User login
Benign Pneumatosis Intestinalis
A 9‐year‐old male, with a history of immune thrombocytopenic purpura (ITP) and hypoplastic left heart syndrome (HLHS) repaired by total cavopulmonary shunt, presented to the Emergency Department with a 4‐day history of crampy abdominal pain with defecation and a 4‐day history of nonbloody diarrhea with intermittent nonbilious vomiting. The abdominal pain was diffuse and nonspecific without any radiation. He had a history of encopresis and constipation over the past 3 months. No anorexia was noted and the pain did not keep him from his activities of daily living. Review of all other systems was noncontributory.
His past medical history consisted of HLHS repaired by total cavopulmonary shunt with excellent results. He was diagnosed with ITP about 6 weeks prior to this presentation and had been treated with intravenous immunoglobulin and oral prednisone. His home medications included prednisone (1.5 mg/kg/day), lansoprazole, digoxin, enalapril, furosemide, and warfarin.
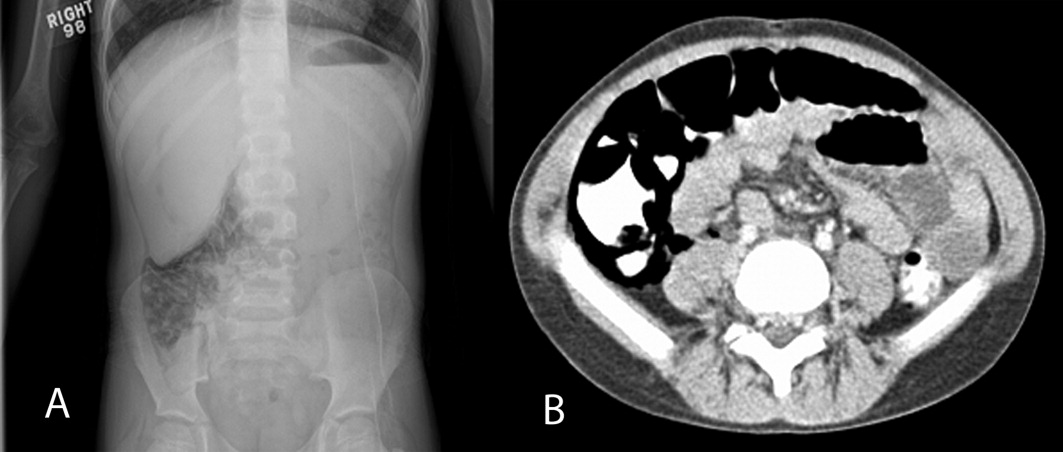
On physical examination, his temperature was 37C, pulse rate 97, respiratory rate 16, unlabored blood pressure was 112/74 mm Hg, oxygen saturation 91% on room air, and weight was 22.8 kg. He had a grade 4/6 holosystolic murmur across the precordium and multiple healed surgical incisions. His abdomen was soft without tenderness or distention with normoactive bowel sounds. The rest of his physical exam was unremarkable. An acute abdominal series was obtained, which showed pneumatosis intestinalis (PI) of the right colon, pneumoperitoneum, and possible portal venous gas (Figure 1A). Initial laboratory evaluation including a complete blood count and a comprehensive metabolic panel; amylase, lipase, lactate, and venous blood gas were all within normal limits, with the exception of a platelet level of 75,000/L (normal, 150,000450,000 cells/mL). Stool was negative for Rotavirus antigen, Clostridium difficile toxin, Helicobacter pylori antigen, and Shiga toxin 1 and 2. Bacterial cultures and trichrome stain for ova and parasites were both negative. Stool analysis for occult blood was negative on admission and became positive during his hospital course. A contrast computed tomography (CT) of the abdomen and pelvis confirmed the findings of pneumatosis intestinalis in the cecum and ascending colon with intraperitoneal and retroperitoneal air, but did not reveal any portal venous gas (Figure 1B).

The patient was admitted to the Children's Hospital and made nil per os (NPO; ie, nothing by mouth), placed on ampicillin/sulbactam and metronidazole prophylaxis, and observed with serial abdominal examinations. Total parenteral nutrition (TPN) was begun on hospital day 2 and continued for 10 days. Surgical intervention was not required at the initial presentation secondary to his clinical and hemodynamic stability. Since immunosuppression from chronic steroid therapy is a known risk factor for the development of PI,1 a slow steroid taper with intravenous methylprednisolone was initiated and was transitioned to oral prednisone after he resumed oral nutrition. He remained NPO for 10 days until the PI radiographically resolved. Oral feeds were reintroduced slowly without complications or recurrence of PI.
Discussion
Three major hypotheses for the origin of bowel wall gas have been proposed: intraluminal gastrointestinal (GI) gas; bacterial production of gas; and pulmonary gas.1 The intralumenal GI gas hypothesis states that intralumenal gas translocates to the bowel wall due to increased intralumenal pressure, mucosal injury from direct trauma, reduction is size of Peyer's patches from immunosuppressive medications, or a combination of factors.1, 2 The bacterial theory proposes direct invasion of the bowel wall by gas‐producing bacteria; this hypothesis is not adequately supported by bacteriologic data.2 The pulmonary gas hypothesis states that alveolar rupture could result in dissection of air through the mediastinum to the retroperitoneum and eventually along vascular channels to the gut.2 Increased intralumenal gut pressure due to coughing also drives this dissection of gas into the bowel wall.2
Chronic immunosuppression with steroids and congenital heart disease are both known risk factors for the development of PI.3 Our patient presented with common complaints of abdominal pain, encopresis, and vomiting, with a benign exam. However, he had a radiographic finding of PI. With bowel rest, antibiotics, and TPN, our patient made a full recovery without requiring surgical intervention. With patients at higher risk for PI, there needs to be a higher index of suspicion for PI in the setting of GI complaints and hemodynamic instability. It has been reported in the literature that patients at higher risk can include those with inflammatory bowel disease, chronic pulmonary disease, immunosuppressive states such as leukemia or acquired immunodeficiency syndrome, short gut syndrome, and malignancies.1, 3 Kurbegov and Sondheimer4 published a series of 32 nonneonatal cases of PI, looking for characteristics that predicted higher risk of poor outcome. Their findings showed low serum bicarbonate and PI with free air and portal venous gas as significant predictors of poor outcome.4 A recent study by Morris et al.5 showed similar results: lactic acidosis is a predictor of poor patient outcome. The clinical examination of this patient, both initially and longitudinally, and the lack of laboratory abnormalities were the key factors in the disposition of this patient in the setting of alarming abdominal radiographs.
- ,,.The spectrum of pneumatosis intestinalis.Arch Surg.2003;138(1):68–75.
- ,,, et al.Pneumatosis intestinalis with pneumoperitoneum mimicking intestinal perforation in a patient with myelodysplastic syndrome after hematopoietic stem cell transplantation.Korean J Intern Med.2007;22(1):40–44.
- ,.Benign pneumatosis in children.Pediatr Radiol.2000;30(11):786–793.
- ,.Pneumatosis intestinalis in non‐neonatal pediatric patients.Pediatrics.2001;108(2):402–406.
- ,,, et al.Management and outcome of pneumatosis intestinalis.Am J Surg.2008;195(5):679–682; discussion 682–683.
A 9‐year‐old male, with a history of immune thrombocytopenic purpura (ITP) and hypoplastic left heart syndrome (HLHS) repaired by total cavopulmonary shunt, presented to the Emergency Department with a 4‐day history of crampy abdominal pain with defecation and a 4‐day history of nonbloody diarrhea with intermittent nonbilious vomiting. The abdominal pain was diffuse and nonspecific without any radiation. He had a history of encopresis and constipation over the past 3 months. No anorexia was noted and the pain did not keep him from his activities of daily living. Review of all other systems was noncontributory.
His past medical history consisted of HLHS repaired by total cavopulmonary shunt with excellent results. He was diagnosed with ITP about 6 weeks prior to this presentation and had been treated with intravenous immunoglobulin and oral prednisone. His home medications included prednisone (1.5 mg/kg/day), lansoprazole, digoxin, enalapril, furosemide, and warfarin.
On physical examination, his temperature was 37C, pulse rate 97, respiratory rate 16, unlabored blood pressure was 112/74 mm Hg, oxygen saturation 91% on room air, and weight was 22.8 kg. He had a grade 4/6 holosystolic murmur across the precordium and multiple healed surgical incisions. His abdomen was soft without tenderness or distention with normoactive bowel sounds. The rest of his physical exam was unremarkable. An acute abdominal series was obtained, which showed pneumatosis intestinalis (PI) of the right colon, pneumoperitoneum, and possible portal venous gas (Figure 1A). Initial laboratory evaluation including a complete blood count and a comprehensive metabolic panel; amylase, lipase, lactate, and venous blood gas were all within normal limits, with the exception of a platelet level of 75,000/L (normal, 150,000450,000 cells/mL). Stool was negative for Rotavirus antigen, Clostridium difficile toxin, Helicobacter pylori antigen, and Shiga toxin 1 and 2. Bacterial cultures and trichrome stain for ova and parasites were both negative. Stool analysis for occult blood was negative on admission and became positive during his hospital course. A contrast computed tomography (CT) of the abdomen and pelvis confirmed the findings of pneumatosis intestinalis in the cecum and ascending colon with intraperitoneal and retroperitoneal air, but did not reveal any portal venous gas (Figure 1B).

The patient was admitted to the Children's Hospital and made nil per os (NPO; ie, nothing by mouth), placed on ampicillin/sulbactam and metronidazole prophylaxis, and observed with serial abdominal examinations. Total parenteral nutrition (TPN) was begun on hospital day 2 and continued for 10 days. Surgical intervention was not required at the initial presentation secondary to his clinical and hemodynamic stability. Since immunosuppression from chronic steroid therapy is a known risk factor for the development of PI,1 a slow steroid taper with intravenous methylprednisolone was initiated and was transitioned to oral prednisone after he resumed oral nutrition. He remained NPO for 10 days until the PI radiographically resolved. Oral feeds were reintroduced slowly without complications or recurrence of PI.
Discussion
Three major hypotheses for the origin of bowel wall gas have been proposed: intraluminal gastrointestinal (GI) gas; bacterial production of gas; and pulmonary gas.1 The intralumenal GI gas hypothesis states that intralumenal gas translocates to the bowel wall due to increased intralumenal pressure, mucosal injury from direct trauma, reduction is size of Peyer's patches from immunosuppressive medications, or a combination of factors.1, 2 The bacterial theory proposes direct invasion of the bowel wall by gas‐producing bacteria; this hypothesis is not adequately supported by bacteriologic data.2 The pulmonary gas hypothesis states that alveolar rupture could result in dissection of air through the mediastinum to the retroperitoneum and eventually along vascular channels to the gut.2 Increased intralumenal gut pressure due to coughing also drives this dissection of gas into the bowel wall.2
Chronic immunosuppression with steroids and congenital heart disease are both known risk factors for the development of PI.3 Our patient presented with common complaints of abdominal pain, encopresis, and vomiting, with a benign exam. However, he had a radiographic finding of PI. With bowel rest, antibiotics, and TPN, our patient made a full recovery without requiring surgical intervention. With patients at higher risk for PI, there needs to be a higher index of suspicion for PI in the setting of GI complaints and hemodynamic instability. It has been reported in the literature that patients at higher risk can include those with inflammatory bowel disease, chronic pulmonary disease, immunosuppressive states such as leukemia or acquired immunodeficiency syndrome, short gut syndrome, and malignancies.1, 3 Kurbegov and Sondheimer4 published a series of 32 nonneonatal cases of PI, looking for characteristics that predicted higher risk of poor outcome. Their findings showed low serum bicarbonate and PI with free air and portal venous gas as significant predictors of poor outcome.4 A recent study by Morris et al.5 showed similar results: lactic acidosis is a predictor of poor patient outcome. The clinical examination of this patient, both initially and longitudinally, and the lack of laboratory abnormalities were the key factors in the disposition of this patient in the setting of alarming abdominal radiographs.
A 9‐year‐old male, with a history of immune thrombocytopenic purpura (ITP) and hypoplastic left heart syndrome (HLHS) repaired by total cavopulmonary shunt, presented to the Emergency Department with a 4‐day history of crampy abdominal pain with defecation and a 4‐day history of nonbloody diarrhea with intermittent nonbilious vomiting. The abdominal pain was diffuse and nonspecific without any radiation. He had a history of encopresis and constipation over the past 3 months. No anorexia was noted and the pain did not keep him from his activities of daily living. Review of all other systems was noncontributory.
His past medical history consisted of HLHS repaired by total cavopulmonary shunt with excellent results. He was diagnosed with ITP about 6 weeks prior to this presentation and had been treated with intravenous immunoglobulin and oral prednisone. His home medications included prednisone (1.5 mg/kg/day), lansoprazole, digoxin, enalapril, furosemide, and warfarin.
On physical examination, his temperature was 37C, pulse rate 97, respiratory rate 16, unlabored blood pressure was 112/74 mm Hg, oxygen saturation 91% on room air, and weight was 22.8 kg. He had a grade 4/6 holosystolic murmur across the precordium and multiple healed surgical incisions. His abdomen was soft without tenderness or distention with normoactive bowel sounds. The rest of his physical exam was unremarkable. An acute abdominal series was obtained, which showed pneumatosis intestinalis (PI) of the right colon, pneumoperitoneum, and possible portal venous gas (Figure 1A). Initial laboratory evaluation including a complete blood count and a comprehensive metabolic panel; amylase, lipase, lactate, and venous blood gas were all within normal limits, with the exception of a platelet level of 75,000/L (normal, 150,000450,000 cells/mL). Stool was negative for Rotavirus antigen, Clostridium difficile toxin, Helicobacter pylori antigen, and Shiga toxin 1 and 2. Bacterial cultures and trichrome stain for ova and parasites were both negative. Stool analysis for occult blood was negative on admission and became positive during his hospital course. A contrast computed tomography (CT) of the abdomen and pelvis confirmed the findings of pneumatosis intestinalis in the cecum and ascending colon with intraperitoneal and retroperitoneal air, but did not reveal any portal venous gas (Figure 1B).

The patient was admitted to the Children's Hospital and made nil per os (NPO; ie, nothing by mouth), placed on ampicillin/sulbactam and metronidazole prophylaxis, and observed with serial abdominal examinations. Total parenteral nutrition (TPN) was begun on hospital day 2 and continued for 10 days. Surgical intervention was not required at the initial presentation secondary to his clinical and hemodynamic stability. Since immunosuppression from chronic steroid therapy is a known risk factor for the development of PI,1 a slow steroid taper with intravenous methylprednisolone was initiated and was transitioned to oral prednisone after he resumed oral nutrition. He remained NPO for 10 days until the PI radiographically resolved. Oral feeds were reintroduced slowly without complications or recurrence of PI.
Discussion
Three major hypotheses for the origin of bowel wall gas have been proposed: intraluminal gastrointestinal (GI) gas; bacterial production of gas; and pulmonary gas.1 The intralumenal GI gas hypothesis states that intralumenal gas translocates to the bowel wall due to increased intralumenal pressure, mucosal injury from direct trauma, reduction is size of Peyer's patches from immunosuppressive medications, or a combination of factors.1, 2 The bacterial theory proposes direct invasion of the bowel wall by gas‐producing bacteria; this hypothesis is not adequately supported by bacteriologic data.2 The pulmonary gas hypothesis states that alveolar rupture could result in dissection of air through the mediastinum to the retroperitoneum and eventually along vascular channels to the gut.2 Increased intralumenal gut pressure due to coughing also drives this dissection of gas into the bowel wall.2
Chronic immunosuppression with steroids and congenital heart disease are both known risk factors for the development of PI.3 Our patient presented with common complaints of abdominal pain, encopresis, and vomiting, with a benign exam. However, he had a radiographic finding of PI. With bowel rest, antibiotics, and TPN, our patient made a full recovery without requiring surgical intervention. With patients at higher risk for PI, there needs to be a higher index of suspicion for PI in the setting of GI complaints and hemodynamic instability. It has been reported in the literature that patients at higher risk can include those with inflammatory bowel disease, chronic pulmonary disease, immunosuppressive states such as leukemia or acquired immunodeficiency syndrome, short gut syndrome, and malignancies.1, 3 Kurbegov and Sondheimer4 published a series of 32 nonneonatal cases of PI, looking for characteristics that predicted higher risk of poor outcome. Their findings showed low serum bicarbonate and PI with free air and portal venous gas as significant predictors of poor outcome.4 A recent study by Morris et al.5 showed similar results: lactic acidosis is a predictor of poor patient outcome. The clinical examination of this patient, both initially and longitudinally, and the lack of laboratory abnormalities were the key factors in the disposition of this patient in the setting of alarming abdominal radiographs.
- ,,.The spectrum of pneumatosis intestinalis.Arch Surg.2003;138(1):68–75.
- ,,, et al.Pneumatosis intestinalis with pneumoperitoneum mimicking intestinal perforation in a patient with myelodysplastic syndrome after hematopoietic stem cell transplantation.Korean J Intern Med.2007;22(1):40–44.
- ,.Benign pneumatosis in children.Pediatr Radiol.2000;30(11):786–793.
- ,.Pneumatosis intestinalis in non‐neonatal pediatric patients.Pediatrics.2001;108(2):402–406.
- ,,, et al.Management and outcome of pneumatosis intestinalis.Am J Surg.2008;195(5):679–682; discussion 682–683.
- ,,.The spectrum of pneumatosis intestinalis.Arch Surg.2003;138(1):68–75.
- ,,, et al.Pneumatosis intestinalis with pneumoperitoneum mimicking intestinal perforation in a patient with myelodysplastic syndrome after hematopoietic stem cell transplantation.Korean J Intern Med.2007;22(1):40–44.
- ,.Benign pneumatosis in children.Pediatr Radiol.2000;30(11):786–793.
- ,.Pneumatosis intestinalis in non‐neonatal pediatric patients.Pediatrics.2001;108(2):402–406.
- ,,, et al.Management and outcome of pneumatosis intestinalis.Am J Surg.2008;195(5):679–682; discussion 682–683.