User login
Biomedical engineering in heart-brain medicine: A review
Biomedical engineering is a rapidly growing field, as indicated by a recent threefold increase in the number of students enrolled in the more than 80 programs granting biomedical engineering degrees.1 The field is known primarily for contributing to the development of devices that aid diagnosis (such as chemical sensors and medical imagers) or help to restore lost function (such as pacemakers, cochlear implants, and artificial limbs). At the same time, biomedical engineering has also contributed to the understanding of physiology and is now a participant in the more recent molecular- and cellular-based discoveries and their potential clinical applications.
This article reviews the contributions of biomedical engineering to heart-brain medicine and looks ahead to where its future contributions may be expected. The focus is on the autonomic control of the heart, with special emphasis on stimulating or blocking the activity of peripheral nerves for therapeutic purposes.
AUTONOMIC MECHANISMS
One way biomedical engineering has contributed to heart-brain medicine is through systems physiology, attempting to characterize complex cardiac control mechanisms by mathematical models ranging from the relatively simple to the very complex.2 In particular, investigation of the effect of the baroreceptor reflex (baroreflex) on heart rate led to recording of efferent vagal activity, demonstrating that the respiratory variations in heart rate are attributable to complete stoppage of vagal efferent activity, at least in anesthetized dogs.3 The results suggested that respiratory sinus arrhythmia was a measure of parasympathetic cardiac control.4 Extensive further work investigated heart rate variability in humans, resulting in the generally accepted concept that rapid variations in heart rate are primarily due to the parasympathetic nervous system, while slower variations are primarily due to sympathetic contributions. 5 It has been amply demonstrated that in a variety disease states, a high degree of parasympathetic control is correlated with improved outcome.6,7
Heart rate variability: Much is still unknown
Because heart rate variability is derived through analysis of easily recorded single-lead electrocardiograms (ECGs), techniques are still being described for determining the degree of variability.8–10 Measurements may be based on heart rate or heart period (interbeat interval); they may be made during spontaneous, deep, or timed periodic breathing; and the recordings may last for a few minutes or for 24 hours. The results are rarely reported for different states, yet variability depends on whether the subjects are awake, in quiet sleep, or in REM sleep.11
The present incomplete understanding of the physiological basis and clinical significance of heart rate variations makes it difficult to judge what the optimum measurement is. For example, recent publications have still debated whether the respiratory variations are primarily of central origin or a result of the baroreflex.12,13 The reviewed evidence leaves little doubt that most respiratory variations are caused by vagal modulation induced by breathing, independently of the baroreflex. On the other hand, breathing affects blood pressure and thus must have some influence on heart rate through the baroreflex as well. These effects are likely to be important when considering low-frequency variations.14 It also has been suggested that the branch of the vagus that contributes to the slow variations may be different from the one responsible for the rapid changes.15
A role for mathematical modeling
Untangling the above relationships requires at least an approximate physiologically based quantitative model of the entire system. What is the “entire system”? It includes all components that significantly affect the clinical condition or physiological/biological phenomenon studied. Developing such models is challenging since they can be misleading if they are too simple. However, if they are too complex, they may obscure rather than illuminate. Even though mathematical modeling has been a major component of biomedical engineering for decades, there is still great need for physiological and clinical studies that are aided by biomedical engineers with mathematical skills and a discerning eye toward the life sciences.
For example, following numerous previous efforts to develop models of cardiorespiratory control systems, a model has been developed to describe changes in heart rate, stroke volume, and blood pressure caused by disordered breathing during sleep.16 The model was initially tested in animals but recently has been evaluated in children with obstructive sleep apnea.17 It was found to be more effective in identifying and characterizing cardiac control abnormalities than was spectrum-derived variability of heart rate or blood pressure alone.
To enhance the usefulness of heart rate variability as a clinical tool, it is necessary to go beyond observing that decreased high-frequency heart rate variations are ominous in a particular disease state because there is an “imbalance” of autonomic control. This gives the physician little guidance as to what to do. Should he or she treat the brain to get more parasympathetic outflow? Should attention be concentrated on making the heart more tolerant to the imbalance? Or should both approaches be tried?
In addition to the building of models, biomedical engineers can also contribute to heart-brain medicine by developing sensors that can measure appropriate physiological variables in animal experiments—and eventually in humans. Such variables include neural activity and chemical signals controlling the heart, as well as neural and chemical signals that arise from the heart and are sensed by the brain. These variables must be included in any model for a comprehensive characterization of heart-brain interactions.
NEURAL INTERVENTIONS
A second and complementary way in which bioengineering can contribute to heart-brain medicine is through the development and evaluation of technology that applies selective electrical, chemical, or mechanical stimulation to the physiological system. External interventions may have therapeutic effects even though the underlying physiological mechanisms are not fully understood. For example, deep brain electrical stimulation is increasingly used or explored to treat epilepsy, Parkinson disease, and depression.18,19 The development of technology to deliver drugs locally is advancing rapidly and is almost certain to play a major role in exploring heart-brain interactions. The remainder of this paper concentrates on interventions applied through peripheral nerves.
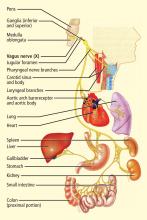
Vagal stimulation
Vagal stimulation is being used to treat epilepsy, but now it is also being explored for the treatment of drug-resistant depression and chronic migraine.20,21 The intensity, frequency, pulse width, and train duration of the apparently single-channel stimulation are set telemetrically. Preliminary data indicate a long-term success rate of about 20% for depression20 and improvement in 2 of 4 patients with chronic migraine.21 Side effects include discomfort caused by the electrical stimulation and vocalization impairments.
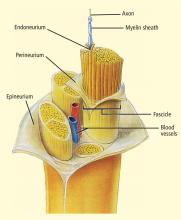
The selective stimulation of nerves has received much attention in rehabilitation engineering since electrical activation of peripheral motor fibers can restore at least some function in patents with spinal cord injury. The stimulation must be selective: different muscles to restore movement need to contract in the appropriate sequence and with appropriate intensity. Thus, fascicles of motor nerves innervating these muscles must be stimulated accordingly.
Electrodes have been developed in a variety of configurations for selectively activating the desired fibers in a nerve bundle.22 Multiple electrodes around the nerve allow targeting of the desired fascicles and preferential stimulation of small nerve fibers.23,24 Gently compressing the nerve using a flat electrode sleeve enhances selectivity by increasing the surface area of the stimulated nerve.25 A tripolar sleeve electrode along the nerve (one cathode at the middle and an anode on each side) may be used to preferentially stimulate small fibers by “anodal blockade” of the propagation of activity in large fibers.26 Mathematical models of nerve excitation, combined with models of the tissue surrounding the nerve fibers, show that positioning the anodes at different distances from the cathode can generate unidirectional propagation.27 Mathematical analysis also shows that the stimulating pulse should have a slowly decaying trailing edge to assure effectiveness of the blockade.27,28
Similar technologies are likely necessary for stimulating the vagus for specific purposes. If the goal is to induce central effects, appropriate afferents should be stimulated. If the goal is to influence the heart directly, cardiac vagal efferents need to be stimulated without confounding the effect by also stimulating afferents. Since cardiac efferent fibers are small and constitute only a small portion of vagal trunk,3 their stimulation requires special care to reverse the normal “largest first” recruitment of stimulated fibers.
The recently reported first pilot study of vagal stimulation in heart failure patients used an electrode that seems to partially satisfy such requirements.29,30 Although the details appear to be proprietary, the implantable electrical stimulator has multiple electrodes and induces anodal blockade to preferentially stimulate efferent rather than afferent nerve fibers.
In the pilot study, 8 patients received intermittent vagal stimulation (2 to 10 sec “on” and 6 to 30 sec “off”) of the right cervical vagus using a pulse delivered 70 msec after each R wave of the ECG. The stimulating current, limited by a threshold or the onset of side effects, was adjusted to achieve a heart rate reduction of 5 to 10 beats/min. Patients were evaluated up to 6 months; no permanent side effects were reported. There was a modest improvement of cardiac function as judged by a reduction in left ventricular volumes, as well as a clear improvement in a measure of quality of life. The study shows feasibility and suggests further investigations.
The primary task seems to be optimization of stimulation parameters. Since natural vagal impulses are distributed through the cardiac cycle,3 artificial stimulation might be more effective if it emulated the natural firing pattern. Since long-term heart rate reduction was minimal, parameters might be tuned further to stimulate small cardiac efferent fibers that may be far from the electrodes. Measurements of heart rate variability during controlled conditions may reveal the pacemaker’s responsiveness to vagal stimulation. Comparison of duty cycles (duration of “on” and “off”) may show whether the study’s choice, to some extent already mimicking the breathing-induced modulation of natural vagal activity, is most effective. Such studies to optimize effectiveness may be best performed in chronically instrumented animals.
Baroreceptor stimulation
An extensively studied mechanism that modulates the autonomic nervous system is the baroreceptor reflex that is known to be depressed in heart failure. Former efforts to use this reflex therapeutically were recently revived in both animal experiments and human studies.31 For example, bilateral carotid sinus stimulation nearly doubled the survival time of dogs with pacing-induced heart failure.32 Although measures of left ventricular function, obtained while the stimulator was turned off, were similar in dogs with stimulated and unstimulated carotid sinuses, plasma norepinephrine was lower in the animals receiving stimulation. This suggests that carotid sinus stimulation led to a general decrease in sympathetic activation. It is noteworthy that the stimulation was applied intermittently (9 min “on”, 1 min “off”) to avoid the resetting (or adaptation) of baroreceptors, a phenomenon that had led to the now-doubted traditional belief that the baroreflex regulates only acute rather than long-term changes of blood pressure.2
Chronic bilateral baroreceptor stimulation was also applied in 21 patients with essential hypertension that could not be controlled by medication.33 Measurements were taken 1 month after implantation with the stimulator turned off and 3 months after chronic stimulation with the stimulator on. Stimulation moderately reduced both blood pressure and heart rate; heart rate variability suggested an increase in parasympathetic activity and a decrease in sympathetic activity.
Renal sympathetic ablation
Technology-based approaches encompass not only the stimulation of nerves but also the abolition of nerve activity. In an exploratory study, bilateral sympathetic denervation was performed in 45 patients with drugresistant hypertension.34 A catheter, introduced through the femoral artery, was positioned at the entrance of each renal artery. Sympathetic denervation was produced by radiofrequeny energy applied for a maximum of 2 minutes. The details of the technology appear proprietary, complete ablation could not be ascertained, and both efferents and afferents are likely to have been stimulated. Nevertheless, the procedure appeared safe and resulted in a significant reduction in both diastolic and systolic pressures over 12 months. In addition to lowering blood pressure, catheter-based sympathetic denervation might also prove therapeutic in heart failure and chronic kidney disease.
AUTOMATED CONTROL OF PHYSIOLOGIC VARIABLES
These initial experiences with baroreceptor stimulation, together with the results obtained by stimulating the vagus and ablating renal sympathetic nerves, indicate that device-based approaches may be useful additions to the treatment of drug-resistant hypertension and heart failure. The incorporation of control features that automatically respond to the changes in physiological states are natural extensions.35 As a recent example, in anesthetized dogs with heart failure and paced heart, systemic arterial pressure, cardiac output, and left atrial pressure were automatically regulated at set levels by a model-based infusion of nitroprusside, dobutamine, and volume expanders.36 When the heart rate was reduced, cardiac energetics (based on a reduction in left ventricular oxygen consumption) improved while the hemodynamic variables remained constant.
CONCLUSIONS
Biomedical engineering played a major role in linking measures of heart rate variation to sympathetic and parasympathetic contributions to cardiac control, as well as in demonstrating that the balance of control was correlated with a variety of disease states of heart and brain. However, much work remains to be done to fully realize the field’s potential for aiding clinicians in preventing or treating diseases. Further studies, some to be performed in chronically prepared animals, are needed to quantitatively characterize the many interacting mechanisms that determine cardiac function. Such studies would benefit from recording naturally occurring neural activity to and from the brain, and are already starting to benefit from the artificial electrical stimulation of nerves in both experimental animals and preliminary trials in patients.
The appropriate use of mathematical modeling is an essential tool for gaining in-depth understanding of physiological function. Mathematical modeling is also essential for developing stimulators that selectively stimulate those nerves that control the function to be influenced. The determination of stimulation parameters, based on understanding rather than on trial and error, is highly desirable. The use of “intelligent” stimulators, automatically controlled by appropriate physiological measurements, is an ambitious but achievable goal for improving human health.
- Katona PG. Biomedical engineering and The Whitaker Foundation: a thirty-year partnership. Ann Biomed Eng 2006; 34:904–916.
- Guyton AC, Coleman TG, Cowley AW, et al Systems analysis of arterial pressure regulation and hypertension. Ann Biomed Eng 1972; 1:254–281.
- Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol 1979; 218:1030–1037.
- Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol 1975; 39:801–805.
- Akselrod S, Gordon D, Ubel FA, et al Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 1981; 213:220–222.
- Carney RM, Freedland KE. Depression and heart rate variability in patients with coronary heart disease. Cleve Clin J Med 2009; 76( suppl 2):S13–S17.
- Lauer MS. Autonomic function and prognosis. Cleve Clin J Med 2009; 76( suppl 2):S18–S22.
- Cnockaert L, Migeotte P-F, Daubigny L, et al A method for the analysis of respiratory sinus arrhythmia using continuous wavelet transforms. IEEE Trans Biomed Eng 2008; 55:1640–1642.
- Castiglioni P, Parati G, Civijian A, et al Local scale exponents of blood pressure and heart rate variability by detrended fluctuation analysis: effects of posture, exercise, and aging. IEEE Trans Biomed Eng 2009; 56:675–684.
- Chen Z, Brown EN, Barbieri R. Assessment of autonomic control and respiratory sinus arrhythmia using point process models of human heart beat dynamics. IEEE Trans Biomed Eng 2009; 56:1791–1802.
- Schmitt DT, Stein PK, Ivanov PC. Stratification pattern of static and scale-invariant dynamic measures of heartbeat fluctuations across sleep stages in young and elderly. IEEE Trans Biomed Eng 2009; 56:1564–1573.
- Eckberg DL. Point:counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 2009; 16:1740–1742.
- Karemaker JM. Counterpoint: respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 2009; 106:1742–1743.
- Moak JP, Goldstein DS, Eldadah BA, et al Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm 2007; 4:1523–1529.
- Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med 2009; 76( suppl 2):S86–S90.
- Khoo MC. Modeling of autonomic control in sleep-disordered breathing. Cardiovasc Eng 2008; 8:30–41.
- Chaicharn J, Kin Z, Chen ML, et al Model-based assessment of cardiovascular autonomic control in children with obstructive sleep apnea. Sleep 2009; 32:927–938.
- Vitek JL. Deep brain stimulation: how does it work? Cleve Clin J Med 2008; 75( suppl 2):S59–S65.
- Lee KH, Blaha CD, Garrris PA. Evolution of deep brain stimulation: human electrometer and smart devices supporting the next generation of therapy. Neuromodulation: Technol Neural Interface 2009; 12:85–103.
- Fitzgerald PB, Daskalaris ZJ. The use of repetitive transcranial magnetic stimulation and vagal nerve stimulation in the treatment of depression. Curr Opin Psych 2008; 21:25–29.
- Cecchini AP, Mea E, Tullo V, et al Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci 2009; 30( suppl 1):S101–S104.
- Grill WM, Norman SE, Bellamkonda RV. Implanted neural interfaces: biochallenges and engineered solutions. Annu Rev Biomed Eng 2009; 11:1–24.
- Tarler MD, Mortimer JT. Selective and independent activation of four motor fascicles using a four-contact nerve-cuff electrode. IEEE Trans Neural Syst Rehab Eng 2004; 12:251–257.
- Lertmanorat Z, Gustafson KJ, Durand DM. Electrode array for reversing the recruitment order of peripheral nerve stimulation: experimental studies. Ann Biomed Eng 2006; 34:152–160.
- Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehab Eng 2002; 10:294–303.
- Fang ZP, Mortimer JT. Selective activation of small motor axons by quasitrapezoidal current pulses. IEEE Trans Biomed Eng 1991; 38:168–174.
- Rijkoff NJM, Holsheimer J, Koledwijn EL, et al Selective stimulation of sacral nerve roots for bladder control: a study in computer modeling. IEEE Trans Biomed Eng 1994; 41:413–424.
- Tosato M, Yoshida K, Toft E, et al Quazi-trapezoidal pulses to selectively block the activation of intrinsic laryngeal muscles during vagal nerve stimulation. J Neural Eng 2007; 41:205–212.
- Schwartz PK, De Ferrari GM, Sanzo A, et al Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Failure 2008; 10:884–891.
- De Ferrari GM, Sanzo A, Borggrefe M, et al Chronic vagus nerve stimulation in patients with chronic heart failure is feasible and appears beneficial. Circulation 2008; 118( suppl):S721. Abstract 2412.
- Durand MT, Fazan R, Salgado MCO, et al Acute and chronic electrical activation of baroreceptor afferents in awake and anesthetized subjects. Braz J Med Biol Res 2009; 42:53–60.
- Zucker IH, Hackley JF, Cornish KG, et al Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension 2007; 50:904–910.
- Wustman K, Kucera JP, Scheffers I, et al Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension 2009; 54:530–536.
- Krum H, Schlaih M, Whitbourn R, et al Catheter-based renal denervation for resistant hypertension: a multicentre safety and proof-of-principle study. Lancet 2009; 373:1228–1239.
- Kawada T, Sugamichi M. Artifical neural interfaces for bionic cardio vascular treatments. J Artific Organs 2009; 12:17–22.
- Uemura K, Sunagawa K, Sugimachi M. Computationally managed bradycardia improved cardiac energetics while restoring normal hemodynamics in heart failure. Ann Biomed Eng 2009; 37:82–93.
Biomedical engineering is a rapidly growing field, as indicated by a recent threefold increase in the number of students enrolled in the more than 80 programs granting biomedical engineering degrees.1 The field is known primarily for contributing to the development of devices that aid diagnosis (such as chemical sensors and medical imagers) or help to restore lost function (such as pacemakers, cochlear implants, and artificial limbs). At the same time, biomedical engineering has also contributed to the understanding of physiology and is now a participant in the more recent molecular- and cellular-based discoveries and their potential clinical applications.
This article reviews the contributions of biomedical engineering to heart-brain medicine and looks ahead to where its future contributions may be expected. The focus is on the autonomic control of the heart, with special emphasis on stimulating or blocking the activity of peripheral nerves for therapeutic purposes.
AUTONOMIC MECHANISMS
One way biomedical engineering has contributed to heart-brain medicine is through systems physiology, attempting to characterize complex cardiac control mechanisms by mathematical models ranging from the relatively simple to the very complex.2 In particular, investigation of the effect of the baroreceptor reflex (baroreflex) on heart rate led to recording of efferent vagal activity, demonstrating that the respiratory variations in heart rate are attributable to complete stoppage of vagal efferent activity, at least in anesthetized dogs.3 The results suggested that respiratory sinus arrhythmia was a measure of parasympathetic cardiac control.4 Extensive further work investigated heart rate variability in humans, resulting in the generally accepted concept that rapid variations in heart rate are primarily due to the parasympathetic nervous system, while slower variations are primarily due to sympathetic contributions. 5 It has been amply demonstrated that in a variety disease states, a high degree of parasympathetic control is correlated with improved outcome.6,7
Heart rate variability: Much is still unknown
Because heart rate variability is derived through analysis of easily recorded single-lead electrocardiograms (ECGs), techniques are still being described for determining the degree of variability.8–10 Measurements may be based on heart rate or heart period (interbeat interval); they may be made during spontaneous, deep, or timed periodic breathing; and the recordings may last for a few minutes or for 24 hours. The results are rarely reported for different states, yet variability depends on whether the subjects are awake, in quiet sleep, or in REM sleep.11
The present incomplete understanding of the physiological basis and clinical significance of heart rate variations makes it difficult to judge what the optimum measurement is. For example, recent publications have still debated whether the respiratory variations are primarily of central origin or a result of the baroreflex.12,13 The reviewed evidence leaves little doubt that most respiratory variations are caused by vagal modulation induced by breathing, independently of the baroreflex. On the other hand, breathing affects blood pressure and thus must have some influence on heart rate through the baroreflex as well. These effects are likely to be important when considering low-frequency variations.14 It also has been suggested that the branch of the vagus that contributes to the slow variations may be different from the one responsible for the rapid changes.15
A role for mathematical modeling
Untangling the above relationships requires at least an approximate physiologically based quantitative model of the entire system. What is the “entire system”? It includes all components that significantly affect the clinical condition or physiological/biological phenomenon studied. Developing such models is challenging since they can be misleading if they are too simple. However, if they are too complex, they may obscure rather than illuminate. Even though mathematical modeling has been a major component of biomedical engineering for decades, there is still great need for physiological and clinical studies that are aided by biomedical engineers with mathematical skills and a discerning eye toward the life sciences.
For example, following numerous previous efforts to develop models of cardiorespiratory control systems, a model has been developed to describe changes in heart rate, stroke volume, and blood pressure caused by disordered breathing during sleep.16 The model was initially tested in animals but recently has been evaluated in children with obstructive sleep apnea.17 It was found to be more effective in identifying and characterizing cardiac control abnormalities than was spectrum-derived variability of heart rate or blood pressure alone.
To enhance the usefulness of heart rate variability as a clinical tool, it is necessary to go beyond observing that decreased high-frequency heart rate variations are ominous in a particular disease state because there is an “imbalance” of autonomic control. This gives the physician little guidance as to what to do. Should he or she treat the brain to get more parasympathetic outflow? Should attention be concentrated on making the heart more tolerant to the imbalance? Or should both approaches be tried?
In addition to the building of models, biomedical engineers can also contribute to heart-brain medicine by developing sensors that can measure appropriate physiological variables in animal experiments—and eventually in humans. Such variables include neural activity and chemical signals controlling the heart, as well as neural and chemical signals that arise from the heart and are sensed by the brain. These variables must be included in any model for a comprehensive characterization of heart-brain interactions.
NEURAL INTERVENTIONS
A second and complementary way in which bioengineering can contribute to heart-brain medicine is through the development and evaluation of technology that applies selective electrical, chemical, or mechanical stimulation to the physiological system. External interventions may have therapeutic effects even though the underlying physiological mechanisms are not fully understood. For example, deep brain electrical stimulation is increasingly used or explored to treat epilepsy, Parkinson disease, and depression.18,19 The development of technology to deliver drugs locally is advancing rapidly and is almost certain to play a major role in exploring heart-brain interactions. The remainder of this paper concentrates on interventions applied through peripheral nerves.
Vagal stimulation
Vagal stimulation is being used to treat epilepsy, but now it is also being explored for the treatment of drug-resistant depression and chronic migraine.20,21 The intensity, frequency, pulse width, and train duration of the apparently single-channel stimulation are set telemetrically. Preliminary data indicate a long-term success rate of about 20% for depression20 and improvement in 2 of 4 patients with chronic migraine.21 Side effects include discomfort caused by the electrical stimulation and vocalization impairments.
The selective stimulation of nerves has received much attention in rehabilitation engineering since electrical activation of peripheral motor fibers can restore at least some function in patents with spinal cord injury. The stimulation must be selective: different muscles to restore movement need to contract in the appropriate sequence and with appropriate intensity. Thus, fascicles of motor nerves innervating these muscles must be stimulated accordingly.
Electrodes have been developed in a variety of configurations for selectively activating the desired fibers in a nerve bundle.22 Multiple electrodes around the nerve allow targeting of the desired fascicles and preferential stimulation of small nerve fibers.23,24 Gently compressing the nerve using a flat electrode sleeve enhances selectivity by increasing the surface area of the stimulated nerve.25 A tripolar sleeve electrode along the nerve (one cathode at the middle and an anode on each side) may be used to preferentially stimulate small fibers by “anodal blockade” of the propagation of activity in large fibers.26 Mathematical models of nerve excitation, combined with models of the tissue surrounding the nerve fibers, show that positioning the anodes at different distances from the cathode can generate unidirectional propagation.27 Mathematical analysis also shows that the stimulating pulse should have a slowly decaying trailing edge to assure effectiveness of the blockade.27,28
Similar technologies are likely necessary for stimulating the vagus for specific purposes. If the goal is to induce central effects, appropriate afferents should be stimulated. If the goal is to influence the heart directly, cardiac vagal efferents need to be stimulated without confounding the effect by also stimulating afferents. Since cardiac efferent fibers are small and constitute only a small portion of vagal trunk,3 their stimulation requires special care to reverse the normal “largest first” recruitment of stimulated fibers.
The recently reported first pilot study of vagal stimulation in heart failure patients used an electrode that seems to partially satisfy such requirements.29,30 Although the details appear to be proprietary, the implantable electrical stimulator has multiple electrodes and induces anodal blockade to preferentially stimulate efferent rather than afferent nerve fibers.
In the pilot study, 8 patients received intermittent vagal stimulation (2 to 10 sec “on” and 6 to 30 sec “off”) of the right cervical vagus using a pulse delivered 70 msec after each R wave of the ECG. The stimulating current, limited by a threshold or the onset of side effects, was adjusted to achieve a heart rate reduction of 5 to 10 beats/min. Patients were evaluated up to 6 months; no permanent side effects were reported. There was a modest improvement of cardiac function as judged by a reduction in left ventricular volumes, as well as a clear improvement in a measure of quality of life. The study shows feasibility and suggests further investigations.
The primary task seems to be optimization of stimulation parameters. Since natural vagal impulses are distributed through the cardiac cycle,3 artificial stimulation might be more effective if it emulated the natural firing pattern. Since long-term heart rate reduction was minimal, parameters might be tuned further to stimulate small cardiac efferent fibers that may be far from the electrodes. Measurements of heart rate variability during controlled conditions may reveal the pacemaker’s responsiveness to vagal stimulation. Comparison of duty cycles (duration of “on” and “off”) may show whether the study’s choice, to some extent already mimicking the breathing-induced modulation of natural vagal activity, is most effective. Such studies to optimize effectiveness may be best performed in chronically instrumented animals.
Baroreceptor stimulation
An extensively studied mechanism that modulates the autonomic nervous system is the baroreceptor reflex that is known to be depressed in heart failure. Former efforts to use this reflex therapeutically were recently revived in both animal experiments and human studies.31 For example, bilateral carotid sinus stimulation nearly doubled the survival time of dogs with pacing-induced heart failure.32 Although measures of left ventricular function, obtained while the stimulator was turned off, were similar in dogs with stimulated and unstimulated carotid sinuses, plasma norepinephrine was lower in the animals receiving stimulation. This suggests that carotid sinus stimulation led to a general decrease in sympathetic activation. It is noteworthy that the stimulation was applied intermittently (9 min “on”, 1 min “off”) to avoid the resetting (or adaptation) of baroreceptors, a phenomenon that had led to the now-doubted traditional belief that the baroreflex regulates only acute rather than long-term changes of blood pressure.2
Chronic bilateral baroreceptor stimulation was also applied in 21 patients with essential hypertension that could not be controlled by medication.33 Measurements were taken 1 month after implantation with the stimulator turned off and 3 months after chronic stimulation with the stimulator on. Stimulation moderately reduced both blood pressure and heart rate; heart rate variability suggested an increase in parasympathetic activity and a decrease in sympathetic activity.
Renal sympathetic ablation
Technology-based approaches encompass not only the stimulation of nerves but also the abolition of nerve activity. In an exploratory study, bilateral sympathetic denervation was performed in 45 patients with drugresistant hypertension.34 A catheter, introduced through the femoral artery, was positioned at the entrance of each renal artery. Sympathetic denervation was produced by radiofrequeny energy applied for a maximum of 2 minutes. The details of the technology appear proprietary, complete ablation could not be ascertained, and both efferents and afferents are likely to have been stimulated. Nevertheless, the procedure appeared safe and resulted in a significant reduction in both diastolic and systolic pressures over 12 months. In addition to lowering blood pressure, catheter-based sympathetic denervation might also prove therapeutic in heart failure and chronic kidney disease.
AUTOMATED CONTROL OF PHYSIOLOGIC VARIABLES
These initial experiences with baroreceptor stimulation, together with the results obtained by stimulating the vagus and ablating renal sympathetic nerves, indicate that device-based approaches may be useful additions to the treatment of drug-resistant hypertension and heart failure. The incorporation of control features that automatically respond to the changes in physiological states are natural extensions.35 As a recent example, in anesthetized dogs with heart failure and paced heart, systemic arterial pressure, cardiac output, and left atrial pressure were automatically regulated at set levels by a model-based infusion of nitroprusside, dobutamine, and volume expanders.36 When the heart rate was reduced, cardiac energetics (based on a reduction in left ventricular oxygen consumption) improved while the hemodynamic variables remained constant.
CONCLUSIONS
Biomedical engineering played a major role in linking measures of heart rate variation to sympathetic and parasympathetic contributions to cardiac control, as well as in demonstrating that the balance of control was correlated with a variety of disease states of heart and brain. However, much work remains to be done to fully realize the field’s potential for aiding clinicians in preventing or treating diseases. Further studies, some to be performed in chronically prepared animals, are needed to quantitatively characterize the many interacting mechanisms that determine cardiac function. Such studies would benefit from recording naturally occurring neural activity to and from the brain, and are already starting to benefit from the artificial electrical stimulation of nerves in both experimental animals and preliminary trials in patients.
The appropriate use of mathematical modeling is an essential tool for gaining in-depth understanding of physiological function. Mathematical modeling is also essential for developing stimulators that selectively stimulate those nerves that control the function to be influenced. The determination of stimulation parameters, based on understanding rather than on trial and error, is highly desirable. The use of “intelligent” stimulators, automatically controlled by appropriate physiological measurements, is an ambitious but achievable goal for improving human health.
Biomedical engineering is a rapidly growing field, as indicated by a recent threefold increase in the number of students enrolled in the more than 80 programs granting biomedical engineering degrees.1 The field is known primarily for contributing to the development of devices that aid diagnosis (such as chemical sensors and medical imagers) or help to restore lost function (such as pacemakers, cochlear implants, and artificial limbs). At the same time, biomedical engineering has also contributed to the understanding of physiology and is now a participant in the more recent molecular- and cellular-based discoveries and their potential clinical applications.
This article reviews the contributions of biomedical engineering to heart-brain medicine and looks ahead to where its future contributions may be expected. The focus is on the autonomic control of the heart, with special emphasis on stimulating or blocking the activity of peripheral nerves for therapeutic purposes.
AUTONOMIC MECHANISMS
One way biomedical engineering has contributed to heart-brain medicine is through systems physiology, attempting to characterize complex cardiac control mechanisms by mathematical models ranging from the relatively simple to the very complex.2 In particular, investigation of the effect of the baroreceptor reflex (baroreflex) on heart rate led to recording of efferent vagal activity, demonstrating that the respiratory variations in heart rate are attributable to complete stoppage of vagal efferent activity, at least in anesthetized dogs.3 The results suggested that respiratory sinus arrhythmia was a measure of parasympathetic cardiac control.4 Extensive further work investigated heart rate variability in humans, resulting in the generally accepted concept that rapid variations in heart rate are primarily due to the parasympathetic nervous system, while slower variations are primarily due to sympathetic contributions. 5 It has been amply demonstrated that in a variety disease states, a high degree of parasympathetic control is correlated with improved outcome.6,7
Heart rate variability: Much is still unknown
Because heart rate variability is derived through analysis of easily recorded single-lead electrocardiograms (ECGs), techniques are still being described for determining the degree of variability.8–10 Measurements may be based on heart rate or heart period (interbeat interval); they may be made during spontaneous, deep, or timed periodic breathing; and the recordings may last for a few minutes or for 24 hours. The results are rarely reported for different states, yet variability depends on whether the subjects are awake, in quiet sleep, or in REM sleep.11
The present incomplete understanding of the physiological basis and clinical significance of heart rate variations makes it difficult to judge what the optimum measurement is. For example, recent publications have still debated whether the respiratory variations are primarily of central origin or a result of the baroreflex.12,13 The reviewed evidence leaves little doubt that most respiratory variations are caused by vagal modulation induced by breathing, independently of the baroreflex. On the other hand, breathing affects blood pressure and thus must have some influence on heart rate through the baroreflex as well. These effects are likely to be important when considering low-frequency variations.14 It also has been suggested that the branch of the vagus that contributes to the slow variations may be different from the one responsible for the rapid changes.15
A role for mathematical modeling
Untangling the above relationships requires at least an approximate physiologically based quantitative model of the entire system. What is the “entire system”? It includes all components that significantly affect the clinical condition or physiological/biological phenomenon studied. Developing such models is challenging since they can be misleading if they are too simple. However, if they are too complex, they may obscure rather than illuminate. Even though mathematical modeling has been a major component of biomedical engineering for decades, there is still great need for physiological and clinical studies that are aided by biomedical engineers with mathematical skills and a discerning eye toward the life sciences.
For example, following numerous previous efforts to develop models of cardiorespiratory control systems, a model has been developed to describe changes in heart rate, stroke volume, and blood pressure caused by disordered breathing during sleep.16 The model was initially tested in animals but recently has been evaluated in children with obstructive sleep apnea.17 It was found to be more effective in identifying and characterizing cardiac control abnormalities than was spectrum-derived variability of heart rate or blood pressure alone.
To enhance the usefulness of heart rate variability as a clinical tool, it is necessary to go beyond observing that decreased high-frequency heart rate variations are ominous in a particular disease state because there is an “imbalance” of autonomic control. This gives the physician little guidance as to what to do. Should he or she treat the brain to get more parasympathetic outflow? Should attention be concentrated on making the heart more tolerant to the imbalance? Or should both approaches be tried?
In addition to the building of models, biomedical engineers can also contribute to heart-brain medicine by developing sensors that can measure appropriate physiological variables in animal experiments—and eventually in humans. Such variables include neural activity and chemical signals controlling the heart, as well as neural and chemical signals that arise from the heart and are sensed by the brain. These variables must be included in any model for a comprehensive characterization of heart-brain interactions.
NEURAL INTERVENTIONS
A second and complementary way in which bioengineering can contribute to heart-brain medicine is through the development and evaluation of technology that applies selective electrical, chemical, or mechanical stimulation to the physiological system. External interventions may have therapeutic effects even though the underlying physiological mechanisms are not fully understood. For example, deep brain electrical stimulation is increasingly used or explored to treat epilepsy, Parkinson disease, and depression.18,19 The development of technology to deliver drugs locally is advancing rapidly and is almost certain to play a major role in exploring heart-brain interactions. The remainder of this paper concentrates on interventions applied through peripheral nerves.
Vagal stimulation
Vagal stimulation is being used to treat epilepsy, but now it is also being explored for the treatment of drug-resistant depression and chronic migraine.20,21 The intensity, frequency, pulse width, and train duration of the apparently single-channel stimulation are set telemetrically. Preliminary data indicate a long-term success rate of about 20% for depression20 and improvement in 2 of 4 patients with chronic migraine.21 Side effects include discomfort caused by the electrical stimulation and vocalization impairments.
The selective stimulation of nerves has received much attention in rehabilitation engineering since electrical activation of peripheral motor fibers can restore at least some function in patents with spinal cord injury. The stimulation must be selective: different muscles to restore movement need to contract in the appropriate sequence and with appropriate intensity. Thus, fascicles of motor nerves innervating these muscles must be stimulated accordingly.
Electrodes have been developed in a variety of configurations for selectively activating the desired fibers in a nerve bundle.22 Multiple electrodes around the nerve allow targeting of the desired fascicles and preferential stimulation of small nerve fibers.23,24 Gently compressing the nerve using a flat electrode sleeve enhances selectivity by increasing the surface area of the stimulated nerve.25 A tripolar sleeve electrode along the nerve (one cathode at the middle and an anode on each side) may be used to preferentially stimulate small fibers by “anodal blockade” of the propagation of activity in large fibers.26 Mathematical models of nerve excitation, combined with models of the tissue surrounding the nerve fibers, show that positioning the anodes at different distances from the cathode can generate unidirectional propagation.27 Mathematical analysis also shows that the stimulating pulse should have a slowly decaying trailing edge to assure effectiveness of the blockade.27,28
Similar technologies are likely necessary for stimulating the vagus for specific purposes. If the goal is to induce central effects, appropriate afferents should be stimulated. If the goal is to influence the heart directly, cardiac vagal efferents need to be stimulated without confounding the effect by also stimulating afferents. Since cardiac efferent fibers are small and constitute only a small portion of vagal trunk,3 their stimulation requires special care to reverse the normal “largest first” recruitment of stimulated fibers.
The recently reported first pilot study of vagal stimulation in heart failure patients used an electrode that seems to partially satisfy such requirements.29,30 Although the details appear to be proprietary, the implantable electrical stimulator has multiple electrodes and induces anodal blockade to preferentially stimulate efferent rather than afferent nerve fibers.
In the pilot study, 8 patients received intermittent vagal stimulation (2 to 10 sec “on” and 6 to 30 sec “off”) of the right cervical vagus using a pulse delivered 70 msec after each R wave of the ECG. The stimulating current, limited by a threshold or the onset of side effects, was adjusted to achieve a heart rate reduction of 5 to 10 beats/min. Patients were evaluated up to 6 months; no permanent side effects were reported. There was a modest improvement of cardiac function as judged by a reduction in left ventricular volumes, as well as a clear improvement in a measure of quality of life. The study shows feasibility and suggests further investigations.
The primary task seems to be optimization of stimulation parameters. Since natural vagal impulses are distributed through the cardiac cycle,3 artificial stimulation might be more effective if it emulated the natural firing pattern. Since long-term heart rate reduction was minimal, parameters might be tuned further to stimulate small cardiac efferent fibers that may be far from the electrodes. Measurements of heart rate variability during controlled conditions may reveal the pacemaker’s responsiveness to vagal stimulation. Comparison of duty cycles (duration of “on” and “off”) may show whether the study’s choice, to some extent already mimicking the breathing-induced modulation of natural vagal activity, is most effective. Such studies to optimize effectiveness may be best performed in chronically instrumented animals.
Baroreceptor stimulation
An extensively studied mechanism that modulates the autonomic nervous system is the baroreceptor reflex that is known to be depressed in heart failure. Former efforts to use this reflex therapeutically were recently revived in both animal experiments and human studies.31 For example, bilateral carotid sinus stimulation nearly doubled the survival time of dogs with pacing-induced heart failure.32 Although measures of left ventricular function, obtained while the stimulator was turned off, were similar in dogs with stimulated and unstimulated carotid sinuses, plasma norepinephrine was lower in the animals receiving stimulation. This suggests that carotid sinus stimulation led to a general decrease in sympathetic activation. It is noteworthy that the stimulation was applied intermittently (9 min “on”, 1 min “off”) to avoid the resetting (or adaptation) of baroreceptors, a phenomenon that had led to the now-doubted traditional belief that the baroreflex regulates only acute rather than long-term changes of blood pressure.2
Chronic bilateral baroreceptor stimulation was also applied in 21 patients with essential hypertension that could not be controlled by medication.33 Measurements were taken 1 month after implantation with the stimulator turned off and 3 months after chronic stimulation with the stimulator on. Stimulation moderately reduced both blood pressure and heart rate; heart rate variability suggested an increase in parasympathetic activity and a decrease in sympathetic activity.
Renal sympathetic ablation
Technology-based approaches encompass not only the stimulation of nerves but also the abolition of nerve activity. In an exploratory study, bilateral sympathetic denervation was performed in 45 patients with drugresistant hypertension.34 A catheter, introduced through the femoral artery, was positioned at the entrance of each renal artery. Sympathetic denervation was produced by radiofrequeny energy applied for a maximum of 2 minutes. The details of the technology appear proprietary, complete ablation could not be ascertained, and both efferents and afferents are likely to have been stimulated. Nevertheless, the procedure appeared safe and resulted in a significant reduction in both diastolic and systolic pressures over 12 months. In addition to lowering blood pressure, catheter-based sympathetic denervation might also prove therapeutic in heart failure and chronic kidney disease.
AUTOMATED CONTROL OF PHYSIOLOGIC VARIABLES
These initial experiences with baroreceptor stimulation, together with the results obtained by stimulating the vagus and ablating renal sympathetic nerves, indicate that device-based approaches may be useful additions to the treatment of drug-resistant hypertension and heart failure. The incorporation of control features that automatically respond to the changes in physiological states are natural extensions.35 As a recent example, in anesthetized dogs with heart failure and paced heart, systemic arterial pressure, cardiac output, and left atrial pressure were automatically regulated at set levels by a model-based infusion of nitroprusside, dobutamine, and volume expanders.36 When the heart rate was reduced, cardiac energetics (based on a reduction in left ventricular oxygen consumption) improved while the hemodynamic variables remained constant.
CONCLUSIONS
Biomedical engineering played a major role in linking measures of heart rate variation to sympathetic and parasympathetic contributions to cardiac control, as well as in demonstrating that the balance of control was correlated with a variety of disease states of heart and brain. However, much work remains to be done to fully realize the field’s potential for aiding clinicians in preventing or treating diseases. Further studies, some to be performed in chronically prepared animals, are needed to quantitatively characterize the many interacting mechanisms that determine cardiac function. Such studies would benefit from recording naturally occurring neural activity to and from the brain, and are already starting to benefit from the artificial electrical stimulation of nerves in both experimental animals and preliminary trials in patients.
The appropriate use of mathematical modeling is an essential tool for gaining in-depth understanding of physiological function. Mathematical modeling is also essential for developing stimulators that selectively stimulate those nerves that control the function to be influenced. The determination of stimulation parameters, based on understanding rather than on trial and error, is highly desirable. The use of “intelligent” stimulators, automatically controlled by appropriate physiological measurements, is an ambitious but achievable goal for improving human health.
- Katona PG. Biomedical engineering and The Whitaker Foundation: a thirty-year partnership. Ann Biomed Eng 2006; 34:904–916.
- Guyton AC, Coleman TG, Cowley AW, et al Systems analysis of arterial pressure regulation and hypertension. Ann Biomed Eng 1972; 1:254–281.
- Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol 1979; 218:1030–1037.
- Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol 1975; 39:801–805.
- Akselrod S, Gordon D, Ubel FA, et al Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 1981; 213:220–222.
- Carney RM, Freedland KE. Depression and heart rate variability in patients with coronary heart disease. Cleve Clin J Med 2009; 76( suppl 2):S13–S17.
- Lauer MS. Autonomic function and prognosis. Cleve Clin J Med 2009; 76( suppl 2):S18–S22.
- Cnockaert L, Migeotte P-F, Daubigny L, et al A method for the analysis of respiratory sinus arrhythmia using continuous wavelet transforms. IEEE Trans Biomed Eng 2008; 55:1640–1642.
- Castiglioni P, Parati G, Civijian A, et al Local scale exponents of blood pressure and heart rate variability by detrended fluctuation analysis: effects of posture, exercise, and aging. IEEE Trans Biomed Eng 2009; 56:675–684.
- Chen Z, Brown EN, Barbieri R. Assessment of autonomic control and respiratory sinus arrhythmia using point process models of human heart beat dynamics. IEEE Trans Biomed Eng 2009; 56:1791–1802.
- Schmitt DT, Stein PK, Ivanov PC. Stratification pattern of static and scale-invariant dynamic measures of heartbeat fluctuations across sleep stages in young and elderly. IEEE Trans Biomed Eng 2009; 56:1564–1573.
- Eckberg DL. Point:counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 2009; 16:1740–1742.
- Karemaker JM. Counterpoint: respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 2009; 106:1742–1743.
- Moak JP, Goldstein DS, Eldadah BA, et al Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm 2007; 4:1523–1529.
- Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med 2009; 76( suppl 2):S86–S90.
- Khoo MC. Modeling of autonomic control in sleep-disordered breathing. Cardiovasc Eng 2008; 8:30–41.
- Chaicharn J, Kin Z, Chen ML, et al Model-based assessment of cardiovascular autonomic control in children with obstructive sleep apnea. Sleep 2009; 32:927–938.
- Vitek JL. Deep brain stimulation: how does it work? Cleve Clin J Med 2008; 75( suppl 2):S59–S65.
- Lee KH, Blaha CD, Garrris PA. Evolution of deep brain stimulation: human electrometer and smart devices supporting the next generation of therapy. Neuromodulation: Technol Neural Interface 2009; 12:85–103.
- Fitzgerald PB, Daskalaris ZJ. The use of repetitive transcranial magnetic stimulation and vagal nerve stimulation in the treatment of depression. Curr Opin Psych 2008; 21:25–29.
- Cecchini AP, Mea E, Tullo V, et al Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci 2009; 30( suppl 1):S101–S104.
- Grill WM, Norman SE, Bellamkonda RV. Implanted neural interfaces: biochallenges and engineered solutions. Annu Rev Biomed Eng 2009; 11:1–24.
- Tarler MD, Mortimer JT. Selective and independent activation of four motor fascicles using a four-contact nerve-cuff electrode. IEEE Trans Neural Syst Rehab Eng 2004; 12:251–257.
- Lertmanorat Z, Gustafson KJ, Durand DM. Electrode array for reversing the recruitment order of peripheral nerve stimulation: experimental studies. Ann Biomed Eng 2006; 34:152–160.
- Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehab Eng 2002; 10:294–303.
- Fang ZP, Mortimer JT. Selective activation of small motor axons by quasitrapezoidal current pulses. IEEE Trans Biomed Eng 1991; 38:168–174.
- Rijkoff NJM, Holsheimer J, Koledwijn EL, et al Selective stimulation of sacral nerve roots for bladder control: a study in computer modeling. IEEE Trans Biomed Eng 1994; 41:413–424.
- Tosato M, Yoshida K, Toft E, et al Quazi-trapezoidal pulses to selectively block the activation of intrinsic laryngeal muscles during vagal nerve stimulation. J Neural Eng 2007; 41:205–212.
- Schwartz PK, De Ferrari GM, Sanzo A, et al Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Failure 2008; 10:884–891.
- De Ferrari GM, Sanzo A, Borggrefe M, et al Chronic vagus nerve stimulation in patients with chronic heart failure is feasible and appears beneficial. Circulation 2008; 118( suppl):S721. Abstract 2412.
- Durand MT, Fazan R, Salgado MCO, et al Acute and chronic electrical activation of baroreceptor afferents in awake and anesthetized subjects. Braz J Med Biol Res 2009; 42:53–60.
- Zucker IH, Hackley JF, Cornish KG, et al Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension 2007; 50:904–910.
- Wustman K, Kucera JP, Scheffers I, et al Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension 2009; 54:530–536.
- Krum H, Schlaih M, Whitbourn R, et al Catheter-based renal denervation for resistant hypertension: a multicentre safety and proof-of-principle study. Lancet 2009; 373:1228–1239.
- Kawada T, Sugamichi M. Artifical neural interfaces for bionic cardio vascular treatments. J Artific Organs 2009; 12:17–22.
- Uemura K, Sunagawa K, Sugimachi M. Computationally managed bradycardia improved cardiac energetics while restoring normal hemodynamics in heart failure. Ann Biomed Eng 2009; 37:82–93.
- Katona PG. Biomedical engineering and The Whitaker Foundation: a thirty-year partnership. Ann Biomed Eng 2006; 34:904–916.
- Guyton AC, Coleman TG, Cowley AW, et al Systems analysis of arterial pressure regulation and hypertension. Ann Biomed Eng 1972; 1:254–281.
- Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol 1979; 218:1030–1037.
- Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol 1975; 39:801–805.
- Akselrod S, Gordon D, Ubel FA, et al Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 1981; 213:220–222.
- Carney RM, Freedland KE. Depression and heart rate variability in patients with coronary heart disease. Cleve Clin J Med 2009; 76( suppl 2):S13–S17.
- Lauer MS. Autonomic function and prognosis. Cleve Clin J Med 2009; 76( suppl 2):S18–S22.
- Cnockaert L, Migeotte P-F, Daubigny L, et al A method for the analysis of respiratory sinus arrhythmia using continuous wavelet transforms. IEEE Trans Biomed Eng 2008; 55:1640–1642.
- Castiglioni P, Parati G, Civijian A, et al Local scale exponents of blood pressure and heart rate variability by detrended fluctuation analysis: effects of posture, exercise, and aging. IEEE Trans Biomed Eng 2009; 56:675–684.
- Chen Z, Brown EN, Barbieri R. Assessment of autonomic control and respiratory sinus arrhythmia using point process models of human heart beat dynamics. IEEE Trans Biomed Eng 2009; 56:1791–1802.
- Schmitt DT, Stein PK, Ivanov PC. Stratification pattern of static and scale-invariant dynamic measures of heartbeat fluctuations across sleep stages in young and elderly. IEEE Trans Biomed Eng 2009; 56:1564–1573.
- Eckberg DL. Point:counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 2009; 16:1740–1742.
- Karemaker JM. Counterpoint: respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol 2009; 106:1742–1743.
- Moak JP, Goldstein DS, Eldadah BA, et al Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm 2007; 4:1523–1529.
- Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med 2009; 76( suppl 2):S86–S90.
- Khoo MC. Modeling of autonomic control in sleep-disordered breathing. Cardiovasc Eng 2008; 8:30–41.
- Chaicharn J, Kin Z, Chen ML, et al Model-based assessment of cardiovascular autonomic control in children with obstructive sleep apnea. Sleep 2009; 32:927–938.
- Vitek JL. Deep brain stimulation: how does it work? Cleve Clin J Med 2008; 75( suppl 2):S59–S65.
- Lee KH, Blaha CD, Garrris PA. Evolution of deep brain stimulation: human electrometer and smart devices supporting the next generation of therapy. Neuromodulation: Technol Neural Interface 2009; 12:85–103.
- Fitzgerald PB, Daskalaris ZJ. The use of repetitive transcranial magnetic stimulation and vagal nerve stimulation in the treatment of depression. Curr Opin Psych 2008; 21:25–29.
- Cecchini AP, Mea E, Tullo V, et al Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci 2009; 30( suppl 1):S101–S104.
- Grill WM, Norman SE, Bellamkonda RV. Implanted neural interfaces: biochallenges and engineered solutions. Annu Rev Biomed Eng 2009; 11:1–24.
- Tarler MD, Mortimer JT. Selective and independent activation of four motor fascicles using a four-contact nerve-cuff electrode. IEEE Trans Neural Syst Rehab Eng 2004; 12:251–257.
- Lertmanorat Z, Gustafson KJ, Durand DM. Electrode array for reversing the recruitment order of peripheral nerve stimulation: experimental studies. Ann Biomed Eng 2006; 34:152–160.
- Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehab Eng 2002; 10:294–303.
- Fang ZP, Mortimer JT. Selective activation of small motor axons by quasitrapezoidal current pulses. IEEE Trans Biomed Eng 1991; 38:168–174.
- Rijkoff NJM, Holsheimer J, Koledwijn EL, et al Selective stimulation of sacral nerve roots for bladder control: a study in computer modeling. IEEE Trans Biomed Eng 1994; 41:413–424.
- Tosato M, Yoshida K, Toft E, et al Quazi-trapezoidal pulses to selectively block the activation of intrinsic laryngeal muscles during vagal nerve stimulation. J Neural Eng 2007; 41:205–212.
- Schwartz PK, De Ferrari GM, Sanzo A, et al Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Failure 2008; 10:884–891.
- De Ferrari GM, Sanzo A, Borggrefe M, et al Chronic vagus nerve stimulation in patients with chronic heart failure is feasible and appears beneficial. Circulation 2008; 118( suppl):S721. Abstract 2412.
- Durand MT, Fazan R, Salgado MCO, et al Acute and chronic electrical activation of baroreceptor afferents in awake and anesthetized subjects. Braz J Med Biol Res 2009; 42:53–60.
- Zucker IH, Hackley JF, Cornish KG, et al Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension 2007; 50:904–910.
- Wustman K, Kucera JP, Scheffers I, et al Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension 2009; 54:530–536.
- Krum H, Schlaih M, Whitbourn R, et al Catheter-based renal denervation for resistant hypertension: a multicentre safety and proof-of-principle study. Lancet 2009; 373:1228–1239.
- Kawada T, Sugamichi M. Artifical neural interfaces for bionic cardio vascular treatments. J Artific Organs 2009; 12:17–22.
- Uemura K, Sunagawa K, Sugimachi M. Computationally managed bradycardia improved cardiac energetics while restoring normal hemodynamics in heart failure. Ann Biomed Eng 2009; 37:82–93.