User login
UPDATE ON: URINARY INCONTINENCE
The authors report no financial relationships relevant to this article.
Four recent studies enhance our understanding of the benefits, efficacy, and risks of the following interventions in women who have urinary incontinence (UI):
- weight loss. Women who were randomized to intensive weight loss reduced the total number of UI episodes in a week by 47.4%—compared with 28.1% in the group randomized to a structured educational program.
- midurethral slings. Treatment of stress UI arising from intrinsic sphincter deficiency was more successful in women randomized to tension-free vaginal tape (TVT) than in women assigned to transobturator tape (TOT). In the first group, urodynamically confirmed stress UI was present in 21% of subjects after treatment, compared with 45% in the TOT group.
- botulinum toxin type A (Botox) injection. Women who had refractory urge incontinence were likely to improve significantly after injection of Botox. Sixty percent of women treated with Botox reported a reduction in incontinence after treatment, with a median response of 373 days, compared with 62 days in the placebo group.
- sacral neuromodulation. The mean number of urge incontinence episodes decreased from 9.6 to 3.9, and the mean number of voids per day decreased from 19.3 to 14.8 in a 5-year follow-up study.
Despite the promise of these findings, all of the studies had limitations, and several identified risks associated with the intervention. These limitations and risks are detailed in the articles that follow.
Urinary incontinence is no small problem. It affects more than 13 million women in the United States alone, and costs more than $20 billion annually in direct health-care costs.1
Despite the high prevalence of urinary incontinence, women are often reluctant to discuss symptoms with their physician. As a result, the condition remains undiagnosed or undertreated in many women.2
The most common types of urinary incontinence include:
- stress incontinence – leakage upon effort, exertion, or increased abdominal pressure
- urge incontinence – leakage accompanied by, or immediately preceded by, urgency
- mixed incontinence – leakage with urgency as well as effort, exertion, or increased abdominal pressure.
Another common problem is overactive bladder syndrome, which involves urgency with or without leakage, and usually increased frequency and nocturia as well.3
Although the midurethral sling revolutionized the treatment of stress urinary incontinence, most women who have incontinence experience mixed symptoms, making it a more challenging condition for the general-practice ObGyn to treat successfully. Furthermore, traditional therapies such as behavior modification, pelvic floor exercise, and medication have had only modest success in certain patient populations.
Weight loss can reduce urinary incontinence
in overweight and obese women
Subak LL, Wing R, Smith West D, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481–490.
Obesity increases intra-abdominal pressure, thereby exerting added force on the bladder, urethra, and pelvic floor and potentially exacerbating urinary incontinence (UI). It has been hypothesized that weight reduction reduces these forces and improves incontinence.
This randomized, clinical trial of 338 women showed that weight loss does have an effect on UI. Investigators compared an intensive 6-month weight loss program—designed to prompt a weight loss of 7% to 9% of body weight—with a structured educational program. The primary outcome was the percentage of change in the number of UI episodes reported in a 7-day voiding diary at 6 months.
After 6 months and a mean weight loss of 8% of baseline body weight, the women in the intensive weight loss group experienced a mean decrease of 47.4% in the total number of UI episodes in a week. Compare this with a mean weight loss of 1.6% (P<.001) and a mean decrease of 28.1% in UI episodes in the control group (P=.01). The reduction in the total number of UI episodes was primarily attributed to a reduction of 57.6% in stress-induced UI in the intensive weight loss group, compared with a reduction of 32.7% in the control group (P=.02).
Women in the weight loss program also perceived incontinence to be less of a problem and reported greater satisfaction with the change in their incontinence at 6 months than did women in the control group (P<.001).
Details of the trial
Women were eligible to participate if they reported 10 or more episodes of UI over 7 days, were at least 30 years old, and had a body mass index (BMI) of 25 to 50 at baseline (normal is 19 to 24). In addition, they had to agree not to initiate any new treatments for UI or weight loss during the 6-month study period.
Subjects were randomized in a 2:1 ratio, with 226 women assigned to the intensive weight loss program and 112 assigned to the structured educational program. Baseline characteristics were similar in both groups, with a mean age of 53±11 years, mean BMI of 36±6 kg/m3, and total mean number of UI episodes of 24±18 per week.
Strengths and limitations
Strengths of this study include the large and varied study population. One important limitation, however, is the fact that the primary outcome measure was based on self-reported UI episodes. Because participants were not blinded to their treatment assignment, bias in self-reporting may have been present. In addition, subjects were selected because of their potential to adhere to the rigorous study protocol.
As the prevalence of obesity reaches pandemic level, it’s imperative that medical science continue to develop novel methods with which we can help patients achieve ideal body weight.
The findings of this study contribute to the growing body of medical literature—across specialties—demonstrating that weight loss can significantly improve the general health of patients, and that it should be part of the first-line treatment for overweight and obese women who complain of UI.
TVT is more effective than TOT
for intrinsic sphincter deficiency
Schierlitz L, Dwyer PL, Rosamilia A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: a randomized controlled trial. Obstet Gynecol. 2008;112:1253–1261.
Many experts consider intrinsic sphincter deficiency (ISD) to be a severe form of stress incontinence. Earlier studies suggested that women who had stress incontinence complicated by ISD had a lower success rate after certain surgical procedures than did women who had stress incontinence alone.
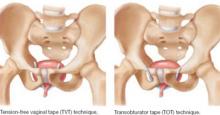
This randomized trial compared tension-free vaginal tape (TVT) with transobturator tape (TOT) in the treatment of stress incontinence with ISD ( FIGURE 1 ). The primary outcome measure was the presence of urodynamically confirmed stress incontinence 6 months after surgery.
At that 6-month mark, stress incontinence was present in 14 of 67 subjects (21%) in the TVT group, compared with 32 of 71 subjects (45%) in the TOT group (P=.004). Nine patients in the TOT group requested a repeat surgical procedure, compared with none in the TVT group.
Although the study was not powered to detect a difference in postoperative complications, there were six bladder perforations in the TVT group and none in the TOT group.
FIGURE 1 TVT and TOT trace different routes
Details of the trial
Women were selected for the trial on the basis of urodynamic parameters and recruited from two academic centers. ISD was defined as maximal urethral closure pressure below 20 cm H2O or Valsalva leak-point pressure less than 60 cm H2O. Subjects in the two groups had similar baseline characteristics.
Surgeons were required to have independently performed at least 15 surgical midurethral sling procedures before the study began.
In addition to placement of TVT or TOT, approximately one third of subjects underwent concomitant prolapse surgery. Postoperatively, subjects were assessed at 6 weeks, 6 months, and 12 months, with repeat urodynamic testing performed at 6 months. Analysis of data was based on intention to treat.
Only 138 of 164 women completed repeat urodynamic testing 6 months after surgery. Seventeen women declined testing, claiming to be “cured,” and nine women withdrew from the study or were lost to follow-up.
Short follow-up was a limitation
The definition of treatment failure as persistent, urodynamically confirmed stress incontinence was another shortcoming of the trial. In addition, the 17 subjects who declined repeat postoperative testing were classified as “cured,” potentially biasing the results.
TVT may be the preferred surgical option for women who have urodynamically confirmed stress incontinence complicated by intrinsic sphincter deficiency (ISD)—on the basis of the data gathered by these researchers. Longer follow-up is needed, however, to determine the long-term, clinical efficacy of midurethral slings in women who have ISD.
Accumulation of more data from future studies will better equip ObGyns to customize surgical treatment options to individual clinical parameters and reduce the risk of surgical failure.
Is Botox a panacea for refractory urge incontinence?
Brubaker L, Richter HE, Visco A, et al: Pelvic Floor Disorders Network. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol. 2008;180:217–222.
Women who fail medical management of urge incontinence have few other options. This multicenter, randomized, double-blind, placebo-controlled trial suggests that there may one day be an effective alternative. Investigators examined the safety and efficacy of botulinum toxin type A (Botox) for the treatment of refractory idiopathic urge incontinence in 43 women—28 of them randomized to injection of 200 U of Botox and 15 to placebo.
Sixty percent of subjects in the Botox arm reported an improvement in symptoms, with a median response of 373 days, compared with 62 days in the placebo arm (P<.0001). Moreover, in the Botox arm, women perceived greater improvement in symptom control and a decrease in the number of self-reported incontinence episodes, compared with the placebo group (P<.0001).
However, 12 of 28 patients (43%) who received Botox developed elevated postvoid residuals (i.e., retention of more than 200 mL of urine), and enrollment was halted for this reason. Median time to initiate intermittent self-catheterization was 30 days, and intermittent self-catheterization lasted a median of 60 days. Nine of the 12 subjects who required self-catheterization developed a urinary tract infection.
Details of the trial
This study was conducted by the Pelvic Floor Disorders Network and sponsored by the National Institute of Child Health and Human Development. To be eligible for the trial, women had to have been diagnosed with refractory urge incontinence, which was defined as persistent symptoms after failing at least two first-line therapies such as anticholinergic medications and behavioral therapy. Also required was documented evidence of detrusor overactivity on urodynamic studies or at least six episodes of urge-related incontinence in a 3-day bladder diary. Investigators determined that a sample size of 210 subjects was needed to test a 50% efficacy rate for Botox, compared with 30% for placebo, at 6 months.
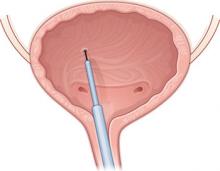
Baseline characteristics were similar between groups. A blinded physician used a cystoscope to inject the agents into the detrusor muscle over the posterior bladder wall ( FIGURE 2 ). Subjects also received an antibiotic before the procedure and for 3 additional days.
The primary outcome measure was treatment failure, defined as the return of symptoms measured at least 2 months after Botox injection or any change in medical therapy.
Enrollment was halted after interval analysis revealed a significantly higher rate of voiding dysfunction in the Botox arm, necessitating intermittent self-catheterization and associated urinary tract infections.
FIGURE 2 Intradetrusor Botox injection
In the trial by Brubaker and colleagues, 200 U of Botox was injected into the detrusor muscle on the posterior bladder wall to treat refractory urge incontinence in 28 women—60% of whom reported improvement.
Is a postvoid residual clinically significant?
Some experts questioned the clinical significance of the elevated postvoid residual reported in this trial, arguing that it was temporary and that many patients were asymptomatic.
Further study is needed to determine the optimal dosing of Botox and a management strategy for postprocedure voiding dysfunction.
When one of your patients considers cystoscopic intradetrusor Botox therapy for refractory urge incontinence, you should:
- counsel her extensively that its use here is not FDA-approved
- caution her that she may need to perform intermittent self-catheterization after injection
- advise her that the procedure and the medication are likely not covered by her health insurance and will be out-of-pocket expenses.
Much-needed and much-anticipated clinical trials are under way with the aim of obtaining FDA approval of Botox for urge incontinence.
At the Cleveland Clinic, we currently offer Botox for refractory urge incontinence.
InterStim therapy may become the gold standard for refractory overactive bladder syndrome
van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029–2034.
Sacral nerve stimulation has been approved for use in patients with refractory voiding dysfunction since 1997. This prospective, worldwide, follow-up study sought to determine the long-term efficacy and safety of sacral neuromodulation for the treatment of refractory urgency, frequency, urge incontinence, and nonobstructive urinary retention.
After 5 years of follow-up, 68% of subjects who had urge incontinence, 56% who had urgency and frequency, and 71% who had nonobstructive urinary retention reported a degree of improvement of 50% or more in their symptoms (relative to baseline). The mean number of urge incontinence episodes decreased from 9.6 to 3.9, and mean voids per day decreased from 19.3 to 14.8 at 5 years (P<.001).
Details of the trial
Eligible patients were previously enrolled in a randomized, clinical trial investigating the efficacy of InterStim. Of the 23 sites that participated in the original study, only 17 elected to participate in the follow-up trial.
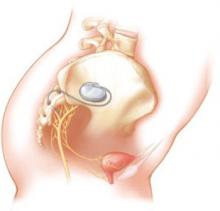
One hundred fifty-two subjects were enrolled—129 of them crossing over from the original study and 23 newly recruited and implanted with the InterStim device ( FIGURE 3 ). Investigators reviewed data from follow-up visits, self-reported symptoms obtained from voiding diaries collected annually for 5 years, and descriptive summaries of adverse events related to the sacral nerve stimulator.
One-year data were available on 138 subjects, and 5-year data were available on 105. Of the 47 participants who did not participate in 5-year follow-up, 16 had the InterStim device removed.
FIGURE 3 Aim of InterStim therapy is to regulate brain–bladder signals
The InterStim device sends a mild electrical impulse through a lead to the sacral nerves to influence the bladder and surrounding muscles. Sacral nerve stimulation helps regulate interaction between the brain and bladder and reduce voiding dysfunction.
Additional surgery was performed in half of patients
who had an adverse event
No life-threatening or irreversible adverse events were reported during the 5 years of follow-up. However, 221 adverse events occurred, of which 110 required a minor surgical procedure—in some cases, more than one. The most commonly reported adverse event was new pain or pain at the implantation site (46.1%). The most common surgical procedure was device exchange (23.7%).
Because many patients who suffer from overactive bladder syndrome fail to respond to conservative therapies, such as behavior modification and pharmacotherapy, novel alternatives are needed. This study:
- contributes to our understanding of sacral nerve stimulation
- provides much needed data on the long-term safety and efficacy of InterStim therapy
- proposes that InterStim therapy is safe and efficacious for women who have refractory overactive bladder syndrome
- suggests that it may become the gold standard for treatment of this condition.
At the Cleveland Clinic, we offer sacral nerve stimulation for refractory overactive bladder and nonobstructive urinary retention.
1. Hu TW, Wagner TH, Bentkover JD, Leblanc K, Zhou SZ, Hunt T. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461-465.
2. Mardon RE, Halim S, Pawlson LG, Haffer SC. Management of urinary incontinence in Medicare managed care beneficiaries: results from the 2004 Medicare Health Outcomes Survey. Arch Intern Med. 2006;166:1128-1133.
3. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167-178.
The authors report no financial relationships relevant to this article.
Four recent studies enhance our understanding of the benefits, efficacy, and risks of the following interventions in women who have urinary incontinence (UI):
- weight loss. Women who were randomized to intensive weight loss reduced the total number of UI episodes in a week by 47.4%—compared with 28.1% in the group randomized to a structured educational program.
- midurethral slings. Treatment of stress UI arising from intrinsic sphincter deficiency was more successful in women randomized to tension-free vaginal tape (TVT) than in women assigned to transobturator tape (TOT). In the first group, urodynamically confirmed stress UI was present in 21% of subjects after treatment, compared with 45% in the TOT group.
- botulinum toxin type A (Botox) injection. Women who had refractory urge incontinence were likely to improve significantly after injection of Botox. Sixty percent of women treated with Botox reported a reduction in incontinence after treatment, with a median response of 373 days, compared with 62 days in the placebo group.
- sacral neuromodulation. The mean number of urge incontinence episodes decreased from 9.6 to 3.9, and the mean number of voids per day decreased from 19.3 to 14.8 in a 5-year follow-up study.
Despite the promise of these findings, all of the studies had limitations, and several identified risks associated with the intervention. These limitations and risks are detailed in the articles that follow.
Urinary incontinence is no small problem. It affects more than 13 million women in the United States alone, and costs more than $20 billion annually in direct health-care costs.1
Despite the high prevalence of urinary incontinence, women are often reluctant to discuss symptoms with their physician. As a result, the condition remains undiagnosed or undertreated in many women.2
The most common types of urinary incontinence include:
- stress incontinence – leakage upon effort, exertion, or increased abdominal pressure
- urge incontinence – leakage accompanied by, or immediately preceded by, urgency
- mixed incontinence – leakage with urgency as well as effort, exertion, or increased abdominal pressure.
Another common problem is overactive bladder syndrome, which involves urgency with or without leakage, and usually increased frequency and nocturia as well.3
Although the midurethral sling revolutionized the treatment of stress urinary incontinence, most women who have incontinence experience mixed symptoms, making it a more challenging condition for the general-practice ObGyn to treat successfully. Furthermore, traditional therapies such as behavior modification, pelvic floor exercise, and medication have had only modest success in certain patient populations.
Weight loss can reduce urinary incontinence
in overweight and obese women
Subak LL, Wing R, Smith West D, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481–490.
Obesity increases intra-abdominal pressure, thereby exerting added force on the bladder, urethra, and pelvic floor and potentially exacerbating urinary incontinence (UI). It has been hypothesized that weight reduction reduces these forces and improves incontinence.
This randomized, clinical trial of 338 women showed that weight loss does have an effect on UI. Investigators compared an intensive 6-month weight loss program—designed to prompt a weight loss of 7% to 9% of body weight—with a structured educational program. The primary outcome was the percentage of change in the number of UI episodes reported in a 7-day voiding diary at 6 months.
After 6 months and a mean weight loss of 8% of baseline body weight, the women in the intensive weight loss group experienced a mean decrease of 47.4% in the total number of UI episodes in a week. Compare this with a mean weight loss of 1.6% (P<.001) and a mean decrease of 28.1% in UI episodes in the control group (P=.01). The reduction in the total number of UI episodes was primarily attributed to a reduction of 57.6% in stress-induced UI in the intensive weight loss group, compared with a reduction of 32.7% in the control group (P=.02).
Women in the weight loss program also perceived incontinence to be less of a problem and reported greater satisfaction with the change in their incontinence at 6 months than did women in the control group (P<.001).
Details of the trial
Women were eligible to participate if they reported 10 or more episodes of UI over 7 days, were at least 30 years old, and had a body mass index (BMI) of 25 to 50 at baseline (normal is 19 to 24). In addition, they had to agree not to initiate any new treatments for UI or weight loss during the 6-month study period.
Subjects were randomized in a 2:1 ratio, with 226 women assigned to the intensive weight loss program and 112 assigned to the structured educational program. Baseline characteristics were similar in both groups, with a mean age of 53±11 years, mean BMI of 36±6 kg/m3, and total mean number of UI episodes of 24±18 per week.
Strengths and limitations
Strengths of this study include the large and varied study population. One important limitation, however, is the fact that the primary outcome measure was based on self-reported UI episodes. Because participants were not blinded to their treatment assignment, bias in self-reporting may have been present. In addition, subjects were selected because of their potential to adhere to the rigorous study protocol.
As the prevalence of obesity reaches pandemic level, it’s imperative that medical science continue to develop novel methods with which we can help patients achieve ideal body weight.
The findings of this study contribute to the growing body of medical literature—across specialties—demonstrating that weight loss can significantly improve the general health of patients, and that it should be part of the first-line treatment for overweight and obese women who complain of UI.
TVT is more effective than TOT
for intrinsic sphincter deficiency
Schierlitz L, Dwyer PL, Rosamilia A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: a randomized controlled trial. Obstet Gynecol. 2008;112:1253–1261.
Many experts consider intrinsic sphincter deficiency (ISD) to be a severe form of stress incontinence. Earlier studies suggested that women who had stress incontinence complicated by ISD had a lower success rate after certain surgical procedures than did women who had stress incontinence alone.
This randomized trial compared tension-free vaginal tape (TVT) with transobturator tape (TOT) in the treatment of stress incontinence with ISD ( FIGURE 1 ). The primary outcome measure was the presence of urodynamically confirmed stress incontinence 6 months after surgery.
At that 6-month mark, stress incontinence was present in 14 of 67 subjects (21%) in the TVT group, compared with 32 of 71 subjects (45%) in the TOT group (P=.004). Nine patients in the TOT group requested a repeat surgical procedure, compared with none in the TVT group.
Although the study was not powered to detect a difference in postoperative complications, there were six bladder perforations in the TVT group and none in the TOT group.
FIGURE 1 TVT and TOT trace different routes
Details of the trial
Women were selected for the trial on the basis of urodynamic parameters and recruited from two academic centers. ISD was defined as maximal urethral closure pressure below 20 cm H2O or Valsalva leak-point pressure less than 60 cm H2O. Subjects in the two groups had similar baseline characteristics.
Surgeons were required to have independently performed at least 15 surgical midurethral sling procedures before the study began.
In addition to placement of TVT or TOT, approximately one third of subjects underwent concomitant prolapse surgery. Postoperatively, subjects were assessed at 6 weeks, 6 months, and 12 months, with repeat urodynamic testing performed at 6 months. Analysis of data was based on intention to treat.
Only 138 of 164 women completed repeat urodynamic testing 6 months after surgery. Seventeen women declined testing, claiming to be “cured,” and nine women withdrew from the study or were lost to follow-up.
Short follow-up was a limitation
The definition of treatment failure as persistent, urodynamically confirmed stress incontinence was another shortcoming of the trial. In addition, the 17 subjects who declined repeat postoperative testing were classified as “cured,” potentially biasing the results.
TVT may be the preferred surgical option for women who have urodynamically confirmed stress incontinence complicated by intrinsic sphincter deficiency (ISD)—on the basis of the data gathered by these researchers. Longer follow-up is needed, however, to determine the long-term, clinical efficacy of midurethral slings in women who have ISD.
Accumulation of more data from future studies will better equip ObGyns to customize surgical treatment options to individual clinical parameters and reduce the risk of surgical failure.
Is Botox a panacea for refractory urge incontinence?
Brubaker L, Richter HE, Visco A, et al: Pelvic Floor Disorders Network. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol. 2008;180:217–222.
Women who fail medical management of urge incontinence have few other options. This multicenter, randomized, double-blind, placebo-controlled trial suggests that there may one day be an effective alternative. Investigators examined the safety and efficacy of botulinum toxin type A (Botox) for the treatment of refractory idiopathic urge incontinence in 43 women—28 of them randomized to injection of 200 U of Botox and 15 to placebo.
Sixty percent of subjects in the Botox arm reported an improvement in symptoms, with a median response of 373 days, compared with 62 days in the placebo arm (P<.0001). Moreover, in the Botox arm, women perceived greater improvement in symptom control and a decrease in the number of self-reported incontinence episodes, compared with the placebo group (P<.0001).
However, 12 of 28 patients (43%) who received Botox developed elevated postvoid residuals (i.e., retention of more than 200 mL of urine), and enrollment was halted for this reason. Median time to initiate intermittent self-catheterization was 30 days, and intermittent self-catheterization lasted a median of 60 days. Nine of the 12 subjects who required self-catheterization developed a urinary tract infection.
Details of the trial
This study was conducted by the Pelvic Floor Disorders Network and sponsored by the National Institute of Child Health and Human Development. To be eligible for the trial, women had to have been diagnosed with refractory urge incontinence, which was defined as persistent symptoms after failing at least two first-line therapies such as anticholinergic medications and behavioral therapy. Also required was documented evidence of detrusor overactivity on urodynamic studies or at least six episodes of urge-related incontinence in a 3-day bladder diary. Investigators determined that a sample size of 210 subjects was needed to test a 50% efficacy rate for Botox, compared with 30% for placebo, at 6 months.
Baseline characteristics were similar between groups. A blinded physician used a cystoscope to inject the agents into the detrusor muscle over the posterior bladder wall ( FIGURE 2 ). Subjects also received an antibiotic before the procedure and for 3 additional days.
The primary outcome measure was treatment failure, defined as the return of symptoms measured at least 2 months after Botox injection or any change in medical therapy.
Enrollment was halted after interval analysis revealed a significantly higher rate of voiding dysfunction in the Botox arm, necessitating intermittent self-catheterization and associated urinary tract infections.
FIGURE 2 Intradetrusor Botox injection
In the trial by Brubaker and colleagues, 200 U of Botox was injected into the detrusor muscle on the posterior bladder wall to treat refractory urge incontinence in 28 women—60% of whom reported improvement.
Is a postvoid residual clinically significant?
Some experts questioned the clinical significance of the elevated postvoid residual reported in this trial, arguing that it was temporary and that many patients were asymptomatic.
Further study is needed to determine the optimal dosing of Botox and a management strategy for postprocedure voiding dysfunction.
When one of your patients considers cystoscopic intradetrusor Botox therapy for refractory urge incontinence, you should:
- counsel her extensively that its use here is not FDA-approved
- caution her that she may need to perform intermittent self-catheterization after injection
- advise her that the procedure and the medication are likely not covered by her health insurance and will be out-of-pocket expenses.
Much-needed and much-anticipated clinical trials are under way with the aim of obtaining FDA approval of Botox for urge incontinence.
At the Cleveland Clinic, we currently offer Botox for refractory urge incontinence.
InterStim therapy may become the gold standard for refractory overactive bladder syndrome
van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029–2034.
Sacral nerve stimulation has been approved for use in patients with refractory voiding dysfunction since 1997. This prospective, worldwide, follow-up study sought to determine the long-term efficacy and safety of sacral neuromodulation for the treatment of refractory urgency, frequency, urge incontinence, and nonobstructive urinary retention.
After 5 years of follow-up, 68% of subjects who had urge incontinence, 56% who had urgency and frequency, and 71% who had nonobstructive urinary retention reported a degree of improvement of 50% or more in their symptoms (relative to baseline). The mean number of urge incontinence episodes decreased from 9.6 to 3.9, and mean voids per day decreased from 19.3 to 14.8 at 5 years (P<.001).
Details of the trial
Eligible patients were previously enrolled in a randomized, clinical trial investigating the efficacy of InterStim. Of the 23 sites that participated in the original study, only 17 elected to participate in the follow-up trial.
One hundred fifty-two subjects were enrolled—129 of them crossing over from the original study and 23 newly recruited and implanted with the InterStim device ( FIGURE 3 ). Investigators reviewed data from follow-up visits, self-reported symptoms obtained from voiding diaries collected annually for 5 years, and descriptive summaries of adverse events related to the sacral nerve stimulator.
One-year data were available on 138 subjects, and 5-year data were available on 105. Of the 47 participants who did not participate in 5-year follow-up, 16 had the InterStim device removed.
FIGURE 3 Aim of InterStim therapy is to regulate brain–bladder signals
The InterStim device sends a mild electrical impulse through a lead to the sacral nerves to influence the bladder and surrounding muscles. Sacral nerve stimulation helps regulate interaction between the brain and bladder and reduce voiding dysfunction.
Additional surgery was performed in half of patients
who had an adverse event
No life-threatening or irreversible adverse events were reported during the 5 years of follow-up. However, 221 adverse events occurred, of which 110 required a minor surgical procedure—in some cases, more than one. The most commonly reported adverse event was new pain or pain at the implantation site (46.1%). The most common surgical procedure was device exchange (23.7%).
Because many patients who suffer from overactive bladder syndrome fail to respond to conservative therapies, such as behavior modification and pharmacotherapy, novel alternatives are needed. This study:
- contributes to our understanding of sacral nerve stimulation
- provides much needed data on the long-term safety and efficacy of InterStim therapy
- proposes that InterStim therapy is safe and efficacious for women who have refractory overactive bladder syndrome
- suggests that it may become the gold standard for treatment of this condition.
At the Cleveland Clinic, we offer sacral nerve stimulation for refractory overactive bladder and nonobstructive urinary retention.
The authors report no financial relationships relevant to this article.
Four recent studies enhance our understanding of the benefits, efficacy, and risks of the following interventions in women who have urinary incontinence (UI):
- weight loss. Women who were randomized to intensive weight loss reduced the total number of UI episodes in a week by 47.4%—compared with 28.1% in the group randomized to a structured educational program.
- midurethral slings. Treatment of stress UI arising from intrinsic sphincter deficiency was more successful in women randomized to tension-free vaginal tape (TVT) than in women assigned to transobturator tape (TOT). In the first group, urodynamically confirmed stress UI was present in 21% of subjects after treatment, compared with 45% in the TOT group.
- botulinum toxin type A (Botox) injection. Women who had refractory urge incontinence were likely to improve significantly after injection of Botox. Sixty percent of women treated with Botox reported a reduction in incontinence after treatment, with a median response of 373 days, compared with 62 days in the placebo group.
- sacral neuromodulation. The mean number of urge incontinence episodes decreased from 9.6 to 3.9, and the mean number of voids per day decreased from 19.3 to 14.8 in a 5-year follow-up study.
Despite the promise of these findings, all of the studies had limitations, and several identified risks associated with the intervention. These limitations and risks are detailed in the articles that follow.
Urinary incontinence is no small problem. It affects more than 13 million women in the United States alone, and costs more than $20 billion annually in direct health-care costs.1
Despite the high prevalence of urinary incontinence, women are often reluctant to discuss symptoms with their physician. As a result, the condition remains undiagnosed or undertreated in many women.2
The most common types of urinary incontinence include:
- stress incontinence – leakage upon effort, exertion, or increased abdominal pressure
- urge incontinence – leakage accompanied by, or immediately preceded by, urgency
- mixed incontinence – leakage with urgency as well as effort, exertion, or increased abdominal pressure.
Another common problem is overactive bladder syndrome, which involves urgency with or without leakage, and usually increased frequency and nocturia as well.3
Although the midurethral sling revolutionized the treatment of stress urinary incontinence, most women who have incontinence experience mixed symptoms, making it a more challenging condition for the general-practice ObGyn to treat successfully. Furthermore, traditional therapies such as behavior modification, pelvic floor exercise, and medication have had only modest success in certain patient populations.
Weight loss can reduce urinary incontinence
in overweight and obese women
Subak LL, Wing R, Smith West D, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481–490.
Obesity increases intra-abdominal pressure, thereby exerting added force on the bladder, urethra, and pelvic floor and potentially exacerbating urinary incontinence (UI). It has been hypothesized that weight reduction reduces these forces and improves incontinence.
This randomized, clinical trial of 338 women showed that weight loss does have an effect on UI. Investigators compared an intensive 6-month weight loss program—designed to prompt a weight loss of 7% to 9% of body weight—with a structured educational program. The primary outcome was the percentage of change in the number of UI episodes reported in a 7-day voiding diary at 6 months.
After 6 months and a mean weight loss of 8% of baseline body weight, the women in the intensive weight loss group experienced a mean decrease of 47.4% in the total number of UI episodes in a week. Compare this with a mean weight loss of 1.6% (P<.001) and a mean decrease of 28.1% in UI episodes in the control group (P=.01). The reduction in the total number of UI episodes was primarily attributed to a reduction of 57.6% in stress-induced UI in the intensive weight loss group, compared with a reduction of 32.7% in the control group (P=.02).
Women in the weight loss program also perceived incontinence to be less of a problem and reported greater satisfaction with the change in their incontinence at 6 months than did women in the control group (P<.001).
Details of the trial
Women were eligible to participate if they reported 10 or more episodes of UI over 7 days, were at least 30 years old, and had a body mass index (BMI) of 25 to 50 at baseline (normal is 19 to 24). In addition, they had to agree not to initiate any new treatments for UI or weight loss during the 6-month study period.
Subjects were randomized in a 2:1 ratio, with 226 women assigned to the intensive weight loss program and 112 assigned to the structured educational program. Baseline characteristics were similar in both groups, with a mean age of 53±11 years, mean BMI of 36±6 kg/m3, and total mean number of UI episodes of 24±18 per week.
Strengths and limitations
Strengths of this study include the large and varied study population. One important limitation, however, is the fact that the primary outcome measure was based on self-reported UI episodes. Because participants were not blinded to their treatment assignment, bias in self-reporting may have been present. In addition, subjects were selected because of their potential to adhere to the rigorous study protocol.
As the prevalence of obesity reaches pandemic level, it’s imperative that medical science continue to develop novel methods with which we can help patients achieve ideal body weight.
The findings of this study contribute to the growing body of medical literature—across specialties—demonstrating that weight loss can significantly improve the general health of patients, and that it should be part of the first-line treatment for overweight and obese women who complain of UI.
TVT is more effective than TOT
for intrinsic sphincter deficiency
Schierlitz L, Dwyer PL, Rosamilia A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: a randomized controlled trial. Obstet Gynecol. 2008;112:1253–1261.
Many experts consider intrinsic sphincter deficiency (ISD) to be a severe form of stress incontinence. Earlier studies suggested that women who had stress incontinence complicated by ISD had a lower success rate after certain surgical procedures than did women who had stress incontinence alone.
This randomized trial compared tension-free vaginal tape (TVT) with transobturator tape (TOT) in the treatment of stress incontinence with ISD ( FIGURE 1 ). The primary outcome measure was the presence of urodynamically confirmed stress incontinence 6 months after surgery.
At that 6-month mark, stress incontinence was present in 14 of 67 subjects (21%) in the TVT group, compared with 32 of 71 subjects (45%) in the TOT group (P=.004). Nine patients in the TOT group requested a repeat surgical procedure, compared with none in the TVT group.
Although the study was not powered to detect a difference in postoperative complications, there were six bladder perforations in the TVT group and none in the TOT group.
FIGURE 1 TVT and TOT trace different routes
Details of the trial
Women were selected for the trial on the basis of urodynamic parameters and recruited from two academic centers. ISD was defined as maximal urethral closure pressure below 20 cm H2O or Valsalva leak-point pressure less than 60 cm H2O. Subjects in the two groups had similar baseline characteristics.
Surgeons were required to have independently performed at least 15 surgical midurethral sling procedures before the study began.
In addition to placement of TVT or TOT, approximately one third of subjects underwent concomitant prolapse surgery. Postoperatively, subjects were assessed at 6 weeks, 6 months, and 12 months, with repeat urodynamic testing performed at 6 months. Analysis of data was based on intention to treat.
Only 138 of 164 women completed repeat urodynamic testing 6 months after surgery. Seventeen women declined testing, claiming to be “cured,” and nine women withdrew from the study or were lost to follow-up.
Short follow-up was a limitation
The definition of treatment failure as persistent, urodynamically confirmed stress incontinence was another shortcoming of the trial. In addition, the 17 subjects who declined repeat postoperative testing were classified as “cured,” potentially biasing the results.
TVT may be the preferred surgical option for women who have urodynamically confirmed stress incontinence complicated by intrinsic sphincter deficiency (ISD)—on the basis of the data gathered by these researchers. Longer follow-up is needed, however, to determine the long-term, clinical efficacy of midurethral slings in women who have ISD.
Accumulation of more data from future studies will better equip ObGyns to customize surgical treatment options to individual clinical parameters and reduce the risk of surgical failure.
Is Botox a panacea for refractory urge incontinence?
Brubaker L, Richter HE, Visco A, et al: Pelvic Floor Disorders Network. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol. 2008;180:217–222.
Women who fail medical management of urge incontinence have few other options. This multicenter, randomized, double-blind, placebo-controlled trial suggests that there may one day be an effective alternative. Investigators examined the safety and efficacy of botulinum toxin type A (Botox) for the treatment of refractory idiopathic urge incontinence in 43 women—28 of them randomized to injection of 200 U of Botox and 15 to placebo.
Sixty percent of subjects in the Botox arm reported an improvement in symptoms, with a median response of 373 days, compared with 62 days in the placebo arm (P<.0001). Moreover, in the Botox arm, women perceived greater improvement in symptom control and a decrease in the number of self-reported incontinence episodes, compared with the placebo group (P<.0001).
However, 12 of 28 patients (43%) who received Botox developed elevated postvoid residuals (i.e., retention of more than 200 mL of urine), and enrollment was halted for this reason. Median time to initiate intermittent self-catheterization was 30 days, and intermittent self-catheterization lasted a median of 60 days. Nine of the 12 subjects who required self-catheterization developed a urinary tract infection.
Details of the trial
This study was conducted by the Pelvic Floor Disorders Network and sponsored by the National Institute of Child Health and Human Development. To be eligible for the trial, women had to have been diagnosed with refractory urge incontinence, which was defined as persistent symptoms after failing at least two first-line therapies such as anticholinergic medications and behavioral therapy. Also required was documented evidence of detrusor overactivity on urodynamic studies or at least six episodes of urge-related incontinence in a 3-day bladder diary. Investigators determined that a sample size of 210 subjects was needed to test a 50% efficacy rate for Botox, compared with 30% for placebo, at 6 months.
Baseline characteristics were similar between groups. A blinded physician used a cystoscope to inject the agents into the detrusor muscle over the posterior bladder wall ( FIGURE 2 ). Subjects also received an antibiotic before the procedure and for 3 additional days.
The primary outcome measure was treatment failure, defined as the return of symptoms measured at least 2 months after Botox injection or any change in medical therapy.
Enrollment was halted after interval analysis revealed a significantly higher rate of voiding dysfunction in the Botox arm, necessitating intermittent self-catheterization and associated urinary tract infections.
FIGURE 2 Intradetrusor Botox injection
In the trial by Brubaker and colleagues, 200 U of Botox was injected into the detrusor muscle on the posterior bladder wall to treat refractory urge incontinence in 28 women—60% of whom reported improvement.
Is a postvoid residual clinically significant?
Some experts questioned the clinical significance of the elevated postvoid residual reported in this trial, arguing that it was temporary and that many patients were asymptomatic.
Further study is needed to determine the optimal dosing of Botox and a management strategy for postprocedure voiding dysfunction.
When one of your patients considers cystoscopic intradetrusor Botox therapy for refractory urge incontinence, you should:
- counsel her extensively that its use here is not FDA-approved
- caution her that she may need to perform intermittent self-catheterization after injection
- advise her that the procedure and the medication are likely not covered by her health insurance and will be out-of-pocket expenses.
Much-needed and much-anticipated clinical trials are under way with the aim of obtaining FDA approval of Botox for urge incontinence.
At the Cleveland Clinic, we currently offer Botox for refractory urge incontinence.
InterStim therapy may become the gold standard for refractory overactive bladder syndrome
van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029–2034.
Sacral nerve stimulation has been approved for use in patients with refractory voiding dysfunction since 1997. This prospective, worldwide, follow-up study sought to determine the long-term efficacy and safety of sacral neuromodulation for the treatment of refractory urgency, frequency, urge incontinence, and nonobstructive urinary retention.
After 5 years of follow-up, 68% of subjects who had urge incontinence, 56% who had urgency and frequency, and 71% who had nonobstructive urinary retention reported a degree of improvement of 50% or more in their symptoms (relative to baseline). The mean number of urge incontinence episodes decreased from 9.6 to 3.9, and mean voids per day decreased from 19.3 to 14.8 at 5 years (P<.001).
Details of the trial
Eligible patients were previously enrolled in a randomized, clinical trial investigating the efficacy of InterStim. Of the 23 sites that participated in the original study, only 17 elected to participate in the follow-up trial.
One hundred fifty-two subjects were enrolled—129 of them crossing over from the original study and 23 newly recruited and implanted with the InterStim device ( FIGURE 3 ). Investigators reviewed data from follow-up visits, self-reported symptoms obtained from voiding diaries collected annually for 5 years, and descriptive summaries of adverse events related to the sacral nerve stimulator.
One-year data were available on 138 subjects, and 5-year data were available on 105. Of the 47 participants who did not participate in 5-year follow-up, 16 had the InterStim device removed.
FIGURE 3 Aim of InterStim therapy is to regulate brain–bladder signals
The InterStim device sends a mild electrical impulse through a lead to the sacral nerves to influence the bladder and surrounding muscles. Sacral nerve stimulation helps regulate interaction between the brain and bladder and reduce voiding dysfunction.
Additional surgery was performed in half of patients
who had an adverse event
No life-threatening or irreversible adverse events were reported during the 5 years of follow-up. However, 221 adverse events occurred, of which 110 required a minor surgical procedure—in some cases, more than one. The most commonly reported adverse event was new pain or pain at the implantation site (46.1%). The most common surgical procedure was device exchange (23.7%).
Because many patients who suffer from overactive bladder syndrome fail to respond to conservative therapies, such as behavior modification and pharmacotherapy, novel alternatives are needed. This study:
- contributes to our understanding of sacral nerve stimulation
- provides much needed data on the long-term safety and efficacy of InterStim therapy
- proposes that InterStim therapy is safe and efficacious for women who have refractory overactive bladder syndrome
- suggests that it may become the gold standard for treatment of this condition.
At the Cleveland Clinic, we offer sacral nerve stimulation for refractory overactive bladder and nonobstructive urinary retention.
1. Hu TW, Wagner TH, Bentkover JD, Leblanc K, Zhou SZ, Hunt T. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461-465.
2. Mardon RE, Halim S, Pawlson LG, Haffer SC. Management of urinary incontinence in Medicare managed care beneficiaries: results from the 2004 Medicare Health Outcomes Survey. Arch Intern Med. 2006;166:1128-1133.
3. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167-178.
1. Hu TW, Wagner TH, Bentkover JD, Leblanc K, Zhou SZ, Hunt T. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461-465.
2. Mardon RE, Halim S, Pawlson LG, Haffer SC. Management of urinary incontinence in Medicare managed care beneficiaries: results from the 2004 Medicare Health Outcomes Survey. Arch Intern Med. 2006;166:1128-1133.
3. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167-178.
Anterior vaginal wall prolapse: Innovative surgical approaches
Does electrical stimulation aid muscle training for stress incontinence?
Objective
To determine whether pelvic floor electrical stimulation (PFES) improves outcomes of multicomponent behavioral training for stress incontinence.
Conclusion
Electrical stimulation did not significantly improve outcomes.
Method
Two hundred community-dwelling women with stress incontinence were randomized to 1 of 3 groups for an 8-week period: 1) biofeedback-assisted pelvic floor muscle training (PFMT), home exercises, bladder control strategies, and self-monitoring with bladder diaries; 2) the same program plus home PFES (15 minutes every other day, alternating with home exercises); or 3) self-administered behavioral training consisting of a self-help booklet and bladder diaries.
Results
Intention-to-treat analysis revealed that frequency of incontinent episodes was reduced by 68.6% in group 1, 71.9% in group 2, and 52.5% in group 3. Attrition rates for the 3 groups were 18.2%, 11.9%, and 37.3%, respectively. Efficacy analysis, which examined only those completing treatment (n = 155), showed no significant differences among the groups on reduction of incontinence episodes. Patients in the PFES group reported more satisfaction with their progress, suggesting some placebo effect.
Expert Commentary
The strength of this timely study is its design: a prospective randomized controlled trial with a large sample size and adequate power. It suffers, however, due to its short-term follow-up and a significantly greater attrition rate in the control group.
Only 2 prior studies have evaluated the effect of electrical stimulation as an adjunct to PFMT for stress incontinence. A study of 14 patients showed that the addition of electrical stimulation improved outcome of physiotherapy.1 Another study found the addition of both biofeedback and electrical stimulation improved symptoms and muscle strength, but the study did not isolate the effects of PFES as a single adjunct.2
Bottom Line
Patient compliance is vital to the success of behavioral therapy. The motivated patient with adequate neuromuscular function will improve with PFMT, with or without adjunctive therapy, and thus may forego surgical intervention. Less motivated patients, those who lack awareness of pelvic floor muscles, or those with decreased pelvic floor function may achieve greater success with adjunctive therapy, be it biofeedback or PFES. Still, long-term efficacy of such therapies is not known.
1. Blowman C, Pickles C, Emery S, et al. Prospective double blind controlled trial of intensive physiotherapy with and without stimulation of the pelvic floor in treatment of genuine stress incontinence. Physiotherapy. 1991;77:661-664.
2. Sung MS, Hong JY, Chol YH, et al. PFES biofeedback versus intensive pelvic floor muscle exercise for the prevention and treatment of genuine stress incontinence. J Korean Med Sci. 2000;15:303-308.
Objective
To determine whether pelvic floor electrical stimulation (PFES) improves outcomes of multicomponent behavioral training for stress incontinence.
Conclusion
Electrical stimulation did not significantly improve outcomes.
Method
Two hundred community-dwelling women with stress incontinence were randomized to 1 of 3 groups for an 8-week period: 1) biofeedback-assisted pelvic floor muscle training (PFMT), home exercises, bladder control strategies, and self-monitoring with bladder diaries; 2) the same program plus home PFES (15 minutes every other day, alternating with home exercises); or 3) self-administered behavioral training consisting of a self-help booklet and bladder diaries.
Results
Intention-to-treat analysis revealed that frequency of incontinent episodes was reduced by 68.6% in group 1, 71.9% in group 2, and 52.5% in group 3. Attrition rates for the 3 groups were 18.2%, 11.9%, and 37.3%, respectively. Efficacy analysis, which examined only those completing treatment (n = 155), showed no significant differences among the groups on reduction of incontinence episodes. Patients in the PFES group reported more satisfaction with their progress, suggesting some placebo effect.
Expert Commentary
The strength of this timely study is its design: a prospective randomized controlled trial with a large sample size and adequate power. It suffers, however, due to its short-term follow-up and a significantly greater attrition rate in the control group.
Only 2 prior studies have evaluated the effect of electrical stimulation as an adjunct to PFMT for stress incontinence. A study of 14 patients showed that the addition of electrical stimulation improved outcome of physiotherapy.1 Another study found the addition of both biofeedback and electrical stimulation improved symptoms and muscle strength, but the study did not isolate the effects of PFES as a single adjunct.2
Bottom Line
Patient compliance is vital to the success of behavioral therapy. The motivated patient with adequate neuromuscular function will improve with PFMT, with or without adjunctive therapy, and thus may forego surgical intervention. Less motivated patients, those who lack awareness of pelvic floor muscles, or those with decreased pelvic floor function may achieve greater success with adjunctive therapy, be it biofeedback or PFES. Still, long-term efficacy of such therapies is not known.
Objective
To determine whether pelvic floor electrical stimulation (PFES) improves outcomes of multicomponent behavioral training for stress incontinence.
Conclusion
Electrical stimulation did not significantly improve outcomes.
Method
Two hundred community-dwelling women with stress incontinence were randomized to 1 of 3 groups for an 8-week period: 1) biofeedback-assisted pelvic floor muscle training (PFMT), home exercises, bladder control strategies, and self-monitoring with bladder diaries; 2) the same program plus home PFES (15 minutes every other day, alternating with home exercises); or 3) self-administered behavioral training consisting of a self-help booklet and bladder diaries.
Results
Intention-to-treat analysis revealed that frequency of incontinent episodes was reduced by 68.6% in group 1, 71.9% in group 2, and 52.5% in group 3. Attrition rates for the 3 groups were 18.2%, 11.9%, and 37.3%, respectively. Efficacy analysis, which examined only those completing treatment (n = 155), showed no significant differences among the groups on reduction of incontinence episodes. Patients in the PFES group reported more satisfaction with their progress, suggesting some placebo effect.
Expert Commentary
The strength of this timely study is its design: a prospective randomized controlled trial with a large sample size and adequate power. It suffers, however, due to its short-term follow-up and a significantly greater attrition rate in the control group.
Only 2 prior studies have evaluated the effect of electrical stimulation as an adjunct to PFMT for stress incontinence. A study of 14 patients showed that the addition of electrical stimulation improved outcome of physiotherapy.1 Another study found the addition of both biofeedback and electrical stimulation improved symptoms and muscle strength, but the study did not isolate the effects of PFES as a single adjunct.2
Bottom Line
Patient compliance is vital to the success of behavioral therapy. The motivated patient with adequate neuromuscular function will improve with PFMT, with or without adjunctive therapy, and thus may forego surgical intervention. Less motivated patients, those who lack awareness of pelvic floor muscles, or those with decreased pelvic floor function may achieve greater success with adjunctive therapy, be it biofeedback or PFES. Still, long-term efficacy of such therapies is not known.
1. Blowman C, Pickles C, Emery S, et al. Prospective double blind controlled trial of intensive physiotherapy with and without stimulation of the pelvic floor in treatment of genuine stress incontinence. Physiotherapy. 1991;77:661-664.
2. Sung MS, Hong JY, Chol YH, et al. PFES biofeedback versus intensive pelvic floor muscle exercise for the prevention and treatment of genuine stress incontinence. J Korean Med Sci. 2000;15:303-308.
1. Blowman C, Pickles C, Emery S, et al. Prospective double blind controlled trial of intensive physiotherapy with and without stimulation of the pelvic floor in treatment of genuine stress incontinence. Physiotherapy. 1991;77:661-664.
2. Sung MS, Hong JY, Chol YH, et al. PFES biofeedback versus intensive pelvic floor muscle exercise for the prevention and treatment of genuine stress incontinence. J Korean Med Sci. 2000;15:303-308.