User login
Obstructive sleep apnea: A diagnostic and treatment guide
CASE 1 Mr. M, age 59, has diabetes, hypertension, hyperlipidemia, and obesity, with a body mass index (BMI) of 37 kg/m2. His hyperlipidemia is well controlled with atorvastatin. He also takes hydrochlorothiazide, lisinopril, metformin, and glyburide, but neither his hypertension nor his glucose levels are well controlled. Mr. M does not exercise, and acknowledges eating a high-calorie diet.
Mr. M reports that his wife has begun complaining about his loud snoring, and that he’s been falling asleep at work. He says he has 2 to 3 alcoholic drinks per week, and doesn’t smoke or take any other drugs.
CASE 2 Ms. C, age 35, is seeking treatment for early morning headaches that began several months ago. She describes a constant dull pain over the frontal area that occurs almost every day and typically resolves in 2 to 3 hours. The pain is not affected by postural changes, she says.
She also reports fatigue, daytime sleepiness, and anxiety. Ms. C has a normal BMI (24 kg/m2), takes no medication, and has no prior history of migraine or tension headaches. She denies any alcohol or recreational drug use.
If Mr. M and Ms. C were your patients, what would your next step be?
Obstructive sleep apnea (OSA) is characterized by repeated cycles of partial airway obstruction, hypoxemia, sympathetic discharge, and arousal to restore ventilation. In addition to fragmented sleep and daytime sleepiness, this common sleep disorder is associated with a decreased functional capacity1-3 and an increased risk for accidents, arrhythmias, myocardial infarction (MI), stroke, and death.4-6 In the Wisconsin Sleep Cohort, an 18-year follow-up study, the estimated hazard ratio for patients with severe OSA was 3.0 for all-cause mortality (95% CI; P-trend <.008) and 5.2 for cardiovascular death (95% CI; P=.003).7
The prevalence of OSA in the United States is 3% to 7% for males and 2% to 5% for females, with higher rates among those older than 65 years. But these figures apply only to those with an OSA diagnosis. An additional 5% of the general population is believed to have undiagnosed OSA.4,8
Arriving at an OSA diagnosis may not be difficult with a patient like Mr. M, who is obese and has classic symptoms. But it is important to consider OSA in patients who, like Ms. C, are not overweight and whose chief complaint appears to be unrelated to sleep.
This review—of risk factors, common (and uncommon) symptoms, diagnostic criteria, and treatment for OSA—highlights key indicators and optimal therapies.
The OSA profile: Risk factors and presenting symptoms
Obesity is perhaps the best-known risk factor for OSA: About 70% of patients with OSA are obese, and 40% of obese individuals have OSA. Approximately 60% of those with OSA have metabolic syndrome.9-12 But it is important to remember that individuals with normal or even low BMI may develop OSA, as well.
Other possible contributing factors include alcohol use, smoking, nasal congestion, menopause, polycystic ovary disease, and a genetic predisposition. And OSA may cause or worsen a wide range of conditions, including hypertension, congestive heart failure, atrial fibrillation, stroke, and nocturnal arrhythmias.8,13
Symptoms may be classic—or not
A bed partner’s complaint about a patient’s snoring, typically followed by arousal and gasping for air, is often the first indication of OSA. Loud snoring and daytime sleepiness are the most common symptoms. But not all patients present with these classic symptoms. Some may complain of irritability, difficulty concentrating, daily headaches, and nocturia.
Because the symptoms of OSA are not highly specific, a wide differential diagnosis must be considered, including numerous causes of excessive daytime sleepiness and conditions, such as panic attacks, pulmonary disease, and gastroesophageal reflux disease, which can interfere with sleep (TABLE 1).8,13
What to include in the medical history and work-up
When you suspect OSA or another sleep disorder, the medical history should include a complete review of systems. The social history needs to include information regarding alcohol intake, use of sedatives, and recreational drugs. If possible, interview the patient’s bed partner, as well.
Examine upper airway anatomy. In performing a physical exam, pay special attention to blood pressure, BMI, and upper airway anatomy. Assess for conditions that can cause obstruction, such as nasal congestion, septal deviation, retrognathia (malocclusion of the mouth due to an abnormal posterior position of the maxilla or mandible), macroglossia (an unusually large tongue), dental malocclusion, enlarged lymphoid tissue and tonsils, large uvula, low hanging soft palate, and a large neck circumference (>40 cm for females and >43 cm for males).13
Order blood tests. Routine blood tests do not support an OSA diagnosis, but they help rule out or identify other conditions associated or mimicking OSA, such as anemia, hypothyroidism, diabetes, liver disease, and kidney disease. Suggested labs include a comprehensive metabolic profile, complete blood count, and thyroid-stimulating hormone test.
Sleep studies are needed for a definitive diagnosis
Conducted overnight in a sleep lab, polysomnography (PSG) uses respiratory effort, respiratory air flow, and peripheral oximetry to identify and quantify episodes of apnea and hypopnea (reported as the apnea-hypopnea index, or AHI). PSG also records brain electrical activity (electroencephalogram), heart rhythm (electrocardiogram), eye movement (electro-oculogram), and muscle activation (electromyogram)—studies used to identify other sleep disorders, such as restless leg syndrome, narcolepsy, parasomnias, and disturbances in rapid-eye movement
(REM) sleep.
Home monitoring. For patients who are unable or unwilling to undergo an overnight sleep study, home portable monitoring is a less expensive alternative. The monitor—a small wireless device—provides data for calculating the AHI and the presence and degree of oxygen desaturation.14 Unlike PSG, which can identify the amount of pressure needed for continuous positive airway pressure (CPAP) therapy, findings from a portable monitor are not sufficient to rule out other sleep disorders to determine whether CPAP is required.15
Evaluating daytime sleepiness. Several tools have been used to evaluate daytime sleepiness. The Epworth Sleepiness Scale (ESS), a quick 8-item screening questionnaire, determines the average person’s level of sleepiness during the day. It ranges from 0 to 24 points, with 10 being normal. Although the ESS has been used extensively in OSA research, recent studies found that it has a low sensitivity (54%) and specificity (57%) for scores >10 and does not correlate well with hypopnea and apnea measurements.16,17
Two additional tools, the Berlin and STOP questionnaires, can also be used to screen for OSA. Both questionnaires have about a 50% positive predictive value and a 70% negative predictive value.18,19
Diagnosing and classifying OSA
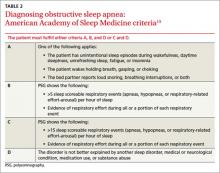
Diagnostic criteria developed by the American Academy of Sleep Medicine (AASM) are based on reported and observed symptoms and PSG recordings of hypopnea and apneic episodes. Of the 4 criteria (A through D), patients must meet either A, B, and D or C and D (TABLE 2).15
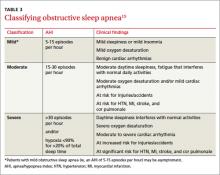
The AASM further classifies OSA as mild, moderate, or severe (TABLE 3)15 based on the AHI as well as on clinical findings, including oxygen desaturation and arrhythmias. Patients with severe OSA have excessive daytime sleepiness (EDS) that interferes with their normal activities, 15 as well as severe oxygen desaturation, moderate to severe cardiac arrhythmias, and significant risk for hypertension, MI, stroke, and cor pulmonale.
CASE 1 An examination of Mr. M’s upper airway anatomy reveals a neck circumference of 44 cm and normal oropharynx. The results of his lab tests were only significant for elevated blood sugar (234 mg/dL) and glycosylated hemoglobin (9.2%). Because he presents with classic symptoms of OSA, he receives a referral for PSG. He is found to have an AHI of 49, consistent with severe sleep apnea.
CASE 2 A system review of Ms. C finds no fever, nausea, vomiting, weakness, vision changes, or neurological symptoms. A Patient Health Questionnaire-9 (depression screen) is normal, as are her lab tests and a brain MRI with and without contrast. After an extensive work-up for headaches finds nothing, OSA is considered, in light of her daytime sleepiness—and she, too, is referred for PSG. This patient has moderate OSA, with an AHI of 27.
Initiating treatment: What’s best?
Ideally, treatment of OSA would reverse EDS and fatigue, restore full cognitive function, reduce the risk of accidents associated with OSA, and minimize its harmful cardiovascular and pulmonary effects. In fact, while OSA can be managed and its effects ameliorated, all available treatments have limitations and a cure remains elusive.
Let patients know that our understanding of OSA is limited, that treatment may not reverse or eliminate all the risks associated with this condition, and that compliance can be challenging. You can also tell them that, while more and better studies are needed, several modalities have been found to successfully treat OSA.
What to expect from lifestyle modification
Recommend lifestyle changes, such as weight loss, regular exercise early in the day, greater emphasis on sleep hygiene (eg, using the bed only for sleeping and sexual activity), and avoidance of sedating drugs and alcohol for patients with OSA.20,21
The beneficial effect of weight loss on OSA has been demonstrated in studies of both bariatric surgery and conventional weight loss therapies.21-23 While early studies of bariatric surgery were often limited by small size, ambiguous classification of OSA, and selection and follow-up biases, more recent trials show that while OSA symptoms frequently improve postoperatively, the disorder typically persists despite significant reductions in both BMI and AHI.24 Weight reduction should be strongly encouraged for obese patients, however, not only to improve OSA symptoms, but also to reduce the risk for other diseases.
Avoidance of alcohol. Alcohol has adverse effects on sleep: It shortens sleep latency, increases slow-wave sleep, suppresses REM and parasympathetic nerve activity,25 and can exacerbate OSA. Driving simulation studies have found that, compared with healthy individuals, those with untreated OSA are more susceptible to the effects of alcohol and at higher risk for accidents after just one drink.26
CPAP improves sleep, but some problems persist
CPAP supplies a flow of positive air pressure, adjusted to the level needed to keep the airway open, delivered through a facial device best suited to the patient’s anatomy, physiology, and comfort.
Multiple studies have demonstrated the effectiveness of CPAP in reducing symptoms of moderate to severe OSA, compared with placebo and other treatment modalities such as oral devices, surgical procedures, and medications. CPAP reduces AHI, blood pressure, and cardiac arrhythmias. It improves sleep efficiency, oxygen saturation, and self-reported sleep and well-being.27-30 While it ameliorates many of the harmful effects of OSA, it does not improve or reverse all of them. (See “Peripheral neuropathy linked to obstructive sleep apnea?”)
A Cochrane review of 36 randomized controlled trials with a combined total of more than 1700 patients demonstrated the superiority of CPAP vs control in several measures, such as subjective daytime sleepiness, quality of life, cognitive function, and blood pressure.28 On specific parameters of OSA, such as snoring and EDS, studies yielded mixed results.
CPAP has been found to decrease work-related injuries and morbidity and mortality associated with motor vehicle accidents linked to EDS.30-32 However, no study of CPAP has demonstrated a long-term reduction in morbidity and mortality. And no standards define the minimum number of sleep hours and/or frequency of CPAP use that is required to obtain specific benefits.
Patient compliance is poor. Part of the problem is that CPAP is difficult to use, which affects compliance. Poor patient compliance is a major barrier to evaluating its long-term benefits. (The video below, "CPAP Patients Tips from the FDA", can help ensure that patients use CPAP safely and effectively.) Studies estimate that 65% to 89% of patients with CPAP devices use them for at least 4 hours a night for 70% of nights, but that about half of those for whom CPAP is prescribed stop using it after 2 to 3 years.33-36 Several risk factors and comorbid conditions, including advanced age, diabetes, obesity, smoking, and especially, depression, are associated with decreased compliance.13
Improving CPAP compliance continues to be a challenge, highlighting the importance of treating not only OSA but all comorbidities, particularly depression. Short-term studies have found behavioral modification to be a promising means of improving CPAP compliance.33-36
Although not a first-line therapy, bilevel positive airway pressure (BiPAP), which delivers both inspiratory and expiratory pressure via a face device, can be tried in patients unable to tolerate CPAP.37 Studies are limited and it has been used in patients with complex OSA.
Oral appliances are a CPAP alternative
Mandibular repositioning devices (MRDs) and tongue-retaining devices are alternatives to CPAP.38 Although both types of oral appliance are beneficial, they are less effective than CPAP.28
MRDs, which are more commonly used than tongue-retaining devices, are available in several models. An MRD can be custom-made to hold the lower jaw in a forward position during sleep, enlarge the space behind the tongue, and put tension on the walls of the pharynx and the palate to reduce collapse.38
Tongue-retaining devices—splints that hold the tongue in place to keep the airway open—can be used for mild to moderate OSA, and for patients unable to tolerate CPAP.38,39
Studies comparing MRDs and tongue-retaining devices found no statistically significant difference in their ability to reduce AHI, but patients tolerated MRDs better.38,39 Nonetheless, both devices can cause dental discomfort, temporomandibular joint pain, dry mouth or excessive salivation, gum irritation, bruxism, and long-term occlusal changes.38
Is surgery an option?
Numerous surgical techniques are available for treating OSA, all aimed at relieving the obstruction by removing or bypassing it or increasing airway size. These include uvulopalatopharyngoplasty, which resects the uvula, retrolingual, and palatine tonsillar tissue; septoplasty; rhinoplasty; nasal turbinate reduction; nasal polypectomy palatal advancement pharyngoplasty; tonsillectomy; adenoidectomy; palatal implants; tongue reduction; genioglossus advancement; and maxillomandibular advancement.
The choice of modality depends on the patient’s anatomy and physiology, and is selected only after a full evaluation by a head and neck surgeon who specializes in surgical treatment of OSA.40,41 There is a paucity of reliable studies on the results of such procedures, but a Cochrane review of the existing literature concluded that surgery is only indicated for severe cases of sleep apnea in patients who have an anatomic obstruction.40
Too little evidence of medications’ efficacy
Numerous drugs have been tested for the treatment of OSA. Two Cochrane reviews looked at multiple trials of more than 20 drugs, in meta-analyses encompassing more than 500 patients.42,43 Ten drugs—eszopiclone, paroxetine, acetazolamide, ondansetron-fluoxetine combination, naltrexone, and fluticasone nasal spray among them—showed a statistically significant reduction in AHI, and a few showed a subjective benefit in daytime sleepiness. However, the studies were very small and of short duration and the reviewers concluded that evidence is insufficient to recommend drug therapy for OSA.
CASE 1 CPAP was prescribed for Mr. M. He tolerated it well and after 2 weeks, he reported feeling refreshed upon awakening, having less daytime somnolence and being better able to concentrate at work. His hypertension and glucose control improved, but he continues to struggle with his weight.
CASE 2 Ms. C started CPAP and within a week, her morning headaches and irritability resolved.
CORRESPONDENCE
Cecilia Gutierrez, MD, 200 West Arbor Drive, Mail Code 8809, San Diego, CA 92103; [email protected]
1. Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112.
2. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199-204.
3. Satela MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249-259.
4. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
5. Yaggi HK, Concato J, Kernan W, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
6. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
7. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;3:1071-1078.
8. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
9. Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735-741.
10. Basta M, Vgontzas AN. Metabolic abnormalities in obesity and sleep apnea are in a continuum. Sleep Med. 2007;8:5-7.
11. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-3021.
12. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467-472.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013-2016.
14. Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-base diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257-263.
15. Iber C, Ancoli-Israel S, Chesson AL Jr, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
16. Johns MW. Sensitivity and specificity of the multiple sleep maintenance tests, the maintenance of wakefulness tests and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5-11.
17. Hesselbacher S, Subramanian S, Allen J, et al. Body mass index, gender, and ethnic variations alter the clinical implication of the Epworth sleepiness scale in patients with suspected of obstructive sleep apnea. Open Respir Med J. 2012;6:2020-2027.
18. Ahmadi N, Chung S, Gibbs A, et al. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:38-45.
19. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
20. Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 2001;(1):CD002875.
21. Barvaux VA, Aubert G, Rodenstein DO. Weight loss as treatment for obstructive sleep apnea. Sleep Med Rev. 2000;4:435-452.
22. Buchwald H, Avidor H, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
23. Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs. conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142-1149.
24. Greenburg A, Lettieri C, Arn E. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542.
25. Sagawa Y, Kondo H, Matsubuchi N. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35:2093-2100.
26. Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447-455.
27. Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565-571.
28. Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3):CD001106.
29. Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25:1070-1076.
30. Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430-434.
31. Findley L, Smith C, Hooper J, et al. Treatment with nasal CPAP decreases automobile accidents in patient with sleep apnea. Am J Respir Crit Care Med. 2000;161:857-859.
32. Barbe F, Sunyer J, de la Pena A, et al. Effects of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74:44-49.
33. Sin DD, Mayers I, Man GC, et al. Long Term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population based study. Chest. 2002;121:430-435.
34. Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart. 2005;91:1265-1270.
35. Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 2006;7:553-560.
36. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea-hypopnea syndrome (SAHS). Sleep Med. 2003;7:81-99.
37. Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath. 2012;16:773-739.
38. Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693-699.
39. Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240-243.
40. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;(4):CD001004.
41. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407.
42. Smith I, Lasserson TJ, Wright JJ. Drug therapy for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006;():CD003002. Review.
43. Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;(5): CD003002.
CASE 1 Mr. M, age 59, has diabetes, hypertension, hyperlipidemia, and obesity, with a body mass index (BMI) of 37 kg/m2. His hyperlipidemia is well controlled with atorvastatin. He also takes hydrochlorothiazide, lisinopril, metformin, and glyburide, but neither his hypertension nor his glucose levels are well controlled. Mr. M does not exercise, and acknowledges eating a high-calorie diet.
Mr. M reports that his wife has begun complaining about his loud snoring, and that he’s been falling asleep at work. He says he has 2 to 3 alcoholic drinks per week, and doesn’t smoke or take any other drugs.
CASE 2 Ms. C, age 35, is seeking treatment for early morning headaches that began several months ago. She describes a constant dull pain over the frontal area that occurs almost every day and typically resolves in 2 to 3 hours. The pain is not affected by postural changes, she says.
She also reports fatigue, daytime sleepiness, and anxiety. Ms. C has a normal BMI (24 kg/m2), takes no medication, and has no prior history of migraine or tension headaches. She denies any alcohol or recreational drug use.
If Mr. M and Ms. C were your patients, what would your next step be?
Obstructive sleep apnea (OSA) is characterized by repeated cycles of partial airway obstruction, hypoxemia, sympathetic discharge, and arousal to restore ventilation. In addition to fragmented sleep and daytime sleepiness, this common sleep disorder is associated with a decreased functional capacity1-3 and an increased risk for accidents, arrhythmias, myocardial infarction (MI), stroke, and death.4-6 In the Wisconsin Sleep Cohort, an 18-year follow-up study, the estimated hazard ratio for patients with severe OSA was 3.0 for all-cause mortality (95% CI; P-trend <.008) and 5.2 for cardiovascular death (95% CI; P=.003).7
The prevalence of OSA in the United States is 3% to 7% for males and 2% to 5% for females, with higher rates among those older than 65 years. But these figures apply only to those with an OSA diagnosis. An additional 5% of the general population is believed to have undiagnosed OSA.4,8
Arriving at an OSA diagnosis may not be difficult with a patient like Mr. M, who is obese and has classic symptoms. But it is important to consider OSA in patients who, like Ms. C, are not overweight and whose chief complaint appears to be unrelated to sleep.
This review—of risk factors, common (and uncommon) symptoms, diagnostic criteria, and treatment for OSA—highlights key indicators and optimal therapies.
The OSA profile: Risk factors and presenting symptoms
Obesity is perhaps the best-known risk factor for OSA: About 70% of patients with OSA are obese, and 40% of obese individuals have OSA. Approximately 60% of those with OSA have metabolic syndrome.9-12 But it is important to remember that individuals with normal or even low BMI may develop OSA, as well.
Other possible contributing factors include alcohol use, smoking, nasal congestion, menopause, polycystic ovary disease, and a genetic predisposition. And OSA may cause or worsen a wide range of conditions, including hypertension, congestive heart failure, atrial fibrillation, stroke, and nocturnal arrhythmias.8,13
Symptoms may be classic—or not
A bed partner’s complaint about a patient’s snoring, typically followed by arousal and gasping for air, is often the first indication of OSA. Loud snoring and daytime sleepiness are the most common symptoms. But not all patients present with these classic symptoms. Some may complain of irritability, difficulty concentrating, daily headaches, and nocturia.
Because the symptoms of OSA are not highly specific, a wide differential diagnosis must be considered, including numerous causes of excessive daytime sleepiness and conditions, such as panic attacks, pulmonary disease, and gastroesophageal reflux disease, which can interfere with sleep (TABLE 1).8,13
What to include in the medical history and work-up
When you suspect OSA or another sleep disorder, the medical history should include a complete review of systems. The social history needs to include information regarding alcohol intake, use of sedatives, and recreational drugs. If possible, interview the patient’s bed partner, as well.
Examine upper airway anatomy. In performing a physical exam, pay special attention to blood pressure, BMI, and upper airway anatomy. Assess for conditions that can cause obstruction, such as nasal congestion, septal deviation, retrognathia (malocclusion of the mouth due to an abnormal posterior position of the maxilla or mandible), macroglossia (an unusually large tongue), dental malocclusion, enlarged lymphoid tissue and tonsils, large uvula, low hanging soft palate, and a large neck circumference (>40 cm for females and >43 cm for males).13
Order blood tests. Routine blood tests do not support an OSA diagnosis, but they help rule out or identify other conditions associated or mimicking OSA, such as anemia, hypothyroidism, diabetes, liver disease, and kidney disease. Suggested labs include a comprehensive metabolic profile, complete blood count, and thyroid-stimulating hormone test.
Sleep studies are needed for a definitive diagnosis
Conducted overnight in a sleep lab, polysomnography (PSG) uses respiratory effort, respiratory air flow, and peripheral oximetry to identify and quantify episodes of apnea and hypopnea (reported as the apnea-hypopnea index, or AHI). PSG also records brain electrical activity (electroencephalogram), heart rhythm (electrocardiogram), eye movement (electro-oculogram), and muscle activation (electromyogram)—studies used to identify other sleep disorders, such as restless leg syndrome, narcolepsy, parasomnias, and disturbances in rapid-eye movement
(REM) sleep.
Home monitoring. For patients who are unable or unwilling to undergo an overnight sleep study, home portable monitoring is a less expensive alternative. The monitor—a small wireless device—provides data for calculating the AHI and the presence and degree of oxygen desaturation.14 Unlike PSG, which can identify the amount of pressure needed for continuous positive airway pressure (CPAP) therapy, findings from a portable monitor are not sufficient to rule out other sleep disorders to determine whether CPAP is required.15
Evaluating daytime sleepiness. Several tools have been used to evaluate daytime sleepiness. The Epworth Sleepiness Scale (ESS), a quick 8-item screening questionnaire, determines the average person’s level of sleepiness during the day. It ranges from 0 to 24 points, with 10 being normal. Although the ESS has been used extensively in OSA research, recent studies found that it has a low sensitivity (54%) and specificity (57%) for scores >10 and does not correlate well with hypopnea and apnea measurements.16,17
Two additional tools, the Berlin and STOP questionnaires, can also be used to screen for OSA. Both questionnaires have about a 50% positive predictive value and a 70% negative predictive value.18,19
Diagnosing and classifying OSA
Diagnostic criteria developed by the American Academy of Sleep Medicine (AASM) are based on reported and observed symptoms and PSG recordings of hypopnea and apneic episodes. Of the 4 criteria (A through D), patients must meet either A, B, and D or C and D (TABLE 2).15
The AASM further classifies OSA as mild, moderate, or severe (TABLE 3)15 based on the AHI as well as on clinical findings, including oxygen desaturation and arrhythmias. Patients with severe OSA have excessive daytime sleepiness (EDS) that interferes with their normal activities, 15 as well as severe oxygen desaturation, moderate to severe cardiac arrhythmias, and significant risk for hypertension, MI, stroke, and cor pulmonale.
CASE 1 An examination of Mr. M’s upper airway anatomy reveals a neck circumference of 44 cm and normal oropharynx. The results of his lab tests were only significant for elevated blood sugar (234 mg/dL) and glycosylated hemoglobin (9.2%). Because he presents with classic symptoms of OSA, he receives a referral for PSG. He is found to have an AHI of 49, consistent with severe sleep apnea.
CASE 2 A system review of Ms. C finds no fever, nausea, vomiting, weakness, vision changes, or neurological symptoms. A Patient Health Questionnaire-9 (depression screen) is normal, as are her lab tests and a brain MRI with and without contrast. After an extensive work-up for headaches finds nothing, OSA is considered, in light of her daytime sleepiness—and she, too, is referred for PSG. This patient has moderate OSA, with an AHI of 27.
Initiating treatment: What’s best?
Ideally, treatment of OSA would reverse EDS and fatigue, restore full cognitive function, reduce the risk of accidents associated with OSA, and minimize its harmful cardiovascular and pulmonary effects. In fact, while OSA can be managed and its effects ameliorated, all available treatments have limitations and a cure remains elusive.
Let patients know that our understanding of OSA is limited, that treatment may not reverse or eliminate all the risks associated with this condition, and that compliance can be challenging. You can also tell them that, while more and better studies are needed, several modalities have been found to successfully treat OSA.
What to expect from lifestyle modification
Recommend lifestyle changes, such as weight loss, regular exercise early in the day, greater emphasis on sleep hygiene (eg, using the bed only for sleeping and sexual activity), and avoidance of sedating drugs and alcohol for patients with OSA.20,21
The beneficial effect of weight loss on OSA has been demonstrated in studies of both bariatric surgery and conventional weight loss therapies.21-23 While early studies of bariatric surgery were often limited by small size, ambiguous classification of OSA, and selection and follow-up biases, more recent trials show that while OSA symptoms frequently improve postoperatively, the disorder typically persists despite significant reductions in both BMI and AHI.24 Weight reduction should be strongly encouraged for obese patients, however, not only to improve OSA symptoms, but also to reduce the risk for other diseases.
Avoidance of alcohol. Alcohol has adverse effects on sleep: It shortens sleep latency, increases slow-wave sleep, suppresses REM and parasympathetic nerve activity,25 and can exacerbate OSA. Driving simulation studies have found that, compared with healthy individuals, those with untreated OSA are more susceptible to the effects of alcohol and at higher risk for accidents after just one drink.26
CPAP improves sleep, but some problems persist
CPAP supplies a flow of positive air pressure, adjusted to the level needed to keep the airway open, delivered through a facial device best suited to the patient’s anatomy, physiology, and comfort.
Multiple studies have demonstrated the effectiveness of CPAP in reducing symptoms of moderate to severe OSA, compared with placebo and other treatment modalities such as oral devices, surgical procedures, and medications. CPAP reduces AHI, blood pressure, and cardiac arrhythmias. It improves sleep efficiency, oxygen saturation, and self-reported sleep and well-being.27-30 While it ameliorates many of the harmful effects of OSA, it does not improve or reverse all of them. (See “Peripheral neuropathy linked to obstructive sleep apnea?”)
A Cochrane review of 36 randomized controlled trials with a combined total of more than 1700 patients demonstrated the superiority of CPAP vs control in several measures, such as subjective daytime sleepiness, quality of life, cognitive function, and blood pressure.28 On specific parameters of OSA, such as snoring and EDS, studies yielded mixed results.
CPAP has been found to decrease work-related injuries and morbidity and mortality associated with motor vehicle accidents linked to EDS.30-32 However, no study of CPAP has demonstrated a long-term reduction in morbidity and mortality. And no standards define the minimum number of sleep hours and/or frequency of CPAP use that is required to obtain specific benefits.
Patient compliance is poor. Part of the problem is that CPAP is difficult to use, which affects compliance. Poor patient compliance is a major barrier to evaluating its long-term benefits. (The video below, "CPAP Patients Tips from the FDA", can help ensure that patients use CPAP safely and effectively.) Studies estimate that 65% to 89% of patients with CPAP devices use them for at least 4 hours a night for 70% of nights, but that about half of those for whom CPAP is prescribed stop using it after 2 to 3 years.33-36 Several risk factors and comorbid conditions, including advanced age, diabetes, obesity, smoking, and especially, depression, are associated with decreased compliance.13
Improving CPAP compliance continues to be a challenge, highlighting the importance of treating not only OSA but all comorbidities, particularly depression. Short-term studies have found behavioral modification to be a promising means of improving CPAP compliance.33-36
Although not a first-line therapy, bilevel positive airway pressure (BiPAP), which delivers both inspiratory and expiratory pressure via a face device, can be tried in patients unable to tolerate CPAP.37 Studies are limited and it has been used in patients with complex OSA.
Oral appliances are a CPAP alternative
Mandibular repositioning devices (MRDs) and tongue-retaining devices are alternatives to CPAP.38 Although both types of oral appliance are beneficial, they are less effective than CPAP.28
MRDs, which are more commonly used than tongue-retaining devices, are available in several models. An MRD can be custom-made to hold the lower jaw in a forward position during sleep, enlarge the space behind the tongue, and put tension on the walls of the pharynx and the palate to reduce collapse.38
Tongue-retaining devices—splints that hold the tongue in place to keep the airway open—can be used for mild to moderate OSA, and for patients unable to tolerate CPAP.38,39
Studies comparing MRDs and tongue-retaining devices found no statistically significant difference in their ability to reduce AHI, but patients tolerated MRDs better.38,39 Nonetheless, both devices can cause dental discomfort, temporomandibular joint pain, dry mouth or excessive salivation, gum irritation, bruxism, and long-term occlusal changes.38
Is surgery an option?
Numerous surgical techniques are available for treating OSA, all aimed at relieving the obstruction by removing or bypassing it or increasing airway size. These include uvulopalatopharyngoplasty, which resects the uvula, retrolingual, and palatine tonsillar tissue; septoplasty; rhinoplasty; nasal turbinate reduction; nasal polypectomy palatal advancement pharyngoplasty; tonsillectomy; adenoidectomy; palatal implants; tongue reduction; genioglossus advancement; and maxillomandibular advancement.
The choice of modality depends on the patient’s anatomy and physiology, and is selected only after a full evaluation by a head and neck surgeon who specializes in surgical treatment of OSA.40,41 There is a paucity of reliable studies on the results of such procedures, but a Cochrane review of the existing literature concluded that surgery is only indicated for severe cases of sleep apnea in patients who have an anatomic obstruction.40
Too little evidence of medications’ efficacy
Numerous drugs have been tested for the treatment of OSA. Two Cochrane reviews looked at multiple trials of more than 20 drugs, in meta-analyses encompassing more than 500 patients.42,43 Ten drugs—eszopiclone, paroxetine, acetazolamide, ondansetron-fluoxetine combination, naltrexone, and fluticasone nasal spray among them—showed a statistically significant reduction in AHI, and a few showed a subjective benefit in daytime sleepiness. However, the studies were very small and of short duration and the reviewers concluded that evidence is insufficient to recommend drug therapy for OSA.
CASE 1 CPAP was prescribed for Mr. M. He tolerated it well and after 2 weeks, he reported feeling refreshed upon awakening, having less daytime somnolence and being better able to concentrate at work. His hypertension and glucose control improved, but he continues to struggle with his weight.
CASE 2 Ms. C started CPAP and within a week, her morning headaches and irritability resolved.
CORRESPONDENCE
Cecilia Gutierrez, MD, 200 West Arbor Drive, Mail Code 8809, San Diego, CA 92103; [email protected]
CASE 1 Mr. M, age 59, has diabetes, hypertension, hyperlipidemia, and obesity, with a body mass index (BMI) of 37 kg/m2. His hyperlipidemia is well controlled with atorvastatin. He also takes hydrochlorothiazide, lisinopril, metformin, and glyburide, but neither his hypertension nor his glucose levels are well controlled. Mr. M does not exercise, and acknowledges eating a high-calorie diet.
Mr. M reports that his wife has begun complaining about his loud snoring, and that he’s been falling asleep at work. He says he has 2 to 3 alcoholic drinks per week, and doesn’t smoke or take any other drugs.
CASE 2 Ms. C, age 35, is seeking treatment for early morning headaches that began several months ago. She describes a constant dull pain over the frontal area that occurs almost every day and typically resolves in 2 to 3 hours. The pain is not affected by postural changes, she says.
She also reports fatigue, daytime sleepiness, and anxiety. Ms. C has a normal BMI (24 kg/m2), takes no medication, and has no prior history of migraine or tension headaches. She denies any alcohol or recreational drug use.
If Mr. M and Ms. C were your patients, what would your next step be?
Obstructive sleep apnea (OSA) is characterized by repeated cycles of partial airway obstruction, hypoxemia, sympathetic discharge, and arousal to restore ventilation. In addition to fragmented sleep and daytime sleepiness, this common sleep disorder is associated with a decreased functional capacity1-3 and an increased risk for accidents, arrhythmias, myocardial infarction (MI), stroke, and death.4-6 In the Wisconsin Sleep Cohort, an 18-year follow-up study, the estimated hazard ratio for patients with severe OSA was 3.0 for all-cause mortality (95% CI; P-trend <.008) and 5.2 for cardiovascular death (95% CI; P=.003).7
The prevalence of OSA in the United States is 3% to 7% for males and 2% to 5% for females, with higher rates among those older than 65 years. But these figures apply only to those with an OSA diagnosis. An additional 5% of the general population is believed to have undiagnosed OSA.4,8
Arriving at an OSA diagnosis may not be difficult with a patient like Mr. M, who is obese and has classic symptoms. But it is important to consider OSA in patients who, like Ms. C, are not overweight and whose chief complaint appears to be unrelated to sleep.
This review—of risk factors, common (and uncommon) symptoms, diagnostic criteria, and treatment for OSA—highlights key indicators and optimal therapies.
The OSA profile: Risk factors and presenting symptoms
Obesity is perhaps the best-known risk factor for OSA: About 70% of patients with OSA are obese, and 40% of obese individuals have OSA. Approximately 60% of those with OSA have metabolic syndrome.9-12 But it is important to remember that individuals with normal or even low BMI may develop OSA, as well.
Other possible contributing factors include alcohol use, smoking, nasal congestion, menopause, polycystic ovary disease, and a genetic predisposition. And OSA may cause or worsen a wide range of conditions, including hypertension, congestive heart failure, atrial fibrillation, stroke, and nocturnal arrhythmias.8,13
Symptoms may be classic—or not
A bed partner’s complaint about a patient’s snoring, typically followed by arousal and gasping for air, is often the first indication of OSA. Loud snoring and daytime sleepiness are the most common symptoms. But not all patients present with these classic symptoms. Some may complain of irritability, difficulty concentrating, daily headaches, and nocturia.
Because the symptoms of OSA are not highly specific, a wide differential diagnosis must be considered, including numerous causes of excessive daytime sleepiness and conditions, such as panic attacks, pulmonary disease, and gastroesophageal reflux disease, which can interfere with sleep (TABLE 1).8,13
What to include in the medical history and work-up
When you suspect OSA or another sleep disorder, the medical history should include a complete review of systems. The social history needs to include information regarding alcohol intake, use of sedatives, and recreational drugs. If possible, interview the patient’s bed partner, as well.
Examine upper airway anatomy. In performing a physical exam, pay special attention to blood pressure, BMI, and upper airway anatomy. Assess for conditions that can cause obstruction, such as nasal congestion, septal deviation, retrognathia (malocclusion of the mouth due to an abnormal posterior position of the maxilla or mandible), macroglossia (an unusually large tongue), dental malocclusion, enlarged lymphoid tissue and tonsils, large uvula, low hanging soft palate, and a large neck circumference (>40 cm for females and >43 cm for males).13
Order blood tests. Routine blood tests do not support an OSA diagnosis, but they help rule out or identify other conditions associated or mimicking OSA, such as anemia, hypothyroidism, diabetes, liver disease, and kidney disease. Suggested labs include a comprehensive metabolic profile, complete blood count, and thyroid-stimulating hormone test.
Sleep studies are needed for a definitive diagnosis
Conducted overnight in a sleep lab, polysomnography (PSG) uses respiratory effort, respiratory air flow, and peripheral oximetry to identify and quantify episodes of apnea and hypopnea (reported as the apnea-hypopnea index, or AHI). PSG also records brain electrical activity (electroencephalogram), heart rhythm (electrocardiogram), eye movement (electro-oculogram), and muscle activation (electromyogram)—studies used to identify other sleep disorders, such as restless leg syndrome, narcolepsy, parasomnias, and disturbances in rapid-eye movement
(REM) sleep.
Home monitoring. For patients who are unable or unwilling to undergo an overnight sleep study, home portable monitoring is a less expensive alternative. The monitor—a small wireless device—provides data for calculating the AHI and the presence and degree of oxygen desaturation.14 Unlike PSG, which can identify the amount of pressure needed for continuous positive airway pressure (CPAP) therapy, findings from a portable monitor are not sufficient to rule out other sleep disorders to determine whether CPAP is required.15
Evaluating daytime sleepiness. Several tools have been used to evaluate daytime sleepiness. The Epworth Sleepiness Scale (ESS), a quick 8-item screening questionnaire, determines the average person’s level of sleepiness during the day. It ranges from 0 to 24 points, with 10 being normal. Although the ESS has been used extensively in OSA research, recent studies found that it has a low sensitivity (54%) and specificity (57%) for scores >10 and does not correlate well with hypopnea and apnea measurements.16,17
Two additional tools, the Berlin and STOP questionnaires, can also be used to screen for OSA. Both questionnaires have about a 50% positive predictive value and a 70% negative predictive value.18,19
Diagnosing and classifying OSA
Diagnostic criteria developed by the American Academy of Sleep Medicine (AASM) are based on reported and observed symptoms and PSG recordings of hypopnea and apneic episodes. Of the 4 criteria (A through D), patients must meet either A, B, and D or C and D (TABLE 2).15
The AASM further classifies OSA as mild, moderate, or severe (TABLE 3)15 based on the AHI as well as on clinical findings, including oxygen desaturation and arrhythmias. Patients with severe OSA have excessive daytime sleepiness (EDS) that interferes with their normal activities, 15 as well as severe oxygen desaturation, moderate to severe cardiac arrhythmias, and significant risk for hypertension, MI, stroke, and cor pulmonale.
CASE 1 An examination of Mr. M’s upper airway anatomy reveals a neck circumference of 44 cm and normal oropharynx. The results of his lab tests were only significant for elevated blood sugar (234 mg/dL) and glycosylated hemoglobin (9.2%). Because he presents with classic symptoms of OSA, he receives a referral for PSG. He is found to have an AHI of 49, consistent with severe sleep apnea.
CASE 2 A system review of Ms. C finds no fever, nausea, vomiting, weakness, vision changes, or neurological symptoms. A Patient Health Questionnaire-9 (depression screen) is normal, as are her lab tests and a brain MRI with and without contrast. After an extensive work-up for headaches finds nothing, OSA is considered, in light of her daytime sleepiness—and she, too, is referred for PSG. This patient has moderate OSA, with an AHI of 27.
Initiating treatment: What’s best?
Ideally, treatment of OSA would reverse EDS and fatigue, restore full cognitive function, reduce the risk of accidents associated with OSA, and minimize its harmful cardiovascular and pulmonary effects. In fact, while OSA can be managed and its effects ameliorated, all available treatments have limitations and a cure remains elusive.
Let patients know that our understanding of OSA is limited, that treatment may not reverse or eliminate all the risks associated with this condition, and that compliance can be challenging. You can also tell them that, while more and better studies are needed, several modalities have been found to successfully treat OSA.
What to expect from lifestyle modification
Recommend lifestyle changes, such as weight loss, regular exercise early in the day, greater emphasis on sleep hygiene (eg, using the bed only for sleeping and sexual activity), and avoidance of sedating drugs and alcohol for patients with OSA.20,21
The beneficial effect of weight loss on OSA has been demonstrated in studies of both bariatric surgery and conventional weight loss therapies.21-23 While early studies of bariatric surgery were often limited by small size, ambiguous classification of OSA, and selection and follow-up biases, more recent trials show that while OSA symptoms frequently improve postoperatively, the disorder typically persists despite significant reductions in both BMI and AHI.24 Weight reduction should be strongly encouraged for obese patients, however, not only to improve OSA symptoms, but also to reduce the risk for other diseases.
Avoidance of alcohol. Alcohol has adverse effects on sleep: It shortens sleep latency, increases slow-wave sleep, suppresses REM and parasympathetic nerve activity,25 and can exacerbate OSA. Driving simulation studies have found that, compared with healthy individuals, those with untreated OSA are more susceptible to the effects of alcohol and at higher risk for accidents after just one drink.26
CPAP improves sleep, but some problems persist
CPAP supplies a flow of positive air pressure, adjusted to the level needed to keep the airway open, delivered through a facial device best suited to the patient’s anatomy, physiology, and comfort.
Multiple studies have demonstrated the effectiveness of CPAP in reducing symptoms of moderate to severe OSA, compared with placebo and other treatment modalities such as oral devices, surgical procedures, and medications. CPAP reduces AHI, blood pressure, and cardiac arrhythmias. It improves sleep efficiency, oxygen saturation, and self-reported sleep and well-being.27-30 While it ameliorates many of the harmful effects of OSA, it does not improve or reverse all of them. (See “Peripheral neuropathy linked to obstructive sleep apnea?”)
A Cochrane review of 36 randomized controlled trials with a combined total of more than 1700 patients demonstrated the superiority of CPAP vs control in several measures, such as subjective daytime sleepiness, quality of life, cognitive function, and blood pressure.28 On specific parameters of OSA, such as snoring and EDS, studies yielded mixed results.
CPAP has been found to decrease work-related injuries and morbidity and mortality associated with motor vehicle accidents linked to EDS.30-32 However, no study of CPAP has demonstrated a long-term reduction in morbidity and mortality. And no standards define the minimum number of sleep hours and/or frequency of CPAP use that is required to obtain specific benefits.
Patient compliance is poor. Part of the problem is that CPAP is difficult to use, which affects compliance. Poor patient compliance is a major barrier to evaluating its long-term benefits. (The video below, "CPAP Patients Tips from the FDA", can help ensure that patients use CPAP safely and effectively.) Studies estimate that 65% to 89% of patients with CPAP devices use them for at least 4 hours a night for 70% of nights, but that about half of those for whom CPAP is prescribed stop using it after 2 to 3 years.33-36 Several risk factors and comorbid conditions, including advanced age, diabetes, obesity, smoking, and especially, depression, are associated with decreased compliance.13
Improving CPAP compliance continues to be a challenge, highlighting the importance of treating not only OSA but all comorbidities, particularly depression. Short-term studies have found behavioral modification to be a promising means of improving CPAP compliance.33-36
Although not a first-line therapy, bilevel positive airway pressure (BiPAP), which delivers both inspiratory and expiratory pressure via a face device, can be tried in patients unable to tolerate CPAP.37 Studies are limited and it has been used in patients with complex OSA.
Oral appliances are a CPAP alternative
Mandibular repositioning devices (MRDs) and tongue-retaining devices are alternatives to CPAP.38 Although both types of oral appliance are beneficial, they are less effective than CPAP.28
MRDs, which are more commonly used than tongue-retaining devices, are available in several models. An MRD can be custom-made to hold the lower jaw in a forward position during sleep, enlarge the space behind the tongue, and put tension on the walls of the pharynx and the palate to reduce collapse.38
Tongue-retaining devices—splints that hold the tongue in place to keep the airway open—can be used for mild to moderate OSA, and for patients unable to tolerate CPAP.38,39
Studies comparing MRDs and tongue-retaining devices found no statistically significant difference in their ability to reduce AHI, but patients tolerated MRDs better.38,39 Nonetheless, both devices can cause dental discomfort, temporomandibular joint pain, dry mouth or excessive salivation, gum irritation, bruxism, and long-term occlusal changes.38
Is surgery an option?
Numerous surgical techniques are available for treating OSA, all aimed at relieving the obstruction by removing or bypassing it or increasing airway size. These include uvulopalatopharyngoplasty, which resects the uvula, retrolingual, and palatine tonsillar tissue; septoplasty; rhinoplasty; nasal turbinate reduction; nasal polypectomy palatal advancement pharyngoplasty; tonsillectomy; adenoidectomy; palatal implants; tongue reduction; genioglossus advancement; and maxillomandibular advancement.
The choice of modality depends on the patient’s anatomy and physiology, and is selected only after a full evaluation by a head and neck surgeon who specializes in surgical treatment of OSA.40,41 There is a paucity of reliable studies on the results of such procedures, but a Cochrane review of the existing literature concluded that surgery is only indicated for severe cases of sleep apnea in patients who have an anatomic obstruction.40
Too little evidence of medications’ efficacy
Numerous drugs have been tested for the treatment of OSA. Two Cochrane reviews looked at multiple trials of more than 20 drugs, in meta-analyses encompassing more than 500 patients.42,43 Ten drugs—eszopiclone, paroxetine, acetazolamide, ondansetron-fluoxetine combination, naltrexone, and fluticasone nasal spray among them—showed a statistically significant reduction in AHI, and a few showed a subjective benefit in daytime sleepiness. However, the studies were very small and of short duration and the reviewers concluded that evidence is insufficient to recommend drug therapy for OSA.
CASE 1 CPAP was prescribed for Mr. M. He tolerated it well and after 2 weeks, he reported feeling refreshed upon awakening, having less daytime somnolence and being better able to concentrate at work. His hypertension and glucose control improved, but he continues to struggle with his weight.
CASE 2 Ms. C started CPAP and within a week, her morning headaches and irritability resolved.
CORRESPONDENCE
Cecilia Gutierrez, MD, 200 West Arbor Drive, Mail Code 8809, San Diego, CA 92103; [email protected]
1. Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112.
2. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199-204.
3. Satela MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249-259.
4. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
5. Yaggi HK, Concato J, Kernan W, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
6. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
7. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;3:1071-1078.
8. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
9. Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735-741.
10. Basta M, Vgontzas AN. Metabolic abnormalities in obesity and sleep apnea are in a continuum. Sleep Med. 2007;8:5-7.
11. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-3021.
12. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467-472.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013-2016.
14. Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-base diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257-263.
15. Iber C, Ancoli-Israel S, Chesson AL Jr, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
16. Johns MW. Sensitivity and specificity of the multiple sleep maintenance tests, the maintenance of wakefulness tests and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5-11.
17. Hesselbacher S, Subramanian S, Allen J, et al. Body mass index, gender, and ethnic variations alter the clinical implication of the Epworth sleepiness scale in patients with suspected of obstructive sleep apnea. Open Respir Med J. 2012;6:2020-2027.
18. Ahmadi N, Chung S, Gibbs A, et al. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:38-45.
19. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
20. Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 2001;(1):CD002875.
21. Barvaux VA, Aubert G, Rodenstein DO. Weight loss as treatment for obstructive sleep apnea. Sleep Med Rev. 2000;4:435-452.
22. Buchwald H, Avidor H, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
23. Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs. conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142-1149.
24. Greenburg A, Lettieri C, Arn E. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542.
25. Sagawa Y, Kondo H, Matsubuchi N. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35:2093-2100.
26. Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447-455.
27. Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565-571.
28. Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3):CD001106.
29. Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25:1070-1076.
30. Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430-434.
31. Findley L, Smith C, Hooper J, et al. Treatment with nasal CPAP decreases automobile accidents in patient with sleep apnea. Am J Respir Crit Care Med. 2000;161:857-859.
32. Barbe F, Sunyer J, de la Pena A, et al. Effects of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74:44-49.
33. Sin DD, Mayers I, Man GC, et al. Long Term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population based study. Chest. 2002;121:430-435.
34. Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart. 2005;91:1265-1270.
35. Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 2006;7:553-560.
36. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea-hypopnea syndrome (SAHS). Sleep Med. 2003;7:81-99.
37. Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath. 2012;16:773-739.
38. Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693-699.
39. Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240-243.
40. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;(4):CD001004.
41. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407.
42. Smith I, Lasserson TJ, Wright JJ. Drug therapy for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006;():CD003002. Review.
43. Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;(5): CD003002.
1. Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112.
2. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199-204.
3. Satela MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249-259.
4. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
5. Yaggi HK, Concato J, Kernan W, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
6. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
7. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;3:1071-1078.
8. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
9. Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735-741.
10. Basta M, Vgontzas AN. Metabolic abnormalities in obesity and sleep apnea are in a continuum. Sleep Med. 2007;8:5-7.
11. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-3021.
12. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467-472.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013-2016.
14. Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-base diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257-263.
15. Iber C, Ancoli-Israel S, Chesson AL Jr, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
16. Johns MW. Sensitivity and specificity of the multiple sleep maintenance tests, the maintenance of wakefulness tests and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5-11.
17. Hesselbacher S, Subramanian S, Allen J, et al. Body mass index, gender, and ethnic variations alter the clinical implication of the Epworth sleepiness scale in patients with suspected of obstructive sleep apnea. Open Respir Med J. 2012;6:2020-2027.
18. Ahmadi N, Chung S, Gibbs A, et al. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:38-45.
19. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
20. Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 2001;(1):CD002875.
21. Barvaux VA, Aubert G, Rodenstein DO. Weight loss as treatment for obstructive sleep apnea. Sleep Med Rev. 2000;4:435-452.
22. Buchwald H, Avidor H, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
23. Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs. conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142-1149.
24. Greenburg A, Lettieri C, Arn E. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542.
25. Sagawa Y, Kondo H, Matsubuchi N. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35:2093-2100.
26. Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447-455.
27. Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565-571.
28. Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3):CD001106.
29. Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25:1070-1076.
30. Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430-434.
31. Findley L, Smith C, Hooper J, et al. Treatment with nasal CPAP decreases automobile accidents in patient with sleep apnea. Am J Respir Crit Care Med. 2000;161:857-859.
32. Barbe F, Sunyer J, de la Pena A, et al. Effects of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74:44-49.
33. Sin DD, Mayers I, Man GC, et al. Long Term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population based study. Chest. 2002;121:430-435.
34. Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart. 2005;91:1265-1270.
35. Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 2006;7:553-560.
36. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea-hypopnea syndrome (SAHS). Sleep Med. 2003;7:81-99.
37. Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath. 2012;16:773-739.
38. Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693-699.
39. Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240-243.
40. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;(4):CD001004.
41. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407.
42. Smith I, Lasserson TJ, Wright JJ. Drug therapy for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006;():CD003002. Review.
43. Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;(5): CD003002.