User login
Take these steps to improve your flu season preparedness
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
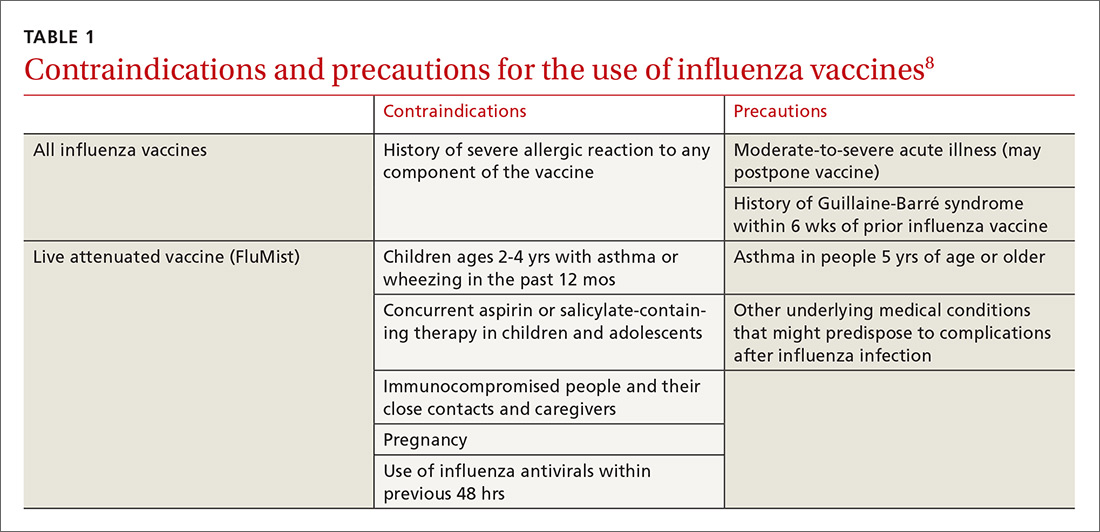
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).
Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.
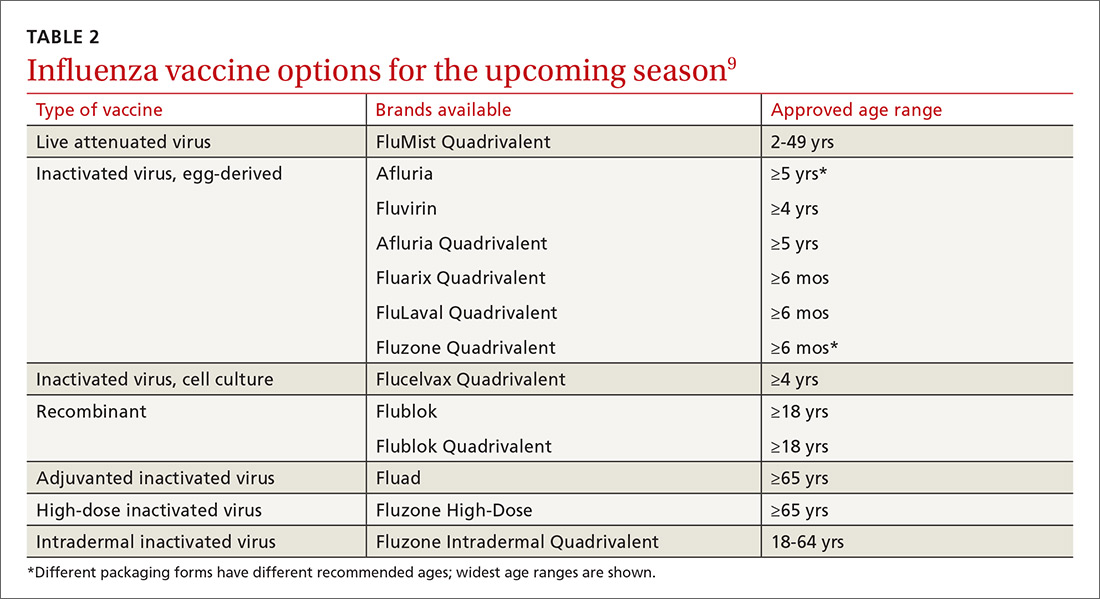
Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
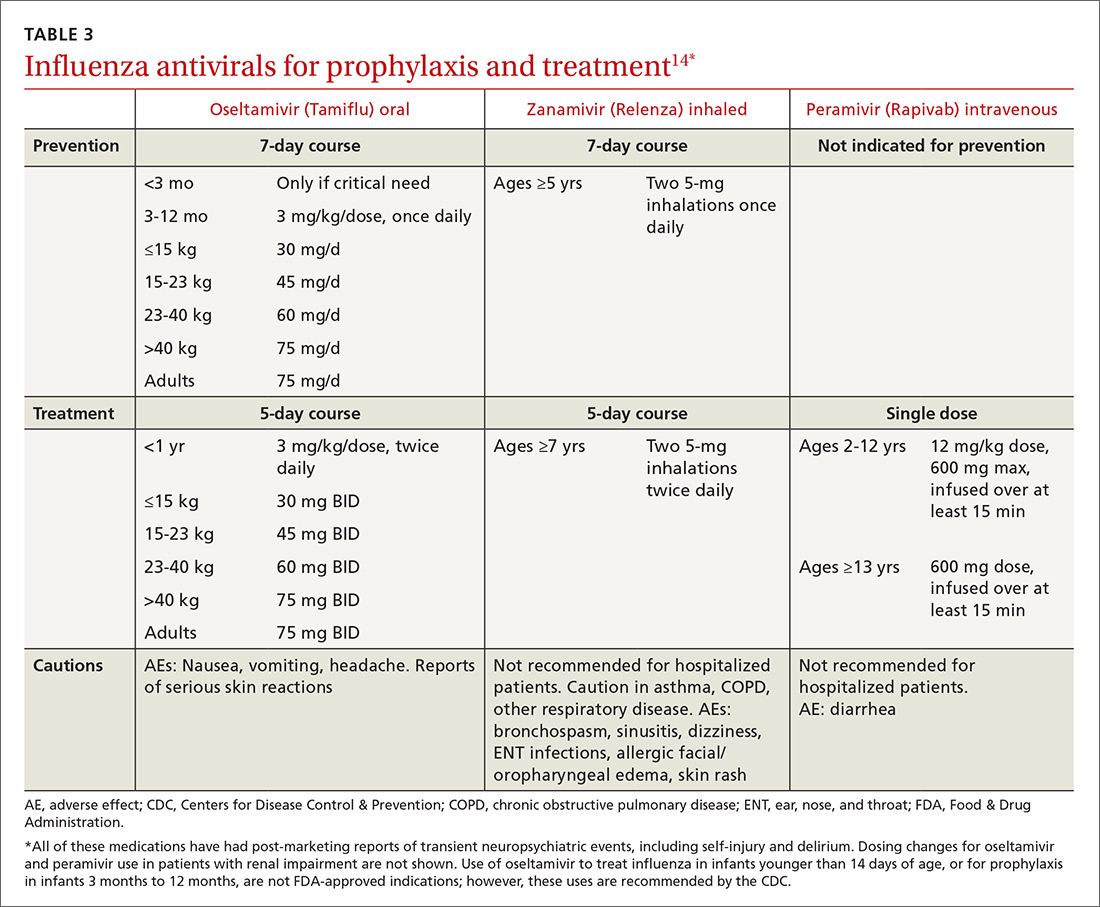
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).
Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
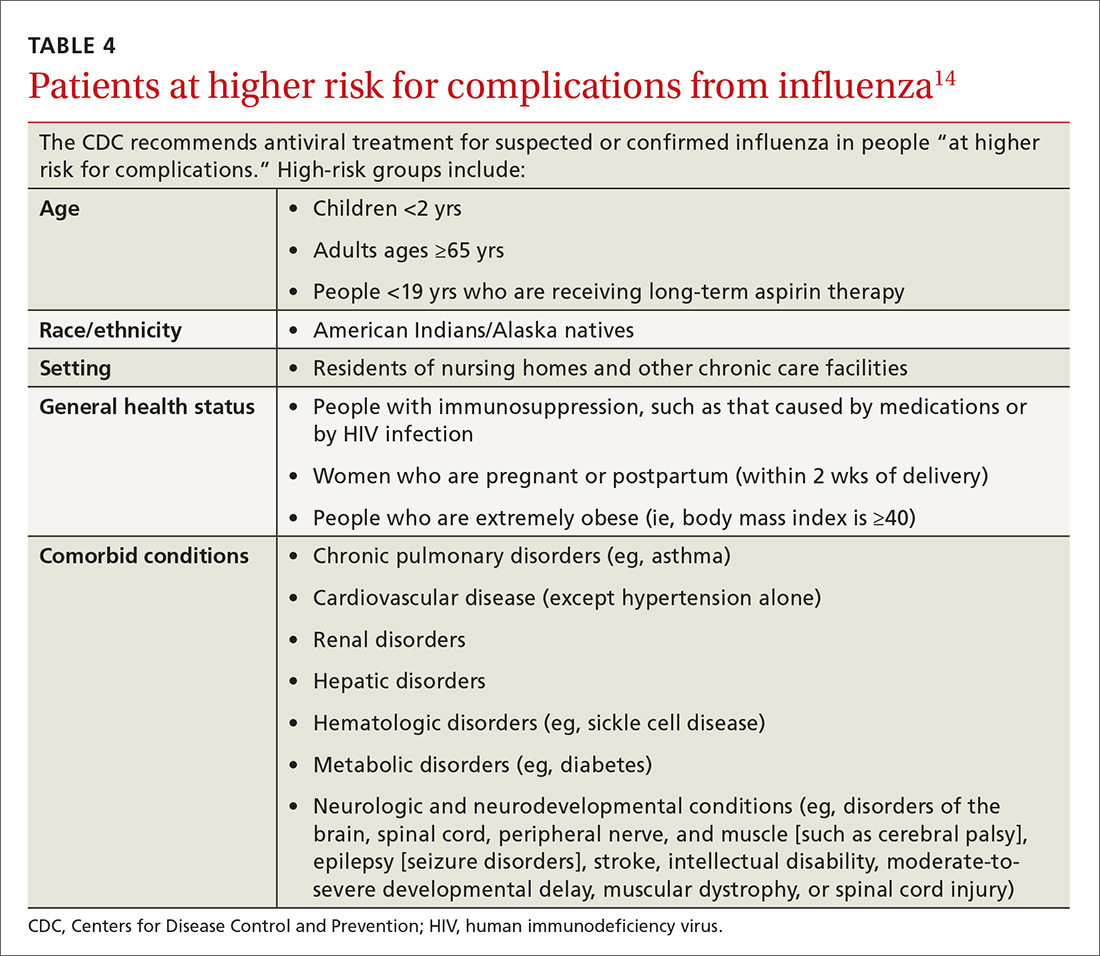
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14
Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).

Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.

Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).

Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14

Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).

Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.

Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).

Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14

Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
PRACTICE RECOMMENDATIONS
› Recommend influenza vaccination for all patients at least 6 months old unless a specific contraindication exists. A
› Recommend pneumococcal vaccination to appropriate patients to reduce the risk for a common complication of influenza. A
› Encourage hygiene-based measures to limit infection, including frequent handwashing or use of a hand sanitizer. B
› Prescribe oseltamivir to hospitalized influenza patients to limit the duration and severity of infection. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
