User login
What Corticosteroid is Most Appropriate for treating Acute Exacerbations of CoPD?
Case
A 66-year-old Caucasian female with moderate chronic obstructive pulmonary disease (COPD) (FEV1 55% predicted), obesity, hypertension, and Type 2 diabetes mellitus on insulin therapy presents to the ED with four days of increased cough productive of yellow sputum and progressive shortness of breath. Her physical exam is notable for an oxygen saturation of 87% on room air, along with diffuse expiratory wheezing with use of accessory muscles; her chest X-ray is unchanged from previous. The patient is given oxygen, nebulized bronchodilators, and one dose of IV methylprednisolone. Her symptoms do not improve significantly, and she is admitted for further management. What regimen of corticosteroids is most appropriate to treat her acute exacerbation of COPD?
Overview
COPD is the fourth-leading cause of death in the United States and continues to increase in prevalence.1 Acute exacerbations of COPD (AECOPD) contribute significantly to this high mortality rate, which approaches 40% at one year in those patients requiring mechanical support.1 An exacerbation of COPD has been defined as an acute change in a patient’s baseline dyspnea, cough, and/or sputum beyond day-to-day variability sufficient to warrant a change in therapy.2 Exacerbations commonly occur in COPD patients and often necessitate hospital admission. In fact, COPD consistently is one of the 10 most common reasons for hospitalization, with billions of dollars in associated healthcare costs.3
The goals for inpatient management of AECOPD are to provide acute symptom relief and to minimize the potential for subsequent exacerbations. These are accomplished via a multifaceted approach, including the use of bronchodilators, antibiotics, supplemental oxygen, noninvasive positive pressure ventilation in certain circumstances, and systemic corticosteroids.
The administration of systemic steroids in AECOPD has been prevalent for several decades, with initial studies showing positive effects on lung function, specifically FEV1.4 Studies have demonstrated the benefit of steroids in prolonging the time to subsequent exacerbation, reducing the rate of treatment failure, and reducing length of stay (LOS).5 Corticosteroids have since become an essential component of the standard of care in AECOPD management.
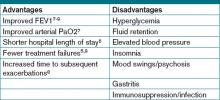
Despite consensus that systemic steroids should be used in COPD exacerbations, a great deal of controversy still surrounds the optimal steroid regimen.6 Steroid use is not without risk, as steroids can lead to adverse outcomes in medically complex hospitalized patients (see Table 1, below). Current guidelines provide limited guidance as to the optimal route of administration, dosing regimen, or length of therapy; clinical practice varies widely.
Review of the Data
Administration route: intravenous (IV) vs. oral. The use of steroids in AECOPD began with such IV formulations as methylprednisolone, and this became the typical method of treating hospitalized patients. This practice was validated in a multicenter Veterans Affairs trial, which demonstrated decreased risk of treatment failure (defined as all-cause mortality, need for intubation, readmission for COPD, or intensification of pharmacologic therapy) for patients randomized to receive an IV-to-oral steroid regimen compared with those randomized to placebo.5 Patients receiving steroids also had shorter LOS and improvements in FEV1 after the first day of treatment. Subsequent randomized controlled trials in patients with AECOPD demonstrated the benefit of oral regimens compared with placebo with regard to FEV1, LOS, and risk of treatment failure.6,7,8
Similarities in the bioavailability of oral and IV steroids have been known for a long time.9 Comparisons in efficacy initially were completed in the management of acute asthma exacerbations, with increasing evidence, including a meta-analysis, demonstrating no difference in improvement in pulmonary function and in preventing relapse of exacerbations for oral compared with IV steroids.10 However, only recently have oral and IV steroids been compared in the treatment of AECOPD. De Jong et al randomized more than 200 patients hospitalized for AECOPD to 60 mg of either IV or oral prednisolone for five days, followed by a week of an oral taper.11 There were no significant differences in treatment failure between the IV and oral groups (62% vs. 56%, respectively, at 90 days; one-sided lower bound of the 95% confidence interval [CI], −5.8%).
A large observational study by Lindenauer et al, including nearly 80,000 AECOPD patients admitted at more than 400 hospitals, added further support to the idea that oral and IV steroids were comparable in efficacy.12 In this study, multivariate analysis found no difference in treatment failure between oral and IV groups (odds ratio [OR] 0.93; 95% CI, 0.84-1.02). The authors also found, however, that current clinical practice still overwhelmingly favors intravenous steroids, with 92% of study patients initially being administered IV steroids.12
Based on the evidence from de Jong and Lindenauer, it appears that there is no significant benefit to the use of IV over oral steroids. Additionally, there is evidence for oral administration being associated with beneficial effects on cost and hospital LOS.12 Oral steroids, therefore, are the preferred route of administration to treat a hospitalized patient with AECOPD, unless the patient is unable to tolerate oral medications. Current guidelines support the practice of giving oral steroids as first-line treatment for AECOPD (see Table 2, above).
High dose vs. low dose. Another important clinical issue concerns the dosing of steroids. The randomized trials examining the use of corticosteroids in AECOPD vary widely in the dosages studied. Further, the majority of these trials have compared steroids to placebo, rather than comparing different dosage regimens. The agents studied have included prednisone, prednisolone, methylprednisolone, and hydrocortisone, or combinations thereof. In order to compare regimens of these different drugs, steroid doses often are converted into prednisone equivalents (see Table 3, below). Though no guidelines define “high dose” and “low dose,” some studies have designated doses of >80 mg prednisone equivalents daily as high-dose and prednisone equivalents of ≤80 mg daily as low-dose.13,14
Starting doses of systemic corticosteroids in the treatment of AECOPD in clinical studies range from prednisone equivalents of 30 mg daily to 625 mg on the first day of treatment.5,8 No randomized studies of high- versus low-dose steroid regimens have been conducted. One retrospective chart review of 145 AECOPD admissions evaluated outcomes among patients who were given higher (mean daily dose >80 mg prednisone equivalent) and lower (mean daily dose of ≤80 mg prednisone) doses.14 The authors found that patients who received higher doses of steroids had significantly longer LOS compared with those who received lower doses, especially among the subset of patients who were admitted to the floor rather than the ICU, though this analysis did not adjust for severity of illness. In this study, the most striking finding noted by the authors was the wide variability in the steroid doses prescribed for the inpatient treatment of AECOPD.
More recently, the study by Lindenauer et al examined outcomes between patients treated with high-dose IV steroids (equivalent of 120 mg-800 mg of prednisone on the first or second day of treatment) compared to low-dose oral steroids (prednisone equivalents of 20 mg-80 mg per day).12 The authors found no differences between the two groups regarding the rate of treatment failure, defined by initiation of mechanical ventilation after the second hospital day, in-hospital mortality, or readmission for COPD within 30 days of discharge. After multivariate adjustment, including the propensity for oral treatment, the low-dose oral therapy group was found to have lower risk of treatment failure, shorter LOS, and lower total hospital cost.
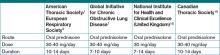
Despite the heterogeneity of the published data and the lack of randomized trials, the existing evidence suggests that low-dose prednisone (or equivalent) is similar in efficacy to higher doses and generally is associated with shorter hospital stays. Recognizing these benefits, guidelines do favor initiating treatment with low-dose steroids in patients admitted with AECOPD. The most recent publications from the American Thoracic Society/European Respiratory Society Task Force (ATS/ERS), the Global Initiative for Chronic Obstructive Lung Disease (GOLD), the National Clinical Guidelines Centre in the United Kingdom, and the Canadian Thoracic Society all recommend equivalent dosing of prednisone in patients admitted with AECOPD who are able to tolerate oral intake (see Table 2).1,2,15,16
Duration. As with the dosing of systemic corticosteroids in AECOPD, the optimal duration of treatment is not well-established. National and international consensus panels vary in their recommendations, as outlined in Table 2. This may be related to the variability in length of treatment found in the literature.
Treatment durations ranging from one day to eight weeks have been studied in inpatients with AECOPD. The landmark randomized controlled trial by Niewoehner and colleagues compared two-week and eight-week courses of systemic corticosteroids and found no difference in the rates of treatment failure, which included death, need for mechanical ventilation, readmission for COPD, and intensification of pharmacologic therapy.5 Based on these results, many experts have concluded that there is no benefit to steroid courses lasting beyond two weeks.
Although improvements in outcomes have been demonstrated with corticosteroid regimens as short as three days compared with placebo, most of the randomized controlled trials have included courses of seven to 14 days.4 Given the risks of adverse events (e.g. hyperglycemia) that are associated with systemic administration of steroids, the shortest effective duration should be considered.
In both clinical practice and clinical studies, steroid regimens often include a taper. A study by Vondracek and Hemstreet found that 79% of hospital discharges for AECOPD included a tapered corticosteroid regimen.14 From a physiologic standpoint, durations of corticosteroid treatment approximately three weeks or less, regardless of dosage, should not lead to adrenal suppression.17 There also is no evidence to suggest that abrupt discontinuation of steroids leads to clinical worsening of disease, and complicated steroid tapers are a potential source of medication errors after hospital discharge.18 Furthermore, the clinical guidelines do not address the tapering of corticosteroids. Therefore, there is a lack of evidence advocating for or against the use of tapered steroid regimens in AECOPD.
Back to the Case
In addition to standard treatment modalities for AECOPD, our patient was administered oral prednisone 40 mg daily. She experienced steroid-induced hyperglycemia, which was corrected with adjustment of her insulin regimen. The patient’s pulmonary symptoms improved within 72 hours, and she was discharged home on hospital day four to complete a seven-day steroid course. At hospital discharge, she was administered influenza and pneumococcal vaccinations, and she was instructed to resume her usual insulin dosing once she finished her prednisone course.
Overview
In the management of AECOPD, there remains a lack of consensus in defining the ideal steroid regimen. Based on current literature, the use of low-dose oral corticosteroids, such as prednisone 40 mg daily, for a seven- to 14-day course is recommended. TH
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is an assistant professor of internal medicine and pediatrics and academic hospitalist at Vanderbilt University School of Medicine.
References
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. Global Initiative for Chronic Obstructive Lung Disease website. Available at: www.goldcopd.org/GuidelineItem.asp?intId=989. Accessed Feb. 21, 2011.
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932-946.
- Morbidity and mortality: 2009 chart book on cardiovascular, lung, and blood diseases. National Institutes of Health’s National Heart, Lung, and Blood Institute website. Available at: www.nhlbi.nih.gov/resources/docs/2009_ChartBook.pdf. Accessed Feb. 24, 2011.
- Albert RK, Martin TR, Lewis SW. Controlled trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med. 1980;92(6):753-758.
- Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340(25):1941-1947.
- Thompson WH, Nielson C, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med. 1996;154:407-412.
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608-1613.
- Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354(9177):456-460.
- Al-Habet S, Rogers HJ. Pharmacokinetics of intravenous and oral prednisolone. Br J Clin Pharmacol. 1980;10(5):503-508.
- Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
- De Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, van den Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: A randomized, controlled, double-blind study. Chest. 2007;132(6):1741-1747.
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359-2367.
- Manser R, Reid D, Abramsom MJ. Corticosteroids for acute severe asthma in hospitalized patients. Cochrane Database Syst Rev. 2000;(2):CD001740.
- Vondracek SF, Hemstreet BA. Retrospective evaluation of systemic corticosteroids for the management of acute exacerbations of chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2006;63:645-652.
- Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Feb. 21, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14 Suppl B:5B-32B.
- Webb J, Clark TJ. Recovery of plasma corticotrophin and cortisol levels after three-week course of prednisolone. Thorax. 1981;36:22-24.
- O’Driscoll BR, Kalra S, Wilson M, Pickering CA, Carroll KB, Woodcock AA. Double-blind trial of steroid tapering in acute asthma. Lancet. 1993; 341:324-7.
Case
A 66-year-old Caucasian female with moderate chronic obstructive pulmonary disease (COPD) (FEV1 55% predicted), obesity, hypertension, and Type 2 diabetes mellitus on insulin therapy presents to the ED with four days of increased cough productive of yellow sputum and progressive shortness of breath. Her physical exam is notable for an oxygen saturation of 87% on room air, along with diffuse expiratory wheezing with use of accessory muscles; her chest X-ray is unchanged from previous. The patient is given oxygen, nebulized bronchodilators, and one dose of IV methylprednisolone. Her symptoms do not improve significantly, and she is admitted for further management. What regimen of corticosteroids is most appropriate to treat her acute exacerbation of COPD?
Overview
COPD is the fourth-leading cause of death in the United States and continues to increase in prevalence.1 Acute exacerbations of COPD (AECOPD) contribute significantly to this high mortality rate, which approaches 40% at one year in those patients requiring mechanical support.1 An exacerbation of COPD has been defined as an acute change in a patient’s baseline dyspnea, cough, and/or sputum beyond day-to-day variability sufficient to warrant a change in therapy.2 Exacerbations commonly occur in COPD patients and often necessitate hospital admission. In fact, COPD consistently is one of the 10 most common reasons for hospitalization, with billions of dollars in associated healthcare costs.3
The goals for inpatient management of AECOPD are to provide acute symptom relief and to minimize the potential for subsequent exacerbations. These are accomplished via a multifaceted approach, including the use of bronchodilators, antibiotics, supplemental oxygen, noninvasive positive pressure ventilation in certain circumstances, and systemic corticosteroids.
The administration of systemic steroids in AECOPD has been prevalent for several decades, with initial studies showing positive effects on lung function, specifically FEV1.4 Studies have demonstrated the benefit of steroids in prolonging the time to subsequent exacerbation, reducing the rate of treatment failure, and reducing length of stay (LOS).5 Corticosteroids have since become an essential component of the standard of care in AECOPD management.
Despite consensus that systemic steroids should be used in COPD exacerbations, a great deal of controversy still surrounds the optimal steroid regimen.6 Steroid use is not without risk, as steroids can lead to adverse outcomes in medically complex hospitalized patients (see Table 1, below). Current guidelines provide limited guidance as to the optimal route of administration, dosing regimen, or length of therapy; clinical practice varies widely.
Review of the Data
Administration route: intravenous (IV) vs. oral. The use of steroids in AECOPD began with such IV formulations as methylprednisolone, and this became the typical method of treating hospitalized patients. This practice was validated in a multicenter Veterans Affairs trial, which demonstrated decreased risk of treatment failure (defined as all-cause mortality, need for intubation, readmission for COPD, or intensification of pharmacologic therapy) for patients randomized to receive an IV-to-oral steroid regimen compared with those randomized to placebo.5 Patients receiving steroids also had shorter LOS and improvements in FEV1 after the first day of treatment. Subsequent randomized controlled trials in patients with AECOPD demonstrated the benefit of oral regimens compared with placebo with regard to FEV1, LOS, and risk of treatment failure.6,7,8
Similarities in the bioavailability of oral and IV steroids have been known for a long time.9 Comparisons in efficacy initially were completed in the management of acute asthma exacerbations, with increasing evidence, including a meta-analysis, demonstrating no difference in improvement in pulmonary function and in preventing relapse of exacerbations for oral compared with IV steroids.10 However, only recently have oral and IV steroids been compared in the treatment of AECOPD. De Jong et al randomized more than 200 patients hospitalized for AECOPD to 60 mg of either IV or oral prednisolone for five days, followed by a week of an oral taper.11 There were no significant differences in treatment failure between the IV and oral groups (62% vs. 56%, respectively, at 90 days; one-sided lower bound of the 95% confidence interval [CI], −5.8%).
A large observational study by Lindenauer et al, including nearly 80,000 AECOPD patients admitted at more than 400 hospitals, added further support to the idea that oral and IV steroids were comparable in efficacy.12 In this study, multivariate analysis found no difference in treatment failure between oral and IV groups (odds ratio [OR] 0.93; 95% CI, 0.84-1.02). The authors also found, however, that current clinical practice still overwhelmingly favors intravenous steroids, with 92% of study patients initially being administered IV steroids.12
Based on the evidence from de Jong and Lindenauer, it appears that there is no significant benefit to the use of IV over oral steroids. Additionally, there is evidence for oral administration being associated with beneficial effects on cost and hospital LOS.12 Oral steroids, therefore, are the preferred route of administration to treat a hospitalized patient with AECOPD, unless the patient is unable to tolerate oral medications. Current guidelines support the practice of giving oral steroids as first-line treatment for AECOPD (see Table 2, above).
High dose vs. low dose. Another important clinical issue concerns the dosing of steroids. The randomized trials examining the use of corticosteroids in AECOPD vary widely in the dosages studied. Further, the majority of these trials have compared steroids to placebo, rather than comparing different dosage regimens. The agents studied have included prednisone, prednisolone, methylprednisolone, and hydrocortisone, or combinations thereof. In order to compare regimens of these different drugs, steroid doses often are converted into prednisone equivalents (see Table 3, below). Though no guidelines define “high dose” and “low dose,” some studies have designated doses of >80 mg prednisone equivalents daily as high-dose and prednisone equivalents of ≤80 mg daily as low-dose.13,14
Starting doses of systemic corticosteroids in the treatment of AECOPD in clinical studies range from prednisone equivalents of 30 mg daily to 625 mg on the first day of treatment.5,8 No randomized studies of high- versus low-dose steroid regimens have been conducted. One retrospective chart review of 145 AECOPD admissions evaluated outcomes among patients who were given higher (mean daily dose >80 mg prednisone equivalent) and lower (mean daily dose of ≤80 mg prednisone) doses.14 The authors found that patients who received higher doses of steroids had significantly longer LOS compared with those who received lower doses, especially among the subset of patients who were admitted to the floor rather than the ICU, though this analysis did not adjust for severity of illness. In this study, the most striking finding noted by the authors was the wide variability in the steroid doses prescribed for the inpatient treatment of AECOPD.
More recently, the study by Lindenauer et al examined outcomes between patients treated with high-dose IV steroids (equivalent of 120 mg-800 mg of prednisone on the first or second day of treatment) compared to low-dose oral steroids (prednisone equivalents of 20 mg-80 mg per day).12 The authors found no differences between the two groups regarding the rate of treatment failure, defined by initiation of mechanical ventilation after the second hospital day, in-hospital mortality, or readmission for COPD within 30 days of discharge. After multivariate adjustment, including the propensity for oral treatment, the low-dose oral therapy group was found to have lower risk of treatment failure, shorter LOS, and lower total hospital cost.
Despite the heterogeneity of the published data and the lack of randomized trials, the existing evidence suggests that low-dose prednisone (or equivalent) is similar in efficacy to higher doses and generally is associated with shorter hospital stays. Recognizing these benefits, guidelines do favor initiating treatment with low-dose steroids in patients admitted with AECOPD. The most recent publications from the American Thoracic Society/European Respiratory Society Task Force (ATS/ERS), the Global Initiative for Chronic Obstructive Lung Disease (GOLD), the National Clinical Guidelines Centre in the United Kingdom, and the Canadian Thoracic Society all recommend equivalent dosing of prednisone in patients admitted with AECOPD who are able to tolerate oral intake (see Table 2).1,2,15,16
Duration. As with the dosing of systemic corticosteroids in AECOPD, the optimal duration of treatment is not well-established. National and international consensus panels vary in their recommendations, as outlined in Table 2. This may be related to the variability in length of treatment found in the literature.
Treatment durations ranging from one day to eight weeks have been studied in inpatients with AECOPD. The landmark randomized controlled trial by Niewoehner and colleagues compared two-week and eight-week courses of systemic corticosteroids and found no difference in the rates of treatment failure, which included death, need for mechanical ventilation, readmission for COPD, and intensification of pharmacologic therapy.5 Based on these results, many experts have concluded that there is no benefit to steroid courses lasting beyond two weeks.
Although improvements in outcomes have been demonstrated with corticosteroid regimens as short as three days compared with placebo, most of the randomized controlled trials have included courses of seven to 14 days.4 Given the risks of adverse events (e.g. hyperglycemia) that are associated with systemic administration of steroids, the shortest effective duration should be considered.
In both clinical practice and clinical studies, steroid regimens often include a taper. A study by Vondracek and Hemstreet found that 79% of hospital discharges for AECOPD included a tapered corticosteroid regimen.14 From a physiologic standpoint, durations of corticosteroid treatment approximately three weeks or less, regardless of dosage, should not lead to adrenal suppression.17 There also is no evidence to suggest that abrupt discontinuation of steroids leads to clinical worsening of disease, and complicated steroid tapers are a potential source of medication errors after hospital discharge.18 Furthermore, the clinical guidelines do not address the tapering of corticosteroids. Therefore, there is a lack of evidence advocating for or against the use of tapered steroid regimens in AECOPD.
Back to the Case
In addition to standard treatment modalities for AECOPD, our patient was administered oral prednisone 40 mg daily. She experienced steroid-induced hyperglycemia, which was corrected with adjustment of her insulin regimen. The patient’s pulmonary symptoms improved within 72 hours, and she was discharged home on hospital day four to complete a seven-day steroid course. At hospital discharge, she was administered influenza and pneumococcal vaccinations, and she was instructed to resume her usual insulin dosing once she finished her prednisone course.
Overview
In the management of AECOPD, there remains a lack of consensus in defining the ideal steroid regimen. Based on current literature, the use of low-dose oral corticosteroids, such as prednisone 40 mg daily, for a seven- to 14-day course is recommended. TH
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is an assistant professor of internal medicine and pediatrics and academic hospitalist at Vanderbilt University School of Medicine.
References
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. Global Initiative for Chronic Obstructive Lung Disease website. Available at: www.goldcopd.org/GuidelineItem.asp?intId=989. Accessed Feb. 21, 2011.
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932-946.
- Morbidity and mortality: 2009 chart book on cardiovascular, lung, and blood diseases. National Institutes of Health’s National Heart, Lung, and Blood Institute website. Available at: www.nhlbi.nih.gov/resources/docs/2009_ChartBook.pdf. Accessed Feb. 24, 2011.
- Albert RK, Martin TR, Lewis SW. Controlled trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med. 1980;92(6):753-758.
- Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340(25):1941-1947.
- Thompson WH, Nielson C, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med. 1996;154:407-412.
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608-1613.
- Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354(9177):456-460.
- Al-Habet S, Rogers HJ. Pharmacokinetics of intravenous and oral prednisolone. Br J Clin Pharmacol. 1980;10(5):503-508.
- Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
- De Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, van den Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: A randomized, controlled, double-blind study. Chest. 2007;132(6):1741-1747.
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359-2367.
- Manser R, Reid D, Abramsom MJ. Corticosteroids for acute severe asthma in hospitalized patients. Cochrane Database Syst Rev. 2000;(2):CD001740.
- Vondracek SF, Hemstreet BA. Retrospective evaluation of systemic corticosteroids for the management of acute exacerbations of chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2006;63:645-652.
- Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Feb. 21, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14 Suppl B:5B-32B.
- Webb J, Clark TJ. Recovery of plasma corticotrophin and cortisol levels after three-week course of prednisolone. Thorax. 1981;36:22-24.
- O’Driscoll BR, Kalra S, Wilson M, Pickering CA, Carroll KB, Woodcock AA. Double-blind trial of steroid tapering in acute asthma. Lancet. 1993; 341:324-7.
Case
A 66-year-old Caucasian female with moderate chronic obstructive pulmonary disease (COPD) (FEV1 55% predicted), obesity, hypertension, and Type 2 diabetes mellitus on insulin therapy presents to the ED with four days of increased cough productive of yellow sputum and progressive shortness of breath. Her physical exam is notable for an oxygen saturation of 87% on room air, along with diffuse expiratory wheezing with use of accessory muscles; her chest X-ray is unchanged from previous. The patient is given oxygen, nebulized bronchodilators, and one dose of IV methylprednisolone. Her symptoms do not improve significantly, and she is admitted for further management. What regimen of corticosteroids is most appropriate to treat her acute exacerbation of COPD?
Overview
COPD is the fourth-leading cause of death in the United States and continues to increase in prevalence.1 Acute exacerbations of COPD (AECOPD) contribute significantly to this high mortality rate, which approaches 40% at one year in those patients requiring mechanical support.1 An exacerbation of COPD has been defined as an acute change in a patient’s baseline dyspnea, cough, and/or sputum beyond day-to-day variability sufficient to warrant a change in therapy.2 Exacerbations commonly occur in COPD patients and often necessitate hospital admission. In fact, COPD consistently is one of the 10 most common reasons for hospitalization, with billions of dollars in associated healthcare costs.3
The goals for inpatient management of AECOPD are to provide acute symptom relief and to minimize the potential for subsequent exacerbations. These are accomplished via a multifaceted approach, including the use of bronchodilators, antibiotics, supplemental oxygen, noninvasive positive pressure ventilation in certain circumstances, and systemic corticosteroids.
The administration of systemic steroids in AECOPD has been prevalent for several decades, with initial studies showing positive effects on lung function, specifically FEV1.4 Studies have demonstrated the benefit of steroids in prolonging the time to subsequent exacerbation, reducing the rate of treatment failure, and reducing length of stay (LOS).5 Corticosteroids have since become an essential component of the standard of care in AECOPD management.
Despite consensus that systemic steroids should be used in COPD exacerbations, a great deal of controversy still surrounds the optimal steroid regimen.6 Steroid use is not without risk, as steroids can lead to adverse outcomes in medically complex hospitalized patients (see Table 1, below). Current guidelines provide limited guidance as to the optimal route of administration, dosing regimen, or length of therapy; clinical practice varies widely.
Review of the Data
Administration route: intravenous (IV) vs. oral. The use of steroids in AECOPD began with such IV formulations as methylprednisolone, and this became the typical method of treating hospitalized patients. This practice was validated in a multicenter Veterans Affairs trial, which demonstrated decreased risk of treatment failure (defined as all-cause mortality, need for intubation, readmission for COPD, or intensification of pharmacologic therapy) for patients randomized to receive an IV-to-oral steroid regimen compared with those randomized to placebo.5 Patients receiving steroids also had shorter LOS and improvements in FEV1 after the first day of treatment. Subsequent randomized controlled trials in patients with AECOPD demonstrated the benefit of oral regimens compared with placebo with regard to FEV1, LOS, and risk of treatment failure.6,7,8
Similarities in the bioavailability of oral and IV steroids have been known for a long time.9 Comparisons in efficacy initially were completed in the management of acute asthma exacerbations, with increasing evidence, including a meta-analysis, demonstrating no difference in improvement in pulmonary function and in preventing relapse of exacerbations for oral compared with IV steroids.10 However, only recently have oral and IV steroids been compared in the treatment of AECOPD. De Jong et al randomized more than 200 patients hospitalized for AECOPD to 60 mg of either IV or oral prednisolone for five days, followed by a week of an oral taper.11 There were no significant differences in treatment failure between the IV and oral groups (62% vs. 56%, respectively, at 90 days; one-sided lower bound of the 95% confidence interval [CI], −5.8%).
A large observational study by Lindenauer et al, including nearly 80,000 AECOPD patients admitted at more than 400 hospitals, added further support to the idea that oral and IV steroids were comparable in efficacy.12 In this study, multivariate analysis found no difference in treatment failure between oral and IV groups (odds ratio [OR] 0.93; 95% CI, 0.84-1.02). The authors also found, however, that current clinical practice still overwhelmingly favors intravenous steroids, with 92% of study patients initially being administered IV steroids.12
Based on the evidence from de Jong and Lindenauer, it appears that there is no significant benefit to the use of IV over oral steroids. Additionally, there is evidence for oral administration being associated with beneficial effects on cost and hospital LOS.12 Oral steroids, therefore, are the preferred route of administration to treat a hospitalized patient with AECOPD, unless the patient is unable to tolerate oral medications. Current guidelines support the practice of giving oral steroids as first-line treatment for AECOPD (see Table 2, above).
High dose vs. low dose. Another important clinical issue concerns the dosing of steroids. The randomized trials examining the use of corticosteroids in AECOPD vary widely in the dosages studied. Further, the majority of these trials have compared steroids to placebo, rather than comparing different dosage regimens. The agents studied have included prednisone, prednisolone, methylprednisolone, and hydrocortisone, or combinations thereof. In order to compare regimens of these different drugs, steroid doses often are converted into prednisone equivalents (see Table 3, below). Though no guidelines define “high dose” and “low dose,” some studies have designated doses of >80 mg prednisone equivalents daily as high-dose and prednisone equivalents of ≤80 mg daily as low-dose.13,14
Starting doses of systemic corticosteroids in the treatment of AECOPD in clinical studies range from prednisone equivalents of 30 mg daily to 625 mg on the first day of treatment.5,8 No randomized studies of high- versus low-dose steroid regimens have been conducted. One retrospective chart review of 145 AECOPD admissions evaluated outcomes among patients who were given higher (mean daily dose >80 mg prednisone equivalent) and lower (mean daily dose of ≤80 mg prednisone) doses.14 The authors found that patients who received higher doses of steroids had significantly longer LOS compared with those who received lower doses, especially among the subset of patients who were admitted to the floor rather than the ICU, though this analysis did not adjust for severity of illness. In this study, the most striking finding noted by the authors was the wide variability in the steroid doses prescribed for the inpatient treatment of AECOPD.
More recently, the study by Lindenauer et al examined outcomes between patients treated with high-dose IV steroids (equivalent of 120 mg-800 mg of prednisone on the first or second day of treatment) compared to low-dose oral steroids (prednisone equivalents of 20 mg-80 mg per day).12 The authors found no differences between the two groups regarding the rate of treatment failure, defined by initiation of mechanical ventilation after the second hospital day, in-hospital mortality, or readmission for COPD within 30 days of discharge. After multivariate adjustment, including the propensity for oral treatment, the low-dose oral therapy group was found to have lower risk of treatment failure, shorter LOS, and lower total hospital cost.
Despite the heterogeneity of the published data and the lack of randomized trials, the existing evidence suggests that low-dose prednisone (or equivalent) is similar in efficacy to higher doses and generally is associated with shorter hospital stays. Recognizing these benefits, guidelines do favor initiating treatment with low-dose steroids in patients admitted with AECOPD. The most recent publications from the American Thoracic Society/European Respiratory Society Task Force (ATS/ERS), the Global Initiative for Chronic Obstructive Lung Disease (GOLD), the National Clinical Guidelines Centre in the United Kingdom, and the Canadian Thoracic Society all recommend equivalent dosing of prednisone in patients admitted with AECOPD who are able to tolerate oral intake (see Table 2).1,2,15,16
Duration. As with the dosing of systemic corticosteroids in AECOPD, the optimal duration of treatment is not well-established. National and international consensus panels vary in their recommendations, as outlined in Table 2. This may be related to the variability in length of treatment found in the literature.
Treatment durations ranging from one day to eight weeks have been studied in inpatients with AECOPD. The landmark randomized controlled trial by Niewoehner and colleagues compared two-week and eight-week courses of systemic corticosteroids and found no difference in the rates of treatment failure, which included death, need for mechanical ventilation, readmission for COPD, and intensification of pharmacologic therapy.5 Based on these results, many experts have concluded that there is no benefit to steroid courses lasting beyond two weeks.
Although improvements in outcomes have been demonstrated with corticosteroid regimens as short as three days compared with placebo, most of the randomized controlled trials have included courses of seven to 14 days.4 Given the risks of adverse events (e.g. hyperglycemia) that are associated with systemic administration of steroids, the shortest effective duration should be considered.
In both clinical practice and clinical studies, steroid regimens often include a taper. A study by Vondracek and Hemstreet found that 79% of hospital discharges for AECOPD included a tapered corticosteroid regimen.14 From a physiologic standpoint, durations of corticosteroid treatment approximately three weeks or less, regardless of dosage, should not lead to adrenal suppression.17 There also is no evidence to suggest that abrupt discontinuation of steroids leads to clinical worsening of disease, and complicated steroid tapers are a potential source of medication errors after hospital discharge.18 Furthermore, the clinical guidelines do not address the tapering of corticosteroids. Therefore, there is a lack of evidence advocating for or against the use of tapered steroid regimens in AECOPD.
Back to the Case
In addition to standard treatment modalities for AECOPD, our patient was administered oral prednisone 40 mg daily. She experienced steroid-induced hyperglycemia, which was corrected with adjustment of her insulin regimen. The patient’s pulmonary symptoms improved within 72 hours, and she was discharged home on hospital day four to complete a seven-day steroid course. At hospital discharge, she was administered influenza and pneumococcal vaccinations, and she was instructed to resume her usual insulin dosing once she finished her prednisone course.
Overview
In the management of AECOPD, there remains a lack of consensus in defining the ideal steroid regimen. Based on current literature, the use of low-dose oral corticosteroids, such as prednisone 40 mg daily, for a seven- to 14-day course is recommended. TH
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is an assistant professor of internal medicine and pediatrics and academic hospitalist at Vanderbilt University School of Medicine.
References
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. Global Initiative for Chronic Obstructive Lung Disease website. Available at: www.goldcopd.org/GuidelineItem.asp?intId=989. Accessed Feb. 21, 2011.
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932-946.
- Morbidity and mortality: 2009 chart book on cardiovascular, lung, and blood diseases. National Institutes of Health’s National Heart, Lung, and Blood Institute website. Available at: www.nhlbi.nih.gov/resources/docs/2009_ChartBook.pdf. Accessed Feb. 24, 2011.
- Albert RK, Martin TR, Lewis SW. Controlled trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med. 1980;92(6):753-758.
- Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340(25):1941-1947.
- Thompson WH, Nielson C, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med. 1996;154:407-412.
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608-1613.
- Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354(9177):456-460.
- Al-Habet S, Rogers HJ. Pharmacokinetics of intravenous and oral prednisolone. Br J Clin Pharmacol. 1980;10(5):503-508.
- Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
- De Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, van den Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: A randomized, controlled, double-blind study. Chest. 2007;132(6):1741-1747.
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359-2367.
- Manser R, Reid D, Abramsom MJ. Corticosteroids for acute severe asthma in hospitalized patients. Cochrane Database Syst Rev. 2000;(2):CD001740.
- Vondracek SF, Hemstreet BA. Retrospective evaluation of systemic corticosteroids for the management of acute exacerbations of chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2006;63:645-652.
- Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Feb. 21, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14 Suppl B:5B-32B.
- Webb J, Clark TJ. Recovery of plasma corticotrophin and cortisol levels after three-week course of prednisolone. Thorax. 1981;36:22-24.
- O’Driscoll BR, Kalra S, Wilson M, Pickering CA, Carroll KB, Woodcock AA. Double-blind trial of steroid tapering in acute asthma. Lancet. 1993; 341:324-7.
What is the proper duration of antibiotic treatment in adults hospitalized with community-acquired pneumonia?
Case
An 83-year-old male with hypertension, coronary artery disease, and obstructive sleep apnea presents with progressive shortness of breath, a productive cough, wheezing, and tachypnea. His blood pressure is 158/70 mm/Hg; temperature is 101.8; respirations are 26 breaths per minute; and oxygen saturation is 87% on room air. He has coarse breath sounds bilaterally, and decreased breath sounds over the right lower lung fields. His chest X-ray reveals a right lower lobe infiltrate. He is admitted to the hospital with a diagnosis of community-acquired pneumonia (CAP), and medical therapy is started. How should his antibiotic treatment be managed?
Overview
Community-acquired pneumonia is the most common infection-related cause of death in the U.S., and the eighth-leading cause of mortality overall.1 According to a 2006 survey, CAP results in more than 1.2 million hospital admissions annually, with an average length of stay of 5.1 days.2 Though less than 20% of CAP patients require hospitalization, cases necessitating admission contribute to more than 90% of the overall cost of pneumonia care.3
During the past several years, the availability of new antibiotics and the evolution of microbial resistance patterns have changed CAP treatment strategies. Furthermore, the development of prognostic scoring systems and increasing pressure to streamline resource utilization while improving quality of care have led to new treatment considerations, such as managing low-risk cases as outpatients.
More recently, attention has been directed to the optimal duration of antibiotic treatment, with a focus on shortening the duration of therapy. Historically, CAP treatment duration has been variable and not evidence-based. Shortening the course of antibiotics might limit antibiotic resistance, decrease costs, and improve patient adherence and tolerability.4 However, before defining the appropriate antibiotic duration for a patient hospitalized with CAP, other factors must be considered, such as the choice of empiric antibiotics, the patient’s initial response to treatment, severity of the disease, and presence of co-morbidities.
Review of the Data
Antibiotic choice. The most widely referenced practice guidelines for the management of CAP patients were published in 2007 by representatives of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).5 Table 1 (above, right) summarizes the recommendations for empiric antibiotics for patients requiring inpatient treatment.
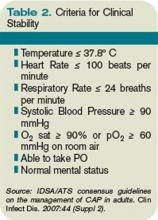
Time to clinical stability. A patient’s clinical response to empiric antibiotic therapy contributes heavily to the decision regarding treatment course and duration. The IDSA/ATS guidelines recommend patients be afebrile for 48 to 72 hours and have no more than one CAP-associated sign of clinical instability before discontinuation of therapy. Although studies have used different definitions of clinical stability, the consensus guidelines refer to six parameters, which are summarized in Table 2 (right).
With appropriate antibiotic therapy, most patients hospitalized with CAP achieve clinical stability in approximately three days.6,7 Providers should expect to see some improvement in vital signs within 48 to 72 hours of admission. Should a patient fail to demonstrate objective improvement during that time, providers should look for unusual pathogens, resistant organisms, nosocomial superinfections, or noninfectious conditions.5 Certain patients, such as those with multilobar pneumonia, associated pleural effusion, or higher pneumonia-severity index scores, also take longer to reach clinical stability.8
Switch to oral therapy. The ability to achieve clinical stability has important implications for hospital length of stay. Most patients hospitalized with CAP initially are treated with intravenous (IV) antibiotics and require transition to oral therapy in anticipation of discharge. Several studies have found there is no advantage to continuing IV medication once a patient is deemed clinically stable and is able to tolerate oral medication.9,10 There are no specific guidelines regarding choice of oral antibiotics, but it is common practice, supported by the IDSA/ATS recommendations, to use the same agent as the IV antibiotic or a medication in the same drug class. For patients started on β-lactam and macrolide combination therapy, it usually is appropriate to switch to a macrolide alone.5 In cases in which a pathogen has been identified, antibiotic selection should be based on the susceptibility profile.
Once patients are switched to oral antibiotics, it is not necessary for them to remain in the hospital for further observation, provided they have no other active medical problems or social needs. A retrospective analysis of 39,232 patients hospitalized with CAP compared those who were observed overnight after switching to oral antibiotics with those who were not and found no difference in 14-day readmission rate or 30-day mortality rate.11 These findings, in conjunction with the strategy of an early switch to oral therapy, suggest hospital length of stay may be safely reduced for many patients with uncomplicated CAP.
Duration of therapy. After a patient becomes clinically stable and a decision is made to switch to oral medication and a plan for hospital discharge, the question becomes how long to continue the course of antibiotics. Historically, clinical practice has extended treatment for up to two weeks, despite lack of evidence for this duration of therapy. The IDSA/ATS guidelines offer some general recommendations, noting patients should be treated for a minimum of five days, in addition to being afebrile for 48 to 72 hours and meet other criteria for clinical stability.5
Li and colleagues conducted a systematic review evaluating 15 randomized controlled trials comparing short-course (less than seven days) with extended (more than seven days) monotherapy for CAP in adults.4 Overall, the authors found no difference in the risk of treatment failure between short-course and extended-course antibiotic therapy, and they found no difference in bacteriologic eradication or mortality. It is important to note the studies included in this analysis enrolled patients with mild to moderate CAP, including those treated as outpatients, which limits the ability to extrapolate to exclusively inpatient populations and more severely ill patients.
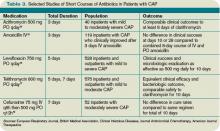
Another meta-analysis, published shortly thereafter, examined randomized controlled trials in outpatients and inpatients not requiring intensive care. It compared different durations of treatment with the same agent in the same dosage. The authors similarly found no difference in effectiveness or safety of short (less than seven days) versus longer (at least two additional days of therapy) courses.12 Table 3 (above) reviews selected trials of short courses of antibiotics, which have been studied in inpatient populations.
The trials summarized in these meta-analyses examined monotherapy with levofloxacin for five days; gemifloxacin for seven days, azithromycin for three to five days; ceftriaxone for five days; cefuroxime for seven days; amoxicillin for three days; or telithromycin for five to seven days. The variety of antibiotics in these studies contrasts the IDSA/ATS guidelines, which recommend only fluoroquinolones as monotherapy for inpatient CAP.
One important randomized, double-blind study of fluoroquinolones compared a five-day course of levofloxacin 750 mg daily, with a 10-day course of levofloxacin, 500 mg daily, in 528 patients with mild to severe CAP.13 The authors found no difference in clinical success or microbiologic eradication between the two groups, concluding high-dose levofloxacin for five days is an effective and well-tolerated alternative to a longer course of a lower dose, likely related to the drug’s concentration-dependent properties.
Azithromycin also offers potential for short courses of therapy, as pulmonary concentrations of azithromycin remain elevated for as many as five days following a single oral dose.14 Several small studies have demonstrated the safety, efficacy, and cost-effectiveness of three to five days of azithromycin, as summarized in a meta-analysis by Contopoulos-Ioannidis and colleagues.15 Most of these trials, however, were limited to outpatients or inpatients with mild disease or confirmed atypical pneumonia. One randomized trial of 40 inpatients with mild to moderately severe CAP found comparable clinical outcomes with a three-day course of oral azithromycin 500 mg daily versus clarithromycin for at least eight days.16 Larger studies in more severely ill patients must be completed before routinely recommending this approach in hospitalized patients. Furthermore, due to the rising prevalence of macrolide resistance, empiric therapy with a macrolide alone can only be used for the treatment of carefully selected hospitalized patients with nonsevere diseases and without risk factors for drug-resistant Streptococcus pneumoniae.5
Telithromycin is a ketolide antibiotic, which has been studied in mild to moderate CAP, including multidrug-resistant strains of S. pneumoniae, in courses of five to seven days.17 However, severe adverse reactions, including hepatotoxicity, have been reported. At the time of the 2007 guidelines, the IDSA/ATS committee waited for additional safety data before making any recommendations on its use.
One additional study of note was a trial of amoxicillin in adult inpatients with mild to moderately severe CAP.18 One hundred twenty-one patients who clinically improved (based on a composite score of pulmonary symptoms and general improvement) following three days of IV amoxicillin were randomized to oral amoxicillin for an additional five days or given a placebo. At days 10 and 28, there was no difference in clinical success between the two groups. The authors concluded that a total of three days of treatment was not inferior to eight days in patients who substantially improved after the first 72 hours of empiric treatment. This trial was conducted in the Netherlands, where amoxicillin is the preferred empiric antibiotic for CAP and patterns of antimicrobial resistance differ greatly from those found in the U.S.
Other considerations. While some evidence supports shorter courses of antibiotics, many of the existing studies are limited by their inclusion of outpatients, adults with mild to moderate CAP, or small sample size. Hence, clinical judgment continues to play an important role in determining the appropriate duration of therapy. Factors such as pre-existing co-morbidities, severity of illness, and occurrence of complications should be considered. Data is limited on the appropriate duration of antibiotics in CAP patients requiring intensive care. It also is important to note the IDSA/ATS recommendations and most of the studies reviewed exclude patients with human immunodeficiency virus (HIV), and it is unknown whether these shorter courses of antibiotics are appropriate in the HIV population.
Lastly, the IDSA/ATS guidelines note longer durations of treatment may be required if the initial therapy was not active against the identified pathogen, or in cases complicated by extrapulmonary infections, such as endocarditis or meningitis.
Back to the Case
Our patient with moderately severe CAP was hospitalized based on his age and hypoxia. He was immediately treated with supplemental oxygen by nasal cannula, IV fluids, and a dose of IV levofloxacin 750 mg. Within 48 hours he met criteria for clinical stability, including defervescence, a decline in his respiratory rate to 19 breaths per minute, and improvement in oxygen saturation to 95% on room air. At this point, he was changed from IV to oral antibiotics. He continued on levofloxacin 750 mg daily and later that day was discharged home in good condition to complete a five-day course.
Bottom Line
For hospitalized adults with mild to moderately severe CAP, five to seven days of treatment, depending on the antibiotic selected, appears to be effective in most cases. Patients should be afebrile for 48 to 72 hours and demonstrate signs of clinical stability before therapy is discontinued. TH
Kelly Cunningham, MD, and Shelley Ellis, MD, MPH, are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn. Sunil Kripalani, MD, MSc, serves as the section chief.
References
1. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56.
2. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5.
3. Niederman MS. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest. 2007;131:1205-1215.
4. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
5. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72.
6. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848-850.
7. Halm EA, Fine MJ, Marrie TJ et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452-1457.
8. Menendez R, Torres A, Rodriguez de Castro F et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783-1790.
9. Siegal RE, Halpern NA, Almenoff PL et al. A prospective randomised study of inpatient IV antibiotics for community-acquired pneumonia: the optimal duration of therapy. Chest. 1996;110:965-971.
10. Oosterheert JJ, Bonten MJ, Schneider MM et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333:1193-1197.
11. Nathan RV, Rhew DC, Murray C et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006;119:512-518.
12. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
13. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
14. Morris DL, De Souza A, Jones JA, Morgan WE. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859-861.
15. Contopoulos-Ioannidis DG, Ioannidis JPA, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for lower respiratory tract infections. J Antimicrob Chemother. 2001;48:691-703.
16. Rizzato G, Montemurro L, Fraioli P et al. Efficacy of a three-day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J. 1995;8:398-402.
17. Tellier G, Niederman MS, Nusrat R et al. Clinical and bacteriological efficacy and safety of 5- and 7-day regimens of telithromycin once daily compared with a 10-day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother. 2004;54:515.
18. El Moussaoui R, de Borgie CA, van den Broek P et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355-1361.
19. Siegel RE, Alicea M, Lee A, Blaiklock R. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized double-blind study. Am J Ther. 1999;6:217-222.
Case
An 83-year-old male with hypertension, coronary artery disease, and obstructive sleep apnea presents with progressive shortness of breath, a productive cough, wheezing, and tachypnea. His blood pressure is 158/70 mm/Hg; temperature is 101.8; respirations are 26 breaths per minute; and oxygen saturation is 87% on room air. He has coarse breath sounds bilaterally, and decreased breath sounds over the right lower lung fields. His chest X-ray reveals a right lower lobe infiltrate. He is admitted to the hospital with a diagnosis of community-acquired pneumonia (CAP), and medical therapy is started. How should his antibiotic treatment be managed?
Overview
Community-acquired pneumonia is the most common infection-related cause of death in the U.S., and the eighth-leading cause of mortality overall.1 According to a 2006 survey, CAP results in more than 1.2 million hospital admissions annually, with an average length of stay of 5.1 days.2 Though less than 20% of CAP patients require hospitalization, cases necessitating admission contribute to more than 90% of the overall cost of pneumonia care.3
During the past several years, the availability of new antibiotics and the evolution of microbial resistance patterns have changed CAP treatment strategies. Furthermore, the development of prognostic scoring systems and increasing pressure to streamline resource utilization while improving quality of care have led to new treatment considerations, such as managing low-risk cases as outpatients.
More recently, attention has been directed to the optimal duration of antibiotic treatment, with a focus on shortening the duration of therapy. Historically, CAP treatment duration has been variable and not evidence-based. Shortening the course of antibiotics might limit antibiotic resistance, decrease costs, and improve patient adherence and tolerability.4 However, before defining the appropriate antibiotic duration for a patient hospitalized with CAP, other factors must be considered, such as the choice of empiric antibiotics, the patient’s initial response to treatment, severity of the disease, and presence of co-morbidities.
Review of the Data
Antibiotic choice. The most widely referenced practice guidelines for the management of CAP patients were published in 2007 by representatives of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).5 Table 1 (above, right) summarizes the recommendations for empiric antibiotics for patients requiring inpatient treatment.
Time to clinical stability. A patient’s clinical response to empiric antibiotic therapy contributes heavily to the decision regarding treatment course and duration. The IDSA/ATS guidelines recommend patients be afebrile for 48 to 72 hours and have no more than one CAP-associated sign of clinical instability before discontinuation of therapy. Although studies have used different definitions of clinical stability, the consensus guidelines refer to six parameters, which are summarized in Table 2 (right).
With appropriate antibiotic therapy, most patients hospitalized with CAP achieve clinical stability in approximately three days.6,7 Providers should expect to see some improvement in vital signs within 48 to 72 hours of admission. Should a patient fail to demonstrate objective improvement during that time, providers should look for unusual pathogens, resistant organisms, nosocomial superinfections, or noninfectious conditions.5 Certain patients, such as those with multilobar pneumonia, associated pleural effusion, or higher pneumonia-severity index scores, also take longer to reach clinical stability.8
Switch to oral therapy. The ability to achieve clinical stability has important implications for hospital length of stay. Most patients hospitalized with CAP initially are treated with intravenous (IV) antibiotics and require transition to oral therapy in anticipation of discharge. Several studies have found there is no advantage to continuing IV medication once a patient is deemed clinically stable and is able to tolerate oral medication.9,10 There are no specific guidelines regarding choice of oral antibiotics, but it is common practice, supported by the IDSA/ATS recommendations, to use the same agent as the IV antibiotic or a medication in the same drug class. For patients started on β-lactam and macrolide combination therapy, it usually is appropriate to switch to a macrolide alone.5 In cases in which a pathogen has been identified, antibiotic selection should be based on the susceptibility profile.
Once patients are switched to oral antibiotics, it is not necessary for them to remain in the hospital for further observation, provided they have no other active medical problems or social needs. A retrospective analysis of 39,232 patients hospitalized with CAP compared those who were observed overnight after switching to oral antibiotics with those who were not and found no difference in 14-day readmission rate or 30-day mortality rate.11 These findings, in conjunction with the strategy of an early switch to oral therapy, suggest hospital length of stay may be safely reduced for many patients with uncomplicated CAP.
Duration of therapy. After a patient becomes clinically stable and a decision is made to switch to oral medication and a plan for hospital discharge, the question becomes how long to continue the course of antibiotics. Historically, clinical practice has extended treatment for up to two weeks, despite lack of evidence for this duration of therapy. The IDSA/ATS guidelines offer some general recommendations, noting patients should be treated for a minimum of five days, in addition to being afebrile for 48 to 72 hours and meet other criteria for clinical stability.5
Li and colleagues conducted a systematic review evaluating 15 randomized controlled trials comparing short-course (less than seven days) with extended (more than seven days) monotherapy for CAP in adults.4 Overall, the authors found no difference in the risk of treatment failure between short-course and extended-course antibiotic therapy, and they found no difference in bacteriologic eradication or mortality. It is important to note the studies included in this analysis enrolled patients with mild to moderate CAP, including those treated as outpatients, which limits the ability to extrapolate to exclusively inpatient populations and more severely ill patients.
Another meta-analysis, published shortly thereafter, examined randomized controlled trials in outpatients and inpatients not requiring intensive care. It compared different durations of treatment with the same agent in the same dosage. The authors similarly found no difference in effectiveness or safety of short (less than seven days) versus longer (at least two additional days of therapy) courses.12 Table 3 (above) reviews selected trials of short courses of antibiotics, which have been studied in inpatient populations.
The trials summarized in these meta-analyses examined monotherapy with levofloxacin for five days; gemifloxacin for seven days, azithromycin for three to five days; ceftriaxone for five days; cefuroxime for seven days; amoxicillin for three days; or telithromycin for five to seven days. The variety of antibiotics in these studies contrasts the IDSA/ATS guidelines, which recommend only fluoroquinolones as monotherapy for inpatient CAP.
One important randomized, double-blind study of fluoroquinolones compared a five-day course of levofloxacin 750 mg daily, with a 10-day course of levofloxacin, 500 mg daily, in 528 patients with mild to severe CAP.13 The authors found no difference in clinical success or microbiologic eradication between the two groups, concluding high-dose levofloxacin for five days is an effective and well-tolerated alternative to a longer course of a lower dose, likely related to the drug’s concentration-dependent properties.
Azithromycin also offers potential for short courses of therapy, as pulmonary concentrations of azithromycin remain elevated for as many as five days following a single oral dose.14 Several small studies have demonstrated the safety, efficacy, and cost-effectiveness of three to five days of azithromycin, as summarized in a meta-analysis by Contopoulos-Ioannidis and colleagues.15 Most of these trials, however, were limited to outpatients or inpatients with mild disease or confirmed atypical pneumonia. One randomized trial of 40 inpatients with mild to moderately severe CAP found comparable clinical outcomes with a three-day course of oral azithromycin 500 mg daily versus clarithromycin for at least eight days.16 Larger studies in more severely ill patients must be completed before routinely recommending this approach in hospitalized patients. Furthermore, due to the rising prevalence of macrolide resistance, empiric therapy with a macrolide alone can only be used for the treatment of carefully selected hospitalized patients with nonsevere diseases and without risk factors for drug-resistant Streptococcus pneumoniae.5
Telithromycin is a ketolide antibiotic, which has been studied in mild to moderate CAP, including multidrug-resistant strains of S. pneumoniae, in courses of five to seven days.17 However, severe adverse reactions, including hepatotoxicity, have been reported. At the time of the 2007 guidelines, the IDSA/ATS committee waited for additional safety data before making any recommendations on its use.
One additional study of note was a trial of amoxicillin in adult inpatients with mild to moderately severe CAP.18 One hundred twenty-one patients who clinically improved (based on a composite score of pulmonary symptoms and general improvement) following three days of IV amoxicillin were randomized to oral amoxicillin for an additional five days or given a placebo. At days 10 and 28, there was no difference in clinical success between the two groups. The authors concluded that a total of three days of treatment was not inferior to eight days in patients who substantially improved after the first 72 hours of empiric treatment. This trial was conducted in the Netherlands, where amoxicillin is the preferred empiric antibiotic for CAP and patterns of antimicrobial resistance differ greatly from those found in the U.S.
Other considerations. While some evidence supports shorter courses of antibiotics, many of the existing studies are limited by their inclusion of outpatients, adults with mild to moderate CAP, or small sample size. Hence, clinical judgment continues to play an important role in determining the appropriate duration of therapy. Factors such as pre-existing co-morbidities, severity of illness, and occurrence of complications should be considered. Data is limited on the appropriate duration of antibiotics in CAP patients requiring intensive care. It also is important to note the IDSA/ATS recommendations and most of the studies reviewed exclude patients with human immunodeficiency virus (HIV), and it is unknown whether these shorter courses of antibiotics are appropriate in the HIV population.
Lastly, the IDSA/ATS guidelines note longer durations of treatment may be required if the initial therapy was not active against the identified pathogen, or in cases complicated by extrapulmonary infections, such as endocarditis or meningitis.
Back to the Case
Our patient with moderately severe CAP was hospitalized based on his age and hypoxia. He was immediately treated with supplemental oxygen by nasal cannula, IV fluids, and a dose of IV levofloxacin 750 mg. Within 48 hours he met criteria for clinical stability, including defervescence, a decline in his respiratory rate to 19 breaths per minute, and improvement in oxygen saturation to 95% on room air. At this point, he was changed from IV to oral antibiotics. He continued on levofloxacin 750 mg daily and later that day was discharged home in good condition to complete a five-day course.
Bottom Line
For hospitalized adults with mild to moderately severe CAP, five to seven days of treatment, depending on the antibiotic selected, appears to be effective in most cases. Patients should be afebrile for 48 to 72 hours and demonstrate signs of clinical stability before therapy is discontinued. TH
Kelly Cunningham, MD, and Shelley Ellis, MD, MPH, are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn. Sunil Kripalani, MD, MSc, serves as the section chief.
References
1. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56.
2. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5.
3. Niederman MS. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest. 2007;131:1205-1215.
4. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
5. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72.
6. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848-850.
7. Halm EA, Fine MJ, Marrie TJ et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452-1457.
8. Menendez R, Torres A, Rodriguez de Castro F et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783-1790.
9. Siegal RE, Halpern NA, Almenoff PL et al. A prospective randomised study of inpatient IV antibiotics for community-acquired pneumonia: the optimal duration of therapy. Chest. 1996;110:965-971.
10. Oosterheert JJ, Bonten MJ, Schneider MM et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333:1193-1197.
11. Nathan RV, Rhew DC, Murray C et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006;119:512-518.
12. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
13. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
14. Morris DL, De Souza A, Jones JA, Morgan WE. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859-861.
15. Contopoulos-Ioannidis DG, Ioannidis JPA, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for lower respiratory tract infections. J Antimicrob Chemother. 2001;48:691-703.
16. Rizzato G, Montemurro L, Fraioli P et al. Efficacy of a three-day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J. 1995;8:398-402.
17. Tellier G, Niederman MS, Nusrat R et al. Clinical and bacteriological efficacy and safety of 5- and 7-day regimens of telithromycin once daily compared with a 10-day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother. 2004;54:515.
18. El Moussaoui R, de Borgie CA, van den Broek P et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355-1361.
19. Siegel RE, Alicea M, Lee A, Blaiklock R. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized double-blind study. Am J Ther. 1999;6:217-222.
Case
An 83-year-old male with hypertension, coronary artery disease, and obstructive sleep apnea presents with progressive shortness of breath, a productive cough, wheezing, and tachypnea. His blood pressure is 158/70 mm/Hg; temperature is 101.8; respirations are 26 breaths per minute; and oxygen saturation is 87% on room air. He has coarse breath sounds bilaterally, and decreased breath sounds over the right lower lung fields. His chest X-ray reveals a right lower lobe infiltrate. He is admitted to the hospital with a diagnosis of community-acquired pneumonia (CAP), and medical therapy is started. How should his antibiotic treatment be managed?
Overview
Community-acquired pneumonia is the most common infection-related cause of death in the U.S., and the eighth-leading cause of mortality overall.1 According to a 2006 survey, CAP results in more than 1.2 million hospital admissions annually, with an average length of stay of 5.1 days.2 Though less than 20% of CAP patients require hospitalization, cases necessitating admission contribute to more than 90% of the overall cost of pneumonia care.3
During the past several years, the availability of new antibiotics and the evolution of microbial resistance patterns have changed CAP treatment strategies. Furthermore, the development of prognostic scoring systems and increasing pressure to streamline resource utilization while improving quality of care have led to new treatment considerations, such as managing low-risk cases as outpatients.
More recently, attention has been directed to the optimal duration of antibiotic treatment, with a focus on shortening the duration of therapy. Historically, CAP treatment duration has been variable and not evidence-based. Shortening the course of antibiotics might limit antibiotic resistance, decrease costs, and improve patient adherence and tolerability.4 However, before defining the appropriate antibiotic duration for a patient hospitalized with CAP, other factors must be considered, such as the choice of empiric antibiotics, the patient’s initial response to treatment, severity of the disease, and presence of co-morbidities.
Review of the Data
Antibiotic choice. The most widely referenced practice guidelines for the management of CAP patients were published in 2007 by representatives of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS).5 Table 1 (above, right) summarizes the recommendations for empiric antibiotics for patients requiring inpatient treatment.
Time to clinical stability. A patient’s clinical response to empiric antibiotic therapy contributes heavily to the decision regarding treatment course and duration. The IDSA/ATS guidelines recommend patients be afebrile for 48 to 72 hours and have no more than one CAP-associated sign of clinical instability before discontinuation of therapy. Although studies have used different definitions of clinical stability, the consensus guidelines refer to six parameters, which are summarized in Table 2 (right).
With appropriate antibiotic therapy, most patients hospitalized with CAP achieve clinical stability in approximately three days.6,7 Providers should expect to see some improvement in vital signs within 48 to 72 hours of admission. Should a patient fail to demonstrate objective improvement during that time, providers should look for unusual pathogens, resistant organisms, nosocomial superinfections, or noninfectious conditions.5 Certain patients, such as those with multilobar pneumonia, associated pleural effusion, or higher pneumonia-severity index scores, also take longer to reach clinical stability.8
Switch to oral therapy. The ability to achieve clinical stability has important implications for hospital length of stay. Most patients hospitalized with CAP initially are treated with intravenous (IV) antibiotics and require transition to oral therapy in anticipation of discharge. Several studies have found there is no advantage to continuing IV medication once a patient is deemed clinically stable and is able to tolerate oral medication.9,10 There are no specific guidelines regarding choice of oral antibiotics, but it is common practice, supported by the IDSA/ATS recommendations, to use the same agent as the IV antibiotic or a medication in the same drug class. For patients started on β-lactam and macrolide combination therapy, it usually is appropriate to switch to a macrolide alone.5 In cases in which a pathogen has been identified, antibiotic selection should be based on the susceptibility profile.
Once patients are switched to oral antibiotics, it is not necessary for them to remain in the hospital for further observation, provided they have no other active medical problems or social needs. A retrospective analysis of 39,232 patients hospitalized with CAP compared those who were observed overnight after switching to oral antibiotics with those who were not and found no difference in 14-day readmission rate or 30-day mortality rate.11 These findings, in conjunction with the strategy of an early switch to oral therapy, suggest hospital length of stay may be safely reduced for many patients with uncomplicated CAP.
Duration of therapy. After a patient becomes clinically stable and a decision is made to switch to oral medication and a plan for hospital discharge, the question becomes how long to continue the course of antibiotics. Historically, clinical practice has extended treatment for up to two weeks, despite lack of evidence for this duration of therapy. The IDSA/ATS guidelines offer some general recommendations, noting patients should be treated for a minimum of five days, in addition to being afebrile for 48 to 72 hours and meet other criteria for clinical stability.5
Li and colleagues conducted a systematic review evaluating 15 randomized controlled trials comparing short-course (less than seven days) with extended (more than seven days) monotherapy for CAP in adults.4 Overall, the authors found no difference in the risk of treatment failure between short-course and extended-course antibiotic therapy, and they found no difference in bacteriologic eradication or mortality. It is important to note the studies included in this analysis enrolled patients with mild to moderate CAP, including those treated as outpatients, which limits the ability to extrapolate to exclusively inpatient populations and more severely ill patients.
Another meta-analysis, published shortly thereafter, examined randomized controlled trials in outpatients and inpatients not requiring intensive care. It compared different durations of treatment with the same agent in the same dosage. The authors similarly found no difference in effectiveness or safety of short (less than seven days) versus longer (at least two additional days of therapy) courses.12 Table 3 (above) reviews selected trials of short courses of antibiotics, which have been studied in inpatient populations.
The trials summarized in these meta-analyses examined monotherapy with levofloxacin for five days; gemifloxacin for seven days, azithromycin for three to five days; ceftriaxone for five days; cefuroxime for seven days; amoxicillin for three days; or telithromycin for five to seven days. The variety of antibiotics in these studies contrasts the IDSA/ATS guidelines, which recommend only fluoroquinolones as monotherapy for inpatient CAP.
One important randomized, double-blind study of fluoroquinolones compared a five-day course of levofloxacin 750 mg daily, with a 10-day course of levofloxacin, 500 mg daily, in 528 patients with mild to severe CAP.13 The authors found no difference in clinical success or microbiologic eradication between the two groups, concluding high-dose levofloxacin for five days is an effective and well-tolerated alternative to a longer course of a lower dose, likely related to the drug’s concentration-dependent properties.
Azithromycin also offers potential for short courses of therapy, as pulmonary concentrations of azithromycin remain elevated for as many as five days following a single oral dose.14 Several small studies have demonstrated the safety, efficacy, and cost-effectiveness of three to five days of azithromycin, as summarized in a meta-analysis by Contopoulos-Ioannidis and colleagues.15 Most of these trials, however, were limited to outpatients or inpatients with mild disease or confirmed atypical pneumonia. One randomized trial of 40 inpatients with mild to moderately severe CAP found comparable clinical outcomes with a three-day course of oral azithromycin 500 mg daily versus clarithromycin for at least eight days.16 Larger studies in more severely ill patients must be completed before routinely recommending this approach in hospitalized patients. Furthermore, due to the rising prevalence of macrolide resistance, empiric therapy with a macrolide alone can only be used for the treatment of carefully selected hospitalized patients with nonsevere diseases and without risk factors for drug-resistant Streptococcus pneumoniae.5
Telithromycin is a ketolide antibiotic, which has been studied in mild to moderate CAP, including multidrug-resistant strains of S. pneumoniae, in courses of five to seven days.17 However, severe adverse reactions, including hepatotoxicity, have been reported. At the time of the 2007 guidelines, the IDSA/ATS committee waited for additional safety data before making any recommendations on its use.
One additional study of note was a trial of amoxicillin in adult inpatients with mild to moderately severe CAP.18 One hundred twenty-one patients who clinically improved (based on a composite score of pulmonary symptoms and general improvement) following three days of IV amoxicillin were randomized to oral amoxicillin for an additional five days or given a placebo. At days 10 and 28, there was no difference in clinical success between the two groups. The authors concluded that a total of three days of treatment was not inferior to eight days in patients who substantially improved after the first 72 hours of empiric treatment. This trial was conducted in the Netherlands, where amoxicillin is the preferred empiric antibiotic for CAP and patterns of antimicrobial resistance differ greatly from those found in the U.S.
Other considerations. While some evidence supports shorter courses of antibiotics, many of the existing studies are limited by their inclusion of outpatients, adults with mild to moderate CAP, or small sample size. Hence, clinical judgment continues to play an important role in determining the appropriate duration of therapy. Factors such as pre-existing co-morbidities, severity of illness, and occurrence of complications should be considered. Data is limited on the appropriate duration of antibiotics in CAP patients requiring intensive care. It also is important to note the IDSA/ATS recommendations and most of the studies reviewed exclude patients with human immunodeficiency virus (HIV), and it is unknown whether these shorter courses of antibiotics are appropriate in the HIV population.
Lastly, the IDSA/ATS guidelines note longer durations of treatment may be required if the initial therapy was not active against the identified pathogen, or in cases complicated by extrapulmonary infections, such as endocarditis or meningitis.
Back to the Case
Our patient with moderately severe CAP was hospitalized based on his age and hypoxia. He was immediately treated with supplemental oxygen by nasal cannula, IV fluids, and a dose of IV levofloxacin 750 mg. Within 48 hours he met criteria for clinical stability, including defervescence, a decline in his respiratory rate to 19 breaths per minute, and improvement in oxygen saturation to 95% on room air. At this point, he was changed from IV to oral antibiotics. He continued on levofloxacin 750 mg daily and later that day was discharged home in good condition to complete a five-day course.
Bottom Line
For hospitalized adults with mild to moderately severe CAP, five to seven days of treatment, depending on the antibiotic selected, appears to be effective in most cases. Patients should be afebrile for 48 to 72 hours and demonstrate signs of clinical stability before therapy is discontinued. TH
Kelly Cunningham, MD, and Shelley Ellis, MD, MPH, are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn. Sunil Kripalani, MD, MSc, serves as the section chief.
References
1. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56.
2. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5.
3. Niederman MS. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest. 2007;131:1205-1215.
4. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
5. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72.
6. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848-850.
7. Halm EA, Fine MJ, Marrie TJ et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452-1457.
8. Menendez R, Torres A, Rodriguez de Castro F et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783-1790.
9. Siegal RE, Halpern NA, Almenoff PL et al. A prospective randomised study of inpatient IV antibiotics for community-acquired pneumonia: the optimal duration of therapy. Chest. 1996;110:965-971.
10. Oosterheert JJ, Bonten MJ, Schneider MM et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomized trial. BMJ. 2006;333:1193-1197.
11. Nathan RV, Rhew DC, Murray C et al. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006;119:512-518.
12. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
13. Dunbar LM, Wunderink RG, Habib MP et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752-760.
14. Morris DL, De Souza A, Jones JA, Morgan WE. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859-861.
15. Contopoulos-Ioannidis DG, Ioannidis JPA, Chew P, Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for lower respiratory tract infections. J Antimicrob Chemother. 2001;48:691-703.
16. Rizzato G, Montemurro L, Fraioli P et al. Efficacy of a three-day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J. 1995;8:398-402.
17. Tellier G, Niederman MS, Nusrat R et al. Clinical and bacteriological efficacy and safety of 5- and 7-day regimens of telithromycin once daily compared with a 10-day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother. 2004;54:515.
18. El Moussaoui R, de Borgie CA, van den Broek P et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355-1361.
19. Siegel RE, Alicea M, Lee A, Blaiklock R. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized double-blind study. Am J Ther. 1999;6:217-222.