User login
Detecting the other reflux disease
• Suspect laryngopharyngeal reflux (LPR) in a patient with chronic laryngitis; 50% to 60% of such cases are related to LPR. B
• Refer patients with risk factors for head and neck cancer or whose symptoms persist despite lifestyle modification and medical management to an otolaryngologist. A
• While symptoms of LPR should show improvement after 6 to 8 weeks of proton pump inhibitor therapy, advise patients to continue treatment for 4 to 6 months to ensure that laryngeal lesions and edema resolve. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laryngopharyngeal reflux (LPR), the retrograde movement of gastric content into the upper aerodigestive tract, is a common—and commonly underdiagnosed—condition. Characterized by inflammation of the laryngopharynx, LPR can coexist with gastroesophageal reflux disease (GERD), but it is a distinct disorder.1 In GERD, the lower esophageal sphincter malfunctions, whereas LPR involves a dysfunctional upper esophageal sphincter.
Because both conditions involve acid reflux, LPR is sometimes mistaken for GERD. Often, too, patients and physicians alike attribute LPR’s signs and symptoms, which are largely nonspecific, to other causes. The hoarseness and laryngitis that are characteristic of LPR may be blamed on vocal cord abuse or smoking, for instance; the chronic cough and throat clearing associated with LPR thought to be caused by allergies; and the sore throat and postnasal drip that often accompany LPR attributed to infection. Another reason LPR is underdiagnosed: Primary care physicians, who are often the first clinicians from whom symptomatic patients seek treatment, are often unfamiliar with this lesser-known reflux disease.2
The failure to recognize and provide timely treatment for LPR may increase patients’ risk for a number of conditions, including laryngeal ulcers, granulomas, subglottic stenosis, chronic sinusitis, laryngospasm, nasal congestion, and asthma.1 Evidence suggests that LPR increases the risk for esophageal and laryngeal carcinomas,3,4 and for laryngeal injury from intubation, as well.1 To minimize these risks, it is important for primary care physicians to promptly identify this disorder, treat it appropriately, and recognize red flags that warrant referral to a specialist.
How LPR develops, what to look for
There is no gold standard for the diagnosis of LPR. Nonetheless, a review of the pathophysiology and clinical presentation of this reflux disorder and the ways in which it differs from GERD will help you identify cases of LPR. The prevalence of LPR in the general population is uncertain. But reports suggest that as many as 10% of otolaryngology referrals are for patients with a classic presentation of LPR, and that 50% to 60% of cases of chronic laryngitis are related to LPR.1,5,6
The laryngopharynx becomes irritated and inflamed
When the physiological barriers protecting the laryngopharynx from the retrograde flow of gastric content break down, gastric contents can directly irritate the ciliated columnar epithelial cells of the upper respiratory tract, leading to ciliary dysfunction. A lack of mucous clearance leads to mucous stasis and, subsequently, to excessive throat clearing and the sensation of postnasal drip.7 In addition, the laryngopharyngeal epithelium becomes inflamed, and this affects the sensitivity of laryngeal sensory endings and leads to laryngospasm and coughing.8 The inflammatory reaction in turn leads to vocal fold edema, contact ulcers, and granulomas. These changes make patients with LPR particularly prone to developing hoarseness, globus pharyngeus—a sensation of a foreign body in the larynx—and sore throat.5,7 The gastric content can also act indirectly by initiating laryngeal reflexes through irritation of the esophagus, leading to vagally mediated changes such as chronic cough and bronchoconstriction.
Enzyme production declines. Under normal circumstances, carbonic anhydrase isoenzyme III (CAIII) is produced in the posterior aspect of the larynx, catalyzing the production of bicarbonate and neutralizing stomach acid.9-11 In LPR, however, the production of CAIII decreases significantly, thereby exposing the larynx to stomach acid without the enzyme’s protective effect.9,10 At the same time, a marked increase in pepsin levels intensifies laryngeal injury.10,12,13
The larynx is highly vulnerable. The laryngopharynx is much more susceptible to pathology from gastric reflux than the esophagus, for a number of reasons. Damage can occur with much less exposure to acid,1 not only because of the decrease in CAIII, but also because of the absence of peristalsis in the larynx.
What’s more, the esophagus has the ability to clear gastric reflux and minimize damage to the epithelial layer.9,10,14,15 In most patients who develop signs and symptoms of LPR, there has been enough gastric reflux to damage the laryngopharynx but not enough to overcome the protective mechanisms of the esophagus. That’s why most LPR patients have little or none of the heartburn and esophagitis that are classic symptoms of GERD.
Common signs and symptoms that signal LPR
LPR is primarily a clinical diagnosis based on signs and symptoms—which are also used to rule out GERD. Notably, less than 50% of patients with LPR suffer from heartburn and regurgitation.16 Those who do have heartburn and regurgitation typically suffer with reflux during the day, when they’re in an upright position, whereas reflux associated with GERD develops primarily at night.16 The results of a recent survey of members of the American Bronchoesophagological Association highlight the most common signs and symptoms of LPR, listed below from the most to the least frequent:17
- throat clearing
- persistent cough
- globus sensation
- hoarseness
- choking episodes.
Additional signs and symptoms include excessive and chronic throat clearing, sore throat, postnasal drip, and dysphagia.
Use a validated symptom index. To further assess the probability and severity of LPR, use the Reflux Symptom Index18 (TABLE 1). A recent cohort study validated the index, with an average score of 21.2 for those with LPR, vs an average of 11.6 for controls (P<.001). A score >13 is suggestive of LPR (odds ratio=9.19), the researchers found.18
If the diagnosis remains uncertain and the patient continues to be troubled by signs and symptoms suggestive of LPR, refer him or her to an otolaryngologist for further investigation. A referral is needed, too, to rule out malignancy in any patient with 3 or more of the following red flags: older than 50 years, otalgia, weight loss, progressive hoarseness, neck mass, a significant history of alcohol use, and a history of smoking.7
TABLE 1
The Reflux Symptom Index for laryngopharyngeal reflux
| Within the last month, how did the following problems affect you? | 0 = NO PROBLEM 5 = SEVERE PROBLEM (CIRCLE THE APPROPRIATE RESPONSE) | |||||
|---|---|---|---|---|---|---|
| 1. Hoarseness or a problem with your voice | 0 | 1 | 2 | 3 | 4 | 5 |
| 2. Clearing your throat | 0 | 1 | 2 | 3 | 4 | 5 |
| 3. Excess throat mucus or postnasal drip | 0 | 1 | 2 | 3 | 4 | 5 |
| 4. Difficulty swallowing food, liquids, or pills | 0 | 1 | 2 | 3 | 4 | 5 |
| 5. Coughing after you ate or after lying down | 0 | 1 | 2 | 3 | 4 | 5 |
| 6. Breathing difficulties or choking episodes | 0 | 1 | 2 | 3 | 4 | 5 |
| 7. Troublesome or annoying cough | 0 | 1 | 2 | 3 | 4 | 5 |
| 8. Sensations of something sticking in your throat or a lump in your throat | 0 | 1 | 2 | 3 | 4 | 5 |
| 9. Heartburn, chest pain, indigestion, or stomach acid coming up | 0 | 1 | 2 | 3 | 4 | 5 |
| TOTAL SCORE= | ||||||
| *A score >13 is considered suggestive of laryngopharyngeal reflux. | ||||||
| Source: Belafsky PC et al. J Voice. 2002.18 Reprinted with permission. | ||||||
Diagnostic tools the specialists will use
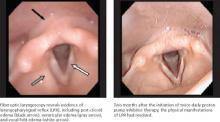
Fiberoptic laryngoscopy is the most common test used by otolaryngologists to confirm LPR and rule out other pathology. The test reveals inflammatory findings (FIGURE), such as erythema, edema, granulomas, and contact ulcers, in several anatomical locations of the larynx—especially the posterior aspect and the true vocal folds. It is important to note, however, that as many as 70% of the general population will have some laryngeal inflammation, so these findings alone are not definitive evidence of LPR.7
FIGURE
Fiberoptic laryngoscopy: Before and after treatment
Ambulatory 24-hour dual sensor pH probe monitoring is sometimes used as an adjunctive test to confirm LPR. In 2005, 2 meta-analyses found that the pH probe is reliable and sensitive and specific enough to identify significantly more acid reflux in patients with LPR than in controls.19,20 However, not everyone agrees: Some clinicians question its use as a diagnostic tool for LPR, citing problems with observer reliability, among other things. Because it increases costs to the patient and is impractical, pH monitoring is not widely used by specialists, but primarily as a research tool.21,22
Barium swallow and esophagogastroduodenoscopy (EGD) are relatively common diagnostic tools used to identify anatomical abnormalities in the gastrointestinal tract, such as a Schatzki’s ring or hiatal hernia, that can lead to symptoms of GERD and/or LPR. Some studies suggest that all patients with symptoms of LPR should undergo EGD to screen for esophageal adenocarcinoma.23,24 Because LPR symptoms are relatively common, however, many clinicians believe that EGD should be considered only when heartburn is a primary complaint in a patient with signs and symptoms of LPR—or when a patient believed to have LPR fails to respond to medical management.24
Treating LPR: Lifestyle changes, drug therapy
For all patients with LPR, dietary and lifestyle modifications have been shown to be both clinically effective and cost effective.25 In addition to dietary restrictions (TABLE 2), advise patients to avoid eating too rapidly or drinking large quantities of fluid. Late night meals—indeed, eating within 3 hours of bedtime—should also be avoided, as should heavy lunches and dinners. Tell patients to eat small, frequent meals instead.7,25,26
Recommend other behavioral changes, as well. Tell patients to avoid tight clothing, lying down immediately after a meal, and applying pressure to the abdomen, whether through exercise, heavy lifting, singing, or bending over. Smoking and overuse (or misuse) of the voice—screaming at a concert or singing for hours, for instance—are contraindicated, as well.7,25,26
Tell patients that weight loss, as needed, is likely to bring some symptom relief. Using wooden blocks to elevate the head of the bed about 4 to 6 inches may also be helpful, particularly for patients who suffer from both LPR and GERD.7,25,26
TABLE 2
Dietary management of LPR: What to tell patients7,14,24
| Avoid* | Enjoy† |
|---|---|
| Caffeine | Meat |
| Alcohol | Poultry |
| Spicy foods | Seafood |
| Tomatoes | Milk |
| Chocolate | Fresh vegetables‡ |
| Fats | |
| Citrus fruits | |
| Carbonated beverages | |
| Jams/jellies | |
| Barbecue sauces | |
| Salad dressings | |
| Hot mustard | |
| Curry | |
| Hot peppers | |
| *Other acidic foods. | |
| †Other foods and beverages that are neither spicy nor acidic. | |
| ‡ Except tomatoes. | |
Drug therapy: Straightforward, but not without controversy
Acid suppression with proton pump inhibitors (PPIs) is the primary treatment for LPR, as it is for GERD. But because the larynx is extremely susceptible to injury from acid reflux, LPR typically requires more aggressive and prolonged treatment, compared with GERD.1,5
Clinical trials have shown that PPIs do not inhibit acid production to an intragastric pH of >4 for more than 16.8 hours.1,27 Thus, most patients need twice-daily dosing (although once-a-day dosing or conservative management may be sufficient for those with mild and intermittent symptoms).27,28 Regardless of dosing, PPIs should be taken on an empty stomach, 30 minutes before a meal to increase bioavailability. For maximum benefits, patients should continue the twice-daily regimen for 4 to 6 months, although the optimal duration is unknown.26
One study found 4 months of therapy to be effective;28 others suggest that while symptom relief should begin after 6 to 8 weeks of treatment, 6 months of PPI therapy is needed for laryngeal lesions and edema to resolve.1,8 Despite the time frame, patients should be weaned gradually to prevent the delayed rebound effect associated with abrupt cessation of PPIs.
The PPI controversy. Not only the length of treatment is controversial, however, but the efficacy of PPIs for LPR. Many studies, including several prospective cohort studies and 9 RCTs, have reported significant improvement in laryngeal symptoms, but evidence that PPIs are significantly better than placebo is weak.25,29,30 In fact, a systematic review and 2 meta-analyses concluded that not only is there a lack of sufficient evidence to draw reliable conclusions about the efficacy of PPIs vs placebo for the treatment of LPR, but there seems to be a significant response to placebo among patients with this condition, as well.25,29,30
The role of adjunctive therapy. Histamine type 2 (H2) blockers have been shown to be helpful in the treatment of GERD. But data showing their efficacy for LPR, either as a single agent or in combination with a PPI, are limited. Indeed, 3 clinical trials have found that H2 blockers do not provide any added benefit to PPI therapy for LPR. All 3 were cohort studies that compared the treatment outcomes of PPI alone vs PPI and H2 blockers, and found no statistically significant difference (P>.05).28,31,32 Despite these findings, recent studies suggest that 300 mg ranitidine twice a day provides added benefit (P<.01).33,34 Given these mixed findings, H2 blockers may be considered as adjuvant therapy to the PPI regimen to further reduce acid production in patients with more severe symptoms. Ant-acids and prokinetic agents are sometimes used for this purpose, as well.
When medical management fails
Surgery has a limited, but useful, role in the treatment of LPR.
Nissen fundoplication—a procedure in which the fundus of the stomach is passed posteriorly behind the esophagus to encircle it and provide mechanical obstruction to the retrograde movement of acid—may be considered for patients with a confirmed diagnosis, severe symptoms, and little response to treatment. However, there is little evidence that this procedure will result in long-term improvement in LPR symptoms. Laryngeal surgery can be used to treat vocal fold sequelae of LPR, such as granulomas—with a higher likelihood of success.35
CORRESPONDENCE Kevin Fung, MD, FRCSC, FACS, University of Western Ontario, London Health Sciences Center-Victoria Hospital, 800 Commissioners Road East, Room B3-427, London, Ontario, Canada, N6A 4G5; [email protected]
1. Koufman JA, Aviv JE, Casiano RR, et al. Laryngopharyngeal reflux: position statement of the Committee on Speech, Voice, and Swallowing Disorders of the American Academy of Otolaryngology–Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32-35.
2. Karkos PD, Thomas L, Temple RH, et al. Awareness of general practitioners towards treatment of laryngopharyngeal reflux: a British survey. Otolaryngol Head Neck Surg. 2005;133:505-508.
3. Morrison MD. Is chronic gastroesophageal reflux a causative factor in glottic carcinoma? Otolaryngol Head Neck Surg. 1988;99:370-373.
4. Ward PH, Hanson DG. Reflux as an etiologic factor of carcinoma of the laryngopharynx. Laryngoscope. 1988;98:1195-1199.
5. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101(4 pt 2 suppl 53):S1-S78.
6. Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg. 2000;123:385-388.
7. Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA. 2005;294:1534-1540.
8. Aviv JE, Liu H, Parides M, et al. Laryngopharyngeal sensory deficits in patients with laryngopharyngeal reflux and dysphagia. Ann Otol Rhinol Laryngol. 2000;109:1000-1006.
9. Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112:481.-
10. Gill GA, Johnston N, Buda A, et al. Laryngeal epithelial defenses against laryngopharyngeal reflux: investigations of E-cadherin, carbonic anhydrase isoenzyme III, and pepsin. Ann Otol Rhinol Laryngol. 2005;114:913-921.
11. Okamura H, Sugai N, Kanno T, et al. Histochemical localization of carbonic anhydrase in the trachea of the guinea pig. Histochem Cell Biol. 1996;106:257-260.
12. Johnston N, Knight J, Dettmar PW, et al. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope. 2004;114:2129-2134.
13. Johnston N, Dettmar PW, Bishwokarma B, et al. Activity/stability of human pepsin: implications for reflux attributed laryngeal disease. Laryngoscope. 2007;117:1036-1039.
14. Axford SE, Sharp N, Ross PE, et al. Cell biology of laryngeal epithelial defenses in health and disease: preliminary studies. Ann Otol Rhinol Laryngol. 2001;110:1099-1108.
15. Toros SZ, Toros AB, Yüksel OD, et al. Association of laryngopharyngeal manifestations and gastroesophageal reflux. Eur Arch Otorhinolaryngol. 2009;266:403-409.
16. Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease Ear Nose Throat J. 2002;81(9 suppl 2):S7-S9.
17. Book DT, Rhee JS, Toohill RJ, et al. Perspectives in laryngopharyngeal reflux: an international survey. Laryngoscope. 2002;112(8 Pt 1):1399-1406.
18. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the Reflux Symptom Index (RSI). J Voice. 2002;16:274-277.
19. Ulualp SO, Roland PS, Toohill RJ, et al. Prevalence of gastroesophagopharyngeal acid reflux events: an evidence-based systematic review. Am J Otolaryngol. 2005;26:239-244.
20. Merati AL, Lim HJ, Ulualp SO, et al. Meta-analysis of upper probe measurements in normal subjects and patients with laryngopharyngeal reflux. Ann Otol Rhinol Laryngol. 2005;114(3):177-82.
21. Vaezi MF, Schroeder PL, Richter JE. Reproducibility of proximal probe pH parameters in 24-hour ambulatory esophageal pH monitoring. Am J Gastroenterol. 1997;92:825-829.
22. Vincent DA, Jr., Garrett JD, Radionoff SL, et al. The proximal probe in esophageal pH monitoring: development of a normative database. J Voice. 2000;142:247-254.
23. Reavis KM, Morris CD, Gopal DV, et al. Laryngopharyngeal reflux symptoms better predict the presence of esophageal adenocarcinoma than typical gastroesophageal reflux symptoms. Ann Surg. 2004;239:849-858.
24. Gupta R, Sataloff RT. Laryngopharyngeal reflux: current concepts and questions. Curr Opin Otolaryngol Head Neck Surg. 2009;17:143-148.
25. Tsunoda K, Ishimoto S, Suzuki M, et al. An effective management regimen for laryngeal granuloma caused by gastro-esophageal reflux: combination therapy with suggestions for lifestyle modifications. Acta Otolaryngol. 2007;127:88-92.
26. Hopkins C, Yousaf U, Pedersen M. Acid reflux treatment for hoarseness [protocol]. Cochrane Database Syst Rev. 2006;(1):CD005054.-
27. Kahrilas PJ, Falk GW, Johnson DA, et al. The Esomeprazole Study Investigators Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. Aliment Pharmacol Ther. 2000;14:1249-1458.
28. Park W, Hicks DM, Khandwala F, et al. Laryngopharyngeal reflux: prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope. 2005;115:1230-1238.
29. Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101:2646-2654.
30. Karkos PD, Wilson JA. Empiric treatment of laryngopharyngeal reflux with proton pump inhibitors: a systematic review. Laryngoscope. 2006;116:144-148.
31. Fackler WK, Ours TM, Vaezi MF, et al. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology. 2002;122:625-632.
32. Ours TM, Fackler WK, Richter JE, et al. Nocturnal acid breakthrough: clinical significance and correlation with esophageal acid exposure. Am J Gastroenterol. 2003;98:545-550.
33. Sato K. Laryngopharyngeal reflux disease with nocturnal gastric acid breakthrough while on proton pump inhibitor therapy. Eur Arch Otorhinolaryngol. 2006;263:1121-1126.
34. Mainie I, Tutuian R, Castell DO. Addition of a H2 receptor antagonist to PPI improves acid control and decreases nocturnal acid breakthrough. J Clin Gastroenterol. 2008;42:676-679.
35. Koufman JA, Rees CJ, Frazier WD, et al. Office-based laryngeal laser surgery: a review of 443 cases using three wavelengths. Otolaryngol Head Neck Surg. 2007;137:146-151.
• Suspect laryngopharyngeal reflux (LPR) in a patient with chronic laryngitis; 50% to 60% of such cases are related to LPR. B
• Refer patients with risk factors for head and neck cancer or whose symptoms persist despite lifestyle modification and medical management to an otolaryngologist. A
• While symptoms of LPR should show improvement after 6 to 8 weeks of proton pump inhibitor therapy, advise patients to continue treatment for 4 to 6 months to ensure that laryngeal lesions and edema resolve. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laryngopharyngeal reflux (LPR), the retrograde movement of gastric content into the upper aerodigestive tract, is a common—and commonly underdiagnosed—condition. Characterized by inflammation of the laryngopharynx, LPR can coexist with gastroesophageal reflux disease (GERD), but it is a distinct disorder.1 In GERD, the lower esophageal sphincter malfunctions, whereas LPR involves a dysfunctional upper esophageal sphincter.
Because both conditions involve acid reflux, LPR is sometimes mistaken for GERD. Often, too, patients and physicians alike attribute LPR’s signs and symptoms, which are largely nonspecific, to other causes. The hoarseness and laryngitis that are characteristic of LPR may be blamed on vocal cord abuse or smoking, for instance; the chronic cough and throat clearing associated with LPR thought to be caused by allergies; and the sore throat and postnasal drip that often accompany LPR attributed to infection. Another reason LPR is underdiagnosed: Primary care physicians, who are often the first clinicians from whom symptomatic patients seek treatment, are often unfamiliar with this lesser-known reflux disease.2
The failure to recognize and provide timely treatment for LPR may increase patients’ risk for a number of conditions, including laryngeal ulcers, granulomas, subglottic stenosis, chronic sinusitis, laryngospasm, nasal congestion, and asthma.1 Evidence suggests that LPR increases the risk for esophageal and laryngeal carcinomas,3,4 and for laryngeal injury from intubation, as well.1 To minimize these risks, it is important for primary care physicians to promptly identify this disorder, treat it appropriately, and recognize red flags that warrant referral to a specialist.
How LPR develops, what to look for
There is no gold standard for the diagnosis of LPR. Nonetheless, a review of the pathophysiology and clinical presentation of this reflux disorder and the ways in which it differs from GERD will help you identify cases of LPR. The prevalence of LPR in the general population is uncertain. But reports suggest that as many as 10% of otolaryngology referrals are for patients with a classic presentation of LPR, and that 50% to 60% of cases of chronic laryngitis are related to LPR.1,5,6
The laryngopharynx becomes irritated and inflamed
When the physiological barriers protecting the laryngopharynx from the retrograde flow of gastric content break down, gastric contents can directly irritate the ciliated columnar epithelial cells of the upper respiratory tract, leading to ciliary dysfunction. A lack of mucous clearance leads to mucous stasis and, subsequently, to excessive throat clearing and the sensation of postnasal drip.7 In addition, the laryngopharyngeal epithelium becomes inflamed, and this affects the sensitivity of laryngeal sensory endings and leads to laryngospasm and coughing.8 The inflammatory reaction in turn leads to vocal fold edema, contact ulcers, and granulomas. These changes make patients with LPR particularly prone to developing hoarseness, globus pharyngeus—a sensation of a foreign body in the larynx—and sore throat.5,7 The gastric content can also act indirectly by initiating laryngeal reflexes through irritation of the esophagus, leading to vagally mediated changes such as chronic cough and bronchoconstriction.
Enzyme production declines. Under normal circumstances, carbonic anhydrase isoenzyme III (CAIII) is produced in the posterior aspect of the larynx, catalyzing the production of bicarbonate and neutralizing stomach acid.9-11 In LPR, however, the production of CAIII decreases significantly, thereby exposing the larynx to stomach acid without the enzyme’s protective effect.9,10 At the same time, a marked increase in pepsin levels intensifies laryngeal injury.10,12,13
The larynx is highly vulnerable. The laryngopharynx is much more susceptible to pathology from gastric reflux than the esophagus, for a number of reasons. Damage can occur with much less exposure to acid,1 not only because of the decrease in CAIII, but also because of the absence of peristalsis in the larynx.
What’s more, the esophagus has the ability to clear gastric reflux and minimize damage to the epithelial layer.9,10,14,15 In most patients who develop signs and symptoms of LPR, there has been enough gastric reflux to damage the laryngopharynx but not enough to overcome the protective mechanisms of the esophagus. That’s why most LPR patients have little or none of the heartburn and esophagitis that are classic symptoms of GERD.
Common signs and symptoms that signal LPR
LPR is primarily a clinical diagnosis based on signs and symptoms—which are also used to rule out GERD. Notably, less than 50% of patients with LPR suffer from heartburn and regurgitation.16 Those who do have heartburn and regurgitation typically suffer with reflux during the day, when they’re in an upright position, whereas reflux associated with GERD develops primarily at night.16 The results of a recent survey of members of the American Bronchoesophagological Association highlight the most common signs and symptoms of LPR, listed below from the most to the least frequent:17
- throat clearing
- persistent cough
- globus sensation
- hoarseness
- choking episodes.
Additional signs and symptoms include excessive and chronic throat clearing, sore throat, postnasal drip, and dysphagia.
Use a validated symptom index. To further assess the probability and severity of LPR, use the Reflux Symptom Index18 (TABLE 1). A recent cohort study validated the index, with an average score of 21.2 for those with LPR, vs an average of 11.6 for controls (P<.001). A score >13 is suggestive of LPR (odds ratio=9.19), the researchers found.18
If the diagnosis remains uncertain and the patient continues to be troubled by signs and symptoms suggestive of LPR, refer him or her to an otolaryngologist for further investigation. A referral is needed, too, to rule out malignancy in any patient with 3 or more of the following red flags: older than 50 years, otalgia, weight loss, progressive hoarseness, neck mass, a significant history of alcohol use, and a history of smoking.7
TABLE 1
The Reflux Symptom Index for laryngopharyngeal reflux
| Within the last month, how did the following problems affect you? | 0 = NO PROBLEM 5 = SEVERE PROBLEM (CIRCLE THE APPROPRIATE RESPONSE) | |||||
|---|---|---|---|---|---|---|
| 1. Hoarseness or a problem with your voice | 0 | 1 | 2 | 3 | 4 | 5 |
| 2. Clearing your throat | 0 | 1 | 2 | 3 | 4 | 5 |
| 3. Excess throat mucus or postnasal drip | 0 | 1 | 2 | 3 | 4 | 5 |
| 4. Difficulty swallowing food, liquids, or pills | 0 | 1 | 2 | 3 | 4 | 5 |
| 5. Coughing after you ate or after lying down | 0 | 1 | 2 | 3 | 4 | 5 |
| 6. Breathing difficulties or choking episodes | 0 | 1 | 2 | 3 | 4 | 5 |
| 7. Troublesome or annoying cough | 0 | 1 | 2 | 3 | 4 | 5 |
| 8. Sensations of something sticking in your throat or a lump in your throat | 0 | 1 | 2 | 3 | 4 | 5 |
| 9. Heartburn, chest pain, indigestion, or stomach acid coming up | 0 | 1 | 2 | 3 | 4 | 5 |
| TOTAL SCORE= | ||||||
| *A score >13 is considered suggestive of laryngopharyngeal reflux. | ||||||
| Source: Belafsky PC et al. J Voice. 2002.18 Reprinted with permission. | ||||||
Diagnostic tools the specialists will use
Fiberoptic laryngoscopy is the most common test used by otolaryngologists to confirm LPR and rule out other pathology. The test reveals inflammatory findings (FIGURE), such as erythema, edema, granulomas, and contact ulcers, in several anatomical locations of the larynx—especially the posterior aspect and the true vocal folds. It is important to note, however, that as many as 70% of the general population will have some laryngeal inflammation, so these findings alone are not definitive evidence of LPR.7
FIGURE
Fiberoptic laryngoscopy: Before and after treatment
Ambulatory 24-hour dual sensor pH probe monitoring is sometimes used as an adjunctive test to confirm LPR. In 2005, 2 meta-analyses found that the pH probe is reliable and sensitive and specific enough to identify significantly more acid reflux in patients with LPR than in controls.19,20 However, not everyone agrees: Some clinicians question its use as a diagnostic tool for LPR, citing problems with observer reliability, among other things. Because it increases costs to the patient and is impractical, pH monitoring is not widely used by specialists, but primarily as a research tool.21,22
Barium swallow and esophagogastroduodenoscopy (EGD) are relatively common diagnostic tools used to identify anatomical abnormalities in the gastrointestinal tract, such as a Schatzki’s ring or hiatal hernia, that can lead to symptoms of GERD and/or LPR. Some studies suggest that all patients with symptoms of LPR should undergo EGD to screen for esophageal adenocarcinoma.23,24 Because LPR symptoms are relatively common, however, many clinicians believe that EGD should be considered only when heartburn is a primary complaint in a patient with signs and symptoms of LPR—or when a patient believed to have LPR fails to respond to medical management.24
Treating LPR: Lifestyle changes, drug therapy
For all patients with LPR, dietary and lifestyle modifications have been shown to be both clinically effective and cost effective.25 In addition to dietary restrictions (TABLE 2), advise patients to avoid eating too rapidly or drinking large quantities of fluid. Late night meals—indeed, eating within 3 hours of bedtime—should also be avoided, as should heavy lunches and dinners. Tell patients to eat small, frequent meals instead.7,25,26
Recommend other behavioral changes, as well. Tell patients to avoid tight clothing, lying down immediately after a meal, and applying pressure to the abdomen, whether through exercise, heavy lifting, singing, or bending over. Smoking and overuse (or misuse) of the voice—screaming at a concert or singing for hours, for instance—are contraindicated, as well.7,25,26
Tell patients that weight loss, as needed, is likely to bring some symptom relief. Using wooden blocks to elevate the head of the bed about 4 to 6 inches may also be helpful, particularly for patients who suffer from both LPR and GERD.7,25,26
TABLE 2
Dietary management of LPR: What to tell patients7,14,24
| Avoid* | Enjoy† |
|---|---|
| Caffeine | Meat |
| Alcohol | Poultry |
| Spicy foods | Seafood |
| Tomatoes | Milk |
| Chocolate | Fresh vegetables‡ |
| Fats | |
| Citrus fruits | |
| Carbonated beverages | |
| Jams/jellies | |
| Barbecue sauces | |
| Salad dressings | |
| Hot mustard | |
| Curry | |
| Hot peppers | |
| *Other acidic foods. | |
| †Other foods and beverages that are neither spicy nor acidic. | |
| ‡ Except tomatoes. | |
Drug therapy: Straightforward, but not without controversy
Acid suppression with proton pump inhibitors (PPIs) is the primary treatment for LPR, as it is for GERD. But because the larynx is extremely susceptible to injury from acid reflux, LPR typically requires more aggressive and prolonged treatment, compared with GERD.1,5
Clinical trials have shown that PPIs do not inhibit acid production to an intragastric pH of >4 for more than 16.8 hours.1,27 Thus, most patients need twice-daily dosing (although once-a-day dosing or conservative management may be sufficient for those with mild and intermittent symptoms).27,28 Regardless of dosing, PPIs should be taken on an empty stomach, 30 minutes before a meal to increase bioavailability. For maximum benefits, patients should continue the twice-daily regimen for 4 to 6 months, although the optimal duration is unknown.26
One study found 4 months of therapy to be effective;28 others suggest that while symptom relief should begin after 6 to 8 weeks of treatment, 6 months of PPI therapy is needed for laryngeal lesions and edema to resolve.1,8 Despite the time frame, patients should be weaned gradually to prevent the delayed rebound effect associated with abrupt cessation of PPIs.
The PPI controversy. Not only the length of treatment is controversial, however, but the efficacy of PPIs for LPR. Many studies, including several prospective cohort studies and 9 RCTs, have reported significant improvement in laryngeal symptoms, but evidence that PPIs are significantly better than placebo is weak.25,29,30 In fact, a systematic review and 2 meta-analyses concluded that not only is there a lack of sufficient evidence to draw reliable conclusions about the efficacy of PPIs vs placebo for the treatment of LPR, but there seems to be a significant response to placebo among patients with this condition, as well.25,29,30
The role of adjunctive therapy. Histamine type 2 (H2) blockers have been shown to be helpful in the treatment of GERD. But data showing their efficacy for LPR, either as a single agent or in combination with a PPI, are limited. Indeed, 3 clinical trials have found that H2 blockers do not provide any added benefit to PPI therapy for LPR. All 3 were cohort studies that compared the treatment outcomes of PPI alone vs PPI and H2 blockers, and found no statistically significant difference (P>.05).28,31,32 Despite these findings, recent studies suggest that 300 mg ranitidine twice a day provides added benefit (P<.01).33,34 Given these mixed findings, H2 blockers may be considered as adjuvant therapy to the PPI regimen to further reduce acid production in patients with more severe symptoms. Ant-acids and prokinetic agents are sometimes used for this purpose, as well.
When medical management fails
Surgery has a limited, but useful, role in the treatment of LPR.
Nissen fundoplication—a procedure in which the fundus of the stomach is passed posteriorly behind the esophagus to encircle it and provide mechanical obstruction to the retrograde movement of acid—may be considered for patients with a confirmed diagnosis, severe symptoms, and little response to treatment. However, there is little evidence that this procedure will result in long-term improvement in LPR symptoms. Laryngeal surgery can be used to treat vocal fold sequelae of LPR, such as granulomas—with a higher likelihood of success.35
CORRESPONDENCE Kevin Fung, MD, FRCSC, FACS, University of Western Ontario, London Health Sciences Center-Victoria Hospital, 800 Commissioners Road East, Room B3-427, London, Ontario, Canada, N6A 4G5; [email protected]
• Suspect laryngopharyngeal reflux (LPR) in a patient with chronic laryngitis; 50% to 60% of such cases are related to LPR. B
• Refer patients with risk factors for head and neck cancer or whose symptoms persist despite lifestyle modification and medical management to an otolaryngologist. A
• While symptoms of LPR should show improvement after 6 to 8 weeks of proton pump inhibitor therapy, advise patients to continue treatment for 4 to 6 months to ensure that laryngeal lesions and edema resolve. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laryngopharyngeal reflux (LPR), the retrograde movement of gastric content into the upper aerodigestive tract, is a common—and commonly underdiagnosed—condition. Characterized by inflammation of the laryngopharynx, LPR can coexist with gastroesophageal reflux disease (GERD), but it is a distinct disorder.1 In GERD, the lower esophageal sphincter malfunctions, whereas LPR involves a dysfunctional upper esophageal sphincter.
Because both conditions involve acid reflux, LPR is sometimes mistaken for GERD. Often, too, patients and physicians alike attribute LPR’s signs and symptoms, which are largely nonspecific, to other causes. The hoarseness and laryngitis that are characteristic of LPR may be blamed on vocal cord abuse or smoking, for instance; the chronic cough and throat clearing associated with LPR thought to be caused by allergies; and the sore throat and postnasal drip that often accompany LPR attributed to infection. Another reason LPR is underdiagnosed: Primary care physicians, who are often the first clinicians from whom symptomatic patients seek treatment, are often unfamiliar with this lesser-known reflux disease.2
The failure to recognize and provide timely treatment for LPR may increase patients’ risk for a number of conditions, including laryngeal ulcers, granulomas, subglottic stenosis, chronic sinusitis, laryngospasm, nasal congestion, and asthma.1 Evidence suggests that LPR increases the risk for esophageal and laryngeal carcinomas,3,4 and for laryngeal injury from intubation, as well.1 To minimize these risks, it is important for primary care physicians to promptly identify this disorder, treat it appropriately, and recognize red flags that warrant referral to a specialist.
How LPR develops, what to look for
There is no gold standard for the diagnosis of LPR. Nonetheless, a review of the pathophysiology and clinical presentation of this reflux disorder and the ways in which it differs from GERD will help you identify cases of LPR. The prevalence of LPR in the general population is uncertain. But reports suggest that as many as 10% of otolaryngology referrals are for patients with a classic presentation of LPR, and that 50% to 60% of cases of chronic laryngitis are related to LPR.1,5,6
The laryngopharynx becomes irritated and inflamed
When the physiological barriers protecting the laryngopharynx from the retrograde flow of gastric content break down, gastric contents can directly irritate the ciliated columnar epithelial cells of the upper respiratory tract, leading to ciliary dysfunction. A lack of mucous clearance leads to mucous stasis and, subsequently, to excessive throat clearing and the sensation of postnasal drip.7 In addition, the laryngopharyngeal epithelium becomes inflamed, and this affects the sensitivity of laryngeal sensory endings and leads to laryngospasm and coughing.8 The inflammatory reaction in turn leads to vocal fold edema, contact ulcers, and granulomas. These changes make patients with LPR particularly prone to developing hoarseness, globus pharyngeus—a sensation of a foreign body in the larynx—and sore throat.5,7 The gastric content can also act indirectly by initiating laryngeal reflexes through irritation of the esophagus, leading to vagally mediated changes such as chronic cough and bronchoconstriction.
Enzyme production declines. Under normal circumstances, carbonic anhydrase isoenzyme III (CAIII) is produced in the posterior aspect of the larynx, catalyzing the production of bicarbonate and neutralizing stomach acid.9-11 In LPR, however, the production of CAIII decreases significantly, thereby exposing the larynx to stomach acid without the enzyme’s protective effect.9,10 At the same time, a marked increase in pepsin levels intensifies laryngeal injury.10,12,13
The larynx is highly vulnerable. The laryngopharynx is much more susceptible to pathology from gastric reflux than the esophagus, for a number of reasons. Damage can occur with much less exposure to acid,1 not only because of the decrease in CAIII, but also because of the absence of peristalsis in the larynx.
What’s more, the esophagus has the ability to clear gastric reflux and minimize damage to the epithelial layer.9,10,14,15 In most patients who develop signs and symptoms of LPR, there has been enough gastric reflux to damage the laryngopharynx but not enough to overcome the protective mechanisms of the esophagus. That’s why most LPR patients have little or none of the heartburn and esophagitis that are classic symptoms of GERD.
Common signs and symptoms that signal LPR
LPR is primarily a clinical diagnosis based on signs and symptoms—which are also used to rule out GERD. Notably, less than 50% of patients with LPR suffer from heartburn and regurgitation.16 Those who do have heartburn and regurgitation typically suffer with reflux during the day, when they’re in an upright position, whereas reflux associated with GERD develops primarily at night.16 The results of a recent survey of members of the American Bronchoesophagological Association highlight the most common signs and symptoms of LPR, listed below from the most to the least frequent:17
- throat clearing
- persistent cough
- globus sensation
- hoarseness
- choking episodes.
Additional signs and symptoms include excessive and chronic throat clearing, sore throat, postnasal drip, and dysphagia.
Use a validated symptom index. To further assess the probability and severity of LPR, use the Reflux Symptom Index18 (TABLE 1). A recent cohort study validated the index, with an average score of 21.2 for those with LPR, vs an average of 11.6 for controls (P<.001). A score >13 is suggestive of LPR (odds ratio=9.19), the researchers found.18
If the diagnosis remains uncertain and the patient continues to be troubled by signs and symptoms suggestive of LPR, refer him or her to an otolaryngologist for further investigation. A referral is needed, too, to rule out malignancy in any patient with 3 or more of the following red flags: older than 50 years, otalgia, weight loss, progressive hoarseness, neck mass, a significant history of alcohol use, and a history of smoking.7
TABLE 1
The Reflux Symptom Index for laryngopharyngeal reflux
| Within the last month, how did the following problems affect you? | 0 = NO PROBLEM 5 = SEVERE PROBLEM (CIRCLE THE APPROPRIATE RESPONSE) | |||||
|---|---|---|---|---|---|---|
| 1. Hoarseness or a problem with your voice | 0 | 1 | 2 | 3 | 4 | 5 |
| 2. Clearing your throat | 0 | 1 | 2 | 3 | 4 | 5 |
| 3. Excess throat mucus or postnasal drip | 0 | 1 | 2 | 3 | 4 | 5 |
| 4. Difficulty swallowing food, liquids, or pills | 0 | 1 | 2 | 3 | 4 | 5 |
| 5. Coughing after you ate or after lying down | 0 | 1 | 2 | 3 | 4 | 5 |
| 6. Breathing difficulties or choking episodes | 0 | 1 | 2 | 3 | 4 | 5 |
| 7. Troublesome or annoying cough | 0 | 1 | 2 | 3 | 4 | 5 |
| 8. Sensations of something sticking in your throat or a lump in your throat | 0 | 1 | 2 | 3 | 4 | 5 |
| 9. Heartburn, chest pain, indigestion, or stomach acid coming up | 0 | 1 | 2 | 3 | 4 | 5 |
| TOTAL SCORE= | ||||||
| *A score >13 is considered suggestive of laryngopharyngeal reflux. | ||||||
| Source: Belafsky PC et al. J Voice. 2002.18 Reprinted with permission. | ||||||
Diagnostic tools the specialists will use
Fiberoptic laryngoscopy is the most common test used by otolaryngologists to confirm LPR and rule out other pathology. The test reveals inflammatory findings (FIGURE), such as erythema, edema, granulomas, and contact ulcers, in several anatomical locations of the larynx—especially the posterior aspect and the true vocal folds. It is important to note, however, that as many as 70% of the general population will have some laryngeal inflammation, so these findings alone are not definitive evidence of LPR.7
FIGURE
Fiberoptic laryngoscopy: Before and after treatment
Ambulatory 24-hour dual sensor pH probe monitoring is sometimes used as an adjunctive test to confirm LPR. In 2005, 2 meta-analyses found that the pH probe is reliable and sensitive and specific enough to identify significantly more acid reflux in patients with LPR than in controls.19,20 However, not everyone agrees: Some clinicians question its use as a diagnostic tool for LPR, citing problems with observer reliability, among other things. Because it increases costs to the patient and is impractical, pH monitoring is not widely used by specialists, but primarily as a research tool.21,22
Barium swallow and esophagogastroduodenoscopy (EGD) are relatively common diagnostic tools used to identify anatomical abnormalities in the gastrointestinal tract, such as a Schatzki’s ring or hiatal hernia, that can lead to symptoms of GERD and/or LPR. Some studies suggest that all patients with symptoms of LPR should undergo EGD to screen for esophageal adenocarcinoma.23,24 Because LPR symptoms are relatively common, however, many clinicians believe that EGD should be considered only when heartburn is a primary complaint in a patient with signs and symptoms of LPR—or when a patient believed to have LPR fails to respond to medical management.24
Treating LPR: Lifestyle changes, drug therapy
For all patients with LPR, dietary and lifestyle modifications have been shown to be both clinically effective and cost effective.25 In addition to dietary restrictions (TABLE 2), advise patients to avoid eating too rapidly or drinking large quantities of fluid. Late night meals—indeed, eating within 3 hours of bedtime—should also be avoided, as should heavy lunches and dinners. Tell patients to eat small, frequent meals instead.7,25,26
Recommend other behavioral changes, as well. Tell patients to avoid tight clothing, lying down immediately after a meal, and applying pressure to the abdomen, whether through exercise, heavy lifting, singing, or bending over. Smoking and overuse (or misuse) of the voice—screaming at a concert or singing for hours, for instance—are contraindicated, as well.7,25,26
Tell patients that weight loss, as needed, is likely to bring some symptom relief. Using wooden blocks to elevate the head of the bed about 4 to 6 inches may also be helpful, particularly for patients who suffer from both LPR and GERD.7,25,26
TABLE 2
Dietary management of LPR: What to tell patients7,14,24
| Avoid* | Enjoy† |
|---|---|
| Caffeine | Meat |
| Alcohol | Poultry |
| Spicy foods | Seafood |
| Tomatoes | Milk |
| Chocolate | Fresh vegetables‡ |
| Fats | |
| Citrus fruits | |
| Carbonated beverages | |
| Jams/jellies | |
| Barbecue sauces | |
| Salad dressings | |
| Hot mustard | |
| Curry | |
| Hot peppers | |
| *Other acidic foods. | |
| †Other foods and beverages that are neither spicy nor acidic. | |
| ‡ Except tomatoes. | |
Drug therapy: Straightforward, but not without controversy
Acid suppression with proton pump inhibitors (PPIs) is the primary treatment for LPR, as it is for GERD. But because the larynx is extremely susceptible to injury from acid reflux, LPR typically requires more aggressive and prolonged treatment, compared with GERD.1,5
Clinical trials have shown that PPIs do not inhibit acid production to an intragastric pH of >4 for more than 16.8 hours.1,27 Thus, most patients need twice-daily dosing (although once-a-day dosing or conservative management may be sufficient for those with mild and intermittent symptoms).27,28 Regardless of dosing, PPIs should be taken on an empty stomach, 30 minutes before a meal to increase bioavailability. For maximum benefits, patients should continue the twice-daily regimen for 4 to 6 months, although the optimal duration is unknown.26
One study found 4 months of therapy to be effective;28 others suggest that while symptom relief should begin after 6 to 8 weeks of treatment, 6 months of PPI therapy is needed for laryngeal lesions and edema to resolve.1,8 Despite the time frame, patients should be weaned gradually to prevent the delayed rebound effect associated with abrupt cessation of PPIs.
The PPI controversy. Not only the length of treatment is controversial, however, but the efficacy of PPIs for LPR. Many studies, including several prospective cohort studies and 9 RCTs, have reported significant improvement in laryngeal symptoms, but evidence that PPIs are significantly better than placebo is weak.25,29,30 In fact, a systematic review and 2 meta-analyses concluded that not only is there a lack of sufficient evidence to draw reliable conclusions about the efficacy of PPIs vs placebo for the treatment of LPR, but there seems to be a significant response to placebo among patients with this condition, as well.25,29,30
The role of adjunctive therapy. Histamine type 2 (H2) blockers have been shown to be helpful in the treatment of GERD. But data showing their efficacy for LPR, either as a single agent or in combination with a PPI, are limited. Indeed, 3 clinical trials have found that H2 blockers do not provide any added benefit to PPI therapy for LPR. All 3 were cohort studies that compared the treatment outcomes of PPI alone vs PPI and H2 blockers, and found no statistically significant difference (P>.05).28,31,32 Despite these findings, recent studies suggest that 300 mg ranitidine twice a day provides added benefit (P<.01).33,34 Given these mixed findings, H2 blockers may be considered as adjuvant therapy to the PPI regimen to further reduce acid production in patients with more severe symptoms. Ant-acids and prokinetic agents are sometimes used for this purpose, as well.
When medical management fails
Surgery has a limited, but useful, role in the treatment of LPR.
Nissen fundoplication—a procedure in which the fundus of the stomach is passed posteriorly behind the esophagus to encircle it and provide mechanical obstruction to the retrograde movement of acid—may be considered for patients with a confirmed diagnosis, severe symptoms, and little response to treatment. However, there is little evidence that this procedure will result in long-term improvement in LPR symptoms. Laryngeal surgery can be used to treat vocal fold sequelae of LPR, such as granulomas—with a higher likelihood of success.35
CORRESPONDENCE Kevin Fung, MD, FRCSC, FACS, University of Western Ontario, London Health Sciences Center-Victoria Hospital, 800 Commissioners Road East, Room B3-427, London, Ontario, Canada, N6A 4G5; [email protected]
1. Koufman JA, Aviv JE, Casiano RR, et al. Laryngopharyngeal reflux: position statement of the Committee on Speech, Voice, and Swallowing Disorders of the American Academy of Otolaryngology–Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32-35.
2. Karkos PD, Thomas L, Temple RH, et al. Awareness of general practitioners towards treatment of laryngopharyngeal reflux: a British survey. Otolaryngol Head Neck Surg. 2005;133:505-508.
3. Morrison MD. Is chronic gastroesophageal reflux a causative factor in glottic carcinoma? Otolaryngol Head Neck Surg. 1988;99:370-373.
4. Ward PH, Hanson DG. Reflux as an etiologic factor of carcinoma of the laryngopharynx. Laryngoscope. 1988;98:1195-1199.
5. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101(4 pt 2 suppl 53):S1-S78.
6. Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg. 2000;123:385-388.
7. Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA. 2005;294:1534-1540.
8. Aviv JE, Liu H, Parides M, et al. Laryngopharyngeal sensory deficits in patients with laryngopharyngeal reflux and dysphagia. Ann Otol Rhinol Laryngol. 2000;109:1000-1006.
9. Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112:481.-
10. Gill GA, Johnston N, Buda A, et al. Laryngeal epithelial defenses against laryngopharyngeal reflux: investigations of E-cadherin, carbonic anhydrase isoenzyme III, and pepsin. Ann Otol Rhinol Laryngol. 2005;114:913-921.
11. Okamura H, Sugai N, Kanno T, et al. Histochemical localization of carbonic anhydrase in the trachea of the guinea pig. Histochem Cell Biol. 1996;106:257-260.
12. Johnston N, Knight J, Dettmar PW, et al. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope. 2004;114:2129-2134.
13. Johnston N, Dettmar PW, Bishwokarma B, et al. Activity/stability of human pepsin: implications for reflux attributed laryngeal disease. Laryngoscope. 2007;117:1036-1039.
14. Axford SE, Sharp N, Ross PE, et al. Cell biology of laryngeal epithelial defenses in health and disease: preliminary studies. Ann Otol Rhinol Laryngol. 2001;110:1099-1108.
15. Toros SZ, Toros AB, Yüksel OD, et al. Association of laryngopharyngeal manifestations and gastroesophageal reflux. Eur Arch Otorhinolaryngol. 2009;266:403-409.
16. Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease Ear Nose Throat J. 2002;81(9 suppl 2):S7-S9.
17. Book DT, Rhee JS, Toohill RJ, et al. Perspectives in laryngopharyngeal reflux: an international survey. Laryngoscope. 2002;112(8 Pt 1):1399-1406.
18. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the Reflux Symptom Index (RSI). J Voice. 2002;16:274-277.
19. Ulualp SO, Roland PS, Toohill RJ, et al. Prevalence of gastroesophagopharyngeal acid reflux events: an evidence-based systematic review. Am J Otolaryngol. 2005;26:239-244.
20. Merati AL, Lim HJ, Ulualp SO, et al. Meta-analysis of upper probe measurements in normal subjects and patients with laryngopharyngeal reflux. Ann Otol Rhinol Laryngol. 2005;114(3):177-82.
21. Vaezi MF, Schroeder PL, Richter JE. Reproducibility of proximal probe pH parameters in 24-hour ambulatory esophageal pH monitoring. Am J Gastroenterol. 1997;92:825-829.
22. Vincent DA, Jr., Garrett JD, Radionoff SL, et al. The proximal probe in esophageal pH monitoring: development of a normative database. J Voice. 2000;142:247-254.
23. Reavis KM, Morris CD, Gopal DV, et al. Laryngopharyngeal reflux symptoms better predict the presence of esophageal adenocarcinoma than typical gastroesophageal reflux symptoms. Ann Surg. 2004;239:849-858.
24. Gupta R, Sataloff RT. Laryngopharyngeal reflux: current concepts and questions. Curr Opin Otolaryngol Head Neck Surg. 2009;17:143-148.
25. Tsunoda K, Ishimoto S, Suzuki M, et al. An effective management regimen for laryngeal granuloma caused by gastro-esophageal reflux: combination therapy with suggestions for lifestyle modifications. Acta Otolaryngol. 2007;127:88-92.
26. Hopkins C, Yousaf U, Pedersen M. Acid reflux treatment for hoarseness [protocol]. Cochrane Database Syst Rev. 2006;(1):CD005054.-
27. Kahrilas PJ, Falk GW, Johnson DA, et al. The Esomeprazole Study Investigators Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. Aliment Pharmacol Ther. 2000;14:1249-1458.
28. Park W, Hicks DM, Khandwala F, et al. Laryngopharyngeal reflux: prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope. 2005;115:1230-1238.
29. Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101:2646-2654.
30. Karkos PD, Wilson JA. Empiric treatment of laryngopharyngeal reflux with proton pump inhibitors: a systematic review. Laryngoscope. 2006;116:144-148.
31. Fackler WK, Ours TM, Vaezi MF, et al. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology. 2002;122:625-632.
32. Ours TM, Fackler WK, Richter JE, et al. Nocturnal acid breakthrough: clinical significance and correlation with esophageal acid exposure. Am J Gastroenterol. 2003;98:545-550.
33. Sato K. Laryngopharyngeal reflux disease with nocturnal gastric acid breakthrough while on proton pump inhibitor therapy. Eur Arch Otorhinolaryngol. 2006;263:1121-1126.
34. Mainie I, Tutuian R, Castell DO. Addition of a H2 receptor antagonist to PPI improves acid control and decreases nocturnal acid breakthrough. J Clin Gastroenterol. 2008;42:676-679.
35. Koufman JA, Rees CJ, Frazier WD, et al. Office-based laryngeal laser surgery: a review of 443 cases using three wavelengths. Otolaryngol Head Neck Surg. 2007;137:146-151.
1. Koufman JA, Aviv JE, Casiano RR, et al. Laryngopharyngeal reflux: position statement of the Committee on Speech, Voice, and Swallowing Disorders of the American Academy of Otolaryngology–Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32-35.
2. Karkos PD, Thomas L, Temple RH, et al. Awareness of general practitioners towards treatment of laryngopharyngeal reflux: a British survey. Otolaryngol Head Neck Surg. 2005;133:505-508.
3. Morrison MD. Is chronic gastroesophageal reflux a causative factor in glottic carcinoma? Otolaryngol Head Neck Surg. 1988;99:370-373.
4. Ward PH, Hanson DG. Reflux as an etiologic factor of carcinoma of the laryngopharynx. Laryngoscope. 1988;98:1195-1199.
5. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101(4 pt 2 suppl 53):S1-S78.
6. Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg. 2000;123:385-388.
7. Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA. 2005;294:1534-1540.
8. Aviv JE, Liu H, Parides M, et al. Laryngopharyngeal sensory deficits in patients with laryngopharyngeal reflux and dysphagia. Ann Otol Rhinol Laryngol. 2000;109:1000-1006.
9. Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112:481.-
10. Gill GA, Johnston N, Buda A, et al. Laryngeal epithelial defenses against laryngopharyngeal reflux: investigations of E-cadherin, carbonic anhydrase isoenzyme III, and pepsin. Ann Otol Rhinol Laryngol. 2005;114:913-921.
11. Okamura H, Sugai N, Kanno T, et al. Histochemical localization of carbonic anhydrase in the trachea of the guinea pig. Histochem Cell Biol. 1996;106:257-260.
12. Johnston N, Knight J, Dettmar PW, et al. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope. 2004;114:2129-2134.
13. Johnston N, Dettmar PW, Bishwokarma B, et al. Activity/stability of human pepsin: implications for reflux attributed laryngeal disease. Laryngoscope. 2007;117:1036-1039.
14. Axford SE, Sharp N, Ross PE, et al. Cell biology of laryngeal epithelial defenses in health and disease: preliminary studies. Ann Otol Rhinol Laryngol. 2001;110:1099-1108.
15. Toros SZ, Toros AB, Yüksel OD, et al. Association of laryngopharyngeal manifestations and gastroesophageal reflux. Eur Arch Otorhinolaryngol. 2009;266:403-409.
16. Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease Ear Nose Throat J. 2002;81(9 suppl 2):S7-S9.
17. Book DT, Rhee JS, Toohill RJ, et al. Perspectives in laryngopharyngeal reflux: an international survey. Laryngoscope. 2002;112(8 Pt 1):1399-1406.
18. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the Reflux Symptom Index (RSI). J Voice. 2002;16:274-277.
19. Ulualp SO, Roland PS, Toohill RJ, et al. Prevalence of gastroesophagopharyngeal acid reflux events: an evidence-based systematic review. Am J Otolaryngol. 2005;26:239-244.
20. Merati AL, Lim HJ, Ulualp SO, et al. Meta-analysis of upper probe measurements in normal subjects and patients with laryngopharyngeal reflux. Ann Otol Rhinol Laryngol. 2005;114(3):177-82.
21. Vaezi MF, Schroeder PL, Richter JE. Reproducibility of proximal probe pH parameters in 24-hour ambulatory esophageal pH monitoring. Am J Gastroenterol. 1997;92:825-829.
22. Vincent DA, Jr., Garrett JD, Radionoff SL, et al. The proximal probe in esophageal pH monitoring: development of a normative database. J Voice. 2000;142:247-254.
23. Reavis KM, Morris CD, Gopal DV, et al. Laryngopharyngeal reflux symptoms better predict the presence of esophageal adenocarcinoma than typical gastroesophageal reflux symptoms. Ann Surg. 2004;239:849-858.
24. Gupta R, Sataloff RT. Laryngopharyngeal reflux: current concepts and questions. Curr Opin Otolaryngol Head Neck Surg. 2009;17:143-148.
25. Tsunoda K, Ishimoto S, Suzuki M, et al. An effective management regimen for laryngeal granuloma caused by gastro-esophageal reflux: combination therapy with suggestions for lifestyle modifications. Acta Otolaryngol. 2007;127:88-92.
26. Hopkins C, Yousaf U, Pedersen M. Acid reflux treatment for hoarseness [protocol]. Cochrane Database Syst Rev. 2006;(1):CD005054.-
27. Kahrilas PJ, Falk GW, Johnson DA, et al. The Esomeprazole Study Investigators Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. Aliment Pharmacol Ther. 2000;14:1249-1458.
28. Park W, Hicks DM, Khandwala F, et al. Laryngopharyngeal reflux: prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope. 2005;115:1230-1238.
29. Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101:2646-2654.
30. Karkos PD, Wilson JA. Empiric treatment of laryngopharyngeal reflux with proton pump inhibitors: a systematic review. Laryngoscope. 2006;116:144-148.
31. Fackler WK, Ours TM, Vaezi MF, et al. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology. 2002;122:625-632.
32. Ours TM, Fackler WK, Richter JE, et al. Nocturnal acid breakthrough: clinical significance and correlation with esophageal acid exposure. Am J Gastroenterol. 2003;98:545-550.
33. Sato K. Laryngopharyngeal reflux disease with nocturnal gastric acid breakthrough while on proton pump inhibitor therapy. Eur Arch Otorhinolaryngol. 2006;263:1121-1126.
34. Mainie I, Tutuian R, Castell DO. Addition of a H2 receptor antagonist to PPI improves acid control and decreases nocturnal acid breakthrough. J Clin Gastroenterol. 2008;42:676-679.
35. Koufman JA, Rees CJ, Frazier WD, et al. Office-based laryngeal laser surgery: a review of 443 cases using three wavelengths. Otolaryngol Head Neck Surg. 2007;137:146-151.