User login
Morbidly adherent placenta: A multidisciplinary approach
The rate of placenta accreta has been rising, almost certainly as a consequence of the increasing cesarean delivery rate. It is estimated that morbidly adherent placenta (placenta accreta, increta, and percreta) occurs today in approximately 1 in 500 pregnancies. Women who have had prior cesarean deliveries or other uterine surgery, such as myomectomy, are at higher risk.
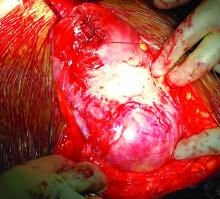
Morbidly adherent placenta (MAP) is associated with significant hemorrhage and morbidity – not only in cases of attempted placental removal, which is usually not advisable, but also in cases of cesarean hysterectomy. Cesarean hysterectomy is technically complex and completely different from other hysterectomies. The abnormal vasculature of MAP requires intricate, stepwise, vessel-by-vessel dissection and not only the uterine artery ligation that is the focus in hysterectomies performed for other indications.
In the last several years, we have demonstrated improved outcomes with such an approach at the University of Maryland, Baltimore. In 2014, we instituted a multidisciplinary complex obstetric surgery program for patients with MAP and others at high risk of intrapartum and postpartum complications. The program brings together obstetric anesthesiologists, the blood bank staff, the neonatal and surgical intensive care unit staff, vascular surgeons, perinatologists, interventional radiologists, urologists, and others.
Since the program was implemented, we have reduced our transfusion rate in patients with MAP by more than 60% while caring for increasing numbers of patients with the condition. We also have reduced the intensive care unit admission rate and improved overall surgical morbidity, including bladder complications. Moreover, our multidisciplinary approach is allowing us to develop more algorithms for management and to selectively take conservative approaches while also allowing us to lay the groundwork for future research.
The patients at risk
Anticipation is important: Identifying patient populations at high risk – and then evaluating individual risks – is essential for the prevention of delivery complications and the reduction of maternal morbidity.
Having had multiple cesarean deliveries – especially in pregnancies involving placenta previa – is one of the most important risk factors for developing MAP. One prospective cohort study of more than 30,000 women in 19 academic centers who had had cesarean deliveries found that, in cases of placenta previa, the risk of placenta accreta went from 3% after one cesarean delivery to 67% after five or more cesarean deliveries (Obstet Gynecol. 2006 Jun;107[6]:1226-32). Placenta accreta was defined in this study as the placenta’s being adherent to the uterine wall without easy separation. This definition included all forms of MAP.
Even without a history of placenta previa, patients who have had multiple cesarean deliveries – and developed consequent myometrial damage and scarring – should be evaluated for placental location during future pregnancies, as should patients who have had a myomectomy. A placenta that is anteriorly located in a patient who had a prior classical cesarean incision should also be thoroughly investigated. Overall, there is a risk of MAP whenever the placenta attaches to an area of uterine scarring.
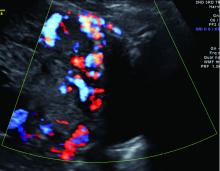
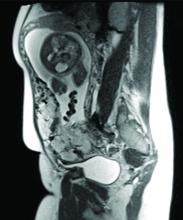
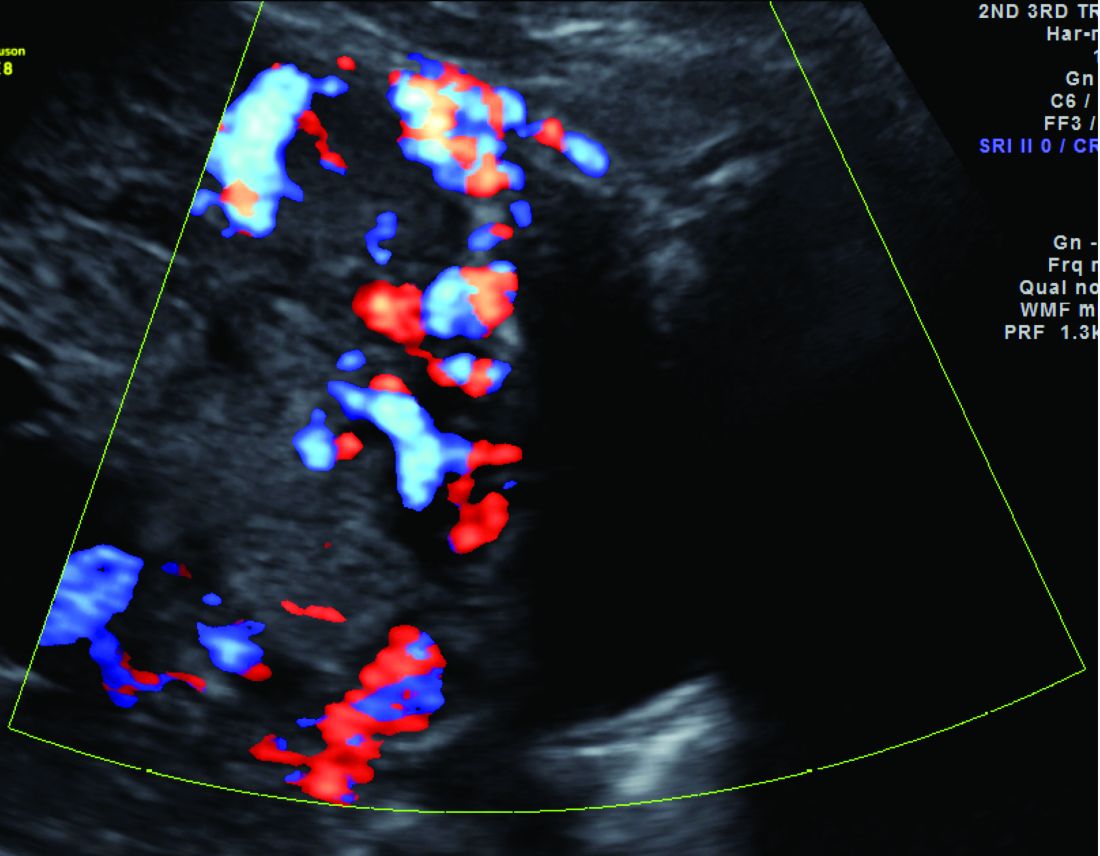
Diagnosis of MAP can be made – as best as is currently possible – by ultrasonography or by MRI, the latter of which is performed in high-risk or ambiguous cases to look more closely at the depth of placental growth.
Our outcomes and process
In our complex obstetric surgery program, we identify and evaluate patients at risk for developing MAP and also prepare comprehensive surgical plans. Each individual’s plan addresses the optimal timing of and conditions for delivery, how the patient and the team should prepare for high-quality perioperative care, and how possible complications and emergency surgery should be handled, such as who should be called in the case of emergency preterm delivery.
Indeed, research has shown that the value of a multidisciplinary approach is greatest when MAP is identified or suspected before delivery. For instance, investigators who analyzed the pregnancies complicated by placenta accreta in Utah over a 12-year period found that cases managed by a multidisciplinary care team had a 50% risk reduction for early morbidities, compared with cases managed with standard obstetric care. The benefits were even greater when placenta accreta (defined in the study to include the spectrum of MAP) was suspected before delivery; this group had a nearly 80% risk reduction with multidisciplinary care (Obstet Gynecol. 2011 Feb;117[2 Pt 1]:331-7).
We recently compared our outcomes before and after the multidisciplinary complex obstetric surgery program was established. For patients with MAP, estimated blood loss has decreased by 40%, and the use of blood products has fallen by 60%-70%, with a corresponding reduction in intensive care unit admission. Moreover, our bladder complication rate fell to 6% after program implementation. This and our reoperation rate, among other outcomes, are lower than published rates from other similar medical centers that use a multidisciplinary approach.
We strive to have two surgeons in the operating room – either two senior surgeons or one senior surgeon and one junior surgeon – as well as a separate “operation supervisor” who monitors blood loss (volume and sources), vital signs, and other clinical points and who is continually thinking about next steps. The operation supervisor is not necessarily a third surgeon but could be an experienced surgical nurse or an obstetric anesthesiologist.
Obstetric anesthesiologists and the blood bank staff have proven to be especially important parts of our multidisciplinary team. At 28-30 weeks’ gestation, each patient has an anesthesia consult and also is tested for blood type and screened for antibodies. Patients also are tested for anemia at this time so that it may be corrected if necessary before surgery.
As determined by our multidisciplinary team, all deliveries are performed under general anesthesia, with early placement of both a central venous catheter and a peripheral arterial line to enable rapid transfusions of blood or fluid. Patients are routinely placed in the dorsal lithotomy position, which enables direct access to the vagina and better assessment of vaginal bleeding. And, when significant blood loss is anticipated, the intensive care unit team prepares a bed, and our surgical colleagues are alerted.
Conservative management
Interest in conservative management – in avoiding hysterectomy when it is deemed to carry much higher risks of hemorrhage or injury to adjacent tissue than leaving the placenta in situ – has resurged in Europe. However, research is still in its infancy regarding the benefits and safety of conservative management, and clear guidance about eligibility and contraindications is still needed (Am J Obstet Gynecol. 2015 Dec;213[6]:755-60).
One patient with the placenta left in situ had an urgent hysterectomy within 2 hours of delivery because of vaginal bleeding, with the total blood loss within an acceptable range and without complications. Another required an urgent hysterectomy 6 weeks after delivery because of severe hemorrhaging. The remaining two had nonurgent hysterectomies at least 6 weeks later, with the total blood loss minimized by the period of recovery and by some spontaneous regression of the placental bulk.
As we have gained more experience with conservative management and spent more time shaping multidisciplinary protocols, it has become clear to us that programs must have in place excellent protocols and strict rules for monitoring and follow-up given the risks of life-threatening hemorrhage and other significant complications when the placenta is left in situ.
A conservative approach also may be preferred by women who desire fertility preservation. Currently, in such cases, we have performed segmental or local resection with uterine repair. We do not yet have any data on subsequent pregnancies.
Research conducted within the growing sphere of complex obstetric surgery should help us to improve decision making and management of MAP. For instance, we need better imaging techniques to more accurately predict MAP and show us the degree of placental invasion. A study published several years ago that blinded sonographers from information about patients’ clinical history and risk factors found significant interobserver variability for the diagnosis of placenta accreta and sensitivity (53.5%) that was significantly lower than previously described (J Ultrasound Med. 2014 Dec;33[12]:2153-8).
Dr. Turan’s stepwise dissection
In addition to a multidisciplinary approach, a meticulous dissection technique can help drive improved outcomes. The morbidly adherent placenta is a hypervascular organ; it recruits a host of blood vessels, largely from the vaginal arteries, superior vesical arteries, and vaginal venous plexus.
Moreover, in most cases, this vascular remodeling exacerbates vascular patterns that are distorted to begin with as a result of the scarring process following previous uterine surgery. Scarred tissue is already hypervascular.
I have found that most of the blood loss during hysterectomy occurs during dissection of the poorly defined interface between the lower uterine segment and the bladder and not during dissection of the uterine artery. Identification of the cleavage plane and ligation of each individual vessel using a bipolar or small hand-held desiccation device are key in reducing blood loss. This can take a significant amount of time but is well worth it.
Managing super morbid obesity
The number of pregnant women who require challenging obstetric surgeries is increasing, and this includes women with super morbid obesity (BMI greater than 50 kg/m2 or weight greater than 350 lb). Cesarean deliveries for these patients have proven to be much more complicated, involving special anesthesia needs, for instance.
In addition to women with placental implantation abnormalities (MAP and placenta previa, for instance) and those with extreme morbid obesity, the complex obstetric surgery program also aims to manage patients with increased risk for surgical morbidities based on previous surgery, patients whose fetuses require ex utero intrapartum treatment, and women who require abdominal cerclage.
Dr. Turan is director of fetal therapy and complex obstetric surgery at the University of Maryland, Baltimore, as well as an associate professor of obstetrics, gynecology, and reproductive sciences. He reported having no relevant financial disclosures.
The rate of placenta accreta has been rising, almost certainly as a consequence of the increasing cesarean delivery rate. It is estimated that morbidly adherent placenta (placenta accreta, increta, and percreta) occurs today in approximately 1 in 500 pregnancies. Women who have had prior cesarean deliveries or other uterine surgery, such as myomectomy, are at higher risk.
Morbidly adherent placenta (MAP) is associated with significant hemorrhage and morbidity – not only in cases of attempted placental removal, which is usually not advisable, but also in cases of cesarean hysterectomy. Cesarean hysterectomy is technically complex and completely different from other hysterectomies. The abnormal vasculature of MAP requires intricate, stepwise, vessel-by-vessel dissection and not only the uterine artery ligation that is the focus in hysterectomies performed for other indications.
In the last several years, we have demonstrated improved outcomes with such an approach at the University of Maryland, Baltimore. In 2014, we instituted a multidisciplinary complex obstetric surgery program for patients with MAP and others at high risk of intrapartum and postpartum complications. The program brings together obstetric anesthesiologists, the blood bank staff, the neonatal and surgical intensive care unit staff, vascular surgeons, perinatologists, interventional radiologists, urologists, and others.
Since the program was implemented, we have reduced our transfusion rate in patients with MAP by more than 60% while caring for increasing numbers of patients with the condition. We also have reduced the intensive care unit admission rate and improved overall surgical morbidity, including bladder complications. Moreover, our multidisciplinary approach is allowing us to develop more algorithms for management and to selectively take conservative approaches while also allowing us to lay the groundwork for future research.
The patients at risk
Anticipation is important: Identifying patient populations at high risk – and then evaluating individual risks – is essential for the prevention of delivery complications and the reduction of maternal morbidity.
Having had multiple cesarean deliveries – especially in pregnancies involving placenta previa – is one of the most important risk factors for developing MAP. One prospective cohort study of more than 30,000 women in 19 academic centers who had had cesarean deliveries found that, in cases of placenta previa, the risk of placenta accreta went from 3% after one cesarean delivery to 67% after five or more cesarean deliveries (Obstet Gynecol. 2006 Jun;107[6]:1226-32). Placenta accreta was defined in this study as the placenta’s being adherent to the uterine wall without easy separation. This definition included all forms of MAP.
Even without a history of placenta previa, patients who have had multiple cesarean deliveries – and developed consequent myometrial damage and scarring – should be evaluated for placental location during future pregnancies, as should patients who have had a myomectomy. A placenta that is anteriorly located in a patient who had a prior classical cesarean incision should also be thoroughly investigated. Overall, there is a risk of MAP whenever the placenta attaches to an area of uterine scarring.
Diagnosis of MAP can be made – as best as is currently possible – by ultrasonography or by MRI, the latter of which is performed in high-risk or ambiguous cases to look more closely at the depth of placental growth.
Our outcomes and process
In our complex obstetric surgery program, we identify and evaluate patients at risk for developing MAP and also prepare comprehensive surgical plans. Each individual’s plan addresses the optimal timing of and conditions for delivery, how the patient and the team should prepare for high-quality perioperative care, and how possible complications and emergency surgery should be handled, such as who should be called in the case of emergency preterm delivery.
Indeed, research has shown that the value of a multidisciplinary approach is greatest when MAP is identified or suspected before delivery. For instance, investigators who analyzed the pregnancies complicated by placenta accreta in Utah over a 12-year period found that cases managed by a multidisciplinary care team had a 50% risk reduction for early morbidities, compared with cases managed with standard obstetric care. The benefits were even greater when placenta accreta (defined in the study to include the spectrum of MAP) was suspected before delivery; this group had a nearly 80% risk reduction with multidisciplinary care (Obstet Gynecol. 2011 Feb;117[2 Pt 1]:331-7).
We recently compared our outcomes before and after the multidisciplinary complex obstetric surgery program was established. For patients with MAP, estimated blood loss has decreased by 40%, and the use of blood products has fallen by 60%-70%, with a corresponding reduction in intensive care unit admission. Moreover, our bladder complication rate fell to 6% after program implementation. This and our reoperation rate, among other outcomes, are lower than published rates from other similar medical centers that use a multidisciplinary approach.
We strive to have two surgeons in the operating room – either two senior surgeons or one senior surgeon and one junior surgeon – as well as a separate “operation supervisor” who monitors blood loss (volume and sources), vital signs, and other clinical points and who is continually thinking about next steps. The operation supervisor is not necessarily a third surgeon but could be an experienced surgical nurse or an obstetric anesthesiologist.
Obstetric anesthesiologists and the blood bank staff have proven to be especially important parts of our multidisciplinary team. At 28-30 weeks’ gestation, each patient has an anesthesia consult and also is tested for blood type and screened for antibodies. Patients also are tested for anemia at this time so that it may be corrected if necessary before surgery.
As determined by our multidisciplinary team, all deliveries are performed under general anesthesia, with early placement of both a central venous catheter and a peripheral arterial line to enable rapid transfusions of blood or fluid. Patients are routinely placed in the dorsal lithotomy position, which enables direct access to the vagina and better assessment of vaginal bleeding. And, when significant blood loss is anticipated, the intensive care unit team prepares a bed, and our surgical colleagues are alerted.
Conservative management
Interest in conservative management – in avoiding hysterectomy when it is deemed to carry much higher risks of hemorrhage or injury to adjacent tissue than leaving the placenta in situ – has resurged in Europe. However, research is still in its infancy regarding the benefits and safety of conservative management, and clear guidance about eligibility and contraindications is still needed (Am J Obstet Gynecol. 2015 Dec;213[6]:755-60).
One patient with the placenta left in situ had an urgent hysterectomy within 2 hours of delivery because of vaginal bleeding, with the total blood loss within an acceptable range and without complications. Another required an urgent hysterectomy 6 weeks after delivery because of severe hemorrhaging. The remaining two had nonurgent hysterectomies at least 6 weeks later, with the total blood loss minimized by the period of recovery and by some spontaneous regression of the placental bulk.
As we have gained more experience with conservative management and spent more time shaping multidisciplinary protocols, it has become clear to us that programs must have in place excellent protocols and strict rules for monitoring and follow-up given the risks of life-threatening hemorrhage and other significant complications when the placenta is left in situ.
A conservative approach also may be preferred by women who desire fertility preservation. Currently, in such cases, we have performed segmental or local resection with uterine repair. We do not yet have any data on subsequent pregnancies.
Research conducted within the growing sphere of complex obstetric surgery should help us to improve decision making and management of MAP. For instance, we need better imaging techniques to more accurately predict MAP and show us the degree of placental invasion. A study published several years ago that blinded sonographers from information about patients’ clinical history and risk factors found significant interobserver variability for the diagnosis of placenta accreta and sensitivity (53.5%) that was significantly lower than previously described (J Ultrasound Med. 2014 Dec;33[12]:2153-8).
Dr. Turan’s stepwise dissection
In addition to a multidisciplinary approach, a meticulous dissection technique can help drive improved outcomes. The morbidly adherent placenta is a hypervascular organ; it recruits a host of blood vessels, largely from the vaginal arteries, superior vesical arteries, and vaginal venous plexus.
Moreover, in most cases, this vascular remodeling exacerbates vascular patterns that are distorted to begin with as a result of the scarring process following previous uterine surgery. Scarred tissue is already hypervascular.
I have found that most of the blood loss during hysterectomy occurs during dissection of the poorly defined interface between the lower uterine segment and the bladder and not during dissection of the uterine artery. Identification of the cleavage plane and ligation of each individual vessel using a bipolar or small hand-held desiccation device are key in reducing blood loss. This can take a significant amount of time but is well worth it.
Managing super morbid obesity
The number of pregnant women who require challenging obstetric surgeries is increasing, and this includes women with super morbid obesity (BMI greater than 50 kg/m2 or weight greater than 350 lb). Cesarean deliveries for these patients have proven to be much more complicated, involving special anesthesia needs, for instance.
In addition to women with placental implantation abnormalities (MAP and placenta previa, for instance) and those with extreme morbid obesity, the complex obstetric surgery program also aims to manage patients with increased risk for surgical morbidities based on previous surgery, patients whose fetuses require ex utero intrapartum treatment, and women who require abdominal cerclage.
Dr. Turan is director of fetal therapy and complex obstetric surgery at the University of Maryland, Baltimore, as well as an associate professor of obstetrics, gynecology, and reproductive sciences. He reported having no relevant financial disclosures.
The rate of placenta accreta has been rising, almost certainly as a consequence of the increasing cesarean delivery rate. It is estimated that morbidly adherent placenta (placenta accreta, increta, and percreta) occurs today in approximately 1 in 500 pregnancies. Women who have had prior cesarean deliveries or other uterine surgery, such as myomectomy, are at higher risk.
Morbidly adherent placenta (MAP) is associated with significant hemorrhage and morbidity – not only in cases of attempted placental removal, which is usually not advisable, but also in cases of cesarean hysterectomy. Cesarean hysterectomy is technically complex and completely different from other hysterectomies. The abnormal vasculature of MAP requires intricate, stepwise, vessel-by-vessel dissection and not only the uterine artery ligation that is the focus in hysterectomies performed for other indications.
In the last several years, we have demonstrated improved outcomes with such an approach at the University of Maryland, Baltimore. In 2014, we instituted a multidisciplinary complex obstetric surgery program for patients with MAP and others at high risk of intrapartum and postpartum complications. The program brings together obstetric anesthesiologists, the blood bank staff, the neonatal and surgical intensive care unit staff, vascular surgeons, perinatologists, interventional radiologists, urologists, and others.
Since the program was implemented, we have reduced our transfusion rate in patients with MAP by more than 60% while caring for increasing numbers of patients with the condition. We also have reduced the intensive care unit admission rate and improved overall surgical morbidity, including bladder complications. Moreover, our multidisciplinary approach is allowing us to develop more algorithms for management and to selectively take conservative approaches while also allowing us to lay the groundwork for future research.
The patients at risk
Anticipation is important: Identifying patient populations at high risk – and then evaluating individual risks – is essential for the prevention of delivery complications and the reduction of maternal morbidity.
Having had multiple cesarean deliveries – especially in pregnancies involving placenta previa – is one of the most important risk factors for developing MAP. One prospective cohort study of more than 30,000 women in 19 academic centers who had had cesarean deliveries found that, in cases of placenta previa, the risk of placenta accreta went from 3% after one cesarean delivery to 67% after five or more cesarean deliveries (Obstet Gynecol. 2006 Jun;107[6]:1226-32). Placenta accreta was defined in this study as the placenta’s being adherent to the uterine wall without easy separation. This definition included all forms of MAP.
Even without a history of placenta previa, patients who have had multiple cesarean deliveries – and developed consequent myometrial damage and scarring – should be evaluated for placental location during future pregnancies, as should patients who have had a myomectomy. A placenta that is anteriorly located in a patient who had a prior classical cesarean incision should also be thoroughly investigated. Overall, there is a risk of MAP whenever the placenta attaches to an area of uterine scarring.
Diagnosis of MAP can be made – as best as is currently possible – by ultrasonography or by MRI, the latter of which is performed in high-risk or ambiguous cases to look more closely at the depth of placental growth.
Our outcomes and process
In our complex obstetric surgery program, we identify and evaluate patients at risk for developing MAP and also prepare comprehensive surgical plans. Each individual’s plan addresses the optimal timing of and conditions for delivery, how the patient and the team should prepare for high-quality perioperative care, and how possible complications and emergency surgery should be handled, such as who should be called in the case of emergency preterm delivery.
Indeed, research has shown that the value of a multidisciplinary approach is greatest when MAP is identified or suspected before delivery. For instance, investigators who analyzed the pregnancies complicated by placenta accreta in Utah over a 12-year period found that cases managed by a multidisciplinary care team had a 50% risk reduction for early morbidities, compared with cases managed with standard obstetric care. The benefits were even greater when placenta accreta (defined in the study to include the spectrum of MAP) was suspected before delivery; this group had a nearly 80% risk reduction with multidisciplinary care (Obstet Gynecol. 2011 Feb;117[2 Pt 1]:331-7).
We recently compared our outcomes before and after the multidisciplinary complex obstetric surgery program was established. For patients with MAP, estimated blood loss has decreased by 40%, and the use of blood products has fallen by 60%-70%, with a corresponding reduction in intensive care unit admission. Moreover, our bladder complication rate fell to 6% after program implementation. This and our reoperation rate, among other outcomes, are lower than published rates from other similar medical centers that use a multidisciplinary approach.
We strive to have two surgeons in the operating room – either two senior surgeons or one senior surgeon and one junior surgeon – as well as a separate “operation supervisor” who monitors blood loss (volume and sources), vital signs, and other clinical points and who is continually thinking about next steps. The operation supervisor is not necessarily a third surgeon but could be an experienced surgical nurse or an obstetric anesthesiologist.
Obstetric anesthesiologists and the blood bank staff have proven to be especially important parts of our multidisciplinary team. At 28-30 weeks’ gestation, each patient has an anesthesia consult and also is tested for blood type and screened for antibodies. Patients also are tested for anemia at this time so that it may be corrected if necessary before surgery.
As determined by our multidisciplinary team, all deliveries are performed under general anesthesia, with early placement of both a central venous catheter and a peripheral arterial line to enable rapid transfusions of blood or fluid. Patients are routinely placed in the dorsal lithotomy position, which enables direct access to the vagina and better assessment of vaginal bleeding. And, when significant blood loss is anticipated, the intensive care unit team prepares a bed, and our surgical colleagues are alerted.
Conservative management
Interest in conservative management – in avoiding hysterectomy when it is deemed to carry much higher risks of hemorrhage or injury to adjacent tissue than leaving the placenta in situ – has resurged in Europe. However, research is still in its infancy regarding the benefits and safety of conservative management, and clear guidance about eligibility and contraindications is still needed (Am J Obstet Gynecol. 2015 Dec;213[6]:755-60).
One patient with the placenta left in situ had an urgent hysterectomy within 2 hours of delivery because of vaginal bleeding, with the total blood loss within an acceptable range and without complications. Another required an urgent hysterectomy 6 weeks after delivery because of severe hemorrhaging. The remaining two had nonurgent hysterectomies at least 6 weeks later, with the total blood loss minimized by the period of recovery and by some spontaneous regression of the placental bulk.
As we have gained more experience with conservative management and spent more time shaping multidisciplinary protocols, it has become clear to us that programs must have in place excellent protocols and strict rules for monitoring and follow-up given the risks of life-threatening hemorrhage and other significant complications when the placenta is left in situ.
A conservative approach also may be preferred by women who desire fertility preservation. Currently, in such cases, we have performed segmental or local resection with uterine repair. We do not yet have any data on subsequent pregnancies.
Research conducted within the growing sphere of complex obstetric surgery should help us to improve decision making and management of MAP. For instance, we need better imaging techniques to more accurately predict MAP and show us the degree of placental invasion. A study published several years ago that blinded sonographers from information about patients’ clinical history and risk factors found significant interobserver variability for the diagnosis of placenta accreta and sensitivity (53.5%) that was significantly lower than previously described (J Ultrasound Med. 2014 Dec;33[12]:2153-8).
Dr. Turan’s stepwise dissection
In addition to a multidisciplinary approach, a meticulous dissection technique can help drive improved outcomes. The morbidly adherent placenta is a hypervascular organ; it recruits a host of blood vessels, largely from the vaginal arteries, superior vesical arteries, and vaginal venous plexus.
Moreover, in most cases, this vascular remodeling exacerbates vascular patterns that are distorted to begin with as a result of the scarring process following previous uterine surgery. Scarred tissue is already hypervascular.
I have found that most of the blood loss during hysterectomy occurs during dissection of the poorly defined interface between the lower uterine segment and the bladder and not during dissection of the uterine artery. Identification of the cleavage plane and ligation of each individual vessel using a bipolar or small hand-held desiccation device are key in reducing blood loss. This can take a significant amount of time but is well worth it.
Managing super morbid obesity
The number of pregnant women who require challenging obstetric surgeries is increasing, and this includes women with super morbid obesity (BMI greater than 50 kg/m2 or weight greater than 350 lb). Cesarean deliveries for these patients have proven to be much more complicated, involving special anesthesia needs, for instance.
In addition to women with placental implantation abnormalities (MAP and placenta previa, for instance) and those with extreme morbid obesity, the complex obstetric surgery program also aims to manage patients with increased risk for surgical morbidities based on previous surgery, patients whose fetuses require ex utero intrapartum treatment, and women who require abdominal cerclage.
Dr. Turan is director of fetal therapy and complex obstetric surgery at the University of Maryland, Baltimore, as well as an associate professor of obstetrics, gynecology, and reproductive sciences. He reported having no relevant financial disclosures.
Detecting and managing monochorionic twin complications
Approximately 20% of all twin pregnancies are monochorionic, with the fetuses sharing a single placenta. Although the majority of these pregnancies are uncomplicated, monochorionic twins are significantly more likely than dichorionic twins to incur complications that can threaten the life and health of one or both fetuses.
The death of one monochorionic twin leaves the other twin with a 15% risk of demise. Survival after the loss of a co-twin is also associated with a 25% incidence of neurologic injury, compared with a 2% incidence in dichorionic pregnancies. Additionally, monochorionic pregnancies carry the risk of unique complications such as twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion.
Increased ultrasonographic surveillance recommended for monochorionic twin pregnancies has been outlined in a recent consensus statement from the North American Fetal Therapy Network (Obstet Gynecol. 2015 Jan;125[1]:118-23). Beginning at 16 weeks’ gestation, monochorionic twins should be assessed every 2 weeks using amniotic fluid balance, presence/absence of fluid within the fetal bladder, and with fetal Doppler (umbilical artery, middle cerebral artery, and ductus venosus) studies. Fetal growth should also be assessed at least every 4 weeks.
Since monochorionic twins are at increased risk for congenital heart disease, echocardiography is also performed between 18 and 22 weeks, with surveillance intervals of 2 weeks or shorter if potential complications are identified. Early detection of these and other complications allows for earlier intervention, earlier referral if necessary, and potentially better outcomes.
Twin-to-twin transfusion syndrome
Twin-to-twin transfusion syndrome (TTTS) is one of the most common and most serious complications, affecting approximately 10% of monochorionic pregnancies. Significant imbalances in blood-flow exchange lead to progressive cardiovascular decompensation that causes one twin to become a “donor” of blood volume, and the other twin to become a “recipient.” Without proper treatment between 16 and 26 weeks’ gestation, the perinatal mortality rate has been estimated to be 70% or higher.
Disease severity is classified according to the Quintero staging system. Stage I is characterized by amniotic fluid discordance. In stage II, the bladder of the donor twin is no longer visible sonographically. Stage III is marked by critically abnormal Doppler waveforms in either twin (absent/reverse end-diastolic velocity in the umbilical artery, reverse flow in the ductus venosus, or pulsatile flow in the umbilical vein). In stage IV, one of the twins has developed hydrops, and stage V is characterized by the death of one or both of the twins.
Amnioreduction to decrease intra-amniotic pressure had been the treatment of choice until a randomized controlled trial, published in 2004, demonstrated that fetoscopic laser coagulation of anastomoses was superior as a first-line treatment for severe TTTS that is diagnosed before 26 weeks. Perinatal mortality and morbidity were significantly lower after the laser treatment (N Engl J Med. 2004 Jul 8;351[2]:136-44).
Outcomes were further improved over the next decade as the laser surgery technique was modified to cover the entire vascular equator rather than selective components of the vasculature. In an open-label randomized controlled trial comparing the two approaches for severe TTTS, fetoscopic laser coagulation of the vascular equator (known as the Solomon technique) reduced the risk of twin anemia polycythemia sequence and recurrence of TTTS – the two main postoperative complications associated with residual anastomoses after selective coagulation (Lancet. 2014 Jun 21;383[9935]:2144-51).
The procedure has many challenges and can be impacted by one’s inability to see the entire vascular equator because of poor access, by the patient’s history of other interventions, and by the stage of TTTS.
Laser coagulation is regarded as the standard treatment for Quintero stage II-IV disease, and it is offered in some cases of stage I disease, such as those involving severe polyhydramnios and shortened cervix. Research currently underway is examining the outcomes of treatment for stage I disease, but data thus far suggest that intervening at stage I is generally better than expectant management.
With laser coagulation treatment, the survival rate in pregnancies complicated by TTTS is about 85%-90% for one fetus, and about 70% for both. TTTS sometimes causes one twin, particularly the recipient, to develop pulmonary valve stenosis, but this is generally a functional problem that resolves when the syndrome is treated.
After treatment, it is important to monitor for the development of twin anemia-polycythemia sequence, which may still occur if full visualization of the vascular equator was not possible or if a fine vessel was missed. Such monitoring involves weekly ultrasound surveillance with middle cerebral artery peak systolic velocity measurements.
Patients should also be monitored for abnormal neurologic development, ventriculomegaly, and other signs of abnormal brain development. Even “perfect” laser treatment with seemingly complete placental separation has been associated with abnormal neurologic development in about 10%-15% of cases.
Maternal complications with TTTS include placental abruption and preterm membrane rupture, the latter of which occurs about 15%-20% of the time.
Currently under much discussion is fetoscopic laser coagulation of TTTS placentas that have “proximate cord insertions.” The surgery in these cases – where the cords are less than 4 cm apart – is much more challenging because of technical difficulties in visualizing the vascular equator, and outcomes are being studied. Some centers will not perform laser surgery on placentas with proximate cord insertions, which fortunately are uncommon. However, the surgery is possible; I have completed three cases thus far, each with dual survival.
Selective fetal growth restriction
Selective fetal growth restriction (sFGR) stems from unequal placental sharing and affects approximately 15%-20% of all monochorionic pregnancies, making it a bit more common than TTTS. Diagnostic criteria vary, but the North American Fetal Therapy Network recommends using either an estimated fetal weight below the 10th percentile, with or without significant growth discordance (greater than 25%), or just growth discordance greater than 25%. Either provides an acceptable definition of sFGR.
With sFGR, in general, the normally growing twin has normal fluid and the growth-restricted twin has less fluid. This makes it different from TTTS, in which the twins may have different sizes but fluid discordance is always present. Also in TTTS, there is a finding of polyhydramnios in the recipient.
There are three types of sFGR, based on umbilical artery Doppler findings. In type I there is no cardiovascular imbalance, and management typically involves weekly monitoring with Doppler ultrasound. If Doppler findings remain normal for some time, monitoring every 2-4 weeks will suffice. Elective delivery is generally set for 35 or 36 weeks.
Type II sFGR involves cardiovascular compromise early in pregnancy, with umbilical artery Doppler showing persistent reversed or absent end-diastolic flow. Treatment options include monitoring closely and, in general, delivering by 32 weeks. In these cases, prematurity may jeopardize the life or health of the normally growing twin while saving the life of the growth-restricted twin.
When type II sFGR is diagnosed early, selective termination of the growth-restricted fetus may be another option. This is a relatively safe procedure overall but it carries risks such as ruptured membrane and damage to the normal twin (10%-35% risk).
Type III sFGR is uniquely unpredictable, with intermittently absent or reversed flow stemming from a large artery-artery anastomosis. The direction of blood flow may suddenly change; in fact, the diagnosis is made by placing the Doppler caliper close to the placenta cord insertion and watching the end-diastolic flow. Present, absent, and reverse flow within a minute of observation demonstrates the presence of a large artery-artery anastomosis.
The risk of unexpected fetal death with severe sFGR is estimated to be 15% or higher, and the spontaneous death of the poorly growing twin threatens both the survival and the neurologic health of the co-twin. The risk of a parenchymal lesion for the co-twin is about 20%-40%.
Management decisions can be extremely difficult. As with type II, one could manage expectantly and generally deliver by 32 weeks. Fetoscopic laser coagulation to achieve complete dichorionization, as done with TTTS, could also be discussed; this approach could save the life of one twin in the event that the co-twin dies. Finally, selective termination may again be an option. None is a perfect treatment, and parents must be thoroughly counseled and supported in understanding the options and risks.
Twin anemia polycythemia sequence
Unlike TTTS, twin anemia polycythemia sequence (TAPS) does not involve a fluid shift. Rather, red blood cells shift from one fetus to the other through extremely small-caliber vessels, leading to severe anemia of one fetus and polycythemia of the other. The chronic and unbalanced transfusion occurs in about 5% of monochorionic twins, generally after 26 weeks’ gestation.
TAPS also occurs after laser treatment for TTTS in about 10%-15% of cases (generally within 4 weeks of treatment), though this incidence is significantly reduced when complete dichorionization is achieved using the Solomon technique for fetoscopic laser coagulation. Diagnosis is made when the middle cerebral artery peak systolic velocity of the red blood cell donor is greater than 1.5 MoM and the peak systolic velocity of the recipient is less than 1.0 MoM, without amniotic fluid discordance.
There are no established preferred treatments, but fetoscopic laser coagulation is an option for some patients. Visibility can be extremely poor when TAPS occurs after a laser treatment and vessels can be difficult to identify, but in selected cases it is possible with an experienced team. When performed, treatment can be followed by delivery or by intrauterine transfusion of the anemic fetus. Intrauterine transfusion has been studied as a primary treatment, but it generally is problematic because the small vessels at the root of TAPS continue to exist.
Twin reversed arterial perfusion
In about 1% of monochorionic pregnancies, an arterial incident prevents one of the twins from developing a heart and upper body. Some research has suggested that the condition is associated with trisomies in about 10% of the cases.
The viable, structurally normal co-twin therefore acts like a pump, continually perfusing the nonviable twin through an abnormal vascular circuit that allows arterial blood to flow in a reverse direction. In the process, the normal twin, or “pump twin,” can develop heart failure and hydrops. Mortality appears to be about 55%.
Diagnosis is straightforward, but it has been challenging to determine which pregnancies will require intervention. Some research has suggested that the risk of hydrops and mortality increases significantly – and favors intervention – when the weight difference is greater than 70%. On the other hand, if the difference is less than 50%, survival of the pump twin approaches 80% and continuing surveillance may be most appropriate.
Radiofrequency ablation of the cord of the nonviable twin is one of the treatment methods and has about an 80% success rate. Another option is coagulation of the blood supply in the abnormal twin using a laser fiber via a fine needle during the first trimester. An ongoing European trial of the procedure is showing success rates of approximately 70%.
Dr. Turan is director of fetal therapy and complex obstetric surgery, and an associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported having no relevant financial disclosures.
Approximately 20% of all twin pregnancies are monochorionic, with the fetuses sharing a single placenta. Although the majority of these pregnancies are uncomplicated, monochorionic twins are significantly more likely than dichorionic twins to incur complications that can threaten the life and health of one or both fetuses.
The death of one monochorionic twin leaves the other twin with a 15% risk of demise. Survival after the loss of a co-twin is also associated with a 25% incidence of neurologic injury, compared with a 2% incidence in dichorionic pregnancies. Additionally, monochorionic pregnancies carry the risk of unique complications such as twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion.
Increased ultrasonographic surveillance recommended for monochorionic twin pregnancies has been outlined in a recent consensus statement from the North American Fetal Therapy Network (Obstet Gynecol. 2015 Jan;125[1]:118-23). Beginning at 16 weeks’ gestation, monochorionic twins should be assessed every 2 weeks using amniotic fluid balance, presence/absence of fluid within the fetal bladder, and with fetal Doppler (umbilical artery, middle cerebral artery, and ductus venosus) studies. Fetal growth should also be assessed at least every 4 weeks.
Since monochorionic twins are at increased risk for congenital heart disease, echocardiography is also performed between 18 and 22 weeks, with surveillance intervals of 2 weeks or shorter if potential complications are identified. Early detection of these and other complications allows for earlier intervention, earlier referral if necessary, and potentially better outcomes.
Twin-to-twin transfusion syndrome
Twin-to-twin transfusion syndrome (TTTS) is one of the most common and most serious complications, affecting approximately 10% of monochorionic pregnancies. Significant imbalances in blood-flow exchange lead to progressive cardiovascular decompensation that causes one twin to become a “donor” of blood volume, and the other twin to become a “recipient.” Without proper treatment between 16 and 26 weeks’ gestation, the perinatal mortality rate has been estimated to be 70% or higher.
Disease severity is classified according to the Quintero staging system. Stage I is characterized by amniotic fluid discordance. In stage II, the bladder of the donor twin is no longer visible sonographically. Stage III is marked by critically abnormal Doppler waveforms in either twin (absent/reverse end-diastolic velocity in the umbilical artery, reverse flow in the ductus venosus, or pulsatile flow in the umbilical vein). In stage IV, one of the twins has developed hydrops, and stage V is characterized by the death of one or both of the twins.
Amnioreduction to decrease intra-amniotic pressure had been the treatment of choice until a randomized controlled trial, published in 2004, demonstrated that fetoscopic laser coagulation of anastomoses was superior as a first-line treatment for severe TTTS that is diagnosed before 26 weeks. Perinatal mortality and morbidity were significantly lower after the laser treatment (N Engl J Med. 2004 Jul 8;351[2]:136-44).
Outcomes were further improved over the next decade as the laser surgery technique was modified to cover the entire vascular equator rather than selective components of the vasculature. In an open-label randomized controlled trial comparing the two approaches for severe TTTS, fetoscopic laser coagulation of the vascular equator (known as the Solomon technique) reduced the risk of twin anemia polycythemia sequence and recurrence of TTTS – the two main postoperative complications associated with residual anastomoses after selective coagulation (Lancet. 2014 Jun 21;383[9935]:2144-51).
The procedure has many challenges and can be impacted by one’s inability to see the entire vascular equator because of poor access, by the patient’s history of other interventions, and by the stage of TTTS.
Laser coagulation is regarded as the standard treatment for Quintero stage II-IV disease, and it is offered in some cases of stage I disease, such as those involving severe polyhydramnios and shortened cervix. Research currently underway is examining the outcomes of treatment for stage I disease, but data thus far suggest that intervening at stage I is generally better than expectant management.
With laser coagulation treatment, the survival rate in pregnancies complicated by TTTS is about 85%-90% for one fetus, and about 70% for both. TTTS sometimes causes one twin, particularly the recipient, to develop pulmonary valve stenosis, but this is generally a functional problem that resolves when the syndrome is treated.
After treatment, it is important to monitor for the development of twin anemia-polycythemia sequence, which may still occur if full visualization of the vascular equator was not possible or if a fine vessel was missed. Such monitoring involves weekly ultrasound surveillance with middle cerebral artery peak systolic velocity measurements.
Patients should also be monitored for abnormal neurologic development, ventriculomegaly, and other signs of abnormal brain development. Even “perfect” laser treatment with seemingly complete placental separation has been associated with abnormal neurologic development in about 10%-15% of cases.
Maternal complications with TTTS include placental abruption and preterm membrane rupture, the latter of which occurs about 15%-20% of the time.
Currently under much discussion is fetoscopic laser coagulation of TTTS placentas that have “proximate cord insertions.” The surgery in these cases – where the cords are less than 4 cm apart – is much more challenging because of technical difficulties in visualizing the vascular equator, and outcomes are being studied. Some centers will not perform laser surgery on placentas with proximate cord insertions, which fortunately are uncommon. However, the surgery is possible; I have completed three cases thus far, each with dual survival.
Selective fetal growth restriction
Selective fetal growth restriction (sFGR) stems from unequal placental sharing and affects approximately 15%-20% of all monochorionic pregnancies, making it a bit more common than TTTS. Diagnostic criteria vary, but the North American Fetal Therapy Network recommends using either an estimated fetal weight below the 10th percentile, with or without significant growth discordance (greater than 25%), or just growth discordance greater than 25%. Either provides an acceptable definition of sFGR.
With sFGR, in general, the normally growing twin has normal fluid and the growth-restricted twin has less fluid. This makes it different from TTTS, in which the twins may have different sizes but fluid discordance is always present. Also in TTTS, there is a finding of polyhydramnios in the recipient.
There are three types of sFGR, based on umbilical artery Doppler findings. In type I there is no cardiovascular imbalance, and management typically involves weekly monitoring with Doppler ultrasound. If Doppler findings remain normal for some time, monitoring every 2-4 weeks will suffice. Elective delivery is generally set for 35 or 36 weeks.
Type II sFGR involves cardiovascular compromise early in pregnancy, with umbilical artery Doppler showing persistent reversed or absent end-diastolic flow. Treatment options include monitoring closely and, in general, delivering by 32 weeks. In these cases, prematurity may jeopardize the life or health of the normally growing twin while saving the life of the growth-restricted twin.
When type II sFGR is diagnosed early, selective termination of the growth-restricted fetus may be another option. This is a relatively safe procedure overall but it carries risks such as ruptured membrane and damage to the normal twin (10%-35% risk).
Type III sFGR is uniquely unpredictable, with intermittently absent or reversed flow stemming from a large artery-artery anastomosis. The direction of blood flow may suddenly change; in fact, the diagnosis is made by placing the Doppler caliper close to the placenta cord insertion and watching the end-diastolic flow. Present, absent, and reverse flow within a minute of observation demonstrates the presence of a large artery-artery anastomosis.
The risk of unexpected fetal death with severe sFGR is estimated to be 15% or higher, and the spontaneous death of the poorly growing twin threatens both the survival and the neurologic health of the co-twin. The risk of a parenchymal lesion for the co-twin is about 20%-40%.
Management decisions can be extremely difficult. As with type II, one could manage expectantly and generally deliver by 32 weeks. Fetoscopic laser coagulation to achieve complete dichorionization, as done with TTTS, could also be discussed; this approach could save the life of one twin in the event that the co-twin dies. Finally, selective termination may again be an option. None is a perfect treatment, and parents must be thoroughly counseled and supported in understanding the options and risks.
Twin anemia polycythemia sequence
Unlike TTTS, twin anemia polycythemia sequence (TAPS) does not involve a fluid shift. Rather, red blood cells shift from one fetus to the other through extremely small-caliber vessels, leading to severe anemia of one fetus and polycythemia of the other. The chronic and unbalanced transfusion occurs in about 5% of monochorionic twins, generally after 26 weeks’ gestation.
TAPS also occurs after laser treatment for TTTS in about 10%-15% of cases (generally within 4 weeks of treatment), though this incidence is significantly reduced when complete dichorionization is achieved using the Solomon technique for fetoscopic laser coagulation. Diagnosis is made when the middle cerebral artery peak systolic velocity of the red blood cell donor is greater than 1.5 MoM and the peak systolic velocity of the recipient is less than 1.0 MoM, without amniotic fluid discordance.
There are no established preferred treatments, but fetoscopic laser coagulation is an option for some patients. Visibility can be extremely poor when TAPS occurs after a laser treatment and vessels can be difficult to identify, but in selected cases it is possible with an experienced team. When performed, treatment can be followed by delivery or by intrauterine transfusion of the anemic fetus. Intrauterine transfusion has been studied as a primary treatment, but it generally is problematic because the small vessels at the root of TAPS continue to exist.
Twin reversed arterial perfusion
In about 1% of monochorionic pregnancies, an arterial incident prevents one of the twins from developing a heart and upper body. Some research has suggested that the condition is associated with trisomies in about 10% of the cases.
The viable, structurally normal co-twin therefore acts like a pump, continually perfusing the nonviable twin through an abnormal vascular circuit that allows arterial blood to flow in a reverse direction. In the process, the normal twin, or “pump twin,” can develop heart failure and hydrops. Mortality appears to be about 55%.
Diagnosis is straightforward, but it has been challenging to determine which pregnancies will require intervention. Some research has suggested that the risk of hydrops and mortality increases significantly – and favors intervention – when the weight difference is greater than 70%. On the other hand, if the difference is less than 50%, survival of the pump twin approaches 80% and continuing surveillance may be most appropriate.
Radiofrequency ablation of the cord of the nonviable twin is one of the treatment methods and has about an 80% success rate. Another option is coagulation of the blood supply in the abnormal twin using a laser fiber via a fine needle during the first trimester. An ongoing European trial of the procedure is showing success rates of approximately 70%.
Dr. Turan is director of fetal therapy and complex obstetric surgery, and an associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported having no relevant financial disclosures.
Approximately 20% of all twin pregnancies are monochorionic, with the fetuses sharing a single placenta. Although the majority of these pregnancies are uncomplicated, monochorionic twins are significantly more likely than dichorionic twins to incur complications that can threaten the life and health of one or both fetuses.
The death of one monochorionic twin leaves the other twin with a 15% risk of demise. Survival after the loss of a co-twin is also associated with a 25% incidence of neurologic injury, compared with a 2% incidence in dichorionic pregnancies. Additionally, monochorionic pregnancies carry the risk of unique complications such as twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion.
Increased ultrasonographic surveillance recommended for monochorionic twin pregnancies has been outlined in a recent consensus statement from the North American Fetal Therapy Network (Obstet Gynecol. 2015 Jan;125[1]:118-23). Beginning at 16 weeks’ gestation, monochorionic twins should be assessed every 2 weeks using amniotic fluid balance, presence/absence of fluid within the fetal bladder, and with fetal Doppler (umbilical artery, middle cerebral artery, and ductus venosus) studies. Fetal growth should also be assessed at least every 4 weeks.
Since monochorionic twins are at increased risk for congenital heart disease, echocardiography is also performed between 18 and 22 weeks, with surveillance intervals of 2 weeks or shorter if potential complications are identified. Early detection of these and other complications allows for earlier intervention, earlier referral if necessary, and potentially better outcomes.
Twin-to-twin transfusion syndrome
Twin-to-twin transfusion syndrome (TTTS) is one of the most common and most serious complications, affecting approximately 10% of monochorionic pregnancies. Significant imbalances in blood-flow exchange lead to progressive cardiovascular decompensation that causes one twin to become a “donor” of blood volume, and the other twin to become a “recipient.” Without proper treatment between 16 and 26 weeks’ gestation, the perinatal mortality rate has been estimated to be 70% or higher.
Disease severity is classified according to the Quintero staging system. Stage I is characterized by amniotic fluid discordance. In stage II, the bladder of the donor twin is no longer visible sonographically. Stage III is marked by critically abnormal Doppler waveforms in either twin (absent/reverse end-diastolic velocity in the umbilical artery, reverse flow in the ductus venosus, or pulsatile flow in the umbilical vein). In stage IV, one of the twins has developed hydrops, and stage V is characterized by the death of one or both of the twins.
Amnioreduction to decrease intra-amniotic pressure had been the treatment of choice until a randomized controlled trial, published in 2004, demonstrated that fetoscopic laser coagulation of anastomoses was superior as a first-line treatment for severe TTTS that is diagnosed before 26 weeks. Perinatal mortality and morbidity were significantly lower after the laser treatment (N Engl J Med. 2004 Jul 8;351[2]:136-44).
Outcomes were further improved over the next decade as the laser surgery technique was modified to cover the entire vascular equator rather than selective components of the vasculature. In an open-label randomized controlled trial comparing the two approaches for severe TTTS, fetoscopic laser coagulation of the vascular equator (known as the Solomon technique) reduced the risk of twin anemia polycythemia sequence and recurrence of TTTS – the two main postoperative complications associated with residual anastomoses after selective coagulation (Lancet. 2014 Jun 21;383[9935]:2144-51).
The procedure has many challenges and can be impacted by one’s inability to see the entire vascular equator because of poor access, by the patient’s history of other interventions, and by the stage of TTTS.
Laser coagulation is regarded as the standard treatment for Quintero stage II-IV disease, and it is offered in some cases of stage I disease, such as those involving severe polyhydramnios and shortened cervix. Research currently underway is examining the outcomes of treatment for stage I disease, but data thus far suggest that intervening at stage I is generally better than expectant management.
With laser coagulation treatment, the survival rate in pregnancies complicated by TTTS is about 85%-90% for one fetus, and about 70% for both. TTTS sometimes causes one twin, particularly the recipient, to develop pulmonary valve stenosis, but this is generally a functional problem that resolves when the syndrome is treated.
After treatment, it is important to monitor for the development of twin anemia-polycythemia sequence, which may still occur if full visualization of the vascular equator was not possible or if a fine vessel was missed. Such monitoring involves weekly ultrasound surveillance with middle cerebral artery peak systolic velocity measurements.
Patients should also be monitored for abnormal neurologic development, ventriculomegaly, and other signs of abnormal brain development. Even “perfect” laser treatment with seemingly complete placental separation has been associated with abnormal neurologic development in about 10%-15% of cases.
Maternal complications with TTTS include placental abruption and preterm membrane rupture, the latter of which occurs about 15%-20% of the time.
Currently under much discussion is fetoscopic laser coagulation of TTTS placentas that have “proximate cord insertions.” The surgery in these cases – where the cords are less than 4 cm apart – is much more challenging because of technical difficulties in visualizing the vascular equator, and outcomes are being studied. Some centers will not perform laser surgery on placentas with proximate cord insertions, which fortunately are uncommon. However, the surgery is possible; I have completed three cases thus far, each with dual survival.
Selective fetal growth restriction
Selective fetal growth restriction (sFGR) stems from unequal placental sharing and affects approximately 15%-20% of all monochorionic pregnancies, making it a bit more common than TTTS. Diagnostic criteria vary, but the North American Fetal Therapy Network recommends using either an estimated fetal weight below the 10th percentile, with or without significant growth discordance (greater than 25%), or just growth discordance greater than 25%. Either provides an acceptable definition of sFGR.
With sFGR, in general, the normally growing twin has normal fluid and the growth-restricted twin has less fluid. This makes it different from TTTS, in which the twins may have different sizes but fluid discordance is always present. Also in TTTS, there is a finding of polyhydramnios in the recipient.
There are three types of sFGR, based on umbilical artery Doppler findings. In type I there is no cardiovascular imbalance, and management typically involves weekly monitoring with Doppler ultrasound. If Doppler findings remain normal for some time, monitoring every 2-4 weeks will suffice. Elective delivery is generally set for 35 or 36 weeks.
Type II sFGR involves cardiovascular compromise early in pregnancy, with umbilical artery Doppler showing persistent reversed or absent end-diastolic flow. Treatment options include monitoring closely and, in general, delivering by 32 weeks. In these cases, prematurity may jeopardize the life or health of the normally growing twin while saving the life of the growth-restricted twin.
When type II sFGR is diagnosed early, selective termination of the growth-restricted fetus may be another option. This is a relatively safe procedure overall but it carries risks such as ruptured membrane and damage to the normal twin (10%-35% risk).
Type III sFGR is uniquely unpredictable, with intermittently absent or reversed flow stemming from a large artery-artery anastomosis. The direction of blood flow may suddenly change; in fact, the diagnosis is made by placing the Doppler caliper close to the placenta cord insertion and watching the end-diastolic flow. Present, absent, and reverse flow within a minute of observation demonstrates the presence of a large artery-artery anastomosis.
The risk of unexpected fetal death with severe sFGR is estimated to be 15% or higher, and the spontaneous death of the poorly growing twin threatens both the survival and the neurologic health of the co-twin. The risk of a parenchymal lesion for the co-twin is about 20%-40%.
Management decisions can be extremely difficult. As with type II, one could manage expectantly and generally deliver by 32 weeks. Fetoscopic laser coagulation to achieve complete dichorionization, as done with TTTS, could also be discussed; this approach could save the life of one twin in the event that the co-twin dies. Finally, selective termination may again be an option. None is a perfect treatment, and parents must be thoroughly counseled and supported in understanding the options and risks.
Twin anemia polycythemia sequence
Unlike TTTS, twin anemia polycythemia sequence (TAPS) does not involve a fluid shift. Rather, red blood cells shift from one fetus to the other through extremely small-caliber vessels, leading to severe anemia of one fetus and polycythemia of the other. The chronic and unbalanced transfusion occurs in about 5% of monochorionic twins, generally after 26 weeks’ gestation.
TAPS also occurs after laser treatment for TTTS in about 10%-15% of cases (generally within 4 weeks of treatment), though this incidence is significantly reduced when complete dichorionization is achieved using the Solomon technique for fetoscopic laser coagulation. Diagnosis is made when the middle cerebral artery peak systolic velocity of the red blood cell donor is greater than 1.5 MoM and the peak systolic velocity of the recipient is less than 1.0 MoM, without amniotic fluid discordance.
There are no established preferred treatments, but fetoscopic laser coagulation is an option for some patients. Visibility can be extremely poor when TAPS occurs after a laser treatment and vessels can be difficult to identify, but in selected cases it is possible with an experienced team. When performed, treatment can be followed by delivery or by intrauterine transfusion of the anemic fetus. Intrauterine transfusion has been studied as a primary treatment, but it generally is problematic because the small vessels at the root of TAPS continue to exist.
Twin reversed arterial perfusion
In about 1% of monochorionic pregnancies, an arterial incident prevents one of the twins from developing a heart and upper body. Some research has suggested that the condition is associated with trisomies in about 10% of the cases.
The viable, structurally normal co-twin therefore acts like a pump, continually perfusing the nonviable twin through an abnormal vascular circuit that allows arterial blood to flow in a reverse direction. In the process, the normal twin, or “pump twin,” can develop heart failure and hydrops. Mortality appears to be about 55%.
Diagnosis is straightforward, but it has been challenging to determine which pregnancies will require intervention. Some research has suggested that the risk of hydrops and mortality increases significantly – and favors intervention – when the weight difference is greater than 70%. On the other hand, if the difference is less than 50%, survival of the pump twin approaches 80% and continuing surveillance may be most appropriate.
Radiofrequency ablation of the cord of the nonviable twin is one of the treatment methods and has about an 80% success rate. Another option is coagulation of the blood supply in the abnormal twin using a laser fiber via a fine needle during the first trimester. An ongoing European trial of the procedure is showing success rates of approximately 70%.
Dr. Turan is director of fetal therapy and complex obstetric surgery, and an associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported having no relevant financial disclosures.