User login
Managing ADHD in children: Are you doing enough?
• Side effects of psychostimulants can often be managed with monitoring, dose adjustment, a switch to another drug, or adjunctive therapy. A
• Weigh and measure a child being treated for ADHD twice a year; aberrant growth may indicate a need for a change in medication regimen. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Untreated attention deficit hyperactivity disorder (ADHD) can have serious academic, social, and psychological consequences, both for young patients and their parents. Diagnosis is based on criteria detailed in the Diagnostic and Statistical Manual of Mental Health Disorders, Fourth Edition Text Revision (DSM-IV-TR), with observations of the child’s behavior obtained from more than one setting.
Physicians should also consider the possibility of coexisting conditions, which could complicate diagnosis and subsequent attempts to treat the signs and symptoms of ADHD. Treatment is multifaceted, and will vary depending on severity, comorbidities, and the degree of compliance with nonpharmacologic modalities.
A comprehensive approach is called for
Managing pediatric ADHD in a primary care setting requires a comprehensive, goal-oriented treatment plan. The primary goal, as noted in the American Academy of Child and Adolescent Psychiatry (AACAP)’s ADHD guideline,1 is to maximize the child’s functioning, both in terms of an improvement in relationships and academic performance and a reduction of disruptive behavior. Parents and children should be integrated into community supports and school resources, the guideline recommends1 (strength of recommendation [SOR]: A).
Additional recommendations focus on patient (and parental) education, and on medication, monitoring, and follow-up (SOR: A). Physicians should:
Educate parents and patients about common ADHD symptoms and treatment strategies.
Initiate pharmacotherapy. Select an agent that is approved by the US Food and Drug Administration (FDA) for ADHD. These include the psychostimulants dextroamphetamine, D- and DL-methylphenidate, and mixed salts amphetamine; and atomoxetine, a noradrenergic reuptake inhibitor. (Central nervous system stimulants should be avoided in children with cardiac abnormalities, who are at increased risk of experiencing sympathomimetic effects.)
Familiarize themselves with medication side effects. Decreased appetite, insomnia, headache, abdominal pain, and irritable mood are the most common side effects of psychostimulants. Common side effects of atomoxetine include somnolence, anorexia, nausea, skin rash, and a mild increase in blood pressure or heart rate. Notably, there is a small risk of suicide associated with atomoxetine.
Monitor patients for the emergence and severity of side effects. Many of the side effects of stimulants are transient and can be managed through monitoring, as long as it does not compromise the patient’s health or interfere with daily living. Side effects can also be managed with dose adjustment, change of drug treatment, or adjunctive therapy.
Measure height and weight of the patient twice yearly. If a child’s height or weight crosses 2 percentiles on his or her growth curve, it may be an indication of aberrant growth—and a drug holiday or switching to a different medication should be considered.
Evaluate treatment success several times a year. The review should include behavior, academic progress, emergence of comorbid disorders, and the need for behavioral therapy and continuing pharmacotherapy. A lack of response to one psychostimulant is not predictive of the patient’s response to another, the AACAP emphasizes, and it is important to keep trying to find another medication until treatment goals are reached.1
If none of the FDA-approved ADHD medications has the desired results, the AACAP recommends (SOR: B):
- a referral to a cognitive behavioral therapist or child psychologist
- a trial with a medication that is not FDA-approved for ADHD, such as bupropion, a tricyclic antidepressant, or an alpha-agonist
- a reevaluation of the ADHD diagnosis, adherence to the treatment plan, and the presence of comorbid conditions.1
AAP stresses hands-on behavioral intervention
The American Academy of Pediatrics (AAP) also has a clinical practice guideline for the treatment of ADHD, issued in 2001.2 Its recommendations are similar to those of the AACAP. But AAP puts additional emphasis on parental training in behavioral therapy and classroom behavioral interventions, and considers both to be more effective than cognitive behavioral therapy (CBT).2
Virtual reality: A viable option?
Although conventional treatment of childhood ADHD has had considerable clinical success, other forms of treatment may be needed in some cases—if a child’s parents reject psychopharmacologic treatment, for example, or medication trials and traditional behavioral therapies, such as CBT, fail to bring the desired results.
Virtual reality (VR), a computer-generated 3-dimensional interactive system, is an emerging clinical tool. VR programs such as The Virtual Classroom3,4—in which a child is “immersed” in a simulated classroom setting—have shown promise for ADHD assessment and treatment.
Perhaps the biggest benefit of VR as an ADHD intervention is the opportunity for a clinician to place a patient in a virtual classroom, with tasks that require the child’s attention as well as distractors, such as conversation, ambient noise, and moving objects. Another advantage is the ability to integrate traditional assessment tools (Continuous Performance Tasks, for example) and treatment modalities, such as CBT.5 This can be accomplished through a graphic display of a child’s performance during a VR session, which the therapist can use as part of the therapeutic process.3 And VR has no side effects.
Several facilities are either using or experimenting with VR for ADHD. More information is available from the Virtual Reality Medical Center at http://www.vrphobia.com/adhd.htm.
CORRESPONDENCE
Keith B. Holten, MD, Berger Health System, 600 North Pickaway Street, Circleville, OH 43113; [email protected]
1. Pliszka S. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894-921.
2. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033-1044.
3. Rizzo AA, Buckwalter JG, Humphrey L, et al. The virtual classroom: a virtual environment for the assessment and rehabilitation of attention deficits. CyberPsych Behav. 2000;3:483-499.
4. Rizzo AA, Klimchuk D, Mitura R, et al. A virtual reality scenario for all seasons: the virtual classroom. CNS Spectr. 2006;11:35-44.
5. Pollak Y, Weiss PL, Rizzo AA, et al. The utility of a continuous performance test embedded in virtual reality in measuring ADHD-related deficits. J Dev Behav Pediatr. 2009;30:2-6.
• Side effects of psychostimulants can often be managed with monitoring, dose adjustment, a switch to another drug, or adjunctive therapy. A
• Weigh and measure a child being treated for ADHD twice a year; aberrant growth may indicate a need for a change in medication regimen. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Untreated attention deficit hyperactivity disorder (ADHD) can have serious academic, social, and psychological consequences, both for young patients and their parents. Diagnosis is based on criteria detailed in the Diagnostic and Statistical Manual of Mental Health Disorders, Fourth Edition Text Revision (DSM-IV-TR), with observations of the child’s behavior obtained from more than one setting.
Physicians should also consider the possibility of coexisting conditions, which could complicate diagnosis and subsequent attempts to treat the signs and symptoms of ADHD. Treatment is multifaceted, and will vary depending on severity, comorbidities, and the degree of compliance with nonpharmacologic modalities.
A comprehensive approach is called for
Managing pediatric ADHD in a primary care setting requires a comprehensive, goal-oriented treatment plan. The primary goal, as noted in the American Academy of Child and Adolescent Psychiatry (AACAP)’s ADHD guideline,1 is to maximize the child’s functioning, both in terms of an improvement in relationships and academic performance and a reduction of disruptive behavior. Parents and children should be integrated into community supports and school resources, the guideline recommends1 (strength of recommendation [SOR]: A).
Additional recommendations focus on patient (and parental) education, and on medication, monitoring, and follow-up (SOR: A). Physicians should:
Educate parents and patients about common ADHD symptoms and treatment strategies.
Initiate pharmacotherapy. Select an agent that is approved by the US Food and Drug Administration (FDA) for ADHD. These include the psychostimulants dextroamphetamine, D- and DL-methylphenidate, and mixed salts amphetamine; and atomoxetine, a noradrenergic reuptake inhibitor. (Central nervous system stimulants should be avoided in children with cardiac abnormalities, who are at increased risk of experiencing sympathomimetic effects.)
Familiarize themselves with medication side effects. Decreased appetite, insomnia, headache, abdominal pain, and irritable mood are the most common side effects of psychostimulants. Common side effects of atomoxetine include somnolence, anorexia, nausea, skin rash, and a mild increase in blood pressure or heart rate. Notably, there is a small risk of suicide associated with atomoxetine.
Monitor patients for the emergence and severity of side effects. Many of the side effects of stimulants are transient and can be managed through monitoring, as long as it does not compromise the patient’s health or interfere with daily living. Side effects can also be managed with dose adjustment, change of drug treatment, or adjunctive therapy.
Measure height and weight of the patient twice yearly. If a child’s height or weight crosses 2 percentiles on his or her growth curve, it may be an indication of aberrant growth—and a drug holiday or switching to a different medication should be considered.
Evaluate treatment success several times a year. The review should include behavior, academic progress, emergence of comorbid disorders, and the need for behavioral therapy and continuing pharmacotherapy. A lack of response to one psychostimulant is not predictive of the patient’s response to another, the AACAP emphasizes, and it is important to keep trying to find another medication until treatment goals are reached.1
If none of the FDA-approved ADHD medications has the desired results, the AACAP recommends (SOR: B):
- a referral to a cognitive behavioral therapist or child psychologist
- a trial with a medication that is not FDA-approved for ADHD, such as bupropion, a tricyclic antidepressant, or an alpha-agonist
- a reevaluation of the ADHD diagnosis, adherence to the treatment plan, and the presence of comorbid conditions.1
AAP stresses hands-on behavioral intervention
The American Academy of Pediatrics (AAP) also has a clinical practice guideline for the treatment of ADHD, issued in 2001.2 Its recommendations are similar to those of the AACAP. But AAP puts additional emphasis on parental training in behavioral therapy and classroom behavioral interventions, and considers both to be more effective than cognitive behavioral therapy (CBT).2
Virtual reality: A viable option?
Although conventional treatment of childhood ADHD has had considerable clinical success, other forms of treatment may be needed in some cases—if a child’s parents reject psychopharmacologic treatment, for example, or medication trials and traditional behavioral therapies, such as CBT, fail to bring the desired results.
Virtual reality (VR), a computer-generated 3-dimensional interactive system, is an emerging clinical tool. VR programs such as The Virtual Classroom3,4—in which a child is “immersed” in a simulated classroom setting—have shown promise for ADHD assessment and treatment.
Perhaps the biggest benefit of VR as an ADHD intervention is the opportunity for a clinician to place a patient in a virtual classroom, with tasks that require the child’s attention as well as distractors, such as conversation, ambient noise, and moving objects. Another advantage is the ability to integrate traditional assessment tools (Continuous Performance Tasks, for example) and treatment modalities, such as CBT.5 This can be accomplished through a graphic display of a child’s performance during a VR session, which the therapist can use as part of the therapeutic process.3 And VR has no side effects.
Several facilities are either using or experimenting with VR for ADHD. More information is available from the Virtual Reality Medical Center at http://www.vrphobia.com/adhd.htm.
CORRESPONDENCE
Keith B. Holten, MD, Berger Health System, 600 North Pickaway Street, Circleville, OH 43113; [email protected]
• Side effects of psychostimulants can often be managed with monitoring, dose adjustment, a switch to another drug, or adjunctive therapy. A
• Weigh and measure a child being treated for ADHD twice a year; aberrant growth may indicate a need for a change in medication regimen. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Untreated attention deficit hyperactivity disorder (ADHD) can have serious academic, social, and psychological consequences, both for young patients and their parents. Diagnosis is based on criteria detailed in the Diagnostic and Statistical Manual of Mental Health Disorders, Fourth Edition Text Revision (DSM-IV-TR), with observations of the child’s behavior obtained from more than one setting.
Physicians should also consider the possibility of coexisting conditions, which could complicate diagnosis and subsequent attempts to treat the signs and symptoms of ADHD. Treatment is multifaceted, and will vary depending on severity, comorbidities, and the degree of compliance with nonpharmacologic modalities.
A comprehensive approach is called for
Managing pediatric ADHD in a primary care setting requires a comprehensive, goal-oriented treatment plan. The primary goal, as noted in the American Academy of Child and Adolescent Psychiatry (AACAP)’s ADHD guideline,1 is to maximize the child’s functioning, both in terms of an improvement in relationships and academic performance and a reduction of disruptive behavior. Parents and children should be integrated into community supports and school resources, the guideline recommends1 (strength of recommendation [SOR]: A).
Additional recommendations focus on patient (and parental) education, and on medication, monitoring, and follow-up (SOR: A). Physicians should:
Educate parents and patients about common ADHD symptoms and treatment strategies.
Initiate pharmacotherapy. Select an agent that is approved by the US Food and Drug Administration (FDA) for ADHD. These include the psychostimulants dextroamphetamine, D- and DL-methylphenidate, and mixed salts amphetamine; and atomoxetine, a noradrenergic reuptake inhibitor. (Central nervous system stimulants should be avoided in children with cardiac abnormalities, who are at increased risk of experiencing sympathomimetic effects.)
Familiarize themselves with medication side effects. Decreased appetite, insomnia, headache, abdominal pain, and irritable mood are the most common side effects of psychostimulants. Common side effects of atomoxetine include somnolence, anorexia, nausea, skin rash, and a mild increase in blood pressure or heart rate. Notably, there is a small risk of suicide associated with atomoxetine.
Monitor patients for the emergence and severity of side effects. Many of the side effects of stimulants are transient and can be managed through monitoring, as long as it does not compromise the patient’s health or interfere with daily living. Side effects can also be managed with dose adjustment, change of drug treatment, or adjunctive therapy.
Measure height and weight of the patient twice yearly. If a child’s height or weight crosses 2 percentiles on his or her growth curve, it may be an indication of aberrant growth—and a drug holiday or switching to a different medication should be considered.
Evaluate treatment success several times a year. The review should include behavior, academic progress, emergence of comorbid disorders, and the need for behavioral therapy and continuing pharmacotherapy. A lack of response to one psychostimulant is not predictive of the patient’s response to another, the AACAP emphasizes, and it is important to keep trying to find another medication until treatment goals are reached.1
If none of the FDA-approved ADHD medications has the desired results, the AACAP recommends (SOR: B):
- a referral to a cognitive behavioral therapist or child psychologist
- a trial with a medication that is not FDA-approved for ADHD, such as bupropion, a tricyclic antidepressant, or an alpha-agonist
- a reevaluation of the ADHD diagnosis, adherence to the treatment plan, and the presence of comorbid conditions.1
AAP stresses hands-on behavioral intervention
The American Academy of Pediatrics (AAP) also has a clinical practice guideline for the treatment of ADHD, issued in 2001.2 Its recommendations are similar to those of the AACAP. But AAP puts additional emphasis on parental training in behavioral therapy and classroom behavioral interventions, and considers both to be more effective than cognitive behavioral therapy (CBT).2
Virtual reality: A viable option?
Although conventional treatment of childhood ADHD has had considerable clinical success, other forms of treatment may be needed in some cases—if a child’s parents reject psychopharmacologic treatment, for example, or medication trials and traditional behavioral therapies, such as CBT, fail to bring the desired results.
Virtual reality (VR), a computer-generated 3-dimensional interactive system, is an emerging clinical tool. VR programs such as The Virtual Classroom3,4—in which a child is “immersed” in a simulated classroom setting—have shown promise for ADHD assessment and treatment.
Perhaps the biggest benefit of VR as an ADHD intervention is the opportunity for a clinician to place a patient in a virtual classroom, with tasks that require the child’s attention as well as distractors, such as conversation, ambient noise, and moving objects. Another advantage is the ability to integrate traditional assessment tools (Continuous Performance Tasks, for example) and treatment modalities, such as CBT.5 This can be accomplished through a graphic display of a child’s performance during a VR session, which the therapist can use as part of the therapeutic process.3 And VR has no side effects.
Several facilities are either using or experimenting with VR for ADHD. More information is available from the Virtual Reality Medical Center at http://www.vrphobia.com/adhd.htm.
CORRESPONDENCE
Keith B. Holten, MD, Berger Health System, 600 North Pickaway Street, Circleville, OH 43113; [email protected]
1. Pliszka S. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894-921.
2. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033-1044.
3. Rizzo AA, Buckwalter JG, Humphrey L, et al. The virtual classroom: a virtual environment for the assessment and rehabilitation of attention deficits. CyberPsych Behav. 2000;3:483-499.
4. Rizzo AA, Klimchuk D, Mitura R, et al. A virtual reality scenario for all seasons: the virtual classroom. CNS Spectr. 2006;11:35-44.
5. Pollak Y, Weiss PL, Rizzo AA, et al. The utility of a continuous performance test embedded in virtual reality in measuring ADHD-related deficits. J Dev Behav Pediatr. 2009;30:2-6.
1. Pliszka S. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894-921.
2. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033-1044.
3. Rizzo AA, Buckwalter JG, Humphrey L, et al. The virtual classroom: a virtual environment for the assessment and rehabilitation of attention deficits. CyberPsych Behav. 2000;3:483-499.
4. Rizzo AA, Klimchuk D, Mitura R, et al. A virtual reality scenario for all seasons: the virtual classroom. CNS Spectr. 2006;11:35-44.
5. Pollak Y, Weiss PL, Rizzo AA, et al. The utility of a continuous performance test embedded in virtual reality in measuring ADHD-related deficits. J Dev Behav Pediatr. 2009;30:2-6.
What’s the best approach to managing chronic pain?
The authors report no financial relationships relevant to this article.
This article is adapted from the December 2008 installment of The Journal of Family Practice’s “Guideline Update” series. The Journal of Family Practice is an NLM-indexed publication of Quadrant HealthCom Inc., publisher of OBG Management.
- What are the critical steps in the assessment of a patient who suffers chronic pain?
- What are the four biologic mechanisms of pain?
- When is referral to a pain specialist recommended?
Answers to these questions are summarized below, and in the 2008 edition of Assessment and Management of Chronic Pain, a practice guideline developed and first published in 2005 by the Institute for Clinical Systems Improvement (ICSI), which also funded the work. ICSI is a collaboration of 57 medical groups sponsored by six Minnesota health plans. A third edition of the guideline, released in August 2008, summarizes current evidence about the assessment and treatment of chronic pain in mature adolescents (16 to 18 years old) and adults.
A distinct challenge to clinicians
Chronic pain—a persistent, life-altering condition—is one of the most challenging disorders for primary care physicians to treat. Unlike the case with acute pain, for which we seek to cure the underlying biologic condition, the goal of chronic pain management is to improve function in the face of pain that may never completely resolve.
Achieving that goal, according to the new guideline, requires a patient-centered, multifaceted approach—often involving a health-care team that includes specialists in behavioral health and physical rehabilitation—that is coordinated by a primary care physician. An effective treatment plan must address biopsychosocial factors as well as spiritual and cultural issues. Patients must be taught self-management skills focused on fitness, stress reduction, and maintaining a healthy lifestyle.
Grade A recommendations
- Develop a physician–patient partnership. This should include a plan of care and realistic goal-setting.
- Begin physical rehabilitation and psychosocial management. This includes an exercise fitness program, cognitive-behavioral therapy, and self-management.
Grade B recommendations
- Obtain a general history, including psychological assessment and spirituality evaluation, and identify barriers to treatment.
- Obtain a thorough pain history.
- Perform a physical examination, including a focused musculoskeletal and neurologic evaluation.
- Perform diagnostic testing as indicated. X-rays, computed tomography, magnetic resonance imaging, electromyography, and nerve conduction studies can help differentiate the biological mechanisms of pain.
- Teach patients to use pain scales for self-reporting.
Grade C recommendations
- Categorize the 4 biological mechanisms of pain (inflammatory, mechanical, musculoskeletal, or neuropathic).
- Consider the following pharmacologic options for Level-I care:
- Consider the following Level-I therapeutic procedures:
- Consider the following Level-II interventions:
Medications may be part of the treatment plan but should not be the sole focus, according to the guideline. Opioids are an option when other therapies fail.
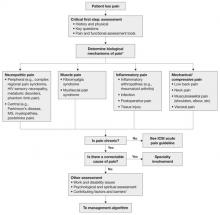
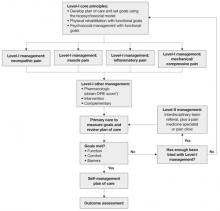
The updated ICSI guideline also addresses the effects of various therapies, the role of psychosocial factors, and the identification of barriers to treatment. The comprehensive guideline, which has 172 references and nine appendices, also features two easy-to-use algorithms. One addresses the assessment of chronic pain ( FIGURE 1 ) and the other deals with chronic pain management ( FIGURE 2 ).
Both algorithms identify Level-I and Level-II strategies that can be readily adapted to primary care practice. They are extremely helpful to physicians who are evaluating and developing a care plan for a patient who has chronic pain.
FIGURE 1 Chronic pain assessment
HIV, human immunodeficiency virus; ICSI, Institute for Clinical Systems Improvement; MS, multiple sclerosis.
*Pain types and contributing factors are not mutually exclusive. Patients frequently have more than one type of pain, as well as overlapping contributing factors.
Source: Institute for Clinical Systems Improvement. Reprinted with permission.
4 objectives
This latest guideline was developed to:
- improve the treatment of adult chronic-pain patients by encouraging physicians to complete an appropriate biopsychosocial assessment (and reassessment)
- improve patients’ function by recommending development and use of a comprehensive treatment plan that includes a multispecialty team
- improve the use of Level-I and Level-II treatment approaches to chronic pain
- provide guidance on the most effective use of nonopioid and opioid medications in the treatment of chronic pain.
With these objectives in mind, the ICSI work group conducted a comprehensive literature review, giving priority to randomized controlled trials (RCTs), meta-analyses, and systematic reviews. The work group used a seven-tier grading system to rate the evidence and a three-category system for the worksheets in the guideline appendices.
For this article, we converted evidence ratings in the guideline into so-called strength-of-recommendation taxonomy, or SORT.1
What aspects of practice have changed?
In addition to reflecting the latest research, the new guideline contains a number of clarifications. For example: The update states that medications are not the “sole” focus of treatment and should be used, when necessary, as part of an overall approach to pain management. (The previous version noted that medications were not the “primary” focus.)
The management algorithm ( FIGURE 2 ) now leads with “core principles”—a term suggesting greater importance than the former term, “general management,” implied. Clinical highlights, a synthesis of key recommendations, have been revised to better align with the guideline’s main components—assessment, functional goals, patient-centered/biopsychosocial care planning, Level-I versus Level-II approaches, and medication and patient selection.
Other changes in the guideline may contribute to clinicians’ understanding of chronic pain and its complex presentation. The guideline now includes a statement about allodynia and hyperalgesia to indicate that both may play an important role in any pain syndrome—not just in complex regional pain syndrome. Information about fibromyalgia symptoms and myofascial pain has been added. The definitions page now has an entry for “biopsychosocial model,” as well as language designed to stress the differences between untreated acute pain and ongoing chronic pain.
FIGURE 2 Chronic pain management
* DIRE, diagnosis, intractability, risk, efficacy.
Source: Institute for Clinical System Improvement. Reprinted with permission.
A limitation, an improvement
A limitation of the guideline is the lack of studies addressing the effectiveness of a comprehensive, multidisciplinary treatment approach to chronic pain management; most studies consider single-therapy management. An improvement, on the other hand, is that the evidence levels for each strategy are now listed within the section describing it—a notable change that makes it easier to identify the quality of individual recommendations.
As has been the case in the past, this latest edition of the guideline offers a number of tools for physicians. The assessment and management algorithms walk clinicians through decision-making. In addition, the following nine appendices provide practical guidance to physicians in various aspects of patient evaluation and care:
- Brief Pain Inventory (Short Form)
- Patient Health Questionnaire (PHQ-9)
- Functional Ability Questionnaire
- Personal Care Plan for Chronic Pain
- DIRE (diagnosis, intractability, risk, efficacy) Score: Patient Selection for Chronic Opioid Analgesia
- Opioid Agreement Form
- Opioid Analgesics
- Pharmaceutical Interventions for Neuropathic Pain
- Neuropathic Pain Treatment Diagram.
As noted, the source document for this guideline is: Assessment and Management of Chronic Pain. 3rd ed. Bloomington (Minn): Institute for Clinical Systems Improvement (ICSI); 2008 July.
The complete guideline is available at: pain__chronic__assessment_and_management_of__guideline_.html " target="_blank"> http://www.icsi.org/pain__chronic__assessment_and_management_of_14399/
pain__chronic__assessment_and_management_of__guideline_.html . (Accessed August 18, 2009.)
Reference
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract. 2004;53:111-120.
The authors report no financial relationships relevant to this article.
This article is adapted from the December 2008 installment of The Journal of Family Practice’s “Guideline Update” series. The Journal of Family Practice is an NLM-indexed publication of Quadrant HealthCom Inc., publisher of OBG Management.
- What are the critical steps in the assessment of a patient who suffers chronic pain?
- What are the four biologic mechanisms of pain?
- When is referral to a pain specialist recommended?
Answers to these questions are summarized below, and in the 2008 edition of Assessment and Management of Chronic Pain, a practice guideline developed and first published in 2005 by the Institute for Clinical Systems Improvement (ICSI), which also funded the work. ICSI is a collaboration of 57 medical groups sponsored by six Minnesota health plans. A third edition of the guideline, released in August 2008, summarizes current evidence about the assessment and treatment of chronic pain in mature adolescents (16 to 18 years old) and adults.
A distinct challenge to clinicians
Chronic pain—a persistent, life-altering condition—is one of the most challenging disorders for primary care physicians to treat. Unlike the case with acute pain, for which we seek to cure the underlying biologic condition, the goal of chronic pain management is to improve function in the face of pain that may never completely resolve.
Achieving that goal, according to the new guideline, requires a patient-centered, multifaceted approach—often involving a health-care team that includes specialists in behavioral health and physical rehabilitation—that is coordinated by a primary care physician. An effective treatment plan must address biopsychosocial factors as well as spiritual and cultural issues. Patients must be taught self-management skills focused on fitness, stress reduction, and maintaining a healthy lifestyle.
Grade A recommendations
- Develop a physician–patient partnership. This should include a plan of care and realistic goal-setting.
- Begin physical rehabilitation and psychosocial management. This includes an exercise fitness program, cognitive-behavioral therapy, and self-management.
Grade B recommendations
- Obtain a general history, including psychological assessment and spirituality evaluation, and identify barriers to treatment.
- Obtain a thorough pain history.
- Perform a physical examination, including a focused musculoskeletal and neurologic evaluation.
- Perform diagnostic testing as indicated. X-rays, computed tomography, magnetic resonance imaging, electromyography, and nerve conduction studies can help differentiate the biological mechanisms of pain.
- Teach patients to use pain scales for self-reporting.
Grade C recommendations
- Categorize the 4 biological mechanisms of pain (inflammatory, mechanical, musculoskeletal, or neuropathic).
- Consider the following pharmacologic options for Level-I care:
- Consider the following Level-I therapeutic procedures:
- Consider the following Level-II interventions:
Medications may be part of the treatment plan but should not be the sole focus, according to the guideline. Opioids are an option when other therapies fail.
The updated ICSI guideline also addresses the effects of various therapies, the role of psychosocial factors, and the identification of barriers to treatment. The comprehensive guideline, which has 172 references and nine appendices, also features two easy-to-use algorithms. One addresses the assessment of chronic pain ( FIGURE 1 ) and the other deals with chronic pain management ( FIGURE 2 ).
Both algorithms identify Level-I and Level-II strategies that can be readily adapted to primary care practice. They are extremely helpful to physicians who are evaluating and developing a care plan for a patient who has chronic pain.
FIGURE 1 Chronic pain assessment
HIV, human immunodeficiency virus; ICSI, Institute for Clinical Systems Improvement; MS, multiple sclerosis.
*Pain types and contributing factors are not mutually exclusive. Patients frequently have more than one type of pain, as well as overlapping contributing factors.
Source: Institute for Clinical Systems Improvement. Reprinted with permission.
4 objectives
This latest guideline was developed to:
- improve the treatment of adult chronic-pain patients by encouraging physicians to complete an appropriate biopsychosocial assessment (and reassessment)
- improve patients’ function by recommending development and use of a comprehensive treatment plan that includes a multispecialty team
- improve the use of Level-I and Level-II treatment approaches to chronic pain
- provide guidance on the most effective use of nonopioid and opioid medications in the treatment of chronic pain.
With these objectives in mind, the ICSI work group conducted a comprehensive literature review, giving priority to randomized controlled trials (RCTs), meta-analyses, and systematic reviews. The work group used a seven-tier grading system to rate the evidence and a three-category system for the worksheets in the guideline appendices.
For this article, we converted evidence ratings in the guideline into so-called strength-of-recommendation taxonomy, or SORT.1
What aspects of practice have changed?
In addition to reflecting the latest research, the new guideline contains a number of clarifications. For example: The update states that medications are not the “sole” focus of treatment and should be used, when necessary, as part of an overall approach to pain management. (The previous version noted that medications were not the “primary” focus.)
The management algorithm ( FIGURE 2 ) now leads with “core principles”—a term suggesting greater importance than the former term, “general management,” implied. Clinical highlights, a synthesis of key recommendations, have been revised to better align with the guideline’s main components—assessment, functional goals, patient-centered/biopsychosocial care planning, Level-I versus Level-II approaches, and medication and patient selection.
Other changes in the guideline may contribute to clinicians’ understanding of chronic pain and its complex presentation. The guideline now includes a statement about allodynia and hyperalgesia to indicate that both may play an important role in any pain syndrome—not just in complex regional pain syndrome. Information about fibromyalgia symptoms and myofascial pain has been added. The definitions page now has an entry for “biopsychosocial model,” as well as language designed to stress the differences between untreated acute pain and ongoing chronic pain.
FIGURE 2 Chronic pain management
* DIRE, diagnosis, intractability, risk, efficacy.
Source: Institute for Clinical System Improvement. Reprinted with permission.
A limitation, an improvement
A limitation of the guideline is the lack of studies addressing the effectiveness of a comprehensive, multidisciplinary treatment approach to chronic pain management; most studies consider single-therapy management. An improvement, on the other hand, is that the evidence levels for each strategy are now listed within the section describing it—a notable change that makes it easier to identify the quality of individual recommendations.
As has been the case in the past, this latest edition of the guideline offers a number of tools for physicians. The assessment and management algorithms walk clinicians through decision-making. In addition, the following nine appendices provide practical guidance to physicians in various aspects of patient evaluation and care:
- Brief Pain Inventory (Short Form)
- Patient Health Questionnaire (PHQ-9)
- Functional Ability Questionnaire
- Personal Care Plan for Chronic Pain
- DIRE (diagnosis, intractability, risk, efficacy) Score: Patient Selection for Chronic Opioid Analgesia
- Opioid Agreement Form
- Opioid Analgesics
- Pharmaceutical Interventions for Neuropathic Pain
- Neuropathic Pain Treatment Diagram.
As noted, the source document for this guideline is: Assessment and Management of Chronic Pain. 3rd ed. Bloomington (Minn): Institute for Clinical Systems Improvement (ICSI); 2008 July.
The complete guideline is available at: pain__chronic__assessment_and_management_of__guideline_.html " target="_blank"> http://www.icsi.org/pain__chronic__assessment_and_management_of_14399/
pain__chronic__assessment_and_management_of__guideline_.html . (Accessed August 18, 2009.)
The authors report no financial relationships relevant to this article.
This article is adapted from the December 2008 installment of The Journal of Family Practice’s “Guideline Update” series. The Journal of Family Practice is an NLM-indexed publication of Quadrant HealthCom Inc., publisher of OBG Management.
- What are the critical steps in the assessment of a patient who suffers chronic pain?
- What are the four biologic mechanisms of pain?
- When is referral to a pain specialist recommended?
Answers to these questions are summarized below, and in the 2008 edition of Assessment and Management of Chronic Pain, a practice guideline developed and first published in 2005 by the Institute for Clinical Systems Improvement (ICSI), which also funded the work. ICSI is a collaboration of 57 medical groups sponsored by six Minnesota health plans. A third edition of the guideline, released in August 2008, summarizes current evidence about the assessment and treatment of chronic pain in mature adolescents (16 to 18 years old) and adults.
A distinct challenge to clinicians
Chronic pain—a persistent, life-altering condition—is one of the most challenging disorders for primary care physicians to treat. Unlike the case with acute pain, for which we seek to cure the underlying biologic condition, the goal of chronic pain management is to improve function in the face of pain that may never completely resolve.
Achieving that goal, according to the new guideline, requires a patient-centered, multifaceted approach—often involving a health-care team that includes specialists in behavioral health and physical rehabilitation—that is coordinated by a primary care physician. An effective treatment plan must address biopsychosocial factors as well as spiritual and cultural issues. Patients must be taught self-management skills focused on fitness, stress reduction, and maintaining a healthy lifestyle.
Grade A recommendations
- Develop a physician–patient partnership. This should include a plan of care and realistic goal-setting.
- Begin physical rehabilitation and psychosocial management. This includes an exercise fitness program, cognitive-behavioral therapy, and self-management.
Grade B recommendations
- Obtain a general history, including psychological assessment and spirituality evaluation, and identify barriers to treatment.
- Obtain a thorough pain history.
- Perform a physical examination, including a focused musculoskeletal and neurologic evaluation.
- Perform diagnostic testing as indicated. X-rays, computed tomography, magnetic resonance imaging, electromyography, and nerve conduction studies can help differentiate the biological mechanisms of pain.
- Teach patients to use pain scales for self-reporting.
Grade C recommendations
- Categorize the 4 biological mechanisms of pain (inflammatory, mechanical, musculoskeletal, or neuropathic).
- Consider the following pharmacologic options for Level-I care:
- Consider the following Level-I therapeutic procedures:
- Consider the following Level-II interventions:
Medications may be part of the treatment plan but should not be the sole focus, according to the guideline. Opioids are an option when other therapies fail.
The updated ICSI guideline also addresses the effects of various therapies, the role of psychosocial factors, and the identification of barriers to treatment. The comprehensive guideline, which has 172 references and nine appendices, also features two easy-to-use algorithms. One addresses the assessment of chronic pain ( FIGURE 1 ) and the other deals with chronic pain management ( FIGURE 2 ).
Both algorithms identify Level-I and Level-II strategies that can be readily adapted to primary care practice. They are extremely helpful to physicians who are evaluating and developing a care plan for a patient who has chronic pain.
FIGURE 1 Chronic pain assessment
HIV, human immunodeficiency virus; ICSI, Institute for Clinical Systems Improvement; MS, multiple sclerosis.
*Pain types and contributing factors are not mutually exclusive. Patients frequently have more than one type of pain, as well as overlapping contributing factors.
Source: Institute for Clinical Systems Improvement. Reprinted with permission.
4 objectives
This latest guideline was developed to:
- improve the treatment of adult chronic-pain patients by encouraging physicians to complete an appropriate biopsychosocial assessment (and reassessment)
- improve patients’ function by recommending development and use of a comprehensive treatment plan that includes a multispecialty team
- improve the use of Level-I and Level-II treatment approaches to chronic pain
- provide guidance on the most effective use of nonopioid and opioid medications in the treatment of chronic pain.
With these objectives in mind, the ICSI work group conducted a comprehensive literature review, giving priority to randomized controlled trials (RCTs), meta-analyses, and systematic reviews. The work group used a seven-tier grading system to rate the evidence and a three-category system for the worksheets in the guideline appendices.
For this article, we converted evidence ratings in the guideline into so-called strength-of-recommendation taxonomy, or SORT.1
What aspects of practice have changed?
In addition to reflecting the latest research, the new guideline contains a number of clarifications. For example: The update states that medications are not the “sole” focus of treatment and should be used, when necessary, as part of an overall approach to pain management. (The previous version noted that medications were not the “primary” focus.)
The management algorithm ( FIGURE 2 ) now leads with “core principles”—a term suggesting greater importance than the former term, “general management,” implied. Clinical highlights, a synthesis of key recommendations, have been revised to better align with the guideline’s main components—assessment, functional goals, patient-centered/biopsychosocial care planning, Level-I versus Level-II approaches, and medication and patient selection.
Other changes in the guideline may contribute to clinicians’ understanding of chronic pain and its complex presentation. The guideline now includes a statement about allodynia and hyperalgesia to indicate that both may play an important role in any pain syndrome—not just in complex regional pain syndrome. Information about fibromyalgia symptoms and myofascial pain has been added. The definitions page now has an entry for “biopsychosocial model,” as well as language designed to stress the differences between untreated acute pain and ongoing chronic pain.
FIGURE 2 Chronic pain management
* DIRE, diagnosis, intractability, risk, efficacy.
Source: Institute for Clinical System Improvement. Reprinted with permission.
A limitation, an improvement
A limitation of the guideline is the lack of studies addressing the effectiveness of a comprehensive, multidisciplinary treatment approach to chronic pain management; most studies consider single-therapy management. An improvement, on the other hand, is that the evidence levels for each strategy are now listed within the section describing it—a notable change that makes it easier to identify the quality of individual recommendations.
As has been the case in the past, this latest edition of the guideline offers a number of tools for physicians. The assessment and management algorithms walk clinicians through decision-making. In addition, the following nine appendices provide practical guidance to physicians in various aspects of patient evaluation and care:
- Brief Pain Inventory (Short Form)
- Patient Health Questionnaire (PHQ-9)
- Functional Ability Questionnaire
- Personal Care Plan for Chronic Pain
- DIRE (diagnosis, intractability, risk, efficacy) Score: Patient Selection for Chronic Opioid Analgesia
- Opioid Agreement Form
- Opioid Analgesics
- Pharmaceutical Interventions for Neuropathic Pain
- Neuropathic Pain Treatment Diagram.
As noted, the source document for this guideline is: Assessment and Management of Chronic Pain. 3rd ed. Bloomington (Minn): Institute for Clinical Systems Improvement (ICSI); 2008 July.
The complete guideline is available at: pain__chronic__assessment_and_management_of__guideline_.html " target="_blank"> http://www.icsi.org/pain__chronic__assessment_and_management_of_14399/
pain__chronic__assessment_and_management_of__guideline_.html . (Accessed August 18, 2009.)
Reference
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract. 2004;53:111-120.
Reference
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract. 2004;53:111-120.
Emergency contraception care
- Does levonorgestrel work better than estrogen-progestin combination in preventing pregnancy?
- Which method is better tolerated, levonorgestrel or estrogen-progestin?
- When should emergency contraception be initiated?
- How often can emergency contraception be used?
This guideline targets women who have had unprotected or inadequately protected intercourse within the past 120 hours and do not desire pregnancy.
Practitioners can make informed decisions about obstetric and gynecologic care, given the evidence in this guideline regarding safety, efficacy, risks and benefits of the use of emergency contraception including progestin-only and combined estrogen-progestin regimen.
The major outcome considered was incidence of unintended pregnancy. The evidence rating is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
This guideline is extremely relevant in light of the recent decision (August 2006) by the US Food and Drug Administration to allow the Plan B (levonorgestrel) to be sold over the counter to women aged 18 and older.2 It is still available by prescription to women aged <18 years.
Forty-nine percent of pregnancies were unintended in 2001. Of these 3.1 million unintended pregnancies, only 44% ended in births.3 Emergency contraception has an important role in reducing the number of unwanted pregnancies.
Lack of cost analysis weakened this guideline. Emergency contraceptive doses for commonly prescribed oral contraceptives are listed in the TABLE.
TABLE
Emergency contraception dosage for commonly prescribed oral contraceptives
| BRAND NAME | ETHINYL ESTRADIOL PER DOSE (MCG) | LEVONORGESTREL PER DOSE (MG) | FIRST DOSE | SECOND DOSE (12 HOURS LATER) |
|---|---|---|---|---|
| Progestin only | ||||
| Plan B | 0 | 1.5 | 2 white pills | none |
| Ovrette | 0 | 1.5 | 20 yellow pills | 20 yellow pills |
| Combined progestin/estrogen pills | ||||
| Alesse | 100 | 0.50 | 5 pink pills | 5 pink pills |
| Lo/Ovral | 120 | 0.60 | 4 white pills | 4 white pills |
| Ovral | 100 | 0.50 | 2 white pills | 2 white pills |
| Seasonale | 120 | 0.60 | 4 pink pills | 4 pink pills |
| Triphasil | 120 | 0.50 | 4 yellow pills | 4 yellow pills |
| Source: adapted from Office of Population Research at Princeton University.4 | ||||
Guideline development and evidence review
A search of the literature was performed using Medline, the Cochrane Library, and the American College of Obstetricians and Gynecologists’ own internal resources and documents. Restrictions included articles published in English between January 1985 and January 2005. Priority was given to meta-analyses and systematic reviews, which were analyzed by an expert panel. Recommendations were graded. Abstracts of research presented at conferences and symposiums were not considered adequate to be considered.
Source for this guideline
American College of Obstetricians and Gynecologists (ACOG). Emergency contraception. ACOG practice bulletin no 69. Washington, DC: American College of Obstetricians and Gynecologists (ACOG); 2005. 10 p. [86 references]
Other guidelines
Emergency Contraception
This 2005 guideline published by the American Academy of Pediatrics is similar to the ACOG Guideline. It is comprehensive, but recommendations are not graded. The focus is on adolescent use of emergency contraception. It has an excellent section on telephone triage of sexually active teens prior to prescribing emergency contraception.
Source. American Academy of Pediatrics. Emergency contraception. Pediatrics 2005 Oct;116(4):1026-35. [101 references] Available at: aappolicy.aappublications.org/cgi/content/full/pediatrics;116/4/1026.
GRADE A RECOMMENDATIONS
- Emergency contraception should be made available to women who have had unprotected or inadequately protected sexual intercourse and who do not desire pregnancy.
- The levonorgestrel-only regime is more effective and is associated with less nausea and vomiting. It should be used in preference to the combined estrogen-progestin regimen.
- The 1.5-mg levonorgestrel-only regimen can be taken in a single dose.
- The two 0.75-mg doses of the levonorgestrel-only regimen are equally effective if taken 12 to 24 hours apart.
- To reduce the chance of nausea with the combined estrogen-progestin regimen, an antiemetic agent may be taken 1 hour prior to the first emergency contraception dose.
- Prescription or provision of emergency contraception in advance of need can increase availability and use.
GRADE B RECOMMENDATIONS
- Treatment with emergency contraception should be initialized as soon as possible after unprotected or inadequately protected intercourse to maximize efficacy.
- Emergency contraception should be made available to patients who request it up to 120 hours after unprotected intercourse.
- No clinician examination or pregnancy testing is necessary before provision or prescription of emergency contraception.
GRADE C RECOMMENDATIONS
- No data specifically examined the risk of using hormonal methods of emergency contraception among women with contraindications to the use of conventional oral contraceptive preparations; nevertheless, emergency contraception may be made available to such women.
- Clinical evaluation is indicated for women who have used emergency contraception if menses are delayed by a week or more after the expected time, if lower abdominal pain occurs, or persistent irregular bleeding develops.
- Information regarding effective contraceptive methods should be made available either at the time emergency contraception is prescribed or at some convenient time thereafter.
- Emergency contraception may be used even if the woman has used it before, even within the same menstrual cycle.
Emergency Contraception
This 2003 Scottish guideline is well written, but lacks information about all current options for emergency contraception. It is strengthened by cost-effectiveness analysis.
Source. Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit. Emergency contraception. Aberdeen, Scotland: Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit; 2003 Jun. 7 p. [53 references] Available at: www.ffprhc.org.uk/admin/uploads/EC%20revised%20PDF%2019.06.03.pdf.
Contraception and Family Planning: A guide to counseling and management
This 2005 guideline was published by Brigham and Women’s Hospital in Boston. In addition to emergency contraception, it provides recommendations on hormonal contraception (oral contraceptive pills, depo-medroxyprogesterone acetate, estrogen-progestin patches, vaginal ring, and levonorgestrel intrauterine), barrier contraception, intrauterine devices, surgical methods for contraception, and pregnancy termination.
Source. New Brigham and Women’s Hospital. Contraception and family planning. A guide to counseling and management. Boston, Mass: Brigham and Women’s Hospital; 2005.15 p. [6 references]. Available at: www.brighamandwomens.org/medical/handbookarticles/ContraceptionGuide.pdf.
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. US Food and Drug Administration. FDA approves over-the-counter access for Plan B for women 18 and older-prescription remains required for those 17 and under. FDA News, August 24, 2006. Available at: www.fda.gov/bbs/topics/NEWS/2006/NEW01436.html. Accessed on November 13, 2006.
3. Finn LB, et al. Disparities in unintentional pregnancy in the United States, 1994 and 2001. Perspect Sexual Reprod Health 2006;38:90-96.
4. The Emergency Contraception Website. Office of Population Research at Princeton University and the Association of Reproductive Health Professionals. 2006. Available at: ec.princeton.edu/questions/dose.html. Accessed on November 13, 2006.
- Does levonorgestrel work better than estrogen-progestin combination in preventing pregnancy?
- Which method is better tolerated, levonorgestrel or estrogen-progestin?
- When should emergency contraception be initiated?
- How often can emergency contraception be used?
This guideline targets women who have had unprotected or inadequately protected intercourse within the past 120 hours and do not desire pregnancy.
Practitioners can make informed decisions about obstetric and gynecologic care, given the evidence in this guideline regarding safety, efficacy, risks and benefits of the use of emergency contraception including progestin-only and combined estrogen-progestin regimen.
The major outcome considered was incidence of unintended pregnancy. The evidence rating is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
This guideline is extremely relevant in light of the recent decision (August 2006) by the US Food and Drug Administration to allow the Plan B (levonorgestrel) to be sold over the counter to women aged 18 and older.2 It is still available by prescription to women aged <18 years.
Forty-nine percent of pregnancies were unintended in 2001. Of these 3.1 million unintended pregnancies, only 44% ended in births.3 Emergency contraception has an important role in reducing the number of unwanted pregnancies.
Lack of cost analysis weakened this guideline. Emergency contraceptive doses for commonly prescribed oral contraceptives are listed in the TABLE.
TABLE
Emergency contraception dosage for commonly prescribed oral contraceptives
| BRAND NAME | ETHINYL ESTRADIOL PER DOSE (MCG) | LEVONORGESTREL PER DOSE (MG) | FIRST DOSE | SECOND DOSE (12 HOURS LATER) |
|---|---|---|---|---|
| Progestin only | ||||
| Plan B | 0 | 1.5 | 2 white pills | none |
| Ovrette | 0 | 1.5 | 20 yellow pills | 20 yellow pills |
| Combined progestin/estrogen pills | ||||
| Alesse | 100 | 0.50 | 5 pink pills | 5 pink pills |
| Lo/Ovral | 120 | 0.60 | 4 white pills | 4 white pills |
| Ovral | 100 | 0.50 | 2 white pills | 2 white pills |
| Seasonale | 120 | 0.60 | 4 pink pills | 4 pink pills |
| Triphasil | 120 | 0.50 | 4 yellow pills | 4 yellow pills |
| Source: adapted from Office of Population Research at Princeton University.4 | ||||
Guideline development and evidence review
A search of the literature was performed using Medline, the Cochrane Library, and the American College of Obstetricians and Gynecologists’ own internal resources and documents. Restrictions included articles published in English between January 1985 and January 2005. Priority was given to meta-analyses and systematic reviews, which were analyzed by an expert panel. Recommendations were graded. Abstracts of research presented at conferences and symposiums were not considered adequate to be considered.
Source for this guideline
American College of Obstetricians and Gynecologists (ACOG). Emergency contraception. ACOG practice bulletin no 69. Washington, DC: American College of Obstetricians and Gynecologists (ACOG); 2005. 10 p. [86 references]
Other guidelines
Emergency Contraception
This 2005 guideline published by the American Academy of Pediatrics is similar to the ACOG Guideline. It is comprehensive, but recommendations are not graded. The focus is on adolescent use of emergency contraception. It has an excellent section on telephone triage of sexually active teens prior to prescribing emergency contraception.
Source. American Academy of Pediatrics. Emergency contraception. Pediatrics 2005 Oct;116(4):1026-35. [101 references] Available at: aappolicy.aappublications.org/cgi/content/full/pediatrics;116/4/1026.
GRADE A RECOMMENDATIONS
- Emergency contraception should be made available to women who have had unprotected or inadequately protected sexual intercourse and who do not desire pregnancy.
- The levonorgestrel-only regime is more effective and is associated with less nausea and vomiting. It should be used in preference to the combined estrogen-progestin regimen.
- The 1.5-mg levonorgestrel-only regimen can be taken in a single dose.
- The two 0.75-mg doses of the levonorgestrel-only regimen are equally effective if taken 12 to 24 hours apart.
- To reduce the chance of nausea with the combined estrogen-progestin regimen, an antiemetic agent may be taken 1 hour prior to the first emergency contraception dose.
- Prescription or provision of emergency contraception in advance of need can increase availability and use.
GRADE B RECOMMENDATIONS
- Treatment with emergency contraception should be initialized as soon as possible after unprotected or inadequately protected intercourse to maximize efficacy.
- Emergency contraception should be made available to patients who request it up to 120 hours after unprotected intercourse.
- No clinician examination or pregnancy testing is necessary before provision or prescription of emergency contraception.
GRADE C RECOMMENDATIONS
- No data specifically examined the risk of using hormonal methods of emergency contraception among women with contraindications to the use of conventional oral contraceptive preparations; nevertheless, emergency contraception may be made available to such women.
- Clinical evaluation is indicated for women who have used emergency contraception if menses are delayed by a week or more after the expected time, if lower abdominal pain occurs, or persistent irregular bleeding develops.
- Information regarding effective contraceptive methods should be made available either at the time emergency contraception is prescribed or at some convenient time thereafter.
- Emergency contraception may be used even if the woman has used it before, even within the same menstrual cycle.
Emergency Contraception
This 2003 Scottish guideline is well written, but lacks information about all current options for emergency contraception. It is strengthened by cost-effectiveness analysis.
Source. Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit. Emergency contraception. Aberdeen, Scotland: Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit; 2003 Jun. 7 p. [53 references] Available at: www.ffprhc.org.uk/admin/uploads/EC%20revised%20PDF%2019.06.03.pdf.
Contraception and Family Planning: A guide to counseling and management
This 2005 guideline was published by Brigham and Women’s Hospital in Boston. In addition to emergency contraception, it provides recommendations on hormonal contraception (oral contraceptive pills, depo-medroxyprogesterone acetate, estrogen-progestin patches, vaginal ring, and levonorgestrel intrauterine), barrier contraception, intrauterine devices, surgical methods for contraception, and pregnancy termination.
Source. New Brigham and Women’s Hospital. Contraception and family planning. A guide to counseling and management. Boston, Mass: Brigham and Women’s Hospital; 2005.15 p. [6 references]. Available at: www.brighamandwomens.org/medical/handbookarticles/ContraceptionGuide.pdf.
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
- Does levonorgestrel work better than estrogen-progestin combination in preventing pregnancy?
- Which method is better tolerated, levonorgestrel or estrogen-progestin?
- When should emergency contraception be initiated?
- How often can emergency contraception be used?
This guideline targets women who have had unprotected or inadequately protected intercourse within the past 120 hours and do not desire pregnancy.
Practitioners can make informed decisions about obstetric and gynecologic care, given the evidence in this guideline regarding safety, efficacy, risks and benefits of the use of emergency contraception including progestin-only and combined estrogen-progestin regimen.
The major outcome considered was incidence of unintended pregnancy. The evidence rating is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
This guideline is extremely relevant in light of the recent decision (August 2006) by the US Food and Drug Administration to allow the Plan B (levonorgestrel) to be sold over the counter to women aged 18 and older.2 It is still available by prescription to women aged <18 years.
Forty-nine percent of pregnancies were unintended in 2001. Of these 3.1 million unintended pregnancies, only 44% ended in births.3 Emergency contraception has an important role in reducing the number of unwanted pregnancies.
Lack of cost analysis weakened this guideline. Emergency contraceptive doses for commonly prescribed oral contraceptives are listed in the TABLE.
TABLE
Emergency contraception dosage for commonly prescribed oral contraceptives
| BRAND NAME | ETHINYL ESTRADIOL PER DOSE (MCG) | LEVONORGESTREL PER DOSE (MG) | FIRST DOSE | SECOND DOSE (12 HOURS LATER) |
|---|---|---|---|---|
| Progestin only | ||||
| Plan B | 0 | 1.5 | 2 white pills | none |
| Ovrette | 0 | 1.5 | 20 yellow pills | 20 yellow pills |
| Combined progestin/estrogen pills | ||||
| Alesse | 100 | 0.50 | 5 pink pills | 5 pink pills |
| Lo/Ovral | 120 | 0.60 | 4 white pills | 4 white pills |
| Ovral | 100 | 0.50 | 2 white pills | 2 white pills |
| Seasonale | 120 | 0.60 | 4 pink pills | 4 pink pills |
| Triphasil | 120 | 0.50 | 4 yellow pills | 4 yellow pills |
| Source: adapted from Office of Population Research at Princeton University.4 | ||||
Guideline development and evidence review
A search of the literature was performed using Medline, the Cochrane Library, and the American College of Obstetricians and Gynecologists’ own internal resources and documents. Restrictions included articles published in English between January 1985 and January 2005. Priority was given to meta-analyses and systematic reviews, which were analyzed by an expert panel. Recommendations were graded. Abstracts of research presented at conferences and symposiums were not considered adequate to be considered.
Source for this guideline
American College of Obstetricians and Gynecologists (ACOG). Emergency contraception. ACOG practice bulletin no 69. Washington, DC: American College of Obstetricians and Gynecologists (ACOG); 2005. 10 p. [86 references]
Other guidelines
Emergency Contraception
This 2005 guideline published by the American Academy of Pediatrics is similar to the ACOG Guideline. It is comprehensive, but recommendations are not graded. The focus is on adolescent use of emergency contraception. It has an excellent section on telephone triage of sexually active teens prior to prescribing emergency contraception.
Source. American Academy of Pediatrics. Emergency contraception. Pediatrics 2005 Oct;116(4):1026-35. [101 references] Available at: aappolicy.aappublications.org/cgi/content/full/pediatrics;116/4/1026.
GRADE A RECOMMENDATIONS
- Emergency contraception should be made available to women who have had unprotected or inadequately protected sexual intercourse and who do not desire pregnancy.
- The levonorgestrel-only regime is more effective and is associated with less nausea and vomiting. It should be used in preference to the combined estrogen-progestin regimen.
- The 1.5-mg levonorgestrel-only regimen can be taken in a single dose.
- The two 0.75-mg doses of the levonorgestrel-only regimen are equally effective if taken 12 to 24 hours apart.
- To reduce the chance of nausea with the combined estrogen-progestin regimen, an antiemetic agent may be taken 1 hour prior to the first emergency contraception dose.
- Prescription or provision of emergency contraception in advance of need can increase availability and use.
GRADE B RECOMMENDATIONS
- Treatment with emergency contraception should be initialized as soon as possible after unprotected or inadequately protected intercourse to maximize efficacy.
- Emergency contraception should be made available to patients who request it up to 120 hours after unprotected intercourse.
- No clinician examination or pregnancy testing is necessary before provision or prescription of emergency contraception.
GRADE C RECOMMENDATIONS
- No data specifically examined the risk of using hormonal methods of emergency contraception among women with contraindications to the use of conventional oral contraceptive preparations; nevertheless, emergency contraception may be made available to such women.
- Clinical evaluation is indicated for women who have used emergency contraception if menses are delayed by a week or more after the expected time, if lower abdominal pain occurs, or persistent irregular bleeding develops.
- Information regarding effective contraceptive methods should be made available either at the time emergency contraception is prescribed or at some convenient time thereafter.
- Emergency contraception may be used even if the woman has used it before, even within the same menstrual cycle.
Emergency Contraception
This 2003 Scottish guideline is well written, but lacks information about all current options for emergency contraception. It is strengthened by cost-effectiveness analysis.
Source. Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit. Emergency contraception. Aberdeen, Scotland: Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit; 2003 Jun. 7 p. [53 references] Available at: www.ffprhc.org.uk/admin/uploads/EC%20revised%20PDF%2019.06.03.pdf.
Contraception and Family Planning: A guide to counseling and management
This 2005 guideline was published by Brigham and Women’s Hospital in Boston. In addition to emergency contraception, it provides recommendations on hormonal contraception (oral contraceptive pills, depo-medroxyprogesterone acetate, estrogen-progestin patches, vaginal ring, and levonorgestrel intrauterine), barrier contraception, intrauterine devices, surgical methods for contraception, and pregnancy termination.
Source. New Brigham and Women’s Hospital. Contraception and family planning. A guide to counseling and management. Boston, Mass: Brigham and Women’s Hospital; 2005.15 p. [6 references]. Available at: www.brighamandwomens.org/medical/handbookarticles/ContraceptionGuide.pdf.
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. US Food and Drug Administration. FDA approves over-the-counter access for Plan B for women 18 and older-prescription remains required for those 17 and under. FDA News, August 24, 2006. Available at: www.fda.gov/bbs/topics/NEWS/2006/NEW01436.html. Accessed on November 13, 2006.
3. Finn LB, et al. Disparities in unintentional pregnancy in the United States, 1994 and 2001. Perspect Sexual Reprod Health 2006;38:90-96.
4. The Emergency Contraception Website. Office of Population Research at Princeton University and the Association of Reproductive Health Professionals. 2006. Available at: ec.princeton.edu/questions/dose.html. Accessed on November 13, 2006.
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. US Food and Drug Administration. FDA approves over-the-counter access for Plan B for women 18 and older-prescription remains required for those 17 and under. FDA News, August 24, 2006. Available at: www.fda.gov/bbs/topics/NEWS/2006/NEW01436.html. Accessed on November 13, 2006.
3. Finn LB, et al. Disparities in unintentional pregnancy in the United States, 1994 and 2001. Perspect Sexual Reprod Health 2006;38:90-96.
4. The Emergency Contraception Website. Office of Population Research at Princeton University and the Association of Reproductive Health Professionals. 2006. Available at: ec.princeton.edu/questions/dose.html. Accessed on November 13, 2006.
Parkinson’s disease: How practical are new recommendations?
This feature reviews guidelines when they are developed with high-quality evidence and are relevant to primary care physicians. Now and then, however, it is instructive to critique recommendations that fall short of this mark. Four Parkinson’s disease practice parameters recently published by the American Academy of Neurology purport to be explicit, evidence-based, and of high quality; however, we feel these guidelines should be used with caution.
These recommendations for care of the Parkinson’s patient were published in Neurology as 4 separate reviews.1-4 The topics covered include diagnosis and prognosis, treatment of motor fluctuations and dyskinesia, neuroprotective strategies, and evaluation and treatment of depression, psychosis, and dementia. There are 201 references. These recommendations were developed by the Quality Standards Subcommittee of the American Academy of Neurology. These guidelines can be accessed on the Web at: www.aan.com/professionals/practice/guideline/index.cfm.
Limitations of these recommendations
In these reviews, terminology regarding effectiveness is not consistently used. Instead of stating that a treatment “is effective,” the authors report that it “should be considered” or “should be offered.”
DIAGNOSIS
- Early falls, poor response to levodopa, symmetry of motor symptoms, and lack of tremor are “probably useful” to suggest other Parkinson-like syndromes, but are not typical for Parkinson’s disease
- Levodopa or apomorphine challenge and olfactory testing are “probably useful” in diagnosing Parkinson’s disease
- Older age at onset, associated comorbidities, rigidity and bradykinesia at onset, and decreased dopamine responsiveness are associated with poorer prognosis
TREATMENT
- Entacapone and rasagiline “should be offered” to reduce off time (periods where medications wear off and Parkinson’s disease symptoms return) (A). Pergolide, pramipexole, ropinirole, and tolcapone “should be considered” (B). Apomorphine, cabergoline, and selegiline “may be considered” (C). Current evidence does not support the use of one medication over another in reducing off time (B). Sustained release carbidopa/levodopa and bromocriptine “may be disregarded” to reduce off time (C)
- Amantadine “may be considered” to reduce dyskinesias (C)
- Deep brain stimulation of the subthalamic nucleus “may be considered” for improving motor function and dyskinesias and reducing off time and medication usage (C)
NEUROPROTECTION
- Levodopa “does not appear” to accelerate disease progression
- No treatment is neuroprotective
- No evidence supports vitamin and food additives for improving motor function
- Exercise “may be helpful” for improving motor function
- Speech therapy “may be helpful” for improving speech volume
- Screening and treatment of depression, psychosis, and dementia
- Depression rating scales “should be considered” to screen for depression (B)
- Dementia screening “should be considered” (B)
- Amitriptyline “may be considered” to treat depression without dementia (C)
- Clozapine “should be considered” (C), quetiapine “may be considered” (C), and olanzapine “should not be considered” (B) for psychosis
- Donepezil or rivastigmine “should be considered” for dementia (B)
There is not consistency between the manuscripts. Abstracts in 2 of the publications2,4 link level of evidence to the summary of recommendations in the abstracts. The other 2 do not.
In all 4 documents the abstracts are written in randomized controlled trial format, which make them difficult to quickly review. They are in question and answer format. There are long blocks of text without figures or tables to aid in learning and retaining the recommendations.
No cost-effectiveness analysis is performed in the reviews. They recommend that deep brain stimulation of the subthalamic nucleus “may be considered” to improve Parkinson’s disease symptoms and reduce medication use. But at what cost?
Because of their perspective from a specialty, these guidelines lack relevance for the family physician. For example, olfactory testing, which they recommend, is impractical for primary care physicians. However, no recommendations discuss dose titration with commonly prescribed medications. There are 3 pages reviewing surgical therapy.
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:968-975.
2. Pahwa R, Factor SA, Lyons KE, et al. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:983-995
3. Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner WJ. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:976-982.
4. Miyasaki JM, Shannon K, Voon V, et al. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:996-1002.
This feature reviews guidelines when they are developed with high-quality evidence and are relevant to primary care physicians. Now and then, however, it is instructive to critique recommendations that fall short of this mark. Four Parkinson’s disease practice parameters recently published by the American Academy of Neurology purport to be explicit, evidence-based, and of high quality; however, we feel these guidelines should be used with caution.
These recommendations for care of the Parkinson’s patient were published in Neurology as 4 separate reviews.1-4 The topics covered include diagnosis and prognosis, treatment of motor fluctuations and dyskinesia, neuroprotective strategies, and evaluation and treatment of depression, psychosis, and dementia. There are 201 references. These recommendations were developed by the Quality Standards Subcommittee of the American Academy of Neurology. These guidelines can be accessed on the Web at: www.aan.com/professionals/practice/guideline/index.cfm.
Limitations of these recommendations
In these reviews, terminology regarding effectiveness is not consistently used. Instead of stating that a treatment “is effective,” the authors report that it “should be considered” or “should be offered.”
DIAGNOSIS
- Early falls, poor response to levodopa, symmetry of motor symptoms, and lack of tremor are “probably useful” to suggest other Parkinson-like syndromes, but are not typical for Parkinson’s disease
- Levodopa or apomorphine challenge and olfactory testing are “probably useful” in diagnosing Parkinson’s disease
- Older age at onset, associated comorbidities, rigidity and bradykinesia at onset, and decreased dopamine responsiveness are associated with poorer prognosis
TREATMENT
- Entacapone and rasagiline “should be offered” to reduce off time (periods where medications wear off and Parkinson’s disease symptoms return) (A). Pergolide, pramipexole, ropinirole, and tolcapone “should be considered” (B). Apomorphine, cabergoline, and selegiline “may be considered” (C). Current evidence does not support the use of one medication over another in reducing off time (B). Sustained release carbidopa/levodopa and bromocriptine “may be disregarded” to reduce off time (C)
- Amantadine “may be considered” to reduce dyskinesias (C)
- Deep brain stimulation of the subthalamic nucleus “may be considered” for improving motor function and dyskinesias and reducing off time and medication usage (C)
NEUROPROTECTION
- Levodopa “does not appear” to accelerate disease progression
- No treatment is neuroprotective
- No evidence supports vitamin and food additives for improving motor function
- Exercise “may be helpful” for improving motor function
- Speech therapy “may be helpful” for improving speech volume
- Screening and treatment of depression, psychosis, and dementia
- Depression rating scales “should be considered” to screen for depression (B)
- Dementia screening “should be considered” (B)
- Amitriptyline “may be considered” to treat depression without dementia (C)
- Clozapine “should be considered” (C), quetiapine “may be considered” (C), and olanzapine “should not be considered” (B) for psychosis
- Donepezil or rivastigmine “should be considered” for dementia (B)
There is not consistency between the manuscripts. Abstracts in 2 of the publications2,4 link level of evidence to the summary of recommendations in the abstracts. The other 2 do not.
In all 4 documents the abstracts are written in randomized controlled trial format, which make them difficult to quickly review. They are in question and answer format. There are long blocks of text without figures or tables to aid in learning and retaining the recommendations.
No cost-effectiveness analysis is performed in the reviews. They recommend that deep brain stimulation of the subthalamic nucleus “may be considered” to improve Parkinson’s disease symptoms and reduce medication use. But at what cost?
Because of their perspective from a specialty, these guidelines lack relevance for the family physician. For example, olfactory testing, which they recommend, is impractical for primary care physicians. However, no recommendations discuss dose titration with commonly prescribed medications. There are 3 pages reviewing surgical therapy.
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
This feature reviews guidelines when they are developed with high-quality evidence and are relevant to primary care physicians. Now and then, however, it is instructive to critique recommendations that fall short of this mark. Four Parkinson’s disease practice parameters recently published by the American Academy of Neurology purport to be explicit, evidence-based, and of high quality; however, we feel these guidelines should be used with caution.
These recommendations for care of the Parkinson’s patient were published in Neurology as 4 separate reviews.1-4 The topics covered include diagnosis and prognosis, treatment of motor fluctuations and dyskinesia, neuroprotective strategies, and evaluation and treatment of depression, psychosis, and dementia. There are 201 references. These recommendations were developed by the Quality Standards Subcommittee of the American Academy of Neurology. These guidelines can be accessed on the Web at: www.aan.com/professionals/practice/guideline/index.cfm.
Limitations of these recommendations
In these reviews, terminology regarding effectiveness is not consistently used. Instead of stating that a treatment “is effective,” the authors report that it “should be considered” or “should be offered.”
DIAGNOSIS
- Early falls, poor response to levodopa, symmetry of motor symptoms, and lack of tremor are “probably useful” to suggest other Parkinson-like syndromes, but are not typical for Parkinson’s disease
- Levodopa or apomorphine challenge and olfactory testing are “probably useful” in diagnosing Parkinson’s disease
- Older age at onset, associated comorbidities, rigidity and bradykinesia at onset, and decreased dopamine responsiveness are associated with poorer prognosis
TREATMENT
- Entacapone and rasagiline “should be offered” to reduce off time (periods where medications wear off and Parkinson’s disease symptoms return) (A). Pergolide, pramipexole, ropinirole, and tolcapone “should be considered” (B). Apomorphine, cabergoline, and selegiline “may be considered” (C). Current evidence does not support the use of one medication over another in reducing off time (B). Sustained release carbidopa/levodopa and bromocriptine “may be disregarded” to reduce off time (C)
- Amantadine “may be considered” to reduce dyskinesias (C)
- Deep brain stimulation of the subthalamic nucleus “may be considered” for improving motor function and dyskinesias and reducing off time and medication usage (C)
NEUROPROTECTION
- Levodopa “does not appear” to accelerate disease progression
- No treatment is neuroprotective
- No evidence supports vitamin and food additives for improving motor function
- Exercise “may be helpful” for improving motor function
- Speech therapy “may be helpful” for improving speech volume
- Screening and treatment of depression, psychosis, and dementia
- Depression rating scales “should be considered” to screen for depression (B)
- Dementia screening “should be considered” (B)
- Amitriptyline “may be considered” to treat depression without dementia (C)
- Clozapine “should be considered” (C), quetiapine “may be considered” (C), and olanzapine “should not be considered” (B) for psychosis
- Donepezil or rivastigmine “should be considered” for dementia (B)
There is not consistency between the manuscripts. Abstracts in 2 of the publications2,4 link level of evidence to the summary of recommendations in the abstracts. The other 2 do not.
In all 4 documents the abstracts are written in randomized controlled trial format, which make them difficult to quickly review. They are in question and answer format. There are long blocks of text without figures or tables to aid in learning and retaining the recommendations.
No cost-effectiveness analysis is performed in the reviews. They recommend that deep brain stimulation of the subthalamic nucleus “may be considered” to improve Parkinson’s disease symptoms and reduce medication use. But at what cost?
Because of their perspective from a specialty, these guidelines lack relevance for the family physician. For example, olfactory testing, which they recommend, is impractical for primary care physicians. However, no recommendations discuss dose titration with commonly prescribed medications. There are 3 pages reviewing surgical therapy.
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:968-975.
2. Pahwa R, Factor SA, Lyons KE, et al. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:983-995
3. Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner WJ. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:976-982.
4. Miyasaki JM, Shannon K, Voon V, et al. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:996-1002.
1. Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:968-975.
2. Pahwa R, Factor SA, Lyons KE, et al. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:983-995
3. Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner WJ. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:976-982.
4. Miyasaki JM, Shannon K, Voon V, et al. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:996-1002.
Endarterectomy for carotid artery stenosis: Who qualifies?
What are the indications for carotid endarterectomy (CE) in the symptomatic patient?
How is symptomatic defined?
What are the indications for CE in the asymptomatic patient?
What is the role of aspirin therapy in these patients?
This guideline reviews the efficacy of carotid endarterectomy for stroke prevention in adults with symptomatic or asymptomatic internal carotid artery stenosis.
Symptomatic is defined as a cerebrovascular event in the carotid distribution (transient ischemic attack or nondisabling stroke) in the previous 6 months.
Indications for or against CE appear in the Practice Recommendations.
GRADE A RECOMMENDATIONS
- Carotid endarterectomy is effective for symptomatic patients with 70% to 99% stenosis. Carotid endarterectomy should not be considered for symptomatic patients with less than 50% stenosis—medical management is preferred. (50%–99% stenosis, Grade B)
- Carotid endarterectomy may be considered for patients between ages 40 to 75 years, with asymptomatic stenosis between 60% to 99%, if there is a 5-year life expectancy and surgical risk is <3%.
- Symptomatic and asymptomatic patients undergoing carotid endarterectomy should receive aspirin (81 or 325 mg) daily prior to surgery and for at least 3 months postoperatively. In the absence of contraindications, it should be continued indefinitely.
GRADE B RECOMMENDATIONS
- Carotid endarterectomy may be considered for symptomatic patients with 50% to 69% stenosis in patients with a 5-year life expectancy and surgical risk <6%.
GRADE C RECOMMENDATION
- Patients with hemispheric transient ischemic attack or cerebrovascular accident had greater benefit from carotid endarterectomy than those with retinal events.
- Patients with severe stenosis and a cerebrovascular event should have carotid endarterectomy within 2 weeks for greatest benefit.
- Contralateral occlusion reduces the benefit of carotid endarterectomy in asymptomatic patients.
- Data are insufficient to recommend other antiplatelet agents perioperatively.
The evidence categories are assessment of therapeutic effectiveness, prevention, and risk assessment. Study outcomes considered are: benefits of carotid endarterectomy for symptomatic patients, benefits for asymptomatic patients, the efficacy within 24 hours in patients with progressing stroke, clinical variables that impact the risk/benefit ratio, the most important radiologic factors impacting the risk/benefit ratio, the ideal dose of aspirin preoperatively, the evidence/practice gap, the likelihood that trial results can be achieved in practice, the benefit of carotid endarterectomy concurrent with or prior to coronary artery bypass grafting, and the optimal time after stroke to perform the surgery. The evidence rating is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Carotid endarterectomy has an important role in the prevention of stroke in patients with internal carotid artery stenosis, since the majority of strokes are ischemic. Strokes can be prevented if a high-grade internal carotid stenosis is corrected. More than 700,000 patients suffer a stroke each year in the US, with 80% related to ischemia (either thrombotic or embolic).2 Most strokes occur after the age of 65, and the risk doubles each decade after the age of 55. Stroke is the third leading cause of death, with only heart disease and cancer killing more people. Strokes cause more serious long-term disabilities than any other illness. The guideline was weakened by lack of cost analysis.
Guideline development and evidence review
A literature search was performed using Ovid Medline for relevant articles published from 1990 to 2001 using the keywords carotid endarterectomy, carotid stenosis, carotid artery diseases, and clinical trials. The Cochrane Library statements on carotid endarterectomy for symptomatic and asymptomatic stenosis from 2004 were reviewed to confirm that additional relevant citations from 2002 to 2004 were identified.
The initial search yielded 1462 citations. This list was reduced by excluding case reports, letters to the editor, review articles without primary data, studies addressing carotid endarterectomy technical issues, case series from a single surgeon, and articles not written in English. Case series from a single institution were included. This narrowed the pertinent list to 186 articles, which were reviewed independently by 2 committee members. The committee also agreed that if a pooled analysis of the major symptomatic carotid endarterectomy studies or if the results of the Asymptomatic Carotid Surgery Trial were published prior to the completion of the committee’s manuscript, these would subsequently be reviewed.
Source for this guideline
Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, Cote R, Hess D, Saver J, Spence JD, Stern B, Wilterdink J. Carotid endarterectomy—an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65:794–801 [27 refs]. Available at: www.neurology.org/cgi/reprint/65/6/794.pdf.
Other guidelines on surgical management of carotid stenosis and stroke
Life after stroke: New Zealand guideline for management of stroke
This comprehensive 2003 guideline is written for New Zealand health care delivery system. It summarizes recommendations for the assessment and management of stroke, transient ischemic attacks, and intracerebral hemorrhage in various locales. The utility of carotid endarterectomy for secondary prevention is reviewed. The recommendations are comparable to the American Academy of Neurology.
Source. New Zealand Guidelines Group. Life after stroke. New Zealand guideline for management of stroke. Wellington, NZ: New Zealand Guidelines Group; 2003: 84 pp [164 refs]. Available at: www.nzgg.org.nz/guidelines/0037/Stroke_Summary.pdf.
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. National Institute of Neurological Disorders and Stroke. Stroke: Hope through research. 2006. Available at: www.ninds.nih.gov/disorders/stroke/detail_stroke.htm#56 051105. Accessed on March 21, 2006.
CORRESPONDENCE: Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
What are the indications for carotid endarterectomy (CE) in the symptomatic patient?
How is symptomatic defined?
What are the indications for CE in the asymptomatic patient?
What is the role of aspirin therapy in these patients?
This guideline reviews the efficacy of carotid endarterectomy for stroke prevention in adults with symptomatic or asymptomatic internal carotid artery stenosis.
Symptomatic is defined as a cerebrovascular event in the carotid distribution (transient ischemic attack or nondisabling stroke) in the previous 6 months.
Indications for or against CE appear in the Practice Recommendations.
GRADE A RECOMMENDATIONS
- Carotid endarterectomy is effective for symptomatic patients with 70% to 99% stenosis. Carotid endarterectomy should not be considered for symptomatic patients with less than 50% stenosis—medical management is preferred. (50%–99% stenosis, Grade B)
- Carotid endarterectomy may be considered for patients between ages 40 to 75 years, with asymptomatic stenosis between 60% to 99%, if there is a 5-year life expectancy and surgical risk is <3%.
- Symptomatic and asymptomatic patients undergoing carotid endarterectomy should receive aspirin (81 or 325 mg) daily prior to surgery and for at least 3 months postoperatively. In the absence of contraindications, it should be continued indefinitely.
GRADE B RECOMMENDATIONS
- Carotid endarterectomy may be considered for symptomatic patients with 50% to 69% stenosis in patients with a 5-year life expectancy and surgical risk <6%.
GRADE C RECOMMENDATION
- Patients with hemispheric transient ischemic attack or cerebrovascular accident had greater benefit from carotid endarterectomy than those with retinal events.
- Patients with severe stenosis and a cerebrovascular event should have carotid endarterectomy within 2 weeks for greatest benefit.
- Contralateral occlusion reduces the benefit of carotid endarterectomy in asymptomatic patients.
- Data are insufficient to recommend other antiplatelet agents perioperatively.
The evidence categories are assessment of therapeutic effectiveness, prevention, and risk assessment. Study outcomes considered are: benefits of carotid endarterectomy for symptomatic patients, benefits for asymptomatic patients, the efficacy within 24 hours in patients with progressing stroke, clinical variables that impact the risk/benefit ratio, the most important radiologic factors impacting the risk/benefit ratio, the ideal dose of aspirin preoperatively, the evidence/practice gap, the likelihood that trial results can be achieved in practice, the benefit of carotid endarterectomy concurrent with or prior to coronary artery bypass grafting, and the optimal time after stroke to perform the surgery. The evidence rating is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Carotid endarterectomy has an important role in the prevention of stroke in patients with internal carotid artery stenosis, since the majority of strokes are ischemic. Strokes can be prevented if a high-grade internal carotid stenosis is corrected. More than 700,000 patients suffer a stroke each year in the US, with 80% related to ischemia (either thrombotic or embolic).2 Most strokes occur after the age of 65, and the risk doubles each decade after the age of 55. Stroke is the third leading cause of death, with only heart disease and cancer killing more people. Strokes cause more serious long-term disabilities than any other illness. The guideline was weakened by lack of cost analysis.
Guideline development and evidence review
A literature search was performed using Ovid Medline for relevant articles published from 1990 to 2001 using the keywords carotid endarterectomy, carotid stenosis, carotid artery diseases, and clinical trials. The Cochrane Library statements on carotid endarterectomy for symptomatic and asymptomatic stenosis from 2004 were reviewed to confirm that additional relevant citations from 2002 to 2004 were identified.
The initial search yielded 1462 citations. This list was reduced by excluding case reports, letters to the editor, review articles without primary data, studies addressing carotid endarterectomy technical issues, case series from a single surgeon, and articles not written in English. Case series from a single institution were included. This narrowed the pertinent list to 186 articles, which were reviewed independently by 2 committee members. The committee also agreed that if a pooled analysis of the major symptomatic carotid endarterectomy studies or if the results of the Asymptomatic Carotid Surgery Trial were published prior to the completion of the committee’s manuscript, these would subsequently be reviewed.
Source for this guideline
Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, Cote R, Hess D, Saver J, Spence JD, Stern B, Wilterdink J. Carotid endarterectomy—an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65:794–801 [27 refs]. Available at: www.neurology.org/cgi/reprint/65/6/794.pdf.
Other guidelines on surgical management of carotid stenosis and stroke
Life after stroke: New Zealand guideline for management of stroke
This comprehensive 2003 guideline is written for New Zealand health care delivery system. It summarizes recommendations for the assessment and management of stroke, transient ischemic attacks, and intracerebral hemorrhage in various locales. The utility of carotid endarterectomy for secondary prevention is reviewed. The recommendations are comparable to the American Academy of Neurology.
Source. New Zealand Guidelines Group. Life after stroke. New Zealand guideline for management of stroke. Wellington, NZ: New Zealand Guidelines Group; 2003: 84 pp [164 refs]. Available at: www.nzgg.org.nz/guidelines/0037/Stroke_Summary.pdf.
What are the indications for carotid endarterectomy (CE) in the symptomatic patient?
How is symptomatic defined?
What are the indications for CE in the asymptomatic patient?
What is the role of aspirin therapy in these patients?
This guideline reviews the efficacy of carotid endarterectomy for stroke prevention in adults with symptomatic or asymptomatic internal carotid artery stenosis.
Symptomatic is defined as a cerebrovascular event in the carotid distribution (transient ischemic attack or nondisabling stroke) in the previous 6 months.
Indications for or against CE appear in the Practice Recommendations.
GRADE A RECOMMENDATIONS
- Carotid endarterectomy is effective for symptomatic patients with 70% to 99% stenosis. Carotid endarterectomy should not be considered for symptomatic patients with less than 50% stenosis—medical management is preferred. (50%–99% stenosis, Grade B)
- Carotid endarterectomy may be considered for patients between ages 40 to 75 years, with asymptomatic stenosis between 60% to 99%, if there is a 5-year life expectancy and surgical risk is <3%.
- Symptomatic and asymptomatic patients undergoing carotid endarterectomy should receive aspirin (81 or 325 mg) daily prior to surgery and for at least 3 months postoperatively. In the absence of contraindications, it should be continued indefinitely.
GRADE B RECOMMENDATIONS
- Carotid endarterectomy may be considered for symptomatic patients with 50% to 69% stenosis in patients with a 5-year life expectancy and surgical risk <6%.
GRADE C RECOMMENDATION
- Patients with hemispheric transient ischemic attack or cerebrovascular accident had greater benefit from carotid endarterectomy than those with retinal events.
- Patients with severe stenosis and a cerebrovascular event should have carotid endarterectomy within 2 weeks for greatest benefit.
- Contralateral occlusion reduces the benefit of carotid endarterectomy in asymptomatic patients.
- Data are insufficient to recommend other antiplatelet agents perioperatively.
The evidence categories are assessment of therapeutic effectiveness, prevention, and risk assessment. Study outcomes considered are: benefits of carotid endarterectomy for symptomatic patients, benefits for asymptomatic patients, the efficacy within 24 hours in patients with progressing stroke, clinical variables that impact the risk/benefit ratio, the most important radiologic factors impacting the risk/benefit ratio, the ideal dose of aspirin preoperatively, the evidence/practice gap, the likelihood that trial results can be achieved in practice, the benefit of carotid endarterectomy concurrent with or prior to coronary artery bypass grafting, and the optimal time after stroke to perform the surgery. The evidence rating is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Carotid endarterectomy has an important role in the prevention of stroke in patients with internal carotid artery stenosis, since the majority of strokes are ischemic. Strokes can be prevented if a high-grade internal carotid stenosis is corrected. More than 700,000 patients suffer a stroke each year in the US, with 80% related to ischemia (either thrombotic or embolic).2 Most strokes occur after the age of 65, and the risk doubles each decade after the age of 55. Stroke is the third leading cause of death, with only heart disease and cancer killing more people. Strokes cause more serious long-term disabilities than any other illness. The guideline was weakened by lack of cost analysis.
Guideline development and evidence review
A literature search was performed using Ovid Medline for relevant articles published from 1990 to 2001 using the keywords carotid endarterectomy, carotid stenosis, carotid artery diseases, and clinical trials. The Cochrane Library statements on carotid endarterectomy for symptomatic and asymptomatic stenosis from 2004 were reviewed to confirm that additional relevant citations from 2002 to 2004 were identified.
The initial search yielded 1462 citations. This list was reduced by excluding case reports, letters to the editor, review articles without primary data, studies addressing carotid endarterectomy technical issues, case series from a single surgeon, and articles not written in English. Case series from a single institution were included. This narrowed the pertinent list to 186 articles, which were reviewed independently by 2 committee members. The committee also agreed that if a pooled analysis of the major symptomatic carotid endarterectomy studies or if the results of the Asymptomatic Carotid Surgery Trial were published prior to the completion of the committee’s manuscript, these would subsequently be reviewed.
Source for this guideline
Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, Cote R, Hess D, Saver J, Spence JD, Stern B, Wilterdink J. Carotid endarterectomy—an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65:794–801 [27 refs]. Available at: www.neurology.org/cgi/reprint/65/6/794.pdf.
Other guidelines on surgical management of carotid stenosis and stroke
Life after stroke: New Zealand guideline for management of stroke
This comprehensive 2003 guideline is written for New Zealand health care delivery system. It summarizes recommendations for the assessment and management of stroke, transient ischemic attacks, and intracerebral hemorrhage in various locales. The utility of carotid endarterectomy for secondary prevention is reviewed. The recommendations are comparable to the American Academy of Neurology.
Source. New Zealand Guidelines Group. Life after stroke. New Zealand guideline for management of stroke. Wellington, NZ: New Zealand Guidelines Group; 2003: 84 pp [164 refs]. Available at: www.nzgg.org.nz/guidelines/0037/Stroke_Summary.pdf.
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. National Institute of Neurological Disorders and Stroke. Stroke: Hope through research. 2006. Available at: www.ninds.nih.gov/disorders/stroke/detail_stroke.htm#56 051105. Accessed on March 21, 2006.
CORRESPONDENCE: Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. National Institute of Neurological Disorders and Stroke. Stroke: Hope through research. 2006. Available at: www.ninds.nih.gov/disorders/stroke/detail_stroke.htm#56 051105. Accessed on March 21, 2006.
CORRESPONDENCE: Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
The Journal of Family Practice ©2006 Dowden Health Media
Revisiting spirometry for the diagnosis of COPD
- The primary usefulness of spirometry is in identifying persons who will benefit from pharmacologic treatment to alleviate exacerbations (by confirming bronchodilator responsiveness).
- Reserve spirometry for those with activity-limited respiratory symptoms to help target bronchodilator therapy (most beneficial in those with forced expiratory volume in 1 second (FEV1) 50% or less of predicted value).
- Spirometry paired with clinical examination improves COPD diagnostic accuracy compared with clinical examination alone.
- Spirometry is useful in diagnosing COPD when patients have suggestive symptoms.
- Evidence does not support widespread use of spirometry to…
- –diagnose new cases of COPD in at-risk patients
- –improve smoking cessation rates
- –monitor the clinical course of COPD, or
- –adjust interventions
A Guideline Update1 published in September 2003 summarized the Global Strategy for the management of chronic obstructive pulmonary disease (COPD).2 The evidence in that report recommended against spirometry to “diagnose or assess severity of COPD.” However, the Agency for Healthcare Research and Quality (AHRQ) recently published new evidence3 that supports limited use of spirometry for assessing the condition of COPD patients.
AHRQ’s Minnesota Evidence-Based Practice Center reviewed articles published from 1966–2005. Pertinent studies assessed outcomes for adults in primary care settings who were at risk for COPD according to race, age, gender, tobacco use, symptoms, and spirometric status. Excluded from the review were children, persons with asthma, and those with alpha-1 antitrypsin deficiency. The 169-page report had 82 references. The evidence was not explicitly graded, which made it difficult to interpret the significance of each recommendation.
The authors cautioned against widespread spirometric testing of COPD patients. They cited expense of spirometry, resulting treatment costs, resource utilization expense, and personnel time. There is risk of labeling a large number of individuals as diseased who would not benefit from treatment.
CORRESPONDENCEKeith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Holten K.B. How should we manage an acute exacerbation of COPD? J Fam Pract 2003;52:780-782.
2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: www.goldcopd.com/revised.pdf.
3. Wilt TJ, Niewoehner D, Kim C, et al. Use of spirometry for case finding, diagnosis, and management of chronic obstructive pulmonary disease. Summary, Evidence Report/Technology Assessment: Number 121. AHRQ Publication Number 05-E017-1, August 2005. Rockville, Md: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/tp/spirotp.htm.
- The primary usefulness of spirometry is in identifying persons who will benefit from pharmacologic treatment to alleviate exacerbations (by confirming bronchodilator responsiveness).
- Reserve spirometry for those with activity-limited respiratory symptoms to help target bronchodilator therapy (most beneficial in those with forced expiratory volume in 1 second (FEV1) 50% or less of predicted value).
- Spirometry paired with clinical examination improves COPD diagnostic accuracy compared with clinical examination alone.
- Spirometry is useful in diagnosing COPD when patients have suggestive symptoms.
- Evidence does not support widespread use of spirometry to…
- –diagnose new cases of COPD in at-risk patients
- –improve smoking cessation rates
- –monitor the clinical course of COPD, or
- –adjust interventions
A Guideline Update1 published in September 2003 summarized the Global Strategy for the management of chronic obstructive pulmonary disease (COPD).2 The evidence in that report recommended against spirometry to “diagnose or assess severity of COPD.” However, the Agency for Healthcare Research and Quality (AHRQ) recently published new evidence3 that supports limited use of spirometry for assessing the condition of COPD patients.
AHRQ’s Minnesota Evidence-Based Practice Center reviewed articles published from 1966–2005. Pertinent studies assessed outcomes for adults in primary care settings who were at risk for COPD according to race, age, gender, tobacco use, symptoms, and spirometric status. Excluded from the review were children, persons with asthma, and those with alpha-1 antitrypsin deficiency. The 169-page report had 82 references. The evidence was not explicitly graded, which made it difficult to interpret the significance of each recommendation.
The authors cautioned against widespread spirometric testing of COPD patients. They cited expense of spirometry, resulting treatment costs, resource utilization expense, and personnel time. There is risk of labeling a large number of individuals as diseased who would not benefit from treatment.
CORRESPONDENCEKeith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
- The primary usefulness of spirometry is in identifying persons who will benefit from pharmacologic treatment to alleviate exacerbations (by confirming bronchodilator responsiveness).
- Reserve spirometry for those with activity-limited respiratory symptoms to help target bronchodilator therapy (most beneficial in those with forced expiratory volume in 1 second (FEV1) 50% or less of predicted value).
- Spirometry paired with clinical examination improves COPD diagnostic accuracy compared with clinical examination alone.
- Spirometry is useful in diagnosing COPD when patients have suggestive symptoms.
- Evidence does not support widespread use of spirometry to…
- –diagnose new cases of COPD in at-risk patients
- –improve smoking cessation rates
- –monitor the clinical course of COPD, or
- –adjust interventions
A Guideline Update1 published in September 2003 summarized the Global Strategy for the management of chronic obstructive pulmonary disease (COPD).2 The evidence in that report recommended against spirometry to “diagnose or assess severity of COPD.” However, the Agency for Healthcare Research and Quality (AHRQ) recently published new evidence3 that supports limited use of spirometry for assessing the condition of COPD patients.
AHRQ’s Minnesota Evidence-Based Practice Center reviewed articles published from 1966–2005. Pertinent studies assessed outcomes for adults in primary care settings who were at risk for COPD according to race, age, gender, tobacco use, symptoms, and spirometric status. Excluded from the review were children, persons with asthma, and those with alpha-1 antitrypsin deficiency. The 169-page report had 82 references. The evidence was not explicitly graded, which made it difficult to interpret the significance of each recommendation.
The authors cautioned against widespread spirometric testing of COPD patients. They cited expense of spirometry, resulting treatment costs, resource utilization expense, and personnel time. There is risk of labeling a large number of individuals as diseased who would not benefit from treatment.
CORRESPONDENCEKeith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Holten K.B. How should we manage an acute exacerbation of COPD? J Fam Pract 2003;52:780-782.
2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: www.goldcopd.com/revised.pdf.
3. Wilt TJ, Niewoehner D, Kim C, et al. Use of spirometry for case finding, diagnosis, and management of chronic obstructive pulmonary disease. Summary, Evidence Report/Technology Assessment: Number 121. AHRQ Publication Number 05-E017-1, August 2005. Rockville, Md: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/tp/spirotp.htm.
1. Holten K.B. How should we manage an acute exacerbation of COPD? J Fam Pract 2003;52:780-782.
2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: www.goldcopd.com/revised.pdf.
3. Wilt TJ, Niewoehner D, Kim C, et al. Use of spirometry for case finding, diagnosis, and management of chronic obstructive pulmonary disease. Summary, Evidence Report/Technology Assessment: Number 121. AHRQ Publication Number 05-E017-1, August 2005. Rockville, Md: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/tp/spirotp.htm.
How should we evaluate and treat constipation in infants and children?
- What are the indications for laboratory studies and imaging?
- What dietary adjustments are most effective?
- What medications are helpful?
- When can enemas be used?
- How effective is behavior modification?
These questions are answered in the recommendations at right, derived from a guideline developed and funded by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The target populations are infants and children with constipation who have no preexisting medical diagnosis.
The evidence categories for this guideline are diagnosis, evaluation, management, and treatment. Outcomes considered are 1) sensitivity and specificity of diagnostic tests; 2) rate of symptomatic relief; 3) prevention and control of symptoms; 4) medication and treatment side effects; 5) quality of life; and 6) bowel movement frequency. Recommendations were grouped by patient age: infants (age <1 year), children (age 1 year and older), and in general for all ages. Functional constipation was defined as fecal retention, unrelated to a medical or anatomic abnormality. Potential benefits and harms of implementing the guidelines were considered in the development. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Constipation is common among infants and children, accounting for 3% to 5% of visits to pediatric outpatient clinics. Males and females are equally affected.2 In one study,3 16% of parents reported constipation in their 2-year-olds.
A long bibliography accompanies this guideline, along with 77 references. The guideline is strengthened by inclusion of 2 algorithms for management of constipation in children aged <1 and >1 year, and consideration of potential harm. It was weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The constipation guideline committee first determined the scope of the guideline. A literature search in Medline from 1966 through 1997 was performed. These were filtered to include only randomized control trials. A second search strategy identified articles on treatment, including drug therapy, surgery, and “therapy.” In total, 160 articles were reviewed for development of the guideline. A systematic review of the literature was performed. Quality and strength of evidence were weighted according to a rating scheme.
The initial guideline was published in 1999. It was reviewed in 2004 and deemed current, after a new literature review and expert committee review.
Source for this guideline
Baker BB, Liptak GS, Colletti RB, et al. Constipation in infants and children: evaluation and treatment. A medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 1999; 29:612–626.
Another similar guideline
Functional constipation and soiling in children
This 2003 guideline presents methods for diagnosis and treatment of functional constipation, associated with soiling in children. It is only pertinent for children with encopresis: voluntary or involuntary passage of formed, semiformed, or liquid stool in places other than the toilet.
Source. University of Michigan Health System. Functional constipation and soiling in children. Ann Arbor: University of Michigan Health System; 2003. 10 pp. [8 references]
GRADE A RECOMMENDATIONS
- Mineral oil and osmotic laxatives are safe and effective for children.
- Medications combined with behavioral management can reduce time to remission in children with functional constipation.
GRADE B RECOMMENDATIONS
- An abdominal radiograph can be useful to diagnose fecal impaction.
- Rectal biopsy and rectal manometry are the only studies to reliably diagnose Hirschsprung disease.
- In infants, rectal disimpaction may be carried out with glycerin suppositories. Enemas should be avoided.
- In children, rectal disimpaction may be carried out with either oral or rectal medications, including enemas.
- In infants, juices that contain sorbitol, such as prune, pear, and apple juice, can decrease constipation.
- Osmotic laxatives (barley malt extract, corn syrup, lactulose, and sorbitol) can be used as stool softeners.
- Stimulant laxatives (senna and bisacodyl) can be useful in more difficult-to-treat cases.
GRADE C RECOMMENDATIONS
- A thorough history and physical is adequate to diagnose functional constipation.
- Stool exam for occult blood should be performed in constipated infants and in children with abdominal pain, failure to thrive, diarrhea, or family history of colon polyps/cancers.
- Mineral oil and stimulant laxatives should not be used in infants.
- In children, a balanced diet, containing whole grains, fruits, and vegetables is recommended.
- Polyethylene glycol electrolyte solution, in low doses, can be effective for difficult to treat patients.
Correspondence
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. Borowitz S. Constipation. eMedicine June 2004. Available at: www.emedicine.com/ped/topic471.htm. Accessed on June 27, 2005.
3. Issenman RM, Hewson S, Pirhonen D, et al. Are chronic digestive complaints the result of abnormal dietary patterns? Diet and digestive complaints in children at 22 and 40 months of age. Am J Dis Child 1987;141:679-682.
- What are the indications for laboratory studies and imaging?
- What dietary adjustments are most effective?
- What medications are helpful?
- When can enemas be used?
- How effective is behavior modification?
These questions are answered in the recommendations at right, derived from a guideline developed and funded by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The target populations are infants and children with constipation who have no preexisting medical diagnosis.
The evidence categories for this guideline are diagnosis, evaluation, management, and treatment. Outcomes considered are 1) sensitivity and specificity of diagnostic tests; 2) rate of symptomatic relief; 3) prevention and control of symptoms; 4) medication and treatment side effects; 5) quality of life; and 6) bowel movement frequency. Recommendations were grouped by patient age: infants (age <1 year), children (age 1 year and older), and in general for all ages. Functional constipation was defined as fecal retention, unrelated to a medical or anatomic abnormality. Potential benefits and harms of implementing the guidelines were considered in the development. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Constipation is common among infants and children, accounting for 3% to 5% of visits to pediatric outpatient clinics. Males and females are equally affected.2 In one study,3 16% of parents reported constipation in their 2-year-olds.
A long bibliography accompanies this guideline, along with 77 references. The guideline is strengthened by inclusion of 2 algorithms for management of constipation in children aged <1 and >1 year, and consideration of potential harm. It was weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The constipation guideline committee first determined the scope of the guideline. A literature search in Medline from 1966 through 1997 was performed. These were filtered to include only randomized control trials. A second search strategy identified articles on treatment, including drug therapy, surgery, and “therapy.” In total, 160 articles were reviewed for development of the guideline. A systematic review of the literature was performed. Quality and strength of evidence were weighted according to a rating scheme.
The initial guideline was published in 1999. It was reviewed in 2004 and deemed current, after a new literature review and expert committee review.
Source for this guideline
Baker BB, Liptak GS, Colletti RB, et al. Constipation in infants and children: evaluation and treatment. A medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 1999; 29:612–626.
Another similar guideline
Functional constipation and soiling in children
This 2003 guideline presents methods for diagnosis and treatment of functional constipation, associated with soiling in children. It is only pertinent for children with encopresis: voluntary or involuntary passage of formed, semiformed, or liquid stool in places other than the toilet.
Source. University of Michigan Health System. Functional constipation and soiling in children. Ann Arbor: University of Michigan Health System; 2003. 10 pp. [8 references]
GRADE A RECOMMENDATIONS
- Mineral oil and osmotic laxatives are safe and effective for children.
- Medications combined with behavioral management can reduce time to remission in children with functional constipation.
GRADE B RECOMMENDATIONS
- An abdominal radiograph can be useful to diagnose fecal impaction.
- Rectal biopsy and rectal manometry are the only studies to reliably diagnose Hirschsprung disease.
- In infants, rectal disimpaction may be carried out with glycerin suppositories. Enemas should be avoided.
- In children, rectal disimpaction may be carried out with either oral or rectal medications, including enemas.
- In infants, juices that contain sorbitol, such as prune, pear, and apple juice, can decrease constipation.
- Osmotic laxatives (barley malt extract, corn syrup, lactulose, and sorbitol) can be used as stool softeners.
- Stimulant laxatives (senna and bisacodyl) can be useful in more difficult-to-treat cases.
GRADE C RECOMMENDATIONS
- A thorough history and physical is adequate to diagnose functional constipation.
- Stool exam for occult blood should be performed in constipated infants and in children with abdominal pain, failure to thrive, diarrhea, or family history of colon polyps/cancers.
- Mineral oil and stimulant laxatives should not be used in infants.
- In children, a balanced diet, containing whole grains, fruits, and vegetables is recommended.
- Polyethylene glycol electrolyte solution, in low doses, can be effective for difficult to treat patients.
Correspondence
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
- What are the indications for laboratory studies and imaging?
- What dietary adjustments are most effective?
- What medications are helpful?
- When can enemas be used?
- How effective is behavior modification?
These questions are answered in the recommendations at right, derived from a guideline developed and funded by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The target populations are infants and children with constipation who have no preexisting medical diagnosis.
The evidence categories for this guideline are diagnosis, evaluation, management, and treatment. Outcomes considered are 1) sensitivity and specificity of diagnostic tests; 2) rate of symptomatic relief; 3) prevention and control of symptoms; 4) medication and treatment side effects; 5) quality of life; and 6) bowel movement frequency. Recommendations were grouped by patient age: infants (age <1 year), children (age 1 year and older), and in general for all ages. Functional constipation was defined as fecal retention, unrelated to a medical or anatomic abnormality. Potential benefits and harms of implementing the guidelines were considered in the development. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Constipation is common among infants and children, accounting for 3% to 5% of visits to pediatric outpatient clinics. Males and females are equally affected.2 In one study,3 16% of parents reported constipation in their 2-year-olds.
A long bibliography accompanies this guideline, along with 77 references. The guideline is strengthened by inclusion of 2 algorithms for management of constipation in children aged <1 and >1 year, and consideration of potential harm. It was weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The constipation guideline committee first determined the scope of the guideline. A literature search in Medline from 1966 through 1997 was performed. These were filtered to include only randomized control trials. A second search strategy identified articles on treatment, including drug therapy, surgery, and “therapy.” In total, 160 articles were reviewed for development of the guideline. A systematic review of the literature was performed. Quality and strength of evidence were weighted according to a rating scheme.
The initial guideline was published in 1999. It was reviewed in 2004 and deemed current, after a new literature review and expert committee review.
Source for this guideline
Baker BB, Liptak GS, Colletti RB, et al. Constipation in infants and children: evaluation and treatment. A medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 1999; 29:612–626.
Another similar guideline
Functional constipation and soiling in children
This 2003 guideline presents methods for diagnosis and treatment of functional constipation, associated with soiling in children. It is only pertinent for children with encopresis: voluntary or involuntary passage of formed, semiformed, or liquid stool in places other than the toilet.
Source. University of Michigan Health System. Functional constipation and soiling in children. Ann Arbor: University of Michigan Health System; 2003. 10 pp. [8 references]
GRADE A RECOMMENDATIONS
- Mineral oil and osmotic laxatives are safe and effective for children.
- Medications combined with behavioral management can reduce time to remission in children with functional constipation.
GRADE B RECOMMENDATIONS
- An abdominal radiograph can be useful to diagnose fecal impaction.
- Rectal biopsy and rectal manometry are the only studies to reliably diagnose Hirschsprung disease.
- In infants, rectal disimpaction may be carried out with glycerin suppositories. Enemas should be avoided.
- In children, rectal disimpaction may be carried out with either oral or rectal medications, including enemas.
- In infants, juices that contain sorbitol, such as prune, pear, and apple juice, can decrease constipation.
- Osmotic laxatives (barley malt extract, corn syrup, lactulose, and sorbitol) can be used as stool softeners.
- Stimulant laxatives (senna and bisacodyl) can be useful in more difficult-to-treat cases.
GRADE C RECOMMENDATIONS
- A thorough history and physical is adequate to diagnose functional constipation.
- Stool exam for occult blood should be performed in constipated infants and in children with abdominal pain, failure to thrive, diarrhea, or family history of colon polyps/cancers.
- Mineral oil and stimulant laxatives should not be used in infants.
- In children, a balanced diet, containing whole grains, fruits, and vegetables is recommended.
- Polyethylene glycol electrolyte solution, in low doses, can be effective for difficult to treat patients.
Correspondence
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. Borowitz S. Constipation. eMedicine June 2004. Available at: www.emedicine.com/ped/topic471.htm. Accessed on June 27, 2005.
3. Issenman RM, Hewson S, Pirhonen D, et al. Are chronic digestive complaints the result of abnormal dietary patterns? Diet and digestive complaints in children at 22 and 40 months of age. Am J Dis Child 1987;141:679-682.
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
2. Borowitz S. Constipation. eMedicine June 2004. Available at: www.emedicine.com/ped/topic471.htm. Accessed on June 27, 2005.
3. Issenman RM, Hewson S, Pirhonen D, et al. Are chronic digestive complaints the result of abnormal dietary patterns? Diet and digestive complaints in children at 22 and 40 months of age. Am J Dis Child 1987;141:679-682.
How should we care for atopic dermatitis?
GRADE A RECOMMENDATIONS
- Long-term, intermittent application of topical corticosteroids is appropriate, effective, and safe. Hydration and occlusion enhance delivery. Data are limited regarding steroid concentration, duration of treatment, and frequency of use.
- Emollients are effective and safe. They are useful for both prevention and treatment of episodes.
- Topical tar is effective, but compliance is reduced due to staining of clothing.
- Topical calcineurin inhibitors (immunomodulators, such as pimecrolimus and tacrolimus) reduce the rash severity and symptoms in children and adults.
- Systemic immunomodulary agents (such as cyclosporin) are effective against severe atopic dermatitis, but of limited value because of adverse effects.
- Oral antibiotics should be used to treat infected skin. They are not helpful for uninfected atopic dermatitis.
- Topical antibiotics are effective for skin infections, but they lead to the development of resistance.
- Oral antihistamines do not relieve pruritis associated with atopic dermatitis. They are indicated for patients with accompanying allergies (rhinitis, conjunctivitis, or urticaria).
- Dietary supplements are not effective.
- Ultraviolet phototherapy is effective.
GRADE B RECOMMENDATIONS
- Dietary restriction is useful only for infants with proven egg allergies.
- Ultraviolet phototherapy coupled with methoxypsoralen (PUVA) is helpful.
GRADE C RECOMMENDATIONS
- Combining education with psychotherapy can reduce symptoms.
- Systemic corticosteroids can be used for short-term treatment. However, there are concerns about rebound flaring and adverse effects.
- Interferon gamma is effective.
- The efficacy of leukotriene inhibitors, desensitization injections, and theophylline is unclear.
- The effectiveness of alternative treatments (herbal therapies, hypnotherapy, acupuncture, massage, or biofeedback) is unclear.
Do topical steroids relieve atopic dermatitis?
What are the indications for pimecrolimus topical therapy?
Is ultraviolet phototherapy useful?
Are systemic corticosteroids indicated?
What is the role of immunomodulary therapies?
Children and adults with atopic dermatitis (eczema) are the target populations of a guideline that was recently funded and developed by the American Academy of Dermatology (AAD). The AAD Work Group and Guideline/Outcomes Task Force created the original document. The entire AAD membership was solicited for review and comment. The final recommendations were reviewed and approved by the AAD board of directors. The intended users are physicians.
The evidence categories for this guideline are therapeutic effectiveness and treatment. Outcomes considered are 1) occurrence of atopic dermatitis; 2) therapeutic effectiveness, as measured by clinical signs and symptoms, blood cortisol levels, symptom scores, bacterial colonization, and serum immunoglobulin E (IgE) levels; and 3) adverse events. Their rating scheme has been updated to comply with the Strength of Recommendation taxonomy (SORT). 1
Guideline relevance and limitations
Atopic dermatitis is a common problem encountered by family physicians. It typically manifests in infants aged 1 to 6 months; approximately 60% of patients experience their first outbreak by age 1 year and 90% by age 5 years. Onset of atopic dermatitis in adolescence or later is uncommon and should prompt consideration of another diagnosis.2 Females usually have a worse prognosis than males.
A lengthy bibliography accompanies this guideline. The guideline is strengthened by use of summary tables and weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The work group was convened and the scope of the guideline was defined. They identified clinical questions to structure the primary issues in diagnosis and management. A literature search in Medline and EMBASE databases spanning the years 1990 to June 3, 2003, was performed. Additional searches were done by hand searching publications, including reviews, meta-analyses and correspondence.
The resultant prospective studies for treatments were screened for outcome evidence. A meta-analysis of patient data and a systematic review of the evidence were performed. Quality and strength of evidence were weighted according to a rating scheme.
Source for this guideline
Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis. J Am Acad Dermatol 2004; 50:391–404.
Other guidelines for atopic dermatitis
Guidelines for the evaluation of food allergies
This guideline from 2001 provides a rational approach to the evaluation of food allergies. Children with atopic dermatitis have a greater risk of food allergies. Allergy testing should be performed, when there is poor response to initial treatments.
Source. American Gastroenterological Association medical position statement: guidelines for the evaluation of food allergies. Gastroenterology 2001; 120:1023–1025.
Rhinitis
This guideline, revised in 2003, contains very little information about atopic dermatitis.
Source. Institute for Clinical Systems Improvement (ICSI). Rhinitis. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2003 May. 34 p. [86 references]
Neonatal skin care
This guideline is mostly directed to routine skin care for infants and does not list separate special instructions for atopic dermatitis.
Source. Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN). Neonatal Skin Care. Evidence-based Clinical Practice Guideline. Washington, DC: AWHONN; 2001. 54 p. [148 references]
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J FamPract 2004;53:111-120.
2. Ghidorzi AJ, Jr. Atopic dermatitis. eMedicine. June 2001. Available at: www.emedicine.com/emerg/topic130.htm. Accessed on April 20, 2005.
GRADE A RECOMMENDATIONS
- Long-term, intermittent application of topical corticosteroids is appropriate, effective, and safe. Hydration and occlusion enhance delivery. Data are limited regarding steroid concentration, duration of treatment, and frequency of use.
- Emollients are effective and safe. They are useful for both prevention and treatment of episodes.
- Topical tar is effective, but compliance is reduced due to staining of clothing.
- Topical calcineurin inhibitors (immunomodulators, such as pimecrolimus and tacrolimus) reduce the rash severity and symptoms in children and adults.
- Systemic immunomodulary agents (such as cyclosporin) are effective against severe atopic dermatitis, but of limited value because of adverse effects.
- Oral antibiotics should be used to treat infected skin. They are not helpful for uninfected atopic dermatitis.
- Topical antibiotics are effective for skin infections, but they lead to the development of resistance.
- Oral antihistamines do not relieve pruritis associated with atopic dermatitis. They are indicated for patients with accompanying allergies (rhinitis, conjunctivitis, or urticaria).
- Dietary supplements are not effective.
- Ultraviolet phototherapy is effective.
GRADE B RECOMMENDATIONS
- Dietary restriction is useful only for infants with proven egg allergies.
- Ultraviolet phototherapy coupled with methoxypsoralen (PUVA) is helpful.
GRADE C RECOMMENDATIONS
- Combining education with psychotherapy can reduce symptoms.
- Systemic corticosteroids can be used for short-term treatment. However, there are concerns about rebound flaring and adverse effects.
- Interferon gamma is effective.
- The efficacy of leukotriene inhibitors, desensitization injections, and theophylline is unclear.
- The effectiveness of alternative treatments (herbal therapies, hypnotherapy, acupuncture, massage, or biofeedback) is unclear.
Do topical steroids relieve atopic dermatitis?
What are the indications for pimecrolimus topical therapy?
Is ultraviolet phototherapy useful?
Are systemic corticosteroids indicated?
What is the role of immunomodulary therapies?
Children and adults with atopic dermatitis (eczema) are the target populations of a guideline that was recently funded and developed by the American Academy of Dermatology (AAD). The AAD Work Group and Guideline/Outcomes Task Force created the original document. The entire AAD membership was solicited for review and comment. The final recommendations were reviewed and approved by the AAD board of directors. The intended users are physicians.
The evidence categories for this guideline are therapeutic effectiveness and treatment. Outcomes considered are 1) occurrence of atopic dermatitis; 2) therapeutic effectiveness, as measured by clinical signs and symptoms, blood cortisol levels, symptom scores, bacterial colonization, and serum immunoglobulin E (IgE) levels; and 3) adverse events. Their rating scheme has been updated to comply with the Strength of Recommendation taxonomy (SORT). 1
Guideline relevance and limitations
Atopic dermatitis is a common problem encountered by family physicians. It typically manifests in infants aged 1 to 6 months; approximately 60% of patients experience their first outbreak by age 1 year and 90% by age 5 years. Onset of atopic dermatitis in adolescence or later is uncommon and should prompt consideration of another diagnosis.2 Females usually have a worse prognosis than males.
A lengthy bibliography accompanies this guideline. The guideline is strengthened by use of summary tables and weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The work group was convened and the scope of the guideline was defined. They identified clinical questions to structure the primary issues in diagnosis and management. A literature search in Medline and EMBASE databases spanning the years 1990 to June 3, 2003, was performed. Additional searches were done by hand searching publications, including reviews, meta-analyses and correspondence.
The resultant prospective studies for treatments were screened for outcome evidence. A meta-analysis of patient data and a systematic review of the evidence were performed. Quality and strength of evidence were weighted according to a rating scheme.
Source for this guideline
Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis. J Am Acad Dermatol 2004; 50:391–404.
Other guidelines for atopic dermatitis
Guidelines for the evaluation of food allergies
This guideline from 2001 provides a rational approach to the evaluation of food allergies. Children with atopic dermatitis have a greater risk of food allergies. Allergy testing should be performed, when there is poor response to initial treatments.
Source. American Gastroenterological Association medical position statement: guidelines for the evaluation of food allergies. Gastroenterology 2001; 120:1023–1025.
Rhinitis
This guideline, revised in 2003, contains very little information about atopic dermatitis.
Source. Institute for Clinical Systems Improvement (ICSI). Rhinitis. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2003 May. 34 p. [86 references]
Neonatal skin care
This guideline is mostly directed to routine skin care for infants and does not list separate special instructions for atopic dermatitis.
Source. Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN). Neonatal Skin Care. Evidence-based Clinical Practice Guideline. Washington, DC: AWHONN; 2001. 54 p. [148 references]
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
GRADE A RECOMMENDATIONS
- Long-term, intermittent application of topical corticosteroids is appropriate, effective, and safe. Hydration and occlusion enhance delivery. Data are limited regarding steroid concentration, duration of treatment, and frequency of use.
- Emollients are effective and safe. They are useful for both prevention and treatment of episodes.
- Topical tar is effective, but compliance is reduced due to staining of clothing.
- Topical calcineurin inhibitors (immunomodulators, such as pimecrolimus and tacrolimus) reduce the rash severity and symptoms in children and adults.
- Systemic immunomodulary agents (such as cyclosporin) are effective against severe atopic dermatitis, but of limited value because of adverse effects.
- Oral antibiotics should be used to treat infected skin. They are not helpful for uninfected atopic dermatitis.
- Topical antibiotics are effective for skin infections, but they lead to the development of resistance.
- Oral antihistamines do not relieve pruritis associated with atopic dermatitis. They are indicated for patients with accompanying allergies (rhinitis, conjunctivitis, or urticaria).
- Dietary supplements are not effective.
- Ultraviolet phototherapy is effective.
GRADE B RECOMMENDATIONS
- Dietary restriction is useful only for infants with proven egg allergies.
- Ultraviolet phototherapy coupled with methoxypsoralen (PUVA) is helpful.
GRADE C RECOMMENDATIONS
- Combining education with psychotherapy can reduce symptoms.
- Systemic corticosteroids can be used for short-term treatment. However, there are concerns about rebound flaring and adverse effects.
- Interferon gamma is effective.
- The efficacy of leukotriene inhibitors, desensitization injections, and theophylline is unclear.
- The effectiveness of alternative treatments (herbal therapies, hypnotherapy, acupuncture, massage, or biofeedback) is unclear.
Do topical steroids relieve atopic dermatitis?
What are the indications for pimecrolimus topical therapy?
Is ultraviolet phototherapy useful?
Are systemic corticosteroids indicated?
What is the role of immunomodulary therapies?
Children and adults with atopic dermatitis (eczema) are the target populations of a guideline that was recently funded and developed by the American Academy of Dermatology (AAD). The AAD Work Group and Guideline/Outcomes Task Force created the original document. The entire AAD membership was solicited for review and comment. The final recommendations were reviewed and approved by the AAD board of directors. The intended users are physicians.
The evidence categories for this guideline are therapeutic effectiveness and treatment. Outcomes considered are 1) occurrence of atopic dermatitis; 2) therapeutic effectiveness, as measured by clinical signs and symptoms, blood cortisol levels, symptom scores, bacterial colonization, and serum immunoglobulin E (IgE) levels; and 3) adverse events. Their rating scheme has been updated to comply with the Strength of Recommendation taxonomy (SORT). 1
Guideline relevance and limitations
Atopic dermatitis is a common problem encountered by family physicians. It typically manifests in infants aged 1 to 6 months; approximately 60% of patients experience their first outbreak by age 1 year and 90% by age 5 years. Onset of atopic dermatitis in adolescence or later is uncommon and should prompt consideration of another diagnosis.2 Females usually have a worse prognosis than males.
A lengthy bibliography accompanies this guideline. The guideline is strengthened by use of summary tables and weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The work group was convened and the scope of the guideline was defined. They identified clinical questions to structure the primary issues in diagnosis and management. A literature search in Medline and EMBASE databases spanning the years 1990 to June 3, 2003, was performed. Additional searches were done by hand searching publications, including reviews, meta-analyses and correspondence.
The resultant prospective studies for treatments were screened for outcome evidence. A meta-analysis of patient data and a systematic review of the evidence were performed. Quality and strength of evidence were weighted according to a rating scheme.
Source for this guideline
Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis. J Am Acad Dermatol 2004; 50:391–404.
Other guidelines for atopic dermatitis
Guidelines for the evaluation of food allergies
This guideline from 2001 provides a rational approach to the evaluation of food allergies. Children with atopic dermatitis have a greater risk of food allergies. Allergy testing should be performed, when there is poor response to initial treatments.
Source. American Gastroenterological Association medical position statement: guidelines for the evaluation of food allergies. Gastroenterology 2001; 120:1023–1025.
Rhinitis
This guideline, revised in 2003, contains very little information about atopic dermatitis.
Source. Institute for Clinical Systems Improvement (ICSI). Rhinitis. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2003 May. 34 p. [86 references]
Neonatal skin care
This guideline is mostly directed to routine skin care for infants and does not list separate special instructions for atopic dermatitis.
Source. Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN). Neonatal Skin Care. Evidence-based Clinical Practice Guideline. Washington, DC: AWHONN; 2001. 54 p. [148 references]
CORRESPONDENCE
Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177. E-mail: [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J FamPract 2004;53:111-120.
2. Ghidorzi AJ, Jr. Atopic dermatitis. eMedicine. June 2001. Available at: www.emedicine.com/emerg/topic130.htm. Accessed on April 20, 2005.
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J FamPract 2004;53:111-120.
2. Ghidorzi AJ, Jr. Atopic dermatitis. eMedicine. June 2001. Available at: www.emedicine.com/emerg/topic130.htm. Accessed on April 20, 2005.
How should we manage Bell’s palsy?
- Do steroids change the course of Bell’s palsy?
- What is the role of surgery for Bell’s palsy?
- Should antiviral therapy be initiated for all patients?
Recommendations for these management issues are found in the guideline that was funded and developed by the American Academy of Neurology. Their Quality Standards Subcommittee, Practice Committee, and Board of Directors approved the recommendations. The target audience is physicians.
Patients with Bell’s palsy are the target population of this guideline. The objective is to summarize evidence regarding effectiveness of steroids, acyclovir, or surgical facial nerve decompression for improved functional outcomes in facial nerve palsy (Bell’s palsy). The evidence categories for this guideline are therapeutic effectiveness and treatment. Outcomes considered are 1) relative rate and 95% confidence interval for goodreturn of facial function, and 2) relative rate and 95% confidence interval for completereturn of facial function. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Bell’s palsy results from damage to the 7th (facial) cranial nerve and affects 40,000 Americans each year. It is seen commonly in pregnant women and diabetics, as well as those with viral illnesses. Besides facial paralysis, other symptoms of Bell’s palsy may include pain, hypersensitivity to sound in the affected ear, and impairment of taste. The common cold sore viruses, herpes simplex virus, and other herpes viruses are the likely pathogens causing many cases of Bell’s palsy. The prognosis for Bell’s palsy is good and most patients get better within 2 weeks. Over 80% recover facial nerve function within 3 months.2
A lengthy bibliography accompanies the guideline. The guideline is weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The authors searched the National Library of Medicine’s Medline database from 1966 to June 2000. The resultant prospective studies for treatments with steroids, acyclovir, or surgery were screened for outcome evidence. There are 25 references. A meta-analysis of patient data and a systematic review of the evidence were performed. Quality and strength of evidence were weighted according to a rating scheme.
Grade B Recommendations
- Treatment with oral corticosteroids improves facial function.
- Treatment with acyclovir, combined with steroids, improves facial function.
Grade C Recommendations
- Facial nerve decompression does not improve facial function.
Source for this guideline
Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:830–836.
Other guidelines on bell’s palsy
- Assessment: Neurologic risk of immunization. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology.
This older guideline refers to a few cases of Bell’s palsy associated with the plasma-derived hepatitis B vaccine used from 1982 to 1988. Since then the recombinant product has replaced the plasma-derived vaccine.
Source: Fenichel GM. Assessment: Neurologic risk of immunization: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology1999; 52:1546–1552. [30 references]
CORRECTION
The photographs in the Figure appearing in last month’s Clinical Inquiry, “Do routine eye exams reduce occurrence of blindness from type 2 dia-betes?” (page 733), were transposed. They appear correctly below.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Ebell M, Siwek J, Weiss BD et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
2. NINDS Bell’s Palsy Information Page National Institute of Neurological Disorders and Stroke.April 2003. Access at: www.ninds.nih.gov/health_and_medical/disorders/bells_doc.htm. Accessed on August 6, 2004.
- Do steroids change the course of Bell’s palsy?
- What is the role of surgery for Bell’s palsy?
- Should antiviral therapy be initiated for all patients?
Recommendations for these management issues are found in the guideline that was funded and developed by the American Academy of Neurology. Their Quality Standards Subcommittee, Practice Committee, and Board of Directors approved the recommendations. The target audience is physicians.
Patients with Bell’s palsy are the target population of this guideline. The objective is to summarize evidence regarding effectiveness of steroids, acyclovir, or surgical facial nerve decompression for improved functional outcomes in facial nerve palsy (Bell’s palsy). The evidence categories for this guideline are therapeutic effectiveness and treatment. Outcomes considered are 1) relative rate and 95% confidence interval for goodreturn of facial function, and 2) relative rate and 95% confidence interval for completereturn of facial function. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Bell’s palsy results from damage to the 7th (facial) cranial nerve and affects 40,000 Americans each year. It is seen commonly in pregnant women and diabetics, as well as those with viral illnesses. Besides facial paralysis, other symptoms of Bell’s palsy may include pain, hypersensitivity to sound in the affected ear, and impairment of taste. The common cold sore viruses, herpes simplex virus, and other herpes viruses are the likely pathogens causing many cases of Bell’s palsy. The prognosis for Bell’s palsy is good and most patients get better within 2 weeks. Over 80% recover facial nerve function within 3 months.2
A lengthy bibliography accompanies the guideline. The guideline is weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The authors searched the National Library of Medicine’s Medline database from 1966 to June 2000. The resultant prospective studies for treatments with steroids, acyclovir, or surgery were screened for outcome evidence. There are 25 references. A meta-analysis of patient data and a systematic review of the evidence were performed. Quality and strength of evidence were weighted according to a rating scheme.
Grade B Recommendations
- Treatment with oral corticosteroids improves facial function.
- Treatment with acyclovir, combined with steroids, improves facial function.
Grade C Recommendations
- Facial nerve decompression does not improve facial function.
Source for this guideline
Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:830–836.
Other guidelines on bell’s palsy
- Assessment: Neurologic risk of immunization. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology.
This older guideline refers to a few cases of Bell’s palsy associated with the plasma-derived hepatitis B vaccine used from 1982 to 1988. Since then the recombinant product has replaced the plasma-derived vaccine.
Source: Fenichel GM. Assessment: Neurologic risk of immunization: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology1999; 52:1546–1552. [30 references]
CORRECTION
The photographs in the Figure appearing in last month’s Clinical Inquiry, “Do routine eye exams reduce occurrence of blindness from type 2 dia-betes?” (page 733), were transposed. They appear correctly below.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- Do steroids change the course of Bell’s palsy?
- What is the role of surgery for Bell’s palsy?
- Should antiviral therapy be initiated for all patients?
Recommendations for these management issues are found in the guideline that was funded and developed by the American Academy of Neurology. Their Quality Standards Subcommittee, Practice Committee, and Board of Directors approved the recommendations. The target audience is physicians.
Patients with Bell’s palsy are the target population of this guideline. The objective is to summarize evidence regarding effectiveness of steroids, acyclovir, or surgical facial nerve decompression for improved functional outcomes in facial nerve palsy (Bell’s palsy). The evidence categories for this guideline are therapeutic effectiveness and treatment. Outcomes considered are 1) relative rate and 95% confidence interval for goodreturn of facial function, and 2) relative rate and 95% confidence interval for completereturn of facial function. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Bell’s palsy results from damage to the 7th (facial) cranial nerve and affects 40,000 Americans each year. It is seen commonly in pregnant women and diabetics, as well as those with viral illnesses. Besides facial paralysis, other symptoms of Bell’s palsy may include pain, hypersensitivity to sound in the affected ear, and impairment of taste. The common cold sore viruses, herpes simplex virus, and other herpes viruses are the likely pathogens causing many cases of Bell’s palsy. The prognosis for Bell’s palsy is good and most patients get better within 2 weeks. Over 80% recover facial nerve function within 3 months.2
A lengthy bibliography accompanies the guideline. The guideline is weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The authors searched the National Library of Medicine’s Medline database from 1966 to June 2000. The resultant prospective studies for treatments with steroids, acyclovir, or surgery were screened for outcome evidence. There are 25 references. A meta-analysis of patient data and a systematic review of the evidence were performed. Quality and strength of evidence were weighted according to a rating scheme.
Grade B Recommendations
- Treatment with oral corticosteroids improves facial function.
- Treatment with acyclovir, combined with steroids, improves facial function.
Grade C Recommendations
- Facial nerve decompression does not improve facial function.
Source for this guideline
Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:830–836.
Other guidelines on bell’s palsy
- Assessment: Neurologic risk of immunization. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology.
This older guideline refers to a few cases of Bell’s palsy associated with the plasma-derived hepatitis B vaccine used from 1982 to 1988. Since then the recombinant product has replaced the plasma-derived vaccine.
Source: Fenichel GM. Assessment: Neurologic risk of immunization: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology1999; 52:1546–1552. [30 references]
CORRECTION
The photographs in the Figure appearing in last month’s Clinical Inquiry, “Do routine eye exams reduce occurrence of blindness from type 2 dia-betes?” (page 733), were transposed. They appear correctly below.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Ebell M, Siwek J, Weiss BD et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
2. NINDS Bell’s Palsy Information Page National Institute of Neurological Disorders and Stroke.April 2003. Access at: www.ninds.nih.gov/health_and_medical/disorders/bells_doc.htm. Accessed on August 6, 2004.
1. Ebell M, Siwek J, Weiss BD et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
2. NINDS Bell’s Palsy Information Page National Institute of Neurological Disorders and Stroke.April 2003. Access at: www.ninds.nih.gov/health_and_medical/disorders/bells_doc.htm. Accessed on August 6, 2004.
How should we manage newly diagnosed atrial fibrillation?
- What is the primary treatment goal of cardiac medication?
- What is the role of digoxin?
- When should medications be used to maintain sinus rhythm after cardioversion?
- Should all patients be anticoagulated with warfarin?
- What are the contraindications to warfarin therapy?
Recommendations for these management issues are found in the guideline developed in a joint effort of the American College of Physicians Clinical Efficacy Assessment Subcommittee and the American Academy of Family Physicians Commission on Clinical Policies and Research. It was funded by both organizations and approved by their Boards before publication. The target audience is internists and family physicians.
The target patients are adults with newly diagnosed atrial fibrillation. The guideline does not apply to postoperative patients, post-myocardial infarction patients, those with class IV heart failure or valvular heart disease, or patients taking antiarrhythmic medications.
The objective of this guideline is to recommend pharmacologic management of newly diagnosed atrial fibrillation. The evidence category for this guideline is management. Outcomes considered are control of heart rate and stroke risk reduction. The committees used the Guyatt method of grading recommendations,1 a qualitative approach to the literature. These were revised to comply with the SORT taxonomy.2
Guideline relevance and limitations
Atrial fibrillation is common, affecting anywhere from 1% of the American population at age 60 to 8% at age 80. It is more common in men than women. Even if patients are asymptomatic, they are at increased risk of stroke (1.9%–18% per year).
A lengthy bibliography accompanies the guideline. Tables of supporting evidence are lacking, which makes it more difficult to analyze the final recommendations. The guideline is weakened by the lack of a cost-effectiveness analysis.
Grade A Recommendations
- Prescribe long-term warfarin at therapeutic levels unless stroke risk is low, as determined by risk factors: congestive heart failure, hypertension, age 75 years older, diabetes mellitus, or history of transient ischemic event/cerebrovascular accident. Warfarin should not be prescribed if there are contraindications of thrombocytopenia, recent trauma, surgery, or alcoholism.
- Atenolol (Tenormin), metoprolol (Lopressor, Toprol-XL), diltiazem, and verapamil are optimal choices for rate control during exercise and at rest. Digoxin (Lanoxin is a second-line agent and is only effective at rest.
Grade B Recommendations
- Rate control with anticoagulation is the primary goal of treatment. Consider rhythm control according to a patient’s symptoms.
- Cardioversion by electrical conversion and pharmacologic conversion are both appropriate.
- For patients who elect cardioversion, options include 1) early antiocoagulation and cardioversion (with transesophageal echocardiography confirming absence of mural thrombus), or 2) delayed cardioversion with pre- and post-anticoagulation.
- Most patients who convert to sinus rhythm do not need maintenance rhythm therapy. When quality of life is threatened, the best agents are amiodarone (Cordarone), disopyramide (Norpace), propafenone (Rythmol), and sotalol (Betapace).
Guideline development and evidence review
This guideline is based on background papers published by McNamara3 and the John Hopkins Evidence-Based Practice Center4 under contract with the Agency for Healthcare Research and Quality. There are 57 references.
The guideline group reviewed the evidence and made graded recommendations regarding rate control versus rhythm control, stroke prevention and anticoagulation, electrical conversion versus pharmacologic conversion, the role of transesophageal echocardiography in guiding therapy, and maintenance therapy.
Source for this guideline
Snow V, Weiss KB, LeFevre M, et al.Management of newly detected atrial fibrillation: A clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med 2003; 139: 1009–1017.
Other guidelines on atrial fibrillation
ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation.
A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Atrial Fibrillation).
This 2001 guideline is the work of an international panel. Algorithms are provided for pharma-cologic management of patients with newly diagnosed atrial fibrillation, pharmacologic management of patients with recurrent paroxysmal atrial fibrillation, antiarrythmic drug therapy to maintain sinus rhythm in patients with recurrent paroxysmal or persistent atrial fibrillation, and pharmacologic management of patients with recurrent persistent or permanent atrial fibrillation. Recent evidence regarding rate control versus rhythm control was not available at publication of this guideline.
Sources: American College of Cardiology, American Heart Association, European Society of Cardiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol 2001; 38:1266i–lxx. (580 references) Fuster V, Ryden LE, Asinger RW, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology. Eur Heart J 2001; 22:1852–1923. (580 references)
Antithrombotic therapy in atrial fibrillation.
In: Sixth ACCP Consensus Conference on Antithrombotic Therapy.
This is an excellent review of a grading scheme for stroke risk and choice of anti-thrombotic agents.
Source: Albers GW, Dalen JE, Laupacis A, et al.Antithrombotic therapy in atrial fibrillation. Chest 2001; 119(1 Suppl):194S–206S. (103 references)
Atrial fibrillation: drug treatment and electric cardioversion.
This Finnish guideline makes recommendations regarding drug treatment, anticoagulation, and cardioversion for patients with atrial fibrillation and atrial flutter. The recommendations are not graded.
Source: Finnish Medical Society Duodecim. Atrial Fibrillation: Drug Treatment and Electric Cardioversion. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 Mar 4. Various pages.
Preventive health care, 2000 update.
Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke.
This guideline is from 2000 and found insufficient evidence to recommend for or against ambulatory electrocardiography to detect atrial fibrillation for patients after stroke or TIA.
Source: Bell C, Kapral M. Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke. Canadian Task Force on Preventive Health Care. Can J Neurol Sc. 2000; 27:25–31. (78 references)
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Guyatt GH, Sackett DL, Sinclair JC, et al. User’s guide to the medical literature:IX.A method for grading health care recommendations. JAMA 1995;274:1800.-
2. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
3. McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Int Med 2003;139:1018-1033.
4. McNamara RL, Bass EB, Miller MR, et al. Evidence report on the management of new onset atrial fibrillation.Agency for Healthcare Research and Quality publication no. AHRQ 01-E026. Rockville, MD: Agency for Healthcare Research and Quality; January 2001.
- What is the primary treatment goal of cardiac medication?
- What is the role of digoxin?
- When should medications be used to maintain sinus rhythm after cardioversion?
- Should all patients be anticoagulated with warfarin?
- What are the contraindications to warfarin therapy?
Recommendations for these management issues are found in the guideline developed in a joint effort of the American College of Physicians Clinical Efficacy Assessment Subcommittee and the American Academy of Family Physicians Commission on Clinical Policies and Research. It was funded by both organizations and approved by their Boards before publication. The target audience is internists and family physicians.
The target patients are adults with newly diagnosed atrial fibrillation. The guideline does not apply to postoperative patients, post-myocardial infarction patients, those with class IV heart failure or valvular heart disease, or patients taking antiarrhythmic medications.
The objective of this guideline is to recommend pharmacologic management of newly diagnosed atrial fibrillation. The evidence category for this guideline is management. Outcomes considered are control of heart rate and stroke risk reduction. The committees used the Guyatt method of grading recommendations,1 a qualitative approach to the literature. These were revised to comply with the SORT taxonomy.2
Guideline relevance and limitations
Atrial fibrillation is common, affecting anywhere from 1% of the American population at age 60 to 8% at age 80. It is more common in men than women. Even if patients are asymptomatic, they are at increased risk of stroke (1.9%–18% per year).
A lengthy bibliography accompanies the guideline. Tables of supporting evidence are lacking, which makes it more difficult to analyze the final recommendations. The guideline is weakened by the lack of a cost-effectiveness analysis.
Grade A Recommendations
- Prescribe long-term warfarin at therapeutic levels unless stroke risk is low, as determined by risk factors: congestive heart failure, hypertension, age 75 years older, diabetes mellitus, or history of transient ischemic event/cerebrovascular accident. Warfarin should not be prescribed if there are contraindications of thrombocytopenia, recent trauma, surgery, or alcoholism.
- Atenolol (Tenormin), metoprolol (Lopressor, Toprol-XL), diltiazem, and verapamil are optimal choices for rate control during exercise and at rest. Digoxin (Lanoxin is a second-line agent and is only effective at rest.
Grade B Recommendations
- Rate control with anticoagulation is the primary goal of treatment. Consider rhythm control according to a patient’s symptoms.
- Cardioversion by electrical conversion and pharmacologic conversion are both appropriate.
- For patients who elect cardioversion, options include 1) early antiocoagulation and cardioversion (with transesophageal echocardiography confirming absence of mural thrombus), or 2) delayed cardioversion with pre- and post-anticoagulation.
- Most patients who convert to sinus rhythm do not need maintenance rhythm therapy. When quality of life is threatened, the best agents are amiodarone (Cordarone), disopyramide (Norpace), propafenone (Rythmol), and sotalol (Betapace).
Guideline development and evidence review
This guideline is based on background papers published by McNamara3 and the John Hopkins Evidence-Based Practice Center4 under contract with the Agency for Healthcare Research and Quality. There are 57 references.
The guideline group reviewed the evidence and made graded recommendations regarding rate control versus rhythm control, stroke prevention and anticoagulation, electrical conversion versus pharmacologic conversion, the role of transesophageal echocardiography in guiding therapy, and maintenance therapy.
Source for this guideline
Snow V, Weiss KB, LeFevre M, et al.Management of newly detected atrial fibrillation: A clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med 2003; 139: 1009–1017.
Other guidelines on atrial fibrillation
ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation.
A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Atrial Fibrillation).
This 2001 guideline is the work of an international panel. Algorithms are provided for pharma-cologic management of patients with newly diagnosed atrial fibrillation, pharmacologic management of patients with recurrent paroxysmal atrial fibrillation, antiarrythmic drug therapy to maintain sinus rhythm in patients with recurrent paroxysmal or persistent atrial fibrillation, and pharmacologic management of patients with recurrent persistent or permanent atrial fibrillation. Recent evidence regarding rate control versus rhythm control was not available at publication of this guideline.
Sources: American College of Cardiology, American Heart Association, European Society of Cardiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol 2001; 38:1266i–lxx. (580 references) Fuster V, Ryden LE, Asinger RW, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology. Eur Heart J 2001; 22:1852–1923. (580 references)
Antithrombotic therapy in atrial fibrillation.
In: Sixth ACCP Consensus Conference on Antithrombotic Therapy.
This is an excellent review of a grading scheme for stroke risk and choice of anti-thrombotic agents.
Source: Albers GW, Dalen JE, Laupacis A, et al.Antithrombotic therapy in atrial fibrillation. Chest 2001; 119(1 Suppl):194S–206S. (103 references)
Atrial fibrillation: drug treatment and electric cardioversion.
This Finnish guideline makes recommendations regarding drug treatment, anticoagulation, and cardioversion for patients with atrial fibrillation and atrial flutter. The recommendations are not graded.
Source: Finnish Medical Society Duodecim. Atrial Fibrillation: Drug Treatment and Electric Cardioversion. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 Mar 4. Various pages.
Preventive health care, 2000 update.
Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke.
This guideline is from 2000 and found insufficient evidence to recommend for or against ambulatory electrocardiography to detect atrial fibrillation for patients after stroke or TIA.
Source: Bell C, Kapral M. Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke. Canadian Task Force on Preventive Health Care. Can J Neurol Sc. 2000; 27:25–31. (78 references)
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- What is the primary treatment goal of cardiac medication?
- What is the role of digoxin?
- When should medications be used to maintain sinus rhythm after cardioversion?
- Should all patients be anticoagulated with warfarin?
- What are the contraindications to warfarin therapy?
Recommendations for these management issues are found in the guideline developed in a joint effort of the American College of Physicians Clinical Efficacy Assessment Subcommittee and the American Academy of Family Physicians Commission on Clinical Policies and Research. It was funded by both organizations and approved by their Boards before publication. The target audience is internists and family physicians.
The target patients are adults with newly diagnosed atrial fibrillation. The guideline does not apply to postoperative patients, post-myocardial infarction patients, those with class IV heart failure or valvular heart disease, or patients taking antiarrhythmic medications.
The objective of this guideline is to recommend pharmacologic management of newly diagnosed atrial fibrillation. The evidence category for this guideline is management. Outcomes considered are control of heart rate and stroke risk reduction. The committees used the Guyatt method of grading recommendations,1 a qualitative approach to the literature. These were revised to comply with the SORT taxonomy.2
Guideline relevance and limitations
Atrial fibrillation is common, affecting anywhere from 1% of the American population at age 60 to 8% at age 80. It is more common in men than women. Even if patients are asymptomatic, they are at increased risk of stroke (1.9%–18% per year).
A lengthy bibliography accompanies the guideline. Tables of supporting evidence are lacking, which makes it more difficult to analyze the final recommendations. The guideline is weakened by the lack of a cost-effectiveness analysis.
Grade A Recommendations
- Prescribe long-term warfarin at therapeutic levels unless stroke risk is low, as determined by risk factors: congestive heart failure, hypertension, age 75 years older, diabetes mellitus, or history of transient ischemic event/cerebrovascular accident. Warfarin should not be prescribed if there are contraindications of thrombocytopenia, recent trauma, surgery, or alcoholism.
- Atenolol (Tenormin), metoprolol (Lopressor, Toprol-XL), diltiazem, and verapamil are optimal choices for rate control during exercise and at rest. Digoxin (Lanoxin is a second-line agent and is only effective at rest.
Grade B Recommendations
- Rate control with anticoagulation is the primary goal of treatment. Consider rhythm control according to a patient’s symptoms.
- Cardioversion by electrical conversion and pharmacologic conversion are both appropriate.
- For patients who elect cardioversion, options include 1) early antiocoagulation and cardioversion (with transesophageal echocardiography confirming absence of mural thrombus), or 2) delayed cardioversion with pre- and post-anticoagulation.
- Most patients who convert to sinus rhythm do not need maintenance rhythm therapy. When quality of life is threatened, the best agents are amiodarone (Cordarone), disopyramide (Norpace), propafenone (Rythmol), and sotalol (Betapace).
Guideline development and evidence review
This guideline is based on background papers published by McNamara3 and the John Hopkins Evidence-Based Practice Center4 under contract with the Agency for Healthcare Research and Quality. There are 57 references.
The guideline group reviewed the evidence and made graded recommendations regarding rate control versus rhythm control, stroke prevention and anticoagulation, electrical conversion versus pharmacologic conversion, the role of transesophageal echocardiography in guiding therapy, and maintenance therapy.
Source for this guideline
Snow V, Weiss KB, LeFevre M, et al.Management of newly detected atrial fibrillation: A clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med 2003; 139: 1009–1017.
Other guidelines on atrial fibrillation
ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation.
A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Atrial Fibrillation).
This 2001 guideline is the work of an international panel. Algorithms are provided for pharma-cologic management of patients with newly diagnosed atrial fibrillation, pharmacologic management of patients with recurrent paroxysmal atrial fibrillation, antiarrythmic drug therapy to maintain sinus rhythm in patients with recurrent paroxysmal or persistent atrial fibrillation, and pharmacologic management of patients with recurrent persistent or permanent atrial fibrillation. Recent evidence regarding rate control versus rhythm control was not available at publication of this guideline.
Sources: American College of Cardiology, American Heart Association, European Society of Cardiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol 2001; 38:1266i–lxx. (580 references) Fuster V, Ryden LE, Asinger RW, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology. Eur Heart J 2001; 22:1852–1923. (580 references)
Antithrombotic therapy in atrial fibrillation.
In: Sixth ACCP Consensus Conference on Antithrombotic Therapy.
This is an excellent review of a grading scheme for stroke risk and choice of anti-thrombotic agents.
Source: Albers GW, Dalen JE, Laupacis A, et al.Antithrombotic therapy in atrial fibrillation. Chest 2001; 119(1 Suppl):194S–206S. (103 references)
Atrial fibrillation: drug treatment and electric cardioversion.
This Finnish guideline makes recommendations regarding drug treatment, anticoagulation, and cardioversion for patients with atrial fibrillation and atrial flutter. The recommendations are not graded.
Source: Finnish Medical Society Duodecim. Atrial Fibrillation: Drug Treatment and Electric Cardioversion. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 Mar 4. Various pages.
Preventive health care, 2000 update.
Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke.
This guideline is from 2000 and found insufficient evidence to recommend for or against ambulatory electrocardiography to detect atrial fibrillation for patients after stroke or TIA.
Source: Bell C, Kapral M. Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke. Canadian Task Force on Preventive Health Care. Can J Neurol Sc. 2000; 27:25–31. (78 references)
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Guyatt GH, Sackett DL, Sinclair JC, et al. User’s guide to the medical literature:IX.A method for grading health care recommendations. JAMA 1995;274:1800.-
2. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
3. McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Int Med 2003;139:1018-1033.
4. McNamara RL, Bass EB, Miller MR, et al. Evidence report on the management of new onset atrial fibrillation.Agency for Healthcare Research and Quality publication no. AHRQ 01-E026. Rockville, MD: Agency for Healthcare Research and Quality; January 2001.
1. Guyatt GH, Sackett DL, Sinclair JC, et al. User’s guide to the medical literature:IX.A method for grading health care recommendations. JAMA 1995;274:1800.-
2. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
3. McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Int Med 2003;139:1018-1033.
4. McNamara RL, Bass EB, Miller MR, et al. Evidence report on the management of new onset atrial fibrillation.Agency for Healthcare Research and Quality publication no. AHRQ 01-E026. Rockville, MD: Agency for Healthcare Research and Quality; January 2001.