User login
Surgical considerations for tremor and dystonia
Over the last decade, several studies have demonstrated that deep brain stimulation (DBS) is among the most effective approaches for the treatment of patients with advanced movement disorders, including chorea, levodopa-induced dyskinesia, tremor, and dystonia.1 The goal of DBS is to restore function or relieve pain by stimulating neuronal activity through surgically implanted electrodes. DBS produces marked and persistent reductions in abnormal movements in patients with common hyperkinetic disorders, with a generally low incidence of serious adverse events in pediatric patients and adults.
DEEP BRAIN STIMULATION FOR ESSENTIAL TREMOR
Improvement with thalamic DBS
The ventral intermediate nucleus (VIM) of the thalamus is the most common target for DBS treatment of essential tremor. Several studies have demonstrated significant long-term improvement in tremor following thalamic DBS.3 Most studies enrolled 20 to 30 patients, who were followed for 1 to 5 years after device implantation. On average, these studies reported an improvement in overall tremor of approximately 50% from baseline with thalamic DBS.
Patient selection and stimulation parameters
Symptoms targeted for DBS treatment include unilateral and sometimes bilateral limb tremor. Some evidence exists for effectiveness in axial and vocal tremor as well. Factors to consider in patient selection for DBS surgery include tremor severity, degree of refractoriness to medication, and type of tremor. In addition, individual patient characteristics should be considered, including age, comorbid conditions, surgical risk, patient preference, social and employment factors, and social support.
DEEP BRAIN STIMULATION FOR DYSTONIA
Dystonia is characterized by involuntary twisting muscle contractions causing abnormal postures sometimes accompanied by jerky or repetitive involuntary movements. It may be classified according to the body part affected as generalized, segmental, or focal; in some cases it may be classified as multifocal dystonia or hemidystonia. Dystonia is also classified as primary or secondary, according to etiology. Primary dystonias are those not caused by any other identifiable condition and not associated with other neurologic abnormalities. These include idiopathic and some genetic dystonias, such as the DYT1 torsinA gene mutation. DBS of the globus pallidus internus (GPi) or subthalamic nucleus (STN) was approved by the US Food and Drug Administration under a humanitarian device exemption in 2003 for the treatment of primary generalized dystonia (PGD) in patients aged 7 years and older; GPi is the more common target).1
Evidence of efficacy
Several clinical studies have demonstrated the efficacy of DBS for patients with disabling PGD that is unresponsive to pharmacotherapy.
Long-term efficacy. Isaias and colleagues examined long-term safety and efficacy of DBS in 30 consecutive patients with PGD who were followed for at least 3 years after pallidal DBS surgery.6 DBS was delivered bilaterally in 28 patients and unilaterally in 2 patients. Clinical rating scales of motor function improved by a mean of 82.5% after 2 years, and dystonia-related disability improved by a mean of 75.2%. Improvement in motor function from baseline was noted for all 30 subjects. In five patients who were followed for 7 years, improvement in motor function remained greater than 80% at the last follow-up visit. Transient regressions were noted for patients with hardware failures or whose batteries had reached the end of life. Stimulation-related adverse events were reported for three patients and included speech difficulties and, in one patient, transient blepharospasm.
Vidailhet and colleagues examined the efficacy of bilateral pallidal stimulation in 22 patients with PGD who were followed prospectively for 3 years.7 Mean improvement from baseline in motor function on a dystonia rating scale was 51% after 1 year and 58% after 3 years (P = .03). Significant improvement was noted for individual ratings of upper and lower limb function scores. Health-related quality of life was also significantly improved at 3-year follow-up (P = .05). Serious adverse events were reported for three patients, including two lead fractures and one infection.
Results from double-blind trial. Kupsch and colleagues performed a randomized, double-blind clinical trial comparing pallidal DBS versus device implantation and sham stimulation in 40 patients with primary segmental or generalized dystonia.8 After 3 months, the mean change from baseline in severity of dystonia was 15.8% for patients who received DBS versus 1.4% with sham stimulation (P < .001). At the conclusion of the double-blind treatment phase, patients entered an open-label extension phase in which all patients received DBS for another 3 months. The initial benefit of treatment was sustained across the entire 6-month study period for patients initially randomized to DBS, whereas patients who were initially randomized to sham stimulation exhibited improved motor function during the open-label extension phase. Ratings of disability and quality of life also improved for patients receiving DBS at the end of the 6-month study. Adverse events included dysarthria (five patients), serious infections (four patients), and lead dislodgement (one patient).
Response with DYT1mutation. Coubes and colleagues examined the long-term efficacy and safety of bilateral DBS in 31 children and adults with PGD.9 PGD is associated with autosomal DYT1 mutations in approximately 30% of cases, and these authors examined the effects of treatment in patients with and without the DYT1 mutation. After 2 years of treatment, mean scores on a dystonia clinical rating scale decreased by 79% from baseline, and mean disability ratings decreased by 65%. The improvement in clinical dystonia rating scale scores was significantly greater for children than adults after 2 years (84.7% vs 70.1%; P = .04). In children, functional improvement was greater after 2 years in the subset of patients with DYT1 mutations than in the subset of patients without (76.1% vs 44.5%; P = .03), whereas in adults, DYT1 mutation status did not significantly influence response to treatment. One case of unilateral infection was noted, which required removal of the implant with successful reimplantation 6 months later. No other adverse events were reported.
Patient selection
Appropriate patients for DBS include those with an unequivocal diagnosis of dystonia and significant disability. Etiology and type of dystonia should also be considered. Patients with secondary dystonia (eg, due to structural brain lesions or heredodegenerative disorders) generally do not respond to DBS as well as patients with primary dystonias. A possible exception is tardive dystonia, which is caused by past exposure to dopamine receptor–blocking drugs. Although it is a secondary dystonia, tardive dystonia may respond well to DBS. Data on this point remain limited. Moreover, with tardive dystonia (as well as Sydenham chorea and poststroke hemiballismus), there may be spontaneous remission. DBS in these conditions should therefore be considered when enough time has elapsed that the likelihood of spontaneous remission is low.1
Not all dystonic symptoms have been shown to respond equally to DBS. Evidence of effectiveness is stronger and more consistent for limb and axial dystonia than for dystonic impairment of speech and swallowing. Phasic dystonia (jerky or rhythmic movements) appears to respond better than fixed postures. A critical point is that fixed postures not caused by electrically active muscle contraction will not respond to DBS. For example, bony deformity of the spine, joint disease, or tendon shortening cannot be expected to improve with DBS. The situation is complicated, since such conditions may develop as secondary consequences of dystonia. The potential for their development may warrant earlier rather than later DBS surgery in childhood-onset PGD.10
UNRESOLVED ISSUES IN DBS FOR DYSTONIA
How aggressively should other therapies be tried before starting DBS?
Pharmacologic options include a range of oral, intramuscular, and intrathecal agents. Injection of botulinum toxin to denervate affected muscles is a mainstay of treatment for focal or segmental dystonia, but often fails to improve symptoms because of the involvement of a large number of muscles, complexity of the movement pattern, or the development of neutralizing antibodies.8 With the exception of levodopa-responsive PGD, other pharmacologic therapy for PGD is generally of limited effectiveness for controlling symptoms of dystonia.9 Oral or intrathecal baclofen may improve symptoms, but often produces unacceptable sedation.
How important is intraoperative microelectrophysiology?
Although contemporary imaging techniques are important in the correct placement of stimulating electrodes, the available techniques do not always provide sufficient resolution to delineate the STN or GPi. The accuracy of electrode placement may also be influenced by distortions caused by lack of homogeneity among magnetic resonance images, brain shift, and signal deflections from cannulae or electrodes.14 These errors may result in significant deviation of electrode placement from the intended target. Microelectrode recording and micro-stimulation may be used to map the target region and refine the surgical target. It is widely, but not universally, held that this strategy contributes to superior accuracy and outcomes; it ordinarily requires an awake procedure, which is not always feasible in patients with severe dystonia or in pediatric patients.11
How should be programming (stimulator adjustment) be performed?
Research continues to refine our understanding of how electrical parameters such as voltage, frequency, and pulse width affect clinical outcomes in patients undergoing DBS for dystonia. Some programming approaches, such as long pulse width and high frequency, that were once generally accepted are now widely questioned. Another major unresolved question is: “How long should it take to see the results of stimulation?” In the clinical studies described above, continued improvement was generally observed over months or even years, and, in most patients, stimulators are incrementally adjusted over an extended period. However, some patients may experience much more rapid onset of benefit.
Long-term DBS management of dystonia
Unlike DBS for Parkinson disease or even essential tremor, DBS for dystonia is performed in young patients. This creates special challenges in pediatric patients, who can be expected to grow and develop after device implantation. As a result, children may require additional surgeries to reposition devices.
In addition, the most widely used devices require repeated battery replacement surgeries, although newer rechargeable devices are becoming available.
Finally, there is a nontrivial incidence of hardware-related complications when devices are used continuously for many years. Although individual dystonia patients vary in the acuity of their response to the cessation of stimulation,6 deterioration can be acute and dramatic. In long-term studies of bilateral pallidal stimulation described above, hardware failures were the most commonly reported adverse events, including unilateral or bilateral lead fracture.7,9 These appear to be more frequent in patients with dystonia than in other movement disorders.
SUMMARY AND CONCLUSIONS
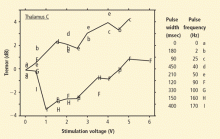
Deep brain stimulation produces marked and long-lasting improvement in motor function and disability in patients with hyperkinetic disorders. In patients with essential tremor, stimulation usually targets the VIM of the thalamus. Reduction in tremor is most closely related to stimulation frequency and voltage, whereas pulse width has little effect on treatment outcome. In patients with dystonia, stimulation typically targets the GPi or STN. Long-term prospective clinical trials demonstrated significant reductions in motor severity rating scale scores. Selecting patients for DBS requires careful consideration of a range of factors, including the specific clinical presentation, treatment history, and social support. Areas of current investigation include optimal stimulation programming, intraoperative mapping, and the long-term efficacy and safety of stimulation.
- Montgomery EB. Deep brain stimulation for hyperkinetic disorders. Neurosurg Focus 2004; 17:E1.
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Scientific Committee. Mov Disord 1998; 13 (suppl 3):2–23.
- Lyons KE, Pahwa R. Deep brain stimulation and tremor. Neurotherapeutics 2008; 5:331–338.
- Koller WC, Lyons KE, Wilkinson SB, Pahwa R. Efficacy of unilateral deep brain stimulation of the VIM nucleus of the thalamus for essential head tremor. Mov Disord 1999; 14:847–850.
- Cooper SE, Kuncel AM, Wolgamuth BR, Rezai AR, Grill WM. A model predicting optimal parameters for deep brain stimulation in essential tremor. J Clin Neurophysiol 2008; 25:265–273.
- Isaias IU, Alterman RL, Tagliati M. Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol 2009; 66:465–470.
- Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral, pallidal, deepbrain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol 2007; 6:223–229.
- Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Coubes P, Cif L, El Fertit H, et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg 2004; 101:189–194.
- Loher TJ, Capelle HH, Kaelin-Lang A, et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol 2008; 255:881–884.
- Lozano AM, Snyder BJ, Hamani C, Hutchison WD, Dostrovsky JO. Basal ganglia physiology and deep brain stimulation. Mov Disord 2010; 25 (suppl 1):S71–S75.
Over the last decade, several studies have demonstrated that deep brain stimulation (DBS) is among the most effective approaches for the treatment of patients with advanced movement disorders, including chorea, levodopa-induced dyskinesia, tremor, and dystonia.1 The goal of DBS is to restore function or relieve pain by stimulating neuronal activity through surgically implanted electrodes. DBS produces marked and persistent reductions in abnormal movements in patients with common hyperkinetic disorders, with a generally low incidence of serious adverse events in pediatric patients and adults.
DEEP BRAIN STIMULATION FOR ESSENTIAL TREMOR
Improvement with thalamic DBS
The ventral intermediate nucleus (VIM) of the thalamus is the most common target for DBS treatment of essential tremor. Several studies have demonstrated significant long-term improvement in tremor following thalamic DBS.3 Most studies enrolled 20 to 30 patients, who were followed for 1 to 5 years after device implantation. On average, these studies reported an improvement in overall tremor of approximately 50% from baseline with thalamic DBS.
Patient selection and stimulation parameters
Symptoms targeted for DBS treatment include unilateral and sometimes bilateral limb tremor. Some evidence exists for effectiveness in axial and vocal tremor as well. Factors to consider in patient selection for DBS surgery include tremor severity, degree of refractoriness to medication, and type of tremor. In addition, individual patient characteristics should be considered, including age, comorbid conditions, surgical risk, patient preference, social and employment factors, and social support.
DEEP BRAIN STIMULATION FOR DYSTONIA
Dystonia is characterized by involuntary twisting muscle contractions causing abnormal postures sometimes accompanied by jerky or repetitive involuntary movements. It may be classified according to the body part affected as generalized, segmental, or focal; in some cases it may be classified as multifocal dystonia or hemidystonia. Dystonia is also classified as primary or secondary, according to etiology. Primary dystonias are those not caused by any other identifiable condition and not associated with other neurologic abnormalities. These include idiopathic and some genetic dystonias, such as the DYT1 torsinA gene mutation. DBS of the globus pallidus internus (GPi) or subthalamic nucleus (STN) was approved by the US Food and Drug Administration under a humanitarian device exemption in 2003 for the treatment of primary generalized dystonia (PGD) in patients aged 7 years and older; GPi is the more common target).1
Evidence of efficacy
Several clinical studies have demonstrated the efficacy of DBS for patients with disabling PGD that is unresponsive to pharmacotherapy.
Long-term efficacy. Isaias and colleagues examined long-term safety and efficacy of DBS in 30 consecutive patients with PGD who were followed for at least 3 years after pallidal DBS surgery.6 DBS was delivered bilaterally in 28 patients and unilaterally in 2 patients. Clinical rating scales of motor function improved by a mean of 82.5% after 2 years, and dystonia-related disability improved by a mean of 75.2%. Improvement in motor function from baseline was noted for all 30 subjects. In five patients who were followed for 7 years, improvement in motor function remained greater than 80% at the last follow-up visit. Transient regressions were noted for patients with hardware failures or whose batteries had reached the end of life. Stimulation-related adverse events were reported for three patients and included speech difficulties and, in one patient, transient blepharospasm.
Vidailhet and colleagues examined the efficacy of bilateral pallidal stimulation in 22 patients with PGD who were followed prospectively for 3 years.7 Mean improvement from baseline in motor function on a dystonia rating scale was 51% after 1 year and 58% after 3 years (P = .03). Significant improvement was noted for individual ratings of upper and lower limb function scores. Health-related quality of life was also significantly improved at 3-year follow-up (P = .05). Serious adverse events were reported for three patients, including two lead fractures and one infection.
Results from double-blind trial. Kupsch and colleagues performed a randomized, double-blind clinical trial comparing pallidal DBS versus device implantation and sham stimulation in 40 patients with primary segmental or generalized dystonia.8 After 3 months, the mean change from baseline in severity of dystonia was 15.8% for patients who received DBS versus 1.4% with sham stimulation (P < .001). At the conclusion of the double-blind treatment phase, patients entered an open-label extension phase in which all patients received DBS for another 3 months. The initial benefit of treatment was sustained across the entire 6-month study period for patients initially randomized to DBS, whereas patients who were initially randomized to sham stimulation exhibited improved motor function during the open-label extension phase. Ratings of disability and quality of life also improved for patients receiving DBS at the end of the 6-month study. Adverse events included dysarthria (five patients), serious infections (four patients), and lead dislodgement (one patient).
Response with DYT1mutation. Coubes and colleagues examined the long-term efficacy and safety of bilateral DBS in 31 children and adults with PGD.9 PGD is associated with autosomal DYT1 mutations in approximately 30% of cases, and these authors examined the effects of treatment in patients with and without the DYT1 mutation. After 2 years of treatment, mean scores on a dystonia clinical rating scale decreased by 79% from baseline, and mean disability ratings decreased by 65%. The improvement in clinical dystonia rating scale scores was significantly greater for children than adults after 2 years (84.7% vs 70.1%; P = .04). In children, functional improvement was greater after 2 years in the subset of patients with DYT1 mutations than in the subset of patients without (76.1% vs 44.5%; P = .03), whereas in adults, DYT1 mutation status did not significantly influence response to treatment. One case of unilateral infection was noted, which required removal of the implant with successful reimplantation 6 months later. No other adverse events were reported.
Patient selection
Appropriate patients for DBS include those with an unequivocal diagnosis of dystonia and significant disability. Etiology and type of dystonia should also be considered. Patients with secondary dystonia (eg, due to structural brain lesions or heredodegenerative disorders) generally do not respond to DBS as well as patients with primary dystonias. A possible exception is tardive dystonia, which is caused by past exposure to dopamine receptor–blocking drugs. Although it is a secondary dystonia, tardive dystonia may respond well to DBS. Data on this point remain limited. Moreover, with tardive dystonia (as well as Sydenham chorea and poststroke hemiballismus), there may be spontaneous remission. DBS in these conditions should therefore be considered when enough time has elapsed that the likelihood of spontaneous remission is low.1
Not all dystonic symptoms have been shown to respond equally to DBS. Evidence of effectiveness is stronger and more consistent for limb and axial dystonia than for dystonic impairment of speech and swallowing. Phasic dystonia (jerky or rhythmic movements) appears to respond better than fixed postures. A critical point is that fixed postures not caused by electrically active muscle contraction will not respond to DBS. For example, bony deformity of the spine, joint disease, or tendon shortening cannot be expected to improve with DBS. The situation is complicated, since such conditions may develop as secondary consequences of dystonia. The potential for their development may warrant earlier rather than later DBS surgery in childhood-onset PGD.10
UNRESOLVED ISSUES IN DBS FOR DYSTONIA
How aggressively should other therapies be tried before starting DBS?
Pharmacologic options include a range of oral, intramuscular, and intrathecal agents. Injection of botulinum toxin to denervate affected muscles is a mainstay of treatment for focal or segmental dystonia, but often fails to improve symptoms because of the involvement of a large number of muscles, complexity of the movement pattern, or the development of neutralizing antibodies.8 With the exception of levodopa-responsive PGD, other pharmacologic therapy for PGD is generally of limited effectiveness for controlling symptoms of dystonia.9 Oral or intrathecal baclofen may improve symptoms, but often produces unacceptable sedation.
How important is intraoperative microelectrophysiology?
Although contemporary imaging techniques are important in the correct placement of stimulating electrodes, the available techniques do not always provide sufficient resolution to delineate the STN or GPi. The accuracy of electrode placement may also be influenced by distortions caused by lack of homogeneity among magnetic resonance images, brain shift, and signal deflections from cannulae or electrodes.14 These errors may result in significant deviation of electrode placement from the intended target. Microelectrode recording and micro-stimulation may be used to map the target region and refine the surgical target. It is widely, but not universally, held that this strategy contributes to superior accuracy and outcomes; it ordinarily requires an awake procedure, which is not always feasible in patients with severe dystonia or in pediatric patients.11
How should be programming (stimulator adjustment) be performed?
Research continues to refine our understanding of how electrical parameters such as voltage, frequency, and pulse width affect clinical outcomes in patients undergoing DBS for dystonia. Some programming approaches, such as long pulse width and high frequency, that were once generally accepted are now widely questioned. Another major unresolved question is: “How long should it take to see the results of stimulation?” In the clinical studies described above, continued improvement was generally observed over months or even years, and, in most patients, stimulators are incrementally adjusted over an extended period. However, some patients may experience much more rapid onset of benefit.
Long-term DBS management of dystonia
Unlike DBS for Parkinson disease or even essential tremor, DBS for dystonia is performed in young patients. This creates special challenges in pediatric patients, who can be expected to grow and develop after device implantation. As a result, children may require additional surgeries to reposition devices.
In addition, the most widely used devices require repeated battery replacement surgeries, although newer rechargeable devices are becoming available.
Finally, there is a nontrivial incidence of hardware-related complications when devices are used continuously for many years. Although individual dystonia patients vary in the acuity of their response to the cessation of stimulation,6 deterioration can be acute and dramatic. In long-term studies of bilateral pallidal stimulation described above, hardware failures were the most commonly reported adverse events, including unilateral or bilateral lead fracture.7,9 These appear to be more frequent in patients with dystonia than in other movement disorders.
SUMMARY AND CONCLUSIONS
Deep brain stimulation produces marked and long-lasting improvement in motor function and disability in patients with hyperkinetic disorders. In patients with essential tremor, stimulation usually targets the VIM of the thalamus. Reduction in tremor is most closely related to stimulation frequency and voltage, whereas pulse width has little effect on treatment outcome. In patients with dystonia, stimulation typically targets the GPi or STN. Long-term prospective clinical trials demonstrated significant reductions in motor severity rating scale scores. Selecting patients for DBS requires careful consideration of a range of factors, including the specific clinical presentation, treatment history, and social support. Areas of current investigation include optimal stimulation programming, intraoperative mapping, and the long-term efficacy and safety of stimulation.
Over the last decade, several studies have demonstrated that deep brain stimulation (DBS) is among the most effective approaches for the treatment of patients with advanced movement disorders, including chorea, levodopa-induced dyskinesia, tremor, and dystonia.1 The goal of DBS is to restore function or relieve pain by stimulating neuronal activity through surgically implanted electrodes. DBS produces marked and persistent reductions in abnormal movements in patients with common hyperkinetic disorders, with a generally low incidence of serious adverse events in pediatric patients and adults.
DEEP BRAIN STIMULATION FOR ESSENTIAL TREMOR
Improvement with thalamic DBS
The ventral intermediate nucleus (VIM) of the thalamus is the most common target for DBS treatment of essential tremor. Several studies have demonstrated significant long-term improvement in tremor following thalamic DBS.3 Most studies enrolled 20 to 30 patients, who were followed for 1 to 5 years after device implantation. On average, these studies reported an improvement in overall tremor of approximately 50% from baseline with thalamic DBS.
Patient selection and stimulation parameters
Symptoms targeted for DBS treatment include unilateral and sometimes bilateral limb tremor. Some evidence exists for effectiveness in axial and vocal tremor as well. Factors to consider in patient selection for DBS surgery include tremor severity, degree of refractoriness to medication, and type of tremor. In addition, individual patient characteristics should be considered, including age, comorbid conditions, surgical risk, patient preference, social and employment factors, and social support.
DEEP BRAIN STIMULATION FOR DYSTONIA
Dystonia is characterized by involuntary twisting muscle contractions causing abnormal postures sometimes accompanied by jerky or repetitive involuntary movements. It may be classified according to the body part affected as generalized, segmental, or focal; in some cases it may be classified as multifocal dystonia or hemidystonia. Dystonia is also classified as primary or secondary, according to etiology. Primary dystonias are those not caused by any other identifiable condition and not associated with other neurologic abnormalities. These include idiopathic and some genetic dystonias, such as the DYT1 torsinA gene mutation. DBS of the globus pallidus internus (GPi) or subthalamic nucleus (STN) was approved by the US Food and Drug Administration under a humanitarian device exemption in 2003 for the treatment of primary generalized dystonia (PGD) in patients aged 7 years and older; GPi is the more common target).1
Evidence of efficacy
Several clinical studies have demonstrated the efficacy of DBS for patients with disabling PGD that is unresponsive to pharmacotherapy.
Long-term efficacy. Isaias and colleagues examined long-term safety and efficacy of DBS in 30 consecutive patients with PGD who were followed for at least 3 years after pallidal DBS surgery.6 DBS was delivered bilaterally in 28 patients and unilaterally in 2 patients. Clinical rating scales of motor function improved by a mean of 82.5% after 2 years, and dystonia-related disability improved by a mean of 75.2%. Improvement in motor function from baseline was noted for all 30 subjects. In five patients who were followed for 7 years, improvement in motor function remained greater than 80% at the last follow-up visit. Transient regressions were noted for patients with hardware failures or whose batteries had reached the end of life. Stimulation-related adverse events were reported for three patients and included speech difficulties and, in one patient, transient blepharospasm.
Vidailhet and colleagues examined the efficacy of bilateral pallidal stimulation in 22 patients with PGD who were followed prospectively for 3 years.7 Mean improvement from baseline in motor function on a dystonia rating scale was 51% after 1 year and 58% after 3 years (P = .03). Significant improvement was noted for individual ratings of upper and lower limb function scores. Health-related quality of life was also significantly improved at 3-year follow-up (P = .05). Serious adverse events were reported for three patients, including two lead fractures and one infection.
Results from double-blind trial. Kupsch and colleagues performed a randomized, double-blind clinical trial comparing pallidal DBS versus device implantation and sham stimulation in 40 patients with primary segmental or generalized dystonia.8 After 3 months, the mean change from baseline in severity of dystonia was 15.8% for patients who received DBS versus 1.4% with sham stimulation (P < .001). At the conclusion of the double-blind treatment phase, patients entered an open-label extension phase in which all patients received DBS for another 3 months. The initial benefit of treatment was sustained across the entire 6-month study period for patients initially randomized to DBS, whereas patients who were initially randomized to sham stimulation exhibited improved motor function during the open-label extension phase. Ratings of disability and quality of life also improved for patients receiving DBS at the end of the 6-month study. Adverse events included dysarthria (five patients), serious infections (four patients), and lead dislodgement (one patient).
Response with DYT1mutation. Coubes and colleagues examined the long-term efficacy and safety of bilateral DBS in 31 children and adults with PGD.9 PGD is associated with autosomal DYT1 mutations in approximately 30% of cases, and these authors examined the effects of treatment in patients with and without the DYT1 mutation. After 2 years of treatment, mean scores on a dystonia clinical rating scale decreased by 79% from baseline, and mean disability ratings decreased by 65%. The improvement in clinical dystonia rating scale scores was significantly greater for children than adults after 2 years (84.7% vs 70.1%; P = .04). In children, functional improvement was greater after 2 years in the subset of patients with DYT1 mutations than in the subset of patients without (76.1% vs 44.5%; P = .03), whereas in adults, DYT1 mutation status did not significantly influence response to treatment. One case of unilateral infection was noted, which required removal of the implant with successful reimplantation 6 months later. No other adverse events were reported.
Patient selection
Appropriate patients for DBS include those with an unequivocal diagnosis of dystonia and significant disability. Etiology and type of dystonia should also be considered. Patients with secondary dystonia (eg, due to structural brain lesions or heredodegenerative disorders) generally do not respond to DBS as well as patients with primary dystonias. A possible exception is tardive dystonia, which is caused by past exposure to dopamine receptor–blocking drugs. Although it is a secondary dystonia, tardive dystonia may respond well to DBS. Data on this point remain limited. Moreover, with tardive dystonia (as well as Sydenham chorea and poststroke hemiballismus), there may be spontaneous remission. DBS in these conditions should therefore be considered when enough time has elapsed that the likelihood of spontaneous remission is low.1
Not all dystonic symptoms have been shown to respond equally to DBS. Evidence of effectiveness is stronger and more consistent for limb and axial dystonia than for dystonic impairment of speech and swallowing. Phasic dystonia (jerky or rhythmic movements) appears to respond better than fixed postures. A critical point is that fixed postures not caused by electrically active muscle contraction will not respond to DBS. For example, bony deformity of the spine, joint disease, or tendon shortening cannot be expected to improve with DBS. The situation is complicated, since such conditions may develop as secondary consequences of dystonia. The potential for their development may warrant earlier rather than later DBS surgery in childhood-onset PGD.10
UNRESOLVED ISSUES IN DBS FOR DYSTONIA
How aggressively should other therapies be tried before starting DBS?
Pharmacologic options include a range of oral, intramuscular, and intrathecal agents. Injection of botulinum toxin to denervate affected muscles is a mainstay of treatment for focal or segmental dystonia, but often fails to improve symptoms because of the involvement of a large number of muscles, complexity of the movement pattern, or the development of neutralizing antibodies.8 With the exception of levodopa-responsive PGD, other pharmacologic therapy for PGD is generally of limited effectiveness for controlling symptoms of dystonia.9 Oral or intrathecal baclofen may improve symptoms, but often produces unacceptable sedation.
How important is intraoperative microelectrophysiology?
Although contemporary imaging techniques are important in the correct placement of stimulating electrodes, the available techniques do not always provide sufficient resolution to delineate the STN or GPi. The accuracy of electrode placement may also be influenced by distortions caused by lack of homogeneity among magnetic resonance images, brain shift, and signal deflections from cannulae or electrodes.14 These errors may result in significant deviation of electrode placement from the intended target. Microelectrode recording and micro-stimulation may be used to map the target region and refine the surgical target. It is widely, but not universally, held that this strategy contributes to superior accuracy and outcomes; it ordinarily requires an awake procedure, which is not always feasible in patients with severe dystonia or in pediatric patients.11
How should be programming (stimulator adjustment) be performed?
Research continues to refine our understanding of how electrical parameters such as voltage, frequency, and pulse width affect clinical outcomes in patients undergoing DBS for dystonia. Some programming approaches, such as long pulse width and high frequency, that were once generally accepted are now widely questioned. Another major unresolved question is: “How long should it take to see the results of stimulation?” In the clinical studies described above, continued improvement was generally observed over months or even years, and, in most patients, stimulators are incrementally adjusted over an extended period. However, some patients may experience much more rapid onset of benefit.
Long-term DBS management of dystonia
Unlike DBS for Parkinson disease or even essential tremor, DBS for dystonia is performed in young patients. This creates special challenges in pediatric patients, who can be expected to grow and develop after device implantation. As a result, children may require additional surgeries to reposition devices.
In addition, the most widely used devices require repeated battery replacement surgeries, although newer rechargeable devices are becoming available.
Finally, there is a nontrivial incidence of hardware-related complications when devices are used continuously for many years. Although individual dystonia patients vary in the acuity of their response to the cessation of stimulation,6 deterioration can be acute and dramatic. In long-term studies of bilateral pallidal stimulation described above, hardware failures were the most commonly reported adverse events, including unilateral or bilateral lead fracture.7,9 These appear to be more frequent in patients with dystonia than in other movement disorders.
SUMMARY AND CONCLUSIONS
Deep brain stimulation produces marked and long-lasting improvement in motor function and disability in patients with hyperkinetic disorders. In patients with essential tremor, stimulation usually targets the VIM of the thalamus. Reduction in tremor is most closely related to stimulation frequency and voltage, whereas pulse width has little effect on treatment outcome. In patients with dystonia, stimulation typically targets the GPi or STN. Long-term prospective clinical trials demonstrated significant reductions in motor severity rating scale scores. Selecting patients for DBS requires careful consideration of a range of factors, including the specific clinical presentation, treatment history, and social support. Areas of current investigation include optimal stimulation programming, intraoperative mapping, and the long-term efficacy and safety of stimulation.
- Montgomery EB. Deep brain stimulation for hyperkinetic disorders. Neurosurg Focus 2004; 17:E1.
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Scientific Committee. Mov Disord 1998; 13 (suppl 3):2–23.
- Lyons KE, Pahwa R. Deep brain stimulation and tremor. Neurotherapeutics 2008; 5:331–338.
- Koller WC, Lyons KE, Wilkinson SB, Pahwa R. Efficacy of unilateral deep brain stimulation of the VIM nucleus of the thalamus for essential head tremor. Mov Disord 1999; 14:847–850.
- Cooper SE, Kuncel AM, Wolgamuth BR, Rezai AR, Grill WM. A model predicting optimal parameters for deep brain stimulation in essential tremor. J Clin Neurophysiol 2008; 25:265–273.
- Isaias IU, Alterman RL, Tagliati M. Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol 2009; 66:465–470.
- Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral, pallidal, deepbrain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol 2007; 6:223–229.
- Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Coubes P, Cif L, El Fertit H, et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg 2004; 101:189–194.
- Loher TJ, Capelle HH, Kaelin-Lang A, et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol 2008; 255:881–884.
- Lozano AM, Snyder BJ, Hamani C, Hutchison WD, Dostrovsky JO. Basal ganglia physiology and deep brain stimulation. Mov Disord 2010; 25 (suppl 1):S71–S75.
- Montgomery EB. Deep brain stimulation for hyperkinetic disorders. Neurosurg Focus 2004; 17:E1.
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Scientific Committee. Mov Disord 1998; 13 (suppl 3):2–23.
- Lyons KE, Pahwa R. Deep brain stimulation and tremor. Neurotherapeutics 2008; 5:331–338.
- Koller WC, Lyons KE, Wilkinson SB, Pahwa R. Efficacy of unilateral deep brain stimulation of the VIM nucleus of the thalamus for essential head tremor. Mov Disord 1999; 14:847–850.
- Cooper SE, Kuncel AM, Wolgamuth BR, Rezai AR, Grill WM. A model predicting optimal parameters for deep brain stimulation in essential tremor. J Clin Neurophysiol 2008; 25:265–273.
- Isaias IU, Alterman RL, Tagliati M. Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol 2009; 66:465–470.
- Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral, pallidal, deepbrain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol 2007; 6:223–229.
- Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Coubes P, Cif L, El Fertit H, et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg 2004; 101:189–194.
- Loher TJ, Capelle HH, Kaelin-Lang A, et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol 2008; 255:881–884.
- Lozano AM, Snyder BJ, Hamani C, Hutchison WD, Dostrovsky JO. Basal ganglia physiology and deep brain stimulation. Mov Disord 2010; 25 (suppl 1):S71–S75.