of irritable bowel syndrome. J Clin Pharm Therapeutics 2002;27:343-352.
27. Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2002;16:1877-1888.
28. Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther 2001;15:1655-1666.
29. Kellow J, Lee OY, Chang FY, et al. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut 2003;52:671-676.
30. Jones RH, Holtmann G, Rodrigo L, Ehsanullah, Crompton PM, Jacques LA, Mills JG. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther 1999;13:1419-1427.
31. Jailwala J, Imperiale TF, Kroeneke K. Pharmacologic treatment of the irritable bowel syndrome: A systematic review of randomized, controlled trials. Ann Intern Med 2000;133:136-147.
32. Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2002;15:79-86.
33. Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet 2000;355:1035-1040.
34. Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther 1999;13:1149-1159.
35. Lembo T, Wright RA, Bagby B, et al. Lotronex Investigator Team. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predom-inant irritable bowel syndrome. Am J Gastroenterol 2001;96:2662-2670.
36. Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomized control trials. Gut 2001;48:272-282.
37. Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroeneke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a metaanalysis. Am J Med 2000;108:65-72.
38. Myren J, Lovland B, Larssen SE, Larsen S. A double-blind study of the effect of trimipramine in patients with the irritable bowel syndrome. Scand J Gastroenterol 1984;19:835-843.
39. Greenbaum DS, Mayle JE, Vanegeren LE, et al. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Dig Dis Sci 1987;32:257-266.
40. Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci 1984;29:239-247.
41. Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl 1987;130:81-84.
42. Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol 1996;31:463-468.
43. Ritchie JA, Truelove SC. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br Med J 1979;1:376-378.
44. Parisi GC, Zilli M, Miani MP, E, et al. High-fiber diet supplementation in patients with irritable bowel syndrome. A multi-center, randomized, open trial comparison between wheat bran diet and partially hydrolyzed guar gum. Dig Dis Sci 2002;47:1697-1704.
45. Golechha AC, Chadda VS, Chadda S, Sharma SK, Mishra SN. Role of ispaghula husk in the management of irritable bowel syndrome (A randomized double-blind crossover study). JAPI 1982;30:353-354.
46. Arthurs Y, Fielding JF. Double blind trial of ispaghula/poloxamer in the irritable bowel syndrome. BMJ 1983;76:253.-
47. Jalihal A, Kurian G. Ispaghula therapy in irritable bowel syndrome: Improvement in overall well being is related to reduction in bowel dissatisfaction. J Gastroenterol Hep 1990;5:507-513.
48. Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in irritable bowel syndrome. Arch Intern Med 2003;163:265-274.
49. Pittler MH, Ernst E. Peppermint Oil for irritable bowel syndrome: A critical review and meta-analysis. Am J Gastroenterol 1998;93:1131-1135.
50. Creed F, Fernandes L, Guthrie E, et al. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003;124:303-317.
51. Kuiken SD, Tytgat GNJ, Boeckxstaens GEE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: A double blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol 2003;1:219-228.
52. Heymann-Monnikes I, Arnold R, Florin I, et al. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
53. Talley NJ, Owen BK, Boyce P, Paterson K. Psychological treatments for irritable bowel syndrome: A critique of controlled treatment trials. Am J Gastroenterol 1996;91:277-286.
54. Svedlund J, Sjodin I, Ottoson JO, Dotevall G. Controlled study of psychotherapy in irritable bowel syndrome. Lancet 1983;2:589-592.
55. Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consulting Clinic Psychology 1994;62:576-582.
56. Guthrie E, Creed F, Dawson D, Tomenson B. A ramdomized controlled trial of psychotherapy in patients with refractory irritable bowel syndrome. B J Psychiatry 1993;163:315-321.
57. Heymann-Monnikes I, Arnold R, Florin I, Herda C, Melfsen S, Monnikes H. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
- For patients aged <50 years without alarm symptoms, diagnostic testing is unnecessary. Consider celiac sprue testing for patients with diarrhea (C).
- Treatment is indicated when both the patient with irritable bowel syndrome and the physician agree that quality of life has been diminished (C). The goal of therapy is to alleviate global IBS symptoms (abdominal discomfort, bloating, and altered bowel habits that are life-impacting) (C).
- Tegaserod, a 5HT4 receptor agonist, is more effective than placebo at relieving global IBS symptoms in women with constipation (A). Its effectiveness in men is unknown.
- Alosetron, a 5HT3 receptor antagonist, is more effective than placebo at relieving global IBS symptoms in women with diarrhea (A).
- Behavior therapy–relaxation therapy, hypnotherapy, or cognitive therapy–is more effective than placebo at relieving individual symptoms, but no data are available for quality-of-life improvement (B).
An extensive and expensive evaluation for irritable bowel syndrome (IBS) can be avoided if your patient is aged <50 years and is not experiencing alarm symptoms (hematochezia, 10 lbs weight loss, fever, anemia, nocturnal or severe diarrhea), has not recently taken antibiotics, and has no family history of colon cancer. An algorithm ( Figure ) indicates when work-up is needed and what it should entail.
Newer medications that act on 5HT receptors have proven effective in improving quality of life (global symptom reduction). Evidence supports the use of several traditional medications to reduce individual symptoms of IBS, but not for global symptom reduction.
FIGURE
Evaluating possible irritable bowel syndrome
Who gets irritable bowel syndrome?
Ten percent to 15% of the North American population has IBS, and twice as many women as men have it.1 Symptoms usually begin before the age of 35 years, and many patients can trace their symptoms back to childhood.2 Onset in the elderly is rare.3 The disorder is responsible for approximately 50% of referrals to gastroenterologists.4
The company IBS keeps
Comorbid psychiatric illness is common with IBS, but few patients seek psychiatric care.5 Depression, anxiety, and somatoform disorders are seen in 94% of patients with IBS. IBS is common in patients with chronic fatigue syndrome (51%), fibromyalgia (49%), temporomandibular joint syndrome (64%), and chronic pelvic pain (50%).6 IBS often follows stressful life events,5,7,8 such as a death in the family or divorce. It tends to be chronic, intermittent, and relapsing.3
The symptoms of IBS can overlap with those of other illnesses, including thyroid dysfunction (diarrhea or constipation), functional dyspepsia (abdominal pain), Crohn’s disease or ulcerative colitis (diarrhea, abdominal pain), celiac sprue (diarrhea), polyps and cancers (constipation or abdominal pain), and infectious diarrhea.
Elusive physiologic mechanism
Several physiologic mechanisms have been proposed for IBS symptoms: altered gut reactivity in response to luminal stimuli, hypersensitive gut with enhanced pain response, and altered brain-gut biochemical axis.9 Though the symptoms of irritable bowel syndrome appear to have a physiologic basis, there are no structural or biochemical markers for the disease.
Use symptom-based criteria for diagnosis
Consider a diagnosis of IBS when a patient complains of abdominal discomfort and altered bowel habits. In the absence of a structural or biochemical marker, IBS must be diagnosed according to symptom-based criteria–such as Manning, Rome I, or Rome II–which have been developed for research and epidemiologic purposes. Though their clinical utility remains unproven, these criteria (delineated in Table 1 ) are the crux of clinical diagnosis for IBS.4,10-14 Subtypes of IBS have been described (diarrheapredominant IBS or constipation-predominant IBS), but they are not diagnostically useful, since the treatment goal is improved quality of life.
TABLE 1
Symptom-based criteria for irritable bowel syndrome
| Symptom-based criteria | Symptoms | Sn | Sp | PV+ |
|---|---|---|---|---|
| Manning4,10,13,14 |
| 42%–90% | 70%–100% | 74% |
| Rome I4,10,13 |
| 65%–84% | 100% | 69%–100% |
| Rome II11-13 |
| 49%-65%* | 100%* | 69%-100%* |
| Supportive symptoms | ||||
| Fewer than 3 bowel movements per week | ||||
| More than 3 bowel movements per day | ||||
| Hard or lumpy stools | ||||
| *Found to have similar sensitivity and specificity to Rome I.14 | ||||
| Sn, sensitivity; Sp, specificity; PV+, positive predictive value | ||||
Dubious value of diagnostic tests
The literature regarding the value of diagnostic testing for IBS is controversial. Symptom-based criteria have varied in many studies, as have the criteria used to enroll patients and the measured outcomes of treatment (reduction in abdominal pain, in diarrhea, or in constipation, or improvement in quality of life). Because of these discrepancies, it is difficult to apply the literature clinically. Of the 6 landmark studies that considered the value of diagnostic testing for IBS patients,15-20 only 2 compared IBS patients with groups of healthy controls.17,19
Test results yield little. Most research in this area has compared the prevalence of specific illnesses in the general population with the yield of positive test results for these illnesses among persons meeting the symptom-based diagnostic criteria for IBS.
Two studies15,16 determined the incidence of abnormal test results in patients who met the Manning or Rome I criteria for IBS. In these studies, most diagnostic tests yielded positive results in 2% (range, 0%-8.2%) of patients or less, except for thyroid and lactose intolerance testing. That is equivalent to the incidence in the general population. The prevalence of thyroid disorders and lactose malabsorption was higher in IBS patients (6% and 22%-26%, respectively), but prevalence in the general population is similarly higher (5%-9% and 25%). Based on these results, testing for thyroid disease or lactose malabsorption is indicated only for patients exhibiting symptoms of these disorders (fatigue/weight change or diarrhea related to diertary intake of dairy products, respectively).
An exception. Some clinicians propose that diagnostic testing for patients with IBS symptoms should be driven by the pretest probability of organic disease (prevalence) compared with the general population. Cash21 found the pretest probability of inflammatory bowel disease, colorectal cancer, and infectious diarrhea is less than 1% among IBS patients without alarm symptoms ( Table 2 ). He demonstrated that patients with IBS had a 5% pretest probability of celiac sprue compared with healthy patients (<1% prevalence). Therefore, testing for celiac sprue (eg, complete blood count, antiendomysial antibody, and antigliadin antibody) may be considered for patients with diarrhea.6,21,22 Sigmoidoscopy,15,17 rectal biopsy,17 and abdominal ultrasound18 have low positive yield in patients meeting the diagnostic criteria for IBS.
TABLE 2
Probability of organic disease in irritable bowel syndrome patients
| Disease | Pretest probability-IBS patients (%) | Prevalence-general population (%) | Comments |
|---|---|---|---|
| Colitis/inflammatory inflammatory bowel disease | 0.51-0.98 | 0.3-1.2 | Structural colon lesions were detected with barium enema, colonscopy, sigmoidoscopy |
| Colon cancer | 0-0.51 | 4-6 | Structural colon lesions were detected with barium enema, colonoscopy, sigmoidoscopy |
| Celiac disease | 4.67 | 0.25-0.5 | Note: celiac disease prevalence higher than in general population. |
| Gastrointestinal infection | 0-1.7 | N/A | |
| Thyroid dysfunction | 6 | 5-9 | Prevalence high in both groups |
| Lactose malabsorption | 22-26 | 25 | Prevalence high in both groups |
| Adapted from: Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002; 97:2812-2819. | |||
| Results are from multiple studies: n=125-306. | |||
How to proceed
Those under 50 years of age who have no alarm symptoms can forgo further testing. Testing for celiac sprue and lactose malabsorption might be considered for patients with diarrhea that improves or worsens with change in diet (strength of recommendation [SOR]: C).
Threshold for treatment
Treatment for IBS is indicated when both patient and physician believe global symptoms (abdominal discomfort, bloating, altered bowel habits) have diminished the quality of life (SOR: C). The goal of treatment is to alleviate all IBS symptoms (SOR: C). Treating altered bowel habits (constipation, diarrhea, and fecal urgency) without addressing other IBS symptoms (eg, abdominal pain) is inferior treatment.23,24
Treatment options for IBS
Treatments for IBS include medications, behavior therapy, and complimentary and alternative therapies. Medications traditionally prescribed include bulking agents, anticholinergics/antispasmodics, antidiarrheals, and antidepressants. A 5HT3 receptor antagonist and a 5HT4 receptor partial agonist are now available. Table 3 summarizes the traditional treatments in terms of efficacy, strength of recommendations, and outcomes measured. Alternative and complimentary therapies appear in Table 4 .
As Brandt24 has noted, the evidence for treatment effectiveness is difficult to review and summarize, because the quality of studies has been poor. Most studies did not use healthy control groups, and the numbers of participants were small. Many studies did not use blinded placebo groups. Outcomes measured varied among the studies, with most of them measuring reductions of individual bowel symptoms (eg, diarrhea or constipation). Quality-of-life tools were used in other studies to measure reduction in global IBS symptoms (eg, IBS Quality of Life 25 ). Because of these discrepancies, there is no sound evidence for traditional therapies.
TABLE 3
Treatments for irritable bowel syndrome
| Treatment | Efficacy (NNT) | SOR (studies) | Outcomes measured | Comments |
|---|---|---|---|---|
| 5HT4 receptor agonist (tegaserod)23,24,26-30 | More effective than placebo at relieving global IBS symptoms in women with constipation (3.9-17) | A (4) | Global IBS symptoms, individual IBS symptoms | 83%-100% of study participants were women with IBS and constipation. Rome I and II criteria for entry. May cause diarrhea |
| 5HT4 receptor agonist(alosetron)23,24,26- 35 | More effective than placebo at relieving global IBS symptoms in women with diarrhea (2.5-8.3) | A (4) | Global IBS symptoms, individual IBS symptoms, adverse events | 82%-93% of study participants were women. Rome I and II criteria for entry. May cause severe constipation; restricted use |
| Tricylic antidepressants (trimipramine, desipramine)23,24,36- 39 | Reduces abdominal pain. No more effective than placebo at relieving gloal IBS symptoms (3.2-5) | B (6) | GI symptoms | May cause constipation; no studies done with SSRIs |
| Loperamide23,24,36-39 | Relieves diarrhea. No more effective than placebo at relieving global IBS symptoms (3.2-5) | B (3) | Global IBS symptoms, diarrhea | Constipation or paralytic ileus can occur |
| Bulking agents (corn fiber, wheat bran, psyllium, ispaghula husks, calcium polycabophil)23,24,31,40-42 | Improves constipation. No more effective than placebo in studies considering global symptom improvement (2.2-8.6) | B (13) | GI symptoms, global IBS symptoms | May increase bloating. All studiessmall numbers of patients |
| Anti-spasmodics (hyoscyamine dicyclomine)23,24,26-30 | No evidence on improvement of global IBS symptoms (5.9) | B (3) | Individual IBS and global symptoms | Studies were short, small numbers, inconsistent effectiveness. Could worsen constipation; 15 additional studies done on drugs not available in the US |
| Behavioral therapies (hypnotherapy, relaxation therapy, psychotherapy, biofeedback)23,24,44, 52-57 | More effective than placebo at relieving individuals IBS symptoms (1.4-1.9) | B (16) | GI symptoms, psychological sypmtoms | None measures global IBS symptom improvement. Small numbers of patients |
| SSRI antidepressants (paroxtetine, fluoxetine)23,24, 50-51 | Improved quality of life, decreased abdominal pain | B (16) | Abdominal | One study severe IBS, other study only 10 participants quality of life |
| SOR, strength of recommendation; IBS, irritable bowel syndrome; GI, gastrointestinal; SSRI, selective serotonin reuptake inhibitor. For an explanation of SORs. | ||||
TABLE 4
Complementary and alternative treatments for irritable bowel syndrome
| Treatment | Efficacy | SOR | Outcomes measured | Comments |
|---|---|---|---|---|
| Neomycin 20 | Treatment for 1 week improved symptoms of abdominal pain, diarrhea, and constipation | A | Abdominal pain, diarrhea, or constipation | Studies measuring global symptom improvement lacking |
| Peppermint oil 31,4849 | Some demonstrated improvement in abdominal pain | B | Individual IBS symptoms | Studies measuring global symptom improvement lacking |
| Guar gum 44 | Improved abdominal pain and bowel alterations | B | Study compared fiber to guar gum–equal affect on abdominal pain. Gum was better toleratEd | No placebo-controlled trials |
| Probiotics48 (lactobacillus) | Improvement of abdominal pain and flatulence | C | Abdominal pain, flatulence | Two studies with small numbers |
| Elimination diets 48 | Improvement of diarrhea | C | Diarrhea | Milk, wheat, eggs eliminated; 15%-71% improvement of diarrhea |
| Lactose and fructose avoidance 48 | Conflicting evidence results | D | No controlled studies available | |
| Pancreatic enzymes 48 | No evidence | D | Evidence lacking | |
| Ginger 48 | No evidence | D | No studies |
Medications
Strength of recommendation: A. The recently approved 5HT4 receptor agonist tegaserod (Zelnorm) is more effective than placebo at relieving global symptoms in women with constipation (number needed to treat [NNT]=3.9-17).26-30 Diarrhea can be a serious side effect.
The 5HT3 receptor antagonist alosetron (Lotronex) is more effective than placebo at relieving global IBS symptoms in women with diarrhea (NNT=2.5-8.3).31-35 Severe constipation can be an adverse effect. The prescribing of alosetron is currently restricted to physicians who participate in the manufacturer’s risk management program.
In addition to these serotoninergic agents, others in this class are being developed and undergoing clinical trials. The knowledge being gained about 5HT receptors may revolutionize the care of patients with IBS.
Strength of recommendation: B. Tricyclic antidepressants are no more effective than placebo at relieving global IBS symptoms, but they do decrease abdominal pain (NNT=3.2-5).36-39
Loperamide is no more effective than placebo at relieving IBS global symptoms, but it may be used to treat diarrhea (NNT=2.3-5).31,40-42
Bulking agents (such as calcium polycarbophil or psyllium) are no more effective than placebo at relieving IBS global symptoms, but they may decrease constipation (NNT=2.2-8.6).31,36,43-47
Peppermint oil may be helpful for abdominal pain, but global symptom reduction has not been demonstrated.31,48-49 Only a few studies have looked at the use of antispasmodic agents for IBS. They are of poor quality and used small numbers with no placebo controls.23,31,36,43
Strength of recommendation: C. There are limited studies evaluating the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and paroxetine. Paroxetine was shown in 1 study to improve quality of life.50 Fluoxetine reduced abdominal pain, but did not improve quality of life.51
Behavioral and complementary/alternative therapies
Relaxation therapy, hypnotherapy, and cognitive therapy are effective at relieving individual IBS symptoms, but have not been shown to reduce global IBS symptoms (SOR: B).52-57 Other alternative therapies (eg, guar gum44 [SOR: B], ginger48 [SOR: B], and pancreatic enzymes48 [SOR: C]) have been studied, but high-quality studies considering global improvement have not been published.
The position statement of the American College of Gastroenterology on the management of IBS23 and Brandt’s systematic review of this subject24 were the starting points for this review. The majority of the references from these sources were reviewed and a Medline search was completed to identify new evidence. The Oxford Centre for Evidence-Based Medicine grades of recommendations were applied to this evidence, a care algorithm was created, summary tables were developed, and numbers needed to treat were calculated.
Promote self-awareness
Quality-of-life assessment should be done routinely in the care of IBS patients. Provide support, empathy, and basic behavior modification tools. Educate patients and their families on the theoretical biochemical basis of this illness, and help them connect symptoms with stressors, to facilitate lifestyle modification.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- For patients aged <50 years without alarm symptoms, diagnostic testing is unnecessary. Consider celiac sprue testing for patients with diarrhea (C).
- Treatment is indicated when both the patient with irritable bowel syndrome and the physician agree that quality of life has been diminished (C). The goal of therapy is to alleviate global IBS symptoms (abdominal discomfort, bloating, and altered bowel habits that are life-impacting) (C).
- Tegaserod, a 5HT4 receptor agonist, is more effective than placebo at relieving global IBS symptoms in women with constipation (A). Its effectiveness in men is unknown.
- Alosetron, a 5HT3 receptor antagonist, is more effective than placebo at relieving global IBS symptoms in women with diarrhea (A).
- Behavior therapy–relaxation therapy, hypnotherapy, or cognitive therapy–is more effective than placebo at relieving individual symptoms, but no data are available for quality-of-life improvement (B).
An extensive and expensive evaluation for irritable bowel syndrome (IBS) can be avoided if your patient is aged <50 years and is not experiencing alarm symptoms (hematochezia, 10 lbs weight loss, fever, anemia, nocturnal or severe diarrhea), has not recently taken antibiotics, and has no family history of colon cancer. An algorithm ( Figure ) indicates when work-up is needed and what it should entail.
Newer medications that act on 5HT receptors have proven effective in improving quality of life (global symptom reduction). Evidence supports the use of several traditional medications to reduce individual symptoms of IBS, but not for global symptom reduction.
FIGURE
Evaluating possible irritable bowel syndrome
Who gets irritable bowel syndrome?
Ten percent to 15% of the North American population has IBS, and twice as many women as men have it.1 Symptoms usually begin before the age of 35 years, and many patients can trace their symptoms back to childhood.2 Onset in the elderly is rare.3 The disorder is responsible for approximately 50% of referrals to gastroenterologists.4
The company IBS keeps
Comorbid psychiatric illness is common with IBS, but few patients seek psychiatric care.5 Depression, anxiety, and somatoform disorders are seen in 94% of patients with IBS. IBS is common in patients with chronic fatigue syndrome (51%), fibromyalgia (49%), temporomandibular joint syndrome (64%), and chronic pelvic pain (50%).6 IBS often follows stressful life events,5,7,8 such as a death in the family or divorce. It tends to be chronic, intermittent, and relapsing.3
The symptoms of IBS can overlap with those of other illnesses, including thyroid dysfunction (diarrhea or constipation), functional dyspepsia (abdominal pain), Crohn’s disease or ulcerative colitis (diarrhea, abdominal pain), celiac sprue (diarrhea), polyps and cancers (constipation or abdominal pain), and infectious diarrhea.
Elusive physiologic mechanism
Several physiologic mechanisms have been proposed for IBS symptoms: altered gut reactivity in response to luminal stimuli, hypersensitive gut with enhanced pain response, and altered brain-gut biochemical axis.9 Though the symptoms of irritable bowel syndrome appear to have a physiologic basis, there are no structural or biochemical markers for the disease.
Use symptom-based criteria for diagnosis
Consider a diagnosis of IBS when a patient complains of abdominal discomfort and altered bowel habits. In the absence of a structural or biochemical marker, IBS must be diagnosed according to symptom-based criteria–such as Manning, Rome I, or Rome II–which have been developed for research and epidemiologic purposes. Though their clinical utility remains unproven, these criteria (delineated in Table 1 ) are the crux of clinical diagnosis for IBS.4,10-14 Subtypes of IBS have been described (diarrheapredominant IBS or constipation-predominant IBS), but they are not diagnostically useful, since the treatment goal is improved quality of life.
TABLE 1
Symptom-based criteria for irritable bowel syndrome
| Symptom-based criteria | Symptoms | Sn | Sp | PV+ |
|---|---|---|---|---|
| Manning4,10,13,14 |
| 42%–90% | 70%–100% | 74% |
| Rome I4,10,13 |
| 65%–84% | 100% | 69%–100% |
| Rome II11-13 |
| 49%-65%* | 100%* | 69%-100%* |
| Supportive symptoms | ||||
| Fewer than 3 bowel movements per week | ||||
| More than 3 bowel movements per day | ||||
| Hard or lumpy stools | ||||
| *Found to have similar sensitivity and specificity to Rome I.14 | ||||
| Sn, sensitivity; Sp, specificity; PV+, positive predictive value | ||||
Dubious value of diagnostic tests
The literature regarding the value of diagnostic testing for IBS is controversial. Symptom-based criteria have varied in many studies, as have the criteria used to enroll patients and the measured outcomes of treatment (reduction in abdominal pain, in diarrhea, or in constipation, or improvement in quality of life). Because of these discrepancies, it is difficult to apply the literature clinically. Of the 6 landmark studies that considered the value of diagnostic testing for IBS patients,15-20 only 2 compared IBS patients with groups of healthy controls.17,19
Test results yield little. Most research in this area has compared the prevalence of specific illnesses in the general population with the yield of positive test results for these illnesses among persons meeting the symptom-based diagnostic criteria for IBS.
Two studies15,16 determined the incidence of abnormal test results in patients who met the Manning or Rome I criteria for IBS. In these studies, most diagnostic tests yielded positive results in 2% (range, 0%-8.2%) of patients or less, except for thyroid and lactose intolerance testing. That is equivalent to the incidence in the general population. The prevalence of thyroid disorders and lactose malabsorption was higher in IBS patients (6% and 22%-26%, respectively), but prevalence in the general population is similarly higher (5%-9% and 25%). Based on these results, testing for thyroid disease or lactose malabsorption is indicated only for patients exhibiting symptoms of these disorders (fatigue/weight change or diarrhea related to diertary intake of dairy products, respectively).
An exception. Some clinicians propose that diagnostic testing for patients with IBS symptoms should be driven by the pretest probability of organic disease (prevalence) compared with the general population. Cash21 found the pretest probability of inflammatory bowel disease, colorectal cancer, and infectious diarrhea is less than 1% among IBS patients without alarm symptoms ( Table 2 ). He demonstrated that patients with IBS had a 5% pretest probability of celiac sprue compared with healthy patients (<1% prevalence). Therefore, testing for celiac sprue (eg, complete blood count, antiendomysial antibody, and antigliadin antibody) may be considered for patients with diarrhea.6,21,22 Sigmoidoscopy,15,17 rectal biopsy,17 and abdominal ultrasound18 have low positive yield in patients meeting the diagnostic criteria for IBS.
TABLE 2
Probability of organic disease in irritable bowel syndrome patients
| Disease | Pretest probability-IBS patients (%) | Prevalence-general population (%) | Comments |
|---|---|---|---|
| Colitis/inflammatory inflammatory bowel disease | 0.51-0.98 | 0.3-1.2 | Structural colon lesions were detected with barium enema, colonscopy, sigmoidoscopy |
| Colon cancer | 0-0.51 | 4-6 | Structural colon lesions were detected with barium enema, colonoscopy, sigmoidoscopy |
| Celiac disease | 4.67 | 0.25-0.5 | Note: celiac disease prevalence higher than in general population. |
| Gastrointestinal infection | 0-1.7 | N/A | |
| Thyroid dysfunction | 6 | 5-9 | Prevalence high in both groups |
| Lactose malabsorption | 22-26 | 25 | Prevalence high in both groups |
| Adapted from: Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002; 97:2812-2819. | |||
| Results are from multiple studies: n=125-306. | |||
How to proceed
Those under 50 years of age who have no alarm symptoms can forgo further testing. Testing for celiac sprue and lactose malabsorption might be considered for patients with diarrhea that improves or worsens with change in diet (strength of recommendation [SOR]: C).
Threshold for treatment
Treatment for IBS is indicated when both patient and physician believe global symptoms (abdominal discomfort, bloating, altered bowel habits) have diminished the quality of life (SOR: C). The goal of treatment is to alleviate all IBS symptoms (SOR: C). Treating altered bowel habits (constipation, diarrhea, and fecal urgency) without addressing other IBS symptoms (eg, abdominal pain) is inferior treatment.23,24
Treatment options for IBS
Treatments for IBS include medications, behavior therapy, and complimentary and alternative therapies. Medications traditionally prescribed include bulking agents, anticholinergics/antispasmodics, antidiarrheals, and antidepressants. A 5HT3 receptor antagonist and a 5HT4 receptor partial agonist are now available. Table 3 summarizes the traditional treatments in terms of efficacy, strength of recommendations, and outcomes measured. Alternative and complimentary therapies appear in Table 4 .
As Brandt24 has noted, the evidence for treatment effectiveness is difficult to review and summarize, because the quality of studies has been poor. Most studies did not use healthy control groups, and the numbers of participants were small. Many studies did not use blinded placebo groups. Outcomes measured varied among the studies, with most of them measuring reductions of individual bowel symptoms (eg, diarrhea or constipation). Quality-of-life tools were used in other studies to measure reduction in global IBS symptoms (eg, IBS Quality of Life 25 ). Because of these discrepancies, there is no sound evidence for traditional therapies.
TABLE 3
Treatments for irritable bowel syndrome
| Treatment | Efficacy (NNT) | SOR (studies) | Outcomes measured | Comments |
|---|---|---|---|---|
| 5HT4 receptor agonist (tegaserod)23,24,26-30 | More effective than placebo at relieving global IBS symptoms in women with constipation (3.9-17) | A (4) | Global IBS symptoms, individual IBS symptoms | 83%-100% of study participants were women with IBS and constipation. Rome I and II criteria for entry. May cause diarrhea |
| 5HT4 receptor agonist(alosetron)23,24,26- 35 | More effective than placebo at relieving global IBS symptoms in women with diarrhea (2.5-8.3) | A (4) | Global IBS symptoms, individual IBS symptoms, adverse events | 82%-93% of study participants were women. Rome I and II criteria for entry. May cause severe constipation; restricted use |
| Tricylic antidepressants (trimipramine, desipramine)23,24,36- 39 | Reduces abdominal pain. No more effective than placebo at relieving gloal IBS symptoms (3.2-5) | B (6) | GI symptoms | May cause constipation; no studies done with SSRIs |
| Loperamide23,24,36-39 | Relieves diarrhea. No more effective than placebo at relieving global IBS symptoms (3.2-5) | B (3) | Global IBS symptoms, diarrhea | Constipation or paralytic ileus can occur |
| Bulking agents (corn fiber, wheat bran, psyllium, ispaghula husks, calcium polycabophil)23,24,31,40-42 | Improves constipation. No more effective than placebo in studies considering global symptom improvement (2.2-8.6) | B (13) | GI symptoms, global IBS symptoms | May increase bloating. All studiessmall numbers of patients |
| Anti-spasmodics (hyoscyamine dicyclomine)23,24,26-30 | No evidence on improvement of global IBS symptoms (5.9) | B (3) | Individual IBS and global symptoms | Studies were short, small numbers, inconsistent effectiveness. Could worsen constipation; 15 additional studies done on drugs not available in the US |
| Behavioral therapies (hypnotherapy, relaxation therapy, psychotherapy, biofeedback)23,24,44, 52-57 | More effective than placebo at relieving individuals IBS symptoms (1.4-1.9) | B (16) | GI symptoms, psychological sypmtoms | None measures global IBS symptom improvement. Small numbers of patients |
| SSRI antidepressants (paroxtetine, fluoxetine)23,24, 50-51 | Improved quality of life, decreased abdominal pain | B (16) | Abdominal | One study severe IBS, other study only 10 participants quality of life |
| SOR, strength of recommendation; IBS, irritable bowel syndrome; GI, gastrointestinal; SSRI, selective serotonin reuptake inhibitor. For an explanation of SORs. | ||||
TABLE 4
Complementary and alternative treatments for irritable bowel syndrome
| Treatment | Efficacy | SOR | Outcomes measured | Comments |
|---|---|---|---|---|
| Neomycin 20 | Treatment for 1 week improved symptoms of abdominal pain, diarrhea, and constipation | A | Abdominal pain, diarrhea, or constipation | Studies measuring global symptom improvement lacking |
| Peppermint oil 31,4849 | Some demonstrated improvement in abdominal pain | B | Individual IBS symptoms | Studies measuring global symptom improvement lacking |
| Guar gum 44 | Improved abdominal pain and bowel alterations | B | Study compared fiber to guar gum–equal affect on abdominal pain. Gum was better toleratEd | No placebo-controlled trials |
| Probiotics48 (lactobacillus) | Improvement of abdominal pain and flatulence | C | Abdominal pain, flatulence | Two studies with small numbers |
| Elimination diets 48 | Improvement of diarrhea | C | Diarrhea | Milk, wheat, eggs eliminated; 15%-71% improvement of diarrhea |
| Lactose and fructose avoidance 48 | Conflicting evidence results | D | No controlled studies available | |
| Pancreatic enzymes 48 | No evidence | D | Evidence lacking | |
| Ginger 48 | No evidence | D | No studies |
Medications
Strength of recommendation: A. The recently approved 5HT4 receptor agonist tegaserod (Zelnorm) is more effective than placebo at relieving global symptoms in women with constipation (number needed to treat [NNT]=3.9-17).26-30 Diarrhea can be a serious side effect.
The 5HT3 receptor antagonist alosetron (Lotronex) is more effective than placebo at relieving global IBS symptoms in women with diarrhea (NNT=2.5-8.3).31-35 Severe constipation can be an adverse effect. The prescribing of alosetron is currently restricted to physicians who participate in the manufacturer’s risk management program.
In addition to these serotoninergic agents, others in this class are being developed and undergoing clinical trials. The knowledge being gained about 5HT receptors may revolutionize the care of patients with IBS.
Strength of recommendation: B. Tricyclic antidepressants are no more effective than placebo at relieving global IBS symptoms, but they do decrease abdominal pain (NNT=3.2-5).36-39
Loperamide is no more effective than placebo at relieving IBS global symptoms, but it may be used to treat diarrhea (NNT=2.3-5).31,40-42
Bulking agents (such as calcium polycarbophil or psyllium) are no more effective than placebo at relieving IBS global symptoms, but they may decrease constipation (NNT=2.2-8.6).31,36,43-47
Peppermint oil may be helpful for abdominal pain, but global symptom reduction has not been demonstrated.31,48-49 Only a few studies have looked at the use of antispasmodic agents for IBS. They are of poor quality and used small numbers with no placebo controls.23,31,36,43
Strength of recommendation: C. There are limited studies evaluating the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and paroxetine. Paroxetine was shown in 1 study to improve quality of life.50 Fluoxetine reduced abdominal pain, but did not improve quality of life.51
Behavioral and complementary/alternative therapies
Relaxation therapy, hypnotherapy, and cognitive therapy are effective at relieving individual IBS symptoms, but have not been shown to reduce global IBS symptoms (SOR: B).52-57 Other alternative therapies (eg, guar gum44 [SOR: B], ginger48 [SOR: B], and pancreatic enzymes48 [SOR: C]) have been studied, but high-quality studies considering global improvement have not been published.
The position statement of the American College of Gastroenterology on the management of IBS23 and Brandt’s systematic review of this subject24 were the starting points for this review. The majority of the references from these sources were reviewed and a Medline search was completed to identify new evidence. The Oxford Centre for Evidence-Based Medicine grades of recommendations were applied to this evidence, a care algorithm was created, summary tables were developed, and numbers needed to treat were calculated.
Promote self-awareness
Quality-of-life assessment should be done routinely in the care of IBS patients. Provide support, empathy, and basic behavior modification tools. Educate patients and their families on the theoretical biochemical basis of this illness, and help them connect symptoms with stressors, to facilitate lifestyle modification.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Saito YA, Schoenfeld P, Locke R. The epidemiology of irritable bowel syndrome in North America: A systematic review. Am J Gastrenterol 2002;97:1910-1915.
2. Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 1995;109:1736-1741.
3. Maxwell PR, Mendall MA, Kumar D. Irritable bowel syndrome. Lancet 1997;350:1691-1695.
4. Fass R, Longstreth GF, Pimental M, et al. Evidence- and consensus-based practice guidelines for the diagnosis of irritable bowel syndrome. Arch Intern Med 2001;161:2081-2088.
5. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry 1997;42:835-840.
6. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology 2002;122:1140-1156.
7. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am 2000;84:1313-1327.
8. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 2000;160:221-227.
9. Camilleri M, Prather CM. The irritable bowel syndrome: mechanisms and a practical approach to management. Ann Internal Med 1992;116:1001-1008.
10. Paterson WG, Thompson WG, Vanner SJ, et al. Recommendations for the management of irritable bowel syndrome in family practice. JAMC 1999;161:154-160.
11. Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology 2002;122:1701-1714.
12. Tosetti C, Stanghellini V, Corinaldesi R. The Rome II criteria for patients with functional gastroduodenal disorders. J Clin Gastroenterol 2003;37:92-93.
13. Chey WD, Olden K, Carter E, Boyle J, Drossman D, Chang L. Utility of the Rome I and Rome II criteria for irritable bowel syndrome in US women. Am J Gastroenterol 2002;97:2803-2811.
14. Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol 1999;94:2912-2917.
15. Hamm LR, Sorrells SC, Harding JP, et al. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am J Gastroenterol 1999;94:1279-1282.
16. Tolliver BA, Herrara JL, DiPalma JA. Evaluation of patients who meet clinical criteria for irritable bowel syndrome. Am J Gastroenterol 1994;89:176-178.
17. MacIntosh DG, Thompson WG, Patel DP, Barr R, Guindi M. Is rectal biopsy necessary in irritable bowel syndrome? Am J Gastroenterol 1992;87:1407-1409.
18. Francis CY, Duffy JN, Whorwell PJ, Martin DF. Does routine ultrasound enhance diagnostic accuracy in irritable bowel syndrome? Am J Gastroenterol 1996;91:1348-1350.
19. Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult celiac disease with irritable bowel syndrome: a case-control study in patients fulfilling Rome II criteria referred to secondary care. Lancet 2001;358:1504-1508.
20. Pimental M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome; a double blind, randomized, placebo-controlled trial. Am J Gastroenterol 2003;98:412-419.
21. Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002;97:2812-2819.
22. O’Leary CO, Wieneke P, Buckley S, et al. Celiac disease and irritable bowel-type symptoms. Am J Gastroenterol 2002;97:1463-1467.
23. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastrenterol 2002;97:s1-s5.
24. Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, et al. Systematic review on the management of irritable bowel syndrome in north America. Am J Gastroenterol 2002;97:s7-s26.
25. Drossman DA, Patrick DL, Whitehead WE, NE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol 2000;95:999-1007.
26. Jones BW, Moore DJ, Robinson SM, Song F. A systematic review of tegaserod for the treatment of irritable bowel syndrome. J Clin Pharm Therapeutics 2002;27:343-352.
27. Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2002;16:1877-1888.
28. Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther 2001;15:1655-1666.
29. Kellow J, Lee OY, Chang FY, et al. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut 2003;52:671-676.
30. Jones RH, Holtmann G, Rodrigo L, Ehsanullah, Crompton PM, Jacques LA, Mills JG. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther 1999;13:1419-1427.
31. Jailwala J, Imperiale TF, Kroeneke K. Pharmacologic treatment of the irritable bowel syndrome: A systematic review of randomized, controlled trials. Ann Intern Med 2000;133:136-147.
32. Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2002;15:79-86.
33. Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet 2000;355:1035-1040.
34. Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther 1999;13:1149-1159.
35. Lembo T, Wright RA, Bagby B, et al. Lotronex Investigator Team. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predom-inant irritable bowel syndrome. Am J Gastroenterol 2001;96:2662-2670.
36. Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomized control trials. Gut 2001;48:272-282.
37. Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroeneke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a metaanalysis. Am J Med 2000;108:65-72.
38. Myren J, Lovland B, Larssen SE, Larsen S. A double-blind study of the effect of trimipramine in patients with the irritable bowel syndrome. Scand J Gastroenterol 1984;19:835-843.
39. Greenbaum DS, Mayle JE, Vanegeren LE, et al. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Dig Dis Sci 1987;32:257-266.
40. Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci 1984;29:239-247.
41. Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl 1987;130:81-84.
42. Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol 1996;31:463-468.
43. Ritchie JA, Truelove SC. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br Med J 1979;1:376-378.
44. Parisi GC, Zilli M, Miani MP, E, et al. High-fiber diet supplementation in patients with irritable bowel syndrome. A multi-center, randomized, open trial comparison between wheat bran diet and partially hydrolyzed guar gum. Dig Dis Sci 2002;47:1697-1704.
45. Golechha AC, Chadda VS, Chadda S, Sharma SK, Mishra SN. Role of ispaghula husk in the management of irritable bowel syndrome (A randomized double-blind crossover study). JAPI 1982;30:353-354.
46. Arthurs Y, Fielding JF. Double blind trial of ispaghula/poloxamer in the irritable bowel syndrome. BMJ 1983;76:253.-
47. Jalihal A, Kurian G. Ispaghula therapy in irritable bowel syndrome: Improvement in overall well being is related to reduction in bowel dissatisfaction. J Gastroenterol Hep 1990;5:507-513.
48. Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in irritable bowel syndrome. Arch Intern Med 2003;163:265-274.
49. Pittler MH, Ernst E. Peppermint Oil for irritable bowel syndrome: A critical review and meta-analysis. Am J Gastroenterol 1998;93:1131-1135.
50. Creed F, Fernandes L, Guthrie E, et al. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003;124:303-317.
51. Kuiken SD, Tytgat GNJ, Boeckxstaens GEE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: A double blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol 2003;1:219-228.
52. Heymann-Monnikes I, Arnold R, Florin I, et al. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
53. Talley NJ, Owen BK, Boyce P, Paterson K. Psychological treatments for irritable bowel syndrome: A critique of controlled treatment trials. Am J Gastroenterol 1996;91:277-286.
54. Svedlund J, Sjodin I, Ottoson JO, Dotevall G. Controlled study of psychotherapy in irritable bowel syndrome. Lancet 1983;2:589-592.
55. Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consulting Clinic Psychology 1994;62:576-582.
56. Guthrie E, Creed F, Dawson D, Tomenson B. A ramdomized controlled trial of psychotherapy in patients with refractory irritable bowel syndrome. B J Psychiatry 1993;163:315-321.
57. Heymann-Monnikes I, Arnold R, Florin I, Herda C, Melfsen S, Monnikes H. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
1. Saito YA, Schoenfeld P, Locke R. The epidemiology of irritable bowel syndrome in North America: A systematic review. Am J Gastrenterol 2002;97:1910-1915.
2. Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 1995;109:1736-1741.
3. Maxwell PR, Mendall MA, Kumar D. Irritable bowel syndrome. Lancet 1997;350:1691-1695.
4. Fass R, Longstreth GF, Pimental M, et al. Evidence- and consensus-based practice guidelines for the diagnosis of irritable bowel syndrome. Arch Intern Med 2001;161:2081-2088.
5. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry 1997;42:835-840.
6. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology 2002;122:1140-1156.
7. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am 2000;84:1313-1327.
8. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 2000;160:221-227.
9. Camilleri M, Prather CM. The irritable bowel syndrome: mechanisms and a practical approach to management. Ann Internal Med 1992;116:1001-1008.
10. Paterson WG, Thompson WG, Vanner SJ, et al. Recommendations for the management of irritable bowel syndrome in family practice. JAMC 1999;161:154-160.
11. Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology 2002;122:1701-1714.
12. Tosetti C, Stanghellini V, Corinaldesi R. The Rome II criteria for patients with functional gastroduodenal disorders. J Clin Gastroenterol 2003;37:92-93.
13. Chey WD, Olden K, Carter E, Boyle J, Drossman D, Chang L. Utility of the Rome I and Rome II criteria for irritable bowel syndrome in US women. Am J Gastroenterol 2002;97:2803-2811.
14. Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol 1999;94:2912-2917.
15. Hamm LR, Sorrells SC, Harding JP, et al. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am J Gastroenterol 1999;94:1279-1282.
16. Tolliver BA, Herrara JL, DiPalma JA. Evaluation of patients who meet clinical criteria for irritable bowel syndrome. Am J Gastroenterol 1994;89:176-178.
17. MacIntosh DG, Thompson WG, Patel DP, Barr R, Guindi M. Is rectal biopsy necessary in irritable bowel syndrome? Am J Gastroenterol 1992;87:1407-1409.
18. Francis CY, Duffy JN, Whorwell PJ, Martin DF. Does routine ultrasound enhance diagnostic accuracy in irritable bowel syndrome? Am J Gastroenterol 1996;91:1348-1350.
19. Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult celiac disease with irritable bowel syndrome: a case-control study in patients fulfilling Rome II criteria referred to secondary care. Lancet 2001;358:1504-1508.
20. Pimental M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome; a double blind, randomized, placebo-controlled trial. Am J Gastroenterol 2003;98:412-419.
21. Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002;97:2812-2819.
22. O’Leary CO, Wieneke P, Buckley S, et al. Celiac disease and irritable bowel-type symptoms. Am J Gastroenterol 2002;97:1463-1467.
23. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastrenterol 2002;97:s1-s5.
24. Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, et al. Systematic review on the management of irritable bowel syndrome in north America. Am J Gastroenterol 2002;97:s7-s26.
25. Drossman DA, Patrick DL, Whitehead WE, NE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol 2000;95:999-1007.
26. Jones BW, Moore DJ, Robinson SM, Song F. A systematic review of tegaserod for the treatment of irritable bowel syndrome. J Clin Pharm Therapeutics 2002;27:343-352.
27. Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2002;16:1877-1888.
28. Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther 2001;15:1655-1666.
29. Kellow J, Lee OY, Chang FY, et al. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut 2003;52:671-676.
30. Jones RH, Holtmann G, Rodrigo L, Ehsanullah, Crompton PM, Jacques LA, Mills JG. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther 1999;13:1419-1427.
31. Jailwala J, Imperiale TF, Kroeneke K. Pharmacologic treatment of the irritable bowel syndrome: A systematic review of randomized, controlled trials. Ann Intern Med 2000;133:136-147.
32. Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2002;15:79-86.
33. Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet 2000;355:1035-1040.
34. Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther 1999;13:1149-1159.
35. Lembo T, Wright RA, Bagby B, et al. Lotronex Investigator Team. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predom-inant irritable bowel syndrome. Am J Gastroenterol 2001;96:2662-2670.
36. Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomized control trials. Gut 2001;48:272-282.
37. Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroeneke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a metaanalysis. Am J Med 2000;108:65-72.
38. Myren J, Lovland B, Larssen SE, Larsen S. A double-blind study of the effect of trimipramine in patients with the irritable bowel syndrome. Scand J Gastroenterol 1984;19:835-843.
39. Greenbaum DS, Mayle JE, Vanegeren LE, et al. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Dig Dis Sci 1987;32:257-266.
40. Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci 1984;29:239-247.
41. Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl 1987;130:81-84.
42. Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol 1996;31:463-468.
43. Ritchie JA, Truelove SC. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br Med J 1979;1:376-378.
44. Parisi GC, Zilli M, Miani MP, E, et al. High-fiber diet supplementation in patients with irritable bowel syndrome. A multi-center, randomized, open trial comparison between wheat bran diet and partially hydrolyzed guar gum. Dig Dis Sci 2002;47:1697-1704.
45. Golechha AC, Chadda VS, Chadda S, Sharma SK, Mishra SN. Role of ispaghula husk in the management of irritable bowel syndrome (A randomized double-blind crossover study). JAPI 1982;30:353-354.
46. Arthurs Y, Fielding JF. Double blind trial of ispaghula/poloxamer in the irritable bowel syndrome. BMJ 1983;76:253.-
47. Jalihal A, Kurian G. Ispaghula therapy in irritable bowel syndrome: Improvement in overall well being is related to reduction in bowel dissatisfaction. J Gastroenterol Hep 1990;5:507-513.
48. Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in irritable bowel syndrome. Arch Intern Med 2003;163:265-274.
49. Pittler MH, Ernst E. Peppermint Oil for irritable bowel syndrome: A critical review and meta-analysis. Am J Gastroenterol 1998;93:1131-1135.
50. Creed F, Fernandes L, Guthrie E, et al. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003;124:303-317.
51. Kuiken SD, Tytgat GNJ, Boeckxstaens GEE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: A double blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol 2003;1:219-228.
52. Heymann-Monnikes I, Arnold R, Florin I, et al. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
53. Talley NJ, Owen BK, Boyce P, Paterson K. Psychological treatments for irritable bowel syndrome: A critique of controlled treatment trials. Am J Gastroenterol 1996;91:277-286.
54. Svedlund J, Sjodin I, Ottoson JO, Dotevall G. Controlled study of psychotherapy in irritable bowel syndrome. Lancet 1983;2:589-592.
55. Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consulting Clinic Psychology 1994;62:576-582.
56. Guthrie E, Creed F, Dawson D, Tomenson B. A ramdomized controlled trial of psychotherapy in patients with refractory irritable bowel syndrome. B J Psychiatry 1993;163:315-321.
57. Heymann-Monnikes I, Arnold R, Florin I, Herda C, Melfsen S, Monnikes H. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
Irritable bowel syndrome and psychiatric illness: Three clinical challenges
Psychiatrists often treat patients with irritable bowel syndrome (IBS) and an accompanying mental illness. Knowledge of available treatments and communication with the referring doctor are crucial to treating both the IBS symptoms and the comorbidity.
This article presents three cases that illustrate the challenges of identifying target symptoms, avoiding drug-drug interactions, ruling out serious underlying medical problems, and formulating treatment.
WHO GETS IBS?
Approximately 12% of the United States population reports IBS symptoms (abdominal pain, bloating, altered bowel habits).1 These symptoms begin before age 35 in most patients and during childhood in some. Onset after age 65 is rare.
IBS is common among patients with alcohol abuse disorder (32%),2 chronic fatigue syndrome (92%), fibromyalgia (77%), or temporomandibular joint syndrome (64%).3 Seventy percent of patients with IBS are women.4 Chronic pelvic pain, dyspareunia, dysmenorrhea, or a history of abdominal surgeries are risk factors for IBS in women.
LINK BETWEEN IBS AND MENTAL ILLNESS
Although mental illness often coexists with IBS, no cause-effect relationship has been shown.5
IBS is often preceded by stressful life events, such as family death or divorce,3 and some believe IBS is a precursor to numerous psychiatric disorders. Generalized anxiety disorder, major depression, panic disorder, social phobia, somatization disorder, or dysthymia have been diagnosed in most IBS patients.2
CASE 1: IBS AND DEPRESSION
Ms. R, age 55, has had IBS for 10 years. She has occasional diarrhea and abdominal cramps relieved by bowel movements. She is taking a bulking agent but still sometimes suffers abdominal pain.
She is referred to a psychiatrist after complaining of fatigue, loss of interest in hobbies, and crying spells for 2 months. She denies suicidal ideations. Her referring physician reports that she is taking conjugated estrogens to manage menopause symptoms. She denies any recent stressful life events. Thyroid function, glucose, and CBC are normal.
The challenge: Deciding which to treat first—the IBS symptoms or the depression—and how.
Discussion: The predominant symptom (in Ms. R’s case, abdominal pain) can help determine choice of medication. Bulk-forming agents, antispasmodics, barbiturates, benzodiazepines, and serotonin reuptake inhibitors have historically been used to treat IBS,6 but scant evidence supports their use.
Obtaining a thorough prescription history from the primary care physician, OB/GYN, and other treatment team members is critical before formulating a treatment plan. Ms. R’s estrogen use will not affect the choice of psychotropic or IBS medication because there are no significant interactions between estrogen and these classes of drugs.
Ms. R’s abdominal pain and depression can be treated simultaneously. Randomized, controlled trials have demonstrated that tricyclic antidepressants reduce abdominal pain and that behavioral therapy (relaxation therapy, hypnotherapy, and cognitive-behavioral therapy) may relieve individual IBS symptoms.7
Case 1 concluded: After reviewing Ms. R’s medications, the psychiatrist starts:
- desipramine, 50 mg at bedtime, to minimize anticholinergic side effects
- and short-term psychotherapy, which helped her identify support mechanisms and ways to better balance her life stresses.
After 6 weeks, her Beck Depression Inventory score improved from 30 at baseline to 8. She reports her abdominal pain is “the best it has been in 10 years.” Six months after diagnosis, she continues to take desipramine and is doing well.
CASE 2: IBS, DEPRESSION, AND PSYCHOSIS
Ms. H, age 32, is referred to a psychiatrist for treatment of depression with paranoid features.
Four years ago, a gastroenterologist diagnosed her as having IBS. She experiences frequent diarrhea and lower abdominal cramping. For 2 years she has been taking the antimuscarinic dicyclomine, 10 mg tid, which has provided some relief from her cramps. An estimated 20 diarrhea attacks per day leaves her housebound much of the time, however.
She reports fatigue, loss of interest in hobbies across 2 months, and paranoid thinking. She denies hallucinations or delusions but believes that her teenage children are discussing her “sickness” and plotting to “drive her crazy.” She is not suicidal.
The challenge: Treating Ms. H’s depression and paranoia while avoiding drug-drug interactions.
Discussion: Adverse drug-drug interactions can occur when prescribing psychotropics to patients with IBS (Table 1). Additive constipation, diarrhea, abdominal pain, and sedation are common interactions between psychotropics and the 5-HT3 antagonists and 5HT4 agonists commonly prescribed for IBS.
Table 1
Interactions between psychotropics and agents prescribed for IBS
| Antispasmodics | Benzodiazepines | SSRIs | Tricyclics | |
|---|---|---|---|---|
| MAOIs | Additive sedation | Additive dizziness, sedation, dry mouth, | Contraindicated–hyperpyrexia and severe neurologic effects | Contraindicated–hyperpyrexia, seizures, and death |
| SSRIs | Additive sedation | Additive sedation | —- | Increased tricyclic levels with concurrent use |
| Tricyclics | Additive sedation, dry mouth | Additive sedation | Additive sedation, dry mouth, increased tricyclic levels | —- |
| Anticonvulsants | Additive sedation | Additive sedation | Increased levels of anticonvulsants | Additive sedation, dry mouth, constipation |
| Benzodiazepines | Additive sedation | —- | Additive sedation and dry mouth | Additive sedation |
| Buspirone | Additive sedation, dizziness | Additive sedation | Additive sedation, dizziness, nausea | Additive sedation, dry mouth, constipation, increased tricyclic level |
| Traditional antipsychotics | Additive sedation, CNS effects | Additive sedation, CNS effects | Additive sedation, dizziness | Additive sedation and anticholinergic effects; increased tricyclic level |
| Atypical antipsychotics | Additive sedation, CNS effects | Contraindicated–respiratory and cardiovascular collapse | Elevated antipsychotic levels | Levels of both drugs increased |
| Aripiprazole | Somnolence,constipation | Additive sedation | Increased blood levels of aripiprazole | Increased sedation and anticholinergic effects |
| Psychotropics and 5-HT3 antagonists taken concomitantly typically lead to additive constipation and abdominal pain. | ||||
| Psychotropics and 5-HT4 agonists taken concomitantly typically lead to additive diarrhea and/or abdominal pain. | ||||
| Source: Physician’s Desk Reference. Mobile PDR release version 32. Database version 437. Montvale, NJ: Thomson Healthcare 2003. | ||||
- Hematochezia
- Weight loss < 10 pounds
- Family history of colon cancer
- Recurrent fever
- Anemia
- Chronic severe diarrhea
Source: American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Am J Gastroenterol. 2002;97:S1-S5.
Other than fiber supplements, most traditional IBS medications are sedating and are associated with anticholinergic side effects. In Ms. H’s case, extreme caution is necessary before prescribing an antidepressant or antipsychotic because of dicyclomine’s additive sedating effects.
Case 2 concluded: After a thorough initial patient interview, the psychiatrist elects to treat Ms. H’s major depression with an antidepressant but delays the use of an antipsychotic to avoid additive sedation.
After talking with Ms. H’s family physician, the psychiatrist stops her dicyclomine and starts sertraline, 100 mg/d. She tolerates the sertraline well and the dosage is titrated across 1 month to 200 mg/d.
Four weeks later, Ms. H’s Beck Depression Inventory score has improved from 26 at baseline to 5, but her paranoid thoughts and frequent diarrhea persist. The psychiatrist adds low-dose olanzapine (5 mg at bedtime) to minimize extrapyramidal side effects. One month later, her depression and paranoia have resolved.
Ms. H’s gastroenterologist instructs her to begin taking alosetron, 1 mg bid, for her continued frequent diarrhea. Adding this agent to her sertraline/olanzapine regimen can lead to additive constipation and abdominal pain, so the psychiatrist monitors her psychiatric medications. One month later, she reports that her affect is much improved and her diarrhea is “gone.”
CASE 3: DEPRESSION AND ABDOMINAL PAIN
Mr. J, age 52, has had depression for 1 year. His depressive symptoms have improved significantly on fluoxetine, 20 mg/d; he once again enjoys life and has a more positive outlook.
The patient was in reasonably good health until about 1 month ago, when he began to experience abdominal pain. He has lost 14 lbs over the past month. He is not taking other medications.
The challenge: Find the cause of Mr. J’s persistent abdominal pain without undermining depression therapy.
Discussion: Although Mr. J’s symptoms might be side effects of fluoxetine, his abdominal pain and weight loss >10 lbs within 1 month are cause for concern. The American College of Gastroenterology has identified six alarm symptoms that could point to a serious medical problem in patients with severe abdominal pain (Box).7
Patients who exhibit any of these symptoms should be referred for endoscopic and stool studies. Colon cancer screening should be considered for all patients age 50 and older.
Patients with IBS usually present first to their primary care physicians with abdominal pain and altered bowel habits. These symptoms can occur in many gastrointestinal and systemic illnesses (Table 2).8
Table 2
Diagnosing irritable bowel syndrome: What to rule out
| Differential diagnosis | Examples |
|---|---|
| Inflammatory bowel disease | Crohn’s disease, ulcerative colitis |
| Medication effects | Laxatives, constipating agents |
| Infections | Parasitic, bacterial, viral, opportunistic |
| Malabsorption syndromes | Celiac disease, pancreatic insufficiency |
| Endocrine disorders | Hypothyroidism, hyperthyroidism, diabetes, Addison’s disease |
| Endocrine tumors (extremely uncommon) | Gastrinoma, carcinoid |
| Colorectal carcinoma | Adenocarcinoma, villous adenoma |
| Intestinal pseudo-obstruction | Diabetes, scleroderma |
| Lactose intolerance | —- |
| Psychiatric disorders | Depression, anxiety, somatization disorders |
| Source: Dalton CB, Drossman D. Am Fam Physician. 1997;55(3):875-80. | |
Case 3 concluded: The psychiatrist and primary care physician consult a gastroenterologist, who performs a colonoscopy and identifies a resectable Duke’s Class B adenocarcinoma in the transverse colon. A partial colectomy is performed.
Three years later, Mr. J is cancer-free and his depression is stable. The psychiatrist advises him to keep taking fluoxetine, 20 mg/d, because the stress of his cancer therapy increases the risk of depression recurrence.
Related resources
- National Institute of Diabetes and Digestive and Kidney Diseases —Irritable Bowel Syndrome www.niddk.nih.gov/health/digest/pubs/irrbowel/irrbowel.htm
Drug brand names
- Alosetron • Lotronex
- Aripiprazole • Abilify
- Buspirone • BuSpar
- Desipramine • Norpramin
- Dicyclomine • Bentyl
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Sertraline • Zoloft
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Locke GR, 3rd. The epidemiology of functional gastrointestinal disorders in North America. Gastroenterol Clin North Am. 1996;25:1-19.
2. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry. 1997;42:835-40.
3. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221-7.
4. Smith RP. Lower gastrointestinal disease in women. Obstet Gynecol Clin North Am. 2001;28:351-62.
5. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am. 2000;84:1313-276.
6. Mobile PDR Release Version 32. Database Version 437. An abbreviated, up-to-date version of the PDR onto computing devices. Thomson Healthcare, Ortho-Biotech Oncology, 2003.
7. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S1-S5.
8. Dalton CB, Drossman D. Diagnosis and treatment of irritable bowel syndrome. Am Fam Physician. 1997;55(3):875-80.
Psychiatrists often treat patients with irritable bowel syndrome (IBS) and an accompanying mental illness. Knowledge of available treatments and communication with the referring doctor are crucial to treating both the IBS symptoms and the comorbidity.
This article presents three cases that illustrate the challenges of identifying target symptoms, avoiding drug-drug interactions, ruling out serious underlying medical problems, and formulating treatment.
WHO GETS IBS?
Approximately 12% of the United States population reports IBS symptoms (abdominal pain, bloating, altered bowel habits).1 These symptoms begin before age 35 in most patients and during childhood in some. Onset after age 65 is rare.
IBS is common among patients with alcohol abuse disorder (32%),2 chronic fatigue syndrome (92%), fibromyalgia (77%), or temporomandibular joint syndrome (64%).3 Seventy percent of patients with IBS are women.4 Chronic pelvic pain, dyspareunia, dysmenorrhea, or a history of abdominal surgeries are risk factors for IBS in women.
LINK BETWEEN IBS AND MENTAL ILLNESS
Although mental illness often coexists with IBS, no cause-effect relationship has been shown.5
IBS is often preceded by stressful life events, such as family death or divorce,3 and some believe IBS is a precursor to numerous psychiatric disorders. Generalized anxiety disorder, major depression, panic disorder, social phobia, somatization disorder, or dysthymia have been diagnosed in most IBS patients.2
CASE 1: IBS AND DEPRESSION
Ms. R, age 55, has had IBS for 10 years. She has occasional diarrhea and abdominal cramps relieved by bowel movements. She is taking a bulking agent but still sometimes suffers abdominal pain.
She is referred to a psychiatrist after complaining of fatigue, loss of interest in hobbies, and crying spells for 2 months. She denies suicidal ideations. Her referring physician reports that she is taking conjugated estrogens to manage menopause symptoms. She denies any recent stressful life events. Thyroid function, glucose, and CBC are normal.
The challenge: Deciding which to treat first—the IBS symptoms or the depression—and how.
Discussion: The predominant symptom (in Ms. R’s case, abdominal pain) can help determine choice of medication. Bulk-forming agents, antispasmodics, barbiturates, benzodiazepines, and serotonin reuptake inhibitors have historically been used to treat IBS,6 but scant evidence supports their use.
Obtaining a thorough prescription history from the primary care physician, OB/GYN, and other treatment team members is critical before formulating a treatment plan. Ms. R’s estrogen use will not affect the choice of psychotropic or IBS medication because there are no significant interactions between estrogen and these classes of drugs.
Ms. R’s abdominal pain and depression can be treated simultaneously. Randomized, controlled trials have demonstrated that tricyclic antidepressants reduce abdominal pain and that behavioral therapy (relaxation therapy, hypnotherapy, and cognitive-behavioral therapy) may relieve individual IBS symptoms.7
Case 1 concluded: After reviewing Ms. R’s medications, the psychiatrist starts:
- desipramine, 50 mg at bedtime, to minimize anticholinergic side effects
- and short-term psychotherapy, which helped her identify support mechanisms and ways to better balance her life stresses.
After 6 weeks, her Beck Depression Inventory score improved from 30 at baseline to 8. She reports her abdominal pain is “the best it has been in 10 years.” Six months after diagnosis, she continues to take desipramine and is doing well.
CASE 2: IBS, DEPRESSION, AND PSYCHOSIS
Ms. H, age 32, is referred to a psychiatrist for treatment of depression with paranoid features.
Four years ago, a gastroenterologist diagnosed her as having IBS. She experiences frequent diarrhea and lower abdominal cramping. For 2 years she has been taking the antimuscarinic dicyclomine, 10 mg tid, which has provided some relief from her cramps. An estimated 20 diarrhea attacks per day leaves her housebound much of the time, however.
She reports fatigue, loss of interest in hobbies across 2 months, and paranoid thinking. She denies hallucinations or delusions but believes that her teenage children are discussing her “sickness” and plotting to “drive her crazy.” She is not suicidal.
The challenge: Treating Ms. H’s depression and paranoia while avoiding drug-drug interactions.
Discussion: Adverse drug-drug interactions can occur when prescribing psychotropics to patients with IBS (Table 1). Additive constipation, diarrhea, abdominal pain, and sedation are common interactions between psychotropics and the 5-HT3 antagonists and 5HT4 agonists commonly prescribed for IBS.
Table 1
Interactions between psychotropics and agents prescribed for IBS
| Antispasmodics | Benzodiazepines | SSRIs | Tricyclics | |
|---|---|---|---|---|
| MAOIs | Additive sedation | Additive dizziness, sedation, dry mouth, | Contraindicated–hyperpyrexia and severe neurologic effects | Contraindicated–hyperpyrexia, seizures, and death |
| SSRIs | Additive sedation | Additive sedation | —- | Increased tricyclic levels with concurrent use |
| Tricyclics | Additive sedation, dry mouth | Additive sedation | Additive sedation, dry mouth, increased tricyclic levels | —- |
| Anticonvulsants | Additive sedation | Additive sedation | Increased levels of anticonvulsants | Additive sedation, dry mouth, constipation |
| Benzodiazepines | Additive sedation | —- | Additive sedation and dry mouth | Additive sedation |
| Buspirone | Additive sedation, dizziness | Additive sedation | Additive sedation, dizziness, nausea | Additive sedation, dry mouth, constipation, increased tricyclic level |
| Traditional antipsychotics | Additive sedation, CNS effects | Additive sedation, CNS effects | Additive sedation, dizziness | Additive sedation and anticholinergic effects; increased tricyclic level |
| Atypical antipsychotics | Additive sedation, CNS effects | Contraindicated–respiratory and cardiovascular collapse | Elevated antipsychotic levels | Levels of both drugs increased |
| Aripiprazole | Somnolence,constipation | Additive sedation | Increased blood levels of aripiprazole | Increased sedation and anticholinergic effects |
| Psychotropics and 5-HT3 antagonists taken concomitantly typically lead to additive constipation and abdominal pain. | ||||
| Psychotropics and 5-HT4 agonists taken concomitantly typically lead to additive diarrhea and/or abdominal pain. | ||||
| Source: Physician’s Desk Reference. Mobile PDR release version 32. Database version 437. Montvale, NJ: Thomson Healthcare 2003. | ||||
- Hematochezia
- Weight loss < 10 pounds
- Family history of colon cancer
- Recurrent fever
- Anemia
- Chronic severe diarrhea
Source: American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Am J Gastroenterol. 2002;97:S1-S5.
Other than fiber supplements, most traditional IBS medications are sedating and are associated with anticholinergic side effects. In Ms. H’s case, extreme caution is necessary before prescribing an antidepressant or antipsychotic because of dicyclomine’s additive sedating effects.
Case 2 concluded: After a thorough initial patient interview, the psychiatrist elects to treat Ms. H’s major depression with an antidepressant but delays the use of an antipsychotic to avoid additive sedation.
After talking with Ms. H’s family physician, the psychiatrist stops her dicyclomine and starts sertraline, 100 mg/d. She tolerates the sertraline well and the dosage is titrated across 1 month to 200 mg/d.
Four weeks later, Ms. H’s Beck Depression Inventory score has improved from 26 at baseline to 5, but her paranoid thoughts and frequent diarrhea persist. The psychiatrist adds low-dose olanzapine (5 mg at bedtime) to minimize extrapyramidal side effects. One month later, her depression and paranoia have resolved.
Ms. H’s gastroenterologist instructs her to begin taking alosetron, 1 mg bid, for her continued frequent diarrhea. Adding this agent to her sertraline/olanzapine regimen can lead to additive constipation and abdominal pain, so the psychiatrist monitors her psychiatric medications. One month later, she reports that her affect is much improved and her diarrhea is “gone.”
CASE 3: DEPRESSION AND ABDOMINAL PAIN
Mr. J, age 52, has had depression for 1 year. His depressive symptoms have improved significantly on fluoxetine, 20 mg/d; he once again enjoys life and has a more positive outlook.
The patient was in reasonably good health until about 1 month ago, when he began to experience abdominal pain. He has lost 14 lbs over the past month. He is not taking other medications.
The challenge: Find the cause of Mr. J’s persistent abdominal pain without undermining depression therapy.
Discussion: Although Mr. J’s symptoms might be side effects of fluoxetine, his abdominal pain and weight loss >10 lbs within 1 month are cause for concern. The American College of Gastroenterology has identified six alarm symptoms that could point to a serious medical problem in patients with severe abdominal pain (Box).7
Patients who exhibit any of these symptoms should be referred for endoscopic and stool studies. Colon cancer screening should be considered for all patients age 50 and older.
Patients with IBS usually present first to their primary care physicians with abdominal pain and altered bowel habits. These symptoms can occur in many gastrointestinal and systemic illnesses (Table 2).8
Table 2
Diagnosing irritable bowel syndrome: What to rule out
| Differential diagnosis | Examples |
|---|---|
| Inflammatory bowel disease | Crohn’s disease, ulcerative colitis |
| Medication effects | Laxatives, constipating agents |
| Infections | Parasitic, bacterial, viral, opportunistic |
| Malabsorption syndromes | Celiac disease, pancreatic insufficiency |
| Endocrine disorders | Hypothyroidism, hyperthyroidism, diabetes, Addison’s disease |
| Endocrine tumors (extremely uncommon) | Gastrinoma, carcinoid |
| Colorectal carcinoma | Adenocarcinoma, villous adenoma |
| Intestinal pseudo-obstruction | Diabetes, scleroderma |
| Lactose intolerance | —- |
| Psychiatric disorders | Depression, anxiety, somatization disorders |
| Source: Dalton CB, Drossman D. Am Fam Physician. 1997;55(3):875-80. | |
Case 3 concluded: The psychiatrist and primary care physician consult a gastroenterologist, who performs a colonoscopy and identifies a resectable Duke’s Class B adenocarcinoma in the transverse colon. A partial colectomy is performed.
Three years later, Mr. J is cancer-free and his depression is stable. The psychiatrist advises him to keep taking fluoxetine, 20 mg/d, because the stress of his cancer therapy increases the risk of depression recurrence.
Related resources
- National Institute of Diabetes and Digestive and Kidney Diseases —Irritable Bowel Syndrome www.niddk.nih.gov/health/digest/pubs/irrbowel/irrbowel.htm
Drug brand names
- Alosetron • Lotronex
- Aripiprazole • Abilify
- Buspirone • BuSpar
- Desipramine • Norpramin
- Dicyclomine • Bentyl
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Sertraline • Zoloft
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychiatrists often treat patients with irritable bowel syndrome (IBS) and an accompanying mental illness. Knowledge of available treatments and communication with the referring doctor are crucial to treating both the IBS symptoms and the comorbidity.
This article presents three cases that illustrate the challenges of identifying target symptoms, avoiding drug-drug interactions, ruling out serious underlying medical problems, and formulating treatment.
WHO GETS IBS?
Approximately 12% of the United States population reports IBS symptoms (abdominal pain, bloating, altered bowel habits).1 These symptoms begin before age 35 in most patients and during childhood in some. Onset after age 65 is rare.
IBS is common among patients with alcohol abuse disorder (32%),2 chronic fatigue syndrome (92%), fibromyalgia (77%), or temporomandibular joint syndrome (64%).3 Seventy percent of patients with IBS are women.4 Chronic pelvic pain, dyspareunia, dysmenorrhea, or a history of abdominal surgeries are risk factors for IBS in women.
LINK BETWEEN IBS AND MENTAL ILLNESS
Although mental illness often coexists with IBS, no cause-effect relationship has been shown.5
IBS is often preceded by stressful life events, such as family death or divorce,3 and some believe IBS is a precursor to numerous psychiatric disorders. Generalized anxiety disorder, major depression, panic disorder, social phobia, somatization disorder, or dysthymia have been diagnosed in most IBS patients.2
CASE 1: IBS AND DEPRESSION
Ms. R, age 55, has had IBS for 10 years. She has occasional diarrhea and abdominal cramps relieved by bowel movements. She is taking a bulking agent but still sometimes suffers abdominal pain.
She is referred to a psychiatrist after complaining of fatigue, loss of interest in hobbies, and crying spells for 2 months. She denies suicidal ideations. Her referring physician reports that she is taking conjugated estrogens to manage menopause symptoms. She denies any recent stressful life events. Thyroid function, glucose, and CBC are normal.
The challenge: Deciding which to treat first—the IBS symptoms or the depression—and how.
Discussion: The predominant symptom (in Ms. R’s case, abdominal pain) can help determine choice of medication. Bulk-forming agents, antispasmodics, barbiturates, benzodiazepines, and serotonin reuptake inhibitors have historically been used to treat IBS,6 but scant evidence supports their use.
Obtaining a thorough prescription history from the primary care physician, OB/GYN, and other treatment team members is critical before formulating a treatment plan. Ms. R’s estrogen use will not affect the choice of psychotropic or IBS medication because there are no significant interactions between estrogen and these classes of drugs.
Ms. R’s abdominal pain and depression can be treated simultaneously. Randomized, controlled trials have demonstrated that tricyclic antidepressants reduce abdominal pain and that behavioral therapy (relaxation therapy, hypnotherapy, and cognitive-behavioral therapy) may relieve individual IBS symptoms.7
Case 1 concluded: After reviewing Ms. R’s medications, the psychiatrist starts:
- desipramine, 50 mg at bedtime, to minimize anticholinergic side effects
- and short-term psychotherapy, which helped her identify support mechanisms and ways to better balance her life stresses.
After 6 weeks, her Beck Depression Inventory score improved from 30 at baseline to 8. She reports her abdominal pain is “the best it has been in 10 years.” Six months after diagnosis, she continues to take desipramine and is doing well.
CASE 2: IBS, DEPRESSION, AND PSYCHOSIS
Ms. H, age 32, is referred to a psychiatrist for treatment of depression with paranoid features.
Four years ago, a gastroenterologist diagnosed her as having IBS. She experiences frequent diarrhea and lower abdominal cramping. For 2 years she has been taking the antimuscarinic dicyclomine, 10 mg tid, which has provided some relief from her cramps. An estimated 20 diarrhea attacks per day leaves her housebound much of the time, however.
She reports fatigue, loss of interest in hobbies across 2 months, and paranoid thinking. She denies hallucinations or delusions but believes that her teenage children are discussing her “sickness” and plotting to “drive her crazy.” She is not suicidal.
The challenge: Treating Ms. H’s depression and paranoia while avoiding drug-drug interactions.
Discussion: Adverse drug-drug interactions can occur when prescribing psychotropics to patients with IBS (Table 1). Additive constipation, diarrhea, abdominal pain, and sedation are common interactions between psychotropics and the 5-HT3 antagonists and 5HT4 agonists commonly prescribed for IBS.
Table 1
Interactions between psychotropics and agents prescribed for IBS
| Antispasmodics | Benzodiazepines | SSRIs | Tricyclics | |
|---|---|---|---|---|
| MAOIs | Additive sedation | Additive dizziness, sedation, dry mouth, | Contraindicated–hyperpyrexia and severe neurologic effects | Contraindicated–hyperpyrexia, seizures, and death |
| SSRIs | Additive sedation | Additive sedation | —- | Increased tricyclic levels with concurrent use |
| Tricyclics | Additive sedation, dry mouth | Additive sedation | Additive sedation, dry mouth, increased tricyclic levels | —- |
| Anticonvulsants | Additive sedation | Additive sedation | Increased levels of anticonvulsants | Additive sedation, dry mouth, constipation |
| Benzodiazepines | Additive sedation | —- | Additive sedation and dry mouth | Additive sedation |
| Buspirone | Additive sedation, dizziness | Additive sedation | Additive sedation, dizziness, nausea | Additive sedation, dry mouth, constipation, increased tricyclic level |
| Traditional antipsychotics | Additive sedation, CNS effects | Additive sedation, CNS effects | Additive sedation, dizziness | Additive sedation and anticholinergic effects; increased tricyclic level |
| Atypical antipsychotics | Additive sedation, CNS effects | Contraindicated–respiratory and cardiovascular collapse | Elevated antipsychotic levels | Levels of both drugs increased |
| Aripiprazole | Somnolence,constipation | Additive sedation | Increased blood levels of aripiprazole | Increased sedation and anticholinergic effects |
| Psychotropics and 5-HT3 antagonists taken concomitantly typically lead to additive constipation and abdominal pain. | ||||
| Psychotropics and 5-HT4 agonists taken concomitantly typically lead to additive diarrhea and/or abdominal pain. | ||||
| Source: Physician’s Desk Reference. Mobile PDR release version 32. Database version 437. Montvale, NJ: Thomson Healthcare 2003. | ||||
- Hematochezia
- Weight loss < 10 pounds
- Family history of colon cancer
- Recurrent fever
- Anemia
- Chronic severe diarrhea
Source: American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Am J Gastroenterol. 2002;97:S1-S5.
Other than fiber supplements, most traditional IBS medications are sedating and are associated with anticholinergic side effects. In Ms. H’s case, extreme caution is necessary before prescribing an antidepressant or antipsychotic because of dicyclomine’s additive sedating effects.
Case 2 concluded: After a thorough initial patient interview, the psychiatrist elects to treat Ms. H’s major depression with an antidepressant but delays the use of an antipsychotic to avoid additive sedation.
After talking with Ms. H’s family physician, the psychiatrist stops her dicyclomine and starts sertraline, 100 mg/d. She tolerates the sertraline well and the dosage is titrated across 1 month to 200 mg/d.
Four weeks later, Ms. H’s Beck Depression Inventory score has improved from 26 at baseline to 5, but her paranoid thoughts and frequent diarrhea persist. The psychiatrist adds low-dose olanzapine (5 mg at bedtime) to minimize extrapyramidal side effects. One month later, her depression and paranoia have resolved.
Ms. H’s gastroenterologist instructs her to begin taking alosetron, 1 mg bid, for her continued frequent diarrhea. Adding this agent to her sertraline/olanzapine regimen can lead to additive constipation and abdominal pain, so the psychiatrist monitors her psychiatric medications. One month later, she reports that her affect is much improved and her diarrhea is “gone.”
CASE 3: DEPRESSION AND ABDOMINAL PAIN
Mr. J, age 52, has had depression for 1 year. His depressive symptoms have improved significantly on fluoxetine, 20 mg/d; he once again enjoys life and has a more positive outlook.
The patient was in reasonably good health until about 1 month ago, when he began to experience abdominal pain. He has lost 14 lbs over the past month. He is not taking other medications.
The challenge: Find the cause of Mr. J’s persistent abdominal pain without undermining depression therapy.
Discussion: Although Mr. J’s symptoms might be side effects of fluoxetine, his abdominal pain and weight loss >10 lbs within 1 month are cause for concern. The American College of Gastroenterology has identified six alarm symptoms that could point to a serious medical problem in patients with severe abdominal pain (Box).7
Patients who exhibit any of these symptoms should be referred for endoscopic and stool studies. Colon cancer screening should be considered for all patients age 50 and older.
Patients with IBS usually present first to their primary care physicians with abdominal pain and altered bowel habits. These symptoms can occur in many gastrointestinal and systemic illnesses (Table 2).8
Table 2
Diagnosing irritable bowel syndrome: What to rule out
| Differential diagnosis | Examples |
|---|---|
| Inflammatory bowel disease | Crohn’s disease, ulcerative colitis |
| Medication effects | Laxatives, constipating agents |
| Infections | Parasitic, bacterial, viral, opportunistic |
| Malabsorption syndromes | Celiac disease, pancreatic insufficiency |
| Endocrine disorders | Hypothyroidism, hyperthyroidism, diabetes, Addison’s disease |
| Endocrine tumors (extremely uncommon) | Gastrinoma, carcinoid |
| Colorectal carcinoma | Adenocarcinoma, villous adenoma |
| Intestinal pseudo-obstruction | Diabetes, scleroderma |
| Lactose intolerance | —- |
| Psychiatric disorders | Depression, anxiety, somatization disorders |
| Source: Dalton CB, Drossman D. Am Fam Physician. 1997;55(3):875-80. | |
Case 3 concluded: The psychiatrist and primary care physician consult a gastroenterologist, who performs a colonoscopy and identifies a resectable Duke’s Class B adenocarcinoma in the transverse colon. A partial colectomy is performed.
Three years later, Mr. J is cancer-free and his depression is stable. The psychiatrist advises him to keep taking fluoxetine, 20 mg/d, because the stress of his cancer therapy increases the risk of depression recurrence.
Related resources
- National Institute of Diabetes and Digestive and Kidney Diseases —Irritable Bowel Syndrome www.niddk.nih.gov/health/digest/pubs/irrbowel/irrbowel.htm
Drug brand names
- Alosetron • Lotronex
- Aripiprazole • Abilify
- Buspirone • BuSpar
- Desipramine • Norpramin
- Dicyclomine • Bentyl
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Sertraline • Zoloft
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Locke GR, 3rd. The epidemiology of functional gastrointestinal disorders in North America. Gastroenterol Clin North Am. 1996;25:1-19.
2. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry. 1997;42:835-40.
3. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221-7.
4. Smith RP. Lower gastrointestinal disease in women. Obstet Gynecol Clin North Am. 2001;28:351-62.
5. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am. 2000;84:1313-276.
6. Mobile PDR Release Version 32. Database Version 437. An abbreviated, up-to-date version of the PDR onto computing devices. Thomson Healthcare, Ortho-Biotech Oncology, 2003.
7. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S1-S5.
8. Dalton CB, Drossman D. Diagnosis and treatment of irritable bowel syndrome. Am Fam Physician. 1997;55(3):875-80.
1. Locke GR, 3rd. The epidemiology of functional gastrointestinal disorders in North America. Gastroenterol Clin North Am. 1996;25:1-19.
2. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry. 1997;42:835-40.
3. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221-7.
4. Smith RP. Lower gastrointestinal disease in women. Obstet Gynecol Clin North Am. 2001;28:351-62.
5. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am. 2000;84:1313-276.
6. Mobile PDR Release Version 32. Database Version 437. An abbreviated, up-to-date version of the PDR onto computing devices. Thomson Healthcare, Ortho-Biotech Oncology, 2003.
7. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S1-S5.
8. Dalton CB, Drossman D. Diagnosis and treatment of irritable bowel syndrome. Am Fam Physician. 1997;55(3):875-80.
Management of the Patient with Otitis Externa
Otitis externa (OE), also referred to as external otitis, is inflammation of the auricle, external ear, or tympanic membrane. The severity can range from mild inflammation to life-threatening infection.1 It is commonly seen by family physicians and affects 4 out of each 1000 Americans every year.2 In most cases the significant pain of OE compels the patient to seek care urgently.
OE can be categorized as localized or diffuse. When it persists for more than 6 months, it is considered chronic and is more commonly bilateral. It is thought to be caused by local trauma to the external canal, diabetes, high humidity, loss of the canal’s protective coating of cerumen, eczema, use of a hearing aid or stethoscope, or glandular obstruction. It is commonly seen in swimmers, particularly in the summer months.1 The most frequent symptoms are discharge, pain, hearing loss, itching, and tinnitus.
Necrotizing (malignant) otitis externa (NOE) is the most severe form of OE and is most often seen in elderly patients with diabetes. One case series in a referral population found a mortality rate of 53%.3 Pain, purulent discharge, bilateral involvement, and external canal granulation tissue are common symptoms.
Pathophysiology
The ear canal is a blind sac with an anterior recess. Trauma to the canal, accumulation of keratin, or a change in pH can trigger inflammation and infection. One study4 found that aerobic bacteria account for 91% of bacterial causes; anaerobes, 4%; and mixed infections, 4%. The most common offending organisms are Pseudomonas aeruginosa (50%), Staphylococcus aureus (23%), anaerobes and gram-negative organisms (12.5%), and yeast, such as Aspergillus and Candida (12.5%). The increased pH of pool water is believed to make infection more likely, since bacteriologic studies fail to show a direct link between swimming pool contamination and the organisms of OE.
Diagnosis
There are no published studies of the accuracy of the medical history, physical examination, or office laboratory tests for the diagnosis of OE. Diagnosis is usually made based on physical examination findings: pain on movement of the auricle, edema, redness, and foul-smelling discharge.5 Swelling often obscures the tympanic membrane.
NOE is also a clinical diagnosis and requires a high index of suspicion. A study by Zaky and colleagues3 considered 2 new cases and 32 that were retrospectively reviewed from the literature. They found the following frequencies of symptoms: pain (100%), purulent discharge (97%), bilateral involvement (21%), and a polyp in the external canal (88%). Diabetes was common in this group of patients (82%), confirming that it is consistently a predisposing risk factor, and 91% of these patients were aged 55 years and older.
Few papers have been published concerning diagnostic studies for NOE. One found that temporal radiographs and tomograms are positive in most cases of NOE but were not necessary for diagnosis.6 The erythrocyte sedimentation rate is usually increased,3 but this is true in many other illnesses. Many studies3,8-9,31-35 have reported that Pseudomonas is the primary offending organism for NOE. Because most ear cultures are positive for Pseudomonas, these cultures are of questionable value.10
There are 4 studies that consider more aggressive diagnostic testing for NOE using conventional temporal radiographs, tomographic temporal radiographs, qualitative and single photon emission computed tomography67 gallium scans, and qualitative and quantitative99 technetium bone scans.6-9 These studies were limited by the use of unclear7 or poor quality6,8 reference standards (radiograph or poor response to antibiotics). The diagnosis of NOE does not require additional studies. Expert opinion supports a diagnosis based on the history and physical examination and poor response to treatment. The most common symptom of NOE is persistent pain that is constant and severe. The leukocyte count may be normal or mildly elevated.3 Physicians should consider the diagnosis of NOE in any patient with diabetes who has OE, particularly older patients. Characteristics of OE and NOE are presented in Table 1.
Treatment
Otitis Externa
The main principles of treatment are local cleansing of debris, drainage of the infection, re-establishment of the normal acidic environment, use of topical and systemic antimicrobials, and prevention of recurrent infections. The evidence regarding these treatments is summarized in Table 2. The best evidence (grade of evidence: A) demonstrates equivalent results with ear cleaning, an ear wick, and any of the choices of topical agents12-13—acidifying agents, antibiotics, antibiotic and steroid combinations, or antifungal agents. Frequent dosing (3 to 4 times daily) for at least 4 days is supported by the studies. Two studies demonstrated equivalent efficacy with topical ciprofloxacin or ofloxacin dosed twice daily compared with antibiotic and steroid combinations dosed 4 times daily.16-17 However, these agents are also more expensive than older topical antibiotics. The evidence for single topical treatments and oral antibiotics is weaker14-29 (grade of evidence: B).
Physicians should treat patients with one of the following regimens for at least 4 days:
- ear cleaning + ear wick + acidifying agent dosed 4 times daily
- ear cleaning + ear wick + topical antibiotic dosed 4 times daily (twice daily if quinolone)
- ear cleaning + ear wick + topical antibiotic/steroid combination dosed 4 times daily (twice daily if quinolone)
The ear is best cleaned by simply irrigating the canal. Be sure to look for a foreign body, particularly in younger patients. For a wick, use either the Pope ear wick (Merocel Corporation, Mystic, Conn) or a fourth-inch sterile gauze. The wick helps draw topical medications into the affected canal, particularly when it is obstructed. The patient should return in approximately 2 days for removal of the wick and reassessment. Do not forget analgesics; this is a painful condition.
Necrotizing Otitis Externa
The treatment for NOE must be aggressive. As the infection invades through the soft tissues into surrounding bony structures, it can be life threatening.10 The evidence for various treatments is presented in Table 3. The studies are weaker than those for treatment of otitis externa (grade of recommendations: B and C). They support twice daily dosing with oral ofloxacin 400 mg orally twice daily or ciprofloxacin 750 mg orally twice daily for up to 3 months.31-33 There is weak evidence that hyperbaric oxygen is effective.34 Intravenous anti-Pseudomonal antibiotics are often used initially, based on expert opinion (grade: D) and the high mortality rate.
The oral fluoroquinolones (ofloxacin and ciprofloxacin) show promise for treating NOE. The choice of parenteral or oral antibiotics still rests with clinician judgment, based on the patient’s clinical presentation. If oral antibiotics are started, the length of treatment should be based on the severity of illness.
Prognosis
The prognosis for cure of OE is excellent, although the actual natural history of untreated disease has not been studied. OE complicated by NEO is more common in persons with diabetes who have a persistent course and granulation tissue visualized in the external auditory canal. Untreated NEO can lead to osteomyelitis and paralysis of cranial nerves. Death can result from sepsis and central nervous system infection.
1. Agius AM, Pickles JM, Burch KL. A prospective study of otitis externa. Clin Otolaryngol 1992;17:150-54.
2. Diagnosis and treatment of acute otitis media: an interdisciplinary update. Proceedings of a roundtable discussion. Ann Otol Rhinol Laryngol 1999;108:2-23.
3. Zaky DA, Bentley DW, Lowy K, Betts RF, Douglas RG. Malignant external otitis: a severe form of otitis in diabetic patients. Am J Med 1976;61:298-302.
4. Clark WB, Brook I, Bianki D, Thompson DH. Microbiology of otitis externa. Otolaryngol Head Neck Surg 1997;116:23-25.
5. Leung AKC, Fong JHS, Leong AG. Otalgia in children. J Natl Med Assoc 2000;92:254-60.
6. Kim BH. Roentgenographic findings of malignant external otitis. Am J Roentgenol Radium Ther Nucl Med 1971;112:366-72.
7. Stokkel MPM, Boot ICN, vanEck-Smit BLF. SPECT gallium scintigraphy in malignant external otitis: initial staging and follow-up: case reports. Laryngoscope 1996;106:338-40.
8. Uri N, Gips S, Front A, Meyer SW, Hardoff R. Quantitative bone and 67Ga scintigraphy in the differentiation of necrotizing external otitis from severe external otitis. Arch Otolarngol Head Neck Surg 1991;117:623-26.
9. Hardoff R, Gips S, Uri N, Front A, Tamir A. Semiquantitative skull planar and SPECT bone scintigraphy in diabetic patients: differentiation of necrotizing (malignant) external otitis from severe external otitis. J Nucl Med 1994;35:411-15.
10. Brook I. Treatment of otitis externa in children. Pediatric Drugs 1999;1:283-89.
11. Densert O, Toremalm NG. Pain relief with indomethacin in external otitis. Arch Otolaryng 1972;95:460-63.
12. Ordonez GE, Kime CE, Updegraff WR, Glassman JM, Soyka JP. Effective treatment of acute diffuse otitis externa: I. a controlled comparison of hydrocortisone-acetic acid, non-aqueous and hydrocortisone-neomycin-polymyxin b-colistin otic solutions. Curr Ther Res 1978;23:ss3-14.
13. Kime CE, Ordonez GU, Updegraff WR, Glassman JM, Soyka JP. Effective Treatment of acute diffuse otitis externa: II. a controlled comparison of hydrocortisone-acetic acid, non-aqueous and hydrocortisone-neomycin-polymyxin b otic solutions. Curr Ther Res 1978;23:ss15-28.
14. Freedman R. Versus placebo in treatment of acute otitis externa. Ear Nose Throat J 1978;57:28-37.
15. Gyde MC, Norris D, Kavalec EC. The weeping ear: clinical re-evaluation of treatment. J Int Med Res 1982;10:333-40.
16. Arnes A, Dibb WL. Otitis externa: clinical comparison of local ciprofloxacin versus local oxytetracycline, polymyxin B, hydrocortisone combination treatment. Curr Med Res Opin 1993;13:182-86.
17. Jones RN, Milazzo J, Seidlin M. Ofloxacin otic solution for treatment of otits externa in children and adults. Arch Otolaryngol Head Neck Surg 1997;123:1193-200.
18. Wadston CJ, Bertilsson CA, Sieradzki H, Edstrom S. A randomized clinical trial of two topical preparations (framycitin/gramicidin and oxytetracycline/hydrocortisone with polymyxin b) in the treatment of external otitis. Arch Otorhinolaryngol 1985;242:135-39.
19. Barton RPE, Wright JLW, Gray RFE. The clinical evaluation of a new clobetasol propionate preparation in the treatment of otitis externa. J Laryngol Otol 1979;93:703-06.
20. Treatment of otitis externa: a clinical trial of local applications. Br J Clin Pract 1967;21:507-10.
21. Bain DJG. A double-blind comparative study of otoseptil ear drops and otosporin: ear drops in otitis externa. J Int Med Res 1976;4:79-81.
22. Worgan D. Treatment of otitis externa: report of a clinical trial. Practitioner 1969;202:817-20.
23. Yelland MJ. The efficacy of oral cotrimoxazole in the treatment of otitis externa in general practice. Med J Aust 1993;158:697-99.
24. Barr GD, Al-Khabori M. A randomized prospective comparison of two methods of administering topical treatment in otitis externa. Clinical Otolaryng Allied Sci 1991;16:547-48.
25. Clayton MI, Osborne JE, Rutherford D, Rivron RP. A double-blind, randomized, prospective trial of a topical antiseptic versus a topical antibiotic in the treatment of otorrhea. Clin Otolaryngol 1990;15:7-10.
26. Cannon S. External otitis: controlled therapeutic trial. Eye Ear Nose Throat Monthly 1970;49:56-61.
27. Slack RWT. A study of three preparations in the treatment of otitis externa. J Laryngol Otol 1987;101:533-35.
28. Smith RB, Moodie J. A general practice study to compare the efficacy and tolerability of a spray (“Otomize”) versus a standard drop formulation (“Sofradex”) in the treatment of patients with otitis externa. Curr Med Res Opin 1990;12:12-18.
29. McGarry GW, Swan IRC. Endoscopic photographic comparison of drug delivery by ear-drops and by aerosol spray. Clin Otolaryngol 1992;17:359-60
30. Wilde AD, England J, Jones AS. An alternative to reguler dressings for otitis externa and chronic supperative otitis media? J Laryngol Otol 1995;109:101-03.
31. Gehanno P. Ciprofloxacin in the treatment of malignant external otitis. Chemotherapy 1994;40:35-40.
32. Levy R, Shpitzer T, Shvero J, Pitlik SD. Oral ofloxacin as treatment of malignant external otitis: a study of 17 cases. Laryngoscope 1990;100:548-51
33. Zikk D, Rapoport Y, Redianu C, Shalit I, Himmelfarb MZ. Oral ofloxacin therapy for invasive external otitis. Ann Otol Rhinol Laryngol 1991;100:632-37.
34. Mader JT, Love JT. Malignant external otitis: cure with adjunctive hyperbaric oxygen therapy. Arch Otolaryngol 1982;108:38-40.
35. Neu HC. Contemporary antibiotic therapy in otolaryngology. Otolaryngol Clin N Am 1984;17:745-60.
Otitis externa (OE), also referred to as external otitis, is inflammation of the auricle, external ear, or tympanic membrane. The severity can range from mild inflammation to life-threatening infection.1 It is commonly seen by family physicians and affects 4 out of each 1000 Americans every year.2 In most cases the significant pain of OE compels the patient to seek care urgently.
OE can be categorized as localized or diffuse. When it persists for more than 6 months, it is considered chronic and is more commonly bilateral. It is thought to be caused by local trauma to the external canal, diabetes, high humidity, loss of the canal’s protective coating of cerumen, eczema, use of a hearing aid or stethoscope, or glandular obstruction. It is commonly seen in swimmers, particularly in the summer months.1 The most frequent symptoms are discharge, pain, hearing loss, itching, and tinnitus.
Necrotizing (malignant) otitis externa (NOE) is the most severe form of OE and is most often seen in elderly patients with diabetes. One case series in a referral population found a mortality rate of 53%.3 Pain, purulent discharge, bilateral involvement, and external canal granulation tissue are common symptoms.
Pathophysiology
The ear canal is a blind sac with an anterior recess. Trauma to the canal, accumulation of keratin, or a change in pH can trigger inflammation and infection. One study4 found that aerobic bacteria account for 91% of bacterial causes; anaerobes, 4%; and mixed infections, 4%. The most common offending organisms are Pseudomonas aeruginosa (50%), Staphylococcus aureus (23%), anaerobes and gram-negative organisms (12.5%), and yeast, such as Aspergillus and Candida (12.5%). The increased pH of pool water is believed to make infection more likely, since bacteriologic studies fail to show a direct link between swimming pool contamination and the organisms of OE.
Diagnosis
There are no published studies of the accuracy of the medical history, physical examination, or office laboratory tests for the diagnosis of OE. Diagnosis is usually made based on physical examination findings: pain on movement of the auricle, edema, redness, and foul-smelling discharge.5 Swelling often obscures the tympanic membrane.
NOE is also a clinical diagnosis and requires a high index of suspicion. A study by Zaky and colleagues3 considered 2 new cases and 32 that were retrospectively reviewed from the literature. They found the following frequencies of symptoms: pain (100%), purulent discharge (97%), bilateral involvement (21%), and a polyp in the external canal (88%). Diabetes was common in this group of patients (82%), confirming that it is consistently a predisposing risk factor, and 91% of these patients were aged 55 years and older.
Few papers have been published concerning diagnostic studies for NOE. One found that temporal radiographs and tomograms are positive in most cases of NOE but were not necessary for diagnosis.6 The erythrocyte sedimentation rate is usually increased,3 but this is true in many other illnesses. Many studies3,8-9,31-35 have reported that Pseudomonas is the primary offending organism for NOE. Because most ear cultures are positive for Pseudomonas, these cultures are of questionable value.10
There are 4 studies that consider more aggressive diagnostic testing for NOE using conventional temporal radiographs, tomographic temporal radiographs, qualitative and single photon emission computed tomography67 gallium scans, and qualitative and quantitative99 technetium bone scans.6-9 These studies were limited by the use of unclear7 or poor quality6,8 reference standards (radiograph or poor response to antibiotics). The diagnosis of NOE does not require additional studies. Expert opinion supports a diagnosis based on the history and physical examination and poor response to treatment. The most common symptom of NOE is persistent pain that is constant and severe. The leukocyte count may be normal or mildly elevated.3 Physicians should consider the diagnosis of NOE in any patient with diabetes who has OE, particularly older patients. Characteristics of OE and NOE are presented in Table 1.
Treatment
Otitis Externa
The main principles of treatment are local cleansing of debris, drainage of the infection, re-establishment of the normal acidic environment, use of topical and systemic antimicrobials, and prevention of recurrent infections. The evidence regarding these treatments is summarized in Table 2. The best evidence (grade of evidence: A) demonstrates equivalent results with ear cleaning, an ear wick, and any of the choices of topical agents12-13—acidifying agents, antibiotics, antibiotic and steroid combinations, or antifungal agents. Frequent dosing (3 to 4 times daily) for at least 4 days is supported by the studies. Two studies demonstrated equivalent efficacy with topical ciprofloxacin or ofloxacin dosed twice daily compared with antibiotic and steroid combinations dosed 4 times daily.16-17 However, these agents are also more expensive than older topical antibiotics. The evidence for single topical treatments and oral antibiotics is weaker14-29 (grade of evidence: B).
Physicians should treat patients with one of the following regimens for at least 4 days:
- ear cleaning + ear wick + acidifying agent dosed 4 times daily
- ear cleaning + ear wick + topical antibiotic dosed 4 times daily (twice daily if quinolone)
- ear cleaning + ear wick + topical antibiotic/steroid combination dosed 4 times daily (twice daily if quinolone)
The ear is best cleaned by simply irrigating the canal. Be sure to look for a foreign body, particularly in younger patients. For a wick, use either the Pope ear wick (Merocel Corporation, Mystic, Conn) or a fourth-inch sterile gauze. The wick helps draw topical medications into the affected canal, particularly when it is obstructed. The patient should return in approximately 2 days for removal of the wick and reassessment. Do not forget analgesics; this is a painful condition.
Necrotizing Otitis Externa
The treatment for NOE must be aggressive. As the infection invades through the soft tissues into surrounding bony structures, it can be life threatening.10 The evidence for various treatments is presented in Table 3. The studies are weaker than those for treatment of otitis externa (grade of recommendations: B and C). They support twice daily dosing with oral ofloxacin 400 mg orally twice daily or ciprofloxacin 750 mg orally twice daily for up to 3 months.31-33 There is weak evidence that hyperbaric oxygen is effective.34 Intravenous anti-Pseudomonal antibiotics are often used initially, based on expert opinion (grade: D) and the high mortality rate.
The oral fluoroquinolones (ofloxacin and ciprofloxacin) show promise for treating NOE. The choice of parenteral or oral antibiotics still rests with clinician judgment, based on the patient’s clinical presentation. If oral antibiotics are started, the length of treatment should be based on the severity of illness.
Prognosis
The prognosis for cure of OE is excellent, although the actual natural history of untreated disease has not been studied. OE complicated by NEO is more common in persons with diabetes who have a persistent course and granulation tissue visualized in the external auditory canal. Untreated NEO can lead to osteomyelitis and paralysis of cranial nerves. Death can result from sepsis and central nervous system infection.
Otitis externa (OE), also referred to as external otitis, is inflammation of the auricle, external ear, or tympanic membrane. The severity can range from mild inflammation to life-threatening infection.1 It is commonly seen by family physicians and affects 4 out of each 1000 Americans every year.2 In most cases the significant pain of OE compels the patient to seek care urgently.
OE can be categorized as localized or diffuse. When it persists for more than 6 months, it is considered chronic and is more commonly bilateral. It is thought to be caused by local trauma to the external canal, diabetes, high humidity, loss of the canal’s protective coating of cerumen, eczema, use of a hearing aid or stethoscope, or glandular obstruction. It is commonly seen in swimmers, particularly in the summer months.1 The most frequent symptoms are discharge, pain, hearing loss, itching, and tinnitus.
Necrotizing (malignant) otitis externa (NOE) is the most severe form of OE and is most often seen in elderly patients with diabetes. One case series in a referral population found a mortality rate of 53%.3 Pain, purulent discharge, bilateral involvement, and external canal granulation tissue are common symptoms.
Pathophysiology
The ear canal is a blind sac with an anterior recess. Trauma to the canal, accumulation of keratin, or a change in pH can trigger inflammation and infection. One study4 found that aerobic bacteria account for 91% of bacterial causes; anaerobes, 4%; and mixed infections, 4%. The most common offending organisms are Pseudomonas aeruginosa (50%), Staphylococcus aureus (23%), anaerobes and gram-negative organisms (12.5%), and yeast, such as Aspergillus and Candida (12.5%). The increased pH of pool water is believed to make infection more likely, since bacteriologic studies fail to show a direct link between swimming pool contamination and the organisms of OE.
Diagnosis
There are no published studies of the accuracy of the medical history, physical examination, or office laboratory tests for the diagnosis of OE. Diagnosis is usually made based on physical examination findings: pain on movement of the auricle, edema, redness, and foul-smelling discharge.5 Swelling often obscures the tympanic membrane.
NOE is also a clinical diagnosis and requires a high index of suspicion. A study by Zaky and colleagues3 considered 2 new cases and 32 that were retrospectively reviewed from the literature. They found the following frequencies of symptoms: pain (100%), purulent discharge (97%), bilateral involvement (21%), and a polyp in the external canal (88%). Diabetes was common in this group of patients (82%), confirming that it is consistently a predisposing risk factor, and 91% of these patients were aged 55 years and older.
Few papers have been published concerning diagnostic studies for NOE. One found that temporal radiographs and tomograms are positive in most cases of NOE but were not necessary for diagnosis.6 The erythrocyte sedimentation rate is usually increased,3 but this is true in many other illnesses. Many studies3,8-9,31-35 have reported that Pseudomonas is the primary offending organism for NOE. Because most ear cultures are positive for Pseudomonas, these cultures are of questionable value.10
There are 4 studies that consider more aggressive diagnostic testing for NOE using conventional temporal radiographs, tomographic temporal radiographs, qualitative and single photon emission computed tomography67 gallium scans, and qualitative and quantitative99 technetium bone scans.6-9 These studies were limited by the use of unclear7 or poor quality6,8 reference standards (radiograph or poor response to antibiotics). The diagnosis of NOE does not require additional studies. Expert opinion supports a diagnosis based on the history and physical examination and poor response to treatment. The most common symptom of NOE is persistent pain that is constant and severe. The leukocyte count may be normal or mildly elevated.3 Physicians should consider the diagnosis of NOE in any patient with diabetes who has OE, particularly older patients. Characteristics of OE and NOE are presented in Table 1.
Treatment
Otitis Externa
The main principles of treatment are local cleansing of debris, drainage of the infection, re-establishment of the normal acidic environment, use of topical and systemic antimicrobials, and prevention of recurrent infections. The evidence regarding these treatments is summarized in Table 2. The best evidence (grade of evidence: A) demonstrates equivalent results with ear cleaning, an ear wick, and any of the choices of topical agents12-13—acidifying agents, antibiotics, antibiotic and steroid combinations, or antifungal agents. Frequent dosing (3 to 4 times daily) for at least 4 days is supported by the studies. Two studies demonstrated equivalent efficacy with topical ciprofloxacin or ofloxacin dosed twice daily compared with antibiotic and steroid combinations dosed 4 times daily.16-17 However, these agents are also more expensive than older topical antibiotics. The evidence for single topical treatments and oral antibiotics is weaker14-29 (grade of evidence: B).
Physicians should treat patients with one of the following regimens for at least 4 days:
- ear cleaning + ear wick + acidifying agent dosed 4 times daily
- ear cleaning + ear wick + topical antibiotic dosed 4 times daily (twice daily if quinolone)
- ear cleaning + ear wick + topical antibiotic/steroid combination dosed 4 times daily (twice daily if quinolone)
The ear is best cleaned by simply irrigating the canal. Be sure to look for a foreign body, particularly in younger patients. For a wick, use either the Pope ear wick (Merocel Corporation, Mystic, Conn) or a fourth-inch sterile gauze. The wick helps draw topical medications into the affected canal, particularly when it is obstructed. The patient should return in approximately 2 days for removal of the wick and reassessment. Do not forget analgesics; this is a painful condition.
Necrotizing Otitis Externa
The treatment for NOE must be aggressive. As the infection invades through the soft tissues into surrounding bony structures, it can be life threatening.10 The evidence for various treatments is presented in Table 3. The studies are weaker than those for treatment of otitis externa (grade of recommendations: B and C). They support twice daily dosing with oral ofloxacin 400 mg orally twice daily or ciprofloxacin 750 mg orally twice daily for up to 3 months.31-33 There is weak evidence that hyperbaric oxygen is effective.34 Intravenous anti-Pseudomonal antibiotics are often used initially, based on expert opinion (grade: D) and the high mortality rate.
The oral fluoroquinolones (ofloxacin and ciprofloxacin) show promise for treating NOE. The choice of parenteral or oral antibiotics still rests with clinician judgment, based on the patient’s clinical presentation. If oral antibiotics are started, the length of treatment should be based on the severity of illness.
Prognosis
The prognosis for cure of OE is excellent, although the actual natural history of untreated disease has not been studied. OE complicated by NEO is more common in persons with diabetes who have a persistent course and granulation tissue visualized in the external auditory canal. Untreated NEO can lead to osteomyelitis and paralysis of cranial nerves. Death can result from sepsis and central nervous system infection.
1. Agius AM, Pickles JM, Burch KL. A prospective study of otitis externa. Clin Otolaryngol 1992;17:150-54.
2. Diagnosis and treatment of acute otitis media: an interdisciplinary update. Proceedings of a roundtable discussion. Ann Otol Rhinol Laryngol 1999;108:2-23.
3. Zaky DA, Bentley DW, Lowy K, Betts RF, Douglas RG. Malignant external otitis: a severe form of otitis in diabetic patients. Am J Med 1976;61:298-302.
4. Clark WB, Brook I, Bianki D, Thompson DH. Microbiology of otitis externa. Otolaryngol Head Neck Surg 1997;116:23-25.
5. Leung AKC, Fong JHS, Leong AG. Otalgia in children. J Natl Med Assoc 2000;92:254-60.
6. Kim BH. Roentgenographic findings of malignant external otitis. Am J Roentgenol Radium Ther Nucl Med 1971;112:366-72.
7. Stokkel MPM, Boot ICN, vanEck-Smit BLF. SPECT gallium scintigraphy in malignant external otitis: initial staging and follow-up: case reports. Laryngoscope 1996;106:338-40.
8. Uri N, Gips S, Front A, Meyer SW, Hardoff R. Quantitative bone and 67Ga scintigraphy in the differentiation of necrotizing external otitis from severe external otitis. Arch Otolarngol Head Neck Surg 1991;117:623-26.
9. Hardoff R, Gips S, Uri N, Front A, Tamir A. Semiquantitative skull planar and SPECT bone scintigraphy in diabetic patients: differentiation of necrotizing (malignant) external otitis from severe external otitis. J Nucl Med 1994;35:411-15.
10. Brook I. Treatment of otitis externa in children. Pediatric Drugs 1999;1:283-89.
11. Densert O, Toremalm NG. Pain relief with indomethacin in external otitis. Arch Otolaryng 1972;95:460-63.
12. Ordonez GE, Kime CE, Updegraff WR, Glassman JM, Soyka JP. Effective treatment of acute diffuse otitis externa: I. a controlled comparison of hydrocortisone-acetic acid, non-aqueous and hydrocortisone-neomycin-polymyxin b-colistin otic solutions. Curr Ther Res 1978;23:ss3-14.
13. Kime CE, Ordonez GU, Updegraff WR, Glassman JM, Soyka JP. Effective Treatment of acute diffuse otitis externa: II. a controlled comparison of hydrocortisone-acetic acid, non-aqueous and hydrocortisone-neomycin-polymyxin b otic solutions. Curr Ther Res 1978;23:ss15-28.
14. Freedman R. Versus placebo in treatment of acute otitis externa. Ear Nose Throat J 1978;57:28-37.
15. Gyde MC, Norris D, Kavalec EC. The weeping ear: clinical re-evaluation of treatment. J Int Med Res 1982;10:333-40.
16. Arnes A, Dibb WL. Otitis externa: clinical comparison of local ciprofloxacin versus local oxytetracycline, polymyxin B, hydrocortisone combination treatment. Curr Med Res Opin 1993;13:182-86.
17. Jones RN, Milazzo J, Seidlin M. Ofloxacin otic solution for treatment of otits externa in children and adults. Arch Otolaryngol Head Neck Surg 1997;123:1193-200.
18. Wadston CJ, Bertilsson CA, Sieradzki H, Edstrom S. A randomized clinical trial of two topical preparations (framycitin/gramicidin and oxytetracycline/hydrocortisone with polymyxin b) in the treatment of external otitis. Arch Otorhinolaryngol 1985;242:135-39.
19. Barton RPE, Wright JLW, Gray RFE. The clinical evaluation of a new clobetasol propionate preparation in the treatment of otitis externa. J Laryngol Otol 1979;93:703-06.
20. Treatment of otitis externa: a clinical trial of local applications. Br J Clin Pract 1967;21:507-10.
21. Bain DJG. A double-blind comparative study of otoseptil ear drops and otosporin: ear drops in otitis externa. J Int Med Res 1976;4:79-81.
22. Worgan D. Treatment of otitis externa: report of a clinical trial. Practitioner 1969;202:817-20.
23. Yelland MJ. The efficacy of oral cotrimoxazole in the treatment of otitis externa in general practice. Med J Aust 1993;158:697-99.
24. Barr GD, Al-Khabori M. A randomized prospective comparison of two methods of administering topical treatment in otitis externa. Clinical Otolaryng Allied Sci 1991;16:547-48.
25. Clayton MI, Osborne JE, Rutherford D, Rivron RP. A double-blind, randomized, prospective trial of a topical antiseptic versus a topical antibiotic in the treatment of otorrhea. Clin Otolaryngol 1990;15:7-10.
26. Cannon S. External otitis: controlled therapeutic trial. Eye Ear Nose Throat Monthly 1970;49:56-61.
27. Slack RWT. A study of three preparations in the treatment of otitis externa. J Laryngol Otol 1987;101:533-35.
28. Smith RB, Moodie J. A general practice study to compare the efficacy and tolerability of a spray (“Otomize”) versus a standard drop formulation (“Sofradex”) in the treatment of patients with otitis externa. Curr Med Res Opin 1990;12:12-18.
29. McGarry GW, Swan IRC. Endoscopic photographic comparison of drug delivery by ear-drops and by aerosol spray. Clin Otolaryngol 1992;17:359-60
30. Wilde AD, England J, Jones AS. An alternative to reguler dressings for otitis externa and chronic supperative otitis media? J Laryngol Otol 1995;109:101-03.
31. Gehanno P. Ciprofloxacin in the treatment of malignant external otitis. Chemotherapy 1994;40:35-40.
32. Levy R, Shpitzer T, Shvero J, Pitlik SD. Oral ofloxacin as treatment of malignant external otitis: a study of 17 cases. Laryngoscope 1990;100:548-51
33. Zikk D, Rapoport Y, Redianu C, Shalit I, Himmelfarb MZ. Oral ofloxacin therapy for invasive external otitis. Ann Otol Rhinol Laryngol 1991;100:632-37.
34. Mader JT, Love JT. Malignant external otitis: cure with adjunctive hyperbaric oxygen therapy. Arch Otolaryngol 1982;108:38-40.
35. Neu HC. Contemporary antibiotic therapy in otolaryngology. Otolaryngol Clin N Am 1984;17:745-60.
1. Agius AM, Pickles JM, Burch KL. A prospective study of otitis externa. Clin Otolaryngol 1992;17:150-54.
2. Diagnosis and treatment of acute otitis media: an interdisciplinary update. Proceedings of a roundtable discussion. Ann Otol Rhinol Laryngol 1999;108:2-23.
3. Zaky DA, Bentley DW, Lowy K, Betts RF, Douglas RG. Malignant external otitis: a severe form of otitis in diabetic patients. Am J Med 1976;61:298-302.
4. Clark WB, Brook I, Bianki D, Thompson DH. Microbiology of otitis externa. Otolaryngol Head Neck Surg 1997;116:23-25.
5. Leung AKC, Fong JHS, Leong AG. Otalgia in children. J Natl Med Assoc 2000;92:254-60.
6. Kim BH. Roentgenographic findings of malignant external otitis. Am J Roentgenol Radium Ther Nucl Med 1971;112:366-72.
7. Stokkel MPM, Boot ICN, vanEck-Smit BLF. SPECT gallium scintigraphy in malignant external otitis: initial staging and follow-up: case reports. Laryngoscope 1996;106:338-40.
8. Uri N, Gips S, Front A, Meyer SW, Hardoff R. Quantitative bone and 67Ga scintigraphy in the differentiation of necrotizing external otitis from severe external otitis. Arch Otolarngol Head Neck Surg 1991;117:623-26.
9. Hardoff R, Gips S, Uri N, Front A, Tamir A. Semiquantitative skull planar and SPECT bone scintigraphy in diabetic patients: differentiation of necrotizing (malignant) external otitis from severe external otitis. J Nucl Med 1994;35:411-15.
10. Brook I. Treatment of otitis externa in children. Pediatric Drugs 1999;1:283-89.
11. Densert O, Toremalm NG. Pain relief with indomethacin in external otitis. Arch Otolaryng 1972;95:460-63.
12. Ordonez GE, Kime CE, Updegraff WR, Glassman JM, Soyka JP. Effective treatment of acute diffuse otitis externa: I. a controlled comparison of hydrocortisone-acetic acid, non-aqueous and hydrocortisone-neomycin-polymyxin b-colistin otic solutions. Curr Ther Res 1978;23:ss3-14.
13. Kime CE, Ordonez GU, Updegraff WR, Glassman JM, Soyka JP. Effective Treatment of acute diffuse otitis externa: II. a controlled comparison of hydrocortisone-acetic acid, non-aqueous and hydrocortisone-neomycin-polymyxin b otic solutions. Curr Ther Res 1978;23:ss15-28.
14. Freedman R. Versus placebo in treatment of acute otitis externa. Ear Nose Throat J 1978;57:28-37.
15. Gyde MC, Norris D, Kavalec EC. The weeping ear: clinical re-evaluation of treatment. J Int Med Res 1982;10:333-40.
16. Arnes A, Dibb WL. Otitis externa: clinical comparison of local ciprofloxacin versus local oxytetracycline, polymyxin B, hydrocortisone combination treatment. Curr Med Res Opin 1993;13:182-86.
17. Jones RN, Milazzo J, Seidlin M. Ofloxacin otic solution for treatment of otits externa in children and adults. Arch Otolaryngol Head Neck Surg 1997;123:1193-200.
18. Wadston CJ, Bertilsson CA, Sieradzki H, Edstrom S. A randomized clinical trial of two topical preparations (framycitin/gramicidin and oxytetracycline/hydrocortisone with polymyxin b) in the treatment of external otitis. Arch Otorhinolaryngol 1985;242:135-39.
19. Barton RPE, Wright JLW, Gray RFE. The clinical evaluation of a new clobetasol propionate preparation in the treatment of otitis externa. J Laryngol Otol 1979;93:703-06.
20. Treatment of otitis externa: a clinical trial of local applications. Br J Clin Pract 1967;21:507-10.
21. Bain DJG. A double-blind comparative study of otoseptil ear drops and otosporin: ear drops in otitis externa. J Int Med Res 1976;4:79-81.
22. Worgan D. Treatment of otitis externa: report of a clinical trial. Practitioner 1969;202:817-20.
23. Yelland MJ. The efficacy of oral cotrimoxazole in the treatment of otitis externa in general practice. Med J Aust 1993;158:697-99.
24. Barr GD, Al-Khabori M. A randomized prospective comparison of two methods of administering topical treatment in otitis externa. Clinical Otolaryng Allied Sci 1991;16:547-48.
25. Clayton MI, Osborne JE, Rutherford D, Rivron RP. A double-blind, randomized, prospective trial of a topical antiseptic versus a topical antibiotic in the treatment of otorrhea. Clin Otolaryngol 1990;15:7-10.
26. Cannon S. External otitis: controlled therapeutic trial. Eye Ear Nose Throat Monthly 1970;49:56-61.
27. Slack RWT. A study of three preparations in the treatment of otitis externa. J Laryngol Otol 1987;101:533-35.
28. Smith RB, Moodie J. A general practice study to compare the efficacy and tolerability of a spray (“Otomize”) versus a standard drop formulation (“Sofradex”) in the treatment of patients with otitis externa. Curr Med Res Opin 1990;12:12-18.
29. McGarry GW, Swan IRC. Endoscopic photographic comparison of drug delivery by ear-drops and by aerosol spray. Clin Otolaryngol 1992;17:359-60
30. Wilde AD, England J, Jones AS. An alternative to reguler dressings for otitis externa and chronic supperative otitis media? J Laryngol Otol 1995;109:101-03.
31. Gehanno P. Ciprofloxacin in the treatment of malignant external otitis. Chemotherapy 1994;40:35-40.
32. Levy R, Shpitzer T, Shvero J, Pitlik SD. Oral ofloxacin as treatment of malignant external otitis: a study of 17 cases. Laryngoscope 1990;100:548-51
33. Zikk D, Rapoport Y, Redianu C, Shalit I, Himmelfarb MZ. Oral ofloxacin therapy for invasive external otitis. Ann Otol Rhinol Laryngol 1991;100:632-37.
34. Mader JT, Love JT. Malignant external otitis: cure with adjunctive hyperbaric oxygen therapy. Arch Otolaryngol 1982;108:38-40.
35. Neu HC. Contemporary antibiotic therapy in otolaryngology. Otolaryngol Clin N Am 1984;17:745-60.