User login
Subphrenic abscess from a perforated duodenal ulcer
A 55-year-old man presented after 3 weeks of sharp epigastric pain radiating to the right upper quadrant, fever, and generalized weakness. He had a history of significant heroin and cocaine abuse and was currently in a methadone maintenance program. He was not taking any nonsteroidal anti-inflammatory drugs.
His temperature was 38.7°C (101.7°F). He had tenderness in the right upper quadrant but no rebound tenderness.
Laboratory studies revealed an elevated white blood cell count of 14.9 × 109/L (reference range 4.5–11.0) with 13% band cells, a normal lipase level, and normal liver function tests. Urine toxicology testing was positive for cocaine.
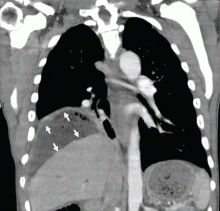
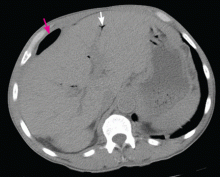
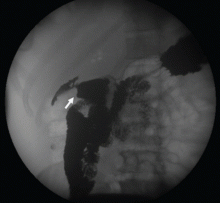
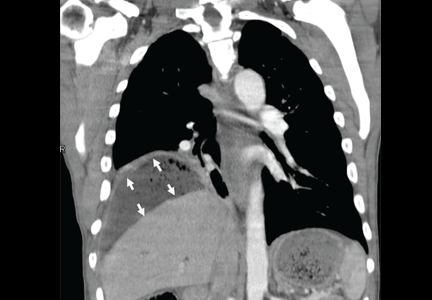
To investigate the cause of the abscess, we obtained an upper gastrointestinal radiographic series with oral contrast. This showed leaking of contrast from a perforated ulcer in the proximal duodenum (Figure 3).
TREATING PERFORATED ULCER AND ITS COMPLICATIONS
Perforated ulcers usually present with an acute abdomen and are life-threatening unless immediately recognized and treated. A more insidious presentation, as reported by Wong et al1 and as seen in our patient, presents additional diagnostic challenges. Our patient’s presentation may have been insidious and relatively benign because the perforation was not a free perforation (ie, the bowel contents were not freely spilling into the abdominal cavity), and because the spillage and inflammation were confined to an abscess cavity. Also, he was taking methadone 60 mg per day, which may have further masked the symptoms.
Perforated duodenal ulcers are usually treated surgically. Nonoperative management—ie, fluid resuscitation, nasogastric tube aspiration, and intravenous antibiotics—has been described,2 but the hemodynamic status and abdominal findings need to be continually monitored to detect any deterioration in the patient’s condition.
Initial management of the abscess with early percutaneous drainage and empiric intravenous antibiotics to cover enteric flora and anaerobes may suffice if there is no fistula.3 Also, in a series of 62 patients with subphrenic abscess managed by percutaneous drainage, Mueller et al4 found that this method was successful in most of the patients, noting that small-bowel perforation required the longest drainage period, generally more than 10 days.4
Helicobacter pylori infection and nonsteroidal anti-inflammatory drugs are responsible for the vast majority of duodenal ulcers. Serum H pylori antibody testing should be done, and if it is positive, treatment should be started. This patient did not have H pylori infection as an inpatient. He received metronidazole to cover the anaerobes and a proton pump inhibitor to treat the ulcer.
Although upper endoscopy was contraindicated in our patient because of his perforation, it was warranted for follow-up. Medical management with a proton pump inhibitor in high doses helped ulcer healing by reducing acid and gastric secretions. Octreotide had similar benefits, including reducing pancreatic and biliary secretion. This conservative management resulted in healing of the ulcer and early closure of the enteric fistula.
- Wong CH, Chow PK, Ong HS, Chan WH, Khin LW, Soo KC. Posterior perforation of peptic ulcers: presentation and outcome of an uncommon surgical emergency. Surgery 2004; 135:321–325.
- Berne TV, Donovan AJ. Nonoperative treatment of perforated duodenal ulcer. Arch Surg 1989; 124:830–832.
- Flancbaum L, Nosher JL, Brolin RE. Percutaneous catheter drainage of abdominal abscesses associated with perforated viscus. Am Surg 1990; 56:52–56.
- Mueller PR, Simeone JF, Butch RJ, et al. Percutaneous drainage of subphrenic abscess: a review of 62 patients. AJR Am J Roentgenol 1986; 147:1237–1240.
A 55-year-old man presented after 3 weeks of sharp epigastric pain radiating to the right upper quadrant, fever, and generalized weakness. He had a history of significant heroin and cocaine abuse and was currently in a methadone maintenance program. He was not taking any nonsteroidal anti-inflammatory drugs.
His temperature was 38.7°C (101.7°F). He had tenderness in the right upper quadrant but no rebound tenderness.
Laboratory studies revealed an elevated white blood cell count of 14.9 × 109/L (reference range 4.5–11.0) with 13% band cells, a normal lipase level, and normal liver function tests. Urine toxicology testing was positive for cocaine.
To investigate the cause of the abscess, we obtained an upper gastrointestinal radiographic series with oral contrast. This showed leaking of contrast from a perforated ulcer in the proximal duodenum (Figure 3).
TREATING PERFORATED ULCER AND ITS COMPLICATIONS
Perforated ulcers usually present with an acute abdomen and are life-threatening unless immediately recognized and treated. A more insidious presentation, as reported by Wong et al1 and as seen in our patient, presents additional diagnostic challenges. Our patient’s presentation may have been insidious and relatively benign because the perforation was not a free perforation (ie, the bowel contents were not freely spilling into the abdominal cavity), and because the spillage and inflammation were confined to an abscess cavity. Also, he was taking methadone 60 mg per day, which may have further masked the symptoms.
Perforated duodenal ulcers are usually treated surgically. Nonoperative management—ie, fluid resuscitation, nasogastric tube aspiration, and intravenous antibiotics—has been described,2 but the hemodynamic status and abdominal findings need to be continually monitored to detect any deterioration in the patient’s condition.
Initial management of the abscess with early percutaneous drainage and empiric intravenous antibiotics to cover enteric flora and anaerobes may suffice if there is no fistula.3 Also, in a series of 62 patients with subphrenic abscess managed by percutaneous drainage, Mueller et al4 found that this method was successful in most of the patients, noting that small-bowel perforation required the longest drainage period, generally more than 10 days.4
Helicobacter pylori infection and nonsteroidal anti-inflammatory drugs are responsible for the vast majority of duodenal ulcers. Serum H pylori antibody testing should be done, and if it is positive, treatment should be started. This patient did not have H pylori infection as an inpatient. He received metronidazole to cover the anaerobes and a proton pump inhibitor to treat the ulcer.
Although upper endoscopy was contraindicated in our patient because of his perforation, it was warranted for follow-up. Medical management with a proton pump inhibitor in high doses helped ulcer healing by reducing acid and gastric secretions. Octreotide had similar benefits, including reducing pancreatic and biliary secretion. This conservative management resulted in healing of the ulcer and early closure of the enteric fistula.
A 55-year-old man presented after 3 weeks of sharp epigastric pain radiating to the right upper quadrant, fever, and generalized weakness. He had a history of significant heroin and cocaine abuse and was currently in a methadone maintenance program. He was not taking any nonsteroidal anti-inflammatory drugs.
His temperature was 38.7°C (101.7°F). He had tenderness in the right upper quadrant but no rebound tenderness.
Laboratory studies revealed an elevated white blood cell count of 14.9 × 109/L (reference range 4.5–11.0) with 13% band cells, a normal lipase level, and normal liver function tests. Urine toxicology testing was positive for cocaine.
To investigate the cause of the abscess, we obtained an upper gastrointestinal radiographic series with oral contrast. This showed leaking of contrast from a perforated ulcer in the proximal duodenum (Figure 3).
TREATING PERFORATED ULCER AND ITS COMPLICATIONS
Perforated ulcers usually present with an acute abdomen and are life-threatening unless immediately recognized and treated. A more insidious presentation, as reported by Wong et al1 and as seen in our patient, presents additional diagnostic challenges. Our patient’s presentation may have been insidious and relatively benign because the perforation was not a free perforation (ie, the bowel contents were not freely spilling into the abdominal cavity), and because the spillage and inflammation were confined to an abscess cavity. Also, he was taking methadone 60 mg per day, which may have further masked the symptoms.
Perforated duodenal ulcers are usually treated surgically. Nonoperative management—ie, fluid resuscitation, nasogastric tube aspiration, and intravenous antibiotics—has been described,2 but the hemodynamic status and abdominal findings need to be continually monitored to detect any deterioration in the patient’s condition.
Initial management of the abscess with early percutaneous drainage and empiric intravenous antibiotics to cover enteric flora and anaerobes may suffice if there is no fistula.3 Also, in a series of 62 patients with subphrenic abscess managed by percutaneous drainage, Mueller et al4 found that this method was successful in most of the patients, noting that small-bowel perforation required the longest drainage period, generally more than 10 days.4
Helicobacter pylori infection and nonsteroidal anti-inflammatory drugs are responsible for the vast majority of duodenal ulcers. Serum H pylori antibody testing should be done, and if it is positive, treatment should be started. This patient did not have H pylori infection as an inpatient. He received metronidazole to cover the anaerobes and a proton pump inhibitor to treat the ulcer.
Although upper endoscopy was contraindicated in our patient because of his perforation, it was warranted for follow-up. Medical management with a proton pump inhibitor in high doses helped ulcer healing by reducing acid and gastric secretions. Octreotide had similar benefits, including reducing pancreatic and biliary secretion. This conservative management resulted in healing of the ulcer and early closure of the enteric fistula.
- Wong CH, Chow PK, Ong HS, Chan WH, Khin LW, Soo KC. Posterior perforation of peptic ulcers: presentation and outcome of an uncommon surgical emergency. Surgery 2004; 135:321–325.
- Berne TV, Donovan AJ. Nonoperative treatment of perforated duodenal ulcer. Arch Surg 1989; 124:830–832.
- Flancbaum L, Nosher JL, Brolin RE. Percutaneous catheter drainage of abdominal abscesses associated with perforated viscus. Am Surg 1990; 56:52–56.
- Mueller PR, Simeone JF, Butch RJ, et al. Percutaneous drainage of subphrenic abscess: a review of 62 patients. AJR Am J Roentgenol 1986; 147:1237–1240.
- Wong CH, Chow PK, Ong HS, Chan WH, Khin LW, Soo KC. Posterior perforation of peptic ulcers: presentation and outcome of an uncommon surgical emergency. Surgery 2004; 135:321–325.
- Berne TV, Donovan AJ. Nonoperative treatment of perforated duodenal ulcer. Arch Surg 1989; 124:830–832.
- Flancbaum L, Nosher JL, Brolin RE. Percutaneous catheter drainage of abdominal abscesses associated with perforated viscus. Am Surg 1990; 56:52–56.
- Mueller PR, Simeone JF, Butch RJ, et al. Percutaneous drainage of subphrenic abscess: a review of 62 patients. AJR Am J Roentgenol 1986; 147:1237–1240.
Short‐term Femoral Vein Catheterization
Central venous catheters (CVC) are routinely used to deliver medications and monitor intravascular pressures of critically ill patients. Experts and national regulatory bodies have questioned the safety of femoral vein catheterization (FVC), and currently recommend against venous access at this site whenever possible. 13 However, a large prospective nonrandomized study has suggested that rates of FVC infections are not higher than jugular or subclavian sites. 4 Some authors have suggested that increased risk of deep vein thrombosis (DVT) also relatively contraindicates the femoral site. 5 No study has prospectively examined rates of DVT in patients receiving FVC for short durations (72 hours). In this brief report, we prospectively examined the rates of catheter‐related bloodstream infections (CRBI) and DVT in critically ill patients receiving CVC.
Methods
This prospective observational cohort study was conducted in the medical intensive care unit (MICU) of Bridgeport Hospital, a 350‐bed community teaching hospital. The hospital's Institutional Review Board approved the study and waived the informed consent requirement because it has been the practice for the past decade to favor use of the femoral site for initial resuscitations with very low complication rates. All patients admitted to the MICU between September 1, 2008 and March 31, 2009 were eligible. VC were defined as catheters placed in the jugular, subclavian or femoral veins or peripherally inserted and guided to a central intrathoracic vein (PICC). CVC refers to catheters placed directly into central veins. In early 2008, a hospital‐wide initiative was introduced to insert all CVC using the Pronovost check‐list. 1 VC sites were chosen at the discretion of caregivers in the emergency department and MICU. The policy of our intensive care units is to use only saline flushes of VCs.
Demographic data including age, gender, and body mass index, were collected on all patients. In addition the following parameters were monitored for the duration of ICU stay for the purpose of this study: (1) site and duration of installation of all intravascular catheters, (2) level of training of clinician inserting CVC, (3) catheter/blood culture results. For the purposes of this study, bilateral femoral Doppler compression ultrasound studies were expected to be performed by radiology house officers within 24 hours of removing and again 5 to 7 days following removal of FVC. Local VC complications, methods of thromboprophylaxis and risk factors for venous thromboembolism (VTE) were recorded. Patient outcomes and disposition destinations were also recorded.
CRBI were defined using the Centers for Disease Control definitions. 2 CRBI were identified by daily review of all positive blood cultures and review of patients' medical records. In addition, Infection Control Committee data were reviewed to corroborate contemporaneously determined CRBI during the study period and for 1 year prior to the study period. Patients with FVC were examined each day for signs or symptoms of thrombosis (tenderness along the vein, leg swelling, pitting edema or visible collateral superficial veins). Patients were followed up until death or hospital discharge for clinical signs, symptoms or diagnosis of thromboembolic disease.
Bedside Duplex ultrasounds of bilateral common femoral and superficial femoral veins were performed using graded compression and color Doppler techniques. The leg without FVC served as the control. Evaluations were conducted by senior radiology residents (>100 hours training) utilizing a high‐resolution (>7.5 MHz) linear array transducer. Frame capture images were digitally stored and subsequently reviewed by a Board‐certified radiologist, who was blinded to side of insertion and clinical outcomes, and rendered a final interpretation.
Values are listed as means standard deviations. Comparisons of group means were performed using nonpaired Student's t tests. A P value of 0.05 signified statistical significance.
Results
During the study period, 675 patients were admitted to the MICU. VCs were inserted in 238 (35% of) patients. During their MICU stay, 182 (77% of) patients had 1 VC, 48 (20%) had 2 VC, and 8 (3%) had 3 VC. On admission, 38 patients (6%) had preexisting VC (tunneled catheter 58%, PICC 32%, and dialysis catheters 10%). Additional VCs were placed in 10 of these patients (26%).
Of the 302 VC, 85 (28%) were PICCs and 217 were CVC (107, 49% FVC; 82, 38% internal jugular; 28, 13% subclavian). A total of 151 (28%) patients had radial arterial catheters placed around the time of admission. The types of CVC included triple lumen in 164 (75%), dialysis catheters in 29 (13%), single‐lumen large bore catheters in 17 (8%), and tunneled catheters in 4 patients (2%). The average duration (standard deviation [SD]) of CVC was 2.7 2.2 days for FVC, 5.7 9.6 days for internal jugular and 3.6 3.1 days for subclavian vein catheters.
During these seven months, including 1200 catheter‐days, only 1 CRBI was identified in a patient who only had a PICC, yielding an infection rate of 0.83 CRBI per 1000 catheter‐days. No femoral, subclavian or internal jugular catheter infections were detected. Hospital epidemiologic data confirmed this finding, and demonstrated only 1 other CRBI during 3721 line‐days, in the 7 months of this study and 12 months before, yielding an average of 0.40 CRBI/1000 catheter‐days.
Of 107 FVC, 101 were placed during initial resuscitations and 6 as second‐access sites, (2 for dialysis, 4 triple lumen catheters). Thromboprophylaxis was administered to 104 (97% of) patients with FVC. Thromboprophylaxis was pharmacological (heparins) in 63 (59% of) patients and mechanical (pneumatic compression) in 46 (43%). Five patients had both mechanical and pharmacological prophylaxis. Catheters were placed by a critical care or emergency department attending in 11%, critical care fellows in 11%, and residents in 78%. Ultrasound studies of the legs were performed in 57 patients; 56 had studies within 24 hours of removing FVC. Of these 56 patients, 53 studies were interpreted as negative and 3 were considered incomplete. The 3 initially incomplete studies were repeated, and found to be negative. Six patients were discharged from the hospital before the post‐FVC‐removal ultrasound could be performed. Of the 50 patients who had both ultrasounds (initial and follow up 57 days after removal of FVC), none had a DVT on the side of the catheter or in the control leg. Of the 50 patients with no ultrasound follow‐up, no patient developed clinically detected VTE; these patients had FVC for shorter duration (2.4 2.4 vs. 3.4 1.9 days for those with 2 Duplex; P = 0.02) and their ICU length of stay was shorter (3.8 4.6 vs. 6.6 5.6 days for those with 2 Duplex; P = 0.01).
Since no VTE or CRBI were detected further analyses regarding risks for these complications was not possible.
Discussion
Contrary to regulatory guidelines suggesting a poor safety profile, we found that short‐term FVC was associated with no episode of DVT or CRBI. While the incidence of complications is lower in more experienced operators, 6 most FVC in our hospital were placed by resident‐trainees (78%) with or without supervision from an attending physician. There were no immediate or subacute (ie, thrombosis, infection) major complications. There are a number of features that favor short‐term FVC for initial resuscitation of critically ill patients. Subclavian and intrajugular CVC require prolonged Trendelenburg position, which may not be well tolerated by some patients. FVC does not require Trendelenburg position. Major bleeding1.0% to 1.5% for all the CVCis minimized because direct compression of femoral vessels is possible. Compression of subclavian hemorrhage is impossible while compression of the jugular vessels is uncomfortable. Pneumothorax, while uncommon in the subclavian and intrajugular approaches, 7 has serious consequences for an unstable patient, whereas FVC obviates the risk. Some might argue that FVC cannot accurately reflect cardiovascular filling thereby defeating 1 of the important purposes of the catheter. While this is certainly true in patients with raised intraabdominal pressures, a small case series suggests that (longer‐than‐normal) FVC can accurately measure central filling pressures. 8 Another potential shortcoming of FVC is that if used only for short durations during initial resuscitationsas in this studysome patients will require a second CVC or PICC with incumbent risks.
Our study differs from previous studies that have shown infection rates ranging from 1.5/1000 to 20/1000 catheter‐days 4, 9, 10 and thrombosis rates of 6.6% to 25%. 5, 1013 Some previous studies have suggested higher rates of infection of FVC relative to internal jugular or subclavian sites (3.7/1000 vs. 20/1000 catheter‐days) 9 while others found similar infection or colonization rates between femoral and nonfemoral sites. 4, 10 Our 0.83 CRBSI per 1000 catheter‐day rate is similar to that of Pronovost et al. 1 who avoided FVC, whereas it was the preferred site (nearly half of all CVC) in our MICU. The incidence of VTE in critically ill patients ranges from 9% to 33 %, 14, 15 and CVC are a well recognized risk factor of VTE. 5 The reported incidence of DVT in patients with CVC varies widely from 3% to 10% in subclavian catheters 9 to 6.6% to 25% in FVC. 11, 12 We attribute the remarkable difference in our results to the fact that FVC was used for brief durations (mean 2.7 days, range 116 days) for the primary purpose of resuscitating critically ill patients. Also, techniques introduced by Pronovost et al. 1 to reduce CRBI had permeated our institutional practices by the time of this study; our results match his, of very low rates of CRBI when checklists are employed. In previous studies, FVC was used for extended durations similar to other CVC sites (ranging from 4 to 9.6 days). 5, 9, 12, 13, 16 Additionally, almost all of our patients received VTE prophylaxis whereas rates were variable in previous studies.
This study has several limitations. First, catheter insertion sites were not randomly assigned. This can introduce selection bias. For example, often femoral access is used in more unstable patients 4 who are less tolerant of Trendelenberg position whereas it is often avoided in obese patients. Another important limitation is that ultrasound studies were not performed in 47% of patients who had FVC. While these missed cases were not advertent (eg, CVC on weekends when no study personnel available), we cannot exclude the possibility of bias. However, no FVC patients who did not have ultrasounds developed clinically detected VTE. It is also possible that DVT could have appeared >5 to 7 days after our follow‐up ultrasound, though later development might favor spontaneous DVT unrelated to CVC. Finally, this was a relatively small study, but it appears that the rate of DVT from FVC, if placed for short durations and accompanied by thromboprophylaxis, is very low.
In conclusion, short‐term FVC was used safelywith no major complicationsin our MICU. Our data support that short‐term FVC (with thromboprophylaxis) has a reasonable safety profile for initial resuscitation of critically ill patients. Notwithstanding the limitations of our study, we suggest that it may be premature to abandon entirely 3, 17 the use of FVC for resuscitation of critically ill patients. We propose that our data suggest the need for a larger study to examine more definitively the safety profile of short‐term FVC.
- , , , et al. An intervention to decrease catheter related bloodstream infections in the ICU. N Engl J Med. 2006; 355: 2725– 2732.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infection. MMWR Website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5110a1.htm. Accessed February 2010.
- Joint Commission. National Accreditation: Hospital Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4‐423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed February 2010.
- , , , et al. The incidence of infectious complications of central venous catheters at the subclavian, internal jugular and femoral sites in an intensive care unit population. Crit Care Med. 2005; 33: 13– 20.
- , , , . Femoral deep vein thrombosis associated with central venous catheterization: Results from a prospective, randomized trial. Crit Care Med. 1995; 23: 52– 59.
- , , , , . Central vein catheterization. Failure and complicagtion rates by three percutaneous approaches. Arch Intern Med. 1986; 146: 259– 261.
- , , . Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002; 30: 454– 460.
- , , , , , . Comparison of intrathoracic and intra‐abdominal measurements of central venous pressure. Lancet. 1996; 347: 1155– 1157.
- , , , et al. Complications of femoral and subclavian venous catheterization in critically ill patients. A randomized controlled trial. JAMA. 2001; 286: 700– 707.
- , , , et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy. A randomized trial. JAMA. 2008; 299: 2413– 2422.
- , , , , , . A prospective evaluation of the use of femoral venous catheters in critically ill adults. Crit Care Med. 1997; 25: 1986– 1989.
- , , , , . Lower extremity deep vein thrombosis: a prospective, randomized, controlled trial in comatose or sedated patients undergoing femoral vein catheterization. Crit Care Med. 1997; 25: 1982– 1985.
- , , , , . Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients. Chest. 2000; 117: 178– 183.
- , , . The incidence of deep venous thrombosis in ICU patients. Chest. 1997; 111: 661– 664.
- , , , et al. Deep venous thrombosis in medical‐surgical critically il patients: prevalence, incidence and risk factors. Crit Care Med. 2005; 33: 1565– 1571.
- , , , et al. Central vein catheter related thrombosis in intensive care patients: incidence, risk factors and relationship with catheter related sepsis. Chest. 1998; 114: 207– 213.
- Institute for Healthcare Improvement. Optimal catheter site selection, with avoidance of the femoral vein for central venous access in adults. Available at: http://www.ihi.org/IHI/Topics/CriticalCare/IntensiveCare/Changes/IndividualChanges/OptimalCatheterSiteSelectionwithAvoidanceofFemoralVeinforCentralVenousAccessinAdultPatients.htm. Accessed February 2010.
Central venous catheters (CVC) are routinely used to deliver medications and monitor intravascular pressures of critically ill patients. Experts and national regulatory bodies have questioned the safety of femoral vein catheterization (FVC), and currently recommend against venous access at this site whenever possible. 13 However, a large prospective nonrandomized study has suggested that rates of FVC infections are not higher than jugular or subclavian sites. 4 Some authors have suggested that increased risk of deep vein thrombosis (DVT) also relatively contraindicates the femoral site. 5 No study has prospectively examined rates of DVT in patients receiving FVC for short durations (72 hours). In this brief report, we prospectively examined the rates of catheter‐related bloodstream infections (CRBI) and DVT in critically ill patients receiving CVC.
Methods
This prospective observational cohort study was conducted in the medical intensive care unit (MICU) of Bridgeport Hospital, a 350‐bed community teaching hospital. The hospital's Institutional Review Board approved the study and waived the informed consent requirement because it has been the practice for the past decade to favor use of the femoral site for initial resuscitations with very low complication rates. All patients admitted to the MICU between September 1, 2008 and March 31, 2009 were eligible. VC were defined as catheters placed in the jugular, subclavian or femoral veins or peripherally inserted and guided to a central intrathoracic vein (PICC). CVC refers to catheters placed directly into central veins. In early 2008, a hospital‐wide initiative was introduced to insert all CVC using the Pronovost check‐list. 1 VC sites were chosen at the discretion of caregivers in the emergency department and MICU. The policy of our intensive care units is to use only saline flushes of VCs.
Demographic data including age, gender, and body mass index, were collected on all patients. In addition the following parameters were monitored for the duration of ICU stay for the purpose of this study: (1) site and duration of installation of all intravascular catheters, (2) level of training of clinician inserting CVC, (3) catheter/blood culture results. For the purposes of this study, bilateral femoral Doppler compression ultrasound studies were expected to be performed by radiology house officers within 24 hours of removing and again 5 to 7 days following removal of FVC. Local VC complications, methods of thromboprophylaxis and risk factors for venous thromboembolism (VTE) were recorded. Patient outcomes and disposition destinations were also recorded.
CRBI were defined using the Centers for Disease Control definitions. 2 CRBI were identified by daily review of all positive blood cultures and review of patients' medical records. In addition, Infection Control Committee data were reviewed to corroborate contemporaneously determined CRBI during the study period and for 1 year prior to the study period. Patients with FVC were examined each day for signs or symptoms of thrombosis (tenderness along the vein, leg swelling, pitting edema or visible collateral superficial veins). Patients were followed up until death or hospital discharge for clinical signs, symptoms or diagnosis of thromboembolic disease.
Bedside Duplex ultrasounds of bilateral common femoral and superficial femoral veins were performed using graded compression and color Doppler techniques. The leg without FVC served as the control. Evaluations were conducted by senior radiology residents (>100 hours training) utilizing a high‐resolution (>7.5 MHz) linear array transducer. Frame capture images were digitally stored and subsequently reviewed by a Board‐certified radiologist, who was blinded to side of insertion and clinical outcomes, and rendered a final interpretation.
Values are listed as means standard deviations. Comparisons of group means were performed using nonpaired Student's t tests. A P value of 0.05 signified statistical significance.
Results
During the study period, 675 patients were admitted to the MICU. VCs were inserted in 238 (35% of) patients. During their MICU stay, 182 (77% of) patients had 1 VC, 48 (20%) had 2 VC, and 8 (3%) had 3 VC. On admission, 38 patients (6%) had preexisting VC (tunneled catheter 58%, PICC 32%, and dialysis catheters 10%). Additional VCs were placed in 10 of these patients (26%).
Of the 302 VC, 85 (28%) were PICCs and 217 were CVC (107, 49% FVC; 82, 38% internal jugular; 28, 13% subclavian). A total of 151 (28%) patients had radial arterial catheters placed around the time of admission. The types of CVC included triple lumen in 164 (75%), dialysis catheters in 29 (13%), single‐lumen large bore catheters in 17 (8%), and tunneled catheters in 4 patients (2%). The average duration (standard deviation [SD]) of CVC was 2.7 2.2 days for FVC, 5.7 9.6 days for internal jugular and 3.6 3.1 days for subclavian vein catheters.
During these seven months, including 1200 catheter‐days, only 1 CRBI was identified in a patient who only had a PICC, yielding an infection rate of 0.83 CRBI per 1000 catheter‐days. No femoral, subclavian or internal jugular catheter infections were detected. Hospital epidemiologic data confirmed this finding, and demonstrated only 1 other CRBI during 3721 line‐days, in the 7 months of this study and 12 months before, yielding an average of 0.40 CRBI/1000 catheter‐days.
Of 107 FVC, 101 were placed during initial resuscitations and 6 as second‐access sites, (2 for dialysis, 4 triple lumen catheters). Thromboprophylaxis was administered to 104 (97% of) patients with FVC. Thromboprophylaxis was pharmacological (heparins) in 63 (59% of) patients and mechanical (pneumatic compression) in 46 (43%). Five patients had both mechanical and pharmacological prophylaxis. Catheters were placed by a critical care or emergency department attending in 11%, critical care fellows in 11%, and residents in 78%. Ultrasound studies of the legs were performed in 57 patients; 56 had studies within 24 hours of removing FVC. Of these 56 patients, 53 studies were interpreted as negative and 3 were considered incomplete. The 3 initially incomplete studies were repeated, and found to be negative. Six patients were discharged from the hospital before the post‐FVC‐removal ultrasound could be performed. Of the 50 patients who had both ultrasounds (initial and follow up 57 days after removal of FVC), none had a DVT on the side of the catheter or in the control leg. Of the 50 patients with no ultrasound follow‐up, no patient developed clinically detected VTE; these patients had FVC for shorter duration (2.4 2.4 vs. 3.4 1.9 days for those with 2 Duplex; P = 0.02) and their ICU length of stay was shorter (3.8 4.6 vs. 6.6 5.6 days for those with 2 Duplex; P = 0.01).
Since no VTE or CRBI were detected further analyses regarding risks for these complications was not possible.
Discussion
Contrary to regulatory guidelines suggesting a poor safety profile, we found that short‐term FVC was associated with no episode of DVT or CRBI. While the incidence of complications is lower in more experienced operators, 6 most FVC in our hospital were placed by resident‐trainees (78%) with or without supervision from an attending physician. There were no immediate or subacute (ie, thrombosis, infection) major complications. There are a number of features that favor short‐term FVC for initial resuscitation of critically ill patients. Subclavian and intrajugular CVC require prolonged Trendelenburg position, which may not be well tolerated by some patients. FVC does not require Trendelenburg position. Major bleeding1.0% to 1.5% for all the CVCis minimized because direct compression of femoral vessels is possible. Compression of subclavian hemorrhage is impossible while compression of the jugular vessels is uncomfortable. Pneumothorax, while uncommon in the subclavian and intrajugular approaches, 7 has serious consequences for an unstable patient, whereas FVC obviates the risk. Some might argue that FVC cannot accurately reflect cardiovascular filling thereby defeating 1 of the important purposes of the catheter. While this is certainly true in patients with raised intraabdominal pressures, a small case series suggests that (longer‐than‐normal) FVC can accurately measure central filling pressures. 8 Another potential shortcoming of FVC is that if used only for short durations during initial resuscitationsas in this studysome patients will require a second CVC or PICC with incumbent risks.
Our study differs from previous studies that have shown infection rates ranging from 1.5/1000 to 20/1000 catheter‐days 4, 9, 10 and thrombosis rates of 6.6% to 25%. 5, 1013 Some previous studies have suggested higher rates of infection of FVC relative to internal jugular or subclavian sites (3.7/1000 vs. 20/1000 catheter‐days) 9 while others found similar infection or colonization rates between femoral and nonfemoral sites. 4, 10 Our 0.83 CRBSI per 1000 catheter‐day rate is similar to that of Pronovost et al. 1 who avoided FVC, whereas it was the preferred site (nearly half of all CVC) in our MICU. The incidence of VTE in critically ill patients ranges from 9% to 33 %, 14, 15 and CVC are a well recognized risk factor of VTE. 5 The reported incidence of DVT in patients with CVC varies widely from 3% to 10% in subclavian catheters 9 to 6.6% to 25% in FVC. 11, 12 We attribute the remarkable difference in our results to the fact that FVC was used for brief durations (mean 2.7 days, range 116 days) for the primary purpose of resuscitating critically ill patients. Also, techniques introduced by Pronovost et al. 1 to reduce CRBI had permeated our institutional practices by the time of this study; our results match his, of very low rates of CRBI when checklists are employed. In previous studies, FVC was used for extended durations similar to other CVC sites (ranging from 4 to 9.6 days). 5, 9, 12, 13, 16 Additionally, almost all of our patients received VTE prophylaxis whereas rates were variable in previous studies.
This study has several limitations. First, catheter insertion sites were not randomly assigned. This can introduce selection bias. For example, often femoral access is used in more unstable patients 4 who are less tolerant of Trendelenberg position whereas it is often avoided in obese patients. Another important limitation is that ultrasound studies were not performed in 47% of patients who had FVC. While these missed cases were not advertent (eg, CVC on weekends when no study personnel available), we cannot exclude the possibility of bias. However, no FVC patients who did not have ultrasounds developed clinically detected VTE. It is also possible that DVT could have appeared >5 to 7 days after our follow‐up ultrasound, though later development might favor spontaneous DVT unrelated to CVC. Finally, this was a relatively small study, but it appears that the rate of DVT from FVC, if placed for short durations and accompanied by thromboprophylaxis, is very low.
In conclusion, short‐term FVC was used safelywith no major complicationsin our MICU. Our data support that short‐term FVC (with thromboprophylaxis) has a reasonable safety profile for initial resuscitation of critically ill patients. Notwithstanding the limitations of our study, we suggest that it may be premature to abandon entirely 3, 17 the use of FVC for resuscitation of critically ill patients. We propose that our data suggest the need for a larger study to examine more definitively the safety profile of short‐term FVC.
Central venous catheters (CVC) are routinely used to deliver medications and monitor intravascular pressures of critically ill patients. Experts and national regulatory bodies have questioned the safety of femoral vein catheterization (FVC), and currently recommend against venous access at this site whenever possible. 13 However, a large prospective nonrandomized study has suggested that rates of FVC infections are not higher than jugular or subclavian sites. 4 Some authors have suggested that increased risk of deep vein thrombosis (DVT) also relatively contraindicates the femoral site. 5 No study has prospectively examined rates of DVT in patients receiving FVC for short durations (72 hours). In this brief report, we prospectively examined the rates of catheter‐related bloodstream infections (CRBI) and DVT in critically ill patients receiving CVC.
Methods
This prospective observational cohort study was conducted in the medical intensive care unit (MICU) of Bridgeport Hospital, a 350‐bed community teaching hospital. The hospital's Institutional Review Board approved the study and waived the informed consent requirement because it has been the practice for the past decade to favor use of the femoral site for initial resuscitations with very low complication rates. All patients admitted to the MICU between September 1, 2008 and March 31, 2009 were eligible. VC were defined as catheters placed in the jugular, subclavian or femoral veins or peripherally inserted and guided to a central intrathoracic vein (PICC). CVC refers to catheters placed directly into central veins. In early 2008, a hospital‐wide initiative was introduced to insert all CVC using the Pronovost check‐list. 1 VC sites were chosen at the discretion of caregivers in the emergency department and MICU. The policy of our intensive care units is to use only saline flushes of VCs.
Demographic data including age, gender, and body mass index, were collected on all patients. In addition the following parameters were monitored for the duration of ICU stay for the purpose of this study: (1) site and duration of installation of all intravascular catheters, (2) level of training of clinician inserting CVC, (3) catheter/blood culture results. For the purposes of this study, bilateral femoral Doppler compression ultrasound studies were expected to be performed by radiology house officers within 24 hours of removing and again 5 to 7 days following removal of FVC. Local VC complications, methods of thromboprophylaxis and risk factors for venous thromboembolism (VTE) were recorded. Patient outcomes and disposition destinations were also recorded.
CRBI were defined using the Centers for Disease Control definitions. 2 CRBI were identified by daily review of all positive blood cultures and review of patients' medical records. In addition, Infection Control Committee data were reviewed to corroborate contemporaneously determined CRBI during the study period and for 1 year prior to the study period. Patients with FVC were examined each day for signs or symptoms of thrombosis (tenderness along the vein, leg swelling, pitting edema or visible collateral superficial veins). Patients were followed up until death or hospital discharge for clinical signs, symptoms or diagnosis of thromboembolic disease.
Bedside Duplex ultrasounds of bilateral common femoral and superficial femoral veins were performed using graded compression and color Doppler techniques. The leg without FVC served as the control. Evaluations were conducted by senior radiology residents (>100 hours training) utilizing a high‐resolution (>7.5 MHz) linear array transducer. Frame capture images were digitally stored and subsequently reviewed by a Board‐certified radiologist, who was blinded to side of insertion and clinical outcomes, and rendered a final interpretation.
Values are listed as means standard deviations. Comparisons of group means were performed using nonpaired Student's t tests. A P value of 0.05 signified statistical significance.
Results
During the study period, 675 patients were admitted to the MICU. VCs were inserted in 238 (35% of) patients. During their MICU stay, 182 (77% of) patients had 1 VC, 48 (20%) had 2 VC, and 8 (3%) had 3 VC. On admission, 38 patients (6%) had preexisting VC (tunneled catheter 58%, PICC 32%, and dialysis catheters 10%). Additional VCs were placed in 10 of these patients (26%).
Of the 302 VC, 85 (28%) were PICCs and 217 were CVC (107, 49% FVC; 82, 38% internal jugular; 28, 13% subclavian). A total of 151 (28%) patients had radial arterial catheters placed around the time of admission. The types of CVC included triple lumen in 164 (75%), dialysis catheters in 29 (13%), single‐lumen large bore catheters in 17 (8%), and tunneled catheters in 4 patients (2%). The average duration (standard deviation [SD]) of CVC was 2.7 2.2 days for FVC, 5.7 9.6 days for internal jugular and 3.6 3.1 days for subclavian vein catheters.
During these seven months, including 1200 catheter‐days, only 1 CRBI was identified in a patient who only had a PICC, yielding an infection rate of 0.83 CRBI per 1000 catheter‐days. No femoral, subclavian or internal jugular catheter infections were detected. Hospital epidemiologic data confirmed this finding, and demonstrated only 1 other CRBI during 3721 line‐days, in the 7 months of this study and 12 months before, yielding an average of 0.40 CRBI/1000 catheter‐days.
Of 107 FVC, 101 were placed during initial resuscitations and 6 as second‐access sites, (2 for dialysis, 4 triple lumen catheters). Thromboprophylaxis was administered to 104 (97% of) patients with FVC. Thromboprophylaxis was pharmacological (heparins) in 63 (59% of) patients and mechanical (pneumatic compression) in 46 (43%). Five patients had both mechanical and pharmacological prophylaxis. Catheters were placed by a critical care or emergency department attending in 11%, critical care fellows in 11%, and residents in 78%. Ultrasound studies of the legs were performed in 57 patients; 56 had studies within 24 hours of removing FVC. Of these 56 patients, 53 studies were interpreted as negative and 3 were considered incomplete. The 3 initially incomplete studies were repeated, and found to be negative. Six patients were discharged from the hospital before the post‐FVC‐removal ultrasound could be performed. Of the 50 patients who had both ultrasounds (initial and follow up 57 days after removal of FVC), none had a DVT on the side of the catheter or in the control leg. Of the 50 patients with no ultrasound follow‐up, no patient developed clinically detected VTE; these patients had FVC for shorter duration (2.4 2.4 vs. 3.4 1.9 days for those with 2 Duplex; P = 0.02) and their ICU length of stay was shorter (3.8 4.6 vs. 6.6 5.6 days for those with 2 Duplex; P = 0.01).
Since no VTE or CRBI were detected further analyses regarding risks for these complications was not possible.
Discussion
Contrary to regulatory guidelines suggesting a poor safety profile, we found that short‐term FVC was associated with no episode of DVT or CRBI. While the incidence of complications is lower in more experienced operators, 6 most FVC in our hospital were placed by resident‐trainees (78%) with or without supervision from an attending physician. There were no immediate or subacute (ie, thrombosis, infection) major complications. There are a number of features that favor short‐term FVC for initial resuscitation of critically ill patients. Subclavian and intrajugular CVC require prolonged Trendelenburg position, which may not be well tolerated by some patients. FVC does not require Trendelenburg position. Major bleeding1.0% to 1.5% for all the CVCis minimized because direct compression of femoral vessels is possible. Compression of subclavian hemorrhage is impossible while compression of the jugular vessels is uncomfortable. Pneumothorax, while uncommon in the subclavian and intrajugular approaches, 7 has serious consequences for an unstable patient, whereas FVC obviates the risk. Some might argue that FVC cannot accurately reflect cardiovascular filling thereby defeating 1 of the important purposes of the catheter. While this is certainly true in patients with raised intraabdominal pressures, a small case series suggests that (longer‐than‐normal) FVC can accurately measure central filling pressures. 8 Another potential shortcoming of FVC is that if used only for short durations during initial resuscitationsas in this studysome patients will require a second CVC or PICC with incumbent risks.
Our study differs from previous studies that have shown infection rates ranging from 1.5/1000 to 20/1000 catheter‐days 4, 9, 10 and thrombosis rates of 6.6% to 25%. 5, 1013 Some previous studies have suggested higher rates of infection of FVC relative to internal jugular or subclavian sites (3.7/1000 vs. 20/1000 catheter‐days) 9 while others found similar infection or colonization rates between femoral and nonfemoral sites. 4, 10 Our 0.83 CRBSI per 1000 catheter‐day rate is similar to that of Pronovost et al. 1 who avoided FVC, whereas it was the preferred site (nearly half of all CVC) in our MICU. The incidence of VTE in critically ill patients ranges from 9% to 33 %, 14, 15 and CVC are a well recognized risk factor of VTE. 5 The reported incidence of DVT in patients with CVC varies widely from 3% to 10% in subclavian catheters 9 to 6.6% to 25% in FVC. 11, 12 We attribute the remarkable difference in our results to the fact that FVC was used for brief durations (mean 2.7 days, range 116 days) for the primary purpose of resuscitating critically ill patients. Also, techniques introduced by Pronovost et al. 1 to reduce CRBI had permeated our institutional practices by the time of this study; our results match his, of very low rates of CRBI when checklists are employed. In previous studies, FVC was used for extended durations similar to other CVC sites (ranging from 4 to 9.6 days). 5, 9, 12, 13, 16 Additionally, almost all of our patients received VTE prophylaxis whereas rates were variable in previous studies.
This study has several limitations. First, catheter insertion sites were not randomly assigned. This can introduce selection bias. For example, often femoral access is used in more unstable patients 4 who are less tolerant of Trendelenberg position whereas it is often avoided in obese patients. Another important limitation is that ultrasound studies were not performed in 47% of patients who had FVC. While these missed cases were not advertent (eg, CVC on weekends when no study personnel available), we cannot exclude the possibility of bias. However, no FVC patients who did not have ultrasounds developed clinically detected VTE. It is also possible that DVT could have appeared >5 to 7 days after our follow‐up ultrasound, though later development might favor spontaneous DVT unrelated to CVC. Finally, this was a relatively small study, but it appears that the rate of DVT from FVC, if placed for short durations and accompanied by thromboprophylaxis, is very low.
In conclusion, short‐term FVC was used safelywith no major complicationsin our MICU. Our data support that short‐term FVC (with thromboprophylaxis) has a reasonable safety profile for initial resuscitation of critically ill patients. Notwithstanding the limitations of our study, we suggest that it may be premature to abandon entirely 3, 17 the use of FVC for resuscitation of critically ill patients. We propose that our data suggest the need for a larger study to examine more definitively the safety profile of short‐term FVC.
- , , , et al. An intervention to decrease catheter related bloodstream infections in the ICU. N Engl J Med. 2006; 355: 2725– 2732.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infection. MMWR Website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5110a1.htm. Accessed February 2010.
- Joint Commission. National Accreditation: Hospital Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4‐423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed February 2010.
- , , , et al. The incidence of infectious complications of central venous catheters at the subclavian, internal jugular and femoral sites in an intensive care unit population. Crit Care Med. 2005; 33: 13– 20.
- , , , . Femoral deep vein thrombosis associated with central venous catheterization: Results from a prospective, randomized trial. Crit Care Med. 1995; 23: 52– 59.
- , , , , . Central vein catheterization. Failure and complicagtion rates by three percutaneous approaches. Arch Intern Med. 1986; 146: 259– 261.
- , , . Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002; 30: 454– 460.
- , , , , , . Comparison of intrathoracic and intra‐abdominal measurements of central venous pressure. Lancet. 1996; 347: 1155– 1157.
- , , , et al. Complications of femoral and subclavian venous catheterization in critically ill patients. A randomized controlled trial. JAMA. 2001; 286: 700– 707.
- , , , et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy. A randomized trial. JAMA. 2008; 299: 2413– 2422.
- , , , , , . A prospective evaluation of the use of femoral venous catheters in critically ill adults. Crit Care Med. 1997; 25: 1986– 1989.
- , , , , . Lower extremity deep vein thrombosis: a prospective, randomized, controlled trial in comatose or sedated patients undergoing femoral vein catheterization. Crit Care Med. 1997; 25: 1982– 1985.
- , , , , . Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients. Chest. 2000; 117: 178– 183.
- , , . The incidence of deep venous thrombosis in ICU patients. Chest. 1997; 111: 661– 664.
- , , , et al. Deep venous thrombosis in medical‐surgical critically il patients: prevalence, incidence and risk factors. Crit Care Med. 2005; 33: 1565– 1571.
- , , , et al. Central vein catheter related thrombosis in intensive care patients: incidence, risk factors and relationship with catheter related sepsis. Chest. 1998; 114: 207– 213.
- Institute for Healthcare Improvement. Optimal catheter site selection, with avoidance of the femoral vein for central venous access in adults. Available at: http://www.ihi.org/IHI/Topics/CriticalCare/IntensiveCare/Changes/IndividualChanges/OptimalCatheterSiteSelectionwithAvoidanceofFemoralVeinforCentralVenousAccessinAdultPatients.htm. Accessed February 2010.
- , , , et al. An intervention to decrease catheter related bloodstream infections in the ICU. N Engl J Med. 2006; 355: 2725– 2732.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infection. MMWR Website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5110a1.htm. Accessed February 2010.
- Joint Commission. National Accreditation: Hospital Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4‐423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed February 2010.
- , , , et al. The incidence of infectious complications of central venous catheters at the subclavian, internal jugular and femoral sites in an intensive care unit population. Crit Care Med. 2005; 33: 13– 20.
- , , , . Femoral deep vein thrombosis associated with central venous catheterization: Results from a prospective, randomized trial. Crit Care Med. 1995; 23: 52– 59.
- , , , , . Central vein catheterization. Failure and complicagtion rates by three percutaneous approaches. Arch Intern Med. 1986; 146: 259– 261.
- , , . Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002; 30: 454– 460.
- , , , , , . Comparison of intrathoracic and intra‐abdominal measurements of central venous pressure. Lancet. 1996; 347: 1155– 1157.
- , , , et al. Complications of femoral and subclavian venous catheterization in critically ill patients. A randomized controlled trial. JAMA. 2001; 286: 700– 707.
- , , , et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy. A randomized trial. JAMA. 2008; 299: 2413– 2422.
- , , , , , . A prospective evaluation of the use of femoral venous catheters in critically ill adults. Crit Care Med. 1997; 25: 1986– 1989.
- , , , , . Lower extremity deep vein thrombosis: a prospective, randomized, controlled trial in comatose or sedated patients undergoing femoral vein catheterization. Crit Care Med. 1997; 25: 1982– 1985.
- , , , , . Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients. Chest. 2000; 117: 178– 183.
- , , . The incidence of deep venous thrombosis in ICU patients. Chest. 1997; 111: 661– 664.
- , , , et al. Deep venous thrombosis in medical‐surgical critically il patients: prevalence, incidence and risk factors. Crit Care Med. 2005; 33: 1565– 1571.
- , , , et al. Central vein catheter related thrombosis in intensive care patients: incidence, risk factors and relationship with catheter related sepsis. Chest. 1998; 114: 207– 213.
- Institute for Healthcare Improvement. Optimal catheter site selection, with avoidance of the femoral vein for central venous access in adults. Available at: http://www.ihi.org/IHI/Topics/CriticalCare/IntensiveCare/Changes/IndividualChanges/OptimalCatheterSiteSelectionwithAvoidanceofFemoralVeinforCentralVenousAccessinAdultPatients.htm. Accessed February 2010.