User login
Total Laparoscopic Segmental Resection With Transanal NOSE for Deep Colorectal Endometriosis
What to Know About NOSE
Traditionally, a laparoscopic approach to bowel resection secondary to endometriosis means enlargement of the port site or more commonly, a Pfannenstiel incision to introduce an anvil. Both incisions raise the risk of postoperative complications including pain and incisional hernia. Moreover, recovery time is prolonged.
Natural orifice specimen extraction (NOSE) makes use of a natural orifice, the vagina or anus, to extract the specimen and introduce the anvil. Transanal extraction for the management of endometriosis-related bowel disease was first described by the late David Redwine, MD, in his 1996 publication in Fertility and Sterility.1
The concern with use of vaginal NOSE surgery is rectovaginal fistula secondary to two incision lines in close opposition. Both transvaginal NOSE and transanal NOSE have been criticized for longer dissection of the mesorectum and thus longer resected specimen. Despite these concerns, over the past 10 years, articles have been published showing not only the feasibility of the NOSE technique, but excellent comparative outcomes, as well.2-4
A recent systematic review and meta-analysis by Kar et al. comparing NOSE extraction to minilaparotomy in bowel resection due to endometriosis revealed no significant difference in complication rate, but significantly shorter operation duration, reduced length of stay, and less blood loss in the NOSE surgical arm.5
The most recent study to be published in the Journal of Minimally Invasive Gynecology involving total laparoscopic segmental resection with transanal NOSE for the treatment of colorectal endometriosis was written by the guest author of this edition of the Master Class in Gynecologic Surgery, Professor Mario Malzoni, Scientific Director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. In the JMIG article, and in this Master Class, Professor Malzoni, a world-renowned endometriosis specialist recognized for his extraordinary skill and multi-organ surgical work, discusses the difference between the standard bowel resection technique with that of NOSE, and reports on his experience with NOSE.6
It is a pleasure and honor to welcome my friend and colleague, Professor Mario Malzoni, to this edition of the Master Class in Gynecologic Surgery.
Dr. Charles E. Miller is Professor, Obstetrics & Gynecology, Department of Clinical Sciences, Rosalind Franklin University of Medicine and Science, North Chicago, and Director, Minimally Invasive Gynecologic Surgery, Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at [email protected].
References
1. Redwine DB et al. Laparoscopically assisted transvaginal segmental resection of the rectosigmoid colon for endometriosis. Fertil Steril. 1996 Jan;65(1):193-7.
2. Akladios C et al. Totally laparoscopic intracorporeal anastomosis with natural orifice specimen extraction (NOSE) techniques, particularly suitable for bowel endometriosis. J Minim Invasive Gynecol. 2014 Nov-Dec;21(6):1095-102.
3. Bokor A et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018 Sep-Oct;25(6):1065-74.
4. Grigoriadis G et al. Natural orifice specimen extraction colorectal resection for deep endometriosis: A 50 case series. J Minim Invasive Gynecol. 2022 Sep;29(9):1054-62.
5. Kar E et al. Natural orifice specimen extraction as a promising alternative for minilaparotomy in bowel resection due to endometriosis: A systematic review and meta-analysis. J Minim Invasive Gynecol. 2024 Jul;31(7):574-83.e1.
6. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024 Oct;18:S1553-4650(24)01453-5.
Laparoscopic Resection With Transanal NOSE for Deep Colorectal Endometriosis
When segmental sigmoid colon/rectal resection is indicated for treatment of deep infiltrating bowel endometriosis, a totally laparoscopic resection with intracorporeal anastomosis and transanal natural orifice specimen extraction (NOSE) can be safe, effective, and advantageous compared with an abdominal mini-laparotomy for extracorporeal anastomosis and specimen retrieval.
With advanced laparoscopic surgical skills, accurate preoperative ultrasound evaluation, and a high-quality perioperative care protocol (including high-quality bowel preparation), segmental resection with NOSE can offer better preservation of vascularization, smaller resection sizes, lower rates of postoperative pain, a shorter time for gas passage after surgery, decreased hospital stays, and lower rates of wound infection and incisional hernia.
We have seen such improved results in our high-volume center since shifting in April 2021 from our classical technique for segmental rectosigmoid resection1 to a totally laparoscopic approach with NOSE. We first reported on this new technique in 2022 in a video article in the Journal of Minimally Invasive Gynecology,2and in October 2024, outcomes of 81 patients who underwent segmental sigmoid colon/rectal resection with intracorporeal anastomosis and transanal NOSE at our institution from April 2021 through March 2024 were reported in Journal of Minimally Invasive Gynecology.3Given our experience with this approach for deep endometriosis, I am convinced that NOSE should be preferred to transabdominal specimen extraction for segmental rectosigmoid resection. This is where the future lies for deep infiltrating endometriosis. Here, I share our outcomes and describe our technique.
Our Prior Approach With A Mini-Laparotomy
Our traditional laparoscopic approach for segmental colon/rectal resection of deep infiltrating endometriosis involved an abdominal mini-laparotomy (no more than 4 cm in length) for specimen retrieval, resection of the proximal rectum, and positioning of the head of a circular stapler for end-to-end anastomosis. (Connection of the two parts of the circular stapler for anastomosis was achieved via laparoscopy.)1
Several factors allowed us to minimize potential complications, including standardization of our technique, the use of three-dimensional endoscopes, and indocyanine green fluorescence angiography to assess the perfusion of the bowel after the completion of anastomosis. Still, an unpublished analysis of 1050 segmental bowel resections performed over more than 17 years using our traditional technique showed that hospital stays averaged 8 days, time to first defecation averaged 7 days, and time to first passage of flatus averaged 1 day.
Per the Clavien-Dindo classification system for surgical complications that was used at the time, 1.9% of these 1050 patients had grade 1 (minor) or grade 2 complications and 6% had grade 3 complications. Grade 2 complications were defined as needing intervention or a hospital stay more than twice the median for the procedure. Grade 3 complications were defined as leading to lasting disability or organ resection.
In the meantime, in colorectal cancer surgery, published studies on NOSE versus conventional laparoscopy with transabdominal specimen extraction have consistently pointed to the benefits of NOSE. A 2022 review 4 of 19 studies involving over 3400 patients and a 2022 meta-analysis 5 of 21 randomized controlled trials involving more than 2000 patients both showed significantly reduced postoperative morbidity and no differences in oncologic outcomes. Among the notable differences in the 2022 meta-analysis was a relative risk of postoperative infection of 0.34 for patients treated with NOSE compared with conventional laparoscopic techniques.
Among patients with deep rectal endometriosis, reported experience with intracorporeal anastomosis and NOSE for segmental resection is limited, with a few studies published between 2014 and 2022. Excluding anecdotal reports, only about 140 patients have been reported in the literature as having intracorporeal anastomosis and transanal NOSE for deep infiltrating bowel endometriosis. 3
Our Outcomes With A Totally Laparoscopic Approach
Our approach to evaluating our experience with totally laparoscopic resection with transanal NOSE has been to systematically collect data from consecutive patients and to analyze 1) complications of the technique, 2) conversion to the traditional technique/open surgery, and 3) endometriosis-free bowel resection margins and recurrence. Secondarily, we look at intraoperative blood loss, operative time, recovery of gastrointestinal function, hospital stay length, and reproductive outcomes.
Across 81 patients from our TrEnd study database (Surgical TReatment of women with deep ENDometriosis infiltrating the sigmoid colon and/or rectum) who received a totally laparoscopic standardized procedure involving intracorporeal anastomosis and transanal NOSE, we had no conversions even to mini-laparotomy, and no cases of protective colostomy or ileostomy.
Complete endometriosis removal was achieved in 100% of cases, with final pathology showing endometriosis-free resection margins, and patients remained free of bowel endometriosis at a median follow-up of 21 months.3Our analysis also shows improved gastrointestinal function recovery (median time to first defecation of 4 days, compared to 7 days previously, with the same 1-day median time to first passage of flatus) and a significantly improved hospital length of stay to a median of 3 days (interquartile range, 3-4.5 days); the latter reflects in part the absence of infection.3There were no intra-operative complications. Postoperative Clavien-Dindo grade 3 complications occurred in three patients (3.7%), two of whom required reoperation (one for hemoperitoneum and one for ureteral injury) and one who experienced rectal anastomotic bleeding on postoperative day 1. (The two requiring reoperation had significant right lateral parametrial involvement and underwent nerve-sparing parametrectomy at the time of bowel resection).3Notably, 7 of the 81 patients had totally laparoscopic double segmental colorectal resection — 6 with simultaneous ileocecal valve and rectal resection, and 1 with simultaneous ileal and rectal resection. None of these seven patients had postoperative complications. (A report on the six surgeries for deep endometriosis infiltrating the ileocecal valve and rectum was also published this year in the journal Colorectal Disease.6)
Median blood loss in the 81-patient cohort was 20 mL (IQR, 20-30 mL), and the median length of surgery was 160 minutes (IQR, 130-210 minutes).
Bowel endometriotic nodules had a mean maximum diameter of 4.3 cm (3.5-5.5 cm), a mean depth of invasion of 9 mm (7-9 mm), a mean estimated stenosis of 40% (35%-50%), and a mean distance from the anal verge of 13 cm (10-15 cm).3 (The cohort in this analysis did not include patients who received concomitant hysterectomy and transvaginal NOSE.)
Specifics of the New Technique, Including Totally Intracorporeal Anastomosis
As in our previous technique, the surgical procedure starts with left and dorsal rectal mobilization. The left pelvic sidewall dissection begins with incision of the parietal peritoneum along the pelvic portions of the psoas muscle. The retroperitoneal connective tissue is dissected in order to identify the ureter, the hypogastric nerve, and the presacral parietal pelvic fascia (PPPF). Dissection is carried downward in front of the PPPF and medially to the left hypogastric nerve.
Right and ventral rectal mobilization begins with incision of the peritoneum along the gray avascular line, which is seen with upward retraction of the rectum.
Again, for preservation of bowel and bladder function, the hypogastric nerve must be identified and preserved through dissection that displaces the nerve dorsally and laterally.
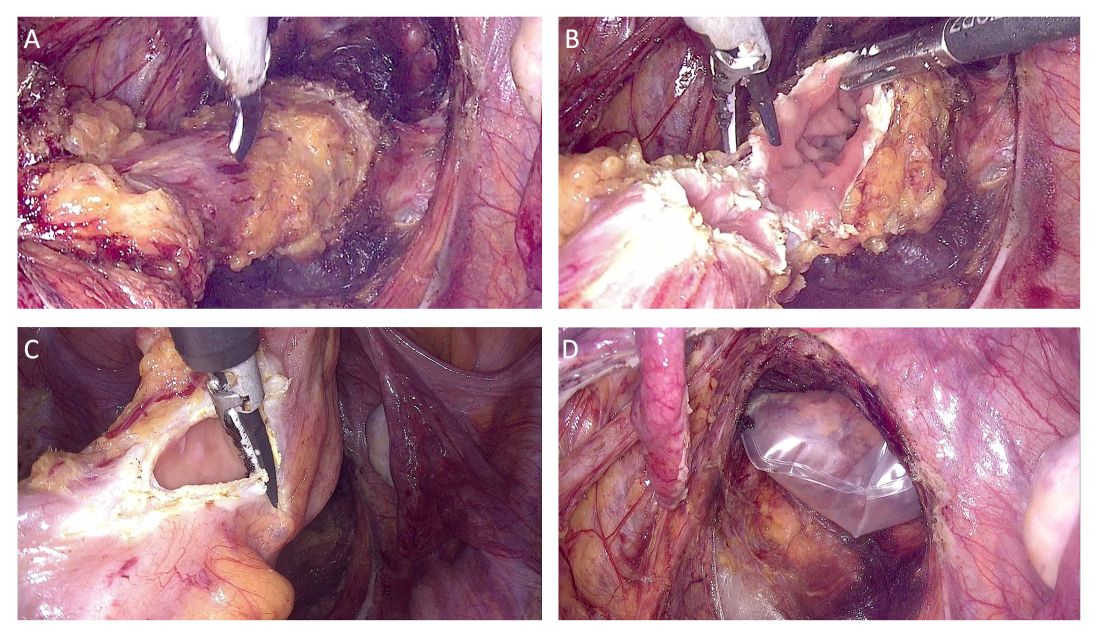
Dissection proceeds to sharply divide the nodule into two parts (rectal and rectocervical components). The caudal boundary of ventral dissection is reached by incising the septum and opening the rectovaginal space. The lymphovascular fatty tissue surrounding the rectum is then excised and the rectal tube is denuded at the level of the distal resection margin.
The distal and proximal resection margins are prepared and transected using a tissue-sealing device, and the resected rectal specimen is placed into a retrieval bag and pulled out through the anus (Figure 1).
The totally intracorporeal side-to-end anastomosis is performed using a traditional circular stapler. Its anvil is inserted through the proximal resection line and exteriorized forward on the antimesenteric side of the sigmoid colon/rectum. The proximal and distal resection lines are closed with a 60-mm linear endo-stapler, and finally, the shaft of the circular stapler is inserted into the rectum, and the two parts of the stapler are joined. The stapler is then closed and fired (Figure 2).
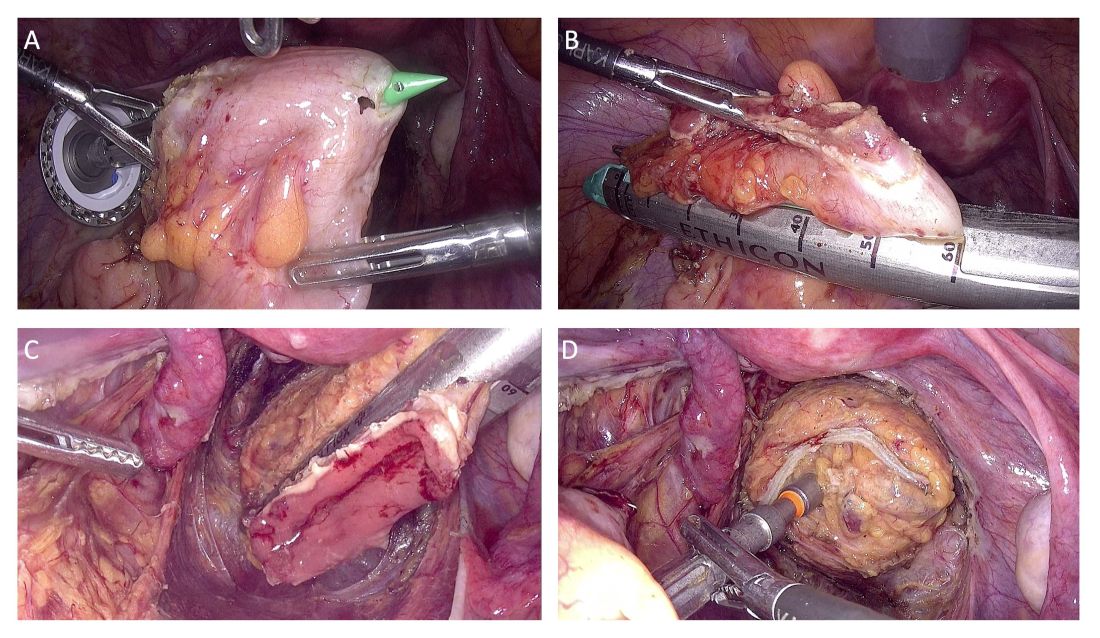
An air leak test is performed to check the quality of the rectal sutures. However, regardless of the results, we routinely apply interrupted stitches to the so-called “dog ears” in order to reduce tension along the anastomotic line.
We also evaluate the microvascularization of the two parts of the bowel at the level of the reanastomosis using indocyanine green fluorescence angiography. Doing so allows us to avoid complications caused by anastomotic leakage.
Patient Selection And Peri-Surgical Care
The decision to perform segmental resection as opposed to other more conservative laparoscopic excision techniques is made after skilled preoperative imaging reveals the number of lesions, the largest nodule diameter, the infiltration depth, and the circumference of bowel involvement.
A recently published international consensus statement7 on noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems concludes there is Level 1a evidence that preoperative imaging with transvaginal ultrasound (TVUS) can predict with good precision the size and degree of infiltration of deep endometriosis of the rectum (a Grade A statement, per the Oxford Centre for Evidence-Based Medicine levels of evidence).
The consensus document was published across seven different journals by international and European societies, and the American Association of Gynecologic Laparoscopists (AAGL), each of which contributed to a multidisciplinary panel of gynecological surgeons, sonographers, and radiologists.
At our center, we achieve precise preoperative evaluations with TVUS (other centers also employ MRI) and have long set clear preoperative indications for segmental resection. Based on our experience,8 a nodule ≥ 3 cm and infiltration of the tunica muscularis of the sigmoid colon/rectum ≥ 7 mm indicate the need for segmental resection. It is impossible to achieve surgical goals in such cases through shaving or discoid resection.
Our perioperative care protocol for total laparoscopic segmental resection with transanal NOSE, described in our new paper,3 includes several components: antibiotic therapy (metronidazole 500 mg 12 hours before surgery; cefazolin 2 g plus metronidazole 500 mg 1 hour before skin incision; and cefazolin 1 g plus metronidazole 500 mg 12 hours after surgery); good intraoperative bowel preparation (mechanical with oral polyethylene glycol solution preoperatively, and rectal irrigation with a mixed solution of povidine-iodine and normal saline intraoperatively); and venous thromboembolism prophylaxis postoperatively for 28 days.
Moving Forward, A Word On Classification
Multicenter trials and research with longer follow-up times will be helpful in advancing the use of totally laparoscopic segmental colorectal resection for deep endometriosis. To enable better sharing of diagnostic and therapeutic results — and to advance the credibility of research — my hope is that the field will reach further agreement on endometriosis classification systems. This would also help with the development of standards for postgraduate education.
We have made significant strides in recognizing imaging as a standard for diagnosis and treatment, and I believe we currently have two very good endometriosis classification systems — the #Enzian classification system issued in 2021 and the AAGL 2021 Endometriosis Classification — that can be used in combination with imaging to reliably and consistently describe deep endometriosis.
Mario Malzoni, MD, is scientific director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. He reported having no disclosures relevant to this Master Class.
References
1. Malzoni M et al. Surgical principles of segmental rectosigmoid resection and reanastomosis for deep infiltrating endometriosis. J Minim Invasive Gynecol. 2020;27(2):258.
2. Malzoni M et al. Totally laparoscopic resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the rectum. J Minim Invasive Gynecol. 2022;29(1):19.
3. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024. (in press). 4. Brincat SD et al. Natural orifice versus transabdominal specimen extraction in laparoscopic surgery for colorectal cancer: meta-analysis. BJS Open 2022;6(3):zrac074.
5. Zhou Z et al. Laparoscopic natural orifice specimen extraction surgery versus conventional surgery in colorectal cancer: A meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2022 Jan 18;2022:6661651.
6. Malzoni M et al. Simultaneous total laparoscopic double segmental resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the ileocaecal valve and rectum—A video vignette. Colorectal Dis 2024 Jul 25.
7. Condous G et al. Noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An international consensus. J. Minim Invasive Gynecol. 2024;31(7):557-73.
8. Malzoni M et al. Preoperative ultrasound indications determine excision technique for bowel surgery for deep infiltrating endometriosis: A single, high-volume center. J Minim Invasive Gynecol. 2020;27(5):1141-7.
What to Know About NOSE
Traditionally, a laparoscopic approach to bowel resection secondary to endometriosis means enlargement of the port site or more commonly, a Pfannenstiel incision to introduce an anvil. Both incisions raise the risk of postoperative complications including pain and incisional hernia. Moreover, recovery time is prolonged.
Natural orifice specimen extraction (NOSE) makes use of a natural orifice, the vagina or anus, to extract the specimen and introduce the anvil. Transanal extraction for the management of endometriosis-related bowel disease was first described by the late David Redwine, MD, in his 1996 publication in Fertility and Sterility.1
The concern with use of vaginal NOSE surgery is rectovaginal fistula secondary to two incision lines in close opposition. Both transvaginal NOSE and transanal NOSE have been criticized for longer dissection of the mesorectum and thus longer resected specimen. Despite these concerns, over the past 10 years, articles have been published showing not only the feasibility of the NOSE technique, but excellent comparative outcomes, as well.2-4
A recent systematic review and meta-analysis by Kar et al. comparing NOSE extraction to minilaparotomy in bowel resection due to endometriosis revealed no significant difference in complication rate, but significantly shorter operation duration, reduced length of stay, and less blood loss in the NOSE surgical arm.5
The most recent study to be published in the Journal of Minimally Invasive Gynecology involving total laparoscopic segmental resection with transanal NOSE for the treatment of colorectal endometriosis was written by the guest author of this edition of the Master Class in Gynecologic Surgery, Professor Mario Malzoni, Scientific Director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. In the JMIG article, and in this Master Class, Professor Malzoni, a world-renowned endometriosis specialist recognized for his extraordinary skill and multi-organ surgical work, discusses the difference between the standard bowel resection technique with that of NOSE, and reports on his experience with NOSE.6
It is a pleasure and honor to welcome my friend and colleague, Professor Mario Malzoni, to this edition of the Master Class in Gynecologic Surgery.
Dr. Charles E. Miller is Professor, Obstetrics & Gynecology, Department of Clinical Sciences, Rosalind Franklin University of Medicine and Science, North Chicago, and Director, Minimally Invasive Gynecologic Surgery, Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at [email protected].
References
1. Redwine DB et al. Laparoscopically assisted transvaginal segmental resection of the rectosigmoid colon for endometriosis. Fertil Steril. 1996 Jan;65(1):193-7.
2. Akladios C et al. Totally laparoscopic intracorporeal anastomosis with natural orifice specimen extraction (NOSE) techniques, particularly suitable for bowel endometriosis. J Minim Invasive Gynecol. 2014 Nov-Dec;21(6):1095-102.
3. Bokor A et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018 Sep-Oct;25(6):1065-74.
4. Grigoriadis G et al. Natural orifice specimen extraction colorectal resection for deep endometriosis: A 50 case series. J Minim Invasive Gynecol. 2022 Sep;29(9):1054-62.
5. Kar E et al. Natural orifice specimen extraction as a promising alternative for minilaparotomy in bowel resection due to endometriosis: A systematic review and meta-analysis. J Minim Invasive Gynecol. 2024 Jul;31(7):574-83.e1.
6. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024 Oct;18:S1553-4650(24)01453-5.
Laparoscopic Resection With Transanal NOSE for Deep Colorectal Endometriosis
When segmental sigmoid colon/rectal resection is indicated for treatment of deep infiltrating bowel endometriosis, a totally laparoscopic resection with intracorporeal anastomosis and transanal natural orifice specimen extraction (NOSE) can be safe, effective, and advantageous compared with an abdominal mini-laparotomy for extracorporeal anastomosis and specimen retrieval.
With advanced laparoscopic surgical skills, accurate preoperative ultrasound evaluation, and a high-quality perioperative care protocol (including high-quality bowel preparation), segmental resection with NOSE can offer better preservation of vascularization, smaller resection sizes, lower rates of postoperative pain, a shorter time for gas passage after surgery, decreased hospital stays, and lower rates of wound infection and incisional hernia.
We have seen such improved results in our high-volume center since shifting in April 2021 from our classical technique for segmental rectosigmoid resection1 to a totally laparoscopic approach with NOSE. We first reported on this new technique in 2022 in a video article in the Journal of Minimally Invasive Gynecology,2and in October 2024, outcomes of 81 patients who underwent segmental sigmoid colon/rectal resection with intracorporeal anastomosis and transanal NOSE at our institution from April 2021 through March 2024 were reported in Journal of Minimally Invasive Gynecology.3Given our experience with this approach for deep endometriosis, I am convinced that NOSE should be preferred to transabdominal specimen extraction for segmental rectosigmoid resection. This is where the future lies for deep infiltrating endometriosis. Here, I share our outcomes and describe our technique.
Our Prior Approach With A Mini-Laparotomy
Our traditional laparoscopic approach for segmental colon/rectal resection of deep infiltrating endometriosis involved an abdominal mini-laparotomy (no more than 4 cm in length) for specimen retrieval, resection of the proximal rectum, and positioning of the head of a circular stapler for end-to-end anastomosis. (Connection of the two parts of the circular stapler for anastomosis was achieved via laparoscopy.)1
Several factors allowed us to minimize potential complications, including standardization of our technique, the use of three-dimensional endoscopes, and indocyanine green fluorescence angiography to assess the perfusion of the bowel after the completion of anastomosis. Still, an unpublished analysis of 1050 segmental bowel resections performed over more than 17 years using our traditional technique showed that hospital stays averaged 8 days, time to first defecation averaged 7 days, and time to first passage of flatus averaged 1 day.
Per the Clavien-Dindo classification system for surgical complications that was used at the time, 1.9% of these 1050 patients had grade 1 (minor) or grade 2 complications and 6% had grade 3 complications. Grade 2 complications were defined as needing intervention or a hospital stay more than twice the median for the procedure. Grade 3 complications were defined as leading to lasting disability or organ resection.
In the meantime, in colorectal cancer surgery, published studies on NOSE versus conventional laparoscopy with transabdominal specimen extraction have consistently pointed to the benefits of NOSE. A 2022 review 4 of 19 studies involving over 3400 patients and a 2022 meta-analysis 5 of 21 randomized controlled trials involving more than 2000 patients both showed significantly reduced postoperative morbidity and no differences in oncologic outcomes. Among the notable differences in the 2022 meta-analysis was a relative risk of postoperative infection of 0.34 for patients treated with NOSE compared with conventional laparoscopic techniques.
Among patients with deep rectal endometriosis, reported experience with intracorporeal anastomosis and NOSE for segmental resection is limited, with a few studies published between 2014 and 2022. Excluding anecdotal reports, only about 140 patients have been reported in the literature as having intracorporeal anastomosis and transanal NOSE for deep infiltrating bowel endometriosis. 3
Our Outcomes With A Totally Laparoscopic Approach
Our approach to evaluating our experience with totally laparoscopic resection with transanal NOSE has been to systematically collect data from consecutive patients and to analyze 1) complications of the technique, 2) conversion to the traditional technique/open surgery, and 3) endometriosis-free bowel resection margins and recurrence. Secondarily, we look at intraoperative blood loss, operative time, recovery of gastrointestinal function, hospital stay length, and reproductive outcomes.
Across 81 patients from our TrEnd study database (Surgical TReatment of women with deep ENDometriosis infiltrating the sigmoid colon and/or rectum) who received a totally laparoscopic standardized procedure involving intracorporeal anastomosis and transanal NOSE, we had no conversions even to mini-laparotomy, and no cases of protective colostomy or ileostomy.
Complete endometriosis removal was achieved in 100% of cases, with final pathology showing endometriosis-free resection margins, and patients remained free of bowel endometriosis at a median follow-up of 21 months.3Our analysis also shows improved gastrointestinal function recovery (median time to first defecation of 4 days, compared to 7 days previously, with the same 1-day median time to first passage of flatus) and a significantly improved hospital length of stay to a median of 3 days (interquartile range, 3-4.5 days); the latter reflects in part the absence of infection.3There were no intra-operative complications. Postoperative Clavien-Dindo grade 3 complications occurred in three patients (3.7%), two of whom required reoperation (one for hemoperitoneum and one for ureteral injury) and one who experienced rectal anastomotic bleeding on postoperative day 1. (The two requiring reoperation had significant right lateral parametrial involvement and underwent nerve-sparing parametrectomy at the time of bowel resection).3Notably, 7 of the 81 patients had totally laparoscopic double segmental colorectal resection — 6 with simultaneous ileocecal valve and rectal resection, and 1 with simultaneous ileal and rectal resection. None of these seven patients had postoperative complications. (A report on the six surgeries for deep endometriosis infiltrating the ileocecal valve and rectum was also published this year in the journal Colorectal Disease.6)
Median blood loss in the 81-patient cohort was 20 mL (IQR, 20-30 mL), and the median length of surgery was 160 minutes (IQR, 130-210 minutes).
Bowel endometriotic nodules had a mean maximum diameter of 4.3 cm (3.5-5.5 cm), a mean depth of invasion of 9 mm (7-9 mm), a mean estimated stenosis of 40% (35%-50%), and a mean distance from the anal verge of 13 cm (10-15 cm).3 (The cohort in this analysis did not include patients who received concomitant hysterectomy and transvaginal NOSE.)
Specifics of the New Technique, Including Totally Intracorporeal Anastomosis
As in our previous technique, the surgical procedure starts with left and dorsal rectal mobilization. The left pelvic sidewall dissection begins with incision of the parietal peritoneum along the pelvic portions of the psoas muscle. The retroperitoneal connective tissue is dissected in order to identify the ureter, the hypogastric nerve, and the presacral parietal pelvic fascia (PPPF). Dissection is carried downward in front of the PPPF and medially to the left hypogastric nerve.
Right and ventral rectal mobilization begins with incision of the peritoneum along the gray avascular line, which is seen with upward retraction of the rectum.
Again, for preservation of bowel and bladder function, the hypogastric nerve must be identified and preserved through dissection that displaces the nerve dorsally and laterally.
Dissection proceeds to sharply divide the nodule into two parts (rectal and rectocervical components). The caudal boundary of ventral dissection is reached by incising the septum and opening the rectovaginal space. The lymphovascular fatty tissue surrounding the rectum is then excised and the rectal tube is denuded at the level of the distal resection margin.
The distal and proximal resection margins are prepared and transected using a tissue-sealing device, and the resected rectal specimen is placed into a retrieval bag and pulled out through the anus (Figure 1).

The totally intracorporeal side-to-end anastomosis is performed using a traditional circular stapler. Its anvil is inserted through the proximal resection line and exteriorized forward on the antimesenteric side of the sigmoid colon/rectum. The proximal and distal resection lines are closed with a 60-mm linear endo-stapler, and finally, the shaft of the circular stapler is inserted into the rectum, and the two parts of the stapler are joined. The stapler is then closed and fired (Figure 2).

An air leak test is performed to check the quality of the rectal sutures. However, regardless of the results, we routinely apply interrupted stitches to the so-called “dog ears” in order to reduce tension along the anastomotic line.
We also evaluate the microvascularization of the two parts of the bowel at the level of the reanastomosis using indocyanine green fluorescence angiography. Doing so allows us to avoid complications caused by anastomotic leakage.
Patient Selection And Peri-Surgical Care
The decision to perform segmental resection as opposed to other more conservative laparoscopic excision techniques is made after skilled preoperative imaging reveals the number of lesions, the largest nodule diameter, the infiltration depth, and the circumference of bowel involvement.
A recently published international consensus statement7 on noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems concludes there is Level 1a evidence that preoperative imaging with transvaginal ultrasound (TVUS) can predict with good precision the size and degree of infiltration of deep endometriosis of the rectum (a Grade A statement, per the Oxford Centre for Evidence-Based Medicine levels of evidence).
The consensus document was published across seven different journals by international and European societies, and the American Association of Gynecologic Laparoscopists (AAGL), each of which contributed to a multidisciplinary panel of gynecological surgeons, sonographers, and radiologists.
At our center, we achieve precise preoperative evaluations with TVUS (other centers also employ MRI) and have long set clear preoperative indications for segmental resection. Based on our experience,8 a nodule ≥ 3 cm and infiltration of the tunica muscularis of the sigmoid colon/rectum ≥ 7 mm indicate the need for segmental resection. It is impossible to achieve surgical goals in such cases through shaving or discoid resection.
Our perioperative care protocol for total laparoscopic segmental resection with transanal NOSE, described in our new paper,3 includes several components: antibiotic therapy (metronidazole 500 mg 12 hours before surgery; cefazolin 2 g plus metronidazole 500 mg 1 hour before skin incision; and cefazolin 1 g plus metronidazole 500 mg 12 hours after surgery); good intraoperative bowel preparation (mechanical with oral polyethylene glycol solution preoperatively, and rectal irrigation with a mixed solution of povidine-iodine and normal saline intraoperatively); and venous thromboembolism prophylaxis postoperatively for 28 days.
Moving Forward, A Word On Classification
Multicenter trials and research with longer follow-up times will be helpful in advancing the use of totally laparoscopic segmental colorectal resection for deep endometriosis. To enable better sharing of diagnostic and therapeutic results — and to advance the credibility of research — my hope is that the field will reach further agreement on endometriosis classification systems. This would also help with the development of standards for postgraduate education.
We have made significant strides in recognizing imaging as a standard for diagnosis and treatment, and I believe we currently have two very good endometriosis classification systems — the #Enzian classification system issued in 2021 and the AAGL 2021 Endometriosis Classification — that can be used in combination with imaging to reliably and consistently describe deep endometriosis.
Mario Malzoni, MD, is scientific director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. He reported having no disclosures relevant to this Master Class.
References
1. Malzoni M et al. Surgical principles of segmental rectosigmoid resection and reanastomosis for deep infiltrating endometriosis. J Minim Invasive Gynecol. 2020;27(2):258.
2. Malzoni M et al. Totally laparoscopic resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the rectum. J Minim Invasive Gynecol. 2022;29(1):19.
3. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024. (in press). 4. Brincat SD et al. Natural orifice versus transabdominal specimen extraction in laparoscopic surgery for colorectal cancer: meta-analysis. BJS Open 2022;6(3):zrac074.
5. Zhou Z et al. Laparoscopic natural orifice specimen extraction surgery versus conventional surgery in colorectal cancer: A meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2022 Jan 18;2022:6661651.
6. Malzoni M et al. Simultaneous total laparoscopic double segmental resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the ileocaecal valve and rectum—A video vignette. Colorectal Dis 2024 Jul 25.
7. Condous G et al. Noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An international consensus. J. Minim Invasive Gynecol. 2024;31(7):557-73.
8. Malzoni M et al. Preoperative ultrasound indications determine excision technique for bowel surgery for deep infiltrating endometriosis: A single, high-volume center. J Minim Invasive Gynecol. 2020;27(5):1141-7.
What to Know About NOSE
Traditionally, a laparoscopic approach to bowel resection secondary to endometriosis means enlargement of the port site or more commonly, a Pfannenstiel incision to introduce an anvil. Both incisions raise the risk of postoperative complications including pain and incisional hernia. Moreover, recovery time is prolonged.
Natural orifice specimen extraction (NOSE) makes use of a natural orifice, the vagina or anus, to extract the specimen and introduce the anvil. Transanal extraction for the management of endometriosis-related bowel disease was first described by the late David Redwine, MD, in his 1996 publication in Fertility and Sterility.1
The concern with use of vaginal NOSE surgery is rectovaginal fistula secondary to two incision lines in close opposition. Both transvaginal NOSE and transanal NOSE have been criticized for longer dissection of the mesorectum and thus longer resected specimen. Despite these concerns, over the past 10 years, articles have been published showing not only the feasibility of the NOSE technique, but excellent comparative outcomes, as well.2-4
A recent systematic review and meta-analysis by Kar et al. comparing NOSE extraction to minilaparotomy in bowel resection due to endometriosis revealed no significant difference in complication rate, but significantly shorter operation duration, reduced length of stay, and less blood loss in the NOSE surgical arm.5
The most recent study to be published in the Journal of Minimally Invasive Gynecology involving total laparoscopic segmental resection with transanal NOSE for the treatment of colorectal endometriosis was written by the guest author of this edition of the Master Class in Gynecologic Surgery, Professor Mario Malzoni, Scientific Director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. In the JMIG article, and in this Master Class, Professor Malzoni, a world-renowned endometriosis specialist recognized for his extraordinary skill and multi-organ surgical work, discusses the difference between the standard bowel resection technique with that of NOSE, and reports on his experience with NOSE.6
It is a pleasure and honor to welcome my friend and colleague, Professor Mario Malzoni, to this edition of the Master Class in Gynecologic Surgery.
Dr. Charles E. Miller is Professor, Obstetrics & Gynecology, Department of Clinical Sciences, Rosalind Franklin University of Medicine and Science, North Chicago, and Director, Minimally Invasive Gynecologic Surgery, Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at [email protected].
References
1. Redwine DB et al. Laparoscopically assisted transvaginal segmental resection of the rectosigmoid colon for endometriosis. Fertil Steril. 1996 Jan;65(1):193-7.
2. Akladios C et al. Totally laparoscopic intracorporeal anastomosis with natural orifice specimen extraction (NOSE) techniques, particularly suitable for bowel endometriosis. J Minim Invasive Gynecol. 2014 Nov-Dec;21(6):1095-102.
3. Bokor A et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018 Sep-Oct;25(6):1065-74.
4. Grigoriadis G et al. Natural orifice specimen extraction colorectal resection for deep endometriosis: A 50 case series. J Minim Invasive Gynecol. 2022 Sep;29(9):1054-62.
5. Kar E et al. Natural orifice specimen extraction as a promising alternative for minilaparotomy in bowel resection due to endometriosis: A systematic review and meta-analysis. J Minim Invasive Gynecol. 2024 Jul;31(7):574-83.e1.
6. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024 Oct;18:S1553-4650(24)01453-5.
Laparoscopic Resection With Transanal NOSE for Deep Colorectal Endometriosis
When segmental sigmoid colon/rectal resection is indicated for treatment of deep infiltrating bowel endometriosis, a totally laparoscopic resection with intracorporeal anastomosis and transanal natural orifice specimen extraction (NOSE) can be safe, effective, and advantageous compared with an abdominal mini-laparotomy for extracorporeal anastomosis and specimen retrieval.
With advanced laparoscopic surgical skills, accurate preoperative ultrasound evaluation, and a high-quality perioperative care protocol (including high-quality bowel preparation), segmental resection with NOSE can offer better preservation of vascularization, smaller resection sizes, lower rates of postoperative pain, a shorter time for gas passage after surgery, decreased hospital stays, and lower rates of wound infection and incisional hernia.
We have seen such improved results in our high-volume center since shifting in April 2021 from our classical technique for segmental rectosigmoid resection1 to a totally laparoscopic approach with NOSE. We first reported on this new technique in 2022 in a video article in the Journal of Minimally Invasive Gynecology,2and in October 2024, outcomes of 81 patients who underwent segmental sigmoid colon/rectal resection with intracorporeal anastomosis and transanal NOSE at our institution from April 2021 through March 2024 were reported in Journal of Minimally Invasive Gynecology.3Given our experience with this approach for deep endometriosis, I am convinced that NOSE should be preferred to transabdominal specimen extraction for segmental rectosigmoid resection. This is where the future lies for deep infiltrating endometriosis. Here, I share our outcomes and describe our technique.
Our Prior Approach With A Mini-Laparotomy
Our traditional laparoscopic approach for segmental colon/rectal resection of deep infiltrating endometriosis involved an abdominal mini-laparotomy (no more than 4 cm in length) for specimen retrieval, resection of the proximal rectum, and positioning of the head of a circular stapler for end-to-end anastomosis. (Connection of the two parts of the circular stapler for anastomosis was achieved via laparoscopy.)1
Several factors allowed us to minimize potential complications, including standardization of our technique, the use of three-dimensional endoscopes, and indocyanine green fluorescence angiography to assess the perfusion of the bowel after the completion of anastomosis. Still, an unpublished analysis of 1050 segmental bowel resections performed over more than 17 years using our traditional technique showed that hospital stays averaged 8 days, time to first defecation averaged 7 days, and time to first passage of flatus averaged 1 day.
Per the Clavien-Dindo classification system for surgical complications that was used at the time, 1.9% of these 1050 patients had grade 1 (minor) or grade 2 complications and 6% had grade 3 complications. Grade 2 complications were defined as needing intervention or a hospital stay more than twice the median for the procedure. Grade 3 complications were defined as leading to lasting disability or organ resection.
In the meantime, in colorectal cancer surgery, published studies on NOSE versus conventional laparoscopy with transabdominal specimen extraction have consistently pointed to the benefits of NOSE. A 2022 review 4 of 19 studies involving over 3400 patients and a 2022 meta-analysis 5 of 21 randomized controlled trials involving more than 2000 patients both showed significantly reduced postoperative morbidity and no differences in oncologic outcomes. Among the notable differences in the 2022 meta-analysis was a relative risk of postoperative infection of 0.34 for patients treated with NOSE compared with conventional laparoscopic techniques.
Among patients with deep rectal endometriosis, reported experience with intracorporeal anastomosis and NOSE for segmental resection is limited, with a few studies published between 2014 and 2022. Excluding anecdotal reports, only about 140 patients have been reported in the literature as having intracorporeal anastomosis and transanal NOSE for deep infiltrating bowel endometriosis. 3
Our Outcomes With A Totally Laparoscopic Approach
Our approach to evaluating our experience with totally laparoscopic resection with transanal NOSE has been to systematically collect data from consecutive patients and to analyze 1) complications of the technique, 2) conversion to the traditional technique/open surgery, and 3) endometriosis-free bowel resection margins and recurrence. Secondarily, we look at intraoperative blood loss, operative time, recovery of gastrointestinal function, hospital stay length, and reproductive outcomes.
Across 81 patients from our TrEnd study database (Surgical TReatment of women with deep ENDometriosis infiltrating the sigmoid colon and/or rectum) who received a totally laparoscopic standardized procedure involving intracorporeal anastomosis and transanal NOSE, we had no conversions even to mini-laparotomy, and no cases of protective colostomy or ileostomy.
Complete endometriosis removal was achieved in 100% of cases, with final pathology showing endometriosis-free resection margins, and patients remained free of bowel endometriosis at a median follow-up of 21 months.3Our analysis also shows improved gastrointestinal function recovery (median time to first defecation of 4 days, compared to 7 days previously, with the same 1-day median time to first passage of flatus) and a significantly improved hospital length of stay to a median of 3 days (interquartile range, 3-4.5 days); the latter reflects in part the absence of infection.3There were no intra-operative complications. Postoperative Clavien-Dindo grade 3 complications occurred in three patients (3.7%), two of whom required reoperation (one for hemoperitoneum and one for ureteral injury) and one who experienced rectal anastomotic bleeding on postoperative day 1. (The two requiring reoperation had significant right lateral parametrial involvement and underwent nerve-sparing parametrectomy at the time of bowel resection).3Notably, 7 of the 81 patients had totally laparoscopic double segmental colorectal resection — 6 with simultaneous ileocecal valve and rectal resection, and 1 with simultaneous ileal and rectal resection. None of these seven patients had postoperative complications. (A report on the six surgeries for deep endometriosis infiltrating the ileocecal valve and rectum was also published this year in the journal Colorectal Disease.6)
Median blood loss in the 81-patient cohort was 20 mL (IQR, 20-30 mL), and the median length of surgery was 160 minutes (IQR, 130-210 minutes).
Bowel endometriotic nodules had a mean maximum diameter of 4.3 cm (3.5-5.5 cm), a mean depth of invasion of 9 mm (7-9 mm), a mean estimated stenosis of 40% (35%-50%), and a mean distance from the anal verge of 13 cm (10-15 cm).3 (The cohort in this analysis did not include patients who received concomitant hysterectomy and transvaginal NOSE.)
Specifics of the New Technique, Including Totally Intracorporeal Anastomosis
As in our previous technique, the surgical procedure starts with left and dorsal rectal mobilization. The left pelvic sidewall dissection begins with incision of the parietal peritoneum along the pelvic portions of the psoas muscle. The retroperitoneal connective tissue is dissected in order to identify the ureter, the hypogastric nerve, and the presacral parietal pelvic fascia (PPPF). Dissection is carried downward in front of the PPPF and medially to the left hypogastric nerve.
Right and ventral rectal mobilization begins with incision of the peritoneum along the gray avascular line, which is seen with upward retraction of the rectum.
Again, for preservation of bowel and bladder function, the hypogastric nerve must be identified and preserved through dissection that displaces the nerve dorsally and laterally.
Dissection proceeds to sharply divide the nodule into two parts (rectal and rectocervical components). The caudal boundary of ventral dissection is reached by incising the septum and opening the rectovaginal space. The lymphovascular fatty tissue surrounding the rectum is then excised and the rectal tube is denuded at the level of the distal resection margin.
The distal and proximal resection margins are prepared and transected using a tissue-sealing device, and the resected rectal specimen is placed into a retrieval bag and pulled out through the anus (Figure 1).

The totally intracorporeal side-to-end anastomosis is performed using a traditional circular stapler. Its anvil is inserted through the proximal resection line and exteriorized forward on the antimesenteric side of the sigmoid colon/rectum. The proximal and distal resection lines are closed with a 60-mm linear endo-stapler, and finally, the shaft of the circular stapler is inserted into the rectum, and the two parts of the stapler are joined. The stapler is then closed and fired (Figure 2).

An air leak test is performed to check the quality of the rectal sutures. However, regardless of the results, we routinely apply interrupted stitches to the so-called “dog ears” in order to reduce tension along the anastomotic line.
We also evaluate the microvascularization of the two parts of the bowel at the level of the reanastomosis using indocyanine green fluorescence angiography. Doing so allows us to avoid complications caused by anastomotic leakage.
Patient Selection And Peri-Surgical Care
The decision to perform segmental resection as opposed to other more conservative laparoscopic excision techniques is made after skilled preoperative imaging reveals the number of lesions, the largest nodule diameter, the infiltration depth, and the circumference of bowel involvement.
A recently published international consensus statement7 on noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems concludes there is Level 1a evidence that preoperative imaging with transvaginal ultrasound (TVUS) can predict with good precision the size and degree of infiltration of deep endometriosis of the rectum (a Grade A statement, per the Oxford Centre for Evidence-Based Medicine levels of evidence).
The consensus document was published across seven different journals by international and European societies, and the American Association of Gynecologic Laparoscopists (AAGL), each of which contributed to a multidisciplinary panel of gynecological surgeons, sonographers, and radiologists.
At our center, we achieve precise preoperative evaluations with TVUS (other centers also employ MRI) and have long set clear preoperative indications for segmental resection. Based on our experience,8 a nodule ≥ 3 cm and infiltration of the tunica muscularis of the sigmoid colon/rectum ≥ 7 mm indicate the need for segmental resection. It is impossible to achieve surgical goals in such cases through shaving or discoid resection.
Our perioperative care protocol for total laparoscopic segmental resection with transanal NOSE, described in our new paper,3 includes several components: antibiotic therapy (metronidazole 500 mg 12 hours before surgery; cefazolin 2 g plus metronidazole 500 mg 1 hour before skin incision; and cefazolin 1 g plus metronidazole 500 mg 12 hours after surgery); good intraoperative bowel preparation (mechanical with oral polyethylene glycol solution preoperatively, and rectal irrigation with a mixed solution of povidine-iodine and normal saline intraoperatively); and venous thromboembolism prophylaxis postoperatively for 28 days.
Moving Forward, A Word On Classification
Multicenter trials and research with longer follow-up times will be helpful in advancing the use of totally laparoscopic segmental colorectal resection for deep endometriosis. To enable better sharing of diagnostic and therapeutic results — and to advance the credibility of research — my hope is that the field will reach further agreement on endometriosis classification systems. This would also help with the development of standards for postgraduate education.
We have made significant strides in recognizing imaging as a standard for diagnosis and treatment, and I believe we currently have two very good endometriosis classification systems — the #Enzian classification system issued in 2021 and the AAGL 2021 Endometriosis Classification — that can be used in combination with imaging to reliably and consistently describe deep endometriosis.
Mario Malzoni, MD, is scientific director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. He reported having no disclosures relevant to this Master Class.
References
1. Malzoni M et al. Surgical principles of segmental rectosigmoid resection and reanastomosis for deep infiltrating endometriosis. J Minim Invasive Gynecol. 2020;27(2):258.
2. Malzoni M et al. Totally laparoscopic resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the rectum. J Minim Invasive Gynecol. 2022;29(1):19.
3. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024. (in press). 4. Brincat SD et al. Natural orifice versus transabdominal specimen extraction in laparoscopic surgery for colorectal cancer: meta-analysis. BJS Open 2022;6(3):zrac074.
5. Zhou Z et al. Laparoscopic natural orifice specimen extraction surgery versus conventional surgery in colorectal cancer: A meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2022 Jan 18;2022:6661651.
6. Malzoni M et al. Simultaneous total laparoscopic double segmental resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the ileocaecal valve and rectum—A video vignette. Colorectal Dis 2024 Jul 25.
7. Condous G et al. Noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An international consensus. J. Minim Invasive Gynecol. 2024;31(7):557-73.
8. Malzoni M et al. Preoperative ultrasound indications determine excision technique for bowel surgery for deep infiltrating endometriosis: A single, high-volume center. J Minim Invasive Gynecol. 2020;27(5):1141-7.
Maternal Immunization to Prevent Serious Respiratory Illness
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Managing Cancer in Pregnancy: Improvements and Considerations
Introduction: Tremendous Progress on Cancer Extends to Cancer in Pregnancy
The biomedical research enterprise that took shape in the United States after World War II has had numerous positive effects, including significant progress made during the past 75-plus years in the diagnosis, prevention, and treatment of cancer.
President Franklin D. Roosevelt’s 1944 request of Dr. Vannevar Bush, director of the then Office of Scientific Research and Development, to organize a program that would advance and apply scientific knowledge for times of peace — just as it been advanced and applied in times of war — culminated in a historic report, Science – The Endless Frontier. Presented in 1945 to President Harry S. Truman, this report helped fuel decades of broad, bold, and coordinated government-sponsored biomedical research aimed at addressing disease and improving the health of the American people (National Science Foundation, 1945).
Discoveries made from research in basic and translational sciences deepened our knowledge of the cellular and molecular underpinnings of cancer, leading to advances in chemotherapy, radiotherapy, and other treatment approaches as well as continual refinements in their application. Similarly, our diagnostic armamentarium has significantly improved.
As a result, we have reduced both the incidence and mortality of cancer. Today, some cancers can be prevented. Others can be reversed or put in remission. Granted, progress has been variable, with some cancers such as ovarian cancer still having relatively low survival rates. Much more needs to be done. Overall, however, the positive effects of the U.S. biomedical research enterprise on cancer are evident. According to the National Cancer Institute’s most recent report on the status of cancer, death rates from cancer fell 1.9% per year on average in females from 2015 to 2019 (Cancer. 2022 Oct 22. doi: 10.1002/cncr.34479).
It is not only patients whose cancer occurs outside of pregnancy who have benefited. When treatment is appropriately selected and timing considerations are made, patients whose cancer is diagnosed during pregnancy — and their children — can have good outcomes.
To explain how the management of cancer in pregnancy has improved, we have invited Gautam G. Rao, MD, gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to write this installment of the Master Class in Obstetrics. As Dr. Rao explains, radiation is not as dangerous to the fetus as once thought, and the safety of many chemotherapeutic regimens in pregnancy has been documented. Obstetricians can and should counsel patients, he explains, about the likelihood of good maternal and fetal outcomes.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Managing Cancer in Pregnancy
Cancer can cause fear and distress for any patient, but when cancer is diagnosed during pregnancy, an expectant mother fears not only for her own health but for the health of her unborn child. Fortunately, ob.gyn.s and multidisciplinary teams have good reason to reassure patients about the likelihood of good outcomes.
Cancer treatment in pregnancy has improved with advancements in imaging and chemotherapy, and while maternal and fetal outcomes of prenatal cancer treatment are not well reported, evidence acquired in recent years from case series and retrospective studies shows that most imaging studies and procedural diagnostic tests – and many treatments – can be performed safely in pregnancy.
Decades ago, we avoided CT scans during pregnancy because of concerns about radiation exposure to the fetus, leaving some patients without an accurate staging of their cancer. Today, we have evidence that a CT scan is generally safe in pregnancy. Similarly, the safety of many chemotherapeutic regimens in pregnancy has been documented in recent decades,and the use of chemotherapy during pregnancy has increased progressively. Radiation is also commonly utilized in the management of cancers that may occur during pregnancy, such as breast cancer.1
Considerations of timing are often central to decision-making; chemotherapy and radiotherapy are generally avoided in the first trimester to prevent structural fetal anomalies, for instance, and delaying cancer treatment is often warranted when the patient is a few weeks away from delivery. On occasion, iatrogenic preterm birth is considered when the risks to the mother of delaying a necessary cancer treatment outweigh the risks to the fetus of prematurity.1
Pregnancy termination is rarely indicated, however, and information gathered over the past 2 decades suggests that fetal and placental metastases are rare.1 There is broad agreement that prenatal treatment of cancer in pregnancy should adhere as much as possible to protocols and guidelines for nonpregnant patients and that treatment delays driven by fear of fetal anomalies and miscarriage are unnecessary.
Cancer Incidence, Use of Diagnostic Imaging
Data on the incidence of cancer in pregnancy comes from population-based cancer registries, and unfortunately, these data are not standardized and are often incomplete. Many studies include cancer diagnosed up to 1 year after pregnancy, and some include preinvasive disease. Estimates therefore vary considerably (see Table 1 for a sampling of estimates incidences.)
It has been reported, and often cited in the literature, that invasive malignancy complicates one in 1,000 pregnancies and that the incidence of cancer in pregnancy (invasive and noninvasive malignancies) has been rising over time.8 Increasing maternal age is believed to be playing a role in this rise; as women delay childbearing, they enter the age range in which some cancers become more common. Additionally, improvements in screening and diagnostics have led to earlier cancer detection. The incidence of ovarian neoplasms found during pregnancy has increased, for instance, with the routine use of diagnostic ultrasound in pregnancy.1
Among the studies showing an increased incidence of pregnancy-associated cancer is a population-based study in Australia, which found that from 1994 to 2007 the crude incidence of pregnancy-associated cancer increased from 112.3 to 191.5 per 100,000 pregnancies (P < .001).9 A cohort study in the United States documented an increase in incidence from 75.0 per 100,000 pregnancies in 2002 to 138.5 per 100,000 pregnancies in 2012.10
Overall, the literature shows us that the skin, cervix, and breast are also common sites for malignancy during pregnancy.1 According to a 2022 review, breast cancer during pregnancy is less often hormone receptor–positive and more frequently triple negative compared with age-matched controls.11 The frequencies of other pregnancy-associated cancers appear overall to be similar to that of cancer occurring in all women across their reproductive years.1
Too often, diagnosis is delayed because cancer symptoms can be masked by or can mimic normal physiological changes in pregnancy. For instance, breast cancer can be difficult to diagnose during pregnancy and lactation due to anatomic changes in the breast parenchyma. Several studies published in the 1990s showed that breast cancer presents at a more advanced stage in pregnant patients than in nonpregnant patients because of this delay.1 Skin changes suggestive of melanoma can be attributed to hyperpigmentation of pregnancy, for instance. Several observational studies have suggested that thicker melanomas found in pregnancy may be because of delayed diagnosis.8
It is important that we thoroughly investigate signs and symptoms suggestive of a malignancy and not automatically attribute these symptoms to the pregnancy itself. Cervical biopsy of a mass or lesion suspicious for cervical cancer can be done safely during pregnancy and should not be delayed or deferred.
Fetal radiation exposure from radiologic examinations has long been a concern, but we know today that while the imaging modality should be chosen to minimize fetal radiation exposure, CT scans and even PET scans should be performed if these exams are deemed best for evaluation. Embryonic exposure to a dose of less than 50 mGy is rarely if at all associated with fetal malformations or miscarriage and a radiation dose of 100 mGy may be considered a floor for consideration of therapeutic termination of pregnancy.1,8
CT exams are associated with a fetal dose far less than 50 mGy (see Table 2 for radiation doses).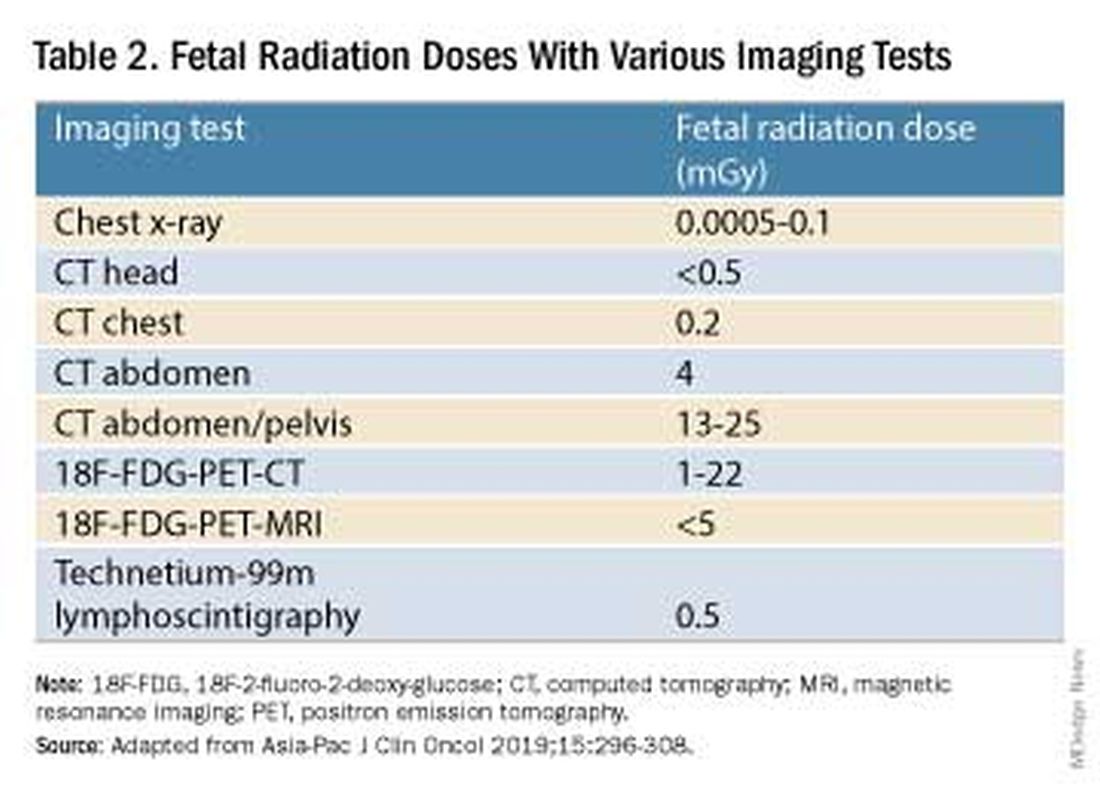
Magnetic resonance imaging with a magnet strength of 3 Tesla or less in any trimester is not associated with an increased risk of harm to the fetus or in early childhood, but the contrast agent gadolinium should be avoided in pregnancy as it has been associated with an increased risk of stillbirth, neonatal death, and childhood inflammatory, rheumatologic, and infiltrative skin lesions.1,8,12
Chemotherapy, Surgery, and Radiation in Pregnancy
The management of cancer during pregnancy requires a multidisciplinary team including medical, gynecologic, or radiation oncologists, and maternal-fetal medicine specialists (Figure 1). Prematurity and low birth weight are frequent complications for fetuses exposed to chemotherapy, although there is some uncertainty as to whether the treatment is causative. However, congenital anomalies no longer are a major concern, provided that drugs are appropriately selected and that fetal exposure occurs during the second or third trimester.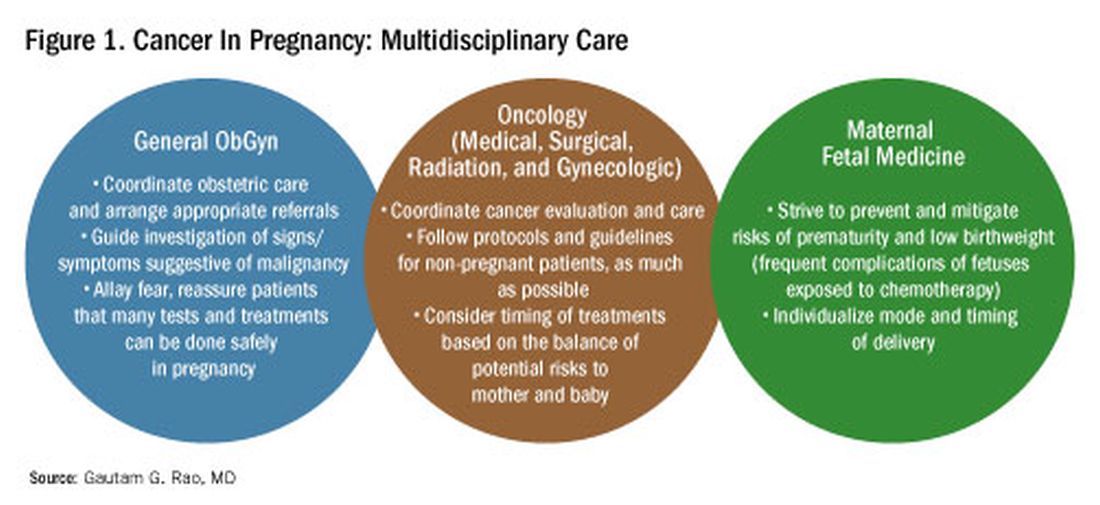
For instance, alkylating agents including cisplatin (an important drug in the management of gynecologic malignancies) have been associated with congenital anomalies in the first trimester but not in the second and third trimesters, and a variety of antimetabolites — excluding methotrexate and aminopterin — similarly have been shown to be relatively safe when used after the first trimester.1
Small studies have shown no long-term effects of chemotherapy exposure on postnatal growth and long-term neurologic/neurocognitive function,1 but this is an area that needs more research.
Also in need of investigation is the safety of newer agents in pregnancy. Data are limited on the use of new targeted treatments, monoclonal antibodies, and immunotherapies in pregnancy and their effects on the fetus, with current knowledge coming mainly from single case reports.13
Until more is learned — a challenge given that pregnant women are generally excluded from clinical trials — management teams are generally postponing use of these therapies until after delivery. Considering the pace of new developments revolutionizing cancer treatment, this topic will likely get more complex and confusing before we begin acquiring sufficient knowledge.
The timing of surgery for malignancy in pregnancy is similarly based on the balance of maternal and fetal risks, including the risk of maternal disease progression, the risk of preterm delivery, and the prevention of fetal metastases. In general, the safest time is the second trimester.
Maternal surgery in the third trimester may be associated with a risk of premature labor and altered uteroplacental perfusion. A 2005 systematic review of 12,452 women who underwent nonobstetric surgery during pregnancy provides some reassurance, however; compared with the general obstetric population, there was no increase in the rate of miscarriage or major birth defects.14
Radiotherapy used to be contraindicated in pregnancy but many experts today believe it can be safely utilized provided the uterus is out of field and is protected from scattered radiation. The head, neck, and breast, for instance, can be treated with newer radiotherapies, including stereotactic ablative radiation therapy.8 Patients with advanced cervical cancer often receive chemotherapy during pregnancy to slow metastatic growth followed by definitive treatment with postpartum radiation or surgery.
More research is needed, but available data on maternal outcomes are encouraging. For instance, there appear to be no significant differences in short- and long-term complications or survival between women who are pregnant and nonpregnant when treated for invasive cervical cancer.8 Similarly, while earlier studies of breast cancer diagnosed during pregnancy suggested a poor prognosis, data now show similar prognoses for pregnant and nonpregnant patients when controlled for stage.1
Dr. Rao is a gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported no relevant disclosures.
References
1. Rao GG. Chapter 42. Clinical Obstetrics: The Fetus & Mother, 4th ed. Reece EA et al. (eds): 2021.
2. Bannister-Tyrrell M et al. Aust N Z J Obstet Gynaecol. 2014;55:116-122.
3. Oehler MK et al. Aust N Z J Obstet Gynaecol. 2003;43(6):414-420.
4. Ruiz R et al. Breast. 2017;35:136-141. doi: 10.1016/j.breast.2017.07.008.
5. Nolan S et al. Am J Obstet Gynecol. 2019;220(1):S480. doi: 10.1016/j.ajog.2018.11.752.
6. El-Messidi A et al. J Perinat Med. 2015;43(6):683-688. doi: 10.1515/jpm-2014-0133.
7. Pellino G et al. Eur J Gastroenterol Hepatol. 2017;29(7):743-753. doi: 10.1097/MEG.0000000000000863.
8. Eastwood-Wilshere N et al. Asia-Pac J Clin Oncol. 2019;15:296-308.
9. Lee YY et al. BJOG. 2012;119(13):1572-1582.
10. Cottreau CM et al. J Womens Health (Larchmt). 2019 Feb;28(2):250-257.
11. Boere I et al. Best Pract Res Clin Obstet Gynaecol. 2022;82:46-59.
12. Ray JG et al. JAMA 2016;316(9):952-961.
13. Schwab R et al. Cancers. (Basel) 2021;13(12):3048.
14. Cohen-Kerem et al. Am J Surg. 2005;190(3):467-473.
Introduction: Tremendous Progress on Cancer Extends to Cancer in Pregnancy
The biomedical research enterprise that took shape in the United States after World War II has had numerous positive effects, including significant progress made during the past 75-plus years in the diagnosis, prevention, and treatment of cancer.
President Franklin D. Roosevelt’s 1944 request of Dr. Vannevar Bush, director of the then Office of Scientific Research and Development, to organize a program that would advance and apply scientific knowledge for times of peace — just as it been advanced and applied in times of war — culminated in a historic report, Science – The Endless Frontier. Presented in 1945 to President Harry S. Truman, this report helped fuel decades of broad, bold, and coordinated government-sponsored biomedical research aimed at addressing disease and improving the health of the American people (National Science Foundation, 1945).
Discoveries made from research in basic and translational sciences deepened our knowledge of the cellular and molecular underpinnings of cancer, leading to advances in chemotherapy, radiotherapy, and other treatment approaches as well as continual refinements in their application. Similarly, our diagnostic armamentarium has significantly improved.
As a result, we have reduced both the incidence and mortality of cancer. Today, some cancers can be prevented. Others can be reversed or put in remission. Granted, progress has been variable, with some cancers such as ovarian cancer still having relatively low survival rates. Much more needs to be done. Overall, however, the positive effects of the U.S. biomedical research enterprise on cancer are evident. According to the National Cancer Institute’s most recent report on the status of cancer, death rates from cancer fell 1.9% per year on average in females from 2015 to 2019 (Cancer. 2022 Oct 22. doi: 10.1002/cncr.34479).
It is not only patients whose cancer occurs outside of pregnancy who have benefited. When treatment is appropriately selected and timing considerations are made, patients whose cancer is diagnosed during pregnancy — and their children — can have good outcomes.
To explain how the management of cancer in pregnancy has improved, we have invited Gautam G. Rao, MD, gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to write this installment of the Master Class in Obstetrics. As Dr. Rao explains, radiation is not as dangerous to the fetus as once thought, and the safety of many chemotherapeutic regimens in pregnancy has been documented. Obstetricians can and should counsel patients, he explains, about the likelihood of good maternal and fetal outcomes.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Managing Cancer in Pregnancy
Cancer can cause fear and distress for any patient, but when cancer is diagnosed during pregnancy, an expectant mother fears not only for her own health but for the health of her unborn child. Fortunately, ob.gyn.s and multidisciplinary teams have good reason to reassure patients about the likelihood of good outcomes.
Cancer treatment in pregnancy has improved with advancements in imaging and chemotherapy, and while maternal and fetal outcomes of prenatal cancer treatment are not well reported, evidence acquired in recent years from case series and retrospective studies shows that most imaging studies and procedural diagnostic tests – and many treatments – can be performed safely in pregnancy.
Decades ago, we avoided CT scans during pregnancy because of concerns about radiation exposure to the fetus, leaving some patients without an accurate staging of their cancer. Today, we have evidence that a CT scan is generally safe in pregnancy. Similarly, the safety of many chemotherapeutic regimens in pregnancy has been documented in recent decades,and the use of chemotherapy during pregnancy has increased progressively. Radiation is also commonly utilized in the management of cancers that may occur during pregnancy, such as breast cancer.1
Considerations of timing are often central to decision-making; chemotherapy and radiotherapy are generally avoided in the first trimester to prevent structural fetal anomalies, for instance, and delaying cancer treatment is often warranted when the patient is a few weeks away from delivery. On occasion, iatrogenic preterm birth is considered when the risks to the mother of delaying a necessary cancer treatment outweigh the risks to the fetus of prematurity.1
Pregnancy termination is rarely indicated, however, and information gathered over the past 2 decades suggests that fetal and placental metastases are rare.1 There is broad agreement that prenatal treatment of cancer in pregnancy should adhere as much as possible to protocols and guidelines for nonpregnant patients and that treatment delays driven by fear of fetal anomalies and miscarriage are unnecessary.
Cancer Incidence, Use of Diagnostic Imaging
Data on the incidence of cancer in pregnancy comes from population-based cancer registries, and unfortunately, these data are not standardized and are often incomplete. Many studies include cancer diagnosed up to 1 year after pregnancy, and some include preinvasive disease. Estimates therefore vary considerably (see Table 1 for a sampling of estimates incidences.)
It has been reported, and often cited in the literature, that invasive malignancy complicates one in 1,000 pregnancies and that the incidence of cancer in pregnancy (invasive and noninvasive malignancies) has been rising over time.8 Increasing maternal age is believed to be playing a role in this rise; as women delay childbearing, they enter the age range in which some cancers become more common. Additionally, improvements in screening and diagnostics have led to earlier cancer detection. The incidence of ovarian neoplasms found during pregnancy has increased, for instance, with the routine use of diagnostic ultrasound in pregnancy.1
Among the studies showing an increased incidence of pregnancy-associated cancer is a population-based study in Australia, which found that from 1994 to 2007 the crude incidence of pregnancy-associated cancer increased from 112.3 to 191.5 per 100,000 pregnancies (P < .001).9 A cohort study in the United States documented an increase in incidence from 75.0 per 100,000 pregnancies in 2002 to 138.5 per 100,000 pregnancies in 2012.10
Overall, the literature shows us that the skin, cervix, and breast are also common sites for malignancy during pregnancy.1 According to a 2022 review, breast cancer during pregnancy is less often hormone receptor–positive and more frequently triple negative compared with age-matched controls.11 The frequencies of other pregnancy-associated cancers appear overall to be similar to that of cancer occurring in all women across their reproductive years.1
Too often, diagnosis is delayed because cancer symptoms can be masked by or can mimic normal physiological changes in pregnancy. For instance, breast cancer can be difficult to diagnose during pregnancy and lactation due to anatomic changes in the breast parenchyma. Several studies published in the 1990s showed that breast cancer presents at a more advanced stage in pregnant patients than in nonpregnant patients because of this delay.1 Skin changes suggestive of melanoma can be attributed to hyperpigmentation of pregnancy, for instance. Several observational studies have suggested that thicker melanomas found in pregnancy may be because of delayed diagnosis.8
It is important that we thoroughly investigate signs and symptoms suggestive of a malignancy and not automatically attribute these symptoms to the pregnancy itself. Cervical biopsy of a mass or lesion suspicious for cervical cancer can be done safely during pregnancy and should not be delayed or deferred.
Fetal radiation exposure from radiologic examinations has long been a concern, but we know today that while the imaging modality should be chosen to minimize fetal radiation exposure, CT scans and even PET scans should be performed if these exams are deemed best for evaluation. Embryonic exposure to a dose of less than 50 mGy is rarely if at all associated with fetal malformations or miscarriage and a radiation dose of 100 mGy may be considered a floor for consideration of therapeutic termination of pregnancy.1,8
CT exams are associated with a fetal dose far less than 50 mGy (see Table 2 for radiation doses).
Magnetic resonance imaging with a magnet strength of 3 Tesla or less in any trimester is not associated with an increased risk of harm to the fetus or in early childhood, but the contrast agent gadolinium should be avoided in pregnancy as it has been associated with an increased risk of stillbirth, neonatal death, and childhood inflammatory, rheumatologic, and infiltrative skin lesions.1,8,12
Chemotherapy, Surgery, and Radiation in Pregnancy
The management of cancer during pregnancy requires a multidisciplinary team including medical, gynecologic, or radiation oncologists, and maternal-fetal medicine specialists (Figure 1). Prematurity and low birth weight are frequent complications for fetuses exposed to chemotherapy, although there is some uncertainty as to whether the treatment is causative. However, congenital anomalies no longer are a major concern, provided that drugs are appropriately selected and that fetal exposure occurs during the second or third trimester.
For instance, alkylating agents including cisplatin (an important drug in the management of gynecologic malignancies) have been associated with congenital anomalies in the first trimester but not in the second and third trimesters, and a variety of antimetabolites — excluding methotrexate and aminopterin — similarly have been shown to be relatively safe when used after the first trimester.1
Small studies have shown no long-term effects of chemotherapy exposure on postnatal growth and long-term neurologic/neurocognitive function,1 but this is an area that needs more research.
Also in need of investigation is the safety of newer agents in pregnancy. Data are limited on the use of new targeted treatments, monoclonal antibodies, and immunotherapies in pregnancy and their effects on the fetus, with current knowledge coming mainly from single case reports.13
Until more is learned — a challenge given that pregnant women are generally excluded from clinical trials — management teams are generally postponing use of these therapies until after delivery. Considering the pace of new developments revolutionizing cancer treatment, this topic will likely get more complex and confusing before we begin acquiring sufficient knowledge.
The timing of surgery for malignancy in pregnancy is similarly based on the balance of maternal and fetal risks, including the risk of maternal disease progression, the risk of preterm delivery, and the prevention of fetal metastases. In general, the safest time is the second trimester.
Maternal surgery in the third trimester may be associated with a risk of premature labor and altered uteroplacental perfusion. A 2005 systematic review of 12,452 women who underwent nonobstetric surgery during pregnancy provides some reassurance, however; compared with the general obstetric population, there was no increase in the rate of miscarriage or major birth defects.14
Radiotherapy used to be contraindicated in pregnancy but many experts today believe it can be safely utilized provided the uterus is out of field and is protected from scattered radiation. The head, neck, and breast, for instance, can be treated with newer radiotherapies, including stereotactic ablative radiation therapy.8 Patients with advanced cervical cancer often receive chemotherapy during pregnancy to slow metastatic growth followed by definitive treatment with postpartum radiation or surgery.
More research is needed, but available data on maternal outcomes are encouraging. For instance, there appear to be no significant differences in short- and long-term complications or survival between women who are pregnant and nonpregnant when treated for invasive cervical cancer.8 Similarly, while earlier studies of breast cancer diagnosed during pregnancy suggested a poor prognosis, data now show similar prognoses for pregnant and nonpregnant patients when controlled for stage.1
Dr. Rao is a gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported no relevant disclosures.
References
1. Rao GG. Chapter 42. Clinical Obstetrics: The Fetus & Mother, 4th ed. Reece EA et al. (eds): 2021.
2. Bannister-Tyrrell M et al. Aust N Z J Obstet Gynaecol. 2014;55:116-122.
3. Oehler MK et al. Aust N Z J Obstet Gynaecol. 2003;43(6):414-420.
4. Ruiz R et al. Breast. 2017;35:136-141. doi: 10.1016/j.breast.2017.07.008.
5. Nolan S et al. Am J Obstet Gynecol. 2019;220(1):S480. doi: 10.1016/j.ajog.2018.11.752.
6. El-Messidi A et al. J Perinat Med. 2015;43(6):683-688. doi: 10.1515/jpm-2014-0133.
7. Pellino G et al. Eur J Gastroenterol Hepatol. 2017;29(7):743-753. doi: 10.1097/MEG.0000000000000863.
8. Eastwood-Wilshere N et al. Asia-Pac J Clin Oncol. 2019;15:296-308.
9. Lee YY et al. BJOG. 2012;119(13):1572-1582.
10. Cottreau CM et al. J Womens Health (Larchmt). 2019 Feb;28(2):250-257.
11. Boere I et al. Best Pract Res Clin Obstet Gynaecol. 2022;82:46-59.
12. Ray JG et al. JAMA 2016;316(9):952-961.
13. Schwab R et al. Cancers. (Basel) 2021;13(12):3048.
14. Cohen-Kerem et al. Am J Surg. 2005;190(3):467-473.
Introduction: Tremendous Progress on Cancer Extends to Cancer in Pregnancy
The biomedical research enterprise that took shape in the United States after World War II has had numerous positive effects, including significant progress made during the past 75-plus years in the diagnosis, prevention, and treatment of cancer.
President Franklin D. Roosevelt’s 1944 request of Dr. Vannevar Bush, director of the then Office of Scientific Research and Development, to organize a program that would advance and apply scientific knowledge for times of peace — just as it been advanced and applied in times of war — culminated in a historic report, Science – The Endless Frontier. Presented in 1945 to President Harry S. Truman, this report helped fuel decades of broad, bold, and coordinated government-sponsored biomedical research aimed at addressing disease and improving the health of the American people (National Science Foundation, 1945).
Discoveries made from research in basic and translational sciences deepened our knowledge of the cellular and molecular underpinnings of cancer, leading to advances in chemotherapy, radiotherapy, and other treatment approaches as well as continual refinements in their application. Similarly, our diagnostic armamentarium has significantly improved.
As a result, we have reduced both the incidence and mortality of cancer. Today, some cancers can be prevented. Others can be reversed or put in remission. Granted, progress has been variable, with some cancers such as ovarian cancer still having relatively low survival rates. Much more needs to be done. Overall, however, the positive effects of the U.S. biomedical research enterprise on cancer are evident. According to the National Cancer Institute’s most recent report on the status of cancer, death rates from cancer fell 1.9% per year on average in females from 2015 to 2019 (Cancer. 2022 Oct 22. doi: 10.1002/cncr.34479).
It is not only patients whose cancer occurs outside of pregnancy who have benefited. When treatment is appropriately selected and timing considerations are made, patients whose cancer is diagnosed during pregnancy — and their children — can have good outcomes.
To explain how the management of cancer in pregnancy has improved, we have invited Gautam G. Rao, MD, gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to write this installment of the Master Class in Obstetrics. As Dr. Rao explains, radiation is not as dangerous to the fetus as once thought, and the safety of many chemotherapeutic regimens in pregnancy has been documented. Obstetricians can and should counsel patients, he explains, about the likelihood of good maternal and fetal outcomes.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Managing Cancer in Pregnancy
Cancer can cause fear and distress for any patient, but when cancer is diagnosed during pregnancy, an expectant mother fears not only for her own health but for the health of her unborn child. Fortunately, ob.gyn.s and multidisciplinary teams have good reason to reassure patients about the likelihood of good outcomes.
Cancer treatment in pregnancy has improved with advancements in imaging and chemotherapy, and while maternal and fetal outcomes of prenatal cancer treatment are not well reported, evidence acquired in recent years from case series and retrospective studies shows that most imaging studies and procedural diagnostic tests – and many treatments – can be performed safely in pregnancy.
Decades ago, we avoided CT scans during pregnancy because of concerns about radiation exposure to the fetus, leaving some patients without an accurate staging of their cancer. Today, we have evidence that a CT scan is generally safe in pregnancy. Similarly, the safety of many chemotherapeutic regimens in pregnancy has been documented in recent decades,and the use of chemotherapy during pregnancy has increased progressively. Radiation is also commonly utilized in the management of cancers that may occur during pregnancy, such as breast cancer.1
Considerations of timing are often central to decision-making; chemotherapy and radiotherapy are generally avoided in the first trimester to prevent structural fetal anomalies, for instance, and delaying cancer treatment is often warranted when the patient is a few weeks away from delivery. On occasion, iatrogenic preterm birth is considered when the risks to the mother of delaying a necessary cancer treatment outweigh the risks to the fetus of prematurity.1
Pregnancy termination is rarely indicated, however, and information gathered over the past 2 decades suggests that fetal and placental metastases are rare.1 There is broad agreement that prenatal treatment of cancer in pregnancy should adhere as much as possible to protocols and guidelines for nonpregnant patients and that treatment delays driven by fear of fetal anomalies and miscarriage are unnecessary.
Cancer Incidence, Use of Diagnostic Imaging
Data on the incidence of cancer in pregnancy comes from population-based cancer registries, and unfortunately, these data are not standardized and are often incomplete. Many studies include cancer diagnosed up to 1 year after pregnancy, and some include preinvasive disease. Estimates therefore vary considerably (see Table 1 for a sampling of estimates incidences.)
It has been reported, and often cited in the literature, that invasive malignancy complicates one in 1,000 pregnancies and that the incidence of cancer in pregnancy (invasive and noninvasive malignancies) has been rising over time.8 Increasing maternal age is believed to be playing a role in this rise; as women delay childbearing, they enter the age range in which some cancers become more common. Additionally, improvements in screening and diagnostics have led to earlier cancer detection. The incidence of ovarian neoplasms found during pregnancy has increased, for instance, with the routine use of diagnostic ultrasound in pregnancy.1
Among the studies showing an increased incidence of pregnancy-associated cancer is a population-based study in Australia, which found that from 1994 to 2007 the crude incidence of pregnancy-associated cancer increased from 112.3 to 191.5 per 100,000 pregnancies (P < .001).9 A cohort study in the United States documented an increase in incidence from 75.0 per 100,000 pregnancies in 2002 to 138.5 per 100,000 pregnancies in 2012.10
Overall, the literature shows us that the skin, cervix, and breast are also common sites for malignancy during pregnancy.1 According to a 2022 review, breast cancer during pregnancy is less often hormone receptor–positive and more frequently triple negative compared with age-matched controls.11 The frequencies of other pregnancy-associated cancers appear overall to be similar to that of cancer occurring in all women across their reproductive years.1
Too often, diagnosis is delayed because cancer symptoms can be masked by or can mimic normal physiological changes in pregnancy. For instance, breast cancer can be difficult to diagnose during pregnancy and lactation due to anatomic changes in the breast parenchyma. Several studies published in the 1990s showed that breast cancer presents at a more advanced stage in pregnant patients than in nonpregnant patients because of this delay.1 Skin changes suggestive of melanoma can be attributed to hyperpigmentation of pregnancy, for instance. Several observational studies have suggested that thicker melanomas found in pregnancy may be because of delayed diagnosis.8
It is important that we thoroughly investigate signs and symptoms suggestive of a malignancy and not automatically attribute these symptoms to the pregnancy itself. Cervical biopsy of a mass or lesion suspicious for cervical cancer can be done safely during pregnancy and should not be delayed or deferred.
Fetal radiation exposure from radiologic examinations has long been a concern, but we know today that while the imaging modality should be chosen to minimize fetal radiation exposure, CT scans and even PET scans should be performed if these exams are deemed best for evaluation. Embryonic exposure to a dose of less than 50 mGy is rarely if at all associated with fetal malformations or miscarriage and a radiation dose of 100 mGy may be considered a floor for consideration of therapeutic termination of pregnancy.1,8
CT exams are associated with a fetal dose far less than 50 mGy (see Table 2 for radiation doses).
Magnetic resonance imaging with a magnet strength of 3 Tesla or less in any trimester is not associated with an increased risk of harm to the fetus or in early childhood, but the contrast agent gadolinium should be avoided in pregnancy as it has been associated with an increased risk of stillbirth, neonatal death, and childhood inflammatory, rheumatologic, and infiltrative skin lesions.1,8,12
Chemotherapy, Surgery, and Radiation in Pregnancy
The management of cancer during pregnancy requires a multidisciplinary team including medical, gynecologic, or radiation oncologists, and maternal-fetal medicine specialists (Figure 1). Prematurity and low birth weight are frequent complications for fetuses exposed to chemotherapy, although there is some uncertainty as to whether the treatment is causative. However, congenital anomalies no longer are a major concern, provided that drugs are appropriately selected and that fetal exposure occurs during the second or third trimester.
For instance, alkylating agents including cisplatin (an important drug in the management of gynecologic malignancies) have been associated with congenital anomalies in the first trimester but not in the second and third trimesters, and a variety of antimetabolites — excluding methotrexate and aminopterin — similarly have been shown to be relatively safe when used after the first trimester.1
Small studies have shown no long-term effects of chemotherapy exposure on postnatal growth and long-term neurologic/neurocognitive function,1 but this is an area that needs more research.
Also in need of investigation is the safety of newer agents in pregnancy. Data are limited on the use of new targeted treatments, monoclonal antibodies, and immunotherapies in pregnancy and their effects on the fetus, with current knowledge coming mainly from single case reports.13
Until more is learned — a challenge given that pregnant women are generally excluded from clinical trials — management teams are generally postponing use of these therapies until after delivery. Considering the pace of new developments revolutionizing cancer treatment, this topic will likely get more complex and confusing before we begin acquiring sufficient knowledge.
The timing of surgery for malignancy in pregnancy is similarly based on the balance of maternal and fetal risks, including the risk of maternal disease progression, the risk of preterm delivery, and the prevention of fetal metastases. In general, the safest time is the second trimester.
Maternal surgery in the third trimester may be associated with a risk of premature labor and altered uteroplacental perfusion. A 2005 systematic review of 12,452 women who underwent nonobstetric surgery during pregnancy provides some reassurance, however; compared with the general obstetric population, there was no increase in the rate of miscarriage or major birth defects.14
Radiotherapy used to be contraindicated in pregnancy but many experts today believe it can be safely utilized provided the uterus is out of field and is protected from scattered radiation. The head, neck, and breast, for instance, can be treated with newer radiotherapies, including stereotactic ablative radiation therapy.8 Patients with advanced cervical cancer often receive chemotherapy during pregnancy to slow metastatic growth followed by definitive treatment with postpartum radiation or surgery.
More research is needed, but available data on maternal outcomes are encouraging. For instance, there appear to be no significant differences in short- and long-term complications or survival between women who are pregnant and nonpregnant when treated for invasive cervical cancer.8 Similarly, while earlier studies of breast cancer diagnosed during pregnancy suggested a poor prognosis, data now show similar prognoses for pregnant and nonpregnant patients when controlled for stage.1
Dr. Rao is a gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported no relevant disclosures.
References
1. Rao GG. Chapter 42. Clinical Obstetrics: The Fetus & Mother, 4th ed. Reece EA et al. (eds): 2021.
2. Bannister-Tyrrell M et al. Aust N Z J Obstet Gynaecol. 2014;55:116-122.
3. Oehler MK et al. Aust N Z J Obstet Gynaecol. 2003;43(6):414-420.
4. Ruiz R et al. Breast. 2017;35:136-141. doi: 10.1016/j.breast.2017.07.008.
5. Nolan S et al. Am J Obstet Gynecol. 2019;220(1):S480. doi: 10.1016/j.ajog.2018.11.752.
6. El-Messidi A et al. J Perinat Med. 2015;43(6):683-688. doi: 10.1515/jpm-2014-0133.
7. Pellino G et al. Eur J Gastroenterol Hepatol. 2017;29(7):743-753. doi: 10.1097/MEG.0000000000000863.
8. Eastwood-Wilshere N et al. Asia-Pac J Clin Oncol. 2019;15:296-308.
9. Lee YY et al. BJOG. 2012;119(13):1572-1582.
10. Cottreau CM et al. J Womens Health (Larchmt). 2019 Feb;28(2):250-257.
11. Boere I et al. Best Pract Res Clin Obstet Gynaecol. 2022;82:46-59.
12. Ray JG et al. JAMA 2016;316(9):952-961.
13. Schwab R et al. Cancers. (Basel) 2021;13(12):3048.
14. Cohen-Kerem et al. Am J Surg. 2005;190(3):467-473.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).
(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).
(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).

(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).

(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).

(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).

(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).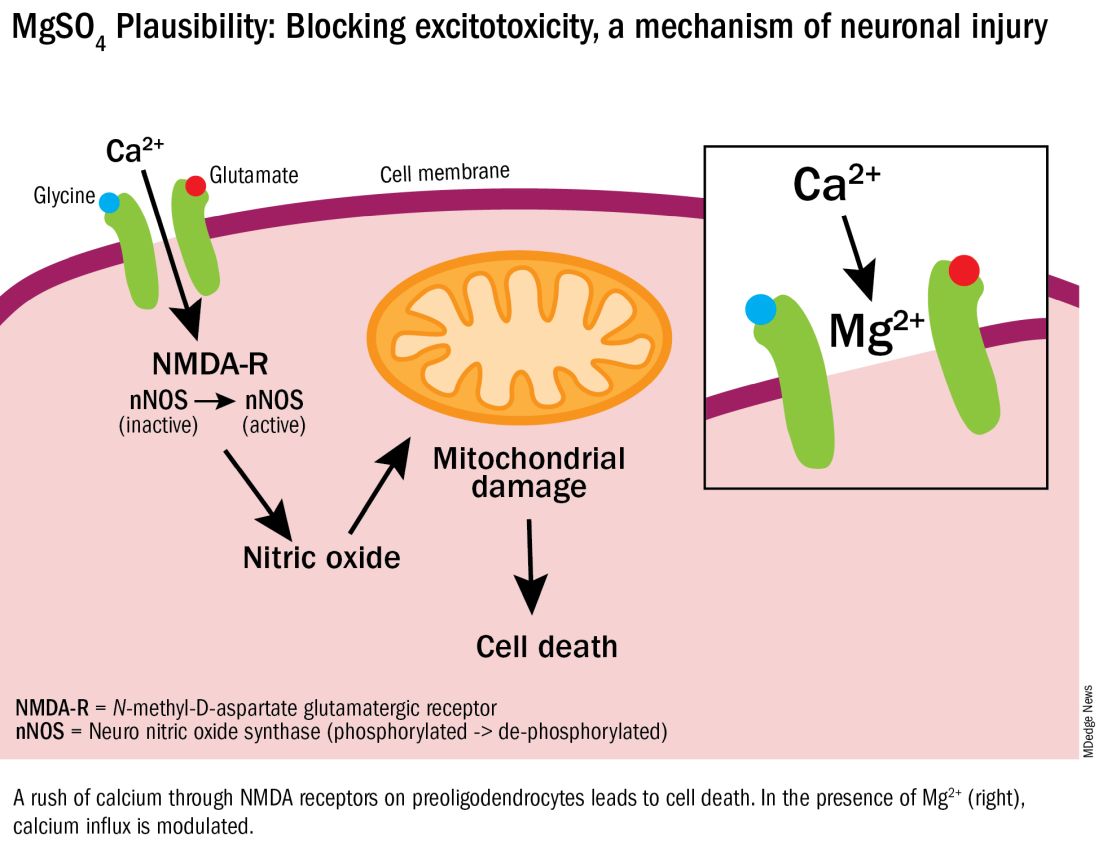
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.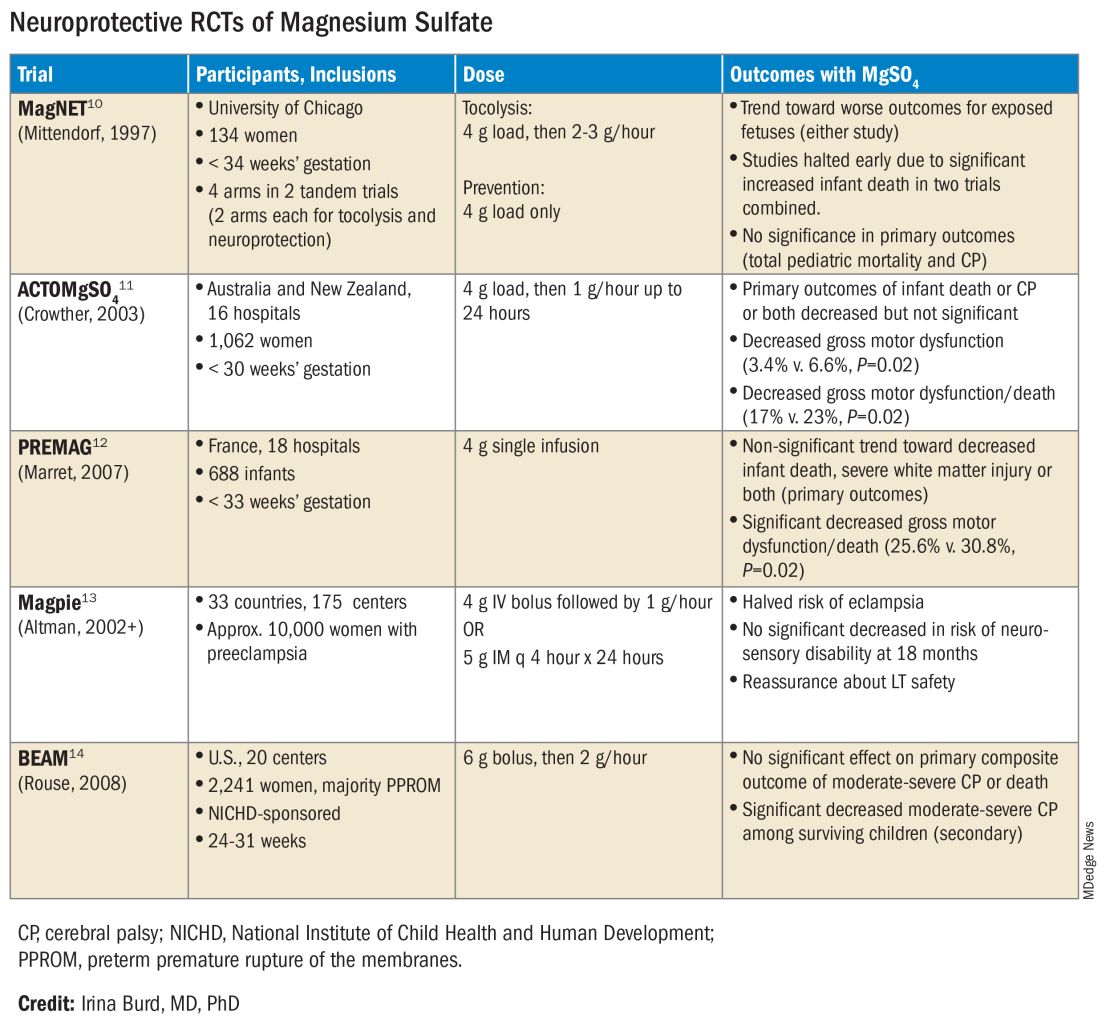
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Investigating the etiology of recurrent pregnancy loss
With attention to the timing of loss
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.
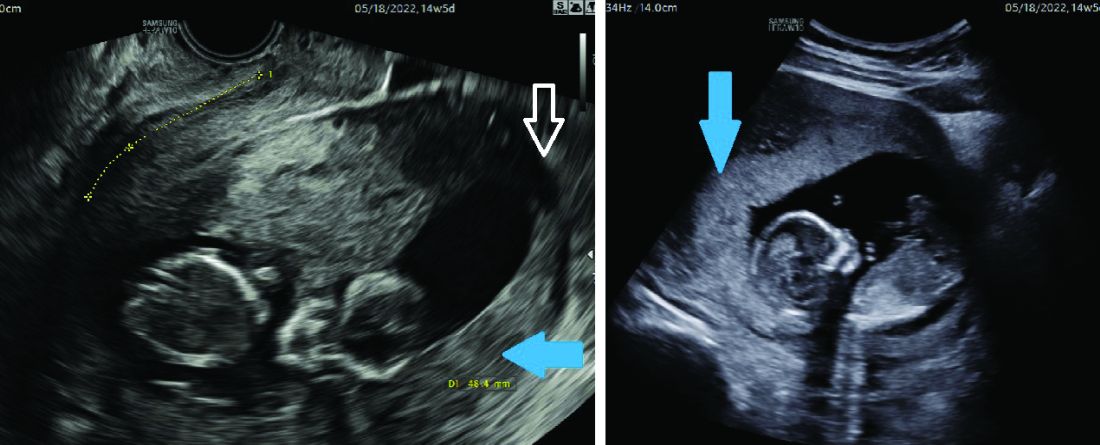
Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
With attention to the timing of loss
With attention to the timing of loss
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.

Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.

Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
Prepare for endometriosis excision surgery with a multidisciplinary approach
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Decoding mechanisms of diabetic embryopathy suggests therapeutic targets
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.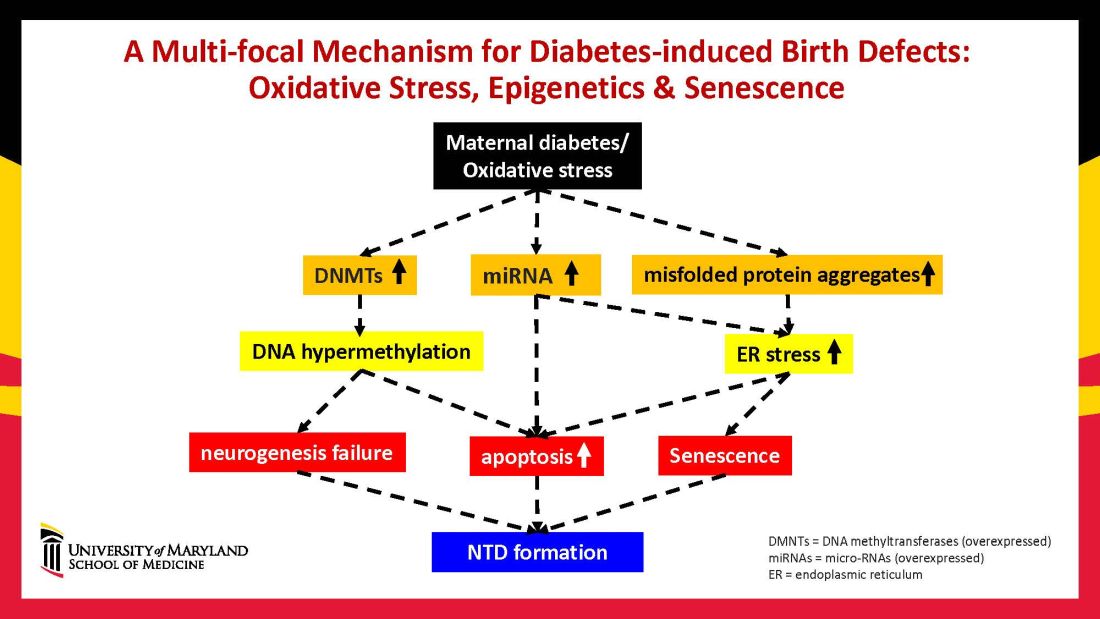
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.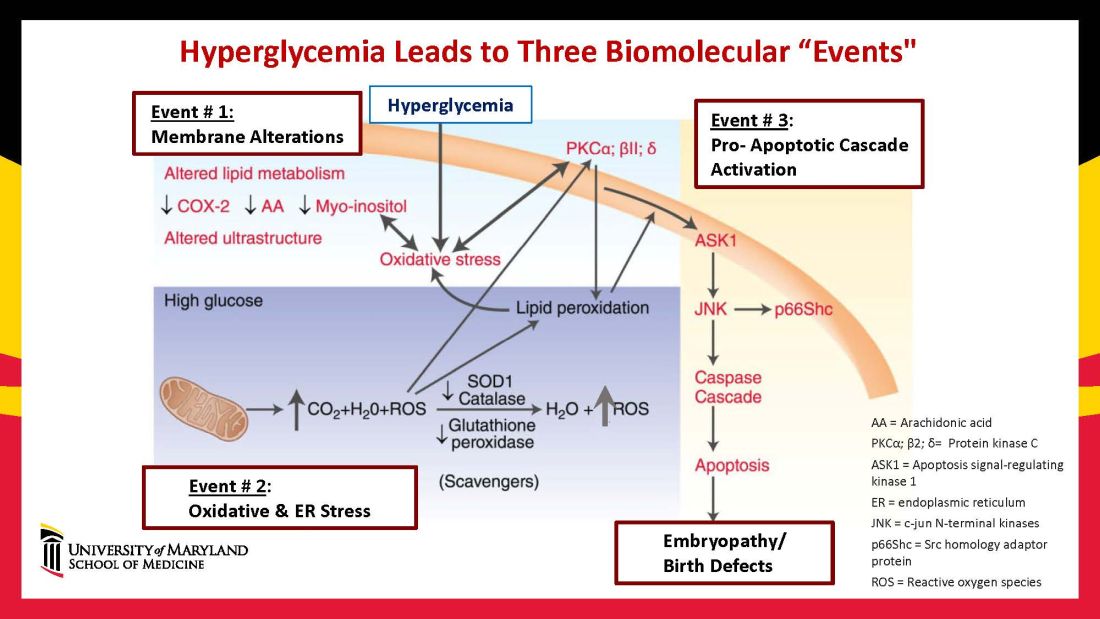
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9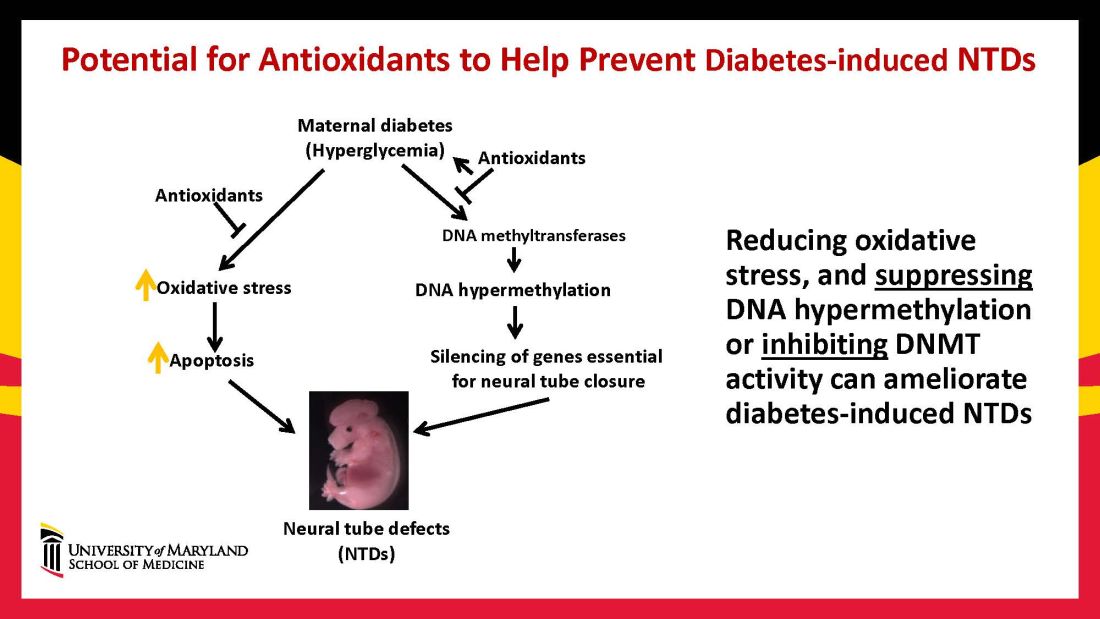
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Discoveries in diabetic embryogenesis
Many issues surrounding pregnancy care of women with preexisting diabetes remain challenging, especially in light of the relentless increase in maternal morbidity and mortality in the United States and globally. Rising rates of death and severe morbidity in diabetic women have continued despite significant advances in insulin pharmacology and administration technology.
However, despite these advances in glucose monitoring and insulin administration, fetal mortality and childhood morbidity rates continue to climb. This is because critical fetal structural anomalies arise from developmental errors occurring in the embryonic period – between 2 and 13 weeks of gestation – a time when most women with preexisting diabetes are just entering into prenatal care, often with suboptimal glycemic control.
Thus, significant future progress in reducing fetal mortality and childhood disability in infants of diabetic mothers will depend upon effective interventions in the first trimester while embryogenesis and critical organ formation are underway.
In this issue of Ob.Gyn. News, the editor of Master Class in Obstetrics, E. Albert Reece MD, PhD, MBA, steps into the role of coauthor. He and his research colleague Peixin Yang, PhD, present exciting insights into the cellular mechanisms underlying structural birth defects in infants of diabetic mothers – especially cardiac and neural tube defects – and also provide a glimpse into some potentially effective maternal pharmacologic interventions. After appropriate human trials, these interventions could be effectively applied from the time of a positive pregnancy test with potentially dramatic results.
Dr. Reece and Dr. Yang, who lead the Center for the Study of Birth Defects at the University of Maryland School of Medicine, share their impressive accumulation of data from embryos of pregnant diabetic rodents. They demonstrate convincingly that, in first-trimester rodent embryos, maternal hyperglycemia induces excessive apoptosis, which in turn leads to structural defects in critical fetal organs. They further found that maternal hyperglycemia reduces embryonic autophagosomes – the developmentally essential organelles that remove abnormal or damaged cells during embryo formation.
These investigators also identified reactivators of these organelles which, when administered maternally in the first trimester, significantly reduced the incidence of neural tube defects. Thus, for optimal development of diabetes-affected embryos, first-trimester administration of reactivators of autophagy could offer a significant, life-changing intervention in the foreseeable future.
Dr. Moore is professor emeritus of maternal-fetal medicine and chair emeritus in the department of obstetrics, gynecology, and reproductive sciences at UC San Diego Health. He reported no disclosures.
*This story was updated on Nov. 3, 2022.
Many issues surrounding pregnancy care of women with preexisting diabetes remain challenging, especially in light of the relentless increase in maternal morbidity and mortality in the United States and globally. Rising rates of death and severe morbidity in diabetic women have continued despite significant advances in insulin pharmacology and administration technology.
However, despite these advances in glucose monitoring and insulin administration, fetal mortality and childhood morbidity rates continue to climb. This is because critical fetal structural anomalies arise from developmental errors occurring in the embryonic period – between 2 and 13 weeks of gestation – a time when most women with preexisting diabetes are just entering into prenatal care, often with suboptimal glycemic control.
Thus, significant future progress in reducing fetal mortality and childhood disability in infants of diabetic mothers will depend upon effective interventions in the first trimester while embryogenesis and critical organ formation are underway.
In this issue of Ob.Gyn. News, the editor of Master Class in Obstetrics, E. Albert Reece MD, PhD, MBA, steps into the role of coauthor. He and his research colleague Peixin Yang, PhD, present exciting insights into the cellular mechanisms underlying structural birth defects in infants of diabetic mothers – especially cardiac and neural tube defects – and also provide a glimpse into some potentially effective maternal pharmacologic interventions. After appropriate human trials, these interventions could be effectively applied from the time of a positive pregnancy test with potentially dramatic results.
Dr. Reece and Dr. Yang, who lead the Center for the Study of Birth Defects at the University of Maryland School of Medicine, share their impressive accumulation of data from embryos of pregnant diabetic rodents. They demonstrate convincingly that, in first-trimester rodent embryos, maternal hyperglycemia induces excessive apoptosis, which in turn leads to structural defects in critical fetal organs. They further found that maternal hyperglycemia reduces embryonic autophagosomes – the developmentally essential organelles that remove abnormal or damaged cells during embryo formation.
These investigators also identified reactivators of these organelles which, when administered maternally in the first trimester, significantly reduced the incidence of neural tube defects. Thus, for optimal development of diabetes-affected embryos, first-trimester administration of reactivators of autophagy could offer a significant, life-changing intervention in the foreseeable future.
Dr. Moore is professor emeritus of maternal-fetal medicine and chair emeritus in the department of obstetrics, gynecology, and reproductive sciences at UC San Diego Health. He reported no disclosures.
*This story was updated on Nov. 3, 2022.
Many issues surrounding pregnancy care of women with preexisting diabetes remain challenging, especially in light of the relentless increase in maternal morbidity and mortality in the United States and globally. Rising rates of death and severe morbidity in diabetic women have continued despite significant advances in insulin pharmacology and administration technology.
However, despite these advances in glucose monitoring and insulin administration, fetal mortality and childhood morbidity rates continue to climb. This is because critical fetal structural anomalies arise from developmental errors occurring in the embryonic period – between 2 and 13 weeks of gestation – a time when most women with preexisting diabetes are just entering into prenatal care, often with suboptimal glycemic control.
Thus, significant future progress in reducing fetal mortality and childhood disability in infants of diabetic mothers will depend upon effective interventions in the first trimester while embryogenesis and critical organ formation are underway.
In this issue of Ob.Gyn. News, the editor of Master Class in Obstetrics, E. Albert Reece MD, PhD, MBA, steps into the role of coauthor. He and his research colleague Peixin Yang, PhD, present exciting insights into the cellular mechanisms underlying structural birth defects in infants of diabetic mothers – especially cardiac and neural tube defects – and also provide a glimpse into some potentially effective maternal pharmacologic interventions. After appropriate human trials, these interventions could be effectively applied from the time of a positive pregnancy test with potentially dramatic results.
Dr. Reece and Dr. Yang, who lead the Center for the Study of Birth Defects at the University of Maryland School of Medicine, share their impressive accumulation of data from embryos of pregnant diabetic rodents. They demonstrate convincingly that, in first-trimester rodent embryos, maternal hyperglycemia induces excessive apoptosis, which in turn leads to structural defects in critical fetal organs. They further found that maternal hyperglycemia reduces embryonic autophagosomes – the developmentally essential organelles that remove abnormal or damaged cells during embryo formation.
These investigators also identified reactivators of these organelles which, when administered maternally in the first trimester, significantly reduced the incidence of neural tube defects. Thus, for optimal development of diabetes-affected embryos, first-trimester administration of reactivators of autophagy could offer a significant, life-changing intervention in the foreseeable future.
Dr. Moore is professor emeritus of maternal-fetal medicine and chair emeritus in the department of obstetrics, gynecology, and reproductive sciences at UC San Diego Health. He reported no disclosures.
*This story was updated on Nov. 3, 2022.
A hypogastric nerve-focused approach to nerve-sparing endometriosis surgery
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.
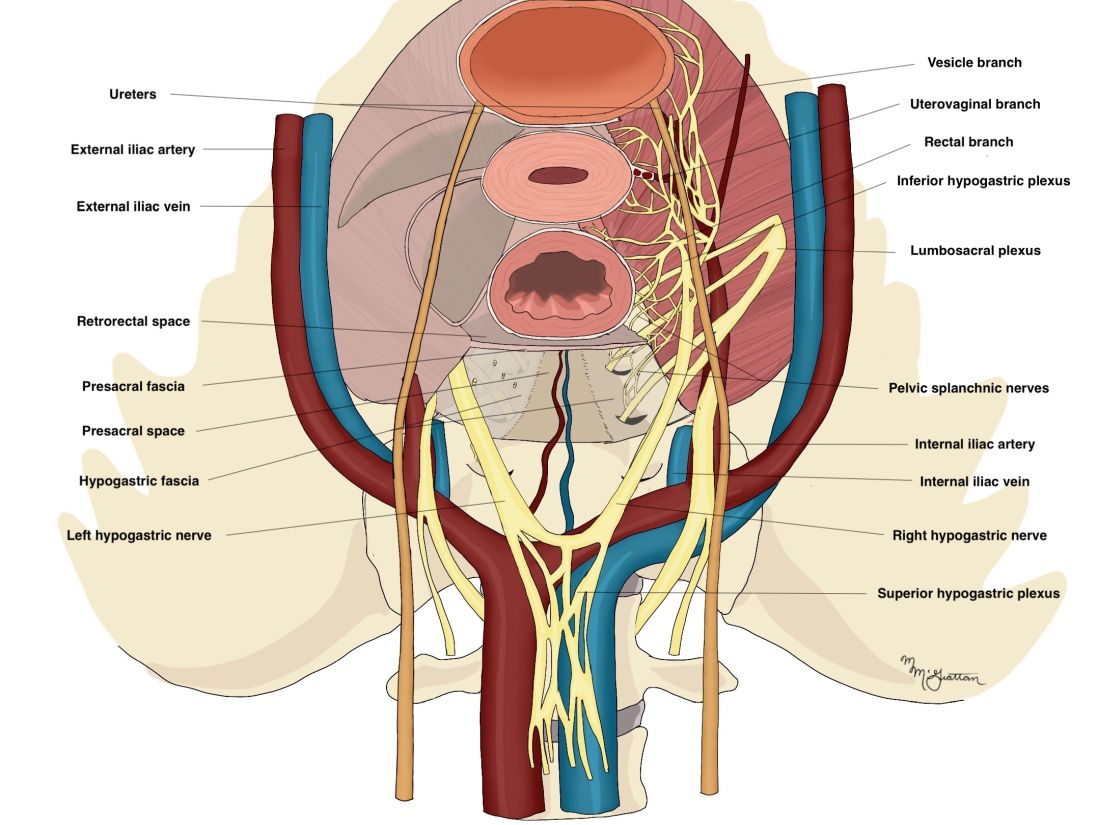
The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7
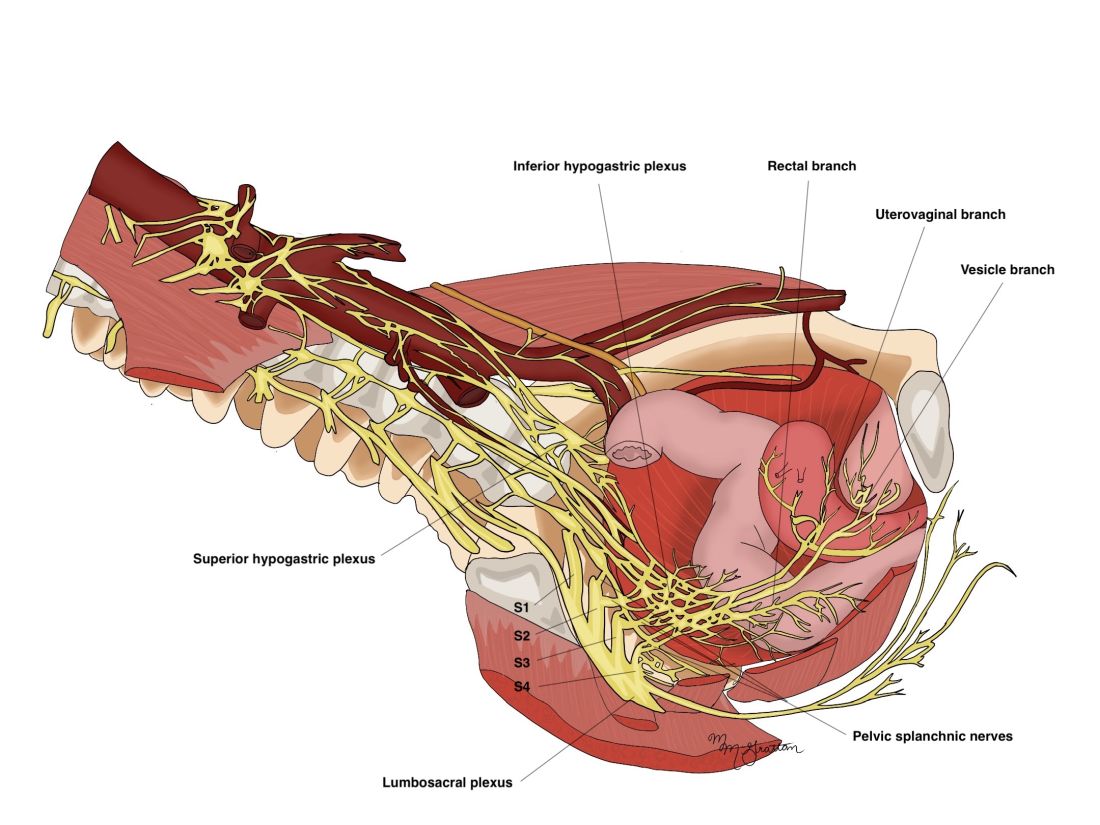
The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.

The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7

The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.

The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7

The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.