User login
Risk prediction models for cardiac morbidity and mortality in noncardiac surgery: A systematic review of the literature
Postoperative gastrointestinal tract dysfunction: An overview of causes and management strategies
Tolerance of an enteral diet is one of the fundamental components of postoperative wellness, along with the ability to mobilize freely without supplemental oxygen and a readiness to be discharged home as soon as possible. Accordingly, postoperative gastrointestinal (GI) tract dysfunction is best defined as intolerance of an enteral diet after having been tolerant of one preoperatively. I prefer the term postoperative GI tract dysfunction over postoperative ileus, as ileus is ill defined, covering a wide spectrum of clinical signs and having a range of published incidences so broad (5%–100%) that it defies useful discussion.
GI DYSFUNCTION: A COMMON POSTOPERATIVE MORBIDITY
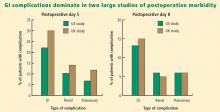
Postoperative GI tract dysfunction is common, as illustrated by a large prospective cohort study at Duke University Medical Center3 that used the Postoperative Morbidity Survey (which has since been validated4) to document complications following major noncardiac surgery (ie, anticipated duration > 2 hours and anticipated blood loss > 500 mL). Hospital discharge was delayed in 27% of the study’s 438 patients as a result of a postoperative complication, and GI dysfunction was the most common type of complication overall and on postoperative days 5, 8, and 15. Episodes of GI dysfunction ranged from intolerance of an enteral diet to ischemic gut resulting in multiple organ failure.3
A MULTIFACTORIAL PATHOGENESIS
The pathophysiology of postoperative GI tract dysfunction can be ischemic, metabolic, toxic, neurogenic, myogenic, pharmacologic, or mechanical.
It is important to recognize that in many cases no single factor explains the whole story behind postsurgical GI tract dysfunction, and none of these factors is an ipso facto cause of such dysfunction. For instance, a “mechanical” pathogenesis refers to any manipulation of the gut that causes an inflammatory response in the gut’s various layers, resulting in injury.5,6 However, GI tract dysfunction commonly occurs after operations (including laparoscopic procedures) in which the gut was not handled at all. Similarly, in terms of a pharmacologic pathophysiology, while opioids can affect GI propulsion and cause constipation,7,8 avoidance of opioid use does not ensure prevention of GI tract dysfunction. Moreover, opioid abusers do not generally exhibit intolerance of enteral nutrition.
A common mechanism that is often ignored is perioperative gut ischemia resulting in low-grade injury. Low-grade hypovolemia can cause loss of perfusion to the tip of the microvillus, triggering apoptosis and potentially necrosis, which typically requires about 3 days for recovery. An experiment among 6 healthy volunteers who underwent elective hemorrhage (25% of blood volume removed) over 1 hour demonstrated that gastric tonometry was an earlier indicator of hypovolemia than were commonly measured hemodynamic variables such as invasive blood pressure, stroke volume, heart rate, and lactate and arterial blood gas measurements.9
FLUID LOADING AIDS GI RECOVERY
A targeted increase of intravascular volume and global blood flow perioperatively has been shown repeatedly to improve surgical outcome.10–24 In clinical trials, the most common intervention to achieve the predetermined hemodynamic goal has been fluid loading. Overall, targeted increases in perioperative global blood flow have been associated with reduced mortality,25 with the presumed mechanism being maintenance of end-organ perfusion.
The role of end-organ perfusion maintenance was confirmed in a controlled study of 60 patients undergoing cardiac surgery in which perioperative fluid loading (with colloid) maintained gut perfusion as measured by gastric tonometry, whereas a control group had a reproducible reduction in gut perfusion.15 Fluid loading was associated with a significant reduction in the incidence of gut mucosal hypoperfusion—from 56% to 7%—and significant reductions in the incidence of minor and major complications, mean days in the hospital, and mean days in the intensive care unit.
Fluid type matters
The type of intraoperative fluid loading is a factor in postoperative recovery.
Colloid vs crystalloid. Moretti et al found that colloid (6% hetastarch in saline or 6% hetastarch in balanced salt) was superior to crystalloid (lactated Ringer’s solution) in preventing nausea, severe pain, vomiting, periorbital edema, and double vision postoperatively (P < .05 for all) despite comparable hemodynamic profiles.26
Ringer’s vs normal saline. Williams et al compared intravenous lactated Ringer’s solution with normal saline (0.9% sodium chloride) in a randomized study of healthy volunteers.27 The group that received normal saline demonstrated central nervous system changes and a much higher incidence of abdominal discomfort, a finding consistent with the toxic properties of chlorine to the gut.
Balanced electrolyte solutions vs saline-based fluids. Wilkes et al compared crystalloid and colloid solutions with physiologically balanced electrolyte formulations (Hextend) against saline-based fluids (Hespan) in elderly surgical patients.28 They found that balanced electrolyte solutions were superior in improving gastric mucosal perfusion and preventing hyperchloremic metabolic acidosis. As a result of a reduction in GI tract perfusion, postoperative vomiting was more frequent in the group receiving saline-based fluids.
Evidence for Doppler-guided fluid management
Use of esophageal Doppler ultrasonography to guide fluid administration intraoperatively is fairly common in the United Kingdom and is based on randomized controlled trials showing that Doppler-guided colloid administration to maximize stroke volume reduces morbidity and length of hospital stay in surgical patients. In one government-supported study of 128 colorectal resection patients, Doppler-guided small boluses of colloid increased stroke volume, cardiac output, and oxygen delivery compared with conventional (central venous pressure–based) fluid management.29 Gut function improved significantly faster with Doppler-guided fluid management as evidenced by a more rapid return of flatus, opening of bowels, and achievement of a full diet, and by faster discharge from the hospital. The incidence of GI complications was reduced from 45.3% in the conventional management group to 14.1% in the Doppler group. The relative risk of GI tract dysfunction was 5.3 times higher with conventional management.
OTHER STRATEGIES TO REDUCE POSTOPERATIVE GI DYSFUNCTION
In addition to fluid loading, a number of other methods have been studied in an attempt to reduce the incidence of postoperative GI tract dysfunction.
Epidural neostigmine: Improvement in some measures
Epidural neostigmine was compared with saline control in a randomized study of 45 patients scheduled for abdominal aortic surgery.30 Time to first bowel sounds and time to first flatus were significantly shorter in the neostigmine group, but time to first defecation and the incidence of postoperative complications were similar between the groups.
Laxatives speed return of GI function
In a study of 53 women undergoing fast-track hysterectomy, recovery of GI tract function was faster in those randomized to receive laxatives (magnesium oxide and disodium phosphate) starting 6 hours postoperatively compared with those receiving placebo.31 Median time to first defecation was reduced from 69 hours in the placebo group to 45 hours in the laxative group (P < .0001), and postoperative hospitalization was shortened by a median of 1 day in the laxative group. There were no significant between-group differences in pain scores, postoperative nausea and vomiting, or the use of morphine or antiemetics.
Fentanyl reduces gastric myoelectrical activity
Intravenous administration of the opioid fentanyl significantly reduced gastric myoelectrical activity in an uncontrolled study of 20 patients undergoing elective surgery, but wide variation in effect was observed among patients.32 There was no correlation between the myoelectrical outcome and the presence of polymorphisms of the mu-opioid receptor gene.
Systemic lidocaine accelerates return of bowel function
Perioperative administration of systemic lidocaine, given as a 1.5-mg/kg bolus followed by continuous infusion at 2 mg/min, accelerated the return of bowel function and shortened the length of hospital stay compared with placebo in a randomized study of 60 colorectal surgery patients.33
Early oral feeding cuts length of stay
A recent meta-analysis of randomized trials found that early oral intake of fluids and food after major abdominal gynecologic surgery was associated with an increased risk of nausea but a reduced length of hospital stay.34 The authors recommended an individualized approach to early feeding, and called for cost-effectiveness and patient satisfaction studies.
Mosapride improves gastric emptying
Mosapride is a 5-HT4 agonist that has been shown to improve gastric emptying in a randomized controlled study of 40 patients undergoing laparoscopic colectomy.35 Time to first postoperative bowel movement, time to maximal gastric emptying rate, and postoperative hospital stay were all significantly shorter in patients receiving mosapride versus control. Mosapride is not currently approved for marketing in the United States.
Mu-opioid antagonists: Some show promise, others don’t
Mu-opioid receptor antagonists have been developed primarily to reverse opioid-induced bowel dysfunction. Commercially available drugs in this class include alvimopan, methylnaltrexone, nalbuphine, and naloxone. A recent meta-analysis of 23 randomized controlled studies of these agents for opioid-induced bowel dysfunction concluded that alvimopan and methylnaltrexone were superior to placebo but that evidence was insufficient for the safety or efficacy of naloxone and nalbuphine.36
Nasogastric decompression: Usually more harm than benefit
Prophylactic nasogastric decompression is an intervention devoid of evidence. A meta-analysis of 33 studies encompassing 5,240 patients randomized to routine nasogastric tube placement, selective nasogastric tube use, or no nasogastric tube placement after abdominal surgery found no advantage to routine nasogastric tube use.37 In fact, patients not receiving routine tube placement had a significantly earlier return of bowel function and a significant decrease in pulmonary complications. The incidence of anastomotic leak was not different among the groups. Routine tube use was associated with a lower incidence of vomiting but more patient discomfort. The clear conclusion is that, in most situations, elective placement of a nasogastric tube only causes harm.
Chewing gum: A simple intervention that works
In a recent meta-analysis of five randomized controlled trials, the simple intervention of gum chewing after colorectal surgery significantly accelerated the time to flatus and time to defecation, and was associated with a nonsignificant trend toward a shorter postoperative hospital stay.38
CONCLUSIONS ON MANAGEMENT
Traditional measures intended to reduce the incidence of postoperative GI tract dysfunction—administration of prokinetic drugs, placement of nasogastric tubes, avoidance of food and fluids—are not beneficial and are often harmful. Administration of targeted amounts of fluid to optimize ventricular filling and end-organ perfusion has repeatedly been demonstrated to improve outcomes, particularly those related to GI tract perfusion and function. Administration of larger volumes of colloid, to achieve predetermined increases in stroke volume, improves gut perfusion and reduces the incidence of GI tract dysfunction.
Many simple, inexpensive, and readily available strategies for preventing or reversing postoperative GI tract dysfunction have some degree of evidence-based support and should be considered. I would recommend a multimodal approach that includes a limited surgical incision, regional local anesthesia without use of opioids, immediate postoperative mobilization, early enteral feeding, and postoperative gum chewing.1 Such an approach promises to reduce GI tract dysfunction and other postoperative complications as well as to shorten hospital stay.
DISCUSSION
Question from the audience: You mentioned the selective use of nasogastric tubes. In which patients would you use them?
Dr. Mythen: For upper GI surgeries—esophagectomy, for example—a nasogastric tube is inevitable. Beyond that, the specific indications for tube placement are very limited. At our institution, we no longer place nasogastric tubes following the vast majority of GI tract operations, with esophagectomy being the exception.
Question from the audience: Would you comment on the selective contribution of thoracic epidural analgesia with respect to early feeding after abdominal or colon surgery?
Dr. Mythen: If you’re an enthusiast for thoracic epidurals, you can present the literature in a way that definitively demonstrates a huge advantage to thoracic epidurals. When they work well for the individual, they are fantastic, but you must have a very effective team and system to deliver success to the whole patient population. At our institution the failure rate 20 to 24 hours postoperatively is about 50%.
Question from the audience: I’m an internist and I’ve never heard of the esophageal Doppler-directed fluid bolus protocol—or of anyone using colloids at all. Is that something that is generally practiced in the United States?
Dr. Mythen: Some institutions are practicing goal-directed fluid management now. If you measure stroke volume and give small boluses of colloid, you need a lot less fluid to achieve a higher intravascular volume and goal. At our institution, we’ve repackaged it as “goal-directed fluid restriction” to gain acceptance among surgeons. Uptake has been slower in the United States, though studies here have reinforced the message and been supported by editorials. Guessing about fluids, which we’ve done historically, is not very smart. One thing that differentiates an anesthesiologist from an anesthetic technician is the ability to give goal-directed fluid therapy. The ability to act in a targeted fashion makes it possible to achieve an appropriate physiological goal, but it is more difficult.
Question from the audience: In terms of maintenance fluids and chloride toxicity, is there an alternative to D5 half-normal saline for maintenance fluid?
Dr. Mythen: We don’t have a very good postoperative maintenance fluid; D5 half-normal with some potassium is probably as good as it gets at present. I emphasize getting patients to drink as quickly as possible. If they’re not drinking (not using the GI tract), they need a very high level of physician input because fluid balance is rocket science. The GI tract is very clever. Once patients are drinking and eating, they’re fine, but if they still have an intravenous line in, close attention is required.
Question from the audience: Would you use lactated Ringer’s solution in a patient who is just not eating or drinking?
Dr. Mythen: I do, actually. I tend to mix it in with some D5 half-normal saline because lactated Ringer’s is a great solution. The body can use the lactate to make sugar if necessary. The brain is one of the few organs that will metabolize lactate.
Follow-up question: Would you use it at a lower rate to prevent volume overload?
Dr. Mythen: Yes, at 60 mL/hr. The important thing is that if intravenous fluids are still required, the patient needs to be in a fairly supervised, high-dependency environment. You must address the real issue: Why aren’t they drinking? If the patient is not drinking postoperatively, someone’s done a bad job or there is something that needs fixing.
Question from audience: In the operating room, do you have a preference between albumin and a high-molecular-weight hetastarch like Hextend?
Dr. Mythen: Europe is slightly different in its choice of colloids. We’ve pretty much abandoned the high-molecular-weight starches. We do not use albumin at our institution for cost reasons, and we can’t find any evidence to support its use. We would have to close one intensive care unit bed to be able to afford using albumin. We use low-molecular-weight hydroxyethyl starches, which I believe are now coming into the United States. They have no major coagulation effect.
- Mythen MG. Postoperative gastrointestinal tract dysfunction. Anesth Analg 2005; 100:196–204.
- Gan TJ, Meyer T, Apfel CC, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg 2003; 97:62–71.
- Bennett-Guerrero E, Welsby I, Dunn TJ, et al. The use of a postoperative morbidity survey to evaluate patients with prolonged hospitalization after routine, moderate-risk, elective surgery. Anesth Analg 1999; 89:514–519.
- Grocott MP, Browne JP, Van der Meulen J, et al. The Postoperative Morbidity Survey was validated and used to describe morbidity after major surgery. J Clin Epidemiol 2007; 60:919–928.
- Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg 1998; 228:652–663.
- Schwarz NT, Beer-Stolz D, Simmons RL, Bauer AJ. Pathogenesis of paralytic ileus: intestinal manipulation opens a transient pathway between the intestinal lumen and the leukocytic infiltrate of the jejunal muscularis. Ann Surg 2002; 235:31–40.
- Manara L, Bianchetti A. The central and peripheral influences of opioids on gastrointestinal propulsion. Annu Rev Pharmacol Toxicol 1985; 25:249–273.
- Manara L, Bianchi G, Ferretti P, Tavani A. Inhibition of gastrointestinal transit by morphine in rats results primarily from direct drug action on gut opioid sites. J Pharmacol Exp Ther 1986; 237:945–949.
- Hamilton-Davies C, Mythen MG, Salmon JB, et al. Comparison of commonly used clinical indicators of hypovolaemia with gastrointestinal tonometry. Intensive Care Med 1997; 23:276–281.
- Bender JS, Smith-Meek MA, Jones CE. Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: results of a prospective, randomized trial. Ann Surg 1997; 226:229–237.
- Berlauk JF, Abrams JH, Gilmour IJ, et al. Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery: a prospective, randomized clinical trial. Ann Surg 1991; 214:289–299.
- Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 1993; 270:2699–2707.
- Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002; 97:820–826.
- Lobo SM, Salgado PF, Castillo VG, et al. Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med 2000; 28:3396–3404.
- Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg 1995; 130:423–429.
- Pölönen P, Ruokonen E, Hippeläinen M, Pöyhönen M, Takala J. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg 2000; 90:1052–1059.
- Sandham JD, Hull RD, Brant RF, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 2003; 348:5–14.
- Shoemaker WC, Appel PL, Kram HB, Nathan RC, Thompson JL. Multicomponent noninvasive physiologic monitoring of circulatory function. Crit Care Med 1988; 16:482–490.
- Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ 1997; 315:909–912.
- Ueno S, Tanabe G, Yamada H, et al. Response of patients with cirrhosis who have undergone partial hepatectomy to treatment aimed at achieving supranormal oxygen delivery and consumption. Surgery 1998; 123:278–286.
- Valentine RJ, Duke ML, Inman MH, et al. Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. J Vasc Surg 1998; 27:203–212.
- Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth 2002; 88:65–71.
- Wilson J, Woods I, Fawcett J, et al. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ 1999; 318:1099–1103.
- Ziegler DW, Wright JG, Choban PS, Flancbaum L. A prospective randomized trial of preoperative “optimization” of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery 1997; 122:584–592.
- Grocott MPW, Hamilton MA, Bennett ED, Harrison D, Rowan K. Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery. Cochrane Database Syst Rev 2006; 2:CD004082. doi:10.1002/14651858.CD004082.pub4.
- Moretti EW, Robertson KM, El-Moalem H, Gan TJ. Intraoperative colloid administration reduces postoperative nausea and vomiting and improves postoperative outcomes compared with crystalloid administration. Anesth Analg 2003; 96:611–617.
- Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg 1999; 88:999–1003.
- Wilkes NJ, Woolf R, Mutch M, et al. The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid-base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg 2001; 93:811–816.
- Wakeling HG, McFall MR, Jenkins CS, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005; 95:634–642.
- Caliskan E, Turkoz A, Sener M, et al. A prospective randomized double-blind study to determine the effect of thoracic epidural neostigmine on postoperative ileus after abdominal aortic surgery. Anesth Analg 2008; 106:959–964.
- Hansen CT, Sørensen M, Møller C, Ottesen B, Kehlet H. Effect of laxatives on gastrointestinal functional recovery in fast-track hysterectomy: a double-blind, placebo-controlled randomized study. Am J Obstet Gynecol 2007; 196:311.e1–311.e7.
- Walldén J, Lindberg G, Sandin M, Thörn SE, Wattwil M. Effects of fentanyl on gastric myoelectrical activity: a possible association with polymorphisms of the mu-opioid receptor gene? Acta Anaesthesiol Scand 2008; 52:708–715.
- Herroeder S, Pecher S, Schönherr ME, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg 2007; 246:192–200.
- Charoenkwan K, Phillipson G, Vutyavanich T. Early versus delayed oral fluids and food for reducing complications after major abdominal gynaecologic surgery. Cochrane Database Syst Rev 2007; 4:CD004508. doi:10.1002/14651858.CD004508.pub3.
- Narita K, Tsunoda A, Takenaka K, et al. Effect of mosapride on recovery of intestinal motility after hand-assisted laparoscopic colectomy for carcinoma. Dis Colon Rectum 2008; 51:1692–1695.
- McNicol ED, Boyce D, Schumann R, Carr DB. Mu-opioid antagonists for opioid-induced bowel dysfunction. Cochrane Database Syst Rev 2008; 2:CD006332. doi:10.1002/14651858.CD006332.pub2.
- Nelson RL, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2007; 3:CD004929. doi:10.1002/14651858.CD004929.pub3.
- de Castro SM, van den Esschert JW, van Heek NT, et al. A systematic review of the efficacy of gum chewing for the amelioration of postoperative ileus. Dig Surg 2008; 25:39–45.
Tolerance of an enteral diet is one of the fundamental components of postoperative wellness, along with the ability to mobilize freely without supplemental oxygen and a readiness to be discharged home as soon as possible. Accordingly, postoperative gastrointestinal (GI) tract dysfunction is best defined as intolerance of an enteral diet after having been tolerant of one preoperatively. I prefer the term postoperative GI tract dysfunction over postoperative ileus, as ileus is ill defined, covering a wide spectrum of clinical signs and having a range of published incidences so broad (5%–100%) that it defies useful discussion.
GI DYSFUNCTION: A COMMON POSTOPERATIVE MORBIDITY
Postoperative GI tract dysfunction is common, as illustrated by a large prospective cohort study at Duke University Medical Center3 that used the Postoperative Morbidity Survey (which has since been validated4) to document complications following major noncardiac surgery (ie, anticipated duration > 2 hours and anticipated blood loss > 500 mL). Hospital discharge was delayed in 27% of the study’s 438 patients as a result of a postoperative complication, and GI dysfunction was the most common type of complication overall and on postoperative days 5, 8, and 15. Episodes of GI dysfunction ranged from intolerance of an enteral diet to ischemic gut resulting in multiple organ failure.3
A MULTIFACTORIAL PATHOGENESIS
The pathophysiology of postoperative GI tract dysfunction can be ischemic, metabolic, toxic, neurogenic, myogenic, pharmacologic, or mechanical.
It is important to recognize that in many cases no single factor explains the whole story behind postsurgical GI tract dysfunction, and none of these factors is an ipso facto cause of such dysfunction. For instance, a “mechanical” pathogenesis refers to any manipulation of the gut that causes an inflammatory response in the gut’s various layers, resulting in injury.5,6 However, GI tract dysfunction commonly occurs after operations (including laparoscopic procedures) in which the gut was not handled at all. Similarly, in terms of a pharmacologic pathophysiology, while opioids can affect GI propulsion and cause constipation,7,8 avoidance of opioid use does not ensure prevention of GI tract dysfunction. Moreover, opioid abusers do not generally exhibit intolerance of enteral nutrition.
A common mechanism that is often ignored is perioperative gut ischemia resulting in low-grade injury. Low-grade hypovolemia can cause loss of perfusion to the tip of the microvillus, triggering apoptosis and potentially necrosis, which typically requires about 3 days for recovery. An experiment among 6 healthy volunteers who underwent elective hemorrhage (25% of blood volume removed) over 1 hour demonstrated that gastric tonometry was an earlier indicator of hypovolemia than were commonly measured hemodynamic variables such as invasive blood pressure, stroke volume, heart rate, and lactate and arterial blood gas measurements.9
FLUID LOADING AIDS GI RECOVERY
A targeted increase of intravascular volume and global blood flow perioperatively has been shown repeatedly to improve surgical outcome.10–24 In clinical trials, the most common intervention to achieve the predetermined hemodynamic goal has been fluid loading. Overall, targeted increases in perioperative global blood flow have been associated with reduced mortality,25 with the presumed mechanism being maintenance of end-organ perfusion.
The role of end-organ perfusion maintenance was confirmed in a controlled study of 60 patients undergoing cardiac surgery in which perioperative fluid loading (with colloid) maintained gut perfusion as measured by gastric tonometry, whereas a control group had a reproducible reduction in gut perfusion.15 Fluid loading was associated with a significant reduction in the incidence of gut mucosal hypoperfusion—from 56% to 7%—and significant reductions in the incidence of minor and major complications, mean days in the hospital, and mean days in the intensive care unit.
Fluid type matters
The type of intraoperative fluid loading is a factor in postoperative recovery.
Colloid vs crystalloid. Moretti et al found that colloid (6% hetastarch in saline or 6% hetastarch in balanced salt) was superior to crystalloid (lactated Ringer’s solution) in preventing nausea, severe pain, vomiting, periorbital edema, and double vision postoperatively (P < .05 for all) despite comparable hemodynamic profiles.26
Ringer’s vs normal saline. Williams et al compared intravenous lactated Ringer’s solution with normal saline (0.9% sodium chloride) in a randomized study of healthy volunteers.27 The group that received normal saline demonstrated central nervous system changes and a much higher incidence of abdominal discomfort, a finding consistent with the toxic properties of chlorine to the gut.
Balanced electrolyte solutions vs saline-based fluids. Wilkes et al compared crystalloid and colloid solutions with physiologically balanced electrolyte formulations (Hextend) against saline-based fluids (Hespan) in elderly surgical patients.28 They found that balanced electrolyte solutions were superior in improving gastric mucosal perfusion and preventing hyperchloremic metabolic acidosis. As a result of a reduction in GI tract perfusion, postoperative vomiting was more frequent in the group receiving saline-based fluids.
Evidence for Doppler-guided fluid management
Use of esophageal Doppler ultrasonography to guide fluid administration intraoperatively is fairly common in the United Kingdom and is based on randomized controlled trials showing that Doppler-guided colloid administration to maximize stroke volume reduces morbidity and length of hospital stay in surgical patients. In one government-supported study of 128 colorectal resection patients, Doppler-guided small boluses of colloid increased stroke volume, cardiac output, and oxygen delivery compared with conventional (central venous pressure–based) fluid management.29 Gut function improved significantly faster with Doppler-guided fluid management as evidenced by a more rapid return of flatus, opening of bowels, and achievement of a full diet, and by faster discharge from the hospital. The incidence of GI complications was reduced from 45.3% in the conventional management group to 14.1% in the Doppler group. The relative risk of GI tract dysfunction was 5.3 times higher with conventional management.
OTHER STRATEGIES TO REDUCE POSTOPERATIVE GI DYSFUNCTION
In addition to fluid loading, a number of other methods have been studied in an attempt to reduce the incidence of postoperative GI tract dysfunction.
Epidural neostigmine: Improvement in some measures
Epidural neostigmine was compared with saline control in a randomized study of 45 patients scheduled for abdominal aortic surgery.30 Time to first bowel sounds and time to first flatus were significantly shorter in the neostigmine group, but time to first defecation and the incidence of postoperative complications were similar between the groups.
Laxatives speed return of GI function
In a study of 53 women undergoing fast-track hysterectomy, recovery of GI tract function was faster in those randomized to receive laxatives (magnesium oxide and disodium phosphate) starting 6 hours postoperatively compared with those receiving placebo.31 Median time to first defecation was reduced from 69 hours in the placebo group to 45 hours in the laxative group (P < .0001), and postoperative hospitalization was shortened by a median of 1 day in the laxative group. There were no significant between-group differences in pain scores, postoperative nausea and vomiting, or the use of morphine or antiemetics.
Fentanyl reduces gastric myoelectrical activity
Intravenous administration of the opioid fentanyl significantly reduced gastric myoelectrical activity in an uncontrolled study of 20 patients undergoing elective surgery, but wide variation in effect was observed among patients.32 There was no correlation between the myoelectrical outcome and the presence of polymorphisms of the mu-opioid receptor gene.
Systemic lidocaine accelerates return of bowel function
Perioperative administration of systemic lidocaine, given as a 1.5-mg/kg bolus followed by continuous infusion at 2 mg/min, accelerated the return of bowel function and shortened the length of hospital stay compared with placebo in a randomized study of 60 colorectal surgery patients.33
Early oral feeding cuts length of stay
A recent meta-analysis of randomized trials found that early oral intake of fluids and food after major abdominal gynecologic surgery was associated with an increased risk of nausea but a reduced length of hospital stay.34 The authors recommended an individualized approach to early feeding, and called for cost-effectiveness and patient satisfaction studies.
Mosapride improves gastric emptying
Mosapride is a 5-HT4 agonist that has been shown to improve gastric emptying in a randomized controlled study of 40 patients undergoing laparoscopic colectomy.35 Time to first postoperative bowel movement, time to maximal gastric emptying rate, and postoperative hospital stay were all significantly shorter in patients receiving mosapride versus control. Mosapride is not currently approved for marketing in the United States.
Mu-opioid antagonists: Some show promise, others don’t
Mu-opioid receptor antagonists have been developed primarily to reverse opioid-induced bowel dysfunction. Commercially available drugs in this class include alvimopan, methylnaltrexone, nalbuphine, and naloxone. A recent meta-analysis of 23 randomized controlled studies of these agents for opioid-induced bowel dysfunction concluded that alvimopan and methylnaltrexone were superior to placebo but that evidence was insufficient for the safety or efficacy of naloxone and nalbuphine.36
Nasogastric decompression: Usually more harm than benefit
Prophylactic nasogastric decompression is an intervention devoid of evidence. A meta-analysis of 33 studies encompassing 5,240 patients randomized to routine nasogastric tube placement, selective nasogastric tube use, or no nasogastric tube placement after abdominal surgery found no advantage to routine nasogastric tube use.37 In fact, patients not receiving routine tube placement had a significantly earlier return of bowel function and a significant decrease in pulmonary complications. The incidence of anastomotic leak was not different among the groups. Routine tube use was associated with a lower incidence of vomiting but more patient discomfort. The clear conclusion is that, in most situations, elective placement of a nasogastric tube only causes harm.
Chewing gum: A simple intervention that works
In a recent meta-analysis of five randomized controlled trials, the simple intervention of gum chewing after colorectal surgery significantly accelerated the time to flatus and time to defecation, and was associated with a nonsignificant trend toward a shorter postoperative hospital stay.38
CONCLUSIONS ON MANAGEMENT
Traditional measures intended to reduce the incidence of postoperative GI tract dysfunction—administration of prokinetic drugs, placement of nasogastric tubes, avoidance of food and fluids—are not beneficial and are often harmful. Administration of targeted amounts of fluid to optimize ventricular filling and end-organ perfusion has repeatedly been demonstrated to improve outcomes, particularly those related to GI tract perfusion and function. Administration of larger volumes of colloid, to achieve predetermined increases in stroke volume, improves gut perfusion and reduces the incidence of GI tract dysfunction.
Many simple, inexpensive, and readily available strategies for preventing or reversing postoperative GI tract dysfunction have some degree of evidence-based support and should be considered. I would recommend a multimodal approach that includes a limited surgical incision, regional local anesthesia without use of opioids, immediate postoperative mobilization, early enteral feeding, and postoperative gum chewing.1 Such an approach promises to reduce GI tract dysfunction and other postoperative complications as well as to shorten hospital stay.
DISCUSSION
Question from the audience: You mentioned the selective use of nasogastric tubes. In which patients would you use them?
Dr. Mythen: For upper GI surgeries—esophagectomy, for example—a nasogastric tube is inevitable. Beyond that, the specific indications for tube placement are very limited. At our institution, we no longer place nasogastric tubes following the vast majority of GI tract operations, with esophagectomy being the exception.
Question from the audience: Would you comment on the selective contribution of thoracic epidural analgesia with respect to early feeding after abdominal or colon surgery?
Dr. Mythen: If you’re an enthusiast for thoracic epidurals, you can present the literature in a way that definitively demonstrates a huge advantage to thoracic epidurals. When they work well for the individual, they are fantastic, but you must have a very effective team and system to deliver success to the whole patient population. At our institution the failure rate 20 to 24 hours postoperatively is about 50%.
Question from the audience: I’m an internist and I’ve never heard of the esophageal Doppler-directed fluid bolus protocol—or of anyone using colloids at all. Is that something that is generally practiced in the United States?
Dr. Mythen: Some institutions are practicing goal-directed fluid management now. If you measure stroke volume and give small boluses of colloid, you need a lot less fluid to achieve a higher intravascular volume and goal. At our institution, we’ve repackaged it as “goal-directed fluid restriction” to gain acceptance among surgeons. Uptake has been slower in the United States, though studies here have reinforced the message and been supported by editorials. Guessing about fluids, which we’ve done historically, is not very smart. One thing that differentiates an anesthesiologist from an anesthetic technician is the ability to give goal-directed fluid therapy. The ability to act in a targeted fashion makes it possible to achieve an appropriate physiological goal, but it is more difficult.
Question from the audience: In terms of maintenance fluids and chloride toxicity, is there an alternative to D5 half-normal saline for maintenance fluid?
Dr. Mythen: We don’t have a very good postoperative maintenance fluid; D5 half-normal with some potassium is probably as good as it gets at present. I emphasize getting patients to drink as quickly as possible. If they’re not drinking (not using the GI tract), they need a very high level of physician input because fluid balance is rocket science. The GI tract is very clever. Once patients are drinking and eating, they’re fine, but if they still have an intravenous line in, close attention is required.
Question from the audience: Would you use lactated Ringer’s solution in a patient who is just not eating or drinking?
Dr. Mythen: I do, actually. I tend to mix it in with some D5 half-normal saline because lactated Ringer’s is a great solution. The body can use the lactate to make sugar if necessary. The brain is one of the few organs that will metabolize lactate.
Follow-up question: Would you use it at a lower rate to prevent volume overload?
Dr. Mythen: Yes, at 60 mL/hr. The important thing is that if intravenous fluids are still required, the patient needs to be in a fairly supervised, high-dependency environment. You must address the real issue: Why aren’t they drinking? If the patient is not drinking postoperatively, someone’s done a bad job or there is something that needs fixing.
Question from audience: In the operating room, do you have a preference between albumin and a high-molecular-weight hetastarch like Hextend?
Dr. Mythen: Europe is slightly different in its choice of colloids. We’ve pretty much abandoned the high-molecular-weight starches. We do not use albumin at our institution for cost reasons, and we can’t find any evidence to support its use. We would have to close one intensive care unit bed to be able to afford using albumin. We use low-molecular-weight hydroxyethyl starches, which I believe are now coming into the United States. They have no major coagulation effect.
Tolerance of an enteral diet is one of the fundamental components of postoperative wellness, along with the ability to mobilize freely without supplemental oxygen and a readiness to be discharged home as soon as possible. Accordingly, postoperative gastrointestinal (GI) tract dysfunction is best defined as intolerance of an enteral diet after having been tolerant of one preoperatively. I prefer the term postoperative GI tract dysfunction over postoperative ileus, as ileus is ill defined, covering a wide spectrum of clinical signs and having a range of published incidences so broad (5%–100%) that it defies useful discussion.
GI DYSFUNCTION: A COMMON POSTOPERATIVE MORBIDITY
Postoperative GI tract dysfunction is common, as illustrated by a large prospective cohort study at Duke University Medical Center3 that used the Postoperative Morbidity Survey (which has since been validated4) to document complications following major noncardiac surgery (ie, anticipated duration > 2 hours and anticipated blood loss > 500 mL). Hospital discharge was delayed in 27% of the study’s 438 patients as a result of a postoperative complication, and GI dysfunction was the most common type of complication overall and on postoperative days 5, 8, and 15. Episodes of GI dysfunction ranged from intolerance of an enteral diet to ischemic gut resulting in multiple organ failure.3
A MULTIFACTORIAL PATHOGENESIS
The pathophysiology of postoperative GI tract dysfunction can be ischemic, metabolic, toxic, neurogenic, myogenic, pharmacologic, or mechanical.
It is important to recognize that in many cases no single factor explains the whole story behind postsurgical GI tract dysfunction, and none of these factors is an ipso facto cause of such dysfunction. For instance, a “mechanical” pathogenesis refers to any manipulation of the gut that causes an inflammatory response in the gut’s various layers, resulting in injury.5,6 However, GI tract dysfunction commonly occurs after operations (including laparoscopic procedures) in which the gut was not handled at all. Similarly, in terms of a pharmacologic pathophysiology, while opioids can affect GI propulsion and cause constipation,7,8 avoidance of opioid use does not ensure prevention of GI tract dysfunction. Moreover, opioid abusers do not generally exhibit intolerance of enteral nutrition.
A common mechanism that is often ignored is perioperative gut ischemia resulting in low-grade injury. Low-grade hypovolemia can cause loss of perfusion to the tip of the microvillus, triggering apoptosis and potentially necrosis, which typically requires about 3 days for recovery. An experiment among 6 healthy volunteers who underwent elective hemorrhage (25% of blood volume removed) over 1 hour demonstrated that gastric tonometry was an earlier indicator of hypovolemia than were commonly measured hemodynamic variables such as invasive blood pressure, stroke volume, heart rate, and lactate and arterial blood gas measurements.9
FLUID LOADING AIDS GI RECOVERY
A targeted increase of intravascular volume and global blood flow perioperatively has been shown repeatedly to improve surgical outcome.10–24 In clinical trials, the most common intervention to achieve the predetermined hemodynamic goal has been fluid loading. Overall, targeted increases in perioperative global blood flow have been associated with reduced mortality,25 with the presumed mechanism being maintenance of end-organ perfusion.
The role of end-organ perfusion maintenance was confirmed in a controlled study of 60 patients undergoing cardiac surgery in which perioperative fluid loading (with colloid) maintained gut perfusion as measured by gastric tonometry, whereas a control group had a reproducible reduction in gut perfusion.15 Fluid loading was associated with a significant reduction in the incidence of gut mucosal hypoperfusion—from 56% to 7%—and significant reductions in the incidence of minor and major complications, mean days in the hospital, and mean days in the intensive care unit.
Fluid type matters
The type of intraoperative fluid loading is a factor in postoperative recovery.
Colloid vs crystalloid. Moretti et al found that colloid (6% hetastarch in saline or 6% hetastarch in balanced salt) was superior to crystalloid (lactated Ringer’s solution) in preventing nausea, severe pain, vomiting, periorbital edema, and double vision postoperatively (P < .05 for all) despite comparable hemodynamic profiles.26
Ringer’s vs normal saline. Williams et al compared intravenous lactated Ringer’s solution with normal saline (0.9% sodium chloride) in a randomized study of healthy volunteers.27 The group that received normal saline demonstrated central nervous system changes and a much higher incidence of abdominal discomfort, a finding consistent with the toxic properties of chlorine to the gut.
Balanced electrolyte solutions vs saline-based fluids. Wilkes et al compared crystalloid and colloid solutions with physiologically balanced electrolyte formulations (Hextend) against saline-based fluids (Hespan) in elderly surgical patients.28 They found that balanced electrolyte solutions were superior in improving gastric mucosal perfusion and preventing hyperchloremic metabolic acidosis. As a result of a reduction in GI tract perfusion, postoperative vomiting was more frequent in the group receiving saline-based fluids.
Evidence for Doppler-guided fluid management
Use of esophageal Doppler ultrasonography to guide fluid administration intraoperatively is fairly common in the United Kingdom and is based on randomized controlled trials showing that Doppler-guided colloid administration to maximize stroke volume reduces morbidity and length of hospital stay in surgical patients. In one government-supported study of 128 colorectal resection patients, Doppler-guided small boluses of colloid increased stroke volume, cardiac output, and oxygen delivery compared with conventional (central venous pressure–based) fluid management.29 Gut function improved significantly faster with Doppler-guided fluid management as evidenced by a more rapid return of flatus, opening of bowels, and achievement of a full diet, and by faster discharge from the hospital. The incidence of GI complications was reduced from 45.3% in the conventional management group to 14.1% in the Doppler group. The relative risk of GI tract dysfunction was 5.3 times higher with conventional management.
OTHER STRATEGIES TO REDUCE POSTOPERATIVE GI DYSFUNCTION
In addition to fluid loading, a number of other methods have been studied in an attempt to reduce the incidence of postoperative GI tract dysfunction.
Epidural neostigmine: Improvement in some measures
Epidural neostigmine was compared with saline control in a randomized study of 45 patients scheduled for abdominal aortic surgery.30 Time to first bowel sounds and time to first flatus were significantly shorter in the neostigmine group, but time to first defecation and the incidence of postoperative complications were similar between the groups.
Laxatives speed return of GI function
In a study of 53 women undergoing fast-track hysterectomy, recovery of GI tract function was faster in those randomized to receive laxatives (magnesium oxide and disodium phosphate) starting 6 hours postoperatively compared with those receiving placebo.31 Median time to first defecation was reduced from 69 hours in the placebo group to 45 hours in the laxative group (P < .0001), and postoperative hospitalization was shortened by a median of 1 day in the laxative group. There were no significant between-group differences in pain scores, postoperative nausea and vomiting, or the use of morphine or antiemetics.
Fentanyl reduces gastric myoelectrical activity
Intravenous administration of the opioid fentanyl significantly reduced gastric myoelectrical activity in an uncontrolled study of 20 patients undergoing elective surgery, but wide variation in effect was observed among patients.32 There was no correlation between the myoelectrical outcome and the presence of polymorphisms of the mu-opioid receptor gene.
Systemic lidocaine accelerates return of bowel function
Perioperative administration of systemic lidocaine, given as a 1.5-mg/kg bolus followed by continuous infusion at 2 mg/min, accelerated the return of bowel function and shortened the length of hospital stay compared with placebo in a randomized study of 60 colorectal surgery patients.33
Early oral feeding cuts length of stay
A recent meta-analysis of randomized trials found that early oral intake of fluids and food after major abdominal gynecologic surgery was associated with an increased risk of nausea but a reduced length of hospital stay.34 The authors recommended an individualized approach to early feeding, and called for cost-effectiveness and patient satisfaction studies.
Mosapride improves gastric emptying
Mosapride is a 5-HT4 agonist that has been shown to improve gastric emptying in a randomized controlled study of 40 patients undergoing laparoscopic colectomy.35 Time to first postoperative bowel movement, time to maximal gastric emptying rate, and postoperative hospital stay were all significantly shorter in patients receiving mosapride versus control. Mosapride is not currently approved for marketing in the United States.
Mu-opioid antagonists: Some show promise, others don’t
Mu-opioid receptor antagonists have been developed primarily to reverse opioid-induced bowel dysfunction. Commercially available drugs in this class include alvimopan, methylnaltrexone, nalbuphine, and naloxone. A recent meta-analysis of 23 randomized controlled studies of these agents for opioid-induced bowel dysfunction concluded that alvimopan and methylnaltrexone were superior to placebo but that evidence was insufficient for the safety or efficacy of naloxone and nalbuphine.36
Nasogastric decompression: Usually more harm than benefit
Prophylactic nasogastric decompression is an intervention devoid of evidence. A meta-analysis of 33 studies encompassing 5,240 patients randomized to routine nasogastric tube placement, selective nasogastric tube use, or no nasogastric tube placement after abdominal surgery found no advantage to routine nasogastric tube use.37 In fact, patients not receiving routine tube placement had a significantly earlier return of bowel function and a significant decrease in pulmonary complications. The incidence of anastomotic leak was not different among the groups. Routine tube use was associated with a lower incidence of vomiting but more patient discomfort. The clear conclusion is that, in most situations, elective placement of a nasogastric tube only causes harm.
Chewing gum: A simple intervention that works
In a recent meta-analysis of five randomized controlled trials, the simple intervention of gum chewing after colorectal surgery significantly accelerated the time to flatus and time to defecation, and was associated with a nonsignificant trend toward a shorter postoperative hospital stay.38
CONCLUSIONS ON MANAGEMENT
Traditional measures intended to reduce the incidence of postoperative GI tract dysfunction—administration of prokinetic drugs, placement of nasogastric tubes, avoidance of food and fluids—are not beneficial and are often harmful. Administration of targeted amounts of fluid to optimize ventricular filling and end-organ perfusion has repeatedly been demonstrated to improve outcomes, particularly those related to GI tract perfusion and function. Administration of larger volumes of colloid, to achieve predetermined increases in stroke volume, improves gut perfusion and reduces the incidence of GI tract dysfunction.
Many simple, inexpensive, and readily available strategies for preventing or reversing postoperative GI tract dysfunction have some degree of evidence-based support and should be considered. I would recommend a multimodal approach that includes a limited surgical incision, regional local anesthesia without use of opioids, immediate postoperative mobilization, early enteral feeding, and postoperative gum chewing.1 Such an approach promises to reduce GI tract dysfunction and other postoperative complications as well as to shorten hospital stay.
DISCUSSION
Question from the audience: You mentioned the selective use of nasogastric tubes. In which patients would you use them?
Dr. Mythen: For upper GI surgeries—esophagectomy, for example—a nasogastric tube is inevitable. Beyond that, the specific indications for tube placement are very limited. At our institution, we no longer place nasogastric tubes following the vast majority of GI tract operations, with esophagectomy being the exception.
Question from the audience: Would you comment on the selective contribution of thoracic epidural analgesia with respect to early feeding after abdominal or colon surgery?
Dr. Mythen: If you’re an enthusiast for thoracic epidurals, you can present the literature in a way that definitively demonstrates a huge advantage to thoracic epidurals. When they work well for the individual, they are fantastic, but you must have a very effective team and system to deliver success to the whole patient population. At our institution the failure rate 20 to 24 hours postoperatively is about 50%.
Question from the audience: I’m an internist and I’ve never heard of the esophageal Doppler-directed fluid bolus protocol—or of anyone using colloids at all. Is that something that is generally practiced in the United States?
Dr. Mythen: Some institutions are practicing goal-directed fluid management now. If you measure stroke volume and give small boluses of colloid, you need a lot less fluid to achieve a higher intravascular volume and goal. At our institution, we’ve repackaged it as “goal-directed fluid restriction” to gain acceptance among surgeons. Uptake has been slower in the United States, though studies here have reinforced the message and been supported by editorials. Guessing about fluids, which we’ve done historically, is not very smart. One thing that differentiates an anesthesiologist from an anesthetic technician is the ability to give goal-directed fluid therapy. The ability to act in a targeted fashion makes it possible to achieve an appropriate physiological goal, but it is more difficult.
Question from the audience: In terms of maintenance fluids and chloride toxicity, is there an alternative to D5 half-normal saline for maintenance fluid?
Dr. Mythen: We don’t have a very good postoperative maintenance fluid; D5 half-normal with some potassium is probably as good as it gets at present. I emphasize getting patients to drink as quickly as possible. If they’re not drinking (not using the GI tract), they need a very high level of physician input because fluid balance is rocket science. The GI tract is very clever. Once patients are drinking and eating, they’re fine, but if they still have an intravenous line in, close attention is required.
Question from the audience: Would you use lactated Ringer’s solution in a patient who is just not eating or drinking?
Dr. Mythen: I do, actually. I tend to mix it in with some D5 half-normal saline because lactated Ringer’s is a great solution. The body can use the lactate to make sugar if necessary. The brain is one of the few organs that will metabolize lactate.
Follow-up question: Would you use it at a lower rate to prevent volume overload?
Dr. Mythen: Yes, at 60 mL/hr. The important thing is that if intravenous fluids are still required, the patient needs to be in a fairly supervised, high-dependency environment. You must address the real issue: Why aren’t they drinking? If the patient is not drinking postoperatively, someone’s done a bad job or there is something that needs fixing.
Question from audience: In the operating room, do you have a preference between albumin and a high-molecular-weight hetastarch like Hextend?
Dr. Mythen: Europe is slightly different in its choice of colloids. We’ve pretty much abandoned the high-molecular-weight starches. We do not use albumin at our institution for cost reasons, and we can’t find any evidence to support its use. We would have to close one intensive care unit bed to be able to afford using albumin. We use low-molecular-weight hydroxyethyl starches, which I believe are now coming into the United States. They have no major coagulation effect.
- Mythen MG. Postoperative gastrointestinal tract dysfunction. Anesth Analg 2005; 100:196–204.
- Gan TJ, Meyer T, Apfel CC, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg 2003; 97:62–71.
- Bennett-Guerrero E, Welsby I, Dunn TJ, et al. The use of a postoperative morbidity survey to evaluate patients with prolonged hospitalization after routine, moderate-risk, elective surgery. Anesth Analg 1999; 89:514–519.
- Grocott MP, Browne JP, Van der Meulen J, et al. The Postoperative Morbidity Survey was validated and used to describe morbidity after major surgery. J Clin Epidemiol 2007; 60:919–928.
- Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg 1998; 228:652–663.
- Schwarz NT, Beer-Stolz D, Simmons RL, Bauer AJ. Pathogenesis of paralytic ileus: intestinal manipulation opens a transient pathway between the intestinal lumen and the leukocytic infiltrate of the jejunal muscularis. Ann Surg 2002; 235:31–40.
- Manara L, Bianchetti A. The central and peripheral influences of opioids on gastrointestinal propulsion. Annu Rev Pharmacol Toxicol 1985; 25:249–273.
- Manara L, Bianchi G, Ferretti P, Tavani A. Inhibition of gastrointestinal transit by morphine in rats results primarily from direct drug action on gut opioid sites. J Pharmacol Exp Ther 1986; 237:945–949.
- Hamilton-Davies C, Mythen MG, Salmon JB, et al. Comparison of commonly used clinical indicators of hypovolaemia with gastrointestinal tonometry. Intensive Care Med 1997; 23:276–281.
- Bender JS, Smith-Meek MA, Jones CE. Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: results of a prospective, randomized trial. Ann Surg 1997; 226:229–237.
- Berlauk JF, Abrams JH, Gilmour IJ, et al. Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery: a prospective, randomized clinical trial. Ann Surg 1991; 214:289–299.
- Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 1993; 270:2699–2707.
- Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002; 97:820–826.
- Lobo SM, Salgado PF, Castillo VG, et al. Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med 2000; 28:3396–3404.
- Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg 1995; 130:423–429.
- Pölönen P, Ruokonen E, Hippeläinen M, Pöyhönen M, Takala J. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg 2000; 90:1052–1059.
- Sandham JD, Hull RD, Brant RF, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 2003; 348:5–14.
- Shoemaker WC, Appel PL, Kram HB, Nathan RC, Thompson JL. Multicomponent noninvasive physiologic monitoring of circulatory function. Crit Care Med 1988; 16:482–490.
- Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ 1997; 315:909–912.
- Ueno S, Tanabe G, Yamada H, et al. Response of patients with cirrhosis who have undergone partial hepatectomy to treatment aimed at achieving supranormal oxygen delivery and consumption. Surgery 1998; 123:278–286.
- Valentine RJ, Duke ML, Inman MH, et al. Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. J Vasc Surg 1998; 27:203–212.
- Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth 2002; 88:65–71.
- Wilson J, Woods I, Fawcett J, et al. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ 1999; 318:1099–1103.
- Ziegler DW, Wright JG, Choban PS, Flancbaum L. A prospective randomized trial of preoperative “optimization” of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery 1997; 122:584–592.
- Grocott MPW, Hamilton MA, Bennett ED, Harrison D, Rowan K. Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery. Cochrane Database Syst Rev 2006; 2:CD004082. doi:10.1002/14651858.CD004082.pub4.
- Moretti EW, Robertson KM, El-Moalem H, Gan TJ. Intraoperative colloid administration reduces postoperative nausea and vomiting and improves postoperative outcomes compared with crystalloid administration. Anesth Analg 2003; 96:611–617.
- Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg 1999; 88:999–1003.
- Wilkes NJ, Woolf R, Mutch M, et al. The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid-base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg 2001; 93:811–816.
- Wakeling HG, McFall MR, Jenkins CS, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005; 95:634–642.
- Caliskan E, Turkoz A, Sener M, et al. A prospective randomized double-blind study to determine the effect of thoracic epidural neostigmine on postoperative ileus after abdominal aortic surgery. Anesth Analg 2008; 106:959–964.
- Hansen CT, Sørensen M, Møller C, Ottesen B, Kehlet H. Effect of laxatives on gastrointestinal functional recovery in fast-track hysterectomy: a double-blind, placebo-controlled randomized study. Am J Obstet Gynecol 2007; 196:311.e1–311.e7.
- Walldén J, Lindberg G, Sandin M, Thörn SE, Wattwil M. Effects of fentanyl on gastric myoelectrical activity: a possible association with polymorphisms of the mu-opioid receptor gene? Acta Anaesthesiol Scand 2008; 52:708–715.
- Herroeder S, Pecher S, Schönherr ME, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg 2007; 246:192–200.
- Charoenkwan K, Phillipson G, Vutyavanich T. Early versus delayed oral fluids and food for reducing complications after major abdominal gynaecologic surgery. Cochrane Database Syst Rev 2007; 4:CD004508. doi:10.1002/14651858.CD004508.pub3.
- Narita K, Tsunoda A, Takenaka K, et al. Effect of mosapride on recovery of intestinal motility after hand-assisted laparoscopic colectomy for carcinoma. Dis Colon Rectum 2008; 51:1692–1695.
- McNicol ED, Boyce D, Schumann R, Carr DB. Mu-opioid antagonists for opioid-induced bowel dysfunction. Cochrane Database Syst Rev 2008; 2:CD006332. doi:10.1002/14651858.CD006332.pub2.
- Nelson RL, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2007; 3:CD004929. doi:10.1002/14651858.CD004929.pub3.
- de Castro SM, van den Esschert JW, van Heek NT, et al. A systematic review of the efficacy of gum chewing for the amelioration of postoperative ileus. Dig Surg 2008; 25:39–45.
- Mythen MG. Postoperative gastrointestinal tract dysfunction. Anesth Analg 2005; 100:196–204.
- Gan TJ, Meyer T, Apfel CC, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg 2003; 97:62–71.
- Bennett-Guerrero E, Welsby I, Dunn TJ, et al. The use of a postoperative morbidity survey to evaluate patients with prolonged hospitalization after routine, moderate-risk, elective surgery. Anesth Analg 1999; 89:514–519.
- Grocott MP, Browne JP, Van der Meulen J, et al. The Postoperative Morbidity Survey was validated and used to describe morbidity after major surgery. J Clin Epidemiol 2007; 60:919–928.
- Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg 1998; 228:652–663.
- Schwarz NT, Beer-Stolz D, Simmons RL, Bauer AJ. Pathogenesis of paralytic ileus: intestinal manipulation opens a transient pathway between the intestinal lumen and the leukocytic infiltrate of the jejunal muscularis. Ann Surg 2002; 235:31–40.
- Manara L, Bianchetti A. The central and peripheral influences of opioids on gastrointestinal propulsion. Annu Rev Pharmacol Toxicol 1985; 25:249–273.
- Manara L, Bianchi G, Ferretti P, Tavani A. Inhibition of gastrointestinal transit by morphine in rats results primarily from direct drug action on gut opioid sites. J Pharmacol Exp Ther 1986; 237:945–949.
- Hamilton-Davies C, Mythen MG, Salmon JB, et al. Comparison of commonly used clinical indicators of hypovolaemia with gastrointestinal tonometry. Intensive Care Med 1997; 23:276–281.
- Bender JS, Smith-Meek MA, Jones CE. Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: results of a prospective, randomized trial. Ann Surg 1997; 226:229–237.
- Berlauk JF, Abrams JH, Gilmour IJ, et al. Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery: a prospective, randomized clinical trial. Ann Surg 1991; 214:289–299.
- Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 1993; 270:2699–2707.
- Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002; 97:820–826.
- Lobo SM, Salgado PF, Castillo VG, et al. Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med 2000; 28:3396–3404.
- Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg 1995; 130:423–429.
- Pölönen P, Ruokonen E, Hippeläinen M, Pöyhönen M, Takala J. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg 2000; 90:1052–1059.
- Sandham JD, Hull RD, Brant RF, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 2003; 348:5–14.
- Shoemaker WC, Appel PL, Kram HB, Nathan RC, Thompson JL. Multicomponent noninvasive physiologic monitoring of circulatory function. Crit Care Med 1988; 16:482–490.
- Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ 1997; 315:909–912.
- Ueno S, Tanabe G, Yamada H, et al. Response of patients with cirrhosis who have undergone partial hepatectomy to treatment aimed at achieving supranormal oxygen delivery and consumption. Surgery 1998; 123:278–286.
- Valentine RJ, Duke ML, Inman MH, et al. Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. J Vasc Surg 1998; 27:203–212.
- Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth 2002; 88:65–71.
- Wilson J, Woods I, Fawcett J, et al. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ 1999; 318:1099–1103.
- Ziegler DW, Wright JG, Choban PS, Flancbaum L. A prospective randomized trial of preoperative “optimization” of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery 1997; 122:584–592.
- Grocott MPW, Hamilton MA, Bennett ED, Harrison D, Rowan K. Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery. Cochrane Database Syst Rev 2006; 2:CD004082. doi:10.1002/14651858.CD004082.pub4.
- Moretti EW, Robertson KM, El-Moalem H, Gan TJ. Intraoperative colloid administration reduces postoperative nausea and vomiting and improves postoperative outcomes compared with crystalloid administration. Anesth Analg 2003; 96:611–617.
- Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg 1999; 88:999–1003.
- Wilkes NJ, Woolf R, Mutch M, et al. The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid-base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg 2001; 93:811–816.
- Wakeling HG, McFall MR, Jenkins CS, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005; 95:634–642.
- Caliskan E, Turkoz A, Sener M, et al. A prospective randomized double-blind study to determine the effect of thoracic epidural neostigmine on postoperative ileus after abdominal aortic surgery. Anesth Analg 2008; 106:959–964.
- Hansen CT, Sørensen M, Møller C, Ottesen B, Kehlet H. Effect of laxatives on gastrointestinal functional recovery in fast-track hysterectomy: a double-blind, placebo-controlled randomized study. Am J Obstet Gynecol 2007; 196:311.e1–311.e7.
- Walldén J, Lindberg G, Sandin M, Thörn SE, Wattwil M. Effects of fentanyl on gastric myoelectrical activity: a possible association with polymorphisms of the mu-opioid receptor gene? Acta Anaesthesiol Scand 2008; 52:708–715.
- Herroeder S, Pecher S, Schönherr ME, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg 2007; 246:192–200.
- Charoenkwan K, Phillipson G, Vutyavanich T. Early versus delayed oral fluids and food for reducing complications after major abdominal gynaecologic surgery. Cochrane Database Syst Rev 2007; 4:CD004508. doi:10.1002/14651858.CD004508.pub3.
- Narita K, Tsunoda A, Takenaka K, et al. Effect of mosapride on recovery of intestinal motility after hand-assisted laparoscopic colectomy for carcinoma. Dis Colon Rectum 2008; 51:1692–1695.
- McNicol ED, Boyce D, Schumann R, Carr DB. Mu-opioid antagonists for opioid-induced bowel dysfunction. Cochrane Database Syst Rev 2008; 2:CD006332. doi:10.1002/14651858.CD006332.pub2.
- Nelson RL, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2007; 3:CD004929. doi:10.1002/14651858.CD004929.pub3.
- de Castro SM, van den Esschert JW, van Heek NT, et al. A systematic review of the efficacy of gum chewing for the amelioration of postoperative ileus. Dig Surg 2008; 25:39–45.
KEY POINTS
- GI tract dysfunction is the most common type of postoperative morbidity and frequently delays hospital discharge.
- Low-grade hypovolemia leading to gut ischemia is a common but neglected mechanism of postoperative GI tract dysfunction.
- Administration of colloid to achieve target levels of cardiac output improves gut perfusion and lowers the incidence of GI tract dysfunction.
- Doppler-guided fluid management reduces GI morbidity and length of hospital stay in surgical patients.