User login
Understanding current guidelines for colorectal cancer screening: A case-based approach
Fewer than half of all people in the United States who should be screened for colorectal cancer have actually been screened. But at the same time, many people who have no or low-risk polyps on colonoscopy may be returning unnecessarily soon. Utilizing current screening and surveillance guidelines to direct patient care can reduce the number of unnecessary colonoscopies and improve surveillance of patients who may be at greater-than-average risk of colorectal cancer.
In this paper, we use several case examples to clarify the current guidelines on who should be screened, why, how, and how often.
WHY SCREEN?
Approximately 6% of American men and women develop an invasive colorectal neoplasm in their lifetime. Colorectal cancer is the second-leading cause of cancer death in the United States. In 2007, an estimated 153,760 people were newly diagnosed with colorectal cancer, and 52,180 people died of it.1
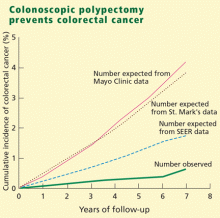
Yet, colorectal cancer is one of the few preventable cancers. Screening has been advocated as a way of preventing deaths by removing precancerous adenomas and detecting colorectal cancer early.2 Medicare has paid for screening colonoscopy since 1998, and since that time demand for this procedure has increased 112%.3,4 (See “Colonoscopy is the preferred test”.2,4–17)
START SCREENING AT AGE 50 FOR PEOPLE AT AVERAGE RISK
The US Multi-Society Task Force on Colorectal Cancer19 suggests that people at average risk undergo one of the following:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- Fecal occult blood testing every year
- An air-contrast barium enema or computed tomographic (CT) colonography every 5 years
- Fecal DNA testing, interval uncertain.
Anyone who has a positive result with any test other than colonoscopy should subsequently undergo colonoscopy.
Start screening sooner in people at higher risk
African Americans should undergo screening for colorectal cancer under an average-risk strategy starting at age 45, according to a position paper from the American College of Gastroenterology.4 Reasons for starting sooner are that African Americans have the highest incidence of colorectal cancer of any racial or ethnic group, and that they present with it at a younger age. In the years 1970–1994, 10.7% of cases of colorectal cancer in African Americans were detected before age 50 compared with 5.5% of cases in white people.22 In addition, compared with other ethnic groups, African Americans have a more proximal distribution of colorectal neoplasms, present with later-stage disease, and have lower survival rates.4
People with a family history of colorectal polyps or cancer should also start screening earlier—as early as age 40, or 10 years younger than the age at which the relative was affected—and some should be tested more often than every 10 years (see below).
Patients with ulcerative colitis or Crohn’s colitis. Current multisociety guidelines for colorectal cancer screening and surveillance in patients with ulcerative colitis or Crohn’s colitis are based on expert consensus and recommend a systematic biopsy protocol in some patients. When to begin surveillance in these patients and the specifics of the biopsy protocol are beyond the scope of this paper but are discussed in detail elsewhere.19
FAMILY HISTORY INCREASES RISK
Case 1: A woman with a family history of cancer
A 55-year-old woman comes in for a routine physical examination. Her medical history is not remarkable, but her family history is: her maternal grandmother was diagnosed with colon cancer at age 75, her sister was diagnosed with endometrial cancer at age 34, and her mother was diagnosed with colon cancer at age 60. The patient underwent colonoscopy 5 years ago, and a 1.2-cm villous adenoma was removed from her right colon. She had been advised to have her next colonoscopy in 3 years.
Current recommendations for screening and surveillance differ based upon the number, age, and relationship of relatives affected with colorectal neoplasia (Table 1). The patient described above began screening at age 50 in accordance with the guidelines for people at average risk, but her extended family history was not taken into account.
Hereditary nonpolyposis colorectal cancer
Our patient’s family history meets the criteria for hereditary nonpolyposis colorectal cancer,23 ie, she has three family members with hereditary nonpolyposis colorectal cancer-associated cancers (colorectal cancer or cancer of the endometrium, small bowel, ureter, or renal pelvis), and one family member (her mother) is a first-degree relative of the other two affected relatives. Two successive generations of her family are affected, and one family member (her sister) was diagnosed before the age of 50.
People in families like this have an 80% lifetime risk of colorectal cancer, so it is imperative to review every patient’s family history. Patients who meet the criteria should be referred for genetic counseling and possibly genetic testing. In addition, they should begin screening—with colonoscopy, not the other tests—between the ages of 21 and 25 or at an age 10 years younger than when the youngest family member was diagnosed with colorectal cancer, whichever is earlier. They should subsequently undergo colonoscopy every 1 to 2 years.
These patients also have an increased risk of certain extracolonic cancers, including a 40% to 60% lifetime risk of endometrial adenocarcinoma. They and their physicians need to be aware of consensus screening recommendations for ovarian, endometrial, and transitional cell cancers.24
Familial adenomatous polyposis
Patients with familial adenomatous polyposis develop hundreds to thousands of adenoma-tous colorectal polyps, usually in their teens, and have a 100% risk of developing colon cancer if the colon is not removed. Patients with a family history of this disorder should undergo screening at 10 to 12 years of age.
OVERCOMING BARRIERS TO SCREENING
In 2004, an estimated 70.1 million Americans were 50 years of age and older and at average risk of colorectal cancer.25 Of these, only 28.3 million (40.4%) had undergone screening, and 41.8 million had not.
We could view this as an opportunity to make a significant impact on the disease, but resources are limited. Seeff et al25 estimated that it would take 10 years to perform screening colonoscopy on unscreened Americans if one-half of all current endoscopic capacity were used for screening alone.
Barriers to screening also exist on an individual level. A recent study26 found that only 50% of patients referred for screening colonoscopy actually underwent the procedure; patients were significantly less likely to make an appointment and keep it if they were younger or female or if they were on Medicaid. Reasons cited by patients for not following through with colonoscopy after referral included fear of pain or perforation, dislike of the bowel preparation, and misperceptions about colorectal cancer risk.
Understanding these barriers and improving patient-physician communication about the procedure and the risk of colorectal cancer in the general population, even in the absence of a family history, may help improve adherence to screening colonoscopy.

POST-POLYPECTOMY SURVEILLANCE: OFTEN TOO SOON, TOO FREQUENT
After a polyp or polyps are discovered on colonoscopy, many patients are being told to come back for repeat colonoscopy unnecessarily soon,27,28 thus diverting a scarce resource away from patients who may derive the most benefit—ie, those with high-risk polyps, those with a strong family history of colon cancer or an inherited predisposition to colon cancer, and those who have never undergone screening.
The following cases illustrate how current evidence-based guidelines can be applied to several different patients.
Case 2: ‘Three benign polyps’
A 51-year-old woman with no personal or family history of colorectal neoplasia calls her primary care physician after undergoing her first colonoscopy. The patient noted that she had had “three benign polyps removed.” She would like to know when her next colonoscopy should be.
The primary care physician obtains the patient’s colonoscopy report, which reveals that three polyps measuring 5 mm, 4 mm, and 4 mm were removed from the patient’s descending colon. The pathology report reveals that two of these polyps were tubular adenomas, and one of the 4-mm polyps was hyperplastic.
Case 3: A large tubulovillous polyp
A 46-year-old African American man with no personal or family history of colorectal neoplasia underwent his first colonoscopy 1 year ago. He had had a 1.5-cm pedunculated polyp removed in toto from his ascending colon. The pathologist characterized the polyp as “tubulovillous.”
Not all polyps are precancerous
Adenomas are precursors to colorectal cancer, progressing via the widely recognized adenoma-carcinoma sequence.29 It is not unusual that both of our patients would have adenomatous polyps, since the prevalence of these polyps increases with age.30 Adenomas are detected in 11% of average-risk people ages 50 to 54, increasing to 33% to 50% in people 65 to 75 years old.31,32
Small, left-sided hyperplastic polyps, on the other hand, are considered nonneoplastic and do not require follow-up unless a patient meets the criteria for hyperplastic polyposis (Table 2). While current guidelines do not take into account hyperplastic polyps when determining postpolypectomy surveillance, the clinical significance and possible neoplastic potential of large and right-sided hyperplastic polyps is an area of active research.
Often, hyperplastic polyps are erroneously spoken of as “benign” when in fact they are not precancerous and are clinically insignificant. In fact, Boolchand et al27 found that 61% of primary care physicians would bring a patient with a single 6-mm hyperplastic polyp back for surveillance colonoscopy in 5 years or sooner. Current consensus guidelines do not recommend surveillance colonoscopy for the majority of patients with hyperplastic polyps. These individuals are not at an increased risk of colorectal cancer and should go back to average-risk screening recommendations, ie, colonoscopy in 10 years, the same interval as for the average-risk individual.33
Adenomas: How many? How big? What features?
If adenomas are discovered, three key questions affect how soon the patient should undergo colonoscopy again (Table 2):
Other studies also found that the number of adenomas predicts the subsequent development of more adenomas, and in particular advanced colorectal neoplasia.35–38
How big? Noshirwani et al35 retrospectively analyzed data from their adenoma registry and found that polyps 1 cm or larger were significantly associated with the finding of advanced adenomas 3 years later.
What features? Tubulovillous or villous features in an adenoma have been shown to increase the risk of future advanced adenomas and cancer.39,40 Similarly, high-grade dysplasia is associated with the subsequent development of advanced adenomas. In the Veterans Affairs Cooperative Study,41 10.9% of patients who had a polyp of any size with high-grade dysplasia developed an advanced neoplasm within 5 years, compared with only 0.6% of those with small polyps that did not harbor high-grade dysplasia.
Recognizing advanced adenomas is important when interpreting a patient’s colonoscopy results because multiple studies have shown them to predict recurrent advanced neoplasms or colorectal cancer.35,39–42
What does this mean for our patients?
If a patient (like our patient in case 2) who is otherwise at average risk is found to have an adenoma or adenomas without advanced features, the postpolypectomy surveillance interval should be dictated by the number of adenomas found. Current guidelines recommend that patients like this one—with one or two small tubular adenomas without features of advanced colorectal neoplasia—have a low risk of recurrent advanced adenomas and should undergo colonoscopy again in 5 to 10 years (Table 2).33
In contrast, in case 3, the polyp (which was completely removed) had two characteristics of advanced neoplasia: size larger than 1 cm and a villous component. This patient should come back in 3 years.
In colonoscopy, quality matters
An important caveat is that current post-polypectomy surveillance recommendations are based on the assumption that the bowel has been prepared adequately and that the entire colon is examined thoroughly up to the level of the cecum. Therefore, when deciding on the proper surveillance interval, one must take into account certain factors regarding the patient’s colonoscopy. Patients who have had an inadequate bowel preparation, incomplete examination, or large lesions removed piecemeal should be recalled at a shorter interval.
A final observation: another possible reason that patients are being sent back for repeat colonoscopy sooner than recommended is the concern for missed polyps. Nonpolypoid adenomas, which include flat and depressed lesions, can be easily missed using conventional endoscopy.43 A systematic review of six studies involving 465 patients who underwent tandem colonoscopy found a pooled miss rate of 26% for adenomas 1 to 5 mm.44 One way endoscopists can improve adenoma detection is to perform a slow endoscopic withdrawal over at least 6 minutes.45
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007; 57:43–66.
- Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 137:132–141.
- Prajapati DN, Saeian K, Binion DG, et al. Volume and yield of screening colonoscopy at a tertiary medical center after change in Medicare reimbursement. Am J Gastroenterol 2003; 98:194–199.
- Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol 2005; 100:515–523.
- Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. Am J Gastroenterol 2000; 95:868–877.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology 1987; 93:1009–1013.
- Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992; 326:658–662.
- Gloeckler-Ries LA, Hankey BF, Edwards BK. Cancer Statistics Review, 1973–1987. Bethesda, Md.: Department of Health and Human Services, 1990. (DHHS publication no. (NIH) 90-2789.)
- Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005; 365:305–311.
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328:1365–1371.
- Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348:1467–1471.
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348:1472–1477.
- Allison JE, Feldman R, Tekawa IS. Hemoccult screening in detecting colorectal neoplasm: sensitivity, specificity, and predictive value. Long-term follow-up in a large group practice setting. Ann Intern Med 1990; 112:328–333.
- Young GP, St John DJ, Winawer SJ, Rozen P WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy). Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies: a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. Am J Gastroenterol 2002; 97:2499–2507.
- Lieberman DA, Weiss DG Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 2001; 345:555–560.
- Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med 2005; 352:2061–2068.
- Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001—testing for early lung cancer detection. CA Cancer J Clin 2001; 51:38–75.
- Levin B, Lieberman D, McFarland B, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin, first published on Mar 5, 2008 as doi: doi:10.3322/CA.2007.0018
- Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology 2003; 124:544–560.
- U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med 2002; 137:129–131.
- Theuer CP, Wagner JL, Taylor TH, et al. Racial and ethnic colorectal cancer patterns affect the cost-effectiveness of colorectal cancer screening in the United States. Gastroenterology 2001; 120:848–856.
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology 1999; 116:1453–1456.
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006; 296:1507–1517.
- Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology 2004; 127:1661–1669.
- Denberg TD, Melhado TV, Coombes JM, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med 2005; 20:989–995.
- Boolchand V, Olds G, Singh J, Singh P, Chak A, Cooper GS. Colorectal screening after polypectomy: a national survey study of primary care physicians. Ann Intern Med 2006; 145:654–659.
- Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med 2004; 141:264–271.
- Hill MJ, Morson BC, Bussey HJ. Aetiology of adenoma-carcinoma sequence in large bowel. Lancet 1978; 1:245–247.
- Squillace S, Berggreen P, Jaffe P, et al. A normal initial colonoscopy after age 50 does not predict a polyp-free status for life. Am J Gastroenterol 1994; 89:1156–1159.
- Khullar SK, DiSario JA. Colon cancer screening. Sigmoidoscopy or colonoscopy. Gastrointest Endosc Clin North Am 1997; 7:365–386.
- Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut 1982; 23:835–842.
- Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006; 130:1872–1885.
- van Stolk RU, Beck GJ, Baron JA, Haile R, Summers R. Adenoma characteristics at first colonoscopy as predictors of adenoma recurrence and characteristics at follow-up. The Polyp Prevention Study Group. Gastroenterology 1998; 115:13–18.
- Noshirwani KC, van Stolk RU, Rybicki LA, Beck GJ. Adenoma size and number are predictive of adenoma recurrence: implications for surveillance colonoscopy. Gastrointest Endosc 2000; 51:433–437.
- Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993; 328:901–906.
- Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology 2005; 129:34–41.
- Bonithon-Kopp C, Piard F, Fenger C, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum 2004; 47:323–333.
- Loeve F, van Ballegooijen M, Boer R, Kuipers EJ, Habbema JD. Colorectal cancer risk in adenoma patients: a nation-wide study. Int J Cancer 2004; 111:147–151.
- Yang G, Zheng W, Sun QR, et al. Pathologic features of initial adenomas as predictors for metachronous adenomas of the rectum. J Natl Cancer Inst 1998; 90:1661–1665.
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Cheifec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000; 343:162–168.
- Martinez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology 2001; 120:1077–1083.
- Lieberman D. Nonpolypoid colorectal neoplasia in the United States: the parachute is open. JAMA 2008; 299:1068–1069.
- van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006; 101:343–350.
- Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006; 355:2533–2541.
Fewer than half of all people in the United States who should be screened for colorectal cancer have actually been screened. But at the same time, many people who have no or low-risk polyps on colonoscopy may be returning unnecessarily soon. Utilizing current screening and surveillance guidelines to direct patient care can reduce the number of unnecessary colonoscopies and improve surveillance of patients who may be at greater-than-average risk of colorectal cancer.
In this paper, we use several case examples to clarify the current guidelines on who should be screened, why, how, and how often.
WHY SCREEN?
Approximately 6% of American men and women develop an invasive colorectal neoplasm in their lifetime. Colorectal cancer is the second-leading cause of cancer death in the United States. In 2007, an estimated 153,760 people were newly diagnosed with colorectal cancer, and 52,180 people died of it.1
Yet, colorectal cancer is one of the few preventable cancers. Screening has been advocated as a way of preventing deaths by removing precancerous adenomas and detecting colorectal cancer early.2 Medicare has paid for screening colonoscopy since 1998, and since that time demand for this procedure has increased 112%.3,4 (See “Colonoscopy is the preferred test”.2,4–17)
START SCREENING AT AGE 50 FOR PEOPLE AT AVERAGE RISK
The US Multi-Society Task Force on Colorectal Cancer19 suggests that people at average risk undergo one of the following:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- Fecal occult blood testing every year
- An air-contrast barium enema or computed tomographic (CT) colonography every 5 years
- Fecal DNA testing, interval uncertain.
Anyone who has a positive result with any test other than colonoscopy should subsequently undergo colonoscopy.
Start screening sooner in people at higher risk
African Americans should undergo screening for colorectal cancer under an average-risk strategy starting at age 45, according to a position paper from the American College of Gastroenterology.4 Reasons for starting sooner are that African Americans have the highest incidence of colorectal cancer of any racial or ethnic group, and that they present with it at a younger age. In the years 1970–1994, 10.7% of cases of colorectal cancer in African Americans were detected before age 50 compared with 5.5% of cases in white people.22 In addition, compared with other ethnic groups, African Americans have a more proximal distribution of colorectal neoplasms, present with later-stage disease, and have lower survival rates.4
People with a family history of colorectal polyps or cancer should also start screening earlier—as early as age 40, or 10 years younger than the age at which the relative was affected—and some should be tested more often than every 10 years (see below).
Patients with ulcerative colitis or Crohn’s colitis. Current multisociety guidelines for colorectal cancer screening and surveillance in patients with ulcerative colitis or Crohn’s colitis are based on expert consensus and recommend a systematic biopsy protocol in some patients. When to begin surveillance in these patients and the specifics of the biopsy protocol are beyond the scope of this paper but are discussed in detail elsewhere.19
FAMILY HISTORY INCREASES RISK
Case 1: A woman with a family history of cancer
A 55-year-old woman comes in for a routine physical examination. Her medical history is not remarkable, but her family history is: her maternal grandmother was diagnosed with colon cancer at age 75, her sister was diagnosed with endometrial cancer at age 34, and her mother was diagnosed with colon cancer at age 60. The patient underwent colonoscopy 5 years ago, and a 1.2-cm villous adenoma was removed from her right colon. She had been advised to have her next colonoscopy in 3 years.
Current recommendations for screening and surveillance differ based upon the number, age, and relationship of relatives affected with colorectal neoplasia (Table 1). The patient described above began screening at age 50 in accordance with the guidelines for people at average risk, but her extended family history was not taken into account.
Hereditary nonpolyposis colorectal cancer
Our patient’s family history meets the criteria for hereditary nonpolyposis colorectal cancer,23 ie, she has three family members with hereditary nonpolyposis colorectal cancer-associated cancers (colorectal cancer or cancer of the endometrium, small bowel, ureter, or renal pelvis), and one family member (her mother) is a first-degree relative of the other two affected relatives. Two successive generations of her family are affected, and one family member (her sister) was diagnosed before the age of 50.
People in families like this have an 80% lifetime risk of colorectal cancer, so it is imperative to review every patient’s family history. Patients who meet the criteria should be referred for genetic counseling and possibly genetic testing. In addition, they should begin screening—with colonoscopy, not the other tests—between the ages of 21 and 25 or at an age 10 years younger than when the youngest family member was diagnosed with colorectal cancer, whichever is earlier. They should subsequently undergo colonoscopy every 1 to 2 years.
These patients also have an increased risk of certain extracolonic cancers, including a 40% to 60% lifetime risk of endometrial adenocarcinoma. They and their physicians need to be aware of consensus screening recommendations for ovarian, endometrial, and transitional cell cancers.24
Familial adenomatous polyposis
Patients with familial adenomatous polyposis develop hundreds to thousands of adenoma-tous colorectal polyps, usually in their teens, and have a 100% risk of developing colon cancer if the colon is not removed. Patients with a family history of this disorder should undergo screening at 10 to 12 years of age.
OVERCOMING BARRIERS TO SCREENING
In 2004, an estimated 70.1 million Americans were 50 years of age and older and at average risk of colorectal cancer.25 Of these, only 28.3 million (40.4%) had undergone screening, and 41.8 million had not.
We could view this as an opportunity to make a significant impact on the disease, but resources are limited. Seeff et al25 estimated that it would take 10 years to perform screening colonoscopy on unscreened Americans if one-half of all current endoscopic capacity were used for screening alone.
Barriers to screening also exist on an individual level. A recent study26 found that only 50% of patients referred for screening colonoscopy actually underwent the procedure; patients were significantly less likely to make an appointment and keep it if they were younger or female or if they were on Medicaid. Reasons cited by patients for not following through with colonoscopy after referral included fear of pain or perforation, dislike of the bowel preparation, and misperceptions about colorectal cancer risk.
Understanding these barriers and improving patient-physician communication about the procedure and the risk of colorectal cancer in the general population, even in the absence of a family history, may help improve adherence to screening colonoscopy.

POST-POLYPECTOMY SURVEILLANCE: OFTEN TOO SOON, TOO FREQUENT
After a polyp or polyps are discovered on colonoscopy, many patients are being told to come back for repeat colonoscopy unnecessarily soon,27,28 thus diverting a scarce resource away from patients who may derive the most benefit—ie, those with high-risk polyps, those with a strong family history of colon cancer or an inherited predisposition to colon cancer, and those who have never undergone screening.
The following cases illustrate how current evidence-based guidelines can be applied to several different patients.
Case 2: ‘Three benign polyps’
A 51-year-old woman with no personal or family history of colorectal neoplasia calls her primary care physician after undergoing her first colonoscopy. The patient noted that she had had “three benign polyps removed.” She would like to know when her next colonoscopy should be.
The primary care physician obtains the patient’s colonoscopy report, which reveals that three polyps measuring 5 mm, 4 mm, and 4 mm were removed from the patient’s descending colon. The pathology report reveals that two of these polyps were tubular adenomas, and one of the 4-mm polyps was hyperplastic.
Case 3: A large tubulovillous polyp
A 46-year-old African American man with no personal or family history of colorectal neoplasia underwent his first colonoscopy 1 year ago. He had had a 1.5-cm pedunculated polyp removed in toto from his ascending colon. The pathologist characterized the polyp as “tubulovillous.”
Not all polyps are precancerous
Adenomas are precursors to colorectal cancer, progressing via the widely recognized adenoma-carcinoma sequence.29 It is not unusual that both of our patients would have adenomatous polyps, since the prevalence of these polyps increases with age.30 Adenomas are detected in 11% of average-risk people ages 50 to 54, increasing to 33% to 50% in people 65 to 75 years old.31,32
Small, left-sided hyperplastic polyps, on the other hand, are considered nonneoplastic and do not require follow-up unless a patient meets the criteria for hyperplastic polyposis (Table 2). While current guidelines do not take into account hyperplastic polyps when determining postpolypectomy surveillance, the clinical significance and possible neoplastic potential of large and right-sided hyperplastic polyps is an area of active research.
Often, hyperplastic polyps are erroneously spoken of as “benign” when in fact they are not precancerous and are clinically insignificant. In fact, Boolchand et al27 found that 61% of primary care physicians would bring a patient with a single 6-mm hyperplastic polyp back for surveillance colonoscopy in 5 years or sooner. Current consensus guidelines do not recommend surveillance colonoscopy for the majority of patients with hyperplastic polyps. These individuals are not at an increased risk of colorectal cancer and should go back to average-risk screening recommendations, ie, colonoscopy in 10 years, the same interval as for the average-risk individual.33
Adenomas: How many? How big? What features?
If adenomas are discovered, three key questions affect how soon the patient should undergo colonoscopy again (Table 2):
Other studies also found that the number of adenomas predicts the subsequent development of more adenomas, and in particular advanced colorectal neoplasia.35–38
How big? Noshirwani et al35 retrospectively analyzed data from their adenoma registry and found that polyps 1 cm or larger were significantly associated with the finding of advanced adenomas 3 years later.
What features? Tubulovillous or villous features in an adenoma have been shown to increase the risk of future advanced adenomas and cancer.39,40 Similarly, high-grade dysplasia is associated with the subsequent development of advanced adenomas. In the Veterans Affairs Cooperative Study,41 10.9% of patients who had a polyp of any size with high-grade dysplasia developed an advanced neoplasm within 5 years, compared with only 0.6% of those with small polyps that did not harbor high-grade dysplasia.
Recognizing advanced adenomas is important when interpreting a patient’s colonoscopy results because multiple studies have shown them to predict recurrent advanced neoplasms or colorectal cancer.35,39–42
What does this mean for our patients?
If a patient (like our patient in case 2) who is otherwise at average risk is found to have an adenoma or adenomas without advanced features, the postpolypectomy surveillance interval should be dictated by the number of adenomas found. Current guidelines recommend that patients like this one—with one or two small tubular adenomas without features of advanced colorectal neoplasia—have a low risk of recurrent advanced adenomas and should undergo colonoscopy again in 5 to 10 years (Table 2).33
In contrast, in case 3, the polyp (which was completely removed) had two characteristics of advanced neoplasia: size larger than 1 cm and a villous component. This patient should come back in 3 years.
In colonoscopy, quality matters
An important caveat is that current post-polypectomy surveillance recommendations are based on the assumption that the bowel has been prepared adequately and that the entire colon is examined thoroughly up to the level of the cecum. Therefore, when deciding on the proper surveillance interval, one must take into account certain factors regarding the patient’s colonoscopy. Patients who have had an inadequate bowel preparation, incomplete examination, or large lesions removed piecemeal should be recalled at a shorter interval.
A final observation: another possible reason that patients are being sent back for repeat colonoscopy sooner than recommended is the concern for missed polyps. Nonpolypoid adenomas, which include flat and depressed lesions, can be easily missed using conventional endoscopy.43 A systematic review of six studies involving 465 patients who underwent tandem colonoscopy found a pooled miss rate of 26% for adenomas 1 to 5 mm.44 One way endoscopists can improve adenoma detection is to perform a slow endoscopic withdrawal over at least 6 minutes.45
Fewer than half of all people in the United States who should be screened for colorectal cancer have actually been screened. But at the same time, many people who have no or low-risk polyps on colonoscopy may be returning unnecessarily soon. Utilizing current screening and surveillance guidelines to direct patient care can reduce the number of unnecessary colonoscopies and improve surveillance of patients who may be at greater-than-average risk of colorectal cancer.
In this paper, we use several case examples to clarify the current guidelines on who should be screened, why, how, and how often.
WHY SCREEN?
Approximately 6% of American men and women develop an invasive colorectal neoplasm in their lifetime. Colorectal cancer is the second-leading cause of cancer death in the United States. In 2007, an estimated 153,760 people were newly diagnosed with colorectal cancer, and 52,180 people died of it.1
Yet, colorectal cancer is one of the few preventable cancers. Screening has been advocated as a way of preventing deaths by removing precancerous adenomas and detecting colorectal cancer early.2 Medicare has paid for screening colonoscopy since 1998, and since that time demand for this procedure has increased 112%.3,4 (See “Colonoscopy is the preferred test”.2,4–17)
START SCREENING AT AGE 50 FOR PEOPLE AT AVERAGE RISK
The US Multi-Society Task Force on Colorectal Cancer19 suggests that people at average risk undergo one of the following:
- Colonoscopy every 10 years
- Flexible sigmoidoscopy every 5 years
- Fecal occult blood testing every year
- An air-contrast barium enema or computed tomographic (CT) colonography every 5 years
- Fecal DNA testing, interval uncertain.
Anyone who has a positive result with any test other than colonoscopy should subsequently undergo colonoscopy.
Start screening sooner in people at higher risk
African Americans should undergo screening for colorectal cancer under an average-risk strategy starting at age 45, according to a position paper from the American College of Gastroenterology.4 Reasons for starting sooner are that African Americans have the highest incidence of colorectal cancer of any racial or ethnic group, and that they present with it at a younger age. In the years 1970–1994, 10.7% of cases of colorectal cancer in African Americans were detected before age 50 compared with 5.5% of cases in white people.22 In addition, compared with other ethnic groups, African Americans have a more proximal distribution of colorectal neoplasms, present with later-stage disease, and have lower survival rates.4
People with a family history of colorectal polyps or cancer should also start screening earlier—as early as age 40, or 10 years younger than the age at which the relative was affected—and some should be tested more often than every 10 years (see below).
Patients with ulcerative colitis or Crohn’s colitis. Current multisociety guidelines for colorectal cancer screening and surveillance in patients with ulcerative colitis or Crohn’s colitis are based on expert consensus and recommend a systematic biopsy protocol in some patients. When to begin surveillance in these patients and the specifics of the biopsy protocol are beyond the scope of this paper but are discussed in detail elsewhere.19
FAMILY HISTORY INCREASES RISK
Case 1: A woman with a family history of cancer
A 55-year-old woman comes in for a routine physical examination. Her medical history is not remarkable, but her family history is: her maternal grandmother was diagnosed with colon cancer at age 75, her sister was diagnosed with endometrial cancer at age 34, and her mother was diagnosed with colon cancer at age 60. The patient underwent colonoscopy 5 years ago, and a 1.2-cm villous adenoma was removed from her right colon. She had been advised to have her next colonoscopy in 3 years.
Current recommendations for screening and surveillance differ based upon the number, age, and relationship of relatives affected with colorectal neoplasia (Table 1). The patient described above began screening at age 50 in accordance with the guidelines for people at average risk, but her extended family history was not taken into account.
Hereditary nonpolyposis colorectal cancer
Our patient’s family history meets the criteria for hereditary nonpolyposis colorectal cancer,23 ie, she has three family members with hereditary nonpolyposis colorectal cancer-associated cancers (colorectal cancer or cancer of the endometrium, small bowel, ureter, or renal pelvis), and one family member (her mother) is a first-degree relative of the other two affected relatives. Two successive generations of her family are affected, and one family member (her sister) was diagnosed before the age of 50.
People in families like this have an 80% lifetime risk of colorectal cancer, so it is imperative to review every patient’s family history. Patients who meet the criteria should be referred for genetic counseling and possibly genetic testing. In addition, they should begin screening—with colonoscopy, not the other tests—between the ages of 21 and 25 or at an age 10 years younger than when the youngest family member was diagnosed with colorectal cancer, whichever is earlier. They should subsequently undergo colonoscopy every 1 to 2 years.
These patients also have an increased risk of certain extracolonic cancers, including a 40% to 60% lifetime risk of endometrial adenocarcinoma. They and their physicians need to be aware of consensus screening recommendations for ovarian, endometrial, and transitional cell cancers.24
Familial adenomatous polyposis
Patients with familial adenomatous polyposis develop hundreds to thousands of adenoma-tous colorectal polyps, usually in their teens, and have a 100% risk of developing colon cancer if the colon is not removed. Patients with a family history of this disorder should undergo screening at 10 to 12 years of age.
OVERCOMING BARRIERS TO SCREENING
In 2004, an estimated 70.1 million Americans were 50 years of age and older and at average risk of colorectal cancer.25 Of these, only 28.3 million (40.4%) had undergone screening, and 41.8 million had not.
We could view this as an opportunity to make a significant impact on the disease, but resources are limited. Seeff et al25 estimated that it would take 10 years to perform screening colonoscopy on unscreened Americans if one-half of all current endoscopic capacity were used for screening alone.
Barriers to screening also exist on an individual level. A recent study26 found that only 50% of patients referred for screening colonoscopy actually underwent the procedure; patients were significantly less likely to make an appointment and keep it if they were younger or female or if they were on Medicaid. Reasons cited by patients for not following through with colonoscopy after referral included fear of pain or perforation, dislike of the bowel preparation, and misperceptions about colorectal cancer risk.
Understanding these barriers and improving patient-physician communication about the procedure and the risk of colorectal cancer in the general population, even in the absence of a family history, may help improve adherence to screening colonoscopy.

POST-POLYPECTOMY SURVEILLANCE: OFTEN TOO SOON, TOO FREQUENT
After a polyp or polyps are discovered on colonoscopy, many patients are being told to come back for repeat colonoscopy unnecessarily soon,27,28 thus diverting a scarce resource away from patients who may derive the most benefit—ie, those with high-risk polyps, those with a strong family history of colon cancer or an inherited predisposition to colon cancer, and those who have never undergone screening.
The following cases illustrate how current evidence-based guidelines can be applied to several different patients.
Case 2: ‘Three benign polyps’
A 51-year-old woman with no personal or family history of colorectal neoplasia calls her primary care physician after undergoing her first colonoscopy. The patient noted that she had had “three benign polyps removed.” She would like to know when her next colonoscopy should be.
The primary care physician obtains the patient’s colonoscopy report, which reveals that three polyps measuring 5 mm, 4 mm, and 4 mm were removed from the patient’s descending colon. The pathology report reveals that two of these polyps were tubular adenomas, and one of the 4-mm polyps was hyperplastic.
Case 3: A large tubulovillous polyp
A 46-year-old African American man with no personal or family history of colorectal neoplasia underwent his first colonoscopy 1 year ago. He had had a 1.5-cm pedunculated polyp removed in toto from his ascending colon. The pathologist characterized the polyp as “tubulovillous.”
Not all polyps are precancerous
Adenomas are precursors to colorectal cancer, progressing via the widely recognized adenoma-carcinoma sequence.29 It is not unusual that both of our patients would have adenomatous polyps, since the prevalence of these polyps increases with age.30 Adenomas are detected in 11% of average-risk people ages 50 to 54, increasing to 33% to 50% in people 65 to 75 years old.31,32
Small, left-sided hyperplastic polyps, on the other hand, are considered nonneoplastic and do not require follow-up unless a patient meets the criteria for hyperplastic polyposis (Table 2). While current guidelines do not take into account hyperplastic polyps when determining postpolypectomy surveillance, the clinical significance and possible neoplastic potential of large and right-sided hyperplastic polyps is an area of active research.
Often, hyperplastic polyps are erroneously spoken of as “benign” when in fact they are not precancerous and are clinically insignificant. In fact, Boolchand et al27 found that 61% of primary care physicians would bring a patient with a single 6-mm hyperplastic polyp back for surveillance colonoscopy in 5 years or sooner. Current consensus guidelines do not recommend surveillance colonoscopy for the majority of patients with hyperplastic polyps. These individuals are not at an increased risk of colorectal cancer and should go back to average-risk screening recommendations, ie, colonoscopy in 10 years, the same interval as for the average-risk individual.33
Adenomas: How many? How big? What features?
If adenomas are discovered, three key questions affect how soon the patient should undergo colonoscopy again (Table 2):
Other studies also found that the number of adenomas predicts the subsequent development of more adenomas, and in particular advanced colorectal neoplasia.35–38
How big? Noshirwani et al35 retrospectively analyzed data from their adenoma registry and found that polyps 1 cm or larger were significantly associated with the finding of advanced adenomas 3 years later.
What features? Tubulovillous or villous features in an adenoma have been shown to increase the risk of future advanced adenomas and cancer.39,40 Similarly, high-grade dysplasia is associated with the subsequent development of advanced adenomas. In the Veterans Affairs Cooperative Study,41 10.9% of patients who had a polyp of any size with high-grade dysplasia developed an advanced neoplasm within 5 years, compared with only 0.6% of those with small polyps that did not harbor high-grade dysplasia.
Recognizing advanced adenomas is important when interpreting a patient’s colonoscopy results because multiple studies have shown them to predict recurrent advanced neoplasms or colorectal cancer.35,39–42
What does this mean for our patients?
If a patient (like our patient in case 2) who is otherwise at average risk is found to have an adenoma or adenomas without advanced features, the postpolypectomy surveillance interval should be dictated by the number of adenomas found. Current guidelines recommend that patients like this one—with one or two small tubular adenomas without features of advanced colorectal neoplasia—have a low risk of recurrent advanced adenomas and should undergo colonoscopy again in 5 to 10 years (Table 2).33
In contrast, in case 3, the polyp (which was completely removed) had two characteristics of advanced neoplasia: size larger than 1 cm and a villous component. This patient should come back in 3 years.
In colonoscopy, quality matters
An important caveat is that current post-polypectomy surveillance recommendations are based on the assumption that the bowel has been prepared adequately and that the entire colon is examined thoroughly up to the level of the cecum. Therefore, when deciding on the proper surveillance interval, one must take into account certain factors regarding the patient’s colonoscopy. Patients who have had an inadequate bowel preparation, incomplete examination, or large lesions removed piecemeal should be recalled at a shorter interval.
A final observation: another possible reason that patients are being sent back for repeat colonoscopy sooner than recommended is the concern for missed polyps. Nonpolypoid adenomas, which include flat and depressed lesions, can be easily missed using conventional endoscopy.43 A systematic review of six studies involving 465 patients who underwent tandem colonoscopy found a pooled miss rate of 26% for adenomas 1 to 5 mm.44 One way endoscopists can improve adenoma detection is to perform a slow endoscopic withdrawal over at least 6 minutes.45
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007; 57:43–66.
- Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 137:132–141.
- Prajapati DN, Saeian K, Binion DG, et al. Volume and yield of screening colonoscopy at a tertiary medical center after change in Medicare reimbursement. Am J Gastroenterol 2003; 98:194–199.
- Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol 2005; 100:515–523.
- Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. Am J Gastroenterol 2000; 95:868–877.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology 1987; 93:1009–1013.
- Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992; 326:658–662.
- Gloeckler-Ries LA, Hankey BF, Edwards BK. Cancer Statistics Review, 1973–1987. Bethesda, Md.: Department of Health and Human Services, 1990. (DHHS publication no. (NIH) 90-2789.)
- Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005; 365:305–311.
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328:1365–1371.
- Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348:1467–1471.
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348:1472–1477.
- Allison JE, Feldman R, Tekawa IS. Hemoccult screening in detecting colorectal neoplasm: sensitivity, specificity, and predictive value. Long-term follow-up in a large group practice setting. Ann Intern Med 1990; 112:328–333.
- Young GP, St John DJ, Winawer SJ, Rozen P WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy). Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies: a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. Am J Gastroenterol 2002; 97:2499–2507.
- Lieberman DA, Weiss DG Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 2001; 345:555–560.
- Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med 2005; 352:2061–2068.
- Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001—testing for early lung cancer detection. CA Cancer J Clin 2001; 51:38–75.
- Levin B, Lieberman D, McFarland B, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin, first published on Mar 5, 2008 as doi: doi:10.3322/CA.2007.0018
- Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology 2003; 124:544–560.
- U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med 2002; 137:129–131.
- Theuer CP, Wagner JL, Taylor TH, et al. Racial and ethnic colorectal cancer patterns affect the cost-effectiveness of colorectal cancer screening in the United States. Gastroenterology 2001; 120:848–856.
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology 1999; 116:1453–1456.
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006; 296:1507–1517.
- Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology 2004; 127:1661–1669.
- Denberg TD, Melhado TV, Coombes JM, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med 2005; 20:989–995.
- Boolchand V, Olds G, Singh J, Singh P, Chak A, Cooper GS. Colorectal screening after polypectomy: a national survey study of primary care physicians. Ann Intern Med 2006; 145:654–659.
- Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med 2004; 141:264–271.
- Hill MJ, Morson BC, Bussey HJ. Aetiology of adenoma-carcinoma sequence in large bowel. Lancet 1978; 1:245–247.
- Squillace S, Berggreen P, Jaffe P, et al. A normal initial colonoscopy after age 50 does not predict a polyp-free status for life. Am J Gastroenterol 1994; 89:1156–1159.
- Khullar SK, DiSario JA. Colon cancer screening. Sigmoidoscopy or colonoscopy. Gastrointest Endosc Clin North Am 1997; 7:365–386.
- Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut 1982; 23:835–842.
- Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006; 130:1872–1885.
- van Stolk RU, Beck GJ, Baron JA, Haile R, Summers R. Adenoma characteristics at first colonoscopy as predictors of adenoma recurrence and characteristics at follow-up. The Polyp Prevention Study Group. Gastroenterology 1998; 115:13–18.
- Noshirwani KC, van Stolk RU, Rybicki LA, Beck GJ. Adenoma size and number are predictive of adenoma recurrence: implications for surveillance colonoscopy. Gastrointest Endosc 2000; 51:433–437.
- Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993; 328:901–906.
- Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology 2005; 129:34–41.
- Bonithon-Kopp C, Piard F, Fenger C, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum 2004; 47:323–333.
- Loeve F, van Ballegooijen M, Boer R, Kuipers EJ, Habbema JD. Colorectal cancer risk in adenoma patients: a nation-wide study. Int J Cancer 2004; 111:147–151.
- Yang G, Zheng W, Sun QR, et al. Pathologic features of initial adenomas as predictors for metachronous adenomas of the rectum. J Natl Cancer Inst 1998; 90:1661–1665.
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Cheifec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000; 343:162–168.
- Martinez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology 2001; 120:1077–1083.
- Lieberman D. Nonpolypoid colorectal neoplasia in the United States: the parachute is open. JAMA 2008; 299:1068–1069.
- van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006; 101:343–350.
- Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006; 355:2533–2541.
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007; 57:43–66.
- Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 137:132–141.
- Prajapati DN, Saeian K, Binion DG, et al. Volume and yield of screening colonoscopy at a tertiary medical center after change in Medicare reimbursement. Am J Gastroenterol 2003; 98:194–199.
- Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol 2005; 100:515–523.
- Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. Am J Gastroenterol 2000; 95:868–877.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology 1987; 93:1009–1013.
- Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992; 326:658–662.
- Gloeckler-Ries LA, Hankey BF, Edwards BK. Cancer Statistics Review, 1973–1987. Bethesda, Md.: Department of Health and Human Services, 1990. (DHHS publication no. (NIH) 90-2789.)
- Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005; 365:305–311.
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328:1365–1371.
- Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348:1467–1471.
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348:1472–1477.
- Allison JE, Feldman R, Tekawa IS. Hemoccult screening in detecting colorectal neoplasm: sensitivity, specificity, and predictive value. Long-term follow-up in a large group practice setting. Ann Intern Med 1990; 112:328–333.
- Young GP, St John DJ, Winawer SJ, Rozen P WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy). Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies: a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. Am J Gastroenterol 2002; 97:2499–2507.
- Lieberman DA, Weiss DG Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 2001; 345:555–560.
- Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med 2005; 352:2061–2068.
- Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001—testing for early lung cancer detection. CA Cancer J Clin 2001; 51:38–75.
- Levin B, Lieberman D, McFarland B, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin, first published on Mar 5, 2008 as doi: doi:10.3322/CA.2007.0018
- Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology 2003; 124:544–560.
- U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med 2002; 137:129–131.
- Theuer CP, Wagner JL, Taylor TH, et al. Racial and ethnic colorectal cancer patterns affect the cost-effectiveness of colorectal cancer screening in the United States. Gastroenterology 2001; 120:848–856.
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology 1999; 116:1453–1456.
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006; 296:1507–1517.
- Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology 2004; 127:1661–1669.
- Denberg TD, Melhado TV, Coombes JM, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med 2005; 20:989–995.
- Boolchand V, Olds G, Singh J, Singh P, Chak A, Cooper GS. Colorectal screening after polypectomy: a national survey study of primary care physicians. Ann Intern Med 2006; 145:654–659.
- Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med 2004; 141:264–271.
- Hill MJ, Morson BC, Bussey HJ. Aetiology of adenoma-carcinoma sequence in large bowel. Lancet 1978; 1:245–247.
- Squillace S, Berggreen P, Jaffe P, et al. A normal initial colonoscopy after age 50 does not predict a polyp-free status for life. Am J Gastroenterol 1994; 89:1156–1159.
- Khullar SK, DiSario JA. Colon cancer screening. Sigmoidoscopy or colonoscopy. Gastrointest Endosc Clin North Am 1997; 7:365–386.
- Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut 1982; 23:835–842.
- Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006; 130:1872–1885.
- van Stolk RU, Beck GJ, Baron JA, Haile R, Summers R. Adenoma characteristics at first colonoscopy as predictors of adenoma recurrence and characteristics at follow-up. The Polyp Prevention Study Group. Gastroenterology 1998; 115:13–18.
- Noshirwani KC, van Stolk RU, Rybicki LA, Beck GJ. Adenoma size and number are predictive of adenoma recurrence: implications for surveillance colonoscopy. Gastrointest Endosc 2000; 51:433–437.
- Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993; 328:901–906.
- Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology 2005; 129:34–41.
- Bonithon-Kopp C, Piard F, Fenger C, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum 2004; 47:323–333.
- Loeve F, van Ballegooijen M, Boer R, Kuipers EJ, Habbema JD. Colorectal cancer risk in adenoma patients: a nation-wide study. Int J Cancer 2004; 111:147–151.
- Yang G, Zheng W, Sun QR, et al. Pathologic features of initial adenomas as predictors for metachronous adenomas of the rectum. J Natl Cancer Inst 1998; 90:1661–1665.
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Cheifec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000; 343:162–168.
- Martinez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology 2001; 120:1077–1083.
- Lieberman D. Nonpolypoid colorectal neoplasia in the United States: the parachute is open. JAMA 2008; 299:1068–1069.
- van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006; 101:343–350.
- Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006; 355:2533–2541.
KEY POINTS
- All polyps do not pose the same risk. Small, left-sided hyperplastic polyps are nonneoplastic and require no increased follow-up. Adenomas are precancerous, and follow-up is determined by size, number, and histologic features.
- The American College of Gastroenterology recommends that African Americans undergo screening under an average-risk strategy starting at age 45, as they have the highest incidence of colorectal cancer of any racial or ethnic group and present with it at a younger age.
- People with a family history of colorectal polyps or cancer are recommended to start screening earlier—at age 40 or 10 years younger than the age of the relative that was affected (whichever is younger)—-and some of them should have colonoscopy more often than every 10 years
- When deciding on the proper surveillance interval, one must take into account several details regarding the patient’s colonoscopy. Patients who have had an inadequate preparation, incomplete examination, or large lesions removed piecemeal should be recalled sooner.