User login
When should you consider combining 2 long-acting injectable antipsychotics?
Ms. S, age 39, with a 15-year history of schizophrenia and severe paranoid delusions, is admitted after physically assaulting a staff member at a group home. She is receiving paliperidone palmitate, 234 mg every 4 weeks. This has reduced the severity of her symptoms, but she continues to have persistent delusions that affect her ability to accept redirection from staff. Ms. S frequently accuses staff and peers of sexual assault, says that she is pregnant, and does not adhere to treatment recommendations for laboratory monitoring because the “staff uses her blood for experiments.”
Ms. S frequently requires administration of oral and IM haloperidol, as needed, when she becomes aggressive with the staff. She has poor insight into her mental illness and does not believe that she needs medication. Ms. S has a long history of stopping her oral antipsychotic after a few days, reporting that it is “harming her baby.” Monotherapy has been tried with various long-acting injectable antipsychotics (LAIAs), but she still exhibits persistent delusions. The treatment team decides to add a second LAIA, haloperidol decanoate, 200 mg every 4 weeks, to her regimen.
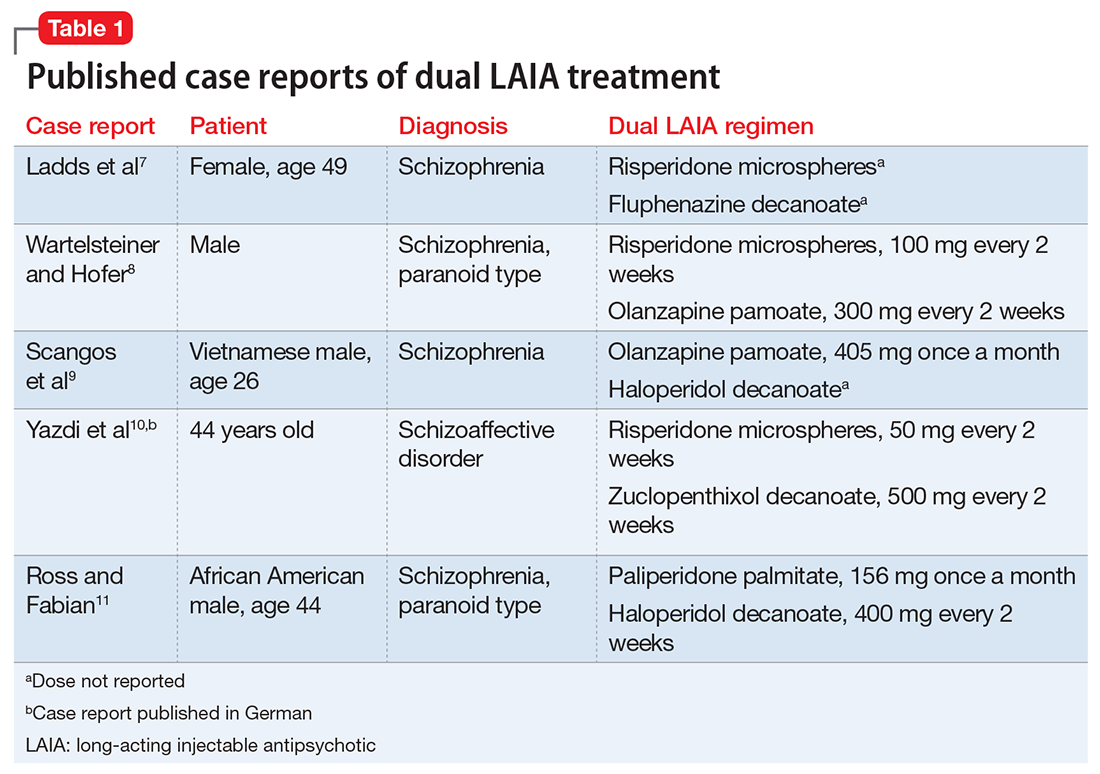
Ladds et al.7 A 49-year-old woman with schizophrenia who was hospitalized for aggressive and bizarre behavior and had been institutionalized for 20 years stopped taking her medication regimen.7 She started taking 8-hour showers with bleach, talking incoherently, and believing that someone was poisoning her. She had poor response to oral risperidone monotherapy; however, 2 months after adding oral fluphenazine and benztropine to her regimen, her symptoms substantially improved (doses not reported). Because she had impaired insight into the need for daily medication, she was started on depot fluphenazine decanoate and risperidone microspheres (doses not reported) before discharge. No substantial adverse effects were noted with this regimen.
Wartelsteiner and Hofer.8 A man who had been diagnosed with paranoid schizophrenia at age 20 presented with thought blocking, incoherence, persecutory delusions, and uncontrolled self-damaging behavior.8 He had been admitted 27 times over 7 years; during this time he received many antipsychotic monotherapies and combination regimens. A total of 8 oral antipsychotics (including clozapine) and 5 LAIAs had been administered during these trials. He significantly improved with the combination of olanzapine and risperidone. Both medications were switched to LAIA formulations to address medication nonadherence. His symptoms remained stable with risperidone microspheres, 100 mg, and olanzapine pamoate, 300 mg, each administered every 2 weeks. He did not experience any adverse effects with this combination therapy.
Scangos et al.9 A 26-year-old Vietnamese man with schizophrenia and an extensive history of unprovoked, psychotically driven assaults was given multiple antipsychotics (including clozapine) during hospitalizations, and his medication regimen consistently included 2 antipsychotics. After contracting viral gastroenteritis, he refused oral medications and required short-acting IM administration of both haloperidol, 5 mg, twice a day, and olanzapine, 10 mg, twice a day. Because of concerns about continuing this regimen, he was switched to haloperidol decanoate (dose not reported) and olanzapine pamoate, 405 mg, administered once per month. The injections were scheduled to alternate so that the patient would receive 1 injection every 2 weeks. The patient’s assaultive behavior was significantly reduced, and no adverse effects were reported.
Ross and Fabian.11 An African American man, age 44, was receiving haloperidol decanoate, 400 mg every 2 weeks, and oral haloperidol, 20 mg/d.11 Because of residual symptoms, a history of nonadherence, and concerns about increasing the haloperidol decanoate dose or frequency, oral haloperidol was discontinued and paliperidone palmitate, 156 mg every 4 weeks, was started. The patient was able to transition into a step-down unit, and no adverse effects were reported.
What to consider before initiating dual LAIA treatment
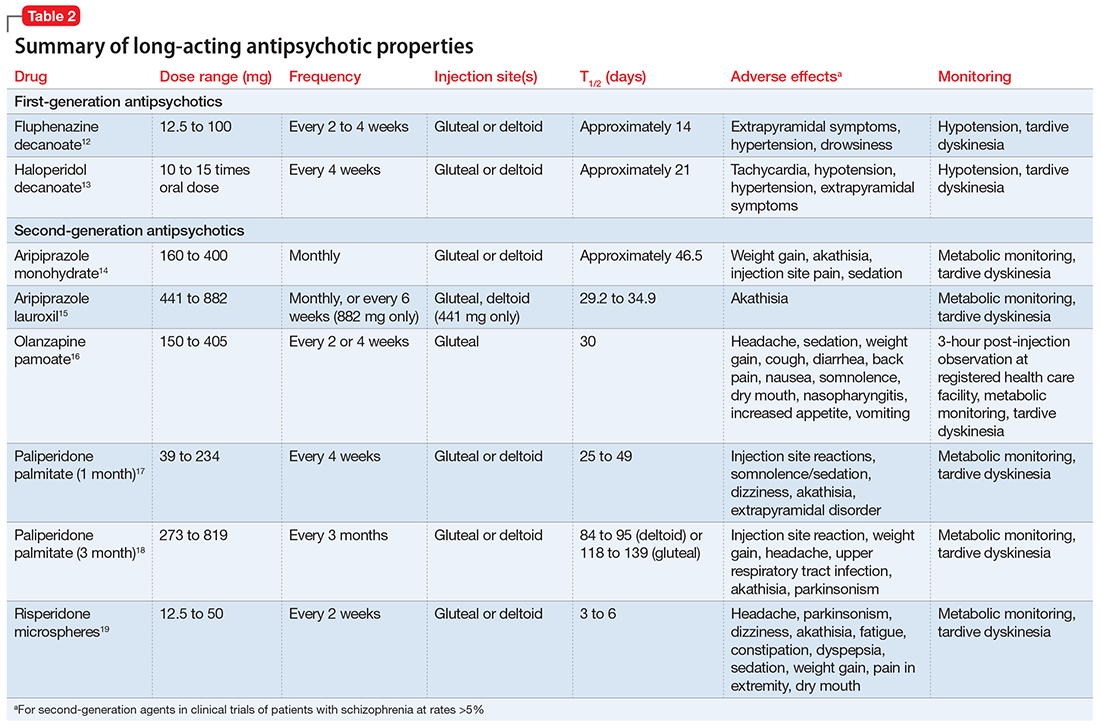
Evaluate the frequency of administration, flexibility of dosing, administration site, adverse effects, and monitoring requirements of each LAIA (Table 212-19) to ensure the patient’s optimal tolerability of the regimen. Previous tolerability of each medication must be confirmed by evaluating the patient’s medication history or oral or IM administration of each agent prior to initiating the LAIA.
When choosing 2 agents that are each administered once every 4 weeks, consider administering the medications together every 4 weeks or alternating administration so that the patient receives an injection every 2 weeks. Receiving an injection once every 2 weeks might be beneficial for patients who need close follow-up or are more sensitive to injection site reactions, whereas a regimen of once every 4 weeks might be beneficial for patients who are more resistant to receiving the injections, so there is potentially less time spent agitated or anxious leading up to the date of the injection.
Use the lowest effective dose of each LAIA to limit adverse effects and improve tolerability of the regimen. Monitor patients closely for adverse reactions and discontinue the regimen as soon as possible if a severe adverse reaction occurs.
Cost may influence the decision to use 2 LAIAs. The majority of LAIAs in the United States are available only as branded formulations. Insurance companies may require prior authorization for the use of 2 LAIAs.
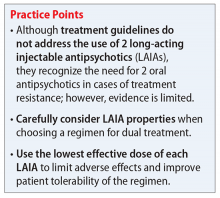
Although there are no treatment guidelines for combining 2 LAIAs, this practice has been used. A few case reports have described successful use of dual LAIA treatment, but one should consider the risk of the publication’s bias. Overall, the decision to use 2 LAIAs is difficult because there is lack of a large evidence base supporting the practice or direction from treatment guidelines. Because of this, dual LAIA treatment should not be used for most patients. In cases of treatment-resistant schizophrenia where clozapine is not an option and adherence is a concern, it is reasonable to consider this strategy on a case-by-case basis.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Hasan A, Falkai P, Wobrock T, et al; the WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-78.
4. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620. 5. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
6. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103.
7. Ladds B, Cosme R, Rivera F. Concurrent use of two depot antipsychotic medications in schizophrenia. The Internet Journal of Psychiatry. 2009;1(1):1-3.
8. Wartelsteiner F, Hofer A. Treating schizophrenia with 2 long-acting injectable antipsychotic drugs: a case report. J Clin Psychopharmacol. 2015;35(4):474-475.
9. Scangos KW, Caton M, Newman WJ. Multiple long-acting injectable antipsychotics for treatment-resistant schizophrenia: case report. J Clin Psychopharmacol. 2016;36(3):283-285.
10. Yazdi K, Rosenleitner J, Pischinger B. Combination of two depot antipsychotic drugs [in German]. Nervenarzt. 2014;85(7):870-871.
11. Ross C, Fabian T. High dose haloperidol decanoate augmentation with paliperidone palmitate. Presented at: College of Psychiatric and Neurologic Pharmacists 16th Annual Meeting; April 21-24, 2013; Colorado Springs, CO.
12. Fluphenazine decanoate [package insert]. Schaumburg, IL: APP Pharmaceuticals, LLC; 2010.
13. Haloperidol decanoate [package insert]. Rockford, IL: Mylan; 2014.
14. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2016.
15. Aristada [package insert]. Waltham, MA: Alkermes; 2016.
16. Zyprexa Relprevv [package insert]. Indianapolis, IN: Lilly USA, LLC; 2016.
17. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2009.
18. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2015.
19. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2007.
Ms. S, age 39, with a 15-year history of schizophrenia and severe paranoid delusions, is admitted after physically assaulting a staff member at a group home. She is receiving paliperidone palmitate, 234 mg every 4 weeks. This has reduced the severity of her symptoms, but she continues to have persistent delusions that affect her ability to accept redirection from staff. Ms. S frequently accuses staff and peers of sexual assault, says that she is pregnant, and does not adhere to treatment recommendations for laboratory monitoring because the “staff uses her blood for experiments.”
Ms. S frequently requires administration of oral and IM haloperidol, as needed, when she becomes aggressive with the staff. She has poor insight into her mental illness and does not believe that she needs medication. Ms. S has a long history of stopping her oral antipsychotic after a few days, reporting that it is “harming her baby.” Monotherapy has been tried with various long-acting injectable antipsychotics (LAIAs), but she still exhibits persistent delusions. The treatment team decides to add a second LAIA, haloperidol decanoate, 200 mg every 4 weeks, to her regimen.

Ladds et al.7 A 49-year-old woman with schizophrenia who was hospitalized for aggressive and bizarre behavior and had been institutionalized for 20 years stopped taking her medication regimen.7 She started taking 8-hour showers with bleach, talking incoherently, and believing that someone was poisoning her. She had poor response to oral risperidone monotherapy; however, 2 months after adding oral fluphenazine and benztropine to her regimen, her symptoms substantially improved (doses not reported). Because she had impaired insight into the need for daily medication, she was started on depot fluphenazine decanoate and risperidone microspheres (doses not reported) before discharge. No substantial adverse effects were noted with this regimen.
Wartelsteiner and Hofer.8 A man who had been diagnosed with paranoid schizophrenia at age 20 presented with thought blocking, incoherence, persecutory delusions, and uncontrolled self-damaging behavior.8 He had been admitted 27 times over 7 years; during this time he received many antipsychotic monotherapies and combination regimens. A total of 8 oral antipsychotics (including clozapine) and 5 LAIAs had been administered during these trials. He significantly improved with the combination of olanzapine and risperidone. Both medications were switched to LAIA formulations to address medication nonadherence. His symptoms remained stable with risperidone microspheres, 100 mg, and olanzapine pamoate, 300 mg, each administered every 2 weeks. He did not experience any adverse effects with this combination therapy.
Scangos et al.9 A 26-year-old Vietnamese man with schizophrenia and an extensive history of unprovoked, psychotically driven assaults was given multiple antipsychotics (including clozapine) during hospitalizations, and his medication regimen consistently included 2 antipsychotics. After contracting viral gastroenteritis, he refused oral medications and required short-acting IM administration of both haloperidol, 5 mg, twice a day, and olanzapine, 10 mg, twice a day. Because of concerns about continuing this regimen, he was switched to haloperidol decanoate (dose not reported) and olanzapine pamoate, 405 mg, administered once per month. The injections were scheduled to alternate so that the patient would receive 1 injection every 2 weeks. The patient’s assaultive behavior was significantly reduced, and no adverse effects were reported.
Ross and Fabian.11 An African American man, age 44, was receiving haloperidol decanoate, 400 mg every 2 weeks, and oral haloperidol, 20 mg/d.11 Because of residual symptoms, a history of nonadherence, and concerns about increasing the haloperidol decanoate dose or frequency, oral haloperidol was discontinued and paliperidone palmitate, 156 mg every 4 weeks, was started. The patient was able to transition into a step-down unit, and no adverse effects were reported.
What to consider before initiating dual LAIA treatment
Evaluate the frequency of administration, flexibility of dosing, administration site, adverse effects, and monitoring requirements of each LAIA (Table 212-19) to ensure the patient’s optimal tolerability of the regimen. Previous tolerability of each medication must be confirmed by evaluating the patient’s medication history or oral or IM administration of each agent prior to initiating the LAIA.

When choosing 2 agents that are each administered once every 4 weeks, consider administering the medications together every 4 weeks or alternating administration so that the patient receives an injection every 2 weeks. Receiving an injection once every 2 weeks might be beneficial for patients who need close follow-up or are more sensitive to injection site reactions, whereas a regimen of once every 4 weeks might be beneficial for patients who are more resistant to receiving the injections, so there is potentially less time spent agitated or anxious leading up to the date of the injection.
Use the lowest effective dose of each LAIA to limit adverse effects and improve tolerability of the regimen. Monitor patients closely for adverse reactions and discontinue the regimen as soon as possible if a severe adverse reaction occurs.
Cost may influence the decision to use 2 LAIAs. The majority of LAIAs in the United States are available only as branded formulations. Insurance companies may require prior authorization for the use of 2 LAIAs.
Although there are no treatment guidelines for combining 2 LAIAs, this practice has been used. A few case reports have described successful use of dual LAIA treatment, but one should consider the risk of the publication’s bias. Overall, the decision to use 2 LAIAs is difficult because there is lack of a large evidence base supporting the practice or direction from treatment guidelines. Because of this, dual LAIA treatment should not be used for most patients. In cases of treatment-resistant schizophrenia where clozapine is not an option and adherence is a concern, it is reasonable to consider this strategy on a case-by-case basis.
Ms. S, age 39, with a 15-year history of schizophrenia and severe paranoid delusions, is admitted after physically assaulting a staff member at a group home. She is receiving paliperidone palmitate, 234 mg every 4 weeks. This has reduced the severity of her symptoms, but she continues to have persistent delusions that affect her ability to accept redirection from staff. Ms. S frequently accuses staff and peers of sexual assault, says that she is pregnant, and does not adhere to treatment recommendations for laboratory monitoring because the “staff uses her blood for experiments.”
Ms. S frequently requires administration of oral and IM haloperidol, as needed, when she becomes aggressive with the staff. She has poor insight into her mental illness and does not believe that she needs medication. Ms. S has a long history of stopping her oral antipsychotic after a few days, reporting that it is “harming her baby.” Monotherapy has been tried with various long-acting injectable antipsychotics (LAIAs), but she still exhibits persistent delusions. The treatment team decides to add a second LAIA, haloperidol decanoate, 200 mg every 4 weeks, to her regimen.

Ladds et al.7 A 49-year-old woman with schizophrenia who was hospitalized for aggressive and bizarre behavior and had been institutionalized for 20 years stopped taking her medication regimen.7 She started taking 8-hour showers with bleach, talking incoherently, and believing that someone was poisoning her. She had poor response to oral risperidone monotherapy; however, 2 months after adding oral fluphenazine and benztropine to her regimen, her symptoms substantially improved (doses not reported). Because she had impaired insight into the need for daily medication, she was started on depot fluphenazine decanoate and risperidone microspheres (doses not reported) before discharge. No substantial adverse effects were noted with this regimen.
Wartelsteiner and Hofer.8 A man who had been diagnosed with paranoid schizophrenia at age 20 presented with thought blocking, incoherence, persecutory delusions, and uncontrolled self-damaging behavior.8 He had been admitted 27 times over 7 years; during this time he received many antipsychotic monotherapies and combination regimens. A total of 8 oral antipsychotics (including clozapine) and 5 LAIAs had been administered during these trials. He significantly improved with the combination of olanzapine and risperidone. Both medications were switched to LAIA formulations to address medication nonadherence. His symptoms remained stable with risperidone microspheres, 100 mg, and olanzapine pamoate, 300 mg, each administered every 2 weeks. He did not experience any adverse effects with this combination therapy.
Scangos et al.9 A 26-year-old Vietnamese man with schizophrenia and an extensive history of unprovoked, psychotically driven assaults was given multiple antipsychotics (including clozapine) during hospitalizations, and his medication regimen consistently included 2 antipsychotics. After contracting viral gastroenteritis, he refused oral medications and required short-acting IM administration of both haloperidol, 5 mg, twice a day, and olanzapine, 10 mg, twice a day. Because of concerns about continuing this regimen, he was switched to haloperidol decanoate (dose not reported) and olanzapine pamoate, 405 mg, administered once per month. The injections were scheduled to alternate so that the patient would receive 1 injection every 2 weeks. The patient’s assaultive behavior was significantly reduced, and no adverse effects were reported.
Ross and Fabian.11 An African American man, age 44, was receiving haloperidol decanoate, 400 mg every 2 weeks, and oral haloperidol, 20 mg/d.11 Because of residual symptoms, a history of nonadherence, and concerns about increasing the haloperidol decanoate dose or frequency, oral haloperidol was discontinued and paliperidone palmitate, 156 mg every 4 weeks, was started. The patient was able to transition into a step-down unit, and no adverse effects were reported.
What to consider before initiating dual LAIA treatment
Evaluate the frequency of administration, flexibility of dosing, administration site, adverse effects, and monitoring requirements of each LAIA (Table 212-19) to ensure the patient’s optimal tolerability of the regimen. Previous tolerability of each medication must be confirmed by evaluating the patient’s medication history or oral or IM administration of each agent prior to initiating the LAIA.

When choosing 2 agents that are each administered once every 4 weeks, consider administering the medications together every 4 weeks or alternating administration so that the patient receives an injection every 2 weeks. Receiving an injection once every 2 weeks might be beneficial for patients who need close follow-up or are more sensitive to injection site reactions, whereas a regimen of once every 4 weeks might be beneficial for patients who are more resistant to receiving the injections, so there is potentially less time spent agitated or anxious leading up to the date of the injection.
Use the lowest effective dose of each LAIA to limit adverse effects and improve tolerability of the regimen. Monitor patients closely for adverse reactions and discontinue the regimen as soon as possible if a severe adverse reaction occurs.
Cost may influence the decision to use 2 LAIAs. The majority of LAIAs in the United States are available only as branded formulations. Insurance companies may require prior authorization for the use of 2 LAIAs.
Although there are no treatment guidelines for combining 2 LAIAs, this practice has been used. A few case reports have described successful use of dual LAIA treatment, but one should consider the risk of the publication’s bias. Overall, the decision to use 2 LAIAs is difficult because there is lack of a large evidence base supporting the practice or direction from treatment guidelines. Because of this, dual LAIA treatment should not be used for most patients. In cases of treatment-resistant schizophrenia where clozapine is not an option and adherence is a concern, it is reasonable to consider this strategy on a case-by-case basis.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Hasan A, Falkai P, Wobrock T, et al; the WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-78.
4. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620. 5. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
6. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103.
7. Ladds B, Cosme R, Rivera F. Concurrent use of two depot antipsychotic medications in schizophrenia. The Internet Journal of Psychiatry. 2009;1(1):1-3.
8. Wartelsteiner F, Hofer A. Treating schizophrenia with 2 long-acting injectable antipsychotic drugs: a case report. J Clin Psychopharmacol. 2015;35(4):474-475.
9. Scangos KW, Caton M, Newman WJ. Multiple long-acting injectable antipsychotics for treatment-resistant schizophrenia: case report. J Clin Psychopharmacol. 2016;36(3):283-285.
10. Yazdi K, Rosenleitner J, Pischinger B. Combination of two depot antipsychotic drugs [in German]. Nervenarzt. 2014;85(7):870-871.
11. Ross C, Fabian T. High dose haloperidol decanoate augmentation with paliperidone palmitate. Presented at: College of Psychiatric and Neurologic Pharmacists 16th Annual Meeting; April 21-24, 2013; Colorado Springs, CO.
12. Fluphenazine decanoate [package insert]. Schaumburg, IL: APP Pharmaceuticals, LLC; 2010.
13. Haloperidol decanoate [package insert]. Rockford, IL: Mylan; 2014.
14. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2016.
15. Aristada [package insert]. Waltham, MA: Alkermes; 2016.
16. Zyprexa Relprevv [package insert]. Indianapolis, IN: Lilly USA, LLC; 2016.
17. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2009.
18. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2015.
19. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2007.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Hasan A, Falkai P, Wobrock T, et al; the WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-78.
4. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620. 5. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
6. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103.
7. Ladds B, Cosme R, Rivera F. Concurrent use of two depot antipsychotic medications in schizophrenia. The Internet Journal of Psychiatry. 2009;1(1):1-3.
8. Wartelsteiner F, Hofer A. Treating schizophrenia with 2 long-acting injectable antipsychotic drugs: a case report. J Clin Psychopharmacol. 2015;35(4):474-475.
9. Scangos KW, Caton M, Newman WJ. Multiple long-acting injectable antipsychotics for treatment-resistant schizophrenia: case report. J Clin Psychopharmacol. 2016;36(3):283-285.
10. Yazdi K, Rosenleitner J, Pischinger B. Combination of two depot antipsychotic drugs [in German]. Nervenarzt. 2014;85(7):870-871.
11. Ross C, Fabian T. High dose haloperidol decanoate augmentation with paliperidone palmitate. Presented at: College of Psychiatric and Neurologic Pharmacists 16th Annual Meeting; April 21-24, 2013; Colorado Springs, CO.
12. Fluphenazine decanoate [package insert]. Schaumburg, IL: APP Pharmaceuticals, LLC; 2010.
13. Haloperidol decanoate [package insert]. Rockford, IL: Mylan; 2014.
14. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2016.
15. Aristada [package insert]. Waltham, MA: Alkermes; 2016.
16. Zyprexa Relprevv [package insert]. Indianapolis, IN: Lilly USA, LLC; 2016.
17. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2009.
18. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2015.
19. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2007.