User login
Short of breath, not short of diagnoses
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 71‐year‐old African‐American woman presented to the emergency department with chest pain, shortness of breath, and cough. She had initially presented to her primary care physician 2 weeks previously complaining of worsening cough and shortness of breath and was told to continue her inhaled albuterol and glucocorticoids and was prescribed a prednisone taper and an unknown course of antibiotics. She noted no improvement in her symptoms despite compliance with this treatment. Three days prior to admission she described the gradual onset of left‐sided pleuritic chest pain with continued cough, associated with yellow sputum and worsening dyspnea. Review of systems was remarkable for generalized weakness and malaise. She denied fever, chills, orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema, diarrhea, nausea, vomiting, or abdominal pain.
Her past medical history included a diagnosis of chronic obstructive pulmonary disease (COPD) but pulmonary function tests 7 years prior to admission showed an forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) ratio of 81%. She had a 30 pack‐year history of smoking, but quit 35 years ago. The patient also carried a diagnosis of heart failure, but an echocardiogram done 1 year ago demonstrated a left ventricular ejection fraction of 65% to 70% without diastolic dysfunction but mild right ventricular dilation and hypertrophy. Additionally, she had known nonobstructive coronary atherosclerotic heart disease, dyslipidemia, hypertension, morbid obesity, depression, and a documented chronic right hemidiaphragm elevation.
At this point the history suggests that the patient does not have a clear diagnosis of COPD. The lack of definitive spirometry evidence of chronic airway obstruction concerns me; I think that she may have been mistakenly treated with chronic inhaled steroids and doses of antibiotics for an acute exacerbation of chronic lung disease. Additional review of her history gives some indication of advanced lung disease, with her recent echocardiogram showing strain on the right ventricle with right ventricular hypertrophy and dilation, but there is no mention of the presence or severity of pulmonary hypertension. Nonetheless, I would be concerned that she probably has underlying significant cor pulmonale.
The patient now re‐presents with a worsening of her pulmonary symptoms. Her left‐sided pleuritic pain would make me concerned that she had a pulmonary embolus (PE). This morbidly obese patient with new pulmonary symptoms, right ventricular strain on her previous echocardiogram, and a persistent elevated right hemidiaphragm suggests a presentation of another PE.
At this time I cannot rule out other common possibilities such as infectious pneumonia. If she does have pneumonia, I would be concerned she could be harboring a multidrug‐resistant bacterial infection given her recent course of antibiotics in addition to her use of both chronic inhaled and intermittent oral glucocorticoids.
After gathering the rest of her full medical history, I would focus my physical exam on looking for evidence of parenchymal lung disease, signs of pulmonary hypertension, and pneumonia.
Her surgical history includes a previous hysterectomy, cholecystectomy, hernia repair, and left hepatic lobectomy for a benign mass. Her outpatient medications were ibuprofen, bupropion, fluvastatin, atenolol, potassium, aspirin, clopidogrel, albuterol inhaler, fluticasone/salmeterol inhaler, and omeprazole. She reports an allergy to penicillin and to sulfa drugs. Her mother died of an unknown cancer at age 77 years. She denied any international travel and she has always lived in Georgia.
The patient has been retired since 1992, having previously worked for the U.S. Postal Service. She admits to occasional alcohol intake (2 to 3 drinks a month). No recent travel, surgery, or prolonged immobilization was noted.
On initial examination she was alert and mentally appropriate, but appeared to be in mild respiratory distress with a respiratory rate of 28 breaths/minute. Her blood pressure (BP) was 99/70, heart rate 102, temperature of 38.2C, and oxygen saturation of 93% on room air and 97% on 2 L of oxygen via nasal cannula. Auscultation of her lungs revealed crackles over her left anterior lung field, bronchial breath sounds in the left posterior midlung, and bibasilar crackles. No wheezing was noted. Her cardiovascular exam and the remainder of her physical exam were unremarkable except for morbid obesity.
While my initial thoughts were leaning toward an exacerbation of chronic lung disease or possibly a new PE, at this moment, infection seems more likely. Indeed, her pulmonary findings suggest a left‐sided inflammatory process, and her vital signs meet criteria for systemic inflammatory response syndrome (SIRS). My primary concern is sepsis due to a drug‐resistant bacterial infection, including Staphylococcus aureus or gram‐negative bacteria or possibly more unusual organisms such as Nocardia or fungi, due to her recent use of antibiotics and chronic inhaled steroid use and recent course of oral glucocorticoids.
Conversely, the SIRS could be a manifestation of a noninfectious lung process such as acute interstitial pneumonia or an eosinophilic pneumonia. Given the diagnostic complexity, I would strongly consider consulting a pulmonologist if the patient did not improve quickly. At this point, I would like to review a posterior‐anterior (PA) and lateral chest radiograph, and room air arterial blood gas (ABG) in addition to basic laboratory test values.
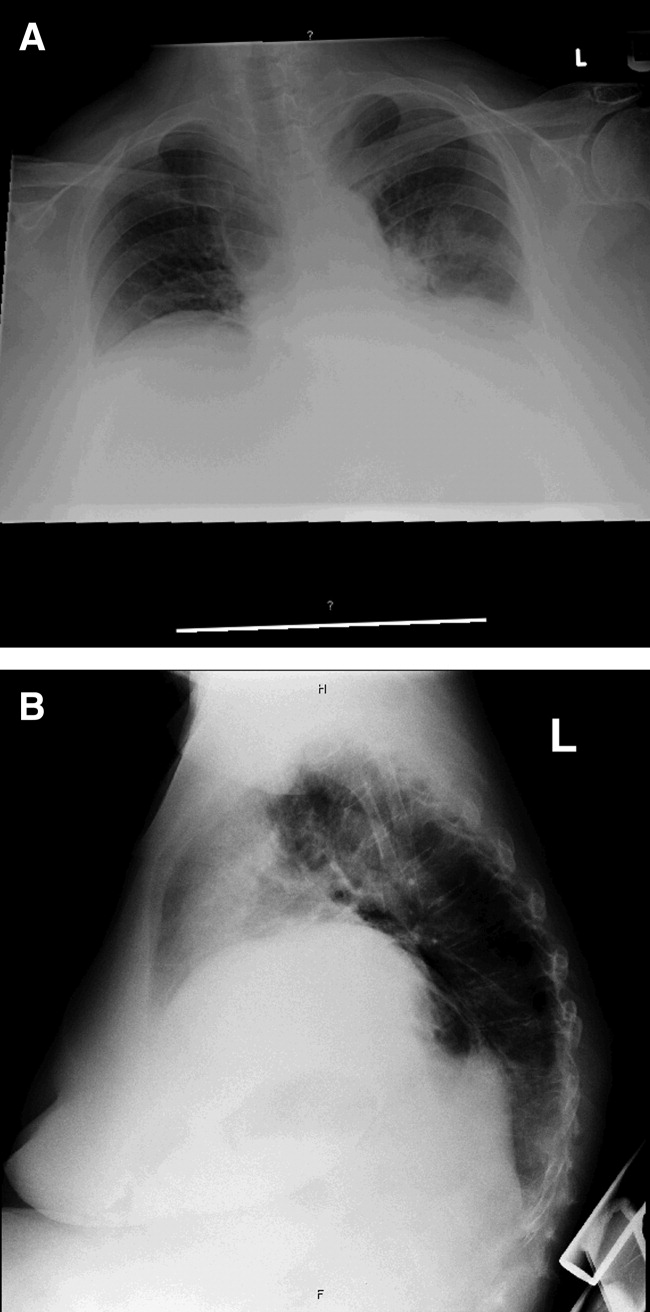
Laboratory data obtained on admission was remarkable for a white blood cell (WBC) count of 26,500/L with 75% neutrophils and 6% eosinophils. Hemoglobin was 14.4 gm/dL. Platelet count was 454,000/L. Serum chemistries showed a sodium of 137 mEq/dL, potassium 4.3 mEq/dL, Cl 108 mEq/dL, bicarbonate 19 mEq/dL, blood urea nitrogen (BUN) 8 mg/dL, creatinine 1.0 mg/dL, and glucose 137 mg/dL. Cardiac enzymes were normal. Calcium was 9.8 mg/dL, albumin 2.7 gm/dL, total protein 6.9 gm/dL, AST 36 U/L, ALT 54 U/L and the bilirubin was normal. Chest radiograph (Figure 1) demonstrated a left perihilar infiltrate with air bronchograms and marked right hemidiaphragm elevation as seen on previous films. Unchanged increased interstitial markings were also present. Her electrocardiogram (ECG) showed normal sinus rhythm, normal axis, and QRS duration with nonspecific diffuse T‐wave abnormalities.
Given her presentation, I am worried about how well she is oxygenating and ventilating. An ABG should be done to assess her status more accurately. An albumin of 2.7 gm/dL indicates that she is fairly sick. I would not hesitate to consider testing the patient for human immunodeficiency virus (HIV) given how this information would dramatically change the differential diagnoses of her pulmonary process.
I am still most concerned about sepsis secondary to pneumonia in this patient with multiple chronic comorbidities, underlying chronic lung disease, receiving chronic inhaled glucocorticoids and a recent course of oral glucocorticoids and antibiotics. While I would initiate hydration I do not see a clear indication for early goal‐directed therapy for severe sepsis. In addition to obtaining an ABG and starting intravenous fluids, I would also draw blood cultures, send sputum for gram stain, culture, and sensitivity, and perform a urinalysis. I would also administer empiric antibiotics as quickly as possible based on a number of pneumonia clinical studies suggesting improved outcomes with early antibiotic administration. Because of her use of antibiotics and both inhaled and oral glucocorticoids, she is at higher risk for potentially multidrug‐resistant bacterial pathogens, including Staphyloccocus aureus and gram‐negative bacteria such as Pseudomonas and Klebsiella (Table 1). Therefore, I would initially cover her broadly for these organisms.
| Meets Any of the Following |
|---|
| Antimicrobial therapy in the preceding 90 days |
| Current hospitalization of 5 days or more |
| High frequency of antibiotic resistance in the community or in the specific hospital unit |
| Presence of risk factors for healthcare‐associated pneumonia (HCAP) |
| Hospitalization for >2 days in the preceding 90 days |
| Residence in nursing home or long‐term care facility (LTAC) for at least 5 days in last 90 days |
| Home infusion therapy including intravenous antibiotics within 30 days |
| Home wound care within 30 days |
| Chronic hemodialysis in hospital or clinic within 30 days |
| Family member with multidrug‐resistant pathogen |
| Immunosuppressive disease and/or therapy |
In addition to initial treatment choice, the inpatient triage decision is another important issue, especially at a community hospital where intensive care unit (ICU) resources are rare and often the admission decision is between sending a moderately sick patient to a regular floor bed or the medical ICU. Both the American Thoracic Society and Infectious Diseases Society of America support an ICU triage protocol in their guidelines for the management of community‐acquired pneumonia in adults that utilizes the following 9 minor criteria, of which the presence of at least 3 should support ICU admission: respiratory rate 30 breaths/minute; oxygenation index (pressure of oxygen [PaO2]/fraction of inspired oxygen [FiO2] ratio) 250; multilobar infiltrates; confusion/disorientation; uremia (BUN level 20 mg/dL); leukopenia (WBC count 4,000 cells/mm3); thrombocytopenia (platelet count 100,000 cells/mm3); hypothermia (core temperature 36C); and hypotension requiring aggressive fluid. Despite the absence of these criteria in this patient, it is important to note that no triage protocol has been adequately prospectively validated. Retrospective study of the minor criteria has found that the presence of at least 2 of the following 3 clinical criteria to have the highest specificity for predicting cardiopulmonary decompensation and subsequent need for ICU care: (1) initial hypotension (BP 90/60) on presentation with response to initial intravenous fluids to a BP >90/60; (2) oxygenation failure as indicated by PaO2/FiO2 ratio less than 250; or (3) the presence of multilobar or bilateral infiltrates on chest radiography.
I also want to comment on the relative elevation of her calcium, especially given the low albumin. This may simply be due to volume depletion, as many older patients have asymptomatic mild primary hyperparathyroidism. However, this elevated calcium may be a clue to the underlying lung process. Granulomatous lung disease due to tuberculosis or fungal infection could yield elevated calcium levels via increases in macrophage production of the active vitamin D metabolite calcitriol. This will need to be followed and a parathormone (PTH) level would be the best first test to request if the calcium level remains elevated. If the PTH level is suppressed, granulomatous disease or malignancy would be the more likely cause.
The patient was admitted with a presumptive diagnosis of community‐acquired pneumonia, was started on ceftriaxone and azithromycin, and given intravenous fluids, oxygen, and continued on inhaled salmeterol/fluticasone. Sputum was ordered for gram stain, culture, and sensitivity, and blood cultures were obtained. Urinalysis showed 1‐5 WBCs/high‐power field. Venous thromboembolism prophylaxis was initiated with subcutaneous heparin 5,000 units 8 hours. Her blood pressure normalized rapidly and during the next few days she stated she was feeling better. Despite continued significant wheezing her oxygen saturation remained at 98% on 2 L of oxygen via nasal cannula and she was less tachypneic. Attempts at obtaining an ABG were unsuccessful, and the patient subsequently refused additional attempts. Over the first few days her WBC count remained elevated above 20,000/L, with worsening bandemia (11%), and fever ranging from 38C to 39C. Sputum analysis was initially unsuccessful and blood cultures remained negative.
I am concerned about the persistent fever and elevated WBC count, and want to emphasize that I might have treated her with broader spectrum antibiotics to cover additional multidrug‐resistant bacterial organisms. I would have initially ordered vancomycin to cover methicillin resistant Staphylococcus aureus (MRSA) plus 2 additional antibiotics that cover multidrug‐resistant gram negative pathogens including Pseudomonas aeruginosa.
On the fifth hospital day, her WBC count dropped to 13,400/L and she defervesced. However, her respiratory status worsened during that same day with increased tachypnea. Of note, no results were reported from the initial sputum cultures and they were reordered and a noncontrast chest computed tomography (CT) was also ordered.
I think at this point, even though she has remained stable hemodynamically and oxygenating easily with supplemental oxygen, the question of whether or not her primary process is infectious or noninfectious lingers. I agree with obtaining a chest CT scan.
I am not surprised that sputum was not evaluated despite the orders. Among hospitalized patients with pneumonia, we frequently find that about a third of the time sputum cannot be obtained, about a third of the time it is obtained but the quality is unsatisfactory, and only a third of the time does the sputum sample meet criteria (less than 5 squamous epithelial cells per high‐power field) for adequate interpretation of the gram‐stain and culture result. Unfortunately, no one has developed a better way to improve this process. Nonetheless, I believe we do not try hard enough to obtain sputum in the first hours of evaluating our patients. I joke with our internal medicine residents that they should carry a sputum cup with them when they evaluate a patient with possible pneumonia. One recent prospective study of the value of sputum gram‐staining in community‐acquired pneumonia has found it to be highly specific for identifying Streptococcus pneumoniae or Haemophilus influenzae pneumonia.
The CT scan (Figure 2) performed on hospital day 6 demonstrated consolidation in the left upper lobe with areas of cavitation. There was also interstitial infiltrate extending into the lingula. Elevation of the right hemidiaphragm with atelectasis in both lung bases was also noted. A small effusion was present on the left and possibly a minimal effusion on the right as well. There was no pericardial effusion and only a few small pretracheal and periaortic lymph nodes were noted.

Given her failure to improve significantly after 6 days of antibiotic treatment, and her recent use of glucocorticoids, I would expand my diagnostic considerations to include other necrotizing bacterial infections, tuberculosis, fungus, and Nocardia.
Given the results of the CT scan she was placed in respiratory isolation to rule out active pulmonary tuberculosis. Though tachypneic, her blood pressure and pulse remained stable. However, her oxygen saturation deteriorated, declining to 92% on 2 L of oxygen via nasal cannula during hospital days 6 and 7. Subsequent successful attempts at collecting sputum yielded rapid growth of yeast (not Cryptococcus spp.). Pulmonary and infectious disease consultations were obtained and vancomycin was added to her regimen. The patient subsequently agreed to undergo diagnostic bronchoscopy.
I agree with obtaining input from expert consultants. I think we too often underutilize consultation in patients that are better but not completely better when we are not entirely sure what is going on. Evidence of noncryptococcus yeast in sputum may sometimes indicate colonization with Candida spp. without any significant clinical consequence. This finding may alternatively suggest the possibility of a true fungal pneumonia caused by 1 of the dimorphic fungi, including Histoplasma capsulatum, Paracoccidioides brasiliensis, Blastomyces dermatitides, or Coccidioides immitis. However, in this case there is not a strong epidemiologic patient history of exposure to any of these types of fungi.
Three sputum smears were negative for acid fast bacilli (AFB). Bronchoscopy revealed grossly abnormal mucosa in the left upper lobe and bronchomalacia, but no obstructive lesions. A transthoracic echocardiogram was ordered to evaluate her degree of pulmonary hypertension.
The 3 sputum specimens that were negative for AFB despite cavitary lung disease have high sensitivity for ruling out pulmonary tuberculosis. In addition, given the absence of any bacterial pathogen isolated from these specimens, I would pursue the possibility of other potential fungal pathogens given the patient's subacute course, history of using inhaled and oral corticosteroids, sputum results, and the presence of a cavitary lesion on her CT scan images.
Cytologic examination of the bronchoalveolar lavage (BAL) sample showed a cell differential of 1% bands, 58% neutrophils, 9% lymphs, and 27% eosinophils. The routine postbronchoscopy chest radiograph showed complete opacification of the left lung. The patient's WBC count rose to 26,000/L but she remained afebrile. Echocardiogram was reported to be of very poor quality due to her obesity. The cardiologist reviewing the echocardiogram called the attending physicians and stated there was possibly something in the left pulmonary artery and aortic dissection could not be ruled out.
The presence of eosinophilia on BAL may be a very important clue as to what lung pathology she has. In fact, eosinophilia in this setting may indicate the possibility of parasitic or fungal infection of the lung, or inflammation of the airway associated to drug toxicity, asthma, or environmental toxin exposure. With this additional information, I am concerned that she may be harboring an atypical infection such as an invasive fungus. The echocardiogram results are unclear to me but will need to be clarified with additional testing.
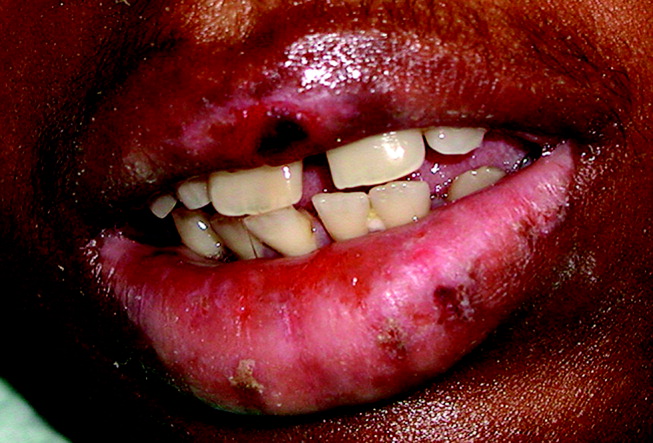
The interpretation of the transbronchial biopsy specimen was limited but suggested invasive pseudomembranous tracheal bronchitis due to aspergillosis. The routine hematoxylin and eosin stain showed portions of alveolar lung tissue and some collapsed submucosal bronchial glands with relatively normal‐looking lung tissue but along the edge of the spaces were obvious fungal organisms. The Gomori's methenamine silver (GMS) stain suggested the presence of Aspergillus organisms (Figure 3). Fungal cultures were also negative for any of the other dimorphic fungi or for molds.

Despite the negative culture results, the overall clinical picture suggests a necrotizing pneumonia caused by an invasive Aspergillus affecting both the bronchial tree and the lower respiratory tract. Generally, necrotizing pneumonias usually have a slow response to antimicrobial therapy. Given the inherent difficulty in differentiating clearly between invasive and noninvasive disease based on a transbronchial biopsy specimen, initiating antifungal therapy for invasive aspergillosis is appropriate in this patient. This patient's recent use of oral glucocorticoids and chronic use of inhaled glucocorticoids are both potential risk factors that predisposed this patient to develop invasive aspergillosis.
Many times we simply follow treatment guidelines for different categories of pneumonia, and have limited or inadequate clinical information to make more definitive diagnoses. While we need these treatment protocols, physicians must avoid falling into the trap that antibiotics treat all infectious etiologies in the lung and we should make reasonable efforts to pin down the etiology. All of us have been fooled by atypical presentations of tuberculosis, fungus, and noninfectious diseases of the lung. I think it behooves us to be vigilant about alternative diagnoses and consider pursuing additional studies whenever the clinical response to initial treatment does not meet our expectations.
Subsequently, the patient's additional cultures remained negative. The official echocardiogram report was read as questionable PE in the pulmonary artery. A spiral CT angiogram revealed a pulmonary artery embolus in the left upper lobe and she was treated with anticoagulation. Her shortness of breath improved steadily and she was successfully discharged after receiving 9 days of oral voriconazole. Outpatient pulmonary function testing documented the presence of chronic obstructive lung disease. She completed a 5‐month course of voriconazole therapy with significant clinical and radiologic improvement of her pulmonary infiltrate. She also completed a 12‐month treatment with warfarin for the concomitant pulmonary embolism. On follow‐up at 12 months she was doing well.
COMMENTARY
Aspergillosis caused particularly by Aspergillus fumigatus is considered an emerging infectious disease that frequently produces significant morbidity and mortality among immunocompromised patients.1, 2 The most frequently‐affected organs by this fungal pathogen include the lung and the central nervous system. There are 3 pathogenic mechanisms of Aspergillus infection of the lung: colonization, hypersensitivity reaction, and invasive aspergillosis.1
Invasive pulmonary aspergillosis is predominantly seen among individuals with severe degrees of immunosuppression as a result of solid‐organ transplantation, immunosuppressive therapies for autoimmune diseases, systemic glucocorticoids, and chemotherapy for hematologic malignancies. Mortality due to invasive aspergillosis continues to be very high (>58%) despite our improved ability to diagnose this condition and newer therapies to treat immunocompromised individuals.1 Invasive aspergillosis can manifest clinically in multiple ways. These include: (1) an invasive vascular process in which fungal organisms invade blood vessels, causing a rapidly progressive and often fatal illness; (2) necrotizing pseudomembranous tracheal bronchitis; (3) chronic necrotizing aspergillosis; (4) bronchopleural fistula; or (5) empyema.35 In our case, while the pathologic findings were most suggestive of an invasive pseudomembranous tracheal bronchitis, the overall clinical picture was most compatible with a necrotizing pneumonia due to invasive aspergillosis.
In addition to the traditional identified risk factors for invasive pulmonary aspergillosis, a number of reports during the last decade have demonstrated the occurrence of invasive aspergillosis in patients with COPD.14 A systematic review of the literature demonstrated that among 1,941 patients with invasive aspergillosis, 26 (1.3%) had evidence of COPD as the main risk factor for developing invasive aspergillosis.1 A single report has associated the potential use of inhaled steroids with the occurrence of invasive aspergillosis in this patient population.2 However, other factors that may promote increased susceptibility to invasive fungal infection among patients with COPD include the use of long‐term or repeated short‐term glucocorticoid treatments, and the presence of multiple additional comorbidities, which may be found in this same population such as diabetes, malnutrition, or end‐stage renal disease.3, 4 Most reported series have demonstrated a high mortality rate of invasive pulmonary aspergillosis in patients with COPD.14
The diagnosis of invasive pulmonary aspergillosis represents a significant clinical challenge. Diagnostic algorithms incorporating CT, antigen detection testing (for serum galactomannan and ‐glucan) as well as polymerase chain reaction diagnostic testing appear to be beneficial in the early diagnosis of invasive aspergillosis in particular settings such as in allogeneic hematopoietic stem cell transplantation.5 The role of antigen testing to identify early invasive aspergillosis in patients with COPD remains uncertain since it has been evaluated in a limited number of patients and therefore clinical suspicion is critical to push clinicians to pursue invasive tissue biopsy and cultures to confirm the diagnosis.3, 4
Based on the available clinical case series and in our case, invasive pulmonary aspergillosis should be suspected in COPD patients with rapidly progressive pneumonia not responding to antibacterial therapy and who have received oral or inhaled glucocorticoids in the recent past. In addition, this case also illustrates that occasionally, patients present with more than 1 life‐threatening diagnosis. This patient was also diagnosed with PE despite adequate prophylaxis. In addition to the well‐known clinical risk factors of obesity and lung disease, the underlying infection may have contributed to a systemic or local hypercoagulable condition that further increased her risk for venous thromboembolism.
KEY TEACHING POINTS
-
Clinicians should remember to consider a broad differential in patients presenting with pneumonia, including the possibility of fungal pathogens in patients with known risk factors and in patients with multiple, potentially immunosuppressive comorbidities, or in patients who do not improve on standard antibiotic therapy.
-
There is some evidence of an association between COPD and invasive aspergillosis, likely due to the frequent use of oral corticosteroids and/or chronic inhaled steroids in this population.
- ,,.Aspergillosis case‐fatality rate: systematic review of the literature.Clin Infect Dis.2001;32:358–366.
- ,,,,.Invasive pulmonary filamentous fungal infection in a patient receiving inhaled corticosteroid therapy.Clin Infect Dis.2002;35:e54–e56.
- ,,, et al.Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease: an emerging fungal pathogen.Clin Microbiol Infect.2005;11:427–429.
- ,,,,,.Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: report of eight cases and review.Clin Infect Dis.1998;26:1473–1475.
- ,.Current approaches to diagnosis and treatment to invasive aspergillosis.Am J Respir Crit Care Med.2006;173:707–717.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 71‐year‐old African‐American woman presented to the emergency department with chest pain, shortness of breath, and cough. She had initially presented to her primary care physician 2 weeks previously complaining of worsening cough and shortness of breath and was told to continue her inhaled albuterol and glucocorticoids and was prescribed a prednisone taper and an unknown course of antibiotics. She noted no improvement in her symptoms despite compliance with this treatment. Three days prior to admission she described the gradual onset of left‐sided pleuritic chest pain with continued cough, associated with yellow sputum and worsening dyspnea. Review of systems was remarkable for generalized weakness and malaise. She denied fever, chills, orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema, diarrhea, nausea, vomiting, or abdominal pain.
Her past medical history included a diagnosis of chronic obstructive pulmonary disease (COPD) but pulmonary function tests 7 years prior to admission showed an forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) ratio of 81%. She had a 30 pack‐year history of smoking, but quit 35 years ago. The patient also carried a diagnosis of heart failure, but an echocardiogram done 1 year ago demonstrated a left ventricular ejection fraction of 65% to 70% without diastolic dysfunction but mild right ventricular dilation and hypertrophy. Additionally, she had known nonobstructive coronary atherosclerotic heart disease, dyslipidemia, hypertension, morbid obesity, depression, and a documented chronic right hemidiaphragm elevation.
At this point the history suggests that the patient does not have a clear diagnosis of COPD. The lack of definitive spirometry evidence of chronic airway obstruction concerns me; I think that she may have been mistakenly treated with chronic inhaled steroids and doses of antibiotics for an acute exacerbation of chronic lung disease. Additional review of her history gives some indication of advanced lung disease, with her recent echocardiogram showing strain on the right ventricle with right ventricular hypertrophy and dilation, but there is no mention of the presence or severity of pulmonary hypertension. Nonetheless, I would be concerned that she probably has underlying significant cor pulmonale.
The patient now re‐presents with a worsening of her pulmonary symptoms. Her left‐sided pleuritic pain would make me concerned that she had a pulmonary embolus (PE). This morbidly obese patient with new pulmonary symptoms, right ventricular strain on her previous echocardiogram, and a persistent elevated right hemidiaphragm suggests a presentation of another PE.
At this time I cannot rule out other common possibilities such as infectious pneumonia. If she does have pneumonia, I would be concerned she could be harboring a multidrug‐resistant bacterial infection given her recent course of antibiotics in addition to her use of both chronic inhaled and intermittent oral glucocorticoids.
After gathering the rest of her full medical history, I would focus my physical exam on looking for evidence of parenchymal lung disease, signs of pulmonary hypertension, and pneumonia.
Her surgical history includes a previous hysterectomy, cholecystectomy, hernia repair, and left hepatic lobectomy for a benign mass. Her outpatient medications were ibuprofen, bupropion, fluvastatin, atenolol, potassium, aspirin, clopidogrel, albuterol inhaler, fluticasone/salmeterol inhaler, and omeprazole. She reports an allergy to penicillin and to sulfa drugs. Her mother died of an unknown cancer at age 77 years. She denied any international travel and she has always lived in Georgia.
The patient has been retired since 1992, having previously worked for the U.S. Postal Service. She admits to occasional alcohol intake (2 to 3 drinks a month). No recent travel, surgery, or prolonged immobilization was noted.
On initial examination she was alert and mentally appropriate, but appeared to be in mild respiratory distress with a respiratory rate of 28 breaths/minute. Her blood pressure (BP) was 99/70, heart rate 102, temperature of 38.2C, and oxygen saturation of 93% on room air and 97% on 2 L of oxygen via nasal cannula. Auscultation of her lungs revealed crackles over her left anterior lung field, bronchial breath sounds in the left posterior midlung, and bibasilar crackles. No wheezing was noted. Her cardiovascular exam and the remainder of her physical exam were unremarkable except for morbid obesity.
While my initial thoughts were leaning toward an exacerbation of chronic lung disease or possibly a new PE, at this moment, infection seems more likely. Indeed, her pulmonary findings suggest a left‐sided inflammatory process, and her vital signs meet criteria for systemic inflammatory response syndrome (SIRS). My primary concern is sepsis due to a drug‐resistant bacterial infection, including Staphylococcus aureus or gram‐negative bacteria or possibly more unusual organisms such as Nocardia or fungi, due to her recent use of antibiotics and chronic inhaled steroid use and recent course of oral glucocorticoids.
Conversely, the SIRS could be a manifestation of a noninfectious lung process such as acute interstitial pneumonia or an eosinophilic pneumonia. Given the diagnostic complexity, I would strongly consider consulting a pulmonologist if the patient did not improve quickly. At this point, I would like to review a posterior‐anterior (PA) and lateral chest radiograph, and room air arterial blood gas (ABG) in addition to basic laboratory test values.
Laboratory data obtained on admission was remarkable for a white blood cell (WBC) count of 26,500/L with 75% neutrophils and 6% eosinophils. Hemoglobin was 14.4 gm/dL. Platelet count was 454,000/L. Serum chemistries showed a sodium of 137 mEq/dL, potassium 4.3 mEq/dL, Cl 108 mEq/dL, bicarbonate 19 mEq/dL, blood urea nitrogen (BUN) 8 mg/dL, creatinine 1.0 mg/dL, and glucose 137 mg/dL. Cardiac enzymes were normal. Calcium was 9.8 mg/dL, albumin 2.7 gm/dL, total protein 6.9 gm/dL, AST 36 U/L, ALT 54 U/L and the bilirubin was normal. Chest radiograph (Figure 1) demonstrated a left perihilar infiltrate with air bronchograms and marked right hemidiaphragm elevation as seen on previous films. Unchanged increased interstitial markings were also present. Her electrocardiogram (ECG) showed normal sinus rhythm, normal axis, and QRS duration with nonspecific diffuse T‐wave abnormalities.

Given her presentation, I am worried about how well she is oxygenating and ventilating. An ABG should be done to assess her status more accurately. An albumin of 2.7 gm/dL indicates that she is fairly sick. I would not hesitate to consider testing the patient for human immunodeficiency virus (HIV) given how this information would dramatically change the differential diagnoses of her pulmonary process.
I am still most concerned about sepsis secondary to pneumonia in this patient with multiple chronic comorbidities, underlying chronic lung disease, receiving chronic inhaled glucocorticoids and a recent course of oral glucocorticoids and antibiotics. While I would initiate hydration I do not see a clear indication for early goal‐directed therapy for severe sepsis. In addition to obtaining an ABG and starting intravenous fluids, I would also draw blood cultures, send sputum for gram stain, culture, and sensitivity, and perform a urinalysis. I would also administer empiric antibiotics as quickly as possible based on a number of pneumonia clinical studies suggesting improved outcomes with early antibiotic administration. Because of her use of antibiotics and both inhaled and oral glucocorticoids, she is at higher risk for potentially multidrug‐resistant bacterial pathogens, including Staphyloccocus aureus and gram‐negative bacteria such as Pseudomonas and Klebsiella (Table 1). Therefore, I would initially cover her broadly for these organisms.
| Meets Any of the Following |
|---|
| Antimicrobial therapy in the preceding 90 days |
| Current hospitalization of 5 days or more |
| High frequency of antibiotic resistance in the community or in the specific hospital unit |
| Presence of risk factors for healthcare‐associated pneumonia (HCAP) |
| Hospitalization for >2 days in the preceding 90 days |
| Residence in nursing home or long‐term care facility (LTAC) for at least 5 days in last 90 days |
| Home infusion therapy including intravenous antibiotics within 30 days |
| Home wound care within 30 days |
| Chronic hemodialysis in hospital or clinic within 30 days |
| Family member with multidrug‐resistant pathogen |
| Immunosuppressive disease and/or therapy |
In addition to initial treatment choice, the inpatient triage decision is another important issue, especially at a community hospital where intensive care unit (ICU) resources are rare and often the admission decision is between sending a moderately sick patient to a regular floor bed or the medical ICU. Both the American Thoracic Society and Infectious Diseases Society of America support an ICU triage protocol in their guidelines for the management of community‐acquired pneumonia in adults that utilizes the following 9 minor criteria, of which the presence of at least 3 should support ICU admission: respiratory rate 30 breaths/minute; oxygenation index (pressure of oxygen [PaO2]/fraction of inspired oxygen [FiO2] ratio) 250; multilobar infiltrates; confusion/disorientation; uremia (BUN level 20 mg/dL); leukopenia (WBC count 4,000 cells/mm3); thrombocytopenia (platelet count 100,000 cells/mm3); hypothermia (core temperature 36C); and hypotension requiring aggressive fluid. Despite the absence of these criteria in this patient, it is important to note that no triage protocol has been adequately prospectively validated. Retrospective study of the minor criteria has found that the presence of at least 2 of the following 3 clinical criteria to have the highest specificity for predicting cardiopulmonary decompensation and subsequent need for ICU care: (1) initial hypotension (BP 90/60) on presentation with response to initial intravenous fluids to a BP >90/60; (2) oxygenation failure as indicated by PaO2/FiO2 ratio less than 250; or (3) the presence of multilobar or bilateral infiltrates on chest radiography.
I also want to comment on the relative elevation of her calcium, especially given the low albumin. This may simply be due to volume depletion, as many older patients have asymptomatic mild primary hyperparathyroidism. However, this elevated calcium may be a clue to the underlying lung process. Granulomatous lung disease due to tuberculosis or fungal infection could yield elevated calcium levels via increases in macrophage production of the active vitamin D metabolite calcitriol. This will need to be followed and a parathormone (PTH) level would be the best first test to request if the calcium level remains elevated. If the PTH level is suppressed, granulomatous disease or malignancy would be the more likely cause.
The patient was admitted with a presumptive diagnosis of community‐acquired pneumonia, was started on ceftriaxone and azithromycin, and given intravenous fluids, oxygen, and continued on inhaled salmeterol/fluticasone. Sputum was ordered for gram stain, culture, and sensitivity, and blood cultures were obtained. Urinalysis showed 1‐5 WBCs/high‐power field. Venous thromboembolism prophylaxis was initiated with subcutaneous heparin 5,000 units 8 hours. Her blood pressure normalized rapidly and during the next few days she stated she was feeling better. Despite continued significant wheezing her oxygen saturation remained at 98% on 2 L of oxygen via nasal cannula and she was less tachypneic. Attempts at obtaining an ABG were unsuccessful, and the patient subsequently refused additional attempts. Over the first few days her WBC count remained elevated above 20,000/L, with worsening bandemia (11%), and fever ranging from 38C to 39C. Sputum analysis was initially unsuccessful and blood cultures remained negative.
I am concerned about the persistent fever and elevated WBC count, and want to emphasize that I might have treated her with broader spectrum antibiotics to cover additional multidrug‐resistant bacterial organisms. I would have initially ordered vancomycin to cover methicillin resistant Staphylococcus aureus (MRSA) plus 2 additional antibiotics that cover multidrug‐resistant gram negative pathogens including Pseudomonas aeruginosa.
On the fifth hospital day, her WBC count dropped to 13,400/L and she defervesced. However, her respiratory status worsened during that same day with increased tachypnea. Of note, no results were reported from the initial sputum cultures and they were reordered and a noncontrast chest computed tomography (CT) was also ordered.
I think at this point, even though she has remained stable hemodynamically and oxygenating easily with supplemental oxygen, the question of whether or not her primary process is infectious or noninfectious lingers. I agree with obtaining a chest CT scan.
I am not surprised that sputum was not evaluated despite the orders. Among hospitalized patients with pneumonia, we frequently find that about a third of the time sputum cannot be obtained, about a third of the time it is obtained but the quality is unsatisfactory, and only a third of the time does the sputum sample meet criteria (less than 5 squamous epithelial cells per high‐power field) for adequate interpretation of the gram‐stain and culture result. Unfortunately, no one has developed a better way to improve this process. Nonetheless, I believe we do not try hard enough to obtain sputum in the first hours of evaluating our patients. I joke with our internal medicine residents that they should carry a sputum cup with them when they evaluate a patient with possible pneumonia. One recent prospective study of the value of sputum gram‐staining in community‐acquired pneumonia has found it to be highly specific for identifying Streptococcus pneumoniae or Haemophilus influenzae pneumonia.
The CT scan (Figure 2) performed on hospital day 6 demonstrated consolidation in the left upper lobe with areas of cavitation. There was also interstitial infiltrate extending into the lingula. Elevation of the right hemidiaphragm with atelectasis in both lung bases was also noted. A small effusion was present on the left and possibly a minimal effusion on the right as well. There was no pericardial effusion and only a few small pretracheal and periaortic lymph nodes were noted.

Given her failure to improve significantly after 6 days of antibiotic treatment, and her recent use of glucocorticoids, I would expand my diagnostic considerations to include other necrotizing bacterial infections, tuberculosis, fungus, and Nocardia.
Given the results of the CT scan she was placed in respiratory isolation to rule out active pulmonary tuberculosis. Though tachypneic, her blood pressure and pulse remained stable. However, her oxygen saturation deteriorated, declining to 92% on 2 L of oxygen via nasal cannula during hospital days 6 and 7. Subsequent successful attempts at collecting sputum yielded rapid growth of yeast (not Cryptococcus spp.). Pulmonary and infectious disease consultations were obtained and vancomycin was added to her regimen. The patient subsequently agreed to undergo diagnostic bronchoscopy.
I agree with obtaining input from expert consultants. I think we too often underutilize consultation in patients that are better but not completely better when we are not entirely sure what is going on. Evidence of noncryptococcus yeast in sputum may sometimes indicate colonization with Candida spp. without any significant clinical consequence. This finding may alternatively suggest the possibility of a true fungal pneumonia caused by 1 of the dimorphic fungi, including Histoplasma capsulatum, Paracoccidioides brasiliensis, Blastomyces dermatitides, or Coccidioides immitis. However, in this case there is not a strong epidemiologic patient history of exposure to any of these types of fungi.
Three sputum smears were negative for acid fast bacilli (AFB). Bronchoscopy revealed grossly abnormal mucosa in the left upper lobe and bronchomalacia, but no obstructive lesions. A transthoracic echocardiogram was ordered to evaluate her degree of pulmonary hypertension.
The 3 sputum specimens that were negative for AFB despite cavitary lung disease have high sensitivity for ruling out pulmonary tuberculosis. In addition, given the absence of any bacterial pathogen isolated from these specimens, I would pursue the possibility of other potential fungal pathogens given the patient's subacute course, history of using inhaled and oral corticosteroids, sputum results, and the presence of a cavitary lesion on her CT scan images.
Cytologic examination of the bronchoalveolar lavage (BAL) sample showed a cell differential of 1% bands, 58% neutrophils, 9% lymphs, and 27% eosinophils. The routine postbronchoscopy chest radiograph showed complete opacification of the left lung. The patient's WBC count rose to 26,000/L but she remained afebrile. Echocardiogram was reported to be of very poor quality due to her obesity. The cardiologist reviewing the echocardiogram called the attending physicians and stated there was possibly something in the left pulmonary artery and aortic dissection could not be ruled out.
The presence of eosinophilia on BAL may be a very important clue as to what lung pathology she has. In fact, eosinophilia in this setting may indicate the possibility of parasitic or fungal infection of the lung, or inflammation of the airway associated to drug toxicity, asthma, or environmental toxin exposure. With this additional information, I am concerned that she may be harboring an atypical infection such as an invasive fungus. The echocardiogram results are unclear to me but will need to be clarified with additional testing.
The interpretation of the transbronchial biopsy specimen was limited but suggested invasive pseudomembranous tracheal bronchitis due to aspergillosis. The routine hematoxylin and eosin stain showed portions of alveolar lung tissue and some collapsed submucosal bronchial glands with relatively normal‐looking lung tissue but along the edge of the spaces were obvious fungal organisms. The Gomori's methenamine silver (GMS) stain suggested the presence of Aspergillus organisms (Figure 3). Fungal cultures were also negative for any of the other dimorphic fungi or for molds.

Despite the negative culture results, the overall clinical picture suggests a necrotizing pneumonia caused by an invasive Aspergillus affecting both the bronchial tree and the lower respiratory tract. Generally, necrotizing pneumonias usually have a slow response to antimicrobial therapy. Given the inherent difficulty in differentiating clearly between invasive and noninvasive disease based on a transbronchial biopsy specimen, initiating antifungal therapy for invasive aspergillosis is appropriate in this patient. This patient's recent use of oral glucocorticoids and chronic use of inhaled glucocorticoids are both potential risk factors that predisposed this patient to develop invasive aspergillosis.
Many times we simply follow treatment guidelines for different categories of pneumonia, and have limited or inadequate clinical information to make more definitive diagnoses. While we need these treatment protocols, physicians must avoid falling into the trap that antibiotics treat all infectious etiologies in the lung and we should make reasonable efforts to pin down the etiology. All of us have been fooled by atypical presentations of tuberculosis, fungus, and noninfectious diseases of the lung. I think it behooves us to be vigilant about alternative diagnoses and consider pursuing additional studies whenever the clinical response to initial treatment does not meet our expectations.
Subsequently, the patient's additional cultures remained negative. The official echocardiogram report was read as questionable PE in the pulmonary artery. A spiral CT angiogram revealed a pulmonary artery embolus in the left upper lobe and she was treated with anticoagulation. Her shortness of breath improved steadily and she was successfully discharged after receiving 9 days of oral voriconazole. Outpatient pulmonary function testing documented the presence of chronic obstructive lung disease. She completed a 5‐month course of voriconazole therapy with significant clinical and radiologic improvement of her pulmonary infiltrate. She also completed a 12‐month treatment with warfarin for the concomitant pulmonary embolism. On follow‐up at 12 months she was doing well.
COMMENTARY
Aspergillosis caused particularly by Aspergillus fumigatus is considered an emerging infectious disease that frequently produces significant morbidity and mortality among immunocompromised patients.1, 2 The most frequently‐affected organs by this fungal pathogen include the lung and the central nervous system. There are 3 pathogenic mechanisms of Aspergillus infection of the lung: colonization, hypersensitivity reaction, and invasive aspergillosis.1
Invasive pulmonary aspergillosis is predominantly seen among individuals with severe degrees of immunosuppression as a result of solid‐organ transplantation, immunosuppressive therapies for autoimmune diseases, systemic glucocorticoids, and chemotherapy for hematologic malignancies. Mortality due to invasive aspergillosis continues to be very high (>58%) despite our improved ability to diagnose this condition and newer therapies to treat immunocompromised individuals.1 Invasive aspergillosis can manifest clinically in multiple ways. These include: (1) an invasive vascular process in which fungal organisms invade blood vessels, causing a rapidly progressive and often fatal illness; (2) necrotizing pseudomembranous tracheal bronchitis; (3) chronic necrotizing aspergillosis; (4) bronchopleural fistula; or (5) empyema.35 In our case, while the pathologic findings were most suggestive of an invasive pseudomembranous tracheal bronchitis, the overall clinical picture was most compatible with a necrotizing pneumonia due to invasive aspergillosis.
In addition to the traditional identified risk factors for invasive pulmonary aspergillosis, a number of reports during the last decade have demonstrated the occurrence of invasive aspergillosis in patients with COPD.14 A systematic review of the literature demonstrated that among 1,941 patients with invasive aspergillosis, 26 (1.3%) had evidence of COPD as the main risk factor for developing invasive aspergillosis.1 A single report has associated the potential use of inhaled steroids with the occurrence of invasive aspergillosis in this patient population.2 However, other factors that may promote increased susceptibility to invasive fungal infection among patients with COPD include the use of long‐term or repeated short‐term glucocorticoid treatments, and the presence of multiple additional comorbidities, which may be found in this same population such as diabetes, malnutrition, or end‐stage renal disease.3, 4 Most reported series have demonstrated a high mortality rate of invasive pulmonary aspergillosis in patients with COPD.14
The diagnosis of invasive pulmonary aspergillosis represents a significant clinical challenge. Diagnostic algorithms incorporating CT, antigen detection testing (for serum galactomannan and ‐glucan) as well as polymerase chain reaction diagnostic testing appear to be beneficial in the early diagnosis of invasive aspergillosis in particular settings such as in allogeneic hematopoietic stem cell transplantation.5 The role of antigen testing to identify early invasive aspergillosis in patients with COPD remains uncertain since it has been evaluated in a limited number of patients and therefore clinical suspicion is critical to push clinicians to pursue invasive tissue biopsy and cultures to confirm the diagnosis.3, 4
Based on the available clinical case series and in our case, invasive pulmonary aspergillosis should be suspected in COPD patients with rapidly progressive pneumonia not responding to antibacterial therapy and who have received oral or inhaled glucocorticoids in the recent past. In addition, this case also illustrates that occasionally, patients present with more than 1 life‐threatening diagnosis. This patient was also diagnosed with PE despite adequate prophylaxis. In addition to the well‐known clinical risk factors of obesity and lung disease, the underlying infection may have contributed to a systemic or local hypercoagulable condition that further increased her risk for venous thromboembolism.
KEY TEACHING POINTS
-
Clinicians should remember to consider a broad differential in patients presenting with pneumonia, including the possibility of fungal pathogens in patients with known risk factors and in patients with multiple, potentially immunosuppressive comorbidities, or in patients who do not improve on standard antibiotic therapy.
-
There is some evidence of an association between COPD and invasive aspergillosis, likely due to the frequent use of oral corticosteroids and/or chronic inhaled steroids in this population.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 71‐year‐old African‐American woman presented to the emergency department with chest pain, shortness of breath, and cough. She had initially presented to her primary care physician 2 weeks previously complaining of worsening cough and shortness of breath and was told to continue her inhaled albuterol and glucocorticoids and was prescribed a prednisone taper and an unknown course of antibiotics. She noted no improvement in her symptoms despite compliance with this treatment. Three days prior to admission she described the gradual onset of left‐sided pleuritic chest pain with continued cough, associated with yellow sputum and worsening dyspnea. Review of systems was remarkable for generalized weakness and malaise. She denied fever, chills, orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema, diarrhea, nausea, vomiting, or abdominal pain.
Her past medical history included a diagnosis of chronic obstructive pulmonary disease (COPD) but pulmonary function tests 7 years prior to admission showed an forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) ratio of 81%. She had a 30 pack‐year history of smoking, but quit 35 years ago. The patient also carried a diagnosis of heart failure, but an echocardiogram done 1 year ago demonstrated a left ventricular ejection fraction of 65% to 70% without diastolic dysfunction but mild right ventricular dilation and hypertrophy. Additionally, she had known nonobstructive coronary atherosclerotic heart disease, dyslipidemia, hypertension, morbid obesity, depression, and a documented chronic right hemidiaphragm elevation.
At this point the history suggests that the patient does not have a clear diagnosis of COPD. The lack of definitive spirometry evidence of chronic airway obstruction concerns me; I think that she may have been mistakenly treated with chronic inhaled steroids and doses of antibiotics for an acute exacerbation of chronic lung disease. Additional review of her history gives some indication of advanced lung disease, with her recent echocardiogram showing strain on the right ventricle with right ventricular hypertrophy and dilation, but there is no mention of the presence or severity of pulmonary hypertension. Nonetheless, I would be concerned that she probably has underlying significant cor pulmonale.
The patient now re‐presents with a worsening of her pulmonary symptoms. Her left‐sided pleuritic pain would make me concerned that she had a pulmonary embolus (PE). This morbidly obese patient with new pulmonary symptoms, right ventricular strain on her previous echocardiogram, and a persistent elevated right hemidiaphragm suggests a presentation of another PE.
At this time I cannot rule out other common possibilities such as infectious pneumonia. If she does have pneumonia, I would be concerned she could be harboring a multidrug‐resistant bacterial infection given her recent course of antibiotics in addition to her use of both chronic inhaled and intermittent oral glucocorticoids.
After gathering the rest of her full medical history, I would focus my physical exam on looking for evidence of parenchymal lung disease, signs of pulmonary hypertension, and pneumonia.
Her surgical history includes a previous hysterectomy, cholecystectomy, hernia repair, and left hepatic lobectomy for a benign mass. Her outpatient medications were ibuprofen, bupropion, fluvastatin, atenolol, potassium, aspirin, clopidogrel, albuterol inhaler, fluticasone/salmeterol inhaler, and omeprazole. She reports an allergy to penicillin and to sulfa drugs. Her mother died of an unknown cancer at age 77 years. She denied any international travel and she has always lived in Georgia.
The patient has been retired since 1992, having previously worked for the U.S. Postal Service. She admits to occasional alcohol intake (2 to 3 drinks a month). No recent travel, surgery, or prolonged immobilization was noted.
On initial examination she was alert and mentally appropriate, but appeared to be in mild respiratory distress with a respiratory rate of 28 breaths/minute. Her blood pressure (BP) was 99/70, heart rate 102, temperature of 38.2C, and oxygen saturation of 93% on room air and 97% on 2 L of oxygen via nasal cannula. Auscultation of her lungs revealed crackles over her left anterior lung field, bronchial breath sounds in the left posterior midlung, and bibasilar crackles. No wheezing was noted. Her cardiovascular exam and the remainder of her physical exam were unremarkable except for morbid obesity.
While my initial thoughts were leaning toward an exacerbation of chronic lung disease or possibly a new PE, at this moment, infection seems more likely. Indeed, her pulmonary findings suggest a left‐sided inflammatory process, and her vital signs meet criteria for systemic inflammatory response syndrome (SIRS). My primary concern is sepsis due to a drug‐resistant bacterial infection, including Staphylococcus aureus or gram‐negative bacteria or possibly more unusual organisms such as Nocardia or fungi, due to her recent use of antibiotics and chronic inhaled steroid use and recent course of oral glucocorticoids.
Conversely, the SIRS could be a manifestation of a noninfectious lung process such as acute interstitial pneumonia or an eosinophilic pneumonia. Given the diagnostic complexity, I would strongly consider consulting a pulmonologist if the patient did not improve quickly. At this point, I would like to review a posterior‐anterior (PA) and lateral chest radiograph, and room air arterial blood gas (ABG) in addition to basic laboratory test values.
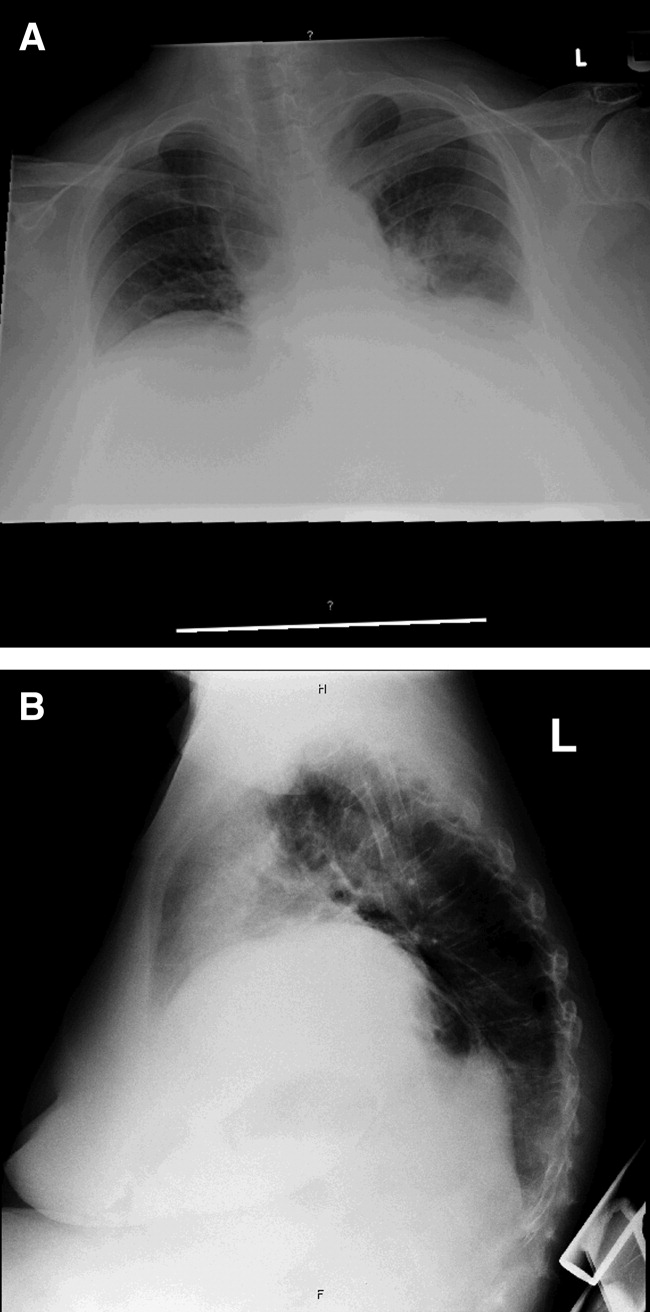
Laboratory data obtained on admission was remarkable for a white blood cell (WBC) count of 26,500/L with 75% neutrophils and 6% eosinophils. Hemoglobin was 14.4 gm/dL. Platelet count was 454,000/L. Serum chemistries showed a sodium of 137 mEq/dL, potassium 4.3 mEq/dL, Cl 108 mEq/dL, bicarbonate 19 mEq/dL, blood urea nitrogen (BUN) 8 mg/dL, creatinine 1.0 mg/dL, and glucose 137 mg/dL. Cardiac enzymes were normal. Calcium was 9.8 mg/dL, albumin 2.7 gm/dL, total protein 6.9 gm/dL, AST 36 U/L, ALT 54 U/L and the bilirubin was normal. Chest radiograph (Figure 1) demonstrated a left perihilar infiltrate with air bronchograms and marked right hemidiaphragm elevation as seen on previous films. Unchanged increased interstitial markings were also present. Her electrocardiogram (ECG) showed normal sinus rhythm, normal axis, and QRS duration with nonspecific diffuse T‐wave abnormalities.

Given her presentation, I am worried about how well she is oxygenating and ventilating. An ABG should be done to assess her status more accurately. An albumin of 2.7 gm/dL indicates that she is fairly sick. I would not hesitate to consider testing the patient for human immunodeficiency virus (HIV) given how this information would dramatically change the differential diagnoses of her pulmonary process.
I am still most concerned about sepsis secondary to pneumonia in this patient with multiple chronic comorbidities, underlying chronic lung disease, receiving chronic inhaled glucocorticoids and a recent course of oral glucocorticoids and antibiotics. While I would initiate hydration I do not see a clear indication for early goal‐directed therapy for severe sepsis. In addition to obtaining an ABG and starting intravenous fluids, I would also draw blood cultures, send sputum for gram stain, culture, and sensitivity, and perform a urinalysis. I would also administer empiric antibiotics as quickly as possible based on a number of pneumonia clinical studies suggesting improved outcomes with early antibiotic administration. Because of her use of antibiotics and both inhaled and oral glucocorticoids, she is at higher risk for potentially multidrug‐resistant bacterial pathogens, including Staphyloccocus aureus and gram‐negative bacteria such as Pseudomonas and Klebsiella (Table 1). Therefore, I would initially cover her broadly for these organisms.
| Meets Any of the Following |
|---|
| Antimicrobial therapy in the preceding 90 days |
| Current hospitalization of 5 days or more |
| High frequency of antibiotic resistance in the community or in the specific hospital unit |
| Presence of risk factors for healthcare‐associated pneumonia (HCAP) |
| Hospitalization for >2 days in the preceding 90 days |
| Residence in nursing home or long‐term care facility (LTAC) for at least 5 days in last 90 days |
| Home infusion therapy including intravenous antibiotics within 30 days |
| Home wound care within 30 days |
| Chronic hemodialysis in hospital or clinic within 30 days |
| Family member with multidrug‐resistant pathogen |
| Immunosuppressive disease and/or therapy |
In addition to initial treatment choice, the inpatient triage decision is another important issue, especially at a community hospital where intensive care unit (ICU) resources are rare and often the admission decision is between sending a moderately sick patient to a regular floor bed or the medical ICU. Both the American Thoracic Society and Infectious Diseases Society of America support an ICU triage protocol in their guidelines for the management of community‐acquired pneumonia in adults that utilizes the following 9 minor criteria, of which the presence of at least 3 should support ICU admission: respiratory rate 30 breaths/minute; oxygenation index (pressure of oxygen [PaO2]/fraction of inspired oxygen [FiO2] ratio) 250; multilobar infiltrates; confusion/disorientation; uremia (BUN level 20 mg/dL); leukopenia (WBC count 4,000 cells/mm3); thrombocytopenia (platelet count 100,000 cells/mm3); hypothermia (core temperature 36C); and hypotension requiring aggressive fluid. Despite the absence of these criteria in this patient, it is important to note that no triage protocol has been adequately prospectively validated. Retrospective study of the minor criteria has found that the presence of at least 2 of the following 3 clinical criteria to have the highest specificity for predicting cardiopulmonary decompensation and subsequent need for ICU care: (1) initial hypotension (BP 90/60) on presentation with response to initial intravenous fluids to a BP >90/60; (2) oxygenation failure as indicated by PaO2/FiO2 ratio less than 250; or (3) the presence of multilobar or bilateral infiltrates on chest radiography.
I also want to comment on the relative elevation of her calcium, especially given the low albumin. This may simply be due to volume depletion, as many older patients have asymptomatic mild primary hyperparathyroidism. However, this elevated calcium may be a clue to the underlying lung process. Granulomatous lung disease due to tuberculosis or fungal infection could yield elevated calcium levels via increases in macrophage production of the active vitamin D metabolite calcitriol. This will need to be followed and a parathormone (PTH) level would be the best first test to request if the calcium level remains elevated. If the PTH level is suppressed, granulomatous disease or malignancy would be the more likely cause.
The patient was admitted with a presumptive diagnosis of community‐acquired pneumonia, was started on ceftriaxone and azithromycin, and given intravenous fluids, oxygen, and continued on inhaled salmeterol/fluticasone. Sputum was ordered for gram stain, culture, and sensitivity, and blood cultures were obtained. Urinalysis showed 1‐5 WBCs/high‐power field. Venous thromboembolism prophylaxis was initiated with subcutaneous heparin 5,000 units 8 hours. Her blood pressure normalized rapidly and during the next few days she stated she was feeling better. Despite continued significant wheezing her oxygen saturation remained at 98% on 2 L of oxygen via nasal cannula and she was less tachypneic. Attempts at obtaining an ABG were unsuccessful, and the patient subsequently refused additional attempts. Over the first few days her WBC count remained elevated above 20,000/L, with worsening bandemia (11%), and fever ranging from 38C to 39C. Sputum analysis was initially unsuccessful and blood cultures remained negative.
I am concerned about the persistent fever and elevated WBC count, and want to emphasize that I might have treated her with broader spectrum antibiotics to cover additional multidrug‐resistant bacterial organisms. I would have initially ordered vancomycin to cover methicillin resistant Staphylococcus aureus (MRSA) plus 2 additional antibiotics that cover multidrug‐resistant gram negative pathogens including Pseudomonas aeruginosa.
On the fifth hospital day, her WBC count dropped to 13,400/L and she defervesced. However, her respiratory status worsened during that same day with increased tachypnea. Of note, no results were reported from the initial sputum cultures and they were reordered and a noncontrast chest computed tomography (CT) was also ordered.
I think at this point, even though she has remained stable hemodynamically and oxygenating easily with supplemental oxygen, the question of whether or not her primary process is infectious or noninfectious lingers. I agree with obtaining a chest CT scan.
I am not surprised that sputum was not evaluated despite the orders. Among hospitalized patients with pneumonia, we frequently find that about a third of the time sputum cannot be obtained, about a third of the time it is obtained but the quality is unsatisfactory, and only a third of the time does the sputum sample meet criteria (less than 5 squamous epithelial cells per high‐power field) for adequate interpretation of the gram‐stain and culture result. Unfortunately, no one has developed a better way to improve this process. Nonetheless, I believe we do not try hard enough to obtain sputum in the first hours of evaluating our patients. I joke with our internal medicine residents that they should carry a sputum cup with them when they evaluate a patient with possible pneumonia. One recent prospective study of the value of sputum gram‐staining in community‐acquired pneumonia has found it to be highly specific for identifying Streptococcus pneumoniae or Haemophilus influenzae pneumonia.
The CT scan (Figure 2) performed on hospital day 6 demonstrated consolidation in the left upper lobe with areas of cavitation. There was also interstitial infiltrate extending into the lingula. Elevation of the right hemidiaphragm with atelectasis in both lung bases was also noted. A small effusion was present on the left and possibly a minimal effusion on the right as well. There was no pericardial effusion and only a few small pretracheal and periaortic lymph nodes were noted.

Given her failure to improve significantly after 6 days of antibiotic treatment, and her recent use of glucocorticoids, I would expand my diagnostic considerations to include other necrotizing bacterial infections, tuberculosis, fungus, and Nocardia.
Given the results of the CT scan she was placed in respiratory isolation to rule out active pulmonary tuberculosis. Though tachypneic, her blood pressure and pulse remained stable. However, her oxygen saturation deteriorated, declining to 92% on 2 L of oxygen via nasal cannula during hospital days 6 and 7. Subsequent successful attempts at collecting sputum yielded rapid growth of yeast (not Cryptococcus spp.). Pulmonary and infectious disease consultations were obtained and vancomycin was added to her regimen. The patient subsequently agreed to undergo diagnostic bronchoscopy.
I agree with obtaining input from expert consultants. I think we too often underutilize consultation in patients that are better but not completely better when we are not entirely sure what is going on. Evidence of noncryptococcus yeast in sputum may sometimes indicate colonization with Candida spp. without any significant clinical consequence. This finding may alternatively suggest the possibility of a true fungal pneumonia caused by 1 of the dimorphic fungi, including Histoplasma capsulatum, Paracoccidioides brasiliensis, Blastomyces dermatitides, or Coccidioides immitis. However, in this case there is not a strong epidemiologic patient history of exposure to any of these types of fungi.
Three sputum smears were negative for acid fast bacilli (AFB). Bronchoscopy revealed grossly abnormal mucosa in the left upper lobe and bronchomalacia, but no obstructive lesions. A transthoracic echocardiogram was ordered to evaluate her degree of pulmonary hypertension.
The 3 sputum specimens that were negative for AFB despite cavitary lung disease have high sensitivity for ruling out pulmonary tuberculosis. In addition, given the absence of any bacterial pathogen isolated from these specimens, I would pursue the possibility of other potential fungal pathogens given the patient's subacute course, history of using inhaled and oral corticosteroids, sputum results, and the presence of a cavitary lesion on her CT scan images.
Cytologic examination of the bronchoalveolar lavage (BAL) sample showed a cell differential of 1% bands, 58% neutrophils, 9% lymphs, and 27% eosinophils. The routine postbronchoscopy chest radiograph showed complete opacification of the left lung. The patient's WBC count rose to 26,000/L but she remained afebrile. Echocardiogram was reported to be of very poor quality due to her obesity. The cardiologist reviewing the echocardiogram called the attending physicians and stated there was possibly something in the left pulmonary artery and aortic dissection could not be ruled out.
The presence of eosinophilia on BAL may be a very important clue as to what lung pathology she has. In fact, eosinophilia in this setting may indicate the possibility of parasitic or fungal infection of the lung, or inflammation of the airway associated to drug toxicity, asthma, or environmental toxin exposure. With this additional information, I am concerned that she may be harboring an atypical infection such as an invasive fungus. The echocardiogram results are unclear to me but will need to be clarified with additional testing.
The interpretation of the transbronchial biopsy specimen was limited but suggested invasive pseudomembranous tracheal bronchitis due to aspergillosis. The routine hematoxylin and eosin stain showed portions of alveolar lung tissue and some collapsed submucosal bronchial glands with relatively normal‐looking lung tissue but along the edge of the spaces were obvious fungal organisms. The Gomori's methenamine silver (GMS) stain suggested the presence of Aspergillus organisms (Figure 3). Fungal cultures were also negative for any of the other dimorphic fungi or for molds.

Despite the negative culture results, the overall clinical picture suggests a necrotizing pneumonia caused by an invasive Aspergillus affecting both the bronchial tree and the lower respiratory tract. Generally, necrotizing pneumonias usually have a slow response to antimicrobial therapy. Given the inherent difficulty in differentiating clearly between invasive and noninvasive disease based on a transbronchial biopsy specimen, initiating antifungal therapy for invasive aspergillosis is appropriate in this patient. This patient's recent use of oral glucocorticoids and chronic use of inhaled glucocorticoids are both potential risk factors that predisposed this patient to develop invasive aspergillosis.
Many times we simply follow treatment guidelines for different categories of pneumonia, and have limited or inadequate clinical information to make more definitive diagnoses. While we need these treatment protocols, physicians must avoid falling into the trap that antibiotics treat all infectious etiologies in the lung and we should make reasonable efforts to pin down the etiology. All of us have been fooled by atypical presentations of tuberculosis, fungus, and noninfectious diseases of the lung. I think it behooves us to be vigilant about alternative diagnoses and consider pursuing additional studies whenever the clinical response to initial treatment does not meet our expectations.
Subsequently, the patient's additional cultures remained negative. The official echocardiogram report was read as questionable PE in the pulmonary artery. A spiral CT angiogram revealed a pulmonary artery embolus in the left upper lobe and she was treated with anticoagulation. Her shortness of breath improved steadily and she was successfully discharged after receiving 9 days of oral voriconazole. Outpatient pulmonary function testing documented the presence of chronic obstructive lung disease. She completed a 5‐month course of voriconazole therapy with significant clinical and radiologic improvement of her pulmonary infiltrate. She also completed a 12‐month treatment with warfarin for the concomitant pulmonary embolism. On follow‐up at 12 months she was doing well.
COMMENTARY
Aspergillosis caused particularly by Aspergillus fumigatus is considered an emerging infectious disease that frequently produces significant morbidity and mortality among immunocompromised patients.1, 2 The most frequently‐affected organs by this fungal pathogen include the lung and the central nervous system. There are 3 pathogenic mechanisms of Aspergillus infection of the lung: colonization, hypersensitivity reaction, and invasive aspergillosis.1
Invasive pulmonary aspergillosis is predominantly seen among individuals with severe degrees of immunosuppression as a result of solid‐organ transplantation, immunosuppressive therapies for autoimmune diseases, systemic glucocorticoids, and chemotherapy for hematologic malignancies. Mortality due to invasive aspergillosis continues to be very high (>58%) despite our improved ability to diagnose this condition and newer therapies to treat immunocompromised individuals.1 Invasive aspergillosis can manifest clinically in multiple ways. These include: (1) an invasive vascular process in which fungal organisms invade blood vessels, causing a rapidly progressive and often fatal illness; (2) necrotizing pseudomembranous tracheal bronchitis; (3) chronic necrotizing aspergillosis; (4) bronchopleural fistula; or (5) empyema.35 In our case, while the pathologic findings were most suggestive of an invasive pseudomembranous tracheal bronchitis, the overall clinical picture was most compatible with a necrotizing pneumonia due to invasive aspergillosis.
In addition to the traditional identified risk factors for invasive pulmonary aspergillosis, a number of reports during the last decade have demonstrated the occurrence of invasive aspergillosis in patients with COPD.14 A systematic review of the literature demonstrated that among 1,941 patients with invasive aspergillosis, 26 (1.3%) had evidence of COPD as the main risk factor for developing invasive aspergillosis.1 A single report has associated the potential use of inhaled steroids with the occurrence of invasive aspergillosis in this patient population.2 However, other factors that may promote increased susceptibility to invasive fungal infection among patients with COPD include the use of long‐term or repeated short‐term glucocorticoid treatments, and the presence of multiple additional comorbidities, which may be found in this same population such as diabetes, malnutrition, or end‐stage renal disease.3, 4 Most reported series have demonstrated a high mortality rate of invasive pulmonary aspergillosis in patients with COPD.14
The diagnosis of invasive pulmonary aspergillosis represents a significant clinical challenge. Diagnostic algorithms incorporating CT, antigen detection testing (for serum galactomannan and ‐glucan) as well as polymerase chain reaction diagnostic testing appear to be beneficial in the early diagnosis of invasive aspergillosis in particular settings such as in allogeneic hematopoietic stem cell transplantation.5 The role of antigen testing to identify early invasive aspergillosis in patients with COPD remains uncertain since it has been evaluated in a limited number of patients and therefore clinical suspicion is critical to push clinicians to pursue invasive tissue biopsy and cultures to confirm the diagnosis.3, 4
Based on the available clinical case series and in our case, invasive pulmonary aspergillosis should be suspected in COPD patients with rapidly progressive pneumonia not responding to antibacterial therapy and who have received oral or inhaled glucocorticoids in the recent past. In addition, this case also illustrates that occasionally, patients present with more than 1 life‐threatening diagnosis. This patient was also diagnosed with PE despite adequate prophylaxis. In addition to the well‐known clinical risk factors of obesity and lung disease, the underlying infection may have contributed to a systemic or local hypercoagulable condition that further increased her risk for venous thromboembolism.
KEY TEACHING POINTS
-
Clinicians should remember to consider a broad differential in patients presenting with pneumonia, including the possibility of fungal pathogens in patients with known risk factors and in patients with multiple, potentially immunosuppressive comorbidities, or in patients who do not improve on standard antibiotic therapy.
-
There is some evidence of an association between COPD and invasive aspergillosis, likely due to the frequent use of oral corticosteroids and/or chronic inhaled steroids in this population.
- ,,.Aspergillosis case‐fatality rate: systematic review of the literature.Clin Infect Dis.2001;32:358–366.
- ,,,,.Invasive pulmonary filamentous fungal infection in a patient receiving inhaled corticosteroid therapy.Clin Infect Dis.2002;35:e54–e56.
- ,,, et al.Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease: an emerging fungal pathogen.Clin Microbiol Infect.2005;11:427–429.
- ,,,,,.Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: report of eight cases and review.Clin Infect Dis.1998;26:1473–1475.
- ,.Current approaches to diagnosis and treatment to invasive aspergillosis.Am J Respir Crit Care Med.2006;173:707–717.
- ,,.Aspergillosis case‐fatality rate: systematic review of the literature.Clin Infect Dis.2001;32:358–366.
- ,,,,.Invasive pulmonary filamentous fungal infection in a patient receiving inhaled corticosteroid therapy.Clin Infect Dis.2002;35:e54–e56.
- ,,, et al.Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease: an emerging fungal pathogen.Clin Microbiol Infect.2005;11:427–429.
- ,,,,,.Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: report of eight cases and review.Clin Infect Dis.1998;26:1473–1475.
- ,.Current approaches to diagnosis and treatment to invasive aspergillosis.Am J Respir Crit Care Med.2006;173:707–717.
Erythema multiforme secondary to HSV labialis precipitating sickle cell pain crisis
A 28‐year‐old man with sickle cell anemia was admitted with generalized pain. He noted an upper lip lesion 2 weeks prior to admission. He subsequently developed generalized pain in his legs, chest, and back typical of his pain crises. At admission he noted subjective fevers without chills for a week. Vital signs revealed a blood pressure of 135/80, a pulse of 81, a respiratory rate of 16, and an initial temperature of 37.7C. On examination he had scleral icterus and a large upper lip ulcer (Fig. 1). His hospital course was complicated by persistent fevers, a hepatic sequestration crisis, persistent hemolytic anemia requiring blood transfusion, and ultimately the identification of iris‐shaped targetoid lesions on the palms (Fig. 2).These lesions were believed to be consistent with erythema multiforme (EM) secondary to his recent HSV labialis, confirmed by a herpes culture. The patient recovered uneventfully after a 10‐day hospitalization. Erythema multiforme is an acute, self‐limited, but sometimes recurrent dermatologic condition considered to be a distinct hypersensitivity reaction.1 It is associated with certain infections such as herpes simplex 1 and 2, Mycoplasma pneumoniae and fungal infections, and a number of medications in the classes barbiturates, nonsteroidal anti‐inflammatory drugs, penicillins, hydantoins, phenothiazines, and sulfonamides.2 EM is diagnosed clinically by the characteristic rash on the hands and feet, with some cases involving the oral cavity. Treatment is typically focused on resolving the underlying infection or removing the offending drug. Dermatologic manifestations usually improve over 3‐5 weeks without residual sequelae.
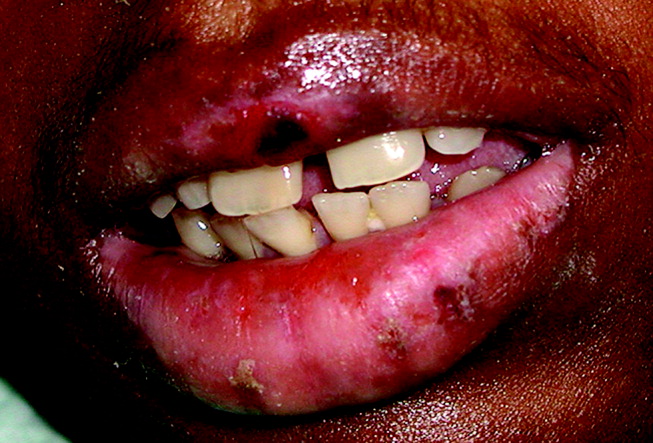
- ,,.Understanding the pathogenesis of HSV‐associated erythema multiforme.Dermatology.1998;197:219–222.
- ,,.Erythema multiforme.Am Fam Physician.2006;74:1883–1888.
A 28‐year‐old man with sickle cell anemia was admitted with generalized pain. He noted an upper lip lesion 2 weeks prior to admission. He subsequently developed generalized pain in his legs, chest, and back typical of his pain crises. At admission he noted subjective fevers without chills for a week. Vital signs revealed a blood pressure of 135/80, a pulse of 81, a respiratory rate of 16, and an initial temperature of 37.7C. On examination he had scleral icterus and a large upper lip ulcer (Fig. 1). His hospital course was complicated by persistent fevers, a hepatic sequestration crisis, persistent hemolytic anemia requiring blood transfusion, and ultimately the identification of iris‐shaped targetoid lesions on the palms (Fig. 2).These lesions were believed to be consistent with erythema multiforme (EM) secondary to his recent HSV labialis, confirmed by a herpes culture. The patient recovered uneventfully after a 10‐day hospitalization. Erythema multiforme is an acute, self‐limited, but sometimes recurrent dermatologic condition considered to be a distinct hypersensitivity reaction.1 It is associated with certain infections such as herpes simplex 1 and 2, Mycoplasma pneumoniae and fungal infections, and a number of medications in the classes barbiturates, nonsteroidal anti‐inflammatory drugs, penicillins, hydantoins, phenothiazines, and sulfonamides.2 EM is diagnosed clinically by the characteristic rash on the hands and feet, with some cases involving the oral cavity. Treatment is typically focused on resolving the underlying infection or removing the offending drug. Dermatologic manifestations usually improve over 3‐5 weeks without residual sequelae.


A 28‐year‐old man with sickle cell anemia was admitted with generalized pain. He noted an upper lip lesion 2 weeks prior to admission. He subsequently developed generalized pain in his legs, chest, and back typical of his pain crises. At admission he noted subjective fevers without chills for a week. Vital signs revealed a blood pressure of 135/80, a pulse of 81, a respiratory rate of 16, and an initial temperature of 37.7C. On examination he had scleral icterus and a large upper lip ulcer (Fig. 1). His hospital course was complicated by persistent fevers, a hepatic sequestration crisis, persistent hemolytic anemia requiring blood transfusion, and ultimately the identification of iris‐shaped targetoid lesions on the palms (Fig. 2).These lesions were believed to be consistent with erythema multiforme (EM) secondary to his recent HSV labialis, confirmed by a herpes culture. The patient recovered uneventfully after a 10‐day hospitalization. Erythema multiforme is an acute, self‐limited, but sometimes recurrent dermatologic condition considered to be a distinct hypersensitivity reaction.1 It is associated with certain infections such as herpes simplex 1 and 2, Mycoplasma pneumoniae and fungal infections, and a number of medications in the classes barbiturates, nonsteroidal anti‐inflammatory drugs, penicillins, hydantoins, phenothiazines, and sulfonamides.2 EM is diagnosed clinically by the characteristic rash on the hands and feet, with some cases involving the oral cavity. Treatment is typically focused on resolving the underlying infection or removing the offending drug. Dermatologic manifestations usually improve over 3‐5 weeks without residual sequelae.


- ,,.Understanding the pathogenesis of HSV‐associated erythema multiforme.Dermatology.1998;197:219–222.
- ,,.Erythema multiforme.Am Fam Physician.2006;74:1883–1888.
- ,,.Understanding the pathogenesis of HSV‐associated erythema multiforme.Dermatology.1998;197:219–222.
- ,,.Erythema multiforme.Am Fam Physician.2006;74:1883–1888.