User login
Perioperative management of diabetes: Translating evidence into practice
Diabetes confers an increased risk of perioperative morbidity and mortality, mostly from infection and cardiovascular events. It is not unusual for surgical patients with diabetes to have a number of comorbidities or underlying chronic vascular complications that put them at risk for cardiovascular events or an infectious complication. Silent ischemia, coronary artery disease, and autonomic neuropathy are common among patients with diabetes, and each can contribute to perioperative morbidity and mortality. These are important considerations since nearly one-fifth of surgical patients have diabetes and since a person with diabetes has a 50% risk of undergoing surgery at some point in his or her lifetime.1
This article reviews the preoperative evaluation of patients with diabetes, discusses the relation between glycemic control and perioperative outcomes, and examines targets and strategies for glycemic control in patients with type 1 and type 2 diabetes throughout the perioperative period.
PREOPERATIVE EVALUATION
The preoperative evaluation must consider first and foremost the status of the patient’s diabetes and his or her surgical risk factors. Also important are the characteristics of the procedure to be performed, the method of anesthesia to be used, and select laboratory values.
Diabetes status
The type of diabetes and its treatment must be considered. Type 1 diabetes requires continuous insulin therapy to prevent ketoacidosis; patients with type 2 diabetes are usually treated with oral medications with or without insulin. Baseline control of blood glucose is a predictor of morbidity following surgery. Hypoglycemia is associated with increased morbidity in the inpatient setting, so a history of severe hypoglycemic events or of difficulty recognizing hypoglycemia (hypoglycemia unawareness) should be elicited in the preoperative evaluation. Complications of diabetes and other comorbidities also must be evaluated, along with their treatments.
Surgical risk factors
Patients with diabetes have surgical risk factors specific to their health—namely, cardiovascular risk factors that may or may not have been previously diagnosed. Patients with diabetes may have silent ischemia, atypical manifestations of coronary ischemia, or underlying cardiomyopathy. Many patients with type 2 diabetes have hypertension, which may complicate perioperative management. Other common surgical risk factors in this population include obesity, chronic kidney disease, and undiagnosed autonomic dysfunction, which may compromise hemodynamic stability in the perioperative period. Additionally, patients with long-standing diabetes experience reductions in pulmonary function (eg, forced expiratory volume, peak expiratory flow, and diffusion capacity for carbon monoxide) related to disease duration and vascular injury,2 which may complicate weaning from ventilatory support.
Characteristics of the procedure and anesthetic
Both surgery and anesthesia may induce an increase in levels of stress hormones (epinephrine, cortisol, growth hormone) and inflammatory cytokines (interleukin-6 and tumor necrosis factor–alpha), resulting in insulin resistance and impaired insulin secretion (even among patients who present with adequate insulin secretion). These in turn contribute to lipolysis and protein catabolism, leading to hyperglycemia and, if a patient is severely insulin deficient, ketoacidosis. Other factors that particularly affect insulin resistance and secretion include cardiovascular bypass surgery, sepsis, the need for total parenteral nutrition, and steroid therapy.
The characteristics of the surgical procedure, including the type of surgery as well as its urgency, duration, and timing (morning vs later in the day), are important in planning for perioperative glycemic management. For example, a short, minor procedure may require only observation, whereas more extensive procedures warrant periodic monitoring and active glycemic management with insulin infusions.
The type of anesthesia should also be considered. Compared with epidural anesthesia, general anesthesia is associated with greater stimulation of the sympathetic nervous system and increased catecholamine levels, resulting in more pronounced hyperglycemia.3
Preoperative tests
Preoperative testing and laboratory evaluation should include, at minimum, an electrocardiogram, a basic metabolic panel to assess renal function, electrolyte levels, and hemoglobin A1c measurement. For low-risk procedures in patients with adequate exercise tolerance, no diagnostic tests might be needed. In any case, knowledge of the hemoglobin A1c level may help not only to classify perioperative risk but also to determine postoperative care, including the choice of antiglycemic medications at discharge.
IMPORTANCE OF GLYCEMIC CONTROL
Preoperative glycemic control has a significant impact on the risk of infectious complications—including pneumonia, wound infection, urinary tract infection, and sepsis—in patients with diabetes across a variety of surgical procedures.4 Similarly, postoperative glycemic control—to a mean blood glucose level less than 200 mg/dL in the immediate postoperative period—significantly reduces the incidence of deep sternal wound infection after open heart surgery.5
Among patients undergoing cardiothoracic surgery, both cardiac-related and overall mortality are greater with increasing postoperative blood glucose levels, although a cause-and-effect relationship has not been established.6
Glycemic control matters regardless of diabetes status
Hyperglycemia affects mortality regardless of diabetes status. In a study of 779 consecutive patients admitted for acute myocardial infarction, mortality at 180 days was highly associated with hyperglycemia on admission independent of a history of diabetes; the highest mortality was among hyperglycemic patients without previously known diabetes.7 Similarly, a large study of glycemic control in intensive care unit (ICU) patients receiving insulin found that mortality in nondiabetic patients increased with median glucose level and was higher than mortality in diabetic patients.8 These findings suggest a need for vigilance in the perioperative and critical care management of all patients with hyperglycemia, regardless of preadmission diabetes diagnosis, as they carry significant morbidity and mortality risk.
GLYCEMIC CONTROL IN THE CRITICALLY ILL: SOME SUPPORT FOR A MODIFIED TARGET, BUT VIGILANCE FOR HYPOGLYCEMIA NEEDED
The landmark study by Van den Berghe et al of intensive insulin therapy in surgical ICU patients demonstrated significant reductions in morbidity and mortality when glucose levels were controlled aggressively (80 to 110 mg/dL; average, 103 mg/dL) compared with conventional control (180 to 200 mg/dL).9 The benefit of intensive glycemic control was evident on outcomes such as the occurrence of sepsis, need for dialysis, need for blood transfusion, and development of acute polyneuropathy. Intensive insulin therapy was also associated with cost savings compared with conventional insulin therapy in mechanically ventilated patients.10
However, a number of subsequent studies have clearly shown that as blood glucose levels approach normoglycemia, the risks of hypoglycemia, especially severe hypoglycemia, can offset the benefits of tight blood glucose control.
A follow-up study by Ven den Berghe et al in a medical ICU failed to show a mortality benefit from tight glycemic control, though patients in the intensive control arm experienced less renal injury, faster weaning from ventilation, and earlier discharge from the ICU and hospital.11
The recent NICE-SUGAR study of aggressive glucose control in the ICU randomized patients to a target blood glucose of 81 to 108 mg/dL (intensive group) or 180 mg/dL or less (control group).12 At study’s end, the groups’ mean blood glucose levels were 115 mg/dL and 144 mg/dL, respectively, while rates of severe hypoglycemia (blood glucose < 40 mg/dL) were 6.8% and 0.5%, respectively. Mortality rates were higher in the intensive therapy group (27.5%) than in the control group (24.9%), driven by severe hypoglycemic events. Notably, blood glucose monitoring in this and other studies was conducted at a frequency of anywhere between 1 and 4 hours.
The conclusions of the available data would support, for the time being, a modified glycemic target in critically ill patients, with strict avoidance of severe hypoglycemia. The recent consensus statement from the American Association of Clinical Endocrinologists and the American Diabetes Association recommends using insulin therapy if blood glucose levels exceed 180 mg/dL, with target glucose levels less than 180 mg/dL in critically ill patients and less than 140 mg/dL in non–critically ill patients.13 Development and implementation of safer insulin infusion algorithms and more frequent and accurate blood glucose monitoring in this setting should enable us to achieve better glycemic targets with lower risk.
ELEMENTS OF PHYSIOLOGIC INSULIN REPLACEMENT
In hospitalized patients with hyperglycemia, three different components of insulin replacement require management1:
Basal insulin replacement consists of a long-acting insulin preparation administered regardless of the patient’s oral intake status, with the premise of matching hepatic (endogenous) glucose production
Prandial insulin replacement requires a rapid-acting insulin preparation given to cover nutritional needs
Supplemental (or correction) insulin replacement requires a rapid-acting preparation (usually the same insulin type as for prandial coverage) to correct blood glucose values that exceed predetermined glycemic targets.
For most patients, basal insulin replacement might be appropriate preoperatively to control fasting glucose, whereas during surgery, especially if prolonged or high risk, an intravenous (IV) insulin drip is the most effective means of glucose control. The postoperative transition from the IV insulin drip usually involves basal insulin replacement plus supplemental rapid-acting insulin. Prandial or nutritional insulin should be started once the patient begins to receive nutrition (oral, enteral, or hyperalimentation).
GOALS OF PERIOPERATIVE GLYCEMIC CONTROL
Perioperative glycemic management has several key objectives:
- Avoidance of clinically significant hyper- or hypoglycemia
- Maintenance of electrolyte and fluid balance
- Prevention of ketoacidosis, which is imperative in patients with type 1 diabetes, who require insulin at all times
- Achievement of specific glycemic targets, as discussed above—ie, less than 180 mg/dL in critically ill patients and less than 140 mg/dL in stable patients.13
Strategies differ across the perioperative timeline
Strategies for perioperative glycemic control differ before, during, and after surgery, as summarized immediately below and detailed in the following sections.
Preoperatively, glycemia should be stabilized, typically with subcutaneous insulin, if there is enough time to do so. For patients who have not previously been on insulin, placing them on an insulin supplemental scale to correct glycemia to desired targets might be a first step. In the setting of hyperglycemia, these patients may also be started on a low dose of basal insulin, with preference given to basal insulin analogs, given their consistent and relatively peakless action profile and lower risk of hypoglycemia. A starting dose of 0.2 to 0.4 U/kg is appropriate and carries a low risk of hypoglycemia. For patients already using insulin on an outpatient basis, continuing their basal insulin dose, possibly at a reduced dosage (25% less), together with supplemental-scale insulin coverage, should stabilize blood glucose levels. For patients on combination insulin or premixed insulin types, the basal insulin dose for preoperative management can be estimated by taking the patient’s usual total daily dose and delivering 40% to 50% of that dose as a basal insulin analog injection. Clearly, a supplemental scale should be implemented along with basal insulin replacement.
Intraoperatively, switching to IV insulin may be appropriate for stabilizing glycemia, depending on the type of surgery. A number of IV insulin protocols have been proposed, although no consistent comparisons of efficacy or safety among these protocols have been published.
Postoperatively, patients eventually should be transitioned from IV to subcutaneous insulin when glycemic control stabilizes. This transition may be complicated for many reasons. Oral intake may be inconsistent. The surgery and surrounding environment can induce stressors, promote susceptibility to infection, and increase insulin resistance. Additionally, some patients may be on hyperalimentation. Specific instructions for the transition from IV to subcutaneous insulin are covered later in this article.
PREOPERATIVE GLYCEMIC MANAGEMENT
In patients with type 2 diabetes, oral agents pose certain safety risks and should be discontinued prior to surgery.
Sulfonylureas may induce hypoglycemia in patients who are placed on NPO (“nothing by mouth”) orders and should be held in patients who are fasting.
Metformin can induce lactic acidosis if kidney function declines and should be withheld 1 to 2 days before planned surgery if a need for IV contrast is anticipated or the procedure could potentially lead to hemodynamic instability and reduced renal perfusion.
Thiazolidinediones may cause fluid retention that can complicate the postoperative period; they can be discontinued several days prior to a planned surgery.
GLP-1 agonists, such as exenatide, can slow gastric motility and potentially delay gastrointestinal recovery after major surgery; they should be held the day of surgery.
DPP-4 inhibitors (incretin enhancers), such as sitagliptin, do not have significant side effects and, if need be, can be continued. Because incretin therapies act via a glucose-dependent mechanism, they are unlikely to cause hypoglycemia, even in a patient whose oral intake is held or delayed. On the other hand, since their effect is mostly in reducing postprandial glycemia, there may be little need to use them in a patient who is NPO.
Patients with type 1 diabetes must continue basal insulin replacement preoperatively (0.2 to 0.3 U/kg/day of a long-acting insulin). Patients with type 2 diabetes may benefit from basal insulin replacement, as previously noted.
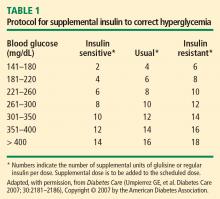
Supplemental insulin scales are used to correct hyperglycemia regardless of a patient’s oral intake status. They can be individualized based on the estimated total daily insulin dose and require glycemic targets to be established. Fingerstick glucose monitoring should be done every 4 to 6 hours in a patient who is NPO, and supplemental-scale insulin should be used to correct glucose values that exceed target. For supplemental-scale coverage, rapid-acting insulin analogs have a shorter duration of action than human regular insulin and may be given subcutaneously every 4 to 6 hours, whereas regular insulin should not be given more often than every 6 hours to correct hyperglycemia. These differences in action duration should be kept in mind to minimize the potential for insulin stacking.
INTRAOPERATIVE GLYCEMIC MANAGEMENT
Procedure length is an important determinant
Strategies for intraoperative glucose management vary according to the length of the procedure.
For minor, short procedures, the preoperative glucose management orders may be continued.
For longer, more complex procedures, a switch to an IV insulin drip is safe and allows rapid adjustments in dosing and plasma glucose levels. Ideally, IV insulin is started prior to the procedure so that the glucose level is stable once the patient arrives in the operating room. Given the logistics of IV insulin management, including the need for frequent monitoring (hourly) and dose adjustments, this type of treatment should be reserved for environments with adequate numbers of trained staff.
IV regular insulin is therapy of choice
Several different algorithms for IV regular insulin therapy are in use. Some are static, such as those of Markovitz et al14 and Stockton et al,15 while others are dynamic (ie, doses are self-adjusted based on changes in blood glucose level), such as the “Yale protocol” of Goldberg et al (Figure 1).16
POSTOPERATIVE GLYCEMIC MANAGEMENT
Start subcutaneous transition before stopping IV drip
Transitioning from IV to subcutaneous insulin is often complicated. Nonoral nutrition options (ie, parenteral nutrition or enteral supplementation) must be considered. As noted, insulin must be replaced according to physiologic needs, which requires that a long-acting basal insulin be used regardless of oral intake status, a rapid-acting insulin be given to cover prandial or nutritional needs, and supplemental rapid-acting insulin be used to correct hyperglycemia.
In the transition from IV insulin, basal insulin replacement can begin at any time. I recommend starting the transition from IV to subcutaneous insulin about 12 to 24 hours before discontinuing the insulin drip. In type 1 diabetes, this transition ensures basal insulin coverage and minimizes the risk of developing ketones and ketoacidosis. In type 2 diabetes, it can ensure a more stable transition and better glycemic control.
Determining the basal insulin dose
Switching to subcutaneous supplemental insulin
Instructions must be given for switching to subcutaneous supplemental doses of insulin. Glycemic targets, generally from less than 130 to 150 mg/dL, must be established, as must the frequency of fingerstick testing:
- If the patient is being fed enterally or parenterally, fingerstick testing is recommended every 4 to 6 hours if a rapid-acting insulin analog is used and every 6 hours if regular insulin is used.
- If the patient is eating, fingerstick testing should be performed before meals and at bedtime.
Covering nutritional requirements
Nutrition-related insulin needs depend on the type of caloric intake prescribed:
In patients receiving total parenteral nutrition (TPN), start 1 U of regular insulin (placed in the bag) for every 10 to 15 g of dextrose in the TPN mixture.
In patients receiving enteral nutrition, use regular insulin every 6 hours or a rapid-acting insulin analog every 4 hours. Start 1 U of insulin subcutaneously for every 10 to 15 g of delivered carbohydrates. For example, if a patient is receiving 10 g of carbohydrates per hour, a rapid-acting analog given at a dose of 4 U every 4 hours (1 U per 10 g of carbohydrates) should adequately cover enteral feedings. For any bolus feedings, give the injection as a full bolus 15 to 20 minutes in advance, based on the carbohydrate content of the feeding.
In patients who are eating, use regular insulin or a rapid-acting insulin analog before meals. Again, start 1 U of insulin subcutaneously for every 10 to 15 g of carbohydrates, or use the prandial portion of the Miami 4/12 rule (Figure 2). For example, in a 60-kg patient one would start with 5 U (60 ÷ 12) of a rapid-acting insulin before each meal.
Basal/bolus replacement outperforms supplemental-scale regular insulin
Use of a basal/bolus insulin regimen appears to be more beneficial than supplemental-scale regular insulin in hospitalized patients with type 2 diabetes, according to a recent randomized trial comparing the two approaches in 130 such patients with blood glucose levels greater than 140 mg/dL.17 In the group randomized to basal/bolus insulin, the starting total daily dose was 0.4 to 0.5 U/kg/day, with half the dose given as basal insulin (insulin glargine) once daily and half given as a rapid-acting insulin analog (glulisine) in fixed doses before every meal. A rapid-acting analog was used for supplemental insulin in the basal/bolus regimen. By study’s end, patients in the basal/bolus group were receiving a higher total daily insulin dose than those in the supplemental-scale group (mean of 42 U/day vs 13 U/day).
Mean daily blood glucose levels were 27 mg/dL lower, on average, in patients who received basal/bolus therapy compared with the supplemental-scale group, yet there was no difference between groups in the risk of hypoglycemia. More patients randomized to basal/bolus therapy achieved the glycemic goal of less than 140 mg/dL (66% vs 38%). Fourteen percent of patients assigned to supplemental-scale insulin had values persistently greater than 240 mg/dL and had to be switched to the basal/bolus regimen.17
SUMMARY
Perioperative glycemic control can reduce morbidity, particularly the incidence of infectious complications, in surgical patients, even in those without diagnosed diabetes. Optimal management of glycemia in the perioperative period involves applying principles of physiologic insulin replacement. Postoperatively, the transition from IV to subcutaneous insulin can be achieved through the use of basal insulin for coverage of fasting insulin needs, regardless of the patient’s feeding status, and the use of rapid-acting insulin to cover hyperglycemia and nutritional needs. Management of hospitalized patients exclusively with supplemental-scale regular insulin should be abandoned.
DISCUSSION
Question from the audience: As an attending physician in a preoperative clinic I’m never sure what to do with NPH insulin the morning of surgery. What guidance can you give?
Dr. Meneghini: NPH is a peaking basal insulin, and the peak can induce hypoglycemia in a patient who is NPO. If we have the opportunity, we try to switch patients previously receiving insulin therapy to a long-acting basal insulin analog, which has a much flatter action profile and is safer in the fasting state. If there is no opportunity for switching, we instruct the patient to take two-thirds of his or her usual morning dose of insulin and we initiate a D5 drip when the patient arrives at the hospital.
Question from the audience: How do you handle perioperative insulin in patients on insulin pumps?
Dr. Meneghini: The pumps provide a subcutaneous basal insulin infusion, which should, if set correctly, maintain stable blood glucose levels when the patient is NPO. Supplemental doses of insulin to correct hyperglycemia can be delivered via the usual subcutaneous practice with a syringe or insulin pen. If you are uncomfortable with pump function, or if the pump insertion site interferes with the surgery site, simply replace the 24-hour basal amount delivered via pump with an injection of glargine or detemir divided into twice-daily injections. Correct hyperglycemia with supplemental-scale insulin as per usual protocol.
Question from the audience: The manufacturer of insulin glargine makes no recommendations for its use the night before or morning of surgery. What do you recommend?
Dr. Meneghini: It depends on whether the glargine is dosed appropriately. Most patients with type 2 diabetes require 0.4 to 0.6 U/kg/day of a long-acting insulin. If they’re on much more, they may be overdosed, and I would cut the basal dose by about half. Otherwise, 75% to 100% of the usual basal amount is appropriate. In type 1 diabetes, the usual replacement dose of basal insulin is 0.2 to 0.3 U/kg/day. If a patient is in this range, the basal insulin can be continued. Patients who experience hypoglycemia, or a substantial fall in blood glucose if meals are skipped or delayed, may be getting too much basal insulin and might benefit from a dose reduction when placed on NPO status.
Question from the audience: Metformin has a black-box warning advising that it be stopped at least 48 hours before surgery, but patients often come to surgery having taken metformin within the prior 12 to 24 hours. How should we manage such patients coming for elective surgery?
Dr. Meneghini: Metformin is cleared exclusively by the kidneys; its accumulation as a result of impaired kidney function (eg, due to hemodynamic instability or radiology studies using IV iodine) can result in increased lactic acid production by the liver and lactic acidosis. A patient who has taken metformin within the prior 48 hours but doesn’t have a risk of hemodynamic dysfunction is at low risk of lactic acidosis if hydrated appropriately. There’s not much choice if a patient needs urgent surgery and has recently taken metformin; in that case, just ensure maintenance of adequate glomerular filtration via fluid repletion to clear the drug.
Question from the audience: What’s the evidence for tight glycemic control or any type of glycemic control in patients undergoing outpatient surgery or “same-day” patients who will be admitted to a regular surgical floor? Also, what would you consider maximal glucose values for a patient going into elective surgery?
Dr. Meneghini: I haven’t seen any guidelines for glycemic control in patients undergoing outpatient surgery. If a patient has poor glycemic control coming into surgery, even for a minor procedure, the risk of an infectious complication may be increased. Keeping blood glucose below 180 mg/dL and avoiding electrolyte imbalances is likely sufficient in such patients. On the second question, if it’s an elective procedure and can be delayed a few hours, you can certainly institute IV insulin therapy to correct hyperglycemia rapidly—just ensure adequate replacement of fluids since the patient may have had volume depletion or dehydration as a result of the preceding osmotic diuresis. Once glycemic control is improved (blood glucose < 180–200 mg/dL), the patient can proceed to surgery.
Question from the audience: What are your recommendations for resuming oral diabetes medications after surgery?
Dr. Meneghini: Once patients are tolerating their meals and being considered for discharge, you may want to resume their oral medications, assuming their admission hemoglobin A1c levels were near goal. If glycemic control was inadequate preoperatively, this may be a good opportunity to adjust their prior regimen to more appropriate therapy. In some cases, this might include some form of insulin, either basal therapy or basal and supplemental insulin.
- Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004; 27:553–591.
- Kaparianos A, Argyropoulou E, Sampsonas F, et al. Pulmonary complications in diabetes mellitus. Chron Respir Dis 2008; 5:101–108.
- Grigoleit HG. Anesthesia and blood glucose. Acta Diabetologica 1973; 10:569–574.
- Dronge AS, Perkal MF, Kancir S, et al. Long-term glycemic control and postoperative infectious complications. Arch Surg 2006; 141:375–380.
- Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg 1997; 63:356–361.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003; 125:1007–10021.
- Ainla T, Baburin A, Tessalu R, et al. The association between hyperglycaemia on admission and 180-day mortality in acute myocardial infarction patients with and without diabetes. Diabet Med 2005; 22:1321–1325.
- Rady MY, Johnson DJ, Patel BM, et al. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc 2005; 80:1558–1567.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Van den Berghe G, Wouters PJ, Kesteloot K, Hilleman DE. Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med 2006; 34:612–616.
- Van den Berghe G, Wawilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. New Engl J Med 2006; 354:449–461.
- NICE-SUGAR Study Investigators. Intensive vs conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Moghissi ES, Korytowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- Markovitz LJ, Wiechmann RJ, Harris N, et al. Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery. Endocr Pract 2002; 8:10–18.
- Stockton L, Baird M, Cook CB, et al. Development and implementation of evidence-based guidelines for IV insulin: a statewide collaborative approach. Insulin 2008; 3:67–77.
- Goldberg PA, Siegel MD, Sherwin RS, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care 2004; 27:461–467.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
Diabetes confers an increased risk of perioperative morbidity and mortality, mostly from infection and cardiovascular events. It is not unusual for surgical patients with diabetes to have a number of comorbidities or underlying chronic vascular complications that put them at risk for cardiovascular events or an infectious complication. Silent ischemia, coronary artery disease, and autonomic neuropathy are common among patients with diabetes, and each can contribute to perioperative morbidity and mortality. These are important considerations since nearly one-fifth of surgical patients have diabetes and since a person with diabetes has a 50% risk of undergoing surgery at some point in his or her lifetime.1
This article reviews the preoperative evaluation of patients with diabetes, discusses the relation between glycemic control and perioperative outcomes, and examines targets and strategies for glycemic control in patients with type 1 and type 2 diabetes throughout the perioperative period.
PREOPERATIVE EVALUATION
The preoperative evaluation must consider first and foremost the status of the patient’s diabetes and his or her surgical risk factors. Also important are the characteristics of the procedure to be performed, the method of anesthesia to be used, and select laboratory values.
Diabetes status
The type of diabetes and its treatment must be considered. Type 1 diabetes requires continuous insulin therapy to prevent ketoacidosis; patients with type 2 diabetes are usually treated with oral medications with or without insulin. Baseline control of blood glucose is a predictor of morbidity following surgery. Hypoglycemia is associated with increased morbidity in the inpatient setting, so a history of severe hypoglycemic events or of difficulty recognizing hypoglycemia (hypoglycemia unawareness) should be elicited in the preoperative evaluation. Complications of diabetes and other comorbidities also must be evaluated, along with their treatments.
Surgical risk factors
Patients with diabetes have surgical risk factors specific to their health—namely, cardiovascular risk factors that may or may not have been previously diagnosed. Patients with diabetes may have silent ischemia, atypical manifestations of coronary ischemia, or underlying cardiomyopathy. Many patients with type 2 diabetes have hypertension, which may complicate perioperative management. Other common surgical risk factors in this population include obesity, chronic kidney disease, and undiagnosed autonomic dysfunction, which may compromise hemodynamic stability in the perioperative period. Additionally, patients with long-standing diabetes experience reductions in pulmonary function (eg, forced expiratory volume, peak expiratory flow, and diffusion capacity for carbon monoxide) related to disease duration and vascular injury,2 which may complicate weaning from ventilatory support.
Characteristics of the procedure and anesthetic
Both surgery and anesthesia may induce an increase in levels of stress hormones (epinephrine, cortisol, growth hormone) and inflammatory cytokines (interleukin-6 and tumor necrosis factor–alpha), resulting in insulin resistance and impaired insulin secretion (even among patients who present with adequate insulin secretion). These in turn contribute to lipolysis and protein catabolism, leading to hyperglycemia and, if a patient is severely insulin deficient, ketoacidosis. Other factors that particularly affect insulin resistance and secretion include cardiovascular bypass surgery, sepsis, the need for total parenteral nutrition, and steroid therapy.
The characteristics of the surgical procedure, including the type of surgery as well as its urgency, duration, and timing (morning vs later in the day), are important in planning for perioperative glycemic management. For example, a short, minor procedure may require only observation, whereas more extensive procedures warrant periodic monitoring and active glycemic management with insulin infusions.
The type of anesthesia should also be considered. Compared with epidural anesthesia, general anesthesia is associated with greater stimulation of the sympathetic nervous system and increased catecholamine levels, resulting in more pronounced hyperglycemia.3
Preoperative tests
Preoperative testing and laboratory evaluation should include, at minimum, an electrocardiogram, a basic metabolic panel to assess renal function, electrolyte levels, and hemoglobin A1c measurement. For low-risk procedures in patients with adequate exercise tolerance, no diagnostic tests might be needed. In any case, knowledge of the hemoglobin A1c level may help not only to classify perioperative risk but also to determine postoperative care, including the choice of antiglycemic medications at discharge.
IMPORTANCE OF GLYCEMIC CONTROL
Preoperative glycemic control has a significant impact on the risk of infectious complications—including pneumonia, wound infection, urinary tract infection, and sepsis—in patients with diabetes across a variety of surgical procedures.4 Similarly, postoperative glycemic control—to a mean blood glucose level less than 200 mg/dL in the immediate postoperative period—significantly reduces the incidence of deep sternal wound infection after open heart surgery.5
Among patients undergoing cardiothoracic surgery, both cardiac-related and overall mortality are greater with increasing postoperative blood glucose levels, although a cause-and-effect relationship has not been established.6
Glycemic control matters regardless of diabetes status
Hyperglycemia affects mortality regardless of diabetes status. In a study of 779 consecutive patients admitted for acute myocardial infarction, mortality at 180 days was highly associated with hyperglycemia on admission independent of a history of diabetes; the highest mortality was among hyperglycemic patients without previously known diabetes.7 Similarly, a large study of glycemic control in intensive care unit (ICU) patients receiving insulin found that mortality in nondiabetic patients increased with median glucose level and was higher than mortality in diabetic patients.8 These findings suggest a need for vigilance in the perioperative and critical care management of all patients with hyperglycemia, regardless of preadmission diabetes diagnosis, as they carry significant morbidity and mortality risk.
GLYCEMIC CONTROL IN THE CRITICALLY ILL: SOME SUPPORT FOR A MODIFIED TARGET, BUT VIGILANCE FOR HYPOGLYCEMIA NEEDED
The landmark study by Van den Berghe et al of intensive insulin therapy in surgical ICU patients demonstrated significant reductions in morbidity and mortality when glucose levels were controlled aggressively (80 to 110 mg/dL; average, 103 mg/dL) compared with conventional control (180 to 200 mg/dL).9 The benefit of intensive glycemic control was evident on outcomes such as the occurrence of sepsis, need for dialysis, need for blood transfusion, and development of acute polyneuropathy. Intensive insulin therapy was also associated with cost savings compared with conventional insulin therapy in mechanically ventilated patients.10
However, a number of subsequent studies have clearly shown that as blood glucose levels approach normoglycemia, the risks of hypoglycemia, especially severe hypoglycemia, can offset the benefits of tight blood glucose control.
A follow-up study by Ven den Berghe et al in a medical ICU failed to show a mortality benefit from tight glycemic control, though patients in the intensive control arm experienced less renal injury, faster weaning from ventilation, and earlier discharge from the ICU and hospital.11
The recent NICE-SUGAR study of aggressive glucose control in the ICU randomized patients to a target blood glucose of 81 to 108 mg/dL (intensive group) or 180 mg/dL or less (control group).12 At study’s end, the groups’ mean blood glucose levels were 115 mg/dL and 144 mg/dL, respectively, while rates of severe hypoglycemia (blood glucose < 40 mg/dL) were 6.8% and 0.5%, respectively. Mortality rates were higher in the intensive therapy group (27.5%) than in the control group (24.9%), driven by severe hypoglycemic events. Notably, blood glucose monitoring in this and other studies was conducted at a frequency of anywhere between 1 and 4 hours.
The conclusions of the available data would support, for the time being, a modified glycemic target in critically ill patients, with strict avoidance of severe hypoglycemia. The recent consensus statement from the American Association of Clinical Endocrinologists and the American Diabetes Association recommends using insulin therapy if blood glucose levels exceed 180 mg/dL, with target glucose levels less than 180 mg/dL in critically ill patients and less than 140 mg/dL in non–critically ill patients.13 Development and implementation of safer insulin infusion algorithms and more frequent and accurate blood glucose monitoring in this setting should enable us to achieve better glycemic targets with lower risk.
ELEMENTS OF PHYSIOLOGIC INSULIN REPLACEMENT
In hospitalized patients with hyperglycemia, three different components of insulin replacement require management1:
Basal insulin replacement consists of a long-acting insulin preparation administered regardless of the patient’s oral intake status, with the premise of matching hepatic (endogenous) glucose production
Prandial insulin replacement requires a rapid-acting insulin preparation given to cover nutritional needs
Supplemental (or correction) insulin replacement requires a rapid-acting preparation (usually the same insulin type as for prandial coverage) to correct blood glucose values that exceed predetermined glycemic targets.
For most patients, basal insulin replacement might be appropriate preoperatively to control fasting glucose, whereas during surgery, especially if prolonged or high risk, an intravenous (IV) insulin drip is the most effective means of glucose control. The postoperative transition from the IV insulin drip usually involves basal insulin replacement plus supplemental rapid-acting insulin. Prandial or nutritional insulin should be started once the patient begins to receive nutrition (oral, enteral, or hyperalimentation).
GOALS OF PERIOPERATIVE GLYCEMIC CONTROL
Perioperative glycemic management has several key objectives:
- Avoidance of clinically significant hyper- or hypoglycemia
- Maintenance of electrolyte and fluid balance
- Prevention of ketoacidosis, which is imperative in patients with type 1 diabetes, who require insulin at all times
- Achievement of specific glycemic targets, as discussed above—ie, less than 180 mg/dL in critically ill patients and less than 140 mg/dL in stable patients.13
Strategies differ across the perioperative timeline
Strategies for perioperative glycemic control differ before, during, and after surgery, as summarized immediately below and detailed in the following sections.
Preoperatively, glycemia should be stabilized, typically with subcutaneous insulin, if there is enough time to do so. For patients who have not previously been on insulin, placing them on an insulin supplemental scale to correct glycemia to desired targets might be a first step. In the setting of hyperglycemia, these patients may also be started on a low dose of basal insulin, with preference given to basal insulin analogs, given their consistent and relatively peakless action profile and lower risk of hypoglycemia. A starting dose of 0.2 to 0.4 U/kg is appropriate and carries a low risk of hypoglycemia. For patients already using insulin on an outpatient basis, continuing their basal insulin dose, possibly at a reduced dosage (25% less), together with supplemental-scale insulin coverage, should stabilize blood glucose levels. For patients on combination insulin or premixed insulin types, the basal insulin dose for preoperative management can be estimated by taking the patient’s usual total daily dose and delivering 40% to 50% of that dose as a basal insulin analog injection. Clearly, a supplemental scale should be implemented along with basal insulin replacement.
Intraoperatively, switching to IV insulin may be appropriate for stabilizing glycemia, depending on the type of surgery. A number of IV insulin protocols have been proposed, although no consistent comparisons of efficacy or safety among these protocols have been published.
Postoperatively, patients eventually should be transitioned from IV to subcutaneous insulin when glycemic control stabilizes. This transition may be complicated for many reasons. Oral intake may be inconsistent. The surgery and surrounding environment can induce stressors, promote susceptibility to infection, and increase insulin resistance. Additionally, some patients may be on hyperalimentation. Specific instructions for the transition from IV to subcutaneous insulin are covered later in this article.
PREOPERATIVE GLYCEMIC MANAGEMENT
In patients with type 2 diabetes, oral agents pose certain safety risks and should be discontinued prior to surgery.
Sulfonylureas may induce hypoglycemia in patients who are placed on NPO (“nothing by mouth”) orders and should be held in patients who are fasting.
Metformin can induce lactic acidosis if kidney function declines and should be withheld 1 to 2 days before planned surgery if a need for IV contrast is anticipated or the procedure could potentially lead to hemodynamic instability and reduced renal perfusion.
Thiazolidinediones may cause fluid retention that can complicate the postoperative period; they can be discontinued several days prior to a planned surgery.
GLP-1 agonists, such as exenatide, can slow gastric motility and potentially delay gastrointestinal recovery after major surgery; they should be held the day of surgery.
DPP-4 inhibitors (incretin enhancers), such as sitagliptin, do not have significant side effects and, if need be, can be continued. Because incretin therapies act via a glucose-dependent mechanism, they are unlikely to cause hypoglycemia, even in a patient whose oral intake is held or delayed. On the other hand, since their effect is mostly in reducing postprandial glycemia, there may be little need to use them in a patient who is NPO.
Patients with type 1 diabetes must continue basal insulin replacement preoperatively (0.2 to 0.3 U/kg/day of a long-acting insulin). Patients with type 2 diabetes may benefit from basal insulin replacement, as previously noted.
Supplemental insulin scales are used to correct hyperglycemia regardless of a patient’s oral intake status. They can be individualized based on the estimated total daily insulin dose and require glycemic targets to be established. Fingerstick glucose monitoring should be done every 4 to 6 hours in a patient who is NPO, and supplemental-scale insulin should be used to correct glucose values that exceed target. For supplemental-scale coverage, rapid-acting insulin analogs have a shorter duration of action than human regular insulin and may be given subcutaneously every 4 to 6 hours, whereas regular insulin should not be given more often than every 6 hours to correct hyperglycemia. These differences in action duration should be kept in mind to minimize the potential for insulin stacking.
INTRAOPERATIVE GLYCEMIC MANAGEMENT
Procedure length is an important determinant
Strategies for intraoperative glucose management vary according to the length of the procedure.
For minor, short procedures, the preoperative glucose management orders may be continued.
For longer, more complex procedures, a switch to an IV insulin drip is safe and allows rapid adjustments in dosing and plasma glucose levels. Ideally, IV insulin is started prior to the procedure so that the glucose level is stable once the patient arrives in the operating room. Given the logistics of IV insulin management, including the need for frequent monitoring (hourly) and dose adjustments, this type of treatment should be reserved for environments with adequate numbers of trained staff.
IV regular insulin is therapy of choice
Several different algorithms for IV regular insulin therapy are in use. Some are static, such as those of Markovitz et al14 and Stockton et al,15 while others are dynamic (ie, doses are self-adjusted based on changes in blood glucose level), such as the “Yale protocol” of Goldberg et al (Figure 1).16
POSTOPERATIVE GLYCEMIC MANAGEMENT
Start subcutaneous transition before stopping IV drip
Transitioning from IV to subcutaneous insulin is often complicated. Nonoral nutrition options (ie, parenteral nutrition or enteral supplementation) must be considered. As noted, insulin must be replaced according to physiologic needs, which requires that a long-acting basal insulin be used regardless of oral intake status, a rapid-acting insulin be given to cover prandial or nutritional needs, and supplemental rapid-acting insulin be used to correct hyperglycemia.
In the transition from IV insulin, basal insulin replacement can begin at any time. I recommend starting the transition from IV to subcutaneous insulin about 12 to 24 hours before discontinuing the insulin drip. In type 1 diabetes, this transition ensures basal insulin coverage and minimizes the risk of developing ketones and ketoacidosis. In type 2 diabetes, it can ensure a more stable transition and better glycemic control.
Determining the basal insulin dose
Switching to subcutaneous supplemental insulin
Instructions must be given for switching to subcutaneous supplemental doses of insulin. Glycemic targets, generally from less than 130 to 150 mg/dL, must be established, as must the frequency of fingerstick testing:
- If the patient is being fed enterally or parenterally, fingerstick testing is recommended every 4 to 6 hours if a rapid-acting insulin analog is used and every 6 hours if regular insulin is used.
- If the patient is eating, fingerstick testing should be performed before meals and at bedtime.
Covering nutritional requirements
Nutrition-related insulin needs depend on the type of caloric intake prescribed:
In patients receiving total parenteral nutrition (TPN), start 1 U of regular insulin (placed in the bag) for every 10 to 15 g of dextrose in the TPN mixture.
In patients receiving enteral nutrition, use regular insulin every 6 hours or a rapid-acting insulin analog every 4 hours. Start 1 U of insulin subcutaneously for every 10 to 15 g of delivered carbohydrates. For example, if a patient is receiving 10 g of carbohydrates per hour, a rapid-acting analog given at a dose of 4 U every 4 hours (1 U per 10 g of carbohydrates) should adequately cover enteral feedings. For any bolus feedings, give the injection as a full bolus 15 to 20 minutes in advance, based on the carbohydrate content of the feeding.
In patients who are eating, use regular insulin or a rapid-acting insulin analog before meals. Again, start 1 U of insulin subcutaneously for every 10 to 15 g of carbohydrates, or use the prandial portion of the Miami 4/12 rule (Figure 2). For example, in a 60-kg patient one would start with 5 U (60 ÷ 12) of a rapid-acting insulin before each meal.
Basal/bolus replacement outperforms supplemental-scale regular insulin
Use of a basal/bolus insulin regimen appears to be more beneficial than supplemental-scale regular insulin in hospitalized patients with type 2 diabetes, according to a recent randomized trial comparing the two approaches in 130 such patients with blood glucose levels greater than 140 mg/dL.17 In the group randomized to basal/bolus insulin, the starting total daily dose was 0.4 to 0.5 U/kg/day, with half the dose given as basal insulin (insulin glargine) once daily and half given as a rapid-acting insulin analog (glulisine) in fixed doses before every meal. A rapid-acting analog was used for supplemental insulin in the basal/bolus regimen. By study’s end, patients in the basal/bolus group were receiving a higher total daily insulin dose than those in the supplemental-scale group (mean of 42 U/day vs 13 U/day).
Mean daily blood glucose levels were 27 mg/dL lower, on average, in patients who received basal/bolus therapy compared with the supplemental-scale group, yet there was no difference between groups in the risk of hypoglycemia. More patients randomized to basal/bolus therapy achieved the glycemic goal of less than 140 mg/dL (66% vs 38%). Fourteen percent of patients assigned to supplemental-scale insulin had values persistently greater than 240 mg/dL and had to be switched to the basal/bolus regimen.17
SUMMARY
Perioperative glycemic control can reduce morbidity, particularly the incidence of infectious complications, in surgical patients, even in those without diagnosed diabetes. Optimal management of glycemia in the perioperative period involves applying principles of physiologic insulin replacement. Postoperatively, the transition from IV to subcutaneous insulin can be achieved through the use of basal insulin for coverage of fasting insulin needs, regardless of the patient’s feeding status, and the use of rapid-acting insulin to cover hyperglycemia and nutritional needs. Management of hospitalized patients exclusively with supplemental-scale regular insulin should be abandoned.
DISCUSSION
Question from the audience: As an attending physician in a preoperative clinic I’m never sure what to do with NPH insulin the morning of surgery. What guidance can you give?
Dr. Meneghini: NPH is a peaking basal insulin, and the peak can induce hypoglycemia in a patient who is NPO. If we have the opportunity, we try to switch patients previously receiving insulin therapy to a long-acting basal insulin analog, which has a much flatter action profile and is safer in the fasting state. If there is no opportunity for switching, we instruct the patient to take two-thirds of his or her usual morning dose of insulin and we initiate a D5 drip when the patient arrives at the hospital.
Question from the audience: How do you handle perioperative insulin in patients on insulin pumps?
Dr. Meneghini: The pumps provide a subcutaneous basal insulin infusion, which should, if set correctly, maintain stable blood glucose levels when the patient is NPO. Supplemental doses of insulin to correct hyperglycemia can be delivered via the usual subcutaneous practice with a syringe or insulin pen. If you are uncomfortable with pump function, or if the pump insertion site interferes with the surgery site, simply replace the 24-hour basal amount delivered via pump with an injection of glargine or detemir divided into twice-daily injections. Correct hyperglycemia with supplemental-scale insulin as per usual protocol.
Question from the audience: The manufacturer of insulin glargine makes no recommendations for its use the night before or morning of surgery. What do you recommend?
Dr. Meneghini: It depends on whether the glargine is dosed appropriately. Most patients with type 2 diabetes require 0.4 to 0.6 U/kg/day of a long-acting insulin. If they’re on much more, they may be overdosed, and I would cut the basal dose by about half. Otherwise, 75% to 100% of the usual basal amount is appropriate. In type 1 diabetes, the usual replacement dose of basal insulin is 0.2 to 0.3 U/kg/day. If a patient is in this range, the basal insulin can be continued. Patients who experience hypoglycemia, or a substantial fall in blood glucose if meals are skipped or delayed, may be getting too much basal insulin and might benefit from a dose reduction when placed on NPO status.
Question from the audience: Metformin has a black-box warning advising that it be stopped at least 48 hours before surgery, but patients often come to surgery having taken metformin within the prior 12 to 24 hours. How should we manage such patients coming for elective surgery?
Dr. Meneghini: Metformin is cleared exclusively by the kidneys; its accumulation as a result of impaired kidney function (eg, due to hemodynamic instability or radiology studies using IV iodine) can result in increased lactic acid production by the liver and lactic acidosis. A patient who has taken metformin within the prior 48 hours but doesn’t have a risk of hemodynamic dysfunction is at low risk of lactic acidosis if hydrated appropriately. There’s not much choice if a patient needs urgent surgery and has recently taken metformin; in that case, just ensure maintenance of adequate glomerular filtration via fluid repletion to clear the drug.
Question from the audience: What’s the evidence for tight glycemic control or any type of glycemic control in patients undergoing outpatient surgery or “same-day” patients who will be admitted to a regular surgical floor? Also, what would you consider maximal glucose values for a patient going into elective surgery?
Dr. Meneghini: I haven’t seen any guidelines for glycemic control in patients undergoing outpatient surgery. If a patient has poor glycemic control coming into surgery, even for a minor procedure, the risk of an infectious complication may be increased. Keeping blood glucose below 180 mg/dL and avoiding electrolyte imbalances is likely sufficient in such patients. On the second question, if it’s an elective procedure and can be delayed a few hours, you can certainly institute IV insulin therapy to correct hyperglycemia rapidly—just ensure adequate replacement of fluids since the patient may have had volume depletion or dehydration as a result of the preceding osmotic diuresis. Once glycemic control is improved (blood glucose < 180–200 mg/dL), the patient can proceed to surgery.
Question from the audience: What are your recommendations for resuming oral diabetes medications after surgery?
Dr. Meneghini: Once patients are tolerating their meals and being considered for discharge, you may want to resume their oral medications, assuming their admission hemoglobin A1c levels were near goal. If glycemic control was inadequate preoperatively, this may be a good opportunity to adjust their prior regimen to more appropriate therapy. In some cases, this might include some form of insulin, either basal therapy or basal and supplemental insulin.
Diabetes confers an increased risk of perioperative morbidity and mortality, mostly from infection and cardiovascular events. It is not unusual for surgical patients with diabetes to have a number of comorbidities or underlying chronic vascular complications that put them at risk for cardiovascular events or an infectious complication. Silent ischemia, coronary artery disease, and autonomic neuropathy are common among patients with diabetes, and each can contribute to perioperative morbidity and mortality. These are important considerations since nearly one-fifth of surgical patients have diabetes and since a person with diabetes has a 50% risk of undergoing surgery at some point in his or her lifetime.1
This article reviews the preoperative evaluation of patients with diabetes, discusses the relation between glycemic control and perioperative outcomes, and examines targets and strategies for glycemic control in patients with type 1 and type 2 diabetes throughout the perioperative period.
PREOPERATIVE EVALUATION
The preoperative evaluation must consider first and foremost the status of the patient’s diabetes and his or her surgical risk factors. Also important are the characteristics of the procedure to be performed, the method of anesthesia to be used, and select laboratory values.
Diabetes status
The type of diabetes and its treatment must be considered. Type 1 diabetes requires continuous insulin therapy to prevent ketoacidosis; patients with type 2 diabetes are usually treated with oral medications with or without insulin. Baseline control of blood glucose is a predictor of morbidity following surgery. Hypoglycemia is associated with increased morbidity in the inpatient setting, so a history of severe hypoglycemic events or of difficulty recognizing hypoglycemia (hypoglycemia unawareness) should be elicited in the preoperative evaluation. Complications of diabetes and other comorbidities also must be evaluated, along with their treatments.
Surgical risk factors
Patients with diabetes have surgical risk factors specific to their health—namely, cardiovascular risk factors that may or may not have been previously diagnosed. Patients with diabetes may have silent ischemia, atypical manifestations of coronary ischemia, or underlying cardiomyopathy. Many patients with type 2 diabetes have hypertension, which may complicate perioperative management. Other common surgical risk factors in this population include obesity, chronic kidney disease, and undiagnosed autonomic dysfunction, which may compromise hemodynamic stability in the perioperative period. Additionally, patients with long-standing diabetes experience reductions in pulmonary function (eg, forced expiratory volume, peak expiratory flow, and diffusion capacity for carbon monoxide) related to disease duration and vascular injury,2 which may complicate weaning from ventilatory support.
Characteristics of the procedure and anesthetic
Both surgery and anesthesia may induce an increase in levels of stress hormones (epinephrine, cortisol, growth hormone) and inflammatory cytokines (interleukin-6 and tumor necrosis factor–alpha), resulting in insulin resistance and impaired insulin secretion (even among patients who present with adequate insulin secretion). These in turn contribute to lipolysis and protein catabolism, leading to hyperglycemia and, if a patient is severely insulin deficient, ketoacidosis. Other factors that particularly affect insulin resistance and secretion include cardiovascular bypass surgery, sepsis, the need for total parenteral nutrition, and steroid therapy.
The characteristics of the surgical procedure, including the type of surgery as well as its urgency, duration, and timing (morning vs later in the day), are important in planning for perioperative glycemic management. For example, a short, minor procedure may require only observation, whereas more extensive procedures warrant periodic monitoring and active glycemic management with insulin infusions.
The type of anesthesia should also be considered. Compared with epidural anesthesia, general anesthesia is associated with greater stimulation of the sympathetic nervous system and increased catecholamine levels, resulting in more pronounced hyperglycemia.3
Preoperative tests
Preoperative testing and laboratory evaluation should include, at minimum, an electrocardiogram, a basic metabolic panel to assess renal function, electrolyte levels, and hemoglobin A1c measurement. For low-risk procedures in patients with adequate exercise tolerance, no diagnostic tests might be needed. In any case, knowledge of the hemoglobin A1c level may help not only to classify perioperative risk but also to determine postoperative care, including the choice of antiglycemic medications at discharge.
IMPORTANCE OF GLYCEMIC CONTROL
Preoperative glycemic control has a significant impact on the risk of infectious complications—including pneumonia, wound infection, urinary tract infection, and sepsis—in patients with diabetes across a variety of surgical procedures.4 Similarly, postoperative glycemic control—to a mean blood glucose level less than 200 mg/dL in the immediate postoperative period—significantly reduces the incidence of deep sternal wound infection after open heart surgery.5
Among patients undergoing cardiothoracic surgery, both cardiac-related and overall mortality are greater with increasing postoperative blood glucose levels, although a cause-and-effect relationship has not been established.6
Glycemic control matters regardless of diabetes status
Hyperglycemia affects mortality regardless of diabetes status. In a study of 779 consecutive patients admitted for acute myocardial infarction, mortality at 180 days was highly associated with hyperglycemia on admission independent of a history of diabetes; the highest mortality was among hyperglycemic patients without previously known diabetes.7 Similarly, a large study of glycemic control in intensive care unit (ICU) patients receiving insulin found that mortality in nondiabetic patients increased with median glucose level and was higher than mortality in diabetic patients.8 These findings suggest a need for vigilance in the perioperative and critical care management of all patients with hyperglycemia, regardless of preadmission diabetes diagnosis, as they carry significant morbidity and mortality risk.
GLYCEMIC CONTROL IN THE CRITICALLY ILL: SOME SUPPORT FOR A MODIFIED TARGET, BUT VIGILANCE FOR HYPOGLYCEMIA NEEDED
The landmark study by Van den Berghe et al of intensive insulin therapy in surgical ICU patients demonstrated significant reductions in morbidity and mortality when glucose levels were controlled aggressively (80 to 110 mg/dL; average, 103 mg/dL) compared with conventional control (180 to 200 mg/dL).9 The benefit of intensive glycemic control was evident on outcomes such as the occurrence of sepsis, need for dialysis, need for blood transfusion, and development of acute polyneuropathy. Intensive insulin therapy was also associated with cost savings compared with conventional insulin therapy in mechanically ventilated patients.10
However, a number of subsequent studies have clearly shown that as blood glucose levels approach normoglycemia, the risks of hypoglycemia, especially severe hypoglycemia, can offset the benefits of tight blood glucose control.
A follow-up study by Ven den Berghe et al in a medical ICU failed to show a mortality benefit from tight glycemic control, though patients in the intensive control arm experienced less renal injury, faster weaning from ventilation, and earlier discharge from the ICU and hospital.11
The recent NICE-SUGAR study of aggressive glucose control in the ICU randomized patients to a target blood glucose of 81 to 108 mg/dL (intensive group) or 180 mg/dL or less (control group).12 At study’s end, the groups’ mean blood glucose levels were 115 mg/dL and 144 mg/dL, respectively, while rates of severe hypoglycemia (blood glucose < 40 mg/dL) were 6.8% and 0.5%, respectively. Mortality rates were higher in the intensive therapy group (27.5%) than in the control group (24.9%), driven by severe hypoglycemic events. Notably, blood glucose monitoring in this and other studies was conducted at a frequency of anywhere between 1 and 4 hours.
The conclusions of the available data would support, for the time being, a modified glycemic target in critically ill patients, with strict avoidance of severe hypoglycemia. The recent consensus statement from the American Association of Clinical Endocrinologists and the American Diabetes Association recommends using insulin therapy if blood glucose levels exceed 180 mg/dL, with target glucose levels less than 180 mg/dL in critically ill patients and less than 140 mg/dL in non–critically ill patients.13 Development and implementation of safer insulin infusion algorithms and more frequent and accurate blood glucose monitoring in this setting should enable us to achieve better glycemic targets with lower risk.
ELEMENTS OF PHYSIOLOGIC INSULIN REPLACEMENT
In hospitalized patients with hyperglycemia, three different components of insulin replacement require management1:
Basal insulin replacement consists of a long-acting insulin preparation administered regardless of the patient’s oral intake status, with the premise of matching hepatic (endogenous) glucose production
Prandial insulin replacement requires a rapid-acting insulin preparation given to cover nutritional needs
Supplemental (or correction) insulin replacement requires a rapid-acting preparation (usually the same insulin type as for prandial coverage) to correct blood glucose values that exceed predetermined glycemic targets.
For most patients, basal insulin replacement might be appropriate preoperatively to control fasting glucose, whereas during surgery, especially if prolonged or high risk, an intravenous (IV) insulin drip is the most effective means of glucose control. The postoperative transition from the IV insulin drip usually involves basal insulin replacement plus supplemental rapid-acting insulin. Prandial or nutritional insulin should be started once the patient begins to receive nutrition (oral, enteral, or hyperalimentation).
GOALS OF PERIOPERATIVE GLYCEMIC CONTROL
Perioperative glycemic management has several key objectives:
- Avoidance of clinically significant hyper- or hypoglycemia
- Maintenance of electrolyte and fluid balance
- Prevention of ketoacidosis, which is imperative in patients with type 1 diabetes, who require insulin at all times
- Achievement of specific glycemic targets, as discussed above—ie, less than 180 mg/dL in critically ill patients and less than 140 mg/dL in stable patients.13
Strategies differ across the perioperative timeline
Strategies for perioperative glycemic control differ before, during, and after surgery, as summarized immediately below and detailed in the following sections.
Preoperatively, glycemia should be stabilized, typically with subcutaneous insulin, if there is enough time to do so. For patients who have not previously been on insulin, placing them on an insulin supplemental scale to correct glycemia to desired targets might be a first step. In the setting of hyperglycemia, these patients may also be started on a low dose of basal insulin, with preference given to basal insulin analogs, given their consistent and relatively peakless action profile and lower risk of hypoglycemia. A starting dose of 0.2 to 0.4 U/kg is appropriate and carries a low risk of hypoglycemia. For patients already using insulin on an outpatient basis, continuing their basal insulin dose, possibly at a reduced dosage (25% less), together with supplemental-scale insulin coverage, should stabilize blood glucose levels. For patients on combination insulin or premixed insulin types, the basal insulin dose for preoperative management can be estimated by taking the patient’s usual total daily dose and delivering 40% to 50% of that dose as a basal insulin analog injection. Clearly, a supplemental scale should be implemented along with basal insulin replacement.
Intraoperatively, switching to IV insulin may be appropriate for stabilizing glycemia, depending on the type of surgery. A number of IV insulin protocols have been proposed, although no consistent comparisons of efficacy or safety among these protocols have been published.
Postoperatively, patients eventually should be transitioned from IV to subcutaneous insulin when glycemic control stabilizes. This transition may be complicated for many reasons. Oral intake may be inconsistent. The surgery and surrounding environment can induce stressors, promote susceptibility to infection, and increase insulin resistance. Additionally, some patients may be on hyperalimentation. Specific instructions for the transition from IV to subcutaneous insulin are covered later in this article.
PREOPERATIVE GLYCEMIC MANAGEMENT
In patients with type 2 diabetes, oral agents pose certain safety risks and should be discontinued prior to surgery.
Sulfonylureas may induce hypoglycemia in patients who are placed on NPO (“nothing by mouth”) orders and should be held in patients who are fasting.
Metformin can induce lactic acidosis if kidney function declines and should be withheld 1 to 2 days before planned surgery if a need for IV contrast is anticipated or the procedure could potentially lead to hemodynamic instability and reduced renal perfusion.
Thiazolidinediones may cause fluid retention that can complicate the postoperative period; they can be discontinued several days prior to a planned surgery.
GLP-1 agonists, such as exenatide, can slow gastric motility and potentially delay gastrointestinal recovery after major surgery; they should be held the day of surgery.
DPP-4 inhibitors (incretin enhancers), such as sitagliptin, do not have significant side effects and, if need be, can be continued. Because incretin therapies act via a glucose-dependent mechanism, they are unlikely to cause hypoglycemia, even in a patient whose oral intake is held or delayed. On the other hand, since their effect is mostly in reducing postprandial glycemia, there may be little need to use them in a patient who is NPO.
Patients with type 1 diabetes must continue basal insulin replacement preoperatively (0.2 to 0.3 U/kg/day of a long-acting insulin). Patients with type 2 diabetes may benefit from basal insulin replacement, as previously noted.
Supplemental insulin scales are used to correct hyperglycemia regardless of a patient’s oral intake status. They can be individualized based on the estimated total daily insulin dose and require glycemic targets to be established. Fingerstick glucose monitoring should be done every 4 to 6 hours in a patient who is NPO, and supplemental-scale insulin should be used to correct glucose values that exceed target. For supplemental-scale coverage, rapid-acting insulin analogs have a shorter duration of action than human regular insulin and may be given subcutaneously every 4 to 6 hours, whereas regular insulin should not be given more often than every 6 hours to correct hyperglycemia. These differences in action duration should be kept in mind to minimize the potential for insulin stacking.
INTRAOPERATIVE GLYCEMIC MANAGEMENT
Procedure length is an important determinant
Strategies for intraoperative glucose management vary according to the length of the procedure.
For minor, short procedures, the preoperative glucose management orders may be continued.
For longer, more complex procedures, a switch to an IV insulin drip is safe and allows rapid adjustments in dosing and plasma glucose levels. Ideally, IV insulin is started prior to the procedure so that the glucose level is stable once the patient arrives in the operating room. Given the logistics of IV insulin management, including the need for frequent monitoring (hourly) and dose adjustments, this type of treatment should be reserved for environments with adequate numbers of trained staff.
IV regular insulin is therapy of choice
Several different algorithms for IV regular insulin therapy are in use. Some are static, such as those of Markovitz et al14 and Stockton et al,15 while others are dynamic (ie, doses are self-adjusted based on changes in blood glucose level), such as the “Yale protocol” of Goldberg et al (Figure 1).16
POSTOPERATIVE GLYCEMIC MANAGEMENT
Start subcutaneous transition before stopping IV drip
Transitioning from IV to subcutaneous insulin is often complicated. Nonoral nutrition options (ie, parenteral nutrition or enteral supplementation) must be considered. As noted, insulin must be replaced according to physiologic needs, which requires that a long-acting basal insulin be used regardless of oral intake status, a rapid-acting insulin be given to cover prandial or nutritional needs, and supplemental rapid-acting insulin be used to correct hyperglycemia.
In the transition from IV insulin, basal insulin replacement can begin at any time. I recommend starting the transition from IV to subcutaneous insulin about 12 to 24 hours before discontinuing the insulin drip. In type 1 diabetes, this transition ensures basal insulin coverage and minimizes the risk of developing ketones and ketoacidosis. In type 2 diabetes, it can ensure a more stable transition and better glycemic control.
Determining the basal insulin dose
Switching to subcutaneous supplemental insulin
Instructions must be given for switching to subcutaneous supplemental doses of insulin. Glycemic targets, generally from less than 130 to 150 mg/dL, must be established, as must the frequency of fingerstick testing:
- If the patient is being fed enterally or parenterally, fingerstick testing is recommended every 4 to 6 hours if a rapid-acting insulin analog is used and every 6 hours if regular insulin is used.
- If the patient is eating, fingerstick testing should be performed before meals and at bedtime.
Covering nutritional requirements
Nutrition-related insulin needs depend on the type of caloric intake prescribed:
In patients receiving total parenteral nutrition (TPN), start 1 U of regular insulin (placed in the bag) for every 10 to 15 g of dextrose in the TPN mixture.
In patients receiving enteral nutrition, use regular insulin every 6 hours or a rapid-acting insulin analog every 4 hours. Start 1 U of insulin subcutaneously for every 10 to 15 g of delivered carbohydrates. For example, if a patient is receiving 10 g of carbohydrates per hour, a rapid-acting analog given at a dose of 4 U every 4 hours (1 U per 10 g of carbohydrates) should adequately cover enteral feedings. For any bolus feedings, give the injection as a full bolus 15 to 20 minutes in advance, based on the carbohydrate content of the feeding.
In patients who are eating, use regular insulin or a rapid-acting insulin analog before meals. Again, start 1 U of insulin subcutaneously for every 10 to 15 g of carbohydrates, or use the prandial portion of the Miami 4/12 rule (Figure 2). For example, in a 60-kg patient one would start with 5 U (60 ÷ 12) of a rapid-acting insulin before each meal.
Basal/bolus replacement outperforms supplemental-scale regular insulin
Use of a basal/bolus insulin regimen appears to be more beneficial than supplemental-scale regular insulin in hospitalized patients with type 2 diabetes, according to a recent randomized trial comparing the two approaches in 130 such patients with blood glucose levels greater than 140 mg/dL.17 In the group randomized to basal/bolus insulin, the starting total daily dose was 0.4 to 0.5 U/kg/day, with half the dose given as basal insulin (insulin glargine) once daily and half given as a rapid-acting insulin analog (glulisine) in fixed doses before every meal. A rapid-acting analog was used for supplemental insulin in the basal/bolus regimen. By study’s end, patients in the basal/bolus group were receiving a higher total daily insulin dose than those in the supplemental-scale group (mean of 42 U/day vs 13 U/day).
Mean daily blood glucose levels were 27 mg/dL lower, on average, in patients who received basal/bolus therapy compared with the supplemental-scale group, yet there was no difference between groups in the risk of hypoglycemia. More patients randomized to basal/bolus therapy achieved the glycemic goal of less than 140 mg/dL (66% vs 38%). Fourteen percent of patients assigned to supplemental-scale insulin had values persistently greater than 240 mg/dL and had to be switched to the basal/bolus regimen.17
SUMMARY
Perioperative glycemic control can reduce morbidity, particularly the incidence of infectious complications, in surgical patients, even in those without diagnosed diabetes. Optimal management of glycemia in the perioperative period involves applying principles of physiologic insulin replacement. Postoperatively, the transition from IV to subcutaneous insulin can be achieved through the use of basal insulin for coverage of fasting insulin needs, regardless of the patient’s feeding status, and the use of rapid-acting insulin to cover hyperglycemia and nutritional needs. Management of hospitalized patients exclusively with supplemental-scale regular insulin should be abandoned.
DISCUSSION
Question from the audience: As an attending physician in a preoperative clinic I’m never sure what to do with NPH insulin the morning of surgery. What guidance can you give?
Dr. Meneghini: NPH is a peaking basal insulin, and the peak can induce hypoglycemia in a patient who is NPO. If we have the opportunity, we try to switch patients previously receiving insulin therapy to a long-acting basal insulin analog, which has a much flatter action profile and is safer in the fasting state. If there is no opportunity for switching, we instruct the patient to take two-thirds of his or her usual morning dose of insulin and we initiate a D5 drip when the patient arrives at the hospital.
Question from the audience: How do you handle perioperative insulin in patients on insulin pumps?
Dr. Meneghini: The pumps provide a subcutaneous basal insulin infusion, which should, if set correctly, maintain stable blood glucose levels when the patient is NPO. Supplemental doses of insulin to correct hyperglycemia can be delivered via the usual subcutaneous practice with a syringe or insulin pen. If you are uncomfortable with pump function, or if the pump insertion site interferes with the surgery site, simply replace the 24-hour basal amount delivered via pump with an injection of glargine or detemir divided into twice-daily injections. Correct hyperglycemia with supplemental-scale insulin as per usual protocol.
Question from the audience: The manufacturer of insulin glargine makes no recommendations for its use the night before or morning of surgery. What do you recommend?
Dr. Meneghini: It depends on whether the glargine is dosed appropriately. Most patients with type 2 diabetes require 0.4 to 0.6 U/kg/day of a long-acting insulin. If they’re on much more, they may be overdosed, and I would cut the basal dose by about half. Otherwise, 75% to 100% of the usual basal amount is appropriate. In type 1 diabetes, the usual replacement dose of basal insulin is 0.2 to 0.3 U/kg/day. If a patient is in this range, the basal insulin can be continued. Patients who experience hypoglycemia, or a substantial fall in blood glucose if meals are skipped or delayed, may be getting too much basal insulin and might benefit from a dose reduction when placed on NPO status.
Question from the audience: Metformin has a black-box warning advising that it be stopped at least 48 hours before surgery, but patients often come to surgery having taken metformin within the prior 12 to 24 hours. How should we manage such patients coming for elective surgery?
Dr. Meneghini: Metformin is cleared exclusively by the kidneys; its accumulation as a result of impaired kidney function (eg, due to hemodynamic instability or radiology studies using IV iodine) can result in increased lactic acid production by the liver and lactic acidosis. A patient who has taken metformin within the prior 48 hours but doesn’t have a risk of hemodynamic dysfunction is at low risk of lactic acidosis if hydrated appropriately. There’s not much choice if a patient needs urgent surgery and has recently taken metformin; in that case, just ensure maintenance of adequate glomerular filtration via fluid repletion to clear the drug.
Question from the audience: What’s the evidence for tight glycemic control or any type of glycemic control in patients undergoing outpatient surgery or “same-day” patients who will be admitted to a regular surgical floor? Also, what would you consider maximal glucose values for a patient going into elective surgery?
Dr. Meneghini: I haven’t seen any guidelines for glycemic control in patients undergoing outpatient surgery. If a patient has poor glycemic control coming into surgery, even for a minor procedure, the risk of an infectious complication may be increased. Keeping blood glucose below 180 mg/dL and avoiding electrolyte imbalances is likely sufficient in such patients. On the second question, if it’s an elective procedure and can be delayed a few hours, you can certainly institute IV insulin therapy to correct hyperglycemia rapidly—just ensure adequate replacement of fluids since the patient may have had volume depletion or dehydration as a result of the preceding osmotic diuresis. Once glycemic control is improved (blood glucose < 180–200 mg/dL), the patient can proceed to surgery.
Question from the audience: What are your recommendations for resuming oral diabetes medications after surgery?
Dr. Meneghini: Once patients are tolerating their meals and being considered for discharge, you may want to resume their oral medications, assuming their admission hemoglobin A1c levels were near goal. If glycemic control was inadequate preoperatively, this may be a good opportunity to adjust their prior regimen to more appropriate therapy. In some cases, this might include some form of insulin, either basal therapy or basal and supplemental insulin.
- Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004; 27:553–591.
- Kaparianos A, Argyropoulou E, Sampsonas F, et al. Pulmonary complications in diabetes mellitus. Chron Respir Dis 2008; 5:101–108.
- Grigoleit HG. Anesthesia and blood glucose. Acta Diabetologica 1973; 10:569–574.
- Dronge AS, Perkal MF, Kancir S, et al. Long-term glycemic control and postoperative infectious complications. Arch Surg 2006; 141:375–380.
- Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg 1997; 63:356–361.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003; 125:1007–10021.
- Ainla T, Baburin A, Tessalu R, et al. The association between hyperglycaemia on admission and 180-day mortality in acute myocardial infarction patients with and without diabetes. Diabet Med 2005; 22:1321–1325.
- Rady MY, Johnson DJ, Patel BM, et al. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc 2005; 80:1558–1567.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Van den Berghe G, Wouters PJ, Kesteloot K, Hilleman DE. Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med 2006; 34:612–616.
- Van den Berghe G, Wawilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. New Engl J Med 2006; 354:449–461.
- NICE-SUGAR Study Investigators. Intensive vs conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Moghissi ES, Korytowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- Markovitz LJ, Wiechmann RJ, Harris N, et al. Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery. Endocr Pract 2002; 8:10–18.
- Stockton L, Baird M, Cook CB, et al. Development and implementation of evidence-based guidelines for IV insulin: a statewide collaborative approach. Insulin 2008; 3:67–77.
- Goldberg PA, Siegel MD, Sherwin RS, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care 2004; 27:461–467.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
- Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004; 27:553–591.
- Kaparianos A, Argyropoulou E, Sampsonas F, et al. Pulmonary complications in diabetes mellitus. Chron Respir Dis 2008; 5:101–108.
- Grigoleit HG. Anesthesia and blood glucose. Acta Diabetologica 1973; 10:569–574.
- Dronge AS, Perkal MF, Kancir S, et al. Long-term glycemic control and postoperative infectious complications. Arch Surg 2006; 141:375–380.
- Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg 1997; 63:356–361.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003; 125:1007–10021.
- Ainla T, Baburin A, Tessalu R, et al. The association between hyperglycaemia on admission and 180-day mortality in acute myocardial infarction patients with and without diabetes. Diabet Med 2005; 22:1321–1325.
- Rady MY, Johnson DJ, Patel BM, et al. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc 2005; 80:1558–1567.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Van den Berghe G, Wouters PJ, Kesteloot K, Hilleman DE. Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med 2006; 34:612–616.
- Van den Berghe G, Wawilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. New Engl J Med 2006; 354:449–461.
- NICE-SUGAR Study Investigators. Intensive vs conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Moghissi ES, Korytowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009; 15:353–369.
- Markovitz LJ, Wiechmann RJ, Harris N, et al. Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery. Endocr Pract 2002; 8:10–18.
- Stockton L, Baird M, Cook CB, et al. Development and implementation of evidence-based guidelines for IV insulin: a statewide collaborative approach. Insulin 2008; 3:67–77.
- Goldberg PA, Siegel MD, Sherwin RS, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care 2004; 27:461–467.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
KEY POINTS
- Surgery and anesthesia can induce hormonal and inflammatory stressors that increase the risk of complications in patients with diabetes.
- Elevated blood glucose levels are associated with worse outcomes in surgical patients, even among those not diagnosed with diabetes.
- The perioperative glycemic target in critically ill patients is 140 to 180 mg/dL. Evidence for a target in patients who are not critically ill is less robust, though fasting levels less than 140 mg/dL and random levels less than 180 mg/dL are appropriate.
- Postoperative nutrition-related insulin needs vary by nutrition type (parenteral or enteral), but ideally all regimens should incorporate a basal/bolus approach to insulin replacement.