User login
Cardiac risk stratification for noncardiac surgery
In patients undergoing noncardiac surgery, preoperative intervention for a cardiac condition is rarely needed simply to reduce the risk of the surgery unless such intervention is indicated separate from the preoperative context.
This is the overriding message of the 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery issued by the American College of Cardiology (ACC) and American Heart Association (AHA),1 for which I was privileged to chair the writing committee. This article outlines current best practices in cardiac risk stratification for noncardiac surgery, highlighting key recommendations from the ACC/AHA 2007 perioperative guidelines.
PURPOSE OF THE PREOPERATIVE CARDIAC EVALUATION
Provide clinical judgment, not clearance for surgery
A proper cardiac evaluation prior to noncardiac surgery involves a comprehensive patient assessment that draws on clinical findings, the clinical experience of the consulting physician (typically a cardiologist or internist), and an assessment of the literature. The purpose is not to give medical clearance for surgery but rather to provide informed clinical judgment to the anesthesiologist and the surgical team in terms of the following1:
- The patient’s current medical status
- Recommendations regarding the management and risk of cardiac problems during the perioperative period
- The patient’s clinical risk profile, to assist with treatment decisions that may affect short- or long-term cardiac outcomes.
Order tests only when results may change management
The consulting physician’s clinical judgment is critical in determining the need to order any specific tests. In general, a test to further define cardiac risk is valid only when its results could change the planned management and lead to a specific intervention. Potential interventions that may result from knowledge gained through testing include:
- Delaying the operation because of unstable symptoms
- Coronary revascularization
- Attempting medical optimization before surgery
- Involving additional specialists or providers in the patient’s perioperative care
- Modification of intraoperative monitoring
- Modification of postoperative monitoring
- Modification of the surgical location, particularly when the procedure is scheduled for an ambulatory surgical center.
The cardiac evaluation should result in an estimation of cardiac risk. If the consulting physician’s estimation of risk is not clearly above or below the threshold for a potential intervention, then further testing may be indicated to further define the need for interventions (ie, reaching the threshold for action).
WHAT TO WORRY ABOUT FIRST: HIGH-RISK CONDITIONS THAT REQUIRE EVALUATION AND TREATMENT
In a recommendation categorized as a Class I, Level B endorsement,* the ACC/AHA 2007 perioperative guidelines specify four active cardiac conditions for which an evaluation and treatment are required before noncardiac surgery1:
- Unstable coronary syndromes, including unstable or severe angina or recent myocardial infarction (MI). These syndromes should be the first and most important consideration. Unstable angina is a hypercoagulable state, as is recent MI. The hypercoagulability of these conditions is compounded by the hypercoagulability induced by the perioperative setting itself. As a result, the rate of perioperative MI or death in the setting of unstable angina is as high as 28%.2 In the case of unstable coronary syndromes, delaying surgery is appropriate if the risks of the surgery are deemed greater than its potential benefits.
- Decompensated heart failure, defined as New York Heart Association functional class IV disease or worsening or new-onset heart failure.
- Significant arrhythmias, defined as high-grade or Mobitz II atrioventricular block, third-degree atrioventricular heart block, symptomatic ventricular arrhythmias, supraventricular arrhythmias with uncontrolled ventricular rate, symptomatic bradycardia, and newly recognized ventricular tachycardia.
- Severe valvular disease, defined as severe aortic stenosis and symptomatic mitral stenosis.
(*The ACC/AHA 2007 perioperative guidelines make recommendations by classifying the magnitude of benefit versus risk [I = the intervention should be undertaken; IIa = the intervention is reasonable to undertake; IIb = the intervention may be considered; III = the intervention should not be undertaken] and assigning a level of supporting evidence [A = highest level of evidence; B = limited evidence; C = very limited evidence].)
CARDIAC RISK STRATIFICATION: INITIAL PATIENT ASSESSMENT
Clinical risk factors and functional capacity
The Revised Cardiac Risk Index of Lee et al3 remains the general paradigm for stratifying cardiac risk before noncardiac surgery. This validated index consists of six independent predictors of cardiac complications:
- High-risk surgery (intraperitoneal, intrathoracic, or suprainguinal vascular procedures)
- Ischemic heart disease
- History of congestive heart failure
- History of cerebrovascular disease
- Insulin therapy for diabetes mellitus
- Preoperative creatinine level greater than 2.0 mg/dL.
The more predictors a patient has, the greater the risk of perioperative complications. Thus, the Revised Cardiac Risk Index is a good tool for establishing a baseline risk level for use in determining whether a preoperative or perioperative intervention is likely to make a difference in the patient’s surgical outcome. For the purpose of the algorithmic approach to testing, the surgical procedure is not considered a risk factor. Additionally, type 2 diabetes mellitus is also considered a risk factor.
Another important determinant of risk is the patient’s functional capacity. A study of 600 patients undergoing major noncardiac procedures found that poor self-reported exercise capacity, defined as an inability to walk four blocks or climb two flights of stairs, was associated with significantly more perioperative complications than was good exercise capacity.4 Simple instruments such as the Duke Activity Status Index5 can be used to estimate the patient’s functional capacity.
Procedure-specific risk
In addition to patient-specific factors, surgery-specific cardiac risk can be important, especially in patients with more than two clinical risk factors. The ACC/AHA 2007 perioperative guidelines identify three categories of surgery-specific risk1:
- Vascular surgery (the highest-risk category and also the most extensively studied), which has been associated with cardiac morbidity rates of greater than 5% in many reports. Examples include aortic and other major vascular surgery, as well as peripheral vascular surgery.
- Intermediate-risk surgery, for which reported cardiac morbidity rates range from 1% to 5%. Examples include intraperitoneal and intrathoracic procedures, carotid endarterectomy, head and neck surgery, orthopedic surgery, and prostate surgery.
- Low-risk surgery, for which reported cardiac morbidity rates are generally below 1%. Examples include endoscopic and superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Patients undergoing these procedures do not generally require further preoperative cardiac testing.1
Of course, some variability exists within each risk level as a result of institutional differences in surgical volume and expertise as well as in preoperative evaluation and other processes of care. Endovascular surgery is considered intermediate risk from a perioperative perspective but is in the same risk category as vascular surgery from a 1-year perspective.
Risk stratification promotes good perioperative outcomes
Appropriate risk stratification can make the day of surgery among the safest times for patients undergoing outpatient procedures. A retrospective analysis of Medicare claims from the late 1990s for more than 500,000 elderly patients undergoing low-risk procedures in various outpatient settings found that the mortality rate was only 1 in 50,000 on the day of surgery but increased substantially over the following 7 days and 30 days.6 This was likely a reflection of the diligence applied to managing patient-specific risk factors before proceeding to outpatient surgery.
HEART RATE CONTROL
Chronic beta-blockade can obviate need for cardiac testing
The DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Strees Echo) II trial assessed the value of cardiac testing before major vascular surgery in intermediate-risk patients (ie, with one or two cardiac risk factors) receiving chronic beta-blocker therapy begun 7 to 30 days prior to surgery.7 Among the study’s 770 intermediate-risk patients, the primary outcome—cardiac death or MI at 30 days—was no different between those randomized to receive stress testing or no stress testing. The investigators concluded that cardiac testing can safely be omitted in intermediate-risk patients if beta-blockers are used with the aim of tight heart rate control.
Continue ongoing beta-blocker therapy, start in select high-risk patients
The ACC/AHA 2007 perioperative guidelines recommend continuing beta-blocker therapy in patients who are already receiving these agents (Class I, Level C). For patients not already taking beta-blockers, their initiation is recommended in those undergoing vascular surgery who have ischemia on preoperative testing (Class I, Level B). The guidelines designate beta-blockers as “probably” recommended (Class IIa, Level B) for several other patient subgroups with high cardiac risk, mainly in the setting of vascular surgery.1
Notably, the guidelines were written before publication of the Perioperative Ischemic Evaluation (POISE),8 which questioned the risk/benefit profile of perioperative beta-blockade in patients with or at high risk of atherosclerotic disease (see the Poldermans–Devereaux debate on page S84 of this supplement), and therefore may require revision (an update is scheduled for release in November 2009).
LIMITED ROLE FOR CORONARY REVASCULARIZATION
Until recently, no randomized trials had assessed the benefit of prophylactic coronary revascularization to reduce the perioperative risk of noncardiac surgery. The first large such trial was the Coronary Artery Revascularization Prophylaxis (CARP) study, which randomized 510 patients scheduled for major elective vascular surgery to undergo or not undergo coronary artery revascularization before the procedure.9 The study found that revascularization failed to affect any outcome measure, including mortality or the development of MI, out to 6 years of follow-up. Notably, the CARP population consisted mostly of patients with single-, double-, or mild triple-vessel coronary artery disease, so the study was limited in that it did not include patients with strong indications for coronary artery bypass graft surgery (CABG).7
A reanalysis of the CARP results by the type of revascularization procedure—CABG or percutaneous coronary intervention (PCI)—revealed that patients undergoing CABG had lower rates of death, MI, and additional revascularization procedures compared with those undergoing PCI, despite the presumably more extensive disease of the CABG recipients.10
Benefit apparently limited to left main disease
Further analysis of patients in the CARP trial who underwent coronary angiography found that one subgroup—patients with left main disease—did experience an improvement in survival with preoperative coronary revascularization.11
In a subsequent randomized pilot study, Poldermans et al found no advantage to preoperative coronary revascularization among patients with extensive ischemia who underwent major vascular surgery.12 While this study was not adequately sized to definitively address the value of preoperative revascularization in these high-risk patients, its results are consistent with those of the CARP trial.
In a retrospective cohort study of patients who underwent noncardiac surgery, Posner and colleagues found that rates of adverse cardiac outcomes among patients who had recent PCI (≤ 90 days before surgery) were similar to rates among matched controls with nonrevascularized coronary disease.13 Patients who had had remote PCI (> 90 days before surgery) had a lower risk of poor outcomes than did matched controls with nonrevascularized disease, but had a higher risk than did controls without coronary disease.13
PATIENTS WITH CORONARY STENTS: STENT TYPE AND TIME SINCE PLACEMENT ARE KEY
The lack of benefit from prophylactic PCI prior to noncardiac surgery also applies to PCI procedures that involve coronary stent placement. For instance, a propensity-score analysis found no benefit from prophylactic PCI (using stents in the vast majority of cases) in patients with coronary artery disease in terms of adverse coronary events or death following aortic surgery.14
In patients who have undergone prior PCI, noncardiac surgery poses special challenges, especially in relation to stents. Restenosis is a particular concern with the use of bare-metal stents, and development of stent thrombosis is a particular risk with the use of drug-eluting stents.15 The use of drug-eluting stents requires intensive antiplatelet therapy for at least 1 year following stent implantation to prevent stent thrombosis.16
Time interval to surgery after bare-metal stent placement
The effect of prior PCI with bare-metal stents on outcomes following noncardiac surgery was examined in a recent large retrospective study by Nuttall et al.17 The incidence of major cardiac events was found to be lowest when noncardiac surgery was performed more than 90 days after PCI with bare-metal stents. Using patients who had a greater than 90-day interval before surgery as the reference group, propensity analysis showed that performing surgery within 30 days of PCI was associated with an odds ratio of 3.6 for major cardiac events. The odds ratio was reduced to 1.6 when surgery was performed 31 to 90 days after PCI. These findings suggest that 30 days may be an ideal minimum time interval, from a risk/benefit standpoint, between PCI with bare-metal stents and noncardiac surgery.
Time interval to surgery after drug-eluting stent placement
A recent retrospective study by Rabbitts et al examined patients who had noncardiac surgery after prior PCI with drug-eluting stents, focusing on the relationship between the timing of the procedures and major cardiac events during hospitalization for the surgery.18 Although the frequency of major cardiac events was not statistically significantly associated with the time between stent placement and surgery, the frequency was lowest—3.3%—when surgery followed drug-eluting stent placement by more than 365 days (versus rates of 5.7% to 6.4% for various intervals of less than 365 days).
ACC/AHA recommendations
Timing of antiplatelet interruption
Results from a prospectively maintained Dutch registry19 are consistent with the findings reviewed above: patients who underwent noncardiac surgery less than 30 days after bare-metal stent implantation or less than 6 months after drug-eluting stent implantation (early surgery group) had a significantly elevated rate of major cardiac events compared with patients in whom the interval between stenting and noncardiac surgery was longer (late surgery group). Notably, this report also found that the rate of major cardiac events within the early surgery group was significantly higher in patients whose antiplatelet therapy was discontinued during the preoperative period than in those whose antiplatelet therapy was not stopped.19
A hypercoagulable state develops within 7 to 10 days after interruption of antiplatelet therapy, at which time the patient is vulnerable to thrombosis. In general, surgery should not proceed during this time without antiplatelet coverage.
From my perspective, giving ketorolac or aspirin the morning of surgery may be beneficial for patients whose antiplatelet therapy has been stopped 7 to 10 days previously, although no data from randomized trials exist to support this practice. Theoretically, it is reasonable to stop antiplatelet therapy 4 to 5 days before surgery in patients with an increased risk of bleeding without exposing them to the hypercoagulability that would set in if therapy were stopped earlier.
A FRAMEWORK FOR CARDIAC EVALUATION
The following are among the algorithm’s key recommendations:
- Patients requiring urgent noncardiac surgery should proceed to the operating room with perioperative surveillance (Class I, Level C).
- Patients with active cardiac conditions who are undergoing nonurgent surgery should be evaluated and treated per ACC/AHA guidelines before proceeding to the operating room is considered (Class I, Level B).
- Patients scheduled for a low-risk procedure can proceed to surgery without testing (Class I, Level B).
- Patients scheduled for intermediate-risk surgery or vascular surgery are to be assessed by functional capacity and clinical risk factors. Proceeding with planned surgery is appropriate in patients with good functional capacity (Class IIa, Level B). In patients with poor or unknown functional capacity undergoing vascular surgery who have three or more clinical risk factors, testing should be considered if the results would change management (Class IIa, Level B).
- Patients with one or more clinical risk factors undergoing intermediate-risk surgery and those with fewer than three clinical risk factors undergoing vascular surgery may proceed to planned surgery with control of heart rate to diminish the stress response perioperatively (Class IIa, Level B), or they may undergo noninvasive testing, but only if the results would change management (Class IIb, Level B).
- Patients undergoing intermediate-risk or vascular surgery who have poor or unknown functional capacity but no clinical risk factors may proceed to surgery without testing (Class I, Level B).
DISCUSSION
Question from the audience: The POISE study showed a 30% reduction in nonfatal MI with routine perioperative beta-blockade but an overall increase in mortality. Since most MIs occur immediately postoperatively and sepsis occurs a bit later, would you consider continuing beta-blocker therapy for a few days to prevent an MI but then stopping it before sepsis develops?
Dr. Fleisher: I’ve had discussions with sepsis experts about the link between beta-blocker therapy and sepsis and death in POISE, and the belief is that beta-blockers do not cause sepsis. I think that a septic patient on acute high-dose beta-blocker therapy can’t respond appropriately because of an inability to increase cardiac output. I believe we should titrate beta-blockers more closely. Information on preoperative dose titration in POISE is not available because of the way the trial was designed. Sepsis developed in only 53 of the 8,351 patients randomized in the study.
I would not start an acute beta-blocker protocol just to get a patient through surgery. I would start a perioperative hemodynamic protocol with the goal of maintaining the patient’s heart rate at lower than 80 beats per minute. Because I don’t believe that beta-blockers cause sepsis, if I initiated a beta-blocker preoperatively, I would not stop it at 2 days.
Question from the audience: Is there a time period during which a patient with a bare-metal stent could have back surgery or knee replacement surgery while not on aspirin?
Dr. Fleisher: The guidelines say that if a patient is on aspirin, it should be continued indefinitely. The issue is one of risk versus benefit. For back surgery, if bleeding is a concern, stopping aspirin for 6 or 7 days after the 30-day period following PCI is not unreasonable, but I would not stop it during the first 30 days following PCI.
Question from the audience: I don’t assess for vascular surgery but rather for the Whipple procedure [radical pancreatoduodenectomy], and I use the Revised Cardiac Risk Index to assess the number of risk factors. I believe the Whipple procedure is a high-risk operation, but it appears to be considered an intermediate-risk operation by the ACC/AHA guidelines. Is my approach to risk assessment appropriate?
Dr. Fleisher: If the rates of morbidity and mortality with the Whipple procedure are low at your institution, you might risk worsening your outcomes by applying someone else’s paradigms to your institution. There’s a big difference in risk between a surgeon who does a Whipple in 5 hours with 0.5 to 1.0 U of blood loss and a surgeon who does a 12-hour Whipple with 20 U of blood loss, necessitating a stay in the intensive care unit for multiple days. You need to consider the risk associated with your institution and specifically with the surgeon.
Question from the audience: Peripheral vascular disease is considered a coronary heart disease risk equivalent, so why is it not one of the criteria in the Revised Cardiac Risk Index?
Dr. Fleisher: The criteria are not hard and fast. The index was devised at one institution, Brigham and Women’s Hospital, in about 4,000 patients, and it has been used differently. It assigns 1 point to ischemic heart disease. It would not be inappropriate to assume that any atherosclerotic class of disease is equivalent to ischemic heart disease for risk purposes.
Question from the audience: You mentioned a 4-day window for withholding clopidogrel. Do you factor into the decision the duration of therapy? Some cardiologists go beyond the 1-year recommendation to continue clopidogrel after stenting because they believe there is still benefit.
Dr. Fleisher: The key is to confer with the cardiologist who implanted the stent, who knows the stenosis for which the stent was implanted. A problem we’ve had for years is that a practitioner will stop the antiplatelet agent without having spoken to the surgeon or the anesthesiologist. As an anesthesiologist, I need to know that someone has done a risk/benefit assessment of whether to continue antiplatelet agents in a given patient.
Question from the audience: The Revised Cardiac Risk Index of Lee et al3 includes the type of surgery in its total point system while the ACC/AHA guidelines do not. Can you explain the discrepancy?
Dr. Fleisher: We on the writing committee for the ACC/AHA 2007 perioperative guidelines made a decision to pull out the type of surgery and use the other five risk factors of Lee et al. It was a consensus of the committee because we believed that the complexity of the surgery itself is a separate consideration for risk. That’s why we included the medical risk factors and considered the surgical factors separately.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol 2008; 52:794–797]. J Am Coll Cardiol 2007; 50:1707–1732.
- Shah KB, Kleinman BS, Rao TLK, Jacobs HK, Mestan K, Schaafsma M. Angina and other risk factors in patients with cardiac diseases undergoing noncardiac operations. Anesth Analg 1990; 70:240–247.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Reilly DF, McNeely MJ, Doerner D, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med 1999; 159:2185–2192.
- Nelson CL, Herndon JE, Mark DB, et al. Relation of clinical and angiographic factors to functional capacity as measured by the Duke Activity Status Index. Am J Cardiol 1991; 68:973–975.
- Fleisher LA, Pasternak LR, Herbert R, Anderson GF. Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg 2004; 139:67–72.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- POISE Study Group, Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomized controlled trial. Lancet 2008; 371:1839–1847.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
- Ward HB, Kelly RF, Thottapurathu L, et al. Coronary artery bypass grafting is superior to percutaneous coronary intervention in prevention of perioperative myocardial infarctions during subsequent vascular surgery. Ann Thorac Surg 2006; 82:795–801.
- Garcia S, Moritz TE, Ward HB, et al. Usefulness of revascularization of patients with multivessel coronary artery disease before elective vascular surgery for abdominal aortic and peripheral occlusive disease. Am J Cardiol 2008; 102:809–813.
- Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol 2007; 49:1763–1769.
- Posner KL, Van Norman GA, Chan V. Adverse cardiac outcomes after noncardiac surgery in patients with prior percutaneous transluminal coronary angioplasty. Anesth Analg 1999; 89:553–560.
- Godet G, Riou B, Bertrand M, et al. Does preoperative coronary angioplasty improve perioperative cardiac outcome? Anesthesiology 2005; 102:739–746.
- Shuchman M. Debating the risks of drug-eluting stents. N Engl J Med 2007; 356:325–328.
- King SB III, Smith SC Jr, Hirshfeld JW Jr, et al. ACC/AHA/SCAI. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Nuttall GA, Brown MJ, Stombaugh JW, et al. Time and cardiac risk of surgery after bare-metal stent percutaneous coronary intervention. Anesthesiology 2008; 109:588–595.
- Rabbitts JA, Nuttall GA, Brown MJ, et al. Cardiac risk of noncardiac surgery after percutaneous coronary intervention with drug-eluting stents. Anesthesiology 2008; 109:596–604.
- Schouten O, van Domburg RT, Bax JJ, et al. Noncardiac surgery after coronary stenting: early surgery and interruption of antiplatelet therapy are associated with an increase in major adverse cardiac events. J Am Coll Cardiol 2007; 49:122–124.
- Correction to Fleisher et al, J Am Coll Cardiol 2007; 50:1707–1732. J Am Coll Cardiol 2008; 52:794–797.
In patients undergoing noncardiac surgery, preoperative intervention for a cardiac condition is rarely needed simply to reduce the risk of the surgery unless such intervention is indicated separate from the preoperative context.
This is the overriding message of the 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery issued by the American College of Cardiology (ACC) and American Heart Association (AHA),1 for which I was privileged to chair the writing committee. This article outlines current best practices in cardiac risk stratification for noncardiac surgery, highlighting key recommendations from the ACC/AHA 2007 perioperative guidelines.
PURPOSE OF THE PREOPERATIVE CARDIAC EVALUATION
Provide clinical judgment, not clearance for surgery
A proper cardiac evaluation prior to noncardiac surgery involves a comprehensive patient assessment that draws on clinical findings, the clinical experience of the consulting physician (typically a cardiologist or internist), and an assessment of the literature. The purpose is not to give medical clearance for surgery but rather to provide informed clinical judgment to the anesthesiologist and the surgical team in terms of the following1:
- The patient’s current medical status
- Recommendations regarding the management and risk of cardiac problems during the perioperative period
- The patient’s clinical risk profile, to assist with treatment decisions that may affect short- or long-term cardiac outcomes.
Order tests only when results may change management
The consulting physician’s clinical judgment is critical in determining the need to order any specific tests. In general, a test to further define cardiac risk is valid only when its results could change the planned management and lead to a specific intervention. Potential interventions that may result from knowledge gained through testing include:
- Delaying the operation because of unstable symptoms
- Coronary revascularization
- Attempting medical optimization before surgery
- Involving additional specialists or providers in the patient’s perioperative care
- Modification of intraoperative monitoring
- Modification of postoperative monitoring
- Modification of the surgical location, particularly when the procedure is scheduled for an ambulatory surgical center.
The cardiac evaluation should result in an estimation of cardiac risk. If the consulting physician’s estimation of risk is not clearly above or below the threshold for a potential intervention, then further testing may be indicated to further define the need for interventions (ie, reaching the threshold for action).
WHAT TO WORRY ABOUT FIRST: HIGH-RISK CONDITIONS THAT REQUIRE EVALUATION AND TREATMENT
In a recommendation categorized as a Class I, Level B endorsement,* the ACC/AHA 2007 perioperative guidelines specify four active cardiac conditions for which an evaluation and treatment are required before noncardiac surgery1:
- Unstable coronary syndromes, including unstable or severe angina or recent myocardial infarction (MI). These syndromes should be the first and most important consideration. Unstable angina is a hypercoagulable state, as is recent MI. The hypercoagulability of these conditions is compounded by the hypercoagulability induced by the perioperative setting itself. As a result, the rate of perioperative MI or death in the setting of unstable angina is as high as 28%.2 In the case of unstable coronary syndromes, delaying surgery is appropriate if the risks of the surgery are deemed greater than its potential benefits.
- Decompensated heart failure, defined as New York Heart Association functional class IV disease or worsening or new-onset heart failure.
- Significant arrhythmias, defined as high-grade or Mobitz II atrioventricular block, third-degree atrioventricular heart block, symptomatic ventricular arrhythmias, supraventricular arrhythmias with uncontrolled ventricular rate, symptomatic bradycardia, and newly recognized ventricular tachycardia.
- Severe valvular disease, defined as severe aortic stenosis and symptomatic mitral stenosis.
(*The ACC/AHA 2007 perioperative guidelines make recommendations by classifying the magnitude of benefit versus risk [I = the intervention should be undertaken; IIa = the intervention is reasonable to undertake; IIb = the intervention may be considered; III = the intervention should not be undertaken] and assigning a level of supporting evidence [A = highest level of evidence; B = limited evidence; C = very limited evidence].)
CARDIAC RISK STRATIFICATION: INITIAL PATIENT ASSESSMENT
Clinical risk factors and functional capacity
The Revised Cardiac Risk Index of Lee et al3 remains the general paradigm for stratifying cardiac risk before noncardiac surgery. This validated index consists of six independent predictors of cardiac complications:
- High-risk surgery (intraperitoneal, intrathoracic, or suprainguinal vascular procedures)
- Ischemic heart disease
- History of congestive heart failure
- History of cerebrovascular disease
- Insulin therapy for diabetes mellitus
- Preoperative creatinine level greater than 2.0 mg/dL.
The more predictors a patient has, the greater the risk of perioperative complications. Thus, the Revised Cardiac Risk Index is a good tool for establishing a baseline risk level for use in determining whether a preoperative or perioperative intervention is likely to make a difference in the patient’s surgical outcome. For the purpose of the algorithmic approach to testing, the surgical procedure is not considered a risk factor. Additionally, type 2 diabetes mellitus is also considered a risk factor.
Another important determinant of risk is the patient’s functional capacity. A study of 600 patients undergoing major noncardiac procedures found that poor self-reported exercise capacity, defined as an inability to walk four blocks or climb two flights of stairs, was associated with significantly more perioperative complications than was good exercise capacity.4 Simple instruments such as the Duke Activity Status Index5 can be used to estimate the patient’s functional capacity.
Procedure-specific risk
In addition to patient-specific factors, surgery-specific cardiac risk can be important, especially in patients with more than two clinical risk factors. The ACC/AHA 2007 perioperative guidelines identify three categories of surgery-specific risk1:
- Vascular surgery (the highest-risk category and also the most extensively studied), which has been associated with cardiac morbidity rates of greater than 5% in many reports. Examples include aortic and other major vascular surgery, as well as peripheral vascular surgery.
- Intermediate-risk surgery, for which reported cardiac morbidity rates range from 1% to 5%. Examples include intraperitoneal and intrathoracic procedures, carotid endarterectomy, head and neck surgery, orthopedic surgery, and prostate surgery.
- Low-risk surgery, for which reported cardiac morbidity rates are generally below 1%. Examples include endoscopic and superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Patients undergoing these procedures do not generally require further preoperative cardiac testing.1
Of course, some variability exists within each risk level as a result of institutional differences in surgical volume and expertise as well as in preoperative evaluation and other processes of care. Endovascular surgery is considered intermediate risk from a perioperative perspective but is in the same risk category as vascular surgery from a 1-year perspective.
Risk stratification promotes good perioperative outcomes
Appropriate risk stratification can make the day of surgery among the safest times for patients undergoing outpatient procedures. A retrospective analysis of Medicare claims from the late 1990s for more than 500,000 elderly patients undergoing low-risk procedures in various outpatient settings found that the mortality rate was only 1 in 50,000 on the day of surgery but increased substantially over the following 7 days and 30 days.6 This was likely a reflection of the diligence applied to managing patient-specific risk factors before proceeding to outpatient surgery.
HEART RATE CONTROL
Chronic beta-blockade can obviate need for cardiac testing
The DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Strees Echo) II trial assessed the value of cardiac testing before major vascular surgery in intermediate-risk patients (ie, with one or two cardiac risk factors) receiving chronic beta-blocker therapy begun 7 to 30 days prior to surgery.7 Among the study’s 770 intermediate-risk patients, the primary outcome—cardiac death or MI at 30 days—was no different between those randomized to receive stress testing or no stress testing. The investigators concluded that cardiac testing can safely be omitted in intermediate-risk patients if beta-blockers are used with the aim of tight heart rate control.
Continue ongoing beta-blocker therapy, start in select high-risk patients
The ACC/AHA 2007 perioperative guidelines recommend continuing beta-blocker therapy in patients who are already receiving these agents (Class I, Level C). For patients not already taking beta-blockers, their initiation is recommended in those undergoing vascular surgery who have ischemia on preoperative testing (Class I, Level B). The guidelines designate beta-blockers as “probably” recommended (Class IIa, Level B) for several other patient subgroups with high cardiac risk, mainly in the setting of vascular surgery.1
Notably, the guidelines were written before publication of the Perioperative Ischemic Evaluation (POISE),8 which questioned the risk/benefit profile of perioperative beta-blockade in patients with or at high risk of atherosclerotic disease (see the Poldermans–Devereaux debate on page S84 of this supplement), and therefore may require revision (an update is scheduled for release in November 2009).
LIMITED ROLE FOR CORONARY REVASCULARIZATION
Until recently, no randomized trials had assessed the benefit of prophylactic coronary revascularization to reduce the perioperative risk of noncardiac surgery. The first large such trial was the Coronary Artery Revascularization Prophylaxis (CARP) study, which randomized 510 patients scheduled for major elective vascular surgery to undergo or not undergo coronary artery revascularization before the procedure.9 The study found that revascularization failed to affect any outcome measure, including mortality or the development of MI, out to 6 years of follow-up. Notably, the CARP population consisted mostly of patients with single-, double-, or mild triple-vessel coronary artery disease, so the study was limited in that it did not include patients with strong indications for coronary artery bypass graft surgery (CABG).7
A reanalysis of the CARP results by the type of revascularization procedure—CABG or percutaneous coronary intervention (PCI)—revealed that patients undergoing CABG had lower rates of death, MI, and additional revascularization procedures compared with those undergoing PCI, despite the presumably more extensive disease of the CABG recipients.10
Benefit apparently limited to left main disease
Further analysis of patients in the CARP trial who underwent coronary angiography found that one subgroup—patients with left main disease—did experience an improvement in survival with preoperative coronary revascularization.11
In a subsequent randomized pilot study, Poldermans et al found no advantage to preoperative coronary revascularization among patients with extensive ischemia who underwent major vascular surgery.12 While this study was not adequately sized to definitively address the value of preoperative revascularization in these high-risk patients, its results are consistent with those of the CARP trial.
In a retrospective cohort study of patients who underwent noncardiac surgery, Posner and colleagues found that rates of adverse cardiac outcomes among patients who had recent PCI (≤ 90 days before surgery) were similar to rates among matched controls with nonrevascularized coronary disease.13 Patients who had had remote PCI (> 90 days before surgery) had a lower risk of poor outcomes than did matched controls with nonrevascularized disease, but had a higher risk than did controls without coronary disease.13
PATIENTS WITH CORONARY STENTS: STENT TYPE AND TIME SINCE PLACEMENT ARE KEY
The lack of benefit from prophylactic PCI prior to noncardiac surgery also applies to PCI procedures that involve coronary stent placement. For instance, a propensity-score analysis found no benefit from prophylactic PCI (using stents in the vast majority of cases) in patients with coronary artery disease in terms of adverse coronary events or death following aortic surgery.14
In patients who have undergone prior PCI, noncardiac surgery poses special challenges, especially in relation to stents. Restenosis is a particular concern with the use of bare-metal stents, and development of stent thrombosis is a particular risk with the use of drug-eluting stents.15 The use of drug-eluting stents requires intensive antiplatelet therapy for at least 1 year following stent implantation to prevent stent thrombosis.16
Time interval to surgery after bare-metal stent placement
The effect of prior PCI with bare-metal stents on outcomes following noncardiac surgery was examined in a recent large retrospective study by Nuttall et al.17 The incidence of major cardiac events was found to be lowest when noncardiac surgery was performed more than 90 days after PCI with bare-metal stents. Using patients who had a greater than 90-day interval before surgery as the reference group, propensity analysis showed that performing surgery within 30 days of PCI was associated with an odds ratio of 3.6 for major cardiac events. The odds ratio was reduced to 1.6 when surgery was performed 31 to 90 days after PCI. These findings suggest that 30 days may be an ideal minimum time interval, from a risk/benefit standpoint, between PCI with bare-metal stents and noncardiac surgery.
Time interval to surgery after drug-eluting stent placement
A recent retrospective study by Rabbitts et al examined patients who had noncardiac surgery after prior PCI with drug-eluting stents, focusing on the relationship between the timing of the procedures and major cardiac events during hospitalization for the surgery.18 Although the frequency of major cardiac events was not statistically significantly associated with the time between stent placement and surgery, the frequency was lowest—3.3%—when surgery followed drug-eluting stent placement by more than 365 days (versus rates of 5.7% to 6.4% for various intervals of less than 365 days).
ACC/AHA recommendations
Timing of antiplatelet interruption
Results from a prospectively maintained Dutch registry19 are consistent with the findings reviewed above: patients who underwent noncardiac surgery less than 30 days after bare-metal stent implantation or less than 6 months after drug-eluting stent implantation (early surgery group) had a significantly elevated rate of major cardiac events compared with patients in whom the interval between stenting and noncardiac surgery was longer (late surgery group). Notably, this report also found that the rate of major cardiac events within the early surgery group was significantly higher in patients whose antiplatelet therapy was discontinued during the preoperative period than in those whose antiplatelet therapy was not stopped.19
A hypercoagulable state develops within 7 to 10 days after interruption of antiplatelet therapy, at which time the patient is vulnerable to thrombosis. In general, surgery should not proceed during this time without antiplatelet coverage.
From my perspective, giving ketorolac or aspirin the morning of surgery may be beneficial for patients whose antiplatelet therapy has been stopped 7 to 10 days previously, although no data from randomized trials exist to support this practice. Theoretically, it is reasonable to stop antiplatelet therapy 4 to 5 days before surgery in patients with an increased risk of bleeding without exposing them to the hypercoagulability that would set in if therapy were stopped earlier.
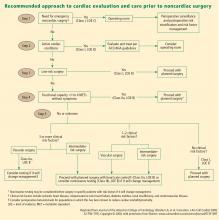
A FRAMEWORK FOR CARDIAC EVALUATION
The following are among the algorithm’s key recommendations:
- Patients requiring urgent noncardiac surgery should proceed to the operating room with perioperative surveillance (Class I, Level C).
- Patients with active cardiac conditions who are undergoing nonurgent surgery should be evaluated and treated per ACC/AHA guidelines before proceeding to the operating room is considered (Class I, Level B).
- Patients scheduled for a low-risk procedure can proceed to surgery without testing (Class I, Level B).
- Patients scheduled for intermediate-risk surgery or vascular surgery are to be assessed by functional capacity and clinical risk factors. Proceeding with planned surgery is appropriate in patients with good functional capacity (Class IIa, Level B). In patients with poor or unknown functional capacity undergoing vascular surgery who have three or more clinical risk factors, testing should be considered if the results would change management (Class IIa, Level B).
- Patients with one or more clinical risk factors undergoing intermediate-risk surgery and those with fewer than three clinical risk factors undergoing vascular surgery may proceed to planned surgery with control of heart rate to diminish the stress response perioperatively (Class IIa, Level B), or they may undergo noninvasive testing, but only if the results would change management (Class IIb, Level B).
- Patients undergoing intermediate-risk or vascular surgery who have poor or unknown functional capacity but no clinical risk factors may proceed to surgery without testing (Class I, Level B).
DISCUSSION
Question from the audience: The POISE study showed a 30% reduction in nonfatal MI with routine perioperative beta-blockade but an overall increase in mortality. Since most MIs occur immediately postoperatively and sepsis occurs a bit later, would you consider continuing beta-blocker therapy for a few days to prevent an MI but then stopping it before sepsis develops?
Dr. Fleisher: I’ve had discussions with sepsis experts about the link between beta-blocker therapy and sepsis and death in POISE, and the belief is that beta-blockers do not cause sepsis. I think that a septic patient on acute high-dose beta-blocker therapy can’t respond appropriately because of an inability to increase cardiac output. I believe we should titrate beta-blockers more closely. Information on preoperative dose titration in POISE is not available because of the way the trial was designed. Sepsis developed in only 53 of the 8,351 patients randomized in the study.
I would not start an acute beta-blocker protocol just to get a patient through surgery. I would start a perioperative hemodynamic protocol with the goal of maintaining the patient’s heart rate at lower than 80 beats per minute. Because I don’t believe that beta-blockers cause sepsis, if I initiated a beta-blocker preoperatively, I would not stop it at 2 days.
Question from the audience: Is there a time period during which a patient with a bare-metal stent could have back surgery or knee replacement surgery while not on aspirin?
Dr. Fleisher: The guidelines say that if a patient is on aspirin, it should be continued indefinitely. The issue is one of risk versus benefit. For back surgery, if bleeding is a concern, stopping aspirin for 6 or 7 days after the 30-day period following PCI is not unreasonable, but I would not stop it during the first 30 days following PCI.
Question from the audience: I don’t assess for vascular surgery but rather for the Whipple procedure [radical pancreatoduodenectomy], and I use the Revised Cardiac Risk Index to assess the number of risk factors. I believe the Whipple procedure is a high-risk operation, but it appears to be considered an intermediate-risk operation by the ACC/AHA guidelines. Is my approach to risk assessment appropriate?
Dr. Fleisher: If the rates of morbidity and mortality with the Whipple procedure are low at your institution, you might risk worsening your outcomes by applying someone else’s paradigms to your institution. There’s a big difference in risk between a surgeon who does a Whipple in 5 hours with 0.5 to 1.0 U of blood loss and a surgeon who does a 12-hour Whipple with 20 U of blood loss, necessitating a stay in the intensive care unit for multiple days. You need to consider the risk associated with your institution and specifically with the surgeon.
Question from the audience: Peripheral vascular disease is considered a coronary heart disease risk equivalent, so why is it not one of the criteria in the Revised Cardiac Risk Index?
Dr. Fleisher: The criteria are not hard and fast. The index was devised at one institution, Brigham and Women’s Hospital, in about 4,000 patients, and it has been used differently. It assigns 1 point to ischemic heart disease. It would not be inappropriate to assume that any atherosclerotic class of disease is equivalent to ischemic heart disease for risk purposes.
Question from the audience: You mentioned a 4-day window for withholding clopidogrel. Do you factor into the decision the duration of therapy? Some cardiologists go beyond the 1-year recommendation to continue clopidogrel after stenting because they believe there is still benefit.
Dr. Fleisher: The key is to confer with the cardiologist who implanted the stent, who knows the stenosis for which the stent was implanted. A problem we’ve had for years is that a practitioner will stop the antiplatelet agent without having spoken to the surgeon or the anesthesiologist. As an anesthesiologist, I need to know that someone has done a risk/benefit assessment of whether to continue antiplatelet agents in a given patient.
Question from the audience: The Revised Cardiac Risk Index of Lee et al3 includes the type of surgery in its total point system while the ACC/AHA guidelines do not. Can you explain the discrepancy?
Dr. Fleisher: We on the writing committee for the ACC/AHA 2007 perioperative guidelines made a decision to pull out the type of surgery and use the other five risk factors of Lee et al. It was a consensus of the committee because we believed that the complexity of the surgery itself is a separate consideration for risk. That’s why we included the medical risk factors and considered the surgical factors separately.
In patients undergoing noncardiac surgery, preoperative intervention for a cardiac condition is rarely needed simply to reduce the risk of the surgery unless such intervention is indicated separate from the preoperative context.
This is the overriding message of the 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery issued by the American College of Cardiology (ACC) and American Heart Association (AHA),1 for which I was privileged to chair the writing committee. This article outlines current best practices in cardiac risk stratification for noncardiac surgery, highlighting key recommendations from the ACC/AHA 2007 perioperative guidelines.
PURPOSE OF THE PREOPERATIVE CARDIAC EVALUATION
Provide clinical judgment, not clearance for surgery
A proper cardiac evaluation prior to noncardiac surgery involves a comprehensive patient assessment that draws on clinical findings, the clinical experience of the consulting physician (typically a cardiologist or internist), and an assessment of the literature. The purpose is not to give medical clearance for surgery but rather to provide informed clinical judgment to the anesthesiologist and the surgical team in terms of the following1:
- The patient’s current medical status
- Recommendations regarding the management and risk of cardiac problems during the perioperative period
- The patient’s clinical risk profile, to assist with treatment decisions that may affect short- or long-term cardiac outcomes.
Order tests only when results may change management
The consulting physician’s clinical judgment is critical in determining the need to order any specific tests. In general, a test to further define cardiac risk is valid only when its results could change the planned management and lead to a specific intervention. Potential interventions that may result from knowledge gained through testing include:
- Delaying the operation because of unstable symptoms
- Coronary revascularization
- Attempting medical optimization before surgery
- Involving additional specialists or providers in the patient’s perioperative care
- Modification of intraoperative monitoring
- Modification of postoperative monitoring
- Modification of the surgical location, particularly when the procedure is scheduled for an ambulatory surgical center.
The cardiac evaluation should result in an estimation of cardiac risk. If the consulting physician’s estimation of risk is not clearly above or below the threshold for a potential intervention, then further testing may be indicated to further define the need for interventions (ie, reaching the threshold for action).
WHAT TO WORRY ABOUT FIRST: HIGH-RISK CONDITIONS THAT REQUIRE EVALUATION AND TREATMENT
In a recommendation categorized as a Class I, Level B endorsement,* the ACC/AHA 2007 perioperative guidelines specify four active cardiac conditions for which an evaluation and treatment are required before noncardiac surgery1:
- Unstable coronary syndromes, including unstable or severe angina or recent myocardial infarction (MI). These syndromes should be the first and most important consideration. Unstable angina is a hypercoagulable state, as is recent MI. The hypercoagulability of these conditions is compounded by the hypercoagulability induced by the perioperative setting itself. As a result, the rate of perioperative MI or death in the setting of unstable angina is as high as 28%.2 In the case of unstable coronary syndromes, delaying surgery is appropriate if the risks of the surgery are deemed greater than its potential benefits.
- Decompensated heart failure, defined as New York Heart Association functional class IV disease or worsening or new-onset heart failure.
- Significant arrhythmias, defined as high-grade or Mobitz II atrioventricular block, third-degree atrioventricular heart block, symptomatic ventricular arrhythmias, supraventricular arrhythmias with uncontrolled ventricular rate, symptomatic bradycardia, and newly recognized ventricular tachycardia.
- Severe valvular disease, defined as severe aortic stenosis and symptomatic mitral stenosis.
(*The ACC/AHA 2007 perioperative guidelines make recommendations by classifying the magnitude of benefit versus risk [I = the intervention should be undertaken; IIa = the intervention is reasonable to undertake; IIb = the intervention may be considered; III = the intervention should not be undertaken] and assigning a level of supporting evidence [A = highest level of evidence; B = limited evidence; C = very limited evidence].)
CARDIAC RISK STRATIFICATION: INITIAL PATIENT ASSESSMENT
Clinical risk factors and functional capacity
The Revised Cardiac Risk Index of Lee et al3 remains the general paradigm for stratifying cardiac risk before noncardiac surgery. This validated index consists of six independent predictors of cardiac complications:
- High-risk surgery (intraperitoneal, intrathoracic, or suprainguinal vascular procedures)
- Ischemic heart disease
- History of congestive heart failure
- History of cerebrovascular disease
- Insulin therapy for diabetes mellitus
- Preoperative creatinine level greater than 2.0 mg/dL.
The more predictors a patient has, the greater the risk of perioperative complications. Thus, the Revised Cardiac Risk Index is a good tool for establishing a baseline risk level for use in determining whether a preoperative or perioperative intervention is likely to make a difference in the patient’s surgical outcome. For the purpose of the algorithmic approach to testing, the surgical procedure is not considered a risk factor. Additionally, type 2 diabetes mellitus is also considered a risk factor.
Another important determinant of risk is the patient’s functional capacity. A study of 600 patients undergoing major noncardiac procedures found that poor self-reported exercise capacity, defined as an inability to walk four blocks or climb two flights of stairs, was associated with significantly more perioperative complications than was good exercise capacity.4 Simple instruments such as the Duke Activity Status Index5 can be used to estimate the patient’s functional capacity.
Procedure-specific risk
In addition to patient-specific factors, surgery-specific cardiac risk can be important, especially in patients with more than two clinical risk factors. The ACC/AHA 2007 perioperative guidelines identify three categories of surgery-specific risk1:
- Vascular surgery (the highest-risk category and also the most extensively studied), which has been associated with cardiac morbidity rates of greater than 5% in many reports. Examples include aortic and other major vascular surgery, as well as peripheral vascular surgery.
- Intermediate-risk surgery, for which reported cardiac morbidity rates range from 1% to 5%. Examples include intraperitoneal and intrathoracic procedures, carotid endarterectomy, head and neck surgery, orthopedic surgery, and prostate surgery.
- Low-risk surgery, for which reported cardiac morbidity rates are generally below 1%. Examples include endoscopic and superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Patients undergoing these procedures do not generally require further preoperative cardiac testing.1
Of course, some variability exists within each risk level as a result of institutional differences in surgical volume and expertise as well as in preoperative evaluation and other processes of care. Endovascular surgery is considered intermediate risk from a perioperative perspective but is in the same risk category as vascular surgery from a 1-year perspective.
Risk stratification promotes good perioperative outcomes
Appropriate risk stratification can make the day of surgery among the safest times for patients undergoing outpatient procedures. A retrospective analysis of Medicare claims from the late 1990s for more than 500,000 elderly patients undergoing low-risk procedures in various outpatient settings found that the mortality rate was only 1 in 50,000 on the day of surgery but increased substantially over the following 7 days and 30 days.6 This was likely a reflection of the diligence applied to managing patient-specific risk factors before proceeding to outpatient surgery.
HEART RATE CONTROL
Chronic beta-blockade can obviate need for cardiac testing
The DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Strees Echo) II trial assessed the value of cardiac testing before major vascular surgery in intermediate-risk patients (ie, with one or two cardiac risk factors) receiving chronic beta-blocker therapy begun 7 to 30 days prior to surgery.7 Among the study’s 770 intermediate-risk patients, the primary outcome—cardiac death or MI at 30 days—was no different between those randomized to receive stress testing or no stress testing. The investigators concluded that cardiac testing can safely be omitted in intermediate-risk patients if beta-blockers are used with the aim of tight heart rate control.
Continue ongoing beta-blocker therapy, start in select high-risk patients
The ACC/AHA 2007 perioperative guidelines recommend continuing beta-blocker therapy in patients who are already receiving these agents (Class I, Level C). For patients not already taking beta-blockers, their initiation is recommended in those undergoing vascular surgery who have ischemia on preoperative testing (Class I, Level B). The guidelines designate beta-blockers as “probably” recommended (Class IIa, Level B) for several other patient subgroups with high cardiac risk, mainly in the setting of vascular surgery.1
Notably, the guidelines were written before publication of the Perioperative Ischemic Evaluation (POISE),8 which questioned the risk/benefit profile of perioperative beta-blockade in patients with or at high risk of atherosclerotic disease (see the Poldermans–Devereaux debate on page S84 of this supplement), and therefore may require revision (an update is scheduled for release in November 2009).
LIMITED ROLE FOR CORONARY REVASCULARIZATION
Until recently, no randomized trials had assessed the benefit of prophylactic coronary revascularization to reduce the perioperative risk of noncardiac surgery. The first large such trial was the Coronary Artery Revascularization Prophylaxis (CARP) study, which randomized 510 patients scheduled for major elective vascular surgery to undergo or not undergo coronary artery revascularization before the procedure.9 The study found that revascularization failed to affect any outcome measure, including mortality or the development of MI, out to 6 years of follow-up. Notably, the CARP population consisted mostly of patients with single-, double-, or mild triple-vessel coronary artery disease, so the study was limited in that it did not include patients with strong indications for coronary artery bypass graft surgery (CABG).7
A reanalysis of the CARP results by the type of revascularization procedure—CABG or percutaneous coronary intervention (PCI)—revealed that patients undergoing CABG had lower rates of death, MI, and additional revascularization procedures compared with those undergoing PCI, despite the presumably more extensive disease of the CABG recipients.10
Benefit apparently limited to left main disease
Further analysis of patients in the CARP trial who underwent coronary angiography found that one subgroup—patients with left main disease—did experience an improvement in survival with preoperative coronary revascularization.11
In a subsequent randomized pilot study, Poldermans et al found no advantage to preoperative coronary revascularization among patients with extensive ischemia who underwent major vascular surgery.12 While this study was not adequately sized to definitively address the value of preoperative revascularization in these high-risk patients, its results are consistent with those of the CARP trial.
In a retrospective cohort study of patients who underwent noncardiac surgery, Posner and colleagues found that rates of adverse cardiac outcomes among patients who had recent PCI (≤ 90 days before surgery) were similar to rates among matched controls with nonrevascularized coronary disease.13 Patients who had had remote PCI (> 90 days before surgery) had a lower risk of poor outcomes than did matched controls with nonrevascularized disease, but had a higher risk than did controls without coronary disease.13
PATIENTS WITH CORONARY STENTS: STENT TYPE AND TIME SINCE PLACEMENT ARE KEY
The lack of benefit from prophylactic PCI prior to noncardiac surgery also applies to PCI procedures that involve coronary stent placement. For instance, a propensity-score analysis found no benefit from prophylactic PCI (using stents in the vast majority of cases) in patients with coronary artery disease in terms of adverse coronary events or death following aortic surgery.14
In patients who have undergone prior PCI, noncardiac surgery poses special challenges, especially in relation to stents. Restenosis is a particular concern with the use of bare-metal stents, and development of stent thrombosis is a particular risk with the use of drug-eluting stents.15 The use of drug-eluting stents requires intensive antiplatelet therapy for at least 1 year following stent implantation to prevent stent thrombosis.16
Time interval to surgery after bare-metal stent placement
The effect of prior PCI with bare-metal stents on outcomes following noncardiac surgery was examined in a recent large retrospective study by Nuttall et al.17 The incidence of major cardiac events was found to be lowest when noncardiac surgery was performed more than 90 days after PCI with bare-metal stents. Using patients who had a greater than 90-day interval before surgery as the reference group, propensity analysis showed that performing surgery within 30 days of PCI was associated with an odds ratio of 3.6 for major cardiac events. The odds ratio was reduced to 1.6 when surgery was performed 31 to 90 days after PCI. These findings suggest that 30 days may be an ideal minimum time interval, from a risk/benefit standpoint, between PCI with bare-metal stents and noncardiac surgery.
Time interval to surgery after drug-eluting stent placement
A recent retrospective study by Rabbitts et al examined patients who had noncardiac surgery after prior PCI with drug-eluting stents, focusing on the relationship between the timing of the procedures and major cardiac events during hospitalization for the surgery.18 Although the frequency of major cardiac events was not statistically significantly associated with the time between stent placement and surgery, the frequency was lowest—3.3%—when surgery followed drug-eluting stent placement by more than 365 days (versus rates of 5.7% to 6.4% for various intervals of less than 365 days).
ACC/AHA recommendations
Timing of antiplatelet interruption
Results from a prospectively maintained Dutch registry19 are consistent with the findings reviewed above: patients who underwent noncardiac surgery less than 30 days after bare-metal stent implantation or less than 6 months after drug-eluting stent implantation (early surgery group) had a significantly elevated rate of major cardiac events compared with patients in whom the interval between stenting and noncardiac surgery was longer (late surgery group). Notably, this report also found that the rate of major cardiac events within the early surgery group was significantly higher in patients whose antiplatelet therapy was discontinued during the preoperative period than in those whose antiplatelet therapy was not stopped.19
A hypercoagulable state develops within 7 to 10 days after interruption of antiplatelet therapy, at which time the patient is vulnerable to thrombosis. In general, surgery should not proceed during this time without antiplatelet coverage.
From my perspective, giving ketorolac or aspirin the morning of surgery may be beneficial for patients whose antiplatelet therapy has been stopped 7 to 10 days previously, although no data from randomized trials exist to support this practice. Theoretically, it is reasonable to stop antiplatelet therapy 4 to 5 days before surgery in patients with an increased risk of bleeding without exposing them to the hypercoagulability that would set in if therapy were stopped earlier.
A FRAMEWORK FOR CARDIAC EVALUATION
The following are among the algorithm’s key recommendations:
- Patients requiring urgent noncardiac surgery should proceed to the operating room with perioperative surveillance (Class I, Level C).
- Patients with active cardiac conditions who are undergoing nonurgent surgery should be evaluated and treated per ACC/AHA guidelines before proceeding to the operating room is considered (Class I, Level B).
- Patients scheduled for a low-risk procedure can proceed to surgery without testing (Class I, Level B).
- Patients scheduled for intermediate-risk surgery or vascular surgery are to be assessed by functional capacity and clinical risk factors. Proceeding with planned surgery is appropriate in patients with good functional capacity (Class IIa, Level B). In patients with poor or unknown functional capacity undergoing vascular surgery who have three or more clinical risk factors, testing should be considered if the results would change management (Class IIa, Level B).
- Patients with one or more clinical risk factors undergoing intermediate-risk surgery and those with fewer than three clinical risk factors undergoing vascular surgery may proceed to planned surgery with control of heart rate to diminish the stress response perioperatively (Class IIa, Level B), or they may undergo noninvasive testing, but only if the results would change management (Class IIb, Level B).
- Patients undergoing intermediate-risk or vascular surgery who have poor or unknown functional capacity but no clinical risk factors may proceed to surgery without testing (Class I, Level B).
DISCUSSION
Question from the audience: The POISE study showed a 30% reduction in nonfatal MI with routine perioperative beta-blockade but an overall increase in mortality. Since most MIs occur immediately postoperatively and sepsis occurs a bit later, would you consider continuing beta-blocker therapy for a few days to prevent an MI but then stopping it before sepsis develops?
Dr. Fleisher: I’ve had discussions with sepsis experts about the link between beta-blocker therapy and sepsis and death in POISE, and the belief is that beta-blockers do not cause sepsis. I think that a septic patient on acute high-dose beta-blocker therapy can’t respond appropriately because of an inability to increase cardiac output. I believe we should titrate beta-blockers more closely. Information on preoperative dose titration in POISE is not available because of the way the trial was designed. Sepsis developed in only 53 of the 8,351 patients randomized in the study.
I would not start an acute beta-blocker protocol just to get a patient through surgery. I would start a perioperative hemodynamic protocol with the goal of maintaining the patient’s heart rate at lower than 80 beats per minute. Because I don’t believe that beta-blockers cause sepsis, if I initiated a beta-blocker preoperatively, I would not stop it at 2 days.
Question from the audience: Is there a time period during which a patient with a bare-metal stent could have back surgery or knee replacement surgery while not on aspirin?
Dr. Fleisher: The guidelines say that if a patient is on aspirin, it should be continued indefinitely. The issue is one of risk versus benefit. For back surgery, if bleeding is a concern, stopping aspirin for 6 or 7 days after the 30-day period following PCI is not unreasonable, but I would not stop it during the first 30 days following PCI.
Question from the audience: I don’t assess for vascular surgery but rather for the Whipple procedure [radical pancreatoduodenectomy], and I use the Revised Cardiac Risk Index to assess the number of risk factors. I believe the Whipple procedure is a high-risk operation, but it appears to be considered an intermediate-risk operation by the ACC/AHA guidelines. Is my approach to risk assessment appropriate?
Dr. Fleisher: If the rates of morbidity and mortality with the Whipple procedure are low at your institution, you might risk worsening your outcomes by applying someone else’s paradigms to your institution. There’s a big difference in risk between a surgeon who does a Whipple in 5 hours with 0.5 to 1.0 U of blood loss and a surgeon who does a 12-hour Whipple with 20 U of blood loss, necessitating a stay in the intensive care unit for multiple days. You need to consider the risk associated with your institution and specifically with the surgeon.
Question from the audience: Peripheral vascular disease is considered a coronary heart disease risk equivalent, so why is it not one of the criteria in the Revised Cardiac Risk Index?
Dr. Fleisher: The criteria are not hard and fast. The index was devised at one institution, Brigham and Women’s Hospital, in about 4,000 patients, and it has been used differently. It assigns 1 point to ischemic heart disease. It would not be inappropriate to assume that any atherosclerotic class of disease is equivalent to ischemic heart disease for risk purposes.
Question from the audience: You mentioned a 4-day window for withholding clopidogrel. Do you factor into the decision the duration of therapy? Some cardiologists go beyond the 1-year recommendation to continue clopidogrel after stenting because they believe there is still benefit.
Dr. Fleisher: The key is to confer with the cardiologist who implanted the stent, who knows the stenosis for which the stent was implanted. A problem we’ve had for years is that a practitioner will stop the antiplatelet agent without having spoken to the surgeon or the anesthesiologist. As an anesthesiologist, I need to know that someone has done a risk/benefit assessment of whether to continue antiplatelet agents in a given patient.
Question from the audience: The Revised Cardiac Risk Index of Lee et al3 includes the type of surgery in its total point system while the ACC/AHA guidelines do not. Can you explain the discrepancy?
Dr. Fleisher: We on the writing committee for the ACC/AHA 2007 perioperative guidelines made a decision to pull out the type of surgery and use the other five risk factors of Lee et al. It was a consensus of the committee because we believed that the complexity of the surgery itself is a separate consideration for risk. That’s why we included the medical risk factors and considered the surgical factors separately.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol 2008; 52:794–797]. J Am Coll Cardiol 2007; 50:1707–1732.
- Shah KB, Kleinman BS, Rao TLK, Jacobs HK, Mestan K, Schaafsma M. Angina and other risk factors in patients with cardiac diseases undergoing noncardiac operations. Anesth Analg 1990; 70:240–247.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Reilly DF, McNeely MJ, Doerner D, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med 1999; 159:2185–2192.
- Nelson CL, Herndon JE, Mark DB, et al. Relation of clinical and angiographic factors to functional capacity as measured by the Duke Activity Status Index. Am J Cardiol 1991; 68:973–975.
- Fleisher LA, Pasternak LR, Herbert R, Anderson GF. Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg 2004; 139:67–72.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- POISE Study Group, Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomized controlled trial. Lancet 2008; 371:1839–1847.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
- Ward HB, Kelly RF, Thottapurathu L, et al. Coronary artery bypass grafting is superior to percutaneous coronary intervention in prevention of perioperative myocardial infarctions during subsequent vascular surgery. Ann Thorac Surg 2006; 82:795–801.
- Garcia S, Moritz TE, Ward HB, et al. Usefulness of revascularization of patients with multivessel coronary artery disease before elective vascular surgery for abdominal aortic and peripheral occlusive disease. Am J Cardiol 2008; 102:809–813.
- Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol 2007; 49:1763–1769.
- Posner KL, Van Norman GA, Chan V. Adverse cardiac outcomes after noncardiac surgery in patients with prior percutaneous transluminal coronary angioplasty. Anesth Analg 1999; 89:553–560.
- Godet G, Riou B, Bertrand M, et al. Does preoperative coronary angioplasty improve perioperative cardiac outcome? Anesthesiology 2005; 102:739–746.
- Shuchman M. Debating the risks of drug-eluting stents. N Engl J Med 2007; 356:325–328.
- King SB III, Smith SC Jr, Hirshfeld JW Jr, et al. ACC/AHA/SCAI. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Nuttall GA, Brown MJ, Stombaugh JW, et al. Time and cardiac risk of surgery after bare-metal stent percutaneous coronary intervention. Anesthesiology 2008; 109:588–595.
- Rabbitts JA, Nuttall GA, Brown MJ, et al. Cardiac risk of noncardiac surgery after percutaneous coronary intervention with drug-eluting stents. Anesthesiology 2008; 109:596–604.
- Schouten O, van Domburg RT, Bax JJ, et al. Noncardiac surgery after coronary stenting: early surgery and interruption of antiplatelet therapy are associated with an increase in major adverse cardiac events. J Am Coll Cardiol 2007; 49:122–124.
- Correction to Fleisher et al, J Am Coll Cardiol 2007; 50:1707–1732. J Am Coll Cardiol 2008; 52:794–797.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol 2008; 52:794–797]. J Am Coll Cardiol 2007; 50:1707–1732.
- Shah KB, Kleinman BS, Rao TLK, Jacobs HK, Mestan K, Schaafsma M. Angina and other risk factors in patients with cardiac diseases undergoing noncardiac operations. Anesth Analg 1990; 70:240–247.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Reilly DF, McNeely MJ, Doerner D, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med 1999; 159:2185–2192.
- Nelson CL, Herndon JE, Mark DB, et al. Relation of clinical and angiographic factors to functional capacity as measured by the Duke Activity Status Index. Am J Cardiol 1991; 68:973–975.
- Fleisher LA, Pasternak LR, Herbert R, Anderson GF. Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg 2004; 139:67–72.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- POISE Study Group, Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomized controlled trial. Lancet 2008; 371:1839–1847.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
- Ward HB, Kelly RF, Thottapurathu L, et al. Coronary artery bypass grafting is superior to percutaneous coronary intervention in prevention of perioperative myocardial infarctions during subsequent vascular surgery. Ann Thorac Surg 2006; 82:795–801.
- Garcia S, Moritz TE, Ward HB, et al. Usefulness of revascularization of patients with multivessel coronary artery disease before elective vascular surgery for abdominal aortic and peripheral occlusive disease. Am J Cardiol 2008; 102:809–813.
- Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol 2007; 49:1763–1769.
- Posner KL, Van Norman GA, Chan V. Adverse cardiac outcomes after noncardiac surgery in patients with prior percutaneous transluminal coronary angioplasty. Anesth Analg 1999; 89:553–560.
- Godet G, Riou B, Bertrand M, et al. Does preoperative coronary angioplasty improve perioperative cardiac outcome? Anesthesiology 2005; 102:739–746.
- Shuchman M. Debating the risks of drug-eluting stents. N Engl J Med 2007; 356:325–328.
- King SB III, Smith SC Jr, Hirshfeld JW Jr, et al. ACC/AHA/SCAI. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Nuttall GA, Brown MJ, Stombaugh JW, et al. Time and cardiac risk of surgery after bare-metal stent percutaneous coronary intervention. Anesthesiology 2008; 109:588–595.
- Rabbitts JA, Nuttall GA, Brown MJ, et al. Cardiac risk of noncardiac surgery after percutaneous coronary intervention with drug-eluting stents. Anesthesiology 2008; 109:596–604.
- Schouten O, van Domburg RT, Bax JJ, et al. Noncardiac surgery after coronary stenting: early surgery and interruption of antiplatelet therapy are associated with an increase in major adverse cardiac events. J Am Coll Cardiol 2007; 49:122–124.
- Correction to Fleisher et al, J Am Coll Cardiol 2007; 50:1707–1732. J Am Coll Cardiol 2008; 52:794–797.
KEY POINTS
- In addition to patient-specific factors, preoperative cardiac assessment should account for the risk of cardiac morbidity related to the procedure itself. Vascular surgery confers the highest risk, with reported rates of cardiac morbidity often greater than 5%.
- Continuation of chronic beta-blocker therapy is prudent during the perioperative period.
- Coronary revascularization prior to noncardiac surgery is generally indicated only in unstable patients and in patients with left main disease.
- Nonurgent noncardiac surgery should be delayed for at least 30 days after PCI using a bare-metal stent and for at least 365 days after PCI using a drug-eluting stent.
- Discontinuing antiplatelet therapy in patients with coronary stents may induce a hypercoagulable state within approximately 7 to 10 days.