User login
Assessment of abnormal uterine bleeding: 3 office-based tools
- Identification and measurement of the endometrial echo and descriptions of the echogenicity and heterogeneity of the endometrium are key to defining endometrial health.
- The introduction of intracervical fluid (saline-infusion sonography) during transvaginal ultrasound is one of the most significant advances in ultrasonography of the past decade.
- Hysteroscopic visualization has several advantages: immediate office evaluation, direct visualization of the endometrium and endocervix, and the ability to detect minute focal endometrial pathology and to perform directed endometrial biopsies.
- Sometimes a combination of procedures may be the best way to determine the cause of abnormal uterine bleeding.
Because office-based physicians tend to feel comfortable relying upon endometrial biopsy or dilation and curettage (D&C) to evaluate abnormal uterine bleeding, newer tools—transvaginal ultrasound (TVUS), saline-infusion sonography (SIS), and hysteroscopy—see far too little utilization. Although these modalities are remarkably user-friendly when employed correctly, only 28% of gynecologists perform office hysteroscopy, and even fewer use SIS.1
This article reviews indications for use, sensitivity and specificity, advantages and disadvantages, special considerations including cost issues, and suggestions for incorporating these modalities into gynecologic practice.
For most patients, neither endometrial biopsy nor D&C is particularly helpful in the assessment of abnormal uterine bleeding (see “Limitations of D&C and endometrial biopsy”). Does a negative biopsy signify health? An absence of intracavitary pathology? Or does it mean that we failed to sample the culprit?
Fortunately, abnormal uterine bleeding usually is attributable to endometrial conditions other than cancer, such as atrophy, hyperplasia, polyps, or fibroids. These pathologies may be benign, but they are still a nuisance. When left undetected and untreated, they can cause the patient much worry, along with weeks or months of unnecessary medical therapy and surgical procedures.
Dilation and curettage (D&C) detects benign pathology in about 80% of patients with menstrual dysfunction.1 It is most likely to detect the problem when pathology affects the endometrium globally.
A recent study reported on 105 postmenopausal women with bleeding and an endometrial echo of more than 5 mm who were evaluated with both hysteroscopy and D&C.2 Although 80% of the women had pathology in the uterine cavity, and 98% of the pathologic lesions manifested a focal growth pattern at hysteroscopy after D&C, the whole or parts of the lesion remained in situ in 87% of the women. In addition, D&C failed to detect 58% of polyps, 50% of hyperplasias, 60% of complex atypical hyperplasias, and 11% of endometrial cancers. When disease was global, D&C detected 94% of abnormalities.
Focal disease therefore mandates operative hysteroscopic-directed biopsy and removal of suspicious pathology.
Endometrial biopsy can be performed in the office without anesthesia—a great advantage. The technique is most helpful in “dating” the endometrium and diagnosing endometrial cancer or hyperplasia. Unfortunately, blind endometrial biopsy studies are frequently returned with pathology reports of insufficient tissue, atrophic changes, mucus and debris, scanty tissue, no visible endometrial tissue, endocervical tissue, or proliferative or secretory endometrium.
Further, when a blindly performed biopsy reveals normal histology, it does not necessarily rule out other pathology. In addition, a biopsy via endometrial suction curette frequently misses focal lesions such as endometrial polyps and submucosal fibroids. Global noninvasive surveillance of the endometrium is more effective at detecting such focal lesions.
Investigators who performed endometrial biopsy prior to hysterectomy in patients with known endometrial cancer demonstrated that the sensitivity of diagnosing endometrial cancer with a biopsy via endometrial suction curette increases when the pathology affects more than 50% of the surface area of the endometrial cavity.3 However, biopsy failed to detect cancer in 11 of 65 patients in whom the malignancy affected less than 50% of the endometrium. These 11 false negatives included 5 cases of endometrial polyps, 3 malignancies that affected less than 5% of the endometrium, and 7 cancers that affected less than 25% of the endometrium.
REFERENCES
1. Holst J, Koskela O, von Schoultz B. Endometrial findings following curettage in 2,018 women according to age and indications. Ann Chir Gynaecol. 1983;72:274-277.
2. Epstein E, Ramirez A, Skoog L, Valentin L. Dilation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136.
3. Guido RS, Kanbour-Shakir A, Rulin MC, Christopherson WA. Pipelle endometrial sampling: sensitivity in the detection of endometrial cancer. J Reprod Med. 1995;40:553-555.
Transvaginal ultrasound
TVUS is quick, convenient, inexpensive, and comfortable for the patient. It can evaluate the endometrium utilizing gray scale, color or power Doppler, contrast media (SIS), or 3-dimensional ultrasound technology. In addition, TVUS permits visualization of the adnexa and pelvic organs, including the bladder and cul de sac. Among the abnormalities detectable with TVUS are fibroids (including submucosal leiomyomas) and endometrial polyps. Not surprisingly, an experienced operator is crucial for a precise diagnosis.
Optimal TVUS evaluation includes endometrial measurements in the sagittal plane, with the bilayer thickness measured from the proximal to distal myometrialendometrial junctions. In the coronal view, measurement should be from the cervix to the fundus. When intraluminal fluid is present, each endometrial thickness should be measured separately (in single layers), and the combined endometrial thickness should be expressed as the sum of the 2 layers.
Characterize the endometrium. Sonographic hallmarks increasingly are used to describe uterine pathology. These include endometrial thickness, morphology, endocavitary lesions, borders, and myometrial invasion. In addition, a number of practitioners have attempted to describe the sonographic texture of the endometrium in post-menopausal women to increase sensitivity in the detection of disease, improve diagnosis, and guide treatment.
The endometrium can be characterized as homogeneous, diffusely inhomogeneous, or associated with focally or diffusely increased echogenicity. Textural inhomogeneity is present in cases of endometrial cancer.2 A homogeneous endometrium less than 6 mm thick is commonly associated with tissue insufficient for diagnosis. If focal or diffuse increased echogenicity occurs with a thin endometrium, SIS or hysteroscopy is more sensitive than TVUS in identifying the endometrial abnormality.
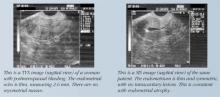
We rely increasingly on descriptions of the echogenicity and heterogeneity of the entire endometrial echo, as well as on echo measurements, to define endometrial health. Most experts use an endometrial echo of 5 mm as the cutoff for significant endometrial disease. A number of investigators have noted that the endometrial thickness in hyperplasia often ranges from 8 mm to 15 mm in post-menopausal women, but the number of reported cases is too low to draw firm conclusions based on endometrial echo alone.3,4
A prospective evaluation of 200 postmenopausal women with endometrial echo ranging from 3 mm to 10 mm noted that homogeneity, thin endometrium, and sonographically demonstrable central endometrium with symmetry were associated with absence of pathology. In contrast, heterogeneity and high echogenicity were indicative of pathology.5
Endometrial echo measurement and morphology increase sensitivity in predicting endometrial disease and can point to the need for ancillary testing with SIS or hysteroscopy. For example, in the postmenopausal patient, an endometrial echo of less than 5 mm, in combination with a negative endometrial biopsy, might be the only evaluation the patient needs if she responds to medical therapy for endometrial atrophy. If symptoms persist, office hysteroscopy or SIS could be performed to rule out endometrial polyps and endometrial hyperplasia (which is less likely).
Saline-infusion sonography offers an exquisite view of the endomyometrial complex that cannot be obtained with transvaginal ultrasound alone.
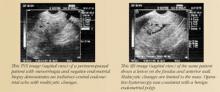
A thickened endometrial echo (more than 5 mm) at the initial TVUS in the postmenopausal patient should be evaluated promptly with SIS. In most of these patients, polyps or fibroids are the cause of bleeding.
Perform imaging at optimal time. In postmenopausal women, the endometrial thickness remains constant unless the patient is taking hormone replacement therapy (HRT) or tamoxifen.6 Thus, it is easier to detect or predict endometrial pathology in this population.
In premenopausal women, TVUS is most likely to detect fibroids during the early follicular stage because the endometrium is thin, usually measuring 2 mm to 4 mm. Endometrial polyps and submucosal fibroids are best viewed when a trilayered midfollicular endometrium is present, while uterine synechiae are visualized most clearly during midcycle. Adhesions appear as hyperechoic irregular structures that vary in size from 2 mm to 6 mm. They interrupt the continuity of the endometrial layer and appear much different from polyps, which tend to be round, symmetrical, and uniform.7
Diagnosing endometrial hyperplasia among premenopausal women based on an absolute endometrial measurement is more difficult than it is in postmenopausal women because of the wide range in thickness associated with the menstrual cycle (TABLE 1). Although endometrial hyperplasia or cancer is more likely when the endometrial echo is thicker than anticipated based on age or menstrual phase, it can only be proven definitively with histologic sampling.
Sensitivity and specificity. Because of the monotonous nature of the endometrium in healthy postmenopausal women, TVUS has higher sensitivity in this age group than in reproductive-age women. In the postmenopausal population, the sensitivity for detecting uterine pathology is 87%, while specificity is 82%.
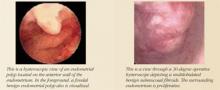
Office hysteroscopy offers immediate evaluation and direct visualization of the endometrium and endocervix.
In premenopausal women, polyps were the pathology most likely to be missed, according to one literature review.8 TVUS identified only 275 of 344 polyps in this population—a sensitivity of 80%. When submucosal fibroids were located near the endometrium, the diagnostic sensitivity rose to 94%. It was difficult to discern location (i.e., submucosal or intramural or polyps) with TVUS alone, however.
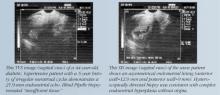
Another investigation compared the sensitivity and specificity of TVUS and endometrial biopsy for the detection of endometrial disease in 448 postmenopausal women who took estrogen alone, cyclic or continuous estrogen-progesterone, or placebo for 3 years.9 Using a threshold value of 5 mm for endometrial thickness, TVUS had a positive predictive value (PPV) of 9% for detecting any abnormality, with 90% sensitivity, 48% specificity, and a negative predictive value (NPV) of 99%. Using this threshold, biopsies would be indicated in more than half of the women, of whom only 4% actually had serious disease.
Using a threshold thickness of 4 mm, Gull et al10 evaluated TVUS for the detection of endometrial cancer and atypical hyperplasia. For endometrial thicknesses exceeding 4 mm, TVUS had a sensitivity of 100%, specificity of 60%, a PPV of 25%, and an NPV of 100%. No woman with an endometrial thickness of 4 mm or less was found to have endometrial cancer.
Special considerations.
- Intracavitary fluid. On occasion, TVUS reveals “naturally occurring SIS”—that is, the spontaneous appearance of intracavitary fluid, which is probably secondary to cervical stenosis. (The name of this phenomenon refers to the iatrogenic fluid that is intrinsic to SIS.) Endometrial fluid also is observed in women who use tamoxifen, diethylstilbestrol, or megestrol acetate and in those who have a hematometra. In addition, about one quarter of patients with an endometrial malignancy have fluid in the endometrial cavity.11
- Endometrial thickness. As noted, TVUS is excellent for ruling out endometrial abnormalities in postmenopausal patients because of the consistent thickness of the endometrium. When the endometrium is especially thickened, however, its usefulness is limited.
Observations in more than 5,000 women consistently note that an endometrium of less than 5 mm, measured as a double-layer, is most often associated with a pathology reading of “tissue insignificant for diagnosis”—hence atrophy.12 When the endometrium is thicker than 5 mm, there is a greater chance of detecting polyps, endometrial hyperplasia, and endometrial cancer. Fewer than 0.12% of cancers were missed in this series.
When a skilled physician performs office hysteroscopy, the complication rate is less than 1%.
If the endometrium is thicker than 6 mm, then SIS or hysteroscopy is more helpful than TVUS in reaching a conclusive etiology for uterine bleeding. Further evaluation (hysteroscopy or SIS) is usually recommended when TVUS measurements of the endometrium are greater than 5 mm.13 Although a low cut-off, such as 4 mm or 5 mm, is associated with improved sensitivity, it sacrifices specificity.
TABLE 1
Transvaginal ultrasound: Endometrial thickness32
| PHASE | THICKNESS (MM) |
|---|---|
| Menstrual | 2-4 |
| Early proliferative | 4-6 |
| Periovulatory | 6-8 |
| Secretory | 8-12 |
| Postmenopausal | 4-8 |
| Postmenopausal on hormone replacement therapy | 4-10 |
Saline-infusion sonography
In SIS, saline is infused into the endometrial cavity during TVUS to enhance the view of the endometrium. This constitutes one of the most significant advances in ultrasonography of the past decade. SIS can provide a wealth of information about the uterus and adnexa in patients with abnormal bleeding. It offers an exquisite view of the endomyometrial complex that cannot be obtained with TVUS alone. It differentiates between focal and global processes and improves overall sensitivity for detecting abnormalities of the endometrium.
Although many terms have been used to describe this technique (echohysteroscopy, sonohysterosalpingography, and hydrosonography, to name a few), the term “saline-infusion sonography,” coined in 1996, most clearly describes the technique.14
Indications for use. SIS has been used to evaluate:15
- menstrual disorders
- endometrium that is thickened, irregular, immeasurable, or poorly defined on conventional TVUS, magnetic resonance imaging, or computed axial tomography studies
- midline endometrial echo with a thickness of more than 8 mm
- endometrium that appears irregular, bizarre, or homogeneous on TVUS in women using tamoxifen
- sessile and pedunculated masses of the endometrium that need to be differentiated
- findings with hysterosalpingogram
- recurrent pregnancy loss
- intracavitary fibroids before surgery to determine the depth of myometrial involvement and operative hysteroscopic resectability (see “Using saline infusion sonography staging to plan for fibroid surgery”)
- the endometrium after surgery
Complications are infrequent. The risk of infection is less than 1%. Practitioners should follow the same protocols for administering antibiotics as they do with hysterosalpingogram or other invasive endometrial procedures.
Possible problems during SIS include cervical stenosis, inability to distend the endometrium, uterine contractions, and heavy vaginal bleeding with resultant artifacts. There is also the risk of performing the procedure in early pregnancy, as well as the theoretic risk of disseminating endometrial cancer.
Sensitivity and specificity. Interpreting SIS images requires experience, correlation with the menstrual history, and careful scanning. In premenopausal women, SIS has an overall sensitivity of 94% and a specificity of 85%.8 In 1 review, its sensitivity for detecting endometrial polyps was 93%, and its specificity was 96%.8 In the detection of submucosal fibroids, it had a sensitivity of 94% and a specificity of 95%.8
Another investigation compared the accuracy and pain of SIS with flexible office hysteroscopy.14 For all findings combined—including polyps, submucous myomas, synechiae, endometrial hyperplasia, and cancer—SIS’s sensitivity was 96% and its specificity was 88%, compared with hysteroscopy. SIS did not miss any intracavitary lesions. It was also less painful than hysteroscopy. False-positive results included diagnosing endometrial polyps as fibroids or vice versa. This is not really a problem, however, since both lesions are associated with bleeding abnormalities and both are removed with the operative hysteroscope.
False-positive results may be caused by blood, debris, clots, thickened endometrial folds, secretory endometrium, detached fragments of endometrium, or endometrium that was sheared during placement of the catheter. (When SIS is performed in the presence of active uterine bleeding, endometrial clots may sometimes be mistaken for polyps, fibroids, or hyperplasia.)
Because false positives may lead to unnecessary surgical intervention,16 SIS should be performed when these are least likely. For premenopausal women, that means scheduling SIS within 1 to 7 days of the menstrual cycle’s completion. In postmenopausal women, the patient should be scanned when she is not bleeding, if possible. If she is taking sequential HRT, SIS is best performed after progesterone withdrawal.
Until insurers realize the advantages of office-based hysteroscopy and reimburse accordingly, women will be unnecessarily subjected to surgery, preoperative testing, lost time, and increased anxiety.
Some have suggested that if SIS detects a lesion smaller than 10 mm, the procedure should be repeated when the patient is not bleeding. If the lesion is still present, operative intervention is appropriate.17,18
Cost issues. The development of clinical algorithms incorporating SIS—particularly those that take into account the differences between evaluation of premenopausal and postmenopausal women—may decrease costs by eliminating unnecessary D&Cs and endometrial biopsies, including those that are hysteroscopically directed.
For example, a study examining almost 1,200 women in 4 Nordic countries suggests that in women with postmenopausal bleeding and an endometrial thickness of 4 mm or less, curettage is unnecessary.19 An algorithm developed after the evaluation of more than 400 perimenopausal women concluded that hysteroscopically directed endometrial biopsy is best for women with focal processes, while endometrial aspiration biopsy is best for those with global disease.20
Although SIS overcomes many of the limitations of TVUS, in some cases the endometrium simply cannot be visualized.
In general, SIS is less costly to perform than hysteroscopy.
For a successful surgical outcome, it is important to preoperatively identify the size, number, location, and depth of intramural extension. Fibroid size and location affect resectability, the number of surgical procedures necessary for complete resection, the duration of surgery, and the potential complications from fluid overload.1
Using saline-infusion sonography (SIS), uterine fibroids can be categorized into 3 classes. An experienced surgeon can successfully resect class 1 (FIGURE 1) or class 2 (FIGURE 2) fibroids hysteroscopically. Class 3 fibroids (FIGURE 3) are less amenable to such surgery because perforation is a significant risk. Fluid overload, contracture of the endometrial cavity, and incomplete resection also are possible complications.2
FIGURE 1 Class 1 fibroids
Class 1 fibroids occur completely within the uterine cavity, with no involvement of myometrium. The base or stalk is visible with SIS.
FIGURE 2 Class 2 fibroids
Class 2 fibroids have a submucosal component involving less than half of the myometruim.
FIGURE 3 Class 3 fibroids
Class 3 fibroids have an intramural component of greater than 50%. They can be transmural, and may be located anywhere from the submucosa to the serosa. These fibroids often appear as a bulge or indentation into the submucosa.
REFERENCES
1. Emanuel MH, Verdel MJ, Wamsteker K. A prospective comparison of transvaginal ultrasonography and diagnostic hysteroscopy in the evaluation of patients with abnormal uterine bleeding: Clinical implications. Am J Obstet Gynecol. 1995;172:547-552.
2. Bradley LD, Falcone T, Magen AB. Radiographic imaging techniques for the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:245-276.
Office hysteroscopy
Hysteroscopy has revolutionized the practice of gynecology. Thanks to improvements in optics and subsequent reduction in hysteroscope diameters in the 1980s and 1990s, office use has become practical. Thin operative hysteroscopes with outer diameters ranging from 3 mm to 5 mm now are available. A study in 417 women undergoing flexible hysteroscopy in an office setting without anesthesia demonstrated the patient acceptability, diagnostic accuracy, and cost-effectiveness of this technology.21
Advantages of hysteroscopic visualization include:
- immediate office evaluation
- direct visualization of the endometrium and endocervix
- the ability to detect minute focal endometrial pathology
- the ability to perform directed endometrial biopsies (with some hysteroscopes) and lyse filmy adhesions with the distal tip of the flexible hysteroscope22
Office hysteroscopy is comfortable and quick and associated with low complication rates. Preprocedural nonsteroidal agents or misoprostol may make the procedure more tolerable.
Disadvantages of office hysteroscopy include the need for expensive office equipment (camera, insufflator, hysteroscope, video equipment, etc.) and a skillful and experienced hysteroscopist, as well as the costliness of the procedure.
Complications. The complication rate is low (less than 1%) when a skilled physician performs the procedure. Complications include uterine perforation, infections, and excessive bleeding; complications related to the distending medium also have been recorded.23
Indications for use. Entities that can be visualized hysteroscopically include:
- endometrial polyps
- submucosal and intramural fibroids
- synechiae
- retained products of conception
- foreign bodies
- endocervical lesions
- endometrial atrophy
- endometrial hyperplasia and cancer
- arteriovenous malformations
- gestational trophoblastic disease
- pregnancy (although hysteroscopy should be avoided when a viable intrauterine pregnancy has been documented)
Infrequently, endometrial gland openings associated with adenomyosis also can be seen.
On occasion, it may be difficult to distinguish hysteroscopically between a sessile polyp and submucosal fibroid if the typical characteristics of a polyp or fibroid are not appreciated. Luckily, treatment with operative hysteroscopy is the same for both findings.
Special considerations.
- Lesion size. The hysteroscopist must remember that it can be difficult to estimate the size of lesions noted. The reason: The eyepiece is focused at infinity, so objects that are closer appear magnified and objects that are further away appear smaller.24 This can lead to surprises in the operating room, especially when the size of a lesion has been underestimated. In this regard, hysteroscopy is not as accurate as TVUS.
- Rigid versus flexible. Some gynecologists may prefer to use a rigid hysteroscope because it permits a greater viewing angle than a flexible hysteroscope. In a rigid instrument, that angle ranges from 0 to 30 degrees. The outer sheaths of most rigid hysteroscopes are 3 mm to 7 mm. Larger-diameter sheaths permit insertion of biopsy instruments and graspers for directed endometrial biopsies.
- The trouble with high pressure. Hysteroscopy can produce a falsely negative view when the operator uses CO2 or high intrauterine pressure, since higher pressures can flatten an endometrial lesion. For this reason, it is wise for the hysteroscopist to get in the habit of slowly deflating the uterine cavity and carefully reinspecting the endometrial surface before concluding the procedure. A note about this maneuver should be included in the procedure report. If this approach is followed consistently, it should increase accuracy, reduce false negatives and medicolegal risk, and lead to better outcomes.
- The specificity and PPV of hysteroscopy in cases of abnormal uterine bleeding should theoretically be 100%. In practice, however, the false-negative rate is 2% to 4%—the result of operator error.25
Cost issues. The CPT code for office hysteroscopy is 58555. Regrettably, poor reimbursement has prevented rapid assimilation of this unique tool into office practice. Until insurance companies realize the marked advantages of this quick, safe, and comfortable office-based procedure and reimburse accordingly, women will be unnecessarily subjected to the rigors of surgical clearance and preoperative testing, loss of time from work and family, and the increased anxiety of having this procedure performed in an operating suite.
Comparing modalities
Investigators compared the accuracy of TVUS, transabdominal sonohysterography, and hysteroscopy in detecting submucosal fibroids (and determining their size) and myometrial growth in 52 premenopausal women scheduled for hysterectomy.26 Transabdominal sonohysterography most accurately predicted size and myometrial ingrowth of fibroids. Hysteroscopy was least accurate in detecting the myoma’s size, perhaps because of the optical refractive index with which it is associated. Still, hysteroscopy is more accurate than blind biopsy alone in detecting intracavitary lesions such as polyps and fibroids.27
SIS versus TVUS. SIS provides a more comprehensive view of the pelvic anatomy than TVUS alone, with more concentrated visualization of the endometrium. However, it can pose some technical difficulties. One group of investigators was unable to complete the procedure in patients with a uterus larger than 12 to 14 weeks, submucosal fibroids greater than 4 cm, polyps that filled the endometrial cavity, or large transmural fibroids that precluded distention of the endometrium.28 Other limitations of SIS include the inability to thread the catheter, iatrogenic introduction of air bubbles into the uterus, and the inability to maintain distension in patients with a patulous cervix.
Evaluation of the endometrium is not ‘either-or.’ Sometimes a combination of procedures aids diagnosis of menstrual dysfunction.
Patients with a distended cervix may require an SIS catheter with a balloon that occludes the lower uterine segment. Placement of the intrauterine catheter may be difficult in patients with cervical stenosis, isthmic synechiae, a markedly retroverted uterus, or intrauterine septa. Women with cervical stenosis may benefit from placement of laminaria tents or misoprostol29 (orally 100 μg 8 to 12 hours before the procedure or vaginally 200 μg to 400 μg 2 to 4 hours before the procedure). Uterine sounding may sufficiently disrupt synechiae. Cervical traction with a single-toothed tenaculum can straighten the uterine axis if marked retroversion is present.
Regardless of the patient’s age, abnormal uterine bleeding requires an aggressive workup.
SIS versus hysteroscopy. Compared with hysteroscopy, SIS more reliably predicts uterine fibroids’ size and the depth of myometrial involvement (TABLE 2).26 In addition,SIS permits classification of location, size, and degree of intramural extension. This allows the clinician to determine the resectability of the lesion and select the appropriate surgical approach.15
However, a recent comparison of SIS and hysteroscopy in diagnosing uterine pathology in 90 women with abnormal uterine bleeding showed that SIS had a high failure rate (34%) among postmenopausal women, usually because of cervical stenosis.30 In premenopausal women, that rate was lower (10.3%). The failure rate of hysteroscopy, meanwhile, was 10.6% in postmenopausal women and 2.9% in premenopausal women. Another investigation of hysteroscopy noted a failure rate of 8% in postmenopausal women.31
Complementary technologies. Although SIS overcomes many of the limitations of TVUS, in some cases the endometrium simply cannot be visualized or is indistinct, or findings are indeterminate. If the endometrium is not well visualized with SIS, then hysteroscopy plays a definitive role in ascertaining endometrial morphology. In addition to evaluating equivocal or indeterminate SIS findings, hysteroscopy is invaluable when menstrual aberrations persist despite a normal SIS.
Fortunately, evaluation of the endometrium is not an “either-or” proposition. Sometimes a combination of procedures may be important in solving the puzzle of menstrual dysfunction.
- Equivocal TVUS results. If the patient had a TVUS that led to an equivocal interpretation of the endometrium, clinicians should proceed with SIS or office hysteroscopy. If the woman continues to experience abnormal uterine bleeding after a normal SIS, diagnostic hysteroscopy should be performed to rule out occult lesions in the cornu, endocervix, or uterus.
- Pap test. It seems prudent to perform a Papanicolaou test, including an endocervical and endometrial biopsy, in high-risk patients. (This includes patients who are chronically anovulatory, obese, or nulliparous; those who use tamoxifen or unopposed estrogen; and women with untreated endometrial hyperplasia.) Keep in mind, however, that only 50% of women with endometrial cancer actually have risk factors for the disease.32
TABLE 2
Comparison of saline-infusion sonography (SIS) and hysteroscopy
| CHARACTERISTIC | SIS | HYSTEROSCOPY |
|---|---|---|
| Pain | Less | More |
| Adnexal imaging | Possible | Impossible |
| Determination of depth of fibroid penetration | Possible (accurate) | Not possible unless lesion is pedunculated |
| Cost | Less | More |
| Office based | Yes | Yes |
| Large uterine size | Difficult if uterus is >14 weeks’ size | Yes |
| Ability to complete exam | >95% cases | >95% cases |
| Complication rate | <1% | <1% |
Aggressive workup is warranted
Regardless of the patient’s age, abnormal uterine bleeding requires an aggressive workup. If we are diligent and find atrophy, we can reassure and educate the patient about the fragility of the menopausal endometrium. In most women, short-term, low-dose hormone replacement therapy is the right antidote. For the perimenopausal woman, “hormonal problems” can best be verified by a negative workup with hysteroscopy or SIS. Treatment usually consists of a low-dose contraceptive or a levonorgestrel intrauterine device, which is associated with shorter, lighter periods.
Dr. Bradley reports that she serves as a consultant to Olympus and Karl Storz.
1. Rogerson L, Duffy S. A national survey of outpatient hysteroscopy. Gynecol Endosc. 2001;10:343-348.
2. Sheikh M, Sawhney S, Khurana A, Al-Yatama M. Alteration of sonographic texture of the endometrium in post-menopausal bleeding. A guide to further management. Acta Obstet Gynecol Scand. 2000;79:1006-1010.
3. Persadie RJ. Ultrasonographic assessment of endometrial thickness: A review. J Obstet Gynaecol Can. 2002;24(2):131-136.
4. Goldstein RB, Bree RL, Benson CB, et al. Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med. 2001;20:1025-1036.
5. Weigel M, Friese K, Strittmatter HJ, Meichert F. Measuring the thickness—is that all we have to do for sonographic assessment of endometrium in postmenopausal women? Ultrasound Obstet Gynecol. 1995;6:97-102.
6. Schwartz LB, Snyder J, Horan C, Porges RF, Nachitgall LE, Goldstein SR. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patient on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
7. Shalev J, Meizner I, Bar-Hava I, Dicker D, Mashiach R, Ben-Rafael Z. Predictive value of transvaginal sonography performed before routine diagnostic hysteroscopy for evaluation of infertility. Fert Steril. 2000;73:412-417.
8. Dueholm M, Lundorf E, Olesen F. Imaging techniques for evaluation of the uterine cavity and endometrium in premenopausal patients before minimally invasive surgery. Obstet Gynecol Surv. 2002;57:389-403.
9. Langer RD, Pierce JJ, O’Hanlan KA, et al. Transvaginal ultrasonography compared with endometrial biopsy for the detection of endometrial disease. N Engl J Med. 1997;337:1792-1798.
10. Gull B, Karlsson B, Milson L, Granberg S. Can ultrasound replace dilation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol. 2003;188:401-408.
11. Carlson JA, Arger P, Thompson S, Carlson EJ. Clinical and pathologic correlation of endometrial cavity fluid detected by ultrasound in the postmenopausal patient. Obstet Gynecol. 1991;77:119-123.
12. Weber A, Belinson J, Bradley LD, Piedmonte M. Vaginal ultrasound versus endometrial biopsy in women with postmenopausal bleeding. Am J Obstet Gynecol. 1997;177:924-929.
13. Laifer-Narin S, Ragavendra N, Parmenter EK, Grant EG. False-normal appearance of the endometrium on conventional transvaginal sonography: Comparison with saline hysterosonography. AJR. 2002;178(1):129-133.
14. Widrich T, Bradley L, Mitchinson AR, Collins R. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol. 1996;174:1327-1334.
15. Bradley LD, Falcone T, Magen AB. Radiographic imaging techniques for the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:245-276.
16. Mihm LM, Quick VA, Brumfield JA, Connors AF, Jr, Finnerty JJ. The accuracy of endometrial biopsy and saline sonohysterography in the determination of the cause of abnormal uterine bleeding. Am J Obstet Gynecol. 2002;186:858-860.
17. Goldstein SR. Saline infusion sonohysterography. Clin Obstet Gynecol. 1996;39(1):248-258.
18. Dijkhuizen FP, DeVries LD, Mol BW, et al. Comparison of transvaginal ultrasonography and saline infusion sonography for the detection of intracavitary abnormalities in premenopausal women. Ultrasound Obstet Gynecol. 2000;15:372-376.
19. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol. 1995;172:1488-1494.
20. Goldstein S. Use of ultrasonohysterography for triage of perimenopausal patients with unexplained uterine bleeding. Obstet Gynecol. 1994;170:565-570.
21. Bradley L, Widrich T. State-of-the-art flexible hysteroscopy for office gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
22. Nagele F, O’Connor H, Davies A, Badawy A, Mohamed H, Magos A. 2,500 outpatient diagnostic hysteroscopies. Obstet Gynecol. 1996;88:87-92.
23. Serden SP. Diagnostic hysteroscopy to evaluate the cause of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:277-286.
24. Apgar B, Dewitt D. Diagnostic hysteroscopy. Am Fam Physician. 1992;46(5 suppl):19S-26S.
25. Gimpelson R, Roppold HA. Comparative study between panoramic hysteroscopy with directed biopsies and dilation and curettage. Am J Obstet Gynecol. 1988;158:489-494.
26. Cincinelli E, Romano F, Anastasio P, et al. Transabdominal sonohysterography, transvaginal sonography, and hysteroscopy in the evaluation of submucous myomas. Obstet Gynecol. 1995;85:42-47.
27. Pasqualotto EB, Margossian H, Price LL, Bradley LD. Accuracy of preoperative diagnostic tools and outcome of hysteroscopic management of menstrual dysfunction. J Am Assoc Gynecol Laparosc. 2000;7:201-209.
28. Kochli, OR ed. Hysteroscopy: State of the Art. Contributions to Gynecology and Obstetrics. Vol. 20. Karger Verlag Basel.
29. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: A randomized controlled trial. Obstet Gynecol. 2000;96:890-894.
30. Rogerson L, Bates J, Weston M, Duffy S. A comparison of outpatient hysteroscopy with saline infusion hysterosonography. BJOG. 2002;109:800-804.
31. Rose PG. Endometrial cancer. N Engl J Med. 1996;335:640-649.
32. Blumenfeld M, Turner P. Role of transvaginal sonography in the evaluation of endometrial hyperplasia and cancer. Clin Obstet Gynecol. 1996;39:641-655.
- Identification and measurement of the endometrial echo and descriptions of the echogenicity and heterogeneity of the endometrium are key to defining endometrial health.
- The introduction of intracervical fluid (saline-infusion sonography) during transvaginal ultrasound is one of the most significant advances in ultrasonography of the past decade.
- Hysteroscopic visualization has several advantages: immediate office evaluation, direct visualization of the endometrium and endocervix, and the ability to detect minute focal endometrial pathology and to perform directed endometrial biopsies.
- Sometimes a combination of procedures may be the best way to determine the cause of abnormal uterine bleeding.
Because office-based physicians tend to feel comfortable relying upon endometrial biopsy or dilation and curettage (D&C) to evaluate abnormal uterine bleeding, newer tools—transvaginal ultrasound (TVUS), saline-infusion sonography (SIS), and hysteroscopy—see far too little utilization. Although these modalities are remarkably user-friendly when employed correctly, only 28% of gynecologists perform office hysteroscopy, and even fewer use SIS.1
This article reviews indications for use, sensitivity and specificity, advantages and disadvantages, special considerations including cost issues, and suggestions for incorporating these modalities into gynecologic practice.
For most patients, neither endometrial biopsy nor D&C is particularly helpful in the assessment of abnormal uterine bleeding (see “Limitations of D&C and endometrial biopsy”). Does a negative biopsy signify health? An absence of intracavitary pathology? Or does it mean that we failed to sample the culprit?
Fortunately, abnormal uterine bleeding usually is attributable to endometrial conditions other than cancer, such as atrophy, hyperplasia, polyps, or fibroids. These pathologies may be benign, but they are still a nuisance. When left undetected and untreated, they can cause the patient much worry, along with weeks or months of unnecessary medical therapy and surgical procedures.
Dilation and curettage (D&C) detects benign pathology in about 80% of patients with menstrual dysfunction.1 It is most likely to detect the problem when pathology affects the endometrium globally.
A recent study reported on 105 postmenopausal women with bleeding and an endometrial echo of more than 5 mm who were evaluated with both hysteroscopy and D&C.2 Although 80% of the women had pathology in the uterine cavity, and 98% of the pathologic lesions manifested a focal growth pattern at hysteroscopy after D&C, the whole or parts of the lesion remained in situ in 87% of the women. In addition, D&C failed to detect 58% of polyps, 50% of hyperplasias, 60% of complex atypical hyperplasias, and 11% of endometrial cancers. When disease was global, D&C detected 94% of abnormalities.
Focal disease therefore mandates operative hysteroscopic-directed biopsy and removal of suspicious pathology.
Endometrial biopsy can be performed in the office without anesthesia—a great advantage. The technique is most helpful in “dating” the endometrium and diagnosing endometrial cancer or hyperplasia. Unfortunately, blind endometrial biopsy studies are frequently returned with pathology reports of insufficient tissue, atrophic changes, mucus and debris, scanty tissue, no visible endometrial tissue, endocervical tissue, or proliferative or secretory endometrium.
Further, when a blindly performed biopsy reveals normal histology, it does not necessarily rule out other pathology. In addition, a biopsy via endometrial suction curette frequently misses focal lesions such as endometrial polyps and submucosal fibroids. Global noninvasive surveillance of the endometrium is more effective at detecting such focal lesions.
Investigators who performed endometrial biopsy prior to hysterectomy in patients with known endometrial cancer demonstrated that the sensitivity of diagnosing endometrial cancer with a biopsy via endometrial suction curette increases when the pathology affects more than 50% of the surface area of the endometrial cavity.3 However, biopsy failed to detect cancer in 11 of 65 patients in whom the malignancy affected less than 50% of the endometrium. These 11 false negatives included 5 cases of endometrial polyps, 3 malignancies that affected less than 5% of the endometrium, and 7 cancers that affected less than 25% of the endometrium.
REFERENCES
1. Holst J, Koskela O, von Schoultz B. Endometrial findings following curettage in 2,018 women according to age and indications. Ann Chir Gynaecol. 1983;72:274-277.
2. Epstein E, Ramirez A, Skoog L, Valentin L. Dilation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136.
3. Guido RS, Kanbour-Shakir A, Rulin MC, Christopherson WA. Pipelle endometrial sampling: sensitivity in the detection of endometrial cancer. J Reprod Med. 1995;40:553-555.
Transvaginal ultrasound
TVUS is quick, convenient, inexpensive, and comfortable for the patient. It can evaluate the endometrium utilizing gray scale, color or power Doppler, contrast media (SIS), or 3-dimensional ultrasound technology. In addition, TVUS permits visualization of the adnexa and pelvic organs, including the bladder and cul de sac. Among the abnormalities detectable with TVUS are fibroids (including submucosal leiomyomas) and endometrial polyps. Not surprisingly, an experienced operator is crucial for a precise diagnosis.
Optimal TVUS evaluation includes endometrial measurements in the sagittal plane, with the bilayer thickness measured from the proximal to distal myometrialendometrial junctions. In the coronal view, measurement should be from the cervix to the fundus. When intraluminal fluid is present, each endometrial thickness should be measured separately (in single layers), and the combined endometrial thickness should be expressed as the sum of the 2 layers.
Characterize the endometrium. Sonographic hallmarks increasingly are used to describe uterine pathology. These include endometrial thickness, morphology, endocavitary lesions, borders, and myometrial invasion. In addition, a number of practitioners have attempted to describe the sonographic texture of the endometrium in post-menopausal women to increase sensitivity in the detection of disease, improve diagnosis, and guide treatment.
The endometrium can be characterized as homogeneous, diffusely inhomogeneous, or associated with focally or diffusely increased echogenicity. Textural inhomogeneity is present in cases of endometrial cancer.2 A homogeneous endometrium less than 6 mm thick is commonly associated with tissue insufficient for diagnosis. If focal or diffuse increased echogenicity occurs with a thin endometrium, SIS or hysteroscopy is more sensitive than TVUS in identifying the endometrial abnormality.
We rely increasingly on descriptions of the echogenicity and heterogeneity of the entire endometrial echo, as well as on echo measurements, to define endometrial health. Most experts use an endometrial echo of 5 mm as the cutoff for significant endometrial disease. A number of investigators have noted that the endometrial thickness in hyperplasia often ranges from 8 mm to 15 mm in post-menopausal women, but the number of reported cases is too low to draw firm conclusions based on endometrial echo alone.3,4
A prospective evaluation of 200 postmenopausal women with endometrial echo ranging from 3 mm to 10 mm noted that homogeneity, thin endometrium, and sonographically demonstrable central endometrium with symmetry were associated with absence of pathology. In contrast, heterogeneity and high echogenicity were indicative of pathology.5
Endometrial echo measurement and morphology increase sensitivity in predicting endometrial disease and can point to the need for ancillary testing with SIS or hysteroscopy. For example, in the postmenopausal patient, an endometrial echo of less than 5 mm, in combination with a negative endometrial biopsy, might be the only evaluation the patient needs if she responds to medical therapy for endometrial atrophy. If symptoms persist, office hysteroscopy or SIS could be performed to rule out endometrial polyps and endometrial hyperplasia (which is less likely).
Saline-infusion sonography offers an exquisite view of the endomyometrial complex that cannot be obtained with transvaginal ultrasound alone.
A thickened endometrial echo (more than 5 mm) at the initial TVUS in the postmenopausal patient should be evaluated promptly with SIS. In most of these patients, polyps or fibroids are the cause of bleeding.
Perform imaging at optimal time. In postmenopausal women, the endometrial thickness remains constant unless the patient is taking hormone replacement therapy (HRT) or tamoxifen.6 Thus, it is easier to detect or predict endometrial pathology in this population.
In premenopausal women, TVUS is most likely to detect fibroids during the early follicular stage because the endometrium is thin, usually measuring 2 mm to 4 mm. Endometrial polyps and submucosal fibroids are best viewed when a trilayered midfollicular endometrium is present, while uterine synechiae are visualized most clearly during midcycle. Adhesions appear as hyperechoic irregular structures that vary in size from 2 mm to 6 mm. They interrupt the continuity of the endometrial layer and appear much different from polyps, which tend to be round, symmetrical, and uniform.7
Diagnosing endometrial hyperplasia among premenopausal women based on an absolute endometrial measurement is more difficult than it is in postmenopausal women because of the wide range in thickness associated with the menstrual cycle (TABLE 1). Although endometrial hyperplasia or cancer is more likely when the endometrial echo is thicker than anticipated based on age or menstrual phase, it can only be proven definitively with histologic sampling.
Sensitivity and specificity. Because of the monotonous nature of the endometrium in healthy postmenopausal women, TVUS has higher sensitivity in this age group than in reproductive-age women. In the postmenopausal population, the sensitivity for detecting uterine pathology is 87%, while specificity is 82%.
Office hysteroscopy offers immediate evaluation and direct visualization of the endometrium and endocervix.
In premenopausal women, polyps were the pathology most likely to be missed, according to one literature review.8 TVUS identified only 275 of 344 polyps in this population—a sensitivity of 80%. When submucosal fibroids were located near the endometrium, the diagnostic sensitivity rose to 94%. It was difficult to discern location (i.e., submucosal or intramural or polyps) with TVUS alone, however.
Another investigation compared the sensitivity and specificity of TVUS and endometrial biopsy for the detection of endometrial disease in 448 postmenopausal women who took estrogen alone, cyclic or continuous estrogen-progesterone, or placebo for 3 years.9 Using a threshold value of 5 mm for endometrial thickness, TVUS had a positive predictive value (PPV) of 9% for detecting any abnormality, with 90% sensitivity, 48% specificity, and a negative predictive value (NPV) of 99%. Using this threshold, biopsies would be indicated in more than half of the women, of whom only 4% actually had serious disease.
Using a threshold thickness of 4 mm, Gull et al10 evaluated TVUS for the detection of endometrial cancer and atypical hyperplasia. For endometrial thicknesses exceeding 4 mm, TVUS had a sensitivity of 100%, specificity of 60%, a PPV of 25%, and an NPV of 100%. No woman with an endometrial thickness of 4 mm or less was found to have endometrial cancer.
Special considerations.
- Intracavitary fluid. On occasion, TVUS reveals “naturally occurring SIS”—that is, the spontaneous appearance of intracavitary fluid, which is probably secondary to cervical stenosis. (The name of this phenomenon refers to the iatrogenic fluid that is intrinsic to SIS.) Endometrial fluid also is observed in women who use tamoxifen, diethylstilbestrol, or megestrol acetate and in those who have a hematometra. In addition, about one quarter of patients with an endometrial malignancy have fluid in the endometrial cavity.11
- Endometrial thickness. As noted, TVUS is excellent for ruling out endometrial abnormalities in postmenopausal patients because of the consistent thickness of the endometrium. When the endometrium is especially thickened, however, its usefulness is limited.
Observations in more than 5,000 women consistently note that an endometrium of less than 5 mm, measured as a double-layer, is most often associated with a pathology reading of “tissue insignificant for diagnosis”—hence atrophy.12 When the endometrium is thicker than 5 mm, there is a greater chance of detecting polyps, endometrial hyperplasia, and endometrial cancer. Fewer than 0.12% of cancers were missed in this series.
When a skilled physician performs office hysteroscopy, the complication rate is less than 1%.
If the endometrium is thicker than 6 mm, then SIS or hysteroscopy is more helpful than TVUS in reaching a conclusive etiology for uterine bleeding. Further evaluation (hysteroscopy or SIS) is usually recommended when TVUS measurements of the endometrium are greater than 5 mm.13 Although a low cut-off, such as 4 mm or 5 mm, is associated with improved sensitivity, it sacrifices specificity.
TABLE 1
Transvaginal ultrasound: Endometrial thickness32
| PHASE | THICKNESS (MM) |
|---|---|
| Menstrual | 2-4 |
| Early proliferative | 4-6 |
| Periovulatory | 6-8 |
| Secretory | 8-12 |
| Postmenopausal | 4-8 |
| Postmenopausal on hormone replacement therapy | 4-10 |
Saline-infusion sonography
In SIS, saline is infused into the endometrial cavity during TVUS to enhance the view of the endometrium. This constitutes one of the most significant advances in ultrasonography of the past decade. SIS can provide a wealth of information about the uterus and adnexa in patients with abnormal bleeding. It offers an exquisite view of the endomyometrial complex that cannot be obtained with TVUS alone. It differentiates between focal and global processes and improves overall sensitivity for detecting abnormalities of the endometrium.
Although many terms have been used to describe this technique (echohysteroscopy, sonohysterosalpingography, and hydrosonography, to name a few), the term “saline-infusion sonography,” coined in 1996, most clearly describes the technique.14
Indications for use. SIS has been used to evaluate:15
- menstrual disorders
- endometrium that is thickened, irregular, immeasurable, or poorly defined on conventional TVUS, magnetic resonance imaging, or computed axial tomography studies
- midline endometrial echo with a thickness of more than 8 mm
- endometrium that appears irregular, bizarre, or homogeneous on TVUS in women using tamoxifen
- sessile and pedunculated masses of the endometrium that need to be differentiated
- findings with hysterosalpingogram
- recurrent pregnancy loss
- intracavitary fibroids before surgery to determine the depth of myometrial involvement and operative hysteroscopic resectability (see “Using saline infusion sonography staging to plan for fibroid surgery”)
- the endometrium after surgery
Complications are infrequent. The risk of infection is less than 1%. Practitioners should follow the same protocols for administering antibiotics as they do with hysterosalpingogram or other invasive endometrial procedures.
Possible problems during SIS include cervical stenosis, inability to distend the endometrium, uterine contractions, and heavy vaginal bleeding with resultant artifacts. There is also the risk of performing the procedure in early pregnancy, as well as the theoretic risk of disseminating endometrial cancer.
Sensitivity and specificity. Interpreting SIS images requires experience, correlation with the menstrual history, and careful scanning. In premenopausal women, SIS has an overall sensitivity of 94% and a specificity of 85%.8 In 1 review, its sensitivity for detecting endometrial polyps was 93%, and its specificity was 96%.8 In the detection of submucosal fibroids, it had a sensitivity of 94% and a specificity of 95%.8
Another investigation compared the accuracy and pain of SIS with flexible office hysteroscopy.14 For all findings combined—including polyps, submucous myomas, synechiae, endometrial hyperplasia, and cancer—SIS’s sensitivity was 96% and its specificity was 88%, compared with hysteroscopy. SIS did not miss any intracavitary lesions. It was also less painful than hysteroscopy. False-positive results included diagnosing endometrial polyps as fibroids or vice versa. This is not really a problem, however, since both lesions are associated with bleeding abnormalities and both are removed with the operative hysteroscope.
False-positive results may be caused by blood, debris, clots, thickened endometrial folds, secretory endometrium, detached fragments of endometrium, or endometrium that was sheared during placement of the catheter. (When SIS is performed in the presence of active uterine bleeding, endometrial clots may sometimes be mistaken for polyps, fibroids, or hyperplasia.)
Because false positives may lead to unnecessary surgical intervention,16 SIS should be performed when these are least likely. For premenopausal women, that means scheduling SIS within 1 to 7 days of the menstrual cycle’s completion. In postmenopausal women, the patient should be scanned when she is not bleeding, if possible. If she is taking sequential HRT, SIS is best performed after progesterone withdrawal.
Until insurers realize the advantages of office-based hysteroscopy and reimburse accordingly, women will be unnecessarily subjected to surgery, preoperative testing, lost time, and increased anxiety.
Some have suggested that if SIS detects a lesion smaller than 10 mm, the procedure should be repeated when the patient is not bleeding. If the lesion is still present, operative intervention is appropriate.17,18
Cost issues. The development of clinical algorithms incorporating SIS—particularly those that take into account the differences between evaluation of premenopausal and postmenopausal women—may decrease costs by eliminating unnecessary D&Cs and endometrial biopsies, including those that are hysteroscopically directed.
For example, a study examining almost 1,200 women in 4 Nordic countries suggests that in women with postmenopausal bleeding and an endometrial thickness of 4 mm or less, curettage is unnecessary.19 An algorithm developed after the evaluation of more than 400 perimenopausal women concluded that hysteroscopically directed endometrial biopsy is best for women with focal processes, while endometrial aspiration biopsy is best for those with global disease.20
Although SIS overcomes many of the limitations of TVUS, in some cases the endometrium simply cannot be visualized.
In general, SIS is less costly to perform than hysteroscopy.
For a successful surgical outcome, it is important to preoperatively identify the size, number, location, and depth of intramural extension. Fibroid size and location affect resectability, the number of surgical procedures necessary for complete resection, the duration of surgery, and the potential complications from fluid overload.1
Using saline-infusion sonography (SIS), uterine fibroids can be categorized into 3 classes. An experienced surgeon can successfully resect class 1 (FIGURE 1) or class 2 (FIGURE 2) fibroids hysteroscopically. Class 3 fibroids (FIGURE 3) are less amenable to such surgery because perforation is a significant risk. Fluid overload, contracture of the endometrial cavity, and incomplete resection also are possible complications.2
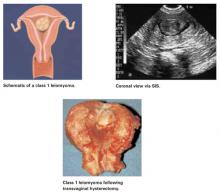
FIGURE 1 Class 1 fibroids
Class 1 fibroids occur completely within the uterine cavity, with no involvement of myometrium. The base or stalk is visible with SIS.
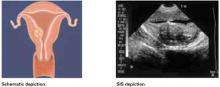
FIGURE 2 Class 2 fibroids
Class 2 fibroids have a submucosal component involving less than half of the myometruim.
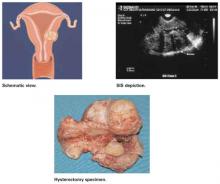
FIGURE 3 Class 3 fibroids
Class 3 fibroids have an intramural component of greater than 50%. They can be transmural, and may be located anywhere from the submucosa to the serosa. These fibroids often appear as a bulge or indentation into the submucosa.
REFERENCES
1. Emanuel MH, Verdel MJ, Wamsteker K. A prospective comparison of transvaginal ultrasonography and diagnostic hysteroscopy in the evaluation of patients with abnormal uterine bleeding: Clinical implications. Am J Obstet Gynecol. 1995;172:547-552.
2. Bradley LD, Falcone T, Magen AB. Radiographic imaging techniques for the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:245-276.
Office hysteroscopy
Hysteroscopy has revolutionized the practice of gynecology. Thanks to improvements in optics and subsequent reduction in hysteroscope diameters in the 1980s and 1990s, office use has become practical. Thin operative hysteroscopes with outer diameters ranging from 3 mm to 5 mm now are available. A study in 417 women undergoing flexible hysteroscopy in an office setting without anesthesia demonstrated the patient acceptability, diagnostic accuracy, and cost-effectiveness of this technology.21
Advantages of hysteroscopic visualization include:
- immediate office evaluation
- direct visualization of the endometrium and endocervix
- the ability to detect minute focal endometrial pathology
- the ability to perform directed endometrial biopsies (with some hysteroscopes) and lyse filmy adhesions with the distal tip of the flexible hysteroscope22
Office hysteroscopy is comfortable and quick and associated with low complication rates. Preprocedural nonsteroidal agents or misoprostol may make the procedure more tolerable.
Disadvantages of office hysteroscopy include the need for expensive office equipment (camera, insufflator, hysteroscope, video equipment, etc.) and a skillful and experienced hysteroscopist, as well as the costliness of the procedure.
Complications. The complication rate is low (less than 1%) when a skilled physician performs the procedure. Complications include uterine perforation, infections, and excessive bleeding; complications related to the distending medium also have been recorded.23
Indications for use. Entities that can be visualized hysteroscopically include:
- endometrial polyps
- submucosal and intramural fibroids
- synechiae
- retained products of conception
- foreign bodies
- endocervical lesions
- endometrial atrophy
- endometrial hyperplasia and cancer
- arteriovenous malformations
- gestational trophoblastic disease
- pregnancy (although hysteroscopy should be avoided when a viable intrauterine pregnancy has been documented)
Infrequently, endometrial gland openings associated with adenomyosis also can be seen.
On occasion, it may be difficult to distinguish hysteroscopically between a sessile polyp and submucosal fibroid if the typical characteristics of a polyp or fibroid are not appreciated. Luckily, treatment with operative hysteroscopy is the same for both findings.
Special considerations.
- Lesion size. The hysteroscopist must remember that it can be difficult to estimate the size of lesions noted. The reason: The eyepiece is focused at infinity, so objects that are closer appear magnified and objects that are further away appear smaller.24 This can lead to surprises in the operating room, especially when the size of a lesion has been underestimated. In this regard, hysteroscopy is not as accurate as TVUS.
- Rigid versus flexible. Some gynecologists may prefer to use a rigid hysteroscope because it permits a greater viewing angle than a flexible hysteroscope. In a rigid instrument, that angle ranges from 0 to 30 degrees. The outer sheaths of most rigid hysteroscopes are 3 mm to 7 mm. Larger-diameter sheaths permit insertion of biopsy instruments and graspers for directed endometrial biopsies.
- The trouble with high pressure. Hysteroscopy can produce a falsely negative view when the operator uses CO2 or high intrauterine pressure, since higher pressures can flatten an endometrial lesion. For this reason, it is wise for the hysteroscopist to get in the habit of slowly deflating the uterine cavity and carefully reinspecting the endometrial surface before concluding the procedure. A note about this maneuver should be included in the procedure report. If this approach is followed consistently, it should increase accuracy, reduce false negatives and medicolegal risk, and lead to better outcomes.
- The specificity and PPV of hysteroscopy in cases of abnormal uterine bleeding should theoretically be 100%. In practice, however, the false-negative rate is 2% to 4%—the result of operator error.25
Cost issues. The CPT code for office hysteroscopy is 58555. Regrettably, poor reimbursement has prevented rapid assimilation of this unique tool into office practice. Until insurance companies realize the marked advantages of this quick, safe, and comfortable office-based procedure and reimburse accordingly, women will be unnecessarily subjected to the rigors of surgical clearance and preoperative testing, loss of time from work and family, and the increased anxiety of having this procedure performed in an operating suite.
Comparing modalities
Investigators compared the accuracy of TVUS, transabdominal sonohysterography, and hysteroscopy in detecting submucosal fibroids (and determining their size) and myometrial growth in 52 premenopausal women scheduled for hysterectomy.26 Transabdominal sonohysterography most accurately predicted size and myometrial ingrowth of fibroids. Hysteroscopy was least accurate in detecting the myoma’s size, perhaps because of the optical refractive index with which it is associated. Still, hysteroscopy is more accurate than blind biopsy alone in detecting intracavitary lesions such as polyps and fibroids.27
SIS versus TVUS. SIS provides a more comprehensive view of the pelvic anatomy than TVUS alone, with more concentrated visualization of the endometrium. However, it can pose some technical difficulties. One group of investigators was unable to complete the procedure in patients with a uterus larger than 12 to 14 weeks, submucosal fibroids greater than 4 cm, polyps that filled the endometrial cavity, or large transmural fibroids that precluded distention of the endometrium.28 Other limitations of SIS include the inability to thread the catheter, iatrogenic introduction of air bubbles into the uterus, and the inability to maintain distension in patients with a patulous cervix.
Evaluation of the endometrium is not ‘either-or.’ Sometimes a combination of procedures aids diagnosis of menstrual dysfunction.
Patients with a distended cervix may require an SIS catheter with a balloon that occludes the lower uterine segment. Placement of the intrauterine catheter may be difficult in patients with cervical stenosis, isthmic synechiae, a markedly retroverted uterus, or intrauterine septa. Women with cervical stenosis may benefit from placement of laminaria tents or misoprostol29 (orally 100 μg 8 to 12 hours before the procedure or vaginally 200 μg to 400 μg 2 to 4 hours before the procedure). Uterine sounding may sufficiently disrupt synechiae. Cervical traction with a single-toothed tenaculum can straighten the uterine axis if marked retroversion is present.
Regardless of the patient’s age, abnormal uterine bleeding requires an aggressive workup.
SIS versus hysteroscopy. Compared with hysteroscopy, SIS more reliably predicts uterine fibroids’ size and the depth of myometrial involvement (TABLE 2).26 In addition,SIS permits classification of location, size, and degree of intramural extension. This allows the clinician to determine the resectability of the lesion and select the appropriate surgical approach.15
However, a recent comparison of SIS and hysteroscopy in diagnosing uterine pathology in 90 women with abnormal uterine bleeding showed that SIS had a high failure rate (34%) among postmenopausal women, usually because of cervical stenosis.30 In premenopausal women, that rate was lower (10.3%). The failure rate of hysteroscopy, meanwhile, was 10.6% in postmenopausal women and 2.9% in premenopausal women. Another investigation of hysteroscopy noted a failure rate of 8% in postmenopausal women.31
Complementary technologies. Although SIS overcomes many of the limitations of TVUS, in some cases the endometrium simply cannot be visualized or is indistinct, or findings are indeterminate. If the endometrium is not well visualized with SIS, then hysteroscopy plays a definitive role in ascertaining endometrial morphology. In addition to evaluating equivocal or indeterminate SIS findings, hysteroscopy is invaluable when menstrual aberrations persist despite a normal SIS.
Fortunately, evaluation of the endometrium is not an “either-or” proposition. Sometimes a combination of procedures may be important in solving the puzzle of menstrual dysfunction.
- Equivocal TVUS results. If the patient had a TVUS that led to an equivocal interpretation of the endometrium, clinicians should proceed with SIS or office hysteroscopy. If the woman continues to experience abnormal uterine bleeding after a normal SIS, diagnostic hysteroscopy should be performed to rule out occult lesions in the cornu, endocervix, or uterus.
- Pap test. It seems prudent to perform a Papanicolaou test, including an endocervical and endometrial biopsy, in high-risk patients. (This includes patients who are chronically anovulatory, obese, or nulliparous; those who use tamoxifen or unopposed estrogen; and women with untreated endometrial hyperplasia.) Keep in mind, however, that only 50% of women with endometrial cancer actually have risk factors for the disease.32
TABLE 2
Comparison of saline-infusion sonography (SIS) and hysteroscopy
| CHARACTERISTIC | SIS | HYSTEROSCOPY |
|---|---|---|
| Pain | Less | More |
| Adnexal imaging | Possible | Impossible |
| Determination of depth of fibroid penetration | Possible (accurate) | Not possible unless lesion is pedunculated |
| Cost | Less | More |
| Office based | Yes | Yes |
| Large uterine size | Difficult if uterus is >14 weeks’ size | Yes |
| Ability to complete exam | >95% cases | >95% cases |
| Complication rate | <1% | <1% |
Aggressive workup is warranted
Regardless of the patient’s age, abnormal uterine bleeding requires an aggressive workup. If we are diligent and find atrophy, we can reassure and educate the patient about the fragility of the menopausal endometrium. In most women, short-term, low-dose hormone replacement therapy is the right antidote. For the perimenopausal woman, “hormonal problems” can best be verified by a negative workup with hysteroscopy or SIS. Treatment usually consists of a low-dose contraceptive or a levonorgestrel intrauterine device, which is associated with shorter, lighter periods.
Dr. Bradley reports that she serves as a consultant to Olympus and Karl Storz.
- Identification and measurement of the endometrial echo and descriptions of the echogenicity and heterogeneity of the endometrium are key to defining endometrial health.
- The introduction of intracervical fluid (saline-infusion sonography) during transvaginal ultrasound is one of the most significant advances in ultrasonography of the past decade.
- Hysteroscopic visualization has several advantages: immediate office evaluation, direct visualization of the endometrium and endocervix, and the ability to detect minute focal endometrial pathology and to perform directed endometrial biopsies.
- Sometimes a combination of procedures may be the best way to determine the cause of abnormal uterine bleeding.
Because office-based physicians tend to feel comfortable relying upon endometrial biopsy or dilation and curettage (D&C) to evaluate abnormal uterine bleeding, newer tools—transvaginal ultrasound (TVUS), saline-infusion sonography (SIS), and hysteroscopy—see far too little utilization. Although these modalities are remarkably user-friendly when employed correctly, only 28% of gynecologists perform office hysteroscopy, and even fewer use SIS.1
This article reviews indications for use, sensitivity and specificity, advantages and disadvantages, special considerations including cost issues, and suggestions for incorporating these modalities into gynecologic practice.
For most patients, neither endometrial biopsy nor D&C is particularly helpful in the assessment of abnormal uterine bleeding (see “Limitations of D&C and endometrial biopsy”). Does a negative biopsy signify health? An absence of intracavitary pathology? Or does it mean that we failed to sample the culprit?
Fortunately, abnormal uterine bleeding usually is attributable to endometrial conditions other than cancer, such as atrophy, hyperplasia, polyps, or fibroids. These pathologies may be benign, but they are still a nuisance. When left undetected and untreated, they can cause the patient much worry, along with weeks or months of unnecessary medical therapy and surgical procedures.
Dilation and curettage (D&C) detects benign pathology in about 80% of patients with menstrual dysfunction.1 It is most likely to detect the problem when pathology affects the endometrium globally.
A recent study reported on 105 postmenopausal women with bleeding and an endometrial echo of more than 5 mm who were evaluated with both hysteroscopy and D&C.2 Although 80% of the women had pathology in the uterine cavity, and 98% of the pathologic lesions manifested a focal growth pattern at hysteroscopy after D&C, the whole or parts of the lesion remained in situ in 87% of the women. In addition, D&C failed to detect 58% of polyps, 50% of hyperplasias, 60% of complex atypical hyperplasias, and 11% of endometrial cancers. When disease was global, D&C detected 94% of abnormalities.
Focal disease therefore mandates operative hysteroscopic-directed biopsy and removal of suspicious pathology.
Endometrial biopsy can be performed in the office without anesthesia—a great advantage. The technique is most helpful in “dating” the endometrium and diagnosing endometrial cancer or hyperplasia. Unfortunately, blind endometrial biopsy studies are frequently returned with pathology reports of insufficient tissue, atrophic changes, mucus and debris, scanty tissue, no visible endometrial tissue, endocervical tissue, or proliferative or secretory endometrium.
Further, when a blindly performed biopsy reveals normal histology, it does not necessarily rule out other pathology. In addition, a biopsy via endometrial suction curette frequently misses focal lesions such as endometrial polyps and submucosal fibroids. Global noninvasive surveillance of the endometrium is more effective at detecting such focal lesions.
Investigators who performed endometrial biopsy prior to hysterectomy in patients with known endometrial cancer demonstrated that the sensitivity of diagnosing endometrial cancer with a biopsy via endometrial suction curette increases when the pathology affects more than 50% of the surface area of the endometrial cavity.3 However, biopsy failed to detect cancer in 11 of 65 patients in whom the malignancy affected less than 50% of the endometrium. These 11 false negatives included 5 cases of endometrial polyps, 3 malignancies that affected less than 5% of the endometrium, and 7 cancers that affected less than 25% of the endometrium.
REFERENCES
1. Holst J, Koskela O, von Schoultz B. Endometrial findings following curettage in 2,018 women according to age and indications. Ann Chir Gynaecol. 1983;72:274-277.
2. Epstein E, Ramirez A, Skoog L, Valentin L. Dilation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136.
3. Guido RS, Kanbour-Shakir A, Rulin MC, Christopherson WA. Pipelle endometrial sampling: sensitivity in the detection of endometrial cancer. J Reprod Med. 1995;40:553-555.
Transvaginal ultrasound
TVUS is quick, convenient, inexpensive, and comfortable for the patient. It can evaluate the endometrium utilizing gray scale, color or power Doppler, contrast media (SIS), or 3-dimensional ultrasound technology. In addition, TVUS permits visualization of the adnexa and pelvic organs, including the bladder and cul de sac. Among the abnormalities detectable with TVUS are fibroids (including submucosal leiomyomas) and endometrial polyps. Not surprisingly, an experienced operator is crucial for a precise diagnosis.
Optimal TVUS evaluation includes endometrial measurements in the sagittal plane, with the bilayer thickness measured from the proximal to distal myometrialendometrial junctions. In the coronal view, measurement should be from the cervix to the fundus. When intraluminal fluid is present, each endometrial thickness should be measured separately (in single layers), and the combined endometrial thickness should be expressed as the sum of the 2 layers.
Characterize the endometrium. Sonographic hallmarks increasingly are used to describe uterine pathology. These include endometrial thickness, morphology, endocavitary lesions, borders, and myometrial invasion. In addition, a number of practitioners have attempted to describe the sonographic texture of the endometrium in post-menopausal women to increase sensitivity in the detection of disease, improve diagnosis, and guide treatment.
The endometrium can be characterized as homogeneous, diffusely inhomogeneous, or associated with focally or diffusely increased echogenicity. Textural inhomogeneity is present in cases of endometrial cancer.2 A homogeneous endometrium less than 6 mm thick is commonly associated with tissue insufficient for diagnosis. If focal or diffuse increased echogenicity occurs with a thin endometrium, SIS or hysteroscopy is more sensitive than TVUS in identifying the endometrial abnormality.
We rely increasingly on descriptions of the echogenicity and heterogeneity of the entire endometrial echo, as well as on echo measurements, to define endometrial health. Most experts use an endometrial echo of 5 mm as the cutoff for significant endometrial disease. A number of investigators have noted that the endometrial thickness in hyperplasia often ranges from 8 mm to 15 mm in post-menopausal women, but the number of reported cases is too low to draw firm conclusions based on endometrial echo alone.3,4
A prospective evaluation of 200 postmenopausal women with endometrial echo ranging from 3 mm to 10 mm noted that homogeneity, thin endometrium, and sonographically demonstrable central endometrium with symmetry were associated with absence of pathology. In contrast, heterogeneity and high echogenicity were indicative of pathology.5
Endometrial echo measurement and morphology increase sensitivity in predicting endometrial disease and can point to the need for ancillary testing with SIS or hysteroscopy. For example, in the postmenopausal patient, an endometrial echo of less than 5 mm, in combination with a negative endometrial biopsy, might be the only evaluation the patient needs if she responds to medical therapy for endometrial atrophy. If symptoms persist, office hysteroscopy or SIS could be performed to rule out endometrial polyps and endometrial hyperplasia (which is less likely).
Saline-infusion sonography offers an exquisite view of the endomyometrial complex that cannot be obtained with transvaginal ultrasound alone.
A thickened endometrial echo (more than 5 mm) at the initial TVUS in the postmenopausal patient should be evaluated promptly with SIS. In most of these patients, polyps or fibroids are the cause of bleeding.
Perform imaging at optimal time. In postmenopausal women, the endometrial thickness remains constant unless the patient is taking hormone replacement therapy (HRT) or tamoxifen.6 Thus, it is easier to detect or predict endometrial pathology in this population.
In premenopausal women, TVUS is most likely to detect fibroids during the early follicular stage because the endometrium is thin, usually measuring 2 mm to 4 mm. Endometrial polyps and submucosal fibroids are best viewed when a trilayered midfollicular endometrium is present, while uterine synechiae are visualized most clearly during midcycle. Adhesions appear as hyperechoic irregular structures that vary in size from 2 mm to 6 mm. They interrupt the continuity of the endometrial layer and appear much different from polyps, which tend to be round, symmetrical, and uniform.7
Diagnosing endometrial hyperplasia among premenopausal women based on an absolute endometrial measurement is more difficult than it is in postmenopausal women because of the wide range in thickness associated with the menstrual cycle (TABLE 1). Although endometrial hyperplasia or cancer is more likely when the endometrial echo is thicker than anticipated based on age or menstrual phase, it can only be proven definitively with histologic sampling.
Sensitivity and specificity. Because of the monotonous nature of the endometrium in healthy postmenopausal women, TVUS has higher sensitivity in this age group than in reproductive-age women. In the postmenopausal population, the sensitivity for detecting uterine pathology is 87%, while specificity is 82%.
Office hysteroscopy offers immediate evaluation and direct visualization of the endometrium and endocervix.
In premenopausal women, polyps were the pathology most likely to be missed, according to one literature review.8 TVUS identified only 275 of 344 polyps in this population—a sensitivity of 80%. When submucosal fibroids were located near the endometrium, the diagnostic sensitivity rose to 94%. It was difficult to discern location (i.e., submucosal or intramural or polyps) with TVUS alone, however.
Another investigation compared the sensitivity and specificity of TVUS and endometrial biopsy for the detection of endometrial disease in 448 postmenopausal women who took estrogen alone, cyclic or continuous estrogen-progesterone, or placebo for 3 years.9 Using a threshold value of 5 mm for endometrial thickness, TVUS had a positive predictive value (PPV) of 9% for detecting any abnormality, with 90% sensitivity, 48% specificity, and a negative predictive value (NPV) of 99%. Using this threshold, biopsies would be indicated in more than half of the women, of whom only 4% actually had serious disease.
Using a threshold thickness of 4 mm, Gull et al10 evaluated TVUS for the detection of endometrial cancer and atypical hyperplasia. For endometrial thicknesses exceeding 4 mm, TVUS had a sensitivity of 100%, specificity of 60%, a PPV of 25%, and an NPV of 100%. No woman with an endometrial thickness of 4 mm or less was found to have endometrial cancer.
Special considerations.
- Intracavitary fluid. On occasion, TVUS reveals “naturally occurring SIS”—that is, the spontaneous appearance of intracavitary fluid, which is probably secondary to cervical stenosis. (The name of this phenomenon refers to the iatrogenic fluid that is intrinsic to SIS.) Endometrial fluid also is observed in women who use tamoxifen, diethylstilbestrol, or megestrol acetate and in those who have a hematometra. In addition, about one quarter of patients with an endometrial malignancy have fluid in the endometrial cavity.11
- Endometrial thickness. As noted, TVUS is excellent for ruling out endometrial abnormalities in postmenopausal patients because of the consistent thickness of the endometrium. When the endometrium is especially thickened, however, its usefulness is limited.
Observations in more than 5,000 women consistently note that an endometrium of less than 5 mm, measured as a double-layer, is most often associated with a pathology reading of “tissue insignificant for diagnosis”—hence atrophy.12 When the endometrium is thicker than 5 mm, there is a greater chance of detecting polyps, endometrial hyperplasia, and endometrial cancer. Fewer than 0.12% of cancers were missed in this series.
When a skilled physician performs office hysteroscopy, the complication rate is less than 1%.
If the endometrium is thicker than 6 mm, then SIS or hysteroscopy is more helpful than TVUS in reaching a conclusive etiology for uterine bleeding. Further evaluation (hysteroscopy or SIS) is usually recommended when TVUS measurements of the endometrium are greater than 5 mm.13 Although a low cut-off, such as 4 mm or 5 mm, is associated with improved sensitivity, it sacrifices specificity.
TABLE 1
Transvaginal ultrasound: Endometrial thickness32
| PHASE | THICKNESS (MM) |
|---|---|
| Menstrual | 2-4 |
| Early proliferative | 4-6 |
| Periovulatory | 6-8 |
| Secretory | 8-12 |
| Postmenopausal | 4-8 |
| Postmenopausal on hormone replacement therapy | 4-10 |
Saline-infusion sonography
In SIS, saline is infused into the endometrial cavity during TVUS to enhance the view of the endometrium. This constitutes one of the most significant advances in ultrasonography of the past decade. SIS can provide a wealth of information about the uterus and adnexa in patients with abnormal bleeding. It offers an exquisite view of the endomyometrial complex that cannot be obtained with TVUS alone. It differentiates between focal and global processes and improves overall sensitivity for detecting abnormalities of the endometrium.
Although many terms have been used to describe this technique (echohysteroscopy, sonohysterosalpingography, and hydrosonography, to name a few), the term “saline-infusion sonography,” coined in 1996, most clearly describes the technique.14
Indications for use. SIS has been used to evaluate:15
- menstrual disorders
- endometrium that is thickened, irregular, immeasurable, or poorly defined on conventional TVUS, magnetic resonance imaging, or computed axial tomography studies
- midline endometrial echo with a thickness of more than 8 mm
- endometrium that appears irregular, bizarre, or homogeneous on TVUS in women using tamoxifen
- sessile and pedunculated masses of the endometrium that need to be differentiated
- findings with hysterosalpingogram
- recurrent pregnancy loss
- intracavitary fibroids before surgery to determine the depth of myometrial involvement and operative hysteroscopic resectability (see “Using saline infusion sonography staging to plan for fibroid surgery”)
- the endometrium after surgery
Complications are infrequent. The risk of infection is less than 1%. Practitioners should follow the same protocols for administering antibiotics as they do with hysterosalpingogram or other invasive endometrial procedures.
Possible problems during SIS include cervical stenosis, inability to distend the endometrium, uterine contractions, and heavy vaginal bleeding with resultant artifacts. There is also the risk of performing the procedure in early pregnancy, as well as the theoretic risk of disseminating endometrial cancer.
Sensitivity and specificity. Interpreting SIS images requires experience, correlation with the menstrual history, and careful scanning. In premenopausal women, SIS has an overall sensitivity of 94% and a specificity of 85%.8 In 1 review, its sensitivity for detecting endometrial polyps was 93%, and its specificity was 96%.8 In the detection of submucosal fibroids, it had a sensitivity of 94% and a specificity of 95%.8
Another investigation compared the accuracy and pain of SIS with flexible office hysteroscopy.14 For all findings combined—including polyps, submucous myomas, synechiae, endometrial hyperplasia, and cancer—SIS’s sensitivity was 96% and its specificity was 88%, compared with hysteroscopy. SIS did not miss any intracavitary lesions. It was also less painful than hysteroscopy. False-positive results included diagnosing endometrial polyps as fibroids or vice versa. This is not really a problem, however, since both lesions are associated with bleeding abnormalities and both are removed with the operative hysteroscope.
False-positive results may be caused by blood, debris, clots, thickened endometrial folds, secretory endometrium, detached fragments of endometrium, or endometrium that was sheared during placement of the catheter. (When SIS is performed in the presence of active uterine bleeding, endometrial clots may sometimes be mistaken for polyps, fibroids, or hyperplasia.)
Because false positives may lead to unnecessary surgical intervention,16 SIS should be performed when these are least likely. For premenopausal women, that means scheduling SIS within 1 to 7 days of the menstrual cycle’s completion. In postmenopausal women, the patient should be scanned when she is not bleeding, if possible. If she is taking sequential HRT, SIS is best performed after progesterone withdrawal.
Until insurers realize the advantages of office-based hysteroscopy and reimburse accordingly, women will be unnecessarily subjected to surgery, preoperative testing, lost time, and increased anxiety.
Some have suggested that if SIS detects a lesion smaller than 10 mm, the procedure should be repeated when the patient is not bleeding. If the lesion is still present, operative intervention is appropriate.17,18
Cost issues. The development of clinical algorithms incorporating SIS—particularly those that take into account the differences between evaluation of premenopausal and postmenopausal women—may decrease costs by eliminating unnecessary D&Cs and endometrial biopsies, including those that are hysteroscopically directed.
For example, a study examining almost 1,200 women in 4 Nordic countries suggests that in women with postmenopausal bleeding and an endometrial thickness of 4 mm or less, curettage is unnecessary.19 An algorithm developed after the evaluation of more than 400 perimenopausal women concluded that hysteroscopically directed endometrial biopsy is best for women with focal processes, while endometrial aspiration biopsy is best for those with global disease.20
Although SIS overcomes many of the limitations of TVUS, in some cases the endometrium simply cannot be visualized.
In general, SIS is less costly to perform than hysteroscopy.
For a successful surgical outcome, it is important to preoperatively identify the size, number, location, and depth of intramural extension. Fibroid size and location affect resectability, the number of surgical procedures necessary for complete resection, the duration of surgery, and the potential complications from fluid overload.1
Using saline-infusion sonography (SIS), uterine fibroids can be categorized into 3 classes. An experienced surgeon can successfully resect class 1 (FIGURE 1) or class 2 (FIGURE 2) fibroids hysteroscopically. Class 3 fibroids (FIGURE 3) are less amenable to such surgery because perforation is a significant risk. Fluid overload, contracture of the endometrial cavity, and incomplete resection also are possible complications.2
FIGURE 1 Class 1 fibroids
Class 1 fibroids occur completely within the uterine cavity, with no involvement of myometrium. The base or stalk is visible with SIS.
FIGURE 2 Class 2 fibroids
Class 2 fibroids have a submucosal component involving less than half of the myometruim.
FIGURE 3 Class 3 fibroids
Class 3 fibroids have an intramural component of greater than 50%. They can be transmural, and may be located anywhere from the submucosa to the serosa. These fibroids often appear as a bulge or indentation into the submucosa.
REFERENCES
1. Emanuel MH, Verdel MJ, Wamsteker K. A prospective comparison of transvaginal ultrasonography and diagnostic hysteroscopy in the evaluation of patients with abnormal uterine bleeding: Clinical implications. Am J Obstet Gynecol. 1995;172:547-552.
2. Bradley LD, Falcone T, Magen AB. Radiographic imaging techniques for the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:245-276.
Office hysteroscopy
Hysteroscopy has revolutionized the practice of gynecology. Thanks to improvements in optics and subsequent reduction in hysteroscope diameters in the 1980s and 1990s, office use has become practical. Thin operative hysteroscopes with outer diameters ranging from 3 mm to 5 mm now are available. A study in 417 women undergoing flexible hysteroscopy in an office setting without anesthesia demonstrated the patient acceptability, diagnostic accuracy, and cost-effectiveness of this technology.21
Advantages of hysteroscopic visualization include:
- immediate office evaluation
- direct visualization of the endometrium and endocervix
- the ability to detect minute focal endometrial pathology
- the ability to perform directed endometrial biopsies (with some hysteroscopes) and lyse filmy adhesions with the distal tip of the flexible hysteroscope22
Office hysteroscopy is comfortable and quick and associated with low complication rates. Preprocedural nonsteroidal agents or misoprostol may make the procedure more tolerable.
Disadvantages of office hysteroscopy include the need for expensive office equipment (camera, insufflator, hysteroscope, video equipment, etc.) and a skillful and experienced hysteroscopist, as well as the costliness of the procedure.
Complications. The complication rate is low (less than 1%) when a skilled physician performs the procedure. Complications include uterine perforation, infections, and excessive bleeding; complications related to the distending medium also have been recorded.23
Indications for use. Entities that can be visualized hysteroscopically include:
- endometrial polyps
- submucosal and intramural fibroids
- synechiae
- retained products of conception
- foreign bodies
- endocervical lesions
- endometrial atrophy
- endometrial hyperplasia and cancer
- arteriovenous malformations
- gestational trophoblastic disease
- pregnancy (although hysteroscopy should be avoided when a viable intrauterine pregnancy has been documented)
Infrequently, endometrial gland openings associated with adenomyosis also can be seen.
On occasion, it may be difficult to distinguish hysteroscopically between a sessile polyp and submucosal fibroid if the typical characteristics of a polyp or fibroid are not appreciated. Luckily, treatment with operative hysteroscopy is the same for both findings.
Special considerations.
- Lesion size. The hysteroscopist must remember that it can be difficult to estimate the size of lesions noted. The reason: The eyepiece is focused at infinity, so objects that are closer appear magnified and objects that are further away appear smaller.24 This can lead to surprises in the operating room, especially when the size of a lesion has been underestimated. In this regard, hysteroscopy is not as accurate as TVUS.
- Rigid versus flexible. Some gynecologists may prefer to use a rigid hysteroscope because it permits a greater viewing angle than a flexible hysteroscope. In a rigid instrument, that angle ranges from 0 to 30 degrees. The outer sheaths of most rigid hysteroscopes are 3 mm to 7 mm. Larger-diameter sheaths permit insertion of biopsy instruments and graspers for directed endometrial biopsies.
- The trouble with high pressure. Hysteroscopy can produce a falsely negative view when the operator uses CO2 or high intrauterine pressure, since higher pressures can flatten an endometrial lesion. For this reason, it is wise for the hysteroscopist to get in the habit of slowly deflating the uterine cavity and carefully reinspecting the endometrial surface before concluding the procedure. A note about this maneuver should be included in the procedure report. If this approach is followed consistently, it should increase accuracy, reduce false negatives and medicolegal risk, and lead to better outcomes.
- The specificity and PPV of hysteroscopy in cases of abnormal uterine bleeding should theoretically be 100%. In practice, however, the false-negative rate is 2% to 4%—the result of operator error.25
Cost issues. The CPT code for office hysteroscopy is 58555. Regrettably, poor reimbursement has prevented rapid assimilation of this unique tool into office practice. Until insurance companies realize the marked advantages of this quick, safe, and comfortable office-based procedure and reimburse accordingly, women will be unnecessarily subjected to the rigors of surgical clearance and preoperative testing, loss of time from work and family, and the increased anxiety of having this procedure performed in an operating suite.
Comparing modalities
Investigators compared the accuracy of TVUS, transabdominal sonohysterography, and hysteroscopy in detecting submucosal fibroids (and determining their size) and myometrial growth in 52 premenopausal women scheduled for hysterectomy.26 Transabdominal sonohysterography most accurately predicted size and myometrial ingrowth of fibroids. Hysteroscopy was least accurate in detecting the myoma’s size, perhaps because of the optical refractive index with which it is associated. Still, hysteroscopy is more accurate than blind biopsy alone in detecting intracavitary lesions such as polyps and fibroids.27
SIS versus TVUS. SIS provides a more comprehensive view of the pelvic anatomy than TVUS alone, with more concentrated visualization of the endometrium. However, it can pose some technical difficulties. One group of investigators was unable to complete the procedure in patients with a uterus larger than 12 to 14 weeks, submucosal fibroids greater than 4 cm, polyps that filled the endometrial cavity, or large transmural fibroids that precluded distention of the endometrium.28 Other limitations of SIS include the inability to thread the catheter, iatrogenic introduction of air bubbles into the uterus, and the inability to maintain distension in patients with a patulous cervix.
Evaluation of the endometrium is not ‘either-or.’ Sometimes a combination of procedures aids diagnosis of menstrual dysfunction.
Patients with a distended cervix may require an SIS catheter with a balloon that occludes the lower uterine segment. Placement of the intrauterine catheter may be difficult in patients with cervical stenosis, isthmic synechiae, a markedly retroverted uterus, or intrauterine septa. Women with cervical stenosis may benefit from placement of laminaria tents or misoprostol29 (orally 100 μg 8 to 12 hours before the procedure or vaginally 200 μg to 400 μg 2 to 4 hours before the procedure). Uterine sounding may sufficiently disrupt synechiae. Cervical traction with a single-toothed tenaculum can straighten the uterine axis if marked retroversion is present.
Regardless of the patient’s age, abnormal uterine bleeding requires an aggressive workup.
SIS versus hysteroscopy. Compared with hysteroscopy, SIS more reliably predicts uterine fibroids’ size and the depth of myometrial involvement (TABLE 2).26 In addition,SIS permits classification of location, size, and degree of intramural extension. This allows the clinician to determine the resectability of the lesion and select the appropriate surgical approach.15
However, a recent comparison of SIS and hysteroscopy in diagnosing uterine pathology in 90 women with abnormal uterine bleeding showed that SIS had a high failure rate (34%) among postmenopausal women, usually because of cervical stenosis.30 In premenopausal women, that rate was lower (10.3%). The failure rate of hysteroscopy, meanwhile, was 10.6% in postmenopausal women and 2.9% in premenopausal women. Another investigation of hysteroscopy noted a failure rate of 8% in postmenopausal women.31
Complementary technologies. Although SIS overcomes many of the limitations of TVUS, in some cases the endometrium simply cannot be visualized or is indistinct, or findings are indeterminate. If the endometrium is not well visualized with SIS, then hysteroscopy plays a definitive role in ascertaining endometrial morphology. In addition to evaluating equivocal or indeterminate SIS findings, hysteroscopy is invaluable when menstrual aberrations persist despite a normal SIS.
Fortunately, evaluation of the endometrium is not an “either-or” proposition. Sometimes a combination of procedures may be important in solving the puzzle of menstrual dysfunction.
- Equivocal TVUS results. If the patient had a TVUS that led to an equivocal interpretation of the endometrium, clinicians should proceed with SIS or office hysteroscopy. If the woman continues to experience abnormal uterine bleeding after a normal SIS, diagnostic hysteroscopy should be performed to rule out occult lesions in the cornu, endocervix, or uterus.
- Pap test. It seems prudent to perform a Papanicolaou test, including an endocervical and endometrial biopsy, in high-risk patients. (This includes patients who are chronically anovulatory, obese, or nulliparous; those who use tamoxifen or unopposed estrogen; and women with untreated endometrial hyperplasia.) Keep in mind, however, that only 50% of women with endometrial cancer actually have risk factors for the disease.32
TABLE 2
Comparison of saline-infusion sonography (SIS) and hysteroscopy
| CHARACTERISTIC | SIS | HYSTEROSCOPY |
|---|---|---|
| Pain | Less | More |
| Adnexal imaging | Possible | Impossible |
| Determination of depth of fibroid penetration | Possible (accurate) | Not possible unless lesion is pedunculated |
| Cost | Less | More |
| Office based | Yes | Yes |
| Large uterine size | Difficult if uterus is >14 weeks’ size | Yes |
| Ability to complete exam | >95% cases | >95% cases |
| Complication rate | <1% | <1% |
Aggressive workup is warranted
Regardless of the patient’s age, abnormal uterine bleeding requires an aggressive workup. If we are diligent and find atrophy, we can reassure and educate the patient about the fragility of the menopausal endometrium. In most women, short-term, low-dose hormone replacement therapy is the right antidote. For the perimenopausal woman, “hormonal problems” can best be verified by a negative workup with hysteroscopy or SIS. Treatment usually consists of a low-dose contraceptive or a levonorgestrel intrauterine device, which is associated with shorter, lighter periods.
Dr. Bradley reports that she serves as a consultant to Olympus and Karl Storz.
1. Rogerson L, Duffy S. A national survey of outpatient hysteroscopy. Gynecol Endosc. 2001;10:343-348.
2. Sheikh M, Sawhney S, Khurana A, Al-Yatama M. Alteration of sonographic texture of the endometrium in post-menopausal bleeding. A guide to further management. Acta Obstet Gynecol Scand. 2000;79:1006-1010.
3. Persadie RJ. Ultrasonographic assessment of endometrial thickness: A review. J Obstet Gynaecol Can. 2002;24(2):131-136.
4. Goldstein RB, Bree RL, Benson CB, et al. Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med. 2001;20:1025-1036.
5. Weigel M, Friese K, Strittmatter HJ, Meichert F. Measuring the thickness—is that all we have to do for sonographic assessment of endometrium in postmenopausal women? Ultrasound Obstet Gynecol. 1995;6:97-102.
6. Schwartz LB, Snyder J, Horan C, Porges RF, Nachitgall LE, Goldstein SR. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patient on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
7. Shalev J, Meizner I, Bar-Hava I, Dicker D, Mashiach R, Ben-Rafael Z. Predictive value of transvaginal sonography performed before routine diagnostic hysteroscopy for evaluation of infertility. Fert Steril. 2000;73:412-417.
8. Dueholm M, Lundorf E, Olesen F. Imaging techniques for evaluation of the uterine cavity and endometrium in premenopausal patients before minimally invasive surgery. Obstet Gynecol Surv. 2002;57:389-403.
9. Langer RD, Pierce JJ, O’Hanlan KA, et al. Transvaginal ultrasonography compared with endometrial biopsy for the detection of endometrial disease. N Engl J Med. 1997;337:1792-1798.
10. Gull B, Karlsson B, Milson L, Granberg S. Can ultrasound replace dilation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol. 2003;188:401-408.
11. Carlson JA, Arger P, Thompson S, Carlson EJ. Clinical and pathologic correlation of endometrial cavity fluid detected by ultrasound in the postmenopausal patient. Obstet Gynecol. 1991;77:119-123.
12. Weber A, Belinson J, Bradley LD, Piedmonte M. Vaginal ultrasound versus endometrial biopsy in women with postmenopausal bleeding. Am J Obstet Gynecol. 1997;177:924-929.
13. Laifer-Narin S, Ragavendra N, Parmenter EK, Grant EG. False-normal appearance of the endometrium on conventional transvaginal sonography: Comparison with saline hysterosonography. AJR. 2002;178(1):129-133.
14. Widrich T, Bradley L, Mitchinson AR, Collins R. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol. 1996;174:1327-1334.
15. Bradley LD, Falcone T, Magen AB. Radiographic imaging techniques for the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:245-276.
16. Mihm LM, Quick VA, Brumfield JA, Connors AF, Jr, Finnerty JJ. The accuracy of endometrial biopsy and saline sonohysterography in the determination of the cause of abnormal uterine bleeding. Am J Obstet Gynecol. 2002;186:858-860.
17. Goldstein SR. Saline infusion sonohysterography. Clin Obstet Gynecol. 1996;39(1):248-258.
18. Dijkhuizen FP, DeVries LD, Mol BW, et al. Comparison of transvaginal ultrasonography and saline infusion sonography for the detection of intracavitary abnormalities in premenopausal women. Ultrasound Obstet Gynecol. 2000;15:372-376.
19. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol. 1995;172:1488-1494.
20. Goldstein S. Use of ultrasonohysterography for triage of perimenopausal patients with unexplained uterine bleeding. Obstet Gynecol. 1994;170:565-570.
21. Bradley L, Widrich T. State-of-the-art flexible hysteroscopy for office gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
22. Nagele F, O’Connor H, Davies A, Badawy A, Mohamed H, Magos A. 2,500 outpatient diagnostic hysteroscopies. Obstet Gynecol. 1996;88:87-92.
23. Serden SP. Diagnostic hysteroscopy to evaluate the cause of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:277-286.
24. Apgar B, Dewitt D. Diagnostic hysteroscopy. Am Fam Physician. 1992;46(5 suppl):19S-26S.
25. Gimpelson R, Roppold HA. Comparative study between panoramic hysteroscopy with directed biopsies and dilation and curettage. Am J Obstet Gynecol. 1988;158:489-494.
26. Cincinelli E, Romano F, Anastasio P, et al. Transabdominal sonohysterography, transvaginal sonography, and hysteroscopy in the evaluation of submucous myomas. Obstet Gynecol. 1995;85:42-47.
27. Pasqualotto EB, Margossian H, Price LL, Bradley LD. Accuracy of preoperative diagnostic tools and outcome of hysteroscopic management of menstrual dysfunction. J Am Assoc Gynecol Laparosc. 2000;7:201-209.
28. Kochli, OR ed. Hysteroscopy: State of the Art. Contributions to Gynecology and Obstetrics. Vol. 20. Karger Verlag Basel.
29. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: A randomized controlled trial. Obstet Gynecol. 2000;96:890-894.
30. Rogerson L, Bates J, Weston M, Duffy S. A comparison of outpatient hysteroscopy with saline infusion hysterosonography. BJOG. 2002;109:800-804.
31. Rose PG. Endometrial cancer. N Engl J Med. 1996;335:640-649.
32. Blumenfeld M, Turner P. Role of transvaginal sonography in the evaluation of endometrial hyperplasia and cancer. Clin Obstet Gynecol. 1996;39:641-655.
1. Rogerson L, Duffy S. A national survey of outpatient hysteroscopy. Gynecol Endosc. 2001;10:343-348.
2. Sheikh M, Sawhney S, Khurana A, Al-Yatama M. Alteration of sonographic texture of the endometrium in post-menopausal bleeding. A guide to further management. Acta Obstet Gynecol Scand. 2000;79:1006-1010.
3. Persadie RJ. Ultrasonographic assessment of endometrial thickness: A review. J Obstet Gynaecol Can. 2002;24(2):131-136.
4. Goldstein RB, Bree RL, Benson CB, et al. Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med. 2001;20:1025-1036.
5. Weigel M, Friese K, Strittmatter HJ, Meichert F. Measuring the thickness—is that all we have to do for sonographic assessment of endometrium in postmenopausal women? Ultrasound Obstet Gynecol. 1995;6:97-102.
6. Schwartz LB, Snyder J, Horan C, Porges RF, Nachitgall LE, Goldstein SR. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patient on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
7. Shalev J, Meizner I, Bar-Hava I, Dicker D, Mashiach R, Ben-Rafael Z. Predictive value of transvaginal sonography performed before routine diagnostic hysteroscopy for evaluation of infertility. Fert Steril. 2000;73:412-417.
8. Dueholm M, Lundorf E, Olesen F. Imaging techniques for evaluation of the uterine cavity and endometrium in premenopausal patients before minimally invasive surgery. Obstet Gynecol Surv. 2002;57:389-403.
9. Langer RD, Pierce JJ, O’Hanlan KA, et al. Transvaginal ultrasonography compared with endometrial biopsy for the detection of endometrial disease. N Engl J Med. 1997;337:1792-1798.
10. Gull B, Karlsson B, Milson L, Granberg S. Can ultrasound replace dilation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol. 2003;188:401-408.
11. Carlson JA, Arger P, Thompson S, Carlson EJ. Clinical and pathologic correlation of endometrial cavity fluid detected by ultrasound in the postmenopausal patient. Obstet Gynecol. 1991;77:119-123.
12. Weber A, Belinson J, Bradley LD, Piedmonte M. Vaginal ultrasound versus endometrial biopsy in women with postmenopausal bleeding. Am J Obstet Gynecol. 1997;177:924-929.
13. Laifer-Narin S, Ragavendra N, Parmenter EK, Grant EG. False-normal appearance of the endometrium on conventional transvaginal sonography: Comparison with saline hysterosonography. AJR. 2002;178(1):129-133.
14. Widrich T, Bradley L, Mitchinson AR, Collins R. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol. 1996;174:1327-1334.
15. Bradley LD, Falcone T, Magen AB. Radiographic imaging techniques for the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:245-276.
16. Mihm LM, Quick VA, Brumfield JA, Connors AF, Jr, Finnerty JJ. The accuracy of endometrial biopsy and saline sonohysterography in the determination of the cause of abnormal uterine bleeding. Am J Obstet Gynecol. 2002;186:858-860.
17. Goldstein SR. Saline infusion sonohysterography. Clin Obstet Gynecol. 1996;39(1):248-258.
18. Dijkhuizen FP, DeVries LD, Mol BW, et al. Comparison of transvaginal ultrasonography and saline infusion sonography for the detection of intracavitary abnormalities in premenopausal women. Ultrasound Obstet Gynecol. 2000;15:372-376.
19. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol. 1995;172:1488-1494.
20. Goldstein S. Use of ultrasonohysterography for triage of perimenopausal patients with unexplained uterine bleeding. Obstet Gynecol. 1994;170:565-570.
21. Bradley L, Widrich T. State-of-the-art flexible hysteroscopy for office gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
22. Nagele F, O’Connor H, Davies A, Badawy A, Mohamed H, Magos A. 2,500 outpatient diagnostic hysteroscopies. Obstet Gynecol. 1996;88:87-92.
23. Serden SP. Diagnostic hysteroscopy to evaluate the cause of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:277-286.
24. Apgar B, Dewitt D. Diagnostic hysteroscopy. Am Fam Physician. 1992;46(5 suppl):19S-26S.
25. Gimpelson R, Roppold HA. Comparative study between panoramic hysteroscopy with directed biopsies and dilation and curettage. Am J Obstet Gynecol. 1988;158:489-494.
26. Cincinelli E, Romano F, Anastasio P, et al. Transabdominal sonohysterography, transvaginal sonography, and hysteroscopy in the evaluation of submucous myomas. Obstet Gynecol. 1995;85:42-47.
27. Pasqualotto EB, Margossian H, Price LL, Bradley LD. Accuracy of preoperative diagnostic tools and outcome of hysteroscopic management of menstrual dysfunction. J Am Assoc Gynecol Laparosc. 2000;7:201-209.
28. Kochli, OR ed. Hysteroscopy: State of the Art. Contributions to Gynecology and Obstetrics. Vol. 20. Karger Verlag Basel.
29. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: A randomized controlled trial. Obstet Gynecol. 2000;96:890-894.
30. Rogerson L, Bates J, Weston M, Duffy S. A comparison of outpatient hysteroscopy with saline infusion hysterosonography. BJOG. 2002;109:800-804.
31. Rose PG. Endometrial cancer. N Engl J Med. 1996;335:640-649.
32. Blumenfeld M, Turner P. Role of transvaginal sonography in the evaluation of endometrial hyperplasia and cancer. Clin Obstet Gynecol. 1996;39:641-655.
Abnormal uterine bleeding: A Quick Guide To Evaluation And Treatment
- Approximately 15% to 20% of office gynecologic visits are for the evaluation of abnormal uterine bleeding (AUB), and 25% to 50% of gynecologic surgeries are performed to address menstrual dysfunction.
- Office hysteroscopy and saline infusion sonography are essential skills for the practicing gynecologist. Learn them and use them liberally.
- Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- Liberal use of endometrial biopsy is encouraged in women over 35 years of age at risk for endometrial hyperplasia and cancer.
- About 20% to 30% of teens with irregular heavy menses have a major bleeding diathesis.
- Medical therapy is the standard unless uterine pathology is present.
Half of all hysterectomies in the United States are performed to treat abnormal uterine bleeding. Of these, approximately 20% are performed in women with a normal uterine size.1 However, when the uterus appears normal, without adenomyosis or uterine pathology, it is imperative that the clinician perform a thorough evaluation before resorting to hysterectomy.
Abnormal uterine bleeding is defined as excessive, erratic, or irregular bleeding in the presence or absence of intracavitary or uterine pathology. It may be associated with structural or systemic abnormalities. In contrast, dysfunctional uterine bleeding (DUB) is associated with anovulatory menstrual cycles. It is not caused by pelvic pathology, medications, systemic disease, or pregnancy.
Abnormal bleeding is associated with an array of symptoms. Frequent complaints include heavy or prolonged menstrual flow, social embarrassment, diminished quality of life, sexual compromise, and the need to alter lifestyle. Pain is not a common presenting symptom unless it is associated with the passage of large blood clots.
The following menstrual patterns are associated with DUB:
- Oligomenorrhea. A cycle length of more than 35 days
- Polymenorrhea. A cycle length of less than 21 days
- Amenorrhea. The absence of menses for 6 months or 3 consecutive cycles
- Menorrhagia. Heavy or increased flow occurring at regular intervals, or a loss of more than 80 mL of blood
- Metrorrhagia. Irregular episodes of bleeding
- Menometrorrhagia. A longer duration of flow occurring at unpredictable intervals
- Postmenopausal bleeding. Bleeding that occurs more than 12 months after the last menstrual cycle
Basic evaluation and management of abnormal uterine bleeding
*If still bleeds, then SIS or hysteroscopy
SIS=saline infusion sonography
Prevalence and pathophysiology
Although we lack precise figures regarding the prevalence of abnormal uterine bleeding, it is estimated that 9% to 30% of reproductive-age women have menstrual irregularities requiring medical evaluation.2 Approximately 15% to 20% of office gynecologic visits are scheduled for the evaluation of abnormal uterine bleeding, which is exceeded only by vaginitis as a chief complaint. In addition, 25% to 50% of gynecologic surgical procedures are performed to address menstrual dysfunction.
Normal menstruation is triggered by fluctuations in the hypothalamic-pituitary-ovarian axis that lead to denudation and sloughing of the endometrium. This hemorrhage is followed by prompt hemostasis and repair. Low physiologic levels of estrogen prime the endometrium, while the normal secretion of progesterone from the corpus luteum stabilizes it, decreasing vascular fragility and supporting the endometrial stroma. Platelets and fibrin are necessary for endometrial hemostasis. Deficiencies in either factor may result in heavier menstruation.
DUB occurs when there is inadequate progesterone secretion to stabilize the endometrium. Anovulatory bleeding can be episodic or continuous. Patients with anovulatory cycles typically do not experience premenstrual tension, breast discomfort, increased mucoid vaginal discharge, or cramping and bloating, all characteristic of ovulatory cycles. Although ovulatory cycles are predictable, erratic bleeding may occur when they coexist with intracavitary lesions, including polyps and fibroids.
Anovulatory cycles typically are associated with puberty and the perimenopausal years. In puberty, the immature hypothalamic-pituitary-ovarian axis has not yet developed the necessary hormonal feedback to sustain the endometrium. In perimenopause, the decline of inhibin and rise in follicle-stimulating hormone (FSH) levels reflect the loss of follicular activity and competence.
In some cases, severe anemia can cause incessant menstrual blood loss. Typical complaints of anemia include fatigue, unusual food cravings (pica), and headaches. Severe anemia also can cause fainting, exercise-induced fatigue, shortness of breath, congestive heart failure, and/or the inability to perform routine activities. Unless it is a chronic condition, DUB is rarely associated with the need for a blood transfusion. Hemorrhagic shock and death are rare sequelae.
Diagnosis
Diagnosis involves 3 main components:
- A detailed medical history and review of systems. This should alert the physician to the possible etiology of a patient’s menstrual dysfunction (Table 1). Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- A physical examination. The exam must be comprehensive, even in the presence of heavy bleeding, focusing on the vagina, cervix, uterus, and adnexa to exclude pathology.
- Appropriate laboratory studies based on the focused clinical history and any physical findings. Pregnancy testing is necessary in all sexually active premenopausal women. In addition, women with profuse menorrhagia and a normal uterine size should be screened for von Willebrand’s disease, since 13% to 20% of women offered surgical intervention have the subtle form of Type I disease. (In women, von Willebrand’s disease most commonly presents as DUB.) Obviously, medical therapy is paramount for women with von Willebrand’s disease; hysterectomy or other surgical treatment should not be the first option.
TABLE 1
Causes of menstrual dysfunction
| ANATOMIC |
| Polyps |
| Fibroids |
| Adenomyosis |
| Vaginitis |
| Endometritis |
| Retained products of conception |
| Endometriosis |
| Hyperplasia |
| Malignancy |
| ENDOCRINE |
| Thyroid dysfunction |
| Elevated prolactin levels |
| Adrenal dysfunction |
| Hypothalamic/pituitary dysfunction |
| Estrogen-producing tumors |
| HEMATOLOGIC |
| Anemia |
Coagulopathy
|
| Leukemia |
| SYSTEMIC |
| Renal impairment |
| Liver disorders |
| Obesity |
| Anorexia |
| Chronic illness |
| Rapid fluctuations in weight |
| MEDICATIONS |
| Anticoagulants |
| Steroids |
| Progesterone withdrawal |
| Herbal and soy products |
| MISCELLANEOUS |
| Smoking |
| Depression |
| Excessive alcohol intake |
| Sexually transmitted diseases |
Special populations
Adolescents. Teens with irregular heavy menses should be evaluated for coagulopathies, since 20% to 30% have a major bleeding diathesis.3 This is especially true if the patient presents with a hemoglobin level of less than 10 g/dL or if hospitalization is required. Specifically, adolescents should be evaluated for von Willebrand’s disease with the ristocetin cofactor assay, the single best screening test for the disease. This prevents false-negative results. Other laboratory tests should include:
- Serum human chorionic gonadotropin (hCG)
- Bleeding time
- Partial time (PT) and partial thromboplastin time (PTT)
- Complete blood count (CBC) with platelets
Successful medical therapies for von Willebrand’s disease include oral contraceptives (OCs), which have an 88% success rate; desmopressin acetate; antifibrinolytic agents; and plasma-derived concentrates rich in the high-molecular-weight multimers of von Willebrand factor (vWf).4
Perimenopausal women. Women entering perimenopause may have recurrent bouts of DUB and associated physical complaints due to changes in the hypothalamic-pituitaryovarian axis. The hormonal milieu is associated with decreased inhibin, variable estradiol, normal FSH, and menstrual cycles that can be episodically ovulatory.5 Many menstrual complaints occur in perimenopausal women, including menometrorrhagia, amenorrhea, and oligomenorrheic cycles. Decreased mental clarity and concentration, vaginal dryness, hot flushes, and night sweats are classic symptoms of perimenopause.
Oral contraceptive (OC) therapy is quite useful in these women and should be the first line of intervention, rather than conventional hormone replacement therapy (HRT).6 The usual postmenopausal doses of HRT do not suppress ovulation or prevent pregnancy, while OCs do. In healthy, nonsmoking women over 35 years of age, OCs regulate menstrual cycles, decrease vasomotor symptoms, improve bone mineral density (BMD), and reduce the need for surgical intervention for DUB. They also reduce endometrial and ovarian cancer rates.
Postmenopausal patients. Bleeding that occurs with HRT or tamoxifen use more than 1 year after the cessation of menses requires thorough evaluation. While the most common cause of postmenopausal bleeding is atrophy, it is important to rule out intracavitary pathology, endometrial hyperplasia, and cancer. Approximately 10% of women with postmenopausal bleeding have endometrial cancer. Because the risk of this cancer increases with each decade of life, its exclusion is critical.
Focal intracavitary lesions, including polyps, submucosal fibroids, and endometrial hyperplasia, account for 20% to 40% of cases of abnormal uterine bleeding in this population.7
Organic diseases. Women with renal or liver disease also may have abnormal uterine bleeding. Patients with liver disease may have higher circulating levels of estrogen due to hepatic dysfunction and an inability to metabolize estrogen. Coagulopathies also may occur with liver disease, while renal failure is associated with hypothalamic-pituitaryovarian axis irregularities due to gonadal resistance to hormones, platelet dysfunction, and abnormal factor VIII activity.
Medical therapy
Once the likely cause of abnormal bleeding is identified, appropriate treatment should be instituted. For anovulatory cycles, medical therapy with OCs or progesterone is the standard. Patients with ovulatory abnormal bleeding should be evaluated for intracavitary uterine pathology, since hormonal dysfunction is probably not the cause. Patients whose abnormal bleeding is anatomic in origin usually are managed surgically.
Medical therapy should be tailored to the individual after reviewing her risks, benefits, contraindications, and individual concerns. It is important to determine which facet of the menstrual cycle the patient wants improved, e.g., length, duration, clotting, pain, quantity, in order to target treatment appropriately.
Objective measurements (alkaline hematin assay) of menstrual blood loss are impractical in an office setting.
Oral contraceptives. OCs have many roles in the treatment of menorrhagia and other forms of DUB. Short-term, high-dose therapy is valuable when excessive bleeding occurs in an emergency situation or when heavy menstrual bleeding occurs in adolescent and perimenopausal women. Any low-dose (30 to 35 mcg) ethinyl estradiol product can be given at 6-hour intervals for 5 days to stabilize bleeding. This should be followed by a tapering regimen of 1 low-dose OC pill at 8-hour intervals for 5 days, 12-hour intervals for 5 more days, and then daily for 5 days. This regimen quickly halts heavy menses and controls bleeding. It also prepares the patient for a withdrawal menses.
After the withdrawal bleed, the patient should continue on a maintenance dose (1 pill daily) to ensure regular menstrual cycles and contraception. Low-dose OCs are safe and effective for women over 35 who do not smoke and lack a history of thromboembolic disease.
Progesterone therapy. Women with anovulatory menstrual cycles also may benefit from progesterone therapy, which stabilizes the proliferative endometrium and establishes regular sloughing. Cyclical progesterone is useful in women with contraindications to estrogen therapy, e.g., women over 35 who smoke or have a history of deep venous thrombosis (DVT) or cardiovascular risk factors. Generally, 10 mg of medroxyprogesterone acetate for 10 to 14 days each month will induce a regular withdrawal bleed, although it does not provide contraception.
Long-acting progesterone therapy in the form of medroxyprogesterone (Depo-Provera; Pharmacia Corp, Peapack, NJ) will stop menses in the majority of patients. Standard dosing is 150 mg administered intramuscularly (IM) every 3 months. Approximately 80% to 90% of patients who complete 12 months of Depo-Provera therapy will be amenorrheic. Potential side effects include weight gain, irregular bleeding, and depression.
Danazol. This pituitary suppressant creates a hypoestrogenic state and decreases menstrual blood loss by 70% to 80%. A daily dose of 50 to 100 mg may be adequate in some cases; otherwise, the conventional 400 to 800 mg is recommended. Potential side effects include weight gain, acne, and alteration of lipids.8
GnRH therapy. Gonadotropin-releasing hormone (GnRH) therapy with leuprolide or nafarelin creates a hypoestrogenic menopause-like condition, with menstruation usually ceasing within 3 months. Menopausal symptoms may include hot flushes, night sweats, vaginal dryness, bone loss, decreased concentration, and diminished libido. Nevertheless, compliance generally is good. Because prolonged therapy can lead to osteoporosis, treatment usually is limited to 6 months unless estrogen “addback” therapy is instituted.
GnRH therapy is a valuable option for the late perimenopausal woman who has significant contraindications to other medical regimens. For most of these women, the cessation of menses is a relief. After therapy, many patients spontaneously transition into the menopause. An intermittent 6-month course of leuprolide is an option for women with uterine fibroids. Data indicate that it provides an additional 9 months of symptom control (range: 2 to longer than 25 months).9
Progesterone intrauterine system. The recently introduced levonorgestrel-releasing intrauterine system (IUS) (Mirena, Berlex Laboratories, Montville, NJ) also is effective therapy for DUB. This IUS causes pseudodecidual changes and amenorrhea, decreasing menstrual blood loss by 65% to 98% within 12 months, with little systemic absorption of progesterone. It is likely to prove quite valuable for women with menorrhagia who need contraception, have a normal uterine size, and wish to avoid surgery.10
NSAIDs. Nonsteroidal anti-inflammatory drugs (NSAIDs) decrease the rate of dysmenorrhea, improve clotting, and reduce menstrual blood loss. Some studies have demonstrated a 50% to 80% reduction in blood loss with proper use.11 Patients are advised to begin therapy 1 to 2 days before their period is expected and continue throughout the menses. NSAIDs may be combined with OCs, if necessary.
Most menstrual cycles occur every 21 to 35 days. Normal menstrual flow lasts 3 to 7 days, with most blood lost within the first 3 days. The typical menstrual flow averages 35 mL and consists of effluent debris and blood. Women with normal menstrual cycles use an average of 5 to 6 pads or tampons each day. Social obligations, sexual activity, hobbies, work, and travel are not interrupted with normal menstrual function.
When menorrhagia is present, a woman may lose more than 80 mL of blood with each menstrual cycle. Since approximately 16 mg of iron is lost in normal cycles, women with menorrhagia often develop anemia. They also typically have an imbalance of prostaglandin levels and increased fibrinolytic activity.
It is important to note that more than 50% of women who complain of menorrhagia do not have heavy menses. Some patients change their sanitary products more often not because of heavy flow, but for reasons concerning hygiene, personal preference, or fear of toxic shock syndrome.—Linda D. Bradley, MD
FIGURE 5
When medical therapy fails
When the patient fails to improve after 3 months of medical therapy, additional evaluation such as endometrial biopsy is warranted. For hemodynamically stable patients with normal laboratory evaluation, imaging may be a valuable adjunct. In fact, imaging is increasingly used during the initial workup.12
Biopsy. The endometrium generally is sampled in an office setting using a Pipelle instrument. The biopsy can be performed quickly and generally is well-tolerated by the patient, with few complications. While it has a high sensitivity for detecting endometrial cancer and hyperplasia, it is not as effective in detecting intracavitary lesions, including polyps and submucosal fibroids. Lesions that encompass a small surface area are likely to be missed, as the instrument samples only 10% to 25% of the endometrial cavity. Patients with persistent symptoms despite a normal biopsy require further evaluation.
Transvaginal sonography (TVS). This imaging modality is extremely helpful in evaluating women with postmenopausal bleeding. TVS enhances the detection of uterine fibroids and aids in determining their size and position. Adnexal pathology also can be assessed. If the uterine size is greater than 12 to 14 gestational weeks, transabdominal scanning is preferred.
Measurement of the endometrial echo is helpful in determining whether endometrial biopsy or further imaging studies are necessary. Normally, the postmenopausal endometrial echo measures less than 5 mm. Greater thicknesses are associated with endometrial hyperplasia, polyps, fibroids, and cancer. When the endometrial echo exceeds 5 mm or is indistinct or indeterminate, an enhanced view using saline infusion sonography (SIS) or hysteroscopy is advised. When the endometrial echo is less than 5 mm, malignancy is present in fewer than 0.5% of cases.
Weigel et al prospectively evaluated 200 postmenopausal women with an endometrial echo of 3 to 10 mm and found that homogeneity of the echo, a low echo, and a “sonographically depictable central echo between symmetrical endometrial leaves” were associated with an absence of pathology, while heterogeneity and high echogenicity were associated with pathology.13
During the reproductive years, TVS also is useful in assessing myometrial echotexture, adnexal pathology, and, less consistently, endometrial echo. The endometrial thickness varies daily during a normal menstrual cycle. It is thinnest during menses, increases during the follicular phase, and achieves the greatest endometrial height (10 to 14 mm) during the secretory phase. By correlating these measurements with ovarian activity (corpus luteum vs follicular activity), the physician is better able to assess endometrial morphology observed in the midfollicular and secretory phases. However, ancillary testing with SIS is more sensitive for the detection of intracavitary pathology in these women.
Saline infusion sonography (SIS). With SIS, saline is infused into the endometrial cavity during TVS to enhance the image. Many terms have been used to describe this technique, but I prefer SIS because it defines the technique more precisely.14
SIS allows for more accurate evaluation of the uterus for intracavitary lesions than TVS and makes it easier to differentiate the causes of increased endometrial thickness. Indications for SIS include:
- Abnormal bleeding in premenopausal or postmenopausal patients
- Evaluation of an endometrium that is thickened, irregular, immeasurable, or poorly defined on conventional TVS
- Evaluation of an endometrium that appears irregular on TVS in women on tamoxifen
- Differentiating between sessile and pedunculated masses of the endometrium
- Preoperative evaluation of intracavitary fibroids
Increasingly, gynecologists are embracing the concept of “one-stop” evaluation for menstrual disorders by combining the physical exam and basic laboratory studies (CBC and thyroid-stimulating hormone [TSH]) with TVS unless the clinical history dictates otherwise. However, when TVS is indeterminate, SIS should be performed. Also, all women over 40 who have a suspicious TVS exam should undergo SIS and endometrial biopsy. When such evaluation suggests endometrial polyps or submucosal fibroids, the patient needs operative intervention instead of medical therapy, the patient can be referred directly to a surgeon.
Hysteroscopy. Office hysteroscopy has revolutionized the practice of gynecology. Thin operative hysteroscopes with outer diameters ranging from 3 to 5 mm can be utilized comfortably in an office setting. The procedure permits full visualization of the endometrial cavity and endocervix and facilitates the accurate diagnosis of atrophy, endometrial hyperplasia, polyps, fibroids, and endometrial cancer. Directed endometrial biopsies are possible with some hysteroscopes.
Office hysteroscopy accurately diagnoses many endometrial lesions associated with abnormal bleeding. When the endometrial cavity appears normal at hysteroscopy, aggressive medical therapy should be considered.
Surgical options
Among the surgical treatments for abnormal uterine bleeding are myomectomy, polypectomy, endometrial ablation, and hysterectomy. Dilatation and curettage (D&C), commonly used in the past to treat menstrual aberrations, is no longer preferred. D&C is highly inaccurate, resulting in missed diagnoses, incomplete removal of intracavitary pathology, and a high false-negative rate.15 Operative hysteroscopy with directed endometrial sampling is now the gold standard for surgical evaluation of the uterine cavity. With it, full evaluation is possible even in the presence of heavy bleeding; coexisting intrauterine pathology also can be removed. Because the range of surgical options has broadened in recent years, hysterectomy should be the last resort.
To a large extent, the best surgical modality for a given patient depends on her specific pathology, fertility, and contraceptive needs.
Normal uterine cavity. Endometrial ablation typically is offered after failed medical therapy in women with a normal uterine cavity and negative laboratory workup, provided they have completed childbearing. Hysteroscopic and global endometrial ablation procedures destroy the endometrium, preventing regeneration. This creates Asherman’s syndrome, which leads to hypomenorrhea, eumenorrhea, or amenorrhea.
Endometrial ablation is an outpatient procedure associated with a rapid return to work, minimal complications, and high patient satisfaction. Approximately 20% to 30% of patients undergoing endometrial ablation will become amenorrheic, 65% to 70% will become hypomenorrheic, and 5% to 10% of the procedures will fail. Approximately 30% of patients treated by endometrial ablation will require a subsequent operation.16
If the woman wants to preserve her fertility, I generally order magnetic resonance imaging (MRI) to rule out adenomyosis, since TVS, SIS, and hysteroscopy usually cannot detect it. Adenomyosis can be focal or diffuse and is associated with irregular menses and dysmenorrhea. I also suggest an aggressive trial of medical therapy for at least 3 or 4 cycles, with extensive laboratory evaluation. That is, if the history is suggestive of systemic disease, I order liver function tests. If renal disease is suspected, blood urea nitrogen (BUN) and creatinine levels are helpful, as are luteinizing hormone (LH), FSH, and androgen levels to diagnose PCOS. Adrenal function tests (cortisol, 17-alpha hydroxyprogesterone [17-OHP]) are useful in the diagnosis of hyperandrogensim with suspected adrenal tumors, and congenital adrenal hyperplasia (CAH) is diagnosed by an abnormal 17-OHP level.
Women who smoke often have difficulties with abnormal bleeding. Nicotine is detrimental to the ovaries and is associated with irregular menses and premature ovarian failure. I pressure smokers to stop, as they seem to fail medical therapy more frequently than any other group. I also encourage overweight patients to lose weight. Stress, depression, eating disorders, and excessive exercise also should be addressed.
Submucosal fibroids and endometrial polyps. These lesions vary in number, location, and size. When they are present, the altered endometrial surface area, increased fragility and vascularity, other endometrial irregularities, and atypical prostaglandin levels contribute to abnormal bleeding. Intracavitary lesions also may coexist with anovulatory and ovulatory cycles.
As I mentioned earlier, office hysteroscopy and SIS are the most accurate methods of detecting these lesions. Treatment generally consists of outpatient hysteroscopic myomectomy or polypectomy, which is quick, safe, eases symptoms, and is associated with high levels of patient satisfaction.17
Intramural fibroids. Intramural fibroids can cause disturbances in menstrual flow. Although the mechanisms of action are unclear, these disturbances probably result from topographic endometrial abnormalities, glandular atrophy overlying the fibroid, venous congestion, increased endometrial surface area, or an alteration in prostaglandin levels.
The type of therapy offered depends on the patient’s desire for pregnancy or preservation of the uterus. Basically, there are 3 options: removing or destroying the fibroids or removing the uterus. When future fertility is desired, the patient typically undergoes abdominal or laparoscopic myomectomy, with the surgical route contingent upon the number, size, and location of fibroids, as well as the surgeon’s level of skill and experience.
Attempts to resect large intramural fibroids hysteroscopically should be avoided in patients who have not completed childbearing, since scarring and/or the obliteration of a significant portion of the endometrium overlying the fibroid can lead to infertility.
When the patient experiences heavy menstrual bleeding and does not wish to preserve her fertility, she may be offered a minimally invasive outpatient procedure called uterine artery embolization (UAE). In this procedure, a catheter is inserted transcutaneously and threaded through the femoral artery into the aortic bifurcation and then to the contralateral uterine artery, which is then occluded. Several products are used for UAE, including Embosphere Microspheres (BioSphere Medical, Rockland, Mass), polyvinyl alcohol (PVA) particles, coils, or gelfoam, cutting off blood flow to the fibroid. The fibroid then necroses and shrinks in size and volume. Of the many symptoms associated with fibroids, menorrhagia is most effectively controlled with UAE. In fact, patients have an 85% to 95% chance of resolving symptoms of menorrhagia after undergoing this procedure.18
Hysterectomy offers definitive therapy for patients who have completed childbearing and have no desire for uterine preservation. Either vaginal, laparoscopic-assisted, or abdominal hysterectomy is an option. Factors influencing selection of the surgical route include the number, size, and location of the fibroids, as well as any concomitant pelvic pathology and the surgeon’s skill. (See “Complex hysterectomy: opting for the vaginal approach”.)
Conclusion
After the initial history, physical examination, and laboratory evaluation, the factors involved in abnormal uterine bleeding usually are well defined. Medical management is the standard unless uterine pathology is present. Most patients respond favorably to hormonal manipulation with OCs or progesterone. Another effective option, particularly for women who cannot tolerate traditional medical therapy, is the levonorgestrel-releasing IUS.
Endometrial ablation offers 90% success for the treatment of menorrhagia and dysfunctional bleeding in women with a normal uterine cavity and negative laboratory workup, provided they do not desire children.
Patients with intrauterine polyps and submucosal fibroids have excellent relief of symptoms following operative hysteroscopy. Also, uterine artery embolization is an excellent nonsurgical intervention for patients with fibroids and menorrhagia who wish to avoid major surgery. Fortunately, in this era of many alternative medical and surgical treatments, hysterectomy is the last resort for abnormal uterine bleeding.
Dr. Bradley reports that she serves as a speaker and consultant/preceptor for Olympus.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;328(12):856-860.
2. Coulter A, Bradlow J, Agass M, et al. Outcomes of referrals to gynecology outpatient clinics for menstrual problems: an audit of general practice records. Br J Obstet Gynaecol. 1991;98:789-796.
3. Claessens E, Cowell CA. Acute adolescent menorrhagia. Am J Obstet Gynecol. 1981;139:277-280.
4. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #263. von Willebrand’s disease in gynecologic practice. Obstet Gynecol. 2001;98:1185-1186.
5. Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril. 1999;71:658-662.
6. Kaunitz A. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2):S32-S37.
7. Jones H. Clinical pathway for evaluating women with abnormal uterine bleeding. Obstet Gynecol Surv. 2002;57(1):22-24.
8. Higham JM, Shaw RW. A comparative study of danazol, a regimen of decreasing doses of danazol and norethindrone in the treatment of objectively proven unexplained menorrhagia. Am J Obstet Gynecol. 1993;169:1134-1139.
9. Scialli A, Levi A. Intermittent leuprolide acetate for the nonsurgical management of women with leiomyomata uteri. Fertil Steril. 2000;74(3):540-546.
10. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomized trial. Lancet. 2001;357:273-277.
11. Vargyas JM, Campeau JD, Mishell DR. Treatment of menorrhagia with meclofenamate sodium. Am J Obstet Gynecol. 1987;157:944-950.
12. Jones K, Bourne T. The feasibility of a “one-stop” ultrasound-based clinic for the diagnosis and management of abnormal uterine bleeding. Ultrasound Obstet Gynecol. 2001;176:517-521.
13. Weigel M, Friese K, Strittmatter HJ, et al. Measuring the thickness—is that all we have to do for sonographic assessment of endometrium in postmenopausal women? Ultrasound Obstet Gynecol. 1995;6:97-102.
14. Widrich T, Bradley L, Mitchinson AR, Collins R. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol. 1996;174:1327-1334.
15. Bettocchi S, Ceci O, Vicino M, et al. Diagnostic inadequacy of dilation and curettage. Fertil Steril. 2001;75(4):803-805.
16. Stabinsky S, Einstein M, Breen J. Modern treatments of menorrhagia attributable to dysfunctional uterine bleeding. Obstet Gynecol Surv. 1999;54(11):251-262.
17. Pasqualotto EB, Margossian H, Priul L, Bradley LD, et al. Accuracy of preoperative diagnostic tools and outcome of hysteroscopic management of menstrual dysfunction. J Am Assoc Gynecol Laparosc. 2000;7(2):201-209.
18. Hurst B, Stackhouse D, Matthews M, Marshburn P. Uterine artery embolization for symptomatic uterine myomas. Fertil Steril. 2000;74(5):855-869.
- Approximately 15% to 20% of office gynecologic visits are for the evaluation of abnormal uterine bleeding (AUB), and 25% to 50% of gynecologic surgeries are performed to address menstrual dysfunction.
- Office hysteroscopy and saline infusion sonography are essential skills for the practicing gynecologist. Learn them and use them liberally.
- Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- Liberal use of endometrial biopsy is encouraged in women over 35 years of age at risk for endometrial hyperplasia and cancer.
- About 20% to 30% of teens with irregular heavy menses have a major bleeding diathesis.
- Medical therapy is the standard unless uterine pathology is present.
Half of all hysterectomies in the United States are performed to treat abnormal uterine bleeding. Of these, approximately 20% are performed in women with a normal uterine size.1 However, when the uterus appears normal, without adenomyosis or uterine pathology, it is imperative that the clinician perform a thorough evaluation before resorting to hysterectomy.
Abnormal uterine bleeding is defined as excessive, erratic, or irregular bleeding in the presence or absence of intracavitary or uterine pathology. It may be associated with structural or systemic abnormalities. In contrast, dysfunctional uterine bleeding (DUB) is associated with anovulatory menstrual cycles. It is not caused by pelvic pathology, medications, systemic disease, or pregnancy.
Abnormal bleeding is associated with an array of symptoms. Frequent complaints include heavy or prolonged menstrual flow, social embarrassment, diminished quality of life, sexual compromise, and the need to alter lifestyle. Pain is not a common presenting symptom unless it is associated with the passage of large blood clots.
The following menstrual patterns are associated with DUB:
- Oligomenorrhea. A cycle length of more than 35 days
- Polymenorrhea. A cycle length of less than 21 days
- Amenorrhea. The absence of menses for 6 months or 3 consecutive cycles
- Menorrhagia. Heavy or increased flow occurring at regular intervals, or a loss of more than 80 mL of blood
- Metrorrhagia. Irregular episodes of bleeding
- Menometrorrhagia. A longer duration of flow occurring at unpredictable intervals
- Postmenopausal bleeding. Bleeding that occurs more than 12 months after the last menstrual cycle
Basic evaluation and management of abnormal uterine bleeding
*If still bleeds, then SIS or hysteroscopy
SIS=saline infusion sonography
Prevalence and pathophysiology
Although we lack precise figures regarding the prevalence of abnormal uterine bleeding, it is estimated that 9% to 30% of reproductive-age women have menstrual irregularities requiring medical evaluation.2 Approximately 15% to 20% of office gynecologic visits are scheduled for the evaluation of abnormal uterine bleeding, which is exceeded only by vaginitis as a chief complaint. In addition, 25% to 50% of gynecologic surgical procedures are performed to address menstrual dysfunction.
Normal menstruation is triggered by fluctuations in the hypothalamic-pituitary-ovarian axis that lead to denudation and sloughing of the endometrium. This hemorrhage is followed by prompt hemostasis and repair. Low physiologic levels of estrogen prime the endometrium, while the normal secretion of progesterone from the corpus luteum stabilizes it, decreasing vascular fragility and supporting the endometrial stroma. Platelets and fibrin are necessary for endometrial hemostasis. Deficiencies in either factor may result in heavier menstruation.
DUB occurs when there is inadequate progesterone secretion to stabilize the endometrium. Anovulatory bleeding can be episodic or continuous. Patients with anovulatory cycles typically do not experience premenstrual tension, breast discomfort, increased mucoid vaginal discharge, or cramping and bloating, all characteristic of ovulatory cycles. Although ovulatory cycles are predictable, erratic bleeding may occur when they coexist with intracavitary lesions, including polyps and fibroids.
Anovulatory cycles typically are associated with puberty and the perimenopausal years. In puberty, the immature hypothalamic-pituitary-ovarian axis has not yet developed the necessary hormonal feedback to sustain the endometrium. In perimenopause, the decline of inhibin and rise in follicle-stimulating hormone (FSH) levels reflect the loss of follicular activity and competence.
In some cases, severe anemia can cause incessant menstrual blood loss. Typical complaints of anemia include fatigue, unusual food cravings (pica), and headaches. Severe anemia also can cause fainting, exercise-induced fatigue, shortness of breath, congestive heart failure, and/or the inability to perform routine activities. Unless it is a chronic condition, DUB is rarely associated with the need for a blood transfusion. Hemorrhagic shock and death are rare sequelae.
Diagnosis
Diagnosis involves 3 main components:
- A detailed medical history and review of systems. This should alert the physician to the possible etiology of a patient’s menstrual dysfunction (Table 1). Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- A physical examination. The exam must be comprehensive, even in the presence of heavy bleeding, focusing on the vagina, cervix, uterus, and adnexa to exclude pathology.
- Appropriate laboratory studies based on the focused clinical history and any physical findings. Pregnancy testing is necessary in all sexually active premenopausal women. In addition, women with profuse menorrhagia and a normal uterine size should be screened for von Willebrand’s disease, since 13% to 20% of women offered surgical intervention have the subtle form of Type I disease. (In women, von Willebrand’s disease most commonly presents as DUB.) Obviously, medical therapy is paramount for women with von Willebrand’s disease; hysterectomy or other surgical treatment should not be the first option.
TABLE 1
Causes of menstrual dysfunction
| ANATOMIC |
| Polyps |
| Fibroids |
| Adenomyosis |
| Vaginitis |
| Endometritis |
| Retained products of conception |
| Endometriosis |
| Hyperplasia |
| Malignancy |
| ENDOCRINE |
| Thyroid dysfunction |
| Elevated prolactin levels |
| Adrenal dysfunction |
| Hypothalamic/pituitary dysfunction |
| Estrogen-producing tumors |
| HEMATOLOGIC |
| Anemia |
Coagulopathy
|
| Leukemia |
| SYSTEMIC |
| Renal impairment |
| Liver disorders |
| Obesity |
| Anorexia |
| Chronic illness |
| Rapid fluctuations in weight |
| MEDICATIONS |
| Anticoagulants |
| Steroids |
| Progesterone withdrawal |
| Herbal and soy products |
| MISCELLANEOUS |
| Smoking |
| Depression |
| Excessive alcohol intake |
| Sexually transmitted diseases |
Special populations
Adolescents. Teens with irregular heavy menses should be evaluated for coagulopathies, since 20% to 30% have a major bleeding diathesis.3 This is especially true if the patient presents with a hemoglobin level of less than 10 g/dL or if hospitalization is required. Specifically, adolescents should be evaluated for von Willebrand’s disease with the ristocetin cofactor assay, the single best screening test for the disease. This prevents false-negative results. Other laboratory tests should include:
- Serum human chorionic gonadotropin (hCG)
- Bleeding time
- Partial time (PT) and partial thromboplastin time (PTT)
- Complete blood count (CBC) with platelets
Successful medical therapies for von Willebrand’s disease include oral contraceptives (OCs), which have an 88% success rate; desmopressin acetate; antifibrinolytic agents; and plasma-derived concentrates rich in the high-molecular-weight multimers of von Willebrand factor (vWf).4
Perimenopausal women. Women entering perimenopause may have recurrent bouts of DUB and associated physical complaints due to changes in the hypothalamic-pituitaryovarian axis. The hormonal milieu is associated with decreased inhibin, variable estradiol, normal FSH, and menstrual cycles that can be episodically ovulatory.5 Many menstrual complaints occur in perimenopausal women, including menometrorrhagia, amenorrhea, and oligomenorrheic cycles. Decreased mental clarity and concentration, vaginal dryness, hot flushes, and night sweats are classic symptoms of perimenopause.
Oral contraceptive (OC) therapy is quite useful in these women and should be the first line of intervention, rather than conventional hormone replacement therapy (HRT).6 The usual postmenopausal doses of HRT do not suppress ovulation or prevent pregnancy, while OCs do. In healthy, nonsmoking women over 35 years of age, OCs regulate menstrual cycles, decrease vasomotor symptoms, improve bone mineral density (BMD), and reduce the need for surgical intervention for DUB. They also reduce endometrial and ovarian cancer rates.
Postmenopausal patients. Bleeding that occurs with HRT or tamoxifen use more than 1 year after the cessation of menses requires thorough evaluation. While the most common cause of postmenopausal bleeding is atrophy, it is important to rule out intracavitary pathology, endometrial hyperplasia, and cancer. Approximately 10% of women with postmenopausal bleeding have endometrial cancer. Because the risk of this cancer increases with each decade of life, its exclusion is critical.
Focal intracavitary lesions, including polyps, submucosal fibroids, and endometrial hyperplasia, account for 20% to 40% of cases of abnormal uterine bleeding in this population.7
Organic diseases. Women with renal or liver disease also may have abnormal uterine bleeding. Patients with liver disease may have higher circulating levels of estrogen due to hepatic dysfunction and an inability to metabolize estrogen. Coagulopathies also may occur with liver disease, while renal failure is associated with hypothalamic-pituitaryovarian axis irregularities due to gonadal resistance to hormones, platelet dysfunction, and abnormal factor VIII activity.
Medical therapy
Once the likely cause of abnormal bleeding is identified, appropriate treatment should be instituted. For anovulatory cycles, medical therapy with OCs or progesterone is the standard. Patients with ovulatory abnormal bleeding should be evaluated for intracavitary uterine pathology, since hormonal dysfunction is probably not the cause. Patients whose abnormal bleeding is anatomic in origin usually are managed surgically.
Medical therapy should be tailored to the individual after reviewing her risks, benefits, contraindications, and individual concerns. It is important to determine which facet of the menstrual cycle the patient wants improved, e.g., length, duration, clotting, pain, quantity, in order to target treatment appropriately.
Objective measurements (alkaline hematin assay) of menstrual blood loss are impractical in an office setting.
Oral contraceptives. OCs have many roles in the treatment of menorrhagia and other forms of DUB. Short-term, high-dose therapy is valuable when excessive bleeding occurs in an emergency situation or when heavy menstrual bleeding occurs in adolescent and perimenopausal women. Any low-dose (30 to 35 mcg) ethinyl estradiol product can be given at 6-hour intervals for 5 days to stabilize bleeding. This should be followed by a tapering regimen of 1 low-dose OC pill at 8-hour intervals for 5 days, 12-hour intervals for 5 more days, and then daily for 5 days. This regimen quickly halts heavy menses and controls bleeding. It also prepares the patient for a withdrawal menses.
After the withdrawal bleed, the patient should continue on a maintenance dose (1 pill daily) to ensure regular menstrual cycles and contraception. Low-dose OCs are safe and effective for women over 35 who do not smoke and lack a history of thromboembolic disease.
Progesterone therapy. Women with anovulatory menstrual cycles also may benefit from progesterone therapy, which stabilizes the proliferative endometrium and establishes regular sloughing. Cyclical progesterone is useful in women with contraindications to estrogen therapy, e.g., women over 35 who smoke or have a history of deep venous thrombosis (DVT) or cardiovascular risk factors. Generally, 10 mg of medroxyprogesterone acetate for 10 to 14 days each month will induce a regular withdrawal bleed, although it does not provide contraception.
Long-acting progesterone therapy in the form of medroxyprogesterone (Depo-Provera; Pharmacia Corp, Peapack, NJ) will stop menses in the majority of patients. Standard dosing is 150 mg administered intramuscularly (IM) every 3 months. Approximately 80% to 90% of patients who complete 12 months of Depo-Provera therapy will be amenorrheic. Potential side effects include weight gain, irregular bleeding, and depression.
Danazol. This pituitary suppressant creates a hypoestrogenic state and decreases menstrual blood loss by 70% to 80%. A daily dose of 50 to 100 mg may be adequate in some cases; otherwise, the conventional 400 to 800 mg is recommended. Potential side effects include weight gain, acne, and alteration of lipids.8
GnRH therapy. Gonadotropin-releasing hormone (GnRH) therapy with leuprolide or nafarelin creates a hypoestrogenic menopause-like condition, with menstruation usually ceasing within 3 months. Menopausal symptoms may include hot flushes, night sweats, vaginal dryness, bone loss, decreased concentration, and diminished libido. Nevertheless, compliance generally is good. Because prolonged therapy can lead to osteoporosis, treatment usually is limited to 6 months unless estrogen “addback” therapy is instituted.
GnRH therapy is a valuable option for the late perimenopausal woman who has significant contraindications to other medical regimens. For most of these women, the cessation of menses is a relief. After therapy, many patients spontaneously transition into the menopause. An intermittent 6-month course of leuprolide is an option for women with uterine fibroids. Data indicate that it provides an additional 9 months of symptom control (range: 2 to longer than 25 months).9
Progesterone intrauterine system. The recently introduced levonorgestrel-releasing intrauterine system (IUS) (Mirena, Berlex Laboratories, Montville, NJ) also is effective therapy for DUB. This IUS causes pseudodecidual changes and amenorrhea, decreasing menstrual blood loss by 65% to 98% within 12 months, with little systemic absorption of progesterone. It is likely to prove quite valuable for women with menorrhagia who need contraception, have a normal uterine size, and wish to avoid surgery.10
NSAIDs. Nonsteroidal anti-inflammatory drugs (NSAIDs) decrease the rate of dysmenorrhea, improve clotting, and reduce menstrual blood loss. Some studies have demonstrated a 50% to 80% reduction in blood loss with proper use.11 Patients are advised to begin therapy 1 to 2 days before their period is expected and continue throughout the menses. NSAIDs may be combined with OCs, if necessary.
Most menstrual cycles occur every 21 to 35 days. Normal menstrual flow lasts 3 to 7 days, with most blood lost within the first 3 days. The typical menstrual flow averages 35 mL and consists of effluent debris and blood. Women with normal menstrual cycles use an average of 5 to 6 pads or tampons each day. Social obligations, sexual activity, hobbies, work, and travel are not interrupted with normal menstrual function.
When menorrhagia is present, a woman may lose more than 80 mL of blood with each menstrual cycle. Since approximately 16 mg of iron is lost in normal cycles, women with menorrhagia often develop anemia. They also typically have an imbalance of prostaglandin levels and increased fibrinolytic activity.
It is important to note that more than 50% of women who complain of menorrhagia do not have heavy menses. Some patients change their sanitary products more often not because of heavy flow, but for reasons concerning hygiene, personal preference, or fear of toxic shock syndrome.—Linda D. Bradley, MD
FIGURE 5
When medical therapy fails
When the patient fails to improve after 3 months of medical therapy, additional evaluation such as endometrial biopsy is warranted. For hemodynamically stable patients with normal laboratory evaluation, imaging may be a valuable adjunct. In fact, imaging is increasingly used during the initial workup.12
Biopsy. The endometrium generally is sampled in an office setting using a Pipelle instrument. The biopsy can be performed quickly and generally is well-tolerated by the patient, with few complications. While it has a high sensitivity for detecting endometrial cancer and hyperplasia, it is not as effective in detecting intracavitary lesions, including polyps and submucosal fibroids. Lesions that encompass a small surface area are likely to be missed, as the instrument samples only 10% to 25% of the endometrial cavity. Patients with persistent symptoms despite a normal biopsy require further evaluation.
Transvaginal sonography (TVS). This imaging modality is extremely helpful in evaluating women with postmenopausal bleeding. TVS enhances the detection of uterine fibroids and aids in determining their size and position. Adnexal pathology also can be assessed. If the uterine size is greater than 12 to 14 gestational weeks, transabdominal scanning is preferred.
Measurement of the endometrial echo is helpful in determining whether endometrial biopsy or further imaging studies are necessary. Normally, the postmenopausal endometrial echo measures less than 5 mm. Greater thicknesses are associated with endometrial hyperplasia, polyps, fibroids, and cancer. When the endometrial echo exceeds 5 mm or is indistinct or indeterminate, an enhanced view using saline infusion sonography (SIS) or hysteroscopy is advised. When the endometrial echo is less than 5 mm, malignancy is present in fewer than 0.5% of cases.
Weigel et al prospectively evaluated 200 postmenopausal women with an endometrial echo of 3 to 10 mm and found that homogeneity of the echo, a low echo, and a “sonographically depictable central echo between symmetrical endometrial leaves” were associated with an absence of pathology, while heterogeneity and high echogenicity were associated with pathology.13
During the reproductive years, TVS also is useful in assessing myometrial echotexture, adnexal pathology, and, less consistently, endometrial echo. The endometrial thickness varies daily during a normal menstrual cycle. It is thinnest during menses, increases during the follicular phase, and achieves the greatest endometrial height (10 to 14 mm) during the secretory phase. By correlating these measurements with ovarian activity (corpus luteum vs follicular activity), the physician is better able to assess endometrial morphology observed in the midfollicular and secretory phases. However, ancillary testing with SIS is more sensitive for the detection of intracavitary pathology in these women.
Saline infusion sonography (SIS). With SIS, saline is infused into the endometrial cavity during TVS to enhance the image. Many terms have been used to describe this technique, but I prefer SIS because it defines the technique more precisely.14
SIS allows for more accurate evaluation of the uterus for intracavitary lesions than TVS and makes it easier to differentiate the causes of increased endometrial thickness. Indications for SIS include:
- Abnormal bleeding in premenopausal or postmenopausal patients
- Evaluation of an endometrium that is thickened, irregular, immeasurable, or poorly defined on conventional TVS
- Evaluation of an endometrium that appears irregular on TVS in women on tamoxifen
- Differentiating between sessile and pedunculated masses of the endometrium
- Preoperative evaluation of intracavitary fibroids
Increasingly, gynecologists are embracing the concept of “one-stop” evaluation for menstrual disorders by combining the physical exam and basic laboratory studies (CBC and thyroid-stimulating hormone [TSH]) with TVS unless the clinical history dictates otherwise. However, when TVS is indeterminate, SIS should be performed. Also, all women over 40 who have a suspicious TVS exam should undergo SIS and endometrial biopsy. When such evaluation suggests endometrial polyps or submucosal fibroids, the patient needs operative intervention instead of medical therapy, the patient can be referred directly to a surgeon.
Hysteroscopy. Office hysteroscopy has revolutionized the practice of gynecology. Thin operative hysteroscopes with outer diameters ranging from 3 to 5 mm can be utilized comfortably in an office setting. The procedure permits full visualization of the endometrial cavity and endocervix and facilitates the accurate diagnosis of atrophy, endometrial hyperplasia, polyps, fibroids, and endometrial cancer. Directed endometrial biopsies are possible with some hysteroscopes.
Office hysteroscopy accurately diagnoses many endometrial lesions associated with abnormal bleeding. When the endometrial cavity appears normal at hysteroscopy, aggressive medical therapy should be considered.
Surgical options
Among the surgical treatments for abnormal uterine bleeding are myomectomy, polypectomy, endometrial ablation, and hysterectomy. Dilatation and curettage (D&C), commonly used in the past to treat menstrual aberrations, is no longer preferred. D&C is highly inaccurate, resulting in missed diagnoses, incomplete removal of intracavitary pathology, and a high false-negative rate.15 Operative hysteroscopy with directed endometrial sampling is now the gold standard for surgical evaluation of the uterine cavity. With it, full evaluation is possible even in the presence of heavy bleeding; coexisting intrauterine pathology also can be removed. Because the range of surgical options has broadened in recent years, hysterectomy should be the last resort.
To a large extent, the best surgical modality for a given patient depends on her specific pathology, fertility, and contraceptive needs.
Normal uterine cavity. Endometrial ablation typically is offered after failed medical therapy in women with a normal uterine cavity and negative laboratory workup, provided they have completed childbearing. Hysteroscopic and global endometrial ablation procedures destroy the endometrium, preventing regeneration. This creates Asherman’s syndrome, which leads to hypomenorrhea, eumenorrhea, or amenorrhea.
Endometrial ablation is an outpatient procedure associated with a rapid return to work, minimal complications, and high patient satisfaction. Approximately 20% to 30% of patients undergoing endometrial ablation will become amenorrheic, 65% to 70% will become hypomenorrheic, and 5% to 10% of the procedures will fail. Approximately 30% of patients treated by endometrial ablation will require a subsequent operation.16
If the woman wants to preserve her fertility, I generally order magnetic resonance imaging (MRI) to rule out adenomyosis, since TVS, SIS, and hysteroscopy usually cannot detect it. Adenomyosis can be focal or diffuse and is associated with irregular menses and dysmenorrhea. I also suggest an aggressive trial of medical therapy for at least 3 or 4 cycles, with extensive laboratory evaluation. That is, if the history is suggestive of systemic disease, I order liver function tests. If renal disease is suspected, blood urea nitrogen (BUN) and creatinine levels are helpful, as are luteinizing hormone (LH), FSH, and androgen levels to diagnose PCOS. Adrenal function tests (cortisol, 17-alpha hydroxyprogesterone [17-OHP]) are useful in the diagnosis of hyperandrogensim with suspected adrenal tumors, and congenital adrenal hyperplasia (CAH) is diagnosed by an abnormal 17-OHP level.
Women who smoke often have difficulties with abnormal bleeding. Nicotine is detrimental to the ovaries and is associated with irregular menses and premature ovarian failure. I pressure smokers to stop, as they seem to fail medical therapy more frequently than any other group. I also encourage overweight patients to lose weight. Stress, depression, eating disorders, and excessive exercise also should be addressed.
Submucosal fibroids and endometrial polyps. These lesions vary in number, location, and size. When they are present, the altered endometrial surface area, increased fragility and vascularity, other endometrial irregularities, and atypical prostaglandin levels contribute to abnormal bleeding. Intracavitary lesions also may coexist with anovulatory and ovulatory cycles.
As I mentioned earlier, office hysteroscopy and SIS are the most accurate methods of detecting these lesions. Treatment generally consists of outpatient hysteroscopic myomectomy or polypectomy, which is quick, safe, eases symptoms, and is associated with high levels of patient satisfaction.17
Intramural fibroids. Intramural fibroids can cause disturbances in menstrual flow. Although the mechanisms of action are unclear, these disturbances probably result from topographic endometrial abnormalities, glandular atrophy overlying the fibroid, venous congestion, increased endometrial surface area, or an alteration in prostaglandin levels.
The type of therapy offered depends on the patient’s desire for pregnancy or preservation of the uterus. Basically, there are 3 options: removing or destroying the fibroids or removing the uterus. When future fertility is desired, the patient typically undergoes abdominal or laparoscopic myomectomy, with the surgical route contingent upon the number, size, and location of fibroids, as well as the surgeon’s level of skill and experience.
Attempts to resect large intramural fibroids hysteroscopically should be avoided in patients who have not completed childbearing, since scarring and/or the obliteration of a significant portion of the endometrium overlying the fibroid can lead to infertility.
When the patient experiences heavy menstrual bleeding and does not wish to preserve her fertility, she may be offered a minimally invasive outpatient procedure called uterine artery embolization (UAE). In this procedure, a catheter is inserted transcutaneously and threaded through the femoral artery into the aortic bifurcation and then to the contralateral uterine artery, which is then occluded. Several products are used for UAE, including Embosphere Microspheres (BioSphere Medical, Rockland, Mass), polyvinyl alcohol (PVA) particles, coils, or gelfoam, cutting off blood flow to the fibroid. The fibroid then necroses and shrinks in size and volume. Of the many symptoms associated with fibroids, menorrhagia is most effectively controlled with UAE. In fact, patients have an 85% to 95% chance of resolving symptoms of menorrhagia after undergoing this procedure.18
Hysterectomy offers definitive therapy for patients who have completed childbearing and have no desire for uterine preservation. Either vaginal, laparoscopic-assisted, or abdominal hysterectomy is an option. Factors influencing selection of the surgical route include the number, size, and location of the fibroids, as well as any concomitant pelvic pathology and the surgeon’s skill. (See “Complex hysterectomy: opting for the vaginal approach”.)
Conclusion
After the initial history, physical examination, and laboratory evaluation, the factors involved in abnormal uterine bleeding usually are well defined. Medical management is the standard unless uterine pathology is present. Most patients respond favorably to hormonal manipulation with OCs or progesterone. Another effective option, particularly for women who cannot tolerate traditional medical therapy, is the levonorgestrel-releasing IUS.
Endometrial ablation offers 90% success for the treatment of menorrhagia and dysfunctional bleeding in women with a normal uterine cavity and negative laboratory workup, provided they do not desire children.
Patients with intrauterine polyps and submucosal fibroids have excellent relief of symptoms following operative hysteroscopy. Also, uterine artery embolization is an excellent nonsurgical intervention for patients with fibroids and menorrhagia who wish to avoid major surgery. Fortunately, in this era of many alternative medical and surgical treatments, hysterectomy is the last resort for abnormal uterine bleeding.
Dr. Bradley reports that she serves as a speaker and consultant/preceptor for Olympus.
- Approximately 15% to 20% of office gynecologic visits are for the evaluation of abnormal uterine bleeding (AUB), and 25% to 50% of gynecologic surgeries are performed to address menstrual dysfunction.
- Office hysteroscopy and saline infusion sonography are essential skills for the practicing gynecologist. Learn them and use them liberally.
- Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- Liberal use of endometrial biopsy is encouraged in women over 35 years of age at risk for endometrial hyperplasia and cancer.
- About 20% to 30% of teens with irregular heavy menses have a major bleeding diathesis.
- Medical therapy is the standard unless uterine pathology is present.
Half of all hysterectomies in the United States are performed to treat abnormal uterine bleeding. Of these, approximately 20% are performed in women with a normal uterine size.1 However, when the uterus appears normal, without adenomyosis or uterine pathology, it is imperative that the clinician perform a thorough evaluation before resorting to hysterectomy.
Abnormal uterine bleeding is defined as excessive, erratic, or irregular bleeding in the presence or absence of intracavitary or uterine pathology. It may be associated with structural or systemic abnormalities. In contrast, dysfunctional uterine bleeding (DUB) is associated with anovulatory menstrual cycles. It is not caused by pelvic pathology, medications, systemic disease, or pregnancy.
Abnormal bleeding is associated with an array of symptoms. Frequent complaints include heavy or prolonged menstrual flow, social embarrassment, diminished quality of life, sexual compromise, and the need to alter lifestyle. Pain is not a common presenting symptom unless it is associated with the passage of large blood clots.
The following menstrual patterns are associated with DUB:
- Oligomenorrhea. A cycle length of more than 35 days
- Polymenorrhea. A cycle length of less than 21 days
- Amenorrhea. The absence of menses for 6 months or 3 consecutive cycles
- Menorrhagia. Heavy or increased flow occurring at regular intervals, or a loss of more than 80 mL of blood
- Metrorrhagia. Irregular episodes of bleeding
- Menometrorrhagia. A longer duration of flow occurring at unpredictable intervals
- Postmenopausal bleeding. Bleeding that occurs more than 12 months after the last menstrual cycle
Basic evaluation and management of abnormal uterine bleeding
*If still bleeds, then SIS or hysteroscopy
SIS=saline infusion sonography
Prevalence and pathophysiology
Although we lack precise figures regarding the prevalence of abnormal uterine bleeding, it is estimated that 9% to 30% of reproductive-age women have menstrual irregularities requiring medical evaluation.2 Approximately 15% to 20% of office gynecologic visits are scheduled for the evaluation of abnormal uterine bleeding, which is exceeded only by vaginitis as a chief complaint. In addition, 25% to 50% of gynecologic surgical procedures are performed to address menstrual dysfunction.
Normal menstruation is triggered by fluctuations in the hypothalamic-pituitary-ovarian axis that lead to denudation and sloughing of the endometrium. This hemorrhage is followed by prompt hemostasis and repair. Low physiologic levels of estrogen prime the endometrium, while the normal secretion of progesterone from the corpus luteum stabilizes it, decreasing vascular fragility and supporting the endometrial stroma. Platelets and fibrin are necessary for endometrial hemostasis. Deficiencies in either factor may result in heavier menstruation.
DUB occurs when there is inadequate progesterone secretion to stabilize the endometrium. Anovulatory bleeding can be episodic or continuous. Patients with anovulatory cycles typically do not experience premenstrual tension, breast discomfort, increased mucoid vaginal discharge, or cramping and bloating, all characteristic of ovulatory cycles. Although ovulatory cycles are predictable, erratic bleeding may occur when they coexist with intracavitary lesions, including polyps and fibroids.
Anovulatory cycles typically are associated with puberty and the perimenopausal years. In puberty, the immature hypothalamic-pituitary-ovarian axis has not yet developed the necessary hormonal feedback to sustain the endometrium. In perimenopause, the decline of inhibin and rise in follicle-stimulating hormone (FSH) levels reflect the loss of follicular activity and competence.
In some cases, severe anemia can cause incessant menstrual blood loss. Typical complaints of anemia include fatigue, unusual food cravings (pica), and headaches. Severe anemia also can cause fainting, exercise-induced fatigue, shortness of breath, congestive heart failure, and/or the inability to perform routine activities. Unless it is a chronic condition, DUB is rarely associated with the need for a blood transfusion. Hemorrhagic shock and death are rare sequelae.
Diagnosis
Diagnosis involves 3 main components:
- A detailed medical history and review of systems. This should alert the physician to the possible etiology of a patient’s menstrual dysfunction (Table 1). Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- A physical examination. The exam must be comprehensive, even in the presence of heavy bleeding, focusing on the vagina, cervix, uterus, and adnexa to exclude pathology.
- Appropriate laboratory studies based on the focused clinical history and any physical findings. Pregnancy testing is necessary in all sexually active premenopausal women. In addition, women with profuse menorrhagia and a normal uterine size should be screened for von Willebrand’s disease, since 13% to 20% of women offered surgical intervention have the subtle form of Type I disease. (In women, von Willebrand’s disease most commonly presents as DUB.) Obviously, medical therapy is paramount for women with von Willebrand’s disease; hysterectomy or other surgical treatment should not be the first option.
TABLE 1
Causes of menstrual dysfunction
| ANATOMIC |
| Polyps |
| Fibroids |
| Adenomyosis |
| Vaginitis |
| Endometritis |
| Retained products of conception |
| Endometriosis |
| Hyperplasia |
| Malignancy |
| ENDOCRINE |
| Thyroid dysfunction |
| Elevated prolactin levels |
| Adrenal dysfunction |
| Hypothalamic/pituitary dysfunction |
| Estrogen-producing tumors |
| HEMATOLOGIC |
| Anemia |
Coagulopathy
|
| Leukemia |
| SYSTEMIC |
| Renal impairment |
| Liver disorders |
| Obesity |
| Anorexia |
| Chronic illness |
| Rapid fluctuations in weight |
| MEDICATIONS |
| Anticoagulants |
| Steroids |
| Progesterone withdrawal |
| Herbal and soy products |
| MISCELLANEOUS |
| Smoking |
| Depression |
| Excessive alcohol intake |
| Sexually transmitted diseases |
Special populations
Adolescents. Teens with irregular heavy menses should be evaluated for coagulopathies, since 20% to 30% have a major bleeding diathesis.3 This is especially true if the patient presents with a hemoglobin level of less than 10 g/dL or if hospitalization is required. Specifically, adolescents should be evaluated for von Willebrand’s disease with the ristocetin cofactor assay, the single best screening test for the disease. This prevents false-negative results. Other laboratory tests should include:
- Serum human chorionic gonadotropin (hCG)
- Bleeding time
- Partial time (PT) and partial thromboplastin time (PTT)
- Complete blood count (CBC) with platelets
Successful medical therapies for von Willebrand’s disease include oral contraceptives (OCs), which have an 88% success rate; desmopressin acetate; antifibrinolytic agents; and plasma-derived concentrates rich in the high-molecular-weight multimers of von Willebrand factor (vWf).4
Perimenopausal women. Women entering perimenopause may have recurrent bouts of DUB and associated physical complaints due to changes in the hypothalamic-pituitaryovarian axis. The hormonal milieu is associated with decreased inhibin, variable estradiol, normal FSH, and menstrual cycles that can be episodically ovulatory.5 Many menstrual complaints occur in perimenopausal women, including menometrorrhagia, amenorrhea, and oligomenorrheic cycles. Decreased mental clarity and concentration, vaginal dryness, hot flushes, and night sweats are classic symptoms of perimenopause.
Oral contraceptive (OC) therapy is quite useful in these women and should be the first line of intervention, rather than conventional hormone replacement therapy (HRT).6 The usual postmenopausal doses of HRT do not suppress ovulation or prevent pregnancy, while OCs do. In healthy, nonsmoking women over 35 years of age, OCs regulate menstrual cycles, decrease vasomotor symptoms, improve bone mineral density (BMD), and reduce the need for surgical intervention for DUB. They also reduce endometrial and ovarian cancer rates.
Postmenopausal patients. Bleeding that occurs with HRT or tamoxifen use more than 1 year after the cessation of menses requires thorough evaluation. While the most common cause of postmenopausal bleeding is atrophy, it is important to rule out intracavitary pathology, endometrial hyperplasia, and cancer. Approximately 10% of women with postmenopausal bleeding have endometrial cancer. Because the risk of this cancer increases with each decade of life, its exclusion is critical.
Focal intracavitary lesions, including polyps, submucosal fibroids, and endometrial hyperplasia, account for 20% to 40% of cases of abnormal uterine bleeding in this population.7
Organic diseases. Women with renal or liver disease also may have abnormal uterine bleeding. Patients with liver disease may have higher circulating levels of estrogen due to hepatic dysfunction and an inability to metabolize estrogen. Coagulopathies also may occur with liver disease, while renal failure is associated with hypothalamic-pituitaryovarian axis irregularities due to gonadal resistance to hormones, platelet dysfunction, and abnormal factor VIII activity.
Medical therapy
Once the likely cause of abnormal bleeding is identified, appropriate treatment should be instituted. For anovulatory cycles, medical therapy with OCs or progesterone is the standard. Patients with ovulatory abnormal bleeding should be evaluated for intracavitary uterine pathology, since hormonal dysfunction is probably not the cause. Patients whose abnormal bleeding is anatomic in origin usually are managed surgically.
Medical therapy should be tailored to the individual after reviewing her risks, benefits, contraindications, and individual concerns. It is important to determine which facet of the menstrual cycle the patient wants improved, e.g., length, duration, clotting, pain, quantity, in order to target treatment appropriately.
Objective measurements (alkaline hematin assay) of menstrual blood loss are impractical in an office setting.
Oral contraceptives. OCs have many roles in the treatment of menorrhagia and other forms of DUB. Short-term, high-dose therapy is valuable when excessive bleeding occurs in an emergency situation or when heavy menstrual bleeding occurs in adolescent and perimenopausal women. Any low-dose (30 to 35 mcg) ethinyl estradiol product can be given at 6-hour intervals for 5 days to stabilize bleeding. This should be followed by a tapering regimen of 1 low-dose OC pill at 8-hour intervals for 5 days, 12-hour intervals for 5 more days, and then daily for 5 days. This regimen quickly halts heavy menses and controls bleeding. It also prepares the patient for a withdrawal menses.
After the withdrawal bleed, the patient should continue on a maintenance dose (1 pill daily) to ensure regular menstrual cycles and contraception. Low-dose OCs are safe and effective for women over 35 who do not smoke and lack a history of thromboembolic disease.
Progesterone therapy. Women with anovulatory menstrual cycles also may benefit from progesterone therapy, which stabilizes the proliferative endometrium and establishes regular sloughing. Cyclical progesterone is useful in women with contraindications to estrogen therapy, e.g., women over 35 who smoke or have a history of deep venous thrombosis (DVT) or cardiovascular risk factors. Generally, 10 mg of medroxyprogesterone acetate for 10 to 14 days each month will induce a regular withdrawal bleed, although it does not provide contraception.
Long-acting progesterone therapy in the form of medroxyprogesterone (Depo-Provera; Pharmacia Corp, Peapack, NJ) will stop menses in the majority of patients. Standard dosing is 150 mg administered intramuscularly (IM) every 3 months. Approximately 80% to 90% of patients who complete 12 months of Depo-Provera therapy will be amenorrheic. Potential side effects include weight gain, irregular bleeding, and depression.
Danazol. This pituitary suppressant creates a hypoestrogenic state and decreases menstrual blood loss by 70% to 80%. A daily dose of 50 to 100 mg may be adequate in some cases; otherwise, the conventional 400 to 800 mg is recommended. Potential side effects include weight gain, acne, and alteration of lipids.8
GnRH therapy. Gonadotropin-releasing hormone (GnRH) therapy with leuprolide or nafarelin creates a hypoestrogenic menopause-like condition, with menstruation usually ceasing within 3 months. Menopausal symptoms may include hot flushes, night sweats, vaginal dryness, bone loss, decreased concentration, and diminished libido. Nevertheless, compliance generally is good. Because prolonged therapy can lead to osteoporosis, treatment usually is limited to 6 months unless estrogen “addback” therapy is instituted.
GnRH therapy is a valuable option for the late perimenopausal woman who has significant contraindications to other medical regimens. For most of these women, the cessation of menses is a relief. After therapy, many patients spontaneously transition into the menopause. An intermittent 6-month course of leuprolide is an option for women with uterine fibroids. Data indicate that it provides an additional 9 months of symptom control (range: 2 to longer than 25 months).9
Progesterone intrauterine system. The recently introduced levonorgestrel-releasing intrauterine system (IUS) (Mirena, Berlex Laboratories, Montville, NJ) also is effective therapy for DUB. This IUS causes pseudodecidual changes and amenorrhea, decreasing menstrual blood loss by 65% to 98% within 12 months, with little systemic absorption of progesterone. It is likely to prove quite valuable for women with menorrhagia who need contraception, have a normal uterine size, and wish to avoid surgery.10
NSAIDs. Nonsteroidal anti-inflammatory drugs (NSAIDs) decrease the rate of dysmenorrhea, improve clotting, and reduce menstrual blood loss. Some studies have demonstrated a 50% to 80% reduction in blood loss with proper use.11 Patients are advised to begin therapy 1 to 2 days before their period is expected and continue throughout the menses. NSAIDs may be combined with OCs, if necessary.
Most menstrual cycles occur every 21 to 35 days. Normal menstrual flow lasts 3 to 7 days, with most blood lost within the first 3 days. The typical menstrual flow averages 35 mL and consists of effluent debris and blood. Women with normal menstrual cycles use an average of 5 to 6 pads or tampons each day. Social obligations, sexual activity, hobbies, work, and travel are not interrupted with normal menstrual function.
When menorrhagia is present, a woman may lose more than 80 mL of blood with each menstrual cycle. Since approximately 16 mg of iron is lost in normal cycles, women with menorrhagia often develop anemia. They also typically have an imbalance of prostaglandin levels and increased fibrinolytic activity.
It is important to note that more than 50% of women who complain of menorrhagia do not have heavy menses. Some patients change their sanitary products more often not because of heavy flow, but for reasons concerning hygiene, personal preference, or fear of toxic shock syndrome.—Linda D. Bradley, MD
FIGURE 5
When medical therapy fails
When the patient fails to improve after 3 months of medical therapy, additional evaluation such as endometrial biopsy is warranted. For hemodynamically stable patients with normal laboratory evaluation, imaging may be a valuable adjunct. In fact, imaging is increasingly used during the initial workup.12
Biopsy. The endometrium generally is sampled in an office setting using a Pipelle instrument. The biopsy can be performed quickly and generally is well-tolerated by the patient, with few complications. While it has a high sensitivity for detecting endometrial cancer and hyperplasia, it is not as effective in detecting intracavitary lesions, including polyps and submucosal fibroids. Lesions that encompass a small surface area are likely to be missed, as the instrument samples only 10% to 25% of the endometrial cavity. Patients with persistent symptoms despite a normal biopsy require further evaluation.
Transvaginal sonography (TVS). This imaging modality is extremely helpful in evaluating women with postmenopausal bleeding. TVS enhances the detection of uterine fibroids and aids in determining their size and position. Adnexal pathology also can be assessed. If the uterine size is greater than 12 to 14 gestational weeks, transabdominal scanning is preferred.
Measurement of the endometrial echo is helpful in determining whether endometrial biopsy or further imaging studies are necessary. Normally, the postmenopausal endometrial echo measures less than 5 mm. Greater thicknesses are associated with endometrial hyperplasia, polyps, fibroids, and cancer. When the endometrial echo exceeds 5 mm or is indistinct or indeterminate, an enhanced view using saline infusion sonography (SIS) or hysteroscopy is advised. When the endometrial echo is less than 5 mm, malignancy is present in fewer than 0.5% of cases.
Weigel et al prospectively evaluated 200 postmenopausal women with an endometrial echo of 3 to 10 mm and found that homogeneity of the echo, a low echo, and a “sonographically depictable central echo between symmetrical endometrial leaves” were associated with an absence of pathology, while heterogeneity and high echogenicity were associated with pathology.13
During the reproductive years, TVS also is useful in assessing myometrial echotexture, adnexal pathology, and, less consistently, endometrial echo. The endometrial thickness varies daily during a normal menstrual cycle. It is thinnest during menses, increases during the follicular phase, and achieves the greatest endometrial height (10 to 14 mm) during the secretory phase. By correlating these measurements with ovarian activity (corpus luteum vs follicular activity), the physician is better able to assess endometrial morphology observed in the midfollicular and secretory phases. However, ancillary testing with SIS is more sensitive for the detection of intracavitary pathology in these women.
Saline infusion sonography (SIS). With SIS, saline is infused into the endometrial cavity during TVS to enhance the image. Many terms have been used to describe this technique, but I prefer SIS because it defines the technique more precisely.14
SIS allows for more accurate evaluation of the uterus for intracavitary lesions than TVS and makes it easier to differentiate the causes of increased endometrial thickness. Indications for SIS include:
- Abnormal bleeding in premenopausal or postmenopausal patients
- Evaluation of an endometrium that is thickened, irregular, immeasurable, or poorly defined on conventional TVS
- Evaluation of an endometrium that appears irregular on TVS in women on tamoxifen
- Differentiating between sessile and pedunculated masses of the endometrium
- Preoperative evaluation of intracavitary fibroids
Increasingly, gynecologists are embracing the concept of “one-stop” evaluation for menstrual disorders by combining the physical exam and basic laboratory studies (CBC and thyroid-stimulating hormone [TSH]) with TVS unless the clinical history dictates otherwise. However, when TVS is indeterminate, SIS should be performed. Also, all women over 40 who have a suspicious TVS exam should undergo SIS and endometrial biopsy. When such evaluation suggests endometrial polyps or submucosal fibroids, the patient needs operative intervention instead of medical therapy, the patient can be referred directly to a surgeon.
Hysteroscopy. Office hysteroscopy has revolutionized the practice of gynecology. Thin operative hysteroscopes with outer diameters ranging from 3 to 5 mm can be utilized comfortably in an office setting. The procedure permits full visualization of the endometrial cavity and endocervix and facilitates the accurate diagnosis of atrophy, endometrial hyperplasia, polyps, fibroids, and endometrial cancer. Directed endometrial biopsies are possible with some hysteroscopes.
Office hysteroscopy accurately diagnoses many endometrial lesions associated with abnormal bleeding. When the endometrial cavity appears normal at hysteroscopy, aggressive medical therapy should be considered.
Surgical options
Among the surgical treatments for abnormal uterine bleeding are myomectomy, polypectomy, endometrial ablation, and hysterectomy. Dilatation and curettage (D&C), commonly used in the past to treat menstrual aberrations, is no longer preferred. D&C is highly inaccurate, resulting in missed diagnoses, incomplete removal of intracavitary pathology, and a high false-negative rate.15 Operative hysteroscopy with directed endometrial sampling is now the gold standard for surgical evaluation of the uterine cavity. With it, full evaluation is possible even in the presence of heavy bleeding; coexisting intrauterine pathology also can be removed. Because the range of surgical options has broadened in recent years, hysterectomy should be the last resort.
To a large extent, the best surgical modality for a given patient depends on her specific pathology, fertility, and contraceptive needs.
Normal uterine cavity. Endometrial ablation typically is offered after failed medical therapy in women with a normal uterine cavity and negative laboratory workup, provided they have completed childbearing. Hysteroscopic and global endometrial ablation procedures destroy the endometrium, preventing regeneration. This creates Asherman’s syndrome, which leads to hypomenorrhea, eumenorrhea, or amenorrhea.
Endometrial ablation is an outpatient procedure associated with a rapid return to work, minimal complications, and high patient satisfaction. Approximately 20% to 30% of patients undergoing endometrial ablation will become amenorrheic, 65% to 70% will become hypomenorrheic, and 5% to 10% of the procedures will fail. Approximately 30% of patients treated by endometrial ablation will require a subsequent operation.16
If the woman wants to preserve her fertility, I generally order magnetic resonance imaging (MRI) to rule out adenomyosis, since TVS, SIS, and hysteroscopy usually cannot detect it. Adenomyosis can be focal or diffuse and is associated with irregular menses and dysmenorrhea. I also suggest an aggressive trial of medical therapy for at least 3 or 4 cycles, with extensive laboratory evaluation. That is, if the history is suggestive of systemic disease, I order liver function tests. If renal disease is suspected, blood urea nitrogen (BUN) and creatinine levels are helpful, as are luteinizing hormone (LH), FSH, and androgen levels to diagnose PCOS. Adrenal function tests (cortisol, 17-alpha hydroxyprogesterone [17-OHP]) are useful in the diagnosis of hyperandrogensim with suspected adrenal tumors, and congenital adrenal hyperplasia (CAH) is diagnosed by an abnormal 17-OHP level.
Women who smoke often have difficulties with abnormal bleeding. Nicotine is detrimental to the ovaries and is associated with irregular menses and premature ovarian failure. I pressure smokers to stop, as they seem to fail medical therapy more frequently than any other group. I also encourage overweight patients to lose weight. Stress, depression, eating disorders, and excessive exercise also should be addressed.
Submucosal fibroids and endometrial polyps. These lesions vary in number, location, and size. When they are present, the altered endometrial surface area, increased fragility and vascularity, other endometrial irregularities, and atypical prostaglandin levels contribute to abnormal bleeding. Intracavitary lesions also may coexist with anovulatory and ovulatory cycles.
As I mentioned earlier, office hysteroscopy and SIS are the most accurate methods of detecting these lesions. Treatment generally consists of outpatient hysteroscopic myomectomy or polypectomy, which is quick, safe, eases symptoms, and is associated with high levels of patient satisfaction.17
Intramural fibroids. Intramural fibroids can cause disturbances in menstrual flow. Although the mechanisms of action are unclear, these disturbances probably result from topographic endometrial abnormalities, glandular atrophy overlying the fibroid, venous congestion, increased endometrial surface area, or an alteration in prostaglandin levels.
The type of therapy offered depends on the patient’s desire for pregnancy or preservation of the uterus. Basically, there are 3 options: removing or destroying the fibroids or removing the uterus. When future fertility is desired, the patient typically undergoes abdominal or laparoscopic myomectomy, with the surgical route contingent upon the number, size, and location of fibroids, as well as the surgeon’s level of skill and experience.
Attempts to resect large intramural fibroids hysteroscopically should be avoided in patients who have not completed childbearing, since scarring and/or the obliteration of a significant portion of the endometrium overlying the fibroid can lead to infertility.
When the patient experiences heavy menstrual bleeding and does not wish to preserve her fertility, she may be offered a minimally invasive outpatient procedure called uterine artery embolization (UAE). In this procedure, a catheter is inserted transcutaneously and threaded through the femoral artery into the aortic bifurcation and then to the contralateral uterine artery, which is then occluded. Several products are used for UAE, including Embosphere Microspheres (BioSphere Medical, Rockland, Mass), polyvinyl alcohol (PVA) particles, coils, or gelfoam, cutting off blood flow to the fibroid. The fibroid then necroses and shrinks in size and volume. Of the many symptoms associated with fibroids, menorrhagia is most effectively controlled with UAE. In fact, patients have an 85% to 95% chance of resolving symptoms of menorrhagia after undergoing this procedure.18
Hysterectomy offers definitive therapy for patients who have completed childbearing and have no desire for uterine preservation. Either vaginal, laparoscopic-assisted, or abdominal hysterectomy is an option. Factors influencing selection of the surgical route include the number, size, and location of the fibroids, as well as any concomitant pelvic pathology and the surgeon’s skill. (See “Complex hysterectomy: opting for the vaginal approach”.)
Conclusion
After the initial history, physical examination, and laboratory evaluation, the factors involved in abnormal uterine bleeding usually are well defined. Medical management is the standard unless uterine pathology is present. Most patients respond favorably to hormonal manipulation with OCs or progesterone. Another effective option, particularly for women who cannot tolerate traditional medical therapy, is the levonorgestrel-releasing IUS.
Endometrial ablation offers 90% success for the treatment of menorrhagia and dysfunctional bleeding in women with a normal uterine cavity and negative laboratory workup, provided they do not desire children.
Patients with intrauterine polyps and submucosal fibroids have excellent relief of symptoms following operative hysteroscopy. Also, uterine artery embolization is an excellent nonsurgical intervention for patients with fibroids and menorrhagia who wish to avoid major surgery. Fortunately, in this era of many alternative medical and surgical treatments, hysterectomy is the last resort for abnormal uterine bleeding.
Dr. Bradley reports that she serves as a speaker and consultant/preceptor for Olympus.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;328(12):856-860.
2. Coulter A, Bradlow J, Agass M, et al. Outcomes of referrals to gynecology outpatient clinics for menstrual problems: an audit of general practice records. Br J Obstet Gynaecol. 1991;98:789-796.
3. Claessens E, Cowell CA. Acute adolescent menorrhagia. Am J Obstet Gynecol. 1981;139:277-280.
4. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #263. von Willebrand’s disease in gynecologic practice. Obstet Gynecol. 2001;98:1185-1186.
5. Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril. 1999;71:658-662.
6. Kaunitz A. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2):S32-S37.
7. Jones H. Clinical pathway for evaluating women with abnormal uterine bleeding. Obstet Gynecol Surv. 2002;57(1):22-24.
8. Higham JM, Shaw RW. A comparative study of danazol, a regimen of decreasing doses of danazol and norethindrone in the treatment of objectively proven unexplained menorrhagia. Am J Obstet Gynecol. 1993;169:1134-1139.
9. Scialli A, Levi A. Intermittent leuprolide acetate for the nonsurgical management of women with leiomyomata uteri. Fertil Steril. 2000;74(3):540-546.
10. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomized trial. Lancet. 2001;357:273-277.
11. Vargyas JM, Campeau JD, Mishell DR. Treatment of menorrhagia with meclofenamate sodium. Am J Obstet Gynecol. 1987;157:944-950.
12. Jones K, Bourne T. The feasibility of a “one-stop” ultrasound-based clinic for the diagnosis and management of abnormal uterine bleeding. Ultrasound Obstet Gynecol. 2001;176:517-521.
13. Weigel M, Friese K, Strittmatter HJ, et al. Measuring the thickness—is that all we have to do for sonographic assessment of endometrium in postmenopausal women? Ultrasound Obstet Gynecol. 1995;6:97-102.
14. Widrich T, Bradley L, Mitchinson AR, Collins R. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol. 1996;174:1327-1334.
15. Bettocchi S, Ceci O, Vicino M, et al. Diagnostic inadequacy of dilation and curettage. Fertil Steril. 2001;75(4):803-805.
16. Stabinsky S, Einstein M, Breen J. Modern treatments of menorrhagia attributable to dysfunctional uterine bleeding. Obstet Gynecol Surv. 1999;54(11):251-262.
17. Pasqualotto EB, Margossian H, Priul L, Bradley LD, et al. Accuracy of preoperative diagnostic tools and outcome of hysteroscopic management of menstrual dysfunction. J Am Assoc Gynecol Laparosc. 2000;7(2):201-209.
18. Hurst B, Stackhouse D, Matthews M, Marshburn P. Uterine artery embolization for symptomatic uterine myomas. Fertil Steril. 2000;74(5):855-869.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;328(12):856-860.
2. Coulter A, Bradlow J, Agass M, et al. Outcomes of referrals to gynecology outpatient clinics for menstrual problems: an audit of general practice records. Br J Obstet Gynaecol. 1991;98:789-796.
3. Claessens E, Cowell CA. Acute adolescent menorrhagia. Am J Obstet Gynecol. 1981;139:277-280.
4. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #263. von Willebrand’s disease in gynecologic practice. Obstet Gynecol. 2001;98:1185-1186.
5. Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril. 1999;71:658-662.
6. Kaunitz A. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2):S32-S37.
7. Jones H. Clinical pathway for evaluating women with abnormal uterine bleeding. Obstet Gynecol Surv. 2002;57(1):22-24.
8. Higham JM, Shaw RW. A comparative study of danazol, a regimen of decreasing doses of danazol and norethindrone in the treatment of objectively proven unexplained menorrhagia. Am J Obstet Gynecol. 1993;169:1134-1139.
9. Scialli A, Levi A. Intermittent leuprolide acetate for the nonsurgical management of women with leiomyomata uteri. Fertil Steril. 2000;74(3):540-546.
10. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomized trial. Lancet. 2001;357:273-277.
11. Vargyas JM, Campeau JD, Mishell DR. Treatment of menorrhagia with meclofenamate sodium. Am J Obstet Gynecol. 1987;157:944-950.
12. Jones K, Bourne T. The feasibility of a “one-stop” ultrasound-based clinic for the diagnosis and management of abnormal uterine bleeding. Ultrasound Obstet Gynecol. 2001;176:517-521.
13. Weigel M, Friese K, Strittmatter HJ, et al. Measuring the thickness—is that all we have to do for sonographic assessment of endometrium in postmenopausal women? Ultrasound Obstet Gynecol. 1995;6:97-102.
14. Widrich T, Bradley L, Mitchinson AR, Collins R. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol. 1996;174:1327-1334.
15. Bettocchi S, Ceci O, Vicino M, et al. Diagnostic inadequacy of dilation and curettage. Fertil Steril. 2001;75(4):803-805.
16. Stabinsky S, Einstein M, Breen J. Modern treatments of menorrhagia attributable to dysfunctional uterine bleeding. Obstet Gynecol Surv. 1999;54(11):251-262.
17. Pasqualotto EB, Margossian H, Priul L, Bradley LD, et al. Accuracy of preoperative diagnostic tools and outcome of hysteroscopic management of menstrual dysfunction. J Am Assoc Gynecol Laparosc. 2000;7(2):201-209.
18. Hurst B, Stackhouse D, Matthews M, Marshburn P. Uterine artery embolization for symptomatic uterine myomas. Fertil Steril. 2000;74(5):855-869.