User login
Hepatitis C: Screening changes, treatment advances
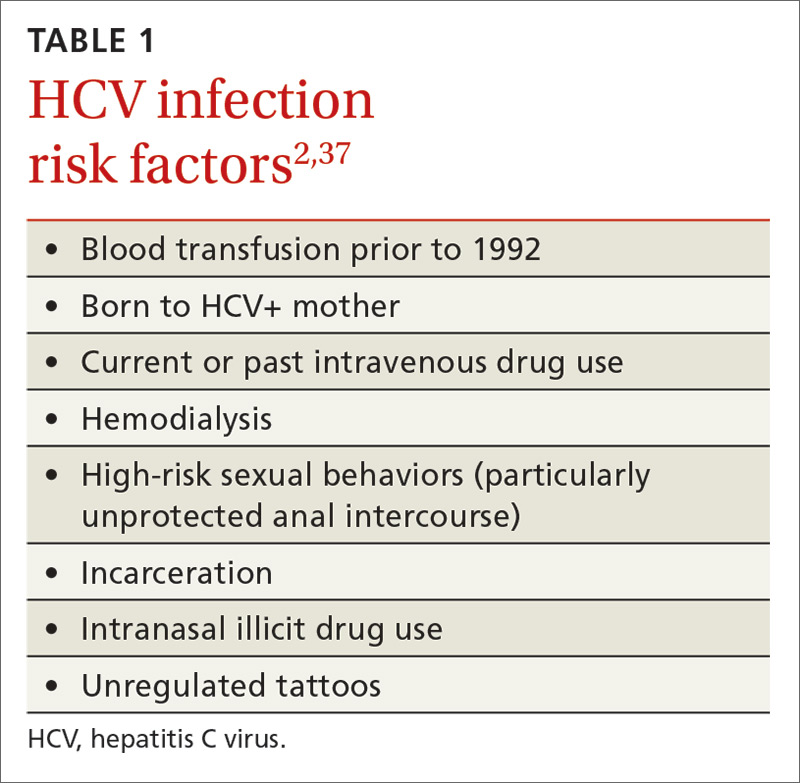
Several recent developments have prompted a renewed focus on the way we screen for, and manage the treatment of, hepatitis C virus (HCV) infection. In 2013, the United States Preventive Services Task Force expanded its HCV screening guidelines to include baby boomers born between 1945 and 1965, regardless of apparent risk factors (TABLE 11).2 The recommendation is based on the high prevalence of chronic HCV in this cohort, estimated to be 4.3%, which is about 4 times higher than that of the general US population.3 It is believed that 75% of chronic HCV infections in the United States are in this cohort. After decades of infection, many in this age group are now presenting with advanced disease, leading to 19,659 HCV-related deaths in America in 2014.4
In addition, while HCV incidence in America had been steadily declining, it is now once again on the rise among young, non-urban whites, mainly because of increasing intravenous drug use in this population.5 On a positive note, new highly-effective and better-tolerated treatments are greatly improving the care we can provide.
In light of these factors, family physicians (FPs) are likely to be screening for HCV more than ever before and must be prepared to provide appropriate counseling and initial clinical management for those with positive test results. This article reviews the evaluation and primary care management of HCV-infected patients, as well as approaches to treatment with the newest direct-acting antivirals (DAAs).
The natural history of hepatitis C (and what we’re seeing as boomers age)
Acute HCV infection is rarely symptomatic, but results in chronic infection approximately 75% of the time.6 While some chronically infected individuals remain unaffected, most develop some degree of hepatic fibrosis, and 20% will develop cirrhosis within 20 years of diagnosis.7-9
The rate of progression is variable; factors that result in more rapid progression of liver disease include coinfection with HIV or HBV, overweight or obesity, insulin resistance, male gender, and use of alcohol.7 As the baby boomer cohort has aged, patients infected in their youth are now presenting with the sequelae of decompensated cirrhosis, including ascites, portal vein thrombosis, and thrombocytopenia.
Extrahepatic manifestations of chronic hepatitis C can include fatigue, membranoproliferative glomerulonephritis, porphyria cutanea tarda, cryoglobulinemia, a higher likelihood of insulin resistance, and possibly lymphoma.10-12
Chronic HCV is also the major contributor to the increased incidence of hepatocellular carcinoma (HCC), which has tripled in the past 2 decades in the United States.13
Although results are inconsistent, studies suggest 5% to 10% of HCV-infected patients will succumb to liver-related death.7
Who you’ll screen
If your patient is at heightened risk of contracting HCV infection (TABLE 11) or was born between 1945 and 1965, you’ll want to screen for infection with an HCV antibody test. A positive antibody test must be followed by testing for hepatitis C viral RNA to confirm whether the patient is chronically infected or is among the approximately 25% of patients who spontaneously clear the virus.6
For the patient with no detectable HCV RNA, no further evaluation or treatment is necessary. HCV viral load itself provides little insight into the rate of progression of the illness, but does correlate with risk of transmission.14 Counseling patients about the full testing protocol before screening can help to reduce anxiety and confusion.
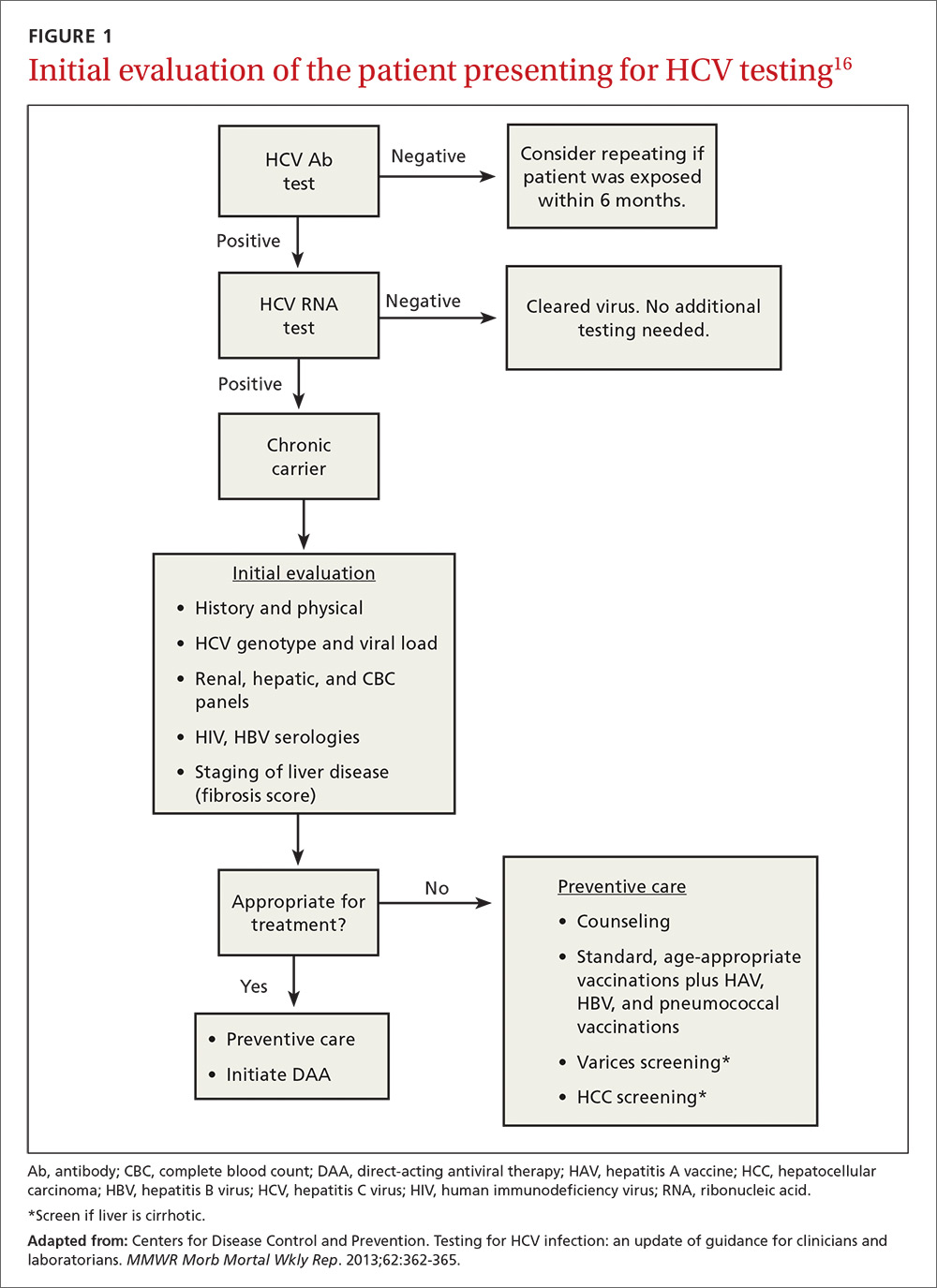
At present, 6 genotypes and multiple subtypes of HCV have been identified; these have important implications for prognosis and treatment.15 HCV genotyping is frequently ordered along with a test for HCV viral load, but may be deferred until after fibrosis staging is performed (more on that in a bit). It may also be deferred if treatment is not planned within the next 12 weeks, as its main clinical use is to guide choice of treatment. Once chronic infection has been confirmed by the presence of HCV viremia, further work-up focuses on evaluating the effects on the host, which, in turn, helps the provider finalize a treatment plan (FIGURE 116).
Follow initial screening with an evaluation including liver disease staging
Following a screen that comes back positive for HCV, you’ll conduct a more thorough history including questioning about previous or ongoing risk factors for HCV, perform a physical examination that includes looking for signs of liver failure or extrahepatic disease, and order more lab work. Laboratory investigations include a complete blood count; renal and hepatic panels; and testing for human immunodeficiency virus (HIV) antibody, hepatitis B virus (HBV) surface antigen, HBV surface antibody, and HBV core antibody.1,17 Finally, the patient must be evaluated for hepatic fibrosis and cirrhosis to quantify the likelihood of developing liver failure and HCC.
Staging liver disease is a prerequisite to treating HCV infection because the extent of liver fibrosis impacts not only prognosis, but also the choice of the treatment regimen and the duration of therapy. The traditional gold standard for diagnosing hepatic fibrosis and cirrhosis has been a liver biopsy; however, a single 1.6-mm biopsy evaluates only a small portion of the liver and can miss affected liver parenchyma. In addition, a liver biopsy carries a small, but not inconsequential, risk of morbidity, and can be costly and complex to arrange.
Several noninvasive options are now available and are typically the preferred methods for staging liver disease. FibroSURE (LabCorp), for example, uses a peripheral venous blood sample and combines the patient’s age, gender, and 6 biochemical markers to generate a range of scores that correspond to the fibrosis component of the well-known METAVIR scoring system and correlate with results of liver biopsies.18,19 (The METAVIR system is a histology-based scoring system that grades fibrosis from F0 [no fibrosis] to F4 [cirrhosis].)
Noninvasive imaging studies assess for fibrosis more directly by assessing liver elasticity, either by ultrasound or magnetic resonance (MR) technology. The ultrasound modality FibroScan (Echosens) is currently the most widely available, although some data suggest the more expensive MR elastography has higher sensitivity (94% sensitive for METAVIR F2 or higher compared to 79% by FibroScan).20,21 While each of these modalities has limitations (eg, body habitus, availability), these tests allow stratification of patients into categories of low, moderate, and high risk for cirrhosis without the risks of biopsy.
A curable viral infection
HCV is one of the few curable chronic viral infections; unlike HBV and HIV, HCV does not create a long-term intra-nuclear reservoir. DAAs have cure rates of more than 95% for many HCV genotypes,22-24 allowing the possibility for dramatic reductions in prevalence in the decades to come.
Cure is defined by reaching a sustained virologic response (SVR), or absence of detectable viral load, 12 weeks after completion of therapy. Patients with HCV infection with advanced fibrosis who achieve SVR have shown benefits beyond improvement in liver function and histology. One large, multicenter, prospective study of 530 patients with chronic HCV, for example, found that those who achieved SVR experienced a 76% reduction in the risk of HCC and a 66% reduction in all-cause mortality (number needed to treat [NNT] was 5.8 to prevent one death or 6 to prevent one case of HCC in 10 years) compared to those without SVR.25 Other extrahepatic manifestations that impair quality of life, such as renal disease, autoimmune disease, and circulatory problems, are likewise reduced.25
Guidelines now recommend treating most patients with HCV infection
Until 2011, HCV treatment included the injectable immune-activating agent interferon and the non–HCV-specific antiviral ribavirin. This regimen had low SVR rates of 40% to 60% and adverse effects that were often intolerable.26 The advent of the first-generation HCV protease inhibitors in 2011 improved SVR rates, which have continued to improve exponentially with the development of combination therapy using DAAs (TABLE 227-29). (In order to stay up to date with the latest options for the treatment of HCV, see The American Association for the Study of Liver Diseases treatment guidelines at: http://hcvguidelines.org.)

What the guidelines say. Due to the tolerability and efficacy of the new DAAs, current guidelines state that HCV treatment should be recommended to most patients with HCV infection—not just those with advanced disease.30 This is a major change from prior guidelines, which were based on more toxic and less effective regimens. Limited data from long-term cohort studies of patients using interferon-based regimens suggest that the benefits of SVR are greatest for those treated at early stages before significant fibrosis develops. At least one analysis involving over 4000 patients found, however, that this approach may be less cost-effective, with an NNT of 20 to prevent one death in 20 years.31
In practice, the decision to treat requires a discussion between the patient and provider, weighing the risks and benefits of treatment in the context of the patients’ comorbidities and overall life expectancy. Such a discussion must also include cost. Many insurance companies will still only cover antiviral therapy for patients with advanced fibrosis, but these restrictions are slowly lifting and are having significant implications for our health care system. By one estimate, treating all patients with HCV at current drug prices would cost approximately $250 billion—about one-tenth of the total annual health care costs in this country.32 As policies change and the cost of drug regimens decreases from increasing competition, access is likely to improve for the majority of Americans.
Which regimen is most likely to be successful?
Many factors influence the choice of regimen and likelihood for SVR. These factors include whether the patient has cirrhosis and any comorbidities, the hepatitis C genotype involved, and any prior treatment the patient may have received. (See TABLE 37,12,18,28,30,33 for a comprehensive list.)

The easiest patients to treat are treatment-naive, with minimal liver disease and a favorable genotype. For example, combination therapy with the NS5B inhibitor sofosbuvir and an NS5A inhibitor (ledipasvir, daclatasvir, or velpatasvir) administered for 12 weeks has an SVR rate of >95% in genotype-1, treatment-naive, non-cirrhotic patients.22-24 Patients with prior treatment failure, especially failure on DAA therapy, or who have genotype 3, may be less responsive to standard therapies and may require more complex regimens or a longer duration of therapy.
Patients requiring special attention. It’s preferable to manage patients with decompensated liver disease in a specialized hepatology center due to the possibility of further decline and need for transplant prior to completion of therapy. Patients with HIV are another population that requires special attention. As many as 25% of HIV-infected patients are co-infected with HCV; their treatment follows the same principles as that in non-HIV patients, with extra attention paid to avoiding drug-drug interactions. Elbasvir/grazoprevir, for example, should not be used with any protease inhibitors, with nevirapine, or with efavirenz, and sofosbuvir should not be used with efavirenz, nevirapine, or tipranavir.34
Beyond medication regimens: The advice you’ll offer
In addition to counseling about antiviral therapy, patients with HCV infection require other types of advice and care that are often best administered by a primary care physician who is familiar with the patient and his or her family and community.
Prevention of transmission
Many patients have concerns about transmission of the virus to family members, co-workers, and sexual partners. You can assure patients that they are not likely to spread the virus in the workplace, even in health care environments.
Close contacts are also not at risk as long as basic prevention measures, such as not sharing toothbrushes or razors, are established to avoid transmission of blood and bodily fluids. Patient handouts can be found at the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc).35
Patients and their sexual partners, however, must be counseled about the risk of sexual transmission. In monogamous relationships between serodiscordant partners who practice vaginal intercourse, there is a low, but clinically important, risk of transmission of HCV—up to 0.6% per year.36 Anal intercourse and co-infection with HIV increase this risk significantly.37 Pregnant women must be advised on the currently non-modifiable risk of transmission to newborns, which is approximately 6% in mono-infected women, but may be at least twice as likely in HIV/HCV co-infected women.38,39
Staying healthy. In addition to pneumococcal and standard age-appropriate vaccines, vaccination against hepatitis A and HBV is recommended for all HCV-infected patients to reduce the risk of a severe acute hepatitis.40,41 Advise patients to avoid alcohol, to consume a healthy diet, and to participate in regular activity and exercise. Review the patient’s medication list for hepatotoxic drugs and counsel the patient on the risks of excessive use of acetaminophen, non-steroidal anti-inflammatory drugs, and herbal medicines such as kava kava. Because obesity and metabolic syndrome are known risk factors for hepatic steatosis, which hastens the progression to cirrhosis and liver failure, counsel overweight and obese patients on the importance of healthy weight loss.42,43
Disease-related screenings. Consider screening all HCV patients for diabetes mellitus (DM) because people with chronic HCV infection have a higher prevalence of insulin resistance than those who are HCV-negative, and patients with type 2 DM are at higher risk for worse outcomes of their HCV infection.44 In addition, screen all patients with a METAVIR score of F3 or higher every 6 months for HCC using liver ultrasound, and recommend upper endoscopy to patients with cirrhosis to screen for esophageal varices.45,46
Health maintenance after treatment
Once patients have achieved SVR 12 weeks after completion of therapy, they are deemed cured. However, those patients who were already METAVIR F3 or higher maintain sufficient risk of HCC to recommend ongoing screening with ultrasound.47,48
CORRESPONDENCE
Mark Shaffer, MD, 3209 Colonial Drive, Columbia, SC 29206; [email protected].
1. AASLD-IDSA. HCV testing and linkage to care. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed August 22, 2016.
2. US Preventive Services Task Force. Hepatitis C: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-C-screening. Accessed August 28, 2016.
3. Denniston MM, Jiles RN, Brobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300.
4. Centers for Disease Control and Prevention. Surveillance for viral hepatitis-United States, 2014. Available at: https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm. Accessed February 6, 2017.
5. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mort Wkly Rep. 2015;64:453-458.
6. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41.
7. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46.
8. Klevens M, Huang X, Yeo AE, et al. The burden of liver disease among persons with hepatitis C in the United States. Conference on Retroviruses and Opportunistic Infections. Seattle, February 23-24, 2015. Abstract 145.
9. Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307-314.
10. Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79:47-56.
11. Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non-Hodgkin’s lymphomas: what do we know? Ther Adv Hematol. 2016;7:94-107.
12. Gastaldi G, Goossens N, Clément S, et al. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res. 2017;8:149-159.
13. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127.
14. Elrazek AE, Amer M, Hawary B, et al. Prediction of HCV vertical transmission: What are factors should be optimized using data mining computational analysis. Liver Int. 2016.
15. Wang LS, D’Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol. 2016;88:1844-1855.
16. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
17. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed December 15, 2016.
18. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
19. Patel K, Friedrich-Rust M, Lurie Y, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589.
20. Shiraishi A, Hiraoka A, Aibiki T, et al. Real-time tissue elastography: non-invasive evaluation of liver fibrosis in chronic liver disease due to HCV. Hepatogastroenterology. 2014;61:2084-2090.
21. Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782.
22. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898.
23. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714-725.
24. Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599-2607.
25. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593.
26. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46.
27. AASLD-IDSA. Initial treatment of HCV infection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. Accessed August 24, 2016.
28. Lexicomp. Wolters Kluwer. Clinical Drug Information, Inc. Available at: http://online.lexi.com/action/home.
29. GoodRx. Available at: https//www.goodrx.com. Accessed January 25, 2017.
30. AASLD-IDSA. When and in whom to initiate HCV therapy. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed August 31, 2016.
31. Jezequel C, Bardou-Jacquet E, Desille Y, et al. Survival of patients infected by chronic hepatitis C and F0F1 fibrosis at baseline after a 15-years follow-up. Poster presented at: 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015; Vienna, Austria.
32. Lin KW. Should family physicians routinely screen patients for hepatitis C? Am Fam Physician. 2016;93:17-18.
33. Center for Medicare and Medicaid Services. Center for Medicaid and CHIP Services. Medicaid drug rebate program notice. Release no. 172. Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/state-releases/state-rel-172.pdf. Accessed August 24, 2016.
34. AASLD-IDSA. Unique patient populations: patients with HIV/HCV coinfection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/unique-patient-populations-patients-hivhcv-coinfection. Accessed February 6, 2017.
35. Centers for Disease Control and Prevention. Viral hepatitis-hepatitis C information. Patient education resources. Available at: http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc. Accessed June 15, 2016.
36. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99-S105.
37. Chan DP, Sun HY, Wong HT, et al. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47-58.
38. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
39. European Paediatric Hepatitis C Virus Network. A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872-1879.
40. Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102-1106.
41. Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525-1531.
42. Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561.
43. Hu KQ, Kyulo NL, Esrailian E, et al. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147-154.
44. Hammerstad SS, Grock SF, Lee HJ, et al. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
45. Lok AS, Seeff LV, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148.
46. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5-10.
47. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337.
Several recent developments have prompted a renewed focus on the way we screen for, and manage the treatment of, hepatitis C virus (HCV) infection. In 2013, the United States Preventive Services Task Force expanded its HCV screening guidelines to include baby boomers born between 1945 and 1965, regardless of apparent risk factors (TABLE 11).2 The recommendation is based on the high prevalence of chronic HCV in this cohort, estimated to be 4.3%, which is about 4 times higher than that of the general US population.3 It is believed that 75% of chronic HCV infections in the United States are in this cohort. After decades of infection, many in this age group are now presenting with advanced disease, leading to 19,659 HCV-related deaths in America in 2014.4

In addition, while HCV incidence in America had been steadily declining, it is now once again on the rise among young, non-urban whites, mainly because of increasing intravenous drug use in this population.5 On a positive note, new highly-effective and better-tolerated treatments are greatly improving the care we can provide.
In light of these factors, family physicians (FPs) are likely to be screening for HCV more than ever before and must be prepared to provide appropriate counseling and initial clinical management for those with positive test results. This article reviews the evaluation and primary care management of HCV-infected patients, as well as approaches to treatment with the newest direct-acting antivirals (DAAs).

The natural history of hepatitis C (and what we’re seeing as boomers age)
Acute HCV infection is rarely symptomatic, but results in chronic infection approximately 75% of the time.6 While some chronically infected individuals remain unaffected, most develop some degree of hepatic fibrosis, and 20% will develop cirrhosis within 20 years of diagnosis.7-9
The rate of progression is variable; factors that result in more rapid progression of liver disease include coinfection with HIV or HBV, overweight or obesity, insulin resistance, male gender, and use of alcohol.7 As the baby boomer cohort has aged, patients infected in their youth are now presenting with the sequelae of decompensated cirrhosis, including ascites, portal vein thrombosis, and thrombocytopenia.
Extrahepatic manifestations of chronic hepatitis C can include fatigue, membranoproliferative glomerulonephritis, porphyria cutanea tarda, cryoglobulinemia, a higher likelihood of insulin resistance, and possibly lymphoma.10-12
Chronic HCV is also the major contributor to the increased incidence of hepatocellular carcinoma (HCC), which has tripled in the past 2 decades in the United States.13
Although results are inconsistent, studies suggest 5% to 10% of HCV-infected patients will succumb to liver-related death.7
Who you’ll screen
If your patient is at heightened risk of contracting HCV infection (TABLE 11) or was born between 1945 and 1965, you’ll want to screen for infection with an HCV antibody test. A positive antibody test must be followed by testing for hepatitis C viral RNA to confirm whether the patient is chronically infected or is among the approximately 25% of patients who spontaneously clear the virus.6
For the patient with no detectable HCV RNA, no further evaluation or treatment is necessary. HCV viral load itself provides little insight into the rate of progression of the illness, but does correlate with risk of transmission.14 Counseling patients about the full testing protocol before screening can help to reduce anxiety and confusion.
At present, 6 genotypes and multiple subtypes of HCV have been identified; these have important implications for prognosis and treatment.15 HCV genotyping is frequently ordered along with a test for HCV viral load, but may be deferred until after fibrosis staging is performed (more on that in a bit). It may also be deferred if treatment is not planned within the next 12 weeks, as its main clinical use is to guide choice of treatment. Once chronic infection has been confirmed by the presence of HCV viremia, further work-up focuses on evaluating the effects on the host, which, in turn, helps the provider finalize a treatment plan (FIGURE 116).

Follow initial screening with an evaluation including liver disease staging
Following a screen that comes back positive for HCV, you’ll conduct a more thorough history including questioning about previous or ongoing risk factors for HCV, perform a physical examination that includes looking for signs of liver failure or extrahepatic disease, and order more lab work. Laboratory investigations include a complete blood count; renal and hepatic panels; and testing for human immunodeficiency virus (HIV) antibody, hepatitis B virus (HBV) surface antigen, HBV surface antibody, and HBV core antibody.1,17 Finally, the patient must be evaluated for hepatic fibrosis and cirrhosis to quantify the likelihood of developing liver failure and HCC.
Staging liver disease is a prerequisite to treating HCV infection because the extent of liver fibrosis impacts not only prognosis, but also the choice of the treatment regimen and the duration of therapy. The traditional gold standard for diagnosing hepatic fibrosis and cirrhosis has been a liver biopsy; however, a single 1.6-mm biopsy evaluates only a small portion of the liver and can miss affected liver parenchyma. In addition, a liver biopsy carries a small, but not inconsequential, risk of morbidity, and can be costly and complex to arrange.
Several noninvasive options are now available and are typically the preferred methods for staging liver disease. FibroSURE (LabCorp), for example, uses a peripheral venous blood sample and combines the patient’s age, gender, and 6 biochemical markers to generate a range of scores that correspond to the fibrosis component of the well-known METAVIR scoring system and correlate with results of liver biopsies.18,19 (The METAVIR system is a histology-based scoring system that grades fibrosis from F0 [no fibrosis] to F4 [cirrhosis].)
Noninvasive imaging studies assess for fibrosis more directly by assessing liver elasticity, either by ultrasound or magnetic resonance (MR) technology. The ultrasound modality FibroScan (Echosens) is currently the most widely available, although some data suggest the more expensive MR elastography has higher sensitivity (94% sensitive for METAVIR F2 or higher compared to 79% by FibroScan).20,21 While each of these modalities has limitations (eg, body habitus, availability), these tests allow stratification of patients into categories of low, moderate, and high risk for cirrhosis without the risks of biopsy.
A curable viral infection
HCV is one of the few curable chronic viral infections; unlike HBV and HIV, HCV does not create a long-term intra-nuclear reservoir. DAAs have cure rates of more than 95% for many HCV genotypes,22-24 allowing the possibility for dramatic reductions in prevalence in the decades to come.
Cure is defined by reaching a sustained virologic response (SVR), or absence of detectable viral load, 12 weeks after completion of therapy. Patients with HCV infection with advanced fibrosis who achieve SVR have shown benefits beyond improvement in liver function and histology. One large, multicenter, prospective study of 530 patients with chronic HCV, for example, found that those who achieved SVR experienced a 76% reduction in the risk of HCC and a 66% reduction in all-cause mortality (number needed to treat [NNT] was 5.8 to prevent one death or 6 to prevent one case of HCC in 10 years) compared to those without SVR.25 Other extrahepatic manifestations that impair quality of life, such as renal disease, autoimmune disease, and circulatory problems, are likewise reduced.25
Guidelines now recommend treating most patients with HCV infection
Until 2011, HCV treatment included the injectable immune-activating agent interferon and the non–HCV-specific antiviral ribavirin. This regimen had low SVR rates of 40% to 60% and adverse effects that were often intolerable.26 The advent of the first-generation HCV protease inhibitors in 2011 improved SVR rates, which have continued to improve exponentially with the development of combination therapy using DAAs (TABLE 227-29). (In order to stay up to date with the latest options for the treatment of HCV, see The American Association for the Study of Liver Diseases treatment guidelines at: http://hcvguidelines.org.)

What the guidelines say. Due to the tolerability and efficacy of the new DAAs, current guidelines state that HCV treatment should be recommended to most patients with HCV infection—not just those with advanced disease.30 This is a major change from prior guidelines, which were based on more toxic and less effective regimens. Limited data from long-term cohort studies of patients using interferon-based regimens suggest that the benefits of SVR are greatest for those treated at early stages before significant fibrosis develops. At least one analysis involving over 4000 patients found, however, that this approach may be less cost-effective, with an NNT of 20 to prevent one death in 20 years.31
In practice, the decision to treat requires a discussion between the patient and provider, weighing the risks and benefits of treatment in the context of the patients’ comorbidities and overall life expectancy. Such a discussion must also include cost. Many insurance companies will still only cover antiviral therapy for patients with advanced fibrosis, but these restrictions are slowly lifting and are having significant implications for our health care system. By one estimate, treating all patients with HCV at current drug prices would cost approximately $250 billion—about one-tenth of the total annual health care costs in this country.32 As policies change and the cost of drug regimens decreases from increasing competition, access is likely to improve for the majority of Americans.
Which regimen is most likely to be successful?
Many factors influence the choice of regimen and likelihood for SVR. These factors include whether the patient has cirrhosis and any comorbidities, the hepatitis C genotype involved, and any prior treatment the patient may have received. (See TABLE 37,12,18,28,30,33 for a comprehensive list.)

The easiest patients to treat are treatment-naive, with minimal liver disease and a favorable genotype. For example, combination therapy with the NS5B inhibitor sofosbuvir and an NS5A inhibitor (ledipasvir, daclatasvir, or velpatasvir) administered for 12 weeks has an SVR rate of >95% in genotype-1, treatment-naive, non-cirrhotic patients.22-24 Patients with prior treatment failure, especially failure on DAA therapy, or who have genotype 3, may be less responsive to standard therapies and may require more complex regimens or a longer duration of therapy.
Patients requiring special attention. It’s preferable to manage patients with decompensated liver disease in a specialized hepatology center due to the possibility of further decline and need for transplant prior to completion of therapy. Patients with HIV are another population that requires special attention. As many as 25% of HIV-infected patients are co-infected with HCV; their treatment follows the same principles as that in non-HIV patients, with extra attention paid to avoiding drug-drug interactions. Elbasvir/grazoprevir, for example, should not be used with any protease inhibitors, with nevirapine, or with efavirenz, and sofosbuvir should not be used with efavirenz, nevirapine, or tipranavir.34
Beyond medication regimens: The advice you’ll offer
In addition to counseling about antiviral therapy, patients with HCV infection require other types of advice and care that are often best administered by a primary care physician who is familiar with the patient and his or her family and community.
Prevention of transmission
Many patients have concerns about transmission of the virus to family members, co-workers, and sexual partners. You can assure patients that they are not likely to spread the virus in the workplace, even in health care environments.
Close contacts are also not at risk as long as basic prevention measures, such as not sharing toothbrushes or razors, are established to avoid transmission of blood and bodily fluids. Patient handouts can be found at the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc).35
Patients and their sexual partners, however, must be counseled about the risk of sexual transmission. In monogamous relationships between serodiscordant partners who practice vaginal intercourse, there is a low, but clinically important, risk of transmission of HCV—up to 0.6% per year.36 Anal intercourse and co-infection with HIV increase this risk significantly.37 Pregnant women must be advised on the currently non-modifiable risk of transmission to newborns, which is approximately 6% in mono-infected women, but may be at least twice as likely in HIV/HCV co-infected women.38,39
Staying healthy. In addition to pneumococcal and standard age-appropriate vaccines, vaccination against hepatitis A and HBV is recommended for all HCV-infected patients to reduce the risk of a severe acute hepatitis.40,41 Advise patients to avoid alcohol, to consume a healthy diet, and to participate in regular activity and exercise. Review the patient’s medication list for hepatotoxic drugs and counsel the patient on the risks of excessive use of acetaminophen, non-steroidal anti-inflammatory drugs, and herbal medicines such as kava kava. Because obesity and metabolic syndrome are known risk factors for hepatic steatosis, which hastens the progression to cirrhosis and liver failure, counsel overweight and obese patients on the importance of healthy weight loss.42,43
Disease-related screenings. Consider screening all HCV patients for diabetes mellitus (DM) because people with chronic HCV infection have a higher prevalence of insulin resistance than those who are HCV-negative, and patients with type 2 DM are at higher risk for worse outcomes of their HCV infection.44 In addition, screen all patients with a METAVIR score of F3 or higher every 6 months for HCC using liver ultrasound, and recommend upper endoscopy to patients with cirrhosis to screen for esophageal varices.45,46
Health maintenance after treatment
Once patients have achieved SVR 12 weeks after completion of therapy, they are deemed cured. However, those patients who were already METAVIR F3 or higher maintain sufficient risk of HCC to recommend ongoing screening with ultrasound.47,48
CORRESPONDENCE
Mark Shaffer, MD, 3209 Colonial Drive, Columbia, SC 29206; [email protected].
Several recent developments have prompted a renewed focus on the way we screen for, and manage the treatment of, hepatitis C virus (HCV) infection. In 2013, the United States Preventive Services Task Force expanded its HCV screening guidelines to include baby boomers born between 1945 and 1965, regardless of apparent risk factors (TABLE 11).2 The recommendation is based on the high prevalence of chronic HCV in this cohort, estimated to be 4.3%, which is about 4 times higher than that of the general US population.3 It is believed that 75% of chronic HCV infections in the United States are in this cohort. After decades of infection, many in this age group are now presenting with advanced disease, leading to 19,659 HCV-related deaths in America in 2014.4

In addition, while HCV incidence in America had been steadily declining, it is now once again on the rise among young, non-urban whites, mainly because of increasing intravenous drug use in this population.5 On a positive note, new highly-effective and better-tolerated treatments are greatly improving the care we can provide.
In light of these factors, family physicians (FPs) are likely to be screening for HCV more than ever before and must be prepared to provide appropriate counseling and initial clinical management for those with positive test results. This article reviews the evaluation and primary care management of HCV-infected patients, as well as approaches to treatment with the newest direct-acting antivirals (DAAs).

The natural history of hepatitis C (and what we’re seeing as boomers age)
Acute HCV infection is rarely symptomatic, but results in chronic infection approximately 75% of the time.6 While some chronically infected individuals remain unaffected, most develop some degree of hepatic fibrosis, and 20% will develop cirrhosis within 20 years of diagnosis.7-9
The rate of progression is variable; factors that result in more rapid progression of liver disease include coinfection with HIV or HBV, overweight or obesity, insulin resistance, male gender, and use of alcohol.7 As the baby boomer cohort has aged, patients infected in their youth are now presenting with the sequelae of decompensated cirrhosis, including ascites, portal vein thrombosis, and thrombocytopenia.
Extrahepatic manifestations of chronic hepatitis C can include fatigue, membranoproliferative glomerulonephritis, porphyria cutanea tarda, cryoglobulinemia, a higher likelihood of insulin resistance, and possibly lymphoma.10-12
Chronic HCV is also the major contributor to the increased incidence of hepatocellular carcinoma (HCC), which has tripled in the past 2 decades in the United States.13
Although results are inconsistent, studies suggest 5% to 10% of HCV-infected patients will succumb to liver-related death.7
Who you’ll screen
If your patient is at heightened risk of contracting HCV infection (TABLE 11) or was born between 1945 and 1965, you’ll want to screen for infection with an HCV antibody test. A positive antibody test must be followed by testing for hepatitis C viral RNA to confirm whether the patient is chronically infected or is among the approximately 25% of patients who spontaneously clear the virus.6
For the patient with no detectable HCV RNA, no further evaluation or treatment is necessary. HCV viral load itself provides little insight into the rate of progression of the illness, but does correlate with risk of transmission.14 Counseling patients about the full testing protocol before screening can help to reduce anxiety and confusion.
At present, 6 genotypes and multiple subtypes of HCV have been identified; these have important implications for prognosis and treatment.15 HCV genotyping is frequently ordered along with a test for HCV viral load, but may be deferred until after fibrosis staging is performed (more on that in a bit). It may also be deferred if treatment is not planned within the next 12 weeks, as its main clinical use is to guide choice of treatment. Once chronic infection has been confirmed by the presence of HCV viremia, further work-up focuses on evaluating the effects on the host, which, in turn, helps the provider finalize a treatment plan (FIGURE 116).

Follow initial screening with an evaluation including liver disease staging
Following a screen that comes back positive for HCV, you’ll conduct a more thorough history including questioning about previous or ongoing risk factors for HCV, perform a physical examination that includes looking for signs of liver failure or extrahepatic disease, and order more lab work. Laboratory investigations include a complete blood count; renal and hepatic panels; and testing for human immunodeficiency virus (HIV) antibody, hepatitis B virus (HBV) surface antigen, HBV surface antibody, and HBV core antibody.1,17 Finally, the patient must be evaluated for hepatic fibrosis and cirrhosis to quantify the likelihood of developing liver failure and HCC.
Staging liver disease is a prerequisite to treating HCV infection because the extent of liver fibrosis impacts not only prognosis, but also the choice of the treatment regimen and the duration of therapy. The traditional gold standard for diagnosing hepatic fibrosis and cirrhosis has been a liver biopsy; however, a single 1.6-mm biopsy evaluates only a small portion of the liver and can miss affected liver parenchyma. In addition, a liver biopsy carries a small, but not inconsequential, risk of morbidity, and can be costly and complex to arrange.
Several noninvasive options are now available and are typically the preferred methods for staging liver disease. FibroSURE (LabCorp), for example, uses a peripheral venous blood sample and combines the patient’s age, gender, and 6 biochemical markers to generate a range of scores that correspond to the fibrosis component of the well-known METAVIR scoring system and correlate with results of liver biopsies.18,19 (The METAVIR system is a histology-based scoring system that grades fibrosis from F0 [no fibrosis] to F4 [cirrhosis].)
Noninvasive imaging studies assess for fibrosis more directly by assessing liver elasticity, either by ultrasound or magnetic resonance (MR) technology. The ultrasound modality FibroScan (Echosens) is currently the most widely available, although some data suggest the more expensive MR elastography has higher sensitivity (94% sensitive for METAVIR F2 or higher compared to 79% by FibroScan).20,21 While each of these modalities has limitations (eg, body habitus, availability), these tests allow stratification of patients into categories of low, moderate, and high risk for cirrhosis without the risks of biopsy.
A curable viral infection
HCV is one of the few curable chronic viral infections; unlike HBV and HIV, HCV does not create a long-term intra-nuclear reservoir. DAAs have cure rates of more than 95% for many HCV genotypes,22-24 allowing the possibility for dramatic reductions in prevalence in the decades to come.
Cure is defined by reaching a sustained virologic response (SVR), or absence of detectable viral load, 12 weeks after completion of therapy. Patients with HCV infection with advanced fibrosis who achieve SVR have shown benefits beyond improvement in liver function and histology. One large, multicenter, prospective study of 530 patients with chronic HCV, for example, found that those who achieved SVR experienced a 76% reduction in the risk of HCC and a 66% reduction in all-cause mortality (number needed to treat [NNT] was 5.8 to prevent one death or 6 to prevent one case of HCC in 10 years) compared to those without SVR.25 Other extrahepatic manifestations that impair quality of life, such as renal disease, autoimmune disease, and circulatory problems, are likewise reduced.25
Guidelines now recommend treating most patients with HCV infection
Until 2011, HCV treatment included the injectable immune-activating agent interferon and the non–HCV-specific antiviral ribavirin. This regimen had low SVR rates of 40% to 60% and adverse effects that were often intolerable.26 The advent of the first-generation HCV protease inhibitors in 2011 improved SVR rates, which have continued to improve exponentially with the development of combination therapy using DAAs (TABLE 227-29). (In order to stay up to date with the latest options for the treatment of HCV, see The American Association for the Study of Liver Diseases treatment guidelines at: http://hcvguidelines.org.)

What the guidelines say. Due to the tolerability and efficacy of the new DAAs, current guidelines state that HCV treatment should be recommended to most patients with HCV infection—not just those with advanced disease.30 This is a major change from prior guidelines, which were based on more toxic and less effective regimens. Limited data from long-term cohort studies of patients using interferon-based regimens suggest that the benefits of SVR are greatest for those treated at early stages before significant fibrosis develops. At least one analysis involving over 4000 patients found, however, that this approach may be less cost-effective, with an NNT of 20 to prevent one death in 20 years.31
In practice, the decision to treat requires a discussion between the patient and provider, weighing the risks and benefits of treatment in the context of the patients’ comorbidities and overall life expectancy. Such a discussion must also include cost. Many insurance companies will still only cover antiviral therapy for patients with advanced fibrosis, but these restrictions are slowly lifting and are having significant implications for our health care system. By one estimate, treating all patients with HCV at current drug prices would cost approximately $250 billion—about one-tenth of the total annual health care costs in this country.32 As policies change and the cost of drug regimens decreases from increasing competition, access is likely to improve for the majority of Americans.
Which regimen is most likely to be successful?
Many factors influence the choice of regimen and likelihood for SVR. These factors include whether the patient has cirrhosis and any comorbidities, the hepatitis C genotype involved, and any prior treatment the patient may have received. (See TABLE 37,12,18,28,30,33 for a comprehensive list.)

The easiest patients to treat are treatment-naive, with minimal liver disease and a favorable genotype. For example, combination therapy with the NS5B inhibitor sofosbuvir and an NS5A inhibitor (ledipasvir, daclatasvir, or velpatasvir) administered for 12 weeks has an SVR rate of >95% in genotype-1, treatment-naive, non-cirrhotic patients.22-24 Patients with prior treatment failure, especially failure on DAA therapy, or who have genotype 3, may be less responsive to standard therapies and may require more complex regimens or a longer duration of therapy.
Patients requiring special attention. It’s preferable to manage patients with decompensated liver disease in a specialized hepatology center due to the possibility of further decline and need for transplant prior to completion of therapy. Patients with HIV are another population that requires special attention. As many as 25% of HIV-infected patients are co-infected with HCV; their treatment follows the same principles as that in non-HIV patients, with extra attention paid to avoiding drug-drug interactions. Elbasvir/grazoprevir, for example, should not be used with any protease inhibitors, with nevirapine, or with efavirenz, and sofosbuvir should not be used with efavirenz, nevirapine, or tipranavir.34
Beyond medication regimens: The advice you’ll offer
In addition to counseling about antiviral therapy, patients with HCV infection require other types of advice and care that are often best administered by a primary care physician who is familiar with the patient and his or her family and community.
Prevention of transmission
Many patients have concerns about transmission of the virus to family members, co-workers, and sexual partners. You can assure patients that they are not likely to spread the virus in the workplace, even in health care environments.
Close contacts are also not at risk as long as basic prevention measures, such as not sharing toothbrushes or razors, are established to avoid transmission of blood and bodily fluids. Patient handouts can be found at the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc).35
Patients and their sexual partners, however, must be counseled about the risk of sexual transmission. In monogamous relationships between serodiscordant partners who practice vaginal intercourse, there is a low, but clinically important, risk of transmission of HCV—up to 0.6% per year.36 Anal intercourse and co-infection with HIV increase this risk significantly.37 Pregnant women must be advised on the currently non-modifiable risk of transmission to newborns, which is approximately 6% in mono-infected women, but may be at least twice as likely in HIV/HCV co-infected women.38,39
Staying healthy. In addition to pneumococcal and standard age-appropriate vaccines, vaccination against hepatitis A and HBV is recommended for all HCV-infected patients to reduce the risk of a severe acute hepatitis.40,41 Advise patients to avoid alcohol, to consume a healthy diet, and to participate in regular activity and exercise. Review the patient’s medication list for hepatotoxic drugs and counsel the patient on the risks of excessive use of acetaminophen, non-steroidal anti-inflammatory drugs, and herbal medicines such as kava kava. Because obesity and metabolic syndrome are known risk factors for hepatic steatosis, which hastens the progression to cirrhosis and liver failure, counsel overweight and obese patients on the importance of healthy weight loss.42,43
Disease-related screenings. Consider screening all HCV patients for diabetes mellitus (DM) because people with chronic HCV infection have a higher prevalence of insulin resistance than those who are HCV-negative, and patients with type 2 DM are at higher risk for worse outcomes of their HCV infection.44 In addition, screen all patients with a METAVIR score of F3 or higher every 6 months for HCC using liver ultrasound, and recommend upper endoscopy to patients with cirrhosis to screen for esophageal varices.45,46
Health maintenance after treatment
Once patients have achieved SVR 12 weeks after completion of therapy, they are deemed cured. However, those patients who were already METAVIR F3 or higher maintain sufficient risk of HCC to recommend ongoing screening with ultrasound.47,48
CORRESPONDENCE
Mark Shaffer, MD, 3209 Colonial Drive, Columbia, SC 29206; [email protected].
1. AASLD-IDSA. HCV testing and linkage to care. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed August 22, 2016.
2. US Preventive Services Task Force. Hepatitis C: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-C-screening. Accessed August 28, 2016.
3. Denniston MM, Jiles RN, Brobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300.
4. Centers for Disease Control and Prevention. Surveillance for viral hepatitis-United States, 2014. Available at: https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm. Accessed February 6, 2017.
5. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mort Wkly Rep. 2015;64:453-458.
6. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41.
7. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46.
8. Klevens M, Huang X, Yeo AE, et al. The burden of liver disease among persons with hepatitis C in the United States. Conference on Retroviruses and Opportunistic Infections. Seattle, February 23-24, 2015. Abstract 145.
9. Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307-314.
10. Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79:47-56.
11. Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non-Hodgkin’s lymphomas: what do we know? Ther Adv Hematol. 2016;7:94-107.
12. Gastaldi G, Goossens N, Clément S, et al. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res. 2017;8:149-159.
13. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127.
14. Elrazek AE, Amer M, Hawary B, et al. Prediction of HCV vertical transmission: What are factors should be optimized using data mining computational analysis. Liver Int. 2016.
15. Wang LS, D’Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol. 2016;88:1844-1855.
16. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
17. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed December 15, 2016.
18. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
19. Patel K, Friedrich-Rust M, Lurie Y, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589.
20. Shiraishi A, Hiraoka A, Aibiki T, et al. Real-time tissue elastography: non-invasive evaluation of liver fibrosis in chronic liver disease due to HCV. Hepatogastroenterology. 2014;61:2084-2090.
21. Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782.
22. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898.
23. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714-725.
24. Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599-2607.
25. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593.
26. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46.
27. AASLD-IDSA. Initial treatment of HCV infection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. Accessed August 24, 2016.
28. Lexicomp. Wolters Kluwer. Clinical Drug Information, Inc. Available at: http://online.lexi.com/action/home.
29. GoodRx. Available at: https//www.goodrx.com. Accessed January 25, 2017.
30. AASLD-IDSA. When and in whom to initiate HCV therapy. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed August 31, 2016.
31. Jezequel C, Bardou-Jacquet E, Desille Y, et al. Survival of patients infected by chronic hepatitis C and F0F1 fibrosis at baseline after a 15-years follow-up. Poster presented at: 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015; Vienna, Austria.
32. Lin KW. Should family physicians routinely screen patients for hepatitis C? Am Fam Physician. 2016;93:17-18.
33. Center for Medicare and Medicaid Services. Center for Medicaid and CHIP Services. Medicaid drug rebate program notice. Release no. 172. Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/state-releases/state-rel-172.pdf. Accessed August 24, 2016.
34. AASLD-IDSA. Unique patient populations: patients with HIV/HCV coinfection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/unique-patient-populations-patients-hivhcv-coinfection. Accessed February 6, 2017.
35. Centers for Disease Control and Prevention. Viral hepatitis-hepatitis C information. Patient education resources. Available at: http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc. Accessed June 15, 2016.
36. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99-S105.
37. Chan DP, Sun HY, Wong HT, et al. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47-58.
38. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
39. European Paediatric Hepatitis C Virus Network. A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872-1879.
40. Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102-1106.
41. Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525-1531.
42. Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561.
43. Hu KQ, Kyulo NL, Esrailian E, et al. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147-154.
44. Hammerstad SS, Grock SF, Lee HJ, et al. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
45. Lok AS, Seeff LV, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148.
46. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5-10.
47. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337.
1. AASLD-IDSA. HCV testing and linkage to care. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. Accessed August 22, 2016.
2. US Preventive Services Task Force. Hepatitis C: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-C-screening. Accessed August 28, 2016.
3. Denniston MM, Jiles RN, Brobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300.
4. Centers for Disease Control and Prevention. Surveillance for viral hepatitis-United States, 2014. Available at: https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm. Accessed February 6, 2017.
5. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mort Wkly Rep. 2015;64:453-458.
6. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41.
7. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46.
8. Klevens M, Huang X, Yeo AE, et al. The burden of liver disease among persons with hepatitis C in the United States. Conference on Retroviruses and Opportunistic Infections. Seattle, February 23-24, 2015. Abstract 145.
9. Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307-314.
10. Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79:47-56.
11. Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non-Hodgkin’s lymphomas: what do we know? Ther Adv Hematol. 2016;7:94-107.
12. Gastaldi G, Goossens N, Clément S, et al. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res. 2017;8:149-159.
13. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127.
14. Elrazek AE, Amer M, Hawary B, et al. Prediction of HCV vertical transmission: What are factors should be optimized using data mining computational analysis. Liver Int. 2016.
15. Wang LS, D’Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol. 2016;88:1844-1855.
16. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
17. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed December 15, 2016.
18. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
19. Patel K, Friedrich-Rust M, Lurie Y, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589.
20. Shiraishi A, Hiraoka A, Aibiki T, et al. Real-time tissue elastography: non-invasive evaluation of liver fibrosis in chronic liver disease due to HCV. Hepatogastroenterology. 2014;61:2084-2090.
21. Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782.
22. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898.
23. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714-725.
24. Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599-2607.
25. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593.
26. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46.
27. AASLD-IDSA. Initial treatment of HCV infection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. Accessed August 24, 2016.
28. Lexicomp. Wolters Kluwer. Clinical Drug Information, Inc. Available at: http://online.lexi.com/action/home.
29. GoodRx. Available at: https//www.goodrx.com. Accessed January 25, 2017.
30. AASLD-IDSA. When and in whom to initiate HCV therapy. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed August 31, 2016.
31. Jezequel C, Bardou-Jacquet E, Desille Y, et al. Survival of patients infected by chronic hepatitis C and F0F1 fibrosis at baseline after a 15-years follow-up. Poster presented at: 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015; Vienna, Austria.
32. Lin KW. Should family physicians routinely screen patients for hepatitis C? Am Fam Physician. 2016;93:17-18.
33. Center for Medicare and Medicaid Services. Center for Medicaid and CHIP Services. Medicaid drug rebate program notice. Release no. 172. Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/state-releases/state-rel-172.pdf. Accessed August 24, 2016.
34. AASLD-IDSA. Unique patient populations: patients with HIV/HCV coinfection. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/full-report/unique-patient-populations-patients-hivhcv-coinfection. Accessed February 6, 2017.
35. Centers for Disease Control and Prevention. Viral hepatitis-hepatitis C information. Patient education resources. Available at: http://www.cdc.gov/hepatitis/hcv/patienteduhcv.htm#cdc. Accessed June 15, 2016.
36. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99-S105.
37. Chan DP, Sun HY, Wong HT, et al. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47-58.
38. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
39. European Paediatric Hepatitis C Virus Network. A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005;192:1872-1879.
40. Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102-1106.
41. Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525-1531.
42. Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561.
43. Hu KQ, Kyulo NL, Esrailian E, et al. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147-154.
44. Hammerstad SS, Grock SF, Lee HJ, et al. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
45. Lok AS, Seeff LV, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148.
46. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5-10.
47. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337.
PRACTICE RECOMMENDATIONS
› Offer hepatitis C virus (HCV) screening to all patients with identified risk factors, as well as anyone born between 1945 and 1965, regardless of risk factors. B
› Offer human immunodeficiency virus and hepatitis B testing, as well as hepatitis A, hepatitis B, and pneumococcal vaccinations, to all patients with chronic HCV infection. C
› Consider treatment with interferon-free direct-acting antiviral therapies for all patients with chronic HCV infection to reduce liver-related and all-cause morbidity and mortality. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series